Note: Descriptions are shown in the official language in which they were submitted.
Cl 02952435 2016-12-14
WO 2015/195809 PCT/US2015/036243
1
MOLECULAR SIEVE CATALYST COMPOSITIONS, CATALYST COMPOSITES,
SYSTEMS, AND METHODS
TECHNICAL FIELD
[0001] The present
invention relates generally to the field of selective catalytic reduction
materials, selective catalytic reduction composites, and to methods of
selectively reducing
nitrogen oxides. More particularly, embodiments of the invention relate to a
SCR catalyst
material comprising a spherical particle including an agglomeration of
crystals of a molecular
sieve.
BACKGROUND
[0002] Over time,
the harmful components of nitrogen oxides (NO) have led to
atmospheric pollution. NO3 is contained in exhaust gases such as from internal
combustion
engines (e.g., automobiles and trucks), from combustion installations (e.g.,
power stations
heated by natural gas, oil, or coal), and from nitric acid production plants.
[0003] Various
methods have been used in the treatment of NON-containing gas mixtures.
One type of treatment involves catalytic reduction of nitrogen oxides. There
are two
processes: (1) a nonselective reduction process wherein carbon monoxide,
hydrogen, or a
lower hydrocarbon is used as a reducing agent, and (2) a selective reduction
process wherein
ammonia or ammonia precursor is used as a reducing agent. In the selective
reduction process,
a high degree of
removal with nitrogen oxide can be obtained with a small amount of reducing
agent.
[0004] The
selective reduction process is referred to as a SCR process (Selective
Catalytic
Reduction). The SCR process uses catalytic reduction of nitrogen oxides with
ammonia in the
presence of atmospheric oxygen with the formation predominantly of nitrogen
and steam:
4N0+4NH3+02 ¨> 4N2+6H20 (standard SCR reaction)
2NO2+4NH3 3N2+6H20 (slow SCR reaction)
NO+NO2+NH3 2N2+3H20 (fast SCR reaction)
[0005] Catalysts
employed in the SCR process ideally should be able to retain good
catalytic activity over the wide range of temperature conditions of use, for
example, 200 C to
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
2
600 C or higher, under hydrothermal conditions. Hydrothermal conditions are
often
encountered in practice, such as during the regeneration of a soot filter, a
component of the
exhaust gas treatment system used for the removal of particles.
100061 Molecular sieves such as zeolites have been used in the selective
catalytic reduction
(SCR) of nitrogen oxides with a reductant such as ammonia, urea, or a
hydrocarbon in the
presence of oxygen. Zeolites are crystalline materials having rather uniform
pore sizes which,
depending upon the type of zeolite and the type and amount of cations included
in the zeolite
lattice, range from about 3 to 10 Angstroms in diameter. Zeolites having 8-
ring pore openings
and double-six ring secondary building units, particularly those having cage-
like structures,
have recently found interest in use as SCR catalysts. A specific type of
zeolite having these
properties is chabazite (CHA), which is a small pore zeolite with 8 member-
ring pore openings
(-3.8 Angstroms) accessible through its 3-dimensional porosity. A cage like
structure results
from the connection of double six-ring building units by 4 rings.
100071 Metal-promoted zeolite catalysts including, among others, iron-
promoted and
copper-promoted zeolite catalysts, for the selective catalytic reduction of
nitrogen oxides with
ammonia are known. Iron-promoted zeolite beta has been an effective commercial
catalyst for
the selective reduction of nitrogen oxides with ammonia. Unfortunately, it has
been found that
under harsh hydrothermal conditions, for example exhibited during the
regeneration of a soot
filter with temperatures locally exceeding 700 C, the activity of many metal-
promoted zeolites
begins to decline. This decline is often attributed to dealumination of the
zeolite and the
consequent loss of metal-containing active centers within the zeolite.
100081 Metal-promoted, particularly copper promoted aluminosilicate
zeolites having the
CHA structure type have recently solicited a high degree of interest as
catalysts for the SCR of
oxides of nitrogen in lean burning engines using nitrogenous reductants. This
is because of the
wide temperature window coupled with the excellent hydrothermal durability of
these
materials, as described in United States Patent Number 7,601,662. Prior to the
discovery of
metal promoted zeolites described in United States Patent Number 7,601,662,
while the
literature had indicated that a large number of metal-promoted zeolites had
been proposed in
the patent and scientific literature for use as SCR catalysts, each of the
proposed materials
suffered from one or both of the following defects: (1) poor conversion of
oxides of nitrogen at
low temperatures, for example 350 C and lower; and (2) poor hydrothermal
stability marked
by a significant decline in catalytic activity in the conversion of oxides of
nitrogen by SCR.
3
Thus, the invention described in United State Patent Number 7,601,662
addressed a compelling,
unsolved need to provide a material that would provide conversion of oxides of
nitrogen at low
temperatures and retention of SCR catalytic activity after hydrothermal aging
at temperatures in excess
of 650 C.
100091 Even though the current catalysts exhibit excellent properties, there
is a continuing desire to
reduce N20 make during the SCR reaction. Accordingly, an SCR catalyst is
needed with improved
NOx conversion efficiency and lower N20 make relative to the current
technologies.
SUMMARY
100101 A first aspect of the invention is directed to a selective catalytic
reduction (SCR) catalyst
material. In a first embodiment, a selective catalytic reduction catalyst
material comprises a spherical
particle including an agglomeration of crystals of a molecular sieve, wherein
the spherical particle has
a median particle size in the range of about 0.5 to about 5 microns. For
instance, the molecular sieve
comprises a zeolitic framework material of silicon and aluminum atoms, wherein
a fraction of the
silicon atoms are isomorphously substituted with a tetravalent metal.
100111 In a second embodiment, the SCR catalyst material of the first
embodiment is modified,
wherein the molecular sieve comprises a d6r unit.
[0012] In a third embodiment, the SCR catalyst material of the first and
second
embodiments is modified, wherein the molecular sieve has a structure type
selected from the
group consisting of AEI, AFT, AFX, CHA, EAB, EMT, ERI, FAU, GME, JSR, KFI,
LEV, LTL,
LTN, MOZ, MSO, MWW, OFF, SAS, SAT, SAV, SBS, SBT, SFW, SSF, SZR, TSC, WEN, and
combinations thereof.
100131 In a fourth embodiment, the SCR catalyst material of the first
through third embodiments
is modified, wherein the molecular sieve has a structure type selected from
AEI, AFT, AFX, CHA,
EAB, ERE, KFI, LEV, SAS, SAT, and SAY.
100141 In a fifth embodiment, the SCR catalyst material of the first
through fourth
embodiments is modified, wherein the molecular sieve has a structure type
selected from AEI, CHA,
and AFX.
100151 In a sixth embodiment, the SCR catalyst material of the first
through fifth embodiments,
wherein the molecular sieve has the CHA structure type.
100161 In a seventh embodiment, the SCR catalyst material of the first
through sixth
embodiments is modified, wherein the molecular sieve having the CHA structure
type is
Date Recue/Date Received 2021-10-20
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
4
selected from an aluminosilicate zeolite, a borosilicate, a gallosilicate, a
SAPO, an A1P0, a
MeAPSO, and a MeAPO.
[0017] In an eighth embodiment, the SCR catalyst material of the first
through seventh
embodiments is modified, wherein the molecular sieve having the CHA structure
type is
selected from the group consisting of SSZ-13, SSZ-62, natural chabazite,
zeolite K-G, Linde
D, Linde R, LZ-218, LZ-235, LZ-236, ZK-14, SAPO-34, SAPO-44, SAPO-47, and ZYT-
6.
[0018] In a ninth embodiment, the SCR catalyst material of the first
through eighth
embodiments is modified, wherein the molecular sieve is selected from SSZ-13
and SSZ-62.
[0019] In a tenth embodiment, the SCR catalyst material of the first
through ninth
embodiments is modified, wherein the molecular sieve is promoted with a metal
selected from
Cu, Fe, Co, Ni, La, Ce, Mn, V. Ag, and combinations thereof.
[0020] In an eleventh embodiment, the SCR catalyst material of the first
through tenth
embodiments is modified, wherein the molecular sieve is promoted with a metal
selected from
Cu, Fe, and combinations thereof.
[0021] In a twelfth embodiment, the SCR catalyst material of the first
through eleventh
embodiments is modified, wherein the SCR catalyst material is effective to
catalyze the
selective catalytic reduction of nitrogen oxides in the presence of a
reductant at temperatures
between 200 C and 600 C.
[0022] In a thirteenth embodiment, the SCR catalyst material of the sixth
embodiment is
modified, wherein the molecular sieve having the CHA structure type has a
silica to alumina
ratio in the range of 10 to 100.
[0023] In a fourteenth embodiment, the SCR catalyst material of the tenth
and eleventh
embodiments is modified, wherein the metal is present in an amount in the
range of about 0.1
to about 10 wt. % on an oxide basis.
[0024] In a fifteenth embodiment, the SCR catalyst material of the first
through fourteenth
embodiments is modified, wherein the spherical particle has a median particle
size in the range
of about 1.2 to about 3.5 microns.
[0025] In a sixteenth embodiment, the SCR catalyst material of the first
through fifteenth
embodiments is modified, wherein the crystals have a crystal size in the range
of about 1 to
about 250 nm.
5
[0026] In a seventeenth embodiment, the SCR catalyst material of the first
through
sixteenth embodiments is modified, wherein the crystals have a crystal size in
the range of
about 100 to about 250 nm.
[0027] In an eighteenth embodiment, the SCR catalyst material of the first
through seventeenth embodiments is modified, wherein the SCR catalyst material
is in the
form of a washcoat.
[0028] In a nineteenth embodiment, the SCR catalyst material of the
eighteenth
embodiment is modified, wherein the washcoat is a layer deposited on a
substrate.
[0029] In a twentieth embodiment, the SCR catalyst material of nineteenth
embodiment
is modified, wherein the substrate comprises a filter.
[0030] In a twenty-first embodiment, the SCR catalyst material of the
twentieth
embodiment is modified, wherein the filter is a wall flow filter.
[0031] In a twenty-second embodiment, the SCR catalyst material of the
twentieth
embodiment is modified, wherein the substrate is a flow through substrate.
[0032] In a twenty-third embodiment, the SCR catalyst material of the first
through
twenty-second embodiments is modified, wherein at least 80% of the spherical
particles have
a median particle size in the range of 0.5 to 2.5 microns.
[0033] In a twenty-fourth embodiment, the SCR catalyst material of the
first through
twenty-third embodiments is modified, wherein the molecular sieve comprises a
zeolitic
framework material of silicon and aluminum atoms, wherein a fraction of the
silicon atoms are
isomorphously substituted with a tetravalent metal.
[0034] In a twenty-fifth embodiment, the SCR catalyst material of the
twenty-fourth
embodiment is modified, wherein the molecular sieve is promoted with a metal
selected from
Cu, Fe, Co, Ni, La, Ce, Mn, V. Ag, and combinations thereof.
[0035] In a twenty-sixth embodiment, the SCR catalyst material of the
twenty-twenty
fourth and twenty-fifth embodiments is modified, wherein the tetravalent metal
comprises a
tetravalent transition metal.
[0036] In a twenty-seventh embodiment, the SCR catalyst material of the
twenty-fourth
through twenty-sixth embodiments is modified, wherein the tetravalent
transition metal is
selected from the group consisting of Ti, Zr, Hf, Ge, and combinations
thereof.
Date recu/Date Received 2020-07-09
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
6
[0037] In a twenty-eighth embodiment, the SCR catalyst material of the
twenty-fourth
through twenty-seventh embodiments is modified, wherein the tetravalent
transition metal
comprises Ti.
[0038] A second aspect of the invention is directed to a method for
selectively reducing
nitrogen oxide (NO,). In a twenty-ninth embodiment, the method for selectively
reducing
nitrogen oxide (NO,) comprises contacting an exhaust gas stream containing NO,
with a SCR
catalyst material comprising a spherical particle including an agglomeration
of crystals of a
molecular sieve, wherein the spherical particle has a median particle size in
the range of about
0.5 to about 5 microns. In other embodiments, the method for selectively
reducing nitrogen
oxide (N01,) comprises contacting an exhaust gas stream containing NO3 with
the SCR catalyst
material of the first through twenty-eighth embodiments.
[0039] A third aspect of the invention is direct to a system for treating
exhaust gas from a
lean burn engine containing NO1. In a thirtieth embodiment, the system for
treating exhaust
gas from a lean burn engine containing NO, comprises the SCR catalyst material
of the first
through twenty-eighth embodiments and at least one other exhaust gas treatment
component.
[0040] A thirty-first embodiment pertains to SCR catalyst comprising a
zeolitic framework
material of silicon and aluminum atoms, wherein a fraction of the silicon
atoms are
isomorphously substituted with a tetravalent metal and the catalyst is
promoted with a metal
selected from Cu, Fe, Co, Ni, La, Ce, Mn, V, Ag, and combinations thereof.
[0041] In a thirty-second embodiment, the SCR catalyst of the thirty-first
embodiment is
modified, wherein the tetravalent metal comprises a tetravalent transition
metal.
[0042] In a thirty-third embodiment, the SCR catalyst of the thirty-first
and thirty-second
embodiments is modified, wherein the tetravalent transition metal is selected
from the group
consisting of Ti, Zr, Hf, Ge, and combinations thereof.
[0043] In a thirty-fourth embodiment, the SCR catalyst of the thirty-first
through thirty-
third embodiments is modified, wherein the tetravalent transition metal
comprises Ti.
[0044] In a thirty-fifth embodiment, the SCR catalyst of the thirty-first
through thirty-
fourth embodiments is modified, wherein the silica to alumina ratio is in the
range of 1 to 300.
100451 In a thirty-sixth embodiment, the SCR catalyst of the thirty-first
through thirty-fifth
embodiments is modified, wherein the silica to alumina ratio is in the range
of 1 to 50.
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
7
[0046] In a thirty-seventh embodiment, the SCR catalyst of the thirty-
first through thirty-
sixth embodiments is modified, wherein the tetravalent metal to alumina ratio
is in the range of
0.0001 to 1000.
[0047] In a thirty-eighth embodiment, the SCR catalyst of the thirty-
first through thirty-
seventh embodiments is modified, wherein the tetravalent metal to alumina
ratio is in the range
of 0.01 to 10.
100481 In a thirty-ninth embodiment, the SCR catalyst of the thirty-first
through thirty-
eighth embodiments is modified, wherein the tetravalent metal to alumina ratio
is in the range
of 0.01 to 2.
[0049] In a fourtieth embodiment, the SCR catalyst of the thirty-first
through thirty-ninth
embodiments is modified, wherein the silica to tetravalent metal ratio is in
the range of 1 to
100.
[0050] In a forty-first embodiment, the SCR catalyst of the thirty-first
through a fourtieth
embodiment is modified, wherein the silica to tetravalent metal ratio is in
the range of 5 to 20.
[0051] In a forty-second embodiment, the SCR catalyst of the thirty-first
through forty-first
embodiments if modified, wherein the zeolitic framework material comprises
ring sizes no
larger than 12.
[0052] In a forty-third embodiment, the SCR catalyst of the thirty-first
through forty-
second embodiments is modified, wherein the zeolitic framework material
comprises a d6r
unit.
[0053] In a forty-fourth embodiment, the SCR catalyst of the thirty-first
through forty-third
embodiments is modified, wherein the zeolitic framework material is selected
from AEI, AFT,
AFX, CHA, EAB, EMT, EM, FAU, GME, JSR, KFI, LEV, LTL, LTN, MOZ, MSO, MWW,
OFF, SAS, SAT, SAY, SBS, SBT, SFW, SSF, SZR, TSC, WEN, and combinations
thereof.
[0054] In a forty-fifth embodiment, the SCR catalyst of the thirty-first
through forty-fourth
embodiments is modified, wherein the zeolitic framework material is selected
from AEI, CHA,
AFX, ERI, KFI, LEV, and combinations thereof.
[0055] In a forty-sixth embodiment, the SCR catalyst of the thirty-first
through forty-fifth
embodiments is modified, wherein the zeolitic framework material is selected
from AEI, CHA,
and AFX.
[0056] In a forty-seventh embodiment, the SCR catalyst of the thirty-
first through forty-
sixth embodiments is modified, wherein the zeolitic framework material is CHA.
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
8
[0057] In a forty-eighth embodiment, the SCR catalyst of the thirty-first
through forty-
seventh embodiments is modified, wherein the catalyst is promoted with Cu, Fe,
and
combinations thereof.
[0058] In a forty-ninth embodiment, the SCR catalyst of the thirty-first
through forty-
eighth embodiments is modified, wherein the catalyst is effective to promote
the formation of
NO'.
[0059] In a fiftieth embodiment, the SCR catalyst of the thirty-first
through forty-ninth
embodiments is modified with the proviso that the zeolitic framework excludes
phosphorous
atoms.
[0060] Embodiments of an additional aspect of the invention are directed to
a method for
selectively reducing nitrogen oxides (NO,). In a fifty-first embodiment, the
method for
selectively reducing nitrogen oxides (NO3) comprises contacting an exhaust gas
stream
containing NO, with a catalyst of the thirty-first through fiftieth
embodiments.
[0061] Embodiments of a further aspect of the invention are directed to
an exhaust gas
treatment system. In a fifty-second embodiment, an exhaust gas treatment
system comprises
an exhaust gas stream containing ammonia and a catalyst in accordance with the
thirty-rust
through fiftieth embodiments.
[0062] In another aspect, a fifty-third embodiment is provided directed
to use of the
catalyst of any of the first through fiftieth embodiments as a catalyst for
the selective catalytic
reduction of NO3 in the presence of ammonia.
[0063] A fifty-fourth embodiment pertains to SCR catalyst composite
comprising a SCR
catalyst material that promotes the reaction of ammonia with nitrogen oxides
to form nitrogen
and H20 selectively over a temperature range of 150 C to 600 C; and an
ammonia storage
material comprising a transition metal having an oxidation state of IV, the
SCR catalyst
material effective to store ammonia at 400 C and above with a minimum NH3
storage of 0.1
g/L at 400 C.
