Note: Descriptions are shown in the official language in which they were submitted.
CA 02947139 2016-10-26
WO 2015/191173
PCMJS2015/027925
NANO-TRIBOLOGY COMPOSITIONS AND
RELATED METHODS INCLUDING HARD PARTICLES
TECHNICAL FIELD
[0001] This
specification relates to compositions and methods in the field of
tribology, solid surface engineering, lubrication, wear, and related functions
such as
corrosion resistance, catalysis, and the like. This specification also relates
to
compositions and methods in the sub-field of nano-tribology and associated
solid
surface nano-engineering, nano-lubrication, and nano-wear.
BACKGROUND
[0002] Tribology
refers to the science and engineering of solid surfaces.
Tribology includes the study and application of surface chemistry and
structure,
friction, lubrication, corrosion, and wear. The tribological interactions of a
solid
surface with interfacing materials and the surrounding environment may result
in the
loss of material from the surface in processes generally referred to as
"wear." Major
types of wear include abrasion, friction (adhesion and cohesion), erosion, and
corrosion. Wear may be reduced by the use of lubricants and/or other anti-wear
agents. Wear may also be reduced by modifying the surface properties of solids
using one or more "surface engineering" processes (i.e., modifying the
chemical
and/or structural properties of solid surfaces).
SUMMARY
[0003] In a non-
limiting embodiment, a composition comprises a plurality of hard
particles and a plurality of lubricant nanoparticles. The lubricant
nanoparticles have
an average primary particle size of less than or equal to about 500 nm and an
open
architecture. An organic medium is intercalated in the lubricant
nanoparticles.
[0004] In another
non-limiting embodiment, a composition comprises a plurality of
hard particles and a plurality of molybdenum disulfide nanoparticles. The
molybdenum disulfide nanoparticles have an average primary particle size of
less
than or equal to about 100 nm and an open architecture. An oil medium is
intercalated in and encapsulates the molybdenum disulfide nanoparticles.
81800862
[0094a] According to one aspect of the present invention, there is provided
a
composition comprising: a plurality of hard particles comprising at least one
of:
synthetic diamond, natural diamond, amorphous carbon, nanocrystalline carbon,
or a
metal compound comprising a metal boride, a metal carbide, a metal nitride, a
metal
oxide, a metal silicide, a metal carbonitride, a solid state solution of any
thereof, or a
particulate mixture of any thereof; a plurality of lubricant nanoparticles
having an
average particle size of less than 500 nm or equal to about 500 nm and an open
architecture; and an organic medium intercalated in the lubricant
nanoparticles;
wherein the metal nitride is selected from the group consisting of cubic boron
nitrides,
silicon nitrides, titanium nitrides, zirconium nitrides, hafnium nitrides,
tungsten
nitrides, solid state solutions of any thereof, and particulate mixtures of
any thereof;
and wherein the metal oxide is selected from the group consisting of aluminum
oxides, cerium oxides, titanium oxides, zirconium oxides, solid state
solutions of any
thereof, and particulate mixtures of any thereof.
[0004b] According to another aspect of the present invention, there is
provided
a composition comprising: a plurality of hard particles comprising at least
one of
metal boride, a metal carbide, a metal nitride, a metal oxide, a metal
silicide, a metal
carbonitride, a solid state solution of any thereof, or a particulate mixture
of any
thereof; a plurality of molybdenum disulfide nanoparticles having an average
particle
size of less than 100 nm or equal to about 100 nm and an open architecture;
and an
oil medium intercalated in and encapsulating the molybdenum disulfide
nanoparticles;
wherein the metal nitride is selected from the group consisting of cubic boron
nitrides,
silicon nitrides, titanium nitrides, zirconium nitrides, hafnium nitrides,
tungsten
nitrides, solid state solutions of any thereof, and particulate mixtures of
any thereof;
and wherein the metal oxide is selected from the group consisting of aluminum
oxides, cerium oxides, titanium oxides, zirconium oxides, solid state
solutions of any
thereof, and particulate mixtures of any thereof.
la
CA 2947139 2018-11-30
CA 02947139 2016-10-26
WO 2015/191173
PCMJS2015/027925
[0005] It is
understood that the invention disclosed and described in this
specification is not limited to the embodiments summarized in this Summary.
BRIEF DESCRIPTION OF THE DRAWINGS
[0006] Various
features and characteristics of the non-limiting and non-exhaustive
embodiments disclosed and described in this specification may be better
understood
by reference to the accompanying figures, in which:
[0007] Figure 1 is
a diagram illustrating a method of producing lubricant
nanoparticles;
[0008] Figure 2 is
a diagram illustrating one method of preparing nanoparticle
based lubricants;
[0009] Figure 3
shows transmission electron microscopy (TEM) micrographs of
molybdenum disulfide particles; Figure 3(A) shows molybdenum disulfide as it
is
available, typically from about a few microns to submicron size; Figure 3(B)
shows
molybdenum disulfide that has been ball milled in air for 48 hours; Figure
3(C) is a
high resolution electron microscopy image that shows molybdenum disulfide that
has
been ball milled in air for 48 hours; Figure 3(D) is a high-resolution
transmission
electron microscopy (HRTEM) image that shows molybdenum disulfide that has
been ball milled in air for 48 hours followed by ball milling in oil for 48
hours;
[0010] Figure 4 is
a graph showing XRD spectra of molybdenum disulfide
particles; Figure 4(A) is the XRD spectra for molybdenum disulfide that has
been ball
milled in air for 48 hours followed by ball milling in oil for 48 hours;
Figure 4(B) is the
XRD spectra for molybdenum disulfide that has been ball milled in air for 48
hours;
Figure 4(C) is the XRD spectra for molybdenum disulfide that has not been ball
milled;
[0011] Figure 5 is
a graph showing XPS spectra of molybdenum disulfide
particles in which the carbon peak for molybdenum disulfide that has not been
ball
milled is shown, as well as the carbon peak for molybdenum disulfide that has
been
ball milled in air for 48 hours, followed by ball milling in oil for 48 hours;
[0012] Figures 6(A)-
6(D) show graphs and bar charts depicting tribological test
data for different additives in paraffin oil; Figure 6(A) shows the average
wear scar
2
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
diameter for a base oil (paraffin oil), paraffin oil with micron sized MoS2,
paraffin oil
with MoS2 that was milled in air for 48 hours, and paraffin oil with MoS2 that
was
milled in air for 48 hours followed by milling in canola oil for 48 hours;
Figure 6(B)
shows the load wear index for paraffin oil without a nanoparticle additive,
paraffin oil
with micron sized MoS2, paraffin oil with MoS2 that was milled in air for 48
hours, and
paraffin oil with MoS2 that was milled in air for 48 hours followed by milling
in canola
oil for 48 hours; Figure 6(C) shows the coefficient of friction for paraffin
oil without a
nanoparticle additive, paraffin oil with micron sized MoS2 (c-MoS2), paraffin
oil with
MoS2 that was milled in air for 48 hours (d-MoS2), and paraffin oil with MoS2
that was
milled in air for 48 hours followed by milling in canola oil for 48 hours (n-
MoS2);
Figure 6(D) shows the extreme pressure data for paraffin oil with micron sized
MoS2
(c-MoS2), paraffin oil with MoS2 that was milled in air for 48 hours (d-MoS2),
and
paraffin oil with MoS2 that was milled in air for 48 hours followed by milling
in canola
oil for 48 hours (n-MoS2); in each test the lubricant nanoparticle additive
was present
in the amount of 1% by weight;
[0013] Figure 7 is
a TEM image showing the architecture of molybdenum disulfide
nanoparticles (15-70 nm average size); Figure 7(A) shows the close caged dense
oval shaped architecture of molybdenum disulfide nanoparticles that have been
ball
milled in air for 48 hours; Figure 7(B) shows the open ended oval shaped
architecture of molybdenum disulfide nanoparticles that have been ball milled
in air
for 48 hours followed by ball milling in canola oil for 48 hours;
[0014] Figure 8 is
a graph depicting a comparison of wear scar diameters for
different additives in paraffin oil; one additive is crystalline molybdenum
disulfide (c-
MoS2); another is molybdenum disulfide nanoparticles that were ball milled in
air (n-
MoS2); another additive is molybdenum disulfide nanoparticles that were ball
milled
in air followed by ball milling in canola oil and to which a phospholipid
emulsifier was
added (n-MoS2+Emulsifier);
[0015] Figure 9 shows photographs and graphs produced using energy
dispersive x-ray analysis (EDS) depicting the chemical analysis of wear scar
diameters in four-ball tribological testing for nanoparticle based lubricants;
Figure
9(A) shows paraffin oil without any nanoparticle composition additive; Figure
9(B)
shows paraffin oil with molybdenum disulfide nanoparticles that have been ball
milled
3
81800862
in air for 48 hours followed by ball milling in oil for 48 hours and treated
with a
phospholipid emulsifier;
[0016] Figures
10(A) and 10(B) show schematic diagrams of the crystal structure
of molybdenum disulfide.
[0017] Figure 11
shows a schematic diagram of the crystal structure of hexagonal
boron nitride.
[0018] The
reader will appreciate the foregoing details, as well as others, upon
considering the following detailed description of various non-limiting and non-
exhaustive embodiments according to this specification.
