Note: Descriptions are shown in the official language in which they were submitted.
CA 02931988 2016-05-27
DESCRIPTION
Title of Invention
HOT-DIP ZN-ALLOY-PLATED STEEL SHEET
Technical Field
[0001] The present invention relates to a hot-dip Zn alloy-plated steel sheet
excellent in
blackening resistance.
Background Art
[0002] As plated steel sheet excellent in corrosion resistance, a hot-dip Zn
alloy-plated
steel sheet having a base steel sheet with a surface coated with a hot-dip Zn
alloy plating
layer including Al and Mg is known. The composition of the plating layer of a
hot-dip Zn
alloy-plated steel sheet includes, for example, 4,0 to 15.0% by mass of Al,
1.0 to 4.0% by
mass of Mg, 0.002 to 0.1% by mass of Ti, 0.001 to 0.045% by mass of B, and the
balance
of Zn and unavoidable impurities. The hot-dip Zn alloy-plated steel sheet
includes a
plating layer of mixed metal structure of [primary crystal Al] and [single
phase Zn] in a
matrix of [A1an/Z,n2Mg ternary eutectic structure], having sufficient
corrosion resistance
and surface appearance as an industrial product.
[0003] The hot-dip Zn alloy-plated steel sheet described above can be
continuously
produced by the following steps. First, a base steel sheet (steel strip) is
passed through a
furnace, dipped in a hot-dip Zn alloy plating bath, and then passed through,
for example, a
gas wiping apparatus, such that the amount of the molten metal adhered to the
surface of
the base steel sheet is adjusted to a specified amount. Subsequently, the
steel strip with
the specified amount of molten metal adhered thereto is passed through an air
jet cooler
and a mist cooling zone, so that the molten metal is cooled to form a hot-dip
Zn alloy
plating layer. Further, the steel strip with the hot-dip Zn alloy plating
layer is passed
1
CA 02931988 2016-05-27
2F14248-PCT
through a water quenching zone, so as to come in contact with cooling water. A
hot-dip
Zn alloy-plated steel sheet is thus obtained.
[0004] The hot-dip Zn alloy-plated steel sheet thus produced, however, allows
the surface
of the plating layer to be blackened over time in some cases. Since the
progress of
blackening of a hot-dip Zn alloy-plated steel sheet spoils the appearance with
a dark gray
color without metallic luster, a method for suppressing the blackening has
been needed.
[0005] As a method for preventing the blackening, adjusting of the temperature
of the
surface of a plating layer in the water quenching zone has been proposed (e.g.
refer to PTL
1). In the invention described in PTL 1, the temperature of the surface of
a plating layer is
adjusted at lower than 105 C when contacted with cooling water in the water
quenching
zone so that blackening of the surface of a plating layer is prevented.
Alternatively,
instead of the temperature control of the surface of a plating layer at lower
than 105 C,
readily oxidizable elements (rare earth elements, Y, Zr or Si) are added into
a plating bath
and the temperature of the surface of a plating layer is adjusted at 105 to
300 C so that
blackening of the surface of the plating layer is prevented.
Citation List
Patent Literature
[0006]
PTL 1
Japanese Patent Application Laid-Open No.2002-226958
Summary of Invention
Technical Problem
[0007] In the invention described in PTL 1, since the surface of a plating
layer is required
to be cooled to a specified temperature before passed through a water
quenching zone, the
2
CA 02931988 2016-05-27
production of a hot-dip Zn alloy-plated steel sheet is restricted in some
cases. For
example, the feed rate of a plated steel sheet having a large thickness is
required to be slow
so that the plated steel sheet is cooled to a specified temperature, resulting
in reduced
productivity. In addition, in the case of adding readily oxidizable elements
into a plating
bath, the readily oxidizable elements tend to form a dross. Consequently,
complicated
concentration control of the readily oxidizable elements is required,
resulting in a
complicated production process, which has been a problem.
[0008] An object of the present invention is to provide a hot-dip Zn alloy-
plated steel
sheet excellent in blackening resistance which can be produced without
reduction in
productivity and without complicated control of the components of a plating
bath.
Solution to Problem
[0009] The present inventors have found that the problem can be solved by
forming a
composite oxide film containing the constituent components of a plating layer
and
vanadium on the surface of the plating layer and reducing the ratio of Zn
hydroxide
contained in the composite oxide film, and accomplished the present invention
through
further study.
[0010] The present invention relates to the following hot-dip Zn alloy-plated
steel sheet.
[0011]
[1] A hot-dip Zn alloy-plated steel sheet comprising: a steel sheet; a hot-dip
Zn
alloy plating layer disposed on a surface of the steel sheet; and a composite
oxide film
disposed on a surface of the hot-dip Zn alloy plating layer; wherein the
composite oxide
film comprises constituent components of the hot-dip Zn alloy plating layer
and vanadium,
and the composite oxide film satisfies, at the whole of a surface of the
composite oxide
film, following Equation 1:
3
S[Hydroxide]
100.5_ 40
, x
S[Hydroxidd+ S[Oxidej (Equation 1)
S[O]tidel is a peak area derived from Zn oxide and centered at approximately
1022
eV in an intensity profile of the 3CPS analysis of the surface of the
composite oxide film;
and S[Hydroxide] is a peak area derived from Zn hydroxide and centred at
approximately
1023 eV in the intensity profile of the 3CPS analysis of the surface of the
composite oxide
film.
[2] The hot-dip Zn alloy-plated steel sheet according to [1], wherein: the
hot-dip Zn alloy plating layer comprises 1.0 to 22.0% by mass of Al, 0.1 to
10.0% by mass
of Mg, and the balance of the hot-dip Zn alloy plating layer being Zn and
unavoidable
impurities.
[3] The hot-dip Zn alloy-plated steer sheet according to [2], wherein: the
hot-dip Zn alloy plating layer further comprises at least one selected from
the group
consisting of 0.001 to 2.0% by mass of Si, 0.001 to 0.1% by mass of Ti, and
0.001 to
0.045% by mass of B.
[4] The hot-dip Zn alloy-plated steel sheet according to any one of [I] to
[3],
wherein the adhering amount of the vanadium contained in the composite oxide
film is in
the range of 0.01 to 10.0 mg/m2.
Advantageous Effects of Invention
[0012] According to the present invention, a hot-dip Zn alloy-plated steel
sheet excellent
in blackening resistance can be easily produced at high productivity.