[0064] In a fifty-fifth embodiment, the SCR catalyst composite of the
fifty-fourth
embodiment is modified, wherein the transition metal is selected from the
group consisting of
Ti, Ce, Zr, Hf, Ge, and combinations thereof.
[0065] In a fifty-sixth embodiment, the SCR catalyst composite the fifty-
fourth and fifty-
fifth embodiments is modified, wherein the SCR catalyst material is
isomorphously substituted
with the ammonia storage material.
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
9
[0066] In a fifty-seventh embodiment, the SCR catalyst composite of the
fifty-fourth and
fifty-fifth embodiments is modified, wherein the ammonia storage material is
dispersed in the
SCR catalyst material.
[0067] In a fifty-eighth embodiment, the SCR catalyst composite of the
fifty-fourth and
fifty-fifth embodiments is modified, wherein the ammonia storage material is
dispersed as a
layer on the SCR catalyst material.
[0068] In a fifty-ninth embodiment, the SCR catalyst composite of the
fifty-fourth and
fifty-fifth embodiments is modified, wherein the ammonia storage material and
the SCR
catalyst material arc arranged in a zoned configuration.
[0069] In a sixtieth embodiment, the SCR catalyst composite of the fifty-
ninth embodiment
is modified, wherein the ammonia storage material is upstream of the SCR
catalyst material.
[0070] In a sixty-first embodiment, the SCR catalyst composite of the
fifty-fourth and
fifty-fifth embodiments is modified, wherein the SCR catalyst material is ion-
exchanged with
the ammonia storage material.
[0071] In a sixty-second embodiment, the SCR catalyst composite of the
fifty-fourth
through sixty-first embodiments is modified, wherein the SCR catalyst material
is disposed on
a filter.
[0072] In a sixty-third embodiment, the SCR catalyst composite of the
sixty-second
embodiment is modified, wherein the filter is a wall flow filter.
[0073] In a sixty-fourth embodiment, the SCR catalyst composite of the
sixty-second
embodiment is modified, wherein the filter is a flow through filter.
[0074] In a sixty-fifth embodiment, the SCR catalyst composite of the
fifty-fourth through
sixty-fourth embodiments is modified, wherein the SCR catalyst material
comprises one or
more of a molecular sieve, a mixed oxide, and an activated refractory metal
oxide support.
[0075] In a sixty-sixth embodiment, the SCR catalyst composite of the sixty-
fifth
embodiment is modified, wherein the mixed oxide is selected from Fe/titania,
Fe/alumina,
Mg/titania, Mg/alumina, Mn/alumina, MnItitania, Cu/titania, Ce/Zr, Ti/Zr,
vanadia/titania, and
mixtures thereof.
[0076] In a sixty-seventh embodiment, the SCR catalyst composite of the
sixty-fifth and
sixty-sixth embodiments is modified, wherein the mixed oxide comprises
vanadia/titania and is
stabilized with tungsten.
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
100771 In a sixty-eighth embodiment, the SCR catalyst composite of the
sixty-fifth
embodiment is modified, wherein the molecular sieve has a framework of
silicon, phosphorus
and aluminum atoms.
100781 In a sixty-ninth embodiment, the SCR catalyst composite of the
sixty-eighth
5 embodiment is modified, wherein the silica to alumina ratio is in the
range of 1 to 300.
100791 In a seventieth embodiment, the SCR catalyst composite of the
sixty-eighth and
sixty-ninth embodiments is modified, wherein the silica to alumina ratio is in
the range of I to
50.
100801 In a seventy-first embodiment, the SCR catalyst composite of sixty-
eighth through
10 seventieth embodiments is modified, wherein the ratio of alumina to the
tetravalent metal is in
the range of 1:10 to 10:1.
100811 In a seventy-second embodiment, the SCR catalyst composite of the
sixty-eigth
through seventy-first embodiments is modified, wherein a fraction of the
silicon ions are
isoinorphously substituted with the metal of the ammonia storage material.
100821 In a seventy-third embodiment, the SCR catalyst composite of the
sixty-fifth
embodiment is modified, wherein the molecular sieve comprises ring sizes no
larger than 12.
[0083] In a seventy-second embodiment, the SCR catalyst composite of the
sixty-fifth
through seventy-third embodiments is modified, wherein the molecular sieve has
a structure
type selected from the group consisting of MFI, BEA, AEI, AFT, AFX, CHA, EAB,
EMT,
ER!, FAU, GME, JSR, KFI, LEV, LTL, LTN, MOZ, MSO, MWW, OFF, SAS, SAT, SAV,
SBS, SBT, SFW, SSF, SZR, TSC, WEN, and combinations thereof.
100841 In a seventy-third embodiment, the SCR catalyst composite of the
seventy-second
embodiment is modified, wherein the molecular sieve has a structure type
selected from the
group consisting of MFI, BEA, CHA, AEI, AFX, ERI, KFI, LEV, and combinations
thereof.
100851 In a seventy-fourth embodiment, the SCR catalyst composite of the
seventy-third
embodiment is modified, wherein the molecular sieve has a structure type
selected from the
group consisting of AEI, CHA, AFX, and combinations thereof.
100861 In a seventy-fifth embodiment, the SCR catalyst composite of the
fifty-fourth
through seventy-fourth embodiments is modified, wherein the SCR catalyst
material is
promoted with a metal selected from Cu, Fe, Co, Ni, La, Ce, Mn, V, Ag, and
combinations
thereof.
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
11
[0087] In a seventy-sixth embodiment, the SCR catalyst composite of the
fifty-fourth
through seventy-fourth embodiments is modified, wherein the SCR catalyst
material is
promoted with Cu, Fe, and combinations thereof.
[0088] In a seventy-seventh embodiment, the SCR catalyst composite of the
sixty-fifth
.. embodiment is modified, wherein the molecular sieve comprises SSZ-13, SSZ-
39, or SAPO-
34.
[0089] In a seventy-eighth embodiment, the SCR catalyst composite of the
sixty-fifth
embodiment is modified, wherein the activated refractory metal oxide support
is selected from
alumina, ceria, zirconia, silica, titania, silica-alumina, zirconia-alumina,
titania-alumina,
lanthana-alumina, lanthana-zirconia-alumina, baria-alumina, baria-lanthana-
alumina, baria-
lanthana-neodynna-alumina, alumina-chomia, alumina-ceria, zirconia-silica,
titania-silica, or
zirconia-titania, and combinations thereof.
[0090] In a seventy-ninth embodiment, the SCR catalyst composite of the
seventy-eighth
embodiment is modified, wherein the activated refractory metal oxide support
is exchanged
with a metal selected from the group consisting of Cu, Fe, Co, Ni, La, Ce, Mn,
V, Ag, and
combinations thereof.
[0091] In an eightieth embodiment, the SCR catalyst composite of the
sixty-fifth
embodiment is modified, wherein the transition metal comprises Ti.
[0092] In an eighty-first embodiment, the SCR catalyst composite of the
eightieth
embodiment is modified, wherein the ratio of alumina to titanium is in the
range of 1:10 to
10:1.
[0093] A further aspect of the present invention is directed to a method.
In an eighty-
second embodiment, a method for simultaneously selectively reducing nitrogen
oxide (NO)
and storing ammonia comprises contacting an exhaust gas stream containing NO3
with the
SCR catalyst composite of the fifty-fourth through eighty-first embodiments.
[0094] In an eighty-third embodiment, the method of the eighty-second
embodiment is
modified, wherein the oxygen content of the exhaust gas stream is from 1 to
30% and the water
content of the exhaust gas stream is from 1 to 20%.
100951 An additional aspect of the present invention is directed to a SCR
catalyst
composite. In an eighty-fourth embodiment, a SCR catalyst composite comprises
a SCR
catalyst material that effectively promotes the reaction of ammonia with
nitrogen oxides to
form nitrogen and H2O selectively over a temperature range of 200 C to 600
C, wherein the
12
SCR catalyst material comprises SSZ-13; and an ammonia storage material
comprising Ti, the
ammonia storage material effective to store ammonia at 400 C and above.
In some aspects, embodiments of the present invention as described herein
include the following
items:
1. A selective catalytic reduction (SCR) catalyst material comprising a
spherical
particle including an agglomeration of crystals of a molecular sieve, wherein
the spherical
particle has a median particle size in a range of about 0.5 to about 5
microns, wherein the
molecular sieve comprises a zeolitic framework material of silicon and
aluminum atoms,
wherein a fraction of the silicon atoms are isomoiphously substituted with a
tetravalent metal.
2. The SCR catalyst material of item 1, wherein the molecular sieve
comprises a d6r
unit.
3. The SCR catalyst material of item 2, wherein the molecular sieve has a
structure
type selected from the group consisting of AEI, AFT, AFX, CHA, EAB, EMT, ERI,
FAU, GME,
JSR, KFI, LEV, LTL, LTN, MOZ, MSO, MWW, OFF, SAS, SAT, SAY, SBS, SBT, SFW,
SSF,
SZR, TSC, WEN, and combinations thereof.
4. The SCR catalyst material of item 3, wherein the molecular sieve has a
structure
type selected from AEI, AFT, AFX, CHA, EAB, EM, KFI, LEV, SAS, SAT, and SAY.
5. The SCR catalyst material of item 4, wherein the molecular sieve has a
structure
type selected from AEI, CHA, and AFX.
6. The SCR catalyst material of item 5, wherein the molecular sieve has the
CHA
structure type.
7. The SCR catalyst material of item 6, wherein the molecular sieve having
the
CHA structure type is selected from an aluminosilicate zeolite, a
borosilicate, a gallosilicate, a
SAPO, an Al PO, a MeAPSO, and a MeAPO.
8. The SCR catalyst material of item 6, wherein the molecular sieve having
the CHA
structure type is selected from the group consisting of SSZ-13, SSZ-62,
natural
Date Recue/Date Received 2022-09-16
12a
chabazite, zeolite K-G, Linde D, Linde R, LZ-218, LZ-235, LZ-236, ZK-14, SAPO-
34, SAPO-
44, SAPO-47, and ZYT-6.
9.
The SCR catalyst material of item 8, wherein the molecular sieve is selected
from SSZ-13 and SSZ-62.
10. The SCR
catalyst material of item 1, wherein the molecular sieve is promoted
with a metal selected from Cu, Fe, Co, Ni, La, Ce, Mn, V, Ag, and combinations
thereof.
11. The SCR catalyst material of item 10, wherein the molecular sieve is
promoted
with a metal selected from Cu, Fe, and combinations thereof.
12. The SCR catalyst material of item 1, wherein the SCR catalyst material
is
effective to catalyze the selective catalytic reduction of nitrogen oxides in
the presence of a
reductant at temperatures between 200 C and 600 C.
13. The SCR catalyst material of item 6, wherein the molecular sieve having
the
CHA structure type has a silica to alumina ratio in the range of 10 to 100.
14. The SCR catalyst material of item 10, wherein the metal is present in
an amount
in a range of about 0.1 to about 10 wt.% on an oxide basis.
15. The SCR catalyst material of item 1, wherein the spherical particle has
a median
particle size in the range of about 1.2 to about 3.5 microns.
16. The SCR catalyst material of item 1, wherein the crystals have a
crystal size in
the range of about 1 to about 250 nm.
17. The SCR
catalyst material of item 16, wherein the crystals have a crystal size in
the range of about 100 to about 250 nm.
18. The SCR catalyst material of item 1, wherein the SCR catalyst material
is in the
form of a washcoat.
19. The SCR catalyst material of item 18, wherein the washcoat is a layer
deposited
on a substrate.
20. The SCR catalyst material of item 19, wherein the substrate comprises a
filter.
Date Recue/Date Received 2022-09-16
12b
21. The SCR catalyst material of item 20, wherein the filter is a wall flow
filter.
22. The SCR catalyst material of item 19, wherein the substrate is a flow
through
substrate.
23. The SCR catalyst material of item 1, wherein at least 80% of the
spherical
particles have a median particle size in the range of 0.5 to 2.5 micron.
24. The SCR catalyst material of any one of items 1 to 23, wherein the
molecular
sieve is promoted with a metal selected from Cu, Fe, Co, Ni, La, Ce, Mn, V,
Ag, and
combinations thereof.
25. The SCR catalyst material of any one of items 1 to 23, wherein the
tetravalent
.. metal comprises a tetravalent transition metal.
26. The SCR catalyst material of item 25, wherein the tetravalent
transition metal is
selected from the group consisting of Ti, Zr, Hf, Ge, and combinations
thereof.
27. The SCR catalyst material of item 25, wherein the tetravalent
transition metal
comprises Ti.
28. A method for selectively reducing nitrogen oxide (NO), the method
comprising
contacting an exhaust gas stream containing NO with a SCR catalyst material as
defined in any
one of items 1 to 27.
29. A method for selectively reducing nitrogen oxide (N0x), the
method comprising:
contacting an exhaust gas stream containing NOx with a SCR catalyst material
comprising a spherical particle including an agglomeration of crystals of a
molecular
sieve, wherein the spherical particle has a median particle size in a range of
about 0.5 to
about 5 microns, wherein the molecular sieve comprises a zeolitic framework
material
of silicon and aluminum atoms, wherein a fraction of the silicon atoms are
isomorphously substituted with a tetravalent metal;
wherein the spherical particle has a monodispersed snowball structure defined
as an
arrangement of crystals;
wherein said crystals have approximately the same crystal size, wherein the
same crystal
size is selected from the range of about 1 to about 250 nm;
Date Recue/Date Received 2022-09-16
12c
wherein the molecular sieve has a structure type selected from the group
consisting of
AEI, AFT, AFX, CHA, EAB, EMT, ERI, GME, JSR, KFI, LEV, LTL, LTN, MOZ,
MSO, MWW, OFF, SAS, SAT, SAV, SBS, SBT, SFW, SSF, SZR, TSC, WEN, and
combinations thereof; and
wherein the molecular sieve is promoted with a metal selected from Cu, Fe, Co,
Ni, La,
Ce, Mn, V, Ag, and combinations thereof.
30. The method of item 29, wherein the molecular sieve comprises a d6r
unit.
31. The method of item 29, wherein the molecular sieve has a structure type
selected
from AEI, AFT, AFX, CHA, EAB, ERI, KFI, LEV, SAS, SAT, and SAV.
32. The method of item 31, wherein the molecular sieve has a structure type
selected
from AEI, CHA, and AFX.
33. The method of item 32, wherein the molecular sieve has the CHA
structure type.
34. The method of item 33, wherein the molecular sieve having the CHA
structure
type is selected from an aluminosilicate zeolite, a borosilicate, a
gallosilicate, a SAPO, an
ALPO, a MeAPSO, and a MeAPO.
35. The method of item 33, wherein the molecular sieve having the CHA
structure
type is selected from the group consisting of SSZ-13, SSZ-62, natural
chabazite, zeolite K-G,
Linde D, Linde R, LZ-218, LZ-235, LZ-236, ZK-14, SAPO-34, SAPO-44, SAPO-47,
and ZYT-
6.
36. The method of item 35, wherein the molecular sieve is selected from SSZ-
13 and
SSZ-62.
37. The method of item 33, wherein the molecular sieve having the CHA
structure
type has a silica to alumina ratio in the range of 10 to 100.
38. The method of item 29, wherein the molecular sieve is promoted with a
metal
selected from Cu, Fe, and combinations thereof.
Date Recue/Date Received 2022-09-16
12d
39. The method of item 29, wherein the selective catalytic reduction
material is
effective to catalyze the selective catalytic reduction of nitrogen oxides in
the presence of a
reductant at temperatures between 200 C. and 600 C.
40. The method of item 29, wherein the metal is present in an amount in a
range of
about 0.1 to about 10 wt. % on an oxide basis.
41. The method of item 29, wherein the spherical particle has a median
particle size
in the range of about 1.2 to about 3.5 microns.
42. The method of item 29, wherein the crystals have a crystal size in the
range of
about 100 to about 250 nm.
43. The method of item 29, wherein the selective catalytic reduction
material is in
the form of a washcoat.
44. The method of item 43, wherein the washcoat is a layer deposited on a
substrate.
45. The method of item 44, wherein the substrate comprises a filter.
46. The method of item 45, wherein the filter is a wall flow filter.
47. The method of item 44, wherein the substrate is a flow through
substrate.
48. The method of item 29, wherein at least 80% of the spherical particles
have a
median particle size in the range of 0.5 to 2.5 micron.
49. The method of item 29, wherein the tetravalent metal comprises a
tetravalent
transition metal.
50. The method of item 49, wherein the tetravalent transition metal is
selected from
the group consisting of Ti, Zr, Hf, Ge, and combinations thereof.
51. The method of item 49, wherein the tetravalent transition metal
comprises Ti.
52. A system for treating exhaust gas from a lean burn engine containing
NO,,, the
system comprising the SCR catalyst material of any one of items 1 to 27 and at
least one other
exhaust gas treatment component.
Date Recue/Date Received 2022-12-12
12e
53. A system for treating exhaust gas from a lean burn engine containing
NO., the
system comprising:
a SCR catalyst material comprising a spherical particle including an
agglomeration of
crystals of a metal-promoted molecular sieve, wherein the spherical particle
has a
median particle size in a range of about 0.5 to about 5 microns, wherein the
molecular
sieve comprises a zeolitic framework material of silicon and aluminum atoms,
wherein
a fraction of the silicon atoms are isomorphously substituted with a
tetravalent metal;
wherein the crystals of the metal-promoted molecular sieve have approximately
the
same crystal size;
wherein the same crystal size is selected from the range of about 1 to about
250 nm; and
wherein the metal-promoted molecular sieve has a structure type selected from
the group
consisting of AEI, AFT, AFX, CHA, EAB, EMT, ERI, FAU, GME, JSR, KFI, LEV,
LTL, LTN, MOZ, MSO, MWW, OFF, SAS, SAT, SAY, SBS, SBT, SFW, SSF, SZR,
TSC, WEN, and combinations thereof; and at least one other exhaust gas
treatment
component.
