Note: Descriptions are shown in the official language in which they were submitted.
CA 02923508 2016-03-10
SPECTROMETER CALIBRATION METHOD AND REFERENCE MATERIAL
The present invention refers to a calibration method for a spectrometer and a
reference
material.
The composition of iron and steel alloys can be measured using different
analytical techniques.
One of these techniques, optical emission spectroscopy involves exciting atoms
of a target
sample of which knowledge of the composition is desired and examining the wave
length of
photons emitted by atoms during transition from an excited state to a lower
energy state. Each
element in the periodic table emits a characteristic set of discrete wave
lengths when its atoms
return from an excited state to a lower energy state. By detecting and
analysing these wave
lengths the elemental composition of a sample can be determined in accordance
with a
calibration curve showing the relation between the spectral intensity ratio
(absolute radiation
power of an element / absolute radiation power of the base metal) and the
concentration of the
element in the standard sample. The spectral light may be produced by
irradiation with
electromagnetic radiation such as by a laser, x-rays, but is generally
produced by a short spark
produced by a spark generator incident upon the target of which knowledge of
its elemental
composition is desired. Irrespective of the energy source, the accuracy and
reliability of such
emission spectrometers is dependent on the accuracy and quality of the
detector and optics
used to receive the radiation emitted from the sample.
The output of a spectrometer may drift with time. Drift correction may be
required due to
changes in the optics, the excitation source, processing electronics and even
ambient room
temperature or humidity. These changes can cause drifts in the intensity
ratios from those
recorded during the initial calibration. In order to guarantee the accuracy,
detector and/or
optics response should be checked and the spectrometer recalibrated, if
necessary, using
reference material with a well-defined composition. The process of drift
correcting the curves
has many names: normalization, standardization, and re-calibration. Regardless
of the name
given the process, the curves are adjusted back to their state at the time of
the original
calibration.
1
CA 02923508 2016-03-10
If a drift correction is not made, errors will occur and false concentration
readings will result.
Timing for implementing drift correction is critical. However, this is
determined by the
individual laboratory considering the criticality of the analysis. Some
laboratories periodically
run check or SPC standards to determine if the instrument is within the
allowed tolerances.
The stability and duty cycle of the instrument determine the period for
checking drift. It should
at least be checked every hour or before a batch of samples are run to assure
quality of
results. If drift detection tolerances are not set up, it is imperative that
drift correction, at a
minimum, is accomplished every shift for laboratory instruments and hourly for
mobile
instruments. If the analyst is unsure about the state of the instrument, drift
correction should
immediately precede the analysis of any samples, for optimum accuracy.
Since the spectral intensity ratio of a target sample to be analysed is
incorporated from a
previously prepared and known reference calibration curve, the precision of
the analysis for
the target sample depends on the accuracy of the previously generated
calibration curve.
Accordingly, a standard reference material of homogeneous and known elemental
content is
necessary for accurate analysis. These standards should be selected to cover
the
concentration ranges of all elements for which the spectrometer is capable.
The standards
should also match the structure and alloy type being evaluated.
One such standard reference material used for the routine calibration is a
circular ingot of for
example 40 to 60 mm diameter. Prior to each use, the surface of the ingot must
be prepared by
grinding or milling in order to obtain a new active surface exhibiting no
oxidation or other
chemical changes in the surface. In this way accurate measuring results can be
obtained and a
consistent calibration curve developed. The procedures and processes to obtain
a
representative analysis of metals are well known in the art. In Dulski, T.R. A
Manual for the
Chemical Analysis of Metals, ASTM International, 1996 or the publication ASTM
E1009 well
describes the analysis of carbon and low alloy steel. Reference materials are
commercially
available and known for example from the MBH catalogue 2014 from MBH
Analytical LTD,
Holland House, Queens Road, Barnet, EN5 4DJ, England.
According to published procedures such as; USA standard ASTM E415 - 14
Standard Test
Method for Analysis of Carbon and Low-Alloy Steel by Spark Atomic Emission
Spectrometry or
2
CA 02923508 2016-03-10
ASTM E716 ¨ 10 Standard Practices for Sampling and Sample Preparation of
Aluminum and
Aluminum Alloys for Determination of Chemical Composition by Spectrochemical
Analysis, in
Japan standard for steel, JIS Z 2611 and JIS Z 2612 , for aluminum JIS H 1305
and European
standards for steel DIN 51009 and for aluminum, DIN 14726 all state grinding
preparation of
the sample and that its surface should be free of contamination. Those in the
analytical field are
accustomed to and train to grind the standard reference material even when
received in a
protective shipping container. The referenced national standard procedures
dictate that the
renewal of the surface of the same sample provides a continuity of results. It
is well known in
the art that once the sample is prepared the surface begins to deteriorate due
to interaction with
the environment.
