Note: Descriptions are shown in the official language in which they were submitted.
MULTI-FUNCTIONAL SURFACTANT COMPLEXES FOR USE IN
SUBTERRANEAN FORMATIONS
BACKGROUND
The present disclosure relates to systems and methods for treating
subterranean
formations using surfactants.
Treatment fluids can be used in a variety of subterranean treatment
operations. As
used herein, the terms "treat," "treatment," "treating," and grammatical
equivalents thereof
refer to any subterranean operation that uses a fluid in conjunction with
achieving a desired
function and/or for a desired purpose. Use of these terms does not imply any
particular
action by the treatment fluid. Illustrative treatment operations can include,
for example,
fracturing operations, gravel packing operations, acidizing operations, scale
dissolution and
removal, consolidation operations, and the like.
Many such treatment fluids include a variety of chemicals to treat common
problems
encountered in the subterranean formation and/or well bore. Commonly
encountered
problems include the production of scale producing compounds, wax buildup and
corrosion.
To solve this wide variety of problems, the oil industry has developed several
categories of
well treatment chemicals. A non-inclusive classification of well treatment
chemicals
includes: scale inhibitors, biocides, corrosion inhibitors, hydrogen sulfide
scavengers, well
tracing materials, de-waxing agents, clay stabilizers, and many others.
Surfactants are also widely used in treatment fluids for drilling operations
and other
well treatment operations, including hydraulic fracturing and acidizing (both
fracture
acidizing and matrix acidizing) treatments. Surfactants may also be used in
enhanced or
improved oil recovery operations. Many variables may affect the selection of a
surfactant for
use in such treatments and operations, such as interfacial surface tension,
wettability,
1
CA 2951289 2018-04-03
CA 02951289 2016-12-09
Attorney Docket: 063718.6746
2014-1P-0887761J1P ipeT
compatibility with other additives (such as other additives used in acidizina
treatments), and
emulsification tendency. Surfactants are often an important component in
treatment fluids
for ensuring higher productivity from unconventional oil and gas formations.
2
SUMMARY
The present disclosure relates to systems and methods for treating
subterranean
formations. More particularly, the present disclosure relates to systems and
methods for creating
and/or using multi-functional surfactant complexes that may enhance surfactant
treatments in
subterranean formations.
In certain embodiments, the present disclosure provides a method comprising:
providing
a first solution comprising at least one surfactant and a second solution
comprising at least one
polymeric additive, wherein the surfactant and the polymeric additive carry
opposite charges;
using a stop-flow mixing apparatus at a well site to mix the first and second
solutions to form
one or more multi-functional surfactant complexes that comprise the surfactant
and the
polymeric additive; using a low-dose pumping apparatus at the well site to
transfer the one or
more multi-functional surfactant complexes from the stop-flow mixing apparatus
to a blending
apparatus at the well site; using the blending apparatus to mix the one or
more multi-functional
surfactant complexes with an aqueous base fluid to form a treatment fluid; and
introducing the
treatment fluid into a well bore at the well site penetrating at least a
portion of a subterranean
formation.
In certain embodiments, the present disclosure provides a system for preparing
multi-
functional surfactant complexes at a well site comprising: a pump and blender
system disposed at
a surface of a well bore penetrating at least a portion of a subterranean
formation; a stop-flow
mixing apparatus having at least a first inlet for receiving a solution
comprising a surfactant, a
second inlet for receiving a solution comprising a polymeric additive, and an
outlet through
which a solution comprising one or more multi-functional surfactant complexes
flows out of the
stop-flow mixing apparatus; a low-dose pumping apparatus coupled between the
outlet of the
stop-flow mixing apparatus and an inlet of the pump and blender system; and a
base fluid source
coupled to an inlet of the pump and blender system.
In certain embodiments, the present disclosure provides a method comprising:
providing
a treatment fluid comprising an aqueous base fluid and one or more multi-
functional surfactant
complexes that comprise at least one surfactant and at least one polymeric
additive, wherein the
surfactant and the polymeric additive carry opposite charges and wherein the
polymeric additive
comprises a clay stabilizer; and introducing the treatment fluid into a well
bore at a well site
penetrating at least a portion of a subterranean formation; using a low-dose
pumping apparatus at
3
CA 2951289 2019-01-23
the well site to transfer the one or more multi-functional surfactant
complexes into a blending
apparatus at the well site; and using the blending apparatus to mix the one or
more multi-
functional surfactant complexes with an aqueous base fluid to form a treatment
fluid.
The features and advantages of the present disclosure will be readily apparent
to those
skilled in the art. While numerous changes may be made by those skilled in the
art, such
changes are within the scope of the disclosure.
4
CA 2951289 2019-01-23
CA 02951289 2016-12-09
Attorney Docket: 063718.6746
2014-1P-088776U1P1PCT
BRIEF DESCRIPTION OF THE DRAWINGS
These drawings illustrate certain aspects of some of the embodiments of the
present
disclosure, and should not be used to limit or define the claims.
Figure 1 is a diagram illustrating an example of a well treatment system that
may be
used in accordance with certain embodiments of the present disclosure.
