Note: Descriptions are shown in the official language in which they were submitted.
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
CATALYST CONFIGURATION FOR INCREASED
HYDROCRACKING ACTIVITY
FIELD
100011 Methods are provided for hydroprocessing of feedstocks to produce
distillate
products.
BACKGROUND
100021 Hydrocracking of hydrocarbon feedstocks is often used to convert
lower
value hydrocarbon fractions into higher value products, such as conversion of
vacuum
gas oil (VG0) feedstocks to various fuels and lubricants. Typical
hydrocracking reaction
schemes can include an initial hydrotreatment step, a hydrocracking step, and
a post
hydrotreatment step, such as d.ewax.ing or hydrofinishing. After these steps,
the effluent
can be fractionated to separate out a desired diesel fuel and/or lubricant oil
base oil.
100031 A process train for hydrocracking a feedstock can be designed to
emphasize
the production of fuels or the production of lubricant base oils. During fuels
hydrocracking, typically the goal of the hydrocracking is to cause conversion
of higher
boiling point molecules to molecules boiling in a desired range, such as the
diesel boiling
range, kerosene boiling range, and/or naphtha boiling range. Many types of
fuels
hydrocracking processes also generate a bottoms component from hydrocracking
that
potentially can be used as a lubricant base oil. However, the lubricant base
oil is
produced in a lesser amount, and often is recycled and/or hydrocracked again
to increase
the fuels yield. In hydrocracking for forming a lubricant base oil the goal of
the
hydrocracking is typically to remove contaminants and/or provide viscosity
index uplift
for the feed. This results in some feed conversion, however, so that a
hydrocracking
process for generating a lubricant base oil typically produces a lesser amount
of fractions
that boil in the diesel boiling range, kerosene boiling range, and/or naphtha
boiling
range. Due to the difference in the desired goals, the overall process
conditions during
fuels hydrocracking of a given feedstock typically differ from the overall
process
conditions during hydrocracking for lubricant base oil production on a similar
type of
feedstock.
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 2 -
[0004] U.S. Patent 7,261,805 describes a method for dewaxing and cracking
of
hydrocarbon streams. A feedstock with an end boiling point exceeding 650 F
(343 C) is
contacted with a hydrocracking catalyst and an isornerization dewaxing
catalyst to
produce an upgraded product with a reduced wax content. The feedstock is
described as
contacting the hydrocracking catalyst first, but it is noted that the order of
the steps can
be changed without a significant decrease in yield.
[00051 U.S.- Patent 6,103,101 describes a method for hydroprocessing of
a feedstock
for either lubricant base oil production or for fuels production. The
hydroprocessing
includes sequentially contacting a feed with a detnetailization catalyst, a
hydrotreating
catalyst that includes Group VIB and Group VIII base metals, a
hydroisomerization /
hydrocracking catalyst that includes Group VIB and/or Group VIII metals, and a
hydrofinishing catalyst that includes Group ViB and Group VIII base metals. in
one
configuration described as being suitable for fuels production, the bottoms
product from
fractionation of the hydroprocessed effluent can be recycled to the beginning
of the
hydroprocessing sequence.
100061 U.S. Patent Application Publication 2012/0248008 describes a method
for
fuels hydrocracking with dewaxing of fuel products. In a first stage, a feed
is
hydrotreated and/or hydrocracked, with the resulting effluent being exposed to
a
dewaxing catalyst. The dewaxing catalyst can be a ZSM-48 catalyst with
supported Pt as
a hydrogenation metal. The hydrotreatcd/hydrocracked and &waxed effluent can
then
be fractionated to form filet product streams. A bottoms portion from the
fractionator
can be passed into a second h:yrdrotreating and/or hydrocracking stage for
further
conversion of the feed.
SUMMARY
100071 In an aspect, a method is provided for processing a feedstock to
form a
distillate product, including exposing a feedstock having a T50 boiling point
of at least
about 430 F (221 C), a first aromatics content of at least about 5 wt%, and a
sulfur
content of about 500 wppm or less (or about 100 wpm or less, or about 50 wppm
or
less, or about 15 wppm or less) to an aromatic saturation catalyst comprising
a Group
VIII noble metal under eMctive aromatic saturation conditions to produce an
aromatic
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- -
saturation effluent, the aromatic saturation diluent having a second aromatics
content of
less than about 10 wt%, the second aromatics content being less than the first
aromatics
content; and exposing at least a portion of the aromatic saturation effluent
to a
hydrocracking catalyst under effective hydrocracking conditions to produce a
hydrocracked effluent, the effective hydrocracking conditions being effective
for
converting at least about 5 wt% of the at least a portion of the aromatic
saturation
effluent relative to a conversion temperature of 430 F (221'C), the
hydrocracking
catalyst comprising at least one Group VIII non-noble metal, at least one
Group V113
metal, or a combination thereof, wherein the hydrocracked effluent comprises
at least a
naphtha fuel product fraction and a distillate fuel product fraction.
[0008] In another aspect, a method is provided for processing a feedstock
to fOrm a
distillate product, including exposing a feedstock having a T50 boiling point
of at least
about 430 F (221 C), a first aromatics content, and a sulfur content of about
500 wppm
or less (or about 100 wppm or less, or about 50 wppm or less, or about 15 wppm
or less)
to an aromatic saturation catalyst comprising a Group VIII noble metal under
effective
aromatic saturation conditions to produce an aromatic saturation effluent, the
aromatic
saturation effluent having a second aromatics content, a ratio of the second
aromatics
content to the first aromatics content being about 0.6 or less; and exposing
at least a
portion of the aromatic saturation effluent to a hydrocracking catalyst under
effective
hydrocracking conditions to produce a hydrocracked effluent, the effective
hydrocracking, conditions being effective for converting at least about 5 wt%
of the at
least a portion of the aromatic saturation effluent relative to a conversion
temperature of
430 F (221 C), the hydrocracking catalyst comprising at least one Group VIII
non-noble
metal, at least one Group yin metal, or a combination -thereof; wherein the
hydrocracked
effluent comprises at least a naphtha fuel product fraction and a distillate
fuel product
fraction.
BRIEF' DESCRIPTION OF THE DRAWINGS
[0009] FIG. II schematically shows an example of a reaction system for
processing a
feedstock to form naphtha and diesel boiling range fractions.
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 4 -
DETAILED DESCRIPTION
[0010] All numerical values within the detailed description and the claims
herein are
modified by "about" or "approximately" the indicated value, and take into
account
experimental error and variations that would be expected by a person having
ordinary
skill in the art.
Overview
[0011] In various aspects, systems and methods are provided for producing
an
improved product slate during hydrocracking of a feedstock for production of
naphtha
and distillate fuels. The methods can include use of stacked beds and/or
sequential
reactors so that a feedstock is exposed to a suitable catalyst under aromatic
saturation
conditions prior to exposing the feedstock to the hydrocracking catalyst. The
catalyst for
performing the aromatic saturation process can be a catalyst including a Group
VIII
noble metal, such as Pt, Pd, or a combination thereof, while the hydrocracking
catalyst
can include Group VIB and Group VIII non-noble metals. In various aspects,
treating a
feed with an initial aromatic saturation catalyst that includes a Group VIII
noble metal
instead of first treating the feed with a hydrocracking catalyst can allow for
production of
a naphtha fraction with increased octane; a diesel, jet, and/or distillate
fuel fraction with
improved cloud point and/or freezing point; and/or an increased amount of
branched C4
alkanes versus linear C4 alkanes, so that the value of the C4 alkanes for use
(for
example) as a alkylati.on feed is increased. Additionally or alternately, the
total liquid
product yield and/or the yield of one or more of the naphtha, distillate, or
C4 products
can be enhanced.
[0012] In a hydroprocessin.g reaction system, one way of characterizing a
reaction
stage is based on the stage being a "sweet" reaction stage or a "sour"
reaction stage. In
this discussion, a reaction stage where the feedstock passed into to the stage
contains at
least about 500 wppm of sulfur, or at least about 1000 wppm of sulfur, can be
referred to
as a "sour" reaction stage. Optionally, the reaction stage can be
characterized based on
the sulfur content of both the feedstock and any treat gas passed into the
reaction stage.
A sour reaction stage can be in contrast to a "sweet" reaction stage, where
the sulfur
content in the feedstock passed into the stage is about 500 wppm or less, or
about 300
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 5 -
wppm or less, or about 100 wppm. or less, or about 50 wppm or less, or about
15 wppm
or less.
100131 In many reaction systems that include a hydrocracking catalyst, one
or more
initial reaction stages of the reaction system can correspond to sour reaction
stages.
Many types of petroleum feeds can have an elevated content of sulfur and/or
nitrogen.