54. The system of item 53, wherein the least one other exhaust gas
treatment
component is selected from a soot filter, a diesel oxidation catalyst, an
ammonia oxidation
catalyst, and combinations thereof.
55. The system of item 54, wherein the system comprises a soot filter, and
wherein
the soot filter is a wall-flow filter substrate upon which the SCR material is
disposed.
56. The system of item 54, wherein the system comprises a diesel oxidation
catalyst
located upstream of the SCR material.
57. The system of item 54, wherein the system comprises an ammonia
oxidation
catalyst located downstream of the SCR material.
58. The system of item 57, wherein the ammonia oxidation catalyst comprises
a
platinum group metal selected from platinum, palladium, rhodium, and
combinations thereof.
59. The system of item 54, wherein the system comprises a soot
filter and a diesel
oxidation catalyst located upstream of the SCR material.
Date Recue/Date Received 2022-09-16
12f
60. The system of item 53, wherein the metal-promoted molecular sieve has a
structure type selected from AEI, AFT, AFX, CHA, EAB, EM, KFI, LEV, SAS, SAT,
and
SAY.
61. The system of item 53, wherein the metal-promoted molecular sieve has a
structure type selected from AEI, CHA, and AFX.
62. The system of item 61, wherein the metal-promoted molecular sieve has
the CHA
structure type.
63. The system of item 62, wherein the metal-promoted molecular sieve
having the
CHA structure type has a silica to alumina ratio in the range of 10 to 100.
64. The system of item 62, wherein the metal-promoted molecular sieve
having the
CHA structure type is selected from an aluminosilicate zeolite, a
borosilicate, a gallosilicate, a
SAPO, an AlP0, a MeAPSO, and a MeAPO.
65. The system of item 62, wherein the metal-promoted molecular sieve
having the
CHA structure type is selected from the group consisting of SSZ-13, SSZ-62,
natural chabazite,
zeolite K-G, Linde D, Linde R, LZ-218, LZ-235, LZ-236, ZK-14, SAPO-34, SAPO-
44, SAPO-
47, and ZYT-6.
66. The system of item 65, wherein the metal-promoted molecular sieve
having the
CHA structure type is selected from SSZ-13 and SSZ-62.
67. The system of item 53, wherein the metal-promoted molecular sieve is
promoted
with a metal selected from Cu, Fe, Co, Ni, La, Ce, Mn, V, Ag, and combinations
thereof.
68. The system of item 67, wherein the metal-promoted molecular sieve is
promoted
with a metal selected from Cu, Fe, and combinations thereof.
69. The system of item 53, wherein the SCR material is effective to
catalyze the
selective catalytic reduction of nitrogen oxides in the presence of a
reductant at temperatures
between 200 C. and 600 C.
70. The system of item 53, wherein the metal-promoted molecular sieve
comprises
metal in an amount in a range of about 0.1 to about 10 wt. % on an oxide
basis.
Date Recue/Date Received 2022-09-16
12g
71. The system of item 53, wherein the spherical particle has a median
particle size
in the range of about 1.2 to about 3.5 microns.
72. The system of item 53, wherein the crystals have a crystal size in the
range of
about 100 to about 250 nm.
73. The system of item 53, wherein the SCR material is in the form of a
washcoat
layer deposited on a substrate.
74. The system of item 53, wherein the tetravalent metal is selected from
the group
consisting of Ti, Zr, Hf, Ge, and combinations thereof.
75. The system of item 74, wherein the tetravalent metal comprises Ti.
BRIEF DESCRIPTION OF DRAWINGS
[0096] FIG. 1 is a schematic of a cross-section of a SCR catalyst material
according to one or
more embodiments;
[0097] FIG. 2 shows a partial cross-sectional view of an SCR catalyst
composite according to
one or more embodiments;
[0098] FIG. 3 shows a partial cross-sectional view of an SCR catalyst
composite according
to one or more embodiments;
[0099] FIG. 4A shows a perspective view of a wall flow filter substrate;
[00100] FIG. 4B shows a cutaway view of a section of a wall flow filter
substrate;
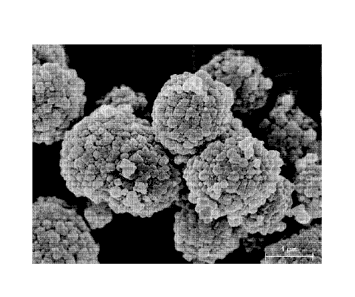
[00101] FIG. 5 is a SEM image showing crystal morphology of a catalyst
material according
to the Examples;
[00102] FIG. 6 is a SEM image showing crystal morphology of a catalyst
material according
to the Comparative Example;
[00103] FIG. 7 is a bar graph comparing NO), conversion for catalysts
according to the
Examples;
[00104] FIG. 8 is a bar graph comparing N20 make for catalysts according to
the Examples;
[00105] FIG. 9 is a graph comparing NO), conversion for catalysts according to
the
Examples;
Date Regue/Date Received 2022-09-16
12h
[00106] FIG. 10 is a graph comparing N20 make for catalysts according to the
Examples;
1001071 FIG. 11 is a bar graph comparing NOx conversion at 20 ppm NH3 slip for
catalysts
according to the Examples;
[00108] FIG. 12 is an ATR analysis for catalysts according to the Examples;
[00109] FIG. 13 is a FTIR analysis for catalysts according to the Examples;
[00110] FIG. 14 is a FTIR analysis for catalysts according to the Examples;
[00111] FIG. 15 is a scanning electron microscope image of material according
to the Examples;
[00112] FIG. 16 compares NO, conversion for catalysts according to the
Examples;
[00113] FIG. 17 compares NOx conversion for catalysts according to the
Examples;
Date Recue/Date Received 2022-09-16
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
13
[00114] FIGS. 18A and 18B are scanning electron microscope images of material
of
materials according to the Examples;
[00115] FIG. 19 is a washcoat porosity measurement for catalysts according to
the
Examples;
[00116] FIG. 20 compares NH3 absorption for catalysts according to the
Examples;
[00117] FIG. 21 compares NH3 absorption for catalysts according to the
Examples;
[00118] FIG. 22 compares NH3 absorption for catalysts according to the
Examples;
[00119] FIG. 23 compares NH3 absorption for catalysts according to the
Examples; and
[00120] FIG. 24 compares NH3 absorption for catalysts according to the
Examples.
110
DETAILED DESCRIPTION
[00121] Before describing several exemplary embodiments of the invention,
it is to be
understood that the invention is not limited to the details of construction or
process steps set forth
in the following description. The invention is capable of other embodiments
and of being
practiced or being carried out in various ways.
[00122] Governmental regulations mandate the use of NO reduction technologies
for light and
heavy-duty vehicles. Selective catalytic reduction (SCR) of NO3 using urea is
an effective and
dominant emission control technology for NO3 control. To meet governmental
regulations, an
SCR catalyst that has improved performance compared to the current Cu-SSZ-13
based
benchmark technology is necessary. Provided is an SCR catalyst material having
improved
NO3 conversion efficiency and lower N20 make relative to the current Cu-SSZ-13
based
benchmark technologies. The SCR catalyst material effectively promotes the
reaction of
ammonia with nitrogen oxides to form nitrogen and H20 selectively over a
temperature range
of 200 to 600 C.
[00123] Embodiments of the invention arc directed to a selective catalytic
reduction material
comprising a spherical particle including an agglomeration of crystals of a
molecular sieve. It
was surprisingly found that spherical particles having an agglomeration of
crystals of a
molecular sieve are particularly suitable in exhaust gas purification catalyst
components, in
particular as SCR catalyst materials.
[00124] With respect to the terms used in this disclosure, the following
definitions are
provided.
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
14
[00125] As used herein, the term "catalyst" or "catalyst composition" or
"catalyst material"
refers to a material that promotes a reaction.
[00126] As used herein, the term "catalytic article" or "catalyst composite"
refers to an
element that is used to promote a desired reaction. For example, a catalytic
article or catalyst
composite may comprise a washcoat containing a catalytic species, e.g, a
catalyst composition,
on a substrate.
[00127] As used herein, the term "selective catalytic reduction" (SCR) refers
to the catalytic
process of reducing oxides of nitrogen to dinitrogen (N2) using a nitrogenous
reductant.
[00128] As used herein, the term "FTIR" refers to Fourier transform infrared
spectroscopy,
which is a technique used to obtain an infrared spectrum of absorption,
emission,
photoconductivity or Raman scattering of a solid, liquid, or gas.
[00129] As used herein, the term "ATR" refers to attenuated total reflectance,
which is a
sampling technique used in conjunction with infrared spectroscopy,
particularly FTIR, which
enables samples to be examined directly in the solid or liquid state without
further preparation.
[00130] According to one or more embodiments, a selective catalytic reduction
catalyst
material comprises a spherical particle including an agglomeration of crystals
of a molecular
sieve, wherein the spherical particle has a median particle size in the range
of about 0.5 to
about 5 microns.
[00131] As used herein, the phrase "molecular sieve" refers to framework
materials such as
zeolites and other framework materials (e.g. isomorphously substituted
materials), which may
in particulate form in combination with one or more promoter metals be used as
catalysts.
Molecular sieves are materials based on an extensive three-dimensional network
of oxygen
ions containing generally tetrahedral type sites and having a substantially
uniform pore
distribution, with the average pore size being no larger than 20 A. The pore
sizes are defined
by the ring size. As used herein, the term "zeolite" refers to a specific
example of a molecular
sieve, including silicon and aluminum atoms. According to one or more
embodiments, it will
be appreciated that by defining the molecular sieves by their structure type,
it is intended to
include the structure type and any and all isotypic framework materials such
as SAPO, ALPO
and MeAPO materials having the same structure type as the zeolite materials.
[00132] In more specific embodiments, reference to an aluminosilicate zeolite
structure type
limits the material to molecular sieves that do not include phosphorus or
other metals
substituted in the framework. However, to be clear, as used herein,
"aluminosilicate zeolite"
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
excludes aluminophosphate materials such as SAPO, ALPO, and MeAPO materials,
and the
broader term "zeolite" is intended to include aluminosilicates and
aluminophosphates. Zeolites
are crystalline materials having rather uniform pore sizes which, depending
upon the type of
zeolite and the type and amount of cations included in the zeolite lattice,
range from about 3 to
5 10 Angstroms in diameter. Zeolites generally comprise silica to alumina
(SAR) molar ratios of
2 or greater.
[00133] The term "aluminophosphates" refers to another specific example of a
molecular
sieve, including aluminum and phosphate atoms. Aluminophosphates are
crystalline materials
having rather uniform pore sizes.
10 [00134] Generally, molecular sieves, e.g. zeolite, are defined as
aluminosilicates with open
3-dimensional framework structures composed of corner-sharing TO4 tetrahedra,
where T is Al
or Si, or optionally P. Cations that balance the charge of the anionic
framework are loosely
associated with the framework oxygens, and the remaining pore volume is filled
with water
molecules. The non-framework cations are generally exchangeable, and the water
molecules
15 removable.
[00135] In an exemplary embodiment, the molecular sieve can be isomorphously
substituted. As used herein, the terms "zeolitic framework" and "zeolitic
framework material"
refer to a specific example of a molecular sieve, further including silicon
and aluminum atoms.
According to embodiments of the invention, the molecular sieve comprises a
zeolitic framework
.. material of silicon (Si) and aluminum (Al) ions, wherein a fraction of the
silicon atoms are
isomorphously substituted with a tetravalent metal. In specific embodiments,
the framework does
not include phosphorous (P) atoms.
[00136] As used herein, the terms "isomorphously substituted" and "isomorphous
substitution" refer to the substitution of one element for another in a
mineral without a
significant change in the crystal structure. Elements that can substitute for
each other generally
have similar ionic radii and valence state. In one or more embodiments, a
fraction of the
silicon atoms are isomorphously substituted with a tetravalent metal. In other
words, a fraction
of the silicon atoms in the zeolitic framework material are being replaced
with a tetravalent
metal. Such isomorophous substitution does not significantly alter the crystal
structure of the
.. zeolitic framework material.
[00137] As used herein, the term "tetravalent metal" refers to a metal having
a state with four
electrons available for covalent chemical bonding in its valence (outermost
electron shell).
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
16
Tetravalent metals include germanium (Ge) and those transition metals located
in Group 4 of
the periodic table, titanium (Ti), zirconium (Zr), and hafnium (Hf). In one or
more
embodiments, the tetravalent metal is selected fiom Ti, Zr, Hf, Ge, and
combinations thereof.
In specific embodiments, the tetravalent metal comprises Ti.
[00138] in other embodiments, a fraction of the silicon atoms are
isomorphously substituted
with a transition metal having an oxidation state of IV. Without intending to
be bound by
theory, it is thought that the presence of an element with a formal oxidation
state of IV helps to
increase ammonia storage at high temperature. In one or more embodiments, the
transition
metal having an oxidation state of IV can either be in oxide form, or
intrinsically embedded in
the SCR catalyst material. As used herein, the term "transition metal having
an oxidation state
of IV" refers to a metal having a state with four electrons available for
covalent chemical
bonding in its valence (outermost electron shell). Transition metals having an
oxidation state
of IV include germanium (Ge), cerium (Ce), and those transition metals located
in Group 4 of
the periodic table, titanium (Ti), zirconium (Zr), and hafnium (Hf). In one or
more
embodiments, the transition metal having an oxidation state of IV is selected
from Ti, Ce, Zr,
Hf, Ge, and combinations thereof. In specific embodiments, the transition
metal having an
oxidation state of IV comprises Ti.
[00139] In one or more embodiments, the zeolitic framework material comprises
M04/SiO4/A104 tetrahedra (where M is a tetravalent metal) and is linked by
common oxygen
atoms to form a three-dimensional network. The isomorphously substituted
tetravalent metals
are embedded into the zeolitic framework material as a tetrahedral atom (M04).
The
isomorphously substituted tetrahedron units together with the silicon and
aluminum
tetrahedron units then form the framework of the zeolitic material. In
specific embodiments,
the tetravalent metal comprises titanium, and the zeolitic framework material
includes
TiO4/SiO4/A104 tetrahedra. Thus, in one or more embodiments, the catalyst
comprises a
zeolitic framework of silicon and aluminum atoms, wherein a fraction of the
silicon atoms are
isomorphously substituted with titanium.
[00140] The isomorphously substituted zeolitic framework material of one or
more
embodiments is differentiated mainly according to the geometry of the voids
which are formed
by the rigid network of the M04/(SiO4)/A104 tetrahedra (where M is a
tetravalent metal).
[00141] In one or more embodiments, the molecular sieve comprises SiO4/A104
tetrahedra
and is linked by common oxygen atoms to form a three-dimensional network. In
other
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
17
embodiments, the molecular sieve comprises SiO4/A104/PO4 tetrahedra. The
molecular sieve
of one or more embodiments is differentiated mainly according to the geometry
of the voids
which are foluied by the rigid network of the (SiO4)/A104, or SiO4/A104/PO4,
tetrahedra. The
entrances to the voids are formed from 6, 8, 10, or 12 ring atoms with respect
to the atoms
which form the entrance opening. In one or more embodiments, the molecular
sieve comprises
ring sizes of no larger than 12, including 6, 8, 10, and 12.
[00142] According to one or more embodiments, the molecular sieve can be based
on the
framework topology by which the structures are identified. Typically, any
structure type of
zeolite can be used, such as structure types of ABW, ACO, AEI, AEL, AEN, AET,
AFG, AFI,
AFN, AFO, AFR, AFS, AFT, AFX, AFY, AHT, ANA, APC, APD, AST, ASV, ATN, ATO,
ATS, ATT, ATV, AWO, AWW, BCT, BEA, BEC, BIK, BOG, BPH, BRE, CAN, CAS, SCO,
CFI, SGF, CGS, CIA, CHI, CLO, CON, CZP, DAC, DDR, DFO, DFT, DOH, DON, EAB,
EDI, EMT, EON, EPI, ERI, ESV, ETR, EUO, FAU, FER, FRA, GIS, GIU, GME, GON,
GOO, HEU, IFR, IHW, ISV, ITE, 1TH, ITW, IWR, IWVV, JBW, KFI, LAU, LEV, LID,
LIT,
LOS, LOV, LTA, LTL, LTN, MAR, MAZ, MEI, MEL, MEP, MER, MFI, MFS, MON, MOR,
MOZ, MSO, MTF, MTN, MU, MTW, MWW, NAB, NAT, NES, NON, NPO, NSI, OBW,
OFF, OSI, OSO, OWE, PAR, PAU, PHI, PON, RHO, RON, RRO, RSN, RTE, RTH, RUT,
RWR, RWY, SAO, SAS, SAT, SAV, SBE, SBS, SBT, SFE, SFF, SFG, SFH, SFN, SFO,
SGT,
SOD, SOS, SSY, STF, STI, STT, TER, THO, TON, TSC, UEI, UFI, UOZ, USI, UTL,
VET,
VFI, VNI, VSV, WIE, WEN, YUG, ZON, or combinations thereof.
[00143] In one or more embodiments, the molecular sieve comprises an 8-ring
small pore
aluminosilicate zeolite. As used herein, "small pore" refers to pore openings
which are smaller
than about 5 Angstroms, for example on the order of ¨3.8 Angstroms. The phrase
"8-ring"
zeolites refers to zeolites having 8-ring pore openings and double-six ring
secondary building
units and having a cage like structure resulting from the connection of double
six-ring building
units by 4 rings. Zeolites are comprised of secondary building units (SBU) and
composite
building units (CBU), and appear in many different framework structures.
