Note: Descriptions are shown in the official language in which they were submitted.
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
METHODS OF WASHING CELLULOSE-RICH SOLIDS FROM BIOMASS
FRACTIONATION TO REDUCE LIGNIN AND ASH CONTENT
PRIORITY DATA
[0001] This patent application is an international patent application
claiming
priority to U.S. Provisional Patent App. No. 61/905,938, filed November 19,
2013,
and to U.S. Patent App. No. 14/546,146, filed November 18, 2014, each of which
is
hereby incorporated by reference herein.
FIELD
[0002] The present invention generally relates to fractionation
processes for
converting biomass into fermentable sugars, cellulose, and lignin, and for
processes
and apparatus to recover the lignin.
BACKGROUND
[0003] Biomass refining (or biorefining) is becoming more prevalent
in
industry. Cellulose fibers and sugars, hemicellulose sugars, lignin, syngas,
and
derivatives of these intermediates are being used by many companies for
chemical
and fuel production. Indeed, we now are observing the commercialization of
integrated biorefineries that are capable of processing incoming biomass much
the
same as petroleum refineries now process crude oil. Underutilized
lignocellulosic
biomass feedstocks have the potential to be much cheaper than petroleum, on a
carbon
basis, as well as much better from an environmental life-cycle standpoint.
[0004] Lignocellulosic biomass is the most abundant renewable
material on
the planet and has long been recognized as a potential feedstock for producing
chemicals, fuels, and materials. Lignocellulosic biomass normally comprises
- 1 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
primarily cellulose, hemicellulose, and lignin. Cellulose and hemicellulose
are
natural polymers of sugars, and lignin is an aromatic/aliphatic hydrocarbon
polymer
reinforcing the entire biomass network. Some forms of biomass (e.g., recycled
materials) do not contain hemicellulose.
[0005] There are many reasons why it would be beneficial to process
biomass
in a way that effectively separates the major fractions (cellulose,
hemicellulose, and
lignin) from each other. Cellulose from biomass can be used in industrial
cellulose
applications directly, such as to make paper or other pulp-derived products.
The
cellulose can also be subjected to further processing to either modify the
cellulose in
some way or convert it into glucose. Hemicellulose sugars can be fermented to
a
variety of products, such as ethanol, or converted to other chemicals. Lignin
from
biomass has value as a solid fuel and also as an energy feedstock to produce
liquid
fuels, synthesis gas, or hydrogen; and as an intermediate to make a variety of
polymeric compounds. Additionally, minor components such as proteins or rare
sugars can be extracted and purified for specialty applications.
[0006] In light of this objective, a major shortcoming of previous
process
technologies is that one or two of the major components can be economically
recovered in high yields, but not all three. Either the third component is
sacrificially
degraded in an effort to produce the other two components, or incomplete
fractionation is accomplished. An important example is traditional biomass
pulping
(to produce paper and related goods). Cellulose is recovered in high yields,
but lignin
is primarily consumed by oxidation and hemicellulose sugars are mostly
degraded.
Approximately half of the starting biomass is essentially wasted in this
manufacturing
process. State-of-the-art biomass-pretreatment approaches typically can
produce high
yields of hemicellulose sugars but suffer from moderate cellulose and lignin
yields.
[0007] There are several possible pathways to convert biomass into
intermediates. One thermochemical pathway converts the feedstock into syngas
(CO
and H2) through gasification or partial oxidation. Another thermochemical
pathway
converts biomass into liquid bio-oils through pyrolysis and separation. These
are both
high-temperature processes that intentionally destroy sugars in biomass.
[0008] Sugars (e.g., glucose and xylose) are desirable platform
molecules
because they can be fermented to a wide variety of fuels and chemicals, used
to grow
- 2 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
organisms or produce enzymes, converted catalytically to chemicals, or
recovered and
sold to the market. To recover sugars from biomass, the cellulose and/or the
hemicellulose in the biomass must be hydrolyzed into sugars. This is a
difficult task
because lignin and hemicelluloses are bound to each other by covalent bonds,
and the
three components are arranged inside the fiber wall in a complex manner. This
recalcitrance explains the natural resistance of woody biomass to
decomposition, and
explains the difficulty to convert biomass to sugars at high yields.
[0009] Fractionation of biomass into its principle components
(cellulose,
hemicellulose, and lignin) has several advantages. Fractionation of
lignocellulosics
leads to release of cellulosic fibers and opens the cell wall structure by
dissolution of
lignin and hemicellulose between the cellulose microfibrils. The fibers become
more
accessible for hydrolysis by enzymes. When the sugars in lignocellulosics are
used as
feedstock for fermentation, the process to open up the cell wall structure is
often
called "pretreatment." Pretreatment can significantly impact the production
cost of
lignocellulosic ethanol.
[0010] One of the most challenging technical obstacles for cellulose
has been
its recalcitrance towards hydrolysis for glucose production. Because of the
high
quantity of enzymes typically required, the enzyme cost can be a tremendous
burden
on the overall cost to turn cellulose into glucose for fermentation. Cellulose
can be
made to be reactive by subjecting biomass to severe chemistry, but that would
jeopardize not only its integrity for other potential uses but also the yields
of
hemicellulose and lignin.
[0011] Many types of pretreatment have been studied. A common
chemical
pretreatment process employs a dilute acid, usually sulfuric acid, to
hydrolyze and
extract hemicellulose sugars and some lignin. A common physical pretreatment
process employs steam explosion to mechanically disrupt the cellulose fibers
and
promote some separation of hemicellulose and lignin. Combinations of chemical
and
physical pretreatments are possible, such as acid pretreatment coupled with
mechanical refining. It is difficult to avoid degradation of sugars. In some
cases,
severe pretreatments (i.e., high temperature and/or low pH) intentionally
dehydrate
sugars to furfural, levulinic acid, and related chemicals. Also, in common
acidic
- 3 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
pretreatment approaches, lignin handling is very problematic because acid-
condensed
lignin precipitates and forms deposits on surfaces throughout the process.
[0012] One type of pretreatment that can overcome many of these
disadvantages is called "organosolv" pretreatment. Organosolv refers to the
presence
of an organic solvent for lignin, which allows the lignin to remain soluble
for better
lignin handling. Traditionally, organosolv pretreatment or pulping has
employed
ethanol-water solutions to extract most of the lignin but leave much of the
hemicellulose attached to the cellulose. For some market pulps, it is
acceptable or
desirable to have high hemicellulose content in the pulp. When high sugar
yields are
desired, however, there is a problem. Traditional ethanol/water pulping cannot
give
high yields of hemicellulose sugars because the timescale for sufficient
hydrolysis of
hemicellulose to monomers causes soluble-lignin polymerization and then
precipitation back onto cellulose, which negatively impacts both pulp quality
as well
as cellulose enzymatic digestibility.
[0013] An acid catalyst can be introduced into organosolv
pretreatment to
hydrolyze hemicellulose into monomers while still obtaining the solvent
benefit.
Conventional organosolv wisdom dictates that high delignification can be
achieved,
but that a substantial fraction of hemicellulose must be left in the solids
because any
catalyst added to hydrolyze the hemicellulose will necessarily degrade the
sugars
(e.g., to furfural) during extraction of residual lignin.
[0014] Solvent cooking chemicals have been attempted as an
alternative to
Kraft or sulfite pulping. The original solvent process is described in U.S.
Patent No.
1,856,567 by Kleinert et al. Groombridge et al. in U.S. Patent No. 2,060,068
showed
that an aqueous solvent with sulfur dioxide is a potent delignifying system to
produce
cellulose from lignocellulosic material. Three demonstration facilities for
ethanol-
water (Alcell), alkaline sulfite with anthraquinone and methanol (ASAM), and
ethanol-water-sodium hydroxide (Organocell) were operated briefly in the
1990s.
