Note: Descriptions are shown in the official language in which they were submitted.
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
DETECTION OF NEUROLOGICAL DISEASES VIA MEASUREMENT OF
NEUROMELANIN IN RECIRCULATING PHAGOCYTES
CROSS REFERENCE
[0001] This application claims priority to U.S. Provisional Patent Application
No.
61/722,441, filed November 5, 2012, the specification(s) of which is/are
incorporated herein in their entirety by reference.
BACKGROUND OF THE INVENTION
100021 In general, when tissue damage occurs, it incites inflammation, which
usually aids in wound healing. For example, one of the normal functions of
inflammation is to recruit phagocytes to clear away the cellular debris and
prepare
the injured site for repair and rebuilding. These phagocytes may be resident
in the
brain (e.g., dendritic cells, microglial cells) or recruited from the blood
stream (e.g.,
monocytes). Cells that engulf debris are thought to enter the brain by
crossing the
blood-brain barrier but are not believed to return to the blood stream. For
example, when phagocytes engulf tissue debris and exit the tissue, it is
thought to
be via the lymph notes.
100031 We have surprisingly discovered that debris-laden phagocytes may re-
enter the blood stream and if sufficient numbers are present, it may be
possible to
measure the debris that is inside the phagocytic cells. For example, we have
found that the CNS antigens Tau and Hippocalcin like-1 are present in PBMC
preparations at a frequency that is statistically different from apparently
healthy
controls. Examining the cargo (e.g., the debris that would only normally be
found
in nerve cells) of these recirculating phagocytes is termed "Window into the
Brain."
This technique may provide close to real-time data on what is happening in the
brain since that particular cargo may only be present in the recirculating
phagocytes for a few days before it is completely digested. The present
invention
features methods of detecting various diseases by examining the debris present
in
such recirculating phagocytes.
100041 The present invention also features methods for detecting Parkinson's
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
disease by the detection of melanin (e.g., neuromelanin, e.g., neuromelanin
from
neurons of substantia nigra) and other neuronal antigens in recirculating
phagocytes.
10005] Any feature or combination of features described herein are included
within the scope of the present invention provided that the features included
in any
such combination are not mutually inconsistent as will be apparent from the
context, this specification, and the knowledge of one of ordinary skill in the
art.
Additional advantages and aspects of the present invention are apparent in the
following detailed description and claims.
SUMMARY OF THE INVENTION
100061 The present invention features a method of detecting Parkinson's
disease
in a mammal. In some embodiments, the method comprises detecting a level of a
biomarker associated with Parkinson's disease in a first sample from outside a
brain tissue of the mammal, the first sample comprising a first circulating
phagocyte; and comparing the level of the biomarker in the first sample with a
level of the biomarker in a second sample, the second sample being either (i)
a
control sample or (ii) a second sample from outside of a brain tissue, the
second
sample comprising a second circulating phagocyte, the second sample being
collected prior to the first fluid sample, wherein if the level of the
biomarker in the
first sample is higher than that of the second sample then Parkinson's disease
is
detected.
100071 In some embodiments, the sample is derived from blood, peripheral blood
mononuclear cells (PBMCs), cerebrospinal fluid (CSF), synovial fluid, cystic
fluid,
lymph fluid, ascites, pleural effusion, interstitial fluid, ocular fluids,
vitreal fluid,
urine, the like, or a combination thereof. In some embodiments, the biomarker
associated with Parkinson's disease comprises neuromelanin or a fragment
thereof. In some embodiments, the circulating phagocyte includes a monocyte, a
macrophage, a lymphocyte, or a combination thereof. In some embodiments,
detecting the biomarker comprises subjecting the first sample and the second
sample each to a peptide that binds to neuromelanin. In some embodiments, the
peptide that binds to neuromelanin comprises 4B4 (SEQ ID NO:1A).
2
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
100081 The present invention also features a kit for detecting Parkinson's
disease, said kit comprising a 484 peptide (SEC) ID NO:1A), the 4B4 peptide is
for
detecting neuromelanin in a recirculating phagocyte. In some embodiments, the
4B4 peptide comprises a label. In some embodiments, the label comprises
biotin.
[00091 The present invention also features the use of a system for detecting
Parkinson's disease. In some embodiments, the system comprises a
neuromelanin-binding peptide for binding to neuromelanin, the neuromelanin-
binding peptide is incubated in a first sample comprising a first circulating
phagocyte from outside of a brain tissue and a second sample comprising a
control sample, wherein if the level of neuromelanin detected in the first
sample
via the neuromelanin-binding peptide is higher than the level of neuromelanin
detected in the second sample via the neuromelanin-binding peptide then
Parkinson's disease is detected. In some embodiments, the neuromelanin-binding
peptide comprises 484 (SEQ ID NO:1A). In some embodiments, the first sample
is derived from blood. In some embodiments, the first sample comprises PBMCs.
10010] The present invention also features a system for detecting Parkinson's
disease, wherein the system comprises a neuromelanin-binding peptide for
binding to neuromelanin, the neuromelanin-binding peptide is incubated in a
first
sample comprising a first circulating phagocyte from outside of a brain tissue
and
a second sample comprising a control sample, wherein if the level of
neuromelanin detected in the first sample via the neuromelanin-binding peptide
is
higher than the level of neuromelanin detected in the second sample via the
neuromelanin-binding peptide then Parkinson's disease is detected. In some
embodiments, the neuromelanin-binding peptide comprises 484 (SEQ ID NO:1A).
In some embodiments, the first sample is derived from blood. In some
embodiments, the first sample comprises PBMCs.
[0011] The present invention also features a method of determining status of
Parkinson's disease. In some embodiments, method comprises detecting a level
of a biomarker associated with Parkinson's disease in a first fluid sample
from
outside a brain tissue of the mammal, the first fluid sample comprising a
first
3
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
circulating phagocyte; comparing the level of the biomarker in the first
sample with
a level of the biomarker in a second sample, the second sample being either
(i) a
control sample or (ii) a second fluid sample from outside of a brain tissue,
the
second fluid sample comprising a second circulating phagocyte, the second
fluid
sample being collected prior to the first fluid sample.
[0012] In some embodiments, if the biomarker level in the first sample is the
same as the level of the biomarker in the second sample or in the control
sample,
then Parkinson's disease activity is the same. In some embodiments, if the
biomarker level in the first sample is higher than the level of the biomarker
in the
second sample or in the control sample, then Parkinson's disease activity is
increased in the first sample. In some embodiments, if the biomarker level in
the
first sample is lower than the level of the biomarker in the second sample or
in the
control sample, then Parkinson's disease activity is decreased in the first
sample.
100131 In some embodiments, the sample is derived from blood, peripheral blood
mononuclear cells (PBMCs), cerebrospinal fluid (CSF), synovial fluid, cystic
fluid,
lymph fluid, ascites, pleural effusion, interstitial fluid, ocular fluids,
vitreal fluid,
urine, the like, or a combination thereof. In some embodiments, the
circulating
phagocyte includes a monocyte, a macrophage, a lymphocyte, or a combination
thereof. In some embodiments, the biomarker comprises neuromelanin or a
fragment thereof.
10014] The present invention also features a method of detecting neuromelanin.
In some embodiments, the method comprises introducing a neuromelanin binding
protein comprising a labeled 4B4 peptide (SEQ ID NO:1A) to a sample; and
detecting the label on the 4B4 peptide. In some embodiments, the sample
comprises a circulating phagocyte. In some embodiments, the sample comprises
a circulating phagocyte derived from serum, plasma, peripheral blood
mononuclear cells (PBMCs), cerebrospinal fluid (CSF), synovial fluid, cystic
fluid,
lymph fluid, ascites, pleural effusion, interstitial fluid, ocular fluids,
vitreal fluid, or a
combination thereof. In some embodiments, the label comprises an enzyme. In
some embodiments, the label comprises biotin. In some embodiments, the
enzyme comprises horseradish peroxidase.
4
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
100151 The present invention also features a method of detecting Parkinson's
disease in a patient. In some embodiments, the method comprises obtaining from
a patient a fluid sample from outside of a brain tissue of the patient, the
fluid
sample comprises peripheral blood mononuclear cells (PBMCs); and detecting
neuromelanin in the fluid sample, wherein when neuromelanin is detected then
Parkinson's disease is detected in the patient. In some embodiments, the fluid
sample comprises a circulating phagocyte. In some embodiments, the circulating
phagocyte includes a monocyte, a macrophage, or a lymphocyte.
BRIEF DESCRIPTION OF THE DRAWINGS
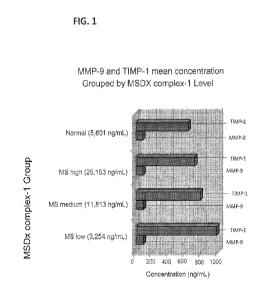
100161 FIG. 1: Groups of plasma samples from multiple sclerosis (MS) patients
that had low, medium or high levels of MSDx complex-1 were selected and then
MMP-9 was measured. FIG. 1 shows the MMP-9 level was elevated in MS
patients relative to normal subjects (p=0.01-0.006) and did not vary by MSDx
complex-1 level in MS subjects (p=not significant). TIMP-1 levels were higher
in
subjects with low MSDx complex -1 levels (consistent with low proteolytic
activity
and potentially lower levels of leukocyte invasion and disease activity). MS
subjects with high MSDX complex-1 level had lower levels of TIMP-1 (consistent
with higher proteolytic activity and potentially higher levels of leukocyte
invasion
and disease activity; TIMP-1 in MSDx complex-1 high vs. Low p=0.0033).
100171 FIG. 2: Image (a) on the left shows neuromelanin-containing
dopaminergic neurons in the human substantia nigra revealed by the Masson-
Fontana stain. Image (b) on the right shows neuromelanin-containing
dopaminergic neurons in the human substantia nigra revealed by the 4B4 peptide
binding (4B4 peptide binds to neuromelanin in substantia nigra tissue
sections).
[0018] FIG. 3 shows the binding of the 4B4 peptide to neuromelanin in extracts
of
retinal pigment epithelium immobilized on ELISA plates in two-fold dilution
series.
DESCRIPTION OF PREFERRED EMBODIMENTS
100191 Referring now to FIG. 1-3, the present invention features the detection
and/or the monitoring of diseases, e.g., neurodegenerative diseases,
Parkinson's
disease, etc., by analysis of phagocylosed central nervous system (CNS) debris
within phagocytes that have re-entered the blood circulation (e.g.,
recirculating
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
phagocytes).
MSDX COMPLEX-I
100201 MSDx complex-1 comprises Fibrinogen, Fibulin-1 and Fibronectin.
Fibronectin and Fibulin-1 are basement membrane proteins, suggesting that the
circulating complex may be generated as a consequence of leukocyte
transmigration into target tissues. Transmigration of leukocytes is mediated
by the
enzyme activity of matrix metalloproteinases (MMPs).
10021] FIG. 1 shows that in plasma samples of Multiple Sclerosis patients,
levels
of MSDx complex-1 may be indirectly related to TIMP-1 levels (TIMPs are tissue
inhibitors of metalloproteinases). For example, higher levels of TIMP-1 (a
specific
inhibitor of MMP-9) may be associated with lower activity of MMP-9 and lower
level of MSDx complex-1 (see FIG. 1). Conversely, a lower level of TIMP-1 may
be associated with a higher level of Mfv1P-9 activity and level of MSDx
complex-1.
Intermediate levels of TIEV1P-1 correlate with intermediate levels of MSDx
complex-
1.
100221 Without wishing to limit the present invention to any theory or
mechanism,
it is believed that the data suggests that MSDx complex-1 may be generated by
cell transmigration into tissues. For example, MSDx complex-1 may be generated
by proteolytic activity of leukocytes (or other cell types) crossing the blood
vessel
wall and tissue barriers in order to enter the target organ.
100231 Further, a disease that is characterized by movement of leukocytes (or
other cell types) into tissues can be monitored by the measurement of MSDx
complex-1. Diseases that may be monitored or detected by measurement of
MSDx complex-1 include but are not limited to: autoimmune diseases, e.g.,
multiple sclerosis, rheumatoid arthritis, lupus, sjogren's syndrome,
thyroiditis,
uveitis. Crohn's disease, ulcerative colitis, psoriasis, type 1 diabetes
mellitus,
autoimmune Addison's disease, autoimmune hepatitis, celiac disease,
pemphigous, chronic inflammatory demyelinating polyneuropathy, acute
disseminated encephalomyelopathy, sarcoidosis, dermatomyositis and behcet's
disease; neurological diseases, e.g., stroke, concussion, chronic traumatic
6
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
encephalopathy, neuromyelitis optica, transverse myelitis, intractable
epilepsy and
CNS infections; Parkinson's disease; primary tumor growth, metastasis of
tumors;
etc.
WINDOW INTO THE BRAIN
100241 Various debris antigens may be found in recirculating phagocytes in the
peripheral blood. Such debris antigens may be used to detect (or monitor)
neurodegenerative or neuroinflammatory diseases (e.g., diseases as described
above). Antigens include but are not limited to: a Tau protein (or fragment
thereof), a Tau protein or fragment thereof comprising a phosphorylated
residue
(e.g., a phosphorylated serine reside, a phosphorylated threonine reside;
e.g.,
serine 214, serine 235, serine 262, serine 356, serine 396, serine 404, serine
413,
serine 46, serine 515, serine 516, serine 519, serine 531, serine 552, serine
610,
serine 622, serine 641, serine 713, serine 721, serine 726, serine 730, serine
739,
threonine 181, threonine 205, threonine 470, threonine 492, threonine 498,
threonine 522, threonine 529, threonine 534, threonine 548; a protein or a
fragment thereof selected from the group consisting of UCHL-1, neuromelanin,
neuroglobin, valosin-containing protein, brain hexokinase, hippocalcin-1,
nestin,
synaptotagmin, myelin associated glycoprotein, transketolase, NS1 associated
protein 1, major vault protein, synaptojanin, enolase, alpha synuclein, glial
fibrillary
acidic protein, S-100 protein, Neu-N, 265 proteasome subunit 9, annexin A2,
annexin A3, annexin AS, annexin A6, annexin All, ubiquitin activating enzyme
ZE1, ubiquitin B precursor, vimentin, glyceraldehyde-3-
phosphate
dehydrogenase, 13-3-3 protein, 14-3-3 protein (e.g., zeta isoform), NOGO-A.
[0025] Another debris antigen that may be found in recirculating phagocytes in
the peripheral blood may include neuromelanin (or a fragment thereof).
Neuromelanin may be used to detect Parkinson's disease. For example,
neuromelanin may be detected in the debris of degenerated dopaminergic
neurons (by recirculating phagocytes).
[0026] Neuromelanin can be measured in several ways, e.g., via the binding of
labeled melanin selective peptides (e.g., 4B4 peptide (SEQ ID NO:1A), e.g.,
biotinylated 4B4 peptide, a control peptide P601G (DGASYSWMYGA (SEQ ID
7
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
NO:2A)) may be used as a control); the binding of monoclonal or polyclonal
antibodies to melanin; measurement of metal binding to melanin; measurement of
the semiconductor properties of melanin; measurement of the fluorescence
properties of melanin; and extraction of melanin from recirculating phagocytes
and
subsequent quantification of melanin, its components or adducts (both natural
or
synthetic); physical methods such as gas chromatography, liquid chromatography
or mass spectrometry; and combinations of these methods. As shown in FIG. 2,
the 4B4 peptide of sequence YERKFWHGRH (SEQ ID NO:1A) binds to
neuromelanin granules in the dopaminergic neurons of the human substantia
nigra.
[0027] As an example of a means of measurement of melanin within cells, FIG.,
3 shows the binding of the 4B4 peptide. Extracts of retinal pigment epithelium
were immobilized on ELISA plates in two-fold dilution series of the extract
and
incubated with biotinylated 4B4 peptide. Unbound peptide was washed off and
bound peptide was detected with streptavidin-HRP. In comparison, PBMCs from
healthy human subjects show little binding of 4B4 peptide.
[0028] In some embodiments, more than one biomarker is detected in the
sample(s). In some embodiments, the biomarker(s) is a neural¨derived
biomarker.
However, the biomarker(s) is not limited to neural-derived biomarkers. In some
embodiments, one or more biomarkers are detected in the sample, wherein the
biomarkers are neural-derived, non-neural-derived biomarkers, or a combination
thereof.
[0001] As used herein, the term "peripheral" refers to anything outside of
brain
tissue. For example, a peripheral phagocyte may be obtained from cerebrospinal
fluid (CSF). Phagocytes may include monocytes, macrophages, and/or
lymphocytes. Such circulating phagocytes may be found in tissues, cells,
and/or
fluids in the body, for example in blood, peripheral blood mononuclear cells
(PBMCs), synovial fluid, cerebrospinal fluid (CSF), central nervous system
tissues, synovial fluid, cystic fluid, lymph fluid, ascites, pleural effusion,
interstitial
fluid, ocular fluids, vitreal fluid, urine the like, or a combination thereof.
In some
embodiments, the biomarker is an intracellular component. For example, the
8
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
biomarker may be obtained from within a macrophage. In some embodiments,
the macrophage sample is permeabilized. In some embodiments, the
macrophage is lysed via various means, e.g., hypotonic solution treatment,
detergent solution treatment, mechanical stress, etc.
DETECTABLE DISEASES AND BIOMARKERS FOR DETECTION
[0029] As previously discussed, the present invention features the detection
of
and/or the monitoring of various diseases via detection/measurement of various
biomarkers in recirculating phagocytes.
100301 For example, in some embodiments, the a disease detected or monitored
includes (but is not limited to): autoimmune diseases, e.g., multiple
sclerosis,
rheumatoid arthritis, lupus, sjogren's syndrome, thyroiditis, uveitis, Crohn's
disease, ulcerative colitis, psoriasis, type 1 diabetes mellitus, autoimmune
addison's disease, autoimmune hepatitis, celiac disease, pemphigous, chronic
inflammatory demyelinating polyneuropathy, acute disseminated
encephalomyelopathy, sarcoidosis, dermatomyositis and behcet's disease;
neurological diseases, e.g., stroke, concussion, chronic traumatic
encephalopathy, neuromyelitis optica, transverse myelitis, intractable
epilepsy and
CNS infections; Parkinson's disease; primary tumor growth, metastasis of
tumors;
etc.
