Note: Descriptions are shown in the official language in which they were submitted.
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
1
HOLISTIC HOSPITAL PATIENT CARE AND MANAGEMENT
SYSTEM AND METHOD FOR TELEMEDICINE
FIELD
[0001] The present disclosure relates to the healthcare industry, and more
particularly
to a holistic hospital patient care and management system and method.
BACKGROUND
[0002] A major challenge facing hospitals today is the timely identification
of disease
and appropriate engagement of patients and families required to offer patients
appropriate care
and treatment in order to avoid the progression of existing disease as well as
the occurrence of
a new adverse event, as well as to ensure that appropriate interventions and
resources are
available and deployed according to patients' needs.
[0003] Many national agencies, such as the Centers for Medicare and Medicaid
Services (CMS), Institute for Healthcare Improvement (IHI), National Quality
Forum (NQF),
Agency for Healthcare Research and Quality (AHRQ), and Joint Commission have
demonstrated their prioritization of high quality patient care through clearly
articulated
performance and quality measurement programs that incorporate disease-focused
and patient-
focused process and outcomes measures. These metrics are tied to standards
that currently and
will continue to impact the national performance-based incentive and penalty
framework
designed to realign efforts and focus on quality of care.
BRIEF DESCRIPTION OF THE DRAWINGS
[0004] FIG. 1 is a simplified block diagram of an exemplary embodiment of a
holistic
hospital patient care and management system and method according to the
present disclosure;
[0005] FIG. 2 is a simplified diagram of an exemplary architecture of the
holistic
hospital patient care and management system and method according to the
present disclosure;
[0006] FIG. 3 is a timeline diagram depicting the application of the holistic
hospital
patient care and management system and method during a patient's progression
from hospital
admission to post-discharge according to the present disclosure;
[0007] FIG. 4 is a simplified logical block diagram of an exemplary embodiment
of a
clinical predictive and monitoring system and method, by detailed inputs and
outputs,
according to the present disclosure;
[0008] FIG. 5 is a simplified logical block diagram illustrating the
conceptual data
integration, disease/risk, and data presentation and system configuration
logic of an exemplary
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
2
embodiment of the holistic hospital patient care and management system and
method
according to the present disclosure;
[0009] FIG. 6 is a simplified flowchart/block diagram, illustrating the
process of
predictive analytics based on data inputs and outputs throughout a patient's
care continuum, of
an exemplary embodiment of a clinical predictive and monitoring method
according to the
present disclosure;
[0010] FIG. 7 is a simplified flowchart/block diagram of an exemplary
embodiment of
a clinical predictive modeling method, describing the application of
predictive analytics across
the different stages of a patient's clinical encounter in various settings of
care, according to the
present disclosure;
[0011] FIG. 8 is a simplified flowchart diagram of an exemplary embodiment of
a
dashboard user interface method according to the present disclosure;
[0012] FIG. 9 is a simplified flowchart of an exemplary embodiment of an
enhanced
predictive modeling method according to the present disclosure;
[0013] FIG. 10 is a simplified flowchart of an exemplary embodiment of a
facial and
biological recognition process according to the present disclosure;
[0014] FIG. 11 is a simplified flowchart of an exemplary embodiment of an
automated
patient monitoring process according to the present disclosure;
[0015] FIG. 12 is a simplified flowchart of an exemplary embodiment of an
automated
healthcare staff monitoring process according to the present disclosure;
[0016] FIG. 13 is a simplified flowchart of an exemplary embodiment of an
automated
resource management process according to the present disclosure;
[0017] FIG. 14 is a simplified flowchart of an exemplary embodiment of a
telemedicine process according to the present disclosure;
[0018] FIG. 15 is a simplified flowchart of an exemplary embodiment of a
patient/family engagement process according to the present disclosure; and
[0019] FIG. 16 is a simplified flowchart of an exemplary embodiment of a
situation
analysis simulation process according to the present disclosure.
DETAILED DESCRIPTION
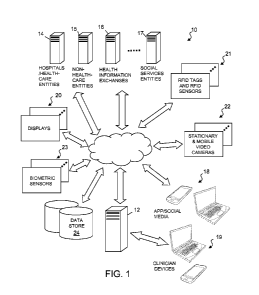
[0020] FIG. 1 is a simplified block diagram of an exemplary embodiment of a
holistic
hospital patient care and management system and method 10 according to the
present
disclosure. The holistic hospital patient care and management system 10
includes a computer
system 12 adapted to receive a variety of clinical and non-clinical data
relating to patients or
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
3
individuals requiring care. The variety of data include real-time data streams
and historical or
stored data from hospitals and healthcare entities 14, non-health care
entities 15, health
information exchanges 16, and social-to-health information exchanges and
social services
entities 17, for example. These data are used to determine the likelihood of
occurrence of an
adverse event or disease classification via a risk score for selected patients
so that they may
receive more targeted intervention, treatment, and care that are better
tailored and customized
to their particular condition(s) and needs. The system 10 is most suited for
identifying
particular patients who require intensive inpatient and/or outpatient care to
avert serious
detrimental effects of certain diseases and to reduce hospital readmission
rates. It should be
noted that the computer system 12 may comprise one or more local or remote
computer servers
operable to transmit data and communicate via wired and wireless communication
links and
computer networks.
[0021] The data received by the holistic hospital patient care and management
system
10 may include electronic medical records (EMR) data that is both clinical and
non-clinical in
nature. The EMR clinical data may be received from entities such as, but not
limited to,
hospitals, clinics, pharmacies, laboratories, and health information
exchanges, and detail things
such as, but limited to, vital signs and other physiological data; data
associated with
comprehensive or focused history and physical exams by a physician, nurse, or
allied health
professional; medical history (including utilization of various medical
services); prior allergy
and adverse medical reactions; family medical history; prior surgical history;
emergency room
records; medication administration records; culture results; dictated clinical
notes and records;
gynecological and obstetric history; mental status examination; vaccination
records;
radiological imaging exams; invasive visualization procedures; psychiatric
treatment history;
prior histological specimens; laboratory data; genetic information;
physician's notes;
networked devices and monitors (such as blood pressure devices and glucose
meters);
pharmaceutical and supplement intake information; and focused genotype
testing.
[0022] The EMR non-clinical data may include, but is not limited to, social,
behavioral,
lifestyle, and economic data; history, type and nature of employment; medical
insurance
information; exercise information; (addictive) substance use; occupational
chemical exposure;
frequency of physician or health system contact; location of residences and
frequency of
residence changes over a specific time period; predictive screening health
questionnaires such
as the patient health questionnaire (PHQ); patient preference survey;
personality tests; census
and demographic data; neighborhood environments; diet; gender; marital status;
education;
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
4
proximity and number of family or care-giving assistants; address; housing
status; social media
data; and educational level. The non-clinical patient data may further include
data entered by
the patients, such as data entered or uploaded to a social media website.
[0023] Additional sources or devices of EMR data may provide, for example,
procedure codes, lab/order results, medication assignments and changes, EKG
results,
radiology notes, daily weight readings, and daily blood sugar testing results.
Data may be
retrieved from sources such as hospitals, clinics, patient care facilities,
patient home
monitoring devices. Additionally, data may be provided by other available and
relevant clinical
or healthcare sources.
[0024] As shown in FIG. 1, patient data sources may include non-healthcare
entities
15. These are entities or organizations that are not thought of as traditional
healthcare
providers. These entities 15 may provide non-clinical data that may include
details around
gender; marital status; education; community and religious organizational
involvement;
proximity and number of family or care-giving assistants; address; census
tract location and
census reported socioeconomic data for the tract; housing status; number of
home address
changes; requirements for governmental living assistance; number of scheduled
(clinical)
appointments which were kept and missed; independence on activities of daily
living; hours of
seeking medical assistance; location of medical services frequently sought
after; sensory
impairments; cognitive impairments; mobility impairments; educational level;
employment;
and economic status in absolute and relative terms to the local and national
distributions of
income; climate data; and health registries. Such data sources may provide
additional insightful
information about patient lifestyle/environment, such as the number of family
members,
marital status, any personal dependents, and health and lifestyle preferences
that may influence
individual health outcomes.
[0025] The holistic hospital patient care and management system 10 may further
receive data from health information exchanges (HIE) 16. HIEs are
organizations that mobilize
healthcare information electronically across groups within a region, community
or hospital
system. HIEs are increasingly developed to share clinical and non-clinical
patient data between
healthcare entities within cities, states, regions, or within umbrella health
systems. Data may be
extracted from numerous sources such as hospitals, clinics, consumers, payers,
physicians,
labs, outpatient pharmacies, ambulatory centers, long-term acute care centers,
skilled nursing
facilities, and state or public health agencies.
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
[0026] A subset of HIEs connect healthcare entities to community organizations
that do
not specifically provide health services, such as non-governmental charitable
organizations,
social service agencies, and city agencies. The holistic hospital patient care
and management
system 10 may receive data from these social services organizations and social-
to-health
5 information exchanges 17, which may include, for example, information on
daily living skills,
availability of transportation to scheduled doctor's appointments, proximity
of healthcare
services, employment assistance, training, substance abuse rehabilitation,
counseling or
detoxification, rent and utilities assistance, homelessness status and receipt
of services, medical
follow-up, mental health services, meals and nutrition, food pantry services,
housing
assistance, temporary shelter, home health visits, domestic violence, medical
appointment
adherence, discharge instructions, prescriptions, medication instructions,
neighborhood of
residence, and ability to track referrals and appointments.
[0027] Another data source may include social media or social network services
18,
such as FACEBOOK, TWITTER, GOOGLE+, and other similar websites. Such
information
sources 18 (represented by mobile phones and laptop computers) can provide
information like
number of family members, educational level, and relationship status, or may
help to identify
individuals who may be directly or indirectly involved with caring for a
specific patient, and
health and lifestyle preferences that may influence health outcomes. These
social media data
may be received from relevant social networking websites, at the expressed
consent of the
individual being evaluated, and some data may come directly from a user's
computing devices
(mobile phones, tablet computers, laptops, etc.) as the user enters status
updates, at the
expressed consent of the individual being evaluated. The above-enumerated non-
clinical
patient data may potentially provide a much more realistic and accurate
depiction of the
patient's overall health status and holistic healthcare environment. Augmented
with such non-
clinical patient data, the analysis and predictive modeling performed by the
present system to
identify patients at high-risk of readmission or an alternate adverse clinical
event become much
more robust and accurate. As always, prior to the collection and use of a
patient's data,
necessary patient consent and authorization are requested and received.
[0028] The system 10 is further adapted to receive and display user
preferences and
system configuration data from clinicians' computing devices (mobile devices,
tablet
computers, laptop computers, desktop computers, servers, etc.) 19 in a wired
or wireless
manner. These computing devices 19 are equipped to display a system dashboard
and/or
another graphical user interface to present data, reports, and alerts. The
system is further in
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
6
communication with a number of display monitors 20 mounted and located in a
number of
locations, including patient rooms, hallways, etc. A clinician (physicians,
nurses, physician
assistants, and other healthcare personnel) may use the system to access a
number of patient
data, including immediately generating a list of patients that have the
highest congestive heart
failure readmission risk scores using real-time data, e.g., top n numbers or
top x %. A display
in a patient's room may be used to provide care plan and/or discharge
information to the
patient and family. The graphical user interfaces are further adapted to
receive the user's
(healthcare personnel) input of preferences and configurations, etc. The data
may be
transmitted, presented, and displayed to the clinician/user in the form of web
pages, web-based
message, text files, video messages, multimedia messages, text messages, e-
mail messages,
and in a variety of suitable ways and formats.
