Note: Descriptions are shown in the official language in which they were submitted.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- -
1-TYDR.OPROCESSING FOR DISTILLATE PRODUCTION
FIELD
[0001] Systems and methods are provided for processing distillate boiling
range
feeds for production of distillate boiling range products.
BACKGROUND
[0002] As methods for recovering natural gas from shale formations and
other
non-conventional sources have improved, the cost of using natural gas has
decreased.
This reduction in natural gas cost means that processes dependent on natural
gas as a
substantial feed are also more economically favorable. One process that can
directly
benefit from a reduced natural gas cost is steam reforming of methane to form
hydrogen
andlor syngas.
[0003] One of the challenges in processing of liquid petroleum feeds is
that the
hydrogen to carbon ratio of the petroleum feed is often lower than the
hydrogen to
carbon ratio of the desired products from a feed. Some refinery processes can
generate
small volumes of excess hydrogen, but in general hydrogen is a limited
resource.
[0004] U.S. Patents 8,722,563 and 8,722,564 describe multi.m.etallic
hydroprocessing
catalysts prepared by forming a catalyst precursor and then heating the
catalyst precursor
to form, the catalyst. The multimetallic catalysts are described as having
improved
activity for hydrodenitrogenation of various types of feeds.
[0005] U.S. Patent 6,582,590 and U.S. Patent 6,929,738 describe various
types of
processing sequences that include hydroprocessing in the presence of a bulk
multimetallic catalyst. The processes are described as being suitable for
production of
various product fractions, including distillate fuels.
SUMMARY
[0006] In an aspect, a hydroprocessing process is provided, comprising:
reacting a
feedstream having a sulfur content of at least about 3000 wppm, or at least
about 4000
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 2 -
wppm, or at least about 5000 wppm (such as up to about 50000 wppm.), and a T90
boiling point of about 900 F (482 C) or less, in a first hydrotreating stage
in the presence
of a hydrogen-containing treat gas and in the presence of at least one first
stage
hydrotreating catalyst, the first hydrotreating stage being operated at first
stage
hydrotreating conditions, to produce a first liquid effluent having a sulfur
content of
about 5000 wppm or less, or about 4000 wppm or less, or about 3000 wppm or
less, the
sulfur content of the first liquid effluent being less than the sulfur content
of the
feedstream; separating the first liquid effluent to produce a first vapor
phase stream and a
first liquid product stream, the first liquid product stream optionally having
a 110 boiling
point and a190 boiling point; reacting at least a portion of the first liquid
product stream
in a second hydrotreating stage in the presence of a hydrogen-containing treat
gas and a
second hydrotreating catalyst, the second hydrotreating stage being operated
at second
stage hydrotreating conditions to produce a second liquid effluent; and
separating the
second liquid effluent to produce a second vapor phase stream. and a second
liquid
product stream having a sulfur content of about 500 wppm or less, or about 250
wppm or
less, or about 100 wppm or less, wherein about 15 wt% or less of the
feedstream is
converted relative to a conversion temperature of 350 F (177 C) during the
reacting in
the first hydrotreating stage and the second hydrotreating stage. Optionally,
the first
liquid effluent can have a sulfur content of at least about 1000 wppm, or at
least about
1500 wppm, or at least about 2000 wppm.
100071 In another aspect, a hydroprocessing process is provided,
comprising:
reacting a feedstream. having a 190 boiling point of about 900 F (482 C) or
less in a first
hydrotreating stage in the presence of a hydrogen-containing treat gas and in
the
presence of at least one first stage hydrotreating catalyst, the first
hydrotreating stage
being operated at first stage hydrotreating conditions, to produce a first
liquid effluent;
separating at least a portion of the first liquid effluent to produce a first
vapor phase
stream and a first liquid product stream, the first liquid product stream
having a sulfur
content of about 1000 wppm to about 20,000 wppm, the first liquid product
stream
having a) a T10 boiling point of at least about 350 F (177"C), b) a T90
boiling point of
about 850 F (454 C) or less, or c) a combination thereof; reacting at least a
portion of
the first liquid product stream in a second hydrotreating stage in the
presence of a
hydrogen-containing treat gas and a mixed metal catalyst, the second
hydrotreating stage
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 3 -
being operated at second stage hydrotreating conditions to produce a second
liquid
effluent, the second stage hydrotreating conditions being effective for
conversion of
about 10 wt% or less of the at least a portion of the first liquid product
stream. relative to
a conversion temperature of about 350 F (177 C); and separating at least a
portion of the
second liquid effluent to produce a second vapor phase stream and a second
liquid
product stream, the second liquid product stream having a sulfur content of
about 250
wppm or less, or about 100 wppm or less.
BRIEF DESCRIPTION OF THE DRAWINGS
0008] FIG. 1 schematically shows an example of a configuration suitable for
processing a feed to produce distillate boiling range products.
[0009] FIG. 2 schematically shows an example of a configuration suitable
for
processing a feed to produce distillate boiling range products.
DETAILED DESCRIPTION
[0010] All numerical values within the detailed description and the claims
herein are
modified by "about" or "approximately" the indicated value, and take into
account
experimental error and variations that would be expected by a person having
ordinary
skill in the art.
Overview
[001I] in various aspects, methods are provided for hydrotreating a feed to
generate
a product with a reduced or minimized aromatics content. For example, a
distillate
boiling range feed having an elevated content of sulfur and/or nitrogen can be
hydrotreated using at least two hydrotreating stages with intermediate
separation to
produce a hydrotreated distillate boiling range product with a reduced or
minimized
aromatics content. Additionally or alternately, a mixed metal catalyst formed
from a
suitable precursor can be used during the hydrotreating. A. mixed metal
catalyst formed
from a suitable precursor can provide an unexpectedly superior activity for
aromatic
saturation. A still further unexpected benefic can be achieved by combining a
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 4 -
multi-stage hydrotreating process with intermediate separation with
hydrotreating in the
presence of a mixed metal catalyst formed from a suitable precursor.
[0012] Some feeds that have an appropriate boiling range for use as a
distillate fuel
correspond to feeds with both a substantial content of heteroatoms, such as
sulfur and
nitrogen, and a substantial content of aromatic compounds. The aromatic
compounds
can optionally include multi-ring aromatic compounds. Such aromatic compounds
have
a high density relative to aliphatic compounds. By increasing the amount of
hydrogenation of aromatics in a distillate boiling range feed, the overall
density of the
resulting liquid product can be reduced at a given level of feed conversion
while also
maintaining a (relatively) constant absolute number of carbon atoms within the
feed.
Using hydrogenation to decrease the density of a petroleum feed can be
referred to as a
"volume swell" for the feed. This type of volume swell can be economically
valuable
for distillate fuel products, due to the fact that many types of distillate
fuel are sold on a
volume basis. By increasing the volume of distillate fuel corresponding to a
given
number of carbon atoms, the overall yield of distillate fuel from a feedstock
can be
increased. It is noted that the benefit from volume swell can be dependent on
the ability
to increase hydrogenation of the feed without increasing conversion of the
distillate
boiling range feed to naphtha boiling range products.
[0013.1 The amount of aromatic saturation that occurs during hydrotreatment
can be
suppressed for feeds that have elevated contents of sulfur and/or nitrogen.
For example,
cycle oils and other cracked distillate feeds can have sulfur contents of at
least about
3000 wppm or greater, such as about 5000 wppm or greater, or even about 10000
wppm
or greater. A conventional hydrotreating process can be suitable for reducing
the sulfur
content of such a feed to a desired level, such as about 500 wppm or less, or
about 250
wppm or less, or about 100 wppm or less. However, the H2S generated during
hydrotreatment can tend to suppress the aromatic saturation activity of a
hydrotreating
catalyst. This can result in an increased level of aromatics in the
hydrotreated product.
[0014] As an example, during a typical hydrotreatment process, the early
(upstream)
portions of a hydrotreatment process typically cause removal of sulfur from
compounds
that have a faster reaction rate. The removal of this more easily removed
sulfur is not
believed to be strongly impacted by the absence or presence of H2S in the
hydrogen treat
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 5 -
gas. Thus, a treat gas containing H2S can be suitable for the initial catalyst
beds and/or
stages of a distillate hydrotreater when removing sulfur from a feed having an
elevated
sulfur content. However, this easily removed sulfur can still generate H2S. As
a result,
in a conventional distillate hydrotreater that does not have interstage
separation, the
downstream stage(s) / catalyst bed(s) / portions of a catalyst bed are exposed
to the feed
in the presence of a treat gas that can contain at least about 1 vol% H2S, or
at least about
2 vol% H2S, depending on the amount of sulfur initially present in the feed.
Thus, even
though a conventional hydrotreater may start with a contaminant free hydrogen
treat gas,
after removal of a portion of the sulfur in the feed, the downstream portions
of the
distillate hydrotreating system. effectively receive a treat gas having an H2S
content of at
least about 1 vol% or more. This H2S content in the downstream portions of a
conventional distillate hydrotreater can suppress the activity of the
downstream portions
of the hydrotreating catalyst for both desulfurization and aromatic saturation
activity.
[0015] In addition to difficulties in performing aromatic saturation in an
environment
containing substantial amounts of H2S, traditionally increasing the amount of
hydrogenation that occurs when forming a distillate fuel product from a
distillate boiling
range feed has not been desirable. Because hydrogen is a limited resource in a
refinery
setting, the cost of hydrogen consumed by saturation of aromatic rings in a
distillate fuel
was difficult to justify based on the resulting increase in value in the
distillate fuel
products. As a result, the amount of aromatic saturation performed on a
distillate feed
was usually limited to be sufficient for meeting regulatory requirements, such
as
specifications for the maximum allowable amounts of polyaromatic compounds.
[0016] In contrast to conventional processes, a catalyst and/or
hydroprocessing
conditions have been identified that allow for increased or improved aromatic
saturation
during hydrotreatment of a distillate feed that contains elevated levels of
sulfur. Use of a
catalyst and/or process conditions that allow for improved aromatic saturation
can allow
for production of increased volumes of distillate fuels while reducing or
minimizing the
amount of "overcracking" or other excess conversion of a feed. In some
aspects, this can
allow processing conditions to be selected based on a desired level of
heteroatom
removal while also providing the volume swell benefit that com.es from
increased
aromatic saturation.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 6 -
[0017] Volume swelling in a product can be characterized in any convenient
manner,
such as by directly measuring the volume, measuring the specific gravity of a
product, or
by measuring the API gravity of a product. Volume swelling due to processing a
feed as
described herein can generally lead to an increase in volume of about 0.25
vol% to about
2.5 vol% (or possibly more). Although an increase in volume of less than 1
vol% may
appear to be small, due to the size of typical commercial processing units,
and based on
the typical continuous (or near-continuous) operation schedule of such
commercial
processing units, an increase in volume of a few tenths of a percent for a
distillate
product can correspond to a substantial and significant increase in total
product
generated and/or in commercial value generated over time.
[0018] In some aspects, the methods for distillate hydrotreating can
include use of a
catalyst formed from a catalyst precursor composition comprising at least one
metal from
Group 6 of the Periodic Table of the Elements, at least one metal from. Groups
8-10 of
the Periodic Table of the Elements, and a reaction product formed from (i) a
first organic
compound containing at least one amine group and at least 10 carbons or (ii) a
second
organic compound containing at least one carboxylic acid group and at least 10
carbons,
but not both (i) and (ii).
[0019] In other aspects, the process can use a catalyst formed from a
catalyst
precursor composition comprising at least one metal from Group 6 of the
Periodic Table
of the Elements, at least one metal from Groups 8-10 of the Periodic Table of
the
Elements, and a reaction product formed from (1) a first organic compound
containing at
least one amine group, and (ii) a second organic compound separate from said
first
organic compound and containing at least one carboxylic acid group. More
broadly, this
aspect of the present invention relates to use of a catalyst formed from a
catalyst
precursor composition comprising at least one metal from Group 6 of the
Periodic Table
of the Elements, at least one metal from Groups 8-10 of the Periodic Table of
the
Elements, and a condensation reaction product formed from (i) a first organic
compound
containing at least one first functional group, and (ii) a second organic
compound
separate from said first organic compound and containing at least one second
functional
group, wherein said first functional group and said second functional group
are capable
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 7 -
of undergoing a condensation reaction and/or a (decomposition) reaction
causing an
additional tmsatwation to form an associated product.
100201 In still other aspects, the process can use a catalyst formed from a
catalyst
precursor composition comprising at least one metal from Group 6 of the
Periodic Table
of the Elements, at least one metal from Groups 8-10 of the Periodic Table of
the
Elem.ents, and a reaction product comprising an amide group. In this type of
aspect, the
reaction product is formed prior to incorporation into the catalyst precursor.
The
reaction product is an amide-containing reaction product formed from an ex-
situ reaction
of (i) a first organic compound containing at least one amine group, and (ii)
a second
organic compound separate from said first organic compound and containing at
least one
carboxylic acid group.
100211 In yet other aspects, a reaction system including a plurality of
reaction stages
with intermediate separation for removal of gases can be used to produce a
hydrotreated
distillate product with reduced aromatic content by processing a distillate
feed in the
presence of a conventional hydrotreating catalyst and/or a mixed metal
catalyst. One or
more initial stages can be used to reduce the sulfur from. an elevated amount
to an
amount less than about 5000 wppm, such as less than about 3000 wppm. Gases can
be
separated from the effluent of the initial stages to reduce or minimize the
H2S and/or
NH3 content prior to one or more additional hydrotreating stages. Reducing or
minimizing the H2S content can allow for increased aromatic saturation
activity in the
additional hydrotreating stages.
Feedstock
100221 In various aspects, methods are provided for improving the yield of
distillate
products from hydrotreatment of distillate feedstocks and/or heavier
feedstocks that have
elevated sulfur content. Examples of suitable feedstocks can include, but are
not limited
to, atmospheric gas oils, vacuum gas oil feeds, cycle oils, and/or other feeds
(such as
cracked feeds) having a similar type of boiling range, during the production
of distillate
fuels. In addition to using a mixed metal catalyst formed from a suitable
precursor, the
methods can involve stripping of gases to separate out contaminant gases (such
as H2S
and/or NH3) during hydrotreatment of a feed. This can allow for an improved
yield of
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 8 -
distillate products at a desired level of heteroatom removal. The improved
yield of
distillate can be achieved while reducing or minimizing production of lower
boiling
compounds, such as light ends or naphtha boiling range products. In some
aspects, the
improved yield can be based in part on increased volume swell of the
distillate products
due to having a reduced or minimized amount of aromatics in the resulting
distillate
products. Particular examples of suitable feeds can include raw virgin
distillate feeds,
such as straight run light vacuum gas oils, and catalytically cracked feeds,
such as
distillate boiling range cycle oils produced during fluid catalytic cracking
or coker
distillate feeds.
[0023] More generally, a wide range of petroleum and chemical feedstocks
can be
hydroprocessed in accordance with the present invention. Suitable feedstocks
include
whole and reduced petroleum crudes, atmospheric and vacuum residua, propane
deasphalted residua, e.g., brightstock, cycle oils, FCC tower bottoms, gas
oils, including
atmospheric and vacuum gas oils and coker gas oils, light to heavy distillates
including
raw virgin distillates, hydrocrackates, hydrotreated oils, dewaxed oils, slack
waxes,
Fischer-Tropsch waxes, raflinates, and mixtures thereof.
[00241 In this discussion, the distillate boiling range is defined as 350 F
(177 C) to
700 F (371 C). Distillate boiling range products can include products suitable
for use as
kerosene products (including jet fuel products) and diesel products, such as
premium
diesel or winter diesel products. Such distillate boiling range products can
be suitable for
use directly, or optionally after further processing. With regard to other
boiling ranges,
the lubricant boiling range is defined as 700 F (371 C) to 950 F (482 C) and
the
naphtha boiling range is defined as 100 F (37 C) to 350 F (177 C).
[0025] One way of defining a feedstock is based on the boiling range of the
feed.
One option for defining a boiling range is to use an initial boiling point for
a feed and/or
a fmal boiling point for a feed. Another option, which in some instances may
provide a
more representative description of a feed, is to characterize a feed based on
the amount
of the feed that boils at one or more temperatures. The amount of a feed that
boils at a
given temperature can be referred to as a fractional weight boiling point. For
example, a
"T5" boiling point for a feed is defined as the temperature at which 5 wt% of
the feed
will boil off. Similarly, a "T95" boiling point is a temperature at which 95
wt% of the
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 9 -
feed. will boil, while a "T99.5" boiling point is a temperature at which 99.5
wt% of the
feed will boil.
[00261 In some aspects, a distillate boiling range feedstock can correspond
to a feed
where at least a substantial portion of the feed has a boiling point in the
distillate boiling
range. In various aspects, a distillate boiling range feedstock can have a T20
boiling
point, or a TIO boiling point, or a T5 boiling point of at least about 350 F
(177 C), or at
least about 400 F (204 C), or at least about 450 F (232"C). Additionally or
alternately,
a distillate boiling range kedstock can have a T95 boiling point, or a T90
boiling point,
or a 175 boiling point of about 900 F (482 C) or less, or about 850 F (454 C)
or less, or
about 800 F (427 C) or less, or about 750 F (399 C) or less, or about 700 F
(371 C) or
less. In still further additional or alternate aspects, a distillate boiling
range feedstock
can have two or more of the above fractional weight boiling points, or three
or more of
the above fractional weight boiling points, or any other convenient
combination.
Examples of distillate boiling range feedstocks having two or more of the
above
fractional weight boiling points include feeds with a T5 boiling point of at
least about
350 F (177 C) and a T20 boiling point of at least about 450 F (232"C), or a T5
boiling
point of at least about 400 F (20.4 C) and a T95 boiling point of 850 F (454
C) or less,
or another convenient combination. It is noted that all combinations of
explicitly recited
fractional weight boiling points are also explicitly contemplated in
conjunction with each
other to provide distillate boiling range feedstocks having two or more of the
above
fractional weight boiling points, or three or more of the above fractional
weight boiling
points.