[0031] In some embodiments, a biomarker detected or measured in recirculating
phagocytes includes (but is not limited to): Neuromelanin (or a fragment
thereof);
a Tau protein (or fragment thereof), a Tau protein or fragment thereof
comprising
a phosphorylated residue (e.g., a phosphorylated serine reside, a
phosphorylated
threonine reside; e.g., serine 214, serine 235, serine 262, serine 356, serine
396,
serine 404, serine 413, serine 46, serine 515, serine 516, serine 519, serine
531,
serine 552, serine 610, serine 622, serine 641, serine 713, serine 721, serine
726,
serine 730, serine 739, threonine 181, threonine 205, threonine 470, threonine
492, threonine 498, threonine 522, threonine 529, threonine 534, threonine
548; a
protein or a fragment thereof selected from the group consisting of UCHL-1,
neuromelanin, neuroglobin, valosin-containing protein, brain hexokinase,
hippocalcin-1, nestin, synaptotagmin, myelin associated glycoprotein,
9
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
transketolase, NS1 associated protein 1, major vault protein, synaptojanin,
enolase, alpha synuclein, glial fibrillary acidic protein, S-100 protein, Neu-
N, 26S
proteasome subunit 9, annexin A2, annexin A3, annexin A5, annexin A6, annexin
All, ubiquitin activating enzyme ZE1, ubiquitin B precursor, vimentin,
glyceraldehyde-3-phosphate dehydrogenase, 13-3-3 protein, 14-3-3 protein
(e.g.,
zeta isoform), NOGO-A, Ubiquitin carboxy-terminal hydrolase Li (UCHL1) also
known as PARKS protein; neuronal-specific protein gene product 9.5; SwissProt
P09936; proteolipid protein; myelin oligodendrocyte glycoprotein.
100321 Table A shows non-limiting examples of biomarkers that may be
associated with disease states (e.g., degenerative disease states) with
various
organs. For example, the present invention may be used to detect a diabetes
condition by detecting somatostatin in a similar manner as described herein,
e.g.,
similar to methods for detecting neuromelanin for Parkinson's disease.
100331 Table A
Damaged Organ
=
=
Associated Markers
Joints (eg Rheumatoid Arthritis) Citrulinated proteins
Carbamylated proteins
Articular cartilage degradation products
Central Nervous System (eg multiple Neuromelanin (or a fragment thereof); a
Tau
sclerosis, Parkinsons disease, Alzheimer's protein (or fragment thereof), a
Tau protein or
disease etc). fragment thereof comprising a
phosphorylated
residue (e.g., a phosphorylated serine reside, a
phosphorylated threonine reside; e.g., serine
214, serine 235, serine 262, serine 356, serine
396, serine 404, serine 413, serine 46, serine
515, serine 516, serine 519, serine 531, serine
552, serine 610, serine 622, serine 641, serine
713, serine 721, serine 726, serine 730, serine
739, threonine 181, threonine 205, threonine
470, threonine 492, threonine 498, threonine
522. threonine 529, threonine 534, threonine
548; a protein or a fragment thereof selected
from the group consisting of UCHL-1,
neuromelanin. neuroglobin, valosin-containing
protein, brain .hexokinase, hippocalcin-1, nestin,
synaptotagmin, myelin associated glycoprotein,
transketolase, NS1 associated protein 1, major
vault protein, synaptojanin, enolase, alpha
synuclein, glial fibrillary acidic protein, 5-100
protein, Neu-N, 265 proteasome subunit 9,
annexin A2, annexin A3, annexin AS, annexin
A6, annexin All, ubiquitin activating enzyme
ZE1, ubiquitin B precursor, vimentin,
glyceraldehyde-3-phosphate dehydrogenase,
13-3-3 protein, 14-3-3 protein (e.g., zeta
isoform), NOGO-A, Ubiquitin carboxy-terminal
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
hydrolase Li (UCHL1) also known as PARK5
protein; neuronal-specific protein gene product
9.5; SwissProt P09936; proteolipid protein;
myelin oligodendrocyte!ycoprotein.
Thyroid (eg Graves disease, Hashimotos Thyroglobulin
thyroiditis) Thyroid peroxidase
Retina (eg macular degeneration, retinitis Rhodopsin
pigmentosa)
Pancreatic islets (Diabetes) Insulin, Glucagon, somatostatin,
pancreatic poiypeptide
Inflammatory bowel disease Microbial glycans
Lung Surfactant proteins A-C)
Sjogrens Syndrome Salivary proiine rich proteins
severe traumatic brain injury patients all-spectrin breakdown products
(SBDPs)
METHODS OF DETECTING A NEUROLOGICAL CONDITION VIA ANALYSIS
OF CIRCULATING PHAGOCYTES
[00341 Multiple Sclerosis (MS) is predominantly a disease of women of northern
European origin and afflicts up to three million people worldwide. In the
United
States it is estimated that 400,000 people are affected. It is thought to be
an
autoimmune disorder and typically strikes young adults, causing a wide variety
of
symptoms that are often mistaken for other diseases. These symptoms stem from
disruption of the central nervous system (CNS) and may include blurred or
double
vision; weakness in the arms or legs; changes or difficulties in balance,
coordination and gait; bladder and/or bowel dysfunction; and emotional
disturbances. Each patient may present a little differently and there may have
been episodes in the past that were barely noticed by the patient at the time.
it is
difficult to firmly diagnose MS, especially if there has been only one
symptomatic
episode. This leaves patients and their doctors waiting months or years for a
relapse to confirm that the symptoms are due to MS.
100351 MS is a demyelinating disease, where myelin, the insulating layer on
nerve fibers, is destroyed in the CNS, which consists of the brain, optic
nerves,
and spinal column. There is an accompanying inflammatory response and the
blood brain barrier (BBB) is breached. Axon damage can occur and the optic
nerve is commonly affected. Myelin damage makes it more difficult for nerves
to
transmit impulses, leading to symptoms of MS. The diagnostic McDonald Criteria
(1) were revised in 2005 to include magnetic resonance imaging (MRI) criteria
of
different types of lesions of the brain and spinal cord in the diagnosis of
MS.
11
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
Prognosis is difficult to determine, and many brain lesions do not necessarily
correlate with severity of disease. There are medications available to
alleviate
some symptoms and a few others to modify and hopefully delay the onset or
severity of relapses of MS.
100361 The most common form of MS is relapsing-remitting multiple sclerosis
(RRMS), which is characterized by symptomatic episodes separated in time, with
partial or complete recovery of an apparently normal state between relapses.
It
often converts to secondary progressive MS after several years, where there is
a
steady worsening of symptoms. A minority of patients have Primary Progressive
MS which presents as a continuous slow worsening of the disease state. An even
smaller minority of patients is diagnosed with Progressive-Relapsing MS, where
in
contrast to RRMS, there is a continuous worsening of their condition between
acute episodes. A first episode is referred to as Clinically Isolated Syndrome
(CIS) pending a more certain diagnosis of MS corresponding to clinical signs
and/or brain lesions visualized by mRi, or possibly a spinal tap to check for
immunoglobulin oligoclonal bands (OCB) in the cerebral spinal fluid (CSF).
None
of these diagnostic methods is 100% specific. (2). Its drawbacks include the
expense and the fact that a patient must wait one to three months between
scans
to determine if new lesions have formed during the intervening period. There
is a
clear need for identification of a biomarker or set of biomarkers that
indicate
presence and/or severity of disease for MS patients. A simple blood test would
be
ideal for diagnosing MS, however at this time, no commercial blood test
exists.
100371 Early diagnosis of MS is thought to be increasingly important, as much
of
the damage occurs early in the disease process. The earlier the diagnosis, the
earlier disease-modifying treatment can begin and progression of the disease
and
associated disability can hopefully be slowed.
100381 The present invention features a method of detecting multiple sclerosis
or
a risk of multiple sclerosis. The present invention also features methods of
determining the status of a disease or condition or monitoring disease
activity and
drug efficacy. The method comprises detecting a multiple sclerosis-associated
biomarker, e.g., an antigen, wherein detecting an elevated level of such
multiple
12
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
sclerosis-associated biomarker indicates the presence of multiple sclerosis or
a
risk of multiple sclerosis. In some embodiments, the antigens detected in
accordance with the present invention includes, for example, Ubiquitin carboxy-
terminal hydrolase LI (UCHL1 ) also known as PARK5 protein; neuronal-specific
protein gene product 9.5; SwissProt P09936.
Inflammatory Conditions
100391 The present invention features a method of detecting an inflammatory
condition. The method comprises providing a first sample (e.g., a fluid
sample)
that contains a peripheral (e.g., circulating) phagocyte, and detecting one or
more
biomarkers, e.g., an antigen, inside a phagocyte of said fluid sample, wherein
the
biomarker is associated with an inflammatory condition. The sample may be
provided from a mammal (e.g., a patient, a mouse, a rat, etc.). The fluid
obtained
does not necessarily directly come into contact with the inflamed tissue being
detected. For example, there may be a barrier between the fluid and the source
of the biomarker. In other words, the fluid obtained may have once directly
come
into contact with the inflamed tissue, but at the time that it is being
extracted in
accordance with the present invention, it is being separated from the inflamed
tissue by a barrier.
100401 The method may further comprise comparing the level of the biomarker in
the first sample with a level of the biomarker in a second sample (e.g., a
fluid
sample). The second sample may be a control sample. In some embodiments,
the second sample is a fluid sample from outside of a brain tissue comprising
a
peripheral (e.g., circulating phagocyte), The second sample may be provided
from
a mammal (e.g., a patient, a mouse, a rat, etc.). The second sample may have
been collected prior to the first fluid sample.
100411 As used herein, the term "peripheral" refers to anything outside of
brain
tissue. For example, a peripheral phagocyte may be obtained from cerebrospinal
fluid (CSF). Phagocytes may include monocytes, macrophages, and/or
lymphocytes. Such circulating phagocytes may be found in tissues, cells,
and/or
fluids in the body, for example in blood, peripheral blood mononuclear cells
(PBMCs), synovial fluid, cerebrospinal fluid (CSF), central nervous system
13
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
tissues, synovial fluid, cystic fluid, lymph fluid, ascites, pleural effusion,
interstitial
fluid, ocular fluids, vitreal fluid, urine the like, or a combination thereof.
In some
embodiments, the biomarker is an intracellular component. For example, the
biomarker may be obtained from within a macrophage. In some embodiments,
the macrophage sample is permeabilized. In some embodiments, the
macrophage is lysed via various means, e.g., hypotonic solution treatment,
detergent solution treatment, mechanical stress, etc.
100421 In some embodiments, the sample is a plasma sample.
100431 Also, as used herein, "a fluid that does not directly come into contact
with
the inflamed tissue" is a fluid that is separated from the inflamed tissue by
at least
one barrier, e.g., a tissue membrane, a layer of cells, etc.
100441 In some embodiments, one or more biomarkers are detected in the
collected fluid sample. For example, a pattern of biomarkers may be detected
in
the sample. Detecting the biomarker or biomarkers indicates the presence of
the
inflammatory condition or a risk of the inflammatory condition. In some
embodiments, detecting an increased level of the biomarker or biomarkers as
compared to the level of the biomarker or biomarkers of a control sample
indicates
the presence of the inflammatory condition or a risk thereof. A control sample
is
discussed below. In some embodiments, detecting a decreased level of the
biomarker or biomarkers as compared to the level of the biomarker or
biomarkers
of a control sample indicates the presence of the inflammatory condition or a
risk
thereof.
[00451 In some embodiments, the inflammation condition that may be monitored
or detected includes Rheumatoid Arthritis, Systemic Lupus Erythematosis,
Shogren's Syndrome, and the like.
100461 In some embodiments, the biomarker(s) is a neural¨derived biomarker.
However, the biomarker(s) is not limited to neural-derived biomarkers. In some
embodiments, one or more biomarkers are detected in the sample, wherein the
biomarkers are neural-derived, non-neural-derived biomarkers, or a combination
14
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
thereof.
100471 The biomarker(s) may be detected using a variety of methods. Methods
may include an immunoassay, a histological assay, a flow cytometry assay, the
like, or a combination thereof. For example, in some embodiments, the step of
detecting the biomarker(s) in the sample may comprise introducing an antibody
to
the sample, wherein the antibody binds to the biomarker or is specific for the
biomarker.
100481 In some embodiments, this method of detecting an inflammatory condition
is used in combination with one or more different methods for detecting the
inflammatory disease. In some embodiments, this method is used to
differentiate
between one or more inflammatory conditions.
Neurological Conditions
10049J The present invention also features a method of detecting a
neurological
condition. The method comprises providing a first sample (e.g., a fluid
sample)
that comprises a peripheral (e.g., circulating) phagocyte. The first sample
may be
derived from outside of a brain tissue. The method further comprises detecting
one or more biomarkers, e.g., an antigen, inside a phagocyte of said sample,
wherein the biomarker is associated with a neurological condition (e.g., a
neurological condition-associated protein). The sample may be provided from a
mammal (e.g., a patient, a mouse, a rat, etc.). Detecting the neurological
condition-associated protein indicates the presence of the neurological
condition
or a risk of the neurological condition.
[00501 The sample (e.g., fluid) obtained does not necessarily directly come
into
contact with the inflamed tissue being detected. For example, in some
embodiments, there may be a barrier between the fluid and the source of the
biomarker. In other words, the fluid obtained may have once directly come into
contact with the inflamed tissue, but at the time that it is being extracted
in
accordance with the present invention, it is being separated from the inflamed
tissue by a barrier.
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
[00511 The method may further comprise comparing the level of the biomarker in
the first sample with a level of the biomarker in a second sample (e.g., a
fluid
sample). The second sample may be a control sample. In some embodiments,
the second sample is a fluid sample from outside of a brain tissue comprising
a
peripheral (e.g., circulating phagocyte), The second sample may be provided
from
a mammal (e.g., a patient, a mouse, a rat, etc.). The second sample may have
been collected prior to the first fluid sample.
100521 In some embodiments, detecting an increased level of the neurological
condition-associated protein as compared to the level of the neurological
condition-associated protein of a control sample indicates the presence of the
neurological condition or a risk thereof. In some embodiments, detecting a
decreased level of the neurological condition-associated protein as compared
to
the level of the neurological condition-associated protein of a control sample
indicates the presence of the neurological condition or a risk thereof. A
control
sample is discussed below.
100531 In some embodiments, the neurological condition that may be monitored
or detected includes Alzheimer's Disease, Parkinson's Disease, Neuromyelitis
Optica, transverse myelitis, Acute and chronic Stroke, Traumatic Brain Injury,
and
the like.
100541 In some embodiments, the neurological condition-associated protein is
derived from a brain source. In some embodiments, the neurological condition-
associated protein is derived from a non-brain source. In some embodiments,
one or more neurological condition-associated proteins is derived from a brain
source, a non-brain source, or a combination thereof.
100551 The neurological condition-associated protein may be present in a
circulating phagocyte. Phagocytes may include monocytes, macrophages, and/or
lymphocytes. Such circulating phagocytes may be found in tissues, cells,
and/or
fluids in the body, for example in blood, peripheral blood mononuclear cells
(PBMCs), cerebrospinal fluid (CSF), central nervous system tissues, synovial
fluid, cystic fluid, lymph fluid, ascites, pleural effusion, interstitial
fluid, ocular fluids,
16
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
vitreal fluid, urine, the like, or a combination thereof. In some embodiments,
the
neurological condition-associated protein is an intracellular component. For
example, the neurological condition-associated protein may be obtained from
within a macrophage. In some embodiments, the macrophage sample is
permeabilized. In some embodiments, the macrophage is lysed via various
means, e.g., hypotonic solution treatment, detergent solution treatment,
mechanical stress, etc.
100561 The neurological condition-associated protein may be detected using a
variety of methods. Methods may include an immunoassay, a histological assay,
a flow cytometry assay, the like, or a combination thereof. In some
embodiments,
the step of detecting the neurological condition-associated protein in the
sample
may comprise introducing an antibody to the sample, wherein the antibody binds
to the protein or is specific for the protein.
100571 In some embodiments, this method is used in combination with one or
more different methods for detecting the neurological condition. In some
embodiments, this method is used to differentiate between one or more
neurological conditions.
Multiple Sclerosis
100581 The present invention also features methods of detecting multiple
sclerosis or a risk of multiple sclerosis. In some embodiments, the methods of
the
present invention may allow for monitoring, detecting and/or predicting a
relapse
or a remission of multiple sclerosis. In some
embodiments, the method of
detecting multiple sclerosis is used in combination with one or more methods
of
detecting multiple sclerosis. For example, the present methods may be used in
conjunction with other modalities to monitor, detect or predicting a relapse
or a
remission of multiple sclerosis.
100591 The method of detecting multiple sclerosis comprises (1) providing a
first
sample (e.g., a fluid sample) that comprises a peripheral (e.g., circulating)
phagocyte. The first sample may be derived from outside of a brain tissue, and
(2) detecting a multiple sclerosis-associated biamarker in the phagocyte. The
17
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
sample may be provided from a mammal (e.g., a patient, a mouse, a rat, etc.).
In
some embodiments, one or more multiple sclerosis-associated biomarkers is
detected in the sample. The multiple sclerosis-associated biomarkers are
associated with multiple sclerosis.
100601 The method may further comprise comparing the level of the biomarker in
the first sample with a level of the biomarker in a second sample (e.g., a
fluid
sample). The second sample may be a control sample. In some embodiments,
the second sample is a fluid sample from outside of a brain tissue comprising
a
peripheral (e.g., circulating phagocyte), The second sample may be provided
from
a mammal (e.g., a patient, a mouse, a rat, etc.). The second sample may have
been collected prior to the first fluid sample. Detecting the multiple
sclerosis-
associated biomarker may indicate the presence of multiple sclerosis or a risk
of
multiple sclerosis.
10061j In some embodiments, detecting an increased level of the multiple
sclerosis-associated biomarker as compared to the level of the multiple
sclerosis-
associated biomarker of a control sample indicates the presence of multiple
sclerosis or a risk thereof. In some embodiments, detecting a decreased level
of
the multiple sclerosis-associated biomarker as compared to the level of the
multiple sclerosis-associated biomarker of a control sample indicates the
presence of multiple sclerosis or a risk thereof. In some embodiments,
detecting
an increased level of one class of multiple sclerosis-associated biomarker and
a
decreased of another class of multiple sclerosis-associated biomarker as
compared to the respective level of the multiple sclerosis-associated
biomarker of
a control sample indicates the presence of multiple sclerosis or a risk
thereof.
