Note: Descriptions are shown in the official language in which they were submitted.
CA 02950582 2016-11-28
WO 2015/184318
PCMJS2015/033275
FEEDER-FREE DERIVATION OF HUMAN-INDUCED PLURIPOTENT STEM CELLS
WITH SYNTHETIC MESSENGER RNA
RELATED APPLICATION
100011 This application is a continuation-in-part of U.S. application
serial number
14/292,317, filed on May 30, 2014, which is a continuation-in-part of, U.S.
application serial
number 13/893,166, filed on May 13, 2013; which claims the benefit of priority
of U.S.
provisional application serial number 61/646,292, filed on May 13, 2012. The
contents of each
of which are hereby incorporated by reference in their entireties.
FIELD OF THE INVENTION
100021 The present disclosure relates generally to novel methods and
compositions for
using engineered reprogramming factor(s) for the creation of induced
pluripotent stem cells
(iPSCs) through a controlled process. Specifically, this disclosure relates to
establishing
combinations of reprogramming factors, including fusions between conventional
reprogramming factors with transactivation domains, optimized for
reprogramming of various
cell types. More specifically, the exemplary methods disclosed herein can be
used for creating
induced pluripotent stem cells from various mammalian cell types, including
non-human
primate cells. Exemplary methods of feeder-free derivation of human induced
pluripotent stem
cells using synthetic messenger RNA are also disclosed.
BACKGROUND
100031 The following includes information that may be useful in
understanding various
aspects and embodiments of the present disclosure. It is not an admission that
any of the
information provided herein is prior art, or relevant, to the presently
described or claimed
inventions, or that any publication or document that is specifically or
implicitly referenced is
prior art.
100041 The therapeutic potential of induced pluripotent stem cells (iPSCs)
has spurred
efforts to develop reprogramming methods that sidestep the need to genetically
modify somatic
cells to effect reprogramming to the pluripotent state. The first "non-
integrating" approaches to
achieve success in this regard¨protein transduction, plasmid transfection and
the use of
adenoviral vectors¨were limited in application due to the low efficiencies of
iPSC conversion
attained. More recently, techniques employing episomal DNA, Sendai virus, and
synthetic
messenger RNA (mRNA) have been shown to generate "footprint-free" iPSCs with
efficiencies
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
comparable to or surpassing those attained using integrating viral vectors.
RNA transfection is
in principle the most attractive of these methods as it affords precise
control over the
reprogramming factor (RF) expression time course, while completely obviating
any requirement
for "clean up" of the reprogrammed cells to purge residual traces of the
vector. Current
protocols for mRNA-based reprogramming are relatively labor- intensive,
however, owing to
the need to retransfect daily for the ¨2 weeks required for the induction of
pluripotency in
human cells. These procedures also rely on the use of feeder cells, adding
complexity and
technical variability to the process while introducing a potential source of
contamination with
non-human-derived ("xeno") biological material.
100051 A major difficulty of producing induced pluripotent stem cells
(iPSCs) has been
the low efficiency of reprogramming differentiated cells into pluripotent
cells. Previously, it
has been reported that 5% of mouse embryonic fibroblasts (MEFs) were
reprogrammed into
iPSCs when they were transduced with a fusion gene composed of 0ct4 and the
transactivation
domain ofiltyoD (called M30), along with Sox2, Klf4 and c- Myc (SKM). In
addition, M30
facilitated chromatin remodeling of pluripotency genes in the majority of
transduced MEFs,
including cells that did not become iPSCs. These observations suggested the
possibility that
more than 5% of cells had acquired the ability to become iPSCs given more
favorable culture
conditions.
SUMMARY OF THE INVENTION
100061 Successful reprogramming of cells from non-human origins using mRNAs
has
been elusive due to potential cellular immune responses induced by RNA
molecules once they
are transfectcd into cells, particularly mammalian cells. In reprogramming
human fibroblast
cells, a common practice is to use a viral protein as a decoy receptor to
blunt the interferons
induced by the transfected mRNA molecules. However, such a strategy of using
decoy receptor
or other similar strategies have not been successful in reprogramming of
fibroblasts from non-
human species, including baboons, horses, and dogs.
100071 Accordingly, to address these deficiencies, the present disclosure
provides
methods and compositions for generating stern cells capable of producing all
the different tissues
of the body. In certain aspects, using messenger RNA molecules and without the
need of viral
vectors or feeder cells, the methods disclosed herein can be used to reprogram
non-human
fibroblasts into induced pluripotent stem cells (iPSCs). The use of the
exemplary methods and
2
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
compositions resulted in a surprising and unexpected improved efficiency over
previously
reported cell reprogramming methodologies and overcame a previously unsolved
problem in
suppressing cellular immune responses in non-human mammalian cells.
100081 Accordingly, methods, agents and/or compositions useful for
accelerating
mRNA-mediated reprogramming by improving the reprogramming factors (RF)
cocktail,
notably through application of engineered variants of conventional
reprogramming factors, such
as 0ct4 (also referred to as 0ct3/4), Sox 2, etc., incorporating
transactivation domains of
known, strong transcription factors, such as VP16 and MyoD, are provided. The
methods and
compositions disclosed herein results in a feeder-free protocol which
dramatically reduces the
time, cost and effort involved in mRNA-based reprogramming.
100091 In one aspect, the present disclosure provides a method for
dedifferentiating or
reprogramming somatic cell comprising: a) transfecting the isolated somatic
cell with a
composition comprising an effective amount of a fusion product between any one
or more of a
synthetic mRNA reprogramming factor selected from 0ct4, Sox2, Klf4, cMyc,
Nanog, and
Lin28 and a transactivation domains whereby the somatic cell is reprogrammed
or de-
differentiated.
100101 In one embodiment, a method of claim 1, wherein the composition
comprises
0ct4 fused to an N-terminal MyoD transactivation domain is provided. In one
embodiment, the
0ct4 is fused to an N-terminal MyoD transactivation domain in tandem
triplicates.
100111 In one aspect, a method for reprogramming mammalian cells by using
any one or
more of the synthetic mRNA of reprogramming factor of claim 1, said method
compromising:
a) growing target cells at a density of 15 k to 500k cells/well of a standard
6-well plate in a
feeder-free surface; b) transfecting cells with varying doses of 50 ng to 800
ng/m1mRNA each
time during reprogramming is provided.
100121 In one embodiment, the target cells are grown at a density of 30 k,
75 k, 100 k, or
150 k cells/well of a standard 6-well plate in a feeder-free surface; b)
transfecting cells with
varying doses of 50 ng to 800 ng/ml mRNA each time during reprogramming,
whereas lower
doses are used at earlier time points than later time points; c) attaining
iPSCs without passaging.
100131 In one embodiment, the target cells are grown at a density of 15 k,
75 k, 100k, or
150 k cells/well of a standard 6-well plate in a feeder-free surface, whereas
the volume of each
3
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
well is adjusted to be as between 0.5 ml to 5 ml of appropriate medium;
transfecting cells with
varying doses of 50 ng to 800 ng/m1mRNA each time during reprogramming,
whereas lower
doses are used at earlier time points than later time points; attaining iPSCs
without passaging.
100141 In certain embodiments using lower doses throughout the
reprogramming
process reduced cellular immune responses and resulted in improved iPSCs
success rate. In
other embodiments using higher purity mRNA molecules, i.e. without
contaminating aberrant
transcripts from in vitro transcription allowed cells to be seeded at lower
density and survive the
repeated tranfections and higher iPSCs yield.
100151 In one embodiment, the mammalian cells are human cells. In one
embodiment,
the method is Xeno-free. In one embodiment, the mammalian cells are non-human
primate
cells. In one embodiment, the non-human primate cells are cynomolgus monkey
cells.
100161 In one embodiment, the one or more factors are selected from the
group
consisting of mRNAs, regulatory RNAs, siRNA miRNA, and combinations thereof.
100171 In one embodiment, the somatic cells are transfected with at least
two different
RNAs. In one embodiment, the somatic cells are selected from the group
consisting of
unipotent, multipotent, pluripotent, and differentiated cells. In one
embodiment, the one or more
RNAs induces de-differentiation of the somatic cells to unipotent,
multipotent, or pluripotent
cells.
100181 In one embodiment, the at least one of the factors is selected from
the group
consisting of OCT4, SOX2, NANOG, LIN28, KLF4 and MYC mRNA. In one embodiment,
the
OCT4, SOX2, NANOG, and LINN mRNA are administered in combination. In one
embodiment, the OCT4, SOX2, KLF4 and MYC mRNA are administered in combination.
