Note: Descriptions are shown in the official language in which they were submitted.
CA 02923454 2016-02-03
WO 2016/010518
PCT/US2014/046649
FLUID MOBILITY MODIFIERS FOR INCREASED PRODUCTION IN
SUBTERRANEAN FORMATIONS
BACKGROUND
[0001] The embodiments herein relate to fluid mobility modifiers for
increased production in subterranean formation operations.
[0002] Subterranean wells (such as hydrocarbon or natural gas
producing wells) may be porous and permeable, affecting their ability to store
hydrocarbons (e.g., oil or natural gas) and the facility with which they can
be
extracted from the formation. To improve recovery of hydrocarbons, such
subterranean wells are often stimulated by hydraulic fracturing treatments. In
hydraulic fracturing treatments, a viscous fracturing fluid, which may also
function as a carrier fluid, is pumped into a portion of a subterranean
formation
at a rate and pressure such that the subterranean formation breaks down and
one or more fractures are formed. Typically, particulates, such as graded
sand,
are suspended in a portion of the fracturing fluid or another fluid and then
deposited into the fractures. These particulates, referred to herein as
"proppant
particulates" or simply "proppant," serve to prevent the fractures from fully
closing once the hydraulic pressure is removed. By keeping the fracture from
fully closing, the proppant aids in forming conductive paths through which
fluids
may flow. As used herein, the term "fluid" refers to a substance that is
capable
of flowing, including particulate solids, liquids, and gases.
[0003] Fracturing fluids are often viscosified using chemicals, such as
gelling agents (e.g., polymers) or gelling agents in combination with
crosslinking
agents. Additional chemicals may also be included in the fracturing fluids so
as
to accommodate the specific properties of a particular subterranean formation,
operation, and the like. In some instances, these chemicals may damage the
subterranean formation by entering into the reservoir rock and blocking pore
throats. Fracturing fluids or the chemicals included therein may also become
trapped in the formation due to capillary end effects in and around the
vicinity of
fractures formed therein. Such fluid invasion, or phase trapping, may lead to
blocking of hydrocarbon production within a formation.
[0004] Fracturing fluids may form emulsions in the subterranean
formation during a treatment operation. Such emulsion tendencies may be due
to immiscibility between two fluids within the fracturing fluid, including
between
1
CA 02923454 2016-02-03
WO 2016/010518 PCT/US2014/046649
base fluids and chemicals added into the fracturing fluid (e.g., gelling
agents).
Such emulsions may associate strongly with the subterranean formation and
result in phase trapping, thus impeding flow and impairing production of the
formation. The emulsion tendency of fracturing fluids may be treated with non-
emulsifiers. However,
only certain fracturing fluid formulations may be
responsive to such non-emulsifiers. As such, non-emulsifiers are often not
capable of universal use for preventing or reducing the emulsion tendency of a
particular fracturing fluid.
BRIEF DESCRIPTION OF THE DRAWINGS
[0005] The following figures are
included to illustrate certain aspects
of the embodiments, and should not be viewed as exclusive embodiments. The
subject matter disclosed is capable of considerable modifications,
alterations,
combinations, and equivalents in form and function, as will occur to those
skilled
in the art and having the benefit of this disclosure.
[0006] FIG. 1 depicts an
embodiment of a system configured for
delivering the treatment fluids of the embodiments described herein to a
downhole location.
DETAILED DESCRIPTION
[0007] The embodiments herein
relate to fluid mobility modifiers for
increased production in subterranean formation operations. Specifically, the
fluid mobility modifiers described herein are capable of reducing the emulsion
tendency of certain treatment fluids (e.g., fracturing fluids, completion
fluids,
and the like). As used herein, the term
"emulsion tendency" refers to the
surface tensions at fluid interfaces within a treatment fluid. That is, the
emulsion tendency of a treatment fluid may be evaluated by determining the
surface tension at the fluid interfaces of the treatment fluid. By using the
fluid
mobility modifiers disclosed in the embodiments herein, the emulsion tendency
of the treatment fluids described herein may be reduced, thus improving the
productivity of the particular subterranean formation being treated.
[0008] Although some embodiments
described herein are illustrated
by reference to hydraulic fracturing operations in subterranean formations,
the
fluid mobility modifiers may be used in any subterranean operation that may
benefit from reducing the emulsion tendency of a treatment fluid. Such
2
CA 02923454 2016-02-03
WO 2016/010518 PCT/US2014/046649
treatment operations may include, but are not limited to, a drilling
operation; a
lost circulation operation; a stimulation operation; an acidizing operation;
an
acid-fracturing operation; a sand control operation; a completion operation; a
scale inhibiting operation; a water-blocking operation; a clay stabilizer
operation; a fracturing operation; a frac-packing operation; a gravel packing
operation; a wellbore strengthening operation; a sag control operation; and
any
combination thereof. Moreover, the fluid mobility modifiers described herein
may be used in any non-subterranean operation that may benefit from reducing
the emulsion tendency of a fluid. Such operations may be performed in any
industry including, but not limited to, oil and gas, mining, chemical, pulp
and
paper, aerospace, medical, automotive, and the like.
