Note: Descriptions are shown in the official language in which they were submitted.
METHODS FOR THE DIRECT ELECTROLYTIC PRODUCTION OF STABLE, HIGH CONCENTRATION
AQUEOUS HALOSULFAMATE OR HALOSULFONAMIDE SOLUTIONS
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims priority to and the benefit of filing of U.S.
Provisional Patent Application
Serial No. 62/089,770, entitled "Methods for the Direct Electrolytic
Production of Stable, High
Concentration Aqueous Halosulfamate or Halosulfonamide Solutions", filed on
December 9, 2014.
BACKGROUND OF THE INVENTION
Field Of The Invention (Technical Field)
The present invention is related to the in situ production of high
concentration stable aqueous
biocidal solutions comprised of mixtures of free halogens, N-halosulfamic acid
compounds, N-
halosulfamate compounds, N,N-dihalosulfamic acid compounds and N,N-
dihalosulfamate compounds,
with or without additional biocidally active components, in any desired
compositional mixture and at a
desired pH through an electrolysis process, where the nature of the biocidal
solution is determined by the
nature of the brine used in the electrolysis process as well as the
operational parameters of the
electrolytic process.
Background Art
Note that the following discussion may refer to a number of publications and
references.
Discussion of such publications herein is given for more complete background
of the scientific principles
and is not to be construed as an admission that such publications are prior
art for patentability
determination purposes.
Aqueous free halogen species such as chlorine, bromine, iodine, hypochlorous
acid,
hypobromous acid, hypoiodous acid, hypochlorite, hypobromite, and hypoiodite
are known to be very
powerful anti-microbial agents and are often used in water disinfection
applications. Along with a high
1
Date recue / Date received 2021-12-14
CA 02968405 2017-05-18
WO 2016/094591 PCT/US2015/064846
level of biocidal activity, aqueous free halogens are also highly chemically
reactive to other species often
present in waters undergoing treatment. These species include natural organic
matter, synthetic and
natural organic chemicals, iron, manganese, hydrogen sulfide, ammonia,
arsenic, and other chemicals.
The presence of these free halogen demanding substances consume free halogens
added to the water
that would otherwise be useful in inactivating microorganisms, thus, this
process could be considered
detrimental to the overall treatment process if the free halogen consuming
chemical reaction is not
desirable. Reactions between aqueous free halogens and organic material
present in the water being
treated can also, in some cases, lead to the undesirable products such as the
formation of halogenated
organic chemicals such as trihalomethanes and haloacetic acids. Therefore, the
treatment of waters
containing high amounts of these halogen-reactive compounds is often
advantageously accomplished
through the use of biocides that are less chemically reactive than aqueous
free halogen species.
Haloamines, which can broadly be considered as chemical species which contain
at least one
nitrogen-halogen bond, are often used in place of oxidizing free halogens to
provide a disinfectant for
water treatment. While haloamines are effective biocidal compounds, they are
also much less chemically
reactive as compared to free halogens. Thus, in waters containing substantial
amounts of free halogen
demanding substances, haloamines have the potential to provide a substantial
benefit to the overall water
disinfection process. Aqueous solutions of haloamines are typically produced
through a chemical reaction
between an aqueous free halogen species and a compound containing at least one
nitrogen-hydrogen
bond whereby the chemical reaction produces a compound containing at least one
nitrogen-halogen
.. bond. Ammonia or ammonium ions are the most common source of the nitrogen
containing compound
used in this process, although other nitrogen containing compounds are often
utilized as well.
One major deterrent to the use of haloamines, and especially haloamines
produced through the
reaction of ammonia or ammonium ions with an aqueous free halogen species, is
that they are highly
unstable, especially at higher concentrations (i.e. greater than 10 mg/L); see
for example Audrieth, L. F.;
Rowe, R. A. The Stability of Aqueous Chloramine Solutions" J. Am. Chem. Soc.
1955, 77, 4726-4728.
Due to the instability of haloamines, it is typically not possible to produce
concentrated haloamine
solutions and ship them to a point of application. As a result, haloamines
derived from ammonia or
ammonium ions are often produced in situ during treatment by the action of
aqueous free halogens on
ammonia or organic amines naturally present or added to water being treated.
Alternatively, it is
-2-
CA 02968405 2017-05-18
WO 2016/094591 PCT/US2015/064846
sometimes desirable to prepare concentrated aqueous haloamine solutions
derived from the reaction
between ammonia or ammonium ions at the point of application and then use
these formed haloamine
solutions as a primary disinfectant. Situations where this could be
advantageous include the treatment of
waters which have a known or highly variable free halogen demand which would
consume free halogens
without the benefit of microbial inactivation but which can be effectively
treated with a haloamine
disinfectant.
Sulfamic acid and its organic compound derivatives, known as sulfonamides (for
example,
toluenesulfonamide), are chemically distinct from ammonia or other organic
amine-containing compounds
used to produce haloamines but, like ammonia or many amines, typically contain
a nitrogen-hydrogend
bond which can react with aqueous halogen species. Products of the reaction
between sulfamic acid,
having the chemical formula H3NS03, and an aqueous halogen species are N-
halosulfamate compounds
(either N-halosulfamate, having the chemical formula HNS03X-, or N-
halosulfamic acid, having the
chemical formula H2NS03X), N,N-dihalosulfamate compounds (N,N-dihalosulfamic
acid, having the
chemical formula HNS03X2, or N,N-dihalosulfamate, having the chemical formula
NS03X2-), or
combinations thereof, where, in all cases, the letter X represents a halogen
(i.e. Cl, Br, l). Products of the
reaction between and organic sulfonamide compounds, having the chemical
formula of RSO2NH2, and an
aqueous halogen species are N-halosulfanomide compounds, having the chemical
formula of RSO2NHX,
N,N-dihalosulfonamide compounds, having the chemical formula of RSO2NX2, or
combinations thereof,
where, in all cases, the letter R represents and organic functional group
comprising at least carbon and
hydrogen and the letter X represents a halogen (i.e. Cl, Br, l). These
compounds can stabilize the
electrolyzed halogen species.
