Note: Descriptions are shown in the official language in which they were submitted.
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
PROTEINS AND PROTEIN CONJUGATES WITH INCREASED
HYDROPHOBICITY
CROSS-REFERENCE TO RELATED APPLICATION
[0001] This application claims the benefit of priority to the following
applications: U.S.
Provisional Application No. 62/086,294, filed December 2, 2014; U.S.
Application No.
14/954,591, entitled "PROTEINS AND PROTEIN CONJUGATES WITH INCREASED
HYDROPHOBICITY," filed November 30, 2015; and U.S. Application No. 14/954,701,
entitled
"PROTEINS AND PROTEIN CONJUGATES WITH INCREASED HYDROPHOBICITY,"
filed November 30, 2015. The content of these applications is incorporated
herein by reference
for all purposes.
BACKGROUND
[0002] Delivery of a drug, hormone, protein, or other medically active agent
into a patient
faces a number of challenges. The medically active agent has to be delivered
into the patient.
Two such ways are ingestion and injection. With ingestion the drug may have to
pass through a
patient's digestive system before reaching the bloodstream or targeted area
for treatment.
Injection may allow the medically active agent to reach the bloodstream or
targeted area for
treatment quickly or directly, but injection may be inconvenient or painful
for the patient. Once
in the body, the concentration of the medically active agent as a function of
time may vary
depending on the type of medically active agent, the attachment of different
functional groups or
molecules on the medically active agent, the encapsulation of the medically
active agent, or other
factors. If the concentration of the medically active agent decreases below a
threshold, the
medically active agent may need to be administered once again. Many medically
active agents
have to be administered frequently, including several times a day. A more
frequent
administration schedule may increase the inconvenience to the patient, may
decrease the
compliance rate by patients, and may lead to less than optimal outcomes for
the patient. If the
medically active agent is administered by injection, another injection
increases the frequency of
pain, the risk of infection, and the probability of an immune response in the
patient. Thus, a need
1
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
for medically active agents that have superior concentration profiles in the
patient exists. The
methods and compositions described herein provide solutions to these and other
needs.
BRIEF SUMMARY
[0003] A medically active agent may be attached to an aliphatic chain, a
polyethylene glycol
(PEG), a hydrophobic anion, or other compounds. The attachment of the
polyethylene glycol
may add molecular weight to the medically active agent and may lead to an
increased half-life of
the medically active agent. Additionally, the attachment of polyethylene
glycol, including
smaller PEG molecules, or a hydrophobic anion to a medically active agent may
increase the
hydrophobicity of the medically active agent and may make the medically active
agent
amphiphilic. The medically active agent may be more easily dissolved in an
organic solvent
with a biodegradable polymer. The biodegradable polymer may encapsulate the
medically active
agent in a microsphere. The encapsulation of the medically active agent may
increase the half-
life of the medically active agent. The formulations described herein may
release the medically
active agent slowly and uniformly over a period of time. The release profile
may result in a
sustained and near peak-less protein level over the intended treatment period,
without the need of
an excipient. The resulting concentration profile of the medically active
agent in a patient may
lead to a more optimal clinical result in the patient. Formulations described
herein may be
administered to a patient as infrequently as once a month. Processes to
manufacture these
formulations may be efficient, high yielding, or not prohibitively expensive.
[0004] Examples may include a method of making a protein-PEG conjugate salt
with increased
hydrophobicity. The method may include providing an aqueous protein solution.
This aqueous
protein solution may include a protein and a pH buffer. The method may also
include reacting a
polyethylene glycol with the protein to form a protein-PEG conjugate. The
method may further
include protonating an amino group on the protein-PEG conjugate with a
hydrophobic organic
acid. The protonation may occur in an organic phase. The protonation may form
the protein-
PEG conjugate salt having a hydrophobic anion that increases the
hydrophobicity-PEG conjugate
salt.
[0005] Examples may include a method of making a protein-PEG conjugate with
increased
hydrophobicity. The method may include providing an aqueous protein solution,
which may
2
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
include a protein and a pH buffer. The method may further include reacting a
polyethylene
glycol with the protein to form a protein-PEG conjugate. The protein-PEG
conjugate may have a
higher hydrophobicity than the protein.
[0006] Examples may include a method of making a protein salt with increased
hydrophobicity. The method may include providing an aqueous protein solution
with a protein
and a pH buffer. The method may further include protonating at least one amino
group on the
protein with a hydrophobic organic acid. The protonation may form the protein
salt having a
hydrophobic anion that increases the hydrophobicity of the protein salt.
[0007] In examples, a method of making controlled-release microspheres
containing a protein-
PEG conjugate salt may include providing an aqueous protein solution. The
aqueous protein
solution may include a protein and a pH buffer. The method may further include
reacting a
polyethylene glycol with the protein to form a protein-PEG conjugate. In
addition, the method
may include protonating an amino group on the protein-PEG conjugate with a
hydrophobic
organic acid. The protonation may form the protein-PEG conjugate salt having a
hydrophobic
anion. Furthermore, the method may include mixing the protein-PEG conjugate
salt in an
organic solvent with a biodegradable polymer. The hydrophobic anion of the
protein-PEG
conjugate salt may increase the solubility of the salt in the organic solvent.
The method may also
include emulsifying the mixture of the protein-PEG conjugate salt and the
biodegradable
polymer in an aqueous solution. Additionally, the method may include hardening
the emulsified
mixture of the protein-PEG conjugate salt and the biodegradable polymer into
the controlled-
release microspheres.
[0008] Examples may include a microsphere. The composition may include a
biodegradable
polymer. Furthermore, the microsphere may include a protein mixture selected
from the group
consisting of a protein-polyethylene glycol conjugate, the protein-
polyethylene glycol conjugate
and the hydrophobic anion of the organic acid, a protein and the hydrophobic
anion of the
organic acid, and combinations thereof.
[0009] Examples may also include a composition that may include a
biodegradable polymer,
an organic solvent, and a protein mixture. The protein mixture may include a
protein, a protein-
polyethylene glycol conjugate, a hydrophobic anion of an organic acid, or
combinations thereof.
The composition may be a solution or a suspension.
3
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
[0010] In examples, methods may include making a solution or suspension of a
biodegradable
polymer and a protein-PEG conjugate salt. The methods may include providing a
protein-PEG
conjugate. The protein-PEG conjugate may be free of the protein-PEG conjugate
salt. The
methods may also include mixing the biodegradable polymer, the protein-PEG
conjugate, a
hydrophobic organic acid, and an organic solvent in a mixture. Methods may
include forming
the protein-PEG conjugate salt, which may include the protein-PEG conjugate
and an anion of
the hydrophobic organic acid. Additionally, methods may include agitating the
mixture to form
the solution or suspension.
[0011] Some examples may include methods of making a solution or suspension of
a
biodegradable polymer and a protein-PEG conjugate salt. The methods may
include dissolving a
biodegradable polymer in an organic solvent to form a mixture. The methods may
also include
adding the protein-PEG conjugate and a hydrophobic organic acid to the
mixture. The methods
may further include protonating an amino group on the protein-PEG conjugate
with the
hydrophobic organic acid. The protonation may form the protein-PEG conjugate
salt having a
hydrophobic anion. Furthermore, the methods may include agitating the mixture
to form the
solution or suspension.