100621 In some embodiments, the sample is obtained from the mammal
immediately following a relapse (e.g., exacerbation of symptoms) before a drug
(e.g., a steroid) treatment has begun. In some embodiments, the sample is
obtained from the mammal before a relapse. In some embodiments, the sample
is obtained during the course of the drug (e.g., steroid) treatment.
[00631 The multiple sclerosis-associated biomarker may be present in a
18
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
circulating phagocyte. Phagocytes may include monocytes, macrophages, and/or
lymphocytes. For example, macrophages are a type of monocyte and are
phagocytic cells important in both specific cell-mediated immunity and non-
specific innate immunity. Circulating phagocytes may be found in tissues,
cells,
and/or fluids in the body, for example in blood, peripheral blood mononuclear
cells
(PBMCs), cerebrospinal fluid (CSF), central nervous system tissues, synovial
fluid, cystic fluid, lymph fluid, ascites, pleural effusion, interstitial
fluid, ocular fluids,
vitreal fluid, urine, the like, or a combination thereof. In some embodiments,
the
neurological condition-associated protein is an intracellular component.
For
example, the neurological condition-associated protein may be obtained from
within a macrophage. In some embodiments, the macrophage sample is
permeabilized.
[00641 As used herein, a mammal includes a human, a mouse, a rat, a llama, a
rabbit, a dog, a primate, a guinea pig, a cat, a hamster, a pig, a chicken, a
goat, a
horse, or a cow.
10065] In some embodiments, the multiple sclerosis-associated biomarker is a
Tau protein (or a fragment thereof) or a Tau protein (or fragment thereof)
comprising a phosphorylated residue (e.g., a phosphorylated serine reside, a
phosphorylated threonine reside). In some embodiments, the phosphorylated
residue is serine 214, serine 235, serine 262, serine 356, serine 396, serine
404,
serine 413, serine 46, serine 515, serine 516, serine 519, serine 531, serine
552,
serine 610, serine 622, serine 641, serine 713, serine 721, serine 726, serine
730,
serine 739, threonine 181, threonine 205, threonine 470, threonine 492,
threonine
498, threonine 522, threonine 529, threonine 534, threonine 548, the like, or
a
combination thereof.
100661 In some embodiments, phosphorylation of Tau can decrease its
solubility.
In some embodiments, the method of detecting multiple sclerosis comprises
detecting a level of insoluble Tau protein in the sample. In some embodiments,
an increased level of insoluble Tau protein as compared to a control level of
insoluble Tau protein is indicative of multiple sclerosis or a risk thereof.
19
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
100671 In some embodiments, the multiple sclerosis-associated biomarker is a
protein or a fragment thereof selected from the group consisting of
neuroglobin,
valosin-containing protein, brain hexokinase, hippocalcin-1,
nestin,
synaptotagmin, myelin associated glycoprotein, myelin basic protein, myelin
oligodendrocyte glycoprotein, myelin proteolipid protein, transketolase, NS1
assocated protein 1, major vault protein, synaptojanin, enolase, alpha
synuclein,
glial fibrillary acidic protein, S-100 proteinNeu-N, 26S proteasome subunit 9,
annexin A2, annexin A3, annexin A5, annexin A6, annexin All, ubiquitin
activating enzyme ZE1, ubiquitin B precursor, vimentin, glyceraldehyde-3-
phosphate dehydrogenase, 13-3-3 protein, 14-4-4 protein, neurofilament heavy
chain and neurofilament light chain.
100681 The multiple sclerosis-associated biomarker (e.g., Tau protein or
fragment
thereof) may be of various lengths. For example, in some embodiments, the
multiple sclerosis-associated biomarker consists of between about 5 to 20
amino
acids. In some embodiments, the multiple sclerosis-associated biomarker
consists of about 20 to 40 amino acids. In some embodiments, the multiple
sclerosis-associated biomarker consists of about 40 to 80 amino acids. In some
embodiments, the multiple sclerosis-associated biomarker consists of about 80
to
150 amino acids. In some embodiments, the multiple sclerosis-associated
biomarker consists of about 150 to 200 amino acids. In some embodiments, the
multiple sclerosis-associated biomarker consists of about 200 to 300 amino
acids.
In some embodiments, the multiple sclerosis-associated biomarker consists of
about 300 to 400 amino acids. In some embodiments, the multiple sclerosis-
associated biomarker consists of about 400 to 500 amino acids. In some
embodiments, the multiple sclerosis-associated biomarker consists of about 500
to 600 amino acids.
100691 The multiple sclerosis-associated biomarker (e.g., Tau protein or
fragment
thereof) may comprise various regions of the full-length protein. For example,
in
some embodiments, the multiple sclerosis-associated biomarker comprises the
amino-terminus (e.g., N-terminus, NH2-terminus, N-terminal end, amine-
terminus). The amino-terminus refers to the amino acid at the end of a protein
or
polypeptide that has a free amine group (-NH2). In some embodiments, the
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
multiple-sclerosis associated biomarker consists of about the first 15 amino
acids.
In some embodiments, the multiple-sclerosis associated biomarker consists of
about the first 25 amino acids. In some embodiments, the multiple-sclerosis
associated biomarker consists of about the first 50 amino acids. In some
embodiments, the multiple-sclerosis associated biomarker consists of about the
first 75 amino acids. In some embodiments, the multiple-sclerosis associated
biomarker consists of about the first 100 amino acids. In some embodiments,
the
multiple-sclerosis associated biomarker consists of about the first 125 amino
acids.
100701 In some embodiments, the multiple-sclerosis associated biomarker or
fragment thereof comprises the carboxy-terminus (e.g., C-terminus, COOH-
terminus, C-terminal end, carboxyl-terminus). The carboxy-terminus refers to
the
amino acid at the end of a protein or polypeptide that has a free carboxylic
acid
group (-COOH). In some embodiments, the multiple-sclerosis associated
biomarker consists of about the last 100 amino acids.
Monitoring Disease Activity and Drug Efficacy
100711 The present invention also features methods of determining the status
of
a disease or condition (e.g., a neurological condition, an inflammatory
condition,
multiple sclerosis, etc.) or determining the status of drug efficacy. The
present
invention may also feature methods of monitoring disease activity and drug
efficacy. For example, biomarkers can be used to detect a disease or condition
and the biomarkers may be used to determine severity of the disease or
condition
(e.g. relapse, remission, etc.).
[0072j in some embodiments, the method comprises providing a sample (e.g., a
fluid, a brain tissue), the sample comprising a circulating phagocyte. The
sample
may be derived from a mammal (e.g., a patient, a mouse, a rat, etc.). A
biomarker
or level thereof associated with a disease or condition (e.g., a multiple
sclerosis-
associated biomarker) may be detected in the sample (e.g., in the phagocyte)
and
compared to the level or presence of the biomarker in control samples. In some
embodiments, the biomarker detected may be compared to the level or presence
of the biomarker in a second sample, the second sample having been collected
21
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
prior to the first sample. By comparing the level or presence of the biomarker
in
the sample to either a control sample or a patient's previous sample, disease
activity may be determined. A biomarker may include but is not limited to Tau
or a
fragment thereof.
100731 In some embodiments, the monitoring of disease activity may be used to
determine drug efficacy. In some embodiments, the monitoring of disease
activity
may be used to determine drug failure and/or breakthrough disease. In some
embodiments, the monitoring of disease activity may be used to determine
patient
compliance with drug therapy. In some embodiments, the monitoring of disease
activity may be used to determine therapeutic non-responders. In some
embodiments, the monitoring of disease activity may be used to aid drug
development.
100741 In some embodiments, the present invention features a method of
monitoring disease activity of a neurological condition, the method comprises
obtaining from a mammal a first fluid sample from outside of a brain tissue of
the
mammal, the first fluid sample comprises a first circulating phagocyte;
detecting a
level of a biomarker associated with the neurological condition in the first
sample;
and comparing the level of the biomarker in the first sample with a level of
the
biomarker in a second sample, the second sample being either a control sample
or a second fluid sample from outside of a brain tissue of the mammal, the
second
fluid sample comprising a second circulating phagocyte, the second fluid
sample
having been taken prior to the first fluid sample.
10075] Table 1 shows the amino acid sequence of full-length human Tau protein.
100761 TABLE 1
Length (#
MW Amino SEQ
of Amino Amino Acid Sequence
(Daltons) Acids ID
NO
Acids)
758 AA 78,878 Da MA??.Qv AZDHAGn:GL C.D?.KDQGC7YT
07his is M7his is the MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA 1_758
the length MW of the
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
of the unprocessed
HVTQEPESGK VVQEM,REP GPPGLSHQLM
unprocessed precursor] SGMPGA1;=
22
CA 02929711 2016-05-04
WO 2014/071359 PCT/US2013/068465
precursor] GizjiA2LLKH QLLGULUQG 2Z.LKAGGi<:6
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VOKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
KKVAVVRTPP KSPSSAKSRL QTAPVPMPDL
KNVKSKIGST ENLKHQPGGG KVQIINKKLD
LSNVOKCGS KDNIKHVPGG GSVQIVYKPV
DLSKVTSKCG SLGNIHHKPG GGQVEVKSEK
LDFKDRVQSK IGSLDNITHV PGGGNKKIET
HKLTFRENAK AKTDHGAEIV YKSPVVSGDT
SPRHLSNVSS TGSIDMVDSP QLATLADEVS
ASLAKQGL
[00771 Table 2 shows examples of some of the possible multiple sclerosis-
associated biomarkers (e.g., Tau protein or a fragment there).
[00781 TABLE 2
Length (# MW Amino Acid Sequence Amino
SEQ ID
of Amino (Daltons) Acids NO
Acids)
1235.4 MAEPRQEFEV 1-10 2
2310.56 MAEPRQEFEV MEDHAGTYGL 1-20 3
3388.69 MAEPRQEFEV MEDHAGTYGL
GDRKDQGGYT 1-30 4
4545.84 MAEPRQEFEV MEDHAGTYGL
GDRKDQGGYT 1-40 5
MHQDQEGDTD
5571.02 MAEPRQEFEV MEDHAGTYGL
GDRKDQGGYT 1-50 6
MHQDQEGDTD AGLKESPLQT
6570 MAEPRQEFEV MEDHAGTYGL GDRKDQGGYT 1-60 7
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
7574.04 MAEPRQEFEV MEDHAGTYGL
GDRKDQGGYT 1-70 8
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP
8571.17 MAEPRQEFEV MEDHAGTYGL
GDRKDQGGYT 1-80 9
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV
9496.14 MAEPRQEFEV MEDHAGTYGL GDRKDQGGYT 1-
90 10
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
100 10556.29 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-100 11
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEI PEG
110 11501.26 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-110 12
MHQDQEGDTD AGLKESPLQT ------------------------------------------------
23
CA 02929711 2016-05-04
WO 2014/071359 PCT/US2013/068465
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD
120 12472.27 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-120 13
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
130 13565.44 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-130 14
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK
140 14720.77 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-140 15
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP
150 15738.98
MAEPRQEFEV MEDHAGTYGL GDRKDQGGYT 1-150 16
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
160 16660.12 MAEPRQEFEV
MEDHAGTYGL 1-160
GDRKDQGGYT MHQDQEGDTD AGLKESPLQT
PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP
170 17782.35 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-170 18
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP
180 18713.25 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-180 19
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
190 19822.58 1-190 20
200 20879.8 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-200 21
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG
210 21847.88 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-210 22
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
24
CA 02929711 2016-05-04
WO 2014/071359 PCT/US2013/068465
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
220 23050.05 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGIT 1-220 23
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAG1GD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE
230 24062.07 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-230 24
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRBAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ
240 25070.18 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-240 25
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHOLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH OLLGDLHOG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
250 26125.36 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-250 26-
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGOLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA
260 27062.44 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGY1 1-260 27
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGOLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP
270 28148.74 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-270 28 ---
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGOLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDR22QAA REATSIPGFP AEGA1PLPVD
280 29145.79 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-280 29
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP
290 30141.88 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-290 30
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG
300 31313.22 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-300 31
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
310 32422.47 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-310 32
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN
320 33510.63 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-320 33
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHOLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE
330 34449.64 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-330 34
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHOLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
26
CA 02929711 2016-05-04
WO 2014/071359 PCT/US2013/068465
ETTHVEI:PN VQKEQAHSEE HLGRAAFPGA
1
340 35433.69 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-340 35
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH OLLGDLHOEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG PAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP
350 36529.87 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-350 36
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD
360 37535.06 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-360 37
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
370 38611.37 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-370 38
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKOPA
AAPRGKPVSR
380 39701.64 MAEPRQEFEV MEDHAGTYGL GDRKDQGGYT 1-380
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
27
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS
390 40719.73 MAEPRQEFEV MEDHAGTYGL GDRKDQGGYT 1-390
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGOLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGA1PLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
400 41806.98 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-400 41
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAG1GD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS
410 42900.36 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-410 42
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH OLLGDLHOEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL
420 43892.47 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-420 43
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAG1GD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
28
CA 02929711 2016-05-04
WO 2014/071359 PCT/US2013/068465
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
430 44857.59 MAEPRQEFEV
MEDHAGTYGL GDRKDOGGYT 1-430 44
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA
440 45867.71 'MAEPROEFEV
MEDHAGTYGL GDRKDQGGYT 1-440
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPOLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK
450 46828.72 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-450 16
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHOLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH OLLGDLHOEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSRTG
460 47858.97 MAEPRQEFEV
MEDHAGTYGL GDRKDOGGYT 1-460 47
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGOLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKOPA
29
CA 02929711 2016-05-04
WO 2014/071359 PCT/US2013/068465
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPL1QPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK
470 48822.13 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-470 48
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQ.DAPLE
FTFHVEITPN VQKEQAH SEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKOPA
AAPPGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT
480 49775.15 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-480 49
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHOLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH OLLGDLHOEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA PEATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGODAr'LE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKOPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
490 50804.42 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT i-490 SU
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPPEATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DPDVDESSPQ DSPPSKASPA
QDGPPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSPTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRIPA
500 51782.51 MAEPRQEFEV MEDHAGTYGL GDRKDQGGYT 1-500
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHOLM
CA 02929711 2016-05-04
WO 2014/071359 PCT/US2013/068465
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP
410 52807.56 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-510 52
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHOLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHOEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKOPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
520 53701.53 MAEPRQEFEV MEDHAGTYGL
GDRKDQGGY 1-520 53
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGL8HQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKOPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP
530 54735.74 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-530 54
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGOLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKOPA
---------------------------------------------------- AAPRGKPVSR VPQLKARMVS
KSKDGTGSDD
31
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
KKAK2S21.33 A17LKNRPCL SPKLPTPGSS
DPL1QPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLY GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPYTPP SSGEPPKSGU
RSGYSS.7'GST, GTPGSRSRTP
540 55677.7 MAEF7,0,-_v
M_J¨A¨,YGL GDRKDQGGY7 1-540 55
MHQDQEGUTD AGLKESPLQT PZEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPH2EiPEG TTAEEAGIGD 2PSLEDEAAG
HVTQEPESGK VVQEGFLREP G22GLSHOLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH OLLGDI_HQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPOTAA REA7SIPGFP AEGAIPLPVD
FLSKVS7EIP ASEPDGPSVG PAKGQDAPLE
FTFHVEL:PN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LREPSEKQ?A
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AK2LKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPE22SSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SL2TPPTREP
56295.63 MAEPROEFEV MEDHAGTYGL
GDIRKDQGGIT 1-550 36
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SG2GPEDTEG
GRHAPELLKH QLLGULHQEG PRLKGAGGKE
RPGSKEEVDE DPDVDESSPQ DSPPSKASPA
QDGRPPQTAA REA2S1PGFP AEGAIPLPVD
FLSKVS2E1P ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VOKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAK7S7RSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVS3VTSP7G
SSGAKEMKLK GADGKZY1AT PRGAAPPGQK
GOANA2RiPA KTPPARKTPP SSGEPPKSGD
RSGYSSRGSP GTPGSRSRTP SL2TPPTREP
KKVAVVR_PP
360 57337.84 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGY_ 57
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPH7EIPEG TTAEEAGIGD 7PSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SG7GPEDTEG
GRHAPELLYH QLLGOLHOEG PPLYGAGGKE
RPGSKEEVDE DRENUESSPQ DSRPSKASPA
QDGRPPQ_AA REATSIPGFP AEGA1PLPVD
1LSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEi_PN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKOPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTR33 A17LKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPP33F1c HVSSVTSRTG
SSGAKEMKLY GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPYTPP SSGEPPKSGU
32
CA 02929711 2016-05-04
WO 2014/071359 PCT/US2013/068465
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
KKVAVVRTPP KSPSSAKSRL
570 58388.1 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-570 58
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAG1GD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
KKVAVVRTPP KSPSSAKSRL QTAPVPMPDL
580 59431.32 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-580 59
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHOLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGA1PLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
KKVAVVRTPP KSPSSAKSRL QTAPVPMPDL
KNVKSKIGST
590 60449.41 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-590 60
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGK2K1AT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
33
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
KKVAVVRTPP KSPSSAKSRL QTAPVPMPDL
KNVKSKIGST ENLKHQPGGG
600 61629.85 MAEPRQEFEV
MEDHAGTYGL GDRKDOGGYT 1-600 61
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPL1QPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
KKVAVVRTPP KSPSSAKSRL QTAPVPMPDL
KNVKSKIGST ENLKHQPGGG KVQIINKKLD
610 62633.98 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-610 r,
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAG1GD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKOPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
KKVAVVRTPP KSPSSAKSRL QTAPVPMPDL
KNVKSKIGST ENLKHQPGGG KVQIINKKLD
LSNVQSKCGS
620 63680.16 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-620 63
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHOLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHOEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGA1PLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKOPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
34
CA 02929711 2016-05-04
WO 2014/071359 PCT/US2013/068465
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
KKVAVVRTPP KSPSSAKSRL QTAPVPMPDL,
KNVKSKIGST ENLKHQPGGG KVQIINKKLD
LSNVOSKCGS KDNIKHVPGG
630 64751.44 MAEPRQEFEV MEDHAGTYGL GDRKDQGGYT 1-630
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGOLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
KKVAVVRTPP KSPSSAKSRL QTAPVPMPDL
KNVKSKIGST ENLKHQPGGG KVQIINKKLD
LSNVQSKCGS KDNIKHVPGG GSVQIVYKPV
640 65770.63 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-640 65
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VOKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTK1AT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
KKVAVVRTPP KSPSSAKSRL QTAPVPMPDL
KNVKSKIGST ENLKHQPGGG KVQIINKKLD
LSNVOKCGS KDNIKHVPGG GSVOIVYKPV
DLSKVTSKCG
650 66811.8 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-650 66
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VOKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA --------------------
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPL1QPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTK1AT PRGAAPPGQK
GQANATRiPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
KKVAVVRTPP KSPSSAKSRL QTAPVPMPDL
KNVKSKIGST ENLKHQPGGG KVQIINKKLD
LSNVOKCGS KDNIKHVPGG GSVQIVYKPV
DLSKVTSKCG SLGNiHHKPG
660 67853.95 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-660 67
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GREAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATS1PGFP AEGAIPLPVD
FLSKVS2E1P ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VOKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSPTG
SSGAKEMKLK GADGKTK1AT PRGAAPPGQK
GQANATR1PA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
KKVAVVRTPP KSPSSAKSRL QTAPVPMPDL
KNVKSKIGST ENLKHQPGGG KVQIINKKLD
LSNVOKCGS KDNIKHVPGG GSVQIVYKPV
DLSKVTSKCG SLGNiHHKPG GGQVEVKSEK
670 69071.34 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-670 60
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPPEATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DPDVDESSPQ DSPPSKASPA
QDGPPPQTAA REATS1PGFP AEGAIPLPVD
FLSKVS2E1P ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPOLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSPTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRiPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
KKVAVVRTPP KSPSSAKSRL QTAPVPMPDL
KNVKSKIGST ENLKHQPGGG KVQIINKKLD
LSNVOSKCGS KDNIKHVPGG GSVQ1VYKPV
DLSKVTSKCG SLGNIHHKPG GGQVEVKSEK
LDFKDRVQSK
680 70121.52 MAEPRQEFEV MEDHAGTYGL GDRKDQGGYT 1-680
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
36
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGOLHOEG PPLKGAGGKF
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKOPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
KKVAVVRTPP KSPSSAKSRL QTAPVPMPDL
KNVKSKIGST ENLKHQPGGG KVQIINKKLD
LSNVQSKCGS KDNIKHVPGG GSVQIVYKPV
DLSKVTSKCG SLGNIHHKPG GGQVEVKSEK
LDFKDRVQSK IGSLDNITHV
690 71103.62 MAEPPQEFEV MEDHAGTYGL GDRKDQGGYT 1-690 70
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPFGLSHOLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH OLLGDLHOEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG PAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPOLKARMVS KSKDGTGSDD
KKAKTSTRSS Ar< '1KNRPCL SPKLPTPGSS
DPLIQPSSPA VCz',L22SSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
KKVAVVRTPP KSPSSAKSRL QTAPVPMPDL
KNVKSKIGST ENLKHQPGGG KVQIINKKLD
LSNVQSKCGS KDNIKHVPGG GSVQIVYKPV
DLSKVTSKCG SLGNIHHKPG GGQVEVKSEK
LDFKDRVQSK IGSLDNITHV PGGGNKKII:
70072329 0 MAEPRQEFEV MEDHAGTYGL GDRKDQGGYT 1-700 71
.
