Note: Descriptions are shown in the official language in which they were submitted.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
1
Covalently linked helicar-anti-helicar antibody conjugates and uses thereof
Herein are reported (covalent) complexes comprising the helicar motif amino
acid
sequence and an anti-helicar antibody whereby the helicar motif amino acid
sequence is present in or conjugated to a payload whereby the helicar motif
amino
acid sequence containing element and the anti-helicar antibody are covalently
linked to each other via a single bond. Also reported are methods for
producing the
covalent complexes and uses thereof
Background of the Invention
Major bottlenecks for therapeutic application of polypeptides are their
limited
solubility, in vivo stability, short serum half-life and fast clearance from
the
bloodstream.
Different approaches are reported to address these drawbacks. However, none of
these technologies provides for a robust and universal platform that enables
pharmacokinetic (PK) modulation without encountering immunogenicity risks or
potential loss of biological activity.
One approach to improve PK/stability and biophysical behavior of therapeutic
polypeptides is to fuse them to entities which stabilized the polypeptide,
keep it in
solution, and extend its half-life. Examples of such entities are human serum
albumin or human immunoglobulin Fc-regions. This approach is applicable to
many linear polypeptides that are composed of naturally occurring amino acid
residues and that tolerate modifications at either their C- or N-terminus
without
losing their biological activity. Polypeptides that are cyclic, stapled,
contain non-
natural amino acid residues, or additional modifications cannot be
recombinantly
produced as fusion polypeptides. However, such polypeptides may be the desired
choice for therapeutic applications because they are frequently superior to
'normal'
linear peptides in terms of protease stability, activity and specificity.
One approach to improve PK/stability and biophysical behavior of therapeutic
polypeptides, which can also be applied to those that are cyclic, stapled, or
contain
non-natural structures, is the chemical or enzymatic conjugation to polymers,
for
example by PEGylation or HESylation. However, such modifications frequently
lead to significant reduction of the biological activity of the polypeptide
and can
under certain circumstances be the reason for safety or toxicity problems.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 2 -
A major disadvantage of most existing chemical coupling technologies for
stabilization or PK modulation of therapeutic polypeptides is their
complexity.
Beside the chemical coupling step the methods result in many cases in a
mixture of
polypeptide derivatives that are connected to the PK-modulating entity with
uncertain stoichiometries and/or at undefined positions. Additionally
currently used
polypeptide modification-technologies often result in strongly reduced or even
complete loss of biological activity of the therapeutic polypeptide. In
addition, it is
difficult to predict pharmacological properties and/or possible degradation
routes of
the chemical conjugates.
The helicar element is composed of a 12-mer amino acids peptide forming an
a-helix. The structural elements of the peptide are described in Nygaard et
al.
reporting also an anti-helicar antibody and the complex structure with the 12-
mer
peptide, part of a yeast leucine zipper protein called GCN4. The antibody
portion
Fv has been affinity matured using the phage display technique to an affinity
of
25 pM (Zahnd, C., et al., J. Biol. Chem. 279 (2004) 18870-18877).
Metz, S., et al. (Proc. Natl. Acad. Sci. USA 108 (2011) 8194-8424) report
bispecific digoxigenin-binding antibodies for targeted payload delivery. PK
modulation of haptenylated peptides via non-covalent antibody complexation is
reported by Hoffmann, E., et al. (J. Contr. Rel. 171 (2013) 48-56). In
WO 2012/093068 a pharmaceutical composition of a complex of an anti-dig
antibody and digoxigenin that is conjugated to a peptide is reported. Directed
in
vitro evolution and crystallographic analysis of a peptide-binding single
chain
antibody fragment (scFv) with low picomolar affinity is reported by Zahnd, C.,
et
al. (J. Biol. Chem. 279 (2004) 18870-18877). Hanes, J., et al. (Proc. Natl.
Acad. Sci.
USA 95 (1998) 14130-14135) report that ribosome display efficiently selects
and
evolves high-affinity antibodies in vitro from immune libraries.
US 5,804,371 reports hapten-labeled peptides and their use in an immunological
method of detection. A digoxigenin-labeled peptide (Bradykinin) and its
application to chemiluminoenzyme immunoassay of Bradykinin in inflamed tissues
are reported by Decarie A., et al. (Peptides 15 (1994) 511-518).
In WO 2004/065569 multi-functional antibodies are reported.
In WO 2014/006124 covalent hapten-anti-hapten antibody complexes are reported.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 3 -
Summary of the Invention
It has been found that helicar-mediated complex formation can be used to
covalently conjugate polypeptides comprising the helicar motif amino acid
sequence either at one of the termini or within the polypeptide sequence. In
case of
an insertion, the 12-mer helicar motif amino acid sequence is either inserted
within
the sequence of the polypeptide or an existing helical motif is modified to
incorporate the essential amino acids that are involved in the anti-helicar
antibody
recognition.
It has been found that by the covalent conjugation of a helicar motif amino
acid
sequence containing compound to an anti-helicar antibody stabilization, PK-
property improvement of the compound or in case of a bispecific antibody an
additional targeting can be achieved.
One aspect as reported herein is a conjugate comprising a helicar motif amino
acid
sequence containing compound and an antibody that specifically binds to the
helicar motif amino acid sequence characterized by a covalent bond between the
helicar motif amino acid sequence containing compound and an amino acid
residue
in the CDR2 of the anti-helicar antibody, whereby the CDR2 is determined
according to Kabat.
It has been found that any compound can be used in the conjugates and methods
as
reported herein upon derivatization with a helicar motif amino acid sequence,
which comprises the functional residue for the formation of the covalent bond
between the helicar motif amino acid sequence containing compound and an amino
acid residue in the CDR2 of the antibody. The location of the functional group
in
the helicar motif amino acid sequence has the advantage that it is not
necessary to
re-engineer the synthesis and the position of the functional group in the CDR2
of
the antibody when the helicar motif-derivatized compound is changed.
One aspect as reported herein is a conjugate comprising a helicar motif amino
acid
sequence containing compound and an antibody that specifically binds to the
helicar motif amino acid sequence of the helicar motif amino acid sequence
containing compound (anti-helicar motif amino acid sequence antibody)
characterized by a covalent bond between the helicar motif amino acid sequence
containing compound and an amino acid residue in the CDR2 of the antibody,
whereby the CDR2 is determined according to Kabat.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 4 -
In one embodiment the CDR2 is the light chain CDR2.
In one embodiment the helicar motif amino acid sequence has the amino acid
sequence AHLENEVARLKK (SEQ ID NO: 01) or is a variant thereof with one
amino acid residue changed to cysteine. In one embodiment the helicar motif
amino acid sequence has the amino acid sequence AHLENEVARLKK (SEQ ID
NO: 01), wherein one amino acid residue of the three C-terminal amino acid
residues is changed to cysteine. In one embodiment the helicar motif amino
acid
sequence has the amino acid sequence of AHLENEVARCKK (SEQ ID NO: 02) or
AHLENEVARLCK (SEQ ID NO: 03).
In one embodiment the helicar motif amino acid sequence containing compound
comprises a helicar motif amino acid sequence, optionally a linker, and a
payload.
In one embodiment the helicar motif amino acid sequence containing compound is
a polypeptide comprising the helicar motif amino acid sequence either fused to
one
of its termini or within the polypeptide sequence. In this embodiment the
payload is
a polypeptide.
One aspect as reported herein is a conjugate comprising i) a compound
comprising
a helicar motif amino acid sequence selected from the group comprising SEQ ID
NO: 01, a variant of SEQ ID NO: 01 wherein one amino acid residue has been
changed to cysteine, SEQ ID NO: 02, and SEQ ID NO: 03, and ii) an antibody
that
specifically binds to the helicar motif amino acid sequence, wherein the
conjugate
comprises a covalent bond between the helicar motif amino acid sequence and an
amino acid residue in the CDR2 of the anti-helicar antibody, whereby the CDR2
is
determined according to Kabat.
In one embodiment the CDR2 is the light chain CDR2.
In one embodiment the helicar motif amino acid sequence containing compound
comprises a helicar motif amino acid sequence, optionally a linker, and a
payload.
In one embodiment of all aspects the covalent bond is between the helicar
motif
amino acid sequence containing compound and an amino acid residue in the CDR2
of the antibody.
In one embodiment the covalent bond is between a functional group in the
helicar
motif amino acid sequence containing compound and the amino acid residue in
the
CDR2 of the antibody.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 5 -
In one embodiment the functional group is in the helicar motif amino acid
sequence.
In one embodiment the covalent bond is between a cysteine residue in the light
chain CDR2 of the antibody and a functional group in the helicar motif amino
acid
sequence containing compound.
In one embodiment the cysteine residue in the light chain CDR2 of the antibody
is
at position 55 or position 51 according to the light chain variable domain
numbering of Kab at.
In one embodiment the cysteine residue in the light chain CDR2 of the antibody
is
at position 55 according to the light chain variable domain numbering of
Kabat.
In one embodiment of all aspects the covalent bond is a disulfide bond.
In one embodiment of all aspects the covalent bond is a disulfide bond between
a
cysteine residue in the helicar motif amino acid sequence and a cysteine
residue in
the CDR2 of the light chain of the anti-helicar antibody. In one embodiment
the
helicar motif amino acid sequence has the amino acid sequence of SEQ ID NO:
02.
In one embodiment of all aspects the antibody is a bispecific antibody
comprising a
first binding specificity to a non-helicar antigen and a second binding
specificity to
helicar motif amino acid sequence. In one embodiment the (non-helicar) antigen
is
a cell surface antigen. In one embodiment the cell surface antigen is a tumor
associated antigen. In one embodiment one heavy chain of the bispecific
antibody
comprises a hole mutation and the respective other chain comprises a knob
mutation. In one embodiment one heavy chain of the bispecific antibody
comprises
the mutations T3665, L368A and Y407V and the respective other chain comprises
the mutation T366W. In one embodiment one heavy chain of the bispecific
antibody further comprises the mutation 5354C and the respective other chain
comprises the mutation Y349C.
In one embodiment the bispecific antibody is a full length antibody to which
at one
or both heavy chain C-termini a scFv or a dsscFv or a scFab or a dsscFab or a
combination thereof has been fused either directly or via a peptidic linker.
In one embodiment the bispecific antibody is a full length antibody. In one
embodiment one heavy chain of the bispecific antibody comprises a hole
mutation
and the respective other chain comprises a knob mutation.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 6 -
In one embodiment the payload is selected from a binding moiety, a labeling
moiety, and a biologically active moiety.
In one embodiment the antibody is a full length antibody.
In one embodiment the antibody is a humanized or a human antibody.
In one embodiment the constant region of the antibody is of the IgG1 subclass
or of
the IgG4 subclass.
In one embodiment the antibody has a constant region of the IgG1 subclass with
an
alanine at position 234 and 235 and with a glycine at position 329 with
numbering
according to the EU index of Kabat.
In one embodiment the antibody has a constant region of the IgG4 class with a
proline at position 228, a glutamic acid at position 235 and a glycine at
position
329 with numbering according to the EU index of Kabat.
In one embodiment the antibody is an antibody fragment. In one embodiment the
fragment is a Fab or a (Fab)2.
In one embodiment of all aspects the conjugate comprises exactly one covalent
bond per light chain CDR2.
In one embodiment the helicar motif amino acid sequence containing compound
comprises a reactive group that can form a covalent bond with the thiol group
of
the cysteine residue in the CDR2 of the antibody. In one embodiment the
reactive
group is a thiol, or a maleimide, or a haloacetyl.
In one embodiment of all aspects the covalent bond is a disulfide bond. In one
embodiment the disulfide bond is formed without the addition of a redox active
agent.
In one embodiment the conjugate comprises a therapeutic or detectable moiety.
In
one embodiment the therapeutic or detectable moiety is covalently conjugated
to
helicar motif amino acid sequence or the helicar motif amino acid sequence is
incorporated into the therapeutic or detectable moiety.
In one embodiment the helicar motif amino acid sequence is conjugated to a
polypeptide consisting of 5 to 500 amino acid residues. In one embodiment the
polypeptide comprises 10 to 450 amino acid residues. In one embodiment the
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 7 -
polypeptide comprises 12 to 450 amino acid residues. In one embodiment the
polypeptide comprises 15 to 400 amino acids residues.
In one embodiment the helicar motif amino acid sequence is conjugated to a
detectable label.
In one embodiment the helicar motif amino acid sequence is conjugated to the
polypeptide, or to the detectable label, or to the payload via a linker. In
one
embodiment the linker is a non-peptidic linker. In one embodiment the linker
is a
peptidic linker.
One aspect as reported herein is an anti-helicar antibody that has in the
light chain a
cysteine residue in the CDR2 whereby the CDRs are determined according to
Kabat.
In one embodiment the cysteine residue in the light chain CDR2 of the antibody
is
at position 55 or position 51 according to the light chain variable domain
numbering of Kabat.
In one embodiment the cysteine residue in the light chain CDR2 of the antibody
is
at position 55 according to the light chain variable domain numbering of
Kabat.
In one embodiment the antibody has in exactly one light chain variable domain
a
cysteine residue at position 55 or position 51 according to the light chain
variable
domain numbering of Kabat.
In one embodiment of all aspects the antibody is a humanized or human
antibody.
In one embodiment the antibody is a full length antibody, or a Fab, or a scFv,
or a
scFv conjugated to an Fc-region.
In one embodiment the cysteine forms a disulfide bond with an isolated
cysteine
residue or an isolated homocysteine residue.
One aspect as reported herein is an immunoconjugate comprising the conjugate
as
reported herein and a cytotoxic agent.
One aspect as reported herein is a pharmaceutical formulation comprising the
conjugate as reported herein and a pharmaceutically acceptable carrier.
The conjugate as reported herein for use as a medicament.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 8 -
The conjugate as reported herein for the treatment of cancer.
The conjugate as reported herein for the treatment of diabetes.
The conjugate as reported herein for the treatment of adiposities.
The conjugate as reported herein for the treatment of an inflammatory disease.
The conjugate as reported herein for the treatment of a metabolic disease.
The conjugate as reported herein for the treatment of a viral disease.
One aspect as reported herein is the use of a conjugate as reported herein in
the
manufacture of a medicament.
One aspect as reported herein is the use of a conjugate as reported herein as
diagnostic agent.
One aspect as reported herein is the use of a conjugate as reported herein
comprising a therapeutic polypeptide to increase the stability of the
therapeutic
polypeptide.
One aspect as reported herein is the use of a conjugate as reported herein
comprising a therapeutic polypeptide to increase the activity of the
therapeutic
polypeptide.
One aspect as reported herein is the use of a conjugate as reported herein
comprising a therapeutic polypeptide to increase the in vivo half-life of the
therapeutic polypeptide.
One aspect as reported herein is the use of a conjugate as reported herein in
the
treatment of a disease.
One aspect as reported herein is a method of treating an individual having a
disease
comprising administering to the individual an effective amount of a conjugate
as
reported herein.
One aspect as reported herein is a method of treating a disease in an
individual
comprising administering to the individual an effective amount of the
conjugate as
reported herein.
In one embodiment the disease is cancer.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 9 -
In one embodiment the disease is diabetes.
In one embodiment the disease is adipositas.
One aspect as reported herein is a method of producing a conjugate as reported
herein comprising the combination of an anti-helicar antibody comprising a
first
reactive group and an helicar motif amino acid sequence containing compound
comprising a second reactive group, whereby the alpha carbon atom of the amino
acid residue that bears the first reactive group is about 10 to 11 Angstrom
apart
from the atom of the helicar motif amino acid sequence containing compound.
One aspect as reported herein is a method of producing a conjugate as reported
herein comprising the steps of
- combining in solution an anti-helicar antibody, which specifically binds
to
a helicar motif amino acid sequence and which comprises a first reactive
group at one amino acid residue in the CDR2, with a helicar motif amino
acid sequence containing compound comprising a second reactive group,
wherein the helicar motif amino acid sequence containing compound
comprises a payload, such as a peptide consisting of 5 to 500 amino acids
or a detectable label, and
- recovering of the conjugate from the solution.
One aspect as reported herein is a method for producing an anti-helicar
antibody
for the formation of a conjugate as reported herein, comprising the step of
- cultivating a cell comprising a nucleic acid encoding the anti-helicar
antibody, and
- recovering the anti-helicar antibody from the cell or the cultivation
medium,
wherein in the anti-helicar antibody the residue in the light chain CDR2 is
mutated
to cysteine that has in the X-ray structure of the non-covalent complex of the
anti-
helicar antibody and the helicar motif amino acid sequence containing compound
a
distance of 10 to 11 Angstrom between the alpha-carbon atom of the amino acid
residue in the antibody CDR2 and the atom of the helicar motif amino acid
sequence containing compound atom between which the covalent bond is to be
formed.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 10 -
One aspect as reported herein is a method for identifying a position in an
anti-
helicar antibody CDR2 that can be mutated to cysteine for the formation of a
covalent bond between the residue in the antibody CDR2 and the bound helicar
motif amino acid sequence containing compound comprising the step of
- providing a crystal structure of the non-covalent complex of the anti-
helicar antibody and the helicar motif amino acid sequence containing
compound, and
-
identifying an amino acid residue in the CDR2 of the anti-helicar antibody
and in the helicar motif amino acid sequence containing compound with a
distance of 10 to 11 Angstrom between the alpha-carbon atoms of the
amino acid residue in the antibody CDR2 and the atom in the helicar motif
amino acid sequence containing compound,
wherein the identified position is the position in an antibody CDR2 that can
be
mutated to cysteine for the formation of a covalent bond between the residue
in the
antibody CDR2 and the bound helicar motif amino acid sequence containing
compound.
One aspect as reported herein is a bispecific anti-helicar antibody for
targeted
delivery of a helicar motif amino acid sequence containing compound to a
target
cell, wherein the bispecific antibody comprises a first binding specificity
(site) that
specifically binds to the helicar motif amino acid sequence containing
compound
and a second binding specificity that specifically binds to a cell surface
marker of
the target cell.
One aspect as reported herein is the use of a complex consisting of a helicar
motif
amino acid sequence containing compound and an antibody that has a first
binding
specificity that specifically binds to a helicar motif amino acid sequence and
a
second binding specificity that specifically binds to a blood brain barrier
receptor
for delivering the helicar motif amino acid sequence containing compound to
the
brain.
In one embodiment the blood brain barrier receptor is selected from the group
consisting of transferrin receptor (TfR), insulin receptor, insulin-like
growth factor
receptor (IGF receptor), low density lipoprotein receptor-related protein 8
(LRP8),
low density lipoprotein receptor-related protein 1 (LRP1), and heparin-binding
epidermal growth factor-like growth factor (HB-EGF).
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 11 -
In one embodiment the bispecific antibody is a full length antibody comprising
two
binding sites.
In one embodiment the bispecific antibody is a full length antibody to which
one or
two scFvs or scFabs have been fused and that comprises three or four binding
sites.
In one embodiment the bispecific antibody is an antibody fragment. In one
embodiment the antibody fragment is selected from F(ab')2 and diabodies.
In one embodiment the bispecific antibody is a humanized or a human antibody.
In one embodiment the bispecific antibody is free of effector function. In one
embodiment the bispecific antibody has no functional Fc-region. In one
embodiment the bispecific antibody has no Fc-region. In one embodiment the
bispecific antibody has an Fc-region of the human IgG1 subclass with the
mutations L234A, L235A and P329G, wherein the positions are determined
according to the Fc-region numbering of Kabat (Kabat EU index). In one
embodiment the bispecific antibody has an Fc-region of the human IgG4 subclass
with the mutations S228P, L235E and P329G, wherein the positions are
determined
according to the Fc-region numbering of Kabat (Kabat EU index).
In one embodiment the bispecific antibody comprises
a) one
binding site for the helicar motif amino acid sequence containing
compound and one binding site for the blood brain barrier receptor, or
b) two binding sites for the helicar motif amino acid sequence containing
compound and one binding site for the blood brain barrier receptor, or
c) one binding site for the helicar motif amino acid sequence containing
compound and two binding sites for the blood brain barrier receptor, or
d) two binding sites for the helicar motif amino acid sequence containing
compound and two binding sites for the blood brain barrier receptor.
In cases b) and c) of the previous embodiment one heavy chain of the
bispecific
antibody comprises a hole mutation and the respective other chain comprises a
knob mutation.
In one preferred embodiment the bispecific antibody comprises two binding
sites
for the helicar motif amino acid sequence containing compound and one or two
binding sites for the blood brain barrier receptor.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 12 -
In one embodiment the helicar motif amino acid sequence containing compound
comprises between the hapten and the payload a linker. In one embodiment the
linker is a peptidic linker. In one embodiment the linker is a chemical linker
(non-
peptidic linker).
It has been found that by the covalent coupling of a helicar motif amino acid
sequence containing compound to an anti-helicar motif amino acid sequence
containing compound antibody a stabilization and PK-property improvement of
the
compound can be achieved.
In one embodiment the bispecific antibody and the helicar motif amino acid
sequence containing compound each comprise a functional group whereby upon
binding of the helicar motif amino acid sequence containing compound by the
bispecific antibody a covalent bond is formed between the helicar motif amino
acid
sequence containing compound and the bispecific antibody.
In one embodiment the bispecific antibody comprises a functional group at an
amino acid residue in the CDR2 of the antibody, whereby the CDR2 is determined
according to Kabat. In one embodiment the functional group at an amino acid
residue in the CDR2 of the antibody is a thiol group. In one embodiment the
bispecific antibody comprises a cysteine amino acid residue in the CDR2 of the
antibody.
In one embodiment of all aspects the helicar motif amino acid sequence
containing
compound comprises a functional group in the helicar motif amino acid sequence
or if present in the linker between the helicar motif amino acid sequence and
the
compound. In one embodiment the functional group is a thiol, or a maleimide,
or a
haloacetyl. In one embodiment the functional group in the helicar motif amino
acid
sequence or if present in the linker is a thiol group.
In one embodiment of all aspects the covalent bond is between a cysteine
residue in
the CDR2 of the antibody and the thiol group in the helicar motif amino acid
sequence containing compound. In one embodiment the covalent bond is a
disulfide bond. In one embodiment the covalent bond is a disulfide bond and it
is
formed without the addition of redox active agents.
In one embodiment of all aspects the CDR2 is the light chain CDR2. In one
embodiment the cysteine residue in the light chain CDR2 of the antibody is at
position 51 or at position 55 according to the light chain variable domain
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 13 -
numbering of Kabat. In one preferred embodiment the cysteine residue in the
light
chain CDR2 of the antibody is at position 55 according to the light chain
variable
domain numbering of Kabat.
It has been found that any compound can be used in the helicar motif amino
acid
sequence containing compound upon derivatization of the helicar amino acid
sequence with a cysteine comprising the functional group for the formation of
the
covalent disulfide bond between the helicar motif amino acid sequence
containing
compound and the cysteine residue in the light chain CDR2 of the antibody. The
location of the cysteine residue (thiol functional group) in the helicar motif
amino
acid sequence has the advantage that it is not necessary to re-engineer the
synthesis
and the position of the cysteine residue in the light chain CDR2 of the
antibody if
the payload is changed.
In one embodiment of all aspects exactly one covalent bond is formed per light
chain CDR2.
In one embodiment of all aspects the compound is selected from a binding
moiety,
a labeling moiety, and a biologically active moiety.
In one embodiment of all aspects the biologically active moiety is selected
from the
group comprising antibodies, polypeptides, natural ligands of one or more CNS
target(s), modified versions of natural ligands of one or more CNS target(s),
aptamers, inhibitory nucleic acids (i.e., small inhibitory RNAs (siRNA) and
short
hairpin RNAs (shRNA)), locked nucleic acids (LNAs), ribozymes, and small
molecules, or active fragments of any of the foregoing.
In one embodiment of all aspects the compound is a nucleic acid or nucleic
acid
derivative. In one embodiment the nucleic acid is an iRNA or a LNA.
In one embodiment of all aspects the compound is a polypeptide.
In one embodiment the compound is a small molecule (non-polypeptide
biologically active moiety).
In one embodiment the biologically active moiety is a polypeptide. In one
embodiment the polypeptide is consisting of 5 to 500 amino acid residues. In
one
embodiment the polypeptide comprises 10 to 450 amino acid residues. In one
embodiment the polypeptide comprises 15 to 400 amino acid residues. In one
embodiment the polypeptide comprises 18 to 350 amino acids residues.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 14 -
In one embodiment the bispecific antibody comprises a first binding
specificity that
specifically binds to a helicar motif amino acid sequence containing compound
(anti-helicar motif amino acid sequence binding specificity) and a second
binding
specificity that specifically binds to the (human) transferrin receptor (anti-
(human)
transferrin receptor binding specificity; anti-(h)TfR binding specificity) or
to low
density lipoprotein receptor-related protein 8 (anti-low density lipoprotein
receptor-
related protein 8 binding specificity; anti-LRP8 binding specificity).
In one embodiment the bispecific antibody has two binding specificities that
specifically bind to the helicar motif amino acid sequence containing compound
(two anti-helicar motif amino acid sequence binding specificities) and two
binding
specificities that specifically bind to the (human) transferrin receptor (two
anti-
(human) transferrin receptor binding specificities) or to low density
lipoprotein
receptor-related protein 8 (anti-low density lipoprotein receptor-related
protein 8
binding specificity).
Description of the Figures
Figure 1:
Introduction of SH functionalities in the hapten as well as in the
antibody at appropriate positions allow the antibody and the
hapten to form a covalent bond in between resulting in a
conjugate.
Figure 2: Scheme of SDS-
PAGE self-fluorescence band pattern (without
further staining of the SDS-PAGE gel):
A: If no covalent bond is formed between the antibody and the
hapten-fluorophore conjugate both under reducing or non-
reducing conditions one self-fluorescent band at the molecular
weight of free hapten-fluorophore conjugate can be detected.
B: If a covalent bond is formed between the antibody and the
hapten-fluorophore conjugate under non-reducing conditions one
self-fluorescent band at the combined molecular weight of the
antibody and the hapten-fluorophore conjugate can be detected.
Under reducing conditions the disulfide bridges in the conjugate
of the antibody and the hapten-fluorophore conjugate
(haptenylated compound) are cleaved and one self-fluorescent
band at the molecular weight of free hapten-fluorophore
conjugate can be detected.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 15 -
Figure 3: Conjugate formation of hapten-binding Cys-mutated
antibodies
with hapten-Cys-fluorescent label conjugates (haptenylated
compound) in the presence of redox active agents: oxidation
agent (glutathione disulfide, GSSG) and reducing agent
(dithioerythritol, DTE): Antibody complexation and subsequent
covalent linkage at defined positions is detected by fluorescence
signals in SDS PAGE analyses. Non-reducing (upper images) and
reducing (lower images) SDS-PAGE analyses were performed as
described in Example 3. Covalently antibody linked haptens are
detectable as larger sized protein bound signals at the appropriate
positions under non-reduced conditions. These signals detach
from protein upon reduction and are visible as small entities
under reducing conditions.
Left: fluorescence image
Right: Coomassie blue staining
Series 1: anti-digoxigenin antibody with 52bC mutation
Series 2: anti-digoxigenin antibody with wild-type residue at
position 52b
(A) covalent coupling with 3 mM DTE and 10 mM GSSG;
(B) covalent coupling with 0.3 mM DTE and 1 mM GSSG;
(C) covalent coupling with 0.03 mM DTE and 0.1 mM GSSG.
Figure 4: Complex formation of hapten-binding Cys mutated
antibodies
with hapten-Cys-fluorescent label conjugates in the presence
solely of an oxidation agent (glutathione disulfide, GSSG) but in
the absence of reducing agents or in the absence of both:
Antibody complexation and subsequent covalent linkage at
defined positions is detected by fluorescence signals in SDS
PAGE analyses. Non-reducing (upper images) and reducing
(lower images) SDS-PAGE analyses were performed as
described in Example 4. Covalently antibody linked haptens are
detectable as larger sized protein bound signals at the appropriate
positions under non-reduced conditions. These signals detach
from protein upon reduction and are visible as small entities
under reducing conditions.
Left: fluorescence image
Right: Coomassie blue staining
Series 1: anti-digoxigenin antibody with 52bC mutation
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 16 -
Series 2: anti-digoxigenin antibody with wild-type residue at
position 52b
(A) no additives
(B) covalent coupling with 1 mM GSSG;
(C) covalent coupling with 0.1 mM GSSG.
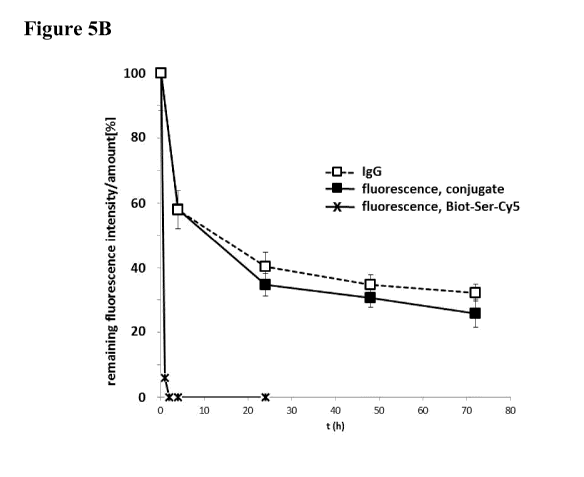
Figure 5: Results of in vivo blood PK study with covalent
conjugates and
non-covalent complexes compared to non-complexed
antigen/hapten; the relative remaining fluorescence intensity (%,
solid marks) of Cy5-mediated fluorescence of Biotin-Cy5 non-
covalent complexes (Figure 5A) and covalent (SS-bridged)
conjugates (Figure 6B), as well as of non-complexed Biotin-Ser-
Cy5 (asterix) is shown; the fluorescence signal at time point
t = 0.08 h was set to 100 %; additionally, the relative remaining
amount of human IgG in the mouse serum samples is shown
(open marks); IgG serum concentration (mg/ml) at t = 0.08 h was
set to 100%.
Figure 6: Western blot of the determination of the amount of
digoxigenylated PYY polypeptide in the serum of mice.
Figure 7: Analysis of affinity-driven complexation of haptenylated
compounds with anti-hapten antibodies.
Antibody complexation and subsequent covalent linkage at
defined positions is directed by fluorescence signals in SDS
PAGE analyses, which were carried out as described in Example
11.
Left: fluorescent image with non-reduced (left side of gel) and
reduced (right side of gel) samples.
Right: Coomassie blue staining.
1: humanized anti-digoxigenin antibody + biotin-Cys-Cy5
2: humanized anti-digoxigenin antibody VH52bC + biotin-Cys-
Cy5
3: humanized anti-biotin antibody + biotin-Cys-Cy5
4: humanized anti-biotin antibody VH53C + biotin-Cys-Cy5
The white arrows mark the excess (uncoupled) biotin-Cys-Cy5,
which is significantly higher when anti-digoxigenin antibody
VH52bC is used, because the conjugation reaction is not affinity
driven in this case.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 17 -
Figure 8:
Relative remaining fluorescence intensity (%) of Cy5-mediated
fluorescence of Dig-Cy5 non-covalent complexes and covalent
(disulfide-bridged) conjugates, as well as of non-complexed Dig-
Cy5; the fluorescence signal at time point t = 0.08 h was set to
100 %; additionally, the relative remaining amount of human IgG
in the mouse serum samples is shown; IgG serum concentration
(mg/ml) at t = 0.08 h was set to 100%.
Figure 9: Pharmacokinetics under in vivo-like conditions of Cy5-
mediated
fluorescence of Biotin-Cy5 of non-covalent complexes and of
covalent (disulfide-bridged) conjugates, as well as of non-
complexed Biotin-Cy5, determined by non-invasive eye imaging;
solid diamond: biotin-Cy5, solid square biotin-Cy5+anti-biotin
antibody (complex); triangle: Cy5-Biotin-anti-biotin antibody
conjugate.
Figure 10: Formation of
covalent complexes between biotin-binding
antibodies and Biotin-Cys-Cy5 is demonstrated by non-reducing
and reducing SDS PAGE; the coupling reaction was performed in
murine serum at 37 C for 1 hr. Cy5 appears coupled to the H-
chain under non-reducing conditions only in samples that
contained Biotin-Cys-Cy5 and Cys-mutated antibody; these
covalent conjugates disintegrate upon reduction (right lanes);
lanes 1: Molecular weight marker; 2-3 non-reducing - 2: anti-
Biotin antibody (without Cys mutation) + Biotin-Cys-Cy5
(complex); 3: anti-Biotin antibody-Cys + Biotin-Cys-Cy5
(conjugate); 4-5 reducing - 5: anti-Biotin antibody (without Cys
mutation) + Biotin-Cys-Cy5 (complex); 6: anti-Biotin antibody-
Cys + Biotin-Cys-Cy5 (conjugate).
Figure 11: In vivo pharmacokinetics of Cy5-mediated fluorescence of
Biotin-Cy5 of non-covalent complexes and of covalent (disulfide-
bridged) conjugates, as well as of non-complexed Biotin-Cy5,
determined by non-invasive eye imaging; solid diamond: biotin-
Cy5, solid circle: biotin-Cy5 administered 24 hours after
administration of anti-biotin antibody (in vivo complex
formation); solid square: biotin-Cys-Cy5 administered 24 hours
after administration of anti-biotin antibody-Cys (in vivo
conjugate formation).
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 18 -
Figure 12: SDS
PAGE gel of the coupling of antibody 0155 with the helicar
motif amino acid sequence cysteine variant 2 using a 2.5 molar
excess of helicar motif amino acid sequence containing
compound form the covalent complex 0156; 1 = helicar motif
amino acid sequence cysteine variant 2; 2 = antibody 0019; 3 =
antibody 0155.
Figure 13: SDS PAGE gel of the coupling of antibody 0157 with the
helicar
motif amino acid sequence cysteine variant 1; 1 = helicar motif
amino acid sequence cysteine variant 1 (oxidized); 2 = control
coupling (oxidized); 3 = covalent conjugate (oxidized); 4 =
molecular weight marker; 5 = covalent conjugate (reduced); 6 =
control coupling (reduced); 7 = helicar motif amino acid sequence
cysteine variant 1 (reduced).
Figure 14: SEC
chromatogram of antibody 0155, the helicar motif amino
acid sequence cysteine variant 1 containing Pseudomonas
exotoxin molecule LR8M with the C-terminal lysine residue
deleted of SEQ ID NO: 28 and the covalent conjugate thereof
Figure 15: Analysis of the conjugation efficiency by SDS-CE, Caliper,
for
the non reduced samples.
Detailed Description of the Invention
I. Definitions
An "acceptor human framework" for the purposes herein is a framework
comprising the amino acid sequence of a light chain variable domain (VL)
framework or a heavy chain variable domain (VH) framework derived from a
human immunoglobulin framework or a human consensus framework, as defined
below. An acceptor human framework "derived from" a human immunoglobulin
framework or a human consensus framework may comprise the same amino acid
sequence thereof, or it may contain amino acid sequence changes. In some
embodiments, the number of amino acid changes are 10 or less, 9 or less, 8 or
less,
7 or less, 6 or less, 5 or less, 4 or less, 3 or less, or 2 or less. In some
embodiments,
the VL acceptor human framework is identical in sequence to the VL human
immunoglobulin framework sequence or human consensus framework sequence.
The term "amino acid" denotes the group of carboxy oc-amino acids, either
occurring naturally, i.e. which directly or in form of a precursor can be
encoded by
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 19 -
a nucleic acid, or occurring non-naturally. The individual naturally occurring
amino acids are encoded by nucleic acids consisting of three nucleotides, so
called
codons or base-triplets. Each amino acid is encoded by at least one codon.