Brief Description of Drawings
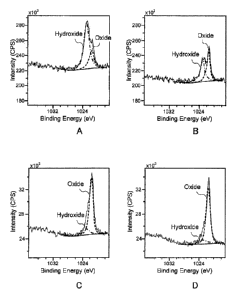
[0013]
FIGS. 1A to 1D illustrate the intensity profiles of the chemical binding
energy
CA 2 9 31 9 8 8 2 0 1 7 ¨ 1 0 ¨1 3
CA 02931988 2016-05-27
corresponding to the 2p orbitals of Zn at the surface of a composite oxide
film.
FIG. 2A illustrates an exemplary method for contacting a cooling aqueous
solution
with the surface of a hot-dip Zn alloy plating layer by a spraying process;
FIG. 2B illustrates an exemplary method for contacting a cooling aqueous
solution
with the surface of a hot-dip Zn alloy plating layer by a dipping process; and
FIG. 3 is a schematic diagram illustrating the configuration of a part of the
production line of a hot-dip Zn alloy-plated steel sheet.
Description of Embodiments
[0014] (Hot-dip Zn alloy-plated steel sheet of the present invention)
The hot-clip Zn alloy-plated steel sheet of the present invention includes a
base steel
sheet, a hot-dip Zn alloy plating layer, and a composite oxide film. The hot-
dip Zn
alloy-plated steel sheet of the present invention is excellent in blackening
resistance, by
virtue of a specified composite oxide film.
[0015] The type of the base steel sheet is not particularly limited. For
example, a steel
sheet made of low-carbon steel, medium-carbon steel, high-carbon steel, alloy
steel or the
like may be used as the base steel sheet. When excellent press formability is
required, a
steel sheet for deep drawing made of low-carbon Ti-alloyed steel, low-carbon
Nb-alloyed
steel or the like is preferably used as the base steel sheet. Alternatively, a
high-strength
steel sheet containing P, Si, Mn and the like may be used.
[0016] The hot-dip Zn alloy plating layer is disposed on the surface of a base
steel sheet.
The composition of the hot-dip Zn alloy plating layer may be appropriately
selected
depending on the purpose. For example, the plating layer includes 1.0 to 22.0%
by mass
of Al, 0.1 to 10.0% by mass of Mg, and the balance of Zn and unavoidable
impurities.
The plating layer may further include at least one selected from the group
consisting of
0.001 to 2.0% by mass of Si, 0.001 to 0.1% by mass of Ti, and 0.001 to 0.045%
by mass of
5
CA 02931988 2016-05-27
B. Examples of the hot-dip Zn alloy plating include a molten Zn-0.18% by mass
of
A1-0.09% by mass of Sb alloy plating, a molten Zn-0.18% by mass of A1-0.06% by
mass of
Sb alloy plating, a molten Zn-0.18% by mass Al alloy plating, a molten Zn-l%
by mass of
A1-1% by mass of Mg alloy plating, a molten Zn-1.5% by mass of A1-1.5% by mass
of Mg
alloy plating, a molten Zn-2.5% by mass of A1-3% by mass of Mg alloy plating,
a molten
Zn-2.5% by mass of A1-3% by mass of Mg-0.4% by mass of Si alloy plating, a
molten
Zn-3.5% by mass of A1-3% by mass of Mg alloy plating, a molten Zn-4% by mass
of
A1-0.75% by mass of Mg alloy plating, a molten Zn-6% by mass of A1-1% by mass
of
Mg-0.05% by mass of Ti-0.003% by mass of B alloy plating, a molten Zn-6% by
mass of
A1-3% by mass of Mg-0.02% by mass of Si-0.05% by mass of Ti-0.003% by mass of
B
alloy plating, a molten Zn-11% by mass of A1-3% by mass of Mg alloy plating, a
molten
Zn-11% by mass of A1-3% by mass of Mg-0.2% by mass of Si alloy plating, and a
molten
Zn-55% by mass of A1-1.6% by mass of Si alloy plating. Although blackening of
a
plating layer can be suppressed by addition of Si as described in PTL 1, in
the case of the
hot-dip Zn alloy-plated steel sheet of the present invention, blackening of a
plating layer
can be suppressed without addition of Si to the plating layer.
[0017] The amount of the hot-dip Zn alloy plating layer adhered is not
specifically
limited. The amount of the plating layer adhered may be, for example,
approximately 60
to 500 g/m2.
[0018] The composite oxide film is disposed on the surface of a hot-dip Zn
alloy plating
layer, preferably on the entire surface. The composite oxide film mainly
contains
constituent components of the hot-dip Zn alloy plating layer (e.g. Zn, Al and
Mg) and
vanadium. The composite oxide film satisfies, at the entire surface, the
following
equation 2.
S[Hydroxide]
,x100 40
S[Hydroxidd+ S[Oxide] (Equation 2)
6
CA 02931988 2016-05-27
wherein S [Oxide] is a peak area derived from the Zn oxide and centered at
approximately 1022 eV in the intensity profile of the XPS analysis of the
surface of a
composite oxide film; and S[Hydroxide] is a peak area derived from the Zn
hydroxide and
centered at approximately 1023 eV in the intensity profile of the XPS analysis
of the
surface of a composite oxide film.
[0019] The equation 2 indicates that the ratio of the peak area derived from
the Zn
hydroxide and centered at approximately 1023 eV (hereinafter referred to as
"hydroxide
ratio") is 40% or less relative to the total of the peak area derived from the
Zn oxide and
centered at approximately 1022 eV and a peak area derived from the Zn
hydroxide and
centered at approximately 1023 eV in the intensity profile measured in the XPS
analysis.
[0020] FIGS. IA to, ID illustrate the intensity profiles of the chemical
bonding energy
corresponding to the 2p orbitals of Zn at the surface of the composite oxide
film of a
hot-dip Zn alloy-plated steel sheet. FIG. lA illustrates the intensity profile
with a Zn
hydroxide ratio of approximately 80%, FIG. IB illustrates the intensity
profile with a Zn
hydroxide ratio of approximately 40%, FIG. 1C illustrates the intensity
profile with a Zn
hydroxide ratio of approximately 15%, and FIG 1D illustrates the intensity
profile with a
Zn hydroxide ratio of approximately 10%. A dotted line is the base line, a
broken line is
the intensity profile derived from Zn oxide (a peak centered at approximately
1022 eV),
arid a dashed dotted line is the intensity profile derived from Zn hydroxide
(a peak centered
at approximately 1023 eV). In the hot-dip Zn alloy-plated steel sheet of the
present
invention, the Zn hydroxide ratio is 40% or less over the entire surface of
the plating layer
as shown in FIGS. 1B to 1D.
