Note: Descriptions are shown in the official language in which they were submitted.
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
CATHETER OR GUIDE WIRE DEVICE INCLUDING FLOW SENSING
AND USE THEREOF
CROSS-REFERENCES TO RELATED APPLICATIONS
[0001] This application is a continuation-in-part of U.S. patent application
serial no.
13/844,677, entitled "CATHETER DEVICE INCLUDING FLOW SENSING", filed on March
15, 2013, which claims priority to U.S. provisional application serial no.
61/668,338, filed on
July 5, 2012, entitled "METHOD AND APPARATUS FOR DENERVATION," U.S.
provisional
application serial no. 61/728,653, filed on November 20, 2012, entitled "RENAL
DENERVATION," and U.S. provisional application serial no. 61/733,575, filed on
December 5,
2012, entitled "INCREASING THE RESOLUTION OF TEMPERATURE MEASUREMENT
FOR FLOW DETECTION IN THE RENAL ARTERY," each of which is hereby incorporated
herein by reference in its entirety, including drawings.
BACKGROUND
[0002] Diseases such as heart disease, stroke and hypertension are global
epidemics that affect
billions of people worldwide. Hypertension underlies the progression of
several debilitating
diseases, including heart disease and stroke. Despite widespread use of anti-
hypertension
medication to counter high blood pressure, the prevalence of hypertension is
alarmingly high and
constitutes a severe economic burden on health care.
[0003] Blood pressure is controlled, in large part, by the sympathetic nervous
system. The
sympathetic nervous system involves several organs that are responsible for
regulating blood
pressure such as the brain, heart and kidneys. The kidney is a key element in
long-term blood
pressure regulation. Hypertension, or high blood pressure, results from
hyperactive renal nerves.
This, in turn, can cause heart, kidney, and blood vessel damage.
[0004] Other systems of the body where nerves activity can affect fluid flow
include the carotid
sinus, the carotid body, the vagal nerve, the pulmonary artery, the celiac
ganglion, and the
bladder trigone.
SUMMARY
[0005] The Inventors have recognized that an ability to monitor procedures
during treatment of
tissue is advantageous. For example, renal ablation represents a useful and
potentially safe
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
technique. Its applicability may be limited due to a lack of sensing
capability following a
procedure such as ablation.
[0006] In view of the foregoing, various examples described herein are
directed generally to
systems, apparatus and methods for facilitating the monitoring and/or
verification of the outcome
of procedures, such as but not limited to a nerve denervation and/or a nerve
pacing procedure.
The result of the monitoring and/or verification can be used to determine a
clinical endpoint of a
denervation and/or a pacing procedure. Systems and methods described herein
also facilitate
establishing a credible endpoint in denervation procedures, including renal
sympathetic
denervation procedures.
[0007] Systems and methods described herein provide novel devices, including
catheter
devices or guidewire devices, with diagnostic capabilities, to assess the
state of the tissue
following each procedure, including each ablation cycle of a series of
ablation cycles.
[0008] Systems and methods described herein provide novel devices, including
catheter
devices, with diagnostic capabilities, to assess the state of the tissue in
other systems, including
in pulmonary veins, coronary arteries, and peripheral blood vessels, following
each procedure in
a series of procedures, such as but not limited to each ablation cycle of a
series of ablation
cycles.
[0009] In an example, a system, apparatus and method herein provide novel
devices that can be
implemented for measuring blood flow, or other fluid flow, coupled with pacing
and/or
denervation of nerves, using a single smart catheter or guidewire device.
[0010] In an example, a system, apparatus and method herein can be implemented
for
facilitating monitoring and/or verifying the outcome of denervation and/or
pacing procedures
performed in one or more systems, such as but not limited to the carotid
sinus, the carotid body,
the vagal nerve, the pulmonary artery, celiac ganglion, the bladder trigone,
or the renal arteries.
[0011] In an example, a system, apparatus and method is provided that is based
on thin device
islands, including integrated circuitry (IC) chips and/or stretchable and/or
flexible interconnects
that are encapsulated in an encapsulant.
[0012] In an example, a system, apparatus and method herein can be implemented
for
performing a procedure on the portion of tissue, and where the procedure is a
denervation
procedure or a nerve stimulation procedure. In an example, the procedure can
be a carotid sinus
denervation, a carotid body disruption, a vagus nerve stimulation, a pulmonary
artery
2
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
denervation, a celiac ganglion disruption, a bladder trigone ablation, or a
renal denervation.
[0013] In an example, a system, apparatus and method is provided for
determining a flow rate
of a fluid proximate to a portion of a tissue. An example device according to
this principle
includes an elongated member having a proximal portion and a distal portion,
and a flow sensor
disposed proximate to the distal portion of the elongated member. The flow
sensor includes at
least one temperature sensor and at least one heating element to heat an area
proximate to the
elongated member, at least a portion of the at least one heating element
forming a cavity. At
least a portion of the at least one temperature sensor is housed in a portion
of the cavity. A
temperature measurement of the temperature sensor provides a first indication
of a flow rate of
the fluid proximate to the flow sensor.
[0014] In an example, the device can further include an inflatable and/or
expandable body
coupled to a portion of the elongated member and having a proximal portion and
a distal portion.
The distal portion of the inflatable and/or expandable body is disposed
proximate to the flow
sensor. The example device can further include an electronic circuit coupled
with the inflatable
and/or expandable body, where the electronic circuit comprises at least one
stretchable
interconnect, and where the electronic circuit is stretchable and conformable
such that the
electronic circuit accommodates an expansion of the inflatable and/or
expandable body. The
electronic circuit can further include at least one passive electronic
component and/or at least one
active electronic component, and wherein the at least one stretchable
interconnect electrically
couples at least two electronic components of the electronic circuit.
[0015] In an example, the device can include at least one heating element is
comprised of a
coiled resistive wire, where a hollow portion of the coiled resistive wire
forms the cavity. In
another example, the at least one heating element can include a thin-film
patterned resistive
element, where the at least one heating element is formed in a substantially
cylindrical
conformation including the cavity. In another example, the thin-film patterned
resistive element
can include a pattern of resistive elements disposed on a stretchable and/or
flexible substrate.
The resistive elements may be formed in a linear pattern, a serpentine
pattern, a boustrophedonic
pattern, a zig-zag pattern, a wavy pattern, a polygonal pattern, or a
substantially circular pattern.
[0016] In an example, a system, apparatus and method herein is provided for
displaying
representations of parameters of an inflatable body and/or expandable body
disposed proximate
to a portion of a tissue. In an example, the inflatable body and/or expandable
body includes a
3
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
plurality of sensors coupled to at least a portion of the inflatable body
and/or expandable body.
An example apparatus can include a user interface, at least one memory to
store processor-
executable instructions, and at least one processing unit communicatively
coupled to the at least
one memory. Upon execution of the processor-executable instructions, the at
least one
processing unit can controls the user interface to display at least one
representation of the
parameters. The at least one representation includes: (A) a first
representation of a state of the
inflatable body and/or expandable body and (B) a second representation of a
state of at least one
sensor of the plurality of sensors. The first representation can include (i) a
first form indicator to
indicate that the inflatable body and/or expandable body is in an inflated
and/or an expanded
state, or (ii) a second form indicator to indicate that the inflatable body
and/or expandable body
is in a deflated and/or a collapsed state. The second representation can
include (i) a first
activation indicator to indicate that the at least one sensor of the plurality
of sensors measures a
signal below a threshold value, or (ii) a second activation indicator to
indicate that the at least
one sensor of the plurality of sensors measures a signal above or about equal
to the threshold
value.
[0017] In an example implementation of the apparatus, a signal below a
specified (threshold)
value indicates that the at least one sensor is not in contact with a portion
of the tissue, and a
signal above or about equal to the specified (threshold) value indicates that
the at least one sensor
is in contact with a portion of the tissue.
[0018] In an example implementation of the apparatus, the first activation
indicator and the
second activation indicator can be displayed as binary visual representations
and/or as
quantitative visual representations that correspond to a magnitude of the
signal.
[0019] In an example implementation of the apparatus, the at least one
processing unit can be
used to control the user interface to cause display of the first
representation and the second
representation in a staged process, such that no second representation is
displayed while the first
representation is the first form indicator, and the second representation is
displayed once the first
representation is the second form indicator.
[0020] In an example implementation of the apparatus, the at least one
processing unit can be
used to control the user interface to further cause display of an indication
of at least one stage of
a procedure being performed on the portion of the tissue and/or an indication
of an endpoint of a
procedure being performed on the portion of the tissue.
4
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
[0021] In an example, a system, apparatus and method herein can be implemented
for
performing a medical treatment procedure. An example method can include
disposing in
proximity to the tissue an apparatus that includes an elongated member having
a proximal
portion and a distal portion, at least one flow sensor disposed proximate to
the distal portion of
the elongated member, and a reference temperature sensor disposed on a
proximal portion of the
elongated member. Each of the at least one flow sensor includes at least one
temperature sensor,
and at least one heating element disposed proximate to the at least one
temperature sensor. The
example apparatus can include a control module coupled to the at least one
flow sensor and the
reference temperature sensor. The example method includes using the control
module to
maintain a temperature difference between the reference temperature sensor and
the temperature
sensor of the at least one flow sensor at a stage of performance of the
medical treatment
procedure. Use of the example control module includes monitoring a value of a
temperature
measurement of the reference temperature sensor and/or a temperature
measurement of the
temperature sensor of the at least one flow sensor, and controlling a first
signal to the at least one
heating element to cause the at least one heating element to emit heat or
discontinue emitting
heat, such that that the temperature difference is maintained.
[0022] In an example implementation of the method, the temperature difference
can be a
constant temperature difference or a time-varying temperature difference. In
an example the
temperature difference can be a constant temperature difference, where the
constant temperature
difference is about 1.5 C, about 2.0 C, about 2.5 C, about 3.0 C, about
3.5 C, about 4.0 C,
or about 4.5 C.
[0023] In an example implementation, the example control module includes a
proportional-
integral-derivative (PID) controller or an anemometer. Where the control
module includes a PID
controller, the method can further include applying the PID controller to
compare the value of
the temperature measurement of the reference temperature sensor to the
temperature
measurement of the temperature sensor of at least one flow sensor, and to
determine a second
signal based on the comparison, and using the control module to determine the
first signal to the
at least one heating element based on the second signal.
[0024] In an example implementation of the method, stages of the method can be
repeated until
the difference falls in a specified range of values.
[0025] In an example, a system, apparatus and method herein can be implemented
for
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
monitoring a hemodynamic effect during a medical treatment procedure performed
on a vascular
tissue. An example method can include disposing in proximity to the tissue an
apparatus that
includes an elongated member having a proximal portion and a distal portion,
at least one flow
sensor disposed proximate to the distal portion of the elongated member, and
at least one
component coupled to the elongated member to perform a medical treatment
procedure on a
portion of the tissue proximate to the elongated member. The example method
can further
include activating the at least one component to perform the medical treatment
procedure on the
portion of the tissue, administering a substance that causes a change in
dimension of the vascular
tissue, using the at least one flow sensor to perform at least one flow
measurement, the at least
one flow measurement providing data indicative of a change in the flow
subsequent to the
medical treatment procedure of a fluid proximate to the apparatus, and
analyzing the data
indicative of the flow of the fluid to determine at least one parameter
indicative of the change in
the hemodynamics of the fluid. A reduction in the change in the hemodynamics
of the fluid is
used to provide an indication of the efficacy of the medical treatment
procedure.
[0026] In an example implementation of the method, stages of the method can be
repeated until
the rate of reduction of the change in the hemodynamics of the fluid falls
below a specified
value. The example method can further include generating an indication of an
endpoint of the
medical treatment procedure when the rate of reduction of the change in the
hemodynamics of
the fluid falls below the specified value.
[0027] In an example implementation of the method, the substance can include
an endogenous
substance or an exogenous substance. For example, the substance can include a
dopamine,
adenosine, prostacyclin, saline, or nitric oxide. The at least one component
can be an ablative
component, where the medical treatment procedure is a denervation procedure.
[0028] An example system, apparatus and method herein provides a catheter or
guidewire
device for performing a procedure on tissue. The catheter or guidewire device
includes an
inflatable and/or expandable body disposed near a distal end of the catheter,
at least one flow
sensor disposed on the inflatable and/or expandable body. At least one
component is coupled to
the catheter or guidewire to perform an ablation procedure on a portion of the
tissue of the renal
artery. Each of the at least one flow sensors includes a heating element to
heat an area proximate
to the inflatable and/or expandable body, the heating element including a
cavity, and a
temperature sensor at least partially disposed in the cavity of the heating
element. Measurement
6
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
of the temperature sensor provides an indication of a flow rate of a fluid
proximate to the
inflatable and/or expandable body.
[0029] The following publications, patents, and patent applications are hereby
incorporated
herein by reference in their entirety:
[0030] Kim et al., "Stretchable and Foldable Silicon Integrated Circuits,"
Science Express,
March 27, 2008, 10.1126/science.1154367;
[0031] Ko et al., "A Hemispherical Electronic Eye Camera Based on Compressible
Silicon
Optoelectronics," Nature, August 7, 2008, vol. 454, pp. 748-753;
[0032] Kim et al., "Complementary Metal Oxide Silicon Integrated Circuits
Incorporating
Monolithically Integrated Stretchable Wavy Interconnects," Applied Physics
Letters, July 31,
2008, vol. 93, 044102;
[0033] Kim et al., "Materials and Noncoplanar Mesh Designs for Integrated
Circuits with
Linear Elastic Responses to Extreme Mechanical Deformations," PNAS, December
2, 2008, vol.
105, no. 48, pp. 18675-18680;
[0034] Meitl et al., "Transfer Printing by Kinetic Control of Adhesion to an
Elastomeric
Stamp," Nature Materials, January, 2006, vol. 5, pp. 33-38;
[0035] U.S. Patent Application publication no. 2010 0002402-Al, published
January 7, 2010,
filed March 5, 2009, and entitled "STRETCHABLE AND FOLDABLE ELECTRONIC
DEVICES;"
[0036] U.S. Patent Application publication no. 2010 0087782-Al, published
April 8, 2010,
filed October 7, 2009, and entitled "CATHETER BALLOON HAVING STRETCHABLE
INTEGRATED CIRCUITRY AND SENSOR ARRAY;"
[0037] U.S. Patent Application publication no. 2010 0116526-Al, published May
13, 2010,
filed November 12, 2009, and entitled "EXTREMELY STRETCHABLE ELECTRONICS;"
[0038] U.S. Patent Application publication no. 2010 0178722-Al, published July
15, 2010,
filed January 12, 2010, and entitled "METHODS AND APPLICATIONS OF NON-PLANAR
IMAGING ARRAYS;" and
[0039] U.S. Patent Application publication no. 2010 027119-Al, published
October 28, 2010,
filed November 24, 2009, and entitled "SYSTEMS, DEVICES, AND METHODS UTILIZING
STRETCHABLE ELECTRONICS TO MEASURE TIRE OR ROAD SURFACE
CONDITIONS."
7
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
[0040] Kim, D. H. et at. (2010). Dissolvable films of silk fibroin for
ultrathin conformal bio-
integrated electronics. Nature Materials, 9, 511-517.
[0041] Omenetto, F.G. and D. L. Kaplan. (2008). A new route for silk. Nature
Photonics, 2,
641-643.
[0042] Omenetto, F. G., Kaplan, D. L. (2010). New opportunities for an ancient
material.
Science, 329, 528 -531.
[0043] Halsed, W. S. (1913). Ligature and suture material. Journal of the
American Medical
Association, 60, 1119 -1126.
[0044] Masuhiro, T., Yoko, G., Masaobu, N., et al. (1994). Structural changes
of silk fibroin
membranes induced by immersion in methanol aqueous solutions. Journal of
Polymer Science,
5, 961-968.
[0045] Lawrence, B. D., Cronin-Golomb, M., Georgakoudi, I., et al. (2008).
Bioactive silk
protein biomaterial systems for optical devices. Biomacromolecules, 9, 1214-
1220.
[0046] Demura, M., Asakura, T. (1989). Immobilization of glucose oxidase with
Bombyx mori
silk fibroin by only stretching treatment and its application to glucose
sensor. Biotechnololgy and
Bioengineering, 33, 598-603.
[0047] Wang, X., Zhang, X., Castellot, J. et al. (2008).Controlled release
from multilayer silk
biomaterial coatings to modulate vascular cell responses. Biomaterials, 29,
894-903.