[0009] One or more illustrative
embodiments disclosed herein are
presented below. Not all features of an actual implementation are described or
shown in this application for the sake of clarity. It is understood that in
the
development of an actual embodiment incorporating the embodiments disclosed
herein, numerous implementation-specific decisions must be made to achieve
the developer's goals, such as compliance with system-related, business-
related,
government-related, and other constraints, which vary by implementation and
from time to time. While a developer's efforts might be mixture and time-
consuming, such efforts would be, nevertheless, a routine undertaking for
those
of ordinary skill the art having benefit of this disclosure.
[0010] It should be noted that
when "about" is provided herein at
the beginning of a numerical list, the term modifies each number of the
numerical list. In some numerical listings of ranges, some lower limits listed
may
be greater than some upper limits listed. One skilled in the art will
recognize
that the selected subset will require the selection of an upper limit in
excess of
the selected lower limit. Unless otherwise indicated, all numbers expressing
quantities of ingredients, properties such as molecular weight, reaction
conditions, and so forth used in the present specification and associated
claims
are to be understood as being modified in all instances by the term "about."
Accordingly, unless indicated to the contrary, the numerical parameters set
forth
in the following specification and attached claims are approximations that may
vary depending upon the desired properties sought to be obtained by the
exemplary embodiments described herein. At the very least, and not as an
attempt to limit the application of the doctrine of equivalents to the scope
of the
3
CA 02923454 2016-02-03
WO 2016/010518 PCT/US2014/046649
claim, each numerical parameter should at least be construed in light of the
number of reported significant digits and by applying ordinary rounding
techniques.
[0011] While compositions and
methods are described herein in
terms of "comprising" various components or steps, the compositions and
methods can also "consist essentially of" or "consist of" the various
components
and steps. When "comprising" is used in a claim, it is open-ended.
[0012] In some embodiments, the
present invention provides a
method comprising providing a treatment fluid comprising a first aqueous base
fluid and a polymeric gelling agent. The treatment fluid comprises a first
surface
tension (i.e., emulsion tendency). A fluid mobility modifier is added into the
treatment fluid so as to cause the treatment fluid to adopt a second surface
tension that is less than the first surface tension. The fluid mobility
modifier
comprises a first surfactant selected from the group consisting of a non-ionic
surfactant; a cationic surfactant; and any combination thereof (which also may
be referred to as "non-ionic surfactant and/or cationic surfactant" herein)
and a
solvent-surfactant blend comprising a second aqueous base fluid, a second
surfactant, a solvent, and a co-solvent. The treatment fluid comprising the
fluid
mobility modifier is then introduced into a subterranean formation in order to
form a subterranean operation.
[0013] The aqueous base fluid
that may be used in the treatment
fluids or fluid mobility modifiers described herein include, but are not
limited to,
fresh water; saltwater (water containing one or more salts dissolved therein);
brine (e.g., saturated salt water; seawater (e.g., naturally occurring water
containing one or more salts dissolved therein); produced water (e.g., water
that is recovered along with oil or gas in a subterranean formation);
reclaimed
water (e.g., treated or untreated wastewater); and any combination thereof.
Generally, the water may be from any source, provided that it does not contain
components that might adversely affect the stability and/or performance of the
treatment fluids and the fluid mobility modifiers described herein.
[0014] The polymeric gelling agent may be cationic or anionic. In
preferred embodiments, the polymeric gelling agent is anionic. Examples of
such suitable polymeric gelling agents may include, but are not limited to, a
derivatized guar gum (e.g., carboxymethyl guar, carboxymethylhydroxyethyl
guar, and carboxymethylhydroxypropyl guar ("CMHPG")); a cellulose derivative
4
,
CA 02923454 2016-02-03
WO 2016/010518
PCT/U52014/046649
(e.g., hydroxyethyl cellulose, carboxyethylcellulose, carboxymethylcellulose,
and
carboxymethylhydroxyethylcellulose); xanthan ;
succinoglycan; alginate;
chitosan; any derivative thereof; and any combination thereof. The term
"derivative" is defined herein any compound that is made from one of the
listed
compounds, for example, by replacing one atom in one of the listed compounds
with another atom or group of atoms, ionizing one of the listed compounds, or
creating a salt of one of the listed compounds. Examples
of suitable
commercially available polymeric gelling agents for use in the methods and
compositions of the present invention include, but are not limited to, WG-
39TM,
available from Halliburton Energy Services, Inc. in Houston, Texas.