[0021] The XPS analysis of the surface of the composite oxide film of a hot-
dip Zn
alloy-plated steel sheet of the present invention is performed using an XPS
analyzer (AXIS
Nova, produced by Kratos Group PLC.). The peak area derived from Zn oxide and
centered at approximately 1022 eV and the peak area derived from Zn hydroxide
and
7
CA 02931988 2016-05-27
2F 14248-P CT
centered at approximately 1023 eV are calculated using software (Vision 2)
attached to the
XPS analyzer.
[0022] The position of the peak derived from Zn oxide is precisely at 1021.6
eV, and the
position of the peak derived from Zn hydroxide is precisely at 1023.3 eV.
These values
may change in some cases due to characteristics of XPS analysis, contamination
of a
sample, and charging of a sample. Those skilled in the art, however, are
capable of
distinguishing the peak derived from Zn oxide from the peak derived from Zn
hydroxide.
[0023] The adhering amount of the vanadium in the composite oxide film is not
specifically limited, but preferably in the range of 0.01 to 10.0 mg/m2. With
an adhering
amount of the vanadium of 0.01 mg/na2 or more, the blackening resistance can
be further
improved. With an adhering amount of the vanadium of 10.0 mg/m2 or less, the
reactivity
with a chemical conversion liquid for chemical conversion treatment can be
improved.
The adhering amount of vanadium in a composite oxide film can be measured
using an ICP
emission analyzer.
[0024] (Producing method of hot-dip Zn alloy-plated steel sheet of the present
invention)
The producing method of a hot-dip Zn alloy-plated steel sheet of the present
invention is not specifically limited. For example, the hot-dip Zn alloy-
plated steel sheet
of the present invention may be produced by: (1) a first step of forming a hot-
dip Zn alloy
plating layer (hereinafter, also referred to as "plating layer") on the
surface of a base steel
sheet; and (2) a second step of contacting a specified aqueous solution with
the surface of
the plating layer for cooling of the base steel sheet and the plating layer at
a raised
temperature through formation of the plating layer, and for forming a
composite oxide film.
Each of the steps is described as follows.
[0025] (1) First step
In the first step, a base steel sheet is dipped in a hot-dip Zn alloy plating
bath, so that
a hot-dip Zn alloy plating layer is formed on the surface of the base steel
sheet.
8
CA 02931988 2016-05-27
2F 14248-PCT
[0026] First, a base steel sheet is dipped in a hot-dip Zn alloy plating bath,
and a
specified amount of molten metal is allowed to adhere to the surface of the
base steel sheet
by gas wiping or the like. As described above, the type of the base steel
sheet is not
specifically limited. The composition of the plating bath is appropriately
selected
depending on the composition of the hot-dip Zn alloy plating layer to be
formed.
[0027] Subsequently, the molten metal adhered to the surface of a base steel
sheet is
cooled to a temperature equal to or more than 100 C and equal to or less than
the
solidifying point of the plating layer so as to be solidified. A plated steel
sheet is thus
formed, having a plating layer with a composition approximately the same as
the
composition of the plating bath, on the surface of the base steel sheet.
[0028] (2) Second step
In the second step, a specified cooling aqueous solution is contacted with the
surface
of the hot-dip Zn alloy plating layer, so that the base steel sheet and the
plating layer at a
raised temperature through formation of the hot-dip Zn alloy plating layer are
cooled. In
this step, a composite oxide film is formed on the surface of the plating
layer. From the
viewpoint of productivity, the second step is performed preferably by water
quenching
(water cooling). In this case, the temperature of the surface of the hot-dip
Zn alloy plating
layer when the cooling aqueous solution is to be contacted with the surfarle
of the hot-dip
Zn alloy plating layer is equal to or more than 1 00 C and approximately equal
to or less
than the solidifying point of the plating layer.
[0029] The cooling aqueous solution is formed of an aqueous solution
containing a
vanadium compound. The concentration of the vanadium compound in the cooling
aqueous solution is preferably 0.01 g/L or more in terms of V element. When a
concentration of the vanadium compound is less than 0.01 g/L in terms of V
element,
blackening of the surface of a composite oxide film may not be sufficiently
prevented.
[0030] The method for preparing the aqueous solution (cooling aqueous
solution)
9
CA 02931988 2016-05-27
2F14248-P CT
containing a vanadium compound is not specifically limited. For example, a
vanadium
compound and a dissolution promoter on an as needed basis, may be dissolved in
water
(solvent). Preferable examples of the vanadium compound include acptylacetone
vanadyl,
vanadium acetylacetonate, vanadium oxysulfate, vanadium pentoxide, and
ammonium
vanadate. These vanadium compounds may be used singly or in combination.
[0031] In the case of adding a dissolution promoter, the amount of the
dissolution
promoter added is not specifically limited. For example, 90 to 130 parts by
mass of the
dissolution promoter may be added to 100 parts by mass of the vanadium
compound.
With an excessively small amount of the dissolution promoter added, the
vanadium
compound cannot be sufficiently dissolved in some cases. On the other hand,
with an
excessively large amount of the dissolution promoter added, the effect is
saturated,
resulting in a cost disadvantage.
[0032] Examples of the dissolution promoter include 2-aminoethano1,
tetraethylarnmonium hydroxide, ethylene diamine, 2,2'-iminodiethanol, and
1 -amino -2-prop anol.
[0033] The method for contacting the cooling aqueous solution with the surface
of a
hot-dip Zn alloy plating layer is not specifically limited. Examples of the
method for
contacting the cooling aqueous solution with the surface of a hot-dip Zn alloy
plating layer
include a spraying process and a dipping process.
[0034] FIGS. 2A and 2B illustrate exemplary methods for contacting a cooling
aqueous
solution with the surface of a hot-dip Zn alloy plating layer. FIG. 2A
illustrates an
exemplary method for contacting a cooling aqueous solution with the surface of
a hot-dip
Zn alloy plating layer by a spraying process. FIG. 2B illustrates an exemplary
method for
contacting a cooling aqueous solution with the surface of a hot-dip Zn alloy
plating layer
by a dipping process.
[0035] As shown in FIG. 2A, cooling apparatus 100 for spraying process
includes a
CA 02931988 2016-05-27
plurality of spray nozzles 110, squeeze rollers 120 disposed downstream of
spray nozzles
110 in the feed direction of a steel strip S, and housing 130 which covers the
nozzles and
the rollers. Spray nozzles 110 are disposed on both sides of the steel strip
S. The steel
strip S is cooled by a cooling aqueous solution supplied from spray nozzles
110 such that a
water film is temporarily formed on the surface of the plating layer, inside
housing 130.