U.S. Patent No. 3,776,825 to Vit entitled "Electrolytic Treatment" discloses
that a brine comprising
a halide ion source and an amine compound both dissolved in water is first pH
adjusted through the
addition of a base such as sodium hydroxide, and then electrolyzed, thereby
producing a solution
containing one or more haloamine compound, including N-chlorosulfamates or N-
chlorosulfonamides
produced from sulfamic acid or organic sulfonamides, respectively. Vit further
teaches that when
electrolysis is used to make these haloamine solutions they are unstable, and
therefore the invention
descried by Vit is focused entirely on the instantaneous production and
immediate subsequent use of the
produced haloamines as opposed to the production of stable haloamine
solutions. Because Vit discloses
-3-
CA 02968405 2017-05-18
WO 2016/094591 PCT/US2015/064846
the addition of NaOH to the brine prior to electrolysis, and discloses that
the pH of the solution is thus
greater than 12, the solution prior to electrolysis no longer comprises
sulfamic acid, but instead comprises
sulfamate ions.
SUMMARY OF THE INVENTION (DISCLOSURE OF THE INVENTION)
The present invention is a method for producing a stable disinfecting solution
comprising a
plurality of halosulfamate and/or halosulfamic acid species and one or more
halogen species, the method
comprising preparing an acidic solution comprising desired concentrations of
halide ions and sulfamic
acid and electrolyzing the solution, wherein the concentrations of the halide
ions and the sulfamic acid in
the acidic solution and the pH of the acidic solution are chosen to produce
the desired concentrations of
the halosulfamate species and/or halosulfamic acid species in the stable
disinfecting solution. The acidic
solution optionally further comprises one or more additional halogen
stabilizing compounds. The one or
more additional halogen stabilizing compounds are preferably selected from the
group consisting of
lithium sulfamate, sodium sulfamate, potassium sulfamate, organic sulfonamide,
methylsulfonamide, o-
toluenesulfonamide, m-toluenesulfonamide, p-toluenesulfonamide, cyanuric acid,
a derivative of cyanuric
acid, succinimide, a derivative of succinimide, hydantoin, a derivative of
hydantoin, and combinations
thereof. The stable disinfecting solution can then optionally comprise N-
halosulfonamide compounds
and/or N,N-dihalosulfonamide compounds. The halosulfamate species preferably
comprise N-
halosulfamate compounds and/or N,N-dihalosulfamate compounds, and the
halosulfamic acid species
preferably comprise N-halosulfamic acid compounds and/or N,N-dihalosulfamic
acid compounds. The
stable disinfecting solution is preferably a high concentration solution. The
acidic solution preferably does
not comprise a non-amine base.
The preparing step optionally comprises providing a salt blend comprising
sulfamic acid and one
or more salts comprising the halide ions and diluting the salt blend with
water. The salt blend preferably
comprises a pellet, briquette, or compacted form, preferably comprises a solid
solution, and optionally
comprises an anti-caking agent. The method preferably further comprises
flowing the acidic solution
through an electrolytic cell at a flow rate selected to produce the desired pH
of the stable disinfecting
solution. The flow rate can optionally be increased, thereby maintaining the
acidity of electrolyte in the
vicinity of cathodes in the electrolytic cell during the electrolyzing step,
which then preferably results in
-4-
CA 02968405 2017-05-18
WO 2016/094591 PCT/US2015/064846
removing scale from surfaces of the cathodes or preventing the formation of
scale on the surfaces of the
cathodes. The pH of the acidic solution is preferably less than 4, and more
preferably less than 1. The
pH of the stable disinfecting solution is optionally greater than 9, or
optionally greater than 11, or
optionally less than 3.
Objects, advantages and novel features, and further scope of applicability of
the present invention
will be set forth in part in the detailed description to follow, taken in
conjunction with the accompanying
drawings, and in part will become apparent to those skilled in the art upon
examination of the following, or
may be learned by practice of the invention. The objects and advantages of the
invention may be realized
and attained by means of the instrumentalities and combinations particularly
pointed out in the appended
claims.
BRIEF DESCRIPTION OF THE DRAWINGS
The accompanying drawings, which are incorporated into and form a part of the
specification,
illustrate several embodiments of the present invention and, together with the
description, serve to explain
the principles of the invention. The drawings are only for the purpose of
illustrating certain embodiments
of the invention and are not to be construed as limiting the invention. In the
drawings:
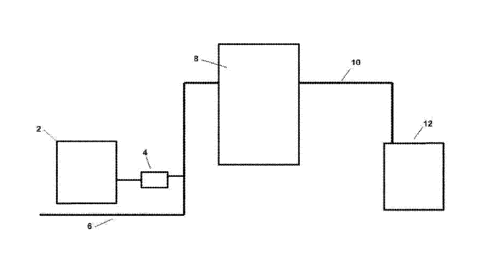
FIG. 1 is a schematic drawing of a system for the production of stable,
concentrated aqueous
solutions comprised of free halogen species, N-halosulfamate compounds, N,N-
dihalosulfamate
compounds, N-halosulfonamide compounds, N,N-dihalosulfonamide compounds from a
single, highly
concentrated brine source that is diluted into a process water.
FIG. 2 is a schematic drawing of a system for the production of stable,
concentrated aqueous
solutions comprised of free halogen species, N-halosulfamate compounds, N,N-
dihalosulfamate
compounds, N-halosulfonamide compounds, N,N-dihalosulfonamide compounds from a
dual, highly
concentrated brine sources that are diluted into a process water.
