Note: Descriptions are shown in the official language in which they were submitted.
CA 02940149 2016-08-18
WO 2015/126584
PCT/US2015/013204
PROCESS FOR THE MANUFACTURE OF
HYDROCHLOROFLUOROOLEFINS
Field of The Invention
The present invention relates to a process for the manufacture of
hydrochlorofluoroolefin s.
Background of the Invention
The Montreal Protocol for the protection of the ozone layer mandates the phase
out of
the use of chlorofluorocarbons (CFCs). Materials more "friendly" to the ozone
layer
such as hydrofluorocarbons (HFCs) e.g. 134a replaced chlorofluorocarbons. The
latter compounds have proven to be greenhouse gases, causing global warming
and
could be regulated by the Kyoto Protocol on Climate Change. Replacement
materials
are needed which are environmentally acceptable i.e. have negligible ozone
depletion
potential (ODP) and acceptable low global warming potential (GWP). The present
invention describes a process for manufacturing of the
hydrochlorofluoroolefin, trans
1233zd (E- 1233zd, 1-chloro-3.3,3-trifluorpropene) which is useful as a low
ODP and
low GWP blowing agent for thermoset and thermoplastic foams, solvent, heat
transfer
fluid such as in heat pumps, and refrigerant such as a low pressure
refrigerant for
chillers.
US patent publications US2008/0051610 and US2008/0103342 disclose a process
that
includes a step of the catalytic isomerization of cis 1234ze to trans 1234ze.
US
7,420,094 discloses the isomerization of 1234ze to 1234yf with a Cr based
catalyst.
US 8,217,208 discloses a process of isomerization of one isomer of 1-chloro-
3,3,3-
trifluoropropene to the other isomer including the step of contacting a feed
stream
with a heated surface. US2008/0051611 discloses the recovery of trans 1234ze
from a
mixture that includes cis 1234ze and trans1234ze via distillation.
Summary of the Invention
The present invention relates a process for the manufacture of the
hydrochlorofluoroolefin, trans 1-chloro-3,3,3-trifluoropropene (E-1233zd). The
1
CA 02940149 2016-10-12
process comprises an isomerization step from cis 1233zd (Z-1233zd) to trans
1233zd
(E- 1233zd).
In one aspect, there is provided a process for manufacturing trans 1- chloro
3,3,3
trifluoro propene (E-1233zd) from 1,1,3,3- tetrachloropropene (1230za) and/ or
1,1,1,3,3-pentachloropropane (240fa) comprising the steps of: fluorination of
1,1,3,3-
tetrachloropropene (1230za) and/ or 1,1,1,3,3-pentachloropropane (240fa) to a
mixture comprising cis 1233zd (Z-1233zd) and trans 1233zd (E- 1233zd);
followed
by separation of said cis 1233zd (Z-1233zd) from said trans 1233zd (E-
1233zd);
followed by isomerization of said cis 1233zd (Z-1233zd) to form trans 1233zd
(E-
1233zd) wherein the improvement comprises forming a chloroalkane via addition
of
an olefin to a starting chloroalkane and reacting the chloroalkane to form the
1,1,3,3-
tetrachlororopropene (1230za, CC12=CH-CHC12) and/or 1,1,1,3,3-
pentachoropropane
(240fa).
Brief Summary of the Drawings
Figure 1 is a schematic of a liquid phase process in accordance with the
present
invention.
Figure 2 is schematic of a gas phase process in accordance with the present
invention.
Detailed Description of the Invention
The present invention provides a process for the manufacture of trans 1-chloro-
3,3,3-
trifluoropropene (E-1233zd). The first step of the process comprises forming a
chloroalkane via addition of an olefin to a starting chloroalkane and reacting
the
chloroalkane to form 1,1,3,3- tetrachlororopropene (1230za, CC12=CH-CHC12)
and/or
1,1,1,3,3-pentachoropropane (240fa). That step is followed by a fluorination
step
comprising the fluorination of 1,1,3,3- tetrachlororopropene (1230za, CC12=CH-
CHC12) and/or 1,1,1,3,3-pentaachloropropane (240fa) to a mixture of cis 1233zd
(Z-
1233zd) and trans 1233zd (E- 1233zd). That step is followed by a separation
step that
comprises a separation of the mixture formed in the first step to isolate cis
1233zd
1233zd) from the mixture. That step is followed by an isomerization step that
comprises isomerization of cis 1233zd (Z-1233zd) to trans 1233zd (E- 1233zd).