[0027] In various aspects, a distillate boiling range feedstock containing
high levels
of sulfur and/or nitrogen can be passed into one or more hydrodesulfurization
reaction
stages to remove sulfur and nitrogen. Suitable distillate boiling range
feedstocks can be
feeds containing at least about 3000 wppm sulfur, or at least about 4000 wppm
sulfur, or
at least about 5000 wppm sulfur, or at least about 7500 wppm sulfur, or at
least about
10,000 wppm sulfur, or at least about 15,000 wppm sulfur, or at least about
20,000
wppm sulfur, such as up to about 50,000 wppm sulfur.
[0028] In some alternative aspects, a feed with a higher boiling range can
be used,
such as a feed with an initial boiling point of at least about 650 F (343 C),
or at least
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 10 -
about 700 F (371 C), or at least about 750 F (399"C). Alternatively, a feed
may be
characterized using a T5 boiling point, such as a feed with a T5 boiling point
of at least
about 650 F (343'C), or at least about 700 F (371 C), or at least about 750 F
(399 C).
Such a feed can have a final boiling point of about 1150 F (621 C), or about
1100 F
(593 C) or less, or about 1050 F (566 C) or less. Alternatively, such a feed
may be
characterized using a T95 boiling point, such as a feed with a T95 boiling
point of about
1150 F (621 C), or about 1100 F (593 C) or less, or about 1050 F (566 C) or
less.
[0029] In some aspects, the aromatics content of the feed prior to
hydroprocessing
can be at least about 30 wt% aromatics, or at least about 40 wt%, or at least
about 50
wt%, or at least about 60 wt%, or at least about 70 wt%, such as up to about
80 we/0 or
more or up to about 90 wt% or more. After hydroprocessing, the aromatics
content of
the distillate boiling range liquid product from the final hydrotreating stage
can be about
60 wt% or less, or about 50 wt% or less, or about 40 wt% or less, or about 30
wt% or
less. Each of the above upper bounds for the aromatics content is explicitly
contemplated herein in combination with each of the above lower bounds for the
aromatics content.
[0030] in some aspects, the content of multi-ring aromatics in the feed
prior to
hydroprocessing can be at least about 2.0 wt% multi-ring aromatics, or at
least about 25
wt%, or at least about 30 wt%, or at least about 35 wt%, or at least about 40
wt%, or at
least about 45 wt%, or at least about 50 wt%, such as up to about 60 wt% or
more. After
hydroprocessing, the multi-ring aromatics content of the distillate boiling
range liquid
product from the final hydrotreating stage can be about 10 wt% or less, or
about 7.5 wt%
or less, or about 5 wt% or less, or about 3 wt% or less. Each of the above
upper bounds
for the multi-ring aromatics content is explicitly contemplated herein in
combination
with each of the above lower bounds for the multi-ring aromatics content,
[0031] In some aspects, at least a portion of the feed can correspond to a
feed derived
from a biocomponent source. In this discussion, a biocomponent feedstock
refers to a
hydrocarbon feedstock derived from a biological raw material component, from
biocomponent sources such as vegetable, animal, fish, and/or algae. Note that,
for the
purposes of this document, vegetable fats/oils refer generally to any plant
based material,
and can include fats/oils derived from a source such as plants of the genus
Jatropha.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
-11-
Generally, the biocomponent sources can include vegetable fats/oils, animal
fats/oils,
fish oils, pyrolysis oils, and algae lipids/oils, as well as components of
such materials,
and in some embodiments can specifically include one or more type of lipid
compounds.
Lipid compounds are typically biological compounds that are insoluble in
water, but
soluble in nonpolar (or fat) solvents. Non-limiting examples of such solvents
include
alcohols, ethers, chloroform, alkyl acetates, benzene, and combinations
thereof.
Process Configuration
10032] in various aspects, methods are provided for improving the yield of
distillate
products from hydrotreatment of distillate feedstocks and/or heavier
feedstocks that have
elevated sulfur content. Examples of suitable feedstocks can include, but are
not limited
to, atmospheric gas oils, vacuum gas oil feeds, cycle oils, and/or other feeds
(such as
cracked feeds) having a similar type of boiling range, during the production
of distillate
fuels. In addition to using a mixed metal catalyst formed from a suitable
precursor, the
methods can involve stripping of gases to separate out contaminant gases (such
as H2S
and/or NH3) during hydrotreatmen.t of a feed. This can allow for an improved
yield of
distillate products at a desired level of heteroatom removal. The improved
yield of
distillate can be achieved while reducing or minimizing production of lower
boiling
compounds, such as light ends or naphtha boiling range products. In some
aspects, the
improved yield can be based in part on increased volum.e swell of the
distillate products
due to having a reduced or minimized amount of aromatics in the resulting
distillate
products.
[0033] In some aspects, a feed can be hydrodesulfurized in a first stage,
which
contains one or more reaction zones, in the presence of hydrogen and a first
hydrotreatin.g catalyst under hydrodesulfurizing conditions. The product
stream can then
be passed to a separation zone wherein a vapor phase stream and a liquid phase
(product)
stream are produced. The liquid phase product stream is a passed to a second
hydrodesulfitrization stage, which contains at least one reaction zone, where
it is further
hydrodesulfitrized in the presence of hydrogen and a second
hydrodesulfurization
catalyst. The liquid product stream from the second hydrodesulfurization stage
is passed
to a second separation zone wherein a vapor product stream is collected for
further
processing or blending. Optionally, the liquid product stream from the second
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 12 -
hydrodesulfurization zone can be passed to a third reaction stage which is
operated in the
presence of a dewaxing catalyst, a hydrogenation catalyst, or another
hydrotreating
catalyst. Optionally, the liquid product stream. from the first
hydrodesulfurization zone
can be passed to an additional intermediate hydrodesulfurization stage between
the first
and second stage. It is within the scope of this invention that at least a
portion of the
vapor product stream from either or both of the first and second reaction
stages can be
recycled to the first reaction stage. Optionally but preferably, the vapor
product stream
from the first reaction stage and/or the second reaction stage is not recycled
to the second
reaction stage. The vapor product stream from a hydrotreating reaction stage
can
typically contain H2S and/or NH3. Recycling such a stream to the second
reaction stage
could reduce or minimize the desired additional aromatic saturation that can
provide
volume swell of the hydrotreated distillate product.
[0034] A. variety of process schemes can be used for hydroprocessing a feed
as
described above. In some aspects, a reaction system can include at least two
hydrotreatment stages. Each hydrotreatment stage can include a hydrotreating
catalyst,
such as a conventional hydrotreating catalyst, a mixed metal catalyst formed
from a
suitable precursor, or a combination thereof. A gas-liquid separation can be
performed
between the hydrotreatment stages to reduce or minimize the content of
contaminant
gases in the second hydrotreatment stage.
[0035] As another example, at least three separate reaction stages can be
used, each
containing one or more reaction zones, with each zone containing at least one
bed of
catalyst. The first two reaction stages can contain hydrodesulfurization
catalysts and the
third reaction stage (and any further downstream stages) can contain a
hydrogenation
catalyst, a dewaxing catalyst, a hydrocracking catalyst, and/or a
hydrotreating catalyst.
Each reaction stage can optionally further include a mixed metal catalyst.
Depending on
the aspect, the mixed metal catalyst can serve as the hydrodesulfitrization
catalyst in a
stage, or the mixed metal catalyst can be present in addition to a
hydrodesulfurization
catalyst (or hydrogenation catalyst or dewaxing catalyst or hydrocracking
catalyst). In
some aspects, when this process scheme is practiced the feedstock introduced
into the
first reaction stage can be a distillate boiling range feedstock. One suitable
type of
feedstock can be a distillate boiling range feedstock from an atmospheric
distillation
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 13 -
tower, such as a raw virgin petroleum distillate. Another example of a
suitable feedstock
can be a cracked feedstock, such as a light cycle oil from a fluid catalytic
cracking
process. Such feedstocks can contain (for example) at least about 3000 wppm
sulfur, or
at least about 4000 wppm sulfur, or at least about 5000 wppm sulfur, or at
least about
10,000 wppm sulfur, or at least about 15,000 wppm sulfur, and optionally can
further
contain a relatively high nitrogen content. in other aspects, such as some
aspects where
the third reaction stage (and/or a later reaction stage) includes a dewaxing
catalyst and/or
a hydrocracking catalyst, a feed having a boiling range suitable for
production of
lubricant base oils can be used in addition to or in place of a distillate
boiling range feed.
[0036] After being hydrodesulfurized in a first hydrodesulfurization stage
the feed
product stream can contain from about 500 to about 20000 wppm sulfur, or about
500 to
about 5000 wppm, or about 500 to about 3000 wppm, or about 750 to about 20000
wpm, or about 750 to about 5000 wppm, or about 750 to about 3000 wppm, or
about
1000 to about 20000 wppm, or about 1000 to about 5000 wppm, or about 1000 to
about
3000 wppm, or about 1500 to about 20000 wppm, or about 1500 to about 5000
wppm, or
about 1500 to about 3000 wppm. This amount of sulfur removal can correspond to
removal of about 40% to about 80% of the sulfur initially present in the
feedstock, and
optionally can correspond to 'removal of about 40% to about 70% of the sulfur,
or about
40% to about 60%. It is preferred that at least one of the reaction zones can
contain a
bed of the mixed metal catalyst. For example, the reactor of the first and/or
second
hydrodesulfurization stage can contain a stacked bed arrangement wherein a
conventional hydrodesulfurization catalyst comprises one or more reaction
zones and a
mixed metal catalyst comprises the other one or more reaction zones. It is
preferred that
if a conventional hydrodesulfurization catalyst and a mixed metal catalyst are
used, the
conventional catalyst can be in the upstream reaction zone or zones. It is
preferred that
the mixed metal catalyst is present in at least the second
hydrodesulfurization stage. In
some aspects, the plurality of reaction stages can correspond to two reaction
stages, with
the second reaction stage preferably containing the mixed metal catalyst.
[0037] The reaction product is passed to a separation zone where a vapor
phase
product stream and a liquid phase product stream is produced. The liquid phase
product
stream (having a reduced sulfur content) can then be introduced into the
second
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 14 -
bydrodesulfurization stage, which also contains one or more reaction zones.
This second
hydrodesulfitrization stage, like the first, can contain, in one or more of
its reaction zones
the mixed metal catalyst. If present, the other catalyst can be a conventional
hydrodesulfurization catalyst. The product stream is passed to a second
separation zone
wherein a vapor phase and liquid phase product streams are produced. The
resulting
liquid phase product stream can then contain less than about 150 wppm sulfur,
or less
than about 100 wppm, or less than about 50 wppm sulfur, or less than about 25
wppm
sulfur, or less than about 10 wppm sulfur. This twice hydrodesulfitrized
product stream
can optionally be passed to a third reaction stage. In some aspects, the twice
hydrodesulfitrized liquid product stream can be reacted in the presence of
hydrogen and a
catalyst capable of further reducing the sulfur and nitrogen levels and
hydrogenating
aromatics. In such aspects, the sulfur level of the final product stream can
be less than
about 10 wppm, preferably less than about 5 wppm, and more preferably less
than about
1 wppm sulfur. In such aspects, the third reaction stage can contain, in at
least one
reaction zone, a hydrogenation catalyst and optionally the mixed metal
catalyst. in other
aspects, the third reaction stage can include a dewaxing catalyst.
[0038] FIGS. 1 and 2 provide a comparison between a conventional
hydrotreating
configuration and a hydrotreating configuration suitable for increasing the
amount of
volume swell during processing of a distillate boiling range feed to form a
distillate
boiling range product. As noted above, examples of suitable feedstocks can
include (but
are not limited to) distillate boiling range feedstocks, gas oil (atmospheric
and/or
vacuum) boiling range feedstocks, or another type of feedstock having a T10
boiling
point of at least about 350 F (177 C) and at least about 3000 wppm of sulfur
prior to
hydrotreatment.
[0039] In the conventional configuration shown in FIG. 1, a feed 105 is
hydrotreated
in multiple stages for removal of sulfur and/or nitrogen. For example, the
feed 105 can
be hydrotreated in two stages (and/or reactors) using hydrotreatment stage
(and/or
reactor) 110 and hydrotreatment stage (and/or reactor) 120. The effluent 115
from
hydrotreatment stage 110 is cascaded into second hydrotreatment stage 120
without
stripping or other intermediate separation. The second hydrotreatment stage
generates a
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 15 -
hydrotreated effluent 122 that can include a distillate boiling range product
with reduced
heteroatom content.
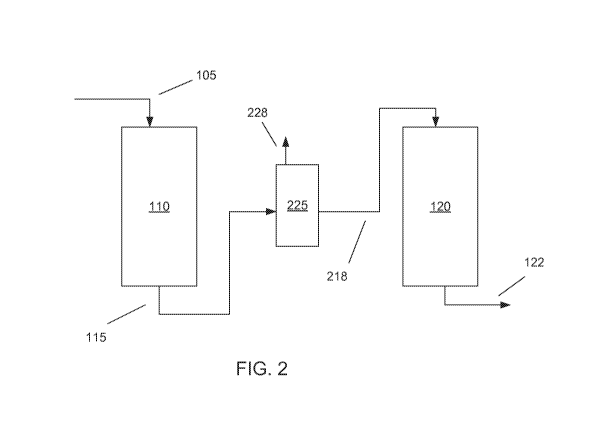
[0040] FIG. 2
shows configuration where the effluent 115 can pass through a
separation stage 225 after hydrotreatment stage 110 and prior to second
hydrotreatment
stage 120. One option is to use a gas-liquid separator or stripper as
separation stage 225.
In this option, contaminant gases 228 formed during hydrotreatment in first
hydrotreatment stage 110, such as H2S and NH3, as well as other light ends,
can be
removed from the effluent prior to second hydrotreatment stage 120.
[0041] The
types of configurations exemplified by FIG. 2 can provide at least two
types of benefits relative to a configuration similar to FIG. 1. For
configurations where
contaminant gases are removed prior to passing the hydrotreated effluent into
the second
hydrotreatment stage, the removal of contaminant gases allows for use of
milder reaction
conditions in the second hydrotreatment stage while achieving a similar level
of
contaminant removal and/or feed conversion. This can be due, for example, to
the
catalysts in the second hydrotreatment stage having a higher effective
catalytic activity
for desulfurization when catalyst suppressants or poisons (such as contaminant
gases) are
removed.
Additionally, for a given level of reaction condition severity for
desulfurization, the amount of aromatic saturation performed can be increased
due to
removal of contaminants that suppress aromatic saturation activity.
[0042] In
various alternative aspects, a mixed metal catalyst formed from a suitable
precursor can be used in one or more reactors of a convenient reaction system,
such as
the reaction system schematically represented in FIG. 1. A mixed metal
catalyst formed
from a suitable precursor can be suitable for hydroprocessing under sour
conditions, such
as for hydrotreating in reactor 110, hydrotreating in reactor 120, or in a
combination
thereof.
[0043] In
this discussion, the severity of hydroprocessing performed on a feed can be
characterized based on an amount of conversion of the feedstock. In various
aspects, the
reaction conditions in the reaction system can be selected to generate a
desired level of
conversion of a feed. Conversion of a feed is defined in terms of conversion
of
molecules that boil above a temperature threshold to molecules below that
threshold.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 16 -
The conversion temperature can be any convenient temperature. Unless otherwise
specified, the conversion temperature in this discussion is a conversion
temperature of
350 F (177 C).
[0044] The amount of conversion can correspond to the total conversion of
molecules within any stage of the reaction system that is used to hydroprocess
the lower
boiling portion of the feed from the vacuum distillation unit. The amount of
conversion
desired for a suitable feedstock can depend on a variety of factors, such as
the boiling
range of the feedstock, the amount of heteroatom contaminants (such as sulfur
and/or
nitrogen) in the feedstock, and/or the nature of the desired lubricant
products. Suitable
amounts of conversion across all hydroprocessing stages can correspond to
about 15
wt% or less conversion of 35097+ (177 C-1-) portions of the feedstock to
portions boiling
below 350 F, such as about 10 wt% or less, or about 5 wt% or less, or about 3
wt% or
less. It is noted that a conversion temperature of 350 F (177 C) is an
indicator of
preserving the distillate boiling range nature of compounds in a feed.
Portions of a feed
that are converted relative to a conversion temperature of 350 F (177 C) can
tend to
correspond to compounds that are more suitable for inclusion in a naphtha
product as
opposed to a distillate product. It is also noted that sulfur and/or nitrogen
in a distillate
boiling range feed can tend to be present primarily in heavi.er and/or higher
boiling
compounds within a feed. During hydrodesulfurization, these sulfur and/or
nitrogen
containing compounds may be altered when sulfur and/or nitrogen is removed,
and this
alteration may lower the boiling point. However, if the boiling point of the
desulfurized
(or denitrogenated) product compound is still greater than the conversion
temperature,
this is not considered "conversion" of the feed relative to the conversion
temperature.
[0045] In this discussion, a stage can correspond to a single reactor or a
plurality of
reactors. Optionally, multiple parallel reactors can be used to perform one or
more of the
processes, or multiple parallel reactors can be used for all processes in a
stage. Each
stage and/or reactor can include one or more catalyst beds containing
hydroprocessing
catalyst. Note that a "bed" of catalyst in the discussion below can refer to a
partial
physical catalyst bed. For example, a catalyst bed within a reactor could be
filled
partially with a hydrocracking catalyst and partially with a dewaxing
catalyst. For
convenience in description, even though the two catalysts may be stacked
together in a
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 17 -
single catalyst bed, the hydrocrackin.g catalyst and dewaxing catalyst can
each be
referred to conceptually as separate catalyst beds.
