Note: Descriptions are shown in the official language in which they were submitted.
CA 02947079 2016-11-01
APPARATUS FOR VOLATILE ORGANIC COMPOUND (VOC) DETECTION
[0001] TECHNICAL FIELD
[0002] The present invention provides an apparatus for detecting and
differentiating volatile
organic compounds (VOC). In particular, this invention relates to gas
detection apparatus
having a coated channel and a gas sensor.
[0003] BACKGROUND
[0004] There is a need for rapid, sensitive and high precision detectors of
volatile organic
compound (VOC) gases for different applications including beverage and food
quality
assessment [1], analytical chemistry [2], biological diagnosis [3-5], and
safety and
environmental monitoring [6]. Numerous approaches have been developed for
detection of
VOCs. Gas chromatography (GC) [7] and mass spectrometry (MS) [8] are the most
commonly
used methods which provide high sensitivity and selectivity. However,
miniaturization of these
methods which is required for numerous emerging applications [9-10] is
challenging due to the
complexity of their fabrication, calibration and sample extraction processes.
Moreover, their
high cost and long processing time hinder the implementation of these
techniques to
applications which require disposable and rapid detection methods [11].
[0005] More recently, electronic noses (e-nose) have been used as an
alternative method of
gas detection. E-nose systems are based on sensor arrays coupled with pattern
recognition
systems. In an e-nose system, the gas sensor array provides a fingerprint
response to a given
odor; then, a pattern recognition software tool is used to perform odor
identification and
discrimination [12-13]. Despite the general success of electronic noses, there
are practical
challenges in adaptation of this technology: in essence, the inevitable
multidimensional drifts of
the components of the sensor array result in frequent replacement of the
expensive parts and
cumbersome recalibrations [14]. Moreover, since general-purpose gas sensors
are not selective
against different gases, the sensor array used in e-noses is required to have
a specific sensor for
detecting each target gas. This makes the drift compensation and sensor
recalibration even
more complicated [15-16].
[0006] Recently, microfluidic-based gas detectors with high selectivity and
sensitivity
features of both traditional methods (GC and MS) and e-noses have been
introduced [17-21].
These systems function based on analyzing the kinetic response of diffused
gases in micro-
channels using a single general purpose gas sensor [18-21]. As each gas has
different diffusion
and physical adsorption rates, microfluidic-based gas detectors successfully
differentiate among
1
CA 02947079 2016-11-01
the components of a mixture (and even binary mixtures of different isomers)
[20]. Although
these devices are selective to different gases, they cannot differentiate
among components of
complex mixtures at low concentrations. Moreover, due to the slow process of
gas diffusion in
the microchannels and also chemical adsorption of gas molecules to the channel
walls, the
recovery process of fabricated sensors takes relatively long time (up to 10
minutes) [20]. It has
been recognized that the diffusion constants of a target gas depends on the
temperature of the
diffusion medium [29] and clearance of a channel may be accomplished by
providing flow of air
or a pure gas in the opposite direction of the diffusion process [29].
However, the design of
microfluidic-based gas detectors must be further optimized to improve their
performance.
[0007] SUMMARY
[0008] The present invention is based in part on the discovery that
different channel coating
materials can have a beneficial effect the performance of the microfluidic-
based gas detectors.
In particular, 11 different coating combinations for the channel were
compared. Moreover, the
geometry of the channel was optimized to study the effect of channel
dimensions on the
selectivity and recovery time of the device. To show the diagnostic power of
the developed
miniaturized gas detector, in terms of differentiating small concentrations
(ppm level) of
different volatile organic compounds (VOCs), a range of different target gases
including alcohol
and ketone vapors; methanol and tetrahydrocannabanol (THC) were tested and
successfully
differentiated. As described herein, the selectivity of microfluidic gas
detectors may be
significantly enhanced by optimizing the micro-channel geometry and surface
treatment.
Moreover, the sensor recovery time may be reduced to 150 seconds, which is
significantly faster
than the recovery time reported in previous studies [20]. Furthermore, the
integration of
heaters along the micro-channels to enhance the diffusion rate of the THC
molecules in the
channel and decreasing the sensor response and recovery time to below 200 s.
Accordingly, the
improvements described herein may advance the state-of-the-art gas analysis
methods, but
especially for applications [22] requiring real-time sensing.
[0009] In accordance with a first embodiment, there is provided a gas
detection apparatus,
the apparatus including: (a) a channel having an inner surface and having at
least one opening,
such that the channel may be in fluid communication with a sample gas through
the opening,
the inner surface having a coating including: (i) a first layer comprising a
non-reactive metal or
non-reactive metalloid compound; (ii) a second layer comprising a moisture
barrier; and (b) a
gas sensor disposed within the channel.
2
CA 02947079 2016-11-01
[0010] In
accordance with a further embodiment, there is provided a gas detection
apparatus, the apparatus including: (a) a channel having an inner surface and
having at least
one opening, such that the channel may be optionally in fluid communication
with a sample gas
when the opening is in an open position and optionally not in fluid
communication when the
opening is in a closed position, the inner surface may have a coating
including: (i) a first layer
comprising a non-reactive metal or non-reactive metalloid compound; (ii) a
second layer
comprising a moisture barrier; and (b) a gas sensor disposed within the
channel.
[0011] In
accordance with a further embodiment, there is provided a gas detection
apparatus, the apparatus including: (a) a channel having an inner surface and
having at least
one opening, such that the channel may be optionally in fluid communication
with a sample gas
when the opening is in an open position and an optional closed position, the
inner surface may
have a coating including: (i) a first layer comprising a non-reactive metal or
non-reactive
metalloid compound; (ii) a
second layer comprising a moisture barrier; and (b) a gas sensor
disposed within the channel.
[0012] In accordance with a further embodiment, there is provided an apparatus
comprising
the gas detection apparatus described herein for use in a Tetrahydrocannabinol
(THC)
breathalyzer.
[0013] In accordance with a further embodiment, there is provided an apparatus
comprising
the gas detection apparatus described herein for use in natural gas leakage
detection.
[0014] In
accordance with a further embodiment, there is provided an apparatus
comprising
the gas detection apparatus described herein for use in nuisance sewer gas
detection.
[0015] The second layer may include a moisture barrier has a gas
permeability sufficient to
absorb the gas particles being sampled. The non-reactive metal may be selected
from one or
more of the following: copper; chromium; ruthenium; rhodium; palladium; gold;
silver;
osmium; iridium; platinum; titanium; niobium; tantalum; bismuth; tungsten;
tin; nickel; cobalt;
manganese; and zinc; or (ii) may be metalloid compound is SiO2. The moisture
barrier with
high porosity may be Parylene or Polydimethylsiloxane (PDMS). The Parylene may
be selected
from Parylene C, Parylene N or Parylene D. The Parylene may be Parylene C. The
non-reactive
metal may be selected from one or more of the following: copper; chromium;
ruthenium;
rhodium; palladium; gold; silver; iridium; platinum; titanium; niobium; and
tantalum. The
coating may be chromium, gold and Parylene C. The channel may further include
a heater. The
heater may be operable to increase the channel temperature to at least 8o C.
The gas sensor
may be a Metal Oxide Semiconductor (MOS). The gas sensor may be a tin oxide-
based
chemoresistive gas sensor. There may be more than one gas sensor in the
channel. There may
3
CA 02947079 2016-11-01
be a pluralitiy of channels with one sensor per channel. There may be a
pluralitiy of channels
with more than one gas sensor in the channel. The channel length to channel
depth ration may
be 15o:i. The channel width to channel depth ration may be 3:1. The channel
length may be
3mm wide, 30 mm long and 200 pLM deep. The first layer may include chromium
and gold.