4 MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPPEATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPOLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSPTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
KKVAVVRTPP KSPSSAKSRL QTAPVPMPDL
37
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
KNVKSKIG3T ENLKHQPGGG KVQIINKKLD
LSNVQSKCGS KDN1KHVPGG GSVQIVYKPV
DLSKVTSKCG SLGNIHHKPG GGQVEVKSEK
LDFKDRVQSK 1GSLDNITHV PGGGNKKIE1
HKLTFRENAK
73351.17 MAEPPQEFEV MEDHAGTYGL
GDRKDQGGYT 1-710 72
710
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH OLLGDLHOEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGP3VG PAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPL1QPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPG3P GTPGSRSRTP SLPTPPTPEP
KKVAVVRTPP KSPSSAK3RL QTAPVPMPDL
KNVKSKIG3T ENLKHQPGGG KVQIINKKLD
LSNVQSKCGS KDN1KHVPGG GSVQIVYKPV
DLSKVTSKCG SLGNIHHKPG GGQVEVKSEK
LDFKDRVQSK 1GSLDNITHV PGGGNKK1Er
HKLTFRENAK AKTDHGAEIV
720 74385.31 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-720 77
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH OLLGDLHOEG PPLKGAGGKE
RPGSKEEVDE DRDVDE33PQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPG3P GTPGSRSRTP SLPTPPTPEP
KKVAVVRTPP KSPSSAK3RL QTAPVPMPDL
KNVKSKIGST ENLKHQPGGG KVQIINKKLD
LSNVQSKCGS KDN1KHVPGG GSVQIVYKPV
DLSKVTSKCG SLGNIHHKPG GGQVEVKSEK
LDFKDRVQSK 1GSLDNITHV PGGGNKK1E_
HKLTFRENAK AKTDHGAE1V YKSPVVSGDT
730 75450.47 MAEPRQEFEV MEDHAGTYGL GDRKDQGGYT 1-730
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
38
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
KKVAVVRTPP KSPSSAKSRL QTAPVPMPDL
KNVKSKIGST ENLKHQPGGG KVQIINKKLD
LSNVOSKCGS KDNIKHVPGG GSVQIVYKPV
DLSKVTSKCG SLGNIHHKPG GGQVEVKSEK
LDFKDRVQSK IGSLDNITHV PGGGNKKIET
HKLTFRENAK AKTDHGAEIV YKSPVVSGDT
SPRHLSNVSS
740 76804.91 MAEPRQEFEV
MEDHAGTYGL GDRKDQGGYT 1-740 75
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHQLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKOPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIQPSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
KKVAVVRTPP KSPSSAKSRL QTAPVPMPDL
KNVKSKIGST ENLKHQPGGG KVQIINKKLD
LSNVQSKCGS KDNIKHVPGG GSVQIVYKPV
DLSKVTSKCG SLGNIHHKPG GGQVEVKSEK
LDFKDRVQSK IGSLDNITHV PGGGNKKIET
HKLTFRENAK AKTDHGAEIV YKSPVVSGDT
SPRHLSNVSS TGSIDMVDSP
750 78109.39 MAEPRQEFEV
MEDHAGTYGL GDRKDOGGYT 1-750 76
MHQDQEGDTD AGLKESPLQT PTEDGSEEPG
SETSDAKSTP TAEDVTAPLV DEGAPGKQAA
AQPHTEIPEG TTAEEAGIGD TPSLEDEAAG
HVTQEPESGK VVQEGFLREP GPPGLSHOLM
SGMPGAPLLP EGPREATRQP SGTGPEDTEG
GRHAPELLKH QLLGDLHQEG PPLKGAGGKE
RPGSKEEVDE DRDVDESSPQ DSPPSKASPA
QDGRPPQTAA REATSIPGFP AEGAIPLPVD
FLSKVSTEIP ASEPDGPSVG RAKGQDAPLE
FTFHVEITPN VQKEQAHSEE HLGRAAFPGA
PGEGPEARGP SLGEDTKEAD LPEPSEKQPA
AAPRGKPVSR VPQLKARMVS KSKDGTGSDD
KKAKTSTRSS AKTLKNRPCL SPKLPTPGSS
DPLIUSSPA VCPEPPSSPK HVSSVTSRTG
SSGAKEMKLK GADGKTKIAT PRGAAPPGQK
39
CA 02929711 2016-05-04
WO 2014/071359 PCT/US2013/068465
GQANATRIPA KTPPAPKTPP SSGEPPKSGD
RSGYSSPGSP GTPGSRSRTP SLPTPPTREP
KKVAVVRTPP KSPSSAKSRL QTAPVPMPDL
KNVKSKIGST ENLKHQPGGG KVQIINKKLD
LSNVOSKCGS KDNIKHVPGG GSVQIVYKPV
DLSKVTSKCG SLGNIHHKPG GGQVEVKSEK
LDFKDRVQSK IGSLDNITHV PGGGNKKIET
HKLTFRENAK AKTDHGAEIV YKSPVVSGDT
SPRHLSNVSS TGSIDMVDSP QLATLADEVS
1815.06 QLATLADEVS ASLAKQGL 749-758 77
2818.17 TGSIDMVDSP QLATLADEVS ASLAKQGL 739-758 78
2992.33 SS TGSIDMVDSP QLATLADEVS 729-758 79
ASLAKQGL
4099.53 DT SPRHLSNVSS TGSIDMVDSP 719-758 80
QLATLADEVS ASLAKQGL
100791 In some embodiments, the step of detecting the multiple sclerosis-
associated biomarker in the sample may comprise introducing an antibody to the
sample, wherein the antibody binds to the multiple sclerosis-associated
biomarker.
100801 In some embodiments, the step of detecting the multiple sclerosis-
associated biomarker in the sample comprises subjecting the sample to a
western
blot, an enzyme-linked immunosorbent assay (ELISA), a lateral flow assay, a
radioimmunoassay, an immunohistochemistry assay, a bioluminescent assay, a
chemiluminescent assay, a mass spectrometry assay, a flow cytometry assay
(e.g., florescence-activated cell sorting (FACS)); or a combination thereof
and the
like. Such assays are well known in the art.
[00811 In some embodiments, the step of detecting the multiple sclerosis-
associated biomarker further comprises contacting the sample with an antibody
that binds to the multiple sclerosis-associated biomarker and detecting an
antibody-biomarker complex. The step
of detecting an antibody-biomarker
complex may comprise subjecting the sample to a micro array, western blot, an
enzyme-linked immunosorbent assay (ELISA), a lateral flow assay, a
radioimmunoassay, an immunohistochemistry assay, a bioluminescent assay, a
chemiluminescent assay, a flow cytometry assay (e.g., florescence-activated
cell
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
sorting (FAGS)), or a combination thereof and the like. In some embodiments,
detecting the antibody-biomarker complex indicates the presence of multiple
sclerosis or a risk of multiple sclerosis.
10082] As described above, in some embodiments, the step of detecting the
multiple sclerosis-associated biomarker may comprise subjecting the sample
florescence-activated cell sorting (FAGS). Fluorescence-activated cell sorting
(FAGS) is a type of flow cytometry that sorts a mixture of biological cells,
one at a
time, into separate containers based upon the specific light scattering and
fluorescent characteristics of each cell. It provides quantitative recording
of
fluorescent signals from individual cells as well as physical separation of
cells of
particular interest. Generally, a current of a rapidly flowing stream of
liquid carries
a suspension of cells through a nozzle. The flow is selected such that there
is a
large separation between cells relative to their diameter. Vibrations at the
tip of
the nozzle cause the stream of cells to break into individual droplets, and
the
system is adjusted so that there is a low probability of more than one cell
being in
a droplet. A monochromatic laser beam illuminates the droplets, which are
electronically monitored by fluorescent detectors. The droplets that emit the
proper fluorescent wavelengths are electrically charged between deflection
plates
in order to be sorted into collection tubes.
10083] As described above, in some embodiments, the step of detecting the
multiple sclerosis-associated biomarker may comprise subjecting the sample to
an
enzyme-linked immunosorbent assay (ELISA). ELISA is an assay used to detect
the presence of an antibody or a biomarker in a sample. Generally, in ELISA, a
sample containing an unknown amount of biomarker, e.g., an antigen, is
affixed/immobilized to a surface (e.g., a polystyrene microtiter plate). Then,
an
antibody that binds to the antigen of interest is washed over the surface so
that it
can bind the antigen and form an antibody/antigen complex. In some cases, this
antibody is covalently linked to an enzyme. In some cases, the antibody is not
covalently linked to an enzyme but can be detected by a secondary antibody
that
is linked to an enzyme. In the final step, a substance (e.g., substrate) that
the
enzyme is capable of converting to a detectable visible signal (e.g., color
signal) is
added to the reaction. Thus, if the antibody/antigen complex is present, the
41
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
substrate will be converted to the detectable visible signal, and then amount
of
antigen in the sample can be measured.
100841 As mentioned above, in some embodiments, an antibody is used to detect
the presence of the multiple sclerosis-associated biomarker. The
multiple
sclerosis-associated biomarker may be detected with a variety of antibodies.
In
some embodiments, the antibody is a monoclonal or a polyclonal antibody. In
some embodiments, the antibody is a humanized antibody. In some
embodiments, the antibody is a chimera. In some embodiments, the antibody is
derived from a human, a mouse, a rat, a llama, a rabbit, a dog, a primate, a
guinea pig, a cat, a hamster, a pig, a chicken, a goat, a horse, or a cow. In
some
embodiments, the antibody is synthetic. In some embodiments, the antibody is a
recombinant antibody.
100851 Frequently, antibodies are labelled either covalently or non-covalently
by
combining the antibody with a second substance that provides for detectable
signal. A wide variety of labels and conjugation techniques are known in the
art
and are reported extensively in both the scientific and patent literature.
Examples
of labels include but are not limited to radioisotopes, enzymes, substrates,
cofactors, inhibitors, fluorescers, chemiluminescers, magnetic particles, and
the
like. In some embodiments of the present invention, the antibody comprises a
label.
100861 In some embodiments, the present invention is used to detect the
presence of multiple sclerosis. For
example, a patient may present with
symptoms of a demyelinating disease. A sample (e.g., derived from the paitent)
may be tested for an elevated level of a multiple sclerosis-associated
biamarker.
If, according to the present invention, the level of a multiple sclerosis-
associated
biomarker is elevated and the patient presents symptoms of a demyelinating
disease, then the patient is diagnosed as having multiple sclerosis.
100871 In some embodiments, the present invention is used to detect a risk of
multiple sclerosis. For example, a patient may present with no symptoms of a
demyelinating disease, but he or she wishes to be tested for a risk of
multiple
42
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
sclerosis. If, according to the present invention, the level of a multiple
sclerosis-
associated biomarker is elevated and the patient does not present symptoms of
a
demyelinating disease, then the patient is diagnosed as having a risk of
multiple
sclerosis.
100881 As used herein, the term "elevated level" refers to a level that is
higher
than the normal level of the multiple sclerosis-associated biomarker (e.g.,
the level
that would be detected in a person who does not have multiple sclerosis). To
identify the level of the multiple sclerosis-associated biomarker that is the
normal
level, samples are pooled from about, for example, 500 patients (or an
appropriate
number of patients that would be statistically meaningful) who do not
experience
any symptoms of multiple sclerosis (or other demyelinating diseases) and who
do
not test positive for multiple sclerosis as detected by MRI. From those pooled
samples, the average level of the multiple sclerosis-associated biomarker can
be
quantified and then defined as being the normal level of the multiple
sclerosis-
associated biomarker. If the normal level of the multiple sclerosis-associated
biomarker is about zero, then an elevated level refers to any level that is
greater
than zero, for example, about 5 units, about 25 units, about 50 units, about
100
units, about 500 units, about 1000 units, about 10,000 units, about 100,000
units,
about 1,000,000 units. In some embodiments, a unit may be an absorbance unit
(e.g., from an ELISA), a percent positive (e.g., from a flow cytometry or FACS
assay), or a fluorescence unit.
10089] If the normal level of the multiple sclerosis-associated biomarker is
some
positive value (e.g., 5 units, 10 units, 50 units, 100 units, 500 units), then
an
elevated level refers to any level that is higher than the normal level. In
some
embodiments, an elevated level of the multiple sclerosis-associated biomarker
may be a level that is about 10-20% higher than the normal level of the
multiple
sclerosis-associated biomarker. In some embodiments, an elevated level of the
multiple sclerosis-associated biomarker may be a level that is about 20-30%
higher than the normal level of the multiple sclerosis-associated biomarker.
In
some embodiments, an elevated level of the multiple sclerosis-associated
biomarker may be a level that is about 30-40% higher than the normal level of
the
multiple sclerosis-associated biomarker. In some embodiments, an elevated
level
43
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
of the multiple sclerosis-associated biomarker may be a level that is about 40-
50%
higher than the normal level of the multiple sclerosis-associated biomarker.
In
some embodiments, an elevated level of the multiple sclerosis-associated
biomarker may be a level that is about 50-60% higher than the normal level of
the
multiple sclerosis-associated biomarker. In some embodiments, an elevated
level
of the multiple sclerosis-associated biomarker may be a level that is about 60-
70%
higher than the normal level of the multiple sclerosis-associated biomarker.
In
some embodiments, an elevated level of the multiple sclerosis-associated
biomarker may be a level that is about 70-80% higher than the normal level of
the
multiple sclerosis-associated biomarker. In some embodiments, an elevated
level
of the multiple sclerosis-associated biomarker may be a level that is about 80-
90%
higher than the normal level of the multiple sclerosis-associated biomarker.
In
some embodiments, an elevated level of the multiple sclerosis-associated
biomarker may be a level that is about 90-100% higher than the normal level of
the multiple sclerosis-associated biomarker. In some embodiments, an elevated
level of the multiple sclerosis-associated biomarker may be a level that is
about 1-
2 fold higher than the normal level of the multiple sclerosis-associated
biomarker.
In some embodiments, an elevated level of the multiple sclerosis-associated
biomarker may be a level that is about 2-3 fold higher than the normal level
of the
multiple sclerosis-associated biomarker. In some embodiments, an elevated
level
of the multiple sclerosis-associated biomarker may be a level that is about 3-
4 fold
higher than the normal level of the multiple sclerosis-associated biomarker.
In
some embodiments, an elevated level of the multiple sclerosis-associated
biomarker may be a level that is about 4-5 fold higher than the normal level
of the
multiple sclerosis-associated biomarker. In some embodiments, an elevated
level
of the multiple sclerosis-associated biomarker may be a level that is about 5-
10
fold higher than the normal level of the multiple sclerosis-associated
biomarker. In
some embodiments, an elevated level of the multiple sclerosis-associated
biomarker may be a level that is about 10-20 fold higher than the normal level
of
the multiple sclerosis-associated biomarker. in some embodiments, an elevated
level of the multiple sclerosis-associated biomarker may be a level that is
about
20-50 fold higher than the normal level of the multiple sclerosis-associated
biomarker.
