Note: Descriptions are shown in the official language in which they were submitted.
CA 02923793 2016-03-09
WO 2015/038092 PCT/US2013/000207
CARBON DIOXIDE AND SALINE NASAL DELIVERY METHODS
AND DELIVERY DEVICES
Attorney Docket No. GTI-131PCT
REFERENCES TO RELATED APPPLICATIONS
[0001] This application is based on previously filed United States Patent
Applications, as
follows: USSN 13/506,425, filed on April 18, 2012, titled "Carbon Dioxide,
Saline and
Additional Active Nasal Delivery Methods and Treatments" ( Docket No. GTI-
132A), same
inventor as herein; USSN13/506,426, filed on April 18, 2012, titled "Carbon
Dioxide and Saline
Nasal Delivery Methods and Treatments" ( Docket No. GTI-131A) same inventor as
herein; and
USSN13/507,112, filed on June 4, 2012, titled "Nasal Treatment Delivery Device
For Carbon
Dioxide and Saline" ( Docket No. GTI-131A) same inventor as herein. No
priority is claimed.
BACKGROUND OF THE INVENTION
a. Field of Invention
[0002] This invention relates generally to healthcare, and specifically to the
treatment of head
ailments. More specifically, the present invention relates to intranasal
delivery methods and
treatments with carbon dioxide and saline; and with carbon dioxide, saline and
other active
components, as well as to devices used in these methods. These treatment
methods and devices
result in reduction of nasal passage swelling and congestion and treatment of
causes of such
swelling and congestion.
2
CA 02923793 2016-03-09
WO 2015/038092 PCT/US2013/000207
b. Description of Related Art
[0003] The following patents and applications are representative of various
types of nasal
medicine delivery devices:
[0004] United States Patent No. 8,007,842 B2 to Rau describes a composition
for providing
aromatherapy, and in particular, symptomatic relief of nasal and sinus
congestion in unit dosage
format. The composition includes a penetrating aromatic vapor whose release
from a preparation
of warm water is augmented by an effervescent component which reacts in the
warm water to
promote release of the aromatic fragrance, or sustained over time by tableting
or gelatin
encapsulation. As the fragrance is inhaled, symptomatic relief is obtained.
The composition of
matter may be rendered ingestible, so that the warm water containing the
composition is
consumed following inhalation. In preferred embodiments, the release of the
penetrating aromatic
fragrance persists over time.
[0005] United States Patent No. 7,959,597 B2 to Baker et al. describes an
irrigation and
aspiration system. The system can be configured to aspirate and irrigate
alone, sequentially or
concurrently. The system can be configured to aspirate and irrigate the nasal
cavity. The system
can be manually controlled. The system can have removable and easily cleanable
reservoirs for
aspirant and irrigant.
[0006] United States Patent No. 7,858,650 B2 to Yamamoto et al. describes a
medicinal
composition for inhalation containing a continuous-release type prodrug of an
EP2 agonist which
topically exhibits a prolonged bronchodilating and antiinflammatory effects.
Namely, the
medicinal composition for inhalation containing a continuous-release type
prodrug of an EP2
agonist is useful as a safe preventive and/or a remedy for respiratory
diseases (for example,
3
CA 02923793 2016-03-09
WO 2015/038092 PCT/US2013/000207
asthma, pulmonary injury, pulmonary fibrosis, pulmonary emphysema, bronchitis,
chronic
obstructive pulmonary disease, adult respiratory distress syndrome, cystic
fibrosis, pulmonary
hypertension or the like) without causing any systemic effect such as lowering
blood pressure.
Thus, a safe and useful remedy for respiratory diseases is provided.
[0007] United States Patent No. 7,845,348 B2 to Rasor et al. describes
apparatus, methods,
and kits for treating symptoms associated with common ailments, such as
headaches, rhinitis,
asthma, epilepsy, nervous disorders and the like. The apparatus comprises
dispensers for carbon
dioxide and other therapeutic gases. The methods comprise delivering small
volumes of these
gases to patients in a manner where the gas infuses into a body region in
order to bathe the
mucous membranes therein. It has been found that even very short exposure of
patients to small
volumes and high concentrations of such gases can provide significant relief
from symptoms.
[0008] United States Patent No. 7,836,883 B2 to Rasor et al. describes
apparatus, methods,
and kits for treating symptoms associated with common ailments, such as
headaches, rhinitis,
asthma, epilepsy, nervous disorders and the like. The apparatus comprises
dispensers for carbon
dioxide and other therapeutic gases. The methods comprise delivering small
volumes of these
gases to patients in a manner where the gas infuses into a body region in
order to bathe the
mucous membranes therein. It has been found that even very short exposure of
patients to small
volumes and high concentrations of such gases can provide significant relief
from symptoms.
[0009] United States Patent No. 7,827,986 B2 to Rasor et al. describes
apparatus, methods,
and kits for treating symptom associated with common ailments, such as
headaches, rhinitis,
asthma, epilepsy, nervous disorders and the like. The apparatus comprises
dispensers for carbon
dioxide and other therapeutic gases. The methods comprise delivering small
volumes of these
gases to patients in a manner where the gas infuses into a body region in
order to bathe the
4
CA 02923793 2016-03-09
WO 2015/038092
PCT/US2013/000207
mucous membranes therein. It has been found that even very short exposure of
patients to small
volumes and high concentrations of such gases can provide significant relief
from symptoms.
[00010] United States Patent Application No. 2008/0169047 Al to Connolly et
al. describes a
hand-held, low-flow dispenser which comprises an enclosure holding a gas
cartridge. A spring-
biased needle is advanced to puncture a septum on the gas cartridge, and a
separate spring-biased
ball valve is used to turn the resulting gas flow off and on as well as to
control the flow rate.
[00011] United States Patent Application No. 2008/0078382 Al to LeMahieu et
al. describes
systems and methods for delivery of a drug to the respiratory system of a
patient in a stream of
purified air are provided. In particular, the drugs are delivered to the
respiratory system of the
patient at a positive air pressure relative to atmospheric pressure. With the
systems and methods
of the present disclosure, medication available in a variety of forms is
introduced in a controlled
fashion into the air stream in aerosol, nebulized, or vaporized form.
[00012] United States Patent Application No. 2008/0066741 Al to LeMahieu et
al. describes
systems and methods for delivery of a drug to the respiratory system of a
patient, where the drug
is supplied in purified air at a positive pressure relative to atmospheric
pressure. With the systems
and methods of the present disclosure, medication available in a variety of
forms is introduced in
a controlled fashion into the purified air stream in aerosol, nebulized, or
vaporized form.
[00013] United States Patent Application No. 2008/0066739 Al to LeMahieu et
al. describes
systems and methods for delivery of a drug to the respiratory system of a
patient where the drug
is supplied at a positive pressure relative to atmospheric pressure. In
particular, the drugs are
delivered to the respiratory system of a patient who is capable of unassisted
breathing. With the
systems and methods of the present disclosure, medication available in a
variety of forms is
introduced in a controlled fashion into the air stream in aerosol, nebulized,
or vaporized form.