The chromium may be applied to the channel prior to the gold. The second layer
may include
Parylene C. The first layer may include Si02. The second layer may include
Parylene C. The
opening may further include a closed position. The opening may further include
a open
position. The opening may include an open and a closed position. The apparatus
may further
include a second opening. The second opening may have both an open and closed
position.
[0016] The apparatus may further include a liquid trap positioned in fluid
communication
with the at least one opening. The apparatus may further include a humidity
filter positioned in
fluid communication with the at least one opening. The apparatus may further
include may
further include a pump which may optionally be in fluid communication with the
second
opening. The apparatus may further include a compressed air source which may
optionally be
in fluid communication with the channel. The apparatus may further include a
compressed gas
source which is optionally in fluid communication with the channel. The
apparatus may further
include a pentane plume which may optionally be in fluid communication with
the channel. The
apparatus may further include a compressed 02 source or N2 source or separate
02 and N2
sources which may optionally be in fluid communication with the channel. The
apparatus may
further include a cleaning solution which may optionally be in fluid
communication with the
channel. The compressed gas source may be selected from one or more of the
following: air;
pentane; CO2; 02; or N2. The more than one compressed gas source, may be
selected from the
following: air; pentane; CO2; 02; or N2*
BRIEF DESCRIPTION OF THE DRAWINGS
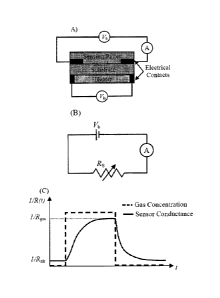
FIGURE 1 shows a schematic of a chemo-resistor (MOS gas sensor) and it's bias
circuit, where
Vb is the bias voltage for the sensor and VII is the voltage across the heater
(A); (B) shows the
equivalent electrical circuit of the sensor in a DC bias; and (C) shows a
typical response of a
sensor exposed to a certain concentration of a certain gas, wherein i/Rair and
i/Rgas are the
conductances of the sensor in clean air and after exposure to a gas,
respectively.
[0017] FIGURE 2 shows a schematic of a MOS gas sensor and its bias circuit
exposed to
two different gases in (A); typical transient responses of the sensor to two
different gases (. and
A) are almost the same in (B); a schematic of a MOS gas sensor integrated with
a micro-channel
and its bias circuit exposed to two different gases in (C); typical transient
responses of the
4
CA 02947079 2016-11-01
microfluidic-based gas sensor to two different gases (= and A) are distinct in
(D); and a
schematic of the gas sensor integrated with a micro-channel is shown in (E),
wherein analyte
molecules diffuse into the channel, and some of the molecules get adsorbed
while some of the
adsorbed molecules get desorbed.
[0018] FIGURE 3 shows a schematic of the experimental setup in (A), wherein
the sensor
is mounted on a chamber, while three different positions (i.e. Bl; B2; and B3)
are overlaid on a
typical normalized transient response of the sensor to a concentration of a
gas, with Bi showing
the analyte injection position; B2 showing the exposure position; and B3
showing the recovery
position.
[0019] FIGURE 4 shows a schematic diagram of an embodiment of a 3D-printed gas
detector, having a channel coated with chromium (Cr), gold (Au), and Parylene
C, wherein the
Cr forms a part of the first layer with Au and the Parylene C forms the second
layer of the
channel.
[0020] FIGURE 5 shows normalized responses from six sensors with six different
coating
material combinations deposited on the channel to 2000 ppm Ethanol (coatings
are as follows:
(1) Si02 and Parylene C; (2) Parylene C alone; (3) Copper and Parylene C; (4)
chromium/gold
and Parylene C; (5) chromium and gold; and (6) chromium/gold and Cytonix).
[0021] FIGURE 6 shows normalized responses for three different analytes
(i.e. ethanol (-);
methanol (0); and acetone( A)) with four different channel coatings, as
follows: (A) Si02 and
Parylene C, (B) Cr and Au, (C) Cu and Parylene C, (D) Cr and Au and Parylene
C.
[0022] FIGURE 7 shows typical normalized responses for three different
analytes (i.e.
ethanol (-); methanol (0); and acetone( A)), wherein the separation factor is
defined to show the
differentiation power of the sensor.
[0023] FIGURE 8 shows a feature space for the sensor with the coating
combination of Cr
and Au and Parylene C, which had the best performance for three VOCs (Acetone:
V, Ethanol: X,
and Methanol: 0) in terms of selectively and recovery time.
[0024] FIGURE 9 shows normalized responses for three different analytes
(i.e. ethanol (-);
methanol (0); and acetone( A)) for four different channel dimensions, as
follows: 1=20 MM;
d=500 1AM (A); 1=30 mm, d=500 pm (B); 1=40 mm, d=500 im (C); and1=30 mm, d=200
pm
(D).
[0025] FIGURE 10 shows recorded transient responses for 8 different
concentrations (250
ppm-4000 ppm) for 6 different targets, including three alcohols: 2-Pentanol
(A), Methanol (B),
Ethanol (C), and three ketone: Acetone (D), 2-butanone (E), 2-pentanone (F).
CA 02947079 2016-11-01
(0026] FIGURE ii shows a feature space presentation for all the responses
shown in
FIGURE 10.
[0027] FIGURE 12 shows a schematic of a breath-analyzer prototype.
[0028] FIGURE 13 shows normalized responses of the sensor to (A) THC-methanol,
and
(B) pure methanol at different temperatures, wherein the 3D feature space is
shown for (C)
THC-methanol and (D) pure Methanol, and features Fi. and F2 are the points in
time at which
the normalized response level reaches 5% and 95% of the maximum level,
respectively, and F3 is
the magnitude of the normalized response at the final read out, wherein (25 C:
V, 40 C: X, and
80 C: 0).
[0029] FIGURE 14 shows normalized responses for two different analytes A (.);
and B (s);
the selectivity factor is defined to examine the differentiation power of the
sensor is shown in (A)
and the sensor response time and selectivity factor between binary mixture of
THC-methanol
and methanol vs. channel temperature is shown in (B).
[0030] FIGURE 15 shows a scanning electron micrograph (SEM) to demonstrate
pore size
of a typical the channel surface coated with parylene C (i.e. pore size is
about 50 nm, with a
range of between 36 nm and 84 nm).
[0031] FIGURE 16 shows a typical transient response of the sensor to a
concentration of a
gas in (A); and the feature extraction method used for identification of the
concentration of the
analyte is presented in (B), wherein the three selected features are the
maximum level of the
transient response (Fl), the response level at the final readout (F2), and the
area under the
transient response curve (F3).
[0032] FIGURE 17 shows the transient response of the sensor to three different
concentrations of ethanol, i.e., moo ppm (X), 2000 ppm (0), and 3000 ppm (V)
in (A); and the
feature space (using the method described in FIGURE 16) is presented for
identification of the
concentration of the analyte in (B).
[0033] FIGURE 18 shows the regression model used for characterization of the
concentration of the analyte (C) with respect to the area underneath the
transient response
curve (A), wherein the relation between the concentration and the average of
the area
underneath the curves is linear and each square marker is the average of 5
points and the error
bars present the deviation from the average.