As stated before, that accuracy of the analysis is dependent upon the quality
of the
recalibration and this is dependent upon the quality of the reference standard
in terms of its
composition and more importantly, its preparation prior to analysis. The
technique of sample
preparation is known in the art. In some instances it is a totally manual
operation that itself is
subject to minute variation. In a laboratory setting this variation can be
controlled to an
acceptable level. In industrial environments where optical emission
spectrographs are
installed near a metallurgical process these devices maybe subject to the same
rigorous
process of recalibration however, a higher frequency of recalibration could be
necessary due
to the environmental stress on the instruments. Routinely, one skilled in the
recalibration
process must travel to the location of the instrument or in the case of mobile
equipment a field
calibration is necessary or a return of the device to a certified calibration
facility. Recalibration
is a labor intensive and necessary endeavor costing a laboratory a
considerable percentage of
the analytical cost involved in the repeated preparation of the reference
materials for
calibration. The sample surface must be grounded or milled flat, free of
residue of this
process, and with a maximum tool impression on the surface. This is can be
manual or
automatic but still requires the loading and retrieval and segregation of the
standard reference
material.
Shipping reactive materials in inert gas, such as bottles or ampoules, is
common in the material
supply industry. The use of protective coatings such a plastic covers, paints,
oils, ceramic and
CVD films are known in the art to prevent oxidation and corrosion protection
of metal parts.
3
CA 02923508 2016-03-10
Metallic coatings are well known in the electrical contact industry for
corrosion resistance. Silver
coatings have been used to improve conductivity and provide corrosion
resistance is disclosed
in U.S. Patent No. 4,189,204 to Brown et al..
It is an object of the present invention to provide a calibration method for a
spectrometer and a
reference material which facilitates the calibration of a spectrometer.
The object of the invention is solved by a method comprising the features of
claim 1 as well as
by the subject matter of the further independent claim. The sub-claims refer
to preferred
embodiments of the invention.
The object is therefore solved by a method for calibration of a spectrometer
wherein a reference
material has a homogeneous content of elements. Further the reference material
is protected by
an inert coating, which protects a repeatable surface of the reference
material. The reference
material is used for calibration of the spectrometer in the as received
condition, without
additional preparation. "Inert" in the sense of the protective coating relates
not to the absolute
involatility of the element but to the practical resistance to environment
degradation and more
particularly to oxidation.
Due to the coating, it is not necessary to prepare a surface of the reference
material by grinding
or milling in order to obtain a new active surface exhibiting no oxidation or
other chemical
changes in the surface. Thus, the invention allows reducing the technical
effort.
Since an elaborate preparation is not required, an automatic transfer of the
reference material
from a dispensing device to the spectrometer can take place for calibration
which allows a
further reduction of the technical effort.
In a preferred embodiment of the invention, the reference material is kept
under an inert gas
atmosphere or vacuum within the dispensing device which allows a long storage
of the
reference material. Preferably Argon may be used as inert gas.
4
CA 02923508 2016-03-10
In a preferred embodiment of the invention, a composition of steel is measured
by the
spectrometer after the calibration. Steel is an alloy of iron and carbon. In a
preferred
embodiment of the invention, a composition of all types of ferrous and
nonferrous metals
including precious metals, alloys and ferroalloys, including as powdered
metals are measured
by the spectrometer after calibration.
In a preferred embodiment of the invention, the inert coating does not
comprise elements to be
measured in the steel or iron alloy in order to avoid falsified results.
It may further be preferred that an inert gas is used to protect the
homogeneous content instead
of or in addition to the inert coating.
In a preferred embodiment of the invention, a plurality of reference
materials, preferably in the
form of coins are inserted into a cassette respectively a container of
cassette comprising a lid
prior to the calibration of a spectrometer.
In a preferred embodiment of the invention, one or more cassettes comprising a
plurality of
reference materials are inserted into a storage and dispensing device prior to
the calibration of a
spectrometer, in which the reference materials maybe kept under vacuum or
under an inert gas
atmosphere from a remote supply. An inert gas (example argon but others are
possible)
protects the recalibration surface and the protective coating of a reference
material from
environmental contamination.