CA 02923793 2016-03-09
WO 2015/038092 PCT/US2013/000207
[00014] United States Patent Application No. 2006/0172017 Al to Rasor etal.
describes an
apparatus and methods to deliver vasoconstrictive agents simultaneously with
capnic gases. The
capnic gases can enhance the effectiveness of the vasoconstrictive agent,
lower the dosage of
drug or concentration of agent necessary to achieve a therapeutic result, or
both. Exemplary
capnic gases include carbon dioxide, nitric oxide, nitrous oxide, and dilute
acid gases.
[00015] United States Patent Application No. 2004/0009126 Al to Pilkiewicz et
al. describes
an inhalation system comprising an anti-infective agent in particle form, the
anti-infective agent
being directed toward prevention and treatment of intracellular infection, and
an inhalation
device, and a method of use of the system.
[00016] United States Patent Application No. 2002/0040205 Al to Rasor et al.
describes
methods and devices for transcutaneous and transmucosal application of carbon
dioxide in the
form of gas and in the form of a capnic solution (such as carbonated water)
for the relief of pain,
including musculoskeletal disorders, neuralgias, rhinitis and other ailments.
Gaseous carbon is
applied to the skin for at least three minutes, and the capnic solution may be
held on the skin for
at least three minutes, which provides relief of symptoms. The capnic solution
may be sprayed
onto mucous membranes such as the nose for relief of symptoms such as allergic
rhinitis.
[00017] Casale, et al., "Nasal Carbon Dioxide for the Symptomatic Treatment of
Perennial
Allergic Rhinitis," Ann Allergy Asthma Immunol., Oct. 2011, pp. 364-370,
examines the safety
and efficacy of nasal carbon dioxide on the symptoms of perennial allergic
rhinitis.
[00018] Baroody et al., "The Effect of Intranasal Carbon Dioxide on the Acute
Response to
No.sal Challenge with Allergen," Allergy Asthma Proc., May¨Jun. 2011, pp. 206-
212 describes a
study in which intranasal carbon dioxide (CO(2)) was shown to reduce symptoms
of seasonal
allergic rhinitis (SAR). This study was designed to evaluate the effect of
CO(2) on nasal allergen
6
CA 02923793 2016-03-09
WO 2015/038092 PCT/US2013/000207
challenge. We conducted a randomized, controlled, crossover trial in 12
subjects with SAR
outside their pollen season. Thirty minutes after a 20-second exposure to
CO(2) or no exposure,
subjects underwent a unilateral, localized, nasal allergen challenge. Filter
paper disks were placed
on the nasal septum to deliver a sham challenge followed by 2 increasing doses
of either grass or
ragweed allergen. Secretions were collected from both sides of the septum to
evaluate the
nasonasal reflex and were assayed for histamine. Nasal and eye symptoms were
recorded. The
primary outcome measure was the contralateral, reflex, secretory response to
allergen as
measured by secretion weights. Secondary outcome measures included ipsilateral
nasal secretion
weights, nasal and eye symptoms, levels of histamine in nasal secretions, and
eosinophils in nasal
scrapings. Subjects reported a transient burning sensation during exposure to
CO(2). Compared
with no treatment, active treatment resulted in a significant reduction in
sneezes (p = 0.05),
contralateral secretion weights (p = 0.04), and bilateral runny nose symptoms
(p = 0.01).
Ipsilateral secretion weights were numerically reduced. Histamine levels in
ipsilateral nasal
secretions increased significantly when the subjects received sham treatment
but did not increase
after pretreatment with CO(2). Treatment with nasal CO(2) resulted in partial
reduction of the
acute response to allergen challenge. Reflex responses were reduced,
supporting an effect on
neuronal mechanisms, which predict usefulness in the treatment of allergic
rhinitis.
1000191 Pagani et al., "Carbon Dioxide-Enriched Water Inhalation in Patients
With Allergic
Rhinitis and its Relationship with Nasal Fluid Cytolcine/Chemokine Release,"
Arch Med Res,
May 2011, pp. 329-333 investigates a possible in vivo effect of carbon dioxide-
enriched water
inhalation in patients with allergic rhinitis.
1000201 Casale, Romero, and Spierings, "Intranasal Noninhaled Carbon Dioxide
for the
Symptomatic Treatment of Seasonal Allergic Rhinitis," J Allergy Clin Immunol.,
Jan. 2008, pp.
7
CA 02923793 2016-03-09
WO 2015/038092
PCT/US2013/000207
105-109, studies whether noninhaled intranasal CO2 would be effective in the
treatment of
seasonal allergic rhinitis.
[00021] Notwithstanding the prior art, the present invention is neither taught
nor rendered
obvious thereby.
SUMMARY OF INVENTION
[00022] The present invention is directed to a method for treating ailments in
a patient in need
thereof. It includes the step of directing a therapeutic, non-inhaled dosage
to at least one nasal
cavity of the patient through a flow regulating device. The dosage includes a
saline fluid and a
gas contnining carbon dioxide, and I some embodiments, at least one additional
active
component, wherein the therapeutic, non-inhaled dosage is delivered at a flow
rate from 1 cc/sec
to 20 cc/sec for a duration of 2 to 30 seconds. The invention includes the
step of having the
patient substantially refrain from inhaling while the fluid is being released.
[00023] In some preferred embodiments of the present invention method for
treating ailments
in a patient in need thereof, the head ailment is rhinitis.
[00024] In some preferred embodiments of the present invention method for
treating ailments
in a patient in need thereof, the head ailment is conjunctivitis.
[00025] In some preferred embodiments of the present invention method for
treating ailments
in a patient in need thereof, the head ailment is the common cold.
[00026] In some preferred embodiments of the present invention method for
treating ailments
in a patient in need thereof, the head ailment is sinusitis.
[00027] In some preferred embodiments of the present invention method for
treating ailments
in a patient in need thereof, the head ailment is a headache.
8
CA 02923793 2016-03-09
WO 2015/038092 PCT/US2013/000207
[00028] In some preferred embodiments of the present invention method for
treating ailments
in a patient in need thereof, the flow regulating device is a single dose
dispenser with a pressure
control valve for released flow regulation.
[00029] In some preferred embodiments of the present invention method for
treating ailments
in a patient in need thereof, the flow regulating device is a multiple dose
dispenser with a
pressure control valve for released flow regulation. In some of these
preferred embodiments of
the present invention method for treating ailments in a patient in need
thereof, the multiple dose
dispenser further includes a dosage amount control mechanism and activator to
limit dosage
release amount for each activation.
[00030] In some preferred embodiments of the present invention method for
treating ailments
in a patient in need thereof, the duration is 5 to 10 seconds per nasal
cavity.
[00031] In some preferred embodiments of the present invention method for
treating ailments
in a patient in need thereof, the duration is 2 to 15 seconds per nasal
cavity.
[00032] In some preferred embodiments of the present invention method for
treating ailments
in a patient in need thereof, the dose is repeated from 1 to 10 times.
