Note: Descriptions are shown in the official language in which they were submitted.
CA 02952464 2016-12-21
MEANS FOR CONTROLLED SEALING OF
=
ENDOVASCULAR DEVICES
FIELD OF THE INVENTION
The present disclosure is directed generally to endoluminal devices and
associated systems and methods, and specifically to a method and devices for
controlled actuation of means for sealing of an endoluminal prosthesis to a
vessel wall.
BACKGROUND OF THE INVENTION
An aneurysm is a localized, blood-filled dilation of a blood vessel caused
by disease or weakening of the vessel wall. Aneurysms affect the ability of
the
vessel to conduct fluids, and can be life threatening if left untreated.
Aneurysms
most commonly occur in arteries at the base of the brain and in the aorta. As
the
size of an aneurysm increases, there is an increased risk of rupture, which
can
result in severe hemorrhage or other complications including sudden death.
Aneurysms are typically treated by surgically removing apart or all of the
aneurysm and implanting a replacement prosthetic section into the body lumen.
Such procedures, however, can require extensive surgery and recovery time.
Patients often remain hospitalized for several days following the procedure,
and
can require several months of recovery time. Moreover, the morbidity and
mortality rates associated with such major surgery can be significantly high.
Another approach for treating aneurysms involves remote deployment of
an endovascular graft assembly at the affected site. Such procedures typically
require intravascular delivery of the endovascular graft assembly to the site
of
the aneurysm. The graft is then expanded or deployed in situ and the ends of
the
CA 02952464 2016-12-21
=
graft are anchored to the body lumen on each side of the aneurysm. In this
way,
= the graft effectively excludes the aneurysm sac from circulation.
One concern with many conventional endovascular graft assemblies,
however, is the long term durability of such structures. Over time, the graft
can
become separated from an inner surface of the body lumen, resulting in
bypassing of the blood between the vessel wall and the graft. As used herein,
endoleak is defined as a persistent blood or other fluid flow outside the
lumen of
the endoluminal graft, but within the aneurysm sac or adjacent vascular
segment
being treated by the device. When an endoleak occurs, it can cause continuous
pressurization of the aneurysm sac and may result in an idcreased risk of
rupture.
In addition to endoleaks, another concern with many conventional
= endovascular graft assemblies is subsequent device migration and/or
dislodgement. For example, after a surgeon has found an optimal location for
the graft, the device must be fixed to the wall of the body lumen and fully
sealed
at each end of the graft to prevent endoleaks and achieve a degree of fixation
that will prevent subsequent device migration and/or dislodgement.
= Aortic stenosis, also known as aortic valve stenosis, is
characterized by =
an abnormal narrowing of the aortic valve. =The narrowing prevents the valve
= 20 from opening fully, which obstructs blood flow from the heart into the
aorta. As =
a result, the left ventricle has to work harder to maintain adequate blood
flow
= through the body. If left untreated, aortic stenosis can lead to life-
threatening =
problems including heart failure, irregular heart rhythms, cardiac arrest, and
chest pain. Aortic stenosis is typically due to age-related progressive
calcification of the normal trileaflet valve, though other predisposing
conditions
include congenital heart defects, calcification of a congenital bicuspid
aortic
= valve, and acute rheumatic fever.
For the last fifty years, open heart surgery for aortic valve replacement
using cardiopulmonary bypass, sternotomy (or mini-sternotomy), aortic cross
=
= = 30 clamping and cardioplegic arrest represents the treatment
of choice and the
2
CA 02952464 2016-12-21
standard of care for patients having severe aortic stenosis with symptoms
=
(Bonow, et al., Circulation, 114:e84-231 (2006), Kvidal, et al., J. Am. Coll.
Cardiol., 35:747-56 (2000), Otto, Heart, 84:211-8 (2000), Schwarz, et al.,
== Circulation, 66:1105-10 (1982)). However, there is a large pool of
patients
affected by severe aortic stenosis who are not candidates for open heart valve
replacement surgery because they are considered too old (nonagenarians,
centenaries) for such an invasive procedure, or because they are also affected
by
= other co-existing conditions that compound their operative risk (Tung, et
al., Eur
Heart J. 26:2714-20 (2005). For these patients, who are at high surgical risk,
a
less invasive treatment is necessary.
Transcatheter aortic-valve implantation (TAV) is a procedure in which a
= bioprosthetic valve is inserted through a catheter and implanted within
the
diseased native aortic valve. The most common implantation routes include the
transapical approach (TA) and transfermoral (TF), though trans-subclavian and.
trans-aortic routes are also being explored (Ferrari, et al., Swiss Med Wkly,
140:w13127 (2010). These percutaneous routes rely on a needle catheter getting
access to a blood vessel, followed by the introduction of a guidewire through
the
lumen of the needle. It is over this wire that other catheters can be placed
into
= the blood vessel, and implantation of the prosthesis is carried out.
Since 2002 when the procedure was first performed, there has been rapid
growth in its use throughout the world for the treatment of severe aortic
stenosis
= in patients who are at high surgical risk, and there is mounting support
to adopt
the therapy as the standard of care for patients that are not at a high risk
for
surgery. Clinical studies have shown that the rate of death from any cause at
the
one-year mark among patients treated with TAV was approximately 25%
(Grube, et al., Circ. Cardiovasc. Interv. 1:167-175 (2008), Himbert et al., J.
Am.
= Coll. Cardiol.. 54:303-311 (2009), Webb, et al., Circulation, 119:3009-
3016
= (2009), Rodes-Cabau, et al., J. Am. Coll. Cardiol., 55:1080-1090 (2010),
and the
results of two parallel prospective, multicenter, randomized, active-treatment-
controlled clinical trials showed that TAV is superior to standard therapy,
when .
3
CA 02952464 2016-12-21
=
comparing the rate of death from any cause at the 1-year mark (30.7% in the
TAV group, as compared with 50.7% in the standard-therapy group) (Leon, et
al., N. Engl. J. Med., 363:1597-1607 (2010)). .
=
Paravalvular leaks are extremely rare in surgical aortic-valve =
replacement¨seen in just 1.5% to 2% of cases. But as experts observed at Euro
PCR 2011, mild padvalvular leaks are relatively connnon in transcatheter =
aortic-valve implantation (TAV), and new data suggest that more severe
= paravalvular aortic regurgitation (AR) is a key reason for prosthetic
valve
= dysfunction. According to Dr. Jan-Malte Sinning (Universitatsklinikum,.
Bonn, .
Germany), moderate to severe periprosthetic aortic regurgitation occurs in
=
approximately 15% of TAV-treated patients, a number drawn from 12
international registries. In 127 consecutive patients treated with TAV at his
center, 21 developed moderate paravalvular AR postprocedure, and this was
associated with a significantly higher rate of 30-day and one-year mortality,
as
well as acute kidney injury, compared with patients with no or mild AR.
Predictors of paravalvular AR included a low baseline left ventricular
ejection
fraction (LVEF) and inadequate sizing of the annulus or device. Dr. Kensuke
Takagi (San Raffaele Hospital, Milan, Italy), reported that at his center, 32
patients developed AR grade 2+ to 4+, out of 79 consecutive patients treated
TM
with the CoreValve (Medtronic). In multivariate analyses, valve-annulus
mismatch, Particularly in larger aortic annuli, was a significant predictor of
developing more severe paravalvular AR; an even stronger predictor was low
implantation of thc valve, which increased the risk by more than threefold.
And
while postdilatation can help treat paravalvular AR, this is appropriate only
in
patients in whom the valve was correctly positioned at the outset, Takagi
said.
See Leon MB, Pia77aN, Nikolsky E, et al. Standardized endpoint definitions for
transcatheter aortic valve implantation clinical trials. ./Am .Coll Cardiol
201 l;
57:253-269; Eur Heart J 2011; 32:205-217 =
=
=
The major potential offered by solving leaks with transcatheter heart
valves is in growing the market to the low risk patient segment. The market
4
=
CA 02952464 2016-12-21
opportunity in the low-risk market segment is double the size of that in the
high
risk segment and therefore it is imperative for a TAV device to have
technology
to provide superior long-term hemodynamic performance so that the physicians
recommend TAV over SAVR.
More than 3 million people in the United States suffer from moderate or
severe mitral regurgitation (MR), with more than 250,000 new patients
diagnosed each year. Functional MR can be found in 84% of patients with
congestive heart failure and in 65% of them the degree of regurgitation is
moderate or severe. The long term prognostic implications of functional mitral
regurgitation have demonstrated a significant increase in risk for heart
failure or
death, which is directly related to the severity of the regurgitation.
Compared to
mild regurgitation, moderate to severe regurgitation was associated with a 2.7
fold risk of death and 3.2 fold risk of heart failure, and thus significantly
higher
= health care cost.
Treatment of mitral valve regurgitation depends on the severity and
progression of signs and symptoms. Left unchecked, mitral regurgitation can
lead to heart enlargement, heart failure and further progression of the
severity of
mitral regurgitation. For mild cases, medical treatment may be sufficient. For
more severe cases, heart surgery might be needed to repair or replace the
valve. =
These open-chest/open-heart procedures carry significant risk, especially for
elderly patients and those with severe co-morbidities. While several companies
are attempting to develop less invasive approaches to repair the mitral valve,
they have found limited anatomical applicability due to the heterogeneous
nature
of the disease and, so far, have had a difficult time demonstrating efficacy
that is
= 25 equivalent to surgical approaches. Innovative approaches to less
invasive heart
valve replacement are a promising alternative and Transcatheter Mitral Valve
= Implantation (TMVI) devices are under development. PVL is likely to be a
major problem with these devices and more critical than it is in the case of
TAV
devices. This is in part due to the lesser degree of calcification observed at
the
= 30 mitral valve replacement site, requiring the device have greater
holding power.
5
CA 02952464 2016-12-21
= TAV and TMVI devices may also be used to treat the disease states of
aortic insufficiency (or aortic regurgitation) and mitral stenosis
respectively,
which are less prevalent compared to the aforementioned valvular disease
states,
yet have sirnilar or worse clinical prognosis/severity. They can also be
implanted
= within failing bioprostheses that are already implanted surgically, referred
to as
a valve-in-valve procedure.
An improved device for treatment of these conditions has been
developed which includes a means for sealing the device at the site of
placement, using a sealing ring that is activated by pressure as it is
expanded in
situ. As the device expands, a swellable material is released into the sealing
means that causes the sealing means to expand and conform to the vessel walls,
securing it in place. See W02010/083558 by Endoluminal Sciences Pty Ltd.
The mechanical constraints of these seals are extremely difficult to achieve-
require rapid activation in situ, sufficient pressure to secure but not to
deform or
.displace the implanted prosthesis, biocompatibility, and retention of
strength and
flexibility in situ over a prolonged period of time.
It is therefore an object of the present invention to provide improved
physician controllable means for sealing endovascular devices such as stents
and
aortic valves in situ.
lt is a further object of the present invention to provide means for active
conformation of the sealing means to the vascular anatomy if any remodeling
occurs after implantation so that any resulting leaks are sealed.
It is a further object of the present invention to provide sealing means to
support fixation, anchoring or landing platform of/for the TAV device,
especially in individuals lacking sufficient calcification in the native valve
and
in individual with aortic insufficiency as a diseased state.
It is a further object of the present invention to provide expandable
materials, such as hydrogels, with the appropriate chemical and physical
properties to permanently seal an endoluminal device to a vessel wall.
6
CA 02952464 2016-12-21
SUMMARY OF THE INVENTION
Expandable sealing means for endoluminal devices have been developed
for controlled activation. These include a means for controlled activation at
the
site where the device is to be secured, and thereby avoids premature
activation
that could result in misplacement or leakage at the site. The sealing means
for
placement at least partially between an endoluminal prosthesis and a wall of a
= body lumen has a first relatively reduced radial configuration and a
second
= relatively increased radial configuration which is activated by means of
a wire or
other similar means, by the pressure of expansion at the site of implantation,
or
simply by virtue of the expansion of.the device, releasing a swellable
material
such as a hydrogel, foal or sponge into the sealing means, for example, by
rupture of a capsule containing the swellable material, which then swells upon
contact with fluid at the site to expand the sealing means into secure contact
with the lumen walls. A semi-permeable membrane is used to prevent the _
_
hydrogel gel material from escaping the seal, yet allows access of the fluid
to the
hydiogel. In preferred embodiments, the swellable material is spray dried onto
the interior of the seal, optionally tethered to the material chemically by
covalent
crosslinking. This material typically has a permeability in the range of five
to
70 microns, most preferably 35 to allow rapid access of the fluid to the
hydrogel.
The sealing means is particularly advantageous since it expands into sites to
. eliminate all prosthetic-annular incongruities, as needed. A major
advantage of
these devices is that the sealing means creates little to no increase in
profile,
since it remains flat/inside or on the device until the sealing means is
activated.
Exemplary endoluminal devices including the sealing means for
controlled activation include stents, stent grafts for aneurysm treatment and
transcutaneously implanted aortic valves (TAV) or Mara], tricuspid or
puhnonary valves. In all embodiments, the sealing means is configured to
maintain the same low profile as the device without the sealing means. In a
preferred embodiment, the sealing means is positioned posterior to the
prosthetic =
implant, and is expanded or pulled up into a position adjacent to the implant
at
7
CA 02952464 2016-12-21
=
the time of placement/deployment or sealing. This is achieved using sutures or
.
elastic means to pull the seal up and around the implant at the time of
placement,
having a seal that expands up around implant, and/or crimping the seal so that
it
= moves up around implant when the implant comes out of introducer sheath.
This is extremely important with large diameter implants such as aortic
valves,
which are already at risk of damage to the blood vessel walls during
transport.
In another embodiment, the seal is placed around the skeleton of the TAV, so
that it expands with the skeleton at the time of implantation of the TAV. In a
variation of this embodiment, the seal is placed between the TAV and the =
skeleton, and expands through the skeleton sections at the time of
implantation
to insure sealing.
In all embodiments, it is absolutely critical that the hydrogel/expandable
material operates under sufficient low pressure so that it does not push the
stent
= away from the wall or alter the device configuration. These materials
must
expand quickly (less than ten minutes, more preferably less than five minutes
to
full swelling), expand to a much greater volume (from two to 100 fold, more
preferably from 50 to 90 fold, most preferably sixty fold) and retain the
desired
mechanical and physiochemical properties for an extended period of time, even
under the stress of being implanted with the vasculature or heart. Gels having
=
the desired mechanical and swellable properties have beendeveloped, as
demonstrated by the examples. =
In yet another embodiment, a mechanism enables both deployment and
retrieval of the system: This is particularly important from the ease of use
and
= placement accuracy perspective. This feature enables the physician to
change/alter the placement of the device in vivo if it was not properly
positioned
in the first attempt. Also, in the event of some complication during the
operation, the physician can completely =retrieve the device out of the
patient =
. ' (even after the "expandable material" has completely expanded).
These devices have the advantages of providing excellent sealing in
combination with a low profile, controlled or contained release, and active
=
8
=
=
CA 02952464 2016-12-21
conforming to leak sites to eliminate prosthetic-annular incongruence. ff
= vascular re-modeling occurs over time, which could lead to leakage, the
seal will
also remodel, preventing leaks from developing. For devices that are at high
risk of leakage, a pleated or accordion-like design provides for even better
coverage and prevents uneven distribution of seal filler.
BRIEF DESCRIPTION OF THE DRAWINGS
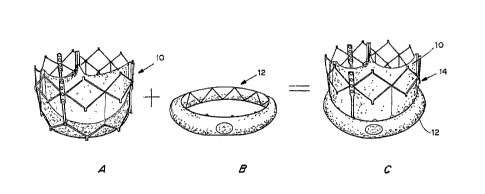
= Figures 1A, 1B and IC are perspective views of a transcatheter aortic
valve (TAV) (Figure IA), a controlled activatable seal (Figure 1B), and the
seal
placed around the TAV (Figure IC).
Figures 2A, 2B and 2C are perspective views of the TAV of Figure 1C
crimped toward the inflow side of the TAV in a telescopic manner (Figure 2A),
= with the TAV and seal in an expanded state with the stent aligned with
the
= bottom section of the TAV, with the activation wire activated to expose
the seal
to fluids (Figure 2B), and post deployment, with the seal expanded by swelling
=
of the hydrogel within the seal when it contacts the blood. =
Figure 3 is a perspective cross-sectional view of the seal, showing the
inner and outer membranes, hydrogel within the inner membrane and the
rupture/activation site.
= Figures 4A, 4B and 4C are perspective views of the seal prior to rupture
and expansion of the seal (Figure 4A), during application of pressure from a
wire to rupture the swelling material container and with partial expansion of
the =
seal (Figure 4B), and after rupture of the swelling material container and
with
= full expansion (Figure 4C).
