Note: Descriptions are shown in the official language in which they were submitted.
CA 02940510 2016-08-23
WO 2015/126655 PCT/US2015/015099
TITLE
IMPLANTABLE LAYERS COMPRISING A PRESSED REGION
BACKGROUND
[0001] The present invention relates to surgical instruments and, in various
arrangements, to surgical stapling and
cutting instruments and staple cartridges therefor that are designed to staple
and cut tissue.
BRIEF DESCRIPTION OF THE DRAWINGS
[0002] The features and advantages of this invention, and the manner of
attaining them, will become more apparent
and the invention itself will be better understood by reference to the
following description of embodiments of the
invention taken in conjunction with the accompanying drawings, wherein:
[0003] FIG. 1 is a left front perspective view of a surgical stapling and
severing instrument with a handle portion;
[0004] FIG. 2 is a perspective view of a two-piece knife and tiring bar ("E-
beam") of the surgical stapling and
severing instrument of FIG. 1;
[0005] FIG. 3 is a perspective view of a wedge sled of a staple cartridge of a
staple applying assembly;
[0006] FIG. 4 is a longitudinal cross-sectional view of an anvil in a closed
position and a staple cartridge comprising
a rigid support portion and a compressible tissue thickness compensator
illustrated with staples being moved from an
unfired position to a fired position during a first sequence;
[0007] FIG. 5 is another cross-sectional view of the anvil and the staple
cartridge of FIG. 4 illustrating the anvil in
an open position after the firing sequence has been completed;
[0008] FIG. 6 is an exploded perspective view of a tissue thickness
compensator and a staple cartridge assembly;
[0009] FIG. 7 is a partial cross-sectional view of the staple cartridge
assembly of FIG. 6, illustrating unfired staples
positioned in staple cavities of a staple cartridge body and partially
embedded in a tissue thickness compensator;
[0010] FIG. 8 is a partial cross-sectional view of the staple cartridge
assembly of FIG. 6, illustrating fired staples
ejected from the staple cavities of the staple cartridge body and formed
against an anvil, and further illustrating the
tissue thickness compensator and tissue captured within the staple entrapment
area of the formed staples;
[0011] FIG. 9 is a partial perspective view of an end effector of a surgical
fastening instrument illustrated with some
portions removed and other portions illustrated in cross-section; moreover, a
cutting member of the end effector is
illustrated in a partially advanced position;
[0012] FIG. 10 is a partial cross-sectional end view of the end effector of
FIG. 9 illustrated with patient tissue
captured between an anvil and a tissue thickness compensator of the end
effector; moreover, staples removably stored
within a cartridge body of the end effector are illustrated in an unfired
position and the cutting member of the end
effector is illustrated in an unadvanced position which is proximal to the
tissue thickness compensator;
[0013] FIG. 11 is a partial cross-sectional end view of the end effector of
FIG. 9 illustrated with the staples in a fired
position and the cutting member in a partially advanced position in which the
patient tissue has been at least partially
transected;
1
SUBSTITUTE SHEET (RULE 26)
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
[0014] FIG. 12 is a partial cross-sectional end view of the end effector of
FIG. 9 illustrated with the staples in a fired
position and the cutting member in an advanced position in which at least a
portion or the tissue thickness
compensator has been transected by the cutting member;
100151 FIG. 13 is a perspective view of a fastener cartridge including a
tissue thickness compensator;
[0016] FIG. 14 is a cross-sectional view of the tissue thickness compensator
of FIG. 13 illustrating a cutting member
positioned relative to a proximal end of the tissue thickness compensator.
100171 FIG. 15 is an exploded view of a tissue thickness compensator assembly;
10018] FIG. 16 is a perspective view of layer of a tissue thickness
compensator assembly;
[0019] FIG. 17 is a cross-sectional view of the tissue thickness compensator
assembly of FIG. 15;
[0020] FIG. 18 is a cross-sectional perspective view of an assembled tissue
thickness compensator assembly, and a
mold for assembling the same;
10021] FIG. 19 is a perspective view of the assembled tissue thickness
compensator assembly of FIG. 18;
(0022] FIG. 20 is a perspective view of a tissue thickness compensator
assembly and a mold for assembling the
same;
100231 FIG. 21 is a perspective view of a tissue thickness compensator
assembly and a mold for assembling the
same;
[0024] FIG. 22 is a cross-sectional perspective view of the tissue thickness
compensator assembly of FIG. 21and the
mold of FIG. 21 for assembling the same;
[0025] FIG. 23 is a perspective view of an end effector comprising a tissue
thickness compensator;
[0026] FIG. 24 is a perspective view of the end effector and the tissue
thickness compensator of FIG. 23 and a
modifying member modifying the tissue thickness compensator;
[0027] FIG. 25 is a perspective view of the end effector of FIG. 23 comprising
the modified tissue thickness
compensator of FIG. 24;
[0028] FIG. 26 is a cross-sectional perspective view of a tissue thickness
compensator;
100291 FIG. 27 is a cross-sectional perspective view of a mold for modifying
the tissue thickness compensator of
FIG. 26;
[0030] FIG. 28 is a cross-sectional perspective view of the tissue thickness
compensator of FIG. 26 after
modification by the mold of FK.1. 27;
[0031] FIG. 29 is a cross-sectional perspective view of a tissue thickness
compensator;
100321 FIG. 30 is a cross-sectional perspective view of a mold for modifying
the tissue thickness compensator of
FIG. 29;
[0033] FIG. 31 is a cross-sectional perspective view of the tissue thickness
compensator of FIG. 29 after
modification by the mold of FIG. 30;
100341 FIG. 32 is a cross-sectional perspective view of a tissue thickness
compensator;
10035] FIG. 33 is a cross-sectional perspective view of a mold for modifying
the tissue thickness compensator of
FIG. 32;
- 2 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
[0036] FIG. 34 is a cross-sectional perspective view of the tissue thickness
compensator of FIG. 32 after
modification by the mold of FIG. 33;
[0037] FIG. 35 is a cross-sectional perspective view of a tissue thickness
compensator including a first height;
100381 FIG. 36 is a cross-sectional perspective view of the tissue thickness
compensator of FIG. 35 after
modification to change the first height to a second height;
100391 FIG. 37 is a cross-sectional view of a mold for modifying the tissue
thickness compensator of FIG. 35;
100401 FIG. 38 is a cross-sectional perspective view of a tissue thickness
compensator;
[0041] FIG. 39 is a cross-sectional perspective view the tissue thickness
compensator of FIG. 38 after modification;
[0042] FIG. 40 is a graph illustrating the effect of compression forces on a
spring rate of a tissue thickness
compensator;
100431 FIG. 41 is a cross-sectional perspective view of a tissue thickness
compensator;
[0044] FIG. 42 is a cross-sectional perspective view of a space creator for
modifying the tissue thickness
compensator of FIG. 41;
100451 FIG. 43 is a cross-sectional perspective view of the tissue thickness
compensator of FIG. 41 after
modification by the space creator of FIG. 42;
[0046] FIG. 44 is a partial cross-sectional devotional view of a fastener
cartridge for use with a surgical instrument
including a tiring member in accordance with at least one embodiment
illustrated with portions removed;
100471 FIG. 45 is a partial cross-sectional devotional view depicting a tissue
thickness compensator of the fastener
cartridge of FIG. 44 being removed from the fastener cartridge and the firing
member of FIG. 44 illustrated in a
locked-out condition;
100481 FIG. 46 is a partial perspective view of the tissue thickness
compensator of FIG. 45;
100491 FIG. 47 is a partial perspective view a tissue thickness compensator in
accordance with at least one
embodiment;
[0050] FIG. 48 is a partial cross-sectional elevational view of an end
effector of a surgical instrument comprising a
fastener cartridge including the tissue thickness compensator of FIG. 47, a
sled, and a firing member supported by the
sled illustrated with portions removed;
100511 FIG. 49 is a partial cross-sectional devotional view of the end
effector of FIG. 48 illustrating the firing
member in a partially-fired position;
100521 FIG. 50 is a partial cross-sectional devotional view of the end
effector of FIG. 48 illustrating the tissue
thickness compensator removed from the fastener cartridge and the firing
member in a locked-out condition;
[0053] FIG. 51 is a partial perspective view of a fastener cartridge in
accordance with at least one embodiment
illustrated with portions removed;
100541 FIG. 52 is a perspective view of a sled of the fa,stener cartridge of
FIG. 51;
100551 FIG. 53 is a partial perspective view of the fastener cartridge of FIG.
31;
100561 FIG. 54 is an devotional view of a sled in accordance with at least one
embodiment;
100571 FIG. 55 is a perspective view of a sled in accordance with at least one
embodiment illustrated in an unlocked
configuration;
- 3 -
[0058] FIG. 56 is a perspective view of the sled of FIG. 55 illustrated in a
locked-out configuration;
[0059] FIG. 57 is a partial cross-sectional elevational view of the sled of
FIG. 55 positioned within a fastener
cartridge illustrating the sled in its unlocked configuration, a firing member
supported by the sled, and a tissue
thickness compensator of the fastener cartridge engaged with the sled;
[0060] FIG. 58 is a partial cross-sectional elevational view of the tissue
thickness compensator of FIG. 57 being
removed from the fastener cartridge of FIG. 57 which has placed the sled of
FIG. 55 in its locked-out configuration
and the firing member of FIG. 57 in a locked-out condition;
[0061] FIG. 59 is a partial cross-sectional elevational view of a sled
positioned at the proximal end of a fastener
cartridge in accordance with at least one embodiment illustrated with portions
removed;
[0062] FIG. 60 is a partial cross-sectional elevational view of the sled of
FIG. 59 illustrated at the distal end of the
fastener cartridge;
[0063] FIG. 61 is a perspective view of a sled in accordance with at least one
embodiment;
[0064] FIG. 62 is a diagram depicting a staple comprising a plurality of barbs
in accordance with at least one
embodiment, wherein the staple is illustrated in an unformed configuration and
a deformed configuration;
[0065] FIG. 63 is an elevational view of a staple comprising a plurality of
barbs in accordance with at least one
embodiment, wherein the staple is positioned within a staple cavity in an
unfired position;
[0066] FIG. 64 is an elevational view of a staple including a plurality of
barbs in accordance with at least one
embodiment;
[0067] FIG. 65 is an elevational view of a staple including a plurality of
barbs in accordance with at least one
embodiment;
[0068] FIG. 66 is an elevational view of a staple including a plurality of
barbs in accordance with at least one
embodiment;
[0069] FIG. 67 is an elevational view of a staple including a plurality of
barbs in accordance with at least one
embodiment;
[0070] FIG. 68 is an elevational view of the staple including a plurality of
barbs in accordance with at least one
embodiment, wherein the staple is positioned within a staple cavity in an
unfired position;
[0071] FIG. 69 is a plan view of the staple and the staple cavity of FIG. 68;
[0072] FIG. 70 is a partial perspective view of a barbed staple leg in
accordance with at least one embodiment;
[0073] FIG. 71 is a partial perspective view of a barbed staple leg of the
staple of FIG. 68;
[0074] FIG. 71A is a cross-sectional plan view of the barbed staple leg of
FIG. 71;
[0075] FIG. 72 is a partial perspective view of a barbed staple leg in
accordance with at least one embodiment; and
[0076] FIG. 73 is a partial perspective view of a barbed staple leg in
accordance with at least one embodiment.
DETAILED DESCRIPTION
[0077] The Applicant of the present application also owns the U.S. Patent
Applications identified below:
4
Date Recue/Date Received 2021-07-12
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
U.S. Patent Application Serial No. 12/894,311, entitled SURGICAL INSTRUMENTS
WITH
RECONFIGURABLE SHAFT SEGMENTS; now U.S. Patent Publication No. 2012/0080496;
U.S. Patent Application Serial No. 12/894,340, entitled SURGICAL STAPLE
CARTRIDGES
SUPPORTING NON-LINEARLY ARRANGED STAPLES AND SURGICAL STAPLING INSTRUMENTS
WITH
COMMON STAPLE-FORMING POCKETS; now U.S. Patent Publication No. 2012/0080482;
U.S. Patent Application Serial No. 12/894,327, entitled JAW CLOSURE
ARRANGEMENTS FOR
SURGICAL INSTRUMENTS; now U.S. Patent Publication No. 2012/0080499;
U.S. Patent Application Serial No. 12/894,351, entitled SURGICAL CUTTING AND
FASTENING
INSTRUMENTS WITH SEPARATE AND DISTINCT FASTENER DEPLOYMENT AND TISSUE CUTTING
SYSTEMS; now U.S. Patent Publication No. 2012/0080502;
U.S. Patent Application Serial No. 12/894,338, entitled IMPLANTABLE FASTENER
CARTRIDGE
HAVING A NON-UNIFORM ARRANGEMENT; now U.S. Patent Publication No.
2012/0080481;
U.S. Patent Application Serial No. 12/894,369, entitled IMPLANTABLE FASTENER
CARTRIDGE
COMPRISING A SUPPORT RETAINER; now U.S. Patent Publication No. 2012/0080344;
U.S. Patent Application Serial No. 12/894,312, entitled IMPLANTABLE FASTENER
CARTRIDGE
COMPRISING MULTIPLE LAYERS; now U.S. Patent Publication No. 2012/0080479;
U.S. Patent Application Serial No. 12/894,377, entitled SELECTIVELY ORTENTABLE
IMPLANTABLE
FASTENER CARTRIDGE; now U.S. Patent No. 8,393,514;
U.S. Patent Application Serial No. 12/894,339, entitled SURGICAL STAPLING
INSTRUMENT WITH
COMPACT ARTICULATION CONTROL ARRANGEMENT; now U.S. Patent Publication No.
2012/0080500;
U.S. Patent Application Serial No. 12/894,360, entitled SURGICAL STAPLING
INSTRUMENT WITH A
VARIABLE STAPLE FORMING SYSTEM; now U.S. Patent Publication No. 2012/0080484;
U.S. Patent Application Serial No. 12/894,322, entitled SURGICAL STAPLING
INSTRUMENT WITH
INTERCHANGEABLE STAPLE CARTRIDGE ARRANGEMENTS; now U.S. Patent Publication No.
2012/0080501;
U.S. Patent Application Serial No. 12/894,350, entitled SURGICAL STAPLE
CARTRIDGES WITH
DETACHABLE SUPPORT STRUCTURES; now U.S. Patent Publication No. 2012/0080478;
U.S. Patent Application Serial No. 12/894,383, entitled IMPLANTABLE FASTENER
CARTRIDGE
COMPRISING BIOABSORBABLE LAYERS; now U.S. Patent Publication No. 2012/0080345;
U.S. Patent Application Serial No. 12/894,389, entitled COMPRESSIBLE FASTENER
CARTRIDGE; now
U.S. Patent Publication No. 2012/0080335;
U.S. Patent Application Serial No. 12/894,345, entitled FASTENERS SUPPORTED BY
A FASTENER.
CARTRIDGE SUPPORT; now U.S. Patent Publication No. 2012/0080483;
U.S. Patent Application Serial No. 12/894,306, entitled COLLAPSIBLE FASTENER
CARTRIDGE; now
U.S. Patent Publication No. 2012/0080332;
- 5 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
U.S. Patent Application Serial No. 12/894,318, entitled FASTENER SYSTEM
COMPRISING A
PLURALITY OF CONNECTED RETENTION MATRIX ELEMENTS; now U.S. Patent Publication
No.
2012/0080480;
U.S. Patent Application Serial No. 12/894,330, entitled FASTENER SYSTEM
COMPRISING A
RETENTION MATRIX AND AN ALIGNMENT MATRIX; now U.S. Patent Publication No.
2012/0080503;
U.S. Patent Application Serial No. 12/894,361, entitled FASTENER SYSTEM
COMPRISING A
RETENTION MATRIX; now U.S. Patent No. 8,529,600;
U.S. Patent Application Serial No. 12/894,367, entitled FASTENING INSTRUMENT
FOR DEPLOYING A
FASTENER SYSTEM COMPRISING A RETENTION MATRIX; now U.S. Patent Publication No.
2012/0080485;
U.S. Patent Application Serial No. 12/894,388, entitled FASTENER SYSTEM
COMPRISING A
RETENTION MATRIX AND A COVER; now U.S. Patent No. 8,474,677;
U.S. Patent Application Serial No. 12/894,376, entitled FASTENER SYSTEM
COMPRISING A
PLURALITY OF FASTENER CARTRIDGES; now U.S. Patent Publication No.
2012/0080486;
U.S. Patent Application Serial No. 13/097,865, entitled SURGICAL STAPLER ANVIL
COMPRISING A
PLURALITY OF FORMING POCKETS; now U.S. Patent Publication No. 2012/0080488;
U.S. Patent Application Serial No. 13/097,936, entitled TISSUE THICKNESS
COMPENSATOR FOR A
SURGICAL STAPLER; now U.S. Patent Publication No. 2012/0080339;
U.S. Patent Application Serial No. 13/097,954, entitled STAPLE CARTRIDGE
COMPRISING A
VARIABLE THICKNESS COMPRESSIBLE PORTION; now U.S. Patent Publication No.
2012/0080340;
U.S. Patent Application Serial No. 13/097,856, entitled STAPLE CARTRIDGE
COMPRISING STAPLES
POSITIONED WITHIN A COMPRESSIBLE PORTION THEREOF; now U.S. Patent Publication
No.
2012/0080336;
U.S. Patent Application Serial No. 13/097,928, entitled TISSUE THICKNESS
COMPENSATOR
COMPRISING DETACHABLE PORTIONS; now U.S. Patent Publication No. 2012/0080490;
U.S. Patent Application Serial No. 13/097,891, entitled TISSUE THICKNESS
COMPENSATOR FOR A
SURGICAL STAPLER COMPRISING AN ADJUSTABLE ANVIL; now U.S. Patent Publication
No. 2012/0080489;
U.S. Patent Application Serial No. 13/097,948, entitled STAPLE CARTRIDGE
COMPRISING AN
ADJUSTABLE DISTAL PORTION; now U.S. Patent Publication No. 2012/0083836;
U.S. Patent Application Serial No. 13/097,907, entitled COMPRESSIBLE STAPLE
CARTRIDGE
ASSEMBLY; now U.S. Patent Publication No. 2012/0080338;
U.S. Patent Application Serial No. 13/097,861, entitled TISSUE THICKNESS
COMPENSATOR
COMPRISING PORTIONS HAVING DIFFERENT PROPERTIES; now U.S. Patent Publication
No. 2012/0080337;
U.S. Patent Application Serial No. 13/097,869, entitled STAPLE CARTRIDGE
LOADING ASSEMBLY;
now U.S. Patent Publication No, 2012/0160721;
U.S. Patent Application Serial No. 13/097,917, entitled COMPRESSIBLE STAPLE
CARTRIDGE
COMPRISING ALIGNMENT MEMBERS; now U.S. Patent Publication No. 2012/0083834;
- 6 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
U.S. Patent Application Serial No. 13/097,873, entitled STAPLE CARTRIDGE
COMPRISING A
RELEASABLE PORTION; now U.S. Patent Publication No. 2012/0083833;
U.S. Patent Application Serial No. 13/097,938, entitled STAPLE CARTRIDGE
COMPRISING
COMPRESSIBLE DISTORTION RESISTANT COMPONENTS; now U.S. Patent Publication No.
2012/0080491;
U.S. Patent Application Serial No. 13%097,924, entitled STAPLE CARTRIDGE
COMPRISING A TISSUE
THICKNESS COMPENSATOR; now U.S. Patent Publication No. 2012/0083835;
U.S. Patent Application Serial No. 13/242,029, entitled SURGICAL STAPLER WITH
FLOATING ANVIL;
now U.S. Patent Publication No. 2012/0080493;
U.S. Patent Application Serial No. 13/242,066, entitled CURVED END EFFECTOR
FOR A STAPLING
INSTRUMENT; now U.S. Patent Publication No. 2012/0080498;
U.S. Patent Application Serial No. 13/242,086, entitled STAPLE CARTRIDGE
INCLUDING
COLLAPSIBLE DECK; now U.S. Patent Publication No. 2013/0075450;
U.S. Patent Application Serial No. 13/241,912, entitled STAPLE CARTRIDGE
INCLUDING
COLLAPSIBLE DECK ARRANGEMENT; now U.S. Patent Publication No. 2013/0075448;
U.S. Patent Application Serial No. 13/241,922, entitled SURGICAL STAPLER WITH
STATIONARY
STAPLE DRIVERS; now U.S. Patent Publication No. 2013/0075449;
U.S. Patent Application Serial No. 13/241,637, entitled SURGICAL INSTRUMENT WM
I TRIGGER
ASSEMBLY FOR GENERATING MULTIPLE ACTUATION MOTIONS; now U.S. Patent
Publication No.
2012/0074201;
U.S. Patent Application Serial No. 13/241,629, entitled SURGICAL INSTRUMENT
WITH SELECTIVELY
ARTICULATABLE END EFFECTOR.; now U.S. Patent Publication No. 2012/0074200;
U.S. Application Serial No. 13/433,096, entitled TISSUE THICKNESS COMPENSATOR
COMPRISING A
PLURALITY OF CAPSULES; now U.S. Patent Publication No. 2012/0241496;
U.S. Application Serial No. 13/433,103, entitled TISSUE THICKNESS COMPENSATOR
COMPRISING A
PLURALITY OF LAYERS; now U.S. Patent Publication No. 2012/0241498;
U.S. Application Serial No. 13/433,098, entitled EXPANDABLE TISSUE THICKNESS
COMPENSATOR;
now U.S. Patent Publication No. 2012/0241491;
U.S. Application Serial No. 13/433,102, entitled TISSUE THICKNESS COMPENSATOR
COMPRISING A
RESERVOIR; now U.S. Patent Publication No. 2012/0241497;
U.S. Application Serial No. 13/433,114, entitled RETAINER ASSEMBLY INCLUDING A
TISSUE
THICKNESS COMPENSATOR; now U.S. Patent Publication No. 201210241499;
U.S. Application Serial No. 13/433,136, entitled TISSUE THICKNESS COMPENSATOR
COMPRISING
AT LEAST ONE MEDICAMENT; now U.S. Patent Publication No. 2012/0241492;
U.S. Application Serial No. 13/433,141, entitled TtssuE THICKNESS COMPENSATOR
COMPRISING
CONTROLLED RELEASE AND EXPANSION; now U.S. Patent Publication No.
2012/0241493;
U.S. Application Serial No. 13/433,144, entitled TISSUE THICKNESS COMPENSATOR
COMPRISING
FIBERS TO PRODUCE A RESILIENT LOAD; now U.S. Patent Publication No.
2012/0241500;
- 7 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
U.S. Application Serial No. 13/433,148, entitled TISSUE THICKNESS COMPENSATOR
COMPRISING
STRUCTURE TO PRODUCE A RESILIENT LOAD; now U.S. Patent Publication No.
2012/0241501;
U.S. Application Serial No. 131433,155, entitled TISSUE THICKNESS COMPENSATOR
COMPRISING
RESILIENT MEMBERS; now U.S. Patent Publication No. 2012/0241502;
U.S. Application Serial No. 13/433,163, entitled METHODS FOR FORMING TISSUE
THICKNESS
COMPENSATOR ARRANGEMENTS FOR SURGICAL STAPLERS; now U.S. Patent Publication
No.
2012/0248169;
U.S. Application Serial No.13/433,167, entitled TISSUE THICKNESS COMPENSATORS;
now U.S. Patent
Publication No. 2012/0241503;
U.S. Application Serial No. 13/433,175, entitled LAYERED TISSUE THICKNESS
COMPENSATOR; now
U.S. Patent Publication No. 2012/0253298;
U.S. Application Serial No. 13/433,179, entitled TISSUE THICKNESS COMPENSATORS
FOR
CIRCULAR SURGICAL STAPLERS; now U.S. Patent Publication No. 2012/0241505;
U.S. Application Serial No. 13/763,028, entitled ADHESIVE, FILM LAMINATE; now
U.S. Patent
Publication No. 2013/0146643;
U.S. Application Serial No. 13/433,115, entitled TISSUE THICKNESS COMPENSATOR
COMPRISING
CAPSULES DEFINING A LOW PRESSURE ENVIRONMENT; now U.S. Patent Publication No.
2013/0256372;
U.S. Application Serial No. 13/433,118, entitled TISSUE THICKNESS COMPENSATOR
COMPRISED OF
A PLURALITY OF MATERIALS; now U.S. Patent Publication No. 2013/0256365;
U.S. Application Serial No. 13/433,135, entitled MOVABLE MEMBER FOR USE WITH A
TISSUE
THICKNESS COMPENSATOR; now U.S. Patent Publication No. 2013/0256382;
U.S. Application Serial No. 131433,140, entitled TISSUE THICKNESS COMPENSATOR
AND METHOD
FOR MAKING THE SAME; now U.S. Patent Publication No. 2013/0256368;
U.S. Application Serial No. 13/433,129, entitled TISSUE THICKNESS COMPENSATOR
COMPRISING A
PLURALITY OF MEDICAMENTS; now U.S. Patent Publication No. 2013/0256367;
U.S. Application Serial No. 11/216,562, entitled STAPLE CARTRIDGES FOR FORMING
STAPLES
HAVING DIFFERING FORMED STAPLE HEIGHTS, now U.S. Patent No. 7,669,746;
U.S. Application Serial No. 111714,049, entitled SURGICAL STAPLING DEVICE WITH
ANVIL
HAVING STAPLE FORMING POCKETS OF VARYING DEPTHS, now U.S. Patent Publication
No.
2007/0194082;
U.S. Application Serial No. 11/711,979, entitled SURGICAL STAPLING DEVICES
THAT PRODUCE
FORMED STAPLES HAVING DIFFERENT LENGTHS, now U.S. Patent No. 8,317,070;
U.S. Application Serial No. 11/711,975, entitled SURGICAL STAPLING DEVICE WITH
STAPLE
DRIVERS OF DIFFERENT HEIGHT, now U.S. Patent Publication No. 2007/0194079;
U.S. Application Serial No. 11/711,977, entitled SURGICAL STAPLING DEVICE WITH
STAPLE
DRIVER THAT SUPPORTS MULTIPLE WIRE DIAMETER STAPLES, now U.S. Patent No.
7,673,781;
- 8 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
U.S. Application Serial No. 11/712,315, entitled SURGICAL STAPLING DEVICE WITH
MULTIPLE
STACKED ACTUATOR WEDGE CAMS FOR DRIVING STAPLE DRIVERS, now U.S. Patent No.
7,500,979;
U.S. Application Serial No. 12/038,939, entitled STAPLE CARTRIDGES FOR FORMING
STAPLES
HAVING DIFFERING FORMED STAPLE HEIGHTS, now U.S. Patent No. 7,934,630;
U.S. Application Serial No. 13/020,263, entitled SURGICAL STAPLING SYSTEMS
THAT PRODUCE
FORMED STAPLES HAVING DIFFERENT LENGTHS, now U.S. Patent Publication No.
2011/0147434;
U.S. Application Serial No. 13/118,278, entitled R0130TICALLY-CONTROLLED
SURGICAL
STAPLING DEVICES THAT PRODUCE FORMED STAPLES HAVING DIFFERENT LENGTHS, now
U.S.
