Note: Descriptions are shown in the official language in which they were submitted.
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
1
METHOD AND APPARATUS FOR CLASSIFICATION OF SEIZURE TYPE AND
SEVERITY USING ELECTROMYOGRAPHY
CROSS REFERENCE TO RELATED APPLICATIONS
100011 This
application claims priority to U.S. Provisional Patent Application No.
62/001,302 filed May 21, 2014, U.S. Provisional Patent Application No.
62/050,054 filed September
12, 2014, U.S. Provisional Patent Application No. 62/032,147 filed August 1,
2014, U.S. Provisional
Patent Application No. 61/979,225 filed April 14, 2014, U.S. Provisional
Patent Application No.
61/969,660 filed March 24, 2014, U.S. Provisional Patent Application No.
61/910,827 filed
December 2, 2013, and is a continuation-in-part of U.S. Patent Application
Setial No. 13/275,309
filed October 17, 2011, which claims priority to U.S. Provisional Patent
Application Serial No.
61/393,747 filed October 15, 2010 and U.S. Patent Application Serial No.
13/542,596 filed July 7,
2012, which claims priority to U.S. Provisional Patent Application Serial No.
61/504,582 filed
July 5, 2011. The disclosure of all of the above are herein fully incorporated
by reference.
BACKGROUND
100021 A
seizure may be characterized as abnormal or excessive synchronous
activity in the brain. At the beginning of a seizure, neurons in the brain may
begin to fire at a
particular location. As the seizure progresses, this firing of neurons may
spread across the
brain, and in some cases, many areas of the brain may become engulfed in this
activity.
Seizure activity in the brain may cause the brain to send electrical signals
through the
peripheral nervous system to different muscles the activation of which may
initiate a
redistribution of ions within muscle fibers. In electromyography (EMG), an
electrode may be
placed on or near the skin and configured to measure changes in electrical
potential resulting
from ion flow during this muscle activation.
100031
Techniques designed for studying and monitoring seizures have typically
relied upon electroencephalography (EEG), which characterizes electrical
signals using
electrodes attached to the scalp or head region of a seizure prone individual
or seizure patient.
Detecting an epileptic seizure using electroencephalography (EEG) typically
requires
attaching many electrodes and associated wires to the head and using
amplifiers to monitor
brainwave activity. The multiple EEG electrodes may be very cumbersome and
generally
require some technical expertise to apply and monitor. Confirmation of a
seizure typically
requires observation in an environment provided with video monitors and video
recording
equipment. Furthermore, when measuring brain activity with EEG, not all
measured activity
of or relating to a seizure may actually be manifested as an event that is
likely to be
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
2
dangerous. And, EEG data without video corroboration may not be suited to
grade or
differentiate some seizures, including those that may be weak or only of
minimal concern,
from other seizures that may be more dangerous.
100041 Unless
used in a staffed clinical environment, EEG equipment is
frequently not intended to determine if a seizure is in progress but rather
provide a historical
record of the seizure after the incident. And, that equipment is usually
designed for hospital-
like environments where a video camera recording or caregiver's observation
may provide
corroboration of the seizure, and is typically used as part of a more
intensive care regimen
such as a hospital stay for patients who experience multiple seizures. A
hospital stay may be
required for diagnostic purposes or to stabilize a patient until suitable
medication can be
administered. Upon discharge from the hospital, a patient may be sent home
with little further
monitoring. However, at any time after being sent home the person may
experience another
seizure, perhaps fatal.
100051 A
patient should in some cases be monitored at home for some length of
time in case another seizure should occur. Seizures with motor manifestations
may have
patterns of muscle activity that include rhythmic contractions of some, most,
or all of the
muscles of the body. A seizure could, for example, result in Sudden
Unexplained Death in
Epilepsy (SUDEP). The underlying causes of SUDEP are not well understood;
however, in
some cases, severe central nervous system depression may follow a seizure.
Following
central nervous system depression, breathings rates may increase and decrease
in a cycle that
may result in cardiac dysrhythmia and death. However, not all seizures have
the same
likelihood of causing or being associated with SUDEP, and in some patients,
some seizure
activity may be present without significant risk of SUDEP. And, without
differentiation of
seizures by type, severity or further classification, it may be difficult to
selectively identify
seizure activity that is most likely to be dangerous.
100061 While
there presently exist ambulatory devices for diagnosis of seizures,
they are EEG-based and are generally not designed or suitable for long-term
home use or
daily wearability. Other seizure alerting systems may operate by detecting
motion of the
body, usually the extremities. Such systems may generally operate on the
assumption that
while suffering a seizure, a person will move erratically and violently.
However, depending
upon the type of seizure, this assumption may or may not be true. Electrical
signals sent from
the brain during the seizure are frequently transmitted to many muscles
simultaneously,
which may result in muscles fighting each other and effectively canceling out
violent
movement. In other words, the muscles may work to make the person rigid rather
than cause
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
3
actual violent movement. Thus, the seizure may not be consistently detected
with
accelerometer-based detectors.
[0007] Accordingly, there is a need for an epileptic seizure detection
method and
apparatus that can be used in a non-institutional or institutional environment
without many of
the cumbersome electrodes to the head or extremities and that accurately
detects seizure
events with motor manifestations but that is not limited to responding to
violent motions.
There is still further, a need for epileptic seizure detection methods that
differentiate for
caregivers weak motor manifestation that may not demand an emergency response
from other
types of seizures including those may demand emergency intervention.
SUMMARY
100081 .A method of monitoring a patient for seizure activity including
collecting
EMG signal data; processing the collected EMG signal data to determine if a
detected event is
present; categorizing the detected event including risk stratification and
selecting, based, for example,
on the categorization and risk assessment, a transmission protocol included
among a group of
selectable transmission protocols. Upon selection of a transmission protocol
an appropriate
transmission, such as an alarm or warning message, may be sent to caregivers
and/or to designated
BRIEF DESCRIPTION OF THE DRAWINGS
100091 Fig. I illustrates one embodiment of a seizure detection system.
1000101 Fig. 2 illustrates one embodiment of a detection unit for a seizure
detection
system.
[0010] Fig. 3 illustrates one embodiment of a base station.
[0011] Fig. 4 illustrates one embodiment of a method for monitoring a
patient for
seizure related activity and selecting a protocol for alarm transmission.
[0012] Fig. 5 illustrates another embodiment of a method for monitoring
a patient
for seizure related activity.
100131 Fig. 6 illustrates an embodiment of a routine for analysis of EMG
signal
for seizure activity.
[0014] Fig. 7 illustrates an embodiment of a routine for analysis of EMG
signal
for seizure activity based on the detection of signal bursts.
[0015] Fig. 8 illustrates another embodiment of a routine for analysis
of EMG
signal for seizure activity based on the detection of signal bursts.
[0016] Fig. 9 illustrates an embodiment of a routine for analysis of EMG
signal
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
4
for seizure activity based on detection of a burst train.
100171 Fig. 10 illustrates an embodiment of a routine for analysis of
EMG signal
for seizure activity based on detection of burst periodicity.
[0018] Fig. 11 illustrates another embodiment of a method for monitoring
a
patient for seizure related activity and selecting a protocol for alarm
transmission.
[0019] Fig. 12 illustrates EMG signal data.
DETAILED DESCRIPTION
[0020] The apparatuses and methods described herein may be used to
detect
seizures and timely alert caregivers of seizure-related events. The
apparatuses may include
sensors attached to a patient or patient's clothing and may be configured for
measurement of
muscle electrical activity using electromyography (EMG). Detection of seizures
using EMG
electrodes is further described, for example, in Applicant's U.S. Patent
Application Nos.
13/275,309 and 13/542,596 and Applicant's U.S. Provisional Patent Application
Nos.
61/875,429, 61/894,793, 61/969,660, and 61/979,225 the disclosures of each of
which are
herein fully incorporated by reference. As described herein, apparatuses and
methods may be
used to monitor a patient for muscle electrical activity using EMG, detect
possible seizure
events, and stratify detected events based on risk, type, and/or severity. If
a detected event,
including, for example, a seizure of a given type or severity is deemed
present, the
monitoring system may then select a certain transmission protocol for warning
of one or more
caregivers. A transmission protocol may, for example, include sending either
of an alarm
message and/or EMG signal data over a network to one or more caregivers or
other
designated individuals.
[0021] in some embodiments, transmitted EMG data may be organized to
encourage verification or review of detected events. A caregiver may, for
example, in
response to detection of some events, be sent information to easily scan and
review time
and/or frequency domain EMG data. In addition to raw signal data, other
information
associated with analyzed EMG signal data may also be transmitted. For example,
in some
embodiments, one or more patterns typical of abnormal muscle movements may be
identified
from among noisy data, and the particular patterns identified may be
communicated to the
caregiver. Other statistical data related to detected events, including, for
example, statistical
data associated with detection of qualified peak data may also be transmitted
to a caregiver.
Tri some embodiments, that information may be sent and/or presented to a user
based on
system or user defined preferences.
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
[0022] In some
embodiments, risk stratification may facilitate transmission of
either or both of an alarm message and/or more data rich information, such as
time and/or
frequency domain EMG data. Moreover, stratification may facilitate selection
of transmission
protocols that minimize power consumption. And, in some embodiments, some
detected
events may be deemed suitable to be safely ignored or communicated to a remote
user as only
demanding a warning alarm status. For example, it may be deemed that a
detected event may
pose only minimal risk of SUDEP, injury from falling, and/or pose only minimal
risk from
other concerns. A caregiver may then, for example, be given a message that the
event was
detected but that an emergency response is not warranted and/or the event may
be logged in a
searchable database for post-hoc review.
[0023] Data
transmitted from a monitoring system, may, in some embodiments,
be customized for a particular individual or recipient group. For example,
transmitted data
may include, an alarm message, subset of statistical information related to
algorithm
detection, time or frequency domain EMG data, other data, and/or combinations
thereof.
And, that information may be useful to a certain subset of data recipients,
but it may not be
useful (or it may be detrimental) to send that information to other
recipients. For example, an
emergency medical technician (EMT) may be sent alarm information related to a
patient
including some sensor data, but the EMT may not be suitably trained to
interpret all EMG
data. And, sending that data may be a burden and/or confuse the caregiver
during an
emergency response. However, other caregivers, such as the doctor of a patient
with epilepsy
or remote individuals trained to more fully interpret EMG signal data, may be
sent a more
extensive portion of available data including, for example, information
suitable to reconstruct
the time dependence of the collected EMG signal or information suitable to
evaluate the
output of algorithms used for identification of one or more patterns of muscle
activity.
[0024] Data
sent from a monitoring system may be related to the status of a
detected event, including, for example, whether the detected event was
classified as an
emergency or warning event. Data may further be organized for transmission to
any of a
group of selected or designated individuals, including, in addition to
caregivers, any number
of other individuals such as, for example, chosen friends and family.
[0025] In some
embodiments, a monitoring system may send data to a remote
database or server. Designated individuals may have access to the data
included therein or to
a certain or restricted portion of data that is stored therein. To view data
an individual may, in
some embodiments, log on to a remote database and infommtion may be sent from
that
database to the individual. Therefore, data may, in some embodiments, be
presented to an
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
6
individual directly from a patient device (e.g., detection unit or base
station), from a remote
database, or from both sources.
[0026] In some
embodiments, methods herein may detect and classify weak
seizures or other events that may be identified, but may not warrant an
emergency. response.
That classification, may, for example, depend on detection of seizure
characteristics
selectively present in either of the tonic and/or clonic phases of a seizure.
Furthermore,
classification may, in some embodiments, include an analysis of the temporal
relationship
between seizure phases and/or attributes of detected EMG signal collected in
intermediate
periods between detected phases. For example, classification may, in some
embodiments,
include determining whether each of a tonic and clonic phase of a seizure are
detected and
whether those phases are present consecutively, such as with or without a
period of decreased
activity between them. And, in some embodiments, as further described herein,
risk
stratification may further include additional sensor and/or other data. For
example, in some
embodiments, risk stratification may include analysis of additional data such
as may be added
from one or more orientation, position, oxygen saturation, or pulse oximeter
sensors.
100271 Systems
described herein may be suitable for monitoring of a patient in an
ambulatory setting, and may include one or more EMG sensors that may be
coupled to skin
on or near one or more muscles of a patient. EMG signals may be collected in a
substantially
continuous manner, but it may be desirable to only send or alert a caregiver
of a subset of the
collected EMG signals. For example, particularly for mobile detection devices,
power
consumption for sending signal data may be significant, and it may, therefore,
be desirable to
limit an amount of the collected signal transmitted through a network. To
accomplish that
objective, risk stratification of detected events may, as described herein, be
used --- a
functionality that is notably absent from other monitoring systems.
[0028] In some
embodiments, thresholds suitable for detection of weak seizure
events may be set. And, those settings may be used without burdening a
monitoring system
with risk of an inordinate number of false positive detections. For example,
thresholds suited
for identification of weak seizure-related events may be set to identify those
events, but
because detected events may be automatically classified and appropriate
transmission
protocols selected only a subset of detection events may automatically
initiate an emergency
response. Therefore, the system may still warn a caregiver of the presence of
those events
and/or link those events to a searchable database, but inappropriate emergency
response or
false-positive-detections may be limited.
100291 EMG may
be ideally suited for this purpose for a number of reasons. For
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
7
example, while a great deal of information may be available from EEG collected
data,
electrical signals in the brain do not always correlate reliably with a true
seizure or with a
seizure of a given type or risk. And, looking at muscle motor manifestations
of brain activity
may provide a more accurate route to classification of seizures by severity
and type. And,
using EMG, as described herein, different parts of seizure activity may be
selectively
identified. For example, signal elevations in EMG may be transient or
sustained, and for
example, by selecting certain detection settings based on the width of
detected signals or
other factors, as also described, for example, in Applicant's Provisional
Application =No.
61/969,660, one may configure a detection routine to be selective for a
particular part of
seizure activity. Importantly, because different types of seizures may be
detected, seizure data
may be risk stratified based on whether parts most likely to demand a certain
response are
detected.
PM An
executed response to a detected event may be tailored based upon
characteristics of the detected event. For example, understanding whether a
sensor signal may
be related to a Tonic-Clonic, Tonic-only, Clonic-only, or other type of
seizure may enable
caregivers to better evaluate detected events and plan an appropriate
response. Furthermore,
some seizures may be brief and/or lack characteristic signatures of more
intense seizures,
such as repetitive motions that may occur in clonic-phase portions of a
seizure. At least some
of those seizures may be detected as an increase in magnitude of EMG signal or
detected
using other more sophisticated algorithms or devices, but while such seizures
may be
detected and may trigger an alarm, they may, for some patients, present only
limited or
insignificant risk of injury. For example, some detected events may generally
not pose a
significant risk of adverse effects of having a seizure including SUDEP. If
such detections are
made without further classification, unnecessary, and cost-prohibitive
signaling of alarms in
response to non-threatening events may be the only way to also respond to
potentially
dangerous events. Methods herein may alleviate such concerns by processing
data to
facilitate a tailored and more cost-effective strategy for patient monitoring
such as by
estimating whether individual detected events pose a significant risk of
adverse effects of a
seizure.
100311 In some
embodiments, methods herein may classify a detected seizure
based on seizure profiles for the patient or for a patient demographic. For
example, a detected
seizure may be classified based on various metrics, including, by way of
nonlimiting
example, type, intensity, seizure duration, duration of a seizure phase, other
metrics, and
combinations thereof. Classification of the severity of a seizure may, for
example, include
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
8
nonnalizing metrics of the seizure against values typical of a patient or
patient demographic.
For example, for a patient, if a measured magnitude of a detected
characteristic is some factor
of a previously measured value for the characteristic (e.g., during another
seizure for the
patient) or some factor of an average value for the characteristic that factor
may be used to
grade the seizures severity. For example, a certain seizure may be detected,
and the
characteristic detected may only have a magnitude that is only 50% (or some
other factor) as
great as in other seizures detected for the patient. That information may, for
example, be sent
to caregivers and/or otherwise used to determine an appropriate response. For
example, it
may be known that the patient may typically have a number of weak seizures and
that for that
patient risk of adverse effects of those seizures may be low. And, at least
some detected
events may be safely ignored or ignored in some situations. For example, if
the patient
experiences only a weak seizure and if the patient is known to be in bed
resting then risk of
both SIJDEP and risk of falling may be low. And, in some embodiments, at least
some
detected events may be ignored or may only be logged as an event that may not
need an
emergency. response.
