Note: Descriptions are shown in the official language in which they were submitted.
ANLPHOLYTE POLYMERS AND METHODS OF TREATING
SUBTERRANEAN FORMATIONS WITH THE SAME
100011 Deleted
BACKGROUND OF THE INVENTION
100021 During the drilling, stimulation, completion, and
production
phases of wells for petroleum or water extraction, the use of compositions
having high viscosities in subterranean formations is important for a wide
variety of purposes. Higher viscosity fluids can more effectively carry
materials
to a desired location in a subterranean formation, such as proppants. The use
of
higher viscosity fluids during hydraulic fracturing generally results in
larger
more dominant fractures. Higher viscosity drilling fluids can more effectively
carry materials away from a drilling location downhole.
100031 One common way to attain high viscosities in drilling
fluids is to
use a mixture of water and a viscosifier, such as guar gum. However, typically
viscosifiers must be added in high concentrations to provide viscosities
sufficient
to suspend a desired proppant or to suspend drill cuttings, which can result
in
high transportation costs and low efficiency preparation of viscous materials.
However, pumping high viscosity materials into a subterranean formation can
require a large amount of energy. Also, the higher temperatures experienced in
a
subterranean formation can limit, reduce, or degrade the effectiveness of
certain
viscosifiers, resulting in the use of larger amounts of viscosifiers to
compensate
for the high temperatures, or the use of expensive temperature-resistant
viscosifiers. In addition, the presence of certain ions in water can limit,
reduce,
or degrade the effectiveness of certain viscosifiers. This limits the use of
certain
ion-containing water, such as sea water, or water recovered from or naturally
produced by some subterranean formations. As a result, the oil and gas
industry
spends substantial amounts of money and energy to use large amounts of
viscosifiers to compensate for salt sensitivity, obtain expensive salt-
resistant
viscosifiers, obtain fresh water used for drilling fluid or fracturing fluid
1
CA 2938279 2018-04-27
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
applications, or to avoid formations having substantial concentrations of
particular ions.
BRIEF DESCRIPTION OF THE FIGURES
[0004] The drawings illustrate generally, by way of example, but not by
way of limitation, various embodiments discussed in the present document.
[0005] FIG. 1 illustrates a drilling assembly, in accordance with various
embodiments.
[0006] FIG. 2 illustrates a system or apparatus for delivering a
composition in a subterranean formation, in accordance with various
embodiments.
[0007] FIG. 3 provides a graph of the viscosity of an ampholyte
polymeric compound at various concentrations over time at an elevated
temperature, in accordance with various embodiments.
[0008] FIG. 4 provides a graph comparing the viscosity of an ampholyte
polymeric compound and a traditional viscosifier in water, in accordance with
various embodiments.
[0009] FIG. 5 provides a graph comparing the viscosity of an ampholyte
polymeric compound and a traditional viscosifier in a high TDS water, in
accordance with various embodiments.
[0010] FIG. 6 provides a graph of percent friction reduction at various
salinities for three friction reducing additives including one ampholyte
polymeric compound, in accordance with various embodiments.
[0011] FIG. 7 provides a graph of viscosity measurements over time at
various temperatures for a fluid including an ampholyte polymeric compound, in
accordance with various embodiments.
[0012] FIG. 8 provides a graph comparing the intrinsic viscosity over
time for a fluid including an ampholyte polymeric compound and a fluid
including a traditional friction reducing agent, in accordance with various
embodiments.
[0013] FIG. 9 provides a graph of viscosity measurements over time at
various TDS concentrations for fluids including an ampholyte polymeric
compound, in accordance with various embodiments.
2
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
[0014] FIG. 10 illustrates a photograph of a crosslinked ampholyte
polymer, in accordance with various embodiments.
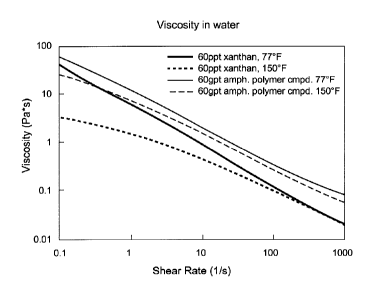
[0015] FIG. 11 illustrates viscosity versus shear rate for a
crosslinked
ampholyte terpolymer, in accordance with various embodiments.
[0016] FIG 12 illustrates frequency sweep data for various
crosslinked
polymers at 77 cF, in accordance with various embodiments.
[0017] FIG. 13 illustrates frequency sweep data for various
crosslinked
polymers at 150 'F., in accordance with various embodiments.
[0018] FIG. 14 illustrates a permeability profile for a crosslinked
polyacrylamide, in accordance with various embodiments.
[0019] FIG. 15 illustrates a permeability profile for a crosslinked
ampholyte terpolymer, in accordance with various embodiments.
DETAILED DESCRIPTION OF THE INVENTION
[0020] Reference will now be made in detail to certain embodiments of
the disclosed subject matter, examples of which are illustrated in part in the
accompanying drawings. While the disclosed subject matter will be described in
conjunction with the enumerated claims, it will be understood that the
exemplified subject matter is not intended to limit the claims to the
disclosed
subject matter.
[0021] Values expressed in a range format should be interpreted in a
flexible manner to include not only the numerical values explicitly recited as
the
limits of the range, but also to include all the individual numerical values
or sub-
ranges encompassed within that range as if each numerical value and sub-range
is explicitly recited. For example, a range of "about 0.1% to about 5%" or
"about 0.1% to 5%" should be interpreted to include not just about 0.1% to
about
5%, but also the individual values (e.g., 1%, 2%, 3%, and 4%) and the sub-
ranges (e.g., 0.1% to 0.5%, 1.1% to 2.2%, 3.3% to 4.4%) within the indicated
range. The statement "about X to Y" has the same meaning as "about X to about
Y," unless indicated otherwise. Likewise, the statement "about X, Y, or about
Z" has the same meaning as "about X, about Y, or about Z," unless indicated
otherwise.
[0022] In this document, the terms "a," "an," or "the" are used to
include
one or more than one unless the context clearly dictates otherwise. The term
3
"or" is used to refer to a nonexclusive "or" unless otherwise indicated. The
statement "at least one of A and B" has the same meaning as "A, B, or A and
B."
In addition, it is to be understood that the phraseology or terminology
employed
herein, and not otherwise defined, is for the purpose of description only and
not
of limitation. Any use of section headings is intended to aid reading of the
document and is not to be interpreted as limiting; information that is
relevant to a
section heading may occur within or outside of that particular section.
[00231 In the methods of manufacturing described herein, the steps
can
be carried out in any order without departing from the principles of the
invention, except when a temporal or operational sequence is explicitly
recited.
Furthermore, specified steps can be carried out concurrently unless explicit
claim language recites that they be carried out separately. For example, a
claimed step of doing X and a claimed step of doing Y can be conducted
simultaneously within a single operation, and the resulting process will fall
within the literal scope of the claimed process.
[0024] Selected substituents within the compounds described herein
are
present to a recursive degree. In this context, "recursive substituent" means
that
a substituent may recite another instance of itself or of another substituent
that
itself recites the first substituent. Recursive substituents are an intended
aspect
of the disclosed subject matter. Because of the recursive nature of such
substituents, theoretically, a large number may be present in any given claim.
One of ordinary skill in the art of organic chemistry understands that the
total
number of such substituents is reasonably limited by the desired properties of
the
compound intended. Such properties include, by way of example and not
limitation, physical properties such as molecular weight, solubility, and
practical
properties such as ease of synthesis. Recursive substituents can call back on
themselves any suitable number of times, such as about I time, about 2 times,
3,
4
CA 2938279 2018-04-27
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
4, 5, 6, 7, 8, 9, 10, 15, 20, 30, 50, 100, 200, 300, CD, 500, 750, 1000, 1500,
2000, 3000, 4000, 5000, 10,000, 15,000, 20,000, 30,000, 50,000, 100,000,
200,000, 500,000, 750,(00, or about 1,000,000 times or more.
[0025] The term "about" as used herein can allow for a degree of
variability in a value or range, for example, within 10%, within 5%, or within
1% of a stated value or of a stated limit of a range.
[0026] The term "substantially" as used herein refers to a majority of, or
mostly, as in at least about 50%, 60%, 70%, 80%, 90%, 95%, 96%, 97%, 98%,
99%, 99.5%, 99.9%, 99.99%, or at least about 99.999% or more.
[0027] The term "organic group" as used herein refers to but is not
limited to any carbon-containing functional group. For example, an oxygen-
containing group such as an alkoxy group, aryloxy group, aralkyloxy group,
oxo(carbonyl) group, a carboxyl group including a carboxylic acid,
carboxylate,
and a carboxylate ester; a sulfur-containing group such as an alkyl and aryl
sulfide group; and other heteroatom-containing groups. Non-limiting examples
of organic groups include OR, 00R, OC(0)N(R)2, CN, CF3, OCF3, R, C(0),
methylenedioxy, ethylenedioxy, N(R)2, SR, SOR, SO2R, SO2N(R)2, SO3R,
C(0)R, C(0)C(0)R, C(0)CH2C(0)R, C(S)R, C(0)0R, OC(0)R, C(0)N(R)2,
OC(0)N(R)2, C(S)N(R)2, (C112)0-2N(R)C(0)R, (CH2)0-2N(R)N(R)2,
N(R)N(R)C(0)R, N(R)N(R)C(0)0R, N(R)N(R)CON(R)2, N(R)S02R,
N(R)S02N(R)2, N(R)C(0)0R, N(R)C(0)R, N(R)C(S)R, N(R)C(0)N(R)2,
N(R)C(S)N(R)2, N(COR)COR, N(OR)R, C(=NH)N(R)2, C(0)N(OR)R, or
C(=NOR)R, wherein R can be hydrogen (in examples that include other carbon
atoms) or a carbon-based moiety, and wherein the carbon-based moiety can
itself
be further substituted.
[0028] The term "substituted" as used herein refers to an organic group
as defined herein or molecule in which one or more hydrogen atoms contained
therein are replaced by one or more non-hydrogen atoms. The term "functional
group" or "substituent" as used herein refers to a group that can be or is
substituted onto a molecule or onto an organic group. Examples of substituents
or functional groups include, but are not limited to, a halogen (e.g., F, Cl,
Br,
and I); an oxygen atom in groups such as hydroxyl groups, alkoxy groups,
aryloxy groups, aralkyloxy groups, oxo(carbonyl) groups, carboxyl groups
including carboxylic acids, carboxylatcs, and carboxylate esters; a sulfur
atom in
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
groups such as thiol groups, alkyl and aryl sulfide groups, sulfoxide groups,
sulfone groups, sulfonyl groups, and sulfonamide groups; a nitrogen atom in
groups such as amines, hydroxylamines, nitrites, nitro groups, N-oxides,
hydrazides, azides, and enamines; and other heteroatoms in various other
groups.
Non-limiting examples of substituents J that can be bonded to a substituted
carbon (or other) atom include F, Cl, Br, I, OR, OC(0)N(R')2, CN, NO, NO2,
0NO2, azido, CF3, OCF3, R, 0 (oxo), S (thiono), C(0), S(0), methylenedioxy,
ethylenedioxy, N(R)2, SR, SOR, SO2R, SO2N(R)2, SO3R, C(0)R, C(0)C(0)R,
C(0)CH2C(0)R, C(S)R, C(0)0R, OC(0)R, C(0)N(R)2, OC(0)N(R)2,
C(S)N(R)2, (CH2)0-2N(R)C(0)R, (CH2)0-2N(R)N(R)2, N(R)N(R)C(0)R,
N(R)N(R)C(0)0R, N(R)N(R)CON(R)2, N(R)S02R, N(R)S02N(R)2,
N(R)C(0)0R, N(R)C(0)R, N(R)C(S)R, N(R)C(0)N(R)2, N(R)C(S)N(R)2,
N(COR)COR, N(OR)R, C(=NH)N(R)2, C(0)N(OR)R, or C(=NOR)R, wherein
R can be hydrogen or a carbon-based moiety, and wherein the carbon-based
moiety can itself be further substituted; for example, wherein R can be
hydrogen,
alkyl, acyl, cycloalkyl, aryl, aralkyl, heterocyclyl, heteroaryl, or
heteroarylalkyl,
wherein any alkyl, acyl, cycloalkyl, aryl, araLkyl, heterocyclyl, heteroaryl,
or
heteroarylalkyl or R can be independently mono- or multi-substituted with J;
or
wherein two R groups bonded to a nitrogen atom or to adjacent nitrogen atoms
can together with the nitrogen atom or atoms form a heterocyclyl, which can be
mono- or independently multi-substituted with J.
[0029] The term "alkyl" as used herein refers to straight chain and
branched alkyl groups and cycloalkyl groups having from 1 to 40 carbon atoms,
1 to about 20 carbon atoms, 1 to 12 carbons or, in some embodiments, from 1 to
8 carbon atoms. Examples of straight chain alkyl groups include those with
from 1 to 8 carbon atoms such as methyl, ethyl, n-propyl, n-butyl, n-pentyl, n-
hexyl, n-heptyl, and n-octyl groups. Examples of branched alkyl groups
include,
but are not limited to, isopropyl, iso-butyl, sec-butyl, t-butyl, neopentyl,
isopentyl, and 2,2-dimethylpropyl groups. As used herein, the term "alkyl"
encompasses n-alkyl, isoalkyl, and anteisoalkyl groups as well as other
branched
chain forms of alkyl. Representative substituted alkyl groups can be
substituted
one or more times with any of the groups listed herein, for example, amino,
hydroxy, cyano, carboxy, nitro, thio, alkoxy, and halogen groups.
6
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
[0030] The term "alkenyl" as used herein refers to straight and
branched
chain and cyclic alkyl groups as defined herein, except that at least one
double
bond exists between two carbon atoms. Thus, alkenyl groups have from 2 to 40
carbon atoms, or 2 to about 20 carbon atoms, or 2 to 12 carbons or, in some
embodiments, from 2 to 8 carbon atoms. Examples include, but are not limited
to vinyl, -C11,---CH(CH3), -CH=C(C113)2, -C(CH3)=C112, -C(CH3)=CH(C113), -
C(CH2CH3)=CH2, cyclohexenyl, cyclopentenyl, cyclohexadienyl, butadienyl,
pentadienyl, and hexadienyl among others.
[0031] The term "alkynyl" as used herein refers to straight and
branched
chain alkyl groups, except that at least one triple bond exists between two
carbon
atoms. Thus, alkynyl groups have from 2 to 40 carbon atoms, 2 to about 20
carbon atoms, or from 2 to 12 carbons or, in some embodiments, from 2 to 8
carbon atoms. Examples include, but are not limited to -
Cm-C(CH2CH3), -CH2C-(CH3), and -
CH2C(CH2CH3) among
others.
[0032] The term "acyl" as used herein refers to a group containing a
carbonyl moiety wherein the group is bonded via the carbonyl carbon atom. The
carbonyl carbon atom is also bonded to another carbon atom, which can be part
of an alkyl, aryl, aralkyl cycloalkyl, cycloalkylalkyl, heterocyclyl,
heterocyclylalkyl, heteroaryl, heteroarylalkyl group or the like. In the
special
case wherein the carbonyl carbon atom is bonded to a hydrogen, the group is a
"formyl" group, an acyl group as the term is defined herein. An acyl group can
include 0 to about 12-20 or 12-40 additional carbon atoms bonded to the
carbonyl group. An acyl group can include double or triple bonds within the
meaning herein. An acryloyl group is an example of an acyl group. An acyl
group can also include heteroatoms within the meaning here. A nicotinoyl group
(pyridy1-3-carbonyl) is an example of an acyl group within the meaning herein.
Other examples include acetyl, benzoyl, phenylacetyl, pyridylac,etyl,
cinnamoyl,
and acryloyl groups and the like. When the group containing the carbon atom
that is bonded to the carbonyl carbon atom contains a halogen, the group is
termed a "haloacyl" group. An example is a trifluoroacetyl group.
[0033] The term "aryl" as used herein refers to cyclic aromatic
hydrocarbons that do not contain heteroatoms in the ring. Thus aryl groups
include, but are not limited to, phenyl, azulenyl, heptalenyl, biphenyl,
indacenyl,
7
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
fluorenyl, phenanthrenyl, triphenylenyl, pyrenyl, naphthacenyl, chrysenyl,
biphenylenyl, anthracenyl, and naphthyl groups. In some embodiments, aryl
groups contain about 6 to about 14 carbons in the ring portions of the groups.
Aryl groups can be unsubstituted or substituted, as defined herein.
Representative substituted aryl groups can be mono-substituted or substituted
more than once, such as, but not limited to, 2-, 3-, 4-, 5-, or 6-substituted
phenyl
or 2-8 substituted naphthyl groups, which can be substituted with carbon or
non-
carbon groups such as those listed herein.
[0034] The term "heterocyclyl" as used herein refers to aromatic and
non-aromatic ring compounds containing 3 or more ring members, of which one
or more is a heteroatom such as, but not limited to, N, 0, and S. Thus, a
heterocyclyl can be a cycloheteroalkyl, or a heteroaryl, or if polycyclic, any
combination thereof. In some embodiments, heterocyclyl groups include 3 to
about 20 ring members, whereas other such groups have 3 to about 15 ring
members. A heterocyclyl group designated as a C2-heterocyclyl can be a 5-ring
with two carbon atoms and three heteroatoms, a 6-ring with two carbon atoms
and four heteroatoms and so forth. Likewise a C4-heterocyclyl can be a 5-ring
with one heteroatom, a 6-ring with two heteroatoms, and so forth. The number
of carbon atoms plus the number of heteroatoms equals the total number of ring
atoms. A heterocyclyl ring can also include one or more double bonds. A
heteroaryl ring is an embodiment of a heterocyclyl group. The phrase
"heterocyclyl group" includes fused ring species including those that include
fused aromatic and non-aromatic groups.
[0035] The term "alkoxy" as used herein refers to an oxygen atom
connected to an alkyl group, including a cycloalkyl group, as are defined
herein.
Examples of linear alkoxy groups include but are not limited to methoxy,
ethoxy, propoxy, butoxy, pentyloxy, hexyloxy, and the like. Examples of
branched alkoxy include but are not limited to isopropoxy, sec-butoxy, tert-
butoxy, isopentyloxy, isohexyloxy, and the like. Examples of cyclic alkoxy
include but are not limited to cyclopropyloxy, cyclobutyloxy, cyclopentyloxy,
cyclohexyloxy, and the like. An alkoxy group can include one to about 12-20 or
about 12-40 carbon atoms bonded to the oxygen atom, and can further include
double or triple bonds, and can also include heteroatoms. For example, an
allyloxy group is an alkoxy group within the meaning herein. A methoxyethoxy
8
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
group is also an alkoxy group within the meaning herein, as is a
methylenedioxy
group in a context where two adjacent atoms of a structure are substituted
therewith.
[0036] The term "amine" as used herein refers to primary, secondary,
and tertiary amines having, e.g., the formula N(group)3 wherein each group can
independently be H or non-H, such as alkyl, aryl, and the like. Amines include
but are not limited to R-NH2, for example, alkylamines, arylamines,
alkylarylamiams; R2NH wherein each R is independently selected, such as
dialkylamines, diarylamines, aralkylamines, heterocyclylamines and the like;
and R3N wherein each R is independently selected, such as trialkylamines,
dialkylarylamines, alkyldiarylamines, triarylamines, and the like. The term
"amine" also includes ammonium ions as used herein.
[0037] The term "amino group" as used herein refers to a substituent of
the form -NH2, -NHR, -NR2, -NR3+, wherein each R is independently selected,
and protonated forms of each, except for -NR3+, which cannot be protonated.
Accordingly, any compound substituted with an amino group can be viewed as
an amine. An "amino group" within the meaning herein can be a primary,
secondary, tertiary, or quaternary amino group. An "alkylamino" group includes
a monoalkylamino, dialkylamino, and trialkylamino group.
[0038] The terms "halo," "halogen," or "halide" group, as used herein,
by themselves or as part of another substituent, mean, unless otherwise
stated, a
fluorine, chlorine, bromine, or iodine atom.
[0039] The term "haloalkyl" group, as used herein, includes mono-halo
alkyl groups, poly-halo alkyl groups wherein all halo atoms can be the same or
different, and per-halo alkyl groups, wherein all hydrogen atoms are replaced
by
halogen atoms, such as fluor . Examples of haloalkyl include trifluoromethyl,
1,1-dichloroethyl, 1,2-dichloroethyl, 1,3-dibromo-3,3-difluoropropyl,
perfluorobutyl, and the like.
[0040] The term "hydrocarbon" as used herein refers to a functional
group or molecule that includes carbon and hydrogen atoms. The term can also
refer to a functional group or molecule that normally includes both carbon and
hydrogen atoms but wherein all the hydrogen atoms are substituted with other
functional groups.
9
CA 02938279 2016-07-28
WO 2015/138018 PCT/U52014/069506
[0041] As used herein, the term "hydrocarbyl" refers to a functional
group derived from a straight chain, branched, or cyclic hydrocarbon, and can
be
alkyl, alkenyl, alkynyl, aryl, cycloalkyl, acyl, or any combination thereof,
[0042] The term "solvent" as used herein refers to a liquid that can
dissolve a solid, liquid, or gas. Nonlimiting examples of solvents are
silicones,
organic compounds, water, alcohols, ionic liquids, and supercritical fluids.
[0043] The term "number-average molecular weight" as used herein
refers to the ordinary arithmetic mean of the molecular weight of individual
molecules in a sample. It is defined as the total weight of all molecules in a
sample divided by the total number of molecules in the sample. Experimentally,
the number-average molecular weight (Ma) is determined by analyzing a sample
divided into molecular weight fractions of species i having ni molecules of
molecular weight 1\41 through the formula MT, = ZMini / En, The number-average
molecular weight can be measured by a variety of well-known methods
including gel permeation chromatography, spectroscopic end group analysis, and
osmometry. If unspecified, molecular weights of polymers given herein are
number-average molecular weights.