Figure 2 is a diagram illustrating a stop-flow mixing apparatus that may be
used in
accordance with certain embodiments of the present disclosure.
Figure 3 is a diagram illustrating an example of a subterranean formation in
which a
fracturing operation may be performed in accordance with certain embodiments
of the
present disclosure.
Figure 4 is a graph illustrating dynamic surface tension data of certain
solutions
according to embodiments of the present disclosure.
Figure 5 is a graph illustrating dynamic surface tension data of certain
solutions
according to embodiments of the present disclosure.
Figures 6A and 6B are graphs illustrating interfacial tension data of certain
solutions
according to embodiments of the present disclosure.
Figure 7 is a bar graph illustrating oil recovery data in tests of certain
solutions
according to embodiments of the present disclosure.
While embodiments of this disclosure have been depicted, such embodiments do
not
imply a limitation on the disclosure, and no such limitation should be
inferred. The subject
matter disclosed is capable of considerable modification, alteration, and
equivalents in form
and function, as will occur to those skilled in the pertinent art and having
the benefit of this
disclosure. The depicted and described embodiments of this disclosure are
examples only,
and not exhaustive of the scope of the disclosure.
5
CA 02951289 2016-12-09
Attorney Docket: 063718.6746
2014-IP-088776U1P1PCT
DESCRIPTION OF EMBODIMENTS
The present disclosure relates to systems and methods for treating
subterranean
formations. More particularly, the present disclosure relates to systems and
methods for
creating and/or using multi-functional surfactant complexes that may enhance
surfactant
treatments in subterranean formations.
The present disclosure provides methods and systems for creating and/or using
multi-
functional surfactant complexes (MSCs) for use in subterranean formations and
subterranean
wells penetrating such formations. The MSCs of the present disclosure
generally comprise a
complex of surfactant molecules and molecules of an oppositely-charged
polymeric additive,
such as a friction reducer, a clay stabilizer, a biocide. a corrosion
inhibitor, a scale inhibitor,
or any combination thereof associated with one another via a non-covalent
(e.g., ionic)
interaction. In certain embodiments, the multi-functional surfactant
complexes of the
present disclosure may be prepared by adding a surfactant and polymeric
additive carrying
opposite electrostatic charges to a stop-flow mixing apparatus and mixed at an
appropriate
speed to form one or more MSCs. The mixture is then transferred to a blender
at a well site
using a low-dose pumping apparatus. The blender then mixes the MSCs into a
base fluid
(and, optionally, additional additives) to prepare a treatment fluid that may
be introduced into
at least a portion of a subterranean formation. In certain embodiments, one or
more of the
aforementioned steps may be performed at a well site, for example,
substantially in or near
real-time with the treatment and/or operation in which the MSCs are used. The
treatment
fluids of the present disclosure thus may comprise an aqueous base fluid and
one or more
multi-functional surfactant complexes. In certain embodiments, the treatment
fluid is a
fracturing fluid. However, the teachings of the present disclosure may be used
in other
treatment or subterranean fluids, including but not limited to, acidizing
fluids and drilling
fluids.
Without limiting the disclosure to any particular theory or mechanism, the
electrostatic attraction between the oppositely charged surfactant and
polymeric additive may
drive the two molecules to form multi-functional surfactant complexes (MSCs).
It is believed
that the MSC is kinetically stable and that surfactant molecules may be
temporarily trapped
by the oppositely-charged polymeric additive. This in turn may minimize any
interactions
between the surfactant / polymeric additive and other components or additives
in a treatment
fluid (e.g., proppants) and/or the formation (e.g., charged rock surfaces in
the formation) as
the additives are pumped downhole. Thus, the surfactant and/or polymeric
additive may be
6
CA 02951289 2016-12-09
Attorney Docket: 063718.6746
2014-IP-088776UIPIPCT
pumped deeper into the reservoir, at which point the MSCs may be disassembled
or inverted
through a variety of mechanisms to release the surfactant molecules and the
polymeric
additive. For example, phase equilibrium of MSC may be associated with the
salinity of its
environment; therefore, change of salinity could lead to disassembly of the
aggregates.
Temperature gradients and/or pH changes may also break up the MSCs and release
the
surfactant molecules and polymeric additive.
Among the many potential advantages to the methods and compositions of the
present
disclosure, only some of which are alluded to herein, the methods,
compositions, and systems
of the present disclosure may enhance the performance of surfactants and/or
polymeric
treatment additives by providing them in a manner that allows for synergistic
interactions
(e.g., delayed release) between the molecules of those components. In some
embodiments,
the methods and systems of the present disclosure may increase the penetration
depth of
certain treatment fluids, enabling the treatment of deep and/or dead-end pores
in certain rock
or formations where conventional treatments may not have been able to deliver
surfactants
and/or other additives as effectively. The methods and systems of the present
disclosure also
may provide a means of preparing MSCs for use at a well site immediately or
soon after their
preparation, which may allow operators to use MSCs in well treatments without
the need to
transport them to the well site and before they degrade or become unstable. In
certain
embodiments, the MSCs and/or the surfactants therein may be used in emulsions
to enhance
and/or prolong their stability. In certain embodiments, the MSCs may alter
the bulk
viscoelastic properties of the fluid and/or may induce turbulent flow therein.
