Note: Descriptions are shown in the official language in which they were submitted.
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Title: imaging Abnormalities in Vascular Response
Field of the invention
The present invention relates to methods for imaging an abnormality of
vascular
reactivity, for example, cerebrovascular reactivity (CVR), broadly defined as
an
abnormality in a vascular response relative to a control population, as
evident
from high resolution imaging. =
Background of the Invention
The measurement of cerebrovascular reactivity (CVR), whereby a strong
vasoactive stimulus is applied to expose occult clinical limitations in
regional
cerebral blood flow (CBF) reactivity constitutes a cerebrovascular stress
test.
Quantitatively, CVR is defined as the change in CBF in response to a
measurable stimulus. A surrogate high resolution measure of changes in CBF
can be obtained by exploiting the Blood Oxygen Level Dependent (BOLD) effect
of magnetic resonance imaging (MRI); and a measurable increase in the end-
tidal (end-exhaled) partial pressure of CO2 (PETCO2) may be used as a
surrogate measure for the true independent stimulus, the partial pressure of
CO2
in arterial blood (PaCO2). CVR is can then optionally be defined as the per
cent
change in BOLD signal (arbitrary units) per mmHg change in PaCO2. CVR
values can be color coded and superimposed, on the corresponding voxel on an
anatomical scan to generate CVR maps. Of particular interest in the CVR maps
are the detection of areas of paradoxical reductions in flow following the
application of a vasodilatory stimulus, termed 'steal'. Steal has been shown
to
exist in deep white matter in healthy people [Mandell, 2008] as well as
associated
with pathology such as arteriovenous malformations [Fierstra, 2011],
vasculitis
[Han, 2008], steno-occlusive vascular disease [Han, 2011]; and associated with
disease in the form of cortical thinning [Fierstra, 2010], cognitive decline
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
[Balucani, 2012; Silvestrini, 2011), and enhanced risk of stroke [Silvestrini,
2000;
Markus, 2001].
Whereas the presence of steal is highly specific for identifying compromised
CVR,
the absence of steal does not necessarily imply normal CVR. For example, CVR
may be considerably reduced, but steal is absent if the stimulated demand
fails to
exceed its supply capacity. Alternatively, if the reduction of CVR is
widespread
and uniform, rather than localized, a differential in vasodilatory capacity
between
vascular territories may not exist and therefore, steal may not occur [Sobczyk
2014]. Steal may also not occur if compromised vessels maintain greater than
some threshold vasodilatory reserve. Under these conditions, the absolute
value
of CVR may be less than 'normal' but the extent of reduction cannot be
assessed
unless the normal range of CVR is known for each anatomical location.
The range of CVR in healthy subjects is large and varies from region to
region. Thus, even substantial reductions in CVR in one region will overlap
with
normal values in another resulting in difficulty in distinguishing reduced CVR
due
to pathophysiology from normally low CVR. Because the interpretation and
assessment of CVR maps currently relies on subjective assessments, it is
difficult
to identify reduced CVR short of that causing 'steal'.
Currently however, the interpretation and assessment of CVR maps relies
on a qualitative review of possible abnormalities, viewed as inhomogeneities
iri
the CVR maps that appear to differ from the CVR maps of healthy individuals.
Such qualitative comparisons require considerable experience for correct
interpretation; areas where blunted CVR is present may be misinterpreted as
healthy responses.
Summary of the Invention
We describe a method of assessing the severity and distribution of an
abnormality or reduction in a subject's vascular response to a vasoactive
stimulus in at least one region of interest (ROI) of the subject's brain.
2
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
The vasoactive stimulus is in the form of at least one change in a subject's
arterial partial pressure of carbon dioxide (each arterial partial termed a
PaCO2).
Measured PetCO2 values are used as a surrogate measure of the true stimulus.
The targeted PaCO2T(s) is maintained during the course of obtaining input of
MR
signals. Accordingly, the stimulus is standardized, allowing the severity and
distribution of abnormal or reduced vascular response values to be assessed by
using statistical scores such as z scores which reveal the severity and
distribution
of abnormal or reduced surrogate measures of blood flow as revealed by MRI.
According to one aspect, the invention is directed to method of assessing the
severity and distribution of an abnormality or reduction in a subject's
vascular
response to a vasoactive stimulus in at least one region of interest (ROI) of
the
subject's brain.
An MRI scanner and a selected MR imaging protocol are used to generate for
members of a group of control subjects, a set of vascular response signals
representing a non-pathological vascular response to at least one change in
the
subject's arterial partial pressure of carbon dioxide (each arterial partial
pressure
of carbon dioxide a PaGO2T) in at least one common ROI of each control
subject's brain.
It will= be appreciated that the control group need not represent a non-
pathological
response since any type of status/criterion/parameter can be controlled for
for the
purposes evaluating a test subject.
The vascular response is quantifiable, from a surrogate measure of blood flow,
on a voxel by voxel basis, with reference to the voxel coordinates, from MR
signals corresponding respectively to each PaCO21. in the form of a response
value per voxel.
3
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
The control subject's respective voxel coordinates are co-registered to a
standardized space based on a set of anatomic landmarks.
A measure of variability of the vascular response values are computed on a
voxel by voxel basis. The vascular response values measure at least one of the
amplitude of the vascular response and the time course of the vascular
response.
For example, a mean and standard deviation of the vascular response values for
voxels corresponding to the at least one ROI are computed to define, for the
control group as a whole, a set of statistical values respectively associated
with
individual voxels corresponding to the ROI (an atlas).
The MR scanner and the selected MR imaging protocol are used to obtain MR
signals per voxel corrresponding to the surrogate measure of blood flow for
each
PaCO2T for a test subject.
By scoring (e.g. as z values) the test subject's response values for
individual
voxels in the at least one ROI (each voxel co-registered to the standarized
space
based on the set of anatomic landmarks), relative to the respective computed
statistical values e.g. means and standard deviations per corresponding voxel,
the severity and distribution of the abnormal or reduced vascular response is
revealed.
The method may be implemented using a MR scanner and a stand alone CPU or
dedicated MR image processor.
The processor obtains input of the abnormal voxel" (pre-defined or user
defined
via a user interface) coordinates and scores.
The processor may employ program code to define a new to ROI.
The processor may employ progam code to compare the scores to a threshold
value.
4
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
The processor may employ progam code to compare the scores to scores
associated with a disease.
According to another aspect, the invention is directed to an imaging system
for
detecting an abnormality in a subject's response to a vasoactive stimulus in
at
least one region of interest (R01) of the subject's brain. The vascular
response
values may measure at least one of the amplitude and time course of the
vascular response.
In one embodiment, the imaging system comprises an MR scanner configurable,
using a pre-selected MR protocol, to capture spatially resolved MR signals
corresponding to the subject's vasoactive response to a standarized
cerebrovascular stimulus comprising at least one targeted change in the
subject's
arterial partial pressure of carbon dioxide (each arterial PCO2 a PaCO21).
Optionally, at least one PaCO2T is attained from an initial steady state PaCO2
value. Optionally, the at least one change in PaCO2 is at least one of a
series of
increments or decrements in the subject's arterial partial pressure of carbon
dioxide.
The imaging system also comprises a computer programmed to obtain input of
the MR signals and implement an algorithm for analyzing the MR signals with
reference to a pre-determined surrogate measure of blood flow in the at least
one
ROI, the pre-determined surrogate measure of blood flow optionally quantifying
at least one of the amplitude of the subject's vascular response and a time
constant of the subject's vascular response to the at least one PetCO21- (at
least
one change from a steady value or two targeted values) The algorithm includes
program code for processing the MR signals with reference to the selected
surrogate measure of blood flow for each PetCO2T including computing a
vasoactive response value per voxel, each voxel co-registered into a
standardized space, and scoring the subject's vascular response values for
respective individual voxels in the ROI, relative to statistical reference
values,
optionally using scores, for example z scores.
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
The imaging system optionally includes a user interface operable to initiate
the
aforesaid algorithm and optionally to map the scores back onto an anatomical
representation of the standardized space to generate a statistical map of the
subject's vascular response to a standardized cerebrovascular stimulus,
wherein
the probability that subject's vascular response to the standardized
cerebrovascular stimulus is pathological is depicted, on a voxel by voxel
basis,
on the statistical map (e.g. a z map) for example using a color scheme wherein
different colors are assigned to different scores such that each color pixel
is
mapped onto its anatomical 3 dimensional origin. The probability that the
vascular response is part of the normal range may be represented by a z score,
where high z scores represent lower probability that they are in the normal
range
and correpondingly higher probability of resulting from underlying pathology.
The reference values are a measure of the amount and variability of the
vasoactive response and optionally comprise a mean and standard deviation of
vascular response values per voxel for a corresponding ROI in a group of
control
subjects, the vascular response values generated using the pre-selected MR
protocol for each same PaCO21- and quantifying, on a voxel by voxel basis, the
statistical scores e.g. the mean and standard deviation of the selected
surrogate
measure of blood flow (amplitude or tau or both). The vascular response values
are generated from a set of MR signals correponding to the control subjects'
respective vascular responses per voxel, the respective voxel coordinates per
subject co-registered to a standardized space based on a set of anatomic
landmarks.
=
The MR scanner captures MR signals from the brain, as surrogates of brain
blood flow, wherein the change in signal corresponds to the subject's
vasoactive
response to the stimulus. The stimulus is standardized with respect to
strength
preferably via induction of at least two levels of arterial partial pressure
of carbon
dioxide (PaCO2), at least one of which is hypercapnic, or greater than the
6
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
baseline resting level of the subject, and the level of which can be
determined
directly by arterial blood sampling or noninvasively by its surrogate, the end
tidal,
or end exhaled partial pressure of carbon dioxide.
For example, where the MR signals quantify the subject's vasoactive response
to
each of a series of targeted increments in the subject's end tidal partial
pressure
of carbon dioxide, each a PetCO2T, the reference values include a statistical
summary of the control subjects' respective vascular response values to each
PetCO2T.
Optionally, the images represent a change in the blood oxygen level dependent
(BOLD) effect of a MR response to a targeted change in a subject's end tidal
PCO2 (PETCO2T)=
Optionally, the images depict a change in the blood flow as measured by
arterial
spin labeling MR response to a targeted change in a subject's end tidal PaCO2.
Optionally, the program code is operable on a dedicated image processor
connected to or forming part of the MR scanner hardware. Alternatively, the MR
signals are recorded in a file, optionally a file according to the DICOM
standard
and processed by a separate computer.
Optionally, the statistical scores are optionally further compared to
threshold
values per voxel associated with a particular disease, on a voxel by voxel
basis.
The statistical scores e.g. .z scores may be used to identify a new ROI, for
example a smaller ROI within an ROI of the subject's brain that was of
interest, a
priori, in virtue of the pathology being assessed or in virtue of a prior,
concurrent
or later assessment. Optionally, the algorithm includes program code for
identifying the new ROI.
7
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
In another aspect, the invention is directed to a computer program product
comprising program code/instructions for executing the above-described
algorithm, and optionally the reference values and/or program code for
accessing
the computer remotely to compare a subject's MR signals corresponding to the
selected surrogate measure of blood flow with reference values and same
targeted arterial partial pressures of carbon dioxide. Optionally, the
computer
program product comprises program code for producing a color coded statistical
map and/or program code for identifying a new ROI.