100321 Along
with or in addition to alarm initiation, apparatuses and method
described herein may also be used to create a log of seizure events to help
medically or
surgically manage a patient. To facilitate organization of detected seizure or
possible seizure-
related events, events may be classified. For example, automatic
classification of seizure
events (e.g., based on type and/or severity) may be used in the creation of
ordered databases
of seizure-related data particularly where video corroboration of events is
absent or where
individual review of sizeable sets of data by trained professionals, such as
medical doctors,
would be inconvenient or prohibitively costly.
100331 In some
embodiments, methods herein may include identification of
regions of EMG signal including processed signal with elevated amplitude and
further
identify regions that are peaks (e.g., regions where signal amplitude,
including processed
signal amplitude, rises and falls). Peaks that rise and fall, and which
include regions of
elevated signal amplitude present for limited time periods of time may be
identified.
Identified peaks may, as further described herein, be qualified against one or
more properties
typically present in the clonic-phase of a seizure, and may be qualified to
increase selectivity
for detection of the clonic-phase of a seizure. For example, a peak may be
compared against
one or more properties of EMG signal data from one or more patients
experiencing a clonic-
phase of a seizure or compared against changes that occur during physiological
transformation into a clonic-phase and qualified to be similar to the
aforementioned clonic-
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
9
phase properties and/or changes. In this disclosure, such a qualified peak may
be referred to
as a "clonic-phase burst." The presence of a critical level of clonic-phase
burst activity may,
for example, be used to detect the presence of clonic-phase activity of a
seizure.
100341 in some
embodiments, methods herein may include identification of
regions of EMG signal including processed signal with elevated amplitude and
further
identify an initial group that are peaks (e.g., regions where signal
amplitude, including
processed signal amplitude, rises and falls). That set of identified peaks may
then be subject
to qualification as may be used to determine the presence of clonic-phase
bursts. Amplitude
may refer to either the magnitude of signal, or absolute value of magnitude,
as may be
appropriate for a given calculation and/or signal form. Signals collected may,
for example, be
rectified, and EMG signal amplitude may refer to the magnitude of rectified
signal from an
EMG sensor. In some embodiments, an EMG signal may be processed to isolate one
or more
frequency bands and the amplitude of a signal may refer to a magnitude of
signal isolated for
the one or more frequency band or to a magnitude of a statistical value
related to levels of
motor activity and processed from isolated signal in the one more frequency
bands. For
example, in some embodiments, a statistical value may be a T-squared
statistical value that is
related to levels of motor activity.
100351
Procedures for determining a group of peaks are described herein, but are
also described, for example, in Applicant's U.S. Patent Application No.
13/275,309, which
claims priority to Provisional Patent Application No. 61/875,429. In brief, in
some
embodiments, a peak-detection program may be executed to identify parts of EMG
signal
data that include one or more peaks. Identification of peaks may, for example,
including
detection of trailing and/or leading edges of peaks a procedure that may
include searching for
portions of EMG data or portions of smoothed EMG data where curvature of the
data
changes. For example, inflection or other critical points in a set of data may
be identified and
used to identify the presence of one or more peaks.
[00361
Qualification may then include identification or selection of peaks that
meet one or more criterion. For example, peaks may be selected that meet
criterion that
increase confidence that the peaks are properly ascribed to patterns
indicative clonic-phase
activity. For example, peaks may be qualified to be clonic-phase bursts. At a
high level,
procedures for qualification of peak data as including one or more clonic-
phase bursts may
include comparison of various peak properties to one or more qualification
thresholds. For
example, if, for a peak, one or more peak property values related to clonic-
phase activity
meets one or more qualification thresholds a qualification criterion may be
deemed satisfied
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
and the peak may then be referred to as a clonic-phase burst.
100371 Some
qualification procedures may operate on individual peaks. That is,
certain properties of a peak such as its height, area, or duration width may
be defined without
including data from other peaks. Therefore, individual values for the property
may be
calculated for each peak in a group. Other properties, as described below, may
be calculated
for more than one peak. Properties of individual peaks tnay include, for
example, peak height,
peak area, signal-to-noise ratio (SNR) (e.g., a ratio of peak amplitude to
estimates of
uncertainty in peak amplitude as may be measured or estimated from background
regions),
duration width, duration of intervening periods of lesser signal on either
side of a peak, other
properties of individual peaks and combinations thereof.
[0038] in some
embodiments of methods herein, each identified peak in an initial
group of peaks (e.g., a set prior to qualification) may be compared against
qualification
thresholds selected from the group of qualification thresholds including a
minimum duration
width, maximum duration width, minimum signal-to-noise ratio (SNR), minimum
duration of
one or more quiet or intervening periods on either said of a peak, maximum
duration of one
or more intervening periods on either said of a peak and/or combinations
thereof An
intervening period may be defined by the duration length of a region of signal
stability or low
amplitude (e.g., low signal variability, RMS noise or signal magnitude) which
may, for
example, be marked by the distance between a peak edge and a nearby region of
signal
increase in magnitude or decrease in signal stability. In some embodiments, a
signal-to-noise
ratio for a peak may be calculated using amplitude data for the peak and an
estimate or
calculation of signal noise. Noise may, for example, be determined by
calculating or
estimating a level of variation or uncertainty in a baseline signal (e.g.,
uncertainty in
measurement of signal amplitude, height, or area that may result from
fluctuations in EMG
data for a region not associated with peak activity of interest) which may,
for example, be
determined from data collected on either side of a peak or from a separately
measured portion
of an EMG signal such as a portion where a patient is at rest. To calculate
noise, for example,
signal may be collected and signal variability may be directly measured.
Alternatively, noise
may, for example, be estimated from a signal magnitude and an estimate of
variability
expected from variations typical of a signal of that magnitude as predicted by
one or more
model functions, including for example, a normal distribution model function.
In some
embodiments, an estimate of variations or uncertainty in a baseline signal or
noise may be
selected or calculated during one or more system calibration routines.
100391 in some
embodiments of peak qualification, a peak may be qualified as
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
11
clonic-phase burst by meeting a threshold SNR, by meeting a minimum threshold
for peak
duration width of about 25 to about 75 milliseconds, and by meeting a maximum
threshold
for peak duration width of about 250 milliseconds to about 500 milliseconds
activity. In some
embodiments, for a peak to qualify as a clonic-phase burst an intervening
sequence of
substantially quiet signal of about 50 milliseconds to about 300 milliseconds
may be detected.
[0040] Some
properties of peak data may be calculated for more than one peak.
And, in some embodiments herein, procedures for qualification of clonic-phase
bursts may
include comparison of a plurality of peaks to one or more qualification
thresholds. That is, a
plurality of peaks may be selected, an aggregate property value for the
plurality of peaks
determined, and the aggregate property value compared to one or more
associated thresholds.
[0041] A
qualification threshold value related to a property of a group of peaks
may be referred to as an aggregate qualification threshold value. For example,
included
among aggregate qualification threshold values that may be used to qualify a
plurality of
peaks are minimum andlor maximum rates of peak repetition and/or thresholds
for variations
in duration of times between peaks.
100421 In some
embodiments, a plurality of peaks may be qualified against a
threshold value for minimum repetition rate of peaks of about one peak per
second and a
threshold value for maximum repetition rate of peaks of about seven peaks per
second. In
some embodiments, for example, if a greater number or lesser number of peaks
than bounded
by the above thresholds is present over an appropriate interval (e.g., an
appropriate interval to
scale a num.ber of peaks as a peak rate), it may be deemed that the peaks may
not be properly
qualified.
100431 Included
among various metrics for characterizing variation in duration of
times between peaks is an average deviation percentage as also described in
Applicant's
related application U.S. 13/275,309. However, other metrics for characterizing
variability of
peak timing such as standard deviation, average deviation or percentage
deviation values are
also described therein. Any of the aforementioned metrics of a plurality of
peaks may be
calculated and may be used as aggregate property values comparable to
aggregate property
threshold values as described herein. In some embodiments, a plurality of
peaks may be
qualified if a minimum average deviation percentage value for time between
peaks is greater
than about 1% or about 5%. That is, an aggregate property threshold value of
minimum
average deviation percentage may, in some embodiments, be between about 1% to
about 5%.
In some embodiments, a plurality of peaks may be qualified as a plurality of
clonic-phase
bursts if a maximum average deviation percentage value for time between peaks
is less than
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
12
about 40% or about 500/0. Routines for determining variations duration of
times between
peaks are further explained in greater detail in various others of Applicant's
copending
applications incorporated herein by reference.
[0044] in some
embodiments, a procedure for peak qualification may include an
initial qualification step based on one or more criterion as described above
(e.g., criterion
based on individual peaks), removal of peaks that fail that initial
qualification, and another
qualification step based on calculation of one or more aggregate property
values for
remaining peaks (e.g., all peaks that meet the initial qualification). For
example, peaks may
be identified, some peaks removed from overall qualification (e.g., peaks may
be removed
because the peaks are too narrow or too wide), and then remaining peaks
qualified if the
remaining peak data as a whole meets one or more aggregate threshold
criterion.
[0045] A
variety of systems may be suitable for collecting large amounts of EMG
and other patient-related data, organizing such data for system optimization,
and for initiating
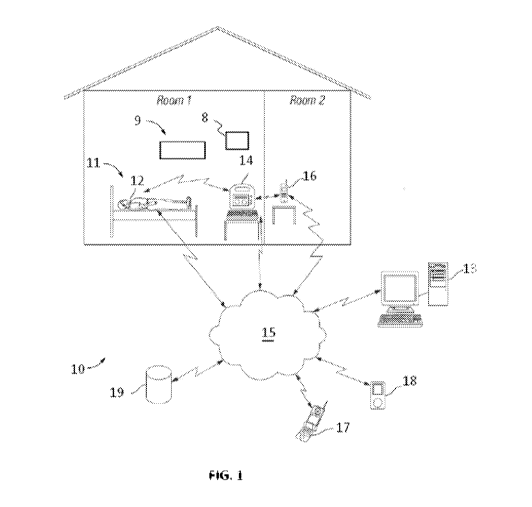
an alarm in response to a suspected seizure. Figure I illustrates an exemplary
embodiment of
such a system. In the embodiment of Figure 1, a seizure detection system 10
may include a
detection unit 12. The detection unit may be configured as a portable and
wearable device
disposed on or near (or even attached to) any suitable muscle or muscle groups
that may be
subject to motor manifestations during a seizure. And, in some embodiments,
the system 10
may include any of various wireless local area network technologies. For
example, a
detection unit 12 may communicate wirelessly to the internet using WiFi,
Bluetooth, or through
another local network. And, using a local network a detection unit 12 may, in
some embodiments,
send data over the internet directly or via an intermediate base station 14.
In some embodiments, a
caregiver may be contacted directly through a local network such as WiFi. A
base station 14 may be
connected to the internet wirelessly (such as through a local network), or may
be linked to the internet
through a hard connection. And, in some embodiments, in addition to a
detection unit 12 or in
addition to a detection unit 12 and base station 14, a system 10 may, for
example, include any of an
acoustic sensor 8, a video camera 9, alert transceiver 16, or combination of
the
aforementioned elements. The detection unit may comprise one or more EMG
electrodes
capable of detecting electrical signals from muscles at or near the skin
surface of a patient,
and delivering those electrical EMG signals to a processor for processing. The
EMG
electrodes may be coupled or attached to a patient, and may, in some
embodiments, be
implanted within the tissue of a patient near a muscle that may be activated
during a seizure.
Implanted devices may, for example, be particularly amenable for some patients
where EMG
signals may typically be weak such as patients with significant adipose
tissue. The base
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
13
station may comprise a computer capable of receiving and processing EMG
signals from the
detection unit, acoustic data from an acoustic sensor, and/or data from other
sensors, and
determining from the processed signals whether a seizure may have occurred,
and sending an
alert to a caregiver. An alert transceiver 16 may be carried by, or placed
near, a caregiver to
receive and relay alerts transmitted by the base station or to the intemet.
Other components
that may be included in the system 10, including for example, wireless device
17, 18, storage
database 19, electronic devices for detecting changes in the integrity of an
electrode skin
interface, and one or more environmental transceivers are also described in
Applicant's U.S.
Patent Application Nos. 13/275,309 and 13/542,596 and Applicant's Provisional
Application
Nos. 61/894,793 and 61/875,429.
100461 In using
the apparatus of FIG. 1, for example, a person 11 susceptible to
epileptic seizures may be resting in bed, or may be at some other location as
daily living may
include, and may have a detection unit 12 in physical contact with or in
proximity to his or
her body. The detection unit 12 may be a wireless device so that a person may
be able to get
up and walk around without having to be tethered to an immobile power source
or to a
bulkier base station 14. For example, the detection unit 12 may be woven into
a shirt sleeve,
may be mounted to an armband or bracelet, or may be an implanted device. In
other
embodiments, one or more detection units 12 or other sensors may be placed or
built into a
bed, a chair, an infant car seat, or other suitable clothing, furniture,
equipment and
accessories used by those susceptible to seizures. The detection unit 12 may
comprise a
simple sensor, such as an electrode, that may send signals to the base station
for processing
and analysis, or may comprise a "smart" sensor having some data processing and
storage
capability. A detection unit 12 may include one or more smart client
applications. In some
embodiments, a simple sensor may be connected via wire or wirelessly to a
battery-operated
transceiver mounted on a belt worn by the person.
100471 The
system may monitor the patient, for example, while resting, such as
during the evening and nighttime hours. If the detection unit 12 on the
patient detects a
seizure, the detection unit 12 may communicate via wire or wirelessly, e.g.,
via a
communications network or wireless link, with the base station 14, to a remote
cell phone or
other hand held or desktop device via bluetooth or simultaneously to a base
station and
remote cell phone or other device. A detection unit 12 may send some signals
to the base
station device for more thorough analysis. For example, the detection unit 12
may process
and use EMG signals (and optionally, or in some embodiments, ECG, temperature,
orientation sensors, saturated oxygen, and/or audio sensor signals) to make an
initial
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
14
assessment regarding the likelihood of occurrence of a seizure, and may send
those signals
and its assessment to the base station 14 for separate processing and
confirmation. If the base
station 14 confirms that a seizure is likely occurring, then the base station
14 may initiate an
alarm for transmission over the network 15 to alert a designated individual by
way of email,
text, or any suitable wired or wireless messaging indicator. It should be
appreciated that the
detection unit 12 may, in some embodiments, be smaller and more compact than
the base
station and it may be convenient to use a power supply with only limited
strength. Therefore,
it may be advantageous, in some embodiments, to control the amount of data
that is
transferred between the detection unit 12 and the base station 14 as this may
increase the
lifetime of any power supply elements integrated in the detection unit 12. In
some
embodiments, if one or more of the detection unit 12, the base station 14, or
a caregiver, e.g.,
a remotely located caregiver monitoring signals provided from the base
station, determines
that a seizure may be occurring a video monitor 9 may be triggered to collect
information.