[0048] U.S. Patent Application Serial No. 12/723,475 entitled "SYSTEMS,
METHODS, AND
DEVICES FOR SENSING AND TREATMENT HAVING STRETCHABLE INTEGRATED
CIRCUITRY," filed March 12, 2010.
[0049] U.S. Patent Application Serial No. 12/686,076 entitled "Methods and
Applications of
Non-Planar Imaging Arrays," filed January 12, 2010.
[0050] U.S. Patent Application Serial No. 12/636,071 entitled "Systems,
Methods, and Devices
Using Stretchable or Flexible Electronics for Medical Applications," filed
December 11, 2009.
[0051] U.S. Patent Application publication no 2012-0065937-Al, published March
15, 2012,
and entitled "METHODS AND APPARATUS FOR MEASURING TECHNICAL
PARAMETERS OF EQUIPMENT, TOOLS AND COMPONENTS VIA CONFORMAL
ELECTRONICS."
[0052] U.S. Patent Application Serial No. 12/616,922 entitled "Extremely
Stretchable
Electronics," filed November 12, 2009.
8
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
[0053] U.S. Patent Application Serial No. 12/575,008 entitled "Catheter
Balloon Having
Stretchable Integrated Circuitry and Sensor Array," filed on October 7, 2009.
[0054] U.S. Patent Application Serial No. 13/336,518 entitled "Systems,
Methods, and Devices
Having Stretchable Integrated Circuitry for Sensing and Delivering Therapy,"
filed December
23, 2011.
[0055] It should be appreciated that all combinations of the foregoing
concepts and additional
concepts described in greater detail below (provided such concepts are not
mutually inconsistent)
are contemplated as being part of the inventive subject matter disclosed
herein. It also should be
appreciated that terminology explicitly employed herein that also may appear
in any disclosure
incorporated by reference should be accorded a meaning most consistent with
the particular
concepts disclosed herein.
BRIEF DESCRIPTION OF THE DRAWINGS
[0056] The skilled artisan will understand that the figures, described herein,
are for illustration
purposes only, and that the drawings are not intended to limit the scope of
the disclosed
teachings in any way. In some instances, various aspects or features may be
shown exaggerated
or enlarged to facilitate an understanding of the inventive concepts disclosed
herein (the
drawings are not necessarily to scale, emphasis instead being placed upon
illustrating the
principles of the teachings). In the drawings, like reference characters
generally refer to like
features, functionally similar and/or structurally similar elements throughout
the various figures.
[0057] FIGs. lA ¨ 1C show example voltage waveforms for stimulating nerves,
according to
the principles described herein.
[0058] FIG. 2 shows a plot of percent changes of renal blood flow as a
function of integrated
voltage being delivered during pacing, according to the principles described
herein.
[0059] FIG. 3A shows an example device that can be used to perform a
procedure, according
to the principles described herein.
[0060] FIG. 3B shows an example flow sensor, according to the principles
described herein.
[0061] FIGs. 4A and 4B show example implementation of an example device,
according to the
principles described herein.
[0062] FIG. 5 shows an example implementation of an electronic circuit and
flow sensor,
according to the principles described herein.
9
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
[0063] FIGs. 6A ¨ 6D show example flow sensors or example heating elements,
according to
the principles described herein,
[0064] FIG. 7A shows another example device, according to the principles
described herein.
[0065] FIG. 7B shows another example device, according to the principles
described herein.
[0066] FIGs. 8A and 8B illustrate an operation of the example flow sensors of
FIG. 7A ¨ 7B,
according to the principles described herein.
[0067] FIG. 9 shows an example simplified schematic of a differential pre-
amplifier, according
to the principles described herein.
[0068] FIG. 10 shows an example operation of a 3-w acquisition system,
according to the
principles described herein.
[0069] FIG. 11 illustrates an operation of an example flow sensor, according
to the principles
described herein.
[0070] FIG. 12 shows an example block diagram of an example PID controller
coupled to an
example flow sensor, according to the principles described herein.
[0071] FIG. 13 shows an example of synchronous demodulation, according to the
principles
described herein.
[0072] FIGs. 14A ¨ 14C show cross-sectional layering structure of various
components of an
example device, according to the principles described herein.
[0073] FIG. 15 shows a flowchart of an example method for performing an
example
assessment, according to the principles described herein.
[0074] FIG. 16 shows example plots of flow measurements, according to the
principles
described herein.
[0075] FIG. 17 shows a block diagram of an example system including an
assessment module
according to the principles described herein.
[0076] FIG. 18A shows an example flow sensor, according to the principles
described herein.
[0077] FIG. 18B shows example measurements using an example flow sensor,
according to the
principles described herein.
[0078] FIG. 19 shows an example method for performing a procedure, according
to the
principles described herein.
[0079] FIG. 20 shows an example architecture of an illustrative computer
system, according to
the principles described herein
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
[0080] FIGs. 21A and 21B show the results of example measurement using an
example device,
according to the principles described herein.
[0081] FIGs. 22A and 22B show the results of example use of an example device,
according to
the principles described herein
[0082] FIGs. 23A-23G illustrates examples of multi-electrode and balloon
catheter devices,
according to the principles described herein.
[0083] FIGs. 24A-24D shows examples of catheter devices.
[0084] FIGs. 24E and 24F shows example forms of sensing, according to the
principles
described herein.
[0085] FIG. 25 shows a non-limiting example of flow sensors on catheters,
according to the
principles described herein.
[0086] FIG. 26 shows an example of flow sensors on a spiral-shaped catheter,
according to the
principles described herein.
[0087] FIG. 27 shows a catheter with bipolar electrodes and metal
interconnects, according to
the principles described herein.
[0088] FIG. 28A ¨ 28D shows example displays of data or analysis, according to
the principles
described herein.
[0089] FIG. 29 shows example displays of data and plots, according to the
principles described
herein.
DETAILED DESCRIPTION
[0090] Following below are more detailed descriptions of various concepts
related to, and
embodiments of, an apparatus and systems for embedding thinned chips in a
flexible polymer. It
should be appreciated that various concepts introduced above and described in
greater detail
below may be implemented in any of numerous ways, as the disclosed concepts
are not limited
to any particular manner of implementation. Examples of specific
implementations and
applications are provided primarily for illustrative purposes.
[0091] As used herein, the term "includes" means includes but is not limited
to, the term
"including" means including but not limited to. The term "based on" means
based at least in part
on. As used herein, the term "disposed on" or "disposed above" is defined to
encompass "at
least partially embedded in."
11
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
[0092] With respect to substrates or other surfaces described herein in
connection with various
examples of the principles herein, any references to "top" surface and
"bottom" surface are used
primarily to indicate relative position, alignment and/or orientation of
various
elements/components with respect to the substrate and each other, and these
terms do not
necessarily indicate any particular frame of reference (e.g., a gravitational
frame of reference).
Thus, reference to a "bottom" of a substrate or a layer does not necessarily
require that the
indicated surface or layer be facing a ground surface. Similarly, terms such
as "over," "under,"
"above," "beneath" and the like do not necessarily indicate any particular
frame of reference,
such as a gravitational frame of reference, but rather are used primarily to
indicate relative
position, alignment and/or orientation of various elements/components with
respect to the
substrate (or other surface) and each other. The terms "disposed on" "disposed
in" and
"disposed over" encompass the meaning of "embedded in," including "partially
embedded in."
In addition, reference to feature A being "disposed on," "disposed between,"
or "disposed over"
feature B encompasses examples where feature A is in contact with feature B,
as well as
examples where other layers and/or other components are positioned between
feature A and
feature B.
[0093] Renal denervation therapy can be used to disrupt the renal nerve
through ablation,
including through applying energy in the form of RF energy, heating, or cryo
(extreme cold) to
the nerves. This can be done by inserting a tube or catheter into the groin
and guiding the device
into the renal artery. The renal denervation procedure ordinarily is not
configured to provide
measurement of the efficacy of the process.
[0094] Other non-limiting examples of ablation energy that can be applied
using a catheter
with flow sensing according to the principles described herein include
radiofrequency (RF),
ultrasound energy, cryoablation, drug-based ablation, alcohol injection,
microwave energy
ablation, and light-based ablation (laser energy).
[0095] While the description of the assessment is described relative to a
procedure on a renal
artery, the assessment of the efficacy of a procedure can be performed in
other systems. For
example, an assessment described herein for determining the efficacy of a
procedure using flow
measurements can be applied to procedures being performed in other tissue
lumen, such as
pulmonary veins, coronary arteries, peripheral blood vessels, cardiac lumen,
and any other lumen
in which flow can be assessed.
12
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
[0096] Denervation therapy can be used to disrupt the nerve through ablation,
including
through applying energy in any of the forms described herein (such as applying
RF energy,
heating, or cryo (extreme cold) to the nerves), in other systems such as but
not limited to the
carotid sinus, the carotid body, the vagal nerve, the pulmonary artery, the
celiac ganglion, or the
bladder trigone.
[0097] An increase in blood flow in the renal artery can be used as an
indicator of the degree of
efficacy of a renal sympathetic denervation (RSDN) procedure. For example, an
indication of an
increase in the rate of blood flow can be considered an indicator that a RSDN
procedure is
effective in achieving the desired degree and/or amount of denervation in the
tissue being
targeted. Such an indication of the degree of efficacy can be extrapolated to
signal an endpoint
to the procedure if the flow-rate of blood is approaching the desired level.
As another example,
an indication of little or no change in the rate of blood flow can be
considered an indicator that a
RSDN procedure is ineffective or marginally effective in achieving the desired
degree and/or
amount of denervation in the tissue being targeted. Such an indication of the
degree of efficacy
can be extrapolated used in a determination of an expected number of
additional procedures to be
performed to achieve the desired outcome, or potential changes that could be
made to make the
RSDN procedure more effective.
[0098] According to the principles described herein, example devices and
methods are
described for determining the efficacy of a denervation procedure, or a pacing
or other
stimulation procedure. Example methods are disclosed that relate to monitoring
changes in
blood flow rates, or other fluid flow rates, before and/or after the
denervation or pacing (or other
stimulation) procedure to monitor the stage of the procedure or to determine
an endpoint for
performance of the procedure.
[0099] This disclosure relates to flow measurement systems that can be
implemented to
determine the efficacy of interventional procedures, including denervation
procedures such as
but not limited to renal denervation or pacing (or other stimulation)
procedures. According to an
example and method described herein, a change in flow rate of blood through
the tissue lumen
can be used to provide an indication of the effectiveness of a procedure
performed on the tissue
(such as but not limited to the renal artery). The procedure can be any
procedure to disrupt the
nerve, e.g., through ablation, including through applying energy in the form
of RF energy,
heating, or cryo (extreme cold) to the nerves. An example application of a
flow measurement
13
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
system, apparatus and method described herein is to provide an indication to a
physician that the
clinical procedure is successful.
[0100] According to the principles described herein, example devices and
methods are
described for use in establishing a clinical endpoint in a procedure in a
renal artery or other
tissue. In an example system and method, a measure of blood flow in a renal
artery or other
tissue prior to the procedure and/or subsequent to the procedure can be used
to provide an
indication of an efficacy of a procedure. In another example, blood flow
measurements during a
pre-procedure cycle and/or during a post-procedure cycle can be used to
establish a clinical
endpoint for the procedure being performed to disrupt the nerve, e.g., through
ablation, including
through applying energy in the form of RF energy, heating, or cryo (extreme
cold) to the nerves.
[0101] Sympathetic nerve activity controls blood pressure and flow by virtue
of
vasoconstriction. Delivery of electrical stimulation to sympathetic nerves
can, in turn, be used to
stimulate the nerves and cause a modulation in blood flow or other fluid flow.
According to the
principles described herein, example devices and methods are described for
measuring changes
in local blood flow and/or pressure during a procedure, such as but not
limited to a RSDN
procedure, .
[0102] At present, most forms of high-performance electronics and electrodes
are rigid, bulky
and have cylindrical cuff-like formats that are inherently low density and
incompatible with the
soft, complex topologies of arteries. In various example implementations,
novel multifunctional
catheter devices are described that include novel microfabrication technology
to build arrays of
soft and flexible nanomembrane flow sensing and electrode elements that can be
used to provide
feedback about renal blood flow, while concurrently delivering pacing energy
and/or ablation
energy. In various examples described herein, novel design strategies and
fabrication techniques
are described that use inorganic semiconductor processes to achieve high
performance flexible
flow sensor and electrode arrays on catheter devices, such as but not limited
to, spiral shaped and
balloon catheters that concurrently measure flow and apply RF energy and
pacing energy inside
a renal artery.
[0103] An example catheter device according to the principles described herein
can include at
least one pacing electrode. In a pacing procedure, a potential is applied to a
portion of tissue
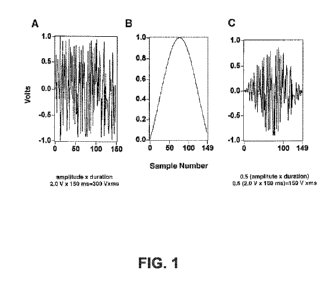
proximate to a nerve to stimulate blood flow. FIGs. lA ¨ 1C show example
voltage waveforms
that can be used to stimulate the nerves. FIG. 2 shows a plot of percent
changes of renal blood
14
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
flow as a function of integrated voltage being delivered during pacing. FIGs.
1A-1C and FIG. 2
demonstrate that blood flow can be changed in the renal artery due to
programmed nerve
stimulation (during pacing). In an example, such pacing can be performed
during a procedure
performed according to the principles described herein. For example, at least
one pacing
electrode can be disposed on an example catheter device described herein to
provide an electrical
stimulation to tissue, e.g., in a region of a nerve source, prior to, during,
and/or following a
procedure. That procedure can be any procedure that disrupts the renal nerve
through ablation,
including through applying energy in the form of RF energy, heating, or cryo
(extreme cold) to
the nerves.
[0104] Example devices and methods are described that combine, on a single
catheter device,
components to perform a procedure on a tissue and components to perform
sensing of the flow
rate of blood, according to the principles described herein. Example devices
and methods are
also described that combine on a single catheter device, components to perform
nerve
stimulation (such as using pacing electrodes) and components to perform
sensing of the flow rate
of blood, according to the principles described herein. In an example, an
indication of the flow
rate of blood based on measurements using the catheter device can be used to
establish a clinical
endpoint during a procedure, including a RSDN procedure.
[0105] FIG. 3A shows an example device 300 that can be used to perform a
procedure
according to the principles described herein. The example device 300 includes
an inflatable
and/or expandable body 302, a flow sensor 304 disposed on a portion of the
inflatable and/or
expandable body 302, and an electronic circuit 306 disposed on the inflatable
and/or expandable
body 302. The electronic circuit 306 includes a number of components that
accommodate
expanding of the inflatable and/or expandable body 302. In FIG. 3A, the flow
sensor 304 is
illustrated as being disposed on a distal portion of the inflatable body. In
another example, the
flow sensor can be disposed on or proximate to a proximal portion of the
inflatable and/or
expandable body.
[0106] In an example implementation, the flow sensor can be a formed as
illustrated in FIG.
3B. FIG. 3B shows an example flow sensor 306' that includes a heating element
307 disposed
proximate to a temperature sensor 308. The heating element 307 and temperature
sensor 308
may be disposed on, or encapsulated in, a support 309. Support 309 can be
formed from a
thermally conductive material. In various examples, the heating element 307
can be separated
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
from the temperature sensor 308 by a separation "x". The parameter "x" can be
about 1 mm,
about 2 mm, about 3 mm, about 5 mm, about 8 mm, about 10 mm, about 12 mm,
about 18 mm,
about 24 mm, about 30 mm or more. Temperature sensor 308 can be a
thermocouple, a
resistance temperature detector (RTD) temperature sensor, a junction potential
temperature
sensor (including sensors that use a voltage measure across a junction as an
indicator of
temperature), a thermistor, an integrated-circuit temperature sensor
(including a LM35-series
temperature sensor), or a semiconductor temperature sensor. Example flow
sensor 306' can
provide a measure of the flow rate of blood in a tissue lumen based on
temperature
measurements of the temperature sensor. In operation, the heating element is
used to maintain
the temperature sensor at a specified temperature measurement value. Any fluid
flowing past the
heating element and temperature sensor can cause some change or fluctuation in
the temperature
measurement of the temperature sensor. The heating element is configured such
that it tried to
maintain the temperature sensor at the stable specified temperature reading. A
change in the
fluid flow rate that causes some fluctuation in the reading of the temperature
sensor causes the
heating element to increase of decrease its heat output to bring the
temperature sensor to its
specified reading. A faster flow rate of the fluid (e.g., the blood) in the
region of the flow sensor
can cause the heating element to increase its heat output. A slower flow rate
of the fluid (e.g.,
the blood) in the region of the flow sensor can cause the heating element to
decrease its heat
output. As a result, a change in the operating point of the heating element
can be used to provide
an indication of the flow rate of the fluid measurement of the temperature
sensor can be used to
provide an indication of the flow rate of fluid proximate to the inflatable
and/or expandable body
302.
