Note: Descriptions are shown in the official language in which they were submitted.
CA 02928325 2016-04-21
DESCRIPTION
TITLE OF THE INVENTION:
METHOD FOR PRODUCING COKE, AND COKE
TECHNICAL FIELD
[0001]
The present invention relates to a method for producing a coke, and a coke.
More specifically, the present invention relates to a method for producing a
coke
suitable as a reducing material for non-ferrous metallurgy, and a coke.
BACKGROUND ART
[0002]
Conventionally, a coke has been used as a reducing material in refining of a
non-ferrous metal such as aluminum and titanium. In particular, a calcine coke
(so-
called calcined coke) obtained by heating a raw petroleum coke is inexpensive
and
therefore, is used for general purposes.
[0003]
The raw petroleum coke as a raw material of the calcine coke is a by-product
generated in the process of refining petroleum from crude oil. Accordingly,
the
character of the calcine coke is dependent on the crude oil. For example, the
impurities (e.g., sulfur, nickel, vanadium, sodium and the like) contained in
the calcine
coke are derived from the crude oil as a raw material of the calcine coke.
These
impurities become a contamination source and therefore, in the case of using
the calcine
coke as a coke for use in refining, the content of the impurities (in
particular, sulfur
content; hereinafter the same) is required to be as small as possible.
However, since
the impurity content in the recently produced crude oil is large, it has been
difficult to
provide a coke with a small impurity content.
[0004]
As regards a carbon material with a small impurity content, studies are being
made to utilize, as the coke raw material, an ashless coal substantially
containing no
1
CA 02928325 2016-04-21
ash. For example, Patent Document 1 discloses a production method of an
ashless
coal to be used for a fuel, a coke raw material, a chemical raw material, etc.
[0005]
However, the ashless coal has high thermal fluidity and has a property of
melting at 200 to 300 C irrespective of the grade of the raw material coal. In
addition,
the ashless coal has a property of expanding when it is heated at around 400
C.
Therefore, when an ashless coal is subjected to forming, followed by dry
distillation by
high-temperature heating, the ashless coal melts, and the shape of the formed
product
cannot be maintained, leading to a problem with thermoplasticity. Furthermore,
expandability is a problem, for example, as follows: the ashless coal may
expand by
undergoing foaming due to high-temperature heating to overflow from a dry
distillation
apparatus or adhere to the inner wall of the dry distillation apparatus,
making its
discharge impossible, or the coke may be obtained as a sponge-like porous body
and
extremely reduced in the bulk specific gravity. In this way, because of the
problem
with thermoplasticity or expandability, the ashless coke could be hardly used
as a coke
raw material.
[0006]
To solve such a problem, the present inventors have proposed a technique for
modification of the ashless coal (Patent Document 2). Specifically, a
production
method of a carbon raw material is disclosed, the method including a slurry
heating step
of heat-treating a slurry containing a coal and an aromatic solvent, a
separation step of
separating the slurry heat-treated in the slurry heating step into a liquid
component
having dissolved therein coal and a solid component composed of an ash and
insoluble
coal, an ashless coal obtaining step of obtaining an ashless coal by removing
the
aromatic solvent from the liquid component, and an ashless coal heating step
of heat-
treating the ashless coal obtained in the ashless coal obtaining step to
provide a carbon
raw material, wherein the volatile content of the carbon raw material obtained
in the
ashless coal heating step, as measured by the method specified in JIS M 8812,
is less
than 35 mass% and 24 mass% or more.
[0007]
According to this technique, by virtue of including a slurry heating step, a
separation step, an ashless coal obtaining step, and an ashless coal heating
step for
2
CA 02928325 2016-04-21
adjusting the volatile content to fall in a predetermined range, a low-ash
carbon material
having excellent self-sinterability can be produced.
PRIOR ART LITERATURE
PATENT DOCUMENTS
[0008]
Patent Document 1: JP-A-2001-26791
Patent Document 2: JP-A-2009-144130
SUMMARY OF THE INVENTION
PROBLEMS THAT THE INVENTION IS TO SOLVE
[0009]
The technique of Patent Document 2 produces an excellent effect in improving
self-sinterability, but since modification of the ashless coal requires a lot
of labor, the
productivity is not necessarily high, and the modified ashless coal is
relatively
expensive.
[0010]
The present invention has been made by focusing on the above-described
circumstances, and an object of the present invention is to provide a method
for
producing a high-purity coke at a lower cost than ever before, and to provide
a high-
purity coke.
MEANS FOR SOLVING THE PROBLEMS
[0011]
The method for producing a coke in the present invention which is capable of
achieving the object includes performing dry distillation of a mixture
containing: an
ashless coal; an oxidized ashless coal obtained by an oxidation treatment of
an ashless
coal; and a raw petroleum coke, in which, relative to 100 parts by mass of a
total of the
ashless coal, the oxidized ashless coal and the raw petroleum coke, a content
of the
ashless coal is from 5 to 40 parts by mass, and a total content of the ashless
coal and the
oxidized ashless coal is from 30 to 70 parts by mass.
[0012]
3
CA 02928325 2016-04-21
Preferable embodiments of the present invention include the case where the
mixture is subjected to forming, and then, the dry distillation is performed,
the case
where a percentage of increase in oxygen of the oxidized ashless coal is from
2 to 10%,
the case where the oxidation treatment is an air oxidation, and the case where
the
oxidation treatment is performed at a temperature of 150 C or more and less
than an
ignition point.
[0013]
In addition, the preferable embodiments include the case where the dry
distillation is performed in a chamber furnace, and the case where the dry
distillation is
performed in a rotary kiln.
An aspect of the present invention includes a coke produced by performing dry
distillation of a mixture, the mixture containing: an ashless coal; an
oxidized ashless
coal obtained by an oxidation treatment of an ashless coal; and a raw
petroleum coke, in
which, relative to 100 parts by mass of a total of the ashless coal, the
oxidized ashless
coal and the raw petroleum coke, a content of the ashless coal is from 5 to 40
parts by
mass, and a total content of the ashless coal and the oxidized ashless coal is
from 30 to
70 parts by mass.