[0048] The base
station 14, which may be powered by a typical household power
supply and contain a battery for backup, may have more processing,
transmission and
analysis power available for its operation than the detection unit 12, may be
able to store a
greater quantity of signal history, and evaluate a received signal against
that greater amount
of data. The base station 14 may communicate with an alert transceiver 16
located remotely
from the base station 14, such as in the bedroom of a family member, or to a
wireless device
17, 18 carried by a caregiver or located at a work office or clinic. The base
station 14 an(Vor
transceiver 16 may send alerts or messages to designated people via any
suitable means, such
as through a network 15 to a cell phone 17, PDA 18 or other client device. The
system 10
may thus provide an accurate log of seizures, which may allow a patient's
physician to
understand more quickly the success or failure of a treatment regimen. Of
course, the base
station 14 may simply comprise a computer having installed a program capable
of receiving,
processing and analyzing signals as described herein, and capable of
transmitting an alert. A
base station 14 may include one or more smart client applications. In other
embodiments, the
system 10 may simply comprise, for example, EMG electrodes as part of a device
configured
to transmit signal data to a smartphone, such as an iPhone, configured to
receive EMG
signals from the electrodes for processing the EMG signals as described herein
using an
installed program application. In further embodiments, so-called "cloud"
computing and
storage may be used via network 15 for storing and processing the EMG signals
and related
data. In yet other embodiments, one or more EMG electrodes could be packaged
together as a
single unit with a processor capable of processing EMG signals as disclosed
herein and
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
sending an alert over a network. In other words, the apparatus may comprise a
single item of
manufacture that may be placed on a patient and that does not require a base
station separate
transceiver. Or the base station may be a smartphone or tablet.
[0049] in the
embodiment of FIG. 1, the signal data may be sent to a remote
database 19 for storage. In some embodiments, signal data may be sent from a
plurality of
patients with epilepsy to a central database 19 and "anonymized" to provide a
basis for
establishing and refining generalized "baseline" sensitivity levels and signal
characteristics of
an epileptic seizure. The database 19 and base station 14 may be remotely
accessed via
network 15 by one or more remote computers 13 to allow updating of detector
unit andior
base station software, and data transmission. And, in some embodiments, the
remote
computer 13 or another computer may also serve to monitor exchange of data
including alarm
signals and EMG signal data between different devices associated with any
number of
designated individuals set to receive the signal. The base station 14 may
generate an audible
alarm, as may a remote transceiver 16 or detection unit 12. All wireless links
may be two-
way for software and data transmission and message delivery confirmation. The
base station
14 may also employ one or all of the messaging methods listed above for
seizure notification.
The base station 14 or detection unit 12 may provide an "alert cancel" button
to terminate the
incident warning.
[0050] In some
embodiments, a transceiver may additionally be mounted within a
unit of furniture or some other structure, e.g., an environmental unit or
object. If a detection
unit is sufficiently close to that transceiver, such a transceiver may be
capable of sending data
to a base station. Thus, the base station may be aware that information is
being received from
that transducer, and therefore the associated environmental unit. In some
embodiments, a
base station may select a specific template file, e.g., such as including
threshold values and
other data as described further herein, that is dependent upon whether or not
it is receiving a
signal from a certain transceiver. Thus, for example, if the base station
receives information
from a detector and from a transducer that is associated with a bed or crib it
may treat the
data differently than if the data is received from a transducer associated
with another
environmental unit, such as, for example, clothing typically worn while an
individual may be
exercising or an item close to a users sink where for example a patient may
brush their teeth.
More generally, a monitoring system may, in some embodiments, be configured
with one or
more elements with global positioning (GPS) capability, and position
information may be
used to adjust one or more routines that may be used in a detection algorithm.
For example,
GPS capability may be included along with or among one or more
microelectromechanical
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
16
sensor elements included in a detection unit.
100511 The
embodiment of FIG. I may be configured to be minimally intrusive to
use while sleeping or minimally interfere in daily activities, may require a
minimum of
electrodes such as one or two, may require no electrodes to the head, may
detect a seizure
with motor manifestations, may alert one or more local ani:Vor remote sites of
the presence of
a seizure, and may be inexpensive enough for home use.
[0052] FIG. 2
illustrates an embodiment of a detection unit 12 or detector. The
detection unit 12 may include EMG electrodes 20, and may also include, in some
embodiments, ECG electrodes 21. The detection unit 12 may further include
amplifiers with
leads-off detectors 22. In some embodiments, one or more leads-off detectors
may provide
signals that indicate whether the electrodes are in physical contact with the
person's body, or
otherwise too far from the person's body to detect muscle activity,
temperature, brain activity
or other patient phenomena. The detection unit may further include one or
elements 28, such
as solid state microelectromechanical (MEMS) structures, configured for
detection of
position and/or orientation of the detection unit. For example, an element 28
may include one
or more micromachined inertial sensors such as may include one or more
gyroscopes,
accelerometers, magnetometers or combinations thereof.
[0053] The
detection unit 12 may further include a temperature sensor 23 to sense
the person's temperature and one or more orientation or position sensitive
elements 28. Other
sensors (not shown) may be included in the detection unit, as well, such as
accelerometers,
microphones, and oximeters. Signals from electrodes 20 and 21, temperature
sensor 23,
orientation and/or position sensors 28 and other sensors may be provided to a
multiplexor 24.
The multiplexor 24 may be part of the detection unit 12 or may be part of the
base station 14
if the detection unit 12 is not a smart sensor. The signals may then be
communicated from the
multiplexor 24 to one or more analog-to-digital converters 25. The analog-to-
digital
converters may be part of the detection unit 12 or may be part of the base
station 14. The
signals may then be communicated to one or more microprocessors 26 for
processing and
analysis as disclosed herein. The microprocessors 26 may be part of the
detection unit 12 or
may be part of the base station 14. The detection unit 12 and/or base station
14 may further
include memory of suitable capacity. The microprocessor 26 may communicate
signal data
and other information using a transceiver 27. Communication by and among the
components
of the detection unit 12 and/or base station 14 may be via wired or wireless
communication.
[0054] Of
course, the exemplary detection unit of FIG. 2 may be differently
configured. Many of the components of the detector of FIG. 2 may be in base
station 14
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
17
rather than in the detection unit 12. For example, the detection unit may
simply comprise an
EMG electrode 20 in wireless communication with a base station 14. In such an
embodiment,
A-D conversion and signal processing may occur at the base station 14. If an
ECG electrode
21 is included, then multiplexing may also occur at the base station 14.
[0055] In
another example, the detection unit 12 of FIG. 2 may comprise an
electrode portion having one or more of the EMG electrode 20, ECG electrode 21
and
temperature sensor 23, in wired or wireless communication with a small belt-
worn
transceiver portion. The transceiver portion may include a multiplexor 24, an
A-D converter
25, microprocessor 26, transceiver 27 and other components, such as memory and
1/0
devices (e.g., alarm cancel buttons and visual display).
[0056] FIG. 3
illustrates an embodiment of a base station 14 that may include one
or more microprocessors 30, a power source 31, a backup power source 32, one
or more I/0
devices 33, and various communications means, such as an Ethernet connection
34 and
transceiver 35. The base station 14 may have more processing and storage
capability than the
detection unit 12, and may include a larger electronic display for displaying
EMU signal
graphs for a caregiver to review EMU signals in real-time as they are received
from the
detection unit 12 or historical EMG signals from memory. The base station 14
may process
EMG signals and other data received from the detection unit 12. If the base
station 14
determines that a seizure is likely occurring, it may send an alert to a
caregiver via transceiver
35.
[0057] Various
devices in the apparatus of FIGS. 1-3 may communicate with each
other via wired or wireless communication. The system 10 may comprise a client-
server or
other architecture, and may allow communication via network 15. Of course, the
system 10
may comprise more than one server and/or client. In other embodiments, the
system 10 may
comprise other types of network architecture, such as a peer-to-peer
architecture, or any
combination or hybrid thereof.
[0058] Figure 4
illustrates an exemplary embodiment of a method 40 of collecting
EMG signals, processing the signals to detect seizure events, and execution of
an alarm
transmission protocol based on the detected event. Detected events may, for
example, include
identification of a part of a seizure or identification of more than one parts
of a seizure. For
example, identification of a clonic-phase part of a seizure may be treated as
an event or
identification of two or more temporally correlated parts of a seizure such as
identification of
a tonic phase part and identification of a clonic phase part of a seizure may
be treated as an
event. That is, the aforementioned detections may be considered detection of
one tonic-clonic
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
18
seizure event. In some embodiments, more than one routine may be executed in a
monitoring
protocol including routines configured for detection of different parts of
seizure activity. For
example, in some embodiments, a first routine may be configured to be
responsive to tonic-
phase seizure activity and a second routine may be selectively responsive for
clonic-phase
seizure activity. And, by combining outputs from various routines different
events may be
detected.
[0059] In the
step 42, one or more EMG sensors may be used to monitor a patient
for seizure activity by collecting EMG signals. In some embodiments,
additional sensor data
may also be collected and used to determine risk of adverse effects of
detected seizure
activity. Signal data may, for example, be collected and analyzed using any of
the various
routines as further described herein. In the step 44, based on the responses
in the various
executed detection routines, different detection events may be identified. For
example, as
shown in Table l, various routines may give different output responses and
based on those
routine responses different events may be identified. in the step 46, detected
events may be
linked to one of several selectable alarm transmission protocols. That is, a
specific alarm
transmission protocol may be selected. The specific form of data determined
for transmission
may, for example, depend upon risk associated with a detected event. Data
determined for
transmission may, in some embodiments, take any of various forms including,
for example, a
form that includes any combination of an alarm message, subset of statistical
information
related to algorithm detection, as well as time and/or frequency domain EMG
data. As shown
in the step 48, a transmission protocol may then be executed, and if deemed to
be warranted,
appropriate data may be transmitted.
100601 In some
embodiments, one or more EMG sensors may be used to monitor
the patient for seizure activity and EMG signals may be collected and analyzed
for the
presence of one or more characteristics of seizure activity. For example, the
presence of a
certain characteristic of seizure activity may be determined by analyzing
collected EMG data
using one or more analysis routines. And, in some embodiments, to facilitate
risk
stratification, at least one routine may be selective for activity of a
particular part of seizure
activity. A routine may be "selective" for a characteristic present in a part
of a seizure and the
characteristic may be detected as a positive response of the routine in a
patient experiencing
that part of a seizure (or when transitioning into that part), but the
characteristic may be
substantially absent, undetectable, or give a substantially different output
response in the
absence of that part of a seizure. And, the likelihood that a positive
response to that routine
(or combination of routines) should properly be associated with the presence
of a certain part
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
19
of a seizure may then be established. Therefore, execution of one or more
selective routines
may encourage identification of a particular part of a seizure and
classification of a detected
seizure may then be based on the presence or absence of that seizure part.
Risk stratification
may then be made accordingly if, for example, the presence of that part of a
seizure is more
or less associated with likelihood of a patient experiencing adverse effects
of a seizure.
[0061] For
example, in some embodiments, one routine may analyze EMG data
for the presence of increased EMG amplitude or sustained increases in EMG
amplitude and
another routine may analyze EMG data for the presence of clonic-phase burst
activity. More
than one detection routine may run simultaneously, and in some embodiments, a
detected
event may be identified based on whether a certain portion of collected EMG
signal data
exhibits a positive response in one routine or exhibits a certain combination
of responses in
more than one routine. And, in some embodiments, a detected event may involve
responses
from one or more routines wherein the routine responses are separated in time.
For example,
two or more routine responses from EMG data collected at different times may
be temporally
correlated and may be treated as being associated with a single detected event
as further
discussed below. Or, if suitably separated in time, two or more routine
responses may be
deemed to be separate events, and those separate events may then, for example,
be linked to
separate event responses.
[0062] A
routine for analysis of EMG signals with increased EMG amplitude
may, for example, include collecting signals over some period of time and
determining if the
collected EMG signal amplitude or an integrated value of signal amplitude
within one or
more time windows within that period is elevated over a certain threshold.
And, in some
embodiments, based, for example, on a number of time windows in which a
certain threshold
amplitude was achieved it may be determined if a level of signal elevation was
sustained for a
threshold duration. Threshold levels of EMG signal amplitude may, in some
embodiments, be
set to make that routine responsive to even weak muscle motor manifestations.
For example,
in some embodiments, a threshold setting tnay be established based on a
measurement of the
maximum signal amplitude an individual may provide during a voluntary muscle
contraction.
For example, for some patients, a value of about 2% to about 50% of a maximum
voluntary
value may be selected to capture weak motor manifestations. Other thresholds
may also be
used, but raising thresholds too high may limit responsivity of the routine to
some motor
manifestations.
[0063] In some
embodiments, signal amplitude may be scaled in units of standard
deviation above a baseline signal. For example, an evaluation of whether
sustained amplitude
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
elevations are present may include scaling the difference between a measured
signal
amplitude and a baseline signal amplitude in units of standard deviations and
assessment of
whether the number of standard deviations exceeds a threshold value (or Z-
factor) (in units of
standard deviations) and/or exceeds that factor over a certain time interval.
To be sustained a
signal or smoothed signal value may be required to maintain a threshold level
for at least a
critical number of times in a time interval or may be required to maintain the
threshold level
over the entirety of the time interval. To improve the signal-to-noise ratio
of a detected signal
it may generally be desired to integrate the signal for a certain interval of
time such as about
100 milliseconds to about 500 milliseconds. Selection of longer integration
intervals may
make the system less susceptible to random fluctuations and sources of signal
noise and may
improve overall detection sensitivity. Therefore, for some routines, including
some routines
responsive to weak motor manifestations and/or certain parts of tonic phase
activity, it may
be advantageous to integrate over windows of time that are relatively long
such as, for
example, by integration over windows of time on the order of hundreds of
milliseconds.
100641 However,
integration of EMG signal over significant durations generally
results in a loss of temporal resolution in the signal data, and routines
designed to be selective
for transient EMG activity typical of clonic-phase bursts (which may only last
for a period of
hundreds of milliseconds) may generally need to break up and measure EMG
signal
amplitude within shorter windows than used in other routines. For example, to
determine if a
signal fluctuates over an interval of time on the order of about a hundred
milliseconds one
needs to measure and analyze the signal at least some number of times over the
time period
of individual fluctuations. in some embodiments, a routine for detection of
clonic-phase
bursts may include identification of peaks and qualification of peaks that
meet criterion to be
qualified as clonic-phase bursts. For example, in some embodiments, EMG data
may be
sampled over various intervals suitable to detect the time variation of signal
during a clonic-
phase burst pattern. The data may be accessed using a peak detection program
and then
subject to qualification as may be used to determine if any identified peaks
may be deemed
clonic-phase bursts. For example, by selectively counting elevations qualified
based on
meeting minimum and/or maximum width requirements typical of clonic-phase
activity an
algorithm may be made selective for clonic-phase burst activity. That is, a
routine suited for
measurement of a characteristic of clonic-phase burst activity, such as clonic-
phase burst
count, may be configured to be selective for the clonic-phase of a seizure.
100651 As
described herein, by analysis of EMG data for signal elevations that
meet minimum and/or maximum width requirements, a routine may be made
selective for a
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
21
pattern that is characteristic of clonic-phase activity. Moreover, because the
clonic-phase of a
seizure may be correlated with seizure risk, including such a routine, i.e.,
one that is selective
for clonic-phase activity, may facilitate risk stratification. A more detailed
description of
some embodiments of routines selective for clonic-phase activity is included
further herein
and in the examples section at the end of this application. Those routines may
be responsive
when the clonic-phase of a seizure is present and/or tnay be responsive during
periods that
typically lead into the clonic-phase.
100661 In some
embodiments, the method 40 may include collecting and
analyzing EMG signals (step 42) for seizure activity using a routine
configured for measuring
sustained EMG activity and with settings and/or thresholds suited to provide a
positive
routine response if tonic-phase seizure activity is present. Another routine
may, in some
embodiments, include settings and/or thresholds suited for selective detection
of the clonic-
phase of a seizure. That is, the routine may indicate a selective response if
clonic-phase
activity is present. In the step 44, a method 40 may then deteintine, based,
for example, on
data from either or both of the aforementioned routines whether a detected
seizure event is
present.
[0067] If, for
example, a first routine is configured to be responsive to tonic-phase
seizure activity and a second routine is selectively responsive for clonic-
phase seizure
activity, an EMG signal may, for example, when analyzed, initiate a positive
first routine
response, but may not initiate a positive second routine response. Those
routine responses
may be used to classify the EMG signal as corresponding with a tonic-phase
detection event.