The cooling aqueous solution is then removed with squeeze roller 120. The
adhering
amount of vanadium in the composite oxide film can be adjusted by controlling
the
opening of squeeze rollers 120.
[0036] As shown in FIG. 2B, cooling apparatus 200 for dipping process includes
dip tank
210 in which a cooling aqueous solution is stored, dip roller 220 disposed
inside dip tank
210, and squeeze rollers 230 disposed downstream of dip roller 220 in the feed
direction of
the steel strip S so as to remove the extra cooling aqueous solution adhered
to the steel strip
S. The steel strip S fed into dip tank 210 is then contacted with the cooling
aqueous
solution so as to be cooled. The steel strip S is then subjected to a turn of
direction by the
rotating dip roller 220, and pulled upward. The cooling aqueous solution is
removed with
squeeze roller 230. The adhering amount of vanadium in the composite oxide
film can be
adjusted by controlling the opening of squeeze rollers 230.
[0037] According to the procedure described above, a hot-dip Zn alloy-plated
steel sheet
of the present invention can be produced.
[0038] Although the composite oxide film was formed through contact with an
aqueous
solution containing a vanadium compound in the water quenching step, it is
conceivable
that a composite oxide film can be also formed by applying an aqueous solution
containing
a vanadium compound to a cooled hot-dip Zn alloy-plated steel sheet and drying
the
applied aqueous solution (post-treatment method). Accordingly, the present
inventors
tried to form a composite oxide film by applying an aqueous solution
containing a
vanadium compound (the same aqueous solution as that used in the producing
method
11
CA 02931988 2016-05-27
described above) to a hot-dip Zn alloy-plated steel sheet cooled to normal
temperature with
a general industrial water, and drying the applied aqueous solution. Although
a composite
oxide film containing constituent components of a plating layer and vanadium
was also
formed on the surface of the plating layer through such a post-treatment
method, the
composite oxide film had a Zn hydroxide ratio of more than 40%. The hot-dip Zn
alloy-plated steel sheet thus produced had no outstanding difference in
blackening
resistance compared with a hot-dip Zn alloy-plated steel plate having no
composite oxide
film.
[0039] The reason is not clear why the hot-dip Zn alloy-plated steel sheet of
the present
invention has higher blackening resistance than a hot-dip Zn alloy-plated
steel sheet having
no composite oxide film. As described above, the hot-dip Zn alloy-plated steel
sheet
produced by the post-treatment method has a Zn hydroxide ratio of more than
40% in the
composite oxide film, which is different from that of the hot-dip Zn alloy-
plated steel sheet
of the present invention. Furthermore, the blackening resistance is notably
different
between the hot-dip Zn alloy-plated steel sheet of the present invention and
the hot-dip Zn
alloy-plated steel sheet produced by the post-treatment method. It is
therefore
conceivable that the stability of Zn contained in the composite oxide film is
different
between the hot-dip Zn alloy-plated steel sheet of the present invention and
the hot-dip Zn
4
alloy-plated steel sheet produced by the post-treatment method, and the Zn
contained in the
composite oxide film of the hot-dip Zn alloy-plated steel sheet of the present
invention is
more difficult to transform into an oxygen-deficient zinc oxide as the source
of blackening.
This may be the reason why the hot-dip Zn alloy-plated steel sheet of the
present invention
has higher blackening resistance.
[0040] (Production line)
The hot-dip Zn alloy-plated steel sheet of the present invention may be
produced, for
example, in the following production line. ,
12
CA 02931988 2016-05-27
[0041] FIG 3 is a schematic diagram illustrating a part of production line 300
of a
hot-dip Zn alloy-plated steel sheet. Production line 300 forms a plating layer
and a
composite oxide film on the surface of a base steel sheet (steel strip), and
can continuously
produce hot-dip Zn alloy-plated steel sheets of the present invention.
Production line 300
may further form a chemical conversion coating on the surface of the composite
oxide film
on an as needed basis, and can continuously produce plated steel sheets with
chemical
conversion treatment.
[0042] As shown in FIG 3, production line 300 includes furnace 310, plating
bath 320,
air jet cooler 340, ,mist cooling zone 350, water quenching zone 360, skin
pass mill 370,
and tension leveler 380.
[0043] The steel strip S fed from a feeding reel not shown in drawing through
a
predetermined step is heated in furnace 310. The heated steel strip S is
dipped in plating
bath 320, so that molten metal is adhered to both sides of the steel strip S.
An excess
amount of molten metal is then removed with a wiping apparatus having wiping
nozzle 330,
allowing a specified amount of molten metal to be adhered to the surface of
the steel strip
S.
[0044] The steel strip S with a specified amount of molten metal adhered
thereto is
cooled to the solidifying point of the molten metal or lower by air jet cooler
340 or in mist
cooling zone 350. Air jet cooler 340 is a facility for cooling the steel strip
S by spraying a
gas. Mist cooling zone 350 is a facility for cooling the steel strip S by
spraying atomized
fluid (e.g. cooling water) and a gas. The molten metal is thereby solidified,
so that a
hot-dip Zn alloy plating layer is formed on the surface of the steel strip S.
When the steel
strip s is cooled in mist cooling zone 350, no water film is formed on the
surface of the
plating layer. The temperature after cooling is not specifically limited, and
may be, for
example, 100 to 250 C.
[0045] The hot-dip Zn alloy-plated steel sheet cooled to a specified
temperature is further
13
cooled in water quenching zone 360. Water quenching zone 360 is a facility for
cooling
the steel strip S through contact with a large amount of cooling water in
comparison with
mist cooling zone 350, supplying an amount of water to form a temporary water
film on
the surface of the plating layer. For example, water quenching zone 360
includm headers
having 10 flat spray nozzles disposed at intervals of 150 ram in the width
direction of the
steel strip S, which are disposed in 7 rows in the feeding direction of the
base steel sheet S.
In water quenching zone 360, an aqueous solution containing a vanadium
compound is
used as cooling aqueous solution. The steel strip S is cooled in water
quenching zone 360,
with the cooling aqueous solution in an amount to temporarily foam a water
film on the
surface of the plating layer being supplied. For example, the cooling aqueous
solution has
a water temperature of approximately 20 C, a water pressure of approximately
2.5 kgf/cm?,
and a water quantity of approximately 150 m3/I1. The phrase "a water film is
temporarily
formed' means a state allowing a water filth in contact with a hot-dip Zn
alloy-plated steer
sheet to be visually observed far approximately one second or more. Through
cooling
using an aqueous solution of a vanadium compound in water quenching zone 360,
a
composite oxide film containing the constituent components of a plating layer
and
vanadium with a Zn hydroxide of 40% or less is formed on the surface of the
plating
layer.