= Figures 5A-5E are perspective views of a method depicting a "method"
to crimp and toad the device with the "activation wire". The "activation wire"
has to be shortened in length during the crimping/loading process so that the
"activation or rupture" can be triggered during deployment/placement of the
device. Before crimping/loading the "activation wire" is long enough:so that
the
"activation mechanism" is far from activation and the hydrogel can remain
completely sealed/de-activated during storage.
9 :=
CA 02952464 2016-12-21
Figures 6A-6B are perspective views of a seal that is placed inside of the
TAV device. Figures 6C-6D are perspective views of a seal that is placed on
the
exterior of the TAV device. Figure 6E shows the seal placed on the inside of
the
= device such that the outer impermeable membrane is moulded to the stent
scaffold and protrudes from within, in alignment with the stent pattern, while
the
inner permeable membrane remains in abutment with the inner circumference of
the device. Hydrogels expand and cause the balloons to pop out.
Figures 7A-7D are perspective views of an impermeable sealing system
= to protect the implantable device during storage in a preservative
Solution such
as glutaraldehyde, seals in place (Figure 7A); exterior seal being removed
(Figure 7B); exterior seal removed and interior seals being removed (Figure
7C,
= 7D).
Figure 8 is a cross-sectional view of the exterior and interior seals of
Figures 7A-7D.
Figures 9A-9D are schematics of the placement of a Sapien valve with
and without the disclosed seaang means. When the Sapien valve is placed too
low into the LVOT leading to the graft skirt not completely apposing against
the
vasculature (Figure 9A), perivalvular leak may occur from the gaps/area above
the skirt and around the device, through the open cells of the stent (Figure
9B).
The Sapien valve with sealing means, even when placed too low into the LVOT,
seals the valve uniformly against the inner wall of the LVOT (Figure 9C).
= Figure 9D shows how no perivalvular leak occurs when the seal is in
place,
preventing the "leaking" blood from going back into the left ventricle.
=Figure 10A shows a correctly placed SJM/Medtronic TAV device.
Figure 10B depicts an incorrectly placed SJM/Medtronic TAV device, resulting
=
in PV leaks. Figure 10C shows how perivascular leaks are prevented with an
incorrectly placed SJM/Medtronic TAV device with sealing means.
= Figures] IA and 11B are prospective views of a self-aligning support
member design for self-expanding TAV prosthesis, which enables system
deployment and retrieval without the use of "activation sutures".
10=
CA 02952464 2016-12-21
Figures 12A-12F are prospective view of the self-aligning support as it is
=
=
deployed, showing how the self-aligning support members are deployed from
the catheter first to align the catheter and subsequently the frame of the
prosthetic exits and extends outwardly and over the support members to
position
the prosthetic.
Figures 13A-13E are photographs of the deployment of the TAV using
the sealing support members to position seal at time of placement.
Figures 14A and 14B are graphs of percent swelling for the various
formulations at 5 min (Figure 14A) and 60 min (Figure 14B).
= 10 Figures 15A-15B show an in vitro model of a paravalvular
leak site due
to device inapposition (Figure 15A) and the leak site sealed with the seal
capsule
without disturbing the base geometry of the device (Figure 15B). The
conformation of the seal happens actively only in places where there are leak
sites. The seal does not decrease the central orifice area of the device not
having
any adverse effect on the blood flow as a result. View from heart into aorta;
device of Figures 2A-2C. =
DETAILED DESCRIPTION OF THE INVENTION
I. Definitions.
"Hydrogel" refers to a substance formed when an organic polymer
(natural or synthetic) is crosslinked via covalent, ionic, or hydrogen bonds
to
create a three-dimensional open-lattice structure which entraps water
molecules
to form a gel.. =
"Biocompatible" generally refers to a material and any metabolites or
degradation products thereof that are generally non-toxic to the recipient and
do
not cause any significant adverse effects to the subject.
"Biodegradable" generally refers to a material that will degrade or erode
by hydrolysis or enzymatic action under physiologic conditions to smaller
units
or chemical species that are capable of being metabolized, eliminated, or
excreted by the subject. The degradation time is a function of material
composition and morphology.
11
=
CA 02952464 2016-12-21
As used herein, "rapidly" expanding refers to a material which reaches
its desired dimensions in less than ten minutes after activation or exposure
to
fluid, more preferably in less than five minutes.
II. Endoluminal Device. Seal
A. Endoluminal Devices
Endoluminal prosthesis and sealing devices are advanced through a body
lumen in a first undeployed and reduced profile configuration. When positioned
in situ, the sealing device expands from its reduced radial profile
configuration
to a second configuration with an increased radial profile. In situ, and in
its
second configuration, the sealing device is configured to be positioned
between
the prosthesis and the wall of the body lumen. In one embodiment, when the
endoluminal prosthesis is at the desired location in the body lumen, it is
typically deployed from an introducer catheter whereupon it may move to an
expanded radial configuration by a number of mechanisms. In some
=
embodiments, the prosthesis may be spring expandable. Alternatively, a balloon
or expandable member can be inflated within the lumen of the prosthesis to
cause it to move to an expanded radial configuration within the vessel. This
= radial expansion, in turn, presses the sealing device against a wall of
the body
lumen. One of the advantages of the seal is that it only fills the gaps, and
does
= 20 not impact the placement and integrity ¨ both physical and functional,
of the
= prosthetic or the implant. =
In one embodiment, the sealing device is configured to fully seal a
= proximal, central and/or distal end of the endoluminal prosthesis for
endovascular aneurysm repair (EVAR) to prevent endoleaks and prevent
=subsequent migration and/or dislodgement of the prosthesis. =
In another embodiment, the sealing device is configured to fully seal a
transcatheter aortic valve. Figures 1A, 1B and 1C are perspective views of a
transcatheter aortic valve (TAV) 10 (Figure IA), a controlled activatable seal
= (Figure 1B) 12, and the seal placed around the TAV 14 (Figure 1C).
12
CA 02952464 2016-12-21
Figures 2A, 2B and 2C are perspective views of the TAV 14 of Figure
IC crimped toward the inflow side of the TAV10 in a telescopic manner (Figure
2A), with the TAV 10 and seal 12 in an expanded state with the stent aligned
with the bottom section of the TAV, with the activation wire 16 activated to
expose the seal 12 to fluids (Figure 2B), and post deployment, with the seal
12
expanded by swelling of the hydrogel within the.seal when it contacts the
blood.
The endoluminal device may be configured such that it moves
independently of the endoluminal prosthesis. Alternatively, the endoluminal
= device may be connected to the prosthesis for delivery to a target site.
The
endoluminal device may be connected to the prosthesis by any number of means =
= including suturing, crimping, elastic members, magnetic or adhesive
connection.
In one embodiment, the sealing means is positioned posterior to the
prosthetic implant, and is expanded and pulled up into a position adjacent to
the
implant at the time of sealing. This is achieved using sutures or elastic
means to =
= 15 pull the seal up and around the implant at the time of placement,
having a seal
that expands up around implant, and/or crimping the'seal so that it moves up
= around implant when implant comes out of introducer sheath. This is
extremely
important with large diameter implants such as aortic valves, which are
already
at risk of damage to the blood vessel walls during transport.
A key feature of the latter embodiment of the seal technology is that it
enables preservation of the crimped profile of the endoluminal prosthesis. The
seal technology is crimped distal or proximal to the prosthesis. In one aspect
of
this technology, the seal is aligned with the prosthesis by expansion of the
seal.
In another aspect, the seal zone of the prosthesis is aligned with the seal
zone
prior to expansion of the prosthesis by use of activation members. In yet
another
embodiment, the seal is aligned with the seal zone of the prosthesis prior to
= prosthesis expansion by use of activation members, which can be made of
an
elastic or non-elastic material.
In additional embodiments, the seal is positioned between the device
skeleton and the device, or on the exterior of the skeleton.
13
CA 02952464 2016-12-21
=
In a further embodiment, the endoluminal device may further include
one.or more engagement members. The one or more engagement members may
include staples, hooks or other means to engage with a vessel Wall, thus
securing
the device thereto.
B. The Seal
The seal includes a flexible component that is configured to conform to.
irregularities between the endoluminal prosthesis and a vessel wall. The seal
includes a generally ring-like structure having a first or inner surface and a
second or outer surface. It contains a material that swells upon contact with
a
= 10 fluid or upon activation of a foam, following placement, to inflate
and conform
the seal around the device.
As shown in Figure 3, the seal 12 is a capsule-within-a capsule. The seal
= i depending on i
12 can be provided n a variety of shapes, dependmg on the device t s to be
used with. A "D" shape is the preferred embodiment, with the flat portion
being
attached to the support structure and/or device to be implanted.
The seal can be composed of a permeable, semi-permeable, or
impenneable material. It may be biostable or biodegradable. For example, the
seal may be composed of natural or synthetic polymers such as polyether or
polyester polyurethanes, polyvinyl alcohol (PVA), silicone, cellulose of low
to
high density, having small, large, or twin pore sizes, and having the
following
features: closed or open cell, flexible or semi-rigid, plain, melamine, or
post-
treated impregnated foams. Additional materials for the seal can inolude
polyvinyl acetal sponge, silicone sponge rubber, closed cell silicone sponges,
silicone foam, and fluorosilicone sponge. Specially designed structures using
vascular graft materials including polytetrafluoroethylene (PTFE),
polyethylterephthalate (PET), polyether ether ketone (PEEK), woven yarns of
nylon, polypropylene (PP), collagen or protein based matrix may also be used.
= PEEK is the preferred material at this time since the strength is high sò
that there
will be no damage leading to failure when the TAV device is expanded against
14
=
CA 02952464 2016-12-21
=
sharp/calcified nodules and at the same time a relatively thin sheet of
material=
can be used, helping maintain a lower profile.
= The seal material may be used independently or in combination with a
mesh made from other types of polymers, titanium, surgical steel or shape
memory alloys.
In other embodiments, the capsule may be segmented to include one or
more compartments. The compartments may be =relatively closely spaced.
Further, thedistance between adjacent compartments may vary. The segmented
capsule of this embodiment may not extend completely around the endoluminal
prosthesis when the support member is in its second increased radial
= configuration. In one embodiment wherein the support member includes a
= capsule, the capsule may be substantially surrounded by the support
npember. In
= other embodiments, however, the capsule may be only partially enveloped
by
= the support member. =
= 15 = The capsule may include an outer wall to hold the
agent therein. The
= outer wall rnay be made of a suitably flexible and biocompatible
material.
Alternatively, the capsule may include a more rigid structure having a pre-
.
designed failure mechanism to allow the release of agent=therefrom. Examples
=
of suitable materials include, but are not limited to, low density
polyethylene,
high density polyethylene, polypropylene, polytetrafluoroethylene, silicone,
or
fluorosilicone. Other fluoropolymers that may be used for the construction of
the capsule include: polytetrafluoroethylene, perfluoroalkoxy polymer resin,
fluorinated ethylene-propylene, polyethylenetetrafluoroethylene,
= polyvinylfluoride, ethylenechlorotrifluoroethylene, polyvinylidene
fluoride,
polylychlorotrifluoroethylene, perfluoropolyether, =fluorinated ethylene
= propylene, terpolymer of tetrafluoroethylene, hexafluoropropylene and
vinylidene fluoride), polysulphone and polyether ether ketone (PEEK). It may
also include non-polymeric materials such as glass, bioglass, ceramic,
platinum
and titanium. It may further include biologically based materials such as
crosslinked collagen or alginates. It will be appreciated that the foregoing
list is
=
=
CA 02952464 2016-12-21
provided merely as an example of suitable materials and is not an exhaustive
list. The capsule may be composed of a material or combination of materials
different from those provided above.
The rate of release of the agent from the support member may vary._ In
some embodiments, pressure exerted on the support member to rupture a capsule
=
rnay release one or more agents. This rate of almost immediate release is
particularly useful for delivering adhesive agents to a vessel to affix a
prosthesis
to a Wall of the vessel. However, other agents may be released at a slower or
at
least a variable rate. Further, the agents may be released after the initial
release
of a primary agent (e.g. the adhesive).
For example, in an embodirnent wherein the support member includes a
segmented capsule, the first agent to be released may be held in one or more
=
"immediate release" sub-compartments which include an outer wall configured
to rupture under a pre-defined initial pressure. The support member may .-
include one or more slow release sub-compartments having outer walls =
configured to withstand the initial pressure but which either rupture when
=
subjected to a greater pressure or which do not rupture but rather degrade
over a
certain period of time to release an agent held therein.
Typically, the capsule is configured to rupture to release one or more
agents at a predetermined range of pressures. The range of rupture pressures
includes between 5 and 250 psi, between 5 and 125 psi, between 10 and 75 psi,
= or at approximately 50 psi.
A variety of different techniques or processes can be used to form
pressure activated capsules or compartments. In one embodiment, for example,
= 25 a process for forming a pressure activated capsule includes pre-
stressing the
capsule during formation. The pre-stressed material will have a limited
capacity
to stretch when subjected to external pressure, and will fail when reaching
critical stress on the stress-strain curve. The first stage of this method
includes
selecting a biocompatible capsule material that is also compatible with its
=
contents= (e.g., the agent which can.include adhesive material or a wide
Variety of =
16
CA 02952464 2016-12-21
=
other types of materials). The capsule material should also have a tensile
= strength suitable for the particular application in which the capsule
will be used.
The next stage of this method includes forming an undersized capsule.
The undersized capsule is essentially shaped as an extruded, elongated tube
(e.g., a "sausage") with one end of the tube sealed (e.g., by dipping, dip
molding,
vacuum forming blow molding, etc). The process continues by expanding the
capsule to its final shape. The capsule can be expanded, for example, by
stretching (e.g., either hot or cold) using appropriate tooling so that the
capsule
material is pre-stressed to within a stress level, and whereby the clinically
=
relevant balloon inflation pressure will exceed the failure stress of the
capsule
material. The method can further include filling the capsule with the desired
contents while the capsule is under pressure so as to achieve pre-stressing in
a
single step. After filling the capsule, the capsule can be sealed (e.g., using
a heat
welding process, laser welding process, solvent welding process, etc.).
In another embodifnent, a capsule can be formed by forming an air =
pillow or bubble wrap-type capsule assembly using a vacuum form process or
other suitable technique. The next stage of this process includes= perforating
a
film at the base of the capsule assembly and filling the individual capsules
with
= the desired contents under an inert atmosphere_ After filling the
capsules, the
puncture hole can be resealed by application of another film over the puncture
hole and localized application of heat and/or solvent. Other methods can be
= used to seal the puncture hole. In several embodiments', the capsule can
be
configured such that the puncture hole re-ruptures at the same pressure as the
capsule itself so that there is some agent (e.g., adhesive material within the
capsule) flowing onto the corresponding portion of the endoluminal prosthesis.
One or more failure points can be created within a capsule. This process
can include creating a capsule shaped as an extruded, elongated tube with one
= end of the tube sealed (e.g., by dipping, dip molding, vacuum forming
blow
molding, etc.). The capsule can be composed of a polymer material (e.g.,
polyethylene, polypropylene, polyolefin, polytetrafluoroethylenes, and
silicone =
17
CA 02952464 2016-12-21
= rubber) or another suitable material. At one or more predetermined
locations
along the elongated tube, the process can include creating areas of
substantially
reduced thickness. These areas can be formed, for example, using a tool (e.g.,
a
core pin with a razor blade finish along the length of the capsule), laser
ablation,
creating partially penetrating holes, creating an axial adhesive joint (e.g.,
tube
from a sheet) that is weaker than the substrate, or other suitable techniques.
The
= method next includes filing the capsule with the desired contents at a
pressure
below that required to rupture the thinned or weakened areas. After filling
the
= capsule, the open end of the capsule' can be sealed using one of the
welding
processes described above=or other suitable processes.
. =
In yet another particular embodiment, one or more stress points can be
created within a capsule. This method can include forming a capsule and
filling
the capsule with the desired contents using any of the techniques described
= above. After forming the capsule and with the capsule in an undeployed
= 15 configuration, the process can further include wrapping a suture
(e.g., a nitinol
wire) about the capsule at a predetermined pitch and tension. When the capsule
is moyed from the undeployed state to a deployed configuration and takes on a
curved or circumferential shape, the suture compresses the capsule at the
predetermined points. Stress points are created in the capsule walls at these
points because of the increased pressure at such points:
In another embodiment the device may include one or more pressure
points on the supporting member such as spikes or other raised areas which
cause the penetration of the capsule once a predetermined pressure is applied
= thereto.
= 25 Still yet another particular embodiment for forming a
pressure activated
capsule or compartment includes creating a double walled capsule in which an =
inner compartment of the capsule is sealed and separated from an outer
=
compartment of the capsule that contains the adhesive or other desired agent.