[0107] As shown in FIG. 3B, the support 309 can be configured to separate
the heating
element 307 from the fluid by a value "y" as shown in FIG. 3B. The parameter
"y" can be about
2 mm, about 3 mm, about 5 mm, about 8 mm, about 10 mm, about 12 mm, about 15
mm or
more. For different example implementations, parameters "x" and "y" can be
modified to vary
the dynamic range of the resulting example flow sensor. For example, "x" can
be made larger
and "y" can be made smaller to increase the overall range of the example flow
sensor. For
smaller values of "y", the heat is able to flow to the region fluid more
easily. With larger values
of "x", it could take more power to generate enough heat to flow to the
position of the
temperature sensor "x" to bring the temperature sensor to a desired specified
measurement
16
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
setpoint. As a result, the flow sensor can be operated over a greater overall
range, including
range of the operation signal to the heating element. For example, as
described hereinbelow, the
level/magnitude of the signal to the heating element can be used to provide an
indication of the
flow rate of the fluid. The greater range of the operation signal to the
heating element according
to this principle can provide a larger range of values and a larger data asset
of values for use in
determining the fluid flow rate. In an example implementation, the system can
include a
plurality of flow sensors, with two or more of the flow sensors configured
with differing values
of "x" and "y" between the heating element and temperature sensor of the
respective flow sensor.
As a result, the example system presents flow sensors displaying a variety of
measurement
ranges.
[0108] The example flow sensors according to the principles described
herein can include a
temperature sensor proximate to a thermal 'radiation' source. According to any
of the example
systems, methods and apparatus described herein, non-limiting examples of
heating elements
that can provide the thermal radiation include any form of heater that can be
coupled with a
catheter, including a resistive heater or a thermoelectric heater.
[0109] In any example device according to the principles described herein,
the temperature
sensor can include at least one of a resistance temperature detector (RTD)
temperature sensor, a
thermocouple, a junction potential temperature sensor (including sensors that
use a voltage
measure across a junction as an indicator of temperature), a thermistor, an
integrated-circuit
temperature sensor (including a LM35-series temperature sensor), and a
semiconductor
temperature sensor. In various examples, a sensor of known impedance is used.
Other non-
limiting examples of sensors that can be used according to any of the systems
and methods
described herein include vapor deposited gold resistors and ceramic
thermistors. In another
example, other materials such as foils can be used.
[0110] In an example, a calibration standard can be developed for the flow
sensor 306', to
correlate the operating point of the heating element to a flow rate. For
example, training samples
can be used to convert a flow measurement, each training sample being a fluid
caused to flow at
a specific flow rate. For a given amount and/or rate of change of operating
point of the heating
element by the heating element, the operating point of the flow sensor is
obtained for each
training sample. The flow rate of each training sample is known (given that it
is pre-set for the
training samples). The amount and/or rate of heating supplied by the heating
element is also
17
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
known. The calibration standard can be developed to correlate the known
heating supplied to the
known flow rate to obtain calibration data. The example calibration standard
can be used to
convert a flow sensor measurement to a flow rate for a fluid having similar
properties as the fluid
used in the training standard.
[0111] In an example implementation, the examples device of FIG. 3A and 3B
can further
include a flow sensor that is disposed on a portion of a catheter that is
coupled to the proximal
portion of the inflatable and/or expandable body.
[0112] In an example, the electronic circuit 306 can include a number of
electrodes disposed
on the inflatable and/or expandable body 302. The electrodes can be used to
perform a
procedure according to the principles described herein. For example, at least
one of the
electrodes can be a radiofrequency (RF) electrode that delivers RF energy to a
portion of the
tissue surface that is proximate to the RF electrode. According to the
principles described
herein, the delivered RF energy is used to modify the tissue, including to
disrupt a renal nerve.
[0113] In another example, the device 300 can include components to perform
a procedure
using other modalities. For example, the device 300 can include components to
disrupt the renal
nerve, e.g., through ablation, including through applying energy in the form
of RF energy,
heating, or cryo (extreme cold) to the nerves.
[0114] In another example, the electronic circuit 306 of device 300 can
include at least one
pacing electrode. The pacing electrode can be implemented to deliver an
electrical stimulation to
a portion of a tissue (such as but not limited to a renal artery) proximate to
the pacing electrode.
As described above, the pacing electrode can be used to stimulate nerve at
different stages of a
procedure. For example, the electrical stimulation from the pacing
electrode(s) can be applied to
the portion of the tissue to stimulate nerves prior to delivery of an energy
to disrupt the nerves,
such as but not limited to through ablation, including through applying energy
in the form of RF
energy, heating, or cryo (extreme cold) to the nerves. In another example, the
electrical
stimulation from the pacing electrode(s) can be applied to the portion of the
tissue to stimulate
nerves subsequent to delivery of an energy to disrupt the nerves, such as but
not limited to
through ablation, including through applying energy in the form of RF energy,
heating, or cryo
(extreme cold) to the nerves.
[0115] In another example, the electronic circuit 306 can also include
temperature sensors,
each temperature being disposed proximate to an electrode of the electronic
circuit 306.
18
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
[0116] In another example, the device 300 can include one or more other
components
disposed on the inflatable and/or expandable body such as, but not limited to,
a pacing electrode,
a light-emitting device, a contact sensor, an image detector, a pressure
sensor, a biological
activity sensors, a temperature sensor, or any combination thereof
[0117] FIGs. 4A and 4B show a non-limiting example implementation of an
example device
400. The example device 400 includes an inflatable and/or expandable body 402,
a flow sensor
404 disposed on a portion of the inflatable and/or expandable body 402, and an
electronic circuit
406 disposed on the inflatable and/or expandable body 402. The electronic
circuit 406 includes a
number of components that accommodate expanding of the inflatable and/or
expandable body
402. As shown in FIGs. 4A and 4B, the flow sensor 404 can be being disposed on
a distal
portion of the inflatable and/or expandable body 402. As a non-limiting
example, a portion of
the distal region of the expandable and/or inflatable structure can be
extended to form a
protrusion. The flow sensor 404 can be mounted on the protrusion. In another
example, the flow
sensor 404 can be disposed on or proximate to a proximal portion of the
inflatable and/or
expandable body.
[0118] In an example implementation, the flow sensor 404 can be a formed as
including a
heating element 407 disposed proximate to a temperature sensor 408. In various
examples, the
heating element 407 can be separated from the temperature sensor 408 by about
1 mm or about 2
mm. As a non-limiting example, the heating element 407 can be a temperature-
controlled
heating element. As a non-limiting example, the temperature sensor 408 can be
a thermistor.
[0119] In the non-limiting example of FIGs. 4A and 4B, the electronic
circuit can include a
number of electrodes 410 disposed on the inflatable and/or expandable body
402. The electrodes
can be used to perform a procedure according to the principles described
herein. For example, at
least one of the electrodes 410 can be a radiofrequency (RF) electrode that
delivers RF energy to
a portion of the tissue surface that is proximate to the RF electrode.
According to the principles
described herein, the delivered RF energy is used to modify the tissue,
including to disrupt a
renal nerve.
[0120] As shown in the non-limiting example of FIGs. 4A and 4B, the
electronic circuit 406
of the example device 400 can include stretchable interconnects 412 disposed
on the surface of
the inflatable and/or expandable body 402. As shown in FIG. 4B, the
stretchable interconnects
can be used to electrically couple at least one of the plurality of electrodes
410 to an external
19
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
circuit.
[0121] As shown in the non-limiting example of FIGs. 4A and 4B, the
electronic circuit 406
of the example device 400 can also include a main bus 414. As shown in FIG.
4B, the
stretchable interconnects 412 electrically couple the electrodes 410 to the
man bus 414. As also
shown in FIG. 4B, the main bus 414 can extend beyond the inflatable and/or
expandable body
402 to facilitate electrical coupling of the electrodes 410 to an external
circuit.
[0122] In another example, the at least one of the electrodes 410 of
electronic circuit 406 of
device 400 can be a pacing electrode. The pacing electrode can be implemented
to deliver an
electrical stimulation to a portion of a tissue (such as but not limited to a
renal artery) proximate
to the pacing electrode. As described above, the pacing electrode can be used
to stimulate nerve
at different stages of a procedure. For example, the electrical stimulation
from the pacing
electrode(s) can be applied to the portion of the tissue to stimulate nerves
prior to delivery of an
energy to disrupt the nerves, such as but not limited to through ablation,
including through
applying energy in the form of RF energy, heating, or cryo (extreme cold) to
the nerves. In
another example, the electrical stimulation from the pacing electrode(s) can
be applied to the
portion of the tissue to stimulate nerves subsequent to delivery of an energy
to disrupt the nerves,
such as but not limited to through ablation, including through applying energy
in the form of RF
energy, heating, or cryo (extreme cold) to the nerves.
[0123] In another example, the device 400 can include components to perform
a procedure
using other modalities. For example, the device 400 can include components to
disrupt the renal
nerve, e.g., through ablation, including through applying energy in the form
of RF energy,
heating, or cryo (extreme cold) to the nerves.
[0124] In another example, the device 400 can include one or more other
components
disposed on the inflatable and/or expandable body such as, but not limited to,
a pacing electrode,
a light-emitting device, a contact sensor, an image detector, a pressure
sensor, a biological
activity sensors, a temperature sensor, or any combination thereof
[0125] In another example, the device 400 can also include temperature
sensors, each
temperature being disposed proximate to an electrode 410 of the electronic
circuit 406.
[0126] FIG. 5 shows a non-limiting example implementation of an electronic
circuit 506 and
flow sensor 504 that can be disposed on a catheter and extend to a shaft of an
example device
according to the principles described herein. The electronic circuit 506
includes a number of
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
electrodes 510. In various examples, the electrodes 510 can be conformable
electrodes that
conform to the surface of the inflatable and/or expandable body. As shown in
FIG. 5, the flow
sensor 504 includes a heating element 507 disposed proximate to a temperature
sensor 808. As a
non-limiting example, the heating element 507 can be a temperature-controlled
heating element.
As a non-limiting example, the temperature sensor 508 can be a thermistor.
[0127] In the non-limiting example of FIG. 5, at least one of the
electrodes 510 can be a
radiofrequency (RF) electrode that delivers RF energy to a portion of the
tissue surface that is
proximate to the RF electrode. According to the principles described herein,
the delivered RF
energy is used to modify the tissue, including to disrupt a renal nerve. At
least one of the
electrodes 510 can be a pacing electrode that delivers an electrical
stimulation to a nerve, as
described herein.
[0128] As shown in the non-limiting example of FIG. 5, the electronic
circuit 506 includes
stretchable interconnects 512 disposed on the surface of the inflatable and/or
expandable body.
The stretchable interconnects 512 can be used to electrically couple at least
one of the plurality
of electrodes 510 to an external circuit.
[0129] As also shown in the non-limiting example of FIG. 5, the electronic
circuit 506 also
includes a main bus 514. As shown in FIG. 5, the stretchable interconnects 512
electrically
couple the electrodes 510 to the main bus 514. As also shown in FIG. 5, the
main bus 514
includes connection pads 516 that facilitate electrical coupling of the
electrodes 510 to an
external circuit.
[0130] FIG. 6A shows a portion of an example device 600 that can be used to
perform a
procedure according to the principles described herein. The example device 300
includes an
elongated member 602, and a flow sensor 604 disposed on a distal portion of
the elongated
member 602. In FIG. 6A, the flow sensor 604 is illustrated as being disposed
on a distal portion
of the elongated member. In another example, the flow sensor can be disposed
on or proximate
to a proximal portion of the elongated member or on a proximal or distal
portion of an inflatable
and/or expandable body that is coupled to the elongated member.
[0131] In this example implementation, the flow sensor can be formed as
illustrated in
FIG.6A, and includes a heating element 606 and a temperature sensor 608. The
heating element
606 includes a cavity 607. As shown in FIG. 6A, at least a portion of the
temperature sensor 608
is housed in a portion of the cavity 607. Similarly to as described in
connection with the flow
21
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
sensor of FIGs. 3A and 3B above, the heating element 606 can be used to heat
an area proximate
to the elongated member 602. A temperature measurement of the temperature
sensor 608 can be
used to provide an indication of the flow rate of a fluid proximate to the
flow sensor 604. For
example, if the fluid has a higher flow rate and wicks away heat from the area
proximate to the
heating element, the temperature sensor can record a different measurement
than obtained if the
fluid flow rate is lower.
[0132] In operation, the heating element is used to maintain the
temperature sensor at a
specified temperature measurement value. Any fluid flowing past the heating
element and
temperature sensor can cause some change or fluctuation in the temperature
measurement of the
temperature sensor. The heating element is configured such that it tries to
maintain the
temperature sensor at the stable specified temperature reading. A change in
the fluid flow rate
that causes some fluctuation in the reading of the temperature sensor causes
the heating element
to increase of decrease its heat output to bring the temperature sensor to its
specified reading. A
faster flow rate of the fluid (e.g., the blood) in the region of the flow
sensor can cause the heating
element to increase its heat output. A slower flow rate of the fluid (e.g.,
the blood) in the region
of the flow sensor can cause the heating element to decrease its heat output.
As a result, a
change in the operating point of the heating element can be used to provide an
indication of the
flow rate of the fluid measurement of the temperature sensor can be used to
provide an indication
of the flow rate of fluid proximate to the inflatable and/or expandable body
302.
[0133] As also described above in connection with the flow sensor of FIGs.
3A and 3B,
temperature sensor 608 can be a thermocouple, a resistance temperature
detector (RTD)
temperature sensor, a junction potential temperature sensor (including sensors
that use a voltage
measure across a junction as an indicator of temperature), a thermistor, an
integrated-circuit
temperature sensor (including a LM35-series temperature sensor), or a
semiconductor
temperature sensor. In various examples, a sensor of known impedance is used.
Other non-
limiting examples of sensors that can be used according to any of the systems
and methods
described herein include vapor deposited gold resistors and ceramic
thermistors. In another
example, other materials such as foils can be used.
[0134] The example flow sensors according to the principles described in
connection with
FIG. 6A can include any type of a thermal 'radiation' source. Non-limiting
examples of heating
elements that can be implemented to provide the thermal radiation include any
form of heater
22
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
that can be coupled with an elongated member and be configured to have a
cavity. As non-
limiting examples, the heating element can be, but is not limited to, a
resistive heater or a
thermoelectric heater.
[0135] FIG. 6B shows an implementation 620 of an example flow sensor 624
according to the
principles of FIG. 6A. The example flow sensor 624 includes a heating element
626 and a
temperature sensor 628. The heating element 626 is formed as a spiral,
helical, or other coiled
resistive wire with a hollow core that provides a cavity. The resistive wire
can be formed from a
high resistivity electrically material to facilitate higher power dissipation.
As shown in FIG. 6A,
at least a portion of the temperature sensor 628 is housed in a portion of the
cavity. As shown in
this example, the heating element 626 and the temperature sensor 628 can be at
least partially
encapsulated in a thermally conductive encapsulant. Similarly to as described
in connection with
the flow sensor of FIGs. 3A and 3B above, the heating element 626 can be used
to heat an area
proximate to an elongated member that the flow sensor is coupled to. A
temperature
measurement of the temperature sensor 628 can be used to provide an indication
of the flow rate
of a fluid proximate to the flow sensor 624.
[0136] FIG. 6C shows another implementation 630 of an example flow sensor
according to
the principles of FIG. 6A. The example flow sensor includes a heating element
636 and a
temperature sensor (not shown). The heating element 636 is formed as a
patterned thin-film of a
resistive material 631 on a flexible and/or stretchable substrate 633. In this
example, the thin-film
is patterned in a boustrophedonic pattern. The heating element 636 can be
rolled into a more
compact form factor, with at least a portion formed with a hollow core that
provides a cavity.
The resistive wire can be formed from a high resistivity electrically
conductive material to
facilitate higher power dissipation. Similarly to as described in connection
with the flow sensor
of FIGs. 3A and 3B above, the heating element 636 can be used to heat an area
proximate to an
elongated member that the flow sensor is coupled to. A temperature measurement
of the
temperature sensor disposed at least partially in the cavity can be used to
provide an indication of
the flow rate of a fluid proximate to the flow sensor 634.