An initial hydrotreatment and/or hydrocracking stage can be used to convert
organically
bound sulfur and nitrogen in the feed to compounds that are readily separated
from. the
hydroprocessed feed, such as H2S and NH3. A separator or fractionator can then
be used
to separate the H2S and NH3 from the hydroprocessed effluent. The resulting
separated
hydroprocessed effluent can then have a sufficiently low sulfur content for
one or more
subsequent reaction stages to be considered as sweet processing stages. In a
processing
stage for reducing the sulfur (and optionally nitrogen) content of a
feedstock, catalysts
including base metals are typically used. This is in part due to the ability
of base metal-
containing catalysts to maintain a desirable level of activity in the presence
of a
feedstock with an elevated sulfur or nitrogen content.
100141 In reaction systems involving multiple types of catalysts in a sweet
reaction
stage, conventional methods for arranging catalysts typically include
arranging the
catalysts so that a feedstock contacts base metal-containing catalysts prior
to precious
metal-containing catalysts. This conventional understanding for arrangement of
catalysts
is based on a variety of considerations. For example, many precious metal-
containing
catalysts have a reduced tolerance for heteroatom. contaminants, such as
sulfur or
nitrogen. Placing precious metal-containing catalysts downstream in a reaction
system
can reduce or minimize the amount of heteroatom contaminants a precious metal-
catalyst
is exposed to, either during normal operation or during an unexpected event
that causes
downstream catalysts to be exposed to higher levels of heteroatom contaminants
than
normal. Additionally, using precious metal-containing catalysts later in a
reaction
sequence can potentially reduce the amount of catalyst needed, which can be
beneficial
for reducing the overall cost of a reaction system. Having a mixture of
precious metal-
containing catalysts and base metal-containing catalysts in a catalyst bed is
also usually
avoided. For example, during a catalyst changeout, it can be desirable to
recover as
much of a precious metal-containing catalyst as possible for recycle of the
metal
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 6 -
components. Having base metal-containing catalysts mixed with a precious metal-
containing catalyst can make this process more difficult.
[0015] Without being bound by any particular theory, it is believed that
hydrocracking of aromatic compounds in a feedstock under sweet conditions
typically
occurs by first saturating the aromatic compound followed by cracking of ring
structures.
In a conventional hydrocracking process, the hydrocracking conditions can be
suitable
for performing both the aromatic saturation and the subsequent cracking of
ring
structures. However, the sequential nature of the aromatic saturation and ring-
cleaving
processes may create a need for additional severity in the reaction conditions
for a
hydrocracking process in order to achieve a desired level of conversion.
[00161 In various aspects, the severity of reaction conditions for a
hydrocracking
process in a sweet environment can be reduced or minimized by first exposing a
feed
containing aromatic compounds to an aromatic saturation catalyst that includes
a Group
VIII noble metal under aromatic saturation conditions. Under the aromatic
saturation
conditions, at least a portion of the aromatic compounds in the feed can be
saturated
based on the use of a catalyst with strong hydrogenation activity. Including a
Group VHI
noble metal as a hydrogenation metal on an aromatic saturation catalyst can
provide
enhanced aromatic saturation activity, as compared with typical aromatic
saturation
activity for a catalyst containing a Group VIB and/or Group VIII non-noble
metal. The
effluent from aromatic saturation can then be exposed to a hydrocracking
catalyst that
includes one or more base metals such as Group VIB and/or Group VIII non-noble
metals. Because a substantial amount of aromatic saturation has already been
performed,
the severity of the hydrocracking conditions can be reduced while still
achieving a
desired level of conversion. The reduction in the severity of hydrocracking
conditions
for a desired level of conversion can allow for forming of one or more
products with
increased isom.erization during hydrocracking, which can lead to improved
product
properties. Without being bound by any particular theory, it is believed that
the
increased isomerization at a given level of conversion represents a reduction
in thermal
cracking of a feed at a given level of conversion in favor of hydrocracking.
[0017] In this discussion, the severity of hydroprocessing performed on a
feed can be
characterized based on the temperature, pressure, and/or liquid hourly space
velocity. In
CA 02960399 2017-03-06
WO 2016/0692247 PCT/US2015/054427
- -
general, higher temperatures and pressures correspond to increased severity
for
processing conditions, while higher space velocities tend to correspond to
lower severity
conditions.
Although treat gas rate is specified as a processing condition, the
relationship between treat gas rate and/or hydrogen consumption relative to
process
severity is not as straightforward. Instead, in some aspects, the treat gas
rate and/or
hydrogen consumption for a process can represent an additional cost for
performing a
process. Thus, reducing or minimizing hydrogen consumption can be a benefit
from
performing a process, as opposed to an indication of a change in the severity
of
processing conditions.
[0018] In
various aspects, the hydrocracking conditions in the reaction system can be
selected to generate a desired level of conversion of a feed. Conversion of a
feed is
defined in terms of conversion of molecules that boil above a temperature
threshold to
molecules below that threshold. The conversion temperature can be any
convenient
temperature. For example, for a fuels hydrocracking process, a suitable
conversion
temperature can be from about 390 F (199 C) to about 470 F (243 C). Unless
otherwise
specified, the conversion temperature in this discussion is a conversion
temperature of
430 F (221 C).
[0019] In
this discussion, the amount of conversion can correspond to the total
conversion of molecules within a stage of the reaction system. where the
feedstock (or
optionally the feedstock plus the treat gas) has a sulfur content of 500 wppm
or less.
Suitable amounts of conversion across the sweet reaction stage(s) can
correspond to at
least about 5 wt% conversion of 430 F+ (221 C+) portions of the feedstock to
portions
boiling below 430 F, or at least about 10 wt%, or at least about 15 wt%, or at
least about
20 wt%. In various aspects, the amount of conversion is about 90 wt% or less,
or about
75 wt% or less, or about 50 wt% or less. This conversion in the sweet reaction
stage(s)
is in addition to any conversion that may occur in one or more prior sour
reaction stages.
It is noted that the amount of conversion refers to conversion during a single
pass
through the sweet reaction stage(s). For example, in a fuels hydrocracking
system, a
portion of the unconverted feed that boils above the distillate fuel range can
be recycled
to the beginning of the sweet reaction stage(s) and/or to another earlier
point i.n the
reaction system for further hydroprocessing.
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
-8-
100201 In this discussion, a Group VIII noble metal refers to a metal
selected from
Pt, Pd, Rh, IR, Ru, or Os, preferably Pt, Pd, or a combination thereof. A
Group VIII
non-noble metal refers to Fe, Co, or Ni, preferably Co, Ni, or a combination
thereof. A
Group VIB metal refers to Cr, Mo, or W, preferably Mo, W, or a combination
thereof.
[0021.1 In this discussion, a stage can correspond to a single reactor or a
plurality of
reactors. Optionally, multiple parallel reactors can be used to perform one or
more of the
processes, or multiple parallel reactors can be used for all processes in a
stage. Each
stage and/or reactor can include one or more catalyst beds containing
hydroprocessing
catalyst. Note that a "bed" of catalyst in the discussion below can refer to a
partial
physical catalyst bed. For example, a catalyst bed within a reactor could be
filled
partially with a hydrocracking catalyst and partially with a dewaxing
catalyst. For
convenience in description, even though the two catalysts may be stacked
together in a
single catalyst bed, the hydrocracking catalyst and dewaxing catalyst can each
be
referred to conceptually as separate catalyst beds.
100221 In this discussion, unless otherwise specified a distillate fuel
product fraction,
such as a diesel product fraction, corresponds to a product fraction having a
boiling range
from about 177 C (350 F) to about 370 C (700 F). Thus, distillate fuel product
fractions have initial boiling points (or alternatively T5 boiling points) of
at least about
177 C and final boiling points (or alternatively T95 boiling points) of about
370 C or
less. A naphtha fuel product fraction corresponds to a product fraction having
a boiling
range from about 36 C (97 F) to about 177 C (350 F). Thus, naphtha fuel
product
fractions have initial boiling points (or alternatively T5 boiling points) of
at least about
36 C and final boiling points (or alternatively T95 boiling points) of about
177 C or less.
It is noted that 36 C roughly corresponds to a boiling point for the various
isomers of a
C5 alkane. A kerosene fuel product fraction (which could alternatively be
referred to as
a jet product fraction) corresponds to a product fraction having a boiling
range from
about 143 C (290 F) to about 221 C (430 F). Thus, kerosene fuel product
fractions
have initial boiling points (or alternatively 15 boiling points) of at least
about143 C and
final boiling points (or alternatively T95 boiling points) of about 221 C or
less. When
determining a boiling point or a boiling range for a feed or product fraction,
an
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 9 -
appropriate ASTM test method can be used, such as the procedures described in
ASTM
D2887 or D86.