= The inner compartment can be composed of a compliant or flexible
material,
and the outer compartment can be composed of a substantially less compliant
=
18
=
CA 02952464 2016-12-21
material. The outer compartment may or may not have failure points. The inner
= compartment is in fluid communication via a one way valve with a low
compliance reservoir. The reservoir is configured to be pressurized by
inflation =
of an expandable member or balloon to a high pressure, thereby allowing the
=
valve to open and pressurize and expand the inner compartment. This process in
= turn pressurizes the outer compartment (that contains the adhesive) until
the
outer compartment ruptures. One advantage of this particular embodiment iS
that it can increase the pressure within the capsule to a value higher than
otherwise possible with an external expandable member or balloon alone.
In a still further embodiment, the capsule has an inner compartment
made from a relatively rigid material or mesh and an outer compartment made
from a relatively flexible material. In =this embodiment, the inner
compartment
= acts as a reservoir, containing the agent and is designetito break or
rupture at a =
predetermined pressure: The outer compartment may also have a failure
1.5 pressitre point to allow release of the agent.. The rigidity of
the inner
compartment may provide a longer-term stability and shelf life of the
encapsulated agent. The application of rupture pressure may be carried out
either locally or remotely, e.g. via a tube directly connected to the capsule
that is
connected to an external source at the delivery device entry site (e.g.
femoral
artery). ==
Expandable Capsule
In one embodiment, a seal entirely surrounds the capsule such that the
capsule is "suspended" within the seal. In one specific embodiment, for
example, the seal 12 can include a porous material configured to prevent any
. 25 embolization (distal or proximal) of released agent(s) 108 from the
capsule 106.
The seal may have a graded degree of relative porosity from relatively porous
to
relatively non-porous. Preferred porosity size is from five to seventy
microns,
more preferably about 35 microns so that the fluid can rapidly access the
swellable material.
19
=
CA 02952464 2016-12-21
In the preferred embodiment, the capsule is a single annular =
compartment within the seal, and extends completely around the periphery of
the endoluminal prosthesis. In other embodiments, however, the capsule may
include one or more additional compartments or sections, and may not extend
completely around the endoluminal prosthesis. Moreover, the capsuie may or
=
may not be contained within the seal, and can be positioned at a different
location on the apparatus relative to the seal. In addition, the capsule can
have a
variety of different shapes and/or sizes depending upon the particUlar
application, the agent(s), the configuration of the endoluminal prosthesis,
and a
number of other factors. = =
Permeable and Impermeable Membranes =
In a preferred embodiment, shown in Figure 3, the seal 12 includes two
= = membranes, an inner membrane 18 and an outer membrane 20. An
expandable
material such as a foam =or hydrogel 22 is placed within the inner membrane
18.
The inner membrane 18 is semi-permeable (allowing fluid ingress but not egress
of entrapped hydrogel or foam) while the outer membrane 20 is impermeable =
except at an optional pre-determined rupture point 24. The outer membrane 20
=
is designed to be impermeable to fluid during storage and transport and during
any pre-procedural preparations e.g. rinsing or washing of the device, to
protect
= , 20
the polymer 22 from premature swelling. The outer membrane 20 is also =
designed to be strong and puncture resistant so that it does not tear or is
punctured or pierced by the sharp edges of the native calcification even when
subject to pressures up to 14atm. This prevents the rupture of the inner
membrane 18, mitigating any risk of embolization of the expandable material or
hydrogel 22. The rupture point 24 allows fluid such as blood to penetrate into
the expandable seal only when the seal is expanded in place, thereby
preventing =
=leaks.
= Permeable membranes may be made from a variety of polymer or =
organic materials, including polyimides, phospholipid bilayer, thin film
composite membranes (TFC or TFM), cellulose ester membranes (CEM),
_
CA 02952464 2016-12-21
charge mosaic membranes (CMM), bipolar membranes (BPM), and anion
= exchange membranes
(AEM). =
A preferred pore size range for allowing fluid in but not hydrogel to
= escape is
from five to =seventy microns, more preferably about 35 to seventy =
microns, most preferably about 35 microns, so that the fluid can rapidly
access
= the swellable material.
The permeable membrane may be formed only of permeable material, or
may have one or more areas that are impermeable. This may be used to insure
that swelling does not disrupt the shape of the seal in an undesirable area,
such
as on the interior of the device where it abuts the implant or prosthesis, or
where
it contacts the device support members.
In some embodiments, the second impermeable membrane is applied =
with plasma vapour deposition, vacuum deposition, co-extrusion, or press
lamination.
Expandable.Materials
Expandable materials which swell in contact with an aqueous fluid are
preferred. Most preferably, these materials expand from two to 100 times; more
= preferably from 50 to 90 fold, most preferablY about 60 fold. Blood
and/or other
= fluids at the site of implantation can penetrate into the seal after it
is breached,
= 20 causing dried or expandable materials. to absorb the fluid and swell
or react to
expand due to formation or release of gas reaction products. The semi-
permeable inner membrane 18 prevents the expandable material 22 from
escaping the seal 12, but allows fluid to enter. By expanding in volume, the
material seals the endoluminal space.
= 25 Any expandable material having suitable physical and
chemical
properties may be used. In certain embodiments, the expandable material is a
hydrogel. Other suitable materials include foams and sponges formed at the
time of activation.
Expandable materials are chosen to be stable at both room temperature
30 and 37-40 C and to be sterilizable by one or more means such as
radiation or
21
CA 02952464 2016-12-21
steam. Sponges or foams can be made from biocompatible materials that allow
= tissue ingrowth or endothelialisation of the matrix. Such
endothelialisation or
tissue ingrowth can be facilitated either through selection of appropriate
polymeric materials or by coating of the polymeric scaffold with suitable
growth
promoting factors or proteins.
1. =
Hydrogels =
Hydrogels are selected to provide rapid swelling as well as to be =
=
. biocompatible in the event of a breach of capsule integrity. Two or more
hydrogels or other materials that swell may be used.
Expandable gels have been developed that are stronger and more
resilient than current expandable gels. These gels are able to expand rapidly
to at
least 10x, 20x, 25x, 30, or 40x of the dry state and more preferably up to 50
x
their dry state when exposed to physiological liquids in less than 25, 24, 23,
22,
21, 20, 19, 18, 17, 16, 15,=14, 13, 12, 11, 10, 9, 8, 7, 6, 5, or 4 minutes.
These
stronger gels are synthesized using long chain cross-linkers, typically
molecules
with more than 20 carbon atoms and/or a molecular weight greater than 400Da,
more preferably more than 40 carbon atoms and/or a molecular weight greater
=
than 800 Da, that will act as molecular reinforcement molecules, creating a
more
resilient and longer lasting gel while maintaining excellent swelling
properties.
The swelling force of these gels can also be adjusted to not exert more radial
force than necessary, typically around 0.0005N/mm2 to 0.025N/mtn2, preferably
= 0.002N/mm2 to 0.012N/mm2.
In some embodiments, these gels can be spray dried onto,nr covalently
attached to, abase membrane or mesh used to encapsulate the gel before being
fitted to the surgical device. The gels can be covalently attached by
introducing
one or more functional groups that can form covalent bonds to one or more
functional groups on the base membrane or mesh. Suitable functional groups
include, but are not limited to, allylic, vinyl or acrylic groups. The
functional =
groups can be introduced directly onto the gel and/or membrane or mesh or as
part of a longer/larger chemical moiety. "Allyl", as used herein, refers to a
group
= 22
CA 02952464 2016-12-21
having the structural formula H2C=CH-CH2R, where R is the point of
= connection to the rest of the molecule, i.e., hydrogel and/or base
membrane or
mesh. "Acrylic", as used herein, refers to a group having the structure
H2C=CH¨C(=0)--. The preferred IUPAC name for the group is prop-2-enoyl,
and it is also (less correctly) known as acrylyl or simply acryl. Compounds
containing an acryloyl group can be referred to as "acrylic compounds".
"Vinyl", as used herein, refers to a group containing the moiety ¨CH=CH2,
which is a derivatives of ethenc, CH2=CH2, with one hydrogen atom replaced
with some other group or bond, such as a bond to the base substrate or
membrane. Vinyl groups can be introduced directly onto the hydrogel and/or
base membrane or mesh or can be part of a longer/larger chain.
=
The long chain hydrophilic crosslinking agents described above have at
= least two and preferably more than two reactive functional groups (e.g.,
allyI,
acrylic, vinyl, etc.) capable of participating in a free radical
polymerization
= 15 reaction or additional reaction, such as Michael addition, and where
at least part
of the molecule is attached to a subStrate, anchoring the gel to the substrate
to
prevent release of smaller gel particles in case of gel fracture.
Long-chain cross-linkers and/or the chemical attachment of the gels to a
porous substrate result in gels that are more capable of withstanding cyclic
loads. These seals containing gels can be made in any shape, including annular
=or strip shape.= The principle behind these cross-linkers is that rather than
having
= a short cross-linker with only two polymerizable groups, the crosslinking
agents
=
described herein includes long chain hydrophilic polymer (such as PVA, PEG,
= PVAc, natural polysaccharides such as dextran, HA, agarose, and starch)
with
multiple polymerizable/reactive groups. The long chain crosslinking agents
result in a hydrogel which is less susceptible to "fragmenting" which is
important as it minimizes any risk of small gel particles breaking off and
embolizing to the brain. The long chain crosslinking agents also result in
increased integrity of the hydrogel, making it more pliable and thereby
= increasingly resilient under cyclic loads, an important factor for long-term
23
=
CA 02952464 2016-12-21
durability of the hydrogel. The benefits are a much stronger hydrogel,
approximately 0.0005N/mm2 to 0.025N/mm2, more preferably between
0.002N/mm2 to 0.012N/mm2, as compared to hydrogels crosslinked with short
= chain divalent linkers, as noted above, less than 20 carbon atoms and/or
a
molecular weight of less than 400 Da with two active groups that can be used
for cross-linking (e.g. vinyl, acrylic, allylic). Interestingly, while these
gels are
very firm, they at the same time possess very good swelling characteristics.
Very
strong gels do not swell as much and/or as rapidly. As used herein, very
strong
refers generally to hydrogels having a strength greater than about 0.0005N/mm2
to 0.025N/mm2. Desired rates of swelling are 30x or greater, with an ideal
range
of 50x - 80x. The greater the swelling rate, the smaller the introduction
profile of
the device, allowing treatment of a greater number of patients who have
smaller
access vessels (femoral arteries, radial arteries, etc.).
Suitable components of such gels include, but are not limited to, acrylic
acid, acrylamide or other polymerizable monomers; cross-linkers such as
polyvinyl alcohols as well as partially hydrolyzed poly vinyl acetates, 2-
.
hydroxyethyl methacrylates (HEMA) or various other polymers with reactive
side groups such as acrylic, allylic, and vinyl groups, can be used. In
addition, a
wide range of natural hydrocolloids such as dextran, cellulose, agarose,
starch,
galactomannans, pectins, hyaluronic acid etc. can be used. Reagents such as
ally' glycidyl ether, allyl bromide, allyl chloride etc. can be used to
incorporate
the necessary double bonds to participate in a free radical polymerization
= reaction or addition reaction, such as those containing acrylic, allylic
and vinyl
groups, into the backbones of these polymers. Depending on the chemistry
employed, a number of other reagents can be used to incorporate reactive
double
bonds.
= Studies to identify hydrogels having substantial swelling in a short time
were.performed, as described in examples 1 and 2. The main factors that
influence swelling of a hydrogel based on polymerisation and cross-linking of
synthetic monomers are: =
24
CA 02952464 2016-12-21
(1) type of monomer;
(2) type of cross-linker;
(3) concentration of monomer and cross-linker in the gel; and
(4) the ratio of monomer to cross-linker.
Examples of rapidly swelling hydrogels include, but are not limited to,
acrylic acid polymers and copolymers, particularly crosslinked acrylic acid
polymer and copolymers. Suitable crosslinking agents include acrylamide,
di(ethylene glycol) diacrylate, poly(ethylene glycol) diacrylate, and long-
chain
hydrophilic polymers with multiple polymerizable groups, such as poly vinyl
alcohol (PVA) derivatized with allyl glycidyl ether. Additional examples of
materials which can be used to form a suitable hydrogel include
polysaccharideS
such as alginate, polyphosphazines, poly(acrylic acids), poly(methacrylic
acids),
poly(alkylene oxides), poly(vinyl acetate), polyvinylpyrrolidone (PVP), and
copolymers and blends of each. See, for example, U.S. Patent No. 5,709,854,
6,129,761 and 6,858,229.
= In general, these polymers are at least partially soluble in aqueous
solutions, such as water, buffered salt solutions, or aqueous alcohol
solutions. In
some embOdiments, the polymers have charged side groups or are monovalent
=
ionic salts thereof. Examples of polymers with acidic side groups that can be=
reacted with cations are poly(phosphazenes), poly(acrylic acids),
= poly(methacrylic acids), poly(vinyl acetate), and sulfonated polymers,
such as
sulfonated polystyrene. Copolymers having acidic side groups formed by
= reaction of acrylic or methacrylic acid and vinyl ether monomers or
polymers
can also be used. Examples of acidic groups are carboxylic acid groups and
=
sulfonic acid groups. =
= Examples of polymers with basic side groups that can be reacted with
=
anions are poly(vinyl amines), poly(vinyl pyridine), poly(vinyl imidazole),
and
some imino substituted polyphosphazenes. The ammonium or quaternary salt of
the polymers can also be formed from the backbone nitrogens or pendant imino
groups. Examples of basic side groups are amino and imino groups.
= 25 '
=
CA 02952464 2016-12-21
A water-soluble gelling agent such as a polysaccharide gum, more
preferably a polyartionic polymer like alginate can be cross-linked with a
polycationic polymer (e.g., an amino acid polymer such as polylysine) to form
a
shell. See e.g., U.S. Patent Nos. 4,806,355, 4,689,293 and 4,673,566 to Goosen
=
et al.; U.S. Patent Nos. 4,409,331, 4,407,957, 4,391,909 and 4,352,883 to Lim
et
al.; U.S. Patent Nos. 4,749,620 and 4,744,933 to Rha et al.; and U.S.
PatentNo.
5,427,935 to Wang et al. Amino acid polymers that may be used to crosslink
hydrogel forming polymers such as alginate include the cationic poly(amino
acids) such as polylysine, polyarginine, polyornithine, and copolymers and
= 10 blends thereof.
Other exemplary polysaccharides include chitosan, hyaluronan (HA),
=and chondroitin sulfate. Alginate and chitosan form crosslinked hydrogels
under
certain solution conditions, while HA and chondroitin sulfate are preferably
modified to contain crosslinkable groups to form a hydragel. Alginate forms a
gel in the presence of divalent cations via ionic crosslinking. Although the
properties of the hydrogel can be controlled to some degree through changes in
the alginate precursor (molecular weight, composition, and macromer
= concentration), alginate does not degrade, but rather dissolves when the
divalent
cations are replaced by monovalent ions. In addition, alginate does not
promote
cell interactions. See U.S. Patent No. 4,391,909 to Lim et al. for description
of =
alginate hydrogel crosslinked with polylysine. Other cationic polymers
suitable .
for use as a cross-linker in place of polylysine include poly( 13-amino
alcohols) =
(PBAAs) (Ma M, et al. Adv. Mater. 23:H189-94 (2011).
Chitosan is made by partially deacetylating chitin, a natural
= 25 nonmammalian polysaccharide, which exhibits a close resemblance to
mammalian polysaccharides, making it attractive for cell encapsulation.
Chitosan degrades predominantly by lysozyme through hydrolysis of the
= acetylated residues. Higher degrees of deacetylation lead to slower
degradation
times, but better cell adhesion due to increased hydrophobicity. Under dilute
=acid conditions (pH < 6), chitosan is positively charged and water soluble,
while
26
=
CA 02952464 2016-12-21
at physiological pH, chitosan is neutral and hydrophobic, leading to the
formation of a solid physically crosslinked hydrogel. The addition of polyol
salts
enables encapsulation of cells at neutral pH,. where gelation becomes
temperature dependent:
Chitosan has many amine and hydroxyl groups that can be modified. For
example, chitosan has been modified by grafting methacrylic acid to create a
crosslinkable macromer while also grafting lactic acid to enhance its water
= solubi% at physiological pH. This crosslinked chitosan hydrogel= degrades
in
the presence of lysozyme and chondrocytes. Photopolymerizable chitosan
= 10 macromer can be synthesized by modifying chitosan with photoreactive
azidobenzoic acid groups. Upon exposure to UV in the absence of any initiator,
=
reactive nitrene groups are formed that react with each other or other amine
=
. = groups on the chitosan to form an azo crosslink. =
Hyaluronan (HA) is a glycosaminoglycan present in many tissues
throughout the body that Plays an important role in embryonic development,
wound healing, and angiogenesis. In addition, HA. interacts with cells through
=
cell-surface receptors to influence intracellular signaling pathways.