[0015] In some embodiments, the polymeric gelling agent may be
present in an amount in the range of from a lower limit of about 1 pounds per
thousand gallons ("ppt"), 5 ppt, 10 ppt, 15 ppt, 20 ppt, 25 ppt, 30 ppt, 35
ppt,
40 ppt, 45 ppt, and 50 ppt to an upper limit of about 100 ppt, 95 ppt, 90 ppt,
85
ppt, 80 ppt, 75 ppt, 70 ppt, 65 ppt, 60 ppt, 55 ppt, and 50 ppt of the
treatment
fluid. The concentration of the polymeric gelling agent may be dependent upon
a number of factors such as, for example, the type of polymeric gelling agent
used, the type of subterranean formation operation being performed, the
conditions of the subterranean formation itself (e.g., pH, temperature, etc.),
and
the like. One of ordinary skill in the art, with the benefit of this
disclosure, will
recognize the appropriate concentration of the polymeric gelling agent to
achieve
a particular result.
[0016] The fluid
mobility modifiers described herein comprise a
nonionic surfactant and/or a cationic surfactant and a solvent-surfactant
blend.
The non-ionic surfactant and/or cationic surfactant may aid in reducing the
emulsion tendency of the treatment fluid in combination with the solvent-
surfactant blend. The non-ionic surfactant and/or cationic surfactant and the
solvent-surfactant blend operate synergistically with each other to reduce the
emulsion tendency of the treatment fluids described herein, such that neither
alone is capable of achieving such emulsion tendency reductions. Indeed, in
some cases, neither the non-ionic surfactant and/or the cationic surfactant
nor
the solvent-surfactant blend alone is capable of achieving any emulsion
tendency
reduction in the treatment fluids.
[0017] Suitable
non-ionic surfactants may include, but are not
limited to, an alkyoxylate (e.g., an alkoxylated nonylphenol condensate, such
as
5
.,
= CA 02923454 2016-02-03
WO 2016/010518
PCT/US2014/046649
poly(oxy-1,2-ethanediy1), alpha-(4-nonylphenyI)-omega-hydroxy-,branched); an
alkylphenol; an ethoxylated alkyl amine; an ethoxylated oleate; a tall oil; an
ethoxylated fatty acid; and combinations thereof. A suitable commercially
available non-ionic surfactant may include, but is not limited to, LoSurf-
300DTM
from Halliburton Energy Services, Inc. In Houston, Texas. Suitable cationic
surfactants may include, but are not limited to, a trimethylcocoammonium
chloride; a trimethyltallowammonium chloride; a dimethyldicocoammonium
chloride; a bis(2-hydroxyethyl)tallow amine; a bis(2-hydroxyethyl)erucylamine;
a bis(2-hydroxyethyl)coco-amine; a cetylpyridinium chloride; and combination
thereof.
[0018] In some
embodiments, the non-ionic surfactant and/or
cationic surfactant may be suspended in a solvent medium comprising any
combination of an aqueous base fluid, a solvent (e.g. heavy aromatic
petroleum,
naphthalene, and the like), and an alcohol (e.g., ethanol). One of ordinary
skill
in the art, with the benefit of this disclosure, will recognize when such
carrier
fluid is needed and what formulation such carrier fluid should possess based
on
factors, such as the type of non-ionic surfactant and/or cationic surfactant
chosen, the composition of the solvent-surfactant blend, the type of polymeric
gelling agent selected, and the like.
[0019] The solvent-surfactant blends described in some
embodiments herein form oil-in-water microemulsions. As used herein, the term
"microemulsion" is given its ordinary meaning in the art and refers to
dispersions
of one immiscible liquid in another, in the form of droplets, with diameters
approximately in the range of between a lower limit of about 1 nanometer
("nm"), 10 nm, 20 nm, 30 nm, 40 nm, 50 nm, 60 nm, 70 nm, 80 nm, 90 nm,
and 100 nm, to an upper limit of about 200 nm, 190 nm, 180 nm, 170 nm, 160
nm, 150 nm, 140 nm, 130 nm, 120 nm, 110 nm, and 100 nm. Microemulsions
are clear or transparent because they contain particles smaller than the
wavelength of visible light. In addition, microemulsions are homogeneous,
thermodynamically stable single phases that form spontaneously and, thus,
differ markedly from thermodynamically unstable emulsions, which generally
depend upon intense mixing energy for their formation. Solvent-surfactant
blends may comprise vast specific formulations having vast performance
characteristics. For that
reason, solvent-surfactant blends are not
interchangeable and those blends having the formulations described herein are
6
CA 02923454 2016-02-03
WO 2016/010518 PCT/US2014/046649
capable of reducing the emulsion tendency of the treatment fluids described
herein, where others may not be so capable.
[0020] The solvent-surfactant
blends described herein may comprise
a second aqueous base fluid, a second surfactant, a solvent, and a co-solvent.
The aqueous base fluids may be any aqueous base fluid described herein. In
preferred embodiments, the aqueous base fluid used in the solvent-surfactant
blend is fresh water. The second surfactant may be used to form an interfacial
film on the solvent dispersed phase in the microemulsion.