[0034] FIGURE 19A shows a schematic of an embodiment for a pentane detector
using a
single microfluidic sensor, wherein the sensor uses solenoid valves to expose
the sensor to the
6
CA 02947079 2016-11-01
pentane plume prior to recovery with compressed on-board gas and the purging
air exits
through the exhaust valve.
[0035] FIGURE 19B shows a schematic of an embodiment for a UAV-mountable
detector
for NG leakage monitoring, having a valve network and sensor array ensures
rapid sampling of
surrounding air for fugitive NG, with compressed air or another source of
clean air
(uncontaminated with target gases) to recover the sensor.
[0036] FIGURE 20 shows a schematic of an embodiment for nuisance sewer gas
detector
including the supporting systems and sensing unit.
DETAILED DESCRIPTION
[0037] Any terms not directly defined herein shall be understood to have the
meanings
commonly associated with them as understood within the present field of art.
Certain terms are
discussed below, or elsewhere in the specification, to provide additional
guidance to the
practitioner in describing the compositions, devices, methods and the like of
embodiments, and
how to make or use them. It will be appreciated that the same thing may be
said in more than
one way. Consequently, alternative language and synonyms may be used for any
one or more of
the terms discussed herein. No significance is to be placed upon whether or
not a term is
elaborated or discussed herein. Some synonyms or substitutable methods,
materials and the
like are provided. Recital of one or a few synonyms or equivalents does not
exclude use of other
synonyms or equivalents, unless it is explicitly stated. Use of examples in
the specification,
including examples of terms, is for illustrative purposes only and does not
limit the scope and
meaning of the embodiments described herein.
[0038] The most widely-used type of gas sensors is Metal Oxide Semiconductor
(MOS) gas
sensors [23]. In the basic configuration of MOS sensors, which is shown in
FIGURE IA, a
chemo-resistor is made by deposition of a thick film metal oxide sensing
pallet and a thick film
thermo-resistor micro-heater on the opposite surfaces of a millimeter-scale
ceramic substrate
[23].
[0039] The electrical behavior of a MOS sensor in a DC bias can be modeled as
a variable
resistance Rs (see FIGURE 1B). The value of this resistance depends on the
type of the gas
molecule, the gas concentration, and the temperature of the sensing pallet.
The resistance of the
sensor in the clean air is called baseline resistance (Rail.). The sensitivity
(S) of such a sensor is
defined by
Rair
= - , (1)
Rgas
7
CA 02947079 2016-11-01
where Rair and Rgas are the resistances of the sensing pallet measured in the
clean air and target
gas, respectively (see FIGURE iC). The selectivity of a sensor between two
gases (i, j) is defined
by
s =
Sel(i, j) = , (2)
S =
where Si and Sj are the sensitivity of the gas sensor to gas i and j,
respectively.
[0040] Current off-the-shelf gas sensors are inexpensive and durable,
however, they are
either made to be evenly sensitive to different gases or fabricated for
detecting a single specific
target. Hence, differentiating among different gases or gas mixtures using a
single sensor is very
challenging, as the transient responses of the sensor to two different gases
are almost the same.
The schematic of a MOS gas sensor and its bias circuit and responses of the
sensor to two
different gases are depicted in FIGURES 2A and 2B. To enhance the selectivity
of the gas
sensor, it can be integrated into a microfluidic channel. The schematic of a
MOS gas sensor
equipped with a channel and its bias circuit is shown in FIGURE 2C. The
microfluidic-based
gas sensor can provide distinct kinetic responses for different gases (see
FIGURE 2D). The
response of such a sensor is dependent on (a) the analyte diffusivity in the
surrounding media
(air), and (b) the physical adsorption/desorption rate of the gas molecules
to/from the channel
walls (see FIGURE 2E).
[0041] The analyte concentration, C(x, t), changes along the channel over
time as a result of
diffusion of the gas molecules into the channel. The gas concentration can
mathematically be
predicted by the solving the diffusion¨ physical adsorption (physisorption)
equation [213] of
(1 2Ca a )ac(x,t) D 02c(x,t)
(3)
d (i+a c(x,t))) at a X2
[0042] where Ca is the number of the surface adsorption sites available per
unit volume of
the channel, a is a modified Langmuir constant, d is the effective
microfluidic channel depth,
and D is the analyte diffusion coefficient (diffusivity) in air [24].
[0043] As used herein "gas permeability" refers to the rate at which a gas or
vapor passes
through the channel coating. The gas permeability process includes absorption
of the gas or
gases into the channel coating and subsequent desorption of the of the gas or
gases from the
channel coating. The second layer may include a moisture barrier having a gas
permeability
sufficient to absorb and desorb the gas particles being sampled. Accordingly,
the coatings may
be optimized for the testing of a particular sample. Factors which may affect
permeability of a
polymer include the following: chain packing; side group complexity; polarity;
crystallinity,
orientation; fillers; humidity; and plasticization. Furthermore, the non-
reactive metals and
8
CA 02947079 2016-11-01
non-reactive metalloid compounds used are non-porous and have very low
permeability as
compared to parylene C, which will stop the gas from going down and reaching
to the substrate
or the channel and facilitate desorption of the VOC.
[0044] Gas permeability is significant, since sufficient permeability is
needed to adsorb and
desorb the gas molecules. The molecular dimensions of most VOCs are couple of
angstroms so
they can diffuse into the voids of Parylene C (which are on average about 50
nm, see FIGURE
15) and reach to the first layer of the channel.
[0045] TABLE 1: Properties of Parylene N, C and D
Parylene Barrier Parylene N Parylene C Parylene D
Properties
Nitrogen Gas 7.7 0.95 4.5
Permeability (cm3-
mil/loo in2-24hr-atm
(23 C))
Oxygen Gas 30 7.1 32
Permeability (cm3-
mil/ioo in2-24hr-atm
(23 C))
Carbon Dioxide Gas 214 7.7 13
Permeability (cm3-
mil/loo in2-24hr-atm
(23 C))
Hydrogen Sulfide Gas 795 13 1.45
Permeability (cm3-
mil/100 in2-24hr-atm
(23 C))
Sulphur Dioxide Gas 1,890 11 4.75
Permeability (cm3-
milhoo in2-24hr-atm
(23 C))
Chlorine Gas 74 0.35 0.55
Permeability (cm3-
mil/loo in2-24hr-atm
(23 C))
Moisture Vapor 1.50 0.14 0.25
Transmission
(g-mil/loo in2
-24hr, 37 C, 90% RH)
Data from Para Tech Parylene Property Data Sheet and gathered following
appropriate ASTM methods
[0046] As used herein "porosity" refers to the "void fraction" which is a
measure of the void
or empty spaces in a material, and is calculated as a fraction of the volume
of voids over the total
volume of the material (i.e. between o and 1, or as a percentage between o and
l00%). The
9
CA 02947079 2016-11-01
porosity may be measured with a BET (Brunauer¨Emmett¨Teller) measurement
device or other
surface analysis device. As used herein "porosity" may be a measure of the
"accessible void"
(i.e. the total amount of void space accessible from the surface) or "total
void" as known in the
art. Accordingly, "porosity" may be used as an alternative measure for
determining the
suitability of a particular coating to make up the second layer which includes
a moisture barrier.
[0047] As used herein "moisture barrier" refers to a water impermeable
material or
compound. In some embodiments, a parylene (i.e. poly(p-xylylene) polymers) may
be used to
form the moisture barrier, in part because the parylene polmers may be added
in a thin uniform
layer that is chemically inert. Some common gas permeabilities and moisture
vapor
transmission for Paylenes N, C and D are given in TABLE 1. There are a number
of parylenes
commonly used.