[0012] The examples described herein may provide for a superior concentration
profile. The
protein may retain its activity after PEGylation and encapsulation. The
protein may have a low
burst release in vitro, in vivo, and/or in situ. The concentration release may
have a near zero
order kinetic profile, with the concentration release varying little with time
or the concentration
of medically active agent left in the microsphere. The protein may be
essentially completely
released from the microsphere when administered to the patient.
BRIEF DESCRIPTION OF THE DRAWINGS
[0013] The present technology is described in conjunction with the appended
figures:
[0014] FIG. 1 shows a block diagram of a method of making a protein-PEG
conjugate salt
with increased hydrophobicity according to examples;
[0015] FIG. 2 shows a block diagram of a method of making a protein-PEG
conjugate
according to examples;
4
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
[0016] FIG. 3 shows a method of making a protein salt with increased
hydrophobicity
according to examples;
[0017] FIG. 4 shows a block diagram of a method of making controlled-release
microspheres
containing a protein-PEG conjugate salt according to examples;
[0018] FIG. 5 shows a block diagram of a method of making a solution or
suspension of a
biodegradable polymer and a protein-PEG conjugate salt according to examples;
[0019] FIG. 6 shows a block diagram of a method of making a solution or
suspension of a
biodegradable polymer and a protein-PEG conjugate salt according to examples;
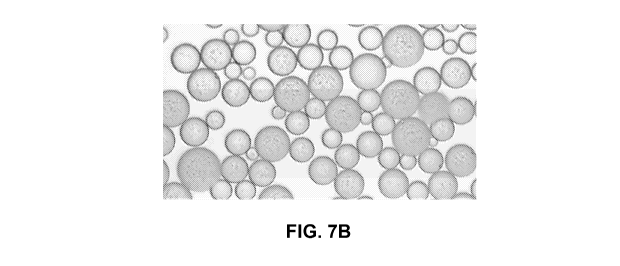
[0020] FIGS. 7A and 7B show light microscopy images of PLGA microspheres
loaded with
medically active agents;
[0021] FIG. 8 shows a graph of results of an extended release study on
microspheres
according to examples;
[0022] FIG. 9 shows a graph of results of an extended release study on
microspheres
according to examples;
[0023] FIG. 10 shows pharmacokinetic analysis of GLP-1 in rat plasma samples
after
microsphere dosing according to examples; and
[0024] FIG. 11 shows pharmacokinetic analysis of insulin in rat plasma samples
after
microsphere dosing according to examples.
DETAILED DESCRIPTION
[0025] Medically active agents that may need to be administered to a patient
include drugs,
hormones, and proteins. One such protein is human growth hormone. Human growth
hormone
(hGH), a 191 amino acid peptide, is a hormone that increases cell growth and
regeneration. hGH
may be used to treat growth disorders and deficiencies. For instance, hGH may
be used to treat
short stature in children or growth hormone deficiencies in adults.
Conventional methods of
5
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
administering hGH include daily subcutaneous injection. A study' has shown
that most patients
are only occasionally compliant or non-compliant with their hGH treatments.
[0026] Another protein that may be used as a medically active agent is
glucagon-like peptide-1
(GLP-1). GLP-1, a 31 amino acid peptide, is an incretin, a hormone that can
decrease blood
glucose levels. GLP-1 may affect blood glucose by stimulating insulin release
and inhibiting
glucagon release. GLP-1 also may slow the rate of absorption of nutrients into
the bloodstream
by reducing gastric emptying and may directly reduce food intake. The ability
of GLP-1 to
affect glucose levels has made GLP-1 a potential treatment for type 2 diabetes
and other
afflictions. In its unaltered state, GLP-1 has an in vivo half-life of less
than two minutes as a
result of proteolysis. GLP-1 receptor agonist treatments can be improved by
minimizing side
effects, increasing effectiveness, and extending the duration of the effect.
[0027] Conventional treatment of diabetes and other afflictions may result in
side effects.
Such side effects may include hypoglycemia, weight gain, an immune response,
inflammation of
the pancreas, increased risk of thyroid cancer, nausea, or pain related to
injection of a treatment.
In addition, conventional treatment may fail to achieve the target glycaemia
in diabetic patients.
Certain formulations may result in an uneven administration of the protein to
the patient, which
may include an initial burst of the drug. The process of formulating the
protein into an
administrable treatment may also result in denaturation or aggregation. The
process of
manufacturing an effective formulation may have high costs or low yields.
[0028] Similar to hGH and GLP-1, enfuvirtide (Fuzeon0) is a medically active
agent that may
face challenges when administered to patients. Enfuvirtide may help treat HIV
and AIDS.
However, enfuvirtide may have to be injected subcutaneously twice a day.
Injections may result
in skin sensitivity reaction side effects, which may discourage patients from
continuing use of
enfuvirtide. A enfuvirtide treatment with less frequent administrations or
extended duration may
be needed to increase patient compliance, lower cost, and enhance the quality
of life for patients
with HIV and AIDS.
[0029] Another medically active agent is parathyroid hormone (PTH) or a
fragment of PTH.
PTH is an anabolic (bone forming) agent. PTH may be secreted by the
parathyroid glands as a
I Rosenfeld R.G., Bakker B. 2008. Compliance and persistence in pediatric and
adult patients receiving growth
hormone therapy. Endocr. Pract. 14(2):143-154.
6
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
polypeptide containing 84 amino acids with a molecular weight of 9,425 Da. The
first 34 amino
acids may be the biologically active moiety of mineral homeostasis. A
synthetic, truncated
version of PTH is marketed by Lilly as Forteo0 Teriparatide. PTH or a fragment
of PTH may be
used to treat osteoporosis. Teriparatide may often be used after other
treatments as a result of its
high cost and required daily injections. As with other medically active
agents, a PTH treatment
with less frequent administrations or extended duration may be desired.
[0030] Unaltered proteins may not have the desired concentration profiles and
other favorable
characteristics. PEGylation, the process of attaching polyethylene glycol to a
molecule, can aid
in the administration of peptides and proteins, which may lead to improved
pharmacological
properties and increased effectiveness. PEG is a linear polymer composed of
subunits of
ethylene glycol and is soluble in both water and many organic solvents. PEG is
flexible,
biocompatible, and non-toxic. As a result of PEG properties, PEGylation may
increase half-life
and/or solubility of a protein or peptide. PEG may be attached to a
monomethoxy group. The
PEG may be a polyethylene glycol aldehyde, including a methoxy polyethylene
glycol aldehyde.
[0031] Another way of altering the concentration profile of a medically active
agent may be to
encapsulate the medically active agent in a biodegradable material. As the
material degrades
gradually in the patient, the medically active agent may be released
gradually. The process of
encapsulating the medically active agent may include an organic solvent. The
medically active
agent may be hydrophilic and insoluble in the organic solvent. The
hydrophobicity of the
medically active agent may be increased to facilitate encapsulation.
[0032] To increase the hydrophobicity of the medically active agent, the
medically active
agent may be PEGylated. Not all medically active agents will increase in
solubility and retain
their biological activity when PEGylated. For example, small PEG molecules may
not be
enough to enhance the solubility of a protein. Adding longer chain PEG
molecules may
eventually increase the hydrophobicity and solubility of the protein, but
these longer chains may
be too large relative to the protein and compromise the protein's biological
activity. As an
example, increasing the PEGylation with hGH was found to linearly reduce the
affinity of hGH
for its receptor.2 Additionally, PEGylation of interferon-a (IFN-a) may result
in lower in vitro
2 Clark R. et al. 1996. Long-acting growth hormones produced by conjugation
with polyethylene glycol¨J. Biol.