[0021] Suitable second
surfactants for use in the solvent-surfactant
blends described herein may preferably be degradable and may have a
hydrophile-lipophile balance in the range of from a lower limit of about 8, 9,
10,
11, and 12 to an upper limit of about 18, 17, 16, 15, 14, 13, and 12. Suitable
surfactants may include, but are not limited to, a polyoxyethylene sorbitan
monopalmitate; a polyoxyethylene sorbitan monostearate; a polyoxyethylene
sorbitan monooleate; a linear alcohol alkoxylate; an alkyl ether sulfate; a
dodecyl benzene sulfonic acid; a sodium dodecyl benzene sulfonate; an
alkoxylated nonyl-phenol; an ethoxylated castor oil (e.g., PEG castor oil);
dIpalmItoylphosphatidylcholine; a sodium 4-(1' heptylnonyl)benzenesulfonate; a
polyoxyethylene(8.6) nonyl-phenol ether; a sodium bis-2-
ethylhexylsulphosuccinate; a tetraethyleneglycol dodecylether; a sodium
octlylbenzenesulfonate; an alkyl propoxy-ethoxysulfate; an alkylarylpropoxy-
ethoxysulfate; a highly substituted benzene sulfonate; and any combinations
thereof. A highly
substituted benzene includes, but is not limited to,
substitutions of xylene, toluene, and naphthalene sulfonates.
[0022] Suitable solvents for use
in the solvent-surfactant blends
described herein may include, but are not limited to, a terpene; an alkyl
ester;
an aryl ester; a short chain alcohol; and any combination thereof. As used
herein, the term "short chain alcohol" refers to alcohols having alkyl chains
of 1
to 3 carbons. Terpenes are unsaturated aliphatic cyclic hydrocarbons and in
the
embodiments described herein include monoterpenes and diterpenes. Specific
terpenes may include, but are not limited to, d-limonene (C101-116). Specific
alkyl,
cyclic, or aryl esters may include, but are not limited to, ethyl lactate and
hexyl
ester.
[0023] The co-solvents described
herein may serve as a coupling
agent between the solvent and the surfactant, ensuring flexibility of the
7
= CA 02923454 2016-02-03
WO 2016/010518 PCT/US2014/046649
interfacial film, thus reducing interfacial tension and aiding the stability
of the
microemulsion. Suitable co-solvents for use in the solvent-surfactant blends
described herein may include, but are not limited to, t-butanol; n-butanol; n-
pentanol; n-hexanol; 2-ethyl-hexanol; and any combination thereof. Any
midrange primary, secondary and tertiary alcohols with between 1 and 20
carbon atoms may be additionally used as the co-solvent described herein.
[0024] One of ordinary skill in
the art, with the benefit of this
disclosure will recognize the appropriate ratios of water:surfactant,
water: solvent, water: co-solvent, surfactant:solvent, surfactant: co-solvent,
and
co-solvent:solvent in the solvent-surfactant blend to achieve the desired
results.
In some preferred embodiments, the ratio of the surfactant to solvent in the
solvent-surfactant blend may be in the range of from a lower limit of about
2:1,
3:1, 4:1, 5:1, 6:1, 7:1, 8:1, 9:1, and 10:1 to an upper limit of about 20:1,
19:1, 18:1, 17:1, 16:1, 15:1, 14:1, 13:1, 12:1, 11:1, and 10:1. In some
preferred embodiments, the ratio of co-solvent to solvent in the solvent-
surfactant blend may be in the range of from a lower limit of about 1:3,
1:2.5,
1:2, 1:1.5, and 1:1 to an upper limit of about 3:1, 2.5:1, 2:1, 1.5:1, and
1:1. A
suitable commercially available solvent-surfactant blend may include, but is
not
limited to, GasPerm1000 , available from Halliburton Energy Services, Inc. In
Houston, Texas.
[0025] In some embodiments, the
non-ionic surfactant and/or
cationic surfactant and the solvent-surfactant blend are present in the fluid
mobility modifier in a ratio in the range of from about 1:5, 1:4, 1:3, 1:2,
1:1,
and 2:1 to an upper limit of about 5:1, 4:1, 3:1, and 2:1. The exact ratio of
non-ionic surfactant and/or cationic surfactant to solvent-surfactant blend to
include in the fluid mobility modifier may depend on a number of factors
including, but not limited to, the type of non-ionic surfactant and/or
cationic
surfactant chosen, the composition of the solvent-surfactant blend, the type
of
polymeric gelling agent selected, and the like.
[0026] The treatment fluids of the present invention may additionally
comprise an additive selected from the group consisting of a proppant
particulate; a weighting agent; an inert solid; a fluid loss control agent; an
emulsifier; a dispersion aid; a corrosion inhibitor; an emulsion thinner; an
emulsion thickener; a breaker; a pH control agent; a lost circulation
material; a
8
CA 02923454 2016-02-03
WO 2016/010518
PCT/US2014/046649
foaming agent; a gas; a biocide; a scale inhibitor; a friction reducer; a clay
stabilizing agent; and any combination thereof.