\
(H2C . CH2 1.---
[0048] Parylene N In
[0049] Parylene N has the highest dielectric strength of the three
versions, and a dielectric
constant value independent of frequency. It is able to penetrate crevices more
effectively than
the other two versions because of the higher level of molecular activity that
occurs during
deposition. Parylene N is commonly used in high frequency applications because
of its low
dissipation factor and dielectric constant values.
CI
--(H2C 40 CH2)
[0050] Parylene C n
[0051] Parylene C differs chemically, having a chlorine atom on the benzene
ring that results
in a useful combinationof electrical and physical properties including
particularly low moisture
and gas permeability. This version deposits on substrates faster is than
Parylene N, with a
consequent reduction in crevice penetration activity.
/ CI
H2c 4. CH2)7
\
[0052] Parylene D CI
[0053] Parylene D has two chlorine atoms added to the benzene ring. This
gives the
resulting film greater thermal stability than either Parylene N or C, but
Prylene D has reduced
ability to penetrate crevices as compared to Parylenes N and C.
[0054] As used herein "reactivity" refers to the tendency of a substance
(i.e. an element or
compound) to undergo a chemical reaction, either by itself or with other
substances. However,
CA 02947079 2016-11-01
all elements and compounds (except helium) undergo at least some chemical
reactions under
the proper conditions.
[0055] As used herein "non-reactive" refers to a reduced or limited tendency
of a substance
(i.e. an element or compound) to undergo a chemical reaction, either by itself
or with other
substances and not a complete absence of reactivity. Furthermore, a non-
reactive element or
compound will still undergo physical reactions (adsorption and desorption)
with the VOCs
diffusing through the channel.
[0056] A non-reactive metal may be selected from one or more of the following:
copper;
chromium; ruthenium; rhodium; palladium; gold; silver; osmium; iridium;
platinum; titanium;
niobium; tantalum; bismuth; tungsten; tin; nickel; cobalt; manganese; and
zinc. The non-
reative metalloid compound may be Si02.
[0057] METHODS AND MATERIALS
[0058] Gas Detector Setup
[0059] The schematic diagram of the experimental setup is shown in FIGURE 3A.
The
device consists of a gas chamber, three-dimensional (3D) printed microfluidic
channel and gas
sensor. The sample in liquid phase is injected into the chamber through its
opening using a
precise Pipet-Lite XLSTM microsampler (analyte injection stage shown in FIGURE
3B1). After
a few minutes, the sample is evaporated into the chamber. The sensor is
rotated around the
hinge and exposed to the gas inside the 1 L polymethyl methacrylate (PMMA)
chamber for 40
seconds (exposure stage shown in FIGURE 3B2). The gas molecules diffuse into
the micro-
channel and reach the sensing pallet of the sensor, which is placed at the
other end of the
channel. The competition between the diffusion process and adsorption of the
gas molecules to
the available adsorption sites on the channel walls creates a unique response
of the sensor (also
known as the smell-print). The different smell-prints of gases result in
selective sensing of
different gases. Finally, the sensor is rotated back to its original position
where it is exposed to
clean air again and the gas molecules diffuse out from the channel (recovery
stage shown in
FIGURE 3B3). Alternatively, the channels could be flushed with clean air or
gas (for example,
02 or CO,) to shorten the recovery time. The data may be collected (using a
microprocessor) for
100 seconds. The device remains in this position for 150 seconds or less where
the channel is
flushed before the sensor becomes fully recovered and ready for the next test.
Most of the
experiments were all carried out at the room temperature (25 1 C), and
relative humidity of
40 5%.
11
CA 02947079 2016-11-01
[0060] Feature Extraction
[0061] The typical normalized response of the sensor to a typical gas
concentration is shown
in FIGURE 3. Note that the normalization process eliminates the effects of the
analyte
concentration and baseline variations from the responses. Using equation (2),
the sensor
conductance (G(t) = VR(t)) change is normalized as
G G(t)¨min(G(t))
t) ,( (4)
max(G(t))¨min(G(tV
where Gn(t), min(G(t)) and max(G(t)) are the normalized conductance, minimum
value of the
measured conductance and the maximum value of the measured conductance,
respectively.
Three significant features are extracted and used from each response [20]: a)
tr which is the
time at which the normalized response level reaches 0.05, b) tm which is the
time at which the
normalized response level reaches 0.95, and c) Rf which is the magnitude of
the normalized
response at the final read out. A 3D feature space coordinate is defined based
on tr, tm, and Rf,
where each response is depicted as a point (tr, tm, Re. The regular atmosphere
of the laboratory
is the background media for all the experiments.
[0062] Fabrication Process
[0063] The fabrication process for each component of the system is explained
below: Gas
sensor: A commercially available tin oxide-based chemoresistive gas sensor
(SP3-AQ2, FIS
Inc.TM, Japan) was used in this study. The nominal operating temperature is
300 C was
maintained by applying 5 V DC to the microheater. The bias circuit for the
sensor is depicted in
FIGURE 1.
[0064] Microchannel: The microchannels and micro-chambers were printed with a
3D-
printer (ConnexTM 500), using the material VeroClear RGD81oTM (see FIGURE 4).
To study
the effect of channel dimensions and channel surface treatment on the
selectivity and recovery
time of the sensors, different devices were printed with different channel
sizes. Channels with
six different dimensions including three lengths (2 cm, 3 cm, and 4 cm) and
two heights (200
mm and 500 jam) were fabricated. The width of the channel, which was limited
to the
dimensions of the sensor chamber, was kept at 3 mm for different channel
dimensions.
[0065] Channel Coating: The inner surfaces of the micro-channels were coated
with single
layers and multi-layer combinations of different materials including: gold
(with chromium
under for adhesion), copper, CytonixTM (Cytonix LLCTM, Product: PFCM 1104V),
and Parylene C
(poly (p-xylylene) polymer, CAS No: 28804-46-8). The total number of ii
sensors (listed in
12
CA 02947079 2016-11-01
TABLE 2) were fabricated using different material combinations for the channel
coating. For
some of the targets (such as Au, Cr, Cu, and Si02) the channel surfaces were
coated using
Physical Vapor Deposition (PVD) sputtering machine (Angstrom EngineeringTM,
NexdepTM
deposition system). Parylene C was coated using a Chemical Vapor Deposition
(CVD) Parylene
C coating machine (SCSTM. PDS 2010 LabcoaterTm), and for the CytonixTM the dip
in and spin
coating methods were both used. Inner surfaces of the microchannel shown in
FIGURE 4 were
coated with multi-layer materials including 65 nm gold (with 35 nm chromium
under for
adhesion) and 4 [tm Parylene C.
[0066] TABLE 2 Different Channel Coating Used for Sensor Fabrication
Number Single Layer/Multilayer Coating Coating Method
1 VeroClear RGD8i0 No coating (3D printed
material)
2 Copper (Cu) Sputtering
3 Chromium (Cr) & Gold (Au) Sputtering
4 Parylene C CVD
Si02 Sputtering
6 Cytonix Spin Coating
7 Cu & Cytonix
Sputtering (Cu) & Spin coating (Cytonix)
8 Cr &Au & Cytonix Sputtering (Cr and Au) &
Spin coating
(Cytonix)
9 Cu & Parylene C Sputtering (Cu) & CVD (Parylene C)
113 Cr & Au & Parylene C
Sputtering (Cr and Au) 8z CVD (Parylene C)
11 Si02 & Parylene C Sputtering (Si02) & CVD
(Parylene C)
[0067] Chamber: A small opening on the chamber (made of PMMA) was provided for
both
analyte injection and purging clean air into the chamber. An electric fan (DC
Brushess. DC24V.
