Note: Descriptions are shown in the official language in which they were submitted.
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
Electromagnetic Therapy Device and Methods
BACKGROUND
The following description relates to an electromagnetic field radiator that
influences the metabolic characteristics of living systems. The techniques may
be used
to therapeutically promote healing of tissue and treat diseases.
Therapeutic value may be achieved by applying an electromagnetic field to
injured bodily tissue. Application of a high-frequency electromagnetic field
at a
sufficiently low field strength so as not to produce tissue heating may result
in a
beneficial effect on healing of the tissue.
In some cases effectiveness of the therapeutic effect of the electromagnetic
field
may be improved by extending the duration of application of the field. The
power
requirements of the applied field may be reduced and the effectiveness of the
treatment
increased by extending the treatment duration.
SUMMARY OF THE DISCLOSURE
The present application discloses various systems and techniques for applying
an
electromagnetic field to bodily tissue.
In on aspect, a system includes an electromagnetic stimulation module. The
electromagnetic stimulation module includes an electromagnetic field
generator, an
antenna coupled to the generator and arranged to radiate the electromagnetic
field, a
power source coupled to the generator, and an activator to initiate radiation
of the
electromagnetic field. The system also includes a negative pressure module.
The
negative pressure module includes a patch, a tubing coupled of the patch, and
a negative
pressure generator coupled to the tubing and arranged to induce a negative
pressure on
an underside of the patch.
Implementations of this aspect may include one or more of the following
features:
In some implementations, the electromagnetic field can have a carrier
frequency
of 27.1 MHz.
1
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
In some implementations, the electromagnetic field generator can include an
adjustment module for adjusting a property of the electromagnetic field. The
property
can be a pulse frequency. The adjustment module can be configured to adjust
the pulse
frequency of the electromagnetic field between 100 Hz and 50 kHz. The property
can be
a duty cycle. The adjustment module can be configured to adjust the duty cycle
between
1% and 50%.
In some implementations, the system can be configured to deliver less than 100
pW/cm2 of energy into a wound site.
In some implementations, the system can be configured to deliver between 100
pW/cm2 and 2 mW/cm2 of energy into a wound site.
In some implementations, the system can be configured to reduce pain at a
wound site.
In some implementations, the system can be configured to reduce inflammation
at a wound site.
In some implementations, the system can be configured to accelerate healing at
a
wound site.
In some implementations, the system can be configured to stimulate blood flow
at a wound site. The system can be configured to stimulate blood flow by
inducing a
stochastic resonance.
BRIEF DESCRIPTION OF DRAWINGS
FIG. 1 is an implementation of a therapeutic electromagnetic device depicting
an
arrangement of the components.
FIG. 2 is an implementation of a therapeutic electromagnetic patch depicting
components in layers.
FIG. 3 is a block diagram of an implementation of a therapeutic
electromagnetic
device.
FIGS. 4A-B illustrate a control waveform and resulting RF waveform.
FIGS. 5A-I illustrate alternative antenna configurations.
FIG. 6 depicts an alternative configuration of a therapeutic electromagnetic
device.
2
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
FIGS. 7A-D depict various applications of a therapeutic electromagnetic
device.
FIGS. 8A-B depict an implementation of an enhanced antenna.
FIG. 9 depicts anatomical locations for placement of a therapeutic device.
FIG. 10 shows an example therapeutic electromagnetic device used in
combination with a negative pressure therapy device.
FIG. 11 shows a hypothetical relationship between a pulse rate of a carrier
signal
and the repetition rate of afferent nerve fiber stimulation in a subject.
FIGS. 12A-B show another example therapeutic electromagnetic device.
Like reference symbols in the various drawings indicate like elements.
DETAILED DESCRIPTION
The systems and techniques described here relate to promoting therapeutic
healing of tissue, providing prophylaxis for, and treatment of disorders and
diseases
through the application of an electromagnetic field. The techniques include
providing a
self-contained miniaturized electromagnetic field generating device that may
be applied
to bodily tissue. In some implementations the techniques and systems include
devices
that are disposable and portable.
The generated electromagnetic field can induce alternating current in bodily
tissue. The alternating current may be subjected to non-linear electrical
characteristics
(for example, diode-like rectification) and so generate low frequency
electrical potentials
having a time dependence the same as the pulse modulation. The low frequency
electrical potentials may stimulate cellular communication by, for example,
altering the
frequency of cellular activation potentials. Cellular communication may
promote the
healing of inflammation and the reduction of edema.
These techniques also may provide a method of transmission and utilization of
the body's capacitance by affixing a transmitting element of the device to
conform and
fit closely over the bodily tissue, provide a small space and low weight
device for field
transport and emergency use. Patient compliance with a therapeutic regimen may
be
important to promote healing of bodily tissue. Patient compliance may be
improved by
providing a therapeutic device that is self-contained and portable.
Some or all of the components of a therapeutic electromagnetic energy delivery
device may be integrated into a control circuit chip to miniaturize the
device. The device
3
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
may be affixed to various parts of the body for prolonged electromagnetic
therapy.
Patient compliance to the therapeutic regimen may be improved by embedding or
concealing the device into a patch, bandage, pad, wrap, brace, cast, or other
injury
support device and affixed to the body or taped over the bodily tissue.
The effectiveness of electromagnetic therapy may be improved by extending the
treatment duration. Lower power electromagnetic radiation may be applied for a
longer
period of time than may be necessary for shorter periods of application. The
self-
contained unit disclosed may promote patient compliance with periods of
therapy that
may extend over weeks.
io FIG. 1
illustrates an implementation of a therapeutic electromagnetic device 26.
A control circuit chip 18 may provide the functionality for the therapeutic
electromagnetic device to operate. An implementation of a control chip 18 is
disclosed
in association with the description of FIG. 3 and includes a radio frequency
(RF)
generator. A power source 10 coupled directly or indirectly to the control
chip may be
used to power the therapeutic electromagnetic device. The power source may
include a
battery, photovoltaic cell or an electro-chemical cell. An activator 12 is
used to activate
the device. The activator may include a switch that is a single-use or
multiple use type
and may be momentary or alternate-action. Actuation of the activator may be
accomplished in various ways including by use of pressure, light or electronic
signal
either remotely or proximately. An antenna 16 is used to emit electromagnetic
radiation
and a deflector shield 14 may be used to deflect the electromagnetic radiation
to the
bodily tissue. In an implementation, the antenna 16 and/or deflector 14 may be
tuned for
electromagnetic energy in the frequency range of 27 0.5Mhz. The therapeutic
electromagnetic device also may include a tuning coil 20 which may be used to
match
the impedance of the antenna 16 to the RF signal generator within the control
circuit
chip 18. A circuit board 22 may be used to mount the elements of the device
and, in
some cases, provide coupling between the elements of the device. The circuit
board may
be comprised of a rigid or flexible material. The assembled device weighs less
than 12
grams.
