Note: Descriptions are shown in the official language in which they were submitted.
CA 02932670 2016-06-06
-
,
ASSESSMENT OF RESERVOIR HETEROGENEITY BY USING PRODUCED
WATER CHEMISTRY
FIELD OF THE INVENTION
[0001] The present invention is generally related to the field of oil and gas
subsurface earth formation evaluation techniques. In particular, the present
invention is directed to methods of estimating properties of a subterranean
formation.
BACKGROUND OF THE INVENTION
[0002] A key consideration in the extraction of oil from oil sands reservoirs
is the
viscosity of the oil, which can be typically between 100,000 and several
million cP.
Thermal recovery methods, such as the Steam Assisted Gravity Drainage (SAGD)
process, is presently a method of choice for the Athabasca oil sands
reservoirs,
located in northeastern Alberta, Canada. In SAGD, in order to reduce the
viscosity of the oil, high pressure (typically between about 1 to 5 MPa) and
high
temperature (typically between about 185 C and 250 C) steam is injected into
the
reservoir. At original conditions, the viscosity of bitumen can be in the
hundreds
of thousands to millions of cP, whereas at elevated temperature, it can drop
to
less than 10 cP and then drain within the reservoir under the action of
gravity.
The SAGD process can incorporate, for example, two horizontal wells, one atop
the other. Steam can be injected into the reservoir through the top well,
whereas
fluids, including steam condensate and mobilized bitumen, may then be produced
through the lower well to the surface.
[0003] An important concern with regard to SAGD is the amount of carbon
dioxide created in the process when fuel, most often natural gas, is combusted
to
generate steam. A high carbon dioxide-to-oil intensity can be the result of
poor
steam conformance in the reservoir. It has been shown that poor steam
conformance may be caused by geological heterogeneities of the reservoir. This
can especially be the case in systems where there may be extensive shale
layers
which can block steam ascension and oil drainage. In real time, with current
1
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
technology, the only means may be to estimate the extent of the steam chamber
around a SAGD wellpair from thermocouple and distributed temperature sensor
data or 4D seismic data interpretation. Larter et al. (2008) analysed the
spatial
arrangement of bitumen composition within the reservoir, from core samples
prior
to steam injection and used the spatial mapping of the compositions to
allocate
the origins of produced oil samples with the reservoir to estimate steam
chamber
conformance. Although they found they could use the data to roughly estimate
steam conformance, these methods can be complex and may be subject to
uncertainty, especially in reservoirs where the composition of bitumen is
essentially the same at different locations within the reservoir.
[0004] The existence and influence of chemical reactions in oil-steam systems
have been extensively studied. Hyne (1986) showed that in temperature range of
around 170 C to 300 C, aquathermolysis (hydrous pyrolysis) is the dominant
reaction class. Thermal cracking (pyrolysis) typically occurs at temperatures
greater than about 300 C. Clark and Hyne (1984) devised a reaction scheme to
represent aquathermolysis that produced mainly methane, light hydrocarbons,
hydrogen sulfide, hydrogen and carbon oxides. Ng (1997) investigated the
kinetics of water gas shift reaction in a temperature range of 320 C to 380 C
and
proposed reaction rate constant and reaction profile depicting the generation
of
hydrogen, hydrogen sulfide and carbon oxides. Kapadia et al. (2012, 2013)
constructed a new reaction model for aquathermolysis and applied it to
understand the generation of hydrogen sulphide in the SAGD process. The
results demonstrated that SAGD may not only be a physical process but also a
chemically reactive one in which the reaction zones of the process may
predominantly be found at the edges of the depletion chamber where there is a
combination of elevated temperature, hot mobile bitumen, and steam condensate.
[0005] Beyond the water gas shift reaction and aquathermolysis reactions
occurring during steam-based processes such as SAGD, another class of
reactions occur between reservoir rock minerals and steam. The products of
steam-rock reactions may significantly affect reservoir properties such as
2
WSLEGAL\ 045074 \00165\13492436v3
CA 02932670 2016-06-06
mineralogy, porosity, and permeability, thus the recovery efficiency. For
example,
there is potential for deposition of scale in the reservoir and at the
injection and
production wells due to steam-rock reactions. Scale buildup within the system,
especially at the wells, may lower the efficiency and productivity of the
recovery
processes significantly. At this point, reactive thermal reservoir simulation
models
of steam-based recovery processes may not take rock-steam reactions into
account.
[0006] Sjoberg (1978) studied calcite dissolution mechanisms with associated
kinetics and found that the dissolution reaction depends on the form of the
calcite
present (powder or crystal) due to the difference in their available surface
area
and pH. He also described that magnesium and phosphate ions act as inhibitors
for calcite dissolution. Boon et al. (1983), in a series of experiments,
determined
the effect of different parameters on calcite dissolution rate and found that
steam-
rock reactions were controlled by pH, salinity and temperature and slightly
affected by bitumen interaction. They concluded that high temperature and high
pH were in favor of calcite dissolution. Abercrombie and Hutcheon (1986), by
studying carbon isotopes, showed that CO2 released after steam flooding may
not
solely be sourced from aquathermolysis reactions between steam and oil but
also
from inorganic rock-steam reactions.
[0007] Gunter and Bird (1988) described that during thermal enhanced oil
recovery, the presence of steam and steam condensate at elevated temperature
enhances reactions that involve rock minerals and that the products may
significantly affect reservoir permeability. They investigated the calcite
dissolution
reaction at 265 C and presented a three stage reaction model where condensate
and calcite react relatively quickly to generate a calcium-saturated smectite,
which
in turn can react to produce CO2.
[0008] Gunter and Perkins (1993) studied CO2 disposal in a carbonate aquifer
in
central Alberta. They concluded the brine water may have less capacity to
absorb
CO2 due to its salt content. They also divided the CO2, water and carbonate
3
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
reactions into two stages. The first stage consisted of calcite dissolution
whereas
the second one was adsorption of dissolved calcium and other ions onto the
solid
matrix (clay) of the aquifer. Using numerical modeling, they demonstrated that
the
amount of CO2 adsorption strongly depended on the mineral type and that the
higher the amounts of calcium and magnesium, the higher the CO2 storage in the
aquifer.
[0009] Previous research has shown that steam injection can promote calcite
decomposition. Maclntire and Stansel (1953) reported that the temperature at
which steam catalyzed dissolution occurs is equal to 973K (-700 C). Wang and
Thomson (1994) studied the effect of steam on calcite decomposition and
developed a reaction rate kinetic model based on their experimental results
and
showed that the presence of steam can significantly promote CO2 generation.
They concluded that steam weakened the bond between CaO and CO2 in calcite
molecules that can lead to calcite dissolution.
[00010] Dreybrodt et al. (1997), in a series of experiments, demonstrated
three
processes in the calcite, water, and CO2 reaction system: 1. precipitation or
dissolution of the reacting species at the surface of the calcite, 2. mass
transport
of reactants through the aqueous phase, and 3. slow reaction rate of CO2 and
water ¨ this reaction being the rate limiting step. They found that when the
solution is sufficiently far from equilibrium, for instance in the presence of
inhibitors such as phosphate, the kinetics may strongly depend on the ratio of
the
solution volume to reactive surface area. They also devised a kinetic model
that
matched the experimental data. Liu and Dreybrodt (1997) presented two
elementary reversible reactions that described changes in carbon dioxide
concentration when steam is reacted with calcite. They concluded that when the
aqueous solution with dissolved CO2 is supersaturated, the mechanism of
precipitation may be the same as that of dissolution of calcite. Emberley et
al.