This is
known as "degeneration of the genetic code". The term "amino acid" as used
within this application denotes the naturally occurring carboxy a-amino acids
comprising alanine (three letter code: ala, one letter code: A), arginine
(Arg, R),
asparagine (Asn, N), aspartic acid (Asp, D), cysteine (Cys, C), glutamine
(Gln, Q),
glutamic acid (Glu, E), glycine (Gly, G), histidine (His, H), isoleucine (Ile,
I),
leucine (Leu, L), lysine (Lys, K), methionine (Met, M), phenylalanine (Phe,
F),
proline (Pro, P), serine (Ser, S), threonine (Thr, T), tryptophane (Trp, W),
tyrosine
(Tyr, Y), and valine (Val, V). Examples of non-naturally occurring amino acids
include, but are not limited to, Aad (alpha-aminoadipic acid), Abu
(aminobutyric
acid), Ach (alpha-aminocyclohexane-carboxylic acid), Acp (alpha-
amino cyclop entane-c arb oxylic acid), Acpc (1 -Amino cycloprop ane-1 -
carboxylic
acid), Aib (alpha-aminoisobutyric acid), Aic (2-Aminoindane-2-carboxylic acid;
also called 2-2-Aic), 1-1-Aic (1-aminoindane-1-carboxylic acid), (2-
aminoindane-
2-carboxylic acid), allylglycine (ally1Gly), alloisoleucine (allo-Ile), Asu
(alpha-
aminosuberic acid, 2-aminooctanedioc acid), Bip (4-phenyl-phenylalanine-
carboxylic acid), BnHP ((2S,4R)-4-hydroxyproline), Cha (beta-
cyclohexylalanine),
Cit (citrulline), cyclohexylglycine (Chg), cyclopentylalanine, beta-
cyclopropyl
alanine, Dab (1,4-Diaminobutyric acid), Dap (1,3-Diaminopropionic acid), p
(3,3-
diphenylalanine-carboxylic acid), 3,3-Diphenylalanine, Di-n-propylglycine
(Dpg),
2-Furylalanine, Homocyclohexylalanine (HoCha), Homocitrulline (HoCit),
Homocycloleucine, Homoleucin (HoLeu), Homoarginine (HoArg), Homoserine
(HoSer), Hydroxyproline, Lys(Ac), (1) Nal (1-Naphtyl Alanine), (2) Nal (2-
Naphtyl Alanine), 4-Me0-Apc (1-amino-4-(4-methoxypheny1)-cyclohexane-1-
carboxylic acid), Nor-leucine (Nle), Nva (Norvaline), Omathine, 3-Pal (alpha-
amino-3-pyridylalanine-carboxylic acid), 4-Pal (alpha-amino-4-pyridylalanine-
carboxylic acid), 3,4,5 ,F3 -Phe (3,4,5 -Trifluoro-phenylalanine), 2,3,4,5
,6,F5 -Phe
(2,3,4,5 ,6-P entafluoro-phenylalanine), Pqa (4-oxo-
6-(1 -pip eraziny1)-3 (4H)-
quinazoline-acetic acid (CAS 889958-08-1)), Pyridylalanine, Quinolylalanine,
Sarcosine (Sar), Thiazolylalanine, Thienylalanine, Tic (alpha-amino-
1,2,3,4,tetrahydroisoquinoline-3-carboxylic acid), Tic(OH), Tle
(tertbutylGlycine),
and Tyr(Me).
The term "amino acid sequence variant" refers to polypeptides having amino
acid
sequences that differ to some extent from a native sequence polypeptide.
Ordinarily,
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 20 -
amino acid sequence variants will possess at least about 70 % sequence
identity
with the native sequence polypeptide. In one embodiment the variant has about
80 % or more sequence identity with the native sequence polypeptide. In one
embodiment the variant has about 90 % or more sequence identity with the
native
sequence polypeptide. In one embodiment the variant has about 95 % or more
sequence identity with the native sequence polypeptide. In one embodiment the
variant has about 98 % or more sequence identity with the native sequence
polypeptide. The amino acid sequence variants possess substitutions,
deletions,
and/or insertions at certain positions within the amino acid sequence of the
native
amino acid sequence. Amino acids are designated by the conventional names, one-
letter and three-letter codes.
The term "antibody" herein is used in the broadest sense and encompasses
various
antibody structures, including but not limited to monoclonal antibodies,
polyclonal
antibodies, multispecific antibodies (e.g., bispecific antibodies), and
antibody
fragments so long as they exhibit the desired antigen-binding activity.
The term "antibody fragment" denotes a molecule other than an intact antibody
that
comprises a portion of an intact antibody that binds the antigen to which the
intact
antibody binds. Examples of antibody fragments include but are not limited to
Fv,
Fab, Fab', Fab'-SH, F(ab')2; diabodies; linear antibodies; single-chain
antibody
molecules (e.g. scFv); and multispecific antibodies formed from antibody
fragments.
The term "biotin", short "BI", denotes 5-[(3aS,4S,6aR)-2-oxohexahydro-1H-
thieno[3,4-d]imidazol-4-yl]pentanoic acid. Biotin is also known as vitamin H
or
coenzyme R.
The term "bispecific antibodies" denotes antibodies which have two different
(antigen/helicar) binding specificities. In one embodiment bispecific
antibodies as
reported herein are specific for two different antigens, i.e. a helicar motif
amino
acid sequence containing compound and a non- helicar motif amino acid sequence
containing antigen.
The term "chimeric" antibody refers to an antibody in which a portion of the
heavy
and/or light chain is derived from a particular source or species, while the
remainder of the heavy and/or light chain is derived from a different source
or
species.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
-21 -
The "class" of an antibody refers to the type of constant domain or constant
region
possessed by its heavy chain. There are five major classes of antibodies: IgA,
IgD,
IgE, IgG, and IgM, and several of these may be further divided into subclasses
(isotypes), e.g., IgG1 , IgG2, IgG3, IgG4, IgAl, and IgA2. The heavy chain
constant domains that correspond to the different classes of immunoglobulins
are
called a, 8, e, 7, and , respectively.
The term "cytotoxic agent" as used herein refers to a substance that inhibits
or
prevents a cellular function and/or causes cell death or destruction.
Cytotoxic
agents include, but are not limited to, radioactive isotopes (e.g., At211,
1131, 1125,
Y90, Re186, Re188, Sm153, Bi212, P32, Pb212 and radioactive isotopes of Lu);
chemotherapeutic agents or drugs (e.g., methotrexate, adriamicin, vinca
alkaloids
(vincristine, vinblastine, etoposide), doxorubicin, melphalan, mitomycin C,
chlorambucil, daunorubicin or other intercalating agents); growth inhibitory
agents;
enzymes and fragments thereof such as nucleolytic enzymes; antibiotics; toxins
such as small molecule toxins or enzymatically active toxins of bacterial,
fungal,
plant or animal origin, including fragments and/or variants thereof; and the
various
antitumor or anticancer agents disclosed below.
The term "digoxigenin", short "DIG", denotes 3-
[(3S,5R,8R,9S,10S,12R,13S,14S,17R)-3,12,14-trihydroxy-10,13-dimethyl-
1,2,3,4,5,6,7,8,9,11,12,15,16,17-tetradecahydro-cyclopenta[a]-phenanthren-17-
y1]-
2H-furan-5-one (CAS number 1672-46-4). Digoxigenin (DIG) is a steroid found
exclusively in the flowers and leaves of the plants Digitalis purpurea,
Digitalis
orientalis and Digitalis lanata (foxgloves) (Polya, G., Biochemical targets of
plant
bioactive compounds, CRC Press, New York (2003) p. 847).
The term "effector functions" denotes those biological activities attributable
to the
Fc-region of an antibody, which vary with the antibody class. Examples of
antibody effector functions include: Cl q binding and complement dependent
cytotoxicity (CDC); Fc receptor binding; antibody-dependent cell-mediated
cytotoxicity (ADCC); phagocytosis; down regulation of cell surface receptors
(e.g.
B cell receptor); and B cell activation.
The term "effective amount" of an agent, e.g., a pharmaceutical formulation,
denotes an amount effective, at dosages and for periods of time necessary, to
achieve the desired therapeutic or prophylactic result.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 22 -
Papain digestion of antibodies produces two identical antigen-binding
fragments,
called "Fab" fragments, each with a single antigen-binding site, and a
residual "Fc"
fragment, whose name reflects its ability to crystallize readily. Pepsin
treatment
yields an F(ab')2 fragment that has two antigen-binding sites and is still
capable of
cross-linking antigen.
The Fab fragment also contains the constant domain of the light chain and the
first
constant domain (CH1) of the heavy chain. Fab' fragments differ from Fab
fragments by the addition of a few residues at the carboxy terminus of the
heavy
chain CH1 domain including one or more cysteines from the antibody hinge
region.
Fab'-SH is the designation herein for Fab' in which the cysteine residue(s) of
the
constant domains bear at least one free thiol group. F(ab')2 antibody
fragments
originally were produced as pairs of Fab' fragments which have hinge cysteines
between them. Other chemical couplings of antibody fragments are also known.
"Fv" is the minimum antibody fragment which contains a complete antigen-
recognition and antigen-binding site. This region consists of a dimer of one
heavy
chain and one light chain variable domain in tight, non-covalent association.
It is in
this configuration that the three hypervariable regions of each variable
domain
interact to define an antigen binding site on the surface of the VH-VL dimer.
Collectively, the six hypervariable regions confer antigen-binding specificity
to the
antibody. However, even a single variable domain (or half of an Fv comprising
only three hypervariable regions specific for an antigen) has the ability to
recognize
and bind antigen, although at a lower affinity than the entire binding site.
The term "Fc-region" herein is used to define a C-terminal region of an
immunoglobulin heavy chain that contains at least a portion of the constant
region.
The term includes native sequence Fc-regions and variant Fc-regions. In one
embodiment, a human IgG heavy chain Fc-region extends from Cys226, or from
Pro230, to the carboxyl-terminus of the heavy chain. However, the C-terminal
lysine (Lys447) of the Fc-region may or may not be present. Unless otherwise
specified herein, numbering of amino acid residues in the Fc-region or
constant
region is according to the EU numbering system, also called the EU index, as
described in Kabat, E.A. et al., Sequences of Proteins of Immunological
Interest,
5th ed., Public Health Service, National Institutes of Health, Bethesda, MD
(1991),
NIH Publication 91-3242.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 23 -
The term "fluorescein", short "FLUO", denotes 6-hydroxy-9-(2-carboxypheny1)-
(3H)-xanthen-3-on, alternatively 2-(6-hydroxy-3-oxo-(3H)-xanthen-9-y1)-benzoic
acid. Fluorescein is also known as resorcinolphthalein, C.I. 45350, solvent
yellow
94, D & C yellow no. 7, angiofluor, Japan yellow 201, or soap yellow.
The term "framework", short "FR", denotes heavy and light chain variable
domain
amino acid residues other than hypervariable region (HVR) residues. The FR of
a
variable domain generally consists of four FR domains: FR1, FR2, FR3, and FR4.
Accordingly, the HVR and FR sequences generally appear in the following
sequence in VH (or VL): FR1-H1(L1)-FR2-H2(L2)-FR3-H3(L3)-FR4.
The term "free cysteine amino acid" denotes a cysteine amino acid residue
which
has been engineered into a parent antibody, has a thiol functional group (SH),
and
is not paired as an intramolecular disulfide bridge. Nevertheless, a free
cysteine
amino acid can be pair as intramolecular disulfide bridge, e.g. with
glutathione.
The term "full length antibody" denotes an antibody having a structure
substantially similar to a native antibody structure or having heavy chains
that
contain an Fc-region as defined herein. Native IgG antibodies are
heterotetrameric
glycoproteins of about 150,000 daltons, composed of two identical light chains
and
two identical heavy chains that are disulfide-bonded. From N- to C-terminus,
each
heavy chain has a variable region (VH), also called a variable heavy domain or
a
heavy chain variable domain, followed by three constant domains (CH1, CH2, and
CH3). Similarly, from N- to C-terminus, each light chain has a variable region
(VL), also called a variable light domain or a light chain variable domain,
followed
by a constant light (CL) domain. The light chain of an antibody may be
assigned to
one of two types, called kappa (x) and lambda (X), based on the amino acid
sequence of its constant domain.
A "full length antibody" is an antibody comprising a VL and VH domain, as well
as a light chain constant domain (CL) and heavy chain constant domains, CH1,
CH2 and CH3. The constant domains may be native sequence constant domains
(e.g., human native sequence constant domains) or an amino acid sequence
variant
thereof The full length antibody may have one or more "effector functions"
which
refer to those biological activities attributable to the Fc constant region (a
native
sequence Fc-region or amino acid sequence variant Fc-region) of an antibody.
Examples of antibody effector functions include C 1 q binding; complement
dependent cytotoxicity; Fc receptor binding; antibody-dependent cell-mediated
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 24 -
cytotoxicity (ADCC); phagocytosis; and down regulation of cell surface
receptors
such as B-cell receptor and BCR.
The term "hapten" denotes a small molecule that can elicit an immune response
only when attached to a large carrier such as a protein. Exemplary haptens are
aniline, o-, m-, and p-aminobenzoic acid, quinone, histamine-succinyl-glycine
(HSG), hydralazine, halothane, indium-DTPA, fluorescein, biotin, digoxigenin,
theophylline and dinitrophenol. In one embodiment the hapten is biotin or
digoxigenin or theophylline or carborane or bromodeoxyuridine.
The term "helicar motif amino acid sequence" denotes an amino acid sequence
that
has the amino acid sequence of SEQ ID NO: 01 or is a variant thereof that is
specifically bound by an anti-helicar antibody that has a variable heavy chain
domain of SEQ ID NO: 04 and a light chain variable domain of SEQ ID NO: 05.
The term "a helicar motif amino acid sequence that is conjugated to" or
"helicar
motif amino acid sequence containing compound" denotes to a helicar motif
amino
acid sequence which is covalently linked to a further moiety such as a
polypeptide
or a label. An activated helicar motif amino acid sequence derivative can be
used as
starting material for the formation of such conjugates. In one embodiment the
linker comprises a) one or more (in one embodiment three to six) methylene-
carboxy-methyl groups (-CH2-C(0)-), and/or b) from 1 to 10 (in one embodiment
from 1 to 5) amino acid residues (in one embodiment selected from glycine,
serine,
glutamate, 13-alanine, y-aminobutyric acid, 8-aminocaproic acid or lysine),
and/or c)
one or more (in one embodiment one or two) compounds having the structural
formula NH2-[(CH2)õ0]xCH2-CH2-COOH in which n is 2 or 3 and x is 1 to 10, in
one embodiment 1 to 7. The last element results (at least partly) in a linker
(part) of
the formula -NH-[(CH2)õ0]xCH2-CH2-C(0)-. One example of such a compound is
e.g. 12-amino-4,7,10-trioxadodecanoic acid (results in a TEG
(triethylenglycol)
linker). In one embodiment the linker further comprises a maleimido group. The
linker has a stabilizing and solubilizing effect since it contains charges
or/and can
form hydrogen bridges. In addition it can sterically facilitate the binding of
the
anti-helicar antibody to the helicar motif amino acid sequence containing
compound. In one embodiment the linker is located at a side chain of an amino
acid
of the helicar motif amino acid sequence (e.g. conjugated to a lysine or
cysteine
side chain via an amino or thiol group). In one embodiment the linker is
located at
the amino terminus or at the carboxy terminus of the helicar amino acid
sequence.
The position of the linker on the conjugated compound (=payload) is typically
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 25 -
chosen at a region where the biological activity of the payload is not
affected.
Therefore the attachment position of the linker depends on the nature of the
payload and the relevant structure elements which are responsible for the
biological
activity. The biological activity of the payload to which the helicar motif
amino
acid sequence is attached can be tested in an in vitro assay.
The terms "host cell", "host cell line", and "host cell culture" are used
interchangeably and refer to cells into which exogenous nucleic acid has been
introduced, including the progeny of such cells. Host cells include
"transformants"
and "transformed cells," which include the primary transformed cell and
progeny
derived therefrom without regard to the number of passages. Progeny may not be
completely identical in nucleic acid content to a parent cell, but may contain
mutations. Mutant progeny that have the same function or biological activity
as
screened or selected for in the originally transformed cell are included
herein.
A "human antibody" is one which possesses an amino acid sequence which
corresponds to that of an antibody produced by a human or a human cell or
derived
from a non-human source that utilizes human antibody repertoires or other
human
antibody-encoding sequences. This definition of a human antibody specifically
excludes a humanized antibody comprising non-human antigen-binding residues.
A "humanized" antibody refers to a chimeric antibody comprising amino acid
residues from non-human HVRs and amino acid residues from human FRs. In
certain embodiments, a humanized antibody will comprise substantially all of
at
least one, and typically two, variable domains, in which all or substantially
all of
the HVRs (e.g., CDRs) correspond to those of a non-human antibody, and all or
substantially all of the FRs correspond to those of a human antibody. A
humanized
antibody optionally may comprise at least a portion of an antibody constant
region
derived from a human antibody. A "humanized form" of an antibody, e.g., a non-
human antibody, refers to an antibody that has undergone humanization.
The term "hypervariable region" or "HVR", as used herein, refers to each of
the
regions of an antibody variable domain which are hypervariable in sequence
("complementarity determining regions" or "CDRs") and/or form structurally
defined loops ("hypervariable loops"), and/or contain the antigen-contacting
residues ("antigen contacts"). Generally, antibodies comprise six HVRs; three
in
the VH (H1, H2, H3), and three in the VL (L1, L2, L3).
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 26 -
HVRs herein include
(a)
hypervariable loops occurring at amino acid residues 26-32 (L1), 50-52
(L2), 91-96 (L3), 26-32 (H1), 53-55 (H2), and 96-101 (H3) (Chothia, C.
and Lesk, A.M., J. Mol. Biol. 196 (1987) 901-917);
(b) CDRs occurring at amino acid residues 24-34 ( L1), 50-56 (L2), 89-97
(L3), 31-35b (H1), 50-65 (H2), and 95-102 (H3) (Kabat, E.A. et al.,
Sequences of Proteins of Immunological Interest, 5th ed. Public Health
Service, National Institutes of Health, Bethesda, MD (1991), NIH
Publication 91-3242);
(c) antigen contacts occurring at amino acid residues 27c-36 (L1), 46-55 (L2),
89-96 (L3), 30-35b (H1), 47-58 (H2), and 93-101 (H3) (MacCallum et al.
J. Mol. Biol. 262: 732-745 (1996)); and
(d)
combinations of (a), (b), and/or (c), including HVR amino acid residues
46-56 (L2), 47-56 (L2), 48-56 (L2), 49-56 (L2), 26-35 (H1), 26-35b (H1),
49-65 (H2), 93-102 (H3), and 94-102 (H3).
An "individual" or "subject" is a mammal. Mammals include, but are not limited
to,
domesticated animals (e.g. cows, sheep, cats, dogs, and horses), primates
(e.g.,
humans and non-human primates such as monkeys), rabbits, and rodents (e.g.,
mice
and rats). In certain embodiments, the individual or subject is a human.
An "isolated" antibody is one which has been separated from a component of its
natural environment. In some embodiments, an antibody is purified to greater
than
95% or 99% purity as determined by, for example, electrophoretic (e.g., SDS-
PAGE, isoelectric focusing (IEF), capillary electrophoresis) or
chromatographic
(e.g., ion exchange or reverse phase HPLC). For review of methods for
assessment
of antibody purity, see, e.g., Flatman, S. et al., J. Chrom. B 848 (2007) 79-
87.
An "isolated" nucleic acid refers to a nucleic acid molecule that has been
separated
from a component of its natural environment. An isolated nucleic acid includes
a
nucleic acid molecule contained in cells that ordinarily contain the nucleic
acid
molecule, but the nucleic acid molecule is present extrachromosomally or at a
chromosomal location that is different from its natural chromosomal location.
The term "monoclonal antibody" as used herein refers to an antibody obtained
from
a population of substantially homogeneous antibodies, i.e., the individual
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
-27 -
antibodies comprising the population are identical and/or bind the same
epitope,
except for possible variant antibodies, e.g., containing naturally occurring
mutations or arising during production of a monoclonal antibody preparation,
such
variants generally being present in minor amounts. In contrast to polyclonal
antibody preparations, which typically include different antibodies directed
against
different determinants (epitopes), each monoclonal antibody of a monoclonal
antibody preparation is directed against a single determinant on an antigen.
Thus,
the modifier "monoclonal" indicates the character of the antibody as being
obtained
from a substantially homogeneous population of antibodies, and is not to be
construed as requiring production of the antibody by any particular method.
For
example, the monoclonal antibodies to be used in accordance with the present
invention may be made by a variety of techniques, including but not limited to
the
hybridoma method, recombinant DNA methods, phage-display methods, and
methods utilizing transgenic animals containing all or part of the human
immunoglobulin loci, such methods and other exemplary methods for making
monoclonal antibodies being described herein.
The term "monospecific antibody" denotes an antibody that has one or more
binding sites each of which has the same binding specificity, i.e. binds to
the same
antigen or helicar motif amino acid sequence.
A "naked antibody" refers to an antibody that is not conjugated to a
heterologous
moiety (e.g., a cytotoxic moiety) or radiolabel. The naked antibody may be
present
in a pharmaceutical formulation.
The term "package insert" is used to refer to instructions customarily
included in
commercial packages of therapeutic products, that contain information about
the
indications, usage, dosage, administration, combination therapy,
contraindications
and/or warnings concerning the use of such therapeutic products.
A "parent antibody" is an antibody comprising an amino acid sequence from
which
one or more amino acid residues are replaced by one or more cysteine residues.
The parent antibody may comprise a native or wild-type sequence. The parent
antibody may have pre-existing amino acid sequence modifications (such as
additions, deletions and/or substitutions) relative to other native, wild-
type, or
modified forms of an antibody. The parent antibody binds specifically to a
helicar
motif amino acid sequence. A parent antibody may be directed additionally also
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 28 -
against a target antigen of interest, e.g. a biologically important
polypeptide.
Antibodies directed against non-polypeptide antigens are also contemplated.
The term "payload" denotes any molecule or combination of molecules whose
activity it is desired to be delivered (in)to and/or localize at a cell.
Payloads include,
but are not limited to labels, cytotoxins (e.g. Pseudomonas exotoxin, ricin,
abrin,
Diphtheria toxin, and the like), enzymes, growth factors, transcription
factors,
drugs, radionuclides, ligands, antibodies, liposomes, nanoparticles, viral
particles,
cytokines, and the like.
A "chemotherapeutic agent" is a chemical compound useful in the treatment of
cancer. Examples of chemotherapeutic agents include alkylating agents such as
thiotepa and cyclosphosphamide (CYTOXANTm); alkyl sulfonates such as
busulfan, improsulfan and piposulfan; aziridines such as benzodopa,
carboquone,
meturedopa, and uredopa; ethylenimines and methylamylamines including
altretamine, triethylenemelamine,
trietylenephosphoramide,
triethylenethiophosphoramide and trimethylomelamine; nitrogen mustards such as
chlorambucil, chlornaphazine, chlorophosphamide, estramustine, ifosfamide,
mechlorethamine, mechlorethamine oxide hydrochloride, melphalan, novembichin,
phenesterine, prednimustine, trofosfamide, uracil mustard; nitroureas such as
carmustine, chlorozotocin, fotemustine, lomustine, nimustine, ranimustine;
antibiotics such as aclacinomysins, actinomycin, authramycin, azaserine,
bleomycins, cactinomycin, calicheamicin, carabicin, carminomycin,
carzinophilin,
chromomycins, dactinomycin, daunorubicin, detorubicin, 6-diazo-5-oxo-L-
norleucine, doxorubicin, epirubicin, esorubicin, idarubicin, marcellomycin,
mitomycins, mycophenolic acid, no galamycin, olivomycins, peplomycin,
potfiromycin, puromycin, quelamycin, rodorubicin, streptonigrin, streptozocin,
tubercidin, ubenimex, zinostatin, zorubicin; anti-metabolites such as
methotrexate
and 5-fluorouracil (5-FU); folic acid analogues such as denopterin,
methotrexate,
pteropterin, trimetrexate; purine analogs such as fludarabine, 6-
mercaptopurine,
thiamiprine, thioguanine; pyrimidine analogs such as ancitabine, azacitidine,
6-
azauridine, carmofur, cytarabine, dideoxyuridine, doxifluridine, enocitabine,
floxuridine, 5-FU; androgens such as calusterone, dromostanolone propionate,
epitiostanol, mepitiostane, testolactone; anti-adrenals such as
aminoglutethimide,
mitotane, trilostane; folic acid replenisher such as frolinic acid;
aceglatone;
aldophosphamide glycoside; amino levulinic acid; amsacrine; bestrabucil;
bisantrene; edatraxate; defo famine ; demecolcine; diaziquone; elfornithine;
elliptinium acetate; etoglucid; gallium nitrate; hydroxyurea; lentinan;
lonidamine;
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 29 -
mito guazone; mitoxantrone; mopidamol; nitracrine; pentostatin; phenamet;
pirarubicin; podophyllinic acid; 2-ethylhydrazide; procarbazine; PSKO;
razoxane;
sizofiran; spirogermanium; tenuazonic
acid; triaziquone; 2,2',2"-
trichlorotriethylamine; urethan; vinde sine;
dacarbazine; mannomustine;
mitobronitol; mitolactol; pipobroman; gacytosine; arabinoside ("Ara-C");
cyclophosphamide; thiotepa; taxanes, e.g. paclitaxel (TAXOLO, Bristol-Myers
Squibb Oncology, Princeton, NJ) and docetaxel (TAXOTEREO, Rh6ne-Poulenc
Rorer, Antony, France); chlorambucil;
gemcitabine; 6-thio guanine ;
mercaptopurine; methotrexate; platinum analogs such as cisplatin and
carboplatin;
vinblastine; platinum; etoposide (VP-16); ifosfamide; mitomycin C;
mitoxantrone;
vincristine; vinorelbine; navelbine; novantrone; teniposide; daunomycin;
aminopterin; xeloda; ibandronate; CPT-II; 35 topoisomerase inhibitor RFS 2000;
difluoromethylornithine (DMF0); retinoic acid; esperamicins; capecitabine; and
pharmaceutically acceptable salts, acids or derivatives of any of the above.
Also
included in this definition are anti-hormonal agents that act to regulate or
inhibit
hormone action on tumors such as anti-estrogens including for example
tamoxifen,
raloxifene, aromatase inhibiting 4(5)-imidazoles, 4-hydroxytamoxifen,
trioxifene,
keoxifene, LY117018, onapristone, and toremifene (Fareston); and anti-
androgens
such as flutamide, nilutamide, bicalutamide, leuprolide, and goserelin; and
pharmaceutically acceptable salts, acids or derivatives of any of the above.
An "anti-angiogenic agent" refers to a compound which blocks, or interferes
with
to some degree, the development of blood vessels. The anti-angiogenic agent
may,
for instance, be a small molecule or an antibody that binds to a growth factor
or
growth factor receptor involved in promoting angiogenesis. The anti-angiogenic
factor is in one embodiment an antibody that binds to Vascular Endothelial
Growth
Factor (VEGF).
The term "cytokine" is a generic term for proteins released by one cell
population
which act on another cell as intercellular mediators. Examples of such
cytokines
are lymphokines, monokines, and traditional polypeptide hormones. Included
among the cytokines are growth hormone such as human growth hormone,
N-methionyl human growth hormone, and bovine growth hormone; parathyroid
hormone; thyroxine; insulin; proinsulin; relaxin; prorelaxin; glycoprotein
hormones
such as follicle stimulating hormone (FSH), thyroid stimulating hormone (TSH),
and luteinizing hormone (LH); hepatic growth factor; fibroblast growth factor;
prolactin; placental lactogen; tumor necrosis factor-a and -P; mullerian-
inhibiting
substance; mouse gonadotropin-associated peptide; inhibin; activin; vascular
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 30 -
endothelial growth factor; integrin; thrombopoietin (TP0); nerve growth
factors
such as NGF-p; platelet growth factor; transforming growth factors (TGFs) such
as
TGF-a and TGF-p; insulin-like growth factor-I and -II; erythropoietin (EPO);
osteoinductive factors; interferons such as interferon-a, -P, and -y; colony
stimulating factors (CSFs) such as macrophage-CSF (M-CSF); granulocyte-
macrophage-CSF (GM-CSF); and granulocyte-CSF (GCSF); interleukins (ILs)
such as IL-I, IL-la, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-I0, IL-
II,
IL-12; a tumor necrosis factor such as TNF-a or TNF-P; and other polypeptide
factors including LIF and kit ligand (KL). As used herein, the term cytokine
includes proteins from natural sources or from recombinant cell culture and
biologically active equivalents of the native sequence cytokines.
The term "fMLP" denotes the tripeptide consisting of N-formylmethionine,
leucine
and phenylalanine. In one embodiment the effector moiety is fMLP or a
derivative
thereof
The term "prodrug" refers to a precursor or derivative form of a
pharmaceutically
active substance that is less cytotoxic to tumor cells compared to the parent
drug
and is capable of being enzymatically activated or converted into the more
active
parent form. See, e.g., Wilman, "Prodrugs in Cancer Chemotherapy" Biochemical
Society Transactions, Vol. 14, 615th Meeting Belfast (1986) pp. 375-382 and
Stella,
et al., "Prodrugs: A Chemical Approach to Targeted Drug Delivery", Directed
Drug
Delivery, Borchardt, et al., (eds.), pp. 247-267, Humana Press (1985). The
prodrugs that can be used as effector moiety include, but are not limited to,
phosphate-containing prodrugs, thiophosphate-containing prodrugs, sulfate-
containing prodrugs, peptide-containing prodrugs, D-amino acid-modified
prodrugs, glycosylated prodrugs, b-lactam-containing prodrugs, optionally
substituted phenoxyacetamide-containing prodrugs or optionally substituted
phenylacetamide-containing prodrugs, 5-fluorocytosine and other 5-
fluorouridine
prodrugs which can be converted into the more active cytotoxic free drug.
Examples of cytotoxic drugs that can be derivatized into a prodrug form for
use in
this invention include, but are not limited to, those chemotherapeutic agents
described herein.
The term "cytotoxic moiety" refers to a substance that inhibits or prevents a
cellular
function and/or causes cell death or destruction. Cytotoxic agents include,
but are
/131, /125, y 90 Re 186, Re 188, sm153,
not limited to, radioactive isotopes (e.g., At211,
.212 32 212
Bi , P , Pb and radioactive isotopes of Lu); chemotherapeutic agents or drugs
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
-31 -
(e.g., methotrexate, adriamicin, vinca alkaloids (vincristine, vinblastine,
etoposide),
doxorubicin, melphalan, mitomycin C, chlorambucil, daunorubicin or other
intercalating agents); growth inhibitory agents; enzymes and fragments thereof
such as nucleolytic enzymes; antibiotics; toxins such as small molecule toxins
or
enzymatically active toxins of bacterial, fungal, plant or animal origin,
including
fragments and/or variants thereof; and the various antitumor or anticancer
agents
disclosed herein.
"Percent (%) amino acid sequence identity" with respect to a reference
polypeptide
sequence is defined as the percentage of amino acid residues in a candidate
sequence that are identical with the amino acid residues in the reference
polypeptide sequence, after aligning the sequences and introducing gaps, if
necessary, to achieve the maximum percent sequence identity, and not
considering
any conservative substitutions as part of the sequence identity. Alignment for
purposes of determining percent amino acid sequence identity can be achieved
in
various ways that are within the skill in the art, for instance, using
publicly
available computer software such as BLAST, BLAST-2, ALIGN or Megalign
(DNASTAR) software. Those skilled in the art can determine appropriate
parameters for aligning sequences, including any algorithms needed to achieve
maximal alignment over the full length of the sequences being compared. For
purposes herein, however, % amino acid sequence identity values are generated
using the sequence comparison computer program ALIGN-2. The ALIGN-2
sequence comparison computer program was authored by Genentech, Inc., and the
source code has been filed with user documentation in the U.S. Copyright
Office,
Washington D.C., 20559, where it is registered under U.S. Copyright
Registration
No. TXU510087. The ALIGN-2 program is publicly available from Genentech,
Inc., South San Francisco, California, or may be compiled from the source
code.
The ALIGN-2 program should be compiled for use on a UNIX operating system,
including digital UNIX V4.0D. All sequence comparison parameters are set by
the
ALIGN-2 program and do not vary.
In situations where ALIGN-2 is employed for amino acid sequence comparisons,
the % amino acid sequence identity of a given amino acid sequence A to, with,
or
against a given amino acid sequence B (which can alternatively be phrased as a
given amino acid sequence A that has or comprises a certain % amino acid
sequence identity to, with, or against a given amino acid sequence B) is
calculated
as follows:
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 32 -
100 times the fraction X/Y
where X is the number of amino acid residues scored as identical matches by
the
sequence alignment program ALIGN-2 in that program's alignment of A and B,
and where Y is the total number of amino acid residues in B. It will be
appreciated
that where the length of amino acid sequence A is not equal to the length of
amino
acid sequence B, the % amino acid sequence identity of A to B will not equal
the %
amino acid sequence identity of B to A. Unless specifically stated otherwise,
all %
amino acid sequence identity values used herein are obtained as described in
the
immediately preceding paragraph using the ALIGN-2 computer program.
The term "pharmaceutical formulation" refers to a preparation which is in such
form as to permit the biological activity of an active ingredient contained
therein to
be effective, and which contains no additional components which are
unacceptably
toxic to a subject to which the formulation would be administered.
A "pharmaceutically acceptable carrier" refers to an ingredient in a
pharmaceutical
formulation, other than an active ingredient, which is nontoxic to a subject.,
A
pharmaceutically acceptable carrier includes, but is not limited to, a buffer,
excipient, stabilizer, or preservative.
A "polypeptide" is a polymer consisting of amino acids joined by peptide
bonds,
whether produced naturally or synthetically. Polypeptides of less than about
20
amino acid residues may be referred to as "peptides", whereas molecules
consisting
of two or more polypeptides or comprising one polypeptide of more than 100
amino acid residues may be referred to as "proteins". A polypeptide may also
comprise non-amino acid components, such as carbohydrate groups, metal ions,
or
carboxylic acid esters. The non-amino acid components may be added by the
cell,
in which the polypeptide is expressed, and may vary with the type of cell.
Polypeptides are defined herein in terms of their amino acid backbone
structure or
the nucleic acid encoding the same. Additions such as carbohydrate groups are
generally not specified, but may be present nonetheless.
All polypeptide sequences are written according to the generally accepted
convention whereby the alpha-N-terminal amino acid residue is on the left and
the
alpha-C-terminal amino acid residue is on the right. As used herein, the term
"N-terminus" refers to the free alpha-amino group of an amino acid in a
polypeptide, and the term "C-terminus" refers to the free a-carboxylic acid
terminus
of an amino acid in a polypeptide. A polypeptide which is N-terminated with a
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 33 -
group refers to a polypeptide bearing a group on the alpha-amino nitrogen of
the
N-terminal amino acid residue. An amino acid which is N-terminated with a
group
refers to an amino acid bearing a group on the alpha-amino nitrogen.
Unless indicated otherwise by a "D" prefix, e.g., D-Ala or N-Me-D-Ile, or
written
in lower case format, e.g., a, i, 1, (D versions of Ala, Ile, Leu), the
stereochemistry
of the alpha-carbon of the amino acids and aminoacyl residues in polypeptides
described in this specification and the appended claims is the natural or
configuration. The Cahn-Ingold-Prelog "R" and "S" designations are used to
specify the stereochemistry of chiral centers in certain acyl substituents at
the
N-terminus of the polypeptides. The designation "R,S" is meant to indicate a
racemic mixture of the two enantiomeric forms. This nomenclature follows that
described in Cahn, R.S., et al., Angew. Chem. Int. Ed. Engl. 5 (1966) 385-415.
The term "single-chain Fv", short "scFv", denotes an antibody fragment that
comprise the VH and VL domains of antibody, wherein these domains are present
in a single polypeptide chain. In one embodiment, the Fv polypeptide further
comprises a polypeptide linker between the VH and VL domains which enables the
scFv to form the desired structure for antigen binding. For a review of scFv,
see
Plueckthun in The Pharmacology of Monoclonal Antibodies, vol. 113, Rosenburg
and Moore (Eds), Springer-Verlag, New York, pp. 269-315 (1994).
The term "theophylline", short "THEO", denotes 1,3-dimethy1-7H-purine-2,6-
dione. Theophylline is also known as dimethylxanthine.
The term "treatment" (and grammatical variations thereof such as "treat" or
"treating") denotes a clinical intervention in an attempt to alter the natural
course of
the individual being treated, and can be performed either for prophylaxis or
during
the course of clinical pathology. Desirable effects of treatment include, but
are not
limited to, preventing occurrence or recurrence of disease, alleviation of
symptoms,
diminishment of any direct or indirect pathological consequences of the
disease,
preventing metastasis, decreasing the rate of disease progression,
amelioration or
palliation of the disease state, and remission or improved prognosis. In some
embodiments, antibodies of the invention are used to delay development of a
disease or to slow the progression of a disease.
The term "x-valent", e.g. "mono-valent" or "bi-valent" or "tri-valent" or
"tetra-
valent", denotes the presence of a specified number of binding sites, i.e.
"x", in an
antibody molecule. As such, the terms "bivalent", "tetravalent", and
"hexavalent"
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 34 -
denote the presence of two binding site, four binding sites, and six binding
sites,
respectively, in an antibody molecule. The bispecific antibodies as reported
herein
are at least "bivalent" and may be "trivalent" or "multivalent" (e.g.
"tetravalent" or
"hexavalent"). In one embodiment the bispecific antibody as reported herein is
bivalent, trivalent, or tetravalent. In one embodiment the bispecific antibody
is
bivalent. In one embodiment the bispecific antibody is trivalent. In one
embodiment the bispecific antibody is tetravalent.