In another aspect, the invention is directed to a non-transitory computer
readable
medium comprising program code for executing the above-described algorithm,
and optionally the reference values and/or program code for accessing the
computer remotely to compare a subject's MR signals corresponding to the
selected surrogate measure of blood flow with reference values and same
targeted arterial partial pressures of carbon dioxide. Optionally, the
computer
program product comprises program code for producing a color coded statistical
map and/or program code for identifying a new ROI.
In one embodiment the reference scores are part of an atlas prepared for each
a
series of targeted increments in a subject's arterial partial pressure of
carbon
dioxide.
Thus, according to another aspect, the invention is directed to a method of
characterizing an abnormality in a subject's vascular response to a vasoactive
stimulus in at least one region of interest (ROI) of the subject's brain
comprising
the steps of:
a) using an MRI scanner and a selected MR imaging protocol to generate for
members of a group of control subjects, a set of vascular response signals
representing a non-pathological vascular response to at least one change in
the
subject's arterial partial pressure of carbon dioxide (each arterial partial
pressure
of carbon dioxide a PaCO2T) in at least one common ROI of each control
8
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
subject's brain, wherein the vascular response is quantifiable, from a
surrogate
measure of blood flow, on a voxel by voxel basis, with reference to the voxel
coordinates, from MR signals corresponding respectively to each PaCO21- in the
form of a response value per voxel;
b) co-registering the respective voxel coordinates in the at least one ROI for
each
control subject to a standardized space based on a set of anatomic landmarks;
c) computing, on a voxel by voxel basis, a mean and standard deviation of the
vascular response values for voxels corresponding to the at least one ROI to
define, for the control group as a whole, a set of statistical values
respectively
associated with individual voxels corresponding to the ROI (an atlas);
d) using the MR scanner and the selected MR imaging protocol to obtain MR
signals per voxel corresponding to the surrogate measure of blood flow for
each
PaCO2T for a test subject, by scoring the test subject's response values for
individual voxels in the at least one ROI (each voxel co-registered to the
standardized space based on the set of anatomic landmarks), relative to the
respective computed means and standard deviations per corresponding voxel, as
z values.
Optionally, the method further comprises the step of color-coding the z values
and mapping the color-coded values back onto an anatomical representation of
the standardized space to produce a z map. The invention is also directed to
such z maps and their use as a diagnostic tool.
Optionally, the co-registered MR images are full brain images defining a
substantially full set of potential ROls.
Optionally, the standardized cerebrovascular stimulus is a vasodilatory
stimulus.
Optionally, the vasodilatory stimulus is at least one targeted increase in the
subject's end tidal PCO2, optionally from a steady state PetCO2 or a
previously
targeted value.
9
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Optionally, the stimulus is a series of increment or decrements in a subject's
arterial partial pressure of carbon dioxide (a so-called ramp sequence).
Optionally, the reference values in an atlas are generated using a ramp
sequence.
As described below, statistical maps such as z maps can be used to interpret
interval differences and values for gain, phase and coherence emerging from a
transfer function analysis.
Optionally, the images represent a change in a blood oxygen level dependent
(BOLD) magnetic resonance imaging (MRI) response to a targeted increase in a
subject's end tidal PCO2 (PETCO2), the vascular response values representing,
for example, a change in BOLD MRI signal (A S), in response to a standardized
increase in the PETCO2 (CVR S / PETCO2).
Optionally, the set of control subjects are selected on the basis that they
report
being free of neurological disease.
Optionally, the control subjects are matched for a parameter that is
appropriate
for the condition being examined in a patient. The term patient is used
broadly to
define a subject being tested with reference a selected control population.
Optionally, the set of control subjects are matched for at least one of age
and
gender.
According to one embodiment, the invention is directed to a method of
assessing
the severity and distribution of an abnormality or reduction in a subject's
vascular
response to a vasoactive stimulus in at least one region of interest (ROI) of
the
subject's brain, comprising the steps of:
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
a) using an MRI scanner and a selected MR imaging protocol to generate for
members of a group of control subjects, a set of vascular response signals
representing a control (e.g. non-pathological) vascular response to at least
one
change in the subject's arterial partial pressure of carbon dioxide (each
arterial
partial pressure of carbon dioxide a PaCO2T) in at least one common ROI of
each
control subject's brain, wherein the vascular response is quantifiable, from a
surrogate measure of blood flow, on a voxel by voxel basis, with reference to
the
voxel coordinates, from MR signals corresponding respectively to each PaCO2T
in the form of a response value per voxel;
b) co-registering the control subject's respective voxel coordinates to a
standardized space based on a set of anatomic landmarks;
c) computing, for the set of control subjects, on a voxel by voxel basis, at
least
one statistical value describing the quantity and variability of vascular
response
values associated with corresponding voxels of the standardized space to
define
at least for the region of interest, at least one statistical value per voxel
in the ROI
for the control group as a whole (an atlas);
d) using the MRI device and the selected imaging protocol to obtain MR signals
per voxel corresponding to the surrogate measure of blood flow for each PaCO2T
for a test subject, by scoring the test subject's response values for
individual
voxels in the at least one ROI (each voxel co-registered to the standardized
space based on the set of anatomic landmarks), relative to the at least
statistical
value per voxel computed in step c), wherein the scoring yields a score per
voxel
describing the manner in which the patient's vascular response values rank in
comparison with the corresponding atlas values.
In one embodiment, the method excludes the MR scans (for one of or for both
the test and control subjects) and optionally also excludes preparation of the
reference value atlas from the MR signal data (D1COM), the method comprising,
for example, the steps required to compute z scores, namely: (a) obtaining
input
of the test subject's vascular response values per voxel; (b) obtaining input
of the
11
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
statistical values per voxel (the reference values); and (c) computing the z
values.
The reference values comprise statistical values, for example, a mean and
standard deviation of respective control subject's vascular response values
per
voxel for a corresponding ROI in each member of the group of control subjects,
the reference values and the test subject's vascular response values per voxel
derived from MR signals obtained from an MR scanner using a pre-selected MR
protocol and including respective voxel coordinates co-registered to a
standardized space based on a set of anatomic landmarks; the vascular
response values corresponding to and quantifying an individual subject's
(control
or test subject) vascular response to at least one change in the individual
subject's arterial partial pressure of carbon dioxide (each arterial partial
pressure
of carbon dioxide a PaCO2T) in at least one common RO1 of each individual
subject's brain, wherein the vascular response is quantified, from a surrogate
measure of blood flow, on a voxel by voxel basis, the MR signals quantifying
at
least one of the amplitude of the individual subject's vascular response and a
time constant of the individual subject's vascular response to the each PaCO21-
,
wherein the scores e.g. z scores, identify the severity and distribution of an
abnormality or reduction in the test subject's vascular response to the
vasoactive
stimulus.
After obtaining input of the test-subject's vascular response values
corresponding
to at least one region of interest (ROI) of the test subject's brain,
obtaining input
of reference values for each voxel in the ROI (an atlas) for a group of
control
subjects (e.g. by interrogating a database), and scoring the test subject's
vascular response values for respective individual voxels in the ROI, relative
to
the corresponding reference values per voxel using the scores, the scores and
voxel coordinates may be compared to a threshold value, for example to define
the extent and distribution of an abnormality.
12
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Optionally, the vascular response values are a measure of a delay in vascular
response to the at least one change in the subject's arterial partial pressure
of
carbon dioxide, wherein at least one statistical value is determined for each
respective voxel using a transfer function analysis. For example, a polynomial
function may be computed to match the MR signals constituting the vascular
response.
Optionally, the at least one statistical value is tau, a standardized transfer
function analysis for all subjects optionally employing a mono-exponential
dispersion function to generate an atlas of tau values.
Optionally, the atlas response values are rank ordered on a voxel by voxel
basis.
For example, the test subject response values per voxel are assigned a rank
score following the rank order to generate a rank score map.
Optionally, a log transformation of the respective (voxel by voxel) vascular
response values for the individual control subjects shows that the values are
generally normally distributed. The individual control subject vascular
response
values and patient vascular response values are transformed, on a voxel by
voxel basis, by taking the log of the values, and wherein a mean and SD of the
control subjects respective vascular responses log transformed values is
computed on a voxel by voxel basis to generate an atlas, and wherein the
patient's respective vascular responses log transformed values are
respectively
scored with a z value.
Optionally, the method further comprises the step of color coding the scores
and
mapping the color-coded scores back onto an anatomical representation of the
standardized space.
Optionally, the test subject and the control subjects are each scanned on one
occasion to obtain a set of response values per voxel (A) and then each re-
13
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
scanned at least once after an interval to obtain another set of response
values
per voxel (B). Optionally, a voxel mean and standard deviation with respect
the
quantum and variability of the respective differences (e.g. consistently A
image
values per voxel minus B image values per voxel, or consistently B image
values
minus A image values) between the test and re-test vascular response values
for
the control group of subjects, wherein the differences between the patient's
test
response and re-test response for respective voxels corresponding to the at
least
one ROI are scored, relative to the voxel means and standard deviations
(optionally the means and standard deviation per voxel of the respective
computed differences), using z values.
Thus, according to another aspect, the invention is directed to a method of
assessing the severity and distribution of an abnormality or reduction in a
subject's vascular response to a vasoactive stimulus, in at least one region
of
interest (ROI) of the subject's brain, comprising the steps of:
a) using an MRI scanner and a selected MR imaging protocol to generate for
members of a group of control subjects, a first set (A) of vascular response
signals representing a control (e.g. non-pathological) vascular response to at
least one change in the subject's arterial partial pressure of carbon dioxide
(each
arterial partial pressure of carbon dioxide a PaCO21-) in at least one common
ROI
of each control subject's brain, wherein the vascular response is
quantifiable,
from a surrogate measure of blood flow, on a voxel by voxel basis, with
reference
to the voxel coordinates, from MR signals corresponding respectively to each
PaCO2T in the form of a response value per voxel;
b) re-testing each control subject at least once after an interval at each
PaCO21-
using the MRI scanner and the selected MR imaging protocol to obtain at least
one second set (B) of the vascular response signals representing at least one
additional measurement of each control subject's (e.g.non-pathological)
vascular
response per voxel;
14
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
c) optionally, computing a value representing the difference between the
respective test vascular response values and the re-test vascular response
values for each individual control subject (consistently A image values minus
B
image values, or consistently B image value minus A image values), on a
subject
by subject and voxel by voxel basis, for voxels corresponding to the at least
one
ROI;
d) co-registering the control subject's respective voxel to a standardized
space
based on a set of anatomic landmarks;
e) computing for the control group as a whole, on a voxel by voxel basis, a
statistical value describing the quantum and variability of the test and re-
test
vascular response values, optionally a statistical value describing the
quantum
and variability of the computed differences between the test and re-test
vascular
response values for individual respective voxels corresponding to the at least
one
ROI and assigning those values to the standardized space (atlas);
f) using the MR scanner and the selected MR imaging protocol to measure a test
vascular response and at least one re-test vascular response obtained after an
interval, for a subject in need of an assessment of a vascular response (a
test
subject), at each PaCO2T , by scoring the difference between the test
subject's
test vascular response values and re-test vascular response values for
respective voxels corresponding to the at least one ROI against the control
group
variability in vascular response for the corresponding voxels.