[0137] FIG. 6C shows another implementation 630 of an example flow sensor
according to
the principles of FIG. 6A. The example flow sensor includes a heating element
636 and a
temperature sensor (not shown). The heating element 636 is formed as a
patterned thin-film of a
resistive material 631 on a flexible and/or stretchable substrate 633. In this
example, the thin-film
23
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
is patterned in a boustrophedonic pattern. In another example, other linear
patterns can be used.
The heating element 636 can be formed into a compact form factor, with at
least a portion
formed with a cavity 637. The thin-film can be formed from a high resistivity
electrically
conductive material to facilitate higher power dissipation. Similarly to as
described in
connection with the flow sensor of FIGs. 3A and 3B above, the heating element
636 can be used
to heat an area proximate to an elongated member that the flow sensor is
coupled to. A
temperature measurement of the temperature sensor disposed at least partially
in the cavity can
be used to provide an indication of the flow rate of a fluid proximate to the
flow sensor.
[0138] FIG. 6D shows another implementation 640 of an example flow sensor
coupled to a
portion of an elongated member 642 according to the principles of FIG. 6A. The
example flow
sensor includes a heating element 646 and a temperature sensor (not shown).
The heating
element 646 is formed as a patterned thin-film of a resistive material 641 on
a flexible and/or
stretchable substrate 643. As shown in this example, the thin-film can be
patterned in a
stretchable pattern. The stretchable pattern allows more bending of the
elongated member while
compensating for surface strain. While the example of FIG. 6D shows a
serpentine pattern, the
stretchable pattern may be other stretchable pattern, including a zig-zag
pattern, a wavy pattern
or a rippled pattern. The heating element 646 can be formed into a compact
form factor, with at
least a portion formed with a cavity. The thin-film can be formed from a high
resistivity
electrically conductive material to facilitate higher power dissipation.
Similarly to as described
in connection with the flow sensor of FIGs. 3A and 3B above, the heating
element 646 can be
used to heat an area proximate to an elongated member that the flow sensor is
coupled to. A
temperature measurement of the temperature sensor disposed at least partially
in the cavity can
be used to provide an indication of the flow rate of a fluid proximate to the
flow sensor.
[0139] FIG. 7A shows another example device 700 that can be used to perform a
procedure
according to the principles described herein. The example device 700 includes
an inflatable
and/or expandable body 702, a pair of flow sensors 704-a and 704-b, and an
electronic circuit
706 disposed on the inflatable and/or expandable body 702. The device 700 is
coupled to a distal
portion of a shaft 708. The electronic circuit 706 includes a number of
components that
accommodate expanding of the inflatable and/or expandable body 702. In FIG.
7A, one of the
flow sensor 704-a is shown to be disposed on a proximal portion of the
inflatable body. The
other flow sensor (reference flow sensor 704-b) is shown to be disposed on a
portion of shaft 708
24
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
at some distance away from the inflatable and/or expandable body 702. In the
example
implementation of FIG. 7A, the flow rate can be measured based on comparison
of the
measurement of the pair of flow sensors 706-a and 706-b. For example, the flow
rate can be
measured based on comparison of voltage measurements of the pair of flow
sensors 706-a and
706-b.
[0140] FIG. 7B shows another example device 700' that can be used to
perform a procedure
according to the principles described herein. Example device 700' includes an
inflatable and/or
expandable body 702, a pair of flow sensors 704-a and 704-b, an electronic
circuit 706 disposed
on the inflatable and/or expandable body 702, and a shaft 708, the same
components as example
device 700, and they are not repeated. Example device 700' also includes a
shaft 710 that can be
disposed over reference electrode 704-b during a procedure or a flow
measurement. The
example device 710 also can be retracted to such an extent that reference flow
sensor 704-b is
exposed.
[0141] In the various examples described herein, the reference sensor can
be positioned on the
shaft at a location that can be covered by a sheath. FIG. 7B shows a non-
limiting example of a
catheter device that includes a sheath member that can be positioned to cover
at least a portion of
the reference sensor. In an example implementation, this distance can be
determined as greater
than or equal to about 10 cm away from the proximal end of the balloon. In
another example
implementation, this distance can be determined as less than about 10 cm away
from the
proximal end of the balloon. For example, the reference flow sensor 704-b can
be positioned at a
distance away on the shaft of the catheter that is at least about 5 cm, at
least about 8 cm, at least
about 10 cm, at least about 13 cm, at least about 15 cm or more. In an example
implementation,
a sheath can be included and used to guide, introduce and steer the catheter.
The sheath can be a
member that surrounds at least a portion of the circumference of the shaft of
the catheter and/or
can be co-axial with the shaft of the catheter. During a procedure, the
reference sensor can be
maintained under the sheath. The sheath can be configured to provide a stable
known
environment and provide a chamber that can include blood in the absence of
flow. In this
example, blood enters the sheath but flow can be halted by the presence of a
stopcock or flow
switch. The blood can be maintained at body temperature but does not flow,
thereby providing a
useful comparison in proximity of the reference sensors. The measurement
performed using the
reference sensor in this environment of blood that is not flowing can serve as
a reference for
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
comparison to the renal artery sensor.
[0142] The internal shaft near the reference sensor can include surface
features in the form of
bumps or tracks that provide channels for the blood. The blood forms an
insulating layer on the
reference sensor, allowing a reference quiescent blood temperature to be
measured. The surface
features can be designed and configured to allow blood to circulate as freely
and prevent the
shaft from making contact with the sheath in that area. For example, the shaft
may include one or
more spacers (also referred to as protuberances) to maintain the shaft spaced
apart from a portion
of the surface of the sheath. The spacing apart of the shaft from the sheath
helps to maintain a
static layer of blood static between the sheath and the shaft.
[0143] FIGs. 8A and 8B illustrate an operation of the flow sensors of FIG.
7A ¨ 7B. FIG. 8A
shows an example device 800 that includes an inflatable and/or expandable body
802, a pair of
flow sensors 804-a and 804-b, an electronic circuit 806 disposed on the
inflatable and/or
expandable body 802, a shaft 808 and a sheath 810. Flow sensor 804-b is
coupled to the shaft
and covered by the sheath 810. In the example of FIG. 8B, blood flows in the
aorta and renal
artery, yet blood remains static in the sheath due to a stopcock or flow
switch. This allows a
differential measurement of flow in the renal artery versus static flow in the
sheath. It also allows
for better use of dynamic range since the measurement is limited between the
two sensors. As
also shown in FIG. 8B, the inflatable or expandable structure can be deflated
or retracted at the
time of flow sensor measurement.
[0144] In an example implementation, the method in connection with FIGs. 8A
and 8B can be
used to resolve small changes in temperature in the body. An example system,
apparatus and
method according to the principles described herein can be used to measure the
signal of interest
in connection with a procedure and reject information that does not relate to
the signal of
interest, thereby increasing resolution and reducing the requirements for
expensive signal
processing.
[0145] An example system, apparatus and method according to the principles
described
herein can be used to measure differential flow, such as described in
connection with FIGs. 8A
and 8B. In an example implementation, two (or more) sensors are used to
measure flow via a
change in flow sensor operating point. As shown in FIG. 8A and 8B, at least
one reference
sensor can be placed on the shaft of a catheter used to perform a measurement
described herein.
The non-limiting example catheter can include one or more renal artery flow
sensors and/or one
26
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
or more other sensors, including one or more ablation components and/or one or
more pacing
electrodes. The reference sensor can be disposed about the inflatable or
expandable member of
the catheter, such as but not limited to a balloon, an expandable mesh, or a
deployable netting.
The reference sensor can be disposed at a sufficient separation distance away
from the proximal
end of the balloon so that the reference sensor is covered by the sheath of
the catheter when it is
proximate to the tissue of the body. One or more renal artery sensors can be
positioned at or near
the proximal end of the balloon. Each measurement taken can be compared or
displayed in
reference to a measurement of the reference sensor.
[0146] Systems, methods and apparatus are described herein that can be used
to increase the
dynamic range of measurements by focusing on the signal of interest. In an
example
implementation, each flow sensor, including any reference sensor, can be
excited using the same
controlled current source. In an example implementation, each sensor can be
measured using an
instrumentation amplifier. FIG. 9 shows a non-limiting example simplified
schematic of a
differential pre-amplifier that can be used to measure differences in
voltages. The example
differential pre-amplifier circuitry can be implemented to compare
measurements of flow sensors
according to the principles of FIGs. 8A ¨ 8B. In this non-limiting example,
the flow sensors can
include thermistors. In other examples, the flow sensors can include at least
one of a resistance
temperature detector (RTD) temperature sensor, a thermocouple, a junction
potential temperature
sensor (including sensors that use a voltage measure across a junction as an
indicator of
temperature), an integrated-circuit temperature sensor (including a LM35-
series temperature
sensor), and a semiconductor temperature sensor. In various examples, a sensor
of known
impedance is used. Other non-limiting examples of sensors that can be used
according to any of
the systems and methods described herein include vapor deposited gold
resistors and ceramic
thermistors. In another example, other materials such as foils can be used.
[0147] In the example of FIG. 9, the difference in the voltage can be
measured between the
flow sensors (such as but not limited to the thermistor) that are driven by an
excitation current.
Signal C is the difference between the signal from the flow sensor proximate
to the inflatable
and/or expandable body (renal flow sensor measurement - signal A) and the
signal from the
reference flow sensor (reference flow sensor measurement - signal B).
[0148] The instrumentation amplifiers can be used to reject common-mode
signals, thereby
providing a higher fidelity signal. In a non-limiting example, an apparatus or
system described
27
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
herein can include thermistors that are well matched (used as the flow sensors
in this example).
The absolute values of the thermistor measurements can be used. A benefit of
measuring a
reference thermistor and renal artery thermistor can be improvement of the
dynamic range by
measuring the difference of the values between the sensors as compared to
using the absolute
values. Limiting the measurements between the reference and the renal artery
sensor can
facilitate improvement of the dynamic range of the measurements.
[0149] An example implementation to perform a measurement is described. In an
example,
the flow sensors are of known impedances, and application of an excitation
current using the
flow sensors creates a voltage that is measured using instrumentation
amplifiers. The amplifiers
are used to measure a voltage correlating to a flow rate. Changes in blood
flow can result in a
change in operating set point in at least one of the flow sensors. By
comparing the value of
voltage measured using the reference sensor to the value of voltage measured
using a flow sensor
disposed proximate to the expandable and/or inflatable body, the flow rate can
be quantified.
Through this comparison, the instrument voltage in the absence of flow also
can be removed. In
an example, the value of voltage measured using the reference sensor is
subtracted from the renal
artery sensor voltage to provide an indication of the instrument voltage in
the absence of flow.
In an example where a reference flow sensor is surrounded by a sheath, blood
in the sheath is
physically static. That is, it is not flowing and remains at body temperature.
Blood in the renal
artery is also at body temperature but flows at some rate (desired to be
measured).
[0150] The differential voltage comparison can be computed based on the flow
sensor
measurement data as follows:
Differential Measurement (C) = Renal Artery Sensor Voltage (A) - Reference
Sensor Voltage(B)
It also can be expressed as: C = A ¨ B
Effectively, in an example implementation, the equation can be expressed as:
Differential Measurement = (VoltagenodyTemp + VoltageRenanow) ¨
(VoltagenodyTemp + 0)
where Vsheathnow ¨ 0.
Differential Measurement = VoltageR.anow
A gain can be added at any of the instrumentation amplifiers to increase the
amplitude of the
signal.
[0151] In an example implementation, one or more flow sensors can be
calibrated. An offset
value between the flow sensors disposed proximate to the inflatable and/or
expandable body and
28
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
the reference flow sensor(s) can be eliminated by placing the catheter in a
known temperature
and flow rate, and measuring the difference between the two sets of sensors.
The measurement
can be performed and/or the offset value can be derived at the time of
manufacture of the
catheter and/or time of assembly of the flow sensors with the inflatable
and/or expandable body
of the catheter. The offset value can be stored and/or indicated as a written
value, or a barcode
or other form of identification (ID). In an example, an integrated circuit or
memory device or
other means can be used to provide this value and ID to a console that is in
communication with
the catheter (including with the flow sensors disposed on or proximate to the
inflatable and/or
expandable body). This offset value can be programmed into the catheter. When
the catheter is
coupled with the console, the console can use the offset value to compensate
for an offset in the
measurements when calculating flow.
[0152] Detecting a change in reading of a thermistor, such as in an example
of the
implementation described in connection with FIGs. 7A - 7B and 8, can provide
an indication of
the rate of fluid flow. Detecting flow rate change in the renal artery can
require high-resolution
measurement.
[0153] For example, the differential measurement described in connection
with FIGs. 7A - 7B
and 8 can be used in conjunction with other methods, such as peak-to-peak
measurements,
synchronous-demodulation (lock-in), and three omega (303) methods. In
different example
implementations, the differential measurement can be used to measure peak-to-
peak output, or it
can be input into a lock-in amplifier or 303 acquisition system as shown in
FIG. 10. The 3w
method can be implemented using a micro-fabricated metal pattern acting as a
resistive heater.
An alternating current (AC) voltage signal energizes the resistive element at
a frequency w. The
periodic heating generates oscillations in the electrical resistance of the
metal line at a frequency
of 2w. In turn, this leads to a third harmonic (303) in the voltage signal.
The third harmonic is
used according to an example implementation to determine the magnitude of the
temperature
oscillations. The temperature oscillations can be used to provide an
indication of the flow rate of
a fluid. For example, the frequency dependence of these temperature
oscillations can be used to
derive the thermal properties of the specimen (e.g., the fluid). The data
indicative of the thermal
properties of the specimen can be used to derive data indicative of the flow
rate of the fluid.
[0154] In any of the examples described herein, the flow sensor (including
the 3-omega
sensor) can be disposed on the example device such that the flow sensor is
disposed within a
29
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
mid-point of a tissue lumen when the example device is disposed within the
lumen. The mid-
point of the lumen in tissue (including the renal artery lumen) can be the
location of maximum
flow velocity). The central positioning of the flow sensor can facilitate more
accurate measured
of fluid flow rate by sampling the area of maximum flow.
[0155] FIG. 11 illustrates an example device that is comprised of a flow
sensor 1154 disposed
on a distal portion of a rod catheter or guidewire 1152, a reference
temperature sensor 1156
disposed on a proximal portion of the rod catheter or guidewire 1152, and a
sheath 1158.
Reference temperature sensor 1156 is coupled to the shaft and may be covered
by the sheath
1158 or may remain uncovered. In the example of FIG. 11, the system can be
operated such that
a specified difference can be maintained between the value of the temperature
measurement of
the reference temperature sensor 1156 and the value of the temperature
measurement of the
temperature sensor of the flow sensor 1154. As described in greater detail
below, the
temperature difference can be maintained at a value of temperature difference
of about 1.5 C,
about 2.0 C, about 2.5 C, about 3.0 C, about 3.5 C, about 4.0 C, or about
4.5 C. While the
examples of FIGs. 7A through 8B are described as having a reference flow
sensor (704-b or
810), each system can be operated as described in connection with FIG. 11, but
with the
reference flow sensor replaced with a reference temperature sensor.
[0156] In an example implementation, a device according to any of the
principles herein and
in any of the figures, including FIGs. 3A through 8B or FIG. 11, can be
implemented to
performing a medical treatment procedure on a tissue as follows. The example
device includes
an elongated member, a flow sensor disposed proximate to a distal portion of
the elongated
member, and a reference temperature sensor disposed proximate to a proximal
portion of the
elongated member. The flow sensor and the reference temperature sensor are in
communication
with a control module. In this example, the control module is used to maintain
a temperature
difference between the measurement of the reference temperature sensor and the
measurement of
the temperature sensor of the flow sensor. For example, the control module can
be used to
monitor the temperature measurement(s) of the reference temperature sensor
and/or the
temperature measurement(s) of the temperature sensor of the flow sensor at
various stages of the
procedure being performed. Based on the monitoring, the control module can
generate signals to
the heating element to cause it to emit heat or discontinue emitting heat,
such that that the
temperature difference is maintained. The signal(s) applied to the heating
element can be stored
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
to a memory, transmitted using a communication interface or a communication
protocol, and/or
read out to a user interface (such as a display).
