Note: Descriptions are shown in the official language in which they were submitted.
83999029
SYSTEMS AND METHODS FOR HEART VALVE THERAPY
TECHNICAL FIELD
This document relates to prosthetic heart valves, such as prosthetic mitral
valves that can
be implanted using transcatheter techniques.
BACKGROUND
The long-term clinical effect of valve regurgitation is recognized as a
significant
contributor to cardiovascular related morbidity and mortality. Thus, for many
therapies intended
to treat the mitral valve, one primary goal is to significantly reduce or
eliminate regurgitation. By
eliminating the regurgitation at the mitral valve, the destructive volume
overload effects on the left
ventricle can be attenuated. The volume overload of mitral regurgitation (MR)
relates to the
excessive kinetic energy required during isotonic contraction to generate
overall stroke volume in
an attempt to maintain forward stroke volume and cardiac output. It also
relates to the pressure
potential energy dissipation of the leaking valve during thc most energy-
consuming portion of the
cardiac cycle, isovolumetric contraction. Additionally, therapies for MR
reduction can have the
effect of reducing the elevated pressures in the left atrium and pulmonary
vasculature reducing
pulmonary edema (congestion) and shortness of breath symptomatology. Such
therapies for MR
reduction may also have a positive effect on the filling profile of the left
ventricle (LV) and the
restrictive LV physiology that can result with MR. These pathophysiologic
issues indicate the
potential benefits of MR therapy, but also indicate the complexity of the
system and the need for a
therapy to focus beyond the MR level or grade.
1
Date Regue/Date Received 2022-04-04
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
Some therapies for treating MR may worsen other (non-MR) existing pathologic
conditions or create new pathologic conditions. One of the conditions to be
managed is
mitral stenosis or creation of an inflow gradient. That is, if a prosthetic
valve system is
used that does not allow for sufficient LV inflow without elevated filling
pressures, then
some benefits of MR reduction may be dissipated or lost. An additional
condition to be
managed is left ventricular outflow tract (LVOT) obstruction or creation of
high LVOT
pressure gradients. That is, if a prosthetic valve system is used that does
significantly
obstructs the LVOT, then some benefits of MR reduction may be dissipated or
lost. Also,
if the procedure results in damage to atrial tissue at surgery, it can
increase the likelihood
of the negative physiologic effect of atrial fibrillation. Further, some
prosthetic valve
systems may increase the risk of higher LV wall stress through an increase in
LV size (LV
geometry). Due to the integral relationship of the mitral valve with LV
geometry through
the papillary and chordal apparatus, LV wall stress levels can be directly
affected
resulting in alterations of LV filling and contraction mechanics. Accordingly,
in some
circumstances, a prosthetic valve system that worsens the geometry of the LV
can counter
the benefits of MR reduction because of the alteration of contractile
physiology.
SUMMARY
This document describes prosthetic heart valves, such as prosthetic mitral
valves
that can be implanted using transcatheter techniques. For example, some
embodiments of
a transcatheter mitral valve delivery system and method described herein can
be deployed
to interface and anchor in cooperation with the native anatomical structures
of a mitral
valve. In addition, this document describes prosthetic heart valve systems
that interface
with native mitral valve structures to create a fluid seal, thereby minimizing
MR and
paravalvular leaks after implantation. Further, this document describes
prosthetic heart
valve systems and techniques that, in particular embodiments, are configured
to manage
blood flow through the left ventricular outflow tract (LVOT) and to thereby
reduce the
risk of full or partial blockages of the LVOT. In addition, some embodiments
of the
prosthetic heart valve systems and techniques described herein may be
configured to
reduce the risk of interference between the prosthetic valves and chordac
tendineae of the
2
83999029
native mitral valve leaflets, which can advantageously facilitate or preserve
the geometry of the
LV.
According to an aspect of the present disclosure, there is provided a mitral
valve
replacement system for a heart, comprising: an expandable anchor assembly
configured to implant
at a native mitral valve, the expandable anchor assembly including a first
expandable frame that is
adjustable from a delivery condition to an expanded condition; a first
delivery sheath device
having a distal end insertable into a left atrium and being configured to
express the anchor
assembly out from the distal end such that the anchor assembly expands within
the left atrium to
the expanded condition; a pusher instrument releasably attachable to the
expandable anchor frame
and being configured to longitudinally advance the anchor assembly within the
left atrium towards
an annulus of the native mitral valve while the anchor assembly is in the
expanded condition; and
an artificial valve assembly comprising a second expandable frame that is
slidably engaged with
an exterior of the pusher instrument and adjustable from a compressed
condition to a deployed
condition to selectively engage with the anchor assembly while the anchor
assembly is in the
.. expanded condition.
Particular embodiments described herein include a mitral valve replacement
system for a
heart. The system may include an expandable anchor assembly configured to
implant at a native
mitral valve, and the expandable anchor assembly may include a first
expandable frame that is
adjustable from a delivery condition to an expanded condition. The system may
also include a
first delivery sheath device having a distal end insertable into a left atrium
and being configured to
express the anchor assembly out from the distal end such that the anchor
assembly expands within
the left atrium to the expanded condition. Optionally, the system may further
include a pusher
instrument releasably attachable to the expandable anchor frame and being
configured to
longitudinally advance the anchor assembly within the left atrium towards an
annulus of the native
mitral valve while the anchor assembly is in the expanded condition. Also, the
system may
include an artificial valve assembly comprising a second expandable frame that
is adjustable from
a compressed condition to a deployed condition to selectively engage with the
anchor assembly
while the anchor assembly is in the expanded condition.
Some embodiments described herein include a method for deploying a prosthetic
mitral
valve system within a native mitral valve of a patient. The method may include
navigating a first
delivery sheath within the patient such that a distal end of the first
delivery sheath is positioned
within a left atrium. The method may also include expressing an anchor
assembly of the
3
Date Recue/Date Received 2022-12-28
83999029
prosthetic heart valve system from the distal end of the first delivery sheath
such that the anchor
assembly at least partially expands while located within the left atrium.
Further, the method may
include, after expressing the anchor assembly within the left atrium, moving
the anchor assembly
towards an annulus of the native mitral valve.
Various embodiments described herein include a prosthetic mitral valve system.
The
system may include a valve assembly, which may include a frame member defining
an outer
profile and an interior frame member space, and an occluder disposed within
the interior frame
member space. The occluder may have an open configuration and a closed
configuration. The
frame member comprises a proximal end frame portion and a distal end frame
portion. Optionally,
an outer periphery of the distal end frame portion may
3a
Date Recue/Date Received 2022-12-28
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
include a generally flat region and a generally round region, and at least
some portions of
the generally flat region may extend toward the interior frame member space.
Particular embodiments described herein include a method of using a prosthetic
mitral valve system. The method may include advancing a valve assembly of the
prosthetic mitral valve system toward an annulus of a native mitral valve.
Optionally, the
valve assembly may include a frame member defining an outer profile and an
interior
frame member space, and occluder disposed within the interior frame member
space.
The frame member may include a proximal end frame portion and a distal end
frame
portion. An outer periphery of the distal end frame portion may optionally
include a
.. generally flat region and a generally round region, and at least some
portions of the
generally flat region extend toward the interior frame member space. The
method may
also include anchoring the valve assembly at the native mitral valve such that
the
generally flat region is adjacent to an anterior native leaflet of the native
mitral valve.
Some embodiments described herein include a prosthetic mitral valve system
that
is implantable at a native mitral valve. The prosthetic mitral valve system
may include an
anchor assembly defining an interior anchor assembly space and longitudinal
axis. The
anchor assembly may include an expandable anchor frame including a hub and a
sub-
annular support arm extending from the hub. The sub-annular support aim may
extend to
an anchor foot having a surface configured for engagement with a sub-annular
gutter of
the native mitral valve. The system may further include a valve assembly that
includes
an expandable valve frame defining an outer profile and an interior frame
member space,
and an oecluder disposed within the interior frame member space. The valve
assembly
may be releasably engageable with the anchor assembly within the interior
anchor
assembly space. Optionally, a distance measured parallel to the longitudinal
axis from a
distal-most end of the anchor assembly to the surface is at least 14
millimeters.
Various embodiments described herein include a method of using a prosthetic
mitral valve system. The method may include advancing an anchor assembly of
the
prosthetic mitral valve system toward an annulus of a native mitral valve. The
anchor
assembly may an interior anchor assembly space and longitudinal axis, and the
anchor
assembly may include an expandable anchor frame including a hub and one or
more sub-
annular support arms extending from the hub. Each of the one or more sub-
annular
4
CA 02964935 2017-04-15
WO 2016/065158
PCT/US2015/056935
support arm may extend to an anchor foot configured to engage with a sub-
annular gutter
of the native mitral valve. The method may further include engaging the anchor
assembly of the prosthetic mitral valve system with tissue proximate the
native mitral
valve such that each anchor foot is engaged with the sub-annular gutter, and
(optionally)
such that the hub is positioned distal of the distal-most area of coaptation
between
anterior and post leaflets of the native mitral valve.
Particular embodiments described herein include a method of sealing between a
prosthetic mitral valve system and native leaflets of a mitral valve. The
method may
include anchoring an anchor assembly of the prosthetic mitral valve system
with tissue
proximate to an annulus of a native mitral valve. Optionally, the anchor
assembly defines
an interior anchor assembly space and longitudinal axis, and the anchor
assembly may
include an expandable anchor frame including a hub and one or more sub-annular
support
arms extending from the hub. Each of the one or more sub-annular support arm
may
extend to an anchor foot that engages with a sub-annular gutter of the native
mitral valve.
The method may further include delivering a valve assembly of the prosthetic
mitral
valve system to engage with the anchor assembly. Optionally, the valve
assembly may
include: an expandable valve frame defining an outer profile and an interior
frame
member space, a tissue layer disposed over at least a portion of the outer
profile, and an
occluder disposed within the interior frame member space. The tissue layer of
the valve
assembly can abut with native leaflets of the mitral valve while each anchor
foot of the
anchor assembly is engaged with the sub-annular gutter.
Some embodiments described herein include a prosthetic mitral valve system.
The system may include an anchor assembly comprising an expandable anchor
frame and
a set of sub-annular anchor feet configured to engage with a sub-annular
gutter of the
native mitral valve. The system may further include a valve assembly that
includes: an
expandable valve frame defining an outer profile and an interior frame member
space, a
tissue layer disposed over at least a portion of the outer profile, and an
occluder mounted
within the interior frame member space. Optionally, an outwardly facing
periphery of the
tissue layer along the valve assembly is positioned to abut native leaflets of
the mitral
valve when the set of anchor feet of the anchor assembly is engaged with the
sub-annular
gutter.
5
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
Various embodiments described herein include a method for deploying a
prosthetic mitral valve system within a native mitral valve of a patient. The
method may
comprise navigating a delivery sheath such that a distal end of the delivery
sheath is
positioned within a left atrium of the patient. Also, the method may include
expressing, in
the left atrium, an anchor assembly of the prosthetic mitral valve system. A
distal pusher
instrument may be releasably engaged with the anchor assembly. The method may
further include engaging the anchor assembly with the native mitral valve
while the distal
pusher instrument remains engaged with the anchor assembly. The method may
also
include expressing, in the left atrium, a valve assembly of the prosthetic
mitral valve
system. Optionally, the valve assembly may slidably engaged with an exterior
of the
distal pusher instrument. The method may further include moving the valve
assembly
into an interior space defined by the anchor assembly. The moving may
optionally
include sliding the valve assembly along the exterior of the distal pusher
catheter while
the distal pusher catheter remains engaged with to the anchor assembly. The
method may
also include, after moving the valve assembly, mounting the valve assembly
with the
anchor assembly. Further, the method may include, after mounting the valve
assembly,
decoupling the distal pusher instrument from the anchor assembly.
Particular embodiments described herein include an implantable medical device
delivery system. The system may include a first deflectable catheter defining
a first
lumen therethrough, and a distal end portion of the first deflectable catheter
may be
controllably laterally deflectable. The system may also include a first device
delivery
sheath slidably disposable within the first lumen, and the first device
delivery sheath may
define a second lumen therethrough. The system may further include a first
device
control sheath slidably disposable within the second lumen, and the first
device control
sheath may define a third lumen therethrough and one or more first device
control wire
lumens. The system may also include a second deflectable catheter slidably
disposable
within the third lumen, and the second deflectable catheter may define a
fourth lumen
therethrough. A distal end portion of the second deflectable catheter may be
controllably
laterally deflectable. The system may further include a device pusher catheter
slidably
disposable within the fourth lumen, and the device pusher catheter may define
a fifth
6
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
lumen therethrough. A distal end portion of the device pusher catheter may be
configured
to releasably couple with a first implantable medical device.
Some embodiments described herein include a method for deploying a prosthetic
mitral valve system within a native mitral valve of a patient. The method may
include
expanding an anchor assembly of the prosthetic heart valve system within a
left atrium,
while the anchor assembly is releasably secured to a first delivery catheter,
such that the
anchor assembly at least partially expands while located within the left
atrium. The
method may optionally include, after expressing the anchor assembly within the
left
atrium, panning or rotating the anchor assembly within the left atrium by
articulating a tip
portion of the first delivery catheter.
Various embodiments described herein include a method for deploying a
prosthetic mitral valve system within a native mitral valve of a patient. The
method may
include expressing a valve assembly of the prosthetic heart valve system
within a left
atrium, while the valve assembly is releasably secured to a valve delivery
catheter, such
that the valve assembly at least partially expands while located within the
left atrium.
The method may optionally include, after expressing the valve assembly within
the left
atrium, panning or rotating the valve assembly within the left atrium by
articulating a tip
portion of the valve delivery catheter.
Some or all of the embodiments described herein may provide one or more of the
following advantages. First, some embodiments of the prosthetic mitral valve
systems
provided herein can be used in a completely percutaneous/transcatheter mitral
replacement procedure that is safe, reliable, and repeatable by surgeons of a
variety of
different skill levels. For example, in some implementations the prosthetic
mitral valve
system can establish a reliable and consistent anchor/substrate to which the
valve/occluder structure subsequently engages. Thus, the prosthetic mitral
valve system
can be specifically designed to make use of the geometry/mechanics of the
native mitral
valve to create sufficient holding capability. In one particular aspect, the
anatomical
gutter found below a native mitral valve annulus can be utilized as a site for
anchoring
the prosthetic mitral valve system, yet the anchoring structure can be
deployed in a matter
that maintains native leaflet function of the mitral valve, thereby providing
the ability to
completely separate and stage the implantation of the components of the
prosthetic mitral
7
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
valve system. Accordingly, some embodiments of the prosthetic mitral valve
systems
described herein are configured to be implanted in a reliable, repeatable, and
simplified
procedure that is broadly applicable to a variety of patients and physicians,
while also
employing a significantly less invasive method.