[0044] The term "weight-average molecular weight" as used herein
refers to IN/Iõõ which is equal to EIVI?ni / IMini, where ni is the number of
molecules of molecular weight M. In various examples, the weight-average
molecular weight can be determined using light scattering, small angle neutron
scattering, X-ray scattering, and sedimentation velocity.
[0045] The term "room temperature" as used herein refers to a
temperature of about 15 C to 28 C.
[0046] The term "standard temperature and pressure" as used herein
refers to 20 C and 101 kPa.
[0047] As used herein, "degree of polymerization" is the number of
repeating units in a polymer.
[0048] As used herein, the term "polymer" refers to a molecule having at
least one repeating unit and can include copolymers.
[0049] The term "copolymer" as used herein refers to a polymer that
includes at least two different monomers. A copolymer can include any suitable
number of monomers.
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
[0050] The term "downhole" as used herein refers to under the surface of
the earth, such as a location within or fluidly connected to a wellbore.
[0051] As used herein, the term "drilling fluid" refers to fluids,
slurries,
or muds used in drilling operations downhole, such as during the formation of
the wellbore.
[0052] As used herein, the term "stimulation fluid" refers to fluids or
slurries used downhole during stimulation activities of the well that can
increase
the production of a well, including perforation activities. In some examples,
a
stimulation fluid can include a fracturing fluid or an acidizing fluid.
[0053] As used herein, the term "clean-up fluid" refers to fluids or
slurries used downhole during clean-up activities of the well, such as any
treatment to remove material obstructing the flow of desired material from the
subterranean formation. In one example, a clean-up fluid can be an
acidification
treatment to remove material formed by one or more perforation treatments. In
another example, a clean-up fluid can be used to remove a filter cake.
[0054] As used herein, the term "fracturing fluid" refers to fluids or
slurries used downhole during fracturing operations.
[0055] As used herein, the term "spotting fluid" refers to fluids or
slurries used downhole during spotting operations, and can be any fluid
designed
for localized treatment of a downhole region. In one example, a spotting fluid
can include a lost circulation material for treatment of a specific section of
the
wellbore, such as to seal off fractures in the wellbore and prevent sag. In
another example, a spotting fluid can include a water control material. In
some
examples, a spotting fluid can be designed to free a stuck piece of drilling
or
extraction equipment, can reduce torque and drag with drilling lubricants,
prevent differential sticking, promote wellbore stability, and can help to
control
mud weight.
[0056] As used herein, the term "completion fluid" refers to fluids or
slurries used downhole during the completion phase of a well, including
cementing compositions.
[0057] As used herein, the term "remedial treatment fluid" refers to
fluids or slurries used downhole for remedial treatment of a well. Remedial
treatments can include treatments designed to increase or maintain the
production rate of a well, such as stimulation or clean-up treatments.
11
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
[0058] As used herein, the term "abandonment fluid" refers to fluids or
slurries used downhole during or preceding the abandonment phase of a well.
[0059] As used herein, the term "acidizing fluid" refers to fluids or
slurries used downhole during acidizing treatments. In one example, an
acidizing fluid is used in a clean-up operation to remove material obstructing
the
flow of desired material, such as material formed during a perforation
operation.
In some examples, an acidizing fluid can be used for damage removal.
[0060] As used herein, the term "cementing fluid" refers to fluids or
slurries used during cementing operations of a well. For example, a cementing
fluid can include an aqueous mixture including at least one of cement and
cement kiln dust. In another example, a cementing fluid can include a curable
resinous material such as a polymer that is in an at least partially uncured
state.
[0061] As used herein, the term "water control material" refers to a solid
or liquid material that interacts with aqueous material downhole, such that
hydrophobic material can more easily travel to the surface and such that
hydrophilic material (including water) can less easily travel to the surface.
A
water control material can be used to treat a well to cause the proportion of
water
produced to decrease and to cause the proportion of hydrocarbons produced to
increase, such as by selectively binding together material between water-
producing subterranean formations and the wellbore while still allowing
hydrocarbon-producing formations to maintain output.
[0062] As used herein, the term "packing fluid" refers to fluids or
slurries that can be placed in the annular region of a well between tubing and
outer casing above a packer. In various examples, the packing fluid can
provide
hydrostatic pressure in order to lower differential pressure across the
sealing
element, lower differential pressure on the wellbore and casing to prevent
collapse, and protect metals and elastomers from corrosion.
[0063] As used herein, the term "fluid" refers to liquids and gels, unless
otherwise indicated.
[0064] As used herein, the term "subterranean material" or "subterranean
formation" refers to any material under the surface of the earth, including
under
the surface of the bottom of the ocean. For example, a subterranean formation
or
material can be any section of a wellbore and any section of a subterranean
petroleum- or water-producing formation or region in fluid contact with the
12
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
wellbore. Placing a material in a subterranean formation can include
contacting
the material with any section of a wellbore or with any subterranean region in
fluid contact therewith. Subterranean materials can include any materials
placed
into the wellbore such as cement, drill shafts, liners, tubing, or screens;
placing a
material in a subterranean formation can include contacting with such
subterranean materials. In some examples, a subterranean formation or material
can be any below-ground region that can produce liquid or gaseous petroleum
materials, water, or any section below-ground in fluid contact therewith. For
example, a subterranean formation or material can be at least one of an area
desired to be fractured, a fracture or an area surrounding a fracture, and a
flow
pathway or an area surrounding a flow pathway, wherein a fracture or a flow
pathway can be optionally fluidly connected to a subterranean petroleum- or
water-producing region, directly or through one or more fractures or flow
pathways.
[0065] As used herein, "treatment of a subterranean formation" can
include any activity directed to extraction of water or petroleum materials
from a
subterranean petroleum- or water-producing formation or region, for example,
including drilling, stimulation, hydraulic fracturing, clean-up, acidizing,
completion, cementing, remedial treatment, abandonment, and the like.
[0066] As used herein, a "flow pathway" downhole can include any
suitable subterranean flow pathway through which two subterranean locations
are in fluid connection. The flow pathway can be sufficient for petroleum or
water to flow from one subterranean location to the wellbore, or vice-versa. A
flow pathway can include at least one of a hydraulic fracture, a fluid
connection
across a screen, across gravel pack, across proppant, including across resin-
bonded proppara or proppant deposited in a fracture, and across sand. A flow
pathway can include a natural subterranean passageway through which fluids
can flow. In some embodiments, a flow pathway can be a water source and can
include water. In some embodiments, a flow pathway can be a petroleum source
and can include petroleum. In some embodiments, a flow pathway can be
sufficient to divert from a wellbore, fracture, or flow pathway connected
thereto
at least one of water, a downhole fluid, or a produced hydrocarbon.
[0067] As used herein "gpt" refers to gallons per thousand gallons.
13
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
[0068] In various embodiments, the present invention provides a method
of treating a subterranean formation. The method includes obtaining or
providing a composition including a crosslinkable ampholyte polymer. The
crosslinkable ampholyte polymer includes an ethylene repeating unit including
a
-C(0)NH2 group, an ethylene repeating unit including an -S(0)20R' group, and
an ethylene repeating unit including an -N-V23X- group. At each occurrence, RI
is independently selected from the group consisting of -H and a counterion. At
each occurrence, R2 is independently substituted or unsubstituted (C1-
C20)hydrocarbyl. At each occurrence, X- is independently a counterion. The
composition includes at least one crosslinker. The method includes placing the
composition in a subterranean formation.
[0069] In various embodiments, the present invention provides a method
of treating a subterranean formation. The method includes obtaining or
providing a composition. The composition includes a reaction product of a
mixture. The mixture includes a crosslinkable ampholyte polymer including an
ethylene repeating unit including a -C(0)NH2 group, an ethylene repeating unit
including an -S(0)20R' group, and an ethylene repeating unit including an -
N+1223X- group. At each occurrence, RI is independently selected from the
group
consisting of -H and a counterion. At each occurrence, R2 is independently
substituted or unsubstituted (Ci-C20)hydrocarbyl. At each occurrence, X- is
independently a counterion. The mixture also includes at least one cmsslinker.
The method also includes placing the composition in a subterranean formation.
[0070] In various embodiments, the present invention provides a method
of treating a subterranean formation. The method includes obtaining or
providing a composition including a crosslinkable ampholyte polymer. The
crosslinkable ampholyte polymer includes repeating units having the structure:
14
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
- - _
-n - -z
__________________________ 0 ______ 0 ______ 0
NH NH NH2
---==S-=- 0
IOR1 H30 ¨N-0 H3 Xe
CH3
At each occurrence, RI is independently selected from the group consisting of -
H
and a counterion. The repeating units are in a block, alternate, or random
configuration, and each repeating unit is independently in the orientation
shown
or in the opposite orientation. The crosslinkable ampholyte polymer has a
molecular weight of about 100,000 g/mol to about 20,000,000 g/mol. The
crosslinkable ampholyte polymer has about 30 wt% to about 50 wt% of the
ethylene repeating unit including the -C(0)NH2 group, about 5 wt% to about 15
wt% of the ethylene repeating unit including the -S(0)20R1 group, and about 40
wt% to about 60 wt% of the ethylene repeating unit including the -INFIR23X-
group. The composition also includes a crosslinker including
polyethyleneimine. The composition also includes a downhole fluid including at
least one of a drilling fluid, a fracturing fluid, a diverting fluid, and a
lost
circulation treatment fluid. The method also includes placing the composition
in
a subterranean formation. About 0.001 wt% to about 30 v/v% of the
composition is the crosslinkable ampholyte polymer and the crosslinker.
[0071] In various embodiments, the present invention provides a system.
The system includes a composition including a crosslinkable ampholyte
polymer. The crosslinkable ampholyte polymer has about Z" wt% of an
ethylene repeating unit including the -C(0)NH2 group, about N 4 wt% of an
ethylene repeating unit including a -S(0)20R1 group, and about M" wt% of an
ethylene repeating unit including an -N+R23X- group. At each occurrence 121 is
independently selected from the group consisting of -H and a counterion. At
each occurrence, R2 is independently substituted or unsubstitutecl (C1-
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
C20)hydrocarbyl. At each occurrence, X is independently a counterion. The
repeating units are in block, alternate, or random configuration. The variable
Z"
is about 10% to about 70%, N" is about 1% to about 40%, and M" is about 20%
to about 80%. The crosslinkable ampholyte polymer has a molecular weight of
about 100,000 g/mol to about 20,000,030 g/mol. The composition also includes
at least one crosslinker. The system also includes a subterranean formation
including the composition therein.
[0072] In various embodiments, the present invention provides a
composition for treatment of a subterranean formation. The composition
includes a crosslinkable ampholyte polymer having about Z" wt% of an
ethylene repeating unit including the -C(0)NH2 group, about N" wt% of an
ethylene repeating unit including a -S(0)20R1 group, and about M" wt% of an
ethylene repeating unit including an -1\11R23X- group. At each occurrence R1
is
independently selected from the group consisting of -H and a counterion. At
each occurrence, R2 is independently substituted or unsubstitutal (Ci-
C20)hydrocarbyl. At each occurrence, X is independently a counterion. The
repeating units are in block, alternate, or random configuration. The variable
Z"
is about 10% to about 70%, N" is about 1% to about 40%, and M" is about 20%
to about 80%. The crosslinkable ampholyte polymer has a molecular weight of
about 100,000 g/mol to about 20,000,000 g/mol. The composition includes at
least one crosslinker. The composition also includes a downhole fluid.
[0073] In various embodiments, the present invention provides a
composition for treatment of a subterranean formation. The composition
includes a reaction product of a mixture. The mixture includes a crosslinkable
ampholyte polymer having about Z" wt% of an ethylene repeating unit
including the -C(0)N112 group, about N" wt% of an ethylene repeating unit
including a -S(0)20121 group, and about M" wt% of an ethylene repeating unit
including an -N1R.23X group. At each occurrence R1 is independently selected
from the group consisting of -H and a counterion. At each occurrence, R2 is
independently substituted or unsubstituted (Ci-C20)hydrocarbyl. At each
occurrence, X is independently a counterion. The repeating units are in block,
alternate, or random configuration. The variable Z" is about 10% to about 70%,
N" is about 1% to about 40%, and M" is about 20% to about 80%. The
crosslinkable ampholyte polymer has a molecular weight of about 100,000 g/mol
16
CA 02938279 2016-07-28
WO 2015/138018
PCT/U52014/069506
to about 20,000,000 g/mol. The mixture also includes at least one crosslinker.
The composition also includes a downhole fluid.
[0074] In various embodiments, the present invention provides a
composition for treatment of a subterranean formation. The composition
includes a crosslinkable ampholyte polymer including repeating units having
the
structure:
___________________________ 0 _____ 0
NH NH NH2
0=s =z0 ED
H3C¨N¨C H3
I Xe
OR1
CH3
At each occurrence 121 is independently selected from the group consisting of -
H
and a counterion. The repeating units are in a block, alternate, or random
configuration, and each repeating unit is independently in the orientation
shown
or in the opposite orientation. The crosslinkable ampholyte polymer has a
molecular weight of about 100,000 g/mol to about 20,000,000 g/mol. The
crosslinkable ampholyte polymer has about 30 wt% to about 50 wt% of the
ethylene repeating unit including the -C(0)NH2 group, about 5 wt% to about 15
wt% of the ethylene repeating unit including the -S(0)20R1 group, and about 40
wt% to about 60 wt% of the ethylene repeating unit including the -N+1223X-
group. The composition includes a crosslinker including polyethyleneimine.
The composition also includes a downhole fluid including at least one of a
drilling fluid, a fracturing fluid, a diverting fluid, and a lost circulation
treatment
fluid. About 0.001 wt% to about 30 v/v% of the composition is the
crosslinkable
ampholyte polymer and the crosslinkcr.
[0075] In various embodiments, the present invention provides a
method
of preparing a composition for treatment of a subterranean formation. The
method includes forming a composition including a crosslinkable ampholyte
17
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
polymer including an ethylene repeating unit including a -C(0)NH2 group, an
ethylene repeating unit including an -S(0)20R1 group, and an ethylene
repeating
unit including an -N+1223X" group. At each occurrence, le is independently
selected from the group consisting of -H and a counterion. At each occurrence,
R2 is independently substituted or unsubstituted (Ci-C20)hydrocarbyl. At each
occurrence, X- is independently a counterion. The composition also includes at
least one crosslinker.
[0076] Various embodiments of the present invention provide certain
advantages over other compositions including viscosifiers and methods of using
the same, at least some of which are unexpected. For example, in some
embodiments, the uncrosslinked crosslinkable ampholyte polymer can act as a
friction reducer before crosslinking (e.g., for sfickwater fracturing or other
uses),
and can act as a viscosifier after crosslinking. In some embodiments, the
uncrosslinked crosslinkable ampholyte polymer can act as a viscosifier before
crosslinking, and can provide an even greater viscosity increase or even a
solidification after crosslinking. In some embodiments, the ability of the
crosslinkable ampholyte polymer to provide multiple uses in addition to
viscosification or gelation in a subterranean formation, such as friction
reduction
and viscosification, can at least one of: simplify a subterranean operation,
reduce
transportation costs, reduce the costs of storing and blending multiple
materials
at a worksite, reduce the amount of equipment needed at a worksite (e.g.,
reduce
footprint), and reduce the equipment cost overall.
[0077] In some embodiments, the crosslinkable ampholyte polymer can
provide a greater increase in viscosity of a downhole fluid per mass (e.g.,
via at
least partially crosslinking the crosslinkable ampholyte polymer) than other
viscosifiers. Compared to the viscosity of a downhole fluid having a given
concentration of a viscosifier (or, e.g., a downhole fluid formed by at least
partially crosslinking a given concentration of a viscosifier), a
corresponding
downhole fluid having the same or lower concentration of various embodiments
of the crosslinkable ampholyte polymer (or, e.g., formed by at least partially
crosslinking the crosslinkablc ampholyte polymer) can have a higher viscosity.
In some embodiments, by enabling a higher viscosity with the use of less
viscosifier, the crosslinkable ampholyte polymer can provide lower
18
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
transportation costs and shorter preparation time, making operations more
efficient overall.
[0078] In various embodiments, the crosslinkable ampholyte polymer
can be less expensive per unit mass as compared to conventional viscosifiers.
In
various embodiments, the crosslinkable ampholyte polymer can provide a
greater viscosity increase or a higher gel strength per unit cost as compared
to
other viscosifiers. In various embodiments, the crosslinkable ampholyte
polymer can provide a greater viscosity increase or a higher gel strength per
unit
cost in the presence of various salts or under high temperature conditions, as
compared to other viscosifiers.
[0079] Conventional viscosifiers provide viscosification of a
composition before and during transport to a desired location in a
subterranean
formation, requiring the energy-intensive pumping of a high viscosity
composition through tubular conduits to reach the desired location in the
subterranean formation. In various embodiments, the crosslinkable ampholyte
polymer partially or fully avoids providing a viscosity increase until the
composition reaches or becomes near a desired subterranean location, and in
some embodiments provides a reduction in friction en route to the desired
location. In various embodiments, the viscosity increase provided by the
crosslinkable ampholyte polymer can he triggered by heat, such as the higher
temperature of the desired location in a subterranean formation. In various
embodiments, by delaying the viscosity increase, the crosslinkable ampholyte
polymer can provide a more efficient method of providing high viscosity
compositions to a desired location in a subterranean formation. In some
embodiments, the crosslinkable ampholyte polymer can be optimized for use at a
particular temperature by varying the structure or concentration of at least
one of
the viscosifier and the crosslinker to provide a desired viscosity in a
desired
location.
[0080] Many conventional viscosifiers suffer a decrease in the viscosity
or gel strength provided when used under high temperature conditions such as
the conditions found in many subterranean formations. In some embodiments,
under high temperature conditions, the crosslinkable ampholyte polymer can
provide a higher viscosity or higher gel strength, or can provide less or no
decrease in viscosity or gel strength, as compared to the viscosity provided
by
19
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
other conventional viscosifiers under corresponding conditions. In various
embodiments, the higher temperature stability of the crosslinkable ampholyte
polymer can allow a desired level of viscosification or gelation with the use
of
less viscosifier, or can allow a higher viscosity or gel strength to be
achieved in a
subterranean formation, as compared to other conventional viscosifiers,
thereby
providing a more versatile, more cost effective, or more efficient
viscosification
or gelation in the subterranean formation than other methods and compositions.
[00811 Many conventional viscosifiers suffer a decrease in the viscosity
or gel strength provided when used with liquids such as water having certain
ions present at particular concentrations. For example, many viscosifiers
suffer
a decrease in the viscosity or gel strength provided when used with liquids
having certain amounts of salts dissolved therein such as sodium chloride or
potassium chloride. In some embodiments, the crosslinkable ampholyte polymer
can be used with liquids having ions dissolved therein and can suffer less or
no
negative effects from the ions, as compared to conventional methods and
compositions for use in subterranean formations, such as less or no decrease
in
the viscosity provided. By being able to retain the viscosity or gel strength
provided or suffer less reduction in viscosity or gel strength in the presence
of
various ions or in the presence of larger amounts of particular ions than
other
methods and compositions, various embodiments can avoid the need for ion-free
or ion-depleted water, or can avoid a need to add greater amounts of
viscosifier
to achieve a desired effect in a subterranean formation, and can thereby be
more
versatile, more cost effective, or more efficient than other methods and
compositions for subterranean use.
[0082] In various embodiments, by providing a higher viscosity or higher
gel strength under high temperature conditions or high salinity conditions,
the
crosslinkable ampholyte polymer can provide a more effective subterranean or
downhole fluid, such as a more effective drilling fluid that has greater
cutting
carrying capacity, sag resistance, or equivalent circulating density, a more
effective hydraulic fracturing fluid that can more effectively carry proppant
or
form more dominant fractures, or a more effective divertcr or lost circulation
material that more effectively seals off flow pathways or controls
permeability.
In various embodiments, by providing a higher viscosity or gel strength under
high temperature conditions or high salinity conditions, the crosslinkable
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
ampholyte polymer can provide a more effective sweeping agent (e.g., for
removing cuttings from the wellbore), improved equivalent circulating density
management, and improved fluid loss control (e.g., the higher viscosity can
reduce fluid flow in pore spaces).
Method of treating a subterranean formation.
[0083] In various embodiments, the present invention provides a method
of treating a subterranean formation. In some embodiments, the method
includes obtaining or providing a composition including a crosslinker and a
crosslinkable ampholyte polymer including an ethylene repeating unit including
a -C(0)NH2 group, an ethylene repeating unit including an -S(0)20R1 group,
and an ethylene repeating unit including an -WR23X- group. At each
occurrence, Ri can be independently selected from the group consisting of -H
and a counterion. At each occurrence, R2 can be independently substituted or
unsubstituted (Ci-C20)hydrocarbyl, and at each occurrence, X- can be
independently a counterion. In some embodiments, the method includes
obtaining or providing a composition including a reaction product of a
crosslinker and the crosslinkable ampholyte polymer (e.g., a product of a
crosslinking reaction between the crosslinker and the crosslinkable ampholyte
polymer). The obtaining or providing of the composition can occur at any
suitable time and at any suitable location. The obtaining or providing of the
composition can occur above the surface. The obtaining or providing of the
composition can occur in the subterranean formation (e.g., downhole). The
method also includes placing the composition in a subterranean formation. The
placing of the composition in the subterranean formation can include
contacting
the composition and any suitable part of the subterranean formation, or
contacting the composition and a subterranean material, such as any suitable
subterranean material. The subterranean formation can be any suitable
subterranean formation. In some embodiments, the method is a method of
drilling the subterranean formation. In some embodiments, the method is a
method of fracturing the subterranean formation. For example, the composition
can be used as or with a drilling fluid, a hydraulic fracturing fluid, a
diverting
fluid, and a lost circulation treatment fluid.
