Note: Descriptions are shown in the official language in which they were submitted.
USE OF A COMBINATION OF LOPINAVIR AND RITONAVIR IN THE TREATMENT OF
HUMAN PAPILLOMA VIRUS (HPV) AND CANCERS ARISING THEREFROM
Provided herein are methods and compositions for treating and/or inhibiting
the
development or progression of cancers (particularly HPV induced cancers) and
benign
proliferative disorders. In particular, provided are compositions comprising
lopinavir and
ritonavir for use in treating and/or inhibiting the progression of HPV related
dysplasia of the
cervix.
BACKGROUND
Many different forms of cancer exist, and it is believed that there are many
different
causes of the disease. The incidence of cancer varies, but it represents the
second highest cause
of mortality, after heart disease, in most developed countries. Current
estimates suggest that one
in three Americans alive at present will suffer from some form of cancer.
There is a well-
recognised need to develop new and improved therapies for treating cancers.
Furthermore, there
is also a requirement to develop therapeutic agents that could be used to
inhibit the development
of cancer in the general population, susceptible high-risk individuals or as
an agent to prevent re-
occurrence of disease in individuals already affected.
Human tumour viruses are recognised to be a major cause of human cancer, and
there is a
great deal of evidence which supports the contention that these viruses cause
cancer by inducing
genetic instability in infected cells. Indeed, both the human T-cell leukemia
virus type 1
(HTLV1) Tax and the human papilloma virus type 16 (HPV16) E6 oncoproteins are
known to
induce genetic instability producing abnormal numbers of centrosomes,
multinucleation and
nuclear atypia.
One approach to the treatment of cancers caused by viruses is disclosed in
International
Publication No. W02005/053694, which outlines the use of certain HIV protease
inhibitors
(which had previously been proposed for use as orally ingested medicaments for
the systemic
clinical management of retroviral infections such as HIV) as being clinically
useful for topical
administration to tissues to prevent or treat malignancies caused by human
papilloma virus. The
authors recognised that HIV protease inhibitors, such as indinavir, were
useful for treating
human papilloma virus (HPV) infections and particularly cancers associated
with such
infections. The work was based on the author's realisation that a chymotryptic
activity of the
proteasome may be the preferred target for treatment of HPV infections and it
is known that
Indinavir and related inhibitors can suppress the chymotryptic activity of the
26S proteasome.
-1-
4166272
Date Recue/Date Received 2020-08-24
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
Thus it was speculated that indinavir and related compounds act to inhibit the
chymotryptic
activity of the proteasome and could thereby be useful for treating HPV
infections and in
particular HPV infections that lead to the development of cancer (e.g. in the
cervix, mouth, anus,
vagina and penis).
International Publication No. W02005/053694 went on to contemplate that a
number of
HIV protease inhibitors could be used in the treatment of cancer (indinavir
was identified as the
most preferred) but also highlighted that certain HIV protease inhibitors
(particularly ritonavir)
did not appear to have the same activity as inhibitors such as indinavir.
Indeed combinations of
ritonavir and other protease inhibitors were actually shown to be less
effective in this regard and
therefore ritonavir fell outside the definition of the efficacious inhibitors
contemplated in that
specification.
A number of clinical trials for cancer have been conducted using ritonavir
alone or
ritonavir with lopinavir. However, each of these trials suggested the
compounds would not be
useful in the field of oncology. For instance, ritonavir has shown activity
against pre-invasive
cells derived from the cervix but was not effective against more advanced
invasive cervical
disease (Barillari, G., Iovane, A., Bacigalupo, I., Palladino, C., Bellino,
S., Leone, P., Monini, P.,
Ensoli, B., Ritonavir or saquinavir impairs the invasion of cervical
intraepithelial neoplasia cells
via a reduction of MMP expression and activity. AIDS, 2012, 26(8):909-19.).
This finding would
potentially lead a skilled person to discount using ritonavir since
elimination of early stage
neoplastic cells (without being effective against advanced cells) could
promote the evolution of
more invasive folins of the disease. Another clinical trial with ritonavir
(Laurent, N., de Bollard,
S., Guillamo, J.Sõ Christov, C., Zini, R., Jouault, H., Andre, P., Lotteau,
V., Peschanski, M.
Effects of the proteasome inhibitor ritonavir on glioma growth in vitro and in
vivo. Mol Cancer
Ther. 2004, 3(2):129-36) reported that ritonavir alone against glioma had no
effect in vivo. A
subsequent trial of ritonavir/lopinavir in humans had very little effect
against glioma (Ahluwalia,
M.Sõ Patton, C., Stevens, G., Tekautz, T., Angelov, L., Vogelbaum, M.A., Weil,
R.J., Chao, S.,
Elson, P., Suh, J.H., Barnett, G.H., Peereboom, D.M., Phase II trial of
ritonavir/lopinavir in
patients with progressive or recurrent high-grade gliomas. J, Neurooncol.
2011, 102(2):317-21)
Infection with high-risk types of HPV has now been established as the main
aetiological
agent for invasive cervical cancer (ICC) and globally there are >270,000
deaths from this disease
per annum with over 85% of these occurring in low resource countries. For
example, in Kenya it is
the most common cancer accounting for between 18-23% of all diagnosed cases of
cancer.
- 2 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
The development of ICC can take 10-20 years and is preceded by HPV related pre-
invasive
pathology which is characterised as either low-grade (CIN1) or high-grade
cervical intraepithelial
neoplasia (CIN2/3). Lesions can be screened for by cervical cytology testing
where they are
diagnosed (or graded) as either borderline atypical squamous cells of
undetermined significance
(ASCUS), low-grade squamous intraepithelial lesions (LSIL) or high-grade
squamous
intraepithelial lesions (HS1L).
The reduction in ICC related mortality in the developed world has been largely
dependent
on organised cytology screening and similar trends in cervical cancer
mortality have been
achieved by organised single screen and treatment in the third world. However,
in the poorer
nations lack of resources and health education means that most pre-invasive
cervical disease
remains undiagnosed and untreated. Thus, where resources are limited, low-cost
screening and
treatment options are clearly a high priority.
Current treatment options in clinical practice are either by ablative
(destructive) or
excisional modalities. Systematic reviews have demonstrated that these
treatment modalities have
similar success rates but have different morbidities. In the developed world,
Large Loop Excision
of the Transformation Zone LLETZ (aka loop electrosurgical excision procedure
¨ LEEP) is used
in the majority of colposcopy clinics. Over 80% of these procedures are
performed under local
analgesia and the whole of the transformation zone is available for subsequent
histological
examination. The procedure is associated with a risk of primary/secondary
haemorrhage,
prolonged discharge, infection and a risk of preterm delivery in subsequent
pregnancies. The
former side effects can be problematic particularly in low resource countries.
Ablative treatment
in the form of cold coagulation and cryotherapy are often advocated for use in
these settings since
these are low cost, require minimal infrastructure and can be carried out by
trained non-medical
health professionals. However, some studies have suggested that cryotherapy
has a higher failure
rate compared to other treatment modalities.
There are a variety of locally-applied, non-surgical approaches which have
been evaluated
for the treatment of cervical dysplasia including; photodynamic therapy (PDT);
off-licence use of
the anti cytomegalovirus (CMV) drug cidofovir; local application of the immune
activator
lmiquimod and direct application of the cytotoxic drug 5 flurouracil (5FU).
Although some of
these alternative treatment modalities show promise, their treatment outcomes
are inferior to the
reported 80-95% success rates obtained in quality assured colposcopy units.
- 3 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
An effective, inexpensive, non-surgical, self-applied treatment for HPV
related cervical
dysplasia would have great potential particularly in low resource settings.
Furthermore, improved
compliance with topical treatment would be enhanced, if the side effects are
minimised.
SUM_MARY
Disclosed herein are compositions comprising lopinavir alone or in combination
with
ritonavir for use as a medicament in the treatment of cancer or benign
proliferative disorders
(warts) or in the prevention of the development of cancer.
Pharmaceutical compositions formulated for topical application comprising a
therapeutically effective amount of lopinavir or a therapeutically effective
amount lopinavir and
ritonavir in a pharmaceutically acceptable vehicle are also provided.
Also disclosed are methods of treating a patient having an HPV related
dysplasia of the
cervix comprising administering to said patient a therapeutically effective
dose of the disclosed
pharmaceutical compositions.
BRIEF DESCRIPTION OF THE DRAWINGS
The summary, as well as the following detailed description, is further
understood when
read in conjunction with the appended drawings. For the purpose of
illustrating the disclosed
compositions and methods, there are shown in the drawings exemplary
embodiments of the
compositions and methods; however, the compositions and methods are not
limited to the
specific embodiments disclosed In the drawings:
FIG. 1, comprising FIGS. 1A-1D, illustrate. A) regression of a HSIL lesion
stained with
Lugol's iodine 1 & 3 months post-treatment - the patient became HPV negative;
B) regression of
a HSIL lesion stained with Lugol's iodine at 1 & 3 months post-treatment - the
patient became
HPV negative at 1 month and cytologically normal at 3 months; C) regression of
a HSIL lesion
stained with Lugol's iodine at 1 & 3 months post treatment - the patient was
HPV negative at 1
& 3 months and had borderline cytology at 3 months; and D) an LS1L lesion
stained with
Lugol's iodine, which showed clearance of HPV and normal cytology at 1 and 3
months post-
treatment.
FIG. 2, illustrates an exemplary patient screening, testing and management
flow chart
used for the study described in Example 2.
- 4 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
FIG. 3, comprising FIGS. 3A-3C, illustrates the combined results of Cervista
HPV test,
cytology and PCR HPV genotype analysis for women initially diagnosed with HSIL
in Example
2. The Cervista HPV titre is indicated by the height of the bar graph above
the cut off and the
cytology status is indicated by colour and shading with the HPV type shown
above the bar. (A)
Represents the results before treatment with lopinavir; (B) Represents results
after 4 and (C) 12
weeks after treatment.
FIG 4, comprising FIGS. 4A-4C, illustrates the combined results of the
Cervista HPV
test, cytology and PCR HPV genotype analysis for women initially diagnosed
with
ASCUS/LSIL in Example 2. The Cervista HPV titre is indicated by the height of
the bar graph
above the cut off and the cytology status is indicated by colour and shading
with the HPV type
shown above the bar. (A) Represents the results before treatment with
lopinavir; (B) Represents
results after 4 and (C) 12 weeks after treatment.
FIG. 5 illustrates the results of colposcopy with VILI before (left column), 4
weeks
(middle column) and 12 weeks (right column) after treatment of patients E02,
E03, E06, E14 and
E12 in Example 2. Magnification x5 was used with the Cervista HPV status and
cytology
shown under each picture and final pathology (CIN status) also shown under the
12 weeks post-
treatment images.
FIG 6, illustrates an agarose gel electrophoresis of ethidium bromide stained
low-risk
HPV 6 or HPV 11 specific PCR products amplified from DNA extracted from LBC
smears
obtained from patients diagnosed with high-risk HPV positive cervical
dysplasia both before and
after treatment with Lopimune.
FIG. 7, comprising FIGS 7A-7B, illustrate the results of AQ96 Growth Assays
for SiHa
(A) and Hela (B) cells.
FIG. 8, illustrates scatter plot profiles of unstained, annexin-only and PI-
only stained
HeLa cells used to optimise the cytometer and set gates appropriately. This
also showed that very
little background was observed in the R4 quadrant where advanced stage
apoptotic cells should
be detected.
FIG. 9, illustrates scatter plot analysis of triplicate cultures treated with
1:1(12.5 uM
lopinavir + 12.5 p.M ritonavir) with an additional analysis of one culture
repeated 3 times to
determine the intra cytometer assay variance. (Note: Cells in the R6 quadrant
are in earlier stages
of apoptosis).
- 5 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
FIG. 10, illustrates scatter plot analysis of triplicate cultures treated with
4:1(20 M
lopinavir + 5 1.1M ritonavir) plus cultures treated with decreasing amounts of
ritonavir (21 1.1M
lopinavir + 4 p.M ritonavir and 23 [tM lopinavir + 2 [tM ritonavir).
FIG. 11, illustrates scatter plot showing duplicate cultures treated with 25
1.IM lopinavir
as a single agent and triplicate cultures treated with same amount of DMSO
used for all the drug
assays shown.
FIG. 12 is a graph illustrating the analysis of intra and inter sample
variance which
occurred within and between flow cytometry apoptotic assays carried out on
1:1(12.5 1.1M Lop+
12.5 p.M Rit) treatments.
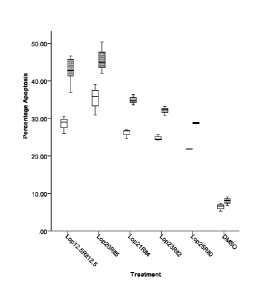
FIG. 13 is a box plot comparing the effects of the various drug treatments on
levels of
apoptosis.
FIG. 14 illustrates total cell death in Hela cell as measured by Trypan blue
dead cell
assay.
DETAILED DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
The disclosed compositions and methods may be understood more readily by
reference to
the following detailed description taken in connection with the accompanying
figures, which
form a part of this disclosure. It is to be understood that the disclosed
compositions and methods
are not limited to the specific compositions and methods described and/or
shown herein, and that
the terminology used herein is for the purpose of describing particular
embodiments by way of
example only and is not intended to be limiting of the claimed compositions
and methods.
Reference to a particular numerical value includes at least that particular
value, unless the
context clearly dictates otherwise. When a range of values is expressed,
another embodiment
includes from the one particular value and/or to the other particular value.
Further, reference to
values stated in ranges include each and every value within that range. All
ranges are inclusive
and combinable.
When values are expressed as approximations, by use of the antecedent "about,"
it will be
understood that the particular value forms another embodiment.
It is to be appreciated that certain features of the disclosed compositions
and methods
which are, for clarity, described herein in the context of separate
embodiments, may also be
provided in combination in a single embodiment. Conversely, various features
of the disclosed
compositions and methods that are, for brevity, described in the context of a
single embodiment,
may also be provided separately or in any subcombination.
- 6 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
As used herein, the singular forms "a," "an," and "the" include the plural.
The following abbreviations are used herein: human papilloma virus (HPV);
Atypical
squamous cells of undetermined significance (ASC-US); Low grade squamous
intraepithelial
lesion (LSIL); Atypical squemous cells ¨ cannot exclude HSIL (ASC-H); High
grade squamous
intraepithelial lesion (HSIL); Squamous cell carcinoma (SCC); Abnormal
glandular cells (AGC);
Cervical intraepithelial neoplasia 1 (CIN1); Cervical Intraepithelial
neoplasia 2 (CIN2); Cervical
intraepithelial neoplasia 3 (CIN3); Carcinoma in situ (CIS); Invasive Cervical
Carcinoma (ICC).