ADVANTAGE OF THE INVENTION
[0014]
According to the production method in the present invention, a high-purity
coke can be produced at a low cost by using a raw petroleum coke. In addition,
in the
present invention, a high-purity coke can be provided.
BRIEF DESCRIPTION OF THE DRAWINGS
[0015]
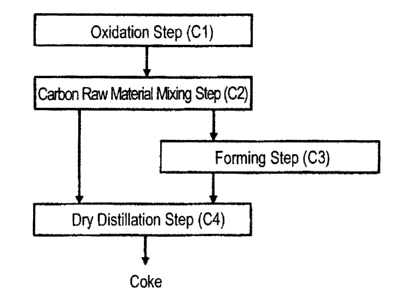
[FIG. 1] FIG. 1 is a flowchart for explaining one example of the production
process of an ashless coal.
[FIG. 2] FIG. 2 is a flowchart for explaining one example of the production
process of a coke in the present invention.
MODE FOR CARRYING OUT THE INVENTION
4
CA 02928325 2016-04-21
[0016]
The present inventors have made many intensive studies to provide a high-
purity coke at a low cost by using a raw petroleum coke as a carbon raw
material, and
found the followings.
[0017]
The impurity content of the ashless coal is very small, and mixing of the
ashless coal with the raw petroleum coke is useful for reducing the impurity
content of
the coke. However, as pointed out in conventional techniques, an ashless coal
has a
problem with thermoplasticity or expandability.
[0018]
Studies of the present inventors revealed that when the ashless coal is
subjected
to an oxidation treatment, the thermoplasticity and expandability of the
ashless coal can
be improved. However, the oxidized ashless coal is in the form of fine powder
and
exhibits low caking, and dry distillation of a mixture of the oxidized ashless
coal and
the raw petroleum coke causes a problem that the coke obtained becomes powdery
and
readily scatters out of a dry distillation apparatus and in addition, the bulk
specific
gravity of the coke is reduced. Intensive studies have been made to solve such
a
problem, and as a result, it has been found that when a mixture of an ashless
coal, an
oxidized ashless coal and a raw petroleum is made, the ashless coal functions
as a
binder for binding the oxidized ashless coal and the raw petroleum coke and a
problem
such as powdering of the coke can be suppressed.
[0019]
It has been then found that when a mixture containing the ashless coal, the
oxidized ashless coal and the raw petroleum coke each with a predetermined
content
described later is used, the coke obtained can be prevented from melting or
expanding
and a high-purity coke could be provided at a low cost.
[0020]
The coke production method in the present invention is described below by
referring to the flowcharts illustrated in FIGs. 1 and 2.
[0021]
First, the ashless coal used in the present invention is described.
[0022]
CA 02928325 2016-04-21
An ashless coal indicates a coal having an ash content of 5 mass% or less,
preferably 3 mass% or less. The ashless coal is preferably a coal in which the
ash
concentration of a residual inorganic material (e.g., silicic acid, alumina,
iron oxide,
lime, magnesia, alkali metal or the like) when heated at 815 C to thereby form
an ashed
body thereof is very low. Specifically, the ash concentration is more
preferably 5,000
ppm or less (on the mass basis), still more preferably 2,000 ppm or less. In
addition,
the ashless coal is absolutely water-free and exhibits higher thermal fluidity
than the
raw material coal.
[0023]
<Production Process of Ashless Coal>
The ashless coal can be obtained by various conventional production methods
and, for example, may be obtained by removing a solvent from a solvent extract
of a
coal. For example, the ashless coal can be obtained through the following
steps S1 to
S3 (see, FIG. 1), but the ashless coal production process (S1 to S3) described
below
may be appropriately changed, and various treatment steps may be added, if
desired.
[0024]
For example, in the production of an ashless coal, as long as each of the
above-
described steps is not adversely affected, other steps, e.g., a coal
pulverization step of
pulverizing the raw material coal, a removal step of removing an unwanted
material
such as refuse, or a drying step of drying the obtained ashless coal, may be
provided
between respective steps above or before or after each step.
[0025]
<Slurry Heating Step: Sl>
The slurry heating step (S1) is a treatment of mixing a coal and an aromatic
solvent to prepare a slurry and heat-treating the slurry to extract a coal
component in the
aromatic solvent.
The kind of the coal as a raw material (hereinafter, sometimes referred to as
"raw material coal") is not particularly limited. For example, various known
coals
such as bituminous coal, subbituminous coal, brown coal and lignite can be
used. In
view of profitability, it is more preferable to use a low-rank coal such as
subbituminous
coal, brown coal and lignite, instead of using an expensive high-grade coal
such as
bituminous coal.
6
CA 02928325 2016-04-21
7
[0026]
The aromatic solvent is not particularly limited as long as it is a solvent
having
a property of dissolving a coal. Examples of the aromatic solvent include a
monocyclic aromatic compound such as benzene, toluene and xylene, and a
bicyclic
aromatic compound such as naphthalene, methylnaphthalene, dimethylnaphthalene
and
trimethylnaphthalene. In addition, examples of the bicyclic aromatic compound
include aliphatic side chain-containing naphthalenes, biphenyl, and a long-
chain
aliphatic side chain-containing alkylbenzene. In the present invention, a
bicyclic
aromatic compound that is a non-hydrogen-donating solvent, is preferred.
[0027]
The non-hydrogen-donating solvent is a coal derivative that is a solvent
primarily purified from a carbonization product of a coal and mainly composed
of a
bicyclic aromatic compound. The reason why a non-hydrogen-donating solvent is
preferred is that the non-hydrogen-donating solvent is stable even in a heated
state and
excellent in the affinity for a coal and therefore, the ratio of a coal
component in the
solvent (hereinafter, sometimes referred to as "extraction percentage") is
high and in
addition, because the solvent can be easily recovered by distillation or other
methods
and furthermore, the solvent recovered can be cyclically used.
[0028]
If the boiling point of the aromatic solvent is too low, the pressure required
during heating extraction or in the later-described separation step (S2) would
be high,
and the loss due to volatilization in the step of recovering the aromatic
solvent is
increased, leading to a decrease in the recovery ratio of the aromatic
solvent.
