Note: Descriptions are shown in the official language in which they were submitted.
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
TITLE
ENZYMATIC HYDROLYSIS OF DISACCHARIDES AND 01. IGOSACCHARIDES
USING ALPHA-GLUCOSIDASE ENZYMES
This application claims the benefit of U.S, Provisional Application Nos.
61/945,233 (filed February 27, 2014), 61/945,241 (filed February 27, 2014),
62/004,290 (filed May 29, 2014), 62/004,308 (filed May 29, 2014), 62/004,312
(filed May 29, 20141), 62/004,300 (filed May 29, 2014), 62/004,314 (filed May
29,
2014), and 62/004,305 (filed May 29, 2014), all of which are incorporated
herein
by reference in their entireties.
FIELD OF INVENTION
The invention is in the field of enzymatic hydrolysis of small sugar
polymers. Specifically, this invention pertains to hydrolyzing disaccharides
and
oligosaccharides comprising one or more alpha-1,5 glucosyl-fructose linkages
with an alpha-glucosidase enzyme.
REFERENCE TO SEQUENCE LISTING SUBMITTED ELECTRONICALLY
The official copy of the sequence listing is submitted electronically via
EFS-Web as an ASCII formatted sequence listing with a file named
CL6115USNP_SequenceListing_ST25.txt created on February 10, 2015, and
having a size of 266 kilobytes and is filed concurrently with the
specification. The
sequence listing contained in this ASCII-formatted document is part of the
specification and is herein incorporated by reference in its entirety.
BACKGROUND
Glucoamylases (EC 3.2.1,3, alpha-1,4-glucan glucohydrolase) are exo-
acting enzymes that catalyze hydrolysis of both alpha-1,4 and alpha-1,6
glycosidic linkages from non-reducing ends of glucose-containing di-, oligo-
and
poly-saccharides, releasing glucose units one at a time (1960, Pazur and Ando,
J. Biol. Chem. 235:297-302). Cleavage occurs at the glycosidic bond connecting
the anonieric carbon with oxygen (1962, Fleetwood and Weigel, Nature
196:984). Alpha-1,4, alpha-1,6, and alpha-1,3 bonds are the only linkages
hydrolyzed at significant rates by glucoamylase (1957, Barker et al., J. Chem.
Soc. 4865-4871).
1
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Glucoamylase is also capable of hydrolyzing the glycosidic bond between
the two glucosyl units linked by alpha-1,2 (e.g., Kojibiose) or alpha-1,1
(e.g.,
trehalose). However, this enzymatic activity occurs at a much lower rate and
at
more dilute substrate concentrations compared to glucoamylase activity toward
disaccharides with alpha-1,4 (maltose) or alpha-1,6 (isornaltose) linkages.
Glucoamylase has been widely used for producing high-glucose syrup
from starch. High-glucose syrup is useful as a feedstock for producing various
value-added compounds such as fuel alcohol, high-fructose corn syrup, organic
acids, amino acids and vitamins. Glucoamylase has been isolated from
numerous rnicroorganisms, animals and plants, and among microorganisrns,
many fungi are good sources of this enzyme. Glucoamylase produced in fungal
organisms, such as Aspergillus niger, is commonly used for commercial
applications such as high-glucose syrup production.
Transglucosidases (EC.2.4.1.24, 1,4-alpha-glucan 6-alpha-
glucosyltransferase) are D-glucosyltransferase enzymes that catalyze both
hydrolytic and transfer reactions on incubation with alpha-D-gluco-
oligosaccharides (1951, Pazur and French, J. Amer. Chem. Soc. 73:3536).
Maltose is the most preferred substrate for transglucosylation reactions with
this
enzyme. Transfer occurs most frequently to HO-6, producing isomaltose from D-
glucose, or panose (6-0-alpha-glucosyl maltose) from maltose.
Transglucosidase can also transfer a glucosyl residue to the HO-2 or HO-3 of
another D-glucosyl unit to form Kojibiose or Nigerose. This enzyme can further
transfer a D-glucosyl unit back to HO-4 to reform maltose.
As a result of transglucosylation reactions with transglucosidase, malto-
oligosaccharide residues are converted to isomalto-oligosaccharides (IMO)
containing a higher proportion of glucosyl residues linked by alpha-D-1,6
glycosidic linkages from the non-reducing end. IMO sugars are used in many
food and beverage formulations in Asia. Brier et al. (U.S. Patent Appl. Publ.
No.
2003/0167929) disclosed using transglucosidase to produce IMO from barley
wort.
2
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Poulose et al. (U.S. Patent Appl. Publ. No. 2008/0229514) disclosed using
transglucosidase to degrade polysaccharides such as xanthan and guar gums.
Xanthan gum comprises a cellulosic backbone in which alternate glucoses are
1,3-linked to branches containing mannose and glucuronic acid. The backbone
of guar gum comprises beta-1,4-linked mannose residues to which galactose
residues are alpha-1,6-linked at every other mannose.
Lantero et al. (U.S. Patent No. 5770437) disclosed using a
transglucosidase to degrade sucrose, melezitose and trehalulose. These sugars
comprise glucose linked to fructose via 1,2- (sucrose), 1,3- (melezitose), or
1,1-
(trehalulose) linkages.
Although various hydrolytic activities of glucoamylase and
transglucosidase have been disclosed, these enzymes are generally considered
to be alpha-glucosidases, given their ability to hydrolyze alpha-linkages
between
two glucosyl residues. For example, both glucoamylase and transglucosidase
are associated with having maltase activity (hydrolysis of the alpha-1,4
glycosidic
link between the two glucosyl residues of maltose), which is a type of alpha-
glucosidase activity.
Notwithstanding the foregoing disclosures, surprisingly, it has now been
found that alpha-glucosidases such as transglucosidase (EC 2.4.1.24),
glucoamylase (EC 3.2.1.3), and other alpha-glucosidases can hydrolyze alpha-
1,5 glycosidic linkage of glucosyl-fructose. Alpha-glucosidases are disclosed
herein as being useful for degrading disaccharides and oligosaccharides
containing glucosyl-alpha-1,5-fructose.
SUMMARY OF INVENTION
In one embodiment, the invention concerns a method of hydrolyzing an
alpha-1,5 glucosyl-fructose linkage in a saccharide comprising at least one
alpha-1,5 glucosyl-fructose linkage, wherein the saccharide is a disaccharide
or
oligosaccharide, and wherein the method comprises: contacting the saccharide
with an alpha-glucosidase enzyme under suitable conditions, wherein the alpha-
glucosidase enzyme hydrolyzes at least one alpha-1,5 glucosyl-fructose linkage
of the saccharide, and wherein the amount of the saccharide is reduced
3
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
compared to the amount of the saccharide that was present prior to the
contacting step.
In another embodiment, the alpha-glucosidase enzyme of the hydrolysis
method is immobilized.
In another embodiment, the saccharide of the hydrolysis method is
leucrose. In another embodiment, the concentration of leucrose after the
contacting step is less than 50% of the concentration of leucrose that was
present prior to the contacting step.
In another embodiment, the suitable conditions of the hydrolysis method
comprise (i) a glucan synthesis reaction, or (ii) a fraction obtained from the
glucan synthesis reaction; wherein the saccharide is a byproduct of the glucan
synthesis reaction. The glucan synthesis reaction produces at least one
insoluble alpha-glucan product in another embodiment. The fraction is a
filtrate
of the glucan synthesis reaction in another embodiment. In another embodiment,
the glucan synthesis reaction produces at least one soluble alpha-glucan
product
that is (I) a product of a glucosyltransferase, or (ii) a product of the
concerted
action of both a glucosyltransferase and an alpha-glucanohydrolase capable of
hydrolyzing glucan polymers having one or more alpha-1,3-glycosidic linkages
or
one or more alpha-1,6-glycosidic linkages. The fraction is a chromatographic
fraction of the glucan synthesis reaction in another embodiment in which the
glucan synthesis reaction produces at least one soluble alpha-glucan product.
In another embodiment, the alpha-glucosidase enzyme is a
transglucosidase or glucoarnylasein another embodiment, (i) the
transglucosidase comprises an amino acid sequence that is at least 90%
identical to SEQ ID NO:1; or (ii) the glucoamylase comprises an amino acid
sequence that is at least 90% identical to SEQ ID NO:2.
In another embodiment, the invention concerns a composition produced
by contacting a saccharide with an alpha-glucosidase enzyme, wherein the
saccharide is a disaccharide or oligosaccharide and comprises at least one
alpha-1,5 glucosyl-fructose linkage, wherein the alpha-glucosidase enzyme
hydrolyzes at least one alpha-1,5 glucosyl-fructose linkage of the saccharide,
4
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
and wherein the composition comprises a reduced amount of the saccharide
compared to the amount of the saccharide that was present prior to the
contacting step.
In another embodiment, the saccharide of the composition is leucrose.
The concentration of the leucrose in the composition is less than 50% of the
concentration of leucrose that was present prior to the contacting, for
example.
In another embodiment, the saccharide of the composition is in (i) a
glucan synthesis reaction, or (ii) a fraction obtained from the glucan
synthesis
reaction; wherein the saccharide is a byproduct of the glucan synthesis
reaction.
In another embodiment, the fraction is a filtrate of the glucan synthesis
reaction
or a chromatographic fraction of the glucan synthesis reaction.
In another embodiment, the invention concerns a method of enriching
fructose present in a fraction of a glucan synthesis reaction, comprising: (a)
contacting a fraction obtained from a glucan synthesis reaction with an alpha-
glucosidase enzyme under suitable conditions, wherein the alpha-glucosidase
enzyme hydrolyzes at least one alpha-1,5 glucosyl-fructose linkage of a
disaccharide or oligosaccharide comprised within the fraction; and (b)
separating
fructose from the hydrolyzed fraction of step (a) to obtain a composition
having a
higher concentration of fructose compared to the fructose concentration of the
fraction of step (a).
In another thirteenth embodiment, the invention concerns a fermentation
method comprising: (a) contacting a fraction obtained from a glucan synthesis
reaction with an alpha-glucosidase enzyme under suitable conditions, wherein
the alpha-glucosidase enzyme hydrolyzes at least one alpha-1,5 giucosyl-
fructose linkage of a disaccharide or oligosaccharide comprised within the
fraction; (b) fermenting the fraction of step (a) with a microbe to yield a
product,
wherein the fermenting can be performed after step (a) or simultaneously with
step (a); and (c) optionally, isolating the product of (b); wherein the yield
of the
product of (b) is increased compared to the product yield of fermenting a
fraction
of the glucan synthesis reaction that has not been contacted with the alpha-
glucosidase enzyme.
5
CA 02940778 2016-08-25
WO 2015/130883 PCT/US2015/017648
BRIEF DESCRIPTION OF THE DRAWINGS AND SEQUENCES
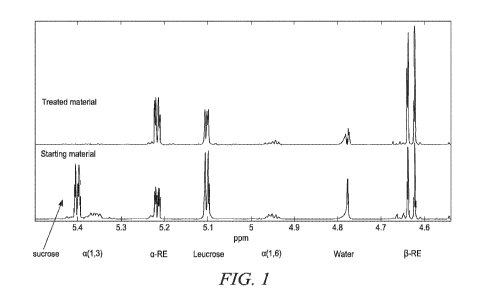
Figure I: 1H NMR spectra of glucan reaction filtrate material before (starting
material) and after (treated material) hydrolysis treatment with NOVO 188
enzyme (see Examples 2-3).
Figure 2: 1H NMR spectra of glucan reaction filtrate material before (starting
material) and after (treated material) hydrolysis treatment with TG L-2000
transglucosidase (see Examples 2-3).
Table I. Summary of Nucleic Acid and Protein Sequence Identification Numbers
Protein
Nucleic acid SEQ ID
Description SEQ ID NO. NO.
"TG L-2000", A. niger transglucosidase 1
(mature form without signal peptide) (965 aa)
"GC 321 Glucoamylase", T. reesei
glucoamylase (TrGA) (mature form 2
without signal peptide) (599 aa)
"gtfJ", Streptococcus safivarius
glucosyltransferase. The first 42 amino
acids of the protein are deleted
compared to GENBANK Identification
No. 47527; a start methionine is 3
included. (1477 aa)
"Aciglul", Aspergillus clavatus alpha-
glucosidase, full-length precursor form 4 5
including signal peptide. (3147 bases) (990 aa)
"AcIglul", Aspergillus clavatus alpha-
glucosidase, mature form lacking signal 6
peptide. (971 aa)
"Nfiglul", Neosartoiya fischeri alpha-
glucosidase, full-length precursor form 7 8
including signal peptide. (3158 bases) (988 aa)
"Nfiglul", Neosartoiya fischeri alpha-
glucosidase, mature form lacking signal 9
peptide. (969 aa)
"Ncrglul", Neurospora crassa alpha-
glucosidase, full-length precursor form 10 11
including signal peptide. (3385 bases) (1044 aa)
"Ncrgiul", Neurospora crassa alpha-
glucosidase, mature form lacking signal 12
peptide. (1022 aa)
6
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
"TauSec098", Rasamsonia composticola
alpha-glucosidase, full-length precursor 13 14
form including signal peptide. (3293 bases)
(1035 aa)
"TauSec098", Rasamsonia composticola
alpha-glucosidase, mature form lacking 15
signal peptide. (1013 aa)
"TauSec099", Rasamsonia composticola
alpha-glucosidase, full-length precursor 16 17
form including signal peptide. (3162 bases) (990 aa)
"TauSec099", Rasamsonia composticola
alpha-glucosidase, mature form lacking 18
signal peptide. (973 aa)
"Blo Bifidobacterium longum
(subsp. longum JD1301) alpha- 19 20
glucosidase (wild type). (1815 bases)
(604 aa)
"BloGlu1", Bifidobacterium longum
(subsp. longum JDM301) alpha- 21
glucosidase, codon-optimized sequence. (1812 bases)
"BloGlu2", Bifidobacterium longurn 22
alpha-glucosidase (wild type). (604 aa)
"BloGiu2", Bifidobacterium longum
alpha-glucosidase, codon-optimized
sequence encoding amino acid 23 24
sequence. (1812 bases)
(604 aa)
"BloGlu3", Bifidobacterium longum
(subsp. F8) alpha-glucosidase (wild 25 26
type) (1815 bases)
(604 aa)
"BloGlu3", Bifidobacterium longum
(subsp. F8) alpha-glucosidase, codon-
optimized sequence encoding amino 27
acid sequence. (1812 bases)
"BpsGlul", Bifidobacterium
pseudolongum alpha-glucosidase (wild 28
type). (585 aa)
"BpsGlul". Bifidobacterium
pseudolongum alpha-glucosidase,
codon-optimized sequence encoding 29 30
amino acid sequence. (1755 bases)
(586 aa)
"BthGlul", Bifidobacterium thermophilum 31 32
R8L.67 alpha-glucosidase (wild type). (1806 bases)
(601 aa)
"BthGlul", Bifidobacterium thermophilum
R8L67 alpha-glucosidase, codon- 33
optimized sequence. (1803 bases)
7
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
"BbrGiu2", Bifidobacterium breve alpha- 34
glucosidase (wild type). (662 aa)
"BbrGiu2", Bifidobacterium breve alpha-
glucosidase, codon-optimized sequence 35 36
encoding amino acid sequence. (1812
bases) (604 aa)
"BbrGlu5", Bifidobacterium breve ACS-
071-V-Sch8b alpha-glucosidase (wild 37 38
type). (1821
bases) (606 aa)
"BbrG1u5", Bifidobacterium breve ACS-
071-V-Sch8b alpha-glucosidase, codon- 39
optimized sequence. (1818 bases)
"Gtf-S", Streptococcus sp. C150
glucosyltransferase, GENBANK GI No. 40
321278321. (1570 aa)
"GTF0459", Streptococcus sp. C150
glucosyltransferase, N-terminal-
truncated version of GENBANK GI No. 41 42
321278321. (4179
bases) (1392 aa)
"Gtf-C", Streptococcus mutans MT-4239
glucosyltransferase, GENBANK GI No. 43
3130088. (1455 aa)
"GTF0088BsT1", Streptococcus mutans
MT-4239 glucosyltransferase, N- and C-
terminal-truncated version of GENBANK 44 45
GI No. 3130088. (2715
bases) (904 aa)
"MUT3325", Penicillium mameffei ATCC
18224 mutanase, GENBANK GI No. 46 47
212533325. (1308
bases) (435 aa)
DETAILED DESCRIPTION OF THE INVENTION
The disclosures of all cited patent and non-patent literature are
incorporated herein by reference in their entirety.
As used herein, the term "invention" or "disclosed invention" is not meant
to be limiting, but applies generally to any of the inventions defined in the
claims
or described herein. These terms are used interchangeably herein.
The terms "saccharide", "saccharide molecule" and "carbohydrate" are
used interchangeably herein and refer to a disaccharide or oligosaccharide,
'10 unless
otherwise noted. A "disaccharide" herein refers to a carbohydrate having
two monosaccharides joined by a glycosidic linkage. An "oligosaccharide"
herein
8
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
refers to a carbohydrate that consists of 2 to 9 monosaccharides, for example,
joined by glycosidic linkages. An oligosaccharide can also be referred to
herein
as an "oligomer". Monosaccharides that are comprised within a disaccharide or
oligosaccharide can be referred to as "monosaccharide units" or "monomeric
units", for example. Preferred monosaccharides herein are fructose and
glucose.
The terms "glycosidic linkage" and "glycosidic bond" are used
interchangeably herein and refer to the type of covalent bond that joins a
carbohydrate molecule to another carbohydrate molecule.
The terms "alpha-1,3 glucosyl-glucose linkage", "alpha-1,3 glucose-
glucose linkage" and "glucose-alpha 1,3-glucose" herein refers to an alpha-1,3-
glycosidic linkage between two alpha-D-glucose molecules. The terms "alpha-
1,6 glucosyl-glucose linkage", "alpha-1,6 glucose-glucose linkage" and
"glucose-
alpha 1,6-glucose" herein refers to an alpha-1,6-glycosidic linkage between
two
alpha-D-glucose molecules. Alpha-1,3 glucosyl-glucose linkage(s) and/or alpha-
1,6 glucosyl-glucose linkage(s) herein are comprised within a disaccharide or
oligosaccharide in certain embodiments.
The terms "alpha-1,5 glucosyl-fructose linkage", "alpha-1,5 glucose-
fructose linkage" and "glucose-alpha-1,5-fructose" herein refers to an alpha-
1,5-
glycosidic linkage between an alpha-D-glucose molecule and a fructose
molecule. An alpha-1,5 glucosyl-fructose linkage herein is comprised within a
disaccharide or oligosaccharide in certain embodiments.
"Alpha-D-glucose" herein can also be referred to as "glucose".
A disaccharide containing an alpha-1,5 glucosyl-fructose linkage is
referred to herein as leucrose. The terms "leucrose and "D-glucopyranosyl-
alpha(1-5)-D-fructopyranose" are used interchangeably herein. Leucrose has the
following structure:
r
Ho--\........
....,;....õ,...õ..t.sk.
HO, \
OH OH
0-- Z\AH
z
9
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
The terms "alpha-glucosidase", "alpha-1,4-glucosidase", and "alpha-D-
glucoside glucohydrolase" are used interchangeably herein. Alpha-glucosidases
(EC 3.2.1.20) ("EC" refers to Enzyme Commission number) have previously been
recognized as enzymes that catalyze hydrolytic release of terminal, non-
reducing
(1,4)-linked alpha-D-glucose residues frorn oligosaccharide (e.g.,
disaccharide)
and polysaccharide substrates. Alpha-glucosidases are now disclosed herein to
also have hydrolytic activity toward alpha-1,5 glucosyl-fructose linkages, and
hydrolytic activity toward alpha-1,3 and alpha-1,6 glucosyl-glucose linkages.
Transglucosidase and glucoamylase enzymes are examples of alpha-
glucosidases with such activity.
The terms "transglucosidase" (TG), "transglucosidase enzyme", and "1,4-
alpha-glucan 6-alpha-glucosyltransferase" are used interchangeably herein.
Transglucosidases (EC 2.4.1.24) have previously been recognized as D-
glucosyltransferase enzymes that catalyze both hydrolytic and transfer
reactions
on incubation with certain alpha-D-gluco-oligosaccharides. Transglucosidases
are now disclosed herein to also have hydrolytic activity toward alpha-1,5
glucosyl-fructose linkages, and hydrolytic activity toward alpha-1,3 and alpha-
1,6
glucosyl-glucose linkages.
The terrns "glucoamylase" (GA), "glucoarnylase enzyme", and "alpha-1,4-
glucan glucohydrolase" are used interchangeably herein. Glucoamylases (EC
3.2.1.3) have previously been recognized as exo-acting enzymes that catalyze
hydrolysis of both alpha-1,4 and alpha-1,6 glycosidic linkages from non-
reducing
ends of glucose-containing di-, oligo- and poly-saccharides. Glucoamylases are
now disclosed herein to also have hydrolytic activity toward alpha-1,5
glucosyl-
fructose linkages.
Enzymatic hydrolysis is a process in which an enzyme facilitates the
cleavage of bonds in molecules with the addition of the elements of water.
"Hydrolyzing", "hydrolysis of', or "hydrolytic activity toward" an alpha-1,5
glucosyl-
fructose linkage herein refers to enzymatic hydrolysis of the alpha-1,5
glycosidic
linkage between glucose and fructose by an alpha-glucosidase such as a
glucoamylase or transglucosidase. Such hydrolysis occurs when contacting a
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
disaccharide or oligosaccharide containing an alpha-1,5 glucosyl-fructose
linkage
with an alpha-glucosidase herein under suitable conditions. Thus, a
"hydrolysis
reaction" herein comprises at least (i) a disaccharide or oligosaccharide
containing an alpha-1,6 glucosyl-fructose linkage, and (ii) an alpha-
glucosidase.
The term "saccharification" herein refers to a process of breaking a
saccharide (disaccharide or oligosaccharide) into its monosaccharide
components. A saccharide can be saccharified in a hydrolysis reaction herein.
"Suitable conditions" for contacting a saccharide (disaccharide or
oligosaccharide) comprising at least one alpha-1,5 glucosyl-fructose linkage
with
an alpha-glucosidase herein refer to those conditions (e.g., temperature, pH,
time) that support the hydrolysis of one or more alpha-1,5 glucosyl-fructose
linkages in the saccharide by the alpha-glucosidase. Suitable conditions can
comprise "aqueous conditions", for example, comprising at least 20 wt% water.
Aqueous conditions may characterize a solution or mixture. The solution or
mixture in which a saccharide comprising at least one alpha-1,5 glucosyl-
fructose
linkage is contacted with an alpha-glucosidase can be referred to as an alpha-
glucosidase reaction, for example (e.g., a transglucosidase or glucoamylase
reaction).
An "immobilized" enzyme herein refers to an enzyme that is attached to an
inert, insoluble material. Methods for preparing immobilized enzymes are
disclosed, for example, in U.S. Patent No. 5541097, which is incorporated
herein
by reference.
The terms "glucan" and "glucan polymer" are used interchangeably herein
and refer to a polysaccharide of glucose monomers linked by glycosidic bonds.
An "alpha-glucan" herein refers to a glucan polymer comprising at least about
80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%,
94%, 96%, 96%, 97%, 98%, 99%, or 100% alpha-glycosidic linkages.
An "insoluble glucan" herein refers to a glucan polymer that is not soluble
in aqueous conditions. An example of insoluble glucan herein is poly alpha-1,3-
glucan with a DP of at least 8 or 9. A glucosyltransferase reaction in certain
11
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
embodiments as presently disclosed produces at least one insoluble glucan
product.
The terms "soluble glucan", "soluble alpha-glucan", "soluble fiber-, "soluble
glucan fiber", "soluble dietary fiber" and the like are used interchangeably
herein
to refer to a glucan polymer that is soluble in aqueous conditions. Examples
of
soluble glucan herein are certain oligosaccharides, such as poly alpha-1,3-
glucan with a DP less than 8, and certain oligosaccharides disclosed in the
Examples provided below. A glucosyltransferase reaction in certain
embodiments as presently disclosed produces at least one soluble glucan
product. Another set of features that characterizes soluble alpha-glucan
compounds in certain embodiments herein is that they are (i) water-soluble
glucose oligomers having a degree of polymerization of 3 or more, (ii)
digestion-
resistant (i.e., exhibit very slow or no digestibility) with little or no
absorption in
the human small intestine, and (iii) at least partially fermentable in the
lower
gastrointestinal tract. Digestibility of a soluble glucan fiber composition
can be
measured using AOAC method 2009.01, for example.
The terms "poly alpha-1,3-glucan" and "alpha-1,3-glucan polymer" are
used interchangeably herein. Poly alpha-1,3-glucan is a polymer comprising
glucose monomeric units linked together by glycosidic linkages, wherein at
least
about 50% of the glycosidic linkages are alpha-1,3-glycosidic linkages. The
term
"alpha-1,3-glycosidic linkage- as used herein refers to the type of covalent
bond
that joins alpha-D-glucose molecules to each other through carbons 1 and 3 on
adjacent alpha-D-glucose rings.
The "molecular weight" of a glucan herein can be represented as number-
average molecular weight (Mõ) or as weight-average molecular weight (Mw).
Alternatively, molecular weight can be represented as Daltons, grams/mole, DPw
(weight average degree of polymerization), or DPõ (number average degree of
polymerization). Various means are known in the art for calculating these
molecular weight measurements such as with high-pressure liquid
chromatography (HPLC), size exclusion chromatography (SEC), or gel
permeation chromatography (GPC).
12
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
The terms "glucosyltransferase enzyme", "gtf enzyme", "gtf enzyme
catalyst", "gtf", "glucansucrase" and the like are used interchangeably
herein.
The activity of a gtf enzyme herein catalyzes the reaction of sucrose
substrate to
make the products glucan and fructose. Other products (byproducts) of a gtf
reaction can include glucose (results from when glucose is hydrolyzed from the
glucosyl-gtf enzyme intermediate complex), various soluble oligosaccharides
(e.g., DP2-DP7), and leucrose (results from when glucose of the glucosyl-gtf
enzyme intermediate complex is linked to fructose). Wild type forms of
glucosyltransferase enzymes generally contain (in the N-terminal to C-terminal
direction) a signal peptide, a variable domain, a catalytic domain, and a
glucan-
binding domain. A glucosyltransferase herein is classified under the glycoside
hydrolase family 70 (GH70) according to the CAZy (Carbohydrate-Active
EnZymes) database (Cantarel et al., Nucleic Acids Res. 37:D233-238, 2009).
The term "sucrose" herein refers to a non-reducing disaccharide
composed of an alpha-D-glucose molecule and a beta-D-fructose molecule linked
by an alpha-1,2-glycosidic bond. Sucrose is known commonly as table sugar.
The terms "glucan synthesis reaction", "glucan reaction" "gtf reaction" and
the like are used interchangeably herein and refer to a reaction that is
performed
by a glucosyltransferase enzyme. A glucan synthesis reaction as used herein
generally refers to a solution comprising at least one active
glucosyltransferase
enzyme in a solution comprising sucrose and water, and optionally other
components. Other components that can be in a glucan synthesis reaction
herein include fructose, glucose, leucrose, soluble oligosaccharides (e.g.,
DP2-
DP7), and soluble glucan product(s), for example. Also, one or more alpha-
glucanohydrolase enzymes can be comprised in a glucan synthesis reaction in
some aspects. It would be understood that certain glucan products, such as
poly
alpha-1,3-glucan with a degree of polymerization (DP) of at least 8 or 9, are
water-insoluble and thus are not dissolved in a glucan synthesis reaction, but
rather may be present out of solution.
The terms "alpha-glucanohydrolase" and "glucanohydrolase" are used
interchangeably herein and refer to an enzyme capable of hydrolyzing an alpha-
13
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
glucan oligomer. An alpha-glucanohydrolase can be defined by its
endohydrolysis activity towards certain alpha-D-glycosidic linkages. Examples
of
alpha-glucanohydrolase enzymes herein include dextranases (EC 3.2.1.11;
capable of endohydrolyzing alpha-1,6-linked glycosidic bonds), mutanases (EC
3.2.1.59; capable of endohydrolyzing alpha-1,3-linked glycosidic bonds), and
altemanases (EC 3.2.1.-; capable of endohydrolytically cleaving alternan).
Various factors including, but not limited to, level of branching, the type of
branching, and the relative branch length within certain alpha-glucans may
adversely impact the ability of an alpha-glucanohydrolase to endohydrolyze
some glycosidic linkages.
The "percent dry solids" of a glucan synthesis reaction refers to the wt% of
all the sugars in a glucan synthesis reaction. The percent dry solids of a gtf
reaction can be calculated, for example, based on the amount of sucrose used
to
prepare the reaction.
A "fraction" of a glucan synthesis reaction herein refers to a liquid solution
portion of a glucan synthesis reaction. A fraction can be a portion of, or all
of, the
liquid solution from a glucan synthesis reaction, and has been separated from
a
soluble or insoluble glucan product synthesized in the reaction. A fraction
can
optionally be referred to as a "mother liquor" in embodiments in which the
product
is an insoluble (solid) glucan product. An example of a fraction is a filtrate
of a
glucan synthesis reaction. Since a fraction can contain dissolved sugars such
as
sucrose, fructose, glucose, leucrose, soluble oligosaccharides (e.g., DP2-
DP7), a
fraction can also be referred to as a "mixed sugar solution" derived from a
glucan
synthesis reaction. A "hydrolyzed fraction" herein refers to a fraction that
has
been treated with an alpha-glucosidase herein to hydrolyze leucrose and/or
oligosaccharides present in the fraction.
The terms "filtrate", "glucan reaction filtrate", "glucan filtrate" and the
like
are used interchangeably herein and refer to a fraction that has been filtered
away from a solid glucan product synthesized in a glucan synthesis reaction. A
"hydrolyzed filtrate" herein refers to a filtrate that has been treated with
an alpha-
14
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
glucosidase herein to hydrolyze leucrose and/or oligosaccharides present in
the
filtrate.
The terms "percent by volume", "volume percent", "vol %", "v/v %- and the
like are used interchangeably herein. The percent by volume of a solute in a
solution can be determined using the formula: [(volume of solute)/(volume of
solution)] x 100%.
The terms "percent by weight", "weight percentage (wt%)", "weight-weight
percentage (% w/w)" and the like are used interchangeably herein. Percent by
weight refers to the percentage of a material on a mass basis as it is
comprised
in a composition, mixture, or solution. All percentages herein are weight
percentages, unless otherwise noted.
As used herein, "polydispersity index", "FD t", "heterogeneity index",
"dispersity" and the like refer to a measure of the distribution of molecular
mass
in a given polymer (e.g., a glucose oligomer such as a soluble alpha-glucan)
sample and can be calculated by dividing the weight average molecular weight
by the number average molecular weight (PEA= Mw/Mn).
The terms "increased", "enhanced" and "improved" are used
interchangeably herein. These terms refer to a greater quantity or activity
such
as a quantity or activity slightly greater than the original quantity or
activity, or a
quantity or activity in large excess compared to the original quantity or
activity,
and including all quantities or activities in between. Alternatively, these
terms
may refer to, for example, a quantity or activity that is at least 1%, 2%, 3%,
4%,
5%, 6%, 7%, 8%, 9%, 10%, 11%, 12%, 13%, 14%, 15%, 16%, 17%, 18%, 19%
or 20% more than the quantity or activity for which the increased quantity or
activity is being compared.
