Note: Descriptions are shown in the official language in which they were submitted.
CA 02949264 2016-11-16
WO 2016/026437 PCT/CN2015/087500
METHOD AND SYSTEM OF DETERMINING PROBE POSITION
IN SURGICAL SITE
FIELD OF THE INVENTION
(00011 The present disclosure relates to a guiding and positioning system, and
more
particularly a method and system of determining the position of a probe in a
surgical
site during a surgical procedure.
BACKGROUND
[0002] Common brain diseases, such as brain tumors, Parkinson's disease and
epilepsy, not only adversely affect the patients' quality of life but
sometimes can also
directly contribute to the patients' death. Invasive surgical procedures are
usually
performed after conservative treatments, such as medicines or physical
therapies,
failed to relieve the patients' symptoms. In such procedures, given the
anatomy of
the brain, a surgeon has limited space to maneuver a surgical instrument.
[0003] Currently, a surgeon can only rely on the pre-operative data before
performing a brain surgery, but even the minor shift of the brain during the
surgical
procedure or the improper operation of the stereotactic positioning system
often
renders the position data of the surgical site inaccurate.
[0004] In addition, any pre-operative planned pathway based on the pre-
operative
data may change due to a number of factors, such as the movement of the
patient's
position, the change in the patient's condition, or the insertion of the
surgical probe
itself. Any such deviation from the pre-operative planned pathway often leads
to
further complications or an increased mortality rate.
SUMMARY
[0005] In accordance with one embodiment of the present disclosure, a method
to
determine a position of a probe in a surgical site with a plurality of
reference
structures is disclosed. The method includes receiving a three-dimensional
image of
the surgical site generated before the probe enters the surgical site and
receiving a
first two-dimensional image generated by the probe from a position within the
1
CA 02949264 2016-11-16
WO 2016/026437 PCT/CN2015/087500
surgical site. The three-dimensional image is associated with a first
coordinate
system, and the first two-dimensional image is associated with a second
coordinate
system. The method also includes acquiring registration to the plurality of
reference
structures based on the first two-dimensional image to obtain a permissible
set of
probe pose parameters, extracting a second two-dimensional image from the
three-
dimensional image based on the permissible set of probe pose parameters, and
computing a correlation between the first two-dimensional image and the
extracted
second two-dimensional image to map the position of the probe represented by
the
second coordinate system to a position represented by the first coordinate
system
with respect to the three-dimensional image.
[0006] In accordance with one embodiment of the present disclosure, a machine-
readable medium embodying a set of instructions, which in response to
execution by
a computing device, cause the computing device to determine a position of a
probe
in a surgical site is disclosed. The method includes receiving a three-
dimensional
image of the surgical site generated before the probe enters the surgical site
and
receiving a first two-dimensional image generated by the probe from a position
within the surgical site. The three-dimensional image is associated with a
first
coordinate system, and the first two-dimensional image is associated with a
second
coordinate system. The method also includes acquiring registration to the
plurality
of reference structures based on the first two-dimensional image to obtain a
permissible set of probe pose parameters, extracting a second two-dimensional
image from the three-dimensional image based on the permissible set of probe
pose
parameters, and computing a correlation between the first two-dimensional
image
and the extracted second two-dimensional image to map the position of the
probe
represented by the second coordinate system to a position represented by the
first
coordinate system with respect to the three-dimensional image.
[0007] In accordance with one embodiment of the present disclosure, a system
configured to determine a position of a probe in a surgical site with a
plurality of
reference structures is disclosed. The system includes a processor, a first
table, a
second table, and a memory. The memory embodies a set of executable
instructions, which in response to execution by the processor, cause the
processor
to acquire registration to the plurality of reference structures based on a
first two-
2
CA 02949264 2016-11-16
WO 2016/026437 PCT/CN2015/087500
dimensional image generated by the probe from a position within the surgical
site
during a surgical procedure and a three-dimensional image of the surgical site
before the surgical procedure to obtain a permissible set of probe pose
parameters,
wherein the three-dimensional image is associated with a first coordinate
system,
and the first two-dimensional image is associated with a second coordinate
system,
extract a second two-dimensional image from the three-dimensional image based
on
the permissible set of probe pose parameters, select a first set of pixels
from the first
two-dimensional image using the first table, select a second set of pixels
from the
extracted second two-dimensional image using the first table and the second
table
and based on one of the permissible set of probe pose parameters, and compute
a
correlation between the first set of pixels and the second set of pixels to
map the
position of the probe represented by the second coordinate system to a
position
represented by the first coordinate system with respect to the three-
dimensional
image.