Feedstocks
100231 A wide range of petroleum and chemical feedstocks can be
hydroprocessed in
accordance with the present invention. Some suitable feedstocks include gas
oils, such
as vacuum gas oils. More generally, suitable feedstocks include whole and
reduced
petroleum crudes, atmospheric and vacuum residua, solvent deasphalted residua,
cycle
oils, FCC tower bottoms, gas oils, including atmospheric and vacuum gas oils
and coker
gas oils, light to heavy distillates including raw virgin distillates,
hydrocrackates,
hydrotreated oils, dewaxed oils, slack waxes, Fischer-Tropsch waxes,
raffinates, and
mixtures of these materials.
[00241 One way of defining a feedstock is based on the boiling range of the
feed.
One option for defining a boiling range is to use an initial boiling point for
a feed and/or
a final boiling point for a feed. Another option, which in some instances may
provide a
more representative description of a feed, is to characterize a feed based on
the amount
of the feed that boils at one or more temperatures. For example, a "T5"
boiling point for
a feed is defined as the temperature at which 5 wt% of the feed will boil off.
Similarly, a
¨no" boiling point is a temperature at which 10 wt% of the feed will boil, a
"T50"
boiling point is a temperature at which 50 wt% of the feed will boil, a "195"
boiling
point is a temperature at which 95 wt% of the feed will boil, while a "T99.5"
boiling
point is a temperature at which 99.5 wt% of the feed will boil.
[00251 The feedstock used can vary depending on a desired mix of output
products.
In some aspects, any feed suitable for use a fuels hydrocracking feed can be
used as a
feedstock. In some aspects, production of distillate products can be
preferred, although
the methods described herein are also suitable for processing of feeds with
substantial
amounts of naphtha boiling range compounds and/or feeds with substantial
amounts of
naphtha after processing. For example, suitable feeds can include feeds with a
150
boiling point of at least about 430 F (221 C) (i.e., where at least about 50
wt% of the
feed has a boiling point greater than 430 F), or at least about 473 F (245 C),
or at least
about 527 F (275 C), or at least about 572 F (300 C), or at least about 600 F
(316 C).
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 10 -
Alternatively, a feed may be characterized using a T5 boiling point, such as a
feed with a
T5 boiling point of at least 430 F (221 C), or at least about 473 F (245 C),
or at least
about 527 F (275 C), or at least about 572 F (300 C), or at least about 600 F
(316 C).
Feeds with higher boiling ranges, such as feeds with higher T5 boiling points,
can be
increasingly suitable for generating higher yields of distillate fuel
products. In some
aspects, feeds can be selected with a boiling range corresponding to an
atmospheric gas
oil or less. Such a feed can have a T95 boiling point of about 800 F (427 C)
or less, or
about 750 F (399 C) or less, or about 700 F (371 C) or less, or about 650 F
(343 C) or
less. Alternatively, a heavier feed can be used that includes vacuum gas oil
boiling range
components. Such a feed can have a T95 boiling point of about 1100 F (593 C)
or less,
or about 1050 F (566 C) or less, or about 1000 F (538 C) or less, or about 950
F
(510 C) or less, or about 900 F (482 C) or less.
[0026] In embodiments involving an initial sulfur removal stage (or stages)
prior to
hydrocracking, the sulfur content of the feed (prior to sulfur removal) can be
at least
about 100 ppm by weight of sulfur, or at least about 500 wppm, or at least
about 1000
wppm, or at least about 2000 wppm, or at least about 4000 wppm, or at least
about
20,000 wppm, such as up to about 40,000 wppm or more. After the initial sulfur
removal stages, the desulfurized effluent can have a sulfur content of about
500 wpm or
less, or about 300 wppm or less, or about 100 wppm or less, or about 50 wpm or
less, or
about 15 wppin or less. In other embodiments, including some embodiments where
a
previously hydrotreated and/or hydrocracked feed is used, the sulfur content
can be about
500 wppm or less, or about 300 wppm or less, or about 100 wppm or less, or
about 50
wppm or less, or about 15 wppm or less.
[0027] In some embodiments, at least a portion of the feed can correspond
to a feed
derived from a biocomponent source. In this discussion, a biocomponent
feedstock
refers to a hydrocarbon feedstock derived from a biological raw material
component,
from biocomponent sources such as vegetable, animal, fish, and/or algae. Note
that, for
the purposes of this document, vegetable fats/oils refer generally to any
plant based
material, and can include fat/oils derived from a source such as plants of the
genus
Jatropha. Generally, the biocomponent sources can include vegetable fats/oils,
animal
fats/oils, fish oils, pyrolysis oils, and algae lipids/oils, as well as
components of such
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 11 -
materials, and in some embodiments can specifically include one or more type
of lipid
compounds. Lipid compounds are typically biological compounds that are
insoluble in
water, but soluble in nonpolar (or fat) solvents. Non-limiting examples of
such solvents
include alcohols, ethers, chloroform, alkyl acetates, benzene, and
combinations thereof.
Hydroprocessing with Improved Product Properties and Yields
[0028] Various types of hydroprocessing can be used in the production of
distillate
products. Typical processes include initial hydrotreating and/or hydrocracking
processes
to remove contaminant heteroatoms, such as sulfur, nitrogen, and/or oxygen
from a feed.
The hydrotreated and/or hydrocracked feed can then be fractionated to form one
or more
fuel fractions, such as a naphtha boiling range fraction, a jet or kerosene
boiling range
fraction, or a diesel boiling range fraction. Optionally, multiple fractions
in a given
boiling range can be formed, such as a light naphtha fraction and a heavy
naphtha
fraction. Typically a light ends fraction and a bottoms fraction can also be
formed.
Optionally, the diesel fraction can correspond to the bottoms fraction.
Optionally, a C4
(butane) fraction can be separated from the light ends fraction, either during
or after the
fractionation.
[0029] The bottoms fraction from the fractionation, having a sulfur content
of about
500 wppm or less, or about 300 wppm or less, preferably about 100 wppm or
less, or
about 50 wppm or less, or about 15 wppm or less, can then be exposed to an
aromatics
saturation catalyst that includes a Group VIII noble metal under effective
aromatic
saturation conditions. The effluent from the aromatic saturation step can then
be
hydrocracked under effective hydrocracking conditions in the presence of a
catalyst
including one or more base metals, such as one or more Group VIB or Group VIII
non-noble metals. The aromatic saturated, hydrocracked effluent can then be
passed into
the same fractionator or a different fractionator for separation of desired
fuel products.
This effectively sets up a recycle loop between the fractionator and the
aromatic
saturation / hydrocracking stage(s), as components from the aromatics
saturation
hydrocracking stage that correspond to the bottoms from the fractionator can
be returned
to the aromatics saturation hydrocracking stage for multiple passes. In some
aspects,
the recycle loop may only correspond to a portion of the bottoms from the
fractionator,
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 12 -
while a second portion of the bottoms is drawn off as a product stream, such
as a diesel
fuel product.
[0030] In some aspects, the effluent from the aromatic saturation /
hydrocracking
stage(s) may also be passed through or more dewaxing catalyst beds and/or
additional
aromatic saturation catalyst beds prior to fractionation. This can allow for
further
upgrading of product properties, such as further improvement of the cold flow
properties
for a diesel fuel fraction.
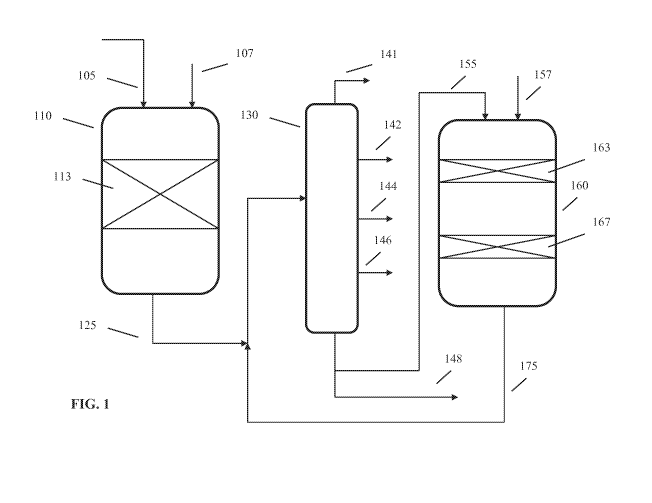
100311 FIG. 1 schematically shows an example of a configuration for
performing
fuels hydrocracking. In FIG. 1, a feed 105 and a hydrogen-containing treat gas
107 are
passed into a reactor 110. Reactor 110 is shown. as having a single catalyst
bed 113, but
any convenient number of catalyst beds can be used. Similarly, a single
reactor 110 is
shown, but any convenient number of reactors could be used, optionally with
gas-liquid
separators (not shown) between reactors. The feed is exposed to the catalyst
in catalyst
bed(s) 113 under effective conditions for reducing the heteroatom content of
the feed.