[0157] The temperature difference can be maintained as a constant
temperature difference or
a time-varying temperature difference. For example, a constant temperature
difference can be
maintained at about 1.5 C, about 2.0 C, about 2.5 C, about 3.0 C, about
3.5 C, about 4.0 C,
or about 4.5 C.
[0158] In an example, the control module includes a proportional-integral-
derivative (PID)
controller. FIG. 12 shows an example control system for implementing a PID
controller control
loop. As shown in FIG. 11, the PID controller receives as input a voltage
signal 1202 from the
temperature sensor of the flow sensor and a voltage signal 1204 from the
reference temperature
sensor. At 1206, the PID controller applies an algorithm and associated method
to determine the
three-term controls: the proportional (P), the integral (I), and the
derivative (D) values. Based
on these computations, the PID controller determines the error or degree of
deviation of the
measured signals from the temperature sensors from the expected values that
would maintain the
desired temperature difference between them. The PID controller applies a
heating element
control algorithm (and associated method) to the combination of P, I, and D
values to determine
a signal to send to a voltage controlled current source 1210 to the heating
element 1212. The
signal sent to the voltage controlled current source 1210 causes adjustments
to the power to the
heating element, to cause it to emit heat, or discontinue emitting heat. That
is, applying the PID
controller involves comparing the value of the temperature measurement of the
reference
temperature sensor to the temperature measurement of the temperature sensor of
the flow sensor,
determining a signal, e.g., a PID correction signal, based on the comparison,
and using the
control module to determine the signal to the heating element based on the PID
correction signal.
As a result of the feedback from the temperature sensors to the PID
controller, the system can
minimize the deviation of the measured signals from the temperature sensors
from the expected
values that would maintain the desired temperature difference between them. In
an example, the
system can include hardware to generate a sinusoidal current with a fixed
frequency but varying
amplitude for the heating element.
[0159] In an example implementation, the control module can be configured
to maintain a
constant temperature difference, such as but not limited to about 2 C, between
the reference
temperature sensor and the temperature measured at the temperature sensor of
the flow sensor.
31
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
For example, the heating element can be powered to heats the temperature
sensor of the flow
sensor (e.g., a thermistor) to about 39 C. The use of the control module as
described herein
maintains this temperature difference, such that the reference temperature
sensor is maintained at
a constant temperature difference of about 2 C, i.e., at about 37 C. The
signal applied to the
heating element can be stored to a memory, transmitted using a communication
interface, and/or
read out to a user interface (such as a display).
[0160] In another example implementation, the control module can be
configured to maintain
a constant temperature difference while the example device is being used
during a procedure,
e.g., nerve stimulation, ablation or other denervation procedure. If fluid
flow rate increases
during the procedure, the increased fluid flow rate removes heat from the
region of the flow
sensor (the coupled heating element and temperature sensor). The control
module determines
from the control loop that the temperature difference is deviating from the
desired value (e.g., a
temperature difference falling below about 2 C). The control module generates
a signal to cause
the heating element to emit heat, to return the temperature difference to the
desired value (e.g., a
value of about 2 C). The signal applied to the heating element can be stored
to a memory,
transmitted using a communication interface, and/or read out to a user
interface (such as a
display). In this case, the signal could show an increase, since as it sending
controls to cause the
heating element to emit heat.
[0161] In an example implementation where a stage of the procedure causes
the fluid flow
rate to decrease, the decreased fluid flow rate removes less heat from the
region of the flow
sensor (the coupled heating element and temperature sensor). The control
module determines
from the control loop that the temperature difference is deviating from the
desired value (e.g., a
temperature difference may be increasing to above about 2 C). The control
module generates a
signal to cause the heating element to cease emitting heat, to return the
temperature difference to
the desired value (e.g., a value of about 2 C). The signal applied to the
heating element can be
stored to a memory, transmitted using a communication interface, and/or read
out to a user
interface (such as a display). In this case, the signal could show a decrease,
since as it sending
controls to cause the heating element to cease emitting heat.
[0162] More precise temperature measurement, i.e., measurements that avoid
the influence of
changes in body temperature, may be obtained through use of differential
temperature
measurement according to the principles herein. The differential temperature
measurement can
32
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
be performed using two or more temperature sensors, including a reference
temperature sensor
that is not coupled to a heating element; and a sense temperature sensor that
is coupled to and
heated by a heating element (forming a flow sensor). The temperature sensor
coupled with the
heating element may be disposed on a distal portion or a proximal portion of
an elongated
member such as but not limited to a rod catheter or a guidewire, or a distal
portion or a proximal
portion of an elongated member that includes an inflatable and/or expandable
body. In an
example, the reference temperature sensor can be disposed at least about 0.5
cm, at least about 1
cm, at least about 1.5 cm, or at least about 2 cm or more distance spaced
apart from the flow
sensor.
[0163] In an example, a device according to any of the principles herein
and in any of the
figures, including FIGs. 3A through 8B or FIG. 11, can include two or more
flow sensors,
including a plurality of flow sensors. The two or more flow sensors can be
coupled via the
control module to a single reference temperature sensor, or each flow sensor
may be coupled to a
respective reference temperature sensor.
[0164] In an example implementation, the signal to the heating element can
be a time-varying
voltage signal. For example, the excitation of the heating element and the
temperature sensor of
the flow sensor can be using an AC frequency greater than the inverse of
tissue response time.
As non-limiting examples, signals at AC frequencies ranging from about lkHz to
about 100kHz
can be used to drive the flow sensor to reduce the risk of accidentally
causing fibrillation.
Operating at frequencies in this range also allows for higher current leakage.
[0165] In an example implementation, the signal to the heating element can
be a voltage
signal, a current signal, a digital signal, or any other signal that transmits
instructions to cause the
heater to heat at a desired temperature to maintain the desired temperature
difference. The signal
can be read out and/or plotted, stored to a memory, or otherwise communicated
or transmitted.
[0166] In an example implementation, the control signal can be mapped to a
flow rate through
the analysis of multiple data runs and measurements, to generate a standard or
other calibration
chart that relates a value of a control signal to the physiological flow rate.
[0167] In an example, novel signal processing algorithms and associated
methods, and control
modules (including PID controller software) are provided for sensing and/or
quantifying fluid
flow rates.
[0168] FIG. 13 shows an example demodulation that can be implemented to
extract the signal
33
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
to the heating element from the noise in the signal. As a non-limiting
example, processor-
executable instructions that create a phase-locked loop (PLL) can be applied
with a synchronous
demodulation method to reject noise and derive the signal. At least one phase-
locked loop can
be used to lock-in the sensing signal to the control signal, and the sensing
signal to the heater
signal for the synchronous-demodulation. Since the same frequency is used to
demodulate the
data, the desired signal is extracted as a DC signal (representing the
amplitude modulation). The
synchronous demodulation provides a narrow band filter to reject noise
injected by the
environment of the device, which can interfere with the desired signal.
[0169] The capability of the temperature difference measurements and the
control module
facilitate measurement of pulsatile flow over a broad dynamic range. As a
result, systems and
methods herein provide a way to determine a clinical endpoint during a
procedure, such as but
not limited to a carotid sinus denervation, a carotid body disruption, a vagus
nerve stimulation, a
pulmonary artery denervation, a celiac ganglion disruption, a bladder trigone
ablation, or a renal
denervation procedure.
[0170] In an example, any system or device according to the principles
described herein may
be entirely or at least partially encapsulated by an encapsulating material,
such as a polymer
material (including any of the polymer materials described herein). An
encapsulating material
can be any material that can be used to laminate, planarize, or encase at
least one component of a
system or device described herein, including any electronic or other type of
component. For
example, a method of fabricating any system or device according to the
principles described
herein can further include encapsulating the system or device. In an example,
an encapsulating
material can be disposed over, or otherwise applied to, an device that
includes an inflatable
and/or expandable body and the electronic circuit or a plurality of
electrodes. In an example, a
polyurethane can be used as the encapsulating material. In another example,
the encapsulating
material can be the same material as the material for the inflatable and/or
expandable body.
Encapsulating any portion of the systems or device described herein can be
useful to enhance the
mechanical stability and robustness of the system or device, or to maintain
electronic
performance of the electronic components of the system or device against a
stress or strain
applied to the system or device during use.
[0171] In any of the example devices according to the principles described
herein, the
encapsulating material can be formed from any material having elastic
properties. For example,
34
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
the encapsulating can be formed from a polymer or polymeric material. Non-
limiting examples
of applicable polymers or polymeric materials include, but are not limited to,
a polyimide, a
polyethylene terephthalate (PET), a silicone, or a polyeurethane. Other non-
limiting examples of
applicable polymers or polymeric materials include plastics, elastomers,
thermoplastic
elastomers, elastoplastics, thermostats, thermoplastics, acrylates, acetal
polymers, biodegradable
polymers, cellulosic polymers, fluoropolymers, nylons, polyacrylonitrile
polymers, polyamide-
imide polymers, polyarylates, polybenzimidazole, polybutylene, polycarbonate,
polyesters,
polyetherimide, polyethylene, polyethylene copolymers and modified
polyethylenes,
polyketones, poly(methyl methacrylate, polymethylpentene, polyphenylene oxides
and
polyphenylene sulfides, polyphthalamide, polypropylene, polyurethanes,
styrenic resins,
sulphone based resins, vinyl-based resins, or any combinations of these
materials. In an
example, a polymer or polymeric material herein can be a DYMAXO polymer (Dymax
Corporation, Torrington, CT).or other UV curable polymer, or a silicone such
as but not limited
to ECOFLEXO (BASF, Florham Park, NJ).
[0172] For applications in biomedical devices, the encapsulant should be
biocompatible. The
stretchable interconnects can be embedded in a polyimide that also acts as a
mechanical
reinforcement.
[0173] In an example, any of the systems or device according to the
principles herein can be
disposed on the inflatable and/or expandable body such that a functional layer
of the system or
device lies at a neutral mechanical plane (NMP) or neutral mechanical surface
(NMS) of the
system or device. The NMP or NMS lies at the position through the thickness of
the device
layers for the system or device where any applied strains are minimized or
substantially zero. In
an example, the functional layer of a system or device according to the
principles described
herein includes the plurality of sensing elements, the coupling bus, and/or
the stretchable
electronic system that includes the flexible annular interconnect and the
plurality of electrodes.
[0174] The location of the NMP or NMS can be changed relative to the layer
structure of the
system or device through introduction of materials that aid in strain
isolation in various layers of
the system or device. In various examples, polymer materials described herein
can be introduced
to serve as strain isolation materials. For example, the encapsulating
material described
hereinabove can be used to position the NMP or NMS, e.g., by varying the
encapsulating
material type and/or layer thickness. For example, the thickness of
encapsulating material
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
disposed over the functional layers described herein may be modified (i.e.,
decreased or
increased) to depress the functional layer relative to the overall system or
device thickness,
which can vary the position of the NMP or NMS relative to the functional
layer. In another
example, the type of encapsulating, including any differences in the elastic
(Young's) modulus
of the encapsulating material.
[0175] In another example, at least a partial intermediate layer of a
material capable of
providing strain isolation can be disposed between the functional layer and
the inflatable and/or
expandable body to position the NMP or NMS relative to the functional layer.
In an example,
the intermediate layer can be formed from any of the polymer materials
described herein, aerogel
materials or any other material with applicable elastic mechanical properties.
[0176] Based on the principles described herein, the NMP or NMS can be
positioned
proximate to, coincident with or adjacent to a layer of the system or device
that includes the
strain-sensitive component, such as but not limited to the functional layer.
The layer can be
considered "strain-sensitive" if it is prone to fractures or its performance
can be otherwise
impaired in response to a level of applied strain. In an example where the NMP
or NMS is
proximate to a strain-sensitive component rather than coincident with it, the
position of the NMP
or NMS may still provide a mechanical benefit to the strain-sensitive
component, such as
substantially lowering the strain that would otherwise be exerted on the
strain-sensitive
component in the absence of strain isolation layers. In various examples, the
NMS or NMP layer
is considered proximate to the strain-sensitive component that provides at
least 10%, 20%, 50%
or 75% reduction in strain in the strain-sensitive component for a given
applied strain, e.g.,
where the inflatable body is inflated.
[0177] In various examples, the encapsulating material and/or the
intermediate layer material
may be disposed at positions coincident with the strain-sensitive component,
including in the
functional layer. For example, portions of the encapsulating material and/or
the intermediate
layer material may be interspersed with the strain-sensitive component,
including at positions
within the functional layer.
[0178] In any of the example devices according to the principles described
herein, portions of
the stretchable interconnects, the electrodes and portions of the main bus can
be formed from a
conductive material. In any of the examples described herein, the conductive
material can be but
is not limited to a metal, a metal alloy, a conductive polymer, or other
conductive material. In an
36
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
example, the metal or metal alloy of the coating may include but is not
limited to aluminum,
stainless steel, or a transition metal (including copper, silver, gold,
platinum, zinc, nickel,
titanium, chromium, or palladium, or any combination thereof) and any
applicable metal alloy,
including alloys with carbon. In other non-limiting example, suitable
conductive materials may
include a semiconductor-based conductive material, including a silicon-based
conductive
material, indium tin oxide or other transparent conductive oxide, or Group III-
IV conductor
(including GaAs). The semiconductor-based conductive material can be doped.
[0179] In any of the example structures described herein, the stretchable
interconnects can
have a thickness of about 0.1 um, about 0.3 um, about 0.5 um, about 0.8 um,
about 1 um, about
1.5 um, about 2 [tm or greater. The buffer structure and/or flexible base can
have a thickness of
about 5 um, about 7.5 um, about 9 um, about 12 [tm or greater. In any example
herein, the
encapsulant can have a thickness of about 100 um, about 125 um, about 150 um,
about 175 um,
about 200 um, about 225 um, about 250 um, about 300 [tm or greater.
[0180] FIGs. 14A and 14B show cross-sectional layering structure of various
components of
the example devices described herein, which can be microfabricated. FIG. 14A
shows the
layering structure of an electrode, which includes a polymer layer 1402, a
layer of conductive
material 1404, and an annular structure of a polymer 1406 about a perimeter of
the electrode.
FIG. 14B shows the layering structure of a stretchable interconnect, which
includes a polymer
layer 1402, a layer of conductive material 1404, and a layer of a polymer
1406. FIG. 14C shows
the layering structure of a flow sensor disposed on the inflatable and/or
expandable body, which
includes a polymer layer 1402, a layer of conductive material 1404, a layer of
a polymer 1406, a
flow sensor 1408, and an encapsulating layer 1410. In an example, the
components can be
fabricated on a carrier substrate, released from the carrier substrate, and
disposed on the
inflatable and/or expandable body.
[0181] A non-limiting example fabrication process for the example device of
FIGs. 14A and
14B is as follows. The electrode can be fabricated using a microfabrication
and transfer printing
process to be between about 1 micron and about 5 microns thick. The sensors
can be 3-omega
sensors (described below) and the surface mount components (including the flow
sensors) can be
fabricated using use pure gold or Cu-Au-Ni fabrication techniques. The
fabricated electronic
structure are integrated on the surface of an inflatable and/or expandable
body (such as but not
limited to a balloon of a catheter). In the example device structures of FIG.
14A and 14B, the
37
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
polyimide can be about 25 microns in thickness. A polyurethane, formed of a
resin and a
solvent, can be used as an encapsulant to planarize the array of electrodes
and other components
on the surface of the inflatable and/or expandable body. The encapsulant helps
to provide
durability during sheath insertion of the example device into a tissue lumen.
[0182] In an example, the micro-fabricated flow sensors, electrode arrays
(including ablation
RF electrodes), electronics and other components of the example device are
ultrathin, and have
mechanical properties substantially similar or matched with the mechanical
properties of the
inflatable or expandable surface.
[0183] Systems and methods are described for performing a procedure on a
tissue, including a
renal artery, using any example device described herein. The example method
includes
disposing an example device in proximity to the tissue, applying the treatment
to be applied to
the tissue, and recording the flow measurement of the flow sensor as described
herein to provide
an indication of the flow rate of a fluid in proximity to the example device.
[0184] In an example, the treatment can include applying an ablation or
applying energy in
the form of RF energy, heating, or cryo (extreme cold) to the tissue. In an
example, the
treatment is performed to disrupt nerves in proximity to the tissue.
[0185] In an example, the method can be performed with an example device
that includes a
flow sensor element configured as a heating element in proximity to a
temperature sensor. In
this example, the operating point of the heating element can be monitored to
provide an
indication of flow rate. In an example, the recording of the flow measurement
of the flow sensor
can be performed subsequent to applying the RF energy to the surface of the
tissue proximate to
the RF electrode. In another example, the recording of the flow measurement of
the flow sensor
can be performed prior to the applying of the RF energy to the surface of the
tissue proximate to
the RF electrode.
