Note: Descriptions are shown in the official language in which they were submitted.
CA 2967846 2017-05-18
CENTRIFUGAL SYRINGE AND METHOD FOR BLOOD FRACTIONATION
BACKGROUND OF THE INVENTION
This invention relates to blood fractionation, devices used for blood
fractionation and components of such devices.
Blood platelets contain various growth factors and other cytokines that are
known to stimulate the healing of both bone and soft tissue. Accordingly,
there is an
inherent need for a device that allows for the concentration of such platelets
in a
blood fraction as well as the ability to remove and separate various blood
fractions
without cross contaminating a selected fraction with an immediate adjacent
fraction.
Since the Platelet Rich Plasma (PRP) process leading to a concentrated
source of generally autologous, platelets requires the fractionation of blood
into
basically three broad fractions: the most dense fraction of the whole blood
being
erythrocytes (red blood cells) at a volume of roughly 45%; the second most
dense
fraction being the buffy coat (leukocytes and platelets) at a volume of less
than 1%;
and the lightest density fraction being plasma at a volume of 55%, the use of
a
centrifuge is a preferred method of fractionation. The resulting fractions are
shown in
FIG. 15 which illustrates a column of blood together with an arrow indicating
the
direction of centrifugal force.
Once the whole blood is centrifuged into its component fractions, the most
critical issue then becomes: how does one classify, or otherwise separate,
fraction
from an adjacent fraction? As persons skilled in the art will be aware, the
sought after
fraction in the PRP process (e.g. the platelets fraction shown in FIG. 15) is
typically
comprised of less than 1% of the entire volume of whole blood. To loose some
of that
fraction to, or to have that fraction gain volume from, either the
erythrocytes fraction
shown in FIG. 15 or the plasma fraction shown in FIG. 15 through comingling,
is to
defeat the process of removing the highest level of platelet concentration
available.
Traditionally, when the need arises to extract and separate blood into
component parts, such as the segregation of plasma, platelets and
erythrocytes, the
- 1 -
CA 2967846 2017-05-18
blood is drawn from an individual into a blood bag which is then centrifuged
to stratify
the blood into differing fractions based on their bulk densities. From this
point, the
fractions are either extracted by inserting an object, such as a needle, into
the blood
bag whereby the fractions then can be withdrawn one at a time or the bag is
gently
compressed to force the blood out of an exit point in the bag with the
extraction
process stopped at the best achievable point between each transition from one
fraction to another.
Two major problems exist with the foregoing methodology.
1. Since the bag possesses pliable walls, and since the line of delineation
between the blood fractions upon centrifuge completion should remain sharp,
any
movement between the fractions or distortion in the bag wall may cause the
points of
delineation between fractions to blend and become comingled. When comingling
takes place, the need for accurate segregations, as is required when raw
platelets
are sought, is lost to mechanical disruption. Then, an attempt to segregate
can often
prove futile.
2. At any point of component blood transfer, whereby either an object is
inserted into a holding chamber for the purpose of extraction or an object is
fixed to
the outlet of the chamber, there exists the possibility of introducing a
contaminant or
pathogen into the blood reserve. For obvious reasons, blood that has been
tainted
with an external contaminant may have adverse consequences. Therefore, much
effort has been engaged in by medical science to both increase the quality and
delineation of segregated blood fractions while at the same time maintaining
sterile
controls.
In response to the problem of flexible containment as found with blood bags, a
number of patents exist wherein a rigid wall containment is used for
centrifuging the
blood fractionations. One of the more effective solutions to the comingling
problem
which occurs with blood segregations in a flexible walled containment is to
carry out
the centrifuge and extraction process within a rigid-walled chamber. The idea
is that
when blood is moved in a linear fashion in a containment with fixed and rigid
sides
then there will be considerably less turbulence taking place between blood
- 2 -
CA 2967846 2017-05-18
fractionations. Patent documents such as U.S. Pat. Appin. Pub. No.