(2005) investigated 002-steam-rock reactions in a carbonate reservoir in
Saskatchewan, Canada and found that the dissolution of carbonate minerals,
especially calcite, can change the mineral concentration (such as calcium
ions)
4
WSLEGAL \ 045074 \ 00165 \ 13492436v3
CA 02932670 2016-06-06
and the total dissolved solid. The CO2 reacts with calcium to form a calcite
precipitate. The changes in CO2 content alter pH, which in turn can affect the
CO2
dissolution reaction.
[00011] Kaufmann and Dreybrodt (2007) described the calcite-water-0O2
reaction system. They divided the kinetics into three different regimes with
respect to the concentration of calcium ions: a low reaction rate regime, the
fast
reaction rate regime, and the high order kinetic regime. The results can
demonstrate that the higher the calcium ion concentration, the lower the pH
and
the higher the CO2 generated in CaCO3-H20-0O2 system. Brantley (2008) found
that for pH lower than 3.5, calcite dissolution can be mass transport-
controlled
whereas it can be reaction rate limited (at the interface) at higher pH. They
mentioned, from a rate-limiting step perspective, that since the activation
energies
of interface reactions are generally high leading to surface reactions being
the
rate-limiting step over than of mass transport, increasing the temperature
might
change the process from being reaction rate limited to transport limited.
Pokrovsky et al. (2009) investigated the effect of pH and partial pressure of
CO2
on kinetics of calcite dissolution in the temperature range from 25 C to 150
C.
They found that the higher the pH, the lower the activation energy of calcite
dissolution. They also concluded that an increase of CO2 partial pressure can
decrease the dissolution rate. They also found that changes of temperature may
not significantly affect the dissolution rate of calcium carbonate.
[00012] During the SAGD process, the steam and produced CO2 from
aquathermolysis and rock-steam reactions within the formation can lead to a
002-
steam-condensate-rock system that can affect heat transfer (partial pressure
effects due to generated gases), oil mobility (002 dissolution in oil phase
leading
to reduction of oil viscosity), oil swelling (002 dissolution in oil phase),
and
formation porosity and permeability (due to precipitate formation in the pore
space), which consequently can affect the growth of the steam chamber and oil
recovery. It would therefore be advantageous for a method to consider such
5
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
,
,
interactions occurring in the steam chamber for establishing the possible
relationships between steam chamber growth and the intensity of the reactions.
[00013] Steam-rock reactions, water geochemistry, and how the produced water
composition varies as the thermal recovery process evolves are issues which
may
not have gained significant attention. As the steam interacts with oil and the
multiple rock types within the reservoir, reaction products formed within the
reservoir accumulate within the steam chamber and are produced with the
produced oil and steam condensate. While the shale layers can contain
concentrations of calcium carbonate, the oil sand typically does not. It would
therefore be advantageous for there to be a method that considered such
produced water compositions so as to provide information about the evolution
of
the steam chamber within the reservoir and to help characterize the shale
layer
heterogeneity of the reservoir.
SUMMARY OF THE INVENTION
[00014] The present invention generally relates to the field of oil and gas
subsurface earth formation evaluation techniques and methods for estimating
properties of a subterranean formation.
[00015] In one aspect, the present invention comprises a method for using
produced water analysis during a thermal heavy oil and bitumen recovery
technique to provide information about the evolution of the steam chamber
within
the reservoir and to help characterize the reservoir.
[00016] In a particular aspect, the present invention comprises methods for
using
the produced water compositions for detecting shale barriers and contact of
the
steam chamber with the overburden in the reservoir.
[00017] In a further aspect, the present invention provides a method for
analysing
produced water compositions so as to provide information about one or more of
the size and extent of any shale layer in the reservoir, the proximity of the
shale
6
WSLEGAL \045074 \ 00 I 65 \13492436v3
CA 02932670 2016-06-06
,
layer to the production well, the thickness of the shale layer, the number of
shale
layers in the reservoir and/or contact of the steam chamber with the
overburden.
[00018] Additional aspects and advantages of the present invention will be
apparent in view of the description, which follows. It should be understood,
however, that the detailed description and the specific examples, while
indicating
preferred embodiments of the invention, are given by way of illustration only,
since
various changes and modifications within the spirit and scope of the invention
will
become apparent to those skilled in the art from this detailed description.
BRIEF DESCRIPTION OF THE DRAWINGS
[00019] The subject matter which is regarded as the invention is particularly
pointed out and distinctly claimed in the concluding portion of the
specification.
The invention, however, may best be understood by reference to the following
detailed description of various embodiments and accompanying drawings in
which:
[00020] FIG. 1 depicts a reservoir simulation model for eight reservoir cases
including SAGD well pair position and shale layer location and geometry;
[00021] FIG. 2 is a chart of cumulative oil production and steam to oil ratios
over
time for each of the eight cases of FIG. 1;
[00022] FIG. 3 is a chart of the calcium production rate for cases 1, 2 and 3;
[00023] FIG. 4a depicts a steam chamber (water phase mole density) at the
beginning of calcium production time for case 2;
[00024] FIG. 4b depicts a steam chamber (water phase mole density) at the
beginning of calcium production time for case 3;
[00025] FIG. 5 is chart of the calcium production rate for cases 3, 4 and 5;
7
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
[00026] FIG. 6 is a chart of the first peak response versus shale layer volume
for
cases 3, 4 and 5;
[00027] FIG. 7 is a chart of the calcium production rate for cases 4 and 6;
[00028] FIG. 8 is a chart of the calcium production rate for cases 5 and 7;
[00029] FIG. 9 is a chart of the calcium production rate for cases 4 and 8;
[00030] FIG. 10 is a chart of the cumulative produced amount of calcium versus
shale volume at day 900;
[00031] FIG. 11 is a chart of the cumulative methane production for each of
cases 1 to 8;
[00032] FIG. 12 is a chart of the cumulative carbon monoxide production for
each
of cases 1 to 8;
[00033] FIG. 13 is a chart of the cumulative carbon dioxide production for
each of
cases 1 to 8;
[00034]FIG. 14 is a chart of the cumulative hydrogen production for each of
cases
1 to 8;
[00035] FIG. 15 is a chart of the cumulative hydrogen sulphide production for
each of cases 1 to 8;
[00036] FIG. 16 depicts the steam chamber development for a system having
three shale layers;
[00037]FIG. 17 is a chart of the calcium production rate for a case with three
shale layers; and
[00038] FIG. 18 is a flow chart illustrating one embodiment of a method for
determining a property of subterranean formation.
DESCRIPTION OF PREFERRED EMBODIMENTS
8
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
[00039] The present invention generally relates to the field of oil and gas
subsurface earth formation evaluation techniques and particular methods for
estimating properties of a subterranean formation during a thermal heavy oil
and
bitumen recovery technique. Suitable recovery techniques for use in
conjunction
with the present invention can generally include a thermal process in which
steam
is injected into a reservoir so as to heat the hydrocarbon product within,
such as
bitumen, and lower its viscosity sufficiently to enable oil flow to a
production well.
Examples of such thermal recovery methods can include SAGD, Cyclic Steam
Stimulation (CSS), steam flood, etc. The thermal recovery technique can also
include steam-solvent processes in which solvent is used in the presence of
steam.
[00040] Before the present invention is described in further detail, it is to
be
understood that the invention is not limited to the particular embodiments or
examples described, and as such may, of course, vary. It is also to be
understood that the terminology used herein is for the purpose of describing
particular embodiments only, and is not intended to be limiting, since the
scope of
the present invention will be limited only by the appended claims.
[00041] Unless defined otherwise, all technical and scientific terms used
herein
have the same meaning as commonly understood by one of ordinary skill in the
art to which this invention belongs. Although any methods and materials
similar
or equivalent to those described herein can also be used in the practice or
testing
of the present invention, a limited number of the exemplary methods and
materials are described herein.
[00042] It must be noted that as used herein and in the appended claims, the
singular forms "a", "an", and "the" include plural referents unless the
context
clearly dictates otherwise.