Process Conditions Hydmtreatment
100461 in various aspects, hydrotreating of a feed can be performed by
exposing the
feed to a hydrotreatin.g catalyst and/or a mixed metal catalyst formed from a
suitable
precursor in the presence of hydrogen. A hydrogen stream is, therefore, fed or
injected
into a vessel or reaction zone or hydroprocessing zone in which the
hydroprocessing
catalyst is located. Hydrogen, which is contained in a hydrogen-containing
"treat gas,"
is provided to the reaction zone. Treat gas, as referred to in this invention,
can be either
pure hydrogen or a hydrogen-containing gas, which is a gas stream containing
hydrogen
in an amount that is sufficient for the intended reaction(s), optionally
including one or
more other gasses (e.g., nitrogen and I.igh.t hydrocarbons such as methane),
and which
will not adversely interfere with or affect either the reactions or the
products. Impurities,
such as H2S and NH3 are undesirable and would typically be removed from. the
treat gas
before it is conducted to the reactor. The treat gas stream introduced into a
reaction stage
will preferably contain at least about 50 vol. % and more preferably at least
about 75 vol.
% hydrogen.
[00471 Hydrotreating conditions can include temperatures of about 200 C to
about
450 C, or about 315 C to about 425 C; pressures of about 250 psig (1.8 MPag)
to about
5000 psig (34.6 MPag) or about 300 psig (2.1 MPag) to about 3000 psig (20.8
MPag);
liquid hourly space velocities (LHSV) of about 0.1 lir-1 to about 10 hi'; and
hydrogen
treat rates of about 200 scf/B (35.6 m3/m3) to about 10,000 scf/B (1781
m3/m3), or about
500 (89 m3/m3) to about 10,000 scf/B (1781 m3/m3).
[00481 The catalysts used for hydrotreatment can include conventional
hydroprocessing catalysts, such as those that comprise at least one Group VIII
non-noble
metal (Columns 8 ¨ 10 of IUPAC periodic table), preferably Fe, Co, and/or Ni,
such as
Co and/or Ni; and at least one Group VIB metal (Column 6 of IUPAC periodic
table),
preferably Mo and/or W. Such hydroprocessing catalysts can optionally include
transition metal sulfides. These metals or mixtures of metals are typically
present as
oxides or sulfides on refractory metal oxide supports. Suitable metal oxide
supports
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 18 -
include low acidic oxides such as silica, alumina, titania, silica-titania,
and titania-
alumina. Suitable aluminas are porous aluminas such as gamma or eta having
average
pore sizes from 50 to 200 A., or 75 to 150 A.; a surface area from 100 to 300
m2/g, or 150
to 250 m2/g; and a pore volume of from 0.25 to 1.0 cm3/g, or 0.35 to 0.8
cm3/g. The
supports are preferably not promoted with a halogen such as fluorine as this
generally
increases the acidity of the support.
100491 The at least one Group VIII non-noble metal, in oxide form., can
typically be
present in an amount ranging from about 1 wt% to about 40 wt%, preferably from
about
4 wt% to about 15 wt%. The at least one Group VIB metal, in oxide form, can
typically
be present in an amount ranging from about 2 wt% to about 70 wt%, preferably
for
supported catalysts from about 6 wt% to about 40 wt% or from about 10 wt% to
about 30
wt%. These weight percents are based on the total weight of the catalyst.
Suitable metal
catalysts include cobalt/molybdenum (1-10% Co as oxide, 10-40% Mo as oxide),
nickel/molybdenum (1-10% Ni as oxide, 10-40% Co as oxide), or nickel/tungsten
(1-10% Ni as oxide, 10-40% Was oxide) on alumina, silica, silica-alumina, or
titania.
100501 Alternatively, the hydrotreating catalyst can be a bulk m.etal
catalyst, or a
combination of stacked beds of supported and bulk metal catalyst. By bulk
metal, it is
meant that the catalysts are unsupported wherein the bulk catalyst particles
comprise 30-
100 wt. % of at least one Group VIII non-noble metal. and at least one Group
V113 metal,
based on the total weight of the bulk catalyst particles, calculated as metal
oxides and
wherein the bulk catalyst particles have a surface area of at least 10 m2/g.
It is
furthermore preferred that the bulk metal hydrotreating catalysts used herein
comprise
about 50 to about 100 wt%, and even more preferably about 70 to about 100 wt%,
of at
least one Group V.1.11 non-noble metal and at least one Group -VIB metal,
based on the
total weight of the particles, calculated as metal oxides. The amount of Group
VIB and
Group Viii non-noble metals can easily be determined -VIB TEm-EDx.
10051.1 Bulk catalyst compositions comprising one Group VIII non-noble
metal and
two Group VIB metals are preferred. It has been found that in this case, the
bulk catalyst
particles are sintering-resistant. Thus the active surface area of the hulk
catalyst particles
is maintained during use. The molar ratio of Group VIB to Group VIII non-noble
metals
ranges generally from 10:1-1:10 and preferably from 3:1-1:3. In the case of a
core-shell
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 19 -
structured particle, these ratios of course apply to the metals contained in
the shell. If
more than one Group VIB metal is contained in the bulk catalyst particles, the
ratio of
the different Group VIB metals is generally not critical. The same holds when
more than
one Group VIII non-noble metal is applied. In the case where molybdenum and
tungsten
are present as Group VIB metals, the molybdenum:tungsten ratio preferably lies
in the
range of 9:1-1:9. Preferably the Group VIII non-noble metal comprises nickel
and/or
cobalt. It is further preferred that the Group VIB metal comprises a
combination of
molybdenum and tungsten. Preferably, combinations of
nickel/molybdenum/tungsten
and cobalt/molybdenum/tungsten and nickelkobalt/molybdenum/tungsten are used.
These types of precipitates appear to be sinter-resistant. Thus, the active
surface area of
the precipitate is maintained during use. The metals are preferably present as
oxidic
compounds of the corresponding metals, or if the catalyst composition has been
sulfided,
sulfidic compounds of the corresponding metals.
[0052] It is also preferred that the bulk metal hydrotreating catalysts
used herein
have a surface area of at least 50 m2/g and more preferably of at least 100
m2/g. It is also
desired that the pore size distribution of the bulk metal hydrotreating
catalysts be
approximately the same as the one of conventional hydrotreating catalysts.
Bulk metal
hydrotreating catalysts have a pore volume of 0.05-5 ml/g, or of 0.1-4 ml/g,
or of 0.1-3
ml/g, or of 0.1-2 mug determined by nitrogen adsorption. Preferably, pores
smaller than
1 nm are not present. The bulk metal hydrotreating catalysts can have a median
diameter
of at least 50 nm, or at least 100 nm. The bulk metal hydrotreating catalysts
can have a
median diameter of not more than 5000 p.m, or not more than 3000 p.m. In an
embodiment, the median particle diameter lies in the range of 0.1-50 p.m and
most
preferably in the range of 0.5-50 p.m.
Process Conditions - Dewaxing
[0053] In some aspects, a dewaxing catalyst may also be included in a
reaction
system for dewaxing a hydrotreated effluent or liquid product. Typically, the
dewaxing
catalyst is located in a bed downstream from any hydrotreating catalyst stages
and/or any
hydrotreating catalyst present in a stage. This can allow the dewaxing to
occur on
molecules that have already been hydrotreated to remove a significant fraction
of organic
sulfur- and nitrogen-containing species. In some configurations, the effluent
from a
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 20 -
reactor containing hydrotreating catalyst, optionally after a gm-liquid
separation, can be
fed into a separate stage or reactor containing the dewaxing catalyst.
[0054] Suitable dewaxing catalysts can include molecular sieves such as
crystalline
aluminosilicates (zeolites). In an embodiment, the molecular sieve can
comprise, consist
essentially of, or be ZSM-5, ZSM-22, ZSM-23, ZSM-35, ZSM-48, zeolite Beta,
ZSM-57, or a combination thereof, for example ZSM-23 and/or ZSM-48, or ZSM-48
and/or zeolite Beta. Optionally but preferably, molecular sieves that are
selective for
dewaxing by isomerization as opposed to cracking can be used, such as ZSM-48,
zeolite
Beta, ZSM-23, or a combination thereof. Additionally or alternately, the
molecular sieve
can comprise, consist essentially of, or be a 10-member ring 1-D molecular
sieve.
Examples include EU-1, ZSM-35 (or ferrierite), ZSM-11, ZSM-57, NU-87, SAPO-I
1,
ZSM-48, ZSM-23, and ZSM-22. Preferred materials are EU-2, EU-11, ZBM-30,
ZSM-48, or ZSM-23. ZSM-48 is most preferred. Note that a zeolite having the
ZSM-23
structure with a silica to alumina ratio of from about 20:1 to about 40:1 can
sometimes
be referred to as SSZ-32. Other molecular sieves that are isostructural with
the above
materials include Theta-1, NU-10, EU-13, KZ-1, and NU-23. Optionally but
preferably,
the dewaxing catalyst can include a binder for the molecular sieve, such as
alumina,
titania, silica, silica-alumina, zirconia, or a combination thereof, for
example alumina
and/or titania or silica and/or zirconia and/or titania.
[0055] Preferably, the dewaxing catalysts used in processes according to
the
invention are catalysts with a low ratio of silica to alumina. For example,
for ZSM-48,
the ratio of silica to alumina in the zeolite can be less than 200:1, or less
than 110:1, or
less than 100:1, or less than 90:1, or less than 80:1. In various embodiments,
the ratio of
silica to alumina can be from 30:1 to 200:1, 60:1 to 110:1, or 70:1 to 100:1.
[0056.1 In various embodiments, the catalysts according to the invention
further
include a metal hydrogenation component. The metal hydrogenation component is
typically a Group VI and/or a Group VIII metal. Preferably, the metal
hydrogenation
component is a Group VIII noble metal. Preferably, the metal hydrogenation
component
is Pt, Pd, or a mixture thereof. in an alternative preferred embodiment, the
metal
hydrogenation component can be a combination of a non-noble Group VIII metal
with a
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 21 -
Group VI metal. Suitable combinations can include Ni, Co, or Fe with Mo or W,
preferably Ni with Mo or W.
100571 The metal hydrogenation component may be added to the catalyst in
any
convenient manner. One technique for adding the metal hydrogenation component
is by
incipient wetness. For example, after combining a zeolite and a binder, the
combined
zeolite and binder can be extruded into catalyst particles. These catalyst
particles can
then be exposed to a solution containing a suitable metal precursor.
Alternatively, metal
can be added to the catalyst by ion exchange, where a metal precursor is added
to a
mixture of zeolite (or zeolite and binder) prior to extrusion.
[0058] The amount of metal in the catalyst can be at least 0.1 wt% based on
catalyst,
or at least 0.15 wt%, or at least 0.2 wt%, or at least 0.25 wt%, or at least
0.3 wt%, or at
least 0.5 wt% based on catalyst. The amount of metal in the catalyst can be 20
wt% or
less based on catalyst, or 10 wt% or less, or 5 wt% or less, or 2.5 wt% or
less, or 1 wt%
or less. For embodiments where the metal is Pt, Pd, another Group VIII noble
metal, or a
combination thereof, the amount of metal can be from 0.1 to 5 wt%, preferably
from 0.1
to 2 wt%, or 0.25 to 1.8 wt%, or 0.4 to 1.5 wt%. For embodiments where the
metal is a
combination of a non-noble Group VIII metal with a Group VI metal, the
combined
amount of metal can be from 0.5 wt% to 20 wt%, or 1 wt% to 15 wt%, or 2.5 wt%
to 10
wt%.
[0059] Dewaxing catalysts can also include a binder. In some embodiments,
the
dewaxing catalysts used in process according to the invention are formulated
using a low
surface area binder, a low surface area binder represents a binder with a
surface area of
100 m2/g or less, or 80 m2/g or less, or 70 m2/g or less.
[0060] A zeolite can be combined with binder in any convenient manner. For
example, a bound catalyst can be produced by starting with powders of both the
zeolite
and binder, combining and mulling the powders with added water to form a
mixture, and
then extruding the mixture to produce a bound catalyst of a desired size.
Extrusion aids
can also be used to modify the extrusion flow properties of the zeolite and
binder
mixture. The amount of framework alumina in the catalyst may range from 0.1 to
3.33
wt%, or 0.1 to 2.7 wt%, or 0.2 to 2 wt%, or 0.3 to 1 wt%.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 22 -
[0061] In yet another embodiment, a binder composed of two or more metal
oxides
can also be used. In such an embodiment, the weight percentage of the low
surface area
binder is preferably greater than the weight percentage of the higher surface
area binder.
Alternatively, if both metal oxides used for forming a mixed metal oxide
binder have a
sufficiently low surface area, the proportions of each metal oxide in the
binder are less
important. When two or more metal oxides are used to form a binder, the two
metal
oxides can be incorporated into the catalyst by any convenient method. For
example,
one binder can be mixed with the zeolite during formation of the zeolite
powder, such as
during spray drying. The spray dried zeolite/binder powder can then be mixed
with the
second metal oxide binder prior to extrusion. in yet another embodiment, the
dewaxing
catalyst is self-bound and does not contain a binder.
[0062] A bound dewaxing catalyst can also be characterized by comparing the
micropore (or zeolite) surface area of the catalyst with the total surface
area of the
catalyst. These surface areas can be calculated based on analysis of nitrogen
porosimetry
data using the BET method for surface area measurement. Previous work has
shown that
the amount of zeolite content versus binder content in catalyst can be
determined from
BET measurements (see, e.g., Johnson, M.F.L., Jour. Catut., (1978) 52, 425).
The
micropore surface area of a catalyst refers to the amount of catalyst surface
area provided
due to the molecular sieve and/or the pores in the catalyst in the BET
measurements.
The total surface area represents the micropore surface plus the external
surface area of
the bound catalyst. In one embodiment, the percentage of micropore surface
area
relative to the total surface area of a bound catalyst can be at least about
35%, for
example at least about 38%, at least about 40%, or at least about 45%.
Additionally or
alternately, the percentage of micropore surface area relative to total
surface area can be
about 65% or less, for example about 60% or less, about 55% or less, or about
50% or
less.
[0063] Additionally or alternately, the dewaxing catalyst can comprise,
consist
essentially of, or be a catalyst that has not been dealuminated. Further
additionally or
alternately, the binder for the catalyst can include a mixture of binder
materials
containing alumina.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 23 -
[0064] Process conditions in a catalytic dewaxing zone can include a
temperature of
about 200 C to about 450 C, preferably about 270 C to about 400 C, a hydrogen
partial
pressure of about 1.8 MPag to about 34.6 MPag (250 psig to 5000 psig),
preferably about
4.8 MPag to about 20.8 MPag, and a hydrogen treat gas rate of about 35.6 m3/m3
(200
SCF/B) to about 1781 m3/m3 (10,000 scf/B), preferably about 178 m3/m3 (1000
SCF/B)
to about 890.6 m3/m3 (5000 SCF/B). In still other embodiments, the conditions
can
include temperatures in the range of about 600 F (343 C) to about 815 F (435
C),
hydrogen partial pressures of from. about 500 psig to about 3000 psig (3.5
MPag-20.9
MPag), and hydrogen treat gas rates of from about 213 m3/m3 to about 1068
m3/m3 (1200
SCF. The LHSV can be from about 0.1 WI to about 10 WI, such as from about 0.5
WI to
about 5 1-11 and/or from about 1111 to about 4 If'.
Process Conditions - Hydrofinishing and/or Aromatic Saturation Processes
0065] in various aspects, a hydrofinishing stage, an aromatic saturation
stage, or a
hydrofinishing and an aromatic saturation stage may also be provided. The
hydrofinishing and/or aromatic saturation stage(s) or reaction zones can occur
after the
last hydrotreating stage, and before and/or after any hydrocracking or
dewaxing stages.
The hydrofinishing and/or aromatic saturation can occur either before or after
fractionation. If hydrofmishing and/or aromatic saturation occurs after
fractionation, the
hydrofinishing can be performed on one or more portions of the fractionated
product,
such as being performed on one or more lubricant base oil portions.
Alternatively, the
entire effluent from the last hydrocracking or dewaxing process can be
hydrofinished
and/or undergo aromatic saturation.
[0066] In some situations, a hydrofinishing process and an aromatic
saturation
process can refer to a single process performed using the same catalyst.
A.ltematively,
one type of catalyst or catalyst system can be provided to perform aromatic
saturation,
while a second catalyst or catalyst system can be used for hydrofinishing. As
still
another alternative, aromatic saturation sometimes refers to a higher
temperature range
of processing than a hydrofinishing process. In such an alternative, a
hydrofinishing
process may be suitable for removing (for example) color bodies from a
product, but
otherwise result in a lower amount of aromatic saturation than an aromatic
saturation
process. Typically a hydrofinishing and/or aromatic saturation process will be
performed
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 24 -
in a separate reactor from dewaxi.ng or hydrocracking processes for practical
reasons,
such as facilitating use of a lower temperature for the hydrofinishing or
aromatic
saturation process.
[0067] Hydrofinishing and/or aromatic saturation catalysts can include
catalysts
containing Group VI metals, Group VIII metals, and mixtures thereof. In an
embodiment, preferred metals include at least one metal sulfide having a
strong
hydrogenation function. In another embodiment, the hydrofinishing catalyst can
include
a Group VIII noble metal, such as Pt, Pd, or a combination thereof. The
mixture of
metals may also be present as bulk metal catalysts wherein the amount of metal
is about
30 wt. % or greater based on catalyst. Suitable metal oxide supports include
low acidic
oxides such as silica, alumina, silica-aluminas or titania, preferably
alumina. The
preferred hydrofinishing catalysts for aromatic saturation can comprise at
least one metal
having relatively strong hydrogenation function on a porous support. The
support
materials may also be modified, such as by halogenation, or in particular
fluorination.