2014/0054246
(Fojtik); U.S. Pat. Appin. Pub. No. 2010/0025342 - now U.S. Pat. No. 9,050,403
(Morimoto et al.); U.S. Pat. Appin. Pub. No. 2005/0261620 - now U.S. Pat. No.
7,195,606 (Bailin); U.S. Pat. Appin. Pub. No. 2004/0256331 (Arking et al.);
U.S. Pat.
Appin. Pub. No. 2004/0167004 - now U.S. Pat. No. 7,452,344 (Jorgensen et al.);
U.S. Pat. No. 7,976,796 (Smith et al.); U.S. Pat. No. 6,716,187 (also
Jorgensen et
al.); U.S. Pat. No. 5,577,513 (Van Vlasselaer); U.S. Pat. No. 4,492,634 (Villa-
Real);
U.S. Pat. No. 4,459,997 (Sarstedt); U.S. Pat. No. 4,020,831 (Adler); and U.S.
Pat.
No. 3,965,889 (Sachs), disclose the use of rigid wall containment. From the
point of
view of flexible verses rigid wall containment, these patent documents offer
an
improved option.
However, other limitations can be found in the prior art. For example, when
blood fractions are evacuated, discharged, or otherwise removed from a blood
chamber, one of two problems can arise. First, an inherent deficiency exists
because
the cross sectional area of the delineation between blood fractions is
relatively large
in relation to the length and volume of the chamber in which the fractions
have been
centrifuged. The larger the cross sectional area between, for example, the
erythrocytes fraction and the platelet fraction, the more difficult it is to
either draw off
or excrete one fraction from the other. In an attempt to reduce the cross
sectional
area between fractions, U.S. Pat. Appin. Pub. No. 2014/0371048 (Ra et al.)
discloses a narrowed hour glass shaped region between the erythrocytes
fraction
and the platelets fraction. The hour glass shaped region provides a
significantly
reduced cross sectional area between the two fractions and with a
significantly
reduced transition in which to segregate the fractions. Ra et al. further
disclose a
plunger/sealer device in which the blood fractions can be mechanically
separated.
Although the approach taken by Ra et al. can be seen as an advancement
over the prior art mentioned above (viz. in terms of creating a better
delineation
between blood fractions), it lacks in two significant areas:
- 3 -
CA 2967846 2017-05-18
1. it relies on an external extraction process which requires the blood to be
injected into a separation chamber from an intermediary device. This extra
step
creates an added risk of introducing contamination into the blood.
2. it relies on the use of an external device (presumably a needle) to extract
each of the blood fractions thus creating further possibilities of
contaminating the
blood.
Generally, there are two distinct negative issues present in prior art,
either:
1. an inability to evacuate, discharge, or otherwise remove one fraction from
another with a high degree of accuracy; or,
2. an inherent inability to maintain a low chance of external contaminant
introduction through a reduced number of mechanical transitions, such as a
plurality
of blood chambers, needle exchanges, needle penetrations, etc.
The present invention addresses such issues.
BRIEF SUMMARY OF THE INVENTION
The present invention relates to a multi-purpose syringe which possesses the
broad functionality to allow the extraction and containment of blood, the
centrifuging
of blood into various blood fractions while yet in the syringe, and then,
without using
external extraction methodologies, the discharge of such blood fractions into
finely
delineated segregations.
In accordance with the present invention there is provided a centrifugable
syringe for use in blood fractionation, the syringe comprising a substantially
transparent barrel, a substantially transparent, elongated delineation neck, a
plunger
for drawing blood into and expelling blood from the barrel, and an adapter
attached
to the neck for enabling releasable connection of a selected device over an
inlet/outlet opening of the neck.
The barrel has an axial bore defined by a bore wall and extends from a mouth
opening at a proximal end of the barrel to a distal end opening at a distal
end of the
- 4 -
CA 2967846 2017-05-18
barrel. Each of the openings has an associated cross sectional area, the cross
sectional area of the distal end opening being substantially less than the
cross
sectional area of the mouth opening.