100191 In one embodiment, the transfected cells are maintained in culture
as induced
pluripotent stem (iPS) cells. In one embodiment, the transfected cells form
induced pluripotent
stem cells, further comprising inducing the iPS cells to form differentiated
cells.
100201 In one aspect, a method for treating or inhibiting one or more
symptoms of a
disease or disorder in a patient comprising de-differentiating cells in vitro
and administering the
cells to the patient is provided. In one embodiment, the composition further
comprises Rarg
4
CA 2950582
and LrH-1 transaction activation domains. In one embodiment, the composition
comprises OCT4
fused to a VP16 transactivation domain.
[0021] In one embodiment, the disclosure provides iPSCs derived from
non-human
primate cells which were used to establish disease model systems because they
can produce in-
integration-free, feeder-free testing environment without other animal
products. Thus, the non-
human iPSCs described herein are useful for preclinical testing.
[0022] The inventions described and claimed herein have many
attributes and
embodiments including, but not limited to, those set forth or described or
referenced in this Brief
Summary. It is not intended to be all-inclusive and the inventions described
and claimed herein are
not limited to or by the features or embodiments identified in this Brief
Summary, which is included
for purposes of illustration only and not restriction. Additional embodiments
may be disclosed in the
Detailed Description below.
[0022A] Various embodiments of the claimed invention relate to a method for
dedifferentiating or reprogramming primate somatic cells, the method
comprising transfecting the
isolated non-human primate somatic cells with a composition, the composition
comprising: a purified
synthetic mRNA encoding reprogramming factor 0ct4 fused to an N-terminal MyoD
transactivation
domain; and purified synthetic mRNAs encoding reprogramming factors Sox2,
Klf4, cMyc, Nanog,
and Lin28, whereby the somatic cells are reprogrammed or de-differentiated,
wherein the synthetic
mRNAs are purified using size-exclusion chromatography.
[0022B] Various embodiments of the claimed invention also relate to a method
for
reprogramming primate somatic cells, wherein the method comprises: growing
target primate
somatic cells at a density of 25 k to 250 k cells/well in a standard 6-well
plate on a feeder-free
surface; and transfecting the cells once daily with purified synthetic mRNAs
in doses ranging from
about 50 ng to about 800 ng/ml, wherein the purified synthetic mRNAs encode
reprogramming factor
0ct4 fused to an N-terminal MyoD transactivation domain, reprogramming factor
Sox2,
reprogramming factor Klf4, reprogramming factor cMyc, reprogramming factor
Nanog, and
reprogramming factor Lin28; wherein the synthetic mRNAs are purified using
size-exclusion
chromatography.
- 5 -
CA 2950582 2019-10-15
CA 2950582
[0022C] Various embodiments of the claimed invention also relate to a method
for
reprogramming primate somatic cells, wherein the method comprises: growing
target primate
somatic cells at a density selected from any one of the following: 15K, 30K,
50 k, 75 k, 100 k, 150 k,
250 K, and 500 K cells/well in a standard 6-well plate in a feeder-free
surface, wherein the volume of
each well is adjusted to be between 0.5 ml to 5 ml of appropriate medium;
transfecting the cells once
daily with purified synthetic mRNAs in doses ranging from about 50 ng to about
800 ng/ml, wherein
the purified synthetic mRNAs encode reprogramming factor 0ct4 fused to an N-
terminal MyoD
transactivation domain, reprogramming factor Sox2, reprogramming factor Klf4,
reprogramming
factor cMyc, reprogramming factor Nanog, and reprogramming factor Lin28,
wherein lower doses
are used at earlier time points than later time points; and attaining iPSCs
without passaging the cells.
[0022D] Various embodiments of the claimed invention also relate to a method
for
dedifferentiating or reprogramming primate somatic cells, the method
comprising transfecting the
isolated non-human primate somatic cells with a composition, the composition
comprising:
a purified synthetic mRNA encoding reprogramming factor 0ct4 fused to an N-
terminal MyoD
transactivation domain; and purified synthetic mRNAs encoding reprogramming
factors Sox2, Klf4,
cMyc, Nanog, and Lin28, wherein the purified synthetic mRNA contains 2-thio-
uracil; whereby the
somatic cells are reprogrammed or de-differentiated, and wherein the synthetic
mRNAs are purified
using size-exclusion chromatography.
[0022E] Various embodiments of the claimed invention also relate to a method
for
reprogramming primate somatic cells, wherein the method comprises: growing
target primate
somatic cells at a density of 25 k to 250 k cells/well in a standard 6-well
plate on a feeder-free
surface; and transfecting the cells once daily with purified synthetic mRNAs
in doses ranging from
about 50 ng to about 800 ng/ml, wherein the purified synthetic mRNAs encode
reprogramming factor
0ct4 fused to an N-terminal MyoD transactivation domain, reprogramming factor
Sox2,
reprogramming factor Klf4, reprogramming factor cMyc, reprogramming factor
Nanog, and
reprogramming factor Lin28, wherein the purified synthetic mRNA contains 2-
thio-uracil.
[0022F] Various embodiments of the claimed invention also relate to a method
for
reprogramming primate somatic cells, wherein the method comprises: growing
target primate
somatic cells at a density selected from any one of the following: 15K, 30K,
50 k, 75 k, 100 k, 150 k,
250 K, and 500 K cells/well in a standard 6-well plate in a feeder-free
surface, wherein the volume of
each well is adjusted to be between 0.5 ml to 5 ml of appropriate medium;
transfecting the cells once
- 5a -
CA 2950582 2019-10-15
CA 2950582
daily with purified synthetic mRNAs in doses ranging from about 50 ng to about
800 ng/ml, wherein
the purified synthetic mRNAs encode reprogramming factor 0ct4 fused to an N-
terminal MyoD
transactivation domain, reprogramming factor Sox2, reprogramming factor Klf4,
reprogramming
factor cMyc, reprogramming factor Nanog, and reprogramming factor Lin28,
wherein lower doses
are used at earlier time points than later time points; wherein the purified
synthetic mRNA contains
2-thio-uracil and attaining iPSCs without passaging the cells.
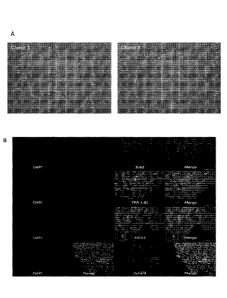
BRIEF DESCRIPTION OF THE DRAWINGS
[0023] Figure 1. iPSC Colonies Derived with M30-Based mRNA
Reprogramming
Cocktail. (A) 10x bright-field images of two of the expanded iPSC clones
derived from the first
M30-based BJ reprogramming trial. (B) Immunostaining of expanded clones for
pluripotency
markers.
[0024] Figure 2. Feeder-Free Reprogramming Using M30-Based Cocktail.
(A)
Immunofluorescence imaging showing the TRA-1-60+ colony yield from feeder-free
derivations on
50K XFF fibroblasts comparing c-Myc and L-Myc-based cocktails and 4- hour and
24-hour
transfection regimens. All wells were transfected for 9 days. 4-hour
transfection cultures were fixed
for staining on day 15 of the experiment, 24-hour transfection cultures on day
11. (B) 10x bright-
field imaging of the 400 ng/ml Stemfect well from the same experiment showing
near-confluent
hESC-like colonies overtaking the culture on day 9 of the derivation. (C) 10x
bright-field time
course of a marked field in a follow-up trial in which 100K XFFs were again
transfected for 9 days
using a 400 ng/ml Stemfect regimen, showing epithelialization and subsequent
emergence of hESC-
like colonies.
[0025] Figure 3. Comparison of Reprogramming Efficiency Using 4
Different
mRNA Cocktails. Flowchart summarizing the four-cocktail comparison experiment.
- 5b -
CA 2950582 2019-10-15
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
100261 Figure 4. hESC-Like Colonies in an HDF-a Feeder-Free Reprogramming
Culture. 10x bright-field images of emergent hESC-like colonies at day 9 of a
feeder-free
derivation from 75K HDF-a adult fibroblasts, treated for 9 days with 400 ng/ml
mRNA cocktail
(M30+ c-Myc+ Nanog+) delivered as a media supplement using Stemfect
transfection reagent.
100271 Figure 5. Generation of Synthetic mRNA Cocktails. (A) Schematic
summarizing the procedure for making mRNA reprogramming cocktails. (B)
Synthetic
mRNAs encoding a number of RFs and fluorescent reporters on a SYBR E-gel. 500
ng of RNA
was loaded per lane.