[00033] In some preferred embodiments of the present invention method for
treating ailments
in a patient in need thereof, the method is for treating rhinitis and the
rhinitis is allergic rhinitis.
[00034] In some preferred embodiments of the present invention method for
treating ailments
in a patient in need thereof, the flow rate is from 2 cc/sec to 10 cc/sec.
[00035] In some preferred embodiments of the present invention method for
treating ailments
in a patient in need thereof, the flow rate is from 1 cc/sec to 5 cc/sec.
[00036] In some preferred embodiments of the present invention method for
treating ailments
in a patient in need thereof, the flow rate is from 4 cc/sec to 5 cc/sec.
9
CA 02923793 2016-03-09
WO 2015/038092
PCT/US2013/000207
[00037] In some preferred embodiments of the present invention method for
treating ailments
in a patient in need thereof, the gas includes at least 50% carbon dioxide.
[00038] In some preferred embodiments of the present invention method for
treating ailments
in a patient in need thereof, the gas includes at least 70% carbon dioxide.
[00039] In some preferred embodiments of the present invention method for
treating ailments
in a patient in need thereof, the gas includes at least 95% carbon dioxide.
[00040] In some preferred embodiments of the present invention method for
treating ailments
in a patient in need thereof, the gas includes at least 100% carbon dioxide.
[00041] The present invention is directed to a nasal treatment delivery
device for mixed carbon
dioxide and saline for treating head ailments in a patient in need thereof,
comprising: a) a main
housing having a proximal and a distal end and having a hollow central area
containing a dosage
that includes a saline fluid and a gas containing carbon dioxide; b) a dosage
dispenser head
located at the distal end of the main housing, the dosage dispenser head
having at least one flow
channel for movement of the dosage from the main housing through the dosage
dispenser head
and to external of the dosage dispenser head; c) a dosage release control
component located
between the main housing and the dosage dispenser head adapted to permit flow
of the dosage
from the main housing and through the dosage dispenser head in response to
increased pressure
against the dosage; and d) a pressure-changing moveable component located on
the main
housing. When the dosage dispenser head of the device is placed in a nasal
cavity and the
pressure-changing moveable component is activated by movement toward the
dosage, the dosage
is at least partially forced through the dosage release control component and
through the dosage
dispenser head for application of the dosage to a nasal cavity wall.
CA 02923793 2016-03-09
WO 2015/038092
PCT/US2013/000207
[00042] In some embodiments of the present invention, the nasal treatment
delivery device for
mixed carbon dioxide and saline for treating head ailments in a patient in
need thereof, the
dosage release control component is selected from the group consisting of a
frangible member, a
puncturable member and a one-way valve.
[00043] In some embodiments of the present invention, the nasal treatment
delivery device for
mixed carbon dioxide and saline for treating head ailments in a patient in
need thereof, the main
housing is an open ended tube with the dosage release control component and
the dosage
dispenser located at the distal end of the main housing and the pressure-
changing moveable
component is located at the proximal end of the main housing.
[00044] In some embodiments of the present invention, the nasal treatment
delivery device for
mixed carbon dioxide and saline for treating head ailments in a patient in
need thereof, the
pressure-changing moveable component is a flexible squeeze member and a seal
float.
[00045] In some embodiments of the present invention, the nasal treatment
delivery device for
mixed carbon dioxide and saline for treating head ailments in a patient in
need thereof, the
pressure-changing moveable component is a push-up piston.
[00046] In some embodiments of the present invention, the nasal treatment
delivery device for
mixed carbon dioxide and saline for treating head ailments in a patient in
need thereof, the
dosage release control component is selected from the group consisting of a
frangible member, a
puncturable member and a one-way valve.
[00047] In some embodiments of the present invention, the nasal treatment
delivery device for
mixed carbon dioxide and saline for treating head ailments in a patient in
need thereof, the main
housing is a tube having an open distal end and a closed proximal end, with
the dosage release
control component and the dosage dispenser head being located at the distal
end of the main
11
CA 02923793 2016-03-09
WO 2015/038092 PCT/US2013/000207
housing, and at least a portion of the tube is flexible and constitutes the
pressure-changing
moveable component.
[00048] In some embodiments of the present invention, the nasal treatment
delivery device for
mixed carbon dioxide and saline for treating head ailments in a patient in
need thereof, the
dosage release control component is selected from the group consisting of a
frangible member, a
puncturable member and a one-way valve.
[00049] In some embodiments of the present invention, the nasal treatment
delivery device for
mixed carbon dioxide and saline for treating head ailments in a patient in
need thereof, the
dosage dispenser head has a plurality of flow channels.
[00050] In some embodiments of the present invention, the nasal treatment
delivery device for
mixed carbon dioxide and saline for treating head ailments in a patient in
need thereof, the
dosage dispenser head has a circular shape from a top viewpoint.
[00051] In some other embodiments of the present invention, the nasal
treatment delivery
device for mixed carbon dioxide and saline for treating head ailments in a
patient in need thereof,
the nasal treatment delivery device for mixed carbon dioxide and saline for
treating head
ailments in a patient in need thereof, comprising: a) a main housing having a
proximal and a
distal end and having a hollow central area containing a dosage that includes
a saline fluid and a
gas containing carbon dioxide; b) a dosage dispenser head located at the
distal end of the main
housing, the dosage dispenser head having at least one flow channel for
movement of the dosage
from the main housing through the dosage dispenser head and to external of the
dosage dispenser
head; c) a nose guard flange connected to and extending from at least one of
the main housing
and the dosage dispenser head; d) a dosage release control component located
between the main
housing and the dosage dispenser head adapted to permit flow of the dosage
from the main
12
_
CA 02923793 2016-09-30
housing and through the dosage dispenser head in response to increased
pressure against the
dosage; e) a pressure-changing moveable component located on the main housing;
wherein, when
the dosage dispenser head of the device is placed in a nasal cavity and the
pressure-changing
moveable component is activated by movement toward the dosage, the dosage is
at least partially
forced through the dosage release control component and through the dosage
dispenser head for
application of the dosage to a nasal cavity wall. In this particular
embodiment with the nose
guard flange, all of the other details and features set forth in the previous
paragraphs, may be
included.
100051a1 According to one particular aspect, the invention relates to a
releasable nasal
treatment delivery device for mixed carbon dioxide and saline for treating
head ailments in a
patient in need thereof, comprising:
a) a main housing having a proximal and a distal end and having a hollow
central area
containing a releasable nasal treatment dosage that includes a saline fluid
and a gaseous carbon
dioxide, wherein said gaseous carbon dioxide is dissolved in said saline
fluid;
b) a dosage dispenser head located at said distal end of said main housing,
said dosage
dispenser head having at least one flow channel for movement of said dosage
from said main
housing through said dosage dispenser head and externally of said dosage
dispenser head;
c) a dosage release control component located between said main housing and
said dosage
dispenser head adapted to permit flow of the dosage comprising said saline
fluid and said gaseous
carbon dioxide from said main housing and through said dosage dispenser head
in response to
increased pressure against said dosage release control component wherein said
permitted flow has
controlled release at a flow rate of between 1.0 cubic centimeter per second
and 5.0 cubic
centimeters per second and wherein said flow rate is a total flow rate of said
dosage:
d) a pressure-changing moveable component located on said main housing,
wherein;
when said dosage dispenser head of said device is placed in a nasal cavity and
said
13
CA 02923793 2016-09-30
pressure-changing moveable component is activated by movement toward said
dosage, said dosage
is at least partially forced through said dosage release control component at
the permitted flow rate
and through said dosage dispenser head for application of said dosage to a
nasal cavity wall.