The term "about" when used in reference to numerical ranges, cutoffs, or
specific values
is used to indicate that the recited values may vary by up to as much as 10%
from the listed
value. As many of the numerical values used herein are experimentally
determined, it should be
understood by those skilled in the art that such determinations can, and often
times will, vary
among different experiments. The values used herein should not be considered
unduly limiting
by virtue of this inherent variation. Thus, the tem) "about" is used to
encompass variations of
100/0 or less, variations of 5% or less, variations of 1% or less,
variations of 0.5% or less, or
variations of 0.1% or less from the specified value.
As used herein, "treating" and like terms refer to reducing the severity
and/or frequency
of HPV induced symptoms, eliminating HPV induced symptoms and/or the
underlying cause of
said symptoms, reducing the frequency or likelihood of HPV induced symptoms
and/or their
underlying cause, delaying, preventing and/or slowing the progression of HPV
induced cancers
or benign proliferative disorders, and improving or remediating damage caused,
directly or
indirectly, by HPV infections. As used herein, the phrase "therapeutically
effective dose" refers
to an amount of a composition comprising lopinavir, or more preferably
lopinavir and ritonavir,
as described herein, effective to achieve a particular biological or
therapeutic result such as, but
not limited to, biological or therapeutic results disclosed, described, or
exemplified herein. The
therapeutically effective dose may vary according to factors such as the
disease state, age, sex,
and weight of the individual, and the ability of the composition to cause a
desired response in a
subject. Such results include, but are not limited to, the reduction,
remission, and/or regression
of the malignant disease or prevention of the development of malignant
disease, as determined
by any means suitable in the art.
As used here, "subject" includes a vertebrate, mammal, domestic animal or
preferably a
human being.
The "pharmaceutically acceptable vehicle" may be any physiological vehicle
known to
those of ordinary skill in the art useful in formulating pharmaceutical
compositions.
- 7 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
As used herein, "ovule" refers to a cream or gel containing solid or semi-
solid
suppository configured for insertion into the vagina.
Disclosed herein are compositions comprising lopinavir and ritonavir for use
as a
medicament in the treatment of cancer or benign proliferative disorders
(warts) or in the
prevention of the development of cancer. Contrary to what was known in the
prior art, the
inventors have found that while ritonavir was previously thought to have
little or known efficacy
in treating HPV-related conditions (see, e.g., W02005/053694), lopinavir and
ritonavir act
synergistically to treat and inhibit progression of such conditions, with the
combination of active
agents producing unexpectedly superior results over the use of either active
agent alone.
The compositions according to the first aspect of the invention are useful in
the treatment
of cancer and particularly useful for preventing the development of cancers.
Accordingly, normal
subjects (i.e. subjects with no detectable cancer), subjects with pre-
malignant cells or particularly
cancer prone subjects may be treated according to the invention (preferably by
topical
administration of the inhibitors) with a view to preventing the development of
cancer.
The invention, to the extent that it is applicable to the prevention and
treatment of cancer,
may be applied to a wide range of cancers such as ovarian carcinoma, breast
carcinoma, lung
carcinoma, uterine carcinoma, cervical carcinoma and thyroid carcinoma. It is
also applicable to
cancer prone conditions. The invention is applicable particularly, but by no
means exclusively, to
pre-cancerous conditions and cancers caused by oncogenic viruses, e.g. high-
risk or even low-
risk forms of human papilloma viruses (HPVs).
According to a preferred embodiment of the invention, the compositions may be
administered to treat, and particularly prevent, the development of cervical
cancer. It is most
preferred that the inhibitors are used to treat, or prevent the development of
cervical cancers
caused by HPV (particularly high-risk types of HPV such as HPV16). The
surprising efficacy of
compositions used according to the first aspect of the invention against HPV
related HSIL/CIN
in humans could thus not be predicted from any of the in vitro, in vivo or
clinical trial studies
carried out to date.
In the developed nations that have cervical screening programs, HPV testing
and cervical
cytology are currently in a state of flux. At present, the cervical smear (or
Pap test) is usually
carried our prior to HPV testing with follow on procedures depending on the
results obtained.
However, depending on geographical location the HPV test may be given first.
Table 1 shows a
typical recommended management protocol based on the combined results of HPV
and Pap tests.
- 8 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
Table 1: Recommended Management of combined HPV and Pap tests
HPV test Pap test Management
Negative Negative Repeat testing in 5 years
Any Negative Repeat testing in 3 years
Negative ASC-US Repeat testing in 3 years
Negative LSIL Repeat testing in 6-12 months
Not performed ASC-US Repeat testing in 6-12 months
Positive Negative Repeat testing in 6-12 months
Not performed LSIL Immediate colposcopy
Positive LSIL Immediate colposcopy
Any ASC-H Immediate colposcopy
Positive ASC-US Immediate colposcopy
Any HSIL Immediate colposcopy
Any SCC Immediate colposcopy
Any AGC Immediate colposcopy
Table 1 Acronyms: Atypical squamous cells of undetermined significance (ASC-
US); Low
grade squamous intraepithelial lesion (LSIL); Atypical squemous cells ¨ cannot
exclude HSIL
(ASC-H); High grade squamous intraepithelial lesion (HSIL); Squamous cell
carcinoma (SCC);
Abnormal glandular cells (AGC). Taken from Schiffman, M., Solomon, D,,
Clinical practice.
Cervical-cancer screening with human papillomavirus and cytologic cotesting. N
Engl J Med.
2013, 369(24):2324-31
Pathology results obtained from biopsies taken at colposcopy are described as:
Cervical
intraepithelial neoplasia 1 (CIN1); Cervical Intraepithelial neoplasia 2
(CIN2); Cervical
intraepithelial neoplasia 3 (CIN3); Carcinoma in situ (CIS); Invasive Cervical
Carcinoma (ICC).
HPV negative CIN1 is clinically equivalent to LSIL and is currently a rescreen
in 6-12 months
(watch and wait).
This recommended management protocol represents the current best clinical
practice for
testing women aged >25. It can be seen that immediate colposcopy is
recommended for any women
with LSIL cytology or greater (HSIL, etc.) in the absence of a HPV test. Women
with LSIL who are
shown to be HPV negative, are rescreened in 6-12 months.
A preferred window of opportunity for use of compositions according to the
first aspect of
the invention is between the time of initial diagnosis with HPV positive
disease (ASC-US, LSIL,
ASC-H, HSIL) until colposcopy, which usually takes approximately 2 weeks or
longer, depending
on waiting times. Based on what is observed at colposcopy, the decision is
then made to either treat
with surgery at this visit (See and Treat) or take biopsies for pathology
which necessitates a further
colposcopy visit approximately one month later. Clearly, if the colposcopy
visits are timed
- 9 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
appropriately, it may advantageously be that treatment according to the
invention removes the need
for surgery.
It is preferred that the compositions are folinulated in a medicament that is
suitable for
topical application. In a most preferred embodiment, the medicament is
formulated such that it is
suitable for topical delivery of the active ingredients to the cervix (e.g. as
a gel, cream, soft capsule,
or pessary) for preventing the development of, or treating cervical cancer
(e.g. caused by high-risk
types of HPV such as HPV16).
Lopinavir (CA S# 192725-17-0) is a protease inhibitor chemically designated as
[1S-
[1R*(R*), 3R*, 4R*]]-N44-[(2,6-dimethylphenoxy0acetyl]amino]-3-hydroxy-5-
pheny1-1-
(phenylmethyl)pentyl]tetrahydro-alpha-(1-methylethyl)-2-oxo-1(2H)-
pyrimidineacetamide. It
has the molecular formula C37H48N405 and a molecular weight of 628.80.
Ritonavir (CAS# 155214-67-5) is a protease inhibitor chemically designated as
10-
Hydroxy-2-methy1-5-(1-methyleth1)-1-[2-(1-methylethyl)-4-thiazoly1]-3,6-dioxo-
8,
1lbis(phenylmethyl)-2,4,7,12-tetraazatridecan-13-oic acid, 5-
thiazolylmethylester, [5S-
(5R*,8R*,10R8,11R*)]. It has the molecular formula C37H4gN605S2 and a
molecular weight of
720.95.
Pharmaceutical products are known that combine lopinavir and ritonavir. For
instance
LOPIMUNE is marketed by Cipla. Another example is KALETRA which is marketed
by
Abbott/Abbvie for the treatment of HIV infections. Other products are
manufactured by
Emcure, Ranbaxy, Hetero and Matrix.
By way of example, KALETRA is used orally and is available for oral
consumption as a
solution comprising 80 mg lopinavir and 20 mg ritonavir per millilitre or as a
soft capsule for
oral administration that comprises 133.3 mg lopinavir and 33.3 mg ritonavir.
Unexpectedly, it has been found that soft capsule versions of pharmaceutical
products
comprising lopinavir and ritonavir (e.g. LOPIMUNE or KALETRA ) can be
administered
topically (e.g. inserted into the vagina for treatment of the cervix) for the
prevention or treatment
of cancerous conditions or for the prevention or treatment of oncogenic viral
infections.
Accordingly, the soft capsule formulation of KALETRA is an example of a
formulation that
may be used for topical application according to the invention, although hard
tablet forms of the
drug exist which may also be useful for direct topical application
KALETRA soft capsules comprise oleic acid, propylene glycol, PEG 35 castor
oil,
purified water, gelatin, sorbitol, special polyol, titanium dioxide, sunset
Yellow FCF CI15985,
- 10 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
medium chain trigylcerides, lecithin and a black printing ink (e.g. Opacode
WBNSP-78-17734
black with ARTG No. 3791). It will be appreciated that these ingredients may
be varied.
However, KALETRA soft capsules are formulated for oral ingestion and in
preferred
embodiments it is preferred that lopinavir and ritonavir are formulated to
suit the way in which
they will be topically administered as discussed in more detail below. For
instance, in preferred
formulations for topical application to the cervix, the composition does not
include pigments,
dyes, or inks. In exemplary embodiments, the formulations for topical
application to the cervix
do not include titanium dioxide, Yellow FCF CI15985 or a black printing ink.
Preferred compositions for use according to the invention are vaginal
suppositories and
ovules that comprise lopinavir and ritonavir which at least preclude the use
of KALETRA soft
capsules. Such vaginal suppositories, or ovules, may be between about 2 and 8
grams. Such
vaginal suppositories, or ovules, typically may use polyethylene glycol as a
main carrier for the
inhibitors. The balance of the carrier may be made up of Oleic acid, PEG 35,
castor oil, purified
water, gelatin and sorbitol special po1yol etc. Lopinavir and Ritonavir are
virtually insoluble in
water and it is preferred that such organic bases (or equivalents thereof) are
formulated in such
vaginal suppositories.
The disclosed compositions are not only useful for treating actual cancers but
are also
surprisingly useful for preventing the development of cancer, particularly in
patients that may
exhibit any of the previously described pre-cancerous lesions in HPV-positive
patients.
Accordingly, compositions comprising lopinavir and ritonavir may be
advantageously used as a
prophylactic.
The compositions may be given to subjects with a genetic disposition to
developing
cancer (most particularly cervical carcinoma) or even those facing
environmental risk (e.g.
people exposed to carcinogens). In a preferred embodiment, the compositions
may be given to
women who are at risk of developing cancer. Such women can include those who
have been
diagnosed as having a high risk HPV infection of the urino-genital tract (and
particularly the
cervix). At the time of diagnosis, there may not be any clinical evidence that
such women have a
cervical carcinoma or even precancerous cells of the cervix, yet women with
such infections are
believed to be at risk of developing cervical cancer. The compositions may be
topically applied
to the cervix of women with a viral infection of the cervix with a view to
treating the viral
infection and thereby preventing the development of cancer at a future date.
-11-
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
The compositions may be used to prevent or treat cancer as a monotherapy (i.e.
use of the
two inhibitors alone) or in combination with other compounds or treatments
used in cancer
therapy (e.g. chemotherapeutic agents, radiotherapy).
It is most preferred that the compositions are used to treat humans (e.g.
women with or at
risk of developing cervical cancer). However, it will be appreciated that the
compositions may
also have some veterinary use.
The medicaments used according to the invention may take a number of different
forms
depending, in particular, on the manner in which the medicament is to be
applied topically.
Thus, for example, the medicament may be in the form of a powder, tablet,
capsule, liquid,
ointment, cream, gel, hydrogel, ovule, suppository, aerosol, spray, micelle,
liposome or any other
suitable form that may be administered to a person or animal. It will be
appreciated that the
vehicle of the medicament of the invention should be one which is well
tolerated by the subject
to whom it is given and enables delivery of the inhibitors to the effected or
target site.
In some aspects, the compositions can be formulated for topical use (e.g. as
gels, creams
or ointments). For instance, when used to treat (or prevent the development
of) cervical cancer,
the compositions can be formulated as gels, creams or ointments that may be
applied directly to
the cervix by techniques known to the art. Alternatively, the compositions may
be formulated as
a vaginal suppository (or incorporated within a pessary) according to
techniques known to the
art.
In other aspects, the medicaments may be in the form of an ovule. The ovule
can
comprise a cream or gel located within a coating, the coating configured to
melt and release the
cream or gel upon being administered intravaginally. Alternatively, the ovule
can consist of a
cream or gel that is configured to melt upon being administered
intravaginally. The compositions
may also be formulated as a soft capsule wherein the outer layer of the
capsule suitably dissolves
at the site of application to allow the release of the compositions. For
instance, unexpectedly, it
has been established that KALETRA (or similar) soft capsules may be
administered to the
cervix of a woman in need of treatment. Accordingly, such capsules are
effective for topical
delivery of therapeutically effective amounts of lopinavir and ritonavir to
the cervix when
inserted into the vagina of a subject. However, as mentioned previously,
preferred embodiments
of the invention exclude the use of lopinavir/ritonavir compositions such as
KALETRA (or
similar) that have actually been formulated for oral administration and
preferred embodiments
include lopinavir/ritonavir compositions formulated for topical administration
(e.g. to the
cervix).
- 12 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
It will be appreciated that the amount of lopinavir and ritonavir required is
determined by
biological activity and bioavailability, which in turn depends, in part, on
the precise mode of
administration, the physicochemical properties of the composition employed,
and whether the
compositions are being used as a monotherapy or in a combined therapy with
other oral or
topical anti-cancer or anti HIV agents. Indeed it is also possible that
topical lopinavir/ritonavir
could be applied in addition to oral dosing of the same compounds or other
anti HIV protease
inhibitors. The frequency of administration will also be influenced by the
abovementioned
factors and particularly the half-life of the compound within the subject
being treated.