2
CA 02940149 2016-10-12
The 1,1,1,3,3-pentachloropropane (240fa) or 1,1,3,3-tetrachloropropene
(1230za)
feedstocks are chlorinated compounds generally manufactured using addition
reactions followed by a step such as chloroination or dehydrohalogenation.
The production of chloroalkanes such as 240fa, 250th, 240db, and 230da by the
addition of olefin to a starting chloroalkane is well-known in the art. For
example,
240fa is generally manufactured by the addition of carbon tetrachloride to
vinyl
chloride using a catalyst and a co-catalyst. The co-catalyst is typically an
organic
ligand capable of forming a complex with the catalyst to bringing the catalyst
into
solution. The process may be carried out batch wise or preferably in a
continuous
mode. The production system may be closed to provide complete recycle of the
unreacted feed material haloalkane and haloalkene.
2a
CA 02940149 2016-08-18
WO 2015/126584
PCT/US2015/013204
Useful starting chlorooalkane raw materials for the preparation of the
1,1,1.3,3-
pentachloropropane (240fa) or 1,1,3.3-tetrachloropropene (1230za) feedstocks
of the
present invention include carbon tetrachloride, chloroform and methyl chloride
while
useful olefins include ethylene, vinyl chloride, 1.1-dichloroethene, 1,2-
dichloroethene, trichloroethylene, tetrachlorethylene and
chlorofluoroethylene.
Exemplary reactions of a starting chloroalkane and olefin include:
= addition of carbon tetrachloride to ethylene to provide 1,1.1,3-
tetrachloropropane (250th)
= addition of carbon tetrachloride to vinyl chloride to provide 1,1,1.3,3-
pentachloropropane (240fa)
= addition of methylene chloride to trichloroethylene to provide 1,1,1,2,3-
pentachloropropane (240db)
= addition of carbon tetrachloride to 1,2-dichloroethylene to provide
1,1,1,2,3,3-
hexachloropropane (230da)
The catalysts useful in reactions of a starting chloroalkane and olefin
include cuprous
compounds, iron powder, iron chloride, organonickel compound catalyst or
organocopper catalyst. Co-catalysts can be selected from chelating agents and
may
serve as solvents. Suitable ligands are selected from amines, amides, nitriles
and
organophosphate solvents.
The chloroalkanes produced, such as 240fa, 250fb, 240db, and 230da are further
reacted such as via chlorination or dehydrochlorination to produce the desired
1, 1,1,3,3-pentachloropropane (240fa) or 1,1,3 .3-tetrachloropropene (1230z a)
feedstocks .
In one embodiment, the further reaction is a chlorination step. More
specifically,
250th can be chlorinated to produce a mixture of 1,1,1,3,3-pentachloropropane
(240fa) and 1,1,1,2,3-pentachloropropane (240db). This chlorination may be
accomplished via photochlorination.
3
CA 02940149 2016-08-18
WO 2015/126584
PCT/US2015/013204
In a second embodiment, the further reaction is a dehydrochlorination step.
More
specifically, 240fa can be dehydrochlorinated to obtain 1230za. The
dehydrochlorination step can be carried out with gas or liquid phase process
in the
presence or absence of a catalyst.
In a third embodiment, the further reaction is a dechlorination step. More
specifically,
230da can be dechlorinated to provide 1230za. Methods known in the art can be
used
to complete this dechlorination, such as the use of zinc dust to complete this
dechlorination reaction.