DESCRIPTION
[0019] Various
embodiments are described and illustrated in this specification to
provide an overall understanding of the composition, function, operation, and
application of the disclosed compositions and methods. It is understood that
the
various embodiments described and illustrated in this specification are non-
limiting
and non-exhaustive. Thus, the invention is not necessarily limited by the
description
of the various non-limiting and non-exhaustive embodiments disclosed in this
specification. The
features and characteristics illustrated and/or described in
connection with various embodiments may be combined with the features and
characteristics of other embodiments. Such modifications and variations are
intended to be included within the scope of this specification. As such, the
claims
may be amended to recite any features or characteristics expressly or
inherently
described in, or otherwise expressly or inherently supported by, this
specification.
Further, Applicant reserves the right to amend the claims to affirmatively
disclaim
features or characteristics that may be present in the prior art. The
various embodiments disclosed and described in this specification can
comprise,
consist of, or consist essentially of the features and characteristics as
variously
described in this specification.
[0020] Also, any
numerical range recited in this specification is intended to
include all sub-ranges of the same numerical precision subsumed within the
recited
range. For example, a range of "1.0 to 10.0" is intended to include all sub-
ranges
4
CA 2947139 2018-11-30
81800862
between (and including) the recited minimum value of 1.0 and the recited
maximum
value of 10.0, that is, having a minimum value equal to or greater than 1.0
and a
maximum value equal to or less than 10.0, such as, for example, 2.4 to 7.6.
Any
maximum numerical limitation recited in this specification is intended to
include all
lower numerical limitations subsumed therein and any minimum numerical
limitation
recited in this specification is intended to include all higher numerical
limitations
subsumed therein.
Accordingly, Applicant reserves the right to amend this
specification, including the claims, to expressly recite any sub-range
subsumed
within the ranges expressly recited in this specification. All such ranges are
intended
to be inherently described in this specification.
[0021]
[0022] The
grammatical articles "one", "a", "an", and "the", as used in this
specification, are intended to include "at least one" or "one or more", unless
otherwise indicated. Thus, the articles are used in this specification to
refer to one or
more than one (i.e., to "at least one") of the grammatical objects of the
article. By
way of example, "a component" means one or more components, and thus,
possibly,
more than one component is contemplated and may be employed or used in an
implementation of the described embodiments. Further, the use of a singular
noun
CA 2947139 2018-11-30
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
includes the plural, and the use of a plural noun includes the singular,
unless the
context of the usage requires otherwise.
[0023] In the field
of tribology, corrosion generally refers to the undesired
chemical reaction of material surfaces with components in the surrounding
environment. In this regard, corrosion is a type of chemical degradation and
the
term is usually used in connection with the electrochemical oxidation of metal
and
alloy surfaces. For
instance, the rusting of iron and steel alloys (i.e., the
electrochemical oxidation of elemental iron atoms to iron oxide compounds) is
a well-
known type of corrosion. Corrosion is a diffusion-controlled process;
therefore,
corrosion occurs on material surfaces exposed to an oxidative external
environment.
Corrosion can develop more or less uniformly over exposed material surfaces.
[0024] Pitting
corrosion is a form of localized corrosion that results in the
formation of small holes or "pits" in the metal or alloy forming the surface
of an
article. Pitting corrosion is typically encountered in passivating metals and
alloys,
i.e., alloys such as stainless steels, aluminum and aluminum-base alloys,
titanium
and titanium-base alloys, and nickel-base alloys, for example, that oxidize in
air to
form a stable, adherent, and inert metal oxide layer that provides a surface
barrier to
corrosive attack of the underlying metallic atoms. Pitting corrosion may also
be
encountered in other metal and alloy materials such as non-stainless steels
that
have been passivated by special treatments such as chromate conversion,
phosphate conversion, and galvanization. Metals and alloys such as untreated
carbon steels, for example, which are susceptible to uniform corrosion because
of a
lack of a passivating surface layer, do not generally develop pitting
corrosion. Thus,
a carbon steel article may corrode uniformly in sea water, while a stainless
steel
article may develop localized pitting corrosion in the same environment.
[0025] The chemical
driving force for pitting corrosion is the depassivation of a
small localized area of a metal or alloy article surface where the passivating
layer is
breached or otherwise compromised. The depassivated area becomes anodic (i.e.,
electrochemically oxidative) while an adjacent area becomes cathodic (i.e.,
electrochemically reductive), leading to localized galvanic corrosion at the
depassivated area. The
localized galvanic corrosion penetrates through the
passivating layer and into the sub-surface region of the metal or alloy
article, thereby
forming the small holes or "pits" characteristic of pitting corrosion. Pitting
corrosion
6
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
may be particularly problematic because the localization of the pitting
results in a
relatively small wear effect on material surfaces, but produces significant
material
wear deep into the sub-surface regions of the material.
[0026] Pitting
corrosion may be initiated by a small surface defect such as
damage to a passivating surface layer (e.g., a surface scratch), a local
change in
surface material composition, or other non-uniformities in a material surface
such as
high levels of surface roughness. Accordingly, polished metal and alloy
surfaces
may exhibit higher resistance to pitting corrosion.
[0027]
Additionally, pitting corrosion is a concern in many mechanical systems
comprising mechanically engaging components (e.g., gears) that are made of
metals
or alloys such as stainless steels, aluminum, or titanium, for example. This
is
because the mechanical contact between the engaging components may cause
localized frictional wearing, abrasion, and/or erosion of the metallic
surfaces, thereby
forming localized surface non-uniformities and/or damaging passivating surface
layers, thereby providing localized areas for the development of pitting
corrosion.
Lubrication processes and compositions are intended to reduce frictional
wearing of
the surfaces of mechanically engaging components. However,
conventional
lubrication processes and compositions do not address the removal of surface
non-
uniformities and nascent pitting corrosion.
[0028] Various non-
limiting embodiments described in this specification are
directed to compositions and methods that simultaneously provide synergistic
lubrication and polishing of mechanically-engaging components, such as, for
example, gears and other mechanical components commonly fabricated from metals
and alloys. The
compositions may comprise hard particles and lubricant
nanoparticles. The hard particles comprise micron-scale or nano-scale
particles that
provide micro-polishing or nano-polishing action to the surfaces of
mechanically-
engaging components, thereby removing surface non-uniformities and nascent
pitting corrosion that may form on the components. The lubricant nanoparticles
provide enhanced lubrication to the surfaces of mechanically-engaging
components,
thereby reducing the coefficient of friction and attendant wear. The
combination of
hard particles and lubricant nanoparticles provides the synergistic
combination of
simultaneous surface polishing and surface lubrication that reduces,
minimizes, or
7
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
eliminates pitting corrosion in mechanical systems, in situ, under operating
conditions.
[0029] In various
embodiments, a composition may comprise a plurality of hard
particles and a plurality of lubricant nanoparticles. The hard particles may
have an
average primary particle size of 10 micrometers or less. The lubricant
nanoparticles
may have an average primary particle size of less than or equal to 1000
nanometers,
and in some embodiments, less than or equal to 500 nanometers, less than or
equal
to 400 nanometers, less than or equal to 300 nanometers, less than or equal to
200
nanometers, less than or equal to 100 nanometers, less than or equal to 75
nanometers, less than or equal to 50 nanometers, or less than or equal to 25
nanometers.
[0030] As used in
this specification, including the claims, the term "average
primary particle size" refers to a particle size as determined by visually
examining a
microscopy image of a sample of particles, measuring the largest length
dimension
of the individual particles in the image (i.e., the diameters of the smallest
spheres
that completely surround the individual particles in the image), and
calculating the
average of the length dimensions (diameters) based on the magnification of the
image. A person having ordinary skill in the art will understand how to
prepare a
microscopy image (e.g., light microscopy, transmission electron microscopy,
and the
like) of the particles comprising a composition and determine the average
primary
particle size of constituent particles (or a subset of the constituent
particles based on
like particle composition) based on the magnification of the microscopy image.
As
used in this specification, the term "average primary particle size" refers
the size of
individual particles as opposed to agglomerations of two or more individual
particles.
[0031] In various
embodiments, the hard particles may comprise at least one of
ceramic particles such as hard carbon particles, boride particles, carbide
particles,
nitride particles, oxide particles, silicide particles, carbo-nitride
particles, oxy-nitride
particles, and combinations of any thereof. For example, the hard particles
may
comprise one or more of a metal boride, a metal carbide, a metal nitride, a
metal
oxide, or a metal silicide. The hard particles may also comprise a solid state
solution
of any two or more metal borides, metal carbides, metal nitrides, metal
oxides, or
metal silicides. For example, the hard particles may comprise a mixed metal
oxide
(e.g., an aluminum-zirconium oxide), a mixed metal nitride (e.g., an aluminum-
8
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
titanium nitride or aluminum chromium nitride), a mixed metal carbide (e.g.,
titanium-
zirconium carbide), a mixed metal boride, or a mixed metal silicide. The hard
particles may also comprise particulate mixtures of any two or more metal
borides,
metal carbides, metal nitrides, metal oxides, or metal silicides (e.g., a
combination of
silicon carbide particles and aluminum oxide particles, or a combination of
cubic
boron nitride particles and cubic zirconia particles).