Daily doses may be given as a single administration (e.g. as a soft capsule,
vaginal
suppository, ovule or pessary). Alternatively, administration may be twice or
more times during
a day. As an example, the compositions (for preventing the development of
cervical cancer) may
be topically administered twice a day.
Optimal dosages to be administered may be determined by those skilled in the
art, and
will vary with the strength of the preparation, the mode of administration,
and the advancement
of the disease condition. Additional factors depending on the particular
subject being treated
will result in a need to adjust dosages, including, for example, subject age,
weight, gender, diet,
and time of administration.
Suitable amounts of lopinavir in the disclosed compositions include from about
0.1 mg to
about 2.0 g. In some embodiments, the amount of lopinavir in the composition
can be from
about 10 mg to about 1.5g. In some embodiments, the amount of lopinavir in the
composition
can be from about 100 mg to about 1.0 g. In some embodiments, the amount of
lopinavir in the
composition can be from about 150 mg to about 900 mg. In some embodiments, the
amount of
lopinavir in the composition can be from about 250 mg to about 800 mg. In some
embodiments,
the amount of lopinavir in the composition can be from about 350 mg to about
700 mg. In some
embodiments, the amount of lopinavir in the composition can be from about 500
mg to about
600 mg.
Suitable amount of ritonavir in the disclosed compositions include from about
0.1 mg to
about 500 mg. In some embodiments, the amount of ritonavir in the composition
can be from
about 10 mg to about 400 mg. In some embodiments, the amount of ritonavir in
the composition
can be from about 20 mg to about 300 mg. In some embodiments, the amount of
ritonavir in the
composition can be from about 30 mg to about 200 mg. In some embodiments, the
amount of
ritonavir in the composition can be from about 50 mg to about 100 mg.
- 13 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
For a human adult, between about 0.1 mg and about 2.0 g of lopinavir, and
about 0.1 mg
and about 500 mg of ritonavir can be preferably topically administered to the
target tissue per
day. Preferably between about 10 mg and about 1.0 g of lopinavir and about 5
mg and about 200
mg of ritonavir can be topically administered to the target tissue per day.
More preferably
between about 100 mg and about 600 mg of lopinavir and between about 30 mg and
about 175
mg of ritonavir can be topically administered to the target tissue per day.
In one embodiment, about 266.6 mg of lopinavir and about 66.6 mg ritonavir per
day
may be administered to the cervix of a woman. This may be achieved by
inserting, twice a day, a
soft gel capsule of Lopimune containing 133.3 mg of lopinavir and 33.3 mg of
ritonavir in the
vagina of the woman being treated.
In another embodiment, about 533 mg of lopinavir and about 126 mg ritonavir
per day
may be administered to treat the cervix of a woman. This may be achieved by
inserting, twice a
day, two soft gel capsules of Lopimune containing 133.3 mg of lopinavir and
33.3 mg of
ritonavir in the vagina of the woman being treated.
In a further embodiment, between about 400 mg to about 600 mg of lopinavir and
about
100 mg to about 150 mg ritonavir per day may be administered to treat the
cervix of a woman.
This may be administered as a single vaginal suppository, or ovule, once a day
(preferably last
thing at night) and should ideally be applied for between 2 ¨ 4 weeks.
The ratio of lopinavir to ritonavir in the medicament may be varied. Suitable
ratios of
lopinavir to ritonavir in the composition include, for example, from about
1:10 to about 10:1. In
some embodiments, the ratio of lopinavir to ritonavir in the composition is
about 1:1. In some
embodiments, the ratio of lopinavir to ritonavir in the composition is about
2:1. In some
embodiments, the ratio of lopinavir to ritonavir in the composition is about
3:1. In some
embodiments, the ratio of lopinavir to ritonavir in the composition is about
4:1. In some
embodiments, the ratio of lopinavir to ritonavir in the composition is about
5:1. In some
embodiments, the ratio of lopinavir to ritonavir in the composition is about
10:1. In some
embodiments, the ratio of lopinavir to ritonavir in the composition is from
about 1:1 to about 4.1.
In other embodiments, the ratio of lopinavir to ritonavir in the composition
is from about 1:1 to
less than 4:1. In some aspects, it is preferred that the ratio of lopinavir to
ritonavir is about 4:1.
In other aspects, it is preferred that the ratio of lopinavir to ritonavir is
less than 4:1. Preferably,
the ratio of lopinavir:ritonavir is one which exhibits synergistic efficacy
above and beyond that
which would have been expected from the use of either active agent alone.
- 14 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
The medicament may be administered to a subject for as long as treatment is
required.
The length of time for which treatment will be required will depend upon the
exact condition
being treated or prevented and its severity. A skilled person will appreciate
that treatment should
be maintained in view of a number of factors which will include any
requirement to eradicate
any oncogenic virus (e.g. HPV); to reduce or eradicate cells with a
precancerous or cancerous
phenotype; or to shrink or eradicate any tumour. Typically a course of
treatment should be for 2 -
4 weeks, 7-21 days or for about 14 days. After this time a clinician may
assess whether the
course of treatment has been successful. A decision may then be made whether
or not to continue
treatment.
It will be appreciated that a clinician may wish to take into account
menstruation when
deciding on a treatment regimen for conditions relating to the cervix.
Accordingly, a preferred
treatment regimen may be for about 14 - 21 days and can be administered
between menses. A
clinician may elect to stop topical treatment of the cervix during menses and
recommence a new
course of treatment in the next menstrual cycle. By way of example, a
preferred treatment
regimen can be: (1) 14 - 21 days of administration; (2) followed by 1 - 14
days without treatment
(during which menses may occur if treating the cervix); and (3) a further
cycle of 14 -21 days of
treatment if this is considered medically necessary. In a most preferred
embodiment, the cervix
of a women may be treated such that she receives about 266.6 mg of lopinavir
and about 66.6 mg
ritonavir per day for 14 -21 days; treatment can then be stopped for 1 - 14
days and a clinical
reassessment can be conducted; then, if necessary a second treatment cycle of
about 533 mg of
lopinavir and about 126 mg ritonavir per day can be administered for a further
14 -21 days. After
the second cycle a further clinical assessment can be made and a decision made
about whether or
not subsequent treatment cycles are required.
Unexpectedly, one treatment cycle of 14 days is very effective for eradicating
oncogenic
viral infection and reducing or eradicating precancerous or cancerous lesions
(see the Examples).
By way of further example, and without intending to be limiting, a preferred
treatment
regimen for treating the cervix of a woman in need of treatment can be: (1) 14
- 21 days of
administration; (2) optionally followed by 1 - 14 days without treatment
(during which menses
may occur); and (3) a further cycle of 14 - 21 days of treatment if this is
considered medically
necessary. The women may be treated such that she receives between about 400
mg and about
600 mg of lopinavir and between about 100 mg and about 150 mg ritonavir per
day as a single
dose which is ideally self-administered last thing at night (i.e. just before
retiring for sleep).
- 15 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
Also disclosed herein are pharmaceutical compositions for topical application
comprising
a therapeutically effective amount of lopinavir and ritonavir and a
pharmaceutically acceptable
vehicle.
In one embodiment, the pharmaceutically acceptable vehicle can be a liquid and
the
composition can be a solution. In another embodiment, the vehicle can be a gel
and the
composition can be a suppository or pessary. In a further embodiment, the
vehicle can be an
emulsion (or other pharmaceutically acceptable base) and the composition can
be a cream.
Liquid vehicles are used in preparing solutions, suspensions, emulsions,
syrups, elixirs
and pressurized compositions. The active ingredient can be dissolved or
suspended in a
pharmaceutically acceptable liquid vehicle such as water, an organic solvent,
a mixture of both
or pharmaceutically acceptable oils or fats. The liquid vehicle can contain
other suitable
pharmaceutical additives such as solubilizers, emulsifiers, buffers,
preservatives, suspending
agents, thickening agents, colors, viscosity regulators, stabilizers or osmo-
regulators. Suitable
examples of liquid vehicles include water (partially containing additives as
above, e.g. cellulose
derivatives, preferably sodium carboxymethyl cellulose solution), alcohols
(including
monohydric alcohols and polyhydric alcohols, e.g. glycols) and their
derivatives, and oils (e.g.
fractionated coconut oil and arachis oil). The vehicle can also be an oily
ester such as ethyl
oleate and isopropyl myristate. The liquid vehicle for pressurized
compositions can be
halogenated hydrocarbon or other pharmaceutically acceptable propellent.
It is preferred that the pharmaceutical composition is a vaginal suppository.
Conventional
vehicles, coatings and other constituents of vaginal suppositories may be
chosen by one skilled
in the art to form a suppository that is characterised by the fact it
comprises therapeutically
effective amounts of lopinavir and ritonavir.
Preferred vaginal suppositories typically may use polyethylene glycol as a
main vehicle
for lopinavir and ritonavir. The balance of the vehicle may be made up of, for
example, Oleic
acid, PEG 35, castor oil, purified water, gelatin, sorbitol special polyol, or
any combination
thereof. Lopinavir and ritonavir are virtually insoluble in water and it is
preferred that such
organic bases (or equivalents thereof) are formulated in such vaginal
suppositories.
Previously published in vitro work on the potential activity of lopinavir
(alone) against
HPV positive cervical cancer cell lines suggested that lopinavir might have
some activity against
this cell type (Intl Publ. No. W02005/053694). However, most compounds which
show
potential anticancer activity from in vitro studies usually fail to show
activity either in vivo or in
clinical trials in humans. For example, "[d]espite their importance for drug
testing, in vitro
- 16 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
methods are beset by pitfalls and inherent limitations" (Zips et al. (2005: In
Vivo Jan-Feb 19(1)
p1-7). Indeed since formal screening began in 1955, many thousands of drugs
have shown
activity in either cell culture based or animal models, but only 39 that are
used exclusively for
chemotherapy, as opposed to supportive care, have won approval from the U.S.
Food and Drug
Administration. "The fundamental problem in drug discovery for cancer is that
model systems
are not predictive at all" (Alan Oliff (Executive Director, Merck Research
Laboratories, West
Point, Pennsylvania).
The typical cancer drug testing scheme is to proceed with a variety of in
vitro methods
which, if successful, subsequently leads to testing in a variety of
experimental in vivo models
prior to any clinical studies in humans (See Zips et al. supra). The failure
rate of compounds as
they move through this testing cascade is very high. Thus, since only limited
in vitro studies
were carried out on the activity of lopinavir (alone) against HPV positive
cervical cancer cell
lines, the results of the clinical trial of the compositions according to the
first aspect of the
invention against HPV related cervical neoplasia in humans (See Examples 1 &
2) are entirely
novel, unexpected and remarkable. Furthermore, the efficacy of lopinavir alone
can also be
considered to be unexpected, and according to a further aspect of the
invention, there is provided
a composition comprising lopinavir for use as a medicament in the treatment of
cancer or benign
proliferative disorders (warts) or in the prevention of the development of
cancer. Accordingly,
lopinavir may be used as contemplated above but without the inclusion of
ritonavir.
Preferably, the pharmaceutical composition comprises a synergistic amount of
lopinavir
and ritonavir. In some embodiments, for example, the synergistic amount of
lopinavir and
ritonavir can be from about 1:1 to about 4.1 lopinavir to ritonavir. In other
embodiments, the
synergistic amount of lopinavir to ritonavir can be from about 1:1 to less
than 4:1 lopinavir to
ritonavir.
The disclosed pharmaceutical compositions can be formulated for intravaginal
delivery.
Suitable formulations for intravaginal delivery include, but are not limited
to, a gel, cream,
ointment, lotion, ovule, soft capsule, suppository, pessary, or any
combination thereof. In some
aspects, the pharmaceutical composition can be formulated as a gel. In some
aspects, the
pharmaceutical composition can be formulated as a cream. In some aspects, the
pharmaceutical
composition can be formulated as an ointment In some aspects, the
pharmaceutical composition
can be formulated as a lotion. In some aspects, the pharmaceutical composition
can be
formulated as an ovule. In some aspects, the pharmaceutical composition can be
formulated as a
soft capsule. In some aspects, the pharmaceutical composition can be
formulated as a
- 17 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
suppository. In some aspects, the pharmaceutical composition can be formulated
as a pessary.
In some aspects, the pharmaceutical composition can be formulated as any
combination of the
above formulations.
In a preferred embodiment, the formulation is substantially free of pigments,
dyes, and/or
inks. As used herein, "substantially free" refers to less than 10%, preferably
less than 5%,
preferably less than 1%, preferably less than 0.1%, and preferably less than
0.05%.
Also disclosed herein are methods of treating a patient having an HPV related
dysplasia
of the cervix comprising administering to said patient a therapeutically
effective dose of the
disclosed pharmaceutical compositions.
As used herein, "dysplasia" encompasses pre-invasive lesions and cancer. HPV
related
pre-invasive lesions include high grade squamous intraepithelial lesion
(HSIL), atypical
squamous cells of undetermined significance (ASCUS), and low grade squamous
intraepithelial
lesion (LSIL). HPV related cancers include, for example, cervical
intraepithelial neoplasia
(CIN) and invasive cervical cancer (ICC).
The disclosed methods can be used to treat HPV related dysplasia. In some
aspects, for
example, the disclosed methods can be used to treat HSIL. In some aspects, the
disclosed
methods can be used to treat ASCUS. In other aspects, the disclosed methods
can be used to
treat LSIL. In other aspects, the disclosed methods can be used to treat CIN.
In yet other
embodiments, the disclosed methods can be used to treat ICC Additionally, the
disclosed
methods can be used to inhibit the progression of HPV related dysplasia. In
some aspects, for
example, the disclosed methods can be used to inhibit the progression of HSIL.
In some aspects,
the disclosed methods can be used to inhibit the progression of ASCUS. In
other aspects, the
disclosed methods can be used to inhibit the progression of LSIL. In other
aspects, the disclosed
methods can be used to inhibit the progression of CIN. In yet other
embodiments, the disclosed
methods can be used to inhibit the progression of ICC.
The pharmaceutical composition can reduce the severity of the HPV related
dysplasia.
Severity of the HPV related dysplasia can be measured and graded by, for
example, changes in
histology. Methods of performing histology on biopsies of HPV-related lesions
are well known
in the art. In some embodiments, for example, the disclosed methods can reduce
the severity of
CIN 3. In some aspects, the disclosed methods can reduce the severity of CIN3
to CIN2. In
other aspects, the disclosed methods can reduce the severity of CIN3 to CIN1.