In certain aspects and embodiments the antibodies as reported herein have two
or
more binding sites and are bispecific. That is, the antibodies may be
bispecific even
in cases where there are more than two binding sites (i.e. that the antibody
is
trivalent or multivalent). The term bispecific antibodies includes, for
example,
multivalent single chain antibodies, diabodies and triabodies, as well as
antibodies
having the constant domain structure of full length antibodies to which
further
antigen-binding sites (e.g., single chain Fv, a VH domain and/or a VL domain,
Fab,
or (Fab)2,) are linked via one or more peptide-linkers. The antibodies can be
full
length from a single species, or be chimerized or humanized. For an antibody
with
more than two antigen binding sites, some binding sites may be identical, so
long
as the protein has binding sites for two different antigens. That is, whereas
a first
binding site is specific for a helicar motif amino acid sequence, a second
binding
site is specific for a non-helicar motif amino acid sequence antigen, and vice
versa.
The term "variable region" denotes the domain of an antibody heavy or light
chain
that is involved in binding the antibody to its antigen. The variable domains
of the
heavy chain and light chain (VH and VL, respectively) of a native antibody
generally have similar structures, with each domain comprising four conserved
framework regions (FRs) and three hypervariable regions (HVRs). (See, e.g.,
Kindt,
T.J. et al. Kuby Immunology, 6th ed., W.H. Freeman and Co., N.Y. (2007), page
91) A single VH or VL domain may be sufficient to confer antigen-binding
specificity. Furthermore, antibodies that bind a particular antigen may be
isolated
using a VH or VL domain from an antibody that binds the antigen to screen a
library of complementary VL or VH domains, respectively. See, e.g., Portolano,
S.
et al., J. Immunol. 150 (1993) 880-887; Clackson, T. et al., Nature 352 (1991)
624-
628).
The term "vector" denotes a nucleic acid molecule capable of propagating
another
nucleic acid to which it is linked. The term includes the vector as a self-
replicating
nucleic acid structure as well as the vector incorporated into the genome of a
host
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 35 -
cell into which it has been introduced. Certain vectors are capable of
directing the
expression of nucleic acids to which they are operatively linked. Such vectors
are
referred to herein as "expression vectors".
II. Conjugates as reported herein
In one aspect the invention is based on the finding that a covalent conjugate
comprising an helicar motif amino acid sequence containing compound and an
anti-helicar motif amino acid sequence antibody that specifically binds to the
helicar motif amino acid sequence can be obtained by the formation of a
covalent
bond between a properly placed functional group in the helicar motif amino
acid
sequence and a functional group in a variable domain of the antibody,
especially in
the CDR2 of the antibody, whereby the CDR2 is determined according to the
variable domain numbering according to Kabat.
In one embodiment the helicar motif amino acid sequence containing compound is
a conjugate comprising a helicar motif amino acid sequence, a linker and a
payload.
In certain embodiments the functional group may contain electron deficient
double
bonds or a thiol. In one embodiment the functional group is a maleimide or a
cysteine.
As the conjugates as reported herein can be used a therapeutic agent in humans
also
a humanized variant of the above antibody that specifically binds to the
helicar
motif amino acid sequence is provided herein.
Covalent conjugates of a helicar motif amino acid sequence containing compound
and an anti-helicar motif amino acid sequence antibody may confer benign
biophysical behavior and improved PK properties to the helicar motif amino
acid
sequence containing compound. Furthermore, in case a bispecific antibody is
used,
the conjugates can be used to target the helicar motif amino acid sequence
containing compound to cells which display the antigen that is recognized by
the
second binding specificity of the bispecific antibody. Such conjugates are
composed of one anti-helicar motif amino acid sequence binding specificity and
one further (non-helicar motif amino acid sequence) antigen binding
specificity.
The stoichiometric ratio of antibody to helicar motif amino acid sequence
containing polypeptide depends on the format of the bispecific antibody and
can be
1:1, 1:2, 2:1, 2:2, 2:4 and 4:2 (antibody: helicar motif amino acid sequence
containing polypeptide).
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 36 -
It is desired that the biologically active compound in the helicar motif amino
acid
sequence containing compound retains good biological activity despite being
conjugated to the helicar motif amino acid sequence, as well as being
conjugated to
the antibody. It is also desired (in case of bispecific targeting modules)
that the cell
surface target binding site of the bispecific antibody retains its binding
specificity
and affinity in the presence of the covalently conjugated helicar motif
containing
compound.
The reactive group in the helicar motif amino acid sequence may be any
reactive
group, such as e.g. a maleimide, e.g. N-ethyl maleimide (NEM), a
iodoacetamide, a
pyridyl disulfide, or other reactive conjugation partner (see e.g. Haugland,
2003,
Molecular Probes Handbook of Fluorescent Probes and Research Chemicals,
Molecular Probes, Inc.; Brinkley, 1992, Bioconjugate Chem. 3:2; Garman, 1997,
Non-Radioactive Labeling: A Practical Approach, Academic Press, London;
Means (1990) Bioconjugate Chem. 1:2; Hermanson, G. in Bioconjugate
Techniques (1996) Academic Press, San Diego, pp. 40-55 and 643-671).
The reactive group on the antibody is limited to those that can be
selectively, i.e.
position specifically, generated. Therefore, it is limited to the side chain
groups of
the amino acid residues cysteine, serine, asparagine, glutamine, tyrosine,
lysine,
arginine, aspartic acid, and glutamic acid.
For the formation of a covalent conjugate between the anti-helicar motif amino
acid sequence antibody and the helicar motif amino acid sequence containing
compound both have to be modified by the introduction of a reactive group.
Upon
binding of the helicar motif amino acid sequence by the anti-helicar motif
amino
acid sequence antibody the two reactive groups are brought in close proximity
allowing the formation of a covalent bond. In one embodiment the modification
is
the introduction of a thiol functionality in each of the compounds. In one
embodiment the thiol compound is a cysteine residue.
The position to be mutated must simultaneously meet two requirements: (i) the
coupling positions should be in proximity to the binding region to utilize the
helicar
motif amino acid sequence positioning effect for directed coupling, and (ii)
the
mutation and coupling position must be positioned in a manner that helicar
motif
amino acid sequence binding by itself is not affected. These requirements for
finding a suitable position are de facto 'contradicting' each other because
requirement (i) is best served by a position close to the binding site, while
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 37 -
requirement (ii) is most safely achieved by positions that are distant from
the
binding site.
Despite these virtually excluding requirements, a position was identified that
can
be mutated without affecting helicar motif amino acid sequence positioning,
and
which nevertheless simultaneously allow directed covalent coupling.
One position is located at position VL55 according to the Kabat numbering of
the
light chain variable domain.
One position is located at position VL5 1 according to the Kabat numbering of
the
light chain variable domain.
These positions are applicable to the helicar motif amino acid sequence
antibody
and, thus, it is not required to start from scratch every time a new covalent
conjugate has to be made. Only the helicar motif amino acid sequence as to be
introduced in/conjugate to the payload.
The antibodies modified as reported herein retain the helicar motif amino acid
sequence binding capability of their parent (i.e. wild-type) antibody
counterparts.
Thus, the engineered antibody is capable of binding, in one embodiment it is
capable of specifically binding, to the helicar motif amino acid sequence.
The terms "binding specificity" or "an antibody that binds to" denote that the
molecule comprising the binding specificity or an antibody can form a complex
with a further molecule in a specific manner. The binding can be detected in
an in
vitro assay, such as in a plasmon resonance assay (BIAcore, GE-Healthcare
Uppsala, Sweden). The affinity of the complex formation is defined by the
terms ka
(rate constant for the association of the compounds to form the complex), kp
(dissociation constant, dissociation of the complex), and KD (1(D/ka). Binding
or
specifically binding means a binding affinity (KD) of about 10-7 M or less, in
one
embodiment of about 10-8 M to about 10-13 M, in one embodiment of about 10-9 M
to about 10-13 M. Thus, an antibody that binds to the helicar motif amino acid
sequence to form a complex as reported herein specifically binds to the
helicar
motif amino acid sequence with a binding affinity (KD) of about 10-8 mo1/1 or
less,
in one embodiment of about 10-8 mo1/1 to about 10-13 mo1/1.
It has been found that the formation of a covalent bond between a cysteine-
modified anti-helicar motif amino acid sequence antibody and a cysteine-
modified
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 38 -
helicar motif amino acid sequence containing compound bearing the cysteine
residue in the helicar motif amino acid sequence takes place upon binding of
the
antibody to the helicar motif amino acid sequence without the requirement of
the
addition of reducing and/or oxidizing agents if the formed bond is a disulfide
bond.
Thus, the disulfide bridge between the two compounds is formed spontaneously
upon formation of the non-covalent complex. Therefore, a method for the
formation of a covalent complex as reported herein simply requires the mixing
of
the two compounds. The only pre-requisite for the formation of the disulfide
bond
is a proper orientation of the two compounds with respect to each other.
The engineered antibodies as reported herein may be site-specifically and
efficiently covalently conjugated (coupled) with a helicar motif amino acid
sequence comprising a reactive group.
Replacement of the amino acid residue at position VL55 or VL5 1, respectively,
(according to the Kabat numbering scheme) with a cysteine residue resulted in
anti-
helicar motif amino acid sequence antibody derivatives with heavy chain
variable
region sequences that are listed in SEQ ID NO: 06 and SEQ ID NO: 07.
One aspect as reported herein is an anti-helicar motif amino acid sequence
antibody
that is a humanized antibody. In one embodiment the anti-helicar motif amino
acid
sequence antibody comprises a humanized heavy chain variable domain derived
from the heavy chain variable domain that has the amino acid sequence of SEQ
ID
NO: 04 and a humanized light chain variable domain derived from a light chain
variable domain that has the amino acid sequence of SEQ ID NO: 05.
One aspect as reported herein is an anti-helicar motif amino acid sequence
antibody
that is a humanized antibody. In one embodiment the anti-helicar motif amino
acid
sequence antibody comprises a humanized heavy chain variable domain derived
from the heavy chain variable domain that has the amino acid sequence of SEQ
ID
NO: 04 and a humanized light chain variable domain derived from a light chain
variable domain that has the amino acid sequence of SEQ ID NO: 06.
One aspect as reported herein is an anti-helicar motif amino acid sequence
antibody
that is a humanized antibody. In one embodiment the anti-helicar motif amino
acid
sequence antibody comprises a humanized heavy chain variable domain derived
from the heavy chain variable domain that has the amino acid sequence of SEQ
ID
NO: 04 and a humanized light chain variable domain derived from a light chain
variable domain that has the amino acid sequence of SEQ ID NO: 07.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 39 -
One aspect as reported herein is an anti-helicar motif amino acid sequence
antibody
that comprises CDRs as in the variable domain of SEQ ID NO: 04 for the heavy
chain and as in any of the variable domains of SEQ ID NO: 05, or SEQ ID NO:
06,
or SEQ ID NO: 07 for the light chain variable domain and further comprises an
acceptor human framework, e.g. a human immunoglobulin framework or a human
consensus framework.
One aspect as reported herein is an anti-helicar motif amino acid sequence
antibody
that comprises hypervariable loops as in the variable domain of SEQ ID NO: 04
for
the heavy chain and as in any of the variable domains of SEQ ID NO: 05, or SEQ
ID NO: 06, or SEQ ID NO: 07 for the light chain variable domain and further
comprises an acceptor human framework, e.g. a human immunoglobulin
framework or a human consensus framework.
For example, PYY was modified to comprise the helicar motif amino acid
sequence and complexed by an anti-helicar motif amino acid sequence antibody
in
order to get advantage of the pharmacokinetic properties of the antibody and
to
avoid the intrinsic instability of the PYY.
The structural investigation of the PYY3_36 peptide (Nygaard, R., et al.,
Biochem.
45 (2006) 8350-8357; SEQ ID NO: 26) reveals a helical motif (helicar-like
motif
amino acid sequence) for the central amino acids. As the N-terminal isoleucine
and
the modified C- terminus have been described as essential for the functional
activity of the peptide, the central helix was modified in order to reflect
the amino
acids in the helicar motif amino acid sequence.
PYY(3-36) 3 36
(SEQ ID NO. 26) IKPEAPGEDASPEELNRYYASLRHYLNLVTRQRYNH2
Helicar motif AHLENEVARLKK
PYY helicar IKPEAPGEDASPEAHLANEVARLHYLNLVTRQRYNH2
_
(SEQ ID NO: 27) (YNH2 = tyrosine amide)
binding soluble
[Ka] in PBS
PYY(3 -36) - + PYY wild-type
(SEQ ID NO: 26)
PYY helicar 12 nM + helicar motif engineered
(SEQ ID NO: 27) PYY
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 40 -
The full IgG1 anti-helicar motif amino acid sequence antibody 0019 and the
modified PYY peptide PYY helicar was obtained in vitro by applying a small
excess of the peptide to the antibody solution. The complex 0052 was formed.
The
stoichiometry of the complex was determined by SEC-Malls analytical
experiments
to be 1.6 peptides complexed on one bivalent antibody.
The antibody 0019, the PYY(3-36) wild-type, the PYY helicar and the complex
0052 were tested for their effect on to the Y2Receptor family.
NPY2R NPY1R NPY4R NPY5R
Ac-Ile-Lys-Pqa-Arg-His-Tyr-Leu-Asn- 1.0 nM inactive inactive
inactive
Trp-Val-Thr-Arg-Gln-(NMe)-Arg-Try-
NH2 * 4 HOAc
PYY helicar 6.38 nM inactive inactive
inactive
(IKPEAPGEDASPEAHLANEVARLH
YLNLVTRQRYNH2) (SEQ ID NO: 27)
PYY(3 -36)
0.05 nM 168 nM 162 nM 170 nM
(IKPEAPGEDASPEELNRYYASLRHY
LNLVTRQRYNH2) (SEQ ID NO: 26)
charge 1
PYY(3 -36)
0.05 nM 160 nM 131 nM 202 nM
(IKPEAPGEDASPEELNRYYASLRHY
LNLVTRQRYNH2) (SEQ ID NO: 26)
charge 2
anti-helicar motif amino acid sequence inactive inactive inactive
inactive
antibody (0019)
anti-helicar motif amino acid sequence 0.93 nM inactive inactive
inactive
antibody-PYY helicar complex (0052)
As demonstrated (Hoffmann, E., et al., J. Cont. Rel. 171 (2013) 48-56.) the
peptides complexed by an antibody have a prolonged half-life in vivo. Moreover
and surprisingly, the complex demonstrates a slightly better affinity for the
NPY2R
receptor compared to the non-complexed peptide; the antibody stabilizes the
polypeptide and presents the peptide in its fixed biologically active
conformation.
In order to increase the in vitro and in vivo stability of the complex between
the
anti-helicar motif amino acid sequence antibody and the helicar motif amino
acid
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
-41 -
sequence containing compound, the formation of a disulfide bridge upon binding
has been used.
The first step is a specific recognition step (high affinity interaction),
i.e. the
formation of the helicar motif amino acid sequence containing compound-anti-
helicar motif amino acid sequence antibody complex. This is followed in the
second step by a spontaneous shuffling of a disulfide bridge to form the
stability
improved covalent complex.
As the 12-mer peptide (helicar motif amino acid sequence) is a relatively
rigid
entity (at least when complexed by a specific anti-helicar motif amino acid
sequence antibody) it has been found that a structurally specific design for
the
disulfide bridge has to be used. As the complex formation and the thereafter
effected covalent coupling is between two recombinantly produced entities, the
artificial cysteine residues introduced for the formation of a covalent
disulfide bond
are not produced necessarily as free cysteine residues but are expressed in a
reduced from, i.e. conjugated to a free cysteine or homo cysteine amino acid.
The position in the amino acid sequence of the anti-helicar motif amino acid
sequence antibody variable domain where the artificial free cysteine residue
is
introduced is critical. A non-exposed cysteine in the antibody variable domain
amino acid sequence has more probability to be expressed as a free cysteine
(not
conjugated), whereas an exposed cysteine residue close to the binding pocket
can
abolish the binding of the 12-mer peptide (helicar motif amino acid sequence)
due
to a steric hindrance induced by the cysteine conjugation to an additional
moiety
like a free cysteine.
In order to identify a suitable position which has minimum risk of steric
hindrance
and strong affinity reduction, different positions for the introduction of the
artificial
cysteine residue in the helicar motif amino acid sequence have been tested.
The
cysteine residue has been introduced at the C-terminal end of the 12mer
(helicar
motif amino acid sequence) in order to have the major part of the paratope
unchanged. The peptides have been synthesized and fused to a fluorescent
motif.
wild-type: AHLENEVARLKK (SEQ ID NO: 01)
cysteine variant 1: AHLENEVARCKK (SEQ ID NO: 02)
-> AHLENEVARCKK(5-Fluo)-OH
cysteine variant 2: AHLENEVARLCK (SEQ ID NO: 03)
-> AHLENEVARLCK(5-Fluo)-OH x TFA
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 42 -
On the antibody, a structural design has been done to allow the formation of
the
disulfide bridge for both designed peptides including each a cysteine in
different
3D environment.
The 12-mer helical peptide AHLENEVARLKK (helicar motif amino acid
sequence) is modeled into the VH and the VH domains. At the C-terminus of the
peptide the residues L10 and Kll are identified as possible position and in
the light
chain variable domain the positions N55 and G51 according to the light chain
numbering of Kabat are identified.
The bivalent antibody 0155 (N55C) was coupled to the helicar motif amino acid
sequence cysteine variant 2. On the SDS page (denaturing condition, see Figure
12)
the fluorescence is seen only on the antibody 0155; in the reducing condition,
only
the small peptide is visible.
The covalent conjugation of the helicar motif amino acid sequence containing
fluorescent compound to the anti-helicar motif amino acid sequence antibody
was
successful.
The coupling of antibody 0157 (G51C) with the helicar motif amino acid
sequence
cysteine variant 1 was not resulting in the expected covalent complex. The
fluorescence is not seen in the expected lane but on the reference which
should be
negative in this experiment (see Figure 13).
The covalent conjugation of the helicar motif amino acid sequence containing
fluorescent compound to the anti-helicar motif amino acid sequence antibody
0157
was not successful. Without being bound by this theory it is assumed that in
this
case the antibody cysteinylation is too deep in the binding pocket to allow
the
helicar motif amino acid sequence containing fluorescent compound to bind
efficiently and deliver the nucleophilic thiol group in an appropriate
position to
attack the C51.
The helicar based methodology becomes particularly attractive when considering
the formation of a covalent complex with a recombinantly produced helicar
motif
amino acid sequence containing polypeptide.
As the conjugation of the antibody 0155 containing the VL-N55C mutation with
the helicar motif amino acid sequence cysteine variant 1 (AHLENEVARLCK;
SEQ ID NO: 02) has much better performed compared to the alternative (G51C on
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 43 -
VL with helicar motif amino acid sequence cysteine variant 2
(AHLENEVARCKK; SEQ ID NO: 03)), the conjugation of 0155 with a helicar
motif amino acid sequence cysteine variant 1 containing polypeptide was
further
investigated. The polypeptide contained the helicar motif amino acid sequence
cysteine variant 1 (AHLENEVARLCK; SEQ ID NO: 02) fused to the N-terminus.
Antibody 0155 is covalently conjugated with the helicar motif amino acid
sequence
cysteine variant 1 containing Pseudomonas exotoxin molecule LR8M with the
C-terminal lysine residue deleted of SEQ ID NO: 28. The SEC chromatogram is
shown in Figure 14. The conjugation efficiency is analyzed by SDS-CE, Caliper,
for the non reduced samples (see Figure 15).
In conclusion, the anti-helicar motif amino acid sequence monoclonal antibody
can
be used to complex peptides, small molecules with peptidic linker, and
recombinant proteins via a high affinity recognition of a 12-mer helicar motif
amino acid sequence. Peptides with propensity to fold as helix can be modified
to
mimic the original 12-mer helicar motif amino acid sequence AHLENEVARLKK
(SEQ ID NO: 01) and are thereafter complexable with the anti-helicar motif
amino
acid sequence monoclonal antibody. In addition to the high affinity
complexation,
covalent conjugation is enabled with a cysteine variant of SEQ ID NO: 01
containing a cysteine and a modified anti-helicar motif amino acid sequence
antibody containing a cysteine in the CDRs via formation a stable disulfide
bond.
Recombinant proteins expressed by different system can be conjugated
afterwards
in vitro without particular reactions conditions but via spontaneous disulfide
bridge
shuffling.
Antibody Affinity
In certain embodiments, the anti-helicar motif amino acid sequence antibody as
reported herein itself or the anti-helicar motif amino acid sequence antibody
in the
complex as reported herein has a dissociation constant (Kd) of < 1 M, < 100
nM,
< about 10 nM (e.g. of about 10-6M or less, e.g. from about 10-6M to about 10-
13M,
e.g., from about 10-8M to about 10-10 M).
In one embodiment, Kd is measured by a radiolabeled antigen binding assay
(RIA)
performed with the Fab version of an antibody of interest and its antigen as
described by the following assay. Solution binding affinity of Fabs for
antigen is
measured by equilibrating Fab with a minimal concentration of (125I)-labeled
antigen in the presence of a titration series of unlabeled antigen, then
capturing
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 44 -
bound antigen with an anti-Fab antibody-coated plate (see, e.g., Chen, Y. et
al.,
J. Mol. Biol. 293 (1999) 865-881). To establish conditions for the assay,
MICROTITER multi-well plates (Thermo Scientific) are coated overnight with
g/m1 of a capturing anti-Fab antibody (Cappel Labs) in 50 mM sodium
5
carbonate (pH 9.6), and subsequently blocked with 2% (w/v) bovine serum
albumin in PBS for two to five hours at room temperature (approximately 23 C).
In
a non-adsorbent plate (Nunc #269620), 100 pM or 26 pM
['251]-antigen are mixed
with serial dilutions of a Fab of interest (e.g., consistent with assessment
of the
anti-VEGF antibody, Fab-12, in Presta, L.G. et al., Cancer Res. 57 (1997) 4593-
4599). The Fab of interest is then incubated overnight; however, the
incubation
may continue for a longer period (e.g., about 65 hours) to ensure that
equilibrium is
reached. Thereafter, the mixtures are transferred to the capture plate for
incubation
at room temperature (e.g., for one hour). The solution is then removed and the
plate
washed eight times with 0.1 % polysorbate 20 (TWEEN-20 ) in PBS. When the
plates have dried, 150 l/well of scintillant (MICROSCINT-20 TM; Packard) is
added, and the plates are counted on a TOPCOUNT TM gamma counter (Packard)
for ten minutes. Concentrations of each Fab that give less than or equal to 20
% of
maximal binding are chosen for use in competitive binding assays.
According to another embodiment, Kd is measured using surface plasmon
resonance assays using a BIACORE -2000 or a BIACORE -3000 (BIAcore, Inc.,
Piscataway, NJ) at 25 C with immobilized antigen CM5 chips at ¨10 response
units (RU). Briefly, carboxymethylated dextran biosensor chips (CM5, BIACORE,
Inc.) are activated with N-ethyl-N'- (3-dimethylaminopropy1)-carbodiimide
hydrochloride (EDC) and N-hydroxysuccinimide (NHS) according to the supplier's
instructions. Antigen is diluted with 10 mM sodium acetate, pH 4.8, to 5 g/m1
(about 0.2 M) before injection at a flow rate of 5 1/minute to achieve
approximately 10 response units (RU) of coupled protein. Following the
injection
of antigen, 1 M ethanolamine is injected to block non-reacted groups. For
kinetics
measurements, two-fold serial dilutions of Fab (0.78 nM to 500 nM) are
injected in
PBS with 0.05% polysorbate 20 (TWEEN-20Tm) surfactant (PBST) at 25 C at a
flow rate of approximately 25 1/min. Association rates (kon) and dissociation
rates
(koff) are calculated using a simple one-to-one Langmuir binding model
(BIACORE Evaluation Software version 3.2) by simultaneously fitting the
association and dissociation sensorgrams. The equilibrium dissociation
constant
(Kd) is calculated as the ratio koff/kon. See, e.g., Chen, Y. et al., J. Mol.
Biol. 293
(1999) 865-881. If the on-rate exceeds 106 M-1 5-1 by the surface plasmon
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 45 -
resonance assay above, then the on-rate can be determined by using a
fluorescent
quenching technique that measures the increase or decrease in fluorescence
emission intensity (excitation = 295 nm; emission = 340 nm, 16 nm band-pass)
at
25 0C of a 20 nM anti-antigen antibody (Fab form) in PBS, pH 7.2, in the
presence
of increasing concentrations of antigen as measured in a spectrometer, such as
a
stop-flow equipped spectrophotometer (Aviv Instruments) or a 8000-series SLM-
AMINCO TM spectrophotometer (ThermoSpectronic) with a stirred cuvette.
Antibody Fragments
In certain embodiments, an anti-helicar motif amino acid sequence antibody
provided herein or in a conjugate as reported herein is an anti-helicar motif
amino
acid sequence antibody fragment. Antibody fragments include, but are not
limited
to, Fab, Fab', Fab'-SH, F(ab')2, Fv, and scFv fragments, and other fragments
described below. For a review of certain antibody fragments, see Hudson, P.J.
et al.,
Nat. Med. 9 (2003) 129-134. For a review of scFy fragments, see, e.g.,
Plueckthun,
A., In; The Pharmacology of Monoclonal Antibodies, Vol. 113, Rosenburg and
Moore (eds.), Springer-Verlag, New York (1994), pp. 269-315; see also WO
93/16185; and U.S. Patent Nos. 5,571,894 and 5,587,458. For discussion of Fab
and F(ab')2 fragments comprising salvage receptor binding epitope residues and
having increased in vivo half-life, see US 5,869,046.
Diabodies are antibody fragments with two antigen-binding sites that may be
bivalent or bispecific. See, for example, EP 0 404 097; WO 93/01161; Hudson,
P.J.
et al., Nat. Med. 9 (2003) 129-134; and Holliger, P. et al., Proc. Natl. Acad.
Sci.
USA 90 (1993) 6444-6448. Triabodies and tetrabodies are also described in
Hudson, P.J. et al., Nat. Med. 9 (20039 129-134).
Single-domain antibodies are antibody fragments comprising all or a portion of
the
heavy chain variable domain or all or a portion of the light chain variable
domain
of an antibody. In certain embodiments, a single-domain antibody is a human
single-domain antibody (Domantis, Inc., Waltham, MA; see, e.g., US 6,248,516).
Antibody fragments can be made by various techniques, including but not
limited
to proteolytic digestion of an intact antibody as well as production by
recombinant
host cells (e.g. E. coli or phage), as described herein.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 46 -
Chimeric and Humanized Antibodies
In certain embodiments, an anti-helicar motif amino acid sequence antibody
provided herein or the anti-helicar motif amino acid sequence antibody in a
conjugate as reported herein is a chimeric antibody. Certain chimeric
antibodies are
described, e.g., in US 4,816,567; and Morrison, S.L. et al., Proc. Natl. Acad.
Sci.
USA 81(1984) 6851-6855). In one example, a chimeric antibody comprises a non-
human variable region (e.g., a variable region derived from a mouse, rat,
hamster,
rabbit, or non-human primate, such as a monkey) and a human constant region.
In a
further example, a chimeric antibody is a "class switched" antibody in which
the
class or subclass has been changed from that of the parent antibody. Chimeric
antibodies include antigen-binding fragments thereof
In certain embodiments, a chimeric antibody is a humanized antibody.
Typically, a
non-human antibody is humanized to reduce immunogenicity to humans, while
retaining the specificity and affinity of the parental non-human antibody.
Generally,
a humanized antibody comprises one or more variable domains in which HVRs,
e.g., CDRs, (or portions thereof) are derived from a non-human antibody, and
FRs
(or portions thereof) are derived from human antibody sequences. A humanized
antibody optionally will also comprise at least a portion of a human constant
region.
In some embodiments, some FR residues in a humanized antibody are substituted
with corresponding residues from a non-human antibody (e.g., the antibody from
which the HVR residues are derived), e.g., to restore or improve antibody
specificity or affinity.
Humanized antibodies and methods of making them are reviewed, e.g., in
Almagro,
J.C. and Fransson, J., Front. Biosci. 13 (2008) 1619-1633, and are further
described,
e.g., in Riechmann, I. et al., Nature 332 (1988) 323-329; Queen, C. et al.,
Proc.
Natl. Acad. Sci. USA 86 (1989) 10029-10033; US Patent Nos. 5,821,337,
7,527,791, 6,982,321, and 7,087,409; Kashmiri, S.V. et al., Methods 36 (2005)
25-
34 (describing SDR (a-CDR) grafting); Padlan, E.A., Mol. Immunol. 28 (1991)
489-498 (describing "resurfacing"); Dall'Acqua, W.F. et al., Methods 36 (2005)
43-60 (describing "FR shuffling"); and Osbourn, J. et al., Methods 36 (2005)
61-68
and Klimka, A. et al., Br. J. Cancer 83 (2000) 252-260 (describing the "guided
selection" approach to FR shuffling).
Human framework regions that may be used for humanization include but are not
limited to: framework regions selected using the "best-fit" method (see, e.g.,
Sims,
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
-47 -
M.J. et al., J. Immunol. 151 (1993) 2296-2308; framework regions derived from
the consensus sequence of human antibodies of a particular subgroup of light
or
heavy chain variable regions (see, e.g., Carter, P. et al., Proc. Natl. Acad.
Sci. USA
89 (1992) 4285-4289; and Presta, L.G. et al., J. Immunol. 151 (1993) 2623-
2632);
human mature (somatically mutated) framework regions or human germline
framework regions (see, e.g., Almagro, J.C. and Fransson, J., Front. Biosci.
13
(2008) 1619-1633); and framework regions derived from screening FR libraries
(see, e.g., Baca, M. et al., J. Biol. Chem. 272 (1997) 10678-10684 and Rosok,
M.J.
et al., J. Biol. Chem. 271 (19969 22611-22618).
Library-Derived Antibodies
Anti-helicar motif amino acid sequence antibodies of the invention or anti-
helicar
motif amino acid sequence antibodies in the conjugate as reported herein may
be
isolated by screening combinatorial libraries for antibodies with the desired
activity
or activities. For example, a variety of methods are known in the art for
generating
phage display libraries and screening such libraries for antibodies possessing
the
desired binding characteristics. Such methods are reviewed, e.g., in
Hoogenboom,
H.R. et al., Methods in Molecular Biology 178 (2001) 1-37 and further
described,
e.g., in the McCafferty, J. et al., Nature 348 (1990) 552-554; Clackson, T. et
al.,
Nature 352 (1991) 624-628; Marks, J.D. et al., J. Mol. Biol. 222 (1992) 581-
597;
Marks, J.D. and Bradbury, A., Methods in Molecular Biology 248 (2003) 161-175;
Sidhu, S.S. et al., J. Mol. Biol. 338 (2004) 299-310; Lee, C.V. et al., J.
Mol. Biol.
340 (2004) 1073-1093; Fellouse, F.A., Proc. Natl. Acad. Sci. USA 101 (2004)
12467-12472; and Lee, C.V. et al., J. Immunol. Methods 284 (2004) 119-132.
In certain phage display methods, repertoires of VH and VL genes are
separately
cloned by polymerase chain reaction (PCR) and recombined randomly in phage
libraries, which can then be screened for antigen-binding phage as described
in
Winter, G. et al., Ann. Rev. Immunol. 12 (1994) 433-455. Phage typically
display
antibody fragments, either as single-chain Fv (scFv) fragments or as Fab
fragments.
Libraries from immunized sources provide high-affinity antibodies to the
immunogen without the requirement of constructing hybridomas. Alternatively,
the
naive repertoire can be cloned (e.g., from human) to provide a single source
of
antibodies to a wide range of non-self and also self-antigens without any
immunization as described by Griffiths, A.D. et al., EMBO J. 12 (1993) 725-
734.
Finally, naive libraries can also be made synthetically by cloning non-
rearranged
V-gene segments from stem cells, and using PCR primers containing random
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 48 -
sequence to encode the highly variable CDR3 regions and to accomplish
rearrangement in vitro, as described by Hoogenboom, H.R. and Winter, G., J.
Mol.
Biol. 227 (1992) 381-388. Patent publications describing human antibody phage
libraries include, for example: US 5,750,373, US 2005/0079574, US
2005/0119455,
US 2005/0266000, US 2007/0117126, US 2007/0160598, US 2007/0237764,
US 2007/0292936, and US 2009/0002360.
Antibodies or antibody fragments isolated from human antibody libraries are
considered human antibodies or human antibody fragments herein.
Antibody formats
The above outlined anti-helicar motif amino acid sequence antibodies and anti-
helicar motif amino acid sequence antibody fragments can be combined in
multiple
ways to generate different antibody formats.
For example, one or more scFv antibody fragments can be fused to the C-
terminus
of one or more polypeptide chains of a complete antibody. Especially to each
heavy chain C-terminus or to each light chain C-terminus a scFv antibody
fragment
can be fused.
For example, one or more antibody Fab fragments can be fused to the C-terminus
of one or more polypeptide chains of a complete antibody. Especially to each
heavy chain C-terminus or to each light chain C-terminus an antibody Fab
fragment can be fused.
For example, one scFv and one antibody Fab fragment can be fused to the
N-termini of an antibody Fc-region.
For example one scFv or antibody Fab fragment can be fused to an N-terminus of
an antibody Fc-region and one scFv or antibody Fab fragment can be fused to
the
C-terminus of the respective other chain of an antibody Fc-region.
Multispecific Antibodies
A wide variety of recombinant antibody formats have been developed, e.g.
tetravalent bispecific antibodies by fusion of, e.g., an IgG antibody format
and
single chain domains (see e.g. Coloma, M.J., et al., Nature Biotech 15 (1997)
159-
163; WO 01/077342; and Morrison, S.L., Nature Biotech 25 (2007) 1233-1234).
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 49 -
Also several other formats wherein the antibody core structure (IgA, IgD, IgE,
IgG
or IgM) is no longer retained such as dia-, tria- or tetrabodies, minibodies,
several
single chain formats (scFv, Bis-scFv), which are capable of binding two or
more
antigens, have been developed (Holliger, P., et al., Nature Biotech 23 (2005)
1126-
1136; Fischer, N., Leger, 0., Pathobiology 74 (2007) 3-14; Shen, J., et al.,
Journal
of Immunological Methods 318 (2007) 65-74; Wu, C., et al., Nature Biotech. 25
(2007) 1290-1297).
All such formats use linkers either to fuse the antibody core (IgA, IgD, IgE,
IgG or
IgM) to a further binding protein (e.g. scFv) or to fuse e.g. two Fab
fragments or
scFvs (Fischer, N. and Leger, 0., Pathobiology 74 (2007) 3-14). It has to be
kept in
mind that one may want to retain effector functions, such as e.g. complement-
dependent cytotoxicity (CDC) or antibody dependent cellular cytotoxicity
(ADCC),
which are mediated through the Fc receptor binding, by maintaining a high
degree
of similarity to naturally occurring antibodies.
In WO 2007/024715 are reported dual variable domain immunoglobulins as
engineered multivalent and multispecific binding proteins. A process for the
preparation of biologically active antibody dimers is reported in US
6,897,044.
Multivalent FV antibody construct having at least four variable domains which
are
linked with each over via peptide linkers are reported in US 7,129,330.
Dimeric
and multimeric antigen binding structures are reported in US 2005/0079170. Tri-
or
tetra-valent monospecific antigen-binding protein comprising three or four Fab
fragments bound to each other covalently by a connecting structure, which
protein
is not a natural immunoglobulin are reported in US 6,511,663. In WO
2006/020258
tetravalent bispecific antibodies are reported that can be efficiently
expressed in
prokaryotic and eukaryotic cells, and are useful in therapeutic and diagnostic
methods. A method of separating or preferentially synthesizing dimers which
are
linked via at least one interchain disulfide linkage from dimers which are not
linked
via at least one interchain disulfide linkage from a mixture comprising the
two
types of polypeptide dimers is reported in US 2005/0163782. Bispecific
tetravalent
receptors are reported in US 5,959,083. Engineered antibodies with three or
more
functional antigen binding sites are reported in WO 2001/077342.
Multispecific and multivalent antigen-binding polypeptides are reported in
WO 97/001580. WO 92/004053 reports homoconjugates, typically prepared from
monoclonal antibodies of the IgG class which bind to the same antigenic
determinant are covalently linked by synthetic cross-linking.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 50 -
WO 91/06305 whereby the oligomers, typically of the IgG class, are secreted
having two or more immunoglobulin monomers associated together to form
tetravalent or hexavalent IgG molecules. Sheep-derived antibodies and
engineered
antibody constructs are reported in US 6,350,860, which can be used to treat
diseases wherein interferon gamma activity is pathogenic. In US 2005/0100543
are
reported targetable constructs that are multivalent carriers of bi-specific
antibodies,
i.e., each molecule of a targetable construct can serve as a carrier of two or
more
bi-specific antibodies. Genetically engineered bispecific tetravalent
antibodies are
reported in WO 95/009917. In WO 2007/109254 stabilized binding molecules that
consist of or comprise a stabilized scFy are reported.