In some embodiments, a first routine may be associated with threshold settings
wherein a
positive routine response may be used to detect an event that may correspond
to either of a
tonic-phase seizure or weak motor manifestation including manifestations that
may not be
related to a seizure. And, for some patients, such events may only carry
minimal risk of
adverse effects of a having a seizure and may be given a warning event status.
For example,
in some embodiments, isolated detection of weak motor manifestations or tonic-
phase
activity may only qualify an event as demanding a warning status and an
associated warning
transmission protocol may be selected.
100681 In the
step 46, it may be determined if transmission of EMG data or an
event message is warranted. For example, an event may be assigned a warning
status and
may, in some embodiments, initiate a transmission protocol that sends a
message that a
warning event was identified. Such a message may, for example, be
advantageously
transmitted (as shown in step 48) using only minimal power consumption. The
message may,
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
22
in some embodiments, be limited to include a time stamp of the event and
describe that a
warning was reached or tnay include a description that a warning threshold was
reached
together with summary data including, for example, an amplitude value reached
or how long
a threshold value of amplitude was maintained. in other embodiments, more
extensive EMG
signal data may also be transmitted together with a warning message, but
generally for events
that pose only limited risk the extent of transmitted data may be limited.
[0069] A
monitoring system may also initiate transmission of an emergency
response such as may include, for example, an emergency message. For example,
in some
embodiments, each of the aforementioned routines (e.g., a first routine for
increased or
sustained activity and a second routine configured for transient EMG
activity), may be
responsive to an EMG signal collected during some collection time period and a
detected
EMG signal event may then be classified as associated with the presence of a
clonic-phase
portion of a seizure. That classification may, for example, initiate an
emergency response
message. That is, a detected clonic-phase event may be linked to alarm
protocol that includes
an emergency response. A clonic-phase event may also be deemed present if, for
example, an
EMG signal only responds to the second routine. To that point, a first routine
may, in some
embodiments, be responsive to tonic-phase activity, but may also respond to
clonic-phase
activity. However, lack of selectivity for that first routine may not prevent
selective
identification of seizure activity and classification based on the presence of
either the tonic or
clonic phase because such ambiguity may be resolved by consideration of the
other routine.
For example, because the second routine may be selective for clonic-phase
activity, the
second routine would not respond if only tonic-phase activity were present.
Therefore, both
tonic and clonic activity may, in some embodiments, be selectively detected.
100701 Based on
detection of clonic-phase activity, an event may be risk-
stratified, and a suitable transmission protocol then be selected as shown in
the step 46. For
example, in some embodiments, a clonic-phase event may be deemed an emergency
event
and a transmission protocol may be selected such as may include an alarm
message being
directly sent to EMT personnel. An ambulance may then be sent to the patient's
house or
another appropriate emergency response may be made. For example, another
caregiver may
alternatively or additionally be contacted and instructed to locate and check
on the patient. In
some embodiments, detection of a clonic-phase event may initiate a
transmission protocol in
which time and/or frequency dependent data is remotely sent to a system user.
The remote
user may then be given an option to evaluate the data, and may execute an
appropriate
response such as may include terminating an emergency response or initiation
of an
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
23
emergency message. If a remote user is sent data and required to actively
initiate an
emergency message, that data may be considered part of a warning protocol,
i.e., data or a
message that still requires initiation by a user to raise the event to
emergency status may be
considered herein as part of a warning protocol. In some embodiments, a
recipient may be
sent data, but after some time period, the system may automatically route an
emergency
message to a caregiver. Therefore, some transmission protocols may be
predetermined to
initiate contact with a caregiver and may be considered to be part of an
emergency protocol.
And, as used herein, unless otherwise noted, an "emergency transmission
protocol" may refer
to a transmission protocol where an emergency message is sent or predetermined
to be sent to
a caregiver including, but not limited to an EMT caregiver, with instructions
to physically
assist the patient, e.g., to actively move to the patient's location. And, in
some embodiments,
an emergency protocol may still be interrupted and canceled by a remote user.
[0071] Continuing with the example of a first routine configured for
identification
of increased or sustained EMG activity and a second routine configured for
identification of
transient EMG activity, in some embodiments, classification and risk
stratification for various
detected events may be selected as shown in Table 1.
[0072] Table I
Event Routine 1 Routine 2 Classification Status / Transmission Protocol
-- Status - Status
A) negative negative non-seizure no
transmission
B) positive negative tonic-phase event or warning
protocol (automatic message only)
tonic / non-seizure
event
C) negative positive clonic =--= phase event
emergency protocol (Que alarm message
and send data - enable review of EMG data
by a remote user and/or verification of
event status)
D) positive positive clonic --- phase event
emergency status (automatic message along
with EMG data to qualified individual)
[0073] Table 1 shows an embodiment of how responses for routines may be
combined and associated with seizure events, and may be applied for EMG data
collected at
about the same time; i.e., where each routine is applied to EMG data collected
at about the
same time. However, a monitoring system may also collect and analyze EMG
signal using
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
24
one or more routines that may be responsive to EMG signal data over time. And,
for
example, a routine or group of routines may respond to different portions of
EMG signal
separated in time. If the routine responses are derived from suitably isolated
EMG data, the
routine responses may be treated as corresponding to separate events. For
example, in some
embodiments, if two routine responses are associated with portions of EMG
signal data that
are separated in time from each other by greater than about 1 minute to about
10 minutes the
two routine responses may be treated as being from isolated events.
Individually detected
events may be classified based on risk, and an appropriate transmission
protocol linked to the
event. Likewise, in some embodiments, if two routine responses are separated
by less than a
certain duration they may, for example, be deemed to be temporally related and
considered
together as one event. That is, the temporal relationship between two or more
routine
responses associated with different portions of signal data may be determined,
and for
example, the signal data may be deemed to be part of a multi-stage seizure
event. For
example, routine responses associated with each of a tonic phase and a clonic
phase of a
seizure may be deemed temporally related and treated to likely be the result
of a single tonic-
clonic seizure event. For example, in some embodiments, if the responses of
one or more
routines shows that a tonic phase is identified and if, within about 2 seconds
to about 60
seconds after that identification is made, one or more routines show that a
clonic phase is
present a tonic-clonic seizure may be deemed likely. Detection of a tonic-
clonic seizure event
may then be treated as a single event for purposes of classification and risk-
stratification. For
example, a tonic-clonic event may, in some embodiments, be deemed to be an
event that
warrants execution of an emergency transmission protocol.
100741 The
systems herein may, in some embodiments, analyze collected EMG
signals based on combinations of responses derived from individual routines.
And, in some
embodiments, a number of responses to signal data, including combinations of
responses of
multiple routines over multiple time intervals, may be considered when
classifying detected
data based on risk.
[0075] In some
embodiments, if a number of routine responses are detected over
time the system may, for example, execute one or more additional actions. For
example, the
system may execute an algorithm suited to evaluate intervening periods between
positive
routine responses and examine the intervening periods for signatures of
different activity. In
some embodiments, the amplitude of EMG data in intervening periods between
positive
routine responses may be analyzed for the presence of EMG signal that may be
elevated,
including, for example, signal that may be elevated yet still below a
threshold level suitable
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
to trigger a response in individual routines. And, for example, based on
whether a certain
EMG signal level is or is not reached within one or more intervening periods
between the
responses, an algorithm may treat a series of responses in different ways. For
example, the
responses may be treated as individual events or different algorithms may
consider whether
the individual responses are likely to be part of one or more multi-stage
seizure events. And,
in some embodiments, patterns of multi-stage seizures tnay be based on
collected data for a
certain patient or certain patient demographic.
[0076I In some
embodiments, the amplitude of EMG data in intervening periods
between positive routine responses may be analyzed for the presence of EMG
signal that may
be either elevated or depressed. For example, some patients may experience a
series of
seizure events and following those events they may experience a general state
of central
nervous system depression (CNS). That depression may be related to a high risk
of SUDEP.
.And, if CNS depression follows a series of seizure events such may be
particularly important
to detect. And, some multi-stage algorithms may look for the presence of
multiple events that
pose a risk of SUDEP. In some embodiment, those algorithms may examine whether
signal
between routine responses meets any of various profiles that may reflect CNS
depression.
Such patterns may include the presence of a trend in relative signals for
baseline periods
between the responses. For example, variability and/or trends in the EMG data
may be
considered.
[0077] And in
some embodiments, data from either of saturated oxygen levels or
heart rate may also be considered. A change in heart rate or oxygen levels may
be a
particularly important consideration in determining the status of a patient.
However, for some
patients, a significant period of time with only minor suppression of heart
rate may be present
before rapid changes in heart rate during SUDEP. Likewise, oxygen saturation
levels may
change only slowly during the progression of SUDEP. Measurement of levels of
carbon
dioxide may be more diagnostic of the onset of SUDEP because those levels may
change
more rapidly during initial periods of SUDEP progression. However, measurement
of carbon
dioxide levels in an ambulatory setting may be difficult. And, by analyzing
the above sensor
elements together with EMG data detection of initial signatures of SUDEP may
be improved.
In some embodiments, EMG data may be used to trigger execution of or adjust
one or more
other sensor routines. For example, one or more responses may be used to
trigger collection
of data using a pulse oximeter or to adjust thresholds and sensitivity of
responses based on
heart rate and/or saturated oxygen levels. And, trends or variability in pulse
oximeter data
may then be considered in determining an overall response to the available set
of data. For
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
26
example, in some embodiments, an emergency event or several suitable warning
events
related by some intervening time interval may be used to trigger collection of
data using one
or more additional sensors including a pulse oximeter.
f0078] in some
embodiments, if over a certain time period, one or more
responses, including, for example, positive responses that may individually be
deemed
warning events, are detected, an appropriate message may be sent to a remote
user informing
the user of the presence of the one or more responses. The remote user may
then, for
example, be able to request that additional EMG information is transmitted
such as, for
example, transmission of data that may be used to display the time and/or
frequency
dependence of relevant EMG signals. In addition, the user may initiate any of
various
calibration procedures or other procedures as may be useful to further
evaluate the collected
EMG signal data andlor the state of sensors during a certain time period.
Other appropriate
actions may also be taken including calling the patient or another caregiver.
In some
embodiments, a monitoring system may treat a series of responses in a manner
that may
depend upon whether the system is in one of several different selectable
states. For example,
a patient may be given an option to select one or more different monitoring
settings based, for
example, on whether the patient is in bed sleeping, whether the patient is at
home alone, or if
the patient is at home with another person. And, depending on whether the
patient is in one or
another of those states, for a certain response or group of responses, a
monitoring system
may, for example, select a transmission protocol associated with either a
warning of
emergency status.
100791 The
method 40 may be useful in organizing whether, for a certain event, it
is desirable to transmit detected data and/or what form the transmitted data
may take. For
example, a transmission protocol may be selected that only sends a message of
the patient
status (and not bandwidth intensive time and/or frequency domain EMG data),
and a remote
user may be given the option to further analyze and review EMG data and
discount the data
or take one or more other actions as described herein. Importantly, at least
fur some detected
events, that analysis may take place before an emergency message is
transmitted. For
example, in some embodiments, further analysis or review may be required
before an
emergency message is sent or review may be enabled because an automatic alarm
message is
queued for transmission but not immediately sent. Because some patients with
epilepsy may
tend to trigger responses from non-seizure or weak events more or less often
and since the
cost and/or inconvenience of initiating an inappropriate response to an event
may be
substantial, risk stratification of events and review of events, including
those that may be
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
27
difficult to access, may be highly desired. In addition, in some embodiments,
analysis and
review of data may be made without manual intervention by a remote user.
[0080] For
example, in some embodiments, in addition or alternatively to
execution of a warning transmission protocol, detection of a warning event may
automatically initiate further analysis and review of collected EMG data based
on application
of one or more algorithms, including, for example, algorithms that examine
sensors for
proper calibration and/or for proper surface contact integrity with skin. And,
that analysis and
review may, in some embodiments, be initiated by either or both of a remote
user and/or by
the detection system automatically. In addition to EMG data associated with
one or more
detected event, that further analysis may include processing of EMG data that
was collected
prior to a detected event and/or after the detected event.
[0081] In some
embodiments, in response to a detected event, a period for further
EMG collection may be triggered. At the completion of the period or at some
other time
during the period, one or more algorithms may be executed that again considers
the status of
the patient. For example, in response to some events, a warning period of
further analysis
may be triggered. At the end of a warning period, a selected algorithm may
direct the system
to either initiate a transmission protocol or clear the warning status and
return monitoring to a
standard measurement protocol. Importantly, at the time of warning period
completion, the
system may have access to both the event that triggered the warning period and
further
collected EMG data and may consider all of the available data to determine an
appropriate
response. Execution of a warning period may, in some embodiments, be triggered
in response
to a warning event and may be executed alternatively or additionally to
execution of a
warning transmission protocol as described in the method 40.
100821 For
example, an event suitable to initiate a warning may be detected, and a
monitoring system may then continue monitoring the patient and collect further
information
to more fully evaluate the state of the patient. Upon further evaluation, one
or more routines
may be responsive to further collected EMG signal and another event (e.g., an
event that
changes the status of the patient) may be detected. Ideally, that event may be
detected with
greater certainty than the initial warning event. And, therefore, by waiting
until a more
certain event detection, the system may determine a more appropriate response
for the
patient.
[0083] In
addition, in some embodiments, other routines may be triggered to
specifically probe data for signatures of non-seizure activity and such data
routines may be
used to avoid false positive detections by discounting the significance of a
warning event. In
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
28
some embodiments, dedicated routines that negatively weight EMG data,
including, for
example, one or more periodicity routines that look for artificially periodic
or regular signals,
may be triggered when a warning period is initiated. Other routines that may
negatively
weight EMG data may include comparison of data to a non-seizure waveform. For
example,
comparison of EMG waveform data to a database of other spectral profiles
(e.g., model
profiles for seizures and/or non-seizure events that may be patient specific)
may be executed
and this information may be used to discount a warning event. In some
embodiments, it may
be beneficial to execute at least some routines at a base station. Therefore,
in some
embodiments, a response to detection of a warning event may be the sending of
data to a base
station.
100841 Some of
the embodiments described herein that include instructions for
further characterization of warning events, may include one or more routines
that are highly
sensitive for even weak motor manifestations. And, for example, by including
those routines
and instructions, some patients, including some patients that are particularly
difficult to
monitor, may be successfully monitored in an ambulatory setting. For example,
for some
patients that may have significant levels of adipose tissue, it may be
difficult to discriminate
seizure activity from non-seizure activity. And, for those patients, it may be
difficult to set
thresholds based on EMG amplitude or sustained EMG amplitude that fully
discriminate
seizure event data from non-seizure events. To alleviate that concern, it may
be desirable to
collect further information after a warning event such as may be executed to
more fully
evaluate the event, and after that further evaluation an emergency alarm may
be initiated if a
dangerous event was suspected to have occurred.
100851 In some
embodiments, methods of detection may include execution of one
routine (or group of routines) that analyzes EMG data for the presence of
clonic-phase bursts
and further include execution of another routine suitable to detect initial or
weak motor
manifestations. Those embodiments may include instructions wherein if a
routine suited for
detection of weak motor manifestations is detected a warning flag may be
tripped and a
warning period may be established. Within the warning period, additional EMG
may be
collected. And, for example, the EMG signal may be analyzed for signatures of
clonic-phase
activity. If clonic-phase activity is detected during the warning period, then
an alarm may be
immediately executed. In some embodiments, such as when extended periods of
elevated
EMG activity are found in the warning period or where the warning signal does
not attenuate,
with or without the presence of clonic-phase activity, a response may be
initiated. For
example, in some embodiments, if motor components of general seizure activity
are detected
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
29
and elevated EMG amplitude exists for at least about 15 to about 30 seconds an
alarm may be
initiated even without detection of clonic-phase activity. In some
embodiments, a method
may, even if seizure or clonic-phase seizure activity is detected during a
warning period,
delay triggering of an alarm. For example, the warning period may run to
completion and
only after the warning period terminates may an alarm be initiated. For
example, a method
may wait until the end of the warning period to collect further data and
increase confidence
that the emergency alarm is proper to send. In addition, within a warning
period, another
event may be detected that qualifies the detection to likely be a false
positive detection. For
example, a routine may be triggered that indicates that the sensor may have
lost calibration or
it may be determined that the signal is artificially periodic and likely to be
from non-seizure
sources.