[0029] The holistic hospital patient care and management system 10 further
receives
input and data from a number of additional sources, including RFID (Radio
Frequency
Identification) tags 21 that are worn, associated with, or affixed to
patients, medical staff,
hospital equipment, hospital instruments, medical devices, supplies, and
medication. A
plurality of RFID sensors 21 are distributed in the hospital rooms, hallways,
equipment rooms,
supply closets, etc. that are configured to detect the presence of RFID tags
so that movement,
usage, and location can be easily determined and monitored. Further, a
plurality of stationary
and mobile video cameras 22 are distributed in various strategic locations in
the hospital to
enable patient monitoring and identify biological changes in the patient. A
plurality of sensors
23 including biometric sensors are also located in the hospital rooms.
Additionally, the system
10 may receive input of ambient temperature and humidity of rooms and
locations in the
hospital, as well as the ability to control some aspects of the patient's
environment, such as
temperature and humidity.
[0030] Another source of location data may include Global Position System
(GPS) data
from a clinician's or patient's mobile telephones. The GPS coordinates may be
received from
the mobile devices and used to pinpoint a person's location if RFID data is
not available. Using
GPS data, a patient may be tracked and monitored during clinical visits,
social services
appointments, and visits and appointments with other care providers. The
patient's location
information may be used to monitor and predict patient utilization patterns of
clinical services
(e.g., emergency department, urgent care clinic, specialty clinic), social
service organizations
(e.g., food pantries, homeless shelters, counseling services), and the
frequency of use of these
services. These data may be used for analysis by the predictive model of the
system.
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
7
[0031] As shown in FIG. 1, the holistic hospital patient care and management
system
may receive data streamed real-time, or from historic or batched data from
various data
sources. Further, the system 10 may store the received data in a data store 24
or process the
data without storing it first. The real-time and stored data may be in a wide
variety of formats
5 according to a variety of protocols, including CCD, XDS, HL7, SSO, HTTPS,
EDI, CSV, etc.
The data may be encrypted or otherwise secured in a suitable manner. The data
may be pulled
(polled) by the system 10 from the various data sources or the data may be
pushed to the
system 10 by the data sources. Alternatively or in addition, the data may be
received in batch
processing according to a predetermined schedule or on-demand. The data store
24 may
10 include one or more secure local servers, memory, drives, and other
suitable storage devices.
Alternatively or in addition, the data may be stored in a data center in the
cloud.
[0032] The computer system 12 may comprise a number of computing devices,
including servers, that may be located locally or in a cloud computing farm.
The data paths
between the computer system 12 and the data store 24 may be encrypted or
otherwise protected
with security measures or transport protocols now known or later developed.
[0033] FIG. 2 is a simplified diagram of an exemplary architecture 30 of the
holistic
hospital patient care and management system and method 10 according to the
present
disclosure. At the bottom layer, data 32 from the information sources are in a
plurality of
EMR-specific data definitions 33, and social service data definitions 34. Each
clinical or non-
clinical (social service) institution or entity may define the format for its
own data and
database, which is typically different from that of other entity or
organization's database
formats. The EMR-specific data definitions 33 are mapped or translated to a
number of data
models 36 used by the system 10. It is preferable that the system's data
models 36 are
normalized, or in other words, organized or arranged to minimize redundancy.
The system's
data models 36 are further converted or mapped to a number of application-
specific data
models 37 that are developed for the system's software applications, such as
real time
applications 38 and reporting applications 39. The system further continuously
perform
ongoing model maintenance to ensure that optimal performance is achieved.
[0034] FIG. 3 is a timeline diagram of an exemplary embodiment of a clinical
predictive and monitoring subsystem 40 of the holistic hospital patient care
and management
system and method 10 according to the present disclosure. The timeline diagram
is used to
illustrate how the holistic hospital patient care and management system and
method 10 may be
applied to a typical patient experiencing congestive heart failure as an
example. A majority of
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
8
U.S. hospitals struggle to contain readmission rates related to congestive
heart failure. Though
numerous studies have found that some combination of careful discharge
planning, care
provider coordination, and intensive counseling can prevent subsequent re-
hospitalizations,
success is difficult to achieve and sustain at the typical U.S. hospital.
Enrolling all heart failure
patients into a uniform, high intensity care transition program requires a
depth of case
management resources that is out of reach for many institutions, particularly
safety-net
hospitals. The clinical predictive and monitoring subsystem and method 40 is
adapted to
accurately stratify risk for certain diseases and conditions such as 30-day
readmission among
congestive heart failure patients.
[0035] When Emergency Medical Technicians (EMTs) are summoned upon a patient
complaining of chest pains in their home, the ideal protocol is that the EMTs
assess the patient,
takes vital signs, and via video cameras worn by the EMT (using, e.g., glasses-
mounted camera
or shoulder-mounted camera), transmits a video of the patient to appropriate
medical personnel
at the hospital. Together with the physician, the EMTs recognize and validate
that the patient
may be suffering from a heart attack, and prepares to administer care to
stabilize the patient.
All past medical history and data of the patient become accessible from the
hospital's EMR to
the EMT personnel, who notes a patient allergy to aspirin prior to
administration of any
therapy. The EMT is able to deliver appropriate care to the patient, and is in
constant
communication with the on-site physician who is awaiting the patient's
arrival. Within a
certain time of a patient's admission to the hospital, stored historical and
real-time patient data
are analyzed by the clinical predictive and monitoring system and method to
confirm both the
likelihood of diagnosis of a specific disease(s) and the likelihood of
occurrence of certain
subsequent adverse events related to the patient, such as congestive heart
failure (readmission),
taking into account the most recent adverse event as well. The processes for
disease
identification and risk score calculation are described in more detail below.
Bypass surgery
may be identified by physicians as necessary to alleviate angina and reduce
the risk of death.
During surgery, the system transmits the patient's conditions and status on a
real time basis to
the patient's family. Therefore, throughout the patient's stay in the hospital
as well as after
discharge, the holistic hospital patient care and management system 10
continually monitors
the patient's condition, collects patient data in real-time, arranges for
efficient delivery of care,
manages the hospital's resources and supplies, and communicates timely or real
time
information to healthcare providers and the patient's family.
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
9
[0036] FIG. 4 is a simplified logical block diagram further illustrating the
information
input into and output from the holistic hospital patient care and management
system and
method 10. As noted above, the system 10 retrieves and uses patient data that
include real-time
and historical clinical and non-clinical data 40. When a patient first
presents at a medical
facility, such as an emergency department of a hospital, his or her symptoms
and information
41 such as height, weight, personal habits (e.g., smoking/non-smoking),
current medications,
etc. are noted and entered by the medical staff into the system 10.
Additionally, the system 10
regularly receives the patient's clinical information, including vital signs
42, (e.g., blood
pressure, pulse rate, and body temperature). The healthcare staff may order
lab tests and these
results 43 are also transmitted or entered into the system 10. The healthcare
staff's input 44,
including notes, diagnosis, and prescribed treatment are entered into the
system 10 as well.
Further, the patient and/or family member may be given a tablet, laptop
computer or use a
mobile telephone to access custom applications designed to facilitate input 45
around the
patient's preferences (dietary preferences, preferred rounding time,
complaints about
medications, etc.), comments, feedback, and current (clinical) status during
the patient's stay at
the hospital, as well as after discharge from the hospital. Additionally, the
hospital is equipped
with a variety of tools, equipment and technology that are configured to
monitor the patient's
vital signs, wellbeing, presence, location, and other parameters. These may
include RFID tags
and sensors, or GPS systems, for example, for location monitoring.
Additionally, cameras may
be mounted in the patient room, hallways, emergency department, radiology
department, and
other parts of the hospital to generate still and moving video images of the
patient. The patient
monitoring, location tracking, and image data 46 from these devices are also
provided as input
to the system.
[0037] Healthcare staff, such as physicians and nurses may also carry ID
badges with
embedded RFID tags that enable their location, movement, and availability
within the hospital
to be tracked. This healthcare staff tracking information 47 is provided as
input to the system.
Further, for resource management, the availability of certain hospital
resources is also tracked
and monitored, with occupied and free resources noted appropriately. Other
resources such as
equipment, medication, supplies may include RFID tags that are used to track
their location
(shelf, room, storage, department, etc.), use, and availability. The system 10
also receives this
resource tracking data 48 from the various sensors distributed throughout the
facilities.
[0038] In addition to the above data that are received by the system 10,
another input
includes "What-If" scenarios 49 intended to simulate outcomes given specific
parameters and
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
conditions as entered by a member of the operations group of the hospital or
health facility.
The user may select one or more constraints, such as staffing level, hours of
operation, the
number of new patients, the number of available patient beds, the availability
of certain
medical equipment, the amount of supplies, and simulation time period, varying
values to
5 create a simulated scenario for purposes of generating possible outcomes.
The system 10 may
further generate recommendations based on the simulated outcome to avoid
adverse events or
unfavorable results.
[0039] All of the above-described input data including the clinical and non-
clinical
patient data are continually received, collected, and/or polled by the system
10 whenever they
10 become available and are used in analysis for a number of output data
and results. The data
may be presented in numerical format, graphical format, textual format, etc.
The system 10 is
configured to provide disease identification 50, risk identification 51,
adverse event
identification 52, and recommended treatment and therapy 53 on a real-time or
near real-time
basis. The information presented by the system 10 preferably includes an
identification of one
or more diseases that the patient has, whether the patient is at risk for
readmission due to a
particular condition, and whether there is a risk of the occurrence of one or
more adverse
events. The system 10 includes a predictive model that provides treatment or
therapy
recommendations based on the patient's data (e.g., medical history, symptoms,
current vital
signs, lab results, and the clinician's notes, comments, and diagnosis), and
forms the
fundamental technology for identification of diseases, readmission risk,
adverse events, and
situation simulation. Additionally, the system 10 is configured to generate a
course of
treatment or therapy recommendations for the patient based on disease, risk,
and adverse event
identification. Disease identification, risk identification, adverse event
identification, and
patient care surveillance information are displayed, reported, transmitted, or
otherwise
presented to healthcare personnel based on the user's identity or in a role-
based manner. In
other words, a patient's data and analysis is available to a particular user
if that user's identity
and/or role is relevant to the patient's care and treatment. For example, the
attending physician
and the nursing staff may access the patient data as well as receive
automatically-generated
alerts regarding the patient's status, and missed or delayed treatment. An
attending physician
may only have access to information for patients under his/her care, but an
oncology
department head may have access to data related to all of the cancer patients
admitted at the
facility, for example. As another example, the hospital facility's chief
medical officer and chief
nursing officer may have access to all of the data about all of the patients
treated at the facility
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
11
so that innovative procedures or policies may be implemented to prevent or
minimize adverse
events.
[0040] Further, the system 10 provides information on the availability of the
healthcare
staff 54, such as current nurse load for efficient resource allocation
purposes. The system 10
also has an inventory of available equipment, supplies, and other resources
55, and can quickly
pinpoint the location of available and required medical resources.
[0041] Another form of information or data presented by the system 10 is
information
about the disease, therapy, and care plan useful to the patient and family 56.
The patient and
family may also have access to the patient's medical information, lab results,
prescriptions, etc.
[0042] The system 10 also provides what-if simulation results 57 in response
to the
variations on some input parameters including staffing level, hours of
operation, resource
availability, current patient census, etc.
[0043] The system 10 also outputs various notifications and alerts 58 to the
appropriate
personnel so that proper action can be taken regarding the patient's treatment
and care. Any of
the functions described above may include an alert and notification output
that can
immediately present and push information to a user. For example, if a
patient's lab results or
vitals became available and it suggests that the patient's condition is
deteriorating, an alert is
immediately generated and transmitted to the attending physician and/or
nursing staff
[0044] FIG. 5 is a simplified logical block diagram of an exemplary embodiment
of the
holistic hospital patient care and management system and method 10 according
to the present
disclosure. The holistic hospital patient care and management system and
method 10 receives
and extracts data from many disparate sources in myriad formats pursuant to
different
protocols, the incoming data must first undergo a multi-step process before
they may be
properly analyzed and utilized. The holistic hospital patient care and
management system and
method 10 includes a data integration logic module 60 that further includes a
data extraction
process 62, a data cleansing process 63, and a data manipulation process 64.