In other aspects,
the disclosed methods can reduce the severity of CIN3 to HPV negative. In
other aspects, the
disclosed methods can reduce the severity of CIN2 to CIN1. In other aspects,
the disclosed
- 18 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
methods can reduce the severity of CIN2 to HPV negative. In other aspects, the
disclosed
methods can reduce the severity of CIN1 to HPV negative.
In some aspects of the methods, the patient has a cervical cytology (e.g.,
from a PAP
smear) of HSIL, ASCUS, or LSIL. Administration of the pharmaceutical
composition to the
patient can reduce the cervical cytology. In some aspects, the cervical
cytology is reduced from
HSIL to a normal cytology. In some aspects, the cervical cytology is reduced
from HSIL to
ACSUS. In some aspects, the cervical cytology is reduced from HSIL to LSIL. In
some aspects,
the cervical cytology is reduced from ACSUS to a normal cytology. In some
aspects, the
cervical cytology s reduced from LS11, to a normal cytology.
Histological assessments to evaluate and/or grade the severity of the HPV
related
dysplasia and cytological screening can be performed at any suitable time
period prior to, during,
and/or post-treatment with the disclosed compositions. In some embodiments,
the methods
further comprise post-treatment monitoring of the patient. Suitable time-
frames for post-
treatment monitoring include, but are not limited to, 4 weeks, 8 weeks, 12
weeks, 16 weeks, 20
weeks, 24 weeks, 28 weeks, 32 weeks, 36 weeks, 40 weeks, 44 weeks, 48 weeks,
or 52 weeks
following treatment with the disclosed pharmaceutical compositions. In some
aspects, for
example, a histological assessment can be performed at baseline (prior to
treatment) and 6
months post-treatment to assess changes in CIN status. In other aspects, a
cytological screen can
be performed at baseline and 6 months post-treatment to assess changes in
cervical cytology. In
yet other aspects, a histological assessment and cytological screen can be
performed at baseline
and 6 months post-treatment to assess changes in CIN status and cervical
cytology, respectively.
The extent and grade of an HPV related dysplasia can be reduced during a
period of from
about 4 weeks to about 52 weeks following administering said composition. In
some aspects, the
extent and histological grade of the dysplasia can be reduced from about 4
weeks to about 46
weeks following administering said composition. In some aspects, the extent
and histological
grade of the dysplasia can be reduced from about 4 weeks to about 40 weeks
following
administering said composition. In some aspects, the extent and histological
grade of the
dysplasia can be reduced from about 4 weeks to about 34 weeks following
administering said
composition. In some aspects, the extent and histological grade of the
dysplasia can be reduced
from about 4 weeks to about 28 weeks following administering said composition.
In some
aspects, the extent and histological grade of the dysplasia can be reduced
from about 4 weeks to
about 24 weeks following administering said composition. In some aspects, the
extent and
histological grade of the dysplasia can be reduced from about 4 weeks to about
18 weeks
- 19 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
following administering said composition. In some aspects, the extent and
histological grade of
the dysplasia can be reduced from about 4 weeks to about 12 weeks following
administering said
composition. In some aspects, the extent and histological grade of the
dysplasia can be reduced
from about 6 weeks to about 10 weeks following administering said composition.
In some
aspects, the extent and histological grade of the dysplasia can be reduced
from about 8 weeks to
about 52 weeks following administering said composition. In some aspects, the
extent and
histological grade of the dysplasia can be reduced from about 12 weeks to
about 52 weeks
following administering said composition. In some aspects, the extent and
histological grade of
the dysplasia can be reduced from about 18 weeks to about 52 weeks following
administering
said composition. In some aspects, the extent and histological grade of the
dysplasia can be
reduced from about 24 weeks to about 52 weeks following administering said
composition. In
some aspects, the extent and histological grade of the dysplasia can be
reduced from about 30
weeks to about 52 weeks following administering said composition. In some
aspects, the extent
and histological grade of the dysplasia can be reduced from about 36 weeks to
about 52 weeks
following administering said composition. In some aspects, the extent and
histological grade of
the dysplasia can be reduced about 42 weeks to about 52 weeks following
administering said
composition. In some aspects, the extent and histological grade of the
dysplasia can be reduced
from about 48 weeks to about 52 weeks following administering said
composition.
In some aspects, the extent and histological grade of the dysplasia can be
reduced within
about 4 weeks following administering said composition. In some aspects, the
extent and
histological grade of the dysplasia can be reduced within about 5 weeks
following administering
said composition. In some aspects, the extent and histological grade of the
dysplasia can be
reduced within about 6 weeks following administering said composition. In some
aspects, the
extent and histological grade of the dysplasia can be reduced within about 7
weeks following
administering said composition. In some aspects, the extent and histological
grade of the
dysplasia can be reduced within about 8 weeks following administering said
composition. In
some aspects, the extent and histological grade of the dysplasia can be
reduced within about 9
weeks following administering said composition. In some aspects, the extent
and histological
grade of the dysplasia can be reduced within about 10 weeks following
administering said
composition. In some aspects, the extent and histological grade of the
dysplasia can be reduced
the extent and histological grade of the dysplasia can be reducedwithin about
11 weeks following
administering said composition. In some aspects, the extent and histological
grade of the
dysplasia can be reduced within about 12 weeks following administering said
composition. In
- 20 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
some aspects, the extent and histological grade of the dysplasia can be
reduced within about 16
weeks following administering said composition. In some aspects, the extent
and histological
grade of the dysplasia can be reduced within about 20 weeks following
administering said
composition. In some aspects, the extent and histological grade of the
dysplasia can be reduced
within about 24 weeks following administering said composition. In some
aspects, the extent
and histological grade of the dysplasia can be reduced within about 28 weeks
following
administering said composition. In some aspects, the extent and histological
grade of the
dysplasia can be reduced within about 32 weeks following administering said
composition. In
some aspects, the extent and histological grade of the dysplasia can be
reduced within about 36
weeks following administering said composition. In some aspects, the extent
and histological
grade of the dysplasia can be reduced within about 40 weeks following
administering said
composition. In some aspects, the extent and histological grade of the
dysplasia can be reduced
within about 44 weeks following administering said composition. In some
aspects, the extent
and histological grade of the dysplasia can be reduced within about 48 weeks
following
administering said composition. In some aspects, the extent and histological
grade of the
dysplasia can be reduced within about 52 weeks following administering said
composition.
The cervical cytology grade can similarly be reduced from about 4 weeks to
about 52
weeks following administering said composition. In some aspects, the cervical
cytology grade
be reduced from about 4 weeks to about 46 weeks following administering said
composition. In
some aspects, the cervical cytology grade be reduced from about 4 weeks to
about 40 weeks
following administering said composition. In some aspects, the cervical
cytology grade be
reduced from about 4 weeks to about 34 weeks following administering said
composition. In
some aspects, the cervical cytology grade be reduced from about 4 weeks to
about 28 weeks
following administering said composition. In some aspects, the cervical
cytology grade be
reduced from about 4 weeks to about 24 weeks following administering said
composition. In
some aspects, the cervical cytology grade be reduced from about 4 weeks to
about 18 weeks
following administering said composition. In some aspects, the cervical
cytology grade be
reduced from about 4 weeks to about 12 weeks following administering said
composition. In
some aspects, the cervical cytology grade be reduced from about 6 weeks to
about 10 weeks
following administering said composition. In some aspects, the cervical
cytology grade be
reduced from about 8 weeks to about 52 weeks following administering said
composition. In
some aspects, the cervical cytology grade be reduced from about 12 weeks to
about 52 weeks
following administering said composition. In some aspects, the cervical
cytology grade be
-21-
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
reduced from about 18 weeks to about 52 weeks following administering said
composition. In
some aspects, the cervical cytology grade be reduced from about 24 weeks to
about 52 weeks
following administering said composition. In some aspects, the cervical
cytology grade be
reduced from about 30 weeks to about 52 weeks following administering said
composition. In
some aspects, the cervical cytology grade be reduced from about 36 weeks to
about 52 weeks
following administering said composition. In some aspects, the cervical
cytology grade be
reduced from about 42 weeks to about 52 weeks following administering said
composition. In
some aspects, the cervical cytology grade be reduced from about 48 weeks to
about 52 weeks
following administering said composition.
In some aspects, the cervical cytology grade reduced within about 4 weeks
following
administering said composition. In some aspects, the cervical cytology grade
reduced within
about 5 weeks following administering said composition. In some aspects, the
cervical cytology
grade reduced within about 6 weeks following administering said composition.
In some aspects,
the cervical cytology grade reduced within about 7 weeks following
administering said
composition. In some aspects, the cervical cytology grade reduced within about
8 weeks
following administering said composition. In some aspects, the cervical
cytology grade reduced
within about 9 weeks following administering said composition. In some
aspects, the cervical
cytology grade reduced within about 10 weeks following administering said
composition. In
some aspects, the cervical cytology grade reduced within about 11 weeks
following
administering said composition. In some aspects, the cervical cytology grade
reduced within
about 12 weeks following administering said composition. In some aspects, the
cervical
cytology grade reduced within about 16 weeks following administering said
composition. In
some aspects, the cervical cytology grade reduced within about 20 weeks
following
administering said composition. In some aspects, the cervical cytology grade
reduced within
about 24 weeks following administering said composition. In some aspects, the
cervical
cytology grade reduced within about 28 weeks following administering said
composition. In
some aspects, the cervical cytology grade reduced within about 32 weeks
following
administering said composition. In some aspects, the cervical cytology grade
reduced within
about 36 weeks following administering said composition. In some aspects, the
cervical
cytology grade reduced within about 40 weeks following administering said
composition. In
some aspects, the cervical cytology grade reduced within about 44 weeks
following
administering said composition. In some aspects, the cervical cytology grade
reduced within
- 22 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
about 48 weeks following administering said composition. In some aspects, the
cervical
cytology grade reduced within about 52 weeks following administering said
composition.
The therapeutically effective dose of the disclosed pharmaceutical
compositions can be
administered for about 1 week to about 4 weeks. After this time a clinician
may assess whether
the course of treatment has been successful. A decision may then be made
whether or not to
continue treatment.
It will be appreciated that a clinician may wish to take into account
menstruation when
deciding on a treatment regimen for conditions relating to the cervix
Accordingly, a preferred
treatment regimen may be for about 14 - 21 days and can be administered
between menses. A
clinician may elect to stop topical treatment of the cervix during menses and
recommence a new
course of treatment in the next menstrual cycle. By way of example, a
preferred treatment
regimen can be: (1) 14 - 21 days of administration; (2) followed by 1 - 14
days without treatment
(during which menses may occur if treating the cervix); and (3) a further
cycle of 14 -21 days of
treatment if this is considered medically necessary. In a most preferred
embodiment, the cervix
of a women may be treated such that she receives about 266.6 mg of lopinavir
and about 66.6 mg
ritonavir per day for 14 -21 days; treatment can then be stopped for 1 - 14
days and a clinical
reassessment can be conducted; then, if necessary a second treatment cycle of
about 533 mg of
lopinavir and about 126 mg ritonavir per day can be administered for a further
14 -21 days. After
the second cycle a further clinical assessment can be made and a decision made
about whether or
not subsequent treatment cycles are required.
Unexpectedly, one treatment cycle of 14 days is very effective for eradicating
oncogenic
viral infection and reducing or eradicating precancerous or cancerous lesions
(see the Examples).
By way of further example, and without intending to be limiting, a preferred
treatment
regimen for treating the cervix of a woman in need of treatment can be: (1) 14
- 21 days of
administration; (2) optionally followed by 1 - 14 days without treatment
(during which menses
may occur); and (3) a further cycle of 14 - 21 days of treatment if this is
considered medically
necessary. The women may be treated such that she receives between about 400
mg and about
600 mg of lopinavir and between about 100 mg and about 150 mg ritonavir per
day as a single
vaginal dose which is ideally self-administered last thing at night (i.e. just
before retiring for
sleep).
In some embodiments, the composition can be administered twice daily for 14
days.
In some embodiments, the composition induces apoptosis of HPV infected cells.
-23 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
EXAMPLES
EXAMPLE 1 - combination of lopinavir and ritonavir are useful for preventing
or treating
malignant conditions caused by HPV infections
Experiments were conducted to demonstrate that a combination of lopinavir and
ritonavir
were useful for preventing or treating malignant conditions caused by HPV
infections.
Lopimune (Kaletre) soft gel capsules were used to illustrate the efficacy of
lopinavir and
ritonavir combination therapy.
EXPERIMENTAL METHODS
Patient Characteristics: Subject to approval by Kenyatta National Hospital
Ethics board,
more than 800 Kenyan women were provided with the opportunity for a free Human
Papillomavirus (HPV) test (Cervistat HR Hologic Inc., USA) followed by a
liquid based
cervical (LBC) cytology test (ThinPrep , Hologic Inc., USA). Study subjects
were recruited
from patients attending Kenyatta National Hospital's Family Planning Clinic
and Gynaecology
Out-patient Clinics in Nairobi according to the following criteria:
Inclusion:
1. Must be aged above 18 yrs.
2. Freely agreed to join the study after extensive information and counselling
and agreed
to give written informed consent.
3. Were capable of receiving and understand verbal and written information
about the
study.
4. Ready and willing to comply with the study follow-up schedule.
Exclusion:
1. Under 18 yrs of age.
2. Do not fulfil the above inclusion criteria.
3. Pre-existing conditions in which blood sampling may increase risk of
complications
e.g. sickle cell disease.
4. A positive HIV test.
5. Patients who are too ill to give informed consent
- 24 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
ThinPrep LBC samples were collected from >800 women who satisfied the above
inclusion criteria in Nairobi between 1st March and 30th August 2013 and these
were shipped to
the University of Manchester for laboratory analysis.
The Cervista HPV Test: The Cervista HR HPV test identifies whether any of 14
different high-risk types of HPV (Types 16, 18, 31, 33, 35, 39, 45, 51, 52,
56, 58, 59, 66, 68) are
present and was the first test of its type to be approved by the FDA. The
Cervista HPV RR test
does not determine the specific type of high risk HPV present.
The Cervista medium Throughput Automation (MTA) machine is a fully automated
CPU controlled device providing a 'sample in ¨ results out' system with
minimal user input.