In some implementations, an adhesive material 24 may be used for affixing the
therapeutic electromagnetic device to bodily tissue. Adhesive material 24
includes, for
example, pharmaceutical grade adhesives. The therapeutic electromagnetic
device may
be affixed using other single or multiple usage therapeutic delivery devices,
which
4
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
include a patch, a bandage, a pad, a brace, a strap, tape, adhesive and a
cast. In some
implementations, an indicator 28 can be used to provide indicia that the
therapeutic
electromagnetic device is active. The indicator 28 may include one or more of
the
following: a visual indicator such as a light emitting diode (LED), lamp or
electro-
luminescent display; an auditory indicator such as noise generator; or a
tactile indicator
such as a vibrator. In an implementation, the indicator may be coupled to an
electromagnetic field detector in the control circuit chip 18 and indicate the
presence or
lack of electromagnetic radiation from the device. In various implementations
the
indicator may be steady, intermittent or pulsed.
The therapeutic electromagnetic device may be enclosed or encapsulated in
encapsulants or other potting compounds to reduce the vulnerability of the
device to
foreign materials including moisture, fluids, fungus, static charges, dirt,
particulate
matter and dust. The encapsulants, including insulating resins such as
epoxies,
polyurethanes, and polyesters, may be cast into cavities containing the device
components, to insulate, protect, and hold the components in place. The
encapsulant also
may reduce the vulnerability of the device to environmental factors including
air, heat,
sunlight, ultraviolet light and spurious electromagnetic fields. In some
implementations,
a conformal coating may be applied to the device components and couplings to
reduce
the vulnerability of the device to moisture, fluids, fungus, static charges,
dirt, particulate
matter and dust.
FIG. 2 illustrates an exploded view of an implementation of the therapeutic
electromagnetic device having the components in a layered form. An activation
switch
206, a control circuit chip 208, a power source 210, a visual indicator 212
and a tuning
coil 204 may be mounted on a top layer and attached to a circuit board 202 to
provide
coupling between the components. A deflecting shield 218 may be layered under
the
circuit board 202. Or deflecting shield is a layer or coating of material,
having high
magnetic permeability, applied directly to circuit board 202. An antenna 214
to radiate
electromagnetic energy may be layered under deflecting shield 218 and coupled
to the
circuit board 202. The deflecting shield 218 may deflect some of the energy
radiated
from the antenna 214 away from components mounted on the circuit board and
toward
the bodily tissue. The shape of the antenna is not restricted and some common
shapes
are depicted in FIGS. 5A-I. The antenna may also comprise separate conductors
that do
not make electrical contact with each other. In some implementations, the
antenna may
5
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
have a thickness of less than 5 millimeters and diameter of less than 9
centimeters or in
other implementations, a length of less than 27 centimeters. The antenna may
be
incorporated into the circuit board 202.
The shape of the circuit board 202 and deflecting shield 218 may be altered to
adapt the therapeutic device to particular applications. The thickness of the
device is less
than 10 millimeters. In one implementation, an adhesive material 216 such as a
pharmaceutical adhesive may be mounted to the bottom layer under antenna 214
to
adhere the device to bodily tissue. Other therapeutic delivery devices
including a patch,
a bandage, a pad, a brace, a strap, tape, adhesive and a cast also may be
used. In some
implementations, the components may be selected and arranged for specific
applications.
Referring to FIG. 6, for example, the therapeutic device 600 may have a
generally
annular shape in a therapeutic application such as post-operative healing over
an eye or
breast. In this case, the annular shape defines a hole 602 through which a
patient may
see while the device is in place.
FIG. 3 is a block diagram of the circuitry of one implementation of a control
circuit chip 300 used in a therapeutic electromagnetic device. Optionally, a
tuning coil
302 may be included within the control circuit chip 300 or mounted separately.
The
components of the control circuit chip 300 may be integrated into one part or
may be
assembled from discrete components. The control circuit chip 300 includes an
electromagnetic field generator 304 comprised of an oscillator 306 and a
driver 308.
Logic circuitry 316 coupled to the generator 304 provides an enable signal 312
to the
generator 304. The logic circuitry also may provide an LED signal 318 to an
indicator
circuit 320, which, in turn, may be coupled to an indicator (not shown). Logic
circuitry
316 may include discrete components, a programmable logic device (PLD), a
microprocessor or other micro-controller unit (MCU). A power source 324 may be
used
to supply power to the electromagnetic therapy device. An activator 326
controls the
flow of power from the power source to a DC to DC converter 328. The activator
includes a switch that can provide for a one-time activation and then sustain
activation
for the duration of life of the power source. The DC to DC converter 328
provides
power to the control chip components including the logic circuitry 316, the
electromagnetic field generator 304 and an optional RF feedback circuit 314.
The RF
feedback circuit provides an RF radiation signal 330 to the logic circuitry
316. The logic
6
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
circuitry also may provide an LED signal 318 to an LED indicator circuit and a
lock
signal 322 to the activator 326.
The electromagnetic field generator 304 comprises an oscillator 306 to
generate
an electromagnetic field, a driver circuit 308 to receive the electromagnetic
field,
amplify the wave and to provide the amplified wave to the optional tuning coil
302. The
tuning coil 302 may be used to match the impedance of the driver 308 to an
antenna 310,
which is arranged to radiate the amplified electromagnetic energy. The
oscillator 306
may be arranged to produce electromagnetic waves, including sinusoidal waves,
at a
carrier frequency of 27 +/- 0.5 megahertz (MHz). In an implementation, the
io electromagnetic therapeutic device has an average available power of
less than
approximately 1 milliwatt and a peak available radiated power density of less
than 100
microwatts per square centimeter ( W/cm2) measured substantially at the
surface of the
tissue. The electrical efficiency of average available radiated power
generation also may
be greater than 20%. Average available power is the power that the device can
dissipate
into a resistive load. The average available power is distinguished from the
power of the
carrier within each pulse, which is termed the "peak" power. The peak
available radiated
power density is the maximum carrier wave power as if it was continuous and
not
pulsed, divided by the loop area of the antenna. A high voltage generator (not
shown)
may be included to increase the intensity of the radiated field. The high
voltage
generator may produce less than 30 VDC and may be synchronized to allow energy
transforming action between therapy pulses, so that therapy pulses are not
affected by
the energy transformation action. Energy transformation could comprise
connecting the
battery to an inductive coil for a brief duration, and then switching the coil
into a diode
or rectifier and capacitor. The capacitor accumulates charge at a higher
voltage than the
battery. When voltage on the capacitor reaches a predetermined value, the
capacitor may
be discharged into the frequency generator for producing a therapy pulse.