These
viscoelastic properties may be tailored, among other reasons, to engineer flow
that increases
the contact of a treatment fluid of the present disclosure with oil globules
in a subterranean
formation (thereby enhancing oil recovery) and/or divert treatment fluids into
deeper pores in
a subterranean formation. In certain embodiments, charged MSCs may create
electric fields
in pore spaces in a formation that may act as "micropumps" that enhance
diffusiooemotic
flow in those pores.
The surfactant in the MSCs may comprise any surfactant (or blend of multiple
surfactants) known in the art. In some embodiments the surfactant may be
anionic, while in
.. other embodiments it may be cationic, or in yet other embodiments,
amphoteric, zwitterionic,
or non-ionic, respectively. In some embodiments, the desired ionization, if
any, of the
surfactant may be determined based at least in part upon one or more
characteristics of the oil
and/or gas of a subterranean formation. For example, the charge of a
surfactant of some
7
CA 02951289 2016-12-09
Attorney Docket: 063718.6746
2014-IP-088776U1P1PCT
embodiments of the treatment fluid may allow the surfactant to induce pair
interactions (e.g.,
electrostatic interactions) with one or more molecules of oil and/or gas in
the subterranean
formation.
Thus, where the oil and/or gas of a subterranean formation contains
predominantly
alkaline compounds, which are typically positively charged in nature, the
surfactant of some
embodiments of the present disclosure may be anionic to allow the surfactant
to induce
electrostatic pair interactions with positively-charged oil and/or gas
molecules. In some
instances, the oil and/or gas of a subterranean formation may contain a
mixture of alkaline
and acidic compounds. In such a circumstance, it may be advantageous to use an
amphoteric
and/or zwitterionic surfactant according to some embodiments of the present
disclosure.
Furthermore, the amphoteric and/or zwitterionic surfactants of some
embodiments may
exhibit different charge and/or reactivity at different ranges of pII. For
instance, some
surfactants that are amphoteric and/or zwitterionic at pH less than about 2
may become
anionic, cationic, or non-ionic at pH greater than about 2. Because the
downhole pH may
change during acidization (for example, pH may rise from levels of from about
0-1 to about
4, as the acid is spent), the characteristics of surfactants of some
embodiments may change
during the process of an acidization treatment. Other characteristics of oil
and/or gas within
the formation that might affect the determination of desired surfactant charge
include, but are
not limited to: weight percentages of saturates, aromatics, resins and
asphaltenes.
Examples of anionic surfactants that may be suitable in certain embodiments
may
include, but are not limited to: sodium, potassium, and ammonium salts of long
chain alkyl
sulfonates and alkyl aryl sulfonates (such as sodium dodecylbenzene
sulfonate); dialkyl
sodium sulfosuccinates (such as sodium dodecylbenzene sulfonate or sodium bis-
(2-
ethylthioxyl)-sulfosuccinate); alkyl sulfates (such as sodium lauryl sulfate);
alkyl sulfonates
(such as methyl sulfonate, heptyl sulfonate, decylbenzene sulfonate,
dodecylbenzene
sulfonate); and alkoxylated sulfates. Certain embodiments of the present
disclosure may
include a combination of anionic surfactants. Examples of non-ionic
surfactants that may be
suitable in certain embodiments may include, but are not limited to:
ethoxylated alcohols and
polyglucosides. In some embodiments, non-ionic surfactants may include
ethoxylated long-
chain alcohols (e.g., ethoxylated dodecanol). Ethoxylation may take place at
any point along
the alcohol. Examples of cationic surfactants that may be suitable in certain
embodiments
may include, but are not limited to: alkyl ammonium bromides. In some
embodiments, the
alkyl chain of the alkyl ammonium bromide may be anywhere from Ito 50 carbons
long, and
8
CA 02951289 2016-12-09
Attorney Docket: 063718.6746
2014-1P-088776U IP I PCT
be branched or un-branched. Thus, an example embodiment may include an alkyl
ammonium bromide that comprises a 16-carbon chain alkyl component (e.g., cetyl
trimethy 1
ammonium bromide). Examples of amphoteric and/or zwitterionic surfactants that
may be
suitable in certain embodiments may include, but are not limited to,
hydroxysultaines (e.g.,
.. cocoamidopropyl hydroxysultaine, lauramidopropyl hydroxysultaine, lauryl
hydroxysultaine,
etc.).
The polymeric additive in the MSCs of the present disclosure may comprise any
any
treatment additive (or blend of multiple additives) known in the art that
carries a charge
opposite that of the surfactant and is capable of performing a particular
treatment or function
in a well bore or subterranean formation. Examples of additives that may be
suitable in
certain embodiments of the present disclosure include, but are not limited to,
friction
reducers, clay stabilizers, biocides, corrosion inhibitors, scale inhibitors,
and any combination
thereof. Examples of polymeric clay stabilizers that may be used to form MSCs
of the
present disclosure include, but are not limited to poly
diallyldimethylammonium chloride
(DADMAC), polyacrylamide-co-diallydimetylammonium chloride (AMD1 and AMD2),
polyacrylic acid-co-di al lydi methylammonium chloride (AAD),
and
dodecyltrimethylammonium bromide (DDAB). In certain embodiments, multiple
different
polymeric additives may be used to form MSCs with a single surfactant and/or
with multiple
different surfactants. In certain
embodiments, MSCs comprising different polymeric
additives may be formed separately and combined in a single treatment fluid.