FIG. 3 is a schematic drawing of a system for the production of stable,
concentrated aqueous
solutions comprised of free halogen species, N-halosulfamate compounds, N,N-
dihalosulfamate
compounds, N-halosulfonamide compounds, N,N-dihalosulfonamide compounds from a
single source
brine.
-5-
CA 02968405 2017-05-18
WO 2016/094591 PCT/US2015/064846
DETAILED DESCRIPTION OF EMBODIMENTS OF THE INVENTION
Embodiments of the present invention are simplified electrochemically-driven
processes whereby
controlled formation and electrolysis of specifically designed aqueous brine
blends comprising metal
halide salts combined with at least sulfamic acid either alone or in
combination with other halogen
stabilizing compounds in a single electrolysis step are used to produce
stable, high concentration
aqueous solutions comprised of at least halogens, N-halosulfamate compounds,
N,N-dihalosulfamate
compounds, or other stabilized compounds, in any desired compositional blend.
Production of these
solutions is preferably achieved through precise control of the composition of
the brines, used in the
electrolysis process as well as electrolytic conditions used to electrolyze
the brines.
As used throughout the specification and claims, the term "high concentration"
means a solution
with a total halogen content of at least 2100 mg/L. Total halogen content
includes free halogen species,
N-halosulfamate compounds, and N,N-dihalosulfamate compounds. As used
throughout the specification
and claims, the term "stable" means a solution with less than a 5% loss in
total halogen content over a 24
hour time period. As used throughout the specification and claims, the term
"non-amine base" means any
basic compound that does not comprise an amine, such as sodium hydroxide,
potassium hydroxide,
sodium bicarbonate, or calcium carbonate. The present invention can be used to
provide halogen-based
disinfectant solutions wherever such solutions can be useful for
microbiological control, and will be
especially useful in the disinfection of highly challenging waters such as
those found in oil and gas
production processes, industrial cooling systems, pulp and paper production
facilities, and food and
beverage production processes. Embodiments of the present invention are
directed to electrolysis
processes of brines comprising mixtures of sodium chloride, sodium bromide,
and sulfamic acid, although
other metal halide sources can be used in place of sodium chloride or sodium
bromide, and other halogen
stabilizing compounds can be used instead of or in addition to sulfamic acid.
Metal halide brines subjected to electrolysis undergo several electrolytic and
chemical
transformations, starting with the electrolytic oxidation of halide ions to
produce halogens:
2 X- - 2e- ¨> X2
where X- is the halide ion and X2 is the diatomic, molecular halogen. Here, X-
can be Cl-, Br-, I-, or any
combination thereof while X2 can be C12, Br2, 12, BrCI, BrI, ICI, or any
combination thereof. Once the X2
-6-
CA 02968405 2017-05-18
WO 2016/094591 PCT/US2015/064846
halogen species is formed by the electrolysis process, it will react with
water also present in the brine to
produce a combination of acids:
X2 + H210 HX + HOX
where H20 is water, HX is a hydrohalic acid, and HOX is a hypohalous acid.
Here, HX can be HCI, HBr,
HI, or any combination thereof while HOX can be HOCI, HOBr, HOI, or any
combination thereof.
Depending on the pH of the brine during electrolysis and/or the pH of the
electrolyzed solution, the
hypohalous acid component of the electrolyzed solution can disassociate to
yield hypohalite ions
according to:
HOX H+ + X0-
where X0- is the hypohalite ion, such as 010-, Br0-, 10-, or any combination
thereof, depending on the
halide ion content of the brine. The relative amount of HOX and X0- present in
the brine during
electrolysis and the electrolyzed solution varies according to the acid
disassociation constants of the
various hypohalous acids. In this process, X2, HOX, and X0- are all considered
to be free halogen
species.
In the presence of sulfamic acid, these chlorine species will participate in a
sequence of chemical
reactions that will yield N-chlorosulfamic acid, N-chlorosulfamate, N,N-
dichlorosulfamic acid, and N,N-
dichlorosulfamate. An example of this is the reaction between hypochlorous
acid and sulfamic acid:
HOCI + H2NSO3H CIHNSO3H + H20
HOCI + CIHNS03H Cl2NS03H + H20.
In other words, when sulfamic acid, having a chemical formula of H3NS03, is
present in the brine during
electrolysis, it will react with the free halogen species present in the
electrolyzed brine to produce N-
halosulfamic acids, having chemical formulas of H2NS03X, N-halosulfamates,
having chemical formulas
of HNS03X-, N,N-dihalosulfamic acids, having chemical formulas of HNS03X2, and
N,N-dihalosulfamates,
having chemical formulas of NS03X2- where, in all cases, X represents halogens
such as chlorine (Cl),
bromine (Br), and/or iodine (I). Depending on the composition of the brine
used in the present invention,
the biocidal composition of the electrolyzed solutions can contain chlorine
(012), bromine (Br2), iodine (12),
bromine monochloride (BrCI), bromine monoiodide (BrI), iodine monochloride
(101) hypochlorous acid
(HOCI), hypobromous acid (HOBr), hypoiodous acid (H01), hypochlorite (C10-),
hypobromite (Br0-),
hypoiodite (10), N-chlorosulfamic acid (H2NS0301), N-bromosulfamic acid
(H2NSO3Br), N-iodosulfamic
-7-
CA 02968405 2017-05-18
WO 2016/094591 PCT/US2015/064846
acid (H2NS031), N-chlorosulfamate (HNS03C1-), N-bromosulfamate (HNSO3B(), N-
iodosulfamate
(HNS031-), N,N-dichlorosulfamic acid (HNSO3C12), N,N-dibromosulfamic acid
(HNSO3Br2), N,N-
diiodosulfamic acid (HNS0312), N-bromo-N-chlorosulfamic acid (HNSO3BrCI), N-
bromo-N-idodsulfamic
acid (HNSO3BrI), N-chloro-N-iodosulfamic acid (HNSO3C11), N,N-
dichlorosulfamate (NS03C12-), N,N-
dibromosulfamate (NSO3Br2), N,N-diiodosulfamate (NS0312), N-bromo-N-
chlorosulfamate (NSO3BrCI-),
N-bromo-N-iodosulfamate (NSO3Br1-), N-chloro-N-iodosulfamate (NSO3C11-), or
any combination of these
components thereof.