Alternatively,
a transformer connected to a rectifier and capacitor as a flyback transformer
may replace
the inductive coil.
The enable signal 312 may be used to initiate or curtail radiation of the
electromagnetic energy. The RF feedback circuit 314 is arranged to detect RF
radiation
from the antenna 310 and to provide RF radiation signal 330 to logic circuitry
316.
Based on the level of the RF radiation signal 330, the logic circuitry
provides the LED
signal 318 to enable/disable the LED indicator circuit 320, which drives the
indicator
7
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
(not shown) and provides an indication that the antenna is radiating
electromagnetic
energy. The logic circuitry 316, the LED indicator circuit 320 or the
indicator may be
arranged so that the indicator is either indicating continuously,
intermittently or
pulsating. The logic circuitry also may provide the enable signal 312 to
enable/disable
the electromagnetic field generator 304.
In an embodiment, the energy radiated by the antenna 310 may be pulsed. PEMF
may be used to provide electromagnetic field therapy over long periods of time
and
reduce heating of the bodily tissue. FIG. 4A illustrates that an enable signal
410 that
may be provided from the logic circuit 316 to enable the generation and
radiation of
io electromagnetic energy. In this example, the enable signal goes to a
logic level high
every millisecond. The enable pulse level is shown as a logic high but
alternatively may
be a logic low. In some implementations, the logic high level may be the power
source,
or regulated non-zero, voltage although other voltages are possible. The
illustrated duty
cycle is approximately 8% to 10%. In some implementations, the electromagnetic
therapeutic device may operate in the frequency range of 3-30 MHz and
application of
the electromagnetic energy may be pulsed to maximize the therapeutic effect of
the field.
Pulses of 100 microsecond (0) pulse duration at intervals of 1 millisecond
(mS) (a
pulse repetition rate of 1000 Hz) may be preferable. In order to reduce
heating of the
tissue, the electromagnetic field strength may be limited to less than 100
micro-Watts per
square centimeter ( Wcm-2) as measured proximate the surface of the tissue.
FIG. 4B
illustrates a resulting output 412 from the antenna. The electromagnetic field
414 is
radiated from the antenna only when the enable signal 410 is at a logic high.
Referring again to FIG. 3, the power source 324 may be direct current (DC) and
preferably less than approximately 10 VDC. The power source may be
rechargeable.
The rechargeable power source may be a battery of the lithium metal hydride or
lithium
ion or lithium polymer technology that may be recharged from an external
source,
including a sine wave field generator proximate the antenna 310 or separate
coil (not
shown) for the non-contacting induction of power from the external source into
the
therapeutic device. Current induced in the antenna or separate coil may be
rectified and
supplied as a reverse current to the rechargeable power source until the power
source
reaches a predetermined terminal voltage or case temperature.
The power source 324 is coupled to the activator 326. When the activator is
actuated, power is coupled to the DC to DC converter which may boost and
regulate the
8
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
power source voltage level. Regulated output voltage from the DC to DC
converter 328
is supplied to the logic circuitry 316, electromagnetic field generator 304
and RF
feedback circuit 314. A lock signal 322 may be provided by the logic circuitry
316 to
lock the activator in the "on" position when the activator is actuated at
least once.
Optionally, extra input signals 332 and extra output signals 334 may be
received
and/or provided by the logic circuitry 316 for additional functionality. For
example, an
output signal may be provided that provides indicia of the level of the
voltage level of
the power source 324. The output signal may activate a visual or auditory
alarm when
the power source requires replacement. An output signal may be provided that
provides
indicia of a state of the bodily tissue. The electrical permittivity and
conductivity of
tissue affects the frequency of the carrier wave in the device. The ratio of
conductivity
(6) to permittivity multiplied by angular frequency (co) determines the
polarity of the
frequency change. If (3 exceeds cog then the carrier frequency decreases. If
cog exceeds (3
then the carrier frequency increases. As conductivity is related to pH and
free ion
concentration, while permittivity is related to abundance of polar molecules
and cell
membrane charge, the bioelectrical state of the tissue may be assessed by
determining
the carrier frequency change from that at initial application of the device.
Optionally, the extra output signal 334 may provide control by enhancing the
electromagnetic field for directed movement of chemical or pharmaceutical
molecules in
tissue, such as silver ions, for infection control. The enhanced
electromagnetic field may
be non-uniform in such a way as to direct movement of polar molecules, a
method
known as dielectrophoresis. Alternatively, the enhanced electromagnetic field
may
induce an electric field, which directs the movement of ions, a method known
as
iontophoresis.
An input 332 may be provided to receive external signals, for example, that
alter
the electromagnetic pulse duration, duty-cycle or pulse repetition rate of the
electromagnetic field generated.
FIGS. 7A-D depict some applications of the therapeutic electromagnetic device.
FIG. 7A depicts a therapeutic electromagnetic device affixed to a knee of a
human leg
702. The device may be applied to aid in healing of, for example, a cracked
knee, a cut,
a sprain or strain. FIG. 7B depicts a therapeutic electromagnetic device 710
affixed to a
muscle of a human arm 712 to aid in the healing of, for example, a sprain, a
strain or a
cut. FIG. 7C depicts a therapeutic electromagnetic device 720 affixed to a
human
9
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
abdomen 722 where, for example, lipo-suction procedures were performed. FIG.
7D
depicts a human face 730 where a therapeutic electromagnetic device 732 is
affixed on a
left side of the face to aid in healing of an injury such as a tooth cavity.
FIGS. 8A-B depict an implementation of an enhanced antenna comprising wires
802 wound around an annular ring 804 mounted on a printed circuit board 810.
The ring
may be a ferrite or magnetic, electrically-insulating ring. The ring may be
arranged to
support a battery 806 around the periphery. The battery 806 may be held in
place by a
retaining clip 808 to retain the battery adjacent the printed circuit board
810. Conductors
812 on the printed circuit board may be arranged to function as a main antenna
for the
o therapeutic electromagnetic device and may be coupled to an
electromagnetic field
generator (not shown) as described above.
The annular turns of the wires 802 can convey current in phase and frequency
with the main antenna 812. The number of turns of wire 802 on the annular ring
are
arranged to provide a larger magnetic flux than that of the main antenna 812.
