Note: Descriptions are shown in the official language in which they were submitted.
CA 02927984 2017-01-27
PRODUCTION OF CHROMIUM IRON ALLOYS
DIRECTLY FROM CHROMITE ORE
BACKGROUND OF THE INVENTION
[0002] This invention pertains to the production of chromium iron alloys
directly
from chromite ore.
100031 Chromium
is an irreplaceable ingredient in all grades of stainless steels. It is
the ingredient that renders steel "stainless". It is present in alloys in
amounts from 12% to about
35% Cr, with generally the more Cr the more corrosion resistant. It is also a
key ingredient in
the high end "super alloys" used for turbines and jet engines. Chromite ores
are the only source
of chromium. The majority of chromite ores are processed into an intermediate
product called
high carbon ferro-chrome, or charge chrome, an alloy containing greater than
50% Cr, about
6-8% C, varying amounts of Si (0-4%, depending on the process used), with the
balance Fe.
[0004I This material is the feed stock for the Argon Oxygen Decarburiser (AOD)
process, which is a modified steel converter and the first step in producing a
low carbon melt
of Cr and Fe to which other alloying elements such as Ni are added before the
liquid steel is cast
- 1 -
CA 02927984 2017-01-27
into plates and then rolled into sheet which is the bulk of the stainless
steel market, and the
feed for the myriad of stainless products such as pipes, tanks, containers,
flanges, valves etc.
required for industry and domestic consumers. Prior to the development of the
AOD process,
and its various derivatives and hybrids, stainless steel was very expensive to
produce because
the intermediate low carbon product required a tricky and time consuming
decarburisation step
using chromite ores.
[0005] The production of stainless and low alloy steels containing chromium
has
rapidly expanded, particularly in Asia. The source of the chromium in the
stainless steel is
partly from the recycling of scrap, but this is limited by the availability of
such materials,
particularly in developing countries. Chromium in stainless steels is not open
to substitution
by other metals. It is essential for the corrosion and heat resistance of the
material. The short
fall in the chromium additions required during the steel making process is met
by the addition
of alloys of chromium and iron, collectively known as "ferro chrome". These
alloys are
produced by the smelting of chromite ores, using solid carbonaceous reductants
in a
Submerged Electric Arc Furnace, (SAF). This process is extremely energy and
carbon
intensive. Existing plants using "best world practices" consume between three
and four
megawatt hours (MWH) of electricity and two hundred to three hundred kilograms
of carbon
per ton of ferro chrome alloy produced. Comprehensive gas cleaning systems are
required to
meet clean air standards. Large quantities of slag are produced and placed in
long term storage
in above ground dumps.
[0006] A small amount of metallic Cr is produced by reacting chemical grade
chromic oxide with metallic aluminum, analogous to the common thermite
reaction between
iron oxide and aluminum to produce molten iron. Production of low carbon FeCr
alloy by
aluminathermic reduction directly from chromite ores has not generally been
practiced because
of a generally unfavorable energy requirement, especially with low grade ores.
[0007] There are no commercially viable deposits of chromite ore in the USA
and
all ferro chrome used in the production of steel is imported, typically from
South Africa and
Kazakhstan. Recent discoveries of very large deposits of such ores have been
made in Canada
in a geographic area known as the "Ring of Fire" (ROF).
- 2 -
CA 02927984 2017-01-27
[0008] The development of huge deposits of natural shale gas in the USA and
Canada has led to a decrease in the long term cost of natural gas and the
prospect of stable
pricing for many years to come. The present invention exploits the
availability of the Ring of
Fire chromite and low cost natural gas.
[0009] Large quantities of Directly Reduced Iron (DRI) are currently produced
in
many countries using existing processes. The present invention uses a
modification of this
basic and well established technology to produce a chromium iron alloy by
using natural gas
to reduce both oxides of chromium and iron contained within the ROF chromite
ore, the
morphology of which has been shown in testing to facilitate the progress of
the reduction
reactions.