The relative composition of free halogens, N-halosulfamic acids, N-
halosulfamates, N,N-
dihalosulfamic acids, and N,N-dihalosulfamates present in the electrolyzed
solution will typically be
determined by factors including, but not limited to, the relative composition
of halide ions and sulfamic
acid in the brine, and the pH of the electrolyzed solution. For example, the
ratio of halide ion (and
therefore eventual halogen content after electrolysis) to sulfamic acid in the
brine will impact the relative
distribution of free halogen, monohalogenated sulfamate species (N-
halosulfamic acid and N-
halosulfamate), and dihalogenated sulfamate species (N,N-dihalosulfamic acid
and N,N-dihalosulfamate).
In brines where there is an excess of sulfamic acid relative to the halide
ion, the primary initial product will
be monohalogenated sulfamate species; conversely, when the halide ion is in
large excess of the
sulfamic acid, the primary initial product of electrolysis will primarily be
free halogen and dihalogenated
sulfamate species. Similarly, the electrolyzed solution's pH can dictate the
relative concentration of
halosulfamic acids versus halosulfamate species, typically with higher
halosulfamic acid composition at
lower electrolyzed solution pH. Therefore, by varying the pH of brine prior to
electrolysis, for example by
varying the relative concentration of sulfamic acid and halides, the
composition of the electrolyzed
solution can be tailored as desired.
Organic sulfonamide, having a chemical formula of RSO2NH2 where R indicates
the presence of
an organic functional group such as, but not limited to, o-tolyl, m-tolyl, or
p-tolyl, will behave in a similar
fashion to sulfamic acid, in that N-halosulfonamide compounds having a
chemical formula of RSO2NHX,
and N,N-dihalosulfonamide compounds having a chemical formula of RSO2NX2 and
where X in both
formulations represents a halogen, can be produced through this process.
Similarly, mixtures of sulfamic
acid and various organic sulfonamides can be used to produce mixtures of
halosulfamate compounds
and halosulfonamide compounds along with free halogen species of any desired
composition. As with the
-8-
CA 02968405 2017-05-18
WO 2016/094591 PCT/US2015/064846
above process, control over the composition of these complex solutions is
preferably achieved by
controlling the composition of the mixed brines used in the electrolysis
process.
Alternatively, this process can utilize other halogen stabilizing chemical
compounds besides or in
addition to sulfamic acid and its derivatives. Typically, these chemicals
comprise a molecule containing at
least one nitrogen atom wherein the at least one nitrogen atom has a chemical
bond to at least one
hydrogen atom and the same nitrogen also has at least one chemical bond to an
atom other than
nitrogen, hydrogen, or a halogen. Examples of halogen stabilizing compounds
that can be used for these
purposes include, but are not limited to, cyanuric acid and its derivatives,
succinimide and its derivatives,
and hydantoin and its derivatives. Another example of a halogen stabilizing
compound, phosphoramidic
acid (a chemical having a formula of H4NP03), can be used in place of or in
addition to sulfamic acid in
the practice of the present invention. Electrolysis of solutions containing at
least phosphoramidic acid and
at least one metal halide ion as described by the practice of the present
invention can result in the
production of free halogens, N-halophosphoramidic acid compounds (having a
chemical formula of
H3NP03X), N-halophosphoramidate compounds (having a chemical formula of
H2NP03X- or HNP03X2-
),N,N-dihalophosphoramidic acid compounds (having a chemical formula of
H2NP03X2), N,N-
dihalophosphoramidate compounds (having a chemical formula of HNP03X2- or
NP03X22-), or
combinations thereof.
In the practice of the present invention, electrolysis is accomplished using
an electrolytic cell
comprising at least one cathode and at least one anode, although some
embodiments of the present
invention will also include several intermediate electrode plates to form a
bipolar cell. Electrodes can be
of any suitable material, but preferably Dimensionally Stable Anodes (which
can be used as both the
anode and cathode) are used in the present invention. Voltage applied to the
electrolytic cell is preferably
approximately 6V.
In the embodiment of the present invention shown in FIG. 1, tank 2 is a brine
generator that is
charged with a blended salt and water from a source (not shown). Here, the
brine generator and a
blended salt are used to produce a brine with continuous, near uniform
composition which is transferred
using pump 4 into line 6, which preferably also contains water. The diluted
mixed brine is then transferred
into generation system 8, which comprises an electrolytic cell along with a
plurality of sensors and
controls which monitor and alter the electrochemical process as needed to
provide the desired product.
-9-
CA 02968405 2017-05-18
WO 2016/094591 PCT/US2015/064846
The electrolyzed brine solution is then transferred through line 10 into tank
12, where the solution is
stored until it is transferred to the application point.