44
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
10090j The present invention also provides a method of monitoring the
progression of multiple sclerosis and/or monitoring the treatment of multiple
sclerosis. For example, in some embodiments, the present invention may be
used to measure the level of the multiple sclerosis-associated biomarker in
order
to detect a change in the level (e.g., an increase in the level, a decrease in
the
level, a maintaining of the level). Without wishing to limit the present
invention to
any theory or mechanism, a change in the level of the multiple sclerosis-
associated biomarker may correlate with a change in the patient's status
(e.g.,
remission, progression, worsening). For example, a decrease in the level of
the
multiple sclerosis-associated biomarker may indicate the patient has entered
or
will enter a remission period. In some embodiments, the present invention may
be used to monitor the level of the multiple sclerosis-associated biomarker in
a
patient while the patient is on a treatment regimen (e.g., a drug). Without
wishing
to limit the present invention to any theory or mechanism, a treatment regimen
(e.g., a drug) that is effective at inhibiting the progression of multiple
sclerosis
and/or reducing the symptoms of multiple sclerosis may decrease the level of
the
multiple sclerosis-associated biomarker in the patient.
100911 As mentioned above, in some embodiments, the method of the present
invention for detecting multiple sclerosis is used in combination with one or
more
different methods for detecting multiple sclerosis. For example, in some
cases, a
combination of family history, a physical exam, and magnetic resonance imaging
(MRI) findings are used to diagnose multiple sclerosis. Currently, 1V1R1 is
the most
sensitive radiographic technique for the imaging of multiple sclerosis.
Multiple
sclerosis plaques are commonly seen as round or void discrete lesions in the
periventricular white matter. Other common locations for multiple sclerosis
plaques include the corpus callosum, corona radiate, internal capsule, and
centrum semiovale. In some embodiments, the present invention is used to
measure a multiple sclerosis-associated biomarker, and the level of the
multiple
sclerosis-associated biomarker is correlated with a magnetic resonance imaging
(MRI) measurement. Without wishing to limit the present invention to any
theory
or mechanism, it is believed that elevated levels of the multiple sclerosis-
associated biomarker correlate with a 1V1R1 scan showing the presence of
multiple
sclerosis plaques in the brain.
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
100921 The method of the present invention for detecting multiple sclerosis
may
be used in combination with one or more methods for detecting a different
condition. For example, the method of the present invention may also help to
distinguish multiple sclerosis from other diseases with similar clinical
manifestations. For example, neuromyelitis optica (NMO), also known as Devic's
syndrome, is a neurological disorder regarded as a severe variant of multiple
sclerosis. The characteristic inflammatory demyelinating lesions of NMO
selectively and repeatedly affect the optic nerves and the spinal cord,
causing
blindness and paralysis. A marker (e.g., aquaporin-4 antibodies) has been
identified in serum and cerebrospinal fluid of patients with NMO, and the
presence
of a NMO marker (e.g., aquaporin-4 antibodies) may be used to distinguish NMO
from multiple sclerosis. In some embodiments, the method of detecting the
presence of multiple sclerosis or a risk of multiple sclerosis comprises
detecting
the presence or absence of at least two biomarkers (e.g., proteins, antigens,
or
the like) wherein at least one biomarker is detected in order to distinguish
multiple
sclerosis from a disease with similar clinical manifestations.
100931 In some embodiments, the method of detecting the presence of multiple
sclerosis or a risk of multiple sclerosis comprises detecting an elevated
level of
two or more multiple sclerosis-associated biomarkers. In some embodiments, the
method of detecting the presence of multiple sclerosis or a risk of multiple
sclerosis comprises detecting an elevated level of three or more multiple
sclerosis-associated biomarkers.
100941 The present invention also provides a method of diagnosing multiple
sclerosis at an early stage of the disease before all clinical criteria are
fulfilled,
thus justifying early initiation of a multiple sclerosis-appropriate therapy.
100951 The present invention also features a kit for detecting the status of a
disease or condition (e.g., an inflammatory condition, a neurological
condition,
multiple sclerosis, etc.). The kit may comprise an antibody specific for a
biomarker (e.g., a multiple sclerosis-associated biomarker), wherein the
biomarker
is a protein selected from the group consisting of Tau or a fragment thereof,
46
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
phosphorylated Tau or a fragment thereof, neuroglobin, valosin-containing
protein,
brain hexokinase, hippocalcin-1, nestin, synaptotagmin, myelin associated
glycoprotein, Pv1yelin Basic Protein (MBP), Proteolipid Protein, Pv1yelin
Oligodendrocyte Glycoprotein, transketolase, NS1 assocated protein 1, major
vault protein, synaptojanin, enolase, alpha synuclein, glial fibrillary acidic
protein,
S-100 proteinNeu-N, 26S proteasome subunit 9, annexin A2, annexin A3, annexin
A5, annexin A6, annexin All, ubiquitin activating enzyme ZE1, ubiguitin B
precursor, vimentin, glyceraldehyde-3-phosphate dehydrogenase, 13-3-3 protein,
or fragments thereof. In some embodiments, the kit further comprises a means
for detecting the binding of the antibody to the multiple sclerosis-associated
biomarker. In some embodiments, the antibody is a monoclonal or a polyclonal
antibody.
100961 In some embodiments, the detection of perforin is used in combination
with detection of a marker (e.g., MBP) in phagocyltes. For example, it has
been
surprisingly discovered that perforin levels can decline in CD16 cells as MBP
levels increase.
Kit for Detecting Multiple Sclerosis
100971 The present invention also features a kit for detecting the presence of
multiple sclerosis or a risk of multiple sclerosis in a circulating phagocyte
sample
derived from a mammal. The kit comprises an antibody that binds to a multiple
sclerosis-associated biomarker. In some embodiments, the kit further comprises
a means for detecting the binding of the antibody to the multiple sclerosis-
associated biomarker/antigen in the sample (e.g., an antibody-antigen
complex).
In some embodiments, the detecting of an elevated level of an antibody-antigen
complex indicates presence of multiple sclerosis or a risk of multiple
sclerosis.
100981 In some embodiments, the kit comprises an antibody, wherein the
antibody is a monoclonal or a polyclonal antibody. In some embodiments, the
antibody is derived from a human, a mouse, a rat, a llama, a rabbit, a dog, a
primate, a guinea pig, a cat, a hamster, a pig, a chicken, a goat, a horse, or
a cow.
In some embodiments, the antibody is humanized. In some embodiments, the
antibody is a chimera. In some embodiments, the antibody is specific for the
47
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
multiple sclerosis-associated biomarker.
EXAMPLE I - Detecting Multiple Sclerosis in a Patient
100991 The following example describes the detection of multiple sclerosis in
a
patient according to two methods disclosed in the present invention. A 24-year-
old male patient presents to his primary care physician complaining of changes
in
vision, limb weakness, and extreme fatigue. He mentions his symptoms have
been recurring over the last 3 months. The physician suspects the possibility
of a
tumor in the central nervous system (CNS) or a CNS disease, as well as
multiple
sclerosis. The physician obtains a blood sample to be sent to a diagnostic
laboratory for multiple sclerosis testing, and also refers the patient to a
neurologist.
1001001 The laboratory receives the patient's blood sample collected in a CPT
tube. PBMCs are obtained from a BD VacutainerTM CPT tube using a cell
separation procedure. The cells are washed three times in 1X PBS and
centrifuged in a horizontal rotor (swing-out head) for a minimum of 5 minutes
at
1200 to 1500 RCF (Relative Centrifugal force). The supernatant is removed and
the cells are resuspended in 1X PBS. After the final wash, extracts of the
PBMCs
are prepared by lysing with a hypotonic solution or other method. Then the
lysate
is subjected to assay involving an antibody that binds to Tau protein fragment
comprising the phosphorylated serine residue Ser-404. The assay indicates that
an elevated level of said Tau protein fragment is present in the PBMCs. The
assay is the assay of example 2 or example 3. Thus, the results of the assay
indicate that the patient has multiple sclerosis. The physician notifies the
patient,
who then begins treatment immediately.
1001011 The laboratory receives the patient's blood sample collected in a CPT
tube. PBMCs are obtained from a BD Vacutainerrm CPT tube using a cell
separation procedure. The cells are washed three times in 1X PBS and
centrifuged in a horizontal rotor (swing-out head) for a minimum of 5 minutes
at
1200 to 1500 RCF (Relative Centrifugal force). The supernatant is removed and
the cells are resuspended in lx PBS. The cells are then subjected to assay
involving an antibody that binds to Tau protein fragment comprising the
48
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
phosphorylated serine residue Ser-404. The assay is the assay of example 4.
EXAMPLE 2 ¨ Direct ELISA Assay Protocol
1001021 The following example describes a direct ELISA assay used for
detecting
a multiple sclerosis-associated antigen in a sample. The protein concentration
of
the sample is determined using the BioRadTM (Bradford method) assay.
MicroELISA plates are coated by addition of 100 pL of a 5- 20 pgimL solution
of
the sample, which is then incubated for 1 hour at 210. The wells are washed
out
with phosphate buffered solution (PBS) with 0.05% polysorbate (Tween 20Tm).
The wells are then filled with 0.1 M glycine in PBS and incubated for 1 hour
at
21 C to block unoccupied binding sites. After rewashing the wells, 100 pL of
an
appropriate dilution of antibody in PBS-0.05% Tween TM 20 with 1% bovine serum
albumin (BSA) is added and incubated for 1 hour at 21C. The unbound antibody
is then washed out with three exchanges of PBS-0.05% TweenTm 20. One
hundred pL of an appropriately diluted horse radish peroxidase conjugated anti-
immunoglobulin G (IgG) in PBS-0.05% TweenT" 20-1% BSA is then added to
each well and incubated for 1 hour at 21'C. The wells are then washed twice
with
PBS-0.05% TweenT"" 20 and finally with PBS. One hundred pL of soluble MTB
substrate solution is added to each well and incubated for 30 minutes at 21'C
after which 100 pL of 1V1TB stop reagent is added and the color intensity is
measured at 450nm using an ELISA plate reader.
1001031 Appropriate dilutions of the antigen and antibody are established by
performing checkerboard titrations. Antigen concentrations in samples are
interpolated from standard curves.
EXAMPLE 3 ¨ Indirect ELISA Assay Protocol
1001041 The following example describes an indirect ELISA assay used for
detecting a multiple sclerosis-associated antigen in various samples. This
assay
is constructed using polyclonal and monoclonal antibodies. ELISA wells are
coated with polyclonal antibody at an appropriate concentration and the wells
are
washed and blocked as described above. Various dilutions of antigen containing
samples are added to the wells and incubated for 1 hour at 21 C, after which
the
wells are washed 3 times with PBS-0.05% TweenT"' 20. The monoclonal antibody
49
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
is then added at an appropriate dilution in PBS-0.05% Tweenrm20 - 1% BSA and
incubated for 1 hour at 21 C. The wells are then washed 3 times and an
appropriately diluted horse radish peroxidase conjugated anti-mouse Igrv1 in
PBS-
0.05% TweenTm20-1% BSA is then added to each well and incubated for 1 hour at
21'C. The wells are then washed twice with PBS-0.05% TweenTm20 and finally
with PBS. One hundred pL of soluble MTB substrate solution is added to each
well and incubated for 30 minutes at 21 C after which 100 pL of MTB stop
reagent
is added and the color intensity is measured at 450nm using an ELISA plate
reader.
1001051 Appropriate dilutions of antigen and antibody are established by
performing checkerboard titrations. Antigen concentrations in samples are
interpolated from standard curves.
EXAMPLE 4 ¨ Flow Cytometry Protocol
1001061 The following example describes a flow cytometry assay used for
detecting a multiple sclerosis-associated antigen in various samples. PBrv1Cs
from multiple sclerosis (MS) subjects and control subjects are stained with
fluorescent antibodies to the multiple sclerosis-associated antigen (e.g., Tau
protein) and also with fluorescent labeled antibodies to cluster designation
(CD) 3
T-lymphocyte marker or CD 19 B-Lymphocyte marker, CD68 intracellular
monocyte marker and CD14 monocytei macrophage cell surface marker. The
labeled cells are analyzed by flow cytometry for qualitative or quantitative
differences.
1001071 PBMCs are obtained from a BD Vacutainerry CPT tube using a cell
separation procedure. The cells are washed three times in 1X PBS and
centrifuged in a horizontal rotor (swing-out head) for a minimum of 5 minutes
at
1200 to 1500 RCF (Relative Centrifugal force). The supernatant is removed and
the cells are resuspended in lx PBS. After the final wash, the cells are
resuspended to approximately 4.0 mL in 1X PBS. Approximately 50 pL of the cell
suspension to be analyzed is transferred into tubes for double staining with
selected antibody pairs. Ten pL of 40mgimL normal human IgG (Sigma-Aldrich)
for a total of 400 pg is added to each tube to block FC binding. The
appropriate
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
cell surface monoclonal antibodies CD3 PE, CD19 PE or CD14 PE are added at
this time and incubated for 20 minutes at room temperature.
1001081 One hundred pi of Dako Intrastain TM Reagent A (fixative) is added to
each
tube and then mixed gently with a vortex mixer to ensure that the cells are in
suspension. Cells are incubated at room temperature for 15 minutes. Two mL of
1X PBS working solution is added to each test tube and mixed gently. The tubes
are centrifuged at 300 X g for 5 minutes. Supernatant is aspirated leaving
about
50 pl of fluid. The fluid is mixed thoroughly to ensure that the cells are in
suspension.
1001091 One hundred pL of Dako intrastain Ty Reagent B (permeabilization) is
added to each tube. The appropriate amount of the antibody specific for the
multiple sclerosis-associated antigen is added to the appropriate tubes. The
tubes are mixed gently to ensure that the cells are in suspension and
incubated at
room temperature for 15-60 minutes. Two mL of 1X PBS working solution is
added to each test tube and mixed gently. The tubes are centrifuged at 300 X g
for 5 minutes, and then the supernatant is aspirated, leaving approximately 50
pl
of fluid. The fluid is mixed thoroughly to ensure that the cells are in
suspension.
1001101 One hundred pL of Dako lntrastainTM Reagent B (permeabilization) is
added to each tube. The appropriate volume of the 2nd step antibody conjugated
to FITC (specific to the multiple sclerosis-associated antigen) is added to
the
appropriate tubes. The tubes are mixed gently to ensure that the cells are in
suspension and incubated at room temperature for 15-60 minutes. To each tube,
2.0 mLs of 1XPBS working solution is added. The tubes are mixed gently then
centrifuged at 300 X g for 5 minutes. The supernatant is aspirated, leaving
approximately 50 pl of fluid. The tubes are mixed thoroughly to ensure that
the
cells are in suspension.
1001111 The pellet is resuspended in an appropriate volume of fluid for flow
cytometry analysis. The sample is analyzed on a flow cytometer within 24 -48
hours. For analysis, the gate is on the monocyte population and the data is
collected in list mode. Qualitative and or quantitative differences are
determined
51
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
between normal and MS patients using the analysis software. Optimization steps
include varying incubation time with antibodies, fixation time and
permeabilization
time.
METHODS FOR MEASURING HIGH MOLECULAR WEIGHT COMPLEXES OF
FIBRINOGEN WITH FIBRONECTIN AND FIBULIN-1 (MSDX COMPLEX-I)
[00112l It has been surprisingly discovered that the expression of a protein
complex (e.g. an aggregate, a complex) termed "MSDX Complex-1" is elevated in
multiple sclerosis patients as compared to healthy controls. MSDX Complex-1 is
a
high molecule weight complex comprising fibrinogen, fibronectin, and fibulin-
1.
MSDX Complex-1 alone or in combination with other markers may be useful as an
indicator of multiple sclerosis or other diseases or conditions, for example
for an
inflammatory condition, a neurodegenerative disease or condition, cancer,
stroke,
or other diseases. rvlSDx complex-1 alone or in combination with one or more
other biomarkers may help monitor disease activity (e.g., relapse, remission,
etc.).
Monitoring disease activity may be useful for detecting a response (e.g.,
positive
response, negative response, lack of response) to a therapy, for detecting
patient
compliance with a therapy, or for providing useful clinical information for
disease
management.
100113j The present invention features methods for measuring high molecular
weight complexes of fibrinogen with fibronectin and fibulin-1 ("MSDx Complex-
1")
and applications thereof. The methods may be used to monitor disease activity
and therapeutic efficacy in diseases or conditions that have an inflammatory
component, for example autoimmune diseases, neurodegenerative diseases,
cancers and metabolic diseases such as type 2 diabetes mellitus. The present
invention is not limited to the aforementioned diseases and conditions or the
aforementioned applications.
1001141 The present invention features methods for measuring high molecular
weight complexes of MSDX Complex-1, e.g., fibrinogen with fibronectin and
fibulin-1, in a sample. As used herein, the term "MSDx Complex-1" refers to a
high
molecular weight complex of fibrinogen, fibronectin, and fibulin-1. The
detection of
MSDX Complex-1 may be used for a variety of purposes, for example for
52
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
detecting a disease or condition, for monitoring a disease or condition, for
monitoring a therapy, etc.
1001151 A circulating high molecular weight protein complex has been found to
bind certain small peptides selectively. For example, by sephacryl 5200 gel
filtration chromatography, the binding activity was found in a broad peak of
400,000-900,000 kD. This peak was collected and shown by LC/MS, after in
solution protease digestion, to consist of Fibrinogen, Fibronectin and Fibulin-
1.
The present invention features a unique competitive ELISA assay format to
measure the amount of Pv15Dx Complex-1 in a sample, e.g., plasma, by its
ability
to compete with an anti-peptide antibody for binding of the labeled peptide
(e.g.,
biotinylated peptide). In some embodiments, the method comprises introducing a
labeled peptide and an anti-peptide antibody to a sample to create an antibody-
sample mixture. The anti-peptide antibody can bind to at least the labeled
peptide
and MSDX Complex-1. The labeled peptide comprises a label molecule (e.g.,
biotin). The label molecule is not limited to biotin but may include any
appropriate
label. Labels are well known to one of ordinary skill in the art.
[00116I In some embodiments, the method further comprises providing a well
(e.g., Et.ISA well) coated with a "well antibody". The well antibody is
specific for a
complex of labeled peptide and anti-peptide antibody. The method further
comprises introducing the antibody-sample mixture to the well and introducing
a
substrate to the antibody-sample mixture in the well. The label molecule of
the
labeled peptide and the substrate interact to provide a signal. The level of
the
signal is compared to a control. If the level of the signal is higher than
that of the
control, then MSDX Complex-1 is not detected. If the level of the signal is
lower
than that of the control then MSDX Complex-1 is detected.
1001171 In some embodiments, the labeled peptide is or comprises SEQ ID
NO:3A. In some embodiments, the labeled peptide is or comprises SEC) ID NO:
4A. In some embodiments, the labeled peptide is or comprises SEC) ID NO: 5A.
