Note: Descriptions are shown in the official language in which they were submitted.
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
USE OF REGENERATIVE CELLS IN MITIGATING BURN PROGRESSION
AND IMPROVING SKIN GRAFT INCORPORATION AND HEALING
STATEMENT REGARDING FEDERALLY SPONSORED R&D
The present invention was made with government support under the following
contract: 1111S0100201200008C awarded by the Department of Health and Human
Services.
The United States government has certain rights in the invention.
BACKGROUND
Skin, or "cutis" is a bilayer organ that includes an outer, epidermal layer,
and the inner,
dermal layer. The epidermal layer itself comprises an outer layer of dead
cells and keratin, and
a basal layer of multiplying keratinocytes. The epidermal layer provides a
physical barrier to
toxins (e.g., bacterial, and environmental), prevents loss of moisture, and
maintains body
temperature. The inner, dermal layer is located between the epidermal layer
and subcutaneous
tissues. The dermal layer is divided into the papillary dermis, which is
composed of collagen
fibers, and the reticular dermis, which is composed of collagen fibers as well
as cells including
fibroblasts, macrophages, mast cells and adipocytes. The dermal layer also
contains the
microcirculation, a complex vascular plexus of arterioles, venules, and
capillaries. The dermis
functions to provide support for the epidermal layer, cushion the body from
stress and strain,
provide nutrients to and remove waste from, the epidermis and dermal layers.
Cutaneous burns are one of the most destructive insults to the skin, causing
damage,
scarring and even death of cutaneous (and, in some cases, subcutaneous)
tissue. Burns
account for over 2 million medical procedures every year in the United States.
Of these,
150,000 subjects are hospitalized and as many as 10,000 subjects die
(Bronzino, 1995, The
Biomedical Engineering Handbook (CRC Press: Florida)).
Burns are classified depending on the lesion severity into four categories:
(1)
superficial or first degree (2) partial thickness or second degree burns (3)
full-thickness or
third degree burns, wherein the lesion involves the subcutaneous layer, and
which associated
with no sensitivity and white coloring; and (4) subdermal or fourth-degree
burns. Partial
thickness burns are further subdivided into (a) superficial partial thickness
burns (b) mid
-1-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
partial thickness burns, and (c) deep partial thickness burns.
Superficial/first-degree burns
affect only the epidermis, and resolve without intervention in 3-5 days
without scarring.
Superficial partial-thickness burns extend through the epidermis into the
papillary dermis.
Superficial partial-thickness burns initially appear red and blister and are
characterized by
hypersensitivity and pain. Typically, superficial partial thickness burns are
not associated with
scarring. Deep partial-thickness burns extend into the reticular layer of the
dermis. Deep
partial thickness burns appear yellow or white, and may exhibit blistering. In
contrast to
superficial partial thickness burns, deep partial-thickness burns are
associated with scarring
and contracture, and often require excision and grafting. Full thickness burns
extend through
the entire dermal layer. Full thickness burns are characterized by scarring
and contractures
Burn excision (and in some rare cases amputation), is standard in full-
thickness burns.
Subdermal or fourth degree burns extend through epidermal and dermal layers
and into
underlying fat, muscle and bone.
Primary tissue loss in burn injury arises from protein denaturation following
thermal,
chemical, electrical, friction, or radiation-induced burns. Post-burn, in
partial and full
thickness burns, necrosis occurs at the focal point of the burn source, and
becomes
progressively less severe at the periphery. The burn area is categorized into
three zones: the
zone of coagulation, the zone of stasis and the zone of hyperemia. The zone of
coagulation/necrosis refers to the nonviable burn eschar nearest to the burn
source. The zone
of stasis surrounds the zone of coagulation, and is characterized by decreased
tissue perfusion,
a mixture of viable and non-viable cells, capillary vasoconstriction and
ischemia. The zone of
hyperemia, which surrounds the zone of stasis, comprises non-injured tissue
that is
characterized by increased blood flow as a compensatory reaction to the burn.
Tissue in the
zone of hyperemia invariably recovers. Tissue in zone of stasis is potentially
salvageable,
given proper intervention. If not properly treated, however, the tissue in the
zone of stasis
dies (e.g., as a result of necrosis and/or apoptosis), as release of
inflammatory mediators,
tissue edema, and/or infection further compromises blood flow to already
critically
injured/ischemic tissues.
The three zones of a burn are three dimensional, and loss of tissue in the
zone of stasis
will lead to the wound deepening as well as widening. This phenomenon is
referred to as burn
-2-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
progression" or "burn conversion." Hence, a burn that initially is assessed as
partial thickness
may progress to full-thickness with time. Both apoptosis (an active process
requiring protein
synthesis, i.e., energy dependent process) and necrosis (energy independent.
"passive" process
leading to cell death) are observed in the conversion of tissue in the zone of
ischemia to non-
viable tissue. See, Singer, et al (2008) Academic Emergency Medicine 15:549-
554.
Tangential excision of burn wounds, escharectomy, or debridement, is regarded
as the
standard of care for burns that are not anticipated to heal within 3 weeks.
Such burns include
deep partial thickness burns and full thickness burns. Choi, et al. (2008) J
Craniofac. Surg.
19:1056-60. Tissue that is already non-viable, or that is expected to become
non-viable is
excised in order to reduce the likelihood of infection, as non-viable, non-
perfiised tissue is a
nidus for bacteria and fungi. Wound debridement is also widely used outside of
the burn
context, e.g., in cases dead, damaged, or infected tissue is present, in order
to improve the
healing potential of the remaining healthy tissue. As loss of the epidermal
layer that normally
functions to shield the individual from exposure to bacteria, fungi, and
environmental toxins,
the risk of infection in burn subjects is extremely high. Non-viable cells and
cell debris are
also a source of toxic products, thereby inciting an inflammatory response.
Burn debridement
has been demonstrated to reduce mortality, reduce hospital stay, and is
associated with
improved rates of wound healing, and reduction in subsequent scarring.
Using debridement alone, the risk of infection is still extremely high. As
such, skin
grafts are often used to promote healing of, and to prevent contracture and
scarring of, the
debrided area. Ideally, skin grafts are taken from the patient's own skin
(donor sites).
However, in cases where a large sized graft is needed, or where the patient is
not stable,
autografts may not be feasible. Furthermore, obtaining donor skin is painful,
and involves
risks such as infection, and destabilization of a subject whose overall health
is already
compromised due to the initial burn injury. In such cases, allografts (i.e.,
taken from other
subjects of the same species), xenografts (i.e., taken from different
species), and synthetic
grafts are used as alternatives. Other potential complications with skin
grafts include: graft
failure; rejection of the skin graft; infections at donor or recipient sites;
or autograft donor
sites oozing fluid and blood as they heal. Certain of these complications
(e.g., graft failure and
-3-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
rejection of the skin graft) may be somewhat mitigated by using an autograft
instead of an
allograft or a xenograft.
Depending upon the depth and severity of the wound/burn, the either a full
thickness
skin graft or a split thickness skin graft is recommended. Split-thickness
skin grafts, or
"STSGs" contain the epidermis and only a portion of the underlying dermis.
Full thickness
skin grafts contain the epidermis and the entire thickness of the dermis.
Split-thickness flaps
are hampered by the low degree of surgical "take." Typically, only about 20%
to 40% of the
transplanted skin successfully reestablishes itself in its new position. Full-
thickness flaps are
even more difficult to reestablish in a new site. See, U.S. Patent No.
4810693. Graft failure
can arise as a consequence of one or several reasons, including inadequate
excision of the
wound bed, that results in non-viable tissue beneath the skin graft;
inadequate vascular supply
to the wound bed; hematomas and seromas forming a barrier between the bed and
skin graft;
shearing or displacement of the graft that prevents revascularization of the
graft; and infection,
which can give rise to disintegration of the graft or excessive exudate that
prevents the graft
from adhering to the bed. Wounds that develop secondary to radiation are less
likely to
support split-thickness skin grafts (STSGs) and often require adjunctive
measures to optimize
survival. Likewise, subjects with diabetes and other conditions that
compromise the vascular
system (e.g., peripheral vascular disease and the like) are also more likely
to have lower skin
graft "take" compared to subjects not affected by conditions that compromise
the vascular
system. In addition to the inherent risks associated with skin grafting, skin
grafts are
expensive and often are limited in supply. Accordingly, it is highly desirable
to minimize (or
even eliminate) tissue excision, and to minimize the amount of graft tissue
used in the
procedure. Burn wound progression creates a "moving target" situation in which
the total
body surface area ("TBSA") of necrotic tissue requiring excision and grafting
can
progressively increase in the first several days after thermal trauma. On top
of this, once the
extent of burn requiring excision and closure is demarcated, due to the
limited supply of
various skin grafts, expansion of the area in which graft is required further
prolongs time to
complete definitive wound closure. The need for therapies that minimize burn
wound
progression/conversion and/or enhance skin graft incorporation and healing is
evident, as
reducing conversion/progression would minimize and/or prevent the need for
tissue excision
-4-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
altogether, thereby enhancing wound closure success rates and accelerating
recovery and
decreasing the morbidity and mortality of burn patients. Furthermore, reducing
or eliminating
burn wound progression will minimize the amount of skin graft material
required. Finally, the
desirability of improving graft "take," in the context of burns or other
wounds which require
grafting, is evident, as improved graft take improves the subject's outcome,
and minimizes
risks and further expenses associated with failed grafts and the need for
secondary repeat graft
harvest and application.
Another major concern in wound healing (e.g., healing of burn and other types
of
wounds) and the healing of skin grafts is the development of pathological
scars, such as
hypertrophic scars. Hypertrophic scarring is a cutaneous condition
characterized by deposits
of excessive amounts of collagen which gives rise to a raised scar.
Hypertrophic scarring
generally develops after thermal or traumatic injury that involves the deep
layers of the dermis.
When present over joints, hypertrophic scarring can cause severe joint
contracture and
eventually lead to erosion of the underlying bone, secondary to disuse. See,
Aarabi, et al.
PLOS Medicine (2007) 4(9):1464-1470. Efforts to limit scar formation, e.g., in
burn patients
have relied largely on immediate skin replacement with human split-thickness
autografts or
allografts or with synthetic dermal analogs such as IntegraTM. Even with skin
grafting,
however, clinicians recognize that hypertrophic scarring remains a terrible
clinical problem.
See, e.g., Sheridan, et al. (2004)1 Am. Col. Surg. 198:243-263.
Clinical experience suggests that hypertrophic scarring is an aberrant form of
the
normal processes of wound healing. Singer, et al. (1999) N Engl J Med. 341:738-
746.
However, the etiology of the overexuberant fibrosis is unknown. The
pathophysiology of
hypertrophic scar formation involves a constitutively active proliferative
phase of wound
healing and disordered production of collagen (for example, excessive
production and
disorganized orientation of collagen). Scar tissue has a unique structural
makeup that is
highly vascular, with inflammatory cells and fibroblasts contributing to an
abundant and
disorganized matrix structure Although the pathogenesis is not well
understood, high
expression of TIMP-1 and inhibition of MMP-1 activity have been implicated in
causing a
decrease in the degradation of collagen during wound repair, and are thought
to contribute to
the formation of hypertrophic scars. The net result is that the original skin
defect is replaced
-5-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
by a dysfunctional mass of tissue. For example, while the scar may maintain,
to a sufficient
extent, the barrier function of normal skin, it does not maintain the
flexibility and softness
required to permit normal motion of the underlying and adjacent structures.
This can lead to
sequelae such as debilitating limited range of motion of a joint and to facial
immobility and
associated inability to achieve facial expressions. The ratio of type III
collagen to type I
collagen has also been reported to be altered/elevated in hypertrophic scars
when compared to
non-pathological scars. Oliviera, et al. (2009) Int. Wound J. 6(6):445-452.
Another hallmark
of hypertrophic scars is elevated levels of a-smooth muscle actin (a-SMA). In
contrast to
non-pathological scars and keloid scars, hypertrophic scars have
characteristic prominent,
vertical vessels present in the scar tissue. Given the adverse consequences
resulting from
hypertrophic scarring ¨ including loss of function, restriction of movement,
disfigurement and
the like, preventative and therapeutic options are desirable.
S UMMARY
Disclosed herein are compositions and methods useful for the treatment of
wounds. In
one aspect, the embodiments disclosed herein relate to the treatment of burns.
Accordingly,
some embodiments relate to compositions and methods for preventing or
mitigating wound
progression. In such embodiments, a subject having a burn, and at risk of
developing burn
progression can be identified. A therapeutically effective amount of a
composition comprising
regenerative cells sufficient to mitigate progression of the burn can be
administered to the
subject. The methods can also include the step of debriding or performing an
escharectomy
on the burn, and/or measuring or calculating burn progression.
In a second aspect, the embodiments disclosed herein relate to compositions
and
methods for enhancing incorporation of a skin graft into a recipient wound
site. Such
embodiments can include the steps of providing a skin graft, administering to
the skin graft a
composition comprising regenerative cells to create a fortified skin graft;
and applying the
fortified skin graft to the recipient wound site. In an alternative
embodiment, the methods can
include the steps of providing a skin graft, administering a composition
comprising
regenerative cells systemically to the subject and/or locally to the wound
site. The skin graft
-6-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
can be applied to the recipient wound site either before or after application
of the regenerative
cells.
A third aspect of the embodiments disclosed herein relate to compositions and
methods for preventing or minimizing the formation of hypertrophic scar in a
deep partial
thickness or full thickness wound. Such embodiments can include the steps of
identifying a
subject having a deep partial thickness or full thickness wound, and
administering to the
subject, e.g., systemically or locally to the deep partial thickness or full
thickness wound, a
composition comprising regenerative cells.
In a fourth aspect, the embodiments disclosed herein provide compositions and
methods of reducing or eliminating a hypertrophic scar. The methods can
include the step of
identifying a subject having a hypertrophic scar; and administering a
composition comprising
regenerative cells to the subject, e.g., systemically and/or locally to the
hypertrophic scar. The
methods can include the further steps of debriding the scar tissue prior to
administration of the
composition comprising regenerative cells.
In a fifth aspect, the embodiments disclosed herein relate to compositions and
methods
of treating contracture in a subject in need thereof A subject with a joint or
muscle
contracture can be identified, and a composition comprising regenerative cells
can be
administered to the subject, thereby treating the contracture. The methods can
include the
steps of assessing range of motion, scarring, and the like.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a chart showing the experimental process flow for the combined
radiation
and thermal injury experiments described in Example 1, below.
Figure 2 is an illustration depicting local injection sites into burn tissue,
as performed
in the experiments in Example 1, below.
Figure 3 is an image showing the areas of (a) contraction (the total area not
covered
by unwounded skin); and (b) epithelialization (the area within the wound
showing evidence of
neo-epithelialization), of an exemplary burn wound analyzed in Example 1. The
thick, solid
line indicates the area of biopsy. The inner dotted line indicates the
boundary of re-
-7-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
epithelialization. The outer dotted line shows the wound boundary for
assessment of
contraction.
Figure 4 is an illustration outlining the scheduling and processing of burn
wounds
(immunohistochemistry [IHC] or snap-freezing for molecular analysis) for wound
biopsy (2 or
4 biopsy collection configuration), as performed in the experiments in Example
1, below.
Figures 5A-5C are graphs showing measurement of hematology parameters over
time in
control animals (Grl a), animals receiving locally administered adipose-
derived regenerative
cells (Grlb), and animals receiving intravenously administered adipose-derived
regenerative
cells (Gr 1 c), as described in Example 1. Figure 5A shows absolute white
blood cell count.
Figure 5B shows absolute neutrophil count. Figure 5C shows absolute platelet
count.
Figure 5D shows absolute lymphocyte count.
Figures 6A-6B show phase contrast photomicrographs (magnification 100X) of
porcine adipose-derived regenerative cells plated under angiogenic conditions
as described in
Example 1. Micrograph of cells from study animal # 5341010 (Figure 6A).
Micrograph of
cells from animal # 5344302 (Figure 6B). The arrows point to tube-like
structures.
Figures 7A and 7B are representative phase contrast micrographs (magnification
100x) showing cells from animal # 5341010 prior to (Figure 7A) and after
(Figure 7B) oil
red 0 staining, in the adipogenesis assay described in Example 1.
Figure 8 is a graph showing percent wound contraction at various time points
for
animals in Group 1 a (control) Group lb (locally delivered adipose-derived
regenerative cells)
and Group 1C (intravenously delivered adipose-derived regenerative cells) as
described in
Example 1
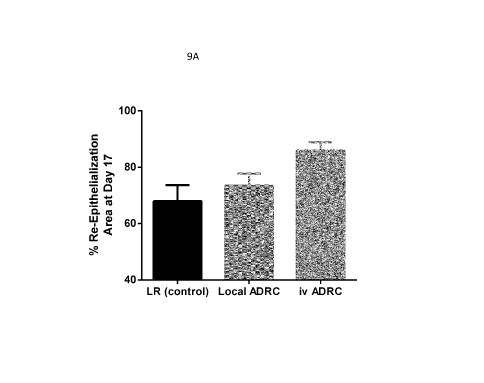
Figures 9A-9D are bar graphs showing the percent re-epithelialization 7 days
post-
injury (Figure 9A); the percent epithelial coverage 7 days post-injury (Figure
9B); the
activated epithelium area ([1m2) (Figure 9C); and the percent proliferating
epithelium (Figure
9D) in animals in Group la (LR control), Group lb (local adipose-derived
regenerative cell
delivery), and Group 1 c (intravenous adipose-derived regenerative cell
delivery), as described
in Example 1
-8-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
Figure 10A is a photograph of masson trichrome staining on biopsy of a sample
of
eschar as described in Example 2. Figures 10B and 10C are details of Figure
10A. The
black arrows indicate hemorrhage in adipose tissue.
Figure 11 Is a photograph (magnification 200x) of Oil Red 0 staining on
adipose-
derived regenerative cells from eschar tissue subjected to the adipogenesis
assay as described
in Example 2.
Figures 12A-12C are photographs of immunostained vessel-like structures formed
in
angiogenic cultures of adipose derived regenerative cells isolated from an
exemplary eschar
sample as described in Example 2.
Figure 13 is a chart showing the experimental process flow for the combined
radiation
and thermal injury experiments described in Example 3, below.
Figure 14 is a graph showing the percentage of open wound area of individual
wounds at day 14 post-burn induction in Group D control (LR) and test (ADRC)-
treated
wounds, as described in Example 3, below.
Figure 15 is a scatter plot showing the percentage of wound epithelialization
for all
wounds in LR and ADRC-treated animals in Group D at study day 14. N=16 wounds
per
control and test cohorts, as described in Example 3, below.
Figures 16A-16D: Figures 16A and D: representative images showing
neovascularization of deep granulation tissue at day 14 and 21, respectively,
in animals in
Group D receiving vehicle alone. Figures 16B and 16D are representative images
showing
neovascularization of deep granulation tissue at day 14 and 21, respectively,
in animals in
Group D receiving ADRCs. Wound biopsies collected from animals receiving
vehicle alone or
local ADRCs were stained with CD31 (endothelial marker). Arrows show CD31-
positive
blood vessels. Scale bar = 300[tm. Bottom panel: quantification of microvessel
density at day
14 and 21 in LR- and ADRCs-treated animals. n=4 animals per group; 6 wounds
total each
treatment condition, as described in Example 3, below.
Figure 17 is a graph showing epithelial thickness in LR- and ADRCs-treated
wounds
of Group D animals, as described in Example 3, below.
Figures 18A-B Histological Assessment of Granulation Tissue Maturation. Figure
18A depicts the scale used for biopsy histology, used in the experiments
described in
-9-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
Example 3, below. Figure 18B is a graph of tissue organization over time in
wounds treated
with INTEGRA or INTEGRA supplemented with ADRC. Figure 18C is a graph
showing
granulation tissue thickness over time in wounds treated with INTEGRA or
INTEGRA
supplemented with ADRC. Mean granulation tissue thickness was greater ADRC +
Integra
treated wounds by Day 21 than Integra controls.
Figures 19A and 19B are graphs showing the microvessel density at days 14 and
21 in
wounds treated with INTEGRA or INTEGRA supplemented with ADRC. Figures 19C
and 19D are graphs showing the total CD312 stain at days 14 and 21 in wounds
treated with
INTEGRA or INTEGRA supplemented with ADRC. Figures 19E and 19F are graphs
showing the total lumen area at days 14 and 21 in wounds treated with INTEGRA
or
INTEGRA supplemented with ADRC.
Figures 20A and 20B are graphs showing the percent of INTEGRA matrix filled
and the number of cells per mm2 in wounds treated with INTEGRA or INTEGRA
supplemented with ADRC. Figure 20C is a graph showing the number of
vessels/mm2 in
wounds treated with INTEGRA or INTEGRA supplemented with ADRC.
Figure 21 is a graph showing epithelial coverage on biopsies collected at day
21 in
Group C, as described in Example 3 below (n=4 animals per group; 6
wounds/group).
Figure 22 is a graph showing quantification of Microvessel Density (MVD) at
day 7,
14 and 21 in animals receiving TISSEEL +vehicle or TISSEEL +ADRCs within
superficial
granulation tissue, as described in Example 3, below (n=4 animals per group; 6
wounds/group).
Figure 23 scattergram from sample ft E5 showing the scatter distribution of
cells
regarding CD34 vs CD90 staining as described in Example 2.
DETAILED DESCRIPTION
The embodiments disclosed herein are based, in part, upon the discovery that
compositions that include regenerative cells can function to mitigate, reduce
and prevent burn
progression/conversion, and/or secondary injury and scarring arising from
burn. The
embodiments also are based, in part, upon the finding that regenerative cells
could be readily
obtained from adipose tissue from subjects suffering from thermal burn injury,
including the
-10-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
adipose from eschar tissue. The embodiments are further based, in part, upon
the finding that
regenerative cells could be readily obtained from adipose tissue from subjects
suffering from
radiation injury. Finally, the embodiments disclosed herein are also based, in
part, upon the
discovery that compositions that include regenerative cells are useful in
preventing and/or
treating pathological scarring, e.g., hypertrophic scarring following a deep-
partial thickness or
full thickness wound (such as a burn or the like).
Definitions
As used herein, the term "about," when referring to a stated numeric value,
indicates a
value within plus or minus 10% of the stated numeric value.
As used herein, the term "derived" means isolated from or otherwise purified
or
separated from. For example, adipose-derived stem and other regenerative cells
are isolated
from adipose tissue. Similarly, the term "derived" does not encompass cells
that are
extensively cultured (e.g., placed in culture conditions in which the majority
of dividing cells
undergo 3, 4, 5 or less, cell doublings), from cells isolated directly from a
tissue, e.g., adipose
tissue, or cells cultured or expanded from primary isolates. Accordingly,
"adipose derived
cells," including adipose-derived stem and other regenerative cells and
combinations thereof,
refers to cells obtained from adipose tissue, wherein the cells are not
extensively cultured, e.g.,
are in their "native" form as separated from the adipose tissue matrix.