Salt blends used in this embodiment of the present invention may include one
or more metal
halide compounds and at least sulfamic acid, but may also or alternatively
include an organic sulfonamide
or any other halogen stabilizing compound in any desired combination. Metal
halide compounds useful
here include, but are not limited to, sodium chloride, potassium chloride,
sodium bromide, potassium
bromide, sodium iodide, and potassium iodide. In addition to sulfamic acid,
the organic sulfonamide
component or halogen stabilizing component of the salt blend used in the
present invention can include,
but is not limited to, methylsulfonamide, o- toluenesulfonamide, m-
toluenesulfonamide, p-
.. toluenesulfonamide, cyanuric acid and its derivatives, succinimide and its
derivatives, and hydantoin and
its derivatives or any combination of these compounds. The ratio of metal
halides to halogen stabilizing
compounds in the blended salt used to prepare the brines is preferably
carefully controlled in order to
produce the desired aqueous solution comprising free halogen species, N-
halosulfamate compounds,
N,N-dihalosulfamate compounds, N-halosulfonamide compounds, N,N-
dihalosulfonamide compounds,
and any combination thereof in a highly stable, concentrated form.
Control over the brine content is preferably achieved by producing the salt
blend as a pellet,
briquette, or other compacted form, such that the individual components of the
salt pellet or briquette are
introduced into the brine at a predictable rate. Here, the salt blend
preferably comprises at least one
halide-containing salt combined with sulfamic acid and/or other halogen
stabilizing agents as desired. The
halide containing salt is preferably sodium chloride, although other halide
containing salts can be used
alone or in combination with sodium chloride. These salts include, but are not
limited to, lithium chloride,
potassium chloride, lithium bromide, sodium bromide, potassium bromide,
lithium iodide, sodium iodide,
and potassium iodide. Additional halogen stabilizing compounds that could be
used in this embodiment of
the present invention in addition to sulfamic acid include, but are not
limited to, lithium sulfamate, sodium
sulfamate, potassium sulfamate, o- toluenesulfonamide, m-toluenesulfonamide, p-
toluenesulfonamide,
cyanuric acid and its derivatives, succinimide and its derivatives, and
hydantoin and its derivatives. In one
embodiment a metal halide salt is preferably combined with sulfamic acid to
form a solid solution. The
sulfamic acid is preferably no more than approximately 30% by weight of the
solid solution and the metal
halide is preferably at least approximately 70% by weight of the solid
solution. The components are
-10-
CA 02968405 2017-05-18
WO 2016/094591 PCT/US2015/064846
preferably thoroughly mixed and evenly dispersed throughout the solid
solution. Additionally, other
components, such as anticaking components, can be added to this mixture. When
diluted, this salt blend
produces a brine with a predictable and steady composition which can be used
to produce, through
electrolysis and the subsequent chemical transformations described above, the
desired product biocidal
.. solutions.
Alternatively, brines with a desired composition can be formulated and used as
an aqueous
solution. In such embodiments the brine preferably comprises a halide
containing salt and sulfamic acid
dissolved in water at the desired concentrations and in the desired ratio of
halide ion to sulfamic acid. The
halide containing salt is preferably sodium chloride, although any other
halide containing salt could be
used in this embodiment of the present invention either alone or in
combination with sodium chloride.
These salts include, but are not limited to, lithium chloride, potassium
chloride, lithium bromide, sodium
bromide, potassium bromide, lithium iodide, sodium iodide, and potassium
iodide. Additionally, other
halogen stabilizing compounds can be added to these brines in addition to the
sulfamic acid. These
halogen stabilizing compounds can include, but are not limited to, lithium
sulfamate, sodium sulfamate,
potassium sulfamate, o- toluenesulfonamide, m-toluenesulfonamide, p-
toluenesulfonamide, cyanuric acid
and its derivatives, succinimide and its derivatives, and hydantoin and its
derivatives.
The present invention preferably does not require the addition of a non-amine
base to produce
the desired halosulfamate or halosulfamic acid solution. In U.S. Patent No.
3,776,825, Vit discloses that
electrolytically produced aqueous solutions of chloramines having a pH in the
range of 8 to 12 require the
.. use of a brine made from a combination of a salt containing at least one
halide ion, at least one amine
compound and, importantly, a hydroxide compound used in molar excess of the
amine compound which
is required for pH control. In the examples disclosed by Vit, the pH of the
brines used to prepare the
desired aqueous organic haloamine solutions were all greater than 12
(calculated from the numbers
presented by Vit). In contrast, the present invention preferably is performed
with an initial brine pH of less
.. than 12, and more preferably less than 10, and even more preferably less
than 7, and even more
preferably less than 4, and even more preferably less than 2, and even more
preferably less than 1.
An alternative embodiment of the present invention is shown in FIG. 2. In this
embodiment of the
present invention, tank 20 is a brine generator where the brine formed by the
generator comprises metal
halide salts substantially without sulfamic and is fed with water from a
source (not shown). Similarly, tank
-11-
CA 02968405 2017-05-18
WO 2016/094591 PCT/US2015/064846
26 is also a brine generator where the brine formed by the generator sulfamic
acid substantially without
metal halide salts, organic sulfonamide compounds, or combinations thereof.
Brine produced in tank 20 is
transferred to line 24 through the action of pump 22 while the brine produced
in tank 26 is also transferred
to line 24 through the action of pump 28. Line 24 also preferably contains
water from a source not shown
here, which serves to dilute the brines from tank 20 and tank 24 to the
desired concentration. The diluted
brine stream then enters generation system 30 wherein the desired biocidal
solution is produced and is
then transferred through line 32 to tank 34. The electrolyzed solution is then
stored in tank 34 until it is
transferred to the application point.
The embodiment depicted in FIG. 2 could optionally utilize a multitude of
brine generator tanks
and injection pumps to provide a further degree of control over the
composition of the brine solution used
in this process. For example, separate brine generation tanks could contain
singular metal halide salts,
sulfamic acid, and organic sulfonamide compounds which could be selectively
and individually injected
into the brine stream entering generator system 30 in any desired composition,
thereby producing a
biocidal solution of any desired composition. This embodiment of the present
invention would be
advantageous in applications were several biocidal solutions or different
compositions are required for
different water treatment applications within a single facility, with the
desired solution begin able to be
produced on demand by simply varying the amounts of the different components
of the brine solution. A
plurality of product tanks could optionally be utilized so that each
specifically desired biocidal solution
could be stored and applied separately.