[0086] As noted
above, within the time period of triggering of a warning state the
EMG data may be further characterized, e.g., clonic-phase EMG data may be
detected or
routines may specifically discount a warning trigger. Decisions based on a
warning threshold
detection may also, in some embodiments, be linked to detection of other
corroborating data
including data collected by other sensors such as implanted or others sensors
that may
provide information about the physiological state of the patient.
Alternatively, after a warning
time period is triggered, the threshold event triggering the period may have
not yet been
further characterized. In the event that a first threshold event is not yet
further characterized,
the method may then initiate a response based on the available collected data.
For example,
an emergency response may be triggered or some other response may be made.
Decisions
based on those first threshold detections may, as discussed above, be patient-
specific or may
be state specific. For example, a patient who is deemed to be at high risk for
adverse
consequences from weak seizure events may be treated differently than another
patient
deemed to be at a lower risk. Or, a patient who is home alone or sleeping may
be treated
differently from a patient known to be in the presence of another person or
caregiver.
[0087] An
exemplary embodiment of an embodiment of a method 50 in which a
warning period may be established is shown in Figure 5. As shown in the step
52, EMG data
may be collected and analyzed for the presence of characteristic of seizure
activity. For
example, a routine may be configured to detect sustained EMG signal amplitude
which may
be characteristics of seizure activity, and may, in some embodiments, include
threshold
settings that make the routine highly sensitive to even weak muscle motor
manifestations. If
upon execution of the routine, a positive response is determined, a warning
flag may be
tripped. For example, tripping the warning flag may start a timer defining a
warning period.
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
A warning period may, for example, be configured to last for a period of time
suitable to
further evaluate EMG data. A warning period may, for example, in some
embodiments, last
for about 15 seconds to about 30 seconds. As shown in the step 56, further EMG
data may be
collected during the warning period as may be used to analyze the collected
EMG data for
additional signatures of seizure activity. In some embodiments, at least one
routine applied to
analyze that further EMG data may be responsive to clonic-phase seizure
activity. At the end
of the warning period, an appropriate response may then be executed.
100881 In some
embodiments, a routine may be selective for clonic-phase seizure
activity and may include collecting EMG signal and evaluation of the EMG
signal data for
the presence of transient EMG signal elevations or bursts. Methods for
identifying bursts and
for collection and counting of bursts are further described herein and in
Applicant's U.S.
Patent Application 13/275,309 and Applicant's Provisional Patent Application
61/969,660.
And, for example, the routines therein for detection of seizures, including,
for example, those
that determine either burst activity, burst count, or the presence of a burst
train, may be
applied as a routine that may be selective for clonic-phase burst activity and
selectively
related to clonic-phase activity. In addition, some of the routines therein
may describe a
supervisory algorithm wherein the input of different routines may be weighted
and used to
determine if a seizure is present. And, by suitably weighting individual
routines based on
clonic-phase burst activity a supervisory algorithm may be made more or less
selective for
the clonic phase of a seizure. Therefore, the output of a supervisory
algorithm as described
therein may also be used, in some embodiments, as a routine that may be
selective for the
presence of clonic-phase activity.
100891 As
described therein, EMG signal data that meets various requirements or
thresholds may be qualified to be a clonic-phase burst. For example, a clonic-
phase burst may
be qualified based on minimum and/or maximum width requirements. Processing
operations
such as signal rectification, filtering, and/or other processing operations
may be executed as
may be appropriate to identify clonic-phase burst data and determine a clonic-
phase burst
count. For example, an analysis routine may include a peak detection program,
which, for
example, after band-pass filtering and rectification may identify and shape
data. Once
processed, generation of burst statistics, comparison to thresholds, and
qualification of data
may be more readily accomplished. Any suitable peak detection technique may be
used (e.g.,
continuous wavelet transform). For example, in some embodiments, peak
detection may
include data smoothing techniques (e.g., moving average filter, Savitzlcy-
Golay filter,
Gaussian filter, Kaiser Window, various wavelet transforms, and the like),
baseline correction
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
31
processes (e.g., monotone minimum, linear interpolation, Loss normalization,
moving
average of minima, and the like) and application of one or more peak-finding
criteria (SNR,
detection/intensity threshold, slopes of peaks, local maximum, shape ratio,
ridge lines, model-
based criterion, peak width, and the like).
[0090] In some
embodiments, peaks may be qualified against properties
associated with the clonic-phase of a seizure or properties associated with a
transition to a
clonic-phase of a seizure such as may occur when tonic activity transitions
into a clonic
portion of a seizure. Peaks may, for example, be qualified based on an
associated waveform,
regularity, periodicity, width (which may be referred to as a duration width),
amplitude, or
other factors. Qualification may facilitate differentiation of seizures from
non-seizure events
and increase confidence that detected signal elevations are properly
associated with seizure
activity. And because other sources of EMG signal elevations (e.g., background
interference,
noise, and non-seizure movements) may be differentiated from qualified burst
activity,
monitoring EMG signals for that activity provides for high sensitivity
detection of seizures.
[0091] In some
embodiments, a portion of EMG signal may be transformed to the
frequency domain and analyzed for the presence of clonic-phase burst activity.
For example,
using a frequency transform collected signal data may be converted to the
frequency domain
and the integrated intensity of signal within one or more frequency bands may
be calculated
for a given time period. And, by repeating that treatment over adjacent time
periods one may
analyze EMG signal data for data features that meet temporal width
requirements associated
with clonic-phase burst data. Alternatively appropriate bandpass filters may
be used to isolate
activity in one or more bands. For example, in some embodiments, signal within
one or more
bands ranging from about 2 Hz to about 120 Hz or about 240 Hz may be isolated
and
analyzed for the presence of clonic-phase bursts. And, at least for some
patients, specific
frequency bands may be selected that encourage differentiation of seizure
events from non-
seizure sources. Alternatively, EMG signal associated with all collected
frequencies may
analyzed for the presence of clonic-phase bursts, and collected EMG signal
data may, for
example, be analyzed without execution of a frequency transform.
[0092] In some
embodiments, identified peaks may, as further described herein,
be qualified against one or more properties typically present in the clonic-
phase of a seizure,
and may be qualified to increase selectivity for detection of the clonic-phase
of a seizure.
Peaks may, for example, be qualified based on an associated waveform,
regularity,
periodicity, width (which may be referred to as a duration width), amplitude,
or other factors
described herein. And, by selection of thresholds that excludes activity from
other parts of a
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
32
seizure, a routine may, for example, be made selective for the clonic-phase of
a seizure. And,
because non-seizure sources and other sources of noise may also be
discriminated from
qualified peaks, qualification of peak data may not only facilitate selective
identification of
the clonic-phase, but also enhance discrimination of burst data from non-
seizure sources, and
therefore, may be used to enhance overall system performance for seizure
detection.
[0093] In some
embodiments, regions of elevation of EMG signal amplitude may
be qualified as meeting one or more conditions related to the duration of a
signal elevation.
That is, region of signal elevation or elevation over background by at least a
SNR may be
qualified as maintaining a requisite condition for a minimum duration, maximum
duration or
both.
[0094] For
example, in some embodiments, to qualify signal data as suitable for
clonic-phase burst identification, analysis of burst statistics, and or
counting of bursts, signal
data may be qualified based on fulfilling of a minimum burst width and/or
maximum burst
width criterion, and if some number of bursts is detected over some period of
time a positive
response may be logged. That is, a routine may count clonic-phase bursts or
determine a
clonic-phase burst rate and if the number or rate exceeds a threshold a
positive response may
be logged. In some embodiments, a burst envelope may be generated and the
burst envelope
may impact a SNR threshold that may be used to identify bursts. For example,
with a simple
peak detect method, clonic-phase bursts may be qualified by meeting a
threshold SNR of
about 1.25 to about 20 and by meeting a minimum threshold for burst width of
about 25 to
about 75 milliseconds and maximum burst width threshold of no greater than
about 250
milliseconds to about 400 milliseconds. Clonic-phase burst may then be counted
and a
number of bursts or rate of bursts may be determined. For example, a positive
routine
response may then, for some patients, be triggered if between about 2 to about
6 clonic-phase
bursts are measured within a time window of about 1 second or if another
suitable number of
clonic-phase bursts are counted in some other appropriate time window.
[0095] In some
embodiments, positive routine response may be made if at least
about 2 to about 6 clonic-phase bursts are measured within a time window of
about 2 to about
seconds. A threshold number of clonic-phase burst count for a patient may be
the same or
different than an expected number of physiological events that may produce
clonic-phase
bursts. For example, depending on a SNR threshold level and or other
thresholds at least
some of the physiological events associated bursts may not be detected, but a
threshold
number of clonic-phase bursts may still be detected and may be used to trigger
a positive
routine response.
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
33
[0096] To
further qualify signal data, other properties of clonic-phase activity,
e.g., in addition to minimum and/or maximum widths of duration, may, in some
embodiments, also be included in evaluation of EMG data. For example, in some
embodiments, a portion of EMG signal may be identified to include one or more
suspected
bursts, and the portion of EMG signal may be transformed to the frequency
domain and
analyzed fur the presence of frequency characteristics associated with clonic-
phase activity.
That is, frequency data may be compared to a model frequency waveform typical
of clothe-
phase activity. If the comparison does not support a finding that the
frequency data may be
associated with clonic-phase activity, the suspected bursts may, for example,
not be included
in the burst count characteristic. That is, the identified activity may fail
qualification and may
not be counted. Model waveforms may be based on EMG data for a particular
patient or for a
patient demographic, and in some embodiments, waveform data may be updated as
historical
EMG data is collected.
[0097] In some
embodiments, regions identified as likely clonic-phase bursts may
be further qualified based on additional criteria. For example, identified
data, e.g., likely
clonic-phase bursts, may be further analyzed based on one or more
characteristic including
periodicity, amplitude regularity, waveform regularity, other criteria
described herein and
combinations thereof. For example, in some embodiments, if identified data is
detemined to
be artificially periodic the data may fail qualification and may not be then
counted. Upon
suitable qualification as meeting criteria of clonic-phase activity, clonic-
phase burst count
may, for example, be used, to evaluate whether seizure activity or the clonic-
phase of a
seizure is present.
100981 In some
embodiments, for example, to be further qualified as a clothe-
phase burst, identified data, e.g., meeting the above minimum ancUor maximum
duration
requirements, may include an adjacent period of substantially quiet signal,
the quiet period
lasting for a duration of about 50 milliseconds to about 300 milliseconds.
That is, the
presence of an adjacent quiet period of threshold duration may be used to
qualify a burst as
related to clonic-phase activity. Upon final qualification as meeting criteria
of clonic-phase
activity, clonic-phase burst count may be used to evaluate whether seizure
activity or the
clonic-phase of a seizure is present. For example, a positive routine response
may then, for
some patients, be triggered if between about 2 to about 6 clonic-phase bursts
are measured
within a time window of about 1 second or if another suitable number of clonic-
phase bursts
are counted in some other appropriate time window.
[0099] In some
embodiments, identified data as likely derived from clonic-phase
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
34
activity may be further determined to be a burst train. A burst train may
include any number
of bursts that are temporally related to each other (present in a certain time
window) and that
may be pooled together to generate burst statistics and/or to look at trends
in burst data over
time. For example, a burst train may include a number of bursts such as about
3 to about 30.
A burst train may include any portion of the total number of bursts present in
the clonic-phase
of a seizure. For example, a number of bursts selected to be included in a
burst train may be
made as appropriate to focus on one portion of the clonic-phase of a seizure
such as the start
of the clonic-phase. To that point, a burst train or burst included therein
may (as described
further in relation to Figure 10 and routine 130) be qualified based on a
distribution of time
periods between individual bursts of the burst train and whether the
distribution is typical of
seizure or non-seizure activity. That is, a metric (such as e.g., standard
deviation or average
percentage deviation) related to the distribution of time periods between
bursts may be
calculated and used to qualify a burst train or bursts included in the burst
train. if too great a
number of bursts are included in a burst train then the metric may be high
because the time
period over which the burst train is measured may extend over a time frame
where the
average length of the time periods between bursts has increased. And, in some
embodiments,
bursts may be collected from each of a front, middle, and back portion of the
clonic-phase of
a seizure. And, in some embodiments, to determine statistics, such as may be
used to identify
a standard deviation or other burst statistics, a pooled value (based on data
from each portion)
may then be calculated.
(00M) In some embodiments, to be further qualified as a clonic-phase burst
train,
a qualified burst train, e.g., a number of nearby individual burst members of
which may meet
the above minimum and/or maximum duration requirements, may meet a threshold
level for
percentage burst deviation for the periods between individual bursts of the
train. The presence
of one or more clonic-phase burst trains may be used to detect seizure
activity, initiate
execution of an alarm or log a positive response in a routine.
(00101) More generally, any number of steps of identification of suspected
clonic-
phase burst data and qualification of that data to increase the confidence
that the data should
properly be associated with the clonic-phase may be made. Figure 6 illustrates
an exemplary
embodiment of a routine 60 of monitoring EMG data for characteristics of
seizure activity
including burst identification, and which may be used to initiate a response.
That response
may be used individually as a method of monitoring a patient or may be used in
combination
with other routines to more fully characterize a seizure and to execute risk
stratification. in
the routine 60, peak data may be identified and qualified in one or more steps
to produce a
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
value (such as clonic-phase burst count, clonic-phase burst count rate or
other clonic-phase
burst statistics), and that value may be compared directly to a threshold
value (such as count,
rate or other statistics threshold) and used to initiate a positive response
in the routine. And,
for example, if used individually as a method of monitoring, a positive
response in the routine
60 may be used to directly trigger one or more transmission protocols.
(00102) In the step 62 of the method 60, an EMG signal may be collected and a
portion of the collected signal may be selected for processing. In the step 64
one or more
routines may be executed to identify if peaks are present in the selected
data. For example,
any of the routines 72 (Fig. 7), 96 (Fig. 8), and 120 (Fig. 9) or combinations
of those routines
may be executed. As shown in the step 66, in some embodiments, the routine 60
may include
further evaluation of whether identified regions meet one or more properties
associated with
the clonic-phase of a seizure, and depending upon how many of the identified
features meet
qualification, a clonic-phase burst count may be determined. In the step 68,
the determined
burst count or rate may be compared to a threshold value. And, in the step 70,
if the threshold
value is met a positive response may be logged for the routine or an alarm may
be executed.
After a positive response is logged the system may, for example, then collect
a next signal
sample. More generally, EMG data may meet an initial screen to be identified,
and any
number of different properties may be compared to the identified data and may
be used to
qualify or further qualify the data as related to clonic-phase activity. When
qualified, clonic-
phase burst count may serve as a highly sensitive and selective seizure
variable to analyze
EMG data for the presence of seizure activity or for a clonic-phase portion of
a seizure.
(00103) In some embodiments, as explained in further detail in the routines 72
(Fig. 7) and 96 (Fig. 8), a step 64 of method 60 may involve identification of
EMG data that
includes periods of short-lived elevations of EMG amplitude and which is a
candidate for
further qualification, e.g., further qualification as exhibiting clonic-phase
behavior. For
example, as further explained in step 90 of the more detailed routine 72 (Fig.
7), data may be
qualified as including amplified signal that is maintained for a minimum
threshold duration.
Likewise, as further explained in step 110 of routine 96 (Fig. 8), data may be
qualified as
including amplified signal that is maintained for no longer than a maximum
threshold
duration. Based on the above criteria, EMG signal may be identified as likely
including one
or more clonic-phase bursts or series of clonic-phase bursts, and depending,
fur example, on
the selection of above minimum and/or maximum threshold levels of duration,
the identified
signal may, at least for some patients, be highly selective for clonic-phase
activity. For
example, the identified EMG signal data may be suitably qualified, e.g., based
on suitable
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
36
minimum and/or maximum duration thresholds, that the data is likely to
correlate with clonic-
phase activity and may be directly incorporated into an algorithm for
identification of seizure
activity. For example, short-lived elevations or bursts may be counted and the
burst count
compared to a threshold count for evaluating whether a seizure or clonic-phase
part of a
seizure is present.