The delineation neck extends away from the distal end of the barrel to a
distal
end of the neck and has an axial bore in fluid flow communication with the
axial bore
of the barrel. The axial bore of the neck extends lengthwise through the neck
to the
aforementioned inlet/outlet opening and has a cross sectional area
substantially the
same as the cross sectional area of the distal end opening of the barrel.
The plunger comprises a fluid sealing member for slidably bearing against the
bore wall of the barrel to prevent the flow of blood from the barrel through
the barrel
mouth and is advanceable and retractable within the axial bore of the barrel.
It
further comprises a handle releasably connect to the sealing member for
enabling
the advancement and retraction of the sealing member within the axial bore of
the
barrel. Preferably, the handle is releasably attachable to the syringe barrel.
The delineation neck may be formed integrally with the syringe barrel, or be
releasably attachable to the syringe barrel, and is an important feature of
the present
invention. Since the axial bore of the neck has a cross sectional area
substantially
less than that of the syringe barrel, a much finer delineation can be made
between
any two blood fractions within the neck as centrifuged blood is slowly
discharged
from the syringe through the inlet/outlet opening in the neck.
The selected device which is connectable over the inlet/outlet opening of the
delineation neck may be one of various devices, including:
- a capping device releasably engageable with the aforementioned adapter to
removably cap and seal the inlet/outlet opening in the neck. Such a device
would be used to contain blood within the syringe during centrifuge
operations;
- a needle device releasably engageable with the aforementioned adapter to
enable the withdrawal of blood from a subject into the syringe;
- 5 -
s CA 2967846 2017-05-18
,
- a hose device releasably engageable with the aforementioned adapter to
carry away blood expelled from the syringe.
The adapter and the selected device which is releasably engageable with the
adapter obviously require cooperating parts to enable suitable engagement.
Those
skilled in art will recognize that cooperative male and female luer fittings
may be
ideal for this purpose. However, they will also recognize that suitable
engagement
may be achieved by other (possibly less desirable) means.
Preferably, the fluid sealing member comprises a framework, a flexible seal
supported by the framework for slidably bearing against the bore wall of the
syringe
barrel, and an adapter supported by the framework for enabling releasable
connection of the plunger handle to the member.
Advantageously, the flexible seal comprises a flexible side wall for bearing
against the bore wall and a flexible conical face projecting from the side
wall forward
of the framework. The conical face serves to translate axial forces applied to
the face
to a lateral outward force on the bore wall.
To further advantage, the fluid sealing member further comprises a plurality
of
flexible locking tabs peripherally supported by the framework, the tabs for
slidably
bearing outwardly from the framework against the bore wall and for engaging a
perimetric locking groove in the bore wall to restrain egress of the sealing
member
through the mouth of the syringe barrel during centrifuge operations.
To still further advantage, the syringe may include a plunger handle lock for
engaging both the handle and a flange extending outwardly from the mouth
opening
of the barrel to hold the fluid sealing member at a selected position within
the barrel
during centrifuge operations. This is useful when it is desired to position
the fluid
sealing member at a location within the barrel where the locking tabs are
unable to
engage the locking groove.
In another aspect of the present invention, there is provided for use in
combination with a syringe barrel having an axial bore for holding blood, the
bore
itself having an associated cross sectional area measured transverse to the
bore:
- 6 -
CA 2967846 2017-05-18
a substantially transparent elongated delineation neck having an axial bore
extending therethrough for viewing centrifuged blood fractions following the
centrifuge of blood while carried within the bore of the barrel and the bore
of
the neck, the bore of the neck being in fluid flow communication with the bore
of the barrel during centrifuge operations and having an associated cross
sectional area measured transverse to the bore of the neck which is
substantially less than the cross sectional area of the bore of the barrel.