In certain embodiments, the polymeric additives and/or surfactants may be
mixed in
any amount and/or concentration that causes them to form one or more MSCs. In
certain
embodiments, the relative concentrations of polymeric additive and surfactant
may be varied,
among other reasons, to control the size and/or number of the MSCs formed, to
make the
MSCs more stable, to increase the reaction rate, and other factors. For
example, in certain
embodiments, the number of MSCs may be increased by increasing the
concentration of the
polymeric additive relative to the concentration of the surfactant. A person
of skill in the art
with the benefit of this disclosure will recognize how to vary the amounts
and/or
concentrations of the polymeric additives and/or surfactants to produce MSCs
having the
.. desired properties.
In certain embodiments, the polymeric additives and/or surfactants may be
provided
in solutions prior to mixing, for example, using the stop-flow mixing
apparatus to form the
MSCs. The treatment fluids of some embodiments may be aqueous or organic. In
certain
9
CA 02951289 2016-12-09
Attorney Docket: 063718.6746
2014-IP-088776U1P1PCT
embodiments, water may be used as a solvent for hydrophilic polymeric
additives. In other
embodiments, organic solvents may be used as a solvent for hydrophobic
polymeric
additives. Examples of organic solvents that may be suitable for certain
embodiments
include, but are not limited to, methanol, ethanol, ethylene glycol, xylene,
toluene, aromatics,
butyl glycol, and any combination thereof. In certain embodiments, the
solutions comprising
the polymeric additive or the surfactant, or the solutions or treatment fluids
comprising the
MSCs, may further comprise one or more salts, among other reasons, to
facilitate the
formation and/or maintenance of the MSCs. In these embodiments, any salt known
in the art
(e.g., NaCl) may be used.
The methods and systems of the present disclosure may be used to form
compositions
(e.g., treatment fluids) that may be used to treat a portion of a subterranean
formation. The
treatment fluids of the present disclosure generally comprise an aqueous base
fluid and one or
more MSCs. The aqueous base fluid used in some embodiments of the treatment
fluids of the
present disclosure may comprise fresh water, saltwater (e.g., water containing
one or more
salts dissolved therein), brine (e.g., saturated saltwater), seawater, or any
combination
thereof. Generally, the water may be from any source, provided that it does
not contain
components that might adversely affect the stability of the treatment fluids
of the present
disclosure. One of ordinary skill in the art, with the benefit of this
disclosure, will recognize
what components might adversely affect the stability and/or performance of the
treatment
fluids of the present disclosure.
In forming a treatment fluid comprising MSCs of the present disclosure, the
MSCs
may be included in an amount sufficient to release a sufficient amount of
surfactant and
polymeric additive to perform the desired treatment in the subterranean
formation (e.g., to
form one or more relatively short-lived oil-in-acid or oil-in-water emulsions
within a
subterranean formation). For example, in some embodiments, sufficient MSCs may
be
included in the treatment fluid to release an amount of surfactant of from
about 0.1 to 50
gallons of surfactant per thousand gallons of acid, water, and/or other
aqueous base fluid
("gpt"), or put another way, approximately 100 to 50,000 ppm. In other example
embodiments, sufficient MSCs may be included in the treatment fluid to release
an amount of
surfactant of from about 2 to 40 gpt (approximately 2,000 ppm to 40,000 ppm),
or in other
embodiments, from about 3 to 25 gpt (approximately 3,000 ppm to about 25.000
ppm). In
some embodiments, sufficient MSCs may be included in the treatment fluid to
release an
CA 02951289 2016-12-09
Attorney Docket: 063718.6746
2014-IP-088776U1P1PCT
amount of surfactant of from about 4 gpt to about 18 gpt (approximately 4,000
ppm to 18,000
PPm).
The treatment fluids of the present disclosure may optionally include other
components such as acids, salts, solvents, particulates, or other compounds as
long as these
components do not interfere with the surfactant or the ability of the
polymeric additive to
delay release of the surfactant. A person of skill in the art with the benefit
of this disclosure
would be able to select the appropriate other components depending on the
desired treatment
fluid. For example, the person of skill in the art might include an acid if it
is desired to
produce an acidizing treatment fluid. A person of skill in the art might also
include
particulates if it is desired to produce a fracturing fluid with proppant
particles.
The treatment fluids of some embodiments may include solvents, such as
methanol,
ethanol, ethylene glycol, xylene, toluene, aromatics, or butyl glycol. Thus,
for example, a
treatment fluid of some embodiments may include ethylene glycol mono-butyl
ether. The
treatment fluids of some embodiments may further include salts, among other
reasons, to
stabilize the MSCs.