In certain embodiments, an anti-helicar motif amino acid sequence antibody
provided herein or the anti-helicar motif amino acid sequence antibody in a
conjugate as reported herein is a multispecific antibody, e.g. a bispecific
antibody.
Multispecific antibodies are monoclonal antibodies that have binding
specificities
for at least two different sites. In certain embodiments, one of the binding
specificities is for a helicar motif amino acid sequence and the other is for
any
other (non-helicar motif amino acid sequence) antigen. Bispecific antibodies
may
also be used to localize cytotoxic agents to cells. Bispecific antibodies can
be
prepared as full length antibodies or antibody fragments.
Techniques for making multispecific antibodies include, but are not limited
to,
recombinant co-expression of two immunoglobulin heavy chain-light chain pairs
having different specificities (see Milstein, C. and Cuello, A.C., Nature 305
(1983)
537-540, WO 93/08829, and Traunecker, A. et al., EMBO J. 10 (1991) 3655-3659),
and "knob-in-hole" engineering (see, e.g., U.S. Patent No. 5,731,168). Multi-
specific antibodies may also be made by engineering electrostatic steering
effects
for making antibody Fc-heterodimeric molecules (WO 2009/089004); cross-linking
two or more antibodies or fragments (see, e.g., US Patent No. 4,676,980, and
Brennan, M. et al., Science 229 (1985) 81-83); using leucine zippers to
produce bi-
specific antibodies (see, e.g., Kostelny, S.A. et al., J. Immunol. 148 (1992)
1547-
1553; using "diabody" technology for making bispecific antibody fragments
(see,
e.g., Holliger, P. et al., Proc. Natl. Acad. Sci. USA 90 (1993) 6444-6448);
and
using single-chain Fv (scFv) dimers (see, e.g. Gruber, M et al., J. Immunol.
152
(1994) 5368-5374); and preparing trispecific antibodies as described, e.g., in
Tutt,
A. et al., J. Immunol. 147 (1991) 60-69).
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
-51 -
In one embodiment the CH3 domains of the heavy chains of the bispecific
antibody
are altered by the "knob-into-holes" technology which is described in detail
with
several examples in e.g. WO 96/027011, WO 98/050431, Ridgway J.B., et al.,
Protein Eng. 9 (1996) 617-621, Merchant, A.M., et al., Nat Biotechnol 16
(1998)
677-681. In this method the interaction surfaces of the two CH3 domains are
altered to increase the heterodimerization of both heavy chains containing
these
two CH3 domains. Each of the two CH3 domains (of the two heavy chains) can be
the "knob", while the other is the "hole". The introduction of a disulfide
bridge
stabilizes the heterodimers (Merchant, A.M, et al., Nature Biotech 16 (1998)
677-681, Atwell, S., et al. J. Mol. Biol. 270 (1997) 26-35) and increases the
yield.
In one embodiment of all aspects the bispecific antibody is characterized in
that
- the CH3 domain of one heavy chain and the CH3 domain of the other heavy
chain each meet at an interface which comprises an original interface
between the antibody CH3 domains,
wherein said interface is altered to promote the formation of the bispecific
antibody, wherein the alteration is characterized in that
a) the CH3 domain of one heavy chain is altered,
so that within the original interface the CH3 domain of one heavy chain
that meets the original interface of the CH3 domain of the other heavy
chain within the bispecific antibody,
an amino acid residue is replaced with an amino acid residue having a
larger side chain volume, thereby generating a protuberance within the
interface of the CH3 domain of one heavy chain which is positionable in a
cavity within the interface of the CH3 domain of the other heavy chain
and
b) the CH3 domain of the other heavy chain is altered,
so that within the original interface of the second CH3 domain that meets
the original interface of the first CH3 domain within the bispecific
antibody
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 52 -
an amino acid residue is replaced with an amino acid residue having a
smaller side chain volume, thereby generating a cavity within the interface
of the second CH3 domain within which a protuberance within the
interface of the first CH3 domain is positionable.
Thus, the bispecific antibodies as reported herein are in one embodiment
characterized in that
- the
CH3 domain of the first heavy chain of the full length antibody and the
CH3 domain of the second heavy chain of the full length antibody each
meet at an interface which comprises an alteration in the original interface
between the antibody CH3 domains,
wherein i) in the CH3 domain of the first heavy chain
an amino acid residue is replaced with an amino acid residue having a larger
side chain volume, thereby generating a protuberance within the interface of
the CH3 domain of one heavy chain which is positionable in a cavity within
the interface of the CH3 domain of the other heavy chain
and wherein ii) in the CH3 domain of the second heavy chain
an amino acid residue is replaced with an amino acid residue having a
smaller side chain volume, thereby generating a cavity within the interface of
the second CH3 domain within which a protuberance within the interface of
the first CH3 domain is positionable.
In one embodiment the amino acid residue having a larger side chain volume is
selected from the group consisting of arginine (R), phenylalanine (F),
tyrosine (Y),
tryptophane (W).
In one embodiment the amino acid residue having a smaller side chain volume is
selected from the group consisting of alanine (A), serine (S), threonine (T),
valine
(V).
In one embodiment both CH3 domains are further altered by the introduction of
cysteine (C) as amino acid in the corresponding positions of each CH3 domain
such that a disulfide bridge between both CH3 domains can be formed.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 53 -
In one embodiment the bispecific antibody comprises a T366W mutation in the
CH3 domain of the "knobs chain" and T366S, L368A, Y407V mutations in the
CH3 domain of the "hole chain". An additional interchain disulfide bridge
between
the CH3 domains can also be used (Merchant, A.M, et al., Nature Biotech 16
(1998) 677-681) e.g. by introducing a Y349C mutation into the CH3 domain of
the
"knobs chain" and a E356C mutation or a S354C mutation into the CH3 domain of
the "hole chain" (numbering according to the EU index of Kabat et al.,
Sequences
of Proteins of Immunological Interest, 5th ed., Public Health Service,
National
Institutes of Health, Bethesda, MD (1991)).
In one embodiment the bispecific antibody comprises Y349C, T366W mutations in
one of the two CH3 domains and E356C, T3665, L368A, Y407V mutations in the
other of the two CH3 domains. In one embodiment the bispecific antibody
comprises Y349C, T366W mutations in one of the two CH3 domains and 5354C,
T3665, L368A, Y407V mutations in the other of the two CH3 domains (the
additional Y349C mutation in one CH3 domain and the additional E356C or
5354C mutation in the other CH3 domain forming a interchain disulfide bridge)
(numbering according to EU index of Kabat; (Kabat, E.A., et al., Sequences of
Proteins of Immunological Interest, 5th ed., Public Health Service, National
Institutes of Health, Bethesda, MD (1991))). Further knobs-in-holes
technologies
as described by EP 1 870 459 Al, can be used alternatively or additionally.
Thus
another example for the bispecific antibody are R409D, K370E mutations in the
CH3 domain of the "knobs chain" and D399K, E357K mutations in the CH3
domain of the "hole chain" (numbering according to EU index of Kabat; (Kabat,
E.A., et al., Sequences of Proteins of Immunological Interest, 5th ed., Public
Health
Service, National Institutes of Health, Bethesda, MD (1991)).
In one embodiment the bispecific antibody comprises a T366W mutation in the
CH3 domain of the "knobs chain" and T3665, L368A, Y407V mutations in the
CH3 domain of the "hole chain" and additionally R409D, K370E mutations in the
CH3 domain of the "knobs chain" and D399K, E357K mutations in the CH3
domain of the "hole chain".
In one embodiment the bispecific antibody comprises Y349C, T366W mutations in
one of the two CH3 domains and 5354C, T3665, L368A, Y407V mutations in the
other of the two CH3 domains or the bispecific antibody comprises Y349C,
T366W mutations in one of the two CH3 domains and 5354C, T3665, L368A,
Y407V mutations in the other of the two CH3 domains and additionally R409D,
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 54 -
K370E mutations in the CH3 domain of the "knobs chain" and D399K, E357K
mutations in the CH3 domain of the "hole chain". Such knob and hole mutations
in
the CH3 domain are typically used in human heavy chain constant regions of SEQ
ID NO: 08, SEQ ID NO: 09, SEQ ID NO: 10, or SEQ ID NO: 11 (human IgG1
subclass allotypes (Caucasian and Afro-American or mutants L234A/L235A, and
L234A/L235A/P329G), SEQ ID NO: 12, SEQ ID NO: 13, or SEQ ID NO: 14
(human IgG4 subclass or mutants 5228P, L235E, and 5228P/L235E/P329G)
(numbering according to the EU index of Kabat et al., Sequences of Proteins of
Immunological Interest, 5th ed., Public Health Service, National Institutes of
Health, Bethesda, MD (1991).
In one embodiment the bispecific antibody comprises human heavy chain constant
regions of SEQ ID NO: 08, SEQ ID NO: 09, SEQ ID NO: 10, or SEQ ID NO: 11,
SEQ ID NO: 12, SEQ ID NO: 13, or SEQ ID NO: 14 further including such
"knob" and "hole" mutations in the CH3 domain (e.g. Y349C, T366W mutations in
one of the two CH3 domains and 5354C, T3665, L368A, Y407V mutations in the
other of the two CH3 domains) (numbering according to the EU index of Kabat et
al., Sequences of Proteins of Immunological Interest, 5th ed., Public Health
Service,
National Institutes of Health, Bethesda, MD (1991).
In one embodiment the bispecific antibody comprises human light chain constant
regions of SEQ ID NO: 15 or SEQ ID NO: 16.
Engineered antibodies with three or more functional antigen binding sites,
including "Octopus antibodies," are also included herein (see, e.g.
US 2006/0025576).
The antibody or fragment herein also includes a "Dual Acting Fab" or "DAF"
comprising an antigen binding site that binds to a helicar motif amino acid
sequence as well as another, different antigen (see US 2008/0069820, for
example).
The antibody or fragment herein also includes multispecific antibodies
described in
WO 2009/080251, WO 2009/080252, WO 2009/080253, WO 2009/080254,
W02010/112193, W02010/115589, W02010/136172, W02010/145792, and
W02010/145793.
In one embodiment the first binding specificity of the bispecific antibody is
to a
helicar motif amino acid sequence and the second binding specificity is to a
non-
helicar motif amino acid sequence antigen. In one embodiment the non-helicar
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 55 -
motif amino acid sequence antigen is selected from the leukocyte markers, CD2,
CD3, CD4, CD5, CD6, CD7, CD8, CD11a,b,c, CD13, CD14, CD18, CD19, CD22,
CD23, CD27 and its ligand, CD28 and its ligands B7.1, B7.2, B7.3, CD29 and its
ligand, CD30 and its ligand, CD40 and its ligand gp39, CD44, CD45 and
isoforms,
CD56, CD58, CD69, CD72, CTLA-4, LFA-1 and TCR; the histocompatibility
antigens, MHC class I or II, the Lewis Y antigens, SLex, SLey, SLea, and SLeb;
the integrins, VLA-1, VLA-2, VLA-3, VLA-4, VLA-5, VLA-6, aV133, and LFA-1,
Mac-1, and p150,95, aV131, gpIIbIIIa, aR 133, a6134, aV13 5, aV136, and aV 62
7; the
selectins, L-selectin, P-selectin, and E-selectin and their counter receptors
VCAM-
1, ICAM-1, ICAM-2, and LFA-3; the interleukins, IL-1, IL-2, IL-3, IL-4, IL-5,
IL-
6, IL-7, IL-8, IL-9, IL-10, IL-11, IL-12, IL-13, IL-14, and IL-15; the
interleukin
receptor is selected from the group consisting of IL-1R, IL-2R, IL-3R, IL-4R,
IL-
5R, IL-6R, IL-7R, IL-8R, IL-9R, IL-10R, IL-11R, IL-12R, IL-13R, IL-14R, and
IL-15R; the chemokine is selected from the group consisting of PF4, RANTES,
MIPla, MCP1, NAP-2, Groa, Gro13, and IL-8; the growth factor is selected from
the group consisting of TNFalpha, TGFbeta, TSH, VEGFNPF, VEGFA, VEGFB,
VEGF111, VEGF121, VEGF165, VEGF189, VEGF206, PTHrP, EGF family,
PDGF family, endothelin, Fibrosin (FSF-1), human Laminin, and gastrin
releasing
peptide (GRP), PLGF, HGH, HGHR; the growth factor receptor is selected from
the group consisting of TNFalphaR, RGFbetaR, TSHR, VEGFR/VPFR, EGFR,
PTHrPR, PDGFR family, EPO-R, GCSF-R and other hematopoietic receptors; the
interferon receptor is selected from the group consisting of IFNCaR, IFN13R,
and
IFN2A; the Ig and its receptor is selected from the group consisting of IgE,
FcyRI,
and FcyRII; the tumor antigen is selected from the group consisting of her2-
neu,
mucin, CEA and endosialin; the allergen is selected from the group consisting
of
house dust mite antigen, lol pl (grass) antigens, and urushiol; the viral
polypeptide
is selected from the group consisting of CMV glycoproteins B, H, and gCIII,
HIV-
1 envelope glycoproteins, RSV envelope glycoproteins, HSV envelope
glycoproteins, HPV envelope glycoproteins, Hepatitis family surface antigens;
the
toxin is selected from the group consisting of pseudomonas endotoxin and
osteopontin/uropontin, snake venom, spider venom, and bee venom conotoxin; the
blood factor is selected from the group consisting of complement C3b,
complement
C4a, complement C4b-9, Rh factor, fibrinogen, fibrin, and myelin associated
growth inhibitor; and the enzyme is selected from the group consisting of
cholesterol ester transfer polypeptide, membrane bound matrix
metalloproteases,
and glutamic acid decarboxylase (GAD).
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 56 -
Antibody Variants
In certain embodiments, amino acid sequence variants of the antibodies
provided
herein are contemplated. For example, it may be desirable to improve the
binding
affinity and/or other biological properties of the anti-helicar motif amino
acid
sequence antibody. Amino acid sequence variants of an antibody may be prepared
by introducing appropriate modifications into the nucleotide sequence encoding
the
antibody, or by peptide synthesis. Such modifications include, for example,
deletions from, and/or insertions into and/or substitutions of residues within
the
amino acid sequences of the antibody. Any combination of deletion, insertion,
and
substitution can be made to arrive at the final construct, provided that the
final
construct possesses the desired characteristics, e.g., antigen-binding.
a) Substitution, Insertion, and Deletion Variants
In certain embodiments, antibody variants having one or more amino acid
substitutions are provided. Sites of interest for substitutional mutagenesis
include
the HVRs and FRs. Amino acid substitutions may be introduced into an antibody
of interest and the products screened for a desired activity, e.g.,
retained/improved
antigen binding, decreased immunogenicity, or improved ADCC or CDC.
Table 1.
Original Exemplary Conservative
Residue Substitutions Substitutions
Ala (A) Val; Leu; Ile Val
Arg (R) Lys; Gln; Asn Lys
Asn (N) Gln; His; Asp, Lys; Arg Gln
Asp (D) Glu; Asn Glu
Cys (C) Ser; Ala Ser
Gln (Q) Asn; Glu Asn
Glu (E) Asp; Gln Asp
Gly (G) Ala Ala
His (H) Asn; Gln; Lys; Arg Arg
Ile (I) Leu; Val; Met; Ala; Phe; Norleucine Leu
Leu (L) Norleucine; Ile; Val; Met; Ala; Phe Ile
Lys (K) Arg; Gln; Asn Arg
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 57 -
Original Exemplary Conservative
Residue Substitutions Substitutions
Met (M) Leu; Phe; Ile Leu
Phe (F) Trp; Leu; Val; Ile; Ala; Tyr Tyr
Pro (P) Ala Ala
Ser (S) Thr Thr
Thr (T) Val; Ser Ser
Trp (W) Tyr; Phe Tyr
Tyr (Y) Trp; Phe; Thr; Ser Phe
Val (V) Ile; Leu; Met; Phe; Ala; Norleucine Leu
Amino acids may be grouped according to common side-chain properties:
(1) hydrophobic: Norleucine, Met, Ala, Val, Leu, Ile;
(2) neutral hydrophilic: Cys, Ser, Thr, Asn, Gin;
(3) acidic: Asp, Glu;
(4) basic: His, Lys, Arg;
(5) residues that influence chain orientation: Gly, Pro;
(6) aromatic: Trp, Tyr, Phe.
Non-conservative substitutions will entail exchanging a member of one of these
classes for another class.
One type of substitutional variant involves substituting one or more
hypervariable
region residues of a parent antibody (e.g. a humanized or human antibody).
Generally, the resulting variant(s) selected for further study will have
modifications
(e.g., improvements) in certain biological properties (e.g., increased
affinity,
reduced immunogenicity) relative to the parent antibody and/or will have
substantially retained certain biological properties of the parent antibody.
An
exemplary substitutional variant is an affinity matured antibody, which may be
conveniently generated, e.g., using phage display-based affinity maturation
techniques such as those described herein. Briefly, one or more HVR residues
are
mutated and the variant antibodies displayed on phage and screened for a
particular
biological activity (e.g. binding affinity).
Alterations (e.g., substitutions) may be made in HVRs, e.g., to improve
antibody
affinity. Such alterations may be made in HVR "hotspots," i.e., residues
encoded
by codons that undergo mutation at high frequency during the somatic
maturation
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 58 -
process (see, e.g., Chowdhury, P.S., Methods Mol. Biol. 207 (2008) 179-196),
and/or SDRs (a-CDRs), with the resulting variant VH or VL being tested for
binding affinity. Affinity maturation by constructing and reselecting from
secondary libraries has been described, e.g., in Hoogenboom, H.R. et al. in
Methods in Molecular Biology 178 (2002) 1-37. In some embodiments of affinity
maturation, diversity is introduced into the variable genes chosen for
maturation by
any of a variety of methods (e.g., error-prone PCR, chain shuffling, or
oligonucleotide-directed mutagenesis). A secondary library is then created.
The
library is then screened to identify any antibody variants with the desired
affinity.
Another method to introduce diversity involves HVR-directed approaches, in
which several HVR residues (e.g., 4-6 residues at a time) are randomized. HVR
residues involved in antigen binding may be specifically identified, e.g.,
using
alanine scanning mutagenesis or modeling. Heavy chain CDR3 and light chain
CDR3 in particular are often targeted.
In certain embodiments, substitutions, insertions, or deletions may occur
within one
or more HVRs so long as such alterations do not substantially reduce the
ability of
the antibody to bind antigen. For example, conservative alterations (e.g.,
conservative substitutions as provided herein) that do not substantially
reduce
binding affinity may be made in HVRs. Such alterations may be outside of HVR
"hotspots" or SDRs. In certain embodiments of the variant VH and VL sequences
provided above, each HVR either is unaltered, or contains no more than one,
two or
three amino acid substitutions.
A useful method for identification of residues or regions of an antibody that
may be
targeted for mutagenesis is called "alanine scanning mutagenesis" as described
by
Cunningham, B.C. and Wells, J.A., Science 244 (1989) 1081-1085. In this
method,
a residue or group of target residues (e.g., charged residues such as Arg,
Asp, His,
Lys, and Glu) are identified and replaced by a neutral or negatively charged
amino
acid (e.g., alanine or polyalanine) to determine whether the interaction of
the
antibody with antigen is affected. Further substitutions may be introduced at
the
amino acid locations demonstrating functional sensitivity to the initial
substitutions.
Alternatively, or additionally, a crystal structure of an antigen-antibody
complex to
identify contact points between the antibody and antigen. Such contact
residues and
neighboring residues may be targeted or eliminated as candidates for
substitution.
Variants may be screened to determine whether they contain the desired
properties.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 59 -
Amino acid sequence insertions include amino- and/or carboxyl-terminal fusions
ranging in length from one residue to polypeptides containing a hundred or
more
residues, as well as intrasequence insertions of single or multiple amino acid
residues. Examples of terminal insertions include an antibody with an N-
terminal
methionyl residue. Other insertional variants of the antibody molecule include
the
fusion to the N- or C-terminus of the antibody to an enzyme (e.g. for ADEPT)
or a
polypeptide which increases the serum half-life of the antibody.
b) Glycosylation variants
In certain embodiments, an antibody provided herein or comprised in a
conjugate
as reported herein is altered to increase or decrease the extent to which the
antibody
is glycosylated. Addition or deletion of glycosylation sites to an antibody
may be
conveniently accomplished by altering the amino acid sequence such that one or
more glycosylation sites is created or removed.
Where the antibody comprises an Fc-region, the carbohydrate attached thereto
may
be altered. Native antibodies produced by mammalian cells typically comprise a
branched, biantennary oligosaccharide that is generally attached by an N-
linkage to
Asn297 of the CH2 domain of the Fc-region. See, e.g., Wright, A. and Morrison,
S.L., TIBTECH 15 (1997) 26-32. The oligosaccharide may include various
carbohydrates, e.g., mannose, N-acetyl glucosamine (G1cNAc), galactose, and
sialic acid, as well as a fucose attached to a GlcNAc in the "stem" of the
biantennary oligosaccharide structure. In some embodiments, modifications of
the
oligosaccharide in an antibody of the invention may be made in order to create
antibody variants with certain improved properties.
In one embodiment, antibody variants are provided having a carbohydrate
structure
that lacks fucose attached (directly or indirectly) to an Fc-region. For
example, the
amount of fucose in such antibody may be from 1 % to 80 %, from 1 % to 65 %,
from 5 % to 65 % or from 20 % to 40 %. The amount of fucose is determined by
calculating the average amount of fucose within the sugar chain at Asn297,
relative
to the sum of all glycostructures attached to Asn 297 (e. g. complex, hybrid
and
high mannose structures) as measured by MALDI-TOF mass spectrometry, as
described in WO 2008/077546, for example. Asn297 refers to the asparagine
residue located at about position 297 in the Fc-region (EU numbering of Fc-
region
residues); however, Asn297 may also be located about 3 amino acids upstream
or
downstream of position 297, i.e., between positions 294 and 300, due to minor
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 60 -
sequence variations in antibodies. Such fucosylation variants may have
improved
ADCC function. See, e.g., US 2003/0157108; US 2004/0093621. Examples of
publications related to "defucosylated" or "fucose-deficient" antibody
variants
include: US 2003/0157108; WO 2000/61739; WO 2001/29246; US 2003/0115614;
US 2002/0164328; US 2004/0093621; US 2004/0132140; US 2004/0110704;
US 2004/0110282; US 2004/0109865; WO 2003/085119; WO 2003/084570;
WO 2005/035586; WO 2005/035778; WO 2005/053742; WO 2002/031140;
Okazaki, A. et al., J. Mol. Biol. 336 (2004) 1239-1249; Yamane-Ohnuki, N. et
al.,
Biotech. Bioeng. 87 (2004) 614-622. Examples of cell lines capable of
producing
defucosylated antibodies include Lec13 CHO cells deficient in protein
fucosylation
(Ripka, J. et al., Arch. Biochem. Biophys. 249 (1986) 533-545; US
2003/0157108;
and WO 2004/056312, especially at Example 3), and knockout cell lines, such as
alpha-1,6-fucosyltransferase gene, FUT8, knockout CHO cells (see, e.g., Yamane-
Ohnuki, N. et al., Biotech. Bioeng. 87 (2004) 614-622; Kanda, Y. et al.,
Biotechnol.
Bioeng. 94 (2006) 680-688; and WO 2003/085107).
Antibodies variants are further provided with bisected oligosaccharides, e.g.,
in
which a biantennary oligosaccharide attached to the Fc-region of the antibody
is
bisected by GlcNAc. Such antibody variants may have reduced fucosylation
and/or
improved ADCC function. Examples of such antibody variants are described,
e.g.,
in WO 2003/011878; US Patent No. 6,602,684; and US 2005/0123546. Antibody
variants with at least one galactose residue in the oligosaccharide attached
to the
Fc-region are also provided. Such antibody variants may have improved CDC
function. Such antibody variants are described, e.g., in WO 1997/30087;
WO 1998/58964; and WO 1999/22764.
c) Fc-region variants
In certain embodiments, one or more amino acid modifications may be introduced
into the Fc-region of an antibody provided herein, thereby generating an Fc-
region
variant. The Fc-region variant may comprise a human Fc-region sequence (e.g.,
a
human IgGl, IgG2, IgG3 or IgG4 Fc-region) comprising an amino acid
modification (e.g. a substitution) at one or more amino acid positions.
In certain embodiments, the invention contemplates an antibody variant that
possesses some but not all effector functions, which make it a desirable
candidate
for applications in which the half-life of the antibody in vivo is important
yet
certain effector functions (such as complement and ADCC) are unnecessary or
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
-61 -
deleterious. In vitro and/or in vivo cytotoxicity assays can be conducted to
confirm
the reduction/depletion of CDC and/or ADCC activities. For example, Fc
receptor
(FcR) binding assays can be conducted to ensure that the antibody lacks FcyR
binding (hence likely lacking ADCC activity), but retains FcRn binding
ability.
The primary cells for mediating ADCC, NK cells, express Fc(RIII only, whereas
monocytes express FcyRI, FcyRII and FcyRIII. FcR expression on hematopoietic
cells is summarized in Table 3 on page 464 of Ravetch, J.V. and Kinet, J.P.,
Annu.
Rev. Immunol. 9 (1991) 457-492. Non-limiting examples of in vitro assays to
assess ADCC activity of a molecule of interest is described in U.S. Patent No.
5,500,362 (see, e.g. Hellstrom, I. et al., Proc. Natl. Acad. Sci. USA 83
(1986) 7059-
7063; and Hellstrom, I. et al., Proc. Natl. Acad. Sci. USA 82 (1985) 1499-
1502);
U.S. Patent No. 5,821,337 (see Bruggemann, M. et al., J. Exp. Med. 166 (1987)
1351-1361). Alternatively, non-radioactive assays methods may be employed
(see,
for example, ACTITm non-radioactive cytotoxicity assay for flow cytometry
(CellTechnology, Inc. Mountain View, CA; and CytoTox 96 non-radioactive
cytotoxicity assay (Promega, Madison, WI). Useful effector cells for such
assays
include peripheral blood mononuclear cells (PBMC) and Natural Killer (NK)
cells.
Alternatively, or additionally, ADCC activity of the molecule of interest may
be
assessed in vivo, e.g., in an animal model such as that disclosed in Clynes,
R. et al.,
Proc. Natl. Acad. Sci. USA 95 (1998) 652-656. C 1 q binding assays may also be
carried out to confirm that the antibody is unable to bind Clq and hence lacks
CDC
activity. See, e.g., Clq and C3c binding ELISA in WO 2006/029879 and
WO 2005/100402. To assess complement activation, a CDC assay may be
performed (see, for example, Gazzano-Santoro, H. et al., J. Immunol. Methods
202
(1996) 163-171; Cragg, M.S. et al., Blood 101 (2003) 1045-1052; and Cragg,
M.S.
and M.J. Glennie, Blood 103 (2004) 2738-2743). FcRn binding and in vivo
clearance/half-life determinations can also be performed using methods known
in
the art (see, e.g., Petkova, S.B. et al., Int. Immunol. 18 (2006: 1759-1769).
Antibodies with reduced effector function include those with substitution of
one or
more of Fc-region residues 238, 265, 269, 270, 297, 327 and 329 (U.S. Patent
No. 6,737,056). Such Fc mutants include Fc mutants with substitutions at two
or
more of amino acid positions 265, 269, 270, 297 and 327, including the so-
called
"DANA" Fc mutant with substitution of residues 265 and 297 to alanine (US
Patent No. 7,332,581).
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 62 -
Certain antibody variants with improved or diminished binding to FcRs are
described. (See, e.g., U.S. Patent No. 6,737,056; WO 2004/056312, and Shields,
R.L. et al., J. Biol. Chem. 276 (2001) 6591-6604).
In certain embodiments, an antibody variant comprises an Fc-region with one or
more amino acid substitutions which improve ADCC, e.g., substitutions at
positions 298, 333, and/or 334 of the Fc-region (EU numbering of residues).
In some embodiments, alterations are made in the Fc-region that result in
altered
(i.e., either improved or diminished) C 1 q binding and/or Complement
Dependent
Cytotoxicity (CDC), e.g., as described in US Patent No. 6,194,551, WO
99/51642,
and Idusogie, E.E. et al., J. Immunol. 164 (2000) 4178-4184.
Antibodies with increased half-lives and improved binding to the neonatal Fc
receptor (FcRn), which is responsible for the transfer of maternal IgGs to the
fetus
(Guyer, R.L. et al., J. Immunol. 117 (1976) 587-593, and Kim, J.K. et al., J.
Immunol. 24 (1994) 2429-2434), are described in US 2005/0014934. Those
antibodies comprise an Fc-region with one or more substitutions therein which
improve binding of the Fc-region to FcRn. Such Fc variants include those with
substitutions at one or more of Fc-region residues: 238, 256, 265, 272, 286,
303,
305, 307, 311, 312, 317, 340, 356, 360, 362, 376, 378, 380, 382, 413, 424 or
434,
e.g., substitution of Fc-region residue 434 (US Patent No. 7,371,826).
See also Duncan, A.R. and Winter, G., Nature 322 (1988) 738-740; US 5,648,260;
US 5,624,821; and WO 94/29351 concerning other examples of Fc-region variants.
d) Cysteine engineered antibody variants
In certain embodiments, it may be desirable to create cysteine engineered
antibodies, e.g., "thioMAbs," in which one or more residues of an antibody are
substituted with cysteine residues. In particular embodiments, the substituted
residues occur at accessible sites of the antibody. By substituting those
residues
with cysteine, reactive thiol groups are thereby positioned at accessible
sites of the
antibody and may be used to conjugate the antibody to other moieties, such as
drug
moieties or linker-drug moieties, to create an immunoconjugate, as described
further herein. In certain embodiments, any one or more of the following
residues
may be substituted with cysteine: V205 (Kabat numbering) of the light chain;
A118
(EU numbering) of the heavy chain; and S400 (EU numbering) of the heavy chain
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 63 -
Fe-region. Cysteine engineered antibodies may be generated as described, e.g.,
in
U.S. Patent No. 7,521,541.
e) Antibody Derivatives
In certain embodiments, an antibody provided herein may be further modified to
contain additional non-proteinaceous moieties that are known in the art and
readily
available. The moieties suitable for derivatization of the antibody include
but are
not limited to water soluble polymers. Non-limiting examples of water soluble
polymers include, but are not limited to, polyethylene glycol (PEG),
copolymers of
ethylene glycol/propylene glycol, carboxymethylcellulose, dextran, polyvinyl
alcohol, polyvinyl pyrrolidone, poly-1, 3-dioxolane, poly-1,3,6-trioxane,
ethylene/maleic anhydride copolymer, polyaminoacids (either homopolymers or
random copolymers), and dextran or poly(n-vinyl pyrrolidone)polyethylene
glycol,
polypropylene glycol homopolymers, polypropylene oxide/ethylene oxide co-
polymers, polyoxyethylated polyols (e.g., glycerol), polyvinyl alcohol, and
mixtures thereof Polyethylene glycol propionaldehyde may have advantages in
manufacturing due to its stability in water. The polymer may be of any
molecular
weight, and may be branched or non-branched. The number of polymers attached
to the antibody may vary, and if more than one polymer is attached, they can
be the
same or different molecules. In general, the number and/or type of polymers
used
for derivatization can be determined based on considerations including, but
not
limited to, the particular properties or functions of the antibody to be
improved,
whether the antibody derivative will be used in a therapy under defined
conditions,
etc.
In another embodiment, conjugates of an antibody and non-proteinaceous moiety
that may be selectively heated by exposure to radiation are provided. In one
embodiment, the non-proteinaceous moiety is a carbon nanotube (Kam, N.W. et
al.,
Proc. Natl. Acad. Sci. USA 102 (2005) 11600-11605). The radiation may be of
any
wavelength, and includes, but is not limited to, wavelengths that do not harm
ordinary cells, but which heat the non-proteinaceous moiety to a temperature
at
which cells proximal to the antibody-non-proteinaceous moiety are killed.
Helicar motif amino acid sequence containing compounds
The helicar motif amino acid sequence in a conjugate as reported herein may be
conjugated to a therapeutic agent (drug), a cytotoxic agent (e.g. a toxin such
as
doxorubicin or pertussis toxin), a fluorophores such as a fluorescent dye like
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 64 -
fluorescein or rhodamine, a chelating agent for an imaging or radiotherapeutic
metal, a peptidyl or non-peptidyl label or detection tag, or a clearance-
modifying
agent such as various isomers of polyethylene glycol, a peptide that binds to
a third
component, or another carbohydrate or lipophilic agent. Such a conjugate is
denoted as helicar motif containing compound. The conjugation can be either
directly or via an intervening linker.
a) Therapeutic agent
The therapeutic agent (drug) of the conjugate can be any compound, moiety or
group which has a cytotoxic or cytostatic effect. Drug moieties include: (i)
chemotherapeutic agents, which may function as microtubule inhibitors, mitosis
inhibitors, topoisomerase inhibitors, or DNA intercalators; (ii) protein
toxins,
which may function enzymatically; and (iii) radioisotopes.
Exemplary therapeutic agents include, but are not limited to, a maytansinoid,
an
auristatin, a dolastatin, a trichothecene, CC1065, a calicheamicin and other
enediyne antibiotics, a taxane, an anthracycline, and stereoisomers, isosters,
analogs or derivatives thereof
Protein toxins include diphtheria-A chain, non-binding active fragments of
diphtheria toxin, exotoxin A chain (from Pseudomonas aeruginosa), ricin A
chain
(Vitetta et al (1987) Science, 238:1098), abrin A chain, modeccin A chain,
alpha-
sarcin, Aleurites fordii proteins, dianthin proteins, Phytolaca americana
proteins
(PAPI, PAPII, and PAP -5), momordica charantia inhibitor, curcin, crotin,
sapaonaria officinalis inhibitor, gelonin, mitogellin, restrictocin,
phenomycin,
enomycin, and the tricothecenes (WO 93/21232).
Therapeutic radioisotopes include 32P, 33P, 90Y, 1251, 1311, 131In, 1535m,
186Re, 188Re, 211At, 212B, 212Pb, and radioactive isotopes of Lu.
The radioisotope or other labels may be incorporated in known ways (Fraker et
al
(1978) Biochem. Biophys. Res. Commun. 80: 49-57; "Monoclonal Antibodies in
Immunoscintigraphy" Chatal, CRC Press 1989). Carbon-14-labeled 1 -
isothio cyanatob enzy1-3 -methyldiethylene triamine pentaacetic acid (MX-DTPA)
is
an exemplary chelating agent for conjugation of a radionuclide to the complex
(WO 94/11026).
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 65 -
b) Labels
The helicar motif amino acid sequence containing compound can be a helicar
motif
amino acid sequence containing compound containing an additional label moiety.
Any label moiety which can be covalently attached to the helicar motif amino
acid
sequence can be used (see e.g. Singh et al (2002) Anal. Biochem. 304:147-15;
Harlow E. and Lane, D. (1999) Using Antibodies: A Laboratory Manual, Cold
Springs Harbor Laboratory Press, Cold Spring Harbor, N.Y.; Lundblad R. L.
(1991) Chemical Reagents for Protein Modification, 2nd ed. CRC Press, Boca
Raton, Fla.). The label may function to: (i) provide a detectable signal; (ii)
interact
with a second label to modify the detectable signal provided by the first or
second
label, e.g. to give FRET (fluorescence resonance energy transfer); (iii)
affect
mobility, e.g. electrophoretic mobility or cell-permeability, by charge,
hydrophobicity, shape, or other physical parameters, or (iv) provide a capture
moiety, e.g. to modulate ionic complexation.
Conjugates comprising a helicar motif amino acid sequence and containing a
label
as reported herein may be useful in diagnostic assays, e.g., for detecting
expression
of an antigen of interest in specific cells, tissues, or serum. For diagnostic
applications, a bispecific antibody will be used wherein the first binding
specificity
binds to a target and the second binding specificity binds to a helicar motif
amino
acid sequence containing label. The helicar motif amino acid sequence will
typically be labeled with a detectable moiety. Numerous labels are available
which
can be generally grouped into the following categories:
(a) Radioisotopes (radionuclides), such as 3H, 11C, 14C, 18F, 32P, 35S, 64Cu,
68Gn, 86Y, 89Zr, 99TC, 111In, 1231, 1241, 1251, 1311, 133Xe, 177Lu, 211At, or
131Bi. Radioisotope labeled conjugates are useful in receptor targeted imaging
experiments. The helicar motif amino acid sequence can be labeled with ligand
reagents that bind, chelate or otherwise complex a radioisotope metal using
the
techniques described in Current Protocols in Immunology, (1991) Volumes 1 and
2,
Coligen et al, Ed. Wiley-Interscience, New York, N.Y., Pubs. Chelating ligands
which may complex a metal ion include DOTA, DOTP, DOTMA, DTPA and
TETA (Macrocyclics, Dallas, Tex.). Radionuclides can be targeted via
complexation with the complex as reported herein (Wu et al, Nature
Biotechnology
23(9) (2005) 1137-1146). Receptor target imaging with radionuclide labeled
complexes can provide a marker of pathway activation by detection and
quantification of progressive accumulation of complexes or corresponding
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 66 -
therapeutic antibodies in tumor tissue (Albert et al (1998) Bioorg. Med. Chem.