21
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
[0084] In some examples, the placing of the composition in the
subterranean formation (e.g., downhole) includes contacting the composition
with or placing the composition in at least one of a fracture, at least a part
of an
area surrounding a fracture, a flow pathway, an area surrounding a flow
pathway, and an area desired to be fractured. The placing of the composition
in
the subterranean formation can be any suitable placing and can include any
suitable contacting between the subterranean formation and the composition.
The placing of the composition in the subterranean formation can include at
least
partially depositing the composition in a fracture, flow pathway, or area
surrounding the same.
[0085] The method can include hydraulic fracturing, such as a method
of
hydraulic fracturing to generate a fracture or flow pathway. The placing of
the
composition in the subterranean formation or the contacting of the
subterranean
formation and the hydraulic fracturing can occur at any time with respect to
one
another; for example, the hydraulic fracturing can occur at least one of
before,
during, and after the contacting or placing. In some embodiments, the
contacting or placing occurs during the hydraulic fracturing, such as during
any
suitable stage of the hydraulic fracturing, such as during at least one of a
pre-pad
stage (e.g., during injection of water with no proppant, and additionally
optionally mid- to low-strength acid), a pad stage (e.g., during injection of
fluid
only with no proppant, with some viscosifier, such as to begin to break into
an
area and initiate fractures to produce sufficient penetration and width to
allow
proppant-laden later stages to enter), or a slurry stage of the fracturing
(e.g.,
viscous fluid with proppant). The method can include performing a stimulation
treatment at least one of before, during, and after placing the composition in
the
subterranean formation in the fracture, flow pathway, or area surrounding the
same. The stimulation treatment can be, for example, at least one of
perforating,
acidizing, injecting of cleaning fluids, propellant stimulation, and hydraulic
fracturing. In some embodiments, the stimulation treatment at least partially
generates a fracture or flow pathway where the composition is placed or
contacted, or the composition is placed or contacted to an area surrounding
the
generated fracture or flow pathway.
[0086] The method can include diverting or fluid loss control. The
composition can be delivered to the subterranean formation to a flowpath
22
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
causing fluid loss or undesired introduction of water. The composition can be
crosslinked, such that the flowpath is at least partially sealed by the
reaction
product of the ampholyte polymer and the crosslinker, at least partially
stopping
fluid loss or preventing water from entering the wellbore and contaminating
fluids such as production fluids.
[0087] In some embodiments, in addition to the crosslinkable
ampholyte
polymer and the crosslinker, or a reaction product thereof, the composition
can
include an aqueous liquid. The method can further include mixing the aqueous
liquid with the polymer viscosifler. The mixing can occur at any suitable time
and at any suitable location, such as above surface or in the subterranean
formation. The aqueous liquid can be any suitable aqueous liquid, such as at
least one of water, brine, produced water, flowback water, brackish water, and
sea water. In some embodiments, the aqueous liquid can include at least one of
an aqueous drilling fluid, aqueous fracturing fluid, aqueous diverting fluid,
and
an aqueous fluid loss control fluid. In some embodiments, the aqueous liquid
can be the aqueous phase of an emulsion (e.g., the composition can include an
emulsion having as the aqueous phase the aqueous liquid).
[0088] The composition can include any suitable proportion of the
aqueous liquid, such that the composition can be used as described herein. For
example, about 0.000,1 wt% to 99.999,9 wt% of the composition can be the
aqueous liquid, or about 0.01 wt% to about 99.99 wt%, about 0.1 wt% to about
99.9 wt%, or about 20 wt% to about 90 wt%, or about 0.000,1 wt% or less, or
about 0.000,001 wt%, 0.000,1, 0.001, 0.01, 0.1, 1,2, 3,4, 5, 10, 15, 20,
30,40,
50, 60, 70, 80, 85, 90, 91, 92, 93, 94, 95, 96,97, 98, 99, 99.9, 99.99, 99.999
wt%, or about 99.999,9 wt% or more of the composition can be the aqueous
liquid.
[0089] The aqueous liquid be a salt water. The salt can be any
suitable
salt, such as at least one of NaBr, CaCl2, CaBr2, ZnBr2, KO, NaC1, a magnesium
salt, a bromide salt, a formate salt, an acetate salt, and a nitrate salt. The
crosslinkable ampholyte polymer and crosslinker can effectively provide
increased viscosity in aqueous solutions having various total dissolved solids
levels, or having various ppm salt concentrations. The crosslinkable ampholyte
polymer and crosslinker can provide effective increased viscosity of a salt
water
having any suitable total dissolved solids level (e.g., wherein the dissolved
solids
23
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
correspond to dissolved salts), such as about 1,000 mg/L to about 250,000
mg/L,
or about 1,000 mg/L or less, or about 5,000 mg,/L, 10,0(0, 15,000, 20,000,
25,000,30,000, 40,000, 50,000, 75,000, 100,000, 125,000, 150,000, 175,000,
200,000, 225,000, or about 250,000 mg/L or more. The crosslinkable ampholyte
polymer and crosslinker can provide effective increased viscosity of a salt
water
having any suitable salt concentration, such as about 1,000 ppm to about
300,000 ppm, or about 1,000 ppm to about 150,000 ppm, or about 1,000 ppm or
less, or about 5,000 ppm, 10,000, 15,000, 20,000, 25,000,30,000, 40,030,
50,000,75,000, 100,000, 125,000, 150,000, 175,000, 200,000, 225,000, 250,000,
275,000, or about 300,000 ppm or more. In some examples, the aqueous liquid
can have a concentration of at least one of NaBr, CaC12, CaBr2, ZnBr2, KC1,
and
NaC1 of about 0.1% w/v to about 20% w/v, or about 0.1% w/v or less, or about
0.5% w/v, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19,
20, 21,
22, 23, 24, 25,26, 27, 28, 29, or about 30% w/v or more.
[0090] The crosslinkable ampholyte polymer and crosslinker, or a
reaction product thereof, can be sufficient to provide effective increased
viscosity to an aqueous liquid at various high temperatures. For example, the
crosslinkable ampholyte polymer and crosslinker, or a reaction product
thereof,
can provide effective increased viscosity at up to about 500 F, or up to
about
490 F, 480, 470, 460, 450, 440, 430, 420, 410, 400, 390, 380, 370, 360, 350,
340, 330, 320, 310, 300, 290, 280, 270, 260, 250, 240, 230, 220, 210, 200,
190,
180, 170, 160, 150 , 140, 130, 120, 110, or up to about 100 F.
[0091] The method can include at least partially crosslinking the
crosslinkable ampholyte polymer to provide a crosslinked ampholyte polymer.
The crosslinking can include at least partially reacting the crosslinkable
ampholyte polymer with at least the crosslinker to provide an at least
partially
crosslinked ampholyte polymer. The crosslinking can occur in any suitable
location and at any suitable time. For example, the crosslinking can occur
above-surface, in the subterranean formation, or a combination thereof. In
some
embodiments, the crosslinking can be triggered by a suitable event, for
example,
chemical triggering (e.g., contacting with one or more chemicals that initiate
or
catalyze the crosslinking reaction), temperature triggering (e.g., raising the
temperature of the composition such that the crosslinking reaction occurs), or
a
combination thereof.
24
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
[0092] Temperature triggering can include exposing the composition to
suitably high temperature in the subterranean formation wherein a higher
viscosity is desired. Temperature-triggered erosslinking can include exposing
the composition to a temperature of about 100 F to about 500 F, 125 F to
about 350 F, 125 'V to about 250 F, 175 F to about 250 F, or about 450 F
or
more, or about 440 F, 430, 420, 410, 400, 390, 380, 370, 360, 350, 340, 330,
320, 310, 300, 290, 280, 270, 260, 250, 240, 230, 220, 210, 200, 190, 180,
170,
160, 150, 140, 130, 120, 110, or about 100 F or less.
[0093] The composition can have any suitable viscosity above surface
and in the subterranean formation, such that the composition can be used as
described herein. The viscosity can he affected by any suitable component,
such
as one or more crosslinkable ampholyte polymers, one or more crosslinkers, one
or more crosslinked products of the crosslinkable ampholyte polymer and the
crosslinker, one or more secondary viscosifiers, one or more secondary
crosslinkers, one or more crosslinked products of a secondary viscosifier and
a
secondary crosslinker, or any combination thereof. In some embodiments, the
viscosity is affected by one or more crosslinked products of the crosslinkable
ampholyte polymer. In some embodiments, the viscosity of the composition, at
standard temperature and pressure and at a shear rate of about 50 s-I to about
500
or about 50 s or less to about 1000 s-I or more, is about 0.01 cP to about
10,000,000 cP, or about 0.01 cP or less, or about 0.1 cP, 1, 2, 3, 4, 5, 10,
15, 20,
25, 50,75, 100, 150, 200, 250, 500, 750, 1,000, 1,250, 1,500,2,000, 2,500,
5,000, 10,000, 15,000,20,000, 25,000, 50,000, 75,000, 100,000, 125,000,
150,000, 175,000, 200,000, 225,000, 250,000, 500,000, 1,000,000, 1,250,000,
1,500,000, 2,000,000, 2,500,000, 5,000,000, 7,500,000, or about 10,000,000 cP
or more. In some embodiments, the viscosity of the composition, at standard
temperature and pressure and at a shear rate of about 0 s4 to about 1 s-I, or
about
0.1 s1 or less to about 1 s-I or more, is about 0.01 cP to about 1,000,000 cP,
or
about 0.01 cP or less, or about 0.1 cP, 1, 2, 3, 4, 5, 10, 15,20, 25, 50, 75,
100,
150, 200, 250, 500, 750, 1,000, 1,250, 1,500, 2,000, 2,500, 5,000, 10,000,
15,000, 20,000, 25,000, 50,000, 75,000, 100,000, 125,000, 150,000, 175,000,
200,000, 225,000, 250,000, 500,000, 1.000,000, 1,250,000, 1,500,000,
2,000,000, 2,500,000, 5,000,000, 7,500,000, or about 10,000,000 cP or more.
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
[0094] Prior to the at least partial crosslinking of the composition, the
composition can have any suitable viscosity. In some embodiments, the
viscosity of the composition, at standard temperature and pressure and at a
shear
rate of about 50 s-1 to about 500 s-1, or about 50 s-1 or less to about 1000 s-
1 or
more, is about 0.01 cP to about 1,000,000 cP, about 0.01 cP to about 10,000
cP,
or about 0.01 cP or less, or about 0.1 cP, 1,2, 3, 4, 5, 10, 15, 20, 25, 50,
75, 100,
150, 200, 250, 503, 750, 1,000, 1,250, 1,500, 2,000, 2,500, 5,000, 10,000,
15,000,20,000, 25,000, 50,000, 75,000, 100,003, 125,000, 150,000, 175,000,
200,000, 225,000, 250,000, 500,000, or about 1,000,000 cP or more.
[0095] After the at least partial crosslinking of the composition, the
composition can have any suitable viscosity. In some embodiments, after the
crosslinking, the viscosity of the composition, at standard temperature and
pressure and at a shear rate of about 50 s-1 to about 500 s-1, or about 50 s-1
or less
to about 1000 s-1 or more, can be about 10 cP to about 10,000,000 cP (e.g.,
the
composition can be a gel with essentially infinite viscosity), about 1,000 cP
to
about 500,000 cP, or about 10 cP or less, or about 15 cP, 20, 25, 50, 75, 100,
150, 200, 250, 500, 750, 1,000, 1,250, 1,500, 2,000, 2,500, 5,000, 10,000,
15,000,20,000, 25,000, 50,000, 75,003, 100,000, 125,000, 150,000, 175,000,
200,000,225,000, 250,000,500,000, 1,000,000, 1,250,000, 1,500,000,
2,000,000, 2,500,030, 5,000,000, 7,503,000, or about 10,000,000 cP or more. In
some embodiments, after the crosslinking, the viscosity of the composition, at
standard temperature and pressure and at a shear rate of about 0 s-1 to about
1 s-1,
or about 0.1 s-1 or less to about 1 s-1 or more, can be about 10 cP to about
1,000,000 cP, about 1,000 cP to about 500,000 cP, or about 10 cP or less, or
about 15 cP, 20, 25, 50, 75, 100, 150, 200, 250, 500, 750, 1,000, 1,250,
1,500,
2,000,2,500, 5,000, 10,000, 15,003, 20,000, 25,000, 50,000, 75,000, 100,000,
125,000, 150,000, 175,000,200,000, 225,000, 250,000,500,000, 1,000,000,
1,250,000, 1,500,000, 2,000,000, 2,500,030, 5,000,000,7,500,000, or about
10,000,000 cP or more.
[0096] After the at least partial crosslinking of the composition, the
composition can have any suitable shear stress (e.g., the composition can be a
gel with essentially infinite viscosity). In some embodiments, after the
crosslinking, the shear stress of the composition can be about 0.1 Pa to about
500,000 Pa, about 1 Pa to about 1,000 Pa, about 1 Pa to about 500 Pa, about
0.1
26
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
Pa or less, about 0.5 Pa, 1, 2, 3, 4, 5, 10, 15, 20, 25, 50, 75, 1(0, 150,
200, 250,
500, 750, 1,000, 1,250, 1,500, 1,750, 2,000,2,500, 5,000, 10,000, 20,000,
25,000, 50,090, 75,000, 100,000, 250,000, 500,000, 750,000, or about 1,000,000
Pa or more.
[0097] In some embodiments, the crosslinkable ampholyte polymer is
sufficient such that, when crosslinked in an aqueous solution at a
concentration
of about 40 gpt with a polyethyleneimine crosslinker at a concentration of
about
ppt to form a crosslinked ampholyte polymer, at 77 T and standard pressure,
with a strain of about 10%, at a frequency of about 0.1 rad/s to about 100
rad/s,
or about 0.1 rad/s or less to about 1000 rad/s or more, the aqueous solution
comprising the crosslinked ampholyte polymer has a loss modulus G" of about
0.1 Pa to about 1000 Pa, about 0.1 Pa to about 100 Pa, about 0.1 Pa to about
10
Pa, or about 0.1 Pa or less, or about 0.5 Pa, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10,
15, 20, 30,
40, 50, 60, 70, 80, 90, 100, 150, 200, 250, 300, 350, 400, 450, 500, 550, 600,
700, 800, 900, or about 1,000 Pa or more. In some embodiments, the
crosslinkable ampholyte polymer is sufficient such that, when crosslinked in
an
aqueous solution at a concentration of about 40 gpt with a polyethyleneimine
crosslinker at a concentration of about 10 ppt to form a crosslinked ampholyte
polymer, at 150 F and standard pressure, with a strain of about 10%, at a
frequency of about 0.1 rad/s to about 100 rad/s, or about 0.1 rad/s or less to
about 1000 rad/s or more, the aqueous solution comprising the crosslinked
polymer has a storage modulus G' of about 10 Pa to about 1000 Pa, or about 10
Pa to about 100 Pa, or about 10 Pa or less, or about 20 Pa, 30, 40, 50, 60,
70, 80,
90, 100, 150, 200, 250, 300, 350, 400, 450, 500, 550, 600, 700, 800, 900, or
about 1,000 Pa or more. In some embodiments, the crosslinkable ampholyte
polymer is sufficient such that, when crosslinked in an aqueous solution at a
concentration of about 40 gpt with a polyethyleneimine crosslinker at a
concentration of about 10 ppt to form a crosslinked ampholyte polymer, at 150
IF and standard pressure, with a strain of about 10%, at a frequency of about
0.1
rad/s to about 100 rad/s, or about 0.1 rad/s or less to about 1000 rad/s or
more,
the aqueous solution comprising the crosslinked polymer has a loss modulus G"
of about 0.5 Pa to about 10 Pa, or about 0.5 to about 5 Pa, or about 0.5 Pa or
less,
or about 1 Pa, 2, 3, 4, 5, 6, 7, 8, 9, or about 10 Pa or more.
27
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
Crosslinkable ampholyte polymer.
[0098] The composition includes at least one crosslinkable ampholyte
polymer, or a crosslinked reaction product thereof (e.g., a reaction product
of a
crosslinldng reaction between the crosslinkable ampholyte polymer and a
crosslinker). The crosslinkable ampholyte polymer can include an ethylene
repeating unit including a -C(0)N112 group, an ethylene repeating unit
including
an -S(0),0121 group, and an ethylene repeating unit including an -1\11Z23X-
group. At each occurrence, R1 can be independently selected from the group
consisting of -H and a counterion. At each occurrence, R2 can be independently
substituted or unsubstituted (CI-C20)hydrocarbyl, and at each occurrence, K
can
be independently a counterion.
[0099] Any suitable concentration of the crosslinkable ampholyte
polymer can be present in the composition, such that the composition can be
used as described herein. In some embodiments, about 0.001 wt% to about 95
wt% of the composition is the one or more crosslinkable ampholyte polymers, or
about 30 wt% to about 95 wt%, or about 70 wt% to about 90 wt%, or about
0.001 wt% or less, or about 0.01 wt%, 0.1, 1, 2, 3,4, 5, 10, 15, 20, 25, 30,
35,
40, 45, 50, 55, 60, 65, 70, 75, 80, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94,
95,96,
97, 98, 99, 99.9, 99.99, or about 99.999 wt% or more of the composition is the
one or more crosslinkable ampholyte polymers. In some examples, for a
composition including the crosslinkable ampholyte polymer and an aqueous
component, about 0.001 wt% to about 50 wt% of the composition is the one or
more crosslinkable ampholyte polymers, or about 0.01 wt% to about 10 wt% of
the composition, about 0.01 wt% to about 30 wt%, or about 0.001 wt% or less,
or about 0.01 wt%, 0.1, 1, 2, 3,4, 5, 10, 15, 20, 25, 30, 35, 40, 45, or about
50
wt% or more of the composition is the one or more crosslinkable ampholyte
polymers. In some examples, for a composition including the crosslinkable
ampholyte polymer or a reaction product thereof and an aqueous component,
about 0.001 vol% to about 30 vol% of the composition is the one or more
crosslinkable ampholyte polymers or a reaction product thereof, or is the
combined volume of the crosslinkablc ampholyte polymers and the crosslinker,
or about 0.001 vol% or less, or about 0.01 vol%, 0.1, 1, 2, 3, 4, 5, 6, 8, 10,
12,
14, 16, 18, 20, 22, 24, 26, 28, or about 30 vol% or more.
28
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
[0000] The crosslinkable ampholyte polymer can have about Z" wt% of
the ethylene repeating unit including the -C(0)NH2 group, wherein Zwt is any
suitable wt%, such as about 10% to about 70%, about 30% to about 50%, or
about 10% or less, or about 15%, 20,25, 30, 31, 32, 33, 34,35, 36, 37, 38, 39,
40, 41, 42, 43,44, 45, 46, 47, 48, 49, 50, 55, 60, 65%, or about 70% or more.
The crosslinkable ampholyte polymer can have about Zm0l mol% of the ethylene
repeating unit including the -C(0)NH2 group, wherein Z""lis any suitable mol%,
such as about 5% to about 50%, about 10% to about 25%, or about 5% or less, or
about 10%, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 30, 35,
40,
45, or about 50% or more.
[00101] The crosslinkable ampholyte polymer can have about N" wt% of
the ethylene repeating unit including the -S(0)20R1 group, wherein N" wt% is
any suitable wt%, such as about 1% to about 40%, 5% to about 15%, or about
1% or less, or about 5%, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 20, 25, 30, 35,
or about
40% or more. The crosslinkable ampholyte polymer can have about Nn'i mol%
of the ethylene repeating unit including the -S(0)20R1 group, wherein Nin01
mol% is any suitable mol%, such as about 1% to about 40%, 5% to about 20%,
or about 1% or less, 5%, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18,
19,20, 25,
30, 35, or about 40% or more.
[00102] The crosslinkable ampholyte polymer can have about M" wt% of
the ethylene repeating unit including the -Nr1223X- group, wherein M" wt% is
any suitable wt%, such as about 20% to about 80%, 40% to about 60%, or about
20% or less, 25%, 30, 35, 40, 41, 42, 43, 44,45, 46, 47, 48, 49, 50, 51, 52,
53,
54, 55, 56, 57, 58, 59, 60, 65, 70, 75, or about 80% or more. The
crosslinkable
ampholyte polymer can have about M' ' mol% of the ethylene repeating unit
including the -N-FR23X- group, wherein IVI'lmol% is any suitable mol%, such as
about 40% to about 90%, 55% to about 70%, or about 40% or less, 45, 50, 55,
56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 75, 80, 85, or
about 90%
or more.
[00103] In various embodiments, the crosslinkable ampholyte polymer is
a terpolymcr, e.g., Z" + NM + M" is about 100%, and Zni01+ 1\1'1+ Mm0l is
about 100%.
[00104] The crosslinkable ampholyte polymer can have any suitable
molecular weight, such as about 100,000 g/mol to about 20,000,000 g/mol,
29
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
2,000,000 g/mol to about 20,000,000 g/mol, about 5,000,000 g/mol to about
15,000,000 g/mol, or about 100,000 g/mol or less, or about 200,000 g/mol,
300,000,400,000, 500,000,750,000, 1,000,000, 2,000,000,3,000,000,
4,000,000, 6,000,000, 8,000,000, 10,000,000, 12,000,000, 14,000,000,
16,000,000, 18,000,000, or about 20,000,000 g/mol or more.
[00105] In various embodiments, the crosslinkable ampholyte polymer
includes repeating units having the structure:
_ _
R3 R3 R3
R5 R5 R5
R4 R4 R4
L2
0=-S=--0 R2-N9--R2
I Xe
OR1 R2 NH2
The repeating units are in a block, alternate, or random configuration, and
each
repeating unit is independently in the orientation shown or in the opposite
orientation.