100281 Figure 6. Generation of synthetic mRNA Cocktails using 2-Thio-Uracil
for
reprogramming cocktails. Synthetic mRNAs encoding a number of RFs on a SYBR E-
gel.
The mRNAs have 10% of their Uracil bases replaced by 2-Thio-Uracils. 100 ng of
RNA was
loaded per lane.
100291 Figure 7. Human iPSCs created using mRNA cocktails with mRNAs
modified with 2-Thio-Uracil. Populations of human iPSCs after (A) 7 days or
(B) 11 days of
reprogramming during different reprogramming runs in Pluriton (A) or Allele
Reprogramming
media (B).
100301 Figure 8. Cynomolgus Monkey iPSC colonies created using mRNA
cocktail
in feeder-free reprogramming culture. Shown in the pictures are 10x bright-
field images of
emergent hESC-like colonies on day 10 and day 13. In the day 10 panel, the
cells near the
center exhibit morphology changes from fibroblast to stem cells. In the day 13
panel, the cells
formed a large colony of fully transformed monkey iPSCs.
DETAILED DESCRIPTION
100311 When describing the present invention, all terms not defined herein
have their
common meanings recognized in the art. To the extent that the following
description is of a
specific embodiment or a particular use of the invention, it is intended to be
illustrative only,
and not limiting of the claimed invention. The following description is
intended to cover all
alternatives, modifications and equivalents that are included in the spirit
and scope of the
invention.
6
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
100321 Differentiated cells can be reverted to a pluripotent state by the
expression of a
select group of transcription factors opened up the prospect that patient-
specific cells might be
used to generate cells of any desired type for the study of genetic disease in
vitro and ultimately
for cell-replacement therapy. Expression of the reprogramming factors can be
achieved through
the application of viral vectors which integrate into the genome, and iPSC
derivations are still
usually performed with integrating retrovirus or lentivirus. The attendant
modification of the
genome represents an important hurdle to therapeutic application of iPSCs,
while the possibility
of reactivated expression from integrated viral cassettes is a concern even
for in vitro studies.
Considerable progress has been made recently in the application of novel
expression vectors
that alleviate or obviate the genome-modification problem. Lentiviral vectors
are now available
which encode the multiple factors required for iPSC induction in a single
polycistronic cassette
flanked by lox recombination sites, which allows for almost-seamless excision
of the transgene
after reprogramming through transient expression of Cre recombinase. Transgene
insertion
with subsequent excision can also be effected by using a transposon vector
followed by brief
expression of transposase. Several different types of non-integrating DNA
vector have been
employed which can transiently express reprogramming factors for enough time
to induce
pluripotency, including adenovirus, plasrnid and episomal DNA. It has also
proved possible to
generate iPSC by repeated transduction of cells with recombinant RF proteins
incorporating
cell-penetrating peptides, albeit with low efficiency. Relatively efficient
iPSC conversion can
now be achieved using Sendai virus, which has a completely RNA-based
reproductive cycle,
and by sustained transfection of synthetic mRNA transcripts encoding the
Yamanaka factors.
100331 The application of mRNA transfection to reprogramming (and
potentially to
directed differentiation and transdifferentiation) is appealing as this system
allows the
expression of reprogramming cocktails and even individual component factors to
be modulated
on a daily basis simply by changing which transcripts are added to the cell
culture media. Once
transfection of a particular factor is terminated, ectopic expression within
the target cells ceases
in short order due to the rapid decay of mRNA in the cytoplasm. In contrast to
non-integrating
DNA vectors or RNA viruses, no cleanup is required with mRNA transfection, nor
is there any
risk of random genomic integration or persistent viral infection. These
advantages assume
greater significance if we envisage that multiple rounds of ectopic RF
expression may
ultimately be employed to go from a patient biopsy to specialized cells of a
desired type via an
iPSC intermediate. Nonetheless, there are drawbacks to mRNA-based
reprogramming as
7
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
currently practiced. While the expression of RFs is typically robust for on
the order of 24 hours
after mRNA is transfected, it takes about two weeks of factor expression to
induce pluripotency
in human cells, so the hands-on time required to reprogram cells with this
technique is relatively
high. Not all cell types and culture media are equally conducive to efficient
mRNA delivery,
and this is currently an impediment to mRNA-based reprogramming of certain
cell types of
interest, including blood cells. It has also so far proved necessary to employ
a feeder layer of
mitotically-arrested fibroblasts in order to successfully reprogram cells into
iPSCs using the
mRNA method. These feeder cells buffer the population density of the culture
as the target
cells grow out from a low starting density over the extended time course
required for iPSC
induction, evening out the delivered dose of RNA and transfection reagent
(both of which have
associated toxicities) and supporting the viability of the target cells in the
face of the pro-
apoptotic and cytostatic forces engendered by the reprogramming process. This
requirement
adds complexity and hands-on time to the procedure and introduces an important
source of
technical variability, especially given that the feeders are themselves
subject to transfection.
The presence of a feeder layer also impedes monitoring and analysis of the
reprogramming
process. Finally, although human feeder cells are currently the standard for
mRNA
reprogramming, even these cells are a potential source of xeno-biological
contamination when
non-human animal products are used in their derivation and expansion.
100341 Accordingly, in view of the problems associated with the previously
known
procedure, novel methods, materials, and protocols are provided herein to
produce iPSCs with
improved efficiency of reprogramming and improved quality of the resultant
cells. The current
invention embodiments were used successfully to achieve significant surprising
and unexpected
improvements through potentiation of the RF cocktail delivered to the cells.
The current
invention embodiments also provide a novel protocol(s) which compresses and
streamlines the
mRNA reprogramming process, and which support the production of footprint-free
iPSCs from
human fibroblasts without the use of feeder cells or any other potentially
xeno-contaminated
reagents. The novel methods and compositions provided herein will extend the
benefits of the
previously known mRNA method and help clear remaining roadblocks to the
therapeutic
application of iP SC technology.
100351 The present disclosure relates generally to methods of using
engineered
reprogramming factor(s) for the creation of induced pluripotent stem cells
(iPSCs) through a
kinetically controlled process. More specifically, this invention relates to
establishing
8
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
combinations of reprogramming factors, including fusions between conventional
reprogramming factors with transactivation domains, optimized for
reprogramming different
types of cells; introducing these factors as synthetic messenger RNA (mRNA)
into cultured
mammalian cells at the preferred density by methods that result in appropriate
levels of
transgene expression; maintaining cell under defined conditions to result in
previously
unattainable efficiency of reprogramming. Compared to other methods that are
known in the
art, the current invention dramatically reduces the time, cost and effort
involved in
reprogramming, with the options to be completely feeder-free and Xeno-free,
and without
passaging. The materials and procedures disclosed herein are useful for
creating induced
pluripotent stem cells from different types of mammalian cells, including
human fibroblasts.
100361 Aspects of the disclosure also provide methods for generating stem
cells capable
of producing variety of different tissues of the human body by using messenger
RNA molecules
without the need of viral vectors, animal products or feeder cells. The novel
methods disclosed
herein can be used to reprogram human fibroblasts into induced pluripotent
stem cells (iPSCs)
with surprising and unexpected efficiency under optimal conditions.
100371 The present disclosure provides useful processes and compositions
for treating
non-human mammalian cells (e.g. fibroblasts) with cocktails of "cleaned-up"
rnRNAs encoding
exemplary reprogramming factors described herein, The mRNA composition can be
used
without causing the treated cells to undergo cell death associated with
introduction of the
mRNA molecules. In one embodiment, the non-human mammalian cells are
cynomolgus
monkey cells. In another embodiment, the cynomolgus monkey cells are seeded in
a gradient in
a well so that cells grow at varying local densities.
DEFINITIONS
100381 As used herein, cells suitable for use with the method include, but
are not limited
to, primary cells and established cell lines, embryonic cells, immune cells,
stem cells, and
differentiated cells including, but not limited to, cells derived from
ectoderm, endoderm, and
mesoderm, including fibroblasts, parenchymal cells, hematopoietic cells, and
epithelial cells.
As used herein, stern cells include unipotent cells, multipotent cells, and
pluripotent cells;
embryonic stem cells, and adult stem cells such as hematopoietic stem cells,
mesenchymal stem
cells, epithelial stem cells, and muscle satellite cells. In one embodiment,
somatic cells are de-
differentiated or reprogrammed. Any suitable somatic cell can be used.