[0186] In an example, the temperature measurement may be performed before and
after
application of the RF energy, to obtain an indication of the flow rate of the
fluid (such a blood)
prior to and subsequent to the treatment procedure being performed.
[0187] In an example, systems, methods and devices for monitoring an
efficacy of,
determining a clinical endpoint for, a procedure. According to the principles
described herein,
the procedure can be any procedure to disrupt the renal nerves, such as but
not limited to an
ablation, including through applying energy in the form of RF energy, heating,
or cryo (extreme
38
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
cold) to the nerves. The procedure is not performed completed blindly with no
feedback on the
success of the procedure, with potential risk of damage to tissue. The example
systems, methods
and devices described herein provide an assessment of a renal denervation
procedure based on
renal hemodynamics (including based on the measures of fluid flow rate).
[0188] In any example described herein, an assessment module is provided
according to the
systems and methods described herein, where the assessment module includes a
processor and a
memory storing processor executable instructions. Execution of the processor
executable
instructions causes the assessment module to perform the activities associated
with any method
described herein, including using the data indicative of flow rate to provide
an indication of the
efficacy of a clinical procedure.
[0189] In an example, the example method can include using an indication of
an increase in
the flow rate of the fluid subsequent to the performance of the treatment as
an indicator of the
efficacy of the treatment procedure to disrupt the nerves (including the
efficacy of applying the
RF energy to the tissue). For example, a pre-set value of fluid flow rate or
clinically desired
percentage increase in flow rate can be used as an indicator of the efficacy
of the procedure,
including being used as an indication of an end-point of performance of the
procedure. As a
non-limiting example, a baseline flow rate can be measured using the flow
sensors described
herein prior to performing the procedure to disrupt the nerves. A desired pre-
set value of fluid
flow rate or clinically desired percentage increase in flow rate can be
determined based on the
baseline flow rate. For example, the pre-set value of fluid flow rate or
clinically desired
percentage increase in flow rate can be set as the amount needed to return the
flow rate to an
average, mean or median range of values for renal blood flow rate. In a
feedback assessment, the
procedure can be performed, the flow rate subsequently re-measured/re-
determined based on
flow sensor measurement data according to the principles described herein, and
the re-measured
flow rate compared to the pre-set value of fluid flow rate or clinically
desired percentage
increase in flow rate. If the desired pre-set value of fluid flow rate or
clinically desired
percentage increase in flow rate is not attained, the procedure can be
repeated and the flow rate
re-measured. If the desired pre-set value of fluid flow rate or clinically
desired percentage
increase in flow rate is attained, it signals the endpoint, and the procedure
can be discontinued.
In an example, the example method can include using an indication of little or
no increase in the
flow rate of the fluid subsequent to the performance of the treatment as an
indicator of a lack of
39
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
the efficacy of the treatment (including the efficacy of applying the RF
energy to the tissue), or
as an indication that the treatment procedure should be repeated, discontinued
or modified. If the
desired pre-set value of fluid flow rate or clinically desired percentage
increase in flow rate is not
attained, the procedure can be modified to achieve the desired outcome. In an
example, the
feedback of performing the procedure, re-measuring the flow rate and comparing
to the pre-set
value of fluid flow rate or clinically desired percentage increase in flow
rate can be repeated until
the endpoint is signaled.
[0190] While the assessment is described relative to a procedure on a renal
artery, the
assessment of the efficacy of a procedure can be performed in other systems.
For example, an
assessment described herein for determining the efficacy of a procedure using
flow
measurements can be applied to procedures being performed in other tissue
lumen, such as
pulmonary veins, coronary arteries, peripheral blood vessels, cardiac lumen,
and any other lumen
in which flow can be assessed.
[0191] In an example, the method can include activating at least one pacing
electrode of the
example device to deliver an electrical stimulation to a portion of the tissue
proximate to the
pacing electrode. For example, the method can include delivering the
electrical stimulation to
the portion of the tissue proximate to the pacing electrode prior to recording
a flow measurement
(including recording a flow measurement of a flow sensor).
[0192] A non-limiting example process sequence, for performance of a
procedure on renal
artery tissue using an example device that is configured as a balloon
catheter, is as follows:
= Perform initial measurement (e.g., obtain a baseline flow)
= Inflate catheter balloon to block blood flow
= Measure renal flow using any of the example devices or methods described
herein
= Pace the renal artery (e.g., apply electrical signals to tissue using
electrodes of the
system)
= Deflate catheter balloon
= Measure "pre-ablation" flow using any of the example devices or methods
described
herein
= Inflate catheter balloon
= Perform ablation of the renal artery (e.g., apply energy to tissue to
induce lesions and
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
necrosis, including RF energy, heating, and cryoablation)
= Deflate catheter balloon
= Measure "post-ablation" flow using any of the example devices or methods
described
herein
[0193] A non-limiting example process sequence, for renal denervation on
renal artery tissue
using an example device that is configured as a balloon catheter, is as
follows:
= Pace the renal artery (e.g., apply electrical signals to tissue using
electrodes of the
system)
= Measure "pre-ablation" flow rate using any of the example devices or
methods described
herein
= Perform ablation of the renal artery (e.g., apply energy to tissue to
induce lesions and
necrosis, including RF energy, heating, and cryoablation)
= Pace the renal artery (e.g., apply electrical signals to tissue using
electrodes of the
system)
= Measure "post-ablation" flow rate using any of the example devices or
methods described
herein
[0194] The flowchart of FIG. 15 shows another non-limiting example method for
performing
an assessment during performance of a procedure, including an ablation
procedure. In this
example, a two-fold or three-fold increase in blood flow rate in the renal
artery is the pre-set
condition used as an indicator of an endpoint of applying the procedure in a
feedback
assessment. In block 1502, a baseline flow rate is measured. In block 1504, a
procedure is
performed on the tissue, such as but not limited to, a procedure performed
using an example
device described herein. In block 1506, the flow rate subsequently re-
measured/re-determined
based on flow sensor measurement data according to the principles described
herein. In block
1508, in a feedback assessment, the re-measured flow rate is compared to the
pre-set value of
fluid flow rate or clinically desired percentage increase in flow rate. If the
desired pre-set value
of fluid flow rate or clinically desired percentage increase in flow rate is
attained (block 1510), it
signals the endpoint (1512), and the procedure can be discontinued. If the
desired pre-set value
of fluid flow rate or clinically desired percentage increase in flow rate is
not attained (block
1514), the procedure can be repeated (block 1516) and the flow rate re-
measured and compared
41
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
to the pre-set value of fluid flow rate or clinically desired percentage
increase in flow rate
(1518). If the desired pre-set value of fluid flow rate or clinically desired
percentage increase in
flow rate is attained (block 1520), it signals the endpoint (1512), and the
procedure can be
discontinued. If the desired pre-set value of fluid flow rate or clinically
desired percentage
increase in flow rate is not attained (block 1522), the procedure can be
modified to achieve the
desired outcome. For example, as shown in block 1524, the position of the
instrument can be
changed to some other region of the tissue and the treatment procedure
repeated. In an example,
the feedback of performing the procedure, re-measuring the flow rate and
comparing to the pre-
set value of fluid flow rate or clinically desired percentage increase in flow
rate can be repeated
until the endpoint is signaled.
[0195] In an example, an assessment module is provided according to the
systems and
methods described herein, where the assessment module includes a processor and
a memory
storing processor executable instructions. Execution of the processor
executable instructions
causes the assessment module to perform any of the example methods described
herein,
including in connection with FIG. 15.
[0196] The example systems, methods and apparatus herein can be used to
improve the
monitoring of stages of performance of a procedure as well as the completion
of the procedure.
Measurement of pulsatile fluid flow prior to performance of a procedure being
performed can be
used to provide quantitative values for parameters indicative of a baseline
fluid flow.
Measurement of pulsatile fluid flow during pre-cycle and post-cycles during
performance of a
procedure can be used to provide feedback, e.g., to a clinician or other
practitioner, on the
efficacy of the procedure. Based on an analysis of the flow rate measurements,
the end-point of
a procedure can be determined. For example, the method can be implemented for
sensing and
treatment in a tissue lumen. For example, in a renal denervation procedure,
the elongated body
can be inserted into a sheath and guided through the femoral vein until it
reaches the renal artery.
Sensors can be used to map or image renal nerves, deliver ablation energy, or
monitor tissue
properties, in conjunction with measurement of fluid flow rates, before,
during, and after an
ablation procedure or other procedure.
[0197] In an example, the efficacy of the performance of stages of a
procedure can be
monitored through an analysis of the time-dependence of the fluid flow rates.
For example, a
change in a time constant associated with the flow rate can be used as a
measure of the efficacy a
42
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
given stage in the performance of procedure. An example method based on
analysis of the time-
constant is as follows. Any example device herein, comprising any of the flow
sensors, can be
disposed proximate to the tissue. In this example, the device includes at
least one component
configured to apply the pacing, ablative, denervation, or any other treatment
procedure being
performed. The at least one component to perform the treatment procedure on
the portion of the
tissue is activated, and the at least one flow sensor is used to perform at
least one flow
measurement. Each of the at least one flow measurement could be used to
provide data
indicative of a change in the flow subsequent to the treatment procedure of a
fluid proximate to
the apparatus. An analysis of the data indicative of the flow of the fluid can
be used to determine
at least one time-constant associated with the data. For example, as shows in
FIG. 16, a time
constant (To) may be associated with a flow rate response prior to performance
of a procedure,
while obtaining flow having a different time constant ('td) can be deemed an
indicator of the end-
point of the procedure. The at least one time-constant associated with the
data can be compared
to a time-constant indicative of the flow of the fluid prior to performance of
the treatment
procedure, Any observed differences can be used to provide an indication of
the efficacy of the
treatment procedure.
[0198] In an example, multiple stages of the procedure can be repeated
until the difference is
low, or falls in a previously-specified range of values. Based on this
analysis, an indication of an
endpoint of a treatment procedure can be provided or displayed.
[0199] In an example, the time constant can be analyzed to provide a
measure of the rate of
change in the flow from a highest value following the treatment procedure to a
steady-state value
at a later time. An example method can include determining a first order rate
of change with
time of the at least one time constant and/or a second order rate of change
with time of the at
least one time constant. Another example method can include comparing the
first order rate of
change with time of the at least one time constant to a standard for the first
order rate of change,
where the comparison provides a second indication of the efficacy of the
medical treatment
procedure. Comparing the second order rate of change with time of the at least
one time constant
to a standard for the second order rate of change can be used to provide an
indication of the
efficacy of the medical treatment procedure.
[0200] In an example, the values of time constant and/or flow rate data
gathered from a
plurality of subject having a known condition can be used to provide an
indication of a degree of
43
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
success of a procedure or a potential for recovery from the procedure of an
unclassified subject.
The analysis of the values of time constant and/or flow rate data gathered
from a plurality of
previously classified subject can be used to provide parameter indicative of a
likelihood of
success of the procedure, the projected recovery time, and/or a risk of
relapse of subject. The
classified subjects have previously undergone any one or more procedures. The
values of time
constant and/or flow rate data may have been gathered prior to, during, and/or
after completion
of the one or more procedures. The values of time constant can include the
first order measure
of the time constant, the first-order rate of change (first derivative) of the
time constant, and/or
the second-order rate of change (second derivative) of the time constant. Any
number of the
plurality of known subjects may have been classified as to the degree of
success of the procedure
performed, the observed recovery time, and/or the incidence of relapse of
subject(s). The values
of time constant and/or flow rate data gathered from the plurality of
subjects, and the classified
parameters of their known conditions, can be used to train a classifier. The
classifier can be
generated as a look-up table, a calibration standard, or a machine-learning
tool. For example, the
values of time constant and/or flow rate data gathered from the plurality of
subjects, and the
classified parameters of known conditions, can be used to train a machine-
learning tool to
provide the subject classifier (or patient classifier).
[0201] The classifier can be used to take as input data from an
unclassified subject, and
generate as output an indication of a classification of a condition of that
subject. For example,
the classifier could be used to classify the subject as to a likelihood of
success of the procedure,
the projected recovery time, and/or a risk of relapse. In operation, data
indicative of a flow rate
of an unclassified subject can be gathered prior to, during, and/or after
completion of a
procedure, and provided as input to the classifier. The classifier can output
the indication of the
classification of the subject. In an example, the result from the
classification of the unclassified
subject may result in modification of the procedure while it is being
performed (e.g., if the
classifier indicates a potential for a relapse), used as an indication of the
endpoint of the
procedure, and/or result in a determination of an optimal recovery or
rehabilitation regimen. In
an example, the classification of the subject using the classifier can be used
to determine at least
one drug, biologic, or other substance to be administered to the subject.
[0202] The example machine-learning tools can be supervised learning tools
(including
support vector machines), unsupervised learning tools (including clustering
analysis), or semi-
44
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
supervised learning tools. As non-limiting examples, the learning tool can be
an artificial neural
network (ANN), a Bayesian network, a decision tree, or any other applicable
tool.
[0203] In an example, a system, apparatus and method is provided for
monitoring a
hemodynamic effect during a medical treatment procedure performed on a
vascular tissue.
Parameters indicative of the hemodynamics of the fluid flow indicate the
motion or equilibrium
state of the fluid. The method can include disposing in proximity to the
tissue an example device
according to the principles described herein (such as but not limited to an
example device as
described in connection with any of FIGs. 3A ¨ 8B, 11, or 23 -27). At least
one component of
the example device to perform a medical treatment procedure on the portion of
the tissue is
activated. A substance that causes a change in dimension of the vascular
tissue is administered.
At least one flow sensor of the example device is used to perform at least one
flow measurement.
The at least one flow measurement provides data indicative of a change in the
flow of a fluid
proximate to the example device subsequent to the medical treatment procedure.
The data
indicative of the flow of the fluid is analyzed to determine at least one
parameter indicative of
the change in the hemodynamics of the fluid. A reduction in the change in the
hemodynamics of
the fluid can be used as an indicator of the efficacy of the medical treatment
procedure.
[0204] The at least one component can be, but is not limited to, an
ablative component. The
medical treatment procedure can be, but is not limited to, a denervation
procedure.
[0205] In an example, several stages of the method can be repeated until
the rate of reduction
of the change in the hemodynamics of the fluid falls below a specified value.
In an example, an
indication of an endpoint of the medical treatment procedure can be generated
when the rate of
reduction of the change in the hemodynamics of the fluid falls below the
specified value.
The example method can include displaying the indication of the endpoint of
the medical
treatment procedure on a display, as described in greater detail below.
[0206] The substance can include an endogenous substance and/or an exogenous
substance.
For example, the substance can include a calcium channel blocker, a cAMP-
mediated stimulant,
or a nitrovasodilator. In another example, the substance can include a
dopamine, adenosine,
prostacyclin, saline, or nitric oxide. The substance can include a
vasodilation substance or a
vasoconstriction substance.
[0207] FIG. 17 shows a block diagram of an example system including an
assessment
module, according to the systems and methods described herein. A non-limiting
example of the
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
system 1700 according to the principles described herein is illustrated in
FIG. 17. The system
1700 includes at least one communication interface 1711, at least one memory
1712, and at least
one processing unit 1713. The at least one processing unit 1713 is
communicatively coupled to
the at least one communication interface 1711 and the at least one memory
1712. The at least
one memory 1712 is configured to store processor-executable instructions 1714
and an
assessment module 1715. As described in greater detail herein, the assessment
module 1715 can
be applied to determine, based on the flow sensor measurement data 1716, the
indication of the
flow rate of fluid in the tissue lumen, including to perform a differential
comparison of flow
sensor measurements or using the measures of flow rate to provide an
indication of the efficacy
of a procedure being performed on the tissue (such as but not limited to a
procedure to disrupt
nerves). In a non-limiting example, the at least one processing unit 1713
executes the processor-
executable instructions 1714 stored in the memory 1712 at least to provide the
feedback
described herein during performance of a procedure. The at least one
processing unit 1713 also
executes processor-executable instructions 1714 to control the memory 1712 to
store, or to
control the communication interface 1711 to transmit 1717 to, e.g., a user
interface or to a
controller for any of the example devices described herein, at least one of an
indication of the
flow rate, an indication of an endpoint for the procedure, an indication of an
efficacy of the
procedure, and a suggested modification of the procedure.
