Note: Descriptions are shown in the official language in which they were submitted.
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
1
RNA TRANSCRIPTION VECTOR AND USES THEREOF
FIELD OF THE INVENTION
.. The present invention in general relates to an improved RNA transcription
vector, which is very
suitable for the production of mRNA for in vivo therapeutic purposes. The
improvements in the
vector in particular reside in the presence of a translation enhancer and a
nuclear retention
element.
.. BACKGROUND TO THE INVENTION
Although our immune system is capable of discriminating healthy cells from
tumor cells and
infectious agents, it sometimes fails in appropriately recognising and
reacting to the problem.
Therefore, medical science has focused on the development of several
strategies that aid the
immune system in the surveillance and elimination of tumor cells and
infectious agents.
Dendritic cells (DCs) are antigen-presenting cells (APCs) which are known as
key players in
the instigation of immune responses and much effort has been put in the
exploitation of DCs in
immunotherapy. In the case of cancer for example, the aim is the induction and
perpetuation of
a tumor specific immune response by eliciting effector T cells that can
specifically decrease
tumor load and induce immunological memory to control tumor relapse. Once
targetable tumor
associated antigens (TAA) have been identified, they can be used to load the
professional
APCs, i.e. the dendritic cells, either in vivo or ex vivo.
Different antigen formats have been assessed with regards to DC for in vivo or
ex vivo
immunotherapy such as peptides, proteins, whole tumor cell extracts, plasmid
DNA or mRNA.
Among these approaches, antigen-encoding mRNA is emerging as particularly
promising. The
advantage over the classical vaccination with peptides is that mRNA encodes
the genetic
information for the whole antigen. The full-length antigen is processed and
all available
epitopes are presented in the MHC molecules of the patient, without the need
to determine
HLA specific peptides. No patients need to be excluded from the treatment
because the
available peptides do not match their HLA type. In addition, mRNA does not
pose the risk of
genomic integration giving it a favourable safety profile compared to DNA or
viral vectors. Due
to its transient nature, mRNA is only expressed during a short period of time
and is eventually
degraded into natural products. Furthermore, mRNA acts as its own adjuvant,
prompting co-
.. stimulatory signals, which is advantageous in the context of mRNA-based
immunotherapy.
Two routes for exogenous mRNA delivery into DCs have been applied: either ex
vivo with
subsequent adoptive transfer of transfected DCs or by direct administration of
mRNA and
uptake in vivo.
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
2
A study performed by Diken et al. (2011) highlights that the maturation
stimulus and/or timing
of its delivery have to be selected carefully as the uptake of mRNA is
dependent on
macropinocytosis, a function of immature DCs that is lost upon DC maturation.
Consequently,
co-delivery of classical maturation stimuli, such as lipopolysaccharide (LPS),
with TAA mRNA
has a negative impact on the bioavailability of the antigen, a parameter that
co-determines the
induction of antigen-specific T cell responses (Van Lint 2012; Diken 2011). To
date two
different strategies have been explored to simultaneously load the DCs with
TAA mRNA and
activate them in vivo.
Fotin-Mleczek et at. (2011) described a two-component system containing free-
and protamin-
complexed mRNA, providing an antigen source for adaptive immunity together
with enhanced
triggering of the pathogen recognition receptor, TLR7. This immunization
strategy resulted in
the induction of a strong anti-tumor immune response and in sustained memory
responses,
which is important, as memory T cells should avoid tumor re-appearance.
Bonehill et al., 2008 evaluated the use of specific combinations of mRNA for
adjuvant
purposes, initially for the activation of ex vivo generated DCs but equally
applicable for direct
administration and uptake in vivo (Bonehill, 2008). This has lead to a patent
application
(W02009034172) in which the inventors describe that the T cell stimulatory
capacity of
antigenic-peptide pulsed antigen presenting cells or antigen presenting cells
(co-)
electroporated with an mRNA encoding a TAA can be greatly enhanced by
providing them with
different molecular adjuvants through electroporation with a mixture of mRNA
or DNA
molecules encoding two or more immunostimulatory factors. Proof of concept is
provided that
such modified antigen presenting cells pulsed with a target-specific peptide
or co-
electroporated with mRNA encoding a target-specific antigen can stimulate
antigen-specific T
cells both in vitro and after vaccination and thus form a promising new
approach for anti-tumor,
anti-viral, anti-bacterial or anti-fungal immunotherapy. A preferred
combination of
immunostimulatory factors used in the invention is CD4OL and caTLR4, or CD4OL
and CD70.
In other preferred embodiments, the combination of CD4OL, CD70 and caTLR4
immunostimulatory molecules is used, which is called "TriMix" hereinafter.
The present invention relates to an RNA transcription vector containing a 5'
translation
enhancer sequence and a 3' nuclear retention sequence. The vector according to
the present
invention, shows an unexpected improvement in expression of the proteins
encoded by the in
vitro transcribed mRNA in comparison with an empty pUC vector, or with vectors
that contain
either a translation enhancer or a nuclear retention sequence. These
improvements are in
particular due to the simultaneous presence of the two components: a
translation enhancer
and a RNA stabilizing sequence in the vector, and the incorporation thereof in
the thus
obtained expression product. Furthermore, in vivo application of TriMix mRNA
obtained from
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
3
the vector of the present invention in a mouse cancer model results in a
slower growth of
tumors and an increased life expectance of said mice.
SUMMARY OF THE INVENTION
In a first aspect the present invention provides a nucleic acid vector
comprising a translation
enhancer (TE) sequence having at least 80% sequence identity to SEQ ID N 1, a
transcribable
nucleic acid sequence and a nuclear retention sequence represented by SEQ ID
N'4.
In a specific embodiment, the transcribable nucleic acid sequence is selected
from the list
comprising mRNA encoding CD4OL, CD70, caTLR4 or antigen/disease specific mRNA.
In a preferred embodiment, the translation enhancer is represented by anyone
of SEQ ID N 1,
2, or 3, more in particular SEQ ID N 1.
In a further aspect, the present invention provides a method of increasing
stability and/or
translation efficiency of in vitro transcribed RNA; said method comprising the
steps of:
(i) providing a vector according to this invention, wherein said transcribable
nucleic acid
sequence is a transcribable DNA sequence, which corresponds to said RNA to be
transcribed; and
(ii) transcribing in vitro the transcribable DNA sequence;
In yet a further embodiment, the present invention provides an RNA molecule
comprising a
translation enhancer (TE) having at least 80% sequence identity to SEQ ID N 1,
a
transcribable nucleic acid sequence, and a nuclear retention sequence
represented by SEQ ID
N 4.
Said RNA molecule may further comprise a poly-A tail.
In the context of the RNA molecules of the present invention, said
translatable nucleic acid
sequence may be selected from the list comprising mRNA encoding CD4OL, CD70,
caTLR4 or
antigen/disease specific mRNA.
In a preferred embodiment of the RNA molecules, the translation enhancer is
represented by
any one of SEQ ID N 1, 2 or 3; more in particular SEQ ID N 1.
The present invention further provides a composition comprising one or more
RNA molecules
according to this invention; more in particular said one or more RNA molecules
represent
mRNA molecules which encode CD4OL, CD70 and caTLR4.
4
The composition according to the present invention, may further comprise mRNA
encoding
antigen/disease specific mRNA.
The present invention further provides the use of the RNA molecule(s) and/or
composition(s)
comprising one or more of said RNA molecules for multiple purposes, such as
for example for
in vivo or in vitro introduction in a host cell; or for use in medicine.
It is also an aspect of the present invention to provide a kit comprising one
or more vectors;
one or more RNA molecules; or a composition according to the present
invention.
The present invention also provides a method for treating a patient in need
thereof with one or
more RNA molecules or a composition according to the present invention;
wherein said RNA
molecules can be administered simultaneously or sequentially with intervals.
The RNA molecules or compositions according to the present invention may be
administered
to a patient in need thereof by any suitable administration route such as for
example
intranodal, intradermal, intralymphatic and intratumoral. Furthermore, when
treating for
example cancer patients, the administration of the RNA molecules or
compositions according
to the present invention may be used in combination with methods for releasing
tumor mRNA
from the tumor in the patient, such as for example ablation or sonoporation.
In accordance with an aspect of the present invention, there is provided a
nucleic acid vector
comprising a translation enhancer (TE) sequence having at least 80% sequence
identity to the
full-length sequence of SEQ ID NO:1, a transcribable nucleic acid sequence and
a nuclear
retention sequence represented by SEQ ID NO:4.
In accordance with a further aspect of the present invention, there is
provided an RNA
molecule comprising a translation enhancer (TE) having at least 80% sequence
identity to the
full-length sequence of SEQ ID NO:1, a transcribable nucleic acid sequence,
and a nuclear
retention sequence represented by SEQ ID NO:4.
In accordance with a further aspect, is an RNA transcription vector comprising
a transcribable
nucleic acid sequence, a 5' translation enhancer (TE) sequence and a 3'
nuclear retention
sequence (ENE).
Date Recue/Date Received 2021-06-10
4a
NUMBERED STATEMENTS OF THE INVENTION
1. A nucleic acid vector comprising a translation enhancer (TE) sequence
having at least 80%
sequence identity to SEQ ID NO.1, a transcribable nucleic acid sequence and a
nuclear
retention sequence represented by SEQ ID NO.4.
2. The nucleic acid vector according to claim 1, wherein said transcribable
nucleic acid
sequence is selected from the list comprising mRNA encoding CD4OL, CD70,
caTLR4 or
antigen/disease specific nnRNA.
3. The nucleic acid vector according to anyone of claims 1-2, wherein said
translation
enhancer is represented by anyone of SEQ ID NO. 1, 2 or 3; more in particular
SEQ ID NO. 1.