As used herein, a cell is "positive" for a particular marker when that marker
is
detectable. For example, an adipose derived regenerative cell is positive for,
e.g., CD73
because CD73 is detectable on an adipose derived stem or regenerative cell in
an amount
detectably greater than background (in comparison to, e.g., an isotype control
or an
experimental negative control for any given assay). A cell is also positive
for a marker when
that marker can be used to distinguish the cell from at least one other cell
type, or can be used
to select or isolate the cell when present or expressed by the cell.
As used herein, "regenerative cells" refers to any heterogeneous or
homogeneous
population of cells obtained using the systems and methods of embodiments
disclosed herein
which cause or contribute to complete or partial regeneration, restoration, or
substitution of
structure or function of an organ, tissue, or physiologic unit or system to
thereby provide a
therapeutic, structural or cosmetic benefit. Examples of regenerative cells
include: adult stem
-11-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
cells, endothelial cells, endothelial precursor cells, endothelial progenitor
cells, macrophages,
fibroblasts, pericytes, smooth muscle cells, preadipocytes, differentiated or
de-differentiated
adipocytes, keratinocytes, unipotent and multipotent progenitor and precursor
cells (and their
progeny), and lymphocytes.
Accordingly, adipose-derived regenerative cells ("ADRCs") as used herein
refers to
any heterogeneous or homogeneous cell population that contains one or more
types of
adipose-derived regenerative cells including adipose-derived stem cells,
endothelial cells
(including blood and lymphatic endothelial cells), endothelial precursor
cells, endothelial
progenitor cells, macrophages, fibroblasts, pericytes, smooth muscle cells,
preadipocytes,
kertainocytes, unipotent and multipotent progenitor and precursor cells (and
their progeny),
and lymphocytes. Adipose-derived stem cells comprise at least 0.1% of the
cellular
component of adipose-derived regenerative cells.
Similarly, "bone marrow-derived regenerative cells" (13MRCs") refers to any
heterogeneous or homogeneous cell population that contains one or more types
of bone
marrow-derived regenerative cells including bone marrow-derived stem cells,
endothelial cells
(including blood and lymphatic endothelial cells), endothelial precursor
cells, endothelial
progenitor cells, macrophages, fibroblasts, pericytes, smooth muscle cells,
preadipocytes,
keratinocytes, unipotent and multipotent progenitor and precursor cells (and
their progeny),
and lymphocytes.
In some contexts, the term "progenitor cell" refers to a cell that is
unipotent, bipotent,
or multipotent with the ability to differentiate into one or more cell types,
which perform one
or more specific functions and which have limited or no ability to self-renew.
Some of the
progenitor cells disclosed herein may be pluripotent.
As used herein the phrase "adherent cells" refers to a homogeneous or
heterogeneous
population of cells which are anchorage dependent, i.e., require attachment to
a surface in
order to grow in vitro.
In some contexts, the term "adipose tissue-derived cells" refers to cells
extracted from
adipose tissue that has been processed to separate the active cellular
component (e.g., the
cellular component that does not include adipocytes and/or red blood cells)
from the mature
adipocytes and connective tissue. Separation may be partial or full. That is,
the "adipose
-12-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
tissue-derived cells" may or may not contain some adipocytes and connective
tissue and may
or may not contain some cells that are present in aggregates or partially
disaggregated form
(for example, a fragment of blood or lymphatic vessel comprising two or more
cells that are
connected by extracellular matrix). This fraction is referred to herein as
"adipose tissue-
derived cells," "adipose derived cells," "adipose derived regenerative cells"
or "ADC."
Typically, ADC refers to the pellet of cells obtained by washing and
separating the cells from
the adipose tissue. The pellet is typically obtained by concentrating a
suspension of cells
released from the connective tissue and adipose tissue matrix. By way of
example, the pellet
can be obtained by centrifuging a suspension of adipose-derived cells so that
the cells
aggregate at the bottom of a centrifuge container, e.g., the stromal vascular
fraction. In some
embodiments, the adipose-derived cell populations described herein include,
among other cell
types, leukocytes. In some embodiments, the adipose-derived cell populations
described
herein include, among other regenerative cell types, endothelial cells.
In some contexts, the term "adipose tissue" refers to a tissue containing
multiple cell
types including adipocytes and vascular cells. Adipose tissue includes
multiple regenerative
cell types, including adult stem cells (ASCs), endothelial progenitor and
precursor cells,
pericytes and the like. Accordingly, adipose tissue refers to fat, including
the connective
tissue that stores the fat.
In some contexts, the term "unit of adipose tissue" refers to a discrete or
measurable
amount of adipose tissue. A unit of adipose tissue may be measured by
determining the
weight and/or volume of the unit. In reference to the disclosure herein, a
unit of adipose
tissue may refer to the entire amount of adipose tissue removed from a
subject, or an amount
that is less than the entire amount of adipose tissue removed from a subject.
Thus, a unit of
adipose tissue may be combined with another unit of adipose tissue to form a
unit of adipose
tissue that has a weight or volume that is the sum of the individual units.
In some contexts, the term "portion" refers to an amount of a material that is
less than
a whole. A minor portion refers to an amount that is less than 50%, and a
major portion
refers to an amount greater than 50%. Thus, a unit of adipose tissue that is
less than the
entire amount of adipose tissue removed from a subject is a portion of the
removed adipose
tissue.
-13-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
As used herein, the term "ROS" and "RNS" refer to reactive oxygen species and
reactive nitrogen species, respectively. ROS and RNS include compounds such as
hydrogen
peroxide, peroxynitrate, hydroxyl radical (.0H), nitrogen dioxide radical
(NO2) and carbonate
radical (CO3). As used herein, the term "lipid peroxidation," or "lipid
peroxidation products"
or "LPPs" can include, but are not limited to malondialdehyde (MDA) and 4-
hydroxynonenal
(HNE), acrolein, and the like.
As used herein, the term "skin substitute" or "skin graft" refers to anything
that
substitutes for any of the skin functions provided by the native skin at that
site prior to injury
or development of a wound. Skin substitutes or skin grafts can be allografts
(e.g., cadaveric
grafts, or the like), or xenografts. Skin grafts can also be autografts (ie:
grafts obtained from
the patient receiving the graft). In certain embodiments, the graft can be in
a dispersed form
(e.g., a skin graft that has been meshed or treated enzymatically to create a
completely or
partially dispersed suspension of skin cells including keratinocytes that is
then applied to the
area in need of coverage). In certain embodiments, the graft can comprise
cultured cells (e.g.,
cultured keratinocytes and/or dermal cells with or without a supportive
scaffold). Preferably, a
skin substitute should in some way be incorporated into the healing wound.
Cultured or
artificial dressings, therefore, may be used as a substitute for the epidermal
layer, the dermal
layer, or both layers simultaneously. Some grafts are used to provide skin
function for a
limited period (temporary coverage). For example, allografts and xenografts
are usually
removed prior to definitive wound treatment or skin grafting.
The compositions and embodiments disclosed herein are useful for treating
subjects
with burn injury, and/or in subjects in need of a skin graft (e.g., skin
graft, skin substitute, or
the like). Accordingly, the term "subject" can refer to any mammal including,
but not limited
to mice, rats, rabbits, guinea pigs, pigs, dogs, cats, sheep, goats, cows,
horses, primates, such
as monkeys, chimpanzees, and apes, and humans. In some embodiments, the
subject is a
human. The term "subject" can be used interchangeably with the terms
"individual" and
"patient" herein. As explained in further detail below, in some embodiments,
the subject has
radiation injury (e.g., acute radiation injury), and a deep partial thickness
or full thickness
wound, such as a burn.
Burn Progression
-14-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
Burn wounds continue to mature for several days following initial insult,
confounding
burn classification and treatment protocols. Damage to the skin continues
several days post-
insult, as tissue in the zone of stasis undergoes necrosis and/or apoptosis.
Both apoptosis and
necrosis occurs in the ischemic zone of burns. Apoptotic dermal cells are
found at a much
higher frequency in deep partial-thickness burns compared to superficial
partial thickness
burns, and persist over 20 days. See, e.g., Gravante, et al. (2006) Surgery
139:854-855.
Burn progression involves a complex concert of events, which include oxidative
stress,
persistent inflammation, and compromised perfusion. See, e.g., Shupp, et al.
(2010) J. Burn
Care & Res. 31:849-873. As discussed in further detail below, the methods and
compositions
disclosed herein function to ameliorate one or more of these pathways, thereby
minimizing
and/or preventing burn progression. As such, the methods and compositions
disclosed herein
can advantageously reduce or minimize the area of the recipient site of a skin
graft, in some
instances, eliminate the need for a skin graft altogether following burn.
Oxidative stress transpires as a result of an imbalance between the systemic
generation
of reactive oxygen species and a biological system's ability to readily
detoxify the reactive
intermediates and/or to repair the resulting damage. Various different
pathways converge to
create oxidative stress and an over-abundance of free radicals in burn. First,
thermal burns
can directly generate free radicals by hemolytic bond fission caused by heat.
Burn also causes
an increased activity of xanthine oxidase and NADPH oxidase, as well as
increased nitric
oxide ("NO") production, e.g, in proliferating keratinocytes, capillary
endothelial cells and
arterial smooth muscle cells. See, e.g., Shupp, et al. (2010) J. Burn Care &
Res. 31:849-873.
Xanthine oxidase and NADPH oxidase generate the damaging ROS hydrogen peroxide
and
superoxide. NO in turn interacts with superoxide radicals to produce the
highly reactive
peroxynitrite compound, a reactive nitrogen species. The increase in reactive
oxygen species
("ROS") and reactive nitrogen species ("RNS") is compounded by reductions in
oxidative
defenses, including reductions in superoxide dismutase ("SOD"), glutathione,
ascorbic acid,
and a-tocopherol associated with burn.
Excessive ROS and RNS cause multiple deleterious effects, including cellular
damage,
e.g., to DNA, proteins, lipids (generating lipid peroxidation products, or
"LPPs"), and other
structural cellular components, and can ultimately lead to apoptosis, thereby
causing and/or
-15-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
worsening burn progression. As such, ROS and RNS are key players in burn
progression.
LLPs have also been shown to play a role in macroscopic interspace necrosis,
neutrophil
infiltration, and thrombosis, thereby promoting burn progression. See, e.g.,
Taira, et al.
(2009) i Burn Care Res. 30:499-504.
In concert with the cellular damage, oxidative stress exacerbates and
contributes to
persistent inflammation, which is also implicated in burn progression. ROS
induce the
expression of pro-inflammatory cytokines through the action of NF-kB. For
example, damage
to cell membranes (e.g., arising from apoptosis or necrosis due to the initial
burn insult and/or
consequent ROS and/or RNS damage), results in a dynamic cascade of
inflammatory
mediators. Prolonged or persistent inflammation in turn results in collagen
degradation and
keratinocyte apoptosis, thereby furthering burn progression.
In addition to the pro-inflammatory effects arising from oxidative stress and
damage,
devitalized tissue, e.g., arising from an initial burn insult, is also pro-
inflammatory.
Devitalized tissue has exposed C3b binding sites as well as self-antigens, and
serves as a
powerful activator of the alternate complement system. In addition, bacteria
that colonize the
necrotic tissues are also powerful activators of the complement system.
Activation of the
complement cascade is known to be involved in burn wound progression. See,
e.g., Henze, et
al. (1997) Burns 23:473-477. Activation of the complement cascade leads to the
diffusion of
chemotactic factors in the surrounding blood stream. Complement split factors,
in turn,
activate neutrophils, leading to regional endothelial cell adhesion and
migration. At the same
time, lymphokines originally stored in the tissues or subsequently produced by
invading cells
are released in the wound itself This stimulates monocyte invasion and
potentiates their
maturation into tissue macrophages, which are the central cells responsible
for wound clearing
of devitalized tissues, bacteria, and large amounts of self-antigens by the
process of
phagocytosis. This process is further enhanced by the opsonizing properties of
the
complement factors. Oxygen free radicals, lysosomes, and inflammatory
cytokines are all
elevated as a result of phagocytosis Complement activation and intravascular
stimulation of
neutrophils result in the production of cytotoxic free radicals.
Cellular release of pro-inflammatory cytokines such as TNFa, IL-1, IL-6, IL-8,
and
IL-10 occurs following burn injury. See, e.g., Dorst, et al. (1993)1 Trauma
35(3): 335-339;
-16-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
Molloy, et al. (1993) i Immunol. 151: 2142-2149. Abnormal levels of
proinflammatory
mediators, such as tumor necrosis factor alpha (TNFa), interleukin- lb (IL-
1b), interleukin-6
(IL-6), interleukin-8 (IL-8), and interleukin-10 (IL-10), have been reported
both systemically
and locally in burn patients. Necrotic expansion in burn progression/
conversion is driven by a
microenvironment characterized by elevated levels of pro-inflammatory
mediators and
reduction of pro-inflammatory cytokines. Blocking of pro-inflammatory
molecules has been
demonstrated to advantageously reduce or mitigate burn progression. Sun, et
al. (2012)
Wound Repair Regen. 20(4):563-72. Leukocyte infiltration is also involved in
burn
progression. Blocking neutrophil adhesion to the endothelium, e.g., via
systemic
administration of blocking antibodies, has been demonstrated to reduce wound
conversion in
an animal model. Choi, et al. (1995) Plastic Reconst. Surg. 96(5): 1007-1250.
The
embodiments disclosed herein are based, in part, on the discovery that the
regenerative cells
disclosed herein can advantageously function to alter the microenvironment of
partial and full
thickness burns, thereby preventing and/or minimizing necrotic and/or
apoptotic expansion.
The compositions disclosed herein can advantageously function to stop or
inhibit the
expansion of the zone of coagulation or necrotic tissue of a burn, or to
minimize the
expansion of the zone of coagulation or necrotic tissue of a burn.
Accordingly, in some
embodiments, provided herein are methods for minimizing and/or preventing
wound
progression in a subject in need thereof The methods can include administering
a
composition comprising regenerative cells to a subject at risk of burn
progression, e.g., a
subject having a deep partial thickness wound or a full thickness wound, such
as a burn, or the
like. Accordingly, in some embodiments, the methods disclosed herein eliminate
the need for
skin grafting. Without being limited by a particular theory, the regenerative
cells disclosed
herein (e.g., mesenchymal stromal cells) can prevent burn progression by one
or several
mechanisms, including, but not limited to minimizing or reducing oxidative
stress and/or
damage following burn injury, modulating the inflammatory response following
burn injury
(e.g., by dampening or reducing proinflammatory cytokines), modulating
leukocyte infiltration
into the zone of stasis, and enhancing, increasing, or restoring bloodflow in
the zone of stasis.
Accordingly, provided herein are methods to reduce or minimize oxidative
stress
and/or damage following burn injury e.g., in the zone of stasis, in a subject
in need thereof,
-17-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
that includes administration of a composition that includes regenerative cells
as described
herein. Other methods relate to the modulation of inflammation following burn
injury (e.g.,
dampening or reducing the local concentration of inflammatory cytokines in the
zone of stasis,
dampening or reducing the infiltration and/or extravasation of inflammatory
leukocytes in the
zone of stasis, modulating polarization of immune cells to an anti-
inflammatory phenotype,
and the like), in a subject in need thereof, that includes administering
regenerative cells as
described herein. Provided herein are methods of increasing or enhancing blood
flow, e.g., in
the zone of stasis, following burn injury, that includes administering
regenerative cells as
described herein to the subject.
Methods of Mitigating Burn Progression / Conversion
In some embodiments provided are methods for reducing burn progression in
subjects
in need thereof In certain embodiments, the subject may be a mammal, e.g.,
preferably a
mouse, rat, rabbit, pig, minipig, dog, cat, horse, monkey ape, human, or the
like. In some
embodiments, the subject may have concomitant radiation injury. Some
embodiments provide
methods for reducing or preventing burn progression in a subject with
radiation injury that has
a deep partial thickness or full thickness burn injury. In some embodiments,
the radiation
injury is acute radiation injury. In some embodiments, the burn injury covers
more than 5%,
more than 10%, more than 15%, more than 20%, more than 25%, more than 30%, or
more,
of the total body surface area of the subject.
In some embodiments, the methods described herein can completely prevent burn
progression. That is, the zone of coagulation of the burn does not expand past
its initial area
following the burn injury. In some embodiments, the zone of coagulation of the
burn does not
expand past its area prior to treatment with a composition as disclosed
herein. In some
embodiments, the zone of coagulation does not expand more than 5%, 10%, 15%,
20%, 25%,
30%, 35%, 40%, 50%, or more, following treatment with a composition as
disclosed herein.
In some embodiments, the zone of stasis remains unchanged following
administration of a
composition as disclosed herein. In some embodiments, the zone of stasis
exhibits less than
5%, less than 10%, less than 15%, less than 20%, less than 25%, less than 30%,
less than
35%, less than 40%, less than 45%, less than 50%, or so, conversion to
devitalized tissue.
Accordingly, in some embodiments, administration of the compositions disclosed
herein can
-18-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
preserve 100%, 95%, 90%, 85%, 80%, 75%, 70%, 65%, 60%, 55%, 50%, or so, of
tissue in
the zone of stasis of the burn.
In some embodiments, mitigating or reducing burn progression, or "treating" a
patient
as disclosed herein, can reduce the amount of tissue necrosis and/or apoptosis
compared to
the amount of tissue necrosis expected in the absence of regenerative cell
administration. For
example, where a patient has received a thermal burn, the administered
regenerative cells can
reduce the progression of burn injury in the zone of ischemia and inhibit the
conversion of
partial thickness injuries into full thickness necrosis. In some embodiments,
the methods
disclosed herein can eliminate burn progression or conversion.
The various zones of partial and full thickness burns (i.e., the zone of
coagulation, the
zone of stasis and the zone of hyperemia) were first described in 1953.
Jackson, et al. (1953)
Br. .1. Surg. 40:588. Accordingly, identification of the zone of coagulation,
the zone of stasis
and the zone of hyperemia of a burn are widely known. Non-limiting examples of
methods
useful for identifying the different zones of burns include, but are not
limited to, those
described in US Patent Application Publication No. 2007/0197895, U.S. Patent
No. 8435750,
and International Patent Application No's WO 2013/110021 and WO
2007/130,423A2, and
the like.
In some embodiments, administration of the compositions as disclosed herein
prevent
or minimize conversion of a superficial partial-thickness burn to a mid
partial thickness burn, a
deep partial thickness burn, a full thickness burn, or a fourth-degree burn.
Superficial second-
degree burns involve the entire epidermis to the basement membrane and no more
than the
upper third of the dermis. Mid-dermal burns involve destruction of the
epidermis through the
middle third of the dermis. Deep second-degree burns involve the entire
epidermis, and at
least two thirds of the dermis. Fourth-degree burns extend through the
epidermal and dermal
layers of the skin, and into underlying tissue (e.g., muscle, tendon,
ligament, bone, or the like).
In some embodiments, administration of the compositions as disclosed herein
prevent or
minimize conversion or progression of (or the amount of tissue converted) a
mid partial-
thickness burn to a deep partial thickness burn, a full thickness burn, or a
fourth-degree burn.
In some embodiments, administration of the compositions as disclosed herein
prevent or
minimize conversion or progression of a deep partial thickness burn to a full
thickness burn or
-19-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
a fourth-degree burn. In some embodiments, the compositions disclosed herein
prevent or
minimize the conversion or progression of a full thickness wound to a fourth
degree burn.
In some embodiments, a subject at risk of burn conversion or burn progression
is
identified, e.g., self-identified, or identified by another person.
Accordingly, in some
embodiments, an individual with a second-degree, or partial thickness burn is
identified. It is
recognized that many patients will exhibit heterogeneous burn depth with
certain areas of the
injury constituting, for example, full thickness injury and other areas
constituting deep partial
and/or partial thickness wounds, and/or fourth degree wounds. In some
embodiments, the
subject has a superficial second-degree burn. In some embodiments, the subject
has a mid
second-degree burn, or mid-dermal burn. Mid-dermal wounds exhibit larger zones
of stasis
than superficial second-degree burns. Subjects with mid-dermal burns are at
high risk of burn
progression/burn conversion. In some embodiments, the subject has a deep
second-degree, or
deep dermal burn. In some embodiments, the subject has a full-thickness or
third-degree burn,
extending through the entire dermal layer. In some embodiments, the subject
has a fourth-
degree, or sub-dermal burn. In some embodiments, the subject has radiation
injury, e.g.,
cutaneous or acute radiation injury. For example, in some embodiments, the
subject at risk of
burn progression has been exposed to 2 gray or more. In some embodiments, the
subject has
radiation injury and has suffered from a thermal, electrical or chemical burn.
The skilled artisan will appreciate that any art-accepted technique to
classify burn
depth is useful in the embodiments disclosed herein. For example, in some
embodiments, burn
depth is assessed visually. In some embodiments, burn depth is classified by
one or more
biopsies followed by histological examination. See, e.g. Chvapil et al, 1984,
Plast. Reconstr.
Surg. 73:438-441. Other methods of classifying burn depth useful in the
embodiments
disclosed herein include, but are not limited to, those described in U.S. Pat.
No's 7860554,
5701902; 4170987, Canadian Patent Application 2,287,687, Mason et al. (1981),
Burns
7:197-202, Park et al. (1998) Plast. Reconstr. Surg. 101:1516-1523, Brink et
al. (1986)
Invest. Radiol. 21:645-651, and Afromowitz et al. (1987) IEEE Trans Biomed Eng
BME34:114-127, each of which is herein incorporated by reference.
Once identified, the subject can be administered a composition comprising
regenerative cells according to the disclosure herein. In some embodiments,
wound
-20-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
progression or conversion can be analyzed or measured prior to and/or
following
administration of regenerative cells as disclosed herein. For example, in some
embodiments,
the viability of tissue in the zone of stasis can be measured. The skilled
artisan will readily
appreciate that any art-accepted methods of determining tissue viability ¨
either known or
discovered in the future ¨ are useful in the embodiments disclosed herein. For
example, the
area of devitalized tissue can be assessed visually, histologically (using
biopsies, for example),
or using other methods, including but not limited to those described in
International Patent
Application Publication No. WO 2001/078587, WO 2001/054580, WO 2005/002425A2,
WO
1991/012766A, U.S. Patent No. 8221989, and the like.
In some embodiments, the level of oxidative stress or oxidative damage or
lipid
peroxidation can be measured prior to and/or following administration of
regenerative cells as
described herein. Oxidative damage and lipid peroxidation can be measured
using art-
recognized methods or methods discovered in the future. By way of example, the
methods
described in Bosken, et al., "Assessments of Oxidative Damage and Lipid
Peroxidation After
Traumatic Brain Injury and Spinal Cord Injury" in Animal Models of Acute
Neurological
Injuries II, Chen, et al. Ed., (c) 2012, Humana Press, New York, NY, pp. 347-
375; Pratico, et
al. (2002) J. Neuro. 80(5): 894-898 can be used to measure lipid peroxidation.
