Note: Descriptions are shown in the official language in which they were submitted.
NOVEL COMPOSITION AND SOLUTION WITH CONTROLLED CALCIUM ION
LEVEL, AND RELATED METHOD AND USE FOR REPERFUSION
[0001]
[0002]
FIELD
[0003] The present invention relates to novel compositions and solutions
suitable for reperfusion and also relates to post-harvest preservation and
protection
of harvested donor hearts prior to their resuscitation and transplantation
into recipient
subjects.
BACKGROUND
[0004] Heart failure affects 10% of North Americans and is the leading
hospital discharge diagnosis. The diagnosis of heart failure is accompanied by
a
survival outlook that is comparable to a major cancer. There are limited
rehabilitation options available to patients who are suffering with heart
failure, and
few strategies actually rehabiliate the heart. Cardiac transplantation remains
the
gold-standard therapeutic intervention for patients with end-stage heart
failure, with
1
Date Recue/Date Received 2022-02-03
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
an increasing number of individuals being added to the transplant waiting list
every
year. However, wider application of this life-preserving intervention is
limited by the
availability of donors. Data from the International Society of Heart and Lung
Transplantation Registry shows that cardiac transplantation is in progressive
decline in suitable donors (2007, Overall Heart and Adult Heart
Transplantation
Statistics). Two hundred and fifty eight Canadians have died during the last
decade
(2000 - 2010; Heart and Stroke Foundation of Canada) while waiting for heart
transplantation. Similarly, in the United States, 304 patients died in 2010
alone
while waiting for heart transplantation (Organ Procurement and Transplantation
Network, U.S. Dept. of Health & Human Services). This phenomenon is primarily
due to a shortage of suitable organ donors, and it is being experienced across
the
globe.
[0005] Time is of the essence for removal of a heart from a donor and its
successful transplantation into a recipient. The following conventional
principles
generally apply for optimal donor heart preservation for the period of time
between
removal from the donor and transplantation: (i) minimization of cell swelling
and
edema, (ii) prevention of intracellular acidosis, (iii) prevention of injury
caused by
oxygen free radicals, and (iv) provision of substrate for regeneration of high-
energy phosphate compounds, particularly adenosine triphosphate (ATP), during
reperfusion. There are two main sources of donor hearts for transplantation.
First,
breathing patients who have suffered irreversible loss of brain function as a
result
of blunt head trauma or intracerebral hemorrhage. Such a patient is referred
to as
a "brainstem-dead" donor or a donor after brain death ("DBD"). Second,
patients
who have suffered circulatory death. Such a patient is referred to as a "non-
heart-
beating" donor, a "cardiac dead" donor, a donor after cardiac death, or a
donor
after circulatory death (DCD).
[0006] Brainstem-dead donors can be maintained under artificial respiration
for extended periods of time to provide hemodynamic stability throughout their
bodies until the point of organ retrieval. Cardiac perfusion is uncompromised
and
organ functionality is theoretically maintained. However, brainstem death
itself can
2
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
profoundly affect cardiac function. The humoral response to brainstem death is
characterized by a marked rise in circulating catecholamines. Physiological
responses to this "catecholamine storm" include vasoconstriction, hypertension
and
tachycardia, all of which increase myocardial oxygen demand. Increased levels
of
catecholamine circulating throughout the vascular system induce
vasoconstriction,
which, in turn, compromises myocardial oxygen supply and can lead to
subendocardial ischemia. This imbalance between myocardial oxygen supply and
demand is one factor implicated in the impairment of cardiac function
following
brainstem death (Halejcio-Delophont et al., 1998, Increase in myocardial
interstitial
adenosine and net lactate production in brain-dead pigs: an in vivo
microdialysis
study. Transplantation 66(10):1278-1284; Halejcio-Delophont et al., 1998,
Consequences of brain death on coronary blood flow and myocardial metabolism.
Transplant Proc. 30(6):2840-2841. Structural myocardial damage occurring after
brainstem death is characterized by myocytolysis, contraction band necrosis,
sub-
endocardial hemorrhage, edema and interstitial mononuclear cell infiltration
(Baroldi et al., 1997, Type and extent of myocardial injury related to brain
damage
and its significance in heart transplantation: a morphometric study. J. Heart
Lung
Transplant 16(10):9941000). In spite of no direct cardiac insult, brainstem-
dead
donors often exhibit reduced cardiac function, and the current understanding
is
that only 40% of hearts can be recovered from this donor population for
transplantation.
[0007] Numerous perfusion apparatus, systems and methods have been
developed for ex vivo maintenance and transportation of harvested organs. Most
employ hypothermic conditions to reduce organ metabolism, lower organ energy
requirements, delay the depletion of high energy phosphate reserves, delay the
accumulation of lactic acid, and retard morphological and functional
deteriorations
associated with disruption of oxygenated blood supply. Harvested organs are
generally perfused in these systems with solutions comprising antioxidants and
pyruvate under low temperatures to maintain their physiological functionality.
[0008] The short-comings of hypothermic apparatus, systems and methods
3
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
have been recognized by those skilled in these arts, and alternative
apparatus,
systems and methods have been developed for preservation and maintenance of
harvested organs at temperatures in the range of about 25 C to about 35 C
(this
can be referred to as "normothermic" temperatures, though normothermic more
conventionally means a normal body temperature, i.e., an average of about 37
C). Normothermic systems typically use perfusates based on the ViaspanTM
formulation (also known as the University of Wisconsin solution or UW
solution)
supplemented with one or more of the following: serum albumin as a source of
protein and colloid; trace elements to potentiate viability and cellular
function;
pyruvate and adenosine for oxidative phosphorylation support; transferrin as
an
attachment factor; insulin and sugars for metabolic support; glutathione to
scavenge
toxic free radicals as well as a source of impermeant; cyclodextrin as a
source of
impermeant, scavenger, and potentiator of cell attachment and growth factors;
a
high Mg2+ concentration for microvessel metabolism support;
mucopolysaccharides
for growth factor potentiation and hemostasis; and endothelial growth factors.
For
instance, Viaspan comprises potassium lactobionate, KH2PO4, MgSO4, raffinose,
adenosine, glutathione, allopurinol, and hydroxyethyl starch. Other
normothermic
perfusion solutions have been developed and used (Muhlbacher et al., 1999,
Preservation solutions for transplantation. Transplant Proc. 31(5):2069-2070).
While harvested kidneys and livers can be maintained beyond twelve hours in
normothermic systems, normothermic bathing and maintenance of harvested
hearts by perfusion beyond 12 hours results in deterioration and irreversible
debilitation of the hearts' functionality. Another disadvantage of using
normothermic, continuous-pulsed-perfusion systems for maintenance of harvested
hearts is the time required to excise a heart from a donor, mount it into the
normothermic perfusion system and then initiate and stabilize the perfusion
process.
[0009] After the excised donor heart has been stabilized, its physiological
functionality is determined and, if transplantation criteria are met, the
excised heart
is transported as quickly as possible to a transplant facility.
4
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
[0010] In the case
of brainstem-dead donors, the heart generally is warm and
beating when it is procured. It is then stopped, cooled, and put on ice until
it is
transplanted. Chilling the harvested heart reduces its metabolic activity and
related
demands by about 95%. However, some metabolic activity continues with the
consequence that the heart muscle begins to die, and clinical data have shown
that
once the period of chilling of a harvested heart is prolonged beyond 4 hours,
the risk
of 1-year mortality post-transplant starts to rise. For example, risk of death
at 1-year
post-transplant for a recipient receiving a heart that has been preserved by
chilling
for six hours more than doubles compared to a recipient receiving a heart that
has
been chilled for less than 1 hour (Taylor et al., 2009, Registry of the
International
Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Heart
Transplant Report- 2009. JHLT 28(10):1007-1022).
[0011] Well-
defined criteria have been developed for harvesting organs for
transplantation from non-heart-beating donors (Kootstra et al., 1995,
Categories of
non-heart-beating donors. Transplant Proc. 27(5):2893-2894; Bos, 2005, Ethical
and legal issues in non-heart-beating organ donation. Transplantation, 2005.
79(9): p. 1143-1147). Non-heart-beating donors have minimal brain function but
do not meet the criteria for bra instem death, and therefore such donors
cannot be
legally declared brainstem dead. When it is clear that there is no hope for
meaningful recovery of the patient, the physicians and family must be in
agreement to withdraw supportive measures. Up to this point in care, non-heart-
beating patients are often supported with mechanical ventilation as well as
intravenous inotropic or vasopressor medication. However, only those patients
with single system organ failure, namely failure of the neurologic system, can
be
considered for organ donation. Withdrawal of life support, most commonly the
cessation of mechanical ventilation, is followed by anoxic cardiac arrest,
after
which the patient must remain asystolic for five minutes before organ
procurement is allowed. Consequently, the organs of non-heart-beating donors
are necessarily exposed to variable periods of warm ischemia after cardiac
arrest,
which may result in varying degrees of organ damage. However, provided that
the
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
duration of warm ischemia is not excessive, many types of organs, such as
kidneys, livers, and lungs, can be harvested from non-heart-beating donors and
are able to recover function after transplantation with success rates that
approximate those for transplanted organs from brainstem-dead donors. While
hearts harvested from brain-dead donors are exposed to an ischemic period
limited to the time from organ procurement to transplant, hearts harvested
from
donors after cardiac death are exposed to much greater ischemic insult events,
including a hypoxemic arrest event, warm ischemic injury occurring during the
mandatory five-minute stand-off period before organ harvesting may be
commenced, and further ischemic injury occurring during reperfusion of the
heart
after it is harvested. Because of the ischemic damage that occurs before organ
harvesting commences, hearts from non-heart-beating donors are not used for
transplantation.