[0015] Contrary to conventional wisdom, it has been found that
fractionation
with a solution of ethanol (or another solvent for lignin), water, and sulfur
dioxide
(S02) can simultaneously achieve several important objectives. The
fractionation can
be achieved at modest temperatures (e.g., 120-160 C). The SO2 can be easily
recovered and reused. This process is able to effectively fractionation many
biomass
- 4 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
species, including softwoods, hardwoods, agricultural residues, and waste
biomass.
The SO2 hydrolyzes the hemicelluloses and reduces or eliminates troublesome
lignin-
based precipitates. The presence of ethanol leads to rapid impregnation of the
biomass, so that neither a separate impregnation stage nor size reduction
smaller than
wood chips are needed, thereby avoiding electricity-consuming sizing
operations.
The dissolved hemicelluloses are neither dehydrated nor oxidized (Iakovlev,
"S02-
ethanol-water fractionation of lignocellulosics," Ph.D. Thesis, Aalto Univ.,
Espoo,
Finland, 2011). Cellulose is fully retained in the solid phase and can
subsequently be
hydrolyzed to glucose. The mixture of hemicellulose monomer sugars and
cellulose-
derived glucose may be used for production of biofuels and chemicals.
[0016] In view of the state of the art, what is desired is to
efficiently
fractionate any lignocellulosic-based biomass (including, in particular,
softwoods)
into its primary components so that each can be used in potentially distinct
processes.
While not all commercial products require pure forms of cellulose,
hemicellulose, or
lignin, a platform biorefinery technology that enables processing flexibility
in
downstream optimization of product mix, is particularly desirable. An
especially
flexible fractionation technique would not only separate most of the
hemicellulose
and lignin from the cellulose, but also render the cellulose highly reactive
to cellulase
enzymes for the manufacture of fermentable glucose.
[0017] The AVAPO fractionation process developed by American Process,
Inc. and its affiliates is able to economically accomplish these objectives.
Improvements are still desired in the area of washing of pulp to reduce lignin
and ash
content.
- 5 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
SUMMARY
[0018] The present invention addresses the aforementioned needs in
the art.
[0019] In some variations, the invention provides a process for
fractionating
lignocellulosic biomass, the process comprising:
(a) digesting a lignocellulosic biomass feedstock under effective conditions
in
the presence of a solvent for lignin, an acid or acid precursor, and water, to
produce
cellulose-rich solids in a digestor liquor;
(b) separating the cellulose-rich solids from the digestor liquor and washing
the cellulose-rich solids with a first wash liquid comprising a wash solvent
for lignin,
to generate first washed cellulose-rich solids;
(c) washing the first washed cellulose-rich solids with a second wash liquid
comprising water, to generate second washed cellulose-rich solids and a wash
liquor
comprising fines, wherein the wash liquor is introduced to or in contact with
a
classifier to remove at least a portion of the fines in a liquid fines-
containing stream;
(d) recovering the second washed cellulose-rich solids; and
(e) optionally separating the fines from the fines-containing stream and
recycling water contained in the fines-containing stream back to step (c).
[0020] The lignocellulosic biomass feedstock is a hardwood or an
annual plant
or agricultural residue, in some embodiments. The solvent for lignin may be
ethanol,
and the wash solvent for lignin may be the same (e.g., ethanol) or different.
The acid
or acid precursor is preferably sulfur dioxide.
[0021] In some embodiments, the classifier comprises a screen with
mesh size
in the range of 10 to 500, such as a range of 100 to 325 or 150 to 250. In
certain
embodiments, the classifier comprises a screen with mesh size of 200. The
classifier
may also comprise a centrifuge or other separation device.
[0022] In some embodiments, during step (b) and/or step (c), a
disperser is
utilized to liberate the fines from the second washed cellulose-rich solids.
During step
(c), a portion of the fines contained in the wash liquor are removed into the
liquid
fines-containing stream. The portion of fines removed may be at least 50%, at
least
75%, or at least 95% of the fines contained in the wash liquor removed into
the liquid
fines-containing stream.
- 6 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
[0023] In some embodiments, during step (b) and/or step (c), one or
more
additives are introduced to remove minerals remaining in the first washed
cellulose-
rich solids and/or the second washed cellulose-rich solids.
[0024] The second washed cellulose-rich solids will typically have a
lower
Kappa number compared to cellulose-rich solids from an otherwise-identical
process
without a classifier to remove at least a portion of the fines. In some
embodiments,
the second washed cellulose-rich solids have a lower ash content compared to
cellulose-rich solids from an otherwise-identical process without a classifier
to
remove at least a portion of the fines. In some of these embodiments, the
second
washed cellulose-rich solids have a lower hemicellulose content compared to
cellulose-rich solids from an otherwise-identical process without a classifier
to
remove at least a portion of the fines.
[0025] For example, the second washed cellulose-rich solids may
contain
about 75% or more cellulose, about 7 wt% or less lignin, about 5 wt% or less
hemicellulose, and about 10 wt% or less ash. In certain embodiments, the
second
washed cellulose-rich solids contain about 80% or more cellulose, about 3 wt%
or less
lignin, about 5 wt% or less hemicellulose, and about 8 wt% or less ash.
[0026] The process may be continuous or semi-continuous, or batch. In
some
embodiments, steps (a) and (b) are conducted countercurrently. In some
embodiments, steps (a)-(c) are conducted countercurrently. In certain
embodiments,
the process is batch or semi-continuous, wherein step (b) and/or step (c) is
conducted
in simulated countercurrent fashion, and wherein multiple wash streams are
generated.
[0027] In some embodiments, the process further comprises hydrolyzing
the
second washed cellulose-rich solids to produce glucose. In some embodiments,
the
process further comprises feeding the second washed cellulose-rich solids to a
pulping
operation.
[0028] The process may further include separating and recycling
unreacted
acid or acid precursor from the digestor liquor. In some embodiments, the
process
further comprises further treating the digestor liquor to generate fermentable
sugars.
[0029] Some variations provide a method of separating fines from
cellulose-
rich solids, the method comprising:
- 7 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
(a) obtaining a biomass digestor liquor comprising cellulose-rich solids;
(b) separating the cellulose-rich solids from the digestor liquor and washing
the cellulose-rich solids with a first wash liquid, to generate first washed
cellulose-
rich solids;
(c) washing the first washed cellulose-rich solids with a second wash liquid,
to
generate second washed cellulose-rich solids and a wash liquor comprising
fines,
wherein the wash liquor is introduced to or in contact with a classifier to
remove at
least a portion of the fines in a liquid fines-containing stream;
(d) recovering the second washed cellulose-rich solids; and
(e) optionally separating the fines from the fines-containing stream and
recycling water contained in the fines-containing stream back to step (c).
[0030] Some variations provide a method of separating fines from
cellulose-
rich solids, the method comprising:
(a) obtaining a biomass digestor liquor comprising cellulose-rich solids;
(b) separating the cellulose-rich solids from the digestor liquor and washing
the cellulose-rich solids with a wash liquid, to generate washed cellulose-
rich solids
and a wash liquor comprising fines, wherein the wash liquor is introduced to
or in
contact with a classifier to remove at least a portion of the fines in a
liquid fines-
containing stream;
(c) recovering the second washed cellulose-rich solids; and
(d) optionally separating the fines from the fines-containing stream and
recycling water contained in the fines-containing stream back to step (b).
[0031] In some embodiments, the classifier comprises a screen with
mesh size
in the range of 10 to 500, such as 100 to 325 or 150 to 250 (e.g., 200). In
some
embodiments, a disperser is utilized to liberate the fines from the washed
cellulose-
rich solids. At least 50%, 75%, or 95% of the fines contained in the wash
liquor may
be removed into the liquid fines-containing stream.
[0032] Optionally, one or more additives are introduced to remove
minerals
remaining in the washed cellulose-rich solids.