The metal content of the catalyst is often as high as about 20 weight percent
for
non-noble metals. In some optional aspects, hydrotreating catalysts as
described above
can be used as hydrotreating catalysts. In other optional aspects, a preferred
hydrofinishing catalyst can include a crystalline material belonging to the M4
IS class or
family of catalysts. The M41S family of catalysts are mesoporous materials
having high
silica content. Examples include MCM-41, MCM-48 and MCM-50. A preferred
member of this class is MCM-41. If separate catalysts are used for aromatic
saturation
and hydrofinishing, an aromatic saturation catalyst can be selected based on
activity
and/or selectivity for aromatic saturation, while a hydrofinishing catalyst
can be selected
based on activity for improving product specifications, such as product color
and
polynuclear aromatic reduction.
10068] Hydrofinishing conditions can include temperatures from about 125 C
to
about 425 C, preferably about 180 C to about 280 C, total pressures from about
500
psig (3.4 MPa) to about 3000 psig (20.7 MPa), preferably about 1500 psig (10.3
MPa) to
about 2500 psig (17.2 MPa), and liquid hourly space velocity from about 0.1
ht.1 to
about 5 hr 1 LHSV, preferably about 0.5 hr' to about 1.5 hr.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 25 -
[0069] In aspects where aromatic saturation is contemplated as a distinct
process
from hydrofmishing, aromatic saturation conditions can include temperatures
from about
175 C to about 425 C, or about 200 C to about 425 C, preferably about 225 C to
about
325 C, or about 225 C to about 280 C, total pressures from about 500 psig (3.4
MPa) to
about 3000 psig (20.7 MPa), preferably about 1500 psig (10.3 MPa) to about
2500 psig
(17.2 MPa), and liquid hourly space velocity from about 0.1 hr I to about 5 hr
l LHSV,
preferably about 0.5 hfl to about 1.5 hfl.
Alternative Configurations - Hydrocracking Conditions
[0070] In some alternative configurations, the plurality of hydrotreating
stages
described above, including separation between the stages, can be used to
prepare a feed
for subsequent hydrocracking for further conversion of the feed. Hydrocracking
catalysts typically contain sul.fided base metals on acidic supports, such as
amorphous
silica alumina, cracking zeolites or other cracking molecular sieves such as
USY, or
acidified alumina. In some preferred aspects, a hydrocracking catalyst can
include at
least one molecular sieve, such as a zeolite. Often these acidic supports are
mixed or
bound with other metal oxides such as alumina, titania or silica. Non-limiting
examples
of supported catalytic metals for hydrocracking catalysts include nickel,
nickel-cobalt-
molybdenum, cobalt-molybdenum, nickel-tungsten, nickel-molybdenum, and/or
nickel-molybdenum-tungsten. Additionally or alternately, hydrocracking
catalysts with
noble metals can also be used. Non-limiting examples of noble metal catalysts
include
those based on platinum, and/or palladium. Support materials which may be used
for both
the noble and non-noble metal catalysts can comprise a refractory oxide
material such as
alumina, silica, alumina-silica, ki.eselguhr, diatomaceous earth, magnesia,
zirconia, or
combinations thereof, with alumina, silica, alumina-silica being the most
common (and
preferred, in one embodiment).
[0071] In some aspects, a hydrocracking catalyst can include a large pore
molecular
sieve that is selective for cracking of branched hydrocarbons and/or cyclic
hydrocarbons.
Zeolite Y, such as ultrastable zeolite Y (USY) is an example of a zeolite
molecular sieve
that is selective for cracking of branched hydrocarbons and cyclic
hydrocarbons.
Depending on the aspect, the silica to alumina ratio in a USY zeolite can be
at least about
10, such as at least about 15, or at least about 25, or at least about 50, or
at least about 100.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 26 -
Depending on the aspect, the unit cell size for a USY zeolite can be about
24.50 Angstroms
or less, such as about 24.45 Angstroms or less, or about 24.40 Angstroms or
less, or about
24.35 Angstroms or less, such as about 24.30 Angstroms.
[0072] In
various embodiments, the conditions selected for hydrocracking can
depend on the desired level of conversion, the level of contaminants in the
input feed to
the hydrocracking stage, and potentially other factors. A hydrocracking
process
performed under sour conditions, such as conditions where the sulfur content
of the input
feed to the hydrocracking stage is at least 500 wppm, can be carried out at
temperatures
of about 550 F (288 C) to about 840 F (449 C), hydrogen partial pressures of
from
about 250 psig to about 5000 psig (1.8 MPag to 34.6 MPag), liquid hourly space
velocities of from 0.05 h.-1 to 10 hA, and hydrogen treat gas rates of from
35.6 m.3/m3 to
1781 m3/m3 (200 SCF/B to 10,000 SCF/B). In other embodiments, the conditions
can
include temperatures in the range of about 600 F (343 C) to about 815 F (435
C),
hydrogen partial pressures of from about 500 psig to about 3000 psig (3.5 MPag-
20.9
MPag), liquid hourly space velocities of from about 0.2 to
about 2 111 and hydrogen
treat gas rates of from about 213 m3/m3 to about 1068 m3/m.3 (1200 SC EB to
6000
SCF/B).
[0073] A
hydrocracking process performed under non-sour conditions can be
performed under conditions similar to those used for sour conditions, or the
conditions
can be different. Alternatively, a non-sour hydrocracking stage can have less
severe
conditions than a similar hydrocracking stage operating under sour conditions.
Suitable
hydrocracking conditions can include temperatures of about 550 F (288 C) to
about
840 F (449 C), hydrogen partial pressures of from. about 250 psig to about
5000 psig
(1.8 MPag to 34.6 MPag), liquid hourly space velocities of from 0.05 11-1 to
10 1-11, and
hydrogen treat gas rates of from 35.6 m3,/m3 to 1781 m3/m3 (200 SCF/B to
10,000
SCEB). In other embodiments, the conditions can include temperatures in th.e
range of
about 600 F (343 C) to about 815 F (435 C), hydrogen partial pressures of from
about
500 psig to about 3000 psig (3.5 MPag-20.9 MPag), liquid hourly space
velocities of
from about 0.2 11-1 to about 2 If' and hydrogen treat gas rates of from about
213 m3/m3 to
about 1068 m3/m3 (1200 SCF/B to 6000 SCF/B),
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 27 -
[0074] After such a hydrocracking process, a suitable feed can undergo
further
additional processing, such as dewaxing and/or hydrofinishing and/or aromatic
saturation. This type of process can be suitable for formation of both
distillate fuel and
lubricant base oil products with increased yield.
Multimetallic Catalyst and Forming Multimetalli.c Catalyst from a Precursor
[0075] As used herein, the term "bulk", when describing a mixed metal oxide
catalyst composition, indicates that the catalyst composition is self-
supporting in that it
does not require a carrier or support. It is well understood that bulk
catalysts may have
some minor amount of carrier or support material in their compositions (e.g.,
about 20 wt
% or less, about 15 wt % or less, about 10 wt % or less, about 5 wt % or less,
or
substantially no carrier or support, based on the total weight of the catalyst
composition);
for instance, bulk hydroprocessing catalysts may contain a minor amount of a
binder,
e.g., to improve the physical and/or thermal properties of the catalyst. In
contrast,
heterogeneous or supported catalyst systems typically comprise a carrier or
support onto
which one or more catalytically active materials are deposited, often using an
impregnation or coating technique. Nevertheless, heterogeneous catalyst
systems
without a carrier or support (or with a minor amount of carrier or support)
are generally
referred to as bulk catalysts and are frequently formed by co-precipitation or
solid-solid
reactions in slurries.
[0076] In some aspects, the m.ethods described herein can include use of a
catalyst
formed from a catalyst precursor composition comprising at least one metal
from Group
6 of the Periodic Table of the Elements, at least one metal from Groups 8-10
of the
Periodic Table of the Elements, and a reaction product formed from (i) a first
organic
compound containing at least one amine group and at least 10 carbons or (ii) a
second
organic compound containing at least one carboxylic acid group and at least 10
carbons,
but not both (i) and (ii), wherein the reaction product contains additional
unsaturated
carbon atoms, relative to (i) the first organic compound or (ii) the second
organic
compound, wherein the metals of the catalyst precursor composition are
arranged in a
crystal lattice, and wherein the reaction product is not located within the
crystal lattice.
This catalyst precursor composition can be a bulk metal catalyst precursor
composition
or a supported metal catalyst precursor composition. When it is a bulk mixed
metal
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 28 -
catalyst precursor composition, the reaction product can be obtained by
heating the
composition (though specifically the amine-containing compound or the
carboxylic acid-
containing compound) to a temperature from about 195 C to about 260 C for a
time
sufficient for the first or second organic compounds to react to form
additional in situ
unsaturated carbon atoms and/or become more oxidized than the first or second
organic
compounds, but not for so long that more than 50% by weight of the first or
second
organic compound is volatilized, thereby forming a catalyst precursor
composition that
contains in situ formed unsaturated carbon atoms and/or that is further
oxidized.
[0077] Other aspects can relate to using a catalyst formed from a catalyst
precursor
composition containing in situ formed unsaturated carbon atoms. The catalyst
can be
formed from the precursor by a process comprising: (a) treating a catalyst
precursor
composition comprising at least one metal from Group 6 of the Periodic Table
of the
Elements, at least one metal from. Groups 8-10 of the Periodic Table of the
Elements,
with a first organic compound containing at least one amine group and at least
10 carbon
atoms or a second organic compound containing at least one carboxylic acid
group and at
least 10 carbon atoms, to form an organically treated precursor catalyst
composition; and
(b) heating said organically treated precursor catalyst composition at a
temperature from
about 195 C to about 260 C for a time sufficient for the first or second
organic
compounds to react to form additional in situ unsaturated carbon atoms and/or
become
more oxidized, but not for so long that more than 50% by weight of the first
or second
organic compound is volatilized, thereby forming a catalyst precursor
composition that
contains in situ formed unsaturated carbon atoms and/or that is further
oxidized. This
process can be used to make a bulk metal catalyst precursor composition or a
supported
metal catalyst precursor composition. When used to m.ake a bulk mixed metal
catalyst
precursor composition, the catalyst precursor composition containing in situ
formed
unsaturated carbon atoms can, in one embodiment, consist essentially of the
reaction
product, an oxide form of the at least one metal from Group 6, an oxide form
of the at
least one metal from Groups 8-10, and optionally about 20 wt % or less of a
binder.
[0078] As an example, when the catalyst precursor is a bulk mixed metal
catalyst
precursor composition, the reaction product can be obtained by heating the
composition
(though specifically the first or second organic compounds, or the amine-
containing or
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 29 -
carboxylic acid-containing compound) to a temperature from about 195 C to
about
260 C for a time sufficient to effectuate a dehydrogenation, andlor an at
least partial
decomposition, of the first or second organic compound to form an additional
=saturation and/or additional oxidation in the reaction product in situ.
Accordingly, a
bulk mixed metal hydroprocessing catalyst composition can be produced from
this bulk
mixed metal catalyst precursor composition by sulfiding it under sufficient
sulfiding
conditions, which sulfiding should begin in the presence of the in situ
additionally
unsaturated reaction product (which may result from at least partial
decomposition, e.g.,
via oxidative dehydrogenation in the presence of oxygen and/or via non-
oxidative
dehydrogenation in the absence of an appropriate concentration of oxygen, of
typically-
unfimctionalized organic portions of the first or second organic compounds,
e.g., of an
aliphatic portion of an organic compound and/or through
conjugationlaromatization of
unsaturations expanding upon an unsaturated portion of an organic compound).
[00791 In still other aspects, a feed can be processed in a reaction system
that
includes a catalyst formed from a catalyst precursor composition comprising at
least one
metal from Group 6 of the Periodic Table of the Elements, at least one metal
from
Groups 8-10 of the Periodic Table of the Elements, and a reaction product
formed from
(i) a first organic compound containing at least one amine group, and (ii) a
second
organic compound separate from said first organic compound and containing at
least one
carboxylic acid group. When this reaction product is an amide, the presence of
the
reaction product in any intermediate or final composition can be determined by
methods
well known in the art, e.g., by infrared spectroscopy (RIR) techniques. When
this
reaction product contains additional unsaturation(s) not present in the first
and second
organic compounds, e.g., from at least partial decomposition/dehydrogenation
at
conditions including elevated temperatures, the presence of the additional
unsaturation(s)
in any intermediate or final composition can be determined by methods well
known in
the art, e.g., by FTIR and/or nuclear magnetic resonance (13C NMR) techniques.
This
catalyst precursor composition can be a bulk metal catalyst precursor
composition or a
heterogeneous (supported) metal catalyst precursor composition.
10080] More broadly, this type of aspect relates to use of a catalyst
formed from a
catalyst precursor composition comprising at least one metal from Group 6 of
the
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 30 -
Periodic Table of the Elements, at least one metal from Groups 8-10 of the
Periodic
Table of the Elements, and a condensation reaction product formed from (i) a
first
organic compound containing at least one first functional group, and (ii) a
second
organic compound separate from said first organic compound and containing at
least one
second functional group, wherein said first functional group and said second
functional
group are capable of undergoing a condensation reaction and/or a
(decomposition)
reaction causing an additional unsaturation to form an associated product.
Though the
description above and herein often refers specifically to the condensation
reaction
product being an amide, it should be understood that any in situ condensation
reaction
product formed can be substituted for the amide described herein. For example,
if the
first functional group is a hydroxyl group and the second functional group is
a carboxylic
acid or an acid chloride or an organic ester capable of undergoing
transesterification with
the hydroxyl group, then the in situ condensation reaction product formed
would be an
ester.
[0081] As an example, when the catalyst precursor is a bulk mixed metal
catalyst
precursor composition, the reaction product can be obtained by heating the
composition
(such as the condensation reactants, or the amine-containing compound and/or
the
carboxylic acid-containing compound) to a temperature from about 195 C to
about
260 C for a time sufficient for the first and second organic compounds to form
a
condensation product, such as an amide, and/or an additional (decomposition)
unsaturation in situ. Accordingly, a bulk mixed metal hydroprocessing catalyst
composition can be produced from this bulk mixed metal catalyst precursor
composition
by sulfiding it under sufficient sulfiding conditions, which sulfiding should
begin in the
presence of the in situ product, e.g., the amide (i.e., when present, the
condensation
product moiety, or amide, can be substantially present and/or can preferably
not be
significantly decomposed by the beginning of the sulfiding step), and/or
containing
additional unsatttrations (which may result from at least partial
decomposition, e.g., via
oxidative dehydrogenation in the presence of oxygen and/or via non-oxidative
dehydrogenation in the absence of an appropriate concentration of oxygen, of
typically-
unfunctionalized organic portions of the first and/or second organic
compounds, e.g., of
an aliphatic portion of an organic compound and/or through
conjugationlaromatization of
=saturations expanding upon an unsaturated portion of an organic compound or
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 31 -
stemming from an interaction of the first and second organic compounds at a
site other
than their respective functional groups).
100821 In yet other aspects, a feed can be processed using a catalyst
formed from a
catalyst precursor composition containing an ex-situ formed reaction product.
The
catalyst can be formed from the precursor by a process comprising: (a)
treating a catalyst
precursor composition comprising at least one metal from Group 6 of the
Periodic Table
of the Elements, at least one metal from Groups 8-10 of the Periodic Table of
the
Elements, with an amide-containing reaction product formed from a first
organic
compound containing at least one amine group and at least 10 carbon atoms or a
second
organic compound containing at least one carboxylic acid group and at least 10
carbon
atoms, to form an organically treated precursor catalyst composition; and (b)
heating said
organically treated precursor catalyst composition at a temperature from about
195 C to
about 260 C for a time sufficient for the amide-containing reaction product to
form
additional in situ unsaturated carbon atoms and/or become more oxidized, but
not for so
long that more than 50% by weight of the first or second organic compound is
volatilized, thereby forming a catalyst precursor composition that contains in
situ formed
unsaturated carbon atoms and/or that is further oxidized. This process can be
used to
make a bulk metal catalyst precursor composition or a supported metal catalyst
precursor
composition. When used to make a bulk mixed metal catalyst precursor
composition, the
catalyst precursor composition can, in one embodiment, consist essentially of
the
reaction product containing further unsaturated carbon atoms and/or further
oxidation, an
oxide form of the at least one metal from Group 6, an oxide form of the at
least one
metal from Groups 8-10, and optionally about 20 wt % or less of a binder.
[00831 When the catalyst precursor is a bulk mixed metal catalyst precursor
composition, the thermal treatment of the amide-impregnated metal oxide
component is
carried out by heating the impregnated composition to a temperature and for a
time
which does not result in gross decomposition of the amide, although additional
unsaturation may arise from partial in situ decomposition; the temperature is
typically
from about 195 C to about 250 C (or optionally about 195 C to about 260 C),
but higher
temperatures, e.g. in the range of 250 to 280 C, can be used in order to
abbreviate the
duration of the heating although due care is required to avoid the gross
decomposition of
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 32 -
the pre-formed amide, as discussed further below. The
bulk mixed metal
hydroprocessing catalyst can be produced from this precursor by sulfiding it
with the
sulfiding taking place with the amide present on the metal oxide component
(i.e., when
the thermally treated amide, is substantially present and/or preferably not
significantly
decomposed by the beginning of the sulfiding step). Additional unsaturation
may be
present in the organic component of the catalyst precursor resulting from a
variety of
mechanisms including partial decomposition, (e.g., via oxidative
dehydrogenation in the
presence of oxygen and/or via non-oxidative dehydrogenation in the absence of
an
appropriate concentration of oxygen), of typically-unfunctionalized organic
portions of
the amide and/or through conjugation/aromatization of unsaturations expanding
upon an
unsaturated portion the amide. The treated organic component may also contain
additional oxygen in addition to the unsaturation when the treatment is
carried out in an
oxidizing atmosphere.