Lett.
8:1207-1210).
Metal-chelate complexes suitable as labels for imaging experiments
(US 2010/0111856; US 5,342,606; US 5,428,155; US 5,316,757; US 5,480,990;
US 5,462,725; US 5,428,139; US 5,385,893; US 5,739,294; US 5,750,660;
US 5,834,456; Hnatowich et al, J. Immunol. Methods 65 (1983) 147-157; Meares
et al, Anal. Biochem. 142 (1984) 68-78; Mirzadeh et al, Bioconjugate Chem. 1
(1990) 59-65; Meares et al, J. Cancer (1990), Suppl. 10:21-26; Izard et al,
Bioconjugate Chem. 3 (1992) 346-350; Nikula et al, Nucl. Med. Biol. 22 (1995)
387-90; Camera et al, Nucl. Med. Biol. 20 (1993) 955-62; Kukis et al, J. Nucl.
Med.
39 (1998) 2105-2110; Verel et al., J. Nucl. Med. 44 (2003) 1663-1670; Camera
et
al, J. Nucl. Med. 21(1994) 640-646; Ruegg et al, Cancer Res. 50 (1990) 4221-
4226; Verel et al, J. Nucl. Med. 44 (2003) 1663-1670; Lee et al, Cancer Res.
61
(2001) 4474-4482; Mitchell, et al, J. Nucl. Med. 44 (2003) 1105-1112;
Kobayashi
et al Bioconjugate Chem. 10 (1999) 103-111; Miederer et al, J. Nucl. Med. 45
(2004) 129-137; DeNardo et al, Clinical Cancer Research 4 (1998) 2483-90;
Blend
et al, Cancer Biotherapy & Radiopharmaceuticals 18 (2003) 355-363; Nikula et
al J.
Nucl. Med. 40 (1999) 166-76; Kobayashi et al, J. Nucl. Med. 39 (1998) 829-36;
Mardirossian et al, Nucl. Med. Biol. 20 (1993) 65-74; Roselli et al, Cancer
Biotherapy & Radiopharmaceuticals, 14 (1999) 209-20).
(b) Fluorescent labels such as rare earth chelates (europium chelates),
fluorescein
types including FITC, 5-carboxyfluorescein, 6-carboxy fluorescein; rhodamine
types including TAMRA; dansyl; Lissamine; cyanines; phycoerythrins; Texas Red;
and analogs thereof. The fluorescent labels can be conjugated to the helicar
motif
amino acid sequence using the techniques disclosed in Current Protocols in
Immunology, supra, for example. Fluorescent dyes and fluorescent label
reagents
include those which are commercially available from Invitrogen/Molecular
Probes
(Eugene, Oregon, USA) and Pierce Biotechnology, Inc. (Rockford, Ill.).
Detection labels such as fluorescent dyes and chemiluminescent dyes (Briggs et
al
"Synthesis of Functionalised Fluorescent Dyes and Their Coupling to Amines and
Amino Acids," J. Chem. Soc., Perkin-Trans. 1 (1997) 1051-1058) provide a
detectable signal and are generally applicable for labeling, especially with
the
following properties: (i) the labeled conjugate should produce a very high
signal
with low background so that small quantities of conjugate can be sensitively
detected in both cell-free and cell-based assays; and (ii) the labeled
conjugate
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 67 -
should be photostable so that the fluorescent signal may be observed,
monitored
and recorded without significant photo bleaching. For applications involving
cell
surface binding of labeled conjugates to membranes or cell surfaces,
especially live
cells, the labels should (iii) have good water-solubility to achieve effective
conjugate concentration and detection sensitivity and (iv) are non-toxic to
living
cells so as not to disrupt the normal metabolic processes of the cells or
cause
premature cell death.
(c) Various enzyme-substrate labels are available or disclosed (see e.g.
US 4,275,149). The enzyme generally catalyzes a chemical alteration of a
chromogenic substrate that can be measured using various techniques. For
example,
the enzyme may catalyze a color change in a substrate, which can be measured
spectrophotometrically. Alternatively, the enzyme may alter the fluorescence
or
chemiluminescence of the substrate. The chemiluminescent substrate becomes
electronically excited by a chemical reaction and may then emit light which
can be
measured (using a chemiluminometer, for example) or donates energy to a
fluorescent acceptor. Examples of enzymatic labels include luciferases (e.g.,
firefly
luciferase and bacterial luciferase; US 4,737,456), luciferin, 2,3-
dihydrophthalazinediones, malate dehydrogenase, urease, peroxidase such as
horseradish peroxidase (HRP), alkaline phosphatase (AP), (3-galactosidase,
glucoamylase, lysozyme, saccharide oxidases (e.g., glucose oxidase, galactose
oxidase, and glucose-6-phosphate dehydrogenase), heterocyclic oxidases (such
as
uricase and xanthine oxidase), lactoperoxidase, microperoxidase, and the like.
Techniques for conjugating enzymes to polypeptides are described in O'Sullivan
et
al. "Methods for the Preparation of Enzyme-Antibody Conjugates for use in
Enzyme Immunoassay", in Methods in Enzym. (ed. by J. Langone & IT Van
Vunakis), Academic Press, New York, 73 (1981) 147-166.
Examples of enzyme-substrate combinations (US 4,275,149; US 4,318,980)
include, for example:
(i) Horseradish peroxidase (HRP) with hydrogen peroxidase as a substrate,
wherein
the hydrogen peroxidase oxidizes a dye precursor (e.g., orthophenylene diamine
(OPD) or 3,3',5,5'-tetramethylbenzidine hydrochloride (TMB));
(ii) alkaline phosphatase (AP) with para-nitrophenyl phosphate as chromogenic
substrate; and
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 68 -
(iii) (3-D-galactosidase ((3-D-Gal) with a chromogenic substrate (e.g., p-
nitro
phenyl-(3-D-galactosidase) or fluorogenic substrate 4-methylumbellifery1-(3-D-
galactosidase.
The labeled conjugate as reported herein may be employed in any known assay
method, such as ELISA, competitive binding assays, direct and indirect
sandwich
assays, and immunoprecipitation assays (Zola, Monoclonal Antibodies: A Manual
of Techniques (1987) pp. 147-158, CRC Press, Inc.).
Labeled conjugates as reported herein are useful as imaging biomarkers and
probes
by the various methods and techniques of biomedical and molecular imaging such
as: (i) MRI (magnetic resonance imaging); (ii) MicroCT (computerized
tomography); (iii) SPECT (single photon emission computed tomography); (iv)
PET (positron emission tomography) Tinianow, J. et al Nuclear Medicine and
Biology, 37(3) (2010) 289-297; Chen et al, Bioconjugate Chem. 15 (2004) 41-49;
US 2010/0111856 (v) bioluminescence; (vi) fluorescence; and (vii) ultrasound.
Immunoscintigraphy is an imaging procedure in which conjugates labeled with
radioactive substances are administered to an animal or human patient and a
picture
is taken of sites in the body where the conjugate localizes (US 6,528,624).
Imaging
biomarkers may be objectively measured and evaluated as an indicator of normal
biological processes, pathogenic processes, or pharmacological responses to a
therapeutic intervention. Biomarkers may be of several types: Type 0 markers
are
natural history markers of a disease and correlate longitudinally with known
clinical indices, e.g. MRI assessment of synovial inflammation in rheumatoid
arthritis; Type I markers capture the effect of an intervention in accordance
with a
mechanism-of-action, even though the mechanism may not be associated with
clinical outcome; Type II markers function as surrogate endpoints where the
change in, or signal from, the biomarker predicts a clinical benefit to
"validate" the
targeted response, such as measured bone erosion in rheumatoid arthritis by
CT.
Imaging biomarkers thus can provide pharmacodynamic (PD) therapeutic
information about: (i) expression of a target protein, (ii) binding of a
therapeutic to
the target protein, i.e. selectivity, and (iii) clearance and half-life
pharmacokinetic
data. Advantages of in vivo imaging biomarkers relative to lab-based
biomarkers
include: non-invasive treatment, quantifiable, whole body assessment,
repetitive
dosing and assessment, i.e. multiple time points, and potentially transferable
effects
from preclinical (small animal) to clinical (human) results. For some
applications,
bioimaging supplants or minimizes the number of animal experiments in
preclinical studies.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 69 -
Peptide labeling methods are well known. See Haugland (2003) Molecular Probes
Handbook of Fluorescent Probes and Research Chemicals, Molecular Probes, Inc.;
Brinkley (1992) Bioconjugate Chem. 3:2; Garman, (1997) Non-Radioactive
Labeling: A Practical Approach, Academic Press, London; Means (1990)
Bioconjugate Chem. 1:2; Glazer et al Chemical Modification of Proteins.
Laboratory Techniques in Biochemistry and Molecular Biology (T. S. Work and E.
Work, Eds.) American Elsevier Publishing Co., New York; Lundblad, R. L. and
Noyes, C. M. (1984) Chemical Reagents for Protein Modification, Vols. I and
II,
CRC Press, New York; Pfleiderer, G. (1985) "Chemical Modification of
Proteins",
Modern Methods in Protein Chemistry, H. Tschesche, Ed., Walter DeGruyter,
Berlin and New York; and Wong (1991) Chemistry of Protein Conjugation and
Cross-linking, CRC Press, Boca Raton, Fla.); DeLeon-Rodriguez et al, Chem.
Eur. J. 10 (2004) 1149-1155; Lewis et al, Bioconjugate Chem. 12(2001) 320-324;
Li et al, Bioconjugate Chem. 13 (2002) 110-115; Mier et al Bioconjugate Chem.
16
(2005) 240-237.
Antibody conjugates
The antibody in a conjugate as reported herein may be further conjugated, if
it is
not by itself one of the molecules, to a therapeutic agent (drug), a cytotoxic
agent
(e.g. a toxin such as doxorubicin or pertussis toxin), a fluorophores such as
a
fluorescent dye like fluorescein or rhodamine, a chelating agent for an
imaging or
radiotherapeutic metal, a peptidyl or non-peptidyl label or detection tag, or
a
clearance-modifying agent such as various isomers of polyethylene glycol, a
peptide that binds to a third component, or another carbohydrate or lipophilic
agent.
Immunoconjugates
The invention also provides immunoconjugates comprising an antibody as
reported
herein or a conjugate as reported herein conjugated to one or more cytotoxic
agents,
such as chemotherapeutic agents or drugs, growth inhibitory agents, toxins
(e.g.,
protein toxins, enzymatically active toxins of bacterial, fungal, plant, or
animal
origin, or fragments thereof), or radioactive isotopes.
In one embodiment, an immunoconjugate is an antibody-drug conjugate (ADC) in
which an antibody is conjugated to one or more drugs, including but not
limited to
a maytansinoid (see US 5,208,020, US 5,416,064 and EP 0 425 235 B1); an
auristatin such as monomethyl auristatin drug moieties DE and DF (MMAE and
MMAF) (see US 5,635,483, US 5,780,588, and US 7,498,298); a dolastatin; a
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 70 -
calicheamicin or derivative thereof (see US 5,712,374, US 5,714,586,
US 5,739,116, US 5,767,285, US 5,770,701, US 5,770,710, US 5,773,001, and
US 5,877,296; Hinman, L.M. et al., Cancer Res. 53 (1993) 3336-3342; and Lode,
H.N. et al., Cancer Res. 58 (1998) 2925-2928); an anthracycline such as
daunomycin or doxorubicin (see Kratz, F. et al., Curr. Med. Chem. 13 (2006)
477-
523; Jeffrey, S.C. et al., Bioorg. Med. Chem. Lett. 16 (2006) 358-362; Torgov,
M.Y. et al., Bioconjug. Chem. 16 (2005) 717-721; Nagy, A. et al., Proc. Natl.
Acad.
Sci. USA 97 (2000) 829-834; Dubowchik, G.M. et al., Bioorg. & Med. Chem.
Letters 12 (2002) 1529-1532; King, H.D. et al., J. Med. Chem. 45 (2002) 4336-
4343; and U.S. Patent No. 6,630,579); methotrexate; vindesine; a taxane such
as
docetaxel, paclitaxel, larotaxel, tesetaxel, and ortataxel; a trichothecene;
and
CC 1065.
In another embodiment, an immunoconjugate comprises an antibody as described
herein or a complex as reported herein conjugated to an enzymatically active
toxin
or fragment thereof, including but not limited to diphtheria A chain,
nonbinding
active fragments of diphtheria toxin, exotoxin A chain (from Pseudomonas
aeruginosa), ricin A chain, abrin A chain, modeccin A chain, alpha-sarcin,
Aleurites fordii proteins, dianthin proteins, Phytolaca americana proteins
(PAPI,
PAPII, and PAP-S), momordica charantia inhibitor, curcin, crotin, sapaonaria
officinalis inhibitor, gelonin, mitogellin, restrictocin, phenomycin,
enomycin, and
the tricothecenes.
In another embodiment, an immunoconjugate comprises an antibody as described
herein or a complex as reported herein conjugated to a radioactive atom to
form a
radioconjugate. A variety of radioactive isotopes are available for the
production of
radioconjugates. Examples include At211, 11315 11255 y905 Reim, Rem, smi535
Bi2125
P32, Pb212 and radioactive isotopes of Lu. When the radioconjugate is used for
detection, it may comprise a radioactive atom for scintigraphic studies, for
example
TC99m or 1123, or a spin label for nuclear magnetic resonance (NMR) imaging
(also
known as magnetic resonance imaging, MRI), such as iodine-123 again, iodine-
131,
indium-111, fluorine-19, carbon-13, nitrogen-15, oxygen-17, gadolinium,
manganese or iron.
Conjugates of an antibody and a cytotoxic agent may be made using a variety of
bifunctional protein coupling agents such as N-succinimidy1-3-(2-
pyridyldithio)
propionate (SPDP), succinimidy1-4-(N-maleimidomethyl) cyclohexane-1-
carboxylate (SMCC), iminothiolane (IT), bifunctional derivatives of
imidoesters
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 71 -
(such as dimethyl adipimidate HC1), active esters (such as disuccinimidyl
suberate),
aldehydes (such as glutaraldehyde), bis-azido compounds (such as bis (p-
azidobenzoyl) hexanediamine), bis-diazonium derivatives (such as bis-(p-
diazoniumbenzoy1)-ethylenediamine), diisocyanates (such as toluene 2,6-
diisocyanate), and bis-active fluorine compounds (such as 1,5-difluoro-2,4-
dinitrobenzene). For example, a ricin immunotoxin can be prepared as described
in
Vitetta, E.S. et al., Science 238 (1987) 1098-1104. Carbon-14-labeled 1-
isothiocyanatobenzy1-3-methyldiethylene triamine pentaacetic acid (MX-DTPA) is
an exemplary chelating agent for conjugation of radionucleotide to the
antibody.
See WO 94/11026. The linker may be a "cleavable linker" facilitating release
of a
cytotoxic drug in the cell. For example, an acid-labile linker, peptidase-
sensitive
linker, photolabile linker, dimethyl linker or disulfide-containing linker
(Chari, R.V.
et al., Cancer Res. 52 (1992) 127-131; U.S. Patent No. 5,208,020) may be used.
The immunoconjugates or ADCs herein expressly contemplate, but are not limited
to such conjugates prepared with cross-linker reagents including, but not
limited to,
BMPS, EMCS, GMBS, HBVS, LC-SMCC, MBS, MPBH, SBAP, SIA, SIAB,
SMCC, SMPB, SMPH, sulfo-EMCS, sulfo-GMBS, sulfo-KMUS, sulfo-MBS,
sulfo-SIAB, sulfo-SMCC, and sulfo-SMPB, and SVSB (succinimidy1-(4-
vinylsulfone)benzoate) which are commercially available (e.g., from Pierce
Biotechnology, Inc., Rockford, IL., U.S.A).
Linker
The term "linker" denotes a bifunctional or multifunctional moiety which can
be
used to conjugate (link) the antibody or helicar motif amino acid sequence to
other
compounds, such as detectable labels or drugs. Helicar motif amino acid
sequence
conjugates can be conveniently prepared using a linker having reactive
functionality for binding to the further compound and to the helicar motif
amino
acid sequence.
In one embodiment, a linker has a reactive site which has an electrophilic
group
that is reactive to a nucleophilic group present on the helicar motif amino
acid
sequence or the antibody or the further compound. A cysteine thiol group for
example is reactive with an electrophilic group on a linker and forms a
covalent
bond to a linker. Useful electrophilic groups include, but are not limited to,
another
thiol, maleimide and haloacetamide groups (see e.g. conjugation method at page
766 of Klussman et al, Bioconjugate Chemistry 15(4) (2004) 765-773).
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 72 -
Examples of thiol-reaction functional groups include, but are not limited to,
thiol,
maleimide, alpha-haloacetyl, activated esters such as succinimide esters, 4-
nitrophenyl esters, pentafluorophenyl esters, tetrafluorophenyl esters,
anhydrides,
acid chlorides, sulfonyl chlorides, isocyanates and isothiocyanates.
The linker may comprise amino acid residues which link the antigen (helicar
motif
amino acid sequence) to the payload. The amino acid residues may form a
dip eptide, trip eptide, tetrapeptide, pentapeptide, hexapeptide,
heptapeptide,
octapeptide, nonapeptide, decapeptide, undecapeptide or dodecapeptide unit.
Amino acid residues include those occurring naturally, as well as non-
naturally
occurring amino acid analogs, such as e.g. citrulline or 13¨amino acids, such
as e.g.
13-alanine, or w-amino acids such as 4-amino-butyric acid.
In another embodiment, the linker has a reactive functional group which has a
nucleophilic group that is reactive to an electrophilic group present on the
helicar
motif amino acid sequence or the antibody (anti-helicar motif amino acid
sequence
antibody). Useful electrophilic groups include, but are not limited to,
aldehyde and
ketone carbonyl groups. The heteroatom of a nucleophilic group of a linker can
react with an electrophilic group on the helicar motif amino acid sequence or
the
antibody and form a covalent bond to an antigen (helicar motif amino acid
sequence) or the antibody. Useful nucleophilic groups on a linker include, but
are
not limited to, hydrazide, oxime, amino, hydrazine, thiosemicarbazone,
hydrazine
carboxylate, and arylhydrazide. The electrophilic group on an antigen (helicar
motif amino acid sequence) provides a convenient site for attachment to a
linker.
Typically, peptide-type linkers can be prepared by forming a peptide bond
between
two or more amino acids and/or peptide fragments. Such peptide bonds can be
prepared, for example, according to the liquid phase synthesis method (E.
Schroder
and K. Lubke "The Peptides", volume 1 (1965) 76-136, Academic Press) which is
well known in the field of peptide chemistry.
In another embodiment, the linker may be substituted with groups which
modulated solubility or reactivity. For example, a charged substituent such as
sulfonate (503-) or ammonium or a polymer such as PEG, may increase water
solubility of the reagent and facilitate the coupling reaction of the linker
reagent
with the antigen (helicar motif amino acid sequence) or the drug moiety, or
facilitate the coupling reaction depending on the synthetic route employed.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
-73 -
The conjugates comprising a drug or label as reported herein expressly
contemplate,
but are not limited to, complexes prepared with linker reagents: BMPEO, BMPS,
EMCS, GMBS, HBVS, LC-SMCC, MBS, MPBH, SBAP, SIA, SIAB, SMCC,
SMPB, SMPH, sulfo-EMCS, sulfo-GMBS, sulfo-KMUS, sulfo-MBS, sulfo-SIAB,
sulfo-SMCC, and sulfo-SMPB, and SVSB (succinimidy1-(4-vinylsulfone)
benzoate), and including bis-maleimide reagents: DTME, BMB, BMDB, BMH,
BMOE, BM(PEO)3, and BM(PEO)4, which are commercially available from Pierce
Biotechnology, Inc. Bis-maleimide reagents allow the attachment of e.g. a
thiol
group to a thiol-containing drug moiety, label, or linker intermediate, in a
sequential or concurrent fashion. Other functional groups besides maleimide,
which
are reactive with e.g. a thiol group include iodoacetamide, bromoacetamide,
vinyl
pyridine, disulfide, pyridyl disulfide, isocyanate, and isothiocyanate.
Exemplary linker include a valine-citrulline (val-cit or vc) dipeptide linker
reagent
having a maleimide stretcher and a para-aminobenzylcarbamoyl (PAB) self-
immolative spacer, and a phe-lys(Mtr) dipeptide linker reagent having a
maleimide
Stretcher unit and a p-amino benzyl self-immolative spacer.
Cysteine thiol groups are nucleophilic and capable of reacting to form
covalent
bonds with electrophilic groups on linker reagents and helicar motif
containing
compounds including: (i) active esters such as NHS esters, HOBt esters,
haloformates, and acid halides; (ii) alkyl and benzyl halides, such as
haloacetamides; (iii) aldehydes, ketones, carboxyl, and maleimide groups; and
(iv)
disulfides, including pyridyl disulfides, via sulfide exchange. Nucleophilic
groups
on a helicar motif containing compound include, but are not limited to: amine,
thiol,
hydroxyl, hydrazide, oxime, hydrazine, thiosemicarbazone, hydrazine
carboxylate,
and arylhydrazide groups capable of reacting to form covalent bonds with
electrophilic groups on linker moieties and linker reagents.
III. Nucleic acid
The DNA encoding the amino acid sequence of the antibody as reported herein or
of the compounds or part of the compounds as comprised in a conjugate as
reported
herein can be prepared by a variety of methods known in the art. These methods
include, but are not limited to, preparation by site-directed (or
oligonucleotide-
mediated) mutagenesis, PCR mutagenesis, and cassette mutagenesis of an earlier
prepared DNA encoding the polypeptide.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 74 -
Variants of recombinant antibodies may be constructed also by restriction
fragment
manipulation or by overlap extension PCR with synthetic oligonucleotides.
Mutagenic primers encode the cysteine codon replacement(s). Standard
mutagenesis techniques can be employed to generate DNA encoding such modified
engineered antibodies. General guidance can be found in Sambrook et al.,
Molecular Cloning, A Laboratory Manual, Cold Spring Harbor Laboratory Press,
Cold Spring Harbor, N.Y., 1989; and Ausubel et al Current Protocols in
Molecular
Biology, Greene Publishing and Wiley-Interscience, New York, N.Y., 1993.
IV. Expression and purification
Antibodies may be produced using recombinant methods and compositions, e.g.,
as
described in US 4,816,567. In one embodiment, isolated nucleic acid encoding
an
antibody described herein is provided. Such nucleic acid may encode an amino
acid
sequence comprising the VL and/or an amino acid sequence comprising the VH of
the antibody (e.g., the light and/or heavy chains of the antibody). In a
further
embodiment, one or more vectors (e.g., expression vectors) comprising such
nucleic acid are provided. In a further embodiment, a host cell comprising
such
nucleic acid is provided. In one such embodiment, a host cell comprises (e.g.,
has
been transformed with): (1) a vector comprising a nucleic acid that encodes an
amino acid sequence comprising the VL of the antibody and an amino acid
sequence comprising the VH of the antibody, or (2) a first vector comprising a
nucleic acid that encodes an amino acid sequence comprising the VL of the
antibody and a second vector comprising a nucleic acid that encodes an amino
acid
sequence comprising the VH of the antibody. In one embodiment, the host cell
is
eukaryotic, e.g. a Chinese Hamster Ovary (CHO) cell or lymphoid cell (e.g.,
YO,
NSO, Sp20 cell). In one embodiment, a method of making an antibody as reported
herein is provided, wherein the method comprises culturing a host cell
comprising
a nucleic acid encoding the antibody, as provided above, under conditions
suitable
for expression of the antibody, and optionally recovering the antibody from
the
host cell (or host cell culture medium).
For recombinant production of an antibody as reported herein, nucleic acid
encoding an antibody, e.g., as described above, is isolated and inserted into
one or
more vectors for further cloning and/or expression in a host cell. Such
nucleic acid
may be readily isolated and sequenced using conventional procedures (e.g., by
using oligonucleotide probes that are capable of binding specifically to genes
encoding the heavy and light chains of the antibody).
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 75 -
Suitable host cells for cloning or expression of antibody-encoding vectors
include
prokaryotic or eukaryotic cells described herein. For example, antibodies may
be
produced in bacteria, in particular when glycosylation and Fc effector
function are
not needed. For expression of antibody fragments and polypeptides in bacteria,
see,
e.g., US 5,648,237, US 5,789,199, and US 5,840,523. (See also Charlton, K.A.,
In:
Methods in Molecular Biology, Vol. 248, Lo, B.K.C. (ed.), Humana Press,
Totowa,
NJ (2003), pp. 245-254, describing expression of antibody fragments in E.
coli.)
After expression, the antibody may be isolated from the bacterial cell paste
in a
soluble fraction and can be further purified.
In addition to prokaryotes, eukaryotic microbes such as filamentous fungi or
yeast
are suitable cloning or expression hosts for antibody-encoding vectors,
including
fungi and yeast strains whose glycosylation pathways have been "humanized,"
resulting in the production of an antibody with a partially or fully human
glycosylation pattern. See Gerngross, T.U., Nat. Biotech. 22 (2004) 1409-1414;
and Li, H. et al., Nat. Biotech. 24 (2006) 210-215.
Suitable host cells for the expression of glycosylated antibody are also
derived
from multicellular organisms (invertebrates and vertebrates). Examples of
invertebrate cells include plant and insect cells. Numerous baculoviral
strains have
been identified which may be used in conjunction with insect cells,
particularly for
transfection of Spodoptera frugiperda cells.
Plant cell cultures can also be utilized as hosts. See, e.g., US Patent Nos.
5,959,177,
6,040,498, 6,420,548, 7,125,978, and 6,417,429 (describing PLANTIBODIESTm
technology for producing antibodies in transgenic plants).
Vertebrate cells may also be used as hosts. For example, mammalian cell lines
that
are adapted to grow in suspension may be useful. Other examples of useful
mammalian host cell lines are monkey kidney CV1 line transformed by 5V40
(COS-7); human embryonic kidney line (293 or 293 cells as described, e.g., in
Graham, F.L. et al., J. Gen Virol. 36 (1977) 59-74); baby hamster kidney cells
(BHK); mouse sertoli cells (TM4 cells as described, e.g., in Mather, J.P.,
Biol.
Reprod. 23 (1980) 243-252); monkey kidney cells (CV1); African green monkey
kidney cells (VERO-76); human cervical carcinoma cells (HELA); canine kidney
cells (MDCK; buffalo rat liver cells (BRL 3A); human lung cells (W138); human
liver cells (Hep G2); mouse mammary tumor (MMT 060562); TRI cells, as
described, e.g., in Mather, J.P. et al., Annals N.Y. Acad. Sci. 383 (1982) 44-
68;
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 76 -
MRC 5 cells; and FS4 cells. Other useful mammalian host cell lines include
Chinese hamster ovary (CHO) cells, including DHFR- CHO cells (Urlaub, G. et
al.,
Proc. Natl. Acad. Sci. USA 77 (1980) 4216-4220); and myeloma cell lines such
as
YO, NSO and 5p2/0. For a review of certain mammalian host cell lines suitable
for
antibody production, see, e.g., Yazaki, P. and Wu, A.M., Methods in Molecular
Biology, Vol. 248, Lo, B.K.C. (ed.), Humana Press, Totowa, NJ (2004), pp. 255-
26 8 .
V. Methods and Compositions for Diagnostics and Detection
In certain embodiments, any of the antibodies, especially the bispecific
antibodies,
and conjugates as reported herein is useful for detecting the presence of one
or
more target molecules in a biological sample. The term "detecting" as used
herein
encompasses quantitative or qualitative detection. In one embodiment a
biological
sample comprises a cell or tissue.
In one embodiment, an antibody or conjugate as reported herein for use in a
method of diagnosis or detection is provided. In certain embodiments, the
method
comprises contacting the biological sample with an antibody or conjugate as
reported herein under conditions permissive for binding of the antibody or the
conjugate to the target, and detecting whether a complex is formed between the
antibody or the conjugate and the target. Such method may be an in vitro or in
vivo
method.
In certain embodiments, labeled antibodies or conjugates are provided. Labels
include, but are not limited to, labels or moieties that are detected directly
(such as
fluorescent, chromophoric, electron-dense, chemiluminescent, and radioactive
labels), as well as moieties, such as enzymes or ligands, that are detected
indirectly,
e.g., through an enzymatic reaction or molecular interaction. Exemplary labels
include, but are not limited to, the radioisotopes 32p, 14C5 12515 3H5 and
1311,
fluorophores such as rare earth chelates or fluorescein and its derivatives,
rhodamine and its derivatives, dansyl, umbelliferone, luciferases, e.g.,
firefly
luciferase and bacterial luciferase (US 4,737,456),
luciferin, 2,3 -
3 0 dihydrophthalazinediones, horseradish peroxidase (HRP), alkaline
phosphatase, 0-
galactosidase, glucoamylase, lysozyme, saccharide oxidases, e.g., glucose
oxidase,
galactose oxidase, and glucose-6-phosphate dehydrogenase, heterocyclic
oxidases
such as uricase and xanthine oxidase, coupled with an enzyme that employs
hydrogen peroxide to oxidize a dye precursor such as HRP, lactoperoxidase, or
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 77 -
microperoxidase, biotin/avidin, spin labels, bacteriophage labels, stable free
radicals, and the like.
VI. Pharmaceutical Formulations
Pharmaceutical formulations of an antibody or conjugate as reported herein are
prepared by mixing such antibody or conjugate having the desired degree of
purity
with one or more optional pharmaceutically acceptable carriers (Remington's
Pharmaceutical Sciences, 16th edition, Osol, A. (ed.) (1980)), in the form of
lyophilized formulations or aqueous solutions. Pharmaceutically acceptable
carriers
are generally nontoxic to recipients at the dosages and concentrations
employed,
and include, but are not limited to: buffers such as phosphate, citrate, and
other
organic acids; antioxidants including ascorbic acid and methionine;
preservatives
(such as octadecyl dimethylbenzyl ammonium chloride; hexamethonium chloride;
benzalkonium chloride; benzethonium chloride; phenol, butyl or benzyl alcohol;
alkyl parabens such as methyl or propyl paraben; catechol; resorcinol;
cyclohexanol; 3-pentanol; and m-cresol); low molecular weight (less than about
10
residues) polypeptides; proteins, such as serum albumin, gelatin, or
immunoglobulins; hydrophilic polymers such as poly(vinylpyrrolidone); amino
acids such as glycine, glutamine, asparagine, histidine, arginine, or lysine;
monosaccharides, disaccharides, and other carbohydrates including glucose,
mannose, or dextrins; chelating agents such as EDTA; sugars such as sucrose,
mannitol, trehalose or sorbitol; salt-forming counter-ions such as sodium;
metal
complexes (e.g. Zn-protein complexes); and/or non-ionic surfactants such as
polyethylene glycol (PEG). Exemplary pharmaceutically acceptable carriers
herein
further include interstitial drug dispersion agents such as soluble neutral-
active
hyaluronidase glycoproteins (sHASEGP), for example, human soluble PH-20
hyaluronidase glycoproteins, such as rhuPH20 (HYLENEX , Baxter International,
Inc.). Certain exemplary sHASEGPs and methods of use, including rhuPH20, are
described in US Patent Publication Nos. 2005/0260186 and 2006/0104968. In one
aspect, a sHAS E GP is combined with one or more additional
glycosaminoglycanases such as chondroitinases.
Exemplary lyophilized antibody formulations are described in US 6,267,958.
Aqueous antibody formulations include those described in US 6,171,586 and
WO 2006/044908, the latter formulations including a histidine-acetate buffer.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 78 -
The formulation herein may also contain more than one active ingredients as
necessary for the particular indication being treated, preferably those with
complementary activities that do not adversely affect each other. Such active
ingredients are suitably present in combination in amounts that are effective
for the
purpose intended.
Active ingredients may be entrapped in microcapsules prepared, for example, by
coacervation techniques or by interfacial polymerization, for example,
hydroxymethylcellulose or gelatin-microcapsules and poly-(methyl methacrylate)
microcapsules, respectively, in colloidal drug delivery systems (for example,
liposomes, albumin microspheres, microemulsions, nanoparticles and
nanocapsules) or in macroemulsions. Such techniques are disclosed in
Remington's
Pharmaceutical Sciences, 16th edition, Osol, A. (ed.) (1980).
Sustained-release preparations may be prepared. Suitable examples of sustained-
release preparations include semi-permeable matrices of solid hydrophobic
polymers containing the antibody or conjugate, which matrices are in the form
of
shaped articles, e.g. films, or microcapsules.
The formulations to be used for in vivo administration are generally sterile.
Sterility may be readily accomplished, e.g., by filtration through sterile
filtration
membranes.
VII. Therapeutic Methods and Compositions
Any of the antibodies or conjugates reported herein may be used in therapeutic
methods.
In one aspect, an antibody or a conjugate as reported herein for use as a
medicament is provided. In further aspects, an antibody or a conjugate as
reported
herein for use in treating a disease is provided. In certain embodiments, an
antibody
or a conjugate as reported herein for use in a method of treatment is
provided. In
certain embodiments, the invention provides an antibody or a conjugate as
reported
herein for use in a method of treating an individual comprising administering
to the
individual an effective amount of the antibody or the conjugate as reported
herein.
In one such embodiment, the method further comprises administering to the
individual an effective amount of at least one additional therapeutic agent,
e.g., as
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 79 -
described below. An "individual" according to any of the above embodiments may
be a human.
In a further aspect, the invention provides for the use of an antibody or a
conjugate
as reported herein in the manufacture or preparation of a medicament. In one
embodiment, the medicament is for treatment of a disease. In a further
embodiment,
the medicament is for use in a method of treating a disease comprising
administering to an individual having a disease an effective amount of the
medicament. In one such embodiment, the method further comprises administering
to the individual an effective amount of at least one additional therapeutic
agent,
e.g., as described below. An "individual" according to any of the above
embodiments may be a human.
In a further aspect, the invention provides a method for treating a disease.
In one
embodiment, the method comprises administering to an individual having such a
disease an effective amount of an antibody or a conjugate as reported herein.
In one
such embodiment, the method further comprises administering to the individual
an
effective amount of at least one additional therapeutic agent, as described
below.
An "individual" according to any of the above embodiments may be a human.
In a further aspect, the invention provides pharmaceutical formulations
comprising
any of the antibodies or conjugates as reported herein, e.g., for use in any
of the
above therapeutic methods. In one embodiment, a pharmaceutical formulation
comprises any of the antibodies or conjugates as reported herein and a
pharmaceutically acceptable carrier. In another embodiment, a pharmaceutical
formulation comprises any of the antibodies or conjugates as reported herein
and at
least one additional therapeutic agent, e.g., as described below.
Antibodies and conjugates as reported herein can be used either alone or in
combination with other agents in a therapy. For instance, an antibody or
conjugate
as reported herein may be co-administered with at least one additional
therapeutic
agent.
Such combination therapies noted above encompass combined administration
(where two or more therapeutic agents are included in the same or separate
formulations), and separate administration, in which case, administration of
the
antibody of the invention can occur prior to, simultaneously, and/or
following,
administration of the additional therapeutic agent and/or adjuvant. Antibodies
and
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 80 -
conjugates as reported herein can also be used in combination with radiation
therapy.
An antibody or conjugate as reported herein (and any additional therapeutic
agent)
can be administered by any suitable means, including parenteral,
intrapulmonary,
and intranasal, and, if desired for local treatment, intralesional
administration.
Parenteral infusions include intramuscular, intravenous, intraarterial,
intraperitoneal, or subcutaneous administration. Dosing can be by any suitable
route, e.g. by injections, such as intravenous or subcutaneous injections,
depending
in part on whether the administration is brief or chronic. Various dosing
schedules
including but not limited to single or multiple administrations over various
time-
points, bolus administration, and pulse infusion are contemplated herein.
Antibodies or conjugates as reported herein would be formulated, dosed, and
administered in a fashion consistent with good medical practice. Factors for
consideration in this context include the particular disorder being treated,
the
particular mammal being treated, the clinical condition of the individual
patient, the
cause of the disorder, the site of delivery of the agent, the method of
administration,
the scheduling of administration, and other factors known to medical
practitioners.