[0008] The foregoing summary is illustrative only and is not intended to be in
any
way limiting. In addition to the illustrative aspects, embodiments, and
features
described above, further aspects, embodiments, and features will become
apparent
by reference to the drawings and the following detailed description.
BRIEF DESCRIPTION OF THE DRAWINGS
[0009] Fig. 1 is block diagram showing the configuration of a surgical guiding
and
positioning system;
[0010] Fig. 2 is a schematic view showing an example arrangement of one or
more
sensors on a probe;
poll] Fig. 3 shows an example three-dimensional image of a patient's head
prior to
performing a surgical procedure;
[0012] Fig. 4 is a simplified block diagram illustrating the extracting of a
two-
dimensional image from a volume image;
[0013] Fig. 5 is a flow diagram illustrating an example process of determining
the
position of a probe during a surgical procedure;
3
CA 02949264 2016-11-16
WO 2016/026437 PCT/CN2015/087500
[0014] Fig. 6 is a flow diagram illustrating an example method 600 to acquire
registration to reference structures;
[0015] Fig. 7 shows an example display with ultrasound images superimposed on
a
slice image extracted from a CT volume image;
100161 Fig. 8 shows an example display with filtered ultrasound images
superimposed on a slice image extracted from a processed CT volume image;
Novi Fig. 9 is a block diagram of an example table-based system configured to
compute a multi-dimensional correlation surface; and
100181 Fig. 10 is a block diagram illustrating a computer program product to
implement a method to determine a position of a probe in a surgical site, all
arranged in accordance with at least some embodiments described herein.
DETAILED DESCRIPTION
[0019] In the following detailed description, reference is made to the
accompanying
drawings, which form a part hereof. In the drawings, similar symbols typically
identify similar components, unless context dictates otherwise. The
illustrative
embodiments described in the detailed description, drawings, and claims are
not
meant to be limiting. Other embodiments may be utilized, and other changes may
be made, without departing from the spirit or scope of the subject matter
presented
here. It will be readily understood that the aspects of the present
disclosure, as
generally described herein, and illustrated in the Figures, can be arranged,
substituted, combined, and designed in a wide variety of different
configurations, all
of which are explicitly contemplated herein.
[0020] This disclosure is drawn, inter alia, to methods, apparatuses, and
systems
related to determine the position of a probe in a surgical site during a
surgical
procedure. Throughout the disclosure, the terms "three-dimensional image" and
"volume image" are used interchangeably.
[0021] Fig. 1 is block diagram showing the configuration of a surgical guiding
and
positioning system 100, in accordance with one embodiment of the present
disclosure. The surgical guiding and positioning system 100 mainly includes a
4
CA 02949264 2016-11-16
WO 2016/026437 PCT/CN2015/087500
global information device 102, a focal information device 104, a computing
device
106, a monitoring device 108, and an operating device 110.
[0022] The global information device 102 is capable of collecting overall
information
of a surgical site, such as a brain, before a surgical procedure begins. In
some
embodiments, the overall information can be acquired through computed
tomography (CT), magnetic resonance imaging (MRI), surface scan, X-ray scan,
ultrasound scan, and etc. With the overall information (e.g. the intracranial
anatomy,
the target or lesion location, or the surface land markings) of the surgical
site, a
surgeon may plan a surgical pathway before a surgical procedure begins.
[0023] One embodiment of the local information device 104 includes a probe 112
with at least one sensor 114 directly disposed on the probe 112.
[0024] A surgeon may also control the probe 112 via the operating device 108.
One
embodiment of the operating device 108 may include a robotic arm 116 via which
the surgeon can control the probe 112.
[0025] During the surgical procedure, the one or more sensors 114 disposed on
the
probe 112 are configured to obtain local data near the probe 112 itself. This
collected local data, in conjunction with the global data from the global
information
device 102, may be processed by the computing device 106.
[0026] In one embodiment, the computing device 106 is capable of determining a
position data of the probe 112 in the surgical site in relation to the global
data. The
global data is obtained before the surgical procedure begins, and the local
data is
obtained during the surgical procedure. Additional details will be provided in
subsequent paragraphs.
[0027] One embodiment of the monitoring device 110 includes a display device
118
and a warning device 120. The display device 118 is capable of displaying a 3D
image based on the aforementioned overall information from the global
information
device 102 before the surgical procedure begins. During the surgical
procedure, the
display device 118 is capable of displaying a real-time position of the probe
112
superimposed on the 3D image based on the position data calculated by the
computing device 106. In such an embodiment, a surgeon may learn the real-time
5
CA 02949264 2016-11-16
WO 2016/026437 PCT/CN2015/087500
position of the probe 112 relative to the 3D image and adjust the procedure
accordingly.