Catalyst bed(s) 113 can include any convenient combination of hydrotreati.ng
and/or
hydrocracking catalysts for converting a sour feedstock to form an effluent
that can be
used at least in part in a sweet hydroprocessin.g stage. The effluent 125 is
then passed
into a fractionator 130. Fractionator 130 can represent any convenient number
of
separators and/or fractionator for generating a desired number of fractions.
FIG. 1
schematically shows fractionator 130 as generating a light ends fraction 141,
a light
naphtha fraction 142, a heavy naphtha fraction 144, a jet or kerosene fraction
146, and a
bottoms (diesel) fraction 148. Optionally, a gas-liquid separation (not shown)
can be
performed on effluent 125, so that only a liquid portion of effluent 125 is
passed into
fractionator 130. In this type of alternative, one or more of the output
streams from
fractionator 130 might instead be generated from the gas-liquid separator.
Optionally, a
diesel fraction (not shown) separate from bottoms fraction 148 can be
generated. In
various aspects, the liquid portion of effluent 125 can have a desired sulfur
content, such
as a sulfur content of 500 wppm or less, or 300 wppm or less, or 100 wppm or
less, or 50
wppm or less, or 15 wppm or less.
[0032] A portion 155 of bottoms fraction 148 is then passed into a second
reactor
160, along with a second hydrogen-containing treat gas stream. 157. The input
feeds to
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 13 -
second reactor 160 are first exposed to one or more beds of aromatic
saturation catalyst
163, followed by one or more beds of a hydrocracking catalyst 167. The
resulting
hydrocracked effluent 175 is then passed into a fractionator. In the
configuration shown
in FIG. 1, hydrocracked effluent 175 is returned to the fractionator 130 that
is used for
fractionation of first stage effluent 125. In alternative configurations, a
separate
fractionator can be used for fractionation of hydrocracked effluent 175.
10033] it is noted that the configuration shown in FIG. 1 corresponds to a
configuration for processing of a feed with a sulfur content of at least 100
wppm, such as
at least about 500 wppm. If a feed has a suitably low sulfur content, reactor
110 could be
omitted and the low sulfur feed could be directly introduced into reactor 160.
[0034] It is noted that the configuration in FIG. 1 shows a recycle of a
portion of the
bottoms fraction to the second reactor 160. In various aspects, a recycle loop
is not
required to achieve the benefits of the catalyst configuration described
herein. Instead,
the second reactor 160 can be operated in a single pass mode, so that the
effluent from.
reactor 160 is fractionated into various types of product streams that can be
used as
products and/or passed to other reaction systems. In various aspects, it can
be
convenient to use a first separation stage for separation or fractionation of
the effluent
from a sour stage (such as reactor 110) and a second, different separation
stage for
fractionation of the sweet stage product effluent.
Hydrotreatment and Sour Hydrocracking Conditions
[0035] Hydrotreatment is typically used to reduce the sulfur, nitrogen, and
aromatic
content of a feed. The catalysts used for hydrotreatment can include
conventional
hydroprocessing catalysts, such as those that comprise at least one Group VIII
non-noble
metal (Columns 8 ¨ 10 of ILTPAC periodic table), preferably Fe, Co, and/or Ni,
such as
Co and/or Ni; and at least one Group VIB metal (Column 6 of IUPAC periodic
table),
preferably Mo and/or W. Such hydroprocessing catalysts can optionally include
transition metal sulfides. These metals or mixtures of metals are typically
present as
oxides or sulfides on refractory metal oxide supports. Suitable metal oxide
supports
include low acidic oxides such as silica, alumina, titania, silica-titania,
and titania-
alumina. Suitable aluminas are porous aluminas such as gamma or eta having
average
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 14 -
pore sizes from 50 to 200 A, or 75 to 150 A; a surface area from 100 to 300
m2/g, or 150
to 250 m2/g; and a pore volume of from 0.25 to 1.0 cm3/g, or 0.35 to 0.8
cm3/g. The
supports are preferably not promoted with a halogen such as fluorine as this
generally
increases the acidity of the support.
[0036] The at least one Group VIII non-noble metal, in oxide form, can
typically be
present in an amount ranging from about 2 wt% to about 40 wt%, preferably from
about
4 wt% to about 15 wt%. The at least one Group VIB metal, in oxide form, can
typically
be present in an amount ranging from about 2 wt% to about 70 wt%, preferably
for
supported catalysts from about 6 wt% to about 40 wt% or from about 10 wt% to
about 30
wt%. These weight percents are based on the total weight of the catalyst.
Suitable metal
catalysts include cobalt/molybdenum (1-10% Co as oxide, 10-40% Mo as oxide),
nickel/molybdenum (1-10% Ni as oxide, 10-40% Co as oxide), or nickel/tungsten
(1-
10% Ni as oxide, 10-40% W as oxide) on alumina, silica, silica-alumina, or
titania.
[0037] Alternatively, the hydrotreating catalyst can be a bulk metal
catalyst, or a
combination of stacked beds of supported and bulk metal catalyst. By bulk
metal, it is
meant that the catalysts are unsupported wherein the bulk catalyst particles
comprise
30-100 wt. % of at least one Group VIII non-noble metal and at least one Group
VIB
metal, based on the total weight of the bulk catalyst particles, calculated as
metal oxides
and wherein the bulk catalyst particles have a surface area of at least 10
m2/g. It is
furthermore preferred that the bulk metal hydrotreating catalysts used herein
comprise
about 50 to about 100 wt%, and even more preferably about 70 to about 100 wt%,
of at
least one Group VIII non-noble metal and at least one Group VIB metal, based
on the
total weight of the particles, calculated as metal oxides. The amount of Group
VIB and
Group VIII non-noble metals can easily be determined VIB TEM-EDX.
[0038] Bulk catalyst compositions comprising one Group VIII non-noble metal
and
two Group VIB metals are preferred. It has been found that in this case, the
bulk catalyst
particles are sintering-resistant. Thus the active surface area of the bulk
catalyst particles
is maintained during use. The molar ratio of Group VIB to Group VIII non-noble
metals
ranges generally from 10:1-1:10 and preferably from 3:1-1:3. In the case of a
core-shell
structured particle, these ratios of course apply to the metals contained in
the shell. If
more than one Group VIB metal is contained in the bulk catalyst particles, the
ratio of
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 15 -
the different Group VIB metals is generally not critical. The same holds when
more than
one Group VIII non-noble metal is applied. In the case where molybdenum and
tungsten
are present as Group VIB metals, the molybdenum:tungsten ratio preferably lies
in the
range of 9:1-1:9. Preferably the Group VIII non-noble metal comprises nickel
and/or
cobalt. It is further preferred that the Group VIB metal comprises a
combination of
molybdenum and tungsten. Preferably, combinations of
nickel/molybdenum/tungsten
and cobalt/molybdenum/tungsten and nickel/cobalt/molybdenum/tungsten are used.
These types of precipitates appear to be sinter-resistant. Thus, the active
surface area of
the precipitate is maintained during use. The metals are preferably present as
oxidic
compounds of the corresponding metals, or if the catalyst composition has been
sulfided,
sulfidic compounds of the corresponding metals.
[0039] It is also preferred that the bulk metal hydrotreating catalysts
used herein
have a surface area of at least 50 m2/g and more preferably of at least 100
m2/g. It is also
desired that the pore size distribution of the bulk metal hydrotreating
catalysts be
approximately the same as the one of conventional hydrotreating catalysts.
Bulk metal
hydrotreating catalysts have a pore volume of 0.05-5 ml/g, or of 0.1-4 ml/g,
or of 0.1-3
ml/g, or of 0.1-2 ml/g determined by nitrogen adsorption. Preferably, pores
smaller than
1 nm are not present. The bulk metal hydrotreating catalysts can have a median
diameter
of at least 50 nm, or at least 100 nm. The bulk metal hydrotreating catalysts
can have a
median diameter of not more than 5000 pm, or not more than 3000 gm. In an
embodiment, the median particle diameter lies in the range of 0.1-50 pm and
most
preferably in the range of 0.5-50 pm.
