Note: Descriptions are shown in the official language in which they were submitted.
CA 02928419 2016-04-29
COMPOSITION FOR REMOVING NATURALLY OCCURRING RADIOACTIVE
MATERIAL (NORM) SCALE
BACKGROUND OF THE INVENTION
In recent years, oil and gas producers have employed new methods to reach
otherwise
inaccessible oil and gas formations and to enhance stimulation. These new
methods,
some of which include hydraulic fracturing, can result in the production of
increased
amounts of radioactive wastes. Geologic formations that contain oil and gas
deposits
also contain naturally-occurring radionuclides, which are referred to as
Naturally
Occurring Radioactive Materials (NORM).
NORM contamination is typically
encountered as a complex mixture of inorganic scales plated on the equipment
surface.
Because the extraction process concentrates the naturally occurring
radionuclides and
exposes them to the surface environment and human contact, these wastes are
classified as Technologically Enhanced Naturally Occurring Radioactive
Material
(TENORM). TENORM is a significant problem in the petroleum industry.
Highly mineralized formation waters contain highly radiotoxic Radium isotopes
from
Uranium decay and from Thorium decay. Primary concerns in oil production are
radium-
226 and radium-228 nuclides. They decay into various radioactive progeny,
before
becoming stable lead. Radium-226 belongs to the Uranium-238 decay series and
Radium-228 to the Thorium-232 decay series. These radium isotopes appear in
the
water produced with oil and gas production. These toxic isotopes, amongst
others,
deposit on surface equipment such as downhole tubulars, surface vessels,
pumps,
valves, separators and others, as scale and sludge.
Sulfate scales are formed when formation water is mixed with injected sea
water. Many
subterranean waters contain alkaline earth metal cations, such as barium,
strontium,
calcium and magnesium. Sea water has high concentration of S042- and formation
waters, with high concentrations of Ca2+, Ba2+ and Sr2+.The injection of
seawater into
oilfield reservoirs is necessary to maintain reservoir pressure and improve
secondary
recovery. When two incompatible waters are mixed such as seawater and
formation
E2841827 DOCX;1 82801629.DOCX;1
CA 02928419 2016-04-29
water and interact chemically, a precipitate (scale) is formed. Two waters are
called
incompatible if they interact chemically and precipitate minerals when mixed.
Mixing of
these waters, therefore, could cause precipitation of CaSO4, BaSO4 and SrSO4.
When
the concentrations of the barium and sulfate ions exceed the solubility
product of barium
sulfate, a solid phase of barium sulfate will form as a precipitate. Radium is
chemically
similar to barium (Ba), strontium (Sr) and calcium (Ca), hence radium co-
precipitates
with Sr, Ba or Ca scale forming radium sulfate, radium carbonate.
The most common NORM containing scales are barium sulfate BaSO4, since
radionuclide do not precipitate directly, but are incorporated in to the
crystal lattice in the
barium sulfate scale causing the scale to be radioactive. Strontium sulfate co-
precipitates radium in a similar way to barium sulfate but less completely. Of
all the
alkaline earth sulfates, radium sulfate is the least soluble. The
concentration of radium
in the brine is not high, but once precipitated in scale deposits, radiation
level can be
higher than regulated limits.
As these reaction products precipitate on the surfaces of the water-carrying
or water-
containing system, they form adherent deposits or scale. Scale may prevent
effective
heat transfer, interfere with fluid flow, facilitate corrosive processes, or
harbor bacteria.
Scale is an expensive problem in many industrial water systems, in production
systems
for oil and gas, in pulp and paper mill systems, and in other systems, causing
delays
and shutdowns for cleaning and removal.
It is generally acknowledged that barium sulfate scale is extremely difficult
to remove
chemically, especially within reasonably short periods of time: the solvents
which have
been found to work generally take a long time to reach an equilibrium
concentration of
dissolved barium sulfate, which itself is usually of a relatively low order.
Consequently,
barium sulfate must be removed mechanically or the equipment, e.g. pipes,
etc.,
containing the deposit must be discarded.
The scale may occur in many different places, including production tubing,
well bore
perforations, the area near the well bore, gathering lines, meters, valves and
in other
production equipment. Barium sulfate scale may also form within subterranean
82841827.DOCX;1 F2801629.DOCX1
2
CA 02928419 2016-04-29
formations such as in disposal wells. Scales and deposits can be formed to
such an
extent that the permeability of the formation is impaired resulting in lower
flow rates,
higher pump pressures, and ultimately abandonment of the well.