[00043] The recovery of heavy oil and bitumen can be a complex process,
requiring the use of particular products and services built for specific
conditions,
as these hydrocarbon products can be extremely viscous at reservoir
conditions.
9
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
Heavy oil and bitumen viscosity decreases significantly with increases in
temperature, and as such, thermal recovery methods such as SAGD, are
commonly employed.
[00044] In order to reduce the viscosity of the oil using the SAGD process,
steam
at high pressure, typically between about 1 to about 5 MPa, and at high
temperature, typically between about 185 C and about 250 C, is injected into
the
reservoir. Steam can be injected into the reservoir through a top well whereas
fluids, including steam condensate and mobilized bitumen, may then be produced
through a lower well to the surface.
[00045] During the SAGD process evolution, geochemical reactions between
rock and steam can arise with variations to the geochemistry and the produced
water composition occurring. The changes in the mineral content of the
produced
water can be due, for example, to the different sets of chemical reactions
between
the steam chamber and formation layers, which represent the reservoir
heterogeneity.
[00046] In accordance with an embodiment of the present invention, methods are
provided for the retrieval and analysis of the produced water during a thermal
heavy oil and bitumen recovery process, such as SAGD. Such methods can
generally provide information about the evolution of the steam chamber within
the
reservoir and assist in characterizing the reservoir. Given that many oil
sands
reservoirs are strongly heterogeneous with shale layers within, the use of
water
geochemical data to provide information about the heterogeneity of the
reservoir
aids in understanding steam conformance within the reservoir. In a particular
aspect, the present invention can comprise methods of using water geochemical
data to identify and index shale layers in an oil sands reservoir.
[00047] In a particular embodiment, the present invention can include methods
for providing information about the size and extent of a shale layer in the
reservoir. In another embodiment, methods of the present invention can be used
to provide an index as to the proximity of the shale layer to the production
well. In
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
another embodiment, the present invention can include methods for providing an
index as to the thickness of a shale layer in the reservoir.
In a further
embodiment, the present invention can include methods for determining the
number of shale layers in a reservoir. In other aspects, the methods of the
present invention may be used to relate the time point at which the steam
chamber reaches the caprock.
[00048] In a SAGD steam chamber, geochemical reactions between rock and
steam in the presence of CO2 (due to aquathermolysis reactions) can take place
which can affect the growth and shape of the steam chamber and alter the
porosity and permeability and oil and water saturations within the chamber.
Two
sets of reactions that can occur are thermal cracking of the oil and
aquathermolysis. However, as thermal cracking becomes significant above
300 C, it is thus not a major contributor to SAGD at typical steam injection
conditions (usually <250 C). On the other hand, aquathermolysis typically
occurs
between about 180 C and 300 C and thus it should be included in the set of
reactions. The aquathermolysis reactions as used herein are as disclosed by
Kapadia et al. (2012, 2013).
[00049] As the steam chamber interacts with the reservoir, the mineralogical
difference between shale layer baffles or barriers and the bitumen bearing
sand
can yield different water compositions, which can drain as condensed water
vapor
with different mineral contents to the production well. A common component of
shale is quartz and calcite. Thus, a key differentiator between the sand
intervals
and shale in an oil sands deposit can be the presence of calcite in the shale
layers.
[00050] The major reactions between 002, calcium carbonate, and hot water
(formation water and steam condensate) can be described as follows:
002+ H2O 4-7) H2003 t H + HCO3-
(1)
CO2 + Oft t; HCO3- (2)
11
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
CO2 + H20 + CaCO3 Ca2+ + 2HCO3-
(3)
[00051] Reactions (1) may be more pronounced when the pH value of the water
is less than 7. Reaction (2) may be dominant for systems when the pH value is
greater than 7. H2CO3 in reaction (1) may be dealt with as an intermediate
product and was not considered for the kinetic model. The reaction rate
constants
for reactions (1) and (2) can be described as follows:
ki = 10-3 exp(934.69 ¨ 9252 T1)
(4)
7H+ 7HCO3- (Ko/K5)
(5)
log k2 = 14.072 ¨ 3025 T-1
(6)
k-2 = k2 (21-1, I 7Hc03) Kw (Ko/K5) (7)
Ko = K5/K6
(8)
K5 = 1.707x10-4
(9)
log 1<6 = -356.3094 + 21834.37T-1¨ 0.060919964 T+ 126.8339 log T¨ 1684915
T-2 (10)
log Kw = 22.801 ¨4787.3 T1¨ 0.010365 T-7.1321 log T (11)
12
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
[00052] Ko, 1<5, and ic are empirical mass balance constants (based on
activities
rather than concentration) which depend on temperature, described by Buhmann
and Dreybrodt (1985). y is the ionic activity coefficient given by Kiel land
(1937).
[00053] Reaction (3) presents the dissolution and precipitation reactions
associated with Ca2+ conversion from CaCO3. Under the assumption that the CO2
partial pressure is higher than 0.05 atmospheres and there are no mass
transfer
limitations (sufficient diffusion due to relatively high porosity and
permeability in
the reservoir sand), the reaction rate constants for reaction (3) can be
described
as follows:
log k3 = 0.198 - 444 T-1 (12)
log k'3 = 2.84 - 2177 T1
(13)
log k"3 = -5.86-317 T1
(14)
log k_3 = -2.375 + 0.025 7-,
(15)
[00054] k, is the reaction rate constant for the forward reaction of reaction
(1) and
k1 is the reaction rate constant for the reverse reaction of reaction (1), T
is
temperature in Kelvin and 7-, is temperature in degrees Celsius. For reaction
(3),
k3, k'3 and k"3 shows the reaction rate constants at slightly acidic, basic
and
neutral medium of forward reaction respectively and k-3 represents the general
reaction rate constant for reverse reaction. According to Buhmann and
Dreybrodt
(1985), the left side of Reaction (1) may be slow, whereas the right side may
be
fast. Reactions (1), (2) and (3) can be written in expanded form as listed in
Table
1.
TABLE 1
Major reactions in CO2-rock-steam media
Reaction Reaction
Reaction rate
number
constant
1 CO2 + H20 H+ + HCO3-
k1
2 H+ + HCO3- -o CO2 + H20 k-
1
3 CO2 + OH- -0 HCO3-
k2
4 HCO3- --o CO2 + OH- k-
2
5 CO2 + H20 + CaCO3 Ca2+ + 2HCO3-
k3
13
WSLEGAL \ 045074 \ 00165 \ 13492436v3
CA 02932670 2016-06-06
6 CO2 + H20 + Ca003 Ca' + 2H003- k'3
7 CO2 + H20 + Ca003 Ca2+ + 2HCO3- k"3
8 Ca2+ + 2HCO3 CO2 + H20 + CaCO3 k_3
[00055] The rate constants can be given by the Arrhenius equation as follows:
k= A e-EIRT
(16)
[00056] Table 2 lists the kinetic parameters which were estimated from the
literature mentioned above.
TABLE 2
Kinetic parameters for Equations (4) to (11)
Reaction Activation energy (J/.mol) Pre-exponential
factor
number (1/day)
1 76921.1 7.43x10-8
2 75082.9 8.96x10-14
3 57909.5 7.32x10-10
4 108464.4 4.57x10-11
5 8499.4 5.48x104
6 41669.8 1.25x102
7 6068.6 6.26x1010
8 48955.3 1.36x10-2
14
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
[00057] The activation energy for the precipitation reaction listed in Table 2
estimated here is comparable with the apparent activation energy for the
calcite
precipitation reaction equal to 46 4 kJ/mol reported by Nancollas and Reddy
(1971).
EXAMPLES
[00058] A two-dimensional reactive-thermal SAGD model was constructed to
evaluate the effect of rock-0O2-wet steam reactions on water composition and
steam chamber evolution. The reaction scheme, as provided in Table 1 and their
calculated kinetic parameters, as provided in Table 2, were encoded within a
commercial thermal reservoir simulator (CMG, 2013) to construct the two-
dimensional reactive-thermal SAGD model.