[0040] The hydrotreatment is carried out in the presence of hydrogen. A
hydrogen
stream is, therefore, fed or injected into a vessel or reaction zone or
hydroprocessing
zone in which the hydroprocessing catalyst is located. Hydrogen, which is
contained in a
hydrogen-containing "treat gas," is provided to the reaction zone. Treat gas,
as referred
to in this invention, can be either pure hydrogen or a hydrogen-containing
gas, which is a
gas stream containing hydrogen in an amount that is sufficient for the
intended
reaction(s), optionally including one or more other gasses (e.g., nitrogen and
light
hydrocarbons such as methane), and which will not adversely interfere with or
affect
either the reactions or the products. Impurities, such as H2S and NH3 are
undesirable
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 16 -
and would typically be removed from the treat gas before it is conducted to
the reactor.
The treat gas stream introduced into a reaction stage will preferably contain
at least about
50 vol. % and more preferably at least about 75 vol. % hydrogen.
100411
Hydrotreating conditions can include temperatures of about 200 C to about
450 C, or about 315 C to about 425 C; pressures of about 250 psig (1.8 MPag)
to about
5000 psig (34.6 MPag) or about 300 psig (2.1 MPag) to about 3000 psig (20.8
MPag);
liquid hourly space velocities (LHSV) of about 0.1 hr-1 to about 10 hr-I; and
hydrogen
treat rates of about 200 scf7B (35.6 m3/m3) to about 10,000 scf7B (1781
m3./m3), or about
500 (89 m3/m3) to about 10,000 scf/B (1781 m3/m3).
100421 in
some aspects, hydrocracking can be used in place of or in addition to
hydrotreating for removal of heteroatom contaminants. Suitable hydrocracking
catalysts
for heteroatom removal can include hydrotreating catalysts as described above,
as well as
hydrocracking catalysts as described below. A hydrocracking process performed
under
sour conditions, such as conditions where the sulfur content of the input feed
to the
hydrocracking stage is at least 500 wppm, can be carried out at temperatures
of about
550 F (288 C) to about 840 F (449 C), hydrogen partial pressures of from about
250
psig to about 5000 psig (1.8 MPag to 34.6 MPag), liquid hourly space
velocities of from
0.05 to 10 and
hydrogen treat gas rates of from 35.6 m3/m.3 to 1781 m3/m.3 (200
SCF/B to 10,000 SCF/B). In
other embodiments, the conditions can include
temperatures in the range of about 600 F (343 C) to about 815 F (435T),
hydrogen
partial pressures of from about 500 psig to about 3000 psig (3.5 MPag-20.9 M
Pag),
liquid hourly space velocities of from about 0.2 11-1 to about 2 1-1-1 and
hydrogen treat gas
rates of from about 213 m3/m3 to about 1068 m3/m3 (1200 SCF/B to 6000 SCF/B).
Hydrofinishing and/or _Aromatic Saturation Process
[0043] In
aspects where an initial stage is used for heteroatom removal, the effluent
from the initial stage can be fractionated. At least a portion of the bottoms
from the
fractionator can then be exposed to an aromatic saturation catalyst under
effective
aromatic saturation conditions.
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 7 -
[0044] In various aspects, for aromatic saturation under sweet conditions
prior to
hydrocracking, suitable aromatic saturation catalysts can correspond to
catalysts
containing one or more Group VIII noble metals, such as Pt, Pd, or
combinations thereof.
Suitable metal oxide supports include low acidic oxides such as silica,
alumina, silica-
aluminas or fitania, preferably alumina. The support materials may also be
modified,
such as by halogenation, or in particular fluorination. The amount of
supported Group
VIII noble metal can be about 0.1 wt% to about 2.0 wt% based on the weight of
the
catalyst, such as about 0.1 wt% to about 1.8 wt%, or about 0.1 wt% to about
1.5 wt%, or
about 0.1 wt% to about 1.2 wt%, or about 0.1 wt% to about 0.9 wt%, or about
0.3 wt% to
about 1.8 wt%, or about 0.3 wt% to about 1.5 wt%, or about 0.3 wt% to about
1.2 wt%,
or about 0.3 wt% to about 0.9 wt%, or about 0.6 wt% to about 1.8 wt%, or about
0.6
wt% to about 1.5 wt%, or about 0.6 wt% to about 1.2 wt%. Optionally, if an
aromatic
saturation catalyst includes both Pt and Pd, the Pt and Pd can be included in
any
convenient ratio, such as 90: 10 Pt to Pd, or 75 : 25, or 60 : 40, or 50: 50,
or 40 : 60, or
25 : 75, or 10 : 90. In an aspect, a preferred hydrofinishing catalyst can
include a
crystalline material belonging to the M41S class or family of catalysts. The
M4 1S
family of catalysts are mesoporous materials having high silica content.
Examples
include MCM-41, MCM-48 and MCM-50. A preferred member of this class is MCM-41.
[0045] Optionally, aromatic saturation or hydrofinishing catalysts may be
located at
other locations within a reaction system. in general, aromatic saturation
catalysts can
include catalysts containing Group VIB metals, Group VIII metals, and mixtures
thereof.
In an aspect, preferred metals include at least one metal sulfide having a
strong
hydrogenation function. In another aspect, the hydrofinishing catalyst can
include a
Group VIII noble metal, such as Pt, Pd, or a combination thereof, in an amount
as
described above. The mixture of metals may also be present as bulk metal
catalysts
wherein the amount of metal is about 30 wt. % or greater based on catalyst.
Suitable
metal oxide supports include low acidic oxides such as silica, alumina, silica-
alumin.as or
titania, preferably alumina. The support materials may also be modified, such
as by
halogenation, or in particular fluorination. The metal content of the catalyst
is often as
high as about 20 weight percent for non-noble metals. In an embodiment, a
preferred
hydrofinishing catalyst can include a crystalline material belonging to the
M41. S class or
family of catalysts. The M41S family of catalysts are mesoporous materials
having high
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 18 -
silica content. Examples include MCM-41, MCM-48 and MCM-50. A preferred
member of this class is MCM-41.
[0046] Aromatic saturation conditions can include temperatures from about
125 C to
about 425 C, preferably about 180 C to about 280 C, total pressures from about
300
psig (2.1 M Pa) to about 3000 psig (20.7 MPa), preferably about 1000 psig (6.9
MPa) to
about 2500 psig (17.2 MPa), liquid hourly space velocities from about 0.1 hr-1
to about
30 hr-1 LHSV, or about 0.5 hr-1 to about 30 hr-1, or about 0.5 hr-1 to about
20 hr-I, or
about 1.0 hr-1 to about 20 hr-1, preferably about 1.0 hr-.1 to about 15 hr-1,
about 1.5 hr-1 to
about 15 hr-1, or about 1.0 hr-1 to about 10 he', or about 1.5 hr-1 to about
10 he', or about
2.0 hr-1 to about 20 hr-1, or about 2.0 hr-1 to about 15 ht-1, and treat gas
rates of from 35.6
m3/m3 to 1781 m3/m3 (200 SCF/B to 10,000 SCF/B), preferably 213 m3/m3 to about
1068
m3/m3 (1200 SCF/B to 6000 SCF/B) of a hydrogen-containing treat gas. The
hydrogen-
containing treat gas can contain at least about 80 vol% H2, or at least about
90 vol%, or
at least about 95 vol%, or at least about 98 vol%.
[0047] The aromatic saturation conditions can be effective for reducing the
aromatics
content of a feed. A feed to the aromatics saturation step can have an
aromatics content
of at least about 5 wt%, or at least about 10 wt%, or at least about 15 wt%,
or at least
about 20 wt% or at least about 25 wt%, or at least about 30 wt%, such as up to
about 60
wt% or more. In some aspects, the amount of aromatics in the effluent from an
aromatics saturation step can be characterized based on a weight percent of
aromatics in
the effluent. The aromatics content after aromatics saturation can be less
than about 10
wt%, or less than about 7.5 wt%, or less than about 5 wt%, or less than about
3 wt%. In
other aspects, the amount of aromatics in the effluent can be characterized
relative to the
amount of aromatics in the feed to the aromatics saturation step. For example,
a ratio of
aromatics in the effluent from aromatics saturation to aromatics in the feed
can be about
0.6 or less, or about 0.5 or less, or about 0.4 or less, or about 0.3 or less,
or about 0.2 or
less, or about 0.15 or less, or about 0.1 or less.
Hydrocracking Conditions after Aromatic Saturation
[0048] Hydrocracking catalysts typically contain suffided base metals on
acidic
supports, such as amorphous silica alumina, cracking zeolites or other
cracking
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 19 -
molecular sieves such as .USY, or acidified alumina. In some preferred
aspects, a
hydrocracking catalyst can include at least one molecular sieve, such as a
zeolite. Often
these acidic supports are mixed or bound with other metal oxides such as
alumina, titania
or silica. Non-limiting examples of supported catalytic metals for
hydrocracking catalysts
include combinations of Group V113 and/or Group VIII non-noble metals,
including nickel,
nickel-cobalt-molybdenum, cobalt-molybdenum, nickel-tungsten, nickel-
molybdenum,
and/or nickel-molybdenum-tungsten. Support materials which may be used can
comprise a
refractory oxide material such as alumina, silica, alumina-silica,
.kieselguhr, diatomaceous
earth, magnesia, zirconia, or combinations thereof, with alumina, silica,
alumina-silica
being the most common (and preferred, in one embodiment).