Together,
these qualities make HA attractive for tissue engineering scaffolds. HA can be
modified with crosslinkable moieties,.such as methacrylates and thiols, for
cell
= 20 encapsulation. Crosslinked HA gels remain susceptible to degradation
by
hyaluronidase, which breaks HA into oligosaccharide fragments of varying
molecular weights. Auricular chondrocytes can be encapsulated in
= photopolymerized HA hydrogels where the gel structure is controlled by
the
macromer concentration and macromer molecular weight. In addition,
photopolymerized HA and dextran hydrogels maintain long-term culture of
= undifferentiated human embryonic stem cells. HA hydrogels have also
been == =
fabricated through Michael-type addition reaction mechanisms where either
acrylated HA is reacted with PEG-tetrathiol, or thiol-modified HA is reacted
with PEG diacrylate.
= =
=
= 27
=
CA 02952464 2016-12-21
Chondroitin sulfate makes up a large percentage of structural
proteoglycans found in many tissues, including skin, cartilage, tendons, and
heart valves, making it an attractive biopolymer for a range of tissue
engineering
applications. Photocrosslinked chondroitin sulfate hydrogels can be been
prepared by modifying chondroitin sulfate with methacrylate groups. The
hydrogel properties were readity controlled by the degree of methacrylate
substitution and macromer concentration in solution prior to polymerization.
Further, the negatively charged polymer creates increased swelling pressures
allowing the gel to imbibe more water without sacrificing its mechanical
properties. Copolymer hydrogels of chondroitin sulfate and an inert polyrner,
such as PEG or PVA, may also be used.
Biodegradable PEG hydrogels can be been prepared from triblock
copolymers of poly(a-hydroxy esters)-b-poly (ethylene glycol)-b-poly(a-
-
= hydroxy esters) endcapped with (meth)acrylate functional groups to enable
crosslinking. PLA and poly(8-caprolactone) (PCL) have been the most
commonly used poly(a-hydroxy esters) in creating =biodegradable PEG
macromers for cell encapsulation. The degradation profile and rate are
controlled through the length of the degradable block and the chemistry. The
ester bonds may also degrade by esterases present in serum, which accelerates
= 20 degradation. Biodegradable PEG hydrogels can also be fabricated from
precursors of PEG-bis-[2-acryloyloxy propanoate]. As an alternative to linear
PEG macromers, PEG-based dendrimers of poly(glycerol-succinic acid)-PEG,
which contain muttiple reactive vinyl groups per PEG molecule, can be used. An
attractive feature of these materials is the ability to control the degree of
branching, which consequently affects the overall structural properties of the
hydrogel and its degradation. Degradation will occur through the ester
linkages
= present in the dendrimer backbone.
The biocompatible, hydrogel-forming polymer can contain =
polyphosphoesters or polyphosphates where the phosphoester linkage is
susceptible to hydrolytic degradation resulting in the release of phosphate.
For
28
=
=
CA 02952464 2016-12-21
example, a phosphoester can be incorporated into the backbone of a
crosslinkable PEG macromer, poly(ethylene glycol)-di-[ethylphosphatidyl
(ethylene glycol) methacrylate] (PhosPEG-dMA), to form a biodegradable
hydrogel. The addition of alkaline phosphatase, an ECM component synthesized
= 5 by bone cells, enhances degradation. The degradation product,
phosphoric acid,
reacts with calcium ions in the medium to produce insoluble calcium phosphate
inducing autocalcification within the hydrogel. Poly(6-aminoethyl propylene
phosphate), a polyphosphoester, can be modified with methacrylates to create
multivinyl macromers where the degradation rate was controlled by the degree
of derivitization of the polyphosphoester polymer.
= Polyphosphazenes are polymers with backbones consisting of nitrogen
and phosphorous separated. by alternating single and double bonds. Each
phosphorous atom is covalently bonded to two side chains. The
polyphosphazenes suitable for cross-linking have a majority of side chain
groups
= 15 which are acidic and capable of forming salt bridges with di- or
trivalent cations.
Examples of preferrecfacidic side groups are carboxylic acid groups and
sulfonic acid groups. Hydrolytically stable polyphosphazenes are formed of
monomers having carboxylic acid side groups that are crosslinked by divalent
or
trivalent cations such as Cal+ or Al3+. Polymers can be synthesized that
degrade
by hydrolysis by incorporating monomers having imidazole, amino acid ester, or
glycerol side groups. Bioerodible polyphosphazines have at least two differing
= types of sick chains, acidic side groups capable of forming salt bridges
with
multivalent cations, and side groups that hydrolyze under in vivo conditions,
e.g., imidazole groups, amino acid esters, glycerol and glucosyl. Hydrolysis
of
the side chain results in erosion of the polymer. Examples of hydrolyzing side
chains are unsubstituted and substituted imidizoles and amino acid esters in
=
which the group is bonded to the phosphorous atom through an amino linkage
(polyphosphazene polymers in which both R groups are attached in this manner
are known as polyaminophosphazenes). For polyimidazolephosphazenes, some
29
CA 02952464 2016-12-21
of the "R" groups on the polyphosphazene backbone are imidazole rings,
attached to phosphorous in the backbone through a ring nitrogen atom.
[nail embodiments, it is absolutely critical that the hydrogel/expandable
material operates under sufficient low pressure so that it does not push the
stent
away from the wall or alter the device configuration. ln summary, the
expandable material is contained within a material, such as a semi-permeable
or
impermeable material so that it is retained at the site Where it is needed to
seal a
leak. The material is selected based on the means for activation. If the
material
is expanded by mechanical shear or exposure to a foaming agent, these
materials
are provided internally within the seal, allowing an external activating agent
such as an activation wire to disrupt the.means for isolating the activation
agent
from the expandable material. If the material is activated by contact with
fluid,
no additional means for isolation are required if the device is stored dry
prior to
tise, since it will activate in situ when exposed to body fluids. If the
material is
stored wet prior to use, a second impermeable membrane is required to keep the
expandable material dry prior to activation. This will typically include a
rupture
site which is opened at the time of implantation to allow biological fluid to
reach
the expandable material through the semi-permeable material (i.e., where Semi-
permeable refers to a material retaining the expandable material but allowing
fluid to pass). Alternatively the impermeable material may not include a
rupture
site but simply be removed after the device is removed from storage and washed
with saline, prior to loading into the catheter, so that once the device is
deployed, in situ liquid will cause the hydrogel to swell.
The properties of the different materials complement each other. For
example, in the time immediately after valve deployment it is important that
the
material swells quickly to seal perivalvular leaks as soon as possible.
Mechanical strength may be compromised in the short term to enable fast
swelling. In the long term, however, it is paramount that the seal has high
mechanical strength. In some embodiments, the mechanical strength of the
hydrogel(s) is from about 0.0005 N/mm2 to about 0.025 N/mm2, preferably from
CA 02952464 2016-12-21
about 0.002 N/mm2 to about 0.012 N/rnm2. The mechanical strength should be
high enough to allow swelling and thereby "actively" conform to the gaps .
leading to leakage but not high enough to disturb the physical or functional
integrity of the prosthesis or implant or to push the prosthesis or implant
away
from the wall. Another important consideration is that the mechanical strength
should not be so high as to exert excess pressure on the anatomy, particularly
around the Left Bundle Branch (LBB), which is responsible for the cardiac
conduction. If excess pressure is exerted a cardiac conduction abnormality
known as the Left Bundle Branch Block (LBBB) may occur. Typically, it is
taken into consideration that the outward pressure exerted on the anatomy by
the
swelling of the hydrogel is less than that exerted by the prosthesis or
implant.
A degradable material, which may be a hydrogel, that swells quickly,
may be used in conjunction with a nondegradable material, which may be a
hydrogel, that swells slower but has higher mechanical strength. In the short
term, the degradable material capable of rapid swelling will quickly seal the=
= perivalvular leak. Over time, this material degrades and will be replaced
by the
=
material exhibiting slower swelling and higher mechanical strength.
Eventually,
the seal will be composed of the slower swelling nondegradable material. It is
also possible to use only one material in the seal, but in two or more
different
forms. For example, two different crystal sizes of hydrogels may be used in
the =
= seal, because different particle sizes of hydrogel may exhibit different
=
properties. =
2. Foams and Sponges
Alternatively, a foam generated prior to implantation can also be used as
a swellable material to form a seal. For example, a suitable matrix, such as a
biocompatible polymer or crosslinkable prepolymer, may be blended with one
or more foaming agents. Foaming agents include compounds or mixtures of
= compounds which generate a gas in response to a stimulus. When dispersed
= within a matrix and exposed to a stimulus, the foaming agents evolve a
gas,
causing the matrix to expand as fine gas bubbles become dispersed within the
=31 =
CA 02952464 2016-12-21
matrix. Examples of suitable foaming agents include compounds which evolve
=
a gas when hydrated with biological fluids, such as mixture of a
physiologically
acceptableacid (e.g., citric acid or acetic acid) and a physiologically
acceptable
= base (e.g., sodium bicarbonate or calcium carbonate). Other suitable
foaming
agents are known in the art, and include dry particles containing pressurized
gas,
such as sugar particles containing carbon dioxide (see, U.S. Patent No.
3,012,893) or other physiologically acceptable gases (e.g., nitrogen or
argon),
and pharmacologically acceptable peroxides. =
Other examples include changing the morphology of known hydrogel =
materials in order to decrease swelling times. Means for changing the
morphology include increasing the porosity of the material, for example, by
freeze-drying or porogen techniques. For example, particles can be produced by
= spray drying by dissolving a biocompatible material such as a polymer and
surfactant or lipid in an appropriate solvent, dispersing a pore forming agent
as a
1.5 solid or as a solution into the solution, and then spray drying the
solution and the
. pore forming agent, to form particles. The polymer solution and pore
forming
agent are atomized to form a fine mist and dried by direct contact with hot
carrier gases. Using spray dryers available in the art, the polymer solution
and
. pore forming agent may be atomized at the inlet port of the spray dryer,
passed
through at least one drying chamber, and then collected as a powder. The
temperature may be varied depending on the gas or polymer used. The
temperature of the inlet and outlet ports can be controlled to produce the
desired =
products. The size and morphology of the particles formed during spray drying
is a function of the nozzle used to spray the solution and the pore forming
agent,
the nozzle pressure, the flow rate of the solution with the pore forming
agent, the
polymer used, the concentration of the polymer in solution, the type of
polymer
solvent, the type and the amount of pore forming agent, the temperature of
= = =spraying (both inlet and outlet temperature) and the polymer molecular
weight.
= Generally, the higher the polymer molecular weight, the larger the
particle size,
assuming the polymer solution concentration is the same. =
32
=
CA 02952464 2016-12-21
=
Typical process parameters for spray drying are as follows: inlet
temperature=30-200 C, outlet temperature=5-100 C, and polymer flow rate=10
=
-
.
5,000 ml/min. Pore forming agents are included in the polymer solution in an
amount of between 0.01% and 90% weight to volume of polymer solution, to
increase pore formation. For example, in spray drying, a pore forming agent
such as a volatile salt, for example, ammonium bicarbonate, ammonium acetate,
ammonium carbonate, ammonium chloride or ammonium benzoate or other
volatile salt as either a solid or as a solution in a solvent such as water
can be
used. The solid pore forming agent or the solution containing the pore forming
agent is then emulsified with the polymer solution to create a dispersion or
droplets of the pore forming agent in the polymer. This dispersion or emulsion
is
- then spray dried to remove both the polymer solvent and the pore forming
agent.
After the polymer is preCipitated, the hardened particles can be frozen and
= lyophilized to remove any pore forming agent not removed during the
polymer
precipitation step.
Fast swelling can be achieved by preparing small particles of dried
hydrogels. The extremely short diffusion path length of microparticles makes
it
possible.to complete swelling in a matter of minutes. Large dried
hydrogels.can
= be made to swell rapidly regardless of their size and shape by creating
pores that
are interconnected to each other throughout the hydrogel matrix. The
interconnected pores allow for fast absorption of water by capillary force. A
simple method of making porous hydrogel is to produce gas bubbles during
polymerization. Completion of polymerization while the foam is still stable
results in formation of superporous hydrogels. Superporous hydrogels can be
=
synthesized in any =molds, and thus, three-dimensional structure of any shape
can
be easily made. The size of pores produced by the gas blowing (or foaming)
==
method is in the order of 100 mm and larger.
= If any portion of a superporous hydrogel is in contact with water or an
aqueous medium, water is absorbed immediately through the open channels to
33
CA 02952464 2016-12-21
fill the whole space. This process makes the dried superporous hydrogels-swell
very quickly.
Expandable sponges or foams can also be used for sealing of surgical
implantations. These sponges or foams and be cut into a strips or annular
shapes
and either dried down or dehydrated by other means and then be allowed to
rapidly re-hydrate once the device is in place. Alternatively, such materials
can
be hydrated and then squeezed to reduce their volume to allow these to be
attached to the surgical implement and then allowed to expand to form a seal
once the surgical implement is in place. Such swelling would be nearly
instant.
One further benefit of sealing material in the form of sponges or foams is
that =
their expansion can be reversible so that they can easier be retracted from
their
implanted position back into the delivery catheter and thereby enable complete
re-positioning of the device multiple times and/or complete retrievability of
the
device. Such sponges and foams can be made from a range of materials
- 15 including, but not limited to, synthetic polymers, natural polymers or
mixtures
thereof. Such materials can be formed by including pore forming substances
= such as gas or immiscible solvents in the monomer/polymer mix prior to
= polymerization and/or cross-linking. By using the appropriate monomers
and/or
polymeric cross-linkers such sponges/foams can be made to withstand cyclic
stress; such materials could also further be reinforced with compatible fibres
or
whiskers to increase strength and reduce the probability for breakage. =
In some embodiments, these sponges or foams can be chemically
attached to a base membrane or mesh used to encapsulate the sponge/foam
before being fated to the surgical device. This could be done by attaching
either
allylic or acrylic groups to the base substrate, either as small molecules or
as
= long chain tentacles anchoring the expandable to the substrate preventing
release
of smaller particles in case of fracture.
Foams may be designed to expand without the need for the semi-
= permeable membrane.
34
CA 02952464 2016-12-21
C. The support member or skeleton
The seal may be sufficiently flexible to conforrn to irregularities between
= the endoluminal prosthesis and a vessel wall. The band of material may
include
a mesh-like or a generally ring-like structure configured to receive at least
a
portion of an endoluminal prosthesis such that it is positioned between the
portion of the prosthesis and a vessel wall. This is usually referred to as a
skeleton or support member.
As shown in Figures 4A-4C, the seal 12 has a stent/metal backing or
skeleton 26. The skeleton 26 provides structure and enables crimping, loading
and deployment. The skeleton 26 can be either a balloon expanding or a self-
expanding stent. The skeleton 26 is attached to the surface of the outer
membrane 20.
When the support member is in the second reduced radial configuration,
it may form a substantially helical configuration. The helical structure of
the
support member provides an internal passage therein to receive at least a
portion
= of an endotuminal prosthesis. The support member may include steel such
as
= MP35N, SS316L'VM, or L605, a shape memory material or a plastically
expandable material. The shape memory material may include one or more
shape memory alloys.. In this embodiment, movement of the shape memory
material in a pre-determined manner causes the support member to move from
the first reduced radial configuration to the second increased radial
configuration. The shape memory material may include Nickel-Titanium alloy
(Nitinol). Alternatively, the shape memory material may include alloys of any
one of the following combinations of metals: copper-zinc-aluminium, copper-
aluminium-nickel, copper-aluminium-nickel, iron-manganese-silicon-
chromium-Manganese and copper-zirconium. Additionally, titanium-palladium-
.
nickel, nickel-titanium-copper, gold-cadmium, iron-zinc-copper-aluminium,
titanium-niobium-aluminium, uranium-niobium, hafnium-titanium-nickel, iron-
manganese-silicon, nickel-iron-zinc-aluminium, copper-aluminium-iron,
titanium-niobium, zirconium-copper-zinc, nickel-zirconium-titanium.
CA 02952464 2016-12-21
=
At least part of the support member may also include any one of the
= following combinations of metals: Ag-Cd 44/49 at.% Cd; Au-Cd 46.5/50 at.%
Cd; Cu-Al-Ni 14/14.5 wt.% Al and 3/4.5 wt.% Ni, Cu-Sn approx. 15 at.% Sri,
Cu-Zn 38.5/41.5 wt.% Zn, Cu-Zn-X (X= Si, Al, SO, Fe-Pt approximately 25 at
% Pt, Mn-Cu 5/35 at.% Cu, Pt allqs, Co-Ni-Al, Co-Ni-Ga, Ni-Fe-Ga, Ti-Pd in
various concentrations, Ni-Ti (approximately 55% Ni). The shape memory
material of the support member may act as a spine along the length of the
support member.
The plastically-expandable or balloon-expandable materials may include
stainless steel (316L, 316LVM, etc.), Elgiloy, titanium alloys, platinum-
iridium
alloys, cobalt chromium alloys (MP35N, L605, etc.), tantalum alloys, niobium
alloys and other stent materials.