[00841
Catalyst precursor compositions and hydroprocessing catalyst compositions
useful in various aspects of the present invention can advantageously comprise
(or can
have metal components that consist essentially of) at least one metal from
Group 6 of the
Periodic Table of Elements and at least one metal from Groups 8-10 of the
Periodic
Table of Elements, and optionally at least one metal from Group 5 of the
Periodic Table
of Elements. Generally, these metals are present in their substantially fully
oxidized
form, which can typically take the form of simple metal oxides, but which may
be
present in a variety of other oxide forms, e.g., such as hydroxides,
oxyhydroxides,
oxycarbonates, carbonates, oxynitrates, oxysulfates, or the like, or some
combination
thereof. In one preferred embodiment, the Group 6 metal(s) can be Mo and/or W,
and
the Group 8-10 metal(s) can be Co and/or Ni. Generally, the atomic ratio of
the Group 6
metal(s) to the metal(s) of Groups 8-10 can be from about 2:1 to about 1:3,
for example
from about 5:4 to about 1:2, from about 5:4 to about 2:3, from about 5:4 to
about 3:4,
from about 10:9 to about 1:2, from about 10:9 to about 2:3, from about 10:9 to
about 3:4,
from about 20:19 to about 2:3, or from about 20:19 to about 3:4. When the
composition
further comprises at least one metal from Group 5, that at least one metal can
be V
and/or Nb. When present, the amount of Group 5 metal(s) can be such that the
atomic
ratio of the Group 6 metal(s) to the Group 5 metal(s) can be from about 99:1
to about
1:1, for example from about 99:1 to about 5:1, from about 99:1 to about 10:1,
or from
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 33 -
about 99:1 to about 20:1. Additionally or alternately, when Group 5 metal(s)
is(are)
present, the atomic ratio of the sum of the Group 5 metal(s) plus the Group
(6) metal(s)
compared to the metal(s) of Groups 8-10 can be from about 2:1 to about 1:3,
for example
from about 5:4 to about 1:2, from about 5:4 to about 2:3, from about 5:4 to
about 3:4,
from about 10:9 to about 1:2, from about 10:9 to about 2:3, from about 10:9 to
about 3:4,
from about 20:19 to about 2:3, or from about 20:19 to about 3:4.
[00851 The
metals in the catalyst precursor compositions and in the hydroprocessing
catalyst compositions according to the invention can be present in any
suitable form prior
to suffiding, but can often be provided as metal oxides. When provided as bulk
mixed
metal oxides, such bulk oxide components of the catalyst precursor
compositions and of
the hydroprocessing catalyst compositions according to the invention_ can be
prepared by
any suitable method known in the art, but can generally be produced by forming
a slurry,
typically an aqueous slurry, comprising (1) (a) an oxyanion of the Group 6
metal(s), such
as a tungstate and/or a molybdate, or (b) an insoluble (oxide, acid) foul' of
the Group 6
metal(s), such as tungstic acid and/or molybdenum trioxide, (2) a salt of the
Group 8-10
metal(s), such as nickel carbonate, and optionally, when present, (3) (a) a
salt or
oxyanion of a Group 5 metal, such as a vanad.ate and/or a niobate, or (b)
insoluble
(oxide, acid) form of a Group 5 metal, such as niobic acid andior diniobium.
pentoxide.
The slurry can be heated to a suitable temperature, such as from about 60 C to
about
150 C, at a suitable pressure, e.g., at atmospheric or autogenous pressure,
for an
appropriate time, e.g., about 4 hours to about 24 hours.
[00861 Non.-
limiting examples of suitable mixed metal oxide compositions can
include, but are not limited to, nickel-tungsten oxides, cobalt-tungsten
oxides, nickel-
molybdenum oxides, cobalt-molybdenum oxides, nickel-molybdenum-tungsten
oxides,
cobalt-molybdenum-tungsten oxides, cobalt-nickel-
tungsten oxides,
cobalt-nickel-molybdenum oxides, cobalt-nickel-tungsten-molybdenum oxides,
nickel-tungsten-niobium oxides, nickel-tungsten-
vanadium oxides,
cobalt-tungsten-vanadium oxides, cobalt-tungsten-niobium oxides, nickel-
molybdenum-
niobium oxides, nickel-molybdenum-vanadium oxides, nickel-molybdenum-tungsten-
niobium oxides, nickel-molybdenum-tungsten-vanadium oxides, and the like, and
combinations thereof.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 34 -
[0087] Suitable mixed metal oxide compositions can advantageously exhibit a
specific surface area (as measured via the nitrogen BET method using a
Quantachrome
Autosorb.IM. apparatus) of at least about 20 m2/g, for example at least about
30 m2/g, at
least about 40 m21g, at least about 50 m2/g, at least about 60 m2/g, at least
about 70 m2/g,
or at least about 80 m2/g. Additionally or alternately, the mixed metal oxide
compositions can exhibit a specific surface area of not more than about 500
m2/g, for
example not more than about 400 m2/g, not more than about 300 m2/g, not more
than
about 250 m2/g, not more than about 200 m2/g, not more than about 175 m2/g,
not more
than about 150 m2/g, not more than about 125 m2/g, or not more than about 100
m2/g.
[0088] In some aspects, after separating and drying the mixed metal oxide
(slurry)
composition, it can be treated, generally by impregnation, with (i) an
effective amount of
a first organic compound containing at least one amine group or (ii) an
effective amount
of a second organic compound separate from the first organic compound and
containing
at least one carboxylic acid group, but not both (i) and (ii).
100891 In other aspects, after separating and drying the mixed metal oxide
(slurry)
composition, it can be treated, generally by impregnation, with (i) an
effective amount of
a first organic compound containing at least one amine group, and (ii) an
effective
amount of a second organic compound separate from the first organic compound
and
containing at least one carboxylic acid group.
[0090] In still other aspects, after separating and drying the mixed metal
oxide
(slurry) composition, it can be treated, generally by impregnation, with the
pre-formed
amide derived from (i) an effective amount of a first organic compound
containing at
least one amine group, and (ii) an effective amount of a second organic
compound
separate from the first organic compound and containing at least one
carboxylic acid
group. The amide is formed by a condensation reaction between the amine
reactant and
the carboxylic acid reactant; this reaction, carried out ex situ, is usually
accomplished at
mildly elevated temperatures.
10091] in aspects where either a first or second organic compound is used,
the first
organic compound can comprise at least 10 carbon atoms, for example can
comprise
from 10 to 20 carbon atoms or can comprise a primary monoamine having from 10
to 30
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 35 -
carbon atoms. Additionally or alternately, the second organic compound can
comprise at
least 10 carbon atoms, for example can comprise from 10 to 20 carbon atoms or
can
comprise only one carboxylic acid group and can have from 10 to 30 carbon
atoms.
[00921 In other aspects where both a first and second organic compound are
used
(including aspects where a first and second organic compound are reacted ex
situ to form
an amide), the first organic compound can comprise at least 10 carbon atoms,
for
example can comprise from 10 to 20 carbon atoms or can comprise a primary
monoamine having from 10 to 30 carbon atoms. Additionally or alternately, the
second
organic compound can comprise at least 10 carbon atoms, for example can
comprise
from 10 to 20 carbon atoms or can comprise only one carboxylic acid group and
can
have from 10 to 30 carbon atoms. Further additionally or alternately, the
total number of
carbon atoms comprised among both the first and second organic compounds can
be at
least 15 carbon atoms, for example at least 20 carbon atoms, at least 25
carbon atoms, at
least 30 carbon atoms, or at least 35 carbon atoms. Although in such
embodiments there
may be no practical upper limit on total carbon atoms from both organic
compounds, in
some embodiments, the total number of carbon atoms comprised among both the
first
and second organic compounds can be 100 carbon atoms or less, for example 80
carbon
atoms or less, 70 carbon atoms or less, 60 carbon atoms or less, or 50 carbon
atoms or
less.
[0093] Representative examples of organic compounds containing amine groups
can
include, but are not limited to, primary and/or secondary, linear, branched,
and/or cyclic
amines, such as triacontanylamine, octacosanylamine, hexacosanylamine,
tetracosanylamine, docosanylamine, eru.cylami.ne, eicosanylam.ine,
octadecylamine,
oleylamine, linoleylamine, hexadecylamine, sapienylamine, palmitoleylamine,
tetradecylamine, myristoleylamine, dodecylamine, decylamine, nonylamine,
cyclooctyl amine, octylamine, cyc I oheptyl ami.ne, heptylamine, cyc lohex y I
amine,
n-hexylamine, isopentylamine, n-pentylamine, t-butylamine, n-butylamine,
isopropylamine, n-propylam.ine, adamantanamine, adamantanemethylamine,
pyrrolidine,
piperidine, piperazine, imidazole, pyrazole, pyrrole, pyrrolidine, pyrroline,
indazole,
indole, carbazol.e, norbomylamine, aniline, pyridylamine, benzylamin.e,
aminotoluene,
alanine, arginine, aspartic acid, glutamic acid, glutamine, glycine,
histidine, isoleucine,
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 36 -
leuci.ne, lysine, phenylalanine, serine, threonin.e, valine, 1-amino-2-
propanol, 2-amino-
1-propanol, diaminoeicosane, diaminooctadecane,
diaminohexadecane,
diaminotetradecane, di.aminododecane, di.aminodecane, 1,2-di aminocyclohex an
e,
1,3-diaminocyclohexane, 1,4-diarninocyclohexane, ethylenediamine,
ethanolamine,
p-phenylenediamine, o-phenylenediamine, m-phenylenediamine, 1,2-
propylenediamine,
1,3-propylenediamine, 1,4-diaminobutane, 1,3diamino-2-propanol, and the like,
and
combinations thereof. In an embodiment, the molar ratio of the Group 6
metal(s) in the
composition to the first organic compound during treatment can be from about
1:1 to
about 20:1.
[0094]
Additionally or alternately, in some aspects the amine portion of the first
organic compound can be a part of a larger functional group in that compound,
so long as
the amine portion (notably the amine nitrogen and the constituents attached
thereto)
retains its operability as a Lewis base. For instance, the first organic
compound can
comprise a urea, which functional group comprises an amine portion attached to
the
carbonyl portion of an amide group. In such an instance, the urea can be
considered
functionally as an "amine-containing" functional group for the purposes of the
present
invention herein, except in situations where such inclusion is specifically
contradicted.
Aside from ureas, other examples of such amine-containing functional groups
that may
be suitable for satisfying the at least one amine group in the first organic
compound can
generally include, but are not limited to, hydrazides, sulfonamides, and the
like, and
combinations thereof.
100951 The
amine functional group from the first organic compound can include
primary or secondary amines, as mentioned above, but generally does not
include
quaternary amines, and in some instances does not include tertiary amines
either.
Furthermore, the first organic compound can optionally contain other
functional groups
besides amines. For instance, the first organic compound can comprise an
aminoacid,
which possesses an amine functional group and a carboxylic acid functional
group
simultaneously. Aside from carboxylic acids, other examples of such secondary
functional groups in amine-containing organic compounds can generally include,
but are
not limited to, hydroxyls, al.dehydes, anhydrides, ethers, esters, imin.es,
imi.des, ketones,
thiols (mercaptans), thioesters, and the like, and combinations thereof.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 37 -
[0096] Additionally or alternately, in other aspects involving formation of
a
condensation product (including aspects involving ex situ formation of an
amide), the
mule functional group from. the first organic compound can include primary or
secondary amines, as mentioned above, but generally does not include tertiary
or
quaternary amines, as tertiary and quaternary amines tend not to be able to
form amides.
Furthermore, the first organic compound can contain other functional groups
besides
amines, whether or not they are capable of participating in forming an amide
or other
condensation reaction product with one or more of the functional groups from
second
organic compound. For instance, the first organic compound can comprise an
aminoacid, which possesses an amine functional group and a carboxylic acid
functional
group simultaneously. In such an instance, the aminoacid would qualify as only
one of
the organic compounds, and not both.; thus, in such an instance, either an
additional
amine-containing (first) organic compound would need to be present (in the
circumstance where the aminoacid would be considered the second organic
compound)
or an additional carboxylic acid-containing (second) organic compound would
need to be
present (in the circumstance where the aminoacid would be considered the first
organic
compound). Aside from carboxylic acids, other examples of such secondary
functional
groups in amine-containing organic compounds can generally include, but are
not limited
to, hydroxyls, aldehydes, anhydrides, ethers, esters, imines, imides, ketones,
thiols
(mercaptans), thioesters, and the like, and combinations thereof.
[0097] Additionally or alternately, the amine portion of the first organic
compound
can be a part of a larger functional group in that compound, so long as the
amine portion
(notably the amine nitrogen and the constituents attached thereto) retains the
capability
of participating in forming an amide or other condensation reaction product
with one or
more of the functional groups from second organic compound. For instance, the
first
organic compound can comprise a urea, which functional group comprises an
amine
portion attached to the carbonyl portion of an amide group. In such an
instance, provided
the amine portion of the urea functional group of the first organic compound
would still
be able to undergo a condensation reaction with the carboxylic acid functional
group of
the second organic compound, then the urea can be considered functionally as
an
"amine-containing" functional group for the purposes of the present invention
herein,
except in situations where such inclusion is specifically contradicted. Aside
from ureas,
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 38 -
other examples of such amine-containing functional groups that may be suitable
for
satisfying the at least one amine group in the first organic compound can
generally
include, but are not limited to, hydrazides, sulfonamides, and the like, and
combinations
thereof.
[0098.1
Representative examples of organic compounds containing carboxylic acids
can include, but are not limited to, primary and/or secondary, linear,
branched, and/or
cyclic amines, such as triacontanoic acid, octacosanoic acid, hexacosanoic
acid,
tetracosanoic acid, docosanoic acid, erucic acid, docosahexanoic acid,
eicosanoic acid,
eicosapen.tanoic acid, arachidoni.c acid, octadecanoi.c acid, oleic acid,
elaidic acid,
stearidonic acid, linoleic acid, alpha-linolenic acid, hexadecanoic acid,
sapienic acid,
palmitoleic acid, tetradecanoic acid, myristolei.c acid, dodecanoic acid,
d.ecanoic acid,
nonanoic acid, cyclooctanoic acid, octanoic acid, cycloheptanoic acid,
heptanoic acid,
cyclohexanoic acid, hexanoic acid, adamantanecarboxylic acid, norbornaneacetic
acid,
benzoic acid, salicylic acid, acetylsalicylic acid, citric acid, maleic acid,
malonic acid,
glutaric acid, lactic acid, oxalic acid, tartaric acid, cinnamic acid,
vanillic acid, succinic
acid, adipic acid, phthalic acid, i sopht ha I ic
acid, tereph thalic acid,
ethylenediaminetetracarboxylic acids (such as EDTA), fumaric acid, alanine,
arginine,
aspartic acid, glutam.ic acid, gl.utamine, glyci.ne, histidi.ne, i.soleucin.e,
leucine, lysine,
phenylalanine, serine, threonine, valine, 1,2-cyclohexanedicarboxylic acid,
1,3-cyclohexanedicarboxylic acid, 1,4-cycloh.exanedicarboxylic acid, and the
like, and
combinations thereof. In an embodiment, the molar ratio of the Group 6
metal(s) in the
composition to the second organic compound during treatment can be from about
3:1 to
about 20:1.
[00991 In
some aspects, the second organic compound can optionally contain other
functional groups besides carboxylic acids. For instance, the second organic
compound
can comprise an ami.noacid, which possesses a carboxylic acid functional group
and an
amine functional group simultaneously. Aside from amines, other examples of
such
secondary functional groups in carboxylic acid-containing organic compounds
can
generally include, but are not limited to, hydroxyls, aldehydes, anhydrides,
ethers, esters,
imines, imides, ketones, thiols (m.ercaptans), thi.oesters, and the like, and
combinations
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 39 -
thereof. In some embodiments, the second organic compound can contain no
additional
amine or alcohol functional groups in addition to the carboxylic acid
functional group(s).
1001001 Additionally or alternately, the reactive portion of the second
organic
compound can be a part of a larger functional group in that compound and/or
can be a
derivative of a carboxylic acid that behaves similarly enough to a carboxylic
acid, such
that the reactive portion and/or derivative retains its operability as a Lewis
acid. One
example of a carboxylic acid derivative can include an alkyl carboxylate
ester, where the
alkyl group does not substantially hinder (over a reasonable time scale) the
Lewis acid
functionality of the carboxylate portion of the functional group.
[001011 In other aspects involving formation of a condensation product
(including
aspects involving ex situ formation of an amide), the second organic compound
can
contain other functional groups besides carboxylic acids, whether or not they
are capable
of participating in forming an amide or other condensation reaction product
with one or
more of the functional groups from first organic compound. For instance, the
second
organic compound can comprise an aminoacid, which possesses a carboxylic acid
functional group and an amine functional group simultaneously. In such an
instance, the
aminoacid would qualify as only one of the organic compounds, and not both;
thus, in
such an instance, either an additional amine-containing (first) organic
compound would
need to be present (in the circumstance where the aminoacid would be
considered the
second organic compound) or an additional carboxylic acid-containing (second)
organic
compound would need to be present (in the circumstance where the aminoacid
would be
considered the first organic compound). Aside from amines, other examples of
such
secondary functional groups in carboxylic acid-containing organic compounds
can
generally include, but are not limited to, hydroxyls, aldehydes, anhydrides,
ethers, esters,
imines, imides, ketones, thiols (mercaptans), thioesters, and the like, and
combinations
thereof.