100051b1 According to another particular aspect, the invention relates to a
nasal treatment
delivery device for mixed carbon dioxide and saline for treating head ailments
in a patient in need
thereof, comprising:
a) a main housing having a proximal and a distal end and having a hollow
central area
containing a dosage that includes a saline fluid and gaseous carbon dioxide
wherein said gaseous
carbon dioxide is dissolved in said saline fluid;
b) a dosage dispenser head located at said distal end of said main housing,
said dosage
dispenser head having at least one flow channel for movement of said dosage
from said main
housing through said dosage dispenser head and externally of said dosage
dispenser head;
c) a nose guard flange connected to and extending from at least one of said
main housing
and said dosage dispenser head;
d) a dosage release control component located between said main housing and
said dosage
dispenser head adapted to permit flow of said dosage that comprises both the
saline fluid and said
gaseous carbon dioxide from said main housing and through said dosage
dispenser head in
response to increased pressure against said dosage release component;
e) a pressure-changing moveable component located on said main housing,
wherein;
when said dosage dispenser head of said device is placed in a nasal cavity and
said
pressure-changing moveable component is activated by movement toward said
dosage, said dosage
is at least partially forced through said dosage release control component for
application of said
dosage to a nasal cavity wall and wherein said dosage is at least partially
forced through said
dosage release control component and through said dosage dispenser head at a
flow rate of'
between 1.0 cubic centimeter per second and 5.0 cubic centimeters per second
wherein said flow
rate is a total flow rate of said dosage.
13a
CA 02923793 2016-09-30
100051CI
According to another particular aspect, the invention relates to the use a
device as
defined above for delivering under pressure a therapeutic, non-inhaled nasal
treatment dosage to at
least one nasal cavity of a patient in need thereof.
[00051d] According to another particular aspect, the invention relates to
the use a device as
defined above for directing at least a portion of said nasal treatment dosage
to at least one nasal
cavity of a patient in need thereof.
100051e1 According to another particular aspect, the invention relates to
the use of a flow
regulating device for delivering a therapeutic, non-inhaled nasal treatment
dosage to at least one
nasal cavity of a patient in need thereof, wherein said dosage comprises a
saline fluid and a gas
containing carbon dioxide, and wherein said dosage is delivered under pressure
to said at least one
nasal cavity at a flow rate of from 1 cc/sec to 5 cc/sec.
1000521
Additional features, advantages, and embodiments of the invention may be set
forth
or apparent from consideration of the following detailed description,
drawings, and claims.
Moreover, it is to be understood that both the foregoing summary of the
invention and the
following detailed description are exemplary and intended to provide further
explanation without
limiting the scope of the invention as claimed.
BRIEF DESCRIPTION OF THE DRAWINGS
[00053] The accompanying drawings, which are included to provide a further
understanding of the invention and are incorporated in and constitute a part
of this specification,
illustrate preferred embodiments of the invention and together with the detail
description serve
to explain the principles of the invention. In the drawings:
1000541 Figure la and Figure lb are block diagrams of embodiments of the
present
invention carbon dioxide and saline nasal delivery methods and treatments,
with and without
one or more additional active components;
1 3 b
CA 02923793 2016-03-09
WO 2015/038092
PCT/US2013/000207
[00055] All of the following Figures are described as carbon dioxide and
saline solutions, it
being understood that this means with and without at least one additional
active component;
[00056] Figure 2 is a block diagram showing head ailments treated by various
embodiments of
the present invention carbon dioxide and saline nasal delivery methods and
treatments;
[00057] Figure 3 is a block diagram showing durations for therapeutic non-
inhaled dosage in
some preferred embodiments of the present invention carbon dioxide and saline
nasal delivery
methods and treatments;
[00058] Figure 4 is a block diagram of another embodiment of the present
invention carbon
dioxide and saline nasal delivery methods and treatments, showing the
additional step of
repeating the other steps;
[00059] Figure 5 is a block diagram showing flow rates in some additional
preferred
embodiments of the present invention carbon dioxide and saline nasal delivery
methods and
treatments;
[00060] Figure 6 is a block diagram showing the percentage of carbon dioxide
present in the
gas in some preferred embodiments of the present invention carbon dioxide and
saline nasal
delivery methods and treatments;
[00061] Figure 7 illustrates a block diagram showing monodose and multidose
dispensers that
may be used in the present invention methods;
[00062] Figure 8 illustrates a front partially cut view of one embodiment
of a present invention
nasal treatment delivery device with a pressure release mechanism;
[00063] Figure 9 illustrates a view of one embodiment of a present invention
nasal treatment
delivery device that is a squeeze to release device;
14
CA 02923793 2016-03-09
WO 2015/038092
PCT/US2013/000207
[00064] Figure 10 shows a front partially cut view of a present invention
nasal treatment
delivery device with a piercing channel, with the device being held in a hand
using two fingers
and a thumb to activate release of the medicinal treatment;
[00065] Figures 11, 12 and 13 illustrate front partially cut views of one
embodiment of a
present invention nasal treatment delivery device with a frangible internal
medicine capsule that
may be used for a monodose or multidose using replacement cartridges. The
three Figures show
the device in different stages of use; and,
[00066] Figures 14 and 15 show alternative types of dosage dispenser heads
that may be used
in present invention devices, one showing multiple release ports and the other
showing multiple
release ports with a soft contact sheath.
DETAILED DESCRIPTION OF THE EMBODIMENTS
[00067] "Saline" and "saline solution" as used herein means water
containing salt. Saline
solutions are used in a wide variety of medical applications. For example,
"normal saline" is the
commonly used term for a solution of 0.90% w/v of sodium chloride (NaC1).
Normal saline is
frequently used in intravenous drips for patients unable to take fluids orally
to prevent
dehydration. Normal saline is also used to flush wounds and skin abrasions.
Another application
of saline solution is as a rinse for contact lenses.
[00068] Saline solution also is frequently used in nasal washes to treat some
of the symptoms
of the common cold or other ailments adversely affecting the nasal cavities.
By irrigating the
nasal passages with saline, inflammation can be reduced. Also, more
concentrated
("hypertonic") solutions of NaC1, can have therapeutic uses. For example, 7%
NaCl/water
CA 02923793 2016-03-09
WO 2015/038092 PCT/US2013/000207
solutions are considered mucoactive agents and as such are used to hydrate
thick secretions
(mucous) in order to make it easier to cough up and out (expectorate).