Various approaches are used to fight equipment scaling from prediction (using
software
simulations), prevention of scale crystal growth, mechanical removal and
chemicals
solution (to dissolve scale deposits) the first two methods are called
proactive and the
latter-reactive approaches.
Scale inhibitors are designed to block the scale crystal growth or by
chelating or
keeping reactants in soluble form. These treatments are sensitive to the
changes in
production systems resulting in failing efficiency. In addition, chelants have
high cost
constraint. Most scale inhibitors are phosphate compounds: inorganic
polyphosphates,
organic phosphate esters, organic phosphonates, organic amino-phosphates, and
organic polymers. They are retained in the formation either by adsorbing to
the pore
walls or precipitating in the pore spaces, and have lifetime ranging from 3
months to 2
years.
Explosives have been used to rattle pipes and break off brittle deposits, but
can cause
excessive damage in the system.
Water jetting is effective on soft scale while, but it's less effective on
hard scale such as
barite. Adding small concentration of solid such as sand or glass beads can
promote
scale removal, but can damage the tubular walls.
Typical equipment decontamination processes have included both chemical and
mechanical efforts, such as milling, high pressure water jetting, sand
blasting, cryogenic
immersion, and chemical chelants and solvents. Water jetting using pressures
in excess
of 140 MPa (with and without abrasives) has been the predominant technique
used for
NORM removal. However, use of high pressure water jetting generally requires
that
each pipe or piece of equipment be treated individually with significant
levels of manual
intervention, which is both time consuming and expensive, but sometimes also
fails to
thoroughly treat the contaminated area.
E2841 827.DOCX; 1 E280 1 629 DOCX, 1
3
CA 02928419 2016-04-29
Primarily one class of chemicals is consistently used for dissolving hard
barium scale,
and is diethylenetriamine pentaacetic acid (DTPA). While chemical chelants,
such as
EDTA (ethylenediaminetetraacetic acid) or DTPA, have long been used to remove
scale
from oil field equipment, once EDTA becomes saturated with scale metal
cations, the
spent solvent is generally disposed of, such as by re-injection into the
subsurface
formation. However, because the process requires that disposal of the solvents
once
saturated, the large amounts of a fairly expensive solvent necessary for
decontamination renders the process economically prohibitive.
US patents 4,215,000 and 4,190,462 and 6,924,253 reveal new barite scale
dissolvers.US Patent no. 5,824,159 aims to treat NORM scale (once removed from
the
equipment and stored) by separating/extracting alkaline earth metal scales,
particularly
barium sulfate and strontium sulfate (with entrained NORM) into aqueous
solution. The
process is done at temperature range: 150-200 F. US Patent No. 7,470,330 B2
exposes
the scale to the chelating agent (EDTA), to cause the scale to dissolve by
complexing
with the alkaline earth metal of the scale salt. Once the chelating agent
becomes
saturated with the metal cations from the scale, the solution is acidified
increasing the
availability of anions with which the sequestered cations may react and
allowing the
cations to be released from the chelated complex to form an insoluble salt
that will
precipitate out of solution.
SUMMARY
A composition is provided for the treatment of scaling and deposits due to
naturally
occurring radioactive material, said composition forming one or more
attractants that
preferentially attract radioactive elements over other alkaline earth cations.
A
composition is further provided wherein said attractants take the form of
polydentate
ligands with one or more central metal groups that act as carriers for
radioactive
elements. The provide composition comprises, one or more metallic salts, one
or more
chelating agents that act synergistically to aid in polydentate ligand
formation and one
E2841827 DOCX.1 E2801629 DOCX;1
4
or more carboxylic acids that enhance stabilization of coordination
complexation in the
formation of polydentate ligands
It is to be understood that other aspects of the present invention will become
readily
apparent to those skilled in the art from the following detailed description,
wherein various
embodiments of the invention are shown and described by way of illustration.
As will be realized, the invention is capable for other and different
embodiments and its
several details are capable of modification in various other respects, all
without departing
from the spirit and scope of the present invention.
BRIEF DESCRIPTION OF THE DRAWINGS
A further, detailed, description of the disclosure will follow by reference to
the following
drawings. The drawings depict only typical embodiments of the disclosure and
are therefore
not to be considered limiting of its scope. In the drawings:
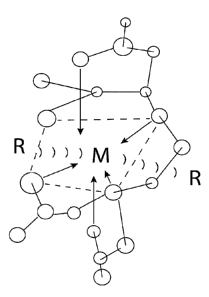
Figure 1 is a diagram of one embodiment of a composition of the present
disclosure.