In some embodiments, the level of bloodflow in the zone of ischemia can be
measured
prior to and/or following administration of regenerative cells as described
herein. In some
embodiments, modulation of an immune response (e.g., either local or
systemic), can be
measured, e.g., using any method now known or discovered in the future, prior
to and/or
following administration of the regenerative cells as described herein.
Accordingly, in some
embodiments, the level of bloodflow is assessed using Laser Doppler imaging,
or any other
technique known or discovered in the future. In some embodiments, the methods
include
analysis of vascular structures, e.g., in the zone of ischemia. For example,
in some
embodiments, the amount or number of CD31-positive structures can be
determined.
In some embodiments, modulation of the immune response can be measured prior
to
and/or following administration of regenerative cells as described herein. For
example, in
some embodiments, the level of proinflammatory modulators (e.g., TNFa, IFNy,
IL-1, IL-2,
IL-3, IL-6, IL-12, IL-18, and the like) can be determined (e.g., in tissue
samples, in whole
-21-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
blood, in plasma, or the like) using any art-accepted method, or any method
discovered in the
future. In some embodiments, number and/or types of leukocytes in the zone of
stasis can be
measured or analyzed prior to and/or following administration of regenerative
cells as
described herein. The numbers of infiltrating macrophages and T cells within
the burned area
can be readily determined, e.g., by analysis using anti-F4/80 and anti-CD3
antibodies,
respectively. In some embodiments, the ratio of different immune cells can be
measured prior
to and/or following administration of regenerative cells as described herein.
By way of
example only, in some embodiments, the methods include the step of determining
the ratio of
MIMI macrophages prior to and/or following administration of regenerative
cells as
described herein. The ratio of M2:M1 cells can be readily determined using art-
accepted
means, including for example, measuring the ratio of CD206/CD11cell surface
markers (e.g.,
in the blood) as described in Fujisaka (2009) Diabetes 58(11): 2574-2582.
Methods of Improving Skin Grafting and Skin Graft Healing
Also provided herein are methods for improving skin grafting, incorporation of
a graft
into underlying tissue, or "take" of a skin graft. The skilled person will
readily appreciate that
the embodiments disclosed herein are useful in the treatment of a variety of
types of wounds
involving the placement of a graft to aid in the healing of the wound, e.g.,
in instances where
the area of skin loss is too big to be closed using local skin and stitches
alone. For example,
the embodiments disclosed herein are useful in the treatment of burns, e.g.,
including those in
which burned tissue is excised. Other exemplary types of wounds in which the
methods and
compositions disclosed herein are used include, but are not limited to non-
healing wounds,
e..g., including chronic wounds and ulcers (for example pressure wounds,
wounds and ulcers
associated with diabetes, peripheral vascular disease, trauma, and the like),
various traumatic
wounds, e.g., caused by mechanical, chemical, insect or other animal sources,
and the like.
For example, the methods described herein are useful in incorporation of
grafts following
surgical removal of cancerous, devitalized, or infected tissue and following
injury from
exposure to chemical agents including chemical warfare agents (e.g., vesicants
and alkylating
agents) where exposure could occur in the course of industrial accident,
warfare, terrorist
attack, or other means.
-22-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
The ultimate success of a skin graft, or its "take," depends on nutrient
uptake and
vascular ingrowth from the recipient bed, which occurs in 3 phases. The first
phase takes place
during the first 24-48 hours. The graft is initially bound to the recipient
site through formation
of a fibrin layer and undergoes diffusion of nutrients by capillary action
from the recipient bed
by a process called plasmatic imbibition. The second phase involves the
process of
inosculation, in which the donor and recipient end capillaries are aligned and
establish a
vascular network. Revascularization of the graft is accomplished through those
capillaries as
well as by ingrowth of new vessels through neovascularization in the third and
final phase,
which is generally complete within 4-7 days. Reinnervation of skin grafts
begins
approximately 2-4 weeks after grafting and occurs by ingrowth of nerve fibers
from the
recipient bed and surrounding tissue. Sensory return is greater in full-
thickness grafts because
they contain a higher content of neurilemmal sheaths. Similarly, hair
follicles may be
transferred with a graft, which allows the graft to demonstrate the hair
growth of the donor
site.
In some embodiments, the methods disclosed herein relate to improving the
incorporation of a graft into the underlying tissue of a wound, such as a burn
(e.g., following
escharectomy, or the like), a chronic non-healing wound, or the like. Some
embodiments
relate to reducing the time between wound debridement and application of a
skin graft, skin
substitute or other scaffold. Regenerative cells as described herein can be
administered to a
debrided wound bed to create a fortified wound bed, and a skin graft can
subsequently be
applied to the fortified wound bed. By way of example only, a composition
comprising
regenerative cells as described herein can be injected into (e.g., at one or
more sites) the
debrided wound bed. In some embodiments, a composition comprising regenerative
cells can
be sprayed onto the debrided wound bed. In some embodiments, a composition
comprising
regenerative cells can be dripped or painted onto a debrided wound bed. In
some
embodiments, the composition comprising regenerative cells is administered
systemically, or
according to any of the methods of administration discussed herein below. In
some
embodiments, the composition comprising regenerative cells is administered
both locally (e.g.,
topically or by local injection) and systemically (e.g., intravascularly,
intralymphatically, or the
like). In some embodiments the regenerative cells are delivered in a simple
vehicle such as a
-23-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
physiologic saline or buffered solution. In other embodiments they are
delivered in a biologic
vehicle such as a fibrin glue. In still further embodiments, the regenerative
cells are delivered
within the graft. In certain embodiments the regenerative cells are mixed with
or delivered in
temporal association with other cells types such as keratinocytes and/or
dermal cells.
Some embodiments provide methods of reducing the time between wound
debridement and application of an autograft to the wound. For example, in some
embodiments, method can include application of a composition comprising
regenerative cells
as disclosed herein to a temporary graft which is applied to the debrided
wound. A permanent
graft (e.g., an autograft or other type of permanent graft), can be
subsequently applied to the
debrided wound In some embodiments, the method includes the step of removing
all or part
of the graft, prior to application of the autograft. By way of example only,
in some
embodiments, a composition comprising regenerative cells as disclosed herein
can be applied
to a graft such as INTEGRA skin substitute to create a temporary, fortified
graft. After a
period of time (e.g., 12 hours, 1 day, 2 days, 3 days, 4 days, 5 days, 6 days,
7 days, 8 days, 9
days, 10 days, 11 days, 12 days, 13 days, 14 days, 15 days, 16 days, 17 days,
18 days, 19
days, 20 days, 21 days, 22 days, 23 days, 24 days, 25 days, 26 days, 27 days,
28 days, 29
days, 30 days or more) part (or all) of the INTEGRA graft is removed (e.g.,
the silicone
backing). An autograft is subsequently applied to the wound. Fortification of
the
INTEGRA skin substitute with regenerative cells as described herein
accelerates
vascularization of the wound tissue within and beneath the INTEGRA skin
substitute, and
shortens the time required before the wound (e.g., debrided burn) is ready to
receive an
autograft. In some embodiments, the time required before application of an
autograft is
reduced by 5%, 10% 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, or more. In some
embodiments, applying a composition comprising regenerative cells to a skin
substitute (e.g.,
INTEGRA or the like), reduces the time required before application of an
autograft by 12
hours, 1 day, 2 days, 3 days, 4 days, 5 days, 6 days, 7 days, 8 days, 9 days,
10 days, 11 days,
12 days, 13 days, 14 days, or more. In some embodiments, application of the
composition
comprising regenerative cells improves remodeling of the autograft.
Some embodiments relate to methods of improving healing of autografts. For
example, some embodiments disclosed herein relate to a method of improving
epithelialization
-24-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
of dispersed or meshed autografts. The method can include the steps of
applying a
composition comprising regenerative cells as disclosed herein (e.g., a
composition comprising
adipose-derived regenerative cells or the like), to a meshed autograft or a
fully or partially
disaggregated suspension of skin cells. In some embodiments, the composition
is applied to
the meshed autograft or cell suspension to create a fortified graft that is
then placed onto a
recipient wound bed (e.g., a debrided burn wound or the like). In some
embodiments, the
meshed autograft or cell suspension is placed onto a recipient wound bed
(e.g., a debrided
wound or the like) and the composition comprising regenerative cells is
applied to the meshed
autograft or cell suspension that is already placed in the recipient site. In
some embodiments,
application of the composition comprising regenerative cells to the meshed
autograft or cell
suspension improves epithelialization. In some embodiments, application of the
composition
comprising regenerative cells improves vascularization of the meshed autograft
and/or healing
wound bed. Epithelialization and vascularization can be readily assessed using
any art-
accepted methods, including but not limited to, those described in Pomahac, et
al. (2007)
Regional Anesthesia and Pain Medicine 32(5): 377-381, Greenwood, et al.
(2009)1 Plastic
Surg. 9: 309-318, and the like. In some embodiments, application of the
composition
comprising regenerative cells improves remodeling of the meshed autograft. In
some
embodiments, application of the composition comprising regenerative cells
prevents
"ghosting" of the graft. As used herein, the term "ghosting" refers to the
phenomenon
whereby integrated grafts subsequently "dissolve" over time, often as a result
of infection. In
some embodiments, application of the composition comprising regenerative cells
promotes
maturation of vessels incorporating into the graft.
Accordingly, provided are embodiments that include the steps of: (1) applying
to a
graft an effective amount of the compositions including regenerative cells as
disclosed herein
(e.g., to create a "fortified graft"), (2) contacting the underlying tissue of
the wound with the
fortified graft; and (3) securing the graft to the underlying tissue, whereby
incorporation of the
graft into said underlying tissue is promoted. As such, in some embodiments,
the regenerative
cells can be applied to a skin graft or skin substitute to create a "fortified
graft," which is
subsequently administered to a recipient site in a subject in need thereof In
some
embodiments, the methods disclosed herein provide for the step of debriding a
burn, and
-25-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
administering the fortified graft to the subject. The methods can also include
the steps of
measuring, analyzing or assessing the incorporation of the fortified graft
into the recipient site.
In some embodiments, the fortified grafts heal more rapidly than non-fortified
grafts. In some
embodiments, the subject has radiation injury, e.g., cutaneous radiation
injury or acute
radiation injury. In some embodiments, the subject has radiation injury and a
thermal,
chemical, or electrical burn requiring a graft. In some embodiments the
subject has an acute
or chronic wound arising from a cause other than burn and in which treatment
of said wound
includes application of a graft.
Also provided are embodiments that include the steps of (1) applying to a
recipient
wound bed a composition comprising regenerative cells to create a fortified
recipient site; and
(2) contacting the recipient site with a graft, whereby incorporation of the
graft into the
recipient wound site is improved. In some embodiments, the subject has
radiation injury, e.g.,
cutaneous radiation injury or acute radiation injury. In some embodiments, the
subject has
radiation injury and a thermal, chemical, or electrical burn requiring a
graft.
In some embodiments, the methods disclosed herein include the step of applying
the
compositions including regenerative cells disclosed herein to the underlying
tissue of a wound
(e.g., a debrided burn or wound) topically, or by injection, prior to
administration of a graft
onto the recipient site, i.e., the underlying tissue of the wound.
Accordingly, provided are
embodiments that include the steps of: (1) applying to a recipient wound site
(e.g., a debrided
wound, such as a debrided burn wound, a debrided ulcer, or the like), an
effective amount of
regenerative cells as disclosed herein, (2) contacting the graft and the
underlying tissue of the
wound; and (3) securing the graft to the recipient wound site, whereby
incorporation of the
graft into the recipient wound site is promoted. In some embodiments, the
subject has
radiation injury, e.g., cutaneous radiation injury or acute radiation injury.
In some
embodiments, the subject has radiation injury and a thermal, chemical, or
electrical burn
requiring a graft.
The skilled person will readily appreciate that securing the graft can be
accomplished
using any acceptable method, including but not limited to, suturing, stapling,
gluing (e.g., with
a biologically compatible glue such as fibrin or the like), or bandaging.
-26-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
The methods can include the step of analyzing the graft incorporation into the
recipient
site. Non-limiting ways to assess graft incorporation include, but are not
limited to, those
described in Dong, et al. (1993) Ann. Biomed. Eng. 21(1):51-55 (measurement of
adherence
of graft to the skin surface), Greenhalgh, et al. (1992) J. Burn Care Rehab.
13(3) 334-339
(transcutaneous oxygen and carbon dioxide measurements), as well as other
methods,
including but not limited to analysis of vascularization and/or necrosis,
analysis of the degree
of granulation, assessment of wound size (e.g., assessment of
epithelialization, assessment of
neodermis formation, or both), and the like. In some embodiments, planimetry
is used to
analyze epithelialization and/or contraction of the recipient site. In some
embodiments,
administration of the composition comprising regenerative cells improves graft
incorporation
and healing by increasing vascularization of a graft, by increasing the
average lumen size of
vessels within the graft, by increasing or accelerating vessel maturation, or
the like.
Vascularization and lumen size can be readily assessed using art-accepted
methods, including
histology and the like.
In some embodiments, the methods provided herein prevent or reduce contraction
of
the wound, e.g., in wounds receiving a fortified graft as discussed herein
(regenerative cells
and skin graft or skin substitute), or in wounds receiving regenerative cells
alone.
Accordingly, a subject is identified that has a wound at risk of development
of contracture. In
some embodiments, the wound at risk of development of contracture is a deep
partial
thickness wound. In some embodiments, the wound at risk of development of
contracture is a
full thickness wound. Deep partial thickness and full thickness wounds can be
assessed using
art-accepted methods described elsewhere herein. In some embodiments, the
subject is
administered a composition comprising regenerative cells. The
composition can be
administered systemically, locally, or both. In some embodiments, the wound at
risk of
development of contracture is contacted with a composition comprising
regenerative cells, as
described elsewhere herein. In some embodiments, the composition includes a
scaffold, e.g., a
tissue scaffold (such as adipose tissue or the like). In some embodiments, the
composition
includes a dermal substitute. In some embodiments, the composition includes a
skin graft.
Accordingly, the regenerative cells can be mixed with or applied to the
surface of, the
scaffold. In some embodiments, the composition is applied to the recipient
wound site, and a
-27-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
scaffold, e.g., a dermal substitute or skin graft is subsequently applied to
the wound site. In
some embodiments, the administration of the composition comprising
regenerative cells,
whether administered systemically or locally, or whether applied in
combination with a
scaffold or not, slows the rate of contraction of the recipient wound. In some
embodiments,
the administration of the composition comprising regenerative cells slows the
rate of
contraction such that the development of contractures is prevented or
minimized. In some
embodiments, the administration of the composition comprising regenerative
cells slows the
rate of contraction such that the development of hypertrophic scars is
prevented or minimized.
Methods of Preventing, Minimizing, or Treating Hypertrophic Scarring
Provided herein are methods for preventing and/or reducing hypertrophic
scarring at a
deep-partial thickness or full thickness wound site. The skilled person will
readily appreciate
that the embodiments disclosed herein are useful in the treatment of a variety
of types of
wounds involving the placement of a graft to aid in the healing of the wound,
e.g., in instances
where the area of skin loss is too big to be closed using local skin and
stitches alone. For
example, the embodiments disclosed herein are useful in the treatment of
burns, e.g., including
those in which burned tissue is excised. Other exemplary types of wounds in
which the
methods and compositions disclosed herein are used include, but are not
limited to non-
healing wounds, e.g., including ischemic wounds and ulcers (for example
pressure wounds,
wounds and ulcers associated with diabetes, wounds and ulcers associated with
peripheral
vascular disease, and the like), various traumatic wounds, e.g., caused by
mechanical,
chemical, insect or other animal sources, and the like. For example, the
methods described
herein are useful in incorporation of grafts following surgical removal of
cancerous,
devitalized, or infected tissue.
Methods of preventing or minimizing hypertrophic scarring can include the
steps of (1)
identifying a subject having a deep partial thickness or full thickness wound;
and (2)
administering to the deep partial thickness or full thickness wound a
composition comprising
regenerative cells. In some embodiments, the subject has radiation injury,
e.g., cutaneous
radiation injury or acute radiation injury. In some embodiments, the subject
has radiation
injury and a thermal, chemical, or electrical burn or other deep partial
thickness or full
thickness wound.
-28-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
In some embodiments, the regenerative cells are applied directly to the wound
site. In
some embodiments, the regenerative cells are applied in a "fortified graft,"
e.g., in
combination with a scaffold as described elsewhere herein, including but not
limited to fat
grafts, skin grafts, or other biological (autologous or non-autologous) or
synthetic skin
substitutes. In some embodiments, a scaffold is applied to the deep partial
thickness or full
thickness wound site, and the regenerative cells are subsequently applied to
the wound site.
In some embodiments, the regenerative cells are mixed together with a scaffold
as described
herein (e.g., unprocessed adipose tissue or the like), and the mixture is
applied to the wound
site. In some embodiments, the regenerative cells are mixed together with a
scaffold as
described herein to produce a fortified scaffold, which is administered to a
recipient site, and a
skin graft or skin substitute is subsequently applied to the recipient site
that has already
received the fortified scaffold. In some embodiments, the composition
comprising
regenerative cells is applied topically to the recipient site. As discussed
elsewhere herein,
topical administration can include dripping a liquid vehicle comprising the
regnerative cells
onto the recipient wound site, spraying a vehicle comprising the regenerative
cells onto the
recipient wound site, or the like. In some embodiments, the composition
comprising
regenerative cells is injected in or around the wound site (e.g., in a single
or multiple
inj ections).
In some embodiments, wherein the wound is a burn, the methods can further
include
the step of debriding the burn to create a debrided recipient site, and
administering the
composition comprising regenerative cells, or composition comprising
regenerative cells and
scaffold (fortified scaffold) to the debrided recipient site of the deep
partial thickness or full
thickness wound. In some embodiments, a skin graft or dermal substitute is
subsequently
applied to the recipient site that has received the fortified scaffold,
whereby hypertrophic scar
formation is prevented or inhibited.
The methods can also include the steps of measuring, analyzing or assessing
the
formation of hypertrophic scar formation at the wound site
In some embodiments, the methods disclosed herein include the step of applying
the
compositions including regenerative cells disclosed herein to the underlying
tissue of a wound
(e.g., a debrided burn or wound) topically, or by injection, prior to
administration of a graft
-29-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
onto the recipient site, i.e., the underlying tissue of the wound.
Accordingly, provided are
embodiments that include the steps of: (1) applying to a recipient wound site
(e.g., a debrided
wound, such as a debrided burn wound, a debrided diabetic and/or peripheral
vascular
disease-associated ulcer wound, a debrided pressure sore, or the like), a
composition
comprising an effective hypertrophic scar inhibiting amount of regenerative
cells as disclosed
herein, and (2) securing the graft to the recipient wound site, whereby
hypertrophic scar
formation is prevented or inhibited.
Some embodiments relate to methods of preventing or slowing wound contraction.
In
some embodiments, prevention of hypertrophic scarring comprises prevention or
slowing
wound contraction. Accordingly, in some embodiments, a subject having a deep
partial
thickness or full thickness wound (e.g., a wound that is at risk of developing
hypertrophic
scarring if wound contraction proceeds too rapidly), is identified. A
composition comprising
regenerative cells (e.g., concentrated populations of adipose-derived
regenerative cells or the
like), can be administered to the subject. In some embodiments, the
composition is
administered directly to the wound site, e.g., by topical administration or
local injection. In
some embodiments, the composition is administered systemically, e.g.,
intravascularly or
intralymphatically. In some embodiments, compositions comprising regenerative
cells are
administered to the subject both locally and systemically. In some
embodiments, the methods
include the step of measuring wound contraction. Wound contraction can be
readily assessed
using any method known to those in the art, including, planimetry, e.g., as
described in
Rogers, et al. (2010) J. Diabetes Sci. Tech, 4(4):799-802. In some
embodiments, e.g.,
wherein the composition comprising regenerative cells is administered locally,
the composition
includes a scaffold such as a tissue scaffold (e.g., adipose tissue, PRP, or
the like), or a
biological or biocompatible scaffold (including skin grafts, skin substitutes,
and the like). In
some embodiments, wound contraction is reduced by more than 3%, 4%, 5%, 6%,
7%, 8%,
9%, 10%, 15%, 20%, or more, by at least 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12,
13, 14, 15, 16, 17,
18, 19, 20, or more days post-injury.
Other embodiments provided herein relate to methods of treating hypertrophic
scars.
that have already developed or that are in the process of developing. For
example, treating a
hypertrophic scar can refer to minimizing and/or eliminating existing scar
tissue, minimizing
-30-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
and/or eliminating hypertrophic scar contracture, improving range of motion
over a scarred
area, eliminating or minimizing pruritus, improving pliability of hypertrophic
scarred tissue,
improving firmness of hypertrophic scarred tissue, improving score in one or
more art-
accepted scar scales (see, e.g., Fearmonti, et al. (2010) J. Plastic Surg. 10:
354-364),
decreasing mast cell number and/or myofibroblast cell numbers in hypertrophic
scarred tissue,
and the like.
A subject having a hypertrophic scar, i.e., a hypertrophic scar that has
existed for more
than 14 days, more than 30 days, more than 45 days, more than 60 days, more
than 90 days,
more than 120 days, more than 1 year, more than 5 years, or longer is
identified. In some
embodiments, the subject has radiation injury, e.g., cutaneous radiation
injury or acute
radiation injury. In some embodiments, the subject has radiation injury and a
hypertrophic
scar. In some embodiments, the methods include administration of a composition
comprising
regenerative cells to a hypertrophic scar, e.g., by local or systemic
injection, or any other
route of administration described herein. In some embodiments, the
regenerative cells are
administered with a scaffold, such as the scaffolds described herein below
(e.g., an autologous
fat graft, an autologous skin graft, allograft, dermal substitute, or any
combination thereof, or
any other biologic or synthetic scaffold). In some embodiments, the
compositions include a
scaffold. In some embodiments, the methods include the step of removing the
hypertrophic
scar tissue using any art accepted method to create a recipient site, and
administering the
composition comprising regenerative cells to the recipient site. In some
embodiments,
hypertrophic scar tissue is not removed prior to administering the composition
comprising
regenerative cells.
In some embodiments relating to minimizing or treating hypertrophic scars, the
method includes the step of performing an adjunct treatment or therapy to
ameliorate the
hypertrophic scar, in combination with the administration of the composition
comprising the
regenerative cells. For example, in some embodiments, the methods can include,
for example,
the step of perforating the scar tissue, e.g., as described in U.S. Patent
Application Publication
No. 2008/0119781, using mechanical force (see, e.g., Costa, et al.: (1999)
Mechanical Force
Induce Scar Remodeling: Am J Pathol. 155: 1671-1679), surgical removal of the
scar tissue
(see, e.g., Suzuki, S. (1996): Operation: Operation of keloid and/or
hypertrophic scar. 50:
-31-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
1557-1561), application of a silicone sheet to the lesion (see, e.g., Perkins,
et al., (1983)
Silicone gel: a new treatment for burn scars and contractures. Burns 9; 201-
204), laser and
pulsed light treatment of the lesion (see, e. g., Vrijman, et al. (2011) Laser
and Intense pulsed
Light Therapy for the Treatment of Hypertrophic Scars, British J. Derm.