An alternative embodiment of the present invention is shown in FIG. 3. In this
embodiment, brine
from a source not shown here is transferred through the action of pump 40 into
generation system 42. In
this embodiment, the brine is at a ready-to-use concentration and does not
need to be diluted by process
water as shown in the other embodiments of the present invention. Once the
brine is in generation
system 42, the brine is electrolyzed and transferred out of generation system
42 along line 44 and into
tank 46, where the electrolyzed solution is stored until needed and
transferred out of tank 46 using a
mechanism not shown here.
The generation systems described above preferably comprise the electrochemical
cell(s) in which
the electrochemical oxidation of halide ions to aqueous halogens occurs. Once
the aqueous halogen
species is formed, it is free to react with sulfamic acid, producing the
desired biocidal solution containing
-12-
CA 02968405 2017-05-18
WO 2016/094591 PCT/US2015/064846
a combination of free halogen species, N-halosulfamate, N-halosulfamic acid,
N,N-dihalosulfamate, and
N,N-dihalosulfamic acid. A plurality of sensors and control systems (not
shown) are preferably used by
the present invention to ensure that the proper brine composition is utilized
by generator system 8 or
generator system 30 to produce the desired biocidal solution.
In the practice of all embodiments of the present invention, it is possible to
control the
composition of the electrolyzed solution by varying the composition of the
brine used in the production
process as well as the overall flow through the electrolytic cell. For
example, by varying the molar ratio of
the total halide ion content of the brine to the sulfamic acid or total
sulfamic acid and other stabilizer
component of the brine, it is possible to produce solutions which comprise the
desired amount of free
halogen and stabilized halogen species. Moreover, it is also possible to
achieve control over the pH of the
electrolyzed solution by varying this ratio as well, so that low pH oxidant
solutions can be produced when
the ratio of sulfamic acid to total halide ion content is high enough.
Similarly, in cases where a biocidal
solution comprising two or more halogen species is desirable, it is possible
to control the ratio of halogen
in the biocidal solution by varying the ratio of different halide ions in the
brine.
Electrolysis operational parameters can also be used to vary the composition
of the electrolyzed
solution, primarily through adjusting the flow rate through the electrolytic
cell. Under low flow conditions,
electrolyzed solutions at high pH and increased combined halogen content are
typically produced, while
at higher flow rates, the electrolyzed solution is typically produced at low
pH and decreased combined
halogen content, even though the brine concentration is the same. This
unexpected finding can be very
useful in the practice of the present invention, for example by providing a
method by which the electrolytic
cell can be self-cleaned. It is well known in the art that the electrolysis of
halide containing brines will
result in the formation of scales on the cathode surfaces. These scales
typically comprise calcium
carbonate, although magnesium hydroxide, iron oxide, and manganese oxide
scales can also form on the
cathode. Scale formation on the cathodes is primarily driven by the low pH
environment at the cathode
surface, and it is well known in the art that acid can be used to remove
scales from the cathode surfaces.
In the practice of the present invention, it is possible to automatically
remove the scale from the electrode
surface while continuing the production of the desired biocidal solution by
simply increasing the flow of
the brine through the cell such that an acidic oxidant solution is produced,
thereby cleaning the scale from
the electrode surfaces. The composition of the electrolyzed product produced
during the cleaning cycle
-13-
CA 02968405 2017-05-18
WO 2016/094591 PCT/US2015/064846
would be different than the composition of the electrolyzed product produced
during normal operation of
the system.
In general, embodiments of the present invention can produce a solution that
has a total halogen
content of less than 500 mg/L, more preferably less than 1000 mg/L, even more
preferably less than 2100
mg/L, even more preferably more than 21 00 mg/L, even more preferably more
than 2500, and even more
preferably more than 3000. In addition, embodiments of the present invention
can be tailored so that the
pH of the electrolyzed solution can be quite high even though the pH of the
brine prior to electrolysis is
very low.
The following examples demonstrate that varying the brine composition as well
as the electrolysis
operational parameters define the composition of the electrolyzed solution.
Example 1
Using an electrochemical system similar to the one depicted in FIG.3, brines
containing a mixture
of sodium chloride and sulfamic acid were electrolyzed at an applied plate-to-
plate voltage of 6 V to
produce solutions comprising free chlorine species, N-chlorosulfamate, N-
chlorosulfamic acid, N,N-
dichlorosulfamate, and N,N-dichlorosulfamic acid. Brines used in this example
were prepared with a
sodium chloride content of 30 g/L in every brine along with a sulfamic acid
content of between 1 and 12
g/L. All brines prepared for this test had a pH of 2.12 or less. After
electrolysis, the free chlorine content
(representing only chlorine, hypochlorous acid, and hypochlorite ions) was
measured alongside the total
chlorine content, which measured free chlorine species as well as N-
chlorosulfamate, N-chlorosulfamic
acid, N,N-dichlorosulfamate, and N,N-dichlorosulfamic acid. These results are
given in Table 1 below. As
can be seen, the relative composition of the electrolyzed solution varies as a
function of the sulfamic acid
content in the initial brine. Moreover, when the sulfamic acid content of the
brine was ¨12% and higher,
the electrolyzed solution primarily comprised N-chlorosulfamate, N-
chlorosulfamic acid, N,N-
dichlorosulfamate, and N,N-dichlorosulfamic acid, with very few free chlorine
species present.