[00106] At each occurrence, RI can be independently selected from the
group consisting of -H and a counterion. At each occurrence RI can be
independently selected from the group consisting of -H, Na, IC+, Lit, NH4,
Znt,
Calf, Zn2+, A13+, and Mg2+. At each occurrence, R' can be -H.
[00107] At each occurrence, R2 can be independently substituted or
unsubstituted (Ci-C20)hydrocarbyl. At each occurrence R2 can be independently
(C1-C20)alkyl. At each occurrence R2 can be independently (Ci-Cio)alkyl. At
each occurrence R2 can be independently selected from the group consisting of
methyl, ethyl, propyl, butyl, and pentyl. At each occurrence, R2 can be
methyl.
[00108] At each occurrence, X- can independently be a counterion. For
example, the counterion can be a halide, such as fluoro, chloro, iodo, or
bromo.
In other examples, the counterion can be nitrate, hydrogen sulfate, dihydrogen
phosphate, bicarbonate, nitrite, perchlorate, iodate, chlorate, bromate,
chlorite,
hypochlorite, hypobroinite, cyanide, amide, cyanate, hydroxide, permanganate.
The counterion can be a conjugate base of any carboxylic acid, such as acetate
or
formate. In some embodiments, a counterion can have a negative charge greater
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
than -1, which can in some embodiments complex to multiple ionized groups,
such as oxide, sulfide, nitride, arsenate, phosphate, arsenite, hydrogen
phosphate,
sulfate, thiosulfate, sulfite, carbonate, chromate, dichromate, peroxide, or
oxalate. At each occurrence, X- can be cr.
[00109] At each occurrence R3, R4, and R5 can each independently be
selected from the group consisting of -H and a substituted or unsubstituted C1-
05
hydrocarbyl. At each occurrence R3, R4, and R5 can be independently selected
from the group consisting of -H and a C1-05 alkyl. At each occurrence R3, R4,
and R5 can be independently selected from the group consisting of -H and a Cr
C3 alkyl (e.g., methyl, ethyl, or propyl). At each occurrence R3, R4, and R5
can
be each -H.
[00110] At each occurrence L1, L2, and L3 can be each independently
selected from the group consisting of a bond and a substituted or
unsubstituted
Ci-C20 hydrocarbyl interrupted or terminated with 0, 1, 2, or 3 of at least
one of -
NR3-, -S-, and -0,
[00111] At each occurrence Ll can be independently selected from the
group consisting of a bond and -(substituted or unsubstituted C1-C20
hydrocarbyl)-NR3-(substituted or unsubstituted C1-C20 hydrocarbyl)-. At each
occurrence LI can be independently -C(0)-NH-(substituted or unsubstituted
C19 hydrocarbyl)-. At each occurrence LI can be independently -C(0)-NH-(Ci-
05 hydrocarbyl)-. The variable L1 can be -C(0)-NH-CH(CH3)2-CH2-.
[00112] At each occurrence, L2 can be independently selected from the
group consisting of -0-(CI-C20)hydrocarbyl- and -NR3-(CI-C20)hydrocarbyl-. At
each occurrence, L2 can be independently selected from -0-(Ci-Cio)alkyl- and -
NH-(C1-C10)alkyl-. At each occurrence, L2 can be independently selected from -
0-CH2-CH2- and -NH-CH2-CH2.
[00113] At each occurrence L3 can be independently selected from the
group consisting of a bond and CI-Cm hydrocarbyl. At each occurrence L3 can
be independently selected from the group consisting of a bond and C1-05 alkyl.
At each occurrence L3 can be a bond.
[00114] The variable n can be about 4 to about 40,000, about 90 to
about
40,000, about 450 to about 14,500, or about 4 or less, or about 5, 6, 7, 8, 9,
10,
15, 20, 25, 30, 40, 50, 60, 70, 80, 90, 100, 200, 250, 500, 750, 1,000, 1,250,
1,500, 1,750, 2,000,2,250, 2,500, 3,000, 3,500, 4,000, 4,500, 5,000, 6,000,
31
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
7,000, 8,000,9,000, 10,030, 11,000, 12,000, 13,000, 14,000, 15,000, 20,000,
25,000,30,000, 35,000, or about 40,000 or more.
[00115] The variable m can be about 100 to about 83,000, about 2,000
to
about 83,000, about 4,000 to about 62,000, or about 100 or less, or about 200,
300, 400, 500, 750, 1,000, 1,500, 2,000,3,000, 4,000, 7,500, 10,000, 15,000,
20,000,25,000, 30,000,35,000, 40,000,45,000, 50,000, 55,000, 60,000,65,000,
70,000,75,000, 80,000, or about 85,000 or more.
[00116] The variable z can be about 125 to about 200,000, about 2,500
to
about 200,000, about 8,500 to about 140,000, or about 125 or less, 150, 175,
200, 250, 300, 400, 500, 750, 1,000, 1,500, 2,000, 2,500, 3,000, 4,000, 5,000,
10,000, 15,000, 20,000, 25,000, 30,000,40,000, 50,000, 60,000, 70,000, 80,000,
90,000, 100,(00, 110,000, 120,000, 130,000, 140,000, 150,000, 160,000,
170,000, 180,000, 190,000, or about 200,000 or more.
[00117] In some embodiments, the crosslinkable ampholyte polymer can
be derived from acrylamide, acryloyloxyethyl trimethylammonium chloride, and
2-acrylamido-2-methylpropane sulfonic acid (AMPS) or a salt thereof, and
includes repeating units having the structure:
_
-n - -m - -z
NH 0 H2
0=S=
HH3C-N-C 3
I Xe
OR1
CH3
The repeating units are in a block, alternate, or random configuration, and
each
repeating unit is independently in the orientation shown or in the opposite
orientation.
[00118] In some embodiments, the crosslinkable ampholyte polymer can
be derived from acrylamide, methacrylamidopropyl trimethylammonium
32
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
chloride, and 2-acrylamido-2-methylpropane sulfonic acid (AMPS) or a salt
thereof, and includes repeating units having the structure:
_ vw2t..
NH NH NH2
0=s=0
H3C-N-CH3
I Xe
OR'
CH3
The repeating units are in a block, alternate, or random configuration, and
each
repeating unit is independently in the orientation shown or in the opposite
orientation.
Crosslinker.
[00119] The composition including the crosslinkable ampholyte polymer
can include one or more crosslinkers. The crosslinker can be any suitable
crosslinker, such that the composition can be used as described herein.
[00120] In some embodiments, the crosslinker can be at least one of a
poly(amino(C2-Cm)hydrocarbylene) crosslinker and a (C6-C20)aryl alcohoL-(Ct-
C20)aldehyde crosslinker. In some examples, the crosslinker can be at least
one
of polyethyleneimine, phenol-formaldehyde, and glyoxal. In some
embodiments, the crosslinker is polyethyleneimine.
[00121] In some embodiments, the crosslinker can be a molecule
including at least one of chromium, aluminum, antimony, zirconium, titanium,
calcium, boron, iron, silicon, copper, zinc, magnesium, and an ion thereof.
The
crosslinker can be at least one of boric acid, borax, a borate, a (C1-
C30)hydrocarbylboronic acid, a (Ci-C30)hydrocarbyl ester of a (Cr
C3o)hydrocarbylboronic acid, a (Ci-C30)hydrocarbylboronic acid-modified
polyacrylamide, ferric chloride, disodium octaborate tetrahydrate, sodium
metaborate, sodium diborate, sodium tetraboratc, disodium tetraborate, a
33
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
pentaborate, ulexite, colemanite, magnesium oxide, zirconium lactate,
zirconium
triethanol amine, zirconium lactate triethanolamine, zirconium carbonate,
zirconium acetylacetonate, zirconium malate, zirconium citrate, zirconium
diisopropylamine lactate, zirconium glycolate, zirconium triethanol amine
glycolate, zirconium lactate glycolate, titanium lactate, titanium malate,
titanium
citrate, titanium ammonium lactate, titanium triethanolamine, titanium
acetylacetonate, aluminum lactate, and aluminum citrate.
[00122] In some embodiments, the crosslinker includes zirconium or a
zirconium derivative. The crosslinker can include at least one of zirconium
lactate, zirconium triethanol amine, zirconium lactate triethanolamine,
zirconium
carbonate, zirconium acetylacetonate, zirconium malate, zirconium citrate,
zirconium diisopropylamine lactate, zirconium glycolate, zirconium triethanol
amine glycolate, and zirconium lactate glycolate.
[00123] The composition can include any suitable concentration of the
one or more crosslinkers. For example, 0.000,1 wt% to about 80 wt% of the
composition can be the one or more crosslinkers, or about 0.001 wt% to about
80
wt%, 10 wt% to about 30 wt%, or about 0.000,1 wt% or less, or about 0.001
wt%, 0.01,0.1, 1, 2, 3, 4, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65,
70, 75,
or about 80 wt% or more of the composition can be the one or more
crosslinkers.
In some examples, in embodiments of the composition including an aqueous
composition, about 0.000,1 wt% to about 50 wt% of the composition is the
crosslinker, or about 0.001 wt% to about 1 wt%, or about 0.000,1 wt% or less,
or
about 0.001 wt%, 0.01, 0.1, 1, 2, 3,4, 5, 10, 15, 20, 25, 30, 35, 40, 45, or
about
50 wt% or more of the composition.
Other components.
[00124] In various embodiments, the composition including the
crosslinkable ampholyte polymer and the crosslinker can further include one or
more suitable additional components. The additional components can be any
suitable additional components, such that the composition can be used as
described herein.
[00125] The composition can further include one or more fluids. The
composition can include a fluid including at least one of water, an organic
solvent, and an oil. The composition can include a fluid including at least
one of
34
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
dipropylene glycol methyl ether, dipropylene glycol dimethyl ether, dimethyl
formamide, diethylene glycol methyl ether, ethylene glycol butyl ether,
diethylene glycol butyl ether, propylene carbonate, D-fimonene, a C2-C40 fatty
acid C1-C10 alkyl ester, 2-butoxy ethanol, butyl acetate, furfuryl acetate,
dimethyl sulfoxide, dimethyl formamide, diesel, kerosene, mineral oil, a
hydrocarbon including an internal olefin, a hydrocarbon including an alpha
olefin, xylenes, an ionic liquid, methyl ethyl ketone, and cyclohexanone. The
composition can further include at least onc of water, brine, produced water,
flowback water, brackish water, and sea water. The composition can include
any suitable proportion of the one or more fluids, such as about 0.001 wt% to
99.999 wt%, about 0.01 wt% to about 99.99 wt%, about 0.1 wt% to about 99.9
wt%, or about 20 wt% to about 90 wt%, or about 0.001 wt% or less, or about
0.01 wt%, 0.1, 1, 2, 3, 4, 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, 85, 90, 91,
92, 93,
94, 95, 96, 97, 98, 99, 99.9, 99.99, or about 99.999 wt% or more of the
composition.
[00126] The composition can further include a secondary viscosifier, in
addition to the crosslinkable ampholyte polymer. The secondary viscosifier can
be present in any suitable concentration, such as more, less, or an equal
concentration as compared to the concentration of the crosslinkable ampholyte
polymer. The secondary viscosifier can include at least one of a substituted
or
unsubstituted polysaccharide, and a substituted or unsubstituted
polyalkenylene,
wherein the polysaccharide or polyalkenylene is crosslinked or uncrosslinked.
The secondary viscosifier can include a polymer including at least one monomer
selected from the group consisting of ethylene glycol, acrylamide, vinyl
acetate,
2-acrylamidomethylpropane sulfonic acid or its salts, trimethylammoniumethyl
acrylate halide, and trimethylammoniumethyl methacrylate halide. The
secondary viscosifier can include a crosslinked gel or a crosslinkable gel.
[00127] The secondary viscosifier can affect the viscosity of the
composition at any suitable time and location. In some embodiments, the
secondary viscosifier provides an increased viscosity at least one of before
placement in the subterranean formation, at the time of placement into the
subterranean formation, during travel in the subterranean formation, once the
composition reaches a particular location in the subterranean formation, or
some
period of time after the composition reaches a particular location in the
CA 02938279 2016-07-28
WO 2015/138018 PCT/11520141069506
subterranean formation. In some embodiments, the secondary viscosifier can
provide some or no increased viscosity until the secondary viscosifier reaches
a
desired location, at which point the secondary viscosifier can provide a small
or
large increase in viscosity.
[00128] In some embodiments, the secondary viscosifier includes at least
onc of a linear polysaccharide, and poly((C2-Cio)alkenylene), wherein at each
occurrence, the (C2-Cio)alkenylene is independently substituted or
unsubstituted.
In some embodiments, the secondary viscosifier can include at least one of
poly(acrylic acid) or (Ci-05)alkyl esters thereof, poly(methacrylic acid) or
(C1-
05)alkyl esters thereof, poly(vinyl acetate), poly(vinyl alcohol),
poly(ethylene
glycol), poly(vinyl pyrrolidone), polyacrylamide, poly (hydroxyethyl
methacrylate), alginate, chitosan, curdlan, dextran, emulsan, gellan,
glucuronan,
N-acetyl-glucosamine, N-acetyl-heparosan, hyaluronic acid, kefiran, lentinan,
levan, mauran, pullulan, scleroglucan, schizophyllan, stewartan,
succinoglycan,
xanthan, welan, derivatized starch, tamarind, tragacanth, guar gum,
derivatized
guar (e.g., hydroxypropyl guar, carboxy methyl guar, or carboxymethyl
hydroxylpropyl guar), gum ghatti, gum arabic, locust bean gum, and derivatized
cellulose (e.g., carboxymethyl cellulose, hydroxyethyl cellulose,
carboxymethyl
hydroxyethyl cellulose, hydroxypropyl cellulose, or methyl hydroxyl ethyl
cellulose).
[00129] In some embodiments, the secondary viscosifier can include a
poly(vinyl alcohol) homopolymer, poly(vinyl alcohol) copolymer, a crosslinked
poly(vinyl alcohol) homopolymer, and a crosslinked poly(vinyl alcohol)
copolymer. The secondary viscosifier can include a poly(vinyl alcohol)
copolymer or a crosslinked poly(vinyl alcohol) copolymer including at least
one
of a graft, linear, branched, block, and random copolymer of vinyl alcohol and
at
least one of a substituted or unsubstitued (C2-05o)hydrocarbyl having at least
one
aliphatic unsaturated C-C bond therein, and a substituted or unsubstituted (C2-
050)alkene. The secondary viscosifier can include a poly(vinyl alcohol)
copolymer or a crosslinked poly(vinyl alcohol) copolymer including at least
one
of a graft, linear, branched, block, and random copolymer of vinyl alcohol and
at
least one of vinyl phosphonic acid, vinylidene diphosphonic acid, substituted
or
unsubstituted 2-acrylamido-2-methylpropanesulfonic acid, a substituted or
unsubstituted (Ci-C20)alkenoic acid, propenoic acid, butenoic acid, pentenoic
36
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
acid, hexenoic acid, octenoic acid, nonenoic acid, decenoic acid, acrylic
acid,
methacrylic acid, hydroxypropyl acrylic acid, acrylamide, fumaric acid,
methacrylic acid, hydroxypropyl acrylic acid, vinyl phosphonic acid,
vinylidene
diphosphonic acid, itaconic acid, crotonic acid, mesoconic acid, citraconic
acid,
styrene sulfonic acid, allyl sulfonic acid, methallyl sulfonic acid, vinyl
sulfonic
acid, and a substituted or unsubstituted (C1.-C20)allcyl ester thereof. The
secondary viscosifier can include a poly(vinyl alcohol) copolymer or a
crosslinked poly(vinyl alcohol) copolymcr including at least one of a graft,
linear, branched, block, and random copolymer of vinyl alcohol and at least
one
of vinyl acetate, vinyl propanoate, vinyl butanoate, vinyl pentanoate, vinyl
hexanoate, vinyl 2-methyl butanoate, vinyl 3-ethylpentanoate, and vinyl 3-
ethyllhexano ate, maleic anhydride, a substituted or unsubstituted (C1-
C20)alkenoic substituted or unsubstituted (Ci-C20)alkanoic anhydride, a
substituted or unsubstituted (Ci-C20)alkenoic substituted or unsubstituted (C1-
C20)alkenoic anhydride, propenoic acid anhydride, butenoic acid anhydride,
pentenoic acid anhydride, hexenoic acid anhydride, octenoic acid anhydride,
nonenoic acid anhydride, decenoic acid anhydride, acrylic acid anhydride,
fumaric acid anhydride, methacrylic acid anhydride, hydroxypropyl acrylic acid
anhydride, vinyl phosphonic acid anhydride, vinylidene diphosphonic acid
anhydride, itaconic acid anhydride, crotonic acid anhydride, mesoconic acid
anhydride, citraconic acid anhydride, styrene sulfonic acid anhydride, ally!
sulfonic acid anhydride, methallyl sulfonic acid anhydride, vinyl sulfonic
acid
anhydride, and an N-(Ci-Cio)alkenyl nitrogen containing substituted or
unsubstituted (Ci-Cio)heterocycle. The secondary viscosifier can include a
poly(vinyl alcohol) copolymer or a crosslinked poly(vinyl alcohol) copolymer
including at least one of a graft, linear, branched, block, and random
copolymer
that includes a poly(vinylalcohop-poly(acrylamide) copolymer, a
poly(vinylalcohop-poly(2-acrylamido-2-methylpropanesulfonic acid)
copolymer, or a poly(vinylakohol)-poly(N-vinylpyrrolidone) copolymer. The
secondary viscosifier can include a crosslinked poly(vinyl alcohol)
homopolymer or copolymer including a crosslinker including at least one of
chromium, aluminum, antimony, zirconium, titanium, calcium, boron, iron,
silicon, copper, zinc, magnesium, and an ion thereof. The secondary
viscosifier
can include a crosslinked poly(vinyl alcohol) homopolymcr or copolymer
37
including a crosslinker including at least one of an aldehyde, an aldehyde-
forming compound, a carboxylic acid or an ester thereof, a sulfonic acid or an
ester thereof, a phosphonic acid or an ester thereof, an acid anhydride, and
an
epihalohydrin.
[00130] In some
embodiments, the secondary viscosifier can be a polymer
including at least one of, or the ampholyte polymer can include a monomer
derived from at least one of, 2,2'-azobis(2,4-dimethyl valeronitrile), 2.2'-
azobis(2,4-dimethy1-4-methoxy valeronitrile), acrylamide ethyltrimethyl
ammonium chloride, acrylamide, acrylamido- and methacrylamido-alkyl trialkyl
ammonium salts, acrylamidomethylpropane sulfonic acid, acrylamidopropyl
trimethyl ammonium chloride, acrylic acid, dimethylaminoethyl
methacrylamide, dimethylaminoethyl methacrylate, dimethylaminopropyl
methacrylamide, dimethylaminopropylmethacrylamide,
dimethyldiallylammonium chloride, dimethylethyl acrylate, fumaramide,
methacrylamide, methacrylamidopropyl trimethyl ammonium chloride,
methacrylamidopropyldimethyl-n-dodecylammonium chloride,
methacrylamidopropyldimethyl-n-octylammonium chloride,
methacrylamidopropyltrimethylammonium chloride, methacryloylalkyl trialkyl
ammonium salts, methacryloylethyl trimethyl ammonium chloride,
methacrylylamidopropyldimethylcetylammonium chloride, N-(3-sulfopropyl)-
N-methacrylainidopropyl-N,N-dimethyl ammonium betaine, N,N-
dimethylacrylamide, N-methylacrylamide,
nonylphenoxypoly(ethyleneoxy)ethylmethacrylate, partially hydrolyzed
polyacrylamide, poly 2-amino-2-methyl propane sulfonic acid, polyvinyl
alcohol, sodium 2-acrylamido-2-methylpropane sulfonate, quaternized
dimethylaminoethylacrylate, quaternized dimethylaminoethylmethacrylate, 2-
(methacryloyloxy)ethyltrimethylammonium methyl sulfate or sulfonic acid or a
salt thereof, 2-(methacryloyloxy)ethyltrimethylammonium chloride copolymer,
or any combination thereof. In certain embodiments, the secondary viscosifier
may include a derivatized cellulose that includes cellulose grafted with an
ally'
or a vinyl monomer, such as those disclosed in U.S. Pat. Nos. 4,982,793,
5,067,565, and 5,122,549.
38
CA 2938279 2018-04-27
CA 02938279 2016-07-28
WO 2015/138018
PCIYUS2014/069506
[00131] The composition can include any suitable proportion of the
secondary viscosifier, such as about 0.001 wt% to 99.999 wt%, about 0.01wt%
to about 99.99 wt%, about 0.1 wt% to about 50 wt%, or about 0.1 wt% to about
20 wt%, or about 0.001 wt% or less, or about 0.01 wt%, 0.1, 1, 2, 3, 4, 5, 10,
15,
20, 30, 40, 50, 60, 70, 80, 85, 90, 91, 92, 93,94, 95, 96, 97,98, 99,
99.9,99.99,
or about 99.999 wt% or more of the composition.
[00132] The composition can further include a secondary crosslinker.