Secondary building
units contain up to 16 tetrahedral atoms and are non-chiral. Composite
building units are not
required to be achiral, and cannot necessarily be used to build the entire
framework. For
example, a group of zeolites have a single 4-ring (s4r) composite building
unit in their
framework structure. In the 4-ring, the "4" denotes the positions of
tetrahedral silicon and
aluminum atoms, and the oxygen atoms are located in between tetrahedral atoms.
Other
18
composite building units include, for example, a single 6-ring (s6r) unit, a
double 4-ring (d4r) unit, and
a double 6-ring (d6r) unit. The d4r unit is created by joining two s4r units.
The d6r unit is created by
joining two s6r units. In a d6r unit, there are twelve tetrahedral atoms.
Zeolitic structure types that have
a d6r secondary building unit include AEI, AFT, AFX, CHA, EAB, EMT, ER1, FAU,
GME, JSR, KFI,
LEV, LTL, LTN, MOZ, MSO, MWW, OFF, SAS, SAT, SAY, SE1S, SBT, SFW, SSF, SZR,
TSC, and
WEN.
1001441 In one or more embodiments, the molecular sieve comprises a d6r
unit. Without intending
to be bound by theory, in one or more embodiments, it is thought that the d6r
unit promotes the formation
of NO+. Thus, in one or more embodiments, the molecular sieve has a structure
type selected from AEI,
AFT, AFX, CHA, EAB, EMT, ER!, FAU, GME, JSR, KFI, LEV, LTL, LTN, MOZ, MSO,
MWW,
OFF, SAS, SAT, SAV, SBS, SBT, SFW, SSF, SZR, TSC, WEN, and combinations
thereof. In other
specific embodiments, the molecular sieve has a structure type selected from
the group consisting of
CHA, AEI, AFX, ER!, KFI, LEV, and combinations thereof. In still further
specific embodiments, the
molecular sieve has a structure 15 type selected from CHA, AEI, and AFX. In
one or more very specific
embodiments, the molecular sieve has the CHA structure type.
1001451 Zeolitic chabazite includes a naturally occurring tectosilicate
mineral of a zeolite
group with approximate formula: (Ca,Na2,K2,Mg)Al2Si4012.6H20 (e.g., hydrated
calcium
aluminum silicate). Three synthetic forms of zeolitic chabazite are described
in "Zeolite Molecular
Sieves," by D. W. Breck, published in 1973 by John Wiley & Sons. The three
synthetic forms reported
by Breck are Zeolite K-G, described in J. Chem. Soc., p. 2822 (1956), Barrer
et at; Zeolite D, described
in British Patent No. 868,846 (1961); and Zeolite R, described in U.S. Patent
No. 3,030,181. Synthesis
of another synthetic form of zeolitic chabazite, SSZ-13, is described in U.S.
Pat. No. 4,544,538.
Synthesis of a synthetic form of a molecular sieve having the chabazite
crystal structure,
silicoalurninophosphate 34 (SAPO-34), is described in U.S. Patent 4,440,871
and No. 7,264,789. A
method of making yet another synthetic molecular sieve having chabazite
structure, SAPO-44, is
described in U.S. Patent No. 6,162,415.
1001461 In one or more embodiments, the molecular sieve can include all
aluminosilicate,
borosilicate, gallosilicate, MeAPSO, and MeAPO compositions. These include,
but are not
Date Recue/Date Received 2021-10-20
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
19
limited to SSZ-13, SSZ-62, natural chabazite, zeolite K-G, Linde D, Linde R,
LZ-218, LZ-235.
LZ-236, ZK-14, SAPO-34, SAPO-44, SAPO-47, ZYT-6, CuSAP0-34, CuSAP0-44, and
CuSAP0-47.
[00147] The ratio of silica to alumina of an aluminosilicate molecular sieve
can vary over a
wide range. In one or more embodiments, the molecular sieve component has a
silica to
alumina molar ratio (SAR) in the range of 2 to 300, including 5 to 250; 5 to
200; 5 to 100; and
5 to 50. In one or more specific embodiments, the molecular sieve has a silica
to alumina
molar ratio (SAR) in the range of 10 to 200, 10 to 100, 10 to 75, 10 to 60,
and 10 to 50; 15 to
100, 15 to 75, 15 to 60, and 15 to 50; 20 to 100, 20 to 75, 20 to 60, and 20
to 50. In more
specific embodiments, the molecular sieve having any of the immediately
preceding SAR
ranges, the spherical particle of the molecular sieve has a median particle
size in the range of
about 0.5 to about 5 microns, and more specifically, about 1.0 to about 3.5
microns, and the
individual crystals of a molecular sieve have a crystal size in the range of
about 100 to about
250 nm.
[00148] Isomorphous substitution of silicon with a tetravalent metal will
affect the
silica/alumina ratio of the zeolitic framework material. In one or more
embodiments, the
molecular sieve is isomorphously substituted with a tetravalent metal and has
a silica to
alumina molar ratio (SAR) in the range of 2 to 300, including 5 to 250; 5 to
200; 5 to 100; and
5 to 50. In one or more specific embodiments, the first and second molecular
sieve,
independently, have a silica to alumina molar ratio (SAR) in the range of 10
to 200, 10 to 100,
10 to 75, 10 to 60, and 10 to 50; 15 to 100, 15 to 75, 15 to 60, and 15 to 50;
20 to 100, 20 to 75,
20 to 60, and 20 to 50.
[00149] In embodiments where the molecular sieve is isomorphously substituted
with a
tetravalent metal, the ratio of tetravalent metal to alumina can vary over a
very wide range. It
is noted that this ratio is an atomic ratio, not a molar ratio. In one or more
embodiments, the
tetravalent metal to alumina ratio is in the range of 0.0001 to 10000,
including 0.0001 to
10000, 0.001 to 1000, and 0.01 to 10. In other embodiments, the tetravalent
metal to alumina
ratio is in the range of 0.01 to 10, including 0.01 to 10, 0.01: to 5,0.01 to
2, and 0.01 to 1. In
specific embodiments, the tetravalent metal to alumina ratio is in the range
of 0.01 to 2.
[00150] In specific embodiments where the molecular sieve is isomorphously
substituted
with a tetravalent metal, the tetravalent metal comprises titanium, and the
titania to alumina
ratio is in the range of 0.0001 to 10000, including 0,0001 to 10000, 0.001 to
1000, and 0.01 to
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
10. In other embodiments, the titania to alumina ratio is in the range of 0.01
to 10, including
0.01 to 10, 0.01: to 5, 0.01 to 2, and 0.01 to 1. In specific embodiments, the
titania to alumina
ratio is in the range of 0.01 to 2.
1001511 The ratio of silica to tetravalent metal can vary over a wide range.
It is noted that
5 this ratio is an atomic ratio, not a molar ratio. In one or more
embodiments, the silica to
tetravalent metal ratio is in the range of 1 to 100, including 1 to 50, 1 to
30, 1 to 25, 1 to 20, 5
to 20, and 10 to 20. In specific embodiments, the silica to tetravalent metal
ratio is about 15.
In one or more embodiments, the tetravalent metal comprises titanium, and the
silica to titania
ratio is in the range of 1 to 100, including 1 to 50, 1 to 30, 1 to 25, 1 to
20, 5 to 20, and 10 to
10 20. In specific embodiments, the silica to titania ratio is about 15.
Promoter Metals:
[00152] The molecular sieve of one or more embodiments may be subsequently ion-
15 exchanged with one or more promoter metals such as iron, copper, cobalt,
nickel, cerium or
platinum group metals. Synthesis of zeolites and related micro- and mesoporous
materials
varies according to the structure type of the zeolitic material, but typically
involves the
combination of several components (e.g. silica, alumina, phosphorous, alkali,
organic template
etc.) to than a synthesis gel, which is then hydrothermally crystallized to
form a final product.
20 The structure directing agent can be in the form of an organic, i.e.
tetraethylammonium
hydroxide (TEAOH), or inorganic cation, i.e. Na + or IC+. During
crystallization, the tetrahedral
units organize around the SDA to fonn the desired framework, and the SDA is
often embedded
within the pore structure of the zeolite crystals. In one or more embodiments,
the
crystallization of the molecular sieve can be obtained by means of the
addition of structure-
.. directing agents/templates, crystal nuclei or elements. In some instances,
the crystallization
can be performed at temperatures of less than 100 C.
[00153] As used herein, "promoted" refers to a component that is intentionally
added to the
molecular sieve, as opposed to impurities inherent in the molecular sieve.
Thus, a promoter is
intentionally added to enhance activity of a catalyst compared to a catalyst
that does not have
promoter intentionally added. In order to promote the SCR of oxides of
nitrogen, in one or
more embodiments, a suitable metal is exchanged into the molecular sieve.
According to one
or more embodiments, the molecular sieve is promoted with a metal selected
from Cu, Fe, Co,
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
21
Ni, La, Ce, Mn, V, Ag, and combinations thereof. In specific embodiments, the
molecular
sieve is promoted with Cu, Fe, and combinations thereof.
[00154] The promoter metal content of the molecular sieve, calculated as the
oxide, is, in
one or more embodiments, at least about 0.1 wt.%, reported on a volatile-free
basis. In specific
embodiments, the promoter metal comprises Cu, and the Cu content, calculated
as CuO is in
the range of up to about 10 wt.%, including 9, 8, 7, 6, 5, 4, 3, 2, 1, 0.5,
and 0.1 wt.%, in each
case based on the total weight of the calcined molecular sieve reported on a
volatile free oxide
basis. In specific embodiments, the Cu content, calculated as CuO, is in the
range of about 2 to
about 5 wt.%. In more specific embodiments, the molecular sieve having this
specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
range of about 100 to about 250 nm.
[00155] For specific molecular sieves having an SAR of 2 to 300, the Cu
content may be in
the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4 wt.%,
or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
free oxide basis. In more specific embodiments, the molecular sieve having
this specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
.. about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
range of about 100 to about 250 nm.
[00156] For specific molecular sieves having an SAR of 5 to 250, the Cu
content may be in
the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4 wt.%,
or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
free oxide basis. In more specific embodiments, the molecular sieve having
this specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
range of about 100 to about 250 nm.
.. 1001571 For specific molecular sieves having an SAR of 5 to 200, the Cu
content may be in
the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4 wt.%,
or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
22
free oxide basis. In more specific embodiments, the molecular sieve having
this specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
.. range of about 100 to about 250 nm.
[00158] For specific molecular sieves having an SAR of 5 to 100, the Cu
content may be in
the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4 wt.%,
or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
free oxide basis. In more specific embodiments, the molecular sieve having
this specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
range of about 100 to about 250 nm.
[00159] For specific molecular sieves having an SAR of 5 to 50, the Cu content
may be in
the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4 wt.%,
or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
free oxide basis. In more specific embodiments, the molecular sieve having
this specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
range of about 100 to about 250 nm.
[00160] For specific molecular sieves having an SAR of 10 to 250, the Cu
content may be in
the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4 wt.%,
or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
free oxide basis. In more specific embodiments, the molecular sieve having
this specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
range of about 100 to about 250 nm.
1001611 For specific molecular sieves having an SAR of 10 to 200, the Cu
content may be in
the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4 wt.%,
or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
23
free oxide basis. In more specific embodiments, the molecular sieve having
this specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
range of about 100 to about 250 nm.
[00162] For specific molecular sieves having an SAR of 10 to 100, the Cu
content may be in
the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4 wt.%,
or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
free oxide basis. In more specific embodiments, the molecular sieve having
this specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
range of about 100 to about 250 nm.
[00163] For specific molecular sieves having an SAR of 10 to 75, the Cu
content may be in
the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4 wt.%,
or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
free oxide basis. In more specific embodiments, the molecular sieve having
this specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
range of about 100 to about 250 nm.
[00164] For specific molecular sieves having an SAR of 10 to 60, the Cu
content may be in
the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4 wt.%,
or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
free oxide basis. In more specific embodiments, the molecular sieve having
this specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
range of about 100 to about 250 nm.
1001651 For specific molecular sieves having an SAR of 10 to 50, the Cu
content may be in
the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4 wt.%,
or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
24
free oxide basis. In more specific embodiments, the molecular sieve having
this specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
range of about 100 to about 250 nm.
[00166] For specific molecular sieves having an SAR of 15 to 100, the Cu
content may be in
the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4 wt.%,
or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
free oxide basis. In more specific embodiments, the molecular sieve having
this specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
range of about 100 to about 250 nm.
[00167] For specific molecular sieves having an SAR of 15 to 75, the Cu
content may be in
the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4 wt.%,
or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
free oxide basis. In more specific embodiments, the molecular sieve having
this specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
range of about 100 to about 250 nm.
[00168] For specific molecular sieves having an SAR of 15 to 60, the Cu
content may be in
the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4 wt.%,
or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
free oxide basis. In more specific embodiments, the molecular sieve having
this specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
range of about 100 to about 250 nm.
1001691 For specific molecular sieves having an SAR of 15 to 50, the Cu
content may be in
the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4 wt.%,
or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
free oxide basis. In more specific embodiments, the molecular sieve having
this specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
5 range of about 100 to about 250 nm.
[00170] For specific molecular sieves having an SAR of 20 to 100, the Cu
content may be in
the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4 wt.%,
or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
free oxide basis. In more specific embodiments, the molecular sieve having
this specific
10 combination of SAR and Cu content, the spherical particle of the
molecular sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
range of about 100 to about 250 nm.
[00171] For specific molecular sieves having an SAR of 20 to 75, the Cu
content may be in
15 the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4
wt.%, or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
free oxide basis. In more specific embodiments, the molecular sieve having
this specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
20 about 3.5 microns, and the individual crystals of a molecular sieve have
a crystal size in the
range of about 100 to about 250 nm.
[00172] For specific molecular sieves having an SAR of 20 to 60, the Cu
content may be in
the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4 wt.%,
or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
25 free oxide basis. In more specific embodiments, the molecular sieve
having this specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
range of about 100 to about 250 nm.
[00173] For specific molecular sieves having an SAR of 20 to 50, the Cu
content may be in
the range of 0.1-10 wt.%, or 0.5 to 8 wt.%, or 0.8 to 6 wt.%, or 1 to 4 wt.%,
or even 2-3 wt.%
in each case based on the total weight of the calcined molecular sieve
reported on a volatile
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
26
free oxide basis. In more specific embodiments, the molecular sieve having
this specific
combination of SAR and Cu content, the spherical particle of the molecular
sieve has a median
particle size in the range of about 0.5 to about 5 microns, and more
specifically, about 1.2 to
about 3.5 microns, and the individual crystals of a molecular sieve have a
crystal size in the
range of about 100 to about 250 nm.
[00174] Without intending to be bound by theory, it is thought that when the
molecular
sieve is isomorphously substituted with a tetravalent metal, the tetravalent
metal is embedded
into the zeolitic framework as a tetrahedral atom, allowing for close coupling
to the active
promoter metal center both structurally and electronically. In one or more
embodiments, the
promoter metal can be ion exchanged into the isomorphously substituted
molecular sieve. In
specific embodiments, copper is ion exchanged into the isomorphously
substituted molecular
sieve. The metal can be exchanged after the preparation or manufacture of the
isomorphously
substituted molecular sieve.
Porosity And Particle Shape And Size:
[00175] In one or more embodiments, the catalyst material comprises a
spherical particle
including an agglomeration of crystals of a molecular sieve. As used herein,
the terms
"agglomerate" or "agglomeration" refer to a cluster or collection of primary
particles, i.e.
crystals of molecular sieve.
[00176] In one or more embodiments, the spherical particle has a median
particle size in the
range of about 0.5 to about 5 microns, including 0.5, 0.6, 0.7, 0.8, 0.9, 1.0,
1.1, 1.2, 1.25, 1.3,
1.35, 1.4, 1.45, 1.5, 1.75, 2.0, 2.25, 2.5, 2.75, 3.0, 3.25, 3.5, 3.75, 4,
4.24, 4.5, 4.75, and 5
microns. The particle size of the spherical particle can be measured by a
microscope, and more
particularly a scanning electron microscope (SEM). In one or more specific
embodiments, the
spherical particle has a median particle size in the range of about 1.0 to
about 5 microns,
including a range of about 1.2 to about 3.5 microns. As used herein, the term
"median particle
size" refers to the median cross-sectional diameter of the spherical
particles. In one or more
embodiments, at least 80% of the spherical particles have a median particle
size in the range of
0.5 to 2.5 microns.
[00177] In one or more embodiments, the individual crystals of molecular sieve
have a
crystal size in the range of about 1 to about 250 nm, including 1, 5, 10, 20,
30, 40, 50, 60, 70,
80, 90, 100, 110, 120, 130, 140, 150, 160, 170, 180, 190, 200, 210, 220, 230,
240, and 250 nm.
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
27
The crystal size of the individual crystals of molecular sieve can be measured
by a microscope,
and more particularly a scanning electron microscope (SEM). In specific
embodiments, the
individual crystals of a molecular sieve have a crystal size in the range of
about 100 to about
250 rim, or about 100 to about 200 nm. Generally, there are no specific
restrictions as far as
the shape of the individual crystals of molecular sieve is concerned. In one
or more
embodiments, the individual crystals of molecular sieve, without limitation,
may be cubic,
spherical, platelet, needle-like, isometric, octahedral, tetragonal,
hexagonal, orthorhombic,
trigonal, and the like, or any combination thereof.
[00178] Without intending to be bound by theory, in one or more embodiments,
it is thought
that the catalyst material has a monodispersed snowball structure. As used
herein, a
monodispersed snowball refers to an arrangement or collection of a number of
individual
molecular sieve crystals into a substantially spherical mass. As used herein,
the term
"monodispersed" means that the individual molecular sieve crystals are uniform
and
approximately the same size, having a crystal size in the range of about 1 to
about 250
nanometers, The monodispersed snowball is similar to individual snow particles
forming a
snowball. In other embodiments, the catalyst material has a spherical snowball
structure,
wherein at least 80% of the spherical particle has a median particle size in
the range of 0.5 to
2.5 microns.