[0027] In various embodiments, systems configured for delivering the
treatment fluids (i.e., the treatment fluids comprising at least the fluid
mobility
modifier) described herein to a downhole location are described. In various
embodiments, the systems can comprise a pump fluidly coupled to a tubular, the
tubular containing the treatment fluids described herein. It will be
appreciated
that while the system described below may be used for delivering either or
both
of the temporary sealant slurry and the fracturing fluid, each treatment fluid
is
delivered separately into the subterranean formation.
[0028] The pump may be a high pressure pump in some embodiments.
As used herein, the term "high pressure pump" will refer to a pump that is
capable of delivering a fluid downhole at a pressure of about 1000 psi or
greater.
A high pressure pump may be used when it is desired to introduce the treatment
fluids to a subterranean formation at or above a fracture gradient of the
subterranean formation, but it may also be used in cases where fracturing is
not
desired. In some embodiments, the high pressure pump may be capable of
fluidly conveying particulate matter, such as the non-degradable particulates,
the degradable particulates, and the proppant particulates described in some
embodiments herein, into the subterranean formation. Suitable high pressure
pumps will be known to one having ordinary skill in the art and may include,
but
are not limited to, floating piston pumps and positive displacement pumps.
[0029] In other embodiments, the pump may be a low pressure pump.
As used herein, the term "low pressure pump" will refer to a pump that
operates
at a pressure of about 1000 psi or less. In some embodiments, a low pressure
pump may be fluidly coupled to a high pressure pump that is fluidly coupled to
the tubular. That is, in such embodiments, the low pressure pump may be
configured to convey the treatment fluids to the high pressure pump. In such
embodiments, the low pressure pump may "step up" the pressure of the
treatment fluids before reaching the high pressure pump.
[0030] In some embodiments, the systems described herein can further
comprise a mixing tank that is upstream of the pump and in which the treatment
fluids are formulated. In various embodiments, the pump (e.g., a low pressure
pump, a high pressure pump, or a combination thereof) may convey the
treatment fluids from the mixing tank or other source of the treatment fluids
to
9
=
CA 02923454 2016-02-03
WO 2016/010518
PCT/US2014/046649
the tubular. In other embodiments, however, the treatment fluids may be
formulated offsite and transported to a worksite, in which case the treatment
fluid may be introduced to the tubular via the pump directly from its shipping
container (e.g., a truck, a railcar, a barge, or the like) or from a transport
pipeline. In either case, the treatment fluids may be drawn into the pump,
elevated to an appropriate pressure, and then introduced into the tubular for
delivery downhole.
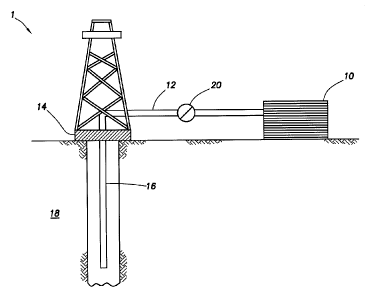
[0031] FIGURE 1 shows an illustrative schematic of a system that can
deliver the treatment fluids of the present disclosure to a downhole location,
according to one or more embodiments. It should be noted that while FIGURE 1
generally depicts a land-based system, it is to be recognized that like
systems
may be operated in subsea locations as well. As depicted in FIGURE 1, system 1
may include mixing tank 10, in which the treatment fluids of the embodiments
herein may be formulated. The treatment fluids may be conveyed via line 12 to
wellhead 14, where the treatment fluids enter tubular 16, tubular 16 extending
from wellhead 14 into subterranean formation 18. Upon being ejected from
tubular 16, the treatment fluids may subsequently penetrate into subterranean
formation 18. Pump 20 may be configured to raise the pressure of the
treatment fluids to a desired degree before introduction into tubular 16. It
is to
be recognized that system 1 is merely exemplary in nature and various
additional components may be present that have not necessarily been depicted
in FIGURE 1 in the interest of clarity. Non-limiting additional components
that
may be present include, but are not limited to, supply hoppers, valves,
condensers, adapters, joints, gauges, sensors, compressors, pressure
controllers, pressure sensors, flow rate controllers, flow rate sensors,
temperature sensors, and the like.
[0032] Although not depicted in FIGURE 1, the treatment fluid may, in
some embodiments, flow back to wellhead 14 and exit subterranean formation
18. In some embodiments, the treatment fluid that has flowed back to wellhead
14 may subsequently be recovered and recirculated to subterranean formation
18.