[0028] One embodiment of the warning device 120 is capable of sending out a
real-
time warning to a surgeon when a blood vessel or vital area is nearby, when
the
probe 112 is in a target position or a risky position, or the probe 112
deviates from
the planned surgical pathway.
10029] Fig. 2 is a schematic view showing an example arrangement of one or
more
sensors on a probe, such as the probe 112 of Fig. 1, in accordance with one
embodiment of the present disclosure. One embodiment of the probe 112 may be
configured as a sheath that wraps around a surgical device 202, and the
surgical
device 202 is moveable therein. Some examples of the surgical device 202 may
include, without limitation, a biopsy needle, a biopsy forceps, a clamp, a
laser fiber,
a brain pressure monitor catheter, and others.
[4:10301 The probe 112 includes one or more sensors 204. An example of the
sensor
204 may be an ultrasound transducer with varying detection ranges. In one
embodiment, the probe 112 may include eight sensors 204, spaced every 45
degrees around the circumference of the probe 112. Each of the sensors 204 may
be configured to collect and generate a two-dimensional (2D) image 206 in a
plane
that includes the probe 112 (e.g., w axis). The data that the probe 112
collects and
generates, in one embodiment, are associated in one coordinate system (e.g.,
u,v,w,
with the w axis aligned with the axis of the probe 112).
(0031] In one embodiment, the ultrasonic transducers are configured to
transmit
pulses of ultrasound into tissues and/or anatomical parts that are within the
ultrasound range of the transducers. The ultrasound may echo off the tissues
and/or anatomical parts, with different types of tissues and anatomical pails
reflecting varying degrees of sounds. The echoes are recorded and displayed as
the 2D image 206. Since the signal strength associated with bones is generally
stronger than the signal strength associated with the soft brain tissues, to
prevent
the bone signal from overpowering the tissue signal, the ultrasound range may
be
adjusted, so that the generated ultrasound images may reveal more information
6
CA 02949264 2016-11-16
WO 2016/026437 PCT/CN2015/087500
associated with the soft brain tissues, which may include the target tissues
and other
vital tissues in the surgical procedure.
[0032] Fig. 3 shows an example three-dimensional (3D) image 300 of a patient's
head prior to performing a surgical procedure. To illustrate, suppose the 3D
image
300 is a CT image. Prior to operation, the 3D image 300 may be ordered by the
surgeon, so that the position of the target area with respect to other tissues
or
structures in the brain can be first evaluated, and a surgical pathway can be
planned.
The 3D image 300 includes voxels, each of which represents a value on a grid
in 3D
space. Here, the voxels are shown to be arranged in a perceptible cube with an
origin 302.
[0033] In one embodiment, the 3D image 300 is associated with one coordinate
system (e.g., x,y,z). For example, with the origin 302 having coordinates (0,
0, 0),
the coordinates for a voxel 304 in the 3D image 300 (e.g., X1. Yi. Z1) in the
same
coordinate system may be obtained.
[0034] To determine where the 2D image data captured and generated by the
probe
112 of Fig. 1 and Fig. 2 in one coordinate system (e.g., u,v,w) can be
appropriately
placed in the 3D image data shown in Fig. 3 in another coordinate system
(e.g,.
x,y,z), one approach is to extract a 2D image from the 3D image data and
compare
the extracted 2D image with the 2D image from the probe 112. Fig. 4 is a
simplified
block diagram illustrating the extracting of a 2D image from a volume image,
in
accordance with one embodiment of the present disclosure. A 2D image 400, with
an origin 402, may correspond to the 2D image 206 of Fig. 2. A volume image
404,
with an origin 406, may correspond to the 3D image 300 and the origin 302 of
Fig. 3,
respectively.
[0035] As discussed earlier, since the 2D image 400 corresponds to a 2D image
that
the probe 112 of Fig. 1 and Fig. 2 captures and generates at a certain
position and
orientation in the surgical site (e.g., brain) during a surgical procedure,
and the
volume image 404 corresponds to the 3D image of the same surgical site before
the
surgical procedure begins, the position and orientation of the probe 112 at
which the
2D image 400 is collected and generated becomes relevant in identifying the
appropriate point in the volume image 404 to extract a 2D image 408 from. For
7
CA 02949264 2016-11-16
WO 2016/026437 PCT/CN2015/087500
simplicity, suppose the origin 402 is determined to map to an origin 410 in
the
volume image 404. In one embodiment, as shown in Fig. 4, the size and/or the
raster scan sequence of the 2D image 400 may be used to extract the 2D image
408.