Patent Publication No. 2011/0290851;
U.S. Application Serial No. 13/369,629, entitled ROBOTICALLY-CONTROLLED CABLE-
BASED
SURGICAL END EFFECTORS, now U.S. Patent Publication No. 2012/0138660;
U.S. Application Serial No. 12/695,359, entitled SURGICAL STAPLING DEVICES FOR
FORMING
STAPLES WITH DIFFERENT FORMED HEIGHTS, now U.S. Patent No. 8,464,923;
U.S. Application Serial No. 13/072,923, entitled STAPLE CARTRIDGES FOR FORMING
STAPLES
HAVING DIFFERING FORMED STAPLE HEIGHTS, now U.S. Patent No.8,567,656;
U.S. Application Serial No. 13/766,325, entitled LAYER OF MATERIAL FOR A
SURGICAL END
EFFECTOR; now U.S. Patent Publication No. 2013/0256380;
U.S. Application Serial No. 13/763,078, entitled ANVIL LAYER ATTACHED TO A
PROXIMAL END OF
AN END EFFECTOR; now U.S. Patent Publication No. 2013/0256383;
U.S. Application Serial No. 13/763,094, entitled LAYER COMPRISING DEPLOYABLE
ATTACHMENT
MEMBERS; now U.S. Patent Publication No. 20:13/0256377;
U.S. Application Serial No. 13/763,106, entitled END EFFECTOR COMPRISING A
DISTAL TISSUE
ABUTMENT MEMBER; now U.S. Patent Publication No. 2013/0256378;
U.S. Application Serial No. 13/433,147, entitled TISSUE 'THICKNESS COMPENSATOR
COMPRISLNG
CHANNELS; now U.S. Patent Publication No. 2013/0256369;
U.S. Application Serial No. 13/763,112, entitled SURGICAL STAPLING CARTRIDGE
WITH LAYER
RETENTION FEATURES; now U.S. Patent Publication No. 2013/0256379;
U.S. Application Serial No. 13/763,035, entitled ACTUATOR FOR RELEASING A
TISSUE THICKNESS
COMPENSATOR FROM A FASTENER CARTRIDGE; now U.S. Patent Publication No.
2013/0214030;
U.S. Application Serial No. 13/763,042, entitled RELEASABLE TISSUE THICKNESS
COMPENSATOR
AND FASTENER CARTRIDGE HAVING THE SAME; now U.S. Patent Publication No.
2013/0221063;
U.S. Application Serial No. 13/763,048, entitled FASTENER CARTRIDGE COMPRISING
A
RELEASABLE TISSUE THICKNESS COMPENSATOR; now U.S. Patent Publication No.
2013/0221064;
U.S. Application Serial No. 13/763,054, entitled FASTENER CARTRIDGE COMPRISING
A CUTTING
MEMBER FOR RELEASING A TISSUE THICKNESS COMPENSATOR;
- 9 -
U.S. Application Serial No. 13/763,065, entitled FASTENER CARTRIDGE COMPRISING
A
RELEASABLY ATTACHED TISSUE THICKNESS COMPENSATOR; now U.S. Patent Publication
No.
2013/0221065;
U.S. Application Serial No. 13/763,021, entitled STAPLE CARTRIDGE COMPRISING A
RELEASABLE
COVER;
U.S. Application Serial No. 13/763,078, entitled ANVIL LAYER ATTACHED TO A
PROXIMAL END OF
AN END EFFECTOR; now U.S. Patent Publication No. 2013/0256383;
U.S. Application Serial No. 13/763,095, entitled LAYER ARRANGEMENTS FOR
SURGICAL STAPLE
CARTRIDGES; now U.S. Patent Publication No. 2013/0161374;
U.S. Application Serial No. 13/463,147, entitled IMPLANTABLE ARRANGEMENTS FOR
SURGICAL
STAPLE CARTRIDGES; now U.S. Patent Publication No. 2013/0292398;
U.S. Application Serial No. 13/763,192, entitled MULTIPLE THICKNESS
IMPLANTABLE LAYERS FOR
SURGICAL STAPLING DEVICES; now U.S. Patent Publication No. 2013/0146642;
U.S. Application Serial No. 13/763,161, entitled RELEASABLE LAYER OF MATERIAL
AND
SURGICAL END EFFECTOR HAVING THE SAME; now U.S. Patent Publication No.
2013/0153641;
U.S. Application Serial No. 13/763,177, entitled ACTUATOR FOR RELEASING A
LAYER OF
MATERIAL FROM A SURGICAL END EFFECTOR; now U.S. Patent Publication No.
2013/0146641;
U.S. Application Serial No. 13/763,037, entitled STAPLE CARTRIDGE COMPRISING A
COMPRESSIBLE PORTION;
U.S. Application Serial No. 13/433,126, entitled TISSUE THICKNESS COMPENSATOR
COMPRISING
TISSUE INGROWTH FEATURES; now U.S. Patent Publication No. 2013/0256366;
U.S. Application Serial No. 13/433,132, entitled DEVICES AND METHODS FOR
ATTACHING TISSUE
THICKNESS COMPENSATING MATERIALS TO SURGICAL STAPLING INSTRUMENTS; now U.S.
Patent
Publication No. 2013/0256373.
U.S. Application Serial No. 13/851,703, entitled FASTENER CARTRIDGE COMPRISING
A TISSUE
THICKNESS COMPENSATOR INCLUDING OPENINGS THEREIN;
U.S. Application Serial No. 13/851,676, entitled TISSUE THICKNESS COMPENSATOR
COMPRISING A
CUTTING MEMBER PATH;
U.S. Application Serial No. 13/851,693, entitled FASTENER CARTRIDGE
ASSEMBLIES; and
U.S. Application Serial No. 13/851,684, entitled FASTENER CARTRIDGE COMPRISING
A TISSUE
THICKNESS COMPENSATOR AND A GAP SETTING ELEMENT.
[0078] Applicant of the present application also owns the following patent
applications that were filed on even date
herewith:
U.S. Patent Application Serial No.14/187,387, entitled STAPLE CARTRIDGE
INCLUDING A BARBED
STAPLE, now U.S. Patent Publication No. 2014/0166724;
U.S. Patent Application Serial No.14/187,395, entitled STAPLE CARTRIDGE
INCLUDING A BARBED
STAPLE, now U.S. Patent Publication No. 2014/0166725;
Date Recue/Date Received 2021-07-12
U.S. Patent Application Serial No.14/187,400, entitled STAPLE CARTRIDGE
INCLUDING A BARBED
STAPLE, now U.S. Patent Publication No. 2014/0166726;
U.S. Patent Application Serial No.14/187,383, entitled IMPLANTABLE LAYERS
COMPRISING A
PRESSED REGION, now U.S. Patent Publication No. 2015/0238185;
U.S. Patent Application Serial No.14/187,386, entitled IMPLANTABLE LAYERS AND
METHODS FOR
ALTERING ONE OR MORE PROPERTIES OF IMPLANTABLE LAYERS FOR USE WITH FASTENING
INSTRUMENTS, now U.S. Patent Publication No. 2015/0239180;
U.S. Patent Application Serial No.14/187,390, entitled IMPLANTABLE LAYERS AND
METHODS FOR
MODIFYING THE SHAPE OF THE IMPLANTABLE LAYERS FOR USE WITH A SURGICAL
FASTENING
INSTRUMENT, now U.S. Patent Publication No. 2015/0238188;
U.S. Patent Application Serial No.14/187,389, entitled IMPLANTABLE LAYER
ASSEMBLIES, now U.S.
Patent Publication No. 2015/0238187; and
U.S. Patent Application Serial No.14/187,384, entitled FASTENING SYSTEM
COMPRISING A FIRING
MEMBER LOCKOUT, now U.S. Patent Publication No. 2015/0238186.
[0079] Certain exemplary embodiments will now be described to provide an
overall understanding of the principles
of the structure, function, manufacture, and use of the devices and methods
disclosed herein. One or more examples
of these embodiments are illustrated in the accompanying drawings. Those of
ordinary skill in the art will understand
that the devices and methods specifically described herein and illustrated in
the accompanying drawings are non-
limiting exemplary embodiments and that the scope of the various embodiments
of the present invention is defined
solely by the claims. The features illustrated or described in connection with
one exemplary embodiment may be
combined with the features of other embodiments. Such modifications and
variations are intended to be included
within the scope of the present invention.
[0080] The terms "comprise" (and any form of comprise, such as "comprises" and
"comprising"), "have" (and any
form of have, such as "has" and "having"), "include" (and any form of include,
such as "includes" and "including")
and "contain" (and any form of contain, such as "contains" and "containing")
are open-ended linking verbs. As a
result, a surgical system, device, or apparatus that "comprises," "has,"
"includes" or "contains" one or more elements
possesses those one or more elements, but is not limited to possessing only
those one or more elements. Likewise, an
element of a system, device, or apparatus that "comprises," "has," "includes"
or "contains" one or more features
possesses those one or more features, but is not limited to possessing only
those one or more features.
[0081] The terms "proximal" and "distal" are used herein with reference to a
clinician manipulating the handle
portion of the surgical instrument. The term "proximal" referring to the
portion closest to the clinician and the term
"distal- referring to the portion located away from the clinician. It will be
further appreciated that, for convenience
and clarity, spatial terms such as "vertical", "horizontal", "up", and "down"
may be used herein with respect to the
drawings. However, surgical instruments are used in many orientations and
positions, and these terms are not
intended to be limiting and/or absolute.
[0082] Various exemplary devices and methods are provided for performing
laparoscopic and minimally invasive
surgical procedures. However, the person of ordinary skill in the art will
readily appreciate that the various methods
11
Date Recue/Date Received 2021-07-12
and devices disclosed herein can be used in numerous surgical procedures and
applications including, for example, in
connection with open surgical procedures. As the present Detailed Description
proceeds, those of ordinary skill in the
art will further appreciate that the various instruments disclosed herein can
be inserted into a body in any way, such as
through a natural orifice, through an incision or puncture hole formed in
tissue, etc. The working portions or end
effector portions of the instruments can be inserted directly into a patient's
body or can be inserted through an access
device that has a working channel through which the end effector and elongated
shaft of a surgical instrument can be
advanced.
[0083] Turning to the Drawings wherein like numerals denote like components
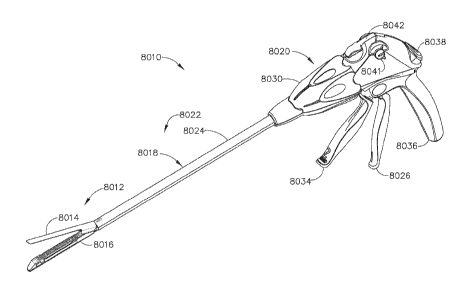
throughout the several views, FIG. 1
illustrates an exemplary surgical stapling and severing instrument 8010
suitable for use with a tissue thickness
compensator assembly as described in greater detail below. The surgical
stapling and severing instrument 8010 can
comprise an anvil 8014 which may be repeatedly opened and closed about its
pivotal attachment to an elongate staple
channel 8016. A staple applying assembly 8012 may comprise the anvil 8014 and
the channel 8016, wherein the
assembly 8012 can be proximally attached to an elongate shaft 8018 forming an
implement portion 8022. When the
staple applying assembly 8012 is closed, or at least substantially closed, the
implement portion 8022 can present a
sufficiently small cross-section suitable for inserting the staple applying
assembly 8012 through a trocar. In various
circumstances, the assembly 8012 can be manipulated by a handle 8020 connected
to the shaft 8018. The handle 8020
can comprise user controls such as a rotation knob 8030 that rotates the
elongate shaft 8018 and the staple applying
assembly 8012 about a longitudinal axis of the shaft 8018. A closure trigger
8026, which can pivot in front of a pistol
grip 8036 to close the staple applying assembly 8012. A closure release button
8038 can be outwardly presented on
the handle 8020 when the closure trigger 8026 is clamped such that the release
button 8038 can be depressed to
unclamp the closure trigger 8026 and open the staple applying assembly 8012,
for example. A firing trigger 8034,
which can pivot in front of the closure trigger 8026, can cause the staple
applying assembly 8012 to simultaneously
sever and staple tissue clamped therein. In various circumstances, multiple
firing strokes can be employed using the
firing trigger 8034 to reduce the amount of force required to be applied by
the surgeon's hand per stroke. In certain
embodiments, the handle 8020 can comprise one or more rotatable indicator
wheels such as, for example, rotatable
indicator wheel 8041which can indicate the firing progress. A manual firing
release lever 8042 can allow the firing
system to be retracted before full firing travel has been completed, if
desired, and, in addition, the firing release lever
8042 can allow a surgeon, or other clinician, to retract the firing system in
the event that the firing system binds and/or
fails. Additional details on the surgical stapling and severing instrument
8010 and other surgical stapling and severing
instruments suitable for use with the present disclosure are described, for
example, in U.S. Patent Application No.
13/851,693, entitled FASTENER CARTRIDGE ASSEMBLY, and filed on March 27, 2013.
Furthermore, powered
surgical stapling and severing instruments can also be utilized with the
present disclosure. See, for example, U.S.
Patent Application Publication No. 2009/0090763 Al, entitled POWERED SURGICAL
STAPLING DEVICE, and
filed on August 8, 2008.
[0084] With reference to FIGS. 2 and 3, a firing assembly such as, for
example, firing assembly 9090 can be utilized
with the surgical stapling and severing instrument 8010 to advance a wedge
sled 9126 which comprises a plurality of
12
Date Recue/Date Received 2021-07-12
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
wedges 9204 configured to deploy staples from the staple applying assembly
8012 into tissue captured between the
anvil 8014 and the elongate staple channel 8016. Furthermore, an E-beam 9102
at a distal portion of the firing
assembly 9090 may facilitate separate closure and tiring as well as spacing of
the anvil 8014 from the elongate staple
channel 8016 during firing. The E-beam 9102 may include a pair of top pins
9110, a pair of middle pins 9112 which
may follow portion 9218 of the wedge sled 9126, and a bottom pin or foot 9114,
as well as a sharp cutting edge 9116
which can be configured to sever the captured tissue as the firing assembly
9090 is advanced distally. In addition,
integrally formed and proximally projecting top guide 9118 and middle guide
9120 bracketing each vertical end of the
cutting edge 9116 may further define a tissue staging area 9122 assisting in
guiding tissue to the sharp cutting edge
9116 prior to being severed. The middle guide 9120 may also serve to engage
and fire the staple applying assembly
8012 by abutting a stepped central member 9124 of the wedge sled 9126 (FIG. 2)
that effects staple formation by the
staple applying assembly 8012.
100851 In various circumstances, a staple cartridge can comprise means for
compensating for thickness of tissue
captured within staples deployed from a staple cartridge. Referring to FIG. 4,
a staple cartridge, such as staple
cartridge 10000, for example, can be utilized with the surgical stapling and
severing instrument 8010 and can include
a rigid first portion, such as support portion 10010, for example, and a
compressible second portion, such as tissue
thickness compensator 10020, for example. The support portion 10010 can
comprise a cartridge body and a plurality
of staple cavities 10012. A staple 10030, for example, can be removably
positioned in each staple cavity 10012.
Referring primarily to FIGS. 4 and 5, each staple 10030 can comprise a base
10031 and one or more legs 10032
extending from the base 1(X)31. Prior to the staples 10030 being deployed, the
bases 10031 oldie staples 10030 can
be supported by staple drivers positioned within the support portion 10010
and, concurrently, the legs 10032 of the
staples 10030 can be at least partially contained within the staple cavities
10012. In various circumstances, the staples
10030 can be deployed between an unfired position and a tired position such
that the legs 10032 move through the
tissue thickness compensator 10020, penetrate through a top surface of the
tissue thickness compensator 10020,
penetrate the tissue T, and contact an anvil positioned opposite the staple
cartridge 10000. As the legs 10032 are
deformed against the anvil, the legs 10032 of each staple 10030 can capture a
portion of the tissue thickness
compensator 10020 and a portion of the tissue I within each staple 10030 and
apply a compressive force to the tissue.
Further to the above, the legs 10032 of each staple 10030 can be deformed
downwardly toward the base 10031 of the
staple to form a staple entrapment area in which the tissue I and the tissue
thickness compensator 10020 can be
captured. In various circumstances, the staple entrapment area can be defined
between the inner surfaces of the
deformed legs 10032 and the inner surface of the base 10031. The size of the
entrapment area for a staple can depend
on several factors such as the length of the legs, the diameter of the legs,
the width of the base, and/or the extent in
which the legs are deformed, for example.
100861 In use, further to the above and referring primarily to FIG. 4, an
anvil, such as anvil 8014 of the surgical
stapling and severing instrument 8010, can be moved into a closed position
opposite the staple cartridge 10000 by
depressing the closure trigger 8026 to advance the E-beam 9102. The anvil 8014
can position tissue against the tissue
thickness compensator 10020 and, in various circumstances, compress the tissue
thickness compensator 10020 against
the support portion 10010, for example. Once the anvil 8014 has been suitably
positioned, the staples 10030 can be
- 13 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
deployed, as also illustrated in FIG. 4. In various circumstances, as
mentioned above, a staple-firing sled 10050,
which is similar in many respects to the sled 9126 (See FIG. 3), can be moved
from a proximal end of the staple
cartridge 10000 toward a distal end 10002, as illustrated in FIG. 5. As the
firing assembly 9090 is advanced, the sled
10050 can contact the staple drivers 10040 and lift the staple drivers 10040
upwardly within the staple cavities 10012.
In at least one example, the sled 10050 and the staple drivers 10040 can each
comprise one or more ramps, or inclined
surface's, which can co-operate to move the staple drivers 10(4() upwardly
from their unfired positions. As the staple
drivers 10040 are lifted upwardly within their respective staple cavities
10012, the staple drivers 10040 can lift the
staples 10030 upwardly such that the staples 10030 can emerge from their
staple cavities 10012. In various
circumstances, the sled 10050 can move several staples upwardly at the same
time as part of a firing sequence.
10087] As discussed above, and referring to FIG. 5, the staple legs 10032 of
the staples 10030 can extend into the
compensator 10020 beyond the support portion 10010 when the staples 10030 are
in their unfired positions. In
various circumstances, the tips of the staple legs 10032, or any other portion
of the staple legs 10032, may not
protrude through a top tissue-contacting surface 10021 of the tissue thickness
compensator 10020 when the staples
10030 are in their unfired positions. In certain circumstances, the tips of
the staple legs 10032 can comprise sharp tips
which can incise and penetrate the tissue thickness compensator 10020.
10088] In various circumstances, it may be preferable to prevent and/or limit
frictional forces between a tissue
thickness compensator and a staple. Referring now to FIGS. 6-8, a tissue
thickness compensator 20220 for use with a
staple cartridge assembly 20200 can include a plurality of clearance apertures
20224 extending at least partially
through the tissue thickness compensator 20220. In various circumstances, the
staple cartridge assembly 20200 can
include a staple cartridge body 20210 and a tissue thickness compensator 20220
releasably secured relative to the
staple cartridge body 20210. The cartridge body 20210 can include a cartridge
deck 20211 and a plurality of staple
cavities 20212 defined through the cartridge deck 20211 and into the body of
the staple cartridge body 20210, for
example. Staples 20230 can be removably positioned in the staple cavities
20212, for example. The tissue thickness
compensator 20220 can include a tissue-contacting surface 20221 (FIG. 7) and a
deck-contacting surface 20222 (FIG.
6). The deck-contacting surface 20222 can be releasably positioned against the
deck 20211 of the cartridge body
20210, for example, and the tissue-contacting surface 20221 can be positioned
against tissue T to be stapled, for
example. Clearance apertures 20224 can extend through the deck-contacting
surface 20222 and into the tissue
thickness compensator 20220 and may comprise holes, slits, gaps, bores,
openings, and/or cleared pathways, for
example, within the tissue thickness compensator 20220.
100891 Referring primarily to FIGS. 7 and 8, staples 20230 can be positioned
in the staple cavities 20212 of the
cartridge body 20210. Each staple 20230 can include a base 20231 and a pair of
staple legs 20232, fbr example,
which can extend from the base 20231. Each. staple leg 20232 can extend from
opposite ends of the base 20231.
Referring primarily to FIG. 7, one or more of the clearance apertures 20224 in
the tissue thickness compensator 20220
can include an opening in the deck-contacting surface 20222. The opening of a
clearance aperture 20224 can be
aligned with a corresponding staple leg 20232 that is positioned in a staple
cavity 20212. For example, a single staple
leg 20232 can be aligned with the opening of a single clearance aperture 20224
when the tissue thickness compensator
20220 is secured relative to the cartridge body 20210. In certain
circumstances, a staple leg 20232 can extend into
- 14 -
each clearance aperture 20224, such that at least a portion of the staple
20230 is embedded in the tissue thickness
compensator 20220, for example. For example, referring primarily to FIG. 7, a
staple 20230 can include a first staple
leg 20232a and a second staple leg 20232b. Furthermore, the tissue thickness
compensator 20220 can include a first
clearance aperture 20224a aligned with the first staple leg 20232a, and a
second clearance aperture 20224b aligned
with the second staple leg 20232b, for example. Prior to deployment of the
staple 20230, the first staple leg 20232a
can extend partially through the first clearance aperture 20224a, and the
second staple leg 20232b can extend partially
through the second clearance aperture 20224b, for example. The tissue
thickness compensator 20220 can include
additional clearance apertures 20224 that are not aligned with staple legs
20232, for example. In certain
circumstances, the staple cartridge assembly 20200 can include additional
staples 20230 and/or staple legs 20232 that
are not aligned with clearance apertures 20224, for example.
[0090] The staples 20230 can be moveable from an unfired configuration (FIG.
7) to a fired configuration (FIG. 8).
Each staple 20230 can be moved along a staple axis when moving between the
unfired configuration and the fired
configuration. When in the unfired configuration, the staple legs 20232 can
extend from the staple cavities 20212 and
into the tissue thickness compensator 20220, for example. The staple legs
20232 can be partially embedded in the
tissue thickness compensator 20220 when the staples 20230 are in the unfired
configuration, for example.
Furthermore, at least a portion of the staple legs 20232 can be aligned with
and/or positioned within the clearance
apertures 20224 of the tissue thickness compensator 20220 when the staples are
in the unfired configuration, for
example. In other circumstances, the staple legs 20232 can be positioned
entirely within the staple cavity 20212 when
in the unfired configuration, and can be aligned with the clearance apertures
20224 positioned above the cartridge
deck 20211 (FIG. 6), for example.
[0091] The staples 20230 can move from the unfired configuration (FIG. 7) to
the fired configuration (FIG. 8)
during a firing stroke, as described herein. A staple driver 20240 can be
positioned within each staple cavity 20212.
The staple driver 20240 within each staple cavity 20212 can be pushed toward
the cartridge deck 20211 (FIG. 6), for
example, to drive the staple 20230 into tissue T and toward an anvil 20260
(FIG. 8) which can be similar in many
respects to other anvils described herein such as, for example, the anvil 8014
(FIG. 1). As each staple 20230 moves
from the unfired configuration to the fired configuration, the staple legs
20232 can move through the clearance
apertures 20224 in the tissue thickness compensator 20220. The clearance
apertures 20224 can have a predefined
trajectory within the tissue thickness compensator 20220. For example, the
clearance apertures 20224 can extend
along an axis that is perpendicular to and/or substantially perpendicular to
the tissue-contacting surface 20221 (FIG. 7)
and/or the deck-contacting surface 20222 (FIG. 6) of the tissue thickness
compensator 20220. In other circumstances,
the clearance apertures 20224 can extend along an axis that is oriented at an
oblique angle relative to the tissue-
contacting surface 20221 and/or the deck-contacting surface 20222 of the
tissue thickness compensator 20220, for
example. In certain circumstances, a group of the clearance apertures 20224
can be parallel. In some circumstances,
all of the clearance apertures 20224 within the tissue thickness compensator
20220 can be parallel, for example. The
clearance apertures 20224 can comprise a partially curved trajectory and/or a
partially linear trajectory. Other
characteristics and features of the clearance apertures 20224 are described in
greater detail in U.S. Patent Application
No.13/851,693, entitled FASTENER CARTRIDGE ASSEMBLY, and filed on March 27,
2013. Methods and
Date Recue/Date Received 2021-07-12
techniques for modifying a tissue thickness compensator to include clearance
apertures such as, for example, the
clearance apertures 20224 are described below in greater detail.
[0092] Referring now to FIGS. 9-12, an end effector 22090 of a surgical
instrument similar in many respects to the
surgical instrument 8010, for example, can comprise a first jaw including a
fastener cartridge assembly 22000 and a
second jaw including an anvil 10060. The first jaw can include a staple
cartridge channel 10070 which can be
configured to removably receive the cartridge assembly 22000. Alternatively,
the staple cartridge channel 10070 and
the cartridge assembly 22000 can comprise an integral unit. In various
circumstances, the anvil 10060 can be moved
between an open position and a closed position (FIGS. 9-12). In the open
position of the anvil 10060, the anvil 10060
can be positioned on a first side of a patient's tissue T (FIGS. 10-12) and
the cartridge assembly 22000 can be
positioned on a second, or opposite, side of the tissue T, for example. When
the anvil 10060 is moved into its closed
position, the anvil 10060 can compress the tissue T against the cartridge
assembly 22000. Alternatively, the first jaw
including the cartridge assembly 22000 can be moved relative to the anvil
10060. A firing member 10052, which is
similar in many respects to the firing assembly 9090 (FIG. 3), can be advanced
distally from a proximal end 22001 of
the cartridge assembly 22000 toward a distal end 22002 of the cartridge
assembly 22000 to eject fasteners, such as
staples 22030, for example, removably stored in a cartridge body 22010 of the
cartridge assembly 22000 as the firing
member 10052 is advanced from the proximal end 22001 toward the distal end
22002 of the cartridge assembly
22000.
[0093] Further to the above, the staples 22030 can be supported by staple
drivers 10040 which are movably
positioned within staple cavities 22012 defined in the cartridge body 22010.
Moreover, the firing member 10052 can
be configured to advance a staple-firing sled 10050 distally within the
cartridge body 22010 as the firing member
10052 is moved from the proximal end 22001 toward the distal end 22002. In
such circumstances, the staple-firing
sled 10050 can be configured to lift the staple drivers 10040, and the staples
22030 supported thereon, toward the
anvil 10060. In essence, further to the above, the staple drivers 10040 can
move the staples 22030 from an unfired
position (FIG. 10) to a fired position (FIGS. 11 and 12) wherein the staples
22030 can contact the anvil 10060 and be
deformed between an undeformed configuration (FIG. 10) and a deformed
configuration (FIGS. 11 and 12). The
anvil 10060 can comprise forming pockets 10062 which can be configured to
receive and deform the staples 22030.
Staples 22030 can be the same as or similar to staples 10030, for example
and/or any other staples disclosed herein,
and, as such, staples 22030 are not described in greater detail herein. The
reader will note, however, that the staples
22030 can comprise any suitable shape and/or suitable dimensions, such as
width and/or height, for example, in their
undeformed configuration and/or their deformed configuration. For instance,
the staples 22030 can, in certain
circumstances, comprise a height which does not extend above a deck surface
22011 of the cartridge body 22010
when the staples 22030 are in their unfired positions while, in other
circumstances, the staples 22030 can comprise a
height in which the legs of the staples 22030 extend upwardly from the deck
surface 22011 when the staples 22030
are in their unfired positions such that the legs of the staples 22030 are at
least partially embedded in a tissue thickness
compensator 22010 of the cartridge assembly 22000.
[0094] With continued reference to the embodiment depicted in FIGS. 9-12,
further to the above, the cartridge
assembly 22000 can comprise a cartridge body 22010 and a tissue thickness
compensator 22020. In various
- 16 -
Date Recue/Date Received 2021-07-12
circumstances, the cartridge body 22010 can be similar to the support portion
10010, for example, in many respects
and, as a result, many of such respects are not repeated herein for the sake
of brevity. Furthermore, the tissue
thickness compensator 22020 can be similar to the tissue thickness compensator
10020, for example, in many
respects. Further to the above, the firing member 10052 can include a cutting
portion 10053 which can be configured
to transect the tissue positioned between the anvil 10060 and the tissue
thickness compensator 22020 as the firing
member 10052 is advanced distally. In various circumstances, as a result, the
firing member 10052 can be configured
to concurrently fire the staples 22030 to staple the tissue T and cut the
tissue T. In certain circumstances, the firing
process can at least partially lead the cutting process. Stated another way,
the cutting process can lag the firing
process. In such circumstances, a portion of the tissue T can be stapled and
then incised.