The terms "sequence identity" or "identity" as used herein with respect to
polynucleotide or polypeptide sequences refer to the nucleic acid bases or
amino
acid residues in two sequences that are the same when aligned for maximum
correspondence over a specified comparison window. Thus, "percentage of
sequence identity" or "percent identity" refers to the value determined by
comparing two optimally aligned sequences over a comparison window, wherein
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
the portion of the polynucleotide or polypeptide sequence in the comparison
window may comprise additions or deletions (i.e., gaps) as compared to the
reference sequence (which does not comprise additions or deletions) for
optimal
alignment of the two sequences. The percentage is calculated by determining
the number of positions at which the identical nucleic acid base or amino acid
residue occurs in both sequences to yield the number of matched positions,
dividing the number of matched positions by the total number of positions in
the
window of comparison and multiplying the results by 100 to yield the
percentage
of sequence identity.
The Basic Local Alignment Search Tool (BLAST) algorithm, which is
available online at the National Center for Biotechnology Information (NCBI)
website, may be used, for example, to measure percent identity between or
among two or more of the polynucleotide sequences (BLASTN algorithm) or
polypeptide sequences (BLASTP algorithm) disclosed herein. Alternatively,
percent identity between sequences may be performed using a Clustal
algorithm (e.g., ClustalW or ClustalV). For multiple alignments using a
Clustal
method of alignment, the default values may correspond to GAP PENALTY=10
and GAP LENGTH PENALTY=10. Default parameters for paimise alignments
and calculation of percent identity of protein sequences using a Clustal
method
may be KTUPLE=1, GAP PENALTY=3, WINDOW=5 and DIAGONALS
SAVED=5. For nucleic acids, these parameters may be KTUPLE=2, GAP
PENALTY=5, WINDOW=4 and DIAGONALS SAVED=4. Alternatively still,
percent identity between sequences may be performed using an EMBOSS
algorithm (e.g., needle) with parameters such as GAP OPEN=10, GAP
EXTEND=0.5, END GAP PENALTY=false, END GAP OPEN=10, END GAP
EXTEND=0.5 using a BLOSUM matrix (e.g., BLOSUM62).
Various polypeptide amino acid sequences are disclosed herein as
features of certain embodiments. Variants of these sequences that are at least
about 70-85%, 85-90%, or 90%-95% identical to the sequences disclosed herein
can be used. Alternatively, a variant amino acid sequence can have at least
70%, 71%, 72%, 73%, 74%, 75%, 76%, 77%, 78%, 79%, 80%, 81%, 82%, 83%,
16
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
84%, 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%,
98% or 99% identity with a sequence disclosed herein. A variant amino acid
sequence herein has the same function/activity of a disclosed sequence, or at
least about 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%,
97%, 98%, or 99% of the function/activity of a disclosed sequence.
The term "isolated" as used in certain embodiments refers to any cellular
component that is completely separated from its native source (e.g., an
isolated
polynucleotide or polypeptide molecule). In some instances, an isolated
polynucleotide or polypeptide molecule is part of a greater composition,
buffer
system or reagent mix. For example, the isolated polynucleotide or polypeptide
molecule can be comprised within a cell or organism in a heterologous manner.
Another example is an isolated alpha-glucosidase (e.g., glucoamylase,
transglucosidase), or glucosyltransferase enzyme. The enzyme reactions (e.g.,
alpha-glucosidase reaction, glucosyltransferase reaction) disclosed herein are
synthetic, non-naturally occurring processes.
Embodiments of the disclosed invention concern a method of hydrolyzing
an alpha-1,5 glucosyl-fructose linkage in a saccharide comprising at least one
alpha-1,5 glucosyl-fructose linkage. The saccharide is a disaccharide or
oligosaccharide. This method comprises contacting the saccharide with an
alpha-glucosidase enzyme under suitable conditions. In the contacting step,
the
alpha-glucosidase enzyme hydrolyzes at least one alpha-1,5 glucosyl-fructose
linkage of the saccharide. Due to this hydrolysis, the amount of the
saccharide is
reduced compared to the amount of the saccharide that was present prior to the
contacting step. Thus, this hydrolysis method can alternatively be referred to
as
a method of reducing the amount of a saccharide in a composition.
Significantly, it is believed to be previously unknown that alpha-
glucosidase enzymes can hydrolyze alpha-1,5 glucosyl-fructose linkages. Alpha-
glucosidase reactions following this hydrolysis method can thus be used to
remove leucrose and other oligosaccharide byproducts containing alpha-1,5
17
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
glucosyl-fructose linkages from a glucan synthesis reaction and/or a fraction
obtained therefrom. Such removal represents an improvement over chemical
processes of byproduct removal, such as acid hydrolysis, which can result in
degradation of glucan product. Finally, a glucan reaction fraction that is
treated
according to the above hydrolysis rnethod is better-suited for downstream
applications such as fermentation, for example, since the level of glucose and
fructose monosaccharides is increased in the fraction. Monosaccharides are
generally more tractable for downstream processes compared to leucrose and
oligosaccharide byproducts.
An alpha-glucosidase (EC 3.2.1.20) is used in embodiments herein to
hydrolyze an alpha-1,5 glucosyl-fructose linkage in a saccharide comprising at
least one alpha-1,5 glucosyl-fructose linkage. Alpha-glucosidase enzymes have
previously been recognized to catalyze hydrolytic release of terminal, non-
reducing (1,4)-linked alpha-D-glucose residues from oligosaccharide (e.g.,
disaccharide) and polysaccharide substrates. These enzymes are now disclosed
herein to also have hydrolytic activity toward alpha-1,5 glucosyl-fructose
linkages, for example.
An alpha-glucosidase can be from any source (e.g., plant, animal, microbe
such as a bacteria or fungus/yeast), for example, such as those sources
disclosed below from which a transglucosidase and/or glucoamylase can be
derived. For example, an alpha-glucosidase can be a fungal alpha-glucosidase.
Other examples of suitable alpha-glucosidases herein include those disclosed
in
U.S. Patent Nos. 6355467, 5922580, 5795766, 5763252, and 8633006, which
are all incorporated herein by reference.
An alpha-glucosidase enzyme in certain embodiments herein may
comprise the amino acid sequence of SEC) ID NO:5, 6, 8, 9, 11, 12, 14, 15, 17,
18, 20, 22, 24, 26, 28, 30, 32, 34, 36, 38, or that of DIAZYME RDF ULTRA
(DuPont Industrial Biosciences). Alternatively, an alpha-glucosidase enzyme
rnay comprise an amino acid sequence that is at least about 90%, 91%, 92%,
93%, 94%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID NO:5, 6, 8, 9, 11,
18
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
12, 14, 15, 17, 18, 20, 22, 24, 26, 28, 30, 32, 34, 36, 38, or to the amino
acid
sequence of DIAZYME RIX' ULTRA, and have hydrolytic activity toward alpha-
1,5 glucosyl-fructose linkages in saccharides. Several of the foregoing
sequences, for example, are mature alpha-glucosidases that lack an N-terminal
signal peptide. For such sequences, it would be understood that an N-terniinal
start-methionine would typically be added (if necessary) (directly or via an
intervening heterologous amino acid sequence such as an epitope) if expressing
it without using a signal peptide (such as with an expression system where the
enzyme is expressed intracellularly and obtained from a cell lysate).
A transglucosidase (EC 2.4.1.24; 1,4-alpha-glucan 6-alpha-
glucasyltransferase) can be used in certain embodiments herein as an alpha-
glucosidase to hydrolyze an alpha-1,5 glucosyl-fructose linkage in a
saccharide
comprising at least one alpha-1,5 glucosyl-fructose linkage. This class of
enzymes has previously been recognized as D-glucosyltransferase enzymes that
catalyze hydrolytic and transfer reactions on incubation with certain alpha-D-
gluco-oligosaccharides. Transglucosidases as now disclosed herein also have
hydrolytic activity toward alpha-1,5 glucosyl-fructose linkages.
A transglucosidase enzyme herein may be derived from any microbial
source, such as a bacteria or fungus. Examples of fungal transglucosidases
include, but are not limited to, those of Trichoderma species (e.g., T.
reesei),
Aspergillus species and Neosartorya species (e.g., N. fischeri). Examples of
Aspergillus species from which a transglucosidase may be derived include, but
are not limited to, A. niger, A. awamori, A. oryzae, A. terreus, A. clavatus,
A.
fumigatus and A. nidulans. Other examples of transglucosidase enzymes useful
herein are described in Barker et al. (1953, J. Chem. Soc. 3588-3593); Pazur
et
al. (1986, Carbohydr. Res. 149:137-147), Nakamura et al. (1997, J. Biotechnol.
53:75-84), and U.S. Patent Appl. Publ. No. 2008/0229514, all of which are
incorporated herein by reference. Still other examples of transglucosidase
enzymes useful herein are those that are thermostable; U.S. Patent No.
4689296, which is incorporated herein by reference, discloses a process for
producing thermostable transglucosidase. Yet more examples of
19
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
transglucosidase enzymes useful herein may be any of those in the GENBANK
database (NCBI), such as accession numbers: D45356 (GID:2645159, A. niger),
BAD06006.1 (GID:4031328, A. awarnori), BAA08125.1 (GID:1054565, A.
oryzae), XP_001210809.1 (GID:115492363, A. terreus), XP_001216899.1
(GID:115433524, A. terreus), XP_001271891.1 (GID:121707620, A. clavatus),
XP 751811.1 (GID:70993928, A. furnigatus), XP 659621.1 (GID:67523121, A.
nidulans). XP 001266999.1 (GID:119500484, N. fischeri) and XP 001258585.1
(GID:119473371, N. fischeri), which are all incorporated herein by reference.
Alternatively, a transglucosidase herein may have an amino acid sequence that
is at least 90% or 95% identical with the amino acid sequence of any of the
foregoing disclosed transglucosidase sequences, and have hydrolytic activity
toward alpha-1,5 glucosyl-fructose linkages in saccharides. All of the
foregoing
transglucosidases, when used in a hydrolysis reaction herein, are preferably
in a
mature form lacking an N-terminal signal peptide.
A transglucosidase enzyme in certain embodiments herein may comprise
the amino acid sequence of SEQ ID NO:1 (Transglucosidase L-2000), which is
an A. niger transglucosidase (U.S. Patent Appl. Publ. No. 2008/0229514).
Alternatively, a transglucosidase may comprise an amino acid sequence that is
at least about 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99%
identical to SEQ ID NO:1 and have hydrolytic activity toward alpha-1,5
glucosyl-
fructose linkages in saccharides. Any of SEQ ID NO:1 or variants thereof can
be
produced following the disclosure of U.S, Patent Appl. Publ. No. 2008/0229514,
for example, which is incorporated herein by reference. SEQ ID NO:1 is a
mature transglucosidase that lacks an N-terminal signal peptide. Since SEQ ID
NO:1 does not begin with a methionine residue, it would be understood that an
N-terminal start-methionine would typically be added to SEQ ID NO:1 (directly
or
via an intervening heterologous amino acid sequence such as an epitope) if
expressing it without using a signal peptide (such as with an expression
system
where the enzyme is expressed intracellularly and obtained from a cell
lysate).
A glucoarnylase (EC 3.2.1.3; alpha-1,4-glucan glucohydrolase) can be
used in certain embodiments herein as an alpha-glucosidase to hydrolyze an
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
alpha-1,5 glucosyl-fructose linkage in a saccharide comprising at least one
alpha-1,5 glucosyl-fructose linkage. This class of enzymes has previously been
recognized as exo-acting enzymes that catalyze hydrolysis of both alpha-1,4
and
alpha-1,6 glycosidic linkages from non-reducing ends of glucose-containing di-
,
oligo- arid poly-saccharides. Glucoamylases as now disclosed herein also have
hydrolytic activity toward alpha-1,5 glucosyl-fructose linkages. In certain
embodiments, an alpha-glucosidase is not a glucoamylase.
A glucoamylase enzyme herein may be derived from any microbial
source, such as a bacteria or fungus. Examples of bacterial glucoamylases
include, but are riot limited to, those of Bacillus species (e.g., B.
alkalophilus, B.
amyloliquefaciens, B. lentus, B. licheniformis, B. stearothermophilus, B.
subtilis,
B. thuringiensis) arid Streptomyces species (e.g., S. !Widens). Examples of
fungal glucoamylases include, but are not limited to, those of Trichoderma
species (e.g., T. reesei, T. longibrachiatum, T. strictipilis, T. asperellum,
T.
konilangbra. T. hazianum), Aspergillus species (e.g., A. niger, A. oryzae. A.
terreus, A. clavatus, A. nidulans, A. kawachi, A. awamori), Rhizopus species
(e.g., R. oryzae, R. niveus), Talaromyces species (e.g., T. emersonii, T.
thermophilus, T. duponti), Mucor species, Hypocrea species (e.g., H.
gelatinosa,
H. orientalis, H. vinosa, H. citrina), Fusariurn species (e.g., F. oxysporum,
F.
roseurn, F. venenatum), Neurospora species (e.g., N. crassa), Humicola species
(e.g.. H. grisea, H. insolens, H. lanuginose), Penicillium species (e.g., P.
notatum, P. chtysogenum) and Saccharomycopsis species (e.g., S. fibuligera).
Examples of these bacterial and fungal glucoamylases for use herein are
disclosed in U.S. Pat. Appl. Publ. No. 2013/0102035, which is incorporated
herein by reference. Other examples of glucoamylase enzymes useful herein
are described in Svensson et al. (1983, Carlsberg Res. Commun. 48:529-544),
Boel et al. (1984, EMBO J. 3:1097-1102); Hayashida et al. (1989, Agric. Biol.
Chem. 53:923-929); U.S. Pat. No. 5024941, U.S. Pat. No. 4794175, U.S. Pat.
No. 4247637, U.S. Pat. No. 6255084, U.S. Pat. No. 6620924, Ashikari et al,
(1986, Agric. Biol. Chem. 50:957-964), Ashikari et al. (1989, Appl. Microbic!.
Biotechnol. 32:129-133), U.S. Pat. No. 4863864; U.S. Pat. No. 4618579,
21
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Houghton-Larsen et al. (2003, Appl. Microbiol. Biotechnot. 62:210-217) and
U.S.
Pat. No. 7413887, all of which are incorporated herein by reference.
Alternatively, a glucoamylase herein may have an amino acid sequence that is
at
least 90% or 95% identical with the amino acid sequence of any of the
foregoing
disclosed glucoamylase sequences, and have hydrolytic activity toward alpha-
1,5
glucosyl-fructose linkages in saccharides. All of the foregoing glucoamylases,
when used in a hydrolysis reaction herein, are preferably in a mature form
lacking an N-terminal signal peptide. Commercially available glucoamylases
useful herein include OPTIDEX L-400, GC 147, GC 321, G ZYME G990 4X,
OPTIMAX 7525, DEXTROZYME, DISTILLASE and GLUCZYME, for example.
A glucoamylase enzyme in certain embodiments herein may comprise the
amino acid sequence of SEQ ID NO:2 (GC 321), which is a T. reesei
glucoamylase. Alternatively, a glucoamylase may comprise an amino acid
sequence that is at least about 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%,
98%, or 99% identical to SEQ ID NO:2 and have hydrolytic activity toward alpha-
1,5 glucosyl-fructose linkages in saccharides. Any of SEQ ID NO:2 or variants
thereof can be produced following the disclosures of U.S. Pat. No. 7413887 or
U.S. Pat. Appl. Publ. Na. 2013/0102035, for example, which are incorporated
herein by reference. SEQ. ID NO:2 is a mature glucoamylase that lacks an N-
terminal signal peptide. Since SEQ ID NO:2 does not begin with a methionine
residue, it would be understood that an N-terniinal start-methionine would
typically be added to SEQ ID NO:2 (directly or via an intervening heterologous
amino acid sequence such as an epitope) if expressing it without using a
signal
peptide (such as with an expression system where the enzyme is expressed
intracellularly and obtained from a cell lysate).
An alpha-glucosidase enzyme herein such as a transglucosidase or
glucoamylase may be from a commercial source (e.g., DuPont Industrial
Biosciences / Genencor, USA; Megazyme International, Ireland; Amano Enzyme
Inc., Japan). Alternatively, such an enzyme may be produced by any means
known in the art, such as described in U.S. Pat. Appl. Publ. No. 2008/0229514,
U.S. Pat. No. 7413887 or U.S. Pat. Appl. Publ. No. 2013/0102035, which are
22
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
incorporated herein by reference. For example, an alpha-glucosidase may be
produced recombinantly in a heterologous expression system, such as a
rnicrobial or fungal heterologous expression system. Examples of heterologous
expression systems include bacterial (e.g., E. coli, Bacillus sp.) and
eukaryotic
systems. Eukaryotic systems can employ yeast (e.g., Pichia sp.,
Saccharomyces sp.) or fungal (e.g., Trichoderma sp. such as T. reesei,
Aspergillus species such as A. niger) expression systems, for example. The
transglucosidase of SEQ ID NO:1 and glucoamylase of SEQ ID NO:2, and
variants thereof, can be expressed in a T. reesei host, for example.
An alpha-glucosidase enzyme when used in a hydrolysis reaction herein is
preferably in a mature form lacking an N-terminal signal peptide. An
expression
system for producing a mature alpha-glucosidase enzyme herein rnay ernploy an
enzyme-encoding polynucleotide that further comprises sequence encoding an
N-terminal signal peptide to direct extra-cellular secretion. The signal
peptide in
such embodiments is cleaved from the enzyme during the secretion process.
The signal peptide may either be native or heterologous to the
transglucosidase
or glucoamylase. Alternatively, an alpha-glucosidase enzyme in a mature form
can be provided by expressing it without using a signal peptide, such as with
an
expression systern where the enzyme is expressed intracellularly and obtained
from a cell lysate. In either scenario (secretion or intracellularly
expressed), a
heterologous amino acid sequence such as an epitope can optionally be included
at the N-terminus of the alpha-glucosidase.
An alpha-glucosidase enzyme in certain embodiments may be provided in
a hydrolysis reaction herein by direct use of a cell that expresses the
enzyme(s).
In other words, an alpha-glucosidase that is contacted with a saccharide can
be
present by virtue of its expression from a cell placed in the suitable
conditions for
hydrolysis. Such a cell could thus be used in place of adding an isolated
alpha-
glucosidase preparation to the hydrolysis reaction. A cell for this purpose
can be
a bacterial, yeast, or fungal cell, for example. Examples of yeast include
those
from the genera Saccharomyces (e.g., S. cerevisiae), Kluyveromyces, Candida,
Pichia, Schizosaccharomyces, Hansenufa, Kloeckera, and Schwanniomyces.
23
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Other expression systems useful herein are disclosed in U.S. Patent. Appl.
Publ.
No. 2013/0323822, which is incorporated herein by reference.
A saccharide herein comprises at least one alpha-1,5 glucosyl-fructose
linkage. Thus, depending on the length of the saccharide, it may contain 1, 2,
3,
4, 5, 6, 7, or 8 alpha-1,5 glucosyl-fructose linkages, for example. A
saccharide
preferably contains 1, 2, or 3 linkages of this type.
Since a saccharide herein comprises at least one alpha-1,5 glucosyl-
fructose linkage, the saccharide comprises at least one glucose unit and at
least
one fructose unit. In certain embodiments, a saccharide herein comprises only
glucose and fructose units. Such a composition may characterize the
disaccharide and oligosaccharide byproducts of a glucan synthesis reaction.
Alternatively, a saccharide herein may contain other monosaccharides in
addition
to glucose and fructose, such as galactose, ribose and xylose.
A saccharide hydrolyzed in certain embodiments of the disclosed
invention can be an oligosaccharide. An oligosaccharide herein can have, for
example, 2, 3, 4, 5, 6, 7, 8, or 9 monosaccharide units. As would be
understood
in the art, an oligosaccharide herein can be referenced with respect to its
degree
of polymerization (DP) number, which specifies the number of monomeric units
in
the oligosaccharide. A DP3 oligosaccharide has 3 monomeric units, for example.
Thus, the oligosaccharide can be a DP3, DP4, DP5, DP6, DP7, DP8, or DP9
oligosaccharide, for example. The DP of a saccharide in certain embodiments is
3 to 7 (i.e., DP 3-7).
An oligosaccharide herein with 3 or more monosaccharide units can
comprise other linkages in addition to at least one alpha-1,5 glucosyl-
fructose
linkage (note that an oligosaccharide with 2 monosaccharide units ¨ i.e., a
disaccharide ¨ is leucrose given that a saccharide in a hydrolysis method
herein
has at least one alpha-1,5 glucosyl-fructose linkage). For example, there may
also be alpha-1,3, alpha-1,6, and/or alpha-1,4 linkages in the
oligosaccharide,
which are also susceptible to hydrolysis by alpha-glucosidases as shown
herein.
24
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
An oligosaccharide in certain embodiments comprises only glucose
monomers linked by alpha-1,3 and/or alpha-1,6 glycosidic linkages. Thus, such
oligosaccharides comprise only alpha-1,3 glucosyl-glucose and/or alpha-1,6
glucosyl-glucose linkages. Examples of such an oligosaccharide contain only
alpha-1,3 linkages or alpha-1,6 linkages. An oligosaccharide can comprise at
least 85%, 86%, 87%, 88%, 89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%,
98%, or 99% glucosyl-glucose linkages in certain embodiments. In other
embodiments, there can be about 75-85% alpha-1,3 glucosyl-glucose linkages
and about 15-25% alpha-1,6 glucosyl-glucose linkages in oligosaccharides
herein. Alternatively, oligosaccharides herein can comprise any percentage
(any
integer value between 1% and 99%) of alpha-1,3 glucosyl-glucose linkages and
any percentage (any integer value between 1% and 99%) of alpha-1,6 glucosyl-
glucose linkages, just so long that the total of these percentages is not
greater
than 100%. Any of these oligosaccharides can be in a fraction from a glucan
synthesis reaction that produces (i) an insoluble alpha-glucan (e.g., poly
alpha-
1,3-glucan), or (ii) a soluble alpha-glucan product, for example. This linkage
content can characterize (i) each oligosaccharide individually, or (ii) a
group of
oligosaccharides (i.e., average linkage content). Oligosaccharides comprising
only glucose rnonomers linked by alpha-1,3 and/or alpha-1,6 glycosidic
linkages
can be DP2-[)P7, or DP3-DP7, for example. It should be understood that the
exact distribution of linkages in oligosaccharides can vary depending on the
conditions of the glucan synthesis reaction (e.g., gtf enzyme) producing
oligosaccharide byproducts. It should further be understood that the exact
linkage distribution is not critical to the presently disclosed methods.
The Examples herein demonstrate that alpha-glucosidases (e.g.,
transglucosidase and glucoamylase enzymes) can hydrolyze both (i) leucrose,
which comprises an alpha-1,5 glucosyl-fructose linkage, and (ii)
oligosaccharides
comprising only alpha-1,3 glucosyl-glucose and/or alpha-1,6 glucosyl-glucose
linkages. Therefore, an alpha-glucosidase can be used, for example, in a
reaction for hydrolyzing alpha-1,5 glucosyl-fructose linkages, alpha-1,3
glucosyl-
glucose linkages and/or alpha-1,6 glucosyl-glucose linkages.
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
At least one alpha-1,5 glucosyl-fructose linkage in a saccharide herein can
be hydrolyzed by an alpha-glucosidase herein. Alternatively, it is believed
that 2,
3, 4, 5, or more alpha-1,5 glucosyl-fructose linkages in a saccharide can be
hydrolyzed by an alpha-glucosidase, for example. Hydrolysis of at least one
alpha-1,5 glucosyl-fructose linkage can occur at the non-reducing-end of the
saccharide in certain embodiments. For example, where the saccharide is the
disaccharide, leucrose, the non-reducing end glucose is cleaved from fructose
yielding free glucose and fructose. As another example, where the saccharide
is
an oligosaccharide with a non-reducing end glucose that is alpha-1,5-linked to
fructose, it is believed that this glucose can be cleaved off, leaving a
fructose
residue at the non-reducing end of the oligosaccharide.
The amount of a saccharide is reduced in the disclosed hydrolysis method
compared to the amount of the saccharide that was present prior to the
contacting step. This reduction results from hydrolytic cleavage of at least
one
alpha-1,5 glucosyl-fructose linkage in the saccharide. The amount (e.g.,
concentration) of a saccharide after the contacting step in a hydrolysis
method
herein can be less than about 1%, 2%, 3%, 4%, 5%, 10%, 20%, 30%, 40%, 50%,
60%, 70%, 80%, or 90% (or any integer value between 1% and 90%) of the
amount of the saccharide that was present prior to the contacting step (prior
to
contacting an alpha-glucosidase herein with a saccharide under suitable
conditions).
A saccharide hydrolyzed in certain embodiments of the disclosed
invention is leucrose, which is a disaccharide with an alpha-1,5 glucosyl-
fructose
linkage. The concentration of leucrose after the contacting step in a
hydrolysis
method herein can be less than about 1%, 2%, 3%, 4%, 5%, 10%, 20%, 30%,
40%, 50%, 60%, 70%, 80%, or 90% (or any integer value between 1% and 90%)
of the concentration of leucrose that was present prior to the contacting step
(prior to contacting an alpha-glucosidase herein with leucrose under suitable
conditions). A hydrolysis method in some aspects herein can alternatively be
referred to as a method of reducing the amount of a leucrose in a composition.
26
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Leucrose can be contacted with a transglucosidase such as one
comprising SEQ ID NO:1 (Transglucosidase L-2000), for example, in a hydrolysis
method herein. The concentration of leucrose after completing such a method
can be less than about 1-3% of the original leucrose concentration in certain
embodiments.
The amount of a saccharide is reduced in the disclosed hydrolysis method
compared to the amount of the saccharide that was present prior to the
contacting step. It would be understood that such a comparison can be made in
any number of ways. For example, the saccharide concentration can be
measured both before and after performing the hydrolysis method.
Alternatively,
the comparison can be made with respect to a control reaction having the same
conditions, except that no alpha-glucosidase as presently disclosed is added
to
the control reaction.
An alpha-glucosidase in certain embodiments herein may be immobilized.
The enzyme may be immobilized using any method and/or means known in the
art, such as those disclosed in U.S. at. Nos. 5541097 and 4713333, both of
which are incorporated herein by reference. For example, one or more enzymes
can be immobilized by contacting the enzyme(s) with a solution of an amine-
reactive material (e.g., glutaraldehyde) to form an adduct (e.g., enzyme-
glutaraldehyde adduct), after which the adduct is bonded to a solid carrier
that
has been treated with a polyamine (e.g., a polyethylenimine such as EPOMIN P-
1050).
A solid carrier (solid support) to which an alpha-glucosidase enzyme can
be immobilized in certain embodiments can be an inorganic or organic material.
Such materials include, for example, gamma-alumina, titania, activated
granular
carbon, granular diatomaceous earth, glass beads, porous glass, pumice-stone,
silica gel, metal oxide and aluminum oxide.
A polyamine can be used to treat a solid carrier such that subsequent
exposure of the solid carrier to an adduct comprising an enzyme and amine-
reactive material leads to attachment of the enzyme to the solid carrier.
27
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Examples of polyamines useful herein include polyethylenediamine, a
polyethylenimine (e.g., polydiethylenetriamine, polytriethylenetetramine,
polypentaethylenehexamine, polyhexarnethylenediamine),
polymethylenedicyclohexylamine, polymethylenedianiline,
polytetraethylenepentamine, polyphenylenediarnine and blends of two or more of
these polyamine compounds. Preferred polyamines are water-soluble and/or
have a molecular weight of about from 500 to 100,000 Da!tons. A
polyethylenimine such as EPOMIN P-1050 can be used in certain embodiments.
An amine-reactive material useful for preparing an adduct comprising an
enzyme herein can be, for example, an aldehyde, organic halide, anhydride, azo
compound, isothiocyanate, and/or isocyanate. Examples of these amine-reactive
rnaterials include glutaraldehyde, succindialdehyde, terephthaldehyde, bis-
diazobenzidine-2,2'-disulfonic acid, 4,4'-difluoro-3,3'-
dinitrodiphenylsulfone,
dipheny1-4,4'-dithiocyanate-2,2'-disulfonic acid, 3-methoxydiphenylmethane-
4,4'-
diisocyanate, toluene-2-isocyanate-4-isothiocyanate, toluene-2,-4-
diisothiocyanate, diazobenzidine, diazobenzidine-3,3'-dianisidine, N,W-
hexamethylene bisiodoacetamide, hexamethylene diisocyanate, cyanuric
chloride, and/or 1,5-difluoro-2,4-dinitrobenzene. Preferably, the amine-
reactive
material is an aldehyde such as glutaraldehyde.
An alpha-glucosidase enzyme adducted with an amine-reactive compound
can be contacted with a polyarnine-treated solid carrier, thereby immobilizing
the
enzyme onto the solid carrier. An immobilized enzyme herein can be employed
in various reactor systems, such as in a column (e.g., packed column) or
stirred
tank reactor, for performing hydrolysis reaction as disclosed herein.
Suitable conditions for contacting a saccharide herein with an alpha-
glucosidase herein (e.g., transglucosidase or glucoamylase) are those
conditions
that support the hydrolysis of one or more alpha-1,5 glucosyl-fructose
linkages in
the saccharide by the alpha-glucosidase. Examples of suitable conditions are
disclosed in the below Examples. Conditions (e.g., ternperature, pH, time) for
contacting an alpha-glucosidase herein with a sugar substrate are also
disclosed
28
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
in U.S. Pat. Appl. Publ. No. 2008/0229514, U.S. Pat. No. 7413887 and U.S. Pat.
Appl. Publ. No. 2013/0102035 (all of which are incorporated herein by
reference),
and may also be applicable to the disclosed hydrolysis method.
The disaccharides and oligosaccharides in the disclosed hydrolysis
method are typically soluble in water or an aqueous solution. Thus, contacting
a
saccharide herein with an alpha-glucosidase is preferably performed under
suitable conditions that are aqueous, in which the saccharide is dissolved.
Aqueous conditions can characterize a solution or mixture comprising at least
about 20 wt% water. Alternatively, aqueous conditions herein are at least
about
20, 30, 40, 50, 60, 70, 80, 85, 90, or 95 wt% water (or any integer value
between
and 95 wt%), for example. Aqueous conditions can further comprise a buffer,
for example, such as an acidic, neutral, or alkaline buffer, at a suitable
concentration and selected based on the pH range provided by the buffer.
Examples of buffers/buffering agents include citrate, acetate (e.g., sodium
15 acetate), KH2PO4, MOPS, CHES, borate, sodium carbonate, and sodium
bicarbonate.
The pH of a hydrolysis reaction herein can be about 3.0 to 9.0, for
example. Hydrolysis reaction pH can be, for example, about 3.0, 3.5, 4.0, 4.5,
5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, or 9Ø Alternatively, the pH can be
about 4-5.
20 Techniques for setting pH include the use of buffers, alkalis, and/or
acids, for
example, and are well known in the art.
The temperature of a hydrolysis reaction herein can be about 20 'C to
about 80 C, for example. Hydrolysis reaction temperature can be, for example,
about 20, 30, 40, 50, 60, 70, or 80 C (or any integer value between 20 and 80
C). A hydrolysis temperature of about 60 C, 65 C, or 60-65 C is preferred
in
certain embodiments.
A hydrolysis reaction herein can be performed for a period of at least
about 10 minutes to about 90 hours, for example. The time of a hydrolysis
reaction can be, for example, at least about 0.5, 1, 2, 3, 4, 8, 12, 16, 20,
24, 30,
36, 42, 48, 54, 60, 66, 72, 78, 84, or 90 hours (or any integer value between
0.5
and 72 hours). In certain embodiments, such as for hydrolyzing leucrose, a
29
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
hydrolysis reaction can be performed in less than 4 hours (e.g., 0.5-4 hours)
for
example. The time period required to achieve a desired level of hydrolysis
will
vary on the exact conditions used, and would be understood by one skilled in
the
art. For example, increasing the amount of enzyme added to a reaction or
immobilized on a solid support used in a reaction will reduce the contact
time.