The support member may be composed of a biocompatible polymer such =
as polyether or polyester, polyurethanes or polyvinyl alcohol. The material
may
further include a natural polymer such as cellulose ranging from low to high
density, having small, large, or twin pore sizes, and having the following
features: closed or open cell, flexible or semi-rigid, plain, melamine, or
post-
treated impregnated foams. Additional materials for the support member
include polyvinyl acetal sponge, silicone sponge rubber, closed cell silicone
sponges, silicone foam, and fluorosilicone sponge.. Specially designed
structures
using vascular graft materials such as PTFE, PET and woven yarns of nylon,
= may also be used.
At least part of the support member may be composed of a permeable
matetial. Alternatively, at least part of the support member may be semi-
permeable. In a further embodiment, at least part of the support member may be
composed of an impermeable material. =
The support member may further include semi-perrneable membranes
made from a number of materials. Example include polyimide, phospholipid
bilayer, thin film composite membranes (TFC or.TFM), cellulose ester =
36
CA 02952464 2016-12-21
= membrane (CEM), charge mosaic membrane (CMM), bipolar membrane (BPM)
or anion exchange membrane (AEM).
The support member may include at least a porous region to provide a
matrix for tissue in-growth. The region may further be impregnated with an
agent to promote tissue in-growth. The support Member itself may be
impregnated with the agent or drug. The support member may further include
individual depots of agent connected to or impregnated in an outer surface
thereof. In one embodiment wherein the support member includes one or more
capsules, the agent may be released by rupturing of the capsule. Whether the
agent is held in capsules, depots, in a coating or impregnated in the material
of
the support member, a number of different agents may be released from the =
support member. For example,' in an embodiment wherein the support member
includes a capsule, the capsule may include an annular compartment divided by
a frangible wall to separate the compartment into two or more sub-
compartments. A different agent may be held in each sub-compartment. In one
embodiment, the annular compartment may be divided longitudinally with at
least one inner sub-compartment and at least one outer sub-compartment.
Alternatively, the capsule may be divided radially into two or more sub-
compartments. The sub-compartments may be concentric relative to one
another. In the embodiment wherein the capsule is segmented, the different
compartments may hold different agents therein. =
The support member may have hooks, barbs or similar/other fixation =
means to allow for improved/enhanced anchoring of the sealing device to the
vasculature. In addition, the support member may serve as the "landing zone"
for the device when there may be the need to position the device in a more
reinforced base structure, for example, in the case of valves where there is
insufficient calcification for adquate anchoring, short and angulated necks of
abdominal and thoracic aortic aneurysms, etc.
In all embodiments, the support member may be connected to a graft or
stent by a tethering member. The tethering member may be made of an
37
CA 02952464 2016-12-21
elastomeric material. Alternatively, the tethering member may be non-.
elastomeric and have a relatively fixed length or an appropriately calculated
One
for desired activation mechanism.
En embodiments where a device support member includes4 capsule, the
capsule may include a single annular compartment within the support member.
In-this embodiment, when the support member is in its second increased radial
configuration, the capsule extends cornpletely around the periphery of the
=
endoluminal prosthesis. Alternatively, the capsule may only partially extend
around the periphery of the prosthesis. Two or more capsules may extend
around the prosthesis.
In other embodiments, shown in Figures 6A-6D, the capsule 80 may =
have an accordion-like structure to allow for wider, deeper expansion into the
=
= potential leak sites and also keep more room for expansion with any
vascular re-
.
modeling and thereby ensure constant and durable sealing. This can be .
positioned within the support structure 82 as shown in Figures 6A-6B or on the
exterior of the support structure 82 as shown in Figures 6C-6D.
D. = Therapeutic, Prophylactic or Diagnostic Agents
It can be advantageous to incorporate one or more therapeutic,
prophylactic or diagnostic agents ("agent") into the device, either by loading
the
agent(s) irito or onto the structural or sealing material. The rate of release
of
agent may be controlled by a number of methods including varying the
= following the ratio of the absorbable material to the agent, the
molecular weight
of the absorbable material, the composition of the agent, the composition of
the .
absorbable polymer, the coating thickness, the number of coating layers and
= their relative thicknesses, the agent concentration, and/or physical or
chemical
=
binding or linking of the agents to the device or sealing material. Top coats
of
polymers and other materials, including absorbable polymers, may also be =
applied to control the rate of release.
Exemplary therapeutic agents include, but are not limited to, agents that
= 30 are anti-inflammatory or immunomodulators., antiproliferative agents,
agents
38
= =
CA 02952464 2016-12-21
which affect migration and extracellular matrix production, agents which
affect
platelet deposition or formation of tbrombis, and agents that promote vascular
healing and re-endothelialization, described in Tanguay et al. Current Status
of =
Biodegradable Stents, Cardiology Clinics, 12:699-713 (1994), J. E. Sousa, P.
W.
= 5 Serruys and M. A. Costa, Circulation 107 (2003) 2274 (Part
I), 2283 (Part II), K.
J. Salu, J. M. Bosmans, H. Bult and C. J. Vrints, Acta Cardiol 59 (2004) 51.
= Examples of antithrombin agents include, but are not limited to, Heparin
(including low molecular heparin), R-Hirudin, Hirulog, Argatroban, Efegatran,
Tick anticoagulant peptide, and Ppack.
Examples of antiproliferative agents include, but are not limited to, =
= Paclitaxel (Taxol), QP-2 Vincristin, Methotrexat, Angiopeptin, Mitomycin,
BCP
678, Antisense c-myc, ABT 578, Actinomycin-D, RestenASE, 1 -Chlor-
deoxyadenosin, PCNA Ribozym, and Celecoxib. =
Agents modulating cell replication/proliferation include targets of
rapamycin (TOR) inhibitors (including sirolimus, everolimus and ABT-578),=
= paclitaxel and antineoplastic agents, including allcylating agents (e.g.,
cyclophosphamide, mechlorethamine, chlorambucil, melphalan, carmustine,
lomustine, ifosfamide, procarbazine, dacarbazine, temozolomide, altretamine,
cisplatin, carboplatin and oxaliplatin), antitumor antibiotics (e.g.,
bleomycin,
actinomycin D, mithramycin, mitomycin C, etoposide, teniposicle, amsacrine,
topotecan, irinotecan, doxorubicin, daunorubicin, idarubicin, epirubicin,
mitoxantrone and mitoxantrone), antimetabolites (e.g., deoxycoformycin, 6-
mercaptopurine, 6-thioguanine, azathioprine, 2-chlorodeoxyadenosine,
hydroxyurea, methotrexate, 5-fluorouracil, capecitabine, cytosine arabinoside,
azacytidine, gemcitabine, fludarabine phosphate and aspariginase), antimitotic
agents (e.g., vincristine, vinblastine, vinorelbine, dotaxel, estramustine)
and
= molecularly targeted agents (e.g., imatinib, tretinoin, bexarotene,
bevacizumab,
gemtuzumab ogomicin and denileukin diftitox).
Examples of anti-restenosis agents include, but are not limited to,
immunomodulators such as Sirolimus (Rapamycin), Tacrolimus, Biorest, =
39
CA 02952464 2016-12-21
Mizoribin, Cyclosporin, Interferon .gamma. I b, Leflunomid, Tranilast,
Corticosteroide, Mycophenolic acid and Biphosphonate.
Examples of anti-migratory agents and extracellular matrix modulators
include, but are not limited to Halofuginone, Propyl-hydroxylase-Inhibitors, C-
Proteinase-Inhibitors, MMP-Inhibitors, Batimastat, Probucol.
Examples of antiplatelet agents include, but are not limited to, heparin.
Examples of wound healing agents and endothelialization promoters
include vascular epithelial growth factor ("VEGF"), 1713-Estradiol, Tkase =
-
Inhibitors, BCP 671, Statins, nitric oxide ("NO")-Donors, and endothelial =
progenitor cell ("EPC")-antibodies.
= Other active agents may be incorporated. For example, in urological
applications, antibiotic agents may be incorporated into the device or device
coating for the prevention of infection. In gastroenterological and urological
applications, active agents may be incorporated into the device or device
coating
for the local treatment of carcinoma.
The agent(s) released from the seal or support member may also include
tissue growth promoting materials, drugs, and biologic agents, gene-delivery
agents and/or gene-targeting molecules, more specifically, vascular
endothelial
growth factor, fibroblast growth factor, hepatocyte growth factor, connective
tissue growth factor, placenta-derived growth factor, angiopoietin-1 or
= granulocyte-macrophage colony-stimulating factor. Agents for
modulating cellular behaviour include microfibrillar collagen, fibronectin,
fibrin
= gels, synthetic Arg-Gly-Asp (RGD) adhesion peptides, tenascin-C, Del-1,
CCN
family (e.g., Cyr6l) hypoxia-inducible factor- l ,- acetyl choline receptor
agonists
and monocyte chemoattractant proteins. Gene delivery agents include viral =
= vectors for gene delivery (e.g., adenoviruses, retroviruses,
lentiviruses, adeno-
.
associated viruses) and non-viral gene delivery agents/methods (e.g.,
polycation
polyethylene imine, functional polycations, consisting of cationic polymers
with
cyclodextrin rings'or DNA within crosslinked hydrogel microparticles, etc.).
=
CA 02952464 2016-12-21
In one embodiment the one or more agents may include monoclonal
antibodies. For example the monoclonal antibody may be an angiogenesis
inhibitor such as Bevacizumab or have anti-inflammatory properties. Further
examples of specific monoclonal antibodies include, but are not limited to,
Adalimumab, Basiliximab, Certolizumab pegol, Cetuximab Daclizumab,
Ecutizumab, Efalizumab, Gemtuzumab, Ibritumomab tiuxetan, Infliximab
Muromonab-CD3, Natalizumab, Omalizumab, Pal ivizumab, Panitumumab,
Ranibizumab, Rituximab, Tositumomab or Trastuzumab.
The agent(s) may be steroids such as corticosteroids, estrogens,
androgens, progestogens and adrenal androgens. The agent(s) may include
antiplatelet, antithrombotic and fibrinolytic agents such as glycoprotein
lIb/lIla
inhibitors, direct thrombin inhibitors, heparins, low molecular weight
heparins,
platelet adenosine diphosphate (ADP) receptor inhibitors, fibrinolytic agents
(e.g., streptokinase, urokinase, recombinant tissue plasminogen activator,
reteplase and tenecteplase, etc).
Additionally, gene targeting molecules such as small interference RNA,
micro RNAs, DNAzymes and antisense oliogonucleotides, or cells such as
progenitor cells (e.g., endothelial progenitor cells, CD34+ or
CD133+monocytes, hemopoietic stem cells, mesenchymal stem cells,
embryonic stem cells, multipotent adult progenitor cells and inducible
pluripotent stem cells) and differentiated cells (e.g., endothelial cells,
fibroblasts,
monocytes and smooth muscle cells) may be agent(s). Furthermore, drug
delivery agents like mucoadhesive polymers (e.g., thiolated polymers), or
pharmacologic agents of local treatment of atherosclerosis such as high
density
lipoprotein cholesterol (HDL), HDL mimetics, heme oxygenase-1 inducers (e.g.
probucol and its analogues, resveratol and its analogues),
hydroxymethylglutaryl
CoA (HMG-CoA) reductase inhibitors and fibrates (including fenofibrate,
gemfibrozil, clofibrate etc) may be included agents.
The agent(s) may also modulate cellular behavior in relation to
bioprosthesis, such as microfibril far collagen, fibronectin, fibrin gels,
synthetic
41
=
CA 02952464 2016-12-21
Arg-Gly-Asp (RGD) adhesion peptides, tenascin-C, Del-I, CCN family (e.g.,
Cyr61) hypoxia-inducible factor-1, acetyl choline receptor agonists and
monocyte chemoattractant proteins.
It may also be advantageous to incorporate in or on the device a contrast
agent, radiopaque markers, or other additives to allow the device to be imaged
in
vivo for tracking, positioning, and other purposes. Such additives could be
added to the absorbable composition used to make the device or device coating,
or absorbed into, melted onto, or sprayed onto the surface of part or all of
the
device. Preferred additives for this purpose include silver, iodine and iodine
labeled compounds, barium sulfate, gadolinium oxide, bismuth derivatives,
zirconium dioxide, cadmium, tungsten, gold tantalum, bismuth, platinum,
iridium, and rhodium. These additives may be, but are not limited to, mircro-
or
nano-sized particles or nano particles. Radio-opacity may be determined by
=
fluoroscopy or by x-ray analysis.
In some embodiments, one or more low molecular weight drug such as
an anti-inflammatory drug are covalently attached to the hydrogel forming
polymer.=
In these cases, the low molecular weight drug such as an anti-
inflammatory drug is attached to the hydrogel forming polymer via a linking
moiety that is designed to be cleaved in vivo. The linking moiety can be
designed to be cleaved hydrolytically, enzymatically, or combinations thereof,
= so as to provide for the sustained release of the low molecular weight
drug in
vivo. Both the composition of the linking moiety and its point of attachment
to
= the drug are selected so that cleavage of the linking moiety releases
either a drug
such as an anti-inflammatory agent, or a suitable prodrug thereof.= The
composition of the linking moiety can also be selected in view of the desired
release rate of the drug.
Linking moieties generally include one or more organic functional
groups. Examples of suitable organic functional groups include secondary
amides (-CONH-), tertiary amides (-CONR-), secondary carbamates (-OCONH-
42
CA 02952464 2016-12-21
= =
; -NHC00-), tertiary carbamates (-000NR-; -NRC00-), ureas (-NHCONH-;
NRCONH-; -NHCONR-, -NRCONR-),.carbinols (-CHOH-, -CROH-), disulfide
- groups, hydrazones, hydrazides, ethers (-OA and esters (-000-,
¨CH202C-,
CHRO2C-), wherein R is an alkyl group, an aryl group, or a heterocyclic group.
=
In general, the identity of the one or more organic functional groups within
the
linking moiety can be chosen.in view of the desired release rate of the anti-
inflammatory agents. In addition, the one or more organic functional groups
can
be chosen to facilitate the covalent attachment of the anti-inflammatory
agents
=
to the hydrogel forming polymer. In preferred embodiments, the linking moiety
contains one or more ester linkages which can be cleaved by simple hydrolysis
in vivo to release the anti-inflammatory agents.
In certain embodiments, the linking moiety includes one or more of the
=
organic functional groups described above in combination with a spacer group.
=
=
The spacer group can be composed of any assembly of atoms, including
oligomeric and polymeric chains; however, the total number of atoms in the
=
spacer group is preferably between 3 and 200 atoms, more preferably between 3
=
and 150 atoms, more. preferably between 3 and 100 atoms, most preferably
= between 3 and 50 atoms. Examples of suitable spacer groups include alkyl
= groups, heteroalkyl groups, alkylaryl groups, oligo- and polyethylene
glycol
chains, and oligo- and poly(amino acid) chains. Variation of the spacer group
provides additional control over the release of the drug in vivo. In
embodiments
where the linking moiety includes a spacer group, one or more organic
functional groups will generally be used to connect the spacer group to loth
the
=
drug and the hydrogel forming polymer.
In certain embodiments, the one or more drugs are covalently attached to
the hydrogel forming polymer via a linking moiety which contains an alkyl.
group, an ester group, and a hydrazide group. By way of exemplification,
conjugation of the anti-inflammatory agent dexamethasone to alginate is via a
linking
moiety containing an alkyl group, an ester group connecting the alkyl group to
the anti-
inflammatory agent, and a hydrazide
43
= =
= =
CA 02952464 2016-12-21
=
group connecting the alkyl group to carboxylic acid groups located on the
alginate. In this embodiment, hydrolysis of the ester group in vivo releases
dexamethasone at a low dose over an extended period of time.
Reactions and strategies useful for the covalent attachment of drugs to
hydrogel forming polymers are known in the art. See, forexample, March,
"Advanced Organic Chemistry." 5th Edition, 2001, Wiley-Interscience
Publication, New York) and Hermanson, "Bioconjugate Techniques," 1996, =
Elsevier Academic Press, U.S.A. Appropriate methods for the covalent
attachment of a given drug can be selected in view of the linking moiety
desired,
= 10 as well as the structure of the anti-inflammatory agents and hydrogel
forming
polymers as a whole as it relates to compatibility of functional groups, '
protecting group strategies, and the presence of labile bondS. =
The seal can further serve as a porous matrix for tissue in-growth and
can aid in promoting tissue in-growth, for example, by adding growth factors,
etc. This should improve the long-term fixation of the endoluminal prosthesis.
For example, the seal can be impregnated with activators (e.g., adhesive =
activator) that induce rapid activation of the agent (e.g., a tissue adhesive)
after
the agent has been released from the capsule. In other embodiments, however,
the seal can be composed of different materials and/or include different
features.