4. A method of increasing stability and/or translation efficiency of in vitro
transcribed RNA; said
method comprising the steps of:
(i) providing a vector according to anyone of claims 1-3, wherein said
transcribable
nucleic acid sequence is a transcribable DNA sequence, which corresponds to
said
Date Recue/Date Received 2021-06-10
CA 02929203 2016-01-29
WO 2015/071295 PCT/EP2014/074349
RNA to be transcribed; and
(ii) transcribing in vitro said transcribable DNA sequence.
5. An RNA molecule comprising a translation enhancer (TE) having at least 80%
sequence
5 identity to SEQ ID N 1, a transcribable nucleic acid sequence, and a
nuclear retention
sequence represented by SEQ ID N 4.
6. An RNA molecule according to claim 5 further comprising a poly-A tail.
7. An RNA molecule according to anyone of claims 5 or 6, wherein said
transcribable nucleic
acid sequence is selected from the list comprising mRNA encoding CD4OL, CD70,
caTLR4 or
antigen/disease specific mRNA.
8. An RNA molecule according to anyone of claims 5-7, wherein said translation
enhancer is
represented by SEQ ID N 1.
9. A composition comprising one or more RNA molecules as claimed in anyone of
claims 5-8.
10. The composition according to claim 9, wherein said one or more RNA
molecules represent
mRNA molecules which encode CD4OL, CD70 and caTLR4.
11. The composition according to c.laim 10 further comprising mRNA encoding
antigen/disease
specific mRNA.
12. The use of an RNA molecule according to anyone of claims 5-8, or the
composition
according to anyone of claims 9-11 for introduction in a host cell.
13. An RNA molecule according to anyone of claims 5-8 or a composition
according to anyone
of claims 9-11 for use in medicine.
14. A kit comprising one or more vectors according to anyone of claims 1-3;
one or more RNA
molecules according to anyone of claims 5-8; or a composition according to
anyone of claims
9-11.
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
6
BRIEF DESCRIPTION OF THE DRAWINGS
With specific reference now to the figures, it is stressed that the
particulars shown are by way
of example and for purposes of illustrative discussion of the different
embodiments of the
present invention only. They are presented in the cause of providing what is
believed to be the
most useful and readily description of the principles and conceptual aspects
of the invention.
In this regard no attempt is made to show structural details of the invention
in more detail than
is necessary for a fundamental understanding of the invention. The description
taken with the
drawings making apparent to those skilled in the art how the several forms of
the invention
may be embodied in practice.
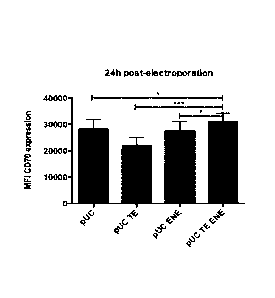
Fig. 1: iDCs were electroporated with TriMix mRNA encoded by the pUC-vector,
the pUC-TE
vector, pUC-ENE vector or the pUC TE ENE-vector. MFI (mean fluorescence
intensity) values
of the positive DC population are shown. Data are presented as mean SEM.
(Paired t test, *
111/4 0,05). N pUC = 6; N pUC-TE = 15; N pUC-ENE = 15; N pUC TE ENE = 19
Fig. 2: WT1 expression in DCs electroporated with WT1 mRNA. iDCs were
electroporated
with WT1 mRNA encoded by the different vectors and analyzed for their WT1
expression by
intracellular staining 4h, 24h, and 48h post-electroporation. A comparison of
MFI values after
electroporation of the iDCs with the different WT1-encoding vectors is shown.
Data are
presented as mean SEM. (Paired t test, * P< 0.05; **P<0.01; *** P<0.001). N
= 6
Fig. 3: Kinetics of eGFP expression of DCs. iDCs were co-electroporated with
eGFP and
TriMix mRNA encoded by the pUC-vector or the pUC TE ENE-vector. eGFP
expression was
analyzed at several time points post-electroporation. The MFI value of the
eGFP positive DC
population was analyzed. Data are presented as mean SEM. (Paired t test, *
P< 0.05;
**P<0.01; *** P<0.001). N = 9
Fig. 4: Phenotype of immature and mature DCs. MFI values of the indicated
molecules were
investigated 24h post-electroporation of iDCs. Data are represented as mean
SEM. (Paired
t test, * P< 0.05; **P<0.01; *** P<0.001). CD40 N = 9; CD70 N = 19; CD80 N =
12; CD 83 N =
12; CCR7 N = 12.
Fig. 5: Two-side tumor model with P815: single treatment of one tumor with
tNGFR as a
control or with pUC TE ENE TriMix. The contralateral, non-treated tumor was
used to evaluate
the systemic anti-tumor immune response. Tumor growth was shown for each
individual
mouse per group (n =6) followed by an overview of the mean tumor volume.
Survival was
visualized in a Kaplan-Meier plot. Differences in survival were analyzed by
the log-rank test.
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
7
Fig. 6: Two-side tumor model with P815: single treatment of one tumor, with
tNGFR or 0.8
volumes of Hartman solution served as a control. The contralateral, non-
treated tumor was
used to evaluate the systemic anti-tumor immune response. Tumor growth was
shown for
each individual mouse per group (n =6) followed by an overview of the mean
tumor volume.
Survival was visualized in a Kaplan-Meier plot. Differences in survival were
analyzed by the
log-rank test.
Fig. 7: The pUC TE ENE-vector with its most important elements.
Fig. 8: Shows a sequence comparison of 3 variable TE sequences (SEQ ID N 1, 2
and 3) as
created by Clustal 2.1 from EMBL.
Fig. 9: (A) WT1 expression in DCs electroporated with WT1 mRNA. iDCs were
electroporated
with 10 lug WT1 mRNA encoded by the different vectors and analyzed for their
WT1
.. expression by intracellular staining 4h, 24h, and 48h post-electroporation.
A comparison of
MEI values after electroporation of the iDCs with the different WT1-encoding
vectors is shown.
N = 3 (B) eGFP expression in DCs electroporated with eGFP mRNA. iDCs were
electroporated with 10 lug eGFP mRNA encoded by the different vectors and
analyzed for their
eGFP expression by intracellular staining 4h, 24h, and 48h post-
electroporation. A comparison
of MEI values after electroporation of the iDCs with the different WT1-
encoding vectors is
shown. N = 3
DETAILED DESCRIPTION OF THE INVENTION
In a first aspect the present invention provides a nucleic acid vector
comprising a translation
enhancer (TE) and a nuclear retention sequence. More in particular, said
nucleic acid vector
comprises a translation enhancer (TE) sequence having at least 80% sequence
identity to
SEQ ID N'1, a transcribable nucleic acid sequence and a nuclear retention
sequence
represented by SEQ ID N'4.
The term "vector" is used here in its most general meaning and comprises any
intermediate
vehicles for a nucleic acid, which, for example, enable said nucleic acid to
be introduced into
prokaryotic and/or eukaryotic host cells and, where appropriate, to be
integrated into a
genome. Such vectors are preferably replicated and/or expressed in the cell.
Vectors comprise
plasmids, phagemids or virus genomes. The term "plasmid", as used herein,
generally relates
to a construct of extrachromosomal genetic material, usually a circular DNA
duplex, which can
replicate independently of chromosomal DNA.
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
8
According to the invention, a nucleic acid molecule or a nucleic acid sequence
refers to a
nucleic acid which is preferably deoxyribonucleic acid (DNA) or ribonucleic
acid (RNA).
According to the invention, nucleic acids comprise genomic DNA, cDNA, mRNA,
recombinantly prepared and chemically synthesized molecules. According to the
invention, a
nucleic acid may be in the form of a single-stranded or double stranded and
linear or
covalently closed circular molecule. The term "nucleic acid" furthermore also
comprises a
chemical derivatization of a nucleic acid on a nucleotide base, on the sugar
or on the
phosphate, and nucleic acids containing non-natural nucleotides and nucleotide
analogs.
The nucleic acids described according to the invention are preferably
isolated. The term
"isolated nucleic acid" means according to the invention that the nucleic acid
has been 1/
amplified in vitro, for example by polymerase chain reaction (FOR) 2/
recombinantly produced
by cloning; 3/ purified, for example by cleavage and gel-electrophoretic
fractionation, or 4/
synthesized, for example by chemical synthesis. An isolated nucleic acid is a
nucleic acid
available to manipulation by recombinant DNA techniques.
5' Translation Enhancer (TE)
Posttranscriptional regulation of translation is mainly controlled at the
translation initiation
phase. A complex of initiation factors binds to the 5'CAP structure and
recruits the ribosomal
subunits. This complex then starts a scanning movement along the mRNA until an
AUG codon
in a suitable context is encountered. The efficiency of this process can be
controlled by
numerous structural features in both 5' and 3' UTRs (UnTranslated Regions) of
the mRNA.
These features include TOP (Terminal OligoPyrimidine tract) regions, IRES
(Internal
Ribosome Entry Site) and Upstream ORF's (Open Reading Frames) in the 5' UTR.
In the 3'
UTR CITE (Cap Independent Translation Enhancer) motifs have been described.
The length of
the poly-A tail has also been shown to play an important role in translation
initiation, since
PABP (Poly-A Binding Protein) needs to associate to both the poly-A tail and
the elF4 complex
on the CAP site.
IRES is a motif that is able to recruit ribosomes independently of interaction
with the 5'CAP
structure. The first IRES elements were described in picornaviruses (e.g.
EMCV,
EncephaloMyoCarditisVirus). During infection CAP dependent translation is shut
down,
yielding an advantage to the CAP independent translation of the viral
proteins. Several
eukaryotic IRES sequences have been described in recent years. In stress
situations CAP
dependent translation is down regulated, while CAP independent translation of
some essential
genes can continue. More specifically, dendritic cells that are activated by
e.g. ligation of LPS
on Toll Like Receptor 4 also shut down CAP dependent translation, while CAP
independent
translation of some genes protects the cells from apoptosis.