SUMMARY OF THE INVENTION
[0010] The present invention provides a process for producing chromium iron
alloy
suitable for steel making directly from chromite ore wherein the fines of
chromite ore with
additions of carbon fines, an accelerant and a binder are agglomerated and
dried, and thereafter
the agglomerates are fed into a reaction vessel with natural gas as a reducing
agent at elevated
temperatures adequate for reduction for thereby producing a chromium iron
alloy suitable for
steel making.
[0011j The accelerant is an alkaline in the form of an oxide, hydroxide or
carbonate,
such as sodium hydroxide or potassium hydroxide. Sodium hydroxide has been
found during
testing to be more effective than other alkaline chemicals in enabling the
reactions required to
rapidly reduce the chrome and iron oxides from the chromite ore concentrates.
[0012] The accelerant is included in an amount sufficient for the
stoichiometric
formation of sodium silicate of silica encapsulating the chromite fines plus
an additional
amount to enable the combination of sodium with the chrome oxide in the
chromite. The
accelerant is included in each agglomerate in the approximate range of 2% to
15% by weight.
However, the range of accelerant inclusion by weight depends upon a number of
variables, one
of which is the silica content of the ore concentrate and the second is the
chrome oxide content.
- 3 -
CA 02927984 2017-01-27
[0013] Carbon is included in the amount sufficient for reduction of the
reduceable
metal oxides of chromium and iron contained in the agglomerate, for example a
carbon
inclusion in each agglomerate in the approximate range of 15% to 25% by
weight.
[0014] The agglomerates may be efficiently dried with furnace off gas and then
charged to the reaction vessel having a temperature range of between 750 and
1,150 C.
[0015] The agglomerates are preferably formed as pellets, and in one
embodiment,
may be swept into the reaction vessel having an elevated temperature in the
range of 750 C
to 1,150 C by reformed natural gas. The fines of chromite ore and carbon for
making up the
pellet agglomerate are preferably in the range of 50 and 250 microns in size,
and the binder is
preferably selected as bentonite or an organic alternative such as corn
starch, which is included
in the amount of 0.5% to 1.5% of the pellet mass.
[0016] The reaction vessel in one embodiment includes a vertical moving bed
process and the natural gas reducing agent is selected as natural gas or
reformed natural gas.
In alternative embodiments the reaction vessel includes a static bed patch
process or a moving
belt process, and the natural gas reducing agent is selected as reformed
natural gas.
According to an aspect of the present invention, there is provided a process
for producing chromium iron alloys suitable for steel making directly from
chromite ore,
comprising:
providing a mixture by combining chromite ore with additions of carbon
fines and an alkali accelerant; and
feeding the mixture into a reaction vessel with natural gas as a reducing
agent
at elevated temperatures adequate for reduction for thereby producing a
chromium iron alloy
suitable for steel making.
According to another aspect of the present invention, there is provided a
chromium iron alloy manufactured in accordance with the process as described
herein.
- 4 -
CA 02927984 2017-01-27
BRIEF DESCRIPTION OF THE DRAWINGS
[0017] Other objects and advantages appear hereinafter in the following
description
and claims. The accompanying drawings show, for the purpose of
exemplification, without
limiting the scope of the present invention or the appended claims, certain
practical
embodiments of the present invention wherein:
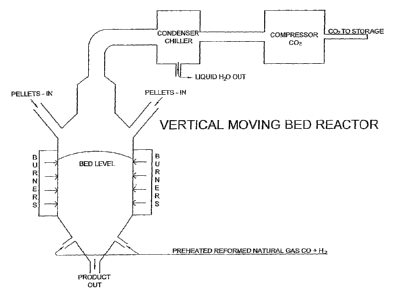
[0018] FIG. I is a schematic diagram illustrating one type of reaction vessel
usable
in the process of the present invention in the form of a vertical moving bed
reactor;
- 4a -
CA 02927984 2016-04-19
WO 2015/060951
PCT/US2014/054644
[0019] FIG. 2 is a schematic diagram illustrating a second type of reaction
vessel which
may be utilized in the process of the present invention in the form of a
vertical static batch
reactor;
[0020] FIG. 3 is a
schematic diagram illustrating a third embodiment of a reaction
vessel usable in the process of the present invention in the form of a
horizontal muffle conveyor
reactor;
[0021] FIG. 4 is a
graphic chart illustrating fossil fuel requirements for existing
processes and that projected for the process of the present invention; and
[0022] FIG. 5 is a graphic chart showing the carbon dioxide emissions from
existing
processes and that projected for the process of the present invention.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0023] The process
for producing chromium iron alloys suitable for steel making
directly from chromite ore in accordance with the teachings of the present
invention is carried
out as described and outlined in the afore-described Summary of the Invention,
preferably
utilizing ROF chromite ore.