It should be noted
that although the data integration logic module 60 is shown to have distinct
processes 62-64,
these are done for illustrative purposes only and these processes may be
performed in parallel,
iteratively, and interactively.
[0045] The data extraction process 62 extracts clinical and non-clinical data
from data
sources in real-time or batch files using hospital-accepted protocols.
Preferably in real-time,
the data cleansing process 63 "cleans" or pre-processes the data, putting
structured data in a
standardized format and preparing unstructured text for natural language
processing (NLP) to
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
12
be performed in the disease/risk logic module 66 described below. The system
10 may also
receive "clean" data or previously processed data and convert them into
desired formats (e.g.,
text date field converted to numeric for calculation purposes).
[0046] The data manipulation process 64 may analyze the representation of a
particular
data feed against a meta-data dictionary and determine if a particular data
feed should be re-
configured or replaced by alternative data feeds. For example, a given
hospital EMR may store
the concept of "maximum creatinine" in different ways. The data manipulation
process 64 may
make inferences in order to determine which particular data feed(s) from the
EMR would most
accurately represent the whole concept of "creatinine" as defined in the meta-
data dictionary
and whether a feed would need particular re-configuration to arrive at the
maximum value
(e.g., select highest value).
[0047] The data integration logic module 60 then passes the pre-processed data
to a
disease/risk logic module 66. The disease/risk logic module 66 is operable to
calculate a risk
score associated with a specific disease or condition for each patient and
subsequently identify
those patients who should receive more targeted intervention and care as a
result of the
assigned risk score (e.g., patient's risk of readmission for a particular
condition, patient's risk
of the occurrence of one or more adverse events). The disease/risk logic
module 66 includes a
de-identification/re-identification process 67 that is adapted to remove all
protected identifying
information according to HIPAA standards before the data is transmitted over
the Internet. It is
also adapted to re-identify the data. Protected identifying information that
may be removed and
added back later may include, for example, name, phone number, facsimile
number, email
address, social security number, medical record number, health plan
beneficiary number,
account number, certificate or license number, vehicle number, device number,
URL, all
geographical subdivisions smaller than a state identifier, including street
address, city, county,
precinct, zip code, and their equivalent geocodes (except for the initial
three digits of a zip
code, if according to the current publicly available data from the Bureau of
the Census),
Internet Protocol number, biometric data, and any other unique identifying
number,
characteristic, or code.
[0048] The disease/risk logic module 66 further includes a disease
identification
process 68. The disease identification process 68 is configured to identify
one or more diseases
or conditions of interest for each patient. The disease identification process
68 considers data
such as, but not limited to, lab orders, lab values, clinical text and
narrative notes, and other
clinical and historical information to determine the probability that a
patient has a particular
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
13
disease. Additionally, during disease identification, natural language
processing is conducted
on unstructured clinical and non-clinical data to determine the potential
disease(s) that the
physician believes are likely to be diagnosed for the patient. This process 68
may be performed
iteratively over the course of multiple days to establish a higher confidence
in identifying the
disease as the attending physician becomes more certain in the diagnosis. When
a patient is
identified to have a particular disease, the patient is identified in a
disease list for that ailment.
Where new or updated patient data may not support a previously identified
disease, the system
would automatically remove the patient from that disease list.
[0049] The disease/risk logic 66 includes a hybrid model of natural language
processing and generation 70, which combines a rule-based model and a
statistically-based
learning model. During natural language processing 70, raw unstructured data,
for example,
physicians' notes and reports, first go through a process called tokenization.
The tokenization
process divides the text, in the form of raw unstructured data, into basic
units of information in
the form of single words or short phrases by using defined separators such as
punctuation
marks, spaces, or capitalizations. Using the rule-based model, these basic
units of information
are identified in a meta-data dictionary and assessed according to predefined
rules that
determine meaning. Using the statistical-based learning model, the disease
identification
process 68 quantifies the relationship and frequency of word and phrase
patterns and then
processes them using statistical algorithms. Using machine learning, the
statistical-based
learning model develops inferences based on repeated patterns and
relationships. The disease
identification process 68 performs a number of complex natural language
processing functions
including text pre-processing, lexical analysis, syntactic parsing, semantic
analysis, handling
multi-word expression, word sense disambiguation, and other functions.
[0050] For example, if a physician's notes include the following: "55 yo m c
h/o dm,
cri. now with adib rvr, chfexac, and rle cellulitis going to 10W, tele." The
data integration logic
60 is operable to translate these notes as: "Fifty-five-year-old male with
history of diabetes
mellitus, chronic renal insufficiency now with atrial fibrillation with rapid
ventricular response,
congestive heart failure exacerbation and right lower extremity cellulitis
going to 10 West and
on continuous cardiac monitoring."
[0051] Continuing with the prior example, the disease identification process
68 is
adapted to further ascertain the following: 1) the patient is being admitted
specifically for atrial
fibrillation and congestive heart failure; 2) the atrial fibrillation is
severe because rapid
ventricular rate is present; 3) the cellulitis is on the right lower
extremity; 4) the patient is on
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
14
continuous cardiac monitoring or telemetry; and 5) the patient appears to have
diabetes and
chronic renal insufficiency.
[0052] The disease component/risk logic module 66 further comprises a
predictive
modeling process 71 that is adapted to predict the risk of being diagnosed
with particular
diseases or developing an adverse event of interest according to one or more
predictive models.
For example, if a hospital desires to determine the level of risk for future
readmission for heart
failure, the heart failure predictive model may be selected for processing
patient data.
However, if the hospital desires to determine the risk levels for readmission
for all internal
medicine patients for any cause, an all-cause readmissions predictive model
may be used to
process the patient data. As another example, if the hospital desires to
identify those patients at
risk for short-term and long-term diabetic complications, the diabetes disease
identification
component may be used to target those patients. Other predictive models may
include HIV
readmission, risk for cardio-pulmonary arrest, kidney disease progression,
acute coronary
syndrome, pneumonia, cirrhosis, colon cancer pathway adherence, and others.
[0053] Continuing to use the prior example, the predictive model for
congestive heart
failure may take into account a set of risk factors, such as laboratory and
vital sign variables
including: albumin, total bilirubin, creatine kinase, creatinine, sodium,
blood urea nitrogen,
partial pressure of carbon dioxide, white blood cell count, troponin-I,
glucose, internationalized
normalized ratio, brain natriuretic peptide, pH, temperature, pulse, diastolic
blood pressure,
and systolic blood pressure. Further, non-clinical factors are also
considered. The predictive
model is configured to each hospital based on a retrospective data analysis
conducted to tune
the model to fit the unique characteristics of each individual hospital. In
this manner, the
system is able to stratify, in real-time, the risk of each patient that
arrives at a hospital or
another healthcare facility. Therefore, those patients at the highest risk are
automatically
identified so that targeted intervention and care may be instituted. One
output from the disease
component/risk logic module 66 includes the risk scores of all the patients
for particular
potential disease diagnosis or adverse event. In addition, the module 66 may
rank the patients
according to the risk scores, and provide a sortable list to facilitate
prioritizing the patients
needing the most resources. For example, a hospital may desire to identify the
top 20 patients
most at highest risk for congestive heart failure readmission, and the top 5%
of patients most at
highest risk for cardio-pulmonary arrest in the next 24 hours. Other diseases
and adverse
events that may be identified through risk stratification using predictive
modeling include, HIV
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
readmission, diabetes identification, kidney disease progression, colorectal
cancer continuum
screening, meningitis management, acid-base management, anticoagulation
management, etc.
[0054] The natural language generation module 70 is adapted to receive the
unstructured clinical information for a patient, and "translate" that data to
present the textual
5 evidence that the patient is at high-risk for a specific disease. In this
manner, the intervention
coordination team may better formulate the targeted inpatient and outpatient
intervention and
treatment plan to address the patient's potential specific situation.
[0055] The disease component/risk logic module 66 further includes an
artificial
intelligence (Al) model tuning process 72. The artificial intelligence model
tuning process 72
10 utilizes adaptive self-learning capabilities using machine learning
technologies. The capacity
for self-reconfiguration enables the system and method 10 to be sufficiently
flexible and
adaptable to detect and incorporate trends or differences in the underlying
patient data or
population that may affect the predictive accuracy of a given algorithm. The
artificial
intelligence model tuning process 72 may periodically retrain a selected
predictive model for
15 improved accurate outcome to allow for selection of the most accurate
statistical methodology,
variable count, variable selection, interaction terms, weights, and intercept
for a local health
system or clinic. The artificial intelligence model tuning process 72 may
automatically modify
or improve a predictive model in three exemplary ways. First, it may adjust
the predictive
weights of clinical and non-clinical variables without human supervision.
Second, it may adjust
the threshold values of specific variables without human supervision. Third,
the artificial
intelligence model tuning process 72 may, without human supervision, evaluate
new variables
present in the data feed but not used in the predictive model, which may
result in improved
accuracy. The artificial intelligence model tuning process 72 may compare the
actual observed
outcome of the event to the predicted outcome then separately analyze the
variables within the
model that contributed to the incorrect outcome. It may then re-weigh the
variables that
contributed to this incorrect outcome, so that in the next reiteration those
variables are less
likely to contribute to a false prediction. In this manner, the artificial
intelligence model tuning
process 72 is adapted to reconfigure or adjust the predictive model based on
the specific
clinical setting or population in which it is applied. Further, no manual
reconfiguration or
modification of the predictive model is necessary. The artificial intelligence
model tuning
process 72 may also be useful to scale the predictive model to different
health systems,
populations, and geographical areas in a rapid timeframe.
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
16
[0056] As an example of how the artificial intelligence model tuning process
72
functions, the sodium variable coefficients may be periodically reassessed to
determine or
recognize that the relative weight of an abnormal sodium laboratory result on
a new population
should be changed from 0.1 to 0.12. Over time, the artificial intelligence
model tuning process
72 examines whether thresholds for sodium should be updated. It may determine
that in order
for the threshold level for an abnormal sodium laboratory result to be
predictive for
readmission, it should be changed from, for example, 140 to 136 mg/dL.
Finally, the artificial
intelligence model tuning process 72 is adapted to examine whether the
predictor set (the list of
variables and variable interactions) should be updated to reflect a change in
patient population
and clinical practice. For example, the sodium variable may be replaced by the
NT-por-BNP
protein variable, which was not previously considered by the predictive model.
[0057] The results from the disease component/risk logic module 66 are
provided to
the designated medical staff, such as the intervention coordination team and
other care
providers, by a data presentation and system configuration logic module 74.
The data
presentation logic module 74 includes a dashboard interface 75 that is adapted
to provide
information on the performance of the system and method 10. A user (e.g.,
medical staff,
administrator, and intervention coordination team) is able to find specific
data they seek
through simple and clear visual navigation cues, icons, windows, and devices.
The interface
may further be responsive to audible commands, for example. Because the number
of patients
a hospital admits each day can be overwhelming, a simple graphical interface
that maximizes
efficiency and reduces user navigation time is especially desirable. The
visual cues are
preferably presented in the context of the problem being evaluated (e.g.,
readmissions, out-of-
ICU, cardiac arrest, diabetic complications, among others).