1001181 In some embodiments, the label of the labeled peptide is located at
the C-
terminus, the N-terminus or at both termini. In some embodiments, the labeled
53
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
peptide is between about 15 to 50 amino acids in length, e.g., 24 amino acids,
between about 15 to 40 amino acids, between about 15 to 30 amino acids,
between about 20 to 30 amino acids, etc. In some embodiments, the labeled
peptide has a pl of about 6.1. In some embodiments, the labeled peptide has a
pl
between about 6 and 7.0, between about 5.5 and 6.5, between about 5.8 and 6.4,
etc. In some embodiments, the labeled peptide has a net charge of about -0.1
at
pH 7Ø In some embodiments, the labeled peptide comprises an epitope tag
disposed at the C-terminus, the N-terminus, or at both termini.
100119] The present invention also features a method of detecting MSDX
Complex-1 comprising introducing a first antibody to a sample to create an
antibody-sample mixture, wherein the first antibody is specific for one of
fibrinogen, fibronectin, or fibulin-1. The first antibody comprises a label
molecule
(e.g., HRP). A well (e.g., ELISA well) is provided. The well is coated with a
second
antibody, wherein the second antibody is specific for one of fibrinogen,
fibronectin,
or fibulin-1. In some embodiments, the method further comprises introducing
the
antibody-sample mixture to the well and introducing a substrate to the
antibody-
sample mixture in the well. The label molecule and the substrate interact to
provide a signal (e.g., a chemiluminescent signal, a fluorescent signal, a
colorimetric signal, a potentiometric signal, an amperometric signal, or a
combination thereof). When the signal is detected then MSDX Complex-1 is
detected.
1001201 In some embodiments, the first antibody is an anti-fibulin-1 antibody
and
the second antibody is an anti-fibrinogen antibody. In some embodiments, the
first
antibody is an anti-fibronectin antibody and the second antibody is an anti-
fibrinogen antibody. In some embodiments, the first antibody is an anti-
fibrinogen
antibody and the second antibody is an anti-fibrinogen antibody. In some
embodiments, the first antibody is an anti-fibulin-1 antibody and the second
antibody is an anti-fibronectin antibody. In some embodiments, the first
antibody is
an anti-fibronectin antibody and the second antibody is an anti-fibronectin
antibody. In some embodiments, the first antibody is an anti-fibrinogen
antibody
and the second antibody is an anti-fibronectin antibody. In some embodiments,
the first antibody is an anti-fibulin-1 antibody and the second antibody is an
anti-
54
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
fibulin-1 antibody. In some embodiments, the first antibody is an anti-
fibronectin
antibody and the second antibody is an anti-fibulin-1 antibody. In some
embodiments, the first antibody is an anti-fibrinogen antibody and the second
antibody is an anti-fibulin-1 antibody.
[00121] In some embodiments, the method further comprises introducing a third
antibody to the antibody-sample mixture prior to introduction to the well, the
third
antibody is specific for one of fibulin-1, fibronectin, or fibrinogen, wherein
the third
antibody has a different specificity than the first antibody. In some
embodiments,
the method further comprises introducing a fourth antibody to the antibody-
sample
mixture prior to introduction to the well, the third antibody is specific for
one of
fibulin-1, fibronectin, or fibrinogen, wherein the third antibody has a
different
specificity than the first antibody and a different specificity than the third
antibody.
[00122] In some embodiments, the label molecule comprises an enzyme. In some
embodiments, the enzyme comprises horseradish peroxidase.
[00123] In some embodiments, the first antibody is a rabbit antibody. The
first
antibody is not limited to rabbit and may be any other appropriate antibody
(e.g.,
mouse, human, etc.). In some embodiments, the second antibody comprises an
anti-rabbit antibody, e.g., a goat anti-rabbit antibody, a mouse anti-rabbit
antibody,
a human anti-rabbit antibody, etc.
[00124] As used herein, the term "about" refers to plus or minus 10% of the
referenced number.
EXAMPLE 1A
[00125] The following example describes an example of a method of detecting
MSDX Complex-1. Anti-Fibrinogen antibodies are immobilized onto an assay
surface (e.g., ELISA well, glass slide, magnetic particle, antibody array
matrix)
and blocked using conventional methods. A biological fluid (e.g., serum,
plasma,
cerebrospinal fluid) is then contacted with the immobilized antibody and
unbound
material is washed off. Then antibodies to fibronectin and/or Fibulin-1 are
contacted with the immobilized material and unbound antibodies are washed off.
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
The bound antibodies are then detected with a labelled anti-immunoglobulin of
the
appropriate specificity to generate a measurable signal (the signal may be
chemiluminescent, fluorescent, colorimetric, potentiometric, amperometric
etc).
EXAMPLE 2A
1001261 The following example describes an example of a method of detecting
MSDX Complex-1. In some embodiments, method is a competitive ELISA assay
format. In some embodiments, the competitive ELISA assay used for detecting
MDSX Complex-1 utilizes a labeled analyte and measures the ability of an
unlabelled native analyte in a biological fluid to compete with the labeled
analyte
for binding to the antibody. In this assay the labeled analyte is bound by an
unrelated binding protein that prevents ifs binding to antibody and is washed
off
before the detection step. A standard curve to quantify binding by mosx
Complex-1 is generated by competition with "cold" peptide.
1001271 ELISA wells coated with goat anti-rabbit IgG(Fc) trap immune complexes
formed between a rabbit anti-peptide antibody and biotinylated peptide. MSDX
Complex-1 in added plasma competes with the rabbit anti-peptide antibody for
binding to biotinylated peptide. The more MSDX Complex-1 that is present in
the
plasma the more biotinylated peptide it binds leaving less available to bind
to
antibody. Thus high levels of MSDX Complex-1 result in low optical density and
vice versa.
1001281 In some embodiments, the peptide is labeled at the C-terminal with
biotin
or another detection agent. In some embodiments, the N-terminal of the peptide
may be amine or amide. In some embodiments, the peptide is 24 amino acids
long. In some embodiments, the peptide sequence is:
CQYRCFQVITNGIGLNLFKDPVAD (SEQ ID NO: 3A). In some embodiments, the
peptide has a pl of 6.1. In some embodiments, the peptide has a net charge of -
0.1 at pH 7Ø In some embodiments, the peptide has an average hydrophilicity
(Hopp & Woods method) of -0.3. In some embodiments, the peptide has a ratio of
hydrophilic residues to total residues of 33%. In some embodiments, an epitope
tag is attached to a terminus, e.g., the N-terminus, to enable the use of
other
capture antibodies, for example a polyHistidine tag (HisTag).
56
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
1001291 In some embodiments, any peptide sequence derived by conservative
amino acid substitution rules such as the Dayhoff matrix and the like of SEQ
ID
NO: 3A may be used. In some embodiments, alternative peptides may be used.
1001301 In some embodiments, the peptide sequence is
CSFKCYSVVINGLGINVFKDPVAD (SEQ ID NO: 4A). In some embodiments, the
peptide has a pl of 6.1. In some embodiments, the peptide has a net charge of -
0.1 at pH 7Ø In some embodiments, the peptide has an average hydrophilicity
(Hopp & Woods method) of -0.3. In some embodiments, the peptide has a ratio of
hydrophilic residues to total residues of 33%.
1001311 In some embodiments, the peptide sequence is
CQYRCFQIITNGIGLNLFKDPVAD (SEQ ID NO: 5A). In some embodiments, the
peptide has a pl of 6.1. In some embodiments, the peptide has a net charge of -
0.1 at pH 7Ø In some embodiments, the peptide has an average hydrophilicity
(Hopp & Woods method) of -0.3. In some embodiments, the peptide has a ratio of
hydrophilic residues to total residues of 33%.
1001321 The various peptides described bind selectively to a macromolecular
complex consisting of Fibrinogen B, Fibronectin and Fibulin 1. The levels of
this
complex have surprisingly been found to be associated with neuroinflammatory
diseases including multiple sclerosis. Addition of the peptide to plasma or
serum
causes the peptide to bind to the complex of Fibrinogen B, Fibronectin and
Fibulin
1 effecting a transformation of matter that results in the formation of the
aggrefatin
complex.
57
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
APPENDIX
ABSTRACT
Several factors currently impede therapy development and clinical study design
for neuroprotective agents for Parkinson's disease. These include the limited
ability to detect early stage PD prior to the onset of motor signs, inadequate
measures of processes or pathways related to disease pathogenesis, and the
lack
of biomarkers that define disease progression.
We propose to develop a new source of circulating biomarkers for use in
diagnosis and monitoring of disease activity by exploiting the ability of
monocytesimacrophages to enter the brain and engulf debris coupled with the
paradigm shifting hypothesis that at least some of these cells re-enter the
circulation. Thus we may exploit these re-circulating cells as a shuttle
vector that
retrieves antigen from the brain so that we may probe these cells for new and
established CNS tissue markers. Unlike circulating antibody markers, which can
remain in the circulation for years after the tissue insult, antigens engulfed
by
phagocytes are degraded within a few days. This means that if these CNS
antigens are detected, they are reflective of recent tissue injury. Given that
70-
80% of striato-nigral neurons are lost before clinical symptoms arise, the
clearance of the dead neurons by recruited and re-circulating mononuclear
phagocytes should be an early indicator of neuronal damage. If we are
successful
in validating this concept, we will be in a position to rapidly identify and
develop
potential biomarkers to improve the efficiency and outcome of Phase H clinical
trials and advance therapeutic development for PD.
PROJECT NARRATIVE:
The work proposed in this application intends to develop biomarkers that
enable
rapid, reproducible and cost effective monitoring of disease activity and
response
to therapy to facilitate phase H drug trials as no such biomarkers are
currently
available for this purpose. We will develop a novel strategy exploiting re-
circulating phagocytes to retrieve biomarkers from the brain for this purpose.
SPECIFIC AIMS
We have postulated, counter to immunological dogma, that phagocytes entering
the brain may re-enter the blood circulation. We have generated preliminary
data
that is consistent with this hypothesis and propose that these phagocytes may
be
exploited as a novel source of biomarkers because they carry CNS debris.
Furthermore, as the debris is degraded within days, this approach may be a
means of monitoring "active" neurodegenerative processes. The goal of this U18
proposal is to apply this innovative concept to monitoring disease activity in
Parkinson's disease by pursuing the following specific aims:
Specific Aim 1; To discover relevant CNS proteins in re-circulating
phagocytes from Parkinson's Disease (PD) Subjects:
Our preliminary studies in multiple sclerosis and Cuprizone fed mice have
shown
that Tau, Hippocalcin like 1, myelin basic protein and proteolipid protein can
be
found by ELISA assay in lysates of peripheral blood mononuclear cells (PBMCs).
Consequently we will probe for these proteins in PBMCs from PD subjects.
58
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
Additionally we will probe lysates for alpha-synuclein, glutamic acid
decarboxylase
(GAD) and ubiquitin carboxyl-terminal esterase Li (UCHL1) proteins. We will
also
attempt to discover potentially informative CNS antigens in PD PBMCs using a
shotgun proteomic analysis described in the approach section of the research
plan. Hippocalcin like 1 was discovered to be a phagocytosed biomarker using
this approach.
Specific Aim 2: To determine marker prevalence in various clinical
phenotypes of Parkinson's disease:
Using an ELISA employing specific antibodies to identified candidate CNS
antigens in PD PBMC lysates we will determine the prevalence of each marker in
50 recently diagnosed (< 3 years) PD subjects and 20 apparently healthy
subjects.
Specific Aim 3; To determine the phenotype of the PBMCs that contain the
neural antigens:
The cells containing the neural markers will be characterized by
immunophenotyping PBMCs. Coexistence of neural antigens with antigens
specific for leukocyte subpopulations will be determined by use of specific
antibodies. This may be achieved by flow cytometry, immunofluorescent
microscopy and/or cell type specific enrichment/depletion using magnetic
beads.
We expect the results to identify known phagocytic cell types (CD14+ monocytes
and/or macrophages (CD 68/CD11b)) to be the source of the neural antigens in
P B MCs
RESEARCH STRATEGY
(a) Significance
As stated in the request for applications, several factors currently impede
therapy
development and clinical study design for neuroprotective agents for
Parkinson's
disease. These include the limited ability to detect early stage PD prior to
the
onset of motor signs, inadequate measures of processes or pathways related to
disease pathogenesis, and the lack of biomarkers that define disease
progression.
We propose to develop a new source of circulating biomarkers for use in
diagnosis and monitoring of disease activity by exploiting the ability of
monocytesimacrophages to enter the brain and engulf debris coupled with the
paradigm shifting hypothesis that at least some of these cells re-enter the
circulation. Thus we may exploit these re-circulating cells as a shuttle
vector that
retrieves antigen from the brain so that we may probe these cells for new and
established CNS tissue markers. Unlike circulating antibody markers, which can
remain in the circulation for years after the tissue insult, antigens engulfed
by
phagocytes are degraded within a few days. This means that if these CNS
antigens are detected, they are reflective of recent tissue injury. Given that
70-
80% of striato-nigral neurons are lost before clinical symptoms arise, the
clearance of the dead neurons by recruited and re-circulating mononuclear
phagocytes should be an early indicator of neuronal damage. If we are
successful
59
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
in validating this concept, we will be in a position to rapidly identify and
develop
potential biomarkers to improve the efficiency and outcome of Phase H clinical
trials and advance therapeutic development for PD.
(b) Innovation
Immunological dogma recognizes that mononuclear phagocytes are recruited to
sites of tissue injury where they perform a number of functions including
clearance
of debris. It is also a matter of dogma that if these debris laden phagocytes
egress from the damaged tissue, they do so into the draining lymph nodes of
the
lymphatic system. We have postulated, counter to immunological dogma, that
phagocytes entering the brain may re-enter the blood circulation, and we have
generated preliminary data in multiple sclerosis (MS) subjects and Cuprizone
fed
mice that is consistent with this hypothesis. We propose that these phagocytes
may be exploited as a novel source of biomarkers because they carry CNS
debris.
Furthermore, as the debris is degraded within days, this approach is a means
of
monitoring "active" neurodegenerative processes. The goal of this U18 proposal
is to validate this innovative concept for monitoring disease activity in
Parkinson's
disease.
(c) Approach
Preliminary Studies
To obtain pilot data to support this concept, we obtained peripheral blood
from 18
subjects with MS and 12 apparently healthy individuals. PBMCs were isolated
and osmotically lysed. The lysates were coated onto ELISA wells at 5ug/mL and
probed for Tau antigen or Hippocalcin like-1 antigen using standard protocols.
Tau is a neuron specific microtubule associated protein that is best known as
the
substrate of neurofibrillary tangles in Alzheimer's disease. Hippocalcin like-
1 is a
neuron specific calcium sequestering protein that is most abundant in the
hippocampus. The ELISA assay results for these 2 antigens in the pilot
population are shown below (Figure ).
CA 02929711 2016-05-04
WO 2014/071359 PCT/US2()13/()68465
.1:1/0512013 1235pin
::::s. ............................................... . === = =
- = :: II:
õ..,
TAU - =''': .. .
õ
:: =:` = , ,,... =
. = k :z
=
:., = 4 .. = = i../ :: . . .... ,
= ..1 = 1 d
=
.3.
ii. ., .4 = . :1 = .
E =:., 1--4::::. i 1:,===1:i ......iv..:::, t: =1/ 1
:il '.. '= ... 1 .
C .. .4 zi a il h il k= 4 41 .. 1 .Z ; q : ; <,. :, k
: === .. .
i' li '.: Z N k=: ..1==,,, =, :. t -- .... - - -.-11-
= .--.:
0 4.- = p= 1 A A ..it :A A.I A l= .µ; 4 = A
i. q
i 1
LO '" 4.1 . i k.:41::,:ii.t.ivi 4 .:
=t: A ,/ 1 = =k11=1'1 11- 1
'1.' .; k...g It k J
.4...4.::::.A::.i.i:....1...11:...1..:h .1. :.1,1 B . .... ib it
1,0 = '
la) <Multiple Sclerosis> <Control>
0
. .. . .....:...-::
= - =
. ... .
....
..... .
. . ...............
-.--:--.-.:... -ke. - - = -:: . HtppocalcilliJ.1: ...::
õ.C2 . = .: :: 11 = .., : ........,
:.
s.... ...õ . .... .. . :.:. . .. 1 =
0 .: := 4 ".:1. = :. == .. A
........- " .. = ' . '''' = ' = ' :.- ' : -====,
ts.) ..:L.= .. ,.. .. = =:=1 = = = '' = = = = =
. . j= = :..: . .:.. A. = .,. =
. =-= = .= :. =
A .s
: k,:.:., :====: . == . . = .=
.=.=== . = = = =
e-1. ,:.....:=.: : .= = z,..
::::. . ... ,õ,...... . ,...=== ...v.,.
... .. . . =
..= . " = -: I == = : :=:.= ==== = =:== == = ==
= ..,,\ =
= = ::: . . ..=== = . . : . = .
: = = .. .
04. .40.: ,..= \:....klt . . :R. . = = . =: .
-,._,4 =
t . : .4 . ..= Z . - = '
= = :.. =.: .. = = :;1 .' . = := . . : .
'' i.'õ 4: . '''' -.4.4. it .. . A = A =p =
...,======= = = = k: = . : i = : .= :..--.
õ=:1...1.....k...4.. ........= .%1 : . , .: . ': µL.r
p..,4 . 0..i..t: : :......-.........--.,..:,
:: ::==== :: A k..p..,i..: 4.....i.k...A. õ.
k..4..: 1 .4..4t..1....4.:.t1...F..4.=,.. =µ=
,,,, õ:-., = ...4..,z=:=g.:.4 'µ'.1. ..-
i-..z:===t.t,'.......' ,, zi. :.... 1 ...4-1...f...ts-t.:4'.1.1.1.= k k' =
.:,. = A R ...b....:4....t...:.k 4..4.A .A.
:11...1=:..k..L..1...t.,.....4....b..:.4. A A.. ,A:.4 .1: ' ',. 4.= =
<=Multiple Sclerosis> <Control>
Figure 1. Tau and Hippocalcitl Like-1 It)vels in PEIMC iysates in 12
apparently heatthy
controls and 16 MS StInjects.
in the control group one individual gave anomalously high results in both the
Tau assay and the
Hic)pecalciri Like-1 assay: Consequently the second highest control was
art)itrarily selected as a
cut-off point for each assay. For each assay this resulteti in 7/18 positive
MS subjects and 1/12
i..)ositive controls. The results were not statistically significant for
either antigen alone. However,
analyzing the results for positivity for either of the two antigei)s resulted
in 9/1f3 positive MS
subjects and 1/12 positive controls (Table 1) which is a statistically
significant difference. -rile
results are shown in the 2x2 contingency table below (Table 1) and are
statistically significant by
the chi squared test, p=0.0235
. ................. .. . ___________________
770-4.Lar=-..flipppl,e,11:1-1..:=::::::::l=:: : '..::::::: =:: Positive
. : : :==:=::::=.= ::.: Native ::: : . ::=.:: : :: ::.