Representative somatic
9
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
cells include fibroblasts, keratinocytes, adipocytes, muscle cells, organ and
tissue cells, and
various blood cells including, but not limited to, hematopoietic cells
including hematopoietic
stem cells, and cells that provide short- or long-term hematopoietic
engraftment. The most
preferred cell types include, but are not limited to, human fibroblasts,
keratinocytes and
hematopoietic stem cells. The methods are particularly useful for de-
differentiating and
optionally re- differentiating cells, without permanent alteration of cell
genomes.
100391 RNAs useful in the disclosed method include mRNAs, regulatory RNAs,
or
small RNAs such as siRNA or miRNA wherein the one or more mRNAs encode
polypeptides
that function to de-differentiate or reprogram the cell. The efficiency of
transfection is high.
Typically more than 90% of the transfected cell population will express the
introduced RNA.
Therefore, it is possible to transfect cells with one or more distinct RNAs.
For example, the
population of cells can be transfected with one or more distinct mRNAs, one or
more distinct
siRNAs, one or more distinct miRNAs, or combinations thereof. The population
of cells can be
transfected with multiple RNAs simultaneously in a single administration, or
multiple
administrations can be staggered minutes, hours, days, or weeks apart.
Transfection of multiple
distinct RNAs may be staggered. For example, if it is desirable for a first
RNA to be expressed
prior to expression of one or more additional RNAs.
100401 The level of expression of the transfected RNA can be manipulated
over a wide
range by changing the amount of input RNA, making it possible to individually
regulate the
expression level of each transfected RNA. The effective amount of input RNA is
determined
based on the desired result. Furthermore, the PCR-based technique of mRNA
production
facilitates the design of mRNAs with different structures and domain
combinations. RNAs
useful in the disclosed methods are known in the art, and will be selected
based on the target
host cell type as well as the pathway or cellular activity to be manipulated,
or the therapeutic
application. Constructs useful for dc- differentiating cells, for example,
converting adult,
differentiated somatic cells into stem cells, can be constructed based on
known genes, mRNAs,
or other nucleotide or protein sequences.
100411 The terms "polynucleotide" and "nucleic acid," used interchangeably
herein,
refer to a polymeric form of nucleotides of any length, either ribonucleotides
or
deoxyribonucleotides. Thus, this term includes, but is not limited to, single-
, double-, or multi-
stranded DNA or RNA, genomic DNA, cDNA, DNA-RNA hybrids, or a polymer
comprising
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
purine and pyrimidine bases or other natural, chemically or biochemically
modified, non-
natural, or derivatized nucleotide bases. "Oligonucleotide" generally refers
to polynucleotides
of between about 5 and about 100 nucleotides of single- or double-stranded
DNA. However,
for the purposes of this disclosure, there is no upper limit to the length of
an oligonucleotide.
Oligonucleotides are also known as oligomers or oligos and may be isolated
from genes, or
chemically synthesized by methods known in the art.
100421 As used herein, the term "microRNA" refers to any type of
interfering RNAs,
including but not limited to, endogenous microRNAs and artificial microRNAs
(e.g., synthetic
miRNAs). Endogenous microRNAs are small RNAs naturally encoded in the genome
which
are capable of modulating the productive utilization of mRNA. An artificial
microRNA can be
any type of RNA sequence, other than endogenous microRNA, which is capable of
modulating
the activity of an mRNA. A microRNA sequence can be an RNA molecule composed
of any
one or more of these sequences.
100431 A "microRNA precursor" (or "pre-miRNA") refers to a nucleic acid
having a
stem-loop structure with a microRNA sequence incorporated therein. A "mature
microRNA"
(or "mature miRNA") includes a microRNA that has been cleaved from a microRNA
precursor
(a "pre-miRNA"), or that has been synthesized (e.g., synthesized in a
laboratory by cell-free
synthesis), and has a length of from about 19 nucleotides to about 27
nucleotides, e.g., a mature
microRNA can have a length of 19 nt, 20 nt, 21 nt, 22 nt, 23 nt, 24 nt, 25 nt,
26 nt, or 27 nt. A
mature microRNA can bind to a target mRNA and inhibit translation of the
target mRNA.
100441 Exemplary genomic, mRNA (cDNA), and protein sequences for OCT4 are
known in the art, see, for example, (OCT4) POU5F1 POU class 5 homeobox [Homo
sapiens]
Gene ID: 5460, which provides mRNA (cDNA) sequences Genbank accession no. NM-
001173531.1 entitled Horno sapiens POU class 5 homeobox 1 (POU5F1), transcript
variant 3,
mRNA; Genbank accession no. NM-002701.4 entitled Homo sapiens POU class 5
homeobox
1 (POU5F1) transcript variant 1, mRNA; and Genbank accession no. NM-203289.4
entitled
Homo sapiens POLE class 5 homeobox 1 (POU5F1), transcript variant 2, mRNA.
Exemplary
genomic, mRNA (cDNA), and protein sequences for SOX2 are also known in the
art, see, for
example, SOX2 SRY (sex determining region Y)-box 2 [Homo sapiens], Gene ID:
6657, which
provides mRNA (cDNA) sequence Genbank Accession no. NM-003106.2 entitled mRNA
sequence Homo sapiens SRY (sex determining region Y)-box 2 (SOX2), mRNA.
Exemplary
11
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
genomic, mRNA (cDNA), and protein sequences for NANOG are also known in the
art, see for
example NANOG Nanog homeobox [Homo sapiens], Gene ID: 79923, which provides
the
mRNA (cDNA) sequence Genbank accession no. NM ____________________ 024865.2
entitled Homo sapiens Nanog
homeobox (NANOG), mRNA. Exemplary genomic, mRNA (cDNA), and protein sequences
for
LIN28 are also known in the art, see for example LIN28A homolog A (C. elegans)
[Homo
sapiens], Gene ID: 79727, which provides the mRNA (cDNA) sequence Genbank
accession no.
NM-024674.4 entitled Homo sapiens lin-28 homolog A (C. elegans) (LIN28A),
mRNA.
Exemplary genomic, mRNA (cDNA), and protein sequences for KLF4 are known in
the art,
see, for example, KLF4 Kruppel- like factor 4 (gut) [Homo sapiens], Gene ID:
9314, which
provides the mRNA (cDNA) sequence Genbank accession no. NM-004235.4 entitled
Homo
sapiens Kruppel-like factor 4 (gut) (KLF4), mRNA. mRNA sequences for MYC are
also
known in the art, see for example MYC v-myc myelocytomatosis viral oncogene
homolog
(avian) [Homo sapiens], Gene ID: 4609, which provides the mRNA (cDNA) sequence
Genbank
accession no. NM 002467.4 entitled Homo sapiens v-myc myelocytomatosis
viral oncogene
homolog avian) (MYC), mRNA.
100451 A "stem-
loop structure" refers to a nucleic acid having a secondary structure that
includes a region of nucleotides which are known or predicted to form a double
strand (step
portion) that is linked on one side by a region of predominantly single-
stranded nucleotides
(loop portion). The terms "hairpin" and "fold-back" structures are also used
herein to refer to
stem-loop structures. Such structures are well known in the art and these
terms are used
consistently with their known meanings in the art. The actual primary sequence
of nucleotides
within the stem-loop structure is not critical to the practice of the
invention as long as the
secondary structure is present. As is known in the art, the secondary
structure does not require
exact base-pairing. Thus, the stem may include one or more base mismatches.
Alternatively,
the base-pairing may be exact, i.e. not include any mismatches.
100461 As used
herein, the term "stem cell" refers to an undifferentiated cell that can be
induced to proliferate. The stem cell is capable of self-maintenance, meaning
that with each cell
division, one daughter cell will also be a stem cell. Stem cells can be
obtained from embryonic,
fetal, post-natal, juvenile or adult tissue. The term "progenitor cell", as
used herein, refers to an
undifferentiated cell derived from a stem cell, and is not itself a stem cell.
Some progenitor
cells can produce progeny that are capable of differentiating into more than
one cell type.
12
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
100471 The term "induced pluripotent stem cell" (or "iPS cell"), as used
herein,
refers to a stem cell induced from a somatic cell, e.g., a differentiated
somatic cell, and that
has a higher potency than said somatic cell. iPS cells are capable of self-
renewal and
differentiation into mature cells, e.g., smooth muscle cells. iPS may also be
capable of
differentiation into smooth muscle progenitor cells.