[0049] The at least one Group VIII non-noble metal, in oxide form., can
typically be
present in an amount ranging from about 2 wt% to about 40 wt%, preferably from
about
4 wt% to about 15 wt%. The at least one Group VIB metal, in oxide form, can
typically
be present in an amount ranging from about 2 wt% to about 70 wt%, preferably
for
supported catalysts from about 6 wt% to about 40 wt% or from about 10 wt% to
about 30
wt%. These weight percents are based on the total weight of the catalyst. In
some
aspects, suitable hydrocracking catalysts can include nickel/molybdenum,
nickel/tungsten, or nickel/molybdenum/tungsten as metals supported on the
hydrocracking catalyst.
[0050] In some aspects, a hydrocracking catalyst can include a large pore
molecular
sieve that is selective for cracking of branched hydrocarbons and/or cyclic
hydrocarbons.
Zeolite Y, such as ultrastable zeolite Y (UM') is an example of a zeolite
molecular sieve
that is selective for cracking of branched hydrocarbons and cyclic
hydrocarbons.
Depending on the aspect, the silica to alumina ratio in a USY zeolite can be
at least about
10, such as at least about 15, or at least about 25, or at least about 50, or
at least about 100.
Depending on the aspect, the unit cell size for a USY zeolite can be about
24.50 Angstroms
or less, such as about 24.45 Angstroms or less, or about 24.40 Angstroms or
less, or about
24.35 Angstroms or less, such as about 24.30 Angstroms. In other aspects, a
variety of
other types of molecular sieves can be used in a hydrocracking catalyst, such
as zeolite Beta
and ZSM-5. Still other types of suitable molecular sieves can include
molecular sieves
having 10-member ring pore channels or 12-member ring pore channels. Examples
of
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 20 -
molecular sieves having 10-member ring pore channels or 12-member ring pore
channels
include molecular sieves having zeolite framework structures selected from
WIRE, IMTT,
EUO, AEI , A FOõSFF, STF, TON, OSI, ATO, (JON, MTW, SEE, SSY, or VET.
[0051] In various embodiments, the conditions selected for hydrocracking
can
depend on the desired level of conversion, the level of contaminants in the
input feed to
the hydrocracking stage, and potentially other factors.
[0052] A hydrocracking process performed under non-sour conditions can be
performed under conditions similar to those used for sour conditions, or the
conditions
can be different. Alternatively, a non-sour hydrocracking stage can have less
severe
conditions than a similar hydrocracking stage operating under sour conditions.
Suitable
hydrocracking conditions can include temperatures of about 450 F (232 C) to
about
840 F (449"C), or about 450 F (232 C) to about 800 F (427 C), or about 450 F
(249 C)
to 750 F (399 C), or about 500 F (260 C) to about 840 F (449 C), or about 500
F
(260 C) to about 800 F (427 C), or about 500 F (260 C) to about 750 F (399 C);
hydrogen partial pressures of from about 250 psig to about 5000 psig (1.8
IVIPag to 34.6
NAPag); liquid hourly space velocities of from 0.05 11-1 to 10 111; and
hydrogen treat gas
rates of from 35.6 m3/m3 to 1781 m3/m3 (200 SCF/B to 10,000 SCF/B), In other
embodiments, the conditions can include temperatures in the range of about 500
F
(260 C) to about 815 F (435 C), or about 500 F (260 C) to about 750 F (399"C),
or
about 500 F (260 C) to about 700 C (371 C); hydrogen partial pressures of from
about
500 psig to about 3000 psig (3.5 NAPag-20.9 MPag); liquid hourly space
velocities of
from about 0.2 11-1 to about 5 h-1; and hydrogen treat gas rates of from about
213 m3/m3 to
about 1068 m3/m3 (1200 SCIF/B to 6000 SCF/B).
Dewa.xing Process
[0053] In some optional aspects, a d.ewaxing catalyst can also be included
for
dewaxing of the hydrocrac.ked effluent in the sweet processing stage. Suitable
dewaxi.ng
catalysts can include molecular sieves such as crystalline aluminosilicates
(zeolites). In
an embodiment, the molecular sieve can comprise, consist essentially of, or be
ZSM-5,
ZSM-22, ZSM-23, ZSM-35, ZSM-48, zeolite Beta, ZSM-57, or a combination
thereof,
for example ZSM-23 and/or ZS:NA-48, or ZSM-48 and/or zeolite Beta. Optionally
but
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 21 -
preferably, molecular sieves that are selective for dewaxing by isomerization
as opposed
to cracking can be used, such as ZSM-48, zeolite Beta, ZSM-23, or a
combination
thereof. Additionally or alternately, the molecular sieve can comprise,
consist essentially
of, or be a 10-member ring 1-D molecular sieve. Examples include EU-1, ZSM-35
(or
ferrierite), ZSM-11, ZSM-57, NU-87, SAPO-11, ZSM-48, ZSM-23, and ZSM-22.
Preferred materials are EU-2, EU-I1, ZBM-30, ZSM-48, or ZSM-23. ZSM-48 is most
preferred. Note that a zeolite having the ZSM-23 structure with a silica to
alumina ratio
of from about 20:1 to about 40:1 can sometimes be referred to as SSZ-32. Other
molecular sieves that are isostructural with the above materials include Theta-
I, NU-10,
EU-13, KZ-1, and NU-23. Optionally but preferably, the dewaxing catalyst can
include
a binder for the molecular sieve, such as alumina, titania, silica, silica-
alumina, zirconia,
or a combination thereof, for example alumina and/or titania or silica and/or
zirconia
and/or titania.
[0054] Preferably, the dewaxing catalysts used in processes according to
the
invention are catalysts with a low ratio of silica to alumina. For example,
for ZSM-48,
the ratio of silica to alumina in the zeolite can be less than 200:1, or less
than 110:1, or
less than 100:1, or less than 90:1, or less than 80:1. In various embodiments,
the ratio of
silica to alumina can be from 30:1 to 200:1, 60:1 to 110:1, or 70:1 to 100:1.
[00551 In various embodiments, the catalysts according to the invention
further
include a metal hydrogenation component. The metal hydrogenation component is
typically a Group VIB and/or a Group VIII metal. Preferably, the metal
hydrogenation
component is a Group VIII noble metal. Preferably, the metal hydrogenation
component
is Pt, Pd, or a mixture thereof. In an alternative preferred embodiment, the
metal
hydrogenation component can be a combination of a non-noble Group VIII metal
with a
Group VIB metal. Suitable combinations can include Ni, Co, or Fe with Mo or W,
preferably Ni with Mo or W.
10056] The amount of metal in the catalyst can be at least 0.1 wt% based on
catalyst,
or at least 0.15 wt%, or at least 0.2 wt%, or at least 0.25 wt%, or at least
0.3 wt%, or at
least 0.5 wt% based on catalyst. The amount of metal in the catalyst can be 20
wt% or
less based on catalyst, or 10 wt% or less, or 5 wt% or less, or 2.5 wt% or
less, or 1 wt%
or less. For embodiments where the metal is Pt, Pd, another Group VIII noble
metal, or a
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 22 -
combination thereof, the amount of metal can be from 0.1 to 5 wt%, preferably
from 0,1
to 2 wt%, or 0.25 to 1.8 wt%, or 0.4 to 1.5 wt%. For embodiments where the
metal is a
combination of a non-noble Group VIII metal with a Group VIB metal, the
combined
amount of metal can be from 0.5 wt% to 20 wt%, or 1 wt% to 15 wt%, or 2.5 wt%
to 10
wt%.
[0057] Process conditions in a catalytic dewaxing zone can include a
temperature of
about 200 C to about 450 C, preferably about 270 C to about 400 C, a hydrogen
partial
pressure of about 1.8 MPag to about 34.6 MPag (250 psig to 5000 psig),
preferably about
4.8 11/1:Pag to about 20.8 l`v1Pag, and a hydrogen treat gas rate of about
35.6 m3/m3 (200
SCF/B) to about 1781 m3/m3 (10,000 scf/B), preferably about 178 m3/m3 (1000
SCF/B)
to about 890.6 m3/m3 (5000 SCF/B). In still other embodiments, the conditions
can
include temperatures in the range of about 600 F (343 C) to about 815 F (435
C),
hydrogen partial pressures of from about 500 psig to about 3000 psig (3.5 MPag-
20.9
Wag), and hydrogen treat gas rates of from about 213 m3/m3 to about 1068 m3/m3
(1200
SCF. The LE1SV can be from about 0.1 11-1 to about 10 WI, such as from about
0.5 111 to
about 5 h-1 and/or from about I hi to about 4 WI,
Product Properties
[0058] Processing a feedstock to produce fuels according to methods
described
herein can provide a variety of advantages. In some aspects, the yield of
total liquid
product from hydrocracking in the sweet processing stage(s) can be increased.