::::=:.==:...:==:=Totai:=::::
ftll=:.::::=::.=::;:,..:;::0:::::.fiti::;:;=::::,:::::;:=.:.t.t::::::::::;:lRt:
I..=:;::::t:;=;:::::::::t:.:;i::;l::::;l:;41::::l::::lll;;:=::;tl;ilia:!:.t....
:;:;:1=1:;l:1::::i..i=til:il.:;:iii:1::::;ll;illll::.:::=:::::;:.=:l.:;:=;::::i
::::::::lt:112::l::::::::::::;::lg=:i:;:i:=,:;:;::::::i
s::::::::::::::::::-
.A:::A::::::::::=:,:=::::....:.::::::=;õ==t;=:::::::=:=:::::õ:=:::::::::=:::=::
::0==ii:::::::::=::::::::::::==::::i=:::::A-
,:m::::::::::::::::::::=1::::0::=:::::::i.R::::=.=::=::::::.=::::::::::::.
=:.=i:=::::::i::.=.=:::::H.:::::::0=:::m::::R:=:?:=:===.:&:::g.::::=ii
MS
Iii;I:I::::;:::.!11:::::=:.liilli;:li.l.liiiiiiiiii!illiiNiiiiii=ill=;::::Ilii!
=il::Ilia.:11;;;:01::;:i.::::!lil:=:.;:i!ll.:;;N::1:,;:ilii::iii:;;;..4:::::=11
;1õ.:1!=;.:Iii.:1:::.:;.:ililii.:IT:;11.li;i::3;=.11':::.l'=iinig;1;i=:ll.11t;.
gii:llilli;li:1;:õIliiii;ll:;1;l::;t1Ill;llii:l;:;:1:::::1;1=:.*:;li;l:;:õ..ill
;:il!;:it;.*:ii=illi;i;lli::::.:::li;.:;ll.:;;Il;;I:t;=.:ltil
. ..... ....................................... . .. ...
.......................... . .............. . ....
......................
,........................................................................
.. ... ......................................... .... ..
... .. .. .. . .. ..... ....... ..... .... ..
..... . ......... .. .... .... ....... ....... .....
.. ..
:.:. . ,.:r.:.:=:.õ,:mi:=.7,-,,:nr,,,,,n,,,n:?....t..:::::.::õ.::::. ...
...:::::..: :: ; :::::::::::::::::::::::.:::: :i:::-
:::;::::::::::::::.::.::::.:.:.:.:...:.:.:......::::::::::::::.:..::::::.::::::
:;::::::::::::::::::,:iii,ii=ii=
..,:::,:õ.:::::,...:,..,:=,:.:ii::::::::::,;::::::ii::::::::::::::::::::,;õ::::
::::i:::::::::::::::::::::::::::::::::::::::,=:::::::::::::;.=::,....::::::::::
=õ::::::::::i:=:::::=::::::::::õ.:::::=:::.::::::::::õJ::::::::::::::::::::::::
:::=:::,:::::::::*ii,::::i.õ-
:=;:::::::::::::::::::::::::::::::i;::::Kõ::::::::::.:.:::::::i;:::::::::::::i:
:::::::::::::::::::::::::::::::::::::.::::
i::::::::::::::::::::::::=::::::i::::::::::=:::.=:::::::::::õ....,::::::::::.=:
.:::::::::::::::a:::::::::::::::::::i
=;;;=-=== .= - -=-= - -; - -;-::::::::::::=,:::::::::::::::-
::::::.:.,:::,:::õ.:-.:,::=::::::::::::::::::::::::::::::=:::::i;:::::::=-
:=::::::::::=::::::::::ii:::::::::::::::::::a:::::::::::::::::::=1::::::::::i;:
::::::::::::::::ii:::a::::.i:::=:ii::::::,::::::::::::,=:::::::i:::::::::::::::
::::i::::::::::::::::::::::::::::::::::::::.=
:::::::=:::::::::::::::::::::::=a::=:::::::::::::::::::::::::::::::::::::::::::
i:::::R:::::::=:::::::::::::::::::::::)
ril.:::!.::::::41.:4::::.;4:::I.4illi'::.:=Ii.;;.;;=;.11%;;'=;l:;:;:ti':::::i;:
:::::-::i::::::;:==:=::::.:::::=:::
::::..,:::::.::::=:::=::::::::::=::::::=:::::i:=.:=::=:.:::::.:::::::::::.õ-
::::::::::::::::::::::::::=:::::=::.:=:::::::::::::.:::::::=:::::i=:::::::,=:::
::::::.=-
:::.=:::::::i0:=::=::::i:::::::i::.:=.:=:::::=::=:::::i::::::::::::=:::::::::::
::::=::::::::a -
::::::;=:i:::=:::::::::::::::.:=:::.:::::=:::::::=:.::::::::::::.::::::::::::::
:::::::::::=::::=:::.;-:::::::::.:::::::::::::.::.:=:t=.::.:=.:=::
1,:iii:jõ,,,,,4,:.õµõ,:4;.:õ:õi.õ:õ::::::::.:õ..:,44:::,,a,,,,,,=,=.õ:::=::::::
:....:.:=:=:=:, :-.:=:::::::-
:::::::::::=:::::::::::::::::::::.::::::::::::::::..;:.:.,....*K.J.:.::::::=:::
::K::::::::1:::::,:::::::::::::::::::,...*:,T;,=4õ;õõ1,::::::::.,,=::,õõiõ:õ:;4
::::4;:.õ:õ=====.:,:,.,,::::::.:,::::::::,:::::=;.,...,::::::::,,,...õ:::::,::=
,:,=;:::õ,:::.,::::::.,::::::::,...,...:::::õ:,,
i==:.:7.:rizilf.:=:::::::::::=::::=:::::n:::::::::::::-õ;t:::=-
=:::::.::::::i::::=:::::,=.::::::=:
=:::::::::::::::::::==::::::::=:.=::::ii:=::::::1.:::::.::::40:::=::::::::::::=
:::::::::::::::=:::,=:::::::::;:i:::::::::::i===::::::::::::=::::::m=;::::2(1::
aa:=-:::::::N:mi.::::::=::::::::,...::::::.,:-
::::::::::::=:.=;.:=::::=::::=====.26:::
.....a...:- .:.:...f.,:iii:......-.,.i.::::.......*:-..:',:-:::i..-.-
:iii:,::.::::::;........=A.-......:
i...i.i::...q.:...i.ii:iift.......iiP......::::::....i..?:'.............t.:::::
:::::::::::'.....:.:N.;............' :'.......n1:,..1......::.*::::::-
..::::::::::.....'.:iiii:"....::::::.......:;:i....---
............::::....:;.:N.........:;:...iii.....N.....:::::=Ki.................
...i..:::::::::-..::::::ii.,:i=-,:::::::....i.:.:::a::-.::-.-:::-.:-
..mii.i.:;:...:i::::::-.::',g,........,.....,::::ii...
IL.:2ILL.Z.i.:L..ii.2...:.,........:::::::::::::'.::::::::,'::::-
,'::.:.....:':'.:::,
:".',..:...,1,''''''',:.::::.:''''';''''',.'j.ki'..:::....-
.*::::..*:::::.A0i'.:::','1..R':4'.....L..L''..Z.....Ll2fi...222.....2.L..2.L.2
.-=.:''.::::::1LLLI:.:::1:.-...'''...Zig:';':::::'.1.:.':i.:MP,.1
61
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
The two-tailed P value equals 0.0235.
Tablet 2x2 contingency table of positivity for Tau or Hippocalcin Like-1.
We also examined PBMC from normal C5761/6 mice and cuprizone fed C5761/6 mice
for
CNS antigens. Cuprizone causes Demyelination at 3-4 weeks and also
neurodegeneration at 4-5 weeks. Discontinuation of Cuprizone feeding at 6
weeks or
earlier results in repair of the CNS damage. Five mice were fed with regular
lab chow,
two groups of five mice were fed with Cuprizone (0.2%) chow for 3 weeks and 4
weeks
and one group of 4 mice were fed with Cuprizone (0.2%) chow for 5 weeks. The
mice
were bled and PBMCs isolated by Ficoll density gradient centrifugation. The
PBMCs
were lysed and the ysates were coated onto EL1SA wells and assayed with
antibodies to
CNS antigens as with the human samples. The mouse lysake.s were probed for
Tau,
Hippocalcin like-1 and myelin basic protein (MBP). Simply by viewing figures 2
to4 it is
apparent that there is no difference in control and Cuprizone treated mice
until 5 weeks of
Cuprizone feeding, a time point when there is extensive histological evidence
of
demyelination and neurodegeneration.
Mouse lysates Tau
0.5
0.4
0.3
0.2
0.1
0
0
rb 'tb '113 4.5.9'hy bt,'Ix = "%- Karp <o< =
,tek*M. .04 k\)=ci4
ce" co' ce-27477
\\3..\\;'\\S/AstAt"tAt`t4cA\cAvAN;'1/2\c;\
Figure 2. Tau in PBMCs of Cuprizone fed mice
62
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
Mouse lysates Hippocalcin Like-1
0.5
0.4
0.3
0.2
1
0.1
0
0 11 11 ill 11 illl fill 11
ffil ill 11 11 11 I 1 I I 1 1 1 1 1 1 1
'''-'13k:4-4e.1s47:4%,114->e,INLQWelV6r-jike,*'¾c*QINL-c
cotN)*(4t-*A'tftb't-44,AAA:/i<DV<A*
CAC-CAvtAc')NbN N C=1., 'Th.n1;v34)`;\()MbP
`t, co'z'coq'co'z'coz'co(642'c-Pcog'46*
Figure 3, Hippocalcin Like-1 in PBMCs of Cuprizone fed mice.
Mouse lysates MBP
0.5
0.4
0.3
0.2
Ls,
0 0.01 11
A , õ
,
--4V'''..-491WeGtkIkc2e4,c-Aqqqqkcelt(-V
co '\ ora),õ,:v0n11)-tbiltb-VV-
b, 1):Vtolo-to-to
co co ro
N N-64-6'1\rcl,b1,(6)rbec,aktc
\.1e0,Z w,0
Figure \;c ,Z,2,7'\"cAvAvAv-\\:\;1/2\%\\;-\\,A 4.
Myelin basic
protein in
PBMCs of Cuprizone fed mice.
Comparison of grouped assay results by unmatched 2 tailed I.-test confirmed
the visual
conclusion that only the mice fed Cuprizone for 5 weeks were different from
the control
mice (Table 2).
Comparison Tau P value (West) Hippocalcin Like-1 P
value (West) MBP P value (West)
63
CA 02929711 2016-05-04
WO 2014/071359 PCT/US2013/068465
Naive vs 3 weeks 0.5204 0.3514 0,4622
Naïve vs 4 weeks 0.4280
0 8364 0,4776
Naive vs 5 weeks 0.0019***
0,0100*** 0,0001***
*** statistically significant difference
Table 2: Comparison of Cuprizone fed mice with control mice using the t-test
(2 tailed P values)
These results are consistent with the hypothesis that some phagocytes that
have engulfed debris
re-enter the blood circulation and suggest that measurement of the phagocyte's
cargo may be
informative in measuring disease activity in the CNS,
The year after we filed our patent application, Joy et al reported that
resident and
recruited phagocytes remove dead photoreceptor cells from the retina in a rat
model of
retinal degeneration. They published the electron micrographs shown below in
figure 5
showing a macrophage loaded with photoreceptor cell debris re-entering the
blood
circulation via a capillary. This cell has to be entering the capillary rather
than leaving it
as photoreceptor cells are unique to the retina and therefore cannot have been
acquired
elsewhere. This study provides incontrovertible evidence that phagocytes do
indeed re-
enter the blood circulation but did not consider the diagnostic implications.
= AVP:K\ \ NiviarMinl
=
1 =
: ====== = ===::;::;ik=::"'.N =
=
:" = "*:**N
= ; = . ---- ' --- " = M.
.M:;':ss. = \
=:>=-=N`= =40K .
=:=;:-
= :: =
= ==
" .
= = ,A;;õ
= :
= \
. " , .õ . = = ...
. = \ ,
gõ
'===:=====
= :
= =
=
,9 _____________________________________ 9
=
Figure 5. From Joy S et al. Resident Microglia and Bone Marrow immigrants
Remove Dead
Photoreceptors in Retinal Lesions. The American Journal of Pathology, Vol.
174, No, 6, June
2009.
Macrophage with engulfed photoreceptor debris in the process of diapedesis
through an
optic nerve head capillary the cell nucleus is inside the capillary whereas
parts of the
debris-containing cytoplasm is still externally located (arrow).
These studies provide "proof of principle".
Our strategy to achieve the specific aims of this proposal is discussed below.
Specific Aim 1; To discover relevant CNS proteins in re-circulating phagocytes
from Parkinson's Disease MD) Subjects.
64
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
While we have generated proof of principle data we do not yet know which
antigens will
be relevant to Parkinson's disease (PD). Consequently, our strategy is to
obtain PBMCs
from Parkinson's disease subjects and test them for the antigens that we have
found to
be relevant in other CNS diseases, namely Tau, Hippocalcin like-1 and myelin
basic
protein. We will also probe for alpha synuclein, glutamic acid decarboxylase
(GAD) and
ubiquitin carboxyl-terminal esterase Li. Alpha synuclein is associated with PD
pathology
and GAD is associated with "stiff man" syndrome which may have some overlap
with
symptoms of PD. Ubiquitin carboxyl-terminal esterase Li (UCHL1) is found in
all neurons
of the brain and should be present in recirculating phagocytes when there is
active
neurodegeneration present. Thus UCHL1 represents a safety net biomarker. UCHL1
polymorphisms have been reported to influence risk of development of PD.
We will also employ a shotgun proteomic approach to discovering new antigens
that may
associate with PD. We have used this method to identify Hippocalcin like-1 as
an antigen
of interest. PBMCs from 10 short duration PD subjects will be utilized for new
phagocytosed antigen discovery. Briefly, a lysate of PD PBMCs will be
subjected to 1-D
PAGE and the gel will be coomassie stained. Coomassie stained protein bands
are cut
out and subjected to in gel trypsin digestion followed by protein
identification by LC-MS-
MS. The list of proteins present will be examined for the presence of CNS
proteins.
Candidate biomarkers found will be studied using either commercial antibodies
if
available or by contracted production of anti peptide antibodies in rabbits by
Genscript
Inc.
The potential biomarkers selected for initial testing will be screened on
lysates from a
further 10, short duration PD, PBMC lysates by ELISA assay. Any potential
biomarker
that is positive on two lysates will be a candidate for further screening in
specific aim 2. A
minimum of three such candidate antigens will be screened in specific aim 2.
By using both a candidate antigen approach and a proteomic discovery approach
we
reduce the risk of not having potentially useful biomarkers for validation in
PD.
Specific Aim 2; To determine marker prevalence in various clinical phenotypes
of
Parkinson's disease:
Candidate biomarkers will be screened on lysates from fifty recently diagnosed
(< 3
years) PD subjects to determine prevalence. Comparisons will also be made to
20
apparently healthy control subjects. A single high prevalence (-80%) antigen
or a
combination of antigens providing high prevalence in aggregate will be sought.
As there is no pre-existing data on which to base power calculations, 50
subjects for each
group has been selected as it will enable the detection of a minimum marker
prevalence
of 2% (1/50)
Specific Aim 3; To determine the phenotype of the PBMCs that contain the
neural
anticiens:
The cell type(s) carrying neural antigens in the PBMCs have not been
specifically
identified but it is assumed that they are bone marrow derived mononuclear
cells. The
cells containing the neural markers will be characterized by immunophenotyping
PBMCs.
Coexistence of neural antigens with antigens specific for leukocyte
subpopulations will be
determined by use of specific antibodies.
This may be achieved by flow cytometry, immunoffuorescent microscopy and/or
cell type
specific enrichment/depletion using magnetic beads. We expect the results to
identify
known phagocytic cell types (CD14+ monocytes and/or macrophages (CD 68/CD11
b)) to
be the source of the neural antigens in PBMCs. Identifying the cell type
carrying neural
antigens will enable additional sample preparation steps that will enrich the
relevant cells
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
and will be expected to increase sensitivity of the assays.
We will collect 2 x 8 mL tubes of blood from PD subjects and prepare PBMCs.
Half of the
PBMC will be used to make a lysates and the other half temporarily
cryopreserved. The
lysates will be assayed to determine which neural biomarkers are present and
then the
cryopreserved cells will be resuscitated for analysis with CD markers for
leukocyte sub-
populations and the relevant neural antigens.
Statistical Analysis:
Statistical analysis will be required to interpret the results of specific aim
2. The
unmatched t-test will be used to determine whether the means of each group are
significantly different from each other. A p-value of 0.05 or less will
indicate statistical
significance.
Apparently healthy controls will be used to determine a cut off value (mean
+2SD) in
order to create categorical data that can be analyzed with 2 x 2 contingency
tables and
the chi squared test. This will also enable expression of the results as
positive and
negative predictive values.
Assay performance characteristics:
Prior to marketing research use only kits, we will perform reagent stability
studies to
determine a minimum shelf life of 6 months. Furthermore, we will provide kits
and PD
blood sample to 3 alpha testing sites to determine inter lab reproducibility
and coefficients
of variation. We will also ship kits to our self to determine transportation
effects on
stability at ambient temperature and on ice.
We have recently launched a research use only assay kit for multiple sclerosis
and have
experience in making these determinations.
Expected results, potential pitfalls and alternative approaches:
The potential pitfall in specific aim one is that the markers we have worked
with to date
have not yet been evaluated in PD and may not be found in phagocytes in PD. To
reduce the risk of not having appropriate antigens to probe for in PD PBMCs we
will also
perform proteomic studies on PD PBMCs to detect potentially relevant CNS
antigens for
PD. We also have included the UCHL1 antigen which is expressed in all neurons
and
should be present in engulfed debris. The tau antigen has also been implicated
in PD
and we expect to find it in PO PBMCs. Some antigens may have regional
differences in
abundance that may affect detect ability and may therefore only be seen in
particular
clinical phenotypes. Given the combination of approaches we expect to have
several
promising candidates.