100481 As used herein the term "isolated" with reference to a cell, refers
to a cell that
is in an environment different from that in which the cell naturally occurs,
e.g., where the
cell naturally occurs in a multicellular organism, and the cell is removed
from the multicellular
organism, the cell is "isolated." An isolated genetically modified host cell
can be present in a
mixed population of genetically modified host cells, or in a mixed population
comprising
genetically modified host cells and host cells that are not genetically
modified. For example,
an isolated genetically modified host cell can be present in a mixed
population of
genetically modified host cells in vitro, or in a mixed in vitro population
comprising
genetically modified host cells and host cells that are not genetically
modified.
100491 A "host cell," as used herein, denotes an in vivo or in vitro cell
(e.g., a eukaryotic
cell cultured as a unicellular entity), which eukaryotic cell can be, or has
been, used as
recipients for a nucleic acid (e.g., an exogenous nucleic acid), and include
the progeny of the
original cell which has been genetically modified by the nucleic acid. It is
understood that the
progeny of a single cell may not necessarily be completely identical in
morphology or in
genomic or total DNA complement as the original parent, due to natural,
accidental, or
deliberate mutation.
100501 The term "genetic modification" and refers to a permanent or
transient genetic
change induced in a cell following introduction of new nucleic acid (i.e.,
nucleic acid
exogenous to the cell). Genetic change ("modification") can be accomplished by
incorporation
of the new nucleic acid into the genome of the host cell, or by transient or
stable maintenance of
the new nucleic acid as an extrachromosomal element. Where the cell is a
eukaryotic cell, a
permanent genetic change can be achieved by introduction of the nucleic acid
into the genome
of the cell. Suitable methods of genetic modification include viral infection,
transfection,
conjugation, protoplast fusion, electroporation, particle gun technology,
calcium phosphate
precipitation, direct microinjection, and the like.
13
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
100511 As used herein, the term "exogenous nucleic acid" refers to a
nucleic acid that is
not normally or naturally found in and/or produced by a cell in nature, and/or
that is introduced
into the cell (e.g., by electroporation, transfection, infection, lipofection,
or any other means of
introducing a nucleic acid into a cell).
[0052] As used herein, the terms "treatment," "treating," and the like,
refer to obtaining
a desired pharmacologic and/or physiologic effect. The effect may be
prophylactic in terms of
completely or partially preventing a disease or symptom thereof and/or may be
therapeutic in
terms of a partial or complete cure for a disease and/or adverse effect
attributable to the disease.
[0053] "Treatment," as used herein, covers any treatment of a disease in a
mammal,
particularly in a human, and includes: (a) preventing the disease from
occurring in a subject
which may be predisposed to the disease but has not yet been diagnosed as
having it; (b)
inhibiting the disease, i.e., arresting its development; and (c) relieving the
disease, i.e., causing
regression of the disease.
[0054] The tern-is "individual," "subject," "host," and "patient," used
interchangeably
herein, refer to a mammal, including, but not limited to, a human, a non-human
primate, a
rodent (e.g., a mouse, a rat. etc.), an ungulate, a canine, a lagomorph, a
feline, etc. In some
embodiments, a subject of interest is a human. In some embodiments, a subject
is a non-
human animal such as a monkey, rodent, or a lagomorph.
100551 A "therapeutically effective amount" or "efficacious amount" means
the amount
of a compound, a nucleic acid, or a number of cells that, when administered to
a subject for
treating a disease, is sufficient to effect such treatment for the disease.
The "therapeutically
effective amount" will vary depending on the compound or the cell, the disease
and its severity
and the age, weight, etc., of the subject to be treated.
[0056] Before the present invention is further described, it is to be
understood that this
invention is not limited to particular embodiments described, as such may, of
course, vary. It is
also to be understood that the terminology used herein is for the purpose of
describing particular
embodiments only, and is not intended to be limiting, since the scope of the
present invention
will be limited only by the appended claims.
[0057] Where a range of values is provided, it is understood that each
intervening value,
to the tenth of the unit of the lower limit unless the context clearly
dictates otherwise,
14
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
between the upper and lower limit of that range and any other stated or
intervening value
in that stated range, is encompassed within the invention. The upper and lower
limits of these
smaller ranges may independently be included in the smaller ranges, and are
also
encompassed within the invention, subject to any specifically excluded limit
in the stated
range. Where the stated range includes one or both of the limits, ranges
excluding either or
both of those included limits are also included in the invention.
100581 Unless defined otherwise, all technical and scientific terms used
herein have the
same meaning as commonly understood by one of ordinary skill in the art to
which this
invention belongs. Although any methods and materials similar or equivalent to
those described
herein can also be used in the practice or testing of the present invention,
the preferred methods
and materials are now described. All publications mentioned herein are
incorporated herein by
reference to disclose and describe the methods and/or materials in connection
with which the
publications are cited.
100591 In one aspect of the current disclosure, mRNA-based reprogramming
could be
enhanced through the use of engineered variants of 0ct4 or Sox 2 incorporating
an N-
terminal MyoD trans activation domain (Hirai et al, Stem Cells, 2011) or a C-
terminal triple
repeat of the VP16 transactivation domain (Wang et al, EMBO Reports, 2011; in
which
synthetic reprogramming factors were prepared by fusing the VP16 trans
activation domain
to OCT4 (also known as Pou5f1), NANOG and SOX2, respectively, these synthetic
factors
could reprogram both mouse and human fibroblasts with enhanced efficiency and
accelerated
kinetics), or by augmenting the "standard" RF cocktail with two additional
factors, Rarg and
Lrh-1 (Wang et al, PNAS, 2011). The contents of each of which are hereby
incorporated in
reference. Strong transcription activators can effectively recruit multiple
chromatin remodeling
complexes when binding to DNA at specific sites. A good example is MyoD, a
master
transcription factor for skeletal myogenesis that can switch the fate of
differentiated cells.
Hirai et al. speculated that since MyoD is such a strong transcription factor,
it can increase
chromatin accessibility to iPS factors if fused together. When mouse or human
cells are
transduced with retroviral vectors carrying an Oct- MyoD TAD fusion gene,
together with Sox2
and Klf4, they increased the number of iPSC colonies by ¨50-fold compared to
canonical
iPS factors. Similarly, VP16, widely known for its being a robust
transcription activator, can
exhibit strong stimulation effects on reprogramming when fused to different
iPS factors.
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
Exemplary Preparation of human iPSCs
100601 The method can also be widely used for re-differentiating or
reprogramming of
cells, for example, to produce iPS cells that can be further modulated to form
hematopoietic
stem cells, mesenchymal stem cells, epithelial stem cells, and muscle
satellite cells, or
differentiated cells of human tissues, including, but not limited to, red
blood cells, white blood
cells including lymphocytes, platelets, stromal cells, fat cells, bone cells
including osteoclasts,
epithelial tissue including skin cells, muscle tissue including smooth muscle,
skeletal muscle,
and cardiac muscle, vascular tissue including endothelial cells, liver tissue
including
hepatocytes, and nervous tissue including neurons. Methods of inducing
differentiation of iPS
cells into various differentiated cells types, including, but not limited to,
cardiomyocytes,
hematopoietic stern cells, bone cells such as, osteoclasts, hepatocytes,
retinal cells, and neurons,
stem cells including, but not limited to, isolated embryonic stem cells,
hematopoietic stem cells,
and induced pluripotent stem cells can be induced to differentiate by
transient transfection with
RNAs that induce differentiation. Additionally, or alternatively, cells can be
re-differentiated
by culturing the cells under cell type-specific conditions. For example, iPS
cells can be
maintained on CF-1 feeders and subsequently adapted to feeder-free conditions.
iPS cells can
be induced to form differentiated retinal cells by culturing the cells in the
presences of noggin,
Dkk-1, and IGF-1
100611 Previously reported methodologies relied on integrating vectors,
i.e. viruses or
plasmids, to carry the modified factors. In one embodiment, a reprogramming
trial was
performed comparing the performance of six different mRNA combination
cocktails comprising
transcripts for five factors for mRNA reprogramming (0ct4, Sox2, Klf4, cMyc-
T58A and
Lin28), or a 7-reprogram factor RF cocktail including Rarg and Lrh-1, each
combination was
tested in three variations based on wild-type 0ct4 and the MyoD- and VP16-Oct4
fusion
constructs (designated M30 and VPx3, respectively). BJ fibroblasts were
transfected in feeder-
based reprogramming cultures for 11 days, by which time advanced morphologies
were
apparent in several of the wells. Over the next few days, colonies with
characteristic hESC
morphologies emerged in wells transfccted with the cocktails based on wild-
type 0ct4 and
M30. The target cells in the VPx3-transfected cultures retained a fibroblastic
morphology,
albeit while showing accelerated growth and some tendency to aggregate into
foci, and no
colonies emerged. The 5-factor and 7-factor embodiments of the wild type 0ct4
and M30-
based cocktails showed similar colony productivity, hence no advantage accrued
from the
16
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
inclusion of Rarg and Lrh-1 in the cocktails. However, the M30 cocktail gave
several times the
number of colonies produced with wild-type 0ct4. In light of this result,
colonies were picked
from the M30 5-factor well for expansion and further analysis (Figure 1).