SUMMARY
[0012] The present disclosure includes a novel solution comprising a
preservation mixture comprising a calcium ion source; anda buffer for
maintaining
a pH of the solution, wherein the molar concentration of calcium ion (Ca2+) in
the
solution is from 0.18 to 0.26 mmol/L, and the pH is lower than 7.4 and higher
than
6.6. The molar concentration of calcium ion (Ca2+) may be 0.22 mmol/L. The pH
may be from 6.8 to 7.0, such as 6.9. The preservation mixture may be a
cardioplegia mixture comprising adenosine, lidocaine, and a magnesium ion
source. The solution may comprise 0.3 to 0.45 mmol/L of adenosine, 0.04 to
0.09
mmol/L of lidocaine, and 11 to 15 mmol/L of Mg2+. The solution may comprise a
sodium ion source and a potassium ion source. The solution may comprise about
130 to about 160 mmol/L of Na + and 4 to 7 mmol/L of K. The solution may
comprise chloride, an osmotic buffer and a reducing agent. The solution may
comprise 70 to 140 or 70 to 180 mmol/L of chloride, 8 to 12.5 mmol/L of
glucose,
7.5 to 12.5 IU/L of insulin, 100 to 140 mmol/L of D-mannitol, 0.75 to 1.25
mmol/L
of pyruvate, and 2.5 to 3.5 mmol/L of reduced glutathione. The solution may
6
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
comprise 0.3 to 0.45 mmol/L of adenosine; 0.04 to 0.09 mmol/L of lidocaine; 8
to
12.5 mmol/L of glucose; 110 to 130 mmol/L of NaCl; 4 to 7 mmol/L of KCl; 16 to
24
mmol/L of NaHCO3; 0.9 to 1.4 mmol/L of NaH2PO4; 0.18 to 0.26 mmol/L of CaCl2;
11 to 15 mmol/L of MgCl2; 7.5 to 12.5 IU/L of insulin; 100 to 140 mmol/L of D-
mannitol; 0.75 to 1.25 mmol/L of pyruvate; and 2.5 to 3.5 mmol/L of reduced
glutathione. The solution may comprise 0.4 mmol/L of adenosine; 0.05 mmol/L of
lidocaine; 10 mmol/L of glucose; 123.8 mmol/L of NaCI; 5.9 mmol/L of KCl; 20
mmol/L of NaHCO3; 1.2 mmol/L of NaH2PO4; 0.22 mmol/L of CaCl2; 13 mmol/L of
MgCl2; 10 IU/L of insulin; 120 mmol/L of D-mannitol; 1 mmol/L of pyruvate; and
3
mmol/L of reduced glutathione.
[0013] A
composition for preparing the solution described in the preceding
paragraph is also provided. The composition may comprise adenosine, lidocaine,
and a calcium source, wherein the molar ratio of adenosine:calcium is from
0.3:0.26 to 0.45:0.18, and the molar ratio of lidocaine:calcium is from
0.04:0.26 to
0.09:0.18. The molar ratio of adenosine:calcium may be 0.4:0.22, and the molar
ratio of lidocaine:calcium may be 0.05:0.22. The composition may further
comprise
a sodium source, a potassium source and a magnesium source, wherein the molar
ratio of calcium:sodium is from 0.26:130 to 0.18:160, the molar ratio of
calcium:potassium is from 0.26:4 to 0.18 to 7, and the molar ratio of
calcium:magnesium is from 0.26:11 to 0.18:15. The molar ratio of
calcium:sodium
may be 0.22:147, the molar ratio of calcium:potassium may be 0.22:5.9, and the
molar ratio of calcium:magnesium may be 0.22:13. The composition may also
comprise chloride, glucose, insulin, D-mannitol, pyruvate, and reduced
glutathione.
[0014] The
solution as described herein may be used to reperfuse a donor
heart and the present disclosure includes a method of reperfusion of a donor
heart
and use of the solution described herein for reperfusion of a donor heart. The
heart may be reperfused with the solution during removal of the heart from the
donor. The heart after removal from the donor may be reperfused in a
reperfusion
device. The heart may be reperfused with the solution for at least 3 minutes
immediately after removal of the heart from the donor. The donor may be a
donor
7
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
after circulatory death. The reperfusion may be at a temperature above about
25
C and below about 37 C. The reperfusion may be at a temperature of about 35
C during reperfusion.
[0015] In such method or use, selected embodiments of the present
disclosure relate to solutions for immersion and bathing of a harvested heart
while
being concurrently flowed through the heart and its vasculature.
[0016] Some embodiments of the present disclosure pertain to use of
solutions for ex vivo maintenance of harvested hearts to reduce and ameliorate
post-harvest ischemic damage.
[0017] Some embodiments of the present disclosure pertain to methods for
ex vivo maintenance of harvested hearts to minimize the occurrence and extent
of
post-harvest ischemic damage.
BRIEF DESCRIPTION OF THE DRAWINGS
[0018] In the figures, which illustrate, by way of examples only,
embodiments of this invention:
[0019] Figure 1 ("FIG. 1) is a schematic flowchart outlining the
experimental
protocols used in Example 1;
[0020] Fig. 2 is a chart showing the myocardial temperature achieved in
harvested pig hearts after an initial 3-minute reperfusion period;
[0021] Fig. 3 is a chart showing the effect of reperfusate temperature on
the
coronary blood flow through harvested pig hearts, measured after the initial 3-
minute reperfusion period;
[0022] Fig. 4 is a chart showing the effect of reperfusate temperature on
coronary vascular resistance to blood flow through harvested pig hearts,
8
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
measured after the initial 3-minute reperfusion period;
[0023] Fig. 5 is a chart showing the effect of reperfusate temperature on
coronary sinus lactate washout from harvested pig hearts, measured after the
initial 3-minute reperfusion period;
[0024] Fig. 6 is a chart showing the effect of reperfusate temperature on
the
accumulation of Troponin I (a marker of myocardial injury) in the perfusate
solution, measured 5 hours after harvest of the pig hearts;
[0025] Fig. 7(A) is a representative micrograph of a section through a
harvested pig heart reperfused at 5 C showing swollen endothelial cells
lining a
capillary, while Fig. 7(B) is a representative micrograph of a section through
a
harvested pig heart reperfused at 35 C showing normal endothelial cells
lining a
capillary;
[0026] Fig. 8 is a chart presenting the average extent of injury to
endothelial
cells and myocytes in harvested pig hearts, as observed in electron-microscopy
micrographs and scored with a scoring system, as a function of reperfusion
temperature;
[0027] Fig. 9 is a chart showing the effect of reperfusate temperature on
the
cardiac index of harvested pig hearts, measured 1 hour ("T1"), 3 hours ("13"),
and
hours ("T5") after harvest of the pig hearts;
[0028] Fig. 10 is a chart showing the effect of reperfusate temperature on
the systolic function of harvested pig hearts, measured 1 hour ("T1"), 3 hours
("T3"), and 5 hours ("T5")after harvest of the pig hearts;
[0029] Fig. 11 is a chart showing the effect of reperfusate temperature on
the
diastolic function of harvested pig hearts, measured 1 hour ("T1"), 3 hours
("T3"),
and 5 hours ("T5") after harvest of the pig hearts;
[0030] Fig. 12 is a schematic chart outlining the temperatures and Ca2+
ion
9
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
concentrations of the cardioplegic solutions used in Example 2;
[0031] Fig. 13 is a schematic flowchart outlining the experimental
protocols
used in Example 2;
[0032] Fig. 14 is a chart showing the effect of Ca2+ ion concentration in
the
reperfusate on weight gain in harvested pig hearts measured 1 hour after
harvest;
[0033] Fig. 15 is a chart showing the effect of Ca2+ ion concentration in
the
reperfusate on the cardiac output of harvested pig hearts measured 1 hour
after
harvest;
[0034] Fig. 16 is a chart showing the effect of Ca2+ ion concentration on
the
contractility of the left ventricle during systole in harvested pig hearts,
measured 1
hour after harvest;
[0035] Fig. 17 is a chart showing the effect of Ca2+ ion concentration on
relaxation of the left ventricle during diastole in harvested pig hearts,
measured 1
hour after harvest;
[0036] Fig. 18 is a schematic chart outlining the temperatures, Ca2+ ion
concentrations, and pH values of the cardioplegic solutions used in Example 3;
[0037] Fig. 19 is a schematic flowchart outlining the experimental
protocols
used in Example 3;
[0038] Fig. 20 is a chart showing the effect of pH of the cardioplegic
reperfusate solution on weight gain in harvested pig hearts, measured 1 hour
after
harvest;
[0039] Fig. 21 is a chart showing the effect of pH of the cardioplegic
reperfusate solution on the cardiac output of harvested pig hearts, measured 1
hour after harvest;
[0040] Fig. 22 is a chart showing the effect of pH of the cardioplegic
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
reperfusate solution on the contractility of the left ventricle during systole
in
harvested pig hearts, measured 1 hour after harvest;
[0041] Fig. 23 is a chart showing the effect of pH of the cardioplegic
reperfusate solution on relaxation of the left ventricle during diastole in
harvested
pig hearts, measured 1 hour after harvest;
[0042] Fig. 24 is a schematic chart outlining the temperatures, Ca2+ ion
concentrations, and pH values of the cardioplegic reperfusate solutions, and
the
duration of reperfusion times used in Example 4;
[0043] Fig. 25 is a schematic flowchart outlining the experimental
protocols
used in Example 4, Part 1;
[0044] Fig. 26 is a chart showing the effect of duration of initial
reperfusion
on weight gain in harvested pig hearts;
[0045] Fig. 27 is a chart showing the effects of duration of initial
reperfusion
on myocardial function of harvested pig hearts, measured 1 hour ("T1"), 3
hours
("T3"), and 5 hours ("T5") after harvest;
[0046] Fig. 28 is a schematic flowchart outlining the experimental
protocols
used in Example 4, Part 2;
[0047] Fig. 29 is a chart showing the effect of an extended initial
reperfusion
with a cardioplegic reperfusate solution having a reduced concentration of
anesthetic on weight gain in harvested pig hearts;
[0048] Fig. 30 is a chart showing the effect of extended initial
reperfusion
with a cardioplegic reperfusate solution having a reduced concentration of
anesthetic on myocardial function of harvested pig hearts, measured 1 hour
("T1"),
3 hours ("T3"), and 5 hours ("15") after harvest; and
[0049] Fig. 31 is a chart showing the effect of anesthetic concentrations
in
cardioplegic reperfusate solutions on myocardial function of harvested pig
hearts,
11
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
measured 1 hour ("T1"), 3 hours ("T3"), and 5 hours ("T5") after harvest.