One or more of alpha-glucosidase enzymes herein may be used in a
hydrolysis reaction in certain embodiments. Both a transglucosidase and
glucoamylase can be used in a reaction, for example. The amount of an alpha-
glucosidase in a hydrolysis reaction herein can be plus/minus 10% to 20% (or
5% to 10%) from any of the amounts used in the Examples below (e.g., Exarnple
2), for example. Alternatively, about 0.1-0.5 vol% or 0.1-1.0 vol% of alpha-
glucosidase can be used in a hydrolysis reaction. Alternatively still, an
alpha-
glucosidase herein can be used at about, or at least about, 1, 2, 3, 4, 5, 6,
7, 8, 9,
10, 11, 12, 13, 14, or 15 ppm in a hydrolysis reaction. A transglucosidase
unit
(TGU) can be defined as the amount of a transglucosidase enzyme that will
produce one micromole of panose per minute under the conditions of the
following assay, for example. Transglucosidase activity can be assayed as
follows, for example: a transglucosidase is brought up in 100 mM sodium
acetate buffer, pH 4.5, containing 4 rnM para-nitrophenyl-alpha-glucoside and
1
mg/m1 bovine serum albumin (BSA). After 30 min incubation at 30 C, the
reaction is terminated by the addition of an equal volurne 1 M sodium
carbonate
and 0D405 is recorded. A glucoamylase unit (GAU) can be defined, for example,
as the amount of glucoamylase enzyme that will produce 1 g of reducing sugar
calculated as glucose per hour from a soluble starch substrate (4% DS [degree
of substitution]) at pH 4.2 and 60 C.
The initial concentration of a saccharide in a hydrolysis reaction in certain
embodiments of the disclosed invention can be about 1 wt% to 50 wt%, for
example. For example, the concentration of leucrose can be about 5, 10, 15,
20,
25, 30, 35, or 40 wt% (or any integer value between 5 and 40 wt%). As another
example, the concentration of one or more oligosaccharides (e.g., DP2, DP3,
DP4, DP2-DP7, DP3-DP7) in a hydrolysis reaction herein can be about 1, 2, 3,
4,
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
5, 6, 7, 8, 9, 10, 11, 12, 13, 14, or 15 wt%. Those skilled in the art would
recognize that the concentration of total sugars (which includes disaccharides
and oligosaccharides) can have an impact on the activity of alpha-glucosidase
enzymes; preferred concentrations of total sugars in a hydrolysis reaction to
maximize enzyme activity can be less than 50 wt% dry solids (DS), with a most
preferred concentration of 20-35 wt% DS in some aspects.
Suitable conditions in certain embodiments for contacting a saccharide
with an alpha-glucosidase herein can comprise (i) a glucan synthesis reaction,
or
(ii) a fraction obtained from a glucan synthesis reaction, where the
saccharide is
a byproduct of the glucan synthesis reaction. In other words, a hydrolysis
reaction herein may be conducted in the context of a glucan synthesis reaction
or
a fraction of a glucan synthesis reaction, though it is typically conducted in
the
latter. A glucan synthesis reaction herein can produce one or more insoluble
and/or soluble alpha-glucan products, for example. Thus, a glucan synthesis
reaction can be characterized in some embodiments herein as an "alpha-glucan
synthesis reaction".
A glucan synthesis reaction generally refers to a solution comprising at
least sucrose, water and one active glucosyltransferase enzyme, and optionally
other components. Other components that can be in a glucan synthesis reaction
include fructose, glucose, leucrose, soluble oligosaccharides (e.g., DP2-DP7),
and soluble glucan product(s). Also, one or more alpha-glucanohydrolase
enzymes can be comprised in a glucan synthesis reaction in some aspects. It
would be understood that certain glucan products, such as poly alpha-1,3-
glucan
with a DP of at least 8 or 9, may be water-insoluble and thus are not
dissolved in
a glucan synthesis reaction, but rather may be present out of solution. Thus,
a
glucan produced by glucan synthesis reaction herein can be insoluble. An alpha-
glucosidase enzyme herein can be added to a glucan synthesis reaction at any
stage thereof, such as during initial preparation of the reaction or when the
reaction is near (e.g., 80 to 90% complete) or at completion, where the latter
two
time points are preferred.
31
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
A glucan synthesis reaction herein may be one that, in addition to
producing a glucan product, produces byproducts such as leucrose and/or
soluble oligosaccharides. A glucan in some aspects is a poly alpha-glucan.
Thus, a glucan synthesis reaction herein can be for producing poly alpha-1,3-
glucan or mutan, for example, which are typically co-produced with at least
leucrose and/or oligosaccharide byproducts in a glucan synthesis reaction.
A glucan synthesis reaction in certain embodiments comprises a
glucosyltransferase enzyme that produces a poly alpha-glucan such as poly
alpha-1,3-glucan. Examples of such glucosyltransferase enzymes useful herein
are disclosed in U.S. Pat. No. 7000000, and U.S. Pat. Appl. Publ. Nos.
2013/0244288, 2013/0244287 and 2014/0087431 (all of which are incorporated
herein by reference.
A glucosyltransferase enzyme herein may be derived from any microbial
source, such as a bacteria or fungus. Examples of bacterial
glucosyltransferase
enzymes are those derived from a Streptococcus species, Leuconostoc species
or Lactobacillus species. Examples of Streptococcus species include S.
salivarius, S. sobrinus, S. dentirousetti, S. downei, S. mutans, S. rails, S.
gallolyticus and S. sanguinis. Examples of Leuconostoc species include L.
rnesenteroides, L. amelibiosurn, L. argentinurn, L. camosum, L. citreum, L.
cremoris, L. dextranicum and L. fructosum. Examples of Lactobacillus species
include L. acidophilus, L. delbrueckii, L. helveticus, L. salivarius, L.
casei, L.
curvatus, L. plantarum, L. sakei, L. brevis, L. buchneri, L. fermentum and L.
reuteri.
A glucosyltransferase enzyme herein can be primer-independent or
primer-dependent. Primer-independent glucosyltransferase enzymes do not
require the presence of a primer to perform glucan synthesis. A primer-
dependent glucosyltransferase enzyme requires the presence of an initiating
molecule in the reaction solution to act as a primer for the enzyme during
glucan
polymer synthesis. The term "primer" as used herein refers to any molecule
that
can act as the initiator for a glucosyltransferase enzyme. Primers that can be
used in certain embodiments include dextran and other carbohydrate-based
32
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
primers, such as hydrolyzed glucan, for example. U.S. Appl. Publ. No.
2013/0244287, which is incorporated herein by reference, discloses preparation
of hydrolyzed glucan using poly alpha-1,3-glucan as the starting material.
Dextran for use as a primer can be dextran T10 (i.e., dextran having a
molecular
weight of 10 kD), for example.
A glucosyltransferase enzyme for a glucan synthesis reaction herein may
be produced by any means known in the art. For example, a glucosyltransferase
enzyme may be produced recombinantly in a heterologous expression system,
such as a microbial heterologous expression system. Examples of heterologous
expression systems include bacterial (e.g., E. co/isuch as TOP10 or MG1655;
Bacillus sp.) and eukaryotic (e.g., yeasts such as Pichia sp. and
Saccharomyces
sp.) expression systems.
A glucosyltransferase enzyme described herein may be used in any
purification state (e.g., pure or non-pure). For example, a
glucosyltransferase
enzyme may be purified and/or isolated prior to its use. Examples of
glucosyltransferase enzymes that are non-pure include those in the form of a
cell
lysate. A cell lysate or extract may be prepared from a bacteria (e.g., E.
roll)
used to heterologously express the enzyme. For example, the bacteria may be
subjected to disruption using a French pressure cell. In alternative
embodiments,
bacteria may be homogenized with a homogenizer (e.g., APV, Rannie, Gaulin).
A glucosyltransferase enzyme is typically soluble in these types of
preparations.
A bacterial cell lysate, extract, or homogenate herein may be used at about
0.15-
(13% (v/v), for example, in a reaction solution for producing a poly alpha-
glucan
such as poly alpha-1,3-glucan from sucrose.
The temperature of a glucan synthesis reaction herein can be controlled, if
desired. In certain embodiments, the temperature of the reaction is between
about 5 C to about 50 'C. The temperature in certain other embodiments is
between about 20 'C to about 40 C.
The initial concentration of sucrose in a glucan synthesis reaction herein
can be about 20 g/L to about 400 g/L, for example. Alternatively, the initial
concentration of sucrose can be about 75 g/L to about 175 g/L, or from about
50
33
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
WI_ to about 150 g/L. Alternatively still, the initial concentration of
sucrose can be
about 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 140, 150, or 160 g/L (or any
integer value between 40 and 160 WO, for example. "Initial concentration of
sucrose" refers to the sucrose concentration in a gtf reaction solution just
after all
the reaction solution components have been added (at least water, sucrose, gif
enzyme).
Sucrose used in a glucan synthesis reaction herein can be highly pure (.?..
99.5%) or be of any other purity or grade. For example, sucrose can have a
purity of at least 99.0%, or can be reagent grade sucrose. As another example,
incornpletely refined sucrose can be used. Incompletely refined sucrose herein
refers to sucrose that has not been processed to white refined sucrose. Thus,
incompletely refined sucrose can be cornpletely unrefined or partially
refined.
Examples of unrefined sucrose are "raw sucrose" ("raw sugar") and solutions
thereof. Examples of partially refined sucrose have not gone through one, two,
three, or more crystallization steps. The ICUMSA (International Commission for
Uniform Methods of Sugar Analysis) of incompletely refined sucrose herein can
be greater than 150, for example. Sucrose herein may be derived from any
renewable sugar source such as sugar cane, sugar beets, cassava, sweet
sorghum, or corn. Suitable forms of sucrose useful herein are crystalline form
or
non-crystalline form (e.g., syrup, cane juice, beet juice), for example.
Additional
suitable forms of incompletely refined sucrose are disclosed in U.S. Appl. No.
61/969,958.
[Methods of determining ICUMSA values for sucrose are well known in the
art and disclosed by the International Commission for Uniform Methods of Sugar
Analysis in ICUMSA Methods of Sugar Analysis: Official and Tentative Methods
Recommended by the International Commission for Uniform Methods of Sugar
Analysis (ICUMSA) (Ed. H.C.S. de Whalley, Elsevier Pub. Co., 1964), for
example, which is incorporated herein by reference. ICUMSA can be measured,
for example, by ICUMSA Method GS1/3-7 as described by 1-Z,J. McCowage, R.M.
Urquhart and M.L. Burge (Determination of the Solution Colour of Raw Sugars,
34
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Brown Suciars and Coloured Syrups at pH 7.0 - Official, Verlag Dr Albert
Bartens, 2011 revision), which is incorporated herein by reference.
The pH of a glucan synthesis reaction in certain embodiments can be
between about 4.0 to about 8Ø Alternatively, the pH can be about 4.0, 4.5,
5.0,
5.5, 6.0, 6.5, 7.0, 7.5, or 8Ø The pH can be adjusted or controlled by the
addition or incorporation of a suitable buffer, including but not limited to:
phosphate, tris, citrate, or a combination thereof. Buffer concentration in a
glucan synthesis reaction can be from 0 mM to about 100 mM, or about 10, 20,
or 50 mM, for example.
Poly alpha-1,3-glucan produced in a glucan synthesis reaction herein may
have at least about 50%, 60%, 70%, 80%, 90%, 95%, 96%, 97%, 98%, 99%, or
100% (or any integer value between 50% and 100%) glycosidic linkages that are
alpha-1,3. In such embodiments, accordingly, the poly alpha-1,3-glucan has
less
than about 50%, 40%, 30%, 20%, 10%, 5%, 4%, 3%, 2%, 1%, or 0% (or any
integer value between 0% and 50%) of glycosidic linkages that are not alpha-
1,3.
Poly alpha-1,3-glucan herein preferably has a backbone that is
linear/unbranched. In certain embodiments, the poly alpha-1,3-glucan has no
branch points or less than about 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, or 1%
branch points as a percent of the glycosidic linkages in the polymer. Examples
of branch points include alpha-1,6 branch points.
The molecular weight of poly alpha-1,3-glucan produced in a glucan
synthesis reaction herein can be measured as number-average molecular weight
(M,) or weight-average molecular weight (Mw). Alternatively, molecular weight
can be measured in Da!tons or grams/mole. It may also be useful to refer to
the
DP,, (weight average degree of polymerization) or DP, (number average degree
of polymerization) of the poly alpha-1,3-glucan polymer.
The M, or Mw, of poly alpha-1,3-glucan herein may be at least about 1000.
Alternatively, the Mõ or Mw can be at least about 1000 to about 600000 (or any
integer value between 1000 and 600000), for example. Alternatively still, poly
alpha-1 ,3-glucan in can have a molecular weight in DPõ or DP w of at least
about
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
100, or of at least about 100 to 1000 (or any integer value between 100 and
1000).
A fraction of a glucan synthesis reaction may constitute suitable conditions
=for contacting a saccharide with an alpha-glucosidase as presently disclosed.
A
fraction can be a portion of, or all of, the liquid solution from a glucan
synthesis
reaction. Typically, a fraction has been separated from soluble or insoluble
glucan product(s) synthesized in the reaction. For example, a fraction can be
separated from one or more glucan products that are insoluble in water (e.g.,
poly alpha-1,3-glucan) which fall out of solution during their synthesis. A
=fraction
in certain preferred embodiments of the present disclosure is from a poly
alpha-
1,3-glucan synthesis reaction.
The volume of a fraction (before optionally diluting or concentrating the
fraction, see below) in certain embodiments can be at least about 10%, 20%,
30%, 40%, 50%, 60%, 70%, 80%, or 90% (or any integer value between 10%
and 90%) of the volume of the glucan synthesis reaction from which it is
obtained. Typically, in glucan synthesis reactions producing an insoluble
glucan
(e.g., poly alpha-1,3-glucan), the fraction will be a portion of (not all of)
the liquid
solution component of the reaction. A fraction can be obtained at any stage of
a
glucan synthesis reaction, but is preferably obtained near (e.g., greater than
80
or 90% complete) or after completion of the reaction.
Examples of a fraction of a glucan synthesis reaction in certain
embodiments include filtrates and supernatants. Thus, in those embodiments in
which an insoluble glucan product is synthesized, a fraction herein can be
obtained (separated) from a glucan synthesis reaction using a funnel, filter
(e.g.,
press filter), centrifuge, or any other method or equipment known in the art
that
allows removal of some or all liquids from solids. Filtration can be by
gravity,
vacuum, or press filtration, =for example. Filtration preferably removes all
or most
of an insoluble glucan; any filter material (e.g., filter paper) with an
average pore
size (e.g., ¨40-50 micron) sufficient to remove solids from liquids can be
used. A
fraction typically retains all or most of its dissolved components, such as
36
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
byproducts of the glucan synthesis reaction. Leucrose is a preferred
saccharide
in a filtrate herein.
A fraction herein can optionally be diluted or concentrated, if desired.
Concentration of a fraction can be performed using any other method or
equipment known in the art suitable for concentrating a solution. For example,
a
fraction can be concentrated by evaporation, such as with a rotary evaporator
(e.g., set at a temperature of about 40-50 C). A fraction in some aspects
herein
can be concentrated down to a volume that is about 75%, 80%, 85%, 90%, or
95% of the original fraction volume. A concentrated fraction (e.g.,
concentrated
=filtrate) can optionally be referred to as a syrup.
A fraction in some aspects can comprise water that replaces the water
that was present in the composition from which the fraction was obtained. For
example, saccharide byproduct(s) from a glucan synthesis reaction can be
separated in certain chromatographic methods in which the original solvent is
replaced with another solvent (e.g.; saccharide byproducts that are bound to a
column [thus removed from the original solvent] can be eluted into a new
solvent).
A fraction in some aspects may be treated in a manner to have any of the
suitable conditions (e.g., temperature, pH and time) disclosed above for
contacting a saccharide with an alpha-glucosidase. For example, a fraction can
be modified to have a pH of about 4 to 5 before an alpha-glucosidase is added
to
the fraction. As another example, the temperature of a hydrolysis reaction
with a
fraction can be about 55-65 C (e.g., about 60 c'C). A fraction that has been
concentrated down to a syrup can be used in a hydrolysis reaction in yet
another
example.
A fraction in certain preferred embodiments herein is from a poly alpha-
1,3-glucan synthesis reaction; such a fraction is preferably a filtrate. A
fraction of
a poly alpha-1,3-glucan synthesis reaction herein comprises at least water,
fructose and one or more types of saccharide (leucrose andior oligosaccharides
such as DP2-DP7). Other components that may be in this type of fraction
include sucrose (i.e., residual sucrose not consumed in the gtf reaction), one
or
37
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
more gtf enzymes, glucose, buffer, salts, FermaSure , borates, sodium
hydroxide, hydrochloric acid, cell lysate components, proteins and/or nucleic
acids, for example. Minimally, the components of a fraction from a poly alpha-
1,3-glucan synthesis reaction include water, fructose, glucose, one or more
types
of saccharide (leucrose and/or oligosaccharides such as DP2-DP7), and
optionally sucrose, for example. It would be understood that the composition
of a
fraction depends, in part, on the conditions of the glucan synthesis reaction
from
which the fraction is obtained. In those fractions containing one or more gtf
enzymes, it is preferable that such one or more gtf enzymes are deactivated
(e.g., heat-deactivated) before using the fraction in a hydrolysis reaction
herein.
It should be understood that the exact distribution of sugar byproducts
produced via polyrnerization of sucrose in a glucan synthesis reaction can
vary
based on the reaction conditions and gtf enzyme used, especially on
temperature
and sucrose concentration. It should also be understood that the exact
composition of sugars in a fraction of a glucan synthesis reaction is not
critical to
the disclosed hydrolysis process. Generally, as the amount of sucrose is
increased, the selectivity of the reaction towards both leucrose and
oligosaccharides will increase. Conversely, as the temperature increases, the
selectivity of the reaction towards leucrose tends to decrease, while the
selectivity towards oligosaccharides is largely unaffected. It should also be
understood that the ratio of sugars to water, i.e., wt% dry solids (DS), which
is
calculated by dividing the mass of sugar to total solution weight, can be
adjusted
either by evaporating water, preferably at temperatures below 50 'IC under
vacuum, or addition of water, without significant impact to the relative
distribution
of sugars in a fraction of a glucan synthesis reaction. It is also possible to
increase the percentage of sucrose in a fraction by stopping the gtf reaction
before complete conversion (to glucan) is achieved, either by reducing the pH
below the active range for the gtf enzyme or by thermal deactivation of the
gtf
enzyme.
38
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
In certain embodiments, a glucan synthesis reaction herein can produce
one or more soluble alpha-glucan products. A soluble alpha-glucan product
("soluble fiber-, alternatively) can be (i) a direct product of a
glucosyltransferase,
or (ii) a product of the concerted action of both a glucosyltransferase and an
alpha-glucanohydrolase capable of hydrolyzing glucan polymers having one or
more alpha-1,3-glycosidic linkages or one or more alpha-1,6-glycosidic
linkages.
A soluble alpha-glucan herein can comprise, for example:
a) at least 75% alpha-1,3-glycosidic linkages;
b) less than 25% alpha-1,6-glycosidic linkages;
c) less than 10% alpha-1,3,6-glycosidic linkages;
d) an Mw of less than 5000 Da!tons;
e) a viscosity of less than 0.25 Pascal second (Pa.$) at 12 wt% in
water at 20 C;
f) a dextrose equivalence (DE) in the range of 4 to 40;
g) a digestibility of less than 10% as measured by the Association of
Analytical Communities (AOAC) method 2009.01;
h) a solubility of at least 20% (w/w) in pH 7 water at 25 C; and
i) a polydispersity index (PDI) of less than 5.
Such a soluble alpha-glucan can be produced as disclosed in U.S. Appl.
No. 62/004,290.
As an example, a soluble alpha-glucan fiber composition can comprise at
least 75%, preferably at least 80%, more preferably at least 85%, even more
preferably at least 90%, and most preferably at least 95% alpha-(1,3)
glycosidic
linkages.
As another example, in addition to the alpha-(1,3) glycosidic linkage
embodiments described above, a soluble alpha-glucan fiber composition can
further comprise less than 25%, preferably less than 10%, more preferably 5%
or
less, and even more preferably less than 1% alpha-(1,6) glycosidic linkages.
As another example, in addition to the alpha-(1,3) and alpha-(1,6)
glycosidic linkage content embodiments described above, a soluble alpha-glucan
39
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
fiber composition can further comprise less than 10%, preferably less than 5%,
and most preferably less than 2.5% alpha-(1,3,6) glycosidic linkages.
As another example, a soluble alpha-glucan fiber composition can
comprise 93 to 97% alpha-(1,3) glycosidic linkages and less than 3% alpha-
(1,6)
glycosidic linkages and has a weight-average molecular weight corresponding to
a DP of 3 to 7 mixture. In a further embodiment, a soluble alpha-glucan fiber
composition can comprise about 95% alpha-(1,3) glycosidic linkages and about
1% alpha-(1,6) glycosidic linkages and has a weight-average molecular weight
corresponding to a DP of 3 to 7 mixture. In a further aspect of the above
ernbodirnent, a soluble alpha-glucan fiber composition can further comprise 1
to
3% alpha-(1,3,6) linkages or preferably about 2 % alpha-(1,3,6) linkages.
As another example, in addition to the above-mentioned glycosidic linkage
content embodiments, a soluble alpha-glucan fiber composition can further
comprise less than 5%, preferably less than 1 %, and most preferably less than
0.5 9/0 alpha-(1,4) glycosidic linkages.
As another example, in addition the above-mentioned glycosidic linkage
content embodiments, a soluble alpha-glucan fiber composition can comprise a
weight average molecular weight (N/1) of less than 5000 Daltons, preferably
less
than 2500 Daltons, more preferably between 500 and 2500 Daltons, and most
preferably about 500 to about 2000 Daltons.
As another example, in addition to any of the above features, a soluble
alpha-glucan fiber composition can comprise a viscosity of less than 250
Gentipoise (0.25 Pa's), preferably less than 10 GP (0.01 Pas), preferably less
than 7 cP (0.007 Pa's), more preferably less than 5 cP (0.005 Pa's), more
preferably less than 4 GP (0.004 Pas), and most preferably less than 3 cP
(0.003
Pa-s) at 12 wt% in water at 20 'C.
A soluble alpha-glucan fiber composition can have, in certain
embodiments, a digestibility of less than 10%, or preferably less than 9%, 8%,
7%, 6%, 5%, 4%, 3%, 2%, or 1% digestibility as measured by the Association of
Analytical Communities (AOAC) method 2009.0'1. In another aspect, the relative
level of digestibility may alternatively be determined using AOAC 2011.25
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
(Integrated Total Dietary Fiber Assay) (McCleary et al., 2012, J. AOAC Int.,
95
(3), 824-844).
In addition to any of the above embodiments, a soluble alpha-glucan fiber
composition can have a solubility of at least 20% ( w/w), preferably at least
30%,
40%, 50%, 60%, or 70% in pH 7 water at 25 C.
In one embodiment, a soluble alpha-glucan fiber composition can
comprise a reducing sugar content of less than 10 wt%, preferably less than 5
wt%, and most preferably 1 wt% or less.
In one embodiment, a soluble alpha-glucan fiber composition can
comprise a caloric content of less than 4 kcal/g, preferably less than 3
kcal/g,
more preferably less than 2.5 kcal/g, and most preferably about 2 kcal/g or
less.
As another example, a soluble alpha-glucan herein can comprise:
a) 10% to 30% alpha-1,3-glycosidic linkages;
b) 65% to 87% alpha-1,6-glycosidic linkages;
c) less than 5% alpha-1,3,6-glycosidic linkages;
d) a weight average molecular weight (Mw) of less than 5000 Daltons;
e) a viscosity of less than 0.25 Pascal second (Pa.'s) at 12 wt% in
water at 20 C;
f) a dextrose equivalence (DE) in the range of 4 to 40, preferably 10
to 40;
g) a digestibility of less than 10% as measured by the Association of
Analytical Communities (AOAC) method 2009.01;
h) a solubility of at least 20% (w/w) in pH 7 water at 25 C; and
i) a polydispersity index (PDI) of less than 5.
Such a soluble alpha-glucan can be produced as disclosed in U.S. Appl.
No. 62/004,308.
As another example, a soluble alpha-glucan herein can comprise:
a) 25-35 alpha-1,3-glycosidic linkages;
b) 55-75% alpha-1,6-glycosidic linkages;
c) 5-15% alpha-1,3,6-glycosidic linkages;
d) a weight average molecular weight of less than 5000 Daltons ;
41
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
e) a viscosity of less than 0.25 Pascal second (Pa.$) at 12 wt% in
water at 20 C;
f) a dextrose equivalence (DE) in the range of 4 to 40;
g) a digestibility of less than 10% as measured by the Association of
Analytical Communities (AOAC) method 2009.01;
h) a solubility of at least 20% (w/w) in water at 25 C; and
i) a polydispersity index of less than 5.
Such a soluble alpha-glucan can be produced as disclosed in U.S. Appl.
No. 62/004,312.
As another example, a soluble alpha-glucan herein can comprise:
a) at least 95% alpha-1,6-glycosidic linkages;
b) 1% or less alpha-1,3-glycosidic linkages;
c) less than 2% alpha-1,3,6-glycosidic linkages;
d) less than 1.5% alpha-1,4-glycosidic linkages;
e) a weight average molecular weight of less than 20000 Daltons;
f) a viscosity of less than 0.25 Pascal second (Pa.$) at 12 wt% in
water at 20 C;
g) a dextrose equivalence (DE) in the range of 1 to 30;
h) a digestibility of less than 10% as measured by the Association of
Analytical Communities (AOAC) method 2009.01;
i) a solubility of at least 20% (w/w) in pH 7 water at 25 C; and
j) a polydispersity index of less than 5.
Such a soluble alpha-glucan can be produced as disclosed in U.S. Appl.
No. 62/004,314.
As another example, a soluble alpha-glucan herein can comprise:
a) a range of:
i) 1% to 50% of alpha-1,3-glycosidic linkages; or
íì) greater than 10% but less than 40 % alpha-1,4-glycosidic
linkages; or
iii) any combination of i) and ii);
b) 1 to 50% alpha-1,2-glycosidic linkages;
42
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
c) 0-25% alpha-1,3,6-glycosidic linkages;
d) less than 98% alpha-1,6-glycosidic linkages;
e) a weight average molecular weight of less than 300 kDa:
f) a viscosity of less than 0.25 Pascal second (Pass) at 12 wt% in
water at 20 C;
g) a digestibility of less than 20% as measured by the Association of
Analytical Communities (AOAC) method 2009.01;
h) a solubility of at least 20% (w/w) in pH 7 water at 25 C; and
i) a polydispersity index of less than 26, preferably less than 5.
Such a soluble alpha-glucan can be produced as disclosed in U.S. Appl.
No. 62/004,305.
In certain embodiments, a soluble alpha-glucan is a direct product of a
glucosyltransferase. Such a glucosyltransferase, and conditions for use
thereof
in a suitable glucan synthesis reaction, can be as disclosed herein, or as
disclosed in any of U.S. Patent Appl. Nos. 62/004,290, 62/004,308, 62/004,312,
62/004,314, and/or 62/004,305, for example.
A soluble alpha-glucan can alternatively be a product, for example, of the
concerted action of both a glucosyltransferase and an alpha-glucanohydrolase
that is capable of hydrolyzing glucan polymers having one or more alpha-1,3-
glycosidic linkages or one or more alpha-1,6-glycosidic linkages. In some
aspects, a glucan synthesis reaction for producing a soluble alpha-glucan
product can comprise both at least one glucosyltransferase and at least one
alpha-glucanohydrolase. In other aspects, a glucan synthesis reaction can
initially comprise one or more glucosyltransferases as the only enzyme
component(s). Such a reaction produces a first alpha-glucan product that has
not yet been subject to modification by an alpha-glucanohydrolase. Then, at
least one alpha-glucanohydrolase is added to the reaction for a suitable
period of
time to allow modification of the first product to a soluble alpha-glucan
product.
Thus, there are different ways by which to synthesize a soluble alpha-glucan
product through the concerted action of both a glucosyltransferase and an
alpha-
glucanohydrolase. Conditions for performing a glucan synthesis reaction in
43
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
which one or more alpha-glucanohydrolase enzymes are included during glucan
synthesis reaction and/or after glucan synthesis can be as disclosed herein,
or as
disclosed in any of U.S. Patent Appl. Nos. 62/004,290, 62/004,308, 62/004,312,
62/004,314, and/or 62/004,305, for example.
An alpha-glucanohydrolase herein can be, for example, a dextranase
(capable of hydrolyzing alpha-1,6-linked glycosidic bonds; E.G. 3.2.1.11), a
mutanase (capable of hydrolyzing alpha-1,3-linked glycosidic bonds; E.C.
3.2.1.59), a mycodextranase (capable of endohydrolysis of (1-4)-alpha-D-
glucosidic linkages in alpha-D-glucans containing both (1-3)- and (1-4)-bonds;
EC 3.2.1.61), a glucan 1,6-alpha-glucosidase (EC 3.2.1.70), and an alternanase
(capable of endohydrolytically cleaving alternan; E.C. 3.2.1.-; see U.S.
Patent
No. 5786196).
A mutanase comprising SEQ ID NOA7 can be used in certain aspects.
Alternatively, a mutanase can comprise an amino acid sequence that is at least
90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99% identical to SEQ ID
NO:47 and have mutanase activity, for example.
A glucan synthesis reaction as presently disclosed for producing one or
more soluble alpha-glucan products can serve directly as suitable conditions
in
which to perform a hydrolysis reaction herein in which an alpha-glucosidase is
used to hydrolyze an alpha-1,5 glucosyl-fructose linkage. Such hydrolysis can
be perforrned following any of the conditions disclosed above regarding
hydrolytic treatment of a glucan synthesis reaction that produces poly alpha-
1,3-
glucan, for example. Alternatively, a fraction (e.g., chromatographic
fraction) of a
glucan synthesis reaction for producing one or more soluble alpha-glucan
products can be used as suitable conditions in which to perform alpha-
glucosidase-mediated hydrolysis of alpha-1,5 glucosyl-fructose linkages.
A fraction in certain embodiments herein can be a chromatographic
=fraction of a glucan synthesis reaction. For example, a fraction can be a
chromatographic fraction of a glucan synthesis reaction that produces one or
rnore soluble alpha-glucan products as disclosed herein. Such a reaction can
optionally include one or more alpha-glucanohydrolases during glucan
synthesis,
44
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
and/or after completion of glucan synthesis. A fraction in any of these types
of
embodiments typically has been obtained for the purpose of separating all of,
or
most of (e.g., at least about 60%, 70%, 80%, 90%, 95%), a soluble alpha-glucan
product from a reaction composition from which it was produced. Once
separated from all or most of a soluble alpha-glucan product, a fraction can
be
subjected to any of the alpha-1,5 glucosyl-fructose hydrolysis processes
disclosed herein using one or more alpha-glucanases.
A chromatographic fraction herein can typically be obtained using a
suitable type of liquid chromatography. Liquid chromatography can be performed
using size-exclusion chromatography (SEC), colurnn chromatography, high-
performance liquid chromatography (HPLC), ion-exchange chromatography,
affinity chrornatography, ultrafiltration, microfiltration, or dialysis, for
example.
