Note: Descriptions are shown in the official language in which they were submitted.
CA 02930949 2016-05-25
N-CARBAMOYLPUTRESCINE TO ENHANCE MUSCLE PROTEIN SYNTHESIS
Description
[0001] The present invention relates to the field of food supplement and
medicament supporting
muscle metabolism, more precisely muscle protein synthesis. In particular, the
invention relates to a
composition comprising N-carbamoylputrescine (NCP). The invention further
relates to the non-
therapeutic use of N-carbamoylputrescine (NCP) to enhance muscle protein
synthesis in a subject.
Moreover, the invention also relates to N-carbamoylputrescine for its use as a
medicament.
[0002] Muscle metabolism is affected by many different processes such as
physical activity and age.
For example, muscle protein synthesis is increased by elevated level of
physical exercise, such as in
the case of athletes. Muscle protein synthesis is also known to be more
important in children during
growth than in adults. Indeed, during growth and until they are adults,
children gain a large mass of
muscle, predominantly of skeletal muscle rich in type I fibers, also called
slow twitch fiber rich
muscles. Muscle enrichment in type I fibers is favored by endurance exercise
and thwarted by
physical inactivity. Muscle enrichment in type I fibers is considered a
favorable factor in the
prevention of metabolic syndrome and related disorders.
[0003] Different products have been found that promote protein synthesis.
However, skeletal muscle
fibers are not all the same. Indeed, in comparison with fast twitch muscles,
slow twitch muscles tend
to have a low activity level of ATPase, a slower speed of contraction with a
less developed glycolytic
capacity. They have been demonstrated to have high concentrations of
mitochondrial enzymes, thus
they are fatigue resistant. Importantly, research has shown that protein
synthesis is differently
regulated depending on the muscle fiber type (Goodman et al.; PLoS One; 7(5);
2012). Despite this
very well-known fact, no product has been discovered yet that can increase
muscle protein synthesis
specifically in slow twitch fiber-rich muscles. Moreover, most of the existing
products have proven
adverse effects, and would probably not be safe for a child.
[0004] EP 2 583 566 for example discloses whey protein micelles, susceptible
to enhance muscle
mass and performance. However, while whey protein micelles represent an
optimized form of
protein supply, they do not promote protein synthesis on the long term and do
not specifically
improve protein synthesis of type I muscle fibers, nor muscle mass and
performance in slow twitch
fiber rich muscles. In addition, high doses of whey protein can cause some
side effects such as
increased bowel movements, nausea, thirst and may in the long term compromise
kidney function.
[0005] WO 2001/056402 discloses alpha lipoic acid-based food supplement able
to increase lean
muscle mass and strength, yet this increase does not seem to occur
specifically in slow twitch fiber-
rich muscles. Moreover, alpha lipoic acid may induce skin rash in humans.
Further, it is recommended
that people with diabetes be particularly cautious when taking alpha lipoic
acid, because it affects
blood sugar regulation.
[0006] Some products intended for increasing muscle mass claim to have low to
no side effects. For
instance, FR 2 907 011 discloses that the use of citrulline increases protein
synthesis in the muscle
with no side effect. Yet, the administration of citrulline does not appear to
enable an increase in
protein synthesis specifically in type I muscle fibers.
1
CA 02930949 2016-05-25
[0007] Therefore, to date, no product has been shown to be able to support or
enhance muscle
protein synthesis specifically in slow twitch fiber-rich muscles, with low to
no toxicity.
[0008] There is thus a need for improved compositions for enhancing muscle
protein synthesis in a
subject, which do not present adverse effects and target preferably skeletal
muscles.
[0009] On the other hand, most muscle-wasting associated disorders, and in
particular wasting which
occurs with ageing, have currently no cure, and patients would obviously
benefit from any
medication capable of preventing or treating those disorders.
[0010] Hence, there is also a need for medicaments for the prevention or
treatment of muscle-
wasting associated disorders.