165(5):934-942),
and the like. The skilled artisan will readily appreciate that any adjunct
therapy that is
performed can be performed prior to, at substantially the same time as, or
subsequently to, the
administration of the composition comprising regenerative cells. In some
embodiments, the
composition comprising regenerative cells is a fortified scaffold, and/or
fortified graft, as
described elsewhere herein that includes regenerative cells in combination
with a scaffold or
graft as described elsewhere herein.
In some embodiments, the methods include the step of assessing treatment of
the
hypertrophic scar. For example, in some embodiments, the size of the
hypertrophic scar is
assessed. In some embodiments, treatment with the compositions comprising
regenerative
cells as described herein results in a 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%,
45%, 50%,
55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, or more, decrease in scar area.
Decrease in scar area can refer to a decrease in the width, height, or depth
of the scar as
assessed using art-accepted techniques. Non-limiting examples of methods to
assess scar area
are described, e.g., in Oliviera, et al. (2005) Dermatot Slug. 31(1): 48-58.
In some
embodiments, treatment with the compositions comprising regenerative cells as
described
herein results in a reduction in scar contracture. Accordingly, some methods
include the step
of assessing the degree or amount of scar contracture. For example, in some
embodiments,
contracture can be measured by one or more of the methods described in Parry,
et al. (2010)
J Burn Care, 31(6): 888-903, or using any number of other art-accepted
techniques. In some
embodiments, treatment with the compositions comprising regenerative cells as
described
herein results in a 5%, 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%,
65%,
70%, 75%, 80%, 85%, 90%, 95%, or more, decrease in scar contracture. In some
embodiments, the range of motion over a scarred area is assessed using art-
accepted methods.
In some embodiments, treatment with the compositions comprising regenerative
cells as
described herein improves the range of motion by at least 2 degrees, 5
degrees, 10 degrees, 15
degrees, 20 degrees, 25 degrees, 30 degrees, 35 degrees, 40 degrees, 45
degrees, 50 degrees,
-32-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
55 degrees, 60 degrees, 65 degrees, 70 degrees, 75 degrees, 80 degrees, 85
degrees, 90
degrees, 95 degrees, 100 degrees, 110 degrees, 120 degrees, or more. Range of
motion can
be assessed using any art-accepted technique, including but not limited to
those described in
Palmieri, et al. (2003) i Burn Care Rehabil. 24:104-108. In some embodiments,
pruritus is
assessed. In some embodiments, treatment with the compositions comprising
regenerative
cells results in improvement in pruritus as measured by one or more art-
accepted techniques,
including but not limited to the tools described in Phan, et al. (2011) Acta
Derm. Venereol.
92: 502-507. In some embodiments, pliability of scar tissue is assessed. In
some
embodiments, treatment of a hypertrophic scar with the compositions comprising
regenerative
cells as described herein results in improvement of pliability of scar tissue.
Pliability can be
assessed using any art-accepted technique, including but not limited to those
described in
Oliviera, et al. (2005) Dermatol. Surg. 31(1): 48-58, Lye et al. (2006)
27(6):82-85, and the
like. In some embodiments, bloodflow within the hypertrophic scar is assessed,
e.g., using
Laser-doppler imaging, or any other art-accepted technique. In some
embodiments, elasticity
of the scar is assessed. In some embodiments, treatment of a hypertrophic scar
with the
compositions comprising regenerative cells as described herein results in
improvement of
elasticity of scar tissue. Elasticity can be assessed using any art-accepted
technique, including
but not limited to those described in Bartell, et al. (1988) i Burn Care
Rehabil. 9(6): 657-
660, and the like. In some embodiments, stiffness of the scar tissue is
assessed. In some
embodiments, treatment of a hypertrophic scar with the compositions comprising
regenerative
cells as described herein results in improvement of stiffness of scar tissue.
Stiffness can be
assessed using any art-accepted technique, including but not limited to those
described in
McHugh, et al. (1997) i Burn Care Rehabil. 18(2): 104-108.
Methods of Administration
In some embodiments, the methods disclosed herein include administering a
therapeutically effective amount of a composition comprising regenerative
cells to a subject.
As used herein, the term "therapeutically effective amount" refers to an
amount sufficient to
mitigate conversion of a burn, and/or to improve graft survival and take.
Determination of the
exact dose of regenerative cells for the embodiments disclosed herein is well
within the ambit
of the ordinary skill in the art.
-33-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
The amount and frequency of administration of the compositions can vary
depending
on, for example, what is being administered, the state of the patient, and the
manner of
administration. In therapeutic applications, compositions can be administered
to a patient
suffering from a burn (e.g., a subject that has been identified as having a
partial thickness burn
and/or a full thickness burn or that is in need of a graft), in an amount
sufficient to relieve or
least partially mitigate burn progression. The compositions can also be
administered to a
patient receiving a graft (e.g., a subject that has a debrided wound or burn)
in an amount
sufficient to improve survival of the graft, once administered to the patient.
The dosage is
likely to depend on such variables as the type and extent of the burn graft,
as well as the age,
weight and general condition of the particular subject, and the route of
administration.
Effective doses can be extrapolated from dose-response curves derived from in
vitro or animal
model test system.
In some embodiments, at least 1 x 102 regenerative cells is a therapeutically
effective
amount. In some embodiments, at least 1 x 103 regenerative cells is a
therapeutically effective
amount. In some embodiments, at least 1 x 104 cells is a therapeutically
effective amount. In
some embodiments, at least 1 x 105 regenerative cells is a therapeutically
effective amount. In
some embodiments, at least 1 x 106 regenerative cells is a therapeutically
effective amount. In
some embodiments, at least 1 x 107 regenerative cells is a therapeutically
effective amount. In
some embodiments, at least 1 x 108 regenerative cells is a therapeutically
effective amount. In
some embodiments, at least 1 x 109 regenerative cells is a therapeutically
effective amount. In
some embodiments, at least 1 x 1019 regenerative cells is a therapeutically
effective amount.
In some embodiments, a greater number of regenerative cells is therapeutically
effective to
treat burns with a larger surface area than to treat burns with a smaller
surface area. In some
embodiments, a greater number of regenerative cells is therapeutically
effective to treat deeper
burns than to treat burns that are not as deep (e.g., a greater number of
regenerative cells may
be therapeutically effective to treat a deep partial thickness wound than to
treat a superficial
partial thickness wound) In some embodiments, a greater number of regenerative
cells is
therapeutically effective to improve the survival or take of a graft that has
a larger surface
area, compared to a smaller graft. In some embodiments, the number of
regenerative cells
that is therapeutically effective depends upon whether the graft is a full
thickness or split-
-34-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
thickness skin graft, or whether the graft is a skin substitute or other
synthetic or biological
scaffold.
In some embodiments, the regenerative cells comprise at least 0.05% stem
cells. For
example, in some embodiments, the regenerative cells comprise at least 0.1%,
0.2%, 0.3%,
0.4%, 0.5%, 0.6%, 0.7%, 0.8%, 0.9%, 1.0%, 2%, 3%, 4%, 5%, 10%, 15%, 20%, 25%,
50%,
or more, stem cells. That is, in some embodiments, at least 0.1%, 0.2%, 0.3%,
0.4%, 0.5%,
0.6%, 0.7%, 0.8%, 0.9%, 1.0%, 2%, 3%, 4%, 5%, 10%, 15%, 20%, 25%, 50%, or
more, of
the nucleated cells within the regenerative cell population are stem cells.
Regenerative Cells
In the embodiments disclosed herein, regenerative cells are used for
mitigating and/or
prevent burn progression/conversion. In various embodiments, regenerative
cells are used for
improving the take or viability of grafts, and or promoting the healing of
grafts. As mentioned
above, a population of "regenerative cells" disclosed herein can be a
homogeneous or
heterogeneous population of cells that cells that which cause or contribute to
complete or
partial regeneration, restoration, or substitution of structure or function of
an organ, tissue, or
physiologic unit or system to thereby provide a therapeutic, structural or
cosmetic benefit.
Examples of regenerative cells include, but are not limited to adult stem
cells, endothelial cells,
endothelial precursor cells, endothelial progenitor cells, macrophages,
fibroblasts, pericytes,
smooth muscle cells, preadipocytes, differentiated or de-differentiated
adipocytes,
keratinocytes, unipotent and multipotent progenitor and precursor cells (and
their progeny),
and lymphocytes.
The regenerative cells disclosed herein can be isolated from various tissues,
including,
but not limited to bone marrow, placenta, adipose tissue, skin, eschar tissue,
endometrial
tissue, adult muscle, corneal stroma, dental pulp, Wharton's jelly, amniotic
fluid, and umbilical
cord. The regenerative cells disclosed herein can be isolated from the tissues
above using any
means known to those skilled in the art or discovered in the future.
By way of example only, regenerative cells can be isolated from adipose tissue
by a
process wherein tissue is excised or aspirated. Excised or aspirated tissue
can be washed, and
then enzymatically or mechanically disaggregated in order to release cells
bound in the adipose
tissue matrix. Once released, the cells can be suspended. By way of example
only,
-35-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
regenerative cells useful in the embodiments disclosed herein can be isolated
using the
methods and/or devices described in U.S. Patent No's. 7390484; 7585670,
7687059,
8309342, 8440440, US Patent Application Publication No's. 2013/0164731,
2013/0012921,
2012/0164113, US2008/0014181. International Patent Application Publication No.
W02009/073724, WO/2013030761, and the like, each of which is herein
incorporated by
reference.
Exemplary, non-limiting methods for isolation of regenerative cells from bone
marrow
useful in the embodiments disclosed herein are described in U.S. Patent No's
5879940, U.S.
Patent Application Publication No's 2013/0101561, 2013/0266541 European Patent
Application Publication No. EP2488632A1, EP0241578A2, EP2624845A2,
International
Patent Application Publication No. W02011047289A1, W01996038482A, each of
which is
herein incorporated by reference.
Exemplary, non-limiting methods for isolation of regenerative cells from
placental
tissue useful in the embodiments disclosed herein are described in U.S. Patent
No. 8580563,
U.S. Patent Application Publication No. 20130040281, International Patent
Application
Publication No. W02003089619A, Klein, et al. (2011) Methods 11/1-ol Biol.
698:75-88,
Vellasamy, et al. (2012) World J Stem Cells 4(6): 53-61; Timmins, et al.
(2012) Biotechnol
Bioeng. 109(7):1817-26; Semenov, et al. (2010) Am J Obstet Gynecol 202:193-
e.13, and the
like, each of which is herein incorporated by reference.
Exemplary, non-limiting methods for isolation of regenerative cells from skin
useful in
the embodiments disclosed herein are described in Toma, et al. (2001), Nat
Cell Biol.
3(9):778-84; Nowak, et al. (2009), Methods Mol Biol. 482:215-32; U.S Patent
Application
Publication No. 2007/0248574, and the like, each of which is herein
incorporated by
reference.
Exemplary, non-limiting methods for isolation of regenerative cells from
eschar tissue
useful in the embodiments disclosed herein are described in Van der Veen, et
al. (2012), Cell
Transplant. 21(5):933-42, and elsewhere herein below.
Exemplary, non-limiting methods for isolation of regenerative cells from
endometrial
tissue useful in the embodiments disclosed herein are described in U.S. Patent
Application
-36-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
Publication No. 2013/0156726, 2008/0241113, and the like, each of which is
herein
incorporated by reference in its entirety.
Exemplary, non-limiting methods for isolation of regenerative cells from
muscle tissue
useful in the embodiments disclosed herein are described in U.S. Patent No.
6337384, U.S.
Patent Application Publication No. 2001/019966, 2011/0033428, 2005/0220775,
and the
like, each of which is herein incorporated by reference.
Exemplary, non-limiting methods for isolation of regenerative cells from
corneal tissue
useful in the embodiments disclosed herein are described in U.S. Patent
Application
Publication No. 2005084119, Sharifi, et al. (2010) Biocell. 34(1):53-5, and
the like, each of
which is herein incorporated by reference.
Exemplary, non-limiting methods for isolation of regenerative cells from
dental pulp
useful in the embodiments disclosed herein are described in U.S. Patent
Application
Publication No. 2012/0251504, Gronthos, et al. (2011) Methods Mol Biol.
698:107-21;
Suchanek, et al. Acta Medica (Hradec Kralove). 2007;50(3):195-201; Yildirm,
Sibel,
"Isolation Methods of Dental Pulp Stem Cells," in Dental Pulp Stem Cells:
Springer Briefs in
Stem Cells, pp. 41-51, 2013, Springer New York, New York, NY, and the like,
each of
which is herein incorporated by reference.
Exemplary, non-limiting methods for isolation of regenerative cells from
Wharton's
jelly useful in the embodiments disclosed herein are described in U.S. Patent
Application
Publication No' s. 2013/0183273, 2011/0151556, International Patent
Application Publication
No. WO 04/072273A1, Sheshareddy, et al. (2008) Methods Cell Biol. 86:101-19,
Mennan,
et al. (2013) BioMed Research International, Article ID 916136, Corotchi, et
al. (2013) Stem
Cell Research & Therapy 4:81, and the like, each of which is herein
incorporated by
reference.
Exemplary, non-limiting methods for isolation of regenerative cells from
amniotic fluid
useful in the embodiments described herein are described in U.S. Patent No.
8021876,
International Patent Application Publication No. WO 2010/033969A1, WO
2012/014247A1,
WO 2009/052132, U.S. Patent Application Publication No. 2013/0230924,
2005/0054093,
and the like, each of which is herein incorporated by reference.
-37-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
Exemplary, non-limiting methods for isolation of regenerative cells from the
umbilical
cord useful in the embodiments described herein are described in U.S. Patent
Application
Publication No. 20130065302, Reddy, et al. (2007), Methods Mol Blot 407:149-
63, Hussain,
et al. (2012) Cell Biol Int. 36(7):595-600, Pham, et al. (2014) Journal of
Translational
Medicine 2014, 12:56, Lee, et al. (2004) Blood 103(5): 1669-1675, and the
like, each of
which is herein incorporated by reference.
The regenerative cells in the methods and compositions described herein can be
a
heterogeneous population of cells that includes stem and other regenerative
cells. In some
embodiments, the regenerative cells in the methods and compositions described
herein can
include stem and endothelial precursor cells. In some embodiments, the
regenerative cells can
include stem and pericyte cells. In some embodiments, the regenerative cells
can include stem
cells and leukocytes. For example, in some embodiments, the regenerative cells
can include
stem cells and macrophages. In some embodiments, the regenerative cells can
include stem
cells and M2 macrophages. ln some embodiments, the regenerative cells can
include pericytes
and endothelial precursor cells. In some embodiments, the regenerative cells
can include
platelets. Preferably, the regenerative cells comprise stem cells and
endothelial precursor
cells. In some embodiments, the regenerative cells can include regulatory
cells such as Treg
cells.
In some embodiments, the regenerative cells are adipose-derived. Accordingly,
some
embodiments provide methods and compositions for mitigating or reducing burn
progression
with adipose-derived regenerative cells, e.g., that include adipose-derived
stem and endothelial
precursor cells.
In some embodiments, the regenerative cells are not cultured prior to use. By
way of
example, in some embodiments, the regenerative cells are for use following
isolation from the
tissue of origin, e.g., bone marrow, placenta, adipose tissue, skin, eschar
tissue, endometrial
tissue, adult muscle, cornea stroma, dental pulp, Wharton's jelly, amniotic
fluid, umbilical
cord, and the like.
In some embodiments, the regenerative cells are cultured prior to use. For
example, in
some embodiments, the regenerative cells are subjected to "limited culture,"
i.e., to separate
cells that adhere to plastic from cells that do not adhere to plastic.
Accordingly, in some
-38-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
embodiments, the regenerative cells are "adherent" regenerative cells. An
exemplary, non-
limiting method of isolating adherent regenerative cells from adipose tissue
are described e.g.,
in Zuk, et al. (2001). Exemplary, non-limiting method of isolating adherent
regenerative cells
from bone marrow are described, e.g., Pereira (1995) Proc. Nat. Acad. Sci. USA
92:4857-
4861, Castro-Malaspina et al. (1980), Blood 56:289-30125; Piersma et al.
(1985) Exp.
Hematol. 13:237-243; Simmons et al., 1991, Blood 78:55-62; Beresford et al.,
1992, 1 Cell.
Sci. 102:341-3 51; Liesveld et al. (1989) Blood 73:1794-1800; Liesveld et al.,
Exp. Hematol
19:63-70; Bennett et al. (1991) i Cell. Sci. 99:131-139), U.S. Patent No.
7056738, and the
like.
In some embodiments, the regenerative cells are cultured for more than 3
passages in
vitro. For example, in some embodiments, the regenerative cells are cultured
for 4, 5, 6, 7, 8,
9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28,
29, 30, 31, 32, 33,
34, 35, 36, 37, 38, 39, 40, or more passages in vitro.
The regenerative cells described herein can be cultured according to
approaches
known in the art, and the cultured cells can be used in several of the
embodied methods. For
example, regenerative cells can be cultured on collagen-coated dishes or 3D
collagen gel
cultures in endothelial cell basal medium in the presence of low or high fetal
bovine serum or
similar product, as described in Ng, et al., (2004), Microvasc. Res. 68(3):258-
64,
incorporated herein by reference. Alternatively, regenerative cells can be
cultured on other
extracellular matrix protein-coated dishes. Examples of extracellular matrix
proteins that may
be used include, but are not limited to, fibronectin, laminin, vitronectin,
and collagen IV.
Gelatin or any other compound or support, which similarly promotes adhesion of
endothelial
cells into culture vessels may be used to culture regenerative cells, as well.
Examples of basal culture medium that can be used to culture regenerative
cells in
vitro include, but are not limited to, EGM, RPMI, M199, MCDB131, DMEM, EMEM,
McCoy's 5A, Iscove's medium, modified Iscove's medium, or any other medium
known in
the art to support the growth of blood endothelial cells. In some embodiments,
the
regenerative cells are cultured in EGM-2MV media. Examples of supplemental
factors or
compounds that can be added to the basal culture medium that could be used to
culture
regenerative cells include, but are not limited to, ascorbic acid, heparin,
endothelial cell
-39-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
growth factor, endothelial growth supplement, glutamine, HEPES, Nu serum,
fetal bovine
serum, human serum, equine serum, plasma-derived horse serum, iron-
supplemented calf
serum, penicillin, streptomycin, amphotericin B, basic and acidic fibroblast
growth factors,
insulin-growth factor, astrocyte conditioned medium, fibroblast or fibroblast-
like cell
conditioned medium, sodium hydrogencarbonate, epidermal growth factor, bovine
pituitary
extract, magnesium sulphate, isobutylmethylxanthine, hydrocortisone,
dexamethasone,
dibutyril cyclic AMP, insulin, transferrin, sodium selenite, oestradiol,
progesterone, growth
hormone, angiogenin, angiopoietin-1, Del-1, follistatin, granulocyte colony-
stimulating factor
(G-CSF), erythropoietin, hepatocyte growth factor (HGF) /scatter factor (SF),
leptin,
midkine , placental growth factor, platelet-derived endothelial cell growth
factor (PD-ECGF),
platelet-derived growth factor-BB (PDGF-BB), pleiotrophin (PTN), progranulin,
proliferin,
transforming growth factor-alpha (TGF-alpha), transforming growth factor-beta
(TGF-beta),
tumor necrosis factor-alpha (TNF-alpha), vascular endothelial growth factor
(VEGF)/vascular
permeability factor (VPF), interleukin-3 (1L-3), interleukin 7 (1L-7),
interleukin-8 (1L-8),
ephrins, matrix metalloproteinases (such as MMP2 and MMP9), or any other
compound
known in the art to promote survival, proliferation or differentiation of
endothelial cells.
Further processing of the cells may also include: cell expansion (of one or
more
regenerative cell types) and cell maintenance (including cell sheet rinsing
and media changing);
sub-culturing; cell seeding; transient transfection (including seeding of
transfected cells from
bulk supply); harvesting (including enzymatic, non-enzymatic harvesting and
harvesting by
mechanical scraping); measuring cell viability; cell plating (e.g., on
microtiter plates, including
picking cells from individual wells for expansion, expansion of cells into
fresh wells); high
throughput screening; cell therapy applications; gene therapy applications;
tissue engineering
applications, therapeutic protein applications, viral vaccine applications,
harvest of
regenerative cells or supernatant for banking or screening, measurement of
cell growth, lysis,
inoculation, infection or induction; generation of cell lines (including
hybridoma cells); culture
of cells for permeability studies; cells for RNAi and viral resistance
studies; cells for knock-out
and transgenic animal studies; affinity purification studies; structural
biology applications;
assay development and protein engineering applications.
-40-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
In some embodiments, methods for isolating regenerative useful in the
embodiments
described herein can include positive selection (selecting the target cells),
negative selection
(selective removal of unwanted cells), or combinations thereof. In addition to
separation by
flow cytometry as described herein and in the literature, cells can be
separated based on a
number of different parameters, including, but not limited to, charge or size
(e.g., by
dielectrophoresis or various centrifugation methods, etc.).
By way of example, the regenerative cells useful in the methods of treatment
disclosed
herein may be identified by different combinations of cellular and genetic
markers. For
example, in some embodiments, the regenerative cells express CD90. In some
embodiments,
the regenerative cells do not express significant levels of lin. In some
embodiments, the
regenerative cells do not express significant levels of ckit. In some
embodiments, the
regenerative cells are CD90+/lin-/ckit-/CD45-.
In some embodiments, the regenerative cells express STRO-1. In some
embodiments,
the regenerative cells express STRO-1 and CD49d. In some embodiments, the
regenerative
cells express STRO-1, CD49d, and one or more of CD29, CD44, CD71, CD90,
C105/SH2
and SH3. In some embodiments, the regenerative cells express STRO-1, CD49d,
and one or
more of CD29, CD44, CD71, CD90, C105/SH2 and SH3, but express low or
undetectable
levels of CD106.
In some embodiments, the regenerative cells express one or more of STRO-1,
CD49d,
CD13, CD29, SH3, CD44, CD71, CD90, and CD105, or any combination thereof. By
way of
example only, in some embodiments, the regenerative cells express each of do
not express
significant levels of CD31, CD34, CD45 and CD104 and do not express detectable
levels of
CD4, CD8, CD11, CD14, CD16, CD19, CD33, CD56, CD62E, CD106 and CD58.
In some approaches, the regenerative cells are CD14 positive and/or CD1 lb
positive.
In some embodiments, the cells are depleted for cells expressing the markers
CD45(+).
In some embodiments, the cells are depleted for cells expressing glycophorin-A
(GlyA). In
some embodiments, the cells are depleted for CD45(+) and GlyA(+) cells.