The present disclosure also concerns a composition produced by
contacting a saccharide with an alpha-glucosidase enzyme (e.g.,
transglucosidase or glucoamylase), wherein (i) the saccharide is a
disaccharide
or oligosaccharide comprising at least one alpha-1,5 glucosyl-fructose
linkage,
and (ii) the alpha-glucosidase enzyme hydrolyzes at least one alpha-1,5
glucosyl-fructose linkage of the saccharide. The composition produced in this
manner comprises a reduced amount of the saccharide compared to the amount
of the saccharide that was present prior to the contacting. Examples of the
composition include any of those disclosed herein, such as a hydrolyzed
filtrate
from a glucan synthesis reaction, or a hydrolyzed fraction of a glucan
synthesis
reaction used to produce soluble alpha-glucan. Any of the features disclosed
above and in the Examples regarding a hydrolysis method and products thereof
can characterize the composition. The following features of the composition
are
examples.
An alpha-glucosidase enzyme in certain embodiments of the composition
can comprise an amino acid sequence that is at least 90%, 91%, 92%, 93%,
94%, 95%, 96%, 97%, 98%, or 99% identical to SEC) D NO:5, 6,8, 9, 11, 12, 14,
15, 17, 18, 20, 22, 24, 26, 28, 30, 32, 34, 36, 38, or that of DIAZYME RDF
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
ULTRA (DuPont Industrial Biosciences). A transglucosidase in certain
embodiments of the composition can comprise an amino acid sequence that is at
least 90% identical to SEQ ID NO:l. A glucoamylase in certain embodiments of
the composition can comprise an amino acid sequence that is at least 90%
identical to SEQ ID NO:2. Alternatively, any of the alpha-glucosidase enzymes
disclosed herein can be used to produce the disclosed composition.
A composition produced by a hydrolysis method herein can have, for
example, a concentration of a saccharide such as leucrose that is less than
50%
of the concentration of leucrose that was present prior to contacting the
saccharide with an alpha-glucosidase.
A composition produced by a hydrolysis method in certain embodiments
herein can be a glucan synthesis reaction, or a fraction thereof, in which a
saccharide byproduct of the glucan synthesis reaction is contacted with an
alpha-
glucosidase. A fraction in this embodiment can be a filtrate of the glucan
synthesis reaction, or a fraction of a glucan synthesis reaction used to
produce
soluble alpha-glucan, for example. A saccharide in this embodiment can be
leucrose, for example.
It would be understood by a skilled artisan that the presently disclosed
embodiments are useful, in part, for saccharifying disaccharides and
oligosaccharides that can otherwise be difficult to breakdown. This feature
can
be taken advantage of to perform enhanced methods of (i) fructose enrichment
and (ii) fermentation, for example.
Example 6 below demonstrates that fructose enrichment by
chromatography is enhanced when using a glucan filtrate hydrolyzed by an
alpha-glucosidase (transglucosidase), as compared to using a filtrate that was
not hydrolyzed.
Thus, the disclosed invention further concerns a method of enriching
fructose that is present in a fraction of a glucan synthesis reaction. This
method
comprises (a) contacting a fraction obtained from a glucan synthesis reaction
with an alpha-glucosidase enzyme (e.g., transglucosidase or glucoamylase)
46
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
under suitable conditions, wherein the enzyme hydrolyzes at least one alpha-
1,5
glucosyl-fructose linkage of a disaccharide or oligosaccharide comprised
within
the fraction; and (b) separating fructose from the hydrolyzed fraction of step
(a) to
obtain a composition having a higher concentration of fructose compared to the
fructose concentration of the fraction of step (a).
The features of the disclosed fructose enrichment method regarding
alpha-glucosidase (e.g., transglucosidase or glucoamylase) enzymes, and
fractions of a glucan synthesis reaction, for example, can be according to any
of
the disclosures provided herein concerning each of these features.
Step (b) of separating fructose can be performed by any means known in
the art. For example, chromatography can be employed as disclosed in the
below Examples, or by following the disclosure of European Patent Publ. No.
EP2292803B1, which is incorporated herein by reference.
A composition (e.g., fructose solution or fructose syrup) having a higher
concentration of fructose resulting from the disclosed enrichment method can
have at least about 90, 91, 92, 93, 94, 95, 96, 97, 98, or 99 wt% fructose.
A fructose enrichment method herein can perform better than one which
utilizes a filtrate that has not been hydrolyzed with an alpha-glucosidase as
presently disclosed. Such increased performance can be measured in terms of a
percent fructose recovery of at least 40%, 45%, or 50%.
The present disclosure further concerns a fermentation method
comprising (a) contacting a fraction obtained from a glucan synthesis reaction
with an alpha-glucosidase enzyme (e.g., transglucosidase or glucoamylase)
under suitable conditions, wherein the alpha-glucosidase enzyme hydrolyzes at
least one alpha-1,5 glucosyl-fructose linkage of a disaccharide or
oligosaccharide
comprised within the fraction; (b) fermenting the fraction of step (a) with a
microbe to yield a product; and (c) optionally, isolating the product of (b).
The
fermenting step of (b) can be performed after step (a) or simultaneously with
step
(a). Significantly, this method can be used to produce ethanol, for example,
by
fermenting a hydrolyzed filtrate of a glucan synthesis reaction. The ethanol
yield
47
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
from such a process is higher than the ethanol yield obtained when fermenting
a
glucan filtrate that has not been hydrolyzed.
The features of the disclosed ferrnentation method regarding alpha-
glucosidase (e.g., transglucosidase or glucoamylase) enzymes, disaccharides
and oligosaccharides, fractions of a glucan synthesis reaction, and suitable
contacting conditions, for example, can be according to any of the disclosures
provided herein concerning each of these features.
A microbe for use in a fermentation method herein can be a bacteria,
yeast, or fungus, for example. Examples of bacteria useful herein include
Lactobacillus species, Streptococcus species, Bifidobacterium species,
Leuconostoc species, Escherichia species (e.g., E. coil) and Bacillus species.
Examples of yeast useful herein include Saccharomyces species such as S.
cerevisiae and S. bayanus.
A fermentation method herein can yield a product such as ethanol or an
acid (e.g., lactic acid). It is believed, however, that other products can be
produced if desired. It would be understood by one of skill in the art that
production of certain products using a fermentation method as disclosed would
depend on various conditions such as the microbe(s) used in the fermentation.
Conditions for fermentation herein can be as disclosed in the below Examples,
or
as disclosed in El-Mansi et al. (2006, Fermentation Microbiolocy and
Biotechnoloov, Second Edition, CRC Press) and Stanbury et at. (1999.
Principles
of Fermentation Technology. Second Edition, Butterworth-Heinemann), for
example, which are both incorporated herein by reference.
The yield of a product in certain embodiments of a fermentation method
herein is higher than the product yield obtained when fermenting a glucan
filtrate
that has not been hydrolyzed with an alpha-glucosidase herein. This comparison
can be with respect to a control fermentation, for example, which used a non-
hydrolyzed fraction of a glucan synthesis reaction. Product yield of a
fermentation herein can be increased by at least about 10%, 20%, 40%, 60%,
80%, or 100% (or any integer value between 10% and 100%), for example. In
addition, the rate of product formation by a fermentation herein can be
increased.
48
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Example 7 below demonstrates that leucrose can be fermented to ethanol
by yeast provided a feed comprising glucan filtrate that had not been
hydrolyzed.
Thus, further disclosed herein is a method of using a microbe to ferment
leucrose
to a product (e.g., ethanol). Such a method can comprise fermenting a glucan
filtrate that (i) has, or (ii) has not been, hydrolyzed with an alpha-
glucosidase as
disclosed herein. Regardless of whether the leucrose is provided in a glucan
filtrate or another form (e.g., semi-purified or enriched form), a method for
fermenting leucrose can comprise adapting a microbe (e.g., yeast such as S.
cerevisiae) for utilizing leucrose. Such adaptation can comprise growing a
microbe in the presence of leucrose, and optionally other sugars, over at
least 2
or 3 growth cycles, for example, after which the microbe utilizes more
leucrose
for fermenting a product. In certain embodiments, a microbe can be (i) grown
in
a first feed comprising leucrose (1 cycle complete), (ii) removed from the
first
feed, (iii) grown in a second feed comprising leucrose (two cycles complete),
(iv)
optionally removed from the second feed, and (v) optionally grown in a third
feed
(three cycles complete). A microbe adapted in this manner can have an
increased capacity to ferment leucrose in certain embodiments.
Example 9 below demonstrates that almost all (e.g., >98% or >99%) the
leucrose present in a glucan filtrate can be used for fermentation by yeast
when
the glucan filtrate is hydrolyzed with a transglucosidase while at the same
time
fermented with yeast. Thus, an enhanced leucrose fermentation method herein
can comprise hydrolysis of leucrose with an alpha-glucosidase (e.g.,
transglucosidase or glucoamylase) while simultaneously fermenting the leucrose
with a microbe.
Non-limiting examples of compositions and methods disclosed herein
include:
1. A method of hydrolyzing an alpha-1,5 glucosyl-fructose linkage in a
saccharide comprising at least one alpha-1,5 glucosyl-fructose linkage,
49
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
wherein the saccharide is a disaccharide or oligosaccharide, and wherein
the method comprises:
contacting the saccharide with an alpha-glucosidase enzyme under
suitable conditions, wherein the alpha-glucosidase enzyme hydrolyzes at
least one alpha-1,5 glucosyl-fructose linkage of the saccharide,
and wherein the amount of the saccharide is reduced compared to the
amount of the saccharide that was present prior to the contacting.
2. The method of embodiment 1, wherein the alpha-glucosidase enzyme is
immobilized.
3. The method of embodiment 1 or 2, wherein the saccharide is leucrose.
4. The method of embodiment 3, wherein the concentration of leucrose after
the contacting step is less than 50% of the concentration of leucrose that
was present prior to the contacting.
5. The method of embodiment 1, 2, 3, or 4, wherein the suitable conditions
comprise:
(i) a glucan synthesis reaction, or
(ii) a fraction obtained from the glucan synthesis reaction;
wherein the saccharide is a byproduct of the glucan synthesis reaction.
6. The method of embodiment 5, wherein the glucan synthesis reaction
produces at least one insoluble alpha-glucan product.
7. The method of embodiment 6, wherein the fraction is a filtrate of the
glucan synthesis reaction.
8. The method of embodiment 5, wherein the glucan synthesis reaction
produces at least one soluble alpha-glucan product that is
(i) a product of a glucosyltransferase, or
(ii) a product of the concerted action of both a glucosyltransferase and an
alpha-glucanohydrolase capable of hydrolyzing glucan polymers having
one or more alpha-1,3-glycosidic linkages or one or more alpha-1 ;6-
glycosidic linkages.
9. The method of embodiment 8, wherein the fraction is a chromatographic
fraction of the glucan synthesis reaction.
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
10. The method of any one of embodiments 1-9, wherein the alpha-
glucosidase enzyme is a transglucosidase or glucoamylase.
11. A composition produced by contacting a saccharide with an alpha-
glucosidase enzyme,
wherein the saccharide is a disaccharide or oligosaccharide and
comprises at least one alpha-1,5 glucosyl-fructose linkage,
wherein the enzyme hydrolyzes at least one alpha-1,5 glucosyl-fructose
linkage of the saccharide,
and wherein the composition comprises a reduced amount of the
saccharide compared to the amount of the saccharide that was present
prior to the contacting.
12. The composition of embodiment 11, wherein the saccharide is leucrose.
13. The composition of embodiment 11 or 12, wherein the saccharide is in
(i)
a glucan synthesis reaction, or (ii) a fraction obtained from the glucan
synthesis reaction;
wherein the saccharide is a byproduct of the glucan synthesis reaction.
14. A method of enriching fructose present in a fraction of a glucan
synthesis
reaction, comprising:
(a) contacting a fraction obtained from a glucan synthesis reaction with
an alpha-glucosidase enzyme under suitable conditions, wherein
the alpha-glucosidase enzyme hydrolyzes at least one alpha-1,5
glucasyl-fructose linkage of a disaccharide or oligosaccharide
comprised within the fraction; and
(b) separating fructose from the hydrolyzed fraction of step (a) to
obtain a composition having a higher concentration of fructose
compared to the fructose concentration of the fraction of step (a).
15. A fermentation method comprising:
(a) contacting a fraction obtained from a glucan synthesis
reaction with
an alpha-glucosidase enzyme under suitable conditions, wherein
the alpha-glucosidase enzyme hydrolyzes at least one alpha-1,5
51
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
glucosyl-fructose linkage of a disaccharide or oligosaccharide
comprised within the fraction;
(b) fermenting the fraction of step (a) with a microbe to yield a product,
wherein the fermenting is performed after step (a) or
simultaneously with step (a); and
(c) optionally, isolating the product of (b);
wherein the yield of the product of (b) is increased compared to the
product yield of fermenting a fraction of the glucan synthesis reaction that
has not been contacted with the alpha-glucosidase enzyme.
EXAMPLES
The disclosed invention is further defined in the following Examples. It
should be understood that these Examples, while indicating certain preferred
aspects of the invention, are given by way of illustration only. From the
above
'15 discussion and these Examples, one skilled in the art can ascertain the
essential
characteristics of this invention, and without departing from the spirit and
scope
thereof, can make various changes and modifications of the invention to adapt
it
to various uses and conditions.
Abbreviations
20 The meaning of some of the abbreviations used herein is as follows: "g"
means gram(s), "h" means hour(s), "mL" means milliliter(s), "psi" means
pound(s)
per square inch, "wt%" means weight percentage, "pm" means micrometer(s),
" /0" means percent, "C" means degrees Celsius, "mg" means milligram(s), "mm"
means millimeter(s), "mL/min" means milliliters per minute, "m" means
meter(s),
25 "pL" means microliter(s), "mmol" means millimole(s), "min" means
minute(s),
"rnol%" means mole percent, "M" means molar, "mg/g" means milligram per
gram, "rpm" means revolutions per minute, "MPa" means megaPascals.
GENERAL METHODS
All reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless
30 stated otherwise. Sucrose was obtained from VWR (Radnor, PA).
52
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Preparation of Crude Extracts of Glucosyltransferase (qtf) Enzymes
The Streptococcus salivarius gtfJ enzyme (SEQ ID NO:3) was expressed
in E. coil strain DH1OB using an isopropyl beta-D-1-thiogalactopyranoside
(IPTG)-induced expression system. SEQ 10 NO:3 has an N-terminal 42-residue
deletion compared to the S. salivarius gtIJ amino acid sequence in GENBANK
Identification No. 47527, but includes a start methionine. Briefly, E. coil
DH1OB
cells were transformed to express SEQ ID NO:3 from a DNA sequence codon-
optimized to express the gtfJ enzyme in E. coll. This DNA sequence was
contained in the expression vector, Nexpress404 (DNA 2.0, Menlo Park CA).
The transformed cells were inoculated to an initial optical density (OD at
600)
of 0.025 in LB medium (10 g/L Tryptone; 5 WI__ yeast extract, 10 WI._ NaCI)
and
allowed to grow at 37 C in an incubator while shaking at 250 rpm. The
cultures
were induced by addition of 1 mM IPTG when they reached an O06o0 of 0.8-1Ø
Induced cultures were left on the shaker and harvested 3 hours post induction.
GtfJ enzyme (SEQ ID NO:3) was harvested by centrifuging cultured cells
(25 C, 16000 rpm) in an Eppendorr centrifuge, re-suspending the cells in 5.0
mM phosphate buffer (pH 7.0) and cooling to 4 'C on ice. The cells were broken
using a bead beater with 0.1-mm silica beads, and then centrifuged at 16000
rpm
at 4 C to pellet the unbroken cells and cell debris. The crude extract
(containing
soluble GtfJ enzyme, SEQ ID NO:3) was separated from the pellet and analyzed
by Bradford protein assay to determine protein concentration (m0114
The Streptococcus sp. C150 gtf-S enzyme (SEQ ID NO:40) was prepared
as follows. SG1184 is a Bacillus subtilis expression strain that expresses a
truncated version of the glycosyltransferase Gtf-S ("GTF0459") from
Streptococcus sp.C150 (GENBANK GI:321278321). The gene (SEQ ID NO:41)
encoding an N-terminal truncated protein GTF0459 (SEQ ID NO:42) from E. coil
expression plasmid pMP79 was cloned into the Nhel and Hindill sites of the
Bacillus subtilis integrative expression plasmid p4JH under the aprE promoter
and fused with the B. subtilis AprE signal peptide on the vector. The
construct
was first transformed into E. coli DH1OB and selected on LB with ampicillin
(100
I.tg/mL) plates. The confirmed construct pDCQ984 expressing GTF0459 was
53
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
then transformed into B. subtilis BG6006 containing nine protease deletions
(amyE::xylRPxylAcomk-ermC, degUHy32, oppA, Aspo11E3501, AaprE, AnprE,
Aepr, AispA, AbprõAvprõAwprA, Ampr-ybfjõAnprB) and selected on LB plates
with chloramphenicol (5 pg/mL). The colonies grown on LB plates with 5 Ittg/mL
chloramphenicol were streaked several times onto LB plates with 25 pg/mL
chloramphenicol. The resulting B. subtilis expression strain, SG1184, was
first
grown in LB medium with 25 p.g/mL chloramphenicol and then subcultured into
Grants11 medium containing 25 pg/mL chloramphenicol grown at 30 'C for 2-3
days. The cultures were spun at 15,000 g for 30 min at 4 'C and the
supernatant
was filtered through 0.22-pm filters. The filtered supernatant was aliquoted
and
frozen at -80 'C.
B. subtilis SG1184 strain, expressing GTF0459 (SEQ ID NO:42), was
grown under an aerobic submerged condition by conventional fed-batch
fermentation. A nutrient medium was used containing 0-0.25% corn steep solids
(Roquette), 5-25 g/L sodium and potassium phosphate, a solution of 0.3-0.6 M
ferrous sulfate, manganese chloride and calcium chloride, 0.5-4 g/L
rnagnesiurn
sulfate, and a solution of 0.01-3.7 g/L zinc sulfate, cuprous sulfate, boric
acid and
citric acid. An antifoam agent, FOAMBLAST 882, at 2-4 mL/L was added to
control foaming. A 10-L fermentation was fed with 50% (Mu) glucose feed when
initial glucose in batch was non-detectable. The glucose feed rate was ramped
over several hours. The fermentation was controlled at 30 C and 20% DO, and
at initial agitation of 750 rpm. The pH was controlled at 7.2 using 50% (Of)
arnmonium hydroxide. Fermentation parameters such as pH, temperature,
airflow, and DO% were monitored throughout the entire 2-day fermentation run.
The culture broth was harvested at the end of the run and centrifuged to
obtain
supernatant. The supernatant containing GTF0459 (SEQ ID NO:42) was then
stored frozen at -80 C.
The S. mutans MT-4239 gtf-C enzyme (SEQ ID NO:43) was prepared as
follows. A gene encoding a truncated version of a glucosyltransferase (gtf)
enzyme identified in GENBANK as GI:3130088 (SEQ ID NO:43; gtf-C from S.
mutans MT-4239) was synthesized using codons optimized for expression in
54
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Bacillus subtilis and synthesized by GenScript. The gene (SEQ ID NO:44)
encoding GTF0088BsT1 with an N-terminal truncation and a C-terminal T1
truncation (SEQ ID NO:45) was amplified from the GENSCRIPT plasmid and
cloned into the Nhel and HindlIl sites of the Bacillus subtilis integrative
expression plasmid p4JH under the aprE promoter and fused with the B. subtilis
AprE signal peptide on the vector. The construct was first transformed into E.
call DH1OB and selected on LB with ampicillin (100 .1gIrriL) plates. The
confirmed construct pDCQ1021 expressing GTF0088BsT1 was then transformed
into B. subtilis BG6006 containing nine protease deletions
(amyE::xylRPxylAcomk-ermC, degUHy32, oppAõAspollE3501, AaprE, AnprE,
Aepr, AispA, AbprõAvprõAwprA, Ampr-ybfjõAnprB) and selected on the LB plates
with chloramphenicol (5 pg/mL). The colonies grown on LB plates with 5 litg/mL
chloramphenicol were streaked several times onto LB plates with 25 i.tg/mL
chloramphenicol. The resulting B. subtilis expression strain SG1221 was first
grown in LB medium with 25 p.g/mL chloramphenicol and then subcultured into
Grants11 medium containing 25 pg/mL chloramphenicol grown at 30 'C for 2-3
days. The cultures were spun at 15,000 g for 30 min at 4 'C and the
supernatant
was filtered through 0.22-pm filters. The filtered supernatant was aliquoted
and
frozen at -80 'C.
B. subtills SG1221 strain, expressing GTF0088BsT1 (SEQ ID NO:45),
was grown under an aerobic submerged condition by conventional fed-batch
fermentation. A nutrient rnediurn was used containing 0-0.25% corn steep
solids
(Roquette), 5-25 g/L sodium and potassium phosphate, a solution of 0.3-0.6 M
ferrous sulfate, manganese chloride and calcium chloride, 0.5-4 g/L
rnagnesiurn
sulfate, and a solution of 0.01-3.7 WI_ zinc sulfate, cuprous sulfate, boric
acid and
citric acid. An antifoam agent, FOAMBLAST 882, at 2-4 mL/L was added to
control foaming. A 2-L fermentation was fed with 50% (w/w) glucose feed when
initial glucose in batch was non-detectable. The glucose feed rate was ramped
over several hours. The fermentation was controlled at 30 "C and 20% DO, and
at an initial agitation of 400 rpm. The pH was controlled at 7.2 using 50%
(v/v)
arnmonium hydroxide. Fermentation parameters such as pH, temperature,
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
airflow, and DO% were monitored throughout the entire 2-day fermentation run.
The culture broth was harvested at the end of run and centrifuged to obtain
supernatant. The supernatant containing GTF088BsT1 (SEQ ID NO:45) was
then stored frozen at -80 'C.
Determination of the Glucosyltransferase GTF0459 and GTF0088BsT1 Activity
Glucosyltransferase activity assay was performed by incubating 1-10%
(v/v) crude protein extract containing GTF enzyme with 200 g/L sucrose in 25
mM or 50 mM sodium acetate buffer at pH 5.5 in the presence or absence of 25
dextran (MW -1500, Sigma-Aldrich, Cat.#313941) at 37 C and 125 rpm
orbital shaking. One aliquot of reaction mixture was withdrawn at 1 h, 2 h and
3
h and heated at 90 C for 5 min to inactivate the GTF. The insoluble material
was removed by centrifugation at 13,000xg for 5 min, followed by filtration
through 0.2-pm RC (regenerated cellulose) membrane. The resulting filtrate was
analyzed by HPLC using two AMINEX HPX-87C columns series at 85 C (Bio-
Rad, Hercules, CA) to quantify sucrose concentration. The sucrose
concentration at each time point was plotted against the reaction time and the
initial reaction rate was determined from the slope of the linear plot. One
unit of
GTF activity was defined as the amount of enzyme needed to consume one
micromole of sucrose in one minute under the assay conditions.
Preparation of a Crude Extract of Alpha-(1,3)-Glucanohydrolase (mutanase)
A gene encoding the Penicilliurn mameffei ATC& 18224T" rnutanase
identified in GENBANK. as GI:212533325 was synthesized by GenScript
(Piscataway, NJ). The nucleotide sequence (SEQ ID NO:46) encoding protein
sequence (MUT3325; SEQ ID NO:47) was subcloned into plasmid pTrex3 at
Sacll and Ascl restriction sites, a vector designed to express the gene of
interest
in Trichoderma reesei, under control of CBI-11 promoter and terminator, with
Aspergillus nigeracetamidase for selection. The resulting plasmid was
transformed into T. reesei by biolistic injection. The detailed method of
biolistic
transformation is described in International PCT Patent Application
Publication
W02009/126773 A1, which is incorporated herein by reference. A 1-cm2 agar
plug with spores from a stable clone, TRM05-3, was used to inoculate the
56
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
production media (described below). The culture was grown in shake flasks for
4-5 days at 28 'C and 220 rpm, To harvest the secreted proteins, the cell mass
was first removed by centrifugation at 4000g for 10 min and the supernatant
was
filtered through 0.2-pm sterile filters. The expression of mutanase MUT3325
(SEQ ID N(3:47) was confirmed by SDS-PAGE.
The production media component is listed below.
NREL-Trich Lactose Defined
Formula Amount Units
ammonium sulfate 5
FPS 33
BD BACTO casamino acid 9
KH2PO4 4.5
CaCl2.2H20 1.32
MgSO4.7H20 1
T. reesei trace elements 2.5 mL
NaOH pellet 4.25
Adjust pH to 5.5 with 50%
NaOH
Bring volume to 920 mL
Add to each aliquot: 5 drops
FOAM BLAST
Autoclave, then add 80 mL
20 % lactose filter sterilized
T. reesei trace elements
Formula Amount Units
citric acid.H20 191.41
FeSO4.7H20 200
ZnSO4.7H20 16
Cu504.5H20 3.2
57
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
MnSO4.H20 1.4
H3B03 (boric acid) 0.8
Bring volume to 1
Fermentation seed culture was prepared by inoculating 0.5 L. of minimal
medium in a 2-L baffled flask with 1.0 rnL frozen spore suspension of the
MUT3325 expression strain TRM05-3 (The minimal medium was composed of 5
gIL ammonium sulfate, 4.5 g/L potassium phosphate monobasic, 1.0 g/L
magnesium sulfate heptahydrate, 14.4 g/L citric acid anhydrous, 1 g/L calcium
chloride dihydrate, 25 g/L glucose and trace elements including 0.4375 g/L
citric
acid, 0.5 WI.. ferrous sulfate heptahydrate, 0.04 git.. zinc sulfate
heptahydrate,
0.008 gIL cupric sulfate pentahydrate, 0.0035 g/L manganese sulfate
monohydrate and 0.002 giL boric acid. The pH was 5.5.). The culture was grown
at 32 QC and 170 rpm for 48 hours before being transferred to 8 L. of the
production medium in a 14-L fermenter. The production medium was composed
of 75 g/L glucose, 4.5 g/L. potassium phosphate monobasic, 0.6 g/L calcium
chloride dehydrate, 1.0 g/L magnesium sulfate heptahydrate, 7.0 g/L ammonium
sulfate, 0.5 g/L citric acid anhydrous, 0.5 g/L ferrous sulfate heptahydrate,
0.04
g/L zinc sulfate heptahydrate, 0.00175 g/L cupric sulfate pentahydrate,
0.0035g/L
manganese sulfate monohydrate, 0.002 g/1... boric acid and 0.3 mL/L
FOAMBLAST 882.
The fermentation was first run with batch growth on glucose at 34 QC, 500
rpm for 24 h. At the end of 24 h, the temperature was lowered to 28 QC and the
agitation speed was increased to1000 rpm. The fermenter was then fed with a
mixture of glucose and sophorose (62% w/w) at a specific feed rate of 0.030 g
glucose-sophorose solids / g biomass / hr. At the end of run, the biomass was
removed by centrifugation and the supernatant containing the MUT3325
mutanase (SEQ lD NO:47) was concentrated about 10-fold by ultrafiltration
using
10-kD Molecular Weight Cut-Off ultrafiltration cartridge (UFP-10-E-35; GE
Healthcare, Little Chalfont, Buckinghamshire, UK). The concentrated protein
was stored at -80 C.
58
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Determination of Alpha-Glucanohydrolase (Mutanase) Activity
Insoluble mutan polymers required for determining mutanase activity were
prepared using secreted enzymes produced by Streptococcus sobrinus ATCC
33478 TM Specifically, one loop of glycerol stock of S. sobrinus ATCC 334781m
was streaked on a BHI agar plate (Brain Heart Infusion agar, Teknova,
Hollister,
CA), and the plate was incubated at 37 C for 2 days. A few colonies were
picked using a loop to inoculate 2X 100 mL BHI liquid medium in the original
medium bottle from Teknova, and the culture was incubated at 37 C, held
static
for 24 h. The resulting cells were removed by centrifugation and the resulting
supernatant was filtered through a 0.2-pm sterile filter; 2X 101 mL of
filtrate was
collected. To the filtrate was added 2X 11.2 mL of 200 giL sucrose (final
sucrose
giL). The reaction was incubated at 37 C with no agitation for 67 h. The
resulting polysaccharide polymers were collected by centrifugation at 5000xg
for
10 min. The supernatant was carefully decanted. The insoluble polymers were
15 washed 4 times with 40 mL of sterile water. The resulting mutan polymers
were
lyophilized for 48 h. Mutan polymer (390 mg) was suspended in 39 mL of sterile
water to make a 10 mg/mL suspension. The mutan suspension was
homogenized by sonication (40% amplitude until large lumps disappear, ¨10 min
in total). The homogenized suspension was aliquoted and stored at 4 'C.
20 A mutanase assay was initiated by incubating an appropriate amount of
enzyme with 0.5 mg/mL mutan polymer (prepared as described above) in 25 rriM
KOAc buffer at pH 5.5 and 37 CC. At various time points, an aliquot of
reaction
mixture was withdrawn and quenched with equal volume of 100 mM glycine
buffer (pH 10). The insoluble material in each quenched sample was removed
by centrifugation at 14,000xg for 5 min. The reducing ends of oligosaccharide
and polysaccharide polymer produced at each time point were quantified by the
p-hydroxybenzoic acid hydrazide solution (PAHBAH) assay (Lever M., Anal.
Biochern., (1972) 47:273-279) and the initial rate was determined from the
slope
of the linear plot of the first three or four time points of the time course.
The
PAHBAH assay was performed by adding 10 pL of reaction sample supernatant
to 100 pL of PAHBAH working solution and heated at 95 C for 5 min. The
59
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
working solution was prepared by mixing one part of reagent A (0.05 g/mL p-
hydroxy benzoic acid hydrazide and 5% by volume of concentrated hydrochloric
acid) and four parts of reagent B (0.05 g/mL NaOH, 0.2 g/mL sodium potassiurn
tartrate). The absorption at 410 nm was recorded and the concentration of the
reducing ends was calculated by subtracting appropriate background absorption
and using a standard curve generated with various concentrations of glucose as
standards.
Analysis of Reaction Profiles by HPLC
Periodic samples from reactions were taken and analyzed using an
Agilente 1260 HPLC equipped with a refractive index detector. An Aminex HP-
87C column (Bic)Rad, Hercules, CA) having deionized water at a flow rate of
0.6
mi../min and 85 C was used to quantitate the level of sucrose, glucose,
leucrose
and fructose in gtf reactions. An Aminex HP-42A column (BioRad) having
deionized water at a flow rate of 0.6 mUrnin and 85 C was used to quantitate
soluble oligosaccharide byproducts (DP2-DP7) in gtf reactions.
A Dionex UltiMaterm 3000 HPLC (Thermo Scientific) equipped with a
refractive index detector was used for samples involving immobilized enzymes
(Example 4). A Phenomenex ReZeXTM calcium monosaccharide column having
deionized water at a flow rate of 0.3 mLimin and 85 C was used to analyze the
sugars.
Analysis of oligosaccharide linkage by NMR
NMR data were acquired on an Agilent DD2 spectrometer operating at
500 MHz for 1H using a 5-mm cryogenic triple-resonance pulsed-field gradient
(PFG) probe. Water suppression was obtained by carefully placing the observe
transmitter frequency on resonance for the residual water signal in a "presat"
experiment, and then using the first slice of a NOESY experiment with a full
phase cycle (multiple of 32) and a mix time of 10 ms. One-dimensional 1H
spectra were acquired with a spectral width of 6410 Hz, acquisition time of
5.1 s,
65536 data points, 4 s presaturation and a 90-degree pulse of 5.85 !.r.s.