[0033] It is also to be recognized that the disclosed treatment fluids
may also directly or indirectly affect the various downhole equipment and
tools
that may come into contact with the treatment fluids during operation. Such
equipment and tools may include, but are not limited to, wellbore casing,
= CA 02923454 2016-02-03
WO 2016/010518 PCT/US2014/046649
wellbore liner, completion string, insert strings, drill string, coiled
tubing,
slickline, wireline, drill pipe, drill collars, mud motors, downhole motors
and/or
pumps, surface-mounted motors and/or pumps, centralizers, turbolizers,
scratchers, floats (e.g., shoes, collars, valves, etc.), logging tools and
related
telemetry equipment, actuators (e.g., electromechanical devices,
hydromechanical devices, etc.), sliding sleeves, production sleeves, plugs,
screens, filters, flow control devices (e.g., inflow control devices,
autonomous
inflow control devices, outflow control devices, etc.), couplings (e.g.,
electro-
hydraulic wet connect, dry connect, inductive coupler, etc.), control lines
(e.g.,
electrical, fiber optic, hydraulic, etc.), surveillance lines, drill bits and
reamers,
sensors or distributed sensors, downhole heat exchangers, valves and
corresponding actuation devices, tool seals, packers, cement plugs, bridge
plugs,
and other wellbore isolation devices, or components, and the like. Any of
these
components may be included in the systems generally described above and
depicted in FIGURE 1.
[0034] Embodiments disclosed herein include:
[0035] A. A method comprising:
providing a treatment fluid
comprising a first aqueous base fluid and a polymeric gelling agent selected
from
the group consisting of a derivatized guar gum, a cellulose derivative,
xanthan,
succinoglycan, alginate, chitosan, and any combination thereof, wherein the
treatment fluid comprises a first surface tension; introducing a fluid
mobility
modifier into the treatment fluid, wherein the fluid mobility modifier
comprises:
a first surfactant selected from the group consisting of a non-ionic
surfactant; a
cationic surfactant; and any combination thereof, and a solvent-surfactant
blend
comprising a second aqueous base fluid, a second surfactant, a solvent, and a
co-solvent, wherein the solvent-surfactant blend is an oil-in-water
microemulsion, wherein the fluid mobility modifier causes the treatment fluid
to
adopt a second surface tension that is less than the first surface tension;
and
introducing the treatment fluid into a subterranean formation.
[0036] B. A method comprising:
providing a treatment fluid
comprising a first aqueous base fluid and a polymeric gelling agent selected
from
the group consisting of a derivatized guar gum, a cellulose derivative,
xanthan,
succinoglycan, alginate, chitosan, and any combination thereof, wherein the
treatment fluid comprises a first surface tension; introducing a fluid
mobility
modifier into the treatment fluid, wherein the fluid mobility modifier
comprises:
11
= CA 02923454 2016-02-03
=
WO 2016/010518 PCT/US2014/046649
a first surfactant selected from the group consisting of a non-ionic
surfactant; a
cationic surfactant; and any combination thereof, and a solvent-surfactant
blend
comprising a second aqueous base fluid, a second surfactant, a solvent, and a
co-solvent, wherein the solvent-surfactant blend is an oil-in-water
microemulsion, wherein the ratio of the first surfactant to the solvent-
surfactant
blend is in the range of between about 1:5 to about 5:1, wherein the fluid
mobility modifier causes the treatment fluid to adopt a second surface tension
that is less than the first surface tension; and introducing the treatment
fluid
into a subterranean formation.
[0037] C. A system comprising: a
wellhead with a tubular extending
therefrom and into a wellbore in a subterranean formation; and a pump fluidly
coupled to the tubular, the tubular containing a treatment fluid comprising a
first
aqueous base fluid, a polymeric gelling agent selected from the group
consisting
of a derivatized guar gum, a cellulose derivative, xanthan, succinoglycan,
alginate, chitosan, and any combination thereof, and a fluid mobility
modifier,
the fluid mobility modifier comprising a first surfactant selected from the
group
consisting of a non-ionic surfactant; a cationic surfactant; and any
combination
thereof and a solvent-surfactant blend comprising a second aqueous base fluid,
a second surfactant, a solvent and a co-solvent.
[0038] Each of embodiments A, B,
and C may have one or more of
the following additional elements in any combination:
[0039] Element 1: Wherein the
ratio of the first surfactant to the
solvent-surfactant blend is in the range of between about 1:5 to about 5:1.
[0040] Element 2: Wherein the
first surfactant is selected from the
group consisting of an alkyoxylate; an alkylphenol; an ethoxylated alkyl
amine;
an ethoxylated oleate; a tall oil; an ethoxylated fatty acid; a
trimethylcocoammonium chloride; a trimethyltallowammonium chloride; a
dimethyldicocoammonium chloride; a bis(2-hydroxyethyl)tallow amine; a bis(2-
hydroxyethyl)erucylamine; a bis(2-hydroxyethyl)coco-amine; a cetylpyridinium
chloride; and any combination thereof.
[0041] Element 3: Wherein the
second surfactant has a hydrophile-
lipophile balance in the range of between about 8 and about 18.