Chem. 217: 21969-21977.
7
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
specific activity, depending on the site of PEGylation and the size of the PEG
molecule.3
Furthermore, PEGylation may not increase solubility. For instance, a PEG-
insulin conjugate
with a small PEG molecule of 2,000 Daltons may not be adequately soluble in
organic solvents.
As another example, PEGylation of granulocyte colony stimulating factor (G-
CSF) was
discovered to increase aggregation of G-CSF and to lower solubility.4
[0033] Alternatively, the hydrophobicity may be increased by attaching a
hydrophobic ion to
the medically active agent. As with PEGylation, attaching a hydrophobic anion
to the medically
active agent does not necessarily increase the hydrophobicity of all medically
active agents. A
protein may not have or may not form enough positively charged sites to pair
with hydrophobic
anions. The number of anions attached to the protein and also the increase in
hydrophobicity
may then be limited. For example, acidic proteins such as serum albumins may
contain more
acidic amino acids than basic amino acids. Such acidic proteins may not be
easily protonated by
hydrophobic acids.
[0034] The combination of PEGylation and attaching a hydrophobic ion to a
medically active
agent may produce synergistic results where the hydrophobicity of the
medically active agent
increases more with the combination than what may be expected from the sum of
the increased
hydrophobicities resulting from PEGylation alone and from attaching a
hydrophobic ion alone.
A protein may achieve superior outcomes in a patient if the protein is made
into a protein-PEG
conjugate salt.
[0035] As shown in FIG. 1, examples of the present technology may include a
method 100 of
making a protein-PEG conjugate salt with increased hydrophobicity. The method
may include
providing an aqueous protein solution 102. This aqueous protein solution may
include a protein
and a pH buffer. The pH buffer may include an inorganic salt of phosphoric
acid.
[0036] The protein may have a molecular weight of 3,000 Daltons or more,
between 3,000
Daltons and 10,000 Daltons, 10,000 Daltons or more, between 10,000 Daltons and
15,000
Daltons, 15,000 Daltons or more, between 15,000 Daltons and 20,000 Daltons, or
20,000
Daltons or more according to examples. In these or other examples, the protein
may include 30
3 Grace M. J. et al. 2005. Site of pegylation and polyethylene glycol molecule
size attenuate interferon-alpha
antiviral and antiproliferative activities through the JAK/STAT signaling
pathway. I Biol. Chem. 280: 6327-6336.
4 Veronese F. M. et al. 2007. Site-specific pegylation of G-CSF by reversible
denaturation. Bioconjug. Chem. 18(6) :
1824-30.
8
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
amino acid units or more, between 30 amino acid units and 100 amino acid
units, 100 amino acid
units or more, between 100 amino acid units and 150 amino acid units, or 150
amino acid units
or more. The protein may include human growth hormone, glucagon-like peptide-1
(GLP-1),
insulin, parathyroid hormone, a fragment of parathyroid hormone, enfuvirtide
(Fuzeon0), or
octreotide (Sandostatin0) in examples. GLP-1 may be a natural extract or
synthetic. The
protein may include analogs or derivatives of GLP-1. A combination of proteins
may be
included in the aqueous solution. For example, both GLP-1 and insulin may be
included in the
aqueous solution.
[0037] Method 100 may include reacting polyethylene glycol with the protein to
form a
protein-PEG conjugate 104. The reaction of the PEG with the protein may form a
protein-PEG
conjugate at an N-terminus of the protein.
[0038] The reaction of the polyethylene glycol with the protein may form a
protein-PEG
conjugate with PEG at specific cysteine sites, or the reaction may not result
in any or
substantially any protein-PEG conjugates with PEG at specific cysteine sites.
The reaction of the
PEG with the protein may include forming at least one of an amine bond, an
amide bond, an
ester bond, or a disulfide bond between the PEG and the protein. The reaction
may exclude the
formation of any bond or groups of bonds. The bonds may form with the reactive
group at the
end of the PEG polymer.
[0039] Reacting the polyethylene glycol with the protein may include attaching
a thiol-reactive
PEG to a cysteine residue of a protein. Thiol-reactive PEGs may include
different reactive
groups, which may include maleimide and vinylsulfone. Thiol-reactive PEGs may
have a
molecular weight from 2 to 40 kDa. PEGylation reactions with thiol-reactive
PEGs may be at a
neutral pH. Cysteine residues in some proteins may participate in disulfide
bonds and may not
be available for derivatization. Through in vitro site-directed mutagenesis
techniques, an
additional cysteine residue can be introduced at any specific site on the
protein. An additional
cysteine residue may serve as a site for the attachment of a PEG molecule.
Using these
additional cysteine residues may avoid product heterogeneity and loss of
activity that may result
from random amine PEGylation reactions.
[0040] In these or other examples, the polyethylene glycol may have a
molecular weight of
5,000 Daltons or less or 2,000 Daltons or less. A larger polyethylene glycol
may increase the
9
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
half-life of the protein. A smaller polyethylene glycol may increase the
solubility of the protein-
PEG conjugate without a long half-life. For example, a polyethylene glycol
with a molecular
weight of 2,000 Daltons may have a half-life of less than an hour. A smaller
polyethylene glycol
or PEG may be used to increase the solubility of the protein-PEG conjugate,
with the
encapsulation of the protein achieving most of the desired increase in half-
life. Method 100 may
not include reacting a polyethylene glycol ester with the protein. A
polyethylene glycol may be
selective for primary amines, while the polyethylene glycol ester may react
with other
functionalities and amino acids.
[0041] Method 100 may also include protonating an amino group on the protein-
PEG
conjugate 106 with a hydrophobic organic acid. Protonating the amino group may
occur in an
organic phase and not an aqueous phase. The molar ratio of the hydrophobic
organic acid to the
protein-PEG conjugate may range from 1:1 to 11:1, from 1:1 to 5:1, from 5:1 to
11:1, from 1:1 to
2:1, or from 3:1 to 8:1 according to examples. The protonation may form the
protein-PEG
conjugate salt having a hydrophobic anion that increases the hydrophobicity-
PEG conjugate salt.
The protein-PEG conjugate salt may include a monoPEGylated salt. The
hydrophobic organic
acid may include pamoic acid, docusate hydrogen, furoic acid, or mixtures
thereof. Organic
acids may include carboxylic acids, sulfonic acids, alcohols, or organic
compounds with thiol
groups. The hydrophobic organic acid may exclude any acid described or any
groups of acids
described.