Second, some embodiments of the prosthetic mitral valve systems described
herein facilitate effective long lasting MR reduction without creating
negative
physiologic consequences to the cardiopulmonary system (heart, lungs,
peripheral
vasculaturc) including stenosis, LV wall stress, and atrial fibrillation.
Also, the system
may provide a safe and durable anchoring effect at the native mitral valve to
provide an
effective mitral regurgitation therapy as well as providing structures that
provide sealing
benefits and avoid significant impairment of the chordal interface of the
native mitral
valve leaflets.
Third, in particular embodiments, the prosthetic mitral valve system can be
delivered to the native mitral valve using a technique in which an expandable
frame of
the anchor component is at least partially expanded in the left atrium prior
to reaching the
mitral valve location. As such, in addition to facilitating the delivery of
the anchor, the
heart surgeon or other user can visualize the expanded component (and its
orientation)
within the heart before it is advanced to the annulus of the mitral valve
(thereby
permitting the user the opportunity to laterally pivot (rotate, pan, re-
orient) the expanded
component prior to reaching the annulus).
Fourth, some embodiments of the prosthetic mitral valve systems described
herein
can be configured to partially extend into the left ventricle after
implantation, yet may
include a profile shape that is configured to reduce the likelihood of
obstructing blood
flow through the LVOT. Accordingly, even though some portions of the
prosthetic mitral
valve systems extend into the left atrium above the mitral valve annulus
(supra-annular)
and other portions extend into the left ventricle below the mitral valve
annulus (sub-
annular), the prosthetic mitral valve system is designed to account for the
natural LVOT
and thereby reduce the risk of full or partial blockages of the LVOT.
Fifth, in particular embodiments, the prosthetic mitral valve system can
include
two different expandable components (e.g., an anchor assembly and a valve
assembly)
that are separately delivered to the implantation site, and both components
can abut and
8
83999029
engage with native heart tissue at the mitral valve. For example, the first
component (e.g., the
anchor assembly) can be configured to engage with the heart tissue that is at
or proximate to the
annulus of the native mitral valve, and the second component (e.g., the valve
assembly) can be
configured to provide a seal interface with native valve leaflets of the
mitral valve.
The details of one or more embodiments of the invention are set forth in the
accompanying
drawings and the description below. Other features, objects, and advantages of
some
embodiments of the invention will be apparent from the description and
drawings.
DESCRIPTION OF DRAWINGS
FIG. 1 is a perspective view of a portion of a prosthetic mitral valve
deployment system in
a cross-sectional view of a native human heart, in accordance with some
embodiments.
FIG. 2 shows a perspective view of a prosthetic mitral valve anchor assembly
in the left
atrium of the heart after the anchor assembly has emerged from an anchor
delivery sheath of the
deployment system of FIG. 1
FIG. 3 shows a perspective view of the anchor assembly of FIG. 2 after being
rotated in
the left atrium so as to orient the anchor assembly generally perpendicular to
the native mitral
valve.
FIG. 4 shows a perspective view of the anchor assembly of FIG, 3 after being
partially
advanced through the native mitral valve so as to position projections of the
anchor assembly
below a sub-annular gutter of the native mitral valve.
FIG. 5 shows a perspective view of the anchor assembly in a similar
arrangement as shown
in FIG. 4, but in a commissural cross-sectional view of the heart (from the
left side of the heart).
FIG. 6 shows a perspective view of the anchor assembly of FIG. 5 after being
retracted so
as to position the projections of the anchor assembly in the sub-annular
gutter of the native mitral
valve.
FIG. 7 shows a perspective view of the anchor assembly of FIG. 6 after the
retraction of
some members of the deployment system.
9
Date Regue/Date Received 2022-04-04
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
FIG. 8 is a top view of a native mitral valve and depicts a gutter perimeter
of the
sub-annular gutter of FIG. 7 (without the anchor assembly).
FIG. 9 shows a perspective top view of an example anchor assembly of FIGS. 2-6
in accordance with some embodiments.
FIG. 10 shows a perspective view of the anchor assembly of FIG. 9 with a
covering material disposed on portions of the anchor frame.
FIG. 11A shows a perspective top view of the anchor assembly of FIG. 9
implanted within a native mitral valve (with the native mitral valve leaflets
in a closed
state), and FIG. 11B shows a corresponding anatomical top view of the anchor
assembly
of FIG. 11A.
FIG. 12A shows a perspective top view of the anchor assembly of FIG. 9
implanted within the native mitral valve of FIG. 11A (with the native mitral
valve leaflets
in an open state), and FIG. 12B shows a corresponding anatomical top view of
the anchor
assembly of FIG. 12A.
FIG. 13 shows a perspective view of the anchor assembly of FIG. 7 implanted
within the native mitral valve and a valve assembly delivery sheath extending
into the left
atrium.
FIG. 14 shows a perspective view of a valve assembly in the left atrium after
partial emergence from the valve assembly delivery sheath of FIG. 13. The
valve
assembly is configured in a first (partially expanded) arrangement.
FIG. 15 shows a perspective view of the valve assembly of FIG. 14 with the
valve
deployment system being manipulated in preparation for the installation of the
valve
assembly into the anchor assembly.
FIG. 16 shows a perspective view of the valve assembly of FIG. 15 (while still
in
the first (partially expanded) arrangement) being positioned within the anchor
assembly.
FIG. 17 shows a perspective view of the valve assembly of FIG. 16 the valve
assembly expanded within the anchor assembly and detached from the deployment
system.
FIG. 18 shows an anterior side view of a valve frame of a valve assembly of
FIG.
17, in accordance with some embodiments.
FIG. 19 shows a bottom view of the valve frame of FIG. 18.
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
FIG. 20 is an exploded posterior side view of an anchor assembly and valve
assembly of FIG. 17, in accordance with some embodiments.
FIG. 21 is a top view of an example prosthetic mitral valve system that
includes a
valve assembly engaged with an anchor assembly, in accordance with some
embodiments.
FIG. 22 is a bottom view of the example prosthetic mitral valve system of FIG.
21.
FIG. 23 shows a top view of the prosthetic mitral valve system of FIG. 21
implanted within a native mitral valve. The occluder portion of prosthetic
mitral valve
system is shown in a closed state.
FIG. 24 shows a top view of the prosthetic mitral valve system of FIG. 21
implanted within a native mitral valve. The occluder portion of the prosthetic
mitral
valve system is shown in an open state.
FIG. 25 is a lateral cross-sectional top view of a heart showing the mitral,
aortic,
tricuspid and pulmonary valves.
FIG. 26 is a schematic diagram of a cross-section of a native mitral valve
including the mitral valve annulus.
FIG. 27 is an anterior side view of a valve assembly, in accordance with some
embodiments. A sealing region of the anterior side of the valve assembly is
demarcated
on the valve assembly.
FIG. 28 is a posterior side view of a valve assembly, in accordance with some
embodiments. A sealing region of the posterior side of the valve assembly is
demarcated
on the valve assembly.
FIG. 29 is a lateral side view of a valve assembly, in accordance with some
embodiments. A sealing region of the lateral side of the valve assembly is
demarcated on
the valve assembly.
FIG. 30 is a schematic depiction of an anterior portion of a valve assembly in
relationship to the annulus of the native mitral valve.
FIG. 31 is a schematic depiction of a commissural region portion of a valve
assembly in relationship to the annulus of the native mitral valve.
11
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
FIG. 32 is a schematic depiction of a posterior portion of a valve assembly in
relationship to the annulus of the native mitral valve.
FIG. 33 is a cross-sectional view of the left side of a heart showing an
example
valve assembly in relationship to the annulus of the native mitral valve and
the annulus of
the aortic root.
FIG. 34 is a fluoroscopic image of a native mitral valve with an example
prosthetic valve therein, an aortic valve, and a left ventricular outflow
track of a heart.
The image also shows blood flowing from the left ventricle to the aorta
through the left
ventricular outflow track.
FIG. 35 is another fluoroscopic image of a native mitral valve with an example
prosthetic valve therein, an aortic valve, and a left ventricular outflow
track of a heart.
The image also shows blood flowing from the left ventricle to the aorta
through the left
ventricular outflow track.
FIG. 36 is a schematic depiction of the annulus of the native mitral valve and
the
annulus of the aortic root.
FIG. 37 is a commissural cross-sectional view of a heart showing an anchor
assembly of a prosthetic mitral valve engaged in the sub-annular gutter of the
native
mitral valve. Chordae tendineae in the left ventricle are also depicted.
FIG. 38 is a lateral cross-section of a left ventricle of a heart showing an
anchor
assembly of a prosthetic mitral valve engaged in the sub-annular gutter of the
native
mitral valve. Chordae tendineae in the left ventricle are also depicted.
FIG. 39 is a perspective view of an anchor assembly showing control wires that
are threaded through portions of the anchor assembly.
FIG. 40 is another perspective view of an anchor assembly showing control
wires
.. that are threaded through portions of the anchor assembly.
FIG. 41 is a side view of a valve assembly frame showing control wires that
are
threaded through portions of the valve assembly frame.
FIG. 42 is a schematic diagram of a threading pattern of a proximal control
wire
corresponding to the valve assembly frame embodiment of FIG. 41.
FIG. 43 is a schematic diagram of a threading pattern of a mid-body control
wire
corresponding to the valve assembly frame embodiment of FIG. 41.
12
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
Like reference symbols in the various drawings indicate like elements.
DETAILED DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
This disclosure describes embodiments of a prosthetic heart valve system, such
as
prosthetic mitral valve systems, and transcatheter systems and methods for
implanting
prosthetic heart valve systems. In some embodiments, the prosthetic mitral
valve system
can be deployed to interface and anchor in cooperation with the native
anatomical
structures of a mitral valve (and, optionally, in a manner that permits the
continued
natural function of the chordae tendineae of the native mitral valve leaflets
even after the
anchor component is deployed). As described herein, the prosthetic mitral
valve system
can be deployed in a manner that interfaces with native mitral valve
structures to create a
fluid seal, thereby minimizing MR and paravalvular leaks after implantation.
As
described in more detail below, FIGS. 1-17 and 39-43 describe a transcatheter
mitral
valve delivery system and method by which the prosthetic mitral valve system
can be
deployed to interface and anchor in cooperation with the anatomical structures
of a native
mitral valve. Also, in FIGS. 18-32, prosthetic mitral valve features are
described by
which the prosthetic valves interface with native mitral valve structures to
create a fluid
seal, thereby reducing the likelihood of MR and paravalvular leaks. In FIGS.
33-36,
prosthetic mitral valve features and techniques are described for managing
blood flow
through the left ventricular outflow tract (LVOT). In FIGS. 37-38, prosthetic
mitral valve
features and techniques are described for reducing the risk of interference
between the
prosthetic valves and chordae tendineae.
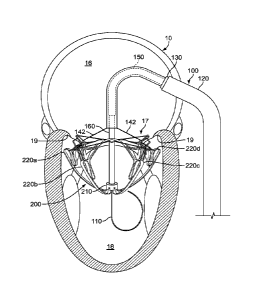
Referring to FIG. 1, an example transcatheter mitral valve delivery system 100
can be navigated through a patient's vasculature to obtain access to the
patient's heart 10.
The transcatheter delivery system 100 facilitates implantation of a prosthetic
mitral valve
in a beating heart 10 using a percutaneous, vessel cutdown, or minimally
invasive
technique (without open-chest surgery). In some implementations, the
transcatheter
delivery system 100 is used in conjunction with one or more imaging modalities
such as
x-ray fluoroscopy, echocardiography, magnetic resonance imaging, computed
tomography (CT), and the like.
13
CA 02964935 2017-04-15
WO 2016/065158
PCT/US2015/056935
The heart 10 (depicted in cross-section from a posterior perspective) includes
a
right atrium 12, a right ventricle 14, a left atrium 16, and a left ventricle
18. A tricuspid
valve 13 separates the right atrium 12 from the right ventricle 14. A mitral
valve 17
separates the left atrium 16 from the left ventricle 18. An atrial septum 15
separates the
right atrium 12 from the left atrium 16. An inferior vena cava 11 is confluent
with the
right atrium 12. It should be understood that this depiction of the heart 10
is somewhat
stylized. The same is true for FIGS. 2-4. FIGS. 1-4 provide general depictions
of the
approach to the mitral valve 17 that is used in some implementations. But, the
commissural cross-sectional views of FIG. 5 and thereafter more accurately
depict the
orientation of the prosthetic mitral valves in relation to the heart 10.
In the depicted embodiment, the delivery system 100 includes a guidewire 110,
a
primary deflectable catheter 120, and an anchor delivery sheath 130.
Additional
components of the delivery system 100 will be described further below. The
anchor
delivery sheath 130 is slidably (and rotationally) disposed within a lumen of
the primary
deflectable catheter 120. The guidewire 110 is slidably disposed within a
lumen of the
anchor delivery sheath 130. In this depiction, the anchor delivery sheath 130
has been
partially extended relative to the primary deflectable catheter 120, allowing
a flared
portion 132 to expand outward, as described further below.
In the depicted implementation, the guidewire 110 is installed into the heart
10
prior to the other components of the delivery system 100. In some embodiments,
the
guidewire 110 has a diameter of about 0.035 inches (about 0.89 mm). In some
embodiments, the guidcwirc 110 has a diameter in a range of about 0.032 inches
to about
0.038 inches (about 0.8 mm to about 0.97 mm). In some embodiments, the
guidcwirc
110 has a diameter smaller than 0.032 inches (about 0.80 mm) or larger than
0.038 inches
(about 0.97 mm). In some embodiments, the guidewire 110 is made of materials
such as,
but not limited to, nitinol, stainless steel, high-tensile-strength stainless
steel, and the like,
and combinations thereof. The guidewire 110 may include various tip designs
(e.g., J-tip,
straight tip, etc.), tapers, coatings, covers, radiopaque (RO) markers, and
other features.
In some implementations, the guidewire 110 is percutaneously inserted into a
femoral vein of the patient. The guidewire 110 is routed to the inferior vena
cava 11 and
into the right atrium 12. After creating an opening in the atrial septum 15
(e.g., a trans-
14
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
septal puncture of the fossa ovalis), the guidewire 110 is routed into the
left atrium 16.
Lastly, the guidewire 110 is routed through the mitral valve 17 and into the
left ventricle
18. In some implementations, the guidewire 110 can be installed into the heart
10 along
other anatomical pathways. The guidewire 110 thereafter serves as a rail over
which
other components of the delivery system 100 are passed.