Description
[0011] The inventors have found that the administration of N-
carbamoylputrescine (compound of
formula (I) hereafter) to mammals can enhance protein synthesis in muscle. The
inventors found that
the administration of N-carbamoylputrescine increases protein synthesis in
muscles, most
particularly in slow twitch fiber-rich muscles. Interestingly, no side effects
were noticed subsequently
to N-carbamoylputrescine administration.
[0012] This result was unexpected and unpredictable in view of the present
state of the art, since no
such properties have been disclosed so far for other compounds. Moreover,
while N-
carbamoylputrescine is known to be present in plants and bacteria (Janowitz et
al., FEBS Lett., 544(1-
3):258-61, 2003), there is no evidence of its presence in mammalian tissues
(Ramani et al.,Anal
Biochem,423(1):54-60, 2012). It was therefore very unlikely that it would play
a physiological role in
muscle protein synthesis.
[0013] The inventors moreover found that N-carbannoylputrescine can
advantageously be used either
for non-therapeutic purpose in healthy subjects, or for therapeutic purposes,
particularly in subjects
suffering from a muscle wastingassociated disorder, and for example for the
protection against the
loss of muscle mass and function.
[0014] A first object of the invention is thus a composition comprising N-
carbamoylputrescine and/or
one of its salts.
[0015] According to the invention, the terms "N-carbamoylputrescine" refer to
N-substituted
putrescine where the Nsubstituent is a carbamoyl group. Preferably, the terms
"N-
carbamoylputrescine" refer to the molecule 1-(4-aminobutyl) urea of formula
(I) (CAS Registry
Number: 6851-51-0).
0
NH.
(I)
2
CA 02930949 2016-05-25
[0016] N-carbamoylputrescine can easily be prepared by methods well known from
the skilled
person. In particular, N-carbamoylputrescine can be chemically synthesized
from putrescine (CAS
Registry Number: 110-60-1) and potassium cyanate (CAS Registry Number: 590-28-
3) by the method
described in Ramani et al. (Anal Biochem, 423(1):54-60, 2012). N-
carbamoylputrescine can also be
produced from barley leaves according to the process described by Smith and
Garraway
(Phytochemistry 1964;3;23-6).
[0017] Alternatively, N-carbamoylputrescine can be produced in microorganisms
expressing
recombinant arginine
decarboxylase and recombinant agmatine iminohydrolase but not the subsequent
enzymes of this
metabolism pathway (in particular N-carbamoylputrescine amidohydrolase and
putrescine
transcarbamylase). Synthesis by recombinant DNA techniques, are well known
from the person
skilled in the art, and are further thoroughly detailed in Sambrook et al.
(CSHL Press, 2001) and
Ausubel et al. (John Wiley & Sons, 1988).
[0018] According to the invention, the terms "salts of N-carbannoylputrescine"
refer to salts of N
carbamoylputrescine derived from inorganic and organic acids. Non-limitative
examples of acids
suitable for the invention include hydrochloric, sulfuric, fumaric, maleic,
phosphoric, glycolic, lactic,
succinic, tartaric, acetic, citric, methanesulfonic acids. The salts of the
invention can easily be
prepared by methods well-known from the art such as disclosed in WO
2012/040139 for instance.
[0019] The composition comprising N-carbamoylputrescine and/or at least one of
its salts can be
formulated according to any method known in the art, depending on its intended
use.
[0020] In a particular embodiment, the composition of the invention is
formulated for oral
administration. For example, for a more pleasurable and convenient
administration, the composition
can advantageously be formulated as an edible product, such as food or
beverage.
[0021] Preferably, the composition of the invention, formulated for oral
administration, is an edible
product. According to the invention, the terms "edible product" refer to
products and compositions
in any physical form which are intended to be consumed by human beings or
lower animals in whole
or part via the oral cavity.
[0022] Preferably, the edible product is chosen from the group consisting of
food products and
beverage products.
[0023] According to the invention, the terms "food product" refer to edible
products in a fluid, semi-
fluid or solid form. Preferably, the term food product encompasses all food-
based compositions, as
well as dietary supplements. According to the invention, the terms "beverage
product" refer to edible
products in a liquid form.
[0024] Most compounds that are taken orally are generally absorbed by the
cells of the intestinal
tract, which require that they overcome the acidic environment of the stomach.