Sample
temperature was maintained at 25 C. Samples were prepared by adding 50 !AL
to a 5-mm NMR. tube along with 450 pl of D20 and 60 pa.. of D20 containing
12.4
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
mM DSS (4,4-dimethy1-4-silapentane-1-sulfonic acid sodium salt) internal
reference with the methyl resonance set to 0 ppm. Chemical shift assignments
for different anomeric linkages were taken from: Goffin et al. (2009, Bull
Korean
Chem. Soc. 30:2535-2541. Peak assignments were 5.35 ppm for alpha(1,3)
linkages, 5.1 ppm for leucrose, and 4.95 for alpha(1,6) linkages. Reducing
ends
(RE) were assigned to be 5.2 for alpha RE and 4.65 for beta RE.
EXAMPLE 1
Production of Sugar Syrup by Polymerization of Sucrose
This example discloses the general manner in which a mixture of soluble
sugars was produced by polymerization of sucrose with a gtf enzyme in a glucan
synthesis reaction. Specifically, a filtrate of a glucan synthesis reaction
was
prepared, which was then concentrated to a syrup.
Sucrose (3000 g) was added to a clean 5-gallon polyethylene bucket.
Water (18.1 L) and FermasureTm (10 mL) were added to the bucket, and the pH
was adjusted to 7.0 by addition of 5 vol% NaOH and 5 vol% H2SO4. The final
volume was ¨20 L and the initial concentration of sucrose as measured by HPLC
was 152.5 g/L. The glucan polymerization reaction was initiated by adding 0.3
vol% of crude gtf enzyme (SEQ ID NO:3) extract prepared as described in the
General Methods section. This extract contained about 2.9 mg/mL of protein.
Agitation to the reaction solution was provided using an overhead mechanical
motor equipped with a glass shaft and PTFE blade.
After 48 hours, HPLC analysis revealed that 96% of the sucrose had been
consumed and the reaction was deemed to be complete. The insoluble poly-
alpha-1,3-glucan product of the reaction was removed by filtration with a
Buchner
filter funnel using 325-mesh steel screen and 40-micron filter paper. The
mother
liquor (filtrate) was then concentrated using a rotary evaporator (bath temp
of 40-
50 C) to a total sugar concentration of about 320 WI__ sugars. The
composition
of the concentrated filtrate is provided in Table 2.
61
CA 02940778 2016-08-25
WO 2015/130883 PCT/US2015/017648
Table 2
Composition of a Concentrated Filtrate of a Glucan Synthesis Reaction
Sucrose Leucrose Glucose Fructose DP2 DP3+ Total
g/L 13.5
130.6 25.5 103.8 18.3 28.3 320.1
wt% 4.2 40.8 8 32.4 5.7 8.9 100
Table 2 indicates that the concentrated filtrate of the glucan synthesis
reaction contains sucrose, fructose, glucose, leucrose and oligosaccharides of
DP2-DP7.
EXAMPLE 2
Effect of Enzymes on Hydrolysis of Sugars in a Filtrate of a Glucan Synthesis
Reaction
This example measures the activity of various glucoamylase (EC 3.2.1.3),
transglucosidase (EC 2.4.1.24), beta-glucosidase (EC 3.2.1.21), alpha-amylase
(EC 3.2.1.1) and glucosidase (EC 3.2.1) enzymes for the purpose of reducing
the
concentration of leucrose and/or oligosaccharide byproducts in a concentrated
filtrate of a glucan synthesis reaction. Certain enzymes such as DIAZYME RDF
ULTRA, transglucosidase (EC 2.4.1.24) and glucoamylase (EC 3.2.1.3), which
are all alpha-glucosidase, were found to be particularly effective at reducing
the
amount of these byproducts, resulting in a corresponding increase in
monosaccharides (glucose and fructose) in the treated filtrate.
A filtrate of a glucan synthesis reaction was first prepared and
concentrated to a syrup according to the procedure outline in Example 1. The
composition of this concentrated filtrate is provided in Table 3. NMR analysis
revealed that the ratio of alpha(1,3) to alpha (1,6) linkages present in the
syrup
was 78:22.
Table 3
Composition of a Concentrated Filtrate of a Glucan Synthesis Reaction
Sucrose Leucrose Glucose Fructose DP2 DP3+ Total
g/L 161 210 93 302 33 61 860
wt% 18.7 24.4 10.8 35.1 3.8 7.1
100.0
62
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
The syrup of Table 3 was used to test the hydrolytic activity of various
enzymes against leucrose and oligosaccharide byproducts of the glucan
synthesis reaction. It was not obvious at the outset of these experiments what
enzyme could be used to hydrolyze both these byproducts, given that leucrose
contains an unusual linkage [alpha(1,5)-glucosyl fructose] and that the
oligosaccharides comprise primarily alpha(1 :3) and alpha(1,6) glucosyl-
glucose
linkages. Enzymes with various activities were selected for this analysis
(Table
4).
Table 4
Enzymes Evaluated for Leucrose and Oligosaccharide Hydrolysis
Activity or protein
Enzyme Source Function
concentration
D1AZYME RDF ULTRA DuPont 113a 1,4-alpha-glucosidase 710 U/g
=
Oligo-1,6-glucosidase Megazyme 1,6-alpha-glucosidase 320 Wmg
SPEZYME FRED DuPont 18 Alpha-amylase 1-5%
SPEZYME RSL DuPont 18 Alpha-amylase 1-5%
OPTIMAX 1-1000 DuPont 18 Pullulanase 1-5%
TRANSGLUCOSIDASE
L-2000 DuPont 18 Transglucosidase >1700 TGU/g
purified
TRANSGLUCOSIDASE DuPont1B Transglucosidase 22.7 mg/mt.
L-2000
ACCELLERASE BG DuPont 1B Beta-glucosidase 3000 U/g
NOVO 188 Sigma-Aldrich Beta-glucosidase > 250 U/g
SUMIZYME BFS-L Shin Nihon Beta-glucosidase 100 U/g
Chemical
SUMIZYME BGA Shin Nihon Beta-glucosidase 2000 U/g
Chemical
ACCELERASE TRIO DuPont 18 Cellulase 5-10%
ACCELERASE 1500 DuPont 18 Cellulase/Beta-
glucosidase
OPT1DEX L-400 DuPont IB Glucoamylase > 350 GAU/g
GC 147 DuPont 1B Glucoamylase 400 GAU/g
, GC 321 DuPont 1B Glucoamylase > 350 GAU/g
a DuPont Industrial Biosciences
Conditions for treating the syrup of Table 3 with each of the above
enzymes are provided in Table 5 (enzyme loading, time, temperature, pH, sugar
concentration). The syrup was diluted with water to reach the sugar
63
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
concentration used in each hydrolysis reaction. Table 5 further provides the
percent hydrolysis of the leucrose and DP3+ (at least [)P3-DP7)
oligosaccharides by each enzyme. Percent DP3+ hydrolysis was calculated as
(1 - (wt% DP3+ oligosaccharides in the final syrup)/ (wt% DP3+
oligosaccharides in the initial syrup)). Similarly, percent leucrose
hydrolysis was
calculated as (1 - (wt% leucrose in the final syrup)/ (wt% leucrose in the
initial
syrup)).
Table 5
Hydrolysis of Le.ucrose and Oligosaccharides in a Concentrated Filtrate by
Various Enzymes
Enzyme.Sugar DP3+1)
Leucrose
Example Enzyme load Temp Tmeing pH concentration hydrolysis
hydrolysis
('C) (hr)
(vol %) (g/IX (%) (%)
DIAZYME
2.1 RDF ULTRA 0.5 60 88 4.0 300 36 13
Olie,o-1,6-
2.2 5 40 72 5.5 400 43 <2
glucosidase . . . .
SPEZYME
2.3 0.5 60 66 4.0 280 <2 <2
2.4 SPEZYME RSL 0.5 60 48 4 290 <2 <2
OPTIIVIAX
2.5 0.5 60 48 4 290 <2 <2
L-1000
2.6 TG L-2000 0.25 60 70 4.0 260 54 >98
2.7 , TG L-2000 2 60 48 4.5 , 300 , 96
>98
PURIFIED
2.8 0.c. 60 48 4.5 260 56 >98
TG L-2000 = =
ACCELERASE
2.9 0.5 60 70 4 300 11 <2
BG
2.10 , NOVO 188 0.25 60 70 4 , 300 ,
49 36
2,11 NOVO 188 5 60 4C) 5.5 , 340 , 93
29
SUNIIZYME
2.12 a5 60 48 4.5 260 55 46
.
SUMIZYME
2.13 0.1 wt% 60 48 4.5 260 26 77
.
ACCELERASE
2.14 a5 60 48 4 290 28 6.6
.
ACCELERASE
2.15 a5 60 66 4 280 26 4
1500
'
2,16 , GC 147 0.5 60 40 4 , 300 55
12
,
2,17 GC 321 5 60 72 5.5 400 74 64
64
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
2.18 OPTIDEX
0.5 60 70 4 300 27 25
L-400
a Sugar concentration (total concentration of sucrose; glucose; fructose,
leucrose
and oligosaccharides) measured by HPLC; reported values are rounded to
nearest 10 g/L increment.
b DP3+ contains DP3-DP7, but may also contain larger soluble oligosaccharides
that have a high ratio of alpha-1,6 linkages to alpha-1,3 linkages, when
produced
using certain gtf enzymes.
Table 5 indicates that 1,4-alpha-glucosidase and 1,6-alpha-glucosidase
showed some (Example 2.1) or very little (Example 2.2) hydrolysis of leucrose,
but did release some glucose from the oligosaccharides. Use of alpha-amylase
(Example 2.3 and Example 2.4) showed very little activity against the
compounds
of interest. Similarly, use of a pullulanase (Example 2.5) showed very little
activity.
Cellulases (Examples 2.14 and 2.15) were largely ineffective at
hydrolyzing leucrose, but did hydrolyze some of the oligosaccharides.
Although the oligosaccharides did not contain beta linkages, surprisingly,
beta-glucosidase enzymes also showed a range of hydrolytic conversion from
very low (ACCELERASE BG, Example 2.9) to very high (NOVO 188, Examples
2.10 and 2.11). The relative efficacy of these enzymes varied quite
dramatically.
In some cases, the amount of oligosaccharide that was hydrolyzed greatly
exceeded (Example 2.11), or was close to (Example 2.12), the percentage of
leucrose that was hydrolyzed. In other cases, leucrose was highly hydrolyzed
by
beta-glucosidase while the oligosaccharides were moderately hydrolyzed
(Example 2.13). The high disparity amongst the results observed with beta-
glucosidase suggests that the presence of other enzymes in the tested beta-
glucosidase formulations, such as glucoarnylase or another type of alpha-
glucosidase, could be responsible for the observed activity.
Conversely, the results in Table 5 indicate that transglucosidase (TG L-
2000, Example 2.6) showed very high activity at hydrolyzing both the
oligosaccharides and leucrose. Leucrose hydrolysis by transglucosidase
appeared quantitative under certain circumstances, and greater than 95% of the
DP3+ material was hydrolyzed to glucose and DP2 at high enzyme loadings
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
(Example 2.7). Use of a purified version of transglucosidase revealed similar
activity (Example 2.8), indicating that the observed hydrolysis is due to the
transglucosidase enzyme and not background activity.
Glucoamylases (Examples 2.16-2.18) showed a range of activity against
leucrose and the oligosaccharides. Only one tested glucoamylase (Example
2.18) gave less than 30% hydrolysis of both the leucrose and oligosaccharides.
The results in Table 5 indicate that alpha-glucosidases such as DIAZYME
RDF ULTRA, glucoamylase and transglucosidase can hydrolyze leucrose
byproduct present in a glucan reaction filtrate. The ability of alpha-
glucosidases
to hydrolyze leucrose indicates that these enzymes can hydrolyze alpha-1,5
glucasyl-fructose linkages. While this activity was shown above using leucrose
as a substrate, it is believed that this activity can also be extended to
oligosaccharides comprising alpha-1,5 glucosyl-fructose linkages.
The results in Table 5 further indicate that alpha-glucosidases such as
glucoamylase and transglucosidase can hydrolyze oligosaccharide byproducts
present in a glucan reaction filtrate. Since these oligosaccharides are mostly
comprised of glucose monomer units linked by alpha-1,3 and/or alpha-1,6
linkages (Example 3), the data in Table 5 indicate that alpha-glucosidase
enzymes can hydrolyze alpha-1,3 glucosyl-glucose and/or alpha-1,6 glucosyl-
glucose linkages.
Since alpha-glucosidase enzymes were generally effective at hydrolyzing
the leucrose and/or oligosaccharide byproducts of a glucan synthesis reaction,
these enzymes can be used alone or in combination to reduce the processing
time necessary to generate a high purity syrup from a glucan reaction filtrate
containing an increased amount of monosaccharides and reduced amount of
sugar byproducts. An example of an effective enzyme combination could be a
transglucosidase such as TG L-2000, for leucrose hydrolysis, and a
glucoamylase (e.g., GC 321) enzyme that efficiently hydrolyzes oligosaccharide
byproducts.
66
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Thus, alpha-glucosidase enzymes can individually hydrolyze (i) alpha-1,5
glucosyl-fructose linkages and (ii) alpha-1,3 and alpha-1,6 glucosyl-glucose
linkages in certain saccharides.
EXAMPLE 3
Comparison of Linkage Distributions of Glucan Reaction Filtrate Components
before and after Enzyme Hydrolysis
This example measures the hydrolytic activity of transglucosidase (EC
2.4.1.24) and beta-glucosidase (EC 3.2.1.21) enzymes against leucrose and
oligosaccharide byproducts present in a concentrated filtrate of a glucan
synthesis reaction. Transglucosidase was found to reduce the amount of these
byproducts, resulting in a corresponding increase in monosaccharides (glucose
and fructose) in the treated filtrate.
The oligosaccharide byproducts present in the filtrate of the above glucan
synthesis reaction comprise >90% glucose-glucose linkages, as determined by
NMR (General Methods). Of the glucose-glucose linkages, -78% represent
alpha-1,3 linkages and -22% represent alpha-1,6 linkages.
NMR was used to determine the linkage profile of material generated in
Example 2.11 above after hydrolysis. As shown in Figure 1, the peak
corresponding to alpha-1,3 linkages was reduced by 86%, the peak
corresponding to alpha-1,6 linkages was reduced by only 2.3%, and the peak
corresponding to leucrose peaks was reduced by 21%. While sucrose was very
nearly quantitatively hydrolyzed by this enzyme, Novo 188 does not appear to
be
capable of hydrolyzing alpha-1,6 linkages.
NMR was similarly used to determine the linkage profile of material
generated using TG L-2000 (SEQ ID NO:1) transglucosidase (Figure 2). 210 ILIL
of concentrated filtrate from the material in Table 3, 300 p.L of D20, and 90
ILIL of
D20 containing 12.4 mM DSS as internal reference were mixed in an NMR tube
to give a total sugar concentration of 300 g/L and heated to 60 C. A time
zero
spectrum (starting material in Figure 2) was acquired after thermal
equilibration
at 60 C, and then 0.5 vol% of enzyme was added. The sample was re-
equilibrated in the probe at 60 C and shimmed, and measurements were taken
67
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
within a few minutes of analysis. After 10 hours of treatment with TG L-2000
enzyme (treated material in Figure 2), the peaks corresponding to alpha-1,3
linkages were reduced by 41%, the peaks corresponding to alpha-1,6 linkages
were reduced by 36%, and the peak corresponding to leucrose was reduced by
>95% (Figure 2). An increase in both the alpha-reducing end and beta-reducing
end peaks was observed, which corresponds to an increase in fructose and
glucose (Figure 2).
These results demonstrate that a transglucosidase can convert
oligosaccharides containing alpha-1,3 and alpha-1,6 linkages into glucose and
can convert leucrose into fructose and glucose. Thus, transglucosidase can
hydrolyze (i) alpha-1,5 glucosyl-fructose linkages and (ii) alpha-1,3 and
alpha-1,6
glucosyl-glucose linkages in certain saccharides.
EXAMPLE 4
Hydrolysis of Leucrose and Oligosaccharides in Glucan Reaction Filtrate Using
Immobilized Enzymes
This Example describes using immobilized glucoamylase (EC 3.2.1.3) and
transglucosidase (EC 2.4.1.24) to hydrolyze leucrose and other
oligosaccharides
present in filtrate obtained from a glucan synthesis reaction. Specifically,
the
effect of immobilized transglucosidase TG L-2000 (SEQ ID NO:1, obtained from
Genencor / DuPont Industrial Biosciences) and immobilized glucoamylase GC-
147 (obtained from Genencor DuPont Industrial Biosciences) on the hydrolysis
of leucrose and oligosaccharides DP2, DP3 and HS (higher sugars, DP4+) in a
filtrate of a glucan synthesis reaction was studied.
Immobilization of the glucoamylase and transglucosidase enzymes was
carried out according to the method described in U.S. Patent No. 5541097,
which
is incorporated herein by reference.
In a typical process for immobilizing the glucoamylase or
transglucosidase, two batches of about 8.0 gibatch of porous granular
diatomaceous earth (EP Minerals, Reno, NV) were hydrated with distilled water
and then transferred to a glass colurnn reactor of 1.5-cm diameter and 30-cm
height. Water was pumped upflow at about 6-7 mL/min to remove fines from all
68
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
three columns. Generally, within an hour the water effluent was free of fines.
Water was drained from the column to the top of the granular diatomaceous
earth beds and replaced with 0.1% w/v aqueous solution of polyethylenirnine
(PEI, EPOMIN P-1050). 3500 mL of the PEI solution was then pumped upflow
and effluent was recycled through the beds for 2 hours. The granular
diatomaceous earth beds were then washed upflow with distilled water for 2
hours to remove free PEI at room temperature. In this manner, granular
diatomaceous earth-PEI carriers were obtained.
In the meantime, 3.5 mL of glucoamylase GC-147 having activity defined
in Table 4 was added to 315 ml of 0.02 M acetate buffer (pH 4.5). 1.575 g of
50% wiw glutaraldehyde (Protectol GA-50) was then slowly added to the
aqueous solution of glucoamylase with gentle mixing, and the glutaraldehyde
was allowed to react with the aqueous glucoamylase solution for 4 hours at a
temperature of 20-25 C with gentle agitation, which resulted in formation of
a
treated enzyme-glutaraldehyde adduct containing treated glucoamylase.
Separately, these steps were repeated using the transglucosidase TG L-2000
having activity defined in Table 4 instead of the glucoamylase, thereby
resulting
in the formation of a treated enzyme-glutaraldehyde adduct containing treated
transglucosidase.
Each of the treated enzyme-glutaraldehyde adducts was then circulated
for 4 hours (20-25 C) in its own column prepared as above containing granular
diatomaceous earth-PEI carrier. Excess treated adduct was then washed out of
the carriers with water. Columns with immobilized glucoamylase or
transglucosidase were thus prepared.
A glucan filtrate having the composition defined in Table 3 was diluted to
180 giL, adjusted to pH 4.5, and passed through a column containing an
immobilized enzyme. Column temperature was controlled to 60 'C. After 16
hours of column equilibration, samples were taken periodically at different
flow
rates. Sugar compositions of hydrolysis reaction products were determined by
HPLC (Table 6). Every time the flow rate setting was changed, the colurnn was
allowed to re-equilibrate for at least 1-2 bed volumes before sampling. The
69
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
degree of hydrolysis of leucrose and oligosaccharides was calculated using the
manner described in Example 2. Three column configurations were tested: 1)
immobilized glucoamylase, 2) immobilized transglucosidase, and 3) immobilized
glucoamylase followed by immobilized transglucosidase.
Table 6
Application of Immobilized Glucoamylase and Transglucosidase Enzymes to
Hydrolyze Oligosaccharides and Leucrose
Mean contact DP3+ Leucrose
immobilized Enzyme time (hr) hydrolysis (%) hydrolysis (%)
GC 147 0.7 16 17
GC 147 1.0 20 22
GC 147 1.3 25 29
GC 147 3.0 39 47
TG L-2000 0.7 28 >95
TG L-2000 1.0 32 = >95
TG L-2000 1.3 37 >95
TG L-2000 3.0 47 >95
GC 147 + TG L-2000 6.0 55 >95
Table 6 indicates that, as the mean contact time (defined as the nominal
column volume divided by the mean flow rate) was increased, the degree of
hydrolysis of leucrose and oligosaccharides generally increased. Use of the
immobilized transglucosidase to hydrolyze leucrose was particularly effective,
as
no significant difference was observed even using the fastest flow rate that
was
tested. While each column individually showed reasonable conversion, the
combination of the glucoamylase and transglucosidase gave the highest
hydrolysis of oligosaccharides.
Thus, use of an immobilized glucoamylase or transglucosidase, or both
types of immobilized enzymes, represents an effective technique to hydrolyze
oligosaccharides containing alpha-1,3 and alpha-1,6 glucosyl-glucose linkages,
as well as leucrose. These results are consistent with those of Example 2.
Immobilization of other alpha-glucosidase enzymes should give similar results.
CA 02940778 2016-08-25
WO 2015/130883 PCT/US2015/017648
EXAMPLE 5
Enrichment of Fructose from a Glucan Reaction Filtrate by Chromatography
This example discloses how fructose in a glucan reaction filtrate can be
further enriched through chromatography.
Generally, when separating sugar molecules by chromatography,
components elute inversely to molecular size so that the largest molecules
elute
first. Thus, with respect to a filtrate of a glucan synthesis reaction,
oligosaccharides elute first, followed by disaccharides, and then
monosaccharides. Separations using a sodium cation resin did not separate
fructose and glucose well, and all of leucrose, sucrose, and DP2 co-eluted.
Use
of ion exchange resins where the cation is calcium are preferred to separate
glucose and fructose.
A filtrate of a glucan synthesis reaction was first prepared and
concentrated to a syrup according to the procedure outline in Example 1. The
composition of this concentrated filtrate is provided in Table 7.
Table 7
Composition of a Concentrated Filtrate of a Glucan Synthesis Reaction
Sucrose Leucrose Glucose Fructose DP2 DP3+ Total
g/L. 126 202 93 295 40 65 821
wt% 15.4 24.6 11.3 36.0 4.8 7.9
100
The syrup of Table 7 was filtered and diluted to 25 g dry solids/100 g
solution with ion-exchanged water, and fed to a column containing a
crosslinked
strong acid ion exchange resin in the calcium form. The physical parameters of
this column appear in Table 8. Diluted syrup (15.8 L) was fed to the column,
which was maintained at 65 'C, after which the column was eluted using water
at
a flow rate of 30 Lihr.
Table 8
Physical Parameters of the Column
Resin Type FINE X CS11GC
Ion form Ca2'
Crosslinking, % divinyl benzene 5.5
Particle size (mm) 0.34
71
CA 02940778 2016-08-25
WO 2015/130883 PCT/US2015/017648
Bed length (m) 5.0
Column diameter (m) 0.225
In this separation, leucrose remained in the column longer than sucrose,
perhaps due to complexation of leucrose with the calcium cation, and in fact,
co-
eluted with glucose. Two fractions containing fructose were isolated. Fraction
5.1 eluted between 47 and 120 minutes, and fraction 5.2 eluted between 120 and
172 minutes. Of the fructose fed to the chromatographic separation, 95.7% of
the fructose was isolated in >90% purity. The product distribution in each
fraction (5.1 and 5.2) as measured by HPLC appears in Table 9.
Table 9
Product Distribution of Chromatographic Fractions Containing Significant
Amounts of Fructose
Fraction Sucrose Leucrose Glucose Fructose DP3+ Others Total Fructose
recovered
5.1 31.9 34.8 20.8 3.9 5.4 4.8 100 3.9
5.2 0.0 1.0 0.8 97.7 0.0 0.6 100 95.7
As the feed composition for this separation comprised 36.0% fructose, a
total of 34.5% of the total stream was recovered as a fructose syrup with >90
wt% DS fructose. If the sucrose in the feed is neglected, 40.7% of the sugars
were recovered as a fructose syrup with >90 wt% DS fructose.
Thus, fructose in a glucan reaction filtrate can be further enriched through
chromatography. Example 6 below demonstrates that this process can be
enhanced using glucan filtrate hydrolyzed with a transglucosidase.
EXAMPLE 6
Enrichment of Fructose from a Hydrolyzed Glucan Reaction Filtrate by
ChromatograDhv
This example demonstrates that fructose isolation from a glucan filtrate in
which the oligosaccharides and leucrose have been hydrolyzed results in an
increased yield of high purity fructose syrup compared to when isolating
fructose
from a non-hydrolyzed glucan filtrate.
72
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
A syrup was prepared by concentrating (vacuum at 50 C) a glucan filtrate
that had been treated with 1 vol% of transglucosidase TG L-2000 (SEQ ID NO:1)
for 24 hr at 60 C and pH 4.5. Some oligosaccharide formation was observed
during the concentration process, which was expected since transglucosidase
enzymes are known to create oligosaccharides at high concentrations of
monosaccharides. The syrup had the final product distribution described in
Table A.
Table A
Composition of a Concentrated Glucan Filtrate that Was Hydrolyzed before
Concentration
Sucrose Leucrose Glucose Fructose DP2 DP3+ Total
g/L 3 <10 294 409 73
81 ¨870
wt% 0.3 1.1 33.7
47.0 8.4 9.3 100
The syrup described in Table A was filtered and diluted to 25.4 g 05/100 g
solution with ion-exchanged water and was fed to a column containing a
crosslinked strong acid cation exchange resin in the calcium form. The
physical
parameters of the column appear in Table B. Diluted syrup (169 g) was then fed
to the column, which was maintained at 65 C, after which the column was
eluted
using water at a flow rate of 50 mUmin.
Table B
Physical Parameters of the Column
Resin Type FINEX CS11GC
Ion form Ca'
Crosslinking, % diyinyl benzene 5.5
Particle size (mm) 0.34
Bed length (m) 1..69
Column diameter (m) 0.093
Two fractions containing fructose were isolated. Fraction 6.1 eluted
between 73 and 103 minutes, and fraction 6.2 eluted between 103 and 120
minutes. Of the fructose fed to the chromatographic separation, 93.0% of the
fructose fed to the column was isolated in fraction 6.2 in >90% purity. The
73
CA 02940778 2016-08-25
WO 2015/130883 PCT/US2015/017648
product distribution in each fraction (6.1 and 6.2) as measured by HPLC
appears
in Table C.
Table C
Product Distribution of Chromatographic Fractions Containing Fructose from a
Hydrolyzed Glucan Filtrate
Fraction Sucrose Leucrose Glucose Fructose DP3+ Others Total Fructose
recovered
6.1 7.7 13.9 63.9 7.3 1.5 5.7 100 5.9
6.2 0.0 0.6 3.0 91.8 0.0 4.6 100 93.0
The reduced separation efficiency in this example compared to Example 5
can be attributed to differences in the scale of the column and the higher
glucose
fraction of the sample. Even so, chromatographic purification of this material
resulted in an increased yield of high purity fructose syrup compared to that
achieved in Example 5, in which syrup was chromatographically prepared from a
glucan filtrate that had not been hydrolyzed by a transglucosidase. As the
feed
composition for this separation comprised 47% fructose (Table A), 43.7% of the
total stream was recovered as a fructose syrup with >90 wt% DS fructose. This
43.7% recovery is significantly better than the 34.5% recovery in Example 5.
Thus, fructose isolation from a glucan filtrate that has been hydrolyzed
with transglucosidase results in an increased yield of fructose compared to
when
isolating fructose from a non-hydrolyzed glucan filtrate.
EXAMPLE 7
Production of Ethanol by Fermenting a Filtrate of a Glucan Synthesis Reaction
This example discloses yeast fermentation of glucan filtrate to ethanol.
Yeast (S. cerevisiae) cream (Tonon mill, Brazil) was washed by
suspending the cream in tap water (2.4 L, optical density of 65 at 600 nm) and
then centrifuging the yeast cream for 5 minutes using a LEGEND XTR centrifuge
(Thermo Scientific) at 4500 g. After decanting the supernatant, the yeast
cells
were resuspended and concentrated by centrifugation two additional times.
After
the third wash, the pH was adjusted to 2 by addition of 5 wt% sulfuric acid.
The
optical density was measured using a GENESYS 20 4001 spectrophotometer
74
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
(Thermo Scientific) and adjusted to 100 at 600 nm by addition of tap water.
The
adjusted yeast cream (1.5 L) was added to a 7.5-L BIOFL0310 fermenter vessel
(New Brunswick). The fermenter was set to maintain temperature at 30 C and
agitation at 100 rpm. Although pH was measured during fermentation, it was not
controlled by the addition of acid or base solutions.
A feed solution containing yeast extract (10 g/L), peptone (20 g/L), and
200 g/L of sugars from a glucan filtrate was prepared and sterilized using a
PHOENIX AV-250 PLUS autoclave at 121 C for 15 minutes. The feed solution
was allowed to cool to 25 C (room temperature) before the fermentation began.
The sterilized feed solution (3.5 L) was added to the fermenter over
approximately 5 hours at a rate of 684 mL/hr, and the fermentation was allowed
to proceed for 22 hours.
Periodic samples were taken during the fermentation and analyzed for
optical density using a GENESYS 20 4001 spectrophotometer, Brix using a PAL-
3 refractometer (Atago), and sugar and ethanol concentrations by HPLC
(General Methods). These results are summarized in Table 10.
Table 10
Feed and Time Course Fermentation Profiles for the First Ethanol Fermentation
Time ( Totalr hr) Sucrose
Leucrose Glucose Fructose DP2 DP3+ Et0H
Suga
Feed 9 70 19 76 7 19 200 0
0 0.2 0.0 0.0 0.1 - 7
1 <1 18 0.3 <1 - - - 15
2 <1 30 0.4 <1 - - - 21
3 <1 40 0.0 <1 - - 25
-
4 <1 46 0.0 <1 - - 28
5 <1 49 0.0 <1 - - 29
6 <1 53 0.0 <1 - - - 32
8 <1 53 0.0 <1 - - 33
22 <1 53 0.0 <1 5 18 76 33
Concentrations (g/L) of ethanol (Et0H) and sugar compounds in the feed and at
various fermentation time points (0-22 hours) are listed.
When the fermentation was over, the yeast cells were separated by
centrifugation using a LEGEND XTR centrifuge at 4500 g for 5 minutes. After
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
decanting the supernatant, the yeast were resuspended and concentrated by
centrifugation two additional times. After the third wash, the pH was adjusted
to
2 by addition of 5 wt% sulfuric acid. The optical density was measured using a
GENESYS 20 4001 spectrophotometer and adjusted to 100 at 600 nm by
addition of tap water. Two additional fermentation cycles, each using fresh
feed,
were performed using recycled yeast cells from the previous fermentation
following the same conditions described above. The fermentation results
obtained using first-time and second-time recycled yeast are provided in
Tables
11 and 12, respectively.
Table 11
Feed and Time Course Fermentation Profiles Using the First Recycle of Yeast
Cells
Total
Time (hr) Sucrose Leucrose Glucose Fructose DP2 DP3+ Et01-1
Sugar
Feed 13 69 21 77 7 18 206 0
0 , 0 4 , 0 0 -- 5
. . .
1 - . <1 19 . 0 <1 - - 18
4 < - 1 35 0 <1 - - 23
4 <1 40 0 <1 - - - 26
5 <1 45 0 <129
-
6 <1 53 0 <1 - . . 32
7 <1 50 0 <1 , -= - 32 _
7 <1 51 0 <1 - - - 33
21 <1 42 0 <1 6 18 65 37
Concentrations (g/L) of ethanol (Et0H) and sugar compounds in the feed and at
various fermentation time points (0-21 hours) are listed.