The
windings cause a magnetic flux to enter/exit the outer perimeter of the
annular ring. A
portion of the (alternating) flux impinges bodily tissue underneath the
therapeutic
electromagnetic device inducing additional alternating current concentric with
the main
antenna. The additional induced current may result in magnetic flux that could
otherwise be generated by a main antenna having a larger diameter. The
magnetic field
lines 814 from the main antenna conductors on the printed circuit board will
take the
path of least magnetic reluctance and pass around the underside of the printed
circuit
board. Only a weak magnetic field impinges the battery 806. The larger portion
of the
field may be restrained near the main antenna conductors. The effect is to
generate
increased magnetic field intensity farther in the bodily tissue. Thus, the
main antenna,
such as a simple loop antenna, with the enhanced antenna windings on the
annular ring
can present as an antenna with a larger effective diameter.
A simple loop antenna can produce a near field of electromagnetism, which can
be confined within a certain volume by the physical geometry of the antenna.
The
magnetic field on the axis of a circular loop antenna diminishes in proportion
to:
1
MagneticField ___________________________________
rz2\15
1+
a1
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
where z is the distance from the center of the loop and a is the radius of the
loop.
Beyond a distance Z, the current induced by the magnetic field in the bodily
tissue may
be ineffective to provide therapeutic value. The distance Z is measured at the
point
where the surface of the volume intersects the axis. A therapy volume wherein
the
electromagnetic field induced in the bodily tissue is adequate to have
therapeutic value
can be determined from the radius, and circularity, of the loop antenna and
the current
flowing in the antenna. Outside of this volume, therapy may be inadequate.
Inside this
volume, therapy may be effective and diminishing on approach to the surface of
the
therapy volume. In some embodiments, the device effects a penetration of
induced
1 o current into the bodily tissue such that a therapeutic response is
elicited at a depth of at
least 2 cm in the bodily tissue.
A larger effective diameter antenna can increase the magnitude of the induced
current and extend the depth of penetration of induced current. Hence, the
main antenna
with the enhanced antenna may result in current induction inside the bodily
tissue over a
larger area and to a greater depth than with the main antenna alone.
Method of Using Pulsed Electromagnetic Field (PEMF) Therapy in Certain
Diseases
Bone and Joint Disorders: The urine of patients with bone and joint disorders
typically shows elevated levels of hydroxyproline, hexosamine, creatinine, and
uronic
acid as a result of metabolic errors in connective tissues surrounding the
affected site.
Not only can these errors be corrected with PEMF therapy, but joint pain and
swelling
can be reduced and mobility of the joint increased. Another major advantage of
PEMF
therapy is that it significantly reduces the time required to heal fractured
bones. It has
also proven to be effective for osteomyelitis, osteoarthritis, rheumatoid
arthritis, cervical
spondylosis, and lower back pain (including that caused by disc displacement).
Diabetes Mellitus: Blood sugar levels may be slowly reduced to normal or near
normal with application of a pulsed electromagnetic field (PEMF). Although the
mechanism of action is not completely understood, the evidence obtained thus
far
indicates that the procedure not only increases the metabolism of glucose in
the tissues
but also increases the production of insulin and enhances insulin binding to
its specific
receptors. The therapy has also proven to be effective for gastritis, peptic
ulcer,
ulcerative colitis, irritable colon, and hemorrhoids.
11
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
Bronchial Asthma: Bronchiolar obstruction can be gradually reduced with PEMF
treatment, which liquifies the mucous and facilitates spontaneous clearance.
PEMF
therapy also has anti-inflammatory action, which helps to ensure that the
airways remain
free and functional. In patients who have undergone the treatment, Forced
Vital
Capacity, Forced Expiratory Volume, and Peak Expiratory Flow Rates have
increased
and wheezing and dyspnea have significantly improved. The treatment is also
effective
for the common cold, tonsillitis, sinusitis, chronic bronchitis,
bronchiectasis
Cardiovascular Diseases: PEMF therapy is useful in the prevention of heart
attacks in hypertensive patients. Treatment helps to lower blood cholesterol
levels and
increase the circulation of blood by centrally mediating vascular dilatation.
This is
particularly important in preventing platelet aggregation and maintaining
adequate
oxygenation and nutrition of cardiovascular and other tissues. PEMF therapy
also
effectively disintegrates atherosclerotic plaques. An additional advantage of
the
procedure is that it blocks the production of free radicals, which play a
major role in
cardiovascular damage at the cellular level. Other vascular conditions for
which PEMF
may be effective are phlebitis, endarteritis, and varicose vein.
Brain and Mind Disorders: Directed through the skull at different points, the
PEMF can, by inductive coupling, produce an electric current in specific areas
of the
brain. It may thus be possible to enhance higher brain functions such as
learning,
memory, and creative thinking by selective stimulation of certain cells. PEMF
may have
broad application as the modality of choice for psychological disorders such
as
depression, aggression, anxiety, and stress as well as for Parkinson's
disease, epilepsy,
migraine, stroke, Alzheimer's and other degenerative brain disorders. In
addition,
cerebral palsy, mental retardation, hyperactivity, learning disabilities may
be improved
by PEMF stimulation of the central nervous system.
PEMF therapy can increase the efficiency of brain cells in synthesizing the
neuro-chemicals required for the transmission of impulses or commands at the
synaptic
level and by improving the electrical activity of these cells. The brain is a
neuro-
chemical complex. The efficiency of the brain or intellectual capacity of the
brain
12
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
depends upon the efficient performance of the brain cells and production of
the
chemicals that are called neurotransmitters.
Too much dopamine can result in hyperactivity, while too little can result in
uncoordinated movements of the limbs (Parkinsonism). Less acetylcholine, a
neuro-
chemical, in the brain is a reason for dementia especially of the Alzheimer's
type. If the
brain cells are stimulated repeatedly, after showing inhibition, they rebound
and become
more active than prior to stimulation. Since PEMF has the ability to stabilize
the genes
and prevent the activity of oxygen free radicals formed in the cells, it helps
to retard the
aging process.
Genitourinary Conditions: PEMF has been successfully used to treat
genitourinary conditions such as menstrual irregularity, sterility,
endometritis, and
endometriosis in women and orchitis, prostatitis, and oligospermia in men.
Preoperative and Prophylactic Therapy: PEMF therapy over the epigastrium can
provide increased blood profusion to the body's extremities to reduce the
inflammatory
response to injury. Preoperative treatment of the surgical site has also been
shown to
accelerate healing.
Post-Operative Recovery: PEMF or TENS over 1.5 inches above the wrist line
may reduce or ease the nausea for post-surgical recovery, motion sickness or
other forms
of nausea symptoms such as vomiting.
Non-Contacting Induction of Electrical Current in Tissue
Devices described herein can induce current at a high frequency. The amount of
current induced by a device is partly proportional to the frequency.