In one embodiment, the method excludes the scans (test and control subjects)
and optionally also excludes preparation of the reference value atlas, the
method
comprising the steps required to compute z scores, namely: (a) obtaining input
of
the A and B values per voxel (or at least the differences per voxel) for the
test
subject; (b) obtaining input of means and standard deviations per voxel of the
differences between the A and B scores for the control group (the reference
values); and computing the z values.
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Optionally, the method further comprises the step of color-coding the z values
and mapping the color-coded values back onto an anatomical representation of
the standardized space to produce a z map.
Optionally, the co-registered voxel coordinates represent full brain images
defining a substantially full set of potential ROls.
Optionally, the standardized cerebrovascular stimulus is a vasodilatory
stimulus.
A vasoactive stimulus can optionally be a vasoconstrictive stimulus
Optionally, the vasodilatory stimulus is at least one targeted increase in the
subject's end tidal PCO2 relative to an steady state baseline PaCO2 or a
previous
targeted value which may optionally be an initial reduction in PCO2.
Optionally, the images represent a change in a blood oxygen level dependent
(BOLD) magnetic resonance imaging (MRI) response to a targeted increase in a
subject's end tidal PCO2 (PETCO2), the CVR response values optionally
representing a change in BOLD MRI signal (A S), in response to a standardized
increase in the PETCO2 (CVR = A S / A PETCO2).
Optionally, the set of control subjects are selected on the basis that they
report
being free of neurological disease.
Optionally, the set of control subjects are matched for at least one
additional
parameter that that defines a preferred subset of control subjects for the
patient
population for whom an assessment of an abnormality in vascular response is
needed.
Optionally, the set of control subjects are matched for at least one of age
and
gender.
16
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Optionally, the set of control subjects are selected on the basis that they
report
being non-smokers.
In another aspect, the invention is directed to a reference atlas of response
values as generated in any manner defined above using a series of increments
in
a subject's arterial partial pressure of carbon dioxide as a stimulus, and to
the
use of such an atlas as a diagnostic tool in aiding of diagnosing a condition
associated with an abnormal vascular response, for example a vascular disease
or disease manifesting an abnormality in a vascular response. Optionally, the
atlas is generated using a sequential gas delivery circuit (physical or
virtual)
wherein end tidal partial pressure of carbon dioxide are used as surrogates
for
targeted arterial partial pressures of carbon dioxide.
According to another aspect, the invention is directed to a neuro-imaging
assessment method in aid of diagnosing at least one of the existence,
location,
deterioration and amelioration of a brain disorder associated with abnormal
vascular reactivity (i.e. any abnormal vascular response including an
abnormality
in the amplitude and/or time course of the response), for example a
cerebrovascular disorder.
The neuro-imaging assessment protocol of the present invention, including any
permutations of the steps defined above or below, enables images to be
produced from which such diagnostic assessments may be carried out and/or
confirmed. According to one embodiment the invention, the organ is brain and
the invention provides a novel cerebrovascular reactivity assessment protocol
for
producing a reference atlas, for example an atlas of non-pathological
cerebrovascular reactivity.
Accordingly in a further embodiment, the invention provides for a method and
for
the use such an atlas of non-pathological cerebrovascular reactivity to
produce
brain imaging results e.g. neuro-imaging results from which a subject in need
of
17
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
assessment of abnormal cerebrovascular reactivity can be assessed for the
abnormality. The method optionally comprises producing a reference atlas and
comparing voxel by voxel test vascular response value of a patient to the
corresponding reference atlas value by scoring those values, preferably in a
manner that accounts for relative departure of the test value from a quantity
describing a characteristic value (e.g. mean/SD for normal distributions of
value
or normal distributions of log values) such as to account for the variability
or
distribution of the control values.
According to another aspect the invention is directed to a diagnostic tools
in the form of a neuro-image and other visual depictions such as graphs
derived
from such images that incorporate statistical transformations of MR signals
generated in response to at least one targeted change in a subject end-tidal
PCO2. According to one embodiment the invention is directed to a
cerebrovascular reactivity response map e.g. in the form a z map, tau z map or
ID z map as described herein.
For example, according to one embodiment the organ is brain and the
invention is directed to a diagnostic tool comprising color-coded z values
mapped
onto an anatomical representation of a standardized 3D map of at least one
region of interest (ROI) of the brain, the z values and 3D map characterized
in
that a standardized set of MR imaging protocols are employed to generate for
members of a group of control subjects, a set of CVR response signals
depicting
a non-pathological CVR response, in at least one common ROI of each control
subject's brain, wherein the CVR response is a reaction to a standardized
vasoactive stimulus, and wherein the CVR response is quantifiable from images
corresponding to the response signals, on a voxel-by-voxel basis, in the form
of
CVR response value per voxel; and wherein
b) a standardized algorithm is used to co-register the respective control
subject
images to a standardized space based on a set of anatomic landmarks;
18
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
c) a computation, for the set of control subjects, on a voxel by voxel basis,
of a
mean and standard deviation of the CVR response values for voxels
corresponding to the at least one ROI is used;
d) the MR scanner and the standardized set of MR imaging protocols is used to
measure a CVR response for a subject in need of an assessment of an
abnormality in CVR, employing the standardized vasoactive stimulus by scoring
the respective responses for individual voxels in the at least one ROI,
relative to
the computed mean and standard deviation, using z values.
Optionally, z values can be generated for test subjects that are based on a
measurement of a plurality of CVR test values, on a voxel by voxel basis, for
each respective control subject. Multiple CVR values per control subject are
obtained from a plurality of imaging tests generated using a standardized
stimulus and therefore reflect expected test/re-test variability in CVR
measurements. The successive tests are preferably conducted on different days
and optionally at different times of day, such that the plurality of variant
values
reflect primarily the inevitable variations corresponding to normal variations
in
physiology and in the technology (even despite using a single scanner), over
time. The different values may also reflect in minor part differences due to
other
categories influences (e.g. unidentified sources of small variation or,
identifiable
sources of small variation of the type not generally subject to practical
control).
The standard CVR atlas may reflect this retest values in the means and
standard deviation per voxel. Alternative the probative value of such re-test
values can be accentuated by generating a specialized reference atlas (an
Interval Difference atlas) in which the control group means and standard
deviations are calculated with respect to intra-subject differences e.g. say
between the two test values for a subject which are subtracted from one
another.
The intra-subject test/re-test variability, however quantified or accounted
for, both
from an intra-control subject perspective and across a group of control
subjects,
19
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
is important for assessing a patient's change in CVR per voxel against a
backdrop of normal re-test variability.
These so-called Interval Difference (ID) variations may be used to compute ID
z
values for a given control or diseased subject, and for creating for the group
of
subjects, an atlas of test-retest value differences, on a voxel by voxel
basis. This
enables an attribution of the statistical probability that changes in CVR to
true
interval change in pathophysiology. Optionally resulting ID-z values may be as
reference maps to monitor progression of the disease over time or responses to
treatment.
Optionally, the standardized cerebrovascular stimulus is a vasodilatory
stimulus.
Optionally, the method is used in aid of diagnosing a neurological disorder
Optionally, the vasodilatory stimulus is a surrogate measure of the subject's
arterial PCO2 (PetCO2), the surrogate measure optionally an end tidal partial
pressure of carbon dioxide measured on a breath by breath basis. The stimulus
is preferably controlled by targeting at least one increase (relative to a
subject's
baseline steady state value or a previously targeted value), in a subject's
end
tidal PCO2.
As described herein, in any of the methods the standardized stimulus
optionally
provides for a subjects baseline PetCO2 to be increased to a targeted value
and
returned to baseline, and optionally increased again to the same targeted
value.
Variations on such standardized protocols would be apparent to those skilled
in
the art of manipulating arterial blood gases.
Several surrogate measures of cerebral blood flow (CBF) are known to persons
skilled in the art.
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Optionally, the images represent a change in a Blood Oxygen Level Dependent
(BOLD) magnetic resonance imaging (MRI) response to a targeted increase in a
subject's end tidal PCO2.
Optionally, the images depict a change in the blood flow as measured by
arterial
spin labeling MR response to a targeted change in a subject's end tidal PaCO2-
The control subjects are preferably free of neurological disease and
optionally
also non-smokers.
Optionally, the control subjects are age and/or gender matched.
The subjects can be matched with respect to a wide variety of parameters
including underlying disease, the use or non-use of certain medications etc.
The z maps or ID standardized z values are optionally employed for the
detection
of areas of paradoxical reductions in blood flow following the application of
the
vasodilatory stimulus ('steal'). In the same connection, parallel increases in
blood
flow elsewhere may also be indicative of an abnormality in a vascular bed.
Optionally, for non-parametric data one can rank order the voxel value in the
reference atlas and then score the test voxel in terms of rank. Also, data can
be
transformed by taking the log of a measure and tested for normal distribution.
If
the logs are normally distributed, then the mean and SD of the logs are
computed. The test voxel is then also transformed to log value and then scored
with a z value.
In a further general aspect, the invention is directed to a method of using
blood
flow correlated high resolution imaging signals for characterizing an
abnormality
in a vascular response to a standardized vasoactive stimulus in at least one
region of interest (ROI) in an organ, the method comprising the steps of:
21
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
a) using an high resolution imaging device and a standardized set of imaging
protocols to generate, for respective members of a group of control subjects,
a
vascular response signal depicting, for each voxel corresponding to the at
least
one ROI, a control group member's vascular response to a standardized
vasoactive stimulus, wherein the vascular response for the ROI for each
control
group member is quantifiable from images corresponding to the respective
individual voxel response signals;
b) using a standardized algorithm to co-register respective control subject
images
to a standardized space based on a set of anatomic landmarks;
c) computing, for the co-registered set of control subject images, on a voxel
by
voxel basis, at least one statistical value describing the quantity and
variability of
vascular response values associated with corresponding voxels of the
standardized space to define at least for the region of interest, at set of
statistical
value respectively associated with individual voxels corresponding to the ROI
for
the control group as a whole (an atlas);
d) using the high resolution imaging device and the standardized set of
imaging
protocols to measure a vascular response for a subject in need of a
comparative
vascular response assessment (patient), employing the standardized vasoactive
stimulus by statistically scoring the patient's respective vascular responses
values for each individual voxel relative to corresponding values in the
atlas, to
generate for each voxel at least one score describing how the patient's
vascular
response values rank in comparison with the corresponding atlas values such
that the quantum and variability of the individual control group member
vascular
response values is taken into account in the score.
Optionally, the method further comprises the step of color-coding the scores
and
mapping the color-coded scores back onto an anatomical representation of the
standardized space to produce a vascular response map of the at least on ROI.
22
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Optionally, the scores are z-scores and wherein the map is a z-map.
Optionally, the organ is brain.
Optionally, the high resolution imaging device is an MRI device, wherein the
co-
registered images are magnetic resonance images.
Optionally, the co-registered MR images are full brain images defining a
substantially full set of potential ROls.
Optionally, the standardized cerebrovascular stimulus is a vasodilatory
stimulus.
Optionally, the vasodilatory stimulus is at least one targeted increase in the
subject's end tidal PCO2 from a steady state PetCO2.
Optionally, the images correspond to signals representing a change in a blood
oxygen level dependent (BOLD) MRI response to a targeted increase in a
subject's end tidal PCO2 (PETCO2), the vascular response values optionally
representing a change in BOLD MRI signal (A S), in response to a standardized
= increase in the PETCO2 (A S / A PETCO2)_ As mentioned above, ASL may be
used in to measure a change in blood flow in response to a standardized P002
stimulus.