1001021 Additionally or alternately, the reactive portion of the second
organic
compound can be a part of a larger functional group in that compound and/or
can be a
derivative of a carboxylic acid that behaves similarly enough to a carboxylic
acid in the
presence of the amine functional group of the first organic compound, such
that the
reactive portion and/or derivative retains the capability of participating in
forming an
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
-40 -
amide or other desired condensation reaction product with one or more of the
functional
groups from first organic compound. One example of a carboxylic acid
derivative can
include an alkyl carboxylate ester, where the alkyl group does not
substantially hinder
(over a reasonable time scale) the condensation reaction between the amine and
the
carboxylate portion of the ester to form an amide.
[00103] For aspects involving formation of a condensation product (including
aspects
involving ex situ formation of an amide), while there is not a strict limit on
the ratio
between the first organic compound and the second organic compound, because
the goal
of the addition of the first and second organic compounds is to attain a
condensation
reaction product, it may be desirable to have a ratio of the reactive
functional groups
within the first and second organic compounds, respectively, from about 1:4 to
about 4:1,
for example from about 1:3 to about 3:1 or from about 1:2 to about 2:1.
[00104] In certain aspects, the organic compound(s)/additive(s) and/or the
reaction
product(s) are not located/incorporated within the crystal lattice of the
mixed metal oxide
precursor composition, e.g., instead being located on the surface and/or
within the pore
volum.e of the precursor composition and/or being associated with (bound to)
one or
more metals or oxides of metals in a manner that does not significantly affect
the
crystalline lattice of the mixed metal oxide precursor composition, as
observed through
XRD and/or other crystallographic spectra. It is
noted that, in these certain
embodiments, a sulfided version of the mixed metal oxide precursor composition
can
still have its sulfided form. affected by the organic compound(s)/additive(s)
and/or the
reaction product(s), even though the oxide lattice is not significantly
affected.
[00105] In some aspects, one way to attain a catalyst precursor composition
containing a decomposition/dehydrogenation reaction product, such as one
containing
additional unsaturations, includes: (a) treating a catalyst precursor
composition, which
comprises at least one metal from Group 6 of the Periodic Table of the
Elements and at
least one metal from Groups 8-10 of the Periodic Table of the Elements, with a
first
organic compound containing at least one amine group or a second organic
compound
separate from said first organic compound and containing at least one
carboxylic acid
group, but not both, to form an organically treated precursor catalyst
composition; and
(b) heating the organically treated precursor catalyst composition at a
temperature
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 41 -
sufficient and for a time sufficient for the first or second organic compounds
to react to
form an in situ product containing additional unsaturation (for example,
depending upon
the nature of the first or second organic compound, the temperature can be
from. about
195 C to about 260 C, such as from about 200 C to about 250 C), thereby
forming the
additionally-unsaturated and/or additionally oxidized catalyst precursor
composition.
[00106] In certain advantageous embodiments, the heating step (b) above can be
conducted for a sufficiently long time so as to form additional
unsaturation(s), which
may result from at least partial decomposition (e.g., oxidative and/or non-
oxidative
dehydrogenation and/or aromatization) of some (typically-unfunctionalized
organic)
portions of the first or second organic compounds, but generally not for so
long that the
at least partial decomposition volatilizes more than 50% by weight of the
first or second
organic compounds. Without being bound by theory, it is believed that
additional
unsaturation(s) formed in situ and present at the point of sulfiding the
catalyst precursor
composition to form a sulfided (hydroprocessing) catalyst composition can
somehow
assist in controlling one or more of the following: the size of sulfided
crystallites; the
coordination of one or more of the metals during sulfidation, such that a
higher
proportion of the one or more types of metals are in appropriate sites for
promoting
desired hydroprocessing reactions (such as hydrotreating,
hydrodenitrogenation,
hydrodesulffirization, hydrodeoxygenation, hydrodemetallation, hydrocracking
including
selective hydrocracking, hydroisomerization, hydrodewaxing, and the like, and
combinations thereof, and/or for reducing/minimizing undesired hydroprocessing
reactions, such as aromatic saturation, hydrogenation of double bonds, and the
like, and
combinations thereof) than for sulfided catalysts made in the absence of the
in situ
formed reaction product having additional unsaturation(s); and
coordination/catalysis
involving one or more of the metals after sulfidation, such that a higher
proportion (or
each) of the one or more types of metals are more efficient at promoting
desired
hydroprocessing reactions (e.g., because the higher proportion of metal sites
can catalyze
more hydrodesulfurization reactions of the same type in a given timescale
and/or because
the higher proportion of the metal sites can catalyze more difficult
hydrodesulfurization
reactions in a similar timescale) than for sulfided catalysts made in the
absence of the in
situ formed reaction product having additional unsaturation(s).
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
-42 -
[00107] In other aspects, one way to attain a catalyst precursor composition
containing a condensation reaction product, such as an amide, and/or a
reaction product
containing additional unsaturations includes: (a) treating a catalyst
precursor
composition, which comprises at least one metal from Group 6 of the Periodic
Table of
the Elements and at least one metal from Groups 8-10 of the Periodic Table of
the
Elements, with a first organic compound containing at least one amine group
and a
second organic compound separate from said first organic compound and
containing at
least one carboxylic acid group to form an organically treated precursor
catalyst
composition; and (b) heating the organically treated precursor catalyst
composition at a
temperature sufficient and for a time sufficient for the first and second
organic
compounds to react to form an in situ condensation product and/or an in situ
product
containing additional unsaturation (for amides made from. amines and
carboxylic acids,
for example, the temperature can be from about 195 C to about 260 C, such as
from
about 200 C to about 250 C), thereby forming the amide-containing and/or
additionally-
unsaturated and/or additionally oxidized catalyst precursor composition.
1001081 Practically, the treating step (a) above can comprise one (or more) of
three
methods: (1) first treating the catalyst precursor composition with the first
organic
compound and second with the second organic compound; (2) first treating the
catalyst
precursor composition with the second organic compound and second with the
first
organic compound; and/or (3) treating the catalyst precursor composition
simultaneously
with the first organic compound and with the second organic compound.
[00109] In certain advantageous embodiments, the heating step (b) above can be
conducted for a sufficiently long time so as to form the amide, but not for so
long that the
amide so formed substantially decomposes. Additionally or alternately in such
advantageous embodiments, the heating step (b) above can be conducted for a
sufficiently long time so as to form additional unsaturation(s), which may
result from at
least partial decomposition (e.g., oxidative and/or non-oxidative
dehydrogenation and/or
aromatization) of some (typically-unfunctionalized organic) portions of the
organic
compounds, but generally not for so long that the at least partial
decomposition (i)
substantially decomposes any condensation product, such as amide, and/or (ii)
volatilizes
more than 50% by weight of the combined first and second organic compounds.
Without
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
-43 -
being bound by theory, it is believed that in situ formed amide and/or
additional
unsaturation(s) present at the point of sulfiding the catalyst precursor
composition to
form a sulfided (hydroprocessing) catalyst composition can somehow assist in
controlling one or more of the following: the size of sulfided crystallites;
the
coordination of one or more of the metals during sulfidation, such that a
higher
proportion of the one or more types of metals are in appropriate sites for
promoting
desired hydroprocessing reactions (such as hydrotreating,
hydrodenitrogenation,
hydrodesulfurization, hydrodeoxygenation, hydrodemetallation, hydrocracking
including
selective hydrocracking, hydroisomerization, hydrodewaxing, and the like, and
combinations thereof, and/or for reducing/minimizing undesired hydroprocessing
reactions, such as aromatic saturation, hydrogenation of double bonds, and the
like, and
combinations thereof) than for sulfided catalysts made in the absence of the
in situ
formed reaction product having an amide (condensation reaction product of
functional
groups) and/or additional unsaturation(s); and coordination/catalysis
involving one or
more of the metals after sulfidation, such that a higher proportion (or each)
of the one or
more types of metals are more efficient at promoting desired hydroprocessing
reactions
(e.g., because the higher proportion of metal sites can catalyze more
hydrodesulfmization reactions of the same type in a given timescale and/or
because the
higher proportion of the metal sites can catalyze more difficult
hydrodesulft.trization
reactions in a similar timescale) than for sulfided catalysts made in the
absence of the in
situ formed reaction product having an amide (condensation reaction product of
functional groups) and/or additional unsaturation(s).
[001101 When used to make a bulk mixed metal catalyst precursor composition,
the in
situ reacted catalyst precursor composition can, in one embodiment, consist
essentially of
the reaction product, an oxide form of the at least one metal from Group 6, an
oxide form
of the at least one metal from Groups 8-10, and optionally about 20 wt % or
less of a
binder (e.g., about 10 wt % or less).
[001111 After treatment of the catalyst precursor containing the at least one
Group 6
metal and the at least one Group 8-10 metal with the first and/or second
organic
compounds, the organically treated catalyst precursor composition can be
heated to a
temperature high enough to form the reaction product and optionally but
preferably high
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
-44 -
enough to enable any dehydrogenation/decomposition/condensation byproduct to
be
easily removed (e.g., in order to drive the reaction equilibrium to the at
least partially
dehydrogenated/decomposed product and/or condensation product). Additionally
or
alternately, the organically treated catalyst precursor composition can be
heated to a
temperature low enough so as to substantially retain the reaction product
containing the
additional unsaturations and/or the condensation product, so as not to
significantly
decompose the reaction product, and/or so as not to significantly volatilize
(more than
50% by weight of) the first and/or second organic compounds (whether reacted
or not).
[001121 It is contemplated that the specific lower and upper temperature
limits based
on the above considerations can be highly dependent upon a variety of factors
that can
include, but are not limited to, the atmosphere under which the heating is
conducted, the
chemical and/or physical properties of the first organic compound, the second
organic
compound, the reaction product, and/or any reaction byproduct, or a
combination
thereof. In one embodiment, the heating temperature can be at least about 120
C, for
example at least about 150 C, at least about 165 C, at least about 175 C, at
least about
185 C, at least about 195 C, at least about 200 C, at least about 210 C, at
least about
220 C, at least about 230 C, at least about 240 C, or at least about 250 C.
Additionally
or alternately, the heating temperature can be not greater than about 400 C,
for example
not greater than about 375 C, not greater than about 350 C, not greater than
about
325 C, not greater than about 300 C, not greater than about 275 C, not greater
than
about 250 C, not greater than about 240 C, not greater than about 230 C, not
greater
than about 220 C, not greater than about 210 C, or not greater than about 200
C.
[001131 In one embodiment, the heating can be conducted in a low- or non-
oxidizing
atmosphere (and conveniently in an inert atmosphere, such as nitrogen). In an
alternate
embodiment, the heating can be conducted in a moderately- or highly-oxidizing
environment. In another alternate embodiment, the heating can include a multi-
step
process in which one or more heating steps can be conducted in the low- or non-
oxidizing atmosphere, in which one or more heating steps can be conducted in
the
moderately- or highly-oxidizing environment, or both. Of course, the period of
time for
the heating in the environment can be tailored to the first or second organic
compound,
but can typically extend from about 5 minutes to about 168 hours, for example
from
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
-45 -
about 10 minutes to about 96 hours, from about 10 minutes to about 48 hours,
from
about 10 minutes to about 24 hours, from about 10 minutes to about 18 hours,
from
about 10 minutes to about 12 hours, from about 10 minutes to about 8 hours,
from about
minutes to about 6 hours, from about 10 minutes to about 4 hours, from about
20
minutes to about 96 hours, from about 20 minutes to about 48 hours, from about
20
minutes to about 24 hours, from about 20 minutes to about 18 hours, from about
20
minutes to about 12 hours, from about 20 minutes to about 8 hours, from about
20
minutes to about 6 hours, from about 20 minutes to about 4 hours, from about
30 minutes
to about 96 hours, from about 30 minutes to about 48 hours, from about 30
minutes to
about 24 hours, from about 30 minutes to about 18 hours, from about 30 minutes
to
about 12 hours, from about 30 minutes to about 8 hours, from about 30 minutes
to about
6 hours, from. about 30 minutes to about 4 hours, from about 45 minutes to
about 96
hours, from about 45 minutes to about 48 hours, from about 45 minutes to about
24
hours, from. about 45 minutes to about 18 hours, from about 45 minutes to
about 12
hours, from about 45 minutes to about 8 hours, from about 45 minutes to about
6 hours,
from about 45 minutes to about 4 hours, from about 1 hour to about 96 hours,
from about
1 hour to about 48 hours, from about 1 hour to about 24 hours, from about 1
hour to
about 18 hours, from about 1 hour to about 12 hours, from about 1 hour to
about 8 hours,
from 1 hour minutes to about 6 hours, or from about 1 hour to about 4 hours.
100114] Additionally or alternately, in aspects where an ex situ formed amide
is used,
the amide can be formed prior to impregnation into the metal oxide component
of the
catalyst precursor by reaction of the amine component and the carboxylic acid
component. Reaction typically takes place readily at mildly elevated
temperatures up to
about 200 C with liberation of water as a by-product of the reaction at
temperatures
above 100 C and usually above 150 C. The reactants can usually be heated
together to
form a melt in which the reaction takes place and the melt impregnated
directly into the
metal oxide component which is preferably pre-heated to the same temperature
as the
melt in order to assist penetration into the structure of the metal oxide
component. The
reaction can also be carried out in the presence of a solvent if desired and
the resulting
solution used for the impregnation step. In certain embodiments, the amide and
its heat
treated derivative may not be located/incorporated within the crystal lattice
of the mixed
metal oxide precursor, e.g., may instead be located on the surface and/or
within the pore
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
-46 -
volume of the precursor and/or be associated with (bound to) one or more
metals or
oxides of metals in a manner that does not significantly affect the
crystalline lattice of the
mixed metal oxide precursor composition, as observed through XRD and/or other
crystallographic spectra. A sulfided version of the mixed metal oxide
precursor
composition can still have its sulfided form affected by the organic
compound(s)/additive(s) and/or the reaction product(s), even though the oxide
lattice is
not significantly affected.
[00115] There is not a strict limit on the ratio between the amine reactant
and the
carboxylic reactant, and accordingly, the ratio of the reactive amine and
carboxylic acid
groups in the two reactants may vary, respectively, from about 1:4 to about
4:1, for
example from about 1:3 to about 3:1 or from about 1:2 to about 2:1. it has
been observed
that catalysts made with amides from equimolar quantities of the amine and
carboxylic
acid reactants compounds show performance improvements in hydroprocessing
certain
feeds and for this reason, amides made with an equimolar ratio are preferred.
[001161 The pre-formed amide is suitably impregnated into the metal oxide
precursor
by incipient wetness impregnation with the amount determined according to the
pore
volume of the metal oxide component. Following impregnation, a heat treatment
is
carried out which first removes any residual water and/or solvent but also
creates a
reaction product containing additional unsaturation sites and possibly
additional oxygen.
The amide-impregnated metal oxide component is then heated at a temperature
sufficient
and for a time sufficient to form a product containing the additional
unsaturation which
is characteristic of the desired organic component; this treatment with the
pre-formed
amide is typically from. about 195 C to about 280 C, for example from about
200 C to
about 250 C).
[001171 The heating step should not be conducted for so long that the amide
becomes
substantially decomposed but is continued for a sufficiently long time to form
additional
unsaturation(s), which may result from at least partial decomposition (e.g.,
oxidative
and/or non-oxidative dehydrogenation and/or aromatization) of some (typically-
unfinictionalized organic) portions of the organic compounds. On the other
hand, the
heating should not be conducted for so long that the decomposition
substantially results
in gross decomposition of the amide or any condensation product. The
impregnated
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
-47 -
catalyst precursor composition can be heated to a temperature high enough to
form the
unsaturated reaction product and typically high enough to enable any
byproducts such as
water to be removed. The temperature to which the impregnated precursor
composition
is heated should, however, maintained low enough so as to substantially retain
the amide
reaction product with the additional unsaturations and any oxygen, and so as
not to
significantly decompose the functionalized reaction product, and/or so as not
to
significantly volatilize (more than 50% by weight of) the amide.
[00118] The specific lower and upper temperature limits based on the above
considerations can be dependent upon a variety of factors that can include,
but are not
limited to, the atmosphere under which the heating is conducted, the chemical
and/or
physical properties of the amide, the amide reaction product, and/or any
functionalized
reaction byproduct as well as the desired duration of the heating with higher
temperatures, e.g. over the optimal temperature range up to 250 C, enabling
shorter
heating durations to be utilized.. The minimum heating temperature can, for
example,
suitably be at least about 120 C, for example at least about 150 C, at least
about 165 C,
at least about 175 C, at least about 185 C, at least about 195 C, at least
about 200 C, at
least about 210 C, at least about 220 C, at least about 230 C, at least about
240 C, or at
least about 250 C. The maximum heating temperature should not be greater than
about
400 C, for example, not greater than about 375 C, not greater than about 350
C, not
greater than about 325 C, not greater than about 300 C, not greater than about
275 C,
not greater than about 250 C, not greater than about 240 C, not greater than
about
230 C, not greater than about 220 C, not greater than about 210 C, or not
greater than
about 200 C. Resort to temperatures above the preferred maximum of 250 C
should be
made with due care to avoid the gross decomposition of the amide as noted
above but a
slightly higher range, for example, 250-280 C, e.g. 260 or 275 C may permit
usefully
shorter heating steps in commercial scale operation. The temperature to be
used should
therefore be selected on an empirical basis depending on the nature of the
amide used in
the impregnation. The progress of the heating can be monitored according to
the
properties of the treated product, including analysis by GC-MS and by its
infrared
spectrum as described below.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 48 -
[001191 In an embodiment, the organically treated catalyst precursor
composition
and/or the catalyst precursor composition containing the reaction product can
contain
from about 4 wt % to about 20 wt %, for example from about 5 wt % to about 15
wt %,
carbon resulting from the first and second organic compounds and/or from the
condensation product, as applicable, based on the total weight of the relevant
composition.