The
crosslinker can be any suitable crosslinker. The secondary crosslinker can be
present in any suitable concentration, such as more, less, or an equal
concentration as compared to the concentration of the crosslinker. In various
embodiments, the secondary crosslinker can include at least one of chromium,
aluminum, antimony, zirconium, titanium, calcium, boron, iron, silicon,
copper,
zinc, magnesium, and an ion thereof. The secondary crosslinkcr can include at
least one of boric acid, borax, a borate, a (Ci-C30)hydrocarbylboronic acid, a
(C1-
C30)hydrocarbyl ester of a (CI-C30)hydrocarbylboronic acid, a (Cr
C30)hydrocarbylboronic acid-modified polyacrylarnide, ferric chloride,
disodium
octaborate tetrahydrate, sodium metaborate, sodium diborate, sodium
tetraborate, disodium tetraborate, a pcntaborate, ulexite, colemanite,
magnesium
oxide, zirconium lactate, zirconium triethanol amine, zirconium lactate
triethanolamine, zirconium carbonate, zirconium acetylacetonate, zirconium
malate, zirconium citrate, zirconium diisopropylamine lactate, zirconium
glycolate, zirconium triethanol amine glycolate, zirconium lactate glycolate,
titanium lactate, titanium malate, titanium citrate, titanium ammonium
lactate,
titanium triethanolamine, titanium acetylacetonate, aluminum lactate, and
aluminum citrate. The composition can include any suitable proportion of the
secondary crosslinker, such as about 0.000,1 wt% to 99.999,9 wt%, about 0.01
wt% to about 99.99 wt%, about 0.1 wt% to about 50 wt%, or about 0.1 wt% to
about 20 wt%, or about 0.000,1 wt% or less, or about 0.001 wt%, 0.01,0.1, 1,2,
3,4, 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, 85, 90, 91, 92, 93,94, 95, 96, 97,
98,
99, 99.9, 99.99, 99.999, or about 99.999,9 wt% or more of the composition.
[00133] Some embodiments of the method can include breaking the
composition including the crosslinkable ampholyte polymers, especially non-
crosslinked ampholyte polymer, but in some embodiments crosslinked
ampholyte polymer can be broken. In some instances, breaking can be achieved
39
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
by partially hydrolyzing the ampholyte polymers. Partial hydrolysis (or
breaking) can be achieved by increasing the temperature, increasing the pH, or
both.
[00134] In some instances, breaking can be achieved by exposure to the
elevated temperatures in the wellbore and/or subterranean formation. For
example, the bottom hole circulating temperature can be about 100 F or greater
(e.g., about 100 F to about 200 F, about 120 F to about 200 F, or about 150 F
to about 200 F). The rate of breaking (or partial hydrolysis and contraction
of
the ampholyte polymer) can depend on the composition of the ampholyte
polymer, the relative ratios of the monomers of the ampholyte polymer, the IDS
of the composition, and the like. Therefore, in some instances, the method can
include breaking the composition including the ampholyte polymer with
minimal to no chemical breaker (e.g., less than about 1% of a chemical
breaker).
[00135] In some instances, breaking can involve increasing the pH of
the
composition including the ampholyte polymeric compounds. Increasing the pH
can be achieved by introducing a suitable breaking fluid or including a
suitable
breaker in the composition (e.g., sodium perborate).
[00136] The composition described herein can, in some instances, be
foamed. As used herein the term "foam" refers to a two-phase composition
having a continuous liquid phase and a discontinuous gas phase. In some
embodiments, the composition described herein can include a base fluid, a gas,
a
foaming agent, and an ampholyte polymeric compound.
[00137] Suitable gases can include, but are not limited to, nitrogen,
carbon
dioxide, air, methane, helium, argon, and any combination thereof. One skilled
in the art, with the benefit of this disclosure, should understand the benefit
of
each gas. By way of nonlimiting example, carbon dioxide foams can have
deeper well capability than nitrogen foams because carbon dioxide emulsions
have greater density than nitrogen gas foams so that the surface pumping
pressure required to reach a corresponding depth is lower with carbon dioxide
than with nitrogen. Moreover, the higher density can impart greater proppant
transport capability, up to about 12 lb of pmppant per gal of composition.
[00138] In some embodiments, the quality of a foamed composition can
range from a lower limit of about 5%, 10%, 25%, 40%, 50%, 60%, or 70% gas
volume to an upper limit of about 95%, 90%, 80%, 75%, 60%, or 50% gas
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
volume, and wherein the quality of the foamed composition can range from any
lower limit to any upper limit and encompasses any subset therebetween. The
foamed composition can have a foam quality from about 85% to about 95%, or
about 90% to about 95%.
[00139] Suitable foaming agents can include, but are not limited to,
cationic foaming agents, anionic foaming agents, amphoteric foaming agents,
nonionic foaming agents, or any combination thereof. Nonlimiting examples of
suitable foaming agents can include, but are not limited to, surfactants like
betaines, sulfated or sulfonated alkoxylates, alkyl quarternary amines,
alkoxylated linear alcohols, alkyl sulfonates, alkyl aryl sulfonates, C10-C20
alkyldiphenyl ether sulfonates, polyethylene glycols, ethers of aLkylated
phenol,
sodium dodecylsulfate, alpha olefin sulfonates such as sodium dodecane
sulfonate, trimethyl hexadecyl ammonium bromide, and the like, any derivative
thereof, or any combination thereof. Foaming agents can be included in
compositions at concentrations ranging typically from about 0.05% to about 2%
of the liquid component by weight (e.g., from about 0.5 to about 20 gallons
per
1000 gallons of liquid).
[00140] The composition including the crosslinkable ampholyte polymer
and the crosslinker, or a crosslinked reaction product thereof, can be
combined
with any suitable downhole fluid before, during, or after the placement of the
composition in the subterranean formation or the contacting of the composition
and the subterranean materiaL In some examples, the composition including the
crosslinkable ampholyte polymer and the crosslinker, or a crosslinked reaction
product thereof, is combined with a downhole fluid above the surface, and then
the combined composition is placed in a subterranean formation or contacted
with a subterranean material. In another example, the composition including
the
crosslinkable ampholyte polymer and the crosslinker, or a crosslinked reaction
product thereof, is injected into a subterranean formation to combine with a
downhole fluid, and the combined composition is contacted with a subterranean
material or is considered to be placed in the subterranean formation. In
various
examples, at least one of prior to, during, and after the placement of the
composition in the subterranean formation or contacting of the subterranean
material and the composition, the composition is used in the subterranean
formation, at least one of alone and in combination with other materials, as a
41
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
drilling fluid, stimulation fluid, fracturing fluid, spotting fluid, clean-up
fluid,
completion fluid, remedial treatment fluid, abandonment fluid, pill, acidizing
fluid, cementing fluid, packer fluid, or a combination thereof.
[00141] In various embodiments, the method includes combining the
composition including the crosslinkable ampholyte polymer and the crosslinker,
or a crosslinked reaction product thereof, with any suitable downhole fluid,
such
as an aqueous or oil-based fluid including a drilling fluid, stimulation
fluid,
fracturing fluid, spotting fluid, clean-up fluid, completion fluid, remedial
treatment fluid, abandonment fluid, pill, acidizing fluid, cementing fluid,
packer
fluid, or a combination thereof, to form a mixture. The placement of the
composition in the subterranean formation can include contacting the
subterranean material and the mixture. The contacting of the subterranean
material and the composition can include contacting the subterranean material
and the mixture. Any suitable weight percent of a mixture that is placed in
the
subterranean formation or contacted with the subterranean material can be the
composition including the crosslinkable ampholyte polymer and the erosslinker,
or a crosslinked reaction product thereof, such as about 0.001 wt% to 99.999
wt%, about 0.01 wt% to about 99.99 wt%, about 0.1 wt% to about 99.9 wt%, or
about 20 wt% to about 90 wt%, or about 0.001 wt% or less, or about 0.01 wt%,
0.1, 1, 2, 3, 4, 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, 85, 90, 91, 92, 93,
94, 95, 96,
97, 98, 99, 99.9, 99.99 wt%, or about 99.999 wt% or more of the mixture or
composition.
[00142] In some embodiments, the composition can include any suitable
amount of any suitable material used in a downhole fluid. For example, the
composition can include water, saline, aqueous base, acid, oil, organic
solvent,
synthetic fluid oil phase, aqueous solution, alcohol or polyol, cellulose,
starch,
alkalinity control agents, acidity control agents, density control agents,
density
modifiers, emulsifiers, dispersants, polymeric stabilizers, crosslinking
agents,
polyacrylamide, a polymer or combination of polymers, antioxidants, heat
stabilizers, foam control agents, foaming agents, solvents, diluents,
plasticizer,
filler or inorganic particle, pigment, dye, precipitating agent, rheology
modifier,
oil-wetting agents, set retarding additives, surfactants, gases, weight
reducing
additives, heavy-weight additives, lost circulation materials, filtration
control
additives, salts, fibers, thixotropic additives, breakers, cmsslinkers,
rheology
42
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
modifiers, curing accelerators, curing retarders, pH modifiers, chelating
agents,
scale inhibitors, enzymes, resins, water control materials, oxidizers,
markers,
Portland cement, pozzolana cement, gypsum cement, high alumina content
cement, slag cement, silica cement, fly ash, metakaolin, shale, zeolite, a
crystalline silica compound, amorphous silica, hydratable clays, microspheres,
pozzolan lime, or a combination thereof. In various embodiments, the
composition can include one or more additive components such as: thinner
additives such as COLDTROLO, ATC , OMC 2TM, and OMC 42TM:
RHEMODTm, a viscosifier and suspension agent including a modified fatty acid;
additives for providing temporary increased viscosity, such as for shipping
(e.g.,
transport to the well site) and for use in sweeps (for example, additives
having
the trade name TEMPERUSTm (a modified fatty acid) and VIS-PLUS , a
thixotropic viscosifying polymer blend); TAU-MODTm, a
viscosifying/suspension agent including an amorphous/fibrous material;
additives for filtration control, for example, ADAPTA , a HTHP filtration
control agent including a crosslinked copolymer; DURATONE HT, a filtration
control agent that includes an organophilic lignite, more particularly
organophilic leonardite; THERMO TONETm, a high temperature high pressure
(HTHP) filtration control agent including a synthetic polymer; BDPm-366, a
HTHP filtration control agent; BDFTm-454, a HTHP filtration control agent;
LIQUITONETm, a polymeric filtration agent and viscosifier; additives for HTHP
emulsion stability, for example, FACTANTTm, which includes highly
concentrated tall oil derivative; emulsifiers such as LE SUPERMULTm and EZ
MUL NT, polyaminated fatty acid emulsifiers, and FORTI-MUL ; DRIL
TREAT , an oil wetting agent for heavy fluids; BARACARB , a sized ground
marble bridging agent; BAROIDO, a ground barium sulfate weighting agent;
BAROLEFT , a hole sweeping agent: SWEEP-WATEO, a sweep weighting
agent; BDF-508, a diarnine dimer rheology modifier; GELTONE II
organophilic clay; BAROFLBRETM 0 for lost circulation management and
seepage loss prevention, including a natural cellulose fiber; STEELSEALO, a
resilient graphitic carbon lost circulation material; HYDRO-PLUG , a
hydratable swelling lost circulation material; lime, which can provide
alkalinity
and can activate certain emulsifiers; and calcium chloride, which can provide
salinity. Any suitable proportion of the composition can include any optional
43
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
component listed in this paragraph, such as about 0.000,1 wt% to 99.999,9 wt%,
about 0.01wt% to about 99.99 wt%, about 0.1 wt% to about 99.9 wt%, or about
20 wt% to about 90 wt%, or about 0.000,1 wt% or less, or about 0.001 wt%,
0.01,0.1, 1, 2, 3, 4, 5, 10, 15, 20, 30, 40, 50, 60, 70, 80, 85, 90, 91, 92,
93, 94,
95, 96, 97, 98, 99, 99.9,99.99, 99.999 wt%, or 99.999,9 wt% or more of the
composition.
[00143] A drilling fluid, also known as a drilling mud or simply
"mud," is
a specially designed fluid that is circulated through a wellbore as the
wellbore is
being drilled to facilitate the drilling operation. The drilling fluid can be
water-
based or oil-based. The drilling fluid can carry cuttings up from beneath and
around the bit, transport them up the annulus, and allow their separation.
Also, a
drilling fluid can cool and lubricate the drill head as well as reduce
friction
between the drill string and the sides of the hole. The drilling fluid aids in
support of the drill pipe and drill head, and provides a hydrostatic head to
maintain the integrity of the wellbore walls and prevent well blowouts.
Specific
drilling fluid systems can be selected to optimize a drilling operation in
accordance with the characteristics of a particular geological formation. The
drilling fluid can be formulated to prevent unwanted influxes of formation
fluids
from permeable rocks and also to form a thin, low permeability filter cake
that
temporarily seals pores, other openings, and formations penetrated by the bit.
In
water-based drilling fluids, solid particles are suspended in a water or brine
solution containing other components. Oils or other non-aqueous liquids can be
emulsified in the water or brine or at least partially solubilized (for less
hydrophobic non-aqueous liquids), but water is the continuous phase. A
drilling
fluid can be present in the mixture with the composition including the
crosslinkable ampholyte polymer and the crosslinker, or a crosslinked reaction
product thereof, in any suitable amount, such as about 1 wt% or less, about 2
wt%, 3, 4, 5, 10, 15, 20, 30,40, 50, 60, 70, 80, 85, 90, 95,96, 97,98, 99,
99.9,
99.99, 99.999, or about 99.999,9 wt% or more of the mixture.
[00144] A water-based drilling fluid in embodiments of the present
invention can be any suitable water-based drilling fluid. In various
embodiments, the drilling fluid can include at least one of water (fresh or
brine),
a salt (e.g., calcium chloride, sodium chloride, potassium chloride, magnesium
chloride, calcium bromide, sodium bromide, potassium bromide, calcium nitrate,
44
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
sodium formate, potassium formate, cesium formate), aqueous base (e.g.,
sodium hydroxide or potassium hydroxide), alcohol or polyol, cellulose,
starches, alkalinity control agents, density control agents such as a density
modifier (e.g., barium sulfate), surfactants (e.g., betaines, alkali metal
alkylene
acetates, sultaines, ether carboxylates), emulsifiers, dispersants, polymeric
stabilizers, crosslinking agents, polyacrylamides, polymers or combinations of
polymers, antioxidants, heat stabilizers, foam control agents, foaming agents,
solvents, diluents, plasticizers, filler or inorganic particles (e.g.,
silica),
pigments, dyes, precipitating agents (e.g., silicates or aluminum complexes),
and
theology modifiers such as thickeners or viscosifiers (e.g., xanthan gum). Any
ingredient listed in this paragraph can be either present or not present in
the
mixture.
[00145] An oil-based drilling fluid or mud in embodiments of the present
invention can be any suitable oil-based drilling fluid. In various embodiments
the drilling fluid can include at least one of an oil-based fluid (or
synthetic fluid),
saline, aqueous solution, emulsifiers, other agents of additives for
suspension
control, weight or density control, oil-wetting agents, fluid loss or
filtration
control agents, and rheology control agents. For example, sec H. C. H. Darley
and George R. Gray, Composition and Properties of Drilling and Completion
Fluids 66-67, 561-562 (5thed. 1988). An oil-based or invert emulsion-based
drilling fluid can include between about 10:90 to about 95:5, or about 50:50
to
about 95:5, by volume of oil phase to water phase. A substantially all oil mud
includes about 100% liquid phase oil by volume (e.g., substantially no
internal
aqueous phase).
[00146] A pill is a relatively small quantity (e.g., less than about 500
bbl,
or less than about 200 bbl) of drilling fluid used to accomplish a specific
task
that the regular drilling fluid cannot perform. For example, a pill can be a
high-
viscosity pill to, for example, help lift cuttings out of a vertical wellbore.
In
another example, a pill can be a freshwater pill to, for example, dissolve a
salt
formation. Another example is a pipe-freeing pill to, for example, destroy
filter
cake and relieve differential sticking forces. In another example, a pill is a
lost
circulation material pill to, for example, plug a thief zone. A pill can
include any
component described herein as a component of a drilling fluid.
CA 02938279 2016-07-28
WO 2015/138018
PCIMS2014/069506
[00147] A cement fluid can include an aqueous mixture of at least one
of
cement and cement kiln dust. The composition including the crosslinkable
ampholyte polymer and the crosslinker, or a crosslinked reaction product
thereof, can form a useful combination with cement or cement kiln dust. The
cement kiln dust can be any suitable cement kiln dust. Cement kiln dust can be
formed during the manufacture of cement and can be partially calcined kiln
feed
that is removed from the gas stream and collected in a dust collector during a
manufacturing process. Cement kiln dust can be advantageously utilized in a
cost-effective manner since kiln dust is often regarded as a low value waste
product of the cement industry. Some embodiments of the cement fluid can
include cement kiln dust but no cement, cement kiln dust and cement, or cement
but no cement kiln dust. The cement can be any suitable cement. The cement
can be a hydraulic cement. A variety of cements can be utilized in accordance
with embodiments of the present invention; for example, those including
calcium, aluminum, silicon, oxygen, iron, or sulfur, which can set and harden
by
reaction with water. Suitable cements can include Portland cements, pozzolana
cements, gypsum cements, high alumina content cements, slag cements, silica
cements, and combinations thereof. In some embodiments, the Portland cements
that are suitable for use in embodiments of the present invention are
classified as
Classes A, C, H, and G cements according to the American Petroleum Institute,
API Specification for Materials and Testing for Well Cements, API
Specification
10, Fifth Ed., Jul. 1, 1990. A cement can be generally included in the
cementing
fluid in an amount sufficient to provide the desired compressive strength,
density, or cost. In some embodiments, the hydraulic cement can be present in
the cementing fluid in an amount in the range of from 0 wt% to about 100 wt%,
0-95 wt%, 20-95 wt%, or about 50-90 wt%. A cement kiln dust can be present
in an amount of at least about 0.01 wt%, or about 5 wt% - 80 wt%, or about 10
wt% to about 50 wt%.
[00148] Optionally, other additives can be added to a cement or kiln
dust-
containing composition of embodiments of the present invention as deemed
appropriate by one skilled in the art, with the benefit of this disclosure.
Any
optional ingredient listed in this paragraph can be either present or not
present in
the composition. For example, the composition can include fly ash, metakaolin,
shale, zeolite, set retarding additive, surfactant, a gas, accelerators,
weight
46
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
reducing additives, heavy-weight additives, lost circulation materials,
filtration
control additives, dispersants, and combinations thereof. In some examples,
additives can include crystalline silica compounds, amorphous silica, salts,
fibers, hydratable clays, microspheres, pozzolan lime, thixotropic additives,
combinations thereof, and the like.
[00149] In various embodiments, the composition or mixture can include
a proppant, a resin-coated proppant, an encapsulated resin, or a combination
thereof. A proppant is a material that keeps an induced hydraulic fracture at
least partially open during or after a fracturing treatment. Proppants can be
transported into the subterranean formation and to the fracture using fluid,
such
as fracturing fluid or another fluid. A higher-viscosity fluid can more
effectively
transport proppants to a desired location in a fracture, especially larger
proppants, by more effectively keeping proppants in a suspended state within
the
fluid. Examples of proppants can include sand, gravel, glass beads, polymer
beads, ground products from shells and seeds such as walnut hulls, and
manmade materials such as ceramic proppant, bauxite, tetrafluoroethylene
materials (e.g., TEFLON' available from DuPont), fruit pit materials,
processed wood, composite particulates prepared from a binder and fine grade
particulates such as silica, alumina, fumed silica, carbon black, graphite,
mica,
titanium dioxide, meta-silicate, calcium silicate, kaolin, talc, zirconia,
boron, fly
ash, hollow glass micmspheres, and solid glass, or mixtures thereof. In some
embodiments, proppant can have an average particle size, wherein particle size
is the largest dimension of a particle, of about 0.001 mm to about 3 mm, about
0.15 mm to about 2.5 mm, about 0,25 mm to about 0.43 mm, about 0.43 mm to
about 0.85 mm, about 0.85 mm to about 1.18 mm, about 1.18 mm to about 1.70
mm, or about 1.70 to about 2.36 mm. In some embodiments, the proppant can
have a distribution of particle sizes clustering around multiple averages,
such as
one, two, three, or four different average particle sizes. The composition or
mixture can include any suitable amount of proppant, such as about 0.000,1 wt%
to about 99.9 wt%, about 0.1 wt% to about 80 wt%, or about 10 wt% to about 60
wt%, or about 0.000,000,01 wt% or less, or about 0.000,001 wt%, 0.000,1,
0.001, 0.01, 0.1, 1, 2, 3, 4, 5, 10, 15, 20, 30, 40, 50, 60,70, 80, 85, 90,
91, 92,93,
94, 95, 96, 97,98, 99, 99.9 wt%, or about 99.99 wt% or more.
47
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
Drilling assembly.
[00150] Embodiments of the composition including the crosslinkable
ampholyte polymer and the crosslinker, or a crosslinked reaction product
thereof, disclosed herein may directly or indirectly affect one or more
components or pieces of equipment associated with the preparation, delivery,
recapture, recycling, reuse, and/or disposal of the composition including
crosslinkable ampholyte polymer and the crosslinker, or a crosslinked reaction
product thereof,. For example, and with reference to FIG. 1, an embodiment of
the composition including the crosslinkable ampholyte polymer and the
crosslinker, or a crosslinked reaction product thereof, and optionally also
including a drilling fluid, may directly or indirectly affect one or more
components or pieces of equipment associated with an exemplary wellbore
drilling assembly 1(0, according to one or more embodiments. It should be
noted that while FIG. 1 generally depicts a land-based drilling assembly,
those
skilled in the art will readily recognize that the principles described herein
are
equally applicable to subsea drilling operations that employ floating or sea-
based
platforms and rigs, without departing from the scope of the disclosure.
[00151] As illustrated, the drilling assembly 100 may include a
drilling
platform 102 that supports a derrick 104 having a traveling block 106 for
raising
and lowering a drill string 108. The drill string 108 may include, but is not
limited to, drill pipe and coiled tubing, as generally known to those skilled
in the
art. A kelly 110 supports the drill string 108 as it is lowered through a
rotary
table 112. A drill bit 114 is attached to the distal end of the drill string
108 and
is driven either by a downhole motor and/or via rotation of the drill string
108
from the well surface. As the bit 114 rotates, it creates a wellbore 116 that
penetrates various subterranean formations 118.