Furthermore, a decrease in the extraction percentage during heating extraction
is caused.
On the other hand, if the boiling point of the aromatic solvent is too high,
separation of
the aromatic solvent from a liquid component or a solid component in the
separation
step (S2) is difficult, and the recovery ratio of the solvent lowers. The
boiling point of
the aromatic solvent is preferably from 180 to 330 C.
[0029]
The coal concentration relative to the aromatic solvent is not particularly
limited. Although it may vary depending on the kind of the raw material coal,
if the
coal concentration relative to the aromatic solvent is low, the ratio of the
coal
7
CA 02928325 2016-04-21
component extracted in the aromatic solvent to the amount of the aromatic
solvent
would be small, and this is not profitable. On the other hand, a higher coal
concentration is better, but if the coal concentration is excessively high,
the slurry
viscosity would be increased, and transfer of the slurry or separation between
a liquid
component and a solid component in the separation step (S2) is likely to
become
difficult. The coal concentration, on the dry coal basis, is preferably 10
mass% or
more, more preferably 20 mass% or more and preferably 50 mass% or less, more
preferably 35 mass% or less.
[0030]
If the heat treatment (heating extraction) temperature of the slurry is too
low,
the bonding between molecules constituting the coal cannot be sufficiently
weakened,
and in the case of using a low-rank coal as the raw material coal, the
resolidification
temperature of the ashless coal obtained in the later-described ashless coal
obtaining
step (S3) cannot be elevated. On the other hand, if the heat treatment
temperature is
too high, the pyrolytic reaction of the coal would be very active to cause
recombination
of pyrolytic radicals produced, leading to a decrease in the extraction rate.
The slurry
heating temperature is preferably 350 C or more, more preferably 380 C or
more, and
preferably 420 C or less.
[0031]
The heating time (extraction time) is not particularly limited, but if the
extraction time is long, the pyrolysis reaction proceeds excessively, allowing
for the
progress of a radical polymerization reaction, and the extraction ratio
lowers. For
example, at the above heating temperature, the heating time is preferably 120
minutes or
less, more preferably 60 minutes or less, still more preferably 30 minutes or
less, and
preferably 10 minutes or more.
[0032]
After the heating extraction, the extract is preferably cooled to 370 C or
less so
as to suppress a pyrolysis reaction. The lower limit of the temperature when
cooling is
preferably 300 C or more. If cooled to less than 300 C, the dissolving power
of the
aromatic solvent is reduced, and reprecipitation of the once extracted coal
component
occurs, leading to a decrease in the yield of ashless coal.
[0033]
8
CA 02928325 2016-04-21
The heating extraction is preferably performed in a non-oxidizing atmosphere.
Specifically, the heating extraction is preferably performed in the presence
of an inert
gas such as nitrogen. This is because contact with oxygen during heating
extraction is
risky due to a fear of ignition and when hydrogen is used, the cost rises.
[0034]
The pressure in the heating extraction may vary depending on the temperature
during heating extraction or the vapor pressure of the aromatic sOlvent to be
used, but if
the pressure is lower than the vapor pressure of the aromatic solvent, the
aromatic
solvent is vaporized and not confined in a liquid phase, and extraction cannot
be
achieved. On the other hand, if the pressure is too high, the equipment cost
and
operation cost are increased, and this is not profitable. The preferable
pressure is
generally from 1.0 to 2.0 MPa.
[0035]
<Separation Step: S2>
The separation step (S2) is a step of separating the slurry heat-treated in
the
slurry heating step (S1) into a liquid component and a solid component. The
liquid
component is a solution containing the coal component extracted in the
aromatic
solvent. The solid component is a slurry containing an ash insoluble in the
aromatic
solvent and an insoluble coal.
[0036]
The method for separating the slurry into a liquid component and a solid
component in the separation step (52) is not particularly limited, and a
conventional
separation method such as filtration method, centrifugal separation method and
gravity
settling method, may be employed. In the present invention, it is preferable
to use a
gravity settling method enabling continuous operation of a fluid and being low-
costly
and suitable for treatment of a large amount. In the case of employing a
gravity
settling method, a liquid component (hereinafter, sometimes referred to as
"supernatant
liquid") that is a solution containing a coal component extracted in the
aromatic solvent
can be obtained from the upper part of a gravity settling tank, and a solid
component
(hereinafter, sometimes referred to as "solid content concentrate") that is a
slurry
containing a solvent-insoluble ash and a coal can be obtained from the lower
part of the
gravity settling tank.
9
CA 02928325 2016-04-21
[0037]
Subsequently, as described below, the aromatic solvent is separated and
recovered from the supernatant liquid by using a distillation method, etc.,
and as a
result, ashless coal having a very low ash concentration can be obtained
(ashless coal
obtaining step (S3)).
[0038]
<Ashless Coal Obtaining step: S3>
The ashless coal obtaining step (S3) is a step of separating the aromatic
solvent
from the supernatant liquid to obtain an ashless coal having a very low ash
concentration.
[0039]
The method for separating the aromatic solvent from the supernatant liquid is
not particularly limited, and a general distillation method, evaporation
method (e.g.,
spray drying method), etc. can be used. The aromatic solvent recovered by
separation
can be repeatedly used. By the separation and recovery of the aromatic
solvent, the
ashless coal can be obtained from the supernatant liquid. The obtained
ashless coal
can be used as a raw material of the mixture in the present invention, and
also can be
used as a raw material of the oxidized ashless coal.
[0040]
(Other Steps>
If desired, a byproduct coal in which the ash is concentrated may be produced
by separating the aromatic solvent from the solid content concentrate
(byproduct coal
obtaining step). As the method for separating the aromatic solvent from the
solid
content concentrate, a general distillation or evaporation method can be used,
similarly
to the above-described ashless coal obtaining step (S3) of obtaining an
ashless coal from
a liquid component.
[0041]
<Production Process of Coke>
The production method of the coke in the present invention is described below
by referring to FIG. 2. In the production of the coke, as long as each step is
not
adversely affected, other steps, e.g., a pulverization step of pulverizing
various raw
materials, etc., a removal step of removing an unwanted material such as
refuse, or a
CA 02928325 2016-04-21
step of applying various treatments to the obtained coke, may be provided
between
respective steps or before or after each step.