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
9
Hu et al. studied a sequence in the 5' leader of the mRNA coding for the mouse
Gtx
homeodomain protein. They described a sequence that is complementary to
sequences in the
18S ribosomal RNA. They found that this motif had a profound influence on the
efficiency of
translation (Hu et al., 1999).
Later, it was shown that this motif functions as an internal ribosome entry
site (IRES) and
showed that shorter nonoverlapping segments of this 5'leader could enhance the
translation of
a second cistron in a dicistronic mRNA. One of these segments was 9
nucleotides in length
and when multiple copies of this IRES module were linked together, IRES
activity was greatly
enhanced. A tandem repeat of the same 9n segment, interspaced by 9n fragments
of the
human beta globin 5' UTR, was shown to function as a Translation Enhancer (TE)
in a
monocistronic mRNA when positioned at the 5'-end of mRNA, in front of the ORF.
Hence, in the context of the present invention, any sequence functioning as a
Translation
Enhancer for mRNA may be used, for example those elements described herein
above. In
particular, a translation enhancer is a sequence in the transcribed RNA that
facilitates
translation. One possible mode of action is through enhancing the binding of
the ribosome to
the 5' end of the mRNA.
In a particular example, the vector according to this invention may yield RNA
that contains a
10x tandem repeat of the wild type 9n sequence from the Gtx leader sequence:
CCGGCGGGT. These motifs are linked by a 9n sequence derived from the 5' UTR of
human
beta globin: TTCTGACAT. This DNA fragment can be cloned in the plasmid between
the
bacteriophage promotor sequence and the ORF (Open Reading Frame).
In a particular embodiment, the translation enhancer according to the present
invention has at
least 80% sequence identity to SEQ ID N 1. As evident from figure 8, SEQ ID N
2 and 3 have
a sequence identity of at least 80% in comparison to SEQ ID N'1, and are thus
suitable to
used in connection with the present invention. More preferably, the
translation enhancer
according to the present invention has at least 85%, 86%, 87%, 88% or 89%
sequence identity
to SEQ ID N 1. As evident from figure 8, SEQ ID N 2 and 3 have a sequence
identity of at
least 85% in comparison to SEQ ID N 1. Even more preferably, the translation
enhancer
according to the present invention has at least 90%, 91%, 92%, 93%, 94%, 95%,
96%, 97%,
98% 99% or 100% sequence identity to SEQ ID N 1. As evident from figure 8, SEQ
ID N 3
has a sequence identity of at least 90% in comparison to SEQ ID N 1. Even more
preferably,
the translation enhancer is represented by anyone of SEQ ID N 1, 2 or 3; most
preferably SEQ
ID N 1.
CA 02929203 2016-04-29
WO 2015/071295
PCT/EP2014/074349
Nuclear retention sequence
The importance of post-transcriptional genetic control processes has become
increasingly
apparent in recent years. Among these processes, one that began to receive
considerable
5 attention is the control of mRNA stability. With the growing recognition
that mRNA degradation
has a profound impact on gene expression and that rates of mRNA decay can be
modulated in
response to environmental and developmental signals, a vigorous research
effort aimed at
understanding this process is now taking place. Significant progress has been
made and
studies over the past 20 years have elucidated a number of general features of
mRNA
10 degradation.
Both cellular and viral mRNAs are subject to robust RNA decay pathways.
Viruses have
developed different methods to protect their mRNA from deadenylation
mechanisms of the
host. The poly-adenylated non-translated RNA (PAN) of Kaposi's sarcoma
associated Herpes
virus (KSHV) is very abundant in the nucleus of infected cells. This RNA is
resistant to
deadenylation and degradation. The accumulation of PAN depends on the activity
of a 79
nucleotide RNA element in the 3' region, called the ENE (Expression and
Nuclear retention
Element). Conrad et al. published in 2005 the first article related to ENE in
describing it as a
Kaposi's sarcoma virus RNA element that gave an increased nuclear abundance of
intronless
transcripts (Conrad, 2005). The ENE fragment contains a specific U-rich
hairpin structure that
interacts with the poly-A tail. As such, a secondary structure is obtained
which results in the
retention of the RNA in the nucleus and hence the name Nuclear Retention
Element. A
secondary effect, is the interaction of the U-rich hairpin structure with the
poly-A tail of mRNA
resulting in a 'shielding' effect from degradation by the host, a trait that
is of particular interest
in the production of mRNA for immunotherapeutic purposes.
Polyadenylated nuclear (PAN) RNA (also known as T1.1 or nut-1 RNA) is a IncRNA
produced
by the oncogenic gammaherpesvirus, Kaposi's sarcoma-associated herpesvirus
(KSHV) (Sun
et al., 1996). PAN RNA accumulates to extraordinarily high levels (¨ 500,000
copies/cell)
during lytic infection and is required for the production of late viral
proteins and infectious virus
(Sun et al., 1996). The expression and nuclear retention element (ENE),
located ¨120 nts
upstream of PAN RNA's polyadenylation site, is essential for this high
accumulation in the
nucleus (Conrad and Steitz, 2005). The ENE inhibits rapid decay of PAN RNA by
blocking
deadenylation (Conrad et al., 2006). PAN RNA does not yield protein
expression. The nuclear
retention keeps the RNA away from the translation machinery in the cytoplasm,
while the
shielding of the poly-A tail prohibits binding to the PABP (polyA binding
protein) which is
essential for efficient translation. Hence, use of this sequence in
transfection is not obvious.
CA 02929203 2016-04-29
WO 2015/071295
PCT/EP2014/074349
11
The KSHV ENE is a 79 nt-long RNA element, composed of a stem-loop structure
with an
asymmetric internal U-rich loop, which in conjunction with adjacent base pairs
constitutes the
ENE's functional core. The crystal structure of the ENE core bound to
oligo(A)9 revealed 5
consecutive U-A-U base triples formed between the U-rich loop and oligo(A)9
(Mitton-Fry et
al., 2010), which are extended by A-minor interactions with three G-C base
pairs of the lower
stem. Genetic and biochemical analyzes indicate similar interactions between
the PAN RNA's
poly(A) tail and the ENE in vivo (Mitten-Fry et al., 2010).
Hence, in the context of the present invention, any sequence functioning as a
nuclear retention
element for mRNA may be used, for example those elements described herein
above. In
particular, a nuclear retention element is a cis acting sequence that has the
capacity to protect
the mRNA to cytoplasmic decay.
In a particular embodiment, the nuclear retention element also functions as an
RNA stabilizing
sequence.
In a particular example, the nuclear retention element is the Expression and
Nuclear retention
Element of KSHV. A 79bp sequence isolated from the PAN (Poly Adenylated Non
translated)
RNA is placed upstream from the A124 stretch in the RNA production plasmid.
The ENE forms
a U rich loop that associates with the polyA tail and protects it from
degradation.
In a particular embodiment, the nuclear retention element according to the
present invention is
represented by SEQ ID N 4.
Further elements in the vector of the present invention
In a further embodiment, the nucleic acid vector of the present invention, may
contain further
elements selected from the list comprising a bacteriophage promoter, a
transcribable nucleic
acid sequence and a poly-A tail.
Messenger RNA or ribonucleic acid (mRNA) consists of a single-stranded polymer
of 4
nucleotides (adenosine, guanosine, cytidine and uridine monophosphate). A 5'-
end
modification or 5'CAP is needed for recognition of the mRNA by the translation
initiation
complex, proper attachment of the mRNA to the ribosomes, as well as protection
from 5'
exonucleases. This modification consists of a 7-methylguanosine nucleotide
added to the first
transcribed nucleotide. The coding region begins with a start codon (usually
AUG) and ends
with a stop codon (usually UAA, UAG or UGA). Before the start codon and after
the stop
codon, mature mRNA contains a 5' untranslated region (UTR) and a 3' UTR. These
regions
contribute to the mRNA stability or instability and translational efficiency.
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
12
A transcribable nucleic acid sequence, in particular a nucleic acid coding for
a peptide or
protein, and an expression control sequence are "functionally" linked to one
another, if they are
covalently linked to one another in such a way that transcription or
expression of the
transcribable and in particular coding nucleic acid is under the control or
under the influence of
the expression control sequence.
The nucleic acids specified herein, in particular transcribable and coding
nucleic acids, may be
combined with any expression control sequence, in particular promoters, which
may be
homologous or heterologous to said nucleic acids, with the term "homologous"
referring to the
fact that a nucleic acid is also functionally linked naturally to the
expression control sequence,
and the term "heterologous" referring to the fact that a nucleic acid is not
naturally functionally
linked to the expression control sequence.
The term "expression control sequences" comprises according to the invention
promoters,
ribosome-binding sequences and other control elements, which control
transcription of a gene
or translation of the derived RNA. In particular embodiments of the invention,
the expression
control sequences can be regulated. The precise structure of expression
control sequences
may vary depending on the species or cell type but usually includes 5'-
untranscribed and 5'-
and 3- untranslated sequences involved in initiating transcription and
translation, respectively,
such as a TATA box, capping sequence, CAAT sequence and the like. More
specifically,
untranscribed expression control sequences include a promoter region which
encompasses a
promoter sequence for transcription control of the functionally linked gene.
Expression control
sequences may also include enhancer sequences or upstream activator sequences.
In particular embodiments, a nucleic acid is functionally linked according to
the invention to
expression control sequences, which may be homologous or heterologous with
respect to the
nucleic acid.
The term "promoter" or "promoter region" refers to a DNA sequence upstream
(5') of the
coding sequence of a gene, which controls expression of said coding sequence
by providing a
recognition and binding site for RNA polymerase. The promoter region may
include further
recognition or binding sites for further factors involved in regulating
transcription of said gene.
A promoter may control transcription of a prokaryotic or eukaryotic gene. A
promoter may be
"inducible" and initiate transcription in response to an inducer, or may be
"constitutive" if
transcription is not controlled by an inducer. An inducible promoter is
expressed only to a very
small extent or not at all, if an inducer is absent. In the presence of the
inducer, the gene is
"switched on" or the level of transcription is increased. This is usually
mediated by binding of a
specific transcription factor.