In the depicted implementation, the primary deflectable catheter 120 is
installed
by pushing it over the guidewire 110. In some implementations, a dilator tip
is used in
conjunction with the primary deflectable catheter 120 as the primary
deflectable catheter
120 is advanced over the guidewire 110. Alternatively, a balloon catheter
could be used
as the initial dilation means. After the distal end of the primary deflectable
catheter 120
reaches the left atrium 16, the dilator tip can be withdrawn. In some
embodiments, the
distal end portion of the primary deflectable catheter 120 is steerable. Using
steering, the
distal end portion of the primary deflectable catheter 120 can be oriented as
desired in
order to navigate the patient's anatomy. For example, the primary deflectable
catheter
120 can be angled within the right atrium 12 to navigate the primary
deflectable catheter
120 from the inferior vena cava 11 to the atrial septum 15.
In some embodiments, the primary deflectable catheter 120 has an outer
diameter
of about 28 Fr (about 9.3 mm). In some embodiments, the primary deflectable
catheter
120 has an outer diameter in the range of about 26 Fr to about 34 Fr (about
8.7 mm to
about 11.3 mm). In some embodiments, the primary deflectable catheter 120 has
an outer
diameter in the range of about 20 Fr to about 28 Fr (about 6.7 mm to about 9.3
mm).
The primary deflectable catheter 120 can comprise a tubular polymeric or
metallic
material. For example, in some embodiments the primary deflectable catheter
120 can be
made from polymeric materials such as, but not limited to,
polytetrafluoroethylene
(PTFE), fluorinated ethylene propylene (FEP), HYTREL , nylon, PICOFLEX ,
PEBAX , TECOFLEXO, and the like, and combinations thereof. In alternative
embodiments, the primary deflectable catheter 120 can be made from metallic
materials
such as, but not limited to, nitinol, stainless steel, stainless steel alloys,
titanium, titanium
alloys, and the like, and combinations thereof. In some embodiments, the
primary
deflectable catheter 120 can be made from combinations of such polymeric and
metallic
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
materials (e.g., polymer layers with metal braid, coil reinforcement,
stiffening members,
and the like, and combinations thereof).
The example delivery system 100 also includes the anchor delivery sheath 130.
In some implementations, after the primary deflectable catheter 120 is
positioned with its
distal end in the left atrium 16, the anchor delivery sheath 130 is installed
into a lumen of
the primary deflectable catheter 120 (over the guidewire 110) and advanced
through the
primary deflectable catheter 120. As described further below, in some
embodiments the
anchor delivery sheath 130 is preloaded with a prosthetic valve anchor
assembly and
other components of the delivery system 100.
In some embodiments, the anchor delivery sheath 130 can be made from the
materials described above in reference to the primary deflectable catheter
120. In some
embodiments, the anchor delivery sheath 130 has an outer diameter in the range
of about
Fr to about 28 Fr (about 6.7 mm to about 9.3 mm). In some embodiments, the
anchor
delivery sheath 130 has an outer diameter in the range of about 14 Fr to about
24 Fr
15 (about 4.7 mm to about 8.0 mm).
In the depicted embodiment, the anchor delivery sheath 130 includes a flared
distal end portion 132. In some embodiments, no such flared distal end portion
132 is
included. The flared distal end portion 132 can collapse to a lower profile
when
constrained within the primary deflectable catheter 120. When the flared
distal end
20 portion 132 is expressed from the primary deflectable catheter 120, the
flared distal end
portion 132 can self-expand to the flared shape. In some embodiments, the
material of
the flared distal end portion 132 includes pleats or folds, may be a
continuous flared end
or may be separated into sections such as flower pedals, and may include one
or more
resilient elements that bias the flared distal end portion 132 to assume the
flared
configuration in the absence of restraining forces (such as from containment
within the
primary deflectable catheter 120). The flared distal end portion 132 can be
advantageous,
for example, for recapturing the anchor assembly within the lumen of the
anchor delivery
sheath 130 after the anchor assembly has been expressed from the flared distal
end
portion 132.
In some embodiments, the maximum outer diameter of the flared distal end
portion 132 is in a range of about 30 Fr to about 34 Fr (about 10.0 mm to
about 11.3
16
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
mm). In some embodiments, the maximum outer diameter of the flared distal end
portion
132 is in a range of about 32 Fr to about 44 Fr (about 10.7 mm to about 14.7
mm). In
some embodiments, the maximum outer diameter of the flared distal end portion
132 is in
a range of about 24 Fr to about 30 Fr (about 8.0 mm to about 10.0 mm). In some
embodiments, the maximum outer diameter of the flared distal end portion 132
is less
than about 24 Fr (about 8.0 mm) or greater than about 44 Fr (about 14.7 mm).
Referring to FIG. 2, additional components of the example delivery system 100
can include a proximal control sheath 140, a secondary deflectable catheter
150, and a
distal pusher catheter 160. The proximal control sheath 140 is slidably
disposed within a
lumen of the anchor delivery sheath 130. The secondary deflectable catheter
150 is
slidably disposed within a lumen of the proximal control sheath 140. The
distal pusher
catheter 160 is slidably disposed within a lumen of the secondary deflectable
catheter
150. These components of the delivery system 100 can be manipulated by a
clinician
operator to control the position and orientation of an anchor assembly 200.
The anchor
assembly 200 is slidably disposed over the guidewire 110.
In some implementations of delivery system 100, one or more of the proximal
control sheath 140, the secondary deflectable catheter 150, the distal pusher
catheter 160,
and the anchor assembly 200 have been loaded into the anchor delivery sheath
130 prior
to the advancement of the anchor delivery sheath 130 into the primary
deflectable
catheter 120 as shown in FIG. 1. That is, in some cases the proximal control
sheath 140,
the secondary deflectable catheter 150, the distal pusher catheter 160, and/or
the anchor
assembly 200 are already installed in the anchor delivery sheath 130 as the
anchor
delivery sheath 130 is distally advanced into the primary deflectable catheter
120 to attain
the arrangement shown in FIG. 1. In other implementations, one or more of the
proximal
control sheath 140, the secondary deflectable catheter 150, the distal pusher
catheter 160,
and the anchor assembly 200 are distally advanced into the anchor delivery
sheath 130
after the anchor delivery sheath 130 has been advanced into the primary
deflectable
catheter 120 to attain the arrangement shown in FIG. 1.
The distal pusher catheter 160 is releasably coupled with a hub 210 of the
anchor
assembly 200. A proximal end of the anchor assembly 200 is also releasably
coupled to
the proximal control sheath 140 by one or more control wires 142. While the
depicted
17
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
embodiment includes one control wire 142, in some embodiments two, three,
four, five,
or more than five control wires are included.
Referring to FIGS. 39 and 40, the control wire 142 is shown in an example
engagement pattern with the anchor assembly 200. In the depicted embodiment,
the
control wire 142 is threaded through a plurality of proximal portions of the
anchor
assembly 200. In the depicted embodiment, the control wire 142 is configured
in a lasso
arrangement. Accordingly, a tensioning of the control wire 142 will cause at
least the
proximal end of the anchor assembly 200 to contract. Conversely, a removal of
tension
from the control wire 142 will allow the anchor assembly 200 to expand. In
some
embodiments, the control wire 142 is threaded through eyelets that are
disposed on
various positions on the anchor assembly 200. In some embodiments, the control
wire
142 is threaded through attachment features that are disposed on various
positions on the
covering or frame of the anchor assembly 200. The control wire 142 can be
tensioned or
relaxed to arrive at a desired extent of expansion of the proximal end of the
anchor
assembly 200 (e.g., the atrial holding features 240a, 240b, 240c, and 240d,
and/or the
undulating supra-annular ring 250). Multiple control wires 142 could also be
used to
achieve asymmetric, controlled expansion of the anchor assembly 300.
Referring again to FIG. 2, the position of the anchor assembly 200 can be
controlled by manipulating the positions of the distal pusher catheter 160
and/or the
proximal control sheath 140. For example, in the depicted embodiment the
anchor
assembly 200 can be expressed out from the anchor delivery sheath 130 (as
shown in
FIG. 2) by moving the distal pusher catheter 160 and/or the proximal control
sheath 140
distally in relation to the anchor delivery sheath 130. In some
implementations, the
expression of the anchor assembly 200 is caused by proximally pulling back the
anchor
delivery sheath 130 while generally maintaining the positions of the distal
pusher catheter
160 and/or the proximal control sheath 140. In some implementations, the
expression of
the anchor assembly 200 is caused by a combination of proximally pulling back
the
anchor delivery sheath 130 while distally extending the positions of the
distal pusher
catheter 160 and/or the proximal control sheath 140.
As the anchor assembly 200 emerges from the confines of the anchor delivery
sheath 130, the anchor assembly 200 expands from a low-profile delivery
configuration
18
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
to a partially expanded configuration (as shown in FIG. 2). The extent of
expansion of
the anchor assembly 200 can be at least partially controlled by the relative
positioning of
the proximal control sheath 140 in relation to the distal pusher catheter 160.
For instance,
as the proximal control sheath 140 is moved proximally in relation to the
distal pusher
catheter 160, the anchor assembly 200 is axially elongated and radially
contracted.
Conversely, as the proximal control sheath 140 is moved distally in relation
to the distal
pusher catheter 160, the anchor assembly 200 is axially shortened and radially
expanded.
In some implementations, this control of the radial size of the anchor
assembly 200 is
used by a clinician during the process of deploying the anchor assembly 200
within the
native mitral valve 17, as described further below. As described further
below, the
control wire 142 can also be used to control some radial expansion of the
anchor
assembly 300 (without changing the relative distance of the proximal control
sheath 140
in relation to the distal pusher catheter 160).
It should be understood that the prosthetic mitral valves provided herein are
comprised of an anchor assembly 200 and a separable valve assembly (e.g.,
refer to
FIGS. 14-20). The anchor assembly 200 is deployed to an arrangement
interfacing
within the native mitral valve 17 prior to deployment of the valve assembly.
Said
differently, after implanting the anchor assembly 200 within the native mitral
valve 17,
the valve assembly can then be deployed within the anchor assembly 200 and
within the
native mitral valve 17 (as described further below). Therefore, it can be said
that the
prosthetic mitral valves provided herein are deployed using a staged
implantation
method. That is, the anchor assembly 200 is deployed in one stage, and the
valve
assembly is deployed in a subsequent stage. In some implementations, the
deployment of
the valve assembly takes place right after the deployment of the anchor
assembly 200
(e.g., during the same medical procedure). In some implementations, the
deployment of
the valve assembly takes place hours, days, weeks, or even months after the
deployment
of the anchor assembly 200 (e.g., during a subsequent medical procedure).
The staged implantation method of the prosthetic mitral valves provided herein
is
facilitated by the fact that when the anchor assembly 200 itself is implanted
within the
native mitral valve 17, the native mitral valve 17 continues to function
essentially as
before the implantation of the anchor assembly 200 without a significant
impact on
19
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
cardiovascular physiology. That is the case because, as described further
below, the
anchor assembly 200 interfaces and anchors within structural aspects of the
native mitral
valve 17 without substantially interfering with the leaflets or chordae
tendineae of the
native mitral valve 17.
Still referring to FIG. 2, in the depicted arrangement the distal end portion
of the
secondary deflectable catheter 150 is located at least partially internally
within the anchor
assembly 200. The secondary deflectable catheter 150 can be manipulated by a
clinician
operator to reversibly bend the distal end portion of the secondary
deflectable catheter
150. As the secondary deflectable catheter 150 is bent by the clinician, other
components
of the delivery system 100 may bend along with the secondary deflectable
catheter 150.
For example, one or more of the distal pusher 160 and the proximal control
sheath 140
may bend in response to the bending of the deflectable catheter 150. Because
the anchor
assembly 200 is coupled to the distal pusher 160 and the proximal control
sheath 140, the
anchor assembly 200 can, in turn, be rotated by bending the secondary
deflectable
catheter 150.
Referring to FIG. 3, as described above, the secondary deflectable catheter
150
can be articulated (also referred to as steered, deflected, bent, curved,
etc.) to pivot
laterally (pan, rotate, etc.) the anchor assembly 200 while the anchor
assembly 200 is
within the left atrium 16. Such rotation of the anchor assembly 200 is
advantageous, for
example, to orient the anchor assembly 200 in a desired relationship to the
native mitral
valve 17 in preparation for implanting the anchor assembly 200 within the
native mitral
valve 17. In some implementations, it is desirable to orient the anchor
assembly 200 so
that its longitudinal axis is generally perpendicular to the native mitral
valve 17. The
lateral pivoting of the partially or fully expanded anchor assembly 200 within
the atrium
16 may be advantageous versus having to pivot laterally the anchor assembly
200 while it
is still constrained within a delivery sheath, as the latter assembly is a
relatively large and
stiff catheter assembly.
In preparation for engaging the anchor assembly 200 with the native mitral
valve
17, the clinician operator may manipulate the radial size of the anchor frame
200 so that
the anchor frame 200 can be passed through the native mitral valve 17 without
damaging
the native mitral valve 17. For example, the clinician can move the proximal
control
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
sheath 140 proximally in relation to the distal pusher catheter 160 to
radially contract the
anchor assembly 200. With the anchor assembly 200 radially contracted, the
anchor
frame 200 can be safely passed through the native mitral valve 17 without
damaging the
native mitral valve 17.
Referring to FIG. 4, while the secondary deflectable catheter 150 is retained
in its
bent configuration as described in reference to FIG. 3, the distal pusher
catheter 160 and
the proximal control sheath 140 can be simultaneously advanced. Because the
distal
pusher catheter 160 is rcleasably coupled to the hub 210 of the anchor
assembly 200, and
because the proximal control sheath 140 is releasably coupled to the proximal
end of the
anchor assembly 200 via the one or more wires 142a and 142b, simultaneous
advancement of the distal pusher catheter 160 and the proximal control sheath
140 results
in advancement of the anchor assembly 200. The anchor assembly 200 is advanced
such
that the distal end of anchor assembly 200 is within the left ventricle 18
while the
proximal end of the anchor assembly 200 is within the left atrium 16. Hence,
some
portions of the anchor assembly 200 are on each side of the native mitral
valve 17.