The present invention is directed toward a process for producing trans 1-
chloro-3,3,3-
trifluoro propene (E-1233zd) from 1,1,3,3 -tetrachlororopropene (1230za,
CC12=CH-
CHC12) and/ or 1,1,1,3,3-tetrachloropropane (240fa) that comprises the steps
of:
a) forming a chloroalkane via addition of an olefin to a starting chloroalkane
and reacting the chloroalkane to form 1,1,3,3- tetrachlororopropene
(1230za, CC12=CH-CHC12) and/or 1,1,1,3,3-pentachoropropane (240fa);
followed by
b) fluorination of 1,1,3,3- tetrachlororopropene (1230za, CC1)=CH-CHCL)
and/or 1,1,1,3,3-pentachoropropane (240fa) in gas phase, or liquid phase
fluorination of 1230za to obtain a mixture of cis (Z) and trans (E) 1-
chloro-3,3,3 trifluoro propene (1233zd, CF3-CH=CHC1); followed by
c) separation of cis (Z) 1-chloro 3,3,3-trifluoropropene (1233zd, CF3-
CH=CHC1) and trans (E) 1-chloro 3,3,3-trifluoropropene (1233zd, CF3-
CH=CHC1); followed by
d) isomerization of the cis 1233zd (Z- 1233zd) from the second step to form
trans 1233zd (E-1233zd).
The fluorination step of the process, gas phase fluorination of 1230za and/or
240fa to
1233zd or liquid phase fluorination of 1230za to 12333zd; can be via any
process
known in the art. For example: the uncatalyzed liquid phase fluorination of
1230za is
disclosed in US Patent No. 5,877,359; the catalyzed gas phase fluorination of
1230za
is disclosed in US Patent No. 5,811,603; US Patent No. 6,166,274 discloses the
fluorination of 1230za to 1233zd in the presence of catalyst such as
trifluoroacetic
4
CA 02940149 2016-08-18
WO 2015/126584
PCT/US2015/013204
acid or triflic acid. Fluorination catalysts such as TiC14, TiF4, SnC14, SnF4,
SbF5,
SbC15, SbFõCly (x+y=5), or an ionic liquid are described in US Patent No.
6,881,698.
When an Sb type catalyst is used, it is preferred to feed low level of C12 to
maintain
the Sb species in an active form.
The separation step of the process comprises the separation of the cis 1233zd
and
trans 1233zd formed in the first step via an appropriate separation means such
as
distillation, liquid phase separation, or extractive separation. The cis
1233zd and
trans 1233zd formed in the first step may contain HF and HC1. Preferably, the
HC1 is
first removed in a first distillation column. Thereafter, liquid phase
separation
coupled with azeotropic distillation can be used to remove HF. The boiling
point
difference of cis 1233zd and trans 1233zd enable them to be separated by
conventional distillation, typically at atmospheric pressures.
The isomerization step of the process involves the isomerization of the cis
1233zd
from the separation step into trans 1233zd. The isomerization step can be
carried out
in the gas phase using a heterogeneous or in the liquid phase uncatalyzed
reaction or a
liquid phase catalyzed reaction using a homogeneous catalyst.
The isomerization step is achievable with a gas phase process in the presence
of a
heterogeneous catalyst. A suitable heterogeneous catalyst is high surface area
Cr(IIe
catalyst, supported or unsupported, that can optionally contains low levels of
one or
more co-catalysts selected from cobalt, nickel, zinc or manganese. For
supported
catalyst, the catalyst support can be selected from materials known in the art
to be
compatible with high temperature and pressure processes. For example,
fluorinated
alumina, HF treated activated carbon or carbon graphite are suitable catalyst
supports. A
preferred catalyst is a high surface area unsupported chromium oxide catalyst
.. that is activated with HF before use, optionally at pressure above 50 psi.
The level of
the co-catalyst, when present, can be varied from 1 to 10 weight %, preferably
from l
to 5 weight % of the catalyst. Co-catalyst can be added to the catalyst by
processes
known in the art such as adsorption from an aqueous or organic solvent,
followed by
solvent evaporation.
Suitable heterogeneous catalyst can also be selected from: Lewis acids
supported
catalysts selected from Sbv, Tilv, ns iv, movi.