[0033] In some embodiments, the washed cellulose-rich solids have a
lower
Kappa number compared to cellulose-rich solids from an otherwise-identical
process
without a classifier to remove at least a portion of the fines. In some
embodiments,
- 8 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
the second washed cellulose-rich solids have a lower ash content compared to
cellulose-rich solids from an otherwise-identical process without a classifier
to
remove at least a portion of the fines. In some embodiments, the second washed
cellulose-rich solids have a lower hemicellulose content compared to cellulose-
rich
solids from an otherwise-identical process without a classifier to remove at
least a
portion of the fines.
BRIEF DESCRIPTION OF THE DRAWINGS
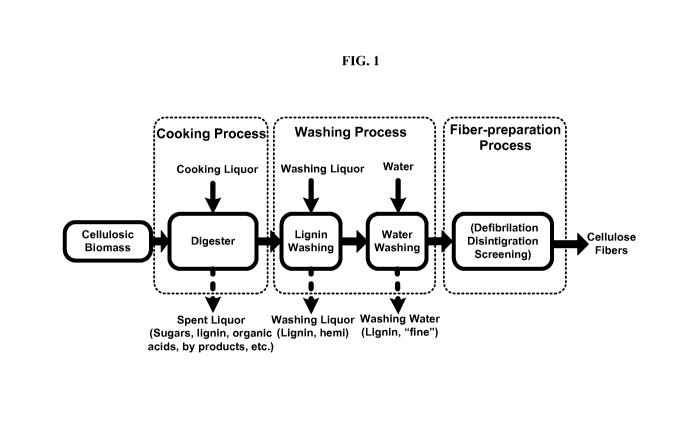
[0034] FIG. 1 depicts a process of fractionating lignocellulosic
biomass, in
some embodiments of the invention.
[0035] FIG. 2 shows photographs of fibers and fines isolated from the
pulp
with a Britt jar assembly, in some embodiments.
[0036] FIG. 3 summarizes an isolation process of fines from washing
water,
according to certain embodiments of the invention.
[0037] FIG. 4 shows exemplary SEM images of pulp produced from
sugarcane straw by AVAPO technology.
[0038] FIG. 5 shows exemplary SEM images of fines isolated from the
pulp
(shown in FIG. 4) during a water-washing stage, according to some embodiments.
[0039] FIG. 6 is a schematic representation of certain improved
washing
procedures, in preferred embodiments.
[0040] These drawings are exemplary in nature and should not be
construed to
limit the invention in any way.
DETAILED DESCRIPTION OF SOME EMBODIMENTS
[0041] This description will enable one skilled in the art to make
and use the
invention, and it describes several embodiments, adaptations, variations,
alternatives,
and uses of the invention. These and other embodiments, features, and
advantages of
the present invention will become more apparent to those skilled in the art
when taken
- 9 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
with reference to the following detailed description of the invention in
conjunction
with any accompanying drawings.
[0042] As used in this specification and the appended claims, the
singular
forms "a," "an," and "the" include plural referents unless the context clearly
indicates
otherwise. For example, reference to "unit" also includes a plurality of units
(e.g.,
reactors or vessels). Unless defined otherwise, all technical and scientific
terms used
herein have the same meaning as is commonly understood by one of ordinary
skill in
the art to which this invention belongs. All composition numbers and ranges
based on
percentages are weight percentages, unless indicated otherwise. All ranges of
numbers or conditions are meant to encompass any specific value contained
within
the range, rounded to any suitable decimal point.
[0043] Unless otherwise indicated, all numbers expressing parameters,
reaction conditions, concentrations of components, and so forth used in the
specification and claims are to be understood as being modified in all
instances by the
term "about." Accordingly, unless indicated to the contrary, the numerical
parameters
set forth in the following specification and attached claims are
approximations that
may vary depending at least upon a specific analytical technique.
[0044] The term "comprising," which is synonymous with "including,"
"containing," or "characterized by" is inclusive or open-ended and does not
exclude
additional, unrecited elements or method steps. "Comprising" is a term of art
used in
claim language which means that the named claim elements are essential, but
other
claim elements may be added and still form a construct within the scope of the
claim.
[0045] As used herein, the phase "consisting of" excludes any
element, step,
or ingredient not specified in the claim. When the phrase "consists of" (or
variations
thereof) appears in a clause of the body of a claim, rather than immediately
following
the preamble, it limits only the element set forth in that clause; other
elements are not
excluded from the claim as a whole. As used herein, the phase "consisting
essentially
of' limits the scope of a claim to the specified elements or method steps,
plus those
that do not materially affect the basis and novel characteristic(s) of the
claimed
subject matter.
[0046] With respect to the terms "comprising," "consisting of," and
"consisting essentially of," where one of these three terms is used herein,
the
- 10 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
presently disclosed and claimed subject matter may include the use of either
of the
other two terms. Thus in some embodiments not otherwise explicitly recited,
any
instance of "comprising" may be replaced by "consisting of" or, alternatively,
by
"consisting essentially of"
[0047] This disclosure describes processes and apparatus to
efficiently
fractionate any lignocellulosic-based biomass into its primary major
components
(cellulose, lignin, and if present, hemicellulose) so that each can be used in
potentially
distinct processes. An advantage of the process is that it produces cellulose-
rich
solids while concurrently producing a liquid phase containing a high yield of
both
hemicellulose sugars and lignin, and low quantities of lignin and
hemicellulose
degradation products. The flexible fractionation technique enables multiple
uses for
the products. The washed cellulose is highly reactive to cellulase enzymes for
the
manufacture of glucose. Other uses for celluloses can be adjusted based on
market
conditions.
[0048] Certain exemplary embodiments of the invention will now be
described. These embodiments are not intended to limit the scope of the
invention as
claimed. The order of steps may be varied, some steps may be omitted, and/or
other
steps may be added. Reference herein to first step, second step, etc. is for
illustration
purposes only.
[0049] In order to produce cellulose fiber (or pulp) from
lignocellulosic
biomass, the biomass along with cooking liquor is first cooked in a digestor
and then
liquid and solid phases are separated. The liquid phase, also called "spent
liquor,"
mainly includes dissolved biomass substances such as lignin, hemicellulosic
and
cellulosic sugars in oligomeric and monomeric form, as well as organic acids
(acetic
acid, uronic acids, formic acid, levulinic acid, lactic acid, etc.), and sugar-
degradation
products (furfural, hydroxymethylfurfural (HMF), etc.). The spent liquor is
typically
sent to downstream processes to recover heat value, cooking chemicals and
other
dissolved products such as organic acids, sugars, furfural, levulinic acid,
formic acid,
lactic acid, and HMF. The solid phase is subjected to subsequent washing and
disintegration to free solid from spent liquor and produce cellulose fibers.
[0050] A schematic representation of a cooking process (or
fractionation
process) of lignocellulosic biomass is shown in FIG. 1. The biomass is cooked
in a
-11-
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
digestor (reactor), and then it is subsequently washed with washing liquors as
shown
in FIG. 1. Cellulose fibers are prepared after defibrillation, disintegration,
and
screening of the cooked biomass. FIG. 1 also shows other streams of the
cooking/fractionation process. The spent liquor mostly contains hemicellulose
and
lignin, while the washing liquor mainly contains lignin and some hemicellulose
as
well as "fines" that are suspended or dissolved in the liquid phase.
[0051] As used herein in some variations, "fines" are defined as
small
particles passing through 200 mesh (or 76 litm in diameter) screen, according
to Tappi
261 cm-10, which is incorporated by reference herein. These particles may
include
both cellulosic and non-cellulosic materials. The fines from annual plants are
mostly
originated from different small vessel elements such as tracheids, parenchyma
cells,
etc. and called "primary fines." The fines generated during chemical pulping
of wood
are mostly as a result of refining and are called "secondary fines." Therefore
the fines
generated during a cooking/fractionation process (such as that depicted in
FIG. 1)
may be either (or both) cellulosic or non-cellulosic in origin. Generally
speaking, fine
components may include (but are not limited to) cellulose, hemicellulose,
lignin, ash,
dirt, dust, metals, and foreign materials (i.e. materials that were not in the
original
biomass).