In the depicted embodiment, the anchor assembly 200 includes four anchor feet:
a
left anterior foot 220a, a left posterior foot 220b, a right posterior foot
220c, and a right
anterior foot 220d. In some embodiments, fewer or more anchor feet may be
included
(e.g., two, three, five, six, or more than six). In some embodiments, the
anchor feet 220a,
220b, 220c, and 220d are portions of the anchor assembly 200 that are
configured for
contact with a sub-annular gutter 19 of the native mitral valve 17, without
penetrating
tissue of the native mitral valve 17. Accordingly, the anchor feet 220a, 220b,
220c, and
220d have atraumatic surfaces that are generally comparable to feet. However,
in some
embodiments one or more of the anchor feet 220a, 220b, 220c, and 220d are
configured
to penetrate tissue and may have anchor features such as barbs, coils, hooks,
and the like.
In the arrangement of FIG. 4, the anchor feet 220a, 220b, 220e, and 220d are
positioned below the sub-annular gutter 19. In this arrangement, the radial
size of the
anchor assembly 200 can be increased to align the anchor feet 220a, 220b,
220c, and
220d with the sub-annular gutter 19. For example, the clinician can move the
proximal
control sheath 140 distally in relation to the distal pusher catheter 160 to
radially expand
the anchor assembly 200 to align the anchor feet 220a, 220b, 220c, and 220d
with the
21
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
sub-annular gutter 19. Such alignment can be performed in preparation for
seating the
anchor feet 220a, 220b, 220c, and 220d within the sub-annular gutter 19.
Referring to FIG. 5, a commissural cross-sectional view of the heart 10
provides
another perspective of the anchor assembly 200 in the same arrangement in
relation to the
native mitral valve 17 as shown in FIG. 4. This commissural cross-sectional
view of the
heart 10 is a cross-sectional view taken through the mitral valve 17 along a
plane through
the left atrium 16 and left ventricle 18 that is parallel to the line that
intersects the two
commissures of the mitral valve 17 (as described further in reference to FIG.
8 below).
In the following FIGS. 5-7 and 13-17, the commissural cross-sectional view of
the heart
10 will be used to describe the delivery system 100 and methods for deploying
the
prosthetic mitral valves provided herein. The view in FIGS. 5-7 and 13-17 is
slightly
tilted so that better visualization of the anchor assembly 200 is provided.
The anchor feet 220a, 220b, 220c, and 220d are positioned below the sub-
annular
gutter 19. In this position, the anchor feet 220a, 220b, 220c, and 220d are
positioned
under the systolic and diastolic excursions of the leaflets of the native
mitral valve 17. In
this orientation, the anchor feet 220a, 220b, 220c, and 220d can be aligned
with the sub-
annular gutter 19 in preparation for seating the anchor feet 220a, 220b, 220c,
and 220d
within the sub-annular gutter 19.
Referring to FIG. 6, the distal pusher 160 and the proximal control sheath 140
can
be simultaneously retracted in relation to the secondary deflectable catheter
150 and the
primary deflectable catheter 120. As a result, the anchor feet 220a, 220b,
220e, and 220d
become seated in the sub-annular gutter 19. In this position, the anchor feet
220a, 220b,
220c, and 220d arc positioned under the systolic and diastolic excursions of
the leaflets of
the native mitral valve 17, and the other structures of the anchor assembly
200 do not
inhibit the movements of the leaflets. Therefore, with the anchor assembly 200
coupled
to the structures of the mitral valve 17 as described, the mitral valve 17 can
continue to
function as it did before the placement of the anchor assembly 200. In
addition, the
manner in which the anchor assembly 200 interfaces with the native mitral
valve 17 does
not result in deformation of the native mitral valve 17. Therefore, the native
mitral valve
17 can continue to function as it did before the placement of the anchor
assembly 200.
22
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
Referring to FIG. 7, with the anchor assembly 200 engaged within the native
mitral valve 17, components of the delivery system 100 can be withdrawn from
the
anchor assembly 200. For example, the control wire 142 can be detached from
the
proximal end of the anchor assembly 200. Thereafter, the proximal control
sheath 140
can be withdrawn. The secondary deflectable catheter 150 can also be
withdrawn. In
fact, if so desired, the proximal control sheath 140, the secondary
deflectable catheter
150, and the anchor delivery sheath 130 can be completely withdrawn from the
primary
deflectable catheter 120. In contrast, in some implementations the distal
pusher catheter
160 is advantageously left attached to the hub 210 of the anchor assembly 200.
As will
be described further below, in some implementations the distal pusher catheter
160 can be
used as a rail on which a valve assembly is deployed into the interior of the
anchor
assembly 200. However, in some implementations the anchor assembly 200 is
completely detached from the delivery system 100, and the delivery system 100
is
removed from the patient. After a period of hours, days, weeks, or months,
subsequent to
the deployment of the anchor assembly 200, a valve assembly can be installed
into the
anchor assembly 200 to complete the installation of the prosthetic mitral
valve.
Referring to FIGS. 8 and 9, the anatomy of the native mitral valve 17 includes
some consistent and predictable structural features across patients that can
be utilized for
engaging the anchor assembly 200 therewith. For example, the native mitral
valve 17
includes the aforementioned sub-annular gutter 19. In addition, the native
mitral valve 17
includes a D-shaped annulus 28, an anterolateral commissure 30a, a
posteromedial
commissurc 30b, a left fibrous frigone 134a, and a right fibrous trigonc 134b.
Further,
the native mitral valve 17 includes an anterior leaflet 20 and a three-part
posterior leaflet
22. The posterior leaflet 22 includes a lateral scallop 24a, a middle scallop
24b, and a
medial scallop 24c. The free edges of the posterior leaflet 22 and the
anterior leaflet 20
meet along a coaptation line 32.
The D-shaped annulus 28 defines the structure from which the anterior leaflet
20
and posterior leaflet 22 extend and articulate. The left and right fibrous
trigones 134a and
134b are located near the left and right ends of the anterior leaflet 20 and
generally
adjacent the lateral and medial scallops 24a and 24c of the posterior leaflet
22. The sub-
23
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
annular gutter 19 runs along the annulus 28 between the left and right fibrous
trigones
134a and 134b along the posterior leaflet 22.
The regions at or near the high collagen annular trigones 134a and 134b can
generally be relied upon to provide strong, stable anchoring locations. The
muscle tissue
in the regions at or near the trigones 134a and 134b also provides a good
tissue ingrowth
substrate for added stability and migration resistance of the anchor assembly
200.
Therefore, the regions at or near the trigones 134a and 134b define a left
anterior anchor
zone 34a and a right anterior anchor zone 34b respectively. The left anterior
anchor zone
34a and the right anterior anchor zone 34b provide advantageous target
locations for
placement of the left anterior foot 220a and the right anterior foot 220d
respectively.
The depicted embodiment of the anchor assembly 200 also includes the left
posterior foot 220b and the right posterior foot 220c. As previously
described, the left
posterior foot 220b and the right posterior foot 220c can also be
advantageously
positioned in the sub-annular gutter 19 in order to provide balanced and
atraumatic
coupling of the anchor assembly 200 to the native mitral valve 17. Therefore,
a left
posterior anchor zone 34b and a right anterior anchor zone 34c are defined in
the sub-
annular gutter 19. The left posterior anchor zone 34b and the right anterior
anchor zone
34c can receive the left posterior foot 220b and the right posterior foot 220c
respectively.
In some implementations, the locations of the left posterior anchor zone 34b
and the right
anterior anchor zone 34c may vary from the depicted locations while still
remaining
within the sub-annular gutter 19. It should be understood that the depicted
anchor
assembly 200 is merely one non-limiting example of the anchor assemblies
provided
within the scope of this disclosure.
In some embodiments, the anchor assembly 200 includes supra-annular structures
and sub-annular structures. For example, the sub-annular structures of the
anchor
assembly 200 include the aforementioned anchor feet 220a, 220b, 220c, and
220d, and
the hub 210. In some embodiments, as described above, the hub 210 functions as
a
connection structure for the delivery system 100 (e.g., refer to FIG. 2). In
addition, the
hub 210 can function as a stabilizing structural component from which a left
anterior sub-
annular support arm 230a, a left posterior sub-annular support arm 230b, a
right posterior
24
CA 02964935 2017-04-15
WO 2016/065158
PCT/US2015/056935
sub-annular support arm 230c, and a right anterior sub-annular support arm
230d extend
to the anchor feet 220a, 220b, 220c, and 220d respectively.
In some embodiments, such as the depicted embodiment, the supra-annular
structures of the anchor assembly 200 include: a left anterior atrial holding
feature 240a,
a left posterior atrial holding feature 240b, a right posterior atrial holding
feature 240c,
and a right anterior atrial holding feature 240d; an anterior anchor arch
250a, a left anchor
arch 250b, a posterior anchor arch 250c, and a right anchor arch 250d; and
connection
bridges 260. The anterior anchor arch 250a, left anchor arch 250b, posterior
anchor arch
250c, and right anchor arch 250d are joined with each other to form an
undulating supra-
.. annular ring 250 that acts as a supra-annular structural element for the
anchor assembly
200. As will be described further below, the supra-annular ring 250 also
defines an
opening to a space within the interior of the anchor assembly 200 that is
configured to
receive and engage with a valve assembly. The atrial holding features 240a,
240b, 240c,
and 240d are configured to contact the shelf-like supra-annular tissue surface
above the
mitral valve annulus, and to thereby stabilize the anchor assembly 200 in
supra-annular
areas that are generally opposite of the anchor feet 220a, 220b, 220c, and
220d
respectively.
In some embodiments, connection bridges 260 provide enhanced stability and
fatigue resistance from vertically oriented forces on a companion artificial
valve
assembly when the valve (not shown) is closed and blocking pressurized blood
during
systole. The anchor assembly 200 can also include one or more holes 226 in
frame
portions adjacent the feet, which arc additional control points for delivery
and retrieval of
the assembly, or could be used to secure a positional delivery frame.
In some embodiments, such as the depicted embodiment, the supra-annular
structures and sub-annular structures of the anchor assembly 200 are
interconnected by a
lateral anterior inter-annular connection 270a, a lateral posterior inter-
annular connection
270b, a medial posterior inter-annular connection 270c, and a medial anterior
inter-
annular connection 270d. For example, the lateral anterior inter-annular
connection 270a
connects the lateral anterior anchor foot 220a with the lateral anterior
atrial holding
feature 240a. In addition, the lateral anterior inter-annular connection 270a
connects the
lateral anterior anchor foot 220a with the anterior anchor arch 250a and the
left anchor
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
arch 250b. In the depicted embodiment, each of the other inter-annular
connections
270b, 270c, and 270d interconnect portions of the supra-annular structures and
sub-
annular structures in manners analogous to that of the lateral anterior inter-
annular
connection 270a. For example, the lateral anterior inter-annular connection
270b
connects the lateral anterior anchor foot 220b with the left anchor arch 250b
and the
posterior anchor arch 250c; the lateral anterior inter-annular connection 270c
connects
the lateral anterior anchor foot 220c with the posterior anchor arch 250c and
the right
anchor arch 250d; and the lateral anterior inter-annular connection 270d
connects the
lateral anterior anchor foot 220d with the right anchor arch 250d and the
anterior anchor
arch 250a.
In some embodiments, the elongate members of the anchor assembly 200 are
formed from a single piece of precursor material (e.g., sheet or tube) that is
cut,
expanded, and connected to the hub 210. For example, some embodiments are
fabricated
from a tube that is laser-cut (or machined, chemically etched, water-jet cut,
etc.) and then
expanded and heat-set into its final expanded size and shape. In some
embodiments, the
anchor assembly 200 is created compositely from multiple elongate members
(e.g., wires
or cut members) that are joined together with the hub 210 and each other to
form the
anchor assembly 200.
The elongate members of the anchor assembly 200 can be comprised of various
materials and combinations of materials. In some embodiments, nitinol (NiTi)
is used as
the material of the elongate members of the anchor assembly 200, but other
materials
such as stainless steel, L605 steel, polymers, MP35N steel, stainless steels,
titanium,
colbalt/chromium alloy, polymeric materials, Pyhnox, Elgiloy, or any other
appropriate
biocornpatible material, and combinations thereof can be used. The super-
elastic
properties of NiTi make it a particularly good candidate material for the
elongate
members of the anchor assembly 200 because, for example, NiTi can be heat-set
into a
desired shape. That is, NiTi can be heat-set so that the anchor assembly 200
tends to self-
expand into a desired shape when the anchor assembly 200 is unconstrained,
such as
when the anchor assembly 200 is deployed out from the anchor delivery sheath
130. A
anchor assembly 200 made of NiTi, for example, may have a spring nature that
allows the
anchor assembly 200 to be elastically collapsed or "crushed" to a low-profile
delivery
26
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
configuration and then to reconfigure to the expanded configuration as shown
in FIG. 9.
The anchor assembly 200 may be generally conformable, fatigue resistant, and
elastic
such that the anchor assembly 200 can conform to the topography of the
surrounding
tissue when the anchor assembly 200 is deployed in a native mitral valve of a
patient.
In some embodiments, the diameter or width/thickness of one or more of the
elongate members forming the anchor assembly 200 may be within a range of
about
0.008" to about 0.015" (about 0.20 mm to about 0.40 mm), or about 0.009" to
about
0.030" (about 0.23 mm to about 0.76 mm), or about 0.01" to about 0.06" (about
0.25 mm
to about 1.52 mm), or about 0.02" to about 0.10" (about 0.51 mm to about 2.54
mm), or
about 0.06" to about 0.20" (about 1.52 mm to about 5.08 mm). In some
embodiments,
the elongate members forming the anchor assembly 200 may have smaller or
larger
diameters or widths/thicknesses. In some embodiments, each of the elongate
members
forming the anchor assembly 200 has essentially the same diameter or
width/thickness.
In some embodiments, one or more of the elongate members forming the anchor
assembly 200 has a different diameter or width/thickness than one or more of
the other
elongate members of the anchor assembly 200. In some embodiments, one or more
portions of one or more of the elongate members forming the anchor assembly
200 may
be tapered, widened, narrowed, curved, radiused, wavy, spiraled, angled,
and/or
otherwise non-linear and/or not consistent along the entire length of the
elongate
members of the anchor assembly 200. Such features and techniques can also be
incorporated with the valve assemblies of the prosthetic mitral valves
provided herein.
In some embodiments, the elongate members forming the anchor assembly 200
may vary in diameter, thickness and/or width so as to facilitate variations in
the forces
that are exerted by the anchor assembly 200 in specific regions thereof, to
increase or
decrease the flexibility of the anchor assembly 200 in certain regions, to
enhance
migration resistance, and/or to control the process of compression
(crushability) in
preparation for deployment and the process of expansion during deployment of
the
anchor assembly 200.