[0042]
<Oxidation Step: Cl>
The oxidation step is a step of applying an oxidation treatment to the ashless
coal to obtain an oxidized ashless coal. By applying an oxidation treatment to
the
ashless coal, the ashless coal is modified, and the thermoplasticity or
expandability can
be improved.
[0043]
The method for oxidizing an ashless coal is not particularly limited. It is
desirable to perform oxidation in an oxidizing atmosphere such as oxygen,
ozone,
nitrogen dioxide and air, and air oxidation using oxygen in air as an oxidizer
is
preferred.
[0044]
The percentage of increase in oxygen of the oxidized ashless coal is not
particularly limited, but if the percentage of increase in oxygen is too low,
the
modification effect on the ashless coal is not sufficient, and a problem
attributable to
thermoplasticity or expandability is sometimes caused during the dry
distillation. On
the other hand, if the percentage of increase in oxygen is too high, the yield
is reduced,
and as a result, this is not profitable. Accordingly, the percentage of
increase in
oxygen is 2% or more, preferably 3% or more, and is preferably 10% or less,
more
preferably 5% or less.
[0045]
In the present invention, when the percentage of increase in oxygen of the
ashless coal is set, an ashless coal having a lower percentage of increase in
oxygen than
the set value is not dealt with as the oxidized ashless coal in the present
invention even
if the ashless coal has been subjected to an oxidation treatment. In addition,
in the
case where an ashless coal having a lower percentage of increase in oxygen
than the set
value is used as a carbon raw material, the ashless coal is dealt with as the
ashless coal
in the present invention,
[0046]
11
CA 02928325 2016-04-21
The percentage of increase in oxygen as used in the present invention is a
value
obtained by measuring the oxygen content percentage of an ashless coal before
and after
oxidation treatment according to JIS M8813 (Calculation Method of Oxygen
Percentage) and making calculation (oxygen content percentage of oxidized
ashless coal
- oxygen content percentage of ashless coal).
[0047]
The temperature kept during oxidation (hereinafter, oxidation temperature)
may be appropriately adjusted so that the desired percentage of increase in
oxygen can
be obtained. If the oxidation temperature is low, the ashless coal may be
insufficiently
oxidized, and the above-described modification effect may not be fully
exerted. In
addition, if the oxidation temperature is low, a long time is required to
achieve the
desired percentage of increase in oxygen, and the productivity is reduced. On
the other
hand, if the oxygen temperature is too high, the oxidation rate is excessively
increased,
and the oxidation degree of the ashless coal can be hardly controlled. The
oxidation
temperature is preferably 150 C or more, more preferably 200 C or more, and is
preferably less than the ignition point of the ashless coal, more preferably
350 C or less.
[0048]
The oxidation time (holding time at a predetermined temperature) may be
appropriately adjusted so that the desired percentage of increase in oxygen
can be
obtained. If the oxidation time is short, the ashless coal may be
insufficiently
oxidized. On the other hand, if the oxidation time is long, the ashless coal
may be
excessively oxidized, and the yield is reduced to cause an increase in the
cost. For
example, the oxidation time in the above-described temperature range is
preferably 0.5
hours or more, more preferably 1 hour or more, and is preferably 6 hours or
less, more
preferably 3 hours or less. After the oxidation, the ashless coal may be
allowed to cool
to room temperature.
[0049]
The particle diameter (equivalent-circle diameter; hereinafter, the same
applies
to the particle diameter) of the ashless coal subjected to an oxidation
treatment is not
particularly limited. If the particle diameter of the ashless coal is too
large, the inside
of the ashless coal may not be sufficiently oxidized, leaving a fear of
occurrence of
melting, etc. during the dry distillation. On the other hand, if the particle
diameter of
12
CA 02928325 2016-04-21
the ashless coal is too small, the handling property is deteriorated. The
average
particle diameter of the ashless coal is preferably 3 mm or less, more
preferably 1 mm
or less, and is preferably 0.2 mm or more, more preferably 0.3 mm or more.
From the
standpoint of accelerating the oxidation, the maximum particle diameter is
also
preferably 3 mm or less, more preferably 1 mm or less, still more preferably
0.5 mm or
less.
[0050]
<Carbon Raw Material Mixing Step: C2>
The carbon raw material mixing step is a step of mixing the ashless coal, the
oxidized ashless coal, and a raw petroleum coke, thereby obtaining a mixture
(hereinafter, referred to as "mixed carbon raw material").
[0051]
The raw petroleum coke is a solid substance by-produced, in the petroleum
refining process, together with light oil in equipment (coker) for producing
light oil by
heating a distillation residue at a high temperature (for example, at 500 C or
more) to
cause pyrolysis. In the present invention, as for the raw petroleum coke,
various
known raw petroleum cokes available on the market can be used. A raw petroleum
coke having a volatile content of 5 to 20 mass% and a sulfur content of 2 to 5
mass% is
preferred.
[0052]
In the present invention, the mixing ratio of the ashless coal in the mixed
carbon raw material and the mixing ratio between the ashless coal and the
oxidized
ashless coal must be appropriately controlled according to the properties of
the ashless
coal (oxidized/non-oxidized, oxidation degree) so as to produce a high-purity
coke.
[0053]
(I) Content of Ashless Coal: from 5 to 40 parts by mass
If the mixing ratio of the ashless coal is too small, the function as a binder
is
not sufficiently exerted, and the coke becomes powdery. On the other hand, if
the
mixing ratio of the ashless coal is too large, thermoplasticization or
expansion due to the
ashless coal becomes excessive and, for example, the coke may be obtained as a
sponge-like porous body and reduced in the bulk specific gravity, or the coke
may
adhere to the inner wall of a dry distillation apparatus, making its discharge
impossible.
13
CA 02928325 2016-04-21
[0054]
In the present invention, the content of the ashless coal is 5 parts by mass
or
more, preferably 10 parts by mass or more, and is 40 parts by mass or less,
preferably
25 parts by mass or less, per 100 parts by mass of the total of the ashless
coal, the
oxidized ashless coal, and the raw petroleum coke.