Optionally, the vascular response values are a measure of a delay in the
vascular
response to the standardized vasoactive stimulus, the at least one statistical
value determined for each respective voxel using a standardized transfer
function
analysis wherein a polynomial function is computed to match the vascular
response signal data.
23
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Optionally, the at least one statistical value is tau, the standardized
transfer
function analysis employing a mono-exponential dispersion function
(exemplified
herein) to generate an atlas of tau values,
Optionally, the atlas response values are rank ordered on a voxel by voxel
basis
and wherein the corresponding patient response values are assigned a rank
score following the rank order to generate a rank score map.
Optionally, a log transformation of the respective (voxel by voxel) vascular
response values for the individual control subjects shows that the values are
generally normally distributed and wherein the individual control subject
vascular
response values and patient vascular response values are transformed, on a
voxel by voxel basis, by taking the log of the values, and wherein a mean and
SD
of the control subjects respective vascular responses log transformed values
is
computed on a voxel by voxel basis to generate an atlas, and wherein the
patient's respective vascular responses log-transformed values are
respectively
scored with a z value.
Optionally, each of the members of the group of control subjects are selected
to
represent healthy individuals exhibiting a non-pathological vascular response
to
the standardized vasoactive stimulus in the at least one ROI. Alternatively,
the
control group can be represented by any number of different criteria.
According to another aspect, the invention is directed to a neuro-imaging
assessment method in aid of diagnosing at least one of the existence,
location,
deterioration and amelioration of a brain disorder associated with abnormal
vascular reactivity, for example a cerebrovascular disorder.
The neuro-imaging assessment protocol of the present invention, including any
permutations of the steps defined above, enables images to be produced from
which such diagnostic assessments may be carried out and/or confirmed.
24
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
According to one embodiment the invention provides a novel cerebrovascular
reactivity assessment protocol for producing an atlas of non-pathological
cerebrovascular reactivity. Accordingly, in a further embodiment, the
invention
provides a method of using such an atlas of non-pathological cerebrovascular
reactivity to produce neuro-imaging results from which a subject in need of
assessment of abnormal cerebrovascular reactivity can be assessed for the
abnormality.
According to another aspect the invention is directed to a diagnostic tool in
the
form of a neuro-image and other visual depictions such as graphs derived from
such images that incorporate statistical values derived from MR signals
generated in response to at least one targeted change in a subject's end-tidal
PCO2.
According to one embodiment the invention is directed to a cerebrovascular
reactivity response map in the form a z map or ID z map, or tau z map as
described herein.
For example, according to one embodiment the invention is directed to a
diagnostic tool comprising color-coded z values mapped onto an anatomical
representation of a standardized 3D map of at least one region of interest
(ROI)
of an organ e.g. brain, the z values and 30 map characterized in that a
standardized set of imaging protocols are employed to generate for members of
a group of control subjects, a set of vascular response signals depicting a
non-
pathological CVR response, in at least one common ROI of each control
subject's organ of interest, wherein the vascular response is a reaction to a
standardized vasoactive stimulus, and wherein the vascular response is
quantifiable from images corresponding to the response signals, on a voxel-by-
voxel basis, in the form of vascular response value per voxel;
b) a standardized algorithm is used to co-register the respective control
subject
images to a standardized space based on a set of anatomic landmarks;
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
c) a computation, for the set of control subjects, on a voxel by voxel basis,
of a
statistical value describing the quantity and variability of vascular response
values associated with corresponding voxels of the standardized space,
optionally a mean and standard deviation of the vascular response values for
voxels corresponding to the at least one ROI is used;
d) the MR scanner and the standardized set of MR imaging protocols is used to
measure a CVR response for a subject in need of an assessment of an
abnormality in CVR, employing the standardized vasoactive stimulus by scoring
the respective responses for individual voxels in the at least one ROI,
relative to
the e.g computed mean and standard deviation, using e.g. z values.
According to another aspect the invention is directed a method of assessing
the
severity and distribution of an abnormality or reduction in a test subject's
vascular
response to a vasoactive stimulus in at least one region of interest (ROI) of
the
subject's brain, cornprising the steps of:
(a) obtaining input of a test-subject's vascular response values per voxel
corresponding to at least one region of interest (ROI) of the test subject's
brain;
(b) obtaining input of reference values for at least each voxel in the ROI (an
atlas)
for a group of control subjects; and
(c) scoring the test subject's vascular response values for respective
individual
voxels in the ROI, relative to the corresponding reference values per voxel
using
z scores;
wherein the reference values comprise a mean and standard deviation of
respective control subject's vascular response values per voxel for a
corresponding ROI in the group of control subjects, the reference values and
the
test subject's vascular response values per voxel derived from MR signals
26
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
obtained from an MR scanner using a pre-selected MR protocol and including
respective voxel coordinates co-registered to a standardized space based on a
set of anatomic landmarks; the vascular response values corresponding to and
quantifying an individual subject's (control or test subject) vascular
response to at
least one change in the individual subject's arterial partial pressure of
carbon
dioxide (each arterial partial pressure of carbon dioxide a PaCO21-) in at
least one
common ROI of each individual subject's brain, wherein the vascular response
is
quantified, from a surrogate measure of blood flow, on a voxel by voxel basis,
the
MR signals quantifying at least one of the amplitude of the individual
subject's
vascular response and a time constant of the individual subject's vascular
response to the each PaCO2T.
Brief Description of the Drawings
Figure 1 is a series of axial slices for a normal cohort atlas displaying the
spatial
distribution of (A) mean CVR values coloured according to the scale shown on
the right in % BOLD change / mmHg PETCO2 change and (B) coefficient of
variation (CV) values with colour scale on right in percent.
Figure 2 is a set of axial slices displaying the spatial p-value results of an
Anderson-Darling normality test. The spatial distribution of the results this
test
was applied to the CVRs of the 46 healthy subjects CVRs graphed onto the MNI
standard brain. At least 60% of the voxels had a p-value greater the 0.05;
these
voxels were fairly evenly distributed throughout the brain.
Figure 3 is a healthy subject's CVR map. An axial slice is shown on the left
displaying the spatial distribution of CVR values coloured according to the
scale
shown on the right in % BOLD change / mmHg PETCO2 change. The
corresponding CVR z-map and its color scale are shown on the right. The CVR
z-map provides a perspective on the (statistical) normality of CVR in the CVR
map. Figure 3 illustrates the extent of expected high statistical abnormality,
as a
27
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
result of physiologic, technical, and anatomical variation in the subject as
well as
errors in matching of voxels during co-registration.
Figure 4 depicts magnetic resonance angiograms, CVR maps and corresponding
z maps for a sample set of 5 patients with varying levels of carotid artery
(CA)
disease. The CVR maps were analyzed by z scoring of the CVR map relative to a
normal atlas. This figure is supplemented with a table, Table 2 (Figure 6)
that
provides additional information and commentary for each subject. (Dx. =
diagnosis; MRA = magnetic resonance angiogram).
Figure 5 depicts magnetic resonance angiograms, CVR maps and corresponding
z maps for a sample set of 4 patients with Moyamoya disease and one patient
with idiopathic intracranial hypertension. The CVR maps were analyzed by z
scoring the CVR map relative to a normal atlas_ Figure 6 (Table 2) provides
additional information and commentary for each subject. (Dx= diagnosis;
MRA=magnetic resonance angiogram).
Figure 6 is a table (Table 2) providing additional information about the
patients for
whom magnetic resonance angiograms, CVR maps and corresponding z maps
are provided in Figures 4 and 5 (Abbreviations: ACA, anterior cerebral artery;
EC-IC, external carotid to internal carotid; GM, gray matter; Hx, History;
ICA,
internal carotid artery; L, Left; R right; MCA, middle cerebral artery; MM,
Moyamoya; PCA, posterior cerebral artery; SD, standard deviation; TIA,
transient
ischemic attack; VA, vertebral artery; WM, white matter)
Figure 7 is summary table (Table 3) comparing CVR maps and z-maps.
Figure 8 shows CVR maps for a male subject tested on two different sessions 14
days apart.
28
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Figure 9 are Bland-Altmann plots of CVR for between-day reproducibility for
gray
(a) and white (b) matter regions. The Bland-Altman analysis compares the CVR
values for gray and white matter obtained on the different days establishing
that
the mean difference between days for gray matter was 0.0013
(A%BOLDSignal/AmmHg), with limits of agreement of -0.0674 and 0.0700 ( 1.96
SD); whereas the mean difference between days for white matter was 0.0078
(A%BOLDSignal/AmmHg) with -0.0449 and 0.0605 ( 1.96 SD) limits of
agreement.
Figure 10 depicts results for the application of a sample ID atlas to assess
the
changes in CVR over time in a healthy control subject (not included in the ID
atlas) demonstrating that the majority of difference between day 1 and day 2
in
this healthy subject < 1.0 SD as expected.
Figure 11 presents angiogram, CVR and ID z maps for an axial slice showing the
spatial distribution of CVR values and the associated z-maps at z value
thresholds of 0.5 and 1Ø Imaging data from a 38 year old female with moya
moya cerebrovascular disease who underwent 2 CVR studies pre, and 6 months
post right EC-IC bypass, 6 months apart. A) The magnetic resonance angiogram
and CVR maps for an axial slice showing the spatial distribution of CVR values
B.
The temporal z-maps of the two CVR maps.
The distribution of changes in both positive and negative directions
consistent
with the history, and the magnitude of voxelwise divergence in interval
differences from the sample ID atlas, establish that these changes were not
due
to technical or physiologic variability.
Figure 12 is a Table describing the distribution of age and sex of the cohort
of 46
control subjects.
29
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Figure 13 presents in an upper left panel a series of deconvolved input
signals -
PETCO2 (red lines) to match BOLD signal (black line) in one voxel (crosshairs)
from which T is calculated. The upper right panel is a CVR map. Lower left
panel
shows the amplitude of response as calculated from the matched deconvolved
function. Lower right panel is the r map.
Figure 14 presents CVR and tau maps and their respective z maps in a patient
with right carotid artery stenosis. Figure 14 shows an abnormal time response
(tau and tau z values) in areas with normal or mildly abnormal CVR amplitude
and z values (outlined, arrows).
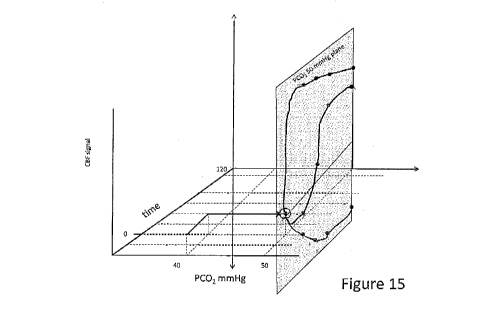
Figure 15 is a graph illustrating a change of amplitude of a BOLD signal (CBF
signal) (Y axis) as a function of PCO2 in mmHg (X axis) and time (z axis) for
robust (blue), dampened (red) and paradoxical (orange) responses.
Figure 16A-E provide illustrations characterizing the use of transfer function
analysis to label each voxel as per gain and phase lag.
Co-registration of such maps for a reference cohort (sometimes described
herein
as a healthy or normal cohort) is accomplished as described below.