In some embodiments, one or more of the elongate members of the elongate
members forming the anchor assembly 200 may have a circular cross-section. In
some
embodiments, one or more of the elongate members forming the anchor assembly
200
27
CA 02964935 2017-04-15
WO 2016/065158
PCT/US2015/056935
may have a rectangular cross-sectional shape, or another cross-sectional shape
that is not
rectangular. Examples of cross-sectional shapes that the elongate members
forming the
anchor assembly 200 may have include circular, C-shaped, square, ovular,
rectangular,
elliptical, triangular, D-shaped, trapezoidal, including irregular cross-
sectional shapes
formed by a braided or stranded construct, and the like. In some embodiments,
one or
more of the elongate members forming the anchor assembly 200 may be
essentially flat
(i.e., such that the width to thickness ratio is about 2:1, about 3:1, about
4:1, about 5:1, or
greater than about 5:1). In some examples, one or more of the elongate members
forming
the anchor assembly 200 may be formed using a center-less grind technique,
such that the
diameter of the elongate members varies along the length of the elongate
members.
The anchor assembly 200 may include features that are directed to enhancing
one
or more desirable functional performance characteristics of the prosthetic
mitral valve
devices. For example, some features of the anchor assembly 200 may be directed
to
enhancing the conformability of the prosthetic mitral valve devices. Such
features may
facilitate improved performance of the prosthetic mitral valve devices by
allowing the
devices to conform to irregular tissue topographies and/or dynamically
variable tissue
topographies, for example. Such conformability characteristics can be
advantageous for
providing effective and durable performance of the prosthetic mitral valve
devices. In
some embodiments of the anchor assembly 200, some portions of the anchor
assembly
200 are designed to be more conformable than other portions of the same anchor
assembly 200. That is, the conformability of a single anchor assembly 200 can
be
designed to be different at various areas of the anchor assembly 200.
In some embodiments, the anchor assembly 200 includes features for enhanced in
vivo radiographic visibility. In some embodiments, portions of the anchor
assembly 200,
such as one or more of the anchor feet 220a, 220b, 220c, and 220d, may have
one or
more radiopaque markers attached thereto. In some embodiments, some or all
portions of
the anchor assembly 200 are coated (e.g., sputter coated) with a radiopaque
coating.
Still referring to FIGS. 8 and 9, as described above the anchor feet 220a,
220b,
220c, and 220d are sized and shaped to engage the sub-annular gutter 19 of the
mitral
valve 17. In some embodiments, the anterior feet 220a and 220d are spaced
apart from
each other by a distance in a range of about 30 mm to about 45 mm, or about 20
mm to
28
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
about 35 mm, or about 40 mm to about 55 mm. In some embodiments, the posterior
feet
220b and 220e are spaced apart from each other by a distance in a range of
about 20 mm
to about 30 mm, or about 10 mm to about 25 mm, or about 25 mm to about 40 mm.
In some embodiments, the anchor feet 220a, 220b, 220c, and 220d have a height
ranging from about 8 mm to about 12 mm, or more than about 12 mm. In some
embodiments, the anchor feet 220a, 220b, 220c, and 220d have a gutter engaging
surface
area (when fabric covered) ranging from about 6 MM2 to about 24 mm2. In some
embodiments, the anchor feet 220a, 220b, 220c, and 220d each have essentially
the same
gutter engaging surface area. In particular embodiments, one or more of the
anchor feet
220a, 220b, 220c, and 220d has a different gutter engaging surface area than
one or more
of the other anchor feet 220a, 220b, 220c, and 220d. The anchor feet 220a,
220b, 220c,
and 220d can have widths ranging within about 1.5 mm to about 4.0 mm or more,
and
lengths ranging within about 3 mm to about 6 mm or more. The anchor feet 220a,
220b,
220c, and 220d are sized and shaped so that the anchor assembly 200 does not
significantly impair the natural function of mitral valve chordae tendineae,
the native
mitral valve leaflets, and papillary muscles even after the anchor assembly is
anchored at
the mitral valve site.
As described previously, the anchor assembly 200 is designed to avoid
interference with the functioning of the native mitral valve 17. Therefore,
the anchor
assembly 200 can be implanted within the native mitral valve 17 some time
prior to the
deployment therein of a replacement valve assembly, without degradation of
valve 17
function during the period of time between the anchor implantation and the
valve
implantation (whether that time is on the order of minutes, or even several
days or
months). To avoid such interference between the anchor assembly 200 and the
native
mitral valve 17, the inter-annular connections 270a, 270b, 270c, and 270d pass
through
the coaptation line 32 approximately. More particularly, the left anterior
inter-annular
connection 270a passes through the coaptation line 32 adjacent to the
anterolateral
commissure 30a. In like manner, the right anterior inter-annular connection
270d passes
through the coaptation line 32 adjacent to the posteromedial commissure 30b.
In some
implementations, the left posterior inter-annular connection 270b and right
posterior
inter-annular connection 270c pass through the native mitral valve 17 in
locations that are
29
CA 02964935 2017-04-15
WO 2016/065158
PCT/US2015/056935
posteriorly biased from the natural coaptation line 32. The posterior leaflet
22 will tend
to compliantly wrap around the left posterior inter-annular connection 270b
and right
posterior inter-annular connection 270c to facilitate sealing of the mitral
valve 17, with
the anchor assembly 200 coupled thereto.
In reference to FIG. 10, in some embodiments the anchor assembly 200 includes
a covering material 270 disposed on one or more portions of the anchor
assembly 200.
The covering material 270 can provide various benefits. For example, in some
implementations the covering material 270 can facilitate tissue ingrowth
and/or
endothelialization, thereby enhancing the migration resistance of the anchor
assembly
200 and preventing thrombus formation on blood contact elements. In another
example,
as described further below, the covering material 270 can be used to
facilitate coupling
between the anchor assembly 200 and a valve assembly that is received therein.
The
cover material 270 also prevents or minimizes abrasion and/or fretting between
the
anchor assembly 200 and valve assembly 300. The cover material 270 also
prevents
valve outer tissue abrasion related wear.
In the depicted embodiment, the covering material 270 is disposed essentially
on
the entire anchor assembly 200. In some embodiments, the covering material 270
is
disposed on one or more portions of the anchor assembly 200, while one or more
other
portions of the anchor assembly 200 do not have the covering material 270
disposed
thereon. While the depicted embodiment includes the covering material 270, the
covering material 270 is not required in all embodiments. In some embodiments,
two or
more portions of covering material 270, which can be separated and/or distinct
from each
other, can be disposed on the anchor assembly 200. That is, in some
embodiments a
particular type of covering material 270 is disposed on some areas of the
anchor assembly
200 and a different type of covering material 270 is disposed on other areas
of the anchor
assembly 200.
In some embodiments, the covering material 270, or portions thereof, comprises
a
fluoropolymer, such as an expanded polytetrafluoroethylene (ePTFE) polymer. In
some
embodiments, the covering material 270, or portions thereof, comprises a
polyester, a
silicone, a urethane, ELAST-EONTm (a silicone and urethane polymer), another
biocompatible polymer, DACRONO, polyethylene terephthalate (PET), copolymers,
or
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
combinations and subcombinations thereof. In some embodiments, the covering
material
270 is manufactured using techniques such as, but not limited to, extrusion,
expansion,
heat-treating, sintering, knitting, braiding, weaving, chemically treating,
and the like. In
some embodiments, the covering material 270, or portions thereof, comprises a
biological
tissue. For example, in some embodiments the covering material 270 can include
natural
tissues such as, but not limited to, bovine, porcine, ovine, or equine
pericardium. In some
such embodiments, the tissues are chemically treated using glutaraldehyde,
formaldehyde, or triglycidylamine (TGA) solutions, or other suitable tissue
crosslinking
agents.
In the depicted embodiment, the covering material 270 is disposed on the
interior
and the exterior of the anchor assembly 200. In some embodiments, the covering
material 270 is disposed on the just the exterior of the anchor assembly 200.
In some
embodiments, the covering material 270 is disposed on the just the interior of
the anchor
assembly 200. In some embodiments, some portions of the anchor assembly 200
are
.. covered by the covering material 270 in a different manner than other
portions of the
anchor assembly 200.
In some embodiments, the covering material 270 is attached to at least some
portions of the anchor assembly 200 using an adhesive. In some embodiments,
FEP
(fluorinated ethylene propylene) is used as an adhesive to attach the covering
material
.. 270 to the anchor assembly 200, or portions thereof. For example, an FEP
coating can be
applied to some or all portions of the anchor assembly 200, and the FEP can
act as a
bonding agent to adhere the covering material 270 to the anchor assembly 200.
In some
embodiments, wrapping, stitching, lashing, banding, and/or clips, and the like
can be used
to attach the covering material 270 to the anchor assembly 200. In some
embodiments, a
combination of techniques are used to attach the covering material 270 to the
anchor
assembly 200.
In some embodiments, the covering material 270, or portions thereof, has a
microporous structure that provides a tissue ingrowth scaffold for durable
sealing and/or
supplemental anchoring strength of the anchor assembly 200. In some
embodiments, the
.. covering material 270 is made of a membranous material that inhibits or
reduces the
passage of blood through the covering material 270. In some embodiments, the
covering
31
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
material 270, or portions thereof, has a material composition and/or
configuration that
inhibits or prevents tissue ingrowth and/or endothelialization to the covering
material
270.
In some embodiments, the covering material 270 can be modified by one or more
chemical or physical processes that enhance certain physical properties of the
covering
material 270. For example, a hydrophilic coating may be applied to the
covering material
270 to improve the wettability and echo translucency of the covering material
270. In
some embodiments, the covering material 270 may be modified with chemical
moieties
that promote or inhibit one or more of endothelial cell attachment,
endothelial cell
migration, endothelial cell proliferation, and resistance to thrombosis. In
some
embodiments, the covering material 270 may be modified with covalently
attached
heparin or impregnated with one or more drug substances that are released in
situ.
In some embodiments, covering material 270 is pre-perforated to modulate fluid
flow through the covering material 270 and/or to affect the propensity for
tissue ingrowth
.. to the covering material 270. In some embodiments, the covering material
270 is treated
to make the covering material 270 stiffer or to add surface texture. For
example, in some
embodiments the covering material 270 is treated with FEP powder to provide a
stiffened
covering material 270 or roughened surface on the covering material 270. In
some
embodiments, selected portions of the covering material 270 are so treated,
while other
portions of the covering material 270 are not so treated. Other covering
material 270
material treatment techniques can also be employed to provide beneficial
mechanical
properties and tissue response interactions. In some embodiments, portions of
the
covering material 270 have one or more radiopaque markers attached thereto to
enhance
in vivo radiographic visualization.
Referring now to FIGS. 11A and 12A, the anchor assembly 200 is shown
implanted within a native mitral valve 17. FIGS. 11B and 12B are photographs
that
correspond to FIGS. 11A and 12A respectively. In FIG. 11A, the mitral valve 17
is
shown in a closed state. In FIG. 12A, the mitral valve 17 is shown in an open
state.
These illustrations are from the perspective of the left atrium looking
towards the mitral
valve 17. For instance, in FIG. 12A chordae tendineae 40 are visible through
the open
leaflets of the mitral valve 17.
32
CA 02964935 2017-04-15
WO 2016/065158
PCT/US2015/056935
These figures illustrate the supra-annular structures and sub-annular
structures of
the anchor assembly 200 in their relationships with the native mitral valve
17. For
example, the closed state of the native mitral valve 17 in FIG. 11A allows
visibility of the
supra-annular structures such as the left anterior atrial holding feature
240a, the left
posterior atrial holding feature 240b, the right posterior atrial holding
feature 240c, and
the right anterior atrial holding feature 240d. In addition, the anterior
anchor arch 250a,
the left anchor arch 250b, the posterior anchor arch 250c, the right anchor
arch 250d, and
the connection bridges 260 are visible. However, the sub-annular structures
are not
visible in FIG. 11A because such structures are obstructed from view by the
anterior
leaflet 20 and the three-part posterior leaflet 24a, 24b, and 24c.
In contrast, in FIG. 12A certain sub-annular structures of the anchor assembly
200
are visible because the native mitral valve 17 is open. For example, sub-
annular support
arms 230a, 230b, 230c, and 230d and hub 210 are in view through the open
mitral valve
17. Nevertheless, the anchor feet 220a, 220b, 220c, and 220d remain out of
view because
of their location within the sub-annular gutter of the mitral valve 17.
Referring to FIG. 13, after implantation of the anchor assembly 200 within the
native mitral valve 17 (as performed, for example, in accordance with FIGS. 1-
7
described above), a valve delivery sheath 170 of the delivery system 100 can
be used to
deploy a valve assembly within the anchor assembly 200. As described above in
reference to FIG. 7, with the distal pusher catheter 160 coupled with the hub
210 of the
anchor assembly 200, the distal pusher catheter 160 can be used to guide the
valve
assembly into the interior of the anchor assembly 200.
In some implementations, with the primary deflectable catheter 120 positioned
with its distal end in the left atrium 16, the valve delivery sheath 170 is
installed into a
lumen of the primary deflectable catheter 120 (over the distal pusher catheter
160) and
advanced through the primary deflectable catheter 120. As described further
below, in
some embodiments the valve delivery sheath 170 is preloaded with a prosthetic
valve
assembly and other components of the delivery system 100. The primary
deflectable
catheter 120 may be the same catheter that was used to deliver the anchor
assembly 200,
or it may be a different catheter (but still referred to here as the primary
deflectable
catheter 120 for simplicity sake).
33
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
In some embodiments, the valve delivery sheath 170 can be made from the
materials described above in reference to the primary deflectable catheter
120. In some
embodiments, the valve delivery sheath 170 has an outer diameter in the range
of about
20 Fr to about 28 Fr (about 6.7 mm to about 9.3 mm). In some embodiments, the
valve
delivery sheath 170 has an outer diameter in the range of about 14 Fr to about
24 Fr
(about 4.7 mm to about 8.0 mm).
In the depicted embodiment, the valve delivery sheath 170 includes a flared
distal
end portion 172. In some embodiments, no such flared distal end portion 172 is
included.
The flared distal end portion 172 can collapse to a lower profile when
constrained within
the primary deflectable catheter 120. When the flared distal end portion 172
is expressed
from the primary deflectable catheter 120, the flared distal end portion 172
can self-
expand to the flared shape. In some embodiments, the material of the flared
distal end
portion 172 includes pleats or folds, may be a continuous flared end or may be
separated
into sections such as flower pedals, and may include one or more resilient
elements that
bias the flared distal end portion 172 to assume the flared configuration in
the absence of
restraining forces (such as from containment within the primary deflectable
catheter 120).