= [0046] The water-cooled hot-dip Zn alloy-plated steel sheet is rolled for
thermal refining
by skin pass mill 370, corrected to fiat by tension leveler 380, and then
wound onto tension
reel 390.
[0047] When a chemical conversion coating is further formed on the surface of
a plating
layer, a specified chemical conversion treatment liquid is applied to the
surface of the
hot-clip Zn alloy-plated steel sheet corrected by tension leveler 380, with
roll coater 400.
The hot-dip Zn alloy-plated steel sheet through the chemical conversion
treatment is dried
and cooled in drying zone 410 and air cooling zone 420, and then wound onto
tension reel
14
CA 2 9 31 9 8 8 2 0 1 7 ¨ 1 0 ¨1 3
CA 02931988 2016-05-27
390.
[0048] As described above, the hot-dip Zn alloy-plated steel sheet of the
present
invention has excellent blackening resistance and can be easily produced at
high
productivity.
[0049] The present invention is described in detail with reference to Examples
as follows.
The present invention is, however, not limited to the Examples.
Examples
[0050] (Experiment 1)
In Experiment 1, the blackening resistance of a hot-clip Zn alloy-plated steel
sheet
was examined for the hot-dip Zn alloy-plated steel sheet cooled using a
cooling water
containing a metal compound after plating.
[0051] 1. Production of hot-dip Zn alloy-plated steel sheet
Using production line 300 shown in FIG. 3, hot-dip Zn alloy-plated steel
sheets were
produced. A hot-rolled steel strip with a sheet thickness of 2.3 mm was
prepared as base
steel sheet (steel strip) S. Plating was applied to the base steel sheet using
the plating bath
compositions and conditions described in Table I, so that 14 types of hot-dip
Zn
alloy-plated steel sheets having different plating layer compositions from
each other were
produced. The composition of the plating bath and the composition of the
plating layer
are approximately the same.
[0052]
[Table 1]
Plating bath composition (balance: Zn) (% by mass) Plating conditions
Sheet
Plating Bath Adhering
passing
No. Al Mg Si Ti B Sb temperature amount
( C) (ghn) speed
(m/min)
1 , 0.18 ¨ ¨ ¨ 0.09 430 90 80
2 0.18 ¨ ¨ ¨ 0.06 430 90 80 _
R
3 0.18 ¨ ¨ ¨ ¨ 430 90 80
,
4 1 1 ¨ 430 90 80
.
0,
0,
1.5 1.5 ¨ ¨ ¨ 430 90 80
.
6 2.5 3 ¨ ¨ ¨ ¨ 430 90 80
.
7 2.5 3 0.4 ¨ 430 90 80
..,
8 3.5 3 _ ¨ ¨ ¨ 430 90 80
9 4 0.75 ¨ ¨ ¨ 430 90 80
-
6 3 0.05 0.003 ¨ 430 90 80
11 6 3 0.02 0.05 0.003_ ¨ 430 = 90
80
12 11 3 ¨ ¨ ¨ 450 90 80
13 11 3 0.2 ¨ ¨ ¨ 450 90 80
14 55 ¨ 1.6 ¨ ¨ ¨ 600 90 80
16
CA 02931988 2016-05-27
[0053] In production of a hot-dip Zn alloy-plated steel sheet, the cooling
conditions in air
jet cooler 340 were changed, such that the temperature of the steel sheet (the
surface of
plating layer) is adjusted at 200 C immediately before passing through water
quenching
zone 360. In water quenching zone 360, any one of the aqueous solution
described in
Table 2 was used as cooling aqueous solution for formation of the composite
oxide film.
Each of the cooing aqueous solutions was prepared by dissolving the metal
compound
described in Table 2 and a dissolution promotor on an as needed basis at a
specified ratio in
a water having a pH of 7.6, and adjusting the water temperature to 20 C. The
concentration of the metal compound in each of the cooling aqueous solutions
was 250
mg/L in terms of metal element in any case. The spray apparatus in water
quenching zone
360 for use includes headers having 10 flat spray nozzles disposed at
intervals of 150 mm
in the width direction, which are disposed in 7 rows in the feeding direction
of the base
steel sheet S. Each of the cooling aqueous solutions supplied from water
quenching zone
360 was under conditions with a water pressure of 2.5 kgf/cm2 and a water
quantity of 150
m3/h.
[0054] As Comparative Example, a composite oxide film was formed by using a
water
containing no metal compound instead of using any one of the aqueous solutions
described
in Table 2 in water quenching zone 360, then applying any of the aqueous
solutions
described in Table 2 by a roll coat method or a spray ringer roll method, and
drying the
applied aqueous solution (post-treatment method).
17
[0055]
[Table 2]
Cooling Metal compound (A) Dissolution promoter
(B)
Category water Amount added Ratio of
amount
Name Name
No. (rng/L) added
(B/A)
Tetraethylanunoniurn
1709
1 Vanadium acetylacetonate 1.1
hydroxide
2 _ Acetylacetonate vanadyl 1301
Ethylene diamine 1.3
Example 3 Ammonium metavanadate 574 ¨
R
4 Sodium metavanadate 598 ¨ ¨
.
,...
,
Divanadium tetroxide _ 407 2,2'-lminodiethanol
0.9 ..
¶,
..
6 Vanadium pentoxide 446 , 1-Amino-2-propanol
1.1 .
7 Ammonium chromate 606 ¨ ¨
.
Lr
,
r,
...,
Comparative 8 Potassium chromate 467 ¨ ¨
Example 9 Sodium silicate 1087 ¨ ¨
18
_
CA 02931988 2016-05-27
[0056] 2. Evaluation of hot-dip Zn alloy-plated steel sheet
(1) Measurement of Zn (OH)2 ratio on surface of composite oxide film
For each of the hot-dip Zn alloy-plated steel sheets, the Zn hydroxide ratio
on the
surface of the composite oxide film was measured using an XPS analyzer (AXIS
Nova,
produced by Kratos Group PLC.). The Zn hydroxide ratio was calculated using
software
(Vision 2) attached to the XPS analyzer.
[0057] (2) Measurement of adhering amount of V on surface of composite oxide
film
For each of the hot-dip Zn alloy-plated steel sheets, the adhering amount of
vanadium on the surface of the composite oxide film was measured using an ICP
emission
analyzer (ICPS-8100, produced by Shimarlzu Corporation).