Pluripotency of
M30-derived colonies was confirmed by immunostaining for nuclear and cell-
surface markers,
and by in vitro differentiation into the three primary germ layers. Six
expanded iPSC clones
were subjected to karyotype analysis and DNA fingerprinting, the cells'
karyotypic normality
and BJ lineage being confirmed in all cases.
100621 The method can also be widely used for re-differentiating or
reprogramming of
cells, for example, to produce iPS cells that can be further modulated to form
hematopoietic
stem cells, mesenchymal stem cells, epithelial stem cells, and muscle
satellite cells, or
differentiated cells of human tissues, including, but not limited to, red
blood cells, white blood
cells including lymphocytes, platelets, stromal cells, fat cells, bone cells
including osteoclasts,
epithelial tissue including skin cells, muscle tissue including smooth muscle,
skeletal muscle,
and cardiac muscle, vascular tissue including endothelial cells, liver tissue
including
hepatocytes, and nervous tissue including neurons. Methods of inducing
differentiation of iPS
cells into various differentiated cells types, including, but not limited to,
cardiomyocytes,
hematopoietic stem cells, bone cells such as, osteoclasts, hepatocytes,
retinal cells, and neurons,
are known in the art (Song at al., Cell Res., 19(11):1233-42 (2009), Lamba et
al., PLoS One,
5(1):e8763 (2010), Gai et al., Cell Biol Int. 200933(10:1184-93 (2009).
Grigoriadis et al.,
Blood, 115(14):2769-76 (2010)). Stem cells including, but not limited to,
isolated embryonic
stem cells, hematopoietic stem cells, and induced pluripotent stem cells can
be induced to
differentiate by transient transfection with RNAs that induce differentiation.
Additionally, or
alternatively, cells can be re-differentiated by culturing the cells under
cell type-specific
conditions. For example, iPS cells can be maintained on CF-1 feeders and
subsequently
adapted to feeder-free conditions. iPS cells can be induced to form
differentiated retinal cells
by culturing the cells in the presences of noggin, Dkk-1, and IGF-1In another
aspect, the
potency of the mRNA cocktail could be further enhanced by the inclusion of
Nanog transcripts.
In this embodiment, four wells containing 50K RI fibroblasts on feeders were
transfected with
wild-type 0ct4 or M30- based 5-factor or 6-factor cocktails for six days, and
each culture was
then passaged 1:6 onto fresh feeders to populate a 6-well plate (4).
Transfection was continued
for 0-5 more days within each plate. The cultures were fixed and stained with
TRA-1-60
antibodies on day 18 (where day 0 corresponds to the first transfection) in
order to assess the
17
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
impact of the different cocktails and transfection time courses on iPSC
productivity. The results
showed that adding Nanog to the cocktail was highly beneficial regardless of
the 0ct4 variant
employed, while the greatest conversion efficiency was attained when M30 and
Nanog were
used together.
100631 In one embodiment, the efficacy of the M30-based 5-factor or 6-
factor
cocktails was confirmed in additional feeder-based experiments using three
additional human
fibroblast lines (HDF-f, HDF-n and XFF). Reprogramming kinetics and efficiency
were
markedly improved with all three of these low-passage lines, which displayed
faster native
population doubling times than BJs in normal expansion culture. In some cases,
we obtained
hESC-like colonies from as little as six days of transfection, although the
yields were much
higher in experiments where transfection was continued for a few more days.
Experiments
involving periodic reinforcement of the feeder layer by addition of fresh
cells suggested that
while this strategy might give some benefits, they would be offset by the
complexity of the
resulting protocol. We therefore decided to focus on applying the more potent
cocktails to
development of a streamlined, feeder- free protocol.
100641 The current disclosure relates to creation of feeder-free iPSCs.
Feeder-
independent iPSC derivation has generally proved somewhat challenging
regardless of the
reprogramming technology employed, but raises special difficulties in the
context of a sustained
transfection regime. There is a lower limit to the density at which
fibroblasts can be plated
without compromising cell viability and proliferative activity. The propensity
of cells to
undergo mitotic arrest or apoptosis in sparse cultures is exacerbated when the
cells are stressed
by transfection and by ectopic expression of reprogramming factors. Moreover,
RNA doses
which are well tolerated at the high cell densities characteristic of feeder-
based reprogramming
produce more severe cytotoxic effects when distributed among fewer cells. At
the same time,
the penetrance of expression which can be achieved with mRNA transfection
declines sharply
after fibroblasts reach confluence, perhaps owing to a down-regulation of
endocytosis
associated with contact inhibition and GI arrest. This reduced permissiveness
to transfection in
crowded cultures seems to be alleviated during reprogramming after cells
undergo
mesenchymal-to-epithelial transition (MET). However, the ¨7 days typically
required for
human fibroblasts to reach MET when using current mRNA cocktails makes it hard
to head off
the problem of fibroblastic overgrowth even if cells are plated at the lowest
survivable initial
densities. Cells can be thinned by passaging to postpone this fate, but the
plating efficiency of
18
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
highly stressed reprogramming intermediates is hard to predict and, in any
case, a passaging-
based derivation protocol would sacrifice convenience and scale poorly to high-
throughput
applications. In certain embodiments, cells can also be seeded at a gradient
in a well so that
different local cell density can facilitate the repeated transfection,
100651 The current disclosure relates to phenotypic indications of MET
(involution of
fibroblastic process, and the emergence of foci and cobblestone morphologies).
In one
embodiment, MET was accelerated by our invention using enhanced cocktails,
enabling feeder-
free reprogramming using the 6-factor M30 cocktail without passaging, seeding
target cells at a
variety of low densities (50K vs. 100K vs. 150K per well). In another
embodiment, the cells are
seeded at a density gradient with a total cell number ranging from about 15K
to 500K cells per
well.
100661 One aspect of the current invention relates to the fate of these
reprogramming
cultures, which proved highly sensitive to the seeding density, presumably
because the effects
of excessive cytotoxicity and of fibroblastic overgrowth are both self-
reinforcing over the
course of the transfection regime. In an experiment using "standard" RNA
dosing (1200 ng per
well), dozens of hESC-like colonies were obtained from HDF-n and XFF
fibroblasts plated at
100K per well, while the corresponding 50K and 150K cultures gave only a few
colonies after
succumbing to a population crash and to fibroblastic overgrowth, respectively.
In derivations
attempted with two other fibroblast lines, BJ and HDF-a, even the most
promising (150K)
cultures became virtually quiescent shortly after reaching confluence,
subsequently yielding
only sporadic colonies with delayed kinetics.
100671 One aspect of the current invention relates to purify the in vitro
transcribed
mRNAs through size exclusion chromatography to remove aberrant RNA species
that may form
dsRNA structures, either by themselves or with mRNAs, and provoke cellular
immune
responses. Using such "cleaned-up" mRNAs - mRNA with reduced cellular
immunogenicity,
effectively allowed reprogramming of non-human primate cells grown in gradient
densities.
100681 In one specific embodiment of the current invention, we switched
from ambient
to 5% oxygen culture and ramped to full RNA dosing from a quarter dose over
the first four
days of transfection in an effort to minimize stress-induced cellular
senescence.
19
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
100691 In one example of the current invention, using these new conditions
the inventors
tested if substitution of L-Myc for c-Myc could further improve the
reprogramming cocktail.
100701 In another embodiment we evaluated a simplified transfection scheme
by which
RNA was added to cells with daily media changes, rather than being delivered
in a separate step
four hours earlier. RNA dosing was scaled down in "24-hour transfection" wells
to compensate
for an expected increase in cytotoxicity, and two different transfection
reagents were tested
(RNAiMAX and Stemfect). XFF cells were plated at 50K per well and transfected
for 9 days.