[00152] A pump 120 (e.g., a mud pump) circulates drilling fluid 122
through a feed pipe 124 and to the kelly 110, which conveys the drilling fluid
122 downhole through the interior of the drill string 108 and through one or
more orifices in the drill bit 114. The drilling fluid 122 is then circulated
back to
the surface via an annulus 126 defined between the drill string 108 and the
walls
of the wellbore 116. At the surface, the recirculated or spent drilling fluid
122
exits the annulus 126 and may be conveyed to one or more fluid processing
unit(s) 128 via an interconnecting flow line 130. After passing through the
fluid
48
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
processing unit(s) 128, a "cleaned" drilling fluid 122 is deposited into a
nearby
retention pit 132 (e.g., a mud pit). While illustrated as being arranged at
the
outlet of the wellbore 116 via the annulus 126, those skilled in the art will
readily appreciate that the fluid processing unit(s) 128 may be arranged at
any
other location in the drilling assembly 100 to facilitate its proper function,
without departing from the scope of the disclosure.
[00153] The composition including the crosslinkable ampholyte polymer
and the crosslinker, or a crosslinked reaction product thereof, may be added
to
the drilling fluid 122 via a mixing hopper 134 communicably coupled to or
otherwise in fluid communication with the retention pit 132. The mixing hopper
134 may include, but is not limited to, mixers and related mixing equipment
known to those skilled in the art. In other embodiments, however, the
composition including the crosslinkable ampholyte polymer and the crosslinker,
or a crosslinked reaction product thereof, may be added to the drilling fluid
122
at any other location in the drilling assembly 100. In at least one
embodiment,
for example, there could be more than one retention pit 132, such as multiple
retention pits 132 in series. Moreover, the retention pit 132 may be
representative of one or more fluid storage facilities and/or units where the
composition including the crosslinkable ampholyte polymer and the crosslinker,
or a crosslinked reaction product thereof, may be stored, reconditioned,
and/or
regulated until added to the drilling fluid 122.
[00154] As mentioned above, the composition including the
crosslinkable
ampholyte polymer and the crosslinker, or a crosslinked reaction product
thereof, may directly or indirectly affect the components and equipment of the
drilling assembly 100. For example, the composition including the
crosslinkable
ampholyte polymer and the crosslinker, or a crosslinked reaction product
thereof, may directly or indirectly affect the fluid processing unit(s) 128,
which
may include, but is not limited to, one or more of a shaker (e.g., shale
shaker), a
centrifuge, a hydrocyclone, a separator (including magnetic and electrical
separators), a dcsilter, a desander, a separator, a filter (e.g., diatomaceous
earth
filters), a heat exchanger, or any fluid reclamation equipment. The fluid
processing unit(s) 128 may further include one or more sensors, gauges, pumps,
compressors, and the like used to store, monitor, regulate, and/or recondition
the
49
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
composition including the crosslinkable ampholyte polymer and the crosslinker,
or a crosslinked reaction product thereof,.
[00155] The composition including the crosslinkable ampholyte polymer
and the crosslinker, or a crosslinked reaction product thereof, may directly
or
indirectly affect the pump 120, which representatively includes any conduits,
pipelines, trucks, tubulars, and/or pipes used to fluidically convey the
composition including the crosslinkable ampholyte polymer and the crosslinker,
or a crosslinked reaction product thereof, downhole, any pumps, compressors,
or
motors (e.g., topside or downhole) used to drive the composition into motion,
any valves or related joints used to regulate the pressure or flow rate of the
composition, and any sensors (e.g., pressure, temperature, flow rate, and the
like), gauges, and/or combinations thereof, and the like. The composition
including the crosslinkable ampholyte polymer and the crosslinker, or a
crosslinked reaction product thereof, may also directly or indirectly affect
the
mixing hopper 134 and the retention pit 132 and their assorted variations.
[00156] The composition including the crosslinkable ampholyte polymer
and the crosslinker, or a crosslinked reaction product thereof, may also
directly
or indirectly affect the various downholc equipment and tools that may come
into contact with the composition including the crosslinkable ampholyte
polymer
and the crosslinker, or a crosslinked reaction product thereof, such as, but
not
limited to, the drill string 108, any floats, drill collars, mud motors,
downhole
motors, and/or pumps associated with the drill string 108, and any measurement
while drilling (MWD)/logging while drilling (LWD) tools and related telemetry
equipment, sensors, or distributed sensors associated with the drill string
108.
The composition including the crosslinkable ampholyte polymer and the
crosslinker, or a crosslinked reaction product thereof, may also directly or
indirectly affect any downhole heat exchangers, valves and corresponding
actuation devices, tool seals, packers and other wellbore isolation devices or
components, and the like associated with the wellbore 116. The composition
including the crosslinkable ampholyte polymer and the crosslinker, or a
crosslinked reaction product thereof, may also directly or indirectly affect
the
drill bit 114, which may include, but is not limited to, roller cone bits,
polycrystalline diamond compact (PDC) bits, natural diamond bits, any hole
openers, reamers, coring bits, and the like.
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
[00157] While not specifically illustrated herein, the composition
including the crosslinkable ampholyte polymer and the crosslinker, or a
crosslinked reaction product thereof, may also directly or indirectly affect
any
transport or delivery equipment used to convey the composition including the
crosslinkable ampholyte polymer and the crosslinker, or a crosslinked reaction
product thereof, to the drilling assembly 100 such as, for example, any
transport
vessels, conduits, pipelines, trucks, tubulars, and/or pipes used to
fluidically
move the composition including the crosslinkable ampholyte polymer and the
crosslinker, or a crosslinked reaction product thereof, from one location to
another, any pumps, compressors, or motors used to drive the composition into
motion, any valves or related joints used to regulate the pressure or flow
rate of
the composition, and any sensors (e.g., pressure and temperature), gauges,
and/or
combinations thereof, and the like.
System or apparatus.
[00158] In various embodiments, the present invention provides a
system.
The system can be any suitable system that can include the use of an
embodiment of the composition including the crosslinkable ampholyte polymer
and the crosslinker described herein, or a crosslinked reaction product
thereof, in
a subterranean formation, or that can include performance of an embodiment of
a method of using the composition described herein. The system can include a
composition including an embodiment of the crosslinkable ampholyte polymer
and the crosslinker, or including a reaction product thereof. The system can
also
include a subterranean formation including the composition therein. In some
embodiments, the composition in the system can also include a downhole fluid,
such as at least one of an aqueous fracturing fluid and an aqueous drilling
fluid.
[00159] In some embodiments, the system can include a drillstring
disposed in a wellbore, the drillstring including a drill bit at a downhole
end of
the drillstring. The system can include an annulus between the drillstring and
the wellbore. The system can also include a pump configured to circulate the
composition through the drill string, through the drill bit, and back above-
surface
through the annulus. The system can include a fluid processing unit configured
to process the composition exiting the annulus to generate a cleaned drilling
fluid for recirculation through the wellbore. In some embodiments, the system
51
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
can include a tubular disposed in a wellbore, and a pump configured to pump
the
composition into the subterranean formation.
[00160] In various embodiments, the present invention provides an
apparatus. The apparatus can be any suitable apparatus that can use an
embodiment of the composition described herein or that can be used to perform
an embodiment of a method described herein.
[00161] Various embodiments provide systems and apparatus configured
for delivering the composition described herein to a subterranean location and
for using the composition therein, such as for drilling or hydraulic
fracturing. In
various embodiments, the system can include a pump fluidly coupled to a
tubular (e.g., any suitable type of oilfield pipe, such as pipeline, drill
pipe,
production tubing, and the like), the tubular containing a composition
including
the crosslinkable ampholyte polymer and the crosslinker, or a crosslinked
reaction product thereof, described herein.
[00162] The pump can be a high pressure pump in some embodiments.
As used herein, the term "high pressure pump" will refer to a pump that is
capable of delivering a fluid downhole at a pressure of about 1000 psi or
greater.
A high pressure pump can be used when it is desired to introduce the
composition to a subterranean formation at or above a fracture gradient of the
subterranean formation, but it can also be used in cases where fracturing is
not
desired. In some embodiments, the high pressure pump can be capable of fluidly
conveying particulate matter, such as proppant particulates, into the
subterranean
formation. Suitable high pressure pumps will be known to one having ordinary
skill in the art and can include, but are not limited to, floating piston
pumps and
positive displacement pumps.
[00163] In other embodiments, the pump can be a low pressure pump. As
used herein, the term "low pressure pump" will refer to a pump that operates
at a
pressure of about 1000 psi or less. In some embodiments, a low pressure pump
can be fluidly coupled to a high pressure pump that is fluidly coupled to the
tubular. That is, in such embodiments, the low pressure pump can be configured
to convey the composition to the high pressure pump. In such embodiments, the
low pressure pump can "step up" the pressure of the composition before it
reaches the high pressure pump.
52
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
[00164] In some embodiments, the systems or apparatuses described
herein can further include a mixing tank that is upstream of the pump and in
which the composition is formulated. In various embodiments, the pump (e.g., a
low pressure pump, a high pressure pump, or a combination thereof) can convey
the composition from the mixing tank or other source of the composition to the
tubular. In other embodiments, however, the composition can be formulated
offsite and transported to a worksite, in which case the composition can be
introduced to the tubular via the pump directly from its shipping container
(e.g.,
a truck, a railcar, a barge, or the like) or from a transport pipeline, hi
either case,
the composition can be drawn into the pump, elevated to an appropriate
pressure,
and then introduced into the tubular for delivery downhole.
[00165] FIG. 2 shows an illustrative schematic of systems and
apparatuses
that can deliver embodiments of the compositions of the present invention to a
subterranean location, according to one or more embodiments. It should be
noted that while FIG. 2 generally depicts a land-based system or apparatus, it
is
to be recognized that like systems and apparatuses can be operated in subsea
locations as well. Embodiments of the present invention can have a different
scale than that depicted in FIG. 2. As depicted in FIG. 2, system or apparatus
1
can include mixing tank 10, in which an embodiment of the composition can be
formulated. The composition can be conveyed via line 12 to wellhead 14, where
the composition enters tubular 16, with tubular 16 extending from wellhead 14
into subterranean formation 18. Upon being ejected from tubular 16, the
composition can subsequently penetrate into subterranean formation 18. Pump
20 can be configured to raise the pressure of the composition to a desired
degree
before its introduction into tubular 16. It is to be recognized that system or
apparatus 1 is merely exemplary in nature and various additional components
can be present that have not necessarily been depicted in FIG. 2 in the
interest of
clarity. Non-limiting additional components that can be present include, but
are
not limited to, supply hoppers, valves, condensers, adapters, joints, gauges,
sensors, compressors, pressure controllers, pressure sensors, flow rate
controllers, flow rate sensors, temperature sensors, and the like.
[00166] Although not depicted in FIG. 2, at least part of the
composition
can, in some embodiments, flow back to wellhead 14 and exit subterranean
formation 18. In some embodiments, the composition that has flowed back to
53
CA 02938279 2016-07-28
WO 2015/138018
PCT/U52014/069506
wellhead 14 can subsequently be recovered, and in some examples reformulated,
and recirculated to subterranean formation 18.
[00167] It is also to be recognized that the disclosed composition can
also
directly or indirectly affect the various downhole equipment and tools that
can
come into contact with the composition during operation. Such equipment and
tools can include, but are not limited to, wellbore casing, wellbore liner,
completion string, insert strings, drill string, coiled tubing, slickline,
wireline,
drill pipe, drill collars, mud motors, downhole motors and/or pumps, surface-
mounted motors and/or pumps, centralizers, turbolizers, scratchers, floats
(e.g.,
shoes, collars, valves, and the like), logging tools and related telemetry
equipment, actuators (e.g., electromechanical devices, hydromechanical
devices,
and the like), sliding sleeves, production sleeves, plugs, screens, filters,
flow
control devices (e.g., inflow control devices, autonomous inflow control
devices,
outflow control devices, and the like), couplings (e.g., electro-hydraulic wet
connect, dry connect, inductive coupler, and the like), control lines (e.g.,
electrical, fiber optic, hydraulic, and the like), surveillance lines, drill
bits and
reamers, sensors or distributed sensors, downhole heat exchangers, valves and
corresponding actuation devices, tool seals, packers, cement plugs, bridge
plugs,
and other wellbore isolation devices or components, and the like. Any of these
components can be included in the systems and apparatuses generally described
above and depicted in FIG. 2.
Composition for treatment of a subterranean formation.
[00168] Various embodiments provide a composition for treatment of a
subterranean formation. The composition can be any suitable composition
including an embodiment of the crosslinkable ampholyte polymer and the
crosslinker, or a crosslinked reaction product thereof, that can be used to
perform
an embodiment of the method for treatment of a subterranean formation
described herein. Various embodiments provide a crosslinked reaction product
of an embodiment of the composition herein, wherein at least some of the
crosslinkable ampholyte polymer has reacted with at least some of the
crosslinker to form a crosslinked product.
[00169] For example, the composition can include a crosslinkable
ampholyte polymer having about Z" wt% of an ethylene repeating unit
54
CA 02938279 2016-07-28
WO 2015/138018 PCT/1JS2014/069506
including the -C(0)NH2 group, about N" wt% of an ethylene repeating unit
including a -S(0)20R1 group, and about M" wt% of an ethylene repeating unit
including an -N-1R23X- group. At each occurrence R1 can be independently
selected from the group consisting of -H and a counterion. At each occurrence,
R2 can be independently substituted or unsubstituted (CI-C20)hydrocarbyl. At
each occurrence, X- can be independently a counterion. The repeating units are
in block, alternate, or random configuration. The variable Z" can be about 10%
to about 70%, N" can be about 1% to about 40%, and M" can be about 20% to
about 80%. The crosslinkable ampholyte polymer can have a molecular weight
of about 100,000 g/mol to about 20,000,000 g/mol. The composition can
include at least one crosslinker. The composition can also include a downhole
fluid. Additionally or alternatively to the composition including the
crosslinkable ampholyte polymer and the crosslinker, the composition can
include a reaction product of the crosslinkable ampholyte polymer and the
crosslinker (e.g., a reaction product of a crosslinking reaction between the
crosslinkable ampholyte polymer and the crosslinker).
[00170] In some embodiments, the crosslinkable ampholyte polymer
includes repeating units having the structure:
NH NH NH2
0 =S=0
H3C ¨N¨C H3
I
0R1 Xe
CH3
At each occurrence le can be independently selected from the group consisting
of -H and a counterion. The repeating units are in a block, alternate, or
random
configuration, and each repeating unit is independently in the orientation
shown
or in the opposite orientation. The crosslinkable ampholyte polymer can have a
molecular weight of about 100,000 g/mol to about 20,000,000 g/mol. The
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
crosslinkable ampholyte polymer can have about 30 wt% to about 50 wt% of the
ethylene repeating unit including the -C(0)N111 group, about 5 wt% to about 15
wt% of the ethylene repeating unit including the -S(0)20R' group, and about 40
wt% to about 60 wt% of the ethylene repeating unit including the -1=11223X-
group. The composition can include a crosslinker including polyethyleneimine.
The composition can also include a downhole fluid including at least one of a
drilling fluid, a fracturing fluid, a diverting fluid, and a lost circulation
treatment
fluid. About 0.001 wt% to about 30 v/v% of the composition is the
crosslinkable
ampholyte polymer and the crosslinker, with the remainder being the downhole
fluid and other optional components. Additionally or alternatively to the
composition including the crosslinkable ampholyte polymer and the crosslinker,
the composition can include a reaction product of the crosslinkable ampholyte
polymer and the crosslinker (e.g., a reaction product of a crosslinking
reaction
between the crosslinkable ampholyte polymer and the crosslinker).
Method for preparing a composition for treatment of a subterranean formation.
[00171] In various embodiments, the present invention provides a
method
for preparing a composition for treatment of a subterranean formation. The
method can be any suitable method that produces an embodiment of the
composition including the crosslinkable ampholyte polymer and the crosslinker,
or a reaction product thereof, described herein. For example, the method can
include forming a composition including an embodiment of the crosslinkable
ampholyte polymer and the crosslinker, or a reaction product thereof. In some
embodiments, the composition further includes a downhole fluid.
Examples
[00172] Various embodiments of the present invention can be better
understood by reference to the following Examples which are offered by way of
illustration. The present invention is not limited to the Examples given
herein.
56
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
Part I. Viscosifier.
Example 1.
[00173] Two samples of an ampholyte polymeric compound including a
terpolymer of acrylamide, 2-acrylamido-2-methylpropane sulfonic acid, and
acryloyloxy ethyl trimethyl ammonium chloride in water were prepared at 5
ga1/1,000 gal and 20 gal/1,000 gal. The ampholyte terpolymer had 40 wt%
monomers from acrylamide, 10 wt% monomers from 2-acrylamido-2-
methylpropane sulfonic acid, and 50 wt% monomers from acryloyloxy ethyl
trimethyl ammonium chloride (AEIAC). The samples were heated from 77 F to
150 F at a rate of 10 F/min and then held at a constant temperature of 150 F
at a
shear rate of 40 s-1. As shown in FIG. 3, the viscosity at the higher
concentration
reduces from about 155 cP to less than about 5 cP in about 90 minutes, while
at
the lower concentration from about 35 cP to less than about 5 cP in about 20-
25
minutes.
[00174] This example illustrates that treatment fluids including the
ampholyte polymeric compounds described herein can reduce in viscosity over
time (e.g., can break over time), which can advantageously allow for the use
of
little to no breaker in the treatment fluids or in subsequent wellbore
operations.
Example 2,
[00175] Samples were prepared with (1) linear xanthan (known to
viscosify high TDS fluids) at 60 lb/1,000 gal and (2) an ampholyte polymeric
compound including a terpolymer of acrylamide, 2-acrylamido-2-methylpropane
sulfonic acid, and acryloyloxy ethyl trimethyl ammonium chloride at 60
ga1/1,000 gal, each in base fluids of (1) water and (2) salt water with an
additional 3% KCI. The ampholyte terpolymer had the same wt% distribution of
monomers as the ampholyte terpolymer used in Example 1. The viscosity of
each sample was analyzed at 77 F and 150 F at a shear rate of 40 s-1. FIG. 4
(water samples) illustrates that the ampholyte polymeric compound provides
higher viscosity than linear xanthan in water. While FIG. 5 (salt water
samples)
illustrates that in a high TDS environment the ampholyte polymeric compound
provides for a comparable viscosity to linear xanthan.
57
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
[00176] This example illustrates that treatment fluids can be
viscosified to
levels comparable to that of traditional viscosifying agents, including in
high
TDS fluids.
Part II. Friction reduction.
Example 3.
[00177] Samples were prepared with individual friction reducers at a
concentration of 1 gallon per thousand gallons (e.g., 0.1% by volume) in
water:
(1) a commercially available friction reducing agent containing
partially hydrolyzed polyacrylamide;
(2) a multi-component, cationic friction reducing agent; and
(3) an ampholyte polymeric compound including a terpolymer of
acrylamide, 2-acrylamido-2-methylpropane sulfonic acid, and
acryloyloxy ethyl trimethyl ammonium chloride. The ampholyte
terpolymer had the same wt% distribution of monomers as the
ampholyte terpolymer used in Example 1.
[00178] The salinity of the samples (measured as ppm of TDS) was then
increased as the percent friction reduction ("%FR") was analyzed by pumping
the sample through a test pipe while measuring the pressure drop with a
pressure
transducer. The %FR was calculated based on the ratio between the measured
pressure drop of the sample and the pressure drop of a fresh water control
sample at the same tested flow rate and ambient temperature and pressure.
[00179] As shown in FIG. 6, the Sample 1 showed an immediate decline
in the %FR with increased salinity and a dramatic drop in %Fli. to essentially
no
friction reduction from about 100,000 to about 150,000 ppm TDS. Samples 2
and 3 showed similar performance over the salinity range tested with only
about
a 5%-10% variations in the %FR from 0 ppm to about 250,000 ppm TDS.
[00180] This example demonstrates that the one-component friction
reducing agent of an ampholyte polymeric compound outperforms other
polymeric friction reducing agents with increased TDS and provides comparable
performance to the more complex friction reducing agents, which tend to be
expensive and complicated to implement.
58
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
Example 4.
[00181] Samples of an ampholyte polymeric compound including a
terpolymer of acrylamide, 2-acrylamido-2-methylpropane sulfonic acid, and
acryloyloxy ethyl trimethyl ammonium chloride in water were analyzed for
degradation rates by analyzing the viscosity of the fluid over time at various
temperatures:
(1) room temperature;
(2) ramp to 150 F; and
(3) ramp to 190 F.
[00182] The ampholyte terpolymer had the same wt% distribution of
monomers as the ampholyte terpolymer used in Example 1. As shown in FIG. 7,
the viscosity of the room temperature sample decreased from about 4.75 cP to
about 1 cP over about 6 hours while the 150 F sample decreased from about 5
cP to about 0.4 cP over about 25 minutes and the 190 F sample decreased from
about 5 cP to about 0.4 cP over about 15 minutes. Reduction in viscosity to
such
levels indicates that the polymer was partially hydrolyzed and contracted. As
shown, the hydrolysis was temperature dependent, indicating that in some
instances the native temperature of the subterranean formation may be such
that
an ampholyte polymeric compound may be capable of breaking with minimal to
no additional breaker.
Example 5.