DETAILED DESCRIPTION
[0050] Unless otherwise defined, all technical and scientific terms used
herein have the same meaning as commonly understood by one of ordinary skill
in
the art to which this invention belongs. In order that the invention herein
described
may be fully understood, the following terms and definitions are provided
herein.
[0051] The word "comprise" or variations such as "comprises" or
"comprising" will be understood to imply the inclusion of a stated integer or
groups
of integers but not the exclusion of any other integer or group of integers.
[0052] The term "about" or "approximately" means within 20%, preferably
within 10%, and more preferably within 5% of a given value or range.
[0053] The term "afterload" means the mean tension produced by a
chamber of the heart in order to contract. It can also be considered as the
`load'
that the heart must eject blood against. Afterload is therefore a consequence
of
aortic large vessel compliance, wave reflection and small vessel resistance
(left
ventricular afterload) or similar pulmonary artery parameters (right
ventricular
afterload).
[0054] The term "preload" refers to the stretching of a single cardiac
myocyte immediately prior to contraction and is therefore related to the
sarcomere
length. Since sarcomere length cannot be determined in the intact heart, other
indices of preload such as ventricular end diastolic volume or pressure are
used.
As an example, preload increases when venous return is increased.
[0055] The term "cardiac myocyte" means a cardiac muscle cell.
[0056] The term "stroke volume" (SV) means the volume of blood ejected by
the right/left ventricle in a single contraction. It is the difference between
the end
12
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
diastolic volume (EDV) and the end systolic volume (ESV). Mathematically, SV =
EDV ¨ ESV. The stroke volume is affected by changes in preload, afterload and
inotropy (contractility). In normal hearts, the SV is not strongly influenced
by
afterload whereas in failing hearts, the SV is highly sensitive to afterload
changes.
[0057] The term "stroke work" (SW) refers to the work performed by the
left
or right ventricle to eject the stroke volume into the aorta or pulmonary
artery,
respectively. The area enclosed by the pressure/volume loop is a measure of
the
ventricular stroke work, which is a product of the stroke volume and the mean
aortic or pulmonary artery pressure (afterload), depending on whether one is
considering the left or the right ventricle.
[0058] The term "ejection fraction" (EF) means the fraction of end
diastolic
volume that is ejected out of the ventricle during each contraction.
Mathematically,
EF = SV/EDV. Healthy ventricles typically have ejection fractions greater than
0.55. Low EF usually indicates systolic dysfunction and severe heart failure
can
result in EF lower than 0.2. EF is also used as a clinical indicator of the
inotropy
(contractility) of the heart. Increasing inotropy leads to an increase in EF,
while
decreasing inotropy decreases EF.
[0059] The term "end systolic pressure volume relationship" (ESPVR)
describes the maximal pressure that can be developed by the left ventricle at
any
given left ventricular volume, or alternatively, by the right ventricle at any
given
right ventricular volume. This implies that the PV loop cannot cross over the
line
defining ESPVR for any given contractile state. The slope of ESPVR (Ees)
represents the end-systolic elastance, which provides an index of myocardial
contractility. The ESPVR is relatively insensitive to changes in preload,
afterload
and heart rate. This makes it an improved index of systolic function over
other
hemodynamic parameters like ejection fraction, cardiac output and stroke
volume.
The ESPVR becomes steeper and shifts to the left as inotropy (contractility)
increases. The ESPVR becomes flatter and shifts to the right as inotropy
decreases.
13
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
[0060] The term "preload recruitable stroke work relationship" (PRSW)
means a measure of cardiac contractility, and is the linear relationship
between
SW and EDV.
[0061] The term "pressure-volume area" (PVA) means the total mechanical
energy generated by ventricular contraction. This is equal to the sum of the
stroke
work (SW), encompassed within the PV loop, and the elastic potential energy
(PE). Mathematically, PVA = PE + SW.
[0062] The term "dP/dt max" is a measure of the global contractility of the
left ventricle. The greater the contractile force exerted during systole, the
greater
the rate of increase in left ventricular pressure.
[0063] The term "dP/dt min" is a measure of the relaxation of the left
ventricle during diastole.
[0064] As used herein, the term "DCD" means donor after circulatory death,
or
donor after cardiac death. As used herein, the term "DBD" means donor after
brain
death.
[0065] The term "Langendorff perfusion" refers to a method of perfusing an
excised heart with a nutrient-rich oxygenated solution in a reverse fashion
via the
aorta. The backwards pressure causes the aortic valve to shut, thereby forcing
the
solution into the coronary vessels that supply the heart tissue with blood.
This
transports nutrients and oxygen to the cardiac muscle, allowing it to continue
beating for several hours after its removal from the animal.
[0066] The term "working heart" as used herein, refers to clinical ex vivo
coronary perfusion throughout an excised heart by ventricular filling via the
left
atrium and ejection from the left ventricle via the aorta driven by the
heart's
contractile function and regular cardiac rhythm. The excised heart is attached
by
cannulae to a perfusate reservoir and circulatory pumps in a Langendorff
preparation. The flow of perfusate through the excised heart in "working
heart"
14
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
mode is in the direction opposite to the flow of perfusate during Langendorff
perfusion.
[0067] The term "ischemia" means a condition that occurs when blood flow
and oxygen are kept from the heart.
[0068] The term "reperfusion" as used herein means passing a solution
through a heart to re-establish supply of oxygen and provide protective or
preservation materials to the heart, such as by pumping the solution through
the
heart in a perfusion device, and optionally immersing the heart in the
solution.
Optionally, during reperfusion the heart may be immersed in an oxygen-rich
perfusate solution, which may be the same as the reperfusion solution or may
be a
different solution.
[0069] The term "reperfusion injury" as used herein refers to tissue damage
in a harvested heart that occurs when a supply of oxygen via a perfusate
solution
is provided to the tissue after a period of ischemia or lack of oxygen.
Depriving the
heart of sufficient oxygen and nutrients during the ischemic period creates a
condition in which the restoration of circulation results in inflammation and
oxidative damage through the induction of oxidative stress, rather than
restoration
of normal function.
[0070] The term ''cardioplegia" as used herein means an intentional and
temporary cessation of, or maintenance of ceased or reduced, cardiac
activities,
such as by arresting or stopping the beating of the heart, for the purpose of
preserving the health of the myocardium, including through a period of
significantly
reduced provision of oxygen and metabolic substrate. Cardioplegia can be
imposed on a beating heart by chilling or by administration of a solution
containing
one or more chemicals that will cause paralysis of the heart muscle, or by
both
concurrently. In embodiments of the present disclosure, cardioplegia may also
be
achieved by providing limited oxygen and other supplies to the myocardium to
preserve its health without fully restoring the cardiac activities of the
heart.
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
[0071] The term "cardioplegic solution" as used herein means a solution
containing chemical components that cause or maintain asystole (paralysis) of
the
heart in a mixture with components to preserve or protect heart cell
functions.
[0072] The term "homeostasis" as used herein means the maintenance of a
fairly stable metabolic equilibrium within and between the muscle cells of a
harvested heart.
[0073] The term "normokalemic" as used herein means having or
characterized by a normal concentration of potassium ion in the blood. Normal
serum potassium ion levels in human blood are in a range between 3.5 mEq/L and
5.0 mEq/L.
[0074] The term "hyperkalemic" as used herein means having or
characterized by a concentration of potassium ion in the blood that is
significantly
elevated over a normokalemic concentration. A hyperkalemic concentration
includes any potassium ion concentration in excess of 6.0 mEq/L.
[0075] The term "hypothermic" as used herein means a temperature that is
less than about 20 C.
[0076] The medically and legally prescribed events that must occur for
ethical
procurement of transplantable hearts from donors after circulatory death (DCD)
inevitably cause an occurrence of cardiac arrest and a sequence of ischemic
events
resulting in damage to the heart muscles. These prescribed events cannot be
modified.
[0077] lschemia is accompanied by significant changes in ion-exchange
patterns into and out of heart muscle cells as a consequence, primarily, of
the loss
of oxygen supply. As the availability of oxygen decreases and stops, the
metabolism of the heart muscle cells shifts from aerobic to anaerobic with an
immediate consequence of rapidly decreasing intracellular pH levels. Low
intracellular pH results in increasing amounts of I-I+ ions being excreted
from the
16
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
muscle cells into the extracellular spaces. At the same time, the ion
potential
across the cellular membranes diminishes due to significantly reduced Na+/Ca2+
ion exchange as a result of lower intracellular ATP levels. The ultimate
result is an
increasing overload in intracellular Ca2+ levels. The increased levels of
intracellular
Ca2+ activate Ca2+-dependent proteases, which disrupt cell structure resulting
in
cell death. The severity of such damage increases with the duration of the
ischemic conditions.
[0078] lschemic damage occurring during the procurement of a donor heart
may be reduced by reperfusion of the harvested heart as soon as possible after
its
harvest in blood or a blood replacement product, as exemplified by Viaspan and
CELSIOR(5 (CELSIOR is a registered trademark of Genzyme Corp., Cambridge,
Massachusetts, U.S.A.). Reperfusion causes a prompt increase in the
extracellular
pH, which results in robust excretion of H+ ions into the extracellular space.
1-1+ ion
movement into the extracellular space drives Na + ions into the cells. Higher
intracellular Na + ion concentrations reverse the Na+/Ca2+ ion exchanger
across the
myocyte cell membranes, resulting in "reverse mode" excretion of accumulated
intracellular Na + ions accompanied by an influx of Ca2 ions, recovery of ATP
synthesis, and a subsequent re-excretion of Ca2+ ions. However, although
reperfusion may re-establish aerobic respiration and metabolism in harvested
hearts, reperfusion commonly results in further damage (known as reperfusion
injury) to the heart muscle cells. For example, the immediate increase in
intracellular pH levels results in the generation of reactive oxygen species
that
activate subcellular signals that in turn activate inflammatory cascades
leading to
apoptosis and cytokine release. Additionally, reactive oxygen species directly
disrupt DNA structures and protein structures, thereby causing cell death.
Another
problem associated with conventional reperfusion techniques is that it is very
difficult in these techniques to modulate the intracellular levels of Ca2+
ions during
the reperfusion process, where reperfusion further increases the intracellular
overload of Ca2+ ions in heart muscle cells.