Using 2 ml of cervical smear sample, stored in ThinPrep Pap Test PreservCyt
solution the
Cervista MTA initially carries out a fully automated DNA extraction before
placing the
extracted ultra-pure DNA into a 96-well assay plate. Subsequently using the
ultra-pure DNA, the
Cervista MTA sets up the Cervista HPV HR assay which uses Invader chemistry,
a FRET-
based signal amplification method for the detection of specific nucleic acid
sequences. By
combining HPV specific signals with control housekeeping reference sequences,
the system is
fully internally controlled and validated.
It is based on signal rather than DNA amplification technology and is 100%
effective at
detecting high-grade squamous intraepithelial lesions (HSIL) of the cervix.
Genomic DNA was
extracted as described and applied to the Cervista machine.
Liquid Based Cytology. ThinPrep LBC slides were then prepared using the
Hologic
Thinprep T2000 Processor from all women. These were then processed with an
automated
staining system for papanicolaou staining and cover-slipping at Central
Manchester NHS
Regional Cytology Laboratories. LBC slides from women identified by Cervista
as positive for
high-risk HPV were then scanned using the ThinPrep Dual Review Imager with
final cytology
reporting carried out by a consultant cytopathologist (Dr Mina Desai CBE).
Treatment with Lopimmune (CIPLA) (Kaletra, Abbvie) Soft Gel Capsules: Women
identified as Cervista positive for high-risk types of HPV who were also
identified as being
positive for HSIL, or low grade disease (LSIL), were enrolled as study
participants. They were
then examined by colposcopy and photographed (Welch Allyn Video Colposcope)
using visual
inspection with Lugol's iodine (VILI - Also known as Schiller's test) VILI
identifies the extent
of pre-cancerous lesions which appear bright yellow saffron in colour whereas
normal squamous
epithelium appears dark brown. They were subsequently given a supply of
Lopimune soft gel
capsules (normally prescribed for oral administration) to cover a treatment
period of two weeks
- 25 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
administering 1 capsule twice daily as an intra-vaginal pessary over this
period during which
they were asked to refrain from sexual activity. Furthermore, women were
interviewed routinely
during the treatment period for any adverse reactions such as itching,
inflammation etc. Two
weeks after the two-week treatment had finished, women were recalled, examined
by colposcope
with VILI, re-photographed and a ThinPrep LBC sample collected which was sent
to
Manchester for Cervista Ent HPV testing and cervical cytology as described
previously.
Two months later women were re-called, examined by colposocope plus VILI, re-
photographed and ThinPrep LBC samples again collected. In addition, punch
biopsies were
also taken and fixed in formalin This material was then sent to Manchester for
Cervista HR
HPV testing plus ThinPrep cervical cytology on LBC's whereas the punch
biopsies were wax
embedded and assessed by histopathology.
RESULTS
FIG. 1A, B, C & D show VILI staining plus HPV status and the cytology report
of
representative examples before and after treatment with Lopimmune as a pessary
and Table 2
summaries the results for 18 patients on the trial.
Table 2: Summary of Lopimmune Treatment Results
Pre-Treatment Post-Treatment Post-Treatment
Diagnosis Cytology HPV Status
1 Month 3 Months 1 Month 3 Month
(1) HPV+ve HSIL Normal Normal Negative Negative
(2) HPV+ve HSIL HSIL Normal Negative Negative
(3) HPV+ve HSIL Normal HSIL Positive Positive
(4) HPV+ve HSIL Normal Normal Negative Negative
(5) HPV+ve LSIL Normal Normal Negative Negative
(6) HPV+ve HSIL HSIL HSIL Positive Positive
(7) HPV+ve HSIL HSIL LSIL Positive Positive
(8) HPV+ve HSIL HSIL ND Positive ND
(9) HPV+ve HSIL HSIL Borderline Positive
Negative
(10) HPV+ve HSIL HSIL ND Positive ND
(11) HPV+ve HSIL Normal ND Negative ND
(12) HPV+ve HSIL Normal ND Positive ND
(13) HPV+ve Normal ND Negative ND
HSIL/LSIL
(14) HPV+ve HSIL Normal ND Positive ND
(15) HPV+ve HSIL Normal ND Negative ND
(16) HPV+ve HSIL Normal ND Negative ND
(17) HPV+ve HSIL Normal ND Positive ND
- 26 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
(18) HPV+ve HSIL Normal ND Negative ND
ND = Not determined at this time.
To date, 11 out of 17 patients (65%) diagnosed as Cervista HPV +ve with HSIL
prior to
treatment became cytologically normal 1 month after the start of Lopimmune
treatment. Patient
was diagnosed with HPV+ve LSIL prior to treatment and was cytologically
nottnal 1 and 3
months after the start of Lopimmune treatment. Six patients with HSIL (No's 2,
6, 7, 8, 9 & 10)
showed no improvement in cytology at 1 month although two of these (2 & 9)
subsequently
became negative for HPV with normal or borderline cytology at 3 months and 7
became LSIL at
3 months. One patient with HSIL (No 3) was cytologically normal but still
positive for HPV at 1
month and was subsequently diagnosed with HSIL at 3 months. 8 out of 18 (44%)
of patients
became Cervista HPV negative at 1 month and although one of these (No 9) was
HPV+ve at 1
month, this patient later became negative at 3 months.
In summary, 15 out of the 18 (85%) of the patients treated showed either
normalisation or
improved cytology during the 3 month follow up and only one of these regressed
to HSIL during
this period. Out of the 16 who responded 10 patients (66%) became HPV negative
during this
period
CONCLUSIONS
There were no adverse reactions reported from any of the patients treated. One
Lopimune soft gel capsule administered as a pessary twice a day for two weeks
is effective for
curative treatment of some HPV positive HSIL lesions within one to three
months of
commencing treatment as exemplified by Figure 1 A & B. At this dose, some
patients became
HPV negative at one month with HSIL cytology which later became normal
cytology at three
months (FIG. IC). At this dose, HPV positive treated LSIL lesions became HPV
negative and
cytology normal at one month (FIG. ID).
Table 2 summarises the results showing that Lopimune (KALETRAR) soft gel
capsules
have a pronounced curative effect against HPV infection of the cervix, and the
lesions this
causes, when administered at a dose of 1 capsule (or equivalent drug content)
twice a day for a
period of 2 weeks.
EXAMPLE 2 ¨ expansion and refinement of the trials described in Example 1
METHOIIS
-27 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
Patients and study centres: Clinical and basic diagnostic tests were done at
University of
Nairobi/Kenyatta National Hospital (KNH), Kenya while scientific aspects of
the study that
required specialized equipment were carried out at the Viral Oncology
Laboratory, St Marys
Hospital in Manchester, UK. The trial was conducted between 01/03/2013 and
01/11/2013 and
patients were recruited from women attending the Family Planning and
Gynaecology Outpatient
clinic at K.N.H. for routine follow up.
Enrollment and procedures: The study protocol is illustrated in FIG. 2.
Briefly, at the
screening stage, potential participants were given the patient information
sheet, counselled
appropriately and those willing to participate gave informed signed consent.
Thereafter, a
questionnaire was used to take both the socio-demographic, sexual histories
and clinical
characteristics. Blood was drawn for a HIV test using Determine TM (Abbott,
USA) and if positive
was confirmed by Unigoldmi (Trinity Biotech, Ireland). All patients were then
given a speculum
examination during which two cervical cyto-brushes samples were taken. The
first of these was
used for LBC ThinPrepg and HPV testing and samples were immediately sent to
Manchester. The
second was used for a conventional smear test as per standard Pap smear
screening procedures
which was examined and reported in Nairobi. Patients were then reviewed after
one month at
which time those that were HIV negative and HPV positive with abnormal
cervical cytology were
enrolled into the Lopimune trial.
At enrolement, a pelvic examination was carried out which included baseline
colposcopy.
Cervical morphology was initially visualised with acetic acid (VIA) followed
by Lugol's Iodine
(VILI) using x5 magnification (Video Coloposcope, Welch Allyn. NY. USA). This
was carried
out according to standard clinical practices and images recorded
(Sankaranarayanan R et al. Int I
Cancer. 2003 Sep 1;106(3):404-8). Blood was drawn for a full blood count,
urea, creatinine,
electrolytes and liver function tests.
Each patient was then issued with a supply of Lopimune soft gels (CIPLA,
India) for
vaginal insertion of one capsule twice daily for 2 weeks. Two additional
visits per week were
scheduled for the two week duration of Lopimune therapy during which a
questionnaire was filled
out to assess occurrence of adverse drug reactions and drug compliance.
Enrolled patients had
serial visits scheduled at 1, 2, 4, 8, 12 and 16 weeks. Follow up LBC ThinPrep
samples were
taken at 4 and 12 weeks and sent to Manchester for cytology and HPV testing.
In addition,
colposcopy with VILI was carried out at 4 and 12 weeks and final punch
biopsies were taken at the
latter time point. Blood was also drawn at these visits for baseline clinical
tests as described
previously. The 8 week visit was to assess any potential drug reactions and to
remind women that
- 28 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
a punch biopsy would be taken from any abnormal areas detected by VIA or VILI
on the cervix at
the 12 week visit. Biopsies were stored in 10% buffered formalin and were sent
to Manchester.
Those patients diagnosed with post-treatment, HPV positive high-grade disease
were referred for
Loop electrosurgical excision procedure (LEEP). These were reviewed again at 5
months and
advised to continue routine Pap smear screening at 6 months thereafter.
Cervista HR-HPV test: All ThinPrep LBC samples sent to Manchester were
analysed
by staff trained and certified by Hologic (Hologic, Bedford, MA). HPV testing
of LBC samples
was carried out using the FDA-approved Cervista HPV HR test (Day et al. J Clin
Virol. 2009
Jul;45 Suppl 1:S63-72) in conjunction with the Cervista MTA (Hologic)
automated platform
according to the manufacturer's instructions. This system provides ultra-pure
DNA extraction and
HPV testing in one sealed unit requiring no user input following initiation.
In brief, Cervista uses
three proprietary oligonucleotide probe master-mixes, designed to detect 14
high risk HPV types
within three familial groups based on phylogenetic similarities: Mix 1 detects
types 51, 56, and 66;
Mix 2 detects types 18, 39, 45, 59 and 68; Mix 3 detects types 16, 31, 33, 35,
52, and 58. A
separate human histone 2 gene probe serves as an internal control for cellular
DNA content within
the LBC sample. A HPV positive signal is indicated by fluorescent signal above
an empirically
derived cut-off value. Prior to and after analysis, all LBC samples were
stored at +4 C in a
monitored refrigerator.
HPV genotyping: Ultrapure DNA isolated from each ThinPrep LBC sample which
was
used for the Cervista test, was then further analysed by PCR to test for
which HPV genotypes
were present in each respective Cervista mastermix positive sample. A novel
hot-start, touch-down
multiplex method was used which simultaneously detects the Li, E6 and E7 ORFs
of HPV type
16, 18, 31, 33, 35, 39, 45, Si, 52, 56, 58, 59, 66, 68, and 70 as described in
Maranga et al. (Open
Virol J. 2013;7:19-27). Each assay was repeated a minimum of three times.
LBC ThinPrep cervical cytology: LBC slide preparation was carried out using
the
ThinPrep-2000 system (Hologic) automated slide preparation unit, according to
the manufactures
directions and Pap stained (Hologic) at the Regional Cytology Laboratories
(Clinical Sciences
Building, Manchester Royal Infirmary, UK). LBC reporting was carried out by Dr
M Desai using
a Dual Review Imaging System (Hologic).
Cervical Pathology: Final 3 mm punch biopsy samples of the cervical
transformation zone
and VILI positive lesions were transported to Manchester, wax embedded and 3-4
i_tm sections cut
at 3 levels according to standard pathology laboratory practices. The sections
were then stained
with haematoxylin and eosin and reported separately by two pathologists (Dr MP
Okemwa -
- 29 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
Kenya and Dr H Stringfellow - a specialist gynaecological pathologist in the
UK). Cases where
there was disagreement were independently reviewed by a third gynaecological
pathologist (Dr B.
Da Gama-Rose).
RESULTS
Primary HPV and Cytology Screening: Out of 805 HIV negative women given the
Cervista HR-HPV test, 164 (20.4%) were positive for high-risk HPV. Out of
these, cytology
showed that 28 (17.1%) had HSIL, 11(6.7%) had LSIL and 21(12.8%) had ASCUS.
Five (3.0%)
were diagnosed with ICC and were sent for immediate biopsy and subsequent
treatment
(hysterectomy). The finding that 20.4% of Kenyan women were positive for HR-
HPV with an
overall incidence of 3.5% for HSIL was entirely consistent with a reported
study (Maranga et el.
supra) and would be predicted from an unscreened population of this type. Out
of the 28 women
identified with HSIL, 5 were lost to follow up with 23 eventually being
enrolled on the trial. In
light of this, an additional 17 women with either ASCUS or LSIL were also
recruited to increase
the power of the study.
Table 3 shows the results of the primary Cervista HR-HPV test, HPV PCR
genotype
analysis, ThinPrepe LBC and conventional smear tests that were carried out on
the 23 women
diagnosed with HSIL. When combined, a relatively high incidence of HPV types
16 and 18 were
detected which together amounted to 10/23 (43.5%) of cases. With 5 infections,
type 52 was the
next most common followed by 4 infections each for types 35, 58 and 33. On one
occasion (E02)
Cervistan detected a positive for more than one master mix whereas PCR only
detected one HPV
genotype present which could be due to differences in sensitivity between
these methods. A good
concordance between HSIL diagnosed by LBC in Manchester and conventional smear
testing
carried out in Kenya was observed with only 3/23 disparities. Patients El and
Ell were diagnosed
with ASC-H by conventional cytology and E7 with LSIL.