[00179] In one or more embodiments, the individual crystals of molecular sieve
form a
microagglomerate, which then forms a macro agglomerated snowball structure. In
one or more
embodiments, the microagglomerates have a size in the range of less than 1.0
micron,
including less than 0.9, less than 0.8, less than 0.7, less than 0.6, less
than 0.5, less than 0.4,
less than 0.3, less than 0.2, and less than 0.1 micron, and the
macroagglomerate spherical
snowball has a particle size in the range of about 0,5 to about 5 microns,
including about 1.2 to
about 3.5 microns. The size of the microagglomerates can be measured by a
microscope, and
more particularly a scanning electron microscope (SEM).
[00180] In one or more embodiments, the molecular sieve comprises an
isomorphously
substituted zeolitic framework material wherein a fraction of the silicon
atoms are
isomorphously substituted with a tetravalent metal. The isomorphously
substituted zeolite
framework material according to embodiments of the invention may be provided
as a
washcoat. The isomorphously substituted zeolitic framework material provides a
washcoat
that is generally very porous. The particle size of the isomorphously
substituted zeolitic
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
28
framework material is generally in the range of 1 to 2 gm. Additionally,
without intending to
be bound by theory, it is thought that the presence of the tetravalent metal,
specifically
titanium, controls the zeolitic crystal such that a mono-dispersed snowball
structure results. In
other words, the molecular sieve includes an agglomeration of crystals of a
molecular sieve
that is isomorphously substituted with a tetravelent metal. As is apparent to
one of ordinary
skill in the art, the particles of the molecular sieve comprising an
isommphously substituted
zeolitic framework material are significantly larger than molecular sieves
having the CHA
structure prepared according to conventional methods known in the art. Such
conventionally
prepared molecular sieves are known to have a particle size less than about
0.5 gm.
[00181] The monodispersed snowball structure of one or more embodiments may be
more
readily understood by the schematic in FIG. 1. Referring to FIG. 1, an
exemplary embodiment
of a catalyst material is shown. The catalyst material comprises a spherical
particle 10
including an agglomeration of molecular sieve crystals 20. The spherical
particle 10 has a
particle size, Sp, of about 0.5 to about 5 microns, including about 1.2 to
about 3.5 microns.
The individual crystals 20 of a molecular sieve have a crystal size S, in the
range of about 1 to
about 250 nanometers, including about 100 to 250 urn, or 100 to 200 nm. hi one
or more
embodiments, the individual crystals 20 of molecular sieve form a
microagglomerate 30, which
then forms the macro agglomerated snowball structure 10. The micro agglomerate
30 has a size
Sn, in the range of less than 1.0 micron and greater than 0 microns.
[00182] As is apparent to one of ordinary skill in the art, the spherical
particles of the
crystals of molecular sieve are significantly different in structure than
molecular sieves having
the CHA structure which do not have an agglomerated snowball structure.
[00183] The catalyst material according to embodiments of the invention may be
provided
in the form of a powder or a sprayed material from separation techniques
including
decantation, filtration, centrifugation, or spraying.
[00184] In general, the powder or sprayed material can be shaped without any
other
compounds, e.g. by suitable compacting, to obtain moldings of a desired
geometry, e.g. tablets,
cylinders, spheres, or the like.
100185] By way of example, the powder or sprayed material is admixed with or
coated by
suitable modifiers well known in the art. By way of example, modifiers such as
silica,
alumina, zeolites or refractory binders (for example a zirconium precursor)
may be used. The
powder or the sprayed material, optionally after admixing or coating by
suitable modifiers,
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
29
may be formed into a slurry, for example with water, which is deposited upon a
suitable
refractory carrier, for example, a flow through honeycomb substrate carrier or
a wall flow
honeycomb substrate carrier.
[00186] The catalyst material according to embodiments of the invention may
also be
.. provided in the form of extrudates, pellets, tablets, or particles of any
other suitable shape, for
use as a packed bed of particulate catalyst, or as shaped pieces such as
plates, saddles, tubes, or
the like.
SCR Catalyst Composite:
.. [00187] Governmental regulations mandate the use of NO1 reduction
technologies for light and
heavy-duty vehicles. Selective catalytic reduction (SCR) of NO1 using ammonia
is an effective
and dominant emission control technology for NO. control. In an exemplary
embodiment,
provided is an SCR catalyst composite having enhanced ammonia storage capacity
at
temperatures of 400 C and above, and the capability to promote ammonia
storage over water.
While the catalyst material of one or more embodiments can be used in any lean
burn engine,
including diesel engines, lean burn gasoline direct injection engines, and
compressed natural
gas engines, in specific embodiments, the catalyst materials are to be used in
lean burn
gasoline direct injection (GDI) engines.
[00188] Embodiments of the invention are directed to a catalyst composite
comprising a SCR
catalyst material and an ammonia storage material comprising a transition
metal having an
oxidation state of IV. The SCR catalyst composite is effective to store
ammonia at 400 C and
above with a minimum NH3 storage of 0.1 g/L at 400 C. In one or more
embodiments, the
SCR catalyst material promotes the reaction of ammonia with nitrogen oxides to
form nitrogen
and H20 selectively over a temperature range of 150 C to 600 C, and the
ammonia storage
material is effective to store ammonia at 400 C and above with a minimum NH3
storage of 0.1
g/L at 400 C. It was surprisingly found that the catalyst composites are
particularly suitable in
exhaust gas purification catalyst components, in particular as SCR catalysts.
[00189] According to one or more embodiments, a SCR catalyst composite
comprises a
SCR catalyst material and an ammonia storage material. In one or more
embodiments, the
SCR catalyst material comprises one or more of a molecular sieve, a mixed
oxide, and an
activated refractory metal oxide support.
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
[00190] In one or more embodiments, the SCR catalyst material comprises a
molecular
sieve. According to one or more embodiments, the ammonia storage material
comprises
transition metal having an oxidation state of IV. Without intending to be
bound by theory, it is
thought that the presence of an element with a formal oxidation state of IV
helps to increase
5 ammonia storage at high temperature. In one or more embodiments, the
transition metal
having an oxidation state of IV can either be in oxide form, or intrinsically
embedded in the
SCR catalyst material. As used herein, the term "transition metal having an
oxidation state of
IV" refers to a metal having a state with four electrons available for
covalent chemical bonding
in its valence (outermost electron shell). Transition metals having an
oxidation state of IV
10 include germanium (Ge), cerium (Cc), and those transition metals located
in Group 4 of the
periodic table, titanium (Ti), zirconium (Zr), and hafnium (HI). In one or
more embodiments,
the transition metal having an oxidation state of IV is selected from Ti, Ce,
Zr, Hf, Ge, and
combinations thereof In specific embodiments, the transition metal having an
oxidation state
of IV comprises Ti.
15 [00191] One or more embodiments of the present invention are directed to
an SCR catalyst
composite comprising an SCR catalyst material and an ammonia storage material
comprising a
transition metal having an oxidation state of IV, wherein the SCR catalyst
material and the
ammonia storage material are in a layered arrangement or relationship. In one
or more
embodiments, the ammonia storage material can be in any flexible font', e.g.
layered or
20 uniformly mixed with the SCR catalyst material, and intrinsically
implemented within the
same SCR catalyst material. According to one or more embodiments, the ammonia
storage
material is dispersed as a layer on top of the SCR catalyst material.
According to one or more
embodiments, the SCR catalyst material is washcoated onto a substrate, and
then the ammonia
storage material is washcoated in a layer overlying the SCR catalyst material.
25 [00192] In other embodiments, the SCR catalyst material and the ammonia
storage material
are arranged in a zoned configuration. In one or more embodiments, the SCR
catalyst material
and the ammonia storage material are arranged in a laterally zoned
configuration, with the
ammonia storage material upstream from the SCR catalyst material. As used
herein, the term
"laterally zoned" refers to the location of the SCR catalyst material and the
ammonia storage
30 material relative to one another. Lateral means side-by-side such that the
SCR catalyst
material and the ammonia storage material are located one beside the other
with the ammonia
storage material upstream of the SCR catalyst material. As used herein, the"
tcmis "upstream"
CA 02952435 2016-12-14
WO 2015/195809 PCT1US2015/036243
31
and "downstream" refer to relative directions according to the flow of an
engine exhaust gas
stream from an engine towards a tailpipe, with the engine in an upstream
location and the
tailpipe and any pollution abatement articles such as filters and catalysts
being downstream
from the engine. According to one or more embodiments, the laterally zoned
ammonia storage
material and SCR catalyst material can be arranged on the same or a common
substrate or on
different substrates separated from each other.
[00193] In still further embodiments, the SCR catalyst material is ion-
exchanged with the
ammonia storage material.
[00194] In one or more embodiments, when in a layered or zoned arrangement,
the
transition metal having an oxidation state of IV can be present in an oxide
form, can be ion-
exchanged, or can be isomorphously substituted at a zeolitic framework
position. For example,
in specific embodiments, the transition metal having an oxidation state of IV
comprises
titanium. In such embodiments where the transition metal having an oxidation
state of TV is
present in the oxide form, the ammonia storage material comprising a
transition metal having
.. an oxidation state of IV is dispersed over a support material.
[00195] Referring to FIG. 2, an exemplary embodiment of a laterally zoned
system is
shown. The SCR catalyst composite 200 is shown in a laterally zoned
arrangement where the
ammonia storage material 210 is located upstream of the SCR catalyst material
220 on a
common substrate 230. The substrate 230 has an inlet end 240 and an outlet end
250 defining
an axial length L. In one or more embodiments, the substrate 230 generally
comprises a
plurality of channels 260 of a honeycomb substrate, of which only one channel
is shown in
cross-section for clarity. The ammonia storage material 210 extends from the
inlet end 240 of
the substrate 230 through less than the entire axial length L of the substrate
230. The length of
the ammonia storage material 210 is denoted as first zone 210a in FIG, 2. The
ammonia
storage material 210 comprises a transition metal having an oxidation state of
IV. The SCR
catalyst material 220 extends from the outlet end 250 of the substrate 230
through less than the
entire axial length L of the substrate 230. The length of the SCR catalyst
material 220 is
denoted as the second zone 220a in FIG. 2. The SCR catalyst material 220
promotes the
reaction of ammonia with nitrogen oxides to form nitrogen and H20 selectively
over a
temperature range of 150 C to 600 C, and the ammonia storage material 210 is
effective to
store ammonia at 400 C and above with a minimum NH3 storage of 0.00001 g/L.
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
32
[00196] It will be appreciated that the length of the first zone 210a and the
second zone 220a
can be varied. In one or more embodiments, the first zone 210a and second zone
220a can be
equal in length. In other embodiments, the first zone can be 20%, 25%, 35% or
40%, 60%,
65%, 75% or 80% of the length L of the substrate, with the second zone
respectively covering
.. the remainder of the length L of the substrate.
[00197] Referring to FIG. 3, another embodiment of a laterally zoned SCR
catalyst
composite 110 is shown. The SCR catalyst composite 110 shown is a laterally
zoned
arrangement where the ammonia storage material 118 is located upstream of the
SCR catalyst
material 120 on separate substrates 112 and 113. The ammonia storage material
118 is
disposed on a substrate 112, and the SCR catalyst material is disposed on a
separate substrate
113. The substrates 112 and 113 can be comprised of the same material or a
different material.
The substrate 112 has an inlet end 122a and an outlet end 124a defining an
axial length Ll.
The substrate 113 has an inlet end 122b and an outlet end 124b defining an
axial length L2. In
one or more embodiments, the substrates 112 and 113 generally comprise a
plurality of
channels 114 of a honeycomb substrate, of which only one channel is shown in
cross-section
for clarity. The ammonia storage material 118 extends from the inlet end 122a
of the substrate
112 through the entire axial length L 1 of the substrate 112 to the outlet end
124a. The length
of the ammonia storage material 118 is denoted as first zone 118a in FIG. 3.
The ammonia
storage material 118 comprises a transition metal having an oxidation state of
IV. The SCR
catalyst material 120 extends from the outlet end 124b of the substrate 113
through the entire
axial length L2 of the substrate 113 to the inlet end 122b. The SCR catalyst
material 120
defines a second zone 120a. The length of the SCR catalyst material is denoted
as the second
zone 20b in FIG. 3. The SCR catalyst material 120 promotes the reaction of
ammonia with
nitrogen oxides to form nitrogen and H20 selectively over a temperature range
of 150 C to
600 C, and the ammonia storage material 118 is effective to store ammonia at
400 C and
above with a minimum NH3 storage of 0.00001 g/L. The length of the zones 118a
and 120a
can be varied as described with respect to FIG. 2.
[00198] In one or more embodiments, the SCR catalyst composite, comprising the
ammonia
storage material and the SCR catalyst material, is coated on a flow through or
wall-flow filter.
FIGS. 4A and 4B illustrate a wall flow filter substrate 35 which has a
plurality of passages 52.
The passages are tubularly enclosed by the internal walls 53 of the filter
substrate. The
substrate has an inlet end 54 and an outlet end 56. Alternate passages are
plugged at the inlet
33
end with inlet plugs 58, and at the outlet end with outlet plugs 60 to foun
opposing checkerboard
patterns at the inlet 54 and outlet 56. A gas stream 62 enters through the
unplugged channel inlet 64,
is stopped by outlet plug 60 and diffuses through channel walls 53 (which are
porous) to the outlet
side 66. The gas cannot pass back to the inlet side of walls because of inlet
plugs 58.
[00199] In one or more embodiments, wall flow filter substrates are
composed of ceramic-
like materials such as cordierite, silicon carbide, silicon nitride, zirconia,
mullite, spodumene, alumina-
silica-magnesia or zirconium silicate, or of porous, refractory metal. In
other embodiments, wall flow
substrates are formed of ceramic Liber composite materials. In specific
embodiments, wall flow
substrates are formed from cordierite and silicon carbide. Such materials are
able to withstand the
environment, particularly high temperatures, encountered in treating the
exhaust streams.
[00200] In one or more embodiments, wall flow substrates include thin
porous walled
honeycombs monoliths through which the fluid stream passes without causing too
great an increase in
back pressure or pressure across the article. Normally, the presence of a
clean wall flow article will
create a back pressure of 1 inch water column to 10 psig. Ceramic wall flow
substrates used in the
system are formed of a material having a porosity of at least 50% (e.g., from
50 to 75%) having a mean
pore size of at least 5 microns (e.g., from 5 to 30 microns). In one or more
embodiments, the substrates
have a porosity of at least 55% and have a mean pore size of at least 10
microns. When substrates with
these porosities and these mean pore sizes are coated with the techniques
described below, adequate
levels of catalyst compositions can be loaded onto the substrates to achieve
excellent NO. conversion
efficiency. These substrates are still able to retain adequate exhaust flow
characteristics, i.e., acceptable
back pressures, despite the SCR catalyst loading. United States Patent No.
4,329,162.
[00201] Typical wall flow filters in commercial use are formed with lower
wall porosities, e.g.,
from about 35% to 50%, than the wall flow filters utilized in the invention.
In general, the pore size
distribution of commercial wall flow filters is typically very broad with a
mean pore size smaller than
17 microns.
[00202] The porous wall flow filter used in one or more embodiments is
catalyzed in that the wall
of said element has thereon or contained therein one or more SCR catalytic
materials. Catalytic materials
may be present on the inlet side of the element wall alone, the outlet side
Date Recue/Date Received 2021-10-20
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
34
alone, both the inlet and outlet sides, or the wall itself may consist all, or
in part, of the
catalytic material. This invention includes the use of one or more layers of
catalytic materials
and combinations of one or more layers of catalytic materials on the inlet
and/or outlet walls of
the element.
[00203] To coat the wall flow substrates with the SCR catalyst composite of
one or more
embodiments, the substrates are immersed vertically in a portion of the
catalyst slurry such that
the top of the substrate is located just above the surface of the slurry. In
this manner slurry
contacts the inlet face of each honeycomb wall, but is prevented from
contacting the outlet face
of each wall. The sample is left in the slurry for about 30 seconds. The
substrate is removed
from the slurry, and excess slurry is removed from the wall flow substrate
first by allowing it
to drain from the channels, then by blowing with compressed air (against the
direction of slurry
penetration), and then by pulling a vacuum from the direction of slurry
penetration. By using
this technique, the catalyst slurry permeates the walls of the substrate, yet
the pores are not
occluded to the extent that undue back pressure will build up in the finished
substrate. As used
herein, the term "permeate" when used to describe the dispersion of the
catalyst slurry on the
substrate, means that the catalyst composition is dispersed throughout the
wall of the substrate.
[00204] The coated substrates are dried typically at about 100 C and calcined
at a higher
temperature (e.g., 300 to 450 C). After calcining, the catalyst loading can
be determined
through calculation of the coated and uncoated weights of the substrate. As
will be apparent to
those of skill in the art, the catalyst loading can be modified by altering
the solids content of
the coating slurry. Alternatively, repeated immersions of the substrate in the
coating slurry can
be conducted, followed by removal of the excess slurry as described above.
[00205] According to one or more embodiments, the ammonia storage material of
the SCR
catalyst composite is dispersed within the SCR catalyst material. Thus,
according to
embodiments of the invention, the SCR catalyst material comprises a molecular
sieve having a
framework of silicon (Si) and aluminum (Al) ions, and, optionally phosphorus
(P) ions, wherein a
fraction of the silicon atoms are isomorphously substituted with the ammonia
storage material
which comprises a transition metal having an oxidation state of IV.
[00206] In one or more embodiments, an ammonia oxidation (AM0x) catalyst may
be
provided downstream of the SCR catalyst composite to remove any slipped
ammonia from the
exhaust gas treatment system. In specific embodiments, the AMOx catalyst may
comprise a
platinum group metal such as platinum, palladium, rhodium, or combinations
thereof.