Means and SD are computed and then a z map is generated for our test subject
of gain and lag phase. Theoretically, these values should correspond to CVR
and T respectively.
Figure 16B-16D images show that indeed they look very similar (compare CVR
line to Gain line in Figure 16B; and phase lag line slide 16B to T line in
Figure
16C. Figure 16D compares images of amplitude from CVR amplitude measured
(first line), amplitude calculated from r dispersion (second line), and gain
using
FTA (fourth line).
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Figure 17a is a flow chart showing a series of steps useful for producing
vascular
response data for a subject (control or patient) according to an embodiment of
the invention.
Figure 17b is table describing the nature and function of each step presented
in
Figure 17a.
Figure 18a is a flow chart showing a series of steps useful for producing z
maps
for a subject according to an embodiment of the invention. These z-map
generation steps may applied to CVR, to amplitude, tau, gain, phase,
coherence,
interval differences etc.
Figure 18b is table describing the nature and function of each step presented
in
Figure 18a.
Figure 19a is a flow chart showing a series of steps useful for producing tau
response values per voxel in at least one ROI for a subject according to an
embodiment of the invention.
Figure 19b is table describing the nature and function of each step presented
in
Figure 19a.
Figure 20a is a flow chart showing a series of steps useful for conducting a
transfer function analysis for a subject according to an embodiment of the
invention.
Figure 20b is table describing the nature and function of each step presented
in
Figure 20a.
Figure 21a is a flow chart showing a series of steps useful for producing
Interval
Difference (ID) z maps for a subject according to an embodiment of the
invention.
Figure 21b is table describing the nature and function of each step presented
in
Figure 21a.
31
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Detailed Description of Preferred Embodiment
A reference atlas can be made for each the vascular response values
exemplified herein, the values matched to a set of particular targeted
arterial
partial pressures of carbon dioxide. For example, the reference atlases can be
made from r and phase lag, for amplitude of CVR , for interval differences
etc. Each can be used to generate a z map_
The terms "vasoactive response" and "vascular response" are used
interchangeably.
The term "co-register" means transforming image data onto common coordinates
of a standard brain using an alignment algorithm that standardizes brain size
while optimizing alignment of a set of key anatomical structures.
The term "vascular reactivity" and the related term cerebrovascular reactivity
(CVR) is used broadly to refer any vascular response to a standardized
vasoactive stimulus, which vascular response may be a change in amplitude of
the response, the time course of the response etc. Vascular response values
may be a measure of the amplitude of the response (i.e a measure of amplitude
alone, wherein amplitude is revealed, for example, by allowing 3 time
constants
in the progress of the response to be attained before modifying the PaCO2T or
where a ramp stimulus is employed e.g. equal size increments in PaCO2T and
equal time intervals, the true amplitude of the response will be substantially
revealed where, for example, two time constants in the progress of the
response
are attained before the next incremental change in PaCO27.
A user may prospectively or retrospectively define a voxel as "abnormal" with
reference to at least one of: (1) the size of the vasoactive stimulus or
change in
stimulus (e.g. the degree of upward departure of the PaCO2T from a normal
baseline value for the subject) used to reveal the abnormality; (2) the size
of the z
32
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
score (e.g. 2 to 3 standard deviations relative to the mean). The smaller the
stimulus required to generate a deviation from the normal distribution of
signals
for the voxel, or the greater the signal change for a given stimulus, the more
indicative of abnormality.
The time course of the response may be revealed with a step change (e.g. a
targeted increase within the range of approximately 5 to 12 mm of Hg, for
example 10 mm of Hg in PetCO2) in the stanadardized vasoactive stimulus by
monitoring the time course of the response to the step change.
Similarly, with respect to the amplitude of the vasoactive response at least
one
step change within this range or a ramp with small increments in PaCO2T from
baseline e.g. to baseline + 10 mm of Hg may be used to assess the amplitude of
the response.
Importantly, each PaCO2T is maintained in the course of obtaining input of the
MR signals. Accordingly the stimulus is standarized for control and test
subjects
and the true nature of the response is revealed. In this manner, comparing
test
subjects with a control subject atlas reveals the severity and distribution of
an
abnormal or reduced vascular response. Thus while a CVR map might show a
mildly abnormal response for a voxel that is hard to judge as a probable
indicator
of disease, the precision of the stimulus allows a more conclusive
determination
of abnormality to be revealed. Herein, statistical maps such as z maps reveal
the
paramount importance of this standardized PaCO2T stimulus. Furthermore a
reduction in the vasoactive response, not visible in a CVR map, will be be
revealed as abnormal and hence as a region that might harbour an underlying
pathology.
The term "high resolution" with used with reference to imaging modality or
device
refers to an imaging modality enjoying a spatial resolution of 1 cubic
centimeter
or smaller. The term includes MRI imaging modalities (for example BOLD, T2*,
33
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
ASL) and other imaging modalities well known as being useful to quantify
surrogate measures of blood flow (CT, SPECT, PET). Proprietary and non-
proprietary software for analyzing images in available to persons skilled in
the
Importantly, a standardized vasoactive stimulus is accomplished in the manner
described herein.
Preferably, the standardized vasoactive stimulus is one or more targeted
arterial
partial pressures of carbon dioxide. Optionally, the standardized vasoactive
stimulus is a series of increments or decrements in a subject's arterial
partial
pressure of carbon dioxide as described in our co-pending U.S. Patent
Application No.: 14/398,034 originally published as WO/2013/163735. One or
more targeted increases in a subject's arterial partial pressure of carbon
dioxide
may also be accomplished in larger steps as described in the examples herein
and more generally in our co-pending US Application No. 14/363,259, originally
published as WO/2013/082703.
A measurable increase in the end-tidal (end-exhaled) partial pressure of CO2
(PETCO2) may be used as a surrogate measure for the true independent
stimulus, the partial pressure of CO2 in arterial blood (PaCO2). Optionally, a
targeted end tidal partial pressure of carbon dioxide is achieved via
sequential
gas delivery using a specialized re-breathing circuit or a virtual sequential
gas
delivery circuit (see our co-pending application No.US/2016/0034085,
originally
published as WO/2013/138910).
CVR may be defined as the per cent change in BOLD signal (arbitrary units) per
mmHg change in PaCO2.
CVR values for subjects in need of assessment of cerebrovascular reactivity in
at
least one ROI are assigned color-coded z values based on computations of
mean (+/- SD) CVR values, preferably computed on a voxel by voxel basis, for a
34
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
group of control individuals using images co-registered to a standardized
space
based on anatomical markers and standardized parameters. The color-coded Z
values representing the number of standard deviations from the mean are then
superimposed, on the corresponding voxel on an anatomical scan to generate Z
maps.
In one embodiment of the method, CVR was measured as the blood oxygen level
dependent (BOLD), magnetic resonance imaging (MRI) response to a
standardized hypercapnic stimulus. CVR maps from 46 healthy subjects were
co-registered into a standard space and mean and standard deviation (SD) was
measured for each voxel to form the normal CVR atlas. CVR maps from 9
patients were assigned a z-score according to the mean and SD of the
corresponding voxel of the atlas. The z-scores were color coded and
superimposed on their anatomical scans to form z-maps, which were assessed to
determine whether they enhanced the interpretation of CVR maps.
The z-maps display of the voxel-by-voxel statistical deviation of CVR from the
mean of the atlas enabled detection of reductions in CVR not apparent in CVR
scans. They identified generalized, symmetrical reductions in CVR as well as
quantifying the extent of abnormality in focal lesions evident on CVR maps.
The inventors have found that z-maps complement CVR maps by detecting,
localizing, and assessing, the deviation from normal vascular responses.
In order to excessive repetition, it is to be understood that the various
optional
features described in connection with one of the various aspects and more
particularized embodiments of the invention described herein, apply to other
aspects / embodiments subject matter described herein including a method as
defined herein, an imaging system as defined herein, an atlas as defined
herein,
a computer program product as defined herein, a non-transitory computer
memory as defined herein etc.
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
The present invention extends the analytic methods of CVR measurement to
determine the region by region normal range of CVR and thereby enable
quantification of abnormality by the assessment of CVR in terms of its
deviation
from a statistical mean. The inventors took an approach similar to that of
Guimond et al. [Guimond A, 2000] and Seitz et al. [Seitz, 1990] who co-
registered scans of healthy subjects into a standard space and determined the
normal mean and variance of CVR, voxel-by-voxel. In one aspect, the present
invention is directed to generating an atlas of images for non-pathological
CVR
response by co-registering CO2 stimulated BOLD MRI CVR maps from a healthy
cohort into a standard space, and calculating the mean and SD of the CVR for
each voxel.
Patient CVR maps were then also co-registered into standard space and
each voxel scored positive or negative relative to the mean, and quantified by
a
z-score of the corresponding voxel in the atlas. These z-scores were then
colour
coded and superimposed on the patient's anatomical scan to generate a z-map.
The inventors determined that z-maps enhance the interpretation of BOLD MRI
CVR maps and highlight brain areas where vessels may have residual reactivity
above the threshold for the development of steal. In particular, by comparing
CVR maps and z-maps in 8 patients with symptomatic cerebrovascular steno-
occlusive disease and one patient with increased intracranial hypertension it
was
determined that z-maps enhance the interpretation of CVR maps.
Example 1
Studies conformed to the standards set by the latest revision of the
Declaration of Helsinki and were approved by the Research Ethics Board (REB)
of the University Health Network, Toronto, Ontario and all subjects gave
written
informed consent, Forty-six healthy volunteers were recruited for the creation
of
36
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
a normal CVR atlas by advertisement and word of mouth. This cohort consisted
of subjects of both sexes and any age who claimed to be in good health, denied
a history of neurologiCal disease, were non-smokers, and were taking no
medication.. They were asked not to engage in heavy exercise or drink
caffeinated drinks on the day of the scan. The characteristics of these
subjects
are presented in Table 1 (Figure 12). We then drew the data from ten patients
from our database of REB-approved CVR studies in patients with known
symptomatic cerebrovascular disease [Spano, 2013]. Sample patients were not
selected for age, sex, diagnosis, or findings on vascular imaging or CVR
studies.
All 10 patients were chosen and grouped before any of their data was analyzed.
None were rejected after analysis.
Experimental Protocol
Hypercapnic stimulus
The implementation of prospective end-tidal gas control has been
described in detail elsewhere [Fierstra, 2013]. In brief, subjects were fitted
with a
face mask, and connected to a sequential gas delivery breathing circuit
[Somogyi,
2005]. The patterns of PETCO2 and PET02 were programmed into the automated
gas blender (RespirActTM , Thornhill Research Inc., Toronto, Canada) running
the
prospective gas targeting algorithm of Slessarev et al. [Slessarev, 2007]. A
standardized step CO2 stimulus was implemented, consisting of the following
sequence: a baseline PETCO2 of 40 mmHg for 60 s, step to a hypercapnia of 50
mmHg for 45 s, baseline for 90 s, hypercapnia for 120 s, and return to
baseline
for 60 s, all during isoxic normoxia. For the healthy cohort the mean (SD)
change in PETCO2 was 9.2 (0.7) mmHg. This methodology wherein the subject
inspires a neutral gas at the end of each breath (implemented via sequential
gas
delivery - see e.g. see our co-pending application No.US/2015/0034085) has
been shown to control the CO2 stimulus such that PETCO2 is equivalent to
PaCO2 [Ito, 2008].