(00104) Figure 7 illustrates one embodiment of a clonic-phase burst
identification
routine 66 that may be used individually or in combination with another
routine to identify a
portion of signal data that may include clonic-phase bursts. The routine 72 is
shown to
include a start trigger 74. The start trigger 74 may, for example, be set
based on a detected
signal change or change with respect to background noise. The start trigger 74
may
alternatively be set to start on predetermined intervals such as may be driven
by a suitable
clock routine. In the step 76, a signal portion may be selected for analysis.
The signal portion
may include any number of data points collected by an EMG sensor. In the step
78, the
routine 72 may establish whether signal selected for analysis is above a
maximum allowed
value or threshold setting. For example, it may be desirable, at least in some
routines, to
determine whether the signal is inappropriately high or achieves a level that
is unlikely to
correspond with actual seizure activity, but rather may correspond, for
example, to an artifact
such as may be present if a sensor or sensor contact has become unstable or if
the detection
device is in need of calibration. In the step 80, another routine may be
instructed that the
signal has exceeded the maximum allowed value. The other routine may, for
example, be a
supervisory algorithm associated with an alami decision or a routine organized
to trigger
recalibration of a detection unit or the routine that is looking for too
regular a signal to be
humanly produceable. As shown in the step 82, the routine 72 may then include
taking a next
signal sample or waiting for a next trigger to re-start the flow shown for the
routine 72.
(00105) In the step 84 (which may additionally or alternatively be included to
execution of the step 78), the routine 66 may determine if the selected signal
sample's SNR is
greater than a selected threshold. If the signal's SNR is greater than
background by at least
the SNR threshold, the routine 72 may determine (as shown in the step 86)
whether the
selected signal sample's SNR has exceeded the SNR threshold for the first time
within the
routine 72. If the signal sample's SNR was greater than the SNR threshold for
the first time in
the routine, a burst timer may be initiated as shown in the step 88. A burst
timer may
establish a threshold level for burst duration as may be useful, for example,
to prevent
transient spikes of activity (e.g., data spikes that may not reflect actual
seizures) from being
qualified as clonic-bursts. For example, the burst timer may run for a period
of about 25 to
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
37
about 100 milliseconds or some other suitable value that is shorter than
typical of a burst of
data originating from seizure activity. Following a start of a burst timer the
routine 72 may
take another signal sample and other steps of the routine may be initiated. In
addition,
appropriate flags may also be tripped such that next signals samples evaluated
for signal
and/or SNR levels are known to include preceding iterations of sample
selection. For
example, a second sample of data may be selected (step 76) and the selected
signal may pass
again through steps of the routine 72. If in the step 84 the selected signal
(which may, for
example, include data from several iterations of taking of signal sample),
does not meet a
threshold SNR, then a step 90 may be executed. In the step 90, it may be
determined if the
time period for the selected signal has exceeded a threshold duration level
and if the duration
level has been exceeded, then the selected signal may be qualified as a clonic-
burst (step 92).
Upon qualification of a clonic-burst the routine 72 may be exited.
[00106) Upon exiting of the routine 72, data may, for example, be further
qualified
for clonic-phase burst characteristics and/or may be passed to a routine that
may evaluate the
data to determine an appropriate response to the detected of burst data. For
example, signal
qualified in the routine 72 may exit the routine if it exceeds a threshold SNR
for a threshold
level of duration and it may, in some embodiments, be useful to determine if
the signal also
does not exceed a maximum threshold duration. To evaluate whether the exiting
signal does
not exceed a maximum threshold duration, the signal exiting the routine 72
may, for example,
be sent to the routine 96 of Figure 8. Also by way of example, burst data may
be sent to a
circular buffer that is periodically evaluated for the detection of a burst
train or each time a
burst is detected a determination may be made of whether or not to trigger an
alarm. If in the
step 90, the selected signal does not exceed a threshold signal duration, it
may be deemed that
the signal data is too short to qualify as a burst, and the routine may
collect a next signal
sample ¨ clearing any appropriate flags ¨ such as may have been tripped
previously.
Alternatively to collecting a next signal sample, EMG data may be collected
and only if a
next trigger is established may the routine 72 be re-initiated.
(00M] Figure 8 illustrates another embodiment of a burst detection routine 96
that
may also be used individually or in combination with another burst detection
routine to
identify a portion of signal data that may include bursts. For example, when
used in
combination with the routine 72, the routine 96 may facilitate selection of
bursts that meet
each of a minimum and a maximum width criterion. Tailoring threshold levels of
burst
duration in the routines 72, 96 may, for example, include the selection of
regions of EMG
data that are elevated over a background level and maintain an elevated level
for between
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
38
about 25 to about 400 milliseconds. In the routine 96, a start signal (step
98) may, for
example, be executed based on a detected signal change or change in signal
with respect to a
background level. A start trigger may alternatively be set to start on a
predetermined interval
such as may be established by a suitable clock routine. In some embodiments,
routine 96 may
automatically start once a pre-qualified burst is found in another burst
detection routine such
as the routine 72 as described in Figure 7, and may be used to further qualify
data also
processed in the routine 72.
1001081 The routine may 96 may include taking a signal sample as shown in the
step 100. In some embodiments, the routine 96 may include the step 102 wherein
the routine
may determine if the sample is greater than a maximum allowed level. If the
maximum level
is exceeded the method may instruct another routine as shown in the step 104,
and in the step
106 a next signal sample may be taken or the method may wait for a next
trigger before
starting over. Alternatively or additionally to the step 102, method 96 may
include step 108
which may include determining if the sample is greater than background by at
least a SNR. If
the sample signal is greater than background by at least the SNR, method 96
may execute the
step 110. In the step 110, the method 96 may determine if a burst duration
timer is greater
than a maximum burst duration level. If routine 96 is executed in combination
with the
routine 72, the burst timer may have been previously tripped as described, for
example, in the
step 86 of the method 72. Alternatively, if, for example, routine 96 is
executed separate of the
routine 72 then a separate burst timer may have been triggered in other steps
(not shown).
PM] If the
maximum burst duration threshold is exceeded, it may be deemed, as
shown in the step 114, that a burst is too long to be indicative of a seizure.
Appropriate flags
may be cleared, and the process may start over by collecting a next signal
sample or waiting
for an appropriate trigger to re-start the process. If in the step 108, it is
determined that this
sample does not exceed the background by the SNR threshold level, a burst may
be deemed
to be over and the burst may be qualified a shown in the step 112. As further
indicated in the
step 112, the signal qualified as a burst may, for example, in some
embodiments, be
characterized by a certainty value, the center of the burst may be determined
and the burst
data may be written into a circular buffer for further analysis. Upon exiting
of the routine 96,
data may, for example, be further qualified for burst characteristics and/or
may be passed to a
routine that may evaluate a response to the detection of burst data. For
example, burst data
may be sent to a circular buffer that is periodically evaluated for the
detection of a burst train
or each time a burst is detected a determination may be made of whether or not
to trigger an
alarm.
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
39
1001101 Figure 9 illustrates an embodiment of a burst train detection routine
120
suitable for selection and/or processing of EMG burst train data. The burst
train detection
routine 120 may, for example, be used in combination with either or both of
the routines 72
and 96.
100111] In a step 122 of the burst train detection routine 120, the routine
may scan
a circular buffer of data which may store information related to bursts such
as may have been
detected in the above routines 72 and 96. In some embodiments, scanning of the
circular
buffer may be initiated at predetermined intervals such as may be set based on
a clock
routine. In other embodiments, scanning may be initiated based on a trigger
signal. For
example, each time that data is input into the buffer, such as may occur each
time one of
routines 72 and/or 96 sends qualified data to the buffer, scanning may be
initiated. As
indicated in the step 124, in some embodiments, the method 120 may evaluate
whether the
data in the circular buffer meets one or more threshold conditions. For
example, in the step
124, the method 120 may eliminate bursts that are too close together or too
far apart from
consideration. As indicated in the step 126, the method 120 may determine if a
suitable
number of bursts are selected to qualify as a burst train. For example, a
number of bursts
suitable to qualify as a burst train may be about 3 to 20 bursts. More
generally, a suitable
number of bursts may be selected to generate statistics and/or to look at
trends in the signal
during the course of a seizure's progression. For example, multiple burst
trains may also be
analyzed and changes in data between adjacent burst trains may also be
calculated. In some
embodiments, burst or burst train information may be collected even after an
alarm may be
initiated. For example, the length of time between bursts may be used to model
or track a
seizure's progression and may provide valuable information to caregivers. That
information
may also be used by other devices or sensors such as those used to treat a
seizure or that may
be used to collect physiological data for a patient. In the step 128,
properties of a detected
bust train may be analyzed for one or more characteristics that may be
indicative of a seizure
or seizure related event and an appropriate response may be executed. The step
128 may, for
example, include analysis of properties of a burst train such as may be
accomplished, for
example, using steps or elements in the routine 130 as shown in Figure 10. By
way of further
example, a response may be to input a value into a supervisory algorithm that
may then be
used in determining whether a seizure is detected. In addition, the routine
120 may be used to
qualify a possible clonic-phase burst train as a clonic-phase burst train, and
the presence of a
detected clonic-phase burst train may then be used, for example, to initiate
an alarm.
100112] In some embodiments, it may be useful to detect one or more burst
trains
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
and determine whether a burst train is indicative of a seizure. For example, a
burst train may,
for example, be analyzed using one or more routines that determine the
periodicity of bursts,
regularity of bursts or both. For example, for some patients, bursts may be
characterized by
some natural variation between individual bursts, and if data is characterized
by a burst
periodicity that is too low it may be deemed that the data is not likely to be
indicative of an
actual seizure. Moreover, for many patients, as a seizure progresses quiet
time periods
between bursts may increase during the seizure. Therefore, bursts may be
evaluated over one
or more time periods, and if the periodicity of bursts increases over time
confidence that the
burst data is indicative of a seizure may increase. A burst train may be
compared to one or
more threshold values related to a characteristic of the bursts, and if a
threshold condition is
met the burst train may be deemed to be indicative of a seizure. Threshold
conditions may be
based on data obtained from a patient experiencing a clonic-phase seizure or
may be based on
clonic-phase data from a patient demographic. Therefore, burst trains may be
qualified burst
trains and bursts within the train may be qualified to be clonic-phase bursts.
In some
embodiments, a burst train may be weighted with a value that is not only
related to detection
of the burst train but also related to the certainty of burst train detection.
[001131 As described herein, it may be beneficial to evaluate bursts over an
extended period because confidence that the seizure is present and of a
certain type or
severity may be increased with statistics from longer time periods. However,
as also
described herein, it may be beneficial to detect a seizure as quickly as
possible. In light of
those criteria, in some embodiments, particularly if pre-clonic detection of
seizures has been
made only recently, then it may be useful to wait a certain duration following
initial burst
detection before initiating an alarm. For example, as long as motor
manifestations have only
been present for about 15 to about 30 seconds (or some other suitable time
period) then a
method may collect further burst data and increase confidence of seizure type
and/or severity
prior to initiating an alarm. And, in some of those embodiments, a periodicity
algorithm or
other algorithms to calculate bursts statistics may be particularly useful
because, for example,
a large number of burst may be measured and if those bursts meet
qualifications for
periodicity such may be highly characteristic of a seizure. Moreover, that
data may, for some
patients, be gathered and may be diagnostic of risk assessment, and may, for
example, be
related, at least in some patients, to future CNS depression. Such embodiments
may be
tailored to specific patients needs, and for some patients, an emergency alarm
may be
executed as soon as seizure or clonic-phase activity of a seizure is detected.
100114] Figure 10 illustrates an embodiment of a routine 130 that may be used
to
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
41
characterize the periodicity of bursts and evaluate whether bursts may be
indicative of a
seizure or stage of a seizure and/or whether the burst should suitably counted
in a burst
detection routine. The periodicity routine 130 may, for example, look at a
circular buffer or
register that may store EMG data identified as including qualified or
prequalified burst data,
and examine, for a selected or determined time period, how regular periods
between bursts
were. A periodicity routine may scan different data values from various time
windows that
the burst detection algorithm wrote into a circular buffer, and examine the
periodicity of
signal characteristics, including those that may not be indicative of a
seizure.
1001151 In the step 132, of the exemplary method 130 of Figure 10, an average
duration of the periods between bursts within a time period may be calculated.
In step 134,
individual durations of periods between bursts may be subtracted from the
average duration,
and the absolute values of the differences used, in step 136, to calculate the
average deviation
of the periods. In this example, the average deviation may be converted to a
percentage
although other suitable metrics may be used to characterize variation between
periods.
1001161 In step 138, the aforementioned percentage (or average deviation
percentage) may be compared to threshold values. Such threshold values may be
taught to the
system in operation and may be customized for a particular patient.
1001171 For example, if in a time period (measuring in seconds), nine bursts
were
detected at the following times:
12, 13, 13.75, 14.35, 15, 15.8, 16.2, 16.5, 17.4
there would be 8 time periods between bursts. So, over a time period including
the foregoing
epoch of 5.4 seconds, there were nine bursts with eight periods between
bursts. The average
period may be calculated as 5.4/8=0.675 seconds per burst. The time periods
between bursts
are as follows:
13-12=1
13.75-13=0.75
14.35-13.75-0.6
15-.14.35=0.65
15.8-15=0.8
16.2-15.8=0.4
16.5-16.2=0.3
17.4-16.5=0.9
[00118) in this example, a simplified method allows the time around which a
burst
is centered to serve as a time stamp for that burst. In other words, each time
the burst
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
42
algorithm qualifies a burst, a time stamp may be written into a circular
buffer for use by the
periodicity algorithm. In other embodiments, real burst width may be used to
calculate the
actual length of the time periods between bursts. For example, if the burst
occurring at 12
seconds lasted for 0.02 seconds, then the time period between the burst
starting at 12 and the
burst starting at 13 would be 0.98 seconds. The absolute value of the
deviations from the
average may be calculated as follows:
I 1.0-0.675 I .325
I 0.75-0.675 I =0.075
I 0.6-0.675 I =0.075
I 0.65-0.675 I =0.025
I 0.8-0.675 I =0.125
I 0.4-0.675 I =0.275
I 0.3-0.675 I =0.375
I 0.9-0.675 I =0.225
[00119] Averaging the absolute values may be accomplished as follows:
Sum of all deviations:
0.325+0.075+0.075+-0.025+ 0.125+0.275+0.375+0.225-1.5
Average deviation: 1.5/8-0.1875
1001201 The average deviation percentage of this average is: 0.1875/0.675-
27.8%.
That is a significant deviation from the average and may be deemed unlikely to
be artificial.
For example, a threshold value of average deviation percentage may be set, for
example, to
15%, and then the periodicity algorithm may declare that it is likely that the
data may be
characteristic of a seizure. For example, as shown in the steps 138 and 140, a
system would
not vote against declaring a seizure. The result may, for example, be placed
in a register for
use by the supervisory algorithm and may vote for declaring an alarm.
Additionally, if the
method 130 were applied to qualify bursts, then the method may support a
finding that the
bursts were related to a seizure, the bursts may then be deemed appropriate
for being counted
and used to support a positive response in a burst detection routine.
[00121] In
another simplified example, the burst train may look like this (in
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
43
seconds):
17, 17.5, 18.02, 18.51, 19.04, 19.56, 20.1, 20.6, 21.13
So, over a periodicity time window including the foregoing epoch of 4.13
seconds, there were
nine bursts with eight periods between bursts. The average period may be
calculated as
4.13/8-0.51625 seconds per burst. The individual times between bursts are as
follows:
17.5-17.5
18.02-17.5=0.52
18.51-18.02=0.49
19.04-18.51=0.53
19.56-19.04=0.52
20.1-19.56=0.45
20.6-20.1=0.5
21.13-20.6.53
1001221 The absolute value of the deviations from the average may then be
determined as follows:
10.5-0.516251=0.01625
10.52-0.51625 1=0.00375
10.49-0.51625 1=0.02625
10.53-0.51625 1=0.01375
10.52-0.51625 1=0.00375
10.45-0.51625 1=0.06625
10.5-0.516251 .01625
10.53-0.51625 1=0.01375
100123) The stmi of all deviations may be calculated as follows:
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
44
0.01625 0.00375+0.02625+0.01375+0.00375+0.06625+0.01625 0.01375=1.