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
[00207] AMOx and/or SCR catalyst material(s) can be coated on the flow through
or wall-
flow filter. If a wall flow substrate is utilized, the resulting system will
be able to remove
particulate matter along with gaseous pollutants. The wall-flow filter
substrate can be made
from materials commonly known in the art, such as cordierite, aluminum
titanate or silicon
5 carbide. It will be understood that the loading of the catalytic
composition on a wall flow
substrate will depend on substrate properties such as porosity and wall
thickness, and typically
will be lower than loading on a flow through substrate.
[00208] In one or more embodiments, a fraction of the silicon atoms are
isomorphously
substituted with a transition metal having an oxidation state of IV. In other
words, a fraction of
10 the silicon atoms in the zcolitic framework material are being replaced
with a transition metal
having an oxidation state of IV. Such isomorophous substitution does not
significantly alter
the crystal structure of the zeolitic framework material.
[00209] Typically, NH3 storage over zeolite SCR catalysts needs to be
suppressed in order
to gain faster NO. conversion response for the highly dynamic engine
operations. Without
15 intending to be bound by theory, it is thought that with the SCR
catalysts of the prior art, it is
impossible to achieve the required high temperature NH3 storage by relying on
the weak NH3
physisorption in the porosity of zeolites, or the Bronsted acidity of unused
exchange sites due
to the presence of relatively high amounts of competing water vapor.
[00210] Therefore, it is necessary to implement a secondary functional
site, i.e., utilization
20 of Lewis acidity, which is capable of performing high temperature NH3
storage and which is
capable of differentiating NH3 and H20 for storage. It is thought that because
NH3, by nature,
is nucleophilic (or, more generally, basic), Lewis acidity can provide an
additional route for
NH3 storage. Accordingly, transition metals with different oxidation states
can provide tunable
strength of Lewis acidity. In general, the higher the oxidation state of the
transition metal, the
25 stronger Lewis acidity is expected. Thus, it is believed that a
transition metal having an
oxidation state of IV will produce catalyst materials where NH3 can be stored
at higher
temperatures.
[00211] In one or more embodiments, the SCR catalyst material comprises a
molecular
sieve which comprises SiO4/A104 tetrahedra. In one or more embodiments, the
SCR catalyst
30 material is isomorphously substituted with the ammonia storage material. In
such
embodiments, the SCR catalyst material comprises M04/SiO4/A104 tetrahedra
(where M is a
transition metal having an oxidation state of IV) and is linked by common
oxygen atoms to
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
36
form a three-dimensional network. The isomorphously substituted transition
metal having an
oxidation state of IV is embedded into the molecular sieve as a tetrahedral
atom (M04). The
isomorphously substituted tetrahedron units together with the silicon and
aluminum
tetrahedron units then form the framework of the molecular sieve. In specific
embodiments,
the transition metal having an oxidation state of IV comprises titanium, and
the SCR catalyst
material then includes TiO4/SiO4/A104 tetrahedra.
[00212] In other embodiments, the SCR catalyst material comprises a molecular
sieve which
comprises SiO4/A104/PO4 tetrahedra. In one or more embodiments, the SCR
catalyst material
is isomorphously substituted with the ammonia storage material. In such
embodiments, the
SCR catalyst material comprises M04/SiO4/A104/PO4 tetrahedra (where M is a
transition metal
having an oxidation state of IV) and is linked by common oxygen atoms to form
a three-
dimensional network. The isomorphously substituted transition metal having an
oxidation
state of IV is embedded into the molecular sieve as a tetrahedral atom (M04).
The
isomorphously substituted tetrahedron units together with the silicon,
aluminum, and
phosphorus tetrahedron units then form the framework of the molecular sieve.
In specific
embodiments, the transition metal having an oxidation state of IV comprises
titanium, and the
SCR catalyst material then includes TiO4/SiO4/A104/PO4 tetrahedra.
[00213] The isomorphously substituted molecular sieve of one or more
embodiments is
differentiated mainly according to the geometry of the voids which are formed
by the rigid
network of the M04/(SiO4)/A104 tetrahedra (where M is a transition metal
having an oxidation
state of IV).
[00214] In one or more embodiments, the molecular sieve of the SCR catalyst
material has a
structure-type selected from any of those previously discussed. In one or more
specific
embodiments, the molecular sieve has a structure type selected from MFI, BEA,
AEI, AFT,
AFX, CHA, EAB, EMT, ERI, FAU, GME, JSR, KFI, LEV, LTL, LTN, MOZ, MSO, MWW,
OFF, SAS, SAT, SAV, SBS, SBT, SFW, SSF, SZR, TSC, WEN, and combinations
thereof. In
other specific embodiments, the molecular material has a structure type
selected from the
group consisting of MFI, BEA, CHA, AEI, AFX, ERI, KFI, LEV, and combinations
thereof. In
very specific embodiments, the molecular sieve has a structure type selected
from CHA, AEI,
and AFX. In very specific embodiments, the molecular sieve comprises SSZ-13,
SSZ-39, or
SAPO-34. In another very specific embodiment, the molecular sieve is an
aluminosilicate
zeolite type and has the AEI structure type, for example, SSZ-39. According to
one or more
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
37
embodiments, it will be appreciated that by defining the molecular sieves by
their structure
type, it is intended to include the structure type and any and all isotypic
framework materials
such as SAPO, AIPO and MeAPO materials having the same structure type.
[00215] The ratio of silica to alumina of a molecular sieve can vary over a
wide range. In
one or more embodiments, the molecular sieve has a silica to alumina molar
ratio (SAR) in the
range of 2 to 300, including 5 to 250; 5 to 200; 5 to 100; and 5 to 50. In one
or more specific
embodiments, the molecular sieve has a silica to alumina molar ratio (SAR) in
the range of 10
to 200, 10 to 100, 10 to 75, 10 to 60, and 10 to 50; 15 to 100, 15 to 75, 15
to 60, and 15 to 50;
20 to 100, 20 to 75, 20 to 60, and 20 to 50.
[00216] The ratio of transition metal having an oxidation state of IV to
alumina can vary
over a very wide range. In one or more embodiments, the transition metal
having an oxidation
state of IV to alumina ratio is in the range of 0.001 to 10000, including
0.001:10000, 0.001 to
1000, 0.01 to 10. In other embodiments, the transition metal having an
oxidation state of IV to
alumina ratio is in the range of 0.01 to 10, including 0.01 to 10, 0.01: to 5,
0.01 to 2, and 0.01
to 1. In specific embodiments, the transition metal having an oxidation state
of IV to alumina
ratio is in the range of 0.01 to 2.
[00217] In specific embodiments, the transition metal having an oxidation
state of IV
comprises titanium, and the titania to alumina ratio is in the range of 0.001
to 10000, including
0.001:10000, 0.001 to 1000, 0.01 to 10. In other embodiments, the titania to
alumina ratio is in
the range of 0.01 to 10, including 0.01 to 10, 0.01: to 5, 0.01 to 2, and 0.01
to 1. In specific
embodiments, the titania to alumina ratio is in the range of 0.01 to 2. In
very specific
embodiments, the titania to alumina ratio is about 1.
[00218] The ratio of silica to transition metal having an oxidation state of
IV can vary over a
wide range. It is noted that this ratio is an atomic ratio, not a molar ratio.
In one or more
embodiments, the silica to transition metal having an oxidation state of IV
ratio is in the range
of 1 to 100, including 1 to 50, 1 to 30, 1 to 25, 1 to 20, 5 to 20, and 10 to
20. In specific
embodiments, the silica to transition metal having an oxidation state of IV
ratio is about 15. In
one or more embodiments, the transition metal having an oxidation state of IV
comprises
titanium, and the silica to titania ratio is in the range of 1 to 100,
including 1 to 50, 1 to 30, 1 to
25, 1 to 20, 5 to 20, and 10 to 20. In specific embodiments, the silica to
titania ratio is about
15.
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
38
[00219] In order to promote the SCR of oxides of nitrogen, in one or more
embodiments, a
suitable metal is exchanged into the SCR catalyst material. According to one
or more
embodiments, the SCR catalyst material is promoted with a metal selected from
Cu, Fe, Co,
Ni, La, Ce, Mn, V, Ag, and combinations thereof. In specific embodiments, the
SCR catalyst
.. material is promoted with Cu, Fe, and combinations thereof.
[00220] The promoter metal content of the SCR catalyst material, calculated as
the oxide, is,
in one or more embodiments, at least about 0.1 wt%, reported on a volatile-
free basis. In
specific embodiments, the promoter metal comprises Cu, and the Cu content,
calculated as
CuO is in the range of up to about 10 wt%, including 9, 8, 7, 6, 5, 4, 3,
2,and 1 wt %, in each
case based on the total weight of the calcined SCR catalyst material reported
on a volatile free
basis. In specific embodiments, the Cu content, calculated as CuO, is in the
range of about 2 to
about 5 wt%.
[00221] Without intending to be bound by theory, it is thought that when the
SCR catalyst
material is isomorphously substituted with the ammonia storage material
comprising a
transition metal having an oxidation state of IV, the transition metal having
an oxidation state
of IV is embedded into the molecular sieve framework as a tetrahedral atom,
allowing for close
coupling to the active promoter metal center both structurally and
electronically. In one or
more embodiments, the promoter metal can be ion exchanged into the SCR
catalyst material.
In specific embodiments, copper is ion exchanged into the SCR catalyst
material. The metal
can be exchanged after the preparation or manufacture of the SCR catalyst
material.
[00222] According to one or more embodiments, the SCR catalyst material
comprises a mixed
oxide. As used herein, the term "mixed oxide" refers to an oxide that contains
cations of more
than one chemical element or cations of a single element in several states of
oxidation. In one
or more embodiments, the mixed oxide is selected from Feititania (e.g.
FeTiO3), Fe/alumina
.. (e.g. FeA1203), Mg/titania (e.g. MgTiO3), Mg/alumina (e.g. MgA1203),
Mn/alumina, Mn/titania
(e.g. MnO/TiO2) (e.g. MnO/Al2O3), Cu/titania (e.g. CuTiO3), Ce/Zr (e.g.
CeZr02), Ti/Zr (e.g.
TiZr02), vanadia/titania (e.g. V205/TiO2), and mixtures thereof. In specific
embodiments, the
mixed oxide comprises vanadia/titania. The vanadia/titania oxide can be
activated or stabilized
with tungsten (e.g. W03) to provide V205/TiO2/ W03. In one or more
embodiments, the SCR
catalyst material comprises titania on to which vanadia has been dispersed.
The vanadia can be
dispersed at concentrations ranging from 1 to 10 wt%, including 1, 2, 3, 4, 5,
6, 7, 8, 9, lOwt%.
In specific embodiments the vanadia is activated or stabilized by tungsten
(W03). The
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
39
tungsten can be dispersed at concentrations ranging from 0.5 to 10 wt%,
including 1, 2, 3, 3. 4,
5, 6, 7, 8, 9, and 10 wt%. All percentages are on an oxide basis,
[00223] According to one or more embodiments, the SCR catalyst material
comprises a
refractory metal oxide support material. As used herein, the terms "refractory
metal oxide
support" and "support" refer to the underlying high surface area material upon
which additional
chemical compounds or elements are carried. The support particles have pores
larger than 20
A and a wide pore distribution. As defined herein, such metal oxide supports
exclude
molecular sieves, specifically, zeolites. In particular embodiments, high
surface area refractory
metal oxide supports can be utilized, e.g., alumina support materials, also
referred to as
"gamma alumina" or "activated alumina," which typically exhibit a BET surface
area in excess
of 60 square meters per gram ("m2/g"), often up to about 200 m2/g or higher.
Such activated
alumina is usually a mixture of the gamma and delta phases of alumina, but may
also contain
substantial amounts of eta, kappa and theta alumina phases. Refractory metal
oxides other than
activated alumina can be used as a support for at least some of the catalytic
components in a
given catalyst. For example, bulk ceria, zirconia, alpha alumina and other
materials are known
for such use. Although many of these materials suffer from the disadvantage of
having a
considerably lower BEI surface area than activated alumina, that disadvantage
tends to be
offset by a greater durability or performance enhancement of the resulting
catalyst. "BET
surface area" has its usual meaning of referring to the Brunauer, Emmett,
Teller method for
determining surface area by N2 adsorption. Pore diameter and pore volume can
also be
determined using BET-type N2 adsorption or desorption experiments.
[00224] One or more embodiments of the present invention include a high
surface area
refractory metal oxide support comprising an activated compound selected from
the group
consisting of alumina, ceria, zirconia, silica, titania, silica-alumina,
zirconia-alumina, titania-
alumina, lanthana-alumina, lanthana-zirconia-alumina, baria-alumina, baria-
lanthana-alumina,
baria-lanthana-neodyrnia-alumina, alumina-chromia, alumina-ceria, zirconia-
silica, titania-
silica, or zirconia-titania, and combinations thereof. In one or more
embodiments, the
activated refractory metal oxide support is exchanged with a metal selected
from the group
consisting of Cu, Fe, Co, Ni, La, Ce, Mn, V. Ag, and combinations thereof.
SCR Activity:
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
[00225] In one or more embodiments, the selective catalytic reduction material
comprising a
spherical particle including an agglomeration of crystals of a molecular sieve
exhibits an aged
NOõ conversion at 200 C of at least 50% measured at a gas hourly space
velocity of 8000010.
In specific embodiments the catalyst exhibits an aged NO, conversion at 450 C
of at least
5 70% measured at a gas hourly space velocity of 80000 11-1. More
specifically the aged NO,
conversion at 200 C is at least 55% and at 450 C at least 75%, even more
specifically the
aged NO, conversion at 200 C is at least 60% and at 450 C at least 80%,
measured at a gas
hourly volume-based space velocity of 80000 1371 under steady state conditions
at maximum
NH3-slip conditions in a gas mixture of 500 ppm NO, 500 ppm NH3, 10% 02, 5%
H20,
10 balance N2. The cores were hydrothcrmally aged in a tube furnace in a
gas flow containing
10%1-120, 10% 02, balance N2 at a space velocity of 4,0000 for 5h at 750 C.
[00226] The SCR activity measurement has been demonstrated in the literature,
see, for
example PCT Application Publication No. WO 2008/106519.
[00227] Furthermore, according to one or more embodiments, the catalyst
material is
15 effective to lower N20 make.
Formation of NO+ and Ammonia Storage:
[00228] Additionally, according to one or more embodiments, particularly when
the
molecular sieve comprises an isomorphously substituted zeolitic framework
material of silicon
20 and aluminum atoms, wherein a fraction of the silicon atoms are
isomorphously substituted
with a tetravalent metal, the material is effective to promote the formation
of NO+. Without
intending to be bound by theory, it is thought that the d6r unit of the
zeolitic framework
material is an important factor in facilitating NO formation due to the fact
that the d6r unit
promotes short-range promoter metal (e.g. Cu) migration/hopping between the
two six-member
25 ring mirror planes to generate suitable vacant positions for NO+, which
requires a stabilizing
coordination environment also provided by the d6r unit.
[00229] Furthermore, according to one or more embodiments, particularly when
the SCR
catalyst composite comprises a SCR catalyst material and an ammonia storage
material
comprising a transition metal having an oxidation state of IV, the SCR
catalyst material
30 promotes the reaction of ammonia with nitrogen oxides to form nitrogen
and H20 selectively
over a temperature range of 150 C to 600 C, and the ammonia storage material
is effective to
store ammonia at temperatures of about 400 C and above with a minimum ammonia
storage
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
41
of 0.00001 g/L. In one or more embodiments, the oxygen content of the exhaust
gas stream is
from 0 to 30% and the water content is from 1 to 20%. The SCR catalyst
composite according
to one or more embodiments adsorbs NH3 even in the presence of H20. The SCR
catalyst
composites of one or more embodiments show more pronounced high temperature
ammonia
storage capacity than reference SCR catalyst materials and catalyst
composites.
[00230] Water, also carrying electron lone pairs as a nucleophile, is the
biggest competitor
towards ammonia storage with Bronsted acid sites. In order to be efficiently
utilized by the
NO3 generated in the lean cycle of lean GDI engines, it is important to
increase the chemically
adsorbed NH3 amount, rather than the physically adsorbed NH3 amount. Without
intending to
be bound by theory, it is thought that the Lewis acidity of a transition metal
having an
oxidation state of IV increases the capability of the SCR catalyst composite
to chemically
adsorb ammonia. Thus, the SCR catalyst composites according to one or more
embodiments
have improved ammonia storage capability at temperatures of about 400 C and
above.
The Substrate:
1002311 In one or more embodiments, the catalyst materials can be applied to a
substrate as a
washcoat. As used herein, the term "substrate" refers to the monolithic
material onto which the
catalyst is placed, typically in the form of a washcoat. A washcoat is formed
by preparing a
slurry containing a specified solids content (e.g., 30-90% by weight) of
catalyst in a liquid
vehicle, which is then coated onto a substrate and dried to provide a washcoat
layer.
[00232] As used herein, the term "washcoat" has its usual meaning in the art
of a thin,
adherent coating of a catalytic or other material applied to a substrate
material, such as a
honeycomb-type carrier member, which is sufficiently porous to permit the
passage of the gas
stream being treated.
[00233] In one or more embodiments, the substrate is a ceramic or metal having
a
honeycomb structure. Any suitable substrate may be employed, such as a
monolithic substrate
of the type having fine, parallel gas flow passages extending there through
from an inlet or an
outlet face of the substrate such that passages are open to fluid flow there
through. The
passages, which are essentially straight paths from their fluid inlet to their
fluid outlet, are
defined by walls on which the catalytic material is coated as a washcoat so
that the gases
flowing through the passages contact the catalytic material. The flow passages
of the
monolithic substrate are thin-walled channels, which can be of any suitable
cross-sectional
42
shape and size such as trapezoidal, rectangular, square, sinusoidal,
hexagonal, oval, circular, etc. Such
structures may contain from about 60 to about 900 or more gas inlet openings
(i.e. cells) per square inch
of moss section.