-14-
CA 02968405 2017-05-18
WO 2016/094591
PCT/US2015/064846
Sulfamic Free Total Percent free
Percent N-
acid chlorine chlorine chlorine in
chlorosulfamate,
content Brine content in content in Electrolyzed
the N-chlorosulfamic
in the pH the the solution pH electrolyzed
acid, N,N-
brine (%) electrolyzed electrolyzed
solution (/0) dichlorosulfamat
solution solution e, and N,N-
(mg/L) (mg/L)
dichlorosulfamic
acid in the
electrolyzed
solution (%)
3 2.12 2675 3950 11.28 68 32
6 1.81 1650 4025 11.48 41 59
9 1.63 400 3925 11.40 10 90
12 1.53 175 3850 10.48 5 95
14 1.45 190 4000 9.96 5 95
17 1.39 85 4300 9.40 2 98
19 1.33 135 4475 2.39 3 97
21 1.26 115 4825 1.90 2 98
23 1.25 130 4950 1.63 3 97
29 1.19 70 5200 1.48 1 99
Table 1
Example 2
Electrolysis of brines containing mixed halide ions and sulfamic acid can be
utilized to produce
complex disinfection solutions containing mixed halogen, halosulfamic acid,
and halosulfamate
compounds. Using an electrochemical system similar to the one depicted in
FIG.3, brines containing a
mixture of sodium chloride and sulfamic acid were electrolyzed at an applied
plate-to-plate voltage of 6 V
to produce solutions comprised of free chlorine species, N-halosulfamate, N-
halosulfamic acid, N,N-
dihalosulfamate, and N,N-dihalosulfamic acid. In this example, N-halosulfamate
can be either N-
chlorosulfamate or N-bromosulfamate, N-halosulfamic acid can be either N-
chlorosulfamic acid or N-
bromosulfamic acid, N,N-dihalosulfamate can be N,N-dichlorosulfamate, N,N-
dibromosulfamate, or N-
bromo-N-chlorosulfamate, and N,N-dihalosulfamic acid can be N,N-
dichlorosulfamic acid, N,N-
dibromosulfamic acid, or N-bromo-N-chlorosulfamic acid. Brines used in this
example were prepared with
a sodium chloride content of 24 g/L and a sodium bromide content of 6 g/L in
every brine along with a
sulfamic acid content of between 0 and 10 g/L, resulting in brines comprising
between 0% and 12% by
weight sulfamic acid, 18% to 20% by weight sodium bromide, and 71% to 80% by
weight sodium chloride.
Brines containing sulfamic acid in this example had a pH of 2.04 or less.
After electrolysis, the free
halogen content, representing the content of chlorine, hypochlorous acid,
hypochlorite ions, bromine,
-15-
CA 02968405 2017-05-18
WO 2016/094591
PCT/US2015/064846
hypobromous acid, and hypobromite ions was measured. The free bromine content,
measuring only the
presence of bromine, hypobromous acid, and hypobromite ions was also measured
along with the total
halogen content, which measured all free halogen species along with the N-
halosulfamate, N-
halosulfamic acid, N,N-dihalosulfamate, and N,N-dihalosulfamic acid content.
These results are given in
Table 2 below. As can be seen in this data, increasing the amount of sulfamic
acid in the brine decreased
the free halogen percentage in the electrolyzed brine while the percentage of
the N-halosulfamate, N-
halosulfamic acid, N,N-dihalosulfamate, and N,N-dihalosulfamic acid content
increased correspondingly.
Unexpectedly, the bulk of the free halogen content in all brines with added
sulfamic acid was found to be
free bromine species.
_____________________________________________________________________
Sodium Sodium Sulfamic Free Free Total
Chloride Bromide acid halogen bromine halogen
content content content content in content of
content in Electrolyzed
in the in the in the Brine the the free
the Solution pH
brine brine brine pH electrolyzed .. halogen in ..
electrolyzed
(%) (%) ( /0) solution the solution
(mg/L (%)) electrolyzed (mg/L (%))
solution
(mg/L(%))
80 20 0 6.94 3600 (100) 2750 (76) Not 9.87
Applicable
77 19 3 2.04 3200 (85) 2400 (75) 3750
(15) 11.18
75 19 6 1.77 2600 (70) 2400 (92) 3700
(30) 11.23
73 18 9 1.58 1925 (53) 1825 (95) 3625
(47) 11.06
71 18 12 1.48 2125 (53) 1850 (87) 4025
(47) 10.53
Table 2
Example 3
Stability of solutions produced through the electrolysis of brines containing
mixed halide ions and
sulfamic acid was examined using the same procedure outlined in Example 2.
Here, using an
electrochemical system similar to the one depicted in Fig. 3, brines
containing a mixture of sodium
chloride, sodium bromide, and sulfamic acid were electrolyzed at an applied
plate-to-plate voltage of 6 V
to produce solutions comprised of free chlorine species, N-halosulfamate, N-
halosulfamic acid, N,N-
dihalosulfamate, and N,N-dihalosulfamic acid. Brines used in this example were
prepared with a sodium
chloride content of 24 g/L and a sodium bromide content of 6 g/L in every
brine along with a sulfamic acid
content of between 0 and 6 g/L, giving brines comprised of between 0% and 9%
by weight sulfamic acid,
-16-
CA 02968405 2017-05-18
WO 2016/094591 PCT/US2015/064846
18% to 20% by weight sodium bromide, and 73% to 80% by weight sodium chloride.