[00059] The reservoir simulator can calculate the material balance equation
for
each component within each phase (oil, water, and gas):
- - = =
a x xoipoSo y jpgSg 111j¨ Mir
¨at 0
mwo mwg mw,
_
(
kõ 'ow
V ____________________ c ( VP y V z)l- DV ___
(17)
koPo
+ c (VPõ x)Vz)+ Def/V P0x0J
14õ mw
\. 0
kgpg eff PgY
gi
+ _______________________________ c \21'g ygVz)+Dg,V
MW,
\ 0
and energy balance accounting for all phases:
a rt
¨ 0)Mr ¨Tref 0(S.põUõ +SopoUo+SgpgU A-EQ.
at
-v.[ ¨km,VT + põuõCõfr pou0C0(T ¨Tjej)+ pgu gC gfr
¨Tõf)]
(18)
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
[00060] Flow of oil, gas and water phases can be governed by multiphase
version of Darcy's law and the governing equations were calculated by using
the
finite volume method (CMG, 2013).
[00061] The reservoir domain was modeled into fifty-eight 0.8 m grid blocks in
the cross-well direction and by sixty 0.5 m grid blocks in the vertical
direction
(Cartesian grid configuration) with the top of the formation at 200 m depth
below
the surface. The length of the SAGD wellpair was equal to 750 m. The oil
column
thickness was set equal to 30 m. Initially, the reservoir water had a pH
around 7
(neutral).
[00062] Tables 3 and 4 list the bulk reservoir properties and properties of
shale
layers within the formation, respectively.
TABLE 3
Input data for bulk reservoir in SAGD reactive reservoir simulation model
Parameters (Reservoir) Value
Two-dimensional grid and dimensions 58 x 0.8 m by 60 x
0.5 m
SAGD wellpair length, m 750
Horizontal permeability, mD 4000
Vertical permeability, mD 2000
Average porosity 0.3
Initial oil saturation 0.75
Initial water saturation 0.25
Irreducible water saturation 0.15
Residual oil saturation with respect to
0.20
water
Relative permeability to oil at irreducible
1.0
water
Relative permeability to water at residual 0.992
oil
Critical gas saturation 0.005
Residual oil saturation with respect to 0.005
gas
Relative permeability to gas at residual 1.0
oil
Relative permeability to oil at critical gas 0.992
Critical gas saturation 0.005
Initial temperature, C 20
Initial pressure, kPa 2000
Rock heat capacity, J/m5 C 2.600x106
Rock thermal conductivity, J/m day C 6.600x105
Water phase thermal conductivity, J/m
5.350x104
day C
16
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
Oil phase thermal conductivity, Jim day
1.150x104
C
Gas phase thermal conductivity, Jim day
5.000x103
C
Bitumen
Molecular weight, kg/kmol 465
Critical temperature, C 903.85
Critical pressure, kPa 792
Viscosity, cP versus Temperature, C 10 1,587,285
100 203.91
200 9.71
TABLE 4
Input data for shale layer in SAGD reactive reservoir simulation model
Parameters (Shale) Value
Horizontal permeability, mD 20
Vertical permeability, mD 10
Average porosity 0.04
Initial oil saturation 0
Initial water saturation 1
Irreducible water saturation 0.35
Initial temperature, C 20
Initial pressure, kPa 2000
Rock heat capacity, J/m3 C 3.328x106
Rock thermal conductivity, Jim day C 1.900x106
Shale water composition, mole fraction
Water 0.3
HCO3- 0.3
H+ 0.1
OH- 0.1
Ca2+ 0.2
[00063] The definitions of the symbols used herein are found below in Table 7.
[00064] Referring now to FIG. 1, in accordance with the present invention, a
cross-sectional view of a reservoir grid for a symmetrical two-dimensional
reservoir simulation model is shown that depicts the positioning of a SAGD
injector and producer well pair and the location and geometry of the shale
layers
within the reservoir. Eight separate reservoir examples are shown to
demonstrate
the effect of shale geometry and its chemical composition on the steam
chamber.
[00065] Beyond the modeled part of the caprock, heat losses were permitted and
were approximated by using Vinesome and Westerveld's (1980) heat loss model.
At the bottom boundary, heat losses were permitted and were also approximated
17
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
by using Vinsome and Westerveld's (1980) model. At the sides of the model,
symmetry conditions were applied (no flow and zero heat transfer). The
physical
properties of the caprock and shale layers were the same.
[00066] To initialize the SAGD wellpair, as is done in field operations with
steam
circulation, a pre-heating period, such as for example 90 days, is typically
conducted so as to heat the oil between the injection and production wells. In
the
model, this was done by using temporary line heaters that were located in the
trajectories of the wells. After the temperature is sufficiently high in the
oil sand
between the wells, the viscosity of the oil between the wells is low enough to
be
mobilized when steam is injected into the upper well and fluids are produced
through the lower well. In the reservoir simulation model, during SAGD
operation,
steam was injected into the upper well at 400 m3/day (expressed as cold water
equivalent, OWE) with steam quality equal to 95%. At the production well, it
was
operated with a maximum steam rate constraint (OWE) equal to 1 m3/day to
mimic steam-trap control.
[00067] Referring again to FIG. 1, of the eight reservoir examples shown, case
1
represents a base case comprising a homogeneous reservoir without a shale
layer. Each of the eight cases represent reservoirs sealed with shale layer
caprock at the top of the reservoir, with the modeled portion of the caprock
having
a thickness equal to about 2.5 m.
[00068] The shale layers have the same thickness, same horizontal extent, and
same horizontal distance from the injection and production well pair with
respect
to cases 2 and 3, while in case 1 there is no shale layer is present. With
regard to
cases 4 and 5, the thickness of the shale layers have been increased by a
factor
of four and eight, respectively, from that of cases 2 and 3. The horizontal
extent
of shale layer, thickness and vertical distance between shale layer or the
well pair
have been changed in cases 6, 7 and 8 versus the previous cases.
[00069] Referring now to FIG. 2, a chart of cumulative oil production and
steam
to oil ratios (SOR) for the eight cases are depicted. The cumulative oil
production
18
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
is depicted by a solid line, whereas a dashed line represents the SOR. It can
generally be seen that the greater the volume of shale in the reservoir, the
greater
the SOR and the lower the cumulative oil produced from the reservoir. This
result
can arise because the steam will heat the volume of shale and yet no oil may
be
produced from it. Doubling of the grid in both vertical and cross-well
directions
can result in changes to the liquid and gas production rates and cumulative
steam-to-oil ratios of less than about 0.001%. Thus the grid was considered to
be
sufficiently converged with respect to the dimensions of the grid blocks.
[00070] The SOR, as shown by FIG. 2, can range from about 3 to about 4.8
m3/m3 for most of the cases during mature production, such as after the
chamber
has become firmly established in the reservoir. The SOR for each of the cases
are generally quite consistent with SAGD operations in the field. This may
imply
that the carbon intensity of these processes can range from about 600 to about
1,000 kgCO2eq/m3 oil produced, respectively. This may also imply that the
energy return from these processes may vary from about 3.8 to 2.4 GJ out, in
the
form of chemical energy, per GJ invested in the process in the form of steam,
respectively.
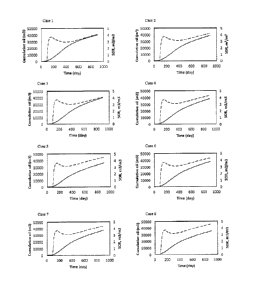
[00071] FIG. 3 is a chart of the calcium ion production rate for cases 1, 2
and 3.