[0042] The hydrophobic anion may include anions associated with the
hydrophobic organic
acids. For example, the hydrophobic anion may include a pamoate anion, a
docusate anion, or a
furoate anion. In these or other examples, the hydrophobic anion may be a
fatty acid anion, a
phospholipid anion, a polystyrene sulfonate anion, or mixtures thereof. The
phospholipid of the
phospholipid anion may include phosphatidylcholine, phosphatidylglycerol,
phosphatidylserine,
phosphatidylinositol, phosphatidylethanolamine, phosphocholine, or mixtures
thereof. The
hydrophobic anion may also exclude any anion described or any group of anions
described. The
hydrophobic anion may attach to a specific side chain on the protein or it may
attach to multiple
side chains on the protein. The hydrophobic anion may have a logP greater than
1. The logP is
the water-octanol partition coefficient and may be defined as the logarithm of
the concentration
of the protein salt in octanol to the concentration of the protein salt in
water. A logP greater than
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
1 may result in a concentration in octanol that is 10 times greater than that
in water. The water-
octanol partition coefficient may be useful in comparing different molecules
for their ability to
partition into a hydrophobic phase, when the molecules themselves may be
amphipathic.
Methods may also include adding cationic detergents, such as dodecylamine
hydrochloride or
cetyltrimethylammonium bromide (CTAB), which may counter the charge of
negatively charged
peptides and may increase the hydrophobicity.
[0043] As illustrated in FIG. 2, examples may include a method 200 of making a
protein-PEG
conjugate with increased hydrophobicity. Method 200 may include providing an
aqueous
protein solution 202, which may include a protein and the pH buffer. The
protein may be any
protein previously described. The aqueous protein solution and the pH buffer
may be any
aqueous protein solution or pH buffer described herein.
[0044] Method 200 may further include reacting a polyethylene glycol with the
protein to form
a protein-PEG conjugate 204. The polyethylene glycol may have any molecular
weight
described herein. The protein may be any protein described herein. The
reaction in forming the
protein-PEG conjugate may proceed in any manner described herein. Method 200
may exclude
protonating an amino group on the protein with a hydrophobic organic acid.
Method 200 may
not result in a protein-PEG conjugate salt.
[0045] Examples, as shown in FIG. 3, may include a method 300 of making a
protein salt with
increased hydrophobicity. Method 300 may include providing an aqueous protein
solution 302
with a protein and a pH buffer. The protein may be separated from water.
Method 300 may
further include protonating an amino group on the protein with a hydrophobic
organic acid 304.
Protonating the amino group may occur in an organic phase and without the
presence of water.
The protonation may form the protein salt having a hydrophobic anion that
increases the
hydrophobicity of the protein salt. Method 300 may not include introducing the
polyethylene
glycol to the aqueous protein solution. Method 300 may not result in a protein-
PEG conjugate or
a protein-PEG conjugate salt. The aqueous protein solution, the protein, the
pH buffer, the
hydrophobic organic acid, and the hydrophobic anion may be any of the
compounds previously
described.
[0046] As in examples depicted in FIG. 4, a method 400 of making controlled-
release
microspheres containing a protein-PEG conjugate salt may include providing an
aqueous protein
11
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
solution 402. The aqueous protein solution may include a protein and a pH
buffer. The protein
may include any protein previously described. Method 400 may further include
reacting a
polyethylene glycol with the protein to form a protein-PEG conjugate 404.
After the protein-
PEG conjugate is formed, the protein-PEG conjugate may be separated from
water. The protein-
PEG conjugate may then be dissolved in an organic solvent.
[0047] In addition, method 400 may include protonating at least one amino
group on the
protein-PEG conjugate with a hydrophobic organic acid 406. The hydrophobic
organic acid may
be added to an organic phase and may not be added to an aqueous phase.
Similarly, protonating
the amino group may occur in the organic phase and without the presence of
water. The
protonation may form the protein-PEG conjugate salt having a hydrophobic
anion. The
hydrophobic organic acid may include any acid described herein, and the
protein-PEG conjugate
salt may be any conjugate salt described herein.
[0048] Furthermore, method 400 may include mixing the protein-PEG conjugate
salt in an
organic solvent with a biodegradable polymer 408. The organic solvent may be
immiscible with
an aqueous phase. The organic solvent may include methylene chloride, benzyl
benzoate,
dichloromethane, chloroform, ethyl ether, ethyl acetate, acetic acid isopropyl
ester (isopropyl
acetate), acetic acid sec-butyl ester, acetophenone, n-amyl acetate, aniline,
benzaldehyde,
benzene, benzophenone, benzyl alcohol, benzyl amine, bromobenzene, bromoform,
n-butyl
acetate, butyric acid methyl ester, caproic acid, carbon disulfide, carbon
tetrachloride, o-
chloroaniline, chlorobenzene, 1-chlorobutane, chloromethane, m-chlorophenol, m-
cresol, o-
cresol, cyanoethane, cyanopropane, cyclohexanol, cyclohexanone, 1,2-
dibromoethane,
dibromomethane, dibutyl amine, m-dichlorobenzene, o-dichlorobenzene, 1,1-
dichloroethane,
1,2-dichloroethane, dichlorofluoromethane, diethyl carbonate, diethyl
malonate, diethyl sulfide,
diethylene glycol dibutyl ether, diisobutyl ketone, diisopropyl sulfide,
dimethyl phthalate,
dimethyl sulfate, dimethyl sulfide, N,N-dimethylaniline, enanthic acid, ethyl
acetoacetate, ethyl
benzoate, ethyl propionate, ethylbenzene, ethylene glycol monobutyl ether
acetate, exxate 600,
exxate 800, exxate 900, fluorobenzene, furan, hexamethylphosphoramide, 1-
hexanol, n-hexyl
acetate, isoamyl alcohol (3-methyl-l-butanol), isobutyl acetate,
methoxybenzene, methyl amyl
ketone, methyl benzoate, methyl formate, methyl isoamyl ketone, methyl
isobutenyl ketone,
methyl isobutyl ketone, methyl n-butyl ketone, methyl propyl ketone, 4-methyl-
2-pentanol, N-
12
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
methylaniline, nitrobenzene, nitroethane, 1-nitropropane, 2-nitropropane, 1-
octanol, 2-octanol, 1-
pentanol, 3-pentanone, 2-phenylethanol, n-propyl acetate, quinoline, styrene,
1,1,2,2-
tetrachloroethane, 1,1,2,2-tetrachloroethylene, toluene, 1,1,1-
trichloroethane, 1,1,2-
trichloroethane, 1,1,2-trichloroethylene, trifluoromethane, valeric acid, m-
xylene, o-xylene, p-
xylene, 2,4-xylenol, or mixtures thereof. The organic solvent may exclude any
solvent or any
groups of solvents.
[0049] Methods may include a mixture of solvents. The mixture of solvents may
include a
solvent that is miscible in water, but the mixture of solvents may be
immiscible in water. For
examples, a water-miscible solvent such as dimethyl sulfoxide (DMSO),
methanol,
dimethylformamide (DMF), acetonitrile, tetrahydrofuran, or mixtures thereof
may be added to
the water immiscible solvent.
[0050] The biodegradable polymer may include a polylactide, a polyglycolide, a
poly(d, 1-
lactide-co-glycolide), a polycaprolactone, a polyorthoester, a copolymer of a
polyester and a
polyether, or a copolymer of polylactide and polyethylene glycol. The
biodegradable polymer
may exclude any of these polymers or groups of these polymers. The molecular
weight of the
biodegradable polymer may be adjusted depending on the size of the PEG to
produce a desired
pharmacokinetic profile.
[0051] Poly(d,l-lactide-co-glycolide) (PLGA) may have a molecular weight from
5,000 Da to
7,000 Da, 7,000 Da to 17,000 Da, 17,000 Da to 20,000 Da, 20,000 Da to 24,000
Da, 24,000 Da
to 38,000 Da, 38,000 Da to 40,000 Da, or 40,000 Da to 50,000 Da, in examples.