1001201 Additionally or alternately, as a result of the heating step, the
reaction product
from the organically treated catalyst precursor can exhibit a content of
unsaturated
carbon atoms (which includes aromatic carbon atom.$), as measured according to
peak
area comparisons using 13C NMR techniques, of at least 29%, for example at
least about
30%, at least about 31%, at least about 32%, or at least about 33%. Further
additionally
or alternately, the reaction product from the organically treated catalyst
precursor can
optionally exhibit a content of unsaturated carbon atoms (which includes
aromatic
carbon atoms), as measured according to peak area comparisons using 13C -MAR
techniques, of up to about 70%, for example up to about 65%, up to about 60%,
up to
about 55%, up to about 50%, up to about 45%, up to about 40%, or up to about
35%.
Still further additionally or alternately, as a result of the heating step,
the reaction
product from the organically treated catalyst precursor can exhibit an
increase in content
of unsaturated carbon atoms (which includes aromatic carbon atoms), as
measured
according to peak area comparisons using 13C NMR techniques, of at least about
17%,
for example at least about 18%, at least about 19%, at least about 20%, or at
least about
21% (e.g., in an embodiment where the first organic compound is oleylarnine
and the
second organic compound is oleic acid, such that the combined unsaturation
level of the
unreacted compounds is about 11.1% of carbon. atoms, a .about.17% increase in
unsaturated carbons upon heating corresponds to about 28.1% content of
unsaturated
carbon atoms in the reaction product). Yet further additionally or
alternately, the
reaction product from the organically treated catalyst precursor can
optionally exhibit an
increase in content of unsaturated carbon atoms (which includes aromatic
carbon atoms),
as measured according to peak area comparisons using 13C NMR techniques, of up
to
about 60%, for example up to about 55%, up to about 50%, up to about 45%, up
to about
40%, up to about 35%, up to about 30%, or up to about 25%.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 49 -
[001211 Again further additionally or alternately, as a result of the
heating step, the
reaction product from the organically treated catalyst precursor can exhibit a
ratio of
unsaturated carbon atoms to aromatic carbon atoms, as measured according to
peak area
ratios using infrared spectroscopic techniques of a deconvoluted peak centered
from
about 1700 cm1 to about 1730 cm- (e.g., at about 1715 cm-1), compared to a
deconvoluted peak centered from about 1380 cm1 to about 1450 cm4 (e.g., from
about
1395 cm-1 to about 1415 cm-1), of at least 0.9, for example at least 1.0, at
least 1.1, at
least 1.2, at least 1.3, at least 1.4, at least 1.5, at least 1.7, at least
2.0, at least 2.2, at least
2.5, at least 2.7, or at least 3Ø Again still further additionally or
alternately, the reaction
product from the organically treated catalyst precursor can exhibit a ratio of
unsaturated
carbon atoms to aromatic carbon atoms, as measured according to peak area
ratios using
infrared spectroscopic techniques of a deconvoluted peak centered from about
1700 cm-1
to about 1730 cm-1 (e.g., at about 1715 cm1), compared to a deconvoluted peak
centered
from about 1380 cni-1 to about 1450 cm.-1 (e.g., from about 1395 cm-1 to about
1415 cm--
`), of up to 15, for example up to 10, up to 8.0, up to 7.0, up to 6.0, up to
5.0, up to 4.5,
up to 4.0, up to 3.5, or up to 3Ø
[00122] A (stinted) hydroprocessing catalyst composition can then be produced
by
sulfiding the catalyst precursor composition containing the reaction product.
SuIfiding is
generally carried out by contacting the catalyst precursor composition
containing the
reaction product with a sulfur-containing compound (e.g., elemental sulfur,
hydrogen
sulfide, polysulfides, or the like, or a combination thereof, which may
originate from a
fossil/mineral oil stream, from a biocomponent-based oil stream, from a
combination
thereof, or from a sulfur-containing stream separate from the aforementioned
oil
stream(s)) at a temperature and for a time sufficient to substantially sulfide
the
composition and/or sufficient to render the sulfided composition active as a
hydroprocessing catalyst. For instance, the suifidation can be carried out at
a
temperature from about 300 C to about 400 C, e.g., from about 310 C to about
350 C,
for a period of time from about 30 minutes to about 96 hours, e.g., from about
1 hour to
about 48 hours or from about 4 hours to about 24 hours. 'The sulfiding can
generally be
conducted before or after combining the metal (oxide) containing composition
with a
binder, if desired, and before or after forming the composition into a shaped
catalyst.
The sulfiding can additionally or alternately be conducted in situ in a
hydroprocessing
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 50 -
reactor. Obviously, to the extent that a reaction product of the first or
second organic
compounds contains additional unsaturations formed in situ, it would generally
be
desirable for the sulfidation (and/or any catalyst treatment after the organic
treatment) to
significantly maintain the in situ formed additional unsaturations of said
reaction
product.
[00123] The sulfided catalyst composition can exhibit a layered structure
comprising a
plurality of stacked YS2 layers, where Y is the Group 6 metal(s), such that
the average
number of stacks (typically for bulk organically treated catalysts) can be
from about 1.5
to about 3.5, for example from about 1.5 to about 3.0, from about 2.0 to about
3.3, from
about 2.0 to about 3.0, or from about 2.1 to about 2.8. For instance, the
treatment of the
metal (oxide) containing precursor composition according to the invention can
afford a
decrease in the average number of stacks of the treated precursor of at least
about 0.8, for
example at least about 1.0, at least about 1.2, at least about 1.3, at least
about 1.4, or at
least about 1.5, as compared to an untreated metal (oxide) containing
precursor
composition. As such, the number of stacks can be considerably less than that
obtained
with an equivalent sulfided mixed metal (oxide) containing precursor
composition
produced without the first or second organic compound treatment. The reduction
in the
average number of stacks can be evidenced, e.g., via X-ray diffraction spectra
of relevant
sulfided compositions, in which the (002) peak appears significantly broader
(as
determined by the same width at the half-height of the peak) than the
corresponding peak
in the spectrum of the sulfided mixed metal (oxide) containing precursor
composition
produced without the organic treatment (and/or, in certain cases, with only a
single
organic compound treatment using an organic compound having less than 10
carbon
atoms) according to the present invention. Additionally or alternately to X-
ray
diffraction, transmission electron microscopy (rEm) can be used to obtain
micrographs
of relevant sulfided compositions, including multiple microcrystals, within
which
micrograph images the multiple microcrystals can be visually analyzed for the
number of
stacks in each, which can then be averaged over the micrograph visual field to
obtain an
average number of stacks that can evidence a reduction in average number of
stacks
compared to a sulfided mixed metal (oxide) containing precursor composition
produced
without the organic treatment (and/or, in certain cases, with only a single
organic
compound treatment) according to the present invention.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 51 -
[00124] The sulfided catalyst composition described above can be used as a
hydroprocessing catalyst, either alone or in combination with a binder. If the
sulfided
catalyst composition is a bulk catalyst, then only a relatively small amount
of binder may
be added. However, if the sulfided catalyst composition is a
heterogeneous/supported
catalyst, then usually the binder is a significant portion of the catalyst
composition, e.g.,
at least about 40 wt %, at least about 50 wt %, at least about 60 wt %, or at
least about 70
wt %; additionally or alternately for heterogeneous/supported catalysts, the
binder can
comprise up to about 95 wt % of the catalyst composition, e.g., up to about 90
wt %, up
to about 85 wt %, up to about 80 wt %, up to about 75 wt %, or up to about 70
wt %.
Non-limiting examples of suitable binder materials can include, but are not
limited to,
silica, silica-alumina (e.g., conventional silica-alumina, silica-coated
alumina, alumina-
coated silica, or the like, or a combination thereof), alumina (e.g.,
boehmite, pseudo-
boehmite, gibbsite, or the like, or a combination thereof), titania, zirconia,
cationic clays
or anionic clays (e.g., saponite, bentonite, kaoline, sepiolite,
hydrotal.cite, or the like, or a
combination thereof), and mixtures thereof. In some preferred embodiments, the
binder
can include silica, silica-alumina, alumina, titania, zirconia, and mixtures
thereof. These
binders may be applied as such or after peptization. It may also be possible
to apply
precursors of these binders that, during precursor synthesis, can be converted
into any of
the above-described binders. Suitable precursors can include, e.g., alkali
metal
alurninates (alumina binder), water glass (silica binder), a mixture of alkali
metal
aluminates and water glass (silica-alumina binder), a mixture of sources of a
di-, tri-,
and/or tetravalent metal, such as a mixture of water-soluble salts of
magnesium,
aluminum, and/or silicon (cationic clay and/or anionic clay), chlorohydrol,
aluminum
sulfate, or mixtures thereof.
100125] Generally, the binder material to be used can have lower catalytic
activity
than the remainder of the catalyst composition, or can have substantially no
catalytic
activity at all (less than about 5%, based on the catalytic activity of the
bulk catalyst
composition being about 100%). Consequently, by using a binder material, the
activity
of the catalyst composition may be reduced. Therefore, the amount of binder
material to
be used, at least in bulk catalysts, can generally depend on the desired
activity of the
final catalyst composition. Binder amounts up to about 25 wt % of the total
composition
can be suitable (when present, from above 0 wt A) to about 25 wt %),
depending on the
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 52 -
envisaged catalytic application. However, to take advantage of the resulting
unusual
high activity of bulk catalyst compositions according to the invention, binder
amounts,
when added, can generally be from about 0.5 wt % to about 20 wt % of the total
catalyst
composition.
[001261 If desired in bulk catalyst cases, the binder material can be
composited with a
source of a Group 6 metal and/or a source of a non-noble Group 8-10 metal,
prior to
being composited with the bulk catalyst composition and/or prior to being
added during
the preparation thereof. Compositing the binder material with any of these
metals may
be carried out by any known means, e.g., impregnation of the (solid) binder
material with
these metal(s) sources.
[001271 A cracking component may also be added during catalyst preparation.
When
used, the cracking component can represent from about 0.5 wt % to about 30 wt
%,
based on the total weight of the catalyst composition. The cracking component
may
serve, for example, as an isomerization enhancer. Conventional cracking
components
can be used, e.g., a cationic clay, an anionic clay, a zeolite (such as ZSM-5,
zeolite Y,
ultra-stable zeolite Y, zeolite X, an AIPO, a SAPO, or the like, or a
combination thereof),
amorphous cracking components (such as silica-alumina or the like), or a
combination
thereof. It is to be understood that some materials may act as a binder and a
cracking
component at the same time. For instance, silica-alumina may simultaneously
have both
a cracking and a binding function.
[00128] If desired, the cracking component may be composited with a Group 6
metal
and/or a Group 8-10 non-noble metal, prior to being composited with the
catalyst
composition and/or prior to being added during the preparation thereof.
Compositing the
cracking component with any of these metals may be carried out by any known
means,
e.g., impregnation of the cracking component with these metal(s) sources. When
both a
cracking component and a binder material are used and when compositing of
additional
metal components is desired on both, the compositing may be done on each
component
separately or may be accomplished by combining the components and doing a
single
compositing step.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 53 -
[001291 The selection of particular cracking components, if any, can depend on
the
intended catalytic application of the final catalyst composition. For
instance, a zeolite
can be added if the resulting composition is to be applied in hydrocracking or
fluid
catalytic cracking. Other cracking components, such as silica-alumina or
cationic clays,
can be added if the final catalyst composition is to be used in hydrotreating
applications.
The amount of added cracking material can depend on the desired activity of
the fmal
composition and the intended application, and thus, when present, may vary
from above
0 wt % to about 80 wt %, based on the total weight of the catalyst
composition. in a
preferred embodiment, the combination of cracking component and binder
material can
comprise less than 50 wt % of the catalyst composition, for example, less than
about 40
wt %, less than about 30 wt %, less than about 20 wt %, less than about 15 wt
%, or less
than about 10 wt %.
[001301 If desired, further materials can be added, in addition to the metal
components
already added, such as any material that would be added during conventional
hydroprocessing catalyst preparation. Suitable examples of such further
materials can
include, but are not limited to, phosphorus compounds, boron compounds,
fluorine-
containing compounds, sources of additional transition metals, sources of rare
earth
metals, fillers, or mixtures thereof.
Additional Embodiments
1001311 Embodiment 1. A hydrotreating process comprising: reacting a
feedstream
having a sulfur content of at least about 3000 wppm, or at least about 4000
wppm, or at
least about 5000 wppm (such as up to about 50000 wppm), and a T90 boiling
point of
about 900 F (482 C) or less, in a first hydrotreating stage in the presence of
a hydrogen-
containing treat gas and in the presence of at least one first stage
hydrotreating catalyst,
the first hydrotreating stage being operated at first stage hydrotreating
conditions, to
produce a first liquid effluent having a sulfur content of about 5000 wppm or
less, or
about 4000 wppm or less, or about 3000 wppm or less, the sulfur content of the
first
liquid effluent being less than the sulfur content of the feedstrearn;
separating the first
liquid effluent to produce a first vapor phase stream and a first liquid
product stream, the
first liquid product stream optionally having a T I 0 boiling point and a T90
boiling point;
reacting at least a portion of the first liquid product stream. in a second
hydrotreating
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 54 -
stage in the presence of a hydrogen-containing treat gas and a second
hydrotreating
catalyst, the second hydrotreating stage being operated at second stage
hydrotreating
conditions to produce a second liquid effluent; and separating the second
liquid effluent
to produce a second vapor phase stream and a second liquid product stream
having a
sulfur content of about 500 wppm or less, or about 250 wppm or less, or about
100
wppm or less, wherein about 15 wt% or less of the feedstream is converted
relative to a
conversion temperature of 350 F (177 C) during the reacting in the first
hydrotreating
stage and the second hydrotreating stage.
1001321 Embodiment 2. The process of Embodiment 1, wherein the first liquid
effluent has a sulfur content of at least about 1000 wppm, or at least about
1500 wppm,
or at least about 2000 wppm.
[00133] Embodiment 3. A hydrotreating process comprising: reacting a
feedstream
having a T90 boiling point of about 900 F (482 C) or less in a first
hydrotreating stage in
the presence of a hydrogen-containing treat gas and in the presence of at
least one first
stage hydrotreating catalyst, the first hydrotreating stage being operated at
first stage
hydrotreating conditions, to produce a first liquid effluent; separating at
least a portion of
the first liquid effluent to produce a first vapor phase stream and a first
liquid product
stream, the first liquid product stream having a sulfur content of about 1000
wppm to
about 20,000 wppm, the first liquid product stream having a) a T10 boiling
point of at
least about 350 F (177 C), b) a T90 boiling point of about 850 F (454 C) or
less, or c) a
combination thereof; reacting at least a portion of the first liquid product
stream in a
second hydrotreating stage in the presence of a hydrogen-containing treat gas
and a
second hydrotreating catalyst, the second hydrotreating stage being operated
at second
stage hydrotreating conditions to produce a second liquid effluent, the second
stage
hydrotreating conditions being effective for conversion of about 10 wt% or
less of the at
least a portion of the first liquid product stream relative to a conversion
temperature of
about 350 F (177 C); and separating at least a portion of the second liquid
effluent to
produce a second vapor phase stream and a second liquid product stream, the
second
liquid product stream having a sulfur content of about 250 wppm or less, or
about 100
wppm or less.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 55 -
[001341 Embodiment 4. The process of any of the above embodiments, wherein the
T90 boiling point of the first liquid product stream is about 800 F (427 C) or
less, or
about 750 F (399 C) or less, or about 700 F (371 C) or less.
[001351 Embodiment 5. The process of any of the above embodiments, wherein the
T10 boiling point of the feedstream is at least about 400 F (204"C), or at
least about
450 F (232"C).
[001361 Embodiment 6. The process of any of the above embodiments, wherein the
T90 boiling point of the feedstream is about 850 F (454 C) or less, or about
800 F
(427 C) or less, or about 750 F (399 C) or less, or about 700 F (371 C) or
less.
[00137] Embodiment 7. The process of any of the above embodiments, wherein the
first stage hydrotreating conditions are effective for conversion of about 10
wt% or less
of the feedstream relative to a conversion temperature of about 350 F (177 C),
or about
wt% or less; or wherein the second stage hydrotreating conditions are
effective for
conversion of about 10 wt% or less of the feedstream relative to a conversion
temperature of about 350 F (177 C), or about 5 wt% or less; or wherein about
10 wt% or
less of the feedstream is converted relative to a conversion temperature of
350 F (177 C)
during the reacting in the first hydrotreating stage and the second
hydrotreating stage, or
about 5 wt% or less, or about 3 wt% or less; or a combination thereof.
1001381 Embodiment 8. The process of any of the above embodiments, further
comprising hydroprocessing at least a portion of the first liquid product
stream in an
intermediate hydrotreating stage.
[00139] Embodiment 9. The process of any of the above embodiments, wherein the
hydrotreating catalyst comprises Mo, W, or a combination thereof, and wherein
the
hydrotreating catalyst comprises Ni, Co, Fe, or a combination thereof, the
hydrotreating
catalyst optionally being a supported catalyst or optionally being a bulk
catalyst.
[001401 Embodiment 10. The process of Embodiment 9, wherein the hydrotreating
catalyst comprises i) about 1 wt% to about 40 wt% of the Mo, W, or a
combination
thereof, ii) Wherein the hydrotreating catalyst comprises about 2 wt% to about
70 wt% of
the Ni, Co, Fe, or a combination thereof, or both i) and ii).
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 56 -
[00141] Embodiment 11. The process of any of the above embodiments, wherein
the
first stage hydrotreating conditions, the second stage hydrotreating
conditions, or a
combination thereof comprise temperatures of about 200 C to about 450 C;
pressures of
about 250 psig (1.8 MPag) to about 5000 psig (34.6 MPag); liquid hourly space
velocities (LHSV) of about 0.1 hr-1 to about 10 hr-1; and hydrogen treat rates
of about
200 scf'S (35.6 m3/m3) to about 10,000 scf/B (1781 m3/m?).