[0095] As illustrated in FIGS. 9-12, the cartridge body 22010 can include a
cartridge knife slot 22015 which can be
configured to receive a portion of the firing member 10052 as the firing
member 10052 is advanced distally. Further
to the above, the anvil 10060 can include an anvil knife slot 10065 which can
be configured to receive a portion of the
firing member 10052 as the firing member 10052 is advanced distally. In
various circumstances, the tissue thickness
compensator 22020 can comprise a tissue thickness compensator knife slot 22025
which can be aligned with the anvil
knife slot 10065 and the cartridge knife slot 22015 such that the firing
member 10052 can pass through the cartridge
knife slot 22015, the anvil knife slot 10065, and the tissue thickness
compensator knife slot 22025 simultaneously. In
various circumstances, the anvil knife slot 10065 can extend over the tissue
thickness compensator knife slot 22025
such that the cutting portion 10053 of the firing member 10052 can pass
through the cartridge knife slot 22015, the
anvil knife slot 10065, and the tissue thickness compensator knife slot 22025
simultaneously. The tissue thickness
compensator knife slot 22025 can define a tissue thickness compensator knife
path for the cutting portion 10053
wherein the tissue thickness compensator knife path can be parallel to the
anvil knife path and the cartridge knife path.
In various circumstances, the tissue thickness compensator knife path can be
longitudinal while, in certain
circumstances, the tissue thickness compensator knife path can be curved.
Further to the above, curved end effectors
and curved fastener cartridges are disclosed in U.S. Patent Application
Publication No. 2008/0169329. In such
circumstances, a tissue thickness compensator can be curved. In at least one
such embodiment, the tissue thickness
compensator can be curved to match the curvature of the cartridge body of the
fastener cartridge. Methods and
techniques for modifying a tissue thickness compensator to include a knife
slot such as, for example, the knife slot
22025 are described below.
[0096] Further to the above, refening primarily to FIG. 9, the tissue
thickness compensator knife slot 22025 can
extend between a first stapling portion 22021a which can be stapled by a first
group of staples 22030 and a second
stapling portion 2202 lb which can be stapled by a second group of staples
22030. The knife slot 22025 can releasably
connect the first stapling portion 22021a to the second stapling portion 2202
lb. In use, as illustrated in FIG. 9, the
cutting portion 10053 can be advanced distally through the knife slot 22025 to
transect the knife slot 22025 and
separate the first stapling portion 22021a and the second stapling portion
2202 lb. In certain circumstances, the knife
slot 22025 can comprise a plurality of connectors, or bridges, 22026 which can
connect the first stapling portion
17
Date Recue/Date Received 2021-07-12
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
22021a and the second stapling portion 22021 b prior to being transectal by
the cutting portion 10053. In various
circumstances, the connectors 22026 can have the same thickness as the first
stapling portion 22021a and/or the
second stapling portion 2202 lb. at least when the tissue thickness
compensator 22020 is in an uncompressed state. In
at least one such circumstance, the connectors 22026, the first stapling
portion 22021a, and/or the second stapling
portion 22021 b can be unitarily and integrally formed from a flat, or at
least substantially flat, piece of material, for
example. In various other circumstances, the first stapling portion 22021a can
comprise a first thickness, the second
stapling portion 22021b can comprise a second thickness, and the connectors
22026 can comprise a third thickness,
wherein one or more of the first thickness, the second thickness, and the
third thickness can be different than the other
thicknesses.
[0097] The knife slot 22025 can firther comprise apertures, such as apertures
22024, for example, defined therein.
For instance, the apertures 22024 can be elongate and can extend
longitudinally along the knife slot 22025. In various
other circumstances, the apertures in the knife slot 22025 can comprise any
suitable arrangement In certain
circumstances, the apertures 22024 can comprise perforations positioned
intermediate the connectors 22026 which can
be formed utilizing a laser cutting operation, for example. In some
circumstances, the apertures 22024 can be cut from
a sheet of material to form the tissue thickness compensator 22020 such that
the apertures 22024 and the connectors
22026 are arranged in an alternating arrangement, for example. In other
instances, the tissue thickness compensator
22020 can be molded with apertures 22024 already formed therein. In various
circumstances, one or more of the
apertures 22024 can comprise through holes, for example. In various
circumstances, one or more of the apertures
22024 can comprise clearance apertures, .for example. In certain instances,
one or more of the apertures 22024 may
not comprise through holes and may instead comprise reductions in the
thickness of the knifr slot 22025, for example.
Methods and techniques for modifying a tissue thickness compensator to include
apertures such as, for example, the
apertures 22024 are described below.
100981 Further to the above, referring again to FIGS. 9-11, patient tissue can
be positioned intermediate the anvil
10060 of the end effector 22090 and the tissue thickness compensator 22020 of
the cartridge assembly 22000 when
the anvil 10060 is in an open position. When the anvil 1(1060 is moved into a
closed posidon, a bottom surface, or
tissue-contacting surface, 10063 of the anvil 10060 can contact the tissue
land push the tissue T toward a deck
surface 22011 of the cartridge body 22010. The tissue T can contact a top
surface, or tissue contacting surface, 22021
of the tissue thickness compensator 22020 wherein, when the anvil 10060 is
moved into its closed position, the anvil
10060 can press the tissue T against the tissue thickness compensator 22020
and, further to the above, compress the
tissue thickness compensator 22020 against the deck surface 22011 of the
cartridge body 22010. In various
circumstances, the tissue thickness compensator 22020 can comprise a bottom
surface 22029 which can abut the deck
surface 22011. In some circumstances, a gap may be present between the bottom
surface 22029 and the deck surface
22011 before the tissue thickness compensator 22020 is compressed against the
cartridge body 22010. In such
circumstances, the tissue thickness compensator 22020 may first translate
toward the cartridge body 22010 before
being compressed thereagainst When the tissue thickness compensator 22020 is
compressed against the cartridge
body 22010, in various circumstances, the first stapling portion 22021a and/or
the second stapling portion 22021 b of
the tissue thickness compensator 22020 may move laterally. For instance, the
first stapling portion 22021a and/or the
- 18-
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
second stapling portion 2202 lb may move laterally away from the cartridge
knife slot 22015. In various
circumstances, the connectors 22026 can be configured to inhibit such lateral
movement between the first stapling
portion 22021a and the second stapling portion 2202 lb. In various
circumstances, referring primarily to FIG. 11, the
connectors 22026 can be configured to stretch to permit some relative lateral
movement between the first stapling
portion 22021a and the second stapling portion 22021 b when the anvil 10060 is
closed. In the event that the anvil
10060 is reopened, the connectors 22026 can be configured to elastically
return, or at least substantially return, to their
unstretched configuration and, as a result, pull the first stapling portion
22021a and the second stapling portion
22021b laterally back toward their original positions, illustrated in FIG. 10.
Moreover, the anvil 10060 can compress
the tissue T when the anvil 10060 is moved into its closed position. In such
circumstances, the tissue T may at least
partially flow into the apertures 22024.
100991 Upon reviewing FIGS. 10-12, the reader will appreciate that the knife
slot 22025 of the tissue thickness
compensator 22020 comprises less material along the longitudinal length
thereof than the first stapling portion 22021a
and/or the second stapling portion 2202 lb. Stated another way, a longitudinal
cross-section through the first stapling
portion 22021a and/or the second stapling portion 2202 lb would transect a
first amount of material while a
longitudinal cross-section through the knife slot 22025 would transect a
second amount of material which is less than
the first amount of material.
[0100) Once the anvil 10060 has been suitably positioned, further to the
above, the firing member 10052 can be
advanced distally to fire the staples, as illustrated in FIG. 11, and incise
the tissue T and the connectors 22026, as
illustrated in FIG. 12. Furthermore, the tissue thickness compensator incision
force, the tissue incision force, the tissue
thickness compensator drag force, and/or the tissue drag three can dull the
cutting portion 10053 of the firing member
10052. A. dull knife may not be able to transect the tissue T and/or the
tissue thickness compensator 22020, for
example, according to a preferred manner. With primary reference to FIG. 12,
the cutting portion 10053 can comprise
a first knife edge zone 10053a, a second knife edge zone 10053b, and/or a
third knife edge zone 10053c, for example,
wherein the first knife edge zone 10053a is positioned vertically above the
second knife edge zone 10053b, and
wherein the second knife edge zone 100531) is positioned vertically above the
third knife edge zone 10053c, for
example. The cutting portion 10053 can comprise any suitable number and/or
location of knife edge zones wherein
the knife edge zones depicted in FIG. 12 have been selected for the purposes
of discussion. Further to the above, the
first knife edge zone 10053a can be configured to transect the tissue T while
the second knife edge zone 10053b can
be con figured to transect the tissue thickness compensator 22020. As a
result, the first knife edge zone 10053a may
experience the tissue incision force and/or the tissue drag force discussed
above. Such forces may wear or dull the
first knife edge zone 10053a at a first rate. The second knife edge zone
10053b may experience the tissue thickness
compensator incision force and/or the tissue thickness compensator drag force
discussed above. Such forces may
wear or dull the second knife edge zone 10053b at a second rate. In various
circumstances, the second rate can be
different than the first rate.
(0101) Turning now to FIGS. 13 and 14, a fastener cartridge 22400 can comprise
a tissue thickness compensator
22420 which can include a first stapling portion 22421a and a second stapling
portion 22421b which are connected by
a knife slot 22425. The knife slot 22425 can comprise an angled longitudinal
connector 22426. The angled
- 19-
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
longitudinal connector 22426 can extend between a proximal end 22401 of the
knife slot 22425 and a distal end 22402
of the knife slot 22425. In some circumstances, the angled longitudinal
connector 22426 can extend the entire length
of the knife slot 22425 while, in other circumstances, the angled longitudinal
connector 22426 can extend less than the
length of the knife slot 22425. The angled longitudinal connector 22426 can
extend between a top surface 22428 of
the tissue thickness compensator 22420 and a bottom surface 22429 of the
tissue thickness compensator 22420. In
some circumstances, the angled longitudinal connector 22426 can extend the
entire distance between the top surface
22428 and the bottom surface 22429 while, in other circumstances, the angled
longitudinal connector 22426 can
extend less than the distance between the top surface 22428 and the bottom
surface 22429. In various circumstances,
the proximal end of the longitudinal connector 22426 can extend from the top
surface 22428 of the tissue thickness
compensator while the distal end of the longitudinal connector 22426 can
extend from the bottom surface 22429.
Alternatively, the distal end of the longitudinal connector 22426 can extend
from the top surface 22428 of the tissue
thickness compensator while the proximal end of the longitudinal connector
22426 can extend from the bottom
surface 22429. In various circumstances, the longitudinal connector 22426 can
comprise a thin bridge (i.e. less than
the full thickness of the tissue thickness compensator 22420) or a series of
thin bridges that join the first stapling
portion 22421a which can be stapled by a first group of staples 22030 to the
second stapling portion 22421 b which
can be stapled by a second group of staples 22030, for example. These thin,
angled bridges, and/or the longitudinal
connector 22426, could distribute the wear across the second knife edge zone
10053b, rather than concentrating it on
one spot. In various circumstances, as a result, the wear occurring on the
second knife edge zone 10053b may be
equal to, or closer to being equal to, the wear occurring at the first knife
edge zone 10053a, for example.
[0102] Referring now to FIGS. 15-17, an exemplary tissue thickness compensator
assembly 1000 may include a first
layer 1002 and a second layer 1004 attachable to the first layer 1002. The
tissue thickness compensator assembly 1000
can be utilized with a surgical instrument such as, for example, the surgical
instrument 8010 (FI(1.1). In addition, the
tissue thickness compensator assembly 1000 can be utilized in a similar manner
as and can replace the tissue thickness
compensator 22020 of the cartridge assembly 22000 of the end effector 22090
(FIG. 9). For example, the second layer
1004 of the tissue thickness compensator assembly 1000 may include a first
portion 1006 which can be positioned on
the deck surface 22011 on a first side of the cartridge knife slot 22015 in a
similar fashion to the first stapling portion
22021a and a second portion 1008 which can be positioned on the deck surface
22011 on a second side, opposite the
first side, of the cartridge knife slot 22015 in a similar fashion to the
second stapling portion 22021 b (FIGS. 9-11). In
various instances, the first portion 1006 and the second portion 1008 of the
second layer 1004 can be spaced apart and
may comprise a gap 1010 therebetween which can comprise a knife path for the
cutting portion 10053 of the firing
member 10052 and may extend at least partially over the cartridge knife slot
22015 when the tissue thickness
compensator assembly 1000 is assembled with the cartridge end effector 22090.
In certain instances, the first layer
1002 can be configured to couple the first portion 1006 and the second portion
1008 and extend at least partially over
the gap 1010, as illustrated in FIG. 17, for example.
101031 In use, tissue T can be captured between the anvil 10060 and a tissue
contacting surface 1012 of the first
layer 1002. As the firing member 10052 is advanced, a first group of staples
20030 can be deployed to staple the first
portion 1006 and a second group of staples can be deployed to staple the
second portion 1008. The first and second
-20 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
groups of staples can be configured to penetrate through a first deck
contacting surface 1007 and a second deck
contacting surface 1009, respectively, of the second layer 1004, then through
the tissue contacting surface 1012 of the
first layer, and then through the captured tissue T to contact the pockets
10062 of the anvil 10060. Furthermore, the
advancement of the firing member 10052 can cause the cutting portion 10053 to
be advanced distally through the gap
1010 of the tissue thickness compensator assembly 1000. The cutting portion
10053 may transect the first layer 1002
while advancing through the gap 1010 thereby separating the first portion 1006
and the second portion 1008 of the
second layer 1004.
10104] Referring again to FIG. 17, the first layer 1002 of the tissue
thickness compensator assembly 1000 may
comprise a first height HI, the first portion 1006 of the second layer 1004
may comprise a second height 112, and the
second portion 1008 of the second layer 1004 may comprise a third height H3.
In certain circumstances, as illustrated
in FIG. 17, the second height H2 and the third height 1-13 can be the same or
substantially the same. In other
circumstances, the second height H2 can be different from the third height H3.
In certain circumstances, the first
height Ill can be less than the second height 112 and/or the third height H3,
as illustrated in FIG. 17. The first layer
1002 of the tissue thickness compensator assembly 1000 may comprise a first
density, the first portion .1006 of the
second layer 1004 may comprise a second density, and the second portion 1008
of the second layer 1004 may
comprise a third density. In certain circumstances, as illustrated in FIG. 17,
the second density and the third density
can he the same or substantially the same. In other circumstances, the second
density can be different from the third
density and/or different from the first density of the first layer 1002. The
material compositions of the first portion
1006 and the second portion 1008 can be the same, or at least substantially
the same. In other circumstances, the
material compositions of the first portion 1006 and the second portion 1008
can he different from each other and/or
can be different from the material composition of the first layer 1002.
101051 As described above, repeated use of the cutting portion 10053 to cut
tissue T and tissue thickness
compensator material may dull the cutting portion 10053. To slow the dulling
process, it may be desirable to reduce
the tissue thickness compensator material that is cut by the cutting portion
10053. An additional benefit can be a
reduction in the forces needed to advance the tiring member 1()052 distally
during a firing stroke. In order to reduce
the dulling of the cutting portion 10053, the first layer 1002 can be
comprised, at least partially, of a thin film, for
example. in such circumstances, the first height UI can be significantly less
than the second height 112 and the third
height I-13, as illustrated in FIG. 17. In certain circumstances, the first
layer 1002 may comprise a uniform, or
substantially uniform, height therethrough, as illustrated in FIG. 17. In
other circumstances, a gap bridging portion
1014 of the first layer 1002 may extend at least partially over the gap 1010
and may be thinner than the remainder of
the first layer 1002. The cutting portion 10053 may transect the gap bridging
portion 1014 of the first layer 1002 while
advancing through the gap 1010 between the first portion 1006 and the second
portion 1008 of the second layer 1004
which may reduce the resistance experienced by the cutting portion 10053
and/or slow the dulling of the cutting
portion 10053. In any event, the first layer 1002 can be configured to
maintain a coupling engagement with the first
portion 1006 and the second portion 1008 of the second layer 1004 prior to
being transected, and to present the cutting
portion 10053 with a reduced resistance as the cutting portion 10053 is
advanced to transect the first layer :1002.
- 21 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
[0106] To further reduce the dulling of the cutting portion 10053 and/or
reduce he resistance experienced by the
cutting portion 10053, the gap bridging portion :1014 may comprise a
perforated segment :1016 along the knife path
defined by the gap 1010, as illustrated in FIG. 16. The perforated segment
1016 can include a plurality of perforations
1018 which can be cut into the first layer 1002 prior to the assembly of the
first layer 1002 to the second layer 1004,
for example. The perforations 1018 can reduce the interaction between the
cutting portion 10053 and the first layer
1002 as the cutting portion 10053 is advanced through the knife path defined
by the gap 1010, which may slow the
dulling of the cutting portion 10053 and/or reduce the resistance experienced
by the cutting portion 10053.
101071 In various circumstances, as described in greater detail below, the
tissue thickness compensator assembly
10(X) can be comprised of one or more biocompatible materials. In certain
circumstance, the first layer 1002 can be
comprised of a biocompatible buttress material and/or plastic material, such
as polydioxanone (PDS) and/or
polyglycolic acid (PGA), for example, and the second layer 1004 can be
comprised of a bioabsorbable foam material
and/or a compressible haemostatic material, such as oxidized regenerated
cellulose (ORC), for example. In certain
circumstances, the first layer 1002 can be a thin film comprising a
bioabsorbable material such as polyglycolic acid
(PGA) which is marketed under the trade name Vicryl, polylactic acid (PLA or
PLLA), polydioxanone (PDS),
polyhydroxyalkanoate (PHA), poliglecaprone 25 (PGCL) which is marketed under
the trade name Monocryl,
polycaprolactoue (PCL), and/or a composite of PGA, PLA, PDS, PHA, PGCL and/or
PCL, for example. In certain
circumstances, the first portion 1006 and/or the second portion 1008 of the
second layer 1004 can be comprised of a
lyophilized foam comprising polylactic acid (PLA) and/or polyglycolic acid
(PGA), for example. In certain
circumstances, the first portion 1006 and/or the second portion 1008 of the
second layer 1004 can be comprised of
biocompatible foam which may comprise a porous, open cell foam and/or a
porous, closed cell foam.
101081 Referring again to FIGS. 15 and 17, the first layer 1002 can be at
least partially disposed over the second
layer 1004 such that the second layer 1004 may be positioned between the first
layer 1002 and the dock surface 22011
(FIG. 9) when the tissue thickness compensator assembly 1000 is assembled with
the end effector 22090 (FIG. 9). In
other circumstances, the first layer 1002 can be positioned beneath the first
portion 1006 and the second portion 1008
(not shown) such that the first layer 1002 may be positioned between the
second layer 1004 and the deck surface
22011 (FIG. 9) when the tissue thickness compensator assembly 1000 is
assembled with the cud effector 22090 (FIG.
9). In any event, the first layer 1002 can be attached to a first contacting
surface 1020 of the first portion 1006 and a
second contacting surface 1022 of the second portion 1008 of the second layer
1004. The first layer 1002 can be
attached to the second layer 1004 via a thermal pressing ixocess involving the
application of heat and/or pressure, as
described in greater detail below. In other circumstances, the first layer
1002 can be attached to the second layer 1004
by a biocompatible adhesive material such as a fibrin and/or protein hydrogel,
for example. Other means for attaching
the first layer 1002 to the second layer 1004 are contemplated by the present
disclosure.
101091 Referring now to FIGS. 21 and 22, the first layer 1002 can be at least
partially embedded into the first
portion 1006 and/or the second portion 1008 of the second layer 1004. In such
circumstances, the tissue thickness
compensator assembly 1000 can be prepared using a mold 1024, for example, as
illustrated in FIG. 21. In various
instances, an organic solution comprising a polymer such as, for example,
polylactic acid (PLA) and/or polyglycolic
acid (PGA) can be poured into the mold 1024. The first layer 1002 can be
immersed into the organic solution. As
- 22 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
illustrated in FIG. 22, a central shelf 1026 and a central beam 1027 of a mold
cover 1028 can trap the first layer 1002
thembetween to ensure that the first layer 1002 remains immersed in the
organic solution which can then be
lyophilized using conventional lyophilization techniques and/or any other
suitable techniques, for example. Upon
completion of the lyophiliz.ation process, and/or any other suitable process,
the .mold cover 1028 can be removed and
the tissue thickness compensator assembly 1000 can be recovered from the mold
1028.
101101 As illustrated in FIG. 21, the first layer 1002 of the tissue thickness
compensator 1()00 can be partially
positioned within the first portion 1006 and the second portion 1008 of the
second layer 1004. In certain
circumstances, the first layer 1002 can be partially positioned within one of
the first portion 1006 and the second
portion 1008 and attached to a top surface or a bottom surface of the other
one of the first portion 1006 and the second
portion 1008.
101111 In certain circumstances, the central beam 1027 and the shelf 1026 can
at least partially extend along an axis
that is parallel or substantially parallel to the first deck contacting
surface 1007 and/or the second deck contacting
surface 1009 when the cover 1028 is in a closed configuration with mold 1024,
as illustrated in FIG. 22.1n such
circumstances, the first layer 1002 can be embedded into the first portion
1006 and/or the second portion 1008 such
that first layer 1002 is positioned or substantially positioned in a parallel
or substantially parallel relationship with the
first deck contacting surface 1007 and/or the second deck contacting surface
1009. In other circumstances, although
not illustrated, the central beam 1027 and the shelf 1026 can at least
partially extend along an axis that is at an oblique
angle with the first deck contacting surface 1007 and/or the second deck
contacting surface 1008 when the cover 1028
is in a closed configuration with mold 1024. In such circumstances, the first
layer 1002 can be embedded into the first
portion 1006 and/or the second portion 1008 such that first layer 1002 is
positioned or substantially positioned at an
oblique angle with respect to the first deck: contacting surface :1007 and/or
the second deck contacting surface 1009.
Other techniques for partially embedding the first layer 1002 into the first
portion 1006 and/or the second portion
1008 are contemplated by the present disclosure.
(0112) Referring now to FIGS. 18 and 19, a tissue thickness compensator
assembly 1033, which is similar in many
respects to the tissue thickness compensator assembly 1000 and the tissue
thickness compensator 20020, is illustrated.
The tissue thickness compensator assembly 1033 can comprise the first portion
1006 and the second portion 1008
which can be spaced apart and separably coupled together by a plurality, of
bridging members or connectors 1030
which may extend across the gap 1010 between the first portion 1006 and the
second portion 1008. In addition, some
or all or the connectors 1030 of the tissue thickness compensator assembly
1033 can be partially embedded into the
first portion 1006 and the second portion 1008, as illustrated in FIG. 19.
Furthermore, some or all of the connectors
1030 can comprise a first end positioned within the first portion 1006, a
second end positioned within the second
portion 1008, and a gap bridging portion 1032 therebetween. The gap bridging
portion 1032 may extend across the
gap 1010 between the first portion 1006 and the second portion 1008, as
illustrated in FIG. 19. The connectors 1030
can be spaced apart along the length of the gap 1010 to separably couple the
first portion 1006 to the second portion
1008.
(0113) In certain circumstances, the connectors 1030 can be evenly distributed
along an axis extending along the
gap 1010, as illustrated in FIG. 19. In other circumstances, although not
illustrated, the connectors 1030 can be
- 23 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
unevenly distributed along the axis extending along the gap 1010. The cutting
portion 10053 can be configured to
transect the gap bridging portions 1032 of the connectors 1030 as the cutting
portion 10053 is advanced between the
first portion 1006 and the second portion 1008 through the knife path defined
by the gap 1010. Where the connectors
1030 are unevenly distributed along the axis extending along the first portion
1006 and the second portion, in at least
one instance, the connectors 1030 can he disposed in greater frequency and/or
in closer proximity to each other at a
distal segment of the gap 1010 than at a proximal segment of the gap 1010 such
that the cutting portion 10053 may
experience an increasing resistance as it is advanced along the knife path
defined by the gap 1010. In other
circumstances, the connectors 1030 can be disposed in greater frequency and/or
in closer proximity to each other at a
proximal segment of the gap 1010 than at a distal segment of the gap 1010 such
that the cutting portion 10053 may
experience a decreasing resistance as it is advanced along the knife path
defined by the gap 1010, for example.
101141 In certain circumstances, the connectors 1030 can extend or
substantially extend in a single plane which can
be parallel or substantially parallel to the first deck contacting portion
1007 and/or the second (Wk contacting portion
1009, as illustrated in FIG. 19. In other circumstances, although not
illustrated, the connectors 1030 can extend or
substantially extend along a plurality of planes which can be parallel or
substantially parallel to each other and/or to
the first deck contacting portion 1007 and/or the second deck contacting
portion 1009.
101151 Further to the above, some or all of the gap bridging portions 1032 of
the connectors 1030 can be thinner
than the remainder of their respective connectors 1030 to present the cutting
portion 10053 with a reduced resistance
as the cutting portion 10053 is advanced to transect the connectors 1030 while
maintaining a coupling engagement
with the first portion 1006 and the second portion 1008 of the second layer
1004. For example, some or all the
connectors 1030 can comprise a dog-bone shape with thicker ends terminating
within the first portion 1006 and the
second portion 1008 of the second layer 1004 and thinner central portions
extending therebetween. In certain
circumstances, the connectors 1030 can each be comprised of a piece of suture
which may be comprised of
bioabsorbable material such as polyglycolic acid (PGA) which is marketed under
the trade name Vicryl, polylactic
acid (PLA or PLLA), polydioxanone (PDS), polyhydroxyalkanoate (PHA),
poliglecaprone 25 (PGCL) which is
marketed under the trade name Monocryl, polycaprolactone (PCL), and/or a
composite of PGA, PLA, PDS, PHA,
PGCL and/or PCL, for example.
101161 Referring again to FIG. 18, the tissue thickness compensator assembly
1033 can be prepared using a mold
1034. Au organic solution comprising a polymer such as, for example,
polyla.ctic acid (PLA) and/or polyglycolic acid
(PGA) can be poured into the mold 1034. The connectors 1030 can he immersed
into the organic solution. As
illustrated in FIG. 18, one or more of the connectors 1030 can each be trapped
in one or more dedicated slots 1040 on
a central shelf 1036 by one or more beams 1039 extending from a mold cover
1038 and configured for mating
engagement with the slots 1040 when the mold cover 1038 is in a closed
configuration with the mold 1034 to ensure
that the connectors 1030 remain immersed in the organic solution. The slots
1040 can be sized to receive or at least
partially receive the bridging portions 1032 which can be secured by the beams
1039 when the mold cover 1038 is in
the closed configuration with the mold 1034. The ends of the connectors 1030
extending from the gap bridging
portions 1032 may freely float in the organic solution. Alternatively, the
ends of the connectors 1030 can be secured to
sides of the mold 1034, for example. In certain circumstances, the connectors
1030 can be stretched in the organic
- 24 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
solution between the sides of the mold 1034. in other circumstances, the
connectors 1030 can be loosely held between
the sides of the mold 1034 to extend through the organic solution in a non-
linear fashion, for example.