[0058] The dashboard user interface 75 allows interactive requests for a
variety of
views, reports and presentations of extracted data and risk score calculations
from an
operational database within the system, including for example, summary views
of a list of
patients in a specific care location; graphical representations of the data
for a patient or
population over time; comparison of incidence rates of predicted events to the
rates of
prediction in a specified time frame; summary text clippings, lab trends and
risk scores on a
particular patient for assistance in dictation or preparation of history and
physical reports, daily
notes, sign-off continuity of care notes, operative notes, discharge
summaries, continuity of
care documents to outpatient medical practitioners; automated order generation
of orders
authorized by a care provider's healthcare environment and state and national
guidelines to be
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
17
returned to the practitioner's office, outside healthcare provider networks or
for return to a
hospital or practices electronic medical record; aggregation of the data into
frequently used
medical formulas to assist in care provision including but not limited to:
acid-base calculation,
MELD score, Child-Pugh-Turcot score, TIMI risk score, CHADS score, estimated
creatinine
clearance, Body Surface area, Body Mass Index, adjuvant, neoadjuvant and
metastatic cancer
survival nomograms, MEWS score, APACHE score, SWIFT score, NIH stroke scale,
PORT
score, AJCC staging; and publishing of elements of the data on scanned or
electronic versions
of forms to create automated data forms.
[0059] The data presentation and system configuration logic module 74 further
includes a messaging interface 76 that is adapted to generate output messaging
code in forms
such as HL7 messaging, text messaging, e-mail messaging, multimedia messaging,
web pages,
web portals, REST, XML, computer generated speech, constructed document forms
containing
graphical, numeric, and text summary of the risk assessment, reminders, and
recommended
actions. The interventions generated or recommended by the system and method
10 may
include: risk score report to the primary physician to highlight risk of
readmission for their
patients; score report via new data field input into the EMR for use by
population surveillance
of entire population in hospital, covered entity, accountable care population,
or other level of
organization within a healthcare providing network; comparison of aggregate
risk of
readmissions for a single hospital or among hospitals within a system to allow
risk-
standardized comparisons of hospital readmission rates; automated
incorporation of score into
discharge summary template, continuity of care document (within providers in
the inpatient
setting or to outside physician consultants and primary care physicians), HL7
message to
facility communication of readmission risk transition to nonhospital
physicians; and
communicate subcomponents of the aggregate social-environmental score,
clinical score and
global risk score. These scores would highlight potential strategies to reduce
readmissions
including, but not limited to: generating optimized medication lists, or
alternate medication
therapy management practices; allowing pharmacies to identify those medication
on formulary
to reduce out-of-pocket cost and improve outpatient compliance with the
pharmacy treatment
plan; flagging patient education around such topics like maintaining a
specific diet, identifying
alternate modes of transportation; identifying alternate housing options
(e.g., nursing home
placement, transitional housing, or Section 8 HHS housing assistance) or
financial assistance
programs.
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
18
[0060] This output may be transmitted wirelessly or via LAN, WAN, the
Internet,
and delivered to healthcare facilities' electronic medical record stores, user
electronic devices
(e.g., pager, text messaging program, mobile telephone, tablet computer,
mobile computer,
laptop computer, desktop computer, and server), health information exchanges,
and other data
stores, databases, devices, and users. The system and method 10 may
automatically generate,
transmit, and present information such as high-risk patient lists with risk
scores, natural
language generated text, reports, recommended therapies, alerts, Continuity of
Care
Documents, flags, appointment reminders, telemedicine video communications,
simulation
results and recommendations, healthcare staff location and availability, and
patient/family
surveys or questionnaires.
[0061] The data presentation and system configuration logic module 74 further
includes a system configuration interface 77. Local clinical preferences,
knowledge, and
approaches may be directly provided as input to the predictive models through
the system
configuration interface 77. This system configuration interface 77 allows the
institution or
health system to set or reset variable thresholds, predictive weights, and
other parameters in the
predictive model directly. The system configuration interface 77 preferably
includes a
graphical user interface designed to minimize user navigation time.
[0062] FIG. 6 is a simplified flowchart of an exemplary embodiment of a
clinical
predictive and monitoring method 80 according to the present disclosure. The
method 80
receives structured and unstructured clinical and non-clinical data related to
specific patients
from a variety of sources and in a number of different formats, as shown in
block 82. These
data may be encrypted or protected using data security methods now known or
later developed.
In block 84, the method 80 pre-processes the received data: data extraction,
data cleansing, and
data manipulation. Other data processing techniques now known and later
developed may be
utilized. In block 86, data processing methods such as natural language
processing and other
suitable techniques may be used to translate or otherwise make sense of the
unstructured data.
In block 88, by analyzing the pre-processed data, one or more potential
diseases or adverse
events of interest as related to each patient are identified. In block 90, the
method 80 applies
one or more predictive models to further analyze the data and calculate one or
more risk scores
for each patient as related to the identified diseases or adverse events. In
blocks 92 and 94, one
or more lists showing those patients with the highest risks for each
identified disease or
adverse event are generated, transmitted, and otherwise presented to
designated medical staff,
such as members of an intervention coordination team. These lists may be
populated in real-
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
19
time, or otherwise regularly according to a recurring schedule depending on
hospital capability
and resources. The intervention coordination team may then prescribe and
follow targeted
intervention and treatment plans for inpatient and outpatient care. In block
96, those patients
identified as high-risk are continually monitored while they are undergoing
inpatient and
outpatient care. The method 80 ends in block 98.
[0063] Not shown explicitly in FIG. 6 is the de-identification process, in
which the
data become disassociated with the patient's identity to comply with HIPAA
regulations. The
data can be de-coupled with the patient's identity whenever they are
transmitted over wired or
wireless network links that may be compromised, and otherwise required by
HIPAA. The
method 80 is further adapted to reunite the patient data with the patient's
identity.
[0064] FIG. 7 is a simplified flowchart/block diagram of an exemplary
embodiment
of a clinical predictive modeling method 100 according to the present
disclosure. A variety of
data are received from a number of disparate data sources 102 related to
particular patients
admitted at a hospital or a healthcare facility. The incoming data may be
received in real-time
or the data may be pulled in batches. The incoming data are stored in a data
store 104. In block
106, the received data undergo a data processing and integration process
following data
extraction (e.g., data cleansing and data manipulation), as described above.
The resultant data
then undergo the disease risk logic process 108 during which disease
identification, and
predictive modeling are performed. The risk score (with specific regard to
high risk) computed
for each patient for a disease of interest is compared to a disease high risk
threshold in block
110. Each disease is associated with its own high risk threshold. If the risk
score is less than the
high risk threshold, then the process determines if the patient's risk score
falls into the medium
or low risk categories, otherwise the process returns to data integration and
is repeated when
new data associated with a patient become available. If the risk score is
greater than or equal to
the high risk threshold, then the identified patient having the high risk
score is identified as
'high risk' and included in a patient list in block 112. In block 114, the
patient list and other
associated information may then be presented to the intervention coordination
team in one or
more possible ways, such as transmission to and display on a desktop or mobile
device in the
form of a text message, e-mail message, web page, etc. In this manner, an
intervention
coordination team is notified and activated to target the patients identified
in the patient list for
assessment, and inpatient and outpatient treatment and care, as shown in block
118. The
process may thereafter provide feedback data to the data sources 102 and/or
return to data
integration 106 that continues to monitor the patient during his/her targeted
inpatient and
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
outpatient intervention and treatment. Data related to the patient generated
during the inpatient
and outpatient care, such as prescribed medicines and further laboratory
results, radiological
images, etc. may be continually monitored to track intervention completion.
[0065] FIG. 8 is a simplified flowchart diagram of an exemplary embodiment of
a
5 dashboard user interface method 120 according to the present disclosure.
The patients' data are
evaluated as described above, and those patients associated with targeted
diseases and
surveillance conditions are identified in block 122. The targeted diseases are
those illnesses
that the patient is at risk for readmission to the healthcare facility. The
monitored conditions
are those patient conditions, e.g., injury and harm, that are indicative of
occurrence of adverse
10 events in the healthcare facility. The patients' inclusion on a
particular disease or surveillance
condition list is further verified by comparison to a predetermined
probability threshold, as
shown in block 124. If the probability threshold is met, then the patient is
classified or
identified as belonging to a disease list or condition list. The display is
also updated so that
when a user selects a particular disease list for display, that patient is
shown in the list, as
15 shown in block 126. In this exemplary screen, the list of patients that
are at risk for 30-day
readmission due to congestive heart failure (CHF) are identified and listed in
the active
congestive heart failure list. Details of the exemplary screen are provided
below.
[0066] The user may use the displayed information acknowledging and adhering
to
patient privacy protocols, and generate standard or custom reports. The
reports may be
20 primarily textual in nature, or include graphical information. For
example, a graphical report
may chart the comparison of expected to observed readmission rates for any
disease type,
condition, or category for patients enrolled or not enrolled in an intensive
intervention
program, the readmission rates for enrolled versus dropped patients over a
period of time for
any disease type, condition, or category. Patients with greater than 95%, for
example,
probability of having heart failure, total versus enrolled in an intervention
program over a
specified time period, and the number of patients not readmitted within 30-day
discharge
readmission window. Additional exemplary standard tracking reports that may
further identify
all enrolled patients for which: post-discharge appointments are scheduled,
post-discharge
phone consults are scheduled, patient has attended follow-up appointment,
patient has received
post-discharge phone consult, patient has received and filled medical
prescriptions, and patient
has received transportation voucher. Further sample reports may include a
comparison of
expected to observed readmission rates for any disease type, adverse event, or
category for
intervention program-enrolled and not enrolled patients, or readmission rates
for intervention-
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
21
enrolled vs. dropped patients over a period of time for any disease type,
adverse event, or
category. Another type of report available is outcome optimization reports.
These are reports
designed to help users (administrators) assess the benefit and efficacy of a
program, establish
benchmarks, and identify needs for change on systematic and population levels
to improve care
outcomes. The report may include data that assist in assessing the
effectiveness of the
identifying high risk patients. Some of the data may demonstrate effort spent,
patients enrolled
in an intervention program following designation as high risk for an adverse
event, and how
often those patents truly are afflicted with the identified diseases. Reports
may include data
that assist in assessing whether interventions are given to the right
patients, at the right time,
etc.
[0067] As new, updated, or additional patient data become available, as shown
in
block 128, the data is evaluated to identify or verify disease/condition. The
patient may be
reclassified if the data now indicate the patient should be classified
differently, for example. A
patient may also be identified as potentially being diagnosed with an
additional disease and be
classified as such. For example, in the first 24 hours of admissions, the
system identifies a
particular patient as having CHF. Upon receiving more information, such as lab
results and
new physician notes, the system identifies this patient as also having AMI.
Thus, this patient is
identified as an AMI candidate and a CHF candidate.
[0068] If there are no new patient data available or accessible to the disease
component/risk logic modules, then there is no change to the patient
classification and the
display reflects the current state of patient classification, as shown in
block 129. Accordingly,
as real-time or near real-time patient data become available, the patients'
disease and adverse
event classifications are re-evaluated and updated as necessary.
[0069] Targeted predictive readmission diseases may include: congestive heart
failure, pneumonia, acute myocardial infarction, diabetes, cirrhosis, and all
cause. Targeted
disease or adverse event identification may include: sepsis, chronic kidney
disease, and
diabetes mellitus. Targeted conditions due to a possible adverse event for
surveillance may
include: sepsis, post-operative pulmonary embolism (PE) or deep vein
thrombosis (DVT),
post-operative sepsis, post-operative shock, unplanned return to surgery,
respiratory failure,
hypertension, unexpected injury, inadequate communication, omission or errors
in assessment,
diagnosis, or monitoring, falls, hospital-acquired infections, medication-
wrong patient, patent
identification issues, out-of-ICU cardiopulmonary arrest and mortality,
chronic kidney disease,
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
22
shock, trigger for narcan, trigger for narcotic (over-sedation), trigger for
hypoglycemia, and
unexpected death.