For instance, the voxel positions in the volume image 404 may be located in a
manner, so that they correspond to a raster scan of the 2D image 400. In some
other embodiments, corrections may be made by interpolating to intermediate
points
between data points represented by the voxels, because the pixels of the
extracted
2D image 408 generally may not align exactly with the voxels of the volume
image
404.
100361 With the extracted 2D image 408, comparisons can be made between the 2D
image 400 and the 2D image 408 to determine whether there is a high
correlation
between the two images. If the correlation is high, then there is higher
confidence
that the mapping between the 2D image 400 and the 2D image 408 is sufficiently
accurate. If the mapping is sufficiently accurate, then a surgeon would be
able to
evaluate the data nearby the probe 112, which are likely the data along the
planned
surgical pathway, in view of the volume image 404 as the surgical procedure is
being performed. Local deformation, including translational and rotation
shifts and
shear distortion in tissues, in the surgical site can thus be estimated and
taken into
consideration during the surgical procedure.
[0037] Although the 2D image 400 and the extracted 2D image 408 are shown in a
square-like shape, it should be apparent to a person skilled in the art to
recognize
that these images can be in any shape (such as a fan slice shown in Fig. 2)
that is
practical to implement.
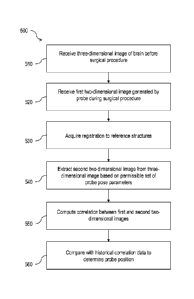
[0038] Fig. 5 is a flow diagram illustrating an example method 500 to
determine the
position of a probe during a surgical procedure, in accordance with one
embodiment
of the present disclosure. The process 500 may include one or more operations,
functions, or actions as illustrated by blocks 510, 520, 530, 540, 550, and/or
560,
which may be performed by hardware, software and/or firmware. The various
blocks are not intended to be limiting to the described embodiments. For
example,
one skilled in the art will appreciate that, for this and other processes and
methods
disclosed herein, the functions performed in the processes and methods may be
8
CA 02949264 2016-11-16
WO 2016/026437 PCT/CN2015/087500
implemented in differing order. Furthermore, the outlined steps and operations
are
only provided as examples, and some of the steps and operations may be
optional,
combined into fewer steps and operations, or expanded into additional steps
and
operations without detracting from the essence of the disclosed embodiments.
Although the blocks are illustrated in a sequential order, these blocks may
also be
performed in parallel, and/or in a different order than those described
herein.
[0039] Processing for the method 500 may begin at block 510, "receive three-
dimensional image of surgical site before surgical procedure." For example,
before
the surgical procedure, some medical imaging techniques may be used to capture
a
snapshot of the patient's conditions, so that an operation plan may be
formulated.
Suppose the surgical site is the brain of the patient. The surgeon may order a
CT
scan of the brain. In conjunction with Fig. 1 and Fig. 2, the computing device
106
may receive this 3D volume image of the patient's brain from the global
information
device 102 (e.g., CT scanner). In addition to soft brain issues, the 3D volume
image
may also include voxels that represent reference structures, such as, without
limitation, the skull of the patient or a base plate coupled to the skull.
[0040] Block 510 may be followed by block 520, "receive first two-dimensional
image
generated by probe during surgical procedure." Here, after the surgical
procedure
begins, the computing device 106 may be configured to receive a first 2D image
generated by the probe 112. As mentioned above, in one embodiment, the
sensors,
or the ultrasound transducers, disposed on the probe 112 may be configured to
capture and generate 2D images from the position and orientation of the probe
112
in the brain.
[0041] Block 520 may be followed by block 530, "acquire registration to
reference
structures," where the acquisition of registration broadly refers to the
determination
of one-to-one mapping between one set of coordinates in one coordinate system
to
another set in another coordinate system, such that the data in the two
coordinate
systems that correspond to the same anatomical part are mapped to one another.
Some examples of the reference structures include, without limitation, the
bone and
certain soft tissues. To acquire registration to such reference structures may
involve
an iterative process, where different types of searches are performed based on
9
CA 02949264 2016-11-16
WO 2016/026437 PCT/CN2015/087500
varying sets of pose parameters (e.g., x,y,z,pitch,yaw,roll) of the probe 112
and
varying search parameters (e.g., the ultrasound range). In one embodiment, one
output of the registration acquisition operation is one or more permissible
sets of
pose parameters in the coordinate system of the 3D volume image. In other
words,
after having acquired registration to the reference structures, the set of
possible
coordinates in the coordinate system of the 3D volume image that could map to
the
location of the probe 112 within the brain becomes more defined. A more
detailed
description of this registration acquisition process is set forth in
subsequent
paragraphs.