In order to increase
intestinal absorption, the skilled person may use delivery vehicles suitable
for gastrointestinal
delivery. One particularly advantageous way to achieve that goal is to coat
the compounds of interest
with an outer enteric vehicle, also called enteric coating.
3
CA 02930949 2016-05-25
[0025] Thus, in a preferred embodiment, the edible product of the invention
comprises at least N
carbamoylputrescine or one of its salts, and an enteric vehicle.
[0026] By "enteric vehicle" it is herein referred to any vehicle that is not
degraded by the fluids and
enzymes in the stomach. According to the invention, suitable enteric vehicle
include pH-triggered
coatings, pressure-sensitive coatings or time-released coatings. Such coatings
are well known from
the skilled person, who can for instance further refer to US 5851579, and G.
Agyilirah and G. S.
Banker (Polymers For Enteric Coating Applications, P. J. Tarcha ed., CRS
Press, 1991). Preferably, an
enteric vehicle is a vehicle that is not degraded at a pH of 5 or below. More
preferably, an enteric
vehicle is a vehicle that is not degraded at pH from 5 to 2.
[0027] Moreover, the edible product of the invention can be improved by the
addition of further
edible compounds of interest, which may either enhance or complete the effect
of N-
carbamoylputrescine.
[0028] Preferably, the edible product according to the invention further
comprises at least one
compound selected from the group consisting of L-amino-acids, polyannines or
carbohydrate
compounds.
[0029] By "L-amino-acid", it is herein referred to an organic levorotatory
compound comprising
amine (-NH2) and carboxylic acid (-COOH) functional groups, along with a side-
chain specific to each
amino acid.
[0030] Preferably, the L-aminoacid according to the invention is selected from
the group consisting of
Histidine, lsoleucine, Leucine, Lysine, Methionine, Phenylalanine, Threonine,
Tryptophan, Valine,
Alanine, Arginine, Citrulline, Asparagine, Aspartic acid, Cysteine, Glutamic
acid, Glutamine, Glycine,
Ornithine, Proline, Serine, Tyrosine, Argininosuccinate, decarboxylated forms
thereof and
deaminated forms thereof.
[0031] By "polyamine", it is herein referred to an organic compound comprising
two or more primary
amino groups (-N H2)
[0032] Preferably, the polyamine according to the invention is selected from
the group consisting of
putrescine, spermine, spermidine, and agmatine.
[0033] By "carbohydrate compound", it is herein referred to an organic
compound that consists only
of carbon, hydrogen, and oxygen atoms. Preferably, the carbohydrate compound
has a
hydrogen:oxygen atom ratio of 2:1.
[0034] Preferably, the carbohydrate compound according to the invention is
selected from the group
consisting of glucose, fructose, galactose, and polysaccharides thereof.
[0035] By "polysaccharides of glucose, fructose, galactose", it is herein
referred to polysaccharides of
glucose, polysaccharides of fructose, polysaccharides of galactose,
polysaccharides of glucose and
fructose, polysaccharides of glucose and galactose, and polysaccharides of
galactose and fructose.
Preferably, the polysaccharide of glucose, fructose, galactose of the
invention is selected from the
4
CA 02930949 2016-05-25
group consisting of saccharose, maltose or lactose. Most preferably, the
polysaccharide of glucose,
fructose, galactose of the invention is saccharose.
[0036] In another embodiment, the composition of the invention is formulated
for topic
administration, more particularly for dermatological administration. Topic
administration can be
obtained by formulating the composition of the invention into forms suitable
for that use. For
instance, the composition of the invention can be formulated into cosmetic
compositions such as
gels, creams or lotions.
[0037] Preferably, the composition of the invention, formulated for topic
administration, is a topical
cosmetic composition.
[0038] By "topical cosmetic composition", it is herein referred to a solid,
liquid or semi-solid
composition, particularly intended for topic administration. Topical cosmetic
compositions according
to the invention can be in solid, liquid or semi-solid forms. Topical cosmetic
compositions in solid
forms can comprise for example powders, aerosols and plasters. Topical
cosmetic compositions in
liquid forms comprise for example lotions, liniments, solutions, emulsions and
suspensions. Cosmetic
compositions in liquid forms can comprise for instance ointments, creams,
paste and gels.