[0058] (3) Treatment for accelerating deterioration of gloss
A test piece was cut out from each of the produced hot-dip Zn alloy-plated
steel
sheets. Each of the test pieces was placed in a thermo-hygrostat (LHU-113,
produced by
Espec Corp.), and subjected to a treatment for accelerating deterioration of
the gloss at a
temperature 70 C, with a relative humidity of 90%, for 72 hours.
[0059] (4) Measurement of degree of blackening
The brightness (L* value) at the surface of the plating layer for each of the
hot-dip
Zn alloy-plated steel sheets was measured before and after the treatment for
accelerating
deterioration of the gloss. The brightness (L* value) at the surface of the
plating layer
was measured using a spectroscopic color difference meter (TC-1800, produced
by Tokyo
Denshoku Co., Ltd), by spectral reflectance measurement in accordance with
.TIS K 5600.
The measurement conditions are as follows:
Optical condition: d/8 method (double beam optical system)
Field of view: 2-degree field of view
Measurement method: reflectometry
Standard illuminant: C
19
CA 02931988 2016-05-27
Color system: CIELAB
Measurement wavelength: 380 to 780 am
Measurement wavelength interval: 5 am
Spectroscope: 1,200/mm diffraction grating
Lighting: halogen lamp (voltage: 12 V, power: 50 W, rating life: 2,000 hours)
Measurement area: 7.25 mm diameter
Detection element: photomultiplier tube (R928 produced by Hamamatsu Photonics
K.K.)
Reflectance: 0 to 150%
Measurement temperature: 23 C
Standard plate: white
[0060] For each of the plated steel sheets, the evaluation was ranked as "A"
for a
difference in L* values (AL*) between before and after the treatment for
accelerating
deterioration of the gloss of less than 1, "B" for a difference of 1 or more
and less than 3,
"C" for a difference of 3 or more and less than 7, and "D" for a difference of
7 or more. It
can be determined that a plated steel sheet evaluated as "A" or "B" has
blackening
resistance.
[0061] (4) Evaluation results
For each of the plated steel sheets, the relation between the type of the
cooling
aqueous solution for use and the method for forming the composite oxide film
(a water
quenching method (WQ), a roll coat method (RC), or a spray ringer roll method
(SP)), and
the Zn hydroxide ratio, the adhering amount of V and the evaluation results of
the degree
of blackening is described in Table 3 to Table 6.
CA 02931988 2016-05-27
[0062]
[Table 3]
Adhering
Test Cooling
Plating Treatment Hydroxide
Amount Blackening
Category piece water
No. method Ratio (%) of V test result
No. No.
(Ingini2)
Ex. 1 11 1 WQ 7 0.004 B
Ex. 2 11 2 WQ 11 0.004 B
Ex. 3 11 3 WQ 7 0.005 B
Ex. 4 11 4 WQ 13 0.004 B
Ex. 5 11 5 WQ 7 0.005 B
Ex. 6 11 6 WQ 25 0.005 B
_
Ex. 7 11 1 WQ 6 0.01 A
Ex. 8 11 2 WQ 11 0.017 A
Ex. 9 11 3 WQ 16 0.013 A
Ex. 10 11 4 WQ 19 0.022 A
Ex. 11 11 , 5 WQ 23 0.029 A
Ex. 12 11 6 WQ 24 0.027 A
Ex. 13 11 1 WQ 8 0.13 A
,
Ex. 14 11 2 WQ 18 0.18 A
Ex. 15 11 3 WQ 21 0.17 A
Ex. 16 11 4 WQ 14 0.12 A
Ex. 17 _ 11 5 WQ 25 0.16 A
Ex. 18 11 6 WQ 18 0.18 A
Ex. 19 11 1 WQ 22 1.02 A
_
Ex. 20 11 2 WQ 7 1.01 A
Ex. 21 11 3 WQ 23 0.96 A
Ex. 22 11 4 WQ 7 0.96 A
_
Ex. 23 11 5 WQ 5 0.98 A
_
Ex. 24 11 6 WQ 19 1.01 A
Ex. 25 11 1 WQ 20 , 7.95 A
Ex. 26 11 2 WQ 16 7.98 A
Ex. 27 11 3 WQ 6 8.02 _ A
Ex. 28 11 4 WQ 21 8.05 A
Ex. 29 11 5 WQ 6 8.01 A
Ex. 30 11 6 WQ 18 8.04 A
21
CA 02931988 2016-05-27
[0063]
[Table 4]
Adhering
Test Cooling
Plating Treatment Hydroxide
Amount Blackening
Category piece water
No. method Ratio (%) of V test result
No. No.
(mg/m2)
Ex. 31 11 1 WQ 13 15.04 A
Ex. 32 11 2 WQ 8 14.97 A
Ex. 33 11 3 WQ 17 14.98 A
Ex. 34 11 4 WQ 5 14.99 A
Ex. 35 11 5 WQ 14 14.97 A
Ex. 36 11 6 WQ 17 14.96 A
Comp. Ex, 37 11 7 WQ 19 0 C
Comp. Ex, 38 11 8 WQ 9 0 C
Comp. Ex. 39 11 9 WQ 24 0 D
Comp. Ex. 40 11 1 RC 76 1.03 D
Comp. Ex. 41 11 2 RC 76 0.96 D
Comp. Ex. , 42 11 3 RC 65 0.99 D
Comp. Ex. 43 11 4 , RC 71 7.96 D
_
Comp. Ex. 44 11 5 RC 83 7.96 D
Comp. Ex. 45 11 6 RC 76 8.01 D
Comp. Ex. 46 11 1 SP 76 1.06 D
Comp. Ex. 47 11 2 _ SP 76 1.05 D
Comp. Ex. 48 11 3 SP 65 1.01 D
Comp. Ex. 49 11 4 SP 71 8.03 D
Comp. Ex. 50 11 5 SP 83 8.03 D
Comp. Ex. 51 11 6 SP 76 8.03 D J
22
CA 02931988 2016-05-27
2F14248-PCT
[0064]
[Table 5]
Adhering
Test Cooling
Plating Treatment Hydroxide
Amount Blackening
Category piece water
No. method Ratio (%) of V test result
No. No.