1.41A. Delta ElectronicsTM) was installed in the chamber to make a uniform
environment inside
the gas chamber. The microchannel was attached to the chamber using a screw
hinge, which
allows the device to rotate on the chamber. The sensor was first exposed to
the clean air.
[0068] The following methods and materials were employed with respect to the
EXAMPLES
described herein.
13
CA 02947079 2016-11-01
EXAMPLES
[0069] EXAMPLE 1: Channel Coating
[0070] The analyte diffusion process was independent of the channel coating
material and
dependent on the analyte type. However, the adsorption and desorption
processes are
dependent on both gas type and the channel surface material. Therefore, it was
expected that
the surface treatment of the channel would results in different transient
response profiles. To
study the effects of channel coating on the sensor response, a set of
materials, as listed in
TABLE 2, were tested.
[0071] Normalized transient responses of six of the sensors (coatings number 3-
4 and 8-11)
to 2000 ppm ethanol are shown in FIGURE 5. The rest of the channel coatings
(coatings
number 1-2 and 5- 7) did not show significant responses as some of the
materials hindered the
diffuse-in process. As a result, these five coatings seemed to trap all the
ethanol molecules
stopping them from travelling along the channel and approaching the sensor. As
it can be seen
in FIGURE 5, the interaction of the gas molecules with different materials was
different
resulting in varying normalized responses.
[0072] Single metal layer coatings: Among all the channels coated and
tested with a single
metal layers, gold (with chromium underlayer and parylene C second layer,
showed the best
response (FIGURES 5 and 6), as it is one of the most non-reactive materials in
nature and was
used here to decrease the chemical cross contamination of the gas molecules to
the channel
walls which eventually results in faster sensor recovery. The chromium layer
was coated to
increase the adhesion of the substrate to gold. Similarly, a Si02 first layer
with a parylene C
second layer showed a good response (volts) and recovery curve (FIGURE 5).
However, the Cr
and Au coated channel without parylene C also showed a reasonable ability to
distinguish
ethanol, methanol and acetone (FIGURE 5).
[0073] First layer (Bottom layer i.e. closest to the channel surface): In
case of channels with
multilayer coatings, it is observed that the channels coated with different
bottom layer materials
(even with the same top layer) provide different responses. For instance, the
channel coated
with three layers of Cr, Au, and Parylene C (with a gold and chromium layer as
the bottom
coating layers) and Cu and Parylene C (with the copper layer as the bottom
coating layer) show
different responses to the same concentration of ethanol. This is due to the
permeation of the
gas molecules through the top layer and reaction with the bottom coating
layer. In choosing a
first layer, it is preferred in some embodiments that the first layer
physically interacts (i.e. non-
14
CA 02947079 2016-11-01
specifically and reversibly via van der Wahl's forces) with the VOC, but does
not chemically
interact with the VOC.
[0074]
Second layer (Top layer i.e. on top of the first layer): The preliminary
experiments
revealed the importance of the porosity of the top coating layer. In essence,
the number of
surface adsorption sites available per unit volume of the channel (Ca in
equation (3)) is greater
in channels with higher porosity. As it is shown in FIGURE 5, the diffuse-in
and diffuse-out
processes of ethanol was more rapid in the channels with the combination of Cr
and Au and
Parylene C coatings, whereas, the coating combination of Cr and Au and Cytonix
shows the
slowest response. This suggests that more physical adsorption occurs in the
case of Cr and Au
and Cytonix channel coating. Thus, Parylene C is a good candidate for the top
layer coating
material as it can be coated as a thin polymer film, which is chemically
inert. It also has high
porosity [25], which increases physical adsorption of the gas molecules to the
channel walls that
eventually increases selectivity of the sensor. In addition, Parylene C
provides a pinhole free
coating and a lower permeability (as compared to other similar polymers) and
has been recently
used in the development of GC columns [26] as well as a material for moisture
barrier in
numerous applications [27]. The latter is potentially significant for gas
sensing, since the gas
sensors are subject to errors as they are vulnerable to ambient fluctuations
such as humidity and
temperature change. Therefore, the response of a sensor depends on not only
the analyte
concentration, but also the ambient conditions (particularly humidity).
Therefore, in high
precision sensing applications, such as breath analyzers, fluctuation in
humidity [28] may result
in false signals. Thus, the use of an effective moisture barrier such as
Parylene C along the
channel may reduce the error caused by humidity. In choosing a second layer,
it is preferred in
some embodiments that the second layer if used has porosity so that the VOC
has access to the
first layer and is also chemically inert. Furthermore, it may also be useful
for the second layer to
have moisture barrier properties.
[0075] Analytes: Three different analytes including ethanol, methanol, and
acetone were
tested to compare the selectivity of the fabricated sensors among different
gases. These gases
were selected to show the capability of the device in differentiating alcohol
and ketone vapors.
Four out of the eleven fabricated sensors showed acceptable selectivity among
the three selected
analytes. The temporal responses obtained from the device are normalized to
fit within the
magnitude range of [o 1], eliminating the influence of the analyte
concentration on the shape of
the responses. Normalized responses for each of the sensors to 2000 ppm of
each of the three
analytes are depicted in FIGURE 6. As it can be seen in FIGURE 6, each of the
four sensors
give unique responses corresponding to different tested analytes such that the
finger-prints of
CA 02947079 2016-11-01
three analytes on each of the four selected sensors were distinct. However,
different sensors
may distinguish these three analytes differently. In other words, it may be
observed that from
one sensor to another the level of segregation between analytes may be
different, showing
different selectivity among the sensors tested. A better quantitative
comparison may be
evaluated based on calculating indicators of selectivity and the recovery time
of the sensor to
find the optimum material for the treatment of the channel of the proposed gas
detector. For
instance, FIGURE 7 shows typical responses of one of the sensors against three
different
analytes. A selectivity factor is defined as S=S1+S2+S3, in which Si, S2, and
S3 are the absolute
values of the distances between the amplitude of responses of methanol-
acetone, ethanol-
methanol, and acetone-ethanol, respectively, at five different time points
(t=2os, t=40s, t=6os,
t=8os, and t=loos). The square root of the sum of square of the selectivity
factors at five points
is used as a measure of selectivity of different sensors. Another factor for
determining the
sensor performance is the recovery time: in essence, the sensor with the lower
recovery time is
preferable.
[0076]
Optimization of coating: The selectivity and recovery time of the fabricated
sensors
are all compared and listed in TABLE 3. In this table, the sensors are listed
based on two major
categories: coating materials and dimensions. The average pick time of each
sensor, which is
the mean of three time points for which the sensors have the maximum readout
for three
different analytes, were also calculated and listed. It is observed that the
smaller the pick time
value the faster the recovery of the sensor. The average pick time was used to
rank (in the order
of 1 to 4, from the lowest average pick time to the highest, respectively),
and hence compare the
speed of the recovery of different detectors. The sensors were also ranked
based on their
selectivity factor (as explained above). The effect of both coating materials
and channel
dimensions are separately investigated through the above ranking schemes. The
results show
that the Cr and Au and Parylene C coated sensor provides the maximum
selectivity and the
minimum recovery time among all the coating materials tested here. This means
that the
proposed coating combination decreases the cross contamination and the
chemical adsorption
and increases the physical adsorption (and hence selectivity). To perform a
quantitative
comparison of the response of the sensor to different analytes three features
(tr, tm, Rf) are
extracted from each normalized response. The feature space for the sensor with
the coating
combination of Cr and Au and Parylene C, which shows the best performance in
terms of
selectively and recovery time, is shown in FIGURE 8. It will be appreciated by
a person of skill
that the optimum coating will depend on the VOCs being tested.