[0079] Contraction of a heart while the heart muscle cells are overloaded
17
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
with intracellular Ca2+ ions during reperfusion inevitably results in a
disruptive type
of necrosis, termed contraction band necrosis, as a result of massive
myofibril
contraction. Contraction band necrosis is considered to be the most severe
form of
reperfusion injury.
[0080] Accordingly, the rationale for chilling donor hearts immediately
after
their procurement and during reperfusion is to reduce metabolic activity
within the
heart muscle cells as quickly as possible to minimize the generation of
reactive
oxygen species during reperfusion and to minimize a subsequent intracellular
overload of Ca2+ ions during reperfusion.
[0081] We have discovered that myocardial injury to donor hearts may be
minimized by a strategy focused on maintaining calcium ion homeostasis in and
about the heart during the harvesting and the reperfusion processes. Our
strategy
comprises two components wherein the first component is an oxygenated
cardioplegic composition for use as reperfusate solution during procurement of
a
harvested heart and for a period of time immediately after harvest during
which the
harvested heart is reperfused, preferably, for at least 3 minutes. The
reperfusate
solution causes an immediate cessation of a donor heart's rhythmic beating
upon
reperfusion. The at-least-3-minute reperfusion period, starting immediately
after
the heart is harvested, is referred to as the immediate ¨ early ("IE") period.
The
second component of our strategy is to avoid chilling the heart during
procurement
process and during the post-harvest reperfusion period, and instead maintain
normothermic conditions during harvest, during IE reperfusion, and during
subsequent ex vivo maintenance of the harvested heart.
[0082] It has been recognized that it would be beneficial to prevent
myocyte
contraction before intracellular calcium overload in a donor heart has been
resolved, and before ATP stores in the heart can be repleted. It is expected
that
after a period of reperfusion or perfusion, as oxygen and energy substrates
are
delivered to the heart, the heart can start beating again. However, if the
heart
starts beating again when there is intracellular calcium overload, it can
result in
18
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
contracture. Thus, it is expected that reducing intracellular calcium ion
concentration to eliminate or prevent intracellular calcium overload before
restarting myocyte contraction, or fully restoring cardiac activities, can
reduce
reperfusion injuries. Our results indicate that intracellular calcium
concentrations
and consequently reperfusion injuries may be reduced by controlling, at least
in
part, the calcium contents in the reperfusion solution.
[0083] When selecting components and their concentrations in a
cardioplegic composition for reperfusion, for example of a DCD heart for
transplant, at temperatures from about 25 to about 37 C, a number of factors
may
need to be considered. To reduce or minimize myocardial injury to such a donor
heart during reperfusion, a balanced approach in view of these factors may be
required. For example, a source of potential complication is that the
intracellular
concentrations of a particular ion, especially the intracellular concentration
of Ca2+
or H+ ions, which if not properly controlled could contribute to myocardial
injury,
can be sensitive to the extracellular concentrations of these ions as well as
other
ions. For instance, the intracellular concentration of Ca2+ in myocytes is
expected
to be affected not only by the extracellular concentration of Ca2+, but also,
as a
result of particular ion exchanges in the plasma membrane, by extracellular
concentrations of other ions, such as I-1+ and Na. Thus, the intracellular
calcium
ion concentration may be adjusted by changing extracellular concentration of
one
or more of Ca2+, Na + and H+. However, changing the extracellular
concentrations
of H.* and Na + may result in other changes which can affect other aspects of
myocardial injury, in addition to optimizing intracellular Ca2+. Another
factor to be
considered is provision of sufficient calcium ions in the reperfusion solution
to
avoid a phenomenon known as "calcium paradox" - where hypocalcemic cardiac
muscles are re-exposed to normal level of Ca2+, the cells can become
overloaded
with Ca2+, which can cause significant cell injuries or damages. To achieve
the
optimal results, these different effects should be considered in a balanced
approach when selecting the components and their respective concentrations.
[0084] In an embodiment, a solution for use as a reperfusion solution may
19
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
include the following components:
- A preservation mixture which may include adenosine to provide
oxidative phosphorylation support, and lidocaine to prevent myocyte
contraction during reperfusion. Additionally, a relatively high
concentration of Mg2+ may also be included, as hypermagnesemia is
also expected to assist in prevention of myocyte contraction during
reperfusion. For example, the mixture may contain 0.3 to 0.45 mmol/L of
adenosine, 0.04 to 0.09 mmol/L of lidocaine and 11 to 15 mmol/L of
mg2+.
Ca2+ at a concentration of 0.18 mmol/L to 0.26 mmol/L, to provide for a
lower than the physiological concentration of extracellular calcium ions
in a normal heart.
Na, such as at a concentration of 130 mmol/L to 160 mmol/L, to provide
for an appropriate concentration of extracellular sodium ion.
- lc in a normakalemic concentration, such as, 4 to 7 mmol/L.
- CI" in a concentration ranging, for example, from 70 to 180 mmol/L.
While in some embodiments, the CI" concentration may be higher, such
as up to about 180 mmol/L in the solution, it may be beneficial in some
embodiments to have a lower CI" concentration such as for example,
from 70 to 140 mmol/L, or up to about 140 mmol/L.
- A pH-buffer for maintaining the pH of the reperfusion solution to be
higher than 6.7 and less than 7.4 at the desired operation temperature
for reperfusion. The pH-buffer may be provided by, for example, a
combination of 16 to 24 mmol/L of HC031" and 0.9 to 1.4 mmol/L of
H2P041 .
- Substrates for energy metabolism, such as a combination of 8 to 12.5
mmol/L of glucose and 0.75 to 1.25 mmol/L of pyruvate.
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
- An osmotic agent in a concentration for obtaining an appropriate
osmolarity, such as, 100 to 140 mmol/L of D-mannitol.
- An antioxidant or reducing agent in a concentration for obtaining an
appropriate degree of protection from reactive oxygen species and
physiological levels of reduction, such as, 2.5 to 3.5 mmol/L of reduced
glutathione.
- Optionally, one or more growth factors, such as, 7.5 to 12.5 IU/L of
insulin.
[0085] During use,
a pre-prepared cardioplegic composition may be titrated
to the desired pH prior to use, such that the composition at the desired
temperature for reperfusion is at the desired pH at the moment of reperfusion.
[0086] A
cardioplegic composition for causing an immediate cessation of a
donor heart's rhythmic beating upon its contact with the cardioplegic
composition
may comprise an adenosine-lidocaine mixture, a normokalemic concentration of
potassium ions, a concentration of Ca2 ions selected to maintain the
intracellular
level of Ca2+ ions in the harvested heart's muscle cells at about 104 mmol/L,
and a
pH of 6.9. A suitable adenosine-lidocaine mixture may comprise 300 pmol/L, 325
pmol/L, 350 pmol/L, 375 pmol/L, 400 pmol/L, 425 pmol/L, 450 pmol/L of
adenosine and 40 pmol/L, 45 pmol/L, 50 pmol/L, 55 pmol/L, 60 pmol/L, 70
pmol/L,
80 pmol/L, 90 pmol/L of lidocaine. The cardioplegic composition may
additionally
comprise 8.012.5 mmol/L of glucose, 120-140 mmol/L of NaCI, 4.0-7.0 mmol/L of
KCL, 12.0-16.0 mmol/L of NaHCO3, 0.9-1.4 mmol/L of NaH2PO4, 0.18-0.26
mmol/L of CaCl2, 11.0-15.0 mmol/L of MgCl2, 7.5-12.5 IU/L of insulin, 100.0-
140.0
mmol/L of D-mannitol, 0.75-1.25 mmol/L of pyruvate, and 2.5-3.5 mmol/L of
reduced glutathione. In a particular embodiment, a cardioplegic composition
may
include 400 pmol/L of adenosine, 50 pmol/L of lidocaine, 10.0 mmol/L of
glucose,
123.8 mmol/L of NaCI, 5.9 mmol/L of KCI, 20 mmol/L of NaHCO3, 1.2 mmol/L of
NaH2PO4, 0.22 mmol/L of CaCl2, 13.0 mmol/L of MgCl2, 10.0 IU/L of insulin,
120.0
mmol/L of D-mannitol, 1.0 mmol/L of pyruvate, and 3.0 mmol/L of reduced
21
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
glutathione.
[0087] The cardioplegic composition may be oxygenated by bubbling a
stream of 02 gas through the cardioplegic composition prior to and during its
use
for bathing and reperfusing a harvested donor.
[0088] Another selected embodiment of the present disclosure pertains to
use of the selected oxygenated cardioplegic composition to reperfuse a
harvested
heart at a temperature of about 35 C. Accordingly, the selected oxygenated
cardioplegic composition is warmed to about 35 C before contacting the heart
during procurement and subsequent IE reperfusion for at least 3 minutes after
procurement has been completed. After the initial IE reperfusion period in the
selected oxygenated cardioplegic composition under normothermic conditions,
the
harvested heart may be resuscitated by installation into a suitable apparatus
for ex
vivo maintenance of a functioning systolic harvested heart, by interconnection
of
conduit infrastructures provided within the apparatus with the heart's aorta,
pulmonary artery, pulmonary vein, and vena cava, and bathing the excised heart
in
a constantly flowing perfusate solution comprising oxygenated blood and/or an
oxygenated blood replacement solution. Additionally the constantly flowing
perfusion solution is flowed through the heart's chambers while it is
maintained in
the apparatus. Such apparatus are generally configured with the following: (i)
a
perfusate pumping system;(ii) flow sensors for monitoring the flow of
perfusate to
and from the installed heart's aorta, pulmonary artery, pulmonary vein, and
vena
cava; (iii) an ECG apparatus interconnectable with the excised heart; (v)
probes
interconnecting the installed heart with instruments for monitoring the
excised
heart's physiological functionality using load independent indices and load
dependent indices; and optionally (vi) pacemakers for initiating or
maintaining
systolic function of the heart.