The agent(s) in the capsule can include adhesive materials, tissue growth
= promoting materials, sealing materials, drugs, biologic agents, gene-
delivery =
=
agents, and/or gene-targeting molecules. In another embodiment, the one or
more agent may be sheathed for delivery to a target site. Once positioned at
the
target site, the one or more agent may be unsheathed to enable release to the
surrounding environment. This embodiment may have particular application for
=
solid or semi-solid state agents.
Adhesives that may be used to aid in securing the seal to the lumen, or to
the device to be implanted include one or more of the following cyanoacrylates
(including 2-octyl cyanoacrylate, n-butyl cyanoacrylate, iso-butyl-
cyanoacrylate
and methyl-2- and ethyl-2-cyanoacrylate), albumin based sealants, fibrin
glues,
44
CA 02952464 2016-12-21
resorcinol-formaldehyde glues (e.g., gelatin-resorcinol-formaldehyde),
ultraviolet-(UV) light-curable glues (e.g., styrene-derivatized (styrenated)
gelatin, poly(ethylene glycol) diacrylate (PEGDA), carboxylated
= camphorquinone in phosphate-buffered saline (PBS), hydrogel sealants-
eosin
based primer consisting of a copolymer of polyethylene glycol with acrylate
end
= caps and a sealant consisting of polyethylene glycol and polylactic acid,
= collagen-based glues and polymethylmethacrylate.
E. Additional encapsulation of sealing means for
increased shelf-
life
l 0 The seal may be sterile packaged for distribution and use.
In the
alternative, it may be packaged as part of, or in a kit with, the device it is
designed to seal, such as a TAV or stent. This additional encapsulation
prevents
= the
activation of the expandable material during storage within solutions (e.g.
=
= glutaraldehyde, alcohol) by acting as a 100% moisture barrier.
= Heart valves, both transcatheter and surgical, are stored in
glutaraldehyde
or similar solutions primarily to preserve the tissue component of the device.
-Before the device is implanted, it is prepared for implantation by removing
it
from the solution and.rinsing it thoroughly so that all the g1ntaraldehyde is
=
= washed off.
Although the outer impermeable layer of the sealing device/capsule is
meant to prevent any penetration of water from the glutaraldehyde into the '
=
=
capsule, there is a likelihood that the thickness may be insufficient given
the
profile constraints and as a result there may only be a limited shelf-life
that may
be obtained. In order to obtain an increased shelf-life where the encapsulated
expandable material remains in its desirable unexpanded state until introduced
within the body, an additional impermeable layer may be needed. This
additional
impermeable layer is not required once the device is removed out of the
storage
solution, and is rinsed to wash all the glutaraldehyde away. This will
typically
be removed after removing the =device from the storage fluid and just before
implantation. =
CA 02952464 2016-12-21
To make the sealing means low profile, the thickness of the outer and =
inner membranes has to be kept to the minimum. If the sealing device is stored
submerged in a solution, as in the case with transcatheter valves, for its
shelf-
life, the low profile, thin membranes may allow moisture to permeate through
= them and thereby risk the premature activation of the sealing means.
Therefore,
an additional means is necessary to ensure the appropriate shelf-life of the
=
sealing device can be obtained.
As shown in Figures 7A-7D and 8, this additional means can be an -
additional layer 92 of encapsulation over the "impermeable" outer membrane
94, This additional layer 92 may be much thicker and may be laminated by
metallic layers several microns in thickness to make it 100% moisture
impermeable.
This additional encapsulation layer is removable and is designed to have
a mechanism which enables easy peeling of the hermetic sealing capsule/layer
so that this layer can be removed just before loading and crimping of the
prosthesis into the delivery catheter, before it is delivered into the
vasculature.
The layer can be removed using different means, including peeling off,
cracking
off, melting off, vapouring off after the rinsing process is complete and the
device is ready to load.
The additional encapsulation layer may be designed with a mechanism
so that it can be attached to the device assembly with the sealing means
during
the assembly process by suturing or other appropriate means such that the
removal process insures that integrity of the sealing means and its assembly
with'
the base device remains completely intact.
A moisture impermeable film composite comprises a combination of
polymer films, metalized polymer films and metal films. The polymer layers can
be comprised of, but not limited to; Polyether ether ketone (PEEK),
= Polyethylene terephthalate (PET), Polypropylene (PP), Polyamide (PI),
Polyetherimide (PEI) or Polytetrafluroethylene (PTFE). Polymer films may or
=may not be mineral filled with either glass or carbon. Polymer films will
have a
= 46 =
CA 02952464 2016-12-21
=
thickness of 6um or above. Metal films and coatings include aluminum,
stainless
steel, gold, mineral filled (glass & carbon) and titanium with a thickness of
9um
or above. Coatings can be applied with processes such as plasma vapor
deposition, press lamination, vacuum deposition, and co-extrusion. Metal films
can be laminated to polymer films via press lamination.
E. Devices for Placement of Devices with Sealing Means
Embodiments which position seal at time of implant
In a preferred embodiment, the sealing means is positioned posterior to
the prosthetic implant, and is expanded or pulled up into a position adjacent
to
the implant at the time of sealing. This is achieved using sutures or elastic
=
means to pull the seal up and around the implant at the time of placement,
having a seal that expands up around implant, and/or crimping the seal so that
it
moves up around implant when implant comes out of introducer sheath. This is
= extremely important with large diameter implants such as aortic valves,
which
are already at risk of damage to the blood vessel walls during transport.
= A key feature of the latter embodiment of the seal teclmology is that it
enables preservation of the crimped profile of the endoluminal prosthesis. The
seal technology is crimped distal or proximal to the prosthesis. In one aspect
of
this technology, the seal is aligned with the prosthesis by expansion of the
seal.
In another aspect, the seal zone of the prosthesis is aligned with the seal
zone
prior to expansion of the prosthesis by use of activation members. In yet
another
embodiment, the seal is aligned with the seal zone of the prosthesis prior to
prosthesis expansion by use of activation members, which can be made of an
elastic or non-elastic material.
In a further embodiment, the endoluminal device may further include
one or more engagement members. The one or more engagement members may
include staples, hooks or other means to engage with a vessel wall, thus
securing
the device thereto. =
As shown in Figures 11A and 11B, self-aligning support members 82
made of Nitinol eliminate the use of attachment sutures within the catheter
80.
47
=
CA 02952464 2016-12-21
The dual-membrane capsule containing the hydrogel can be attached to these
members and is activated with the expansion of the prosthesis. The
selflaligning
members 82 can be directly laser-cut as part of the prosthesis frame 84 or can
be
= connected using sutures. The primary advantage of this mechanism is that
it
. 5 eliminates any failure mode with the "activation member" (sutures,
etc.) that
enables the alignment of the capsule with the distal/proximal/middle section
of
the= prosthesis.
Mechanisms for Deployment and Retrieval
In yet another embodiment, a mechanism enables both deployment and
retrieval of the system. This is particularly important from the ease of use
and
placement accuracy perspective. This feature enables the physician to
change/alter the placement of the device in vivo if it was not properly
positioned
in the first attempt. Also, in the event of some complication during the
= operation, the physician can completely retrieve the device out of the
patient
(even after the "expandable material" has completely expanded).
The key features when used with a self-expanding prosthesis: =
1.. system re-positionability (if the.prosthesis is partially retrieved back
in the
catheter) - that enables accurate/precise placement if the device in the
anatomy
2. system retrievability (both the prosthesis and the els SEAL capsule can be
completely captured back into the catheter and retrieved out of the body).
III. Methods of Use
The device and seal can be utilized for sealing in a variety of tissue
lumens: including cardiac chambers, Cardiac appendages, cardiac walls, cardiac
valves, arteries, veins, nasal passages, sinuses, trachea, bronchi, oral
cavity,
esophagus, small intestine, large intestine, anus; ureters, bladder, urethra,
vagina, uterus, fallopian tubes, biliary tract or auditory canals. In
operation, the
endoluminal prosthesis is positioned intravascularly within a patient so that
the
prosthesis is at a desired location along a vessel wall. A balloon or other
expandable member is then expanded radially from within the endoluminal.
prosthesis to press or force the apparatus against the vessel wall. As the
balloon
48
CA 02952464 2016-12-21
expands, the activation wire is triggered, rupturing the capsule and causing
the
seal to swell, and in some embodiment, releasing agents. In one embodiment,
the agent includes an adhesive material and when the capsule ruptures, the
adhesive material flows through the pores of the seal. As discussed above, the
=
seal can control the flow of the adhesive to prevent embolization of the
adhesive
material.
In specific embodiments, the device may be used to seaI a graft or stent
within an aorta of a patient. In a further embodiment, the device may be used
to
seal an atrial appendage. In this embodiment, the device may deliver an agent
to
effect the seal of a prosthetic component across the opening to the atrial
appendage.
In a further embodiment, the device may be used to seal a dissection in a
vessel. In this embodiment, the support member is positioned adjacent the
=
opening of the false lumen and an intraluminal stent subsequently delivered
thereto. Upon radial expansion of the stent, the support member is caused to
release adhesive therefrom to seal the tissue creating the false lumen against
the
true vessel wall,
In a further embodiment, the device is used to seal one or more
emphysematous vessels.
In a still further embodiment, the device may be used to seal an artificial
valve within a vessel or tissue structure such as the heart. An example
includes
= the sealing of an artificial heart valve such as a TAV. It is envisaged
that the
seal provided by the present device will prevent paravalvular leaks.
As shown in Figures 4A-4C, the activation of the polymer 22 within the
seal 12 takes place when a section of the outer membrane 20 is,ruptured at the
= designated rupture point 24 using the activation wire 16. This is shown
in
Figure 4A prior to rupture where the seal 12 is relatively flat; the
designated
rupture site 24 is opened as shown in Figure 4B, then the seal 12 is-expanded,
as
shown in Figure 4C. The rupture site 24 is formed by weakening the surface of
the membrane 20 at the site 24 using means such as a laser to partially cut
into
49
=
CA 02952464 2016-12-21
=
or perforate the membrane 20. An activation wire 16 is secured to the rupture
==
=
site 24 by means of an adhesive, suture, or restraining means such as a brad,
rivet, staple or clamp. The rupture site 24 is opened at a pre-determined
pressure or location by pulling of the active wire, typically connected to the
prosthesis or a part of the placement catheter.
Figures 5A-5E depict a method to crimp and load the device with the
"activation wire" 16. The activation wire 16 has to be shortened in length
during
the crimping/loading process so that the "activation or rupture" can be
triggered -
during deployment/placement of the device. Before crimping/loading the
activation wire 16 is long enough so that the "activation mechanism" is far
from
activation and the hydrogel in the seal 14 can remain completely sealed/de-
activated during storage and shelf-life. =
The metal crimp is used to shorten the length of the activation wire 16 =
during the crimping/loading procedure. During storage the metal crimp in the
- "uncrimped" state and after the completion of the insertion of the device
into the
catheter it is "crimped" and the excess activation wire 16 is cut off. After
this
step the final steps of completely loading the TAV device in the catheter are
completed and the device is ready to be inserted into the patient.
The device with seal is inserted in a manner typical for the particular
device. After reaching the implantation site, the seal is ruptured and the
seal
expands to seal the site. The guidewire and insertion catheter are then =
= withdrawn and the insertion site closed. ==
Figures 9A-9D are diagrams of the placement of a Sapien valve 50 with
and without the disclosed sealing means 52. When the Sapien valve 50 is placed
=
too low into the LVOT leading to the graft skirt not completely apposing
against
the vaseulature (Figure 9A), perivaivular leaking will occur from the
gaps/area
above the skirt and around the device, through the open cells of the stent
(Figure
98). As shown in Figure 9C, the Sapien valve 50 with sealing means 52, even
when placed too low into the LVOT,.seals the valve 50 uniformly against the
inner wall of the LVOT. Figure 9D shows how no perivalvular leak occurs
CA 02952464 2016-12-21
. when the seal 52 is in place, preventing the "leaking" blood &ongoing back
into the left ventricle.
= Analogous results are obtained with the SIM/Medtronic TAV device.
Figure 10A shows a correctly placed SIM/Medtronic TAV device 60. Figure
10B depicts an incorrectly placed SIM/Medtronic TAV device 60, resulting in
PV leaks. Figure 10C shows how perivascular leaks are prevently with an
incorrectly placed SRA/Medtronic TAV device 60 with sealing means 62.
Figures 6A-6B are perspective views of a seal that is placed
inside of the TAV device. Figures 6C-6D are perspective views of a seal that
is
= 10 placed on the =exterior of the TAV device: Figure 6E shows the seal
placed on
= the inside of the device such that the outer. impermeable membrane is
moulded
to the stent scaffold and protrudes from within, in alignment with the stent
pattern, while the inner permeable membrane remains in abutment with the inner
circumference of the device. Hydrogels expand and cause the balloons to pop
= 15 out.
'Figures 7A-7D are perspective views of an impermeable sealing system
to protect theimplantable device during storage in a preservative solution
such
as glutaraldehyde, seals in place (Figure 7A); exterior seal being removed
(Figure 7B); exterior seal removed and interior seals being removed (Figure
7C,
= 20 7D). Figure '8 is a cross-sectional view of the exterior and interior
seals of
Figures 7A-7D.
As discussed above with reference to Figures 11A and 11B, self-aligning
support members 82 made of Nitinol eliminate the use of attachment sutures
= within the catheter 80. The dual-membrane capsule containing the hydrogel
can
25 be attached to these members and is activated with the expansion of
the
prosthesis. The self-aligning members 82 can be directly laser-cut as part of
the
prosthesis frame 84 or can be connected using sutures. The primary advantage
= of this mechanism is that it eliminates any failure mode with the
"activation
member" (sutures, etc.) that enables the alignment of the capsule with the
30 distal/proximal/middle section of the prosthesis. This embodiment
allows
51
=
CA 02952464 2016-12-21
=
placement of the device and sealing at the same time, and insures proper
alignment of the device at the time of implantation.
As shown in Figures 12A-12F, the self-expanding TAV prosthesis frame
90 is released from the catheter 94 during deployment. Self-aligning support
members 92 after release from the catheter "flip" and align themselves (and
anything attached to it) to the base of the TAV prosthesis. The steps are
followed in the reverse order during retrieval.
Figures 13A-13E show the deployment of a TAV device I 10 using
attachment sutures 112 that pull the seal 114 into place adjacent the device
frame 116 at the time of implantation.
The seal may be sterile packaged for distribution and use. In the
alternative, it may be packaged as part of, or in a kit with, the device it is
designed to seal, such as a TAV or stent. =
The present invention will be further understood by reference to the
following non-limiting examples.
Example 1: Preparation of Hydrogel with Rapid Swelling
Studies to identify hydrogels having substantial swelling in a short time
were performed. The main factors that influence swelling of a hydrogel based
on
polymerisation and cross-linking of synthetic monomers are:
Type of monomer =
Type of cross-linker
Concentration of monomer and cross-linker in the gel
The ratio of monomer to cross-linker
Acrylic acid polymers are capable of rapid swelling and are regarded as
having good biocompatibility. A number of commercially available cross-
linkers can be used to crosslink the polymers to form a hydrogel. These
include
B is acrylamide, di(ethylene glycol) diacrylate, and poly(ethylene glycol)
=
diacrylate (MW 500 Da). =
52
=
CA 02952464 2016-12-21
Materials and Methods
= Studies were conducted to identify appropriate combinations of acrylic
acid concentration, type of cross-linker, concentration. of cross-linker and
ratio
of monomer to cross-linker. The basic composition of the formulations used to
. 5 make the gels is shown in Table 1. These were prepared as
follows:
Mix acrylic acid with cross-linker and 50% of the necessary water, adjust
pH to neutral with 15M sodium hydroxide and adjust the total volume with.
water.
= Degas the solution under vacuum in a desiccator or other suitable
=
=
container.
= Add initiators (APS and TEMED), mix well and leave to gel overnight.
Test for mechanical properties and swelling.
After forming the gels in small beakers or Falcon tubes,=the gels were cut
into small pieces and dried until complete dryness. Small pieces of gel were
then
collected and re-swollen in phosphate buffered saline (PBS). The weight of the
gel pieces were then recorded. at regular intervals. =
Results =
= Compositions and swelling data are shown in Tables 1 and 2. .
=
=
53
. .
=
CA 02 952464 2016-12-21
=
'
Table 1. Swellable Formulations
1Gel = 2 = 3 5 6 21 29 = = 25
AA 40 40 40- 20 20 _15 10
,.
Os = 0.4
. 0.4 0.4 0.2 = 0.1 0.05 -0.02i
._
APS 0.33 0.08 0.08 0_08 0.08 0.08 0.08
=
TEMED 0.33 0.8 = 0.08 0.08 0.1 =
0.1 0.1
STATUS Swelled Swelled =
, Swelled Swelled Swelled = Swelled Swelling
!Gel 17 23 19 26 = 28 =
IAA 20 15 10 10 5 .