[0052] Interestingly, it has been found that the lignin and mineral
content of
cellulose fibers decreases when the amount of washing water used is increased
during
the washing procedure. In order to further investigate this behavior, the
amount of
washing water used during water washing cycle following lignin washing cycle
was
altered as shown in Table 1.
[0053] Four identical cooks with sugarcane straw were conducted in a
digestor. The subsequent washing procedure is almost the same for all four
cooks, the
only difference being the amount of washing water used in the final washing
stage.
Chemical composition of straw used for cooks is summarized in Table 2.
Chemical
composition of cellulose fibers obtained following washing procedure suggested
in
Table 1 is summarized in Table 3.
[0054] According to Table 3, the water washing cycle following lignin
washing cycle has a significant effect on the fiber yield and lignin content
(Kappa
number) as well as on cellulose and hemicellulose content. A high yield of 51%
- 12 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
(Experiment #1) was obtained without water washing cycle, while the lowest
yield
(Experiment #4) of 34% is measured after extensive water washing cycle. The
material loss during extensive water washing step is most likely due to loss
of small
particles with wash water. It should be noted that during washing procedure,
filtering
bags with 200 mesh were used to separate solid and liquid phases by
filtration. The
particles smaller than 200 mesh can go through filtering bags.
[0055] The fines content of pulp produced from sugarcane straws with
washing procedure described by Experiment #3 in Table 1 can be quantified by
Tappi
method 261 cm-90 with Britt jar assembly. The Britt Jar is a single screen
classifier
with 200 mesh screen or a round hole of 76 [tm in diameter. Fibers are
retained while
fines pass through the 200 mesh screen. The fibers and fines isolated from the
pulp
are shown in FIG. 2. The fines content of pulp based on o.d. (oven dried) pulp
is
around 23 %. The amount of fines (23%) determined by Britt Jar is reasonable
when
compared with pulp produced from hardwoods and other annual plants. The Britt
jar
fines reported for variety of pulp produced from hardwoods is between 5% and
21%,
depending on wood species and operation conditions (Nanko et al., 2005).
Sadovnik
et al. (2007) reported that the pulp produced from sugarcane bagasse has high
content
of fines from 35 to 40 %. It is also reported that bleachable-grade wheat
straw pulp
produced by soda pulping process in continuous Pandia digestor has 21.4% of
fines
(Casey, 1980).
[0056] It is clear from Table 3 that increased amount of water used
during
water washing cycle (as seen in Table 1) decreases lignin and ash content of
cellulose
fiber while increases cellulose content of fiber. Without being limited by any
particular hypothesis, this reduction in lignin and ash content may be
attributed to
removal of fines which have high lignin and ash content and relatively low
cellulose
content compared to cellulose fiber during washing procedure. Fines were
separated
during water washing stage and chemical characterization of fines was
determined.
As shown in FIG. 3, the fines were allowed to pass through 200 mesh filtering
bag
into washing water and then they were isolated from washing water by filtering
or
freeze drying following centrifugation. The fines isolated by filtration and
freeze-
drying are shown in FIG. 3.
- 13 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
Table 1. Effect of water washing procedure on fiber yield and kappa number.
Water Exp.# Water washing procedure following lignin wash
1 No water washing following Et0H washing after cooking
:Amount
2 Just rinsed with little water following Et0H after cooking
r of
iiiMashing
water 3 Washed with some water following Et0H washing after
cooking
4 Washed with plenty of water following Et0H washing after cooking
Table 2. The chemical composition of sugarcane straws based on o.d. straws.
Component
Arabinan 2.8 0.0
Galactan 1.1 0.0
Glucan 37.1 0.5
Xylan 20.0 0.2
Mannan 0.6 0.0
Acetyl Groups 1.1 0.0
Uronic Acid Groups 2.2 0.3
Lignin 23.7 0.2
Ash 6.8 0.4
Extractives 2.6 0.0
Total 98.0 1.6
Table 3. Effect of water washing procedure on fiber composition.
% on % on fiber
straw
Exp.# Yield Cellulose Hemicellulose Lignin Ash Kappa #
1 50.9 73.8 5.2 7.9 11.4 52.1
2 43.3 78.8 4.4 5.9 9.3 39.2
3 38.9 81.3 4.4 3.7 8.4 25.0
4 34.4 83.5 4.6 2.8 7.4 19.5
- 14 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
[0057] The chemical composition of freeze-dried fines was determined
and is
summarized in Table 4. It is apparent from Table 4 that fines isolated during
water
washing stage mostly contain cellulose, lignin, and ash. Lignin, ash, and
hemicellulose content of fines are each higher than the corresponding content
in the
fiber (see Table 3, Experiments 2, 3 and 4) while the opposite is true for
cellulose.
Therefore, it can be concluded that depending on the amount of fines removed
during
the washing stage, the cellulose content of fibers can be increased while
hemicellulose, lignin, and ash content of the fiber can be reduced.
Table 4. Sugar and lignin contents of "fines" isolated during water washing
process
following AVAP cooking of the straws and ethanol washing stage.
Component % on od "fines"
Arabinan 0.7
Galactan 0.2
Glucan 57.8
Xylan 6.0
Mannan 0.2
Lignin 15.6
Ash 19.0
Total 99.5
[0058] As explained earlier, lignin content of fines is much higher
than that of
final pulp. This high lignin content of cellulosic fines may be a result of
lignin
precipitation on fines during cooking process or subsequent washing process
due to
higher mobility and surface area of fines in comparison to fibers (Gess,
1998). For
this reason, in order to investigate the precipitation of lignin, scanning
electron
microscopy (SEM) images of both pulp and fines were taken and are displayed in
FIGS. 4 and 5 respectively. More lignin precipitation on cellulosic fines can
be
observed and also it appears that pulp fibers are smoother than fines.
[0059] A schematic representation of an improved washing procedure to
produce cellulose fibers (pulp) with low Kappa number and ash content, along
while
high cellulose content, following cooking of hardwood and/or annual plants is
shown
- 15 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
in FIG. 6. Since fines have high lignin and ash content, Kappa number (lignin
content) and ash content of cellulose fibers can be lowered based on how much
fines
are removed during the washing procedure.
[0060] Some variations provide a process for fractionating
lignocellulosic
biomass, the process comprising:
(a) digesting a lignocellulosic biomass feedstock under effective conditions
in
the presence of a solvent for lignin, an acid or acid precursor, and water, to
produce
cellulose-rich solids in a digestor liquor;
(b) separating the cellulose-rich solids from the digestor liquor and washing
the cellulose-rich solids with a first wash liquid comprising a wash solvent
for lignin,
to generate first washed cellulose-rich solids;
(c) washing the first washed cellulose-rich solids with a second wash liquid
comprising water, to generate second washed cellulose-rich solids and a wash
liquid
comprising fines, wherein the wash liquid is introduced to or in contact with
a
classifier to remove at least a portion of the fines in a liquid fines-
containing stream;
(d) recovering the second washed cellulose-rich solids; and
(e) optionally separating the fines from the fines-containing stream and
recycling water to step (c).
[0061] In some embodiments, the classifier comprises a screen with
mesh size
in the range of 10 to 500. In certain embodiments, the classifier comprises a
screen
with mesh size in the range of 100 to 325, such as 150 to 250. In a particular
embodiment, the classifier comprises a screen with mesh size of 200. Other
screen
sizes may be employed.