[0042] Block 530 may be followed by block 540, "extract second two-dimensional
image from three-dimensional image based on permissible set of probe pose
parameters." As discussed earlier and in conjunction with Fig. 4, with the
coordinates in the coordinate system of the volume image, the second 2D image,
such as the 2D image 408, can be extracted.
[0043] Block 540 may be followed by block 550, "compute correlation between
first
and second two-dimensional images." A high correlation between the two images
would signal that the selected pose parameters for the probe 112 results in a
fairly
accurate mapping between the two coordinate systems, and the surgeon may rely
on the extracted 2D image to evaluate the surgery.
[0044] Block 550 may be followed by block 560, "compare with historical
correlation
data to determine probe position." Here, in one embodiment, the best
correlation
score out the previously computed correlation scores and the associated pose
parameters of the probe are maintained. If a newly computed correlation score
is
higher (i.e., the two images are even more highly correlated), then the newly
.. computed correlation score and the associated pose parameters are kept.
[0045] In one embodiment, to ensure the best computed correlation score is
obtained, all of the permissible set of probe pose parameters may be used to
obtain
the different 2D images and to compute different correlation scores. Also, for
each
of the sensors disposed on the probe 112, a different 2D image is captured and
.. generated from different orientations. All of these different 2D images are
compared
with their corresponding extracted 2D images, and the correlation scores may
be
CA 02949264 2016-11-16
WO 2016/026437 PCT/CN2015/087500
accumulated. Moreover, consistency constraints may be imposed. One constraint
may allow the probe to move continuously along a mostly linear path. Another
constraint may allow the probe to rotate about its axis.
[0046] Fig. 6 is a flow diagram illustrating an example method 600 to acquire
registration to reference structures, in accordance with one embodiment of the
present disclosure. The process 600 may include one or more operations,
functions,
or actions as illustrated by blocks 610, 620, 630, and/or 640, which may be
performed by hardware, software and/or firmware. The various blocks are not
intended to be limiting to the described embodiments. For example, one skilled
in
the art will appreciate that, for this and other processes and methods
disclosed
herein, the functions performed in the processes and methods may be
implemented
in differing order. Furthermore, the outlined steps and operations are only
provided
as examples, and some of the steps and operations may be optional, combined
into
fewer steps and operations, or expanded into additional steps and operations
without detracting from the essence of the disclosed embodiments. Although the
blocks are illustrated in a sequential order, these blocks may also be
performed in
parallel, and/or in a different order than those described herein.
[0047] Processing for the method 600 may begin at block 610, "set probe pose
parameters and search parameters." In one embodiment, one initial probe pose
parameters may be set based on surgical preplanning and/or mechanical
constraints
(e.g., relative to a base plate coupled to the patient's skull). One initial
set of search
parameters may include, without limitation, search interval, increment sizes
for each
pose parameter, ultrasound range limit, and others.
[0048] Block 610 may be followed by block 620, "search for reference
structure(s) in
first two-dimensional image." In one embodiment, the initial ultrasound range
limit is
set to be larger, so that a more exhaustive search in the first 2D image (the
2D
image captured/generated by the probe, such as the 2D image 400 of Fig. 4) to
identify the reference structure(s) may be performed.
[0049] Block 620 may be followed by block 630, "determine whether probe pose
parameters result in an acquisition of identified reference structure(s)." In
other
words, with the probe pose parameters set to certain values, one iteration of
the
11
CA 02949264 2016-11-16
WO 2016/026437 PCT/CN2015/087500
method 600 is to determine whether an agreement can be found between the
identified reference structure(s) in the first 2D image and the corresponding
reference structure(s) in the volume image. If an agreement is found, then the
set of
probe pose parameters leading to the registration of the reference
structure(s) are
maintained. Otherwise, the probe pose parameters may be set to different
values
and block 630 is performed again to determine whether the agreement can be
found.
[0050] Suppose registration to one reference structure, such as the bone, is
acquired in block 630. Block 630 may be followed by block 640, "modify search
parameters." In one embodiment, the ultrasound range limit, as one of the
search
parameters, may be reduced, so that the soft tissue near the probe 112 may be
considered. Different ultrasound range limits may also be utilized, so that
different
distances from the probe 112 may be measured.