_
IPEG 0.1 0.05 = 0.05 0.02 0.025
!APS 0.08 0.08 0.08 0.08 0.08
[TIMED 0.1 0.1 0.1 0.1 0.1 =
-.TATI:J-S Swelled Swelled Swelled Swelling Swelling
Gel 18 = 24 = 27
AA 20 15 16 =
DEG 0.1 0.05 0.02
!APS 0.08 0.08 0.08 ==
`TEMED =0.1 0.1 = 0.1 =
õSTATUS Swelled , Swelled Swelling
. .
. ' .
'
54
= .
. .
. = .
=
= .
Table 2: Analysis of Hydrogels made with the PVA cross-
linker . -
DIMENSIONS AND SUMMARY
Gel 23 Gel 23 = Gel 23 Gel 23A .
Gel 23A Gel 23A
rep 2 rapj. . mi.
. Etp.I
.
.
Approx. rectangular = Approx. Approx.
rectangular Approx. Approx. = trapezoid Approx. trapezoid '
.
Shape . Shape triangle Shape Shape
triangle Shape Shape
,
(-)
'= base side 1
base 1 base i
(mm) 2 side 1 (mm) 1.5 (mm) 2 =(mm) 1 (mm) 1.5
o
n.)
l0
side 1 (mm) 2 height . side 2 =
base 2 base 2 ix
n.)
o.
(mm) = * 5' side 2 (mm) 1.25 (mm) 3 (mm) 1.5
(mm) 2 m
o.
side 2 (mm) 2 thickness thickness
thickness height height n.)
o
thickness (mm) = 0.25 (mm) 0.625 (mm)
0.33 (mm) 1 (mm) = 1
.
m
(mm) = 0.33 height = '
thickness thickness 1-,1
.
. n.)
(mm)
(mm) 0.25 (mm),
0.585 1
n.)
.
1-,
. thickness .
= .
Volume 3.33333 . 1,17187
0:3125 . 1.02375 .
<
.
(mm*3) 3333 1.25 5 I
3.65450 = 6.78654
= Surface = 10.6666 12.8507 =
. =
8.77485 . 8497 = 9883
Area 6667 8106 7.1875. 1773
1.1.6344 6.62910
= (mm *3) =. = 10.2806 =
6.13333 8.77485 27t9 8555
= .
. .
.
.
=
=
-
. ?Or: ,:,--ft,',',,,,,,..-fkle::..1-=:.,,-v,..,=!,,,r'7-:,"`Ar17_õ
Ivr,,1?),:i:. :71,.;:=.-r.147:.,:-,,,..,õ , . ,.., .,/,
71:.., ' Ti,rilt."?..',..':.TA, TAk;e:=-=-=;(:::=: - '2.6. ,..1::;:;.44 =
,.-
4.4:7:1..= ,.::'?. ' .:::;',':c-2(t5:1;
;,:in..:,i';7::::::.:74f*,77.1¨Tf.'1:,,,k1:,.:!:,:?.4'..e.q.:4.4,7:r.... -
....ir:kh..t... r.q:;,-;=!...i.y ...,,,...;.?.:i wi.,.,-.
.71,,..;!:,..g.y..,..,.; .,,... i-,,..,,I.;,,A1.:!,...... .1,.:..."..i.,.
,....,,...1:14..v4.,*õ,,i..., L,;,:1,,, = ; .:*:.,.õ..., ,.-; ,,,i_t,,,.....õ.
...õ..,,....;..,-.T..?!-.....,:-:::, = ..?.4,_
: . = s' .
.. -1.= .1 4.:4'''' .4 ; ..,' " -A=== 1-e,":=' =
.".8...::.i,_''4:i;=:',,',' '=41'.;,: i'll': ",..:.i.::,5;,;=;!====t4',,,",-
,:':-===,-1%:,'i;=.;,...V.::::':',46-.*=...,..i."' ....:11.2 7:,!=-ig-
p=,;,",:::?4,44,-,-..=:=,1.k1r#4. ,`"4.:,,',,:,1t,',.;:,:tt.,,a-
Vssi,,,..7:..,.,,,,::,.;,-;,0.4,::,c = ,..,. - .. ... ::.4:30ifity..44,.!
:=7. % === . : ..4.';=::. ,=-if ;.1t1õ4-;:',1te? ".. =n!" ..' .= ..,E
=:'...Z...-fits,:::=:..;r....;:r-:;.= -...;t::-......,-;4,4.:p _,.--:...: -
õecr.õ,-;..: ;,,;;;44.,....1.,...-!.:;,.......::,.....41., A3A.3,,,..;,,,4,::.
,-.., ..;::.._;kt-oilõ,..,..,al.40.,73:2õ,:,,...,,,,,4, ,,,,,..,=,,,,,,-
.Li:.=:...',::,,,',.-yd:4AtAfci .,...;..,v,f.,,,g.õ.:'7,.::-,- 4`fr,.4..2:.
õ,cõ. = '4,
taA .i,z,: :......;=.,.,..,;.;,..,...,,,....i......õ,... ...,... j:
._;.;:;.. ' :. 4=,.7....4!4'6-4f=-,=4'..;, ,.,,' 4 .=:. = .-; ..r:
..*,:.;fr'..f.i:, ,=.; = ' '...,.!'". 4- 1...?..T.2 ..}1.-;;==';'.:ro ,':; ..
..;:-!...:. -:',': ==== ,^"!.=-;f:'=,'.c \r',...:,....:.' ....\ .;.2ki.4-
.14.P.ri-jig,..1"1".'"":;,...:44.44.- t4.'.11%;,44;;;,-
::.'4...="=:!5'4'....;:et 4gik";:v., . ti= 4; =',.';-=!===:',:: :.-i
'$.1P,,, W:'11/,: f-= = .1===========''i ':'===:!::: - :='= 1 '= =-;='/='
\==='..f,',1.'Z'11.1..0' ,:=====.====,. L,'.?,,i'.i;:::.;:-...,-,T;'.4, `.-
;+2,',4:,=!,=C.,;'!"=.....'%:As':.= ':.' = :., ',F.0'.?.-
=gi'f:'='=4:",=i.7:=i: ''.1-,="$:=,..'4=04.i.!,,-,,,42 'f.`A,',,,
...t,,....V..2:44aVA.' '+'.,. ',. ..:,. ti10' '''..::.4F.,{..",' ". ,..e, =
.'. . :e.. :=. .,..... ....I.. ,..t: :,..
. ...,.., ....1..., Z. = ., :... ..... i ;...
,4=..., .'..N..,' .,..=:4-,=,,,,..47,v,,- .'44r.- .,s4,..,:;.:.. :..
...'''...4V".....'t ' " .-N ...4,: ' 41,:=: - ;-: h. = '..- ....r..= .= 4::; 4-
4:i1.1):,'.1:43, 1.. '4,...., ...' '''=''''''',.-.&===;'''rZ.."-V,a'T =Vcr,-
- 'V.t..e4L'IS.CL*7'r 2r woo n1V--Ii=:=(;,==:'-',..s'=" '- : = -= -= '11/. =-
='=? .
' 4 ;.=;=:='' -
f.c. Pr.1. :.;.r...::-:7;::;=;=7-.1-e:::,,5 ;...'. 5:7.=-'=1= c-4 05=-0- 4 -
:,.-.'-,;:,:::1:4?-,:g=::f., '1": '7:.',,=,, :=g= ==:=, ,,-9'=i',
03''::'..,';',i,:'=!:,.Sx,-:-,õ',...'.,.'."..i'':':='I..-=:.-'=.=.:'''.',µ,-
,.":::'='-'0'4.-.1..i.lit',7,,,==:.:'.*õ'74',,i.':;.===T;gp-;ii-='-.4-,,,4,
õ04,,-,=*_-_-='.= =eiic.', =;74,:, = .Y.,-..i0E:fFI.A1µ, 'A.. '=,. : . :...
,.; _!..g. 911;:-.
6--;b069.,;:,:iirk:i,A-5;.. -,::',4;f4W61g
e-a,-,..,,,.=;,,,......:?:-.,,=;:' =-=.:'-,-V...,.!;eq.,.,:,..-.:,tk-.,,J:-
:..,.:, ,-,,- -,,:-.,;t:A.i..,- g;-;., .::+i-,'-'.:::--.7i,T,547.2-'-',. ,-
".14';.:;;;=:''''',C3."4'ill'"",'-':::41e'r4;4#;'0.7:;µt!':"z,:;'-' ...-:'.
,.i^',61''';11.';': ' ' . '.. 0.9 0.1 9=7'
7-4.=-.1'4!,g ,::',.''.',-P....-.!':.--i:... , ... . - - .::...F.:,,,s-:,;-..,-
,.-'r:,. . ---:,',4'.:;=:..-.':.:v .--;.-: .1-1....,=_.4,:w".Ni-D .0 0
6.76.;:-....-:.::... .,yryd.,,g*,,,,-.'.,...'.:;-...'....,!: -;., - _
.i..÷
' Mass(..
' ''''';'-;1..::.:4j.-: :=1:::'':' :"..' ': 1....':i''.4: :,' = ...:
';'''!....:-?'-'r';',-".f..-:':".:.":1.*........y..::Z:=.'1, = V.:::4; =
.."..e.:17.7:-,,=7 !,...: .....,.=,,,,..::..,=':.,7,..-;
'',.;.=::.:...:;.;i4c...:::i:::=,.7=Mi.:!:=.5'..0,99Z.6"-;1-:.r..,/....=== '
: - - --4481.:
,........ -4;:j!-...;:. -,:. ...'..P00225. (.µ. ';,:=,,,,Z'=..,i
4::-= -4.,:.,=,0,p,.j.4-_,r.,:.. ......# = :=="==:===V.,-,;;-. ....-
.."'i,.. .. ::='.....' Lii=..,0==,:;,...,:".4,!=A'c=-=e:===,' f...t.1==='..-,L-
'......)!-= s.:,::::.::::irw.gifetz=-=:-,-;=-. .....:':/=:".-
.;=,'µ..t;:',,.... = ., ..: -...:- ====!-; .= = ... ,
i= - -- = =
.=== ' ,..b,.....:-=;::....?,=,:'..;..,..:;== ' ;" ..=
,,.i!,....A,:t4,f.;[_:.!4?!=',=;:=:,,..:.;;..:=,=='..:,_;.i'.; .,,::...:-
......:...:-.;..:,,,,,,....fr,-..;: h..: .,...".:µ,..:-.4.,-.:,; 7.-
.,..43i.,,!õ,,..:., 4:4,,...1:1 ,,,....3z.P,,,49:?,5.4=õ;,.,..õ,,:.;.,..,4Q;
'.- ; =.....;.,..,=::::,:ka:,.= .....4.= .., ,.;11,0-x3
..":110gh-4:/ tv,..!,.. õ-rit-f71".=..4., :='1.14;,.....:,.:
: 7,1;:p,:c..;.:,21::,..;:.,..,:,!:., -.:, -"r...7-7,71.`,12-..-
11:1,:, ,..... = 1,,,.., ,7= .4,',......:1,;4...=;,.d.' 161 .'rei- -
.g=Azi=E';;.:;,::: ..,....).=!! =:tu.:41,44--:4t1.;:iMP4._,,,,
_,.'.7?c,;1r.$_,,,,,::::. 7 :7`.',..T.';`.:,,..ci:14..11.1,14;=... . . '
õ . -.. = .. . = .' ".....;=..
. '..."--:'?. . ',.- ' .....' ,j..N',:iY=')'i - ,:', , ::;:;i70¶-:.', .-
..4'=,:rie:','?41.".,5i - '.i.:',,,i-z- '1,rz,,;'.::::, =====,',5;:',-
::i.,:... .. -4.1.t,i3gi I.'-'i'e'A -44 , 1..u.YV.V.-`:7'',:;4iirt-'::'.:r.:-
.' 'r"..'''''. ' '...:-',;`="9,i4923ch y==':4-V:Wje,: k-3".".4" ,116-
1=2;5'',."r=-=,..'::'.=`,...''';A,:. ., = -. =". .1636-Ir.:,
..-,pkt,,...::.=.:1.; ...,i-e,,
,.,..4.7.;:.i.r.:.....õ..t.='.."4.,,f_i.,,,i,.. ;.;==,:',=:!. '....,,, :=:: ,
.i.:-.! ..:4'',. '.1:='....;:'.**.V:q.11=7:: ..',.. '4=;'Y'L.'-':
':9:4":7:"A'IC.' ;:=,77.1,,,*,/,',,,V r,..,%W.,`4.. :.=,...,' -,=,,,q,i,-,,f-
=,,, .Lift, ..
..."...,:.......i,-.,:,..-::A.:?=%,115-,.,-..ii:.::-,=,;%-.t*iv,.6-,,*,:,...k.-
A4,,=;:.,A3;331:7::,':,e.i...!,',.-.:&40.;,,,,.i.,:,,,:,=,:f;..-4:e:i=-
=;j=y),,F.Igy -,41,t;-.,:,_: .:=:.,..,...4--!.e.,t,.-t, ,(=--4-,fit:ip..4.,;
.AdA4N...-..gp.=,-;,,.::;;;2,-.õ..--,õ,..L.,,,=:=.,-.=4=== = . ;....k..
''....,:z.:;,',õf ti'.
;f:'!:::,....7.:µ.'".::''Y..?...!;--0'1:.';14i.17;':=,-:,i'V:-',....": -.;.4.,-
!...-2,='-'.4:,:;14..qTiJit .:.:!"..'i-
,4',..ilri:E_3::!.41;:r.:,:iik.:,4tii.,,f.:;õ,,,..i..µ,:,...%:,:r.,,,:s:-
.1!.1%;,;:,..ot-6,.,--i..9-4".õ4.-:',:li.j1.x.r.,;?!.;q47 23;= T-
p,r,411;112..'A=.'4--.:::.,:.-Wa,.y.::,'...hi':;',Pi'6.-i:::=-="..-; .;
:'',..4.1.?: ......:, .,.....:=,;:e
1,.:44',4it.,!'....µ14.1:',. ''''; '''= '.-== 4:,.11=J:=....-I'''':='''.'-
';'... :'.!''.0,:'...41.';'",i.f;':=il.':."'':' i;t''S':,=.:' '''7=AI
itZµVi:'In.=:+e;,:,''''......:V.V4f44 '411':":4014,1!.$,=;: ,01ritSr.:ftr-,
ttiti:;=4.:7=-..:, ..'..D: ifi-';'%L4':i='... ;:::'= ' ''''.. ., ....':=:.
C=='.= : = 'il.4.,'
=75,i-=':e=4=A.K04!7::;!. =Sf;!':.t1:4'..'=;:-:A.V''';,=;P:==I'..;-
;''';,:`,5:;ft'='f!.:er'F.,' , ....'..4, '''.'::'-:kc;""*IF"!Ki.,.MT.:. i:.'.,
= ...: ' ''.'V4?..krik ,:-:;'3--, , '',5ir;'2A-
":.:A443'..)..Wie.4%...:,:"..;.,.', .'''.1%.,;":.',.?,-,-õ1;',A,=:,::,..7:.'.
. ,,;.........., = ;.:,'''.'....,..",'..,..4 0
i,.,:r.,4-.; :===== . 1. -,67,,,,,rAn ......=,'
.T.: ,..'::: ..=.:,.,......:=::;',.-f,:',:30,71,;-:,;-: it.,,,,:,..,.....Ti--
7e7t,:-:.:5:11,:!,-7.;%.=.cii-, ', '',,,... ..4":,?1",...: ;,...t !,..4==:'
''-....-. -'...,' =-==z4kte:9&.-'' ...= 4: ,...,,r1..iVrr."
'.."1='''P = = = = ;II," - " .4
; ;ratiO = ' ' .' 'L.'
:','':'=''. ' '''''' ' = ''',, === ' = == ' ' .,..!!=-
eiTsi;,,,,!...,<',. i: ,,'" 4 ;;;!'".'.:y".=!..'=''. -..,;,. ;', .--.1' '.."-
:ti';';':.'1"4.' : .-=.::õ=..Z ! ' `;',,:".1-,i' =fV,-,,,,':;.4.
r.',_tiP,'y.t.,:!t;t,./] .:,,::,!^,...4,,,..,^,./.11.;!..''.:
1..E*A'4,1,..:',VT='-: ...-'= ' , ''' .....',4?:. : '.'. .. ev . 7'.-..!;=':==
=:: - ,..'.' . , = r :.2
.
;õ.- : -.1. .ri 4=,;,:c. -:,,, , f .,'. ' - . = .,1;, ' ':
;47:-.... .=,, .i,-.):-,A., ,-... : .:-...*:',....v-'44... --,,e.:
64z.,...,, .L,!...=.._,::..?,.:4,-.:,-,..;%i ,i Ills, =,,,,, -
:,õ,,,?..5;:.:;.-1:=-=,-..i.4.,.. , ,,,,r;.:!,t,,.,1.,r.-:,.,-
.,.:t.A,1,....r.46.;:=%ii.:..i.-;:lot;i:::4"i- 't-L:X.',4?,4 .',.;..1. .;;=.