Negative selection of cells, e.g., depletion of certain cell types from a
heterogeneous
population of cells can done using art-accepted techniques, e.g., utilizing
micromagnetic beads
or the like. In some embodiments, the regenerative cells are CD34+.
-41-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
In some embodiments, the regenerative cells are not cryopreserved. In some
embodiments, the regenerative cells are cryopreserved. For example, in some
embodiments,
the regenerative cells include cryopreserved cells, e.g, as described in Liu,
et al. (2010)
Biotechnol Prog. 26(6):1635-43, Carvalho, et al. (2008) Transplant
Proc.;40(3):839-41,
International Patent Application Publication No. WO 97/039104, WO 03/024215,
WO
2011/064733, WO 2013/020492, WO 2008/09063, WO 2001/011011, European Patent
No.
EP0343217 B 1 , and the like.
In some embodiments, regenerative cells are isolated from a subject having
radiation
injury, e.g., cutaneous or acute radiation injury. Some of the embodiments
described herein
are based, in part, upon the surprising discovery that populations of
regenerative cells isolated
from the adipose tissue of subjects with radiation injury have similar
properties (e.g., cell type,
cell viability, cell frequency, and cell function), as regenerative cells
isolated from adipose
tissue of subjects with no radiation injury.
In some embodiments, the regenerative cells are isolated from adipose tissue
obtained
from eschar. For example, in some embodiments, the regenerative cells are
isolated from
adipose tissue obtained from tangential or en bloc escharectomy. The
embodiments disclosed
herein are based, in part, upon the discovery that regenerative cell
populations isolated from
adipose tissue obtained from eschar have similar properties (e.g., cell type,
cell viability, cell
frequency, and cell function), as regenerative cells isolated from non-eschar
adipose tissue.
Scaffolds
In some embodiments, the regenerative cells disclosed herein can be
administered to a
subject with a scaffold. In some embodiments, the scaffold can be a skin
substitute, e.g., a
biological or synthetic skin substitute. Exemplary skin substitutes useful in
the embodiments
disclosed herein include, but are not limited to, cell-containing skin
substitutes such as
EPICEL skin graft (Genzyme Biosurgery, MA, USA); CELLSPRAY skin graft (Avita
Medical, Perth, Australia), MYSKINTM skin graft (CellTran Ltd., Sheffeild,
UK),
LASERSKIN skin graft (Fidia Advanced Biopolymers, Abano Terme, Italy); RECELL
skin
graft (Avita Medical, Perth, Australia), ORCEL skin graft (Ortec Intl, GA,
USA),
APLIGRAFT skin graft (Organogenesis, MA, USA), POLYACTIVE skin graft (HC
Implants BV, Leiden, Netherlands), and the like. Exemplary non-cellular skin
substitutes
-42-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
useful in the embodiments disclosed herein include, but are not limited
INTEGRA (Integra
NeuroSciences, NJ, USA) scaffold; ALLODERM scaffold (LifeCell Corp., NJ,
USA),
HYALOMATRIX PA scaffold (Fidia Advanced Biopolymers, Abano Terme, Italy),
DERMAGRAFT scaffold (Advanced BioHealing, CT, USA), TRANSCYTE (Advanced
BioHealing, CT, USA), HYALOGRAFT 3DTM scaffold (Fidia Advanced Biopolymers,
Abano
Terme, Italy), DERMAMATRIX scaffold (Synthes, CMF, PA, USA), and the like.
The
skilled person will readily appreciate skin substitutes (whether cellular or
acellular) developed
in the future are useful in the embodiments disclosed herein. Various skin
substitutes useful in
the embodiments disclosed herein are described in US Patent Application
Publication number
U.S. 2011/0245929.
Other, non-limiting examples of scaffolds and matrices useful in the
embodiments
disclosed herein include PURAPLY collagen dressing (Organogensis, Inc. MA,
USA),
ALLEVYN4 matrix (Smith & Nephew, Hull, UK), ACTICOAT matrix (Smith & Nephew,
Hull, UK), CICA-CARE matrix (Smith & Nephew, Hull, UK), DURA-FIBER matrix
(Smith & Nephew, Hull, UK), INTRASITE matrix (Smith & Nephew, Hull, UK),
IODOSORB matrix (Smith & Nephew, Hull, UK), OPSITE matrix (Smith & Nephew,
Hull, UK), PROFORE matrix (Smith & Nephew, Hull, UK), CUTINOVA matrix (Smith
&
Nephew, Hull, UK), JELONET matrix (Smith & Nephew, Hull, UK), BIOBRANE
matrix
(Smith & Nephew, Hull, UK) FORTAFLEX bioengineered collagen matrix
(Organogenesis,
MA, USA), FORTAGEN collagen construct (Organogenesis, MA, USA), and the like.
Accordingly, in some embodiments, the regenerative cells are combined with a
biocompatible matrix such as a mesh, a gauze, a sponge, a monophasic plug, a
biphasic plug, a
paste, a putty, a wrap, a bandage, a patch, a mesh, or a pad. In some
embodiments, the
biocompatible matrix can be resorbable, porous, or both resorbable and porous.
Biocompatible matrices useful in the embodiments disclosed herein can include
one or more of
the following: proteins, polysaccharides, nucleic acids, carbohydrates,
inorganic components
or minerals, and synthetic polymers; or mixtures or combinations thereof. For
example, in
some embodiments, the biocompatible matrix can include one or more of a
polyurethane, e.g.,
NOVOSORBTM biocompatible polyurethane matrices, a siloxane, a polysiloxane, a
collagen, a
glycosaminoglycan, oxidized regenerated cellulose (ORC), an ORC:collagen
composite, an
-43-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
alginate, an alginatexollagen composite, a ethylene diamine tetraacetic acid
(EDTA),a
poly(lactic-co-glycolitic acid (PLGA), a carboxymethylcellulose, a granulated
collagen-
glycosaminoglycan composite, methylcellulose, hydroxypropyl methylcellulose,
or
hydroxyethyl cellulose alginic acid, poly(a- hydroxy acids), poly(lactones),
poly(amino acids),
poly(anhydrides), poly(orthoesters), poly(anhydride-co-imides),
poly(orthocarbonates),
poly(a-hydroxy alkanoates), poly(dioxanones), poly(phosphoesters), poly(L-
lactide) (PLLA),
poly(D,L-lactide) (PDLLA), polyglycolide (PGA), poly(lactide-co-glycolide
(PLGA), poly(L-
lactide-co-D, L-lactide), poly(D,L-lactide-co-trimethylene carbonate),
polyhydroxybutyrate
(PUB), poly(e-caprolactone), poly(5-valerolactone), poly(y-
butyrolactone),
poly(caprolactone), polyacrylic acid, polycarboxylic acid, poly(allylamine
hydrochloride),
poly(diallyldimethylammonium chloride), poly(ethyleneimine), polypropylene
fiimarate,
polyvinyl alcohol, polyvinylpyrrolidone, polyethylene, polymethylmethacrylate,
carbon fibers,
poly(ethylene glycol), poly(ethylene oxide), polyvinyl alcohol),
poly(vinylpyrrolidone),
poly(ethyloxazoline), poly(ethylene oxide)- co-poly(propylene oxide) block
copolymers,
poly(ethylene terephthalate)polyamidearabic gum, guar gum, xantham gum,
gelatin, chitin,
chitosan, chitosan acetate, chitosan lactate, chondroitin sulfate, N,0-
carboxymethyl chitosan,
a dextran, fibrin glue, glycerol, hyaluronic acid, sodium hyaluronate, a
cellulose, a
glucosamine, a proteoglycan, a starch, lactic acid, a pluronic, sodium
glycerophosphate,
glycogen, a keratin, a silk, one or more composites thereof, one or more
mixtures thereof, or
one or more combinations thereof. In some embodiments, comprises calcium
phosphate.
In some embodiments, the biocompatible matrix may comprise a collagen. In
certain
embodiments, the biocompatible matrix comprises a Type I collagen, a Type II
collagen, a
Type III collagen, a Type IV collagen, a Type V collagen, a Type VI collagen,
a Type VII
collagen, a Type VIII collagen, or combinations thereof. Moreover, the
collagen can comprise
bovine collagen, human collagen, porcine collagen, equine collagen, avian
collagen, or
combinations thereof. In certain embodiments, the collagen comprises bovine
Type I collagen
or human Type I collagen. In some embodiments the collagen is in combination
with other
materials (e.g., chondroitin 6 sulfate) and/or is supplemented with materials
that provide
barrier function (e.g., a silicone backing vapor barrier). One example of a
composite collagen-
containing graft is INTEGRA (Integra NeuroSciences, NJ, USA) scaffold.
-44-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
In some embodiments, the regenerative cells can be combined with a FIELISTATS
absorbable collagen hemostatic sponge (Integra Life Sciences, NJ, USA); a
BELITENE
absorbable collagen hemostatic agent (Integra Life Sciences, NJ, USA); Matrix
Collagen
ParticlesTM wound dressing (Collagen Matrix, Inc., NJ, USA); Matrix Collagen
SpongeTm
wound dressing (Collagen Matrix, Inc., NJ, USA); OASIS wound matrix (Smith &
Nephew,
Hull, UK); BIOBLANKETTm surgical mesh (Kensey Nash, Corp.); ZIMMERTm collagen
repair patch (Zimmer, Inc., Swisdon, UK); PROMOGRANTm matrix wound dressing
(Systagenix, MA, USA), FIBROCOL PLUS collagen dressing (Systagenix, MA, USA),
or
the like. Yet other scaffolds and grafts useful in the embodiments disclosed
herein are
described in U.S. Patent No. 6,979,670, 7,972,631, 7,824,711, and
7,358,284U.S. Patent
Application Publication No. 2011/0091515, and the like.
In some embodiments, the regenerative cells are combined with a tissue
scaffold, e.g.,
unprocessed adipose tissue, platelet rich plasma, or other tissue. Mixture of
regenerative cells
with tissue to form a fortified scaffold (e.g., a cell-enriched fat graft)
useful in the
embodiments described herein is disclosed, for example, in U.S. Patent No.
7651684, and
Kakudo, et al. (2013) Journal of Translational Medicine 11:254, and the like.
Combination Therapy
As explained in further detail below, some embodiments provide for treatment
of
subjects with combination therapy, i.e., one or more additional additives
(e.g., pharmaceutical
agents, biologic agents, or other therapeutic agents) in addition to the
regenerative cells as
described herein.
In some embodiments, the one or more additional "agents" described above can
be
administered in a single composition with the regenerative. In some
embodiments, the one or
more additional "agents" can be administered separately from the regenerative
cells. For
example, in some embodiments, one or more additional agents can be
administered just prior
to, or just after, administration of the regenerative cells. As used herein,
the term "just prior"
can refer to within 15 minutes, 30 minutes, an hour, 2 hours, 3 hours, 4
hours, 5 hours, or the
like. Likewise, the phrase "just after administration" can refer to within 15
minutes, 30
minutes, an hour, 2 hours, 3 hours, 4 hours, 5 hours, or the like.
-45-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
Additional agents useful in combination therapy in the methods described
herein
include, for example, growth factors, cytokines, platelet rich plasma,
steroids, non-steroidal
anti-inflammatory agents, anti-bacterial and anti-fungal agents, as well as
other agents known
in the art to have beneficial effects in treatment of burn.
1) Growth Factors, Cytokines, and Hormones
Various growth factors, cytokines, and hormones have been shown to have
beneficial
effects, e.g., in re-epithelialization and recovery in burn injury. See, e.g.,
Wenczak, et al.
(1992)1 Clin. Invest. 90:2392-2401.
In some embodiments, subjects can be administered one or more growth factors,
cytokines or hormones, including combinations thereof, in addition to the
regenerative cells
disclosed herein. For example, in some embodiments, growth factors are
administered
concomitantly with, prior to, or following the administration of the
regenerative cells. Non-
limiting examples of growth factors useful in the embodiments disclosed herein
include, but
are not limited to, angiogenin, angiopoietin-1 (Ang-1), angiopoietin-2 (Ang-
2), brain-derived
neurotrophic factor (BDNF), Cardiotrophin-1 (CT-1), ciliary neurotrophic
factor (CNTF),
Del-1, acidic fibroblast growth factor (aFGF), basic fibroblast growth factor
(bFGF),
follistatin, ganulocyte colony-stimulating factor (G-CSF), glial cell line-
derived neurotrophic
factor (GDNF), hepatocyte growth factor (HGF), scatter factor (SF),
Interleukin-8 (IL-8),
leptin, midkine, nerve growth factor (NGF), neurotrophin-3 (NT-3),
Neurotrophin-4/5,
Neurturin (NTN), placental growth factor, Platelet-derived endothelial cell
growth factor
(PD-ECGF), Platelet-derived growth factor-BB (PDGF-BB), Pleiotrophin (PTN),
Progranulin, Proliferin, PBSF/SDF-1, Transforming growth factor-alpha (TGF-
alpha),
Transforming growth factor-beta (TGF-beta), Tumor necrosis factor-alpha (TNF-
alpha),
Vascular endothelial growth factor (VEGF), vascular permeability factor (VPF),
erythropoietin (see, e.g., Tobalem, et a. (2012) Br. J. Surg. 99(9).1295-
1303), and the like.
2) Anti-Ihflammatoty Agents
In some embodiments, subjects are administered on or more anti-inflammatory
agents,
in addition to the regenerative cells as disclosed herein. As used herein, the
term "anti-
inflammatory agent" refers to any compound that reduces inflammation, and
includes, but is
-46-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
not limited to steroids, non-steroidal anti-inflammatory drugs, and other
biologics that have
been demonstrated to have an anti-inflammatory effect.
Accordingly, in some embodiments, steroids are administered concomitantly
with,
prior to, or following the administration of the regenerative cells. Non-
limiting examples of
steroids useful in the embodiments disclosed herein include, but are not
limited to,
progestegens, e.g., progesterone, and the like; corticosteroids, e.g.,
prednisone, aldosterone,
cortisol, and the like, androgens, e.g., testosterone, and the like, and
estrogens.
Other anti-inflammatory agents useful in the embodiments disclosed herein
include, for
example, antibodies that inhibit action of TNF-a, IL-6 (see, e.g., Sun, et al.
(2012) Repair and
Regeneration, 20(4): 563-572), anti-TNF conjugates, Sun, et al. (2012) Wound
Repair
Regen. 20(4): 563-572, and the like. These anti-inflammatory agents have been
demonstrated
to exhibit beneficial effects in burn recovery.
Non-steroidal anti-inflammatory drugs useful in the embodiments disclosed
herein s
include propionic derivatives; acetic acid derivatives; biphenylcarboxylic
acid derivatives;
fenamic acid derivatives; and oxicams. Examples of anti-inflammatory actives
include without
limitation acetaminophen, diclofenac, diclofenac sodium and other salts,
ibuprofen and its salts
acetaminophen, indomethacin, oxaprozin, pranoprofen, benoxaprofen, bucloxic
acid, elocon;
and mixtures thereof
3) Anti-Oxidants
Anti-oxidants have been shown to be useful in recovery from burn injury. See,
e.g.,
F.H. Al-Jawad, et al. (2008) Ann Burns Fire Disasters 21(4): 186-191.
Accordingly, in some
embodiments, the methods and compositions disclosed herein include
administration of one or
more anti-oxidants in addition to the regenerative cells. Antioxidants useful
in the
embodiments disclosed herein include, but are not limited to, N-
acetylcysteine, curcumarin,
galactomarman, pynivate and other alpha-ketoacids, thioglycollate vitamin A
and derivatives,
including retinoic acid, retinyl aldehyde, retin A, retinyl palmitate,
adapalene, and beta-
carotene; vitamin B (panthenol, provitamin B5, panthenic acid, vitamin B
complex factor);
vitamin C (ascorbic acid and salts thereof) and derivatives such as ascorbyl
palmitate; vitamin
D including calcipotriene (a vitamin D3 analog) vitamin E including its
individual constituents
alpha-, beta-, gamma-, delta-tocopherol and cotrienols and mixtures thereof
and vitamin E
-47-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
derivatives including vitamin E palmitate, vitamin E linolate and vitamin E
acetate; vitamin K
and derivatives; vitamin Q (ubiquinone) and any combination thereof.
4) Platelet-Containing Fluids
Platelet rich plasma ("PRP") has been demonstrated to have beneficial effects
following burn injury. See, e.g., Pallua, et al. (2010) Burns, 36(1):4-8.
Accordingly, in some
embodiments, subjects are administered platelet rich plasma, in addition to
the regenerative
cells disclosed herein. For example, in some embodiments, a platelet
containing fluid is
administered concomitantly with, prior to, or following the administration of
the regenerative
cells. In some embodiments, the regenerative cells as disclosed herein are
combined with a
synergistically effective amount of platelet-containing fluid.
As used herein, the term "platelet-containing fluid" refers to any fluid,
either biological
or artificial, which contains platelets. Non-limiting examples of such fluids
include various
forms of whole blood, blood plasma, platelet rich plasma, concentrated
platelets in any
medium, or the like, derived from human and non-human sources. For example, in
some
embodiments, the platelet-containing fluid refers to blood, platelets, serum,
platelet
concentrate, platelet-rich plasma (PRP), platelet-poor plasma (PPP), plasma,
fresh frozen
plasma (FFP), and the like.
The term "PRP" as used herein refers to a concentration of platelets greater
than the
peripheral blood concentration suspended in a solution of plasma. Methods for
isolating PRP
useful in the embodiments disclosed herein are known in the art. See, e.g., US
Patent No.
8557535, International Patent Application Publication No. WO 09/155069, U.S.
Patent
Application Publication No' s, US20100183561, US20030060352, US20030232712,
US20130216626, U520130273008, U520130233803, US20100025342, European Patent
No. EP1848474B1, and the like. Platelets or PRP can suspended in an excipient
other than
plasma. In some embodiments, the platelet composition can include other
excipients suitable
for administration to a human or non-human animal including, but not limited
to isotonic
sodium chloride solution, physiological saline, normal saline, dextrose 5% in
water, dextrose
30% in water, lactated ringer's solution and the like. Typically, platelet
counts in PRP as
defined herein range from 500,000 to 1,200,000 per cubic millimeter, or even
more. PRP may
be obtained using autologous, allogeneic, or pooled sources of platelets
and/or plasma. PRP
-48-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
may be obtained from a variety of animal sources, including human sources. In
preferred
embodiments, PRP according to the invention is buffered to physiological pH.
Methods of Administration
Compositions administered according to the methods described herein can be
introduced into the subject by, e.g., by intravenous, intra-arterial,
intradermal, intramuscular,
intra-lymphatic, intranodal, intramammary, intraperitoneal, intrathecal,
retrobulbar,
intrapulmonary (e.g., term release); by oral, sublingual, nasal, anal,
vaginal, or transdermal
delivery, or by surgical implantation at a particular site. The introduction
may consist of a
single dose or a plurality of doses over a period of time. In such cases the
plurality of
introductions need not be by the same mechanism. For example, in some
embodiments
introduction at one time might be in the form of a topical spray of the
regenerative cells
whereas at another time the introduction may be regenerative cells combined
with an
autologous fat graft. Vehicles for cell therapy agents are known in the art
and have been
described in the literature. See, for example Remington's Pharmaceutical
Sciences, 18th Ed.
(1990, Mack Publ. Co, Easton Pa. 18042) pp 1435-1712, incorporated herein by
reference.
Sterile solutions are prepared by incorporating the regenerative cells that in
the required
amount in the appropriate buffer with or without various of the other
components described
herein.
In some embodiments, the regenerative cells described herein can be
administered
directly to the burn. For example, in some embodiments, the regenerative cells
disclosed
herein are formulated for injection. Accordingly, in some embodiments, the
compositions
disclosed herein are formulated for intravenous, intraarterial, intradermal,
intramuscular,
intraperitoneal, intrasternal, subcutaneous, intranodal and intra-lymphatic
injection, infusion,
and placement. In some embodiments, the compositions disclosed herein are
formulated for
intra¨lymphatic delivery. Accordingly, in some embodiments, the regenerative
cells can be
injected into the burn site, e.g., within the zone of coagulation, the zone of
stasis, or the zone
of hyperemia of a burn (subcutaneously, intramuscularly, or the like).
In some embodiments, the regenerative cells disclosed herein injected via
subcutaneous or intramuscular injection, adjacent to the zone of coagulation.
In some
embodiments, the regenerative cells disclosed herein are injected adjacent to
the zone of stasis.
-49-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
In some embodiments, the regenerative cells disclosed herein are injected into
and adjacent to
the zone of coagulation. In some embodiments, the regenerative cells disclosed
herein are
injected into and adjacent to the zone of stasis. Accordingly, in some
embodiments, the
regenerative cells are formulated for administration in multiple doses, e.g.,
in multiple
injections in and/or around the burn. In some embodiments, the number of
injections depends
upon the size of the burn. For example, in some embodiments, as the area
(and/or the
severity) of the burn increases, a greater the number of injections of the
regenerative cells is
provided. In some embodiments, for example, the regenerative cells as
disclosed herein are
injected into and around the burn every 0.1 mm2, 0.2 mm2, 0.3 mm2, 0.4 mm2,
0.5 mm2, 0.6
mm2, 0.7 mm2, 0.8 mm2, 0.9 mm2, 1.0 mm2, 2 mm2, 3 mm2, 4 mm2, 5 mm2, 6 mm2, 7
mm2, 8
mm2, 9 mm2, 10 1111112, 20 mm2, 30 mm2, 40 mm2, 50 mm2, 60 mm2, 70 mm2, 80
mm2, 90 mm2,
1 cm2, 5 cm2, 10 cm2, 20 cm2, 30 cm2, 40 cm2, 50 cm2, 60 cm2, 70 cm2, 80 cm2,
90 cm2, 100
cm2 area of the burn, or any value in between. The skilled artisan will
readily appreciate that
various devices, e.g., the JUVAPENTM injection device (Juvaplus, SA,
Switzerland), etc.,
suitable for the injection of multiple doses of regenerative cells, can be
used in the
administration of the regenerative cells according to the embodiments
disclosed herein. In
some embodiments, the regenerative cells are formulated for delivery in a
single injection, e.g.,
a single subcutaneous injection.
In some embodiments, the regenerative cells disclosed herein can be
administered via
one or multiple intravenous injections. For example, in some embodiments, the
regenerative
cells can be administered via a single intravenous infusion over a period of 1
min, 2 min, 3
min, 4 min, 5 min, 10 min, 30 min, 45 min, 1 h, 2 h, or longer.
In some embodiments, the regenerative cells disclosed herein can be
administered by
applying the cells to a scaffold as discussed elsewhere herein (e.g.,
including but not limited to
biocompatible synthetic and non-synthetic matrices, such as skin substitutes),
and applying the
scaffold seeded with the regenerative cells to the burn. In some embodiments,
a scaffold (e.g.,
including but not limited to biocompatible synthetic and non-synthetic
matrices, such as skin
substitutes) is applied to the burn, and the regenerative cells disclosed
herein are applied onto
the scaffold.