The antibody or conjugate need not be, but is optionally formulated with one
or
more agents currently used to prevent or treat the disorder in question. The
effective amount of such other agents depends on the amount of antibody or
conjugate present in the formulation, the type of disorder or treatment, and
other
factors discussed above. These are generally used in the same dosages and with
administration routes as described herein, or about from 1 to 99% of the
dosages
described herein, or in any dosage and by any route that is
empirically/clinically
determined to be appropriate.
For the prevention or treatment of disease, the appropriate dosage of an
antibody or
conjugate as reported herein (when used alone or in combination with one or
more
other additional therapeutic agents) will depend on the type of disease to be
treated,
the type of antibody or conjugate, the severity and course of the disease,
whether
the antibody or conjugate is administered for preventive or therapeutic
purposes,
previous therapy, the patient's clinical history and response to the antibody
or
conjugate, and the discretion of the attending physician. The antibody or
conjugate
is suitably administered to the patient at one time or over a series of
treatments.
Depending on the type and severity of the disease, about 1 ig/kg to 15 mg/kg
(e.g.
0.5 mg/kg - 10 mg/kg) of antibody or conjugate can be an initial candidate
dosage
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 81 -
for administration to the patient, whether, for example, by one or more
separate
administrations, or by continuous infusion. One typical daily dosage might
range
from about 1 ig/kg to 100 mg/kg or more, depending on the factors mentioned
above. For repeated administrations over several days or longer, depending on
the
condition, the treatment would generally be sustained until a desired
suppression of
disease symptoms occurs. One exemplary dosage of the antibody or conjugate
would be in the range from about 0.05 mg/kg to about 10 mg/kg. Thus, one or
more
doses of about 0.5 mg/kg, 2.0 mg/kg, 4.0 mg/kg or 10 mg/kg (or any combination
thereof) may be administered to the patient. Such doses may be administered
intermittently, e.g. every week or every three weeks (e.g. such that the
patient
receives from about two to about twenty, or e.g. about six doses of the
antibody).
An initial higher loading dose, followed by one or more lower doses may be
administered. However, other dosage regimens may be useful. The progress of
this
therapy is easily monitored by conventional techniques and assays.
It is understood that any of the above formulations or therapeutic methods may
be
carried out using an immunoconjugate of the invention in place of or in
addition to
an antibody or a conjugate as reported herein.
VIII. Articles of Manufacture
In another aspect of the invention, an article of manufacture containing
materials
useful for the treatment, prevention and/or diagnosis of the disorders
described
above is provided. The article of manufacture comprises a container and a
label or
package insert on or associated with the container. Suitable containers
include, for
example, bottles, vials, syringes, IV solution bags, etc. The containers may
be
formed from a variety of materials such as glass or plastic. The container
holds
a composition which is by itself or combined with another composition
effective
for treating, preventing and/or diagnosing the condition and may have a
sterile
access port (for example the container may be an intravenous solution bag or a
vial
having a stopper pierceable by a hypodermic injection needle). At least one
active
agent in the composition is an antibody or a complex as reported herein. The
label
or package insert indicates that the composition is used for treating the
condition of
choice. Moreover, the article of manufacture may comprise (a) a first
container
with a composition contained therein, wherein the composition comprises an
antibody or a complex as reported herein; and (b) a second container with a
composition contained therein, wherein the composition comprises a further
cytotoxic or otherwise therapeutic agent. The article of manufacture in this
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 82 -
embodiment of the invention may further comprise a package insert indicating
that
the compositions can be used to treat a particular condition. Alternatively,
or
additionally, the article of manufacture may further comprise a second (or
third)
container comprising a pharmaceutically-acceptable buffer, such as
bacteriostatic
water for injection (BWFI), phosphate-buffered saline, Ringer's solution and
dextrose solution. It may further include other materials desirable from
a commercial and user standpoint, including other buffers, diluents, filters,
needles,
and syringes.
It is understood that any of the above articles of manufacture may include an
immunoconjugate of the invention in place of or in addition to an antibody or
a
conjugate as reported herein.
IX. Specific embodiments
1. A conjugate comprising a helicar motif amino acid sequence containing
compound and an antibody that specifically binds to the helicar motif amino
acid sequence characterized by a covalent bond between the helicar motif
amino acid sequence containing compound and an amino acid residue in the
CDR2 of the anti-helicar antibody, whereby the CDR2 is determined
according to Kabat.
2. A conjugate comprising a helicar motif amino acid sequence containing
compound and an antibody that specifically binds to the helicar motif amino
acid sequence of the helicar motif amino acid sequence containing compound
(anti-helicar motif amino acid sequence antibody) characterized by a covalent
bond between the helicar motif amino acid sequence containing compound
and an amino acid residue in the CDR2 of the antibody, whereby the CDR2
is determined according to Kabat.
3. The conjugate according to any one of items 1 to 2, characterized in
that the
CDR2 is the light chain CDR2.
4. The conjugate according to any one of items 1 to 3, characterized in
that the
helicar motif amino acid sequence has the amino acid sequence
AHLENEVARLKK (SEQ ID NO: 01) or is a variant thereof with one amino
acid residue changed to cysteine.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 83 -
5. The
conjugate according to any one of items 1 to 4, characterized in that the
helicar motif amino acid sequence has the amino acid sequence
AHLENEVARLKK (SEQ ID NO: 01) wherein one amino acid residue of the
three C-terminal amino acid residues is changed to cysteine.
6. The
conjugate according to any one of items 1 to 5, characterized in that the
helicar motif amino acid sequence has the amino acid sequence of
AHLENEVARCKK (SEQ ID NO: 02) or AHLENEVARLCK (SEQ ID
NO: 03).
7. The conjugate according to any one of items 1 to 6, characterized in
that the
helicar motif amino acid sequence containing compound comprises a helicar
motif amino acid sequence, optionally a linker, and a payload.
8. The conjugate according to any one of items 1 to 7, characterized in
that the
helicar motif amino acid sequence containing compound is a polypeptide
comprising the helicar motif amino acid sequence either fused to one of its
termini or within the polypeptide sequence.
9. The conjugate according to any one of items 1 to 8, characterized in
that the
covalent bond is between the helicar motif amino acid sequence containing
compound and an amino acid residue in the CDR2 of the antibody.
10. The conjugate according to any one of items 1 to 9, characterized in
that the
covalent bond is between a functional group in the helicar motif amino acid
sequence containing compound and the amino acid residue in the CDR2 of
the antibody.
11. The conjugate according to any one of items 1 to 10, characterized in
that the
functional group is in the helicar motif amino acid sequence.
12. The conjugate according to any one of items 1 to 11, characterized in that
the
covalent bond is between a cysteine residue in the light chain CDR2 of the
antibody and a functional group in the helicar motif amino acid sequence
containing compound.
13. The
conjugate according to item 12, characterized in that the cysteine residue
in the light chain CDR2 of the antibody is at position 55 or position 51
according to the light chain variable domain numbering of Kabat.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 84 -
14. The conjugate according to any one of items 12 to 13, characterized in
that
the cysteine residue in the light chain CDR2 of the antibody is at position 55
according to the light chain variable domain numbering of Kabat.
15. The conjugate according to any one of items 1 to 14, characterized in
that the
covalent bond is a disulfide bond.
16. The conjugate according to item 15, characterized in that the disulfide
bond
is between a cysteine residue in the helicar motif amino acid sequence and a
cysteine residue in the CDR2 of the light chain of the anti-helicar antibody.
17. The conjugate according to any one of items 1 to 16, characterized in
that the
helicar motif amino acid sequence has the amino acid sequence of SEQ ID
NO: 02.
18. The conjugate according to any one of items 1 to 17, characterized in
that the
antibody is a bispecific antibody comprising a first binding specificity to a
non-helicar antigen and a second binding specificity to helicar motif amino
acid sequence.
19. The conjugate according to item 18, characterized in that the (non-
helicar)
antigen is a cell surface antigen.
20. The conjugate according to item 19, characterized in that the cell surface
antigen is a tumor associated antigen.
21. The conjugate according to any one of items 18 to 20, characterized in
that
the bispecific antibody is a full length antibody to which at one or both
heavy
chain C-termini a scFv or a dsscFv or a scFab or a dsscFab or a combination
thereof has been fused either directly or via a peptidic linker.
22. The conjugate according to any one of items 18 to 21, characterized in
that
the bispecific antibody is a full length antibody.
23. The conjugate according to any one of items 18 to 22, characterized in
that
one heavy chain of the bispecific antibody comprises a hole mutation and the
respective other chain comprises a knob mutation.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 85 -
24. The conjugate according to any one of items 7 to 23, characterized in
that the
payload is selected from a binding moiety, a labeling moiety, and a
biologically active moiety.
25. The conjugate according to any one of items 1 to 24, characterized in
that the
antibody is a full length antibody.
26. The conjugate according to any one of items 1 to 25, characterized in
that the
antibody is a humanized or a human antibody.
27. The conjugate according to any one of items 1 to 26, characterized in
that the
constant region of the antibody is of the IgG1 subclass or of the IgG4
subclass.
28. The conjugate according to any one of items 1 to 27, characterized in
that the
antibody has a constant region of the IgG1 subclass with an alanine at
position 234 and 235 and with a glycine at position 329 with numbering
according to the EU index of Kabat.
29. The conjugate according to any one of items 1 to 27, characterized in that
the
antibody has a constant region of the IgG4 class with a proline at position
228, a glutamic acid at position 235 and a glycine at position 329 with
numbering according to the EU index of Kabat.
30. The conjugate according to any one of items 1 to 24, characterized in
that
embodiment the antibody is an antibody fragment.
31. The conjugate according to item 30, characterized in that the fragment
is a
Fab or a (Fab)2.
32. The conjugate according to any one of items 1 to 31, characterized in
that the
conjugate comprises exactly one covalent bond per light chain CDR2.
33. The conjugate according to any one of items 1 to 32, characterized in that
the
helicar motif amino acid sequence containing compound comprises a reactive
group that can form a covalent bond with the thiol group of the cysteine
residue in the CDR2 of the antibody.
34. The conjugate according to any one of items 1 to 33, characterized
in that the
reactive group is a thiol, or a maleimide, or a haloacetyl.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 86 -
35. The conjugate according to any one of items 1 to 34, characterized in
that the
covalent bond is a disulfide bond.
36. The conjugate according to item 35, characterized in that the disulfide
bond
is formed without the addition of a redox active agent.
37. The conjugate according to any one of items 1 to 36, characterized in that
the
conjugate comprises a therapeutic or detectable moiety.
38. The conjugate according to item 37, characterized in that the
therapeutic or
detectable moiety is covalently conjugated to helicar motif amino acid
sequence or the helicar motif amino acid sequence is incorporated into the
therapeutic or detectable moiety.
39. The conjugate according to any one of items 1 to 38, characterized in
that the
helicar motif amino acid sequence is conjugated to a polypeptide consisting
of 5 to 500 amino acid residues.
40. The conjugate according to item 39, characterized in that the polypeptide
comprises 10 to 450 amino acid residues.
41. The conjugate according to any one of items 39 to 40, characterized in
that
the polypeptide comprises 12 to 450 amino acid residues.
42. The conjugate according to any one of items 39 to 41, characterized in
that
the polypeptide comprises 15 to 400 amino acids residues.
43. The conjugate according to any one of items 1 to 42, characterized in that
the
helicar motif amino acid sequence is conjugated to a detectable label.
44. The
conjugate according to any one of items 1 to 43, characterized in that the
helicar motif amino acid sequence is conjugated to the polypeptide, or to the
detectable label, or to the payload via a linker.
45. The conjugate according to item 44, characterized in that the linker is a
non-
peptidic linker.
46. The conjugate according to item 44, characterized in that the linker is a
peptidic linker.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 87 -
47. An anti-helicar antibody that has in the light chain a cysteine residue
in the
CDR2 whereby the CDRs are determined according to Kabat.
48. The anti-helicar antibody according to item 47, characterized in that the
cysteine residue in the light chain CDR2 of the antibody is at position 55 or
position 51 according to the light chain variable domain numbering of Kabat.
49. The anti-helicar antibody according to any one of items 47 to 48,
characterized in that the cysteine residue in the light chain CDR2 of the
antibody is at position 55 according to the light chain variable domain
numbering of Kabat.
50. The anti-helicar antibody according to any one of items 47 to 49,
characterized in that the antibody has in exactly one heavy chain variable
domain a cysteine residue at position 55 or position 51.
51. The anti-helicar antibody according to any one of items 47 to 50,
characterized in that the antibody is a humanized or human antibody.
52. The anti-helicar antibody according to any one of items 47 to 51,
characterized in that the antibody is a full length antibody, or a Fab, or a
scFv,
or a scFv conjugated to an Fc-region.
53. The anti-helicar antibody according to any one of items 47 to 52,
characterized in that the cysteine forms a disulfide bond with an isolated
cysteine residue or an isolated homocysteine residue.
54. An immunoconjugate comprising the conjugate according to any one of
items
1 to 46 and a cytotoxic agent.
55. A pharmaceutical formulation comprising the conjugate according to any
one
of items 1 to 46 and a pharmaceutically acceptable carrier.
56. The conjugate according to any one of items 1 to 46 for use as a
medicament.
57. The conjugate according to any one of items 1 to 46 for the treatment
of
cancer.
58. The conjugate according to any one of items 1 to 46 for the treatment
of
diabetes.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 88 -
59. The conjugate according to any one of items 1 to 46 for the treatment
of
adiposities.
60. The conjugate according to any one of items 1 to 46 for the treatment
of an
inflammatory disease.
61. The conjugate according to any one of items 1 to 46 for the treatment of a
metabolic disease.
62. The conjugate according to any one of items 1 to 46 for the treatment
of a
viral disease.
63. The use of a conjugate according to any one of items 1 to 46 in the
manufacture of a medicament.
64. The use of a conjugate according to any one of items 1 to 46 as
diagnostic
agent.
65. The use of a conjugate according to any one of items 1 to 46 comprising
a
therapeutic polypeptide to increase the stability of the therapeutic
polypeptide.
66. The use of a conjugate according to any one of items 1 to 46 comprising a
therapeutic polypeptide to increase the activity of the therapeutic
polypeptide.
67. The use of a
conjugate according to any one of items 1 to 46 comprising a
therapeutic polypeptide to increase the in vivo half-life of the therapeutic
polypeptide.
68. The use of a conjugate according to any one of items 1 to 46 in the
treatment
of a disease.
69. A method of
treating an individual having a disease comprising administering
to the individual an effective amount of a conjugate according to any one of
items 1 to 46.
70. A method of treating a disease in an individual comprising administering
to
the individual an effective amount of the conjugate according to any one of
items 1 to 46.
71. The method
according to any one of items 68 to 70, characterized in that the
disease is cancer.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 89 -
72. The method according to any one of items 68 to 70, characterized in
that the
disease is diabetes.
73. The method according to any one of items 68 to 70, characterized in
that the
disease is adipositas.
74. A method of producing a conjugate according to any one of items 1 to 46
comprising the combination of an anti-helicar antibody comprising a first
reactive group and an helicar motif amino acid sequence containing
compound that has a second reactive group whereby the alpha carbon atom of
the amino acid residue that bears the first reactive group is about 10 to 11
Angstrom apart from the atom of the helicar motif amino acid sequence
containing compound to which the linker is fused.
75. A
method of producing a conjugate according to any one of items 1 to 46
comprising the steps of
- combining in solution an anti-helicar antibody that specifically binds to
a helicar motif amino acid sequence and comprises a reactive group at
one amino acid residue in the CDR2 with a helicar motif amino acid
sequence containing compound comprising a reactive group, wherein
the helicar motif amino acid sequence containing compound comprises
a payload, such as a peptide consisting of 5 to 500 amino acids or a
detectable label, and
- recovering of the conjugate from the solution.
76. A method for producing an anti-helicar antibody for the formation of a
conjugate according to any one of items 1 to 46, comprising the step of
- cultivating a cell comprising a nucleic acid encoding the anti-helicar
antibody, and
- recovering the anti-helicar antibody from the cell or the cultivation
medium,
wherein in the anti-helicar antibody the residue in the light chain CDR2 is
mutated to cysteine that has in the X-ray structure of the non-covalent
complex of the anti-helicar antibody and the helicar motif amino acid
sequence containing compound a distance of 10 to 11 Angstrom between the
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 90 -
alpha-carbon atom of the amino acid residue in the antibody CDR2 and the
atom of the helicar motif amino acid sequence containing compound atom
between which the covalent bond is to be formed.
77. A
method for identifying a position in an anti-helicar antibody CDR2 that can
be mutated to cysteine for the formation of a covalent bond between the
residue in the antibody CDR2 and the bound helicar motif amino acid
sequence containing compound comprising the step of
- providing a crystal structure of the non-covalent complex of the anti-
helicar antibody and the helicar motif amino acid sequence containing
compound, and
- identifying an amino acid residue in the CDR2 of the anti-helicar
antibody and in the helicar motif amino acid sequence containing
compound with a distance of 10 to 11 Angstrom between the alpha-
carbon atoms of the amino acid residue in the antibody CDR2 and the
atom in the helicar motif amino acid sequence containing compound,
wherein the identified position is the position in an antibody CDR2 that can
be mutated to cysteine for the formation of a covalent bond between the
residue in the antibody CDR2 and the bound helicar motif amino acid
sequence containing compound.
78. A bispecific anti-helicar antibody for targeted delivery of a helicar
motif
amino acid sequence containing compound to a target cell, wherein the
bispecific antibody comprises a first binding site that specifically binds to
the
helicar motif amino acid sequence containing compound and a second
binding specificity that specifically binds to a cell surface marker of the
target cell.
The disclosure of all references cited herein is herewith incorporated by
reference.
The following examples, figures and sequences are provided to aid the
understanding of the present invention, the true scope of which is set forth
in the
appended claims. It is understood that modifications can be made in the
procedures
set forth without departing from the spirit of the invention.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 91 -
Examples
For the amino acid sequences, synthesis and purification of the anti-hapten
antibodies and haptenylated compounds as used in the examples below please see
WO 2014/006124.
Example 1
Binding of recombinant humanized anti-biotin antibody to biotin-labeled
compound (haptenylated compound)
In order to determine whether the humanization procedure and the subsequent
introduction of cysteine mutations resulted in derivatives that had retained
full
binding activity the following experiments were performed.
The binding properties of the recombinant anti-biotin antibody derivatives
were
analyzed by biolayer interferometry (BLI) technology using an Octet QK
instrument (Fortebio Inc.). This system is well established for the study of
molecule interactions. BLi-technology is based on the measurement of the
interference pattern of white light reflected from the surface of a biosensor
tip and
an internal reference. Binding of molecules to the biosensor tip is resulting
in a
shift of the interference pattern which can be measured. To analyze if the
humanization procedure described above diminished the ability of the anti-
biotin
antibody to bind to biotin, the properties of the chimeric and the humanized
versions of the antibody in their ability to bind to a biotinylated protein
were
compared directly. Binding studies were performed by capturing anti-biotin
antibody on anti-huIgG Fc antibody Capture (AHC) Biosensors (Fortebio Inc.).
First, biosensors were incubated in an antibody solution with a concentration
of
0.5 mg/ml in 20 mM histidine, 140 mM NaC1, pH 6.0 for 1 min. Thereafter, the
biosensors were incubated for 1 min. in lx PBS pH 7.4 to reach a stable
baseline.
Binding was measured by incubating the antibody-coated biosensors in a
solution
containing biotinylated protein with a concentration of 0.06 mg/ml in 20 mM
histidine, 140 mM NaC1, pH 6.0 for 5 min. Dissociation was monitored for 5
min.
in lx PBS pH 7.4. The resulting binding curves for chimeric and humanized anti-
biotin antibodies were compared directly.
The humanized version of the antibody showed equal or even better binding of
the
biotinylated antigen than the chimeric antibody. The same is true for the
humanized
antibody with the Cys mutation at Kabat position VH53. The biotinylated
protein
showed residual unspecific binding to the biosensors which was reduced when
the
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 92 -
biosensors were coated with Herceptin, which does not bind biotin. Thus, the
functionality of the anti-biotin antibody was retained in its humanized
variant
(which is defined by the sequences as depicted in SEQ ID NO: 19 and 20, SEQ ID
NO: 21 and 22).
Surface plasmon resonance
Surface plasmon resonance measurement was performed on a BIAcore0 T200
instrument (GE Healthcare Biosciences AB, Sweden) at 25 C. Around 4300
resonance units (RU) of the capturing system (10 g/ml Anti-human Capture (IgG
Fc) from Human Antibody Capture Kit, BR-1008-39, GE Healthcare Biosciences
AB, Sweden) were coupled on a CM3 chip (GE Healthcare, BR-1005-36) at pH 5.0
by using the standard amine coupling kit supplied by GE Healthcare (BR-1000-
50).
The running buffer for amine coupling was HBS-N (10 mM HEPES, pH 7.4,
150 mM NaC1, GE Healthcare, BR-1006-70). Running and dilution buffer for the
followed binding study was PBS-T (10 mM phosphate buffered saline including
0.05% Tween 20) pH 7.4. The humanized anti-biotin antibody was captured by
injecting a 2 nM solution for 60 sec at a flow rate of 5 1/min. Biotinylated
siRNA
was diluted with PBS-T at concentrations of 0.14 - 100 nM (1:3 dilution
series).
Binding was measured by injecting each concentration for 180 sec at a flow
rate of
30 1/min, dissociation time 600 sec. The surface was regenerated by 30 sec
washing with a 3 M MgC12 solution at a flow rate of 5 1/min. The data were
evaluated using BIAevaluation software (GE Healthcare Biosciences AB, Sweden).
Bulk refractive index differences were corrected by subtracting the response
obtained from an anti-human IgG Fc surface. Blank injections were also
subtracted
(= double referencing). For calculation of KD and kinetic parameters the
Langmuir
1:1 model was used.
Kinetic binding analysis by surface plasmon resonance (SPR) was carried out
for
humanized anti-biotin antibody SEQ ID NO: 19 and 20 and humanized anti-biotin
antibody VH53C SEQ ID NO: 21 and 22. Anti-biotin antibodies at a concentration
of 2 nM were captured by anti-human IgG Fc antibody which was bound to a CM3
sensor chip. Binding of biotinylated siRNA (Mw: 13868 Da) was recorded at the
concentrations 0.41, 1.23, 3.7, 11.1, 33.3, 100 and 300 nM. Measurements were
carried out in duplicates. The calculated KD for humanized anti-biotin
antibody and
humanized anti-biotin antibody VH53C were 0.633 nM and 0.654 nM,
respectively.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 93 -
Example 2
Generation of non-covalent complexes of haptenylated compounds with anti-
hapten antibodies
General method:
The generation of complexes of anti-hapten antibodies with haptenylated
compounds (= haptens conjugated to a payload) shall result in defined
complexes
and it shall be assure that the compound (= payload) in these complexes
retains its
activity. For the generation of complexes of haptenylated compounds with the
respective anti-hapten antibody the haptenylated compound was dissolved in H20
to a final concentration of lmg/ml. The antibody was concentrated to a final
concentration of 1 mg/ml (4.85 M) in 20 mM histidine buffer, 140 mM NaC1,
pH=6Ø Haptenylated payload and antibody were mixed to a 2:1 molar ratio
(compound to antibody) by pipetting up and down and incubated for 15 minutes
at
RT.
Alternatively, the haptenylated compound was dissolved in 100% DMF to a final
concentration of 10 mg/ml. The antibody was concentrated to a final
concentration
of 10 mg/ml in 50 mM Tris-HC1, 1 mM EDTA, pH 8.2. Haptenylated compound
and antibody were mixed to a 2.5:1 molar ratio (compound to antibody) by
pipetting up and down and incubated for 60 minutes at RT and 350 rpm.
Exemplary method for the formation of complexes of haptenylated fluorescent
dyes and anti-hapten antibodies ¨ non-covalent digoxigenin-Cy5 complex
Humanized and murine anti-digoxigenin antibody or bispecific anti-digoxigenin
antibody derivatives were used as antibody components. For the generation of
complexes of digoxigenylated Cy5 with the anti-digoxigenin antibodies the
Cy5-digoxigenin conjugate was dissolved in PBS to a final concentration of
0.5 mg/ml. The antibody was used in a concentration of 1 mg/ml (about 5 M) in
a
buffer composed of 20 mM histidine and 140 mM NaC1, pH 6. Digoxigenylated
Cy5 and antibody were mixed at a 2:1 molar ratio (digoxigenylated Cy5 to
antibody). This procedure resulted in a homogenous preparation of complexes of
defined composition.
The complexation reaction can be monitored by determining the fluorescence
(650/667nm) of the antibody-associated fluorophore on a size exclusion column.
The results of these experiments demonstrate that complexation only occurs if
the
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 94 -
antibody contains binding specificities for digoxigenin. Antibodies without
binding
specificities for digoxigenin do not bind the digoxigenin-Cy5 conjugate. An
increasing signal can be observed for bivalent anti-digoxigenin antibodies
until a
digoxigenin-Cy5 conjugate to antibody ratio of 2:1. Thereafter, the
composition
dependent fluorescence signals reach a plateau.
Exemplary method for the formation of complexes of haptenylated fluorescent
dyes and anti-hapten antibodies ¨ Biotin-Cy5 / chimeric anti-biotin antibody
(human IgG subclass) complex
For the generation of complexes of biotin-derivatized-Cy5 (Biotin-Cys-Cy5)
containing a cysteinylated linker, 0.16 mg of Biotin-Cys-Cy5 were dissolved in
100 % DMF to a concentration of 10 mg/ml. 1 mg of the antibody was used in a
concentration of 10.1 mg/ml (about 69 M) in a buffer composed of 50 mM Tris-
HC1, 1 mM EDTA, pH 8.2. Biotin-Cys-Cy5 and antibody were mixed at a 2.5:1
molar ratio (Biotin-Cys-Cy5 to antibody) and incubated for 60 min at RT,
shaken
at 350 rpm. The resulting conjugate was analyzed by SDS-PAGE as described in
Example 3a. Detection of fluorescence was carried out as described in Example
3a.
Exemplary method for the formation of conjugates of biotinylated fluorescent
dyes
and anti-biotin antibodies ¨ Biotin-Ser-Cy5/ humanized anti-biotin antibody:
For the generation of complexes of biotin-derivatized-Cy5 (Biotin-Ser-Cy5)
containing a serine residue within the linker, 0.61 mg of Biotin-Ser-Cy5 were
dissolved in 20 mM histidine, 140 mM NaC1, pH 6.0 to a concentration of
10 mg/ml. 18.5 mg of the humanized anti-biotin antibody was used in a
concentration of 10 mg/ml (about 69 M) in a buffer composed of 50 mM Tris-
HC1, 1 mM EDTA, pH 8.2. Biotin-Ser-Cy5 and antibody were mixed at a 2.5:1
molar ratio (Biotin-Ser-Cy5 to antibody) and incubated for 60 min at RT,
shaken at
350 rpm. The sample was then subjected to size exclusion chromatography using
Superdex 200 16/60 high load prep grade column (GE Healthcare) with a flow
rate
of 1.5 ml/min and 20 mM histidine, 140 mM NaC1, pH 6.0 as the mobile phase.
Peak fractions were collected and analyzed by SDS-PAGE for purity. The dye to
antibody ratio was calculated by (1) measuring the absorbance of the samples
at the
wavelength 280 nm (protein) and 650 nm (Cy5); (2) using the formula: A650 of
labeled protein/c(Cy5)*protein concentration (M) = moles dye per mole protein,
where c(Cy5) = 250000 M-lcm-1, A650 of the complex = 47.0 and the protein
concentration is 86.67 M. The resulting ratio of dye to antibody molecule was
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 95 -
2.17 which indicates that all antibody paratopes are saturated with Biotin-Cy5
molecules.
Exemplary method for the formation of complexes of haptenylated polypeptides
and anti-hapten antibodies - Digoxigenin-PYY(3-36) / anti-digoxigenin antibody
complex
For the generation of non-covalent complexes of digoxigenylated polypeptides
with an anti-digoxigenin antibody the murine hybridoma-derived antibody
(lyophilisate from 10 mM KPO4, 70 mM NaCl; pH 7.5) was dissolved in 12 ml
water and dialyzed against a solution comprising 20 mM histidine, 140 mM NaC1,
pH 6.0 to yield 300 mg (2 x 10-6 mol) in 11 ml buffer (c = 27.3 mg/ml).
Digoxigenin-PYY(3-36) conjugate (11.57 mg, 4 x 10-6 mol, 2 eq.) was added in
4 portions of 2.85 mg within 1 h and incubated for another hour at room
temperature. After completion of the complexation reaction, the complexes were
purified by size exclusion chromatography via a Superdex 200 26/60 GL column
(320m1) in 20 mM histidine, 140 mM NaC1, at pH 6.0 at a flow rate of 2.5
ml/min.
The eluted complex was collected in 4 ml fractions, pooled and sterilized over
a
0.2 gm filter to give 234 mg of the complex at a concentration of 14.3 mg/ml.
In a
similar manner, for generation of complexes of the humanized anti-digoxigenin
antibody the antibody was adjusted to a concentration of 10.6 mg/ml (9.81 mg,
6.5x10-8 mol in 0.93 ml) in 20 mM histidine, 140 mM NaC1, pH 6Ø 0.57 mg =
1.97x10-7 mol = 3.03 eq. of the digoxigenylated polypeptide (DIG-PYY) were
added to the antibody solution as lyophilisate. Polypeptide and antibody were
incubated for 1.5 hrs. at room temperature. The excess of polypeptide was
removed
by size exclusion chromatography via a Superose 6 10/300 GL column in 20 mM
histidine, 140 mM NaC1, at pH 6.0 at a flow rate of 0.5 ml/min. The eluted
complex was collected in 0.5 ml fractions, pooled and sterilized over a 0.2 gm
filter
to give 4.7 mg of the complex at a concentration of 1.86 mg/ml.
The resulting haptenylated polypeptide-anti-hapten antibody complex was
defined
as monomeric IgG-like molecule via the occurrence of a single peak in a size
exclusion chromatography. The resulting complex was defined as monomeric IgG-
like molecule, carrying two Digoxigenin-PYY derivatives per antibody molecule.
The defined composition of these peptide complexes was confirmed by size
exclusion chromatography, which also indicated the absence of protein
aggregates.
The defined composition (and 2:1 polypeptide to protein ratio) of these
bispecific
peptide complexes was further confirmed by SEC-MALS (Size exclusion
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 96 -
chromatography- Multi Angle Light Scattering). For SEC-MALS analysis,
100-500 g of the respective sample was applied to a Superdex 200 10/300 GL
size
exclusion column with a flow rate of 0.25-0.5 ml/min with 1 x PBS pH 7.4 as
mobile phase. Light scattering was detected with a Wyatt MiniDawn
TREOS/QELS detector, the refractive index was measured with a Wyatt Optilab
rEX-detector. Resulting data was analyzed using the software ASTRA (version
5.3.4.14). The results of SEC MALLS analyses provide information about the
mass, radius and size of the complex. These data were then compared with those
of
the corresponding non-complexed antibody. The results of these experiments
demonstrate that exposure of Digoxigenylated-PYY to the anti-digoxigenin
antibody results in complexes that contain two Digoxigenin-PYY derivatives per
one antibody molecule. Thus, digoxigenylated PYY can be complexed with the
anti-digoxigenin antibody at defined sites (binding region) and with a defined
stoichiometry.
Characterization of the complex by surface plasmon resonance studies provided
additional evidence that the complexation reaction generated defined and
completely complexed molecules. The anti-digoxigenin antibody can be bound to
the SPR chip which results in signal increases. Subsequent addition of
digoxigenin-
PYY conjugate results in further signal increases until all binding sites are
completely occupied. At these conditions, addition of more Digoxigenin-PYY
does
not increase the signal further. This indicates that the complexing reaction
is
specific and that the signals are not caused by non-specific stickiness of the
digoxigenylated polypeptide.
Exemplary method for the formation of complexes of haptenylated polypeptides
and anti-hapten antibodies - Ac-PYY-PEG3-Cys-B-Ala-Biot / chimeric anti-biotin
antibody complex
For the generation of non-covalent complexes of biotinylated-PYY-polypeptide
containing a cysteinylated linker, 0.19 mg of Ac-PYY-PEG3-Cys-B-Ala-Biot were
dissolved in 100% DMF to a concentration of 10 mg/ml. The antibody was used in
a concentration of 10.7 mg/ml (about 73 M) in a buffer composed of 50 mM Tris-
HC1, 1mM EDTA, pH 8.2. Ac-PYY-PEG3-Cys-B-Ala-Biot and antibody were
mixed at a 2.5:1 molar ratio (Ac-PYY-PEG3-Cys-B-Ala-Biot to antibody) and
incubated for 60 min at RT and 350 rpm. The resulting complex was defined as
monomeric IgG-like molecule via the occurrence of a single peak in a size
exclusion chromatography (95% monomer). The resulting complex was further
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 97 -
analyzed by SDS-PAGE and subsequent Western Blot analysis. 10 gg of the
complex were mixed with 4x LDS sample buffer (Invitrogen) and incubated at
95 C for 5 min. The sample was applied to a 4-12% Bis-Tris polyacrylamide-gel
(NuPAGE, Invitrogen) which was run for 35 min at 200V and 120 mA. Molecules
that were separated in the polyacrylamide-gel were transferred to a PVDF
membrane (0.2 gm pore size, Invitrogen) for 40 min at 25V and 160 mA. The
membrane was blocked in 1% (w/v) skim milk in lx PBST (lx PBS + 0.1 %
Tween20) for lh at RT. The membrane was washed 3x for 5 min in lx PBST and
subsequently incubated with a streptavidin-POD-conjugate (2900 U/ml, Roche)
which was used in a 1:2000 dilution. Detection of streptavidin-POD bound to
biotin on the membrane was carried out using Lumi-Light Western Blotting
Substrate (Roche).
Exemplary method for the formation of complexes of haptenylated polypeptides
and anti-hapten antibodies - Ac-PYY-PEG3-Cys-PEG2-Biot)/ chimeric anti-biotin
antibody complex
For the generation of non-covalent complexes of biotinylated-PYY-polypeptide
containing a cysteinylated linker, 0.16 mg of Ac-PYY-PEG3-Cys-PEG2-Biot were
dissolved in 100% DMF to a concentration of 10 mg/ml. The antibody was used in
a concentration of 10.7 mg/ml (about 73 gM) in a buffer composed of 50 mM Tris-
HC1, 1mM EDTA, pH 8.2. Ac-PYY-PEG3-Cys-PEG2-Biot and antibody were
mixed at a 2.5:1 molar ratio (Ac-PYY-PEG3-Cys-PEG2-Biot to antibody) and
incubated for 60 min at RT and 350 rpm. The resulting complex was defined as
63% monomeric IgG-like molecule and 37% dimeric soluble aggregates via size
exclusion chromatography. The resulting complex was further analyzed by SDS-
PAGE and subsequent Western Blot analysis. 10 gg of the complex were mixed
with 4x LDS sample buffer (Invitrogen) and incubated at 95 C for 5 min. The
sample was applied to a 4-12% Bis-Tris polyacrylamide-gel (NuPAGE, Invitrogen)
which was run for 35 min at 200V and 120 mA. Molecules that were separated in
the polyacrylamide-gel were transferred to a PVDF membrane (0.2 gm pore size,
Invitrogen) for 40 min at 25V and 160 mA. The membrane was blocked in 1 %
(w/v) skim milk in lx PBST (lx PBS + 0.1% Tween20) for lh at RT. The
membrane was washed 3x for 5 min in lx PBST and subsequently incubated with a
streptavidin-POD-conjugate (2900 U/ml, Roche) which was used in a 1:2000
dilution. Detection of streptavidin-POD bound to biotin on the membrane was
carried out using Lumi-Light Western Blotting Substrate (Roche).
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 98 -
Exemplary method for the formation of complexes of haptenylated polypeptides
and anti-hapten antibodies - Ac-PYY(PEG3-Cys-PEG2-5-Fluo) / chimeric anti-
fluorescein antibody complex
For the generation of non-covalent complexes of fluorescein-conjugated-PYY-
polypeptide containing a cysteinylated linker, 0.33 mg of Ac-PYY(PEG3-Cys-
PEG2-5-Fluo were dissolved in 100% DMF to a concentration of 10 mg/ml. The
antibody was used in a concentration of 9.99 mg/ml (about 68 M) in a buffer
composed of 50 mM Tris-HC1, 1mM EDTA, pH 8.2. Ac-PYY(PEG3-Cys-PEG2-5-
Fluo and antibody were mixed at a 2.5:1 molar ratio (Ac-PYY(PEG3-Cys-PEG2-5-
Fluo) to antibody) and incubated for 60 min at RT and 350 rpm. The resulting
complex was defined as 76% monomeric IgG-like molecule and 24% dimeric
soluble aggregates via size exclusion chromatography. The resulting complex
was
further analyzed by SDS-PAGE and subsequent detection of fluorescein-related
fluorescence in the polyacrylamide-gel. 8 g of the complex were mixed with 4x
LDS sample buffer (Invitrogen) and incubated at 95 C for 5 min. Fluorescein-
related fluorescence was recorded using a LumiImager F 1 device (Roche) at an
excitation wavelength of 645 nm.