The drawings are not necessarily to scale and in some instances proportions
may have
been exaggerated in order to more clearly depict certain features.
DETAILED DESCRIPTION OF VARIOUS EMBODIMENTS
The description that follows and the embodiments described therein are
provided by way of
illustration of an example, or examples, of particular embodiments of the
principles of
various aspects of the present invention. These examples are provided for the
purposes of
explanation, and not of limitation, of those principles and of the invention
in its various
aspects.
The present invention relates to chemical products for removal of deposits and
scaling from
Naturally Occurring Radioactive Material (NORM) on oil and gas equipment such
as
downhole tubulars, surface vessels, pumps, valves, separators and others, as
scale and
sludge.
The complexation of radium, and other radioactive compounds, in the presence
of other
alkaline earth cations (Na+ , K+, Mg2+ , Ca2+ , Sr2+ , and Ba2+ ) that are in
large excess is
problematic, since Raz+ has the lowest tendency to form complexes. Since Ra2+
cations are
divalent, the charge must be compensated to form neutral complexes.
{16404066-1} 5
Date Recue/Date Received 2022-08-31
The present invention provides a decontamination composition for the removal
of NORM
from surface contaminated equipment. In the present invention, the composition
is
successfully complexed into polydentate ligands that serve to attract
radioactive elements
and capture them.
It has been discovered that mixtures of chelating or sequestering agents such
as polyamino
carboxylic acids, and salts thereof, act to enhance coordination complexation.
Additionally,
it has been found that smaller molecular size carboxylic acids that are non-
nitrogenous, and
salts thereof, act synergistically in the stabilization of the formation of
polydentate ligands
with metals that are otherwise difficult to complex.
These chelating or sequestering agents show a synergistic effect in the
complexation of
radioactive elements into polydentate ligands and in the smaller molecular
size carboxylic
acids then enhance stabilization of this coordination corn plexation.
In the present invention, the choice of composition elements leads to a
composition
designed for the preferential attraction of Ra2+ and other elements such as
alkaline earth
cations Na+ , K+, Mg2+ , Ca2+ , Sr2+ , and Ba2+ , of which a significant
excess exists. Of all the
alkaline earth elements, Raz+ is the most challenging, because it has lowest
affinity for
complex formation.
With reference to Figure 1, this invention relates to compositions that aid in
the formation of
polydentate ligands, illustrated by the structure in general, with various
metals, represented
by M, forming the central metal that act as attractant for radioactive
elements, represented
by R, and specifically radium. In coordination chemistry, a ligand is an ion
or molecule (that
is, a functional group) that binds to a central metal atom to form a
coordination complex.
Ligands with more than one bonded atom are called polydentate or multidentate.
Polydentate ligands range in the number of atoms used to bond to a central
metal atom or
ion. EDTA, a hexadentate ligand, is an example of a polydentate ligand that
has six donor
atoms with electron pairs that can be used to bond to a central metal atom or
ion.
Preferably, in the present invention, the metal can be chosen from elements of
Group IIA
alkaline metal earths and Groups VB, VIB, VIIB and VIIIB transition metals
that may be
functional to attract radium when complexed as a polydentate ligand.
{16404066-1} 6
Date Recue/Date Received 2022-08-31
CA 02928419 2016-04-29
Unlike many prior art methods of radioactive element removal, in which metals
take th
form of solids in packed beds, the present metals are provided in metallic
salt form, as a
liquid or solution, that readily react with the chelating or sequestering
agents to create
polydentate ligands, which can then act as attractants for capture of
radioactive
elements.
These metals are low on the electromotive series of the tendency of a chemical
species
to gain or lose electrons. They therefore have similar tendencies as do the
radioactive
isotopes with respect to how easily they are oxidized. They are similarly
difficult to
complex and tend to destabilize and separate, leading to the metals would
falling out of
solution and re-depositing on the surface of equipment.
Hence the need for stabilization. Chelating or sequestering agents have a
tendency to
form ligands with metals that have higher Log K values, where K is the
stability constant
or formation constant of the metal, and with metals that are higher on the
electromotive
series. However, the inventors have found that by addition of a small
molecular weight
carboxylic acid, the coordination complexation and formation of the ligand
with the
present set of metals, that have an affinity to radioactive elements, is
stabilized. In
formation of the ligand, reactive sites of the chelating agent are now
occupied, and the
chelating agent is prevented from forming a ligand with other metals that have
higher
Log K values.