The yield
of total liquid product from 112,,,,drocracking in the sweet hydroprocessing
stage is defined
as the weight of recovered products that are liquid at 25 C, 1 atm, relative
to the weight
of the input feed to the sweet hydroprocessing stage that is a liquid at 25 C,
1 atm. It is
noted that any portion of the bottoms from fractionation that is recycled to
the sweet
hydroprocessing stage is included within the yield in this definition, so
yield is a measure
of the amount of gas phase product generated during a single pass through the
sweet
processing stage. :In other aspects, the yield of one or yield of jet or
kerosene boiling
range product, or the yield of diesel boiling range product.
[0059] Additionally or alternately, the product quality of one or more
products can
be improved. In aspects where a feed is exposed a precious metal-containing
aromatic
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 23 -
saturation catalyst prior to hydrocracking, without being bound by any
particular theory,
it is believed that a desired level of conversion of the feed can be achieved
using less
severe hydrocracking conditions. As a result, it is believed that a larger
percentage of the
feed conversion is due to hydrocracking mechanisms rather than therrnal
cracking
mechanisms, which leads to an increased amount of branched or isomerized
compounds
in the resulting products. This increase in the amount of branched or
isomerized
compounds can contribute to a variety of product quality improvements. For a
jet or
distillate boiling range fraction, an example of an improvement in product
quality can
correspond to an improvement in a cold flow property, such as a decrease in
the freezing
point of a jet boiling range fraction or the cloud point of a diesel boiling
range fraction.
For a naphtha boiling range fraction, an example of an improvement in product
quality
can correspond to an improvement in octane.
[0060] For a C4 product fraction, an improvement in product quality can
correspond
to an increase in the ratio of isobutane to n-butane generated in the C4
product fraction.
Although a C4 product fraction is not a liquid product at 25 C, 1 atm, a C4
product
fraction with a sufficiently high ratio of iso-C4 to n-C4 can be suitable for
use as a feed
to a reforming process. In a typical fuels hydrocracking process, a C4 product
can have a
ratio of iso-C4 to n-C4 of about 4. In various aspects, a C4 product from a
hydrocracking
process as described herein can have a higher ratio of of iso-C4 to n-C4, such
as a ratio of
iso-C4 to n-C4 of at least about 5, or at least about 5.5, or at least about
6.
Example I ¨ .Aromatic Saturation and Ilydrocracking of a Second Stage
Ilydrocracker
Feed
[0061] In this example, a feed representative of a feed to a second stage
of a fuels
hydrocracking reaction system was processed under various conditions to
demonstrate
the benefit of contacting the feed with a precious metal-containing aromatic
saturation
catalyst prior to hydrocracking. The feed was a hydrocracked gas oil with a
specific
gravity of about 0.877 giml, a sulfur content of about 20 wppm, a nitrogen
content of
about 1.2 wppm, and an initial aromatics content of about 20 wt%.
[0062] The feed was processed by exposing the feed to two catalysts
according to the
reaction conditions shown in Table 1. The two catalysts corresponded to a
commercially
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 24 -
available hydrocracking catalyst and a commercially available aromatic
saturation
catalyst. The hydrocracking catalyst was a bound molecular sieve with nickel
and
tungsten supported on the catalyst as catalytic metals. The aromatic
saturation catalyst
had a combination of platinum and palladium supported on an alumina support.
The
ratio of hydrocracking catalyst to aromatic saturation catalyst was 80 : 20 by
weight. In
a first type of processing run, the feed was exposed to the hydrocracking
catalyst first,
while in the second type of processing run the feed was exposed to the
aromatic
saturation catalyst first. For the processing runs in Table 1, the catalysts
were located in
separate reactors, with the effluent from the first reactor in series being
cascaded into the
second reactor.
[0063] As shown in Table 1, the IRSV, the pressure, and the treat gas rate
were
substantially the same when processing with both types of catalyst
configurations. The
temperature was adjusted in the processing runs to achieve the same level of
feed
conversion across the hydrocracking catalyst. As shown in Table 1, the
temperature
required to achieve 15.6 wt% conversion of the feed (relative to a conversion
temperature of 430 F) differed by about 20 F (11 C) for the catalyst
configuration with
the hydrocracking catalyst as the upstream catalyst as compared to the
configuration with
the aromatic saturation catalyst as the upstream catalyst. This demonstrates
that
substantially less severe hydrocracking conditions could be used to hydrocrack
the feed
to a desired conversion level by first exposing the feed to the precious metal-
containing
aromatic saturation catalyst. Without being bound by any particular theory, it
is believed
that the difference in the hydrocracking temperature to achieve the desired
level of
conversion is due in part to the reduced aromatics content after exposing the
feed to the
aromatics saturation catalyst. In the conventional catalyst configuration, the
feed has
about 20 wt% aromatics content when exposed to the hydrocracking catalyst.
Even after
hydrocracking, in the conventional configuration the hydrocracked feed still
has an
aromatics content of about 12 wt%. By contrast, exposing the feed to the
aromatics
saturation catalyst first reduces the aromatics content to less than about 1.5
wt%. This
allows the hydrocracking step to be performed on a feed with a substantially
reduced
aromatics content. As shown in Table 1, the final effluent from both
configurations has
an aromatics content of less than about 1.5 wt%.
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 25 -
[0064] It is noted that the aromatic saturation temperatures are different
between the
two types of processing runs. For the situation where the feed was first
exposed to the
hydrocracking catalyst, the temperature for aromatic saturation was
constrained based on
the ability to cool the hydrocracking effluent prior to passing the effluent
into the
aromatic saturation reactor. When exposing the feed to the aromatic saturation
catalyst
first, the aromatic saturation temperature was selected so that the resulting
effluent had
approximately the desired temperature for hydrocracking. It is noted that a
wide variety
of aromatic saturation temperatures (both higher and lower than the conditions
in Table
I) would have provided sufficient aromatic saturation.
[0065] Table I also shows the results of characterizing the products from
processing
the feed using the two different types of catalyst systems. As shown in Table
I, the total
liquid yield (C5+ yield) is increased by more than about 0.5 wt% by exposing
the feed
first to the aromatic saturation catalyst. The distillate fuel yield is also
increased by at
least about I wt%. Yield increases on the order of 1 wt% can represent
substantial
efficiency improvements in a commercial refinery context. It is noted that
these yield
improvements are achieved while maintaining a substantially constant amount of
hydrogen consumption.
[0066] The product quality of the resulting products is also improved in
Table 1.
Table I shows the freezing point for a kerosene product with a boiling range
of 190 F
(88 C) to 430 F (221 C). The kerosene product produced by treating the feed
with the
aromatic saturation catalyst first had a freezing point that was at least
about 8 F lower
than the corresponding kerosene product produced by first exposing the feed to
the
hydrocracking catalyst. This improvement in the freezing point in the kerosene
fraction
is believed to be due to the presence of additional branched or isomerized
compounds in
the kerosene fraction. Because the kerosene fraction has overlap with a
traditional
naphtha boiling range fraction, the improvement in freezing point for the
kerosene
boiling range fraction indicates that the corresponding naphtha boiling range
fraction
should have an improved octane rating.
[0067] It is also noted that exposing the feed to the aromatic saturation
catalyst first
resulted in a C4 portion of the light ends that had an unexpectedly high ratio
of
isomerized C4 compounds relative to unbranched C4 compounds. As shown in Table
1,
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 26 -
exposing the feed to the h.ydrocracking catalyst first resulted in a ratio of
isomerized C4
products to unbranched C4 products (iso-C4 / n-C4) of about 4. This is the
ratio of iso-
C4 to n-C4 that would be expected in the products from a fuels hydrocracking
process.
By contrast, exposing the feed to the aromatic saturation catalyst first
produced a C4
product with an iso-C4 to n-C4 ratio of about 6. This unexpected increase in
the iso-C4
to n-C4 ratio makes the C4 product more valuable for use as a feed to an
alkylation unit,
where iso-C4 compounds can be used to generate higher octane naphtha boiling
range
compounds.