16
CA 02947079 2016-11-01
[0077] TABLE 3 Comparison of the Separation Factor and Recovery Time Among the
Fabricated Sensors
Coating Channel Channel Average Peak Selectivity S
Length Depth Peak Time Time Factor (S) Rank
(1) (d) (seconds) Rank
Cr-Au- 59.37 1 1.49 1
Parylene C
Sensors with Cr-Au 154.07 4 1.07 3
Different 8102- 30 mm 500 jim 72.25 2 1.08 2
Coatings Parylene C
Cu- 98.51 3 1.01 4
Parylene C
20 1111T1 500 AM 51.18 1 1.37 4
Sensors with Cr-Au- 30 mm 500 pm 59.37 2 1.49 3
Different Parylene C 40 mm 500 vun 67.25 3 1.52 2
Dimensions 30 MM. 200 pm 68.56 4 1.74 1
[0078] EXAMPLE 2: Channel Dimensions
[0079]
After choosing the preferred coating combination of the tested coatings listed
in
TABLES 2 and 3, for the tested VOCs, Cr and Au and Parylene C were preferred.
This coating
was then tested to study the effect of the channel dimensions on the response
of the sensor,
sensors with three different channel lengths and two different channel depths
are fabricated and
tested (see TABLE 3). The ranking procedure explained above was also used to
quantify the
effect of the channel dimension on the selectivity and recovery time. In
general, there is an
opposite trend in rankings based on the selectivity and recovery time for
sensors with different
dimensions as explained below.
[0080]
Channel depth: Normalized responses for three different analytes (ethanol,
methanol
and acetone) for four different channel dimensions: (i) 1=20 mm, d=500 pm,
(ii) 1=30 mm,
d=500 m, (iii) 1=40 mm, d=500 m, and (iv) 1=30 mm, d=200 pm (1 is the length
and d is the
depth of the channel) are depicted in FIGURE 9. As expected, the sensors with
higher channel
depths are recovered faster. According to equation (3), increasing the depth
of the channel
decreases the effect of physical adoption, which will result in changing the
diffusion-
physisorption equation to only diffusion equation for deep channels. In this
case (which is only
17
CA 02947079 2016-11-01
diffusion-dependent), the only analyte related parameter in the equation is D
(gas diffusivity).
On the other hand, by decreasing the channel depth, the effect of Ca and a in
Equation (3)
increases and more adsorption and desorption dependency will be observed in
the response.
Thus, channels with smaller depths are recommended to differentiate gases with
similar
diffusion coefficients.
[0081]
Channel length: When examining two gases (with different diffusion
coefficients),
increasing the length of the channel increases the diffusion time, which
results in a larger
difference in the temporal responses of the sensor (see FIGURE 9). In other
words, increasing
the length of the channel slows down the diffusion process and increases the
selectivity of the
sensor. However, longer channels result in longer recovery time for the
sensor. Therefore,
considering the trade-off between the selectivity and the recovery time of the
sensor, the
preferred dimension of the channel for the VOCs tested and with the tested
coatings was 1=3o
mm, d=200 gm (see TABLE 3).
[0082] EXAMPLE 3: Analyte Concentration
[0083] After adjusting the sensor coating and dimensions, the coating of Cr
and Au and
Parylene C and the dimensions of 1 = 30 mm and d = 200 gm are used for
verifying the
selectivity of the sensor. A wide range of concentration (250-4000 ppm) of 6
different target
gases were selected among alcohols (including 2-pentanol, ethanol and
methanol) and ketone
vapours (including acetone, 2-butanone and 2-pentanone). As recorded transient
responses for
8 different concentrations for 6 different targets is shown in FIGURE lo; the
sensor
differentiated among different concentration of gases. As presented in FIGURE
ii, the feature
space shows the analytes are successfully separated in the 3D space. The
feature vectors of the
responses related to each analyte at different concentrations form a clear-cut
cluster in the
feature space (see FIGURE 11). No mathematical tool was needed for mapping the
responses
into the feature space, and only one simple feature extraction method [20] was
adequate for the
determination of the positions of the target analytes in the feature space.
The feature space of a
particular device was universal and requires hardly any modification when
applied to different
analytes.
[0084] The gas detector operation is humidity and temperature dependent.
Ambient
temperature and humidity dependence of the responses provided for a specific
analyte may be
considered as sources of error, which causes displacement of the feature
vector related to each
analyte in the feature space. This arises from the fact that the analyte
diffusion/physisorption
along the channel/to the channel walls are both strongly temperature-dependent
processes.
18
CA 02947079 2016-11-01
These errors caused by ambient fluctuations introduce drift-like terms into
the responses of the
sensor, which causes false measurements. Therefore, the ambient temperature
and humidity
are controlled during all the experiments. The apparatus may be further
optimized to minimize
the effect of humidity and temperature fluctuation on the response of the
sensor.
[0085] EXAMPLE 4: Detection of Tetrahydrocannabinol (THC)
[0086] An embodiment of the apparatus was also tested for detection of
cannabis in human
exhaled breath. The tested embodiment was capable of differentiating small
concentrations of
Tetrahydrocannabinol (THC) in presence of other volatile organic compounds
(VOCs). The
main advantage of the proposed device over previous microfluidic-based gas
sensors [30-31] is
the integration of heaters along the micro-channels to enhance the diffusion
rate of the THC
molecules in the channel and decreasing the sensor response and recovery time
from 15 minutes
to below 200 s. Detection of THC in breath has been used as an indicator of
cannabis use [32].
However, as there are traces of other VOCs in the breath, it is important to
differentiate among
different gases, and pinpoint the distinct "smell print" of THC. General
purpose Metal Oxide
Semiconductor (MOS) gas sensors are sensitive and not selective of different
gases [33]. As
described above, micro-channels may be integrated with these sensors to
enhance their
selectively (FIGURE 2A and 2C) [30]. However, these microfluidic gas sensors
are not
suitable for detection of large molecule gases (such as THC) as the diffusion
process is slow and
takes more than few minutes [31]. In this example, the sensor response time
was decreased by
modulating the temperature of the diffusion channel. The sensor assembly was
fabricated using
a similar method as explained in [30]. To control the temperature of the
diffusion channel, a
platinum heater wire is integrated along the channel. The response time and
selectivity of the
sensor for THC-methanol binary mixture (1 mg/mL solution in methanol) and pure
methanol
were studied at different temperatures (25 C, 40 C and 8o C). A method
described in [31]
was used to characterize the sensor response (FIGURE 13). The sensor recovery
time for THC-
methanol mixture at 25 C was approximately 1.5 minutes, and as the
temperature is increased
to 80 C, the recovery time is reduced to under 3 minutes. The slow recovery,
which is
attributed to high molecular weight of THC, was not observed for pure
methanol. Therefore, the
overall sensor response time was decreased drastically for THC detection by
addition of the
heater. Increasing the micro-channel temperature has another important effect:
enhancing the
selectivity. As can be seen in FIGURE 14, the selectivity of the device is
increased at higher
temperatures as bigger molecules of THC in the binary mixture are more
actively involved in the
diffusion process and react with the sensor. It must be noted that the
observed response for the
19
CA 02947079 2016-11-01
binary mixture of THC-methanol is distinct for each THC concentration, and we
have
successfully detected THC concentrations as low as 50 ppm. In contrast to
previous microfluidic
gas sensor designs, the selectivity of the sensor was not compromised when
achieving faster
response times such that the heater embedded channel design would be suitable
for detection of
larger molecules including THC. This embodiment may provide a low-cost breath
analyzer
device, which may provide a powerful tool for roadside testing or also for
personal monitoring
purposes.