Table 3: Patient characteristics of women diagnosed with HSIL prior to
treatment
B HPV status Cytology
irth
E-No Parity Age Conventional
Control Cervista PCR LBC
smear
E01 Cond 2 + 1 29 M3 35 HSIL ASC-H/AGC
E02 TL 2 + 0 39 Ml, 3 52 HSIL HSIL
E03 None 2 + 0 35 M3 16 HSIL HSIL
E04 IUD 4 + 0 42 Ml 70 HSIL HSIL
- 30 -
CA 02928166 2016-04-20
WO 2015/059485
PCT/GB2014/053169
B irth HPV status Cytology
E-No Parity Age Conventional
Control Cervista PCR LBC
smear
E06 None 2 + 0 29 M2, 3 16, 39 HSIL HSIL
E07 IUD 2 + 1 23 M2, 3 18, 52 HSIL LSIL
E08 None 2 + 0 27 M3 52 HSIL HSIL
E09 IUD 2 + 0 38 M2 18 HSIL HSIL
E10 None 2 + 0 37 M2 45 HSIL HSIL
Eli None 2 + 0 37 M3 33,58 HSIL ASC-H
E 1 2 IUD 2 + 0 41 M2 18 HSIL HSIL
E13 None 0 + 0 22 M3 33,58 HSIL HSIL
E14 None 0 + 0 26 M3 52 HSIL HSIL
EIS Depo 3 + 0 40 M2 68 HSIL HSIL
E16 Cond 2 + 0 41 M3 52 HSIL HSIL
E17 None 2 + 0 38 Ml, 3 16, 31, 51 HSIL HSIL
E18 IUD 2 + 0 36 M3 33 HSIL HSIL
E19 PM 7 + 0 69 M3 58 HSIL HSIL
E20 Depo 1 + 1 22 M3 33, 58 HSIL HSIL
E21 None 0 + 1 26 M3 16 HSIL HSIL
E22 Depo 6 + 0 43 M3 16, 35 HSIL HSIL
E23 Jadelle 2 + 0 27 M3 16, 35 HS11, HSIL
E29 Depo 5 + 0 47 M3 16, 35 HSIL HSIL
Table 4 shows the same data for the 17 women initially diagnosed with
LSIL/ASCUS and
demonstrates greater variation between LBC based diagnosis and conventional
smear testing
where 5/17 differential diagnosis were reported. HPV genotype analysis showed
6/17 (35.4%)
were positive for types 16 and 18 followed by type 35 with 5 positives, type
56 with 4 positives
and types 58 and 33 both with 3 positives. Curiously two samples (E33 and E40)
were Cervista
Mix 1 positive but PCR failed to detect any of the Mix 1 genotypes which again
could be related
to sensitivity. The average age of women in table 3 was 34.22 yrs and 33.76
yrs for table 4.
Table 4: Patient characteristics of women diagnosed with ASCUS/LSIL prior to
treatment
B irth HPV status Cytology
E.No Parity Age
Conventional
Control Cervista PCR LBC
smear
E05 Depo 3 + 0 41 M2 18 LSIL LSIL
E24 None 3 + 0 48 M3 16, 35 LSIL HSIL
E25 IUD 3 + 0 46 M 1, 3 33 ASCUS ASCUS
E27 e-pill 1 + 1 25 M3 33, 58 LSIL HSIL
E28 Cond 0 + 1 23 M2, 3 35, 45, 66 LSIL LSIL
-31-
CA 02928166 2016-04-20
WO 2015/059485
PCT/GB2014/053169
B irth HPV status Cytology
E.No Parity Age
Conventional
Control Cervista PCR LBC
smear
E30 Cond 3 + 0 30 M3 16, 35 ASCUS ASCUS
E31 None 3 + 0 37 M1 51 LSIL LSIL
E32 None 0 + 1 27 M3 16, 52 ASCUS ASCUS
E33 None 1 +0 26 MI N ASCUS HSIL
E34 None 4 + 0 56 Ml, 3 56, 58 LSIL LSIL
E35 None 2 + 0 32 M3 16, 31 ASCUS ASCUS
E36 None 0 + 0 21 M1 56 ASCUS ASCUS
E37 None 2 + 0 43 M1 56 ASCUS ASCUS
E38 Depo 3 + 0 27 M3 33, 35, 58 ASCUS Normal
E39 IUD 2 + 0 32 M3 16, 35 LSIL LSIL
E40 None 0 + 0 26 M1 N ASCUS ASCUS
E41 IUD 3 + 1 42 M1 56 ASCUS LSIL
MI: Cervista Mix I (MI) (HPV 51, 56, 66, or 70)
M2: Cervista Mix 2 (M2) (HPV 18, 39, 45, 59, 68)
M3: Cervista Mix 3 (M3) (HPV 16, 31, 33, 35, 52, 58)
ASCUS: Atypical squamous cells of undetermined significance
ASC-H: Atypical squamous cells ¨ cannot exclude HSIL
AGC: Atypical glandular cells
N: Negative
Depo: Depoprovera
TL: Tubal ligation
PM: Post menopausal
None of the women smoked.
Lopimune treatment of women initially diagnosed with HSIL: All of the women
shown
in table 3 were given a two week course of 1 capsule of Lopimune twice daily,
self-administered
as a vaginal suppository. During this treatment period interviews carried out
every 2 days reported
no adverse reactions such as unusual vaginal sensations, discharge, vaginitis,
cervicitis, vulvitis,
itching, burning, vaginal dryness or numbness. Repeat follow up baseline
clinical tests carried out
at 1, 2 and 4 weeks post-treatment also revealed no cause for concern.
The results of repeat ThinPrep LBC and Cervista HPV tests carried out at 4
and 12
weeks post-start of treatment are summarised in FIG. 3A, B and C where the HPV
genotypes
present are shown above each bar. The height of the bar indicates the level of
HPV detected in
each patient and demonstrates that a post-treatment drop in the levels of HPV
infection was
observed in 19/23 (82.6%) with the vinis not detected in 12/23 (52.2%) of
women 12 weeks after
the start of treatment.
- 32 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
After only 4 weeks (FIG. 3B) 14/22 (63.6%) of patients had returned to normal
cytology
and 3/22 (13.6%) now had lower grade disease demonstrating a positive response
in 76.9% of
women. After 12 weeks (FIG. 3C) 14/22 (63.6%) of women still had normal
cytology with 4/22
(18.2%) now presenting with lower grade dysplasia providing an overall
positive response in
81.8% of women. A total of 22 patients were reported at 4 and 12 weeks since
E19 had inadequate
cellularity for HPV testing and cytology at 4 weeks whereas EIS was adequate
for HPV testing but
not cytology at 12 weeks.
Table 5 compares the histopathology results with both cytology and the
Cervista HPV test.
It can be seen that 13/22 (59.1%) had no detectable neoplasia and 4/22 (18.2%)
had CIN1 which
potentially demonstrates a 77.3% positive response to treatment. Only 4/22
(18.2%) had CIN2 or
CIN3 and 1/22 (4.5%) had hCGIN. A total of 22 patients were reported for
pathology since the
biopsies obtained from E19 at 12 weeks were not suitable.
Table 5: Treatment outcome of women with Pre-Treatment HSIL at 3 Months
E-No 3 Month 3 Month Histopath. Histopath.
Cytology Cervista (MPO) (HS)
E01 Negative Positive None None
E02 Negative Negative None CIN2*
E03 HSIL Positive None CIN1 and
HCGIN*
E04 Negative Negative None None
E06 HSIL (mod) Positive CIN1 CIN1 just
E07 LSIL Positive CIN2 CIN1¨CIN2
E08 Negative Negative None CIN1*
E09 ASCUS Negative None None
E10 LSIL (mild) Positive None None
El 1 Negative Negative None None
E12 Negative Positive None None
E13 LSIL (mild) Positive CIN1 CIN1 ¨ CIN2*
E14 Negative Negative None None
El 5 Inadequate Positive None None
E16 Negative Negative None None
E17 HSI:1_, (mod) Positive CIN3 CIN2 + CIN3
El 8 Negative Negative None None
E19 Negative Negative Not suitable Not
suitable
E20 Negative Positive None None
E21 Negative Negative None CIN1 just*
E22 Negative Negative None None
E23 Negative Negative None None
E29 HSIL (sev) Positive None CIN1*
Note: Diagnosis marked with * were confirmed by a 3rd gynaecological
pathologist
- 33 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
Lopimune treatment of women initially diagnosed with ASCUS/LSIL: As for the
patients
diagnosed with HSIL, all of the women shown in table 4 were given a two week
course of 1
capsule of Lopimune twice daily, self-administered as a vaginal suppository
and no adverse
reactions were reported.
The results of repeat ThinPrep LBC and Cervista HPV tests carried out at 4
and 12
weeks post-start of treatment are shown in FIG. 4A, B and C. A post-treatment
drop in the levels
of HPV infection was observed in 11/17 (65.7%) with the virus not detected in
7/17 (41.1%) of
women 12 weeks after treatment.
Out of 16 satisfactory LBC slides, 8 (50%) had returned to normal cytology, 6
(35.3%) still
had ASCUS or LSIL and 2 (12.5%) were reported with HSIL after 4 weeks (FIG.
4B). After 12
weeks it can be seen that 10/16(62.5%) of women had normal cytology, 4/16
(25.0%) ASCUS or
LSIL and 2/16 (12.5%) now had HSIL (FIG. 4C).
Table 6 shows comparison of the histopathology results with both cytology and
the
Cervista HPV test. It can be seen that 9/17 (52.9%) had no evidence of
neoplasia, 6/17 (35.3%)
had CIN1 and 2/17 (11.8%) had CIN1/2.
Table 6: Treatment outcome of women with Pre-Treatment LSIL/ASCUS at 3 Months
E-No 3 Month 3 Month Histopath Histopath
Cytology Cervista (MPO) (HS)
E05 Negative Negative CIN1 CIN1*
E24 HSIL (sev) Positive None None
E25 Negative Negative None CIN1*
E27 Negative Positive None None
E28 Negative Negative CIN1 CIN1
E30 Negative Positive None None
E31 LSIL (mild) Negative None CIN1*
E32 LSIL (mild) Positive None None
E33 Negative Negative None None
E34 Negative Positive CIN2 CIN1*
E35 ASCUS Positive None None
E36 Negative Positive CIN1 ¨ CIN2 CIN1 + CIN2
E37 Negative Positive None None
E38 Inadequate Positive None None
E39 HSIL (sev) Positive CIN1 CIN1 + CIN2*
E40 Negative Negative None None
E41 ASCUS Negative CIN1 CIN1 just
Note: Diagnosis marked with * were confirmed by a 3rd gynaecological
pathologist
- 34 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
Colposcopy with VILI: FIG. 5 shows VILI colposcopic images of the cervix from
5 cases
diagnosed with HSIL taken before, 4 and 12 weeks after treatment with Lopimune
capsules.
Extensive yellow/orange staining characteristic of poorly glycogenated
dysplastic epithelium, was
clearly present in all the pre-treatment images of the ectocervix and the
cases shown were
deliberately chosen to illustrate that types of response observed. The
majority of patients showed a
reduction in dysplastic epithelium, as detected by VILI, which was associated
with reduced
severity of disease as indicated by HPV, cytology and/or pathology status. For
example, patient
E02 became HPV negative at 4 and 12 weeks and also clearly showed a marked
regression of
dysplastic epithelium detected by VILI although a small focus of CIN2 was
still present. Patient
E03 remained HPV positive at 4 and 12 weeks with normal cytology at 4 weeks
and yet showed
HSIL cytology with CINI and hCGIN at 12 weeks. E06 remained HPV positive with
HSIL
cytology after 12 weeks although pathology subsequently showed this to be
CIN1. E14 and El2
both showed return to normal cytology at 4 and 12 weeks with no CIN present at
12 weeks
although E12 remained HPV positive.
DISCUSSION
It was essential to have a suitable foimulation for vaginal delivery of drug
directly to the
cervix in order to translate the preclinical observations into clinical
trials. When used for oral HIV
therapy, lopinavir is normally co-adminstered in a 4:1 ratio with ritonavir
(Lopimune- CIPLA;
Kaletra- Abbvie). It was decided, due to cost and supply issues, to refrain
from specifically
reformulating lopinavir for vaginal use and the inventors opted to use the
commercially available,
orally-administered gelatine capsule form of the drug Lopimune as a vaginal
suppository.
A maximum dose of twice daily for two weeks was chosen on the basis of ease-of-
application and likely patient compliance since the primary objective of this
study was to evaluate
tolerability and any adverse reactions. HSIL was chosen as the primary disease
indication as this is
the most persistent type of HPV-related pre-invasive cervical lesion. Indeed,
although a proportion
of high-risk HPV positive HSIL can regress to normal if left untreated,
previous work has shown
that the mean time for this to occur is approximately 18-30 months. Moreover,
HPV infections are
known to be much more persistent when HSIL is present.
In order to identify approximately 30 women with HSIL, previous work had
indicated it
would be necessary to screen 600-800 women for this purpose (Maranga et
al.supra). The actual
number screened was 821 and the Cervista HPV-HR test was used as a primary
screen followed
- 35 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
by LBC and conventional cytology testing. Cytology was used for pre-treatment
diagnosis in
preference to pathology since this would limit tissue sampling-related damage
to the cervix as this
can affect the natural history of cervical lesions which may, in turn, affect
treatment outcome.
Furthermore, since the study was primarily aimed at assessing the tolerability
of a new treatment,
it was considered desirable to limit pre-treatment damage to the ecto-cervix.
Regarding the initial
diagnosis, two independent cytology reports were used and it is also very
clear that a positive
high-risk HPV test improves the sensitivity of detecting CIN2+.
The results of this study provide evidence which supports the claimed use of
Lopimune and
in particular the use of Lopimune as a locally applied therapy for HPV related
pre-invasive disease
of the cervix. The observation that, after a short 2 week course of treatment,
>60% of women,
initially diagnosed with high-risk HPV positive HSIL, returned to normal
cytology within 12
weeks is remarkable and is further supported by a reduction in disease
severity in others providing
an overall positive response to treatment in >800/0 of women. It is also
interesting that >50% of
these patients were HPV negative after 3 months.
Analysis of histopathology at 12 weeks largely confirmed these observations
but with
some differences. For example, patient E02 had normal cytology and VILI and
was HPV negative
whereas pathology showed a small region of CIN2. E06 and E29 both showed HSIL
at 12 weeks
but were reported as CIN1. E07 and E13 both showed LSIL at 12 weeks but were
reported as
CIN1-CIN2. E08 and E21 were both cytology normal and HPV negative but were
reported as
CIN1. Irrespective of these differences between cytology and pathology the
latter still showed that
¨60% of women had no detectable neoplasia at 12 weeks and others had reduced
severity of
disease (CIN1) indicating a combined positive response in ¨77% of women. Most
significantly
these rates of regression are much higher than would be predicted for
untreated cases of high-risk
HPV positive HSIL.
Although 32 women were identified with ASCUS/LSIL only 17 were chosen at
random for
enrolment on the trial since funding was only available to provide follow on a
maximum of 40
patients. The treatment response of these was perhaps not as striking as for
the patients with HSIL
since the former is known to have a higher rate of spontaneous regression.