The treatment fluids of some embodiments may further comprise additional
surfactants (e.g., in addition to the surfactants provided in the MSCs), among
other reasons,
to lower the surface tension or capillary pressure of the treatment fluid and
allow the fluid to
penetrate deeper into a formation or fracture therein. In certain embodiments,
the additional
surfactant may be included in the treatment fluid in a concentration greater
than the critical
micelle concentration (CMC) of that surfactant in the fluid.
The treatment fluids of some embodiments may include particulates (such as
proppant
particulates or gravel particulates) suitable for use in subterranean
applications. Particulates
suitable for use in the present disclosure may comprise any material suitable
for use in
subterranean operations. Proppant particulates may be used in conjunction with
hydraulic
fracturing to prevent the fractures from fully closing upon the release of
hydraulic pressure,
forming conductive channels through which fluids may flow to the wellbore.
Suitable
particulate materials include, but are not limited to, sand, bauxite, ceramic
materials, glass
materials, polymer materials, Teflon materials, nut shell pieces, cured
resinous particulates
comprising nut shell pieces, seed shell pieces, cured resinous particulates
comprising seed
shell pieces, fruit pit pieces, cured resinous particulates comprising fruit
pit pieces, wood,
composite particulates, and any combination thereof. Suitable composite
particulates may
comprise a binder and a filler material wherein suitable filler materials
include silica,
11
CA 02951289 2016-12-09
Attorney Docket: 063718.6746
2014-1P-088776U1P 1 PCT
alumina, fumed carbon, carbon black, graphite, mica, titanium dioxide, meta-
silicate, calcium
silicate, kaolin, talc, zirconia, boron, fly ash, hollow glass microspheres,
solid glass, and any
combination thereof. The particulate size generally may range from about 2
mesh to about
400 mesh on the U.S. Sieve Series; however, in certain circumstances, other
sizes may be
desired and will be entirely suitable for practice of the present disclosures.
In particular
embodiments, preferred particulates size distribution ranges are one or more
of 6/12, 8/16,
12/20, 16/30, 20/40, 30/50, 40/60, 40/70, or 50/70 mesh. It should be
understood that the
term "particulate," as used in this disclosure, includes all known shapes of
materials,
including substantially spherical materials, fibrous materials, polygonal
materials (such as
cubic materials), and mixtures thereof. Moreover, fibrous materials, that may
or may not be
used to bear the pressure of a closed fracture, are often included in
fracturing and sand
control treatments. In certain embodiments, the particulates included in the
treatment fluids
of some embodiments of the present disclosure may be coated with any suitable
resin or
tackifying agent known to those of ordinary skill in the art.
The treatment fluids of some embodiments may additionally or instead include
one or
more of a variety of well-known additives (in addition to the polymeric
additives included in
the MSCs), such as gel stabilizers, fluid loss control additives, scale
inhibitors, organic
corrosion inhibitors, catalysts, clay stabilizers, biocides, bactericides,
friction reducers, gases,
foaming agents, iron control agents, solubilizers, pH adjusting agents (e.g.,
buffers), and the
like. Those of ordinary skill in the art, with the benefit of this disclosure,
will be able to
determine the appropriate additives for a particular application.
The MSCs and the treatment fluids of the present disclosure may be prepared at
a well
site or at an offsite location. In certain embodiments, a base fluid may be
mixed with a
viscosifying agent first, among other reasons, in order to allow the
viscosifying agent to
hydrate. Then, proppants, MSCs, and/or other additives may be mixed into the
viscosified
fluid. Once prepared, a treatment fluid of the present disclosure may be
placed in a tank, bin,
or other container for storage and/or transport to the site where it is to be
used. In other
embodiments, a treatment fluid of the present disclosure may be prepared on-
site, for
example, using continuous mixing or "on-the-fly" methods, as described below.
The present disclosure in some embodiments provides methods for using the
treatment fluids to carry out a variety of subterranean treatments, including
but not limited to,
hydraulic fracturing treatments, enhanced oil recovery treatments (e.g., water
flooding
treatments, polymer flooding treatments, etc.), acidizing treatments, and
drilling operations.
12
CA 02951289 2016-12-09
Attorney Docket: 063718.6746
2014-IP-088776U1P1PCT
In some embodiments, the treatment fluids of the present disclosure may be
used in treating a
portion of a subterranean formation, for example, in acidizing treatments such
as matrix
acidizing or fracture acidizing. In certain embodiments, a treatment fluid may
be introduced
into a subterranean formation. In some embodiments, the treatment fluid may be
introduced
into a well bore that penetrates a subterranean formation. In some
embodiments, the
treatment fluid may be introduced at a pressure sufficient to create or
enhance one or more
fractures within the subterranean formation (e.g., hydraulic fracturing).
In some embodiments, the treatment fluid further comprising an acid may be
introduced at a pressure sufficient to cause at least a portion of the
treatment fluid to penetrate
.. at least a portion of the subterranean formation, and the treatment fluid
may be allowed to
interact with the subterranean formation so as to create one or more voids in
the subterranean
formation (for example, in acidizing treatments). Introduction of the
treatment fluid may in
some of these embodiments be carried out at or above a pressure sufficient to
create or
enhance one or more fractures within the subterranean formation (e.g.,
fracture acidizing). In
other embodiments, introduction of the treatment fluid may be carried out at a
pressure below
that which would create or enhance one or more fractures within the
subterranean formation
(e.g., matrix acidizing).