[0032] The hard
particles may comprise at least one of a metal boride, a metal
carbide, a metal nitride, a metal oxide, a metal suicide, a solid state
solution of any
thereof, or a particulate mixture of any thereof, wherein the metal comprises
at least
one element selected from groups IIIA, IVB, VB, and VIB of the periodic table
(i.e.,
the boron group, titanium group, vanadium group, and chromium group,
respectively). For example, the hard particles may comprise at least one of a
metal
boride, a metal carbide, a metal nitride, a metal oxide, a metal suicide, a
solid state
solution of any thereof, or a particulate mixture of any thereof, wherein the
metal
comprises at least one element selected from the group consisting of boron,
aluminum, titanium, zirconium, hafnium, vanadium, niobium, tantalum, chromium,
molybdenum, and tungsten.
[0033] In various
embodiments, the hard particles may comprise hard carbon. In
such embodiments, the hard particles may comprise at least one of synthetic
diamond, natural diamond, amorphous carbon, or nanocrystalline carbon. The
hard
particles may comprise single crystal diamond particles or polycrystalline
diamond
particles (i.e., particles comprise more than one crystal grain per particle),
including
single- or poly-crystalline synthetic or natural diamond particles. The hard
particles
may comprise diamond particles alone or in combination with other types of
hard
particles such as at least one of a metal boride, a metal carbide, a metal
nitride, a
metal oxide, or a metal suicide.
[0034] The hard
particles may comprise at least one metal carbide such as, for
example, a tungsten carbide, molybdenum carbide, chromium carbide, tantalum
carbide, niobium carbide, vanadium carbide, hafnium carbide, zirconium
carbide,
titanium carbide, boron carbide, silicon carbide, solid state solutions of any
thereof,
and particulate mixtures of any thereof. While silicon and boron are often
considered
metalloids and not metallic elements per se, in this specification, it is
understood that
silicon and boron are considered metals for purposes of metal carbides, metal
9
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
nitrides, and metal oxides. Silicon is also considered a metal in connection
with
metal borides.
[0035] The hard
particles may comprise at least one metal nitride such as, for
example, cubic boron nitride, a silicon nitride, a titanium nitride, a
zirconium nitride, a
hafnium nitride, a tungsten nitride, solid state solutions of any thereof, and
particulate
mixtures of any thereof.
[0036] The hard
particles may comprise at least one metal oxide such as, for
example, an aluminum oxide (e.g., cx-A1203, i.e., corundum), a cerium oxide, a
titanium oxide, a zirconium oxide, solid state solutions of any thereof, and
particulate
mixtures of any thereof.
[0037] The hard
particles may comprise at least one metal boride such as, for
example, titanium diboride, zirconium diboride, hafnium diboride, a tantalum
boride,
a tungsten boride (e.g., tungsten tetraboride), a silicon boride, solid state
solutions of
any thereof, and particulate mixtures of any thereof.
[0038] In various
embodiments, the hard particles may comprise particle mixtures
of any of the metal boride, a metal carbide, a metal nitride, a metal oxide,
or a metal
suicides described above. The hard particles may also comprise at least one
mixed
non-metal such as a metal carbonitride. For example, the hard particles may
comprise at least one metal carbonitride such as, for example, a boron
carbonitride,
a silicon carbonitride, a titanium carbonitride, a zirconium carbonitride, a
hafnium
carbonitride, a tungsten carbonitride, solid state solutions of any thereof,
and
particulate mixtures of any thereof.
[0039] In various
embodiments, the hard particles may comprise a metal boride, a
metal carbide, a metal nitride, a metal oxide, a metal suicide, solid state
solutions of
any thereof, mixed metal versions of any thereof, mixed non-metal versions of
any
thereof, or particulate mixtures of any thereof, wherein the constituent
compounds
are stoichiometric or non-stoichiometric.
[0040] In various
embodiments, the hard particles may be functionalized. The
hard particles may be functionalized with organic molecules or functional
groups,
inorganic molecules or functional groups, or both organic and inorganic
molecules or
functional groups, forming functionalized hard particles. The hard particles
may be
functionalized with catalysts, antioxidants, anti-corrosion agents, biocidal
agents, or
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
combinations of any thereof. Examples of antioxidants, anticorrosion agents,
and
biocidel agents include, but are not limited to, antioxidants selected from
the group
consisting of hindered phenols, alkylated phenols, alkyl amines, aryl amines,
2,6-di-
tert-butyl-4-methylphenol, 4,4'-di-tert-octyldiphenylamine, tert-butyl
hydroquinone,
tris(2,4-di-tert-butylphenyl)phosphate, phosphites, thioesters, or
combinations of any
thereof; anticorrosion agents selected from the group consisting of alkaline
earth
metal bisalkylphenolsulphonates, dithiophosphates, alkenylsuccinic acid half-
amides,
or combinations thereof; and biocidal agents material selected from the group
consisting of alkyl benzothiazole, hydroxylamine benzothiazole, an amine salt
of an
alkyl succinic acid, an amine salt of an alkenyl succinic acid, a partial
alkyl ester of
an alkyl succinic acid, a partial alkyl ester of an alkenyl succinic acid, or
combinations of any thereof.
[0041] In various
embodiments, the hard particles may be functionalized with a
dispersant agent. Suitable dispersant agents may comprise at least one
material
selected from the group consisting of amide compounds, borate compounds, and
boride compounds. For example, a dispersant agent may comprise at least one of
succinimide and disodium octaborate tetrahydrate.
[0042] As described above, in various embodiments, a composition may
comprise a plurality of hard particles and a plurality of lubricant
nanoparticles. The
lubricant nanoparticles may have an open architecture.
[0043] As used in
this specification, the term "open architecture" or "open-ended
architecture" refers to the morphology of particles comprising fissures,
separations,
or other discontinuities in the particles' outer surfaces which provide
openings to the
internal portions of the individual particles. In embodiments comprising
layered
particles, the terms "open architecture" or "open-ended architecture" refer to
the
morphology of the layered particles comprising inter-layer defects (e.g.,
shearing,
buckling, folding, curling, and dislocating of constituent atomic and/or
molecular
layers) at the surface of the particles, which increase the inter-planar
spacing
between groupings of molecular layers, thereby providing fissures,
separations, or
other discontinuities in the particles' outer surfaces and openings to the
internal
portions of the particles. As used in this specification, the term "layered
particles" or
"layered nanoparticles" refers to particles comprising generally parallel
stacked
molecular layers, wherein the inter-layer bonding comprises relatively weak
bonding
11
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
such van der Waals bonding, and wherein the intra-layer bonding comprises
relatively strong bonding such as covalent bonding. Examples of layered
particles
include, but are not limited to, graphite particles, molybdenum disulfide
particles,
tungsten disulfide particles, niobium diselenide particles, hexagonal boron
nitride
particles, and carbon particles. It is understood that the terms "open
architecture"
and "open-ended architecture" exclude particle morphologies such as closed
nano-
tubes and fullerenes, which are characterized by closed particle surfaces
lacking
fissures, separations, or other discontinuities in the particles' outer
surfaces.
[0044] The term
"closed architecture," as used in this specification, refers to the
morphology of particles lacking fissures, separations, or other
discontinuities in the
particles' outer surfaces and, therefore, lacking openings to the internal
portions of
the individual particles. In various embodiments, the hard particles may
comprise a
closed architecture or an open architecture.
[0045] The
compositions described in this specification may also comprise an
organic medium intercalated in the lubricant nanoparticles. For example, an
organic
medium may be integrated into the internal portions of individual lubricant
nanoparticles by intercalating into the spaces formed by the fissures,
separations, or
other discontinuities in the outer surfaces of lubricant nanoparticles having
an open
architecture. In various
embodiments, the lubricant nanoparticles may be
intercalated and encapsulated with an organic medium.
[0046] The
lubricant nanoparticles may comprise a lubricant material such as, for
example, molybdenum disulfide, tungsten disulfide, hexagonal boron nitride, or
graphite. Thus, the compositions described in this specification may comprise
molybdenum disulfide nanoparticles, tungsten disulfide nanoparticles,
hexagonal
boron nitride nanoparticles, graphite nanoparticles, or combinations of any
thereof,
as lubricant nanoparticles, which may optionally be encapsulated and/or
intercalated
with an organic medium.
[0047] The organic
medium may comprise at least one material selected from the
group consisting of oil mediums, grease mediums, alcohol mediums, composite
oils,
canola oil, vegetable oil, soybean oil, corn oil, rapeseed oil, ethyl and
methyl esters
of rapeseed oil, monoglycerides, distilled monoglycerides, diglycerides,
acetic acid
esters of monoglycerides, organic acid esters of monoglycerides, sorbitan,
sorbitan
12
81800862
esters of fatty acids, propylene glycol esters of fatty acids, polyglycerol
esters of fatty
acids, hydrocarbon oils, n-hexadecane, phospholipids, lecithins, and
combinations of
any thereof. In various embodiments, the organic medium may comprise an oil
medium such as, for example, a composite oil, canola oil, a vegetable oil,
soybean
oil, corn oil, a hydrocarbon oil, a mineral oil, or combinations of any
thereof.
[0048] The
compositions and methods described in this specification may
comprise, among other components, lubricant nanoparticles which may comprise
nano-sheets. Nano-sheets are further described, for example, in U.S. Patent
Application No. 14/173,369, filed February 5, 2014.
As used in this specification, including the claims,
the term "nano-sheets" refers to planar-shaped particles having a thickness
dimension of less than 500 nanometers and an aspect ratio (defined as the
ratio of
the largest length/width dimension to the thickness dimension) of at least 2.
In some
embodiments, for example, nano-sheets may have a thickness dimension of less
than 100 nanometers and an aspect ratio of at least 10. Nano-sheets may have a
thickness dimension of less than 50 nanometers and an aspect ratio of at least
20.