However it is also
possible that women made aware of their diagnosis of ASCUS/LSIL may have been
less compliant
with the treatment than those diagnosed with HSIL. Nonetheless, >60% had
normal cytology at 12
weeks and pathology then confirmed absence of neoplasia in >52%. Since
previous work has
shown the time for natural regression of high risk HPV positive ASCUS or LSIL
is usually
between 12 and 24 months, the rates of regression observed with Lopimune
therapy are greater
- 36 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
than would be predicted by natural causes. Even so, it was noted that two
women (E36 and E39)
progressed to CIN2 over the 3 month period which may indicate that the
treatment dose and
regimen used in the study are sub-optimal. Indeed, the observation that some
women showed a
transient improvement in HPV and/or cytology at 4 weeks but then went on to
develop HPV
positive abnormal cytology/pathology at 12 weeks supports this conclusion (See
FIG. 2 and FIG.
3 E03, E13, E17 and E39). Most preferred treatment dose and regimens are
contemplated in the
description.
Regarding the different HPV genotypes present, there was no significant
correlation with
response to treatment for any of those identified nor was there any obvious
correlation with age,
type of contraception used or parity and none of the women smoked.
How does the current treatment with Lopimune compare to other non-surgical
treatments
for cervical dysplasia? PDT has been extensively evaluated for this purpose
with a large range of
response rates ranging from 0-100% for CIN and 53.4-80% eradication of HPV.
Disadvantages are
that PDT is physician applied with each sequential treatment typically taking
several hours. Also
systemic use of photosensitizers can cause problems with general skin
sensitization to light.
Topical application with the cytotoxic anti CMV drug Cidofovir has been used
to treat CIN2/3 and
showed ¨60% clearance of CIN but did not eliminate HPV infection as assessed
by the hybrid
capture 2 assay. Direct application of the immune activator Imiquimod to the
cervix has been
shown to have some effect against CIN and HPV infections both before and after
LEEP although
the treatment is continued for >8 weeks and the side effects can be quite
severe. A recent study of
topical application of the cytotoxic drug 5FU to the cervix showed this to be
>90% effective
against CIN2 lesions in young women aged 18-29 although this was physician
applied and the
treatment period lasted 16 weeks. Furthermore, there are issues related to the
safety of applying
potent DNA and RNA targeting cytotoxic agents, such as 5FU, to high-risk HPV
positive pre-
cancerous lesions in young women. It is well known that high-risk forms of HPV
induce genetic
instability in infected cells and, when this is combined with a DNA damaging
agent, this may
enhance the acquisition of persistent mutations in surviving cells. Clearly,
these disadvantages
and the potential importance of this for the development of subsequent
neoplasia could only be
determined by long term follow up. A review of the previously discussed
alternative therapeutic
approaches for the treatment of HPV related lesions was carried out by Bernard
in 2004 (J
Antimicrob Chemother. 2004 Feb;53(2):137-9 ) with the conclusion that none of
these are
generally recommended due to side effects and limited efficacy.
- 37 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
The current study illustrates the usefulness of compositions comprising
lopinavir and
ritonavir for use as a medicament in the treatment of cancer or in the
prevention of the
development of cancer. Composition such as Lopimune serve as a surprisingly
effective
alternative to surgery for the treatment of HPV related cervical dysplasia and
has many advantages
over conventional treatment, including:
(a) compositions according to the invention are relatively cheap (¨,E15.00 per
patient for the
current treatment protocol);
(b) compositions according to the invention are not a cytotoxic drug, do not
target DNA and
have very good safety profile with a current license for the long-term
systemic treatment of
pregnant women and children; and
(c) compositions according to the invention can be self-applied and, in
principle, treatment
can be repeated many times since no acute adverse reactions were reported; and
(d) positive antineoplastic/antiviral effects were clearly evident even as
early as 4 weeks post
start of treatment.
All of these factors combine to support the usefulness of the claimed
invention.
SUMMARY
821 women were recruited at Kenyatta National Hospital's Family Planning and
Gynaecology Outpatients Clinics and tested for HIV, HPV (CervistaR) together
with both
conventional and liquid based cervical cytology (LBC -ThinPrep ). Women
identified as HIV
negative and HPV positive with high-grade squamous intraepithelial lesions
(HSIL), atypical
squamous cells of undetermined significance (ASCUS) and low-grade squamous
intraepithelial
lesions (LSIL) were enrolled on the trial and examined by colposcopy. They
were then given a 2
week course of 1 capsule of Lopimune (CIPLA) twice-daily, self-applied as a
vaginal suppository.
Colposcopy, HPV testing and LBC were repeated at 4 and 12 weeks post-start of
treatment with a
final punch biopsy at 3 months for histology.
Out of 821 screened, 16 (1.95%) of women tested HIV positive. Of the remaining
805, 164
(20.4%) were positive for high-risk HEW of which 28 (17.1%) had HSIL, 11(6.7%)
LSIL, 21
(12.8%) ASCUS and 5 (3.0%) were diagnosed with ICC. Of these, Lopimune was
given to 23
women with HSIL and 17 women with ASCUS/LSIL cytology. Post-treatment cytology
at 12
weeks on women initially diagnosed with HSIL, showed 14/22 (63.6%) had no
dysplasia and 4/22
(18.2%) were either LSIL or ASCUS demonstrating a combined positive response
in 81.8% of
women. HPV was no longer detected in 12/23 (52.2%) of women. Histology at 12
weeks showed
- 38 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
absence of neoplasia in 13/22 (59.1%) of cases and 4/22 (18.2%) had low-grade
cervical
intraepithelial neoplasia (CIN1) demonstrating a combined positive response in
77.3% of women.
High-grade neoplasia (CIN2-3) was found in 4/22 (18.2%) and 1/22 had a high
grade glandular
cell neoplasia (hCGIN). A curative treatment response was also seen for women
diagnosed with
ASCUS/LSIL cytology prior to treatment. These data are supported by
colposcopic images which
show regression of cervical lesions and no adverse reactions were reported.
These results further exemplify that compositions according to the invention
serve as a
self-applied therapy for HPV infection and related cervical lesions. Since
there were no adverse
events or detectable post-treatment morbidity, unlike surgery, this treatment
may be repeated many
times.
EXAMPLE 3 treatment with Lopimune eliminates low risk HPV 6 and HPV 11 Genomic
DNA
from Cervical Smears
Samples from subjects in the trials described in Example 1 and Example 2 were
analysed
to examine whether or not Lopimune eliminated HPV from subjects.
Use of endpoint duplex PCR to detect HPV 6 & II viral DNA before and after
treatment with Lopimune: HPV 6 & 11 are low-risk types of HPV which are the
most common
cause of genital warts. In order to identify specific HPV genotypes 6 and 11,
duplex PCR assays
were designed and optimized to simultaneously detect viral Ll or L2 plus E6
and E7 ORFs. A
hot-start PCR kit was used as recommended by the manufacturer (Promega,
Southampton, UK).
The PCR mixtures consisted of 10 pi of 5 X Green GoTaqg Flexi Buffer, 3.0 mM
MgCl2, 0.2
mM dNTPs, 0.1 iuM of each primer, 1.25 unit GoTaqg DNA Polymerase and 50 ng of
genomic
DNA extracted from test ThinPrepg liquid based cytology (LBC) smear samples or
HPV6/11
positive controls, giving a 50 1 reaction volume. The housekeeping gene Beta-
2-microglobulin
(B2M) was used as a sample DNA quality control and PCR was carried out using
the above
reaction mixture with 0.2 M of each B2M primer up to a volume of 50 pl. All
reactions were
set up using a Veriti TM Thermal Cycler (Applied Biosystems, Paisley, UK) with
the conditions
and primers indicated in Table 7. The PCR products were separated by 2.5%
agarose gel
electrophoresis, stained with ethidium bromide and examined/photographed under
UV light.
- 39 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
Table 7. Primers and PCR Conditions Used for HPV6 & 11 genotyping
Amplimer
Primer ID Oligonucleotide Sequence PCR Parameters
Size (bp)
SEQ ID NO!:
6E6/7F 5'-CTAATTCGGTGCTACCTGIGTCA-3'
262 95
SEQ ID NO 2: C x 2 min 8 cycles: 94
C x
45S
6E6/7R 5'-GAATCTTGTCCGTCCACTTCGT-3' , 60 C-1 C/cycle x 45S,
SEQ ID NO 3: 72 C x455; 35 cycles: 94 C
x
6L1F 5'-GACTCGTCTCTTTTCGATCCCACA-3' 45S, 58 C x 45S, 72 C x
45S;
C x min
SEQ ID NO 4: 111 72
6L1R 5'-TAGGAAAGGATGTCCACTTACACCC-3'
SEQ ID NO 5:
11E6/7F 5'-GTGTGCCTGTTGCTTAGAACTGCA-3'
SEQ ID NO 6: 357 95 C x 2 min 8 cycles: 94
C x
11E6/7R 5'-CTIGTCCACCTCATCTTCTGAGCT-3' 45S, 61 C-1 C/cycle x 45S,
SEQ ID NO 7: 72 C x 45S: 35 cycles: 94
C x
11L1/2F 5'-CCTCCACCAAATGGTACACTGGAG-3' 45S, 57 C x 45S, 72 C x
45S;
SEQ ID NO 8: 210 72 C x 7 min
11L1/2R 5'-CCGTCCTCGATATCCACTTTGC-3'
RESULTS
FIG. 6 shows agarose gel electrophoresis of ethidium bromide stained low-risk
HPV 6 or
11 specific PCR products amplified from DNA extracted from LBC smears obtained
from
patients diagnosed with high-risk LIPV positive cervical dysplasi a both
before and after treatment
with Lopimune. It can be seen that two samples (S555 & S410) were positive for
HPV 6 and
four (S359, S744, S321, S394) for HPV 11 DNA prior to treatment giving a total
of 6 patients
infected with low-risk virus. (Note: The duplex assay used simultaneously
detects E6/E7 and Li
but a signal with either one or the other, or both, is classed as positive).
One month post-start of treatment, 4 patients (S555, S410, S744, S394) that
were positive
for low- risk HPV had become negative. Although weak, the Li signal which was
still visible in
S359 at one month became absent at 3 months which indicates that out of 6
patients infected
with low-risk virus, only one (S321) failed to clear the infection after
treatment with Lopimune.
These data show that Lopimune has activity against low-risk genital wart
associated forms of
HPV.
These data illustrate that treatment with Lopimune eliminates Low Risk HPV 6 &
11
Genomic DNA from Cervical Smears. This confirms that treatments according to
the invention
are particularly effective for treating, or preventing the development of,
cancerous conditions
associated with HPV infection (e.g many cervical cancers). Furthermore these
data illustrate that
treatments according to the invention are effective for treating other
conditions associated with
HPV infection such as benign proliferative disorders (e.g. genital warts) as
well as cancers.
- 40 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
EXAMPLE 4 ¨ colorimetric analysis of cell growth
A colorimetric analysis of cell growth was performed to investigate the
activity of
lopinavir and ritonavir against HPV positive cervical carcinoma cell lines
when administered
either separately or combined.
SiHa cells were grown in RPMI plus 10% FBS and HeLa in DMEM plus 10% FBS.
These were seeded at 2000 cells/well for HeLa and 5000 cells/well for SiHa in
triplicate into
standard tissue culture grade 96 well plates and incubated overnight at 37
C/5% CO2. Different
concentrations and ratios of lopinavir and ritonavir were then added in an
identical volume of
DMSO, plus a DMSO only control, and the incubation continued for 0, 24, 48 and
72 hours. At
each time point 20 p.1 of CellTitre 96 AQ reagent (Promega) was then added to
three separate
wells per data point and the absorbance (OD 490) read after 1 hour at 37 C.
The CellTitre 96
AQ reagent is a water soluble MTS formazan version of the well-known MTT
reagent which
produces a colorimetric readout that is directly proportional to the number of
viable cells present.
RESULTS
The results of these experiments are presented in FIG. 7 for SiHa (FIG. 7A)
and Hela
(FIG. 7B) cells. SiHa are HPV16 positive whereas HeLa cells are I-IPV18
positive cervical
carcinoma cell lines and the DMSO control growth curves show that the HeLa
cells have a more
rapid growth rate than do SiHa For SiHa cells it can be seen that 20 p.M
lopinavir plus 5 p.M
ritonavir has the same inhibitory effect on cell growth as 25 M lopinavir,
with 5 !JM ritonavir
having no effect. With HeLa cells, it can be seen that 201.iM lopinavir plus 5
p.M ritonavir was
more growth inhibitory than either 25 p.M lopinavir or 5 p.M ritonavir when
these were applied
as single agents, which illustrates that the combination is more effective at
inhibiting growth than
either compound applied as a single agent. However, although this method
implies that the
additive combination of lopinavir and ritonavir is as effective at killing
cells as 100% lopinavir
in SiHa and more effective in HeLa cells, it does not directly measure cell
death. For this reason
the activity of these compounds was also evaluated by methods which directly
measure either
apoptosis or total cell death (see Example 5).
-41-
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
EXAMPLE 5 - analysis of apoptosis using dual staining with V450 Annexin V and
propidium
iodide.
ME1HODS
Staining with V450 Annexin V (BD Biosciences) was used to analyse the
percentage of
cells within a population that are actively undergoing apoptosis. Propidium
Iodide (PI) is a
standard DNA-staining flow cytometric viability probe and is used to
distinguish viable from
nonviable cells. Cells that stain positive for V450 Annexin V and negative for
PI are undergoing
apoptosis. Cells that stain positive for both V450 Annexin V and PI are either
in the end stages
of apoptosis, are undergoing necrosis, or are already dead. Cells that stain
negative for both
V450 Annexin V and PI are alive and not undergoing measurable apoptosis.
HeLa cells are notoriously robust and yet the previously discussed CellTitre
968 AQ
assays showed they underwent pronounced growth inhibition when treated with
lopinavir and
ritonavir combined. For this reason these cells were chosen to evaluate the
effects of these
compounds on cell death A 2 ml aliquot of growth medium containing 20,000
cells was seeded
into each well of a 6 well plate. After 72 hrs incubation in DMEM (10% FBS and
1% L-
Glutamine) at 376C/5% CO,. various concentrations and ratios of lopinavir and
ritonavir were
added in an identical volume of DMSO and the cells incubated for a further 48
hrs. The growth
medium was removed and retained and the cells washed with PBS followed by
Incubation with 1
ml of Accutase (Life Technologies, Paisley, UK, Catalog Number A11105-01) per
well for 15
mins. The original growth medium was then added back to neutralise the
Accutase, and this
centrifuged at 1,200 rpm for 5 mins to pellet the cells and this repeated
twice with cold PBS. The
cell pellet was finally re-suspended in 1 x binding buffer (10x = 0.1 M Hepes
(pH 7.4), 1.4 M
NaCl, 25 mM CaCl2) at a concentration of 106 cell/ml. A 100 pi aliquot
containing 105 cells was
removed and placed in a 5 ml culture tube and 5 1 of V450 Annexin V added plus
10 1 P1(50
hg/ml) added. The cells were gently mixed and incubated at room temperature
for 15 min in the
dark then 400 p.1 of 1 x binding buffer was added prior to being analysed by
flow cytometry
using a Cyan ADP instrument (Beckman Coulter) and Summit software within one
hour of the
above procedure. The Annexin fluorochrome was excited using a 405 nm laser and
the emission
measured using a 450/50 nm bandpass filter. The PI was excited using a 488 nm
laser and the
emission detected using a 613/20 nm bandpass filter. The data was gated to
select single cells
using a plot of forward scatter against pulse width, and to exclude debris
using forward scatter
plotted against side scatter. All cytometry assays shown were the result of
three separate cultures
- 42 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
and intra-assay variability was also assessed by repeating the same drug
treatment assay (1:1
Lop/Rit) three times. Unstained cells and cells stained separately with either
V450 Annexin V or
PI were used as controls to optimise compensation and gating of the cytometer.