The flared distal end portion 172 can be advantageous, for example, for
recapturing the
valve assembly within the lumen of the valve delivery sheath 170 after the
valve
assembly has been expressed from the flared distal end portion 172.
In some embodiments, the maximum outer diameter of the flared distal end
portion 172 is in a range of about 30 Fr to about 34 Fr (about 10.0 mm to
about 11.3
mm). In some embodiments, the maximum outer diameter of the flared distal end
portion
172 is in a range of about 32 Fr to about 44 Fr (about 10.7 mm to about 14.7
mm). In
some embodiments, the maximum outer diameter of the flared distal end portion
172 is in
a range of about 24 Fr to about 30 Fr (about 8.0 mm to about 10.0 mm). In some
embodiments, the maximum outer diameter of the flared distal end portion 172
is less
than about 24 Fr (about 8.0 mm) or greater than about 44 Fr (about 14.7 mm).
Referring to FIG. 14, in some implementations the valve delivery sheath 170
can
be withdrawn into the primary deflectable catheter 120 while a valve delivery
catheter
180 is held substantially stationary to express a valve assembly 300 from a
lumen of the
valve delivery sheath 170. The valve delivery sheath 170 and the valve
delivery catheter
34
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
180 are additional components in some embodiments of the example delivery
system
100.
The valve assembly 300 can be releasably coupled to the valve delivery
catheter
180 and retained in a low-profile configuration. In some embodiments, both the
distal
and proximal ends of the valve assembly 300 are releasably coupled to the
valve delivery
catheter 180. In some embodiments, just one of the distal end or the proximal
end of the
valve assembly 300 is releasably coupled to the valve delivery catheter 180.
In particular
embodiments, one or more control wires may be included to releasably couple
one or
more portions of the valve assembly 300 to the valve delivery catheter 180.
Referring to FIGS. 41-43, the valve assembly 300 is releasably coupled to the
valve delivery catheter 180 via a proximal control wire 342a and a mid-body
control wire
342b. The control wires 342a and 342b are threaded through one or more lumens
within
the valve delivery catheter 180. The control wires 342a and 342b exit the
valve delivery
catheter 180 and pass through eyelets on the proximal end and mid-body
portions of the
valve assembly 300 respectively. The control wires 342a and 342b are then
threaded
back into the valve delivery catheter 180. By manipulating the control wires
342a and
342b, a clinician operator can control the valve assembly 300. For example,
the
expansion and contraction of the valve assembly 300 can be controlled, and the
detachment of the valve assembly 300 from the valve delivery catheter can be
controlled,
by manipulating the tension and position of the control wires 342a and 342b
within the
delivery catheter 180.
Referring again to FIG. 14, a lumen of the valve delivery catheter 180 can
slidably surround the distal pusher catheter 160. Therefore, advancement of
the valve
delivery catheter 180 results in advancement of the valve assembly 300 over
the distal
pusher catheter 160 towards the anchor assembly 200.
Referring to FIGS. 15 and 16, the delivery system 100 can be manipulated by a
clinician operator to perform a lateral pivot (panning, rotation, etc.) of the
valve assembly
300 within the left atrium 16. The rotation of the valve assembly 300 changes
the
alignment of the valve assembly 300 from being generally axial with the distal
end
portion of the primary deflectable catheter 120 to being generally axial with
the anchor
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
assembly 200 (in preparation for installation of the valve assembly 300 into
the interior of
the anchor assembly 200).
In some implementations, the aforementioned rotation of the valve assembly 300
can be performed as follows. As shown in FIG. 15, because of the influence
from the
primary deflectable catheter 120 on the valve delivery catheter 180, the axis
of the valve
assembly 300 is initially in general alignment with the axis of the distal end
portion of the
primary deflectable catheter 120. From this arrangement, a simultaneous
counter
movement between the distal pusher catheter 160 and the valve delivery
catheter 180 can
be performed by the clinician to rotate the valve assembly 300. That is, as
the distal
pusher catheter 160 is pulled proximally, the valve delivery catheter 180 is
pushed
distally. As a result of that counter movement, the valve assembly 300 rotates
in a
relatively tight radius, as required by the confines of the left atrium 16.
Thereafter, the
valve delivery catheter 180 can be advanced further so that the valve assembly
300 is
coaxially positioned within the interior of the anchor assembly 200 as shown
in FIG. 16.
Referring now also to FIG. 17, in some embodiments the valve assembly 300 and
the anchor assembly 200 become aligned with each other coaxially, linearly
(along their
axes), and rotationally prior to or during the expansion of the valve assembly
300,
resulting in engagement between the valve assembly 300 and the anchor assembly
200.
Thereafter, the delivery system 100 can be withdrawn from the heart 10 and the
prosthetic mitral valve can perform its function.
Coaxial alignment between the valve assembly 300 and the anchor assembly 200,
as described above, is achieved by virtue of the valve delivery catheter 180
being slidably
disposed over the distal pusher catheter 160. Linear alignment between the
valve
assembly 300 and the anchor assembly 200 can be achieved by the interaction of
a distal
end feature 182 of the valve delivery catheter 180 and the hub 210 of the
anchor
assembly 200. For example, in some embodiments an abutting of the distal end
feature
182 and the hub 210 can result in proper linear alignment between the valve
assembly
300 and the anchor assembly 200.
Relative rotational alignment between the valve assembly 300 and the anchor
assembly 200 (about their axes) can be achieved in various manners. For
example, in
some embodiments the valve delivery catheter 180 is mechanically keyed to the
distal
36
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
pusher catheter 160 to slidably fix a desired rotational alignment between the
valve
assembly 300 and the anchor assembly 200. In some embodiments, other types of
mechanical features (e.g., pins/holes, protrusions/receptacles, etc.) can be
included to
facilitate a desired rotational/spin alignment between the valve assembly 300
and the
anchor assembly 200. Alternatively, or additionally, radiopaque markers can be
included
on the valve assembly 300 and on the anchor assembly 200 in locations and/or
patterns
that arc indicative of the relative rotational orientation (about their axes)
of the valve
assembly 300 and the anchor assembly 200. In some embodiments, (e.g., when the
valve
delivery catheter 180 "torqueable") the valve delivery catheter 180 can be
rotated about
its axis until the markers are in proper position relative to the anchor
assembly 200, prior
to final expansion of valve assembly 300. Fluoroscopy can be used to attain a
desired
relative orientation of the radiopaque markers, and of the valve assembly 300
and the
anchor assembly 200 correspondingly.
Referring to FIGS. 18 and 19, an example valve assembly 300 is shown without
any covering or valve/occluder leaflets. Hence, a valve assembly frame 301 of
the valve
assembly 300 is shown. FIG. 18 shows an anterior side view of the valve
assembly frame
301, and FIG. 19 shows a bottom view of the valve assembly frame 301. The
valve
assembly 300 can be constructed using any of the various materials and
manufacturing
techniques described above in reference to the anchor frame 200 (e.g., refer
to FIG. 9). It
should be understood that the depicted valve assembly 300 is merely one non-
limiting
example of the valve assemblies provided within the scope of this disclosure.
The valve assembly 300 includes a proximal end portion 302 and a distal end
portion 304. The valve assembly includes a flared external skirt portion 303
and defines
an interior orifice portion 305. When the valve assembly 300 is implanted in a
native
mitral valve, the proximal end portion 302 is located supra-annular (in the
left atrium)
and the distal end portion 304 is located sub-annular (in the left ventricle).
The proximal
end portion 302 defines the generally circular entrance orifice of the valve
assembly 300,
as described further below.
In the depicted embodiment, the valve assembly 300 generally flares outward
along a distal direction. Said differently, the distal end portion 304 is
flared outward in
comparison to the proximal end portion 302. Accordingly, the proximal end
portion 302
37
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
defines a smaller outer profile in comparison to the distal end portion 304.
However,
some regions of the distal end portion 304 bow inwardly. In particular, for
example, a
posteromedial commissural corner 330a and anterolateral commissural corner
330b of the
valve assembly 300 may bow inwardly. It should be understood that the outward
flare of
the distal end portion 304 in comparison to the proximal end portion 302 is
merely one
example configuration for a profile of the valve assembly 300. In some
embodiments, for
example, a shoulder (a portion of the valve assembly 300 having the largest
outer
periphery) is located proximal of the middle of the valve assembly 300.
The valve assembly 300 also includes an anterior side 306 between the
posteromedial commissural corner 330a and anterolateral commissural corner
330b.
When the valve assembly 300 is implanted in a native mitral valve, the
anterior side 306
faces the anterior leaflet of the native mitral valve. The anterior side 306
of the distal end
portion 304 defines a generally flat surface, whereas the other sides of the
distal end
portion 304 are rounded. Hence, the periphery of the distal end portion 304 is
generally
D-shaped. The D-shaped periphery of the distal end portion 304 provides the
valve
assembly 300 with an advantageous outer profile for interfacing and sealing
with the
native mitral valve. As described further below, sealing is attained by
coaptation between
the D-shaped periphery of the distal end portion 304 and the leaflets of the
native mitral
valve, and, in some embodiments, between the D-shaped periphery in the region
of the
skirt 303 with the native valve annulus.
In the depicted embodiment, the proximal end portion 302 of the valve assembly
300 includes three atrial leaflet arches 310a, 310b, and 310c that together
define an
undulating ring at the proximal end portion 302. Each of the leaflet arches
310a, 310b,
and 310c includes an apex having an attachment hole 312a, 312b, and 312c
respectively.
In some embodiments, the attachment holes 312a, 312b, and 312c are used for
coupling
the proximal end of the valve assembly 300 to a delivery catheter (e.g., valve
delivery
catheter 180 of FIGS. 14-16).
The valve assembly 300 also includes three commissural posts 320a, 320b, and
320c that each extend distally from the intersections of the three leaflet
arches 310a,
310b, and 310c. The commissural posts 320a, 320b, and 320c are disposed at
about 120
apart from each other. The commissural posts 320a, 320b, and 320c each have a
series of
38
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
holes that can be used for attachment of leaflets, such as by suturing. The
three leaflet
arches 310a, 310b, and 310e and the three commissural posts 320a, 320b, and
320c are
areas on the valve assembly 300 to which three prosthetic valve leaflets
become attached
to comprise a tri-leaflet occluder (e.g., refer to FIGS. 22-25).
As best seen in FIG. 19, the three leaflet arches 310a, 310b, and 310c and the
commissural posts 320a, 320b, and 320c define a generally cylindrical frame
for the tri-
leaflet occluder construct. As such, the valve assembly 300 provides a proven
and
advantageous frame configuration for the tii-leaflet occluder. The tri-leaflet
occluder
provides open flow during diastole and occlusion of flow during systole.
Referring to FIG. 20, an exploded depiction of an example prosthetic mitral
valve
400 includes an anchor assembly 200 and a valve assembly 300. This figures
provides a
posterior side view of the anchor assembly 200 and the valve assembly 300.
The valve assembly 300 includes a covering 340. The covering 340 can be made
of any of the materials and constructed using any of the techniques described
above in
reference to covering 270. Additionally, in some embodiments the covering 340
can
comprise natural tissues such as, but not limited to, bovine, porcine, ovine,
or equine
pericardium. In some such embodiments, the tissues are chemically cross-linked
using
glutaraldehyde, formaldehyde, or triglycidyl amine solution, or other suitable
crosslinking agents.
When the valve assembly 300 and the anchor assembly 200 are coupled together,
the valve assembly 300 is geometrically interlocked within the interior of the
anchor
assembly 200 (e.g., in some embodiments by virtue of the tapered shape of the
valve
assembly 300 within the supra-annular ring and interior space of the anchor
assembly
200). In particular, in some embodiments the valve assembly 300 is contained
within the
interior space between the supra-annular ring 250 and the sub-annular support
arms 230a,
230b, 230c, and 230d. As described above, the interlocked arrangement between
the
valve assembly 300 and the anchor assembly 200 is accomplished by positioning
a valve
assembly 300 in a low-profile configuration within the interior of the anchor
assembly
200 and then allowing expansion of the valve assembly 300 within the interior
of the
anchor assembly 200 (e.g., refer to FIGS. 16 and 17).
39
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
Referring to FIGS. 21 and 22, a deployed configuration of the example
prosthetic
mitral valve 400 includes the valve assembly 300 engaged within the anchor
assembly
200. FIG. 21 shows a top (atrial) view of the prosthetic mitral valve 400, and
FIG. 22
shows a bottom (ventricle) view of the prosthetic mitral valve 400.
In some embodiments, such as the depicted embodiment, valve assembly 300
includes three leaflets 350a, 350b, and 350c that perform the occluding
function of the
prosthetic mitral valve 400. The cusps of the three leaflets 350a, 350b, and
350c are
fixed to the three atrial leaflet arches 310a, 310b, and 310c, and to the
three commissural
posts 320a, 320b, and 320c (refer to FIGS. 18 and 19). The free edges of the
three
leaflets 350a, 350b, and 350c can seal by coaptation with each other during
systole and
open during diastole.
The three leaflets 350a, 350b, and 350c can be comprised of natural or
synthetic
materials. For example, the three leaflets 350a, 350b, and 350c can be
comprised of any
of the materials described above in reference to the covering 340, including
the natural
tissues such as, but not limited to, bovine, porcine, ovine, or equine
pericardium. In some
such embodiments, the tissues are chemically cross-linked using
glutaraldehyde,
formaldehyde, or triglycidyl amine solution, or other suitable crosslinking
agents. In
some embodiments, the leaflets 350a, 350b, and 350c have a thickness in a
range of about
0.005" to about 0.020" (about 0.13 mm to about 0.51 mm), or about 0.008" to
about
0.012" (about 0.20 mm to about 0.31 mm). In some embodiments, the leaflets
350a,
350b, and 350e have a thickness that is less than about 0.005" (about 0.13 mm)
or greater
than about 0.020" (about 0.51 mm).
In some embodiments, the occluding function of the prosthetic mitral valve 400
can be performed using configurations other than a tri-leaflet occluder. For
example, bi-
leaflet, quad-leaflet, or mechanical valve constructs can be used in some
embodiments.
Referring to FIGS. 23 and 24, the prosthetic mitral valve 400 is shown
implanted
within a native mitral valve 17. In FIG. 23, the prosthetic mitral valve 400
is shown in a
closed state (occluded). In FIG. 24, the prosthetic mitral valve 400 is shown
in an open
state. These illustrations are from the perspective of the left atrium looking
towards the
mitral valve 17. For instance, in FIG. 24 the hub 210 and the sub-annular
support arms
230a, 230b, 230c, and 230d of the anchor assembly 200 is visible through the
open
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
leaflets 350a, 350b, and 350c of the prosthetic mitral valve 400, whereas in
FIG. 23 the
hub 210 and the sub-annular support arms 230a, 230b, 230e, and 230d are not
visible
because the closed leaflets 350a, 350b, and 350c block the hub 210 from view.