[0051] In one embodiment, the computing device 106 of Fig. 1 may be configured
to
perform the method 500 and the method 600. To achieve more meaningful results
.. and before some of the aforementioned operations are performed, the
computing
device 106 may be configured to process the 3D volume image, the first 2D
image,
and/or the extracted second 2D image.
[0052] Bones are associated with stronger signals than soft brain tissues in
both CT
and ultrasound images. In one embodiment, the computing device 106 may utilize
this signal strength difference between the bone and the soft brain tissues to
differentiate the pixels representing the skull and the pixels representing
the soft
brain tissues in the first 2D image and the extracted second 2D image.
Computing
the correlation between just the pixels representing the soft brain tissues in
the two
2D images may result in more meaningful comparisons.
[0053] More specifically, in one embodiment, the pixels in the 2D image
extracted
from the volume image representing the bone may be assigned a first value, and
the
pixels in the same extracted image representing parts other than the skull may
be
assigned a second value. If a pixel value is closer to the first value than
the second
value, then it may be more likely that such a pixel represents a part in
proximity to
.. the skull but further away from the soft brain tissues. In addition, a mask
may be
12
CA 02949264 2016-11-16
WO 2016/026437 PCT/CN2015/087500
applied to the extracted 2D image to select pixels with an assigned value
below a
threshold to suppress the strong signal associated with the skull.
[0054] In one embodiment, the computing device 106 may apply a spatial
bandpass
filter, such as the Laplacian of Gaussian (LOG) convolution, to the first 2D
image to
suppress finer and coarser textures before the correlation between the first
2D
image and the second extracted 2D image is computed (e.g., block 550 of Fig.
5).
The filter 2D image may have a substantial zero mean with swings both positive
and
negative. The boarders between positive and negative regions in the LOG
filtered
image occur at locations where transitions occur in the original image. In
addition,
the regions of positive and negative may be centered between the transition
regions
and are generally stable. Such regions can be used to acquire registration
between
images of the same subject matter even with significant differences in the
image
capture/generation mechanism such as in the case with ultrasound images and CT
volume images or ultrasound images and MRI volume images.
[0055] In one embodiment, a LOG convolution may be applied to the second
extracted 2D image. Alternatively, the LOG convolution may also be applied to
the
volume image before the second 2D image is extracted from the volume image. A
two-dimensional image extracted from the LOG-processed volume image may be
similar to the second extracted 2D image that is LOG-processed.
[0056] In one embodiment, a Hounsfield Units remapping approach may be applied
to the second extracted 2D image. The Hounsfield Units remapping approach
includes remapping Hounsfield Units to different value ranges to enhance the
tissue
impedances. For example, the Hounsfield Unit ranges associated with brain gray
matters may be assigned to a larger value range than the Hounsfield Unit
ranges
associated with brain white matter.
[0057] Since ultrasound images normally contain significant speckle artifact,
in one
embodiment, the speckle artifact is filtered out of the ultrasound images
before
subsequent processing. One example filter has a zero amplitude at a radial
frequency proportional to the frequency of the ultrasound image. In another
embodiment, the filter is a frequency-domain filter. In yet another
embodiment, the
filter is a sinc function of the radial frequency coordinate:
13
CA 02949264 2016-11-16
WO 2016/026437 PCT/CN2015/087500
sin frit;
s in c (frifs) = fr Ifs
In another embodiment, the scale frequency J. is chosen to give a zero
amplitude at
a desired radial frequency.
[0058] Referring back to Fig. 5, with the probe position determined in block
560, in
one embodiment, the first 2D image (e.g., the 2D image 400 of Fig. 4) and the
second extracted 2D image (e.g., the extracted 20 image 408) may be displayed
on
the display device 118. Fig. 7 shows an example display with ultrasound images
710 and 720 superimposed on a slice image 700 extracted from a CT volume
image,
in accordance with one embodiment of the present disclosure. Here, the
ultrasound
images 710 and 720 are captured and generated from a certain location of a
probe
in the brain, which corresponds to a set of coordinates (e.g., the coordinates
(X0, Yo,
Z0)) in the coordinate system associated with the CT volume image. According
to
the coordinates (Xo, Yo, Zo), the slice image 700 is extracted from the CT
volume
image.
[0059] The slice image 700 shows soft tissues 701 (the region with darker
shading)
confined in a skull 703 (the region with lighter shading), a base plate 705
(the
vertical bar), and a probe axis 707 (the white line through the center) for
this slice
image. Regions 725 and 730 show that the image rendered by the pixels
representing the skull 703 in the slice image 700 are substantially similar
with the
image rendered by the pixels representing the skull 703 in the ultrasound
image 710.