Nbv and Tav= The support itself is
selected from the group such as fluorinated alumina; fluorinated chromia; HF
5
CA 02940149 2016-08-18
WO 2015/126584
PCT/US2015/013204
activated carbon or graphite carbon . Supported antimony halides such as SbF5
are
described in US Patent No. 6,528,691 and are preferred catalysts. Other solid
catalysts such as NAFION type polymer, acidic molecular sieves and, zeolites
can be
also used.
For the gas phase process, the temperature can be varied between 20-500 C,
preferably between 100-400 C. Contact times can vary from 0.5 to 100 seconds.
A
low level of oxidizing agent such as oxygen or oxygen containing gas such as
air or
chlorine gas can be used at between .01- .1 volume percent to prolong the life
of the
catalyst.
The isomerization step is also achievable in a liquid phase process in an
uncatalyzed
reaction or a catalyzed reaction in the presence of a homogenous catalyst
preferably
selected from compounds of group 3, 4, 5, 13, 14 and 15 metal compounds of the
Periodic Table of the elements (IUPAC 1988) and their mixtures (groups of the
Periodic Table of the elements which were previously called MA, IVa, IVb, Va,
Vb
and VIb). The compounds of the metals are intended to include hydroxides,
oxides
and the organic or inorganic salts of these metals, as well as mixtures
thereof.
Preferred are the aluminum, titanium, tantalum, molybdenum, boron, tin and
antimony derivatives such as A1C13, TiC14, TaC15, MoC16, BF3, SnC14. and
SbC15. In
the process according to the invention the preferred derivatives of the metals
are the
.. salts and these are preferably chosen from the halides and more
particularly from
chlorides, fluorides and chlorofluorides such as A1F3, TiF4, TaF5, MoF6, SnF4,
SbF5,
SbFxCly (x+y)=5. The catalyst must be subjected to activation (by HF or any
molecule able to exchange fluorine) prior to the isomerization step. In the
case of
antimony type catalyst, a low level of chlorine gas as oxidizing agent can be
used to
maintain the antimony catalyst in the pentavalent oxidation state. In addition
to the
above mentioned Lewis acids catalyst, an ionic liquid derived from antimony,
titanium, niobium and tantalum is suitable for liquid phase fluorination
processes. A
description of the preparation of such catalysts is disclosed in the US Patent
No.
6,881,698.
The homogenous catalyst for a liquid phase catalyzed process can also be
selected
from the Bronsted type family of acids such as (but not limited to) sulfuric
acid
H2504, sulfonic type acids such as C1S03H or FSO3H or triflic acid CF3S03H or
6
CA 02940149 2016-08-18
WO 2015/126584
PCT/US2015/013204
methane sulfonic acid CH3S03H. For the liquid phase process, the operating
temperature can be varied between about 20-200 C, with a contact time between
about 0.5-50 hours.
The process of the present invention may comprise additional separation steps
between each step. The purpose of theses separations could be:
1. to remove, totally or partially, any hydracid (HF. HC1) from the flow if
required. or
2. to isolate a desired product in order to feed it in a subsequent step,
or
3. to purify a product and removes organic impurities or by products, or
4. to dry a product (H20 removal).
The means used to achieve these additional steps are known in the art and
include but
are not limited to: distillation, extractive distillation or adsorption.
The process of the present invention is exemplified in the figures, which set
forth
block flow diagrams of gas phase and liquid phase processes in accordance with
the
present invention. The processes in the figures are set out in the form of
process
modules designed to achieve a specific purpose and arranged in accordance with
the
process of the present invention. Theses modules comprise:
RFL- comprises a liquid phase fluorination reactor and rectification system
comprising an unagitated, jacketed pressure vessel connected to a
rectification
column. The reactor also acts as the reboiler of the rectification column. The
HF and
organic (1230za) are fed directly to the reactor. The molar feed ratio of HF
to organic
is dictated by the reaction stoichiometry and the amount of HF leaving the
reactor
with the rectification column overhead and liquid phase purges. Mixing is
provided
by the boiling action of the reactor contents. For the most part, the reactor
effluent
leaves the reactor vessel as a gas and enters the bottom of the rectification
column. A
small purge from the liquid phase can remove any non-volatiles that may form
during
the reaction. The rectification column contains either packing or trays
designed to
provide good mass transfer between up flowing gas and down flowing liquid. The
condenser at the top of the column is cooled by either cooling water, chilled
water, or
some type of refrigeration. The condenser is a partial condenser where the
liquid
7
CA 02940149 2016-08-18
WO 2015/126584
PCT/US2015/013204
effluent is refluxed directly back to the column. The vapor effluent consists
of HC1,
HF and organic components.