Table 12
Feed and Time Course Fermentation Profiles Using the Second Recycle of Yeast
Cells
Time Total
Sucrose Leucrose Glucose Fructose DP2 DP3+ Et0F1
(hr) Sugar
Feed 10 70 19 76 6 19 201 0
0 <1 0 0 <1 - - 11
1 <1 32 0 <1 - - - 24
4 <1 40 0 <1 . - . 29
76
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
4 <1 45 0 <3. 31
<1 46 0 <1 33
6 <1 45 0 <1. 34
6 = <1 16 0 <1 48
7 <1 7 0 <1. =- =- 52
21 <1 5 0 <1 5 1.6 27 54
Concentrations (g/L) of ethanol (Et0H) and sugar compounds in the feed and at
various fermentation time points (0-21 hours) are listed.
Very little leucrose was consumed in the first fermentation, although the
5 yeast cells started to acclimate and consume leucrose by the second
recycle.
Ethanol fermentation titers increased from 33 g/L (Table 10, 22 hours) to 54
g/L
(Table 12, 21 hours) after three fermentation cycles with recycled yeast,
although
significant amounts of leucrose were present in the medium, even after the
last
cycle.
Thus, glucan filtrate can be used in a fermentation process to produce
ethanol.
EXAMPLE 8
Production of Ethanol by Fermenting Hydrolyzed Glucan Filtrate
This example demonstrates that fermenting a glucan filtrate in which the
leucrose and oligosaccharide byproduct components have previously been
saccharified results in increased ethanol yields.
Fermentations were performed following the procedure outlined in
Example 7, but using a glucan filtrate that was previously treated with a
transglucosidase (TG L-2000, SEQ ID NO:1). Hydrolyzed glucan filtrate was
prepared as follows. Glucan filtrate was adjusted to 300 g sugars/L and then
the
pH was adjusted to 4.0 using 1.0 M sodium hydroxide and 5 wt% sulfuric acid.
The final volume of this preparation was 6.75 L. The filtrate solution was
then
sterilized using a PHOENIX AV-250 PLUS autoclave at 121 C for 15 minutes,
and then the temperature was adjusted to 60 'C. TG L-2000 enzyme extract as
described in Table 4 (135 mL) was mixed with the sterilized filtrate and the
solution was incubated in an incubator-shaker (IKA KS4000) at 60 C and 100
rpm for 72 hours. Hydrolyzed glucan filtrate was thus prepared.
77
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Yeast (S. cerevisiae) cream (Bom Retiro mill, Brazil) was washed by
suspending the cream in tap water (2.4 L, optical density of 65 at 600 nm) and
then centrifuging the yeast cream for 5 minutes using a LEGEND XTR centrifuge
at 4500 g. After decanting the supernatant, the yeast were resuspended and
concentrated by centrifugation two additional times. After the third wash, the
pH
was adjusted to 4.5 by addition of 5 wt% sulfuric acid and the optical density
was
measured using a GENESYS 20 4001 spectrophotometer and adjusted to 100 at
600 nm by addition of tap water. The adjusted yeast cream (1.5 L) was added to
a 7.5-L BIOFLO310 fermenter vessel. The fermenter was set to maintain
temperature at 30 CC, agitation at 100 rpm, and pH at 4.5 using 4 M aqueous
ammonium hydroxide or 5 wt% aqueous sulfuric acid.
A feed solution containing yeast extract (10 gIL), peptone (20 g/L), and
200 giL of sugars from the hydrolyzed filtrate was prepared and sterilized
using a
PHOENIX AV-250 Plus autoclave at 121 cc for 15 minutes. The feed solution
was allowed to cool to 25 CC (room temperature) before the fermentation began.
The sterilized feed solution (3.5 L) was added to the fermenter over
approximately 5 hours at a rate of 684 mLihr, and the fermentation was allowed
to proceed for 22 hours.
Periodic samples were taken during the fermentation and analyzed for
optical density using a GENESYS 20 4001 spectrophotometer, Brix using a PAL-
3 refractonleter, and sugar and ethanol concentrations by HPLC (General
Methods). These results are summarized in Table 13.
Table 13
Feed and Time Course Fermentation Profiles for the First Ethanol Fermentation
Using Hydrolyzed Glucan Filtrate
Time Total
Sucrose Leucrose Glucose Fructose DP2 DP3+
Et0H
(hr) Sugar
Feed 7 4 65 97 11 3 186 0
0 0 0 0 0 7
1 <1 1 <1 20
2 <1 3 <1 <1. 32
3 <1. 4 1. 1 40
4 <1 4 <1 <1. 49
78
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
<1. 4 <1 <1 - - - 53
6 <1 5 <1 <1. - - - 55
8 <1 5 =<1 <1 - - - 57
,
22 <1 5 <1 <1 8 3 1.6 54
Concentrations (g/L..) of ethanol (Et0H) and sugar compounds in the feed and
at
various fermentation time points (0-22 hours) are listed.
When the fermentation was over, the yeast cells were separated by
5
centrifugation using a LEGEND XTR centrifuge at 4500 g for 5 minutes. After
decanting the supernatant, the yeast cells were resuspended and concentrated
by centrifugation two additional times. After the third wash, the pH was
adjusted
to 2 by addition of 5 wt% sulfuric acid. The optical density was measured
using a
GENESYS 20 4001 spectrophotometer and adjusted to 100 at 600 nm by
addition of tap water. Two additional fermentation cycles, each using fresh
feed,
were performed using recycled yeast cells from the previous fermentation
following the same conditions described above. The fermentation results
obtained using first-time and second-time recycled yeast cells are provided in
Tables 14 and 15, respectively.
Table 14
Feed and Time Course Fermentation Profiles Using the First Recycle of Yeast
Cells with Hydrolyzed Glucan Filtrate
Time Total
Sucrose Leucrose Glucose Fructose DP2 DP3+ Et0H
(hr) Sugar
Feed 7 4 69 104 7 4 194 0
0 <1 i 0 , <1 <3. - - 1.0
1. <1 7 <1 <1- - - 25
4 <1 , 5 <1.-
<1 - - 39
4 <1 4 , <1. <1.- - - 45
5 <1 , 5 <1 <1- - - 51
6 <1 5 , <1 <1- - - 57
6.2 <1 , 5 <1 <1- - - 60
7 <1 5 , <1 <1- - - 59
21. <1 5 <1 <1 9 5 19 58
Concentrations (g/L..) of ethanol (Et0H) and sugar compounds in the feed and
at
various fermentation time points (0-21 hours) are listed.
79
CA 02940778 2016-08-25
WO 2015/130883 PCT/US2015/017648
Table 15
Feed and Time Course Fermentation Profiles Usina the Second Recycle of Yeast
Cells with Hydrolyzed Glucan Filtrate
Time Total
Sucrose Leucrose Glucose Fructose DP2 DP3+ Et0H
(hr) Sugar
Feed 7 4 70 105 7 5 197 0
0 0 0 0 0 - - 1.0
1. <1 7 <1 <1- 25
-
3.5 <1. = 5 <1 <1- - - 39
4 <1 4 <1 <1- 45 .
-
<1 = 5 <1 <1- - - 51
6 <1. 5 <1 <1- - - 57
6.2 <1. 5 <1 <1- - - 60
7 <1 5 <1 <1- - - 59
21. <1 5 <1 <1 9 5 19 58
Concentrations (g/L) of ethanol (Et0H) and sugar compounds in the feed and at
5 various fermentation time points (0-21 hours) are listed.
All of the fermentations were essentially complete within about six hours of
initiating fermentation, and resulted in ethanol titers of 57-60.0 g/L.
Comparing
these fermentations with those in Example 7 demonstrates that hydrolyzing a
glucan filtrate before subjecting it to fermentation results in faster and
greater
ethanol yields than those obtained from fermentations using non-hydrolyzed
glucan filtrate.
Thus, fermenting a glucan filtrate in which the leucrose and
oligosaccharide byproduct components have been saccharified results in
increased ethanol yields at faster rates. This saccharification can be done
using
a transglucosidase, for example.
EXAMPLE 9
Simultaneous Saccharification and Fermentation of a Glucan Filtrate Solution
This example discloses that simultaneous saccharification and
fermentation of a feed containing glucan filtrate can result in enhanced
fermentation properties.
Yeast (S. cerevisiae) cream (Born Retiro mill, Brazil) was washed by
suspending the cream in tap water (2.4 L, optical density of 65 at 600 nm) and
CA 02940778 2016-08-25
WO 2015/130883 PCT/US2015/017648
then centrifuging the yeast cream for 5 minutes using a LEGEND XTR centrifuge
at 4500 g. After decanting the supernatant, the yeast cells were resuspended
and concentrated by centrifugation two additional times. After the third wash,
the
pH was adjusted to 4.5 by addition of 5 wt% sulfuric acid and the optical
density
__ was measured using a GENESYS 20 4001 spectrophotometer and adjusted to
100 at 600 nm by addition of tap water. The adjusted yeast cream (1.5 L) was
added to a 7.5-L BI0FL0310 fermenter vessel. The fermenter was set to
maintain temperature at 30 C, agitation at 100 rpm, and pH at 4.5 using 4 M
aqueous ammonium hydroxide or 5 wt% aqueous sulfuric acid.
A feed solution containing yeast extract (10 g/L), peptone (20 g/L), and
200 g/L. of sugars from a glucan filtrate was prepared and sterilized using a
PHOENIX AV-250 PLUS autoclave at 121 C for 15 minutes. The feed solution
was allowed to cool to 25 C (room temperature) before the fermentation began.
TG L-2000 transglucosidase enzyme extract as described in Table 4 (1% v/v)
__ was added to the sterilized feed solution immediately before adding the
solution
to the fermenter. The feed solution (3.5 L) containing TG L-2000 enzyme was
added to the fermenter over approximately 5 hours at 684 mL/hr, and the
fermentation was allowed to proceed for 48 hours.
Periodic samples were taken during the fermentation and analyzed for
__ optical density using a GENESYS 20 4001 spectrophotometer, Brix using a PAL-
3 refractorneter (Atago), and sugar and ethanol concentrations by HPLC
(General Methods). These results are summarized in Table 16.
Table 16
Feed and Time Course Fermentation Profiles for Simultaneous Saccharification
and Ethanol Fermentation of Glucan Filtrate
Time Total
Sucrose Leucrose Glucose Fructose DP2 DP3+ Et0H
(hr) Sugar
Feed 7 82 12 79 6 20 206 0
0 0 0 0 ___ 0 0 0 0 2
1 <1 13 <1 3 11
2 <1 21 <1 4 30
3 <1 21 <1 3 38
4 <1 20 <1 __ 3 11 16 50 43
81
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
<1 14 <1 2- - - 45
6 <1 <1 <1 2- - 59
22 <1 <1 <1. 1- - 62
-
25 <1 <1 <1 <1- - - 63
27 <1 <1 = <1 <1- - 63
- .
31 <1- - <1 <1. <1 - - 57
- ..
46 <1 <1 <1. <1- - 57
-
48 <1 <1 <1. <1 1 11 12 62
Concentrations (g/t..) of ethanol (Et0H) and sugar compounds in the feed and
at
various fermentation time points (0-48 hours) are listed.
The fermentation was nominally complete in 6 hours, similar to the
5 fermentations where the filtrate was hydrolyzed prior to the fermentation
step
(Example 8), and gave a slightly superior titer of ethanol (62 g/L) compared
to
using unhydrolyzed filtrate (Example 7). In addition, almost all of the
leucrose
was consumed by 6 hours (compare Table 16 with Tables 13-15). In addition to
adding a saccharifying enzyme, such as TG L-2000, to a feed containing glucan
filtrate just prior to fermentation, similar results should be obtained if the
saccharifying enzyme is added to the fermentation directly.
Thus, simultaneous saccharification and fermentation of a feed containing
glucan filtrate can result in enhanced fermentation properties such as
increased
(i) consumption of glucan filtrate components (e.g., leucrose) and (ii)
ethanol
yield and rate of production.
EXAMPLE 10
Preparation of Various Alpha-Glucosidases
This example discloses preparing various alpha-glucosidases in addition
to those alpha-glucosidases (transglucosidase, glucoamylase, DIAZYME RDF
ULTRA) used in some of the foregoing Examples. These additional alpha-
glucosidases were tested for hydrolytic activity against oligosaccharides
comprising alpha-1,5 glucosyl-fructose linkages or alpha-1,3 and/or alpha-1,6
glucosyl-glucose linkages in Examples 11, 12, 15 and 16 provided below.
82
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Discovery of an Aspergillus clavatus alpha-glucosidase (AcIWO)
A strain of Aspergillus clavatus was selected as a potential source of other
enzymes that may be useful in various industrial applications. One of the
genes
identified in Aspergillus clavatus encodes an alpha-glucosidase and the
sequence of this gene, called "AcIglur, is provided in SEQ ID NO:4. The
corresponding protein encoded by SEQ ID NO:4 is provided in SEQ ID NO:5.
Aclglul belongs to Glycosyl hydrolase family 31 based on a PFAM search
(pfam.sanger.ac.uk web link). At the N-terminus, the protein (SEQ ID NO:5) has
a signal peptide with a length of 19 amino acids as predicted by SignalP
version
4.0 (Nordahl Petersen et aL, 2011, Nature Methods, 8:785-786). The presence
of a signal sequence suggests that AcIglul is a secreted enzyme._The amino
acid sequence of the predicted mature form of AcIglul is set forth as SEQ ID
NO:6.
Expression of Aspergillus clavatus alpha-alucosidase Aciglul
A synthetic AcIglul gene was cloned into pTrex3gM expression vector
(described in U.S. Patent Appl. Publ. No. 2011/0136197, incorporated herein by
reference) and the resulting plasmid was designated as pJG294. The sequence
of the Aclglul gene was confirmed by DNA sequencing.
Plasmid pJG294 was transformed into a quad deleted Trichoderma reesei
strain (described in W005/001036) using a biolistic method (Teo VS et al., J
Microbiol Methods, 51:393-9, 2002). The protein, which was predicted to
comprise SEQ ID NO:6, was secreted into the extracellular medium and filtered
culture medium was used to perform SDS-PAGE and alpha-glucosidase activity
assays to confirm enzyme expression.
Discovery of Neosartorya fischeri alpha-alucosidase Nfiglul
A strain of Neosartorya fischeri was selected as a potential source of other
enzymes that may be useful in various industrial applications. One of the
genes
identified in Neosartorya fischeri encodes an alpha-glucosidase and the
sequence of this gene, called "Nfiglul", is provided in SEQ ID NO:7. The
corresponding protein encoded by SEQ ID NO:7 is provided in SEQ ID NO:8.
83
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Nfiglul belongs to Glycosyl hydrolase family 31 based on a PFAM search
(pfam.sangerac.uk web link). At the N-terminus, the protein (SEQ ID NO:8) has
a signal peptide with a length of 19 amino acids as predicted by SignalP
version
4.0 (Nordahl Petersen et al., 2011, Nature Methods, 8:785-786). The presence
of a signal sequence suggests that Nfiglul is a secreted enzyrne. The amino
acid sequence of the predicted mature form of Nfiglul is set forth as SEQ ID
NO:
9.
Expression of Neosartorya fischeri alpha-olucosidase Nfiglul
A synthetic Nfiglul gene was cloned into pTrex3gM expression vector
(described in U.S. Patent Appl. Publ. No. 2011/0136197) and the resulting
plasmid was designated as pJG295. The sequence of the Nfiglul gene was
confirmed by DNA sequencing.
Plasmid pJG295 was transformed into a quad deleted Trichoderma reesei
strain (described in W005/001036) using a biolistic method (Te`o VS et al., J
Microbiol Methods, 51:393-9, 2002). The protein, which was predicted to
comprise SEQ ID NO:9, was secreted into the extracellular medium and filtered
culture medium was used to perform SDS-PAGE and alpha-glucosidase activity
assays to confirm enzyme expression.
Discovery of Neurospora crassa alpha-qlucosidase Ncrdlui
A strain of Neurospora crassa was selected as a potential source of other
enzymes that may be useful in various industrial applications. One of the
genes
identified in Neurospora crassa encodes an alpha-glucosidase and the sequence
of this gene, called "Nctcpui", is provided in SEQ ID NO:10. The corresponding
protein encoded by SEQ ID NO:10 is provided in SEQ ID NO:11. Ncrglul
belongs to Glycosyl hydrolase family 31 based on a PFAM search
(pfam.sanger.ac.uk web link). At the N-terminus, the protein (SEQ ID NO:11)
has a signal peptide with a length of 22 amino acids as predicted by SignalP
version 4.0 (Nordahl Petersen et al., 2011, Nature Methods, 8:785-786). The
presence of a signal sequence suggests that Ncrglul is a secreted enzyme. The
84
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
amino acid sequence of the predicted mature form of Ncrgiul is set forth as
SEQ
ID NO:12.
Expression of Neurospora crassa alpha-glucosidase Ncrglu1
A synthetic Norglul gene was cloned into pTrex3gM expression vector
(described in U.S. Patent Appl. Publ. No. 2011/0136197) and the resulting
plasmid was designated as pJG296. The sequence of the Norglul gene was
confirmed by DNA sequencing.
Plasmid pJG296 was transformed into a quad deleted Trichoderma reesei
strain (described in W005/001036) using a biolistic method (Te`o VS et al., J
Microbiol Methods, 51:393-399, 2002). The protein, which was predicted to
comprise SEQ ID NO:12, was secreted into the extracellular medium and filtered
culture medium was used to perform SDS-PAGE and alpha-glucosidase activity
assays to confirm enzyme expression.
Discovery of Rasamsonia composticola alpha-glucosidase TauSec098
A strain of Rasamsonia composticola was selected as a potential source
of other enzymes that may be useful in various industrial applications. One of
the genes identified in Rasamsonia composticola encodes an alpha-glucosidase
and the sequence of this gene, called "TauSec098", is provided in SEQ ID
NO:13. The corresponding protein encoded by SEQ ID NO:13 is provided in
SEQ ID NO:14. TauSec098 belongs to Glycosyl hydrolase family 31 and
contains an N-terminal CBM 20 domain based on a PFAM search
(pfam.sanger.ac.uk web link). At the N-terminus, the protein (SEQ ID NO:14)
has a signal peptide with a length of 22 amino acids as predicted by SignalP
version 4.0 (Nordahl Petersen et al., 2011, Nature Methods, 8:785-786). The
presence of a signal sequence suggests that TauSec098 is a secreted enzyme.
The amino acid sequence of the predicted mature form of TauSec098 is set forth
as SEQ ID NO:15.
Expression of Rasamsonia composticola alpha-qlucosidase TauSec098
A synthetic TauSec098 gene was cloned into the Trichoderma reesei
expression vector pGXT (a pTTT-derived plasmid) by Generay Biotech Co.
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
(Shanghai: China) and the resulting plasmid was designated as pGX256-
TauSec098. The sequence of the TauSec098 gene was confirmed by DNA
sequencing.
Plasmid pGX256-TauSec098 was transformed into a quad-deleted
Trichoderma reesei strain (described in W005/001036) using protoplast
transformation (Te'o et al., J. Microbiol. Methods 51:393-399, 2002).
Transformants were selected on a medium containing acetamide as a sole
source of nitrogen (acetamide 0.6 g/L; cesium chloride 1.68 g/L; glucose 20
g/L;
potassium dihydrogen phosphate 15 g/L; magnesium sulfate heptahydrate 0.6
g/L; calcium chloride dihydrate 0.6 g/L; iron (II) sulfate 5 mg/L; zinc
sulfate 1.4
mg/L; cobalt (II) chloride 1 mg/L; manganese (II) sulfate 1.6 mg/L; agar 20
g/L;
pH 4.25). Transformed colonies (about 50-100) appeared in about 1 week. After
growth on acetamide plates, the spores of transformants were collected and
transferred into new acetamide agar plates. After 5 days of growth on
acetamide
plates, 1x108 spores were inoculated into 30 ml Glucose/Sophorose defined
media in a 250-mL shake flask. The shake flask was shook at 28 C for 5 days.
Supernatants from these cultures were used to confirm expression (SDS PAGE)
and activity of mature TauSec098 enzyme (SEQ ID NO:15).
Discovery of Rasamsonia composticoia alpha-dlucosidase TauSec099
A strain of Rasamsonia composticola was selected as a potential source
of other enzymes that may be useful in various industrial applications. One of
the genes identified in Rasamsonia composticola encodes an alpha-glucosidase
and the sequence of this gene, called "TauSec099": is provided in SEQ ID
NO:16. The corresponding protein encoded by SEQ ID NO:16 is provided in
SEQ ID NO:17. TauSec099 belongs to Glycosyl hydrolase family 31 based on a
PFAM search (pfam.sanger.ac.uk web link). At the N-terminus, the protein (SEQ
ID NO:17) has a signal peptide with a length of 17 amino acids as predicted by
SignalP version 4.0 (Nordahl Petersen et al., 2011, Nature Methods, 8:785-
786).
The presence of a signal sequence suggests that TauSec099 is a secreted
86
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
enzyme. The amino acid sequence of the predicted mature form of TauSec099
is set forth as SEQ ID NO:18.
Expression of Rasamsonia composficola alpha-glucosidase TauSec099
A synthetic TauSec099 gene was cloned into the Trichoderma reesei
expression vector pGXT (a pTTT-derived plasmid) by Generay Biotech Co.
(Shanghai, China) and the resulting plasmid was designated as pGX256-
TauSec099. The sequence of the TauSec0998 gene was confirmed by DNA
sequencing.
Plasmid pGX256-TauSec099 was transformed into a quad-deleted
Trichoderma reesei strain (described in W005/001036) using protoplast
transformation (Te'co et al., J. Microbiol. Methods 51:393-399, 2002).
Transformants were selected on a medium containing acetamide as a sole
source of nitrogen (acetamide 0.6 g/L; cesium chloride 1.68 g/L; glucose 20
g/L:
potassium dihydrogen phosphate 15 g/L; magnesium sulfate heptahydrate 0.6
g/L; calcium chloride dihydrate 0.6 g/L; iron (H) sulfate 5 mg/L; zinc sulfate
1.4
mg/L; cobalt (H) chloride 1 mg/L; manganese (H) sulfate 1.6 mg/L; agar 20 g/L;
pH 4.25). Transformed colonies (about 50-100) appeared in about 1 week. After
growth on acetamide plates, the spores of transformants were collected and
transferred into new acetamide agar plates. After 5 days of growth on
acetamide
plates, 1x108 spores were inoculated into 30 ml Glucose/Sophorose defined
media in a 250-mL shake flask. The shake flask was shook at 28 'C for 5 days.
Supernatants from these cultures were used to confirm expression (SDS PAGE)
and activity of mature TauSec099 enzyme (SEQ ID NO:18).
Sequences of Bifidobacterium ionqum alpha-qiucosidase BloGlul
An alpha-glucosidase gene, "BloGlul", was identified from Bifidobacterium
long= subsp. longum JDM301. The nucleic acid sequence for the BtoGlul
gene (SEQ ID NO:19, GENBANK Am. No. NC014169.1, complement sequence
from positions 140600 to 142414) and the amino acid sequence of the
hypothetical protein (SEQ ID NO:20) encoded by SEQ ID NO:19 were found in
GENBANK Acc. No. YP 003660432.1.
87
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Expression of Bifidobacteriurn lonqum alpha-glucosidase BloGlul
The DNA sequence encoding the entire BloGlul protein (SEQ ID NO:20)
was optimized for expression in B. subfilis, then synthesized (yielding SEQ ID
NO:21) and inserted into the p3JIVI plasmid by Generay Biotech Co. (Shanghai,
China), resulting in p3JM-BloGlul. The p3JM-BloGlul plasmid contains an aprE
promoter to drive expression of the optimized BloGiul sequence (SEQ ID
NO:21).
Plasmid p3JM-BloGiu1 was used to transform B. subtifis cells (degUHy32,
AnprB, Avpr, Aepr, AscoC, AwprAõAmpr, AispA, Abpr), and the transformed cells
were spread on Luria Agar plates supplemented with 5 ppm chloramphenicol. A
colony with correct insertion, as confirmed by PCR and sequencing, was
selected and subjected to -fermentation in a 250-EnL shake flask with MBD
medium (a MOPS-based defined medium supplemented with an additional 5 mM
CaCl2) to express BloGlu1 protein (SEQ ID NO:20).
Sequences of Bifidobacterium lonqum alpha-glucosidase BloGiu2
An alpha-glucosidase, BloGlu2, was identified from Bifidobacterium
longum. The amino acid sequence (SEQ ID NO:22) of BloGlu2 was found in the
NCBI database (GENBANK Acc. No. WP_007054665.1).
Expression of Bifidobactenum loncium alpha-glucosidase BloGlu2
A DNA sequence encoding BloGlu2 protein was optimized for expression
in B. subtilis, then synthesized (yielding SEQ 10 NO:23) and inserted into the
p3JM plasmid by Generay Biotech Co., resulting in p3JM-BloGiu2. SEQ ID
NO:23 encodes the amino acid sequence of SEQ ID NO:24. The p3JM-BloGlu2
plasmid contains an aprE promoter to drive expression of the optimized BioGiu2
sequence (SEQ ID NO:23).
Plasmid p3JM-BloGlu2 was used to transform B. subfifis cells (degUHy32,
AnprB, Avpr, Aepr, AscoC, AwprA, Ampr, AispA, Abpr), and the transformed cells
were spread on Luria Agar plates supplemented with 5 ppm chloramphenicol. A
colony with correct insertion, as confirmed by PCR and sequencing, was
selected and subjected to fermentation in a 250-mL shake flask with MBD
88
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
medium (a MOPS-based defined medium supplemented with an additional 5 mM
CaCl2) to express BloGlu2 protein (SEQ 10 NO:24).
Sequences of Bifidobacterium lonqum alpha-qlucosidase BloGlu3
An alpha-glucosidase gene, "BloGlu3", was identified from Bifidobacterium
longum subsp. longum F8. The nucleic acid sequence for the BloGlu3 gene
(SEQ ID NO:25, GENBANK Acc. No. NC_021008.1, positions 2130627 to
2132441), and the amino acid sequence of the hypothetical protein (SEQ ID
NO:26) encoded by SEQ ID NO:25 were found in GENBANK Acc. No.
YP 007768249.1.
Expression of Bifidobacterium loncium alpha-qlucosidase BloGlu3
The DNA sequence encoding the entire BloGlu3 protein (SEQ ID NO:26)
was optimized for expression in B. subfilis, then synthesized (yielding SEQ ID
NO:27) and inserted into the p3JM plasmid by Generay Biotech Co., resulting in
p3JM-BloGlu3. The p3JM-BloGlu3 plasmid contains an aprE promoter to drive
expression of the optimized BloGlu3 sequence (SEQ. ID NO:27).
Plasmid p3JM-BloGlu3 was used to transform B. subtilis cells (degUHy32,
AnprB, Avpr, Aepr, AscoC, AwprA, Ampr, AispA, Abpr), and the transformed cells
were spread on Luria Agar plates supplemented with 5 ppm chloramphenicol. A
colony with correct insertion, as confirmed by PCR and sequencing, was
selected and subjected to fermentation in a 250-hiL shake flask with MBD
medium (a MOPS-based defined medium supplemented with an additional 5 mM
CaCl2) to express BloGlu3 protein (SEQ ID NO:26).
Sequences of Bifidobacterium pseudolongum alphaaqlucosidase BpsGlul
An alpha-glucosidase, BpsGlul, was identified from Bifidobacterium
pseudotongum. The amino acid sequence (SEQ ID NO:28) of BpsGlul was
found in the NCB1 database (GENBANK Acc. No. WP 022858408.1).
Expression of Bifidobacterium pseudolonqum alpha-alucosidase BpsGlu1
A DNA sequence encoding BpsGlul protein was optimized for expression
in B. subtilis, then synthesized (yielding SEQ ID NO:29) and inserted into the
89
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
p3JM plasmid by Generay Biotech Co., resulting in p3JM-BpsGlul. SEQ ID
NO:29 encodes the amino acid sequence of SEQ ID NO:30. The p3JM-BpsGlul
plasmid contains an aprE promoter to drive expression of the optimized BpsGlul
sequence (SEQ ID NO:29)
Plasnlid p3JM-BpsGlul was used to transform B. subliiis cells
(degUHy32, AnprB, Avpr, Aepr, AscoC, AwprA, Amp., AispA, Abpr), and the
transformed cells were spread on Luria Agar plates supplemented with 5 ppm
chloramphenicol. A colony with correct insertion, as confirmed by PCR and
sequencing, was selected and subjected to fermentation in a 250-mL shake flask
with MBD medium (a MOPS-based defined medium supplemented with an
additional 5 mM CaCl2) to express BpsGlul protein (SEQ ID NO:30).
Sequences of Bifidobacterium thermophilum alpha-qlucosidase BthGlul
An alpha-glucosidase gene, "BthGlui", was identified from Bificlobacterium
thermophitum RBL67. The nucleic acid sequence of the BthGlul gene (SEQ ID
NO:31, GENBANK Acc. No. NC_020546.1, positions 150690 to 152495), and the
amino acid sequence of the hypothetical protein (SEQ ID NO:32) encoded by
SEQ ID NO:31 were found in GENBANK Acc. No. YP 007592840.1.
Expression of Bifidobacterium thermophilurn alpha-glucosidase BthGlul
The DNA sequence encoding the entire BthGlul protein (SEQ ID NO:32)
was optimized for expression in B. subtilis, then synthesized (yielding SEQ ID
NO:33) and inserted into the p3JIM plasmid by Generay Biotech Co., resulting
in
p3JM-BthGlu1. The p3J11,1-BthGlul plasmid contains an aprE promoter to drive
expression of the optimized BiliG/u/ sequence (SEQ ID NO:33).
Plasmid p3JM-BthGlul was used to transform B. subtilis cells (degUHy32,
AnprE3, Avpr, Aepr, AscoC, AwprA, Ampr, AispA, Abpr), and the transformed
cells
were spread on Luria Agar plates supplemented with 5 ppm chloramphenicol. A
colony with correct insertion, as confirmed by PCR and sequencing, was
selected and subjected to fermentation in a 250-mL shake flask with MBD
medium (a MOPS-based defined medium supplemented with an additional 5 rnM
CaCl2) to express BthGlul protein (SEQ ID NO:32).
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Sequences of Bifidobacterium breve alpha-glucosidase BbrGiu2
An alpha-glucosidase, BbrGlu2, was identified from Bifidobacterium breve.
The amino acid sequence (SEQ ID NO:34) of BbrGlu2 was found in the NCBI
database (GENBANK Acc. No. WP 003827971.1).
Expression of Bifidobactetium breve alpha-glucosidase BbrGiu2
A DNA sequence encoding BbrGiu2 protein was optimized for expression
in B. subtilis, then synthesized (yielding SEQ ID NO:35) and inserted into the
p3JM plasmid by Generay Biotech Co., resulting in p3JM-BbrGlu2. SEQ ID
NO:35 encodes the amino acid sequence of SEQ ID NO:36. The p3JM-BbrGlu2
plasmid contains an aprE promoter to drive expression of the optimized BbrGiu2
sequence (SEQ ID NO:35)
Plasmid p3.1M-BbrGiu2 was used to transform B. subtilis cells (degUHy32,
AnprB, Avpr, AeprõAscoC, AwprA, Ampr, AispA, Abpr), and the transformed cells
were spread on Luria Agar plates supplemented with 5 ppm chloramphenicol. A
colony with correct insertion, as confirmed by PCR and sequencing, was
selected and subjected to fermentation in a 250-mL shake flask with MBD
medium (a MOPS-based defined medium supplemented with an additional 5 mIVI
CaCl2) to express SEQ ID NO:36.
Sequences of Bifidobacterium breve alpha-glucosidase BbrG1u5
An alpha-glucosidase gene, "BbrGlu5", was identified from
Bifidabacterium breve ACS-071-V-Sch8b. The nucleic acid sequence of the
BbrGiu5 gene (SEQ ID NO:37, GENBANK Acc. No. NC017218.1, complement
of sequence from positions 2241075 to 2242895), and the amino acid sequence
of the hypothetical protein (SEQ ID NO:38) encoded by SEQ ID NO:37 were
found in GENBANK Acc. No. YP 005583701.1.