[0055]
(II) Total Content of Ashless Coal and Oxidized Ashless Coal: from 30 to 70
parts by
mass
As described above, the total content of the ashless coal is 40 parts by mass
or
less, but by containing the oxidized ashless coal, the amount of the raw
petroleum coke
used can be more decreased, and the impurity content in the coke can be more
reduced.
The ashless coal and oxidized ashless coal are more expensive than the raw
petroleum
coke and therefore, when the total content of those is increased, the unit
cost of the coke
rises. On the other hand, if the total content of the ashless coal and
oxidized ashless
coal is too low, the effect of reducing impurities is not sufficiently
obtained. For this
reason, the total content of the ashless coal and oxidized ashless coal is 30
parts by mass
or more, preferably 35 parts by mass or more, more preferably 40 parts by mass
or
more, and is 70 parts by mass or less, preferably 65 parts by mass or less,
more
preferably 60 parts by mass or less, per 100 parts by mass of the total of the
ashless
coal, the oxidized ashless coal and the raw petroleum coke.
[0056]
The content of the oxidized ashless coal is not particularly limited, but if
the
content of the oxidized ashless coal is too small, there is caused a problem
that, for
example, the coke expands to have a sponge-like structure or melts and sticks
in an
apparatus. Therefore, the content of the oxidized ashless coal is preferably 5
parts by
mass or more, more preferably 10 parts by mass or more, still more preferably
30 parts
by mass or more, per 100 parts by mass of the total of the ashless coal, the
oxidized
ashless coal and the raw petroleum coke. On the other hand, the upper limit of
the
content of the oxidized ashless coal may be appropriately adjusted to fall in
the above-
described range of the total content of the ashless coal and the oxidized
ashless coal
(from 30 to 70 parts by mass) but it is preferably 50 parts by mass or less,
more
preferably 40 parts by mass or less.
14
CA 02928325 2016-04-21
[0057]
The average particle diameter of the ashless coal is not particularly limited,
but
if the average particle diameter of the ashless coal is too large, a non-
uniformity may be
produced in the mixed state of the mixture, not allowing the ashless coal to
fully exert a
binder effect, etc. On the other hand, if the average particle diameter is too
small, the
handling property may be deteriorated. The average particle diameter of the
ashless
coal is preferably 10 mm or less, more preferably 0.5 mm or less, and is
preferably 0.1
mm or more, more preferably 0.2 mm or more. If the maximum particle diameter
of
the ashless coal is too large, a non-uniformity may be produced in the mixed
state in a
formed product, and for this reason, it is preferably 1.0 mm or less, more
preferably 0.5
mm or less.
[0058]
In addition, the average particle diameter of the ashless coal is preferably
smaller than the average particle diameter of the oxidized ashless coal,
because a gap
between carbon raw materials is filled and the binder effect is more enhanced.
[0059]
The mixture in the present invention may be sufficient if it contains the
ashless
coal, the oxidized ashless coal, and the raw petroleum coke, and the mixture
may
contain other material(s) (for example, a known additive such as a binder and
a
petroleum pitch) as long as the present invention is not adversely affected,
but in the
case of containing other material(s) in the mixture, the impurity content of
the coke may
be increased due to the other material(s). Therefore, the total of the ashless
coal, the
oxidized ashless coal and the raw petroleum coke in the mixture is preferably
90 mass%
or more, more preferably 100 mass%. The 100 mass% indicates that the mixture
consists of the ashless coal, the oxidized coal and the raw petroleum coke and
the
remainder is impurities.
[0060]
The method for mixing the ashless coal, the oxidized ashless coal and the raw
petroleum coke is not particularly limited, and a conventional method ensuring
uniform
mixing may be employed. Examples thereof include a mixer, a kneader, a single-
shaft
mixer, and a double-screw mixer.
[0061]
CA 02928325 2016-04-21
<Forming Step: C3>
The forming step is a step of forming, if desired, the mixture obtained in the
carbon raw material mixing step (C2) into a desired shape to obtain a formed
product.
By making a formed product from the mixture, binding between respective carbon
raw
materials can be more firmly created due to the binder effect of the ashless
coal, and
powdering of the coke or reduction in the bulk specific gravity can be
suppressed.
[0062]
For example, in the case of dry distillation of the mixture in a chamber
furnace,
a load applies in the vertical direction, reducing the distance between
respective carbon
raw materials, and respective carbon materials are bound by the binder effect
of the
ashless coal, so that the coke can be prevented from powdering and the bulk
specific
gravity can be increased. Such an effect can be more enhanced by making a
formed
product.
[0063]
On the other hand, in the case of dry distillation of the mixture by use of a
horizontal furnace in which a sufficient load does not apply in the vertical
direction,
such as rotary kiln, the binder effect is not fully exerted. As a result,
binding between
respective carbon raw materials is weak, and the coke is likely to be
powdered, leading
to a reduction in the bulk specific gravity of the coke. Therefore, the
mixture is
preferably formed into a desired shape before the dry distillation.
[0064]
The method for making a formed product from the mixture is not particularly
limited and examples thereof include, for example, a method using a double
roll (twin
roll)-type forming machine by means of flat rolls or a double roll-type
forming machine
having an almond-shaped pocket, a method using a single-shaft press-type
forming
machine or roller-type forming machine or an extrusion forming machine, and
press
forming by means of a mold, and any of these methods can be employed. Among
them, it is preferable to make a briquette-like formed product or sheet-like
formed
product by means of a double roll-type briquetter, roll compaction, etc.
[0065]
Forming of the mixture may be cold forming that is performed at around room
temperature, but hot forming performed under heating is preferred. When the
mixture
16
CA 02928325 2016-04-21
is formed under pressure at a high temperature, the ashless coal is
plastically deformed
to fill voids between the oxidized ashless coal particles and the raw
petroleum cokes, so
that a more highly densified formed product can be obtained. In turn, a coke
having a
higher bulk specific gravity can be obtained by dry distillation of the highly
densified
formed product. On the other hand, if the forming temperature is too high, the
ashless
coal may be softened and expanded, failing in achieving a high bulk specific
gravity.