[0042] Element 4: Wherein the
second surfactant is selected from
the group consisting of a polyoxyethylene sorbitan monopalmitate; a
polyoxyethylene sorbitan monostearate; a polyoxyethylene sorbitan monooleate;
12
CA 02923454 2016-02-03
=
WO 2016/010518 PCT/US2014/046649
a linear alcohol alkoxylate; an alkyl ether sulfate; a dodecyl benzene
sulfonic
acid; a sodium dodecyl benzene sulfonate; an alkoxylated nonyl-phenol; an
ethoxylated castor oil; dipalmitoylphosphatidylcholine; a sodium 4-(1'
heptylnonyl)benzenesulfonate; a polyoxyethylene(8.6) nonyl-phenol ether; a
sodium bis-2-ethylhexylsulphosuccInate; a tetraethyleneglycol dodecylether; a
sodium octlylbenzenesulfonate; an alkyl propoxy-ethoxysulfate; an
alkylarylpropoxy-ethoxysulfate; a highly substituted benzene sulfonate; and
any
combinations thereof.
[0043] Element 5: Wherein the
solvent in the solvent is selected
from the group consisting of a terpene; an alkyl ester; an aryl ester; a short
chain alcohol; and any combination thereof.
[0044] Element 6: Wherein the co-
solvent is selected from the group
consisting of t-butanol; n-butanol; n-pentanol; n-hexanol; 2-ethyl-hexanol;
and
any combination thereof.
[0045] Element 7: Wherein the co-
solvent is at least one of a
primary alcohol, a secondary alcohol, and a tertiary alcohol having between 1
and 20 carbon atoms.
[0046] Element 8: Wherein the
second surfactant and solvent in the
surfactant-solvent blend are present in a second surfactant:solvent ratio in
the
range of between about 2:1 to about 20:1.
[0047] Element 9: Wherein the co-
solvent and solvent in the
surfactant-solvent blend are present in a co-solvent:solvent ratio in the
range of
between about 1:3 to about 3:1.
[0048] Element 10: Wherein the
treatment fluid further comprises
an additive selected from the group consisting of a proppant particulate; a
weighting agent; an inert solid; a fluid loss control agent; an emulsifier; a
dispersion aid; a corrosion inhibitor; an emulsion thinner; an emulsion
thickener; a breaker; a pH control agent; a lost circulation material; a
foaming
agent; a gas; a biocide; a scale inhibitor; a friction reducer; a clay
stabilizing
agent; and any combination thereof.
[0049] By way of non-limiting
example, exemplary combinations
applicable to A, B, C include: A with 2, 4, and 10; A with 5 and 7; A with 3,
8,
and 9; A with 1 and 4; B with 5, 6, and 10; B with 3 and 8; B with 4 and 7; B
with 10; C with 1 and 10; C with 2, 3, 4, and 7; C with 8 and 9.
13
CA 02923454 2016-02-03
WO 2016/010518 PCT/US2014/046649
[0050] To facilitate a better
understanding of the embodiments of
the present invention, the following examples of preferred or representative
embodiments are given. In no way should the following examples be read to
limit, or to define, the scope of the invention.
EXAMPLE 1
[0051] In this example, the
emulsion tendency reduction of a
treatment fluid comprising a fluid mobility modifier was evaluated. A
demulsification test was performed using a CO2 seltzer device. An example of a
suitable CO2 seltzer device is an ISI Twist'n Sparkle beverage carbonation
system. The device comprises a quart stainless steel vessel equipped with a
hand operated valve and spout to foam the contents of the vessel using a pre-
pressurized CO2 cartridge. Two treatment fluids were evaluated using the
demulsification test.
[0052] A first mixture (M1)
served as a control fluid. M1 was
prepared using 50 mL of crude oil (simulating the oil that may be located
within
a subterranean formation) and 50 mL of a broken treatment fluid (TF1)
comprising 40 ppt WG-39TM polymeric gelling agent in fresh water. TF1 did not
comprise the fluid mobility modifiers described herein. M1 was added to a
blender jar and blended at 12,000 rpm for 30 seconds. Thereafter, M1 was
removed from the blender jar and placed into the vessel of the CO2 seltzer
device. The pressurized CO2 cartridge was released into the vessel and the CO2
seltzer device inverted and vigorously shaken 5 times. Thereafter, M1 was
removed from the vessel of the CO2 seltzer device and into a 500 mL graduated
cylinder. M1 was observed in the graduated cylinder at time 0, 1 minute, and
10
minutes. At time 0, M1 was observed as a highly emulsified fluid, showing a
volume of 200 mL, despite an initial input of only 100 mL. Clear bubble
formation was evident on the surface of M1 and distributed throughout. After 1
minute, the fluid decreased to 100 mL, but remained observably emulsified,
with
apparent foaming on the surface and bubble formation throughout, and visibly
appeared the same after the elapse of 10 minutes.
[0053] The mixture (M2) was
prepared using 50 mL of crude oil and
50 mL of a broken treatment fluid (TF2) comprising 40 ppt WG-39TM polymeric
gelling agent in fresh water and 1 gallons per thousand gallons ("got") fluid
mobility modifier comprising 1:1 LoSurf-300D" non-ionic surfactant to
14
= CA 02923454 2016-02-03
WO 2016/010518 PCT/US2014/046649
GasPerm1000 solvent-surfactant blend. The
demulsification test was
performed on M2 as described above. At time 0, only minimal signs of
emulsification were observed and solely on the surface of the fluid. No
apparent
bubble formation was observed throughout the fluid. After 1 minute, no
emulsification was apparent and no changes were observed after 10 minutes,
demonstrating that the fluid mobility modifier present in TF2 (and M2) was
capable of reducing emulsion tendency.