DH- comprises an HC1 distillation system whereby pure HC1 is removed from the
top
of a distillation column. This column can operate between 100 psig and 300
psig.
More typically, the HC1 is distilled above 120 psig to allow the use of
conventional (-
40C) refrigeration at the top of the HC1 column. The bottoms of this column
contains
HF and organic with a small residual amount of HC1. The ratio of HF to the
organic
component typically is close to the azeotropic composition.
PS- comprises a liquid phase separator to separate two liquid phases, one
consisting
primarily of a hydrochlorofluorocarbon (HCFC) and the other consisting
primarily of
HF. The HF phase is usually the less dense so that it exits from the top of
the phase
separator and the HCFC exits as the bottom phase. There is some HF in the HCFC
phase and some HCFC in the HF phase. However, the compositions of both phases
are far removed from any azeotropic composition. The operating temperature of
the
phase separator can be between ¨40 C and +20 C. However, the lower the
temperature, the better the phase separation.
DA- comprises an azeotropic distillation column which distills overhead an
azeotropic
composition of HF and an organic consisting of one or more HCFC's
(hydrochlorofluorocarbons) and HFC's (hydrofluorocarbons). These organic
compounds can be either saturated or olefinic. The bottoms composition is
either
entirely HF or entirely organic, depending on whether the column feed
composition is
on the HF rich side or the organic rich side of the azeotrope. If the bottoms
are HF,
this stream is normally recycled back to the reactor. If the bottoms steam is
organic, it
is sent to a conventional distillation train.
DS- comprises a straight distillation normally done under pressure.
RI ¨comprises a gas phase isomerization reaction typically done at
temperatures
above 400 C in an adiabatic, packed bed reactor. The module consists of a feed
vaporizer and superheater. It can include an "economizer", whereby hot
effluent is
fed to one side and relatively cold reactor feed gases are fed to another side
of a heat
exchanger. The effluent gases are further cooled before entering a
distillation column.
Isomerization reactions can be run at varying conversions depending on the
8
CA 02940149 2016-08-18
WO 2015/126584
PCT/US2015/013204
equilibrium distribution of isomers. The effluent isomers can have boiling
points very
close together. However, they typically exhibit close to ideal behavior so can
be
separated by conventional distillation. As an alternative to the gas phase,
this reaction
can be done as a homogeneously catalyzed liquid phase reaction. In this
configuration, the reactor would be a continuous stirred tank with the
effluent being
removed as a vapor to effect separation from the catalyst.
RFG- comprises a gas phase fluorination reactor that is an adiabatic packed
bed
reactor that feeds a gas phase over a solid catalyst. No cooling is needed
because of
the reactor has a low conversion per pass and a high HF molar feed ratio. The
adiabatic exotherm is typically less than 100 C. The feed HF and organic are
vaporized in a common vaporizer and superheated to the reactor temperature.
The
common vaporizer allows the 1230za and/or 240fa to be vaporized at a lower
temperature than would be possible if it were vaporized as a pure component,
thereby
minimizing thermal degradation. This module can also include an "economizer",
whereby hot effluent is fed to one side and relatively cold reactor feed gases
are fed to
another side of a heat exchanger. The effluent gases are further cooled before
entering a distillation column. Reaction temperatures are between 200 C and
400 C.
The pressure is high enough to allow the HCl by-product to be distilled with
conventional refrigeration- preferably between 100 psig and 200 psig.
The lower case letter used to identify the modules distinguishes multiple
appearances
of the same type of module in the same process.