A potential pitfall in specific aim 2 is that all potential markers will be
found to have a low
prevalence. This could be due to inadequate sensitivity of the assay. Specific
aim three
will provide the solution to this problem should it arise. Identifying the
phenotype of the
antigen laden cells will enable their enrichment prior to preparing a lysates.
This will
increase the amount of specific protein in the lysates to be assayed. We do
not anticipate
difficulties with specific aim three.
Alignment with PDBP goals:
The goal of the PDBP is to develop new and/or improved PD biomarker
methodologies
and technologies that can help inform Go/NoGo decisions in phase 2 clinical
trials this
includes studies required for moving an assay or method from an exploratory
stage
66
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
towards a validated approach for PD biomarker assessment.
We have developed proof of principle data for a novel approach for antigen
retrieval from
the brain utilizing re-circulating phagocytes as a shuttle vector. Because
these antigens
are short lived in phagocytes, the detection of their presence will indicate
current active
neurodegeneration. This approach is in alignment with PDBP goals as we propose
to
develop this concept beyond the "proof of principle" stage to create a
validated assay for
monitoring disease activity in Parkinson's disease. As drugs that decrease
disease
activity will be expected to decrease CNS antigen laden phagocytes in the
circulation, this
approach will facilitate evaluation of response to therapy and early
evaluation of likelihood
of success of phase II trials.
Usefulness to other PD biomarker researchers:
Progress in the development and use of biomarkers for PD has been limited by
inherent
barriers associated with studying the brain. Blood based biomarkers for PD has
been
unsatisfactory, perhaps because in transport from the brain to blood they are
degraded or
otherwise made inaccessible in many patients. Cerebrospinal fluid (CSF) is
preferred by
many PD biomarker researchers as it is felt that molecules entering the CSF
have not
been as heavily processed as those moving to the blood supply. However, a
simple,
inexpensive and reliable blood based biomarker assay would greatly facilitate
both
research and clinical care. The novel use of recirculating phagocytes as an
antigen
retrieval system coupled with its function in degrading tissue debris may be
developed
into just such a simple, inexpensive and reliable blood based biomarker assay
of active
neurodegeneration.
Data Sharing Plan:
Data will be deposited into the PDBP DMR following completion of data
analysis,
application for provisional patents and/or within one week of publication.
BioSpecimen Requirement:
MSDx will collect sufficient prospective samples from subject that will allow
MSDx to send
a sample (Serum, plasma, PBMC prep) to the NIH PDBP repository for use by
other
researchers. The corresponding patient demographics collected in the Case
Report
Forms will also be supplied.
Proposed Annual Milestones:
Specific AIM 1 Milestones- Candidate biomarkers will be screened on 10 lysates
from
short duration PD subjects. Biomarkers found to be positive in at least 2
subjects will be
selected for further analysis. A minimum of three biomarkers will be selected
for further
study.
67
CA 02929711 2016-05-04
WO 2014/071359 PCT/US2013/068465
11/05/70.13. 1235piti
jecific M 1 Milestones
.01acta for
PhVo:tottie4 IVIOYtgut,.#:.000,004k3.
Take.ftlivord:tholgiusvoiert..
\`. \NA =thect.pnwinttom.. ce.
I-01.pp 4 .c!etrol.zsi*tts:10.r.phogocopo.oti ' ".
Nom s.s,Th$00.,..w; . LAN
. V =
= 'W\.\ \stfft.
eD. .e. Is ======
= .. = = =
=
PrOe0i.tkSitfOrotkedmon0 A:Mk.
Win Pot4'otta
$!:5,e.c4fio AM 7..Milestones..-.=The...9o01 i.s.to dvop a nge phi-
.306cytoeci:bionlarket.of
prevaienCi (or Nghe.r)..Qr a ptainWrmtiot.) ofphagocytoseo biornarkers that
have an
49gregate=prOya.len. at least 70%....
68
CA 02929711 2016-05-04
WO 2014/071359 PCT/US2013/068465
I 1/0512013 12:35pm
- ........................................................................
ii IA .c.4 =v=-, ' .2".,i,,.
...t.p.,..: .,::,1 :: id-
:
,.. :..,.. ::===
:
...+. Y.
, =A:
CA 1 . . ..... i 's a
.... .... .... ....
a e '1 szt
..e t=== -t-0 -t< v.i i<=la
.:.,..: 0... 4.;
i:V 4,4k: M A AA .11:
"
=..... i
.......µ ,z4 :,...
04., W. ;ii ti f0-3 ti
V ill
"4-..... ,... >.,
.,,,...c,..
c,ti ,c,õ) >,..,µ c....,
,...,.
, o.o ,oc, ccc , ,co cv,
r...et .
'...#
..,,5---il-?,1.- e
,-. .õ. *.,..= :Es li: E.:-.
ii.i Ei: if::
cktµ' 'U'i :a': k
>r< =ti.:.* 3ii
....:' ..;.:' ..Z1: 'If'
. Z1.-µ .1? ? g=* ''4
.:
: t :%. t...
2'.
e.5
=,...; <:::= = - = v.;
..!3 C: 4: =?.. ,.4.7. /Tr S.:.
1 Q. r..: 1 .) =3.:, cz c,...: , c:
Q 4.:,
'...S: ',.. Ø -i
..y... ,,.: .m., ,.,...!
.,......,
.....
'-' --' e-' aI __ ;11 i
,õ,,,,..... 10.4 ,I... ,.. .:.=
. co ni V 0 0
W. ,..< co :V.
e c .,- 0 - ...= iret et:. cez
=a r k
A, A A A A A. ...
.,. 1
=t:-.
.....< ...., 1.... ,n, '==. 4::...
4.4 V.. `.....
At
'VS. =V Ø Z
44
,......i < V r" i.V ÷ 4 '("4 ==== 4
___________________________________ . __________ ....-
.,.: .....1 ....... X::
:::..4 I ".. , kt 4:::. k. Y.:=.,
or
CC-c..= .15 ver t .41
C.)1
0t. ..,r r ..,.
tr, r.. a ..t.0 .e.. .õ.-.i.= :
1 ,..::.. "C.. k!... . V.
.,,..:: '1. :::.% =,µ
v:
1 5i1 ti-4 Zi ---r ....! ,Ki:
.,,,1=:::,,,,, :,):: =A 'W
-.,.
A..., ;=..: -,, ..,, .::.4.. : ,= = : : -
`V.
s , .. ..........4.444
. 54.4.
. .7:
................................................................... ......
Sn
....`= .,..,:: ..o.":` *.1 :0.1 V..: 4.7. :
.S., t ::,i4..= ==<,< == = -4;
:..'si.....,!::=,:-.: c C:5 = b .....)3:i
$ .:
,t. I
'-' '-'
..
t ___
; ,
?,
,
4.:.4.a=
...s. 2,
.. 70 is ,,,,;.. =,...:1 1
..e,t. n v
1
..3
...c f cin :KA e I.e.: 2, .,-- =,... ccc
...:,.
. === ....: ..,.0
1.
e
C. t.l ..,
=
= ,.:7. tr. % ZZ =fl : :.E. = ti 1. 5 i 4. tiµa
ma 0, ii. ;Tit. 3 a
==:: h< :.,..
.-= ..1:17 ======== ,..- . :I. ,44 ..,-,. irc :=.1 "-.t.
t.ii <-7. '''.' =<<. ,..;-,, ,...r,,. t=2
,..tc ...
...,;:,.....
ss I. -, .), t.... til 8 n sN=
===== til i.l. -.7i =
-,.. ,..... t....t. '3". :::.: 1,,i+:441 .g.
,=.; gt .-It il= 4 4 ,.. 0 c..,,..
=N., ... .4
P.. .3F= g z1 n
icl, 2> ii.'.
.c. 0. ,,tc, co ' ,,z- ''..0 '44M I.% 00M *7 It =. ''''
.i...',5 r.-µ 41
.,,=11,':-.. t A... kI: .8.: e.4' =A v; .: Z r.7.: t 4; It. t..
,....., 'sIi.
. . 41, 40: 1. 4.4; +1C.; 'i; ti =:.. .Z.
W 0> 1 10 4A. 10
,......,
E3: :si: kj; ,t,'= t .::: ?ci b g x: E. Ez
7- 3,... t
:'=== s:'.4 al a: ' >xi 4s _,=:;:. A.' =a
454.:
. ,- cr= ;0
C ...= . ica ka ciS ,...
:a
PROTECTiON OF HUMAN SUBJECTS: RISK TO HUMAN SUBJECTS
a. i-tuniatl Subjects Involvement end Characteristics
69
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
100 subjects will be enrolled in this study. In this application we propose
three specific
aims. The first specific aim will study 20 short duration PD subjects. Ten
subjects will be
analysed by a shot gun proteomic approach for biomarker discovery. These
biomarkers
along with other candidate markers will be screened by ELBA assay on lysates
from a
further 10 short duration PD subjects
To be eligible for entry into this study, candidates must meet all of the
following eligibility
criteria at the time of entry:
1. Diagnosis of Parkinson's disease within the last 3 years.
2. Male or female, age 50-80 years inclusive.
3. Willing and able to provide written informed consent in compliance with the
regulatory
requirements. If a subject is unable to provide written informed consent,
written informed
consent may be obtained from the subject's legal representative.
Candidates will be excluded from study entry if any of the following exclusion
criteria exist
at the time of entry:
1. Any clinically significant disease other than PD.
2. Unwilling or unable to comply with the requirements of this protocol,
including the
presence of any condition (physical, mental, or social) that is likely to
affect the subject's
ability to comply with the protocol.
3. Any other reasons that, in the opinion of the Investigator, the candidate
is determined to
be unsuitable for entry into the study.
b. Sources of Materials
The clinical PI or authorized staff member will screen patients coming to the
clinic for
entry into the study. Prior to any study-related activity subjects will sign
an informed
consent document that complies with the requirements of 21 CFR Part 50, HIPAA
and all
local regulatory requirements and laws. The following activities will then be
performed:
= Obtain written informed consent.
= Review of inclusion/exclusion criteria.
= Demographics (e.g., age, sex, race).
= Review of medical history.
= Review of concomitant and past medications and therapies.
= Obtain two tubes of blood (total maximum volume approximately 20 mL drawn
in
a BD CPT Tube).
Only the staff at the recruiting Institutions will have access to individually
identifiable
private information about human subjects. All samples and associated data will
have
individually identifiable private information removed and replaced with a code
number
before transmittal to MSDX, Inc.
Blood will be drawn by qualified personnel and associated data will be
extracted from
medical records and recorded on IRB approved data collection forms. The data
on the
collection forms will then be entered into the database created for this
study.
For each subject visit, we enter subject-specific information into the
database including
demographics, medical history, drug therapy history, and symptom severities.
Specimen
samples collected from the subject during each visit are also tracked in the
database,
along with any EUSA, Western blot or Flow cytometry analysis data collected on
the
specimens over time. The database provides detailed single subject reports
that display
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
the data available for any subject as well as multi-subject reports based on
data across all
subjects in the study. Furthermore, since the data is all readily available in
the database,
the data can be directly accessed and analyzed by many third party statistical
analysis
software packages (such as sIMP, etc). The data can also be exported from the
database
into a format compatible with any software statistical analysis package.
c. Potential Risks
Drawing blood involves temporary discomfort (from the needle stick), and may
lead to
bruising at the puncture site, fainting or, very rarely, infection. A blood
draw is considered
to be a low risk procedure. This study involves little to no physical,
psychological,
financial, legal or other risk to the subject.
Protection of Human subjects: Adequacy of Protection Against Risks
a. Recruitment and Informed Consent
The clinical PI or authorized staff member will obtain informed consent. Prior
to any
study-related activity (including screening assessments), all subjects will
sign an informed
consent document that has been approved by the IRB and complies with the
requirements of 21 CFR Part 50, HIPAA, and all local regulatory requirements
and laws.
If a subject is unable to provide written informed consent, written informed
consent may
be obtained from the subject's legal representative. The Investigator will
provide in writing
and explain the nature, purpose, and potential risks and benefits of the study
and provide
the subject with a copy of the informed consent document. The subject will be
given
sufficient time to consider the study's implications before deciding to
participate. Subjects
will be informed that they may withdraw from this study at any time at their
own request
without jeopardizing in any way their access to and quality of treatment they
receive. A
copy of the informed consent document signed by the subject will be given to
the subject.
The clinical PI will retain the original of each subject's signed informed
consent document.
b. Protection against Risk
Only the staff at the recruiting institutions will have access to individually
identifiable
private information about human subjects enrolled into this study by them. All
samples
and associated data will have individually identifiable private information
removed and
replaced with a code number before transmittal to MSDX, Inc. The electronic
database
we use is password protected and the computer that the database resides on is
in a
locked room. Hard copies of data will be kept in locked filing cabinets in a
locked room.
All personnel with access to the data have been trained and certified in
protection of
human subjects from research risks. While absolute guarantees of security of
these data
are not possible it is highly unlikely that a breach of privacy will occur.
Protection of Human subjects: Potential Benefits of the Proposed Research to
Human Subjects and Others
There will not be any direct benefit to the subject by taking part in this
research study.
Knowledge gained from the study may benefit others in the future by leading to
the
development of a simple, inexpensive blood test to monitor new drug
effectiveness in PD.
The general results of the study may be published in scientific journals that
may be
beneficial to further research into PD. The likely benefits of this research
far outweigh the
risks as only a low risk blood draw is involved and adequate measures to
protect subject
confidentiality have been adopted.
71
CA 02929711 2016-05-04
WO 2014/071359 PCT/US2013/068465
Protection of Human subjects: Importance of the Knowledge to be gained
Knowledge gained from the study may benefit others in the future by leading to
the
development of a simple, inexpensive blood test that facilitate phase II
trials of drugs that
would preserve neurological function by limiting damage to the central nervous
system,
slow or prevent disease progression and limit disability thus improving
quality of life for
the patient and reducing the health care costs of PD.
Targeted/Planned Enrollment Table
Study Title: Recirculating Phagocytes: A Shuttle Vector for Retrieval of
Biomarkers
from the Brain.
Total Planned Enrollment 100
Targeted/Planned Enrollment: Number of Subjects
Ethnic Category Sex/Gender
Male Female
Total
Hispanic or Latino
Not Hispanic or Latino 50 50
100
Ethnic Category: Total of all Subjects 50 50
100
Racial Categories
American Indian/ Alaska Native
Asian
Native Hawaiian or other Pacific Islander
Black or African American
White 50 50
100
Racial Categories: Total of All Subjects 50 50
100
Inclusion of Women and Minorities:
A. Gender
Subjects of both genders will be recruited for this study. Parkinson's disease
affects
slightly more men than women.
B. Minority Groups or subaroups
Whether PD is less common in Blacks and other ethnic minorities has been a
matter of
debate. Several epidemiological studies have suggested that Blacks may be less
likely to
develop PD than Whites, but other studies have reported similar disease rates.
In
addition, PD in certain non-White populations may be clinically different.
Neuroclegenerative diseases, PD among them, are clinico-pathological entities
almost
exclusively defined in White populations. It is difficult to determine then
whether the
disease is similar across different ethnic groups, because of a paucity of
studies in non-
Whites. Race and ethnicity modulate diseases via genetic background,
therefore, PD
symptoms and signs may be expected to differ across different ethnicities. In
PD, two
studies from Nigeria observed more frequent atypical features in African Black
PD
patients, but data from the US is lacking. Until the issue of ethnic effects
in PD is
resolved, it is reasonable to assume that all people have a similar
probability of
developing the disease and that the results gained will be applicable to all
ethnicities.
72
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
From the table below it can be seen that Phoenix and the state of Arizona have
a
comparable ethnic and racial distribution of population. The vast majority of
the
population is non-hispanic white and the minority populations are largely
composed of
Hispanic, non-Hispanic black, non-Hispanic Native American and non-Hispanic
Asian.
From the information cited above it is clear that the minority population
available in
Phoenix and the state of Arizona consists of populations at low risk for given
the
time frame of the study and the scarcity of patients of these ethnicities the
study is likely
to be predominantly recruiting patients of white European origin. Data
collection includes
ethnicity/race and where sufficient numbers are available, statistical
analysis by ethnicity
will be performed.
The ethnic and racial breakdown for Arizona and the city of Phoenix
Total Non- Non- Non- Non- Non-
Hispanic Hispanic Hispanic Hispanic Hispanic Hispanic
White Black Native Asian other
American
Arizona 4,961,953 916,017 3,575,201 139,650 255,619 69,749 5,718
City of 1,289,125 258,379 925,079 63,743 20,581 19,566 1,778
Phoenix
Source: Arizona Department of Economic Security, Research Administration
Inclusion of children
Juvenile Parkinson's disease is very rare. Parkinson's disease is more common
in older
people with a prevalence of approximately 1% in people over 60. Consequently
this
proposal will focus on Parkinson's disease in adults
[001331 As used herein, the term "about" refers to plus or minus 10% of the
referenced number.
[001341 Various modifications of the invention, in addition to those described
herein, will be apparent to those skilled in the art from the foregoing
description.
Such modifications are also intended to fall within the scope of the appended
claims. Each reference cited in the present application is incorporated herein
by
reference in its entirety.
[001351 Although there has been shown and described the preferred embodiment
of the present invention, it will be readily apparent to those skilled in the
art that
modifications may be made thereto which do not exceed the scope of the
appended claims. Therefore, the scope of the invention is only to be limited
by the
following claims. Reference numbers recited in the claims are exemplary and
for
73
CA 02929711 2016-05-04
WO 2014/071359
PCT/US2013/068465
ease of review by the patent office only, and are not limiting in any way. In
some
embodiments, the figures presented in this patent application are drawn to
scale,
including the angles, ratios of dimensions, etc. In some embodiments, the
figures
are representative only and the claims are not limited by the dimensions of
the
figures. In some embodiments, descriptions of the inventions described herein
using the phrase "comprising" includes embodiments that could be described as
"consisting of", and as such the written description requirement for claiming
one or
more embodiments of the present invention using the phrase "consisting of" is
met.
1001361 The reference numbers recited in the below claims are solely for ease
of
examination of this patent application, and are exemplary, and are not
intended in
any way to limit the scope of the claims to the particular features having the
corresponding reference numbers in the drawings.
74