37
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
MRI Protocol and CVR Map Generation
Magnetic resonance imaging was performed with a 3.0-Tesla HDx
scanner using an 8-channel phased-array receiver coil (Signa; GE Healthcare,
Milwaukee, Wisconsin), and consisted of BOLD acquisitions with echo planar
imaging (EPI) gradient echo (TRITE = 2000/30 ms, 3.75 x 3.75 x 5 mm voxels,
field of view 24x24 cm, 39 slices, slice thickness 5mm, matrix size 64x64,
number of frames = 254, flip angle (FA) = 85 ).
The acquired MRI and PETCO2 data were analyzed using AFNI software
(National Institutes of Health, Bethesda, Maryland;
http://afni.nimh.nih.gov/afni;
Cox, 1996 #16172]). PETCO2 data was time-shifted to the point of maximum
correlation with the whole brain average BOLD signal. A linear, least-squares
fit
of the BOLD signal data series to the PETCO2 data series (i.e., CVR) was then
performed on a voxel-by-voxel basis. For displaying CVR maps, voxels with a
correlation coefficient between -0.25 and +0.25 were eliminated before color-
coding the remaining CVR values (see spectrum in Figure 3).
BOLD images were then volume registered and slice-time corrected and
co-registered to an axial 3-D T1-weighted Inversion-Recovery prepared Fast
Spoiled Gradient-Echo (IR-FSPGR) volume (TI/TRITE = 450/8/3 ms, voxel size
0.86 x 0.86 x 1.0 mm, matrix size 256 x 256, field of view 22 x 22 cm, slice
thickness = 1mm, FA = 15 ) that was acquired at the same time [Saad, 2009].
This method has been described in greater detail elsewhere [Fierstra, 2010].
Analysis of CVR Maps
Constructing the Atlas (see also Guimond, A 2000, and Seitz, 1990).
38
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Analytical processing software (SPM5; Wellcome Department of Imaging
Neuroscience, University College, London, UK;
http://www.fil.ion.ucl,aauk/spm/software/spm5), was used to co-register each
of
the individual brain volumes from the healthy cohort into MN1 (Montreal
Neurologic Institute) standard space using a 12-parameter [Ashburner,19971
affine transformation followed by nonlinear deformations to warp the brain
volume of interest into an MNI template of identical weighting contrast. The
T1-
weighted FSPGR volume was used to estimate the transformation normalization
into standard space, as defined by a 71-weighted MN1152 standard template
[Ashburner,1999].
A spatial smoothing of Full-Width Half-Maximum (FWHM) 5 mm was
applied to each voxel. Assumption for normality was tested using the Anderson-
Darling test (the statistical test for normality provided in AFNI) with p
values
greater than 0.05 assumed to pass the test. As most voxels (60 %) did pass
this
threshold, and these were diffusely distributed throughout the brain, the
simplifying assumption was made that the CVR for each voxel was normally
distributed. The mean (p) and associated standard deviation (a) of CVR was
calculated (AFN1 software [Cox, 19961). Maps were then constructed for p and
coefficient of variation (a / p) to characterize the atlas.
CVR z-map generation
The generation of an individual's CVR z-map consisted of three steps. First, a
spatial normalization of the individual's anatomical scan and CVR map
[Ashburner,1999] using a MNI152 8PM distributed template was produced.
Second, the CVR of each voxel (x) was scored in terms of a z value (i.e., z =
(x-
p)/a). Finally, a color was assigned to each z score (see scale in Figure 3)
to
indicate the direction and magnitude (in z values) of the differences from the
mean of the corresponding atlas voxel. CVR and CVR z scores were
superimposed on the corresponding anatomical scans to allow comparison of the
CVR and its z score. Note that CVR voxels that are positive but lower than the
39
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
atlas mean for that voxel will have negative z scores. Greater specificity for
identifying underlying vascular pathophysiology was assumed to be connoted by
greater absolute value of z scores and the confluence of similarly scored
voxels
in both CVR and CVR z-maps.
To clarify the colour coding used, it is pointed out that in the resulting z-
map: (1) Patient CVR map voxels that are negative (blue) where the
corresponding atlas CVR map voxels are positive, will have negative z-scores
coded light blue to purple. (2) Patient CVR voxels that are positive but lower
than
the atlas CVR voxels will also have negative z-scores. (3) However, negative
CVR voxels that are greater (towards the positive direction) than the
corresponding atlas CVR voxel will nevertheless have a positive z-score.
Greater specificity is connoted by greater z scores (for z-maps) and the
confluence of similarly scored voxels (both CVR and z-maps).
Z-map
Normal cohort CVR characteristics
Figure 1 shows maps of the mean CVR and coefficient of variation (CV) of
the reference atlas. Voxels over predominantly cortical gray matter (GM) have
mean CVR of 0.20 to 0.30 % ABOLD / A mmHg whereas those over
predominantly white matter (WM) had considerably lower CVR (0.05 to 0.15 %
ABOLD / A mmHg). Many voxels had mixed tissue type content, so that
intermediate CVR values at the interface probably represents voxels that had
greater overlap between WM and GM. Clusters of highest mean CVR values
were found over veins.
With respect to variability, GM areas had the lowest CV values, ranging
between 30-40%, whereas higher CV values, between 50-60%, were found in
WM. The high CV values calculated at the outer margin of the brain result from
the variation in CVR measured where that voxel is predominantly GM, CSF, bone,
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
WM, and blood vessels in different subjects. Similarly, venous sinuses were
difficult to localize consistently.
The spatial distribution of the results of the Anderson-Darling statistical
test of normality applied to the 46 healthy subjects CVRs graphed into the MNI
standard brain is shown in Figure 2. At least 60% of the voxels had a p-value
greater the 0.05; these voxels were fairly evenly distributed throughout the
brain.
For comparison purposes, Figure 3 presents the CVR and its
accompanying z-map from a healthy subject not included in the atlas. The z-
maps of the 10 patients drawn from our database are shown in Figures 4 and 5,
and descriptions related to each patient are presentedTable 2.
Z-scoring the CVR studies in our sample patient cohort provided an
objective, graded demarcation of the reduction in CVR, quantified relative to
the
normal range for the region. The z-map therefore emphasized the CVR changes
attributable to underlying vascular pathophysiology. This approach diverges
significantly from previous practice where CVR was divided into 'steal and non-
steal' territories [Fierstra, 2010] [Balucani, 2012] or compared to normal
atlases
where thresholded CVR values of 2 [Commowick, 2008] and 3 [Kemp, 1995]
standard deviations were required to identify significant differences from
normal.
Our method identifies a graded range of reductions in CVR that do not
meet all of the conditions required for steal, yet nevertheless represent
vascular
pathophysiology [Sobczyk 2014]. Furthermore, we minimized the inter-subject
variability (i.e., 'noise') due to diversity of technical specifications and
brain
physiology by standardizing both MRI sequences and the provocative stimulus
across the atlas and patient studies_ A secondary outcome of the study is that
the calculation of the voxelwise mean and variance, characterizes the
magnitude
and variance of normal CVR in humans, as represented by our sample cohort.
41
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Previous studies examined the deviation from steady state, and therefore
reflected only the combined subject-to-subject, and the test-to-test
variability
[Seitz, 1990]. In contrast, CVR requires the application of a stimulus and the
measurement of a response to that stimulus, both potentially adding variations
to
the CVR values in the atlas. Of these, we' can only address the issue of
variability in the stimulus, leaving the variation of response to be reflected
as a
characteristic of the atlas.
First, we wished to retain the advantage of the high spatial and temporal
resolution provided by BOLD signal [van der Zande, 2005] as the surrogate for
CBF; for this, the application of the stimulus had to be MRI compatible.
Second,
whereas the magnitude of the stimulus¨i.e., the change in PaCO2--is unknown
with other hypercapnic methods (Fierstra, 2013), it is precisely known with
our
method of stimulus generation [Ito, 2008 #14395]. This methodology therefore
enables the reduction of the effects of variations in the stimulus on CBF by
(a)
normalizing the change in BOLD signal for the change in PaCO2 and (b)
implementing a uniform change in PaCO2 [Fierstra, 2013] between patients and
atlas.
Characteristics of the patient cohort
The examination of our patient data illustrated the value added to CVR
interpretation by z-maps suprisingly underscoring the importance of a
standardized vasoactive stimulus. Subjects 3, 6, 8, and 10, in Figures 4 and 6
illustrate the difficulty in confidently interpreting abnormal CVR in areas
not
showing steal. In patients 3 and 6, the reductions in CVR are symmetrical;
there
is little 'steal' as no territory is strong enough to be the 'thief [Sobczyk,
2014].
This mechanism can also explain the small negative CVR values despite
profound reductions in CVR z scores in subjects 8 and 10. In subjects 4, and
5,
the robust CVR is likely due to the recruitment of collateral blood flow, and
had
been interpreted as 'normal' in the original studies. However, the z-map
analysis
42
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
now highlights a previously unappreciated reduction in CVR in the left MCA
territory. A summary of the clinically relevant advantages z-maps provide
beyond
plain CVR maps is presented in Table 3 (Figure 7)
We also note that most of the patients presented with protean transient
symptoms and were otherwise remarkably asymptomatic. We are impressed
that the extent of the neurovascular changes that were provoked by the
hypercapnic challenge are very much out of proportion to the clinical
symptoms,
indicating the considerably greater sensitivity of neuroimaging, including
CVR, in
detecting occult neurovascular disease compared to clinical assessment.
Characteristics of the reference atlas
The CVR atlas represents the distribution of CVR and its variance in the
human brain, as reflected in our sample. It incorporates and reflects the
regional
anatomical differences in the response of the BOLD signal resulting from (a)
=
tissue factors, such as age, sex, 02 consumption, capillary density, changes
in
blood volume, differences in blood arrival time, and vascular response time;
(b)
physiologic factors such as genetic makeup, variations in diet, sleep pattern,
time
of day, hormonal level, physical fitness, blood pressure and blood pressure
response to hypercapnia, state of mind; and (c) unknown technical and
mechanical changes in the MRI system over time. These form the background
"noise", from which a patient's abnormal voxels, their distribution and the
extent
of their deviation, must be discerned.
To optimize sensitivity, the subject-to-subject variability in the atlas can
be
minimized by targeting the atlas to a particular patient group. For example,
matching age, sex, medication, and other physiologic features to the target
study
group (for example young men with multiple sclerosis), and reducing all
technical
and methodological sources of variability--would leave the disease process as
the dominant source of divergence of CVR from that of the reference cohort.
43
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Z-maps to compare CVR across platforms
An important feature that favours the normal atlas and z-map approach is
that its value does not depend on the MRI sequence used or the actual method
of administering the vasodilatory stimulus -- dose of acetazolamide, inspired
concentration of CO2, or breath hold time¨rather it is the consistency of the
acquisition sequence and the stimulus within the atlas population and between
the atlas and the target subjects that is revealed to be important (see
[Fierstra,
2013]). Under these conditions, z values should be comparable across
platforms.
Pooling atlases from multiple scanners may also address this issue but
would also increase the atlas variability and therefore reduce its
sensitivity. We
therefore suggest that at least initially, it is safest to generate a unique
atlas for
each scanner. On the positive side, doing so can be seen as a one time
'calibration'. Since it accounts for between-Subject variability the, z-map
approach provides a robust control group that can be referenced for several
studies, and thereby maximize the statistical power of the subject cohort, and
minimizing the number of subjects required.