Nano-sheets may have a thickness dimension of less than 25 nanometers and an
aspect ratio of at least 40. Nano-sheets may have a thickness dimension of
less
than 10 nanometers and an aspect ratio of at least 100. Nano-sheets may have a
thickness dimension corresponding to approximately one unit cell dimension and
such nano-sheets may be referred to as molecular nano-sheets. Nano-sheets may
have length and width dimensions of less than 1000 nanometers.
[0049] Molecular
nano-sheets are a sub-genus of nano-sheets in which the
thickness dimensions of the nano-sheets correspond to approximately one unit
cell
dimension. Molecular nano-sheets may be, but are not necessarily, crystalline
molecular structures. In some embodiments, molecular nano-sheets may have
length and width dimensions of less than or equal to 1000 nanometers, 500
nanometers, or 100 nanometers. In some embodiments, molecular nano-sheets
may have a thickness dimension corresponding to approximately one unit cell
dimension. Generally, a molecular nano-sheet may comprise a single layer of
any
layered nanoparticle (e.g., a graphite/graphene molecular nano-sheet, a
molybdenum disulfide molecular nano-sheet, a tungsten disulfide molecular nano-
13
CA 2947139 2018-11-30
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
sheet, a niobium diselenide molecular nano-sheet, or a hexagonal boron nitride
molecular nano-sheet).
[0050] The crystal
structure of a material (L e., the spatial arrangement of the
atoms forming a crystal) can be described in terms of the unit cell. A unit
cell is the
smallest molecular unit that a crystal can be divided into using
crystallographic
symmetry operations. In other words, a unit cell is the simplest repeating
unit in a
crystalline material. Unit cells stacked in three-dimensional space describe
the bulk
arrangement of atoms of a crystalline material.
[0051] By way of
example, molybdenum disulfide predominantly exists in a
hexagonal crystal form characterized by MoS2 layers in which the molybdenum
atoms have trigonal prismatic coordination of six sulfur atoms with two
molecules per
unit cell. Thus, the molybdenum disulfide crystal structure comprises a tri-
layer
having one planar hexagonal layer of molybdenum atoms interspersed between two
planar layers of sulfur atoms forming an intra-molecular covalently bonded S-
Mo-S
molecular layer. Bulk
molybdenum disulfide comprises relatively weak inter-
molecular van der Weals bonds between the adjacent sulfur atoms of stacked S-
Mo-
S molecular layers.
[0052] Referring to
Figure 10(A), two intra-molecular covalently bonded S-Mo-S
molecular layers 10 are shown with inter-molecular van der Weals bonds 17
between the adjacent sulfur atoms 11 of the two stacked S-Mo-S molecular
layers
10. Within each S-Mo-S molecular layer 10, the molybdenum atoms 13 and the
sulfur atoms 11 form the tri-layer comprising one planar hexagonal layer of
molybdenum atoms 13 interspersed between two planar layers of sulfur atoms 11
and forming covalent bonds 15. Referring to Figure 10(B), the molybdenum
disulfide
unit cell has a thickness dimension of approximately 3.241 angstroms across
the S-
Mo-S molecular layer.
[0053] Accordingly,
a molybdenum disulfide molecular nano-sheet may comprise
a molybdenum disulfide crystal having a thickness dimension corresponding to
the
thickness of the covalently bonded S-Mo-S molecular layer (without adjoining
inter-
molecular van der Weals bonded layers, i.e., approximately 3.241 angstroms)
and, in
14
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
some embodiments, length and width dimensions of less than or equal to 1000
nanometers.
[0054] The crystal
structure of tungsten disulfide is analogous to that of
molybdenum disulfide and, therefore, a tungsten disulfide molecular nano-sheet
may
comprise a tungsten disulfide crystal having a thickness dimension
corresponding to
the thickness of the covalently bonded S-W-S molecular layer (without
adjoining
inter-molecular van der Waals bonded layers) and, in some embodiments, length
and width dimensions of less than or equal to 1000 nanometers.
[0055] The crystal
structure of hexagonal boron nitride is characterized by
hexagonal coordination between three nitrogen atoms and three boron atoms
forming adjacent six-sided rings that form intra-molecular covalently-bonded
mono-
layers that are atomically thin (L e., having a thickness dimension of a
single atom).
Referring to Figure 11, three intra-molecular covalently bonded B-N molecular
layers
20 are shown with inter-molecular van der Waals bonds 27 between the adjacent
B-
N molecular layers 20. Within each B-N molecular layer 20, the boron atoms 23
and
the nitrogen atoms 21 form the mono-layer comprising the hexagonal atomic
orientation within a single plane and forming the covalent bonds 25. Thus, the
hexagonal boron nitride crystal structure comprises B-N molecular mono-layers,
and
bulk hexagonal boron nitride comprises relatively weak inter-molecular van der
Waals bonds between the adjacent B-N molecular mono-layers. Therefore, a
hexagonal boron nitride molecular nano-sheet may comprise a hexagonal boron
nitride crystal having a single atomic thickness dimension and, in some
embodiments, length and width dimensions of less than or equal to 1000
nanometers.
[0056] The crystal
structure of graphite (carbon) is analogous to that of hexagonal
boron nitride and, therefore, a graphite molecular nano-sheet may comprise a
graphene crystal having a single atomic thickness dimension and, in some
embodiments, length and width dimensions of less than or equal to 1000
nanometers.
[0057] In various
embodiments, nano-sheets may comprise a material such as,
for example, molybdenum disulfide, tungsten disulfide, niobium diselenide,
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
hexagonal boron nitride, graphite/graphene, metals such as copper or silver,
inorganic compounds such as calcium carbonate, polymers such as PTFE, or
dithiophosphate compounds. In some embodiments, the nano-sheets may comprise
molecular nano-sheets comprising a material such as, for example, molybdenum
disulfide, tungsten disulfide, niobium diselenide, hexagonal boron nitride, or
graphene. Thus, the compositions described in this specification may comprise
molybdenum disulfide nano-sheets, tungsten disulfide nano-sheets, niobium
diselenide nano-sheets, hexagonal boron nitride nano-sheets, graphite nano-
sheets,
graphene molecular nano-sheets, metal (e.g., copper) nano-sheets, inorganic
compound (e.g., calcium carbonate) nano-sheets, polymer (e.g., PTFE) nano-
sheets, nano-sheets comprising dithiophosphate compounds, or combinations of
any
thereof.
[0058] It is
important to recognize that layered materials such as, for example,
molybdenum disulfide, tungsten disulfide, niobium diselenide, hexagonal boron
nitride, and graphite, may form nano-sheets (e.g., planar-shaped particles
having a
thickness dimension of less than 500 nanometers and an aspect ratio of at
least 2)
or molecular nano-sheets (e.g., crystalline molecular structures comprising a
thickness dimension corresponding to approximately one unit cell dimension).
In this
regard, molecular nano-sheets are a sub-genus of nano-sheets.
[0059] In various
embodiments, the nano-sheets and/or nanoparticles may be
functionalized. The nano-sheets and/or nanoparticles may be functionalized
with
organic molecules or functional groups, inorganic molecules or functional
groups, or
both organic and inorganic molecules or functional groups, forming
functionalized
nano-sheets and/or nanoparticles. The nano-sheets and/or nanoparticles may be
functionalized with catalysts, antioxidants, anti-corrosion agents, biocidal
agents, or
combinations of any thereof. Examples of antioxidants, anticorrosion agents,
and
biocidal agents include, but are not limited to, antioxidants selected from
the group
consisting of hindered phenols, alkylated phenols, alkyl amines, aryl amines,
2,6-di-
tert-butyl-4-methylphenol, 4,4'-di-tert-octyldiphenylamine, tert-butyl
hydroquinone,
tris(2,4-di-tert-butylphenyl)phosphate, phosphites, thioesters, or
combinations of any
thereof; anticorrosion agents selected from the group consisting of alkaline
earth
metal bisalkylphenolsulphonates, dithiophosphates, alkenylsuccinic acid half-
amides,
16
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
or combinations thereof; and biocidal agents material selected from the group
consisting of alkyl benzothiazole, hydroxylamine benzothiazole, an amine salt
of an
alkyl succinic acid, an amine salt of an alkenyl succinic acid, a partial
alkyl ester of
an alkyl succinic acid, a partial alkyl ester of an alkenyl succinic acid, or
combinations of any thereof.
[0060] In various
embodiments, the nano-sheets and/or nanoparticles may be
functionalized with a dispersant agent. Suitable dispersant agents may
comprise at
least one material selected from the group consisting of amide compounds,
borate
compounds, and boride compounds. For example, a dispersant agent may comprise
at least one of succinimide and disodium octaborate tetrahydrate.