:r-f ' f;:'=:-'-'-'=".. *".....=;= 0
tv
= :..:=, .. . ..: = ,.., õ3,... 4,4,, . . 4 ...= ..
44 4 , .. , .. ,r,ep ,an. .;:: iq, pssais,a
pprgximateecngt.i, .,., , ,....-.1,...õ,-.:.:..-, c''...õ-, -4/,/,..: - -
,!... ',,..4'.....;'''1'.7.e.;',Hfq":.µ.':',. :1.4.õ.,',".1,31.1.,.. :1, .
c 4,`", = , 41..,,,I, -(Froo,.=,;,...V..-V.r..4='? J ...:" - W..!...';`,.
7,c,,;.: j; -.;=7..?41,.. l0
' :. ' ..',4',44V.',: 1,-i.,,',.4'.,
, =-=.' : '1" ,,f 4.-,== ;',1:.:D111j..P.Cr ;,,:r'..J' =
,,,,,,T;=.:,;'4,.., VI. = J .4- :.: ,.'4,,, ,..:....,..e.:..Ø ::' 4
....JAC:" , - v= . ,. i :" 4: ,.'4-,.,..t :4" - = 4 ,- ',, ...-
:::;:='.1i.'.:.,N, =.:',4,!" 1. ,,,,,''..i.r -. ;91,`".1.1,V4WM=ki=j, -.."-
"C' !....:- 0,====;",2,:Th.:'....='. - = ' it .-. .,:' ."-,,,,i=-,:.:.::"
...,' U'l
'', = .i ; . ??.... ' , = : '4".."_....!,,,,ta -',P:,,. ,-,,,,=:5::.,,_-,..,,-
.".: :;..v...;;;ii6.4:1== 40. .VZi ':;,;'*....-:':f''''.-/ `1".::4,-- ='
'.....:'; ' :57'...7-;'''':.;;';.' ' = ' 't'''''' ''''''''' - '-' '.."-
',.5'-e' ':-;,::.-n-''' -::,-'t.1-1.,:'. .L.:=;---:-47,-,,t2P:f-,,!..,.
ft.':,.;.1,,14tt.*:-.,,,--,,,,..,z,-: , =-=-, p-,,:y,:::.;,..,,-
,A=,gi....,,..,:,..--; v ' ',.. :=,% -...:i.....:-,, otysi tv
=
":'''..;.!7...4:1P!P.-
K;,ni=NWS...4::ERCIr.l.e=Pugq;0','47/ai",:;f:
.;T=t,*.::=;*:*=.Y'rlj:.).P.i,-
.V..::`.c1.=Nt...Z:=:7'.'','..:.:._:?'=====I';Ii:3:1:.'*4,,'==V,Z40=P.=-6'j=t-
4.4:-=',. 7: 14.,,,,l'45A,W,,pt51-.4..tilrA-445#4.t.,=;.T...it:;.f:,-
2,::.04'=;.'?';:."=;,:'..1;,1t:.,::",;::::-k:Xit":4.1`, o.
:.
: ... ,:i.....
,:;=,,:44=;.;,,j,.'11:1..,!==(....õ..?_,A4.===ic=:=.;=P?.fi4'.;q4-
,.;',Ykk'51=õ=-3:1t,= :.t.;."=*=4=;====::õ::11.:,.5-
4,4;i4:1';'..,:i,f:`,;,1::,.;;;;.:;#',;';';.:47:.f1.:t.,;=.,{i,-.:;ITVgii,S.i.-
5:-441...!.if=-:4_:=;V:-ZrWir=;:let,;M,,A.,'W.,0-"r:ig,t;.:=P?'W,".-
lige'W.,.*... ":4-=:,..,,,s0=,:4',IF.:4.7,:;:,,,4 cn
..-.4.-..;;;.. *?..= =1/4:'= ''''==''':''''A=44-:='.4>P? :XMT-
WSi1)1S.S110,V..1?74q5IVW,*i:J115.E.*;7.itl*.=e'll ,
l'='..)..ifii:';;Ii,1%4:(, !;i4-0.;=:".=,'.:µ,..'=.4.==%W4-4*4V,M.-
31,41DOPSIgOi'tt.!: i','Ifi.1:i Z===,'::';'4:''''.';'',
'7't341;:*?µ=::!i=i?'41;,',1 o.
4.1...,........:=:,....,:,=.7,','$.::p=,5,?,n,=. =,;;;;.ti,=k?, .-,:...'
..444.'',,,,:i.evattP;O::.';:µ!..-i=,:-.,-,V.i.s.,1-2:,"A'''=,:.-1;!.;.":-:-
.;Nn'X7!;TA-*';'''' ...k.':.ii'lr.: :.=1!:5:::414:::1'1'.::','1:',":- ':::
'=''-1'.;', ''';'..t.r.;,&;',.-.'.;.!'4,k,492,TOK,Iri:': '
?.:',:;:=.11:?4'.;%;-!A*,-;'''.':'.. ' ILL'...;;;.'õ'",:-.:i5t:i;:e:''' tv
41;;..= =
'. - .....:.,-,-sA4:S.f.P.01-
f.a";.3:',,KINI;JATElliOENTN,4:',1,1'..:=' ;.'4::.: s..i.i.:. - -4=1,10-
3;;i.i::::/,..,,;=,=,. j,,Z.-t.1:.1,0...=:X.:,..=;,,:.:7':::,!;'=W-
.....;,,t:v;';i,C.1,44.,.,,e1=44,= lir;e... =,i; .=.17?..,..r.?::i.=
=.õ..,.t....1:-,.. ,,:l....1,-=.!.: i'..,,.,,-;:f.;;:,41 0
:',..;' ''''.......-' .'.. 14 r'''''.; ='='.µ,.:1;-:'=.:-.µ ,''-'7.: /:='.
....7 =- = .': 4; ''',... 1";..-')......;....:...4;:':..'d'===:.'',.'4,.4.-
/...'):::.:;,,;.j.,'= 'µ,..V;I' ,* ',:i:!.'-'.`::P 4 l'.-=. .:;!..:'.'
',..''''-'.= :::.. -''''I?=µ..'.'..Y4.'.','':::'.-"C .: ..: . = -'. . r.'
'.=.r.....",:..1.:;.= - .:,..,-:',4 . .4:',-.';g0,7R,'4r?-7.1.1.7..::k. '.!:
====:ii. = `:=-:;:.:=15;;:q '.' '.-;.r.':*::::=f:.=':,=.Z.;f-.1.'= -.44 1--
,
..:.-s-
.,,.,,. ,.-.-=:-=,--=.,,,,=0:4=;..;i4447-
..c.2,=,......4.,,,,,,g,...1,,t;;;:1-,,i,:::-.,,r,. : ;,p4,,:;.,,,.,,,,,,
..,,,,;,, A.,-1.4....i..,;,,,,,,,r,f1,...,..,,. 515 ..... ...,....
.,,,i,,,t,,,,,..õ.." , ..., t.,....,:44-,...tõ,....:õ,4 : .;..f....,4,,, .
,.,:1;;'.; , .44/04q, = . 4.,,,.3,:r.='''''.'24, -,'.',==4r''',===?:' ..."
.4.11' ,......,,,, ,,_:=?....,-;,..r...1 01
=
M.:P. '17,1:^'`'.ilf.,, ,t; 153;g'.4-,TVIT:rgb
.'2.4:.C.hr.V,....,,Mii-e.,.4-1.:,.. :A,..-1..:14;:qiecizi:Z.I.i.L.
iktr....1"7.::=-.-L'ir-ed,:o wAcs4A2.,tvg:L.,14tck ..,Ni.-..00tt: .4.1. ,-
r....,.. ...a.4447, - .. ... -. = - -.. I
FA
IV
I
IV
FA
r.....E.N.,..5-
V;.16:44
. ' =
.
= .
. .
. .
. .
.
= =
. .
= Gel 23B Gel 23B Gel 23B
Ge123C = Gel 23C = . Gel 23C =
" Lep/ - . rep 3 = rep 1 -
mpl. rap.2
. .
Approx. Approx. " = . Approx.
Approx. . . Approx. = = Approx.
. Shape triangle Shape " . Shape house
Shape square Shape triangle Shape rectangle
base side 1
base side 1
(mm) 4 bottom(mm) 1.5 (mm)
3 (mm) = ' 3 (mm) = 1.5 0
. base (mm) 4.5 height = side 2 = =
height ' side 2 =
.
o
N.)
. (mm) . 3 side (mm) 2.5 = (mm)
0.729 (mm) . 3 ,(m=m) ' 2 l0
Ul
N.)
'height (min) 5 thickness . triangle . thickness =
thickness . thickness o.
(3)
=
thickness (mm) ' 0.441 height (mm)
0.5 (min) 0.448 (in m) = 0,618 o.
N.)
=
(mm) 1.49 thickness
o
1-,
(3)
. .
(mm) . 0.468 =
1
1-,
N.)
1
N.)
Volume.
.
(mm*3) 16.7625 = - 2.646 1.9305 6.561
= 2.016 1.854
Surface Area 45.5441 16.9440 12.1356
.= = 13.3492. =
=
. .
(rnm 2) . 2559 = . ' 9622
99 26.748 = 7536 = 10.326
' = 2,71702 6.40366. = 6.28629 .
4.07681 6.62166 = 5.56957
SA to V ratio .4644 4484 = 8367 7558
= 4366 9288 =
Beginning .= = s
= .. . 0.0014
=
=
.. .
. 57
=
=
= Mass (g) = 0.0177
0.0037 = 0.0015 =0.0034 0.002 0.00075
Density 0.00105 0:00339 = 0.00077
0.00051 = 0.00099 5124
(8imm*) 5928 8337 . 7001 = 8214
2063 = 10.0714
= 5 min swell = 2.54802 =
7.78378 ==11.2666
Ratio = 2599 3784 6667
=9 broke 2857
= before 5
=
min
o
n.)
o(xi
n.)
n.)
n.)
n.)
=
58
CA 02952464 2016-12-21
Swelling data for the various formulations is graphed in Fig. 14A (swelling
within 5 min) and Fig 14B (swelling within 60 min).
As can be seen from the primary data, the quickest swelling gel was gel
No. 23, which swelled 2000% in 5 min, which compares quite well to the 300%
swelling rate for polyacrylamide gels. When allowed to swell for 60 min, gel
No
19 swelled nearly 7000%, while gel No. 23 swelled 4000%.
As the ideal gel has rapid swelling and reaches its maximum swelling
state quickly, gel No. 23 is the best gel based on swelling data alone. Gel
No. 23
consists of 15% Acrylic acid and 0.05% poly(ethylene glycol) diacrylate. Gel
No. 19 consists of 10% Acrylic acid and 0.05% poly(ethylene glycol)
diacrylate.
Example 2: Assessment of Alternative crosslinkers for Hydrogels
The principle behind the selected crosslinkers is that rather than having a
=
short cross-linker with only two polymerizable groups, a polyvalent
crosslinker =
(i.e., a long-chain hydrophilic polymer with multiple polymerizable groups) is
being Used. A much stronger hydrogel is obtained compared to short chain,
divalent crosslinkers. While these gels are very firm, they possess very good
swelling characteristics. Very strong gels do not normally swell very much.
Poly vinyl alcohol (PVA) was derivatized with allyl glycidyl ether under
alkaline conditions. Gels were made by combing acrylic acid with the PVA-
based crosslinker and then polymerizing the mixture by free radical
polymerization using ammonium persulfate and TEMED as initiators.
=
In principle, the crosslinker can be made with a number of different
starting materials: A range of PVAs as well as partially hydrolyzed poly vinyl
acetates, 2-hydroxyethyl methacrylates (1-1EMA) or various other polymers with
reactive side groups can be used as the basic polymeric backbone. In addition,
a
wide range of natural hydrocolloids such as dextran, cellulose, agarose,
starch,
galactomannans, pectins, hyaluronic acid etc. can be used. A range of reagents
such as ally( glycidyl ether, allyl bromide, ally' chloride etc. can be used
to
incorporate the necessary double bonds into this backbone. Depending on the
chemistry employed, a number of other reagents can be used to incorporate
59
CA 02952464 2016-12-21
reactive double bonds.
Preparation of Polyvalent Crosslinker
Polyvinyl alcohol (PVA, 30-70 kDa) was derivatized with ally! glycidyl
ether under alkaline conditions. 2g PVA was dissolved in 190 mL water. Once
fully dissolved, 10 mL 50% NaOH was added, followed by I mL ally1 glycidyl
ether and 0.2g sodium borohydride. The reaction was allowed to proceed for 16
hours. Subsequently, the crosslinker was precipitated from the reaction
mixture =
by addition of isopropanol. The precipitate was collected by filtration,
washed
with isopropanol, and re-dissolved in 50 mL of water. The crosslinker was
utilized for gel formation, as described below without further purification or
characterization.
Gel Formation and Characterization
Gels were formed by combining acrylic acid with the PVA-based
crosslinker prepared above, and then polymerizing the mixture by free radical
polymerization using ammonium persulfate and TEMED as initiators.
Three gels were prepared containing 15% acrylic acid in combination
= with various ratios/concentrations of the PVA-based crosslinker. The
components listed in Table 3 (excluding initiators) were mixed and degassed by
placing the tubes in a desiccator with a vacuum applied. After 10 minutes, the
vacuum was turned off, and the tubes remained in the desiccator for a further
10
minutes under vacuum. The desiccator was opened, and the initiator was added.
= The contents of the tubes were then mixed thoroughly. The tubes were
capped
and left overnight to polymerize, forming hydrogels.
CA 02952464 2016-12-21
Table 3. Composition of gels 23a-c formed using polyvalent PVA-based
crosslinkers.
Gel
Components (mL) 23a 23b 23c
acrylic acid =1.5 1.5 1.5
PVA cross-linker 0.0526 0.526 5.26
= 50% NaOH 1.251 = 2.15 2.35
H20 7.122 5.779 0.795
APS = 0.04 0.04 0.04
TEMED = 0.05 0.05 0.05
total 10.02 10.05 10.00
pEI (pre-initiator addition) 7.416 7.557 7.451
The gel had a faint pink color, and exhibited a pH of approximately 7.7 =
when gelled. An increase in opacity in the gels was observed, with gel 23a
having the lowest opacity, and gel 23c having the highest.opacity. The gels
had
gel strength that was significantly higher than the gels made with the
poly(ethylene glycol) diacrylate as crosslinker. The gels had very good
mechanical properties as well as very good swelling. The swelling rates for
gels .
23a-c were measured, and are shown in Table 4. Percent swelling was
measured after 5 minutes and 60 minutes.
Table 4. Swelling behavior of gels 32a-c formed using polyvalent PVA-based
crosslinkers. .
Gel = 23a 23b 23c
= 5 min swelling* 1000-2000% 250-1100%
900-1000%
60 min swelling* 4000-6000% 1100-2500% 3600-4300% -
*3 repeats were made for each gel swelling experiment
=
61
CA 02952464 2016-12-21
Example 3: Demonstration of Sealing in in vitro model.
Materials and Methods
An in vitro model of a TAV implantation shown in Figures 15A-15B
was constructed using a tube having placed therein a TAV formed of a
= collapsible mesh 102 securing heart leaflets 104. In the model the mesh 102
did
not seat uniformly into the tube, creating a paravalvular leak site 106
between a
region of the mesh 102 and the tube 100. ==
= The TAV includes an expandable seal as described above with reference
=
= to Figures 2A-2C. The seal 12 was expanded using wire 16 to expose seal
12 to
the surrounding:fluid (blood), causing the hydrogel to expand and press the
seal
12 against the interior of the tube 100, causing the seal 12 membrane to seal
the
perivalVular leak site 108.
Results
= Figure 15A shows a paravalvular leak site 106 due to device
inapposition. 'Figure 15B shows the leak site is sealed with the seal capsule
108
without disturbing the base geometry of the device. The conformation of the
seal
happens actively only in places where there are leak sites. The seal does not
decrease the central orifice area of the device not having any adverse effect
on
the blood flow as a result.
= From the foregoing, it will be appreciated that specific embodiments of
the disclosure have been described herein for purposes of illustration, but
that
various modifications may be made from these embodiments. Certain aspects of
the disclosure described in the context of particular embodiments may be
combined or eliminated in other embodiments. For example, a sealing device in
accordance with particular embodiments may include only some of the
= foregoing components and features, and other devices may include other
components and features in addition to those disclosed above. Further, while
advantages associated with certain embodiments have been described in the
= context of those embodiments, other embodiments may also exhibit such
advantages, and not all embodiments need necessarily exhibit such adyantages.
=
62
CA 02952464 2016-12-21
The scope of the claims should not be limited by the preferred embodiment
and examples, but should be given the broadest interpretation consistent with
the
description as a whole.
63