[0208] In any example implementation according to the principles described
herein, readings
of 3-omega sensors can be used as the flow sensor on any of the devices
described herein to
provide an indication of the rate of flow of the fluid. The 3-omega sensors
have similar
fabrication processing steps as the pacing electrodes or the ablation
electrodes. FIG. 18A shows
a non-limiting example of a 3-omega sensor. The 3-omega sensors have intricate
filamentary
patterns, which can survive extreme mechanical bending and twisting and yet
maintain
performance. The 3-omega sensors measure blood flow by assessing minute
changes in local
temperature. Example results collected in a perfusion chamber with preset flow
rates are shown
in FIG. 18B. The 3-omega sensors can be disposed proximate to the inflatable
and/or
expandable body (including at the distal portion of the catheter). The 3-omega
sensor can be
disposed on the example device such that the 3-omega sensor is disposed within
a mid-point of a
tissue lumen (location of maximum flow velocity) and three other locations
near the wall of the
tissue lumen. Data collected across multiple 3-omega sensors in this
configuration can facilitate
46
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
flow rate measurements at multiple positions inside the tissue lumen. The
sensitivity of the 3-
omega sensors (such as the example of FIG. 18A) is in the range compatible
with blood flow
rates that exist in vivo (-5-50 cm/s flow rates).
[0209] In any example implementation according to the principles described
herein, the flow
sensing can be performed using other techniques. For example, an ultrasound
measurement can
be performed to provide an indication of the rate of flow of fluid pre-renal
denervation procedure
and/or post-renal denervation procedure to provide the feedback for
determining the end-point or
a procedure or to determine whether the procedure should be modified. As
another example, an
optical measurement can be used to provide the indication of the rate of flow
of fluid pre-renal
denervation procedure and/or post-renal denervation procedure to provide the
feedback for
determining the end-point or a procedure or to determine whether the procedure
should be
modified. Other applicable flow sensing technology is a time-of-flight
measurement, where the
flow behavior of a tracker fluid introduced into the renal artery is measured
to used to provide
the indication of the rate of flow of fluid pre-renal denervation procedure
and/or post-renal
denervation procedure.
[0210] Fluid flow monitoring before, during and after delivery of nerve
pacing and delivery
of a treatment according to the principles described herein (including
ablation energy) are
powerful capability sets, that when offered in a single spiral catheter, can
enhance the efficacy of
a treatment procedure (including a renal denervation procedure). Variations in
blood flow
change the local steady-state temperature, which is measured with the 3-omega
sensors. Absence
of modulation in renal blood flow during pacing can indicate that ablation was
successful and
enable physicians to determine the end point of the renal denervation
procedure.
[0211] In an example implementation, flow in a perfusion chamber can be
systematically
measured that provides programmable fluid volume velocity to test the
sensitivity of a
measurement system. Fluid flow rates can be systematically characterized at
various ambient
temperatures, ionic strengths, and viscosities to test how heat flux, electro-
osmosis (during
electrical stimulation) and fluid boundary layer thickness affect flow. The
perfusion chamber
can be equipped with electrical sensors that allow concomitant testing of
pacing and ablation.
[0212] An example method for performing a procedure is described in connection
with FIG.
19. The example method includes disposing 1902 an example device according to
the principles
described herein in proximity to a tissue, the device including a catheter, at
least one flow sensor
47
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
disposed on a portion of the catheter, at least one component coupled to the
catheter to perform
an ablation procedure on a portion of a tissue proximate to the catheter, and
an assessment
module coupled to the flow sensor to receive data indicative of at least one
flow measurement
from the at least one flow sensor and provide an indication of the efficacy of
the ablation
procedure based on the data indicative of at least one flow measurement. The
example method
also includes applying 1904 the ablation procedure to the surface of the
tissue proximate to the
catheter and recording 1906 a measurement of the flow sensor to provide an
indication of the
efficacy of the ablation procedure.
[0213] FIG. 20 shows an example architecture of an illustrative computer
system 2000 that
can be employed to implement any of the systems and methods described herein.
The computer
system 2000 of FIG. 20 comprises one or more processors 2020 communicatively
coupled to
memory 2025, one or more communications interfaces 2005, and one or more
output devices
2010 (e.g., one or more display units) and one or more input devices 2015.
[0214] In the computer system 2000 of FIG. 20, the memory 2025 may comprise
any
computer-readable storage media, and may store computer instructions such as
processor-
executable instructions for implementing the various functionalities described
herein for
respective systems, as well as any data relating thereto, generated thereby,
or received via the
communications interface(s) or input device(s). The processor(s) 2020 shown in
FIG. 20 may be
used to execute instructions stored in the memory 2025 and, in so doing, also
may read from or
write to the memory various information processed and or generated pursuant to
execution of the
instructions.
[0215] The example computer system 2000 also includes an assessment module
2030.
Assessment module comprises processor-executable instructions for performing
any of the
methods described herein to, for example, provide an indication of a flow
rate, or to provide an
indication of the efficacy of a procedure to disrupt nerves based on the
measured values of flow
rate. Processor 2020 can be used to execute the processor-executable
instructions in connection
with assessment module 2030.
[0216] The processor 2020 of the computer system 2000 shown in FIG. 20 also
may be
communicatively coupled to or control the communications interface(s) 2005 to
transmit or
receive various information pursuant to execution of instructions. For
example, the
communications interface(s) 2005 may be coupled to a wired or wireless
network, bus, or other
48
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
communication means and may therefore allow the computer system 2000 to
transmit
information to and/or receive information from other devices (e.g., other
computer systems).
Communication interface(s) 2005 also may be in communication with an external
network 2035.
In some implementations, the communications interface(s) may be configured
(e.g., via various
hardware components or software components) to provide a website or
applications program (an
App) on a handheld device as an access portal to at least some aspects of the
computer system
2000. Non-limiting examples of such hand-held devices are tablets, slates,
smartphones,
electronic readers, or other similar hand-held electronic devices.
[0217] The output devices 2010 of the computer system 2000 shown in FIG. 20
may be
provided, for example, to allow various information to be viewed or otherwise
perceived in
connection with execution of the instructions. The input device(s) 2015 may be
provided, for
example, to allow a user to make manual adjustments, make selections, enter
data or various
other information, or interact in any of a variety of manners with the
processor during execution
of the instructions.
[0218] FIGs. 21A and 21B show the results of example measurement using an
example
device according to the principles described herein. FIGs. 21A and 21B show
data from flow
sensor measurements over a dynamic range of flow rates (from about 100 mL/min
to about 600
mL/min) for a flow sensor strategically tuned for renal hemodynamic. FIG. 21A
shows
measurements made for a 50 microAmps sensor. FIG. 21A shows measurements made
for a 20
microAmps sensor.
[0219] FIGs. 22A and 22B show the results of example use of an example
device according to
the principles described herein for use in performing an ablative procedure at
about 0.2 W to
about 0.3 W of power using electrodes for different exposure times (5 sec, 10
sec, 15 sec, 30 sec,
60 sec). The ablation electrodes are shown to generate lesions within about 5
seconds of contact
with tissue, without charring. It is observed that a lesion is generated once
the electrodes are in
contact with the tissue, soft contact was sufficient to generate lesions,
without excess pressure
being exerted.
[0220] An non-limiting example measurement implementation is described. A
system
according to the principles described herein can be used to process
differential measurement. If
one sensor is used, the body temperature of the subject would be taken into
account as well as
static flow of the subject. This may require calibrations that may differ from
patient to patient,
49
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
leading to less accurate results or may require the physician to slow the
procedure to take
separate body temperature static blood flow measurements in addition to the
renal artery flow
measurement.
Non-limiting examples of the innovations described in this disclosure include:
a) Expediting up the clinical procedure;
b) Providing more accurate results to the end point in therapy; and
c) Reducing the amount of computation required.
[0221] In a non-limiting example, temperature sensing devices can be used
in combination
with a catheter to provide flow measurements. Electrical circuits can be used
to provide
differential measurements. Thin, stretchable, flexible and/or conformal
electronics can be used to
provide thin and conformal means to deploy the sensors described herein on the
balloon of the
catheter. The flow sensing systems, device, and methods described herein can
be used for blood
flow quantification and for other types of fluid flows.
[0222] In different example implementations, the change in flow can be
reported to the
clinician via direct values. The changes in flow can be used to show the
stage, the progress, or
the degree of success, of a procedure being performed, such as but not limited
to an ablation
procedure, for example, by indicating on a console or display device the
procedure status. For
example, a change in the flow rate above a defined value or threshold, can be
used to signal or
trigger an action. In an example, the action can be the turning on of an
indicator on the catheter
device, or the display of an icon, numeric value or chart on a display. In an
example, the signal
or trigger of the action can be used to provide the indication of the stage,
the progress, or the
degree of success of a procedure.
[0223] According to the example systems, methods and devices herein,
sensing technology
onboard catheters are described that employ thin, conformal arrays of sensors
that can deform
with the curvilinear structure of various balloon and spiral-shaped catheters.
The ability to
integrate conformal sensors along with silicon-based electronics on spiral-
shaped extrusions and
balloons facilitates, for the first time, ways to integrate multimodal sensory
elements, micro-light
emitting diodes ( LEDs) and integrated circuit building blocks (i.e.
amplifiers and logic gates)
onboard catheters, thereby optimizing sensing while at the same time, not
impacting the
mechanical properties.
[0224] FIGs. 23A-23G illustrates examples of multi-electrode and balloon
catheter devices,
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
according to the principles described herein. FIGS. 23A-23G illustrates
examples of multi-
sensing element (including multielectrode) devices and catheter devices. The
devices in FIGS.
23A-23D include passive wires with polyimide-based encapsulation. The wires
are exposed in
select areas, thus forming electrode contacts. The electrode array can
include, for example, 64
electrodes. FIGS. 23E-23G show the balloon-based ablation catheters that can
be used to apply
cryo-, laser-, and high intensity ultrasound- forms of therapy, respectively,
when deployed
proximate to tissue. Any system according to the principles described herein
can be
implemented using any of the catheters shown in FIGs. 23A-23G.
[0225] Other non-limiting examples of catheters that are applicable to the
systems, methods,
and apparatus described herein include Mallecot catheters, spiral coil
catheter, mesh catheters,
single-Rod catheters, compliant balloon-based catheters, non-compliant balloon-
based catheters,
lasso-shaped catheters, multispline catheters, dilatation balloon catheters,
and angioplasty
balloon catheters.
[0226] Examples of this kind of device are shown in FIGs. 24A-24D.
Electrodes, flow sensors
and LEDs are able to withstand the significant mechanical strains caused by
repetitive inflation
and deflation cycles of the balloon by virtue of their nanomembrane form
factor and the
serpentine interconnect geometries, which help to absorb mechanical strains.
FIGs. 24E and 24F
highlight alternative forms of sensing ¨ temperature sensors, electrodes and
flow sensors on
conformal substrates. The flow sensing and electrode elements are useful for
RSDN catheters,
because assessment of blood flow can be achieved quickly, without the need for
separate
diagnostic devices.
[0227] In an example, 3-omega sensor arrays are used to measure thermal
conductivity and
other related thermal, mechanical and material properties that relate to
thermal conductivity. To
measure flow, the sensors are each positioned perpendicularly to the flow
direction. Such a
configuration can be compatible with the design of a spiral shaped catheter
system. AC current is
applied across each sensor and the resulting AC voltage is measured. This
measured voltage
decreases monotonically as flow rate increases and increases if the blood is
stagnant or slows
down. Computations of measured voltages according to any of the example
devices herein can
be calibrated using a perfusion chamber and the flow is assumed to follow the
Hagen¨Poiseuille
equation and its respective assumptions. Measurements using 3-omega sensor
technology are
versatile because they can be used to extract several other physical
parameters that may be
51
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
relevant to clinicians. This sensing modality can be used to serve as a viable
platform for
catheters, e.g., that can be used to perform a renal denervation.
[0228]
In an example, mechanical modeling of flow sensors and electrodes during
mechanical
stress can be performed. Using modeling simulations, the dynamic material and
mechanical
properties can be characterized for conformal sensor arrays on balloon and
spiral-shaped
catheters that experience significant bending and twisting during operation.
This includes
analytical and finite element modeling of the mechanics of flex electronics
affixed to balloon
catheters. The strain distributions obtained through analytical and
computational modeling
capture, quantitatively, the nature of deformations in the electronics layers.
Characterization of
the effective strain and displacement distributions in the sensor islands and
serpentine
interconnects provide important insights into critical fracture strains and
buckling phenomena.
Such characterization of conformal sensors can dramatically improve the way
nanomembrane
flow sensors and electrodes are designed and implemented on highly deformable
substrates (i.e.
deflectable catheters). Furthermore, the approach holds promise for increasing
the understanding
of the mechanical stresses involved during catheter deployment in vivo.
[0229]
FIG. 25 shows non-limiting examples of flow sensors on rod-shaped catheters
that
include "clover-shaped" flow sensors. The metal rectangles are electrodes on
the catheter. In
some of the example catheters of FIG. 25, the flow sensors include angioplasty
balloons with
ablation electrodes (the circular pads). In the novel example systems herein,
the clover flow
sensors are combined with the balloon electrodes on a single device.
[0230]
According to the systems and methods described herein, ablation electrodes can
be
embedded on angioplasty balloon along with the clover-shaped flow sensors on
the proximal and
distal sides of the balloon on the catheter extrusion. According to the novel
systems and methods
herein, a multifunctional balloon catheter that has (i) array of electrodes is
coupled with (ii) flow
sensors embedded on the catheter's shaft proximate to the balloon. In some
examples, the
balloon catheter may include other sensors on the balloon, such as but not
limited to LEDs,
contact sensors, pressure sensors, biological activity sensors, and
temperature sensors.
[0231]
In an example implementation, the catheter with balloon is deployed proximate
to the
renal tissue (or other portion of the renal system) in a deflated state. For
example, once the
catheter is in the renal artery, fluid flow can be measured (including blood
flow). Once captured,
the balloon can be inflated and the ablation can be performed. Once the
ablation is completed, or
52
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
at selected points during performance of the ablation, the balloon can be
deflated and flow is
sensed again to see what changes are measured. In this example, an increase in
flow can be used
to serve as an indicator of a successful ablation procedure.
[0232]
In another example implementation, the nerve can be paced and the flow can be
measured pre-ablation. The ablation cycle can be performed. Once the ablation
is completed, or
at selected points during performance of the ablation, the nerve can be paced
and the flow can be
measured again (including a post-ablation measurement). If the pacing is
determined to causes a
change in flow, this can be used as an indicator that the nerves are still
active. If the pacing does
not cause a shift in flow, this can be used as an indicator that the nerves
have been successfully
denervated. The flow sensors coupled with ablation electrodes according to the
systems and
methods described herein facilitate this novel analysis and determination of
clinical endpoint.
[0233]
FIG. 26 shows a non-limiting example of flow sensors on a spiral-shaped
catheters.
FIG. 27 shows a catheter with bipolar electrodes and metal interconnects
disposed on its surface.
[0234]
Example design and fabrication are described for examples of 4 flow sensors, 4
pacing
electrodes and 4 ablation electrodes all co-located on spiral-shaped
catheters. A custom data
acquisition system is implemented, and the initial functionality of the flow
sensors and electrodes
is tested by deploying them in flow perfusion chambers. Example combined
functionalities of the
flow sensors, pacing electrodes and ablation electrodes in the renal arteries
of live porcine
models are also described. Spiral catheters containing the sensors and
electrodes are used to
measure blood flow during nerve stimulation immediately pre- and post renal
ablation event. A
comparative analysis is conducted of a catheter system's performance, ease of
use, and
procedure time relative to other renal ablation devices being used in the
clinical setting to gain
insight into how having a clinical endpoint in RSDN helps to improve the
overall procedure
efficacy and safety.
[0235]
Non-limiting examples of flow sensors, pacing and ablation electrodes on
multifunctional spiral catheter in perfusion apparatus is described.
Constrained spaces in the
renal artery can reduce the number of devices that can be positioned inside.
As a result, it can be
challenging to deploy multiple devices in such as a confined space as in the
renal arteries.