[0061] In various
embodiments, the nano-sheets and/or nanoparticles may be
coated and/or encapsulated with an organic medium. For instance, an organic
medium may be chemically or physically adsorbed onto nano-sheets and/or
nanoparticles or otherwise chemically or physically bonded to nano-sheets
and/or
nanoparticles. As described above, nanoparticles may be encapsulated and/or
intercalated with an organic medium. As used herein, the term "organic medium"
refers to hydrophobic/oleophilic substances and carbon-based compounds. For
example, the organic medium may comprise at least one material selected from
the
group consisting of oil media, grease media, alcohol media, composite oils,
mineral
oils, synthetic oils, canola oil, vegetable oil, soybean oil, corn oil,
rapeseed oil, ethyl
and methyl esters of rapeseed oil, monoglycerides, distilled monoglycerides,
diglycerides, acetic acid esters of monoglycerides, organic acid esters of
monoglycerides, sorbitan, sorbitan esters of fatty acids, propylene glycol
esters of
fatty acids, polyglycerol esters of fatty acids, hydrocarbon oils, n-
hexadecane,
phospholipids, lecithins, amide compounds, boron-containing compounds,
dithiophosphate compounds, and combinations of any thereof. Examples of
suitable
dithiophosphate compounds that may comprise an organic medium include, but are
not limited to, zinc dialkyl dithiophosphate (ZDDP) and molybdenum
dithiophosphate
(MoDTP), which may be used alone or in combination with any other organic
medium such as an oil medium. In various embodiments, the organic medium may
comprise an oil medium such as, for example, a composite oil, a mineral oil, a
synthetic oil, canola oil, a vegetable oil, soybean oil, corn oil, a
hydrocarbon oil, a
mineral oil, or combinations of any thereof.
17
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
[0062] In addition
to the lubricant nanoparticles, the compositions described in
this specification may also comprise a secondary solid lubricant material such
as, for
example, polytetrafluoroethylene, soft metals, silver, lead, nickel, copper,
cerium
fluoride, zinc oxide, silver sulfate, cadmium iodide, lead iodide, barium
fluoride, tin
sulfide, zinc phosphate, zinc sulfide, mica, boron nitrate, borax, fluorinated
carbon,
zinc phosphide, boron, or combinations of any thereof. The secondary solid
lubricant material may comprise nanoparticles. The secondary solid lubricant
nanoparticles may have an average primary particle size of less than or equal
to
1000 nanometers, and in some embodiments, less than or equal to 500
nanometers,
less than or equal to 400 nanometers, less than or equal to 300 nanometers,
less
than or equal to 200 nanometers, less than or equal to 100 nanometers, less
than or
equal to 75 nanometers, less than or equal to 50 nanometers, or less than or
equal
to 25 nanometers.
[0063] In various
embodiments, the compositions described in this specification
may also comprise a base lubricant material. The hard particles and the
lubricant
nanoparticles may be dispersed in the base lubricant material. The base
lubricant
material may comprise a material such as, for example, an oil, a grease, a
polymer,
a plastic, a gel, a paste, a wax, a silicone, a hydrocarbon oil, a vegetable
oil, corn oil,
peanut oil, canola oil, soybean oil, a mineral oil, a paraffin oil, a
synthetic oil, a
petroleum gel, a petroleum grease, a hydrocarbon gel, a hydrocarbon grease, a
lithium based grease, a fluorocarbon based grease, silicon based grease,
ethylenebistearamide, or combinations of any thereof. In various embodiments,
the
base lubricant material may comprise at least one material selected from the
group
consisting of an oil, a grease, a plastic, a gel, a wax, a silicone, and
combinations of
any thereof. In various embodiments, the base lubricant material may comprise
an
oil or a grease. In various embodiments, the base lubricant material may
comprise
at least one material selected from the group consisting of a mineral oil, a
paraffin oil,
a synthetic oil, a petroleum grease, a hydrocarbon grease, a lithium based
grease, or
combinations of any thereof.
[0064] In various
embodiments, the compositions described in this specification
may also comprise an emulsifier. The emulsifier may comprise at least one
material
selected from the group consisting of lecithins, phospholipids, soy lecithins,
detergents, distilled monoglycerides, monoglycerides, diglycerides, acetic
acid esters
18
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
of monoglycerides, organic acid esters of monoglycerides, sorbitan esters of
fatty
acids, propylene glycol esters of fatty acids, polyglycerol esters of fatty
acids,
compounds containing phosphorous, compounds containing sulfur, compounds
containing nitrogen, or combinations of any thereof. In various embodiments,
the
emulsifier may comprise a compound containing phosphorous. In various
embodiments, the emulsifier may comprise a phospholipid. In various
embodiments,
the emulsifier may comprise a lecithin.
[0065] In various
embodiments, the compositions described in this specification
may also comprise one or more of an antioxidant, an anticorrosion agent, or a
biocidal. For example, the compositions may comprise at least one antioxidant
material selected from the group consisting of hindered phenols, alkylated
phenols,
alkyl amines, aryl amines, 2,6-di-tert-
butyl-4-methylphenol, 4,41-di-tert-
octyldiphenylamine, tert-butyl hydroquinone, tris(2,4-di-tert-
butylphenyl)phosphate,
phosphites, thioesters, or combinations of any thereof. The compositions may
comprise at least one anticorrosion agent selected from the group consisting
of
alkaline earth metal bisalkylphenolsulphonates, dithiophosphates,
alkenylsuccinic
acid half-amides, or combinations thereof. The compositions may comprise at
least
one biocidal material selected from the group consisting of alkyl
benzothiazole,
hydroxylamine benzothiazole, an amine salt of an alkyl succinic acid, an amine
salt
of an alkenyl succinic acid, a partial alkyl ester of an alkyl succinic acid,
a partial alkyl
ester of an alkenyl succinic acid, or combinations of any thereof.
[0066] The
compositions described in this specification may be used to formulate
a lubricant. For example,
compositions comprising hard particles, lubricant
nanoparticles, and an organic medium intercalated in the lubricant
nanoparticles may
be used as performance-enhancing additives to off-the-shelf liquid based
lubricants.
Additionally, lubricant compositions may comprise hard particles and lubricant
nanoparticles dispersed in a lubricant base material as described above,
wherein a
separate organic medium is intercalated in the lubricant nanoparticles.
Lubricant
compositions comprising hard particles, lubricant nanoparticles, and an
organic
medium intercalated in the lubricant nanoparticles, in accordance with the
embodiments described in this specification, will provide a synergistically
enhanced
combination of simultaneous surface polishing and surface lubrication that
reduces,
19
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
minimizes, or eliminates pitting corrosion in mechanical systems, in situ,
under
operating conditions.
[0067] For
instance, a gear oil for automotive or other mechanical gearing
applications, may be formulated to comprise hard particles, lubricant
nanoparticles,
and an organic medium intercalated in the lubricant nanoparticles, where the
gear oil
provides improved wear protection and reduced pitting, decreasing frictional
energy
losses and improving energy utilization efficiency (e.g., fuel efficiency in
automotive
and racing applications). In lubrication applications, compositions formulated
to
comprise hard particles, lubricant nanoparticles, and an organic medium
intercalated
in the lubricant nanoparticles may effectively operate under high load, high
temperature, and high speed conditions, and may provide improved lubrication
and
pitting resistance under elastohydrodynamic, boundary, and mixed lubrication
regimes. Such improvements may be realized by the addition of additives to off-
the-
shelf liquid phase lubricants, where the additives comprise hard particles,
lubricant
nanoparticles, and an organic medium intercalated in the lubricant
nanoparticles.
[0068] In various
embodiments, a composition may comprise, in weight percent
based on total weight of the particles in the composition, from 1% to 99% hard
particles, and from 1% to 99% lubricant nanoparticles, or any sub-ranges
subsumed
therein, such as, for example, 5% to 95% hard particles, and from 5% to 95%
lubricant nanoparticles.
[0069] The
compositions and methods comprising lubricant nanoparticles and an
organic medium may be made from solid lubricant starting materials. Examples
of
solid lubricants may include, but are not limited to, layered materials such
as, for
example, chalcogenides, like molybdenum disulfide, tungsten disulfide, or a
combination thereof. Other
suitable layered materials include graphite or
intercalated graphite. Other solid
lubricants that may be used alone or in
combination with the layered materials include, but are not limited to
polytetrafluoroethylene (e.g., Teflon ), hexagonal boron nitride, soft metals
(such as,
for example, silver, lead, nickel, copper), cerium fluoride, zinc oxide,
silver sulfate,
cadmium iodide, lead iodide, barium fluoride, tin sulfide, zinc phosphate,
zinc sulfide,
mica, boron nitrate, borax, fluorinated carbon, zinc phosphide, boron, or a
combination thereof. Fluorinated carbons may be, without limitation, carbon-
based
materials such as graphite which has been fluorinated to improve its aesthetic
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
characteristics. Such materials may include, for example, a material such as
CFx
wherein x ranges from about 0.05 to about 1.2. Such a material is produced,
for
example, by Allied Chemical under the trade name Accufluor.
[0070] The methods
of making the lubricant nanoparticles encapsulated and/or
intercalated with the organic medium may include, for example, the milling of
a solid
lubricant feed material. In various embodiments, the solid lubricant feed
material
may be capable of being milled to particles comprising an average primary
particle
size of about 500 nanometers (submicron size) to about 10 nanometers. The
particles may have an average primary particle size of less than or equal to
about
500 nanometers, less than or equal to about 100 nanometers, less than or equal
to
about 75 nanometers, and less than or equal to about 50 nanometers.
Alternatively,
the milling may result in milled lubricant particles comprising a mixture, the
mixture
comprising particles having an average primary particle size of less than or
equal to
about 500 nanometers, plus larger particles. The milling may include, among
other
techniques, ball milling and chemo-mechanical milling. Examples of ball
milling may
include dry ball milling, wet ball milling, and combinations thereof. Ball
milling may
refer to an impaction process that may include two interacting objects where
one
object may be a ball, a rod, 4 pointed pins (jack shape), or other shapes.