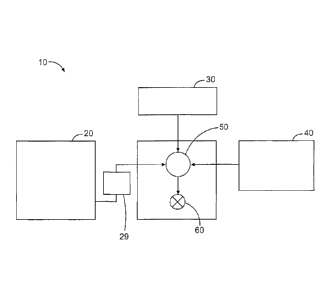
Referring now to Figure 1, an example of a well bore treatment system 10 is
illustrated according to certain embodiments of the present disclosure. System
10 includes a
stop-flow mixing apparatus 20, a low-dose pumping apparatus 29, a base fluid
source 30, a
proppant source 40, and a pump and blender system 50, and is disposed at the
surface at a
well site where a well 60 is located. System 10 may be used to prepare MSCs
and/or
treatment fluids according to the present disclosure and to introduce those
fluids into well 60.
The various apparatus in system 10 may be provided at the well site as
separate components
or equipment, or may be integrated in a single unitary system such as a
fracturing blender
vehicle. The stop-flow mixing apparatus 20 according to some embodiments is
illustrated in
further detail in Figure 2. In certain embodiments, stop-flow mixing apparatus
20 may
include similar components to that of laboratory stop-flow mixing apparatuses
that are
constructed at appropriate scales and with appropriate materials for a well
site application.
.. Referring now to Figure 2, stop-flow mixing apparatus 20 includes at least
two syringes 22
and 23 that inject fluids (e.g., solutions comprising surfactant or polymeric
additive) into one
or more inlets 24a and 24b in mixer 24. Mixer 24 may comprise any mixer,
homogenizer, or
dispersion device that provides sufficient shear to mix relatively small
volumes of fluids,
13
CA 02951289 2016-12-09
Attorney Docket: 063718.6746
2014-1P-088776U1P1PCT
including but not limited to high energy mixing devices and ultrasonic
dispersion devices.
The mixer includes an outlet 24c through which fluid may flow to an
observation cell 26
(through its inlet 26a) and stopping syringe 25. In certain embodiments, as
the solutions
comprising the surfactant and polymeric additive are pushed from syringes 22
and 23,
respectively, and through mixer 24, the molecules of the surfactant and
polymeric additive
associate to form MSCs. Fluid comprising the MSCs then flows into observation
cell 26 and
stopping syringe 25 until stopping syringe 25 reaches a predetermined volume
(e.g., when the
reaction reaches a continuous flow rate). At that volume, the plunger on
stopping syringe
will stop the flow of liquids through the apparatus 20. Apparatus 20 also
includes a
measurement device 27 that is configured to monitor the contents of the
observation cell 26
using one or more known analytical methods (e.g., UV-visible spectroscopy,
FTIR
spectroscopy, etc.) and equipment. This device may be used, among other
purposes to
confirm the formation of MSCs for use in the treatment fluid. The fluid in
observation cell
28 then flows out of the stop-flow mixing apparatus 20 through outlet 26b. In
certain
embodiments, the stop-flow mixing apparatus illustrated in Figure 2 (or
another suitable
device for forming MSCs of the present disclosure) may be located and operated
at a location
other than a well site, and the MSCs formed using that apparatus may be
transported to a well
site for use.
Referring back to Figure 1, fluids comprising MSCs flow out of stop-flow
mixing
apparatus 20, and are then metered into pump and blender system 50 using a low-
dose
pumping apparatus 29 coupled between stop-flow mixing apparatus 20 and an
inlet of pump
and blender system 50. The low-dose pumping apparatus 29 may comprise any
liquid dosing
or metering pump known in the art that is capable of pumping liquids
therethrough in very
low concentrations (e.g., less than about 1 gallon per thousand gallons of
fluid (gpt), or in
.. some cases, less than about 0.1 gpt). Examples of such devices are pumps
equipped with the
Micro Motion meters and measurement devices available from Emerson Process
Management.
The proppant source 40 can include a proppant for combination with the
fracturing
fluid. The pump and blender system 50 receives the base fluid (and any
additives pre-mixed
.. into that fluid) from fluid source 30 and combines it with other
components, including
proppant from the proppant source 40. System 10 optionally may include other
tanks,
hoppers, or pumps (not shown) that are equipped to dispense additional fluids
and/or
additives 70 into pump and blender system 50. The resulting mixture may be
pumped down
14
CA 02951289 2016-12-09
Attorney Docket: 063718.6746
2014-1P-088776U1P 1PCT
the well 60, for example, under a pressure sufficient to create or enhance one
or more
fractures in a subterranean zone, for example, to stimulate production of
fluids from the zone.
Notably, in certain instances, stop-flow mixing apparatus 20, base fluid
source 30, and/or
proppant source 40 may be equipped with one or more metering devices (not
shown) to
control the flow of fluids, proppants, and/or other compositions to the pump
and blender
system 50. Such metering devices may permit the pump and blender system 50 can
source
from one, some or all of the different sources at a given time, and may
facilitate the
preparation of treatment fluids in accordance with the present disclosure
using continuous
mixing or "on-the-fly" methods.