Substantial changes to the rate of calcium production can be seen in each of
the
cases. As the initial 90 days represents the preheating period, changes in the
calcium ion production rate during this period were not expected. With regard
to
case 1 in which no shale layer was present, calcium ion production started at
about day 500, corresponding with the time that the steam chamber reaches the
caprock. For case 2, in which the shale layer was located close to the depth
of
the injection well, calcium production began after about 215 days, or about
125
days after the commencement of the SAGD mode. With respect to case 3, in
which the shale layer was located about 7.25 m above the injection well, the
changes in the calcium production rate began at about day 280, which
corresponds to about 190 days after commencement of SAGD mode.
19
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
[00072] FIGS. 4a and 4b depict the steam chamber (water mole density) at the
beginning of calcium ion production time for cases 2 and 3, respectively. The
onset of calcium ion production corresponds to the time at which the steam
chamber is in contact with the shale layer. FIG. 4a shows case 2 at about day
215, or after about 125 days of SAGD operation. FIG. 4b shows the extent of
the
steam chamber (water mole density) for case 3 at day 280, or after 190 days of
SAGD operation.
[00073] The surge in calcium ion production can be directly associated with
chemical reactions (1), (2) and (3), as disclosed above, which generate
calcium
ions when the steam contacts the shale layers. The ions can then be washed
from the shale layer and drained with steam condensate to the bottom of the
steam chamber to be produced to the surface by the lower well. After the steam
chamber has reached the caprock, the production of calcium ions continues to
grow. Referring back to FIG. 3, it can be seen that calcium ion production
begins
at about day 500 for case 1, which is the day that the steam chamber contacts
the
caprock. With regard to case 2, the shale layer is closer to the injector
well, and
the period of time over which calcium ion extraction occurred was at about day
215 to about day 745. After about day 745, the steam chamber passed the shale
layer and started extracting calcium ions from the caprock until about day
860.
Beyond about day 860, the steam chamber has extracted the calcium ions from
the caprock layer directly exposed to the chamber, and thus the generation
rate
drops. With respect to case 3, the onset of calcium ion production coincides
with
the steam chamber reaching the shale layer. As the shale layer in case 3 is
closer to the caprock and farther from the well pair than case 2, the calcium
production begins later, at about day 280, and the chamber reaches the caprock
before the shale layer is exhausted of freely available calcium ions.
[00074] FIG. 5 depicts the calcium ion production rate for each of cases 3, 4
and
5. Cases 4 and 5 comprise shale layers having a thickness increased by a
factor
of four (case 4) and eight (case 5) from that of case 3. The top location of
the
shale layer is the same for each of cases 3, 4 and 5. However, the bottom
shale
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
,
layer locations each differ, resulting in different distances to the well
pair. The
results demonstrate that the greater the thickness of the shale layer, the
larger the
calcium ion generation response. As well, the closer the shale layer is to the
production well, the sooner is the response.
[00075] FIG. 6 depicts the first peak response against the volume of the shale
layer for each of cases 3, 4, and 5. As shown, the peak correlates with the
volume of the shale layer. Accordingly, this may have important implications
on
the use of produced water geochemical information, in that the peak may be
used
to index the relative sizes of shale layers.
[00076] Referring now to FIG. 7, depicted therein is a chart of the calcium
production rate for each of cases 4 and 6. The main difference between each
case is in regard to the horizontal extent of the shale layers. The nearest
distance
of the shale layer to the production well in each case is the same, and thus,
the
onset of calcium ions is about the same at about Day 200. However, as the
layer
extents differ horizontally, the response profile for case 6 is slightly
elevated over
that of case 4, until the time the steam chamber starts producing calcium ions
from the caprock. At that point, the calcium ion production rate for case 4 is
shown to be slightly higher than that of case 6, as it is easier for the steam
chamber to pass the shorter shale layer in case 4. Accordingly, the gap
between
the curves can provide an index on the horizontal extent of the shale layers.
However, by comparing FIGS. 5 and 7, it can be seen that changes in shale
layer
thickness can affect the calcium production response more significantly than
variations in horizontal extent.
[00077] FIG. 8 depicts a chart for the calcium ion production rate for each of
cases 5 and 7. The main difference between cases 5 and 7 is in the thickness
of
the shale layers. The shale layer is thicker for case 5 than in case 7, while
the
bottom of each shale layer are the same location and distance from the well
pair.
As shown, the commencement day for calcium ion production and the time period
over which the calcium ions are extracted from the shale layers are almost the
21
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
same in each case. However, the peak height for case 5 is about 3 times that
of
the peak height for case 7. Accordingly, this provides additional support that
the
peak height can be directly related to the thickness of the shale layer.
[00078] Referring now to FIG. 9, depicted therein is a chart of the calcium
ion
production rate for each of cases 4 and 8. The main difference between each
case is in the thickness of the shale layers. The thickness of the shale layer
for
case 8 is about two times as thick as that of case 4. As the proximity of the
bottom of the shale layer to the production well is the same for each case,
the
increase in calcium production rate began at about the same time. The peak in
case 8, however, is almost 5.2 times as high than that of case 4, which can
indicate the greater thickness of the shale layer in case 8. By about day 630
in
case 8, the generated calcium ions are mainly sourced from reactions occurring
at
the caprock.
[00079] FIG. 10 depicts the cumulative calcium ion production for each of
cases
2 to 8 against the shale volume at day 900. The cumulative produced amount of
calcium ions, can be calculated by integrating the area under the plot of
calcium
production rate versus time. Case 2 has the same shale volume as Case 3,
whereas case 4 and case 7 also have the same shale volume. As shown, in
cases with the same shale volume, the closer the shale layer is to the well
pair,
the greater the cumulative amount of calcium ions produced. In case 6, a
greater
horizontal extent of shale layer can act as a baffle for the steam chamber to
reach
the caprock, as well as obstructing the complement of reactions within the
shale
layer. Accordingly, such results can be used in conjunction with core, log,
and
seismic data to understand heterogeneity of the reservoir along with its
impact on
steam conformance around the well pair. Also it can be used as a tool to
indicate
the presence of shale layers that the steam chamber is encountering as it
grows
within the reservoir.
[00080] Figures 11 to 15 depict the cumulative production of CH4, CO, 002, H2
and H2S respectively, for each of cases 1 to 8. These gases can be produced as
22
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
a result of aquathermolysis reactions and geochemical reactions as disclosed
in
Table 1. As shown, the lowest cumulative gas productions belong to case 8
followed by case 5. This result can indicate that the larger the thickness of
the
shale layer, the lower the amount of gas production. This result can be due to
the
capability of gas sequestration within shale volume. As shown, case 2 has the
greatest amount of gas production, as it has the closest shale layer to the
well
pair, and there is lesser room for holding gaseous product due to the smaller
thickness of the shale layer. The sequence of cumulative gas production for
each
of the gases shown in FIGS. 11 to 15 are generally the same, with the
exception
of case 1, where there is no shale layer in the reservoir.
[00081] In three-dimensional systems, with discrete shale layers distributed
within the reservoir, as the steam chamber intersects with each shale layer,
the
calcium ion response from the production well will be the superposition of all
of
the responses of each of the steam chamber interactions with the shale layers.
Thus, the signal from the reservoir can become more complex to interpret.
However, the first onset and subsequent step changes of calcium ions
production
can indicate when the first shale layer and then subsequent ones are reached
by
the steam chamber. Thus, providing an index of heterogeneity of the reservoir.
[00082] FIGS. 16 and 17 depict instances in which the reservoir comprises
three
shale layers. FIG. 16 shows the evolution of the steam chambers, as indicated
by
the water mole density. FIG. 17 depicts the calcium ion production profile for
this
case. There are three peaks shown with regard to the calcium ion production
rates. A comparison of the results shown in FIGS. 16 and 17 demonstrate that
the first peak, at about day 150, can be associated with the chamber reaching
the
second shale layer from the bottom. By about day 370, the steam chamber has
not enveloped but has touched the bottommost shale layer, as depicted by the
second peak in FIG. 17. The third peak represents the beginning of the
envelopment of the topmost shale layer at about day 680.