In yet another aspect of the present invention there is provided a method of
blood fractionation, comprising:
(a) providing a syringe described above
(b) releasably connecting a needle device to the delineation neck of the
syringe;
(c) drawing blood from a subject through a needle device and the
delineation neck into the syringe;
(d) disconnecting the needle device from the delineation neck;
(e) capping and sealing the inlet/outlet opening in the delineation neck
with
a removable capping device;
(f) centrifuging the blood within the syringe to separate the blood into
delineable blood fractions;
(g) removing the capping device;
(h) expelling one or more delineated blood fractions through the
inlet/outlet
opening from the syringe.
The foregoing method may further include the step of recentrifuging at least a
portion of the blood before all of the blood is expelled from the syringe.
Advantageously, this may sharpen the delineation between any two blood
fractions.
- 7 -
. CA 2967846 2017-05-18
The foregoing and other features and advantages of the present invention will
now be described with reference to the drawings in which:
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a perspective view of a centrifugable syringe in accordance with the
present invention.
FIG. 2 is a partially exploded side elevation view of the syringe shown in
FIG.
1.
FIG. 3 is a cross sectional view taken along section line 3-3 in FIG. 2.
FIG. 4 is a cross sectional view similar to that shown in FIG. 2 but instead
showing the components in an engaged position.
FIG. 5 is an enlarged exploded perspective view of the piston assembly which
appears in FIGS. 2-4 and which is hidden from view in FIG. 1.
FIG. 6 is an exploded side elevation view of the piston assembly.
FIG. 7 is a cross sectional view, partially cut away, and an accompanying
detail view of the piston assembly inside the syringe barrel with locking tabs
out of
engagement with a locking groove in the syringe barrel.
FIG. 8 shows views similar to FIG. 7 but with the piston assembly moved to a
position where the locking tabs are in engagement with the locking groove.
FIGS. 9 is a pictorial flow chart of steps made possible by characteristics of
the present invention.
FIG. 10 is a pictorial flow chart of additional steps which may be taken
between Steps 6 and 7 shown in FIG. 9 if a finer delineation is desired
between any
two blood fractions. FIG. 10 also illustrates the inclusion of a plunger
handle lock
which engages both a flange extending outwardly from the syringe barrel and
the
plunger handle.
- 8 -
. CA 2967846 2017-05-18
FIG. 11 is an enlarged side elevation view of the plunger handle lock shown in
FIG.10.
FIG. 12 is a top view illustrating engagement between the plunger handle lock
and the plunger.
FIG. 13 is a side elevation view of a modified syringe barrel and a
delineation
neck releasably attachable to the modified barrel.
FIG. 14 is a cross sectional view of the piston assembly showing the action
and translation of centrifugal forces acting on a flexible seal forming part
of the
assembly and bearing against the bore wall of the syringe barrel.
FIG. 15, as previously indicated, illustrates a column of blood together with
an
arrow indicating the direction of centrifugal force.
DETAILED DESCRIPTION OF THE INVENTION
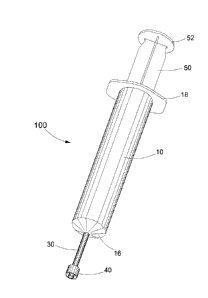
Referring now to the drawings, there is shown a centrifugable syringe
generally designated 100, the syringe comprising a substantially transparent
barrel
10, a substantially transparent, elongated delineation neck 30, an adapter 40
attached to neck 30, and a plunger formed by the combination of a handle 50
and a
fluid sealing member or piston assembly generally designated 70.
As depicted, barrel 10 and neck 30 are generally cylindrical. Other
components are routinely designed to fit with the cylindrical configuration in
a
cooperative manner. However, while a cylindrical configuration is preferred,
it is not
considered essential.
Substantial transparency means transparency which is sufficient to enable
blood and any blood fractions which result after the blood is centrifuged to
be viewed
through the wall of barrel 10 and/or neck 30, as the case may be.
Barrel 10 has an axial bore 11 defined by bore wall 12 and extends from a
mouth opening 14 best seen in FIG. 3 to a distal end opening 15 at distal end
16 of
the barrel. Barrel 10 also includes a flange 18 extending outwardly from
opening 15.