-50-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
Other methods of administering the regenerative cells as disclosed herein
include, but
are not limited to, those described in Gerlach, et al. (2011) Burns 37, e19-
e23. In this
method, the regenerative cells are placed into a sterile syringe with a fitted
nozzle, and
sprayed directly through the nozzle into the burn. Using computer-assisted
delivery, the gun
distributes cells at a uniform velocity throughout the wound. Such a method
could also
readily be used to apply the compositions comprising regenerative cells to a
scaffold as
described herein. The skilled artisan will appreciate that other devices
suitable for
administering the compositions comprising regenerative cells via spraying the
compositions
can be used in the methods described herein, including, but not limited to,
EIBRUET
biomaterial applicators (Nordson Micromedics, St. Paul, MN), EASY SPRAY
applicators
(Baxter, Deerfiled, IL), SMARTJET applicators (Harvest Technologies,
Plymouth, MA),
and the like.
In some embodiments, the compositions including the regenerative cells
disclosed
herein are administered within 5 min, 10 min, 15 min, 20 min, 30 min, 40 min,
50 min, 1 h, 2h,
3h, 4 h, 5 h, 6 h, 7 h, 8 h, 9 h, 10 h, 11 h, 12 h, 24 h, 36 h, 48 h, 60 h, 1
week, 2 weeks, or
less, following the burn injury. In some embodiments, the regenerative cells
are administered
serially over a period of time (e.g., wherein the subject can be administered
regenerative cells
in a single or in a plurality of doses each time). For example, in some
embodiments, the
regenerative cells described herein can be administered every 12 hours, every
day, every 2, 3,
4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 days, every month, or more. The frequency
of treatment
may also vary. The subject can be treated one or more times per day (e.g.,
once, twice, three,
four or more times) or every so-many hours (e.g., about every 2, 4, 6, 8, 12,
or 24 hours).
The time course of treatment may be of varying duration, for example, for two,
three, four,
five, six, seven, eight, nine, ten or more days. For example, the treatment
can be twice a day
for three days, twice a day for seven days, twice a day for ten days. While
our expectation is
that the treatment will continue as the patient's tissues go through a healing
and/or remodeling
process, treatment cycles can be repeated at intervals. For example treatment
can be repeated
weekly, bimonthly or monthly, and the periods of treatment can be separated by
periods in
which no treatment is given. The treatment can be a single treatment or can
last as long as the
life span of the subject (e.g., many years).
-51-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
In some embodiments, the methods disclosed herein include debriding the burned
area,
prior to the administration of the compositions disclosed herein. For example,
in some
embodiments, the methods include a step of removing some, or all, necrotic
tissue present as a
result of the burn, prior to administration of the compositions disclosed
herein. In some
embodiments, the burned area is debrided using surgical or mechanical means.
In some
embodiments, the burned area is debrided using ultrasonic means, e.g., as
described in U.S.
Patent No. 80705503, and the like. In some embodiments, the burned areas is
debrided using
pulsing CO2 lasers are used to debride burn wounds by ablating necrotic
tissue, e.g., as
described in European Patent No. EP0933096 B 1 . Various other methods and
apparatuses
useful for debriding the burned area useful in the embodiments disclosed
herein include, but
are not limited to, those described in U.S. Patent Application Publication
No's. 20130261534,
20130245386, 20130079800, 20130045196, 20100292689, 20100094265, 20090010869,
20070239078, 20040120989, 20040092920, 20030125783, and the like.
In some embodiments, some, or all of the burned or non-viable tissue, e.g., in
the zone
of coagulation, is debrided prior to administration of the regenerative cells
disclosed herein.
In some embodiments, the regenerative cells disclosed herein can be
administered both before
and following debridement of some or all of the burned or non-viable tissue.
Accordingly, in some embodiments, the regenerative cells as disclosed herein
can be
administered immediately following debridement of some or all of the burned or
non-viable
tissue. In some methods, the regenerative cells disclosed herein can be
administered 30 min,
40 min, 50 min, 1 h, 2h, 3h, 4 h, 5 h, 6 h, 7 h, 8 h, 9 h, 10 h, 11 h, 12 h,
24 h, 36 h, 48 h, 60 h,
1 week, 2 weeks, or longer, following the burn injury.
Accordingly, in some embodiments, the regenerative cells as disclosed herein
can be
administered immediately prior to debridement of some or all of the burned or
non-viable
tissue. In some methods, the regenerative cells disclosed herein can be
administered 30 min,
40 min, 50 min, 1 h, 2h, 3h, 4 h, 5 h, 6 h, 7 h, 8 h, 9 h, 10 h, 11 h, 12 h,
24 h, 36 h, 48 h, 60 h,
1 week, 2 weeks, or longer, before debriding some or all of the burned or non-
viable tissue
As disclosed herein, the regenerative cells can be provided to the subject, or
applied
directly to the damaged tissue, or in proximity to the damaged tissue, without
further
processing or following additional procedures to further purify, modify,
stimulate, or
-52-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
otherwise change the cells after isolation from the tissue of origin. For
example, the cells
obtained from a patient may be provided back to said patient without culturing
the cells before
administration. In several embodiments, the collection and processing of
adipose tissue, as
well as, administration of the regenerative cells is performed at a patient's
bedside. In a
preferred embodiment the regenerative cells are extracted from the tissue of
the person into
whom they are to be implanted, thereby reducing potential complications
associated with
antigenic and/or immunogenic responses to the transplant. However, the use of
cells
extracted from or derived from another individual is also contemplated.
EXAMPLES
The following examples are provided to demonstrate particular situations and
settings
in which this technology may be applied and are not intended to restrict the
scope of the
invention and the claims included in this disclosure.
Example I ¨ Viability and Therapeutic Activity of Adipose-Derived Regenerative
Cells Isolated from Subjects with Radiation Injury
The experiments described in this example were performed to assess and
validate a
novel model system useful in studying therapeutics for concomitant radiation
injury and
thermal burn. The experiments also were performed to assess the safety and
efficacy of
freshly isolated adipose-derived regenerative cells, delivered subdermally or
intravenously, in
the treatment of thermal burn in irradiated subjects.
Model System for Radiation Injury and Concomitant Thermal Burn
Pig physiology and skin has been found to be significantly more similar to
humans than
small mammals. Vardaxis et al., (1997) J Anat 190:601-11. The Gottingen
Minipig strain was
specifically chosen for this study because of the considerably greater ease of
handling and
convenience of working with animals that are approximately 10-20kg at maturity
rather than
the >100kg of mature Yorkshire farm swine.
No well-established model evaluating wound healing in the context of non-
lethal
myelosuppressive total body irradiation exists. Therefore, the experiments
described herein
describe the development of a novel model system useful for evaluating
therapies for
-53-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
concomitant radiation injury and thermal burn. Using the model system
developed herein, the
ability of adipose-derived regenerative cells to improve healing in animals
subjected to full
thickness thermal burn injury with concomitant sub-lethal, myelosuppressive
total body
irradiation in the presence or absence of skin graft was assessed.
To assess healing, two major efficacy histological endpoints (contraction and
epithelialization) were evaluated during the course of the study by performing
planimetry
supplemented with a series of biopsies in the wound bed at specified time
points following
injury. Because the process of collecting biopsy samples induces a new injury,
once-sampled
wounds were censored from subsequent analysis. As such, animals were divided
into two
major groups; Group 1 in which wounds were biopsied at early, medium and late
time points
with no skin graft and Group 2 in which the wounds were biopsied only after
application of a
skin graft. The effect of treatment with compositions comprising adipose-
derived regenerative
cells was evaluated by either comparing data within each Group (1 and 2) or in
between the
control groups to the individual cell treatment groups.
Besides the injury to skin, thermal burn injury may also be associated with a
systemic
inflammatory response that can lead to a lethal multi-organ failure. As such,
the experiments
described herein include treatment by local administration of compositions
comprising
adipose-derived regenerative cells as well as intravenous infusion of
compositions comprising
adipose-derived regenerative cells (the latter representing a potential route
of administration
that may provide a more encompassing systemic effect). Furthermore, it is
possible that, in
addition to potential effects on systemic inflammatory markers, adipose-
derived regenerative
cells could also have the potential to positively impact the course of
radiation-induced
neutropenia and thrombocytopenia. In the studies described herein, the safety
and efficacy of a
systemic delivery route (intravenous) was evaluated.
Eighteen animals were randomly assigned to one of the groups set forth in
Table 1,
below:
TABLE 1: EXPERIMENTAL GROUPS AND SUBGROUPS
___________________ 1
Treatment Delivery
Number of Wound Biopsies (days
Group Subgroup
(3 days post Route Animals post-
injury)
-54-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
escharectomy)
la Vehicle only Sub-dermal I 3 10, 17, 23 and 33
1
lb ADRC s Sub-dermal 3 10, 17, 23 and 33
(no STSG) r___ _________________________
c ADRC s Intravenous 6 10, 17, 23 and 33
1-4 4-
2 2a Vehicle only 1- Sub-dermal + 3
17 and 27
(with
2b ADRC s Sub-dermal 3 17 and 27
ST SG)
_______________________________________________________________________________
____ -J
Methods
a. Irradiation A Varian 600c LINAC, was used for animal irradiation. The
instrument was set to 100 MU (machine unit) for the dose to be administered
and with the
dose rate adjusted to 100 MU/min. The LINAC instrument setting needed to
deliver a specific
radiation dose (1.2Gy) The calculations were conducted for each animal using
each animal's
individual dimensions, according to manufacturer's instructions.
For the whole body-irradiation, animals were sedated with 1.1 mg/kg
acepromazine
(IM) and transported to the irradiation room within the LINAC facility. There,
the animal
was placed into a restraint device (v-shaped foam wedge or sling) with their
arms and legs
tucked as closely to the body as possible. A bolus tissue-equivalent material
("SuperFlab")
was wrapped around the animal's entire body. The animal was maintained on
anesthesia with
isoflurane (1-5%) via face mask during the irradiation. Animals received half
the target
radiation dose on both the right and left lateral surfaces. Diode detectors
were placed on each
side of the animal and used to measure the sum of entrance and exit radiation
doses for animal
exposure. Post irradiation, isoflurane was removed, and the animal was
returned to its
holding cage for recovery until it was returned to the housing room.
b. Thermal Burn. A printed wound template sheet was use to ensure that 6 wound
sites in the back of each animal, 3 along each side of the spine, were
correctly placed on each
animal. Each wound was 3 cm apart and located 4 cm from the spine of the
minipig. The
template was directly placed onto the pig skin and from cranial to caudal the
wound sites were
labeled Ll, L2, L3 for wounds on the left side and R1, R2, R3 for wounds on
the right side.
-55-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
A customized burn device (see, U.S. Provisional patent application no.
61/979461)
engineered to control the pressure applied on the animal skin during burn
creation, was used
to induce full thickness burns. At day of injury, animals were cleaned and the
dorsal surface of
each Minipig was clipped with an electric shaver. The skin was cleaned with
chlorhexidine and
prepared with DuraPrep (Betadine/alcohol) prior to injury. Preceding the burn
induction, the
animals were anesthetized by an intramuscular injection of 10-15mg/kg ketamine
with 2mg/kg
xylazine under 3-5% isoflurane via face mask.
For the burn induction, the brass block was heated to approximately 180-200 C,
with
the temperature being verified by a laser thermometer. For this study, the
burn device was
calibrated to apply a pressure of 0.4kg/cm2 on the animal's body. Six (6)
thermal burns
located on the thoracic paravertebral region where the skin surface is flat
and large enough to
ensure complete contact with the burn device were created using the wound
template sheet (3
wounds along each side of the spine). After induction of the burns, the entire
wound area was
bandaged as described in the approved study protocol to protect the burns from
any further
self inflicted injuries and from the environment.
A fentanyl patch (25 i..t.g) placed behind the ear on Day 1 for pain
management. The
patch was changed every other day through Day 11, at which point the study
veterinarian
determined that the patches could be safely removed from the protocol.
To protect the wounds from outside contamination and infections, a multi-layer
dressing was used as follows:
= Layer 1 (placed directly on the burn site) ¨ triple antibiotic ointment
(Bacitracin, Neomycin, and Polymyxine B Sulfate)
= Layer 2 ¨ Tegaderm
= Layer 3 ¨ Ioban2, antimicrobial drapes with an iodophor-impregnated
adhesive
= Layer 4 ¨ the animal was covered with a stocking hose-style shirt
= Layer 5 ¨ the animal wore a Lomir jacket to hold on all the dressings
c. Isolation of adipose-derived regenerative cells. In order to harvest
adipose tissue
for processing, animals were anesthetized and a small incision (about 3
inches) was made near
the inguinal inlet. Approximately 10-25 g adipose tissue was collected
processed using a
-56-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
Cytori Celution cell processing device according to manufacturer's
instructions (Cytori
Therapeutics, Sand Diego, CA), in order to obtain a composition comprising
adipose-derived
regenerative cells. Total cell yield (nucleated cells) and percent viability
were determined as
described below.
d. Escharectomy. Escharectomies were performed on Day 3, approximately 2h
after
adipose tissue collection. Animals were anesthetized by and wound sites were
surgically
excised, down to the muscle layer. The average total size of the resulting
wound was
approximately 14cm2.
To protect the wounds from outside contamination and infection, a multi-layer
dressing was used. Each dressing was changed every 7 days in order to minimize
infection
development throughout the course of the study.
The first dressing layer, placed onto the wound site consisted of silver-
impregnated
soft silicone foam dressing contain (Mepilex-Ag; Molnlycke health Care AB,
Goteborg,
Sweden). A second dressing layer of lobanTM (antimicrobial incise drape with
an iodophor
impregnated adhesive) was applied over the Mepilex to seal off the wound
fields. The third
layer of coverage consisted of a cotton elastic bandage wrap. Finally, a Lomir
jacket was
placed to hold all the dressings.
e. Treatment. On Day 3 post- thermal wound induction and post escharectomy,
compositions comprising freshly isolated adipose-derived regenerative cells
suspended in
Lactated Ringer's solution were injected radially and circumferentially into
the dermal tissue
surrounding the wound (range: 10-16 injections of 0.2 mL/ wound perimeter
region) as well
as directly into the superficial fascia (range: 5-9 injections of 0.2 mL). For
local delivery,
adipose-derived regenerative cells were locally administered at a dose of 0.23-
0.32x106
regenerative cells/cm2 into the excised wound, as illustrated in Figure 2.
For intravenous delivery, freshly isolated adipose-derived regenerative cells
suspended
in Lactated Ringers were administered intravenously in a total volume of 5 mL.
Cell
injections were performed through the ear vein at a rate of 1 mL/min with a
target viable cell
dose of 0.78-3.3 x 106 regenerative cells/kg body weight.
f Wound assessment. On Days 3, 10, 17, 23, 27, or 33 post-injury, standardized
digital photographs were taken of wounds from various study animals. Wounds
were assessed
-57-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
for two parameters: a) contraction (the total area not covered by unwounded
skin) and b)
epithelialization (the area within the wound showing evidence of neo-
epithelialization).
Figure 3 depicts the various areas of the wound as assessed in this study. The
green line
indicates wound boundary for assessment of contraction; the white line shows
boundary of re-
epithelialization; the yellow circle indicates the position of a biopsy.
g. Wound biopsy. 6 mm punch biopsies per wound were collected to coincide with
dressing changes on Days 10, 17, 23 and 33 post-injury (Group 1) or on Days 17
and 27 post-
injury (Subgroups 2a and 2b). If an animal had platelet counts below 50,000/
L, they
received only two (2) biopsies per wound site collected for that day. During
the course of this
study, 7 animals had low platelet counts just prior to a scheduled wound
biopsy and therefore
only 2 biopsies were collected. On the last time point, the entire wound was
collected. Once
collected, biopsies were immediately placed in 10% neutral buffered formalin
or immediately
flash frozen. The absence of a semi-rigid scaffold within the wound led to a
higher than
anticipated rate of wound contraction. Consequently, at day 17 and later times
it was not
practical to obtain all four biopsies inside the wounds as planned. Under
these circumstances,
only two biopsies were collected: one at the center and one at the periphery
of the wound.
Figure 4 illustrates the scheduling and processing (IHC or snap-freezing for
molecular
analysis) for wound biopsy (2 or 4 biopsy collection configuration). Each
biopsy was blindly
evaluated by 2-3 investigators.
h. Histological analysis. Formalin fixed biopsies were dehydrated, embedded in
paraffin, sectioned at 5- m thickness, and stained with Hematoxylin and Eosin
(H&E) by the
testing facility. Seven (7) unstained slides were also provided to Cytori
personnel for
assessment of differential expression of selected markers related to the wound
healing process
(Masson Trichrome, CD31 and Ki67 staining). After staining, slides were
digitally scanned
using the Aperio Scan Scope AT2 Turbo and visualized using the ImageScope
software.
i. Myelosuppression. Myelosuppression was monitored by regular blood cell
counts.
Prior to blood collection all animals were sedated with acepromazine (of 0.5-
1.1mg/Kg, IM).
Blood was collected via the saphenous vein and placed into vacutainers
containing K3EDTA
as anticoagulant.
-58-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
Blood draws were performed 5 days before irradiation injury, and on days 0, 3,
5, 8,
10, 12, 15, 20, 23, 25, 30, and 33 post-irradiation. Blood collected for
hematology was using
the ADVIATM 120 Hematology System (Bayer Corporation). Samples that exhibited
any
evidence of clotting were excluded from the analysis.
Results
a. The model system delivers a consistent, reproducible amount of radiation.
All
animals in this study received total body irradiation using a bilateral
scheme. Based on diode
measurements during irradiation, the actual absorbed doses ranged from 1.184
to 1.328Gy,
corresponding to a range of 98.7 to 110.7% of target doses (1.2Gy). Detailed
actual radiation
dose delivered to each animal is shown in Table 2. These data demonstrate that
consistent
full-body irradiation was delivered.
TABLE 2. INDIVIDUAL RADIATION ABSORBED DOSE.
Diode A Diode B Diode C Diode D
Average T
Animal ID Right Right Hip Left Left Hip 1 `)/0 of
Target
Dose (cGy) 1
Shoulder Shoulder I
5341010 121.6 120.3 128.8 122.9 123.4
102.80% .
....._,__ I ......7__ i
5344302 130.8 130.9 135.6 133.9 132.8 110.70%
- +--1- _
5348057 126.5 118.7 122.9 117.5 121.4 101.20%
5342130i ________________________
126.8 1 , 124.9 , 128.4 __ 125.4 i ---t-
126.4 , 105.30% -1
,
, ,
5345473 127.9, 126.3 , 129.2 127 127.6 , 106.30%
, ,
, , ,
I- 5343136 122 I 118.5 I 120.1 119.5 -I- 120.0
I /00.00%
. .
5344655 135.6 127.1 134.2 128.1 131.3 -I- 109.40%
-i-
5341265 132.1 ! 123.1 122.61 120.6 . 124.6 , 103.80%
,
t"-- 4-- ,
-1 ______________________________________________________________
5340536 118.7 , 118 , 119.5 117.5 118.4 , 98.70%
, ,
I ---1- :
5343063 124.5 i 121.3 , 125.7 i 120.3 123.0 , 102.50%
,
,
5348073i ___________________________________
124.8 i 120.4 , 122.6 , 119.5 121.8 i 101.50%
, ! ,
5346500 122.2 i 117.3 i 119.6 , 117.5 119.2
i 99.30% H
i
5343195 125.2 , 120.1 , 122.4 I 118.7 121.6 , 101.30%
,
5346593 124.7 1 119.3 122.3 ! 120.7 -1.-
-- 121.8 101.50%
-I-
126.2 +, 118.4 itt i, 121.5 , 119.4 121.4 [ /0/./0%
,
,
5343471 120.6 i 119.8 1 118.9 ! 117.6 119.2 i 99.40% ,
, ,
1 i _______ ,
-t-
5349592 125 1 119 , 120.2 i 118.7 120.7 ,
100.60% 1
-59-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
5346534 120.1 118.4 120.8 119.1 119.6 99.70%
Doses are expressed in centigray (cGy); 100cGy = 1Gy
b. The radiation dose administered in the model system was sufficient to
result in
myelosuppression and neutropenia. Figures 5A-5D illustrate the hematolgocial
data (absolute
white blood cells, absolute neutrophil, absolute platelets, and absolute
lymphocytes) for
animals in Group 1, i.e., the "no skin graft" group, including Group la
(control), Group lb
(local ADRC treatment), and Group lc (intravenous ADRC treatment). As shown in
Figures
5A-5D, there were no differences in blood cell counts between the control and
either
treatment group Platelet counts in all control animals remained generally
stable through Day
post-irradiation. Animals exhibited a nadir in platelet count of approximately
100,000/1.11_, or
lower between days 10 and 15 after radiation exposure (Figure 5C). Animals
generally
exhibited a slow return toward normal platelet levels after 15 days. Animals
exhibited a
decline in neutrophil counts to below 1,000/ L with a nadir between days 15
and 23 after
radiation exposure (Figure 5B). The average neutrophil counts were generally
stable through
approximately day 12 post-irradiation. All animals generally exhibited a slow
return toward
normal neutrophil counts after 25 days. By day 3 post-radiation, a substantial
reduction in the
number of circulating lymphocytes was observed in all animals. A gradual
return towards
baseline levels was observed from day 10 onward (Figure 5D).
Similar results were
observed in Group 2 animals that received skin grafts, i.e., Group 2a
(control) and Group 2b
(local regenerative cell treatment). As shown in Figures 6A-6D, there were no
differences in
blood cell counts between the control and treatment group. Further, the
animals exhibited the
same myleosuppression patterns as the Group 1 animals. Together, these data
demonstrate
that transient myelosuppression and lymphosuppression was consistently
achieved with the
selected target radiation dose.
c. Adipose-Derived Regenerative Cells Can Be Obtained From Subjects With
Radiation Injury To assess whether exposure to sublethal radiation affects
the viability and/or
therapeutic efficacy of adipose-derived regenerative cells, the colony forming
units (CFU), cell
composition, and cell differentiation assays were performed on the animal
subjects. Viability
and function of adipose-derived regenerative cells isolated from animals
subjected to 1.2Gy
total body irradiation were assessed.
-60-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
Adipose-derived regenerative cell yield and viability from animals in
subgroups lb, lc
and 2b were assessed. Overall, an average of 1.5 0.4 adipose-derived
regenerative cells were
obtained per gram of adipose tissue processed (range: 0.97-2.16 x 106 cells/g
tissue) with
average viability of 90.2 4.3% (range: 79.1%-94.4%). This number is within
the range of
nucleated cells obtained from tissue obtained from non-irradiated subjects
(comparative data
not shown).
Fluorescence cell sorting analysis for CD45, CD31, CD90 and CD146 was
performed
to determine the constitution of the cell populations isolated from adipose
tissue in the control
and irradiated animals. Table 3 shows the expression profiled used to define
the different cell
sub-populations within the cell populations isolated from adipose tissue.