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
13
In a particular embodiment, the transcribable nucleic acid sequence is
selected from the list
comprising mRNA encoding CD40L (NM_000074), CD70 (NM_001252), caTLR4 ((a
truncated
version of the human TLR4 gene, that contains only the transmembrane and
cytoplasmic
region of the gene, preceded by the signal peptide of LAMP1 (lysosome
associated membrane
protein)) or antigen/disease specific mRNA.
The bacteriophage promotor according to this invention, may be any suitable
promotor for
RNA transcription and is preferably selected from the list comprising T7
promotor, SP6
promotor and T3 promotor; more in particular T7 promotor.
The poly-A tail as used in the context of this invention, preferably consists
of between and
about 100-150 adenosines, more in particular 120-125 adenosines, preferably
about 124
adenosines.
The terms "polyadenyl cassette" or "poly-A sequence" refer to a sequence of
adenyl residues
which is typically located at the 3' end of an RNA molecule. The invention
provides for such a
sequence to be attached during RNA transcription by way of a DNA template on
the basis of
repeated thymidyl residues in the strand complementary to the coding strand,
whereas said
sequence is normally not encoded in the DNA but is attached to the free 3' end
of the RNA by
a template-independent RNA polymerase after transcription in the nucleus.
According to the
invention, a poly(A) sequence of this kind is understood as meaning a
nucleotide sequence of
at least 20, preferably at least 40, preferably at least 80, preferably at
least 100 and preferably
up to 500, preferably up to 400, preferably up to 300, preferably up to 200,
and in particular up
to 150 consecutive A nucleotides, and in particular about 120 consecutive A
nucleotides,
wherein the term "A nucleotides" refers to adenyl residues.
The invention further provides an RNA molecule obtainable by transcription of
the nucleic acid
vector according to this invention.
In a further aspect, the present invention provides a method of increasing
stability and/or
translation efficiency of in vitro transcribed RNA; said method comprising the
steps of:
(i) providing a vector according to this invention wherein said transcribable
nucleic acid
sequence is a transcribable DNA sequence, which corresponds to said RNA to be
transcribed; and
(ii) transcribing in vitro the transcribable DNA sequence;
as well as an RNA molecule obtainable by said method.
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
14
According to the invention, the term "transcription" comprises "in vitro
transcription" wherein
the term "in vitro transcription" relates to a method in which RNA, in
particular mRNA, is
synthesized in vitro in a cell-free manner. The preparation of transcripts
preferably makes use
of cloning vectors which are generally referred to as transcription vectors
and which are
included according to the invention under the term "vector".
The term "nucleic acid sequence transcribed from a nucleic acid sequence"
refers to RNA,
where appropriate as a part of a complete RNA molecule, which is a
transcription product of
the latter nucleic acid sequence.
The term "nucleic acids which can be transcribed to give a common transcript"
means that said
nucleic acids are functionally linked to one another in such a way that, where
appropriate after
linearization such as restriction enzyme cleavage of the nucleic acid molecule
comprising said
nucleic acids, in particular of a closed circular nucleic acid molecule,
transcription under the
control of a promoter results in an RNA molecule comprising the transcripts of
said nucleic
acids covalently bound to one another, where appropriate separated by
sequences located in-
between.
According to the invention, the term "expression" is used in its most general
meaning and
.. comprises production of RNA and/or protein. It also comprises partial
expression of nucleic
acids. Furthermore, expression may be transient or stable. With respect to
RNA, the term
"expression" or "translation" refers in particular to production of peptides
or proteins.
The term 'nucleic acid sequence which is active in order to increase the
translation efficiency
and/or stability of a nucleic acid sequence" means that the first nucleic acid
is capable of
modifying, in a common transcript with the second nucleic acid, the
translation efficiency
and/or stability of said second nucleic acid in such a way that said
translation efficiency and/or
stability is increased in comparison with the translation efficiency and/or
stability of the said
second nucleic acid without said first nucleic acid. In this context, the term
"translation
efficiency" relates to the amount of translation product provided by an RNA
molecule within a
particular period of time and the term "stability" relates to the half-life of
an RNA molecule.
In a particular embodiment, the present invention provides an RNA molecule
comprising a
translation enhancer (TE) and a nuclear retention element (ENE); or a
composition comprising
one or more of said RNA molecules. More in particular, the present invention
provides an RNA
molecule comprising a translation enhancer (TE) having at least 80% sequence
identity to
SEQ ID N 1, a transcribable nucleic acid sequence, and a nuclear retention
sequence
represented by SEQ ID N 4; or a composition comprising said RNA molecule.
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
Said RNA molecule may further comprise one or more elements selected from the
list
comprising a translatable nucleic acid sequence, and a poly-A tail; wherein
said translatable
nucleic acid sequence may be selected from the list comprising mRNA encoding
CD4OL,
CD70, caTLR4 or antigen/disease specific mRNA.
5
In the context of the present invention, the TE element is preferably
positioned at the 5' end of
the transcribable/translatable RNA molecule and the nuclear retention sequence
(ENE)
preferably at the 3' end.
10 "3' end of a nucleic acid" refers according to the invention to that end
which has a free
hydroxyl group."5' end of a nucleic acid" refers according to the invention to
that end which has
a free phosphate group.
In the context of the present invention "mRNA" means "messenger RNA" and
refers to a
15 transcript which is produced using DNA as template and which itself
codes for a peptide or
protein. An mRNA typically comprises a 5'-untranslated region, a protein-
encoding region and
a 3'-untranslated region. mRNA has a limited half time in cells. According to
the invention,
mRNA may be prepared from a DNA template by in vitro transcription. It may be
modified by
further stabilizing modifications and capping, in addition to the
modifications according to the
invention.
In a particular embodiment, the composition according to this invention
comprises mRNA
encoding CD4OL, CD70 and caTLR4 either or not in combination with mRNA
encoding
antigen/disease specific mRNA.
The antigen/disease specific mRNA according to the present invention may be
selected from
the non-limiting list comprising tumor antigens, pathogen derived antigens,
allergens...
The present invention further provides the use of the RNA molecule(s) and/or
composition(s)
comprising one or more of said RNA molecules for multiple purposes, such as
for example for
in vivo or in vitro introduction in a host cell; or for use in medicine.
It is also an aspect of the present invention to provide a kit comprising one
or more vectors;
one or more RNA molecules or a composition according to the present invention.
The present invention also provides a method for treating a patient in need
thereof with one or
more RNA molecules or a composition according to the invention; wherein, said
RNA
molecules can be administered simultaneously or sequentially with intervals.
CA 02929203 2016-04-29
WO 2015/071295
PCT/EP2014/074349
16
The invention provides for nucleic acids in particular RNA to be administered
to a patient.
Nucleic acids can be administered by ex vivo methods, i.e. by removing cells
from a patient,
genetically modifying said cells (e.g. by transfection) and reintroducing the
modified cells into
the patient. Transfection and transduction methods are known to the skilled
worker. The
invention also provides for nucleic acids to be administered in vivo.
According to the invention, the term "transfection" refers to introducing one
or more nucleic
acids into an organism or into a host cell. Various methods may be employed in
order to
introduce according to the invention nucleic acids into cells in vitro or in
vivo. Such methods
include transfection of nucleic acid-CaPO4 precipitates, transfection of
nucleic acids associated
with DEAE, transfection of infection with viruses carrying the nucleic acids
of interest, liposome
mediated transfection, and the like. In particular embodiments, preference is
given to directing
the nucleic acid to particular cells. In such embodiments, a carrier used for
administering a
nucleic acid to a cell (e.g. a retrovirus or a liposome) may have a bound
targeting molecule.
For example, a molecule such as an antibody specific to a surface membrane
protein on the
targeted cell, or a ligand for a receptor on the target cell may be
incorporated into or bound to
the nucleic acid carrier If administration of a nucleic acid by liposomes is
desired, proteins
binding to a surface membrane associated with endocytosis may be incorporated
into the
liposome formulation in order to enable targeting and/or absorption. Such
proteins include
capsid proteins or fragments thereof which are specific to a particular cell
type, antibodies to
proteins that are internalized, proteins targeting an intracellular site, and
the like.
The RNA molecules or compositions according to the present invention may be
administered
to a patient in need thereof by any suitable administration route such as for
example
intranodal, intradermal, intralymphatic and intratumoral. Furthermore, when
treating for
example cancer patients, the administration of the RNA molecules or
compositions according
to this invention, may be used in combination with methods for releasing tumor
mRNA from the
tumor in the patient, such as for example ablation or sonoporation.
According to the invention, standard methods may be used for preparing
recombinant nucleic
acids, culturing cells, in particular electroporation and lipofection.
Enzymatic reactions are
carried out according to the manufacturer's instructions or in a manner known
per se.
According to the invention, a "nucleic acid sequence which is derived from a
nucleic acid
sequence" refers to a nucleic acid containing, in comparison with the nucleic
acid from which it
is derived, single or multiple nucleotide substitutions, deletions and/or
additions and which is
preferably complementary to the nucleic acid from which it is derived, i.e.
there is a certain
degree of homology between said nucleic acids and the nucleotide sequences of
said nucleic
acids correspond in a significant direct or complementary manner.
CA 02929203 2016-04-29
WO 2015/071295
PCT/EP2014/074349
17
According to the invention, a nucleic acid derived from a nucleic acid has a
functional property
of the nucleic acid from which it is derived. Such functional properties
include in particular the
ability to increase, in a functional linkage to a nucleic acid which can be
transcribed into RNA
(transcribable nucleic acid sequence), the stability and/or translation
efficiency of RNA
produced from this nucleic acid in the complete RNA molecule.