In a preferred embodiment of the invention, a new reference material is
removed periodically
from such a cassette and transferred to a spectrometer platen for analysis
respectively
calibration. The entire operation can be performed without human intervention.
A reference material for calibration of a spectrometer has a homogeneous
content of elements.
The content is protected by an inert coating. In this way, there is an active
surface which can be
used for calibration of a spectrometer.
5
CA 02923508 2016-03-10
In a preferred embodiment of the invention, the inert coating is composed of a
metal such as
zinc, nickel, ruthenium, rhodium, palladium, silver, osmium, iridium, platinum
and gold or any
alloys thereof.
In a preferred embodiment of the invention, the inert coating is composed of a
noble metal such
as Ag, Au, Pt, Ir and/or Rh or any alloy thereof. Noble metals are metals that
are resistant to
corrosion and oxidation on one side. As a rule, noble metals are not present
in an iron alloy or in
steel. For these reasons, a noble metal or any alloy thereof is an appropriate
material.
In a preferred embodiment of the invention, the homogeneous content of element
is formed
from steel.
In a preferred embodiment of the invention, the thickness of the inert coating
is 0,1 to 10 pm,
preferably 0,5 to 2 pm.
In a preferred embodiment of the invention, the reference material is a
circular coin in order to
facilitate the handling since an alignment is not required when putting the
circular coin into a
housing or into a spectrometer platen for calibration.
In a preferred embodiment of the invention, the diameter of the coin is 10 to
80 mm diameter
and/or the height of the coin is 1 to 30 mm.
In a preferred embodiment of the invention, the purity of the metal or the
metal alloy is at least
90% and is preferably better than 92.5%.
In another preferred embodiment the reference material is arranged in a
housing, whereby the
housing is preferably closed gas-tightly. It is of advantage, that more than
one piece of the
reference material is arranged in the housing. The pieces can be arranged
separated one from
another. The housing can have at least two parts whereby one part can be
removed at least
partially from the other part.
It can also be preferred that the pieces are arranged in the housing one on
top of the other.
6
CA 02923508 2016-03-10
The reference material is available for immediate use, without preparation due
to an anti-
corrosive package, individually housed in an inert gas or vacuum containing
housing providing
protection from environmental containments in the ambient environment such as
atmosphere,
particulate and contact contamination in handling, storage and use. The
package or housing is
sealed until use. Additionally, the portability of this reference material to
remote sites allows
calibration of mobile units and those installed near the industrial process.
The present invention provides an optical emission spectro-chemical reference
material
especially for metals and metal alloys having a homogeneous content of element
which is
directly usable without preparation. Moreover, the present invention refer to
a disposable
spectro-chemical material that is available for immediate use, protected by an
anti-corrosive
coating and is preferably housed in an inert gas shielded container providing
protection from
environmental containments in the ambient environment such as atmospheric,
particulate and
contact contaminants during handling, storage and use. It is capable for use
in automatic
recalibration routines of analytical equipment in remote locations as well as
laboratory settings.
Especially, the present invention is used for a metallurgical process.
The application of an inert layer allows protecting the recalibration surface
during long term
storage. This solution may also be combined with an inert atmosphere. A
homogeneous coin
of standard reference material according to the industrial standards known in
the art is prepared
with a surface finish according to a known industrial standard for analysis
respectively
calibration. A protective coating is immediately applied to its surface. The
protective coating is
selected in such a way that this coating material does not influence the
analysis of the
recalibration surface for the elements that are of importance for the
analysis. Examples (not
restricted to these only) of suitable materials in the case of reference
material used for steel are
Ag, Au, Pt, Ir, Rh. The coating layer is for example applied using sputtering
technology with
high quality target material in order to obtain a pure layer (so almost
without the elements to be
measured in steel / iron). An appropriate thickness of the layer is 1 pm, but
other layer
thickness in this range can work also.
In order to have enough corrosion resistance with silver (Ag) and further
noble metals, a few pm
is an appropriate layer thickness. Silver is characterized by poor sulfidation
resistance and low
7
CA 02923508 2016-03-10
hardness. However, silver has advantages over other non-oxidizing metals in
that the element is
not routinely analysed as a contaminant in irons based samples and its
dominant spectral
emission line does not interfere with others normally encountered in iron
analysis. As a rule,
this is also true for other noble metals.