Modulating a carrier
waveform, such as the pulse modulation of 27 +/ 0.5Mhz (e.g., 27.1 MHz) in
devices
described herein, allows a larger current to be produced in a tissue than the
pulse
modulation waveform alone. The pulse modulation is selected for time and
amplitude
characteristics appropriate to biological systems. The carrier wave ensures
that induced
current has a magnitude that is maintained coherently within the pulse
modulation. A
varying pulse modulation is sustained by a similar magnitude of induced
current.
Rectification occurring in biological systems, such as across cellular
membranes, causes
13
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
the originating pulse modulation waveform to appear as a low frequency
voltage.
Membrane capacitance allows induced currents to enter cells much more easily
than the
pulse modulation waveform would by itself Shunting of current around cells
rather than
through the cells is also reduced.
No conductive contact of the device with the tissue is required to induce the
electrical current in the tissue. The size of the antenna of the device, being
much smaller
than a wavelength, ensures that the emission is localized to the treatment
area.
Accordingly, there is generally little far-field emission that might interfere
with, for
example, domestic appliances.
o The devices described herein generally induce current at a much higher
frequency than tissue-stimulating devices such as, for example, inductive bone-
healing
stimulators that pulse coils to produce a magnetic field or capacitive
stimulators that
produce a pulsed electric field.
Positioning of Therapeutic Devices
Therapeutic devices such as a PEMF apparatus, a transcutaneous electrical
neural
stimulator (TENS), or a static magnet array can be positioned at particular
points on the
body to achieve an enhanced medical therapeutic effect, e.g., accelerate
healing, reduce
pain, swelling and bruising. TENS operates by causing an electric current to
be passed
between electrodes placed on the skin over, for example, a painful area.
Devices are
described herein that can induce electrical current in a bodily tissue without
the use of
electrodes that are applied to the skin.
A therapeutic device can be positioned and operated at a specific acupuncture
point, including but not limited to the following: the external end of the
elbow
transverse crease; the depression at the lower border of the malleolus
lateralis; below
(e.g., about 1 inch below) the lateral extremity of the clavicle at the level
of the first
intercostals space; between the fourth lumbar vertebra and the fifth lumbar
vertebra; 1
inch to the right or left (horizontally) of the position between the fourth
lumbar vertebra
and the fifth lumbar vertebra; a depression anterior or inferior to the head
of the fibula;
about 1.5 inches above the medial border of the patella; between the radius
and the
palmaris longus; or at a position of pain (e.g., where the pain sensation is
the strongest in
an individual). FIG. 9 depicts specific anatomical locations where a
therapeutic device
14
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
described herein can be placed on an individual as part of a treatment program
(e.g., a
treatment for the reduction or elimination of pain).
The therapeutic devices described herein can be used in combination with
specific acupuncture positioning techniques to reduce or eliminate pain.
Examples of
pain-related disorders include, for example, pain response elicited during
tissue injury
(e.g., inflammation, infection, and ischemia), pain associated with
musculoskeletal
disorders (e.g., joint pain such as that associated with arthritis, toothache,
and
headaches), pain associated with surgery, pain related to irritable bowel
syndrome, and
chest pain.
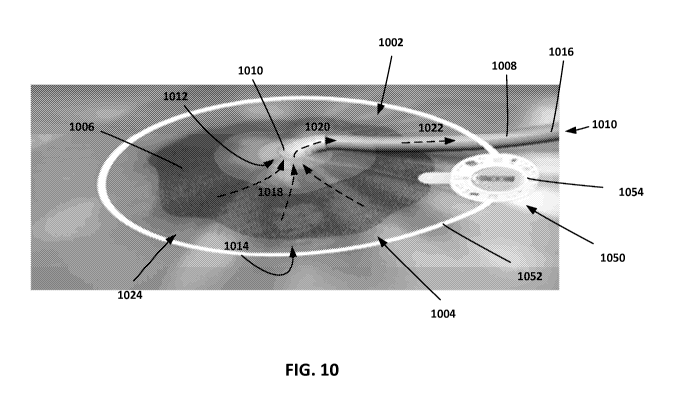
In some cases, implementations of the therapeutic devices described above can
be used in combination with negative pressure therapy. An example
implementation is
shown in FIG. 10. In this example, a negative pressure therapy system 1002 is
positioned over a wound site 1004. Negative pressure therapy system 1002
includes a
patch 1006 and a tubing 1008 coupled via a connecting element 1010. Connecting
element 1010 provides an air-tight connection between patch 1006 and tubing
1008,
such that an air-tight channel 1010 is defined through the center of tubing
1008, through
an aperture 1012 of patch 1006, and through to the underside 1014 of patch
1006. When
a negative pressure (e.g., a vacuum or suction force) is applied to the end
1016 of tubing
1008, air is drawn from the underside 1014 of patch 1006 (indicated by dashed
arrows
1018), through the aperture 1012 (indicated by dashed arrow 1020), through
tubing 1008
(indicated by dashed arrow 1022), and out the end 1016 of tubing 1008.
In an example usage, negative pressure therapy system 1002 is positioned over
a
wound site 1004, such that the patch 1006 fully or partially covers the wound
site 1004.
After the periphery of patch 1006 is securely fastened to the patient's skin
1024 (e.g.,
using an adhesive material such as an adhesive tape, liquid, or gel), negative
pressure is
applied to the end 1016 of tubing 1008, causing air to be drawn from the
underside 1014
of patch 1006, and creating a suction force on the wound site 1004.
Negative pressure can be applied to the end 1016 of tubing 1008 in a variety
of
ways. For example, in some implementations, negative pressure can be applied
through
an air pump (e.g., an electronic and/or mechanical pump that draws air from
tubing
1008), a syringe (e.g., an automated or manually operated syringe that draws
air from
tubing 1008), or any other device capable of exerting a vacuum or suction
force of
tubing 1008. A range of negative pressure can be applied to tubing 1008. For
example,
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
in some implementations, a pressure of approximately -75mmHg to -125mmHG can
be
applied to tubing 1008, such that a similar pressure is applied to the wound
site 1004.
Tubing 1010, patch 1006, and connecting element 1010 can each be made of
similar or different materials. In some implementations, tubing 1010, patch
1006, and
connecting element 1010 are made of materials that are substantially air-
impermeable,
such that air can only enter and exit channel 1010 from the ends of the
channel. As an
example, tubing 1010, patch 1006, and connecting element 1010 can be made of a
synthetic or natural plastic, rubber, or other suitable substance. Tubing
1010, patch
1006, and connecting element 1010 can be secured together in various ways, for
example using an adhesive substance (e.g., an adhesive tape, liquid, or gel),
through
frictional fitting between each of the components, or using other securing
components
(e.g., brackets, clamps, clips, braces, and pins).