The results
demonstrate that as the number of shale layers increases, the calcium ion
profile
can become more complex.
23
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
,
[00083] Goodman et al. (2010) investigated the produced water hardness in
SAGD facilities and reported the average value of 140 to1400 mg/I as
alkalinity as
CaCO3. Table 5 represents the values reported for alkalinity of produced water
as
CaCO3 from field data of SAGD projects. The alkalinity of the produced water
in
Cases 1 to 8 was calculated and compared with the published data by
ConocoPhillips to confirm the reliability of the generated data as produced
ions by
simulation. In the report presented by ConocoPhillips to the Alberta Energy
Regulator, the alkalinity of produced water as CaCO3 was stated to be equal to
228.5 mg/I for the Surmont SAGD project. This alkalinity is close to the
alkalinities
listed in Table 6 obtained from the reactive thermal reservoir simulation
model.
This can demonstrate that the produced amount of calcium ions which strongly
depends on other produced ions, as obtained from the model, can be reliable as
well.
TABLE 5
Alkalinity of produced water as CaCO3 from field data
Reference Alkalinity as CaCO3, mg/I
Goodman et al. 140-1400
ConocoPhillips ¨ Surmont 228.5
SAGD project
TABLE 6
Alkalinity of produced water based on the produced amount of basic ions after
about 2.5 years of production
Cases Produced amount Produced Produced
Alkalinity as
of HCO3, m3 amount of OH-, water, m3
CaCO3, mg/I
m3
Case 1 1107.1 271.6 150158
227.2
Case 2 1144.0 271.5 152070
228.3
Case 3 945.5 240.3 152335
194.5
Case 4 1024.0 248.3 153793
204.1
Case 5 1193.7 262.9 154749
226.3
Case 6 989.2 240.6 153675
197.6
Case 7 1135.9 263.0 153608
221.9
Case 8 1080.7 239.2 155826
203.9
24
WSLEGAL \ 045074 \ 00165 \ I 3492436v3
CA 02932670 2016-06-06
[00084] Referring now to FIG. 18, depicted therein is an example of the
implementation of a method of the present invention.
[00085] Method 100 generally commences at step 102. A hydrocarbon recovery
method for the recovery of a viscous hydrocarbon product, such as for example
bitumen, from a subterranean formation can be initiated at step 104. In a
particular embodiment of the present invention, the recovery method employed
comprises a thermal recovery process, such as a SAGD process. The SAGD
process employed can include, for example, two horizontal wells in the
subterranean formation, located one atop of the other. Steam can be injected
into
the reservoir through the top well, whereas fluids, including steam condensate
and mobilized bitumen, may then be produced through the lower well to the
surface. At step 104, steam at high pressure, for example, between about 1 MPa
to about 5 MPa, and at high temperature, for example between about 185 C to
about 250 C can be injected into the reservoir through the top well. A pre-
heating
step for a period of, for example 90 days, may be initially conducted so as to
heat
the hydrocarbon product between the upper (injection) and lower (production)
well
pair. Once the temperature is sufficiently high, the viscosity of the
hydrocarbon
product between the wells may be low enough so as to be mobilized when the
steam is injected into the upper well and the fluids are produced through the
lower
well.
[00086] As the steam interacts with the viscous hydrocarbon product and the
multiple rock types within the reservoir, reaction products are formed within
the
reservoir and accumulate within the steam chamber and are produced with the
produced oil and steam condensate from the production well.
After commencement of the hydrocarbon recovery process of step 104,
production from the reservoir may be initiated and formation fluid samples may
be
retrieved at step 106. The formation fluid sample can comprise produced water
that further comprises one or more components of interest. Suitable components
in accordance with an embodiment of the present invention can include calcium,
WSLEGAL\ 045074 \ 00165 \13492436v3
CA 02932670 2016-06-06
methane, carbon monoxide, carbon dioxide, hydrogen, hydrogen sulphide, or any
combination thereof. The formation fluid samples may be collected over a
period
of time. In some embodiments, the samples may be collected weekly, or monthly,
for example. In an embodiment, the collection of the samples may occur
throughout the course of the hydrocarbon recovery process, such as in certain
instances, fifteen years or more.
[00087] The formation fluid sample may be analyzed and data derived, at step
108. In an embodiment, the derivation of the fluid sample data may comprise
determining the presence or absence and/or concentration of one or more
components of interest. Any suitable technique recognized by a person skilled
in
the art for determining the presence, absence or concentration of a desired
component may be used. In some embodiments, the technique used may involve
one or more of mass spectrometry (MS), high pressure liquid chromatography-
mass spectrometry (HPLC-MS), titration, ion titration, plasma emission
spectroscopy, ion chromatography, or atomic absorption spectroscopy.
After the analysis and data derivation of step 108, the data may be input into
a
model to determine one or more characteristics of the subterranean formation,
in
accordance with step 110. Additional base line data, can also be input into
the
model. The base line data can include previously determined data regarding the
subterranean formation which may also be used as input for the reservoir
model.
Previously determined data may include any data that provides physical
information regarding a property of the subterranean formation, such as, for
example, SAGD well pair length, initial formation water composition of the
reservoir, initial reservoir pressure, initial reservoir temperature,
horizontal and
vertical permeability, average porosity, initial oil saturation, initial water
saturation,
residual oil saturation with respect to water, bitumen characteristics, etc.
and may
include data related to shale layer.
[00088] In one aspect, the mineralogy of the formation and shale layers can be
determined for making a benchmark of a component assumed to be available only
26
WSLEGAL 045074 \ 00165 \ 13492436v3
CA 02932670 2016-06-06
in the shale layer and not along the reservoir. For example, the initial
formation
water composition may be used in determining the concentration and/or presence
of a particular component of interest, such as calcium, for example.
Accordingly,
base line data can include calcium ion concentration from the initial
reservoir
water, for example.
[00089] The produced water can be retrieved and analyzed, with changes to
mineral or component content being monitored. The monitoring frequency can
vary. For example, in various embodiments, monitoring may take place weekly,
monthly, quarterly, or other frequency. Techniques for monitoring on-line may
be
used, for example. In accordance with the present invention, examples of water
analysis methods can include, for example, laboratory testing of subterranean
fluids and/or core samples, logging techniques, seismic techniques, reservoir
modeling, etc.
[00090] Shale layers may be mapped to some extent from log and core data and
thus, given the position and extent of shale layers and the placement of the
SAGD
well pair, the evolution of the steam chamber may be estimated.
In an
embodiment, the baseline data may be plotted against data for the produced
water samples over a given period of time.
[00091] The usage of water geochemical data, prepared in step 108, may then
be applied at step 110, to provide information about the heterogeneity of the
formation. This can include for example, identifying and indexing shale layers
in
an oil sands reservoir.
[00092] Non-oil sands layers, such as shale layers, can be identified by a
change
of the produced water chemistry by more than about 25% from the base line
value. For example, a change of the concentration of calcium ions in the
produced water by more than 25% from the base line value would demonstrate
that the steam chamber is interacting with a shale layer. If the change grows,
this
can indicate that the shale layer is more extensive since as the steam chamber
is
growing, it is interacting with more of the volume of the shale layer.
27
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
[00093] The severity of the response can be directly related to the size and
extent of the shale layer. In general, the larger the calcium ion generation
response peak the greater the thickness of the shale layer. As well, the
calcium
ion generation response width can provide an index as to the horizontal extent
of
the shale layer. In general, the greater the calcium ion generation response
width
the larger the horizontal extent of the shale layer. The changes in shale
layer
thickness, however, can affect the calcium production response peak. The
beginning of the first peak response of calcium ions can also provide an index
about the distance between shale layer and SAGD well pair. In general, the
larger the peak the greater the thickness of the shale layer. As well, a wider
first
peak can demonstrate a greater horizontal distance of the shale layer.