101171 Further to the above, in various instances, the organic solution can
then be lyophilized using conventional
lyophilization techniques and/or any other suitable techniques. Upon
completion of the lyophilization process, the
mold cover 1036 can be removed and the tissue thickness compensator assembly
1033 can be recovered from the
mold 1034. As illustrated in FIG. 19, the resulting tissue thickness
compensator assembly 1033 includes connectors
1030 partially positioned within the first portion 1006 and the second portion
1008. Other techniques for partially
embedding the connectors 1030 into the first portion 1006 and/or the second
portion 1008 are contemplated by the
present disclosure. The reader will appreciate that the connectors 1030 can be
positioned closer to or farther away
from the deck contacting surfaces 1007 and 1009 by changing the height of the
central shelf 1038 and/or depth of the
slots 1040.
[0118] Referring now to FIG. 20, a tissue thickness compensator assembly 1042,
which may be similar in many
respects to the tissue thickness compensator assembly 1033, the tissue
thickness compensator assembly 1000, and/or
the tissue thickness compensator 20020, is illustrated. The tissue thickness
compensator assembly 1042 may comprise
the first portion 1006 and the second portion 1008 which can be spaced apart
and separably coupled together by a
continuous flexible member 1044 which may form a plurality of bridging members
or connectors 1046 which may
extend across the gap 1010 between the first portion 1006 and the second
portion 1008. The continuous flexible
member 1044 may include a first end 1048, a second end 1050, and a flexible
portion 1052 extending between the
first end 1048 and the second end 1050. The flexible portion 1052 can be
configured to extend through the first
portion 1006 and the second portion 1008 several times, fir example in a
zigzag pattern, to form the connectors 1046,
as illustrated in FIG. 20. The flexible portion 1052 can be passed in a first
direction through a distal segment 1054 of
the first portion 1006 and a distal segment 1056 of the second portion 1008 to
form a first gap bridging portion 1046a
across the gap 1010. The flexible portion 1052 can then be looped and passed
in a second direction, opposite the first
direction, through the second portion 1008 proximal to the distal segment 1056
and through the first portion 1006
proximal to the distal segment 1054 thereby forming a second gap bridging
portion 1046b proximal the first gap
bridging portion 1046a. Additional gap bridging portions 1046c and 1046d, for
example, can be formed in the same
manner across the gap 1010, as illustrated in FIG. 20.
[0119] In certain circumstances, the continuous flexible member 1044 can
comprise a suture and can be comprised
of a suture material such as polyglycolic acid (PGA) which is marketed under
the trade name Vicryl, polylactic acid
(PLA or PLLA), polydioxanone (PDS), polyhydroxyalkanoate (PHA), poliglecaprone
25 (PGCL) which is marketed
under the trade name Monoctyl, polycaprolactone (PCL), and/or a composite of
PGA, PLA, PDS, PHA, PGCL and/or
PCL, for example. In certain circumstances, the tissue thickness compensator
assembly 1042 can be assembled after
the first portion 1006 and the second portion 1008 are manufactured, for
example, via lyophilization. In some
circumstances, a needle (not shown) can be attached to the first end 1048 of
the continuous flexible member 1044 and
can be passed through the first portion 1006 and the second portion 1008, for
example in a zigzag pattern, to couple
the first portion 1006 to the second portion 1008, as described above. The
first end 1048 and/or the second end 1050
of the continuous flexible member 1044 can be secured to the side walls of the
first portion 1006 and/or the second
- 25 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
portion 1008 by tying in one or more knots at the first end 1048 and/or the
second end 1050, for example. The knots
may abut against the side walls of the first portion 1006 and/or the second
portion 1008 to prevent the flexible portion
1052 from unraveling relative to the first portion 1006 and/or the second
portion 1008. In other circumstances, the
first portion 1006 and the second portion 1008 of the tissue thickness
compensator assembly 1042 can be formed
around the continuous flexible member 1044. In such circumstances, as
illustrated in FIG. 20, the continuous flexible
member 1044 can be disposed in a mold 1062, for example in a zigzag pattern,
with slots 1064 defined side walls
1066 and slots 1068 defined in central shelf 1070. An organic solution
comprising a polymer such as, for example,
polylactic acid (PLA) and/or polyglycolic acid (PGA) can be poured into the
mold 1062 until the continuous flexible
member 1044 is immersed in the organic solution. A mold cover 1072 can be used
to ensure that the continuous
flexible member 1044 remains immersed in the organic solution which can then
be lyophilized using conventional
lyophilization techniques and/or any other suitable techniques. The first end
1048 and the second end 1050 of the
continuous flexible member 1044 can be secured at openings 1053 and 1055 of
the mold 1062, respectively, by tying
in one or more knots at the first end 1048 and the second end 1050 after
passing the first end 1048 through the
opening 1053 and the second end 1050 through the opening 1055, for example.
The knots may abut against the side
walls of the mold 1062 to prevent the continuous flexible member 1044 from
unraveling relative to the mold 1066.
After the tissue thickness compensator has been removed from the mold, in
various instances, portions of the
continuous flexible member 1044, such as portions 1048, 1050, and/or 1052, for
example, can then be cut and
removed from the tissue thickness compensator. Other techniques for assembling
the tissue thickness compensator
assembly 1042 are contemplated by the present disclosure.
(0120) In certain circumstances, a tissue thickness compensator assembly such
as, for example, the tissue thickness
compensator assembly 1042 can be compromised when excessive force or pressure
is applied thereto. For instance,
pressure can be applied to a tissue thickness compensator assembly such as,
for example, the tissue thickness
compensator assembly 1042 when the tissue thickness compensator assembly 1042
is loaded onto a staple cartridge
such as, for example, the staple cartridge 10000. The tissue thickness
compensator assembly 1042 can be equipped
with a pressure or force sensitive member that can provide a user with a
warning feedback if the pressure experienced
by the tissue thickness compensator assembly exceeds a threshold. For example,
a pressure or force sensitive film can
be attached to the tissue thickness compensator assembly 1042 and can be
configured to change color upon
experiencing pressure that exceeds the threshold. In certain circumstances,
the pressure or force sensitive film can be
disposed over the first portion 1006 and/or the second portion 1008 and can be
attached thereto via an adhesive, for
example. The pressure or force sensitive film can be biocompatible to permit
implantation of the pressure or force
sensitive film with the tissue thickness compensator assembly 1042 inside a
patient.
[0121] Referring now to FIGS. 23-25, a surgical end effector 1100 is
illustrated. The end effector 1100 is similar in
many respects to various end effectors disclosed elsewhere herein such as, for
example, the end effector 22090 (FIG.
9). As illustrated in FIG. 23, the end effector 1100 can include a staple
cartridge assembly 1.102 which is similar in
many respects to the staple cartridge assembly 20200 (FIG. 6), for example. In
addition, the end effector 1100 may
include a tissue thickness compensator 1104 which is similar in many respects
to other tissue thickness compensators
- 26 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
disclose elsewhere in this document such as the tissue thickness compensator
22020 (FIG. 9), the tissue thickness
compensator 20220 (FIG. 6), and/or the tissue thickness compensator 10020
(FIG. 4), for example.
101221 Further to the above, end effector 1100 can include a tissue thickness
compensator 1104 wherein the tissue
thickness compensator 1104 can be prepared using conventional lyophilization
techniques andlor any other suitable
techniques. In at least one example, the tissue thickness compensator 1104 can
be prepared by dissolving a polymer
such as, for example, polylactic acid (PLA) and/or polyglycolic acid (PGA) in
an organic solvent and lyophilizing the
solution. The tissue thickness compensator 1104 can be comprised of a
biocompatible foam which may comprise a
porous, open cell foam and/or a porous, closed cell foam, for example.
[0123] Further to the above, the tissue thickness compensator 1104 can be
altered or modified for use in a surgical
procedure. For example, upon completion of the lyophiliz.ation process, the
tissue thickness compensator 1104 can be
contacted with a modifying member 1106 to modify the tissue thickness
compensator 1104 for use in a particular
surgical procedure. In certain circumstances, the modi fication can occur
after assembling the tissue thickness
compensator 1104 with the end effector 1100, as illustrated in FIGS. 23-35.
For example, as illustrated in FIG. 23, the
tissue thickness compensator 1104 can be releasably assembled to the cartridge
assembly 1102 and modified while
assembled with the cartridge assembly 1102. In other circumstances, the
modification can occur before assembling the
tissue thickness compensator 1104 with the end effector 1100. In at least one
exarnple, the modification can be
performed as a separate step during manufacturing. In yet another example, the
modification may be performed during
a surgical procedure.
[0124] As described in greater detail below, the modification process can
involve modifying a surface or a plurality
of surfaces of the tissue thickness compensator 1104. In certain
circumstances, the modification process can involve
modifying one or more portions of the tissue thickness compensator 1104. One
or more portions can be modified in a
single modification process. Alternatively, a plurality of portions can each
be modified separately in consecutive
modification processes. In certain circumstances, the modification process can
comprise a thermal pressing process
which can be used to change the shape, size, dimensions, and/or porosity of at
least a portion of the tissue thickness
compensator 1104. Furthermore, the modification process can include means for
creating space within one or more
portions of the tissue thickness compensator 1104.
[0125] Referring again to FIGS. 23-25, in ceitain circumstances, a portion
1107 (FIG. 23) of the tissue thickness
compensator 1104 can be modified by a thermal pressing process which may
include transitioning the portion 1107 to
a glassy state, engaging the portion 1107 with the modifying member 1106,
applying pressure onto the portion 1107
while it is in the glassy state, and allowing the portion 1107 to cool below
the glassy state while the modifying
member 1106 is still engaged with the portion 1107. The modifying member 1106
may be used to maintain the
pressure on the portion 1107 for a time period sufficient to create the
resulting modified portion 1108 (FIG. 25). It is
note worthy that a material's transition into a glassy state can be a
reversible transition from a relatively hard state to a
relatively molten or flexible state in response to an increase in the
temperature of the material to a glass transition
temperature. A glass transition temperature of the material can be a
particular temperature or, in some instances, a
range of temperatures. The tissue thickness compensator modification process
described herein takes advantage of this
phenomenon by modifying a tissue thickness compensator while the tissue
thickness compensator is in the glassy
- 27 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
flexible state and then allowing the tissue thickness compensator to cool
below the glass transition temperature while
maintaining the modification.
101261 Further to the above, referring again to FIGS. 23-25, the portion 1107
of the tissue thickness compensator
1004 can be transitioned into the glassy state by heating at least the portion
1107 to a temperature greater than or equal
to a glass transition temperature of the material from which the portion 1107
is composed but lower than the melting
temperature of the same. For example, the tissue thickness compensator 1104
can be comprised of polyglycolic acid
(PGA) and in such circumstances, the portion 1107 can be transitioned into the
glassy state by heating the portion
1107 to a temperature that is greater than or equal to the glass transition
temperature of polyglycolic acid (PGA) but
lower than the melting temperature of the same. In various instances, the
glass transition temperature of polyglycolic
acid (VGA) can be in the range of 35-40 C, for example, and its melting
temperature can he in the range o1225-230
C, for example. In at least one example, the portion 1107 of the tissue
thickness compensator 1104 can be heated to a
temperature that is greater than or equal to 35 C but lower than 225 C in
order to transition the portion 1107 to the
glassy state. In another example, the portion 1107 can be transitioned to the
glassy state by heating the portion 1107 to
a temperature that is greater than or equal to 40 C but lower than 200 C, for
example.
101271 Further to the above, the modifying member 1106 can then be used to
apply pressure onto the portion 1107
while the portion 1107 is in the glassy state. The portion 1107 can be allowed
to exit the glassy state by cooling the
portion 1107 to a temperature below 35 cC, for example. The pressure may be
maintained for a time period sufficient
to permit the tissue thickness compensator 1104 to retain, or at least
partially retain, the modification imposed by the
modifying member 1106.
101281 In certain examples, the pressure can be maintained for a period of
time from about 30 seconds to about 8
hours, for example, during the time in the glassy state and/or for a period of
time from about 30 seconds to about 8
hours, for example, after exiting the glassy state. In at least one example,
the pressure can be maintained for
approximately 10 minutes during the time in the glassy state and for
approximately 10 minutes after exiting the glassy
state. Other time periods for maintaining the pressure are contemplated by the
present disclosure.
101291 In certain circumstances, the modifying member 1106 can be used to
apply pressure onto the portion 1107
before the portion 1107 is transitioned to the glassy state. In certain
circumstances, the modifying member 1106 may
apply pressure to the portion 1107 while the portion 1107 is heated to reach
the glassy state, while the portion 1107 is
in the glassy state, and/or while the portion 1107 is transitioned or cooled
to a temperature below the glassy state. In
certain circumstances, the pressure applied to the portion 1107 can be
gradually increased toward a threshold as the
temperature of the portion 1107 is gradually increased to transition the
portion 1107 toward the glassy state, for
example. In certain circumstances, the pressure applied to the portion 1107
can be removed, gradually removed, or at
least partially reduced as the portion 1107 exits the glassy state, before the
portion 1107 exits the glassy state, and/or
after the portion 1107 exits the glassy state.
101301 In certain circumstances, the modifying member 1106 can also be a heat
source for transitioning the portion
1107 of the tissue thickness compensator 1104 to the glassy state. For
example, the modifying member 1106 can
comprise a cylindrical distal portion 1110, as illustrated in FIG. 24, which
may include a heating coil (not shown). A
user can may energize the heating coil and engage the portion 1107 of the
tissue thickness compensator 1104 with the
- 28 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
modifying member 1106 to heat the portion 1107 to a temperature that is
greater than or equal the glass transition
temperature of the material composition of the portion 1107. Upon reaching a
desired temperature, the modifying
member may be pressed against the portion 1107, as illustrated in FIG. 24.
Alternatively, the modifying member may
be pressed against the portion 1107 before the modifying member 1106 reaches
the desired temperature. As described
above, the pressure may be maintained for a time period sufficient to permit
the tissue thickness compensator 1104 to
retain, or at least partially retain, the modification imposed by the
modifying member 1106. In addition, the heating
coil of the modifying member 1106 can be turned off to allow the temperature
of the portion 1107 to cool below the
glass transition temperature. The modifying member can then be removed. In
certain circumstances, the pressure
applied by the modifying member 1106 can be initiated prior to the portion
1107 entering the glassy state and
maintained throughout the glassy state. In some circumstances, the pressure
applied by the modifying member 1106
can be removed while the portion 1107 is in the glassy state.
101311 As illustrated in FIGS. 23-25, the modifying member 1106 can be
configured to change the shape, size,
dimensions, density, spring rate, and/or porosity of the portion 1107 of the
tissue thickness compensator 1104. For
example, the modified potion 1108 may comprise a substantially concave top
surface .1114 with a reduced height H.1,
while the remainder of the tissue thickness compensator 1104 may retain a
substantially flat top surface including an
original height H which is greater than the reduced height HI, as illustrated
in FIG. 25. As described above, the
modifying member 1:106 may comprise a cylindrical distal portion 1110. In such
circumstances, the curvature of the
resulting concave surface 1114 can, in part, depend on the curvature of the
cylindrical distal portion 1110 of the
modifying member 1106 in contact with the portion 1107 of the tissue thickness
compensator 1104 during the
modification process. Furthermore, the modified portion 1108 may possess a new
lower porosity compared to the
unmodified portion 1107 which can result, at least in part, from the
compressive forces applied to the portion 1:107 by
the modifying member 1106 during the modification process, as described above.
Said another way, the pressure
applied to the portion 1107 during the modification process may yield a
material redistribution wherein a cross-section
through the modified portion 1108 may comprise a greater material density than
a similar cross section through the
portion 1107 prior to the modification process. Furthermore, the modified
portion 1108 may comprise a different
spring rate from the remainder of the tissue thickness compensator 1104 which
can result, in part, from the changes in
density and porosity realized by the modified portion 1108 during the
modification process, as described in greater
detail below. In at least one instance, the spring rate of the modified
portion 1108 may be less than or greater than the
spring rate of the unmodified portion 1107.
101321 Referring now to FIGS. 26-34, a tissue thickness compensator can be
modified prior to assembly with an end
effector such as, for example, the end effector 22090 (FIG.9). In certain
circumstances, as illustrated in FIGS. 27, 30,
and 33, a mold can be utilized to modify a tissue thickness compensator using
a thermal pressing process, as described
above. For example, as illustrated in FIGS. 26-28, a tissue thickness
compensator 1120 can be modified to include a
longitudinal slot 1122. The tissue thickness compensator 1120 may be similar
in many respects to other tissue
thickness compensators described elsewhere such as, for example, the tissue
thickness compensator 22020 (FIG. 9).
For example, like the compensator 22020, the compensator 1120 can be utilized
with the end effector 22090.
Furthermore, the longitudinal slot 1122 may be similar in many respects to the
knife slot 22025. For example, like the
-29 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
knife slot 22025, the slot 1122 may define a tissue thickness compensator
knife path for the cutting portion 10053
between a first stapling portion 1:124a and a second stapling portion 1124b.
Furthermore, the first stapling portion
1124a and the second stapling portion 1124b can be similar in many respects to
the first stapling portion 22021a (FIG.
9) and the second stapling portion 2202 lb (FIG. 9), respectively, of the
tissue thickness compensator 22020. In
addition, the slot 1122 can be configured to releasably connect the first
stapling portion 1124a and the second stapling
portion I 124b such that, in use with the end effector 22090, the cutting
portion 10053 can be advanced distally
through the slot 1122 to transect the slot 1122 and separate the first
stapling portion 1124a and the second stapling
portion 1124b.
[0133] Referring again to FIGS. 26-28, the tissue thickness compensator 1120
can be prepared using traditional
lyophilization techniques and/or any other suitable techniques. In addition,
the tissue thickness compensator 1120 can
be modified or altered to create the slot 1122 therethrough. Similar to the
tissue thickness compensator 1104, the
tissue thickness compensator 1120 can be comprised at least in part of a
material comprising a glass transition
temperature and can modified by transitioning the material into a glassy
state. In one example, the tissue thickness
compensator 1 120 can be heated in an oven (not shown) to a temperature
greater than or equal to the glass transition
temperature of the material composition of the tissue thickness compensator
1120 but less than the melting
temperature of the same. A mold 1126 comprising a central beam 1128, as
illustrated in FIG. 27, can be utilized to
create the slot 1122 by inserting the central beam 1128 into the tissue
thickness compensator 1120 while the tissue
thickness compensator 1120 is in the glassy state. The tissue thickness
compensator 1120 can then be allowed to cool
to a temperature below the glass transition temperature while the central beam
1128 remains inserted into the tissue
thickness compensator 1120. In some instances, the central beam 1128 can be
removed from the tissue thickness
compensator 1:120 while the tissue thickness compensator 1120 is in its glassy
state.
101341 In certain circumstances, a cooling medium can be utilized to actively
cool the tissue thickness compensator
1120. in some instances, a fan can be used to generate a flow of air over the
tissue thickness compensator 1120 while
the tissue thickness compensator 1120 is in the mold 1126 and/or after the
tissue thickness compensator 1120 has been
removed from the mold. In some instances, a refrigeration process can be
utilized to cool the tissue thickness
compensator 1120 while the tissue thickness compensator 1120 is in the mold
1126 and/or after the tissue thickness
compensator 1120 has been removed from the mold. The central beam 1128 can be
removed after transitioning the
tissue thickness compensator 1120 out of the glassy state. The central beam
1128 can remain inserted into the tissue
thickness compensator 1120 for a time period sufficient to permit the tissue
thickness compensator 1120 to retain, or
at least substantially retain, the space occupied by the central beam 1128. In
certain examples, the central beam 1128
can remain inserted for a period of time from about 30 seconds to about 8
hours, for example, during the time in the
glassy state andlor for a period of time from about 30 seconds to about 8
hours, for example, after exiting the glassy
state. In at least one example, the central beam 1128 can remain inserted for
approximately 10 minutes during the time
in the glassy state and for approximately 10 minutes after exiting the glassy
state. Other time periods for maintaining
the central beam 1128 within the tissue thickness compensator 1120 are
contemplated by the present disclosure.
[0135] Further to the above, as illustrated in FIG. 28, pressure applied by
the central beam 1128 during the
modification process may yield an increased material density at a portion 1130
of the tissue thickness compensator
-30 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
1120. The portion 1130 may connect the first stapling portion 1124a and a
second stapling portion I124b thereby
providing additional stability for the slot 1122. In certain circumstances,
the mold 1126 may comprise edge modifiers
such as, for example, edge modifiers 1132a and 1132b which can modify the
tissue thickness compensator 1120
during the modification process to produce modified edges 1134a and 1134b,
respectively, as illustrated in FIG. 28.
101361 Referring again to FIGS. 26-28, it may be desirable to remove a
significant amount of material from the
tissue thickness compensator 1120 to create the slot 1122.1n such
circumstances, the central beam 1128 can be heated
to a temperature greater than the melting temperature of the material
composition of the tissue thickness compensator
1120. Upon inserting the heated central beam 1128 into the tissue thickness
compensator 1120, the central beam 1128
may melt through the tissue thickness compensator 1120 thereby creating a
space for the slot 1122 within the tissue
thickness compensator 1120, as illustrated in FIG. 28. In certain
circumstances. it may be desirable to gradually
increase the pressure applied by the central beam 1128 against the tissue
thickness compensator 1120 to gradually
insert the central beam 1128 into the tissue thickness compensator 1120.
[0137] in certain circumstances, it can be desirable to increase material
density of one or more surfaces of a tissue
thickness compensator. As illustrated in FIGS. 29-31, a tissue thickness
compensator 1140 can be modified or altered
such that a surface 1142 of the tissue thickness compensator 1140 may comprise
a higher material density than the
remainder of the tissue thickness compensator 1140, which can be achieved, in
certain circumstances, post
lyophilization. The tissue thickness compensator 1140 may be similar in many
respects to other tissue thickness
compensators described elsewhere such as, for example, the tissue thickness
compensator 22020 (FIG. 9) and/or the
tissue thickness compensator 1120 (FIG. 26). A surface modifier 1144 can be
utilized to modify the surface 1142 of
the tissue thickness compensator 1140 using a thermal pressing process which
is similar in many respects to the
thermal pressing processes used to modify the tissue thickness compensator
1104 and/or the tissue thickness
compensator 1120, as described above. For example, the tissue thickness
compensator 1140 can be comprised at least
in part of a material comprising a glass transition temperature and can be
modified after being transitioned into a
glassy state.
1101381 As described above, a tissue thickness compensator such as, for
example, the tissue thickness compensator
1140 can be transitioned to the glassy state where it is heated to a
temperature greater than or equal to the glass
transition temperature of the material composition of the tissue thickness
compensator 1140 but less than the melting
temperature of the same. The surface modifier 1144 can be pressed against the
surface 1142 while the tissue thickness
compensator 1140 is in the glassy state. The pressure applied by the surface
modifier 1144 may compress the surface
1142 thereby increasing the material density of the surface 1142. The increase
in material density can be retained by
the surface 1142 by allowing the surface 1142 to cool to a temperature below
the glass transition temperature.
[0139] In certain instances, the pressure applied by the surface modifier 1144
against the surface 1142 can be
maintained for a period of time from about 30 seconds to about 8 hours, for
example, during the time in the glassy
state and/or for a period of time from about 30 seconds to about 8 hours, for
example, after exiting the glassy state. In
at least one example, the pressure can be maintained for approximately 10
minutes during the time in the glassy state
and for approximately 10 minutes Mier exiting the glassy state. Other time
periods for maintaining the pressure
applied by the surface modifier 1144 against the surface 1142 are contemplated
by the present disclosure.
- 31 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
[0140] In some instances, a fan can be used to generate a flow of air over the
tissue thickness compensator 1140
while the tissue thickness compensator 1140 is in contact with the modifier
1144 and/or after the tissue thickness
compensator 1140 has been removed from the modifier 1144. In some instances, a
refrigeration process can be
utilized to cool the tissue thickness compensator 1140 while the tissue
thickness compensator 1140 is in contact with
the modifier 1144 and/or after the tissue thickness compensator 1140 has been
removed from the modifier 1144.
Upon transitioning the tissue thickness compensator 1140 out of the glassy
state, in various instances, the surface
modifier 1144 can be disengaged from the tissue thickness compensator 1140. In
certain circumstances, the surface
modifier 1 144 can include a heating element which can be utilized to increase
the temperature of the surface 1142 to a
temperature greater than or equal to the glass transition temperature of the
material composition of the tissue thickness
compensator 1140, as described above.
101411 Referring again to FIG. 30, the surface modifier 1144 may comprise a
flat, or at least substantially flat,
contacting surface 1146 for contacting the surface 1142, for example. In other
circumstances, the contacting surface
1146 may comprise various textures such as, for example, protrusions which can
extend into the surface 1142 of the
tissue thickness compensator 1140 during the modification process. In certain
circumstances, the surface modifier
1144 can be used to apply pressure onto the surface 1142 of the tissue
thickness compensator 1140 before the tissue
thickness compensator 1140 is transitioned to the glassy state. In certain
circumstances, the surface modifier 1144
may apply pressure to the surface 1142 while the tissue thickness compensator
1140 is heated to reach the glassy state,
while the tissue thickness compensator 1140 is in the glassy state, and/or
while the tissue thickness compensator 1140
is transitioned or cooled to a temperature below the glassy state. In certain
circumstances, the pressure applied by the
surface modifier 1144 to the surface 1142 can be gradually increased toward a
threshold as the temperature of the
tissue thickness compensator 1140 is gradually increased to transition the
tissue thickness compensator 1140 toward
the glassy state, for example. In certain circumstances, the pressure applied
to the surface 1142 can be removed,
gradually removed, or at least partially reduced as the tissue thickness
compensator 1140 exits the glassy state, before
the tissue thickness compensator 1140 exits the glassy state, and/or after the
tissue thickness compensator 1140 exits
the glassy state.
101421 In certain circumstances, the tissue thickness compensator 1140 can be
modified or altered to include a skin
or a dense outer layer. In certain circumstances, the resulting skin or dense
outer layer may comprise textures such as,
for example, protrusions which can extend into the surface 1142 of the tissue
thickness compensator 1140. In certain
instances, the contacting surface 1146 of the surface modifier 1144 can be
heated to a temperature greater than or
equal to the melting temperature of the material composition of the tissue
thickness compensator 1140. The surface
modifier 1144 and/or the tissue thickness compensator 1140 can be moved to
bring the surface 1142 of the tissue
thickness compensator 1140 into contact with the heated contacting surface
1146 of the surface modifier 1144 thereby
melting, or at least substantially melting, the surface 1142. The surface
modifier 1144 and the tissue thickness
compensator 1140 can then be separated to permit the modified surface 1142 to
cool below its melting temperature
which may create a skin or a dense outer layer onto the tissue thickness
compensator 1140.
- 32 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
[0143] In certain instances, the contacting surface 1146 of the surface
modifier 1144 can be heated prior to coining
in contact with the surface 1142. In other instances, the contacting surface
1146 of th.e surface modifier 1144 can be
heated after coming in contact with the surface 1142.
101441 In certain instances, the contacting surface 1146 of the surface
modifier 1144 can remain in contact with the
surface 1142 of the tissue thickness compensator 1140 for a time period
sufficient to allow the surface 1142 to flow
into a desired geometry. Such a time period can range from about 30 seconds to
about 8 hours, for example; other time
periods are contemplated by the present disclosure. Such a time period can be
sufficient to locally affect and/or melt
the material of the tissue thickness compensator 1140 and have it flow into a
new geometry. As described herein, such
a new geometry can be prescribed by the tooling used to make the tissue
thickness compensator 1140.
[0145] In certain instances, the surface 1142 of the tissue thickness
compensator 1140 can be allowed to cool, or can
be actively cooled, to a temperature below the melting temperature of the
tissue thickness compensator 1140 before
separating the surface modifier 1144 from the tissue thickness compensator
1140. In other instances, the surface 1142
of the tissue thickness compensator 1140 can be allowed to cool, or can be
actively cooled, to a temperature below the
melting temperature of the tissue thickness compensator 1140 after separating
the surface modifier 1144 from the
tissue thickness compensator 1140.