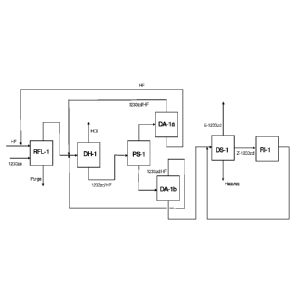
Figure 1 is a block flow diagram of a process in accordance with the present
invention
for converting 1230za to E-1233zd using a liquid phase fluorination step. The
Figure
incorporates the process modules described above. Figure 1 discloses a process
wherein 1230za and HF are fed to reaction module RFL-1. Typically, the
reaction
takes place in a predominantly HF rich medium without a catalyst. The HC1 and
the
1233zd/HF exit the top of the rectification column of RFL-1. The vapor
effluent of
RFL-1 enters DH-1 to remove HCl as a pure overhead product. The bottoms of DH-
1
consists primarily of 1233zd (both E and Z isomers) and HF at a near
azeotropic
composition. This is fed to module PS-1 to effect a liquid phase separation.
The top
HF rich phase is sent to module DA-la, where HF is separated as a bottoms
stream
for recycle to the reactor. The overhead azeotrope of 1233zd and HF is
recycled back
9
CA 02940149 2016-08-18
WO 2015/126584
PCT/US2015/013204
to DH-1 to allow any residual HC1 and light organics to be stripped out in
this column
before the azeotrope gets recycled to phase separation. The bottoms stream
from PS-
1 goes to module DA-lb, which removes an organic stream devoid of HF as a
bottoms stream. The overhead from DA-lb is recycled to DH-1 for the same
reason
that the DA-1a azeotrope was recycled to DH-1. The bottoms of DA-lb is sent to
process module DS-1 that separates any heavies from the 1233zd. The overhead
from
DS-1 is E-1233zd, the desired trans isomer. The Z-1233zd is higher boiling and
is
recovered for feeding to process module RI-I. The effluent from the
isomerization
reactor is recycled to DS-I, which effects the separation of the E and Z
isomers.
Figure 2 is a block flow diagram of a process in accordance with the present
invention
for converting 1230za or 240fa to E-1233zd using a gas phase fluorination
step. The
Figure incorporates the process modules described above. In Figure 2 the
process is
similar to Figure I except, for example, the liquid phase fluorination reactor
(RFL-1)
is replaced by a gas phase fluorination reactor (RFG-1) and azeotropic
distillation
column (DA-2a).
The process as outlined by Figure 2 comprises feeding 1230za and/or 240fa and
HF to
reaction module RFG-2. The reaction takes place in a gas phase with a
catalyst. The
reactor effluent consists of predominantly HC1, 1233zd, unreacted 1230za and
excess
HF. The reactor effluent of RFG-2 enters DA-2a to remove HF and unreacted
F1230za as bottoms that is recycled to the reactor. The overhead, which
consists
predominantly of HCl and the azeotrope of HF and 1233zd (both E and Z
isomers), is
sent to DH-2, which removes HC1 as a pure overhead product. The bottoms of DH-
2
consists of primarily 1233zd (both E and Z isomers) and HF at a near
azeotropic
composition. This is fed to module PS-2 to effect a liquid phase separation.
The top
HF rich phase is sent to module DA-2b, where HF is separated as a bottoms
stream
for recycle to the reactor. The overhead azeotrope of 1233zd and HF is
recycled back
to DH-2 to allow any residual HC1 and light organics to be stripped out in
this column
before the azeotrope gets recycled to phase separation. The bottoms stream
from PS-
2 goes to module DA-2c, which removes an organic stream devoid of HF as a
bottoms
.. stream. The overhead from DA-2c is recycled to DH-2 for the same reason
that the
DA-2b azeotrope was recycled to DH-2. The bottoms of DA-2c is sent to process
module DS-2 that separates any heavies from the 1233zd. The overhead from DS-2
is
E-1233zd- the desired trans isomer. The Z-1233zd is higher boiling and is
recovered
CA 02940149 2016-08-18
WO 2015/126584
PCT/US2015/013204
for feeding to process module RI-2. The effluent from the isomerization
reactor is
recycled to DS-2, which effects the separation of the E and Z isomers.
11