[0089] It is expected that use of an example oxygenated cardioplegic
composition disclosed herein to reperfuse a heart removed from a donor for
transplant may provide a harvested heart with the ionic complement necessary
for
22
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
the ex vivo-maintained heart to continue generating ATP and pumping excess
calcium out of the heart muscles cells while keeping the heart in a paralyzed
condition i.e., a non-beating asystolic condition, thereby minimizing the
potential
for occurrence of contraction band necrosis. While not wishing to be bound by
any
particular theory, it is likely that use of such a cardioplegic composition
for
reperfusion of harvested hearts at temperatures from about 25 to about 35 C
can
facilitate rapid restoration of calcium ion homeostasis and facilitate more
rapid
recovery and functional operation of the harvested heart after transplantation
into
a recipient subject.
[0090] Without being limited to any particular theory, it is also expected
that
when a heart removed from a DCD donor is reperfused immediately after its
removal from the donor with a suitable cardioplegic solution with controlled
calcium ion concentration and pH for a sufficient time, it is possible to
avoid
excessive reperfusion injuries, such as those caused by intracellular calcium
overload, in the heart, without chilling the DCD heart to below about 25 C
before,
during and after reperfusion, and to provide a heart suitable for
transplantation.
[0091] In an embodiment, such a solution may include a cardioplegia
mixture. The mixture contains a calcium ion source and a buffer for
maintaining a
pH of the solution. The molar concentration of calcium ion (Ca2+) in the
solution is
from 0.18 to 0.26 mmol/L and the pH is lower than 7.4 and higher than 6.6. The
molar concentration of calcium ion (Ca2+) in the solution may be 0.22 mmol/L.
The
pH may be from 6.8 to 7.0, such as 6.9. In specific embodiments, the
cardioplegia
mixture may include adenosine, lidocaine, and a magnesium ion source, such as
0.3 to 0.45 mmol/L of adenosine, 0.04 to 0.09 mmol/L of lidocaine, and 11 to
15
mmol/L of Mg2+. The solution may also include a sodium ion source and a
potassium ion source, such as about 130 to about 160 mmol/L of Na + and 4 to 7
mmol/L of K+. The solution may further include chloride, an osmotic buffer and
an
antioxidant or reducing agent. For example, suitable osmotic buffers may
include
D-manitol, lactobionate, dextran, albumin, or the like. Suitable antioxidants
may
include reduced glutathione, resveratrol, apelin analogs or the like. The
solution
23
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
may contain, for example, 70 to 140 mmol/L chloride, 100 to 140 mmol/L of D-
mannitol, and 2.5 to 3.5 mmol/L of reduced glutathione. The solution may
contain
substrates for energy metabolism, such as one or more of glucose, pyruvate,
free
fatty acids (e.g. oleate or palmitate), triglycerides, or the like. For
instance, in some
embodiments, the solution may contain 8 to 12.5 mmol/L of glucose and 0.75 to
1.25 mmol/L of pyruvate. The solution may contain one or more growth factors,
such as insulin, cardiotrophin-1, erythropoietin, platelet-derived growth
factors
(PDGF), various forms of fibroblast growth factors (FGF), or the like. For
example,
the solution may contain 7.5 to 12.51U/L of insulin. Thus, depending on the
application, the solution may contain 0.3 to 0.45 mmol/L of adenosine; 0.04 to
0.09
mmol/L of lidocaine; 8 to 12.5 mmol/L of glucose; 110 to 130 mmol/L of NaCI; 4
to 7
mmol/L of KCl; 16 to 24 mmol/L of NaHCO3; 0.9 to 1.4 mmol/L of NaH2PO4; 0.18
to
0.26 mmol/L of CaC12;11 to 15 mmol/L of MgCl2; 7.5 to 12.5 IU/L of insulin;
100 to
140 mmol/L of D-mannitol; 0.75 to 1.25 mmol/L of pyruvate; and 2.5 to 3.5
mmol/L
of reduced glutathione. More specifically, the solution may contain 0.4 mmol/L
of
adenosine; 0.05 mmol/L of lidocaine; 10 mmol/L of glucose; 123.8 mmol/L of
NaCI;
5.9 mmol/L of KCI; 20 mmol/L of NaHCO3; 1.2 mmol/L of NaH2PO4; 0.22 mmol/L of
CaCl2; 13 mmol/L of MgCl2; 101U/L of insulin; 120 mmol/L of D-mannitol; 1
mmol/L
of pyruvate; and 3 mmol/L of reduced glutathione.
[0092] In different embodiments, a solution for reperfusion of an excised
heart may include a cardioplegia mixture containing an anesthetic agent for
paralyzing the heart and preventing myocyte contraction during reperfusion;
and
agents for protecting or restoring cardiac functions of the heart, the agents
comprising a calcium source, a sodium source, and a potassium source, in
amounts selected to restore and maintain calcium ion homeostasis in the heart
at
a temperature from about 25 to about 35 C. The solution may be at a
temperature
from about 25 to about 35 C, such as about 35 C.
[0093] As can be appreciated by those skilled in the art, a solution
disclosed
herein may be prepared and stored before use, or the solution may be prepared
just before use by mixing pre-packaged compositions or materials, or by adding
a
24
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
solvent such as water or a buffer solution to a pre-formulation to form the
desired
solution. For example, a composition for preparing a reperfusion solution may
include a mixture of adenosine, lidocaine, and a calcium source. The molar
ratio of
adenosine:calcium may be from 0.3:0.26 to 0.45:0.18, such as 0.4:0.22, and the
molar ratio of lidocaine:calcium may be from 0.04:0.26 to 0.09:0.18, such as
0.05:0.22. The composition may also contain a sodium source, a potassium
source and a magnesium source. The molar ratio of calciumsodium may be from
0.26:130 to 0.18:160, such as 0.22:147. The molar ratio of calcium:potassium
may
be from 0.26:4 to 0.18 to 7, such as 0.22:5.9. The molar ratio of
calcium:magnesium may be from 0.26:11 to 0.18:15, such as 0.22:13. The
composition may also contain chloride, and one or more of glucose, insulin, D-
mannitol, pyruvate, and reduced glutathione. The composition may be mixed with
a
suitable pH buffer to prepare the desired reperfusion solution, such as a
selected
reperfusion solution described herein.
[0094] Further embodiments relate to methods of preserving and preparing
hearts for transplantation. For example, in a method for reperfusion of a
heart for
transplant, the heart may be reperfused with a reperfusion solution disclosed
herein in a reperfusion device. The reperfusion device may be similar to a
conventional perfusion device and may be operated similarly except replacing
the
perfusion solution with a reperfusion solution described herein. For example,
the
Quest MPS02 Myocardial Protection System, provided by Quest Medical Inc.,
Allen, TX, USA, may be used as the reperfusion device. A volume infusion pump
may also be used to pump the reperfusion solution. An infuser, such as one
that is
typically used by a trauma patient, or a similar infuser, may be used for
reperfusion. For example, BelmontTM rapid infuser RI-2 may be used in the
reperfusion device.
[0095] The heart may be reperfused with the reperfusion solution for at
least
3 minutes immediately after removal of the heart from the donor of the heart.
The
donor may be a DCD donor, and the DCD heart may be maintained at a
temperature above about 25 C and below about 37 C, such as at about 35 C at
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
any stage of the procurement, reperfusion, perfusion, storage, and
transplantation
procedures.
[0096] Further embodiments are related to methods of maintaining a heart
for transplant. For example, the heart may be treated to maintain calcium ion
homeostasis in the heart at a temperature from about 25 C to about 37 C,
such
as by use of a suitable solution or composition disclosed herein.
[0097] As now can be appreciated, embodiments of solutions disclosed
herein may be used for reperfusion of a donor heart, during removal of the
heart,
or immediately after removal of the heart from the donor, or both. Further,
the
solution may also be used as perfusion solution at other times or for other
purposes as may be appropriate. Conveniently, the heart may be removed from a
donor after circulatory death (DCD) at a temperature from about 25 to 37 C.
In
different embodiments, a solution as described herein may also be used for
reperfusion of other types of hearts such as a heart removed from a donor
after
brain death (DBD). In some embodiments, the solution may also be used at lower
temperatures.
[0098] While some embodiments have been described herein with
reference to reperfusion or cardioplegic solutions or compositions, or
cardioplegia
mixtures, it can be understood that they are preservation compositions,
solutions
or mixtures, which can preserve or protect cell functions and therefore the
health
of the cell in the organ to be transplanted.
[0099] The following examples are provided to more fully describe the
disclosure and are presented for non-limiting, illustrative purposes.
[00100] EXAMPLES
[00101] The sample cardioplegic solutions used in these Examples were
prepared at room temperature and their stated pH was measured at room
temperature. The lidocaine and D-mannitol solutions used to prepare the sample
26
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
solutions were obtained from commercial sources.
[00102] All sample solutions were prepared by adding the component
ingredients to water. The water was double-deionized and sterilized as known
to
those skilled in the art. The sample solutions were oxygenated before use.
[00103] Example 1:
[00104] It is apparent that strategies to minimize post-harvest ex vivo
trauma
and injury to donor hearts require an understanding of ionic changes that
occur in
the heart during ischemia and during/after reperfusion.
[00105] During ischemia, the heart's metabolism shifts from aerobic to
anaerobic with a subsequent production of protons within the cardiac myocytes.
The excess protons efflux through the myocyte cell walls in exchange for
ingressing Nal. ions through Na/K4 pump. As the ATP reserves within the
myocytes are depleted, the myocytes become unable to pump the ingressing Na+
ions back out through the Na+/K+ pump. As a result, as the duration of
ischemia
progresses, there is an accumulation of: (i) Na + ions within the myocytes,
and (ii)
Na + ions and Fl+ ions inside and outside the myocytes.
[00106] During reperfusion, the H.' ions on the outside of the myocytes are
washed away resulting in the occurrence of a large Na+/ H+ gradient across the
myocyte walls resulting in a large influx of Na + ions into the myocytes. The
increased
concentration of Na + ions causes the Na+/Ca2+ pump to work in a reverse mode
resulting in an influx of Ca2+ ions into the myocytes as the Na+/Ca2+ pump
attempts
to equilibrate the levels of Na + ions inside and outside of the myocytes. If
a Ca2+-
overloaded myocyte is allowed to contract, a fatal hypercontracture may occur
(the
hypercontracture is also commonly referred to as "contraction band necrosis").
Consequently, a primary goal of resuscitating a DCD heart is to mitigate a
Ca2+ ion
overload in the myocytes.