[0070] The evaluation may include users inputting observations and comments
about
the patient, for example. As a part of the evaluation process, a user (a
healthcare provider) may
confirm, deny, or express uncertainty about a patient's disease or adverse
event identification
or intervention program enrollment eligibility. For example, the user may
review, via the user
interface, notes and recommendations associated with a particular patient and
confirm the
inclusion of that patient in the congestive heart failure list for
intervention program enrollment,
as shown in block 108. The user may review the clipped clinician's notes that
call attention to
key words and phrases that led to a disease identification by the system. Key
terms such as
"shortness of breath," "BNP was elevated," and "Lasix" may help the user
validate the disease
identification of CHF for that patient, and validate enrollment of the patient
into a specific
intervention program. If the patient's classification, risk level, and
eligibility level are
confirmed, there is no change in the patient's classification and the data
that are displayed
(except to indicate this classification has been confirmed), as shown in block
109. The user
may supply or enter comments associated with the confirmation. The user may
disagree with
the inclusion of the patient in the congestive heart failure list, or express
uncertainty or enter
comments explaining his or her assessment. User comments are stored and can be
seen by
other users, allowing clear and timely communication between team members. The
user may
proceed to select a report or a display parameter, or review and evaluate
particular selected
patients.
[0071] If the user disagrees with the patient's the classification, then the
patient is
removed from the active list of the target disease or condition, and placed on
a drop list. In
response to the user denying the classification, the system may additionally
display or flag
information about the patient that contributed to the inclusion of the patient
on a particular list.
For example, if the user denies the disease identification that John Smith has
heart failure, the
system may further request confirmation wherein which the user is required to
respond to the
query with yes or no. The system may additionally request rationale from the
user for wanting
to remove the patient from the active list. The rationale supplied by the user
may be stored and
displayed as reviewer comments. The user may also indicate uncertainty, which
may result in
the patient being removed from the active list and placed on a watch list for
further evaluation.
The user may then review and evaluate additional patients on the same target
disease list or
review patients included on other disease and adverse event lists. At any
point, the user may
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
23
use, with compliance and adherence to patient privacy protocols, in some form,
the displayed
information, such as generating standard or custom reports.
[0072] As an example, a patient is identified as a CHF patient at the time of
admission. After receiving more data (i.e., new lab results and new physician
notes) during her
hospital stay, the system has identified this patient as having AMI.
[0073] The clinician notes upon admission states: 52 yo female w pmh of CAD,
also
with HTN presents with progressively worsening SOB and edema 1 month. 1.
Dyspnea: likely
CHF with elevated BNP afterload reduction with aCEi and diuresis with Lasix.
02 stats stable
2) elevated troponin: EKG with strain pattern follow serial enzymes to ROMI
and cards
consulted for possible Cath. The clinician notes thereafter states: 52 yo
female with pmh of
CAD, also with HTN presents with progressively worsening SOB and edema 1 month
c CAD
with LHC with stent prox LAD. 1. Elevated troponins -NSTEMI, despite pt
denying CP - pt
with known hx of CAD, mild troponin leak 0.13->0.15->0.09->0.1 ¨ on admission
pt given
325, Plavix load with 300 mg 1, and heparin gtt - Metop increased 50 mg q6,
possibly change
Coreg at later time - LHC today per Cardiology, with PCI. also discuss with EP
for possible
ICD placement 2. Heart failure, acute on chronic - severe diastolic
dysfunction be due HTN off
meds +/- CAD - proBNP elevated 3183 on admission - initially started on lasix
40 tid, edema
much improved, now on lasix 40 po bid - TTE completed showing: 4 chamber
dilatation,
RVH, nml LV thickness, severely depressed LVSF, LVEF 30%,mod MR, mild TR, AR
and
PR; severe diastolic dysfunction, RVSP 52 - continue on Lasix, Lisinopril,
Metop - discuss
AICD evaluation with EP vs initial medical management.
[0074] The reviewer may assess the above admission notes with the disease
identification of CHF compared with a disease identification of AMI by the
system 10 in an
effort to validate this new real-time disease identification. The admission
note indicated CHF
as the primary disease. Key highlighted terms that are indicative of CHF
include "pmh of
CAD" (past medical history of coronary artery disease, "SOB" (shortness of
breath), "edema,"
"elevated BNP." The second note indicates that while the patient has CHF, CAD
is the primary
cause of the CHF. Key highlighted terms such as "elevated troponins" and
"NSTEMI" (Non
ST Segment Myocardial Infarction: heart attack) give the reviewer a snapshot
view of the key
terms the system used to identify AMI as the primary disease. These
highlighted key terms
give the reviewer the tools to validate in real-time or near real-time the
system's recommended
change in disease identification. The reviewer can then confirm, deny, or
express uncertainty
with the new disease identification, and note any validation or rejection in
his or her personal
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
24
notes. In any scenario, the system will provide likely disease diagnosis based
on data inputs,
but can be overruled by the expert opinion of a physician reviewer. Because
the patient's
primary recommended intervention pathway would be for AMI, the patient,
corresponding
disease identification, and risk level would appear in the AMI list.
[0075] The dashboard user interface 75 may also indicate a change in the level
of risk.
For example, upon return of recent lab results (e.g., slightly elevated
creatinine and tox screen
positive for cocaine) and other (updated) social factors that influence risk
(e.g., noncompliance
with sodium restriction due to homelessness) as well as medical pathway
language queues, and
prior admission history, the system may identify a patient initially evaluated
to be at medium
risk of readmission to currently be at high risk for readmission. A reviewer
can follow these
changes in real-time and to validate the change in risk level and take any
additional appropriate
action.
[0076] The holistic hospital patient care and management system and method 10
further include a number of novel features shown in FIGS. 9-16 described
below.
[0077] FIG. 9 is a simplified flowchart of an exemplary embodiment of an
enhanced
predictive modeling method 140 according to the present disclosure. In block
142, the patient's
consent for continued collection and analysis of the patient's data is
requested. Because the
enhanced method will continue to track and monitor the patient's wellbeing and
collect data
associated with the patient for analysis, the patient's consent is sought to
comply with all local,
state, and federal regulations. If the patient's consent is not received or
the patient declined, as
determined in block 144, then the patient's no consent status is recorded in
the system's
database, as shown in block 146. If the consent is received in block 144, then
the patient's
visits to clinical/medical and non-medical/social service appointments are
monitored and
tracked and data recorded, as shown in block 148. This may be done
automatically, such as
tracking the patient's location using, for example, RFID, WiFi, or GPS
methods. Alternatively,
data received or taken at each visit to these scheduled or unscheduled
appointments are
recorded in the system for analysis. The patient's social media data may also
be received and
stored for analysis, upon receipt of patient consent, as shown in block 150.
With regard to
tracking clinical variables post-discharge, the patient's vitals may be
continuously monitored
and taken automatically or otherwise for analysis, as shown in block 152
through an electronic
device (worn by the patient) that is capable of measuring the vitals of the
patient on a periodic
basis, such as once or twice a day. This information may be automatically
relayed or
transmitted to the system 10 directly or via a portal or information exchange.
The enhanced
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
predictive model is capable of serving as a reliable warning tool for the
timely detection and
prevention of adverse events. Its functionality may include patient risk
stratification,
notification of clinical staff of an adverse event, and identification of
health service and
relevant social service organizations based on the patient's location to best
serve the patient's
5 needs. The system 10 may notify caregiver or healthcare provider via, for
example, pages, best
practice alerts, conventional alerts, and visualization reports.
[0078] FIG. 10 is a simplified flowchart of an exemplary embodiment of a
facial and
biological recognition process 140 according to the present disclosure. It is
assumed that the
patient has given all required consent for the enrollment into this program.
One or more video
10 and/or still cameras are placed in strategic locations in the patient's
room. For example, a
camera may be mounted on the ceiling above the patient's bed to be able to
capture unimpeded
visible light and infrared thermal images of the patient's face. In addition,
nurses attending to
the patient may wear a video camera attached to his uniform, glasses, or other
accessories. The
cameras are preferably capable of capturing high definition and high quality
images. These
15 images may be accessible by attending physicians and nurses. In block
142, the system
continually receives images of the patient, and records those images. In block
143, the system
continually analyzes the patient's images to detect biological changes
indicative of an adverse
clinical outcome which may not have physically manifested in the patient yet.
The algorithm
considers abnormalities in variables such as body temperature, conjunctival
color, pupillary
20 responsiveness, facial expression, etc. The system uses facial
recognition and artificial
intelligence software to recognize and detect certain changes in temperature,
color, and
expressions. For example, a change in the patient's conjunctival color may be
identified as a
possibility that the patient is becoming anemic due to anostomotic hemorrhage
post-surgery. A
mild change in the patient's pupillary responsiveness may be detected by the
system as a
25 change in intra-cranial pressure that requires attention. The system may
also recognize an
expression on the patient's face that indicates the patient is experiencing
pain or severe
discomfort.
[0079] In block 144, these biological changes in the patient are recognizes as
requiring
prompt attention by caregivers. In block 146, the attending physician and/or
nurse is notified or
alerted. These alerts may be sent, for example, via page, text message, call,
or the PB system,
and may be preferences set by the individual caregivers. In block 148, prompt
attention and
appropriate intervention and therapy can then be ordered by the healthcare
providers to timely
address the issue(s) that brought on the detected biological change.
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
26
[0080] FIG. 11 is a simplified flowchart of an exemplary embodiment of an
automated
patient monitoring process 150 according to the present disclosure. In block
152, the patient's
consent for the collection and analysis of data is requested. If the patient
does not give consent,
as determined in block 154, then the patient's no consent status is noted in
the system in block
156, and the patient is not enrolled in this program. If the patient does
provide consent, then the
patient is tracked and monitored in a number of ways, including location,
social service
appointments and visits, and vitals, as shown in blocks 158-162.
[0081] The patient's location may be determined using various suitable
technologies,
including RFID, GPS, and WiFi/cell tower triangulation. The patient may be
given an RFID
bracelet or another form of accessory when the patient was first admitted in a
hospital. The
patient's location may then be tracked by a plurality of RFID sensors
distributed within the
hospital. In addition, clinical and social service organizations that
participate in this patient
monitoring program may be outfitted with RFID so that when the patient visits
the
organization for an appointment, his presence is detected. As mobile devices
such as mobile
telephones equipped with GPS capabilities has become ubiquitous, a patient's
location and
movement may also be tracked using the device's GPS functionality and relayed
back to the
system via an application (app) downloaded to the patient's device. The
sensors and mobile
devices are configured to transmit the patient's detected location to the
system for recording
and analysis. The system is able to determine that the patient's location
matches up with the
patient's calendar appointments for healthcare and social services, and is
thus properly
following prescribed therapies and treatment. This functionality combined with
disease and
risk identification functions provide a capability of identifying the highest
priority patients
based on severity of disease and deploying the right resources to the most
vulnerable patients
in timely manner. Patients that repeatedly fail to follow prescribed therapies
may cause an alert
to be generated and sent to healthcare providers or social service providers
so that additional,
more focused assistance or guidance may be given to the patient.
[0082] FIG. 12 is a simplified flowchart of an exemplary embodiment of an
automated
healthcare staff monitoring process 170 according to the present disclosure.
This function is
capable of assessing existing nurse availability and workload and producing
new staff
assignments based on current or expected patient inflow. Healthcare staff such
as nurses are
given ID badges that have embedded RFID tags that respond to RFID sensors
distributed
throughout the hospital facility. In block 172, using RFID technology, each
nurse's location
can be determined, tracked, and recorded. As part of the analysis, a nurse
that is inside a
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
27
patient's room or substantially co-located with a patient is automatically
identified as "busy" or
"with patient." The system may also receive input from the nurses who can
manually indicate
on a user interface (of software application executing on a computing device
such as mobile
telephone, laptop computer, and desktop computer) or in some other manner that
they are
"busy" or "available." The nursing staff for each department may be clearly
marked or
delineated along with the patients assigned to each nurse. The system
continually receives the
nursing staffs location information and makes a determination on whether each
nurse is
"busy" or "available." The nursing staff location and status are displayed on
a graphical user
interface of the system, as shown in block 174. The nurses' location, current
(real-time) status,
and department designation are presented via the graphical user interface at
one glance.