[0039] It is well known to the skilled person that absorption of an active
compound by the skin may
be enhanced by excipients, preferably chosen according to the contemplated way
of administration.
[0040] As it is intended for topical application, the composition of the
invention may further
comprise a cosmetically acceptable carrier or excipient, especially a carrier
or excipient suitable for
topical administration, that is to say a carrier or excipient compatible with
the skin.
[0041] By "cosmetically acceptable excipient" it is herein referred to
excipients suitable with a
cosmetical use, thus excipients compatible with the skin. The cosnnetical
excipient can for example be
chosen among excipients conventionally used in cosmetics, in particular
topical cosmetics, such as
pigments, dyes, polymers, surfactants, rheological agents, fragrances,
electrolytes, pH modifiers,
preservatives and mixtures thereof.
[0042] Another object of the invention is the non-therapeutic use of N-
carbamoylputrescine, or of
one of its salts, or of an edible product or of a topical cosmetic composition
according to the
invention, to enhance muscle protein synthesis in a subject.
[0043] According to the invention, the terms "non-therapeutic use" refer to a
use that does not allow
and/or is not
intended for the prevention nor for the treatment of a pathological state. By
"prevention" is herein
referred to the measures taken for reducing the risk of occurrence of the
pathological state under
consideration.
[0044] The inventors have found that the administration of N-
carbamoylputrescine is particularly
effective for enhancing protein synthesis in slow twitch fiber rich muscles.
[0045] According to the invention, the terms "enhance muscle protein
synthesis" refer to the
increase of protein synthesis in the overall muscle mass of the subject.
Preferably, the terms
CA 02930949 2016-05-25
"enhance muscle protein synthesis" refer to the increase of protein synthesis
in slow twitch fiber rich
muscles of the subject.
[0046] Thus, the invention also relates to the non-therapeutic use of N-
carbamoylputrescine, or of
one of its salts, or of an edible product or of a topical cosmetic composition
according to the
invention, to enhance protein synthesis in slow twitch fiber rich muscles in a
subject.
[0047] By "slow twitch fiber rich muscles", it is herein referred to muscles
rich in type I muscle fiber,
that is to say muscles which fibers comprise more than 50% type I muscle
fibers. By type I muscle
fiber, it is herein referred to muscle fibers comprising the myosin heavy
chain beta (MHC-B) isoform
encoded by the MYH7 gene (NCB! gene reference: 4625).
[0048] Enhancement of said muscle protein synthesis can be assayed by the
person skilled in the art
with stable isotope-based methods, such as described in Dangin et al.(J Nutr.;
132:32285-335; 2002).
[0049] A non-therapeutic use according to the invention can for example be the
enhancement of
physiological aspects or physiological reactions in the subject. Preferably,
the non-therapeutic use of
the invention is to increase muscle mass, muscle strength and/or muscle
performance.
[0050] According to the invention, the terms "increase muscle mass" refer to
the increase of the
overall muscle mass of the subject. The muscle mass can easily be evaluated by
the person skilled in
the art by computerized tomography (CT scan),such as described in Baracos et
al.(Am J Clin Nutr.
2010;91:11335-1137S).
[0051] According to the invention, the terms "increase muscle strength" refer
to the increase in the
force generated by a contraction of said muscle. The force generated by a
contraction can be
measured non-invasively for instance using hand-grip strength determination,
such as described in
Granger et al. (BMC Cancer. 2013;13:135).
[0052] According to the invention, the terms "increase muscle performance"
refer to the increase in
the efficiency of said muscle. In the context of the invention, the efficiency
of a muscle is defined as
the ratio of mechanical work output to the total metabolic cost. The
efficiency of a muscle can be
calculated from oxygen consumption, more particularly from variations of
oxygen consumption when
said muscle is contracted.
[0053] More preferably, the increase in muscle mass, muscle strength and/or
muscle performance is
an increase in muscle mass, muscle strength and/or muscle performance in slow
twitch fiber rich
muscles of the subject.