[0024] Extensive laboratory work has been completed which demonstrates the
validity
of the present invention. Samples of chromite ore concentrates from a deposit
within the ROF
have been successfully reduced in accordance with the teachings of the present
invention to a
highly metallised chromium iron alloy suitable for steel making. The
temperature required for
the reduction of chromium is much higher than that for the reduction of iron
alone. In order to
enable the reduction process to proceed at an acceptable rate at lower
temperatures an accelerator
is used. The chromite ore concentrate is supplied as fines and needs to be
agglomerated prior to
the reduction stage. This may be accomplished by using a disc pelletiser or
other suitable
agglomerating equipment commonly available for the production of iron ore
pellets. It has been
shown that carbon is a required additive to the chromite to facilitate
reduction. The pellet
-5-
CA 02927984 2016-04-19
WO 2015/060951
PCT/US2014/054644
composition is therefore principally of chromite, carbon fines and an
accelerator, typically an
alkaline salt, and an addition of a binder, such as bentonite or an organic
alternative, completes
the pellet composition. The pellets are dried using offgas prior to entering
the reduction reactor.
[0025] The inventive process outlined above has been shown to produce
metalization
levels of chromium and iron of 80% or more. Higher metalization rates for both
chromium and
iron can be expected with process development. The resulting pellets of
reduced chromite are
suitable for stainless and alloy steel making, either as batch or continuously
charged components
of the steel making charge. Substantial cost advantages are expected when
compared to the
usage of conventionally produced ferro chrome alloys.
100261 The carbon content of the reduced chromite is intended to be
considerably lower
than the ferro alloys produced in a SAF. This will result in significant
process advantages for
the steelmaker and therefore lower the cost of production. The reduced
chromite pellets can form
part of the charge of a conventional SAF furnace producing ferro chrome, with
significant cost
benefits.
[00271 Large quantities of Directly Reduced Iron (DRI) are currently produced
in many
countries using several existing processes. The current invention uses
modifications of this basic
and well established technology for direct reduction of iron to produce a
chromium iron alloy by
using reformed natural gas to heat and reduce both oxides of chromium and iron
contained within
the ROF chromite ore, the morphology of which has been shown in testing to
facilitate the
progress of the reduction reactions. The reduction of chromium and iron oxides
in the chromite
ore by carbon monoxide normally requires temperatures in excess of 1,350
Celsius. The present
invention utilizes a controlled addition of an accelerant to reduce the
temperature required for
reduction to occur in the range from 750 to 1,100 Celsius. This lower
temperature requirement
reduces the energy required for the reduction process to around 1/5th of that
needed in the
conventional SAF process of the prior art.
[0028] In the laboratory studies, chromite used for the development work was
sourced
from the Black Horse deposit located within the Ring of Fire region of
Northern Ontario Canada.
-6-
CA 02927984 2016-04-19
WO 2015/060951 PCT/US2014/054644
As received chromite concentrate chemistry is shown Table 1, and the ore
chemistry in elemental
form is shown in Table 2.