Figure 3 shows the well 60 and treatment system 10 during a fracturing
operation in a
portion of a subterranean formation of interest 102 surrounding a well bore
104. The well
bore 104 extends from the surface 106, and the fracturing fluid 108 is applied
to a portion of
the subterranean formation 102 surrounding the horizontal portion of the well
bore. Although
shown as vertical deviating to horizontal, the well bore 104 may include
horizontal, vertical,
slant, curved, and other types of well bore geometries and orientations, and
the fracturing
treatment may be applied to a subterranean zone surrounding any portion of the
well bore.
The well bore 104 can include a casing 110 that is cemented or otherwise
secured to the well
bore wall. The well bore 104 can be uncased or include uncased sections.
Perforations can
be formed in the casing 110 to allow fracturing fluids and/or other materials
to flow into the
subterranean formation 102. In cased wells, perforations can be formed using
shape charges,
a perforating gun, hydro-jetting and/or other tools.
The well is shown with a work string 112 depending from the surface 106 into
the
well bore 104. The pump and blender system 50 is coupled a work string 112 to
pump the
fracturing fluid 108 into the well bore 104. The working string 112 may
include coiled
tubing, jointed pipe, and/or other structures that allow fluid to flow into
the well bore 104.
The working string 112 can include flow control devices, bypass valves, ports,
and or other
tools or well devices that control a flow of fluid from the interior of the
working string 112
into the subterranean zone 102. For example, the working string 112 may
include ports
adjacent the well bore wall to communicate the fracturing fluid 108 directly
into the
subterranean formation 102, and/or the working string 112 may include ports
that are spaced
apart from the well bore wall to communicate the fracturing fluid 108 into an
annulus in the
well bore between the working string 112 and the well bore wall.
CA 02951289 2016-12-09
Attorney Docket: 063718.6746
2014-IP-088776UIPIPCT
The working string 112 and/or the well bore 104 may include one or more sets
of
packers 114 that seal the annulus between the working string 112 and well bore
104 to define
an interval of the well bore 104 into which the fracturing fluid 108 will be
pumped. Figure 3
shows two packers 114, one defining an uphole boundary of the interval and one
defining the
downhole end of the interval. When the fracturing fluid 108 is introduced into
well bore 104
(e.g., in Figure 3, the area of the well bore 104 between packers 114) at a
sufficient hydraulic
pressure, one or more fractures 116 may be created in the subterranean zone
102. The
proppant particulates in the fracturing fluid 108 may enter the fractures 116
where they may
remain after the fracturing fluid flows out of the well bore. These proppant
particulates may
"prop" fractures 116 such that fluids may flow more freely through the
fractures 116.
While not specifically illustrated herein, the disclosed methods and
compositions may
also directly or indirectly affect any transport or delivery equipment used to
convey the
compositions to the fracturing system 10 such as, for example, any transport
vessels,
conduits, pipelines, trucks, tubulars, and/or pipes used to fluidically move
the compositions
from one location to another, any pumps, compressors, or motors used to drive
the
compositions into motion, any valves or related joints used to regulate the
pressure or flow
rate of the compositions, and any sensors (i.e., pressure and temperature),
gauges, and/or
combinations thereof, and the like.
To facilitate a better understanding of the present disclosure, the following
examples
of certain aspects of preferred embodiments are given. The following examples
are not the
only examples that could be given according to the present disclosure and are
not intended to
limit the scope of the disclosure or claims.
EXAMPLES
EXAMPLE lA
Dynamic surface tension tests were performed to determine how the MSCs of the
present disclosure impact the surface tension of the associated surfactants at
the air-water
interface. In these tests, tensiometer was used to determine the requisite
pressure of a gas
(air) pumped into a capillary needle projecting into a solution of the
surfactant / MSC's of the
present disclosure to create a bubble in the solution. Using the maximum
bubble pressure
.. method, the pressure needed to form a bubble is measured and the surface
tension of the
sample is calculated from the pressure difference between inside and outside
the bubble and
the radius of the bubble. Aqueous solutions of two different anionic
surfactants were tested
in this manner, both in samples with the surfactants alone (samples S 1 and
S2) and samples
16
CA 02951289 2016-12-09
Attorney Docket: 063718.6746
2014-IP-088776U1P1PCT
with the surfactants associated with multi-functional surfactant complexes
(samples MSC1
and MSC2). The surfactant in samples S1 and MSC1 comprised a DDBSA anionic
surfactant, and the surfactant in samples S2 and MSC2 comprised a blend of
DDBSA and
ethoxylated surfactants. In samples MSC1 and MSC2, a polyethylenemine cationic
polymer
was used to form the multi-functional surfactant complexes. Figure 4 is a
dynamic surface
tension plot illustrating this data over time. As shown in Figure 4, the
dynamic surface
tension of the solutions containing MSCs maintained a high surface tension for
a longer
period of time and initially decreased less rapidly than the corresponding
solutions of those
surfactants alone. The rate of decrease in the surface tension corresponds to
the diffusion of
the surfactant to the air-water surface.