We accepted the large age range in our atlas. Nevertheless, it was
characteristic of the age range of our patient database. Any discrepancies in
matching would optimize the specificity. Sensitivity in picking up pathology
in our
patient cohort was not a concern as our experiences lead us to expect that the
changes in CVR due to cerebrovascular disease will greatly exceed that between
healthy subjects in an atlas, regardless of the sex and age distribution
[Oudegeest-Sander, 2013]. We did however minimize the variability by using a
single scanner, running the same MRI acquisition sequence for all subjects and
patients, and implementing a uniform stimulus.
These example patients we chose were not intended to represent typical
findings for any particular pathology, but to illustrate the range of images
produced by z-score analysis relative to a reference atlas. We anticipate that
different neurological diseases may call for different stimulus patterns, and
so
specific normal atlases to reveal their pathophysiology. It will be
appreciated that
44
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
certain patterns (square wave, sinusoidal, ramp, pulse, and others) may be
optimum to study certain conditions (vasculitis, traumatic brain injury,
subarachnoid hemorrhage and others). The overarching approach nevertheless
would be the same: compare patient CVR maps to that of a reference atlas.
Construction of the Interval Difference (ID) Atlas
Twelve males with a mean (SD) age of 35(14.3), were selected from the healthy
cohort to repeat their CVR measurement within a two week timeframe, within
which it is assumed that no disease process was initiated and all physiologic
differences are those that occur in healthy people day to day. Construction of
the
ID-atlas proceeded as described for the normal atlas except that in this case
we
first calculated a voxel-by-voxel difference in CVR between the repeated
studies
in each of the 12 subjects.
MRl Protocol and CVR Map Generation
Magnetic resonance imaging was performed with a 3.0-Tesla scanner
(Signe; GE Healthcare, Milwaukee, Wisconsin) and consisted of BOLD
acquisitions with echo planar imaging (EPI) gradient echo (TR 2000, TE 30 ms,
3.75 x 3.75 x 5 mm voxels).
The acquired MRI and PETCO2 data were analyzed using AFNI software (Cox,
1996). PETCO2 data were time-shifted to the point of maximum correlation with
the whole brain average BOLD signal. A linear, least-squares fit of the BOLD
signal data series to the PETCO2 data series was then performed on a voxel-by-
voxel basis. The slope of the relation between the BOLD signal and the PETCO2
was color-coded to a spectrum of colors corresponding to the direction
(positive
or negative) and the magnitude of the correlation to create CVR maps. Voxels
with correlation coefficients between -0.25 to +0.25 were thresholded out of
the
maps. BOLD images were then volume registered and slice-time corrected and
co-registered to an axial 3-D T1-weighted inversion-Recovery prepared Fast
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Spoiled Gradient-Echo (IR-FSPGR) volume (voxel size 0.86 x 0.86 x 1.0 mm)
that was acquired at the same time (Saad et al., 2009). This method has been
described in greater detail by Fierstra et al. (Fierstra et al., 2010).
Analytical processing software (SPM5; Wellcome Department of Imaging
Neuroscience, University College, London, UK; http://www.filion.ucl.ac.uk/
spm/software/spm5), was used to co-register each of the healthy individual
cohort brain volumes into MNI (Montreal Neurologic Institute) standard space
using a 12-parameter (Ashbumer and Friston, 1997) affine transformation
followed by nonlinear deformations to warp the brain volume of interest into
an
MNI template of identical weighting contrast. The T1-weighted FSPGR volume
was used to estimate the transformation normalization into standard space, as
defined by a T1-weighted MNI152 standard template (Ashburner and Friston,
1999). A spatial smoothing of FWHM 5mm was applied to each. Finally, the
mean CVR (T) and associated standard deviation (crr) was calculated for each
voxel (AFNI software (Cox, 1996)).
Repeatability and Construction of the Interval Test Difference (ID) Atlas
Twelve males with a mean (SD) age of 35(14.3), were selected from the
healthy cohort to repeat their CVR measurement within a two week timeframe.
To obtain regional measures of CVR, we segmented the anatomical images into
gray matter and white matter (SPM5; Wellcome Department of Imaging
Neuroscience, Institute of Neurology, University College, London, UK) regions
and spatially normalized to CVR maps. Time comparisons were evaluated by
Bland-Altman plots and the coefficient of variation (CV) for grey and white
matter
as estimates of repeatability (SigmaPlot 12.5, Systat Software, California).
Construction of the 1D-atlas proceed as described for the normal atlas
except that in this case we first calculated a difference CVR map from the two
time points in each of the 12 subjects. Then from the difference maps, we
46
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
calculated a difference mean, and associated standard deviation for each voxel
to produce the test-retest difference probability atlas (ID-atlas).
Z-maps
To compare an individual CVR map with that of the normal or temporal
atlas the spatial CVR information was further analyzed by comparing the
direction and magnitude of the change in BOLD signal of each voxel to that of
the
corresponding voxel in the atlas; the resulting map was called a z-map. This
comparison consisted of three steps. First, a spatial normalization of the
patient
anatomical and CVR scan (Ashburner and Friston, 1999) using a MNI152 SPM
distributed template supplied by the Montreal Neurological Institute was
produced.
Second, the CVR of each voxel was scored in terms of a z value (i.e., the
value
expressed in standard deviations (SD) of the CVR scores of the corresponding
voxel in the atlas, (z )).
Finally, a color was assigned to each z-score; AFNI software (Cox, 1996)
to indicate a magnitude and direction of the differences in z-scores compared
to
the atlas population. Positive scores (where the CVR is greater than the mean
of
the atlas) were coloured green with 15 different shades ranging in intensity
between 0 to 3.08D. Negative scores (where CVR is less than the mean) were
coloured purple with 15 shades ranging in intensity between 0 and -3SD.
The calculated z-scores were superimposed on the anatomical scans to
allow comparison of the patient's CVR to the atlas CVR. As a result: (1)
Patient
CVR map voxels that are negative (blue) where the corresponding atlas CVR
map voxels are positive, will have negative z-scores. (2) Patient CVR voxels
that
are positive but lower than the atlas CVR voxels will also have negative z-
scores.
(3) Negative CVR voxels that are higher than the corresponding atlas CVR voxel
will have a positive z-score. Maps with z-score thresholds < 0.5 SD provide
highest sensitivity and those > 2.0 SD greatest specificity.
47
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
CVR differences over time were calculated for two patients who underwent
more than one CVR study in a year time span (ID z-maps). Z-scores were
calculated voxel-by-voxel by comparing the difference CVR map of the patient
to
the temporal atlas. This allowed us to evaluated changes over time that
differed
significantly from changes over time found in a normal cohort.
We examined the ID z maps generated from our small trial atlas in the
most recent 15 patients in our database that met the search criteria. We
studies
two illustrative cases in detail (one patient who had undergone several scans
before undergoing extracranial-intracranial (EC-IC) bypass, and one patient
with
symptomatic Moyamoya disease that had undergone several CVR studies over a
2 year period).
On average the healthy subjects who participated in the testing for the
temporal atlas were scanned 15 days apart. Figure 8 illustrates the
reproducibility of the CVR map vascular response pattern for one example
subject.
Figure 9 presents the results of a Bland-Altman analysis comparing the
CVR values for gray and white matter obtained on the different days. The mean
difference between days for gray matter was 0.0013 (LABOLDSignal/AmmHg),
with limits of agreement of -0.0674 and 0.0700 ( 1.96 SD). The mean difference
between days for white matter was 0.0078 (A%BOLDSignal/AmmHg) with -
0.0449 and 0.0605 ( 1.96 SD) limits of agreement.
The mean CVR and CV reproducibility measures for gray and white matter
are presented in the Table immediately below. The reproducibility analysis
demonstrates good reproducibility between-day CVR estimates in both gray (Cy
= = 10.25%) and white matter (CV = 9.66%) on average.
48
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Table Mean (SD) CVR differences between days and mean (SD) coefficient of
variation (CV) for the gray and white matter regions.
White
Gray Matter = Matter
Mean CVR difference 0.2179 0.1882
(A%BOLD/AmmHg) (0.021) (0.017)
Mean CV (%) 10.25 (5.19) 9.66 (4.81)
ID Z-Map
Figure 10 represents the application of our sample ID atlas to assess the
changes in CVR over time in a normal subject not included in the ID atlas. We
can see that the majority of difference between day 1 and day 2 in the healthy
subject < 1.0 SD as expected.
Figure 11 represents an example of the application in a patient from our
database. The patient was a 38 year old female who was diagnosed with
bilateral moya moya and had a right EC-IC bypass. A CVR was preformed both
pre- and post-surgery. CVR pre-surgery (Figure 11A) displays severe right side
impairment with decreased CVR in the left MCA territory. Post-surgery CVR
suggests that the bypass on the right side reversed the steal and improved the
flow, resulting in steal from the left MCA territory.
The z-maps provide additional information, suggesting that the areas of
impaired CVR on the left =have in fact improved after surgery when compared to
a
normal cohort. The ID atlas was then applied to determine whether the z-map
changes could be due to variability in the testing over time rather than the
intervention (Figure 11B). The ID z-maps confirmed, and gave an indication of
the extent and distribution of, changes in CVR.
Identifying pathophysiology and distinguishing changes over time
49
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
is capable of determining the voxelwise probability of a true, clinical
interval
change in CVR between two scans. The above described z-maps consisted of a
database of voxelwise mean and standard deviation of CVR suitable for
identifying the probabilities and extent of abnormality of CVR, Z-maps can be
threshokled to alter the balance of sensitivity and specificity in identifying
abnormal voxels. Identifying significant changes in a single subject as the
voxel
statistic does not depend on when the scan is performed, and thus includes the
test to test variability.Therefore according to one aspect of the invention,
we
separately determined, voxel-by-voxel, location-specific statistical
probabilities for
changes between scans not attributable to technological and physiological
variability. This capability of identifying changes between scans is useful
for
carrying out longitudinal studies such as following the progress of disease
and
the effects of interventions. As with z-maps, the range of thresholds from 0.5
to
2.0 would provide a range of high sensitivity, low specificity to high
specificity, low
sensitivity. We used a sample atlas of scan-to-scan differences in 12 healthy
male subjects which we used to evaluate 15 patients in our database with known
cerebrovascular disease. Greater sensitivity would accrue the more the
subjects
used in the atlas reflect factors that affect CVR in the target population;
greater
specificity would accrue from larger cohort numbers and wider inclusion
criteria in
the atlas population.
Technical and physiological sources of variability in z maps
The concept of a voxel-by-voxel comparison of the intensity of an image to
that of
a normal cohort has been extensively explored (Commowick et al., 2008; Kemp
et al., '1995; Laliberte et al., 2004),
but it has not been applied to CVR using BOLD MRI as a surrogate for cerebral
blood flow.