Chemo-
mechanical milling may refer to an impaction process that may form an
integrated
complex between the organic medium and the nanoparticles. As a result of chemo-
mechanical milling, the organic medium may coat, encapsulate, and/or
intercalate
the nanoparticles. In various embodiments, chemo-mechanical milling may be
performed using a ball milling technique.
[0071] In various
embodiments, the solid lubricant feed may be dry milled and
then wet milled. An emulsifier may be mixed with a lubricant base material and
added to the wet milled particles. Dry milling may refer to particles that
have been
milled in the presence of a vacuum, a gas, or a combination thereof. Wet
milling
may refer to particles that have been milled in the presence of a liquid.
[0072] As described
above, the lubricant nanoparticle composition may further
comprise an organic medium. Examples of organic mediums include, but are not
limited to, oil mediums, grease mediums, alcohol mediums, or combinations
thereof.
Specific examples of organic mediums include, but are not limited to,
composite oil,
21
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
canola oil, vegetable oils, soybean oil, corn oil, ethyl and methyl esters of
rapeseed
oil, distilled monoglycerides, monoglycerides, diglycerides, acetic acid
esters of
monoglycerides, organic acid esters of monoglycerides, sorbitan, sorbitan
esters of
fatty acids, propylene glycol esters of fatty acids, polyglycerol esters of
fatty acids, n-
hexadecane, hydrocarbon oils, phospholipids, or a combination thereof. Many of
these organic media may be environmentally acceptable.
[0073] The
compositions described in this specification may contain emulsifiers,
surfactants, or dispersants. Examples of emulsifiers may include, but are not
limited
to, emulsifiers having a hydrophilic-lipophilic balance (HLB) from about 2 to
about 7;
a HLB from about 3 to about 5; or a HLB of about 4. Examples of emulsifiers
may
include, but are not limited to, lecithins, soy lecithins, phospholipids
lecithins,
detergents, distilled monoglycerides, monoglycerides, diglycerides, acetic
acid esters
of monoglycerides, organic acid esters of monoglycerides, sorbitan esters of
fatty
acids, propylene glycol esters of fatty acids, polyglycerol esters of fatty
acids,
compounds containing phosphorous, compounds containing sulfur, compounds
containing nitrogen, or a combination thereof.
[0074] A method of
making a composition, such as, for example, a lubricant
additive or a primary lubricant formulation, is described. The composition may
be
used as an additive dispersed in a lubricant base material or as a component
of a
primary lubricant formulation. Examples of lubricant base materials may
include, but
are not limited to, oils, greases, plastics, gels, sprays, or a combination
thereof.
Specific examples of bases may include, but are not limited to, hydrocarbon
oils,
vegetable oils, corn oil, peanut oil, canola oil, soybean oil, mineral oil,
paraffin oils,
synthetic oils, petroleum gels, petroleum greases, hydrocarbon gels,
hydrocarbon
greases, lithium based greases, fluoroether based greases,
ethylenebistearamide,
waxes, silicones, or a combination thereof.
[0075] Described in
this specification is a method of lubricating or coating an
object that is part of an end application with a composition comprising hard
particles,
lubricant nanoparticles, and an organic medium. Further described is a method
of
lubricating an object by employing the composition comprising hard particles,
lubricant nanoparticles, and an organic medium as a delivery or carrier
mechanism.
22
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
[0076] In various
embodiments, a catalyst delivery or carrier system comprises
hard particles, lubricant nanoparticles, an organic medium, and a catalyst
material.
The catalyst material may be functionalized onto the surface of the hard
particles,
lubricant nanoparticles, or both. The catalyst material may be intercalated or
otherwise integrated into the lubricant nanoparticles.
[0077] The
compositions and methods described in this specification exhibit,
among various advantages, enhanced dispersion stability, resistance to
agglomeration, and corrosion resistance. Figure 1 illustrates a method of
preparing
nanoparticle based lubricants or compositions. A solid lubricant feed is
introduced
via line 210 to a ball milling processor 215. Ball milling is carried out in
the processor
215 and the solid lubricant feed is milled to comprise particles having an
average
primary particle size of less than or equal to about 1000 nanometers, less
than or
equal to about 500 nanometers, less than or equal to about 100 nanometers,
less
than or equal to about 80 nanometers, or less than or equal to about 50
nanometers.
Alternatively, the ball milling may result in milled solid lubricant particles
comprising a
mixture, the mixture comprising particles having an average particle dimension
of
less than or equal to about 1000 nanometers or less than or equal to about 500
nanometers, plus larger particles. The ball milling may be high energy ball
milling,
medium energy ball milling, or combinations thereof.
Additionally, in various
embodiments the ball milling may be carried out in a vacuum, in the presence
of a
gas, in the presence of a liquid, in the presence of a second solid, or
combinations
thereof. The nanoparticle composition may be removed from the processor via
line
220. The nanoparticle composition may be a nanoparticle based lubricant.
[0078] In various
embodiments, the ball milling may comprise a first ball milling
and at least one more subsequent ball millings, or ball milling and/or other
processing as appropriate. The ball milling may comprise dry milling followed
by wet
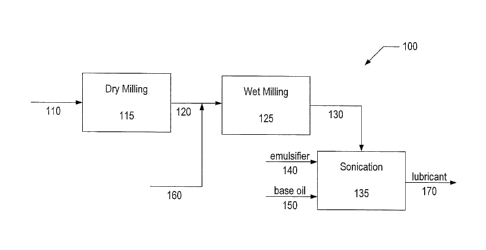
milling. Figure 2 illustrates a further method 100 of preparing nanoparticle
based
lubricants and other compositions where dry milling is followed by wet
milling. Feed
110 introduces a solid lubricant feed into a ball milling processor 115 where
dry ball
milling, such as in a vacuum or in air, reduces the solid lubricant feed to
particles
having an average dimension of the size described above. Line 120 carries the
dry
milled particles to a wet milling processor 125. Via line 160 the dry milled
particles
23
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
are combined with a composite oil or an organic medium prior to entering the
wet
milling processor 125. Alternatively, the organic medium and dry milled
particles
may be combined in the wet milling processor 125. In further
alternative
embodiments (not shown), the dry milling and wet milling may be carried out in
a
single processor where the organic medium is supplied to the single processor
for
wet milling after initially carrying out dry milling. In other alternative
embodiments,
the balls in the ball milling apparatus may be coated with the organic medium
to
incorporate the organic medium in the lubricant nanoparticles.
[0079] After wet
milling, line 130 carries the wet milled particles to a container
135, which may be a sonication device. Alternatively, line 130 may carry a
mixture
comprising lubricant nanoparticles, organic medium, and a complex comprising
the
lubricant nanoparticles combined with an organic medium.
[0080] In another
embodiment, prior to introduction of the wet milled particles into
the container 135, a lubricant base material may be fed to the container 135
via line
150. Alternatively, the base may be supplied to the wet milling processor 125
and
the mixing, which may include sonicating, may be carried out in the wet
milling
processor 125. In such embodiments the lubricant nanoparticle composition may
be
employed as an additive and dispersed in the lubricant base material. A
lubricant
base material may be paired with a lubricant nanoparticle composition
according to
the ability of the base material and the lubricant nanoparticle composition to
blend
appropriately. In such cases the lubricant nanoparticle composition may
enhance
the performance characteristics of the base.
[0081] In various
embodiments, an emulsifier may be mixed with the lubricant
base material. Emulsifiers
may further enhance dispersion of the lubricant
nanoparticle composition in the lubricant base material. The emulsifier may be
selected to enhance the dispersion stability of a nanoparticle composition in
a base.
An emulsifier may also be supplied to the container 135 via line 140. In some
embodiments, the emulsifier and base are combined in the container 135 prior
to
introduction of the wet milled particles. Prior mixing of the emulsifier with
the base
material may enhance dispersion upon addition of complexes of lubricant
nanoparticles and organic medium and/or lubricant nanoparticles by
homogeneously
dispersing the complexes/nanoparticles. In some embodiments, the mixing of the
24
81800862
emulsifier and base may comprise sonicating. The emulsifier may be supplied to
the
wet milling processor 125 and the mixing, which may include sonicating, may be
carried out in the wet milling processor 125. The lubricant removed from the
container 135 via line 170 may be a blend comprising the wet milled particles,
organic medium, and base. The blend may further comprise an emulsifier.
[0082] In various embodiment, antioxidants or anticorrosion agents may be
milled
with the lubricant nanoparticles or added to prior milled lubricant
nanoparticles.
Examples of antioxidants include, but are not limited to, hindered phenols,
alkylated
phenols, alkyl amines, aryl amines, 2,6-di-tert-butyl-4-methylphenol, 4,4*-di-
tert-
octyldiphenylamine, tert-Butyl hydroquinone, tris(2,4-di-tert-
butylphenyl)phosphate,
phosphites, thioesters, or combinations of any thereof. Examples of
anticorrosion
agents include, but are not limited to, alkaline-earth metal
bisalkylphenolsulphonates,
dithiophosphates, alkenylsuccinic acid half-amides, or combinations of any
thereof.
In various embodiments, biocidals may be milled with the lubricant
nanoparticles or
added to prior milled lubricant nanoparticles. Examples of biocidals may
include, but
are not limited to, alkyl or hydroxylamine benzotriazole, an amine salt of a
partial
alkyl ester of an alkyl, alkenyl succinic acid, or a combination thereof.