[00183] Samples were prepared with (1) partially hydrolyzed
polyacrylamide in water (2) an ampholyte polymeric compound including a
terpolymer of acrylamide, 2-acrylamido-2-methylpropane sulfonic acid, and
acryloyloxy ethyl trimethyl ammonium chloride in water. The ampholyte
terpolymer had the same wt% distribution of monomers as the ampholyte
terpolymer used in Example 1. The concentration of each of the polymers was
at infinite dilution. The intrinsic viscosity of the samples were measured
over
about 75 hours. As illustrated in FIG. 8, the ampholyte polymeric compound
sample reduced in intrinsic viscosity from about 95 dUg to about 2 dUg, while
the polyacrylamide sample had a relatively steady intrinsic viscosity of about
100 dLig over the 75 hour time frame. This demonstrates that the ampholyte
polymeric compounds may be capable of breaking over time without the use of
59
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
chemical breakers due, at least in part, to the partial hydrolysis of the
ampholyte
polymeric compound (e.g., the acryloyloxy ethyl trimethyl ammonium chloride
to acrylic acid).
Example 6.
[00184] Samples were prepared with an ampholyte polymeric compound
including a terpolymer of acrylamide, 2-acrylamido-2-methylpropane sulfonie
acid, and acryloyloxy ethyl trimethyl ammonium chloride at 0.1 vol% in (1)
water, (2) 50,000 ppm brine, and (3) 250,000 ppm brine. The ampholyte
terpolymer had the same wt% distribution of monomers as the ampholyte
terpolymer used in Example 1. The samples were heated to 150 F, and the
viscosity of each sample was analyzed at a shear rate of 40 s4. FIG. 9
illustrates
that the sample in water achieved the highest initial viscosity, while both of
the
brine samples achieved about 1/3 the initial viscosity as the water sample.
However, over time, the higher the TDS of the sample the less reduction in the
viscosity (e.g., less hydrolysis and contraction of the ampholyte polymeric
compound).
Example 7.
[00185] Samples were prepared with (1) 0.1 vol% polyaerylamide, (2)
0.1
vol% polyacrylamide and 1 lb/1,000 gal of a chemical breaker, and (3) 0.1 vol%
of an ampholyte polymeric compound including a terpolymer of acrylamide, 2-
acrylamido-2-methylpropane sulfonic acid, and acryloyloxy ethyl trimethyl
ammonium chloride in water. The ampholyte terpolymer had the same wt%
distribution of monomers as the ampholyte terpolymer used in Example 1.
Samples were run through various core/sand pack samples to determine the
regain permeability of the core/sand pack samples after treatment.
[00186] In the regain permeability tests, the initial permeability was
measured by flowing 7% KCl through the core/sand pack sample. Then, the
samples were pumped through the core/sand pack sample at a rate of five pore
volumes. The treated core/sand pack sample was shut-in overnight at 150 F.
The permeability was once again tested by flowing 7% KC1 through the
core/sand pack sample. Table 1 provides the initial permeability and percent
of
permeability regained.
CA 02938279 2016-07-28
WO 2015/138018
PCT/U52014/069506
[00187] Table 1. Initial permeability and percent of permeability
regained.
Fluid Sample Core/Sand Pack Initial Permeability Regain Permeability
100 mesh sand
(2) 1.5 D 96%
pack
100 mesh sand
(3) 1.6 D 98%
pack
(1) Berea core 91 mD 29%
(2) Berea core 106 mD 83%
(3) Berea core 77 mD 80%
(2) Nugget 2.5 mD 54%
(3) Nugget 1.8 mD 61%
[00188] This example demonstrates that the ampholyte polymeric
compound, with no additional chemical breaker, provides for similar or better
regain in permeability to a traditional friction reducer with a chemical
breaker.
Part III. Crosslinked polymer.
Example 8.
[00189] An aqueous solution with 40 gpt ampholyte terpolymer and 10
gpt polyethyleneimine was prepared. The ampholyte terpolymer had the same
wt% distribution of monomers as the ampholyte terpolymer used in Example 1.
The terpolymer was crosslinked by exposing it to elevated temperature (150 F
for about 2 h). FIG.10 shows a photo of the crosslinked ampholyte terpolymer.
[00190] As a comparative sample, an aqueous solution of 25 ppt (parts
per
thousand) guar gum and 2 gpt crosslinker (an instant borate crosslinker) was
crosslinked at room temperature.
Example 9. Viscosity measurement.
[00191] FIG. 11 shows the viscosity curve for the crosslinked
ampholyte
tcrpolyrner of Example 8 at room temperature. It was a pseudoplastic fluid and
exhibited a strong yield-stress. These properties can be helpful for fluid
loss and
diverting applications. The n and K value were 0.2 and 107.1 Pes '2
respectively.
61
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
Example 10. Small amplitude oscillation shear testing,
[00192] Small amplitude oscillation shear (SAOS) testing was performed
to further investigate the structure of the crosslinked materials of Example
8.
FIG. 12 illustrates the results for the crosslinked ampholyte terpolymer and
crosslinked guar gum at room temperature, with a strain of 10% for each test.
[00193] FIG. 12 shows that the storage modulus (1' of the crosslinked
ampholyte terpolymer was relatively constant over a wide range of frequency
and no crossover was observed, indicating a solid-like material, and its CP
was
about 10 times higher than the crosslinked guar.
[00194] FIG. 13 illustrates the SAOS test results for the crosslinked
materials at 150 F, again using 10% strain. At high temperatures, crosslinked
guar became a viscous fluid. As shown in FIG. 13, the G" of the crosslinked
guar gum was greater than G' over the tested frequency, illustrating that the
proppant transport capability decreases due to the reduction in elasticity. In
contrast, not much change was observed for the crosslinked ampholyte
terpolymer. Two conclusions can be drawn from this result. First, crosslinked
ampholyte terpolymer had a much higher elasticity than crosslinked guar, which
can help to suspend the proppant. Second, the crosslinked ampholyte polymer
can tolerate higher temperatures than crosslinked guar, e.g., the gel has
better
temperature stability.
Example 11. Core flow testing.
[00195] Core flow testing was conducted to check the regain
permeability
for crosslinked polyacrylamide and the crosslinked ampholyte terpolymer of
Example 8, The treatment fluid was flown through the core at 5 pore volume,
and the cell was shut-in at 150 17 overnight. 7%,KCI brine was used to flow
through the core and obtain the permeability data. FIG. 14 illustrates the
permeability profile for a crosslinked mixture of 40 gpt polyacrylamide
(having
30 mol% hydrolyzed acrylamide units, having a molecular weight of about
10,000,000) with 10 gpt polyethyleneimine. FIG. 15 illustrates the
permeability
profile for a crosslinked mixture of 40 gpt ampholyte terpolymer with 10 gpt
PEI, where 5PV refers to 5 pore volume, wherein the volume of the fluid is 5
times the pore volume of the core. Table 2 summarizes the results for both
tests.
62
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
[00196] Table 2. Summary of regain permeability for crosslinked
polyacrylamide and crosslinked ainplaolyte, terpolymer
Fluid Initial Regain
permeability permeability
gpt polyacrylamide + 10 gpt PEI, 2.1 Darcy 1.21%
crosslinked
40 gpt ampholyte terpolymer + 10 gpt 7.3 Darcy 0.01%
PEI, crosslinked
[00197] Though the initial permeability for the crosslinked ampholyte
terpolymer test was three times higher than the crosslinked polyacrylamide
test,
it still showed almost 0% regain permeability after the treatment whereas the
crosslinked polyacrylarnide showed 1.2% regain permeability. The crosslinked
ampholytc terpolymer was more effective in reducing the permeability of a
formation.
[00198] The terms and expressions that have been employed are used as
terms of description and not of limitation, and there is no intention in the
use of
such terms and expressions of excluding any equivalents of the features shown
and described or portions thereof, but it is recognized that various
modifications
are possible within the scope of the embodiments of the present invention.
Thus,
it should be understood that although the present invention has been
specifically
disclosed by specific embodiments and optional features, modification and
variation of the concepts herein disclosed may be resorted to by those of
ordinary skill in the art, and that such modifications and variations are
considered to be within the scope of embodiments of the present invention.
Additional Embodiments.
[00199] The following exemplary embodiments are provided, the
numbering of which is not to be construed as designating levels of importance:
[00200] Embodiment 1 provides a method of treating a subterranean
formation, the method comprising:
obtaining or providing a composition comprising
63
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
a crosslinkable ampholyte polymer comprising an ethylene
repeating unit comprising a -C(0)NH2 group, an ethylene repeating unit
comprising an -S(0)20R1 group, and an ethylene repeating unit comprising an -
NtR23X- group, wherein
at each occurrence, R1 is independently selected from the
group consisting of -H and a counterion,
at each occurrence, R2 is independently substituted or
unsubstituted (C1-C20)hydrocarbyl, and
at each occurrence, X- is independently a counterion; and
at least one crosslinker; and
placing the composition in a subterranean formation.
[00201] Embodiment 2 provides the method of Embodiment 1, wherein
the obtaining or providing of the composition occurs above-surface.
[00202] Embodiment 3 provides the method of any one of Embodiments
1-2, wherein the obtaining or providing of the composition occurs in the
subterranean formation.
[00203] Embodiment 4 provides the method of any one of Embodiments
1-3, wherein the method is a method of drilling the subterranean formation.
[00204] Embodiment 5 provides the method of any one of Embodiments
1-4, wherein the method is a method of fracturing the subterranean formation.
[00205] Embodiment 6 provides the method of any one of Embodiments
1-5, wherein the method is a method of fluid loss control or diverting.
[00206] Embodiment 7 provides the method of any one of Embodiments
1-6, wherein the composition comprises an aqueous liquid.
[00207] Embodiment 8 provides the method of Embodiment 7, wherein
the method further comprises mixing the aqueous liquid with the crosslinkable
ampholyte polymer and the crosslinker.
[00208] Embodiment 9 provides the method of Embodiment 8, wherein
the mixing occurs above surface.
[00209] Embodiment 10 provides the method of Embodiment 9, wherein
the mixing occurs in the subterranean formation.
[00210] Embodiment 11 provides the method of any one of Embodiments
7-10, wherein the aqueous liquid comprises at least one of water, brine,
produced water, flowback water, brackish water, and sea water.
64
CA 02938279 2016-07-28
WO 2015/138018
PCPUS2014/069506
[00211] Embodiment 12 provides the method of any one of Embodiments
7-11, wherein the aqueous liquid comprises salt water having a total dissolved
solids level of about 1,000 mg/L to about 300,030 mg/L.
[00212] Embodiment 13 provides the method of any one of Embodiments
7-12, wherein the aqueous liquid comprises at least one of a drilling fluid, a
fracturing fluid, a diverting fluid, and a lost circulation treatment fluid.
[00213] Embodiment 14 provides the method of any one of Embodiments
1-13, further comprising at least partially crosslinking the crosslinkable
ampholyte polymer to provide a crosslinked ampholyte polymer.
[00214] Embodiment 15 provides the method of Embodiment 14, wherein
the crosslinking occurs at least partially above-surface.
[00215] Embodiment 16 provides the method of any one of Embodiments
14-15, wherein the crosslinking occurs at least partially in the subterranean
formation.
[00216] Embodiment 17 provides the method of any one of Embodiments
14-16, wherein the crosslinking is at least partially triggered by an increase
in
temperature.
[00217] Embodiment 18 provides the method of Embodiment 17, wherein
the increase in temperature is at least partially due to placement of the
composition in the subterranean formation.
[00218] Embodiment 19 provides the method of any one of Embodiments
14-18, wherein the crosslinking comprises exposing the composition to a
temperature of about 100 F to about 450 F.
[00219] Embodiment 20 provides the method of any one of Embodiments
14-19, wherein the crosslinking comprises exposing the composition to a
temperature of about 125 F to about 250 F.
[00220] Embodiment 21 provides the method of any one of Embodiments
14-20, wherein after the crosslinking, a viscosity of the composition, at
standard
temperature and pressure and at a shear rate of about 50 s-1 to about 500 s-1,
is
about 10 cP to about 1,000,000 cP.
[00221] Embodiment 22 provides the method of any one of Embodiments
14-21, wherein after the crosslinking, a viscosity of the composition, at
standard
temperature and pressure and at a shear rate of about 50 s-1 to about 500 s-1,
is
about 1,000 cP to about 500,000 cP.
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
[00222] Embodiment 23 provides the method of any one of Embodiments
14-22, wherein after the crosslinking, a viscosity of the composition, at
standard
temperature and pressure and at a shear rate of about 0 s-1 to about 1 s-1, is
about
cP to about 10,000,000 cP.
[00223] Embodiment 24 provides the method of any one of Embodiments
14-23, wherein after the crosslinking, a yield stress of the composition, at
standard temperature and pressure, is about 0.1 Pa and about 1,000 Pa.
[00224] Embodiment 25 provides the method of any one of Embodiments
14-24, wherein after the crosslinking, a yield stress of the composition, at
standard temperature and pressure, is about 1 Pa to about 500 Pa.
[00225] Embodiment 26 provides the method of any one of Embodiments
14-25, wherein prior to the crosslinking, a viscosity of the composition, at
standard temperature and pressure and at a shear rate of about 50 s-1 to about
500
is about 0.01 cP to about 1,000,000 cP.
[00226] Embodiment 27 provides the method of any one of Embodiments
14-26, wherein prior to the crosslinking, a viscosity of the composition, at
standard temperature and pressure and at a shear rate of about 50 s-1 to about
500
-
s1 i , s about 0.01 cP to about 10,000 cP.
[00227] Embodiment 28 provides the method of any one of Embodiments
1-27, wherein the crosslinkable ampholyte polymer is sufficient such that,
when
crosslinked in an aqueous solution at a concentration of about 40 gpt with a
polyethyleneimine crosslinker at a concentration of about 10 ppt to form a
crosslinked ampholyte polymer, at 77 cF and standard pressure, with a strain
of
about 10%, at a frequency of about 0.1 rad/s to about 100 rad/s, the aqueous
solution comprising the crosslinked ampholyte polymer has a loss modulus G"
of about 0.1 Pa to about 100 Pa.
[00228] Embodiment 29 provides the method of any one of Embodiments
1-28, wherein the crosslirtkable ampholyte polymer is sufficient such that,
when
crosslinked in an aqueous solution at a concentration of about 40 gpt with a
polyethyleneimine crosslinker at a concentration of about 10 ppt to form a
crosslinked ampholyte polymer, at 150 F and standard pressure, with a strain
of
about 10%, at a frequency of about 0.1 rad/s to about 100 rad/s, the aqueous
solution comprising the crosslinked polymer has a storage modulus G' of about
10 Pa to about 1000 Pa.
66
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
[00229] Embodiment 30 provides the method of any one of Embodiments
1-29, wherein the crosslinkable ampholyte polymer is sufficient such that,
when
crosslinked in an aqueous solution at a concentration of about 40 gpt with a
polyethyleneimine crosslinker at a concentration of about 10 ppt to form a
crosslinked ampholyte polymer, at 150 F and standard pressure, with a strain
of
about 10%, at a frequency of about 0.1 rad/s to about 100 rad/s, the aqueous
solution comprising the crosslinked polymer has a loss modulus G" of about 0.5
Pa to about 10 Pa.
[00230] Embodiment 31 provides the method of any one of Embodiments
1-30, wherein about 0.001 wt% to about 95 wt% of the composition is the
crosslinkable ampholyte polymer.
[00231] Embodiment 32 provides the method of any one of Embodiments
1-31, wherein about 70 wt% to about 90 wt% of the composition is the
crosslinkable ampholyte polymer.
[00232] Embodiment 33 provides the method of any one of Embodiments
7-32, wherein about 0.01 wt% to about 50 wt% of the composition is the
crosslinkable ampholyte polymer.
[00233] Embodiment 34 provides the method of any one of Embodiments
7-33, wherein about 0.001 to about 30 v/v% of the composition is the
crosslinkable ampholyte polymer.
[00234] Embodiment 35 provides the method of any one of Embodiments
1-34, wherein the crosslinkable ampholyte polymer has about wt% of the
ethylene repeating unit comprising the -C(0)NH2 group, about N" wt% of the
ethylene repeating unit comprising the -S(0)20121 group, and about M" wt% of
the ethylene repeating unit comprising the -N R23X- group, wherein Z" is about
10% to about 70%, 1\T" is about 1% to about 40%, and M" is about 20% to
about 80%.
[00235] Embodiment 36 provides the method of Embodiment 35, wherein
Z" is about 30% to about 50%, N" is about 5% to about 15%, and M" is about
40% to about 60%.
[00236] Embodiment 37 provides the method of any one of Embodiments
35-36, wherein Z" + N" + M" is about 100%.
[00237] Embodiment 38 provides the method of any one of Embodiments
1-37, wherein the crosslinkable ampholyte polymer has about Zin'imol% of the
67
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
ethylene repeating unit comprising the -C(0)NH2 group, about N'Imol% of the
ethylene repeating unit comprising the -S(0)20R' group, and about M"1mol%
of the ethylene repeating unit comprising the -10223X- group, wherein Z146 is
about 5% to about 50%, NI441 is about 1% to about 40%, and Ne 1 is about 40%
to about 90%.
[00238] Embodiment 39 provides the method of Embodiment 38, wherein
Zn" is about 10% to about 25%, N"4 is about 5% to about 20%, and M"I is
about 55% to about 70%.
[00239] Embodiment 40 provides the method of any one of Embodiments
38-39, wherein Z"4 + + 1V1"4 is about 100%.
[00240] Embodiment 41 provides the method of any one of Embodiments
1-40, wherein the crosslinkable ampholyte polymer has a molecular weight of
about 100,000 g/mol to about 20,000,000 g/mol.
[00241] Embodiment 42 provides the method of any one of Embodiments
1-41, wherein the crosslinkable ampholyte polymer has a molecular weight of
about 5,000,000 g/mol to about 15,000,000 g/mol.
[00242] Embodiment 43 provides the method of any one of Embodiments
1-42, wherein the crosslinkable ampholyte polymer comprises repeating units
having the structure:
¨ ¨ _ _
R3 R3 R3
R5 R5 R5
R4 R4 R4
I 9
o R2¨N¨R2 ____________________________________ 0
Xe
R2 NH2 ,
wherein
at each occurrence R3, R4, and R5 are each independently selected
from the group consisting of -H and a substituted or unsubstituted Ci-05
hydrocarbyl,
at each occurrence LI, L2, and L3 are each independently selected
from the group consisting of a bond and a substituted or unsubstituted C1-C20
68
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
hydrocarbyl interrupted or terminated with 0, 1, 2, or 3 of at least one of -
NR3-, -
S-, and -0-, and
the repeating units are in a block, alternate, or random
configuration, and each repeating unit is independently in the orientation
shown
or in the opposite orientation.
[00243] Embodiment 44 provides the method of Embodiment 43, wherein
at each occurrence I) is independently selected from the group consisting of a
bond and -(substituted or unsubstituted Ci-C20 hydrocarby1)-NR3-(substituted
or
unsubstituted Ci-C20 hydrocarby1)-.
[00244] Embodiment 45 provides the method of any one of Embodiments
43-44, wherein at each occurrence LI is independently -C(0)-NH-(substituted or
unsubstituted C1-C19 hydrocarby1)-.
[00245] Embodiment 46 provides the method of any one of Embodiments
43-45, wherein at each occurrence Li is independently -C(0)-NH-(C1-05
hydrocarb y1)-.
[00246] Embodiment 47 provides the method of any one of Embodiments
43-46, wherein Lt is -C(0)-NH-CH(C113)2-CH2-.
[00247] Embodiment 48 provides the method of any one of Embodiments
4347, wherein at each occurence L2 is independently selected from the group
consisting of -0-(C1-C20)hydrocarbyl- and -N123-(C1-C20)hydrocarbyl-.
[00248] Embodiment 49 provides the method of any one of Embodiments
43-48, wherein at each occurrence L2 is independently selected from -0-(C1-
Cio)alkyl- and -NH-(C1-Cio)alkyl-.
[00249] Embodiment 50 provides the method of any one of Embodiments
4349, wherein at each occurrence L2 is independently selected from -0-012-
CH2- and -NH-CH2-C112.
[00250] Embodiment 51 provides the method of any one of Embodiments
43-50, wherein at each occurrence L3 is independently selected from the group
consisting of a bond and C1-C20 hydrocarbyl.
[00251] Embodiment 52 provides the method of any one of Embodiments
43-51, wherein at each occurrence L3 is independently selected from the group
consisting of a bond and C1-05 alkyl.
[00252] Embodiment 53 provides the method of any one of Embodiments
43-52, wherein at each occurrence L3 is a bond.
69
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
[00253] Embodiment 54 provides the method of any one of Embodiments
43-53, wherein at each occurrence R3, R4, and R5 are independently selected
from the group consisting of -H and a C1-05 alkyl.
[00254] Embodiment 55 provides the method of any one of Embodiments
43-54, wherein at each occurrence R3, R4, and R5 are independently selected
from the group consisting of -H and a C1-C3 alkyl.
[00255] Embodiment 56 provides the method of any one of Embodiments
43-55, wherein at each occurrence R3, R4, and R5 are each -IL
[00256] Embodiment 57 provides the method of any one of Embodiments
43-56, wherein at each occurrence Rt is independently selected from the group
consisting of -H, Na, K+, Li4, N114+, Ziff, Ca2+, Zn2+, A134, and Mg2+.
[00257] Embodiment 58 provides the method of any one of Embodiments
43-57, wherein at each occurrence RI is -H.
[00258] Embodiment 59 provides the method of any one of Embodiments
43-58, wherein at each occurrence R2 is independently (Ci-C20)alkyl.
[00259] Embodiment 60 provides the method of any one of Embodiments
43-59, wherein at each occurrence R2 is independently (Ci-Cto)alkyl.
[00260] Embodiment 61 provides the method of any one of Embodiments
43-60, wherein at each occurrence R2 is independently selected from the group
consisting of methyl, ethyl, propyl, butyl, and pentyl.