[0029] Table 1
Cr203 FeO MgO A1203 Si02 CaO TiO2 MnO LO1
45.55 19.08 13.45 13.09 6.1 0.25 0.35 0.33 1.8
[0030] Table 2
Cr Fe Si Mg Ca Al Ti Mn
30.45 14.61 3.41 8.44 0.18 6.93 0.21 0.26
[0031] Experimental results establish than when a suitable catalyst or
accelerant is used,
then the reduction reactions have been shown to occur much more quickly and at
significantly
lower temperatures. This is shown in a comparison of Table 3 with Table 4,
Table 3 showing the
time required to achieve a given percentage reduction at temperature when un-
catalysed, and
Table 4 showing the time required to achieve a given percentage reduction at
temperature when
catalysed.
[0032] Table 3
% Reduction 1200 1250 1300 1400
0 0 0 0 0
20 17 8 4 2
40 44 21 12 6
60 120 50 24 12
80 - 140 67 17
100 - - - 90
-7-
CA 02927984 2017-01-27
100331 Table 4
% Reduction 1000 1150 1200 1250
0 0 0 0 0
20 15 12 10 3
40 23 18 15 7
60 40 30 22 12
80 60 30 23
100 110 60
[0034] The substantial reduction in reaction times demonstrated in these
experiments result in very large increases in specific throughput at a given
temperature. As an
example, at an operating temperature of 1,200 C at atmospheric pressure, the
catalyst system
of the present invention utilizing an accelerant has been shown to have a six
fold increase in
specific output than a similar sized reactor without the benefit of the
accelerant. This in turn
results in a much lower capital expenditure for a given output.
[0035] The rate of reduction of the chromite has been shown to be affected by
the
following variables:
1. Particle size of the ore.
2. Particle size of the reductant.
3. Reactivity of the solid reductant.
4. Temperature.
5. Presence of accelerants.
[0036] The process variations which are available are based on the use of a
carbon
containing pellet of around 12 mm in diameter produced on a disc pelletizer or
a smaller pea sized
product made in a standard industrial agglomerator. The feed for these
operations is typically
- 8 -
CA 02927984 2016-04-19
WO 2015/060951
PCT/US2014/054644
comprised of around 80% chromite concentrate, 17% carbon powder as a partial
reductant, up to
1.5% of bentonite or other suitable organic binder and accelerant.
[0037] Full scale plant configurations capable of processing the agglomerates
or pellets
to the metallized product can utilize reaction vessels of different types to
perform the process of
the present invention. The following is a description of some, but not an
exclusive summary, of
the different types of reaction vessels which may be utilized in the process
of the present
invention.
100381 Referring to FIG. 1, a vertical moving bed reactor as illustrated may
be utilized.
It is indirectly heated by natural gas. Reformed natural gas is fed into the
base of the reactor
column and rises through the bed contained within the reactor. The off gasses
are composed
entirely of water vapor and carbon dioxide. The reduced product is allowed to
flow semi-
continuously from suitable outlets at the base of the reactor into a sealed
atmosphere cooler.
There are no slags or other residual waste streams from this process option.
It has a very small
environmental footprint.
100391 A second reaction vessel which may be utilized in the process of the
present
invention is a high temperature natural gas fired rotary kiln preceded in
series by a lower
temperature kiln of similar design using the off gasses from the hotter kiln
to preheat the pellet
feed.
[0040] A third type of reaction vessel which may be utilized in the process of
the present
invention is illustrated in FIG. 2 as a fixed bed batch reactor. This reactor
is indirectly heated by
natural gas, containing a quantity of pellets produced according to the recipe
hereinbefore
outlined. The reduced product is cooled rapidly immediately after discharge.
[0041] A forth type
of reaction vessel which may be utilized in the process of the
present invention is a moving metal conveyor belt which passes through a
sealed muffle furnace
as illustrated in FIG. 3, which is externally heated by natural gas. The
atmosphere within the
muffle is comprised of reformed natural gas which maintains a slight positive
pressure within the
-9-
muffle. Additionally, a fluidized bed reactor may be utilized in the process
of the present
invention with a feed of small rice sized pellets of the required composition
using natural gas as
the energy source.