[001421 Embodiment 12. The process of any of the above embodiments, further
comprising performing catalytic dewaxing, hydrofinishing, aromatic
saturation, or a
combination thereof on at least a portion of the second liquid product stream.
[001431 Embodiment 13. The process of Embodiment 12, wherein the catalytic
dewaxing is performed at effective catalytic dewaxing conditions comprising
temperatures of about 200 C to about 450 C, hydrogen partial pressures of
about 1.8
MPag to about 34.6 MPag (250 psig to 5000 psig), liquid hourly space
velocities of from
0.05 11-1 to 10 If', and hydrogen treat gas rates of about 35.6 m3/m3 (200
SCF/B) to about
1781 m3/m3 (10,000 scf/B).
[001441 Embodiment 14. The process of Embodiment 12 or 13, wherein the
hydrofinishing is performed at effective hydrofinishing conditions comprise
temperatures from about 125 C to about 425 C, total pressures from about 500
psig (3.4
IV1Pa) to about 3000 psig (20.7 MN), liquid hourly space velocities from about
0.11 hr-1 to
about 5 hr.1 LHSV, and hydrogen treat gas rates of from 500 to 5000 scf/B (89
to 890 m3
m3).
1001451 Embodiment 15. The process of Embodiment 12 or 13 or 14, wherein
the
aromatic saturation is performed at effective aromatic saturation conditions
comprising
temperatures from about 200 C to about 425 C, total pressures from about 500
psig (3.4
IV1Pa) to about 3000 psig (20.7 MPa), liquid hourly space velocities from
about 0.11 hr to
about 5 fir-1 LHSV, and hydrogen treat gas rates of from 500 to 5000 scf/B (89
to 890
m3 / m3).
[001461 Embodiment 16. The process of any of the above embodiments, wherein
the
feedstream has an aromatics content of at least about 60 wt%, or at least
about 70 wt%.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 57 -
[00147] Embodiment 17. The process of any of the above embodiments, wherein
the
feedstream has a multi-ring aromatics content of at least about 40 wt%, or at
least about
45 wt%, or at least about 50 wt%.
[00148] Embodiment 18. The process of any of the above embodiments, wherein
the
first hydrotreating catalyst and the second hydrotreating catalyst are
different, or wherein
the first hydrotreating catalyst and the second hydrotreating catalyst are the
same.
[00149] Embodiment 19. The process of any of the above embodiments, wherein
the
second hydrotreating catalyst comprises a mixed metal catalyst, the mixed
metal catalyst
comprising a sulfided mixed metal catalyst formed by sulfiding a mixed metal
catalyst
precursor composition, the mixed metal catalyst precursor composition being
produced
by a) heating a composition comprising at least one metal from Group 6 of the
Periodic
Table of the Elements, at least one metal from Groups 8-10 of the Periodic
Table of the
Elements, and a reaction product formed from (i) a first organic compound
containing at
least one amine group, and (ii) a second organic compound separate from said
first
organic compound and containing at least one carboxylic acid group to a
temperature
from about 195 C to about 260 C for a time sufficient for the first and second
organic
compounds to form a reaction product in situ that contains an amide moiety,
unsaturated
carbon atoms not present in the first or second organic compounds, oxygen
atoms not
present in the first or second organic compounds, or a combination thereof; b)
heating a
composition comprising one metal from Group 6 of the Periodic Table of the
Elements,
at least one metal from Groups 8-10 of the Periodic Table of the Elements, and
a reaction
product formed from (iii) a first organic compound containing at least one
amine group
and at least 10 carbon atoms or (iv) a second organic compound containing at
least one
carboxylic acid group and at least 10 carbon atoms, but not both (iii) and
(iv), wherein
the reaction product contains additional unsaturated carbon atoms, relative to
(iii) the
first organic compound or (iv) the second organic compound, wherein the metals
of the
catalyst precursor composition are arranged in a crystal lattice, and wherein
the reaction
product is not located within the crystal lattice, to a temperature from about
195 C to
about 260 C for a time sufficient for the first or second organic compounds to
form a
reaction product in situ that contains unsaturated carbon atoms not present in
the first or
second organic compounds, oxygen atoms not present in the first or second
organic
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 58 -
compounds, or a combination thereof; or c) heating a composition comprising at
least
one metal from Group 6 of the Periodic Table of the Elements, at least one
metal from
Groups 8-10 of the Periodic Table of the Elements, and a pre-formed amide
formed from
(v) a first organic compound containing at least one amine group, and (vi) a
second
organic compound separate from said first organic compound and containing at
least one
carboxylic acid group, to form at least one of additional in situ unsaturated
carbon atoms
or in situ added oxygen atoms not present in the first organic compound, the
second
organic compound, or both, but not for so long that the pre-formed amide
substantially
decomposes, thereby forming a catalyst precursor containing at least one of in
situ
formed unsaturated carbon atoms or in situ added oxygen atoms.
Examples
[00150] The following examples illustrate various methods for increasing
distillate
yield based in part on additional aromatic saturation of an appropriate feed.
In some
examples, distillate yield can be improved based on use of a catalyst with
improved
activity for aromatic saturation at a desired level of severity for removal of
heteroatoms.
In other examples, distillate yield can be improved based on using interstage
separation
prior to a second (or subsequent) hydrotreating stage.
Example 1 Distillate Flydrotreating with Interstage Separation
1001511 To demonstrate the benefits of using interstage separation for
distillate
hydrotreating, a light cycle oil was hydrotreated under a series of
conditions. Various
properties of the light cycle oil feed prior to the initial hydrotreatment
stage are shown in
Table 1. In addition to the properties in Table 1, the light cycle oil had a
T5 boiling point
of about 412 F (21PC), a T95 boiling point of about 724 F (384 C), and a final
boiling
point of about 788 F (420 C).
1001521 in an initial stage, the light cycle oil was hydrotreated to reduce
the sulfur
content, nitrogen content, and specific gravity of the liquid product. The
effluent from.
the initial stage was either cascaded into second hydrotreatment stage without
stripping
or other intermediate separation as shown in process configuration FIG. 1, or
was
separated to separate the liquid product from. the gas phase portion of the
effluent and the
liquid phase product was then hydrotreated in a second reaction stage as shown
in
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
-59 -
process configuration FIG. 2. The hydrotreating catalyst in both stages was a
commercially available supported NiMo distillate hydrotreating catalyst.
[00153] The liquid phase effluent from the first stage was hydrotreated using
a treat
gas containing substantially no H2S to simulate the two stage hydroprocessin.g
with
intermediate separation, such as the configuration shown in FIG. 2, and a
treat gas
containing about 2 vol% H2S to simulate the two stage hydroprocessing without
intermediate separation, such as the configuration shown in FIG. 1. As shown
in Table
1, the liquid product from the second hydrotreating stage has a substantially
lower
aromatics content than the feed to the initial hydrotreating stage.
Additionally, the
aromatics present in the liquid product from the second hydrotreating stage
are primarily
1-ring aromatics. This is in contrast to the initial feed, where the majority
of the
aromatics are multi-ring aromatics.
1001541 The reduction in multi-ring aromatics in the final product as H2S is
removed
from the treat gas (as shown in Table 1) is believed to contribute to the
reduced specific
gravity (or increased API gravity) of the liquid products formed during
hydrotreatment
with lower concentrations of H2S and/or no H2S in the treat gas. The change in
specific
gravity shown in Table 1 corresponds to about a 0.44 vol% increase for the
volume of
liquid product generated with no H2S in the second stage treat gas relative to
the volume
of liquid product generated with 2 vol% H2S in the second stage treat gas. The
reduction
in multi-ring aromatics also causes a corresponding increase in the amount of
H2
consumed during the second stage hydrotreatm.ent. It is noted that the net
conversion of
the distillate feed relative to a conversion temperature of 350 F (177 C)
appears to be
relatively unaffected by the amount of H2S present in the second hydrotreating
stage, at
least for H2S amounts of about 2 vol% or less. Thus, the increase in
distillate yield
appears to be achieved at a substantially constant level of process severity.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 60 -
Table 1 ¨ Light Cycle Oil Feed and Hydrotreated Product Properties
Product Product
Feed
1
'Process Conditions
Treat Gas H2S Content, vol% 0
Temperature, F 655 656
Pressure, psi g 1637 1632
.LHSV,hr-J 0.76 0.77
Treat Gas Rate, SCF/13 4431 4405
Treat Gas Purity, % H2 80 78
Product Properties
S, ppm 20100 9.5 19
N, wppm 708 0.4 0.4
API 16.5 28.4 27.7
SpGr, g/ml 0.9561 0.8849 0.8888
Arornatics, wt%
I Ring I 8.7 48.3 52.1
2 Ring 40.9 3.8 4.5
3+ Ring 13.1 0.5 0.4
Total 72.7 52.6 57
Hydrogen Consumption,
1708 1589
scf/bbl
350+% conversion, vvt% 2.22% 2.28%
1001551 As an example of the commercial benefit, an example of a suitable feed
for
commercial distillate hydrotreater can be a feed containing about 30 vol%
light cycle oil,
such as the light cycle oil used for the processes shown in Table 1, with the
remaining
portion of the feed corresponding to a virgin gas oil having a roughly
comparable boiling
range. For this type of feed, a 0.44 vol.% increase in the product resulting
from the light
cycle oil portion (30 vol%) of the feed can correspond to about 23,100 barrels
of
additional distillate product per year generated by a 50,000 barrel per day
distillate
hydrotreater under typical operating conditions.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 61 -
Example 2 ¨ Hydrotreating of Light Vacuum Gas Oil with Mixed Metal Catalyst
[00156] A mixed metal catalyst formed from. a suitable precursor can also be
used to
improve aromatic saturation during distillate hydrotreating. In Examples 2 and
3,
various feeds were hydrotreated in a single processing stage (i.e., no
separation to
remove H2S) using various catalysts or catalyst systems.
[00157] In a first distillate hydrotreating process, a straight run light
vacuum gas oil
feed was hydrotreated in a single stage distillate hydrotreating system. The
catalyst in
the reaction system was a stacked bed of a commercial NiMo supported
hydrotreating
catalyst, a mixed metal catalyst formed from a suitable precursor, and the
commercial
NiMo supported hydrotreating catalyst. About one third of the catalyst volume
corresponded to the mixed metal catalyst, with the mixed metal catalyst being
approximately in the middle of the catalyst bed. For comparison, the straight
run light
vacuum gas oil was hydrotreated in a similar reaction system with a catalyst
bed
composed only of the commercial NiMo supported hydrotreating catalyst.
[00158] As shown in Table 2, the light vacuum gas oil had an initial sulfur
content of
about 0.86 wt% and a specific gravity of about 0.876 g/ml. The light vacuum
gas oil was
exposed to the catalyst or catalyst system at 340 C and at 840 psig (5800 kPa)
of
pressure. The treat gas rate was about 560 scf/B (950 Nm.3/m3) of a gas
containing about
80 vol% hydrogen. The LHSV was about 0.85 hr-1.
[00159] Under the hydrotreating conditions, the stacked bed catalyst including
the
mixed metal catalyst resulted in a liquid product yield with a volume increase
of about
0.29 vol% relative to the product yield from hydrotreating over just the
commercial
supported NiMo catalyst. This increase in volume was achieved with similar
levels of
conversion relative to a 300 F (149 C) conversion temperature. This
demonstrates the
ability of the mixed metal catalyst to improve yield (volume swell) for a feed
having a
sulfur content of less than about 10000 wppm at a roughly constant level of
process
severity.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 62 -
Table 2: Process Conditions for Distillate Hydrotreating of Straight Run Feed
Stacked Bed Including Commercial HDT
Straight Run Feed
Mixed Metal Catalyst catalyst
Feed Properties
S, wt% 0.856
N, wppm 242
SpGr 0.8756
Pliic,:.ssing Conditions
Temp, C 340 340
Pressure, psig 840
TGR, SCF/B 560
TGR purity, vol% 80 80
LHSV, lu=1 0.85 0.85
3004-F Conversion, (.)/0 2.3
Product Properties
ILiquid yield, vol% Base + 0.29% Base
Example 3 ¨ Hydrotreating of High Sulfur Content Feeds with a Mixed Metal
Catalyst
[00160] In this example, the impact of sulfur content on yield when using a
mixed
metal catalyst is further investigated. Two different feeds were hydrotreated
over
hydrotreating catalysts to demonstrate the yield improvement of the mixed
metal
catalyst. One hydrotreating catalyst was a mixed metal catalyst formed from a
suitable
precursor, as described herein. A second catalyst was a bulk NiMoW
hydrotreating
catalyst made according to the methods described in U.S. Patent 6,156,695,
U.S. Patent
6,582,590 and/or U.S. Patent 6,929,738.
[001611 Table 3 below shows the processing conditions used for single stage
hydrotreatment of a feed corresponding to about 20 wt% of a light cycle oil
similar to the
feed in Example 1, with. the remainder of the feed corresponding to a straight
run light
vacuum gas oil similar to the feed described in Example 2. As a result, the
feed had an
initial sulfur content of about 11,000 wppm. The process conditions for
hydrotreatment
are also shown in Table 3.
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 63 -
Table 3 ¨ Process Conditions for Distillate Hydrotreating of Partially Cracked
Feed
20% Cracked Feed (LCO) Mixed Metal Catalyst Comparative Bulk Catalyst
Processing Conditions
Temp ( C) ¨300 ¨300
Pressure (kPa) ¨5800 ¨5800
Treat Gas Rate (Nfm3/m3) ¨250 ¨250
Treat Gas Purity (vol %) ¨100 ¨100
LHSV (hr-1) ¨I.? ¨1,7
Product ¨ Liquid Yield (vol %) Base + ¨0.37% Base
[00162] As shown in Table 3, the mixed metal catalyst provided a liquid
product yield
increase of about 0.37 vol% relative to the yield from the comparative hulk
catalyst. The
amount of conversion of the feed was similar for both catalysts. Thus, based
on the
results in Table 2 and Table 3, for a given level of process severity, the
mixed metal
catalyst formed from a suitable precursor appears to provide a yield advantage
over
various conventional catalysts.
WWI The process conditions and results from processing a feed composed of
only
the light cycle oil are shown in Table 4. As shown in Table 4, the increase in
yield using
the mixed metal catalyst is 0.93 vol%. As indicated by the process conditions,
this yield
increase was again achieved at roughly constant process severity.
Table 4 - Process Conditions for Distillate Hydrotreating of Cracked Feed
100% Cracked Feed (LCO) Mixed Metal Catalyst Comparative Bulk Catalyst
Processing Conditions
Temp ("C) ¨295 ¨295
Pressure (kPa) ¨8300 ¨8300
Treat Gas Rate (NM3,/m3) ¨780 ¨780
Treat Gas Purity (vol %) ¨100 ¨100
LUSA/ (hr-1) ¨2.1 ¨2.1
Product ¨ Liquid Yield (vol %) Base +-O.93% Base
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 64 -
Example 4 ¨ Distillate Hydrotreating with Interstage Separation with Mixed
Metal
Catalyst,
1001641 A mixed metal catalyst formed from a suitable precursor can also be
used in
conjunction with in.terstage separation to achieve still larger increases in
distillate yield.
In this example, a process configuration similar to Example I was used, so
that a light
cycle oil feed could be processed with interstage separation. In this Example,
the initial
hydrotreatment stage included a conventional supported Ni.Mo catalyst to
produce a first
stage hydrotreated liquid product having the properties shown in Table 4. The
first stage
hydrotreated liquid product was then hydrotreated using either the mixed metal
catalyst
formed from a suitable precursor or the comparative bulk NiMoW catalyst made
according to the methods described in 'U.S. Patent 6,156,695, 'U.S. Patent
6,582,590
and/or U.S. Patent 6,929,738. The process conditions and resulting product
properties
are shown in Table 5.
Table 5 ¨ Second Stage Distillate Hydrotreating of Cracked Feed after
Separation
Total Liquid
Product after first Comparative Bulk Mixed Metal
stage Catalyst Catalyst
Conditions
I,HSV (bfI) -1 -1
Temp ( C) -300 -300
Treat Gas Rate ¨820 ¨820
(Nm3/m3)
H2 Pressure (kPa) ¨8300 ¨8300
Product Sulfur (wppm) ¨3940 ¨140 ¨25
Product Nitrogen ¨275 ¨0.3 <0.2
(wppm)
API Gravity -22.12 -27.20 -29.10
[001651 As shown in Table 5, at a similar level of conversion relative to a
conversion
temperature of 350 F (177 C), the mixed metal catalyst produced a liquid
product with a
yield about 1.9 vol% greater than the liquid product from hydrotreating with
the
comparative bulk catalyst. This is almost a doubling of the volume swell
benefit relative
to the processing of the cracked feed as shown in Table 4 of Example 3. Such a
volume
CA 02966880 2017-05-04
WO 2016/081217 PCT/US2015/059815
- 65 -
swell benefit is unexpectedly larger than the benefit that would be expected
based on
mere addition of the volume swell provided by the mixed metal catalyst and the
volume
swell provided by two stage hydrotreatment with interstage separation. This
shows that
the benefits of interstage separation can be synergistically combined with use
of a mixed
metal catalyst to provide an unexpectedly larger yield increase during
distillate
hydrotreating of a high sulfur distillate boiling range feed. This also
demonstrates that
the benefits of interstage separation can be realized for a variety of types
of hydrotreating
catalysts.
[00166] Although the present invention has been described in terms of
specific
embodiments, it is not so limited. Suitable alterations/modifications for
operation under
specific conditions should be apparent to those skilled in the art. it is
therefore intended
that the following claims be interpreted as covering all such
alterations/modifications as
fall within the true spirit/scope of the invention.