FIGS. 25-33 describe additional aspects pertaining to sealing between native
mitral valve structures and the implantable prosthetic mitral valves described
herein.
During systole, ventricle-to-atrium sealing is relevant in order to
effectively treat MR via
implantation of a prosthetic mitral valve. In addition, during diastole,
atrium-to-ventricle
sealing between native mitral valve structures and the prosthetic mitral
valves described
herein is relevant for preventing or reducing paravalvular leakage, and for
good healing
and chronic stability. The prosthetic mitral valves described herein are
designed to have
various features that provide for effective sealing with the native mitral
valve structures.
One feature that enhances the sealing of the prosthetic mitral valves provided
herein pertains to the shape of the prosthetic valve framework in relation to
the shape of
the native mitral valve. As described above, the annulus of a native mitral
valve is
generally D-shaped (e.g., refer to FIG. 8). In addition, as described above,
the distal end
portions of the prosthetic mitral valves described herein are D-shaped (e.g.,
refer to FIG.
19). In other words, the portion of the prosthetic valve that is designed to
interface with
the native valve annulus has a D-shaped profile that is similar to the shape
of the annulus.
This similarity of shapes can provide particular sealing efficacy in the areas
of the lateral
scallop 24a and the medial scallop 24c of the posterior leaflets 22 (refer to
FIG. 8).
Another feature that enhances the sealing of the prosthetic mitral valves
provided
herein pertains to the size of the selected prosthetic valve in relation to
the size of the
native mitral valve, especially during systole. In some implementations, a
selected
prosthetic valve will intentionally have an outer profile (when unconstrained)
that is
equal to or slightly larger than the size of the annulus of the native mitral
valve. That is,
in the area on the valve surface that is intended to be adjacent to the native
valve annulus,
the size of the valve may result in a line-to-line fit or a slight
interference fit with the
native valve annulus. Hence, in some implementations the atrium-to-ventricle
sealing
during diastole is provided by the line-to-line fit or slight interference fit
between the
valve and the native valve annulus.
41
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
Another feature that enhances the sealing of the prosthetic mitral valves
provided
herein pertains to the relative geometric orientation of sealing surfaces on
the prosthetic
valve in relation to the annulus of the native mitral valve. While in some
implementations, some sealing is provided by the mechanical fit between the
outer
profile of the valve and the receiving structure of the native mitral valve,
in some
implementations substantial sealing is provided by coaptation between the
native leaflets
and sealing surfaces on the perimeter of the prosthetic valve (to thereby
create a contact
seal during diastole and a left ventricle pressurized seal during systole).
This type of
sealing may be referred to herein as a leaflet to valve body seal. As
described further
below, the prosthetic mitral valves provided herein have sealing surfaces that
are
geometrically oriented in relation to the native valve annulus so that a
leaflet to valve
body seal is provided. While the leaflet to valve body seal is not entirely a
mechanically
compressive type of seal or an attachment onto the native tissue (active
fixation) type of
seal, in some embodiments such mechanical or attachment type of seals may
alternatively
or additionally be incorporated.
In some implementations, an effective leaflet to valve body seal (not based
entirely on compression or attachment) may necessitate some native leaflet
movement to
the sealing surface of the valve body. Hence, a valve body shape that mimics
the shape
of the native mitral valve is advantageous. As described above, in some
embodiments the
outer periphery of the valve assemblies provided herein have a D-shaped
periphery that
generally correlates with the D-shaped annulus of native mitral valves.
Accordingly, the
movement distance of the native valve leaflets to the sealing surface of the
valve body
can be minimized (or essentially eliminated in some implementations), and
sealing can
thereby be enhanced.
In addition, an effective leaflet to valve body seal exhibits contiguous
coaptation
between the native leaflets and prosthetic valve body around the entire
periphery of the
prosthetic valve body. As described further below, the profiles of the
prosthetic mitral
valves provided herein are designed to interface with the native leaflets so
as to provide
contiguous coaptation around the entire periphery of the prosthetic valve. To
accomplish
this, in some embodiments the profile of some regions of the prosthetic mitral
valves are
42
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
different than the profile of other regions of the same valve (e.g., in
correspondence with
differing anatomical features in various portions of the native mitral valve).
Referring to FIG. 25, a lateral cross-sectional atrial view of a heart 10
shows the
mitral valve 17, aortic valve 510, tricuspid valve, 520 and pulmonary valve
530. As
described above in reference to FIG. 8, the mitral valve 17 includes the
anterior leaflet
20, the posterior leaflet 22 (including the medial scallop 24a, the middle
scallop 24b, and
the lateral scallop 24c), the left fibrous trigone 134a, and the right fibrous
trigonc 134b.
In regard to sealing between a prosthetic mitral valve and a native mitral
valve,
the differing anatomical features of various portions of the native mitral
valve 17 make it
advantageous to consider the mitral valve 17 as having three distinct sealing
regions that
together comprise the entirety of the mitral valve 17. The three distinct
sealing regions
are: an anterior region 25a, a posterior region 25b, and two commissural
regions 25c.
The anterior region 25a extends generally linearly between the left and right
trigones
134a and 134b. The posterior region 25b comprises the middle scallop 24b and
posterior
portions of the lateral scallop 24a and medial scallop 24c. The commissural
regions 25c
extend between the anterior region 25a and the posterior region 25b. The
commissural
regions 25c generally encompass the commissures 30a and 30b, and anterior
portions of
the lateral scallop 24a and medial scallop 24c. These three sealing regions
25a, 25b, and
25e will be referenced again in regard to FIGS. 28-33.
Referring to FIG. 26, a schematic diagram of a cross-section of a native
mitral 17
valve indicates the location of the mitral valve annulus 28. Three geometric
variables (S,
W, and H) that can be used to quantify a relative geometric orientation of
sealing surfaces
on the prosthetic valve in relation to the annulus 28 of the native mitral
valve 17 are also
indicated. As used herein, the term "sealing surfaces" is defined as surface
areas on the
prosthetic valve that are intended to make sealing contact with structures of
the native
mitral valve 17 (especially the leaflets of the native mitral valve 17).
Hence, the sealing
surfaces are the areas on the prosthetic valve that are used to facilitate the
leaflet to valve
body seal.
The geometric variable S quantifies the radial distance from the annulus 28 to
the
adjacent prosthetic valve framework surface. A negative S-value indicates that
the
annulus 28 and the adjacent prosthetic valve surface are spaced apart from
each other.
43
CA 02964935 2017-04-15
WO 2016/065158
PCT/US2015/056935
For example, an S-value of negative 2 mm indicates that there is a 2 mm space
between
the annulus 28 and the adjacent prosthetic valve surface. When S equals zero,
it indicates
that the annulus 28 and the adjacent prosthetic valve surface are in contact
with each
other in a line-to-line fit relationship. When S is a positive number, it
indicates that the
annulus 28 and the adjacent prosthetic valve surface are in an interference
fit relationship.
In other words, when S is a positive number some compressive force is being
applied to
the annulus 28 by the adjacent prosthetic valve surface.
The geometric variable H quantifies the distance from the superior limit
(upper
edge) to the inferior limit (lower edge) of the sealing surface on the
prosthetic valve. H-
values are measured downward (in reference to the illustration). For example,
an H-
value of 10 mm indicates that, for a particular sealing region, the sealing
surface on the
prosthetic valve ends 10 mm below the superior limit of the sealing surface.
In another
example, when the superior limit is at the annulus 28, an H-value of 7 mm
indicates that
the inferior limit of the sealing surface is 7 min below the annulus 28. In
general, the
superior limit of the sealing surface on the prosthetic valve is either at,
slightly above, or
slightly below the annulus 28 (e.g., about 2 mm above or about 2mm below the
annulus
28 in some embodiments).
The geometric variable W quantifies the radial distance from the superior
limit of
the sealing surface to the inferior limit of the sealing surface on the
prosthetic valve. A
negative W-value indicates that the inferior limit of the sealing surface is
positioned
radially inward in comparison to the superior limit of the sealing surface
(e.g., at least a
portion of the scaling surface is flared or bowed inward at the distal end). A
positive W-
value indicates that the inferior limit of the sealing surface is positioned
radially outward
in comparison to superior limit of the sealing surface (e.g., at least a
portion of the sealing
surface is flared or bowed outward at the distal end). A W-value of zero
indicates that the
inferior limit of the sealing surface is positioned at the same radial
position as the
superior limit of the sealing surface.
Referring to FIG. 27, an anterior side view of a valve assembly 300 includes
an
anterior sealing surface 360a in accordance with some embodiments. In the
depicted
embodiment, the anterior sealing surface 360a spans the lower portion of the
anterior side
of valve assembly 300. The anterior sealing surface 360a comprises the surface
area on
44
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
the anterior side of the prosthetic valve assembly 300 that is intended to
make sealing
contact with structures of the native mitral valve. The anterior sealing
surface 360a
consists of structural support from the valve frame 301 as well as a tissue
surface 361a.
The anterior tissue surface 361a provides sealing interface height (H) but its
flexible
nature reduces the amount of LVOT obstruction, as will be described later. For
example,
at least a portion of the anterior sealing surface 360a is intended to make
sealing contact
with the anterior leaflet of the native mitral valve.
Referring to FIG. 28, a posterior side view of a valve assembly 300 includes a
posterior sealing surface 360b in accordance with some embodiments. In the
depicted
embodiment, the posterior sealing surface 360b spans the lower portion of the
posterior
side of valve assembly 300. The posterior sealing surface 360b comprises the
surface
area on the posterior side of the prosthetic valve assembly 300 that is
intended to make
sealing contact with structures of the native mitral valve. For example, at
least a portion
of the posterior sealing surface 360b is intended to make sealing contact with
the
posterior leaflet of the native mitral valve.
Referring to FIG. 29, a commissural (lateral) side view of a valve assembly
300
includes a commissural sealing surface 360c in accordance with some
embodiments.
This view is slightly biased to the anterior side of the valve assembly 300.
In the
depicted embodiment, the commissural sealing surface 360c spans the lower
portion of
the commissural side of valve assembly 300. The commissural sealing surface
360c
comprises the surface area on the lateral side of the prosthetic valve
assembly 300 that is
intended to make sealing contact with structures of the native mitral valve.
For example,
at least a portion of the commissural sealing surface 360c is intended to make
sealing
contact with the medial scallop or the lateral scallop of the posterior
leaflet of the native
mitral valve, and with the leaflet tissue in the commissural regions of the
native mitral
valve.
Referring to FIG. 30, the geometric relationship between a native mitral valve
annulus and an anterior sealing surface of a prosthetic mitral valve in
accordance with
some embodiments can be represented by the S, H, and W values as described
above in
reference to FIG. 26. For example, in some embodiments the S-value of the
anterior
sealing surface of the prosthetic mitral valve is in a range from about zero
millimeters to
CA 02964935 2017-04-15
WO 2016/065158
PCT/US2015/056935
about positive 2 millimeters. In other words, the S-value of the anterior
sealing surface in
relation to the native mitral valve annulus is in a range from about line-to-
line contact to
about 2 millimeters interference. It should be understood that in this context
an
interference fit does not necessarily mean that the native valve annulus is
stretched or
deformed as a result of the interference. More likely, rather, the prosthetic
valve
assembly will be inhibited by the annulus from enlarging to its unconstrained
fully
expanded size. While in the depicted embodiment the S-value is in a range of
about zero
millimeters to about positive 2 millimeters, in some embodiments the S-value
is in a
range of about negative 2 millimeters to about positive 1 millimeter, or about
negative 1
millimeter to about positive 3 millimeters, or about zero millimeters to about
positive 4
millimeters. In some embodiments, the S-value can be more negative than about
negative 2 millimeters or more positive than about positive 4 millimeters.
In some embodiments the H-value of the anterior sealing surface of the
prosthetic
mitral valve is about 14 millimeters. In other words, in some embodiments the
distance
from the superior edge of the anterior sealing surface to the inferior edge of
the anterior
sealing surface is about 14 millimeters. More specifically, the H-value of the
anterior
sealing surface can be divided into two portions: (1) a superior portion,
HEVOT and (2) an
inferior portion, HTISSUE. The Fhvor-value generally corresponds to the
distance from the
superior edge of the anterior sealing surface to the inferior end of the valve
frame 301 at
various places along the anterior sealing surface 360a (refer to FIG. 27). The
HTISSUE-
value corresponds to the distance from the inferior end of the valve frame 301
at various
places along the anterior sealing surface 360a to the inferior end of the
anterior tissue
surface 361a at those places. While in the depicted embodiment, the HD/or-
value and the
HrissuE-value are equal to each other, in some embodiments the ratio between
the Hwor-
value and the lirissuE-value is about 3:1, about 2:1, about 1.5:1, about
1:1.5, about 1:2, or
about 3:1.
While in the depicted embodiment the total H-value is about 14 millimeters, in
some embodiments the H-value is in a range of about 8 millimeters to about 10
millimeters, or about 10 millimeters to about 12 millimeters, or about 12
millimeters to
about 14 millimeters, or about 14 millimeters to about 16 millimeters, or
about 13
46
CA 02964935 2017-04-15
WO 2016/065158
PCT/US2015/056935
millimeters to about 15 millimeters. In some embodiments, the H-value can be
less than
about 8 millimeters or more than about 16 millimeters.
In some embodiments the W-value of the anterior sealing surface of the
prosthetic
mitral valve is about negative 2 millimeters. In other words, in some
embodiments the
radial distance from the superior edge of the anterior sealing surface to the
inferior edge
of the anterior sealing surface on the prosthetic valve is about negative 2
millimeters. A
W-value of negative 2 millimeters indicates that the lower edge of the
anterior sealing
surface is positioned radially inward in comparison to the superior edge of
thc anterior
sealing surface by about 2 millimeters. This also indicates that the sub-
annular anterior
valve assembly is flared or bowed inward, such as indicated by valve body
profile line
362a. While in the depicted embodiment the W-value is about negative 2
millimeters, in
some embodiments the W-value is in a range of about negative 6 millimeters to
about
negative 4 millimeters, or about negative 4 millimeters to about negative 2
millimeters, or
about negative 2 millimeters to about zero millimeters, or about zero
millimeters to about
positive 2 millimeters, or about negative 3 millimeters to about negative 1
millimeter. In
some embodiments, the W-value can be more negative than about negative 6
millimeters
or more positive than about positive 2 millimeters.