Minimizing variability in CVR due to variation in the stimulus
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
The infusion of pharmacologio agents result in a variability of blood levels,
even
to a standard dose; there is also a variation of vasodilatory response to a
given
blood level of drug (see (Fierstra et al., 2013) for discussion). Hypercapnia
may
result in a more reliable response to a blood partial pressure of CO2 (PaCO2),
the
stimulus affecting cerebral blood flow (Kety and Schmidt, 1948); but attaining
a
repeatable PaCO2 is difficult. Infusing CO2 into a face mask, (Markus and
Harrison, 1992) inhaling a fixed concentration of CO2, (van der Zande et al_,
2005)or simply breath holding (Silvestrini et al., 1999) are not reproducible,
and
cannot even provide a reliable measure of the change in the PaCO2 (Hoskins,
1990; Mark et al., 2010; Prisman et aL, 2007; Sasse et al., 1996). In this
study
we used a computer-controlled gas blender to prospectively target PETCO2,
which has equilibrated with the PaCO2 (Ito et al., 2008). This allowed us to
repeatedly administer a standardized stimulus (from baseline PETCO2was 40.2
1.1 (SD) mmHg to 49.9 1.5 mmHg), minimizing the variability in the atlas and
in
the patient scans attributable to variability of the stimulus and optimizing
the
sensitivity of detecting interval changes in CVR.
Accounting for the variability in CVR due to variation in the signal
Despite the precise designation of the MRI scanning sequences and data
analysis, there are technical causes for variation in the CVR. During signal
acquisition, the signal is affected in random ways due to drift; there may be
a drift
in signal over time affecting all voxels and separate drift in individual
voxels. This
51
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
drift is usually described by a polynomial and has no consistent pattern or
direction with time over the long term. Thus ID z maps provide a confidence
interval for identifying changes outside those attributable to technical and
physioiogic (day-to-day physiology or vasodilatory stimulus) signal changes.
As
the technical issues result in highly variable changes voxel by voxel, one
would
expect the observation of systematic changes in contiguous voxels--even if
small
compared to day-to-day variability--to reflect pathophysiologic changes. Thus,
like with the z maps, different balance between sensitivity and specificity
may
occur at different thresholds.
Change in CVR over time in two patients with cerebrovascular disease
We presented the clinical course of two patients with steno-occlusive disease
in
intracranial vessels. In both cases, the symptoms were mild and transient. In
contrast, the stenosis of the intracranial vessels as seen by angiography,
were
relentlessly progressive. The CVR values were more nuanced, reflecting the
balance of blood flow resulting from the establishment of spontaneously
developing, and surgically established, collateral blood flow. The total blood
flow
in both patients apparently remained above the threshold required to sustain
neuronal function and cellular integrity preventing an acute stroke as gauged
by
the absence of ischemia and absence symptoms during follow-up examination. In
these patients, the advanced analysis of the CVR data the ED z maps introduced
in this paper ostensibly improved the resolution of underlying subclinical
pathophysiologic changes not apparent from angiography and CVR maps alone.
52
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
References identified herein are hereby incorporated by reference.
Reference List
Commowick 0, Fillard P, Clatz 0, & WaffleId SK (2008). Detection of DTI white
matter abnormalities in multiple sclerosis patients. Med Image Comput Comput
Assist Interv 11, 975-982.
Cox RW (1996). AFN1: software for analysis and visualization of functional
magnetic resonance neuroimages. Comput Biomed Res 29, 162-173.
Harper AM & Glass HI (1965). Effect of alterations in the arterial carbon
dioxide
tension on the blood flow through the cerebral cortex at normal and low
arterial
blood pressures. J Neurol Neurosurg Psychiatry 28, 449-452.
Hoskin PJ, Abdelath 0, Phillips H, Gilligan S, Saunders MI, Broderick P, &
Baddeley H (1999). Inspired and expired gas concentrations in man during
carbogen breathing. Radiother Oncol 51, 175-177.
Ito S, Mardimae A, Han J, Duffin J, Wells G, Fedorko L, Minkovich L,
Katznelson
R, Meineri M, Arenovich T, Kessler C, & Fisher JA (2008). Non-invasive
prospective targeting of arterial P(CO2) in subjects at rest. J Physiol 586,
3675-
3682.
Kassner A, Winter JD, Poublanc J, Mikulis DJ, & Crawley AP (2010). Blood-
oxygen level dependent MRI measures of cerebrovascular reactivity using a
controlled respiratory challenge: reproducibility and gender differences. J
Magn
Reson Imaging 31, 298-304.
53
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Kazumata K, Tanaka N, Ishikawa T, Kuroda S, Houkin K, & Mitsumori K (1996).
Dissociation of vasoreactivity to acetazolamide and hypercapnia. Comparative
study in patients with chronic occlusive major cerebral artery disease. Stroke
27,
2052-2058.
Kemp PM, Houston AS, Macleod MA, & Pethybridge RJ (1995). Cerebral
perfusion and psychometric testing in military amateur boxers and controls. J
Neural Neurosurg Psychiatry 59, 368-374.
Kety SS & Schmidt CF (1948). The effects of altered arterial tensions of
carbon
dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of
normal young men. J Clin Invest 27, 484-492.
Kleiser B, Krapf H, & Widder B (1991). Carbon dioxide reactivity and patterns
of
cerebral infarction in patients with carotid artery occlusion. J Neurol 238,
392-394.
Kuroda S, Houkin K, Kamiyama H, Mitsumori K, Iwasaki Y, & Abe H (2001).
Long-term prognosis of medically treated patients with internal carotid or
middle
cerebral artery occlusion: can acetazolamide test predict it? Stroke 32, 2110-
2116.
Laliberte JF, Meunier J, Mignotte M, & Soucy JP (2004). Detection of diffuse
abnormal perfusion in SPECT using a normal brain atlas. Neuroimage 23, 561-
568.
Lundar T, Lindegaard KF, Froysaker T, Aaslid R, Grip A, 8, Nornes H (1985).
Dissociation between cerebral autoregulation and carbon dioxide reactivity
during
nonpulsatile cardiopulmonary bypass. Ann Thorac Surg 40, 582-587.
54
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Mandell DM, Han JS, Poublanc J, Crawley AP, Fierstra J, Tymianski M, Fisher
JA, & Mikulis DJ (2011). Quantitative Measurement of Cerebrovascular
Reactivity
by Blood Oxygen Level-Dependent MR Imaging in Patients with Intracranial
Stenosis: Preoperative Cerebrovascular Reactivity Predicts the Effect of
Extracranial-Intracranial Bypass Surgery. AJNR Am J Neuroradiol 32, 721-727.
Mandell DM, Han JS, Poublanc J, Crawley AP, Stainsby JA, Fisher JA, & Mikulis
DJ (2008). Mapping cerebrovascular reactivity using blood oxygen level-
dependent MRI in Patients with arterial steno-occlusive disease: comparison
with
arterial spin labeling MRI. Stroke 39, 2021-2028.
Mark CI, Slessarev M, Ito S, Han J, Fisher JA, & Pike GB (2010). Precise
control
of end-tidal carbon dioxide and oxygen improves BOLD and ASL cerebrovascular
reactivity measures. Magn Reson Med_
Markus H & Cullinane M (2001). Severely impaired cerebrovascular reactivity
predicts stroke and TIA risk in patients with carotid artery stenosis and
occlusion.
Brain 124, 457-467.
Markus HS & Harrison MJ (1992). Estimation of cerebrovascular reactivity using
transcranial Doppler, including the use of breath-holding as the vasodilatory
stimulus
6. Stroke 23, 668-673.
Mikulis DJ, Krolczyk G, Desal H, Logan W, Deveber G, Dirks P, Tymianski M,
Crawley A, Vesely A, Kassner A, Preiss D, Somogyi R, & Fisher JA (2005).
Preoperative and postoperative mapping of cerebrovascular reactivity in
moyamoya disease by using blood oxygen level-dependent magnetic resonance
imaging. J Neurosurg 103, 347-355.
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Nishimura S, Suzuki A, Hatazawa J, Nishimura H, Shirane R, Yasui N, &
Yoshimoto T (1999). Cerebral blood-flow responses to induced hypotension and
to CO2 inhalation in patients with major cerebral artery occlusive disease: a
positron-emission tomography study. Neuroradiology 41, 73-79.
Ogasawara K, Ogawa A, & Yoshimoto T (2002). Cerebrovascular reactivity to
acetazolamide and outcome in patients with symptomatic internal carotid or
middle cerebral artery occlusion: a xenon-133 single-photon emission computed
tomography study. Stroke 33, 1857-1862.
Prisman E, Siessarev M, Han J, Poublanc J, Mardimae A, Crawley A, Fisher J, &
Mikulis D (2007). Comparison of the effects of independently-controlled end-
tidal
PC0(2) and PO(2) on blood oxygen level-dependent (BOLD) MRI. J Magn
Reson Imaging.
Ringelstein EB, Sievers C, Ecker S, Schneider PA, & Otis SM (1988).
Noninvasive assessment of CO2-induced cerebral vasomotor response in normal
individuals and patients with internal carotid artery occlusions. Stroke 19,
963-
969.
Sasse SA, Berry RB, Nguyen TK, Light RVV, & Mahutte CK (1996). Arterial blood
gas changes during breath-holding from functional residual capacity. Chest
110,
958-964.
Silvestrini M, Vernier' F, Troisi E, Passarelli F, Matteis M, Pasqualetti P,
Rossini
PM, & Caltagirone C (1999a). Cerebrovascular reactivity in carotid artery
occlusion: possible implications for surgical management of selected groups of
patients. Acta Neurol Scand 99, 187-191.
56
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Silvestrini M, Vernieri F, Trois' E, Passarelli F, Matteis M, Pasqualetti P,
Rossini
PM, & Caltagirone C (1999b). Cerebrovascular reactivity in carotid artery
occlusion: possible implications for surgical management of selected groups of
patients
17. Acta Neurol Scand 99, 187-191.
van der Zande FH, Hofman PA, & Backes WH (2005). Mapping hypercapnia-
induced cerebrovascular reactivity using BOLD MRI
1. Neuroradiology 47, 114-120.
Vorstrup S, Brun B, & Lassen NA (1986). Evaluation of the cerebral
vasodilatory
capacity by the acetazolamide test before EC-IC bypass surgery in patients
with
occlusion of the internal carotid artery. Stroke 17, 1291-1298.
Webb J, Guimond A, Eldridge P, Chadwick D, Meunier J, Thirion JP, & Roberts N
(1999). Automatic detection of hippocampal atrophy on magnetic resonance
images. Magn Reson Imaging 17, 1149-1161.
Yonas H, Smith HA, Durham SR, Pentheny SL, & Johnson DW (1993). Increased
stroke risk predicted by compromised cerebral blood flow reactivity. J
Neurosurg
79, 483-489.
Supplemental Reference List
Sobczyk 0, Battisti-Charbonney A, Fierstra J, Mandell DM, Poublano J, Crawley
AP, Mikulis DJ, Duffin J, Fisher JA., A conceptual model for CO2-induced
redistribution of cerebral blood flow with experimental confirmation using
BOLD
MRI. Neuroimage. 2014 Feb 5;92C:56-68.
57
CA 02946619 2016-10-21
WO 2015/161363
PCT/CA2015/000274
Y. C. Tzeng, P. N. Ainslie, W. H. Cooke, K. C. Peebles, C. K. Willie, B. A.
MacRae, J. D. Smirl' H. M. Horsman, and C. A. Rickards. Assessment of cerebral
autoregulation: the quandary of quantification. Am J Physiol Heart Circ
Physiol
303: H658¨H671, 2012.
58