[0083] In various embodiments, further processing of wet milled particles
may
comprise removal of oils that are not a part of a complex with the solid
lubricant
particles. Such methods may be suitable for applications that benefit from use
of dry
particles of solid lubricant, such as coating applications. Oil and/or other
liquids can
be removed from wet milled particles to produce substantially dry solid
lubricant
particles and complexes having intercalated organic media. Such wet milling
followed by drying may produce a lubricant with reduced tendency to
agglomerate.
In specific embodiments, an agent, such as acetone or other suitable solvent,
may
be added that dissolves oils that are not a part of a complex with the
particles,
followed by a drying process such as supercritical drying, to produce a
substantially
dry lubricant comprising particles treated by milling in an organic medium.
[0084] Ball milling conditions may vary and, in particular, conditions
such as
temperature, milling time, and size and materials of the balls and vials may
be
manipulated. In various embodiments, ball milling may be carried out from
about 12
hours to about 50 hours, from about 36 hours to about 50 hours, from about 40
CA 2947139 2018-11-30
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
hours to about 50 hours, or for about 48 hours ( 1 hour, 2 hours, or 3
hours).
Ball milling may be conducted at room temperature or elevated temperatures.
The
benefits of increasing milling time may comprise at least one of increasing
the time
for the organic medium and lubricant nanoparticles to interact, integrate, and
complex; and producing finer sizes, better yield of nanoparticles, more
uniform
shapes, and more passive surfaces. An example of ball milling equipment
suitable
for carrying out the described milling includes the SPEX CertiPrep model
8000D,
along with hardened stainless steel vials and hardened stainless steel
grinding balls,
but any type of ball milling apparatus may be used. In some embodiments, a
stress
of 600-650 MPa, a load of 14.9 N, and a strain of 10-3-10-4 per second may be
used.
[0085] The
proportions of the components in a nanoparticle based lubricant or
other composition may contribute to performance of the composition, such as
the
composition's dispersion stability and ability to resist agglomeration. In wet
milling,
suitable ratios of lubricant nanoparticles to organic medium may be about 1
part
particles to about 4 parts organic medium by weight, about 1 part particles to
about 3
parts organic medium by weight, about 3 parts particles to about 8 parts
organic
medium by weight, about 2 parts particles to about 4 parts organic medium by
weight, about 1 part particles to about 2 parts organic medium by weight, or
about 1
part particles to about 1.5 parts organic medium by weight.
[0086] Suitable
ratios of organic medium to emulsifier in a composition including
the lubricant nanoparticles may be about 1 part organic medium to less than or
equal
to about 1 part emulsifier, about 1 part organic medium to about 0.5 parts
emulsifier
by weight, or from about 0.4 to about 1 part emulsifier for about 1 part
organic
medium by weight.
[0087] The amount
of lubricant nanoparticle composition (by weight) sonicated or
dispersed in a lubricant base materials may comprise from about 0.25% to about
5%, about 0.5% to about 3%, about 0.5% to about 2%, or about 0.75% to about
2%,
based on total weight of the composition.
[0088] The amount
of emulsifier (by weight) sonicated or dissolved in a lubricant
base material, depending on the end application, shelf-life, and the like, may
comprise from about 0.5% to about 10%, from about 4% to about 8%, from about
5%
26
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
to about 6%, or from about 0.75% to about 2.25%, based on total weight of the
composition.
[0089] The
compositions described in this specification may be used, without
limitation, as lubricants, coatings, delivery mechanisms, or combinations of
any
thereof. The compositions may be used, without limitation, as an additive
dispersed
in a base oil or other lubricant composition. The compositions may also be
used,
without limitation, to lubricate a boundary lubrication regime. A boundary
lubrication
regime may be a lubrication regime where the average lubricant film thickness
may
be less than the composite surface roughness and the surface asperities may
come
into contact with each other under relative motion. During the relative motion
of two
surfaces with lubricants in various applications, three different lubrication
stages may
occur, and the boundary lubrication regime may be the most severe condition in
terms of temperature, pressure and speed. Mating parts may be exposed to
severe
contact conditions of high load, low velocity, extreme pressure (for example,
1-2
GPa), and high local temperature (for example, 150-300 degrees C.). The
boundary
lubrication regime may also exist under lower pressures and low sliding
velocities or
high temperatures. In the boundary lubrication regime, the mating surfaces may
be
in direct physical contact.
[0090] The
compositions may further be used, without limitation, as a lubricant or
coating in machinery applications, manufacturing applications, mining
applications,
aerospace applications, automotive applications, pharmaceutical applications,
medical applications, dental applications, cosmetic applications, food product
applications, nutritional applications, health related applications, bio-fuel
applications
or a combination thereof. Specific examples of uses in end applications
include,
without limitation, machine tools, bearings, gears, camshafts, pumps,
transmissions,
piston rings, engines, power generators, pin-joints, aerospace systems, mining
equipment, manufacturing equipment, or a combination thereof. Further specific
examples of uses may be, without limitation, as an additive in lubricants,
greases,
gels, compounded plastic parts, pastes, powders, emulsions, dispersions, or
combinations thereof. The composition may also be used as a lubricant that
employs the lubricant nanoparticle composition as a delivery mechanism in
pharmaceutical applications, medical applications, dental applications,
cosmetic
27
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
applications, food product applications, nutritional applications, health
related
applications, bio-fuel applications, or a combination thereof. The various
compositions and methods may also be used, without limitation, in hybrid
inorganic-
organic materials. Examples of
applications using inorganic-organic materials,
include, but are not limited to, optics, electronics, ionics, mechanics,
energy,
environment, biology, medicine, smart membranes, separation devices,
functional
smart coatings, photovoltaic and fuel cells, photocatalysts, new catalysts,
sensors,
smart microelectronics, micro-optical and photonic components and systems for
nanophotonics, innovative cosmetics, intelligent therapeutic vectors that
combined
targeting, imaging, therapy, and controlled release of active molecules, and
nanoceramic-polymer composites.
[0091] In some
embodiments, the ball milling process may create a close caged
dense oval shaped architecture (similar to a football shape or fullerene type
architecture). This may occur when solid lubricant feed materials are milled
in a gas
or vacuum. Figure 7(A) shows the close caged dense oval shaped architecture of
molybdenum disulfide nanoparticles that have been ball milled in air for 48
hours.
[0092] In other
embodiments, the ball milling process may create an open
architecture (as described above), which may be encapsulated and/or
intercalated
with an organic medium. This may occur when solid lubricant feed materials are
milled in a gas or vacuum followed by milling in an organic medium. Figure
7(B)
shows the open architecture of molybdenum disulfide nanoparticles that have
been
ball milled in air for 48 hours followed by ball milling in canola oil for 48
hours.
[0093] As shown in
the examples, the tribological performance of the nanoparticle
based lubricant may be improved. The tribological performance may be measured
by evaluating different properties. An anti-wear property may be a lubricating
fluid
property that has been measured using the industry standard Four-Ball Wear
(ASTM
D4172) Test. The Four-Ball Wear Test may evaluate the protection provided by a
lubricant under conditions of pressure and sliding motion. Placed in a bath of
the
test lubricant, three fixed steel balls may be put into contact with a fourth
ball of the
same grade in rotating contact at preset test conditions. Lubricant wear
protection
properties may be measured by comparing the average wear scars on the three
fixed balls. The smaller the average wear scar, the better the protection.
Extreme
28
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
pressure properties may be lubricating fluid properties that have been
measured
using the industry standard Four-Ball Wear (ASTM D2783) Test. This test method
may cover the determination of the load-carrying properties of lubricating
fluids. The
following two determinations may be made: 1) load-wear index (formerly Mean-
Hertz
load) and 2) weld load (kg). The load-wear index may be the load-carrying
property
of a lubricant. It may be an index of the ability of a lubricant to minimize
wear at
applied loads. The weld load may be the lowest applied load in kilograms at
which
the rotating ball welds to the three stationary balls, indicating the extreme
pressure
level that the lubricants can withstand. The higher the weld point scores and
load
wear index values, the better the anti-wear and extreme pressure properties of
a
lubricant. The coefficient of friction (COF) may be a lubricating fluid
property that
has been measured using the industry standard Four-Ball Wear (ASTM D4172)
Test.
COF may be a dimensionless scalar value which describes the ratio of the force
of
friction between two bodies and the force pressing them together. The
coefficient of
friction may depend on the materials used. For example, ice on metal has a low
COF, while rubber on pavement has a high COF. A common way to reduce friction
may be by using a lubricant which is placed between two surfaces.
[0094] The
compositions described in this specification may have a wear scar
diameter of about 0.4 mm to about 0.5 mm. The composition may have a COF of
about 0.06 to about 0.08. The composition may have a weld load of about 150 kg
to
about 350 kg. The composition may have a load wear index of about 20 to about
40.
The values of these tribological properties may change depending on the amount
of
lubricant nanoparticle composition sonicated or dissolved in the lubricant
base
material.
[0095] The non-
limiting and non-exhaustive examples that follow are intended to
further describe various non-limiting and non-exhaustive embodiments without
restricting the scope of the embodiments described in this specification.
29
81800862
EXAMPLES
Example 1:
TM
[00961 Ball milling was performed in a SPEX 8000D machine using hardened
stainless steel vials and balls. MoS2 (Alfa Aesar, 98% pure, 700 nm average
primary
particle size) and canola oil (Crisco) were used as the starting materials in
a ratio of
1 part MoS2 (10 grams) to 2 parts canola oil (20 grams). The ball to powder
weight
ratio was 2 to 1. MoS2 was ball milled for 48 hours in air followed by milling
in canola
oil for 48 hrs at room temperature. The nanoparticles were about 50 nm after
ball
milling. Table 1 summarizes milling conditions and resultant particle
morphologies.