[00107] Accordingly, our goals were to prevent a harvested DCD heart from
27
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
contracting by reperfusion with an anesthetic-containing cardioplegic solution
while
providing the requisite substrates for regenerating ATP so that the reperfused
heart could restore its homeostasis by pumping Na + ions and Ca2+ ions and
thereby minimize ischemia reperfusion trauma and injury. Because the
generation
of ATP to provide the energy necessary to exchange ions across the Na+/K+
pumps and the Na+/Ca2+ pumps, it was our idea that reperfusion of harvested
donor hearts would facilitate more rapid restoration of ion homeostasis and
recovery of cardiac function. Accordingly, the first study assessed the
effects of
reperfusion temperature on harvested donor hearts.
[00108] Eighteen pigs were separated into three groups and then euthanized
following standard protocols and medical ethics procedures following the
schematic flowchart shown in Fig. 1.
[00109] Six pigs were assigned to the first group ("chilled" group).
Immediately after procurement of each heart was completed, each heart was
installed into a Quest MPS 2 Myocardial Protection System (MPS is a registered
trademark of Quest Medical Inc., Allen, TX, USA) for precise control of the
reperfusion pressure and temperature. The harvested hearts from first group of
pigs were perfused for 3 minutes with a sample oxygenated cardioplegic
composition (see TABLE I) that was chilled to 5 C prior to commencing the
reperfusion process. The cardioplegic composition was initially prepared at
room
temperature and the pH of the composition was measured at room temperature.
The aortic perfusion pressure, coronary artery flow, and myocardial
temperature
were constantly monitored and recorded by the MPS62 apparatus during the 3-
minute initial reperfusion period. Blood gas samples were measured at 0, 30,
60,
120, and 180 seconds of the initial reperfusion period to collect data
pertaining to
changes occurring the partial pressure of 02 (Pa02), partial pressure of CO2
(PaCO2), pH levels, electrolyte levels, lactate levels among others.
28
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
TABLE I Sample I - Cardioplegic solution (pH = 7.35)
Constituent mrnol/L IU/L
Adenosine 0.4 ..
Lidocaine 0.5
Glucose 10
NaCI 1 111.8
KCI 1 5.9
NaHCO3 32
NaH2PO4 1.2
CaCl2 0.22
MgCl2 2.6
D-Mannitol 120 __
Pyruvate 1
Reduced
3 ,
glutathione
Insulin 10
[00110] After the initial 3-minute reperfusion period was completed. Each
heart was removed from the Quest MPS 2 apparatus and transferred into an ex
vivo heart perfusion (EVHP) apparatus where it was perfused with a constantly
flowing supply of a blood-STEEN solution mixture (Hb 45 g/L; XVIVO Perfusion
Inc., Englewood, CO, USA) wherein its systolic function was restored and
maintained in a Langendorff mode at a normothermic temperature of 35 C for 6
hours. The aortic pressure and heart rate were constantly monitored and
processed using the LABCHART software (LABCHART is a registered trademark
of ADInstruments Pty. Ltd., Bella Vista, NSW, Australia). After 1 hour, 3
hours, and
hours of perfusion with the blood-STEEN solution mixture in the EVHP
apparatus, each heart was transitioned from the Langendorff mode to a working
mode by bringing the left atrial pressure from 0 to 8 mmHg and pacing the
heart at
100 beats per minutes ("bpm"). Cardiac output, coronary blood flow, aortic
root,
and coronary sinus blood gases were measured, and cardiac function was
assessed with a pressure-volume loop catheter. After these measurements were
completed, each heart was immediately returned to the Langendorff mode.
29
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
[00111] Five pigs were assigned to the second group ("cooled" group), and
were processed as described above for the first group with the only exception
that
the IE reperfusion was done with a sample oxygenated cardioplegic composition
as shown in TABLE I, which had been cooled to 25 C prior to commencing the
reperfusion process.
[00112] Seven pigs were assigned to the third group ("normothermic" group),
and were processed as described above for the first group with the only
exception
that the IE reperfusion was done with a sample oxygenated cardioplegic
composition as shown in TABLE I, which had been warmed to 35 C prior to
commencing the reperfusion process.
[00113] The data in Fig. 2 show that the myocardial temperatures recorded
in
the hearts receiving the IE reperfusion treatment with the sample oxygenated
cardioplegic composition chilled to 5 C dropped to about 10 C by the end of
the
3-minute IE reperfusion period. The myocardial temperatures recorded in the
hearts that received IE reperfusion with the sample oxygenated cardioplegic
composition cooled to 25 C were about 25 C, while the myocardial
temperatures
recorded in the hearts that received reperfusion with the selected oxygenated
cardioplegic composition were about 35 C.
[00114] Fig. 3 shows that rates of coronary blood flow were reduced by
about
15% in hearts that were reperfused with the sample oxygenated cardioplegic
composition cooled to 25 C compared to coronary blood flow in hearts that
received reperfusion with the sample oxygenated cardioplegic composition.
However, rates of coronary blood flow were reduced by nearly 50% in hearts
that
were reperfused with the sample oxygenated cardioplegic composition chilled to
5
C compared to coronary blood flow in hearts that received reperfusion with the
sample oxygenated cardioplegic composition.
[00115] Fig. 4 shows that the coronary vascular resistance in hearts
reperfused with the cooled oxygenated cardioplegic composition dropped by
about
40% compared to the hearts reperfused with the oxygenated cardioplegic
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
composition, while the chilled oxygenated cardioplegic composition caused a
reduction of more than 50% in the coronary vascular resistance.
[00116] Fig. 5 shows that the coronary sinus lactate dropped by more than
50% in hearts that received the chilled IE reperfusion treatment, and by about
25%
in hearts that received the cooled IE reperfusion treatment, when compared to
the
coronary sinus lactate levels in the hearts receiving the normothermic IE
reperfusion treatment.
[00117] Fig. 6 shows that levels of Troponin I (a marker for myocardial
injury)
increased as the temperature of the IE reperfusion temperature decreased,
relative to the levels observed in hearts receiving the normothermic IE
reperfusion
treatment.
[00118] Fig. 7(A) is an electron micrograph showing a swollen endothelial
cell in a capillary of a heart that received the chilled IE reperfusion
treatment for 3
minutes, while Fig. 7(B) is an electron micrograph showing a typical normal-
appearing endothelial cell in a capillary of a heart that received the
normothermic
IE reperfusion treatment for 3 minutes.
[00119] Fig. 8 is a chart comparing the scores of endothelial injury and
myocyte injury from hearts receiving chilled IF reperfusion for three minutes
and
from hearts receiving normothermic IE reperfusion for three minutes.
[00120] Fig. 9 is a chart showing the effects on cardiac indices of IE
reperfusion with a cooled oxygenated cardioplegic composition and with a
chilled
oxygenated cardioplegic composition, with the effects of IP perfusion with a
normothermic oxygenated cardioplegic composition.
[00121] Fig. 10 is a chart comparing the effects of the initial IE
reperfusion
temperatures on the subsequent systolic functioning of harvested hearts after
1
hour ("Ti"), 3 hours ("T3"), and 5 hours ("T5") of resuscitation and perfusion
of the
hearts with the blood-STEEN solution mixture.
31
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
[00122] Fig. 11 is a chart comparing the effects of the initial IE
reperfusion
temperatures on the subsequent diastolic functioning of harvested after 1 hour
("T1"), 3 hours ("T3"), and 5 hours ("15") of resuscitation and perfusion of
the
hearts with the blood-STEEN solution mixture.
[00123] The data collected in this study demonstrate that the initial
reperfusion conditions, which last only 3 minutes, significantly impact the
severity
of post-harvest trauma and the functional recovery of hearts harvested from
porcine DCD donors.
[00124] Example 2:
[00125] The second study assessed the effects of reducing the Ca2+ ion
concentration in cardioplegic solutions to determine if lowering the Ca2+ ion
levels
on the outside of myocytes would minimize the reverse mode functioning of the
Na+/Ca2+ pump thereby reducing the accumulation of Ca2+ ions within the
myocytes. Accordingly, this study assessed the effects of 50 pmol/L, 220
pmol/L,
500 pmol/L, and 1250 pmol/L of Ca2+ ions in sample oxygenated cardioplegic
solutions (Fig. 12). The components of these sample solutions are shown in
TABLE II. The sample solutions were also prepared at room temperature and
their stated pH values were measured at room temperature, as for sample
solutions in Example I but with different calcium chloride concentrations at
0.05,
0.22, 0.5, or 1.25 mmol/L respectively. All reperfusions in this example were
done
at 35 C.
32
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
TABLE II Sample II - Cardioplegic solutions (pH = 7.35)
Constituent mmol/L 1 IU/L
Adenosine 0.4
Lidocaine 0.5
Glucose 10
NaCI 111.8
KCI 5.9
NaHCO3 32
NaH2PO4 1 1.2
CaCl2 varied
MgCl2 2.6
D-Mannitol 120
Pyruvate 1
Reduced
3
= glutathione
, Insulin 10
[00126] Twenty four pigs were separated into four groups and then
euthanized following standard protocols and medical ethics procedures
following
the schematic flowchart shown in Fig. 13. Immediately after procurement of
each
heart was completed, each heart was installed into a Quest MPSe2 Myocardial
Protection System. The harvested hearts from the first group of pigs were
perfused for 3 minutes with a sample oxygenated cardioplegic composition
containing 50 pmol/L Ca2+ ions, which was warmed to 35 C prior to commencing
the reperfusion process. The harvested hearts from the second group of pigs
were
perfused for 3 minutes with the sample oxygenated cardioplegic composition
containing 220 pmol/L Ca2+ ions, which was warmed to 35 C prior to commencing
the reperfusion process. The harvested hearts from the third group of pigs
were
perfused for 3 minutes with the sample oxygenated cardioplegic composition
containing 500 pmol/L Ca2+ ions, which was warmed to 35 C prior to commencing
the reperfusion process. The harvested hearts from the fourth group of pigs
were
perfused for 3 minutes with the sample oxygenated cardioplegic composition
containing 1,250 pmol/L Ca2+ ions, which was warmed to 35 C prior to
33
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
commencing the reperfusion process.