[00234] The ceramic substrate may be made of any suitable refractory
material, e.g.
cordierite, cordierite-a-alumina, silicon nitride, zircon mullite, spodumene,
alumina-silica- magnesia,
zircon silicate, sillimanite, a magnesium silicate, zircon, petalite, a-
alumina, an aluminosilicate and the
like.
[00235] The substrates useful for the catalyst of embodiments of the
present invention may also
be metallic in nature and be composed of one or more metals or metal alloys.
The metallic substrates
may be employed in various shapes such as pellets, corrugated sheet or
monolithic form. Specific
examples of metallic substrates include the heat-resistant, base-metal alloys,
especially those in which
iron is a substantial or major component. Such alloys may contain one or more
of nickel, chromium,
and aluminum, and the total of these metals may advantageously comprise at
least about 15 wt. % of
the alloy, for instance, about 10 to 25 wt. % chromium, about 1 to 8 wt. % of
aluminum, and about 0 to
20 wt. % of nickel.
Preparation of Catalyst and Catalyst Materials:
Synthesis of Conventional CHA -type Molecular Sieves
[00236] A molecular sieve having the CHA structure may be prepared
according to various
techniques known in the art, for example United States Patent Nos. 4,544,538
(Zones) and 6,709,644
(Zones).
Optionally NH4-exchange to form NH4-Chabazite:
[00237] Optionally, the obtained alkali metal zeolite is NH4-exchanged to
form NH4-
Chabazite. The NH4- ion exchange can be carried out according to various
techniques known
in the art, for example Bleken, F.; Bjorgen, M.; Palumbo, L.; Bordiga, S.;
Svelle, S.; Lillerud,
K.-P.; and Olsbye, U. Topics in Catalysis 52, (2009), 218-228.
Synthesis of Snowball Molecular Sieves
[00238] A molecular sieve having a snowball-type morphology water can be
prepared from
adamantyltrimethylammonium hydroxide (ADAOH), aqueous sodium hydroxide,
aluminum
isopropoxide powder, and colloidal silica.
Synthesis of Isomorphousb, Substituted Zeolitic Framework Materials
Date Recue/Date Received 2021-10-20
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
43
[00239] According to one or more embodiments, methods for the synthesis of
selective
catalytic reduction catalyst materials comprising an isomorphously substituted
zeolitic
framework material are provided. Particularly, the catalyst material comprises
a zeolitic
framework material of silicon and aluminum atoms, wherein a fraction of the
silicon atoms are
isomorphously substituted with a tetravalent metal.
[00240] Generally, the sodium form of the isomorphously substituted zeolitic
framework
material can be prepared from a 0.03A1203:Si02:0.07TiO 2 :
0.06Na20:0.08ATMAOH:2.33H20
gel composition through autoclave hydrothermal synthesis. The product is
recovered by
filtration, and the template is removed by calcination. The final crystalline
material can be
characterized by x-ray diffraction studies.
[00241] The H-form can be prepared by calcination of the ammonia form, which
is obtained
through double Nfl4NO3 exchanges with the sodium form. The Ti level is
unchanged/stable
through the NH4NO3 exchange processes.
[00242] The copper promoted isomorphously substituted zeolitic framework can
be
prepared by ion exchange using the H-form and Cu(OAc)2 to achieve the desired
amount of
promoter metal.
Synthesis of Isotnorphously Substituted Molecular Sieves
[00243] According to one or more embodiments, methods for the synthesis of SCR
catalyst
composites comprising an SCR catalyst material comprising a molecular sieve
isomorphously
substituted with an ammonia storage material comprising a transition metal
having an
oxidation state of IV are provided. Particularly, the SCR catalyst composite
comprises an SCR
catalyst material having a zeolitic framework material of silicon and aluminum
atoms, wherein
a fraction of the silicon atoms are isomorphously substituted with the
transition metal having
an oxidation state of IV of the ammonia storage material.
[00244] Generally, the sodium form of the isomorphously substituted molecular
sieve can
be prepared from a 0.03A1203:Si02:0.07Ti02:0.06Na20:0.08ATMAOH:2.33H20 gel
composition through autoclave hydrothermal synthesis. The product is recovered
by filtration,
and the template is removed by calcination. The final crystalline material can
be characterized
by x-ray diffraction studies.
[00245] The H-form can be prepared by calcination of the ammonia form, which
is obtained
through double NH4NO3 exchanges with the sodium form. The Ti level is
unchanged/stable
through the NH4NO3 exchange processes.
Cl 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
44
[00246] The copper promoted isomorphously substituted molecular sieve can be
prepared
by ion exchange using the H-form and Cu(OAc)2 to achieve the desired amount of
promoter
metal.
Method of Reducing NO3 and Exhaust Gas Treatment System:
[00247] In general, the zeolitic materials that are described above can be
used as a molecular
sieve, adsorbent, catalyst, catalyst support, or binder thereof. In one or
more embodiments, the
materials are used as a catalyst.
[00248] An additional aspect of the invention is directed to a method of
catalyzing a chemical
reaction wherein the spherical particle including an agglomeration of crystals
of a molecular
sieve according to embodiments of the invention is employed as catalytically
active material.
[00249] Another aspect of the invention is directed to a method of catalyzing
a chemical
reaction wherein the zeolitic framework material that is isomorphously
substituted with a
tetravalent metal according to embodiments of the invention is employed as
catalytically active
material.
[00250] A further aspect of the invention is directed to a method of
catalyzing a chemical
reaction wherein the SCR catalyst composite that comprises an SCR catalyst
material and an
ammonia storage material comprising a transition metal having an oxidation
state of IV
according to embodiments of the invention is employed as catalytically active
material.
[00251] Among others, said catalyst materials and catalyst composites may be
employed as
catalysts for the selective reduction (SCR) of nitrogen oxides (NO3); for the
oxidation of NH3,
in particular for the oxidation of NH3 slip in diesel systems; for
applications in oxidation
reactions, in specific embodiments an additional precious metal component
(e.g. Pd, Pt) is
added to the spherical particle including an agglomeration of crystals of a
molecular sieve.
[00252] One or more embodiments provide a method of selectively reducing
nitrogen
oxides (NO.). In one or more embodiments, the method comprises contacting an
exhaust gas
stream containing NO. with the catalyst materials or the catalyst composites
of one or more
embodiments. In particular, the selective reduction of nitrogen oxides wherein
the selective
catalytic reduction catalyst material comprises a spherical particle including
an agglomeration
of crystals of a molecular sieve, wherein the spherical particle has a median
particle size in the
range of about 0.5 to about 5 microns, of embodiments of the invention is
employed as
catalytically active material is carried out in the presence of ammonia or
urea,
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
[00253] While ammonia is the reducing agent of choice for stationary power
plants, urea is
the reducing agent of choice for mobile SCR systems. Typically, the SCR system
is integrated
in the exhaust gas treatment system of a vehicle and, also typically, contains
the following
main components: selective catalytic reduction material comprising a spherical
particle
5 including an agglomeration of crystals of a molecular sieve, wherein the
spherical particle has
a median particle size in the range of about 0.5 to about 5 microns according
to embodiments
of the invention; a urea storage tank; a urea pump; a urea dosing system; a
urea injector/nozzle;
and a respective control unit.
[00254] In other embodiments, the SCR catalyst composite according to one or
more
10 embodiments is employed as an SCR catalyst in an exhaust gas treatment
system for lean-bum
gasoline direct injection engines. In such cases, the SCR catalyst composite
according to one
or more embodiments serves as a passive ammonia-SCR catalyst and is able to
store ammonia
effectively at temperatures of 400 C and above.
[00255] As used herein, the term "stream" broadly refers to any combination of
flowing gas
15 that may contain solid or liquid particulate matter. The term "gaseous
stream" or "exhaust gas
stream" means a stream of gaseous constituents, such as the exhaust of a lean
bum engine,
which may contain entrained non-gaseous components such as liquid droplets,
solid
particulates, and the like. The exhaust gas stream of a lean bum engine
typically further
comprises combustion products, products of incomplete combustion, oxides of
nitrogen,
20 combustible and/or carbonaceous particulate matter (soot), and un-reacted
oxygen and
nitrogen.
[00256] The term nitrogen oxides, NOR, as used in the context of embodiments
of the
invention designates the oxides of nitrogen, especially dinitrogen oxide
(N20), nitrogen
monoxide (NO), dinitrogen trioxide (N203), nitrogen dioxide (NO2), dinitrogen
tetroxide
25 (N204), dinitrogen pentoxide (N205), nitrogen peroxide (NO3).
[00257] A further aspect of the invention is directed to an exhaust gas
treatment system. In
one or more embodiments, the exhaust gas treatment system comprises an exhaust
gas stream
optionally containing a reductant like ammonia, urea, and/or hydrocarbon, and
in specific
embodiments, ammonia and/or urea, and a selective catalytic reduction material
comprising a
30 spherical particle including an agglomeration of crystals of a molecular
sieve, wherein the
spherical particle has a median particle size in the range of about 0.5 to
about 5 microns. The
46
catalyst material is effective for destroying at least a portion of the
ammonia in the exhaust gas stream.
[00258] In one or more embodiments, the SCR catalyst material can be
disposed on a
substrate, for example a soot filter. The soot filter, catalyzed or non-
catalyzed, may be
upstream or downstream of the SCR catalyst material_ In one or more
embodiments, the system can
further comprise a diesel oxidation catalyst. In specific embodiments, the
diesel oxidation catalyst is
located upstream of the SCR catalyst material. In other specific embodiments,
the diesel oxidation
catalyst and the catalyzed soot filter are upstream from the SCR catalyst
material.
[00259] In specific embodiments, the exhaust is conveyed from the engins to
a position
downstream in the exhaust system, and in more specific embodiments, containing
NOR, where a
reductant is added and the exhaust stream with the added reductant is conveyed
to the SCR catalyst
material.
[00260] For example, a catalyzed soot filter, a diesel oxidation catalyst,
and a reductant are
described in WO 2008/106519. In specific embodiments, the soot filter
comprises a wall-flow filter
substrate, where the channels are alternately blocked, allowing a gaseous
stream entering the channels
from one direction (inlet direction), to flow through the channel walls and
exit from the channels from
the other direction (outlet direction).
[00261] An ammonia oxidation (AMOx) catalyst may be provided downstream of
the SCR
catalyst material or catalyst composite of one or more embodiments to remove
any slipped ammonia
from the system. In specific embodiments, the AMOx catalyst may comprise a
platinum group metal
such as platinum, palladium, rhodium, or combinations thereof.
[00262] Such AMOx catalysts are useful in exhaust gas treatment systems
including an SCR
catalyst. As discussed in commonly assigned United States Patent No.
5,516,497, a gaseous stream
containing oxygen, nitrogen oxides, and ammonia can be sequentially passed
through first and second
catalysts, the first catalyst favoring reduction of nitrogen oxides and the
second catalyst favoring the
oxidation or other decomposition of excess ammonia. As described in United
States Patent No.
5,516,497, the first catalysts can be a SCR catalyst comprising a zeolite and
the second catalyst can be
an AMOx catalyst comprising a zeolite.
Date Recue/Date Received 2021-10-20
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
47
[00263] AMOx and/or SCR catalyst composition(s) can be coated on the flow
through or
wall-flow filter. If a wall flow substrate is utilized, the resulting system
will be able to remove
particulate matter along with gaseous pollutants. The wall-flow filter
substrate can be made
from materials commonly known in the art, such as cordierite, aluminum
titanate or silicon
carbide. It will be understood that the loading of the catalytic composition
on a wall flow
substrate will depend on substrate properties such as porosity and wall
thickness, and typically
will be lower than loading on a flow through substrate.
[00264] The invention is now described with reference to the following
examples. Before
describing several exemplary embodiments of the invention, it is to be
understood that the
invention is not limited to the details of construction or process steps set
forth in the following
description. The invention is capable of other embodiments and of being
practiced or being
carried out in various ways.
EXAMPLES
[00265] COMPARATIVE EXAMPLE 1 ¨ Preparation Of Catalyst Compositions And
Articles
[00266] A CuCHA powder catalyst was prepared by crystallization of chabazite
using
ADAOH (Trimethyl-l-adamantylammonium hydroxide) containing synthesis gel,
separation
of the chabazite pioduct, drying and calcination to remove organic template
(ADAOH). Water,
ADAOH solution, and aqueous sodium hydroxide were added into the makedown tank
and
mixed for several minutes. An aluminum source was then added in 3-5 minutes.
Colloidal
silica was then added with stirring in 5 minutes. Mixing was continued for an
additional 30
minutes, resulting in a viscous gel of uniform composition. The gel was
transferred to the
autoclave. The autoclave was heated to 170 C, and crystallization was
continued for 18 hours
while maintaining agitation. The reactor was cooled to <50 C and vented to
atmospheric
pressure prior to unloading. After hydrothermal crystallization, the resultant
suspension had a
pH of 11.5. The suspension was admixed with deionized water and was filtrated
with a
procelain suction filter. The wet product was then heated to a temperature of
120 C in air for 4
hrs. The dried product was then further calcined in air at 600 C for 5 hrs to
remove the
template and ensure a C content less than 0.1 wt.%.
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
48
[00267] As can been observed in the SEM image of the crystal morphology in
FIG. 5, the
as-synthesized material (Comparative Example 1) does not have an agglomerated
morphology,
as identified by SEM analysis (secondary electron imaging) at a scale of
5000x.
[00268] The calcined product was then ready to be ion-exchanged with Cu to
obtain the
metal-containing catalyst.
[00269] An ion-exchange reaction between the Na-form CHA and the copper ions
was
carried out by agitating the slurry at about 60 C for about 1 hour. The
resulting mixture was
then filtered to provide a filter cake, and the filter cake was washed with
deionized water in
three portions until the filtrate was clear and colorless, and the washed
sample was dried.
1002701 The obtained CuCHA catalyst comprised CuO at a range of about 3 to
3.5% by
weight, as determined by 1CP analysis. A CuCHA slurry was prepared to 40%
target solids.
The slurry was milled and a binder of zirconium acetate in dilute acetic acid
(containing 30%
ZrO2) was added into the slurry with agitation.
[00271] The slurry was coated onto 1"Dx3"L cellular ceramic cores, having a
cell density of
400 cpsi (cells per square inch) and a wall thickness of 6.5 mil. The coated
cores were dried at
110 C for 3 hours and calcined at about 400 C for 1 hour. The coating
process was repeated
once to obtain a target washcoat loading of in the range of 2-3 g/in3.
EXAMPLE 2
[00272] The same raw materials as Comparative Example 1 were used to prepare
the
inventive agglomerated (snowball) CHA material, except additional water was
added. The gel
make down procedure was also the same as Comparative Example 1. The autoclave
was
heated to 160 C, and crystallization was continued for 30 hours while
maintaining agitation.
The reactor was cooled to <50 C and vented to atmospheric pressure prior to
unloading. After
hydrothermal crystallization, the resultant suspension had a pH of 12Ø The
suspension was
admixed with deionized water and was filtrated with a procelain suction
filter. The wet product
was then heated to a temperature of 120 C in air for 4 hrs. The dried product
was then further
calcined in air at 600 C for 5 hrs to remove the template and ensure a C
content less than 0.1
wt %.
100273] As can been observed in the SEM image of the crystal morphology in
FIG. 6, the
as-synthesized snowball material (Example 2) has a characteristic secondary
structure of
spheres with a diameter size of 1-2 um, as identified by SEM analysis
(secondary electron
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
49
imaging) at a scale of 5000x. The individual crystals of molecular sieve have
a crystal size in
the range of about 100 to 200 nm.
EXAMPLE 3¨ Cu Promotion
[00274] An ion-exchange reaction between the Na-form CHA of Example 2 and
copper ions
was carried out by agitating the slurry at about 60 C for about 1 hour. The
resulting mixture
was then filtered to provide a filter cake, and the filter cake was washed
with deionized water
in three portions until the filtrate was clear and colorless, and the washed
sample was dried.
[00275] The obtained CuCHA catalysts comprised CuO at a range of about 1.5 to
4% by
weight, as determined by ICP analysis. A CuCHA slurry was prepared to 40%
target solids.
The slurry was milled and a binder of zirconium acetate in dilute acetic acid
(containing 30%
ZrO2) was added into the slurry with agitation.
EXAMPLE 4¨ Preparation Of Washcoats
[00276] The Example 3 slurries were then coated onto a substrate to a washcoat
loading of
2.1 g/in3. The washcoat was dried under air at 130 C for 5 min. After the
final coating, the
substrate was calcined at 450 C for 1 hour.
EXAMPLE 5¨ CuO Loading Study
[00277] Nitrogen oxides selective catalytic reduction (SCR) efficiency and
selectivity of a
fresh catalyst core was measured by adding a feed gas mixture of 500 ppm of
NO, 500 ppm of
NH3, 10% 02, 5% H20, balanced with N2 to a steady state reactor containing a
1"D x 3"L
catalyst core. The reaction was carried at a space velocity of 80,000 hr-'
across a 150 C to
460 C temperature range.
[00278] The samples were hydrothermally aged in the presence of 10% H2O at 750
C for 5
hours, followed by measurement of the nitrogen oxides SCR efficiency and
selectivity by the
same process as outlined above for the SCR evaluation on a fresh catalyst
core.
[00279] Figure 7 is a bar graph showing the NO, conversion (%) versus CuO
loading
(wt.%).
[00280] Figure 8 is a bar graph showing the N20 make (ppm) versus CuO loading
(wt.%).
EXAMPLE 6¨ NOõ Conversion
100281] Nitrogen oxides selective catalytic reduction (SCR) efficiency and
selectivity of a
fresh catalyst core was measured by adding a feed gas mixture of 500 ppm of
NO, 500 ppm of
NH3, 10% 02, 5% 120, balanced with N2 to a steady state reactor containing a
1"D x 3"L
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
catalyst core. The reaction was carried at a space velocity of 80,000 hr l
across a 150 C to
460 C temperature range.
[00282] The samples were hydrothermally aged in the presence of 10% H20 at 750
C for 5
hours, followed by measurement of the nitrogen oxides SCR efficiency and
selectivity by the
5 same process as outlined above for the SCR evaluation on a fresh catalyst
core.