EXAMPLE 2
[0054] In this example, the
emulsion tendency reduction of a
treatment fluid comprising a fluid mobility modifier according to some
embodiments described herein was evaluated. Two test treatment fluids were
prepared and their emulsion tendency tested using surface tension
measurements upon exposure to a sand pack. Two sand packs were prepared
(SP1 and SP2) using washed and dried 70-140 Oklahoma sand. The Oklahoma
sand was packed into a 2.54 cm (1 in) x 25.4 cm (10 in) glass chromatography
column until the sand filled 10.16 cm (4 in) of the column. The pore volume of
the sand pack was determined by measuring the volume of water required to fill
packed column.
[0055] The first treatment fluid
(TF3) served as a control fluid
comprising only a portion of the fluid mobility modifier. TF3 comprised a
broken
treatment fluid comprising 60 ppt WG-39TM polymeric gelling agent in fresh
water and 1 gpt LoSurf-3000TM non-ionic surfactant. TF3 did not comprise a
solvent-surfactant blend and, thus, did not comprise the fluid mobility
modifier
blends described herein. The surface tension of TF3 was measured before
exposure to the sand pack, SP1. Thereafter, three pore volumes of TF3 were
run through SP1 and the surface tension of each effluent was measured.
[0056] The second treatment fluid
(TF4) comprised a complete fluid
mobility modifier. TF4 comprised a broken treatment fluid comprising 60 ppt
WG-39174 polymeric gelling agent in fresh water and 1 gpt fluid mobility
modifier
comprising 1:1 LoSurf-300DTM non-ionic surfactant to GasPerm1000 solvent-
surfactant blend. The surface tension of TF4 was measured before exposure to
the sand pack, SP2. Thereafter, three pore volumes of TF4 were run through
SP2 and the surface tension of each effluent was measured.
CA 02923454 2016-02-03
WO 2016/010518 PCT/US2014/046649
[0057] The surface tension
measurement results are shown in Table
1. The surface tension of TF4 comprising the fluid mobility modifier in the
treatment fluid, as described in embodiments herein, maintained a much
reduced surface tension, indicating its ability to reduce the emulsion
tendency of
a treatment fluid, as compared to TF3 comprising solely the non-ionic
surfactant.
The reduced emulsion tendency was observed even after multiple exposures to
the surface area of the sand pack. Thus, the fluid mobility modifiers
comprising
the non-ionic surfactant or cationic surfactant and the solvent-surfactant
blend
described herein operated synergistically to reduce the emulsion tendency of
the
treatment fluid.
TABLE 1
i!igeMENEMSENNEM WOOVIAMPõWORMFAIg.NOOMAINIANLefa$
:A=Gothigignigmi;iiiii;Nposa=NWAAM:a0M4aquingMteme
gogimmigimpooloommemmiAREWMEN innespwWiggigellini
Treatment Fluid before 31.100 30.030
Exposure to Sand Pack
Pore Volume #1 46.370 31.300
Pore Volume #2 47.530 30.250
Pore Volume #3 50.010 30.130
[0058] Therefore, the present
invention is well adapted to attain the
ends and advantages mentioned as well as those that are inherent therein. The
particular embodiments disclosed above are illustrative only, as the present
invention may be modified and practiced in different but equivalent manners
apparent to those skilled in the art having the benefit of the teachings
herein.
Furthermore, no limitations are intended to the details of construction or
design
herein shown, other than as described in the claims below. It is therefore
evident that the particular illustrative embodiments disclosed above may be
altered, combined, or modified and all such variations are considered within
the
scope and spirit of the present invention. The Invention illustratively
disclosed
herein suitably may be practiced in the absence of any element that is not
specifically disclosed herein and/or any optional element disclosed herein.
While
compositions and methods are described in terms of "comprising," "containing,"
16
or "including" various components or steps, the compositions and methods can
also "consist essentially of" or "consist of" the various components and
steps.
All numbers and ranges disclosed above may vary by some amount. Whenever
a numerical range with a lower limit and an upper limit is disclosed, any
number
and any included range falling within the range is specifically disclosed. In
particular, every range of values (of the form, "from about a to about b," or,
equivalently, "from approximately a to b," or, equivalently, "from
approximately
a-b") disclosed herein is to be understood to set forth every number and range
encompassed within the broader range of values. Also, the terms in the claims
have their plain, ordinary meaning unless otherwise explicitly and clearly
defined
by the patentee. Moreover, the indefinite articles "a" or "an," as used in the
claims, are defined herein to mean one or more than one of the element that it
introduces. If there is any conflict in the usages of a word or term in this
specification and one or more patent or other documents referenced herein, the
definitions that are consistent with this specification should be adopted.
CA 2923454 2017-08-02 17