[0054] The inventors have found that the increase in protein synthesis, and
thus the increase in
muscle mass, muscle strength and/or muscle performance, correlates with an
increase in a specific
amino acid in the muscle of the animals: glutamine, which has been shown to
play a role in the
control of protein synthesis. Thus, the non therapeutic use of
Ncarbamoylputrescine hence directly
increases the concentration of this aminoacid in the muscles of the subject,
more precisely in slow
twitch fiber-rich muscles of the subject.
6
CA 02930949 2016-05-25
[0055] Preferably, the non-therapeutic use of the invention is to increase the
concentration of
glutamine in the muscles of the subject.
[0056] According to the invention, the term "subject" refers to animals.
According to the invention,
the term "animal" refers to members of the animal kingdom. Preferably,
according to the invention,
the term "subject" refers to mammals.
[0057] In an embodiment, the subject according to the invention is a human. It
is to be understood
that, in the context of the non-therapeutic use of the invention, the human
subject is a healthy
subject, more particularly a subject that does not have a muscle wasting-
associated disorder such as
herein defined.
[0058] Among humans, infants, children in the growing phase, and athletes have
in common an
elevated muscle protein synthesis: children because of their rapid growth and
buildup of muscle
tissue, athletes because of further building up muscle mass and performance.
Hence it is those
persons who will benefit the most from the non-therapeutic administration of N-
carbamoylputrescine. In these individuals, the administration of N-
carbamoylputrescine will support
and possibly enhance physiological protein synthesis rather than prevent or
treat a pathological state
of the subject.
[0059] Preferably, the subject according to the invention is a child,
preferably a growing child, or an
athlete.
[0060] According to the invention, the term "child" refers to a human between
the stages of birth
and puberty. According to the invention, the terms "growing child" refer to a
child whose growth is
not yet completed. The growth of a child can be evaluated by the bone
development of said child. A
child is considered as "grown up" when the carpal and tarsal bones have
matured. As a child grows,
some parts of the bones become calcified and appear on the x-rays. For
example, in a growing child,
the carpal and tarsal bones of the hands and feet have not yet completely
matured. On the x-rays,
they appear separated by a layer of invisible cartilage where most of the
growth is occurring. As sex
steroid levels rise during puberty, bone maturation accelerates. As growth
nears conclusion and
attainment of adult height, bones begin to approach the size and shape of
adult bones. The
remaining cartilaginous portions of the epiphyses become thinner. The child is
then considered
"grown up" when these cartilaginous zones become obliterated. Until that
stage, the child is
considered "growing".
[0061] According to the invention, the term "athlete" refers to a person
possessing the natural or
acquired traits, such as strength, agility and endurance that are necessary
for physical exercise or
sports, especially those performed in competitive contexts. Preferably, in the
context of the
invention, the term "athlete" refers to a human being practicing intense
physical exercise or sports
for at least 3 hours a week for at least two consecutive weeks. Metabolic
Equivalents (METs) are
commonly used to express the intensity of physical activities. MET is the
ratio of a person's working
metabolic rate relative to their resting metabolic rate. One MET is defined as
the energy cost of
sitting quietly and is equivalent to a caloric consumption of lkcal/kg/hour.
By intense physical
exercise or sport, it is herein referred to physical exercise of more than 6
METs. It is generally
admitted that 6 METs correspond to physical activities inducing a caloric
consumption of about 7
kcal/min or more for the subject.
7
CA 02930949 2016-05-25
[0062] The non-therapeutic use of the invention can also be beneficial for non-
human animals, for
example for those who are physically active, such as non-human animals taking
part in animal racing
games and competitions.
[0063] In another embodiment, the subject according to the invention is a non-
human animal,
preferably a non human mammal. Preferably, the term "non-human animal"
includes pigs, cows,
poultry, horses, dogs and cats. Even more preferably, the non-human animal
according to the
invention is a cat, a dog or a pig.