The substantially similarity suggests that the pixels representing the skull
703 in the
slice image 700 and the pixels representing the skull 703 in the ultrasound
image
710 correspond to the same part of the skull 703. With the match of the skull
anatomy, the position of coordinates (Xo, Yo, Zo) with respect to the skull
703 may be
determined.
[0060] Fig. 8 shows an example display with filtered ultrasound images 810 and
820
superimposed on a slice image 800 extracted from a processed CT volume image
in
accordance with one embodiment of the present disclosure. Although the
filtered
14
CA 02949264 2016-11-16
WO 2016/026437 PCT/CN2015/087500
ultrasound images 810 and 820 are taken from the same coordinates (X0, Yo, Zo)
of
Fig. 7, the range of the filtered ultrasound images 810 and 820 is more
limited than
the range of ultrasound images 710 and 720, so that ultrasound images 810 and
820 do not include the skull and do not have the regions 725 and 730 shown in
Fig.
7. Also, in this figure, the voxel values of the CT volume image have been
changed
to accentuate soft brain tissues by masking bone boundaries. As a result, the
slice
image 800 only includes pixels representing the soft brain tissues, and none
for the
skull. The pixels representing the soft brain tissues in the slice image 800
may be
compared to the pixels representing the soft brain tissues in the ultrasound
images
810 and 820. Also, correlation between the images rendered by the pixels
representing the soft brain tissues in the slice image 800 and the images
rendered
by the pixels representing the soft brain tissues in the ultrasound image 810
and 820
may be computed. Assuming the slice image 800 and the ultrasound images 810
and 820 are highly correlated, differences between the slice image 800 and the
ultrasound images 810 and 820 would correspond to the shift of the soft brain
tissues. With high confidence in this determination, the surgeon is enabled to
determine whether the target tissues or other vital tissues in the surgical
procedure
are shifted and take appropriate actions during the procedure.
(00611 Fig. 9 is a block diagram of an example table-based system 900
configured to
compute a multi-dimensional correlation surface, in accordance with one
embodiment of the present disclosure.
[0062] The value N is the total number of pixels that will be correlated. N
generally is
smaller than the number of pixels in the ultrasound image. This is because
pixels
beyond a set range from the ultrasound probe are not used, likewise pixels
closer
than a given range are also ignored.
10063] The US mask table contains a list of length N of the memory addresses
(offsets from the beginning of the ultrasound image) of pixels that will be
used for the
correlation. This list follows a raster scan order. This offset output is also
fed to the
fan offset table for selecting the associated voxel addresses in the volume
image.
[0064] The Fan offset tables are a collection of k = ki*k2*k3 fan slice offset
tables
where:
CA 02949264 2016-11-16
WO 2016/026437 PCT/CN2015/087500
k1 is the number of roll orientations (typically 720 for half degree
resolution)
k2 is the number of yaw orientations relative a nominal probe direction
perpendicular to the head plate (typically 40 for a range of 10 degrees
with half degree resolution).
k3 is the number of pitch orientations relative a nominal probe direction
perpendicular to the head plate (typically 40 for a range of 10 degrees with
half
degree resolution).
[0065] Each of the k fan slice tables has a list of offset addresses that
scans a raster
pattern over a fan slice plane in the 3-D image volume. This raster scan has
the
same dimensions as the ultrasound images. Thus in operation, the Fan select
and
Probe axis yaw, pitch and roll boxes provide inputs to the Fan offset tables
box to
select one of the k fan slice offset tables. This selected table receives
input from the
US mask table and outputs an offset address for the 3-D volume image.
[0066] This offset address is summed (ED) with a fixed offset address from the
Probe
.. axis xyz position index offset box. This fixed offset translates the fan
slice in the
image volume. The output of the adder is then fed to the volume image memory
where a value is accessed and output to the correlation multiplier (0).
[0067] The correlation multiplier receives pixel values from the ultrasound
image and
the volume image. It multiplies those values and feeds the result to an
accumulator.
[0068] This entire process is repeated N times as the counter module at the
upper
left steps through its count from 0 to N-1. At the end of this count the
accumulator at
the far right will contain a correlation sum for the six input parameters: fan
index, roll,
pitch, yaw, x, y, and z. A combined correlation for all 8 fan slices is
computed by
incrementing the Fan select register through its range.