[0146] Further to the above, the modified surface 1142 can comprise a density
which is approximately 10% greater
than the density of the remainder of the tissue thickness compensator 1140,
approximately 20% greater than the
density of the remainder of the tissue thickness compensator 1140,
approximately 30% greater than the density of the
remainder of the tissue thickness compensator 1140, approximately 40% greater
than the density of the remainder of
the tissue thickness compensator 1140, approximately 50% greater than the
density of the remainder of the tissue
thickness compensator 1140, approximately 60% greater than the density of the
remainder of the tissue thickness
compensator 1140, approximately 70% greater than the density of the remainder
of the tissue thickness compensator
1140, approximately 80% greater than the density of the remainder of the
tissue thickness compensator 1140,
approximately 90% greater than the density of the remainder of the tissue
thickness compensator 1140, and/or
approximately 100% greater than the density of the remainder of the tissue
thickness compensator 1140, for example.
In various circumstances, the modified surface 1142 can comprise a density
which is more than the density of the
remainder of the tissue thickness compensator 1140 and less than twice the
density of the remainder of the tissue
thickness compensator 1140, for example. In various circumstances, the
modified surface 1142 can comprise a density
which is over twice the density of the remainder of the tissue thickness
compensator 1140, for example.
101471 Referring now to FIGS. 32-34, a tissue thickness compensator 1150 can
be modified to include a plurality of
apertures 1152 which may extend at least partially through the tissue
thickness compensator 1150. The tissue
thickness compensator 1150 may be similar in many respects to other tissue
thickness compensators described herein
such as, for example, the tissue thickness compensator 20220 (FIG. 6). Like
the compensator 20220, the compensator
1150 can be utilized with the cartridge assembly 20200 (FIG. 6) and the
apertures 1152 may be similar in many
respects to the clearance apertures 20224 extending at least partially through
the tissue thickness compensator 20220.
For example, like the apertures 20224, the apertures 1152 can be aligned with
corresponding staple legs 20232 (FIG.
7) when the tissue thickness compensator 1150 is assembled with the cartridge
assembly 20200 such that the staple
- 33 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
legs 20232 may move through the clearance apertures 1152 in the tissue
thickness compensator 1150 when the staple
legs 20232 move from the unfired configuration to the fired configuration, as
described above in greater detail.
101481 Further to the above, referring again to FIGS. 32-34, the tissue
thickness compensator 1150 can be prepared
using traditional lyophilization techniques and/or any other suitable
techniques. In certain circumstances, a polymer
having a glass transition temperature such as, for example, polylactic acid
(PLA) and/or polyglycolic acid (PGA) can
be dissolved in an organic solvent to form a solution which can be lyophilized
to produce the tissue thickness
compensator 1150. Furthermore, the tissue thickness compensator 1150 can be
modified post lyophilization using a
thermal pressing process which is similar in many respects to the thermal
pressing processes used to modify the tissue
thickness compensator 1104, the tissue thickness compensator 1120, and/or the
tissue thickness compensator 1140, for
example, as described above. For example, the tissue thickness compensator
1150 can be modified to include the
apertures 1152 once the tissue thickness compensator 1150 is transitioned to a
glassy state.
101491 As described above, a tissue thickness compensator such as, for
example, the tissue thickness compensator
1150 can be tran.sitioned to a glassy state by being heated in an oven (not
shown) to a temperature greater than or
equal to the glass transition temperature of the material composition of the
tissue thickness compensator 1150 but less
than the melting temperature of the same. A mold 1154 comprising a plurality
of posts, dowels, pins, and/or
protrusions, for example, such as, for example, needles 1156 can be utilized
to create the apertures 1152 by inserting
the needles 1156 into the tissue thickness compensator 1150 while the tissue
thickness compensator 1150 is in the
glassy state. The tissue thickness compensator 1150 can then be allowed to
cool to a temperature below the glass
transition temperature while the needles 1156 remain inserted into the tissue
thickness compensator 1150. In some
instances, the needles 1156 can be removed from the tissue thickness
compensator 1150 while the tissue thickness
compensator 1:150 is in the glassy state. In some instances, a fan can be used
to generate a flow of air over the tissue
thickness compensator 1150 while the tissue thickness compensator 1150 is
engaged with the needles 1156 and/or
after the tissue thickness compensator 1150 has been disengaged from the
needles 1156. In some instances, a
refrigeration process can be utilized to cool the tissue thickness compensator
1150 while the tissue thickness
compensator 1150 is engaged with the needles 1156 and/or after the tissue
thickness compensator 1150 has been
disengaged from the needles 1156. In various instances, the needles 1156 can
be removed after transitioning the
tissue thickness compensator 1150 out of the glassy state. The needles 1156
can remain inserted into the tissue
thickness compensator 1150 for a time period sufficient to permit the tissue
thickness compensator 1150 to retain, or
at least substantially retain, the spaces defining the apertures 1152 which
are occupied by the needles 1156.
101501 In certain examples, the needles 1156 can remain inserted for a period
of time from about 30 seconds to
about 8 hours, for example, during the time in the glassy state and/or for a
period of time from about 30 seconds to
about 8 hours, for example, after exiting the glassy state. In at least one
example, the needles 1156 can remain inserted
for approximately 10 minutes during the time in the glassy state and for
approximately 10 minutes after exiting the
glassy state. Other time periods for maintaining the needles 1156 inserted
into the tissue thickness compensator 1150
are contemplated by the present disclosure.
101511 In certain circumstances, the needles 1156 can be removed from the
tissue thickness compensator 1150 prior
to transitioning the tissue thickness compensator 1150 out of the glassy
state. In other circumstances, the needles 1156
- 34 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
can be gradually removed over time. For example, the needles 1156 can be
partially removed from the tissue thickness
compensator 1150 prior to transitioning the tissue thickness compensator 1:150
out of the glassy state. The needles
1156 can then be fully, removed from the tissue thickness compensator 1150
after transitioning the tissue thickness
compensator 1150 out of the glassy state. The reader will appreciate that the
greater the depth of insertion of the
needles 1156 into the tissue thickness compensator 1150, the greater the depth
of the corresponding apertures 1152
that can be created in the tissue thickness compensator 1150.
101521 Referring again to FIGS. 32-34, in certain instances, the needles 1156
can be heated to a temperature greater
than or equal to the melting temperature of the material composition of the
tissue thickness compensator 1150. In
addition, the needles 1156 can be inserted into the tissue thickness
compensator 1150 to create the apertures 1152 by
melting, or at least partially melting, through the regions of the tissue
thickness compensator 1150 that receive the
needles 1156. In various instances, the needles 1156 can be heated prior to
their insertion into the tissue thickness
compensator 1150. In various instances, the needles 1156 can be heated after
their insertion into the tissue thickness
compensator 1150. In various instances, the needles 1156 can be gradually
heated as the needles 1156 are inserted into
the tissue thickness compensator 1150.
101531 In certain instances, the needles 1156 may remain positioned within the
tissue thickness compensator 1150
for a period of time sufficient to permit the inched material of the tissue
thickness compensator 1150 to flow into a
desired geometry. Such a time period can range from about 30 seconds to about
8 hours, for example; other time
periods are contemplated by the present disclosure. Such a time period can be
sufficient to locally affect and/or melt
the material of the tissue thickness compensator 1150 and have it flow into a
new geometry. As described herein, such
a new geometry can be prescribed by the tooling used to make the tissue
thickness compensator 1150.
(0154) In certain instances, the tissue thickness compensator :1150 can be
allowed to cool, or can be actively cooled,
to a temperature below the melting temperature of the tissue thickness
compensator 1150 before separating the
needles 1156 from the tissue thickness compensator 1150. In other instances,
the tissue thickness compensator 1150
can be allowed to cool, or can be actively cooled, to a temperature below the
melting temperature of the tissue
thickness compensator 1150 after separating the needles 1156 from the tissue
thickness compensator 1150.
101551 Referring again to FIGS. 32-34, the needles 1156 can be arranged in
rows extending longitudinally along a
length of the mold 1154 which may correspond to staple rows in a staple
cartridge such as, for example, the staple
cartridge assembly 20200 (FIG. 6). For example, as illustrated in FIG. 33, the
needles 1156 can are arranged in six
rows which can be configured to create six rows of the apertures 1152 that can
be configured to receive six rows of
the staples 20230 (FIG. 7). In certain circumstances, as illustrated in FIG.
33, the rows of the needles 1156 can be
arranged in two groups which are spaced apart and configured to be received in
two portions 1158 and 1160 of the
tissue thickness compensator 1150 thereby creating two groups of the apertures
1:152 separated by an intermediate
portion 1162. The intermediate portion 1162 can be positioned, at least
partially, over the cartridge knife slot 22015
(FIG. 6), when the tissue thickness compensator 1150 is assembled with staple
cartridge assembly 20200. In use, the
firing member 10052 (FIG. 10) can be advanced distally to push the staple legs
20232 (FIG. 8) through the apertures
1152 within the portions 1158 and 1160 and advance the cutting portion 10053
(FIG. 10) to transect the intermediate
portion 1162 and separate the portions 1158 and 1160.
- 35 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
[0156] Referring again to FIGS. 32-34, the aperturm 1152 can be configured to
extend within the tissue thickness
compensator 1:150 and terminate al: a certain depth within the tissue
thickness compensator 1:150. The apertures :1152
may comprise uniform depths, as illustrated in FIG. 34. In other
circumstances, the apertures 1152 may comprise
different depths (not shown). For example, a first row of the apertures 1152
may comprise a first depth and a second
row of the apertures 1152 may comprise a second depth different from the first
depth and yet a third row of the
apertures 1152 may comprise a third depth different from the first depth and
the second depth. The depths of the
apertures 1152 can be determined, at least in part, by the heights of the
corresponding needles 1156. For example, a
first row of the needles 1156 comprising a first height and a second row of
the needles 1156 comprising second height
greater than first height may create a first row of the apertures 1152
comprising a first depth and a second row of the
apertures 1152 comprising a second depth which is greater than the first
depth.
101571 Referring again to FIGS. 32-34, the needles 1156 can be configured to
define a trajectory for the apertures
1152 within the tissue thickness compensator 1150. In certain circumstances,
the needles 1156 can extend along an
axis that is perpendicular and/or substantially perpendicular to a mold
surface 1164 of the mold 1154, as illustrated in
FIG. 33. Inserting the needles 1156 into the tissue thickness compensator 1150
while maintaining a parallel
relationship between the mold surface 1164 and a surface 1166 of the tissue
thickness compensator 1150 may result in
defining a perpendicular and/or substantially perpendicular trajectory for the
apertures 1152 relative to the surface
1166 of the tissue thickness compensator 1150, as illustrated in FIG. 34. In
other circumstances, the needles 1156 can
extend from the mold surface 1164 at an oblique angle (not shown) and/or the
insertion trajectory of the needles 1156
into the tissue thickness compensator 1150 can be at an angle such that the
needles 1156 may define a non-
perpendicular trajectory for the apertures 1152 relative to the surface 1166
of the tissue thickness compensator 1150.
In certain circumstances, a group of the needles :1156 can be parallel and/or
substantially parallel to each other, as
illustrated in FIG. 33, resulting in a group of the apertures 1152 that may be
parallel and/or substantially parallel to
each other, as illustrated in FIG. 24. In other circumstances, although not
illustrated, a group of non-parallel needles
can extend from the mold surface 1164 and may result in non-parallel apertures
when inserted into the tissue thickness
compensator 1150. In some circumstances, the needles 1156 can be configured to
create apertures within the tissue
thickness compensator 1150 that can comprise a partially curved trajectory
and/or a partially linear trajectory. For
example, the needles 1156 can extend from the mold surface 1164 in a partially
curved trajectory and can be inserted
into the tissue thickness compensator 1150 to create apertures within the
tissue thickness compensator 1150 with a
corresponding partially curved trajectory.
[0158] Referring again to FIGS. 32-34, some or all of the needles 1156 can
comprise blunt distal ends 1168, as
illustrated in FIG. 33. In other circumstances, some or all of the needles
1156 can comprise sharp distal ends (not
shown). Some or all of the needles 1156 can comprise cylindrical, or at least
substantially cylindrical, shapes, for
example, as illustrated in FIG. 33. Other shapes are also contemplated by the
present disclosure. [0159] In various
instances, one or more of the needles :1156 extending from the mold surface
1164 may not be insertable through the
full thickness of the tissue thickness compensator 1150. In certain instances,
one or more of the needles 1156
extending from the mold surface 1164 can be insertable through the full
thickness of the tissue thickness compensator
1150 to create openings an/or holes that extend through the full thickness of
the tissue thickness compensator 1150. In
- 36 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
certain instances, one or more of the needles 1156 extending from the mold
surface 1164 can be inserted through a
first side of the tissue thickness compensator :1150 and exited through a
second side of the tissue thickness
compensator 1150 which may be opposite the first side, for example. In certain
instances, one or more of the needles
1156 may comprise a length greater than the full thickness of the tissue
thickness compensator 1150 to facilitate the
insertion of the one or more needles 1156 through the full thickness of the
tissue thickness compensator 1150.
101601 Referring now to FIGS. 35-37, it may be desirable to resin a tissue
thickness compensator. For example, one
or more dimensions of a tissue thickness compensator may be adjusted to
correspond to dimensions of a staple
cartridge in order to provide a better fit to the staple cartridge when the
tissue thickness compensator is assembled
with the staple cartridge. In certain circumstances, a tissue thickness
compensator 1170 can be resized by changing its
height from a first height HI, as illustrated in FIG. 35, to a second height
H2, as illustrated in FIG. 36. The tissue
thickness compensator 1170 may be similar in many respects to other tissue
thickness compensators described herein
such as, for example, the tissue thickness compensator 22020 (FIG. 9), the
tissue thickness compensator 1140 (FIG.
29), and/or the tissue thickness compensator 1150 (FIG. 32). For example, like
the compensator 22020, the
compensator 1170 can be utilized with the end effector 22090 (FIG. 9).
101611 In various instances, referring again to FIGS. 35-37, the tissue
thickness compensator 1170 can be prepared
using traditional lyophilization techniques and/or any other suitable
techniques. In certain instances, the tissue
thickness compensator 1170 can be resized, as illustrated in FIG. 37, using a
thermal pressing process and a mold
1172, for example. The mold 1172 may comprise a receiver 1174 configured to
receive the tissue thickness
compensator 1170 and an adjustment member 1176 which can be partially
insertable into the receiver 1174. The tissue
thickness compensator 1170 can be resized when the tissue thickness
compensator 1170 is transitioned into a glassy
state. In one embodiment, the tissue thickness compensator 1:170 can be heated
in an oven (not shown) to a
temperature greater than or equal to a glass transition temperature of the
material composition of the tissue thickness
compensator 1170 but less than the melting temperature of the same. In another
embodiment, the receiver 1174 and/or
the adjustment member 1176 may comprise a heating element for transitioning
the tissue thickness compensator 1170
to the glassy state. The adjustment member 1176 can then be inserted into the
receiver 1174 a distance H3, for
example, as illustrated in FIG. 37, thereby compressing the tissue thickness
compensator 1170 and reducing its height
from the first height HI to the second height 112. In some instances, the
adjustment member 1176 can be inserted into
the receiver 1174 before the tissue thickness compensator 1170 enters into the
glassy state or just as the tissue
thickness compensator 1170 enters into the glassy state. The adjustment member
1176 can be held against the tissue
thickness compensator 1170 to compress the tissue thickness compensator 1170
for a time period sufficient to permit
the tissue thickness compensator 1170 to retain, or at least substantially
retain, the second height H2, as illustrated in
FIG. 36. The tissue thickness compensator 1170 can then be allowed to cool to
a temperature below the glass
transition temperature while under compression from the adjustment member
1176. After transitioning the tissue
thickness compensator :1170 out of the glassy state, the adjustment member
1176 can be retracted. In some instances,
the adjustment member 1176 can be retracted before the tissue thickness
compensator 1170 exits the glassy state. In
certain circumstances, the above described resizing process can be utilized to
change another dimension of the tissue
- 37 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
thickness compensator 1170 such as a length or a width of the tissue thickness
compensator 1170, for example. In
some circumstances, these dimensions can be modified simultaneously or
modified sequentially.
101621 In certain examples, the compression from the adjustment member 1176
can be maintained for a period of
time from about 30 seconds to about 8 hours, for example, during the time in
the glassy state and1or for a period of
time from about 30 seconds to about 8 hours, for example, after exiting the
glassy state. In at least one example, the
compression from the adjustment member 1176 can be maintained for
approximately 10 minutes during the time in
the glassy state and for approximately 10 minutes after exiting the glassy
state. Other time periods for maintaining the
compression imposed by the adjustment member 1176 against the tissue thickness
compensator 1170 are
contemplated by the present disclosure.
[0163] In certain circumstances, the adjustment member 1176 can be used to
apply pressure onto the tissue thickness
compensator 1170 before the tissue thickness compensator 1170 is transitioned
to the glassy state. In certain
circumstances, the adjustment member 1176 may apply pressure to the tissue
thickness compensator 1170 while the
tissue thickness compensator 1170 is heated to reach the glassy state, while
the tissue thickness compensator 1170 is
in the glassy state, and/or while the tissue thickness compensator 1170 is
transitioned or cooled to a temperature below
the glassy state. In certain circumstances, the pressure applied to the tissue
thickness compensator 1170 can be
gradually increased toward a threshold as the temperature of the tissue
thickness compensator 1170 is gradually
transitioned toward the glassy state, for example. In certain circumstances,
the pressure applied to the tissue thickness
compensator 1170 can be removed, gradually removed, or at least partially
reduced as the tissue thickness
compensator 1170 exits the glassy state, before the tissue thickness
compensator 1170 exits the glassy state, and/or
after the tissue thickness compensator 1170 exits the glassy state.
[01641 The reader will appreciate that the different molds utilized in the
modification processes described above
such as, for example, the molds 1144, 1154, and/or 1172 are illustrative
examples. Other mold designs and
configurations can also be employed to manipulate tissue thickness
compensators in a variety of ways. Furthermore,
the forces involved in manipulating a tissue thickness compensator need not
only be compressive forces. For example,
tensile forces can also be utilized to modify, reshape, and/or resin a tissue
thickness compensator in similar manners
to those described above. For example, the tissue thickness compensator 1170
can be stretched using tensile forces to
[educe its height from the first height HI (FIG. 35) to the second height 112
(FIG. 36), for example, using a
modification process that is similar in many respects to the modification
processes described above. In certain
circumstances, combinations of tensile and compressive forces can be used to
manipulate a tissue thickness
compensator during a modification process.
[0165] Referring again to FIGS. 35-37, it may be desirable to modify the
porosity of a tissue thickness compensator
for use in a surgical procedure. A tissue thickness compensator may comprise a
porous, open cell foam and/or a
porous, closed cell foam, for example. Traditional lyophilization techniques
may provide some control over a tissue
thickness compensator's porosity but such control may not be easily
reproducible and may need additional fine
adjustments that may not be obtainable by traditional lyophilization
techniques. As illustrated in FIGS. 35-37, the
height of the tissue thickness compensator 1170 can be changed from the first
height III (FIG. 35) to the second
height H2 (FIG. 36), for example, using the modification process described
above. In addition, porosity of the tissue
- 38 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
thickness compensator 1170 can also be modified using the same and/or a
similar modification process. For example,
the tissue thickness compensator 1170 may comprise a first porosity (FIG. 35)
prior to the modification process and a
second porosity (FIG. 36) after completion of the modification process, as
described above. The change in porosity
can be attributed, at least in part, to the compressive forces and/or the
energy applied to the tissue thickness
compensator 1170 by the adjustment member 1176 during the modification process
described above.
101661 Further to the above, the tissue thickness compensator 1170 may
comprise a plurality of pores 1180. Some or
all of the pores 1180 may be altered in position, size, and/or shape, for
example, as a result of the modification process
described above. For example, one or more of the pores 1180 may comprise a
spherical, or substantially spherical,
shape prior to the modification process which may be altered to an oval, or
substantially oval, shape as a result of the
modification process. In at least one example, one or more of the pores 1180
may comprise a fist size prior to the
modification process and a second size different from the first size as a
result of the modification process. In certain
circumstances, as described below in greater detail, the porosity changes can
be localized to one or more regions or
zones of the tissue thickness compensator 1170.
[01671 Furthermore, in certain circumstances, the change in porosity of the
tissue thickness compensator 1170 may
be accompanied by a change in density of the tissue thickness compensator
1170. In other words, as the adjustment
member 1176 is advanced against the tissue thickness compensator 1170,
compressive forces may reduce space
occupied by the tissue thickness compensator 1170 thereby causing material
and/or pore redistribution which may
yield an increase in the density of the tissue thickness compensator 1170
and/or a reduction in its porosity. In certain
circumstances, as described below in greater detail, the density changes can
be localized to one or more regions or
zones of the tissue thickness compensator 1170.
[01681 Further to the above, the change in porosity and/or density of the
tissue thickness compensator 1170 may
yield a change in the spring rate of the tissue thickness compensator 1170. A
tissue thickness compensator's spring
rate can influence its ability to compensate for tissue thickness when the
tissue thickness compensator is deployed
against tissue captured by staples such as, for example, the staples 20230
(FIG. 8), as described above in greater
detail. Furthermore, a tissue thickness compensator's spring rate can also
influence its ability to apply pressure against
tissue captured with the tissue thickness compensator by a staple. In other
words, a change in a tissue thickness
compensator's spring rate may change the pressure exerted by the tissue
thickness compensator against tissue captured
by a staple. Since (Efferent tissue types may respond more positively to
certain pressures, fine control over a tissue
thickness compensator's spring rate can be advantageous.
101691 As illustrated in FIGS. 35-37, the tissue thickness compensator 1170
may comprise a first spring rate (FIG.
35) which may be altered or modified to a second spring rate (FIG. 36)
different from the first spring rate using the
modification process described above. For example, as described above, the
adjustment member 1176 can be
advanced against the tissue thickness compensator 1170 while the tissue
thickness compensator 1170 is in the glassy
state. In response, the tissue thickness compensator 1170 may be compressed
which may cause a change in the spring
rate of the tissue thickness compensator 1170. The adjustment member 1176 can
be retained in the advanced position
for a period of time sufficient to permit the tissue thickness compensator 1
170 to retain, or at least substantially retain,
the change in spring rate. In addition, the tissue thickness compensator 1170
can be allowed to cool below the glass
-39 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
transition temperature of its material composition while maintaining the
pressure applied by the adjustment member
1176 against the tissue thickness compensator 1170.
101701 In certain instances, the adjustment member 1176 can be maintained in
the advanced position against the
tissue thickness compensator 1170 for a period of time from about 30 seconds
to about 8 hours, for example, during
the time in the glassy state and/or for a period of time from about 30 seconds
to about 8 hours, for example, after
exiting the glassy state. In at least one example, the adjustment member 1176
can be maintained in the advanced
position against the tissue thickness compensator 1170 for approximately 10
minutes during the time in the glassy
state and for approximately 10 minutes after exiting the glassy state. Other
time periods for maintaining the
adjustment member 1176 in the advanced position against the tissue thickness
compensator 1170 are contemplated by
the present disclosure.
101711 In certain circumstances, the adjustment member 1176 can be used to
apply pressure onto the tissue thickness
compensator 1170 to change the spring rate of the tissue thickness compensator
1170 before the tissue thickness
compensator 1170 is wansitioned to the glassy state. In certain circumstances,
the adjustment member 1176 may apply
pressure to the tissue thickness compensator 1170 while the tissue thickness
compensator 1170 is heated to reach the
glassy state, while the tissue thickness compensator 1170 is in the glassy
state, and/or while the tissue thickness
compensator 1170 is transitioned or cooled to a temperature below the glassy
state. In certain circumstances, the
pressure applied to the tissue thickness compensator 1170 can be gradually
increased toward a threshold as the
temperature of the tissue thickness compensator 1170 is gradually increased to
transition the tissue thickness
compensator 1170 toward the glassy slate, for example. In certain
circumstances, the pressure applied to the tissue
thickness compensator 1170 can be removed, gradually removed, or at least
partially reduced as the tissue thickness
compensator 1:170 exits the glassy state, before the tissue thickness
compensator 1170 exits the glassy state, andlor
after the tissue thickness compensator 1170 exits the glassy state.
101721 Referring again to FIGS. 35-40, the tissue thickness compensator 1170
may be manufactured with a native
spring rate using traditional lyophilization techniques and/or any other
suitable techniques. As described above, the
spring rate of the tissue thickness compensator 1170 can influence its ability
to apply pressure against tissue captured
with the tissue thickness compensator 1170 by a staple. The modification
process described above may be utilized to
adjust the native spring rate of the tissue thickness compensator 1170 to
adjust its ability to apply pressure against
tissue captured with the tissue thickness compensator 1170 by the staple. In
certain circumstances, the native spring
rate or the tissue thickness compensator 1170 can be increased from a First
spring rate at point A (FIG. 40) to a second
spring rate including and up to a maximum spring rate at point B (FIG. 40). In
certain circumstances, such increase of
the spring rate of the tissue thickness compensator 1170 can be achieved by
applying compression forces to the tissue
thickness compensator 1170 using the adjustment member :1176 while the tissue
thickness compensator 1:170 is in the
glassy state, as explain in the modification process described above. As
illustrated in FIG. 40, the point B represents a
maximum elastic yield of the tissue thickness compensator 1170. As such, any
additional compression applied by the
adjustment member 1176 to the tissue thickness compensator 1170 beyond a
threshold compression at the point B
may produce a decrease in the spring rate of the modified tissue thickness
compensator :1170. For example, as
illustrated in FIG. 40, the spring rate at the point C is lower than the
spring rate at the point B even though the
-40 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
compression force applied by the adjustment member 1176 to the tissue
thickness compensator 1170 at point C is
greater than the compression force applied at the point B.
101731 As discussed above, one or more processes can be used to affect the
spring rate, and/or any other property, of
a material used in conjunction with a fastener cartridge and/or a surgical
fastening instrument, for example. The spring
rate, and/or any other property, of the material may change throughout the
modification process or processes. Such a
change may be gradual in some circumstances, while in other circumstances, the
change may be sudden. In various
instances, one or more of the steps of the modification process may cause an
increase in the spring rate of the material
while one or more steps may cause a decrease in the spring rate of the
material. Ultimately, the net change in the
spring rate can be measured as a comparison between an original spring rate
before the modification process begins
and a subsequent spring rate after the modification process has been
completed. In various instances, a material may
comprise an altered spring rate after the material has been heated and then
cooled.
101741 In certain circumstances, it may be desirable to apply one or more of
the above described modification
processes to a tissue thickness compensator. For example, a first modification
process can be utilized to modify
porosity of the tissue thickness compensator, as described above with respect
to the tissue thickness compensator
1170. A second modification process, following the first modification process,
can be utilized to alter a surface of the
tissue thickness compensator, as described above with respect to the tissue
thickness compensator 1140. Furthermore,
a third modification process can be utilized to modify the tissue thickness
compensator to include a longitudinal slot
similar to the longitudinal slot 1122 of the tissue thickness compensator
1120. In yet a fourth modification process, the
tissue thickness compensator can be modified to include apertures similar to
the apertures 1152 of the tissue thickness
compensator 1150. The reader will appreciate that some of above mentioned
modifications can be combined or
grouped in a single modification process. For example, a mold can be designed
to include the needles 1156 of the
mold 1154 and the central beam 1128 of the mold 1126. Other modification
arrangements are contemplated by the
present disclosure.