(mg/m2) _
_ ____________________________________________________
Ex. 52 9 1 WQ 11 0.005 B
Ex. 53 14 2 WQ 12 0.004 B
Ex. 54 2 3 WQ 7 0.007 B
Ex. 55 10 4 WQ 12 0.005 B
Ex. 56 1 5 WQ 15 0.003 B
_ ____________________________________________________
Ex. 57 12 6 WQ 22 0.005 B
Ex. 58 5 1 WQ 14 0.024 A
Ex. 59 8 2 WQ 8 0.019 A
Ex. 60 13 3 WQ 11 0.022 A
Ex. 61 3 4 WQ 14 0.017 A
Ex. 62 10 5 WQ 8 0.021 A
Ex. 63 4 6 WQ 24 0.023 A .
Ex. 64 13 1 WQ 20 0.221 A
Ex. 65 7 2 WQ 21 0.239 A
Ex. 66 12 3 WQ 6 , 0.217 A
-
Ex. 67 9 4 WQ 5 0.224 A
Ex. 68 7 5 WQ 16 0.189 A
Ex. 69 5 6 WQ 12 0.24 A
_ ____________________________________________________
Ex. 70 12 1 WQ 15 1.08 A
Ex. 71 9 . 2 WQ 6 1.05 A
Ex. 72 4 3 WQ 9 0.98 A
Ex. 73 1 4 WQ 14 0.97 A
Ex. 74 14 5 WQ 8 0.95 A
Ex. 75 3 6 WQ 10 1.04 A
Ex. 76 10 1 WQ 10 7.85 A
Ex. 77 8 2 WQ 6 7.81 A
Ex. 78 13 3 WQ 19 8.19 A
Ex. 79 10 4 WQ , 22 7.81 A _
Ex. 80 6 5 WQ 8 8.12 A
Ex. 81 12 6 WQ 15 8.09 A
23
'
CA 02931988 2016-05-27
[0065]
[Table 6]
Adhering
Test Cooling
Plating Treatment Hydroxide
Amount Blackening
Category piece water
No. method Ratio (%) of V test result
No. No.
(mg/m2)
Ex. 82 5 1 _ WQ , 24 15.16 A
_
Ex. 83 9 2 WQ 24 15.01 A
Ex. 84 1 3 WQ 18 15.08 A
Ex. 85 2 , 4 WQ 6 _ 14.96 A
Ex. 86 13 5 WQ 12 15.05 A
Ex. 87 6 6 WQ 11 15.04 A
Comp. Ex. 88 13 _ 7 WQ 20 0 C
-
Comp. Ex. 89 12 8 WQ 5 0 C
Comp. Ex. 90 10 _ 9 WQ 12 0 D
Comp. Ex. 91 9 1 RC 72 1.02 D
_
Comp. Ex. 92 14 2 RC 70 0.96 D
Comp. Ex. 93 12 3 RC 88 0.91 D
Comp. Ex. 94 8 4 RC 74 0.97 D
-
Comp. Ex. 95 9 5 RC 67 0.91 D
Comp. Ex, 96 5 6 RC 65 1,08 D
Comp. Ex. 97 9 1 SP 72 0.99 D
Comp. Ex. 98 14 2 SP 70 0,96 D
Comp. Ex. 99 12 , 3 SP 88 Q.83 D
Comp. Ex. 100 8 4 SP 74 0.88 D
Comp. Ex. 101 9 5 SP 67 0.81 D
Comp. Ex. 102 5 6 SP 65 1.07 D
[0066] As shown in Table 3 to Table 6, in the case of cooling using an aqueous
solution
containing vanadium in water quenching zone 360, a composite oxide film
containing
vanadium was formed having the surface with a Zn hydroxide ratio of 40% or
less, and
excellent blackening resistance. In contrast, in the case of cooling using an
aqueous
solution containing no vanadium in water quenching zone 360, a composite oxide
film
containing no vanadium was formed, and blackening was insufficiently
suppressed. In
24
CA 02931988 2016-05-27
the case of application of an aqueous solution containing vanadium by a roll
coat method
or a spray ringer roll method, a composite oxide film was formed, having the
surface with a
Zn hydroxide ratio of more than 40%, and blackening was insufficiently
suppressed.
[0067] From the comparison of the blackening resistance of the test pieces
Nos. 1 to 6
and Nos. 52 to 57 with the blackening resistance of the test pieces Nos. 7 to
36 and Nos. 58
to 87, it is found that the blackening resistance is particularly excellent in
the case of an
adhering amount of vanadium in the composite oxide film of 0.01 mg/m2 or more.
[0068] From the results described above, it is found that the cooling using an
aqueous
solution containing vanadium in water quenching zone 360 allows a composite
oxide film
to be formed, which contains vanadium and has the surface with a Zn hydroxide
ratio of
40% or less. The plated steel sheet having such a composite oxide film is
excellent in
blackening resistance.
[0069] (Experiment 2)
In Experiment 2, the 102 types of hot-dip Zn alloy-plated steel sheets
produced in
Experiment 1 were subjected to a chemical conversion treatment under the
following
chemical conversion treatment conditions A to C. Blackening resistance was
measured
when the treatment for accelerating deterioration of the gloss was carried out
in the same
manner as in Experiment 1. The appearance after the chemical conversion
treatment was
also evaluated.
[0070] In chemical conversion treatment conditions A, ZINCHROME 3387N (chrome
concentration: 10 g/L, produced by Nihon Parkerizing Co., Ltd.) was used as
chemical
conversion treatment liquid. The chemical conversion treatment liquid was
applied to
have an adhering amount of chromium of 10 mg/m2 by a spray ringer roll method.
[0071] In chemical conversion treatment conditions B, an aqueous solution
containing 50
g/L of magnesium phosphate, 10 g/L of potassium fluorotitanate, and 3 g/L of
an organic
acid was used as chemical conversion treatment liquid. The chemical conversion
CA 02931988 2016-05-27
treatment liquid was applied to have an adhering amount of metal components of
50 mg/m2
by a roll coat method.
[0072] In chemical conversion treatment conditions C, an aqueous solution
containing 20
g/L of a urethane resin, 3 g/L of ammonium dihydrogen phosphate, and I g/L of
vanadium
pentoxide was used as chemical conversion treatment liquid. The chemical
conversion
treatment liquid was applied to have a dried film thickness of 2 gm by a roll
coat method.
[0073] In the evaluation of the appearance for each of the plated steel sheets
after the
chemical conversion treatment, the evaluation was ranked as "B" for the
chemical
conversion treatment coating having no white turbidity, and "D" for the
chemical
conversion treatment coating having white turbidity.
[0074] For each of the plated steel sheets, the relation between the type of
the original
sheet for the chemical conversion treatment and the type of chemical
conversion treatment,
and the evaluation results of the degree of blackening and the appearance is
described in
Table 7 to Table 10.