In instances in which the horizontal extent of a shale layer can act as a
barrier in a
particular time window, the production of determined ion production can
constantly increase, while when the shale layer acts as a baffle, the ion
production
increases and then decreases. The start of the ion production rate decrease
can
mean that the steam chamber passes the shale layer.
[00094] The onset of calcium production can correspond with the time at which
the steam chamber is in contact with the shale layer. As well, the relative
timing
of the onset of calcium ion production can provide an index as to the
proximity of
the shale well to the production well. Generally, the sooner the calcium ion
response, the closer the shale layer would be to the production well.
[00095] The cumulative production of CH4, CO, CO2, H2 and/or H2S can also be
used to determine the heterogeneity of the reservoir. In general, a lower
amount
of gas production would indicate a greater thickness of the shale layer. This
result
can be due to the capability of gas sequestration within shale volume.
[00096] The number of peaks found in the calcium ion production rate can
correlate with the number of shale layers. For instance, if there are multiple
peaks
within the evolution of the concentrations, this may indicate that the steam
chamber is interacting with multiple shale layers and the number of peaks
would
28
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
be an indicator of the number of shale layers that are being interacted with.
After
the concentrations return back to the base line value, then this can suggest
that
the steam chamber is no longer interacting with the shale layers, for example,
that
the steam chamber has fully surround the shale layer and that the reactions
that
can take place on the surface of the shale are done. The base line may change
as the process evolves in the sense that the steam chamber at start will be
small
and the base line concentrations will have a particular initial set of values
(for
each species). If the chamber encounters a large shale layer, then the water
concentrations can change and a new set of species concentrations will be
achieved which may stay elevated over the initial base line for an extended
period
of time, thus forming a new base line. Thereafter, if the chamber encounters
another shale layer, the concentrations may shift beyond that of the new base
line
indicating the new interaction.
[00097] In an aspect, the greater the peak response of the calcium ions in the
produced water, the greater is the thickness and extent of the shale layer. If
the
response is, for example, over 100% greater than the baseline value, then the
thickness of the shale layer can be estimated to be of order of about several
meters thick. If it is of order of 25% greater than the baseline value, for
example,
the thickness of the shale layer can be an order of less than about 1 m thick.
In
one aspect, if the response curve has a peak and then shifts to a plateau
(with
value greater than that of the baseline) over a period of about 50-100 days,
the
horizontal extent of the shale layer can be estimated to be of order of about
10-20
m. If the decline from the peak value shifts back to the base line, then the
shale
layer may not be extensive and may be an order of about 10 m in extent. In an
aspect, for each reservoir, the water chemistry response can be tuned to the
specific reservoir by examining the log and core data to correlate the
response to
the specific thicknesses of the shale layers.
TABLE 7
NOMENCLATURE
Symbol Definition, SI unit
29
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
A Reaction frequency factor
Cg Specific heat capacity of gas phase, J/(kg.K)
Cgj Molal concentration of component j in gas phase, mol/kg
Co Specific heat capacity of oil phase, J/(kg.K)
coi Molal concentration of component j in oil phase, mol/kg
Cw Specific heat capacity of water phase, J/(kg.K)
cwi Molal concentration of component j in water phase, mol/kg
Deff Effective diffusivity coefficient of component j in gas
phase, m2/s
Deff Effective diffusivity coefficient of component j in oil
phase, m2/s
De ff Effective diffusivity coeff. of component j in water phase,
m2/s
Activation energy, J/mol
Reaction rate constant
kg Gas permeability, m2
ko Oil permeability, m2
kTH Thermal conductivity of formation, W/(m.K)
kw Water permeability, m2
rhi Removal rate of component], kg/(m3.$)
Mir Generation rate of component/ due to reaction, kg/(m3.$)
Empirical mass balance constant based on activity
Mr Volumetric heat capacity, J/(m3.$)
MWg Molecular weight of gas phase, kg/mol
MVVi Molecular weight of component j, kg/mol
MW0 Molecular weight of oil phase, kg/mol
MW w Molecular weight of water phase, kg/mol
Order of reaction
Pg Pressure of gas phase, kg(m.s2)
Po Pressure of oil phase, kg(m.s2)
Pw Pressure of water phase, kg(m.s2)
Input energy from a reaction per unit volume, J/(m3.$)
Q,
Input energy from a source per unit volume, J/(m3.$)
Universal gas constant, J/(mol.K)
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
Sg Gas saturation
So Oil saturation
Sw Water saturation
Temperature at time t, K
To Temperature in degree Celcius, C
Time, s
Tref Reference temperature, K
Ug Darcy velocity of gas phase, m/s
Ug Internal energy of gas phase per unit mass, J/kg
uo Darcy velocity of oil phase, m/s
U0 Internal energy of oil phase per unit mass, J/kg
uw Darcy velocity of water phase, m/s
Uw Internal energy of water phase per unit mass, J/kg
xoj Mole fraction of component j in oil phase
xwj Mole fraction of component j in water phase
Yl Mole fraction of component] in gas phase
Length, m
jig Viscosity of gas phase, kg/(m.$)
po Viscosity of oil phase, kg/(m.$)
pw Viscosity of water phase, kg/(m.$)
Pg Gas phase density, kg/m3
po Oil phase density, kg/m3
Pw Water phase density, kg/m3
Ionic activity coefficient for ion i
Specific gravity of gas phase
7o Specific gravity of oil phase
Specific gravity of water phase
Porosity
v. Divergence operator
V Gradient operator
31
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
[00098] In the preceding description, for purposes of explanation, numerous
details are set forth in order to provide a thorough understanding of the
embodiments of the invention. However, it will be apparent to one skilled in
the
art that these specific details are not required in order to practice the
invention.
[00099] The above-described embodiments of the invention are intended to be
examples only. Alterations, modifications and variations can be effected to
the
particular embodiments by those of skill in the art without departing from the
scope of the invention.
REFERENCES
[000100] All publications mentioned herein are incorporated herein by
reference
(where permitted) to disclose and describe the methods and/or materials in
connection with which the publications are cited. The publications discussed
herein are provided solely for their disclosure prior to the filing date of
the present
application. Nothing herein is to be construed as an admission that the
present
invention is not entitled to antedate such publication by virtue of prior
invention.
Further, the dates of publication provided may be different from the actual
publication dates, which may need to be independently confirmed.
1. Butler RM. GrayDrain's Blackbook: Thermal Recovery of oil and bitumen,
GrayDrain Inc. ISBNO-9682563-0-9. 1997.
2. Gates ID. Basic Reservoir Engineering, first ed. Kendall-Hunt Inc. ISBN:
978-0-
7575-9062-7. 2011.
3. Kapadia PR, Wang J, Kallos MS., Gates ID, New thermal reactive reservoir
engineering model predicts hydrogen sulfide generation in steam assisted
gravity
drainage. Journal of Petroleum Science and Engineering 2012; 94-95:100-111.
4. Gates ID, Larter SR, Energy efficiency and emissions intensity of SAGD.
Fuel
2014; 115:706-713.
32
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
5. Nduagu El, Gates ID, Process analysis of a low emissions hydrogen and steam
generation technology for oil sands operations. Applied Energy 2015; 146:184-
195.
6. Gotawala D, Gates ID, Steam fingering in Steam-Assisted Gravity Drainage.
Canadian Journal of Chemical Engineering 2008; 86:1011-1022.