[0083] When a new patient arrives or is admitted, as determined in block 176,
the
status and location of each nurse working that shift for a particular
department can be clearly
viewed on the user interface. In block 178, an available nurse may be selected
and assigned to
the new patient, an alert or message is sent to the nurse to inform him/her of
this new
assignment, and the nurse's status is immediately updated in the system. A
nurse or another
healthcare staff may also be notified in advance of anticipated need via this
function. For
example, one or more emergency department nurses that are currently
"available" may be
selected to receive notification that seven critically injured patients from a
multi-car accident
are in transit to the hospital with the estimated arrival time. In this
manner, RFID technology is
used to monitor and track the nursing staff at any given moment in order to
identify available
human resources on a real-time basis that are capable to offering care to
incoming patients.
Thus, human resources may be efficiently assigned and utilized.
[0084] FIG. 13 is a simplified flowchart of an exemplary embodiment of an
automated
resource management process 180 according to the present disclosure. This
function is capable
of tracking/monitoring hospital resource availability, deficiencies, and
surpluses. Further, this
function may be used to reserve resources for anticipated use. For example,
the system may be
used to hold a hospital bed for a patient undergoing testing; 2) notify
appropriate staff to
turnover beds for patients who have been discharged/transferred; and 3)
indicate free beds once
necessary cleaning and maintenance has occurred following patient
discharge/transfer. The
system may be used to track and monitor all resources in a hospital, including
patient beds,
medical equipment, medicine, and supplies. All of these resources have an RFID
tag that
communicates with RFID sensors distributed throughout the hospital. For
example, the system
can detect and determine that certain equipment and supplies are located in a
specific storage
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
28
room and/or in a particular storage cabinet. Further, if a patient's RFID tag
is co-located with
the RFID tag of a particular hospital bed, then the system determines that the
patient is
occupying that bed and that bed is not available.
[0085] In block 182, the system receives RFID sensor output that informs the
system of
the location of each resource item. This information is recorded and analyzed.
The resource
information is also presented or displayed via a graphical user interface that
provides an at-a-
glance view of which bed (hospital room) is available for incoming patients,
what equipment
and supplies are available, as shown in block 184. When a status change is
indicated, either
automatically detected (e.g., when an item is moved as detected by RFID
sensors) or by user
input (e.g., when an assignment to a patient is entered by a user), the item's
status is updated in
the system, as shown in block 188. For example, a nurse may use a handheld
barcode scanner
to scan supplies and drugs that are being readied and used for a particular
patient. The
information from the scan would then be transmitted to the system, which would
update the
status and location of these items in the appropriate inventory tracking
module. As another
example, a nurse may scan, via the graphical user interface, that four
emergency beds should
be reserved as four critical patients are being transported to the hospital
from an industrial
accident. This information would be sent back to the system, and the quantity
of required beds
would be held by notification status of HOLD next to the unit/room number in
the bed listing
for the hospital. The process returns to block 182 to continually monitor and
update resource
location and status.
[0086] FIG. 14 is a simplified flowchart of an exemplary embodiment of a
telemedicine process 190 according to the present disclosure. The telemedicine
function is
configured to resolve the issue of competing and high priority demands faced
by clinical staff
Functionality includes the identification of physicians who are able to
provide remote clinical
assessments and validate disease identification. Scenarios in which
telemedicine is initiated are
when the patient is taken to a clinic where specialized medical staff is not
available for consult
for the patient's disease or condition. Alternatively, a telemedicine session
may be initiated
when paramedics are assisting a patient and they need immediate assistance or
consultation
with a physician to deal with a time sensitive condition. In block 192, the
patient's name and/or
other forms of identifier is entered by the attending personnel assisting the
patient. Using the
patient's identification information, the patient's clinical and non-clinical
data are retrieved
from the data store, and displayed if necessary. The patient's current vitals
and other
information taken by the attending personnel are entered into the system and
recorded, as
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
29
shown in block 194. The patient's medical history along with the current
vitals and other
information are used by the predictive model to identify a disease. This
information is used to
select a physician or other telemedicine staff that are available for the
present telemedicine
session. The physicians' medical specialties are considered for the selection.
In block 196, the
available physicians and staff are displayed by specialty area. A selection
may be
recommended by the system taking all data into account or received by a manual
selection by
the attending personnel, as shown in block 198. In block 200, the selected
physician or staff is
alerted or notified by a method preferred by that person. The status of the
selected physician or
staff is updated, as shown in block 202. In block 204, a two-way encrypted
video session
between the telemedicine physician and the attending personnel is initiated to
enable the two
parties to communicate, view the patient, share notes, and attend to the
patient. In this manner,
the best qualified telemedicine physician available may be automatically
selected or
recommended by the system to be consulted for the care of the patient.
[0087] FIG. 15 is a simplified flowchart of an exemplary embodiment of a
patient/family engagement process 210 according to the present disclosure.
This function is
capable of serving as a repository of reference material to inform provider
decision-making
and assist patients/families in self-care and disease management. This
function further allows
patients to describe all medical issues and submit questions to ensure that
physician-patient
communication is as efficient and transparent as possible. A patient's family
is also provided
with opportunities to be notified of patient status in an effort to increase
awareness and shared
decision-making during complicated situations, such as surgery. A software
application may be
provided to the patient or the patient may download the app to a computing
device, such as a
mobile device or laptop computer. The patient and family member may be
provided access to
this function at admission to the hospital, with it remaining accessible even
after discharge
from the hospital, contingent on adequate Internet accessibility. In block
212, the patient and/or
family member that have been given access to this function may enter
authentication data or
login information. Once the access is authenticated, a selective subset of the
patient's data are
retrieved from the data store and displayed, as shown in block 214. Also
displayed are
resources available to the patient, such as information related to a
particular disease that the
patient is being treated for, information related to a therapy or treatment
that the patient is
undergoing, information about available support groups, etc. The system
further displays
queries that solicit the patient's and family's preferences, as shown in block
216. The patient
and family members may provide their preferences by inputting them or
selecting from among
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
available options, as shown in block 218. For example, the patient or family
member may
indicate the preferred rounding time, preferred family notification method,
privacy preferences
for communication, and online health history. The received input are stored
and made available
to healthcare workers and social service workers, where necessary, and are
applied to modify
5 the system configuration (e.g., how the system notifies patient or
family) and the patient's care
plan where suitable, as shown in block 222.
[0088] FIG. 16 is a simplified flowchart of an exemplary embodiment of a
situation
analysis simulation process 230 according to the present disclosure. This
function gives the
hospital administrator the ability to simulate 'what-if scenarios by adjusting
different
10 parameters and observing the expected impact on operations will
facilitate appropriate
planning to optimize existing resources, thereby enhancing operational
efficiency. The use of
real-time data used to run the simulations will provide reasonable confidence
in the application
of simulated results to current and future resource planning. In block 232,
the method displays
input parameters that can be varied to simulate certain scenarios. The
parameters may include
15 the number of available beds, the number of patients, then number of
physicians, the number of
nurses, the number of certain medical equipment, the amount of certain medical
supplies, etc.
In block 234, the user is provided the ability to alter or change these
parameter values to see
what would happen to the operations of the hospital. For example, the user may
increase the
number of patients needing care in the emergency department by two fold due to
a multi-car
20 accident. The user may reduce the number of available beds and decrease
the number of
physicians available to tend to the patients due to a high patient volume day.
The user may
lower the number of physicians, and increase the number of nurses available
due to more
severe cases (e.g., surgeries) requiring physician (rather than nurse)
supervision. The user may
indicate the time period of the simulation in terms of days, weeks, months,
for example. The
25 system receives the user input, as shown in block 234, and uses the
predictive model to
simulate the scenario described by the user input in block 236 in order to
evaluate options
based on potential financial, operational, or clinical outcomes (as selected
by the user) as
demonstrated by the simulation. The system has access to current real-time
data about patient
status, healthcare staff availability, resource and supply availability, and
other information that
30 are modified or influenced by the user simulation input. The system may
identify and display
if, when, where, and how patient care would be compromised with the simulation
input, as
shown in block 238. The system may further identify recommended actions or
advanced
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
31
precautions that can be taken to address shortcomings identified in the
simulation, as shown in
block 240.
[0089] For example, in the case of simulating a large influx of new emergency
department patients, the system may identify one or more patients who are
currently occupying
beds in the emergency department who can be safely discharged or moved to
other
departments of the hospital without compromising their care and treatment.
These are patients
who have been determined by the predictive model to be at low risk for adverse
events (e.g.,
readmission) for example. In this manner, hospital administrators and
physicians may make
advanced informed decisions about staffing mix, adjusting resources and
supplies, and
inpatient care to achieve better efficiencies and outcomes.
[0090] A number of use cases are described below to further illustrate the
operations of
the holistic hospital patient care and management system and method 10.
[0091] Use Case 1 - Cardiology Surgery
[0092] Time-to-surgical repair is an important factor determining outcomes for
patients
identified to be at high-risk of having a ruptured abdominal aortic aneurysm
(AAA). As such,
there is great value and significance derived from a highly sensitive and
specific predictive
model capable of risk stratifying patients with potential AAA rupture due to
the typically
asymptomatic nature of this condition. Specifically, the U.S. Preventive
Services Task Force
(USPSTF) has issued a recommendation for men between the ages of 65 and 75
with a history
of smoking to be screened for AAA due to the common absence of symptoms for
this
condition, and the high potential for adverse outcomes if AAA rupture is left
untreated.
[0093] In this example, the patient is a 68 year-old male who arrives at the
emergency
department complaining of back pain and is found to have hypertension. The
predictive model
detects that this patient is at high risk of having a ruptured AAA, and
transmits an alert to the
physicians, appropriate clinical staff, and blood bank. The patient is rushed
to the CT scanner,
where the CT A/P confirms an AAA with partially contained internal bleeding.
The patient is
taken to the operating room. During surgery the patient's core temperature
drops. In response,
the system 10 automatically alerts the attending healthcare staff to deploy a
warming device to
raise the patient's body temperature, as well as adjust the operating room
temperature and
humidity settings.
[0094] The patient is placed on a ventilator after the surgery is completed.
Four hours
after leaving the operating room, while still on the ventilator, an alert is
fired based on the
patient's conjunctival pallor. The bedside nurse was wearing GOOGLE Glasses
equipped with
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
32
a video camera, which transmits the patient's image to the system 10. The
system's facial
recognition software and other algorithms identified that the patient was
likely becoming more
anemic based on change in conjunctival color. The nurse receives the alert,
and calls the
attending surgeon and the patient is rushed back to the operating room to
control the patient's
anostomotic hemorrhage. The patient then recovers from surgery and is
discharged from the
hospital.
[0095] Cardiology and surgical services are two areas of medicine that can be
aided by
innovative tools to risk stratify patients in real-time to notify the
healthcare providers that
individuals at high risk for developing a specific disease or condition, such
as AAA rupture.
These areas of medicine are highly susceptible to a wide range of adverse
outcomes, such as
readmissions and healthcare-associated infections (HAIs), two adverse clinical
outcomes
hospitals are eager to address and remedy. The system 10 can both accurately
identify patients
at high-risk of AAA rupture in real-time contributes to decreasing delays in
administering/activating evidence-based therapies/interventions aimed at
reducing the
likelihood of poor outcomes due to an unintended or undetected AAA rupture.
[0096] As a result of having a reliable warning tool using the predictive
model, patients
with risk factors for AAA rupture, for example, can be treated in a timely
manner by the
appropriate clinical treatment team to avoid serious and potentially life-
threatening adverse
clinical outcomes, thereby improving population health and costs (through the
avoidance of
unnecessary utilization costs). Further, accurate risk stratification enables
efficient
resource/staff allocation in order to ensure that patients requiring immediate
attention receive
prompt attention and care.