The hot forming temperature (the temperature of a device such as mold or roll)
is
preferably 100 C or more, more preferably 200 C or more, and is preferably 450
C or
less, more preferably 300 C or less. The forming pressure is not particularly
limited,
and conventional conditions may be employed. For example, the forming pressure
is
approximately from 0.5 to 3 ton/cm2.
[0066]
<Dry Distillation Step: C4>
The dry distillation step is a step of performing dry distillation of the
mixture
obtained in the carbon raw material mixing step (C2) or the formed product
obtained in
the forming step (C3), thereby acquiring a coke. The shape of the furnace used
for dry
distillation is not particularly limited, and dry distillation may be
performed batchwise
by using a chamber furnace or dry distillation may be performed continuously
by using
a vertical shaft furnace. In addition, a horizontal rotary furnace such as
rotary kiln
may also be used.
[0067]
As for the dry distillation conditions, conventional conditions may also be
employed, and the dry distillation temperature may be appropriately set and is
not
particularly limited but may be preferably 650 C or more, more preferably 700
C or
more, and preferably 1,200 C or less, more preferably 1,050 C or less. The dry
distillation time at the dry distillation temperature is not particularly
limited as well, and
a desired dry distillation time may be set according to the apparatus
configuration, etc.
and may be preferably 5 minutes or more, more preferably 10 minutes or more,
and
preferably 24 hours or less, more preferably 12 hours or less.
[0068]
The dry distillation atmosphere may be a non-oxidizing gas atmosphere so as
to prevent deterioration of the coke due to oxidation. As the non-oxidizing
gas,
17
CA 02928325 2016-04-21
various known gases may be used, and the gas may be, for example, an inert gas
such as
nitrogen, helium and argon, or a reducing gas such as hydrogen gas.
[0069]
During dry distillation, not only the raw petroleum coke is converted to
calcine
coke (calcined coke) but also the ashless coal acts as a binder between the
oxidized
ashless coal and the calcine coke to firmly bond the oxidized ashless coal to
the calcine
coke, thereby enhancing the coke strength.
[0070]
In the case of subjecting the mixture to dry distillation, respective carbon
raw
materials are bound to each other, and amorphous agglomerate-shaped coke is
obtained.
In addition, when the mixture is formed, the coke having substantially the
same shape
as that of the formed product before dry distillation is obtained. In the coke
in the
present invention, the blending ratio of the ashless coal is appropriately
controlled, so
that the coke can be kept from adhering to the inside of the dry distillation
apparatus,
which makes its discharge impossible, and also from powdering.
[0071]
The thus-obtained coke has higher purity and higher bulk specific gravity than
those of conventionally known coke. Specifically, the content of minerals that
become
impurities is preferably I mass% or less, more preferably 0.5 mass% or less.
The bulk
specific gravity is preferably 0.53 g/cm3 or more, more preferably 0.6 g/cm3
or more,
still more preferably 0.7 g/cm3 or more, and most preferably 0.8 g/cm3 or
more. The
sulfur content is preferably 2 mass% or less.
[0072]
In addition, the mixture is free from the above-described problem attributable
to thermoplasticity or expandability during the dry distillation and in turn,
the coke
obtained is excellent in the appearance and can be discharged from the dry
distillation
apparatus.
[0073]
As described above, the coke produced by performing dry distillation of a
mixture containing the ashless coal, the oxidized ashless coal obtained by an
oxidation
treatment of an ashless coal, and the raw petroleum coke, in which, relative
to 100 parts
by mass of the total of the ashless coal, the oxidized ashless coal and the
raw petroleum
18
CA 02928325 2016-04-21
coke, the content of the ashless coal is from 5 to 40 parts by mass and the
total content
of the ashless coal and the oxidized ashless coal is from 30 to 70 parts by
mass, is a
coke having a high purity and a high bulk density and succeeded in improving
the
above-described thermoplasticity and expandability which may emerge as a
problem in
the case of using an ashless coal.
EXAMPLES
[0074]
The present invention is described more specifically below by referring to
Examples, but the present invention is, of course, not limited to the
following Examples
and may be carried out by appropriately making changes as long as they are in
conformity to the gist described hereinabove and hereinafter, all of which are
included
in the technical scope of the present invention.
[0075]
(Production of Ashless Coal)
(Slurry Heating Step: S1)
With 5 kg of the raw material coal (bituminous coal), an aromatic solvent (1-
methylnaphthalene (produced by Nippon Steel Chemical Co., Ltd.)) in an amount
(20
kg) four times that of the raw material coal was mixed to prepare a slurry.
This slurry
was pressurized with nitrogen of 1.2 MPa and subjected to a heat treatment
(heating
extraction) in an autoclave having an internal volume of 30 liter under the
conditions of
370 C and 1 hour.
[0076]
(Separation Step: S2)
The obtained slurry was separated into a supernatant liquid and a solid
content
concentrate in a gravity settling tank maintained at the same temperature and
pressure.
[0077]
(Ashless Coal Obtaining Step: S3)
The obtained supernatant liquid was further filtered (stainless mesh filter
with
an opening size of 1 pm) to obtain an ashless coal solution. The aromatic
solvent was
separated and recovered from the ashless coal solution by a distillation
method to
19
CA 02928325 2016-04-21
=
produce an ashless coal. The obtained ashless coal was pulverized so as to
pass
through a sieve having an opening size of 3 mm, whereby the ashless coal was
obtained.
[0078]
(Measurement of Sulfur Content)
This ashless coal was measured for the sulfur concentration by the method
specified in JIS M 8122. As a result, the sulfur content of the ashless coal
was 0.5
mass%.
[0079]
(Production of Coke)
[0080]
(Oxidation Step: C1)
A part of the ashless coal was pulverized so as to pass through a sieve having
an opening size of 0.5 mm. The pulverized ashless coal was heated in an air
atmosphere to a predetermined temperature shown in Table 1 and held at the
same
temperature for a predetermined time, thereby performing an oxidation
treatment (in
Table 1, "Oxidation Conditions"). After the oxidation treatment, the ashless
coal was
allowed to cool to room temperature, whereby an oxidized ashless coal was
obtained.