26
CA 02931988 2016-05-27
[0075]
[Table 7]
Original sheet for
Chemical
Test piecechemical conversion Blackening
Category conversion Appearance
No. treatment test result
treatment
(test piece No.)
Ex. 103 A 1 B B
Ex. 104 B 2 B B
_
Ex. 105 C 3 B B
_ Ex. 106 A 4 B ' B
Ex. 107 B 5 B B
Ex. 108 C 6 B B
Ex. 109 A ., 7 A B
Ex. 110 B 8 A B
Ex. 111 C 9 A B
Ex. 112 A 10 A B
Ex_ 113 B 11 A B
Ex. 114 C 12 A B
Ex. 115 A 13 A B
Ex. 116 B 14 A B
Ex. 117 C 15 A B
Ex. 118 , A 16 A B
Ex. 119 B 17 A B ,
Ex. 120 , C 18 A B
Ex. 121 A , 19 , A B
Ex. 122 B 20 A B
Ex. 123 C 21 A B
Ex. 124 A 22 A B
_
Ex. 125 B 23 A B
Ex. 126 C 24 A B
_
Ex. 127 A 25 A B
Ex. 128 B 26 _ A B
_
Ex. 129 C 27 A B
¨
Ex. 130 A 28 A B
Ex. 131 B _ 29 A B
_
Ex. 132 C 30 A B
27
CA 02931988 2016-05-27
[0076]
[Table 8]
Original sheet for
Chemical
Test piece chemical conversion Blackening
Category conversion Appearance
No. treatment test result
treatment
(test piece No.) _
Ex. 133 A 31 A D
_
Ex. 134 B 32 A D
Ex. 135 C 33 A D
Ex. 136 A 34 A _ D
_
Ex. 137 B 35 A D
Ex. 138 C 36 A , D
Comp. Ex. 139 A 37 D B
Comp. Ex. 140 B 38 D B
Comp. Ex. 141 C 39 , D B
Comp. Ex. 142 A 40 D B
. Comp. Ex. 143 ' B 41 _ D B
Comp. Ex. 144 C 42 D B
Comp. Ex. 145 A 43 D , B
Comp. Ex. 146 B 44 D B
Comp. Ex. 147 C 45 D B
Comp. Ex. 148 A 46 D B
Comp. Ex. 149 B 47 D B
Comp. Ex. 150 C 48 D B
-
Comp. Ex. 151 A 49 D B
_ _
Comp. Ex. 152 B 50 D B
Comp. Ex. 153 C 51 D a
28
CA 02931988 2016-05-27
[0077]
[Table 9]
Original sheet for
Chemical
Test piece chemical conversion Blackening
Category conversion Appearance
No. treatment test result
treatment
(test piece No.)
Ex. 154 A 52 B B
Ex. 155 B 53 B B
Ex. 156 C 54 B B
Ex. 157 A 55 B B
Ex. 158 B 56 B B
Ex. 159 C 57 B B
Ex. 160 A 58 A B
Ex. 161 _ B 59 A B
Ex. 162 C 60 A B
Ex. 163 A 61 A B
_
Ex. 164 B 62 A B
Ex. 165 C 63 A B
Ex. 166 A 64 A B
Ex. 167 B 65 A B
Ex. 168 C 66 A B
Ex. 169 A 67 A B
_
Ex. 170 B 68 A B
Ex. 171 C 69 A B
Ex. 172 A 70 A B
Ex. 173 B 71 A B
Ex. 174 C 72 A B
Ex. 175 A 73 A , B
Ex, 176 B 74 A B
Ex. 177 C 75 A B
Ex. 178 A 76 A B
_
Ex. 179 B , 77 A B
Ex. 180 C 78 A B
Ex. 181 A , 79 A B
Ex. 182 B 80 A B
Ex. 183 C 81 A B
29
CA 02931988 2016-05-27
[0078]
[Table 10]
Original sheet for
Chemical
Test piece chemical conversion Blackening
Category conversion Appearance
No. treatment test result
treatment
(test piece No.)
Ex. 184 A 82 A D
Ex. 185 B 83 A D
Ex. 186 C 84 A D
Ex. 187 A 85 A D
Ex. 188 B 86 A D
_
Ex. 189 C 87 A D
_
Ex. 190 A 88 D B
Ex. 191 B 89 D B
Comp. Ex. 192 C 90 D B
- .
Comp. Ex. 193 A 91 D B
Comp. Ex. 194 B 92 D B
Comp. Ex. 195 C 93 D B
-
Comp. Ex. 196 A 94 D B
Comp. Ex. 197 B 95 D B
_
Comp. Ex. 198 C 96 D B
_
Comp. Ex. 199 A 97 D B
Comp. Ex. 200 B 98 D B
Comp. Ex. 201 C 99 D B
_ Comp. Ex. 202 A 100 D B
_ Comp. Ex. 203 B 101 D B
Comp. Ex. 204 C 102 D B
[0079] As shown in Table 7 to Table 10, the plated steel sheets having a
composite oxide
film including vanadium, with the surface having a Zn hydroxide ratio of 40%
or less, had
excellent blackening resistance even when a chemical conversion coating is
formed. In
contrast, in the case of an adhering amount of vanadium in the composite oxide
film of
more than 10.0 mg/m2 (test piece Nos. 31 to 36 and Nos. 82 to 87), the
reactivity between
the chemical conversion treatment liquid and the surface of the plating layer
(composite
oxide film) was decreased, and the chemical conversion treatment coating had
white
turbidity.
[0080] From the results, it is found that in the case of chemical conversion
treatment, the
adhering amount of vanadium in the composite oxide film is preferably adjusted
to 10.0
inghnz or less.
Industrial Applicability
[0082] The hot-clip Zn alloy-plated steel sheet obtained by the production
method of the
present invention is excellent in blackening resistance, and useful as plated
steel sheet for
use in, for example, roof materials and exterior materiaLs for buildings, home
appliances,
and automobiles.
Reference Signs List
[0083]
100,200 Cooling apparatus
110 Spray nozzle
120,230 Squeeze roll
130 Housing
31
CA 2 9 31 9 8 8 2 0 1 7 ¨ 1 0 ¨1 3
CA 02931988 2016-05-27
2F14248-PCT
210 Dip tank
220 Dip roller
300 Production line
310 Furnace
320 Plating bath
330 Wiping nozzle
340 Air jet cooler
350 Mist cooling zone
360 Water quenching zone
370 Skin pass mill
380 Tension leveler
390 Tension reel
400 Roll coater
410 Drying zone
420 Air cooling zone
S: Steel strip
32