101751 Referring now to FIGS. 38 and 39, a tissue thickness compensator such
as, for example, tissue thickness
compensator 1190 can be altered or modified using one or more of the
modification processes described above to
include portions with different spring rates, porosities, and/or densities. In
certain circumstances, the tissue thickness
compensator 1190 can be modified using one or more of the modification
processes described above to include a
gradient pore morphology (i.e. small pores gradually increasing in size to
large pores across the thickness of the tissue
thickness compensator 1190 in one direction). Such morphology could be more
optimal Ibr tissue in-growth or
hemostatic behavior. Further, the gradient could also be compositional with a
varying bio-absorption profile. A short
term absorption profile may be preferred to address hemostasis while a long
term absorption profile may address
better tissue healing without leakages.
101761 Referring again to FIGS. 38 and 39, the tissue thickness compensator
1190 may include one or more zone
geometries that are di fferent from the remainder of the tissue thickness
compensator 1196. For example, as illustrated
in FIG. 38, the tissue thickness compensator 1190 may include one or more
protruding portions such as, for example,
protruding portion 1196. In addition, the tissue thickness compensator 1190
may comprise a uniform, or at least a
substantially uniform, first spring rate, first porosity, and/or first density
through the tissue thickness compensator
- 41 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
1190 including the one or more zone geometries, as illustrated in FIG. 38. In
certain circumstances, the tissue
thickness compensator 1190 can be altered or modified using one or more of the
modification processes described
above to alter or modify the one or more zone geometries and/or to induce
localized changes in the first spring rate,
the first porosity, and/or the first density, for example. The modified tissue
thickness compensator 1190 may comprise
one or more modified zones with different spring rates, porosities, and/or
densities from other modified zones and/or
the first spring rate, the first porosity, and/or the first density,
respectively, of the remainder of the tissue thickness
compensator 1190. In certain circumstances, the resulting one or more modified
zones may correspond to the one or
more zone geometries. For example, a .3 illustrated in FIG. 39, the tissue
thickness compensator 1190 may be altered or
modified to level, or at least substantially level, the protruding portion
1196 and to form a flat, or at least a
substantially flat, surface 1198, for example. The modified tissue thickness
compensator 1190 may include a first
portion 1192 comprising the rust spring rate, the first porosity, and/or the
first density and a second portion 1194
comprising a second spring rate, a second porosity, and/or a second density,
which can be different from the first
spring rate, the first porosity, and/or the first density, respectively. The
second portion 1194 may correspond to the
protruding poition 1196 and can result from the leveling, or at least
substantially leveling, of the protruding portion
1196 to form the flat, or at least substantially flat, surface 1198, for
example. In certain respects, the geometry of the
protruding portion 1196 prior to the modification of the tissue thickness
compensator 1190 mirrors, matches, or
resembles the geometry of the second portion 1194 after the tissue thickness
compensator 1190 has been modified.
101771 Referring again to FIGS. 37-39, the tissue thickness compensator 1190
can be altered or modified using the
mold 1172, in a similar manner to the tissue thickness compensator 1170. For
example, the tissue thickness
compensator 1190 can be heated in the receiver 1174 to a temperature greater
than or equal to a glass transition
temperature of the material composition of the tissue thickness compensator
1190 but less than the melting
temperature of the same. In certain circumstances, the adjustment member 1176
can be advanced against the
protruding portion 1196, while the tissue thickness compensator 1190 is in the
glassy state, thereby compressing the
protruding portion 1196 and rearranging its geometry to form the second
portion 1194, as illustrated in FIG. 39.
Further to the above, the adjustment member 1176 can be configured to maintain
compression against the protruding
portion 1196 for a time period sufficient to permit the tissue thickness
compensator 1190 to retain, or at least
substantially retain, the modification imposed by the adjustment member 1176.
The tissue thickness compensator
1190 can be allowed to cool or can be actively cooled to a temperature below
its glass transition temperature while
under compression from the adjustment member 1176. After transitioning the
tissue thickness compensator 1190 out
of the glassy state, the adjustment member 1190 can be retracted. The tissue
thickness compensator 1190 may retain,
or at least substantially retain, the second portion 1194, as illustrated in
FIG. 39. In certain circumstances, the
adjustment member 1176 may apply pressure onto the protruding portion 1196
while the tissue thickness compensator
1190 is heated to reach the glassy state, while the tissue thickness
compensator 1190 is in the glassy state, and/or
while the tissue thickness compensator 1190 is transitioned or cooled to a
temperature below the glassy state. In
certain circumstances, the pressure applied to the protruding portion 1196 of
the tissue thickness compensator 1190
can be gradually increased toward a threshold as the temperature of the tissue
thickness compensator 1190 is gradually
increased to transition the tissue thickness compensator 1190 toward the
glassy state, for example. In certain
-42 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
circumstances, the pressure applied to the protruding portion 1196 of the
tissue thickness compensator 1190 can be
removed, gradually removed, or at least partially reduced as the tissue
thickness compensator 1190 exits the glassy
state, before the tissue thickness compensator 1190 exits the glassy state,
and/or after the tissue thickness compensator
1190 exits the glassy state.
101781 Referring now to FIGS. 41-43, a tissue thickness compensator such as,
for example, tissue thickness
compensator 1200 can be prepared using traditional lyophilization techniques
and/or any other suitable techniques. In
addition, the tissue thickness compensator 1200 can be modified or altered for
use in a surgical procedure, for
example. The tissue thickness compensator 1200 can be similar in many respects
to other tissue thickness
compensators such as, for example, the tissue thickness compensator 22020
(FIG. 9) and/or the tissue thickness
compensator 1120 (FIG. 26). For example, like the tissue thickness compensator
22020, the tissue thickness
compensator 1200 can be utilized with the end effector 22090. Furthermore, as
illustrated in FIGS. 41-43, the tissue
thickness compensator 1200 can be modified to include a longitudinal slot 1202
which, like the knife slot 22025, may
define a tissue thickness compensator knife path for the cutting portion 10053
between a first stapling portion 1204a
and a second stapling portion 1204b. Furthermore, the first stapling portion
1204a and the second stapling portion
1204b can be similar in many respects to the first stapling portion 22021a
(FIG. 9) and the second stapling portion
22021 b (FIG. 9) of the tissue thickness compensator 22020. In addition, the
slot 1202 can be configured to releasably
connect the first stapling portion 1204a and the second stapling portion 1204b
such that, in use with the end effector
22090. the cutting portion 10053 can be advanced distally through the slot
1202 to transect the slot 1202 and separate
the .first stapling portion I 204a and the second stapling portion 12041,.
101791 Referring again to FIGS. 41-43, the tissue thickness compensator 1200
can be modified prior to assembly
with an end effector such as, for example, the end effector 22090 (FIG.9).
Alternatively, the tissue thickness
compensator 1200 can be modified after it has been assembled with an end
effector. As described above, the tissue
thickness compensator 1200 can be prepared using traditional lyophilization
techniques and/or any other suitable
techniques. A space creator 1206 can be utilized to modify the tissue
thickness compensator 1200 in a thermal
pressing process, as illustrated in FIGS. 41-43. For example, the space
creator 1206 can be heated to a temperature
greater than or equal to a melting temperature of the material composition of
the tissue thickness compensator 1200.
The space creator 1206 can then be aligned with and inserted into the tissue
thickness compensator 1200 to form the
longitudinal slot 1202. The space creator 1206 may melt through the tissue
thickness compensator 1200 to create
spare for the longitudinal slot 1202. The space creator 1206 can be retracted
upon reaching a desired depth within the
tissue thickness compensator 1200. In certain circumstances, the thermal
pressing process can be repeated by
reinserting the heated space creator 1206 through the tissue thickness
compensator 1200 to widen the space created for
the longitudinal slot 1202.
101801 Referring again to FIGS. 41-43, the space creator 1206 may comprise a
hot wire. For example, the space
creator 1206 may comprise a thin, taut metal wire, which can be made of
nichrome or stainless steel, for example, or a
thicker wire preformed into a desired shape. The hot wire can he heated via
electrical resistance to a desired
temperature. As the hot wire of the space creator 1206 is passed through the
material of the tissue thickness
compensator 1200, the heat from the hot wire may vaporize the material just in
advance of contact. In certain
-43 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
circumstances, the hot wire may comprise a cylindrical, or substantially
cylindrical, shape, as illustrated in FIG. 42.
The depth of the longitudinal slot 1202 can depend, in part, on the insertion
depth of the space creator 1206 through
the tissue thickness compensator 1200 and the width of the longitudinal slot
1202 can depend, in part, on the diameter
of the hot wire of the space creator 1206.
101811 in certain instances, the space creator 1206 can be partially inserted
through the full thickness of the tissue
thickness compensator. In certain instances, the space creator 1206 can be
completely inserted through the full
thickness of the tissue thickness compensator 1200 to create openings, holes,
and/or slots extending through the full
thickness oldie tissue thickness compensator 1200. in certain instances, the
space creator 1206 may be inserted
through a first side of the tissue thickness compensator 1200 and exited
through a second side of the tissue thickness
compensator 1200 which may be opposite the first side, for example.
101821 Many processes are disclosed herein which utilize thermal energy to
modify a tissue thickness compensator.
Such processes can be referred to as felting processes. In certain instances,
a felting process may also utilize the
application of compressive and/or tensile forces to a tissue thickness
compensator. In other instances, a felting
process may not utilize the application of compressive and/or tensile forces
to a tissue thickness compensator. In
either event, the felting processes disclosed herein can also be utilized to
modify and suitable implantable layer and/or
buttress material, fur example.
101831 In various circumstances, the tissue thickness compensator assembly may
comprise a polymeric
composition. The polymeric composition may comprise one or more synthetic
polymer and/or one or more non-
synthetic polymer. The synthetic polymer may comprise a synthetic absorbable
polymer and/or a synthetic non-
absorbable polymer. In various circumstances, the polymeric composition may
comprise a biocompatible foam, for
example. The biocompatible foam may comprise a porous, open cell foam and/or a
porous, closed cell foam, for
example. The biocompatible foam can have a uniform pore morphology or may have
a gradient pore morphology (i.e.
small pores gradually increasing in size to large pores across the thickness
of the foam in one direction). In various
circumstances, the polymeric composition may comprise one or more of a porous
scaffold, a porous matrix, a gel
matrix, a hydrogel matrix, a solution matrix, a filamentous matrix, a tubular
matrix, a composite matrix, a
membranous matrix, a biostable polymer, and a biodegradable polymer, and
combinations thereof For example, the
tissue thickness compensator assembly may comprise a foam reinforced by a
filamentous matrix or may comprise a
foam having an additional hydrogel layer that expands in the presence of
bodily fluids to further provide the
compression on the tissue. In various circumstances, a tissue thickness
compensator assembly could also be
comprised of a coating on a material and/or a second or third layer that
expands in the presence of bodily fluids to
further provide the compression on the tissue. Such a layer could be a
hydrogel that could be a synthetic and/or
naturally derived material and could be either bioduxable and/or
biodegradable, for example. In certain circumstances,
a tissue thickness compensator assembly could be reinforced with fibrous non-
woven materials or fibrous mesh type
elements, for example, that can provide additional flexibility, stiffness,
and/or strength. In various circumstances, a
tissue thickness compensator assembly that has a porous morphology which
exhibits a gradient structure such as, for
example, small pores on one surface and larger pores on the other surface.
Such morphology could be more optimal
for tissue in-growth or hemostatic behavior. Further, the gradient could be
also compositional with a varying bio-
- 44 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
absorption profile. A short term absorption profile may be preferred to
address hemostasis while a long term
absorption profile may address better tissue healing without leakages.
101841 Examples of non-synthetic polymers include, but are not limited to,
lyophilized polysaccharide, glycoprotein,
elastin, proteoglycan, gelatin, collagen, and oxidized regenerated cellulose
(ORC). Examples of synthetic absorbable
polymers include, but are not limited to, poly(lactic acid) (PLA), poly(L-
lactic acid) (PLLA), polycaprolactone (PCL),
polyglycolic acid (PGA), poly(trimethylene carbonate) (TMC), polyethylene
terephthalate (PET),
polyhydroxyalkanoate (PHA), a copolymer of glycolide and e-caprolactone
(PGCL), a copolymer of glycolide and-
trimethylene carbonate, poly(glycerol sebacate) (PGS), polydioxanone,
poly(orthoesters), polyanhydrides,
polysaccharides, poly(ester-amides), tyrosine-based polyarylates, tyrosine-
based polyiminocarbonates, tyrosine-based
polycarbonates, poly(D,L-lactide-urethane), poly(B-hydroxybutyrate), poly(E-
caprolactone), polyethyleneglycol
(PEG), poly[bis(carboxylatophenoxy) phosphazene], poly(amino acids), pseudo-
poly(amiuo acids), absorbable
polyurethanes, and combinations thereof. In various circumstances, the
polymeric composition may comprise from
approximately 50% to approximately 90% by weight of the polymeric composition
of PLLA and approximately 50%
to approximately 10% by weight of the polymeric composition of PCL, for
example. In at least one embodiment, the
polymeric composition may comprise approximately 70% by weight of PLLA and
approximately 30% by weight of
PCL, for example. In various circumstances, the polymeric composition may
comprise from approximately 55% to
approximately 85% by weight of the polymeric composition of PGA and 15% to 45%
by weight of the polymeric
composition of PCL, for example. In at least one embodiment, the polymeric
composition may comprise
approximately 65% by weight of PGA and approximately 35% by weight of PCL, for
example. In various
circumstances, the polymeric composition may comprise from approximately 90%
to approximately 95% by weight of
the polymeric composition of PGA and approximately 5% to approximately 10% by
weight of the polymeric
composition of PLA, for example.
101851 In various circumstances, the synthetic absorbable polymer may comprise
a bioabsorbable, biocompatible
elastomeric copolymer. Suitable bioabsorbable, biocompatible elastomeric
copolymers include but are not limited to
copolymers of epsilon-caprolactone and glycolide (preferably having a mole
ratio of epsilon-caprolactone to glycolide
of from about 30:70 to about 70:30, preferably 35:65 to about 65:35, and more
preferably 45:55 to 35:65); elastomeric
copolymers of epsilon-caprolactone and lactide, including L-lactide, D-lactide
blends thereof or lactic acid
copolymers (preferably having a mole ratio of epsilon-caprolactone to lactide
of from about 35:65 to about 65:35 and
more preferably 45:55 to 30:70) elastomeric copolymers of p-dioxanone (1,4-
dioxan-2-one) and lactide including L-
lactide, D-lactide and lactic acid (preferably having a mole ratio of p-
dioxanone to lactide of from about 40:60 to
about 60:40); elastomeric copolymers of epsilon-caprolactone and p-dioxanone
(preferably having a mole ratio of
epsilon-caprolactone to p-dioxanone of from about 30:70 to about 70:30);
elastomeric copolymers of p-dioxanone and
trimethylene carbonate (preferably having a mole ratio of p-dioxanone to
trimethylene carbonate of from about 30:70
to about 70:30); elastomeric copolymers of trimethylene carbonate and
glycolide (preferably having a mole ratio of
trimethylene carbonate to glycolide of from about 30:70 to about 70:30);
elastomeric copolymer of trimethylene
carbonate and lactide including L-lactide, D-lactide, blends thereof or lactic
acid copolymers (Preferably having a
mole ratio of trimethylene carbonate to lactide of from about 30:70 to about
70:30) and blends thereof In one
-45 -
embodiment, the elastomeric copolymer is a copolymer of glycolide and epsilon-
caprolactone. In another
embodiment, the elastomeric copolymer is a copolymer of lactide and epsilon-
caprolactone.
[0187] In various circumstances, the synthetic absorbable polymer may comprise
one or more of 90/10
poly(glycolide-L-lactide) copolymer, commercially available from Ethicon, Inc.
under the trade designation VICRYL
(polyglactic 910), polyglycolide, commercially available from American
Cyanamid Co. under the trade designation
DEXON, polydioxanone, commercially available from Ethicon, Inc. under the
trade designation PDS, poly(glycolide-
trimethylene carbonate) random block copolymer, commercially available from
American Cyanamid Co. under the
trade designation MAXON, 75/25 poly(glycolide-E-caprolactone-
poliglecaprolactone 25) copolymer, commercially
available from Ethicon under the trade designation MONOCRYL, for example.
[0188] Examples of synthetic non-absorbable polymers include, but are not
limited to, foamed polyurethane,
polypropylene (PP), polyethylene (PE), polycarbonate, polyamides, such as
nylon, polyvinylchloride (PVC),
polymethylmetacrylate (PMMA), polystyrene (PS), polyester,
polyetheretherketone (PEEK), polytetrafluoroethylene
(PTFE), polytrifluorochloroethylene (PTFCE), polyvinylfluoride (PVF),
fluorinated ethylene propylene (FEP),
polyacetal, polysulfone, and combinations thereof. The synthetic non-
absorbable polymers may include, but are not
limited to, foamed elastomers and porous elastomers, such as, for example,
silicone, polyisoprene, and rubber. In
various circumstances, the synthetic polymers may comprise expanded
polytetrafluoroethylene (ePTFE),
commercially available from W. L. Gore & Associates, Inc. under the trade
designation GORE-TEX Soft Tissue
Patch and co-polyetherester urethane foam commercially available from
Polyganics under the trade designation
NASOPORE.
[0189] The polymeric composition of a tissue thickness compensator assembly
may be characterized by percent
porosity, pore size, and/or hardness, for example. In various circumstances,
the polymeric composition may have a
percent porosity from approximately 30% by volume to approximately 99% by
volume, for example. In certain
circumstances, the polymeric composition may have a percent porosity from
approximately 60% by volume to
approximately 98% by volume, for example. In various circumstances, the
polymeric composition may have a
percent porosity from approximately 85% by volume to approximately 97% by
volume, for example. In at least one
embodiment, the polymeric composition may comprise approximately 70% by weight
of PLLA and approximately
30% by weight of PCL, for example, and can comprise approximately 90% porosity
by volume, for example. In at
least one such embodiment, as a result, the polymeric composition would
comprise approximately 10% copolymer by
volume. In at least one embodiment, the polymeric composition may comprise
approximately 65% by weight of PGA
and approximately 35% by weight of PCL, for example, and can have a percent
porosity from approximately 93% by
volume to approximately 95% by volume, for example. In various circumstances,
the polymeric composition may
comprise a greater than 85% porosity by volume. The polymeric composition may
have a pore size from
approximately 5 micrometers to approximately 2000 micrometers, for example. In
various circumstances, the
polymeric composition may have a pore size between approximately 10
micrometers to approximately 100
46
Date Recue/Date Received 2021-07-12
micrometers, for example. In at least one such embodiment, the polymeric
composition can comprise a copolymer of
PGA and PCL, for example. In certain circumstances, the polymeric composition
may have a pore size between
approximately 100 micrometers to approximately 1000 micrometers, for example.
In at least one such embodiment,
the polymeric composition can comprise a copolymer of PLLA and PCL, for
example. According to certain aspects,
the hardness of a polymeric composition may be expressed in terms of the Shore
Hardness, which can defined as the
resistance to permanent indentation of a material as determined with a
durometer, such as a Shore Durometer. In
order to assess the durometer value for a given material, a pressure is
applied to the material with a durometer indenter
foot in accordance with ASTM procedure D2240-00, entitled, "Standard Test
Method for Rubber Property-Durometer
Hardness". The durometer indenter foot may be applied to the material for a
sufficient period of time, such as 15
seconds, for example, wherein a reading is then taken from the appropriate
scale. Depending on the type of scale
being used, a reading of 0 can be obtained when the indenter foot completely
penetrates the material, and a reading of
100 can be obtained when no penetration into the material occurs. This reading
is dimensionless. In various
circumstances, the durometer may be determined in accordance with any suitable
scale, such as Type A and/or Type
00 scales, for example, in accordance with ASTM D2240-00. In various
circumstances, the polymeric composition
of a tissue thickness compensator assembly may have a Shore A hardness value
from approximately 4 A to
approximately 16 A, for example, which is approximately 45 00 to approximately
65 00 on the Shore 00 range. In
at least one such embodiment, the polymeric composition can comprise a
PLLA/PCL copolymer or a PGA/PCL
copolymer, for example. In various circumstances, the polymeric composition of
a tissue thickness compensator
assembly may have a Shore A Hardness value of less than 15 A. In various
circumstances, the polymeric composition
of a tissue thickness compensator assembly may have a Shore A Hardness value
of less than 10 A. In various
circumstances, the polymeric composition of a tissue thickness compensator
assembly may have a Shore A Hardness
value of less than 5 A. In certain circumstances, the polymeric material may
have a Shore 00 composition value
from approximately 35 00 to approximately 75 00, for example.
[0190] In various circumstances, the polymeric composition may have at least
two of the above-identified
properties. In various circumstances, the polymeric composition may have at
least three of the above-identified
properties. The polymeric composition may have a porosity from 85% to 97% by
volume, a pore size from 5
micrometers to 2000 micrometers, and a Shore A hardness value from 4 A to 16 A
and Shore 00 hardness value from
45 00 to 65 00, for example. In at least one embodiment, the polymeric
composition may comprise 70% by weight
of the polymeric composition of PLLA and 30% by weight of the polymeric
composition of PCL having a porosity of
90% by volume, a pore size from 100 micrometers to 1000 micrometers, and a
Shore A hardness value from 4 A to 16
A and Shore 00 hardness value from 45 00 to 65 00, for example. In at least
one embodiment, the polymeric
composition may comprise 65% by weight of the polymeric composition of PGA and
35% by weight of the polymeric
composition of PCL having a porosity from 93% to 95% by volume, a pore size
from 10 micrometers to 100
micrometers, and a Shore A hardness value from 4 A to 16 A and Shore 00
hardness value from 45 00 to 65 00, for
example.
[0191] In various circumstances, the polymeric composition may comprise a
pharmaceutically active agent. The
polymeric composition may release a therapeutically effective amount of the
pharmaceutically active agent. In
47
Date Recue/Date Received 2021-07-12
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
various circumstances, the pharmaceutically active agent may be released as
the polymeric composition is
desorbedlabsorbed. In various circumstances, the pharmaceutically active agent
may be released into fluid, such as,
for example, blood, passing over or through the polymeric composition.
Examples of pharmaceutically active agents
may include, but are not limited to, hemostatic agents and drugs, such as, for
example, fibrin, thrombin, and oxidized
regenerated cellulose (ORC); anti-inflammatory drugs, such as, for example,
diclofenac, aspirin, naproxen, sulindac,
and hydrocortisone; antibiotic and antimicrobial drug or agents, such as, for
example, ticlosan, ionic silver,
arapicillin, gentamicin, polymy-xin B, chloramphenicol; and anticancer agents,
such as, for example, cisplatin,
mitomycin, adriamycin.
[0192] Various methods are disclosed herein for altering a tissue thickness
compensator. Such methods could be
used to alter any suitable layer for use with a fastener cartridge and/or a
surgical fastening instrument, for example.
Such a layer can comprise a less than one hundred percent dense composition
which can be created utilizing any
suitable process. For instance, such processes can include, for example,
extruding, injection molding, weaving,
lyophilization, gas-foaming, and/or melt-blowing processes. Some processes may
produce a foam while other
processes may not produce a foam; however, in any event, all such embodiments
are contemplated for use with all of
the embodiments disclosed herein.
[0193] In various embodiments, referring to FIGS. 44-46, an end effector of a
surgical fastening instrument, such as
end effector 100, for example, can be configured to capture, fasten, and/or
incise tissue. The end effector 100 can
include a fastener cartridge 110 and, in addition, a firing member 140 which
can be advanced through the fastener
cartridge 110 to deploy staples removably stored within the staple cartridge
110 into tissue captured within the end
effector 100. In various instances, the firing member 140 can be advanced from
a proximal position (FIG. 44) toward
a distal end of the end effector 100 to simultaneously deploy the staples and
transect the tissue. There are some
circumstances, however, where it may not be desirable to advance the firing
member 140 toward the distal end of the
end effector 100. For instance, the fastener cartridge 110 of the end effector
100 can be removable and/or replaceable
and, in the event that a fastener cartridge 110 is not positioned within the
end effector 100, it may not be desirable for
the thing member 140 to be advanced within the end effector 100. In the event
that the ruing member 140 were to be
advanced through the end effector 100 without a fastener cartridge positioned
within the end effector 100, a knife edge
142 of the firing member 140 may incise tissue captured within the end
effector 100 without simultaneously fastening
the tissue. Similarly, in the event that the fastener cartridge positioned
within the end effector 100 has been previously
used, or expended, and at least some of the fasteners have been deployed from
the fastener cartridge, it may not be
desirable for the firing member 140 to be advanced within the end effector
100. In the event that the firing member
140 were to be advanced through the end effector 100 with a previously
expended fastener cartridge positioned within
the end effector 1()0, the knife edge 142 of the firing member 140 may incise
tissue captured within the end effector
without simultaneously fastening the tissue. In various embodiments, the end
effector 100 can include one or more
lockout systems which can prevent the firing member 140 from being advanced
distally when a fastener cartridge is
not present within the end effector 100 and/or when the fastener cartridge
positioned within the end effector 100 has
been at least partially expended. Various lockout systems are disclosed in
U.S. Patent No. 6,988,649, entitled
SURGICAL STAPLING INSTRUMENT HAVING A SPENT CARTRIDGE LOCKOUT, and issued on
January 24,
-48 -
2006.
[0194] Referring again to FIGS. 44-46, the fastener cartridge 110 can include
a cartridge body and a tissue thickness
compensator 120 wherein, further to the above, the tissue thickness
compensator 120 can be implanted against tissue
captured by the end effector 100 by fasteners removably stored within the
cartridge body. The tissue thickness
compensator 120 can be positioned above a top surface, or deck, of the
cartridge body wherein staples 180 removably
stored within staple cavities defined in the cartridge body can be ejected
from the staple cavities by a firing member,
such as sled 130 and/or firing member 140, for example. In certain
embodiments, the fastener cartridge 110 can
further include drivers configured to support the staples 180 and transmit the
movement of the sled 130 to the staples
180 in order to move the staples 180 between an unfired position and a fired
position. In various instances, the staples
180 can be at least partially embedded in the tissue thickness compensator 120
when the staples 180 are in their
unfired positions and, in certain instances, the staples 180 can hold the
tissue thickness compensator 120 in position
over the cartridge deck when the staples 180 are in their unfired position. In
the event that the tissue thickness
compensator 120 were to be moved relative to the cartridge body and/or the
staples 180 prior to deploying the staples
180 into tissue, in some instances, the tissue thickness compensator 120 may
move the staples 180 relative to or away
from their preferred positions. Moreover, in the event that the tissue
thickness compensator 120 were to be removed
from the cartridge 110 prior to the staples 180 being deployed, the cartridge
110 may no longer be suitable for its
originally intended use. In view of the foregoing, as discussed in greater
detail below, the end effector 100 may
include a lockout configured to prevent the firing member 140 and/or the sled
130 from being advanced distally to
deploy the staples 180 in the event that the tissue thickness compensator 120
is removed from, or becomes at least
partially dislodged from, the cartridge body prior to the staples 180 being
deployed.