- 9 -
T. CA 2967846 2017-05-18
This flange has a conventional shape characteristic of many medical syringes
and is
designed to be easily gripped with fingers. As best seen in FIGS. 7 and 8,
barrel 10
also includes a locking groove 85, the purpose of which is described below in
more
detail.
As will be apparent from FIGS. 3 and 4, the cross sectional area of distal end
opening 16 is substantially less than that of mouth opening 14. In the
cylindrical
embodiment shown, an example would be where mouth opening 14 has a diameter
of about 25 mm. defining an area of about 491 mm2, and where distal end
opening
16 has a diameter of about 3 mm. defining an area of about 7 mm2.
Delineation neck 30 extends away from distal end 16 of the barrel to its own
distal end 32 with an inlet/outlet opening 36. It has an axial bore 34 in
fluid flow
communication with axial bore 11 of the barrel and extends lengthwise through
the
neck to inlet/outlet opening 32. As will be apparent from FIGS. 3 and 4, bore
34 has
a cross sectional area which is substantially the same as the cross sectional
area of
distal end opening 15 of the barrel. Neck 30 not only provides a narrowed down
cross sectional area compared to that of barrel 10, but also allows viewing of
the
delineation between blood fractions as blood is being expelled from the
syringe
through opening 32.
Adapter 40 is attached to neck 30 proximate inlet/outlet opening 32,
preferably in an integral manner, and enables the releasable connection of a
selected device over the opening. The selected device, which should not be
considered as part of the present invention, may be one of various devices
including:
a needle device generally designated 500 comprising a hypodermic needle
502 and fitting 504 as shown in FIG. 9;
a capping device generally designated 600 comprising a cap 602 and fitting
604 as shown in FIGS. 9; or,
a hose device generally designated 700 comprising a hose 702 and fitting 704
as shown in FIG. 9.
- 10-
r CA 2967846 2017-05-18
,
In the present embodiment, adapter 40 and distal end 32 of the neck together
provide a luer male fitting. Fittings 504, 604 and 704 each provide a
cooperative luer
female fitting. Such fittings are well known to those skilled in the art and
are not
described or shown here in any more detail.
Handle 50 extends from a head 52 to an internally threaded connector 55
which is releasably connectable to externally threaded connector 80 forming
part of
piston assembly 70. As best seen in FIGS. 5 and 6, piston assembly 70
comprises
two parts - a flexible rubber seal 75 and a plastic framework 72. The
framework is
integrally formed and includes a retainer ring 74 which permits snap fitting
engagement of the seal with the framework. Connector 80 is integrally formed
with
the framework. The seal includes a flexible side wall 76 for bearing against
bore wall
12 (see FIGS. 3, 4) and a flexible conical face 77 projecting forward of the
framework. As indicated by the right facing horizontal arrows and diagonal
arrows in
FIG. 14, axial forces bearing on the seal are translated by the conical face
and side
wall to a lateral outward force on the bore wall. As pressure is increased
against the
face, it detents slightly by a distance "d" which in turn redirects the force
down the
plane of the face, and inherently produces a lateral outward force against the
wall.
The net effect is to increase sealing pressure and to produce a stronger seal
between the piston assembly and the bore wall.
Piston assembly 70 also includes a plurality (4) of flexible locking tabs 82
which are integrally formed with and thereby supported by the framework. In
use the
tabs slidably bear outwardly from the framework against bore wall 12 and, as
best
seen in FIGS. 7 and 8, are designed to engage aforementioned locking groove 85
in
the bore wall as the piston assembly reaches mouth opening 14 of barrel 10.
Such
engagement serves to restrain egress of the piston assembly through the mouth
opening during centrifuge operations.
At times, and as is discussed below, it is desirable during centrifuge
operations to restrain movement of piston assembly 70 within barrel 10 at a
distance
away from mouth opening 14.