[00069] Another chemical substance useful in medical treatments is carbon
dioxide. One
example is the use of diluted carbon dioxide by inhalation for treating
symptoms related to
headaches, allergies, asthma, nervous disorders, and other common ailments,
which was
demonstrated in the 1940s and 1950s. Another example is the use of high-
concentration, non-
inhaled carbon dioxide, delivered to the nasal passages locally. This type of
treatment may
provide fast relief without the adverse side effects of systemic drugs that
are inhaled, ingested, or
injected.
[00070] By combining the beneficial therapeutic effects of saline treatment
and carbon dioxide
treatment, an improved therapy is created. In this way, the beneficial effects
of the saline, such
as reduced inflammation and expectoration of mucous, are combined with the
beneficial effects
of carbon dioxide therapy, such as relief from headaches, allergies, asthma,
nervous disorders,
and other common ailments. Further, the saline moisturizes the nasal cavities
and acts as a base
host for the carbon dioxide as it acts on the nasal cavity walls. (It is
hypothesized that at least
some of the carbon dioxide is adsorbed by the saline.) In addition, the saline
reduces any slight
burning that might otherwise be felt from the carbon dioxide. The benefits of
saline treatment
are supplemented by the benefits of carbon dioxide treatment, and the benefits
of carbon dioxide
treatment are supplemented by the benefits of saline treatment. This
combination of utilizing the
saline to perform at least moisturizing and other beneficial affects while
carrying and enhancing
the delivery of the carbon dioxide is an unexpected synergistic result
thereof.
[00071] In addition to the benefits listed above, the present invention
carbon dioxide and saline
nasal delivery methods and treatments have other synergistic benefits that are
not available from
16
CA 02923793 2016-03-09
WO 2015/038092
PCT/US2013/000207
either saline treatment or carbon dioxide treatment alone. For example, the
presence of dissolved
carbon dioxide in the saline solution means that the solution will be
carbonated; the effervescent
effect of the carbon dioxide helps the saline solution to mix more
energetically against the
interior surface of the nasal cavity or cavities. This improved mixing allows
the saline treatment
to be more effective. Another potential advantage of combining carbon dioxide
and saline
treatments is that in some embodiments, with sufficient pressure and a proper
nozzle, the carbon
dioxide can act as a carrier gas for the saline, allowing the saline solution
to be aerosolized.
[00072] To summarize the advantages and benefits of the present invention, the
combination of
controlled delivery carbon dioxide and saline provides the following: it
cleanses the nasal cavity
removing allergens and particulates that cause inflammation and congestion;
its special formula
shields nasal mucosa from viruses; it soothes and moisturizes irritated
mucosa; its unique
buffering system neutralizes inhaled irritants such as oxidative free radicals
and endogenous
cytotoxins which cause inflammation and damage to the sensitive mucosa and
muco-cillary hairs
in the nasal cavity; it enhances mucous clearance and flow by reducing mucus
viscosity; its
superior safety profile gives it broader application than corticosteroids and
decongestants and can
be used safely in children 6 months of age and adults, even with co-
morbidities such as diabetes,
hypertension, suppressed immune systems and pregnant and nursing females; and
its exceptional
safety profile allows for flexible dosing.
[00073]
In addition to the beneficial carbon dioxide and saline, as described above,
in some
preferred embodiments of the present invention, there is further included at
least one additional
active component. These active components may be any of one or more beneficial
additions that
are compatible with saline and have some medicinal, curative, pain relieving
or moisturizing
effect on the sinus cavity walls, vascular system or upper respiratory system.
These include, but
17
CA 02923793 2016-03-09
WO 2015/038092 PCT/US2013/000207
are not limited to, moisturizers, humectants, over the counter drugs and
prescription drugs. Such
drugs may be antihistamines, infection treatments, antioxidants, cell growth
accelerators, anti-
inflammatories, vasoconstrictors, nasal decongestants, or other nasal cavity,
wall or upper
respiratory treatments. Preferred actives are moisturizers, decongestants,
antihistamines,
infection treatments and anti-inflammatories. Examples of moisturizers and
humectants are:
glycerin, propylene glycols (MW 400 to 8000), maltodextrins (liquid), honey,
pectin,
hydroxypropyl methylcellulose, and carboxymethylcellulose. Examples of topical
decongestants
are: ephedrine, levomethamphetamine, naphazoline, oxymetazoline,
phenylephrine,
pseudoephedrine, tramazoline, and xylometazoline. The actives may also be
fragrance sensations
or fragrance with other benefits, such as eucalyptus, menthol or lavender.
[00074] Referring now to the drawings, like reference numerals designate
corresponding parts
throughout the several views, various embodiments of the present invention are
shown.
[00075] Figure 1 a is a block diagram of an embodiment of the present
invention carbon
dioxide, saline and additional active component(s) nasal delivery methods and
treatments.
However, because the present invention includes the dosages with and without
other actives, the
additional additives in this Figure 1 a are excluded in Figure 1 b, and both
Figure 1 a and lb are to
be taken as discussed together as Figure la, it being understood that Figure
lb illustrates a the
invention without additional additives, but the Figures are otherwise the
same, and the discussion
below of Figure la, except for the additional actives, applies also to Figure
lb.
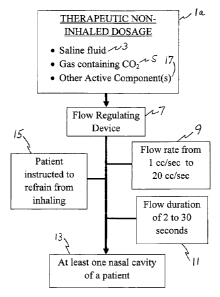
[00076] Figure la illustrates a therapeutic non-inhaled dosage 1, containing
saline fluid 3, a
carbon dioxide-containing gas 5 and at least one additional active 17. The
saline fluid 3 contains
water and at least one salt. In some preferred embodiments of the present
invention, the salt is
sodium chloride. In other embodiments of the present invention, other salts
may be used, but it is
18
CA 02923793 2016-03-09
WO 2015/038092 PCT/US2013/000207
important that any salt used in the saline fluid 3 must be safe for intranasal
use. In some
preferred embodiments of the present invention, the concentration of salt in
the saline fluid is
approximately isotonic with the salt concentration of bodily fluids. In other
preferred
embodiments, the concentration of salt in the saline fluid is less than the
concentration of salt in
bodily fluids. In still other preferred embodiments, the concentration of salt
in the saline fluid is
hypertonic, meaning that it has a salt concentration higher than that of
bodily fluids. In still other
preferred embodiments, the saline solution is saturated with salt.
[00077] The gas 5 contains some portion of carbon dioxide. When the gas 5
containing carbon
dioxide is added to the saline fluid 3, the saline fluid 3 becomes carbonated.
If the therapeutic
non-inhaled dosage 1 containing saline fluid 3 and the gas 5 is kept under
pressure, the pressure
can later be released (for example by opening a valve), which causes some of
the carbon dioxide
to bubble out of the solution. This sudden release of carbon dioxide creates
effervescence in the
therapeutic non-inhaled dosage.