A nucleic acid is "complementary" to another nucleic acid if the two sequences
can hybridize
with one another and form a stable duplex, said hybridization being carried
out preferably
under conditions which allow specific hybridization between polynucleotides
(stringent
conditions). Stringent conditions are described, for example in Molecular
Cloning: A laboratory
manual, J Sambrook et al.
According to the invention, complementary nucleic acids have nucleotides,
which are at least
60%, at least 70%, at least 80%, at least 90%, and preferably at least 95%, at
least 98% or at
least 99% identical.
According to the invention, a first polynucleotide region is considered to be
located
downstream of a second polynucleotide region, if the 5' end of said first
polynucleotide region
is the part of said first polynucleotide region closest to the 3' end of said
second polynucleotide
region.
The 3'-untranslated region typically extends from the termination codon for a
translation
product to the poly-A sequence which is usually attached after the
transcription process. The
3'-untranslated regions of mammalian mRNA typically have a homology region
known as the
AAUAAA hexanucleotide sequence. This sequence is presumably the poly-A
attachment
signal and is frequently located from 10 to 30 bases upstream of the poly-A
attachment site.
3'-untranslated regions may contain one or more inverted repeats which can
fold to give stem-
loop structures, which act as barriers for exoribonucleases or interact with
proteins known to
increase RNA stability (e.g. RNA-binding proteins).
5'- and/or 3'- untranslated regions may, according to the invention, be
functionally linked to a
transcribable and in particular coding nucleic acid, so as for these regions
to be associated
with the nucleic acid in such way that the stability and/or translation
efficiency of the RNA that
is transcribed from said transcribable nucleic acid are increased.
According to the invention, the term "gene" refers to a particular nucleic
acid sequence, which
is responsible for producing one or more cellular products and/or for
achieving one or more
cellular products and/or for achieving one or more intercellular or
intracellular functions. More
CA 02929203 2016-04-29
WO 2015/071295
PCT/EP2014/074349
18
specifically, said term relates to a DNA section, which comprises a nucleic
acid coding for a
specific protein or a functional or structural RNA molecule.
According to the invention, the term "host cell" refers to any cell which can
be transformed or
transfected with an exogenous nucleic acid. The term "host cell" comprises,
according to the
invention prokaryotic (e.g. E. coil) or eukaryotic cells (e.g. yeast cells and
insect cells).
Particular preference is given to mammalian cells such as cells from humans,
mice, hamsters,
pigs, goats, and primates. The cells may be derived from a multiplicity of
tissue types and
comprise primary cells and cell lines. Specific examples include
keratinocytes, peripheral
blood leukocytes, bone marrow stem cells and embryonic stem cells. In other
embodiments,
the host cell is an antigen-presenting cell, in particular a dendritic cell, a
monocyte or a
macrophage. A nucleic acid may be present in the host cell in a single or in
several copies
and, in one embodiment is expressed in the host cell.
According to the invention, a peptide or protein encoded by a nucleic acid may
be a peptide or
protein which is located in the cytoplasm, in the nucleus, in the membrane, in
organelles or in
secreted form. They include structural proteins, regulatory proteins,
hormones,
neurotransmitters, growth-regulating factors, differentiation factors, gene
expression regulating
factors, DNA-associated proteins, enzymes, serum proteins, receptors,
medicaments,
immunomodulators, oncogenes, toxins, tumor antigens or antigens. Said peptides
or proteins
may have a naturally occurring sequence or a mutated sequence in order to
enhance, inhibit,
regulate or eliminate their biological activity.
The term "peptide" refers to substances which comprise two or more, preferably
3 or more,
preferably 4 or more, preferably 6 or more, preferably 8 or more, preferably
10 or more,
preferably 13 or more, preferably 16 or more, preferably 100 or preferably 150
consecutive
amino acids linked to one another via peptide bonds. The term "protein" refers
to large
peptides, preferably peptides having at least 151 amino acids, but the terms
"peptide" and
"protein" are used herein usually as synonyms. The terms "peptide" and
"protein" comprise
according to the invention substances which contain not only amino acid
components but also
non-amino acid components such as sugars and phosphate structures, and also
comprise
substances containing bonds such as ester, thioether or disulphide bonds.
"Reporter" relates to a molecule, typically a peptide or protein, which is
encoded by a reporter
gene and measured in a reporter assay. Conventional systems usually employ an
enzymatic
reporter and measure the activity of said reporter.
According to the invention, two elements, such as nucleotides or amino acids
are consecutive,
if they are directly adjacent to one another, without any interruption.
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
19
"Restriction endonucleases" or "restriction enzymes" refer to a class of
enzymes that cleave
phosphodiester bonds in both strands of a DNA molecule within specific base
sequences.
They recognize specific binding sites, referred to as recognition sequences,
on a double
stranded DNA molecule. The sites at which said phosphodiester bonds in the DNA
are cleaved
by said enzymes are referred to as cleavage sites. In the case of type IIS
enzymes, the
cleavage site is located at a defined distance form the DNA binding site.
AREAS OF APPLICATION
An area of application of the present invention is vaccination, i.e. the use
of modified mRNA for
inoculation or the use of a pharmaceutical composition comprising the modified
mRNA as an
inoculating agent, or the use of modified mRNA in the preparation of a
pharmaceutical
composition for inoculation purposes. Vaccination is based on introducing an
antigen into an
organism or subject, in particular into a cell of the organism or subject. In
the context of the
present invention, the genetic information encoding the antigen is introduced
into the organism
or subject in the form of a modified mRNA encoding the antigen and / or the
different TriMix
mRNA strands. The modified 'antigen' mRNA contained in the pharmaceutical
composition is
translated into an antigen, i.e. the polypeptide or antigenic peptide coded by
the modified
mRNA is expressed and an immune response directed against the polypeptide or
antigenic
peptide is stimulated. For vaccination against a pathogenic organism, e.g, a
virus, a bacterium,
or a protozoan, a surface antigen of such an organism may be used as an
antigen against
which an immune response is elicited. In the context of the present invention,
a
pharmaceutical composition comprising the modified mRNA encoding such a
surface antigen
may be used as a vaccine. In applications wherein a genetic vaccine is used
for treating
cancer, the immune response is directed against tumour antigens by generating
a modified
mRNA encoding a tumour antigen(s), in particular a protein which is expressed
exclusively on
cancer cells. Such a modified mRNA encoding a tumour antigen may be used alone
or as a
component of a pharmaceutical composition according to the invention, wherein
administration
of either the modified mRNA or a composition thereof results in expression of
the cancer
antigen(s) in the organism. An immune response to such a vaccine would,
therefore, confer to
the vaccinate subject a degree of protective immunity against cancers
associated with the
immunizing cancer antigen. Alternatively, such measures could be used to
vaccinate a cancer
patient with a modified mRNA encoding a tumour antigen(s) expressed on the
patient's cancer
cells so as to stimulate the cancer patient's immune response to attack any
cancer cells
expressing the encoded antigen.
For gene therapy applications, for example wherein a pharmaceutical
composition of the
invention is used, the modified mRNA therein codes for at least one biological
active peptide or
polypeptide that is not formed or is only insufficiently or defectively formed
in the patient to be
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
treated. Administration of a modified mRNA encoding the at least one
biologically active
peptide or polypeptide or a composition thereof to such a patient, therefore,
at least partially
restores the expression and/or activity of the at least one biologically
active peptide or
polypeptide in the patient and thereby complements the patient's genetic
defect. The direct
5 introduction of a normal, functional gene into a living animal has been
studied as a means for
replacing defective genetic information. In such studies, nucleic acid
sequences are introduced
directly into cells of a living animal. Accordingly, examples of polypeptides
coded by a
modified mRNA of the invention include, without limitation, dystrophin, the
chloride channel,
which is defectively altered in cystic fibrosis, enzymes that are lacking or
defective in metabolic
10 disorders such as phenylketonuria, galactosaemia, homocystinuria,
adenosine deaminase
deficiency etc.; as well as enzymes that are involved in the synthesis of
neurotransmitters such
as dopamine, norepinephrine and GABA, in particular tyrosine hydroxylase and
DOPA
decarboxylase, and alfa-1-antitrypsin etc. Pharmaceutical compositions of the
invention may
also be used to effect expression of cell surface receptors an/or binding
partners of cell
15 surface receptors of the modified mRNA contained therein encodes for
such biologically active
proteins or peptides. Examples of such proteins that in an extracellular
manner or that bind to
cell surface receptors include for example tissue plasminogen activator (TPA),
growth
hormones, insulin, interferons, granulocyte-macrophage colony stimulation
factor (GM-CFS)
and erythropoietin (EPO) etc.
By choosing suitable growth factors, the pharmaceutical composition of the
present invention
may, for example, be used for tissue regeneration or for interacting with stem
cells. In this way
diseases that are for example characterised by tissue degeneration, among
which
neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease,
etc. and other
degenerative conditions, such as arthrosis, can be treated. In these cases the
modified mRNA,
in particular that contained in the pharmaceutical composition of the present
invention,
preferably encodes without limitation, a TGF-Beta family member, neurotrophic
factors such as
NGF, neurotrophines etc.
METHOD OF TREATMENT
The present invention thus further provides a method for the prevention and/or
treatment of at
least one disease or disorder selected from the non-limiting list comprising
cancer, allergy and
infectious diseases such as bacterial, viral or fungal infections, e.g. HIV
infection or hepatitis.