[0062] In some embodiments, the classifier is a batch or continuous
centrifuge
or hydrocyclone operated to remove fines within one or more selected size
ranges. In
certain embodiments, both a centrifuge and screen(s) may be used, such as
screening
the liquid discharge of a decanting centrifuge. Screen centrifuges, wherein
the
centrifugal acceleration allows the liquid to pass through a screen, include
screen/scroll centrifuges, pusher centrifuges, peeler centrifuges, and
decanter
centrifuges, in which there is no physical separation between the solid and
liquid
phase, rather an accelerated settling due to centrifugal acceleration. Solid
bowl
centrifuges or conical plate centrifuges may also be employed.
- 16 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
[0063] Dispersers may also be added to liberate more fines if
necessary.
During step (b) and/or step (c), a disperser may be utilized to liberate fines
from the
second washed cellulose-rich solids. A disperser may liberate additional fines
that
would not have otherwise been released. In some embodiments, a disperser is a
simple mixing tank, i.e. a stirred tank or vessel. Dispersers may also be in-
line (static)
mixers, high-shear mixers, centrifuges, or other equipment. In some
embodiments,
the disperser is integrated with the classifier; for example, a centrifuge may
be
adapted to both disperse fines from solids as well as classify the fines as
described
above.
[0064] Instead of a disperser, or in addition, other reagents may
also be used
to liberate more fines and/or remove minerals remaining in the pulp at this
stage,
depending on targeted quality of product. During step (b) and/or step (c), one
or more
additives may be introduced to remove minerals remaining in the first washed
cellulose-rich solids and/or the second washed cellulose-rich solids.
Additives
include, but are not limited to, acids, bases, salts, carbon (such as
activated carbon or
carbon foams), metal foams, silica, alumina, or other compounds.
[0065] Cellulose fibers may also be bleached to remove remaining
lignin from
the fiber. Any known bleaching sequence may be utilized.
[0066] The process may be continuous, semi-continuous, or batch. In
some
embodiments, one or more steps are conducted countercurrently. In certain
embodiments, the process is batch or semi-continuous, washing is conducted in
simulated countercurrent fashion, and multiple wash streams (such as two,
three, or
more wash streams) are generated.
[0067] In some embodiments, the solvent for lignin includes an
aliphatic
alcohol, such as ethanol. Preferably, the process further comprises recycling
the
solvent for lignin back to the digestor. Also, the process preferably
comprises
recycling the unreacted acid or acid precursor to the digestor.
[0068] In some embodiments, the acid catalyst (or acid precursor) is
a sulfur-
containing compound or a derivative thereof For example the sulfur-containing
compound may be selected from the group consisting of sulfur dioxide, sulfur
trioxide, sulfurous acid, sulfuric acid, sulfonic acids, lignosulfonic acids,
elemental
sulfur, and combinations thereof
- 17 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
[0069] In some embodiments, the acid catalyst is a nitrogen-
containing
compound (e.g., HNO3) or a derivative thereof In some embodiments, the acid
catalyst is a phosphorous-containing compound (e.g., H3PO4) or a derivative
thereof
In some embodiments, the acid catalyst is one or more hydrogen halides (e.g.,
HBr or
HC1).
[0070] Removal of SO2 may be conducted in a sulfur dioxide separation
unit
selected from the group consisting of a flash vessel, a stripping column, a
distillation
column, and combinations thereof, operated under vacuum or pressure. In some
embodiments, the sulfur dioxide separation unit is a stripping column
employing
steam for stripping the unreacted sulfur dioxide.
[0071] The process may further include dilution with liquid water
during one
or more steps. As intended here, dilution with liquid water may occur via
injection of
a liquid-phase stream comprising water, which may be fresh water or recycled
water
(e.g., process condensate); alternatively, or additionally, dilution with
liquid water
may occur via injection of steam which condenses to form liquid water that
dilutes a
process stream. Dilution with liquid water may assist in the precipitating at
least
some of the lignin in a lignin-containing stream.
[0072] In some embodiments, the process further comprises pH
adjustment
during one or more steps. The pH adjustment may assist in controlling lignin
precipitation in the lignin-containing stream. For example, raising pH may
increase
lignin solubility in aqueous solution, while lowering pH may reduce lignin
solubility
in aqueous solution, in some embodiments.
[0073] In embodiments employing SO2 during fractionation, some amount
of
lignin sulfonation typically occurs. In some embodiments, the process
comprises
further lignin sulfonation during one or more steps. Lignin sulfonation
generally
increases lignin solubility in aqueous solution. Lignin sulfonation may be
accomplished by reaction of soluble lignin or suspended lignin with SO2 or
another
sulfur-containing compound.
[0074] The lignin-containing stream may be in various forms and
phases,
including multiple phases (two, three, or more). For example, the lignin-
containing
stream may be in the form of a slurry. In certain embodiments, lignin-
containing
stream or product contains lignin in substantially solid form, such as lignin
solids
- 18 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
recovered periodically from a semi-continuous process or lignin solids that
form a
filter cake.
[0075] In some embodiments, the lignin-containing stream contains
colloids
of lignin dispersed in the continuous phase (liquor). Colloids of lignin may
be
removed by filtration or centrifugation, for example. To enhance the removal
of
lignin colloids from suspension, it may be desirable to adjust the pH of the
suspension
either during or after dilution with water. Also, additives may be introduced
to
change kinetics or thermodynamics of colloid phase formation. In some
embodiments, the lignin/lignosulfonate ratio is optimized during digestion or
downstream, to adjust the properties of the colloidal suspension.
[0076] The hemicelluloses may be recovered for fermentation or for
further
processing. In some embodiments, the process further comprises a step of
hemicellulose hydrolysis with an acid or enzymes. The acid for hemicellulose
hydrolysis may include lignosulfonic acids that are derived from the initial
fractionation step.
[0077] The cellulose-rich solids may be recovered as a pulp product.
Alternatively, or additionally, the cellulose-rich solids may be hydrolyzed to
produce
glucose.
[0078] The present invention includes apparatus and systems to carry
out the
processes described herein. The present invention also includes products
produced by
the processes described herein. Such products include biomass-derived sugars,
cellulose materials, lignin, lignosulfonates, and other co-products.
[0079] As used herein, "lignocellulosic biomass" means any material
containing cellulose and lignin. Lignocellulosic biomass may also contain
hemicellulose. Mixtures of one or more types of biomass can be used. In some
embodiments, the biomass feedstock comprises both a lignocellulosic component
(such as one described above) in addition to a sucrose-containing component
(e.g.,
sugarcane or energy cane) and/or a starch component (e.g., corn, wheat, rice,
etc.).
[0080] The biomass feedstock may be selected from hardwoods,
softwoods,
forest residues, industrial wastes, pulp and paper wastes, consumer wastes, or
combinations thereof Some embodiments utilize agricultural residues, which
include
lignocellulosic biomass associated with food crops, annual grasses, energy
crops, or
- 19 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
other annually renewable feedstocks. Exemplary agricultural residues include,
but are
not limited to, corn stover, corn fiber, wheat straw, sugarcane bagasse,
sugarcane
straw, rice straw, oat straw, barley straw, miscanthus, energy cane
straw/residue, or
combinations thereof In some embodiments, the biomass feedstock is not
softwood.
[0081] Various moisture levels may be associated with the starting
biomass.
The biomass feedstock need not be, but may be, relatively dry. In general, the
biomass is in the form of a particulate or chip, but particle size is not
critical in this
invention.
[0082] Reaction conditions and operation sequences may vary widely.
Some
embodiments employ conditions described in U.S. Patent No. 8,030,039, issued
Oct.
4,2011; U.S. Patent No. 8,038,842, issued Oct. 11,2011; U.S. Patent No.
8,268,125,
issued Sept. 18, 2012; and U.S. Patent App. Nos. 13/004,431; 12/234,286;
13/585,710; 12/250,734; 12/397,284; 12/304,046; 13/500,916; 13/626,220;
12/854,869; 61/732,047; 61/735,738; 61/739,343; 61/747,010; 61/747,105;
61/747,376; 61/747,379; 61/747,382; and 61/747,408 including the prosecution
histories thereof Each of these commonly owned patent applications is hereby
incorporated by reference herein in its entirety. In some embodiments, the
process is
a variation of the AVAP process technology which is commonly owned with the
assignee of this patent application.