Here, the ashless coal and the oxidized ashless coal were measured for the
oxygen concentration before and after the oxidation treatment, according to
JIS M 8813,
and the percentage of increase in oxygen of the oxidized ashless coal was
calculated.
The results are shown in Table 1 (in Table 1, "Percentage of Increase in
Oxygen").
[0081]
(Raw Petroleum Coke)
Commercially available raw petroleum coke (volatile content: 9.5 mass%,
sulfur content: 3.1 mass%) was pulverized so as to pass through a sieve having
an
opening size of 10 mm.
[0082]
(Carbon Raw Material Mixing Step: C2)
Ashless coal ("A" in the Table), oxidized ashless coal ("B" in the Table), and
raw petroleum coke ("C" in the Table) were mixed in a predetermined ratio
shown in
Table 1 (in Table 1, "Blending Ratio of Raw Materials") to obtain a mixture.
[0083]
CA 02928325 2016-04-21
Here, in No. 16, the ashless coal subjected to the oxidation treatment was
dealt
with as an ashless coal, because the percentage of increase in oxygen was less
than 2%
(1.50%). Accordingly, although the blending ratio of No. 16 was A:B:C=50 (20
mass% of ashless coal not subjected to an oxidation treatment + 30 mass% of
ashless
coal having a percentage of increase in oxygen of 1.5%):0:50, in order to show
details
of the blending ratio of No. 16, for convenience sake, the blending ratio
("20") of the
ashless coal not subjected to an oxidation treatment is shown in column A of
the Table,
and the blending ratio ("30") of ashless coal having a percentage of increase
in oxygen
of 1.5% despite having been subjected to an oxidation treatment is shown in
column B.
[0084]
(Forming Step: C3)
With respect to a part of the mixtures (Nos. 7 to 12 and 15; in the Table,
"Presence or Absence of Forming"=done), a formed product was produced under
the
following conditions:
Forming method: roll compaction method
Roll temperature: 100 C
Roll diameter: 162 mm
Roll width: 60 mm (pyramid-shaped groove)
Inter-roll width: 2 mm
Roll rotational speed: 15 rpm
Linear pressure: 3 ton/cm
[0085]
(Dry Distillation Step: C4)
The mixture (Nos. 1 to 6, 13, 14 and 16 to 25) and the formed product (Nos. 7
to 12 and 15) were subjected to a dry distillation treatment in a chamber
furnace (Nos. 1
to 6 and 15 to 25) or in a kiln (Nos. 7 to 14).
[0086]
(Dry Distillation Treatment in Chamber Furnace)
The mixture (Nos. 1 to 6 and 16 to 25) or the formed product (No. 15) was
added into a graphite crucible having an inner volume of 1,000 mL to provide a
bulk
specific gravity of 0.85 g/cm3, followed by heating to 1,000 C at a rate of 3
C/min in a
21
CA 02928325 2016-04-21
=
nitrogen atmosphere, and held at the same temperature for 5 hours to perform
dry
distillation, thereby producing a coke.
[0087]
(Dry Distillation Treatment in Rotary Kiln)
The mixture (Nos. 13 and 14) or the formed product (Nos. 7 to 12) was
inserted into a heated rotary kiln (diameter: 200 mm, total length: 4,000 mm)
at an
insertion rate of 1 kg/l. As for the heating temperature of the rotary kiln,
the
temperature was adjusted to an inlet temperature of 400 C and an outlet
temperature of
1,000 C. It was held at the temperature above for 60 minutes in a nitrogen
atmosphere
to perform dry distillation, thereby producing a coke.
[0088]
(Evaluation Method)
The obtained coke was measured for bulk specific gravity, sulfur content,
appearance, and presence or absence of adhering to the inside of the
apparatus.
[0089]
(Bulk Specific Gravity) (in the Table, "Bulk Specific Gravity After Dry
Distillation
(g/cm3)")
A wooden cubic container whose one side is 100 mm was filled with the coke,
and the bulk specific gravity was determined from the dry mass (W: g) of the
coke
which had been filled with the container. In this Example, the coke was judged
to be
passed when the bulk specific gravity was 0.53 g/cm3 or more.
[0090]
(Sulfur Content) (in the Table, "Sulfur Content After Dry Distillation (%)")
The sulfur concentration of the coke was measured in the same manner as for
the ashless coal. In this Example, the coke was judged to be passed when the
sulfur
content was 2.0% or less.
[0091]
(Appearance, Presence or Absence of Adhering to Inside of Apparatus) (in the
Table,
"Coke Characteristics")
The appearance of the coke was observed with an eye and evaluated. In the
case of performing dry distillation treatment in a chamber furnace (Nos. 1 to
6 and 15 to
25): the coke that was agglomerate-shaped was judged as "Excellent" (in the
Table,
22
CA 02928325 2016-04-21
A 1
"PE"); the coke that was agglomerate-shaped but slightly expanded (the bulk
specific
gravity: 0.53 g/cm3 or more and less than 0.7 g/cm3) was judged as "Pass" (in
the Table,
"P"); the coke that was powdery was judged as "Fail" (in the Table, "F"); and
the coke
that adhered and could not be discharged (in the Table, "FA") or that expanded
(in the
Table, ''FB"), was also judged as "Fail". The appearance is ranked in the
order of
PE>P>(F, FA, 113).
[0092]
In the case of performing dry distillation treatment in a kiln (Nos. 7 to 14):
the
coke that was flaky and free from occurrence of expansion, cracking, chipping
or
powdering was judged as "Pass" (in the Table, "P"); the coke that was powdery
or
experienced expansion, cracking, chipping or powdering was judged as "Fail"
(in the
Table, "F"); and the coke that adhered and could not be discharged was also
judged as
"Fail" (in the Table, "FA"). The appearance is ranked in the order of P>(F,
FA).