The present invention provides the analytical laboratory with a reference
standard that is
prepared in a predictable fashion, with a predicable surface that is
environmentally stable for
extended periods of time. The elimination of the preparation labor, equipment,
and consumable
supplies provides considerable benefit to the cost of operation of an
analytical instrument. The
environmental stability of the reference standard provides for a means of
recalibration of an
instrument installed at the metallurgical process location with the same
precision as that of one
installed in a controlled laboratory.
The use of an automatic standard reference loading apparatus for the
recalibration of point of
use spectrometers, i.e., shop floor installed analytical equipment does not
exist in the market.
The potential benefit to the users of point of use analysis equipment is
optimized by an
automatic recalibration system and no preparation samples are a key component
to its
realization.
Another distinct point of difference between this and all other standard
reference materials
known in the art is that ready to use prepared surface of the present
invention is two sided.
In the following, the invention is further illustrated by the way of examples.
Fig. 1 is a three dimensional view of a reference material in the form of a
coin.
Fig. 2 is a three dimensional view of a cassette housing for the coins.
Fig. 3 is a sectional view of the cassette housing.
Fig. 4 is a housing for one piece of reference material.
Fig. 5 shows the housing of Fig. 4, opened.
Fig. 6 shows another kind of housing, for multiple coins.
Fig. 7 is another alternative housing.
8
CA 02923508 2016-03-10
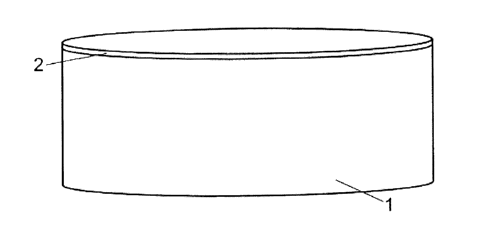
Fig. 1 is a view of a reference material in the form of a circular coin. The
coin consists of known
elemental content 1 and a coating 2 composed of silver or gold. The diameter
of the coin 1, 2 is
between 20 to 60 mm. The height of the coin 1, 2 is between 5 to 30 mm. The
thickness of the
coating 2 is between 0,5 to 5 pm.
The known elemental content 1 consists of steel for example a low alloy steel
comprising Fe, C,
Si, S, P, Mn, Ni, Cr, Mo, Cu, Sn, Al, V, As, Zn, N, a ferritic and martensitic
stainless steel
comprising Fe, C, Si, S, P, Mn, Ni, Cr, Mo, Cu, Sn, V, Co, Nb, W, B, N, a high
nitrogen stainless
steel comprising Fe, C, Si, S, P, Mn, Ni, Cr, Mo, Cu, Al, V, W, Co, Nb, B, N.
Further examples
comprise Fe, C, Si, S, P, Mn, Ni, Cr, Mo, Cu, Sn, Al, As, Pb, N or Fe, C, Si,
S, P, Mn, Ni, Cr, Cu,
Al, Co, Mg, N.
Fig. 2 is a three dimensional view of a cassette housing 3, 4 for the coins 1,
2. Fig. 3 is a
sectional view of the cassette housing 3, 4 comprising a plurality of coins 1,
2. It is possible to
attach the lid 4 to the container 3 by one or more bolts 5.
Each coin 1, 2 is inserted into the cassette housing composed of a container 3
and a lid 4. The
bottom of the container 3 comprises an inlet 6 for inert gas. There remains
slit 7 between the
container 3 and the lid 4 which allows to remove a coin 1, 2 from the cassette
housing 3, 4 in an
automatic manner. Further, the slit 7 may serve as an outlet for inert gas.
The side wall of the
container 3 may comprise a recess 8 which allows to fix the container 3 within
a dispensing
device.
In an alternative embodiment each coin 12 of reference material is inserted
into a housing 10,
Fig. 4. The inert gas (for example argon, but others are possible) purges the
internal space of
the housing and is sealed by a gas tight closure of the housing which protects
the recalibration
surfaces and its protective coating from environmental contamination. An 0-
ring 13, as one
example can be utilized for this purpose. The coin housing 10 is opened just
prior to use, as
shown in Fig. 5. and transferred to the spectrometer for analysis. Use of
plastic bags, Fig. 6, is
also contemplated. Multiple coins 12 can be housed in a foil backed holder 20,
providing
dispensing of individual coins 12 without compromising the inert gas
protection for the remaining
coins 12. This embodiment is similar to a blister used as pill dispenser.
Individual dispensing of
9
CA 02923508 2016-03-10
coins 12 provides the operator with a recalibration surface without the need
for preparation
equipment. Fig. 7 is a familiar package 21 of multiple foils.