The exact mechanism of attraction between complexed metal and radium is
unknown,
however, it is believed that the similarity of the electron configurations of
the outermost
atomic shells may provide insight. While radium has the least tendency of all
the
alkaline metal earth metals to form complexes, radium is attracted to other
electronically
configured metals that are.
The composition of the present invention comprises from about 10 to about 50
percent
by weight of a mixture of a chelating agent which is preferably a polyamino
carboxylic
acid. The polyamino carboxylic acids more preferably include but are not
limited to
ethylenetriaminetetraacetic acid (EDTA), diethylenetriaminepentaacetic acid
(DTPA),
E284 I 827.DOCX; I E2801629 DOCX,1
7
CA 02928419 2016-04-29
polyaspartic acid (PAA), iminodisuccinic acid (IDSA), methylglycinediacetic
acid (MGA)
and alkali metal salts and ammonium salts thereof.
The present invention further comprises from about 1 to about 20 percent by
weight of a
small molecule carboxylic acid selected from the group consisting of alkali
metal salts
and ammonium salts of carboxylic acids selected from ascorbic acid, citric
acid,
glutamic acid, thioctic acid and alpha-lipoic acid.
A neutralizing compound may optionally be used to form the salts of the
polyamino
carboxylic acids and carboxylic acids. While any number neutralizing
compounds, such
as alkali metal hydroxides, may be used to form the salts of the polyamino
carboxylic
acids and carboxylic acids, potassium salt is most preferred.
The present invention further comprises 1 to 10 percent of a metallic salt in
liquid form
that is preferably selected from the group consisting of Group HA and Groups
VB, VIB,
VIIB and VIIIB and salts thereof. More preferably, metals which are good
carriers for
radium are barium, molybdenum, vanadium, manganese and iron.
The present compositions have been seen to be successful in the removal of
BaSO4,
RaSO4, and/or SrSO4 deposits and scale, and more particularly barium sulfate
scale
containing radium sulfate Ba(Ra)SO4.
The present chemical compositions are preferably water (aqueous) based. In
application, a NORM contaminated surface is contacted with the described
composition.
The treatment liquid is applied by any convenient circulation mechanism at a
temperature range of 100 F to 200 F. Preferably the pH of the circulating
liquid of is in
the range 9 to 13. An alkali environment can be created preferably by the
addition of
potassium hydroxide, more preferably a 30 to 50% by weight solution of
potassium
hydroxide. The present chemical compositions are preferably clear liquids that
are water
soluble, non-combustible, biodegradable and non-hazardous by common North
American safety and environment standards.
The additional dilution and rate of application of the present chemical
compositions will
vary with the severity and nature of the scale deposits.
E2841827DOCX,1 E280 I 629 DOCX;1
8
This invention may, however, be embodied in many different forms and should
not be
construed as limited to the embodiment set forth above. It will be recognized
by those
skilled in the art that other suitable polyamino carboxylic acids and
chelating agents may be
interchanged for those materials herein disclosed. In the preferred
embodiment, the
polydentate ligand is formed using manganese (II) sulfate as the metallic
salt. However,
other suitable metal ligands can be created from the present compositions and
would be
well understood by a person of skill in the art as also being functional as
radium and other
radioactive element attractants.
The previous description of the disclosed embodiments is provided to enable
any person
.. skilled in the art to make or use the present invention. Various
modifications to those
embodiments will be readily apparent to those skilled in the art, and the
generic principles
defined herein may be applied to other embodiments without departing from the
spirit or
scope of the invention. Thus, the present invention is not intended to be
limited to the
embodiments shown herein, but is to be accorded the full scope consistent with
the claims,
.. wherein reference to an element in the singular, such as by use of the
article "a" or "an" is
not intended to mean "one and only one" unless specifically so stated, but
rather "one or
more". All structural and functional equivalents to the elements of the
various embodiments
described throughout the disclosure that are known or later come to be known
to those of
ordinary skill in the art are intended to be encompassed by the elements of
the claims.
.. Moreover, nothing disclosed herein is intended to be dedicated to the
public regardless of
whether such disclosure is explicitly recited in the claims.
{16404066-1} 9
Date Recue/Date Received 2022-08-31