6
1001241 The average deviation is therefore: 1.6/8=0.02.
1001251 The percentage deviation of this average is thus: 0.02/0.51625-3.87%.
This example thus shows a very regular pattern. If, for example, a threshold
value of average
deviation were set to 15%, then the algorithm would declare that confidence is
very low that
a true seizure is occurring and would vote against declaring a seizure alarm
(step 142). The
result may be placed in a register for use by a supervisory algorithm.
Likewise, if the method
130 were applied to qualify bursts, then the method may support a finding that
the bursts
were not related to a seizure, the bursts may then be deemed inappropriate for
being counted
and not used to support a positive response in a burst detection routine. Of
course, standard
deviation calculations or other appropriate metrics related to variability may
be substituted
for average deviation calculations. Also the thresholds may be derived from
the particular
patient, averaged model values, or some other method. These thresholds may be
variables in
the detection unit and may be changed when appropriate.
1001261 In some embodiments, data may be automatically formatted and presented
for review in a manner suitable to display a determined risk value. For
example, in some
embodiments, EMG data may be sent and qualified or marked as only
corresponding to a
warning event. That is, the data may be risk stratified and designated with a
non-emergency
or warning status. To identify EMG data as associated with a warning or non-
emergency
status, the EMG data may, for example, be marked with an appropriate status
indicator. For
example, graphical data may be displayed in a color, font, or with some other
marking that
identifies the status of the data as only being associated with a warning.
Likewise, emergency
data may be suitably marked such as in a different color, font, or other
marking.
1001271 While some caregivers may prefer (or prefer for some patients) that
they
are immediately notified and sent EMG data for warning events, for other
caregivers or for
other patients such notification may not be preferred. And, in some
embodiments, marking of
an event as associated with a warning status may include instructions to
buffer the data, e.g.,
store the data in memory in a caregiver's computer, without actively
displaying or notifying
the caregiver. And, in some embodiments, a system may include installing
instructions in a
caregiver or other designated individual's device such as by installing a
smart client
application to facilitate organization of incoming data. For example,
information may, in
some embodiments, or with some device settings, be transmitted to a computer
but not
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
presented to the user until, for example, corroboration is made that the event
demands an
emergency response. In the event that the data is corroborated a signal may be
sent from a
patient detection device (or base station) to update the event status. And,
for example, data
stored "silently" on a caregiver's computer, may then be rapidly displayed
based on an
update signal which may only include instructions to update status and may be
of minimal
data amount. Such embodiments, may, for example, be particularly useful where
a caregiver
may be at a location where connectivity is intermittent and where data
transfer may be
limited. In those cases, it may be difficult to send large amounts of data
rapidly and sending
large amounts of data only after emergency verification may not be desirable
or timely.
1001281 Data transmitted from a monitoring system, may, in some embodiments,
be customized for a particular individual or recipient group. For example,
transmitted data
may include, an alarm message, subset of statistical information related to
algorithm
detection, time or frequency domain EMG data, or combinations thereof. For
example, in
some embodiments, a detection unit may execute as part of a burst detection
routine one or
more peak detection programs. The burst detection routine may be configured to
identify
whether one or more bursts of data are present as may be used to determine
that a clonic-
phase portion of a seizure is present. The burst detection routine, therefore,
may be ideally
suited for risk stratification and automatic classification of detected event
data. And, as part
of organizing the EMG data for transmission, a burst statistics window may be
provided for
presentation of data to a caregiver. The burst statistics window may, for
example, include a
summary of statistical information regarding a detected pattern of bursts. By
way of example
only, data that may be included in a burst statistics window include number of
detected
bursts, rate of burst detection, average signal-to-noise ratio (SNR) of
detected bursts, spread
of SNR of detected bursts, average width for detected bursts, spread of widths
for detected
burst, average length of periods between detected bursts, spread of length of
periods between
detected bursts, deviation of bursts over any number of burst trains,
frequency characteristics
of burst data, and combinations thereof.
1001291 More generally, depending, for example, on a particular routine or set
of
routines used for seizure event detection, analysis information organized
together with other
processed or unprocessed EMG data (e.g., data for reconstructing a raw EMG
data file) may
include a summary of data useful to a caregiver showing what triggered the
transmission.
That is, organization of transmitted data may include a summary of information
to readily
communicate to the caregiver why the particular EMG data was selected, and for
example,
portions of data where a positive response was made for a routine may be
identified. And, for
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
46
example, an output summary of individual detection routines may be presented
including
threshold setting for the routine and/or certainty values for meeting a given
threshold event.
By way of nonlimiting example, some of the routines described herein may
include analysis
of EMG data for sustained amplitude elevations, transient EMG elevations or
bursts, and
spectral isolation of the EMG data, and depending on what seizure
characteristics were
deemed to be present associated information may be organized for presentation.
[001301 In some embodiments, transmitted data may include EMG data and/or one
or more other pieces of data. For example, in some embodiments, a detection
unit may further
include one or more microelectromechanical inertial detection elements, e.g.,
gyroscopes,
magnetometers and/or accelerometers, that may be configured to determine an
orientation of
the detection unit and therefore of the muscle upon which the detection unit
is attached.
Therefore, the system herein may, for example, include a description of
whether a sensor
(and therefore the muscle to which the sensor may be coupled or attached) was
oriented in
one way or another. For example, whether the detection unit was oriented
substantially
vertically, e.g., parallel to a normal vector from the ground as common for a
patient standing,
or show whether the sensor was oriented horizontal to that normal, e.g.,
perpendicular to that
normal vector, as may be common when a patient is lying such as in bed. Other
information
that may be organized together with EMG data may include other sensor data
such as may be
associated with measurement of oxygen saturation in the blood. That
information may, in
some embodiments, be used to further classify an event, e.g., to further
influence a
determined risk stratification. And, that information may be used together
with EMG data to
designate an event as either an emergency or warning event. In addition, in
some
embodiments, a pulsed oximeter may also be used to monitor a patient and may
also be
included with a detection device. In some embodiments, risk stratification may
further
include separate analysis of a patient risk from falling and/or a patient risk
of SUDEP. And,
each of those may be related to a patient state. For example, whether a
patient may be
sleeping, home alone, or home alone with another patient. By way of example
only, if the
patient is sleeping a template file may automatically assign a low risk
assessment from
falling, but may not adjust a risk of SUDEP.
1001311 In some embodiments, organization of information for transmission may
include tailoring data transmittal as appropriate for different designated
individuals including
caregivers, friends, and family. For example, whereas transmittal of data
suitable to display
either or both of the time and/or spectral dependence of EMG data as well as
summary of key
analysis features may be transmitted to a particular caregiver, that
information may be
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
47
unnecessary or detrimental if sent to some friends and family. And, in some
embodiments,
organization of information for transmission may be determined by profiles or
settings
appropriate for the receiver. For example, friends and/or family may be
informed that an
emergency alami or warning was sent, but may not receive raw or processed data
suitable to
reproduce the time and/or spectral dependence of EMG data or other aspects of
data analysis.
And, in some embodiments, information may be sent to one group of caregivers
who are
trained to access from detailed data key seizure characteristics such as, by
way of nonlimiting
example, whether a particular phase of a seizure is present, various aspects
of seizure
semiology, and/or orientation data from a microelectromechanical sensor
element.
Information may also be sent to other caregivers, including some caregivers
involved in the
emergency response, who may not have different specific training in EMG signal
interpretation and may only be sent, for example, information about whether an
emergency
response is warranted or other sensor data for which they are trained to
interpret.
[001321 Data selected and organized for transmission may be sent and received
by
one or more caregivers or other designated individuals. As described above, in
some
embodiments, EMG or other sensor data may be sent from either or both of a
detection unit
12, base station 14, or alert transceiver 16. And, any of the devices 12, 14,
and 16, may, as
shown in Figure 1, communicate information to a device for a designated
individual. In some
embodiments, information may also or alternatively be communicated directly to
a caregiver
or other designated individual over a local network such as WiFi.
[001331 In some embodiments, a caregiver's device may, in some embodiments,
include in hardware or software one or more installed programs or set of
instructions to
facilitate communication and organization of signal data from a transmitting
device. And, in
some embodiments, instructions for executing one or more routines may be
executed using
one or more smart client applications. Instructions for updating a status of a
detected event
may, for example, be included in a caregiver's device and intelligently
updated based one or
more signals or update signals. Also, instructions for performing any of
various routines
including those that may be executed by either of a detection unit 12 or base
station 14 may
also be included in an application on a caregiver's device. And, a caregiver
may, for example,
be able to vary threshold settings and/or manually adjust thresholds or other
routine settings
as may be used to further access EMG and/or other data provided from a
detection unit 12 or
base station 14.
1001341 Figure 11 illustrates another exemplary embodiment of a method 160 of
collecting EMG signals and transmitting selected EMG signal data to monitor a
patient for
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
48
seizure activity. In the step 162, a portion of EMG signal or, in some
embodiments, EMG
signal and other sensor data may be collected. For example, in some
embodiments, EMG
signals may be collected together with either or both of oximeter data and/or
data from one or
more orientation and/or position sensitive devices.
[00135] In the step 164, collected data may be analyzed using one or more
routines
configured to determine whether one or more events were detected. At least one
routine may,
as described above, be selective for a particular portion of a seizure, and if
that portion is
more or less associated with adverse consequences of a possible seizure, risk
stratification
may then be facilitated. In the step 166, a decision may be made, e.g., based
on any number
of EMG analysis routines and/or other information, whether a seizure event was
detected.
Individual routines may be configured to be selective for a given part of a
seizure. For
example, in some embodiments, a first routine may analyze collected data for
the presence of
sustained EMG amplitude and a second routine may analyze collected data for
the presence
of transient elevations of activity or bursts activity that may be selectively
present when a
patient experiences a clonic phase of a seizure. And, in some embodiments, an
event may be
determined using a supervisory algorithm. A supervisory algorithm may, for
example,
execute a number of sub-routines that combine, such as by appropriately
weighting,
contributions of various seizure variables in a routine for determining the
likelihood that a
seizure may be occurring. And, for example, by adjusting a contribution from
one or more
burst detection sub-routines a routine that includes a supervisory algorithm
may be a selective
routine. In some embodiments, other sensor data may be integrated with EMG
sensor data
during the step 164. For example, position and/or orientation data may, in
some
embodiments, be provided by a detection unit or by a detection unit in
combination with a
base station and/or environmental transceiver.
1001361 And, in some embodiments, a system may, for example, be aware that a
patient is likely to be at one location, such as in bed, or at some other
location, such as in a
bathroom. A system may then, for example, apply different routines and/or
routine settings to
determine the likelihood that a patient may be having a seizure or engaged in
some other
activity such as non-seizure moving as dependent upon the location of the
user. And, in some
embodiments, as described, in Applicant's co-pending Provisional Application
No.
61/979,225, settings or thresholds may depend upon whether a detection unit
may be oriented
horizontally or vertically. That is, the system may be particularly calibrated
for whether a
muscle of a patient is in a certain orientation. And may, for example, be
particularly
calibrated based on whether a patient may be lying horizontally, lying
horizontally with the
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
49
patient's weight on the sensor, or oriented vertically. In some embodiments, a
monitoring
system may be configured to enable a patient to update their status based on
one more
selectable profiles. For example, a patient may be given an option to select
one or more
different monitoring settings based, for example, on whether the patient is in
bed sleeping,
whether the patient is at home alone, or if the patient is at home with
another person. And,
depending on whether the patient selects one of those options the system may
select different
settings and/or thresholds. Furthermore, for a certain response or group of
responses, a
monitoring system may, for example, select a transmission protocol associated
with either a
warning of emergency status that depends on a selected option.
1001371 Any of the various routines described herein or, for example, in
Applicant's co-pending Provisional Application No. 61/969,660 may be executed
in order to
determine if events are present in the collected EMG signal (steps 164) and
then determine
whether a seizure was detected (step 1.66). For example, bursts may be
qualified based on
fulfilling of a minimum burst width and/or maximum. burst width criterion, and
if some
number of bursts is detected over some period of time a seizure event or
possible seizure
event may be detected. For example, a burst routine may show a positive
response if between
about 2 to about 6 bursts are measured within a time window of about 1 second
or if another
suitable number of bursts are counted in some other appropriate time window.
In some
embodiments, burst routine may show a positive response if at least about 2 to
about 6 bursts
are measured within a time window of about 2 to about 5 seconds. And, for
example, based
on a positive response in a burst routine a seizure may be detected.
1001381 As shown in the step 168, data may be transferred and stored in one or
more buffers of accessible memory and maintained therein for a desired
duration. A next
portion of EMG and/or EMG and other signals may then be collected. At the
completion of a
desired duration interval of EMG signal collection, buffered data may be
transferred to
another unit of memory, e.g., to permanent memory., as shown in the step 170.
More
generally, the system 160 may be configured so that transfer of data between
one buffer and
other storage maintains a suitable portion of data in accessible memory so
that the data may
be available for transmission if an event is detected. That is, data may be
suitably held
available if it is deemed necessary for seizure analysis and/or categorization
of events or if a
transmission protocol is later selected, and that data then included for
transmission.
1001391 If a seizure or possible seizure event is deemed present, as shown in
the
step 172, the method 160 may include categorizing the collected EMG signal
based on type
and/or intensity. And, in some embodiments, the categorized EMG data may then
be updated
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
with a status indicator such as a warning or an emergency status. As shown in
the method 160
and arrow 161, in some embodiments, one or more portion of data buffered in
memory may
be accessed when categorizing a seizure. That is, data previously sent to a
buffer may be
considered, in categorizing a seizure.
[00140] In some embodiments, categorization of a seizure may include
determining
whether one or more routines were responsive to a portion of EMG signal. For
example, as
shown in Table 1, based on a combination of certain responses to one or more
routines a
detected event may be categorized. In some embodiments, categorization of a
seizure may
include accessing data from any combination of an oximeter, orientation
sensor, or heart rate
sensor. And, for example, in some embodiments, if EMG data may only indicate
the presence
of a warning event, but either a critical level of saturated oxygen was
determined or if the
patient's orientation changed from substantially vertical to substantially
horizontal an
emergency status may instead be determined. For example, risk stratification
may be based
on either a determination that a patient may have fallen or that a patient
shows initial
signatures of SUDEP.
1001411 In some embodiments, a detected seizure or possible seizure event may
be
classified based on various metrics, including, by way of nonlimiting example,
type,
intensity, seizure duration, duration of a seizure phase, other metrics, and
combinations
thereof. Classification of the severity of a seizure may, for example, include
normalizing
metrics of the seizure against values typical of a patient or patient
demographic. For example,
for a patient, if a measured amplitude of a detected characteristic is some
factor of a
previously measured value for the characteristic (e.g., during another seizure
for the patient)
or some factor of an average value for the characteristic that factor may be
used to grade the
seizures severity. That information may, for example, be sent to caregivers
and/or otherwise
used to determine an appropriate response.
1001421 As further described in relation to the step 172, data, including EMG
and/or other sensor data, may be selected for transmission. And, a selected
protocol may be
executed in the step 174. As described above, a decision on whether to select
and transmit
data may depend upon the categorization of the collected data. And, in some
embodiments, a
transmission protocol may include instructions to send information to all or
only some
designated individuals. That is, in some embodiments, an event may be detected
and only
sent or immediately sent to some caregivers. For example, a routine may
identify that a
possible seizure was detected, and data may then be sent to one or more remote
system users,
but not to other designated individuals. The one or more remote system users
may, for
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
51
example, include one or more individuals who are trained to interpret EMG
and/or other
signals and may be able to corroborate that an actual seizure is occurring
using any of various
protocols.