TABLE 3
Antigen CD45 CD31 CD90 CD146
Cell subpopulation
Leukocytes +/- +/- +/-
Endothelial cells +/- +/-
Stromal Cells +/-
Smooth muscle related +/-
Exemplary data from the FACs analysis are presented in Table 4, below.
TABLE 4. RELATIVE FREQUENCY OF THE MAJOR CELL
SUBPOPULATIONS IN ADIPOSE-DERIVED CELLS DERVIED FROM ADIPOSE
TISSUE OF ANIMALS SUBJECT TO RADIATION INJURY
Stromal Endothelial Smooth Muscle-
Animal ID # CD45
Cells Cells related Cells
Group la 23.2 44.6 3.3 17.6
-r
Group la 22.3 24.3 10.2 29.4
Group 2a 1- 14.0 47.6 2.4 + 9.1
Group 2a I *
7 0 35.4 2.2 20.3
-61-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
Average -1D 16.6+7.-7
67
,S 38+10.5
19.1+8.4
The data above demonstrate that adipose-derived regenerative cells isoalted
from irradiated
animals were comprised of the same major populations as adipose-derived
regenerative cells
isolated from non-irradiated farm animals and human specimens as well
(comparative data not
shown).
The colony-forming unit - fibroblast (CFU-F) assay is a well-established assay
used to
quantify functional mesenchymal stem cells. See, Hicok, et al. (2011) Methods
Mol Biol.
702:87.The CFU-F frequency in the animals sampled was shown to be around 4.62
1.38%.
These data demonstrate that adipose-derived regenerative cells isolated from
irradiated
animals contain an adherent population of cells capable of extensive
proliferation in vitro and
in a frequency within the same frequency range as reported to human-derived
cells (1-6%).To
further analyze the functional capability of cells isolated from irradiated
versus non-irradiated
subjects, adipose-derived regenerative cells isolated from irradiated subjects
were assessed in
an in-vitro endothelial tube formation assay as previously described. See,
e.g., Donovan et al.
(2001) Angiogenesis 4:113-121. This assay measures the ability of endothelial
cells, given the
appropriate time and extracellular matrix support, to migrate and form
capillary-like structures
(a.k.a. tubes) in vitro. Adipose-derived regenerative cells were plated at a
density of 125600
cells/cm2 in standard cell culture plates in endothelial cell media (EGM-2,
Lonza, Basel,
Switzerland). Culture media was changed bi-weekly media changes. After 14
days, cells
were allowed to air dry and then fixed using a 50:50 acetone:methanol
solution. Fixed cultures
were then stained using standard inummohistochemistry techniques and reagents.
Exemplary
photographs of the immunohistochemical experiments are shown in Figures 6A-6B.
To
verify the endothelial-related origin of the tube like structures observed in
the cell culture, the
cells were fixed and stained using antibodies against CD146 and CD31. Figure
6C and 6D
below, the tube-like structures shown in Figures 6A and 6B were found to grow
on top of a
confluent fibroblastic monolayer and expressed both CD146 and CD31, indicative
of
endothelial cells.
As another test of cell functionality, adipose-derived regenerative cell
populations
isolated from the animal subjects were assessed for their ability to
differentiate into adipocytes
using the methods described in [Zuk, et al. (2002) Mol. Biol. Cell 13:4279-
4295. Figures 7A
-62-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
and 7B show that adipose-derived regenerative cells isolated from irradiated
subjects are
capable of differentiation into adipocytes.
The foregoing data demonstrate that adipose-derived regenerative can be
isolated from
adipose tissue of subjects receiving a sub-lethal dose of irradiation in
amounts that are similar
to non-irradiated subjects. Further, the data demonstrate that the
constitution of the
regenerative cell populations isolated from adipose tissue of irradiated
subjects mirrors that of
the regenerative cell populations isolated from adipose tissue of non-
irradiated subjects.
Finally, the data demonstrate that the adipose-derived regenerative cells
isolated from
irradiated subjects exhibit the functional capabilities of cells isolated from
non-irradiated
subjects, e.g., CFU-F, capillary-like formation, and differentiation into
adipocytes.
e. Adipose-Derived Regenerative Cells Promote the Healing Process
Wound contraction refers to the movement of the edges of a wound towards the
center to
close it. This process precedes the maturation stage of healing, and generally
occurs between five
and 15 days after the original injury is sustained. One concern with wound
contraction is the
risk of developing a contracture. Ideally, the wound shouldn't tighten too
much, or it might
create heavy scarring that limits range of motion. This can be a particular
concern with full
thickness burn wounds over a large extent of the body. These injuries are so
large that as they
tighten, they may pull against the skin in the region. Patients may need to
use physical therapy
during healing to retain flexibility and keep the skin supple so it doesn't
tighten too much.
To determine the effect of local and intravenous delivery of compositions
comprising
adipose-derived regenerative cells on wound contraction, wounds were assessed
by planimetry
at the time of escharectomy (days after injury) and at day 120, 17, 23 and 33
after injury.
Wounds were assessed for two parameters: 1) contraction ¨ the total area not
covered by
unwounded skin; and 2) epithelialization ¨ the area within the wound showing
evidence of
neo-epithelialization.
Wound contraction was defined as the change in total wound area as a
percentage of
the area immediately following wound excision. Three pairs of full-thickness
thermal burn
wounds were applied to each animal. The wound contraction raw data were
modeled with a
linear-log relationship (time was log-transformed and then a linear regression
was fit to each
wound's data) using a hierarchical mixed-effects model. The data for Group 1
are shown in
-63-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
Figure 8. Importantly, the rate of wound contraction for animals treated with
either local
injection or intravenous injection of composition comprising adipose-derived
regenerative
cells was significantly lower than that observed in the control group. For
animals in Group 2,
no difference in the rate of contraction was observed in the control group and
the group
treated with compositions comprising adipose-derived regenerative cells (data
not shown). It
is recognized that scar contracture is the end result of the process of
contraction (Goel and
Shrivastava, Ind J Plast Surg 2010; 43(Suppl): S63¨S71. "Post-burn scars and
scar
contractures"). Hence, the ability of the regenerative cells to reduce
contraction in this study
is consistent with utility in the treatment, prevention, and/or reduction in
hypertrophic scarring
and/or contracture.
Histological analysis of biopsies collected at the center of the wound showed
an
increase of epithelial coverage in local and intravenously treated animals in
Group 1. See,
Figures 9A and 9B. Furthermore, histological examination showed that local
delivery of
compositions comprising adipose-derived regenerative cells enhanced epithelial
proliferation
at day 7 post-treatment (day 10 post-injury). See, Figures 9C and 9D. These
data
demonstrate that adipose-derived regenerative cells improved wound healing,
and further
suggest that local and intravenous delivery of regenerative cells may function
to improve
wound healing and promote epithelial activation by different mechanisms of
action, e.g., local
delivery of regenerative cells may enhance epithelial proliferation whereas
intravenous delivery
of regenerative cells may accelerate epithelial migration.
Collagen deposition in the later phage of the wound healing process
facilitates greater
tensile strength of the wound is a good parameter to evaluate the healing
process.
Accordingly, collagen deposition in wound biopsies collected at day 33 post-
injury was
determine using ImageScopeTM analysis software using tissue specimens stained
with
Trichrome Masson dye. The software algorithm uses a deconvolution method to
separate
different colors, so that quantification of individual stain is possible
without cross
contamination. The algorithm calculates the percentage of weak (1+), medium
(2+), and
strong (3+) collagen positive staining. As shown in Table 5, below, local
administration of
compositions comprising adipose-derived regenerative cells facilitated
collagen deposition
when compared to control.
-64-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
TABLE 5:
Average of Average of Average of
Percent Percent Percent Weak Average of
Strong Medium Positive Score (0-300)
Positive (+++) Positive (++) (+)
Group la-Control 3.25 2.05 9.32 2.91 32.77 2.14
61.15 13.3
Group lb¨Local
regenerative cell 8.34 4.63 11.89 1.35 31.57 3.77
80.38 12.7
delivery
Group lc ¨
intravenous
5.56 4.09 10.50 3.2 28.12 4.56
65.81 20.13
regenerative cell
delivery
In short, the data illustrate a statistically significant effect in some of
the efficacy
parameters for treatment of wounds, e.g., thermal burns, in the context of
radiation injury.
Specifically, treatment with compositions comprising adipose-derived
regenerative cells
showed a significant decrease in wound contraction and an increase in wound re-
epithelialization compared to animals receiving vehicle alone.
Example 2 ¨ Adipose-Derived Regenerative Cells Can Be Obtained From Eschar
Tissue
Standard treatment for full thickness burn injury involves excision of non-
viable tissue
(eschar) in a process referred to as escharectomy. In practice this involves
excision down to
tissue that exhibits punctate bleeding. Punctate bleeding is clear, visual
evidence that the
excision has reached a viable tissue bed. The excisional nature of
escharectomy thus creates
an additional opportunity to obtain adipose tissue from patients with full
thickness thermal
burns with essentially zero morbidity. For the majority of patients, the
escharectomy is
performed using a layered, tangential approach carefully preserving the viable
tissue
underneath. In cases in which this excision exposes underlying adipose tissue,
it is possible
-65-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
that adipose tissue can be obtained by simply continuing this excision and
excising viable
adipose tissue for processing. For patients where bleeding or surgical time
are of concern, the
eschar is generally excised en bloc down to the fascia in a more aggressive
escharectomy
process ¨ a process in which the sub dermal adipose tissue is frequently
excised along the
denatured burned tissue.
The experiments also demonstrate that regenerative cells can be isolated from
the
adipose obtained from tissue removed during escharectomy ("escharectomized
tissue"), and
further that these regenerative cell populations have the same characteristics
(viability,
constitution [e.g., type and frequency of various cell types], and efficacy)
as regenerative cell
populations isolated from liposuctioned adipose tissue obtained from healthy
volunteers. The
experiments described below were performed to evaluate in detail the freshly
isolated stromal
vascular cells obtained by enzymatic processing of adipose tissue obtained
from escharectomy,
and compare it to the population obtained by processing adipose tissue from
non-burned
individuals. Adipose-derived regenerative cell yield, viability, CFU-F
frequency, cellular
composition and differentiation function were analyzed.
Eschar samples from the Burn Center at the University of California, San Diego
were
transported to Cytori Therapeutics following informed patient consent. Each
sample included
tissue that in which an en bloc excision surgical approach was used to remove
the burned
tissue.
A sample of tissue biopsy from the center of the intact eschar was excised and
prepared for embedding on paraffin and subsequent histological evaluation
prior to dissecting
adipose from the specimen. Tissue sections were stained with hematoxylin-eosin
and/or with
Masson's trichrome following standard histological procedures for histological
evaluation
using these dyes. Eschar tissue-associated adipose was dissected from the
burned skin using
scissors and scalpels in a class II biological safety cabinet. Upon isolation,
the adipose tissue
was weighed and minced into approximately 1-3mm pieces comparable to those of
lipoaspirated adipose using either sterile sharp scissors and/or knifes. The
minced tissue
specimens were processed to prepare adipose-derived regenerative cells in the
Cytori
Celution cell processing device per manufacturer's instructions Nucleated
cell concentration
and viability of adipose-derived regenerative cells were assessed using a
-66-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
NUCLEOCOUNTER cell counting device (Chemometec A/S, Allerod, Denmark), per
manufacturer's instructions.
Fluorescence Activated Cell Sorting using fluorescently labeled antibodies
directed
against, CD31, CD34, CD45, CD90, and CD146 cell membrane proteins was
performed to
determine the identity of the various cell populations within the
heterogeneous population of
freshly isolated adipose-derived regenerative cells.
To assess the adipose-derived stem cell frequency in adipose-derived
regenerative cell
populations isolated from eschar adipose tissue, a CFU-F assay was performed
as described
above. Briefly, cells were seeded at a concentration of 1,000 cells per well
of a standard 6-
well culture plate in DME/F12 culture medium supplemented with 10% fetal
bovine serum
and antibiotic/antimycotic solution. The plates were incubated at 37 C in 5%
CO2 in a
humidified chamber, and the medium was changed once a week. After 12 to 14
days of
culture, the cells were fixed and stained using a standard hematologic dye
(May-Grunwald)
kit. The colonies and clusters were scored using a stereoscope. Six replicate
wells were
plated for each sample evaluated, and the mean of the middle four counts were
used to
determine average CFU-F frequency.
To assess the function of the adipose-derived regenerative cells isolated from
eschar
adipose tissue, the ability of the cells to differentiate into adipocytes was
assessed. Adipose-
derived regenerative cells (25,000 cells/cm2) were first cultured in standard
DME/F12 media
supplemented with 10% fetal bovine serum and antibiotic-antimycotic solution
at 37 C in 5%
CO2. At the first media change the non-adherent cells were removed and after
the remaining
adherent cells had expanded and reached between 70-90% confluence, the
standard growth
medium was replaced by adipocyte differentiation medium (Zenbio, Research
Triangle Park,
NC). Cells were maintained in the differentiation medium for 3 days and then
the media was
replaced with adipocyte maintenance medium. The adipocyte maintenance medium
was
changed every 3 days until mature adipocytes (lipid-containing cells) were
observed (around
7-12 days) After approximately 12 days in culture the cells were fixed in 10%
formalin and
stained with Oil Red 0 following standard procedures. Cells that had undergone
adipocytic
differentiation were evidenced by accumulation of intracellular lipid
visualized by red bright
stain.
-67-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
As another functional test of the adipose-derived regenerative cells isolated
from
eschar adipose, cells were assessed for their ability to differentiate into
capillary-like structures
in an angiogenesis assay. For the angiogenesis assays, adipose-derived
regenerative cells were
plated at a concentration of 25,000 cells/cm2 in endothelial cell media (EGM-
2, Lonza, Basel,
Switzerland) and incubated for 7-21 days at 37 C in 5% CO2. The medium was
changed
twice a week, and the cultures examined weekly for tube formation. After 21
days of culture
in angiogenic medium, the cells were fixed and the tubular structures were
stained with
antibodies directed to endothelial proteins (CD31, CD34, CD146, von
Wilebrand's Factor)
and leukocytic (CD45) markers by immunocytochemistry.
Results:
Histological analysis from eschar tissue showed damaged vessels in the dermis
and the
subdermal adipose tissue as evidenced by clear presence of vascular hemorrhage
in samples
collected from the center or periphery of the specimens. See, Figure10.
Adipose-derived regenerative cell yield and viability was determined for each
eschar
sample. The data are presented in Table 6, below.
TABLE 6: YIELD AND VIABILITY OF ADRC IN ESCHAR SAMPLES
Surface
Adipose Proces ADRC Yield
Area Total Grams
Sample weight s (cells/gram)
Viability
Specimen adipose adipose/cm2
(g) Method (x105)
(cm2)
El 0 192.5 Manual 2.09
92%
508 485.4 0.96
E1Y 157.9 Manual 1.65
90%
E2S 58 Manual 1.77
90%
185 126 0.68
E2D 68 Manual 5.20
92%
E3 1050 42.9 42.9 0.04 Manual 2.93
79%
E5 480 155.1 155.1 0.32 Manual 0.90
91%
E6 114 74.3 74.3 0.65 Manual 1.82
92%
E7M 327.1 Manual 3.00
94%
260 427.1 1.64
E7C 100 CT-X2 1.90
86%
E8 196 216.8 216.8 1.11 Manual 2.49
93%
E9 260 100 100 0.38 Manual 5.00
93%
-68-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
Average 0.72 0.51
2.61 1.37 90 4%
The average yield and viability of adipose-derived regenerative cells obtained
per gram
of eschar adipose tissue processed is similar to that for normal donor tissue
(comparative data
not shown).
Adipose-derived regenerative cell compositions were evaluated by flow
cytometry.
The major populations in adipose-derived regenerative cells isolated from
adipose tissue from
normal, healthy donors (stromal, endothelial cells, smooth-muscle related
cells, and
leukocytes) were defined by a panel of 4 antibodies: CD45, CD31, CD146, and
CD34.
Endothelial cells were defined as cells expressing both CD34 and CD31, but not
CD45;
stromal cells were defined as expressing CD34, but not CD31 or CD45;
leukocytes were
defined as cells expressing the antigen CD45, and smooth muscle-related cells
were defined as
expressing the antigen CD146, but not CD31 or CD45. Cell subpopulations in the
adipose-
derived regenerative cell preparations isolated from adipose obtained from
eschar tissue are
listed in Table 7, below. Importantly, the same major cell populations and
frequencies
observed in the regenerative cell populations obtained from adipose from
normal issue were
observed in the adipose-derived regenerative cells isolated from eschar tissue
In addition, the
same intrapopulation variabity seen in adipose-derived regenerative cells from
non injured
tissues were also observed in cells isolated from eschar tissue. For instance
most but not all, of
the CD34+ cells showed to express the marker CD90 and the population
CD34+/CD90
accounted for an average of 29.70 15.33% of regenerative cells (ranging from
10.10% to
53.40%). See, Figure 23.
-69-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
TABLE 7: MAJOR CELL POPULATIONS IN ADRCS FROM ESCHAR ADIPOSE
TISSUE
Smooth
Leukocyte (in Endothelial Stromal Muscle (in
Sample %) (in %) (in %) 0/0)
E1Y 15.70 15.70 59.20 4.20
E10 17.35 14.30 58.00 5.20
E2S 39.60 7.60 28.00 4.25
E2D 27.00 6.35 43.75 12.90
E3 48.30 12.60 27.15 3.45
E5 26.80 23.55 33.30 5.30
E6 29.75 15.40 34.55 11.45
E7M 46.90 13.10 22.70 5.10
E7C 41.20 13.20 21.60 11.80
E8 44.70 18.70 19.60 5.20
E9 43.00 14.00 27.90 5.80
A VG STD 34.57 1 1. 73 14.05 4.70 34.16 13.84 6.79 3.46
The CFU-F assay performs two functions; it both quantifies the number of
putative
stem cells within the population and it confirms their proliferative capacity.
In this assay,
colonies were defined as containing >50 cells and cell clusters defined as
having more than 4
but less than 50 cells. The average frequency of clusters observed was
approximately 1.71%
and the average number of colonies was around 1%, in the adipose-derived
regenerative cell
populations isolated from adipose tissue from escharectomy. The average
frequency of
colonies from eschar samples was within the range of that reported for adipose-
derived
regenerative cells isolated from normal donors.
Adipogenic capacity was assessed to analyze the functional capacity of the
adipose-
derived regenerative cell populations isolated from eschar tissue. Figure 11
shows Oil Red 0
staining of an exemplary eschar sample processed and tested as described
above. As seen in
Figure 11, an abundant high frequency formation of multilocular adipocytes in
was observed
in regenerative cell populations isolated from the adipose from eschar
samples. This
-70-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
demonstrates that the regenerative cells isolated from adipose from eschar
tissue retained the
capacity to differentiate into adipocytes.
Angiogenic capacity was also assessed as a measurement of functional capacity
of the
adipose-derived regenerative cell populations isolated from eschar tissue.
Donovan et al. have
described an assay for angiogenesis in which endothelial cells growing on a
feeder layer of
fibroblasts-like cells develop a complex network of CD3 lpositive tubes
reminiscent of a
nascent capillary bed. Previously, Cytori has found that plating normal donor
tissue-derived
adipose-derived regenerative cells in similar conditions in the absence of an
exogenous feeder
layer leads to formation of similar structures. Thus, human adipose-derived
regenerative cells
from eschar samples were cultured in tissue culture plates without the
addition of growth
factors or Matrigel to evaluate the angiogenic capacity of adipose-derived
regenerative cells
obtained from the adipose of eschar tissue. An exemplary data set is shown in
figures 12A ¨
12C. These data show that as with regenerative cell populations isolated from
adipose tissue
from unburned subjects, the regenerative cell populations obtained from
adipose derived from
eschar tissue contained cells that are able to migrate and form tubular
structures in the in vitro
angiogenic assay.
The foregoing experiments demonstrate that populations of adipose derived
regenerative cells can be obtained from adipose from eschar tissue. The
cellular composition
and viability of adipose-derived regenerative cells isolated from adipose
obtained from eschar
is similar to that observed in adipose-derived regenerative cells isolated
from healthy (i.e.,
non-burned) tissue. Finally, the adipose-derived regenerative cells isolated
from eschar tissue
retained the same functional capacities observed in adipose-derived
regenerative cells isolated
from healthy tissue. These data demonstrate that regenerative cells can be
obtained from
subcutaneous adipose tissue of patients with thermal burn injury. In this
particular study the
tissue was obtained by excisional means. Subcutaneous adipose tissue can also
be obtained by
aspiration (liposuction) and other means recognized in the art.
Example 3 ¨ Adipose Derived Regenerative Cells Applied with Scaffold Improves
Healing
-71-
CA 02963468 2017-03-31
WO 2016/054592
PCT/US2015/053856
This example demonstrates the utility of adipose-derived regenerative cells in
improving wound healing when applied with a scaffold. INTEGRA and TISSEEL
scaffolds have been used to facilitate wound healing, e.g., the healing of
deep partial thickness
and full thickness burns. The experiments described herein evaluate the
ability of regenerative
cells (specifically adipose-derived regenerative cells), to improve healing
parameters of
INTEGRA collagen-based dermal regeneration template wound matrix and TISSEEL
fibrin-based wound sealant in full thickness thermal burns.
Twenty four animals were randomly assigned to one of the groups set forth in
Table 1,
below:
TABLE 8: EXPERIMENTAL GROUPS AND SUBGROUPS
Group Test Article Control Article
-r
Adipose-Derived Regenerative
A, *D Cells (ADRCs) suspended in
Lactated Ringer's Solution
_____________ Lactated Ringer's Solution
ADRC loaded onto Integra Integra Wound Dressing
ADRCs mixed in TISSEEL TISSEEL/Fibrin Glue
The experimental process flow is outlined in Figure 13. Full thickness burns
were
induced on each animal, and adipose-derived regenerative cells were collected
from each
animal as described in Example 2, above, in the sections "thermal burn" and
"isolation of
adipose-derived regenerative cells." An average of 1.88 x106 + 0.66 x106 ADRCs
was
obtained per gram of processed adipose tissue (range: 0.95 x 106 - 2.4 x 106
cells/g tissue)
from Group A animals that received ADRC injection (n=4). The mean recovered
cell viability
from Group A ADRC preparations was 90.8 3.0% (range: 87.9% - 94.8%) Each
wound
site was surgically debrided by excising the burn site, along with an
approximate 2-mm
margin, to a full-thickness depth.
Immediately following escharectomy the wound beds of animals in Groups A and D
were treated sub-dermal/intrafascial injection with control article 1 (LR
vehicle) or test article
1 (adipose-derived regenerative cells suspended in LR). Control/test article
were administered
as multiple injections delivered radially and circumferentially into the
tissue surrounding the
-72-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
wound (10-16 injections of 0.2 mL each per wound bed) as well as directly into
the superficial
fascia (5-9 injections of 0.2 mL each per wound bed) in a pattern shown in
Figure 2.