[0064] Preferably, in the non-therapeutic use of N-carbamoylputrescine, or one
of its salts, an
effective amount of said compound is provided to the subject. According to the
present invention, an
"effective amount" of a compound is one which is sufficient to achieve the
desired biological effect.
Thus, in the non-therapeutic use according to the invention, an "effective
amount" of N-
carbamoylputrescine, or one of its salts, is one which is sufficient to
enhance muscle protein
synthesis in a subject. It is understood that the effective amount will be
adapted by the skilled person
according to the usual criteria such as for example the age, sex, health of
the subject, and the phylum
(for example human) of the subject.
[0065] Preferably, in the non-therapeutic use according to the invention, N-
carbamoylputrescine, or
one of its salts, is provided to the human subject at a daily dose of at least
0.01 mg/kg of said subject.
More preferably, said daily dose is from 0.1 mg/kg of said subject to 1 mg/kg
of said subject.
[0066] Preferably, in the non-therapeutic use according to the invention, N-
carbamoylputrescine is
provided to the non-human subject in a daily dose of at least 0.01 mg/kg of
said subject. More
preferably, said daily dose is from 0.01 mg/kg of said subject to 10 mg/kg of
said subject.
[0067] In addition to this first non-therapeutic use, the inventors have
surprisingly discovered that N
carbamoylputrescine, or one of its pharmaceutically accepted salts, can
advantageously be used as a
medicament, and is particularly useful when administered to subjects suffering
from a muscle
wasting-associated disorder.
[0068] Another object of the invention is the use of N-carbamoyl putrescine,
or one of its
pharmaceutically accepted salts, for its use as a medicament.
[0069] Thus, the invention also relates to the use of compound of N-
carbamoylputrescine, or one of
its pharmaceutically acceptable salts, for the manufacture of a medicament.
[0070] By "pharmaceutically acceptable salt", it is herein referred to salts
derived from
pharmaceutically acceptable inorganic and organic acids. Examples of suitable
acids include
hydrochloric, sulfuric, nitric, fumaric, maleic, phosphoric, glycolic, lactic,
succinic, tartaric, acetic,
citric, and methanesulfonic acids.
[0071] In an embodiment, said medicament is intended for the prevention and/or
treatment of a
muscle wastingassociated disorder.
8
CA 02930949 2016-05-25
[0072] Thus, another object of the invention is N-carbamoylputrescine or one
of its pharmaceutically
accepted salts for use as a medicament in the prevention and/or treatment of a
muscle wasting-
associated disorder.
[0073] In addition, the invention also relates to a method for the prevention
and/or treatment of a
muscle wastingassociated disorder in a human subject in need thereof,
comprising administering an
effective amount of N-carbamoylputrescine, or one of its pharmaceutically
accepted salts, to said
subject.
[0074] According to the invention, the term "muscle wasting-associated
disorder" refers to disorders
comprising or caused by muscle atrophy. According to the invention, the terms
"muscle wasting"
refer to a decrease in the mass and function of said muscle. Muscle wasting
has been associated with
a large number of disorders, and may for example be present in cachexia, a co-
morbidity phenotype
which results from several common disorders, such as for example cancers,
AIDS, heart failure, COPD
(chronic obstructive pulmonary disease), renal failure, sepsis, severe burns
and any type of trauma,
including post-operative stress. Muscle wasting may also be present in
disorders or syndrome
associated with aging, such as sarcopenia.
[0075] Preferably, the muscle wasting-associated disorder according to the
invention is selected from
the group consisting of: cachexia, cancer, sepsis, chronic heart failure,
rheumatoid arthritis, acquired
immune deficiency syndrome, sarcopenia, diabetes, chronic renal failure, burn,
and head trauma.
[0076] Preferably, in the therapeutic use of N-carbamoylputrescine or one of
its pharmaceutically
accepted salts, an effective amount of N-carbamoylputrescine or of said salt
is provided to the
subject.
[0077] According to the present invention, an "effective amount" of N-
carbamoylputrescine or of one
of its pharmaceutically accepted salt is one which is sufficient to achieve
the desired biological effect.