1001431 For example, a remote user may be able to determine based on the video
monitor 9 the condition of the patient. If, for example, video data is not
available, the remote
user may attempt to contact the patient, e.g., by calling the patient. I-
Towever, in some cases a
remote user may have neither access to video data, may not be able to reach
the patient by
calling the patient, or may not want to contact the patient in this way. For
example, the
detected event, or detected event for the particular patient, may be one of
only low risk, and it
may be likely that the event is a false positive detections. If such
occurrences are common for
that patient, repeated contact of a patient based on false detection may be a
burden and may
not be desired. In some embodiments, the user may do further analysis of the
data and
attempt to conclude if the event is a true emergency, and may thereafter, if
the event is not
deemed dismissible, attempt to contact the patient and/or update the status of
the event. For
example, the user may then contact or initiate contact to other designated
individuals
including other caregivers, friends, and family. Therefore, in some
embodiments, and in some
situations contact of a caregiver device, e.g., cell phone 17, PDA 18 or other
client device,
may be mediated through a remote system user.
[00144] Generally, the devices of a seizure detection system may be of any
suitable
type and configuration to accomplish one or more of the methods and goals
disclosed herein.
For example, a server may comprise one or more computers or programs that
respond to
commands or requests from one or more other computers or programs, or clients.
The client
devices may comprise one or more computers or programs that issue commands or
requests
for service provided by one or more other computers or programs, or servers.
The various
devices in Fig. 1, e.g., 12, 13, 14, 16, 17, 18 and/or 19, may be servers or
clients depending
on their function and configuration. Servers and/or clients may variously be
or reside on, for
example, mainframe computers, desktop computers, PDAs, smartphones (such as
Apple's
iPhoneTM, Motorola's AtrixTm 4G, Motorola's DroidTM, Samsung's Galaxy STM,
Samsung's
Galaxy NOtCTM, and Research in Motion's BlackberryTM devices), tablets (such
as Sony's
XperiaTM, Samsung's Galaxy TabTm, and Arnazon
netbooks, portable computers,
portable media players with network communication capabilities (such as
Microsoft's Zune
HDTM and Apple's iPod TouchTm devices), cameras with network communication
capabilities, smartwatches, wearable computers, and the like.
[00145] A computer may be any device capable of accepting input, processing
the
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
52
input according to a program, and producing output. A computer may comprise,
for example,
a processor, memory and network connection capability. Computers may be of a
variety of
classes, such as supercomputers, mainframes, workstations, microcomputers,
PDAs and
smartphones, according to the computer's size, speed, cost and abilities.
Computers may be
stationary or portable, and may be programmed for a variety of functions, such
as cellular
telephony, media recordation and playback, data transfer, web browsing, data
processing,
data query, process automation, video conferencing, artificial intelligence,
and much more.
1001461 A program may comprise any sequence of instructions, such as an
algorithm, whether in a form that can be executed by a computer (object code),
in a form that
can be read by humans (source code), or otherwise. A program may comprise or
call one or
more data structures and variables. A program may be embodied in hardware or
software, or
a combination thereof A program may be created using any suitable programming
language,
such as C, C-1---F, Java, Peri, PHP, Ruby, SQL, and others. Computer software
may comprise
one or more programs and related data. Examples of computer software include
system
software (such as operating system software, device drivers and
utilities),middleware (such as
web servers, data access software and enterprise messaging software),
application software
(such as databases, video games and media players), firmware (such as device
specific
software installed on calculators, keyboards and mobile phones), and
programming tools
(such as debuggers, compilers and text editors).
(00147) Memory may comprise any computer-readable medium in which
information can be temporarily or permanently stored and retrieved. Examples
of memory
include various types of RAM and ROM, such as SRAM, DRAM, Z-RAM, flash,
optical
disks, magnetic tape, punch cards, EEPROM. Memory may be virtualized, and may
be
provided in, or across one or more devices and/or geographic locations, such
as RAID
technology. An 110 device may comprise any hardware that can be used to
provide
information to and/or receive information from a computer. Exemplary I/0
devices include
disk drives, keyboards, video display screens, mouse pointers, printers, card
readers, scanners
(such as barcode, fingeiprint, iris, QR code, and other types of scanners),
RFID devices, tape
drives, touch screens, cameras, movement sensors, network cards, storage
devices,
microphones, audio speakers, styli and transducers, and associated interfaces
and drivers.
1001481 A network may comprise a cellular network, the Internet, intranet,
local
area network (LAN), wide area network (WAN), Metropolitan Area Network (MAN),
other
types of area networks, cable television network, satellite network, telephone
network, public
networks, private networks, wired or wireless networks, virtual, switched,
routed, fully
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
53
connected, and any combination and subnetwork thereof. The network may use a
variety of
network devices, such as routers, bridges, switches, hubs, repeaters,
converters, receivers,
proxies, firewalls, translators and the like. Network connections may be wired
or wireless,
and may use multiplexers, network interface cards, modems, 1DSN tenninal
adapters, line
drivers, and the like. The network may comprise any suitable topology, such as
point-to-
point, bus, star, tree, mesh, ring and any combination or hybrid thereof.
100149] Wireless technology may take many forms such as person-to-person
wireless, person-to stationary receiving device, person-to-a-remote alerting
device using one
or more of the available wireless technology such as ISM band devices, WiFi,
Bluetooth, cell
phone SMS, cellular (CDMA2000, WCDMA, etc.), WiMAX, WLAN, and the like.
1001501 Communication in and among computers, I/0 devices and network devices
may be accomplished using a variety of protocols. Protocols may include, for
example,
signaling, error detection and correction, data formatting and address
mapping. For example,
protocols may be provided according to the seven-layer Open Systems
Interconnection model
(OSI model), or the TCP/IP model.
1001511 Additional information related to the methods and apparatus described
herein may be understood in connection with the examples provided below.
1001521 Example 1:
[00153] In this Example 1, a number of patients susceptible to seizures were
monitored for seizure activity using EMG electrodes. A sensor was placed on
the patient's
biceps, EMG signal collected, the collected signal analyzed for the presence
of seizure
activity, and a seizure was detected. The data herein in this Example 1
includes a summary of
20 different measured seizures from a total of 11 different patients. The
seizures and patients
herein include a subset of a number of patients in a study to evaluate
different methods of
seizure detection. And, in each of the 20 seizures in this Example 1, a clonic-
phase portion of
the seizure was identified. EMG data for one of the recorded seizures is shown
in Figure 12.
(00154) EMG data from various portions of the recorded seizures was processed
to
determine an average period between bursts as well as an average period of
burst duration.
Burst data was taken from each of several portions of the recorded data. For
example,
average burst width and average timing between bursts were analyzed during
time intervals
near the start, intermediate, and later portions of the clonic-phase. Table 2
shows, for the
period near the start of the clonic phase, the results for the various
patients and seizures in
this study.
[00155] Table 2
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
54
Patient / Seizure Identifier Time between Burst Width (sec.)
bursts (sec.)
1. Aci 0.28 0.07
1 AO 2 0.1.5 -- 0.1.0
2 FoB 0.1.0 0.1.9
3 St' 0.12 0.1.0
4 Loi 0.22 0.21
Rii 0.12 0.1.9
6 Mat. 0.08 0.05
7 Mul-1 0.08 0.13
8 WaA 0.08 0.17
9 PeAl ------------------------- 0.11 0.11
9 PeA2 0.14 0.12
McK1 ------------------------ 0.14 0.12
10 McK2 ------------------------- 0.11 ---------- 0.19
10 McK3 ---------------------- 0.09 ---------- 0.10
--+
10 McK5 ------------------------ 0.11 ---------- 0.11
10 McK6 0.07 0.12
10 McK7 0.07 0.11
10 McK8 0.08 0.07
10 McK9 0.19 0.07
11 ToS 0.21 0.22
1001561 The average period burst duration at initial parts of the clonic phase
for the
patients in this study was about 0.12 seconds (or 120 milliseconds). And, by
selecting
threshold values for minimum burst width of about 25 to about 75 milliseconds
and
maximum burst width threshold of no greater than about 250 milliseconds to
about 400
milliseconds the bursts may be detected. Moreover, other signals that may be
present during
monitoring, e.g., from non-seizure sources and/or from other phases, did not
generally follow
this pattern with significant frequency. And, by selecting elevations that
meet the
aforementioned width requirements bursts may be counted and used to determine
whether
clonic-activity was present. By detection of clonic-phase activity the
seizures may, for
example, be differentiated from other seizure events that do not show clonic-
phase activity.
Furthermore, because clonic-phase activity may be highly indicative of adverse
effects of
having a seizure the seizures may trigger an appropriate response such as an
emergency
status.
1001571 Additional data for the intermediate and later portions of the clonic-
phase
is shown in Table 3 (intermediate portion) and Table 4 (later portion).
[00158] Table 3
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
Patient and Time between Burst Width (sec.)
Recorded Seizure bursts (sec.)
1 Aci 0.51 0.15
1 Aci 2 0.20 0.08
2 FoB 0.26 0.18
3 SU 018 0.13
4 Lai 0.25 0.33
5 RìJ 0.21 0.18
6 Mal 0.17 0.26
7 MaH 0.08 0.14
8 WaA 0.20 016
9 PeAl 0.15 0.12
9 PeA2 0.17 0.12
10 McK1 016 0.13
10 McK2 0.17 0.17
10 McK3 016 0.1.0
10 McK5 0.21. 0.14
10 McK6 0.11 0.13
10 McK7 0.10 0.10
10 McK9 0.31 0.08
11 ToS 0.54 0.20
1001591 Table 4
Patient and Time between Burst Width
Recorded Seizure bursts (sec.) (sec.)
1 Ac1 0.71 0.30
1 AcJ 2 0.14 0.10
2 FOB 0.64 0.24
3 SU 0.27 0.14
4 Lai 0A9 0.33
5 RìJ 0.61 0.21
6 MaL 0.23 0.13
7 Mu1-1 0.12 0.20
8 WaA 0.29 0.17
9 PeAl 0.16 0.14
9 PeA2 0.17 013
10 McK1 0.39 0.1.2
10 McK2 0.13 0.18
10 McK3 0.25 0.11
10 McK5 0.29 0.13
10 McK6 0.14 0.12
10 McK7 0.23 0.09
11 ToS 0.45 0.20
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
56
1001601 For some of the monitored seizures, later portions of activity were
weak
and/or the seizure terminated rapidly. Therefore, in some of the measured
seizures only
earlier periods of activity were measured. That is, for some of the patients
and/or seizures
only initial or intermediate portions were measured.
[001611 Example 2:
(00162) In this Example 2, a specific embodiment of a method of analyzing
collected EMG signals is described. That method may, for example, be used to
analyze the
seizure data collected above for the patients in the aforementioned study. In
Example 2, a
first routine may be executed to analyze EMG data for the presence of
sustained EMG
activity the presence of which may indicate initial motor manifestations of a
seizure. A
second routine may be executed for the presence of sustained EMG activity, but
may include
higher thresholds than the first routine. A third routine configured to be
selective for clonic-
phase activity may also be executed. The responses may be executed
simultaneously on a
given portion of EMG data. And, in some embodiments, if certain conditions are
met a
warning period may be initiated.
1001631 Table 5 shows, by way of example, some settings that may be applied to
monitor the patients in the above study using the first routine of Example 2.
1001641 Table 5
Routine Setting Value
Frequency band selected for routine Full Range / 30-55 Hz / 65-90 Hz / 90 -
120 Hz
Threshold EMG level (% of MVC) 4
Required duration of threshold detection (seconds) 2
Warning time period setting (seconds) 20
(00165) Table 6 shows, by way of example, some settings that may be applied to
monitor the patients in the above study using the second routine of Example 2.
Routine Setting Value
Frequency band selected for routine A Full Range / 30-55 Hz / 65-90 Hz / 90
-120 Hz
Threshold EMG level (% of MVC) 50
Required duration of threshold detection (seconds) 2
Warning time period setting (seconds) 20
(00166) Table 7 shows, by way of example, some settings that may be applied to
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
57
monitor the patients in the above study using the third routine of Example 2.
Routine Setting Value
Frequency band selected for routine Full Range / 30-55 1-1z I 65-90 fiz /
90 -12() Hz
Threshold SNR (EMG amplitude over background) 5
Minimum burst duration threshold (milliseconds) 50
Maximum burst duration threshold (milliseconds) 300
Threshold burst count rate (Bursts/second)
Qualification routine Script ¨ X
Peak fitting routine Script X
1001671 Table 8 shows one embodiment of how a detection of different
combinations of the first, second and third routine may be organized.
1001681 Table 8
Event Routine l Routine 2 Routine 3 Classification Status
Transmission
¨ Status - Status Status Protocol
=
A) Negative Negative negative = non-
seizure no transmission
B) Positive Negative Negative possible
event trigger warning period
C) Positive Positive Negative
tonic-phase = trigger warning period /
warning transmission
protocol
D) positive or positive or Positive
clonic phase emergency transmission
negative negative protocol
1001691 With the routine settings of Example 2, a number of positive responses
may be made, and some of those responses may be from non-seizure sources.
However, an
emergency alarm does not need to be initiated for all combinations of
responses. For
example, detection may only initiate a warning (that may or may not be
provided to an
attendant or remote caregiver) and not an emergency response. Or, the system
may only
trigger a warning period of analysis to some responses, and only after the
completion of that
warning period may an emergency response be triggered. For example, as shown
in Table 8 a
warning period of about 20 seconds may be initiated if either the first or
second routines are
responsive.
1001701 If a warning period is triggered as shown, for example, as shown for
the
events A and B in Table 8, the system may continue capturing EMG data and
further classify
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
58
responses that may be made during the warning period. For example, Table 9
shows one
embodiment of how responses to various events (E-H) obtained during a warning
period may
be considered and possible outcomes to those responses.
Event Routine 1 Routine 2 Routine 3 ¨ Classification Status /
Transmission
Status - Status Status Protocol
E) Negative Negative negative non-seizure
clear warning flag
F) Positive Negative negative non-
seizure execute recalibration
maintain warning flag
G) Positive Positive negative tonic-phase
emergency transmission /
trigger other sensors
1-1) positive or positive or positive tonic-clonic
em.ergen.cy transmission /
negative negative seizure trigger other sensors
1001711 For example, if during a warning period routine 2 maintains one or
more
responses or further responses it may be deemed that an emergency transmission
protocol
should be executed. Likewise, if the routine 3 shows a positive response
within a warning
period a tonic-clonic seizure may be likely and an emergency alarm may be
executed. in
some embodiments, other sensors may be triggered. For example, a pulsed
oximeter sensor
may be triggered as shown in Table 9. And, for example, if a pulsed oximeter
shows that the
patient is under further stress, in some embodiments, the emergency response
may be
adjusted. For example, an emergency transmission may be transmitted to a
caregiver, such as
a family member or other caregiver who may be in another room of the patient's
house.
However, if saturated oxygen levels and/or the patient's pulse indicates that
the patient is
under physiological stress, EMT personnel may be immediately contacted.
[00172] Although the disclosed method and apparatus and their advantages have
been described in detail, it should be understood that various changes,
substitutions and
alterations can be made herein without departing from the invention as defined
by the
appended claims. Moreover, the scope of the present application is not
intended to be limited
to the particular embodiments of the process, machine, manufacture,
composition, or matter,
means, methods and steps described in the specification. Use of the word
"include," for
example, should be interpreted as the word "comprising" would be, i.e., as
open-ended. As
one will readily appreciate from the disclosure, processes, machines,
manufacture,
compositions of matter, means, methods, or steps, presently existing or later
to be developed
that perform substantially the same fiinction or achieve substantially the
sam.e result as the
CA 02931982 2016-05-27
WO 2015/084899
PCT/US2014/068246
59
corresponding embodiments described herein may be utilized. Accordingly, the
appended
claims are intended to include within their scope such processes, machines,
manufacture,
compositions of matter, means, methods or steps.