[0093]
23
[Table 1]
_
Percentage of Blending Ratio of Presence or Bulk
Specific Sulfur Content
Oxidation Dry Distillation
Coke
No. Increase in Raw Materials, A+B Absence of Gravity
After Dry After Dry
ConditionsMethod
Characteristics
Oxygen A/B/C (mass%) Forming
Distillation (g/cm) Distillation (%) ,
' 1 200 C, lh 3% 4/48/48 52 none chamber furnace
0.45 1.6 F
2 200 C, lh 3% 5/47/48 52 none chamber furnace
0.75 1.7 PE
3 200 C. lh 3% 10/40/50 50 none chamber furnace
0.72 1.7 PE
4 200 C, lh 3% 20/30/50 50 none chamber furnace
0.79 1.7 PE
200 C, lh 3% 40/10/50 50 none chamber furnace , 0.63
1.7 P
6 200 C, lh 3% 42/10/48 52 none chamber furnace
0.49 1.5 FB
7 200 C, lh 3% 4/48/48 52 done kiln
0.39 1.6 F
8 200 C, lh 3% 5/47/48 52 done kiln
0.58 1.6 P R
2
9 200 C, lh 3% 10/40/50 50 , done kiln
0.74 1.7 P .
200 C, lh 3% 20/30/50 50 done kiln 0.81
1.6 P
11 200 C, lh 3% 40/10/50 50 done kiln
0.53 1.6 P
.
.
12 200 C, lh 3% 42/10/48 52 done kiln -
1.5 FA .
.r.
,
13 200 C, lh 3% 10/40/50 50 none kiln
0.40 1.7 F r,
,
14 200 C, lh 3% 20/30/50 50 none kiln -
1.6 FA
200 C, lh 3% 20/30/50 50 done chamber furnace 0.81
1.6 PE
16 200 C, 0.3h 1.50% 20/30*/50 50 none chamber furnace
0.52 1.7 FB
17 200 C, 0.5h 2% 20/30/50 50 none chamber furnace
0.67 1.7 P
18 300 C, 0.5h 6% 20/30/50 50 none chamber furnace
0.77 1.6 PE
19 300 C, lh 10% 20/30/50 50 none chamber furnace
0.72 1.5 PE
200 C, lh 3% 20/20/60 40 none chamber furnace 0.79
1.8 PE
21 200 C, lh 3% 20/10/70 30 none chamber furnace
0.82 1.9 PE
22 200 C, lh 3% 20/5/75 25 none chamber furnace_
0.81 2.1 PE
23 200 C, lh 3% 20/40/40 60 none chamber furnace
0.89 1.3 PE
24 200 C, lh 3% 20/50/30 70 none chamber furnace
0.92 1.1 PE
200 C, lh 3% 20/60/20 80 none chamber furnace 0.91
0.8 PE
24
CA 02928325 2016-04-21
[0094]
As shown in Table 1, in Nos. 2 to 5, 8 to 11, 15, 17 to 21, 23 and 24
satisfying
the predetermined requirements of the present invention, the coke was of high
purity
with a sulfur content of 2.0% or less, and the bulk specific gravity thereof
was also high.
In addition, expansion, etc. during the dry distillation treatment were
sufficiently
suppressed, and the coke characteristics were good. Here, in No. 5 where the
blending
ratio of the ashless coal was high, the coke slightly expanded. In No. 17
where the
percentage of increase in oxygen was lower than other cases, modification of
the
oxidized ashless coal was inferior to other cases, and the coke slightly
expanded.
[0095]
No. 1 is the case where the blending ratio of the ashless coal was low. In
this
case, since the content of the ashless coal functioning as a binder was small,
the coke
was powdered by the dry distillation treatment.
[0096]
No. 6 is the case where the blending ratio of the ashless coal was high. In
this
case, since the content of the ashless coal was large, expansion occurred
during the dry
distillation treatment to not only yield sponge-like (porous) coke but also
greatly reduce
the bulk specific gravity.
[0097]
No. 7 is the case where the blending ratio of the ashless coal was low. In
this
case, since the content of the ashless coal was small, powdering occurred in
the kiln
during the dry distillation treatment.
[0098]
No. 12 is the case where the blending ratio of the ashless coal was high. In
this case, not only the ashless coal was melted during the dry distillation
but also the
formed product was foamed and expanded, and as a result, the coke adhered to
the inner
wall of the kiln and could not be discharged.
[0099]
No. 13 is the case where the mixture was not subjected to forming and the
powder was directly subjected to dry distillation in the kiln. In this case,
since an
adequate pressure was not applied to the mixture during the dry distillation,
the oxidized
CA 02928325 2016-04-21
ashless coal and the raw petroleum coke could not be sufficiently bound and
the coke
remained in a powder form.
[0100]
No. 14 is the case where the mixture was not subjected to forming and the
powder was directly subjected to dry distillation in the kiln. In this case,
the oxidized
ashless coal and the raw petroleum coke could not be sufficiently bound,
similarly to the
case of No. 13, and since the ashless coal content was increased and larger
than the case
of No. 13, the coke adhered to the inner wall of the kiln due to melted and
expanded
ashless coal and could not be discharged.
[0101]
No. 16 is the case where the oxidation time was short relative to the
oxidation
temperature and in turn, the percentage of increase in oxygen was low. In this
case,
since the content of the ashless coal (the total of the ashless coal and the
ashless coal
which had been subjected to an oxidation treatment but having a percentage of
increase
in oxygen of less than 2.0%) was too large without containing the oxidized
ashless coal
in which the percentage of increase in oxygen of the ashless coal is 2.0% or
more, the
ashless coal was foamed and expanded during the dry distillation treatment,
and the
bulk specific gravity was reduced.
[0102]
No. 22 is the case where the blending ratio of the raw petroleum coke was
large. In this case, the sulfur content after dry distillation was large, and
the purity of
the coke was low.
[0103]
No. 25 (Reference Example) is the case where the blending ratio of the raw
petroleum coke was small. In this case, the coke having a small sulfur content
and a
high bulk specific gravity was obtained, but since the blending ratio of the
raw
petroleum coke was small, the coke was expensive.
[0104]
While the invention has been described in detail and with reference to
specific
embodiments thereof, it will be apparent to one skilled in the art that
various changes
and modifications can be made therein without departing from the spirit and
scope of
the invention.
26
INDUSTRIAL APPLICABILITY
[0105]
In the present invention, the coke suitable, e.g., as a reducing material for
non-
ferrous metallurgy can be produced at a low cost.
27
CA 2928325 2017-08-25