Thus, in the therapeutic use according to the invention, an "effective amount"
of N-
carbamoylputrescine or of one of its salt is one which is sufficient for the
prevention and/or
treatment of muscle wasting in a subject. It is understood that the effective
amount will be adapted
by the skilled person according to the usual criteria such as for example the
age, sex, health of the
subject, and the phylum (for example human) of the subject.
[0078] Preferably, when N-carbamoylputrescine or one of its salts is used as a
medicament intended
for the prevention and/or treatment of a muscle wasting-associated disorder,
it is to be administered
at a daily dose of at least 1 mg/kg of said subject. More preferably, said
daily dose is from 1 mg/kg of
said subject to 40 mg/kg of said subject, even more preferably from 20 mg/kg
of said subject to 40
mg/kg of said subject.
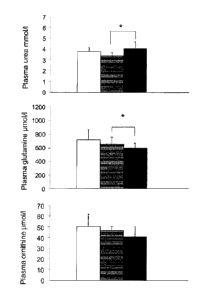
Figure legend
[0079]
Figure 1: Effect of administration of NCP on plasma urea, glutamine and
ornithine in rats.
[0080] Plasma concentrations of urea, glutamine and ornithine were measured in
rats untreated
(control) (white bars) and rats whose diet consisted of 5 mg/ day of NCP (gray
bars) for 10 days (thus
9
CA 02930949 2016-05-25
corresponding to 50 mg/kg of NCP (black bars) The results (mean SD) are
expressed in mmo1/1 urea
and pmo1/1 amino acids * P <0.05.
Examples
Reagents
[0081] Polyamines and other reagents were from Sigma-Aldrich (L'Isle d'Abeau
Chesnes, France).
[0082] N-carbamoylputrescine (NCP; cinoberinerm) was synthetized as described
in Ramani et al.
(Anal. Biochem., 2012, 423(1):54-60)
Animal
[0083] Eight-week old Sprague-Dawley rats (Charles Rivers, Lyon, France) were
used. They were
placed in individual cages and acclimatized for two weeks to our animal
facility. During this period
they received standard rodent chow (A04, UAR, Villemoisson-sur-Orge, France)
and water ad libitum.
[0084] Animal care and experimentation complied with French and European
Community regulations
for animal care and experimentation (Official Journal of the European
Community110 L 358,
18/12/1986). The study protocol has been approved by the Regional ethic
committee of Ile-de-
France.
Animal experimentation
[0085] After acclimatization twenty eight-month old rats were randomized into
three groups in order
to receive for two weeks a standard diet either alone (n=6; control group) or
supplemented with NCP
either 5 mg/kg/d (n=7, NCP5 group) or 50 mg/kg/d (n=7; NCP50 group). Their
weight, behaviour and
mortality were monitored throughout the feeding period.
[0086] At the end of the feeding period, the rats, in the fasted state, were
anesthetized by isoflurane
inhalation and euthanatized by decapitation.
[0087] Mixed blood was collected onto heparinized tubes and rapidly
centrifuged. The liver was
immediately removed and weighed, and a sample was cut off, frozen in liquid
nitrogen, and stored at
-80 C until analysis. For the jejunum and ileum, the intestine was washed with
cold NaC1 (0.9%) and
reverted. Thereafter the intestinal mucosa was scraped, rapidly frozen in
liquid nitrogen, and stored
at -80 C until analysis. Two muscles of the hindlimb, tibialis (rich in type
11 fibres) and soleus (rich in
type 1 fibres), and the right kidney were rapidly removed, weighed, frozen in
liquid nitrogen, and
stored at -80 C until analysis. Body composition was assessed by dissection
and lean mass (carcass),
visceral fat mass, sub-cutaneous fat mass (subcutaneous fat and skin) and mass
of the viscera were
determined.
[0088] Biological parameters studied were:
- in tissues: NCP, protein and amino acid contents
CA 02930949 2016-05-25
- in plasma: amino acids, liver function tests (plasma aspartate
aminotransferase, alanine
aminotransferase, alkaline phosphatase, and bilirubin), muscle enzymes (plasma
creatine
kinase, and lactate dehydrogenase), plasma electrolytes (sodium, potassium,
chloride,
bicarbonates), total protein, urea, creatinine, calcium, phosphate, magnesium,
uric acid,
glucose, cholesterol and triglycerides.