Example 3
Generation of defined covalent conjugates of haptenylated dyes or
polypeptides with an anti-hapten antibody VH52bC/VH53C in the presence of
redox agents
Exemplary method for the formation of conjugates of haptenylated fluorescent
dyes and anti-hapten antibodies - Dig-Cys-Ahx-Cy5/anti-digoxigenin antibody
VH52bC
The generation of covalent conjugates of anti-hapten antibodies and
haptenylated
fluorescent dyes containing a cysteine-linker results in defined conjugates
where a
disulfide bridge is formed at a specific position between VH52bC in the CDR2
of
the anti-hapten antibody and the cysteine in the linker between the hapten and
the
fluorescent dye. The conjugation reaction was carried out in the presence of
redox
reagents. Dig-Cys-Ahx-Cy5 was dissolved in 20 mM histidine, 140 mM NaC1,
pH 6Ø Solubilization was facilitated by drop wise addition of 10% (v/v)
acetic
acid. The final concentration was adjusted to 0.4 mg/ml. The anti-digoxigenin
antibody VH52bC in 20 mM histidine, 140 mM NaC1, pH 6.0 was brought to a
concentration of 10 mg/ml. An anti-digoxigenin antibody was used as a control
and
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 99 -
was treated the same way as anti-digoxigenin antibody VH52bC. 4.7 nmol of each
antibody was mixed with 2.5 molar equivalents of Dig-Cys-Ahx-Cy5. This was
achieved by adding 11.7 nmol of this substance in 4 portions (2.9 nmol each)
every
15 min. In between these additions, the samples were incubated at 25 C while
gently shaking. After addition of the last portion, 0.64 nmol of each antibody-
Dig-
Cys-Ahx-Cy5 complex was transferred to buffer containing the following redox
reagents: 3 mM DTE (Dithioerythritol) + 10 mM GSSG (oxidized Glutathione),
0.3 mM DTE + 1 mM GSSG and 0.03 mM DTE + 0.1 mM GSSG. All samples
were incubated for 15 min in these conditions. After the incubation, samples
were
split into half (0.34 nmol each) and prepared for SDS gel electrophoresis. For
this,
4x LDS sample buffer (Invitrogen) was added. For each sample also a reduced
version was prepared by adding 10x NuPAGE sample reducing agent (Invitrogen).
All samples were incubated at 70 C for 5 min before electrophoresis on a 4-12
%
Bis-Tris polyacrylamide gel (NuPAGE, Invitrogen) with lx MOPS buffer
(Invitrogen). Cy5-related fluorescence in the gel was detected with a
LumiImager
F 1 device (Roche) at an excitation wavelength of 645 nm. After detection of
fluorescence, the gel was stained with SimplyBlue SafeStain (Invitrogen). Gels
are
shown in Figure 3.
Site-specific disulfide bond formation was shown for anti-digoxigenin antibody
VH52bC (Fig. 8, gels on top, lanes 1 A-C) with a low background fluorescence
signal when anti-digoxigenin antibody without a cysteine in CDR2 was used
(lanes
2 A-C). The background signals in the control reactions can be explained by
coupling of Dig-Cys-Ahx-Cy5 to cysteines that are normally involved in the
formation of antibody-interchain disulfide bonds. Increasing amounts of redox
reagents substantially reduce disulfide bridges that connect antibody heavy
and
light chains, producing mainly 3/4 antibodies (- lx LC), HC-dimers (- 2x LC)
and
1/2 antibodies (lx HC + lx LC). On the bottom of the gel fluorescence of Dig-
Cys-
Ahx-Cy5 that was not covalently linked to the antibody can be detected. The
gels
on the bottom of Fig.8 show, that upon reduction of the samples, no Cy5-
related
fluorescence is detectable near the antibody heavy and light chains,
indicating that
the covalent linkage was indeed formed by a disulfide bridge. Coomassie stains
of
each gel show that the total amount of protein in each lane was equal.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 100 -
Exemplary method for the formation of conjugates of haptenylated fluorescent
dyes and anti-hapten antibodies ¨ Dig-Cys-Cy5/ anti-digoxigenin antibody
VH52bC
Dig-Cys-Cy5 was dissolved in 8.3 mM HC1, 10% (v/v) DMF to a final
concentration of 3.25 mg/ml. The anti-digoxigenin antibody VH52bC antibody in
20 mM histidine, 140 mM NaC1, pH 6.0 was brought to a concentration of
mg/ml. anti-digoxigenin antibody was used as a control and was treated the
same way as anti-digoxigenin antibody VH52bC. 13.3 nmol of each antibody was
mixed with 2 molar equivalents of Dig-Cys -Cy5 at a final antibody
concentration
10 of 10 mg/ml in the presence of 1 mM GSH (reduced glutathione) and 5 mM
GSSG
(reduced glutathione). This was achieved by adding 26.6 nmol of this substance
in
2 portions every 5 min. In between these additions, the samples were incubated
at
RT while gently stirred. After addition of the last portion, the samples were
incubated for lh at RT. The efficiency of the coupling reaction was evaluated
by
15 SDS-PAGE and subsequent recording of the Cy5-related fluorescence
signal. 5, 10
and 20 g of each sample were prepared for SDS-PAGE. For this, 4x LDS sample
buffer (Invitrogen) was added. All samples were incubated at 70 C for 5 min
before electrophoresis on a 4-12 % Bis-Tris polyacrylamide gel (NuPAGE,
Invitrogen) with lx MOPS buffer (Invitrogen). Cy5-related fluorescence in the
gel
was detected with a LumiImager Fl device (Roche) at an excitation wavelength
of
645 nm. After detection of fluorescence, the gel was stained with SimplyBlue
SafeStain (Invitrogen).
Exemplary method for the formation of conjugates of haptenylated polypeptides
and anti-hapten antibodies ¨ PEG3-PYY(PEG3-Cys-4Abu-Dig) / humanized anti-
digoxigenin antibody VH52bC
For the generation of conjugates of digoxigenin-derivatized-PYY-polypeptide
containing a cysteinylated linker, 1.4 mg of PEG3-PYY(PEG3-Cys-4Abu-Dig)
were dissolved in 100% DMF to a concentration of 10 mg/ml. 1 mg of the
antibody
was used in a concentration of 10 mg/ml (about 68 M) in a buffer composed of
5 mM Tris-HC1, 1 mM EDTA, 1 mM GSH, 5 mM GSSG, pH 8.2. PEG3-
PYY(PEG3-Cys-4Abu-Dig) and antibody were mixed at a 2:1 molar ratio (PEG3-
PYY(PEG3-Cys-4Abu-Dig) to antibody) and incubated for 60 min at RT, stirred at
100 rpm. The resulting conjugate was analyzed by mass spectrometry. 43% of the
detected species was identified as antibody coupled to 2 polypeptide
molecules,
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 101 -
46% was antibody coupled to 1 polypeptide molecule and 11% was identified as
uncoupled antibody.
Example 4
Generation of defined covalent conjugates of haptenylated dyes and
polypeptides with an anti-hapten antibody VH52bC/VH53C in the absence of
redox agents
For the generation of covalent anti-hapten antibody/haptenylated polypeptide
or
haptenylated dye disulfide-linked conjugates it is necessary to (i) couple the
hapten
(e.g. digoxigenin, fluorescein, biotin or theophylline) via a suitable a
reactive group
(such as e.g. cysteine, maleimide) containing linkers to the polypeptide or
dye that
allows the polypeptide to be exposed above the antibody surface and hence to
retain its activity, and (ii) generate covalent site specific conjugates of
the
haptenylated polypeptides with the anti-hapten antibody with a cysteine
mutation
(= antibody VH52bCNH53C) in which the biological activity of the polypeptide
is
retained, and (iii) to carry out the reaction in the absence of a reducing
agent in
order to avoid the reduction of antibody inter-chain disulfide bridges.
General method:
The generation of conjugates of anti-hapten antibodies with haptenylated
compounds shall result in conjugates with defined stoichiometry and it shall
be
assured that the compound in these conjugates retains its activity. For the
generation of conjugates of haptenylated compounds with the respective anti-
hapten antibody the haptenylated compound was dissolved in 100% DMF to a final
concentration of 10 mg/ml. The anti-hapten antibody VH52bCNH53C was
brought to a concentration of 10 mg/ml in 50 mM Tris-HC1, 1 mM EDTA, pH=8.2.
Haptenylated compound and anti-hapten antibody VH52bCNH53C were mixed in
a 2.5:1 molar ratio (compound to antibody) by pipetting up and down and
incubated for 60 minutes at RT and 350 rpm.
A polypeptide conjugated to the hapten via a cysteine containing linker is
termed
hapten-Cys-polypeptide or polypeptide-Cys-hapten in the following. The
polypeptide may either have a free N-terminus or a capped N-terminus e.g. with
an
acetyl-group (Ac-polypeptide-Cys-hapten) or a PEG-residue (PEG-polypeptide-
Cys-hapten).
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 102 -
A fluorescent dye conjugated to the hapten via a cysteine containing linker is
termed dye-Cys-hapten or hapten-Cys-dye in the following.
Exemplary method for the formation of conjugates of haptenylated fluorescent
dyes and anti-hapten antibodies ¨ Dig-Cys-Ahx-Cy5/ anti-digoxigenin antibody
VH52bC
Samples were prepared exactly as described in Example 3a, with the difference
that
antibody-Dig-Cys-Ahx-Cy5 complexes were transferred to buffer containing
either
no redox compounds, 0.1 mM GSSG (oxidized glutathione) or 1 mM GSSG. The
resulting fluorescence-scanned and Coomassie stained polyacrylamide gels are
shown in Figure 4. All three conditions show a similar specificity for site-
specific
disulfide bond formation (Figure 4, top gels, lanes 1 A-C) with a low level of
background reactions (Figure 4, lanes 2 A-C). This confirms that formation of
the
disulfide bond can be accomplished without the need of reducing agents. This
significantly stabilizes the antibody/reduces antibody disintegration, as only
residual amounts of 3/4 antibodies (- lx LC), HC-dimers (- 2x LC) and 1/2
antibodies
(lx HC + lx LC) are detected in comparison to Example 3.
Exemplary method for the formation of conjugates of haptenylated fluorescent
dyes and anti-hapten antibodies ¨ Dig-Cys-Cy5/ anti-digoxigenin antibody
VH52bC
Samples were prepared exactly as described in Example 3b, with the difference
that
13.3 nmol of antibody was mixed with 2 molar equivalents of Dig-Cys -Cy5 at a
final antibody concentration of 10 mg/ml in the absence of redox reagents.
Exemplary method for the formation of conjugates of haptenylated fluorescent
dyes and anti-hapten antibodies ¨ Biotin-Cys-Cy5/ chimeric anti-biotin
antibody
VH53C
For the generation of conjugates of biotin-derivatized-Cy5 containing a
cysteinylated linker, 0.16 mg of Biotin-Cys-Cy5 were dissolved in 100% DMF to
a
concentration of 10 mg/ml. 1 mg of the anti-biotin antibody VH53C was used in
a
concentration of 9.7 mg/ml (about 68 M) in a buffer composed of 50 mM Tris-
HC1, 1 mM EDTA, pH 8.2. Biotin-Cys-Cy5 and antibody were mixed at a 2.5:1
molar ratio (Ac-Biotin-Cys-Cy5 to antibody) and incubated for 60 min at RT,
shaken at 350 rpm. The resulting conjugate was analyzed by SDS-PAGE as
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 103 -
described in Example 3a. Detection of fluorescence was carried out as
described in
Example 3a.
Exemplary method for the formation of conjugates of haptenylated fluorescent
dyes and anti-hapten antibodies ¨ Biotin-Cys-Cy5/ humanized anti-biotin
antibody
VH53C
For the generation of conjugates of biotin-derivatized-Cy5 containing a
cysteinylated linker, 0.16 mg of Biotin-Cys-Cy5 were dissolved in 100% DMF to
a
concentration of 10 mg/ml. 1 mg of the humanized anti-biotin antibody VH53C
was used in a concentration of 7.4 mg/ml (about 51 M) in a buffer composed of
50 mM Tris-HC1, 1 mM EDTA, pH 8.2. Biotin-Cys-Cy5 and antibody were mixed
at a 2.5:1 molar ratio (Ac-Biotin-Cys-Cy5 to antibody) and incubated for 60
min at
RT, shaken at 350 rpm. The resulting conjugate was analyzed by SDS-PAGE as
described in Example 3a. Detection of fluorescence was carried out as
described in
Example 3a.
Exemplary method for the formation of conjugates of haptenylated polypeptides
and anti-hapten antibodies ¨ Ac-PYY(PEG3-Cys-4Abu-Dig) / humanized anti-
digoxigenin antibody VH52bC
For the generation of conjugates of digoxigenin-derivatized-PYY-polypeptide
containing a cysteinylated linker, 2.4 mg of Ac-PYY(PEG3-Cys-4Abu-Dig) were
dissolved in 20 % acetate to a concentration of 5 mg/ml. 10 mg of the
humanized
anti-digoxigenin antibody VH52bC (68.4 nmol) was used in a concentration of
19.5 mg/ml (about 133 M) in a buffer composed of 20 mM histidine, 140 mM
NaC1, pH 6Ø Ac-PYY(PEG3-Cys-4Abu-Dig) and antibody were mixed at a 2:1
molar ratio (Ac-PYY(PEG3-Cys-4Abu-Dig) to antibody) and incubated for 60 min
at RT, stirred at 100 rpm. The resulting conjugate was analyzed by mass
spectrometry. 7.4 % of the detected species was identified as antibody coupled
to 2
peptide molecules, 40 % was antibody coupled to 1 peptide molecule and 52 %
was
identified as uncoupled antibody.
Exemplary method for the formation of conjugates of haptenylated polypeptides
and anti-hapten antibodies ¨ Ac-PYY(PEG3-Cys-13Ala-Biot) / chimeric anti-
biotin
antibody VH53C
For the generation of conjugates of biotin-derivatized-PYY-polypeptide
containing
a cysteinylated linker, 0.19 mg of Ac-PYY(PEG3-Cys-13A1a-Biot) were dissolved
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 104 -
in 100% DMF to a concentration of 10 mg/ml. 1 mg of the chimeric anti-biotin
antibody VH53C was used in a concentration of 9.7 mg/ml (about 67 M) in a
buffer composed of 50 mM Tris-HC1, 1 mM EDTA, pH 8.2. Ac-PYY[PEG3-Cys-
13Ala-Biot and antibody were mixed at a 2.5:1 molar ratio (Ac-PYY[PEG3-Cys-
13Ala-Biot] to antibody) and incubated for 60 min at RT, shaken at 350 rpm.
The
resulting conjugate was analyzed by mass spectrometry. 87.7 % of the detected
species was identified as antibody coupled to 2 peptide molecules, 12.3 % was
identified as antibody coupled to 1 peptide molecule.
Exemplary method for the formation of conjugates of haptenylated polypeptides
and anti-hapten antibodies ¨ Ac-PYY(PEG3-Cys-PEG2-Biot)/chimeric anti-biotin
antibody VH53C
For the generation of conjugates of biotin-derivatized-PYY-polypeptide
containing
a cysteinylated linker, 0.16 mg of Ac-PYY(PEG3-Cys-PEG2-Biot) were dissolved
in 100% DMF to a concentration of 10 mg/ml. 1 mg of the chimeric anti-biotin
antibody VH53C was used in a concentration of 9.9 mg/ml (about 68 M) in a
buffer composed of 50 mM Tris-HC1, 1 mM EDTA, pH 8.2. Ac-PYY[PEG3-Cys-
PEG2-Biot and antibody were mixed at a 2.5:1 molar ratio (Ac-PYY[PEG3-Cys-
PEG2-Biot] to antibody) and incubated for 60 min at RT, shaken at 350 rpm. The
resulting conjugate was analyzed by mass spectrometry. 100 % of the detected
species was identified as antibody coupled to 2 peptide molecules.
Exemplary method for the formation of conjugates of haptenylated poly peptides
and anti-hapten antibodies ¨ Ac-PYY(PEG3-Cys-13Ala-Biot)/humanized anti-biotin
antibody VH53C
For the generation of conjugates of biotin-derivatized-PYY-polypeptide
containing
a cysteinylated linker, 0.06 mg of Ac-PYY(PEG3-Cys-13Ala-Biot) were dissolved
in 100% DMF to a concentration of 10 mg/ml. 0.8 mg of the humanized anti-
biotin
antibody VH53C was used in a concentration of 9 mg/ml (about 62 M) in a
buffer
composed of 50 mM Tris-HC1, 1 mM EDTA, pH 8.2. Ac-PYY[PEG3-Cys-13Ala-
Biot and antibody were mixed at a 2.5:1 molar ratio (Ac-PYY[PEG3-Cys-13Ala-
Biot] to antibody) and incubated for 60 min at RT, shaken at 350 rpm. The
resulting conjugate was analyzed by mass spectrometry. 62.2 % of the detected
species was identified as antibody coupled to 2 peptide molecules, 33.9 % was
identified as antibody coupled to 1 peptide molecule and 3.9% was identified
as
uncoupled antibody.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 105 -
Exemplary method for the formation of conjugates of haptenylated polypeptides
and anti-hapten antibodies ¨ Ac-PYY(PEG3-Cys-PEG2-Biot)/humanized anti-
biotin antibody VH53C
For the generation of conjugates of biotin-derivatized-PYY-polypeptide
containing
a cysteinylated linker, 0.08 mg of Ac-PYY(PEG3-Cys-PEG2-Biot) were dissolved
in 100% DMF to a concentration of 10 mg/ml. 0.8 mg of the humanized anti-
biotin
antibody VH53C was used in a concentration of 9 mg/ml (about 62 M) in a
buffer
composed of 50 mM Tris-HC1, 1 mM EDTA, pH 8.2. Ac-PYY[PEG3-Cys-PEG2-
Biot and antibody were mixed at a 2.5:1 molar ratio (Ac-PYY[PEG3-Cys-PEG2-
Biot] to antibody) and incubated for 60 min at RT, shaken at 350 rpm. The
resulting conjugate was analyzed by mass spectrometry. 71.4 % of the detected
species was identified as antibody coupled to 2 peptide molecules, 26 % was
identified as antibody coupled to 1 peptide molecule and 2.5% was identified
as
uncoupled antibody.
Exemplary method for the formation of conjugates of haptenylated polypeptides
and anti-hapten antibodies ¨ Ac-PYY(PEG3-Cys-PEG2-Fluo)/anti-fluorescein
antibody VH52bC
For the generation of conjugates of biotin-derivatized-PYY-polypeptide
containing
a cysteinylated linker, 0.33 mg of Ac-PYY[PEG3-Cys-PEG2-Fluo were dissolved
in 100% DMF to a concentration of 10 mg/ml. 1 mg of the anti-fluorescein
antibody VH52bC was used in a concentration of 9.3 mg/ml (about 63 M) in a
buffer composed of 50 mM Tris-HC1, 1 mM EDTA, pH 8.2. Ac-PYY[PEG3-Cys-
PEG2-Fluo and antibody were mixed at a 2.5:1 molar ratio (Ac-PYY[PEG3-Cys-
PEG2-Fluo] to antibody) and incubated for 60 min at RT, shaken at 350 rpm. The
resulting conjugate was analyzed by mass spectrometry. 95 % of the detected
species was identified as antibody coupled to 2 peptide molecules, 5 % was
identified as antibody coupled to 1 peptide molecule.
Exemplary method for the formation of conjugates of haptenylated polypeptides
and anti-hapten antibodies ¨ Ac-PYY(PEG3-Cys-PEG2-Fluo) / anti-fluorescein
antibody VH28C
For the generation of conjugates of biotin-derivatized-PYY-polypeptide
containing
a cysteinylated linker, 0.33 mg of Ac-PYY[PEG3-Cys-PEG2-Fluo were dissolved
in 100% DMF to a concentration of 10 mg/ml. 1 mg of the anti-fluorescein
antibody VH28C was used in a concentration of 9.5 mg/ml (about 63 M) in a
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 106 -
buffer composed of 50 mM Tris-HC1, 1 mM EDTA, pH 8.2. Ac-PYY[PEG3-Cys-
PEG2-Fluo and antibody were mixed at a 2.5:1 molar ratio (Ac-PYY[PEG3-Cys-
PEG2-Fluo] to antibody) and incubated for 60 min at RT, shaken at 350 rpm. The
resulting conjugate was analyzed by mass spectrometry. 100 % of the detected
species was identified as antibody coupled to two peptide molecules.
Example 5
Generation of covalent theophylline-anti-theophylline antibody complexes
To evaluate the formation of covalent antibody complexes that utilize
theophylline
and theophylline-binding antibodies as hapten recognition system, Theophyllin-
Cys-Cy5 was generated as fluorescent payload, applying generally the synthesis
and purification technologies that have been described for Digoxigenin-Cys-Cy5
or
Biotin-Cys-Cy5, with the exception that the hapten has been exchanged against
theophylline. To demonstrate the formation of a covalent disulfide,
theophylline-
binding antibodies were generated which contained a designed Cys at position
54
or 55 of the heavy chain variable region (anti-theophylline antibody-Cys).
These
antibody derivatives were complexed with Theophylline-Cys-Cy5 and
subsequently subjected to SDS-PAGE under non-reducing and reducing conditions
as described in Example 4. Under non-reducing conditions, disulfide-linked
anti-
theophylline-antibody complexed Cy5 was detected by its H-chain associated
fluorescence within the gel in the same manner as described in Example 4.
Covalent complexes had been formed as a consequence of the simple loading
reaction in the same manner as the disulfides that were observed when using
Digoxigenin, Fluorescein or Biotin as hapten. These complexes dissociated as
expected upon reduction, i.e. released the payload from the H-chain only when
the
disulfide became reduced.
Example 6
Generation of covalent hapten-antibody complexes under in-vivo like
conditions, and evidence for directed disulfide-formation in vivo
To evaluate the formation of covalent hapten-antibody complexes under in-vivo
like conditions, anti-Biotin antibodies-Cys were incubated at 37 C in murine
serum with Biotin-Cys-Cy5 for 60 min. Subsequently, the antibody was captured
from the murine serum by protein-A. Thereafter the captured antibodies were
subjected to SDS-PAGE under non-reducing and reducing conditions as described
in Example 4. Disulfide-linked antibody-complexed Cy5 was detected by its H-
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 107 -
chain associated fluorescence within the gel in the same manner as described
in
Example 4. Figure 10 demonstrates that covalent complexes between antibody
form in serum at 37 C, i.e. under conditions that resemble the in-vivo
conditions.
These complexes dissociate as expected upon reduction, i.e. the payload is
released
from the H-chain only when the disulfide becomes reduced (Figure 10). The
observation that upon hapten-positioning a directed disulfide bond between
antibody and payload can be formed even in the presence of serum is unexpected
as serum contains a high amount of proteins, peptides and other compounds
(which
can interfere with disulfide-formation reactions). The observation that upon
hapten-
positioning a directed disulfide bond between antibody and payload can be
formed
in serum at 37 C also opens the possibility to apply this PK-modulation
system in
a pre-targeting setting: separate application of antibody and hapten-payload,
followed by in-vivo assembly of antibody complexes and subsequent disulfide
formation.
To further evaluate potential in vivo 'pre-targeting' applications, the
pharmacokinetics of Biotin-Cy5 was determined under pre-targeting conditions
by
the non-invasive optical imaging technology of the eye of animals as described
in
Example 10. In these experiments, the presence of Cy5 was determined non-
invasive by optical imaging of the eye of animals, which revealed the
fluorescence
of Cy5 in the capillaries. The Cy5-mediated fluorescence values that we
detected in
the eye of mice 10 min. after injection of Biotin-Cy5 were set as 100 % value,
and
fluorescence values measured at subsequent time points were expressed relative
thereto. In this experiment, 1 mg antibody (either anti-Biotin antibody or
anti-
Biotin antibody-Cys (=Cys-mutant of anti-Biotin antibody)) was applied 24
hours
before injection of Biotin-Cy5 and start of the eye imaging. The control group
was
not pre-injected with the anti-biotin antibody.
The results of these experiments are shown in Figure 11: injection of Biotin-
Cy5
into animals that did not receive pre-injected antibody was eliminated with a
low
serum half-life and low exposure levels (diamonds). The serum levels and half-
life
of Biotin-Cy5 that was injected into animals with 24 hours pre-injection of
anti-
Biotin antibody (without Cys mutation) were greatly increased. This shows that
the
antibody captures its antigen (with the payload) in the circulation, and
prolongs the
antigen's (and likewise of the conjugated payload) serum half-life. The
relative
serum level and half-life of Biotin-Cys-Cy5 that was injected into animals
that
were 24 hours pre-injected with the anti-Biotin antibody-Cys (i.e. an antibody
containing the Cys mutation as reported herein for covalent payload coupling)
were
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 108 -
even further increased. In these samples, the relative Cy5 levels were not
only
higher than those of non-complexed compound, but also higher than the levels
of
complexed (but not disulfide-bonded) Cy5. Thus, hapten-complexed disulfide-
linked payloads (which are formed under pre-targeting conditions in vivo) are
more
stable in the circulation, and can reach higher exposure levels, than non-
covalent
complexed payloads.
Example 7
Polypeptides in conjugates and in complexes with anti-hapten antibody retain
functionality
We have previously shown that polypeptides which are part of non-covalent
hapten-polypeptide conjugates and in complexes with anti-hapten antibodies
retain
functionality (W02011/003557, WO 2011/003780 and PCT/EP2011/074273). To
demonstrate that coupled peptides retain functionality also upon covalent
disulfide-
coupling, the biological activity of anti-digoxigenin antibody complexed
polypeptides and their disulfide-conjugates with anti-digoxigenin antibody
VH52bC were compared.
The therapeutically desired functionality of PYY-derived peptides is binding
to and
interfering with the signaling of its cognate receptor NPY2. Signaling via the
NPY2 receptor is involved in and/or regulates metabolic processes.
To evaluate whether complexation or SS-conjugation of the polypeptide Dig-PYY
with the anti-digoxigenin antibody or the conjugation of the polypeptide Dig-
Cys-
PYY with the anti-digoxigenin antibody VH52bC , respectively, affect its
activity,
we evaluated its ability to inhibit the Forskolin stimulated cAMP accumulation
in
HEK293 cells expressing the NPY2 receptor (cAMP assay).
The following Table 2 shows the results of cAMP-assays that were performed to
assess the biological activity of PYY(3-36), its Y2receptor specific modified
analog moPYY, its antibody-complexed Dig-variant and its disulfide-conjugated
Dig-Cys-derivative.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 109 -
Table 2.
day 1 day 2
sample EC50 [nM] EC50 [nM]
PYY,t 0.09 0.1
moPYY 0.14 0.15
moPYY(Cys-Dig)-disulfide conjugated-anti- 5.38 5.33
digoxigenin antibody VH52bC
moPYY(Dig) - anti-digoxigenin antibody 9.26 12.55
complex
For the cAMP agonist assay, the following materials were used: 384-well plate;
Tropix cAMP-Screen Kit; cAMP ELISA System (Applied Biosystems, cat. #T1505;
CS 20000); Forskolin (Calbiochem cat. # 344270); cells: HEK293/hNPY2R;
growth medium: Dulbecco's modified eagle medium (D-MEM, Gibco); 10% Fetal
bovine serum (FBS, Gibco), heat-inactivated; 1 % Penicillin/Streptomycin (Pen
10000 unit/mL: Strep 10000 mg/mL, Gibco); 500 g/mL G418 (Geneticin, Gibco
cat. # 11811-031); and plating medium: DMEM/F12 w/o phenol red (Gibco); 10%
FBS (Gibco, cat. # 10082-147), heat-inactivated; 1 % Penicillin/Streptomycin
(Gibco, cat. # 15140-122); 500 g/mL G418 (Geneticin, Gibco, cat. # 11811-
031).
To perform the assay, on the first day, medium was discarded, and the
monolayer
cells were washed with 10 mL PBS per flask (T225). After decanting with PBS,
5 mL VERSENE (Gibco, cat#1504006) was used to dislodge the cells (5 min @
37 C). The flask was gently tapped and the cell suspension was pooled. Each
flask
was rinsed with 10 mL plating medium and centrifuged at 1000 rpm for 5 min.
The
suspension was pooled and counted. The suspension was resuspended in plating
medium at a density of 2.0 x 105 cells/mL for HEK293/hNPY2R. 50 microliters of
cells (HEK293/hNPY2R ¨ 10,000cells/well) were transferred into the 384-well
plate using Multi-drop dispenser. The plates were incubated at 37 C
overnight. On
the second day, the cells were checked for 75-85 % confluence. The media and
reagents were allowed to come to room temperature. Before the dilutions were
prepared, the stock solution of stimulating compound in dimethyl sulphoxide
(DMSO, Sigma, cat#D2650) was allowed to warm up to 32 C for 5-10 min. The
dilutions were prepared in DMEM/F12 with 0.5 mM 3-Isobuty1-1-methylxanthine
(IBMX, Calbiochem, cat#410957) and 0.5 mg/mL BSA. The final DMSO
concentration in the stimulation medium was 1.1% with Forskolin concentration
of
5 [tM. The cell medium was tapped off with a gentle inversion of the cell
plate on
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 1 1 0 -
a paper towel. 50 [LL of stimulation medium was placed per well (each
concentration done in four replicates). The plates were incubated at room
temperature for 30 min, and the cells were checked under a microscope for
toxicity.
After 30 min of treatment, the stimulation media was discarded and 50 4/well
of
Assay Lysis Buffer (provided in the Tropix kit) was added. The plates were
incubated for 45 min @ 37 C. 201AL of the lysate was transferred from
stimulation
plates into the pre-coated antibody plates (384-well) from the Tropix kit. 10
1AL of
AP conjugate and 20 1AL of anti-cAMP antibody were added. The plates were
incubated at room temperature while shaking for 1 hour. The plates were then
washed 5 times with Wash Buffer, 70 1AL per well for each wash. The plates
were
tapped to dry. 30 1AL /well of CSPD/Sapphire-II RTU substrate/enhancer
solution
was added and incubated for 45 min @ RT (shake). Signal for 1 sec/well in a
Luminometer. (VICTOR-V) was measured.
The results of these assays (Table 2) show that the modified peptide
derivative
moPYY has a neglectable lower activity than the wild-type PYY. The IC50 value
of
the cAMP assay was 0.09 nM for the wild-type PYY and 0.14 nM for the modified
analog. Covalent disulfide-conjugation resulted to a slight reduction in
biological
activity. The IC50 value was 5-36 nM for the conjugate. Surprisingly the
covalent
disulfide-conjugate is 2-fold more active than the non-covalent complex with
an
IC50 value of 10.91 nM.
Example 8
Serum stability of complexes of biotinylated Cy5 with humanized anti-biotin
antibody in comparison to covalent conjugates of biotinylated Cy5 with
humanized anti-biotin antibody VH53C
The objective of the described peptide modification technology is to improve
the
therapeutic applicability of peptides. Major bottlenecks for therapeutic
application
of peptides are currently limited stability in vivo and/or short serum half-
life and
fast clearance. The PK parameters of antibody conjugates of fluorophores were
determined in vivo and compare with the PK of non-covalent antibody-
fluorophore
complexes. Therefore, (i) the anti-biotin antibody VH53C was covalently
conjugated to the biotinylated fluorophore Biot-Cys-Cy5, (ii) a non-covalent
complex of the anti-biotin antibody with biotinylated fluorophore Biot-Cy5 was
generated, (iii) the covalently conjugated and the non-covalently complexed
compounds were applied to animals and (iv) the serum concentrations of the
compounds over time in these animals was measured by determination of the
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 111 -
fluorescence of Cy5 (A650), and that of the corresponding antibody by an ELISA
method that specifically detects the humanized antibody.
Experimental procedure
To analyze the influence on PK parameters of antibody-complexation of a small
fluorescent substrate, 13 nmol of Cy5-biotin/humanized anti-biotin antibody
VH53C-conjugate, or of the corresponding antibody non-covalently complexed
compound, or of the fluorescent compound alone, in 20 mM histidine / 140 mM
NaC1, pH 6.0 were applied to six female mice (strain NRMI) for each substance.
About 0.1 ml blood samples were collected after the following time points:
0.08 h,
4 h and 48 h for Mouse 1, 2, and 3 in a first group, and 0.08 h, 24 h and 72 h
for
Mouse 1, 2 and 3 in a second group. Serum samples of at least 50 1 were
obtained
after 1 h at RT by centrifugation (9300 x g, 3 min, 4 C). Serum samples were
stored at -80 C.
To determine the amount of compound in the serum at the given time points the
fluorescent properties of Cy5 are used: Cy5 related fluorescence in serum
samples
were measured in 120 1 quartz cuvettes at room temperature using a Cary
Eclipse
Fluorescence Spectrophotometer (Varian). Excitation wavelength was 640 nm,
Emission was measured at 667 nm. Serum samples were diluted in 1 x PBS to
reach an appropriate range of Emission intensity. Blood serum of an untreated
mouse in the same dilution in 1 x PBS as the respective sample was used as a
blank
probe and did not show any fluorescence signal.
Figure 5 shows the results of an analysis employing covalent conjugates, non-
covalent complexes and non-complexed hapten-Cy5. The data is shown as relative
(%) levels of Cy5-mediated fluorescence normalized to the (peak) serum levels
5min after injection. For a compound of rather small molecular weight, non-
complexed Biotin-Ser-Cy5 disappears rapidly from the serum. One hour after
injection, only 6 % of the fluorescence that was applied and detectable after
5
minutes in the serum was still detectable. At later time points, 2 hrs., 4
hrs. and 24
hrs. after injection, Cy5-mediated signals were not detectable.
Of the antibody-complexed compound four hours after injection, still approx.
50 %
of the fluorescence that was applied (5 min levels set to 100%) was detectable
in
the serum. Cy5-mediated fluorescence levels were also detectable at later time
points with approx. 22 % of the 5 min values detectable at 2 hrs. and approx.
12 %
detectable 48 hrs. after injection and 8% still detectable after 72 hrs. The
antibody-
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 112 -
conjugated compound shows a significantly longer in vivo half-life than the
antibody-complexed compound. Four hours after injection 58 % of the
fluorescence that was applied (5 min. levels set to 100 %) was still
detectable in the
serum (a factor of 1.16 higher than for the antibody-complexed compound).
After
24 hrs. 35 % (factor 1.6), after 48 hrs. 31 % (factor 2.6) and after 72 hrs.
26 %
(factor 3.3) of the Cy5-mediated fluorescence was detected in serum. The
comparable decrease of fluorescence for complexed and conjugated compounds in
the first 24 hrs. of the experiments can be accounted for the early
distribution
which is similar for complexes and conjugates. After 24 hrs. the in vivo
stability of
antibody-conjugated compounds is responsible for the difference.
To determine the amount of human IgG antibody in the serum at the given time
points, the following assay principle was used: human IgG1 antibodies in serum
samples were captured on a solid phase (Maxisorb0 microtiter plate, NUNC-
ImmunoTM) coated with an anti-human kappa-chain monoclonal IgG antibody.
Serum samples were diluted 1:105 and 1:106 and 100 1 of these dilutions were
added to the wells. After incubation, wells were washed 3-times with 300 I
PBS/0.05 % Tween 20 each. Detection of human IgG antibodies was carried out by
first adding 100 I of anti-human CH1-domain IgG which is digoxigenylated at
the
C-terminus at a concentration of 0.25 ug/ml. After washing 3-times with 300 1
of
1 x PBS/0.05 % Tween 20 each, 100 1 of anti-digoxigenin antibody Fab-fragment
conjugated to horse-radish peroxidase (HRP) was added at a concentration of 25
mU/mL. Finally, per well 100 1 of ABTS were added. After 30 min. incubation
at ambient temperature, the extinction (OD) was measured at 405 nm and 492 nm
11405/4921 in a commercial microtiter plate ELISA Reader (e.g. Tecan Sunrise).