For animals in Group B, within 1/2 hour of cell isolation, ADRCs were directly
loaded
onto the Integra matrix at a concentration of approximately 3 x 106 ADRCs in
500 1 per 10
cm2 of INTEGRA Matrix). The matrix loaded with ADRCs was then placed onto the
excised wound bed so that the side loaded with ADRCs was in direct contact
with the wound
bed.
The INTEGRA matrix loaded with ADRCs was placed onto the excised wound bed
so that the surface loaded with ADRCs was in direct contact with the wound
bed. After
matrix application, the polyethylene sheet was removed. The INTEGRA matrix
for each
wound was then was shaped, securely attached with staples onto the wound. The
silicone
layer was kept in place in the wound throughout the entire course of the
study. Specifically,
animals in Group B received an ADRC dose within the range of 0.25 x 106 cells
/cm2 25%
(i.e. 0.19x106 cells/cm2 - 0.31x106 cells/cm2). 20 x 25 cm Integra sheets were
cut in 6 pieces
of 10 x 8.3 cm (83 cm2). Using a 1000 IA pipette, ADRCs were evenly loaded
onto the
INTEGRA Matrix (2.5 x 105 25 % ADRC per cm2 of INTEGRA , (i.e. 20.8 x 106
25%
ADRCs in 1 mL per 83 cm2 of Integra). Cells were then allowed to soak into and
adhere to
the matrix for 5-10 minutes prior to application onto the wound site.
For animals in Group C, freshly isolated ADRCs were loaded into TISSEEL/Fibrin
Glue at a concentration of 2.5-3x106 ADRCs per 10 cm2 wound). All animals
received an
ADRC dose within the range of 0.25 x 106 cells/cm2 25% (i.e. 0.19 x106
cells/cm2 - 0.31
x106 cells/cm2). Freshly isolated ADRCs loaded into TISSEEL were applied at an
average
dose of 0.24 x 106 0.04 x 106 ADRC per cm2 (range: 0.17 x 106- 0.37 x
106/cm2. The mean
dose of ADRCs in TISSEEL was 0.24 x 106 0.04 x 106 ADRCs per cm2 of wound
area.
Control wounds received an equivalent volume of TIS SEEL with no cells added.
Following administration of test and control article to each animal, treated
sites were
bandaged with the following layers: Mepilex, Ioban, cotton wrapping and a
Lomir jacket.
Follow up measurements, biopsy collection, and blood collection occurred as
the animals
recovered over the next four weeks. Bandaging was changed once weekly (without
-73-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
reapplication of ADRCs or delivery scaffolds) and topical or systemic
antibiotics were applied
as needed.
For biopsy, multiple 6 mm punch biopsies per wound were collected to coincide
with
dressing changes on Days 7, 14, 21, and at in life study phase termination
(Day 28). Once a
wound had been used for a biopsy at any specific time point, it was not
biopsied for the
remainder of the study. Biopsies were taken from the center and the periphery
on Days 7, 14
and 21 of each wound as illustrated in Figure 3. Biopsies were collected and
were fixed in
10% neutral buffered formalin.
At necropsy on day 28, four biopsies were collected from the appropriate wound
(See
Table 6 and Figure 7). Three biopsies 11, 1 and 5 o'clock were fixed in 10%
NBF, and one
biopsy (7 o'clock position) was collected and snap-freezing for future study.
For wound contraction and re-eptithelialization measurements, wounds were
assessed
by planimetry, using the SILHOUETTE CONNECTTm digital imaging software (ARANZ
Medical, Christchurch, NZ). Wounds were assessed for two parameters: 1)
contraction --the
total area not covered by unwounded skin and 2) epithelialization¨the area
within the wound
showing evidence of neo-epithelialization.
For histological analyses, biopsied tissues were fixed in 10% Neutral-Buffered
Formalin (NBF), dehydrated, embedded in paraffin, sectioned at 3- to 5-[tm
thicknesses, and
stained with hematoxylin and eosin (H&E) and Masson Trichrome stain. Slides (2
sections
per biopsy or terminal sample) were qualitatively evaluated via light
microscopy by a board-
certified veterinary pathologist for assessment of tissue structure,
cellularity, and collagen
deposition. Additional histological analyses of H&E, Masson's trichrome,
and
immunohistochemical staining was performed. Slides (one section per biopsy or
terminal
sample) were qualitatively evaluated via light microscopy by 2 board-certified
veterinary
pathologists, by assessment of tissue structure, cellularity, and collagen
deposition.
For immunohistochemical analyses, paraffin sections of biopsied tissues were
deparaffinized and re-hydrated through alcohol to water. Each section was
subjected to an
antigen retrieval step using sodium citrate solution (pH6, Vector) prior
blocking and antibody
incubation.
-74-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
Collagen deposition in wound biopsies collected at day 28 post-injury was
determined
using ImageScope analysis software using tissue specimens stained with
Trichrome Masson.
The software algorithm makes use of a deconvolution method to separate
different colors, so
that quantification of individual stain is possible without cross
contamination. This algorithm
calculates the percentage of weak (1+), medium (2+), and strong (3+) collagen
positive
staining. Thus, a collagen deposition score was calculated by a simple formula
involving the
positive percentages (Score = lx[%weak] + 2x[%Medium] + 3x[%Strong]). In each
biopsy,
annotations were created in order to identify superficial and mid/deep region
of the
granulation tissue Epithelial thickness was evaluated by measuring the length
of the epithelial
layer from the stratum basale to the stratum corneum on Hematoxylin and Eosin
slides. A
total of three-five measurements were performed from the left to the right
border of the
wound scar tissue.
Results
a. ADRCs Improve Wound Closure: Planimetric assessment of Group D control
(LR) and test (ADRCs only) treated wounds found that wound closure rate
increased by an
average of 32% by day 14 post burn induction in animals that received sub-
dermal and fascial
injections of ADRCs. This is illustrated in Figure 14 which plots the
individual open wound
areas of control and test animals. The mean percentage of open wound area in
ADRC treated
animals is significantly reduced compared to control animals on Day 14
(p=0.0004 by
unpaired one-tailed T-test analysis). See, Figure 14. The increase in wound
closure rate
observed in Group D ADRC treated wounds was not due to increased rates of
contraction
since there was no significant difference in mean wound contraction (as
measured by
planimetry) between the control group (local LR injection) and the ADRC-
treated group over
the course of the study (data not shown). Planimetric quantification of the
wound area in
which re-epithelialization had occurred in Group D animals demonstrates an
increased rate of
epithelialization in the ADRC-treated wounds compared to LR-treated animals.
The mean
percentage of epithelialization was 30.3% + 14.9% (range: 7.9% - 53.6%) in
ADRC-treated
wounds versus 14.4% 9.5% (range: 0%-32.7%) LR-treated wounds respectively.
See,
Figure 15. This difference was statistically significant (p=0.0004, by
unpaired one tailed t-
test).
-75-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
To determine the kinetics of neovascularization in the wound granulation
tissue
immunohistochemical analysis of tissue sections was performed on biopsies
collected at day 7,
14 and 21 post-injury. Tissue samples from LR- and ADRCs-treated animals were
subjected
to CD31 (endothelial blood vessel marker) immunostaining to localize neo-
capillaries. Each
stained slide was digitally scanned and then ImageScope analysis software was
applied to
quantify microvessel density (number of blood vessels per mm2). In each
biopsy, annotations
were created in order to identify superficial and deep region of the
granulation tissue.
Wound tissues in biopsies collected at day 7 were noticeably heterogeneous.
Although
CD31 staining was performed, quantification was not feasible due to
inconsistent thickness
and total area of the granulation tissue in harvested biopsies. Microvessel
density (MVD) was
significantly increased (1.47-fold) in deep granulation tissue of animals
receiving local ADRC
treatment compared to LR-treated animals at day 14 (179.6+21.7 versus
121.8+13.3 vessels
per mm2, respectively; p=0.031). See, Figure 16A-16D. No significant
difference was
observed in superficial granulation tissue.
Epithelial thickness was investigated at day 28 in vehicle (Lactated Ringer's,
"LR")-
and ADRCs-treated animals in Group D. Mean epithelial thickness at the center
of the wound
on day 28 was higher in ADRC treated wounds compared to LR treated wounds.
See, Figure
17.
b. Regenerative cells improve INTEGRA healing Turning to the INTEGRA
dermal matrix, histological scoring by a pathologist blinded to treatment
showed accelerated
maturation of granulation tissue beneath the Integra when delivered loaded
with ADRCs
(Figure 18 A, B). As such, the thickness of this granulation tissue was also
assessed.
Histogenesis starts at the base of the matrix where new blood vessels enter.
Layer by layer
from base to silicone, a progressive vascularization allows the process to
occur at higher
levels of the matrix (Gottlied ME, Arimedica, 2005). Interestingly, at day 21,
the thickness of
the granulation tissue beneath the matrix was increased in animals treated
with ADRCs.
Figure 18 C. The majority of biopsies collected on Day 14 did not capture a
core of
INTEGRA within them, and therefore determination of the granulation tissue
thickness was
not performed for this time point.
-76-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
Slides stained with H&E and Masson's Trichrome were evaluated by an outside
veterinary pathologist (ANTECH Diagnostics). This individual was blinded as to
the
treatment applied. By external evaluation, at day 10 post-injury, all biopsies
collected from the
center of wounds treated with ADRCs, whether by local injection or by
intravenous delivery
exhibited moderate mixed suppurative and fibrinous inflammation predominating
in superficial
and mid dermis (score = 2.5). Conversely, control animals had moderate mixed
suppurative
and mononuclear inflammation predominating in superficial and mid dermis
(score = 2.5).
Quantification analysis showed that 100% of the biopsies collected from LR-
treated wounds
(control) exhibited mononuclear inflammation at day 10. In contrary, 100% of
treated wounds
(both local and iv injection) exhibited fibrinous. Interestingly, at day 17,
67% of the LR-
treated wound showed fibrinous inflammation, whereas treated-wounds still
exhibited
fibrinous inflammation. These data demonstrate that the adipose-derived
regenerative cells
modulate the inflammatory response.
lmmunohistological analysis of blood vessel formation was performed to
evaluate
whether the observed increased thickness of granulation tissue involved
accelerated tissue
vascularization. To this end, mean vessel density, mean vessel lumen area, and
total CD31
stained area were determined to assess the relative maturity of granulation
tissue vascularity.
Blood vessel density was measured by quantifying the number of CD31 positive
vessels within
the granulation tissue beneath the Integra sheet on experiment days 14 and 21
post-injury
central wound biopsies using ImagescopeTM (ARANZ Medical, Christchurch, NZ).
Blood
vessel density in the granulation tissue below the INTEGRA matrix was greater
in wounds
receiving INTEGRA matrix supplemented with ADRCs-compared to those covered by
INTEGRA matrix alone. This increase approached statistical significance at
day 14 (p=0.06)
and was statistically significant on day 21 (p=0.024). See, Figures 19A-B. The
mean vessel
lumen area at day 21 in the mid and deep dermis was larger in wounds treated
with
INTEGRA matrix loaded with ADRCs than in Integra loaded with LR Figures 19E-
F. This
difference approached statistical significance (p=0.063) The total CD31
stained area in the
mid and deep dermis was greater in wounds treated with Integra loaded with
ADRCs than in
INTEGRA matrix alone. Figures 19C-D. This difference approached
statistical
significance (p=0.069).
-77-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
Digital image analysis of biopsies stained with Masson Trichrome, Hematoxylin
&
Eosin, and CD31 (blood vessel marker) was performed using color deconvolution,
nuclear,
and vessel density algorithms, respectively (Aperio) to assess the relative
contribution of each
of the biological processes to matrix filling. Digital quantification revealed
that ADRCs loaded
onto INTEGRA matrix increased INTEGRA matrix cellularity at day 21 post-
injury.
Figure 20A-B. INTEGRA matrix filling and cellularity on study day 21 (n=3-4
animals per
group; 6 wounds per treatment). Qualitatively greater cellularity was observed
in ADRC
loaded Integra compared to INTEGRA alone. The observed filling effect (Figure
20A) is
statistically significant (p=0.026 by one-tailed T-test), as is the amount of
cells within the
inter-matrix volume (Figure 20B) with a higher level of cell nuclei found in
ADRC-loaded
INTEGRA (p=0.09 by one-tailed T-testA trend toward increased CD31 positive
vessel
density was observed in ADRC loaded INTEGRA matrix relative to INTEGRA
matrix
that received no cells (n=3-4 animals per group; 6 wounds treated) at day 21.
See, Figure
20C.
Overall, the data with INTEGRA matrix scaffold show that compositions
comprising
adipose-derived regenerative cells improve graft healing. Specifically,
regenerative cells
improve the vascularization, lumen size, and vessel density of INTEGRA
matrix.
Furthermore, the ADRC treated scaffolds exhibit increased cellularity. The
data showed
accelerated maturation of vessels based upon increased mean lumen size in ADRC-
treated
wounds, suggesting that ADRCs can favorably modulate vascular stability and
blood flow to
the new tissue. Indeed, the lumen of a blood vessel is essential for providing
blood to the site
of injury (Axnick J and Lammert E, Curr Optn. Hematol, 2012). Key dynamic
interactions
occur between endothelial cells and mural cells (for example, pericytes) to
affect vessel
remodeling, diameter, and vascular basement membrane matrix assembly, a
fundamental
process necessary for vessel maturation and stabilization. These processes are
critical to
control the development of the functional microcirculation. Our findings
suggest that ADRCs
loaded onto INTEGRA matrix may orchestrate the complex process of
neovascularization
by not only promoting angiogenesis but also blood vessel maturation.
-78-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
c. TISSEEL is an appropriate scaffold for adipose-derived regenerative cell
delivery
The next set of experiments demonstrate that compositions comprising
regenerative cells
beneficially enhance healing in the context of other scaffolds, such as
TISSEEL .
Histological assessment of biopsies collected at day 7 reveals the presence of
TISSEEL/Fibrin above the growing granulation tissue (data not shown).
Furthermore, in
ADRCs-treated animals, the presence of migrating cells was observed at the
interface
granulation tissue/TIS SEEL (data not shown).
As shown in Figure 21, supplementation of TISSEEL with adipose-derived
regenerative cells significantly enhanced the epithelial coverage of the
recipient site at day 21
post-injury. As shown in Figure 22, microvascular density was significantly
increased (1.72-
fold) within the superficial granulation tissue of animals receiving local
ADRC treatment
compared to LR-treated animals at day 14 (103.7+15.25 versus 6O.39,9 vessels
per mm2,
respectively; p=0.0325). At day 21, a trend of increased vascularization was
observed in
ADRCs-treated animals versus LR treatment, (85.8+13.7 versus 62.5+12.9 vessels
per mm2,
respectively) (Figure 22).
These data demonstrate that fibrin glue scaffolds such as TISSEEL are
appropriate
delivery vehicles for administration of adipose-derived regenerative cells to
wound sites, e.g.,
full thickness burn sites.
Example 4 ¨ Adipose-Derived Regenerative Cells Mitigate Burn Progression in
Pigs
This example illustrates the use of adipose-derived regenerative cells as
disclosed
herein for the prevention or mitigation of burn progression in an animal
model.
Animals are randomized to receive treatment with adipose-derived regenerative
cells
(approximately 1 x 105- 1 x 107 nucleated cells) or PBS alone (buffer control)
administered
via subcutaneous injection adjacent to the zone of coagulation approximately 1
hour after
injury.
Briefly, four comb burns are created on the back of each animal, using a brass
comb
preheated in an oven to 100 C, for 5 minutes. This brass comb produces four
distinctive
burns sites separated by three "interspaces" of unburned skin, which were to
undergo
progressive injury. (See, e.g., Singer, et al (2007) Acad. Emergency Med.
14:1125-1129.
-79-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
Two full-thickness excisional wounds per pig with the dimensions identical to
the comb burns
were included as controls.
Full-thickness biopsies from the interspaces 7 days after injury are performed
and
evaluated for evidence of necrosis after H&E staining. The percentages of
interspaces that
progress to necrosis are compared with chi-squared (x2) tests.
At the seventh day, the number of interspaces that processed to full thickness
necrosis
is significantly lower for the burns treated with adipose-derived regenerative
cells compared to
the control group, as determined by histologic analysis and macroscopic
evaluation at days 2,
5, and 7.
Treatment with compositions comprising adipose-derived regenerative cells
(e.g., a
concentrated population of adipose-derived cells including stem and precursor
cells as
disclosed herein), significantly reduces the progression of burn injury in a
pig comb burn
model.
Example 5¨ Adipose-Derived Regenerative Cells Mitigate Burn Progression in
Human
A subject presents with a mid partial-thickness thermal burn over 15% of the
subject's
total body surface area (TBSA). A unit of adipose tissue is obtained from the
subject, and
processed according to the methods disclosed in U.S. Patent No. 7390484,
whereby a
population of adipose-derived regenerative cells is obtained.
Within 24 hours of the burn insult, the subject is administered a composition
comprising approximately 1 x 105 ¨ 1 x 107 adipose-derived regenerative cells
via intravenous
injection.
The mid-partial thickness bum does not progress to a full thickness burn, and
the
surface area of the zone of coagulation decreases, and does not increase, over
time.
Example 6¨ Eschar Tissue-Derived Regenerative Cells Mitigate Burn Progression
in
Human
A subject presents with a full-thickness thermal burn over 10% of the
subject's total
body surface area (TBSA). Devitalized (necrotic and/or apoptotic) tissue of
the burn is
identified and the devitalized tissue is removed via escharectomy.
-80-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
The escharectomized tissue is mechanically disaggregated by mincing the
tissue. The
minced tissue is subsequently subjected to enzymatic digestion, to produce a
cell suspension.
The cell suspension is centrifuged, the resulting cell pellet is resuspended
in a physiologic
solution (e.g. Lactated Ringer's solution), and passed through a 100 [tm
filter, thereby
providing a concentrated population of regenerative cells. Approximately 1 x
105 ¨ 1 x 107
regenerative cells derived from escharectomized tissue is administered to the
subject via
subcutaneous injection around the site of escharectomy within 48 hours of the
initial burn
insult.
The surface area of the full thickness burn and the surface area of the zone
of
coagulation decreases, and does not increase, over time.
Example 7 ¨ Adipose-Derived Regenerative Cells Enhance Engraftment and Healing
ofA utografts
The following example demonstrates that adipose-derived regenerative cells
enhance
graft take.
Pigs are individually housed under standardized conditions with controlled
temperature, humidity and a 12-12 hour day-night light cycle, and are provided
free access to
water and standard mouse chow.
On day 0, the pigs are randomly divided into a "control" group, and a
"treatment"
group. Pigs are anesthetized using isoflorane inhalation anesthesia. The
dorsum of the pig is
shaved, and a circular area with a diameter of 20 mm is outlined on the dorsum
at the midline
An incision is made along the marking using a scalpel, and the outlined skin
is harvested as a
full-thickness graft by separating it from the deep dorsal muscular fascia
layer. In order to
simulate the removal of excess fat from undersurface of the harvested full-
thickness skin grafts
in clinical conditions, panniculus carnosus layer is removed from the
undersurface of the skin
graft. For the control group, the grafts are treated with 0. 5m1 of a
physiological saline
solution. For the treatment group, 1 x 106-8 x 106 regenerative cells obtained
according to
the methods disclosed herein are applied to the graft in a 5m1 volume. The
grafts are placed
back into its donor site by securing the edges with interrupted non-absorbable
sutures. The
pigs are then caged individually as an additional measure to minimize the
trauma to the
surgical site.
-81-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
On day 14 following surgery, the skin grafted areas are macroscopically
assessed by
using planimetry. Areas with healthy graft tissue and areas that have healed
by secondary
intention after graft failure are identified. Regions with hair and/or
follicles are considered to
be healthy graft tissue and areas with a smooth, whitish appearance without
hair or follicles
are considered to be areas that had healed by secondary intention due to full
thickness loss. In
order to calculate the size of the healthy regions and the regions healed by
secondary
intention, these areas are outlined on a transparent paper that was placed on
the skin-grafted
dorsum. The transparency paper is digitally scanned and the ratio of healthy
area to the entire
skin graft area was calculated by using computer software (Image Pro Plus,
Silver Spring,
MD) for each graft.
On day 14, after the macroscopic assessment of the skin grafts, the pigs are
euthanized. The skin grafted area is removed en bloc including the recipient
bed and fixed in
methanol-Carnoy's solution (methanol:choloroform: glacial acetic acid, 6:3:1).
Following this,
representative parts composed of healthy graft areas and secondary intention
healing are cut
out of the main specimens and 4-micron sections were obtained from each
specimen for
histopathological evaluation.
Standard hematoxylin-eosin staining is performed on representative sections
for
histopathological evaluation of epithelialization and granulation tissue
formation. Each of
these parameters was semi-quantitatively evaluated for each representative
slide under low
power (100x) light microscopy by the pathologist (C.Y.F.) blinded to the
source of
specimens, in a four-point scale scoring system (0: absent, 1: mild, 2:
moderate, 3: abundant).
Comparison of data obtained by planimetry for percentage of healthy graft
areas as well as the
data obtained by semi-quantitative scoring for epithelialization and
granulation tissue among
control and treated pigs is performed.
The data show that treatment of the skin graft with adipose-derived
regenerative cells
(e.g., to create a fortified graft), enhances graft take. PIgs with fortified
grafts exhibit
enhanced granulation tissue formation and enhanced epithelialization scores
compared to the
control grafts.
Example 8 ¨ Regenerative Cells Prevent Hypertrophic Scar Formation
-82-
CA 02963468 2017-03-31
WO 2016/054592 PCT/US2015/053856
A subject presents with a deep partial thickness thermal burn between 5%-30%
of the
subject's total body surface area (TBSA). Devitalized (necrotic and/or
apoptotic) tissue of
the burn is identified and the devitalized tissue is removed via escharectomy,
in order to crease
a recipient site.
The escharectomized tissue is mechanically disaggregated by mincing the
tissue. The
minced tissue is subsequently subjected to enzymatic digestion, to produce a
cell suspension.
The cell suspension is centrifuged, the resulting cell pellet is resuspended
in a physiologic
solution (e.g. Lactated Ringer's solution), and passed through a 100 lam
filter, thereby
providing a concentrated population of regenerative cells. Approximately 1 x
105 ¨ 1 x 107
regenerative cells derived from escharectomized tissue is administered to the
recipient site
within 50 days after escharectomy.
The extent and severity of hypertrophic scarring in regions treated with
regenerative
cells as assessed by the Vancouver Scar Scale (VSS), is lower than that of
regions of
equivalent injury that were not treated with regenerative cells. For subjects
in which the entire
region at risk was treated with regenerative cells, the subject does not
develop hypertrophic
scars, as assessed by the Vancouver Scar Scale (VSS), to the same extent and
severity as
would be expected in similar patients not treated with regenerative cells.
-83-