Results
General effects of N-carbamoyl putrescine
[0089] No rats showed abnormal general behavior, and there was no mortality in
the 3 groups during
the study.
[0090] All animals achieve normal weight gain and there was no difference
between rats receiving
NCP compared to controls (Table 1).
[0091] Analysis of body composition showed no difference between the groups.
Lean mass, fat mass,
either total, abdominal or cutaneous, mass of the viscera, and liver mass were
similar in the three
groups (data not shown).
Table 1: Influence of NCP on rat weight. Results are presented as mean SD
Control (n=6) NCP 5 mg/kg (n=7) NCP 50 mg/kg (n=7)
Initial weight (g) 263.3 11.4 264.9 16 264.9 8.2
Final weight (g) 314.2 10.7 313.1 18.1 316.1 13.9
Weight gain (g) 50.8 7.9 48.1 8.1 51.2 8.7
Daily weight gain (g/d) 3.63 0.57 3.44 0.58 3.65 0.62
Biological effects of N-carbamoylputrescine
[0092] No difference was observed between the three groups in terms of plasma
electrolytes levels
(sodium, potassium, chloride, bicarbonates, calcium, phosphate, magnesium), in
hepatic and
muscular enzymes (anninotransferases, creatine kinase, lactate dehydrogenase,
alkaline
phosphatase), in creatinine, glucose, cholesterol, triglycerides and uric acid
levels (data not shown).
Plasma urea was higher in NCP50 group vs NCP5 (Figure 1); however no
difference was shown
between the two NCP-supplemented groups compared to control rats.
[0093] Analysis of amino acid levels showed that plasma glutamine levels were
significantly lower in
the NCP50 group than in controls (Figure 1) and there was a trend for a NCP
dose-effect relationship
(p = 0.06). Plasma ornithine showed a similar dose-effect relationship (p =
0.055) (Figure 1). No
differences were observed for other amino acids. There was no correlation
between plasma urea and
plasma glutamine or ornithine.
[0094] While liver, kidney, tibialis, and jejuna! and Heal mucosa protein
contents were similar
between the three groups, protein level in soleus was higher in NCP5 vs
control group (Control: 12.7
1.8 g/100g, NCP5: 14.5 0.7 g/100g, NCP50: 14.0 0.8 g/100g; p=0.018 NCP5 vs
control).
11
CA 02930949 2016-05-25
,
[0095] Also, in soleus muscle, while the concentration of most amino acids
were similar between the
3 groups, the concentration of Glutamine, alanine and histidine significantly
increased with NCP
administration and there was a positive correlation for glutamine and alanine
with the dose of NCP
(p=0.015 and p=0.013 respectively)(Table 2)
Table 2 : Soleus amino acid content. Results presented as mean SD ; ANOVA
and plsd Fisher a#b : p<0
Amino acid (1.1.mol/g) Control (n=6) (1) NCP 5 mg/kg (n=7)
(2) NCP 50 mg/kg (n=6) (3)
Taurine 22972 3178 23882 2585 25223 1987
Aspartate 3889 612 4195 703 4742 1443
Threonine 481 75 512 109 571 67
Serine 1745 158 1933 384 1964 248
Asparagine 447 57 519 106 538 124
Glutamate 3757 474 3753 684 4074 390
Glutamine 6858 1008a 7605 1016ab 8547 988b
Glycine 1823 190 1932 516 2145 153
Alanine 1539 238a 1735 385ab 2025 182b
Citrulline 321 33 351 53 379 65
Valine 172 38 182 22 194 24
lsoleucine 79 25 83 17 91 22
Leucine 97 34 103 23 113 32
Tyrosine 114 19 106 15 126 21
Phenylalanine 77 14 75 19 77 11
Ornithine 73 21 75 12 74 15
Histidine 406 70a 415 50a 494 68b
Lysine 877 249 933 222 1009 168
Arginine 318 71 381 115 395 62
Proline 207 39 196 42 199 26
12