RESULTS
Scatter plots following analysis in a cell cytometer: The scatter plots of
FIG. 8 show
profiles of unstained, annexin-only and PI-only stained HeLa cells were used
to optimise the
cytometer and set gates appropriately. This also showed that very little
background was observed
in the R4 quadrant where advanced stage apoptotic cells should be detected.
The scatter plots of FIG. 9 show analysis of triplicate cultures treated with
1:1 (12.5 ttM
lopinavir (Lop) + 12.5 uM ritonavir (Rit)) with an additional analysis of one
culture repeated 3
times to determine the intra cytometer assay variance. (Note: Cells in the R6
quadrant are in
earlier stages of apoptosis).
The scatter plots of FIG. 10 show analysis of triplicate cultures treated with
4:1 (20 uM
Lop+5 jiM Rit) plus cultures treated with decreasing amounts of ritonavir (21
uM Lop+ 4 uM
Rit and 23 uM Lop+ 2 uM Rit).
The scatter plots of FIG. 11 show duplicate cultures treated with 25 !AM Lop
as a single
agent and triplicate cultures treated with same amount of DMSO used for all
the drug assays
shown.
Analysis of inter and intra sample variance: FIG. 12 is a graph showing the
analysis of
intra and inter sample variance which occurred within and between the flow
cytometry apoptotic
assays carried out on 1:1 (12.5 uM Lop+ 12.5 uM Rit) treatments. The white box
plots represent
the percentage of cells found in quadrant R4 (Late stage apoptosis) whereas
the shaded box plots
represent the percentage found in R6+R4 (combined early and late stage
apoptosis). Intra-sample
readings demonstrated a maximum of 4 percentage point differences which
indicated that the
scatter plots have a high degree of reproducibility.
Comparison of percentage apoptotic cells between treatments: FIG. 13 is a box
plot
showing comparison of the effects of the various drug treatments on the levels
of late apoptosis
(white boxes R4) and early + late apoptosis (shaded boxes R6+R4) observed in
these cultures. It
can be seen that 4:1(20 uM Lop+5 jiM Rit) has the highest level of apoptosis
followed by 1:1
(12.5 uM Lop+12.5 jiM Rit). Reducing the amount of ritonavir below 5 tiM and
increasing
amounts of lopinavir caused a reduction in the amount of apoptosis observed.
These results
indicate that, with a total dose of 25 uM, the optimal drug ratio lies between
1:1 and 4:1
- 43 -
GA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
lopinavir/ritonavir. Furthermore, 25 JIM lopinavir as a single agent was less
effective than any of
the combination treatments
STATISTICAL ANALYSIS
Table 8 illustrates an a level of 0.05; the replicate readings are acceptable;
and the 95%
confidence intervals do not pass 0. The means are therefore suitable for
ANNOVA which
showed significant difference between sample means (table 9). F score of
59.252 and a p value
of 1.4 x 10-7 (ie < a 0.05)
Table 8:
95% Confidence Interval for
Std. Mean
R4 gate Mean Deviation Std. Error Lower Bound Upper Bound Min Max
DMSO 6.4233 1.07472 .62049 3.7536 9.0931 5.25
7.36
Lop12.5Rit12.5 28.4967 2.29740 1.32640 22.7896 34.2037
25.97 30.46
Lop2ORit5 35.2500 4.13668 2.38831 24.9739 45.5261
30.85 39.06
Lop21Rit4 26.0733 1.28722 .74318 22.8757 29.2710
24.60 26.98
Lop23Rit2 24.7800 .79379 .45829 22.8081 26.7519
24.23 25.69
Lop25Rit0 21.8000 .01414 .01000 21.6729 21.9271
21.79 21.81
Total 23.9218 9.49713 2.30339 19.0388 28.8047
5.25 39.06
Table 9:
Sum of Squares df Mean Square F Sig.
Between Groups 1391.462 5 278.292 59.252
0.000000139935051
Within Groups 51.665 11 4.697
Total 1443.127 16
Table 10 illustrates that Dunnet post-hoc shows that all treatments are
significantly
different to DMSO (p value range: 1.9x10-5 to 1.8x10-8).
Table 10:
95% Confidence Interval
Mean
Treatment Condition Difference * Std. Error Sig.
Lower Bound Upper Bound
Dunnett t Lop12.5Rit12.5 DMSO 22.07333 1.76952 0.000000
17.5805
(>control)b Lop20Rit5 DMSO 28.82667* 1.76952 0.000000
24.3338
Lop21Rit4 DMSO 19.65000 1.76952 0.000001 15.1571
Lop23Rit2 DMSO 18.35667* 1.76952 0.000001 13.8638
Lop25Rit0 DMSO 15.37667 1.97838 0.000019 10.3535
- 44 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
Bonferonni post-hoc analysis (Table 11) also shows that all treatments are
significantly different
to DMSO (expected, not shown) p value range: 1.29x104 to 7.14x10-8.
Table 11
Bonferonni Mean 95% Confidence Interval
Treatment Condition Difference Std. Error Sig. Lower Bound Upper
Bound
Lop12.5Rit12.5 Lop20Rit5 -6.75333 1.76952 0.042907 -
13.3506 -.1561
Lop20Rit5 Lop12.5Rit12.5 6.75333 1.76952 0.042907 .1561
13.3506
Lop21Rit4 9.17667* 1.76952 0.004514 2.5794
15.7739
Lop23Rit2 10.47000* 1.76952 0.001509 3.8727
17.0673
Lop25Rit0 13.45000* 1.97838 0.000444 6.0740
20.8260
Lop23Rit2 Lop20Rit5 -10.47000 1.76952 0.001509 -
17.0673 -3.8727
Lop25Rit0 Lop20Rit5 -13.45000 1.97838 0.000444 -
20.8260 -6.0740
EXAMPLE 6 - analysis of total cell death by Trypan blue staining:-
METHODS
Unlike the previous Annexin V/ PI method, Trypan blue staining does not detect
cells in
the early stages of programmed cell death or distinguish between apoptotic and
necrotic cell
death.
In similar fashion to the Annexin V/PI assay, 2 ml aliquots of growth medium
containing
20,000 cells were seeded into 6 well plates. After 72 hrs incubation in DMEM
(10 % FBS and
1% L-Glutamine) at 37 C15% CO2 various concentrations and ratios of lopinavir
and ritonavir
were added in an identical volume of DMSO and the cells incubated for a
further 48 hrs. The
growth medium was removed and retained and the cells washed with PBS followed
by
Incubation with 1 ml of Accutase (Life Technologies, Paisley, UK, Catalog
Number A11105-01)
per well for 15 mins. The original growth medium was then added back to
neutralise the
Accutase, and this centrifuged at 1,200 rpm for 5 mins to pellet the cells
which were then re-
suspended in PBS. An equal volume of 0.4% w/v Trypan blue solution (Sigma) was
added and
live/dead cells counted using a haemocytometer. All assays were the result of
three separate
cultures and all cell counts were repeated in triplicate.
RESULTS
FIG. 14 illustrates total cell death in Hela cells as measured by the Trypan
Blue dead
cell assay. If the DMSO percentage background cell death is subtracted from
the various drug
treatments, it can be seen that the combination of the 4:1(20 [tM lopinavir
plus 5 p..M ritonavir)
exhibits synergy whereby this was more effective at inducing cell death
(49.18%) than the sum
- 45 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
of both compounds (33.66%) when these were administered separately. Dropping
the ratio of the
two drugs to 1:1 (12.5 [tM Lopinavir plus 12.5 [tM ritonavir) was somewhat
less effective at
inducing cell death than the 4:1 ratio and did not show quite the same level
of synergy.
Accordingly a ratio of 4:1 (lopinavir:ritonavir) represents a preferred ratio
for compositions used
according to the invention.
Those skilled in the art will appreciate that numerous changes and
modifications can be
made to the preferred embodiments of the invention and that such changes and
modifications can
be made without departing from the spirit of the invention. It is, therefore,
intended that the
appended claims cover all such equivalent variations as fall within the true
spirit and scope of the
invention.
The disclosures of each patent, patent application, and publication cited or
described in
this document are hereby incorporated herein by reference, in its entirety
EMBODIMENTS
The following list of embodiments is intended to complement, rather than
displace or
supersede, the previous descriptions.
Embodiment 1. A composition comprising lopinavir, with or without, ritonavir
for use as
a medicament in the treatment of cancer or benign proliferative disorders
(warts) or in the
prevention of the development of cancer.
Embodiment 2. The composition according to Embodiment 1 comprising lopinavir
and
ritonavir.
Embodiment 3. The composition according to Embodiments 1 or 2 used to prevent
the
development of cancer.
Embodiment 4. The composition according to Embodiment 3 used in a dose that is
effective for treating a Human Papilloma Virus (HPV) infection with or without
attendant
abnormal pathology.
Embodiment 5. The composition according to any preceding Embodiments wherein
the
medicament is used to treat or prevent the development of early stage
neoplasias.
- 46 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
Embodiment 6. The composition according to any preceding Embodiments wherein
the
medicament is used to treat or prevent the development of HPV related
cervical, vulval, vaginal,
penile, anal, oral, laryngeal neoplasias and/or warts.
Embodiment 7. The composition according to Embodiment 6 wherein the medicament
is
used to treat or prevent the development of cervical neoplasias.
Embodiment 8. The composition according to any preceding Embodiment wherein
the
medicament is formulated for topical application.
Embodiment 9. The composition according to any preceding Embodiment wherein
the
medicament comprises between about 100-600 mgs of lopinavir with our without
between 30-
175 mg of ritonavir.
Embodiment 10. The composition according to Embodiment 9 wherein the
medicament
comprises about 133.3 mgs of lopinavir and about 33.3 mg of ritonavir.
Embodiment 11. The composition according to any preceding Embodiment wherein
the
medicament is administered either once or twice daily.
Embodiment 12. The composition according to any preceding Embodiment wherein
the
medicament is administered for a period of between 7 and 21 days.
Embodiment 13. The composition according to Embodiment 12 wherein the
medicament
is administered for second or further period of between 7 and 21 days.
Embodiment 14. The composition according to Embodiment 13 wherein the
medicament
is administered in a treatment regimen of a first period of 14 - 21 days;
followed by a period of
1-14 days without treatment; and then followed by a second period of 14 -21
days.
Embodiment 15. A composition comprising lopinavir for use as a medicament in
the
treatment of cancer or benign proliferative disorders (warts) or in the
prevention of the
development of cancer.
Embodiment 16. A pharmaceutical composition that is formulated for topical
application
comprising a therapeutically effective amount of lopinavir or a
therapeutically effective amount
lopinavir and ritonavir in a pharmaceutically acceptable vehicle.
Embodiment 17. The pharmaceutical composition of Embodiment 16, comprising a
synergistic amount of lopinavir and ritonavir.
Embodiment 18. The pharmaceutical composition of Embodiment 17, wherein the
synergistic amount of lopinavir and ritonavir is from about 1.1 to about 4:1
lopinavir to ritonavir.
-47 -
CA 02928166 2016-04-20
WO 2015/059485 PCT/GB2014/053169
Embodiment 19. The pharmaceutical composition of Embodiment 17, wherein the
synergistic amount of lopinavir to ritonavir is from about 1:1 to less than
4:1 lopinavir to
ritonavir.
Embodiment 20. The pharmaceutical composition of any one of Embodiments 16-19,
wherein the pharmaceutical composition is formulated for intravaginal
delivery.
Embodiment 21. The pharmaceutical composition of Embodiment 20, wherein the
formulation comprises a gel, cream, ointment, lotion, ovule, soft capsule,
suppository, pessary, or
any combination thereof
Embodiment 22. The pharmaceutical composition of any one of Embodiments 16-20,
wherein the formulation is substantially free of pigments, dyes, and inks.
Embodiment 23. A method of treating a patient having an HPV related dysplasia
of the
cervix comprising administering to said patient a therapeutically effective
dose of the
pharmaceutical composition of any one of claims 16-22.
Embodiment 24. The method of Embodiment 22, wherein the pharmaceutical
composition reduces the severity of the HPV related dysplasia.
Embodiment 25. The method of Embodiment 24, wherein the severity of the HPV is
reduced from CIN3 to CIN2, from CIN3 to CIN1, from CIN3 to HPV negative, from
CIN2 to
CIN1, from CIN2 to HPV negative, or from CIN1 to HPV negative.
Embodiment 26. The method of Embodiment 25, wherein the severity is reduced
from
about 4 weeks to about 52 weeks following administering said composition.
Embodiment 27. The method according to any one of Embodiments 23 or 26,
wherein
the patient has a cervical cytology of high grade squamous intraepithelial
lesion (HSIL), atypical
squamous cells of undetermined significance (ASCUS), or low grade squamous
intraepithelial
lesion (LSIL).
Embodiment 28. The method of Embodiment 27, wherein the composition reduces
the
cervical cytology.
Embodiment 29. The method of Embodiment 28, wherein the cervical cytology is
reduced from HSIL to a normal cytology, from HSIL to ACSUS, from HSIL to LSIL,
from
ACSUS to a normal cytology, or from LSIL to a normal cytology.
Embodiment 30. The method of Embodiment 29, wherein the cervical cytology is
reduced from about 4 weeks to about 52 weeks following administering said
composition.
Embodiment 31. The method according to any one of Embodiments 23-30, wherein
the
composition is administered twice daily for 14 days.
- 48 -
CA 02928166 2016-04-20
WO 2015/059485
PCT/GB2014/053169
Embodiment 32. The method of any one of Embodiments 23-31, wherein the
composition induces apoptosis of HPV infected cells.
- 49 -