[0097] Use Case 2 - Emergency Department
[0098] Between 2003 and 2009, the mean wait time in U.S. emergency departments
increased 25%, from 46.5 minutes to 58.1 minutes. It has been noted that as
emergency
department wait time increases, patient satisfaction declines. A common
response (as a result
of patient dissatisfaction at longer than anticipated wait times) is the
patient leaving and going
to an alternate institution or location, either leaving before examination or
leaving immediately
after realizing the long wait time. Solutions for improving emergency
department wait time are
necessary to deliver timely care to ill patients, as well as improve staff and
resource allocation
in the emergency department.
[0099] In this example, a patient is a 64 year-old male who has had sudden
onset of
right-sided weakness. Upon observation of the weakness, the patient's wife
calls an
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
33
ambulance, and Emergency medical services (EMS) personnel arrive to provide
assistance.
The EMS paramedic examines the patient and determines that the patient likely
had a stroke.
The paramedic initiates a telemedicine consult with a neurologist who is
available at a hospital.
The neurologist is able to receive needed information from the paramedic about
the patient, ask
questions about the patient's condition, and observe the patient by viewing a
streaming video
of the patient. The neurologist then orders the administration of tissue
plasminogen activator
(TPA) based on the patient's current vitals and a thorough conversation with
the patient's
family regarding the risks and benefits of treatment. The patient is
immediately transported to
the hospital emergency department where the TPA is prepped and immediately
administered.
The patient is then transferred to the Neuro-ICU. In the Neuro-ICU, the
patient may be
monitored by the facial and biological recognition system that is able to
detect a mild change in
pupillary responsiveness signaling an early change in intra-cranial pressure.
This information is
immediately transmitted to the healthcare staff as an alert. The healthcare
staff responds by
taking immediate action to intubate the patient and administer treatment for
increased intra-
cranial pressure. Therefore, early and aggressive treatment aided by the
system 10 helps this
patient regain complete neurologic functioning.
[00100]
The ability to communicate remotely with a trained medical professional
capable of offering sound medical diagnosis and treatment advice in a timely
fashion may
significantly improve patient clinical outcomes, especially for conditions
like stroke, where
time-to-treatment significantly impacts outcomes. Further, as resources such
as clinical staff
face competing and high priority demands, the use of telemedicine services may
reduce the
number of required on-site clinical patient evaluations and assessments,
providing in-house
clinical staff with more time to allocate to those patients requiring in-
person services.
Additionally, tools such as facial recognition capable of detecting biological
changes serves as
warning mechanisms allowing medical professionals to act proactively to
prevent adverse
events and poor outcomes.
[00101]
The emergency department is plagued by prominent issues such as
crowding, delays, and diversions which prevent the delivery of high quality
care. Telemedicine
services may alleviate these burdens by increasing access to care, while
potentially reducing
costs associated with that care. As a result of improved care through the use
of telemedicine
services, the patient experience is enhanced, which as an influencer of
reimbursement
(HCAHPS), will likely positively impact financial payment for the hospital.
[00102] Use Case 3 - Intensive Care Unit
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
34
[00103]
As a result of factors such as the inherently critical and complex nature
of patients who frequent the intensive care unit (ICU), as well the lack of
complete information
about these patients to help inform decision-making, time is a critical
component contributing
significantly to outcomes for patients in this specific setting of care.
Furthermore, due to issues
around ICU bed supply and utilization, time must be adequately factored into
the care plan of
every patient within this unit to ensure that the clinical status of ICU
patients do not
deteriorate, especially when recovery is possible. Therefore, efficient
management of the finite
human and non-human resources within the ICU is vital.
[00104]
In this example, a bus filled with senior citizens turns over on an
interstate highway. Emergency medical services (EMS) dispatch multiple
ambulances to the
scene to bring approximately 30 patients to the emergency department. Upon EMS
dispatch, a
single order is triggered that is transmitted throughout the hospital to
personnel in the
emergency department, operating room, ICU, and on hospital floors. As a result
of the order,
the following actions are carried out in each of the wards: the emergency
department stops
taking new patients, and clears all trauma bay for the accident victims; a
patient waiting for an
elective surgery in the hospital operating room has his case delayed; three
patients who are
flagged for discharge from the ICU are immediately given hospital beds and
moved out of the
ICU; and 10 patients, waiting to be discharged, are expediently given
discharge orders. The
system 10 automatically pages or notifies the on-call nursing staff Current
nurse workload is
calculated and new nursing assignments are immediately generated to properly
handle the
likely surge of new patients as a result of the bus crash. Additionally, the
blood bank is
automatically notified to send '0 Negative' blood to the emergency department
in anticipation
of needed blood transfusions.
[00105] The unique nature of the ICU mandates solutions that assist an already
short-
staffed unit to better manage competing demands. The automated healthcare
staff monitoring
system which accurately communicates existing nurse availability and workload
and produces
new assignments based on expected patient inflow will promote better staff and
resource
planning and patient outcomes. Specifically, an accurate monitoring system
will support the
optimal clinical team necessary to achieve desired patient outcomes through
improvements in
communication and expedited intervention activation/therapy administration.
[00106] Because nurses' patient-related care, treatment, and management
decisions
directly impact patient quality of care, outcomes, and experience, it is
imperative to employ an
efficient clinical staff management solution capable of overcoming existing
medical burdens.
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
Through an automated staff monitoring and patient acuity tracking solution,
better health
outcomes (through better-focused resource allocation and more timely
intervention activation
and therapy administration), improved overall patient experience (through
enhanced
understanding of patient acuity and improved communication), and cost
containment (through
5 better, more efficient utilization) may be realized.
[00107] Use Case 4 - Oncology
[00108] Cancer patients' care is impacted by extrinsic and intrinsic factors.
One recent
national concern around providing effective care for oncology patients is that
patient
preferences are not adequately communicated in a timely manner. Understanding
patient
10 preferences and improving communication are important to promote
opportunities for shared
decision-making that would lead to better patient care. In some disease areas
such as oncology,
patient preferences and feedback are extremely important due to the aggressive
nature of many
therapies and the adverse side effects associated with these treatments.
[00109] In this example, the patient has a scheduled elective mastectomy in 6
days.
15 Challenges associated with this procedure include lack of patient and
family understanding
about the procedure itself, as well as post-op best care practices aimed at
promoting the
individual recovery process.
[00110] Prior to hospital admission, the patient is able to log in and access
an app that
allows her to identify, for example, 1) preferred rounding time, 2) preferred
family notification
20 pathway, 3) privacy preferences for communication, and 4) online health
history. Upon arrival
at the hospital, the patient is checked in and biometric devices (e.g.,
fingerprint scanner, retina
scanner, etc.) may be used to confirm her identity. The patient is given a
bracelet with a RFID
tag that will allow her location to be tracked throughout the hospital.
[00111] The patient is admitted to the hospital for elective mastectomy for
breast
25 cancer. Upon arrival to her floor, the nurse welcomes her and reviews
her pre-populated
answers to the nursing assessment. The nurse confirms the answers. The patient
settles in
comfortably in her room and she is able to view a monitor in her room that has
been
programmed to display more detailed information about her diagnosis and
treatment plan. The
next morning, this patient is prepped for surgery and wheeled to the operating
room. Her
30 family waits is in the waiting room but is able to track the patient's
progress (e.g., anesthesia,
first incision, closing) from an app on their mobile devices. Only those
individuals that have
been explicitly given permission by the patient can access this information.
The patient's
family may also review frequently asked questions (FAQs) regarding her
recovery process on
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
36
the app. The surgery is successful, and the patient is returned to her
inpatient hospital room
after the effects of anesthesia are eliminated. The patient's vitals are
continually monitored.
The next morning, the patient's care team comes to the room at the rounding
time specified by
her in advance of surgery. The doctor checks her surgery wounds, monitors her
vitals, and talks
to her about the surgery, her condition, and her recovery. The doctor also
informs her that all of
the details of her individual care plan, and background on her diagnosis can
be viewed on the
in-room monitor for perusal at her leisure.
[00112] As the patient's care post-mastectomy progresses to her adjuvant
therapy for
breast cancer, educational materials tailored for her primary language, health
literacy level, and
treatment may be offered, during pre-visit check-in and treatment visits. Such
content may be
tailored to be more patient-focused in order to allow for more engaged care.
Potential topics
may discuss improving present symptom control and risks related to
chemotherapy or offer a
future context for the discussion of palliative care and end of life decision-
making as a relevant
concern, even at the outset of curative intent treatment.
[00113] Oncology is an area of medicine where incorporation of patient
preferences
can have a significant and positive impact on clinical outcomes. As a result
of the complexity
of decision-making throughout the oncology patient's care continuum due to the
1) existence
of multiple treatment options, 2) the lack of clinical evidence or
inapplicability of clinical
evidence (due to evidence related to very different populations), or 3)
presence of cultural
beliefs that may impact treatment decisions, innovative solutions should be
focused on
achieving a better patient experience through a coordinated approach including
both the patient
and his/her treatment team.
[00114] Specifically, This solution promotes improved methods of communication
and
increase opportunities for patient/family awareness and engagement. For
example, post-
discharge status remains an area where more active and up-to-date patient
monitoring
mechanisms are required. Through a mobile app that administers surveys,
patients can take a
more participatory role in the communication of their health status and
preferences to the
healthcare providers. This information can help providers develop and deploy
more
personalized care plans targeting specific patient-voiced needs without
patients having to
physically visit the hospital or clinic for care.
[00115] Moreover, this innovative solution is focused on promoting patient
education
around various areas of this disease area to better assist patients/families
understand the
benefits and consequences associated with sometimes extremely aggressive and
harsh therapies
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
37
in order to make the best decision for that particular patient. Specifically,
patient knowledge
around palliative care options is important because the institution of
palliative care
interventions in the early stages of cancer may allow oncologists (with proper
patient input and
feedback) to re-align their focus on simultaneously addressing treatment
concerns, as well as
prominent and widespread issues like poor quality of life, or adverse symptoms
or
psychological distress associated with chemotherapy, radiation therapy, or
other anti-cancer
treatments.
[00116] As patient and family engagement becomes prioritized throughout the
care
process, as demonstrated by the emphasis placed on patient/family feedback by
nationally
recognized quality-focused organizations, such as the National Committee for
Quality
Assurance (NCQA) through their Patient-Centered Medical Home (PCMH)
accreditation
criteria, the functionality described herein will be imperative to incorporate
patient/family
feedback to ensure satisfaction and positive patient experience around areas
of care such as
access, communication, coordination, and individual care/self-management
support.
[00117] Patient educational materials facilitated and presented by this
functionality
help to diminish the common issue of patient-physician information asymmetry.
Adequate
patient education is necessary to ensure patients understand, retain, and are
able to put into
practice the treatment plans physicians prescribe. Additionally, as quality of
life and patient
experience become equally prioritized in care plans, alongside more
conventional treatments,
(especially in areas such as oncology where palliative care consultations have
consistently
demonstrated statistically significant improvements in patients' symptom
control, which may
and oftentimes do lead to better short- and long-term outcomes for those
impacted by cancer) it
will be imperative that patient education focus on the benefits and costs of
both curative and
palliative therapies designed to both eliminate disease and reduce adverse
consequences of that
disease.
[00118] Use Case 5 - Predictive Model
[00119] Poorly coordinated transitions of care may contribute to adverse
outcomes and
added substantial avoidable costs to the U.S. health care system. For example,
poorly planned
care transitions have amounted to unplanned readmission costs to Medicare of
more than $17
billion per year. The reliable predictive model described herein is a very
useful tool to predict
patient utilization patterns based on where patients are going (i.e.,
emergency department,
urgent care clinic, specialty clinic, etc.), the frequency of use of specific
settings, and
utilization of services in each setting, as well as specific patient
complaints. Accurate patient
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
38
utilization patterns will help providers tailor the patient's clinical
assessment and care
coordination plan around relevant patient-specific factors that may likely
facilitate the efficient
utilization of certain health care services and drive down unnecessary costs.