EXAMPLE 1B
Dynamic surface tension tests similar to those described in Example 1 were
performed on a solution of a DDBSA anionic surfactant (sample S3) as well as a
series of
solutions of MSCs of that anionic surfactant with different concentrations of
a dodecyl
.. trimethyl ammonium chloride (C-12 TMAC) cationic polymeric clay stabilizer
additive
(samples MSC3, MSC4, MSC5, and MSC6). Figure 5 is a dynamic surface tension
plot
illustrating this data over time. As shown in Figure 5, similar to the
solutions tested in
Example IA, the dynamic surface tension of the solutions containing MSCs
maintained a
high surface tension for a longer period of time and initially decreased less
rapidly than the
corresponding solution of the surfactant alone, indicating a lower diffusion
rate of the
surfactant. As shown in Figure 5, solutions comprising the MSCs exhibit a
further reduced
surface tension of the solution (i.e., below that of the solution with
surfactant alone) after all
of the surfactant was released, which is a result of the oppositely-charged
polymeric additive
in those solutions released from the MSCs.
The data from Examples IA and 1B demonstrates that, in certain embodiments
where
these MSCs of the present disclosure are included in an aqueous fluid that is
pumped into a
well, the surfactant in the MSC may remain in solution for a longer period of
time than a
solution of the corresponding surfactant alone, instead of adsorbing onto
proppants, well bore
equipment, or other surfaces in the subterranean formation or well bore.
Moreover, it is
noted that, for the surfactant in samples MSC1 and SI, the surface tension of
the solution
with MSCs eventually reaches the same value as the solution without MSCs,
indicating that
the entire surface is occupied by surfactant released from the MSCs.
17
CA 02951289 2016-12-09
Attorney Docket: 063718.6746
2014-IP-088776U 1 P 1PCT
EXAMPLE 2
Dynamic interfacial tension tests were also performed to determine how the
MSCs of
the present disclosure impact the surface tension of the associated
surfactants at the oil-water
interface. In these tests, a fresh oil droplet was created in a U-shaped
needle and an aqueous
continuous phase. The dynamic interfacial tension was obtained from the
pendant shape of
the drop using the Young-Laplace equation. Aqueous solutions of two different
anionic
surfactants were tested in this manner, both in samples with the surfactants
alone (samples SI
and S2) and samples with the surfactants associated with multi-functional
surfactant
complexes (samples MSC1 and MSC2). Figures 6A and 6B are interfacial tension
plots
illustrating this data over time for SI / MSC1 and S2 I MSC2, respectively. As
shown in
Figures 6A and 6B, the interfacial tension decay rate for the solutions of
surfactants alone
was higher than that of the solutions containing MSCs, and the interfacial
tension of the
solutions containing surfactants alone began to decay sooner than in the
solutions containing
MSCs. This data demonstrates that, in certain embodiments where these MSCs of
the present
disclosure are included in an aqueous fluid that is pumped into an oil
reservoir, once pumping
is stopped, the surfactant molecules will still reach out to oil molecules in
contact with the
aqueous fluid and the surfactant inventory in the aqueous fluid will not
deplete with time.
EXAMPLE 3
Oil recovery tests were also performed using an aqueous solution of an anionic
surfactant (comprising a blend of DDBSA and ethoxylated surfactants) and an
aqueous
solution of a corresponding MSC of that anionic surfactant with a poly-DADMAC
cationic
polymer. Concentrations of 1 gallon per thousand gallons (gpt) and 2 gpt of
each type of
solution were tested (the concentration referring to the surfactant or the
MSC). Each solution
was pumped into an high-performance liquid chromatography (HPLC) column packed
with
100 mesh core powders that had been aged with crude oil at reservoir
temperature for two
days. The solutions were injected at a fixed flow rate of 3 ml/hr. The second
pass of the
effluent solution was analyzed using an InfraCal analyzer (available from
Spectro Scientific
of Chelmsford, MA) to determine the oil recovery. The percentage of oil
recovered in each of
these tests was recorded and is shown in Figure 7. As shown, the solutions of
MSCs
generally achieved increased oil recovery as compared to the corresponding
solutions of the
surfactant alone.
Therefore, the present disclosure is well adapted to attain the ends and
advantages
mentioned as well as those that are inherent therein. The particular
embodiments disclosed
18
above are illustrative only, as the present disclosure may be modified and
practiced in
different manners apparent to those skilled in the art having the benefit of
the teachings
herein. While numerous changes may be made by those skilled in the art, such
changes are
encompassed within the subject matter defined herein. Furthermore, no
limitations are
intended to the details of construction or design herein shown. It is
therefore evident that the
particular illustrative embodiments disclosed above may be altered or modified
and all such
variations are considered within the scope of the present disclosure. In
particular, every
range of values (e.g., "from about a to about b," or, equivalently, "from
approximately a to
b," or, equivalently, "from approximately a-b") disclosed herein is to be
understood as
referring to the power set (the set of all subsets) of the respective range of
values. The terms
herein have their plain, ordinary meaning unless otherwise explicitly and
clearly defined by
the patentee.
19
CA 2951289 2018-04-03