[0087] The embodiment shown in FIGURE 12, shows one way to control the
temperature
of the diffusion channel, a heater is shown integrated along the channel. A
benefit of the
embodiment shown in FIGURE 12 is the integration of a heater along the micro-
channels to
enhance the diffusion rate of the THC molecules in the channel and decreasing
the sensor
response and recovery time to below 200S. In contrast, some microfluidic gas
sensor designs do
not have the selectivity of the sensor in combination with a faster response
when used to detect
larger molecules, including THC.
[0088] The sensor selectivity may be further be enhanced by creating a flow
(advection) of
gas inside the micro-channels. Also, a water trap is shown in FIGURE 12 to
minimize large
droplets of moisture entering device. The sample enters an antechamber; the
force of exhalation
drives the sample through a humidity filter and into the sampling chamber. A
one way valve can
be used ensure gas does not escape through the inlet. A small vacuum pump
draws in fresh air
from inlet and out the exhaust port to recover the sensor.
[0089] A 3D-printed microfluidic platform is fabricated by integrating a
chemo-resistor with
a channel. Using a novel coating combination, a surface treatment on the inner
walls of the
microfluidic channel is carried out, which enhances the selectivity power of
the device. Different
coating materials are tested and compared to choose the best material in terms
of giving the
maximum selectivity and the minimum sensor recovery time. The geometry of the
channel is
then optimized after comparison of the results of sensors fabricated with
different channel
dimensions. Embodiments may be developed as low-cost (¨$10), portable and
highly selective
gas detectors, which provide a powerful tool for numerous applications
including personal
monitoring of exhaled breath for patients suffering from different diseases,
biological analysis,
safety and environmental monitoring, and analytical chemistry.
[0090] A different method of feature extraction is also used for
characterization of the
concentration of the analyte. Three different features are extracted from each
transient
response (see FIGURE i6A). The signal maximum response level (F1), the
response level for
the final readout (F2), and the surface area underneath the response (F3) are
the three extracted
CA 02947079 2016-11-01
features from each transient response. The feature vector (0) extracted from
the transient
response is shown in a 3D space in FIGURE 16B. The transient responses of the
sensor with
Cr and Au and Parylene C channel coating and dimensions of 1 = 40 mm, w= 3 mm,
d = 500 m
are shown in FIGURE 17A. The transient responses are shown in FIGURE 17A,
representing
the repeatability of the device for each concentration. Some parts of the
transient responses are
magnified to show the reproducibility of the response for each concentration.
The feature
vectors related to each concentration are segregated (see FIGURE 17B) in the
feature space.
The results show three separated spheres, representing the separation
capability of the device
between different concentrations of the same analyte. A regression model is
used to show the
linear relation between the concentration and the area underneath the curve
(see FIGURE 18).
[0091] EXAMPLE 5: Natural Gas Leakage Detection
[0092] An embodiment of the apparatus was also tested as an automated and
reliable means
for monitoring of natural gas leakage in pipelines and around pump stations.
In particular, a
microfluidic-based sensor as described herein may be deployed using an
unmanned aerial
vehicle (UAV) for timely and precise detection of natural gas leakage at
storage sites and along
pipelines. Such a device may be operated easily by pipeline maintenance
technicians with basic
training to remotely inspect natural gas infrastructure including pumps, tanks
and pipes
wherein the natural gas infrastructure may have limited everyday access. The
sensor can be
used for detection of methane, ethane and pentane.
[0093]
Features of this embodiment may include: a sensor recovery process which is
capable
of automatically regenerating the saturated sensors using a compressed air
recovery chamber
and electrically actuated solenoid valves in order to continuously monitor the
infrastructure for
leakage detection; the slope of the "exposure to pentane", which is
representative of a gas
concentration, may be chosen as the main feature of the response, whereby this
feature
extraction process allows the device to determine the concentration of the
desired analyte; the
capability to switch between multiple channels for an uninterrupted detection
operation
wherein there may be a manifold controlled by micro-valves are used; the
sensor may be
installed in a mobile platform such as a UAV to enable mobile detection of
different gases and to
achieve this goal a novel sampling procedure was developed to enable sampling
consistent
amount of gas as the platform is moving; and an onboard microprocessor may be
used to relate
the UAV flight path to sensor readings of the methane concentration (see
FIGURES 19 A and
B).
21
CA 02947079 2016-11-01
[0094] EXAMPLE 6: Nuisance Sewer Gas Detection
[0095] An embodiment of the apparatus is also envisaged, wherein the sensor
technology
may be used to monitor sewer gases and identify "hotspots" of gas production
for targeted
treatment. Particularly, the gas sensor may be used for detection of nuisance
gases, some of
which are odorous or even hazardous. For example, hydrogen sulfide, ammonia,
carbon
dioxide, methane and nitrous oxide, among other greenhouse gases. The
embodiment may be
relatively independent and low-maintenance, and may have a streamlined data
communications
to collect, transmit, analyze and store data to inform users' mitigation
strategies in real-time.
[0096] Features of this embodiment may include: an aerofoil design is used to
minimize the
risk of obstruction in the turbid environment, wherein the configuration may
be developed to
allow the device to be positioned along the side of the pipe to avoid large
sediments at the
bottom of the pipeline; a shared inlet/outlet channel positioned on the
downstream end of the
apparatus to avoid blockage due to fast-flowing suspended organics and other
waste, which may
be combined with a high pressure air source which may be used to purge the
previous sample
and dislodge any debris build-up and wherein negative pressure may be used to
draw the next
sample through the inlet; a membrane-less microfiltration mechanism may be
used to ensure
that the sensing unit is not in contact with microorganisms or debris that can
interact with the
sample and bias the sensor reading or create nuisance compounds, whereby the
microfiltration
mechanism is based on the use of inertial microfluidic particle sorters; and
since the sensor may
use oxygen (02) to recover between samples, onboard compressed gas may be used
to flush the
micro-chamber and channel, whereby the sensor may recover to the baseline, and
a neutral gas
(N2) may be used to purge 02 and any remaining sample from the sensing unit
and into the
surrounding environment through an exhaust outlet (see FIGURE 20).
[0097] Although embodiments described herein have been described in some
detail by way
of illustration and example for the purposes of clarity of understanding, it
will be readily
apparent to those of skill in the art in light of the teachings described
herein that changes and
modifications may be made thereto without departing from the spirit or scope
of the appended
claims. Such modifications include the substitution of known equivalents for
any aspect of the
invention in order to achieve the same result in substantially the same way.
Numeric ranges are
inclusive of the numbers defining the range. The word "comprising" is used
herein as an open
ended term, substantially equivalent to the phrase "including, but not limited
to", and the word
comprises" has a corresponding meaning. As used herein, the singular forms
"a", "an" and
"the" include plural referents unless the context clearly dictates otherwise.