[00078] The therapeutic non-inhaled dosage travels through a flow-regulating
device 7. In
preferred embodiments, the flow-regulating device 7 controls the flow rate 9
of the therapeutic
non-inhaled dosage 1 at a rate that is safe and comfortable for the patient.
In the embodiment
shown in Figure la, the flow rate 9 of the therapeutic non-inhaled dosage is
between 1 cubic
centimeter per second (cc/sec) and 20 cc/sec. In preferred embodiments of the
present invention
shown in Figure la, the flow rate is adjustable to any value between 1 cc/sec
and 20 cc/sec.
[00079] The therapeutic non-inhaled dosage 1 has a flow duration 11. The flow
duration 11 is
the length of time during which the therapeutic non-inhaled dosage flows
through the flow
regulating device into at least one nasal cavity 13 of a patient. In the
embodiment shown in
Figure la, the flow duration 11 is shown as lasting between 2 and 30 seconds.
In preferred
19
CA 02923793 2016-03-09
WO 2015/038092
PCT/US2013/000207
embodiments of the present invention, the flow duration is adjustable to any
value between 2 and
30 seconds.
1000801 After the therapeutic non-inhaled dosage 1 leaves the flow
regulating device 7, it enters
at least one nasal cavity 13 of a patient. The therapeutic non-inhaled dosage
1 is adsorbed by the
nasal tissue and subsequently absorbed by the body. This adsorption and
subsequent absorption
can have a beneficial effect on many head ailments, some of which are shown in
Figure 2. The
effervescent effect of the gas 5 containing carbon dioxide causes better
contact between the salt
in the saline solution 3 and the nasal tissue.
1000811 The additional step 15 of instructing the patient to refrain from
inhaling protects the
patient from accidently inhaling the therapeutic non-inhaled dosage 1. This is
important, even
critical, when the therapeutic non-inhaled dosage 1 contains a gas 5 that is
substantially 100%
carbon dioxide to prevent carbon dioxide poisoning (hypercapnia). Even mild
hypercapnia can
cause uncomfortable mental and physical effects. Also, when the concentration
of salt in the
saline solution 3 is greater than isotonic (particularly if salts other than
sodium chloride are used),
it is desirable to limit the patient's exposure to the salts. The step 15 of
instructing the patient not
to breathe accomplishes these goals.
1000821 Turning now to Figure 2, a block diagram, block 20, shows some of the
medical
conditions that can be treated using the present invention carbon dioxide and
saline (with and
without additional actives) nasal delivery methods and treatments. In some
embodiments of the
present invention, the carbon dioxide and saline nasal delivery methods and
treatments treat
rhinitis 17, a swelling of some internal parts of the nose. In other
embodiments, the present
invention treats allergic rhinitis 19. In still other embodiments, the present
invention treats
conjunctivitis 21, an inflammation of the conjunctiva also known as pink-eye.
In still other
CA 02923793 2016-03-09
WO 2015/038092
PCT/US2013/000207
embodiments of the present invention, the common cold 23 is treated. In other
embodiments of
the present invention, sinusitis 25, an inflammation of the sinuses, is
treated. In yet other
embodiments, the present invention is used to treat headaches 27. It is
important to recognize
that in some embodiments of the present invention carbon dioxide and saline
nasal delivery
methods and treatments, multiple conditions can be treated simultaneously. For
example, a
patient may be suffering from both sinusitis and headache simultaneously; the
present invention
can alleviate both conditions at the same time. The present invention can
treat any ailment
shown in Figure 2 or any combination of those ailments. It should also be
recognized that the
present invention may be useful in treating other ailments, particularly head
ailments. The
treatment of other ailments on which the present invention carbon dioxide and
saline nasal
delivery methods and treatments is effective are considered to be within the
scope of the
invention.
[00083] Turning now to Figure 3, a block diagram, block 30, shows the
durations of therapeutic
non-inhaled dosage used in some embodiments of the present invention carbon
dioxide and
saline nasal delivery methods and treatments. The durations listed in Figure 3
are ranges, so the
actual duration can be any value between the low end of the range and the high
end of the range,
inclusive. In some embodiments of the present invention, the duration 29 lasts
between 2 and 30
seconds. In other embodiments of the present invention, the duration 31 lasts
between 2 and 15
seconds. In still other embodiments of the present invention, the duration 33
lasts between 5 and
ten seconds. Durations of less than 2 seconds and more than 30 seconds are
also considered
to be within the scope of the invention.
[00084] Turning now to Figure 4, another embodiment of the present invention
carbon dioxide
and saline nasal delivery methods and treatments is shown. Figure 4 is a block
diagram of an
21
CA 02923793 2016-03-09
WO 2015/038092
PCT/US2013/000207
embodiment of the present invention carbon dioxide and saline nasal delivery
methods and
treatments that incorporates many aspects shown in Figures la and 1 b, and
identical blocks are
identically numbered. Figure 4 illustrates a therapeutic non-inhaled dosage 1
containing saline
fluid 3 and a gas 5. The saline fluid 3 contains water and at least one salt.
In some preferred
embodiments of the present invention, the salt is sodium chloride. In other
embodiments of the
present invention, other salts may be used, but it is important that any salt
used in the saline fluid
3 must be safe for intranasal use. In some preferred embodiments of the
present invention, the
concentration of salt in the saline fluid is approximately isotonic with the
salt concentration of
bodily fluids. In other preferred embodiments, the concentration of salt in
the saline fluid is less
than the concentration of salt in bodily fluids. In still other preferred
embodiments, the
concentration of salt in the saline fluid is hypertonic, meaning that it has a
salt concentration
higher than that of bodily fluids. In still other preferred embodiments, the
saline solution is
saturated with salt.
1000851 The gas 5 contains some portion of carbon dioxide. When the gas 5
containing carbon
dioxide is added to the saline fluid 3, the saline fluid 3 becomes carbonated.
If the therapeutic
non-inhaled dosage 1 containing saline fluid 3 and the gas 5 is kept under
pressure, the pressure
can later be released (for example by opening a valve), which causes some of
the carbon dioxide
to bubble out of the solution. This sudden release of carbon dioxide creates
effervescence in the
therapeutic non-inhaled dosage.
[00086] The therapeutic non-inhaled dosage travels through a flow-regulating
device 7. In
preferred embodiments, the flow-regulating device 7 controls the flow rate 9
of the therapeutic
non-inhaled dosage 1 at a rate that is safe and comfortable for the patient.
In the embodiment
shown in Figure la, the flow rate 9 of the therapeutic non-inhaled dosage is
between 1 cubic
22
CA 02923793 2016-03-09
WO 2015/038092
PCT/US2013/000207
centimeter per second (cc/sec) and 20 cc/sec. In preferred embodiments of the
present invention
shown in Figure la, the flow rate is adjustable to any value between 1 cc/sec
and 20 cc/sec.
[00087] The therapeutic non-inhaled dosage 1 has a flow duration 11. The
flow duration 11 is
the length of time during which the therapeutic non-inhaled dosage flows
through the flow
regulating device into at least one nasal cavity 13 of a patient. In the
embodiment shown in
Figure la, the flow duration 11 is shown as lasting between 2 and 30 seconds.