[00120] In this example, a patient has a wide array of medical and social
issues. He is
homeless, requires regular dialysis treatments, and suffers from schizophrenia
and Crohn's
disease. The patient has frequented his local hospital emergency department
approximately 15
times over the last 2 months for dialysis treatments and complications related
to his Crohn's
disease. Additionally, he regularly visits a Dallas social service
organization for his meals,
shelter, and clothing. This organization also provides this patient with the
mental health
services he requires but is unable to afford. Upon arrival at the hospital for
his dialysis
treatment, the patient is given a bracelet equipped with RFID technology that
allows his
location to be tracked as he visits various settings of care, both clinical
and social in nature.
The staff explains the purpose of wearing the bracelet and seeks the patient's
consent for close
monitoring.
[00121] Over the course of the next month, data from the patient's RFID
bracelet are
provided to a predictive model that makes predictions of his future clinical
and social service
utilization based on 1) where he has been going (i.e., emergency department,
urgent care clinic,
specialty clinic, etc.), 2) the frequency of use of these specific settings,
3) utilization of
services in each setting, and 4) complaints. The prediction is electronically
communicated to a
physician at the hospital who administers the patient's dialysis treatments.
The physician may
modify the patient's care plan as a result of the data he has received, and
tailors future care
around the prominent areas observed by the patient's past utilization
patterns.
[00122] RFID technology provides useful information that allows the predictive
model
to forecast, with consistent and reliable accuracy, future clinical and social
service utilization.
This ability allows the care teams to improve care transition plans that focus
on actual patient
needs. Additionally, real-time visibility around patient utilization may
provide opportunities
for clinical organizations to interact with relevant social service
organizations in an effort to
improve long-term patient outcomes and health. For example, the indigent
comprises a large
proportion of the U.S. healthcare system's high-utilizer population, and
understanding the
social and clinical services these patients use enables providers to develop
patient-specific care
plans that have a high potential to both reduce adverse outcomes and improve
the quality of
life for this vulnerable population.
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
39
[00123] By focusing on coordinating care across a patient's care continuum,
providers
can develop care plans that better anticipate the patient's needs, and address
existing patient
concerns across a broad spectrum of issues, such as condition management,
quality of life and
functional status, and psychosocial needs. Further, an evidence-based care
plan can facilitate
shared-decision making, shared accountability, and the collaboration between
clinical and
social service organizations and the entire healthcare system at large to
improve the quality of
patient care and overall patient experience. It is estimated that effective
care coordination may
result in annual healthcare cost savings as high as 240 billion dollars.
[00124] The current system and method are operable to display, transmit, and
otherwise present the list of high risk patients to the intervention
coordination team, which may
include physicians, physician assistants, case managers, patient navigators,
nurses, social
workers, family members, and other personnel or individuals involved with the
patient's care.
The means of presentment may include e-mail, text messages, multimedia
messages, voice
messages, web pages, facsimile, audible or visual alerts, etc. delivered by a
number of suitable
electronic or portable computing devices. The intervention coordination team
may then
prioritize intervention for the highest risk patients and provide targeted
inpatient care and
treatment. The system and method may further automatically present a care plan
to include
recommended intervention and treatment options. Some intervention plans may
include
detailed inpatient clinical assessment as well as patient nutrition, pharmacy,
case manager, and
heart failure education consults starting early in the patient's hospital
stay. The intervention
coordination team may immediately conduct the ordered inpatient clinical and
social
interventions. Additionally, the plan may include clinical and social
outpatient interventions
and developing a post-discharge plan of care and support.
[00125] High-risk patients are also assigned a set of high-intensity
outpatient
interventions. Once a targeted patient is discharged, outpatient intervention
and care begin.
Such interventions may include a follow-up phone call within 48 hours from the
patient's case
manager, such as a nurse; doctors' appointment reminders and medication
updates; outpatient
case management for 30 days; a follow-up appointment in a clinic within 7 days
of discharge; a
subsequent cardiology appointment if needed; and a follow-up primary care
visit. Interventions
that have been found to be successful are based on well-known readmission
reduction
programs and strategies designed to significantly reduce 30-day readmissions
associated with
congestive heart failure.
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
[00126] The clinical predictive and monitoring system and method continue to
receive
clinical and non-clinical data regarding the patient identified as high risk
during the hospital
stay and after the patient's discharge from the hospital to further improve
the diagnosis and
modify or augment the treatment and intervention plan, if necessary.
5
[00127] After the patient is discharged from the hospital, the system and
method
continue to monitor patient intervention status according to the electronic
medical records, case
management systems, social services entities, and other data sources as
described above. The
system and method may also interact directly with caregivers, case managers,
and patients to
obtain additional information and to prompt action. For example, the system
and method may
10
notify a physician that one of his or her patients has returned to the
hospital, the physician can
then send a pre-formatted message to the system directing it to notify a
specific case
management team. In another example, the clinical predictive and monitoring
subsystem and
method 40 may recognize that a patient missed a doctor's appointment and
hasn't rescheduled.
The system may send the patient a text message reminding the patient to
reschedule the
15 appointment.
[00128] Use Case 6 - Situation Analysis Simulator
[00129] Clinical staffing shortages and resource limitations may have
contributed to
the suboptimal operating efficiency observed in many U.S. hospitals and
clinics. While
simulation models have been known to help clinics achieve improvements in
operating
20
efficiency by identifying the required changes necessary to improve patient
experience and
meet future, anticipated demand, conventional simulations may not be as
reliable due to the
incorporation of retrospective data that does not take into account real-time
information.
[00130] For example, Clinic X has experienced a dramatic increase in the
number of
visiting patients over the last week. This clinic normally accepts both walk-
in patients and
25
patients with appointments. Current patient flow is approximately 80%
appointments and 20%
walk-ins. Due to the increase in patients coming to the clinic (both those
with appointments
and those walking-in), the facility has faced issues such as excessive wait
times, inadequate
provider capacity to provide high-quality service to patients, and a greater
than expected
percentage of its patients frequenting the emergency room than other
comparable clinics due to
30
poor or improper care. Additionally, once patients are waiting in examination
rooms to be seen
by providers, insufficient equipment and supplies further extended patient
wait times.
[00131] The clinic administrator may run the situation analysis simulator to
understand, given real-time data, the best mix of staff, exam rooms, clinic
hours, equipment,
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
41
and the optimal service time required for patients to maximize operational
efficiency. Upon
completion of a full simulation using real-time clinic data, the simulation
function determines
that clinic hours should be modified from 8am ¨ 5pm, Monday ¨ Friday to 10am ¨
7pm,
Monday ¨ Friday and additional hours should be added from 10am ¨ 3pm on
Saturdays to
respond to higher patient demands and achieve optimal operating efficiency.
The simulation
function further determines that upward adjustment of the number of
examination rooms would
not substantially reduce the wait time, considering other variable simulation
parameters. The
simulation function further determines the optimal clinical staff mix and
makes a
recommendation of the number of physicians, nurse practitioners, registered
nurses, and
technicians during office hours. The recommendation may further recommend a
staggered
staffing schedule so that more staff are available during the peak hours. In
addition, the
simulation function may recommend adding specific types of equipment based on
existing and
anticipated demand to minimize wait times and move patients through the
examination rooms
to providers more quickly.
[00132] A further clinical illustration of the functionalities of the
situation analysis
simulator is instructional. Many patients' poor outcomes may be attributable
to hospital-
specific factors such as premature discharge, rather than the patient's
inability to properly
manage their condition following departure from the hospital. A "red bed day"
is a common
term used to refer to a hospital that is above capacity and signals the need
to free beds for
incoming patients who may be more critical in nature. As such, hospitals are
at risk of
discharging patients prematurely without a complete understanding of the
impact of their
decision on future patient outcomes. For some patients, early discharge may
not translate to
any adverse event, whereas for other patients, premature discharge may equate
to potentially
avoidable adverse outcomes, such as readmissions or other preventable
conditions.
[00133] In another example, an hospital is experiencing a "red bed day" where
the
hospital is at peak capacity. The clinical staff is alerted of this
unfavorable status and instructed
to prioritize existing patient discharges to free up beds for more critical
incoming patients. A
particular patient, 68 year-old black male, was admitted two days ago with a
diagnosis of
congestive heart failure (CHF). This patient is a recipient of Medicare,
smokes regularly, and
has stable familial support. Additionally, this patient has been previously
identified to have
hypertension and diabetes. Another patient is a 55 year-old white male who was
also admitted
two days ago with a diagnosis of acute myocardial infarction (AMI) and atrial
fibrillation.
Additionally, the second patient is identified as a recipient of Medicaid, has
a history of drug
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
42
abuse, and self-reports that he does not have a permanent address or stable
family support.
Because the need to discharge individuals to make room available for more
severe patients has
escalated in urgency due to the hospital's red bed status, the situation
analysis simulator is used
to analyze current data and generate recommendations. The situation analysis
simulator is
configured to provide real-time identification of patients who are at risk for
an adverse event
due to a specific clinical decision, such as premature discharge. Once the
appropriate
parameters are included in the simulator, the tool is capable of generating
and presenting a risk
score for both patients.
[00134] The situation analysis simulator identifies the first patient as
someone with a
low-risk for readmission. Therefore, the system identifies the first patient
for immediate
discharge. The first patient is thus discharged with appropriate discharge
instructions by the
case manager on shift, including information for a scheduled follow-up
appointment and phone
call. The situation analysis simulator further identifies the second patient
as high-risk for
readmission. Accordingly, despite the dire "red bed" status, the second
patient stays in the
hospital and continues to receive the on-site care he needs to improve his
condition.
[00135] The situation analysis simulator is a tool capable of simulating 'What-
If
scenarios by analyzing the impact of discharging individual patients during
high volume days
will facilitate effective discharge planning in order to reduce the likelihood
of future poor
patient clinical outcomes. The use of real-time data to run the simulations
provides reasonable
confidence in the application of simulated results to current and future
clinical planning (such
as around discharge prioritization). Furthermore, while other existing
solutions are capable of
running a simulation, the novel feature described herein is the ability to
simulate data over a
shorter, more recent period allowing the hospital to behave proactively to
prevent likely
adverse patient events rather than reacting to an adverse outcome that has
occurred, but that
could have been prevented.
[00136] As a result of identifying barriers to effective care through the use
of real-time
data incorporated into the situation analysis simulator tool, the hospital is
able to improve
population health and the overall patient experience by immediately
prioritizing more
vulnerable patients during periods of resource shortages. Specifically with
regard to discharge
planning, hospitals can, reliably and with greater confidence and speed,
deliver more focused
care for individuals at increased risk of adverse outcomes (such as a re-
hospitalization), as
identified by the Situation Analysis Simulator despite hospital-specific
factors, such as red bed
days.
CA 02945138 2016-10-06
WO 2015/157577
PCT/US2015/025207
43
[00137] The system and method as described herein are operable to harness,
simplify,
sort, and present patient information in real-time or near real-time, predict
and identify highest
risk patients, identify adverse events, coordinate and alert practitioners,
and monitor patient
outcomes across time and space. The present system improves healthcare
efficiency, assists
with resource allocation, and presents the crucial information that lead to
better patient
outcomes.
[00138] The features of the present invention which are believed to be novel
are set
forth below with particularity in the appended claims. However, modifications,
variations, and
changes to the exemplary embodiments described above will be apparent to those
skilled in the
art, and the system and method described herein thus encompasses such
modifications,
variations, and changes and are not limited to the specific embodiments
described herein.