Negative pressure therapy system 1002 can be combined with one or more of the
therapeutic electromagnetic devices described above. As shown in FIG. 10, an
example
therapeutic electromagnetic device 1050 can be placed in the vicinity of the
wound site
1004 (e.g., around the wound site 1004 and along the periphery of patch 1006),
such that
electromagnetic radiation is directed into the wound site 1004. Therapeutic
electromagnetic device 1050 can be similar to one or more of the
electromagnetic
devices described above (e.g., device 300 shown in FIG. 3). In this example,
therapeutic
electromagnetic device 1050 includes an antenna 1052 that extends around the
periphery
of patch 1006 and encompasses the wound site 1004. Antenna 1052 is coupled to
a
control module 1054, which houses the other components of the therapeutic
electromagnetic device 1050 (e.g., one or more of the components shown in FIG.
3).
During use, in a similar manner as described above, therapeutic
electromagnetic device
1050 emits electromagnetic radiation into the wound site 1004, increasing
blood
circulation in the region.
This combination of negative pressure and increased blood flow can provide a
variety of benefits. For example, to heal, wounds ideally need to be
maintained in a
moist condition, ideally need to have a robust blood supply to the region, and
ideally
need to be kept warm (i.e., as close to normal body temperature as possible,
for example
37 C). By applying a negative pressure to the wound site 1004 (e.g., by using
negative
pressure therapy system 1002), fluid extravasation from the blood supply in
the vicinity
of the wound site 1004 is enhanced. Due to this increased influx of fluid, the
wound is
16
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
kept moist. Further, by applying electromagnetic radiation to the wound site
1004 (e.g.,
by using therapeutic electromagnetic device 1050), the region is provided with
an
increased supply of blood, which increases oxygen and nutrient delivery to the
wound
site. Further, as blood flow is a major mechanism by which heat is delivered
to the
periphery, enhanced blood flow will result in a warming of the wound region.
Thus, by
combining negative pressure therapy with enhanced blood flow, a synergistic
effect is
obtained which significantly increases the rate of wound healing well beyond
the effect
of either therapy alone, or the expected sum of the effect of the two
individual therapies.
Further, this synergistic effect may be particularly beneficial in certain
circumstances. For example, in chronic (i.e., non-healing) wounds that occur
in the
extremities, maintaining adequate blood flow and warmth at the wound site may
be a
challenge for a healthcare provider. This concern may be compounded if the
patient is
elderly, or otherwise has relatively poor circulation. The negative pressure
therapy
system 1002 and the therapeutic electromagnetic device 1050 can be used in
conjunction
to provide more effective therapy.
In some implementations, negative pressure therapy system 1002 can be used to
remove excess fluid from the wound site 1004. As an example, if the wound site
1004
contains an excess of fluid, the negative pressure provided by negative
pressure therapy
system 1002 may cause a portion of this fluid to be drawn out from the wound
site 1004
and removed through tubing 1008. As above, therapeutic electromagnetic device
1050
also can be used to increase blood circulation to the region. When used in
combination,
these two systems can improve the speed of healing of certain types of wounds
(e.g.,
bedsores) by simultaneously reducing the swelling and pain of the wound.
Hence, by combining the above blood flow enhancement/wound healing short
wave therapy with negative pressure therapy, the wound bed is provided with
sufficient
blood flow (e.g., to provide oxygen and nutrient delivery), is kept moist, and
is
maintained at a warm temperature. The combination of these factors can
potentially
improve the rate of wound healing beyond the rate achieved if only one or two
of these
three conditions are attained in the wound region.
Various therapeutic modalities can be used to treat pain and edema (i.e.,
swelling) of injured tissue. For instance, therapeutic electromagnetic device
1050 can
provide short wave therapy (SWT) to a wound region. In one example
implementation,
therapeutic electromagnetic device 1050 is a self-contained, portable, battery
operated
17
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
therapeutic device that operates at approximately 27 MHz, produces pulses at 1
kHz, has
an 8-10% duty cycle, produces a peak power of less than 1 mW, and produces an
incident radiant power of less than 100 microwatts/cm2. In another example
implementation, therapeutic electromagnetic device 1050 operates at
approximately 27
MHz, produces pulses at 9 kHz, has a 50% duty cycle, produces a peak power of
less
than 1 mW. In some cases, one or both of these parameters are sufficient to
reduce
edema under certain circumstances, suggesting that the therapeutic
electromagnetic
device 1050 is enhancing interstitial fluid (e.g., lymph) return from the
region, resulting
in reduced pain.
While example parameters are provided above, these are only examples. Other
parameters can be used to provide different effects, for example to provide
enhanced
blood flow into a region. Particular parameters can be selected based on
experimentation.
As an example, in some implementations, therapeutic electromagnetic device
1050 operates at 27.1 MHz (+/- 0.5 MHz), produces pulses at a rate of between
approximately 100 Hz ¨ 50kHz (e.g., 100 Hz, 500 Hz, 1 kHz, 2, kHz, 3 kHz, 4
kHz,
5kHz, 6kHz, 7 kHz, 8 kHz, 9kHz, 10 kHz, 11 kHz, 12 kHz, 13 kHz, 14 kHz, 15
kHz, 16
kHz, 17 kHz, 18 kHz, 19 kHz, 20 kHz, 22 kHz, 24 kHz, 26 kHz, 28 kHz, or 50
kHz), has
an 5% to 50% duty cycle (e.g., 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, or
50% duty cycle), produces a peak power of between approximately 100 p.W/cm2 to
5
mW/cm2 (e.g., about 250 pW/cm2, about 500 pW/cm2, about 750 pW/cm2, about 1
mW/cm2, about 2 mW/cm2, about 3 mW/cm2, or about 4 mW/cm2), has a treatment
area
(e.g., antenna area) of between approximately 50 cm2 to 200 cm2, and delivers
a total
power of between approximately 5 mW to 1000 mW (e.g., 10 mW, 50 mW, 100 mW,
200 mW, 300 mW, 400 mW, 500 mW, 600 mW, 700 mW, 800 mW, or 900 mW) to the
tissue (depending on treatment area). In this example, the effects on blood
flow are
detectable within five minutes of initiating of treatment. As above, while
example
parameters are provided, these are only examples. Other parameters can be used
to
provide similar or different effects.
In some cases, the therapeutic electromagnetic device can also operate
according
to different carrier frequencies. As an example, some therapeutic
electromagnetic
devices can operate according to a 1 MHz, 5 MHz, 10 MHz, 15 MHz, 20 MHz, 25,
MHz, 30 MHz, 35 MHz, 40 MHz, 45 MHz, 50 MHz, or any other carrier frequency.
18
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
In some cases, one or more of the parameters can be adjustable by a user or
operator in order to induce different patterns of electromagnetic fields
(e.g., magnetic
fields having different carrier frequencies, pulse frequencies, duty cycles,
and/or power).