100421 The vertical moving bed reactor is flexible and the very latest
installations can
use either natural gas or reformed natural gas. However, most existing DRI
plants have gas
reformers. Natural gas is basically methane, CH4, whereas steam reformed
natural gas is
primarily 112 plus CO. The static bed batch process and belt options require
reformed gas. The
reformed gas has free hydrogen plus carbon monoxide and hydrogen is a much
more effective
reducing gas than is carbon monoxide.
[0043] The existing or prior art processes used to produce chromium iron
alloys from
chromite use large quantities of electricity and carbon containing reductants.
The Submerged Arc
Furnace or SAF is the standard method for producing ferro chrome alloys at
this time. This
process is energy inefficient and produces large quantities of off gas which
need to be captured,
cleaned and eventually emitted to the atmosphere. Substantial quantities of
carbon dioxide are
also discharged. This process produces a liquid metal as the chrome iron alloy
and a large
quantity of chrome containing slag with no beneficial use. This has to be land
filled. By
comparison, the natural gas base solid state process described hereinbefore
emits no off gasses to
the atmosphere. The water produced is condensed to liquid water with a level
of purity close to
that of potable water. Carbon dioxide is the only other gas produced as a
byproduct of the
reduction reactions. This is collected, compressed and sold to industrial
users.
100441 The overall
energy consumption for the gas based process of the present
invention is estimated to be approximately 1/3 of the SAF process and this is
shown in the
equivalent fossel fuel requirements for the existing processes and that
projected for the present
invention in the chart of FIG. 4.
-10-
CA 2927984 2017-09-22
CA 02927984 2016-04-19
WO 2015/060951
PCT/US2014/054644
[0045] FIG. 5 shows
the carbon dioxide emissions from existing processes and that
projected for the process of the present invention.
[0046] Also the land footprint is much lower for the gas based process of the
present
invention than for the SAF process, and no provision is required for the
landfill of slag.
[0047] The process outlined hereinbefore has been shown to produce
metalization levels
of chromium and iron of 80% or more. Higher metallization rates for both
chromium and iron
can be expected with process development. The resulting pellets of reduced
chromite are directly
suitable for stainless and alloy steel making, either as batch or continually
charged components
of the steel making charge.
[0048] The reduced
chrome iron alloy can easily be separated from the unreduced
gangue compounds by established industrial processes using the differences in
density or magnetic
properties, thus producing a highly desirable metallic component of a steel
making charge,
particularly to an Argon Oxygen Decarburisation vessel. The unreduced gangue
may be used as
an inert filler or in the production of building brick or block and as a sand
substitute on roofing
shingles.
[0049] Substantial cost advantages are experienced when compared to the usage
of
conventionally produced ferro chrome alloys. The carbon content of the reduced
chromite is
considerably lower than the ferro alloys produced in an SAF, which are
normally saturated with
carbon. This will result in significant process advantages for the steel maker
and therefore lower
the cost of production. The reduced chromite pellets can form part of the
charge of the
conventional SAF furnace producing ferro chrome, also with significant cost
benefits.
-11-
CA 02927984 2017-01-27
[0050] Some embodiments of the present invention may demonstrate the following
advantages.
1. The need for the installation of a capital intensive smelting step is
eliminated.
2. An intermediate process which upgrades the ore to a saleable intermediate
product is viable.
3. The process of the present invention has lower capital requirements than
that of charge chrome smelting.
4. The process of the present invention effectively utilizes the substantial
cost
and environmental benefits of natural gas for energy.
5. The need for subsidized electrical energy is eliminated.
6. The operating costs for the process of the present invention are
significantly lower than those involving smelting as a primary method of
upgrading.
7. Pollution is greatly reduced.
- 12-