- 11 -
, CA 2967846 2017-05-18
Referring now to FIG. 9 there are shown several typical steps involved when
syringe 100 is being used as an apparatus for blood fractionation.
Step 1 demonstrates the need to attach needle device 500 to adapter 40 of
the syringe.
Step 2 demonstrates whole blood within the syringe after extraction from a
subject.
Step 3 demonstrates a usual preference or need to remove the needle device
and replace it with capping device 600. In addition, it is at this step,
preliminary to
centrifuge operations, that the plunger handle is unthreaded, or otherwise
removed.
Step 4 with arrows indicating alternative directions of centrifuge forces
demonstrates the location of syringe 100 in a centrifuge (not shown) where the
whole blood is spun into density gradients of blood, primarily three
fractions, that of
red blood cells, plasma and a buffy coat (leukocytes and platelets). Because
of the
ability of this invention to stop the piston assembly from exiting the barrel,
the blood
can be fractionated such that upon centrifuging, the red blood cells can be
concentrated at either the end of the barrel or the piston assembly position,
depending on the direction of centrifugal force chosen, thus allowing the
expulsion of
red blood cells either first in the process or last.
Step 5 demonstrates reattachment of the plunger handle in preparation for the
process of expelling the blood from syringe 100 through hose device 700. This
particular example shows the red blood cells centrifuged towards the piston
assembly thus they will be expelled last.
Step 6 demonstrates the first step in the blood expulsion process, that of
expelling the plasma and then the leukocytes and platelets. Expulsion of each
fraction takes place until such time as the delineation between fractions is
visually
observed to be present in the delineation neck. Note: If co-mingling between
any two
fractions has taken place, for example, if the delineation between red blood
cells and
the buffy coat (leukocytes and platelets) is blurred by the two fractions
having
- 12 -
. CA 2967846 2017-05-18
blended, the option is available to take additional steps as is discussed
below with
reference to FIG. 10.
Step 7 demonstrates the expulsion of the last fraction with this particular
example being that of reinjecting the red blood cells into the subject from
whom they
were removed at first instance.
Referring now to FIGS. 10, 11 and 12, there is shown an added component
(plunger handle lock 90) which may be used with and which forms part of the
present
invention for the purpose of achieving a finer delineation between any two
blood
fractions.
Step 6a in FIG. 10 demonstrates the situation where the line of delineation D1
between any two blood fractions, as viewed in the delineation neck and as best
seen
in the expanded view of the transition point, is co-mingled or otherwise
blended
between the two adjoining blood fractions. The delineation may be considered
as
fuzzy. Plunger lock 90 has been placed to engage both the syringe barrel and
the
plunger handle such that that the piston assembly cannot move from it locked
position during centrifuge operation.
As best seen in FIGS. 11 and 12, plunger lock 90 slips over barrel flange 18
and includes a set screw 92 threadable through flange 94 to engage a face 58
of
handle 50 thereby locking the handle and the connected piston assembly in
position.
Step 6b demonstrates the re-centrifuge of the blood sample shown in Step 6a
with the piston assembly restrained in its locked position. As indicated by
the
expanded view in the illustration, re-centrifuging with the direction of
centrifugal
forces indicated by arrows may result in a significantly sharper line of
delineation 02
between blood fractions.
As is shown in various ones of the drawings, barrel 10 and delineation neck
of syringe 100 are integrally formed. However, this is not essential. By way
of
example, FIG. 13 illustrates a centrifugal syringe 100" wherein a delineation
neck
30", now including an externally threaded coupler 39 at its proximal end, is
- 13 -
CA 2967846 2017-05-18
releasably attachable to an internally threaded coupler 19 at the distal end
of the
syringe barrel.
Referring now to FIG. 15, there is shown is a side view of a typical vile
showing the, to-scale, fraction of erythrocytes at approximately 45%,
platelets/leukocytes at less than 1`)/0 and plasma at approximately 55% of
total blood
volume. It is to demonstrate the complexity of attempting to isolate, or
otherwise,
segregate the very narrow band of platelets/leukocytes from the significantly
higher
volume of its adjacent erythrocytes and plasma without losing to or taking
away from
either of the two said adjacent blood components.