The conventional "4-hour transfection" regime gave on the order of a hundred
TRA-1-60+
colonies per well with the c-Myc-based cocktail, while the L-Myc cocktail
performed
comparatively poorly (Figure 2). Results from the "24-hour transfection"
cultures were still
more impressive. In the most productive of these wells (corresponding to the c-
Myc 400 ng/m1
Stemfect condition) the culture had become almost overgrown with hESC-like
cells on day 9,
24 hours after the last transfection. The mechanistic basis for the superior
performance of the
"24-hour transfection" might have had the effect of increasing the effective
density of thin
cultures by concentrating the diffusive factors released by the cells. When
these preferred
protocol conditions were applied in another example experiments, derivation
using HDF-a
fibroblasts, in cultures seeded at 75K cells per well the productivity was
less spectacular than
achieved with the highly proliferative XFF cells, but numerous hESC-like
colonies again
emerged by as early as day 9 of the protocol (Figure 4).
100711 In one particular embodiment, when the cells were seeded at lower
volume of
medium than commonly used volume, e.g. 1 ml, 0.75 ml, 0.5 ml, or the minimum
amount of
medium that can still sustain cell culture, the efficiency of reprogramming
using the above
disclosed conditions is clearly improved, such low volume conditions during
reprogramming is
hereby incorporated in the current invention.
100721 The embodiments that have been described herein by no means are the
only
applications of the current invention. Those skilled in the art will recognize
that the disclosure
is also useful for reprogramming other cells under slightly variable
conditions or with similar
combinations of conventional or engineered factors for reprogramming, directed
differentiation,
or trans-differentiation.
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
100731 In other embodiments, optimization of the factor stoichiometry can
also enhance
the pace of reprogramming the mRNA method provide the opportunity of
defining cocktails
which address early and late phases of ipPSC induction independently. The
gains obtained
from using M30 provide validation for the recent application of novel,
engineered
reprogramming factors to iPSC generation. Such engineered reprogramming
factors include
fusing SOX2, KLF4, CMYC, LMYC, LIN28, NANOG, etc. to transactivation domains
from
factors other than VP16 OR MYOD, SUCH AS GAL4, GATA1, P53, etc. The reagents
and
methodology used to deliver mRNA can also be used to co-transfect siRNA and
miRNA, which
have already proven their worth in iPSC generation. Nonetheless, the feeder-
free protocol
disclosed herein represents a substantial advance over current protocols,
reducing the time
required for reprogramming by as much as a half with an equal or greater
reduction in labor and
materials costs, taking troublesome steps out of the procedure, and allowing
mRNA to be
delivered to cells with almost the same ease as growth factors or
cytokines¨i.e., as a media
supplement.
100741 In some embodiments, cells are reprogrammed to modulate the immune
response. For example, lymphocytes can be reprogrammed into regulatory T cells
which can be
administered to a patient in need thereof to increase or transfer immune
tolerance, especially
self-tolerance. The induction or administration of Foxp3 positive T cells may
be useful in
reducing autoimmune responses such graft rejection, and/or reducing,
inhibiting or mitigating
one or more symptoms of an autoimmune diseases or disorder such as diabetes,
multiple
sclerosis, asthma, inflammatory bowel disease, thyroiditis, renal disease,
rheumatoid arthritis,
systemic lupus erythematosus, alopecia greata, anklosing spondylitis,
antiphospholipid
syndrome, autoimmune Addison's disease, autoimmune hemolytic anemia,
autoimmune
hepatitis, autoimmune inner ear disease, autoimmune lymphoproliferative
syndrome (ALPS),
autoimmune thrombocytopenic purpura (ATP), Behcet's disease, bullous
pemphigoid,
cardiomyopathy, celiac sprue-dermatitis, chronic fatigue syndrome immune
deficiency
syndrome (CFIDS), chronic inflammatory demyelinating polyneuropathy,
cicatricial
pemphigoid, cold agglutinin disease, Crest syndrome, Crohn's disease, Dego's
disease,
dermatomyositis, dermatomyositis¨juvenile, discoid lupus, essential mixed
cryoglobulinemia,
fibromyalgia¨fibromyositis, Grave's disease, Guillain-Barre, Hashimoto's
thyroiditis,
idiopathic pulmonary fibrosis, idiopathic thrombocytopenia purpura (ITP), IgA
nephropathy,
insulin dependent diabetes (Type I), juvenile arthritis, Meniere's disease,
mixed connective
21
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
tissue disease, multiple sclerosis, myasthenia gravis, pcmphigus vulgaris,
pernicious anemia,
polyarteritis nodosa, polychondritis, polyglancular syndromes, polymyalgia
rheumatica,
polymyositis and dermatomyositis, primary agammaglobulinemia, primary biliary
cirrhosis,
psoriasis, Raynaud's phenomenon, Reiter's syndrome, rheumatic fever,
sarcoidosis, scleroderma,
Sjogren's syndrome, stiff-man syndrome, Takayasu arteritis, temporal
arteritis/giant cell
arteritis, ulcerative colitis, uveitis, vasculitis, vitiligo, and Wegener's
granulomatosis.
100751 The methods can be used to generate cells which may be useful in the
treatment
of a variety of diseases and disorders, including, but not limited to,
diseases such as Parkinson's,
Alzheimer disease, wound healing, and multiple sclerosis. The methods are also
useful for
organ regeneration, and for restoration or supplementation of the immune
system. For example,
cells at different stages of differentiation such as iPS cells, hematopoietic
stem cells, multipotent
cells or unipotent cells such as precursor cells, for example, epithelial
precursor cells, and others
can be administered intravenously or by local surgery. The methods can be used
in combination
with other conventional methods, such as a prescription medication regime,
surgery, hormone
therapy, chemotherapy and/or radiotherapy.
100761 In one embodiment, a kit includes RNAs, cells, and a means for
transfecting the
RNA into the cells. The RNAs can be lyophilized or in solution. Kits may
optionally include
other materials such as cell culture reagents. In an alternative embodiment, a
kit provides re-
differentiated, dedifferentiated, or reprogrammed cells prepared according to
the disclosed
methods, and stored and/or shipped refrigerated or frozen for later use. Cells
are typically
stored in a solution maintaining viability. Kits containing cells should be
stored or shipped
using a method consistent with viability such as in a cooler containing dry
ice so that cells are
maintained below 4 C., and preferably below ¨ 20 C.
100771 The kits optionally include one or more of the following: bioactive
agents,
media, excipients and one or more of: a syringe, a needle, thread, gauze, a
bandage, a
disinfectant, an antibiotic, a local anesthetic, an analgesic agent, surgical
thread, scissors, a
scalpel, a sterile fluid, and a sterile vessel. Components of the kit may be
packaged individually
and can be sterile. The kits are generally provided in a container, e.g., a
plastic, cardboard, or
metal container suitable for commercial sale. Any of the kits can include
instructions for use.
The methods can be used to generate cells which may be useful in the treatment
of a variety of
diseases and disorders, including, but not limited to, neurodegenerative
diseases such as
22
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
Parkinson's, Alzheimer disease, and multiple sclerosis. The methods disclosed
herein are also
useful for organ regeneration, and for restoration or supplementation of the
immune system.
For example, cells at different stages of differentiation such as iPS cells,
hematopoietic stem
cells, multipotent cells or unipotent cells such as precursor cells, for
example, epithelial
precursor cells, and others can be administered intravenously or by local
surgery. The methods
can be used in combination with other conventional methods, such as a
prescription medication
regime, surgery, hormone therapy, chemotherapy and/or radiotherapy.
100781 Aspects of the disclosure provide culturing systems of stem cells
and
differentiation methods for producing skin tissue cells for wound treatment,
and stem cell
therapy for the treatment of arthritis, Lupus, and other autoimmune-related
diseases.
EXAMPLES
100791 The invention is now described with reference to the following
Examples. These
Examples are provided for the purpose of illustration only, and the invention
is not limited to
these Examples, but rather encompasses all variations that are evident as a
result of the teaching
provided herein.
EXAMPLE 1- Generation of 1VT Templates
100801 Plasmid constructs for generating linear PCR-product in vitro
transcription
(IVT) templates were constructed using Ligation Independent Cloning (LIC). We
first
constructed a parental plasmid (pIVT) incorporating 5' and 3' untranslated
regions (UTRs)
flanking an insertion site designed to accept an open reading frame (ORF)
insert
encoding a protein of interest. The ORF-flanking sequences are as described in
Warren et
al, Cell Stem Cell, 2010, comprising a low secondary structure leader and
strong Kozak
site (5' UTR) and the murine ct-globin 3' UTR. A linearized version of the
PIVT vector
bearing 5' overhangs was produced by reannealing of two PCR products amplified
from
the plasmid using tailed primers. ORF PCR products with complementary
overhangs were
produced by an analogous procedure, pooled with the vector PCR products and
transformed
into DH5ct bacteria by heat shock to clone gene- specific constructs (pIVT-
KLF4, etc.). The
resulting plasmids were used to template PCR reactions to make linear WT
templates
incorporating a T7 promoter, UTR-flanked ORF and a T120 tail to drive addition
of a
polyA tail, as described in Warren et al, Cell Stem Cell, 2010. The T120 tail
region was
introduced through the use of a tailed reverse primer
23
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
(T120CTICCTACTCAGGCTITATTCAAAGACCA). For the M30 and VPx3 fusion
constructs, the transactivating domain-encoding sequences were appended to the
ORFs by
PCR using tailed primers. PCR product template stocks were maintained at a
concentration of
¨100 ng/uL.