[0083] In some embodiments, a first process step is "cooking"
(equivalently,
"digesting") which fractionates the three lignocellulosic material components
(cellulose, hemicellulose, and lignin) to allow easy downstream removal.
Specifically, hemicelluloses are dissolved and over 50% are completely
hydrolyzed;
cellulose is separated but remains resistant to hydrolysis; and part of the
lignin is
sulfonated into water-soluble lignosulfonates.
[0084] The lignocellulosic material is processed in a solution
(cooking liquor)
of solvent, water, and sulfur dioxide. The cooking liquor preferably contains
at least
wt%, such as at least 20 wt%, 30 wt%, 40 wt%, or 50 wt% of a solvent for
lignin.
By "solvent for lignin," it is meant a chemical that is capable of dissolving
at least
some lignin, in native (non-sulfonated) form, at the conditions of digestion.
[0085] For example, the cooking liquor may contain about 30-70 wt%
solvent,
such as about 50 wt% solvent. The solvent for lignin may be an aliphatic
alcohol,
- 20 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
such as methanol, ethanol, 1-propanol, 2-propanol, 1-butanol, 2-butanol,
isobutanol,
1-pentanol, 1-hexanol, or cyclohexanol. The solvent for lignin may be an
aromatic
alcohol, such as phenol or cresol. Other lignin solvents are possible, such as
(but not
limited to) glycerol, methyl ethyl ketone, or diethyl ether. Combinations of
more than
one solvent may be employed.
[0086] Preferably, enough solvent is included in the extractant
mixture to
dissolve the lignin present in the starting material. The solvent for lignin
may be
completely miscible, partially miscible, or immiscible with water, so that
there may
be more than one liquid phase. Potential process advantages arise when the
solvent is
miscible with water, and also when the solvent is immiscible with water. When
the
solvent is water-miscible, a single liquid phase forms, so mass transfer of
lignin and
hemicellulose extraction is enhanced, and the downstream process must only
deal
with one liquid stream. When the solvent is immiscible in water, the
extractant
mixture readily separates to form liquid phases, so a distinct separation step
can be
avoided or simplified. This can be advantageous if one liquid phase contains
most of
the lignin and the other contains most of the hemicellulose sugars, as this
facilitates
recovering the lignin from the hemicellulose sugars.
[0087] The cooking liquor preferably contains sulfur dioxide and/or
sulfurous
acid (H2S03). The cooking liquor preferably contains S02, in dissolved or
reacted
form, in a concentration of at least 3 wt%, preferably at least 6 wt%, more
preferably
at least 8 wt%, such as about 9 wt%, 10 wt%, 11 wt%, 12 wt%, 13 wt%, 14 wt%,
15
wt%, 20 wt%, 25 wt%, 30 wt% or higher. The cooking liquor may also contain one
or more species, separately from S02, to adjust the pH. The pH of the cooking
liquor
is typically about 4 or less.
[0088] Sulfur dioxide is a preferred acid catalyst, because it can be
recovered
easily from solution after hydrolysis. The majority of the SO2 from the
hydrolysate
may be stripped and recycled back to the reactor. Recovery and recycling
translates
to less lime required compared to neutralization of comparable sulfuric acid,
less
solids to dispose of, and less separation equipment. The increased efficiency
owing to
the inherent properties of sulfur dioxide mean that less total acid or other
catalysts
may be required. This has cost advantages, since sulfuric acid can be
expensive.
Additionally, and quite significantly, less acid usage also will translate
into lower
- 21 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
costs for a base (e.g., lime) to increase the pH following hydrolysis, for
downstream
operations. Furthermore, less acid and less base will also mean substantially
less
generation of waste salts (e.g., gypsum) that may otherwise require disposal.
[0089] The cooking is performed in one or more stages using batch or
continuous digestors. Solid and liquid may flow cocurrently or countercun-
ently, or in
any other flow pattern that achieves the desired fractionation. The cooking
reactor
may be internally agitated, if desired.
[0090] Depending on the lignocellulosic material to be processed, the
cooking
conditions are varied, with temperatures from about 65 C to 175 C, for example
75 C, 85 C, 95 C, 105 C, 115 C, 125 C, 130 C, 135 C, 140 C, 145 C, 150 C,
155 C, 165 C or 170 C, and corresponding pressures from about 1 atmosphere to
about 15 atmospheres in the liquid or vapor phase. The cooking time of one or
more
stages may be selected from about 15 minutes to about 720 minutes, such as
about 30,
45, 60, 90, 120, 140, 160, 180, 250, 300, 360, 450, 550, 600, or 700 minutes.
Generally, there is an inverse relationship between the temperature used
during the
digestion step and the time needed to obtain good fractionation of the biomass
into its
constituent parts.
[0091] The cooking liquor to lignocellulosic material ratio may be
selected
from about 1 to about 10, such as about 2, 3, 4, 5, or 6. In some embodiments,
biomass is digested in a pressurized vessel with low liquor volume (low ratio
of
cooking liquor to lignocellulosic material), so that the cooking space is
filled with
ethanol and sulfur dioxide vapor in equilibrium with moisture. The cooked
biomass is
washed in alcohol-rich solution to recover lignin and dissolved
hemicelluloses, while
the remaining pulp is further processed. In some embodiments, the process of
fractionating lignocellulosic material comprises vapor-phase cooking of
lignocellulosic material with aliphatic alcohol (or other solvent for lignin),
water, and
sulfur dioxide. See, for example, U.S. Patent Nos. 8,038,842 and 8,268,125
which are
incorporated by reference herein.
[0092] A portion or all of the sulfur dioxide may be present as
sulfurous acid
in the extract liquor. In certain embodiments, sulfur dioxide is generated in
situ by
introducing sulfurous acid, sulfite ions, bisulfite ions, combinations
thereof, or a salt
- 22 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
of any of the foregoing. Excess sulfur dioxide, following hydrolysis, may be
recovered and reused.
[0093] In some embodiments, sulfur dioxide is saturated in water (or
aqueous
solution, optionally with an alcohol) at a first temperature, and the
hydrolysis is then
carried out at a second, generally higher, temperature. In some embodiments,
sulfur
dioxide is sub-saturated. In some embodiments, sulfur dioxide is super-
saturated. In
some embodiments, sulfur dioxide concentration is selected to achieve a
certain
degree of lignin sulfonation, such as 1%, 2%, 3%, 4%, 5%, 6%, 7%, 8%, /0 ,s0
z,
v or 10%
sulfur content. SO2 reacts chemically with lignin to form stable lignosulfonic
acids
which may be present both in the solid and liquid phases.
[0094] The concentration of sulfur dioxide, additives, and aliphatic
alcohol (or
other solvent) in the solution and the time of cook may be varied to control
the yield
of cellulose and hemicellulose in the pulp. The concentration of sulfur
dioxide and
the time of cook may be varied to control the yield of lignin versus
lignosulfonates in
the hydrolysate. In some embodiments, the concentration of sulfur dioxide,
temperature, and the time of cook may be varied to control the yield of
fermentable
sugars.
[0095] Once the desired amount of fractionation of both hemicellulose
and
lignin from the solid phase is achieved, the liquid and solid phases are
separated.
Conditions for the separation may be selected to minimize the reprecipitation
of the
extracted lignin on the solid phase. This is favored by conducting separation
or
washing at a temperature of at least the glass-transition temperature of
lignin (about
120 C).
[0096] The physical separation can be accomplished either by
transferring the
entire mixture to a device that can carry out the separation and washing, or
by
removing only one of the phases from the reactor while keeping the other phase
in
place. The solid phase can be physically retained by appropriately sized
screens
through which liquid can pass. The solid is retained on the screens and can be
kept
there for successive solid-wash cycles. Alternately, the liquid may be
retained and
solid phase forced out of the reaction zone, with centrifugal or other forces
that can
effectively transfer the solids out of the slurry. In a continuous system,
countercurrent
flow of solids and liquid can accomplish the physical separation.