Brines used in this
example had a pH of 2.09 or less. Total halogen content of these solutions was
measured immediately
after electrolysis as well as after the electrolyzed solutions were stored for
24 hours. These results are
shown in Table 3, where it can be seen that the addition of even 3% sulfamic
acid to the electrolyzed
brine confers a high degree of stability on the total halogen content of the
electrolyzed solution compared
to solutions where sulfamic acid was not added. Additionally, the compositions
of the chlorine and
bromine components of the free halogen species of these solutions were also
measured both
immediately after electrolysis and after storage for 24 hours. These results,
given in Table 4, show that for
all solutions tested, the relative amount of bromine in the free halogen
content of the electrolyzed brines
increases during aging, indicating that a continuous oxidation process of
bromide ions during storage is
occurring.
Sulfamic Total halogen Total halogen
Percent loss of Electrolyzed
acid content in content in the
the total solution pH
content in Brine the electrolyzed electrolyzed halogen content
the brine pH solution solution after 24 after
storage for
(0/0) immediately after hours of storage 24
hours (%)
electrolysis (mg/L) (mg/L)
0 6.82 3375 450 87 9.91
3 2.09 3400 3400 0
11.12
9 1.62 3350 3200 4
11.13
Table 3
Sulfamic Free halogen content in Free bromine content
in Free bromine content of the
acid the electrolyzed brine the electrolyzed
brine free halogen in the
content in
electrolyzed brine (`)/0)
the brine Immediately After Immediately After
Immediately After
(%) after storage for after storage for
after storage for
electrolysis 24 hours electrolysis 24 hours
electrolysis 24 hours
0 3100 450 2125 375 69 83
3 2850 2975 1925 2350 68 79
9 2050 2275 1825 2175 89 96
Table 4
Example 4
Using an electrochemical system similar to the one depicted in FIG.1, brines
containing a mixture
of sodium chloride and sulfamic acid were combined with process flow water and
electrolyzed at an
-17-
CA 02968405 2017-05-18
WO 2016/094591 PCT/US2015/064846
applied plate-to-plate voltage of 6 V to produce solutions comprising free
chlorine species, N-
chlorosulfamate, N-chlorosulfamic acid, N,N-dichlorosulfamate, and N,N-
dichlorosulfamic acid. Brines
used in this example were prepared with >99% saturated aqueous sodium chloride
with sulfamic acid
added at a concentration of between 0 and 200 g/L. Brines containing sulfamic
acid in this example
typically had a pH of less than 1. After electrolysis, the pH of the
electrolyzed solution was measured
alongside the total chlorine content, which measured free chlorine species as
well as N-chlorosulfamate,
N-chlorosulfamic acid, N,N-dichlorosulfamate, and N,N-dichlorosulfamic acid.
These results are given in
Table 5 below. When the brine sulfamic acid content was 150 g/L or lower, the
pH of the electrolyzed
solution was 9.28 or higher. However, when the sulfamic acid content was
increased to above 150 g/L,
the pH of the electrolyzed solution rapidly decreased to as low as 2.03. Thus
the final composition of the
electrolyzed solution can be varied depending on even small changes in the
sulfamic acid content of the
brine.
Sulfamic acid Total halogen content in pH of the
content in the the electrolyzed solution .. electrolyzed solution
brine (g/L) (mg/L)
0 3100 9.28
10 3300 10.79
3350 11.14
3325 11.41
3475 11.46
3650 11.55
3550 11.59
3525 11.62
3525 11.69
3525 11.64
100 3475 11.44
110 3375 11.18
120 3350 10.99
130 3075 10.43
140 3050 10.16
150 3125 9.76
160 3050 5.34
170 2775 2.33
180 2975 2.68
190 2725 2.16
200 2575 2.03
Table 5
-18-
CA 02968405 2017-05-18
WO 2016/094591 PCT/US2015/064846
Example 5
Using an electrochemical system similar to the one depicted in FIG.1, brines
containing a mixture
of sodium chloride and sulfamic acid were combined with process flow water and
electrolyzed at an
applied plate-to-plate voltage of 6 V to produce solutions comprising free
chlorine species, N-
chlorosulfamate, N-chlorosulfamic acid, N,N-dichlorosulfamate, and N,N-
dichlorosulfamic acid. Brines
used in this example were prepared with >99% saturated aqueous sodium chloride
with sulfamic acid
added to a concentration of 80 g/L, with the brine having a pH of less than 1.
In this example, the total
flow of water through the system was varied between 19.6 and 44.9 gallons per
hour. After electrolysis,
the pH of the electrolyzed solution was measured alongside the total chlorine
content, which measured
free chlorine species as well as N-chlorosulfamate, N-chlorosulfamic acid, N,N-
dichlorosulfamate, and
N,N-dichlorosulfamic acid. These results are given in Table 6 below.
Unexpectedly, the pH of the
electrolyzed solution was highly dependent on total flow rate. Under low flow
conditions (24.4 gal/hr or
lower), the pH of the electrolyzed solution was higher than 10.25. In the
transition flow range of 28.5 to
32.0 gal/hr, the pH of the electrolyzed solution was more moderate with a
range of 3.90 to 8.86. When the
flow was 33.3 gal/hr or higher, the pH of the electrolyzed solution was highly
acidic and lower than 2.81,
and as low as 2.05.
Water Flow pH the electrolyzed Total halogen
Rate (gal/hr) solution content in the
electrolyzed solution
(mg/L)
19.6 11.46 4108
21.5 10.79 3808
24.4 10.25 3400
28.5 8.86 2767
30.9 7.54 2867
32.0 3.90 2667
33.3 2.81 2633
34.3 2.73 2525
36.7 2.13 2392
39.1 2.09 2308
42.0 2.05 2242
44.9 2.06 2042
Table 6
-19-
Although the invention has been described in detail with particular reference
to the disclosed
embodiments, other embodiments can achieve the same results. Variations and
modifications of the
present invention will be obvious to those skilled in the art and it is
intended to cover all such
modifications and equivalents.
Date recue / Date received 2021-12-14