Table I¨ Processing of Second Stage :Hydrocracker Feed
Hydrocracking => Aromatic Saturation
Aromatic Saturation Hydrocracking
Hydrocracking Temperature (I) 555 535
Aromatic Sat Temperature ("F) 557 515
1.11SV (h.(1) 0.94 0.94
Pressure (psig) 1157 1157
Treat. Gas Rate (scf/b) 3717 3725
430 F+ Conversion (wt%) 15.6% 15.6%
H2 Consumption (scf7b) 1317 1319
C1-C4 yield (wt%) 2.15 1.59
C 5 yield (wt%) 1100.07 100.63
Distillate (350"F+) yield (wt%) 78.68 80.76
iC4 / n-C4 3.94 6.24
Jet (290-430 F) Freezing Point ( F:) -68 Less than -76
Aromatics Art% after first catalyst 12.2 < 1.5
Aromatics wt% after both catalysts < 1.5 < 1.5
A.ddi tion al Embodiments
[0068] Embodiment 1. A method fbr processing a feedstock -to form a
distillate
product, comprising: exposing a feedstock haying a T50 boiling point of at
least about
430 F (221 C), a first aromatics content, and a sulfur content of about 500
wppm or less
(Or about 100 wpm or less, or about 50 wppm or less, or about 15 wppm or less)
to an
aromatic saturation catalyst comprising a Group VIII noble metal under
effective
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 27 -
aromatic saturation conditions to produce an aromatic saturation effluent, the
aromatic
saturation effluent having a second aromatics content, a ratio of the second
aromatics
content to the first aromatics content bein.g about 0.6 or less; and. exposing
at least a
portion of the aromatic saturation effluent to a hydrocracking catalyst under
effective
hydrocracking conditions to produce a hydrocracked effluent, the effective
hydrocracking conditions being effective for converting at least about 5 wt%
of the at
least a portion of the aromatic saturation effluent relative to a conversion
temperature of
430 F (221 C), the hydrocracking catalyst comprising at least one Group V111
non-noble
metal, at least one Group VIB metal, or a combination thereof; wherein the
hydrocracked
effluent comprises at least a naphtha fuel product fraction and a distillate
fuel product
fraction.
[0069] Embodiment 2. The method of Embodiment 1, wherein the ratio of the
second aromatics content to the first aromatics content is about 0.5 or less,
or about 0.4
or less, or about 0.3 or less, or about 0.2 or less, or about 0.15 or less, or
about 0.1 or
less.
[0070] Embodiment 3. A method for processing a feedstock to form a
distillate
product, comprising: exposing a feedstock having a T50 boiling point of at
least about
430 F (221 C), a first aromatics content of at least about 5 wt%, and a sulfur
content of
about 500 wppm or less (or about 100 wppm or less, or about 50 wppm or less,
or about
15 wppm or less) to an aromatic saturation catalyst comprising a Group VIII
noble metal
under effective aromatic saturation conditions to produce an aromatic
saturation effluent,
the aromatic saturation effluent having a second aromatics content of less
than about 10
wt%, the second aromatics content being less than the first aromatics content;
and
exposing at least a portion of the aromatic saturation effluent to a
hydrocracking catalyst
under effective hydrocracking conditions to produce a hydrocracked effluent,
the
effective hydrocracking conditions being effective for converting at least
about 5 wt% of
the at least a portion of the aromatic saturation effluent relative to a
conversion
temperature of 430 F (221 C), the hydrocracking catalyst comprising at least
one Group
VIII non-noble metal, at least one Group VIB metal, or a combination thereof,
wherein
the hydrocracked effluent comprises at least a naphtha fuel product fraction
and a
distillate fuel product fraction.
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 28 -
[0071] Embodiment 4. The method of any of the above embodiments, wherein
the
first aromatics content is at least about 10 wt%, or at least about 15 wt%, or
at least about
20 wt%, or at least about 25 wt%, or wherein the second aromatics content is
less than
about 7.5 wt%, or less than about 5 wt%, or less than about 3 wt%, or a
combination
thereof.
[0072] Embodiment 5. The method of any of the above embodiments, wherein
the
feedstock has a I'S boiling point of at least about 430 F (221 C).
[0073] Embodiment 6. The method of any of the above embodiments, wherein
the
feedstock has a T95 boiling point of about 1050 F (566 C) or less, or about
800 F or
less.
[0074] Embodiment 7. The method of any of the above embodiments, further
comprising fractionating at least a portion of the hydrocrac.ked effluent to
form at least a
naphtha product fraction and a bottoms fraction,
[0075] Embodiment 8. The method of Embodiment 7, wherein the feedstock
comprises at least a portion of the bottoms fraction.
[0076] Embodiment 9. The method of any of Embodiments 7 or 8, wherein the
bottoms fraction comprises the distillate fuel product fraction.
[0077] Embodiment 10. The method of any of Embodiments 7 to 9, wherein
fractionating at least a portion of the hydrocrac.ked effluent further
comprises separating
a C4 product stream from the hydrocracked effluent, the separated C4 product
stream
having an iso-C4 to n-C4 ratio of at least about 5.
[0078] Embodiment 11. The method of any of the above embodiments, further
comprising exposing a feed having a sulfur content of greater than about 500
wppm to a
hydroprocessing catalyst under effective hydroproeessing conditions to form a
hydroprocessed effluent; and separating the hydroprocessed effluent to form at
least a
hydroprocessed fraction having a T95 boiling point of about 1050 F (566 C) or
less, or a
T95 boiling point of about 800 F (427 C) or less, wherein the feedstock having
a T50
boiling point of at least about .430 F (221 C) comprises at least a portion of
the
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 29 -
hydroprocessed fraction having a T95 boiling point of about 1050 F (566 C) or
less, or a
T95 boiling point of about 800 F (427 C) or less.
[00791 Embodiment 12. The method of Embodiment 11, wherein separating the
hydroprocessed effluent comprises fractionating the hydroprocessed effluent,
the
fractionating optionally further comprising thrming a second naphtha fuel
product
fraction.
[0080] Embodiment 13. The method of any of Embodiments 11 or 12, wherein
the
hydroprocessed effluent and the hydrocracked effluent are fractionated in a
common
fractionation process.
[0081] Embodiment 14. The method of any of Embodiments 11 to 13, wherein
exposing the feed having a sulthr content of at least about 500 wpm to a
hydroprocessing catalyst under effective hydroprocessing conditions comprises:
exposing the feed having a sulfur content of greater than about 500 wppm to a
hydrotreating catalyst under effective hydrotreating, conditions, exposing the
feed having
a sulfur content of greater than about 500 wppm to a hydrocracking catalyst
under
effective hydrocracking conditions, or a combination thereof.
[0082] Embodiment 15. The method of any of Embodiments 11 to 14, wherein
the
feed having a sulfur content of greater than about 500 wppm has a T5 boiling
point of at
least about 430 F (221 C) and a T95 boiling point of about 1050 F (566 C) or
less, or a
T95 boiling point of about 800 F (427 C) or less.
[00831 Embodiment 16. The method of any of Embodiments 11 to 15, wherein at
least about 50 wt% of the feedstock having a T50 boiling point of at least
about 430 F
(221 C) comprises the at least a portion of the hydroprocessed fraction
having a T95
boiling point of about 1050 F (566 C) or less, or a T95 boiling point of about
800 F or
less.
[0084] Embodiment 17. The method of any of the above embodiments, wherein
aromatic saturation catalyst comprises about 0.1 wt% to about 1.8 wt% of Pt,
Pd, or a
combination thereof
CA 02960399 2017-03-06
WO 2016/069224 PCT/US2015/054427
- 30 -
[0085] Embodiment 18. The method of any of the above claims, further
comprising
dewaxing the hydrocracked effluent prior to fractionating the at least a
portion of the
hydrocracked effluent.
[0086] Embodiment 19. The method of Embodiment 3, wherein a ratio of the
second
aromatics content to the first aromatics content is about 0.6 or less, or
about 0.5 or less,
or about 0.4 or less, or about 0.3 or less, or about 0.2 or less, or about
0.15 or less, or
about 0.1 or less.
10087] When numerical lower limits and numerical upper limits are listed
herein,
ranges from any lower limit to any upper limit are contemplated. While the
illustrative
embodiments of the invention have been described with particularity, it will
be
understood that various other modifications will be apparent to and can be
readily made
by those skilled in the art without departing from. the spirit and scope of
the invention.
Accordingly, it is not intended that the scope of the claims appended hereto
be limited to
the examples and descriptions set forth herein but rather that the claims be
construed as
encompassing all the features of patentable novelty which reside in the
present invention,
including all features which would be treated as equivalents thereof by those
skilled in
the art to which the invention pertains.
10088] The present invention has been described above with reference to
numerous
embodiments and specific examples. Many variations will suggest themselves to
those
skilled in this art in light of the above detailed description. All such
obvious variations
are within the full intended scope of the appended claims.