-23 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
[0097] The recovered solids normally will contain a quantity of
lignin and
sugars, some of which can be removed easily by washing. The washing-liquid
composition can be the same as or different than the liquor composition used
during
fractionation. Multiple washes may be performed to increase effectiveness.
Preferably, one or more washes are performed with a composition including a
solvent
for lignin, to remove additional lignin from the solids, followed by one or
more
washes with water to displace residual solvent and sugars from the solids, as
well as
release fines from the fibers as disclosed in detail herein. Recycle streams,
such as
from solvent-recovery operations, may be used to wash the solids.
[0098] After separation and washing as described, a solid phase and
at least
one liquid phase are obtained. The solid phase contains substantially
undigested
cellulose. A single liquid phase is usually obtained when the solvent and the
water
are miscible in the relative proportions that are present. In that case, the
liquid phase
contains, in dissolved form, most of the lignin originally in the starting
lignocellulosic
material, as well as soluble monomeric and oligomeric sugars formed in the
hydrolysis of any hemicellulose that may have been present. Multiple liquid
phases
tend to form when the solvent and water are wholly or partially immiscible.
The
lignin tends to be contained in the liquid phase that contains most of the
solvent.
Hemicellulose hydrolysis products tend to be present in the liquid phase that
contains
most of the water.
[0099] In some embodiments, hydrolysate from the cooking step is
subjected
to pressure reduction. Pressure reduction may be done at the end of a cook in
a batch
digestor, or in an external flash tank after extraction from a continuous
digestor, for
example. The flash vapor from the pressure reduction may be collected into a
cooking liquor make-up vessel. The flash vapor contains substantially all the
unreacted sulfur dioxide which may be directly dissolved into new cooking
liquor.
The cellulose is then removed to be washed and further treated as desired.
[00100] A process washing step recovers the hydrolysate from the
cellulose.
The washed cellulose is pulp that may be used for various purposes (e.g.,
paper or
nanocellulose production). The weak hydrolysate from the washer continues to
the
final reaction step; in a continuous digestor this weak hydrolysate may be
combined
with the extracted hydrolysate from the external flash tank. In some
embodiments,
- 24 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
washing and/or separation of hydrolysate and cellulose-rich solids is
conducted at a
temperature of at least about 100 C, 110 C, or 120 C. The washed cellulose may
also
be used for glucose production via cellulose hydrolysis with enzymes or acids.
[00101] In another reaction step, the hydrolysate may be further
treated in one
or multiple steps to hydrolyze the oligomers into monomers. This step may be
conducted before, during, or after the removal of solvent and sulfur dioxide.
The
solution may or may not contain residual solvent (e.g. alcohol). In some
embodiments, sulfur dioxide is added or allowed to pass through to this step,
to assist
hydrolysis. In these or other embodiments, an acid such as sulfurous acid or
sulfuric
acid is introduced to assist with hydrolysis. In some embodiments, the
hydrolysate is
autohydrolyzed by heating under pressure. In some embodiments, no additional
acid
is introduced, but lignosulfonic acids produced during the initial cooking are
effective
to catalyze hydrolysis of hemicellulose oligomers to monomers. In various
embodiments, this step utilizes sulfur dioxide, sulfurous acid, sulfuric acid
at a
concentration of about 0.01 wt% to 30 wt%, such as about 0.05 wt%, 0.1 wt%,
0.2
wt%, 0.5 wt%, 1 wt%, 2 wt%, 5 wt%, 10 wt%, or 20 wt%. This step may be carried
out at a temperature from about 100 C to 220 C, such as about 110 C, 120 C,
130 C,
140 C, 150 C, 160 C, 170 C, 180 C, 190 C, 200 C, or 210 C. Heating may be
direct or indirect to reach the selected temperature.
[00102] The reaction step produces fermentable sugars which can then
be
concentrated by evaporation to a fermentation feedstock. Concentration by
evaporation may be accomplished before, during, or after the treatment to
hydrolyze
oligomers. The final reaction step may optionally be followed by steam
stripping of
the resulting hydrolysate to remove and recover sulfur dioxide and alcohol,
and for
removal of potential fermentation-inhibiting side products. The evaporation
process
may be under vacuum or pressure, from about ¨0.1 atmospheres to about 10
atmospheres, such as about 0.1 atm, 0.3 atm, 0.5 atm, 1.0 atm, 1.5 atm, 2 atm,
4 atm,
6 atm, or 8 atm.
[00103] Recovering and recycling the sulfur dioxide may utilize
separations
such as, but not limited to, vapor-liquid disengagement (e.g. flashing), steam
stripping, extraction, or combinations or multiple stages thereof Various
recycle
ratios may be practiced, such as about 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8,
0.9, 0.95, or
- 25 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
more. In some embodiments, about 90-99% of initially charged SO2 is readily
recovered by distillation from the liquid phase, with the remaining 1-10%
(e.g., about
3-5%) of the SO2 primarily bound to dissolved lignin in the form of
lignosulfonates.
[00104] In a preferred embodiment, the evaporation step utilizes an
integrated
alcohol stripper and evaporator. Evaporated vapor streams may be segregated so
as to
have different concentrations of organic compounds in different streams.
Evaporator
condensate streams may be segregated so as to have different concentrations of
organic compounds in different streams. Alcohol may be recovered from the
evaporation process by condensing the exhaust vapor and returning to the
cooking
liquor make-up vessel in the cooking step. Clean condensate from the
evaporation
process may be used in the washing step.
[00105] In some embodiments, an integrated alcohol stripper and
evaporator
system is employed, wherein aliphatic alcohol is removed by vapor stripping,
the
resulting stripper product stream is concentrated by evaporating water from
the
stream, and evaporated vapor is compressed using vapor compression and is
reused to
provide thermal energy.
[00106] The hydrolysate from the evaporation and final reaction step
contains
mainly fermentable sugars but may also contain lignin depending on the
location of
lignin separation in the overall process configuration. The hydrolysate may be
concentrated to a concentration of about 5 wt% to about 60 wt% solids, such as
about
wt%, 15 wt%, 20 wt%, 25 wt%, 30 wt%, 35 wt%, 40 wt%, 45 wt%, 50 wt% or 55
wt% solids. The hydrolysate contains fermentable sugars.
[00107] Fermentable sugars are defined as hydrolysis products of
cellulose,
galactoglucomannan, glucomannan, arabinoglucuronoxylans, arabinogalactan, and
glucuronoxylans into their respective short-chained oligomers and monomer
products,
i.e., glucose, mannose, galactose, xylose, and arabinose. The fermentable
sugars may
be recovered in purified form, as a sugar slurry or dry sugar solids, for
example. Any
known technique may be employed to recover a slurry of sugars or to dry the
solution
to produce dry sugar solids.
[00108] In some embodiments, the fermentable sugars are fermented to
produce biochemicals or biofuels such as (but by no means limited to) ethanol,
isopropanol, acetone, 1-butanol, isobutanol, lactic acid, succinic acid, or
any other
- 26 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
fermentation products. Some amount of the fermentation product may be a
microorganism or enzymes, which may be recovered if desired.
[00109] When the fermentation will employ bacteria, such as Clostridia
bacteria, it is preferable to further process and condition the hydrolysate to
raise pH
and remove residual SO2 and other fermentation inhibitors. The residual SO2
(i.e.,
following removal of most of it by stripping) may be catalytically oxidized to
convert
residual sulfite ions to sulfate ions by oxidation. This oxidation may be
accomplished
by adding an oxidation catalyst, such as FeSO4=7H20, that oxidizes sulfite
ions to
sulfate ions. Preferably, the residual SO2 is reduced to less than about 100
ppm, 50
ppm, 25 ppm, 10 ppm, 5 ppm, or 1 ppm.