Expression of Bifidobacterium breve alpha-glucosidase BbrG1u5
The DNA sequence encoding the entire BbrG1u5 protein (SEQ ID NO:38)
was optimized for expression in B. subtilis, then synthesized (yielding SEQ ID
NO:39) and inserted into the p3JM plasmid by Generay Biotech Co., resulting in
91
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
p3JM-BbrGlu5. The p3JM-BbrGlu5 plasmid contains an aprE promoter to drive
expression of the optimized BbrGIu5 sequence (SEQ ID NO:39).
Plasmid p3JM-BbrG1u5 was used to transform B. subtilis cells (degUHy32,
AnprB, Avpr, Aepr, AscoC, AwprA, Ampr, AispA, Abpr), and the transformed cells
were spread on Luria Agar plates supplemented with 5 ppm chloramphenicol. A
colony with correct insertion, as confirmed by PCR and sequencing, was
selected and subjected to fermentation in a 250-mL shake flask with MBD
medium (a MOPS-based defined medium supplemented with an additional 5 mM
CaCl2) to express BbrG1u5 protein (SEQ. ID NO:38).
Purification of alpha-qlucosidases from expression cultures
AcIGIu1 and NcrGlul
Both AcIGIul (SEQ ID NO:6) and NcrGlul (SEQ ID NO:12) alpha-
glucosidases were purified using two chromatography steps. For each
purification, the crude broth from the shake flask was concentrated, after
which
ammonium sulfate was added to a final concentration of 2 M. The solution was
loaded onto a 50-mL phenyl HP column pre-equilibrated with 20 mM Tris pH 8.0,
2 M ammonium sulfate. The target protein (SEQ ID NO:6 or SEQ ID NO:12) was
eluted from the column with 1 M ammonium sulfate, 20 mM Tris pH 8Ø
Respective fractions were pooled, concentrated and buffer-exchanged into 20
rriM Tris pH 8.0 (buffer A), using a VIVAFLOW 200 ultrafiltration device
(Sartorius
Stedim). The resulting solution was applied to a 40-mL Q HP column pre-
equilibrated with buffer A. The target protein was eluted from the column with
0.3
M NaCI in buffer A. The fractions containing target protein were then pooled
and
concentrated using 10K AMICON ULTRA-15 devices, and stored in 40% glycerol
at -20 'C until usage.
NfiGlul
NfiG1u1 alpha-glucosidase (SEQ ID NO:9) was purified using two
hydrophobic interaction chromatography steps. The crude broth from the shake
flask was concentrated, after which ammonium sulfate was added to a final
concentration of 1 M. The solution was loaded onto a 50-mL phenyl HP column
92
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
pre-equbrated with 20 mM Tris pH 8.0, 1 M ammonium sulfate. The target
protein (SEQ ID NO:9) flowed through the column. Flow-through fractions were
pooled, after which ammonium sulfate was added to a final concentration of 2
M.
The solution was loaded onto the same phenyl HP column pre-equilibrated with
20 mM Tris pH 8.0, 2 M ammonium sulfate. The target protein was eluted from
the column with 1 M ammonium sulfate, 20 mM Tris pH 8Ø The fractions
containing target protein were then pooled and concentrated using 10K AMICON
ULTRA-15 devices, and stored in 40% glycerol at -20 'C until usage.
TauSec098 and TauSec099
Both TauSec098 (SEQ ID NO:15) and TauSec099 (SEQ ID NO:18) alpha-
glucosidases were purified via hydrophobic interaction chromatography. For
each purification, ammonium sulphate was added to about 180 rnL of
concentrated crude broth from a 7-L fermenter to a final concentration of 1 M.
This solution was then loaded onto a 50-mL HIPREP phenyl-FF Sepharose
column (GE Healthcare) pre-equilibrated with 20 mM sodium acetate pH 5.0,1 M
ammonium sulphate (buffer A). After washing with the same buffer with three
column volumes (CVs), the column was eluted stepwise with 75%, 50% and 0%
buffer A using three CVs each, followed by two CVs of MILLIQ H20. All
fractions
were analyzed by SDS-PAGE. The target protein (SEQ ID NO:15 or SEQ ID
NO:18) was mainly present in the flow-through fraction, which was concentrated
and buffer-exchanged to rernove excess ammonium sulfate using 10 KDa
AMICON ULTRA-15 devices. The final product, which was greater than 90%
pure, was stored in 40% glycerol at -80 'C until usage.
BloGlul, BloGiu2 and BloGiu3
BloGlul (SEQ ID NO:20), BloGlu2 (SEQ ID NO:24) and BloGlu3 (SEQ ID
NO:26) alpha-glucosidases were all purified in three steps. For each
purification,
the crude broth from a 1-L DASGIP fermenter was concentrated, after which
arnmonium sulfate was added to 60% saturation. The solution was stirred at 4
C for 1 hr, and then centrifuged at 8000 X g for 30 min. The resulting pellet
was
re-suspended in 20 mM Tris pH 8.0 (buffer A). Ammonium sulfate was added to
the resulting solution to a final concentration of 1 M; this preparation was
then
93
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
loaded onto a 40-mL HiPrepTm Phenyl FF column pre-equilibrated with 20 mM
Tris pH 8.0, 1 M ammonium sulfate (buffer B). After washing, the column was
eluted stepwise with 75%, 50%, and 0% buffer B and H20 in three column
volumes each. All fractions were analyzed using SDS-PAGE and activity assays.
The fractions containing target protein (SEQ ID NO:20, SEQ ID NO:24, or SEQ
ID NO:26) were pooled, concentrated and subsequently loaded onto a HìLoadTM
26/60 SuperdexTm 75 column pre-equilibrated with 20 mM sodium phosphate pH
7.0, 0.15 M NaCI. Flow-through fractions containing the target protein were
then
pooled and concentrated using 10K AMICON ULTRA-15 devices, and stored in
40% glycerol at -20 C until usage.
BpsGlul and BthGlul
Both BpsG1u1 (SEQ ID NO:30) and BthGlul (SEQ ID NO:32) alpha-
glucosidases were purified in two steps. For each purification, the crude
broth
from a 1-L DASGIP fermenter was concentrated, after which ammonium sulfate
was added to 60% saturation. The solution was stirred at 4 C for 1 hr, and
then
centrifuged at 8000 X g for 30 min. The resulting pellet was re-suspended in
20
mM Tris pH 8.0 (buffer A). Ammonium sulfate was added to the resulting
solution to a final concentration of 1 M; this preparation was then loaded
onto a
40-mL HiPrepTM Phenyl FF column pre-equilibrated with 20 mM Tris pH 8.0, 1 M
ammonium sulfate (buffer B). After washing, the column was eluted stepwise
with 75%, 50%, and 0% buffer B and H20 in three column volumes each. All
fractions were analyzed using SDS-PAGE and activity assays. The target
protein (SEQ ID NO:30 or SEQ ID NO:32) was present in the eluate from the 0%
buffer B elution step; this eluate was pooled and concentrated using 10K
AMICON ULTRA-15 devices. The final product, which was greater than 95%
pure, was stored in 40% glycerol at -20 'C until usage.
BbrGlu2 and BbrGlu5
Both BbrGlu2 (SEQ ID NO:36) and BbrGlu5 (SEQ ID NO:38) alpha-
glucosidases were purified in four steps. For each purification, the crude
broth
from a 1-L DASGIP fermenter was concentrated, after which ammonium sulfate
was added to 60% saturation. The solution was stirred at 4 C for 1 hr, and
then
94
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
centrifuged at 8000 X g for 30 min. The resulting pellet was re-suspended in
20
mM HEPES pH 7.0 (buffer A). Ammonium sulfate was added to the resulting
solution to a final concentration of 1 M; this preparation was then loaded
onto a
HiPrepThl Phenyl FF column pre-equilibrated with 20 mM HEPES pH 7.0, 1 M
ammonium sulfate. The target protein (SEQ ID NO:36 or SEQ ID NO:38) was
eluted from the column with 0.5 M ammonium sulfate. Respective fractions were
pooled, concentrated and buffer-exchanged into buffer A using a VIVAFLOW 200
ultrafiltration device (Sartorius Stedim). The resulting solution was applied
to a
HiPrepTm Q FF 1 611 0 column pre-equilibrated with buffer A. Target protein
was
eluted from the column with a linear gradient of 0-0.5 M NaCI in buffer A.
Fractions containing target protein were pooled, concentrated and subsequently
loaded onto a HiLoadTm 26/60 SuperdexTm 75 column pre-equilibrated with 20
mM HEPES pH 7.0, 0.15 M NaCI. The fractions containing target protein were
then pooled and concentrated using 10K AMICON ULTRA-15 devices, and
stored in 40% glycerol at -20 C until usage.
Thus, various additional alpha-glucosidases were expressed and purified.
These alpha-glucosidases were tested for hydrolytic activity against alpha-1,5
glucosyl-fructose linkages and alpha-1,3 and/or alpha-1,6 glucasyl-glucose
linkages in Examples 11, 12, 15 and 16 provided below.
EXAMPLE 11
Testing Alpha-Glucosidases for Hydrolytic Activity Against Various Glycosidic
Linkages
This example discloses testing whether alpha-glucosidases have
hydrolytic activity beyond that previously associated with this class of
enzymes
(EC 3.2.1.20). Alpha-glucosidases from Example 10 were shown to have
hydrolytic activity against alpha-1,5 glucosyl-fructose linkages and alpha-1,3
and
alpha-1,6 glucosyl-glucose linkages.
Substrate Specificity of Alpha-Glucosidases
The substrate specificity of each alpha-glucosidase disclosed in Example
10 was assayed based on the release of glucose from a particular substrate
(isomaltose, maltose, panose, leucrose, or nigerose) when the substrate was
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
incubated with alpha-glucosidase. The rate of glucose release was measured
using a coupled glucose oxidaselperoxidase (GOX/HRP) method (1980, Anal.
Biochern. 105:389-397). Glucose release was quantified as the rate of
oxidation
of 2,2'-azino-bis 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) by peroxide
that
was generated from coupled GOX/HRP enzymes reacted with glucose.
Individual substrate solutions were prepared by mixing a 9 mL solution of
substrate (1 ,,, in water, w/v) with 1 mL of 0.5 M pH 5.0 sodium acetate
buffer and
40 pL of 0.5 M calcium chloride in a 15-mL conical tube. Coupled enzyme
(GOX/HRP) solution with ABTS was prepared in 50 mM sodium acetate buffer
(pH 5.0), with the final concentrations of 2.74 mg/mL ABTS, 0.1 U/mL HRP, and
1 U/mL GOX. Serial dilutions of individual alpha-glucosidase samples and
glucose standard were prepared in MILLIQ water. For nigerose, alpha-
glucosidase samples were tested with only one dosage at 10 ppm due to a
limited stock of substrate solutions. Each alpha-glucosidase sample (10 pL)
was
transferred into a new microtiter plate (Corning 3641) containing 90 pL of
substrate solution pre-incubated at 50 C for 5 min at 600 rpm. Reactions were
carried out at 50 'C for 10 min (for isomaltose, maltose, panose, and nigerose
substrates), or for 60 min (for leucrose substrate) with shaking (600 rpm) in
a
THERMOMIXER (Eppendorf). 10 pL of each reaction mix, as well as 10 pL of
serial dilutions of glucose standard, were then quickly transferred to new
rnicrotiter plates (Coming 3641), respectively, to which 90 pL of ABTS/GOX/HRP
solution was then added accordingly. The microtiter plates containing reaction
mixes were immediately measured at 405 nm at 11 second intervals for 5 min
using a SOFTMAX PRO plate reader (Molecular Devices). The output was the
reaction rate, Vo, for each enzyme concentration. Linear regression was used
to
determine the slope of the plot V, vs. enzyme dose. The specific activity of
each
alpha-glucosidase was calculated based on the glucose standard curve using
Equation 1:
Specific Activity (Unit/mg) = Slope (enzyme) / slope (std) x 1000 (1),
where 1 Unit .1 prnol glucose / min.
96
CA 02940778 2016-08-25
WO 2015/130883 PCT/US2015/017648
For nigerose, the value of the reaction rate with enzyme dosage at 10 ppm was
directly used to indicate enzyme activity.
Using the foregoing method, the specificity of each alpha-glucosidase was
determined against each substrate. The activities of an oligo-1,6-glucosidase
(purchased from Megazyme, see Table 4) and a transglucosidase (TG t..-2000,
see Table 4) against each substrate were also measured. The results of this
analysis are provided in Table 17.
Table 17
Activity of Various Alpha-Glucosidases Against Different Substrates
Enzyme Activity (U/mg) as Measured on:
SE0
lsomaltose Maltose Panose Leucrose Nigerosea
ID
Enzyme NO.
Oligo-1,6- 118.2 0.0 54.3 1.3 19.6
glucosidase
TG L.-2000 1 = 194.0 235.6 = 127.7 68.9 254.0
AciGiul 6 255.7 401.9 180.9 113.7 315.1
NfiGlul 9 521.2 360.0 126.9 89.4 264.3
NcrGtul 12 282.7 34.9 15.9 61.6 200.4
TauSec098 15 = 54.9 123.8 = 23.8 1.8
305.6
TauSec099 18 244.0 97.7 50.8 70.6 184.8
BloGiul 20 71.1 66.9 23.1 2.5 165.0
BloGiu2 24 65.9 86.7 19.9 3.5 217.9
BloGiu3 26 = 120.1 175.5 = 31.4 9.0
272.6
BspGiul 30 64.2 247.9 60.8 27.3 254.6
Maul 32 108.3 93.3 21.1 68.4 128.5
BbrGiu2 36 106.6 167.5 26.9 6.1 258.8
BbrGiu5 38 925.8 0.0 279.7 2.8 22.1
a Each enzyme was used at one dosage (10 ppm) against nigerose.
Interestingly, it was found that alpha-glucosidases, besides exhibiting
hydrolytic activity against alpha-1,4 glucosyl-glucose linkage (maltose), also
exhibit hydrolytic activity against alpha-1,6 glucosyl-glucose linkage
(isomaltose),
97
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
alpha-1,3 glucosyl-glucose linkage (nigerose), and alpha-1,5 glucosyl-fructose
linkage (leucrose) (Table 17).
Thus, alpha-glucosidases have hydrolytic activity beyond that previously
associated with EC 3.2.1.20 enzymes. Specifically, alpha-glucosidases have
hydrolytic activity against alpha-1,5 glucosyl-fructose linkages and alpha-1,3
and
alpha-1,6 glucosyl-glucose linkages.
EXAMPLE 12
I--lydrolysis of Leucrose and Oligosaccharides in Glucan Reaction Filtrate
Using
Alpha-Glucosidase
This Example describes using alpha-glucosidase to hydrolyze leucrose
and other oligosaccharides present in filtrate obtained from a glucan
synthesis
reaction. Specifically, the effect of alpha-glucosidases disclosed in Exarnple
10
on the hydrolysis of leucrose and oligosaccharides DP2, DP3 and HS (higher
sugars, DP4+) in a filtrate of an insoluble glucan (poly alpha-1,3-glucan)
synthesis reaction was studied.
Isolation and Analysis of Oliaosaccharides for Testing Against Alpha-
Glucosidase Activity
First, a concentrated filtrate of a glucan synthesis reaction was prepared
as per Example 1.
Briefly, oligosaccharides were isolated from the concentrated filtrate by
chromatographic separation, and analyzed for glycosidic linkage profile.
Chromatographic separation employing a strong acid cation-exchange resin was
used to isolate the oligosaccharide fraction of the concentrated filtrate. The
physical parameters of the column used for this separation were as follows:
FINEX CS11GC, #227 resin; Na + ion form; 5% divinyl benzene (crosslinking);
0.34 mm particle size; 1.64 m bed length; 0.093 m column diameter.
In more detail, the concentrated sugar solution (i.e., concentrated filtrate)
described in Table 3 was filtered and diluted to 25 g dry solids/100 g
solution
using tap water. Prior to addition of this sugar solution to the column resin,
the
resin was washed with six bed volumes (BV) of sodiurn chloride solution (three
BV at 10 wt% sodium chloride followed by three BV at 5 wt% sodium chloride) to
98
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
convert the resin to the sodium form. The sugar solution (0.6 L) was then fed
to
the column, after which the column was eluted using water at a flow rate of 50
rnLirnin. The run conditions of the chromatographic separation are sumrnarized
as follows: 0.6 L feed size, 25 g dry solids/100 g solution, 65 C column
temperature, 50 EnL/min flow rate. An oligosaccharide solution was eluted
between 11 and 21 minutes. A small amount of salts ¨ indicated by an increase
in conductivity ¨ was eluted at the same time. The oligosaccharide fraction
thus
prepared was analyzed by HPLC to determine its product distribution. In total,
the fraction contained >89% of oligosaccharides containing three or more
hexose
units and less than 1.5% of identifiable mono- and di-saccharides. This
fraction
was concentrated to a total dry weight of 317 g/L using a thin film evaporator
(LC1
Corporation, Charlotte, NC) followed by rotary evaporation with a ROTAVAPOR
(R-151; Buchi, New Castle, DE). The product distribution of the concentrated
fraction as measured by HPLC appears in Table 18.
Table 18
Product Distribution of Concentrated Oligosaccharide Fraction
Sucrose Leucrose Glucose Fructose DP2 DP3 DP4 DP5 DP6 DP7 Total
9/1.. 0.0 2.5 0.0 0.7
31.5 75.9 101.8 62.1 26.9 15.3 316.7
wt% 0.0 0.8 0.0 0.2
9.9 23.9 32.1 19.6 8.5 4.8 100
Primary Screening of Alpha-Glucosidases on Glucan Oligomer Hydrolysis
The activities of eleven different alpha-glucosidases (Example 10), as well
as the activities of two benchmark enzymes, oligo-1,6-glucosidase (purchased
from Megazyme) and transglucosidase (TG L-2000), were individually evaluated
against the purified oligosaccharide fraction prepared above (Table 18). Each
alpha-glucosidase (dosed at 1 mg/mL) was incubated in a solution containing
oligosaccharide substrates (2.9% dry solids) and 2 mM calcium chloride at pH
5.0 at 60 'C. Each reaction was quenched after 24 hours of incubation by
adding
50 pL of 0.5 M NaOH.
The oligosaccharide/monosaccharide contents of the quenched reactions
were determined as follows. A sample of each reaction was diluted 5-fold in
water for HPLC analysis. HPLC separation was done using an Agilent 1200
99
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
series HPLC system with an AMINEX HPX-42A column (300 mm x 7.8 mm) at
85 ''''C. The sample (10 juL) was applied to the HPLC column and separated
with
an isocratic gradient of MILLI-Q water as the mobile phase at a flow rate of
0.6
nillmin. Oligosaccharide products were detected using a refractive index
detector. The numbers provided in Table 19 below reflect the average of peak
area percentages (from duplication of each sample) of each DP n as a fraction
of
the total from DP1 to DP7.
Table 19
Analysis Glucan Filtrate Oligosaccharides Following Treatment with Alpha-
Glucosidase
SEQ
Enzyme ID
NO DP7% DP6% DP5% DP4% = DP3% = DP2% DPI%
Oligo-1,6- ...
_9Iucosidase 0,0 0,6 8,7 27,9 21,9 ::::iiiiii
12,2 28.7
õ,.....................
, TG L-2000 ,
= 0,0 0,0 0,0 0.0 0,0 3.0
, i,i,i,i,i,i,i,,..i::::=.i:::i,i,,,,i,i,i,i,i,i,i,
N.Ifiglul. . 9 OM OM OM OM 0.0 1,..,-.1
Ncrgiul . 12 0.0 0.0 0.0 2.8 1.8 TO ElIftellg
, TauSec098 15 OM OM OM 15,3 0.0 4,5
lltIlØ.4W:i
,
........................
TauSec099 18 0.0 0,0 0,0 15,7 , ,
0,0 =0.5
. .
8IoGhia 20 0.0 0,0 8.7
M316%3:::::::::::::::::::::: m='-.5.5:1".3 0,0 21,3
BloGiu2 24 0,0 0,7 8,6 m31.2g.. m34:::=R 14,0
10,7
BloGiu3 26 0,0 0.6 8,0 28.4UgX.1. 13.9
15,6
:::i:i.......
BspGlul 30 0.0 0.5 5,2 15,4 16,2 8,2
;i:.... &1,5
BthOW. 32 0.0 0.0 13,0 12,6 2,0 1.9
:i:i:i:i:i:i:70,45.
..........:.. .. .,f . ..,,,,,,
BbrGiu2 36 0,0 0,0 21,5 30,7 24,6 0,0 41123an
. BbrGlu5 , 38 0,0 0,0 8,1 23,8 ___ 12,1 15,5
Blank 0.0 0.0 16.2 Ug3 :30,4 0.0
14.8
As indicated with shading in Table 19, the oligosaccharide content of the
reactions generally shifted toward smaller sized sugars, in comparison with
the
control reaction ("Blank") in which there was no enzyme. These results
indicate
that alpha-glucosidase can be used to hydrolyze oligosaccharides comprised
within a glucan synthesis reaction and a fraction thereof. Also, given the
linkage
profile of the oligosaccharides (Examples 3 and 4), and the activity of alpha-
glucosidase against various glycosidic linkages in addition to alpha-1,4
linkages
(Example 11), it is apparent that alpha-glucosidase can be used to break down
100
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
oligosaccharides with alpha-1,5 glucosyl-fructose linkages and/or alpha-1,3
and
alpha-1,6 glucosyl-glucose linkages. The results provided in Table 19 also
suggest that fungal alpha-glucosidases have better hydrolytic activity towards
soluble oligosaccharides compared with the bacterial alpha-glucosidases.
Confirmation of Alpha-Glucosidase Hydrolytic Activity Toward Oliclosaccharide
Products of Glucan Synthesis Reactions
Reactions were prepared comprising one or two alpha-glucosidases and a
concentrated filtrate obtained from a poly alpha-1,3-grucan synthesis reaction
(Table 3). Alpha-glucosidase reactions were dosed with enzyme at 4 ppm, or for
blends, each enzyme was used at a 1:1 ratio with a final dosage of 4 ppm. The
concentrated filtrate was loaded in each reaction at 10% dry solids. Each
reaction further comprised 2 mM calcium chloride at pH 5.0, and was carried
out
at 60 C or 65 'C. The reactions were quenched by adding 50 pi_ of 0.5 M NaOH
after a 23-hour incubation.
The oligosaccharidelmonosaccharide contents of the quenched reactions
were determined as follows. A sample of each reaction diluted 25-fold in water
for HPLC analysis. HPLC separation was done using an Agilent 1200 series
HPLC system with an AMINEX HPX-42A column (300 mm x 7.8 mm) at 85 'C.
The sample (10 pi..) was applied to the HPLC column and separated with an
isocratic gradient of MILLI-Q water as the mobile phase at a flow rate of 0.6
Oligosaccharide products were detected using a refractive index
detector. The numbers provided in Table 20 below reflect the average of peak
area percentages (from duplication of each sample) of each DP, as a fraction
of
the total. The results provided in Table 20 generally confirm the activity of
certain
alpha-glucosidases as discussed above regarding the results provided in Table
19.
101
CL611f.iµNOPCT
=
GO Table 20
_
_
= .tp
v:
t--
=
' = :, t-, " =====,... e
,..! !,.. .2..:-..= .) 2., ....... Ft rate -
... ., ....
Ar 91' .e.:s Clut=an FiltratP Sq.gar.s Following Treatment
o
.
in¨ ........................................... _ , ..............
_ ______________ _. __ ......._.¨..õ,.,.
1.= 3
C
el 1 SEQ ID
i
rn Tertip. Enzyyne NO DP7+% DP7'7 , DPS% DP5*/0 1 DP4%
(P3% I FJP2.; Leur...roN-a,,(;10 GIuco..,s_f.,..7,0_ Fru....,,c,..t.,)sea,fo,
.1 ¨ = ........ = .....,.. õ
! .. - 4 A ii== ".-.i 1 : '6 ,) .:: 0 2y: . = :.-
',=:.w.mr.440.7, ??-'%i.t,..:=-=0:.m
- E-, TO L-2000 = 'l : 5.'...8..:,'Y 0,2
0.4 = i ..4 ::: . i ,P . . 4. Y - ! ''`-= .=,.--.0õõõõ:,
'`" . ::"..'''......:::::44i'. . . .
.:::::::::':::"'=====.'...........'..:.../..1...'õ:./.,,
,.. .. 1 ---..- -7". .. = .. = 7:7--'' i..
:1 .9 .: ... .3 4 :
i.=:.ig1;',i:::':::::',,A&A.'='=..7'..::."'":'''''':::',.'2.6.-'''-"Z."]''':-
,:a=g1".=taRTIME.
a TaAlSec098 15 ::5:.4 = 0;0 ..i.
0.1 .:...4.::.:.:0.2.......:,:',:: Ø, ::.:=:... =-,,. =:.; ._ =
.. ========:.: --.,,z,,,,,õ,õ,,,- = :.... .=...... 7.....;:. ; -",t.
./).:),4=,=>: ======-
0, 5.l'....::...1:.-... -
= == 1...49 .====_._=,',.µ2,'8".
=.':'=:.:.6,,8,..=::::::=::.:=;.:=1.:,...,:, . i.Q.: 0
itto....fi.a.63t!....;:ii4;.:....1.;!!!!!:1,.!ffl_ ;#/.=// .. .....
. 4 ..... .: - - = = ... :
....... = = ===..4..:.......:== -.... 1
=
. " '-':::....:. === =.-.= ....:=========:=:....: ....
.........................
.............................
60 TauSec098+ 15: ' :::: :-.... = . = =
= :' ":===== ....::==. .. . = = : = = :::::::::::=.= -
=:::=::::::: = = ..:. =
.:.....,....2õ.....Ø).....:4',..g,.......:ma:
cC = TauSer099 1-..)" =6:4===== 0,1 0.1
0,2'. Ø5. 2.2 , ,.,..,=,,1 .. 0..0
:,.',=:Ø.....4.4....AM........i.i.....
,
-,-
::..-
.....:....:.:,:.:.:.::.....:.:.:.:::............................:/7/,"
=========-===========-===============================,
= i auSecO9B+ 15. =...
..--...:-!;:=-.: . .
,......!.....................................õ.õ........7
. õ...........................
. ............................
.. õ...õ................................................. ,
. 4
= ' TG L-2000 1= " ' '. '
0 1. ' 0.1 0.2 :0,4 : .= 1.8 1,:.::::8,1 .: 0.0
, .. .
. ....................................... 4., ....... . ,.. . ...
6.4. . . :4 2". .. .
::.,:::.:.:,:.:.=..:,.:,,7::z$/::::.:::-
l.
,-, = : :====:::: . -,,
,
- TG L-2000 i 1 = '.:.8.,3:::
.:.:::... v. '1 = 0,4 11::::::::1',1::.=7- ... 1 ,-
7,.,',.,..,':' '2.2' . "';',7=21..... .. 0.0 . -
1,:',,,.:I4..I....fj.:...?..!!r.., .............".
õ1..?7,......4...............:.
. ¨ i ¨ ' .=-=====-=.- = ==
= ----i , , =::. = = ;-==:::::.: : 4 .-: . . ..,..
q...... = :======..........::".",::.: .4=:.....t:;..:ivi-::f.,...:....':-
:.::'::::::::.,7:7'..7'.2.::::-..,*.A,::::,:olei,.I...ii.::::::::.ii,::::.i
.1 Tau Sec098 4 r ::r. r' :::: ....::: . 0
1 -0,2 .= .' = . 0.2' .1 ...G,....3: . . !
,r; ,..,= :=:;=.....:=:,h=-......:....4 :t.,:!4...:=;...:=.:-
:::......::::.:::,..: ,..f,.:-
..........................................;,...........,.., . . ...
.,..:.........../.......x......
,
,-, TaLissac099 18 i: 1-3,3' 0,2 77,5.5 1
,i1 . T,7 2,1< P.),'1= ' . 0.0 ........:!!!!õ .. r..õ...1,ZA-
"=,i...."9õ:...,.//,'"/õ. = ;.,/ 44
, = -- 1
= == :
, 65 TauSec098+ 15, ..,:- = ' - . - ' : . ..
0
.:. === -:,- / . ////
m"
6 7 '0 =.' : 0 0 2 r' 4
= .1 .77 1 .. ::::.: :7.: 8: -
I -- i,..i czet-099 18 ..:1 = = _,.:._ .. = = .. . _... .
=.= , . _ =1 - = -. = = .= ..õ õ,.....,, :::===
..... = . :',,,:-.':i-r::-:-',.:-..-
:.......,::::::".,:',...:,.....õ'õ!=2=;%,:==,/,,,õ,,,, õõ,.., .,
--- '1 - "" A
0 õ.. .,.. , . .,..,.::
.........
=
= = - --- .
' TauSecO;J8-4, I45, .. .
=:::::::' = :=====.: ...........õ....--.,... = :=:-
.... = i"":"::.::'!!.::.!II.:,',....=!iWI''::::g.).%õ/F7/7 ./,.
0,0
i'...i.".::=::.::::::-..:04=======io-Rigi ;4. /...//z
----= ' ' . ''..P3.''..,........;,%::::::::
, . TG L-2000 1 =:: : :. 6,8i.,.... =
.,:==:,,.="0:',..1 ' = = k,1,2 02 _ =.:0, ,4.1.,,....,...
..'1....=.8. .. .':4::':=7:6. C1=0 r.,=i.:::::::=:::::.
=,,,,.,........
_
Blank -=""
___________________ , ,i = = =..c = A= = v , 4 =-"'v .
____________________ == = ====,v, ,6 .4..., . : 21 2.õõ4
,.:=2:..,. ,4õõg).õ,....õ.,¨.:::.::.::::::::.:,.
............................................. 4. _____
tn
oe
oe
.4.
ii--,
.4.
c.=
eq
0
'102
CA 02940778 2016-08-25
WO 2015/130883 PCT/US2015/017648
Thus, alpha-glucosidase can be used to hydrolyze leucrose and other
oligosaccharides present in a fraction (e.g., filtrate) obtained from a glucan
synthesis reaction, such as a poly alpha-1,3-glucan synthesis reaction.
EXAMPLE 13
Isolation of Olioomer/Leucrose Fraction from etf-S/MUT3325 Reaction
Sucrose (4.50 kg) was dissolved in distilled deionized water to a final total
volume of 9.5 L and the resulting solution was heated with stirring at 80 C
for 5
minutes and then cooled to 47 'C. With stirring, 500 grams of a crude extract
containing 0.6 g/L of gtf-S enzyme (GTF0459, SEQ ID NO:42) and 15.0 mL of a
crude extract containing 10 g/L of mutanase (MUT3325, SEQ ID NO:47) was
added with stirring (see General Methods for enzyme preparations). The pH of
the resulting mixture was immediately adjusted to between pH 5.5 to pH 6.0 by
slowly adding a 1:10 (v/v) dilution of 37 wt% HCI with stirring. The reaction
temperature and pH were maintained at 47 C and pH 5.5-6.0, respectively,
until
sucrose conversion was >95% per HPLC analysis, after which the reaction
mixture was immediately adjusted to pH 7.0 to 7.5 and heated to 90 C for 20
min, then cooled to 25 C for immediate filtration to remove particulates and
precipitate. The resulting filtrate was held at 5 C prior to 'EX/SEC column
chromatography using the following resin and conditions: FINEX CS 11 GC SAC
in Ca2+ form, column i.d = 9.3 cm, resin bed height 1.58 m, T= 70 'C, flow
rate =
51 milmin, linear flow rate = 0.44 m/h, feed size = 0.6 L = 171 g, feed RI-DS
=
25.1 g/100 g, sample interval = 3 min. The column fractions collected between
min and 67 min were combined, concentrated by evaporation to 66%
dissolved solids and analyzed by HPLC as described in the General Methods.