[0195] Referring again to FIGS. 44-46, the tissue thickness compensator 120
can comprise, one, a body 121
configured to be captured by the staples 180 and, two, a lockout pin 122
extending from the body 121. In various
instances, the lockout pin 122 can include a first end 123 embedded in the
body 121 and a second end 124 positioned
intermediate the firing member 140 and the sled 130 when the tissue thickness
compensator 120 has not been removed
from or substantially moved from a suitable position over the cartridge body
deck. In such a position, the second end
124 of the lockout pin 122 can be positioned intermediate a shoulder, or
shelf, 134 defined on the sled 130 and a
protrusion 144 extending distally from the firing bar 140. Stated another way,
when the lockout pin 122 is positioned
intermediate the sled 130 and the firing bar 140, the lockout pin 122 and the
sled 130 can co-operate to support the
firing bar 140 in an unlocked position above a lockout shoulder 112 defined in
the fastener cartridge 110 such that,
when a distal firing force is applied to the firing bar 140, the firing bar
140 can advance the sled 130 distally to fire the
staples 180. When the tissue thickness compensator 120 is removed from the
cartridge 110 and/or sufficiently
dislodged from a desirable position relative to the cartridge body, referring
primarily to FIG. 45, the lockout pin 122
may no longer be positioned intermediate the sled 130 and the firing member
140 and/or may otherwise be unable to
support the firing member 140 in its unlocked position (FIG. 44). In such
circumstances, the firing member 140 may
become positioned in a locked position such that the distal advancement of the
firing member 140 is prevented by the
lockout shoulder 112. In at least one such circumstance, the end effector 100
can further include a biasing member,
49
Date Recue/Date Received 2021-07-12
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
such as a spring, for example, configured to bias the firing member 140 into
its locked condition. In certain
circumstances, the biasing member can bias the firing member 140 into contact
with the sled 130, for instance,
without the lockout pin 122 positioned therebetween which can comprise the
locked position of the firing member
140.
101961 As a result of the above, the cartridge 110 may become inoperable if
the tissue thickness compensator 120 is
prematurely removed from the cartridge 110. In such circumstances, the lockout
pin 122 may comprise a liise which
deactivates the cartridge 110 in the event that the tissue thickness
compensator 120 is removed before the firing
member 140 is advanced distally. In various circumstances, the lockout pin 122
may comprise a key which maintains
the cartridge 110 in an unlocked condition when the key is positioned between
the sled 130 and the firing member 140
and permits the cartridge 110 to enter into a locked condition in the event
that the tissue thickness compensator 120 is
removed from the cartridge 110 before the firing member 140 is advanced
distally, i.e., before the firing member 140
begins its firing stroke. When the firing member 140 is in its locked-out
condition and cannot be advanced distally,
the knife edge 142 of the firing member 140 is unable to incise the tissue
captured within the end effector 100.
Moreover, in such circumstances, the firing member 144) cannot advance the
sled 130 distally to tire the staples 180.
Thus, the tissue thickness compensator lockout can prevent the tissue captured
within the end effector 100 from being
incised and stapled when the tissue thickness compensator 120 is not
positioned on, or properly positioned on, the
cartridge 110. In the event that the firing member 140 is advanced distally
before the tissue thickness compensator
120 is removed, or dislodged, the firing member 140 can complete the firing
stroke, or at least a portion of the firing
stroke, of the end effector 100. In such instances, the sled 130 is advanced
distally so that one or more ramps 132
defined on the sled 130 can lift the staples 180 and that a knife edge 142 of
the firing member 140 can incise the tissue
thickness compensator 120 and/or the tissue captured within the end effector
100. In sonic circumstances, the firing
member 140 can contact the lockout pin 122 and displace it out of the way as
the firing member 140 is advanced
distally. In such circumstances, the lockout pin 122 can be flexible. In
various instances, the lockout pin 122 can be
comprised of a bioabsorbable material and/or a biocompatible material, for
example. In certain circumstances, the
tiring member 140 can incise the lockout pin 122 as the firing member 140 is
advanced distally. In any event, the
purpose of the lockout pin 122 may become obsolete once the firing member 140
has been at least partially advanced.
Stated another way, the tissue thickness compensator lockout can serve as an
initial check to verify that a tissue
thickness compensator is present within the end effector and, once that
initial check has been made, the firing stroke
of the end effector can proceed.
101971 Referring again to FIGS. 47-50, an end effector 200 can comprise an
anvil 260 and, in addition, a fastener
cartridge 210 including a cartridge body 214 and a tissue thickness
compensator 220 wherein, further to the above, the
tissue thickness compensator 220 can be implanted against tissue captured by
the end effector 200 by fasteners
removably stored within the cartridge body 214. The tissue thickness
compensator 220 can be positioned above a top
surface, or deck, 211 of the cartridge body 214 wherein staples removably
stored within staple cavities defined in the
cartridge body 214 can be ejected from the staple cavities by a firing member,
such as a sled 230 and/or a firing
member 240, for example. In certain embodiments, the fastener cartridge 210
can further include drivers configured
to support the staples and transmit the movement of the sled 230 to the
staples in order to move the staples between an
- 50 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
unfired position and a fired position. In various instances, the staples can
be at least partially embedded in the tissue
thickness compensator 220 when the staples are in their unfired positions and,
in certain instances, the staples can hold
the tissue thickness compensator 220 in position when the staples are in their
unfired position. In the event that the
tissue thickness compensator 220 were to be moved relative to the cartridge
body 214 and/or the staples prior to
deploying the staples into the tissue, in some instances, the tissue thickness
compensator 220 may move the staples
relative to or away from their preferred positions. Moreover, in the event
that the tissue thickness compensator 220
were to be removed from the cartridge 210 prior to the staples being deployed,
the cartridge 210 may no longer be
suitable for its originally intended use. In view of the foregoing, as
discussed in greater detail below, the end effector
200 may include a lockout configured to prevent the firing member 240 and/or
the sled 230 from being advanced
distally to deploy the staples in the event that the tissue thickness
compensator 220 is removed from, or becomes at
least partially dislodged from, the cartridge body 214 prior to the staples
being deployed.
[0198] Referring again to FIGS. 44-46, the tissue thickness compensator 220
can comprise, one, a body 221
configured to be captured by the staples and, two, a loop, or tether, 222
extending from the body 221. In various
instances, referring primarily to FIG. 47, the loop 222 can comprise ends
which are at least partially embedded in the
body 221 and an intermediate portion extending between the ends which can be
releasably engaged with the sled 230.
In certain instances, the loop 222 can comprise a suture or flexible thread,
for example. In some instances, the loop
222 can be comprised of a bioabsorbable material and/or a biocompatible
material, for example. Referring primarily
to FIG. 48, the sled 230 can include a longitudinal body portion 236, a hook
238 extending from the body portion 236,
and a slot 237 defined between the body portion 236 and the hook 238. As
illustrated in FIG. 48, the loop 222 is
positioned within the slot 237 when the tissue thickness compensator 220 is
positioned over the cartridge deck 211
and the sled 230 and the firing member 240 are in an unfired position. As also
illustrated in FIG. 48, a distal
projection 244 extending from the firing member 240 is positioned against
and/or above a support shoulder 234
defined on the sled 230 which holds the firing member 240 in an unlocked
position, i.e., in a position in which the
distal movement of the firing member 240 will not be impeded, or at least
substantially impeded, by a lockout
shoulder 212 defined in the end effector 2(X) when a tiring motion is applied
to the firing member 240. Thus, when
the sled 230 holds the firing member 240 in its unlocked position, referring
to FIG. 49, the firing member 240 will
slide past the lockout shoulder 212 to advance the sled 230 distally, fire the
staples removably stored within the
cartridge body 214, and incise the tissue thickness compensator and the tissue
positioned within the end effector 200
with a knife edge 242. As illustrated in FIG. 49, the loop 222 can slide out
of the slot 237 defined in the sled 230
when the sled 230 is advanced distally.
[0199] In the event that the tissue thickness compensator 220 is removed from
the cartridge 210 or substantially
moved from a suitable position over the deck 211 of the cartridge 210,
referring now to FIG. 50, the tissue thickness
compensator 220 can pull the sled 230 distally such that the firing member 240
is no longer supported by the sled 230.
More particularly, the loop 222 of the tissue thickness compensator 220
positioned within the slot 237 can pull the
sled 230 distally from its unfired position such that the support shoulder 234
is no longer positioned under the distal
projection 244 of the firing member 240. In such circumstances, the firing
member 240 may shift downwardly into a
locked position wherein the distal movement of the firing member 240 can be
impeded by the lockout shoulder 212.
- 51 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
In certain circumstances, the end effector 200 can further include a biasing
member, such as a spring, tbr example,
which can bias the firing member 240 into its locked condition. When the
firing member 240 is in its locked
condition, the firing member 240 cannot be moved distally to advance the sled
230, fire the staples from the cartridge
body 210, and/or incise the tissue captured within the end effector 200.
Although the sled 230 may be advanced
distally when the tissue thickness compensator 220 is removed from the
cartridge 210, the sled 230, in various
circumstances, may not be advanced sufficiently to deploy the staples from the
cartridge 210. When the user of the
surgical instrument recognizes that the firing member 240 is in a locked-out
condition, the user can remove the staple
cartridge 210 from the end effector 200 and replace it with a staple cartridge
210, for example, in which the tissue
thickness compensator 220 is correctly positioned over the deck 211 and the
sled 230 has not been advanced distally
from its unfired position. Other embodiments are contemplated in which a
staple cartridge is not removable from the
end effector; in such embodiments, the end effector may be entirely replaced
in the event that the tissue thickness
compensator is removed from the staple cartridge and/or the firing member
enters into a locked-out condition.
[0200] Turning now to FIGS. 51-53, a staple cartridge 310 can include a
cartridge body 314 and a sled 330 movably
positioned within the cartridge body 314. Similar to the above, the cartridge
body 314 can include a plurality of
fastener cavities, such as Fdstener cavities 316, for example, and a
longitudinal slot, such as knife slot 318, for
example, defined therein. The sled 330 can include a central body portion 336
slidably positioned within the knife
slot 318 and a hook 338 extending from the central body portion 336. Referring
primarily to FIG. 51, a tissue
thickness compensator 320 of the cartridge 310 can include a body portion 321
and a catch 322 extending from the
body portion 321 wherein the catch 322 can be releasably retained in a slot
337 defined between the hook 338 and the
central body portion 336 when the sled 330 is in its unfired, or unadvanced,
position. Similar to the above, the catch
322 can include ends 323 mounted within the body 321 and can extend proximally
from the body 321 of the tissue
thickness compensator 320 wherein, in the event that the tissue thickness
compensator 320 is removed from the
cartridge body 314, for instance, the catch 322 can pull the sled 330 distally
such that a support shoulder 334 defined
in the central body portion 336 is no longer able to support a firing member,
such as firing member 240, for example,
thereon and such that the firing member may enter a locked out state. In
various instances, a user of the surgical
instrument may attempt to reassemble or reposition the tissue thickness
compensator 320 over the deck 311 of the
cartridge body 314; however, the firing member 340 will still remain in a
locked out condition as the repositioning of
the tissue thickness compensator 320 will not reset the sled 330. Thus, such
an arrangement can prevent the cartridge
310 from being used if it has been previously tampered with.
102011 In various instances, referring again to FIGS. 51-53, at least a
portion of the hook 338 extending from the
central portion 336 of the sled 330 and/or the slot 337 defined therebetween
can extend above the deck 311. In certain
instances, at least a portion of the hook 338 extending from the central
portion 336 of the sled 330 and/or the slot 337
defined therebetween can extend above the knife slot 318. In such embodiments,
the catch 322 can be easily slid into
the slot 337 when the tissue thickness compensator 32() is assembled to the
cartridge body 314. In certain instances,
the catch 322 can be positioned above or against the deck surface 311 of the
cartridge body 314. In various instances,
referring primarily to FIG. 53, the cartridge body 314 can include a recess or
pocket 319 defined therein within which
the hook 338 can be positioned when the sled 330 is in its unfired, or
unadvanced, position. In such an embodiment,
- 52 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
the top of the hook 338 may be positional below the deck surface 311. In
various instances, the pocket 319 can
further include one or more ramped surfaces 313 which are defined in the
distal end of the pocket 319 and extend
downwardly from the deck surface 311. In some instances, the catch 322 can
abut the ramped surfaces 313 when the
sled 330 is advanced distally and, in such circumstances, the hook 338 can
then separate from the catch 322. In
various instances, the recess 319 can be configured to facilitate the assembly
of the catch 322 to the sled 330 when the
tissue thickness compensator 320 is assembled to the cartridge body 314. In
various embodiments, the slot 337 can
extend longitudinally and can include a closed distal end an open proximal end
wherein the catch 322 can be slid into
the slot 337 from the open proximal end. In the event that the tissue
thickness compensator 320 is not prematurely
removed or dislodged from the cartridge 314, the sled 330 can be advanced
distally such that the catch 322 exits the
slot 337 through the distal end thereof and such that ramps 332 defined on the
sled 330 can eject the staples From the
staple cartridge 310.
[0202] In various instances, a tissue thickness compensator can be adhered to
a sled utilizing at least one adhesive.
In such instances, the adhesive attachment between the tissue thickness
compensator and the sled can be strong
enough to permit the tissue thickness compensator to pull the sled distally in
the event that the tissue thickness
compensator is removed from the cartridge. W'hen the sled is advanced distally
by the firing member as part of the
firing stroke, the adhesive attachment between the tissue thickness
compensator and the sled may fail thereby
permitting the sled to slide distally relative to the tissue thickness
compensator. In various instances, a tissue
thickness compensator can be bonded to a sled utilizing a heat steak process
and/or a thermoform process. In such
instances, the bond between the tissue thickness compensator and the sled can
be strong enough to permit the tissue
thickness compensator to pull the sled distally in the event that the tissue
thickness compensator is removal from the
cartridge. When the sled is advanced distally by the firing member as part of
the firing stroke, the bond between the
tissue thickness compensator and the sled may fail thereby permitting the sled
to slide distally relative to the tissue
thickness compensator.
[0203] In some instances, a loop, a catch, and/or tag, for example, can be
integrally formed with a tissue thickness
compensator. In various instances, the loop, catch, and/or tag, for example,
can comprise a unitary piece of material
with the tissue thickness compensator. In some instances, an additional layer
can be attached to the tissue thickness
compensator. This layer, in various instances, can comprise a mounting portion
engaged with the sled.
[0204] Turning now to FM. 54, a sled 430 can include, similar to the above, a
central body portion 436 and, in
addition, a plurality of ramps 432 which are configured to eject staples
removably stored within a cartridge body, for
example. Also similar to the above, the body portion 436 can include a hook
438 extending therefrom wherein a slot
437 can be defirux1 between the body portion 436 and the hook 438. In certain
instances, the slot 437 can include a
closed distal end 437a and an open proximal end 437d. In various instances,
the slot 437 can further include a first
portion 437b extending in a first direction and a second portion 437c
extending in a second direction. In certain
instances, the first portion 437b can extend along a longitudinal axis and the
second portion 437c can extend along a
second axis which is transverse to the longitudinal axis. In at least one such
instance, the second portion 437e can
extend at an angle relative to the first portion 437b.
- 53 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
[0205] Turning now to FIGS. 55-58, a sled assembly 530 can include a first
portion 535 and, in addition, a second
portion 536 which is movable relative to the first portion 535 between an
unlocked position (FIGS. 55 and 57) and a
locked position (FIGS. 56 and 58). The first portion 535 can include, one, a
central portion configured to slide within
a longitudinal slot, such as a knife slot 518 defined in a staple cartridge
510, for instance, and, two, a plurality of
ramps 532 configured to eject staples removably stored within the cartridge
510. The central portion of the first
portion 535 can include a first slot 533a and a second slot 533b defined
therein. The first slot 533a and the second slot
533b can be configured to receive pins 531a and 531 b, respectively, extending
from the second portion 536. The first
pin 531a can be configured to slide within the first slot 533a and the second
pin 531b can be configured to slide within
the second slot 533b in order to permit the second portion 536 to rotate
relative to the first portion 535. In various
instances, the first pin 531a can be closely received within the first slot
533a such that the first slot 533a can constrain
the motion of the first pin 531a along a first path and, similarly, the second
pin 531b can be closely received within
the second slot 533b such that the second slot 533b can constrain the motion
of the second pin 531b along a second
path. Referring primarily to FIG. 57, the second portion 536 of the sled
assembly 530 can comprise an arm
configured to slide within the knife slot 518 wherein the arm can include a
support shoulder 534 defined on the
proximal end thereof and a hook 538 defined on the distal end thereof Similar
to the above, the support shoulder 534
can be configured to support a firing member 240, for example, in an unlocked
position when the sled assembly 530 is
in a proximal, unfired position and the tissue thickness compensator 220, for
instance, is positioned over and/or
against the deck surface 511 of the cartridge 510. Also similar to the above,
the hook 538 can be configured to
releasably hold the loop 222 of the tissue thickness compensator 220 such
that, in the event that the tissue thickness
compensator 220 were to be removed from and/or substantially displaced
relative to the cartridge body, the loop 222
could pull on the second portion 536 to pivot the second portion 536 into its
locked position as illustrated in FIG. 58.
In such a locked position of the second portion 536, the support shoulder 534
may no longer support the distal
projection 244 of the firing member 240 and the firing member 240 can drop
downwardly into its locked position. As
depicted in FIG. 58, the rotation of the second portion 536 into its locked
position can move the support shoulder 534
distally and/or downwardly away from the firing member 240. As also depicted
in FIG. 58, the firing member 240
can include a lock 541 extending from opposite sides thereof which can be
configured to abut the lockout shoulder
212 when the firing member 240 is in its locked position. When the firing
member 240 is held in its unlocked
position by the sled assembly 530, the locks 541 may not contact the lockout
shoulder 212 and the firing member 240
can be advanced through the cartridge 510.
102061 In various instances, as discussed above, a portion of a staple-driving
sled may extend above the deck surface
of a cartridge body. For instance, referring again to FIGS. 52 and 54, the
hook 338 of the sled 330 (FIG. 52) and/or
the hook 438 of the sled 430, for example, can extend above the deck surface.
In such instances, the hook 338 and/or
the hook 438 can translate distally above the deck surface and, in some
instances, contact the tissue thickness
compensator positioned against or above the deck surface. In certain
instances, the hook 338 and/or the hook 438 can
lift the tissue thickness compensator upwardly away from the cartridge body
and facilitate the progressive release of'
the tissue thickness compensator from the cartridge. For instance, the hook
338 and/or the hook 438 can begin at the
proximal end of the tissue thickness compensator and move toward the distal
end of the tissue thickness compensator
- 54 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
in order to initially lift the proximal end of the tissue thickness
compensator and then progressively lift it away from
the cartridge deck until the distal end of the tissue thickness compensator is
eventually lifted away from the cartridge
body. In other instances, as discussed in greater detail further below. it may
be preferable for the portion of the sled
contacting the tissue thickness compensator to deflect downwardly and/or
otherwise not disturb the tissue thickness
compensator as the sled is advanced distally.
102071 Turning now to FIGS. 59 and 60, a staple cartridge 610 can include a
cartridge body 614, a tissue thickness
compensator 620 releasably retained to the cartridge body 614, and a sled 630
configured to longitudinally traverse
the cartridge body 614 and eject staples removably stored therein. The sled
630 can include a main body portion 635
having a plurality of ramp surfaces defined thereon, a support shoulder 634,
and an arm 636 extending from the body
portion 635. In various instances, the arm 636 can be assembled to the main
body portion 635. For instance, the arm
636 can include a first end embedded in the main body portion 635 and a second
end including a hook 638, for
example. in various instances, the arm 636 can comprise a cantilever beam
extending from the main body portion
635. In certain instances, the arm 636 can be comprised of a resilient and/or
flexible material, for example. Similar to
the above, a slot 637 can be defined between the hook 638 and the arm 636
which can be configured to releasably
hold a portion of the tissue thickness compensator 620 when the sled 630 is in
its proximal, unfired position. In the
event that the tissue thickness compensator 620 is pulled off of the cartridge
body 614, fir example, the tissue
thickness compensator 620 can pull the sled 630 distally away from a firing
member so that the firing member enters
into a locked out condition.
102081 In various instances, further to the above, at least a portion of the
arm 636, such as the hook 638, for
example, can extend above the deck surface 611 of the cartridae body 614. In
certain instances, the arm 636 can be
engaged with a loop, for example, extending from the tissue thickness
compensator 620 when the sled 630 is in its
proximal position (FIG. 59) and, as the sled 630 is advanced distally, the arm
636 can disengage from the loop. As
the sled 630 is advanced distally, in certain instances, the arm 636 can
contact the body portion 621 of the tissue
thickness compensator 620 and flex downwardly. In various instances, the
deflected arm 636 can slide within a
longitudinal knife slot 618 defined in the cartridge body 614 as the sled 630
is advanced distally. In some instances,
referring to FIG. 60, the distal end of the longitudinal slot 618 can be
defined by a nose wall, or roof, 619 wherein,
when the sled 630 reaches a distal end 617 of the cartridge 610, the arm 636
can slide under the nose wall 619 such
that the firing stroke of the end effector can be completed. In some
instances, the arm 636 may not be deflected, or
substantially deflected, downwardly by the tissue thickness compensator 620
wherein, when the arm 636 reaches the
end of the longitudinal slot 618, the arm 636 can contact the nose wall 618
and flex downwardly in order to slide
thereunder as illustrated in FIG. 60. In various circumstances, as a result,
the flexible arm 636 can permit the firing
stroke to be completed and for the sled 630 to be parked at the distal end of
the cartridge.
102091 Turning now to FIG. 61, a sled, such as sled assembly 730, for example,
can include a main body portion 735
and a movable arm 736. Similar to the above, the main body portion 735 can
include one or more staple-driving
ramps 732 and a support shoulder 734 configured to support a firing member in
an unlocked position, as described
above. The arm 736 can include a first end pivotably andlor rotatably mounted
to the main body portion 735 and a
second end including a hook 738 configured to be releasably engaged with a
tissue thickness compensator, as
- 55 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
described above. When the slat assembly 730 is advanced distally, the hook 738
can detach from the tissue thickness
compensator; however, the upper surface of the hook 738 can remain in contact
with the bottom surface of the tissue
thickness compensator. In such circumstances, the arm 736 can pivot dow-
nwardly into the knife slot 318, for
example, in order to slide under the tissue thickness compensator. More
particularly, the arm 736 can pivot from a
raised, or uppermost, position (FIG. 61) to a lowered, or depressed, position.
In various instances, the sled assembly
730 can further include a resilient biasing member, such as a spring 731, for
example, configured to bias the arm 736
into its raised position. When the arm 736 has been rotated downwardly into
its lowered position, the spring 731 can
apply a biasing force to the arm 736 which is transmitted into the tissue
thickness compensator. in certain instances,
the spring 731 can be positioned intermediate the arm 736 and a frame portion
733 defined on the main body portion
735. in various instances, the spring 731 can comprise a cantilever spring or
leaf spring, for example, extending from
the arm 736. When the arm 736 is pushed downwardly, the cantilever spring can
be configured to flex and/or slide
along the frame portion 731, for instance. in various embodiments, the main
body portion 735 can fiwther include a
stop shoulder 739, for example, which can limit the upward rotation, or
travel, of the arm 736. In any event, similar to
the above, the arm 736 can be configured to rotate downwardly when it contacts
the roof 6.19 and permit the firing
stroke to be completed.
102101 In various instances, a staple can comprise a base and one or more legs
extending from the base. In certain
instances, a staple can comprise a base including a first end and a second
end, a first leg extending from the first end,
and a second leg extending from the second end. In some instances, the staple
can be formed from a continuous wire
which comprises the first leg, the base, and the second leg. A first end of
the continuous wire can comprise a tip of
the first staple leg and a second end of the continuous wire can comprise a
tip of the second staple leg. One such
staple, i.e., staple 800, is depicted in FIG. 62, for example. The staple 800
can include a base 802, a first staple leg
804 extending from a first end of the base 802, and a second staple leg 804
extending from a second end of the base
802. The first staple leg 804 can include a first tip 806 and, similarly, the
second staple leg 804 can include a second
tip 806. In various instances, the tips 806 can be configured to penetrate
tissue, such as tissue T depicted in FIG. 62,
for example. In some instances, the tips 806 can be sharp and can be formed by
a coining process, for example. In
various embodiments, the wire can be comprised of titanium and/or stainless
steel, for example.
102111 in various embodiments, the staple 800 can be U-shaped, or at least
substantially U-shaped, for example,
when it is in its unformed configuration. In such embodiments, the legs 804 of
the staple 800 can be parallel, or at
least substantially parallel, to one another. Moreover, in such embodiments,
the legs 804 can be perpendicular, or at
least substantially perpendicular, to the base 802. In certain embodiments,
the staple 800 can be V-shaped, or at least
substantially V-shapocl, for example, when it is in its unformed
configuration. In such embodiments, the legs 804 of
the staple 800 are not parallel to one another; rather, the legs 804 can
extend in non-parallel directions. Moreover, in
such embodiments, one or both of the legs 804 are not perpendicular to the
base 802 wherein one or both of the legs
804 can extend in directions which are oblique to the base 802. In various
instances, the legs 804 may extend, or
splay, outwardly with respect to a center or midline of the staple. In any
event, the staple 800 can be removably stored
within a staple cartridge, ejected from the staple cartridge in penetrate
tissue, as illustrated in FIG. 62, and then contact
an anvil positioned on the opposite side of the tissue. The anvil can be
configured to deform the staple 800 into any
- 56 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
suitable shape, such as a B-form configuration, for example, as also
illustrated in FIG. 62. Various formed staple
configurations, such as the B-form configuration, for example, can define a
tissue entrapment area, such as tissue
entrapment area 807, for example, configured to entrap tissue within the
staple.
102121 As discussed above, a staple can be removably stored within a cavity
defined in a cartridge body. A.
cartridge body 810 is depicted in FIG. 63 which can include one or more staple
cavities 812 defined therein. Referring
to FIGS. 63, 68, and 69, each staple cavity 812 can include a rust end 814 and
a second end 814. In certain
embodiments, such as embodiments including a longitudinal end effector, for
example, the first end 814 can comprise
a proximal end of the staple cavity 812 and the second end 814 can comprise a
distal end of the staple cavity 812. In
various instances, a staple can be positioned within a staple cavity 812 such
that a first leg 804 of the staple 8(X) is
positioned in the first end 814 of the staple cavity 812 and a second leg 804
is positioned in the second end 814. In
various instances, a staple cavity width can be defined between the ends 814
of a staple cavity 812. The base 802 of a
staple can be defined by a base width which can be equal to or shorter than
the staple cavity width, for example. In
certain instances, a staple can comprise a staple width which can be defined
between the tips 806 of the staple legs
804. In some embodiments, the staple width can be equal to the staple cavity
width. In various embodiments, the
staple width can be wider than the staple cavity width. In such embodiments,
the legs 804 can be in contact with the
ends 814 of a staple cavity 812 and can be resiliently biased inwardly by the
ends 814 when the staple is positioned
within the staple cavity 812. When the staple is Li fled upwardly out of the
staple cavity 812, the legs 804 can
resiliently splay outwardly as they emerge from the staple cavity 812. For
example, the staple can be positioned
within the staple cavity 812 such that the tips 806 of the staple legs 804 do
not extend above a top surface, or deck, of
the cartridge body 810 when the staple is in its unfired, or unfitted,
position. In such a position, the tips 806 can be
positioned flush with or recessed below the deck 811 of the cartridge body
810. Alternatively, the tips 806 of the legs
804 can at least partially extend above the deck 811 of the cartridge body
810. In any event, as the staple is lifted
upwardly, the staple tips 806 can emerge above the deck 811 and splay
outwardly as the legs 804 emerge from the
cavity 812. At some point during the lifting of the staple, the legs 804 may
no longer be in contact with the ends 814
of the staple cavity 812 and the legs 804 may no longer be biased inwardly by
the sidewalls of the staple cavity 812.
102131 In various instances, an anvil can include one or more pockets
configured to receive the tips 806 of the staple
legs 804 as the staple 800 is ejected from the staple cartridge. The anvil
pockets can be configured to turn, or bend,
the staple legs 804 inwardly toward one another, for example. In other
instances, the anvil pockets can be configured
to turn, or bend, the staple legs 804 outwardly away from one another, for
example. In some instances, however, one
or more of the staple legs of a staple may miss a staple pocket and may not be
properly deformed. In certain
instances, one or more of the staple legs may not contact the anvil and may
not be deformed at all. In either event, the
staple may not properly capture and/or retain the tissue within its tissue
entrapment area. Moreover, the misformed or
unformed staple may not be able to apply a desired compressive pressure to the
tissue. In some instances, the
misformed or unformed staple may not be retained in the tissue and can become
dislodged from the tissue.