[00127] The aortic perfusion pressure, coronary artery flow, and myocardial
temperature were constantly monitored and recorded by the MPS 2 apparatus
during the 3-minute initial reperfusion period. Blood gas samples were
measured at
0, 30, 60, 120, and 180 seconds of the initial reperfusion period to collect
data
pertaining to changes occurring the partial pressure of 02 (Pa02), partial
pressure of
CO2 (PaCO2), pH levels, electrolyte levels, lactate levels among others.
[00128] After the initial 3-minute reperfusion period was completed. Each
heart
was removed from the Quest MPS 2 apparatus and transferred into an ex vivo
heart
perfusion (EVHP) apparatus where it was perfused with a constantly flowing
supply
of a blood-STEEN solution mixture (Hb 45 g/L; XVIVO Perfusion Inc., Englewood,
CO, USA) wherein its systolic function was restored and maintained in a
Langendorff
mode at a normothermic temperature of 35 C for 1 hour. The aortic pressure
and
heart rate were constantly monitored and processed using the LABCHART
software. At 1 hour of perfusion with the blood-STEEN solution mixture in the
EVHP
apparatus, each heart was transitioned from the Langendorff mode to a working
mode by bringing the left atrial pressure from 0 to 8 mmHg and pacing the
heart at
100 bpm. Cardiac output, coronary blood flow, aortic root, and coronary sinus
blood
gases were measured, and cardiac function was assessed with a pressure-volume
loop catheter. After these measurements were completed, each heart was
immediately returned to the Langendorff mode.
[00129] Fig. 14 shows that the hearts initially reperfused at 35 C with
the
sample oxygenated card ioplegic composition containing 220 pmol/L Ca2+ ions
developed significantly less myocardial edema than the hearts reperfused with
oxygenated cardioplegic compositions containing one of the other three Ca2+
ion
concentrations.
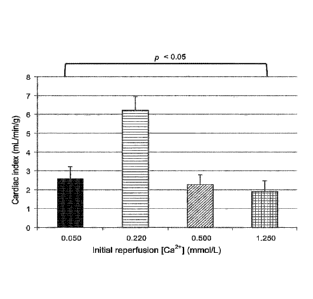
[00130] Fig. 15 shows that the cardiac output (indexed for heart weight) of
reperfused hearts improved as the Ca2+ ion concentration in the oxygenated
card ioplegic compositions was reduced from 1,250 pmol/L to 500 pmol/L to 220
34
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
pmol/L. However, the cardiac output of hearts reperfused with an oxygenated
cardioplegic composition containing 50 pmol/L Ca2+ ions was very poor.
[00131] Fig. 16 shows that the contractility of the left ventricle (as
measured
by dP/dt max) during systole in reperfused hearts improved as the Ca2+ ion
concentration in the oxygenated cardioplegic compositions was reduced from
1,250 pmol/L to 500 pmol/L to 220 pmol/L. However, contractility of the left
ventricle in hearts reperfused with the oxygenated cardioplegic composition
containing 50 pmol/L Ca2+ ions was very poor.
[00132] Fig. 17 shows that the relaxation of the left ventricle (as
measured
by dP/dt min) during diastole in reperfused hearts improved as the Ca2+ ion
concentration in the oxygenated cardioplegic compositions was reduced from
1,250 pmol/L to 500 pmol/L to 220 pmol/L. However, relaxation of the left
ventricle in hearts reperfused with the oxygenated cardioplegic composition
containing 50 pmol/L Ca2+ ions was very poor.
[00133] The data collected during this study demonstrate that hypocalcemic
oxygenated cardioplegic compositions at 35 C significantly improved
myocardial
functional recovery. The best performance in this study was with a Ca2+ ion
concentration of 220 pmol/L. However, it appears that reducing the Ca2+ ion
concentration too low, for instance to 50 pmol/L, may have detrimental
effects, a
phenomenon previously described as the "calcium paradox".
[00134] Example 3:
[00135] The next study assessed if there were potential incremental
benefits
to acidification of a hypocalcemic oxygenated cardioplegic composition.
Accordingly, this study assessed the effects of adjusting the pH of sample
hypocalcemic oxygenated cardioplegic compositions from 7.9 to 7.4, to 6.9, and
to
6.4.
[00136] The components of these sample solutions IIIA to I I ID are shown
in
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
TABLEs IIIA to IIID respectively.
TABLE IIIA Sample IIIA - Cardioplegic solution (pH = 7.9)
Constituent mmol/L ILA
Adenosine 0.4
Lidocaine 0.5
Glucose 10
NaCI 43.8
KCI 5.9
NaHCO3 100
NaH2PO4 _______________________________ 1.2
CaCl2 0.22
MgC12 2.6
D-Mannitol 120
Pyruvate 1
Reduced 3
glutathione
Insulin 10
TABLE IIIB Sample IIIB - Cardioplegic solution (pH = 7.35)
. _ ______________________________________________
Constituent mmol/L Ili&
Adenosine 0.4 !_ __
Lidocaine 0.5 I
Glucose 10
NaCI 111.8
KCI 5.9
NaHCO3 32
NaH2PO4 1.2
CaCl2 0.22
MgC12 2.6
D-Mannitol 120
Pyruvate 1
Reduced
3
glutathione
Insulin 10
36
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
TABLE IIIC Sample IIIC - Cardioplegic solution (pH = 6.9)
Constituent mmol/L I IU/L
Adenosine 0.4
Lidocaine 0.5 1
Glucose 10 j
NaCI 131.8
KCI 5.9
NaHCO3 12
NaH2PO4 1.2
CaCl2 0.22
MgCl2 2.6
D-Mannitol 120
Pyruvate 1
Reduced
3
glutathione
Insulin 10
TABLE IIID Sample IIID - Cardioplegic solution (pH = 6.4)
Constituent mmol/L IU/L
Adenosine 0.4
Lidocaine 0.5
Glucose 10
NaCI 137.8
KCI 5.9
NaHCO3 1 6
NaH2PO4 1.2
CaCl2 0.22
MgCl2 I 2.6
D-Mannitol 120
Pyruvate 1 ____
Reduced
3
glutathione
Insulin 10 j
37
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
[00137] The sample cardioplegic solutions contained 220 pmol/L of Ca2+ ions
and all reperfusions were done at 35 C (Fig. 18).
[00138] Twenty four pigs were separated into four groups and then
euthanized following standard protocols and medical ethics procedures
following
the schematic flowchart shown in Fig. 19. Immediately after procurement of
each
heart was completed, each heart was installed into a Quest MPS62 Myocardial
Protection System. The harvested hearts from the first group of pigs were
perfused for 3 minutes with the sample hypocalcemic oxygenated cardioplegic
composition with a pH of 7.9, which was warmed to 35 C prior to commencing
the
reperfusion process. The harvested hearts from the second group of pigs were
perfused for 3 minutes with a sample hypocalcemic oxygenated cardioplegic
composition adjusted to a pH of 7.4, which was warmed to 35 C prior to
commencing the reperfusion process. The harvested hearts from the third group
of
pigs were perfused for 3 minutes with a sample hypocalcemic oxygenated
cardioplegic composition adjusted to a pH of 6.9, which was warmed to 35 C
prior
to commencing the reperfusion process. The harvested hearts from the fourth
group of pigs were perfused for 3 minutes with the sample hypocalcemic
oxygenated cardioplegic composition adjusted to a pH of 6.4, which was warmed
to 35 C prior to commencing the reperfusion process.
[00139] The aortic perfusion pressure, coronary artery flow, and myocardial
temperature were constantly monitored and recorded by the MPS(8)2 apparatus
during the 3-minute initial reperfusion period. Blood gas samples were
measured
at 0, 30, 60, 120, and 180 seconds of the initial reperfusion period to
collect data
pertaining to changes occurring the partial pressure of 02 (Pa02), partial
pressure
of CO2 (PaCO2), pH levels, electrolyte levels, lactate levels among others.
[00140] After the initial 3-minute reperfusion period was completed. Each
heart was removed from the Quest MPS 2 apparatus and transferred into an ex
vivo heart perfusion (EVHP) apparatus where it was perfused with a constantly
flowing supply of a blood-STEEN solution mixture (Hb 45 g/L; XVIVO Perfusion
38
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
Inc., Englewood, CO, USA) wherein its systolic function was restored and
maintained in a Langendorff mode at a normothermic temperature of 35 C for 1
hour. The aortic pressure and heart rate were constantly monitored and
processed
using the LABCHART software. After 1 hour of perfusion with the blood-STEEN
solution mixture in the EVHP apparatus, each heart was transitioned from the
Langendorff mode to a working mode by bringing the left atrial pressure from 0
to
8 mmHg and pacing the heart at 100 bpm. Cardiac output, coronary blood flow,
aortic root, and coronary sinus blood gases were measured, and cardiac
function
was assessed with a pressure-volume loop catheter. After these measurements
were completed, each heart was immediately returned to the Langendorff mode.
[00141] Fig. 20 shows that the hearts initially reperfused at 35 C with
the
sample hypocalcemic oxygenated cardioplegic compositions that was mildly
acidic(i.e., pH 6.4) exhibited more myocardial edema than those that were
reperfused with the more alkaline (i.e., pH of 7.9, 7.4, 6.9) hypocalcemic
oxygenated cardioplegic compositions.
[00142] Fig. 21 shows that the cardiac outputs (indexed for heart weight)
of
reperfused hearts in a slightly acidic hypocalcemic oxygenated cardioplegic
composition (i.e., pH 6.9) and a slightly alkaline hypocalcemic oxygenated
cardioplegic composition (i.e., pH 7.4) were significantly better that the
cardiac
outputs of hearts reperfused in hypocalcemic oxygenated cardioplegic
compositions adjusted to pH 7.9 or 6.4.
[00143] Fig. 22 shows that the contractility of the left ventricle (as
measured
by dP/dt max) during systole in reperfused hearts in a slightly acidic
hypocalcemic
oxygenated cardioplegic composition (i.e., pH 6.9) and a slightly alkaline
hypocalcemic oxygenated cardioplegic composition (i.e., pH 7.4) were
significantly
better than the left ventricle contractility in hearts reperfused in
hypocalcemic
oxygenated cardioplegic compositions adjusted to pH 7.9 or 6.4.