[00110] The process fermentation and distillation steps are intended
for the
production of fermentation products, such as alcohols or organic acids. After
removal
of cooking chemicals and lignin, and further treatment (oligomer hydrolysis),
the
hydrolysate contains mainly fermentable sugars in water solution from which
any
fermentation inhibitors have been preferably removed or neutralized. The
hydrolysate
is fermented to produce dilute alcohol or organic acids, from 1 wt% to 20 wt%
concentration. The dilute product is distilled or otherwise purified as is
known in the
art.
[00111] When alcohol is produced, such as ethanol, some of it may be
used for
cooking liquor makeup in the process cooking step. Also, in some embodiments,
a
distillation column stream, such as the bottoms, with or without evaporator
condensate, may be reused to wash cellulose. In some embodiments, lime may be
used to dehydrate product alcohol. Side products may be removed and recovered
from the hydrolysate. These side products may be isolated by processing the
vent
from the final reaction step and/or the condensate from the evaporation step.
Side
products include furfural, hydroxymethyl furfural (HMF), methanol, acetic
acid, and
lignin-derived compounds, for example.
[00112] The cellulose-rich material is highly reactive in the presence
of
industrial cellulase enzymes that efficiently break the cellulose down to
glucose
monomers. It has been found experimentally that the cellulose-rich material,
which
generally speaking is highly delignified, rapidly hydrolyzes to glucose with
relatively
low quantities of enzymes. For example, the cellulose-rich solids may be
converted
- 27 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
to glucose with at least 80% yield within 24 hours at 50 C and 2 wt% solids,
in the
presence of a cellulase enzyme mixture in an amount of no more than 15 filter
paper
units (FPU) per g of the solids. In some embodiments, this same conversion
requires
no more than 5 FPU per g of the solids.
[00113] The glucose may be fermented to an alcohol, an organic acid,
or
another fermentation product. The glucose may be used as a sweetener or
isomerized
to enrich its fructose content. The glucose may be used to produce baker's
yeast. The
glucose may be catalytically or thermally converted to various organic acids
and other
materials.
[00114] In some embodiments, the cellulose-rich material is further
processed
into one more cellulose products. Cellulose products include market pulp,
dissolving
pulp (also known as a-cellulose), fluff pulp, purified cellulose, paper, paper
products,
and so on. Further processing may include bleaching, if desired. Further
processing
may include modification of fiber length or particle size, such as when
producing
nanocellulose or nanofibrillated or microfibrillated cellulose. It is believed
that the
cellulose produced by this process is highly amenable to derivatization
chemistry for
cellulose derivatives and cellulose-based materials such as polymers.
[00115] When hemicellulose is present in the starting biomass, all or
a portion
of the liquid phase contains hemicellulose sugars and soluble oligomers. It is
preferred to remove most of the lignin from the liquid, as described above, to
produce
a fermentation broth which will contain water, possibly some of the solvent
for lignin,
hemicellulose sugars, and various minor components from the digestion process.
This
fermentation broth can be used directly, combined with one or more other
fermentation streams, or further treated. Further treatment can include sugar
concentration by evaporation; addition of glucose or other sugars (optionally
as
obtained from cellulose saccharification); addition of various nutrients such
as salts,
vitamins, or trace elements; pH adjustment; and removal of fermentation
inhibitors
such as acetic acid and phenolic compounds. The choice of conditioning steps
should
be specific to the target product(s) and microorganism(s) employed.
[00116] In some embodiments, hemicellulose sugars are not fermented
but
rather are recovered and purified, stored, sold, or converted to a specialty
product.
Xylose, for example, can be converted into xylitol.
- 28 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
[00117] Lignin produced in accordance with the invention can be used
as a
fuel. As a solid fuel, lignin is similar in energy content to coal. Lignin can
act as an
oxygenated component in liquid fuels, to enhance octane while meeting
standards as a
renewable fuel. The lignin produced herein can also be used as polymeric
material,
and as a chemical precursor for producing lignin derivatives. The sulfonated
lignin
may be sold as a lignosulfonate product, or burned for fuel value. In certain
embodiments, the process further comprises combusting or gasifying the
sulfonated
lignin, recovering sulfur contained in the sulfonated lignin in a gas stream
comprising
reclaimed sulfur dioxide, and then recycling the reclaimed sulfur dioxide for
reuse.
[00118] Native (non-sulfonated) lignin is hydrophobic, while
lignosulfonates
are hydrophilic. Hydrophilic lignosulfonates may have less propensity to
clump,
agglomerate, and stick to surfaces. Even lignosulfonates that do undergo some
condensation and increase of molecular weight, will still have an HS03 group
that
will contribute some solubility (hydrophilic). After the evaporative
precipitation or
other method to remove water-insoluble lignin, the remaining water-soluble
lignosulfonates may be precipitated by converting the hydrolysate to an
alkaline
condition (pH higher than 7) using, for example, an alkaline earth oxide,
preferably
calcium oxide (lime). The lignosulfonate precipitate may be filtered. The
lignosulfonate filter cake may be dried as a co-product or burned or gasified
for
energy production. The hydrolysate from filtering may be recovered and sold as
a
concentrated sugar solution product or further processed in a subsequent
fermentation
or other reaction step.
[00119] Lignin with specific property ranges may be obtained by doing
a
multiple-effect evaporative crystallization to purposely create lignin
precipitates with
various properties. Thus in some embodiments, several types of non-sulfonated
lignin
or lignin with low levels of sulfur may be obtained, in addition to one or
more
sulfonated lignins.
[00120] The present invention also provides systems configured for
carrying
out the disclosed processes, and compositions produced therefrom. Any stream
generated by the disclosed processes may be partially or completed recovered,
purified or further treated, and/or marketed or sold.
- 29 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
[00121] In this detailed description, reference has been made to
multiple
embodiments of the invention and non-limiting examples relating to how the
invention can be understood and practiced. Other embodiments that do not
provide
all of the features and advantages set forth herein may be utilized, without
departing
from the spirit and scope of the present invention. This invention
incorporates routine
experimentation and optimization of the methods and systems described herein.
Such
modifications and variations are considered to be within the scope of the
invention
defined by the claims.
[00122] All publications, patents, and patent applications cited in
this
specification are herein incorporated by reference in their entirety as if
each
publication, patent, or patent application were specifically and individually
put forth
herein.
[00123] Where methods and steps described above indicate certain
events
occurring in certain order, those of ordinary skill in the art will recognize
that the
ordering of certain steps may be modified and that such modifications are in
accordance with the variations of the invention. Additionally, certain of the
steps may
be performed concurrently in a parallel process when possible, as well as
performed
sequentially.
[00124] Therefore, to the extent there are variations of the
invention, which are
within the spirit of the disclosure or equivalent to the inventions found in
the
appended claims, it is the intent that this patent will cover those variations
as well.
The present invention shall only be limited by what is claimed.
REFERENCES
Casey, J.P. Pulp and paper, chemistry and chemical technology. 3rd edition,
vol. 1,
John Wily and Sons, Inc., USA, 1980.
Gess, J.M. Retention of fines and fillers during papermaking, Tappi Press.
Atlanta,
GA (1998).
- 30 -
CA 02933827 2016-06-14
WO 2015/077294
PCT/US2014/066333
Nanko, H., Button, A., Hillman, D. The world of market Pulp, Appleton, WI,
WOMP
LLC (2005).
Sadovnik, J.C, Betancourt, L.A, Ramos, J., and Alban, F., "Fines Study in the
Bleached Pulp from the Sugar Cane Bagasse," Tappi 2007 Engineering, Pulping &
Environmental Conference (2007).
Tappi standard T 261 cm-90 "Fines fraction of paper stock by wet screening,"
Tappi
press, Atlanta, GA, USA (1992).
-31 -