The terms "cancer" and/or "tumor" used throughout the description are not
intended to be
limited to the types of cancer or tumors that may have been exemplified. The
term therefore
encompasses all proliferative disorders such as neoplasma, dysplasia,
premalignant or
precancerous lesions, abnormal cell growths, benign tumors, malignant tumors,
cancer or
metastasis, wherein the cancer is selected from the group of: leukemia, non-
small cell lung
CA 02929203 2016-01-29
WO 2015/071295
PCT/EP2014/074349
21
cancer, small cell lung cancer, CNS cancer, melanoma, ovarian cancer, kidney
cancer,
prostate cancer, breast cancer, glioma, colon cancer, bladder cancer, sarcoma,
pancreatic
cancer, colorectal cancer, head and neck cancer, liver cancer, bone cancer,
bone marrow
cancer, stomach cancer, duodenum cancer, oesophageal cancer, thyroid cancer,
hematological cancer, and lymphoma. Specific antigens for cancer can e.g. be
MelanA/MART1, Cancer-germline antigens, gp100, Tyrosinase, CEA, PSA, Her-
2/neu,
survivin, telomerase.
The term "infectious disease" or "infection" used throughout the description
is not intended to
be limited to the types of infections that may have been exemplified herein.
The term therefore
encompasses all infectious agents to which vaccination would be beneficial to
the subject.
Non-limiting examples are the following virus-caused infections or disorders:
Acquired
Immunodeficiency Syndrome - Adenoviridae Infections - Alphavirus Infections -
Arbovirus
Infections - Bell Palsy - Borne Disease - Bunyaviridae Infections -
Caliciviridae Infections -
Chickenpox - Common Cold - Condyloma Acuminata - Coronaviridae Infections -
Coxsackievirus Infections - Cytomegalovirus Infections - Dengue - DNA Virus
Infections -
Contagious Ecthyma, - Encephalitis - Encephalitis, Arbovirus - Encephalitis,
Herpes Simplex -
Epstein-Barr Virus Infections - Erythema Infectiosum - Exanthema Subitum -
Fatigue
Syndrome, Chronic - Hantavirus Infections - Hemorrhagic Fevers, Viral -
Hepatitis, Viral,
Human - Herpes Labialis - Herpes Simplex - Herpes Zoster - Herpes Zoster
Oticus -
Herpesyiridae Infections - HIV Infections - Infectious Mononucleosis -
Influenza in Birds -
Influenza, Human - Lassa Fever - Measles - Meningitis, Viral - Molluscum
Contagiosum -
Monkeypox - Mumps - Myelitis - Papillomavirus Infections - Paramyxoviridae
Infections -
Phlebotomus Fever - Poliomyelitis - Polyomavirus Infections -
Postpoliomyelitis Syndrome -
Rabies - Respiratory Syncytial Virus Infections - Rift Valley Fever - RNA
Virus Infections -
Rubella - Severe Acute Respiratory Syndrome - Slow Virus Diseases - Smallpox -
Subacute
Sclerosing Panencephalitis - Tick-Borne Diseases - Tumor Virus Infections -
Warts - West Nile
Fever - Virus Diseases - Yellow Fever - Zoonoses - Etc. Specific antigens for
viruses can be
HIV-gag, -tat, -rev or -nef, or Hepatitis C-antigens.
Further non-limiting examples are the following bacteria- or fungus-caused
infections or
disorders: Abscess - Actinomycosis - Anaplasmosis - Anthrax - Arthritis,
Reactive -
Aspergillosis - Bacteremia - Bacterial Infections and Mycoses - Bartonella
Infections - Botulism
- Brain Abscess - Brucellosis - Burkholderia Infections - Campylobacter
Infections -
Candidiasis - Candidiasis, Vulvovaginal - Cat-Scratch Disease - Cellulitis -
Central Nervous
System Infections - Chancroid - Chlamydia Infections - Chlamydiaceae
Infections - Cholera -
Clostridium Infections - Coccidioidomycosis - Corneal Ulcer - Cross Infection -
Cryptococcosis
- Dermatomycoses - Diphtheria - Ehrlichiosis - Empyema, Pleural -
Endocarditis, Bacterial -
Endophthalmitis - Enterocolitis, Pseudomembranous - Erysipelas - Escherichia
coli Infections -
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
22
Fasciitis, Necrotizing - Fournier Gangrene - Furunculosis - Fusobacterium
Infections - Gas
Gangrene - Gonorrhea - Gram-Negative Bacterial Infections - Gram-Positive
Bacterial
Infections - Granuloma Inguinale - Hidradenitis Suppurative - Histoplasmosis -
Hordeolum -
Impetigo - Klebsiella Infections - Legionellosis - Leprosy - Leptospirosis -
Listeria Infections -
.. Ludwig's Angina - Lung Abscess - Lyme Disease - Lymphogranuloma Venereum -
Maduromycosis - Melioidosis - Meningitis, Bacterial - Mycobacterium Infections
- Mycoplasma
Infections - Mycoses - Nocardia Infections - Onychomycosis - Osteomyelitis -
Paronychia -
Pelvic Inflammatory Disease - Plague - Pneumococcal Infections - Pseudomonas
Infections -
Psittacosis - Puerperal Infection - Q Fever - Rat-Bite Fever - Relapsing Fever
- Respiratory
Tract Infections - Retropharyngeal Abscess - Rheumatic Fever - Rhinoscleroma -
Rickettsia
Infections - Rocky Mountain Spotted Fever - Salmonella Infections - Scarlet
Fever - Scrub
Typhus - Sepsis - Sexually Transmitted Diseases, Bacterial - Sexually
Transmitted Diseases,
Bacterial - Shock, Septic - Skin Diseases, Bacterial - Skin Diseases,
Infectious -
Staphylococcal Infections - Streptococcal Infections - Syphilis - Syphilis,
Congenital - Tetanus -
Tick-Borne Diseases - Tinea - Tinea Versicolor - Trachoma - Tuberculosis -
Tuberculosis,
Spinal - Tularemia - Typhoid Fever - Typhus, Epidemic Louse-Borne - Urinary
Tract Infections
- Whipple Disease - Whooping Cough - Vibrio Infections - Yaws - Yersinia
Infections -
Zoonoses - Zygomycosis - Etc.
As used herein and unless otherwise stated, the term "solvate" includes any
combination
which may be formed by the RNA molecule(s) of this invention with a suitable
inorganic
solvent (e.g. hydrates) or organic solvent, such as but not limited to water
for injection,
hartmann's solution, PBS, 0,9% NaCl, serum free culture medium
Generally, for pharmaceutical use, the RNA molecule(s) of the invention may be
formulated as
a pharmaceutical preparation or pharmaceutical composition comprising at least
one RNA
molecule of the invention and at least one pharmaceutically acceptable
carrier, diluent or
excipient and/or adjuvant, and optionally one or more further pharmaceutically
active products.
.. By means of non-limiting examples, such a formulation may be in a form
suitable for oral
administration, for parenteral administration (such as by intralymphatic,
intratumoral,
intravenous, intramuscular or subcutaneous injection or intravenous infusion),
for topical
administration (including ocular), for administration by inhalation, by a skin
patch, by an
implant, by a suppository, etc.. Such suitable administration forms ¨ which
may be solid, semi-
solid or liquid, depending on the manner of administration ¨ as well as
methods and carriers,
diluents and excipients for use in the preparation thereof, will be clear to
the skilled person;
reference is again made to for instance US-A-6,372,778, US-A-6,369,086, US-A-
6,369,087
and US-A-6,372,733, as well as to the standard handbooks, such as the latest
edition of
Remington's Pharmaceutical Sciences.
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
23
Some preferred, but non-limiting examples of such preparations include
tablets, pills, powders,
lozenges, sachets, cachets, elixirs, suspensions, emulsions, solutions,
syrups, aerosols,
ointments, creams, lotions, soft and hard gelatin capsules, suppositories, eye
drops, sterile
injectable solutions and sterile packaged powders (which are usually
reconstituted prior to use)
for administration as a bolus and/or for continuous administration, which may
be formulated
with carriers, excipients, and diluents that are suitable per se for such
formulations, such as
lactose, dextrose, sucrose, sorbitol, mannitol, starches, gum acacia, calcium
phosphate,
alginates, tragacanth, gelatin, calcium silicate, microcrystalline cellulose,
polyvinylpyrrolidone,
polyethylene glycol, cellulose, (sterile) water, methylcellulose, methyl- and
propylhydroxybenzoates, talc, magnesium stearate, edible oils, vegetable oils
and mineral oils
or suitable mixtures thereof. The formulations can optionally contain other
pharmaceutically
active substances (which may or may not lead to a synergistic effect with the
products of the
invention) and other substances that are commonly used in pharmaceutical
formulations, such
as lubricating agents, wetting agents, emulsifying and suspending agents,
dispersing agents,
desintegrants, bulking agents, fillers, preserving agents, sweetening agents,
flavoring agents,
flow regulators, release agents, etc.. The compositions may also be formulated
so as to
provide rapid, sustained or delayed release of the active product(s) contained
therein, for
example using liposomes or hydrophilic polymeric matrices based on natural
gels or synthetic
polymers. In order to enhance the solubility and/or the stability of the
products of a
pharmaceutical composition according to the invention, it can be advantageous
to employ a-,
p- or y-cyclodextrins or their derivatives.
More in particular, the compositions may be formulated in a pharmaceutical
formulation
comprising a therapeutically effective amount of particles consisting of a
solid dispersion of the
products of the invention and one or more pharmaceutically acceptable water-
soluble
polymers.
The term "a solid dispersion" defines a system in a solid state (as opposed to
a liquid or
gaseous state) comprising at least two components, wherein one component is
dispersed
more or less evenly throughout the other component or components. When said
dispersion of
the components is such that the system is chemically and physically uniform or
homogenous
throughout or consists of one phase as defined in thermodynamics, such a solid
dispersion is
referred to as "a solid solution". Solid solutions are preferred physical
systems because the
components therein are usually readily bioavailable to the organisms to which
they are
administered.
It may further be convenient to formulate the products in the form of
nanoparticles which have
a surface modifier adsorbed on the surface thereof in an amount sufficient to
maintain an
effective average particle size of less than 1000 nm. Suitable surface
modifiers can preferably
CA 02929203 2016-04-29
WO 2015/071295
PCT/EP2014/074349
24
be selected from known organic and inorganic pharmaceutical excipients. Such
excipients
include various polymers, low molecular weight oligomers, natural products and
surfactants.