PLGA may
have a ratio of lactide to glycolide of 50:50 or 75:25. In some examples, PLGA
may have a ratio
of lactide to glycolide ranging from 40:60 to 50:50, from 50:50 to 60:40, from
60:40 to 70:30,
from 70:30 to 75:25, or from 75:25 to 90:10. The ratio of lactide to glycolide
may be less than or
equal to 50:50, less than or equal to 60:40, or less than or equal to 75:25,
where less than refers
to a smaller proportion of lactide compared to glycolide. The hydrophobic
anion of the organic
acid may improve the release characteristics of some PLGAs but not others.
[0052] Possible PLGAs may include PLGA 502, PLGA 503, PLGA 752, and PLGA 753.
PLGA 502 may be a polymer with a lactide to glycolide ratio of 50:50, an
inherent viscosity
from 0.16 to 0.24 dL/g, and a molecular weight from 7,000 to 17,000 Da. PLGA
503 may be a
polymer with a lactide to glycolide ratio of 50:50, an inherent viscosity from
0.32 to 0.44 dL/g,
13
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
and a molecular weight from 24,000 to 38,000 Da. PLGA 752 may be a polymer
with a lactide
to glycolide ratio of 75:25, an inherent viscosity from 0.14 to 0.22 dL/g, and
a molecular weight
from 4,000 to 15,000 Da. PLGA 753 may be a polymer with a lactide to glycolide
ratio of
75:25, an inherent viscosity from 0.32 to 0.44 dL/g, and a molecular weight
from 24,000 to
38,000 Da. The PLGA polymer may also be acid end-capped or ester end-capped.
[0053] The hydrophobic anion of the protein-PEG conjugate salt may increase
the solubility of
the salt in the organic solvent. Method 400 may also include emulsifying the
mixture of the
protein-PEG conjugate salt and the biodegradable polymer 410 in an aqueous
solution.
Additionally, method 400 may include hardening the emulsified mixture 412 of
the protein-PEG
conjugate salt and the biodegradable polymer into the controlled-release
microspheres. The
microspheres may include the hydrophobic anion of the organic acid. If an
organic acid were
added to an aqueous phase instead of an organic phase, the organic acid and
any anions from the
organic acid may not be included in a microsphere or may be included at a
significantly lower
concentration in the microsphere.
[0054] A method of making controlled-release microspheres may include a
protein-PEG
conjugate or a protein salt instead of a protein-PEG conjugate salt. The
protein-PEG conjugate
or the protein salt may be made by any method described herein.
[0055] Examples may include a microsphere with a biodegradable polymer. A
microsphere
may have a diameter under 1 mm. For example, the microsphere may have a
diameter from 10
to 20 [tm, 20 to 30 [tm, 30 to 40 [tm, 40 to 50 pm, 50 to 60 [tm, 60 to 70 pm,
70 to 80 jim, 80 to
901.tm, or 90 to 100 p.m. In addition, a plurality of microspheres may have a
distribution where
the microsphere diameter is in one of the ranges described herein. The
diameter may be
characterized by the mean, median (D50), 10 percentile (D10), or 90 percentile
(D90) of the
distribution. A microsphere that is too large may not be injectable with a
syringe for treatment of
an individual. Additionally, a microsphere that is too large may also delay
the release of a
medically active agent. On the other hand, if the diameter is too small,
microspheres may be lost
during processing, sieving, and/or screening.
[0056] The microsphere may further include a protein-polyethylene glycol
conjugate, the
protein and a hydrophobic anion of an organic acid, or mixtures thereof The
microsphere may
14
CA 02966207 2017-04-27
WO 2016/089818
PCT/US2015/063102
also exclude a protein-polyethylene glycol conjugate or a hydrophobic anion of
an organic acid.
The protein may be any protein described herein. The composition may include a
combination
of proteins. For example, the composition may include GLP-1 and insulin. The
GLP-1 and
insulin may be present in a microsphere as part of a protein-PEG conjugate.
The term premix
may refer to a microsphere with a combination of proteins or protein-PEG
conjugates.
Alternatively, examples may include a postmix, which refers to a mixture of
microspheres,
where each microsphere may contain only one medically active agent but a
combination of
medically active agents may be included in the mixture of micro spheres. The
organic acid and
the hydrophobic anion of an organic acid may be any compound previously
described.
[0057] A composition may include a biodegradable polymer and may be presented
as a
microsphere. Microspheres may be prepared by first producing an emulsion from
an aqueous
solution and an immiscible solution, and may be followed by solvent extraction
and drying.
Emulsions may be produced by static mixing, dynamic mixing, or packed-bed
emulsifiers.
[0058] Compositions with a biodegradable polymer may be solutions or
suspensions in an
organic solvent. These solutions may be delivered to the human body, and
during the delivery,
the organic solvent may dissolve in body fluid and may deposit the
composition, including the
biodegradable polymer. The organic solvent may be miscible with water and may
not be toxic
so the solvent may be injected into a patient. Additionally, in order to form
a solution, a
PEGylated protein, organic acid, and biodegradable polymer should dissolve in
the organic
solvent. For suspensions, at least one of the PEGylated protein, organic acid,
and the
biodegradable polymer should not be completely soluble in the organic solvent.
Examples of
organic solvents include N-methyl pyrrolidone, dimethyl sulfoxide, propylene
glycol, ethyl
benzoate, benzyl benzoate, triacetin, PEG 400, and mixtures thereof. The
composition may
exclude any organic solvent or groups of organic solvents. Examples of the
present technology
may include methods of making compositions with a biodegradable polymer in
solution with an
organic solvent.
[0059] FIG. 5 shows a method 500 of making a solution or suspension of a
biodegradable
polymer and a protein-PEG conjugate salt. Method 500 may include providing a
protein-PEG
conjugate 502. Method 500 may also include mixing a biodegradable polymer, the
protein-PEG
conjugate, a hydrophobic organic acid, and an organic solvent in a mixture
504. The protein-
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
PEG conjugate may be free of the protein-PEG conjugate salt. The protein-PEG
conjugate may
be glucagon-like peptide- 1 -PEG conjugate. Mixing may include mixing a second
protein-PEG
conjugate, which may be an insulin-PEG conjugate, in the mixture.
[0060] Additionally, method 500 may include forming the protein-PEG conjugate
salt 506.
The protein-PEG conjugate salt may include the protein-PEG conjugate and an
anion of the
hydrophobic organic acid. This conjugate salt may be formed concurrently with
the dissolution
of components in the organic solvent rather than providing an already formed
salt and dissolving
the salt in the solvent. Further, method 500 may include agitating the mixture
to form the
solution or suspension 508. The solution may be a clear solution after
agitating. The protein-
PEG conjugate, the biodegradable polymer, the hydrophobic organic acid, and
the organic
solvent may be any organic solvent described herein. Each component and each
step may be
free of water.
[0061] FIG. 6 shows a method 600 of making a solution or suspension of a
biodegradable
polymer and a protein-PEG conjugate salt. Method 600 may include dissolving a
biodegradable
polymer in an organic solvent to form a mixture 602. Method 600 may exclude
dissolving a
protein-PEG conjugate salt in the organic solvent. Method 600 may also include
adding a
protein-PEG conjugate and a hydrophobic organic acid to the mixture 604.