It was observed that there was a strong effect of milling media on the shape
of the
ball milled nanoparticles. Dry milling showed buckling and folding of the
planes
when the particle size was reduced from micron size to nanometer size.
However,
the dry milling condition used here produced micro clusters embedding several
nanoparticles. On the other hand, wet milling showed no buckling but saw de-
agglomeration.
Table I: Milling conditions and parametric effect on particle size and shape
Dry Milling Shape of the particles Shape of the
clusters
12 hours Plate-like with sharp edges Sharp and irregular
24 hours Plate-like with round edges More or less rounded
48 hours Spherical Globular clusters
Wet Milling Shape of the Particles Shape of the
clusters
12 hours Thin plates with sharp edges Thing plates with sharp edges
24 hours Thin plates with sharp edges Thin plates with sharp edges
48 hours Thin plates with sharp edges Thin plates with sharp edges
Table 2: Effect of milling media on resultant size (starting size sub-micron),
shape, and
agglomeration of particles
Properties Dry Alcohol Oil Dry milled and
oil milled
Clusters size (!tin) 100 300 200 100
Particle size (um) 30 80 80 __________ 30 __
Agglomeration High Very less Very less Very less
Shupe of the particles Spherical Platelet Platelet Sph erical
CA 2947139 2018-11-30
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
[0097] Figure 3
shows TEM micrographs of the as-available (700 nm), air milled,
and hybrid milled (48 hrs in air medium followed by 48 hours in oil medium)
MoS2
nanoparticles. Figure 3(A) represents micron-sized particle chunks of the as-
available MoS2 sample off the shelf. These micrographs, particularly Figure
3(B),
represent agglomerates of lubricant nanoparticles when milled in the air
medium.
Figure 3(B) clearly demonstrates size reduction in air milled MoS2. Higher
magnification (circular regions) revealed formation of the disc shaped
nanoparticles
after milling in the air medium. From Figures 3(C) and 3(D) it may be
concluded that
the particle size was reduced to less than 30 nm after milling in air and
hybrid
conditions. Regardless of the occasionally observed clusters, the average size
of
the clusters is less than or equal to 200 nm.
[0098] Hybrid
milled samples were dispersed in paraffin oil (from Walmart) and
remained suspended without settling. However, the dispersion was not uniform
after
a few weeks. To stabilize the dispersion and extend the anti-wear properties,
phospholipids were added. Around 2% by weight of soy lecithin phospholipids
(from
American Lecithin) was added in the base oil.
[0099] Figures 4
and 5 show the XRD and XPS spectra of MoS2 before and after
ball milling, respectively. XRD spectra revealed no phase change as well as no
observable amorphization in the MoS2 after milling. This observation is
consistent
with the continuous platelets observed throughout the nanoparticle matrix in
TEM
analysis for milled material. Broadening of peaks (FWHM) was observed in XRD
spectra of MoS2 ball milled in air and hybrid media, respectively. The peak
broadening may be attributed to the reduction in particle size. The estimated
grain
size is 6 nm. This follows the theme of ball milling where clusters consist of
grains
and sub-grains of the order of 10 nm. XPS analysis was carried out to study
the
surface chemistry of the as-available and hybrid milled MoS2 nanoparticles. As
shown in Figure 5, a carbon (C) peak observed at 285 eV in the as-available
MoS2
sample shifted to 286.7 eV. Bonding energies of 286 eV and 287.8 eV correspond
to C-0 and C=0 bond formation, respectively. The observed binding energy level
may demonstrate that a thin layer containing mixed C-0 and C=0 groups
encapsulates the MoS2 particles.
31
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
[00100] Preliminary tribological tests on the synthesized nanoparticles were
performed on a four-ball machine by following ASTM 4172. The balls used were
made of AISI 52100 stainless steel and were highly polished. Four Ball Wear
Scar
measurements using ASTM D4172 were made under the following test conditions:
Test Temperature, 'C 75 (111.7)
Test Duration, min 60 ( 1)
Spindle Speed, rpm 1,200 ( 60)
Load, kg 40 ( 0.2)
Wear scar diameter (WSD, mm) of each stationary ball was quantified in both
vertical and horizontal directions. The average value of WSD from 3
independent
tests was reported within 0.03 mm accuracy.
[00101] Four Ball Extreme Pressure measurements using ASTM D2783 were
made under the following test conditions:
Test Temperature, C 23
Test Duration, min 60 ( 1)
Spindle Speed, rpm 1,770 (-1- 60)
Load, kg Varies, 10-sec/stage
Ball Material AIS1-E52100
Hardness 64-66
Grade 25EP
_
[00102] Three different particles (in w/w ratio) were evaluated for their anti-
wear
properties as additives in paraffin oil. Figure 6(A) shows the average wear
scar
measurements for paraffin oil without a nanoparticle additive, paraffin oil
with micron
sized MoS2, paraffin oil with MoS2 that was milled in air for 48 hours, and
paraffin oil
with MoS2 that was milled in air for 48 hours followed by milling in canola
oil for 48
32
CA 02947139 2016-10-26
WO 2015/191173
PCT/US2015/027925
hours. Figure 6(B) shows the load wear index for paraffin oil without a
nanoparticle
additive, paraffin oil with micron sized MoS2, paraffin oil with MoS2 that was
milled in
air for 48 hours, and paraffin oil with MoS2 that was milled in air for 48
hours followed
by milling in canola oil for 48 hours. Figure 6(C) shows the COF for paraffin
oil
without a nanoparticle additive, paraffin oil with micron sized MoS2, paraffin
oil with
MoS2 that was milled in air for 48 hours, and paraffin oil with MoS2 that was
milled in
air for 48 hours followed by milling in canola oil for 48 hours. Figure 6(D)
shows the
extreme pressure data for paraffin oil with micron sized MoS2, paraffin oil
with MoS2
that was milled in air for 48 hours, and paraffin oil with MoS2 that was
milled in air for
48 hours followed by milling in canola oil for 48 hours. In each test the
nanoparticle
additive was present in the amount of 1% by weight.
Test data from nanoparticle composition additive in base oil
Lubricant Four Ball Tests at 40
kg Load Four Ball Extreme Pressure
(ASTM D4172) (ASTM D-2783)
All dispersions diluted to x% WSD (mm) COF Weld Load
Load Wear FIG. 6(A)
by wt. in reference base oil (kg) Index and 6(b)
Paraffin oil 1.033 0.155 126 12.1 A
Nanoparticles of MoS2 without 1.012 0.102 100 16.1
organic medium (0.5%)
Nanoparticles of MoS2 without 0.960 0.114 126 20.8
organic medium (1.0%)
Nanoparticles of MoS2 without 0.915 0.098 126 22.0
organic medium (1.5%)
Conventional available micro 1.009 0.126 160 22.0
particles (0.5%)
Conventional available micro 0.948 0.091 126 19.1
particles (1.0%)
Conventional available micro 0.922 0.106 126 16.5
particles (1.5%)
Nanoparticles of MoS2 with 0.451 0.077 160 24.8 II
organic medium (0.5%)
Nanoparticles of MoS2 with 0.461 0.069 200 25.9
organic medium (1.00/o)
Nanoparticles of MoS2 with 0.466 0.075 315 34.3
organic medium (1.5%)
[00103] Comparison of wear scar diameters for different additives in paraffin
oil are
graphically depicted in Figure 8. One additive is crystalline molybdenum
disulfide (c-
MoS2). Another additive is molybdenum disulfide nanoparticles that were ball
milled
in air (n-MoS2). Another additive is molybdenum disulfide nanoparticles that
were
33
81800862
'ball milled in air followed by ball milling in canola oil and to which a
phospholipid
emulsifier was added (n-MoS2+Emulsifier).
[00104] The transfer film in the wear scar, studied using energy dispersive x-
ray
analysis (EDS), identified the signatures of phosphates in addition to
molybdenum
and sulfur. Figure 9(a) depicts the base case of paraffin oil without a
nanoparticle
additive. Figure 9(b) depicts paraffin oil with the molybdenum disulfide
nanoparticles
and the emulsifier. It shows the early evidences of molybdenum (Mo)-sulfur (S)-
phosphorous (P) in the wear track. Iron (Fe) is seen in Figures 9(a) and 9(b),
as it is
the material of the balls (52100 steel) in the four-ball test. The molybdenum
and
sulfur peaks coincide and are not distinguishable because they have the same
binding energy. Elemental mapping also showed similar results.
[00105] This specification has been written with reference to various non-
limiting
and non-exhaustive embodiments. However, it will be recognized by persons
having
ordinary skill in the art that various substitutions, modifications, or
combinations of
any of the disclosed embodiments (or portions thereof) may be made within the
scope of this specification. Thus, it is contemplated and understood that this
specification supports additional embodiments not expressly set forth in this
specification. Such embodiments may be obtained, for example, by combining,
modifying, or reorganizing any of the disclosed steps, components, elements,
features, aspects, characteristics, limitations, and the like, of the various
non-limiting
and non-exhaustive embodiments described in this specification. In this
manner,
Applicant reserves the right to amend the claims during prosecution to add
features
as variously described in this specification.
34
CA 2947139 2018-11-30