EXAMPLE 2 ¨ Production of mRNA Cocktails
100811 The mRNA
synthesis process is summarized in Figure 5. Synthetic mRNA was
generated in IVT reactions using a 4:1 ratio of ARCA cap analog to GTP to
generate a high
percentage of capped transcripts. Full substitution of 5m-CTP for CTP and
Pseudo-UTP for
UTP in the nucleotide triphosphate (NTP) mix was employed to reduce the
immunogenicity of
the RNA products. Cap analog and modified NTPs were purchased from Trilink
Biotechnologies. A 2.5x NTP mix was prepared (ARCA:ATP:5m- CTP:GTP:Pseudo-UTP
at
15:15:3.75:3.75:3.75 mM) to replace the standard NTPs provided with the
MEGAscript T7 Kit
(Ambion) used to perform IVT reactions. Each 40 uL IVT reaction comprised 16
uL NTP mix,
4 uL 10x T7 Buffer, 16 uL DNA template and 4 uL T7 enzyme. Reactions were
incubated 4-6
hours at 37 C and then treated with 2 uL TURBO DNase for a further 15 minutes
at 37 C
before being purified on MEGAclear (Ambion) spin columns, the RNA products
being eluted in
a volume of 100 uL. To remove immunogenic 5' triphosphate moieties from
uncapped
transcripts, 10 uL of Antarctic Phosphatase reaction buffer and 3 uL of
Antarctic Phosphatase
(NEB) was added to each prep. Phosphatase reactions were incubated for 30
minutes at 37 C
and the IVT products were repurified. RNA yield was quantitated by Nanodrop
(Thermo
Scientific), and the preps were consequently adjusted to a standardized
working concentration
of 100 ng/uL by addition of TE pH 7.0 (Ambion). RNA cocktails were assembled
by pooling
preps representing the various REs in the desired stoichiometric ratios. The
fraction of each RE
used took into account the predicted molecular weight of the respective
transcript, all RFs being
equimolar except for 0ct4 and its derivatives, which were included at 3x molar
concentration.
A 10% spike of mRNA encoding a short-lived nuclearized monomeric LanYFP
fluorescent
protein was added to the cocktails to facilitate monitoring of transfection
efficacy during
reprogramming trials. In other embodiments, mRNAs were also produced by using
2-Thio-
Uracil at 25% or 10% of total Uracil (see Figure 6).
24
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
EXAMPLE 3 ¨ Cells and Culture Media
100821 Cells targeted for reprogramming included BJ neonatal fibroblasts
(ATCC),
HDF-f fetal fibroblasts, HDF-n neonatal fibroblasts and HDF-a adult
fibroblasts (ScienCell),
and XFF xeno-free neonatal fibroblasts (Millipore). Expansion culture was
carried out in BJ
medium (DMEM -h 10% FBS), ScienCell Fibroblast Medium, and FibroGRO Xeno-Free
Human Fibroblast Expansion Medium (Millipore) for the BJ, HDF and XFF cells
respectively.
Feeder cells used were 3001G irradiated neonatal human foreskin fibroblasts
(GlobalStem) and
FibroGRO mitomycin C-inactivated xeno-free human neonatal fibroblasts
(Millipore). Cell
passaging steps pertinent to xeno-free feeder-based and feeder-free
reprogramming trials were
performed using TrypLE Select (Gibco), an animal product-free cell
dissociation reagent.
EXAMPLE 4 ¨ Reprogramming of Human Fibroblasts
100831 All reprogramming experiments described were conducted in 6-well
tissue
culture plates coated with CELLstart (Gibco) xeno-free substrate in accordance
with the
manufacturer's directions. GlobalStem feeders were plated at 250K per well in
the initial BJ
reprogramming experiments, using FBS-containing BJ media. In some of the later
feeder-based
trials, the seeding density was increased and feeders were supplemented ad hoc
during media
changes in an effort to sustain near-confluent feeder layers in response to
the high attrition rates
encountered using novel RE cocktails. Xeno-free feeders, when used, were
plated in Pluriton-
based reprogramming media without serum. Target cells were plated in Pluriton
serum-free
media (Stemgent) plus antibiotics, Pluriton Supplement and 200 ngiml B18R
interferon
inhibitor (eBioscience). Media was replaced daily during and after
reprogramming, with B18R
supplementation being discontinued the day after the final transfection. In
experiments in
which cells were split onto fresh feeders during reprogramming, 10 uM Y27632
(Stemgent) was
included in the media used for replating. Transfections commenced the day
after seeding of
target cells, and were repeated at 24-hour intervals for the durations
indicated in the text. An
RNA dose of 1200 ng was delivered to each well using RNAiMAX (Invitrogen) 4
hours prior to
daily media change, except as otherwise noted. RNAiMAX-based transfection
cocktails were
made up by diluting 100 ng/uL RNA 5x in calcium/magnesium-free DPBS and 5 uL
of
RNAiMAX per ug of RNA 10x in the same diluent, pooling to produce a 10 ng/uL
RNA/vehicle suspension and dispensing to culture media after a 15-minute room
temperature
incubation. For transfections using Stemfect reagent (Stemgent), RNA and
Stemfect (4 uL per
ug of RNA) were mixed in Stemfect buffer to give an RNA concentration of 10
ng/uL. The
CA 02950582 2016-11-28
WO 2015/184318
PCT/US2015/033275
mixture was incubated for 15 minutes, then delivered to culture media or
refrigerated for later
use.
100841 In other embodiments, reprogramming of human fibroblasts were also
performed
in media other than pluriton, e.g. Allele's Reprogramming media (see Figure
7). In some
experiments, B18R was not used at all, in other experiments, it was used only
in some of the
transfections.
EXAMPLE 5. Characterization of iPSC Colonies
100851 To assess iPSC colony productivity, reprogramming cultures were
fixed
using 4% paraformaldehyde in DPBS (with calcium/magnesium) and immunostained
with
StainAlive TRA-1-60 Alexa 488 antibody (Stemgent) diluted 100x in DPBS (with
calcium/magnesium). Colony picking, expansion, and subsequent immunostaining
and
trilineage differentiation assays for molecular and functional validation of
pluripotency were
performed. DNA fingerprinting and karyotype analyses were conducted. Teratoma
formation was performed and confirmed in more than one set of mouse models.
Thereby
demonstrating the pluripotency of the stem cells.
100861 A novel method is disclosed for highly efficient reprogramming of
non- stem
cells into pluripotent stem cells by contacting target cells with combinations
of engineered
reprogramming factors and non-engineered reprogramming factors in such a way
that iPSCs can
be produced in about 9 days, sometimes as short of 6, or even 5 days. These
iPS cells can be
produced as feeder-free, xeno-frce, and footprint-free iPSCs. In addition to
the dramatically
increased efficiency of reprogramming by the invented process, the novel
technology also
differs from all previously known technologies in that the iPSCs so created
are "clean" in that
they have not been in contact with any virus or vector. Utility of the
invention can be found in
virtually all areas that involve stem cell establishment, differentiation,
utility in cellular and
developmental research, as well as clinical applications. Similar procedures
can also be useful
in directed differentiation or transdifferentiation.
EXAMPLE 6 ¨ Reprogramming of Cynomolgus Monkey Fibroblasts
100871 The reprogramming experiments using cynomolgus monkey fibroblasts
were
conducted in 6-well tissue culture plates coated with CELLstart (Gibco)
substrate in accordance
with the manufacturer's directions. Target cells were plated in Allele
Biotech's serum-free
26
CA 02950582 2016-11-28
WO 2015/184318
PCMJS2015/033275
media plus antibiotics. Media was replaced daily during and after
reprogramming with 2-Thio-
modified mRNA/transfection reagent mix delivered together with the fresh media
for 12
consecutive days with or without the use of Bl8R (Figure 8). Colonies of
monkey iPSCs
started to appear in day 9, and reached a stage of mature, compact colony
stage on day 12.
27