Referring to FIG. 31, the geometric relationship between a native mitral valve
annulus and a commissural sealing surface of a prosthetic mitral valve in
accordance with
some embodiments can be represented by the S. H, and W values as described
above in
reference to FIG. 26. For example, in some embodiments the S-value of the
commissural
sealing surface of the prosthetic mitral valve is in a range from about zero
millimeters to
about positive 2 millimeters. In other words, the S-value of the commissural
sealing
surface in relation to the native mitral valve annulus is in a range from
about line-to-line
contact to about 2 millimeters interference. It should be understood that in
this context an
interference fit does not necessarily mean that the native valve annulus is
stretched or
deformed as a result of the interference. More likely, rather, the prosthetic
valve
assembly will be inhibited by the annulus from enlarging to its fully expanded
size.
While in the depicted embodiment the S-value is in a range of about zero
millimeters to
about positive 2 millimeters, in some embodiments the S-value is in a range of
about
negative 2 millimeters to about positive 1 millimeter, or about negative 1
millimeter to
47
CA 02964935 2017-04-15
WO 2016/065158
PCT/US2015/056935
about positive 3 millimeters, or about zero millimeters to about positive 4
millimeters. In
some embodiments, the S-value can be more negative than about negative 2
millimeters
or more positive than about positive 4 millimeters.
In some embodiments, the H-value of the commissural sealing surface of the
prosthetic mitral valve is in a range of about 8 millimeters to about 14
millimeters. In
other words, in some embodiments the distance from the native valve annulus to
the
lower (inferior) edge of the commissural sealing surface is in a range of
about 8
millimeters to about 14 millimeters. This range, from about 8 millimeters to
about 14
millimeters, is at least partly a result of the shape of a commissural comer
364 (refer to
.. FIG. 29) that comprises part of the commissural sealing surface.
Accordingly, the lower
edge of the commissural sealing surface varies across the lateral width of the
commissural sealing surface just by the nature of the shape of the commissural
sealing
surface. While in the depicted embodiment the H-value is in a range of about 8
millimeters to about 14 millimeters, in some embodiments the H-value is in a
range of
about 4 millimeters to about 10 millimeters, or about 6 millimeters to about
12
millimeters, or about 8 millimeters to about 14 millimeters, or about 10
millimeters to
about 16 millimeters, or about 7 millimeters to about 15 millimeters. In some
embodiments, the H-value can be less than about 4 millimeters or more than
about 15
millimeters.
In some embodiments the W-value of the commissural sealing surface of the
prosthetic mitral valve is about negative 2 millimeters. In other words, in
some
embodiments the radial distance from the superior (upper) edge of the
commissural
sealing surface to the inferior (lower) edge of the commissural sealing
surface on the
prosthetic valve is about negative 2 millimeters. A W-value of negative 2
millimeters
indicates that the lower edge of the commissural sealing surface is positioned
radially
inward in comparison to the upper edge of the commissural sealing surface by
about 2
millimeters. This also indicates that the sub-annular commissural valve
assembly is
flared or bowed inward, such as indicated by valve body profile line 362b.
While in the
depicted embodiment the W-value is about negative 2 millimeters, in some
embodiments
the W-value is in a range of about negative 6 millimeters to about negative 4
millimeters,
or about negative 4 millimeters to about negative 2 millimeters, or about
negative 2
48
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
millimeters to about zero millimeters, or about zero millimeters to about
positive 2
millimeters, or about negative 3 millimeters to about negative 1 millimeter.
In some
embodiments, the W-value can be more negative than about negative 6
millimeters or
more positive than about positive 2 millimeters.
Referring to FIG. 32, the geometric relationship between a native mitral valve
annulus and a posterior sealing surface of a prosthetic mitral valve in
accordance with
some embodiments can be represented by the S, H, and W values as described
above in
reference to FIG. 26. For example, in some embodiments the S-value of the
posterior
sealing surface of the prosthetic mitral valve is in a range from about zero
millimeters to
about positive 2 millimeters. In other words, the S-value of the posterior
sealing surface
in relation to the native mitral valve annulus is in a range from about line-
to-line contact
to about 2 millimeters interference. It should be understood that in this
context an
interference fit does not necessarily mean that the native valve annulus is
stretched or
deformed as a result of the interference. More likely, rather, the prosthetic
valve
assembly will be inhibited by the annulus from enlarging to its fully expanded
size.
While in the depicted embodiment the S-value is in a range of about zero
millimeters to
about positive 2 millimeters, in some embodiments the S-value is in a range of
about
negative 2 millimeters to about positive 1 millimeter, or about negative 1
millimeter to
about positive 3 millimeters, or about zero millimeters to about positive 4
millimeters. In
some embodiments, the S-value can be more negative than about negative 2
millimeters
or more positive than about positive 4 millimeters.
In some embodiments, the H-value of the posterior sealing surface of the
prosthetic mitral valve is about 8 millimeters. In other words, in some
embodiments the
distance from the native valve annulus to the lower (inferior) edge of the
posterior sealing
surface is about 8 millimeters. While in the depicted embodiment the H-value
is about 8
millimeters, in some embodiments the H-value is in a range of about 4
millimeters to
about 6 millimeters, or about 6 millimeters to about 8 millimeters, or about 8
millimeters
to about 10 millimeters, or about 10 millimeters to about 12 millimeters, or
about 7
millimeters to about 9 millimeters. In some embodiments, the H-value can be
less than
about 4 millimeters or more than about 12 millimeters.
49
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
In some embodiments, the W-value of the posterior sealing surface of the
prosthetic mitral valve is about positive 2 millimeters. In other words, in
some
embodiments the radial distance from the upper (superior) edge of the
posterior sealing
surface to the lower (inferior) edge of the posterior sealing surface on the
prosthetic valve
is about positive 2 millimeters. A W-value of positive 2 millimeters indicates
that the
lower edge of the posterior sealing surface is positioned radially outward in
comparison
to the upper edge of the posterior sealing surface by about 2 millimeters.
This also
indicates that the sub-annular posterior valve assembly is flared or bowed
outward, such
as indicated by valve body profile line 362c. While in the depicted embodiment
the W-
value is about positive 2 millimeters, in some embodiments the W-value is in a
range of
about negative 4 millimeters to about negative 2 millimeters, or about
negative 2
millimeters to about zero millimeters, or about zero millimeters to about
positive 2
millimeters, or about positive 2 millimeters to about positive 4 millimeters,
or about
positive 1 millimeter to about positive 3 millimeters. In some embodiments,
the W-value
can be more negative than about negative 2 millimeters or more positive than
about
positive 3 millimeters.
Referring to FIG. 33, during systole the aortic valve 510 receives blood
flowing
out from the left ventricle 18. The blood flows to the aortic valve 510 via a
left
ventricular outflow tract (LVOT) 512. In some circumstances, a prosthetic
mitral valve
600 (shown without an anchor assembly for simplicity) implanted in the native
mitral
valve 17 may obstruct the LVOT 512, as represented by an obstruction 514,
resulting in
reduced ejection of blood from the left ventricle 18. As described herein, the
prosthetic
mitral valves provided by this disclosure may be configured to reduce or
eliminate LVOT
obstructions 514.
Referring to FIGS. 34 and 35, a first fluoroscopic image 700 and a second
fluoroscopic image 730 were obtained after fluoroscopic dye was injected into
the left
ventricle to enhance visualization of blood flow and blood flow obstructions.
The images
show blood flowing from the left ventricle to the aorta through the left
ventricular
outflow track (LVOT).
The first fluoroscopic image 700 illustrates an area of reduced blood flow 710
caused by an LVOT obstruction from a prosthetic mitral valve 720. The second
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
fluoroscopic image 730 illustrates improved blood flow 740 through the LVOT.
The
improved blood flow 740 can be a result of less obstruction attributable to
the prosthetic
mitral valve 750. For example, in some embodiments the prosthetic mitral valve
750 can
be positioned or designed so that less structure of the valve 750 is below the
native mitral
valve annulus, resulting in less structure of the valve 750 within the LVOT.
Additionally,
the prosthetic mitral valve 750 can be positioned or designed so that less
structure of the
valve 750 is within the LVOT such as by tapering, bowing, or shaping the
structure away
from the LVOT.
Referring again to FIG. 33, the portion of the prosthetic mitral valve 600
that
faces the aortic valve 510 is the anterior sealing surface 625a. Therefore,
the geometric
orientation of the anterior sealing surface 625a in relation to the LVOT 512
is a factor
relating to whether or not the prosthetic mitral valve 600 will cause
obstructions 514.
Referring also to FIG. 36, the geometric relationships between the LVOT 512,
the
native mitral valve annulus 28, and the anterior sealing surface variables (S-
value, H-
value, and W-value, as described in reference to FIGS. 25, 26, and 30) can be
used to
quantify LVOT obstructions 514. The angle between the LVOT 512 and the native
mitral
valve annulus 28 is identified as 0. The R-value is a variable that accounts
for prosthetic
valve positioning variations from the anticipated/ideal location of the
prosthetic valve
relative to the native valve annulus.
Using geometry, the LVOT obstruction 514 distance (identified as "0" in the
equation below) can be calculated using the following equation:
Equation #1:
0 = Rsine + \IW2 HLVOT 2 sin 0 ¨ tan-1 ¨ ¨ Scos0
where:
0 is the calculated distance of an LVOT obstruction;
R is the distance from the native valve annulus to the top of the anterior
sealing surface;
0 is the angle between the mitral valve annulus and the LVOT;
51
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
W is the radial distance from the upper edge of the sealing surface to the
lower edge of the sealing surface on the prosthetic valve;
HLVOT is the distance from the superior edge of the sealing surface to the
lower structural (frame) edge of the sealing surface on the prosthetic valve:
and
S is the radial distance from the mitral valve annulus to the adjacent
prosthetic valve surface.
Thc following examples arc provided to illustrate Equation #1 above.
HLVOT
Example R (mm) S (mm) (mm) W (mm) 00 O(mm)
1 0 2 8 -2 164 2.2
2 0 0 8 -2 119 6.0
3 0 2 8 -4 119 6.0
4 0 2 5 -4 119 3.4
5 0 0 14 -2 164 1.9
By comparing Examples #1 and #5, with Examples #2, #3, and #4 it can be
ascertained that 0 (the LVOT obstruction) tends to be less when 0 is greater.
By
comparing Example #3 and Example #4, it is seen that a greater TILvoT tends to
result in a
higher 0. By comparing Example #2 and Example #3, it can be determined that
the
effect of a greater S-value can be offset by a more negative W-value. In
summary, one of
ordinary skill in the art can use these teachings to select an R-value, S-
value, FILvo r-
value, and W-value for a given 0 (based on patient anatomy) in effort to
attain an
acceptable 0 (the LVOT obstruction).
Referring to FIGS. 37 and 38, an anchor assembly 200 can be engaged with a
native mitral valve 17 such that the feet 220a, 220b, 220c, and 220d are
seated in the sub-
annular gutter 19 of the native mitral valve 17, while the leaflets 20 and 22
and chordae
tendineae 40 are substantially unhindered by the anchor assembly 200. As
described
above, the anchor assembly 200 is designed to be implanted within a native
mitral valve
17, without substantially interfering with the native valve 17, so that the
native valve 17
can continue to function as it did prior to the placement of the anchor
assembly 200. To
accomplish that, the leaflets 20 and 22 and chordac tendineae 40, especially
the chordae
52
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
tendineae 40 that are attached to the anterior leaflet 20, need to be
substantially
unhindered by the anchor assembly 200.
In some implementations, the positioning of the hub 210 relative to the
anatomical features of the mitral valve 17 is relevant to facilitating
substantially
unhindered leaflets 20 and 22 and chordae tendineae 40. For example, a depth
810 of the
hub 210 in the left ventricle 18 is one relevant consideration. In order to
substantially
prevent hindrances to the leaflets 20 and 22 and chordae tendineae 40, the
depth 810
should be at least slightly below the coaptation depth of the mitral valve 17.
The
coaptation depth is the greatest vertical distance from the annulus of the
mitral valve 17
to an area of coaptation between the native leaflets 20 and 22. Hence,
positioning the hub
210 below the coaptation depth will facilitate substantially unhindered
leaflets 20 and 22
and chordae tendineae 40. In some implementations, the depth 810 is in a range
of about
14 mm to about 20 mm, or about 10 mm to about 16 mm, or about 12 mm to about
18
mm, or about 16 mm to about 22 mm. In some implementations, the depth 810 is
less
than about 10 mm or greater than about 22 mm.
The positioning of the hub 210 relative to the line of coaptation between the
leaflets 20 and 22 (e.g., the line of coaptation 32 shown in FIG. 8) is also
relevant to
facilitating substantially unhindered leaflets 20 and 22 and chordae tendineae
40. For
example, in some implementations positioning the hub 210 generally in vertical
alignment with the line of coaptation will serve to substantially prevent
hindrances to the
leaflets 20 and 22 and the chordae tendineae 40.
In some implementations, the angular positioning of the left anterior sub-
annular
support arm 230a, the left posterior sub-annular support arm 230b, the right
posterior
sub-annular support arm 230c, and the right anterior sub-annular support arm
230d in
relation to the native mitral valve 17 is relevant to facilitating
substantially unhindered
leaflets 20 and 22 and chordae tendineae 40. In some implementations, the sub-
annular
support arms 230a, 230b, 230c, and 230d are arranged symmetrically in relation
to a left
ventricular long axis (LAX) 840. That is, the LAX 840 bisects an anterior
support arm
angle 830 and a posterior support arm angle 820.
To minimize disturbances to the anterior leaflet 20 and chordae tendineae 40,
the
anterior support arms 220a and 220d are positioned essentially between the
chordae
53
CA 02964935 2017-04-18
WO 2016/065158
PCT/US2015/056935
tendineae 40. In some embodiments, the anterior support arm angle 830 is in a
range of
about 100 to about 135 , or about 80 to about 120 , or about 120 to about
1600. To
minimize disturbances to the posterior leaflet 22 and chordae tendineae 40, in
some
implementations the posterior support arms 220b and 220b may extend
essentially
amongst the chordae tendineae 40. In some embodiments, the posterior support
arm
angle 820 is in a range of about 50 to about 120 , or about 40 to about 80 ,
or about 60
to about 100 , or about 80 to about 120 , or about 100 to about 140 .
A number of embodiments of the invention have been described. Nevertheless, it
will be understood that various modifications may be made without departing
from the
scope of the invention. Accordingly, other embodiments are within the scope of
the
following claims.
54