Preferred surface modifiers include nonionic and anionic surfactants.
Yet another interesting way of formulating the products according to the
invention involves a
pharmaceutical composition whereby the products are incorporated in
hydrophilic polymers
and applying this mixture as a coat film over many small beads, thus yielding
a composition
with good bio-availability which can conveniently be manufactured and which is
suitable for
preparing pharmaceutical dosage forms for oral administration. Materials
suitable for use as
.. cores in the beads are manifold, provided that said materials are
pharmaceutically acceptable
and have appropriate dimensions and firmness. Examples of such materials are
polymers,
inorganic substances, organic substances, and saccharides and derivatives
thereof.
The preparations may be prepared in a manner known per se, which usually
involves mixing at
.. least one product according to the invention with the one or more
pharmaceutically acceptable
carriers, and, if desired, in combination with other pharmaceutical active
products, when
necessary under aseptic conditions. Reference is again made to US-A-6,372,778,
US-A-
6,369,086, US-A-6,369,087 and US-A-6,372,733 and the further prior art
mentioned above, as
well as to the standard handbooks, such as the latest edition of Remington's
Pharmaceutical
Sciences.
The pharmaceutical preparations of the invention are preferably in a unit
dosage form, and
may be suitably packaged, for example in a box, blister, vial, bottle, sachet,
ampoule or in any
other suitable single-dose or multi-dose holder or container (which may be
properly labeled);
optionally with one or more leaflets containing product information and/or
instructions for use.
Generally, such unit dosages will contain between 0,1 and 1000 mg.
The products can be administered by a variety of routes including the
intralymphatic,
intratumoral, oral, rectal, ocular, transdermal, subcutaneous, intravenous,
intramuscular or
.. intranasal routes, depending mainly on the specific preparation used and
the condition to be
treated or prevented. The at least one product of the invention will generally
be administered in
an "effective amount", by which is meant any amount of a product that, upon
suitable
administration, is sufficient to achieve the desired therapeutic or
prophylactic effect in the
individual to which it is administered. Usually, depending on the condition to
be prevented or
treated and the route of administration, such an effective amount will usually
be between 0.01
to 1000 mg per kilogram body weight of the patient per day, which may be
administered as a
single daily dose, divided over one or more daily doses, or essentially
continuously, e.g. using
a drip infusion. The amount(s) to be administered, the route of administration
and the further
treatment regimen may be determined by the treating clinician, depending on
factors such as
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
the age, gender and general condition of the patient and the nature and
severity of the
disease/symptoms to be treated. Reference is again made to US-A-6,372,778,US-A-
6,369,086, US-A-6,369,087 and US-A-6,372,733 and the further prior art
mentioned above, as
well as to the standard handbooks, such as the latest edition of Remington's
Pharmaceutical
5 Sciences.
In accordance with the method of the present invention, said pharmaceutical
composition can
be administered separately at different times during the course of therapy or
concurrently in
divided or single combination forms. The present invention is therefore to be
understood as
10 embracing all such regimes of simultaneous or alternating treatment and
the term
"administering" is to be interpreted accordingly.
For an oral administration form, the compositions of the present invention can
be mixed with
suitable additives, such as excipients, stabilizers, or inert diluents, and
brought by means of
15 the customary methods into the suitable administration forms, such as
tablets, coated tablets,
hard capsules, aqueous, alcoholic, or oily solutions. Examples of suitable
inert carriers are
gum arabic, magnesia, magnesium carbonate, potassium phosphate, lactose,
glucose, or
starch, in particular, corn starch. In this case, the preparation can be
carried out both as dry
and as moist granules. Suitable oily excipients or solvents are vegetable or
animal oils, such
20 as sunflower oil or cod liver oil. Suitable solvents for aqueous or
alcoholic solutions are water,
ethanol, sugar solutions, or mixtures thereof. Polyethylene glycols and
polypropylene glycols
are also useful as further auxiliaries for other administration forms. As
immediate release
tablets, these compositions may contain microcrystalline cellulose, dicalcium
phosphate,
starch, magnesium stearate and lactose and/or other excipients, binders,
extenders,
25 disintegrants, diluents and lubricants known in the art.
When administered by nasal aerosol or inhalation, these compositions may be
prepared
according to techniques well-known in the art of pharmaceutical formulation
and may be
prepared as solutions in saline, employing benzyl alcohol or other suitable
preservatives,
absorption promoters to enhance bioavailability, fluorocarbons, and/or other
solubilizing or
dispersing agents known in the art. Suitable pharmaceutical formulations for
administration in
the form of aerosols or sprays are, for example, solutions, suspensions or
emulsions of the
products of the invention or their physiologically tolerable salts in a
pharmaceutically
acceptable solvent, such as ethanol or water, or a mixture of such solvents.
If required, the
formulation can also additionally contain other pharmaceutical auxiliaries
such as surfactants,
emulsifiers and stabilizers as well as a propellant.
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
26
For subcutaneous administration, the product according to the invention, if
desired with the
substances customary therefore such as solubilizers, emulsifiers or further
auxiliaries are
brought into solution, suspension, or emulsion. The products of the invention
can also be
lyophilized and the lyophilizates obtained used, for example, for the
production of injection or
infusion preparations. Suitable solvents are, for example, water,
physiological saline solution in
addition also sugar solutions such as glucose or mannitol solutions, or
alternatively mixtures of
the various solvents mentioned. The injectable solutions or suspensions may be
formulated
according to known art, using suitable non-toxic, parenterally-acceptable
diluents or solvents,
such as mannitol, water, Ringer's solution or isotonic sodium chloride
solution, or suitable
dispersing or wetting and suspending agents, such as sterile, bland, fixed
oils, including
synthetic mono- or diglycerides, and fatty acids, including oleic acid.
When rectally administered in the form of suppositories, these formulations
may be prepared
by mixing the products according to the invention with a suitable non-
irritating excipient, such
as cocoa butter, synthetic glyceride esters or polyethylene glycols, which are
solid at ordinary
temperatures, but liquefy and/or dissolve in the rectal cavity to release the
drug.
In preferred embodiments, the products and compositions of the invention are
used locally, for
instance topical or in both absorbed and non-adsorbed applications.
The compositions are of value in the veterinary field, which for the purposes
herein not only
includes the prevention and/or treatment of diseases in animals, but also ¨
for economically
important animals such as cattle, pigs, sheep, chicken, fish, etc. ¨ enhancing
the growth
and/or weight of the animal and/or the amount and/or the quality of the meat
or other products
obtained from the animal. Thus, in a further aspect, the invention relates to
a composition for
veterinary use that contains at least one product of the invention and at
least one suitable
carrier (i.e. a carrier suitable for veterinary use). The invention also
relates to the use of a
product of the invention in the preparation of such a composition.
EXAMPLES
General material and methods
In vitro experiments: generation of monocyte derived DCs
Peripheral blood mononuclear cells (PBMC) were used as a source of DC
precursors and
isolated from leukapheresis products. Clinical grade DCs were generated in
vitro from the
plastic adherent fraction as follows. On day 0, PBMC were plated at a density
of 10x106
cells/mL in medium suitable for haematopoietic cell culture supplemented with
2% autologous
plasma (AP). The cells were left for 2h to allow plastic adherence of the
monocytes at 37 C.
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
27
Non-adherent cells were removed by washing, and the adherent cells were
cultured in medium
supplemented with 1% AP, 1,000 U/mL GM-CSF and 500 U/mL 1L-4 in the Cell
Factory. On
day 2 and 4, medium containing the cytokine amount of day 0 was added to the
DC culture.
On day 6 of DC culture, the cells were harvested and cryopreserved.
In vitro experiments: electroporation of DCs
On day 6, 4 ¨ 8 x 106 DCs were electroporated with mRNA as indicated. Before
electroporation, the DCs were washed twice, first with PBS without supplements
and secondly
with reduced serum medium without phenol red. After the second wash step, the
DCs were
resuspended in a final volume of 200p1 of reduced serum medium containing the
mRNA.
Electroporation was performed in a 4-mm gap electroporation cuvette. An
exponential decay
pulse was used with the following conditions: voltage, 300V; capacitance,
150pF, and
resistance, co52, resulting in a pulse time of 11 ms. Immediately after
electroporation, the DCs
were diluted in medium supplemented with 1% huAB serum and PS/L-GLU and
incubated at
37 C in a humidified 5% CO2 atmosphere. No additional cytokines were added to
the DCs
after electroporation.
In vivo experiments: mice
Female, 6 to 12 weeks old DBA/2 mice.
In vivo experiments: mouse cell lines
The mastocytoma cell line P815 was obtained from C. Uyttenhove (Universite
Catholique de
Louvain, Louvain-La-Neuve, Belgium).
In vivo experiments: tumor cell inoculation and in situ delivery of mRNA
In order to grow palpable tumors, mice were injected with 5x106 P815 tumor
cells
subcutaneously at both flanks as indicated in the experiment. For intratumoral
delivery of
mRNA, mice were anesthetized with lsoflurane (Abbott). Tumors were injected
with a mixture
containing 10 pg of each TriMix mRNA component in a final volume of 50 pl 0.8
Hartmann's
solution/injected tumor when they reached a volume of about 100 mm3. The same
amount of
mRNA was used between the different groups. mRNA encoding tNGFR produced from
a
pGEM vector served as a control.
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
28
Example 1
Specific material and methods
iDCs generation and electroporation are performed as described in the general
material and
methods part above. iDCs were electroporated with 5pg of each component of
TriMix to allow
maturation of the DCs. All flow cytometric stainings were performed in
PBS/BSA/azide. To
analyze the expression of CD70, anti CD70-fluorescein isothiocyanate (F ITC)
was used. Data
acquisition was performed on a FACSFortessa flow cytometer (BD) and analyzed
using FACS
Diva software.