[0069] By varying the six parameters searching for the best correlation this
mechanism can be used to find the pose of the probe in the image volume that
gives
the best agreement between the pre-recorded volume image and real-time
ultrasound images.
[0070] The system 900 may optionally include a bone mask volume image, so that
bone regions may be excluded from the correlation calculation. In one
embodiment,
16
CA 02949264 2016-11-16
WO 2016/026437 PCT/CN2015/087500
the bone mask volume image includes voxels that indicate whether the
corresponding voxel in the CT/MRI LOG volume are soft tissue or bone. This
bone
mask volume is accessed in parallel with the LOG volume to determine whether
or
not to allow the accessed LOG voxel to contribute to the correlation sum. In
one
embodiment, the mask volume image is derived from the original CT/MR! volume
image using a modality appropriate technique to identify bone voxels. Those
voxel
values are set to 1.0 and non-bone voxels are set to 0Ø A filter means is
then
applied to the volume marked with ones and zeros so that locations marked as
soft
tissue which are near bone get a value greater than zero and less than one.
Furthermore locations closer to bone get a value closer to one. This allows a
threshold to be used to select voxels that are at least a specified distance
from the
nearest bone voxel.
[0071] Fig. 10 is a block diagram illustrating a computer program product 1000
to
implement a method to determine a position of a probe in a surgical site, in
accordance with one embodiment of the present disclosure. The computer program
product 1000 may include a signal bearing medium 1002. Signal bearing medium
1002 may include one or more sets of executable instructions 1004 stored
thereon
that, in response to execution by, for example, the computing device 106 of
Fig. 1,
may provide the features and operations described above.
[0072] In some implementations, the signal bearing medium 1002 may encompass a
non-transitory computer readable medium 1008, such as, but not limited to, a
hard
disk drive, a Compact Disc (CD), a Digital Versatile Disk (DVD), a digital
tape,
memory, etc. In some implementations, the signal bearing medium 1002 may
encompass a recordable medium 1010, such as, but not limited to, memory,
read/write (R/W) CDs, RNV DVDs, etc. In some implementations, signal bearing
medium 1002 may encompass a communications medium 1006, such as, but not
limited to, a digital and/or an analog communication medium (e.g., a fiber
optic cable,
a waveguide, a wired communications link, a wireless communication link,
etc.).
[0073] The foregoing detailed description has set forth various embodiments of
the
devices and/or processes via the use of block diagrams, flowcharts, and/or
examples. Insofar as such block diagrams, flowcharts, and/or examples contain
one
17
CA 02949264 2016-11-16
WO 2016/026437 PCT/CN2015/087500
or more functions and/or operations, it will be understood by those within the
art that
each function and/or operation within such block diagrams, flowcharts, or
examples
can be implemented, individually and/or collectively, by a wide range of
hardware,
software, firmware, or virtually any combination thereof. In some embodiments,
several portions of the subject matter described herein may be implemented via
Application Specific Integrated Circuits (ASICs), Field Programmable Gate
Arrays
(FPGAs), digital signal processors (DSPs), or other integrated formats.
However,
those skilled in the art will recognize that some aspects of the embodiments
disclosed herein, in whole or in part, can be equivalently implemented in
integrated
circuits, as one or more computer programs running on one or more computers
(e.g.,
as one or more programs running on one or more computer systems), as one or
more programs running on one or more processors (e.g., as one or more programs
running on one or more microprocessors), as firmware, or as virtually any
combination thereof, and that designing the circuitry and/or writing the code
for the
software and or firmware would be well within the skill of one of skill in the
art in light
of this disclosure. In addition, those skilled in the art will appreciate that
the
mechanisms of the subject matter described herein are capable of being
distributed
as a program product in a variety of forms, and that an illustrative
embodiment of the
subject matter described herein applies regardless of the particular type of
signal
bearing medium used to actually carry out the distribution. Examples of a
signal
bearing medium include, but are not limited to, the following: a recordable
type
medium such as a floppy disk, a hard disk drive, a Compact Disc (CD), a
Digital
Versatile Disk (DVD), a digital tape, a computer memory, etc.; and a
transmission
type medium such as a digital and/or an analog communication medium (e.g., a
fiber
optic cable, a waveguide, a wired communications link, a wireless
communication
link, etc.).
100741 From the foregoing, it will be appreciated that various embodiments of
the
present disclosure have been described herein for purposes of illustration,
and that
various modifications may be made without departing from the scope and spirit
of
the present disclosure. Accordingly, the various embodiments disclosed herein
are
not intended to be limiting, with the true scope and spirit being indicated by
the
following claims.
18