102141 Referring again to FIG. 62, the staple 800, and/or various other
staples disclosed herein, can include one or
more barbs extending therefrom. In various instances, the barbs can be
configured to engage tissue captured within
and/or surrounding the staple. In certain instances, the barbs can assist in
retaining the staple within the tissue,
- 57 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
especially when the staple has been mistirrrexl or unformed. The staple 800
can include barbs extending from one or
both of the legs 804. For instance, each leg 804 can include one or more barbs
808 which face outwardly from the
center of the staple 800 and/or one or more barbs 809 which face inwardly
toward the center of the staple 800, for
example. In certain instances, the barbs 808 can extend away from the tissue
entrapment area 807 and/or the barbs 809
can extend toward or into the tissue entrapment area 807. As depicted in FIG.
62, both of the staple legs 804 of staple
800 can include barbs 808 and barbs 809. In some instances, the staple legs
804 can include barbs 808, but not barbs
809. A staple 820 is depicted in FIG. 63 which includes barbs 808, but not
baths 809. In some instances, the staple
legs 804 can include barbs 809, but not barbs 808. Staples 830, 840, 850, 860,
and 870 are depicted in FIGS. 64, 65,
66, 67, and 68, respectively, which include barbs 809, but not barbs 808. In
some embodiments, a first leg 804 of a
staple can include barbs 808 while a second leg 804 of the staple can include
barbs 809, for example.
102151 In various instances, the legs 804 and the base 802 of a staple can
define a staple plane when the staple is in
an unformed configuration. The barbs 808 can extend outwardly from the legs
804 within such a staple plane.
Similarly, the barbs 809 can extend inwardly from the legs 804 within such a
plane. In some instances, a staple can
include baths which extend laterally with respect to such a staple plane.
Other embodiments are envisioned in which
the legs 804 and the base 802 do not lie within, or entirely lie within, a
single plane. In such embodiments, the barbs
can extend in any suitable direction. In various embodiments, referring now to
FIG. 67, a staple, such as staple 860,
for example, can include barbs 803 extending from the base 802. In various
instances, the barbs 803 can extend
inwardly toward the tissue entrapment area 807 of the staple 860. In certain
instances, the barbs 803 can extend
outwardly away from the tissue entrapment area 807. As illustrated in FIG. 67,
the barbs 803 can extend within a
staple plane defined by the legs 804 and the base 802. In certain instances,
the barbs 803 can extend laterally with
respect to such. a staple plane. Various exemplary barb configurations are
discussed in greater detail further below.
102161 In various instances, a staple leg 804 can comprise an array of barbs
808 which extends along the entire
length thereof. In some instances, a staple leg 804 can comprise an array of
barbs 808 which extends along less than
the entire length thereof. By way of example, referring to FIG. 62, the legs
804 of the staple 800 each comprise an
array of barbs 808 which extends along less than the entire length of the legs
804. Similarly, referring to FIG. 63, the
legs 804 of the staple 820 each comprise an array of barbs 808 which extends
along less than the entire length of the
legs 804. With regard to the staple 800, for example, an array of barbs 808
can extend along each of the legs 804 from
the base 802 of the staple 800 toward the tips 806 of the legs 804. As
illustrated in FIG. 62, the arrays of barbs 808
may not extend to the tips 806 of the legs 804. In various instances, the
arrays of barbs 808 can extend along half, or
approximately half, the lengths of the legs 804, for example; however, any
suitable length of the barb arrays could be
utilind. For instance, the arrays of barbs 808 can extend along less than half
or more than half of the lengths of the
legs 804. In some embodiments, an array of barbs 808 can extend along each of
the legs 804 from the tips 806 of the
legs 804 toward the base 802. In such embodiments, the array of barbs 808 may
not extend to the base 802. In some
embodiments, a leg 804 can comprise an array of barbs 808 which does not
extend to the tip 806 of the leg 804 or the
base 802. In certain embodiments, a leg 804 can comprise more than one array
of barbs 808.
(0217) In various instances, further to the above, a staple leg 804 can
comprise an array of barbs 809 which extends
along the entire length thereof. By way of example, referring to FIG. 64, the
legs 804 of the staple 830 each comprise
- 58 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
an array of barbs 809 which extends along the entire length of the legs 804.
In some instances, a staple leg 804 can
comprise an array of barbs 809 which extends along less than the entire length
thereof By way of example, referring
to FIG. 65, the legs 804 of the staple 840 each comprise an array of barbs 809
which extends along less than the entire
length of the legs 804. Similarly, referring to FIG. 68, the legs 804 of the
staple 870 each comprise an array of barbs
809 which extends along less than the entire length of the legs 804. With
regard to the staple 840, for example, an
array of barbs 809 can extend along each of the legs 804 from the base 802 of
the staple 840 toward the tips 806 of the
legs 804. As illustrated in FIG. 65, the arrays of barbs 809 may not extend to
the tips 806 of the legs 804. In various
instances, the arrays of barbs 809 can extend along half, or approximately
half, the lengths of the legs 804, for
example; however, any suitable length of the barb arrays could be utilized.
For instance, the arrays of barbs 809 can
extend along less than hair or more than half of the lengths of the legs 804.
In some embodiments, an array of barbs
809 can extend along each of the legs 804 from the tips 806 of the legs 804
toward the base 802. In such
embodiments, the array of barbs 809 may not extend to the base 802. In some
embodiments, as illustrated in FIG. 66,
a leg 804 can comprise an array of barbs 809 which does not extend to the tip
806 of the leg 804 or the base 802. In
certain embodiments, a leg 804 can comprise more than one array of barbs 809.
102181 Various barb configurations are depicted in FIGS. 70-73, although any
suitable barb configuration could be
utilized. Referring to FIG. 70, a staple leg 804 can include at least one barb
809, for example. In various instances,
the barb 809 can comprise a prong. The prong can include a first surface 809a
and a second surface 809b which can
extend from the perimeter 805 of the staple leg 804. The first surface 809a
can comprise an inclined surface, a convex
surface, and/or a concave surface, for example. The second surface 809b can
comprise a flat, or an at least
substantially flat, surface, for example. In various instances, the first
surface 809a and the second surface 809b can
converge at an edge 809c, for example. The barb 809 can be formed utilizing
any suitable process. For instance, the
barb 809 can be formed utilizing a stamping process. In at least one
embodiment, a forming die, for example, can be
utilized to strike the perimeter 805 of the wire comprising the leg 804 in
order to upset, or disturb, enough material to
create the barb 809. In various instances, a barb can comprise any suitable
nib or spur, for example. In various
embodiments, the barb 809 can be tapered. In various instances, the barb 809
can include a base adjacent to the
perimeter 805 which is thicker than a tip of the barb 809.
102191 Referring now to FIGS. 68, 69, 71, and 71A, a staple leg 804 can
include at least one barb 879, for example.
In at least one embodiment, the barb 879 can extend around a portion of the
perimeter 805 of the staple leg 804. In
various instances, the barb 879 can include a first surface 879a and a second
surface 879b which can extend from the
perimeter 805 of the staple leg 804. The first surface 879a can comprise an
inclined surface, a convex surface, and/or
a concave surface, for example. The second surface 879b can comprise a flat,
or an at least substantially flat, surface,
for example. In various instances, the first surface 879a and the second
surface 879b can converge at an edge 879c,
for example. In various instances, the edge 879c can be arcuate, for example.
The barb 879 can be formed utilizing
any suitable process. For instance, the barb 879 can be formed utilizing a
stamping process. In at least one
embodiment, a forming die, for example, can be utilized to strike the
perimeter 805 of the wire comprising the leg 804
in order to upset, or disturb, enough material to create the barb 879.
Referring primarily to FIG. 71A, the wire
comprising the leg 804 can be defined by a diameter 801 and the barb 879 can
be defined by a diameter which is
-59 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
larger than the diameter 801. Correspondingly, the wire comprising the leg 804
can be defined by a radius and the
barb 879 can be defined by a radius which is larger than the wire radius. In
various embodiments, the barb 879 can be
tapered. In various instances, the barb 879 can include a base adjacent to the
perimeter 805 which is thicker than a tip
of the barb 879.
102201 Referring now to FIG. 72, a staple leg 804 can include at least one
barb 889, for example. In at least one
embodiment, the barb 889 can extend around the entirety of the perimeter 805
of the staple leg 804. In various
instances, the barb 889 can include a first surface 88% and a second surface
889b which can extend from the
perimeter 805 of the staple leg 804. The first surface 889a can comprise an
inclined surface, a convex surface, and/or
a concave surface, for example. The second surface 889b can comprise a flat,
or an at least substantially flat, surface.
for example. In various instances, the first surface 889a and the second
surface 889b can converge at an edge 889c,
for example. In various instances, the edge 889c can be arcuate, for example.
The barb 889 can be formed utilizing
any suitable process. For instance, the barb 889 can be formed utilizing a
stamping process. In at least one
embodiment, a forming die, for example, can be utilized to strike the
perimeter 805 of the wire comprising the leg 804
in order to upset, or disturb, enough material to create the barb 889. The
wire comprising the leg 804 can be defined
by a wire diameter and the barb 889 can be defined by a diameter which is
larger than the wire diameter.
Correspondingly, the wire comprising the leg 804 can be defined by a radius
and the barb 889 can be defined by a
radius which is larger than the wire radius. In various embodiments, the barb
889 can be tapered. In various instances,
the barb 889 can include a base adjacent to the perimeter 805 which is thicker
than a tip of the barb 889.
102211 Referring now to FIG. 73, a staple leg 804 can include at least one
barb 899, for example. In various
instances, the barb 899 can comprise a prong. The prong can include a first
surface 899a and a second surface 899b
which can extend from the perimeter of the staple leg 804. The first surface
899a can comprise an inclined surface, a
convex surface, and/or a concave surface, for example. The second surface 899b
can comprise a flat, or an at least
substantially flat, surface, for example. In various instances, the first
surface 899a and the second surface 899b can
converge at an edge 899c, for example. The barb 899 can be formed utilizing
any suitable process. For instance, the
barb 899 can be formed utilizing a stamping process. In at least one
embodiment, a forming die, for example, can be
utilized to strike the perimeter of the wire comprising the leg 804 in order
to upset, or disturb, enough material to
create the barb 899. In various embodiments, the wire comprising the staple
can include one or more flat sides. In at
least one embodiment, the wire can include opposing flat sides 895, for
example. In at least one such embodiment,
the flat sides 895 can be formed into a cylindrical wire. In some instances,
the wire can retain one or more cylindrical
surfaces in addition to the flat sides 895. In various instances, a barb can
comprise any suitable nib or spur, for
example. In various embodiments, the barb 899 can be tapered. In various
instances, the barb 899 can include a base
adjacent to the perimeter of the leg 804 which is thicker than a tip of the
barb 899.
102221 In various instances, the legs of a staple can define a staple plane.
The base of the staple may or may not be
positioned within the staple plane. In either event, one or more barbs
extending from the legs and/or the base may
extend within and/or extend parallel with respect to the staple plane. In some
instances, one or more barbs extending
from the legs and/or the base can extend outwardly from the staple plane. One
or more barbs extending from the legs
and/or the base can extend transversely with respect to the staple plane. In
various instances, a barb can extend
- 60 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
circumferentially around a staple leg. Such a barb can extend within and
outwardly from the staple plane. In some
instances, a barb can extend around the entire circumference of a staple leg.
In certain instances, the barb can extend
less than 360 degrees around a staple leg. A barb extending within a staple
plane can readily control tissue within the
staple plane. A barb extending outwardly from a staple plane can readily
control tissue outside of the staple plane. A
staple, and/or a staple leg, can include one or more barbs extending within
the staple plane and one or more barbs
extending outwardly fixmi the staple plane.
102231 Referring again to FIG. 62, the barbs extending from a staple leg 804
can be configured to retain the staple
leg 804 within tissue. As outline above, the staple legs 804 may be malformed
and/or unformed by an anvil in certain
instances and, owing to the barb, or barbs, extending therefrom, the staple
leg 804 may still be retained in the tissue.
In various instances, the barbs can be configured to trap tissue within the
tissue entrapment area of the staple. In
certain instances, the barbs can be configured to hold the tissue against the
base 802. In such instances, the barbs can
apply a compressive force or pressure to the tissue. As discussed above in
connection with the embodiments depicted
in FIGS. 70-73, a barb can comprise an inclined, convex, and/or concave top
surface, such as surfaces 809a, 879a,
889a, and/or 899a, for example. The top surfaces of the barbs can be
configured to facilitate the insertion of the barbs
and the staple legs 804 into and/or through the tissue. As also discussed
above in connection with the embodiments
depicted in FIGS. 70-73, a barb can comprise a flat, or at least substantially
flat, bottom surface, such as surfaces
809b, 879b, 889b. and/or 899b, for example. The bottom surfaces of the barbs
can be configured to inhibit the
removal of the barbs and the staple legs 804 from the tissue. As a result of
the above, in certain circumstances, the top
surfaces of the barbs can be configured to pierce the tissue while the bottom
surfaces of the barbs can be configured to
abut the tissue. In various circumstances, the tips 806 of the staple legs 804
can be configured to puncture a hole in
the tissue while the staple legs 804 and the barbs extending therefrom can be
configured in resiliently expand the hole
such that such that the tissue can flow around the barbs as the staple legs
804 are being pushed through the tissue and
flow back underneath the bottom surfaces of the barbs.
[0224] In certain embodiments, a first barb can extend from a first leg 804 of
the staple and a second barb can extend
from a second leg 804 of the staple. In various instances, the first barb and
the second barb can be located the same,
or at least substantially the same, distance between from the base 802. In
certain instances, the first barb and the
second barb can be located the same, or at least substantially the same,
vertical distance from the base 802. As
discussed above, a staple leg 804 can include an array of barbs extending
along the length of the staple leg 804. In
various embodiments, referring primarily to FIG. 62, a staple can include a
first leg 804 including a first array of barbs
and a second leg 804 including a second array of barbs wherein the first array
of barbs and the second array of barbs
can be configured to co-operatively hold the staple within the tissue. In
various embodiments, a barb from the first
array and a barb from the second array can comprise a pair of barbs configured
to engage tissue at the same vertical
distance from the base 802, for example. In various instances, a staple can
comprise more than one pair of barbs. In
certain instances, each of the barb pairs can be configured to engage the
tissue at a different vertical distance from the
base 802. In such circumstances, a staple can be suitable for use with
different tissue thicknesses. For instance, when
a staple is used to staple thin tissue, one pair of barbs, or less than all of
the barb pairs, may engage the thin tissue. If
that staple were used to staple thick tissue, however, additional barb pairs,
or all of the barb pairs, may engage the
- 61 -
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
tissue. In cumin embodiments, the barbs extending from the legs 804 can be
arranged in a manner in accordance with
the tissue thickness, or range of tissue thicknesses, that can be stapled by
the staple. For instance, referring again to
FIG. 62, the barbs 808 and 809 can be selectively positioned along the legs
804 such that they are positioned within
and/or adjacent to the tissue captured within the staple. In certain
instances, the portions of the staple legs 804 that are
deformed by, or come into contact with, an anvil may not include barbs
extending therefrom. In at least some
instances, an array of barbs extending from the inwardly-facing side of the
staple legs 804 may be longer than an array
of barbs extending from the outwardly-facing side of the staple legs 804. In
other instances, an array of barbs
extending from the inwardly-facing side of the staple legs 804 may be shorter
than an array of barbs extending from
the outwardly-facing side of the staple legs 804. In yet other instances, an
array of barbs extending from the inwardly-
facing side of the staple legs 804 may be the same length as an array of barbs
extending from the outwardly-facing
side of the staple legs 804.
102251 As discussed above, the barbs extending from the staple legs 804 can
assist in retaining the staple within the
tissue if the staple legs 804 are malformed and/or unintentionally unformed.
Certain circumstances are contemplated,
however, where a staple including one or more of the barbs disclosed herein is
inserted into tissue and remains
intentionally unformed. In any event, staples including one or more of the
barbs disclosed herein can be useful in
stapling thick tissue. More particularly, in some instances, the presence of
thick and/or dense tissue between a staple
cartridge and an anvil and/or the presence of thick and/or dense tissue within
a staple may prevent the staple from
becoming fully formed or closed. For instance, the staple may not be fully
closed into a B-form configuration or the
staple may not be closed at all. In such instances, the barbs of the unclosed
staples may inhibit or prevent the tissue
from being pulled out of the staple, for example. An array of barbs extending
along the length of a staple leg may
permit the leg to remain retained in the tissue regardless of the thickness of
the tissue.
102261 Various embodiments are contemplated in which at least one barbed
staple, such as barbed staple 800, for
example, are removably stored within a staple cartridge, such as the staple
cartridge 22000 illustrated in FIGS. 10-12,
for example. Certain embodiments are envisioned in which a staple cartridge
includes only barbed staples while other
embodiments are envisioned which utilize barbed staples and non-barbed
staples. For instance, a first row of staples
can comprise barbed staples while a second row of staples can comprise non-
barbed staples. In some instances, the
staples stored within a staple cartridge can have the same, or essentially the
same, unformed height. At least with
regard to U-shaped and/or V-shaped staples, for example, the unformed height
of a staple can be defined as the
vertical distance between the bottom of the base of the staple and the tips of
the staple legs. Such a measurement can
be taken before the staples are inserted into the staple cartridge, when the
staples are removably stored within the
staple cartridge, and/or before the staples are deformed against the anvil. In
some instances, barbed staples arranged
in a first row in a staple cartridge can comprise a first unformed height and
barbed staples arranged in a second row in
the staple cartridge can comprise a second unformed height. Barbed staples in
a third row in the staple cartridge can
comprise the first unformed height, the second unformed height, or a third
unformed height. The first row, the second
row, and/or the third row of barbed staples can be positioned on the same side
of a knife slot defined in the staple
cartridge or on opposite sides of the knife slot. In use, the barbed staples
removably stored in a staple cartridge can be
formed to the same formed height or diffeieut formed heights. The formed
height of a staple can be defined as the
- 62 -
overall vertical distance of the staple after it has been deformed against an
anvil. At least with regard to a staple that
has been deformed into a B-form, for example, the formed height of the staple
can be measured between the bottom of
the base of the staple and the top-most portion of the staple legs. In some
instances, barbed staples arranged in a first
row in a staple cartridge can be deformed to a first formed height and barbed
staples arranged in a second row in the
staple cartridge can be deformed to a second formed height. Barbed staples in
a third row in the staple cartridge can
comprise the first formed height, the second formed height, or a third formed
height. The first row, the second row,
and/or the third row of barbed staples can be positioned on the same side of a
knife slot defined in the staple cartridge
or on opposite sides of the staple cartridge. As the reader will appreciate,
the staples depicted in FIGS. 10-12 have
been deformed to different formed heights. Barbed staples 800, for example,
could be utilized in staple cartridges
and/or stapling instruments which create staple rows having different formed
heights. A first row of barbed staples
800 could be deformed to a first formed height and a second row of barbed
staples 800 could be deformed to a second
formed height. In various instances, a third row of barbed staples 800 could
be deformed to a third formed height. In
some instances, the barbed staples 800 deformed to different heights can begin
with the same, or essentially the same,
unformed height. In certain instances, the barbed staples 800 deformed to
different formed heights can begin with
different unformed heights. Various structures can be utilized to form staples
to different formed heights. For
instance, movable drivers supporting the staples can support the staples at
different distances relative to the anvil. In
some instances, the anvil can include staple forming pockets having different
depths. In various instances, a staple
driver can include a cradle configured to support the base of a staple and
push the staple upwardly toward a forming
pocket defined in the anvil. The formed height of a staple can be determined
by the distance between the bottom
surface of the cradle and the top surface of the forming pocket. In certain
instances, the deck of a staple cartridge can
include stepped surfaces, as illustrated in FIG. 1. A first row of staple
cavities can be defined in a first step and a
second row of staple cavities can be defined in a second step wherein the
first step and the second step can be
vertically offset from one another. For instance, the first step can be
positioned vertically above, or closer to, the anvil
than the second step. In certain instances, a wall can be defined between the
first step and the second step. In some
instances, the deck of a staple cartridge can comprise a first step, a second
step positioned vertically above the first
step, and a third step positioned vertically above the second step. Various
embodiments are envisioned in which the
deck of a staple cartridge includes any suitable number of steps and any
suitable number of walls between the steps.
A first row of staple cavities can be defined in the first step, a second row
of staple cavities can be defined in the
second step, and/or a third row of staple cavities can be defined in the third
step, for example. The first row of staple
cavities can include staples having a first unformed height, the second row of
staple cavities can include staples
having a second unformed height, and/or the third row of staple cavities can
include staples having a third unformed
height, for example. Various embodiments are envisioned in which a staple
cartridge includes any suitable number of
staple rows having different unformed heights. The staples in the first row of
staple cavities can be deformed to a first
formed height, the staples in the second row of staple cavities can be
deformed to a second formed height, and/or the
third row of staple cavities can be deformed to a third formed height, for
example. Various embodiments are
63
Date Recue/Date Received 2021-07-12
CA 02990510 2016-09-23
WO 2015/126655 PCT/US2015/015099
envisioned in which a staple cartridge includes any suitable number of staple
rows which are delbrmed to di flermit
formed heights. In addition to or in lieu of having different formed staple
heights, an end effector of a stapling
instrument can have different tissue gaps. For instance, referring generally
to FIGS. 10 and 11, a gap can be defined
between the cartridge deck surface 22011 of a staple cartridge and the anvil
tissue compression surface 10063 of an
anvil. This gap can be configured to receive tissue T. This gap can also be
configured to receive a tissue thickness
compensator; however, a barbed staple may or may not be used with a tissue
thickness compensator and the
discussion provided with respect to barbed staples can be applicable in either
circumstance. In any event, the reader
will appreciate that the anvil tissue compression surface 10063 is stepped.
The anvil tissue compression surface
10063 comprises a first portion positioned vertically above a second portion.
When the anvil and the staple cartridge
of an end effector are in a closed condition, as illustrated in FIG. 11, a
first gap distance is defined between an outer
portion of the anvil tissue compression surface 10063 and the cartridge deck
surface 22011 and a second, different,
gap distance is defined between an inner portion of the anvil tissue
compression surface 10063 and the cartridge deck
surface 22011. The first gap distance is illustrated as being larger than the
second gap distance, but it is possible for
the first gap distance to be shorter than the second gap distance. Tissue
compressed between the anvil and the staple
cartridge in the shorter gap distance can be compressed more than tissue in
the larger gap distance. The barbs of a
barbed staple 800, for example, may engage the tissue differently depending on
whether the tissue is positioned within
a shorter tissue gap or a larger tissue gap. More particularly, tissue
compressed within a shorter tissue gap may seek
to re-expand more after it is released from an end effector than tissue
compressed within a larger tissue gap and the
barbs of a barbed staple may inhibit or resist this re-expansion, depending on
their configuration andior position on the
barbs. In other instances, the barbs may be configured and/or positioned so as
to not inhibit or resist the re-expansion
of the tissue. As the reader will appreciate, anvil tissue compression surface
10063 is stepped and the cartridge deck
surface is flat, or at least substantially flat, and, thus, the difference in
tissue gaps defined within the end effector is a
function of the height of the stepped anvil surfaces. Other embodiments are
envisioned. For instance, the anvil tissue
compression surface can be flat, or at least substantially flat, and the
cartridge deck surface can be stepped. In other
instances, the anvil tissue compression surface and the cartridge deck surface
can both be stepped. In any event,
different gap distances can be defined between the anvil tissue compression
surface and the cartridge deck surface.
While two gap distances have been illustrated in FIGS. 10 and 11, more than
two gap distances may be possible, such
as three gap distances, for example. With further reference to FIGS. 10 and
11, a first longitudinal row of forming
pockets can be arranged within a first portion of an end effector having fitst
tissue gap distance and a second
longitudinal row of forming pockets can be arranged within a second portion of
the end effector having a second
tissue gap distance which is different than the first tissue gap distance. In
some instances, the end effector can
include a third longitudinal row of forming pockets ancanged within a third
portion of the end effector having a third
tissue gap distance which is din-cent than the first tissue gap distance and
the second tissue gap distance. In certain
instances, the end effector can include a third longitudinal row of forming
pockets arranged within a third portion of
the end effixtor having a tissue gap distance which is the same as the first
tissue gap distance or the second tissue gap
distance. The reader will appreciate that an end effector can have different
tissue gap distances and/or different
formed staple heights. An end effector can have one, the other, or both. In
certain instances, shorter formed staple
- 64 -
heights can be associated within shorter tissue gap distances while larger
formed staple heights can be associated with
larger tissue gap distances. In other instances, shorter formed staple heights
can be associated with larger tissue gap
distances while larger formed staple heights can be associated with shorter
tissue gap distances. Further to the above,
a staple can include a U-shape configuration in its unformed state. A U-shape
staple can comprise a base and two
staple legs extending from the base wherein the staple legs extend in parallel
directions to each other. Also further to
the above, a staple can include a V-shape configuration in its unformed state.
A V-shape configuration can comprise a
base and two staple legs extending from the base wherein the staple legs
extend in directions which are not parallel.
[0227] Various embodiments described herein are described in the context of
linear end effectors and/or linear
fastener cartridges. Such embodiments, and the teachings thereof, can be
applied to non-linear end effectors and/or
non-linear fastener cartridges, such as, for example, circular and/or
contoured end effectors. For example, various end
effectors, including non-linear end effectors, are disclosed in U.S. Patent
Application Serial No. 13/036,647, filed
February 28, 2011, entitled SURGICAL STAPLING INSTRUMENT, now U.S. Patent
Application Publication No.
2011/0226837.
[0228] The devices disclosed herein can be designed to be disposed of after a
single use, or they can be designed to
be used multiple times. In either case, however, the device can be
reconditioned for reuse after at least one use.
Reconditioning can include any combination of the steps of disassembly of the
device, followed by cleaning or
replacement of particular pieces, and subsequent reassembly. In particular,
the device can be disassembled, and any
number of the particular pieces or parts of the device can be selectively
replaced or removed in any combination.
Upon cleaning and/or replacement of particular parts, the device can be
reassembled for subsequent use either at a
reconditioning facility, or by a surgical team immediately prior to a surgical
procedure. Those skilled in the art will
appreciate that reconditioning of a device can utilize a variety of techniques
for disassembly, cleaning/replacement,
and reassembly. Use of such techniques, and the resulting reconditioned
device, are all within the scope of the present
application.
[0229] Preferably, the invention described herein will be processed before
surgery. First, a new or used instrument
is obtained and if necessary cleaned. The instrument can then be sterilized.
In one sterilization technique, the
instrument is placed in a closed and sealed container, such as a plastic or
TYVEK bag. The container and instrument
are then placed in a field of radiation that can penetrate the container, such
as gamma radiation, x-rays, or high-energy
electrons. The radiation kills bacteria on the instrument and in the
container. The sterilized instrument can then be
Date Recue/Date Received 2021-07-12
stored in the sterile container. The sealed container keeps the instrument
sterile until it is opened in the medical
facility.
[0231] While this invention has been described as having exemplary designs,
the present invention may be further
modified within the spirit and scope of the disclosure. This application is
therefore intended to cover any variations,
uses, or adaptations of the invention using its general principles. Further,
this application is intended to cover such
departures from the present disclosure as come within known or customary
practice in the art to which this invention
pertains.
66
Date Recue/Date Received 2021-07-12