Thus, for example,
22
CA 02947079 2016-11-01
reference to "a thing" includes more than one such thing. Citation of
references herein is not an
admission that such references are prior art to an embodiment of the present
invention. The
invention includes all embodiments and variations substantially as herein
described and with
reference to the figures.
23
CA 02947079 2016-11-01
[0098] REFERENCES
[1] M. Bunge, et al. "On-line monitoring of microbial volatile metabolites by
proton transfer
reaction-mass spectrometry." Applied and environmental microbiology, vol.
74.7, pp.
2179-2186, 2008.
[2] F. Hossein-Babaei, and V. Ghafarinia. "Gas analysis by monitoring
molecular diffusion in a
microfluidic channel." Analytical chemistry, vol. 82.19, pp. 8349-8355, 2010.
[3] L.C.A. Amorim, and Z.L. Cardeal, "Breath air analysis and its use as a
biomarker in biological
monitoring of occupational and environmental exposure to chemical agents,"
Journal of
Chromatography B, vol. 853, pp. 1-8, 2008.
[4] Xie, Yi, et al. "Three-dimensional ordered ZnO¨CuO inverse opals toward
low concentration
acetone detection for exhaled breath sensing," Sensors and Actuators B:
Chemical, vol.
21, pp. 255-262, 2015.
[5] M. Philips, N. Altorki, J. Austin, R. Cameron, J. Greenberg, R. Kloss, R.
Maxfield, M.
Munawar, and H. Pass, "Prediction of lung cancer using volatile biomarkers in
breath,"
Cancer Biomarkers, vol. 3, no. 2, pp. 95-109, 2007.
[6] L. Zhu, et al. "Integrated microfluidic gas sensor for detection of
volatile organic compounds
in water" Sensors and Actuators B: Chemical, vol. 121.2 pp. 679-688, 2007.
[7] S. Zampolli, et al. "Real-time monitoring of sub-ppb concentrations of
aromatic volatiles
with a MEMS-enabled miniaturized gas-chromatograph." Sensors and Actuators B:
Chemical, vol.141.1, pp. 322-328. 2009.
[8] A. W. Boots, et al. "Identification of microorganisms based on headspace
analysis of volatile
organic compounds by gas chromatography¨mass spectrometry." Journal of breath
research, vol. 8.2, pp. 027106, 2014.
[9] A. Garg, et al. "Zebra GC: A mini gas chromatography system for trace-
level determination of
hazardous air pollutants." Sensors and Actuators B: Chemical, vol. 212, pp.145-
154,
2015.
[10] L. Li, et al. "Mini 12, Miniature Mass Spectrometer for Clinical and
Other Applications,
Introduction and Characterization." Analytical chemistry, vol. 86.6 pp. 2909-
2916,
2014.
[n] W. F. Karasek, and R. E. Clement "Basic gas chromatography-mass
spectrometry: principles
and techniques" Elsevier, 2012.
[12] J. W. Gardner, and P. N. Bartlett. "A brief history of electronic noses."
Sensors and
Actuators B: Chemical, vol. 18.1, pp. 210-211, 1994.
24
CA 02947079 2016-11-01
[13] K. Arshak, et al. "A review of gas sensors employed in electronic nose
applications." Sensor
review, vol. 24.2, pp. 181-198, 2004.
[14] M. Holmberg, et al. "Drift counteraction for an electronic nose." Sensors
and Actuators B:
Chemical 36.1, pp. 528-535, 1996.
[15] W. J. Harper, "The strengths and weaknesses of the electronic nose."
Headspace analysis of
foods and flavors. Springer US, pp. 59-71, 2001.
[16] F. Hossein-Babaei and V. Ghafarinia, "Compensation for the drift-like
terms caused by
environmental fluctuations in the responses of chemoresistive gas sensors,"
Sensors and
Actuators B, vol. 143, pp. 641-648, 2010.
[17] F. Hossein-Babaei, and A. Amini. "Recognition of complex odors with a
single generic tin
oxide gas sensor." Sensors and Actuators B: Chemical, vol. 194, pp. 156-163,
2014.
[18] F. Hossein-Babaei, M. Hemmati, and M. Dehmobed. "Gas diagnosis by a
quantitative
assessment of the transient response of a capillary-attached gas sensor."
Sensors and
Actuators B: Chemical, vol. 107.1, pp. 461-467, 2005.
[19] M. Paknahad, V. Ghafarinia, and F. Hossein-Babaei. "A microfluidic gas
analyzer for
selective detection of biomarker gases" Sensors Applications Symposium (SAS),
2012
IEEE, pp. 1-5. IEEE, 2012.
[20] F. Hossein-Babaei, M. Paknahad, and V. Ghafarinia, "A miniature gas
analyzer made by
integrating a microchannel with a chemoresistor," Lab-on-a-Chip, vol. 12, pp.
1874-
1880, 2012.
[21] V. Ghafarinia, A. Amini, and M. Paknahad. "Gas identification by a single
gas sensor
equipped with microfluidic channels." Sensor Letters, vol. 10.3-4, pp. 845-
849, 2012.
[22] M. Paknahad, Mohammad, et al. "Highly selective multi-target 3D-printed
microfluidic-
based breath analyzer." 2016 IEEE 29th International Conference on Micro
Electro
Mechanical Systems (MEMS). IEEE, pp. 905-908, 2016.
[23] N. Yamazoe, G. Sakai, and K. Shimanoe, "Oxide semiconductor gas sensors."
Catalysis
Surveys from Asia, vol. 7.1, pp. 63-75, 2003.
[24] C. L. Yaws, "Chemical properties handbook.", McGraw Hill Professional,
1998.
[25] Binh-Khiem, Nguyen, Kiyoshi Matsumoto, and Isao Shimoyama. "Porous
Parylene and
effects of liquid on Parylene films deposited on liquid." Micro Electro
Mechanical
Systems (MEMS), 2011 IEEE 24th International Conference on. IEEE, 2011.
[26] H. Noh, P. J. Hesketh, and G. C. Frye-Mason. "Parylene gas
chromatographic column for
rapid thermal cycling." Journal of Microelectromechanical Systems, vol. 11.6,
pp. 718-
725, 2002.
CA 02947079 2016-11-01
[27] 0. Grinberg, et al. "Antibiotic nanoparticles embedded into the Parylene
C layer as a new
method to prevent medical device-associated infections." Journal of Materials
Chemistry B, vol. 3.1, PP. 59-64, 2015.
[28] F. Hossein-Babaei, and S. Rahbarpour. "Alteration of pore size
distribution by sol¨gel
impregnation for dynamic range and sensitivity adjustment in Kelvin
condensationbased
humidity sensors." Sensors and Actuators B: Chemical, vol. 191, pp. 572-578,
2014.
[29] F. HOSSEIN-BABAEI "Novel Device and Method for Gas Analysis" Canadian
Patent
2,395,563.
[30] F. Hossein-Babaei, M. Paknahad, and V. Ghafarinia, Lab on a Chip 12,
1874, (2012).
[31] M. Paknahad, J. S. Bachhal, A. Ahmadi & M. Hoorfar, IEEE MEMS, pp. 905-
908, (2016).
[32] W. Cao, and Y. Duan, Clinical chemistry 52, 800, (2006). 4.
[33] J. W. Gardner, H. Woo Shin, and E. L. Hines., Sensors and Actuators B:
Chemical, 70, 19,
(2000).
26