In preferred
embodiments of the present invention, the flow duration is adjustable to any
value between 2 and
30 seconds.
[00088] After the therapeutic non-inhaled dosage 1 leaves the flow
regulating device 7, it enters
at least one nasal cavity 13 of a patient. The therapeutic non-inhaled dosage
1 is adsorbed by the
nasal tissue. This adsorption can have a beneficial effect on many head
ailments, some of which
are shown in Figure 2. The effervescent effect of the gas 5 containing carbon
dioxide causes
better contact between the salt in the saline solution 3 and the nasal tissue.
[00089] The additional step 15 of instructing the patient to refrain from
inhaling protects the
patient from accidently inhaling the therapeutic non-inhaled dosage 1. This is
important, even
critical, when the therapeutic non-inhaled dosage 1 contains a gas 5 that is
substantially 100%
carbon dioxide to prevent carbon dioxide poisoning (hypercapnia). Even mild
hypercapnia can
cause uncomfortable mental and physical effects. Also, when the concentration
of salt in the
saline solution 3 is greater than isotonic (particularly if salts other than
sodium chloride are used),
it is desirable to limit the patient's exposure to the salts. The step 15 of
instructing the patient not
to breathe accomplishes these goals.
[00090] In the embodiment shown in Figure 4, after the therapeutic non-inhaled
dosage 1
passes through the flow regulating device 7 and into the at least one nasal
cavity 13 of a patient,
23
CA 02923793 2016-03-09
WO 2015/038092
PCT/US2013/000207
the dose is repeated 35. In some preferred embodiments, the dose is repeated
35 between one
and ten times. In still other embodiments, the dose is repeated more than ten
times. The step 35
of repeating the dose can be used if a single application of the therapeutic
non-inhaled dosage 1 is
insufficient to alleviate the head ailment or ailments from which the patient
suffers.
1000911 Turning now to Figure 5, a block diagram, block 50, shows flow
rates used in some
embodiments of the present invention carbon dioxide and saline nasal delivery
methods and
treatments. The flow rates used in Figure 5 are shown as ranges, and the
actual rate of the flow
may any value between the low end of the range and the high end of the range,
inclusive. In
some embodiments, a rate 37 between 1 cc/sec and 20 cc/sec is used. In other
embodiments, a
flow rate 39 between 2 cc/sec and 10 cc/sec is used. In other preferred
embodiments, a flow rate
41 between 1 cc/sec and 5 cc/sec is used. In still other preferred
embodiments, a flow rate 43
between 4 cc/sec and 5 cc/sec is used. In still other preferred embodiments, a
flow rate 45 of
approximately 10 cc/sec is used. Embodiments with flow rates of less than 1
cc/sec or more than
20 cc/sec are also considered to be within the scope of the invention.
[00092] Turning now to Figure 6, a block diagram, block 60, shows levels of
carbon dioxide in
the gases used in some embodiments of the present invention. The levels of
carbon dioxide are
expressed as a percentage of the gas (3 in Figures 1 and 4) used in the
therapeutic non-inhaled
dosage (1 in Figures 1 and 4). In some embodiments of the present invention
carbon dioxide and
saline nasal delivery methods and treatments, the amount of carbon dioxide 47
in the gas is at
least 50%. In other embodiments, the amount of carbon dioxide 49 in the gas is
at least 70%. In
still other embodiments, the amount of carbon dioxide 51 in the gas is at
least 95%. In other
preferred embodiments, the amount of carbon dioxide 53 in the gas is
substantially 100%. Gases
24
CA 02923793 2016-03-09
WO 2015/038092
PCT/US2013/000207
with a percent composition of less than 50% carbon dioxide are also considered
to be within the
scope of the invention.
[000931 Figure 7 illustrates a block diagram showing nasal treatment delivery
devices that may
be used in the present invention methods. Here, block 71 illustrates the
caption of the Figure,
namely, nasal treatment delivery devices. Block 73 shows that the flow
regulating device used in
the present invention methods may be a single dose dispenser (monodose) with a
pressure control
valve for flow rate regulation. The rate of flow is set in accordance with the
ranges set forth
above. In the case of a monodose dispenser, the entire dose is dispensed, so
that time of
dispensing does not need to be controlled- it is just the controlled flow rate
over time it takes to
unload the dose. Thus, a monodose dispenser may controllably release a
pressurized mixture of
the carbon dioxide and the saline, until it stops flowing. The various types
of mechanisms for
driving the contents from the container to the nasal cavity are also
exemplified. These include
squeeze mechanisms where the squeeze component or bulb is below the content so
that external
squeeze pressure forces out the content, much like a turkey baster; squeeze
mechanisms where
the squeeze component is the actual dose holding aspect of the container, like
a nasal
decongestant squeeze spray container; push mechanisms that physically operate
much like
syringes but may have more complex internal aspects, such as piercers or
counter-biased valving;
and others, referring to any known controlled flow mechanism available to the
artisan.
1000941 On the other hand, a plural or multidose dispenser may be used, and
needs dispensing
on/off control, otherwise the entire contents could be unnecessarily released
in one shot. Thus,
block 75 illustrates the use of a multidose dispenser with a pressure control
valve for flow rate
regulation. The rate of flow is set in accordance with the ranges set forth
above. Block 77 shows
one multidose dispenser option wherein the user controls the release time, so
that there is variable
CA 02923793 2016-03-09
WO 2015/038092
PCT/US2013/000207
dosage. For example, there may be an activator, such as a push button or a
squeeze mechanism
to release the dosage, and the user may be directed to dispense for a time,
e.g., dispense fOr eight
to ten seconds. Alternatively, as shown in block 79, an auto-controlled
release mechanism may
be used, e.g., a spring return release that closes a valve based on set
timing, or a dual spring
device with one being reverse spring mechanism that returns a lever to control
the time of
release. Timed valving is well known in the field of medicine dispensing and
any available
multidose fixed time dispensing mechanism may be utilized.
[00095] In
Figure 7, block 73 shows the main housing and dosage. It contains a dosage of
saline fluid and carbon dioxide gas according to parameters as more
specifically set forth above.
Block 79 shows that the main housing 73 may have two open ends or one open
end. In the case
of one open end, the top end would include the release control and dispenser
head mechanisms,
with a closed bottom. In the case of a main housing with two open ends, one
end would have the
release control and dispenser head mechanisms and the other end would contain
a moveable drive
mechanism such as a pressure release mechanism, a piercer or a plunger (drive
piston). Block 81
shows that the main housing 73 may be at least partially flexible or it may be
inflexible. If the
driver is the squeezing of the main housing, it must be flexible. If the
driver a moveable
component attached to the main housing 71 (a push or squeeze mechanism), then
the main
housing 71 is preferably inflexible.