25 Table 21 indicates the oligosacharide and monosaccharide components of
the
isolated fraction thus prepared.
Table 21
Analysis of Oligomer/Leucrose Fraction from gtf-S/MUT3325 Reaction
DP7+ DP6 DP5 DP4 DP3 DP2 sucrose leucrose glucose fructose
(%DS) MDS) (%DS) (%DS) (%DS) (%DS) .(%DS)MDS) (%DS) MDS)
0 1.1 2.9 7.2 16.2 IIIIIIIIINICOMMTIEMME77=HE
103
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
In this Example; a glucan synthesis reaction was used to produce at least
one soluble alpha-glucan product. This soluble product resulted from the
concerted action of both a glucosyltransferase (GTF0459, SEQ ID NO:42) and
an alpha-glucanohydrolase (MUT3325, SEQ ID NO:47) that were both present in
the glucosyltransferase reaction. This Example also demonstrated the
preparation of a chromatographic fraction from the glucan synthesis reaction.
This fraction was used in Examples 15 and 16 below to test the activity of
alpha-
glucosidases thereupon.
EXAMPLE 14
Isolation of Oligorner/Leucrose Fraction from Gtf-C Reaction
Sucrose (4.50 kg) was dissolved in distilled deionized water to a final total
volume of 9.5 L and the resulting solution was heated with stirring at 80 "C
for 5
minutes and then cooled to 47 C . With stirring, 500 grams of a crude extract
containing 0.41 gIL of gtf-C enzyme (GTF0088BsT1, SEQ ID NO:45) was added
with stirring (see General Methods for enzyme preparation). The pH of the
resulting mixture was immediately adjusted to between pH 5.5 to pH 6.0 by
slowly adding a 1:10 (v/v) dilution of 37 wt% HCI with stirring. The reaction
temperature and pH were maintained at 47 C and pH 5.5-6.0, respectively,
until
sucrose conversion was >95% per HPLC analysis, after which the reaction
mixture was immediately adjusted to pH 7.0 to 7.5 and heated to 90 'C for 20
min; then cooled to 25 C for immediate filtration to remove particulates and
precipitate. The resulting filtrate held at 5 C prior to IEX/SEC column
chromatography using the following resin and conditions: FINEX CS 11 GC SAC
in Ca2+ form, column i.d = 9.3 cm, resin bed height 1.58 m, T= 70 'C, flow
rate =
50 mUmin, linear flow rate = 0.44 m/h, feed size = 0.6 L = 171 g, feed RI-DS =
25.8 g/100 g, sample interval = 3 min. The column fractions collected between
34 min and 72 min were combined, concentrated by evaporation to 67%
dissolved solids and analyzed by HPLC as described in the General Methods.
Table 22 indicates the oligosacharide and monosaccharide components of the
isolated fraction thus prepared.
104
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Table 22
Analysis of Oligomer/Leucrose Fraction from Gtf-C Reaction
DP7+ DP6 DP5 DP4 DP3 DP2 sucrose leucrose glucose fructose
MDS) MDS) ( ' DS.2 OluDS) ('YGDS1 ('YGDS) (%DS) (%DS) (`!,10DS)
(%DS)
Mi1.2HE 0.9 .
In this Example, a glucan synthesis reaction was used to produce at least
one soluble alpha-glucan product. This Example also demonstrated the
preparation of a chromatographic fraction from a glucan synthesis reaction
that
produced a soluble alpha-glucan product. This fraction was used in Examples 15
and 16 below to test the activity of alpha-glucosidases thereupon.
EXAMPLE 15
Primary Screening of Alpha-Glucosidases Using Oligomer/Leucrose Fractions
from Gtf-S/MUT3325 and Gtf-C Reactions
This Example describes using alpha-glucosidase to hydrolyze leucrose
and other oligosaccharides present in chromatographic fractions obtained from
glucan synthesis reactions that produced soluble alpha-glucan product.
Specifically, study was made on the effect of alpha-glucosidases disclosed in
Example 10 on the hydrolysis of leucrose and oligosaccharides in the fractions
prepared in Examples 13 and 14.
A total of twelve alpha-glucosidases and two benchmark enzymes (oligo-
1 ,6-glucosidase and TG L-2000 transglucosidase) were screened using
oligomerileucrose fractions from gtf-S/MUT3325 (Example 13) and gtf-C
(Example 14) reactions as substrate material. All the enzymes (alpha-
glucosidases and benchmark enzymes) were dosed at equal protein
concentrations. Each alpha-glucosidase (dosed at 100 ppm) was incubated in a
solution containing oligomerileucrose substrates (10% dry solids) and 2 mM
calcium chloride at pH 5.5 at 47 C. Each reaction was quenched after 21 hours
of incubation by adding 50 pL of 0.5 M NaOH.
The oligosaccharidelmonosaccharide contents of the quenched reactions
were determined as follows. A sample from each reaction was centrifuged and
supernatant therefrom was diluted 25-fold in water for HPLC analysis (General
105
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
Methods). The percentages reported in Table 23 reflect the average of peak
area percentages (from duplicate analyses of each sample) of each OPõ as a
fraction of the total. The results indicate that the fungal alpha-glucosidases
had
better hydrolytic activity towards glucan oligomers when compared to the
bacterial alpha-glucosidases.
106
CL6 I 'I 5VVOPCI
Table 23
=4
0
N
..t-'
....
Su ar Corfloositirin_Anaivsis 0 % ..,
" f Primary Sc err of Air.)ha-Gluco-
iid,,,, OligomtrfLeucrose Fractions :rom
--i _______________ c..,
_
_______________________________________________________________________________
___________________
a
t,--S,KALJ_T3325 and
.
I 1.- 1 õ
,..,
, ............. - __________________ SEQ
ID
rAPYID DP4%
=Substrate ......................... Enzyme NO DP6+% , DP6% ---_
DP3 ',/iõ., DP2.':1* ...:.t......"::sit7!7.7L, ...=::G1:=c-; : brri1.71.7.::
F........:r.,.1-!.!rt .'sH.:/:
.ii.,...i
-
, .4 ---
.., ,t = 6,8 -: igigNii$0aVnikiiii.-..:õ..,.',õ.4---=-=.=-= : -=-;=,-:,-
..:::::.1::.
gOlul,i:/930s-i;11,a6s-e --_,......1: . 0 -1 = . 0.2 4_, ,
!;!;..7 . ' = '. : , --' = ,,,,,,, 1.'" = = 13 8
;:,.= a = = .
.....:..-....-:::::...:g...4.0104::!::::::...'.,;:::::.0,,,-
=!'..::::::;:....::::')
n n n5 = 1,1
0,!= . --2.--; ,:,..-...:v...-;=::....-....-.:,.=,:mo
.:..i':::::;.:W4vr,ftni.:: =
1 = 0.7=== 01 .`i.s* v.¨
.. i . 4 '). 0 '.:: ...
.A.::::........P.4,1i.i-:-n: ;';',.4.4.:,......i.;:-....;...:::;;;:::::t
= TG 1õ-2000 . ; :===.,=,..õ.õ,õ
...,======.====,-=-===õ,-...,,.,,,,====,:::::=.., :i.,.,-
::,:õ.::::,;m:gt.........õ7.==:-...:,..:,,..õ:::::::::::::-..
Acig ill 1 _ 0 '...). . 0.4
= . = = 5 = .. = -.'
----0 . 7 ?,',..--:::::::::,:: *: : ,'"?." -
.'4,;,.',..,':' ,... :,.,:
::::e,::::::,:.:s.:X..?::;!....,...............,..........:,,,
6 =======:-0.=,&=-:-:=-:=!=: ===
O= 1 . .:'==-= .... = ===== = ' " 6 =
= ......= = = ,:::::.....x.''''... : ..-..-......'',........::::;:-
.:"..i3
= - = = i% 1 0 9
0 A,.. ===:.:... :.1...:::- : ... - : -,-7777.,-'--,, --
=,.'...,.]:]]:=...4.6.......i5M....:=... =-=:......:=.....g.:R.....4%ea::i'll
Gti...c, reactiaoUt5sirici
0
a =:== .- 0:7-=== = =%= 5 .,
= =..7.
Nfigl';'1 ¨ ''' -7==::.t''.. ' .= == 0
===== 0.2 ().4.::':.:. "1:-:-"- - -..3. 6
..........'...::.;',=:,.. '-'===.:..,.:===:.:,....:- -: 40J 33
'I'.% = f.1,7 - .. = . ---:.--
== .....-77-"="""n ,,..,õµ-== ^ 9.
=;:::::',;..:16,:::::iii;=zØ::.: - i::-=:.,=,,...=--
.4.:=..,,,,,,;.44,,,,,,,,,.=.... .õ.. A
N,cily 1
-.:.
,,-, -.-4 . 0 4 0. "... = .. õ..,,, z.,-
3.,....,..,..:õ....õ,õ,õõ.:.õ.......::::==
,..=..5,======?,:==,....,.......:::: ==-.- =,: f .,:t.) : : -J
-J
BloGiu I 20 : . = a 1 '1- -- ;.1 P.'.p
it "''""
, .
= :> V:::;:.'...-.-i.i...(.(IP:::::....-'..-.,.:'
l======'"'":"....':'-'"'''''''"E'''l -- .: ' -' ''-
.. 0,9
..-i- ...,,,,_,----;.,H---:',-.."-..::::-
.....:===:...,.....= i.777.71-77::' 'ii..,":-:: - :- : ' . :r?F.3-
A:',.:,:.:. :=::: co
ps,
r
24 = (-1--.8 0.2 ,-.'-, -
- , ,--- ,
-
' .. ' ===,.:='0..'.'-.-:-.1....:-..::..:::::
::::::::-..a:'....i:4514-,:;,::,.ii:,. : =::: ...: 7 .... , ..4.,;:,
o
BloGli,i 2
: p -õ::,:::].,=,-
:,,28-,=.= :=,,.,. .
,
_ .....................
.1,1 _ ,.0,3--- ,-!,-) õ
,....._ ,
=
=== = 27,9 l .:.=::--..:: :=:-
.:..:.,..:*:,v:::,..,:: -: ::',...:::: .:...7.,:=,7:-.=,,,,..' o
co
...................... BloGii,i3
,
.1 4 fl .., 0 , 1 : :,,:-.) , 3 ...,,,J,42.-.5 =====
. :::;..,-;::::: . ,=:.:7:7-F.7..::,,::::;,....,:,-.4,,.i.ti=:,:::.. .
=:,=,,,..,:,::-.28, 4
B ,..1pGlui ........................ 30 :;..C.I.,9:..,::... (1. '.
',...'=--=--------.(7-71f ..;=:ei::=17 .:= = .::: ;.. 2:-..9
-:::',:::::1....:::2:4:..;:.-6,-
.',;:,.....',J:.:',Ir..::::!::.:',:::::::.::=:,,.:',, '-:--::.;'-%;....,.;,777-
7:1
=
, ,.......
. :.....;..;o ....... n 2 :0.0
'... v,'=': s . ::='..'. .:: 1--,..: _ =
,.:-........::';.:-:-1:-Mii,:....;;MR;:.1:::M...3.0:d:.:-... :H,.:::.,--;--
t,,,.-$`4
BthGlui ............................ 32 ::,...-fi..o ==,, -=
= ..- :=.=== .........õ1-.. =:======1= 2=====:::.=
:,. il ./ , ==_:.=,:.-:E=::,:i.',.:;-:*-:,=?='====;-.,...,,,,,..,;.:-
=:..:..'..:-.- -.=:::::::::==i,_,:,: õ:::. = :-.'-==:=..4:,,---,:i.,...-
...:,_.::::
.. i , . u". . 1 ..
. 0 , El.. ;.=,....:::: = :::5.....-- -4...---.-"' -
=....=::::::-..=,,......-.õ:õ...:-.4-...,-...-........*:......;.....-
iiia...,...,.:;:i., :-.....=..........,====,",o=-..-1:;::=::::...::-.::?,=:-
. :3,;.?..,,...7..:,..- ,,,,,,,,,,,,,,,,
..= -õ......- .
========= .....
...............,......:4A,-..:k .,-....i..........ii;=,::::,..........::-
?..::::::::::::::-..........,..,õ,4,:::,...::::=......-..=,,,
...,õ,,,,õõ,õ,õõõ,,,
BbrGiti2 ........................... :36 . ..... 5, - ' . = = .
= ' :" P 4 =
1;,......,:::::::::::::::.:õ.............,,,..õ,=,.....õ õ.õ..... ........
: ..,.,,-,-4 - .= do. = . ...= .= .
= =
.1
,A8 , i A. ..,: v".1
0=2 ...= - -C-1-5 '.. . ::'.'')-'s - 4:1 ,e.../:` ':itr,:%=#A
!'.11:1'...====="17,
õ _
oligonler, BbrGiii5
,. =
7 1 7
= -L = ''''4x' ' :' ./ ------ .,,-
..A.:,....;;:::.-:i:.:'.:::i.:.:::.::.:.:
0.4 -
....;,,,,......A=:.*:=,:n].:=:=.;:i....-
.=,.=-=ii:::=,::========.,..=,==,=,,...a:.4,mkT...:
ie Li Of ose .......
=TauSec098 ,. . ..
15 i s--1 , . =2
1
... ,'
,1 /. = = f ' r 1 1 0.4
8:5
fraction - ,, . , ,,,,,
1 8 ;:' ' ' = ' .J., '....'
' õ, = = = 1 ,...õ,-- i ',-.1. -,9 %. 7.: 5:;,: ...,: %."
/7>X4 -
from c.-4tf.. fau....euutti
. _
,,,.. A 1w.../.....c...,40 ,f/..
/2,/,..nlemo.,,,,,...K..:...,õs... ,..i.,,,,,..:::::*:-..........,-.......:-
:,-.....-...-....,.;;;;,õ, ..... . ._... ,
v
n
C reaction 1 'clank i 1-....: = 0-'.3 Li''''''
. ''- : - . .......,..... -..- --
õ,.,..........õ...=-õ,====477:77::-....,1.....::=.,,.,.:=:,:::-
=.,.....Ø.....:;==.m.,m,::,:::::::::-.:.:
........: ____________________________________________________
,:::::::.......,.... .::: 1..,..:...-:
,::...A..,===..4.=::::7=:' = =
===;:=,'...,.jilliiiiiliilii.:,,i',Jg.ii..!;.;.!,aiiiit:'=-:.:=' .....;i;
::::::-4.-.':'!=:-,.-.1.;-''-':fq5':'=:'::::'-i'
.::::::,:::,i.:::m777..:71,--. :........:.:,:. ...i: - .. -
,..::..::::...:ii:.......,:,;:!V:':':1::::.: 0
.:1..:11::.i.M.:::::2:ta?mg:::.i.:::::.,..H::::1-!%,;:*,:./-:::--
::::!...i...;......:.õ,,..;'.......45 C/2
¨ .
(.31i90-1 :6-
:.....::=======-....:.= =:.-:.:;:====1 :. -;, .;::=. :.:..
.= =.:2==,8 -= . : .7.-,=-,..) ,;:::: = '
q:::?.::.:::.:;:.... = ... ... ' ......... .. ... .. ... = ,
,','.91.a.g././4,/.//*4V ,' ,..",....-..;*::',,,,,;;Fi'it',:;i:i.:::iiiii4
k,)
digorreri
c tucosidase .:.:::,.:.:::.1.0:.:,:a:::.:.::: 1
. ,,...,b .. ... õ. .. .. .. ,, 6 ,. ......:.:;õ
1.....:r,...f. . . . = (..;
i . . = . ...= = : = ,. t .
:, i-, .". = = . .: ...- . .. -
.,i7.......3.*:::- , ...:1,::::5:.:24,.: ::.:. 44:: . 4õ...-......õ, .
. 7....... ----- ' ''......,- '..:-
.....;.:....:;.:....;...................,
,.. ,.,4%...=
I..I
=
...... .
,, . ......
. .,,
' / .!..e,././ .;d1 ..",.//1PA :',=,:::,
:AW..........,..i..i........',1 (A
leuc...rose
TG i ,--2000
(.... '''...:"=5'-''',5- ....5=4,--
e.:=....::.............................-7,55. =
....................................... õ ,.'..' ". ". (2 =
6,9 " -.........1:2,0.....:.:::: ,..1,....r .. '`
::.=::.7.,,2õ,=Avvi)..,,,:..n-X*i'04. -,,,,,..-.?..90,.....4.Mii.i.i..: ---
,
p
m.i
fraction .... r .- 1..8... , = 0,8
2. a
::::::;&,V44"..--(-**tiq . -..:*.:::::::::::::2,.,,;=:.".::=:;:;:,:::
Ad :itil
--..., ' ======:-:::===:i..1=:=====:. = 0:9
.. (-)
frort Gtf- -4 .4 0 8 = . : .2.6 .
.:::?::. ::'===':='::!.0-.=,,. .. = . ::::"."
9 .. : ., = ,
= i ;
.4.
ce
Nififj i u 1
si i s , , . '
=
,
=
107
CA 02940778 2016-08-25
WO 2015/130883 PCT/US2015/017648
i,,p.'.....:., =-:c> :(1....N..:. ::-..µ,.;.,,:i,:..o.:c tc:,: ::-...
:...t. =,..,..*:::...7.--.0
.:.i.-.".: .=5 :.::i N,.;-. :,=.:: .:'..;i =...5. ik$ .
i...,i
si''::,i t: ':7;ii:';'r:'''. ;::''-..t.:::-= 7 7.1...,,l':7'''
1
,:::-.,,...i:i.,:,.:::=,::, t .,,::::,,,,, :: : :::::::
ii,.,..:.:.:1,:: ,-. :.,::::::,=-., '. - :1.1:,=:i.,..,,,.=:,
'::..
,.::::::,.::,:.. :. I::: :: I-. ::::::::::::::::- ...':::.:.,,..H:::.::
:-....= i ::.1a.1:: : .-:
\rõ:::, -:.:::,:=.- sy.,::::,..,:::::::::..,,,.',........,m77,¨,-,,,,:k.,-A,,,
.s>.x. :,.::.......:.:,:'
, .:.:...:,.:........:::,:,:.:,:.=:..:.,......i.,:.:i.i
:i.......::::::...::.:N.,,,. ii::,..0:4:-
..:..ila.,.::::...:4z...i............, :::
0 '.V.i..:.= .:A..:. - -: .:::....1**,. ::.,.:::0::
:'k:''.'''.:..5.,`,.,i;? ''->
..,,A .:::4=:.':,.........:-... iiiiti., .<µ' ' :4.6 :%^,.;'`I ..::.:=,.6
::.s. 'ii.:6 =,(...i.1
Nk:::.:'.i ::'.,'..:.*::::::.:': H.::-....:: ::::::::::.:!::: .::i*:'.::.:
:::::::::'-.... '':'=I:::::.: ';''.0 :P4S. ::
ZN., .i.::::::::::: ::::::::::::: ,:::::.:?.::::: \
:,i:,iii.,ii:µ,.,....=::.:. -1- = i:==.,...,===,
=====µ,õ;=:;:'=====.,::..'õiõ,....õ1
=:' = :.:=========:.:'' : =-=;.=..=:: =.: = 1...', :
:=,:..i.:'.=;::: ' :- =.=-=,'".iaiiss. . ....: \=',.,
. ,.., . .=:.:.::=;=.=;::., :===, :::::: === ::'. :
. ::::::i'.:.:=::::: .:::... :P:';:::::::i= : t';µ,::::'.
:::::::::.:::::::::. :=:::: :- - k= . :f:......i....::::::'
:.,...::::4k's. = = ..'.
. == ..: . -: 1 = ...:::::,:x.: :::: ' ....;:ns, =
.:=:.. `i;;;=i::::.:
==:. . OI:.*::.01::i':.'t:.)
::'6').1.'''''.::::::: ::.:0: ; . ===:::=== :`''':':'=.:
al ::t::61:::::kni
' = . : ',7-'f.:Nt::.1:1''T. :...:7=::::::]:.::::.:...P.'.
'tl'i . :r.:::
1
1::=.=.; .::::.: ':,..'::,.::::i.i...:..=;.: .'::;:::.::::
...t::.:. -..:::::::::,:::: ..2:::::::.;;.':;:')::::::?::::i:: tZi gg
, = : :. = =
, - = = =
: ======== . co :.=, = ::: :.. , =,,,,.: = s.:-.) = :: ...::: A
c..1 = = : = : CNI= C:3 '' t.":1 ::..c)i
N N = N c :: ri , : : : = i
.: =
= .1 : . i..: ::.= : .. = = - k...:..: .
..-..?:....:. =.... ...'s.;..:,... ..,....i:. .....i.::
...t.;:: :: E::... to: ..-;..= .....N I ::t.,...............N. "
i:c...4-',.-:::t:
:--...........=,,".: ===..t. = = .....c..N. :-:',4,.., i-.1.,: = i.V:
i....:6
....t.,.s:: : ,,:-. :::si.-.--= .=,..-,,,... . - :=,,k======
:==tr.,..!= .f.N...=,... =.=,n7'.7. ',"1,7=:
:, =''.":.===: = ====.: ....:: ' ,,,':'i: =:: ==::::.;: .=
:' ========= = :===h==,i,'''...i .:'=.:.,,,.."... ===,::::.:='.
1:=::===========: 1 '====== .======= = 1 1::::'====a4,...= ...........
-.::. =====?== ::===-::.=.' ===:===:=:.=:>=-==-::=-=r-%;
I. = :::: ' ..t; :=..<:==7 1:= .= .=': :::::i 1: ='
=:1=.'==='====.:
. (C) tO '-. K.,===Tµ.... : '.. C \ 3: ==:::'. = CZ f.N.)
a) 1,¨,
=
.;': :=::..:=:= ==
:=.- : t: :... 0:-..:
:..... ..' -..= 1 ..... =::
=s.
. 1...:...
...... .
= .... : .....' =
.....
tr..: = f.47. $r.) :.:(o t.".¨.i= ==
.cr..:-!: ::.=.:
=N: iN ,2µ.: Cs.1/41. :.:..,-'=== : C'.; C-si
c4 C.:'. . N Cr)
. :. .. = ...= = . . =
..... :::::
:. < = - . ::1.= ::::'='= .= =:<:= ::". :...: ::
:.. = =,;
= t.',,: r.'7=== CO CO. :. .C, . N',:- :: Cf.) ts--
t a): ..P.TV
.:.0 . 0,.s.:-....0 p : ass. c.:.:::3 ::.:c.:3 : s.--.1i' . c..z.).::,
=c....).
.:.:.=:=:::::: ::.:... ::.: :...:=:: ===== :.::...k" . i
:: = .:I.:::: ..':
., ...:::: .. . .,, " . .. . ..,, :. ::::%:::=::
.== . ... ........õ....i..... ... , 2.. .
= . = ===:=:: = = = '
t =.µ" = 1.0 Sr) == i'''..: . ''G's := kC . r===== . = CI --te'
..":.t.-
I.:,64 C'i : ,---:. (N ' ,== =1 . ' N cN v-- v--
i ...: =
--4. = i : .............. =
.....i.....;:=
; ' 1 z 1
i.N.I i c I "t co I c> cNi a> cCk I to I vo =
r-- c..1= s-s4 r..f o,..) (,-) (µ) c-:,=) ¨ i .---
. -
,=== r-- t."1 ;..x.) r¨x¨
c-ti c.!.1 el (15 cf) (75 (-5 51 4:15 zi'l µ¨tvi
A...
i' 0 01 o I n= sz 15 .,:cs 1 tf) si) zt
2' ris Fr): Er) (15 En En Fl Fs, =
1----
0
c,
, iN4 c
Li=-:µ, m q
N.--
m D q>
2 '.....
0 ¨
. . .. .
.. .. .. .. .. . . . . . .
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
As indicated with shading in Table 23, the oligosaccharide content of the
reactions generally shifted toward smaller sized sugars, in comparison with
the
control reactions (-Blank") in which there was no enzyme. These results
indicate
that alpha-glucosidase can be used to hydrolyze oligosaccharides comprised
within a glucan synthesis reaction and a fraction thereof, particularly a
chromatographic fraction of a glucan synthesis reaction that produced soluble
alpha-glucan product. Also, given the linkage profile of the oligosaccharides
(Examples 13 and 14), and the activity of alpha-glucosidase against various
glycosidic linkages in addition to alpha-1,4 linkages (Example 11), it is
apparent
that alpha-glucosidase can be used to break down oligosaccharides with alpha-
1,5 glucosyl-fructose linkages and also likely alpha-1,3 and alpha-1,6
glucosyl-
glucose linkages. The results provided in Table 23 also suggest that fungal
alpha-glucosidases have better hydrolytic activity towards soluble
oligosaccharides compared with the bacterial alpha-glucosidases.
Thus, alpha-glucosidase can be used to hydrolyze leucrose and other
oligosaccharides present in a fraction (e.g., chromatographic fraction)
obtained
from a glucan synthesis reaction, such as one that synthesizes a soluble alpha-
glucan product.
EXAMPLE 16
Select Screening of Alpha-Glucosidases using Oligomer/Leucrose Fractions from
Gtf-S/MUT3325 and Gtf-C Reactions
This Example is further to Example 15, describing the use of alpha-
glucosidase to hydrolyze leucrose and other oligosaccharides present in
chromatographic fractions obtained from glucan synthesis reactions that
produced soluble alpha-glucan product.
Evaluation of alpha-glucosidases that were most active for hydrolysis of
oligomer/leucrose fractions from gtf-SIMUT3325 and gtf-C reactions (Example
15) was performed by analyzing sugar compositions resulting in reactions
containing enzymes dosed at equal protein concentrations. Incubations of alpha-
glucosidases (dosed at 4 ppm; for blends, the ratio of the two enzymes was 1:1
and total dosage was 4 ppm) and oligomerileucrose substrate (10% ds) were
109
CA 02940778 2016-08-25
WO 2015/130883
PCT/US2015/017648
performed at pH 5.5 in the presence of 2 mM calcium chloride at 60 'C and 65
C, respectively. The reactions were quenched by adding 50 pL of 0.5 M NaOH
after 23 hours of incubation.
The oligosaccharideimonosaccharide contents of the quenched reactions
were determined as follows. A sample from each reaction was centrifuged and
supernatant therefrom was diluted 25-fold in water for HPLC analysis (General
Methods). The percentages reported in Table 24 (below) reflect the average of
peak area percentages (from duplicate analyses of each sample) of each DP n as
a fraction of the total. The results indicate that TauSe.c098 was efficacious
for
hydrolysis of DP2 to DP7 oligomers and TauSec099 outperformed TG L-2000 for
leucrose hydrolysis when the incubation was performed at 65 C. The blends of
TauSec098 with TauSec099 (or TG L-2000) were effective for hydrolysis of
oligomers and leucrose for DP1 production.
Thus, alpha-glucosidase can be used to hydrolyze leucrose and other
oligosaccharides present in a fraction (e.g.; chromatographic fraction)
obtained
from a glucan synthesis reaction, such as one that synthesizes a soluble alpha-
glucan product.
110
=
CL6115W(3PCT
Table 24 . =
SugalCorivosition Analysis of Select Screenina of Alpha-Gluoosidasf:,,s Using
OligorneriLeurrose Fractions from 0
t.>
0
____________________________________________________________________________
43, 325Dapr;d Gtfp-C6 on
u,
,
o
,
' I SEQG'Ilft;SlfDpc
vi D
rieaDePt5i
=
sDP4 DP3 DP2 Leucrose Glucose 1
Fruct ce
Tem Substrate En
.t. .....yrne NO (% % ''k) PO
(DM VA) S%) ____
C=4
t
_______________________________________________________________________________
_________________________________________
i TC:: L-2000 1 2.5 0.4 0,5
0.9 1.9 5,3 22.9 18.4 21.4 ! 25..
TauSec098 ISM 54 0.3 0.5
1.0 1.4 2,6 6.3 71.8 0.0 4 A "7
;....e
f ,
oligornerl TauSec099 18 2.9 0.3 , 0.5
1.0 1.9 = 4.7 n. ..,,1
3 ;
21.5 , -7
1: . f 94,1
i-,----.
leucrose TauSec098+
TauSec099 15 18 2.7 0.3 0.5
0.9 1.4 3.3 19.9 34.9 18.2 17.8
fraction from ,
--,
Gtf-C reactor . TauSec098+ 1 2.7 MIN 0.8 1.4 4.5
23,7 27.1 18.6 .
TG L-2000
15, _........õ 0
60 ________________ Blank 5,2 0.4 EINI 1.2 1.8 3,2 8.3
70,0 . 0.0 9.2 .
TG L-2000 1 4.1 0.3
1.21 3.2 8.1 17.2 1 13,2 0.0 .
27.9
24.8 .
. , ..,
= doomed TauSec098 15 3.4
0.2 0.0 0.4 1.1 ____ 3.5 12.'3 32.3 35.3 11.5
..,
0
. , .
= !sucrose TauSec099
18 4,2 I 0.0 1.2 ' 3.2 8.2 17.4 15.3 0.0--- 25.4 25.1
.
e
,
fraction from TauSso098+
.
Gtf-S/ TauSec099
35 0.2 04 . 0,9 2.3 6.2 16.8 21..2
31.7 16.9
15, 18 '
.
MUT3325
000 TauSec098+ 3.2 0,1
0.3 = 0.7 2.0 6.0 26M 17.0 29.1 15.6
. reaction. TG L-2 15:1
=
Blank 4.6 0.4 1,2
3.2 7.9 17,5 ' 15.1 36.6 = 0.0 13.5
,
TG L-2000 1 . = 2.5 0.3 0.5
1.0 1.8 4,9 24.9 = 26.0 17,4 20.8
= TauSec098 __ i5 2.8
0.4 0.5 1.0 ......... 1.4. .. 2.5 l 6.6 - T3,6 0.0
11.1
, . ' _ , .. -1
oli clomeri TauSec099 18 2,9 f.i8 0.4 1.0 ___ 2.0
4.9 23,2 17.4 21.6 27.0
.
-
65 !sucrose TauSec098+
i co)
=,-.
4.5 0.3 0.5 0.9 1.4 3.4 2013 28.6 20.1
20,1 -i
%., fraction from TauSec099 15, 18
i .
Gti-C reaction TauSec098+TG L-2000 151
5.1 0.3 ' 0.5
0,9 1.2 2.9 21.1 34.4 18.0 15.7 V
,
.
_______________________________________________________________________________
________________________________________________ u,
,--
Blank 7.0 0,4 0.7 __
1.3 1.8 3.2 7.9 68.4 . = OM OA
t..
.õõ,,,. . .
ei.
4.
= CO
CL6115W0PCT
=
I rot-2)0o 1 2.9 0.2 1,1 r ,&i 18.0 11 7
16,5 19.'3 19.i
gornert TauSec098 15 2.6 0.0 0.1 0.3 0.9 3.3 12.0
33.1 36.6 11. 0
leucrose TauSec099 4.4 0.0 1.2 3.1
7',8 16.1 14.4 0.0 27.4 25..
fraction from TauSec098+
3.9 0.2 0,4 0.8 9.1 5.7 16.2 19.4 33.7 17.;
Gtf-Si' TauSee099 __ 15, 18
MUT3325 TauSec098+
TG L-2000 5 1
3.7 0.2 0.3 0.7 1.8 5.0 24,9 20.5 29.4 13;
reaction . 1,
Blank. 1,1 0,6 '1.1 2.5
6.3 ! 136 13.0 31 1 17.0 11. _
o
o
ul
ps,
9:1
1-3
c/2
k.)
ON
CO
112
=
=