Result:
Twenty-four hours after electroporation, DCs were stained for their CD70
surface expression.
These results show that after electroporation of iDCs with TriMix, the
intensity of CD70
expression - mean fluorescence intensity (MFI) - is significantly higher after
electroporation
with TriMix encoded by the pUC TE ENE plasmid (SEQ ID N 5) containing both
regulatory
elements (TE + ENE), when compared to the pUC-vector (basis of the pUC TE ENE
plasmid),
the pUC TE-vector and the pUC ENE-vector. CD70 expression after
electroporation with
TriMix encoded by the pUC TE ENE plasmid is significantly higher, whereas both
the use of
pUC TE and pUC ENE result in a reduced or at maximum equal levels of CD70
expression
compared to pUC lacking these elements (figure 1). Hence, the presence of both
elements (TE
and ENE) in the vector appear to have an unexpected synergistic effect in
terms of increasing
CD70 expression after electroporation with TriMix encoded by the pUC TE ENE
plasmid.
Example 2
Specific material & methods:
iDCs generation and electroporation conditions are performed as described in
the general
material and methods part above. iDCs were electroporated with 20pg WT1
encoding mRNA
to allow antigen loading. To analyze intracellular WT1 expression, cells were
fixed and
permeabilized, and stained intracellularly with an anti-WT1 monoclonal
antibody (clone 6F-H2;
Dako Cytomation, Carpinteria, CA). An IgG isotype-matched PE-labeled anti-
mouse antibody
was used as secondary Ab (Becton&Dickinson, Erembodegem, Belgium). Non-
reactive
isotype-matched antibody (eBioscience, Vienna, Austria) was used as control.
Data
acquisition was performed on a FACSFortessa flow cytometer (BD) and analyzed
using FAGS
Diva software.
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
29
Result:
These results show that after electroporation of iDC with WTI encoded by the
pUC TE ENE-
vector, a more prolonged WT1 expression is observed when compared to the other
WT1
mRNA-encoding vectors (figure 2). These data nicely demonstrate the different
ways of action
of both 5'TE and 3'ENE segments. While the expression of mRNA from the pUC-TE
vector is
high after 4 hours, it deteriorates rapidly. Translation from the ENE
containing RNA is lower
than all others during the entire period. The pUC TE ENE-vector has the high
translatability of
the TE and the long-lived effect of the ENE sequence. The expression level of
WT1 diminishes
at a significantly slower rate than in the other vectors.
Example 3
Specific material & methods:
iDCs generation and electroporation conditions are performed as described in
the general
material and methods part above. iDCs were co-electroporated with 5pg eGFP
encoding
mRNA and TriMix (5pg of each component) to allow antigen loading and
maturation of the
DCs. eGFP expression was assessed by flow cytometry at several time points.
Result:
eGFP expression was followed-up at several time points after electroporation
(figure 3).
These results show that the expression level of eGFP from both vectors is
comparable 4hours
after electroporation. However on later time points it is clear that
expression from pUC TE ENE
derived mRNA is significantly higher. Again this points at a more stable and
prolonged
expression of the transgene.
Example 4
Specific material & methods:
iDCs generation and electroporation conditions are performed as described
above. iDCs were
electroporated with 5pg of each component of TriMix to allow maturation of the
DCs. All flow
cytometric stainings were performed in PBS/BSA/azide. To analyze the
expression of surface
molecules on the cell surface of the DCs, the following monoclonal antibodies
were used:
CD40-APC (Allophycocyanin), CD7O-F ITC, CD8O-PE, CD83-PE (Phycoerythrin), 0D86-
PE
and CCR7-APC. Data acquisition was performed on a FACS Fortessa flow cytometer
(BD)
and analyzed using FACS Diva software.
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
Result:
Electroporation of iDCs with TriMix mRNA encoded by the pUC TE ENE-vector is
able to
induce maturation of the DCs (figure 4).
5
Example 5: Two-side tumor model with P815: single treatment of one tumor
Specific material & methods:
In order to grow palpable tumors, mice were inoculated with 5x105 P815 tumor
cells
10 subcutaneously at both flanks. Therapy was started when both tumors
reached an injectable
volume of about 100 mm3. By using a two-side tumor model in which only one
tumor was
treated, we aimed to evaluate the systemic effect of the vaccination strategy.
Therefore, only
the left tumor was injected with either control mRNA or pUC TE ENE TriMix mRNA
(10 pg of
each mRNA component) dissolved in 0.8x Hartmann's solution. The systemic anti-
tumor
15 immune response was evaluated by measuring the size of both treated and
non-treated,
contralateral tumor and by survival.
Result:
By using a two-side tumor model, we could evaluate the systemic effect of the
immunization
20 strategy. Single intratumoral delivery of pUC TE ENE TriMix mRNA
resulted in a significantly
reduced tumor growth of both treated and non-treated contralateral tumor
(figure 5). The effect
of vaccination on the distant tumor could be an indication that a single
intratumoral TriMix
injection could be used to treat multiple tumor lesions.
Example 6: Two-side tumor model with P815: single treatment of one tumor,
Hartmann
solution and tNGFR as control
Specific material & methods:
In order to grow palpable tumors, mice were inoculated with 5x105 P815 tumor
cells
subcutaneously at both flanks. Therapy was started when both tumors reached an
injectable
volume of about 100 mm3. By using a two-side tumor model by which only one
tumor was
treated, we aimed to evaluate the systemic effect of the vaccination strategy.
Therefore, only
the left tumor was injected with either Vehicle (0.8 Hartmann's solution),
control mRNA or pUC
TE ENE TriMix mRNA (10 pg of each mRNA component) dissolved in 0.8x Hartmann's
solution, all in a total volume of 50p1/injected tumor. The systemic anti-
tumor immune response
was evaluated by measuring the size of both treated and non-treated,
contralateral tumor and
by survival.
CA 02929203 2016-04-29
WO 2015/071295
PCT/EP2014/074349
31
Result:
By using a two-side tumor model, we could evaluate the systemic effect of the
immunization
strategy. This experiment confirms the previous observations, i.e.
1. Single intratumoral delivery of pUC TE ENE TriMix mRNA resulted in a
significantly
reduced tumor growth of both treated and non-treated contralateral tumor.
2. Single intratumoral devlivery of pUC TE ENE TriMix mRNA resulted in a
prolonged
survival of tumor-bearing mice
3. The effect of vaccination was more pronounced on treated tumors.
Additionally, by taking along a group by which tumors were treated with
vehicle, we could
show the adjuvant effect of mRNA itself.
Example 7: Comparison of different TE sequences
Specific material & methods:
Details regarding the specific assay methods in relation hereto may be found
in examples 2
and 3 as described above.
Result: electroporation of iDC with WTI or eGFP
Electroporation of iDC with WT1 (Fig. 9A) or eGFP (Fig. 9B) encoded by the pUC
TE ENE-
vectors either comprising a TE element represented by SEQ ID N 1, SEQ ID N 2
or SEQ ID
N 3 did not result in a significant difference of WT1 or eGFP expression
respectively.
These data clearly indicate that sequences having at least 80% sequence
identity to SEQ ID
N 1, such as for example SEQ ID N 1, SEQ ID N 2 or SEQ ID N 3, could be used
interchangeably as the translation enhancer element in the vectors according
to this invention.
CA 02929203 2016-04-29
WO 2015/071295 PCT/EP2014/074349
32
REFERENCES
Bonehill A, Tuyaerts S, Van Nuffel AM, Heirman C, Bos TJ, Fostier K, Neyns B,
Thielemans K
- Enhancing the T-cell stimulatory capacity of human dendritic cells by co-
electroporation with
CD4OL, CD70 and constitutively active TLR4 encoding mRNA. - Mol Ther. 2008
Jun;
16(6):1170-80.
Conrad NK, Steitz JA - A Kaposi's sarcoma virus RNA element that increases the
nuclear
abundance of intronless transcripts. - EMBO J. 2005 May 18;24(10):1831-41.
Conrad NK, Mili S, Marshall EL, Shu MD, Steitz JA - Identification of a rapid
mammalian
deadenylation-dependent decay pathway and its inhibition by a viral RNA
element. Mol
Ce11.2006;24:943-953.
Diken M, Kreiter S, Selmi A, Britten CM, Huber C, Tureci 0, Sahin U -
Selective uptake of
naked vaccine RNA by dendritic cells is driven by macropinocytosis and
abrogated upon DC
maturation_ - Gene Ther. 2011 Jul; 18(7)102-8.
Fotin-Mleczek M, Duchardt KM, Lorenz C, Pfeiffer R, Ojkio-Zrna S, Probst J,
Kallen KJ -
Messenger RNA-based vaccines with dual activity induce balanced TLR-7
dependent adaptive
immune responses and provide antitumor activity. - J Immunother. 2011
Jan;34(1):1-15.
Hu MC, Tranque P, Edelman GM, Mauro VP. - rRNA-complementarity in the 5'
untranslated
region of mRNA specifying the Gtx homeodomain protein: evidence that base-
pairing to 18S
rRNA affects translational efficiency. - Proc Nati Acad Sci U S A. 1999 Feb
16;96(4):1339-44.
Mitton-Fry RM, DeGregorio SJ, Wang J, Steitz TA, Steitz JA. - Poly(A) tail
recognition by a
viral RNA element through assembly of a triple helix. - Science. 2010;330:1244-
1247.
Sun R, Lin SF, Gradoville L, Miller G. - Polyadenylylated nuclear RNA encoded
by Kaposi
sarcoma-associated herpesvirus. - Proc Natl Acad Sci USA. 1996;93:11883-11888.
Van Lint Sandra, Goyvaerts Cleo, Maenhout Sarah, et al. - Preclinical
Evaluation of TriMix and
Antigen mRNA-Based Antitumor Therapy - Cancer Res 2012;72:1661-1671.