[00261] Embodiment 62 provides the method of any one of Embodiments
43-61, wherein X- is Cl.
[00262] Embodiment 63 provides the method of any one of Embodiments
43-62, wherein n is about 4 to about 40,000.
[00263] Embodiment 64 provides the method of any one of Embodiments
43-63, wherein n is about 450 to about 14,500.
[00264] Embodiment 65 provides the method of any one of Embodiments
43-64, wherein m is about 100 to about 83,000.
[00265] Embodiment 66 provides the method of any one of Embodiments
43-65, wherein m is about 4,000 to about 62,000.
[00266] Embodiment 67 provides the method of any one of Embodiments
43-66, wherein z is about 125 to about 200,000.
[00267] Embodiment 68 provides the method of any one of Embodiments
43-67, wherein z is about 8,500 to about 140,000.
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
[00268] Embodiment 69 provides the method of any one of Embodiments
1-68, wherein the crosslinkable ampholyte polymer comprises repeating units
having the structure:
-n - -m - -z
__________________________ 0 ______ 0 ______ 0
NH 0 NH2
>K.
0=S =0
H3C N __ CH3
I Xe
OR1
CH3
wherein the repeating units are in a block, alternate, or random
configuration, and each repeating unit is independently in the orientation
shown
or in the opposite orientation.
[00269] Embodiment 70 provides the method of any one of Embodiments
1-69, wherein the crosslinkable ampholyte polymer comprises repeating units
having the structure:
__________________________ 0 ______ 0 ______ 0
NH NH NI-I2
0=s=0
H3C ¨N ¨CH3
0
0 R1 I X
CH3
wherein the repeating units are in a block, alternate, or random
configuration,
and each repeating unit is independently in the orientation shown or in the
opposite orientation.
71
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
[00270] Embodiment 71 provides the method of any one of Embodiments
1-70, wherein about 0.000,1 wt% to about 80 wt% of the composition is the
crosslinker.
[00271] Embodiment 72 provides the method of any one of Embodiments
1-71, wherein about 1 wt% to about 30 wt% of the composition is the
crosslinker.
[00272] Embodiment 73 provides the method of any one of Embodiments
7-72, wherein about 0.000,1 wt% to about 50 wt% of the composition is the
crosslinker.
[00273] Embodiment 74 provides the method of any one of Embodiments
7-73, wherein about 0.001 wt% to about 5 wt% of the composition is the
crosslinker.
[00274] Embodiment 75 provides the method of any one of Embodiments
1-74, wherein the crosslinker comprises at least one of chromium, aluminum,
antimony, zirconium, titanium, calcium, boron, iron, silicon, copper, zinc,
magnesium, and an ion thereof.
[00275] Embodiment 76 provides the method of any one of Embodiments
1-75, wherein the crosslinker comprises at least one of a poly(aminu(C2-
Cm)hydrocarbylene) crosslinker and a (C6-C20)aryl alcohol-(C1-C20)aldehyde
crosslinker.
[00276] Embodiment 77 provides the method of any one of Embodiments
1-76, wherein the crosslinker comprises at least one of polyethyleneimine,
phenol-formaldehyde, and glyoxal.
[00277] Embodiment 78 provides the method of any one of Embodiments
1-77, wherein the crosslinker comprises at least one of boric acid, borax, a
borate, a (Ci-C30)hydrocarbylboronic acid, a (Ci-C30)hydrocarbyl ester of a
(Ci-
C30)hydrocarbylboronic acid, a (Ci-C30)hydrocarbylboronic acid-modified
polyacrylamide, ferric chloride, disodium octaborate tetrahydrate, sodium
metaborate, sodium diborate, sodium tetraborate, disodium tetraborate, a
pentaborate, ulexite, colemanite, magnesium oxide, zirconium lactate,
zirconium
triethanol amine, zirconium lactate triethanolamine, zirconium carbonate,
zirconium acetylacetonate, zirconium malate, zirconium citrate, zirconium
diisopropylamine lactate, zirconium glycolate, zirconium triethanol amine
glycolate, zirconium lactate glycolate, titanium lactate, titanium malate,
titanium
72
CA 02938279 2016-07-28
WO 2015/138018 PC
T/US2014/069506
citrate, titanium ammonium lactate, titanium triethanolamine, titanium
acetylacetonate, aluminum lactate, and aluminum citrate.
[002781 Embodiment 79 provides the method of any one of Embodiments
1-78, wherein the composition further comprises a fluid comprising at least
one
of water, an organic solvent, and an oil.
[00279] Embodiment 80 provides the method of any one of Embodiments
1-79, wherein the composition further comprises a fluid comprising at least
one
of dipropylcne glycol methyl ether, dipropylene glycol dimethyl ether,
dimethyl
formamide, diethylene glycol methyl ether, ethylene glycol butyl ether,
diethylene glycol butyl ether, propylene carbonate, D-limonene, a C2-C40 fatty
acid C1-C10 alkyl ester, 2-butoxy ethanol, butyl acetate, furfuryl acetate,
dimethyl sulfoxide, dimethyl formamide, diesel, kerosene, mineral oil, a
hydrocarbon comprising an internal olefin, a hydrocarbon comprising an alpha
olefin, xylenes, an ionic liquid, methyl ethyl ketone, and cyclohexanone.
[00280] Embodiment 81 provides the method of any one of Embodiments
1-80, wherein the composition further comprises a secondary viscosifier.
[00281] Embodiment 82 provides the method of Embodiment 81, wherein
the secondary viscosifier comprises at least one of a substituted or
unsubstituted
polysaccharide, and a substituted or unsubstituted polyalkenylene, wherein the
polysaccharide or polyalkenylene is crosslinked or uncrosslinked.
[00282] Embodiment 83 provides the method of any one of Embodiments
81-82, wherein the secondary viscosifier comprises a polymer comprising at
least one monomer selected from the group consisting of ethylene glycol,
acrylamide, vinyl acetate, 2-acrylamidomethylpropane sulfonic acid or its
salts,
trimethylammoniumethyl acrylate halide, and trimethylammoniumethyl
methacrylate halide.
[00283] Embodiment 84 provides the method of any one of Embodiments
81-83, wherein the secondary viscosifier comprises a crosslinked gel or a
crosslinkable geL
[00284] Embodiment 85 provides the method of any one of Embodiments
81-84, wherein the secondary viscosifier comprises at least one of a linear
polysaccharide, and poly((C2-Cto)alkenylene), wherein the (C2-Cio)alkenylene
is
substituted or unsubstituted.
73
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
[00285] Embodiment 86 provides the method of any one of Embodiments
81-85, wherein the secondary viscosifier comprises at least one of
poly(acrylic
acid) or (CI-05)alkyl esters thereof, poly(methacrylic acid) or (C1-05)alkyl
esters
thereof, poly(vinyl acetate), poly(vinyl alcohol), poly(ethylene glycol),
poly(vinyl pyrrolidone), polyacrylamide, poly (hydroxyethyl methacrylate),
alginate, chitosan, curdlan, dextran, emulsan, a galactoglucopolysaccharide,
gellan, glucuronan, N-acetyl-glucosamine, N-acetyl-heparosan, hyaluronic acid,
kefiran, lentinan, lcvan, mauran, pullulan, scleroglucan, schizophyllan,
stewartan, succinoglycan, xanthan, welan, derivatized starch, tamarind,
tragacanth, guar gum, derivatized guar, gum ghatti, gum arabic, locust bean
gum, derivatized cellulose, carboxymethyl cellulose, hydroxyethyl cellulose,
carboxymethyl hydroxyethyl cellulose, hydroxypropyl cellulose, methyl
hydroxyl ethyl cellulose, guar, hydroxypropyl guar, carboxy methyl guar, and
carboxymethyl hydroxylpropyl guar.
[00286] Embodiment 87 provides the method of any one of Embodiments
81-86, wherein the secondary viscosifier comprises poly(vinyl alcohol)
homopolymer, poly(vinyl alcohol) copolymer, a crosslinked poly(vinyl alcohol)
homopolymcr, and a crosslinked poly(vinyl alcohol) copolymer.
[00287] Embodiment 88 provides the method of any one of Embodiments
1-87, wherein the composition further comprises a secondary crosslinker
comprising at least one of chromium, aluminum, antimony, zirconium, titanium,
calcium, boron, iron, silicon, copper, zinc, magnesium, and an ion thereof.
[00288] Embodiment 89 provides the method of Embodiment 88, wherein
the secondary crosslinker comprises at least one of boric acid, borax, a
borate, a
(C1-C30)hydrocarbylboronic acid, a (Ci-C30)hydrocarbyl ester of a (Ci-
C30)hydrocarbylboronic acid, a (Cl-C30)hydrocarbylboronic acid-modified
polyacrylamide, ferric chloride, disodium octaborate tetrahydrate, sodium
metaborate, sodium diborate, sodium tetraborate, disodium tetraborate, a
pentaborate, ulexite, colemanite, magnesium oxide, zirconium lactate,
zirconium
triethanol amine, zirconium lactate triethanolamine, zirconium carbonate,
zirconium acetylacctonate, zirconium malate, zirconium citrate, zirconium
diisopropylamine lactate, zirconium glycolate, zirconium triethanol amine
glycolate, zirconium lactate glycolate, titanium lactate, titanium malate,
titanium
74
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
citrate, titanium ammonium lactate, titanium triethanolamine, titanium
acetylacetonate, aluminum lactate, and aluminum citrate.
[00289] Embodiment 90 provides the method of any one of Embodiments
1-89, further comprising combining the composition, or a crosslinked reaction
product thereof, with an aqueous or oil-based fluid comprising a drilling
fluid,
stimulation fluid, fracturing fluid, spotting fluid, clean-up fluid,
completion
fluid, remedial treatment fluid, abandonment fluid, pill, acidizing fluid,
cementing fluid, packer fluid, or a combination thereof, to form a mixture,
wherein the placing the composition in the subterranean formation comprises
placing the mixture in the subterranean formation.
[00290] Embodiment 91 provides the method of any one of Embodiments
1-90, wherein at least one of prior to, during, and after the placing of the
composition in the subterranean formation, the composition, or a crosslinked
reaction product thereof, is used in the subterranean formation, at least one
of
alone and in combination with other materials, as a drilling fluid,
stimulation
fluid, fracturing fluid, spotting fluid, clean-up fluid, completion fluid,
remedial
treatment fluid, abandonment fluid, pill, acidizing fluid, cementing fluid,
packer
fluid, or a combination thereof.
[00291] Embodiment 92 provides the method of any one of Embodiments
1-91, wherein the composition further comprises water, saline, aqueous base,
oil,
organic solvent, synthetic fluid oil phase, aqualus solution, alcohol or
polyol,
cellulose, starch, alkalinity control agent, acidity control agent, density
control
agent, density modifier, emulsifier, dispersant, polymeric stabilizer,
crosslinking
agent, polyacrylamide, polymer or combination of polymers, antioxidant, heat
stabilizer, foam control agent, foaming agent, solvent, diluent, plasticizer,
filler
or inorganic particle, pigment, dye, precipitating agent, theology modifier,
oil-
wetting agent, set retarding additive, surfactant, corrosion inhibitor, gas,
weight
reducing additive, heavy-weight additive, lost circulation material,
filtration
control additive, salt, fiber, thixotropic additive, breaker, crosslinker,
gas,
rheology modifier, curing accelerator, curing retarder, pH modifier,
chelatirtg
agent, scale inhibitor, enzyme, resin, water control material, polymer,
oxidizer, a
marker, Portland cement, pozzolana cement, gypsum cement, high alumina
content cement, slag cement, silica cement, fly ash, metakaolin, shale,
zeolite, a
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
crystalline silica compound, amorphous silica, fibers, a hydratable clay,
microspheres, pozzolan lime, or a combination thereof.
[00292] .. Embodiment 93 provides the method of any one of Embodiments
1-92, wherein placing the composition in the subterranean formation comprises
fracturing at least part of the subterranean formation to form at least one
subterranean fracture.
[00293] Embodiment 94 provides the method of any one of Embodiments
1-93, wherein the composition further comprises a proppant, a resin-coated
proppant, or a combination thereof.
[00294] Embodiment 95 provides the method of any one of Embodiments
1-94, wherein the placing of the composition in the subterranean formation in
the subterranean formation comprises pumping the composition through a drill
string disposed in a wellbore, through a drill bit at a downhole end of the
drill
string, and back above-surface through an annulus.
[00295] Embodiment 96 provides the method of Embodiment 95, further
comprising processing the composition exiting the annulus with at least one
fluid
processing unit to generate a cleaned composition and recirculating the
cleaned
composition through the wellbore.
[00296] .. Embodiment 97 provides a system configured to perform the
method of any one of Embodiments 1-96, the system comprising:
the composition comprising the crosslinkable ampholyte polymer and the
crosslinker; and
the subterranean formation comprising the composition therein.
[00297] Embodiment 98 provides the system of Embodiment 97, further
comprising
a drillstring disposed in a wellbore, the drillstring comprising a drill bit
at
a downhole end of the drillstring;
an annulus between the drillstring and the wellbore; and
a pump configured to circulate the composition through the drill string,
through the drill bit, and back above-surface through the annulus.
[00298] .. Embodiment 99 provides the system of Embodiment 98, further
comprising a fluid processing unit configured to process the composition
exiting
the annulus to generate a cleaned composition for recirculation through the
wellbore.
76
CA 02938279 2016-07-28
WO 2015/138018
PCT/1J52014/069506
[00299] Embodiment 100 provides a method of treating a subterranean
formation, the method comprising:
obtaining or providing a composition comprising
a reaction product of a mixture comprising
a crosslinkable ampholyte polymer comprising an
ethylene repeating unit comprising a -C(0)NH2 group, an ethylene repeating
unit
comprising an -S(0)20121 group, and an ethylene repeating unit comprising an -
N-ER23X- group, wherein
at each occurrence, RI is independently selected
from the group consisting of -H and a counterion,
at each occurrence, R2 is independently substituted
or unsubstituted (C1-C20)hydrocarbyl, and
at each occurrence, X- is independently a
counterion; and
at least one crosslinker; and
placing the composition in a subterranean formation.
[00300] Embodiment 101 provides a method of treating a subterranean
formation, the method comprising:
obtaining or providing a composition comprising
a crosslinkable ampholyte polymer comprising repeating units
having the structure:
NH NH N H2
0 =S="---0
H3c¨ HN¨c
Ixe oR1
cH3
wherein
77
CA 02938279 2016-07-28
WO 2015/138018 PC T/US2014/069506
at each occurrence, R1 is independently selected from the
group consisting of -H and a counterion,
the repeating units are in a block, alternate, or random
configuration, and each repeating unit is independently in the orientation
shown
or in the opposite orientation,
the crosslinkable ampholyte polymer has a molecular
weight of about 100,000 g/mol to about 20,000,000 g/mol, and
the crosslinkable ampholyte polymer has about 30 wt% to
about 50 wt% of the ethylene repeating unit comprising the -C(0)NH2 group,
about 5 wt% to about 15 wt% of the ethylene repeating unit comprising the -
S(0)20R1 group, and about 40 wt% to about 60 wt% of the ethylene repeating
unit comprising the -N+R23X- group;
a crosslinker comprising polyethyleneimine; and
a downhole fluid comprising at least one of a drilling fluid, a
fracturing fluid, a diverting fluid, and a lost circulation treatment fluid;
and
placing the composition in a subterranean formation, wherein about
0.001 wt% to about 30 v/v% of the composition is the crosslinkable ampholyte
polymer and the crosslinker.
[00301] Embodiment 102 provides a system comprising:
a composition comprising
a crosslinkable ampholyte polymer having about 7." wt%
of an ethylene repeating unit comprising the -C(0)NH2 group, about N't wt% of
an ethylene repeating unit comprising a -S(0)20R1 group, and about M" wt% of
an ethylene repeating unit comprising an -N-1223X- group, wherein
at each occurrence le is independently selected
from the group consisting of -II and a counterion,
at each occurrence, R2 is independently substituted
or unsubstituted (C1-C20)hydrocarbyl,
at each occurrence, X- is independently a
counterion,
the repeating units are in block, alternate, or
random configuration,
Z" is about 10% to about 70%, N" is about 1% to
about 40%, and M" is about 20% to about 80%, and
78
CA 02938279 2016-07-28
WO 2015/138018 PCT/US2014/069506
the crosslinkable ampholyte polymer has a
molecular weight of about 100,000 g/mol to about 20,000,000 g/mol; and
at least one crosslinker; and
a subterranean formation comprising the composition therein.
[00302] Embodiment 103 provides the system of Embodiment 102,
further comprising
a drillstring disposed in a wellbore, the drillstring comprising a drill bit
at
a downhole end of the drillstring;
an annulus between the drillstring and the wellbore; and
a pump configured to circulate the composition through the drill string,
through the drill bit, and back above-surface through the annulus.
[00303] Embodiment 104 provides the system of Embodiment 103,
further comprising a fluid processing unit configured to process the
composition
exiting the annulus to generate a cleaned drilling fluid for recirculation
through
the wellbore.
[00304] Embodiment 105 provides the system of any one of Embodiments
102-104, further comprising
a tubular disposed in the subterranean formation; and
a pump configured to pump the composition into the subterranean
formation through the tubular.
[00305] Embodiment 106 provides a composition for treatment of a
subterranean formation, the composition comprising:
a crosslinkable ampholyte polymer having about rt wt% of an ethylene
repeating unit comprising the -C(0)N}12 group, about N" wt% of an ethylene
repeating unit comprising a -S(0)20R' group, and about M" wt% of an ethylene
repeating unit comprising an -Isf1223X- group, wherein
at each occurrence RI is independently selected from the group
consisting of -H and a counterion,
at each occurrence, R2 is independently substituted or
unsubstituted (Ci-C20)hydrocarbyl,
at each occurrence, X- is independently a counterion,
the repeating units are in block, alternate, or random
configuration,
79
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
Z" is about 10% to about 70%, Nw` is about 1% to about 40%,
and le is about 20% to about 80%, and
the crosslinkable ampholyte polymer has a molecular weight of
about 100,000 g/mol to about 20,000,000 g/mol;
at least one crosslinker; and
a downhole fluid.
[00306] Embodiment 107 provides the composition of Embodiment 106,
wherein the downholc fluid comprises at least one of a a drilling fluid, a
fracturing fluid, a diverting fluid, and a lost circulation treatment fluid.
[00307] Embodiment 108 provides a crosslinked reaction product of the
composition of Embodiment 106.
[00308] Embodiment 109 provides a composition for treatment of a
subterranean formation, the composition comprising:
a reaction product of a mixture comprising
a crosslinkable ampholyte polymer having about e wt% of an
ethylene repeating unit comprising the -C(0)NH2 group, about N" wt% of an
ethylene repeating unit comprising a -S(0)20R1 group, and about M" wt% of an
ethylene repeating unit comprising an -N-1R23X group, wherein
at each occurrence 121 is independently selected from the
group consisting of -H and a counterion,
at each occurrence, R2 is independently substituted or
unsubstituted (Ci-C20)hydrocarbyl,
at each occurrence, X- is independently a counterion,
the repeating units are in block, alternate, or random
configuration,
Zw` is about 10% to about 70%, N" is about 1% to about
40%, and M" is about 20% to about 80%, and
the crosslinkable ampholyte polymer has a molecular
weight of about 100,000 g/mol to about 20,000,000 g/mol; and
at least one crosslinker; and
a downhole fluid.
[00309] Embodiment 110 provides a system comprising:
the reaction product of the composition of Embodiment 109; and
a subterranean formation comprising the reaction product therein.
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
[00310] Embodiment 111 provides a composition for treatment of a
subterranean formation, the composition comprising:
a crosslinkable ampholyte polymer comprising repeating units having the
structure:
_
-n - -z
NH NH NH2
0 S =0 =
H3C ________________________________ N __ CH3
I X
OR1
CH3
wherein
at each occurrence RI is independently selected from the group
consisting of -H and a counterion,
the repeating units are in a block, alternate, or random
configuration, and each repeating unit is independently in the orientation
shown
or in the opposite orientation,
the crosslinkable ampholyte polymer has a molecular weight of
about 100,000 g/mol to about 20,000,000 emol, and
the crosslinkable ampholyte polymer has about 30 wt% to about
50 wt% of the ethylene repeating unit comprising the -C(0)NH2 group, about 5
wt% to about 15 wt% of the ethylene repeating unit comprising the -S(0)20R1
group, and about 40 wt% to about 60 wt% of the ethylene repeating unit
comprising the -1=11223X- group; and
a crosslinker comprising polyethyleneimine; and
a downhole fluid comprising at least one of a drilling fluid, a fracturing
fluid, a diverting fluid, and a lost circulation treatment fluid, wherein
about
0.001 wt% to about 30 v/v% of the composition is the crosslinkable ampholyte
polymer and the crosslinker.
81
CA 02938279 2016-07-28
WO 2015/138018
PCT/US2014/069506
[00311] Embodiment 112 provides a crosslinked reaction product of the
composition of Embodiment 111.
[00312] Embodiment 113 provides a method of preparing a composition
for treatment of a subterranean formation, the method comprising:
forming a composition comprising
a crosslinkable ampholyte polymer comprising an ethylene
repeating unit comprising a -C(0)NH2 group, an ethylene repeating unit
comprising an -S(0)20R1 group, and an ethylene repeating unit comprising an -
N R.23X- group, wherein
at each occurrence, R1 is independently selected from the
group consisting of -H and a counterion,
at each occurrence, R2 is independently substituted or
unsubstituted (Ci-C20)hydrocarbyl, and
at each occurrence, X- is independently a counterion; and
at least one crosslinker.
[00313] Embodiment 114 provides the composition, apparatus, method, or
system of any one or any combination of Embodiments 1-113 optionally
configured such that all elements or options recited are available to use or
select
from.
82