[00144] Fig. 23 shows that the relaxation of the left ventricle (as
measured by
dP/dt min) during diastole in reperfused hearts in a slightly acidic
hypocalcemic
39
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
oxygenated cardioplegic composition (i.e., pH 6.9) and a slightly alkaline
hypocalcemic oxygenated cardioplegic composition (i.e., pH 7.4) were
significantly
better than the left ventricle relaxation in hearts reperfused in hypocalcemic
oxygenated cardioplegic compositions adjusted to pH 7.9 or 6.4.
[00145] The data collected during this study demonstrate that initial
alkaline
reperfusion is detrimental and significant acidity (e.g., pH of 6.4) is also
detrimental. However, it appears that mild acidosis (e.g. pH of 6.6 to 6.9) is
beneficial.
[00146] Example 4:
[00147] Part 1: The next study assessed if there were potential incremental
benefits to increasing the duration of reperfusion of harvested donor hearts
with a
mildly acidic hypocalcemic oxygenated cardioplegic composition.
[00148] The sample solutions used for these tests were the same as Sample
solutions IIIC described above.
[00149] Accordingly, this study assessed the effects of 3 minutes or 9
minutes reperfusion with a sample mildly acidic (pH 6.9) hypocalcemic (220
pmol/L Ca2+) oxygenated cardioplegic solution at 35 C (Fig. 24). The
cardioplegic
solution for Part 1 of this study contained 400 pmol/L adenosine and 500
pmol/L
lidocaine.
[00150] Twelve pigs were separated into two groups and then euthanized
following standard protocols and medical ethics procedures following the
schematic flowchart shown in Fig. 25. Immediately after procurement of each
heart
was completed, each heart was installed into a Quest MPS 2 Myocardial
Protection System. The harvested hearts from the first group of pigs were
perfused for 3 minutes with the sample mildly acidic hypocalcemic oxygenated
cardioplegic composition warmed to 35 C prior to commencing the reperfusion
process for 3 minutes. The harvested hearts from the second group of pigs were
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
perfused for 9 minutes with the sample mildly acidic hypocalcemic oxygenated
cardioplegic composition that was warmed to 35 C prior to commencing the
reperfusion process.
[00151] The aortic perfusion pressure, coronary artery flow, and myocardial
temperature were constantly monitored and recorded by the MPS82 apparatus
during the 3-minute or 9-minute initial reperfusion period. Blood gas samples
were
measured at 0, 30, 60, 120, and 180 seconds of the initial reperfusion period
to
collect data pertaining to changes occurring the partial pressure of 02
(Pa02), partial
pressure of CO2 (PaCO2), pH levels, electrolyte levels, lactate levels among
others.
[00152] After the initial 3-minute reperfusion period or the initial 9-
minute
reperfusion period was completed, each heart was removed from the Quest MPS 2
apparatus and transferred into an ex vivo heart perfusion (EVHP) apparatus
where
it was perfused with a constantly flowing supply of a blood-STEEN solution
mixture
(Hb 45 g/L; XVIVO Perfusion Inc., Englewood, CO, USA) wherein its systolic
function was restored and maintained in a Langendorff mode at a normothermic
temperature of 35 C for 1 hour, 3 hours, and 5 hours. The aortic pressure and
heart
rate were constantly monitored and processed using the LABCHART software.
After 1 hour of perfusion with the blood-STEEN solution mixture in the EVHP
apparatus, each heart was transitioned from the Langendorff mode to a working
mode by bringing the left atrial pressure from 0 to 8 mmHg and pacing the
heart at
100 bpm. Cardiac output, coronary blood flow, aortic root, and coronary sinus
blood
gases were measured, and cardiac function was assessed with a pressure-volume
loop catheter. After these measurements were completed, each heart was
immediately returned to the Langendorff mode for an additional 2 hours, after
which
the measurements were repeated (i.e., 3 hours after removal from reperfusion).
After these measurements were completed, each heart was immediately returned
to
the Langendorff mode for an additional 2 hours, after which the measurements
were
repeated (i.e., 5 hours after removal from reperfusion).
[00153] Fig. 26 shows that the hearts initially reperfused for 9 minutes
with
41
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
the sample mildly acidic hypocalcemic oxygenated cardioplegic composition
exhibited more myocardial edema than those that were reperfused for only 3
minutes.
[00154] Fig. 27 shows that the hearts initially reperfused for 9 minutes
trended toward worsening function as ex vivo heart perfusion proceeded from
hour to 3 hours to 5 hours.
[00155] These data suggest that the high (500 pmol/L) concentration of
lidocaine might be toxic.
[00156] Part 2: The next study assessed the effects of reducing the
lidocaine
concentration in the sample mildly acidic hypocalcemic oxygenated cardioplegic
composition. Accordingly, this study assessed the effects of 3 minutes or 9
minutes of reperfusion with a sample mildly acidic (pH 6.9) hypocalcemic (220
pmol/L Ca2+) oxygenated cardioplegic solution at 35 C containing 400 pmol/L
adenosine and 50 pmol/L lidocaine (Fig. 28).
[00157] The components of this sample solution are shown in TABLE IV.
42
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
TABLE IV Sample IV -
Cardioplegic solution (pH = 6.9)
Constituent mmol/L IU/L
Adenosine 0.4
Lidocaine 0.05
Glucose 10 ..
NaCI 123.8
KCI 5.9
NaHCO3 20 ___
NaH2PO4. 1.2
CaCl2 0.22
MgCl2 13
D-Mannitol 120 ..
Pyruvate 1
Reduced
3
glutathione
Insulin = 10
[00158] Twelve pigs
were separated into two groups and then euthanized
following standard protocols and medical ethics procedures following the
schematic
flowchart shown in Fig. 25. Immediately after procurement of each heart was
completed, each heart was installed into a Quest MPS 2 Myocardial Protection
System. The harvested hearts from the first group of pigs were perfused for 3
minutes with the sample mildly acidic hypocalcemic oxygenated cardioplegic
composition warmed to 35 C prior to commencing the reperfusion process for 3
minutes. The harvested hearts from the second group of pigs were perfused for
9
minutes with the sample mildly acidic hypocalcemic oxygenated cardioplegic
composition that was warmed to 35 C prior to commencing the reperfusion
process.
[00159] The aortic
perfusion pressure, coronary artery flow, and myocardial
temperature were constantly monitored and recorded by the MPS 2 apparatus
during the 3-minute or 9-minute initial reperfusion period. Blood gas samples
were
measured at 0, 30, 60, 120, and 180 seconds of the initial reperfusion period
to
collect data pertaining to changes occurring the partial pressure of 02
(Pa02),
partial pressure of CO2 (PaCO2), pH levels, electrolyte levels, lactate levels
among
43
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
others.
[00160] After the initial 3-minute reperfusion period or the initial 9-
minute
reperfusion period was completed, each heart was removed from the Quest
MPS 2 apparatus and transferred into an ex vivo heart perfusion (EVHP)
apparatus where it was perfused with a constantly flowing supply of a blood-
STEEN solution mixture (Hb 45 g/L; XVIVO Perfusion Inc., Englewood, CO, USA)
wherein its systolic function was restored and maintained in a Langendorff
mode at
a normothermic temperature of 35 C for 1 hour, 3 hours, and 5 hours. The
aortic
pressure and heart rate were constantly monitored and processed using the
LABCHART software. At 1 hour of perfusion with the blood-STEEN solution
mixture in the EVHP apparatus, each heart was transitioned from the
Langendorff
mode to a working mode by bringing the left atrial pressure from 0 to 8 mmHg
and
pacing the heart at 100 bpm. Cardiac output, coronary blood flow, aortic root,
and
coronary sinus blood gases were measured, and cardiac function was assessed
with a pressure-volume loop catheter. After these measurements were completed,
each heart was immediately returned to the Langendorff mode for an additional
2
hours, after which the measurements were repeated (i.e., 3 hours after removal
from reperfusion). After these measurements were completed, each heart was
immediately returned to the Langendorff mode for an additional 2 hours, after
which
the measurements were repeated (i.e., 5 hours after removal from reperfusion).
[00161] Fig. 29 shows that there were not any significant differences in
myocardial edema occurring in the hearts initially reperfused for 9 minutes
compared with hearts perfused for 3 minutes in the sample mildly acidic
hypocalcemic oxygenated cardioplegic composition containing 400 pmol/L
adenosine and 50 pmol/L lidocaine.
[00162] Fig. 30 shows that prolonging the initial reperfusion period from 3
minutes to 9 minutes in the sample mildly acidic hypocalcemic oxygenated
cardioplegic composition containing 400 pmol/L adenosine and 50 pmol/L
lidocaine, did not have detrimental effects on the functional recovery of
hearts
44
CA 02965400 2017-04-21
WO 2016/061700
PCT/CA2015/051084
perfused for 1 hour, 3 hours, and 5 hours after reperfusion.
[00163] Fig. 31 combines myocardial functional data from Part 1 (Fig. 27)
and
Part 2 (Fig. 30), wherein it is apparent that the 500 pmol/L concentration of
lidocaine
in the cardioplegic compositions used for initial ex vivo post-harvest
reperfusion has
debilitating effects of donor hearts. This data also demonstrates that
prolonging the
initial reperfusion period beyond 3 minutes is not beneficial for restoration
of
homeostasis and cardiac function in harvested donor hearts.
[00164] The data presented herein indicate that a potentially effective
composition for a card ioplegic solution for initial reperfusion of donor
hearts is
shown in TABLE IV.
[00165] It will be understood that any range of values disclosed herein is
intended to specifically include any intermediate value or sub-range within
the
given range, and all such intermediate values and sub-ranges are individually
and
specifically disclosed.
[00166] It will also be understood that the word "a" or "an" is intended to
mean "one or more" or "at least one", and any singular form is intended to
include
plurals herein.
[00167] It will be further understood that the term "comprise", including
any
variation thereof, is intended to be open-ended and means "include, but not
limited
to," unless otherwise specifically indicated to the contrary.
[00168] When a list of items is given herein with an "or" before the last
item,
any one of the listed items or any suitable combination of two or more of the
listed
items may be selected and used.
[00169] Other modifications to the above-described embodiments are
possible. The invention is therefore defined by the claims, which should be
given a
broad interpretation consistent with the description as a whole.