[00096] Block 83 shows the dosage release control component. Block 85
illustrates the options
for the dosage release control component, which are: frangible, puncturable,
one-way valve, or
gate. Block 87 shows the dosage dispenser head, which Block 89 then shows the
options for,
which are: perforated, hard, soft, or delivery cover (sponge, foam, cotton
batting, or other).
Block 74 shows the optional nose guard flange for the nacal treatment delivery
device 71.
26
CA 02923793 2016-03-09
WO 2015/038092
PCT/US2013/000207
[00097]
Figure 8 illustrates a front partially cut view of one embodiment of a
present invention
nasal treatment delivery device 90. It includes a main housing 91 with a top
93 having a hollow
central area containing a dosage of the present invention medicine. This
storage area may be the
inside of the main housing, or it may be one or more subunits- compartments,
capsules, tanks,
pouches, etc, within the main housing.
[00098]
In this embodiment, the main housing 91 has attached to its distal end a
dosage control
component that is a spray release nozzle 95 that is set for prescribed flow
rates within the ranges
set forth in the present invention claims and as described above. Internal bag
container 105
contains the liquid/ gas mixture of the present invention and external
pressure on bag 105 is
created by pressurized gas located in space 107 inside main housing 91. At top
93 is a dosage
dispenser head, in this case, a push dispenser mechanism 97 that includes
release orifice 101,
actuation tube 99 and push pad 103. A user inserts push dispenser mechanism 97
into a nasal
cavity at its distal end (orifice 101) while holding nasal treatment delivery
device 90 and then
pressing push pad 103 to release the contents. The flow regulation is set to
an acceptable range
so as to be relatively gentle to the user. This may include ranges in the
order of 1 cc/sec to 10 cc
per second. Typically this is a multidose device wherein the user is given
instructions to dispense
for a specified time period while not breathing, e.g., three seconds at full
depression per nostril
twice a day as needed. Alternatively, a built-in timer could automatically
control the dose. For
example, the device could have a slow spring closure that would require reset
and re-push to
reactivate.
[00099]
Figure 9 shows an alternative nasal treatment delivery device 110. This is
an insert and
squeeze device that includes a main body 111 with flexible walls and a
dispensing nozzle 115 at
its top 113. There is a stop 117 and threads 109 and a tapered dispensing tip
119 designed for
27
CA 02923793 2016-03-09
WO 2015/038092
PCT/US2013/000207
nasal cavity insertion. There is a flow control valve 112 that regulates the
rate of delivery.
Additional valving, such as a duck bill valve, may also be included. The
present invention
liquid/gas mixture is contained within the main housing 111 and is dispensed
by a user inserting
and squeezing while holding his/her breath.
[000100] Figure 10 shows a front partially cut view of a present invention
nasal treatment
delivery device 120 being held in a hand using two fingers and a thumb, as
shown. There is a
main housing 121 and a vertically moveable piston 131. A rigid, semi-flexible
or flexible
container or pouch 123 contains the liquid/gas mixture of the present
invention and piercing tube
125 is connected to flow control valve 127. A user holds nasal treatment
delivery device 120 as
shown, inserts it into a nasal cavity, and while not breathing, pushes piston
131 upwardly to force
pouch 123 to rupture via piercing tube 125 for medicine release through valve
127 to the nasal
cavity walls.
[000101] Figures 11, 12 and 13 illustrate front partially cut views of one
embodiment of a
present invention nasal treatment delivery device 150 with a frangible
internal medicine capsule
171 containing medicine 175-the gas and liquid mixtures described above.
Device 150 may be
used for a monodose or multidose using replacement capsules. The three Figures
show the
device in different stages of use. Identical number is used for all three of
the figures and the
device 150 is described collectively for all of these figures.
[000102] Device 150 is a push device that relies upon a frangible capsule
171 to deliver the
medicine 175 by breaking open the top 173 of the frangible capsule 171. Device
105 includes a
main housing 151 designed with both an open top and an open bottom, as shown.
Permanently
inserted into the open top of main housing 151 is a dosage dispensing head
161, with release tube
165 and control valve 153. Dosage dispensing head 161 has a downward
hemispherical end 163
28
CA 02923793 2016-03-09
WO 2015/038092
PCT/US2013/000207
for puncturing the top 173 (e.g., a foil top) of capsule 171. A circular
platform or dual
protrusions, such as platform 167, serves as a finger grip and is attached to
main housing 151.
Capsule 171 may be permanently installed in main housing 151, or it may be
removably placed
therein so that subsequent capsules may be inserted, the former being a
monodose and the latter
being a multidose device.
[000103]
Further, capsule 171 may be fully frangible, but is preferably so only at its
top 173.
Capsule 171 could have different shape, such as a heminspherical bottom to
correspond to the
shape of the end 163 of the dosage dispensing head 161. Or both could have
other shapes and be
the same or different, e.g., a chisel shaped end/bottom. Plunger 157 has a
sealed piston159 at its
distal end and a widened finger rest at its proximal end. Plunger 157 may be
inserted at its distal
end permanently or removably, and its piston 159 may be any shape, but is
preferably the same
or similar to the bottom of the capsule. The piston 159 is used to drive the
capsule 171 into
breaker end 163, as shown sequentially in Figures 11, 12 and 13. In Figure 11,
a user's thumb
and first two fingers are shown embracing the plunger 157 and the platform
167, respectively. By
placing the device 150 in a desired nasal cavity and pushing plunger 157
upwardly while holding
the device steady, and while the user holds his/her breathe, the frangible top
173 is broken and
the gas/liquid medicine begins release from the device 150 (Figure 12). The
medicine is nearly
fully expended by the time the plunger 157 is pushed maximally and the top 163
is near or at the
bottom of the capsule 171 (Figure 13), to deliver the medicine to the user
effectively.
[000104] Figures 14 and 15 show alternative types of dosage dispenser heads
that may be used
in present invention device one has multiple release ports and the other has
multiple release ports
with a soft contact sheath. Figure 14 shows a cut front view of one dosage
dispenser head 180
that may be used in conjunction with a present invention device. It includes a
control valve 181
29
CA 02923793 2016-03-09
WO 2015/038092
PCT/US2013/000207
to regulate release of medicine to be within the proscribed ranges set forth
above. Upstream from
control valve 181 is a main flow channel 183 with branches 185, 187, 189,
191,193 and 195 to
show a diverse multiport manifold head for diverse. This dosage dispensing
head will direct the
gas/liquid medicine in many directions simultaneously to more evenly and
quickly coat the sinus
cavity wall.
[000105] Figure 15 shows a similar present invention dosage dispensing head
200, but with a
soft pad for nasal wall comfort. This pad does not cover the ports and may be
made of soft
pervious or impervious materials such as various foams or skins.
Alternatively, they may be
previous and cover the parts so as to create wetting foams or sponges to
effect broad based
medicine placement in the nasal cavity.
[000106] Although particular embodiments of the invention have been described
in detail herein
with reference to the accompanying drawings, it is to be understood that the
invention is not
limited to those particular embodiments, and that various changes and
modifications may be
effected therein by one skilled in the art without departing from the scope or
spirit of the
invention as defined in the appended claims.