In operation, the present invention typically may be used as follows as
illustrated by FIG. 9:
Step 1. A disposable syringe is removed from its sterile packaging with a
hypodermic needle for the purpose of venipuncture, or otherwise the removal of
blood from a patient by way of inserting the hypodermic needle into a vein and
extracting blood. If needed, a blood anticoagulant may be added prior to
venipuncture as a means of keeping the blood from clotting through potentially
multiple centrifuging of the same sample.
Step 2. A full syringe of blood is extracted to the level of the piston
assembly
engages its stop mechanism within the barrel.
Step 3. Two sub-steps take place within Step 3. The needle is removed from
the syringe and replaced with a sterile cap and the plunger handle is
unthreaded, or
otherwise removed from the piston assembly. The purpose of removing the
plunger
handle is to allow for reduced spin radius within a centrifuge.
Step 4. The full syringe is placed into a centrifuge carriage and pointed in
either of two directions. The syringe chamber can be installed whereby the
centrifugal force exerted on the blood, drives the densest material, that of
red blood
cells, towards the plunger piston assembly or the other way around towards the
discharge/needle end of the syringe. The determining point as to which way the
- 14-
CA 2967846 2017-05-18
,
syringe is to be positioned into the centrifuge has to do with what blood
fraction is to
be expelled through the tip first. In the event that the red blood cells are
needing to
be expelled first, the syringe would be inserted with the capped needle-end
facing
away from the center of centrifuge rotation thus placing the most dense
fractionation,
red blood cells, at the needle end and consequently to be expelled first upon
pushing
the plunger into the barrel.
Step 5. Again, two sub-steps take place within Step 5. The plunger handle is
reattached to the piston assembly to allow for the expelling of the blood
fractions
from the barrel. The blood can then be expelled into either a patient by
attaching
another hypodermic needle or other holding chamber, such as a blood bag, thus
requiring the attachment of a sterile blood transport tube.
Step 6. The blood fractions are now expelled from the syringe using an inward
force, most commonly thumb pressure, on the plunger handle. The point at which
to
stop expelling between blood fractionations has to do with expelling a
particular
fraction, (plasma first, as illustrated in Step 6 of FIG. 9) until such time
as its
delineation point between the two fractions enters the elongated neck whereby
the
much reduced cross sectional area of the neck allows for a much more specific
dividing line between any two fractions. Once the plasma fraction has been
expelled, and/or retained with the platelets, the much less volume of
platelets can
now be expelled until such time as the red blood cells show up in the
elongated neck
and a clear delineation can be seen between the two strata. In the event that
an
even finer line of delineation between either platelets and red blood cells or
platelets
and plasma needs to take place, the syringe can be centrifuged again so that a
finer
line between one fraction and the platelets can be seen in the transparent
neck of
the syringe. This optional auxiliary step, as illustrated in FIG. 10, requires
a retainer
or plunger lock to hold the piston assembly in place through the process of re-
centrifuging.
Step 7. As illustrated in Steps 6 and 7 of FIG. 9, with the option of the red
blood cells being centrifuged to the piston end of the syringe, and thus last
to be
expelled, a change of the attachment to the adapter at the end of the
delineation
- 15 -
CA 2967846 2017-05-18
,
neck may or may not need to take place at this point depending on the ultimate
location of such cells. If the remaining blood, red cells, is needing to be re-
injected
into a patient, then another sterile needle would need to be attached and
those cells
re-introduced into the recipient from which they may or may not have
originated.
As a concluding step, the syringe, needles and applicable hose will be
disposed of under standard medical practice and protocol.
The scope of the claims should not be limited by the specific embodiments
illustrated in the drawings, but should be given the broadest interpretation
consistent
with the description as a whole.
- 1 6 -