Furthermore, method
600 may also protonating an amino group on the protein-PEG conjugate with a
hydrophobic
organic acid 606. The protonation may form the protein-PEG conjugate salt
having a
hydrophobic anion. Additionally, method 600 may include agitating the mixture
to form the
solution or suspension 608. The solution may be a clear solution after
agitating. The protein-
PEG conjugate, the biodegradable polymer, the hydrophobic organic acid, and
the organic
solvent may be any organic solvent described herein. Each component and each
step may be
free of water.
[0062] Solutions produced by method 500, method 600, or similar methods may
produce a
solution. The solution may be injected into an individual. When the solution
contacts water, the
solution may form a solid depot. The depot may then gradually release a
protein or other
medically active agent.
16
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
EXAMPLE 1
[0063] The GLP-1 C-terminal cysteine mutein (GLP-1 [7-36] plus an added
cysteine at
position 37) was cloned as a fusion to a larger polypeptide that contained a
self-cleaving
autoproteolytic sequence between GLP-1 and its fusion partner. The fusion
protein was
expressed in E. coil using an IPTG inducible system under the control of T7
polymerase.
EXAMPLE 2
[0064] The GLP-1 fusion protein was isolated from cell lysates under
denaturing conditions,
renatured, cleaved (autoproteolysis), and further purified using cationic
chromatography.
Characterization assays to confirm the peptides identity included RP-HPLC
analysis, SDS-
PAGE, and mass spectrometry. Commercially available synthetic cysteine mutein
of GLP-1 was
used as the control for these assays.
EXAMPLE 3
[0065] The GLP-1 cysteine mutein (produced either by a recombinant or
synthetic chemical
process) was PEGylated with a 5 kDa or 10 kDa cysteine-reactive maleimide-PEG
by the
following process. The peptide was first dissolved in 20 mM sodium phosphate
buffer, pH 7.5 at
a concentration of 1-5 mg/mL, and an equal molar amount of the maleimide PEG
reagent was
added. The reaction was allowed to continue overnight. The PEGylated GLP-1
peptide was
purified using cation exchange chromatography (SP-HP Sepharose) with an
equilibration buffer
of 10 mM sodium acetate at pH 3.5 and a step elution buffer of 0.02% ammonium
bicarbonate.
The product-containing fractions were pooled, dialyzed against 0.02% ammonium
bicarbonate,
and lyophilized. The concentration of purified PEGylated peptide was
determined by UV
spectroscopy or by Bradford protein assay. Additional analytical assays
performed post-
PEGylation include SEC-HPLC analysis, SDS-PAGE, mass spectral analysis, N-
terminal
analysis, and endotoxin determination. A larger PEG of 20 kDa was also tested.
Even larger
PEGs (e.g., 40 kDa branched) may also be tested.
EXAMPLE 4
[0066] N-terminally PEG-GLP-1 was prepared by the following process. First, 30
mg of GLP-
1 were dissolved in 20 mM sodium acetate at pH 4.5, reaching a GLP-1
concentration of 1 to 5
17
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
mg/mL. Next, 55 mg of 5-kDa propionadehyde PEG were added, followed by 2 mg of
sodium
cyanoborohydride. The reaction was allowed to continue overnight at room
temperature. After
16 hours, the PEG-GLP-1 was purified by cation exchange chromatography (SP-HP
Sepharose).
EXAMPLE 5
[0067] hGH was subcloned in an E. coli IPTG inducible system under the control
of T7
polymerase. Cells were grown to an OD600nm = 0.5, and 1 mM isopropyl-13-D-
thiogalactopyranoside IPTG was added to induce expression. The induced culture
was incubated
overnight for about 16 hr. The cells were pelleted by centrifugation and
stored at -20 C.
[0068] The cell pellet was thawed, suspended in lysis buffer (1 mM EDTA, 150
mM NaC1, 50
mM Tris, 1% Triton X-100, pH 7.5) and homogenized by three passages thru a
microfluidizer.
Insoluble material was recovered by centrifugation, suspended in salt buffer
(500 mM NaC1,
50 mM Tris, pH 7.5), and again collected by centrifugation. The insoluble
pellet (inclusion
bodies) was stored at -20 C.
[0069] A portion of the insoluble pellet (-- 15 mg hGH) was thawed, dissolved
in 10 mL of
8 M urea, 10 mM cysteine, 20 mM BisTris, stirred for 60 min at room
temperature, and then
diluted into 100 mL of 20 mM Tris, 15% glycerol, pH 8.5. The refold mixture
was held at 4 C
for 1 day, centrifuged, and loaded onto a 5 mL Q-Sepharose XL column
equilibrated in 20 mM
BisTris, pH 7.0 (Buffer A). The bound proteins were eluted with a pH/salt
gradient (0-100%)
with Buffer B (50 mM NaC1, 20 mM BisTris, pH 5). Column fractions were
analyzed by RP-
HPLC analysis, and fractions containing renatured hGH were pooled and stored
at -20 C.
EXAMPLE 6
[0070] Refolded, purified hGH was N-terminally PEGylated by the following
protocol. First,
mg of hGH were first dissolved in 20 mM sodium acetate, pH 4.5, reaching an
hGH
concentration of 5 mg/mL. Next, 40 mg of 10-kDa propionadehyde PEG were added
followed
25 by 8 mg of sodium cyanoborohydride. The reaction was allowed to continue
overnight at room
temperature. After 16 hours, the PEG-hGH was purified using a 5 mL Q-Sepharose
HiTrap
column.
18
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
EXAMPLE 7
[0071] GLP-1-loaded PLGA microspheres with a hydrophobic counter ion were
prepared by
the following process. First, 30 mg of 5 kDa-PEG-GLP-1 were dissolved in 2 mL
of
dichloromethane. Next, 50 L of a pamoic acid solution (50 mg/mL in dimethyl
formamide
[DMF]) were added. PLGA 502 at 170 mg was next added to the peptide solution
and mixture
was vortexed until clear. Afterwards, 5 mL of an emulsion stabilizer (1%
polyvinyl alcohol
[PVA]) were added, and the mixture was immediately vortexed at the max speed
on a Genie
vortexer for 7-8 sec. At that point, the emulsion was quickly added to 100 mL
of 0.3% PVA
while rapidly stirring. After 10 min, 150 mL of 2% isopropanol was added, and
the suspension
was stirred for 3-12 hours to allow for solvent evaporation and microsphere
hardening. The
resulting microspheres were isolated by settling, washed three times with 200
mL purified water,
and lyophilized. Different PLGA polymers were used to prepare weekly (e.g.,
PLGA 502) or
monthly formulations (e.g., PLGA 753) of microspheres including GLP-1 and
pamoic acid.
EXAMPLE 8
[0072] Verification of the drug and hydrophobic ion loading of the
microspheres is
accomplished by first dissolving the microspheres in acetonitrile, followed by
precipitation of the
polymer by diluting with water. The resulting supernatant is analyzed by RP-
HPLC. The
analysis showed that PEG-GLP-1 loaded microspheres containing pamoic acid were
produced
with drug loadings ranging from 2% to 18% for PEG-GLP-1 and around 0.3% to
0.5% pamoic
acid. RP-HPLC results confirmed that pamoic acid was incorporated into the
microspheres.