[00283] Figure 9 is a graph showing the NO conversion (%) versus temperature (
C) for the
catalyst of Example 1 (comparative) versus the inventive catalyst of Example
3, having 3.2%
CuO.
[00284] Figure 10 is a graph showing the N20 make (ppm) versus temperature (
C) for the
10 catalyst of Example 1 (comparative) versus the inventive catalyst of
Example 3, having 3.2%
CuO.
[00285] Figure 11 is a bar graph showings the NO3 conversion (%) at 20 ppm NH3
slip for the
catalyst of Exarriple 1 (comparative) versus the inventive catalyst of Example
3, having 3.2%
CuO. The catalyst of Example 3 shows significantly higher NOx conversion
(about 15% greater)
15 at 20 ppm NH3 slip, which is an indication of improved transient
performance during engine
testing conditions.
[00286] As illustrated in Figures 9-11, the snowball morphology results in a
SCR catalyst
material with improved NOx conversion efficiency and lower N20 make versus a
SCR catalyst
material that does not have snowball morphology.
20 Isomorphously Substituted Molecular Sieves
EXAMPLE 7
[00287] An isomorphously substituted zeolitic material (Na- [Ti]CHA) was
prepared from
an 0.03A1203:Si02:0.07Ti02:0.06Na20:0.08ATMAOH:2.33H20 gel composition through
autoclave hydrothermal synthesis at 155 C for 5 days. The product was
recovered by
25 filtration, and the template was removed by calcination at 600 C for 5
hours. The final
crystalline material had an x-ray powder diffraction pattern indicating > 90%
CHA phase and a
silica/alumina ratio (SAR) of 25 by XRF.
EXAMPLE 8
100288] An isomorphously substituted zeolitic material (H-[Ti]CHA) was
prepared by 500
30 C calcination (4 hrs.) of NH4-[Ti]CHA, which was obtained through
double NI-14NO3 (2.4 M)
exchanges with the material of Example 7 (Na-[Ti]CHA). The Ti level is
unchanged through
the NH4NO3 exchange processes, 4.3% vs. 4.5%.
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
51
EXAMPLE 9- Comparative
1002891 The zeolitic material H-CHA was prepared according to the process of
Example 7
(H-[Ti]CHA), but without Ti addition to the synthesis gel.
EXAMPLE 10
[00290] A copper promoted isomorphously substituted zeolitic material (Cu2.72-
[Ti]CHA)
was prepared by ion exchange at 50 C (2 hrs.) using the material of Example 8
(H-[Ti]CHA)
and Cu(OAc)2 (0.06 M), showing a Cu content of 2.72% (ICP).
EXAMPLE 11
[00291] A copper promoted isomorphously substituted zeolitic material (Cu3.64-
[Ti]CHA)
was prepared by ion exchange at 50 C (2 hrs.) using the material of Example 9
(H-[Ti]CHA)
and Cu(OAc)2 (0.125 M), showing a Cu content of 3.64% (ICP)
EXAMPLE 12¨ Comparative
[00292] A standard copper promoted zeolitic material (Cu2.75-CHA) was prepared
according to the process provided in U.S. 8404203B2, with comparable Cu
content (2.75%) to
Example 9. This material is provided as the reference for benchmarking.
EXAMPLE 13¨ Comparative
[00293] A standard copper promoted zeolitic material (Cu3.84-CHA) was prepared
according to the process provided in U.S. 8404203B2, with comparable Cu
content (3.84%) to
Example 10. This material is provided as the reference for aging benchmarking.
EXAMPLE 14
[00294] The incorporation of Ti at the tetrahedral position is evidenced by
fingerprints of Ti
involved framework stretches (Ti-O-Si) at 940 ¨ 980 cm-1, as illustrated in
FIG. 12.
EXAMPLE 15
[00295] In addition to the fingerprint vibrations from Ti involved framework
stretches, the
enhanced acidity of framework due to the high valence framework Ti(IV) is also
evidenced
from the increased intensity of NO, whose formation requires strong Lewis
acidity, as
illustrated in FIG. 13.
EXAMPLE 16
[00296] After Cu was exchanged to acid sites of the isomorphously substituted
zeolitic
material [Ti]CHA providing the compounds of Examples 10 and 11, the formation
of NO is
not affected. As illustrated in FIG. 14, the material of Example 10 (Cu2.72-
[Ti]CHA) shows
superior capability of generating more NO' compared to the unmodified
Comparative Example
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
52
12 (Cu2.75-CHA) at an equilibrium state. Given the nature of high reactivity
of NO towards
nucleophiles, e.g., NH3, it is established that the observed significant
reactivity boost at low
temperatures (e.g., 200 C) from Example 10 (Cu-[Ti]CHA) is due to the improved
generation
and retention of NO over the catalyst.
.. EXAMPLE 17
[00297] As can been observed in the SEM image in FIG. 15, the as-synthesized
[Ti]CHA
(Example 8) has a characteristic secondary structure as spheres with a
diameter size of 1 ¨ 2
gm, as identified by SEM analysis (secondary electron imag'ng) at a scale of
5000x.
EXAMPLE 18
[00298] The material of Example 10 (Cu-[Ti]CHA) was washcoated on a flow-
through
ceramic substrate at a loading of 2.1 g/in3. The typical SCR testing condition
includes
simulated diesel exhaust gas (500 ppm NO, 500 ppm NH3, 10% 02, 5% H20, and
balance N2)
and temperature points from 200 C to 600 'C. Conversion of NO and NH3 at
various
temperatures are monitored by FTIR. An aging condition of 750 C exposure to
10% H20 for
5 hrs. is adopted if desired to evaluate long term hydrothermal durability.
[00299] As illustrated in the SEM images in FIGS. 18A and 18B, the as-
synthesized Cu-
[Ti]CHA produces a washcoat that is very porous (FIG. 18B) when compared to a
standard
copper promoted zeolitic material, Cu-CHA.
EXAMPLE 19
[00300] The porosity and particle size of the materials is presented in FIG.
19. As
illustrated in FIG. 19, shown by Hg intrusion measurement, the washcoat formed
from Cu-
[Ti]CHA (Example 10) has a porosity distribution more towards larger pores
compared to
unmodified Cu-CHA (Example 12).
[00301] In addition to the increased porosity of the washcoat, the as-
synthesized Cu-
.. [Ti]CHA produces particle sizes that are significantly larger than the
particle size of a standard
copper promoted zeolitic material.
EXAMPLE 20
[00302] Catalyst Cu-[Ti]CHA has been washcoated on a flow-through ceramic
substrate at a
loading of 2.1 g/in3. A typical SCR testing condition includes simulated
diesel exhaust gas
.. (500 ppm NO, 500 ppm NH3, 10% 02, 5% H20, and balance N2) and temperature
points from
200 C to 600 C. Conversion of NO and NH3 at various temperatures are monitored
by FTIR.
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
53
An aging condition of 750 C exposure to 10% H20 for 5 hrs. is adopted if
desired to evaluate
long term hydrothermal durability.
[00303] As illustrated in FIG. 16, with the assistance of framework Ti
(Example 10), the
SCR performance at 200 C is significantly improved compared to the analogous
sample
without Ti (Example 6) at comparable Cu%, and no sacrifice of the high
temperature (600 C)
NO3 conversion efficiency is observed.
EXAMPLE 21
100304] As illustrated in FIG. 17, high Cu content (e.g., Cu% > 2.5% SAR
= 30), after
high temperature hydrothermal aging, results in the formation of CuO, which
actively
consumes NH3 leading to a decreased SCR performance at the high temperature
end. The
presence of framework Ti (Example 11) helps to alleviate the NH3 consumption
at the high
temperature region with high Cu loaded sample.
EXAMPLE 22
1003051 An isomorphously substituted zeolitic material (Na-[Ti]AEI) is
prepared
analogously to the material of Example 7. The product is recovered by
filtration, and the
template is removed by calcination at 600 C for 5 hours.
EXAMPLE 23
[00306] An isomorphously substituted zeolitic material (H-[Ti]AEI) is prepared
by 500 C
calcination (4 hrs.) of NH4-[Ti]AEI, which is obtained through double NH4NO3
(2.4 M)
exchanges with the material of Example 21 (Na-[Ti]AEI).
EXAMPLE 24
1003071 A copper promoted isomorphously substituted zeolitic material (Cu-
[Ti]AEI) is
prepared by ion exchange at 50 C (2 hrs.) using the material of Example 22 (H-
[Ti]AEI) and
Cu(OAc)2 (0.06 M).
EXAMPLE 25
[00308] An isomorphously substituted zeolitic material (Na-[Ti]AFX) is
prepared
analogously to the material of Example 7. The product is recovered by
filtration, and the
template is removed by calcination at 600 C for 5 hours.
EXAMPLE 26
[00309] An isomorphously substituted zeolitic material (H-[Ti]AFX) is prepared
by 500 C
calcination (4 hrs.) of NH4-[Ti]AFX, which is obtained through double NI-14NO3
(2.4 M)
exchanges with the material of Example 24 (Na-[Ti]AFX).
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
54
EXAMPLE 27
[00310] A copper promoted isomorphously substituted zeolitic material (Cu-
[Ti]AFX) is
prepared by ion exchange at 50 C (2 hrs.) using the material of Example 25 (I-
1-[Ti]AFX) and
Cu(OAc)2 (0.06 M).
EXAMPLE 28
[00311] An isomorphously substituted zeolitic material (Na-[Ti]CHA) was
prepared from
an 0.03A1203:Si02:0.07Ti02:0.06Na20:0.08ATMAOH:2.33H20 gel composition through
autoclave hydrothermal synthesis at 155 C for 5 days. The product was
recovered by
filtration, and the template was removed by calcination at 600 C for 5 hours.
The final
crystalline material had an X-ray powder diffraction pattern indicating > 90%
CHA phase and
a SAR of 25 by XRF. Other SAR, e.g., 20, can also be obtained by proper
adjustment of Si/A1
ratio in the starting gel.
EXAMPLE 29
[00312] An isomorphously substituted zeolitic material (H-[Ti]CHA) was
prepared by 500
C calcination (4 hrs) of N1-14-[Ti]CHA, which was obtained through double
NH4NO3 (2.4 M)
exchanges with the material of Example 27 (Na-[Ti]CHA). The Ti level was
unchanged through
the NH4NO3 exchange processes, 4.3% vs. 4.5%.
EXAMPLE 30
[00313] The zeolitic material H-CHA was prepared according to the piocess of
Example 28
and 29, but without Ti addition to the initial synthesis sol gel for zeolite
hydrothermal
crystallization.
EXAMPLE 31
1003141 A copper promoted isomorphously substituted zeolitic material (Cu-
[Ti]CHA (SAR
20)) was prepared by ion exchange at 50 C (2 hrs) using the material of
Example 29 (H-
[Ti]CHA) and Cu(OAc)2. Variation of Cu concentration in the exchange process
pioduced a
series of copper zeolite, e.g., Cu2.46-[Ti]CHA (Example 31a), Cu3.03-[Ti]CHA
(Example 3 lb),
Cu3.64-[Ti]CHA (Example 31c), and Cu3.78-[Ti]CHA (Example 31d) (numbers after
Cu denote
Cu percentage).
EXAMPLE 32
1003151 A standard copper promoted zeolitic material (Cu2.75-CHA) was prepared
according
to the process provided in U.S. 8404203B2, and was provided as the reference
for bcnchmarking.
EXAMPLE 33- COMPARATIVE
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
[00316] A Fe-CHA (Fe: 2.5%) was synthesized similarly as Cu-CHA but using
Fe(NO3)3 in
the solution exchange, and was selected as a comparative sample.
EXAMPLE 34- Comparative
[00317] A commercially available Fe-Beta from BASF was selected as a
comparative sample.
5 EXAMPLE 35- Comparative
[00318] A commercially available Fe-MFI (SCP-306) from Siid-Chemie was
selected as a
comparative sample.
EXAMPLE 36
[00319] As illustrated in FIG. 20, in the presence of framework Ti, not only
was the adsorbed
10 NH3 increased from 15.2 to 19.1 cm3/g at the high temperature zone, but
also the desorption
temperature was increased slightly by 10 C (e.g., 470 C to 480 C),
indicating a stronger Lewis
acid site other than acidic proton functioning as the NH3 storage component.
(Example 29 v.
Example 30).
EXAMPLE 37
15 [00320] As illustrated in FIG. 21, after Cu exchange, an increase of Cu
percentage only
boosted the NH3 storage in the mid-temperature zone, e.g., 250 C ¨ 400 C. The
integrated
values for the highest desorption peak were 12.8, 23.8, 28.8, and 23.8 cm3/g
for Cu-CHA
(Example 32), Cu2.46-[Ti]CHA (Example 31a), Cu3.03-[Ti]CHA (Example 31),
Cu3.64-
[Ti]CHA (Example 31c), respectively. The Ti containing Cu4TilCHA samples
consistently
20 showed doubled capacity of NH3 retention above 400 C. (Example 32 v.
Example 31)
EXAMPLE 38
[00321] As illustrated in FIG. 22, the presence of other lower valence
transition metals, e.g.,
Fe(111), however, did not have the effective promotion for NH3 storage above
400 C. The high
temperature (> 400 C) storage capacity for Fe-MFI, Fe-CHA, Fe-Beta were 13.6,
12.8, 7.9
25 cm3/g, respectively, which were at a similar level as the unmodified Cu-
CHA.
EXAMPLE 39
1003221 Both Cu-CHA (Example 32) and Cu3.64-[Ti]CHA (Example 31c) were coated
on
honeycomb with equal washcoat loading, and measured at temperatures (200 C,
300 C,
400 C, 450 C, and 500 C) for NH3 storage in the presence of 5% H20. As
illustrated in FIG.
30 23, being assisted by framework Ti, more chemisorbed NI-13 were found
consistently on Cu-
[Ti]CHA than those on unmodified Cu-CHA up to 400 C.
EXAMPLE 40
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
56
[00323] A commercially available non-zeolitic composite material with TiO2,
A1203, and
SiO2, consisting of Ti, Si, Al based oxides from a co-precipitation process,
also demonstrated
high temperature NI-13 storage feature. As illustrated in FIG. 24, although
the storage capacity
of the commercially available material compared to Cu-CHA (Example 32) was
low, the
desorption temperature was further increased.
EXAMPLE 41
[00324] An isomorphously substituted zeolitic material (Na-[Ti]AEI) is
prepared
analogously to the material of Example 27. The product is recovered by
filtration, and the
template is removed by calcination at 600 C for 5 hours.
EXAMPLE 42
1003251 An isomorphously substituted zeolitic material (H-[TiJAE1) is prepared
by 500 C
calcination (4 hrs) of NH4-[Ti]AEI, which is obtained through double NH4NO3
(2.4 M)
exchanges with the material of Example 41 (Na-[Ti]AEI).
EXAMPLE 43
[00326] A copper promoted isomorphously substituted zeolitic material (Cu-
[Ti]AEI) is
prepared by ion exchange at 50 'V (2 hrs) using the material of Example 42 (H-
[Ti]AEI) and
Cu(OAc)2 (0.06 M).
EXAMPLE 44
[00327] An isomorphously substituted zeolitic material (Na-[Ti]AFX) is
prepared
analogously to the material of Example 27. The product is recovered by
filtration, and the
template is removed by calcination at 600 C for 5 hours.
EXAMPLE 45
1003281 An isomorphously substituted zeolitic material (H-[Ti]AFX) is prepared
by 500 C
calcination (4 hrs) of NH4-[Ti]AFX, which is obtained through double NH4/\103
(2,4 M)
exchanges with the material of Example 44 (Na-[Ti]AFX).
EXAMPLE 46
[00329] A copper promoted isomorphously substituted zeolitic material
(CutTilAFX) is
prepared by ion exchange at 50 C (2 hrs) using the material of Example 45 (H-
[Ti]AFX) and
Cu(OAc)2 (0.06 M).
1003301 The use of the terms "a" and "an" and "the" and similar referents in
the context of
describing the materials and methods discussed herein (especially in the
context of the
following claims) are to be construed to cover both the singular and the
plural, unless
CA 02952435 2016-12-14
WO 2015/195809 PCTMS2015/036243
57
otherwise indicated herein or clearly contradicted by context. Recitation of
ranges of values
herein are merely intended to serve as a shorthand method of referring
individually to each
separate value falling within the range, unless otherwise indicated herein,
and each separate
value is incorporated into the specification as if it were individually
recited herein. All methods
described herein can be performed in any suitable order unless otherwise
indicated herein or
otherwise clearly contradicted by context. The use of any and all examples, or
exemplary
language (e.g., "such as") provided herein, is intended merely to better
illuminate the materials
and methods and does not pose a limitation on the scope unless otherwise
claimed. No
language in the specification should be construed as indicating any non-
claimed element as
essential to the practice of the disclosed materials and methods.
[00331] Reference throughout this specification to "one embodiment," "certain
embodiments," "one or more embodiments" or "an embodiment" means that a
particular
feature, structure, material, or characteristic described in connection with
the embodiment is
included in at least one embodiment of the invention. Thus, the appearances of
the phrases
such as "in one or more embodiments," "in certain embodiments," "in one
embodiment" or "in
an embodiment" in various places throughout this specification are not
necessarily referring to
the same embodiment of the invention. Furthermore, the particular features,
structures,
materials, or characteristics may be combined in any suitable manner in one or
more
embodiments.
[00332] Although the invention herein has been described with reference to
particular
embodiments, it is to be understood that these embodiments are merely
illustrative of the
principles and applications of the present invention. It will be apparent to
those skilled in the
art that various modifications and variations can be made to the method and
apparatus of the
present invention without departing from the spirit and scope of the
invention. Thus, it is
intended that the present invention include modifications and variations that
are within the
scope of the appended claims and their equivalents.