This can be useful, for example, as it allows a patient or other user to move
the device
between different locations on the patient, and induce different patterns of
electromagnetic fields in each of the different treatment area of the
patient's body. As
different treatment areas of a patient's body can, in some cases, respond
differently to
different patterns of electromagnetic fields, this allows a patient or other
user to adjust
the induced electromagnetic field to achieve an optimal therapeutic response
in each
particular location. Likewise, this also can be useful, for example, as it
allows a user to
move the device between multiple patients, and induce different patterns of
electromagnetic fields in each of the different patients. As different
patients can, in
some cases, respond differently to different patterns of electromagnetic
fields, this
allows a user to adjust the induced electromagnetic field to achieve an
optimal
therapeutic response in each particular patient.
In some cases, the pulse frequency can be adjustable between 100 Hz and 50
kHz, the duty cycle can be adjustable between 1% and 99%, and/or the peak
power can
be adjustable between 100 nW/cm2 to 5 mW/cm2. Other adjustment ranges are also
possible, depending on the implementation. For example, in some cases, the
pulse
frequency can be adjustable between 1 kHz and 30 kHz.
The SWT parameters can be adjustable by a user or operator in a variety of
ways.
For example, in some cases, the therapeutic electromagnetic device can include
an
adjustment module having one or more potentiometers that adjustably divide the
voltage
across one or more portions of the circuitry of the therapeutic
electromagnetic device.
As the potentiometer is adjusted, voltages across particular portions of the
circuitry are
correspondingly changed, resulting in a different electromagnetic energy
output. Thus,
the user or operator can adjust the one or more potentiometers until a
particular set of
SWT parameters is achieved (e.g., a particular carrier frequency, pulse
frequency, duty
cycle, and/or power). In some cases, the potentiometer can be access by the
user or
operator through a knob, a slider, a dial, a level, or some other suitable
input device. As
another example, in some cases, the therapeutic electromagnetic device can
include an
adjustment module having one or more microcontrollers that receive one or more
SWT
parameters (e.g., through user input from a key pad, dial, slider, or other
suitable input
19
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
device). In response, the microcontroller can regular the electric energy
applied to the
circuit (e.g., by applying a signal having a particular voltage, current,
frequency, pulse
rate, and so forth) in order to achieve the desired SWT parameters (e.g., a
particular
carrier frequency, pulse frequency, duty cycle, and/or power).
In some cases, parameters can be selected by stimulating a subject using SWT
and varying the SWT parameters (e.g., peak power, pulse rate, duty cycle,
carrier
frequency, feedback jitter frequencies, and other parameters) in order to
achieve a
desirable (or otherwise acceptable) degree of electrical nerve stimulation.
This nerve
stimulation can provide various benefits. For example, in some
implementations,
io inducing localized nerve stimulation at the wound site might stimulate
vibrations in the
tissue of the wound site (e.g., by inducing rapidly cycling periods of muscle
contraction
and muscle relaxation). This vibration can enhance blood flow and circulation
to the
wound site, and as a result, can further improve the rate of healing. In some
implementations, the SWT parameters can be tuned in order to induce a desired
degree
of electrical nerve stimulation and tissue vibration.
As an example, FIG. 11 shows a hypothetical relationship between one SWT
parameter, the pulse rate of the carrier signal, and the repetition rate of
afferent nerve
fiber stimulation in a subject. In this hypothetical example, as pulse rate is
increased, the
repetition rate of afferent nerve fiber stimulation varies over a range of
values. For
example, as the pulse rate increases, the repetition rate may peak at a
particular pulse
rate. This particular pulse rate can be selected for use in SWT. Thus, a
maximal
repetition rate of afferent nerve fiber stimulation is not achieved simply by
maximizing
or minimizing a particular SWT parameter, but rather by "tuning" one or more
SWT
parameters within a particular range to obtain the desired result.
In practice, however, the repetition rate of afferent nerve fiber stimulation
need
not always be maximized. For example, in some cases, the SWT parameters can be
selected such that a particular repetition rate of afferent nerve fiber
stimulation or range
of repetition rates is achieved, or such that a localized maximum repetition
rate is
achieved (as opposed to an absolute maximum repetition rate). This repetition
rate or
range of repetition rates can also vary, depending on the implementation. In
some cases,
this repetition rate or range of repetition rates can vary between different
locations on a
patient or vary between different patients. In some cases, a suitable
repetition rate or
repetition range of rates can be determined experimentally.
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
Although FIG. 11 shows an example of how one SWT parameter can be varied in
order to select a suitable SWT parameter, this is merely an illustrative
example. In
practice, multiple SWT parameters can be similarly varied in order to find a
suitable set
of SWT parameters. In some implementations, SWT parameters can be selected
based
on factors other than the SWT parameters themselves. As an example, each
particular
set of SWT parameters might different based on the temperature of the
subject's tissue.
In some implementations, SWT parameters might be selected in order to enhance
nerve
stimulation by inducing a stochastic response. In a stochastic response, a
signal can be
boosted through the addition of noise (e.g., "white noise," or other noise
from a
io relatively wide spectrum of frequencies). The frequencies in the noise
corresponding to
the original signal's frequencies will resonate with each other, amplifying
the original
signal while not amplifying the rest of the white noise (i.e., inducing a
"stochastic
resonance"). Accordingly, in some implementations, SWT parameters can be
selected in
order to intentionally induce noise (e.g., thermal noise) at a wound site in
order to
amplify the nerve stimulation properties of the induced electromagnetic field.
The amount of noise can be "tuned" in order to provide the desired effect. As
an
example, SWT parameters might be selected to induce a particular amount of
energy
(e.g., up to 100 pW/cm2) into a wound site in order to induce a stochastic
response,
thereby increasing the amount of nerve stimulation induced by the therapeutic
electromagnetic device 1050. As another example, the pulse rate of the carrier
signal
can be increased (e.g., from 1 kHz to 2 kHz, 3 kHz, 4, kHz, 5kKhz, 6 kHz, 9
kHz, 10
kHz, 30 kHz, 50 kHz, and so forth) in order to induce a stochastic response.
As yet
another example, the duty cycle of the pulses can be increased (e.g., from 10%
to 50%,
60%, 70%, and so forth) in order to induce a stochastic response. In some
implementations, one or more parameters can be simultaneously "tuned." For
example,
in some implementations, for a 27.1 MHz carrier signal, the energy level can
be
increased (e.g., from 100 pW/cm2 to 200 pW/cm2, 300 pW/cm2, 400 pW/cm2, 2
mW/cm2 and so forth), the duty cycle can be increased (e.g., from 10% to 70%),
and the
pulse rate can be increased (e.g., from 1 kHz to 10 kHz) in order to induce a
stochastic
response. Other parameters are also possible, depending on the implememtation.