EXAMPLE 9
[0073] Particle sizes were measured using a Beckman particle analyzer. The
particles had
mean diameters between 20 and 45 p.m, which is a range that can be
conveniently dosed
subcutaneously using a 27 gauge syringe. The particles examined by light
microscopy show
smooth spheres with minimal surface artifacts or inclusions. The particles
also have fewer
surface artifacts and inclusions than some conventional particles with
medically active agents.
FIG. 7A shows light microscopy images of PLGA 502 microspheres loaded with PEG-
GLP-1
with pamoic acid. The particles in FIG. 7A have a D10 of 16 pm, a D50 of 33
p.m, and a D90 of
50 m. FIG. 7B shows light microscopy images of PLGA 502 microspheres loaded
with a
19
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
premix of PEG-insulin and PEG-GLP-1 and pamoic acid. The particles in FIG. 7B
have a D10
of 18 turn, a D50 of 30 p.m, and a D90 of 42 p.m. The particle sizes measured
show a tight
distribution around a syringeable diameter.
EXAMPLE 10
[0074] For initial burst release studies, microspheres were prepared with 170
mg of various
PLGA polymers, 30 mg of PEG-GLP-1, and 50 p.L of a pamoic acid solution (50
mg/mL in
dimethyl formamide [DMF]). Control microspheres were also prepared with PLGA,
PEG-GLP-
1, and with and without 50 [tI, DMF. The microspheres, after freeze drying,
were suspended in
0.4% PVA plus 0.05% sodium azide and incubated at 37 C with rotisserie-like
mixing. After 24
hours, the suspensions were centrifuged, and the supernatant analyzed by RP-
HPLC with the
amount of released PEGylated peptide quantified based on standard curves.
Table 1 shows
percentages of the total PEGylated peptide initially present in the
microspheres. A lower initial
burst percentage may be generally desired in treatments. Based on the data in
Table 1, the
pamoic acid minimized the initial burst of PEG-GLP-1 from microspheres that
were produced
using PLGA polymers with a molecular range around 12-18 kDa (PLGA 502 and PLGA
752).
For microspheres with PLGA 503, pamoic acid increased the burst. Table 1 also
shows that the
change in the burst amount was likely a result of the pamoic acid and not the
DMF solvent.
Table 1. Effect of pamoic acid on the burst percentage from microspheres
prepared with
different PLGAs
PLGA No Additive Pamoic acid + DMF DMF
502 24.5 1.2 15.4
503 0 8.5 0
752 24 4.6 18.2
753 0 0 0
EXAMPLE 11
[0075] For the extended release studies, microspheres were prepared with 170
mg of various
PLGA polymers, 30 mg of PEG-GLP-1, and 50 A of a pamoic acid solution (50
mg/mL in
dimethyl formamide [DMF]). Control microspheres were also prepared with PLGA,
PEG-GLP-
1, and with and without 50 p.L DMF. The microspheres, after freeze drying,
were suspended in
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
0.4% PVA and 0.05% sodium azide and incubated at 37 C with rotisserie-like
mixing. After
specific time points, the suspensions were centrifuged, and the supernatant
analyzed by RP-
HPLC with the amount of released PEGylated peptide quantified based on
standard curves.
FIGS. 8 and 9 show graphs of results of these extended release studies on
microspheres. The
figures show release values as percentages of the total PEGylated peptide
initially present in the
microspheres. FIGS. 8 and 9 show that pamoic acid minimized the initial
release of PEG-GLP-1
but had little effect on the final release profile. Rather, the timing of the
full release was mostly
dependent on the hydrophobicity of the PLGA polymer. PLGA 502 has a 50:50
ratio of lactide
to glycolide, and PLGA 752 has a 75:25 ratio of lactide to glycolide. A higher
ratio of lactide to
glycolide results in a more hydrophobic PLGA polymer. Accordingly, PLGA 752
took longer to
release PEG-GLP-1 than PLGA 502.
EXAMPLE 12
[0076] Biacore (GE Healthcare) studies were performed to measure the in vitro
binding
affinities of the PEGylated GLP-1 compounds for the GLP-1 receptor. The GLP-1
receptor was
covalently immobilized to the biosensor surface, and the PEGylated GLP-1
compound or GLP-1
was injected over the surface. The equilibrium dissociation constants (KD )
for GLP-1 receptor
binding were 1.6 [tM for 5 kDa-PEG-GLP-1 (Cys analog), and the KD was 1.0 ttM
for
unmodified GLP-1. The equilibrium constant for N-terminal 5 kDA-PEG-GLP-1
could not be
determined, likely as a result of extremely weak interaction between the
receptor and the N-
terminal PEGylated peptide. Attaching a PEG polymer to the C-terminus may not
significantly
affect the specific activity of GLP-1 whereas attaching a PEG polymer to the N-
terminus may
interfere with receptor binding.
EXAMPLE 13
[0077] The in vitro bioactivities of the PEGylated GLP-1 conjugates were
measured in a cell-
based assay GLP-1 receptor binding assay using CHO-K1/GLP1/Gal5 and monitoring
the GLP-
1-induced concentration-dependent stimulation of intracellular calcium
mobilization. The cells
were loaded with Calcium-4 prior to stimulation with a GLP-1 receptor agonist.
The
intracellular calcium change was measured by FlexStation. The relative
fluorescent units (RFU)
were plotted against the log of the cumulative doses (5-fold dilution) of GLP-
1. Table 2 shows
EC50, the concentration at which 50% of the highest activity level is reached.
A lower EC50
21
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
indicates a higher activity. In agreement with the Biacore data of Example 12,
C-terminally
PEGylated GLP-1 showed close to full activity compared to the unmodified GLP-1
peptide. The
kDa PEG for the C-terminally PEGylated GLP-1 did not appear to significantly
affect the
activity. The specific activities of the N-terminally PEGylated GLP-1 peptides
were
5 significantly reduced compared to the C-terminally PEGylated GLP-1. Using
a 2 kDa PEG
instead of a 5 kDa PEG for N-terminally PEGylated GLP-1 was not observed to
significantly
affect the activity.
Table 2. GLP-1 receptor binding data
Compound ECso (M)
GLP-1 (7-36) 3.80 x 10-7
5 kDa-PEG-Cys(37)-GLP-1 3.36 x 10-7
5 kDa-PEG-N-term-GLP-1 1.3 x 10-5
2 kDa-PEG-N-term-GLP-1 3.41 x 10-5
EXAMPLE 14
[0078] Dual peptide loaded microspheres containing the PEG-GLP-1, PEG-insulin,
and
pamoic acid were prepared using an oil/water single-emulsion solvent
extraction/evaporation
process. The oil phase consisted of 170 mg of PLGA polymer, 10 mg of PEG-
insulin, 20 mg of
PEG-GLP-1, and 2.5 mg of pamoic acid (from a pamoic acid stock of 50 mg/mL in
DMF)
dissolved in 2 mL of dichloromethane. The oil phase was emulsified by
vortexing with 5 mL of
1% w/v PVA, and the primary emulsion was added to a 100 mL of 0.3% PVA
stirring at 300
rpm. Then 150 mL of 2% IPA was added approximately 10 minutes later, and the
suspension
was stirred to facilitate solvent evaporation. After 3 hours, the hardened
microspheres were
washed three times with 200 mL purified water and freeze dried.