Figure 5 shows the Bio-Cy5 serum levels as well as the serum levels of human
IgG
in mice treated with antibody-biotin-Cy5-complexes and -conjugates. The data
is
shown as relative (%) human IgG levels normalized to the (peak) serum levels
5 min. after injection. The relative human IgG serum levels of both antibody-
hapten-complexes and -conjugates are in-line with the relative fluorescence
measured for the antibody-hapten conjugates. Thus, the Biotin-Cys-Cy5 compound
shows a similar in vivo stability as the antibody it is conjugated to, which
means
that antibody-hapten conjugates stay intact in vivo. This is clearly not the
case for
antibody-hapten complexes for which the relative Cy5-mediated fluorescence
decreases faster than the relative human IgG serum levels. This means that the
complexes release the payload over time in vivo.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 113 -
In summary, the in vivo stability of haptenylated compounds is significantly
increased when bound by an anti-hapten antibody. However, antibody-hapten
complexes are not completely stable in vivo as the decrease of the hapten-Cy5
serum levels is faster than the decrease of antibody serum levels. This is not
the
case for antibody-hapten-Cy5 conjugates, which show a similar in vivo behavior
as
normal IgG antibodies.
Dig-Peptide serum kinetic (comparison of non-covalent complex and covalent
conjugate)
To analyze the influence on PK parameters of antibody-complexation and
antibody
conjugation of the digoxigenylated polypeptide, 32.1 nmol of the polypeptide,
or of
the corresponding antibody non-covalently complexed polypeptide in 20 mM
histidine / 140 mM NaC1 pH 6.0 were applied to 2 female mice (strain NRMI) for
each substance. The mice had a weight of 23 g and 25 g for MAK-DIG-PYY and
28 g and 26 g for DIG-PYY. About 0.1 ml blood samples were collected after the
following time points: 0.08 h, 2 h and 24 h for Mouse 1 and 0.08 h, 4 h 24 h
for
Mouse 2. Serum samples of at least 40 gl were obtained after 1 h at RT by
centrifugation (9300 x g, 3 min, 4 C). Serum samples were stored at -80 C.
The determination of the amount of digoxigenylated peptide in the serum at the
given time points was difficult compared to the detection of Dig-Cy5 as no
direct
means to detect the polypeptide in serum samples was available. Therefore, a
Western-Blot related assay to detect digoxigenylated peptide in serum was
established. In a first step, the serum samples were separated on reducing SDS-
PAGE. Because sample preparation included exposure of the serum to high
concentrations of SDS and reducing agents, complexed Dig-polypeptide
conjugates
can become released from the (completely denatured/unfolded) anti-digoxigenin
antibody, whereas covalently conjugates remained bound. To mediate the release
of the polypeptide from the non-covalent antibody complex and separate the
individual components by SDS-PAGE, 2 gl of each serum sample was diluted in
18 gl 20 mM histidine / 140 mM NaC1 pH 6.0, mixed with 6.7 gl of 4x LDS
sample buffer and 3 gl of 10x sample reducing agent (NuPAGE, Invitrogen) for
5 min at 95 C. As a control, 2 gl of serum of an untreated mouse of the same
strain
was used. Samples were applied to a 4-12% Bis-Tris Gel (NuPAGE, Invitrogen)
which was run at 200 V/ 120 mA for 20 minutes using 1xMES (Invitrogen) as a
running buffer. Subsequently, separated polypeptides were blotted onto a PVDF
membrane (0.22 gm pore size, Invitrogen) using the XCell Sure Lock Mini-Cell
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 114 -
system (Invitrogen) for 40 min at 25 V/130 mA. Membranes were blocked in 1 %
skim milk in 1 x PBS + 1 % Tween20 (PBST) for 1 h at RT. Digoxigenylated
polypeptides were subsequently detected on the membrane with an anti-
digoxigenin antibody. For that, anti-digoxigenin antibody was applied to the
membranes in a concentration of 13 ug/m1 in 10 ml of 1 % skim milk/PBST for 2
h
at RT. Membranes were washed for 3 x 5 min in 1 x PBST. Anti-mouse IgG Fab-
fragments coupled to POD from the LumiLightPLus Western Blotting Kit (Roche)
was applied in a 1:25 dilution in 10 ml of 1% skim milk/PBST for 1 h at RT.
Membranes were washed 3 x 5 min with 1 x PBST. Detection was carried out by
incubating the membranes in 4 ml LumiLight Western Blotting substrate for 5
min
at RT. Chemiluminescence was detected with the LumiImager Fl (Roche) with an
exposure time of 20 min.
The results of these analyses are shown in Figure 6A and 6B. The
presence/amount
of the digoxigenin polypeptide in murine serum at different time points has
been
determined. Mice that had received antibody complexed peptides (Figure 6 left)
showed strong signals at the earliest time point (5 min after administration).
These
signals were clearly assignable as shown by the size and location on the blot
of the
controls. In sera of mice that were treated with antibody-complexed
polypeptide,
polypeptide-associated signals were strongest at the early time points and
decreased over time. Nevertheless, polypeptide was still detectable with good
signals at all time points and even 24 hrs. after administration.
In mice that received non-complexed polypeptide, barely any signal associable
to
the small polypeptide was detectable even at the earliest time point. Figure 6
shows
in the right that under normal exposure conditions, no free polypeptide is
visible on
the blot. Contrast enhancement of the blot revealed the presence of some
polypeptide 5 min after administration, however only in trace amounts. At
later
time points, no defined polypeptide band was detectable.
It can be seen that non-complexed polypeptide has a very short half-life in
the
serum of mice. Mice that received the same polypeptides but in antibody
complexed form, show presence of these polypeptides in the serum for an
increased
period of time. Twenty four hours after injection polypeptide can be
determined in
the serum of these mice.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 115 -
Example 9
Serum half-life of covalently linked Digoxigenin-antibody complexes and
Digoxigenin-binding IgGs
To analyze if the covalent complexation further improves the PK-properties in
view of the non-covalently linked hapten complexes, the PK parameters of anti-
digoxigenin antibody-Digoxigenin-Cy5 complexes, as well as of the covalently
linked [anti-digoxigenin antibody-Cys]-[Digoxigenin-Cys-Cy5] conjugates were
determined in vivo. Therefore, Digoxigenin-Cy5 was determined using its
fluorescence (A650), and the corresponding antibody was determined by an ELISA
method that specifically detects the humanized antibody. Digoxigenin-Cy5 was
applied as low molecular weight 'surrogate' for hapten-coupled peptides
because
its fluorescent properties allow easy and accurate detection in the serum.
In the same manner as described for Biotin-Cy5 or Biotin-Cys-Cy5 (see Example
8), Digoxigenin-Cy5 or antibody-complexed or additionally antibody-Cys-linked
Digoxigenin-Cy5 were injected intravenously into female NRMI mice, followed by
collection of blood at 0.08 h, 2 h, 4 h and 24 h. The Cy5-mediated
fluorescence
values detected for/in both mice 5 min. after injection (t = 0.08 hrs.) was
set as
100 % value and fluorescence values measured at subsequent time points were
expressed relative thereto.
The results of these experiments demonstrate that for Digoxigenin-Cy5 less
than
10 % of the fluorescence that was applied (5 min. value) was detectable 2
hours
after injection. At later time points, 4 hrs. and 24 hrs., respectively, after
injection
no Cy5-mediated signals were detectable (see Figure 8). In contrast to non-
complexed compound, antibody-complexed compound was detectable at much
higher levels and at later time points (Figure 8). This indicates that
antibody
complexation significantly increases the serum half-life of the small compound
Digoxigenin-Cy5. Furthermore, covalently linked payloads display a greater PK
prolongation compared to the non-covalently linked complexes. A direct
comparison of the Digoxigenin-Cy5 levels and antibody levels indicated payload
loss from the antibody over time, with Cy5 levels decreasing faster than
antibody
levels. In contrast, covalently linked Digoxigenin-conjugates showed almost
identical Cy5 and IgG serum half-lives (Figure 8). This indicates that the
disulfide-
inked payloads remain stably connected to the antibodies while the non-
covalent
complexes dissociate over time.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 116 -
Example 10
Serum half-life and exposure levels of covalently linked hapten-antibody
complexes and complexes which are only attached via the hapten-binding site
To analyze if the covalent complexation improves the PK-properties of non-
covalently linked hapten complexes, the PK of a complex of anti-biotin
antibody
with Biotin-Cy5, as well as that of the covalently linked conjugate [anti-
biotin-
antibody-Cys]-[Biotin-Cys-Cy5] in vivo were determined. The presence of Cy5
was determined non-invasive by optical imaging of the eye of animals, which
revealed the fluorescence of Cy5 in the capillaries. The Cy5-mediated
fluorescence
values that we detected in the eye of mice 10 min. after injection was set as
100 %
value, and fluorescence values measured at subsequent time points were
expressed
relative thereto. The results of these experiments are shown in Figure 9: non-
complexed Biotin-Cy5 by itself has a low serum half-life and low exposure
levels.
Antibody-complexed compound which was not covalently linked was detectable at
much higher levels and with an extended half-life. Furthermore, covalently
linked
payloads displayed a greater PK prolongation, and higher serum levels compared
to
the non-covalently linked complexes. This indicates that hapten-complexed
disulfide-linked payloads are more stable in the circulation, and can reach
higher
exposure levels, than non-covalent complexed payloads.
Example 11
Peptide-complexation and covalent conjugation with antibodies that bind
different haptens
The application of hapten binding modules to couple haptenylated compounds
(= payloads) to targeting vehicles is one technical possibility by which
hapten-
mediated delivery can be realized. The concept can be expanded to further
haptens
or other entities that capture compounds and connect them to the targeting
module.
For example, for polypeptide delivery or stabilization, mono- or bispecific
antibodies that bind digoxigenin or other haptens can be applied to stabilize
and
PK-optimize therapeutic polypeptides.
Prerequisites for application as polypeptide capturing modules are (i) that
coupling
of compounds to the hapten does not severely interfere with polypeptide
activity
and (ii) the possibility of effective binding/complexation of the antibody to
haptenylated compounds.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 117 -
Hapten-directed binding is a prerequisite for the efficient covalent coupling
of
haptenylated dyes or polypeptides with an anti-hapten cysteinylated antibody.
To show that affinity-driven complexation of haptenylated compounds with anti-
hapten antibodies is a prerequisite for efficient disulfide-bond formation,
Biotin-
Cys-Cy5 was incubated with humanized anti-digoxigenin antibody and humanized
anti-digoxigenin antibody VH53C. Incubation of Biotin-Cys-Cy5 with humanized
anti-biotin antibody and humanized anti-biotin antibody VH53C was carried out
as
a control reaction.
0.13 mg of Biotin-Cys-Cy5 were dissolved in 100% DMF to a concentration of
10 mg/ml. 0.7 mg of each antibody was used in a concentration of 6.7 mg/ml
(about 46 M) in a buffer composed of 50 mM Tris-HC1, 1 mM EDTA, pH 8.2.
Biotin-Cys-Cy5 and antibodies were mixed at a 2.5:1 molar ratio (Ac-Biotin-Cys-
Cy5 to antibody) and incubated for 60 min at RT, shaken at 350 rpm. The
resulting
complex/conjugate was further analyzed by SDS-PAGE and subsequent detection
of Cy5-related fluorescence in the polyacrylamide-gel. 15 g of the
complex/conjugate were mixed with 4x LDS sample buffer (Invitrogen) and
incubated at 95 C for 5 min. Cy5-related fluorescence was recorded using a
LumiImager Fl device (Roche) at an excitation wavelength of 645 nm.
The non-reduced samples show covalent site-specific disulfide bond formation
for
humanized anti-biotin antibody VH53C (Fig. 36, lane 4) with very low
background
fluorescence signal when humanized anti-biotin antibody without a cysteine in
CDR2 was used (Fig. 36, lane 3). Biotin-Cys-Cy5 was also covalently coupled to
humanized anti-digoxigenin antibody VH52bC (Fig. 36, lane 2) with a low
background signal when humanized anti-digoxigenin antibody was used (Fig. 36,
lane 1), but with significantly lower efficiency. This can be deduced from the
excess Biotin-Cys-Cy5 that is detected on the bottom of the gel (arrows). In
the
case of humanized anti-digoxigenin antibody VH52bC significantly more
uncoupled Biotin-Cys-Cy5 can be detected (lane 2) than with humanized anti-
biotin antibody VH53C (lane 4).Upon reduction of the samples, no Cy5-related
fluorescence is detectable near the antibody heavy- and light-chains,
indicating that
the covalent linkage was indeed formed by a disulfide bridge. Coomassie stains
of
each gel show that the total amount of protein in each lane was equal.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 118 -
Example 12
Hapten-directed binding is a prerequisite for the efficient covalent coupling
of
haptenylated dyes or polypeptides with an anti-hapten cysteinylated antibody
To show that affinity-driven complexation of haptenylated compounds with anti-
hapten antibodies is a prerequisite for efficient disulfide-bond formation,
the non-
haptenylated peptide Ac-PYY(PEG3-Cys-4Abu-NH2) (Biosynthan 1763.1, SEQ
ID NO: 23) was incubated with humanized anti-digoxigenin antibody VH52bC and
humanized anti-digoxigenin antibody. 1.4 mg of Ac-PYY(PEG3-Cys-4Abu-NH2)
were dissolved in 100% DMF to a concentration of 10 mg/ml. 2 mg of each
antibody was used in a concentration of 11-13 mg/ml (about 75-89 M) in a
buffer
composed of 50 mM Tris-HC1, 1 mM EDTA, pH 8.2. Ac-PYY(PEG3-Cys-4Abu-
NH2) and antibodies were mixed at a 2.1:1 molar ratio (Ac-PYY(PEG3-Cys-4Abu-
NH2 to antibody)). The peptide was added in 3 portions while the solution was
stirred at 500 rpm with a stirrer bar. Between each addition, samples were
incubated for 5 min at 200 rpm. After addition of the last portion, samples
were
incubated for lh at RT and 200 rpm.
The resulting complex/conjugate was defined as 97% monomeric IgG-like
molecule and 3% dimeric soluble aggregates for the Ac-PYY(PEG3-Cys-4Abu-
NH2): humanized anti-digoxigenin antibody VH52bC conjugate and as 100 %
monomeric for the Ac-PYY(PEG3-Cys-4Abu-NH2): humanized anti-digoxigenin
antibody complex via size exclusion chromatography. Furthermore, the resulting
complex/conjugate was analyzed by mass spectrometry. For the Ac-PYY(PEG3-
Cys-4Abu-NH2): humanized anti-digoxigenin antibody VH52bC conjugate 17 %
of the detected species was identified as antibody coupled to 2 peptide
molecules,
51 % was identified as antibody coupled to 1 peptide molecule and 32 % was
identified as antibody without coupled peptide. For the Ac-PYY(PEG3-Cys-4Abu-
NH2): humanized anti-digoxigenin antibody complex 100% of the antibody was
uncoupled.
Example 13
Disulfide patterns that are required for formation of properly folded
functional hapten-binding antibodies with a cysteine mutation for covalent
payload coupling
Hapten-binding modules for covalent compound/payload coupling may be
composed of 'standard' antibodies such as IgGs which contain extra cysteines
that
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 119 -
enable covalent attachment of haptenylated compounds/payloads. The method as
reported herein introduces the required functionalities (cysteines) within
folded
domains, whose structure and sequence provide the basis for antibody
functionality.
Correct formation of defined disulfide bonds within as well as between the
domains of antibodies is essential for the formation and maintenance of the
correct
structure and functionality. To maintain the proper disulfide pattern, the
additional
cysteine that was introduced in the VH domain must be unoccupied and must not
interfere or react with neighboring cysteines. The fact that the VH52bCNH53C
position is located within the VH domain (and quite close to other cysteines)
aggravates the risk that incorrect disulfides may be formed during the
biosynthesis
of the heavy chain. Another potential problem is that VH and VL domains become
assembled within the secretory pathway to one Fv fragment. The secretory
pathway
involves redox-shuffling conditions and disulfide forming and ¨shuffling
enzymes.
Therefore, the potential to introduce incorrect disulfides by addition of the
VH52bCNH53C mutation may 'spread' also to disulfides of the light chain. This
does further enhance the risk to obtain/generate improperly folded non-
functional
molecules. It is therefore quite surprising that ¨ despite of these risks -
good
amounts of homogeneous functional antibody derivatives that contain the
VH52bCNH53C mutation could be expressed and obtained, and which are capable
to covalently connect to haptenylated compounds/payloads.
Example 14
Composition and generation of anti-hapten disulfide-stabilized single-chain Fv
fragments with a cysteine mutation for covalent coupling
Hapten-binding modules for covalent compound/payload coupling can consist of
'standard' antibodies such as IgGs. Alternatively, they may be modified
entities
such as recombinant Fv or Fab fragments, or derivatives thereof Single-chain
Fv
fragments are frequently applied as alternative to full lengths antibodies,
especially
in applications where small module size is required, or where additional
binding
modules are desired to generate bi- or multispecific antibody derivatives. One
example for anti-hapten Fv-derived entities that have been generated is a
disulfide-
stabilized single-chain Fv which bind to and covalently connects
digoxigenylated
compounds/payloads. The disulfide-stabilized single-chain Fv with Dig-binding
specificity was generated by connecting anti-digoxigenin antibody VH and VL
domains via a flexible Gly and Ser rich linker to each other. These VH and VL
domains harbored in addition cysteine mutations in positions 44 of VH and
position 100 of VL (positions according to Kabat et al.). These additional
cysteines
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 120 -
form a stable intermolecular disulfide bond between VH and VL. This stabilizes
the scFv, as previously described (e.g. Reiter, Y., et al., Nature
Biotechnology 14
(1996) 1239-1245).
In addition to that, another cysteine was introduced into the VH at position
52b or
53, respectively, according to the Kabat numbering to add the covalent linkage
functionality to the Fv fragment.
However, introducing such a mutation into disulfide-stabilized Fv fragments is
far
more challenging than placing them into full length antibodies. Single-chain
Fv
fragments are inherently less stable than full length IgGs or Fab fragments
because
they lack constant domains as stabilizing and heterodimerization forcing
entities.
Stability can be conferred by placing additional cysteine mutations into the
Fvs
such as the VH44-VL100 disulfide. However, this stabilizing principle works
only
if the disulfide forms at the correct positions between correct cysteines.
Thus, in
addition to defined intradomain disulfides (one in VH and one in VL), one
single
defined correct interdomain disulfide needs to be formed. Disulfide
connections
between non-matching cysteines will generate misfolded instable and non-
functional entities. Considering that a disulfide-stabilized Fv fragment
contains 6
cysteines, 21 different disulfide connections can theoretically be formed ¨
but only
the right combination of 3 defined disulfides will form a functional
stabilized
dsscFv. This challenge is aggravated upon addition of another free cysteine
into the
VH domain. The stabilized dsscFv that is desired contains two defined
intradomain
disulfides (one each in VH and VL), one defined interdomain disulfide (between
VH and VL), and furthermore one free cysteine for haptenylated
compound/payload coupling (in VH at position 52b/53). Considering that a
disulfide-stabilized Fv fragment with extra cysteine mutation for covalent
coupling
contains 7 cysteines, many different disulfide connections can theoretically
be
formed but only the right combination of the 3 defined disulfides, with the
exact
free cysteine position at VH52bNH53 will result in a functional stabilized
covalent
coupling competent dsscFv. One additional challenge is the fact that the
additional
free cysteine (VH52bNH53) is located in close proximity to the VH44 cysteine
which is not a naturally occurring cysteine but a mutation introduced for
disulfide
stabilization. VH44C is necessary for forming the correct inter-domain
disulfide,
and this disulfide most likely without being bound by this theory forms after
independent folding and assembly of VH and VL. Proximity of VH44C and
VH52bCNH53C aggravates the risk that the intradomain disulfide does not form
in a correct manner. But it has been found that functional disulfide
stabilized
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 121 -
single-chain Fv modules that bind digoxigenin and that are simultaneously
capable
to covalently connect to digoxigenylated payloads can be produced. The
sequences
that encode the light chain variable regions and the modified heavy chain
variable
regions of this Dig-binding dsscFv with the VH52bC mutation Fv antibody
derivative are listed under SEQ ID NO: 25 (VH) and the corresponding VL under
SEQ ID NO: 24. The successful generation of such dsscFv as modules for the
generation of bispecific antibody derivatives is described in the Example 15
(below).
Example 15
Composition, expression and purification of bispecific anti-hapten antibody
derivatives for targeted delivery of covalently coupled compounds/payloads
Bispecific antibodies were generated that contain hapten-binding antibody
modules
for covalent compound/payload coupling. These antibodies additionally contain
binding modules that enable targeting to other antigens. Applications for such
bispecific antibodies include specific targeting of haptenylated
compounds/payloads to cells or tissues that carry the targeting antigen. One
example for such molecules that was generated is a bispecific antibody with
binding regions that recognize the tumor associated carbohydrate antigen LeY,
and
simultaneously with disulfide-stabilized Fvs which bind and covalently connect
digoxigenylated compounds/payloads. Therefore, disulfide-stabilized single-
chain
Fvs were connected via flexible Gly and Ser rich connector peptides to the
C-termini of the CH3 domains of a LeY antibody, resulting in tetravalent
molecules with two LeY binding arms and additionally two digoxigenin binding
entities. The digoxigenin-binding entities harbored a VH44-VL100 disulfide
bond
which has been previously described (e.g. Reiter, Y., et al., Nature
Biotechnology
14 (1996) 1239-1245). The digoxigenin binding entity contained in addition the
VH52bC mutation for covalent coupling. The sequences that encode the light
chain
and the modified heavy chain of this LeY-Dig antibody derivative are listed
under
SEQ ID NO: 17 and SEQ ID NO: 18.
The bispecific molecules were generated by molecular biology techniques,
expressed by secretion from cultured cells, subsequently purified from culture
supernatants in the same manner as described above. Thus, bispecific
antibodies
which contain targeting modules as well as modules for covalent coupling of
haptenylated compounds/payloads can be generated and purified to homogeneity.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 122 -
Example 16
Helicar motif amino acid sequence containing peptide YY
Peptide YY is a short (36-amino acid) peptide released by cells in the ileum
and
colon in response to feeding. In humans it appears to reduce appetite. The
most
common form of circulating PYY is PYY3_36, which binds to the Y2 receptor
(Y2R) of the Y family of receptors. PYY is found in L cells in the mucosa of
gastrointestinal tract, especially in ileum and colon. Also, a small amount of
PYY,
about 1-10 %, is found in the esophagus, stomach, duodenum and jejunum. In the
circulation, PYY concentration increases after food ingestion and decreases
during
fasting. PYY exerts its action through NPY receptors; it inhibits gastric
motility
and increases water and electrolyte absorption in the colon. PYY and PYY
mimetics have been used to address obesity.
PYY was modified to comprise the helicar motif amino acid sequence and
complexed by an anti-helicar motif amino acid sequence antibody in order to
get
advantage of the pharmacokinetic properties of the antibody and to avoid the
intrinsic instability of the PYY.
Non-covalent complex formation
The structural investigation of the PYY3_36 peptide (Nygaard, R., et al.,
Biochem.
45 (2006) 8350-8357; SEQ ID NO: 26) reveals a helical motif (helicar-like
motif
amino acid sequence) for the central amino acids. As the N-terminal isoleucine
and
the modified C- terminus have been described as essential for the functional
activity of the peptide, the central helix was modified in order to reflect
the amino
acids in the helicar motif amino acid sequence.
PYY (3-36) 3 36
(SEQ ID NO. 26) IKPEAPGEDASPEELNRYYASLRHYLNLVTRQRYNH2
Helicar motif AHLENEVARLKK
PYY helicar IKPEAPGEDASPEAHLANEVARLHYLNLVTRQRYNH2
_
(SEQ ID NO: 27) (YNH2 = tyrosine amide)
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 123 -
binding soluble
[Ka] in PBS
PYY(3-36) - + PYY wild-type
(SEQ ID NO: 26)
PYY helicar 12 nM + helicar motif engineered
(SEQ ID NO: 27) PYY
The full IgG1 anti-helicar motif amino acid sequence antibody was produced in
HEK293 cells by transfecting two plasmids containing the variable regions of
the
heavy and the light chain inserted in a vector containing the constant human
IgG1
and the constant human lambda domain, respectively. The anti-helicar motif
amino
acid sequence antibody (0019) was purified by standard procedures using
protein A
chromatography. A mass spectroscopy experiment confirmed the identity of
antibody 0019.
The complex between antibody 0019 and the modified PYY peptide PYY helicar
was obtained in vitro by applying a small excess of the peptide to the
antibody
solution. The complex 0052 was formed. The stoichiometry of the complex was
determined by SEC-MALLS analytical experiments to be 1.6 peptides complexed
on one bivalent antibody.
The antibody 0019, the PYY(3-36) wild-type, the PYY helicar and the complex
0052 were tested for their effect on to the Y2Receptor family.
NPY2R NPY1R NPY4R NPY5R
Ac-Ile-Lys-Pqa-Arg-His-Tyr-Leu-Asn- 1.0 nM inactive inactive
inactive
Trp-Val-Thr-Arg-Gln-(NMe)-Arg-Try-
NH2 * 4 HOAc
PYY helicar 6.38 nM inactive inactive
inactive
(IKPEAPGEDASPEAHLANEVARLH
YLNLVTRQRYNH2) (SEQ ID NO: 27)
PYY(3-36)
0.05 nM 168 nM 162 nM 170 nM
(IKPEAPGEDASPEELNRYYASLRHY
LNLVTRQRYNH2) (SEQ ID NO: 26)
charge 1
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 124 -
NPY2R NPY1R NPY4R NPY5R
PYY(3-36)
0.05 nM 160 nM 131 nM 202 nM
(IKPEAPGEDASPEELNRYYASLRHY
LNLVTRQRYNH2) (SEQ ID NO: 26)
charge 2
anti-helicar motif amino acid sequence inactive inactive inactive
inactive
antibody (0019)
anti-helicar motif amino acid sequence 0.93 nM inactive inactive
inactive
antibody-PYY helicar complex (0052)
As demonstrated (Hoffmann, E., et al., J. Cont. Rel. 171 (2013) 48-56.) the
peptides complexed by an antibody have a prolonged half-life in vivo. Moreover
and surprisingly, the complex demonstrates a slightly better affinity for the
NPY2R
receptor compared to the non-complexed peptide; the antibody stabilizes the
polypeptide and presents the peptide in its fixed biologically active
conformation.
Covalent complex formation (covalent disulfide bond)
In order to increase the in vitro and in vivo stability of the complex between
the
anti-helicar motif amino acid sequence antibody and the helicar motif amino
acid
sequence containing compound, the formation of a disulfide bridge upon binding
has been used.
The first step is a specific recognition step (high affinity interaction),
i.e. the
formation of the helicar motif amino acid sequence containing compound-anti-
helicar motif amino acid sequence antibody complex. This is followed in the
second step by a spontaneous shuffling of a disulfide bridge to form the
stability
improved covalent complex.
As the 12-mer peptide (helicar motif amino acid sequence) is a relatively
rigid
entity (at least when complexed by a specific anti-helicar motif amino acid
sequence antibody) it has been found that a structurally specific design for
the
disulfide bridge has to be used. As the complex formation and the thereafter
effected covalent coupling is between two recombinantly produced entities, the
artificial cysteine residues introduced for the formation of a covalent
disulfide bond
are not produced necessarily as free cysteine residues but are expressed in a
reduced from, i.e. conjugated to a free cysteine or homo cysteine amino acid.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 125 -
The position in the amino acid sequence of the anti-helicar motif amino acid
sequence antibody variable domain where the artificial free cysteine residue
is
introduced is critical. A non-exposed cysteine in the antibody variable domain
amino acid sequence has more probability to be expressed as a free cysteine
(not
conjugated), whereas an exposed cysteine residue close to the binding pocket
can
abolish the binding of the 12-mer peptide (helicar motif amino acid sequence)
due
to a steric hindrance induced by the cysteine conjugation to an additional
moiety
like a free cysteine.
a) complexes with a helicar motif amino acid sequence containing fluorescent
compound
In order to identify a suitable position which has minimum risk of steric
hindrance
and strong affinity reduction, different positions for the introduction of the
artificial
cysteine residue in the helicar motif amino acid sequence have been tested.
The
cysteine residue has been introduced at the C-terminal end of the 12mer
(helicar
motif amino acid sequence) in order to have the major part of the paratope
unchanged. The peptides have been synthesized and fused to a fluorescent
motif.
wild- type : AHLENEVARLKK (SEQ ID NO: 01)
cysteine variant 1: AHLENEVARCKK (SEQ ID NO: 02)
-> AHLENEVARCKK(5-Fluo) -OH
cysteine variant 2: AHLENEVARLCK (SEQ ID NO: 03)
-> AHLENEVARLCK(5-Fluo)-OH x TFA
On the antibody, a structural design has been done to allow the formation of
the
disulfide bridge for both designed peptides including each a cysteine in
different
3D environment.
The 12-mer helical peptide AHLENEVARLKK (helicar motif amino acid
sequence) is modeled into the VH and the VH domains. At the C-terminus of the
peptide the residues L10 and Kll are identified as possible position and in
the light
chain variable domain the positions N55 and G51 according to the light chain
numbering of Kabat are identified.
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 126 -
The heavy chain variable domain of the anti-helicar motif amino acid sequence
antibody (0019) has the amino acid sequence:
QAVVTQEPSL TVSPGGTVTL TCGSSTGAVT TSNYASWVQQ KPGQAFTGLI
GGTNNRAPWT PARFSGSLLG GKAALTLSGA QPEDEAEYYC ALWYSNHWVF
GGGTKLTVL
(SEQ ID NO: 04).
The light chain variable domain of the anti-helicar motif amino acid sequence
antibody (0019) has the amino acid sequence:
DAVVTQESAL TTSPGETVTL TCRSSTGAVT TSNYASWVQE KPDHLFTGLI
GGTNNRAPGV PARFSGSLIG DKAALTITGA QTEDEAIYFC ALWYSNHWVF
GGGTKLTVL
(SEQ ID NO: 05).
The light chain variable domain N55C variant of the anti-helicar motif amino
acid
sequence antibody (0155) has the amino acid sequence:
DAVVTQESAL TTSPGETVTL TCRSSTGAVT TSNYASWVQE KPDHLFTGLI
GGTNCRAPGV PARFSGSLIG DKAALTITGA QTEDEAIYFC ALWYSNHWVF
GGGTKLTVL
(SEQ ID NO: 06).
The light chain variable domain N51C variant of the anti-helicar motif amino
acid
sequence antibody (0157) has the amino acid sequence:
DAVVTQESAL TTSPGETVTL TCRSSTGAVT TSNYASWVQE KPDHLFTGLI
CGTNNRAPGV PARFSGSLIG DKAALTITGA QTEDEAIYFC ALWYSNHWVF
GGGTKLTVL
(SEQ ID NO: 07).
i) Covalent conjugate of helicar motif amino acid sequence containing compound
with antibody 0155
The bivalent antibody 0155 is expressed in HEK293 cells similarly to its
parent
molecule Y2R(bck)-0019 without free cysteine. The modified antibody is
purified
using the same protocol used for antibody 0019. The mass spectrometry analysis
shows that the experimentally determined mass of the deglycosylated antibody
is
142,001 Da. This exceeds the calculated mass by 259 Da. The reduced chains
have
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 127 -
the experimentally determined mass of 48,167 Da (complete heavy chain,
calculated 48,168 Da, Cys = SH, C-Term= -K) and 22,720 Da (complete light
chain, N55C, calculated 22,720 Da, Cys = SH). The sequences of the chains were
confirmed after reduction.
Antibody 0155 was coupled to the helicar motif amino acid sequence cysteine
variant 2 using a 2.5 molar excess of helicar motif amino acid sequence
containing
compound in 100 % DMF to form the covalent complex 0156.
On the SDS page (denaturing condition, see Figure 12) the fluorescence is seen
only on the antibody 0155; in the reducing condition, only the small peptide
is
visible.
Results:
The covalent conjugation of the helicar motif amino acid sequence containing
fluorescent compound to the anti-helicar motif amino acid sequence antibody
was
successful. A total of about 43 % of the anti-helicar motif amino acid
sequence
antibody was covalently conjugated to two helicar motif amino acid sequences,
about 40 % of the anti-helicar motif amino acid sequence antibody was
covalently
conjugated to one helicar motif amino acid sequence, and about 17 % of the
anti-
helicar motif amino acid sequence was not conjugated.
The conjugate comprising two helicar motif amino acid sequences is modified to
about 50 %. This species has not been taken into account for the
quantification. As
already determined for the starting material the antibody without helicar
motif
amino acid sequence contains two modifications of about 128 Da. The antibody
conjugated to one helicar motif amino acid sequence has only one modification
of
about 128 Da.
ii) Covalent conjugate of the helicar motif amino acid sequence containing
compound with antibody 0157
Similarly to antibody 0155 is antibody 0157 expressed mostly as a
cysteinylated
form. The mass spectrometry analysis shows that the experimentally determined
mass of the deglycosylated antibody is 141,863 Da. This exceeds the calculated
mass by 3 Da. The antibody is mainly present as single or double
homocysteinylated form. The reduced chains have the experimentally determined
mass of 48,168 Da (complete heavy chain, calculated 48,168 Da, Cys = SH, C-
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 128 -
Term= -K) and 22,777 Da (complete light chain, N51C, calculated 22,777 Da, Cys
= SH). The sequences of the chains were confirmed after reduction.
The coupling of antibody 0157 with the helicar motif amino acid sequence
cysteine
variant 1 was not resulting in the expected covalent complex. The fluorescence
is
not seen in the expected lane but on the reference which should be negative in
this
experiment (see Figure 13).
Antibody 0157 was incubated with helicar motif amino acid sequence cysteine
variant 1. As control antibody 0019 was incubated with the same helicar motif
amino acid sequence cysteine variant 1.
Results:
The covalent conjugation of the helicar motif amino acid sequence containing
fluorescent compound to the anti-helicar motif amino acid sequence antibody
was
not successful. Without being bound by this theory it is assumed that in this
case
the antibody cysteinylation is too deep in the binding pocket to allow the
helicar
motif amino acid sequence containing fluorescent compound to bind efficiently
and
deliver the nucleophilic thiol group in an appropriate position to attack the
C51.
b) complexes with helicar motif amino acid sequence containing recombinant
polypeptide
The helicar based methodology becomes particularly attractive when considering
the formation of a covalent complex with a recombinantly produced helicar
motif
amino acid sequence containing polypeptide.
As the conjugation of the antibody 0155 containing the VL-N55C mutation with
the helicar motif amino acid sequence cysteine variant 1 (AHLENEVARLCK;
SEQ ID NO: 02) has much better performed compared to the alternative (G51C on
VL with helicar motif amino acid sequence cysteine variant 2
(AHLENEVARCKK; SEQ ID NO: 03)), the conjugation of 0155 with a helicar
motif amino acid sequence cysteine variant 1 containing polypeptide was
further
investigated. The polypeptide contained the helicar motif amino acid sequence
cysteine variant 1 (AHLENEVARLCK; SEQ ID NO: 02) fused to the N-terminus.
The helicar motif amino acid sequence cysteine variant 1 containing
Pseudomonas
exotoxin molecule LR8M with the C-terminal lysine residue deleted (0236; SEQ
CA 02930154 2016-05-10
WO 2015/101587
PCT/EP2014/079352
- 129 -
ID NO: 28) has been produced in E. coli and purified using a combination of
anion
exchange chromatography and SEC (see e.g. WO 2011/032022).
Antibody 0155 is covalently conjugated with the helicar motif amino acid
sequence
cysteine variant 1 containing Pseudomonas exotoxin molecule LR8M with the
C-terminal lysine residue deleted of SEQ ID NO: 28. The SEC chromatogram is
shown in Figure 14. The conjugation efficiency is analyzed by SDS-CE, Caliper,
for the non reduced samples (see Figure 15).
A total of about 4 % of the anti-helicar motif amino acid sequence antibody
was
covalently conjugated to two polypeptide of SEQ ID NO: 28, about 41 % of the
anti-helicar motif amino acid sequence antibody was covalently conjugated to
one
polypeptide of SEQ ID NO: 28, and about 55 % of the anti-helicar motif amino
acid sequence was not conjugated.
In conclusion, the anti-helicar motif amino acid sequence monoclonal antibody
can
be used to complex peptides, small molecules with peptidic linker, and
recombinant proteins via a high affinity recognition of a 12-mer helicar motif
amino acid sequence. Peptides with propensity to fold as helix can be modified
to
mimic the original 12-mer helicar motif amino acid sequence AHLENEVARLKK
(SEQ ID NO: 01) and are thereafter complexable with the anti-helicar motif
amino
acid sequence monoclonal antibody. In addition to the high affinity
complexation,
covalent conjugation is enabled with a cysteine variant of SEQ ID NO: 01
containing a cysteine and a modified anti-helicar motif amino acid sequence
antibody containing a cysteine in the CDRs via formation a stable disulfide
bond.
Recombinant proteins expressed by different system can be conjugated
afterwards
in vitro without particular reactions conditions but via spontaneous disulfide
bridge
shuffling.