In some cases, the therapeutic electromagnetic device might output
electromagnetic energy having a particular jitter (e.g., a deviation from true
periodicity).
For example, in some cases, counter-electromotive force (commonly known as
"back
21
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
EMF") can be act against the current induced by the therapeutic
electromagnetic device,
resulting in an increase in current draw from device's power source. In some
cases, this
current increase can affect the local oscillator and/or the drive circuit of
the device, and
introduce a jitter.
In some cases, this jitter can introduce additional spectral components of
electromagnetic energy into the subject's tissue (e.g., additional harmonic
frequencies
other than those specified). In some cases, this jitter can have beneficial
effects. For
example, the additional spectral components, in some cases, that increase the
white noise
phenomenon found in stochastic resonance effects. Further, in some cases, the
additional spectral components introduced by jitter may themselves have a
positive
therapeutic effect, either alone or in combination with the spectral
components
associated with otherwise truly periodic electromagnetic energy. Thus, in some
cases,
jitter can be selectively adjusted in order to obtain a desirable therapeutic
result (e.g.,
using a microprocessor feedback circuit that controls the degree of jitter in
the outputted
energy). In some cases, a suitable jitter or range of jitters can be
determined
experimentally.
While several example SWT parameters, tissue temperatures, and spectral
responses are described above, these are only examples. In some
implementations, SWT
parameters, tissue temperatures, and spectral responses can vary, depending on
the
application. Further, while in the above examples, parameters are selected
based on
certain criteria (e.g., to maximize blood flow or nerve stimulation),
parameters may be
selected based on other criteria. For example, parameters may be selected such
that the
therapy remains safe to a patient and is power efficient, while providing a
specified
degree of blood flow, nerve stimulation, and/or heating. Further, blood flow,
nerve
stimulation, and/or heating need not be maximized in order to provide
effective therapy.
As an example, in some implementations, an effective set of SWT parameters
might include a 27.1 MHz carrier signal pulsed at 10 kHz. As the magnitude of
the
carrier signal or pulse duration will have an effect on the heat delivered to
the patient
(and can potentially harm the patient if too much heat is delivered, or if
heat is delivered
too quickly), it might be desirable to use therapeutically effective SWT
parameters that
avoid a "saturation point," above which little or no additional healing
benefits can be
obtained. For example, in some implementations, while inducing a particular
amount of
heat over a relatively short period of time (e.g., 10 minutes) might provide a
desired
22
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
biological effect, in some implementations, it may be preferred to induce this
heat over a
longer period of time (e.g., 30 minutes, 4 hours, or 8 hours). Prolonging the
heating can
also potentially reduce the amount of undesired heat generated by the device
itself (e.g.,
heat generated by batteries or power supply due to high current draw), and can
potentially improve the power efficiency of the device.
Accordingly, suitable SWT parameters can vary, depending on the application.
Once suitable SWT parameters have been selected, they can be implemented in a
variety of ways. As an example, in a pulse rate of approximately 1-3 kHz and a
current
density of 4 p.A/cm2 are desired to induce 1 mV/cm into a subject's tissue,
this results in
io approximately 0.1 V/m. In order to induce this voltage, the magnetic
field required at 3
kHz is approximately 2 Gauss. For a 10 turn coil of 5 cm, this would require
approximately 30 mA, and 24 hour operation would require approximately 500 mAh
of
electric charge. This can be provided, for example, by two 250 mAh AA sized Li-
ion
(3.2V) batteries. This example implementation is provided merely as an
example. Other
implementations are possible, depending on each particular application.
As another example, as an increase in temperature can increase the potential
response, a therapeutic electromagnetic device can be used to warm a subject's
tissue
through RF diathermy. For example, an example therapeutic electromagnetic
device
might operate at approximately 0.3V/cm at a 10% duty cycle in order to produce
approximately 24 [tJ/s/cm3 rms. In this sufficient to produce 15x10-6 C/S, or
0.05 C of
heating per hour in skin tissue in the deep tissue. In another example, the
therapeutic
electromagnetic device might instead operate at approximately three times the
current
with a duty cycle of 100%, resulting in an output power of approximately 2
mJ/s/cm3, or
approximately 4.5 C of heating per hour (assuming not heat loss). As above,
these
example implementations are provided merely as examples. Other implementations
are
possible, depending on each particular application.
While an example therapeutic electromagnetic device 1050 is shown in FIG. 10,
this is merely one example. Other configurations are possible. For instance,
another
example therapeutic electromagnetic device 2000 is shown in FIGS. 12A-B,
showing the
top of the device (FIG. 12A, shown with an antenna 2002) and the bottom of the
device
(FIG. 12B, shown without antenna 2002). In addition to an antenna 2002,
therapeutic
electromagnetic device 2000 includes an enclosure 2004. Enclosure 2004 houses
batteries 2006a-b, a control module 2008, and radio frequency (RF) drive
circuits 2010.
23
CA 02945350 2016-10-07
WO 2015/157725
PCT/US2015/025466
During operation, control module 2008 (using a data processing apparatus, such
as a
computer processor or application-specific integrated circuit (ASIC)) controls
the
operation of RF drive circuits 2010 in order to induce an electromagnetic
field.
Specifically, RF drives circuits 2010 draw electrical power from batteries
2006a-b, and
applies an electrical current to antenna 2002 in order to induce an
electromagnetic field.
Control module 2008 can also control RF drive circuits 2010 such that the
desired
electromagnetic field is induced (e.g., by varying the electric current that
is applied to
antenna 2002). As shown in FIGS. 12A-B, therapeutic electromagnetic device
2000 also
includes a tab 2012, which allows the therapeutic electromagnetic device 2000
to be
affixed to the skin of a patient (e.g., using an adhesive substance applied to
the lower
surface 2014 of tab 2012). After use, therapeutic electromagnetic device 2010
can be
removed by peeling tab 2012 from the skin.
In some implementations, a single loop antenna is sufficient to achieve
enhancement of blood flow. However, in some implementations, antennas with a
multiple loop design may also be effective as long as the antenna is
sufficiently
compliant to conform to the shape of the body tissue. In some implementations,
two or
more antennas can be used simultaneously, or in succession. As an example, a
single
loop antenna might be used for RF diathermy, while a multi-loop antenna might
be used
for electromagnetic stimulation. These antennas can be driven by two or more
different
control units, or by the same control unit. Similarly, these antennas can be
included in a
single device (e.g., in a single shared housing), or in different devices
(e.g., in different
individual housings).
Other implementations are within the scope of the following claims.
24