EXAMPLE 15
[0079] Pharmacokinetic studies were performed in a 20 day study with Sprague
Dawley male
rats. The rats (6 per group) were each given a subcutaneous injections of
either PEG-GLP-1
22
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
loaded microspheres (MS) or dual loaded PEG-GLP-1 and PEG-insulin microspheres
(premix).
Both the PEG-GLP-1 MS and the premix contained pamoic acid and PLGA 502. An
additional
control included a placebo group, which was diluent only (sodium
carboxymethycellulose [1%],
D-mannitol [5%] and polysorbate 20 [0.1%]). The in vivo release profile was
determined by
collection of the plasma samples at predetermined time points followed by
ELISA analysis of the
samples (EM Millipore Anti-GLP-1 kit). FIGS. 10 and 11 show the
pharmacokinetic analyses
of GLP-1 or insulin in rat plasma samples after microsphere dosing. In both
cases, the
PEGylated peptides were released in vivo with a peak concentration occurring
at around 17 days.
The GLP-1 release profile was not observed to be significantly affected by
including PEG-
insulin. The release of insulin with the premix microspheres occurred at about
the same time as
the GLP-1. Thus, premix microspheres were observed to release medically active
agents at
about the same time as non-premix microspheres.
EXAMPLE 16
[0080] PLGA microspheres containing enfuvirtide and pamoic acid were prepared
using an
o/w single-emulsion solvent extraction/evaporation process. Enfuvirtide was
not PEGylated.
The oil phase consisted of 170 mg of PLGA 502, 30 mg of enfuvirtide, 8 mg of
pamoic acid and
2 ml of dichloromethane. The oil phase was emulsified using vortexing with 5
mL of 1% w/v
PVA, and the primary emulsion was added to a 100 mL of 0.3% PVA stirring at
300 rpm. Then
150 mL of 2% IPA were added approximately 10 minutes later, and the suspension
was stirred to
facilitate microsphere hardening. After 3 hours, the hardened microspheres
were filtered,
washed with a large volume of double distilled H20, and freeze dried. A second
lot of
microspheres were also prepared as described above in this example except
pamoic acid was not
added to the mixture. Particle size analysis of the enfuvirtide microspheres
prepared with
pamoic acid present displayed greater homogeneity (mean size: 28
S.D: 9.5 p.M) versus the
enfuvirtide microspheres without pamoic acid (mean size: 32.75 M; S.D: 34.07
p.M).
EXAMPLE 17
[0081] In situ formulations were prepared containing PLGA, PEG-GLP-1, pamoic
acid, and N-
methyl pyrrilidone (NMP). First, 340 mg of PLGA (either PLGA 503, PLGA 752, or
PLGA
753) were dissolved in 1 mL of N-methyl pyrrolidone (NMP). Next, 30 mg of PEG-
GLP-1 were
23
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
added along with 2.5 mg of pamoic acid (from a stock of 200 mg/mL in DMSO).
The mixtures
were swirled gently until clear. For release, each solution was loaded into a
dialysis cassette
(Slide-A-lyzer 3.5 kDa cutoff) and suspended in a beaker of 900 mL of
phosphate buffered saline
and 0.05% polysorbate. The dialysis buffer was gently stirred overnight at
room temperature.
After 16 hours at room temperature, depots had formed within the cassettes
with a layer of liquid
on top. The liquid and solids were separated, and PEG-GLP-1 content was
determined by RP-
HPLC analysis. The calculated burst for the individual depots was 11% for the
PLGA 503
polymer, 62% for the PLGA 752 polymer, and 40% for the PLGA 753 polymer. The
burst was
observed to be affected by the hydrophobicity of the PLGA polymer.
EXAMPLE 18
[0082] In situ formulations were prepared containing PLGA, PEG-GLP-1, pamoic
acid, and a
mixture of FDA-approved solvents. First, 340 mg of PLGA 752 were dissolved in
1 mL of a
mix of 50% N-methyl pyrrolicione (NMP) and 50% benzyl benzoate or a mix of 50%
DMSO and
50% benzyl benzoate. Next, 30 mg of PEG-GLP-1 were added along with 2.5 mg of
pamoic
acid (from a stock of 200 mg/mL in DMSO). The mixture was swirled gently until
clear. For
release, each solution was loaded into a dialysis cassette (Slide-A-lyzer 3.5
kDa cutoff) and
suspended in a beaker of 900 mL of phosphate buffered saline and 0.05%
polysorbate. After 16
hours at room temperature, depots had formed within the cassettes with a layer
of liquid on top.
The liquid and solids were separated, and PEG-GLP-1 content was determined by
RP-HPLC
analysis. The calculated burst for the DMSO:benzyl benzoate mixture was 2.6%
versus 0.2% for
the NMP:benzyl benzoate based in situ formulation. DMSO is more polar than
NMP. The
desired rate of release (weekly versus monthly dosing) may be adjusted based
on the based on
the hydrophobicity and concentration of the PLGA, the water miscibility of the
solvent(s), and/or
the addition of pamoic acid.
EXAMPLE 19
[0083] Examples 1-18 are repeated with human growth hormone, insulin,
enfuvirtide,
parathyroid hormone, a fragment of PTH, octreotide, or other medically active
agent in place of
GLP-1, insulin, and/or enfuvirtide. Examples may also include preparing
microspheres and
compositions with organic solvents for any of the compositions in Examples 1-
19. These
24
CA 02966207 2017-04-27
WO 2016/089818 PCT/US2015/063102
examples showed superior concentration profiles and other characteristics
compared to
conventional compositions and methods.
[0084] In this description, for the purposes of explanation, numerous details
have been set
forth in order to provide an understanding of various examples of the present
technology. It will
be apparent to one skilled in the art, however, that certain examples may be
practiced without
some of these details, or with additional details.
[0085] Having described several examples, it will be recognized by those of
skill in the art that
various modifications, alternative constructions, and equivalents may be used
without departing
from the spirit of the invention. Additionally, a number of well-known
processes and elements
have not been described in order to avoid unnecessarily obscuring the present
invention.
Additionally, details of any specific example may not always be present in
variations of that
example or may be added to other examples.
[0086] Where a range of values is provided, it is understood that each
intervening value, to the
tenth of the unit of the lower limit unless the context clearly dictates
otherwise, between the
upper and lower limits of that range is also specifically disclosed. Each
smaller range between
any stated value or intervening value in a stated range and any other stated
or intervening value
in that stated range is encompassed. The upper and lower limits of these
smaller ranges may
independently be included or excluded in the range, and each range where
either, neither, or both
limits are included in the smaller ranges is also encompassed within the
invention, subject to any
specifically excluded limit in the stated range. Where the stated range
includes one or both of
the limits, ranges excluding either or both of those included limits are also
included.
[0087] As used herein and in the appended claims, the singular forms "a",
"an", and "the"
include plural referents unless the context clearly dictates otherwise. Thus,
for example,
reference to "a method" includes a plurality of such methods and reference to
"the protein"
includes reference to one or more proteins and equivalents thereof known to
those skilled in the
art, and so forth. The invention has now been described in detail for the
purposes of clarity and
understanding. However, it will be appreciated that certain changes and
modifications may be
practice within the scope of the appended claims.