7. Zhang W, Youn S, Doan Q, Understanding reservoir architectures and steam
chamber growth at Christina Lake, Alberta, by using 4D seismic and crosswell
seismic imaging. SPE Reservoir Evaluation Engineering 2007; 10:446-452.
8. Su Y, Wang J, Gates ID, SAGD well orientation in point bar oil sands
deposit
affects performance. Engineering Geology 2013; 157:79-92.
9. Su Y, Wang J, Gates ID, Orientation of a pad of SAGD well pairs in an
Athabasca point bar deposit affects performance. Marine and Petroleum Geology
2014; 54:37-46.
10. Giacchetta Gm, Leporini M, Marchett B., Economic and environmental
analysis of steam-assisted gravity drainage (SAGD) facility for oil recovery
from
Canadian oil sands. Applied Energy 2015; 142:1-9.
11. Larter SR, Adams J, Gates ID, Bennett B, Huang H, The origin, prediction
and
impact of oil viscosity heterogeneity on the production characteristics of tar
sand
and heavy oil reservoirs. Journal of Canadian Petroleum Technology 2008;
47:52-61.
12. Hyne JB,. Aquathermolysis - A synopsis of work on the chemical Reaction
between water (steam) and heavy oil sands during simulated steam stimulation.
Synopsis Report no. 50. AOSTRA Contracts no. 11,103,103B/C, 1986.
13. Kapadia PR, Kallos MS, Gates ID, A new kinetic model for pyrolysis of
Athabasca bitumen. Canadian Journal of Chemical Engineering 2013; 91:889-
901.
33
WSLEGAL \ 045074 \ 00165 1 I 3492436v3
CA 02932670 2016-06-06
14. Clark PD, Hyne JB, Steam¨oil chemical reactions: mechanism for the
aquathermolysis of heavy oils. AOSTRA Journal of Research 1984; 1:15-20.
15. Ng FTT, Upgrading heavy oil/bitumen emulsions via in situ hydrogen
generation. Reprints of symposia-Division of fuel chemistry, American Chemical
Society 1997; 43:456-460.
16. Kapadia PR, Kallos MS, Gates ID, A new reaction model for aquathermolysis
of Athabasca bitumen. Canadian Journal of Chemical Engineering 2013; 91:475-
482.
17. Bin Merdhah AB, Yassin AM, Formation damage due to scale formation in
porous media resulting water injection. Emirates Journal for Engineering
Research 2008; 13 : 69-79
18. Goodman WH, Godfrey MR, Miller TM, Scale and deposit formation in steam
assisted gravity drainage (SAGD) facilities. The International Water
Conference in
San Antonio, Texas, 2010. 19. Sjoberg EL, Kinetics and mechanism of calcite
dissolution in aqueous solutions at low temperatures. Acta Universitatis
Stockholmiensis, Stockholm Contributions in Geology, Doctoral thesis. 1978.
20. Boon JA, Hamilton T, Holloway L, Wiwchar B, Reaction between rock matrix
and injected fluids in Cold Lake oil sand-spotential for formation damage.
Journal
of Canadian Petroleum Technology Reservoir Engineering 1983; 22:55-66.
21. Abercrombie HJ, Hutcheon 1E, Remote monitoring of water-rock-bitumen
interactions during steam assisted heavy oil recovery. International
Association of
Geochemistry and Cosmochemistry 1986; 5th International Symposium on Water
Rock Interaction: 1-4.
22. Gunter WD, Bird GW, CO2 production in tar sand reservoirs under in situ
steam temperatures: reactive calcite dissolution. Chemical Geology 1988;
70:301-
311.
34
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
23. Gunter WD, Perkins EH, Aquifer disposal of CO2 rich gases: reaction design
for added capacity. Energy Conversion and Management 1993; 34:941-984.
24. Burnham AK, Stubblefield CT, Campbell JH, Effect of lime and gas
environment on mineral reactions in Colorado oil shale. Fuel 1980; 59:871-877.
25. Boynton RS, Chemistry and technology of lime and limestone. 2nd ed. New
York: Wiley; 1980.
26. Maclntire WH, Stansel TB, Steam catalysis in calcination of dolomite and
limestone fines. Industrial and Engineering Chemistry 1953; 45:1548-1555.
27. Wang Y, Thomson WJ, The effects of steam and carbon dioxide on calcite
decomposition using dynamic X-ray diffraction. Chemical Engineering Science
1994; 50:1373-1382.
28. Dreybrodt W, Eisenlohr L, Madry B, Ringer S, Precipitation kinetics of
calcite
in the system CaCO3-H20-0O2: The conversion to CO2 by the slow process H+ +
HCO- -) CO2 + H20 as a rate limiting step. Geochimica et Cosmochimica Acta
1997; 61:3897-3904.
29. Liu Z, Dreybrodt W, Dissolution kinetics of calcium carbonate minerals in
H20-
CO2 solutions in turbulent flow: the role of the diffusion boundary layer and
the
slow reaction H20 + CO2 '=. H+ + HCO3-. Geochimica et Cosmochimica Acta
1997; 61:2879-2889.
30. Emberley S, Hutcheon I, Shevalier M, Durocher K, Mayer B, Gunter WD,
Perkins EH, Monitoring of fluid-rock interaction and CO2 storage through
produced fluid sampling at the Weyburn CO2 injection enhanced oil recovery
site,
Saskatchewan, Canada. Applied Geochemistry 2005; 20:1131-1157.
31. Kaufmann G, Dreybrodt W, Calcite dissolution kinetics in the system CaCO3-
H20-0O2 at high undersaturation. Geochimica et Cosmochimica Acta 2007;
71:1398-1410.
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
32. Brantley SL, Kinetics of mineral dissolution. Kinetics of water-rock
interaction,
Chapter 5, Springer 2008;151-210.
33. Pokrovsky OS, Golubev SV, Schott J, Castillo A, Calcite, dolomite and
magnesite dissolution kinetics in aqueous solutions at acid to circumneutral
pH,
25 to 150 C and 1 to 55 atm pCO2: new constraints on CO2 sequestration in
sedimentary basins. Chemical Geology 2009; 265:20-32.
34. Kapadia P, Wang J, Kallos MS, Gates ID, Practical process design for in
situ
gasification of bitumen. Applied Energy 2013; 107:281-296.
35. Buhmann D, Dreybrodt W, The kinetics of calcite dissolution and
precipitation
geologically relevant situations of karst areas, 1. Open system. Chemical
Geology
1985; 48:189-211.
36. Kern MD, The hydration of carbon dioxide. Journal of Chemical Education
1960; 37:14-23.
37. Welch MJ, Lifton JF, Seck JA, Tracer studies with radioactive oxygen -15-
Exchange between carbon dioxide and water. Journal of Physical Chemistry
1969; 73:3351-3356.
38. Kielland J, Activity coefficient of ions in aqueous solution. Journal of
American
Chemical Society 1937; 59:1675-1678.
39. Nancollas GH, Reddy MM, The crystallization of calcium carbonate: II.
Calcite
growth mechanism. Journal of Colloid Interface Science 1971; 37:824-830.
40. Vinsome PKW, Westerveld J, A Simple Method for Predicting Cap and Base
Rock Heat Losses in Thermal Reservoir Simulators. Journal of Canadian
Petroleum Technology 1980; 19:87-90.
41. Akbilgic, 0., Zhu, D., Gates, I.D., and Bergerson, J. Prediction of Steam-
Assisted Gravity Drainage Steam to Oil Ratio from Reservoir Characteristics.
Energy, 93(2):1663-1670, 2015.
36
WSLEGAL\045074\00165\13492436v3
CA 02932670 2016-06-06
42. Alberta Energy Regulator Publications: ConocoPhillips Surmont Project.
Annual Surmont SAGD performance review approvals 9426F and 94600 surface
operations. April 5, 2011, Calgary, AB.
10
20
37
WSLEGAL\045074\00165\13492436v3