Multifunctional RSDN catheters are constructed with electrodes on spiral-
shaped extrusions that
are small enough to conform to the renal artery to enable electrical stimuli
delivery without
affecting measurements. Mechanically optimized nanomembrane electrodes are
incorporated
53
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
with 3-omega flow sensors that interface with the limited space of the renal
artery. In an
example, up to 8 electrodes (0.25x0.25 mm2) and 4 (1x1 mm2) sensors are
fabricated to measure
renal blood flow pre- and post- ablation events. A data acquisition system
(National Instruments
Inc.) is implemented, coupled with an Electrical stimulator console (Medtronic
Inc.) to deliver
the 5-10 W of energy to pace and ablate the renal nerves. This power supply
can be used to apply
pacing energy. Using this new system, fundamental limits of the ablation and
pacing electrodes
with in vitro tissue can be characterized. In addition, a custom perfusion
chamber can be built to
test the flow sensing capabilities. Taken together, these new designs,
microfabrication
approaches and measurements using in-vitro models can provide insight into the
optimal
configuration of electrodes and flow sensors necessary determine changes in
flow rate following
renal denervation.
[0236] Non-limiting example pacing and ablation electrodes on spiral
catheter and test
performance in vitro are described. Ultrathin geometries impart flexibility to
otherwise rigid and
brittle materials. Ultrathin conformal nanomembrane sensors (-250 nm) embedded
in thin
polyimide and elastomeric substrates (-50-100 [tm) in neutral mechanical plane
layouts
accommodate significant mechanical durability with radii of curvature less
than about 1 mm. To
achieve conformal sensors with such designs, arrays of electrodes can be
formed on silicon.
Lithographic processing and vertical trench wet-etching techniques yield
isolated chiplets
(-0.25 x0.25 mm2, and ¨1-5 [tm thick) that remain tethered to the underlying
wafer through
'anchor' structures. This process can be used to yield electrodes that are
referred to as
'printable', due to their ability to be removed and placed onto a target
substrate with a soft,
elastomeric stamp and transferred onto a spiral catheter. The attractive
features of this approach
include: (1) ultrathin circuit layouts for mechanical flexibility to conform
to limited space in the
renal artery, and (2) compatibility with other elements such as contact or
flow sensors.
[0237] The utility of nanomembrane electrodes are tested be perform ablation
measurements
by driving RF energy (5-10 W) to show that renal nerves fibers can be ablated
through arterial
vessel. Histological assessment is performed of the nerves pre- and post
ablation cycles to test
nanomembrane electrode array performance and to see if the surface properties
of the electrodes
change over time (as a result of protein coating and/or electromosis
phenomena). Measurements
performed in the heart and on excised muscle tissue yield promising results on
both pacing and
ablation with this new class of nanomembrane electrodes.
54
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
[0238] A non-limiting example data acquisition system is described.
Stimulation waveforms in
the form of rectified triangular pulses with fixed amplitude of 10-20 V and
100-150 ms durations
can be delivered through the pacing electrodes using instructions programmed
into machine
readable instructions. The waveform patterns are chosen strategically to
induce renal nerve
activity and to give rise to vasoconstriction or changes in local blood flow.
The data acquisition
system includes three modules to measure blood flow, induce nerve stimulation,
and deliver
ablation energy. The data from any of these modules can be transmitted to the
assessment
module to perform an assessment of efficacy as described in connection with
any of the
examples described herein. A National Instruments Inc. PXI-6289 (a
multifunction M Series
data acquisition (DAQ) system), controlled with custom machine readable
instructions
(including in LABVIEWTm software), controls voltages across the sensors.
[0239] Non-limiting example flow sensing, pacing and ablation using
multifunctional spiral
catheters in live animal models are described. Multifunctional balloon and
spiral shaped catheter
described in section 1 above are applied to flow sensing and ablation
measurements in live
animal models. Balloon catheters can be used. In an example, balloon catheters
may have larger
profiles that can affect flow. In a non-limiting example, to minimize effects
of the catheter on
blood flow, spiral-shaped catheters can be used instead of balloons. Flow can
be measured upon
initial deployment in the renal arteries over the course of a few minutes to
determine the initial
average flow rate. Once established, pacing can ensue and flow can be
monitored concurrently.
A 20-30% reduction in flow can be expected during this step if the renal
nerves are functioning
properly. Once this initial calibration is completed, the same set of
procedures can be run
following renal ablation cycles. It is possible that the renal blood flow may
shift to a different
baseline than in the initial measurement. In an example, this is not used as
an indicator of
successful ablation. If ablation is successful, there can be an interpretable
effect that can be
apparent during pacing. That is, there may be little shift in flow during
pacing because the
vasoconstriction properties of the nerves can be dysfunctional, which can
serve as the clinical
endpoint of the procedure.
[0240] In an example, an example method for determining renal denervation
endpoint when
blood pressure and flow are modulated with nitroglycerine is described. To
determine how
changes in flow can be assessed with the sensors described herein, variations
in blood flow
caused by nitroglycerine lead to changes in blood pressure and renal blood
flow rates before and
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
after ablation can be monitored. Systemic injections of nitroglycerine can
cause shifts in blood
pressure that can give rise to changes in renal blood flow. Injections of
nitroglycerine also can be
monitored to determine an affect on blood flow pre-pacing and again following
pacing.
[0241]
In an example, leakage currents and encapsulation are described. Conformal
flow
sensor arrays can be fabricated using a multi-layer process, which has a thin
layer polyimide as
the encapsulating layer. Horizontal and vertical interconnect layers are
insulated using this thin
layer of polyimide. In an example system, leakage currents may escape and lead
to noisy
recordings, bubble formation in the fluid, or sensor deterioration over time.
In an example, to
prevent leakage currents in these systems, additional polymeric encapsulation
(UV curable
polyurethanes or parylenes) can be coated over the sensors, creating an
additional ¨10 m
encapsulating layer to withstand current leakage effects. Over the course of a
few hours (the
extent of RSDN procedures), leakage currents may be manageable with
polyurethane, parylene
and UV curable encapsulation strategies.
[0242] Data visualization and signal fidelity is described. Data acquisition
systems developed
for recording flow, pacing and ablation may not be provided in a single
module. The
visualization of the measurements recordings and stimuli application may
require feedback from
multiple physicians. Interpretation of the flow data in real time can be
challenging. A first
generation data acquisition system is described for measuring and displaying
flow. In an
example, the user interface can be configured to be presented on the same
LABVIEWTm display
as the controls for pacing and ablation, thereby providing all of the catheter
control features on a
single console. This system architecture may be well suited for a product
development
implementation.
[0243]
Renal nerve stimulation is described. In some examples, the nerve pacing
electrodes
may lose contact with the arterial vessel wall. This variability in good
contact may cause poor
denervation results. To counter this effect, x-ray imaging and electrode
impedance recordings
can be monitored to restore proper contact with the vessel wall.
[0244]
Also provided herein is a user interface that can be used during a procedure
to monitor
the progress of the procedure and/or to provide an indication of an endpoint
of the procedure.
The user interface can be provided using an apparatus for displaying
representations of the
parameters of an inflatable body and/or expandable body disposed proximate to
a portion of a
tissue that is being treated. According to the principles herein, the
inflatable body and/or
56
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
expandable body can include a plurality of sensors coupled to at least a
portion of the inflatable
body and/or expandable body. The apparatus can include a user interface, at
least one memory
to store processor-executable instructions, and at least one processing unit
coupled to the at least
one memory. Upon execution of the processor-executable instructions, the at
least one
processing unit controls the user interface to display at least one
representation of the parameters
of the inflatable body and/or expandable body.
[0245] FIGs. 28A and 28B show non-limiting examples of the types of
representation of the
parameters of the inflatable body and/or expandable body that can be shown on
a display. FIG.
28A shows an example first representation of the state of the inflatable body
and/or expandable
body. The inflatable body and/or expandable body can be shown using a first
form indicator
2802 to indicate that it is in an inflated/expanded state , or using a second
form indicator 2804 to
indicate that it is in a deflated/collapsed. FIG. 28B shows examples of the
types of
representations that can be used to indicate the state of at least one sensor
of the plurality of
sensors coupled to the inflatable body and/or expandable body. One or more of
the sensors can
be represented using a first activation indicator 2852 to indicate that
respective sensor measures a
signal below a threshold value, or using a second activation indicator 2854 to
indicate that
respective sensor measures a signal above or about equal to the threshold
value.
[0246] In an example, the first activation indicator 2852 and the second
activation indicator
2854 can be used to indicate a state of contact of a portion of the inflatable
body and/or expandable
body with a tissue A signal below the threshold value can be interpreted as
indicating that the at
least one sensor is not in contact with a portion of the tissue, and a signal
above or about equal to
the threshold value indicates that the at least one sensor is in contact with
a portion of the tissue.
[0247] In an example, the first activation indicator and the second
activation indicator can be
displayed as binary visual representations, e.g., ON/OFF or other binary
indication.
[0248] In an example, the first activation indicator and the second
activation indicator can be
displayed as quantitative visual representations that correspond to a
magnitude of the signal. For
example, as shown in FIG. 28C, the display can show a feature (such as an
arrow or bar) that
changes to indicate a magnitude of a signal to a sensor. The example of FIG.
28C shows values
for "contact" or "no contact". In other examples, the features in the display
could be used to
indicate relative magnitudes of any other parameter measured using a sensor.
In an example, a
graphical plot, such as shown in FIG. 28D also can be used to indicate the
magnitude of the
57
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
signal.
[0249] In an example, the sensor(s) may be flow sensor(s), and the first
activation indicator
and the second activation indicator can be displayed as quantitative visual
representations that
indicate the magnitude of parameters such as but not limited to an
instantaneous velocity, a
volumetric flow, or a vascular resistance of the measured fluid flow rate at
each sensor.
[0250] In an example, the first form indicator and the second form
indicator can be displayed
as color-coded symbols. Each color-coded symbol can be used to indicative of a
range of values
of the magnitudes of the signal. For example, green can be used to indicate a
low range of
values below a first threshold, yellow can be used to indicate a signal
falling in a mid-range of
values up to a second threshold, and red can be used to indicate a signal
falling in a high range of
values above the second threshold.
[0251] In an example, the first form indicator and the second form
indicator could further be
used to provide an indication of a spatial location of the corresponding at
least one sensor
relative to the inflatable body and/or expandable body. For example, the
indicators 2852 and
2854 can be used to indicate the spatial location of the sensor that is
activated.
[0252] In an example, the user interface can be configured to cause display of
the representation
of the inflatable body and/or expandable body and the representation of the
state of activation of
the sensor(s) in a staged process. No representation of the state of
activation of the sensor(s)
would be displayed while the representation of the inflatable body and/or
expandable body is the
first form indicator (indicating a deflated/collapsed state). The
representations of the state of
activation of the sensor(s) would be displayed once the representation of the
inflatable body
and/or expandable body is the second form indicator (indicating an
inflated/expanded state).
That is, the representations of the state of activation of the sensors may not
be allowed to be
display, until the inflatable body and/or expandable body is somewhat
inflated/expanded (even if
not fully inflated or expanded).
[0253] In an example, the user interface can be configured to display
instructions to a
practitioner (including a physician) at various stages of the procedure being
performed. The
example, the display can be configured to indicate when an endpoint of the
procedure has been
reached. If the procedure has not reached an endpoint, the display can be
configured to display
instructions to indicate to the practitioner to continue the procedure or
modify the procedure
(e.g., to move the catheter, guidewire or other elongated body or the
inflatable body and/or
58
CA 02934245 2016-06-16
WO 2015/102951
PCT/US2014/071516
expandable body, or to re-apply the treatment (e.g., the ablation). The user
interface also can be
configured to display of an indication of at least one stage of the procedure
being performed on
the portion of the tissue.
[0254] In
an example shown in FIG. 29, the user interface can be used to display
vascular
resistance value(s) in numerical form and/or as a graph vs. time. The user
interface also can be
used to display data indicative of parameters such as but not limited to the
systolic slope, the
pulsatile flow, and the pulsatile pressure. The user interface can be
configured to display values
indicative of the instantaneous/volumetric flow in a short-time duration graph
that shows
granular flow readings. The user interface can be configured to display values
of the
instantaneous/volumetric flow in a long time-scale graph, e.g., over the
course of the duration of
the procedure (e.g., an hour-long procedure), to discern changes pre- and post-
treatment (such as
but not limited to the ablation.
[0255] While various inventive embodiments have been described and illustrated
herein, those
of ordinary skill in the art will readily envision a variety of other means
and/or structures for
performing the function and/or obtaining the results and/or one or more of the
advantages
described herein, and each of such variations and/or modifications is deemed
to be within the
scope of the inventive embodiments described herein. More generally, those
skilled in the art
will readily appreciate that all parameters, dimensions, materials, and
configurations described
herein are meant to be examples and that the actual parameters, dimensions,
materials, and/or
configurations will depend upon the specific application or applications for
which the inventive
teachings is/are used. Those skilled in the art will recognize, or be able to
ascertain using no
more than routine experimentation, many equivalents to the specific inventive
embodiments
described herein. It is, therefore, to be understood that the foregoing
embodiments are presented
by way of example only and that inventive embodiments may be practiced
otherwise than as
specifically described. Inventive embodiments of the present disclosure are
directed to each
individual feature, system, article, material, kit, and/or method described
herein. In addition, any
combination of two or more such features, systems, articles, materials, kits,
and/or methods, if
such features, systems, articles, materials, kits, and/or methods are not
mutually inconsistent, is
included within the inventive scope of the present disclosure.
[0256] The above-described embodiments of the invention may be implemented in
any of
numerous ways. For example, some embodiments may be implemented using
hardware,
59
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
software or a combination thereof. When any aspect of an embodiment is
implemented at least
in part in software, the software code may be executed on any suitable
processor or collection of
processors, whether provided in a single device or computer or distributed
among multiple
devices/computers.
[0257] Also, the technology described herein may be embodied as a method, of
which at least
one example has been provided. The acts performed as part of the method may be
ordered in
any suitable way. Accordingly, embodiments may be constructed in which acts
are performed in
an order different than illustrated, which may include performing some acts
simultaneously, even
though shown as sequential acts in illustrative embodiments.
[0258] All definitions, as defined and used herein, should be understood to
control over
dictionary definitions, definitions in documents incorporated by reference,
and/or ordinary
meanings of the defined terms.
[0259] The indefinite articles "a" and "an," as used herein in the
specification, unless clearly
indicated to the contrary, should be understood to mean "at least one."
[0260] The phrase "and/or," as used herein in the specification, should be
understood to mean
"either or both" of the elements so conjoined, i.e., elements that are
conjunctively present in
some cases and disjunctively present in other cases. Multiple elements listed
with "and/or"
should be construed in the same fashion, i.e., "one or more" of the elements
so conjoined. Other
elements may optionally be present other than the elements specifically
identified by the
"and/or" clause, whether related or unrelated to those elements specifically
identified. Thus, as a
non-limiting example, a reference to "A and/or B", when used in conjunction
with open-ended
language such as "comprising" can refer, in one embodiment, to A only
(optionally including
elements other than B); in another embodiment, to B only (optionally including
elements other
than A); in yet another embodiment, to both A and B (optionally including
other elements); etc.
[0261] As used herein in the specification, "or" should be understood to have
the same
meaning as "and/or" as defined above. For example, when separating items in a
list, "or" or
"and/or" shall be interpreted as being inclusive, i.e., the inclusion of at
least one, but also
including more than one, of a number or list of elements, and, optionally,
additional unlisted
items. Only terms clearly indicated to the contrary, such as "only one of" or
"exactly one of," or
"consisting of," will refer to the inclusion of exactly one element of a
number or list of elements.
In general, the term "or" as used herein shall only be interpreted as
indicating exclusive
CA 02934245 2016-06-16
WO 2015/102951 PCT/US2014/071516
alternatives (i.e. "one or the other but not both") when preceded by terms of
exclusivity, such as
"either," "one of," "only one of," or "exactly one of."
[0262] As used herein in the specification, the phrase "at least one," in
reference to a list of one
or more elements, should be understood to mean at least one element selected
from any one or
more of the elements in the list of elements, but not necessarily including at
least one of each and
every element specifically listed within the list of elements and not
excluding any combinations
of elements in the list of elements. This definition also allows that elements
may optionally be
present other than the elements specifically identified within the list of
elements to which the
phrase "at least one" refers, whether related or unrelated to those elements
specifically identified.
Thus, as a non-limiting example, "at least one of A and B" (or, equivalently,
"at least one of A or
B," or, equivalently "at least one of A and/or B") can refer, in one
embodiment, to at least one,
optionally including more than one, A, with no B present (and optionally
including elements
other than B); in another embodiment, to at least one, optionally including
more than one, B,
with no A present (and optionally including elements other than A); in yet
another embodiment,
to at least one, optionally including more than one, A, and at least one,
optionally including more
than one, B (and optionally including other elements); etc
61