Note: Descriptions are shown in the official language in which they were submitted.
CA 02968480 2017-05-19
INJECTION PUMP CONTROL METHOD, CONTROL UNIT AND
INJECTION PUMP
Technical Field
The present invention relates to a method of controlling an injection pump,
and further relates to a
related control unit and an injection pump.
Background
Diabetes is a chronic disease that heavily endangers human health in the
modern society. In
practice, it is most effective to use insulin to coptrol blood glucose and
prevent and control various
serious complications. Since insulin cannot be taken orally, it is
conventional to use an injector to
inject insulin. Currently, there are also increasingly more examples of
injection using injection
pumps.
An injection pump is used to accurately inject insulin at a fixed frequency in
a fixed quantity so as
to achieve the purposes of better controlling the blood glucose concentration
in diabetic patients,
reducing the fluctuation of the blood glucose, and reducing the occurrence of
hypoglycemia,
thereby improving the patients' quality of life. The presence of an injection
pump was once
considered as good news for diabetic patients. However, popularity of
injection pumps is at a low
level at present. Apart from factors such as high costs, the trouble caused to
the medical workers
and patients by tedious process of setting an injection dosage of a pump also
restricts the
application and promotion of the injection pumps.
Currently, the dosage of an injection pump is set according to a scheme of
"total dosage - basic
dosage - additional dosage", i.e., the total amount of insulin is calculated
according to the weight
and conditions of a patient, or the past usage and the therapeutic effect of
the insulin; the total
dosage is multiplied by a distribution coefficient to respectively obtain a
basic dosage and a before
meal (during meal) additional dosage. However, the method cannot enable a
patient's blood
glucose concentration to rapidly reach an expected range. Usually, four times
of monitoring of the
blood glucose are needed every day, and the amount of insulin is constantly
adjusted according to
the blood glucose concentration. The operation of initially setting the dosage
of an injection pump
is usually accomplished by medical staff. In order to find out a comparatively
satisfactory amount
of insulin, the diabetic patients are often required to stay in hospital for a
week or so; if the
patients choose not to stay in hospital, then it will take longer time to
accomplish the setting of the
1
CA 02968480 2017-05-19
dosage of the injection pump. Additionally, since the kinds and times of meal
for a patient might
change every day, it then requires the user of the pump to acquire the
calculation method of the
before meal/during meal additional amount. In this regard, it sets out
relatively higher
requirements for users' learning ability. However, many patients abandon pump
treatment because
of disability to accommodate and acquire the treatment. On the other hand,
there are various
insulin preparations that can be chosen by clinical doctors during
conventional treatment,
including rapid-acting, short-acting, intermediate-acting and long-acting
insulin. Each preparation
has its pharmacokinetic/pharmacodynamic characteristics and indications, but
the injection pumps
only employ rapid-acting or short-acting insulin. Such scheme of setting
differs significantly from
daily therapeutic scheme, and the medical staff need to be trained to acquire
the setting method.
Nevertheless, most medical staff do not understand the scheme of setting such
kind of injection
pumps, which also becomes a restrictive factor of the application of injection
pumps.
Summary
With respect to the abovementioned problems, the present invention proposes a
solution.
According to the present invention, the use of rapid-acting or short-acting
preparation in
combination with an injection pump to simulate the
pharmacokinetic/pharmacodynamic
characteristics of long-acting or intermediate-acting preparations extremely
facilitates the medical
staff and the patients to use the injection pumps, thereby promoting
popularization of the injection
pumps and benefiting the majority of patients. Apart from the method of
controlling an injection
pump, the present invention further provides a control unit for the injection
pump and an injection
pump comprising the control unit correspondingly.
According to an aspect of the present invention, a method of controlling an
injection pump for
injecting preparations is provided. The method comprises: (1) acquiring a
current first location
preparation concentration value of an individual to be injected with a
preparation at a current
moment; (2) acquiring an expected concentration value in plasma at a
predetermined next moment
based on a first injection scheme; (3) acquiring a supposed injection value at
a current moment
based on a second injection scheme, an expected concentration value in plasma
at the next
moment, and a current first location preparation concentration value; (4)
injecting a preparation
into an individual using the pump according to the supposed injection value;
and (5) repeating
steps (1) to (4) until the time experienced from a first injection reaches a
lasting time length of the
first injection scheme.
Preferably, when there is no preparation for injection, a first location
preparation concentration
value is 0.
2
CA 02968480 2017-05-19
Preferably, in step (1), a current second location preparation concentration
value at a current
moment is further obtained, and in step (3) a supposed injection value at a
current moment is also
obtained based on the current second location preparation concentration value.
Preferably, when there is no preparation for injection, a second location
preparation concentration
value is 0.
Preferably, the first injection scheme is a long-acting or intermediate-acting
injection scheme.
Preferably, the second injection scheme is a short-acting or rapid-acting
injection scheme.
Preferably, the first location is a subcutaneous location.
Preferably, the second location is interstice.
Preferably, the preparation is insulin.
Preferably, the displacement distance of a pump is monitored in real time when
a pump is used to
inject a preparation into an individual. lii this way, an injection amount of
a pump can be
controlled accurately.
According to another aspect of the present invention, a control unit for
controlling an injection
pump to inject a preparation is provided, which comprises: a current
concentration value acquiring
module, which is configured to obtain a current first location preparation
concentration value of an
individual to be injected with a preparation at a current moment; an expected
concentration value
acquiring module which is configured to obtain an expected concentration value
at a
predetermined next moment based on a first injection scheme; a supposed
injection value
acquiring module which is configured to obtain a supposed injection value at a
current moment
based on a second injection scheme, an expected concentration value at the
next moment, and the
current first location preparation concentration value; an injection
instruction sending module
which is configured to send instructions to inject a preparation into an
individual using the pump
according to the supposed injection value; and a time counting module which is
configured to
send out a signal to stop injection when the time experienced from the first
injection reaches the
lasting time length of the first injection scheme.
Preferably, the expected concentration value acquiring module further acquires
a current second
,
location preparation concentration value at the current moment, and the
supposed injection value
acquiring module further acquires a supposed injection value at the current
moment based on a
current second location preparation concentration value.
3
CA 02968480 2017-05-19
,
Preferably, a time counting module is achieved through a counter, wherein when
the times of
predetermined injection are achieved, the time counting module sends out a
signal to stop
injection.
Preferably, a control unit further comprises a displacement transducer for
monitoring a
displacement distance of a pump in real time.
According to a further aspect of the present invention, an injection pump for
injecting a
preparation is provided, the injection pump comprising a pump body and a
control unit as stated in
the previous paragraph, wherein the control unit is arranged in the pump body.
According to an aspect of the present invention, a method of controlling an
insulin injection pump
is provided. The method comprises: (I) obtaining a current subcutaneous
insulin value and a
current interstitial insulin value of an individual to be injected with
insulin at a current moment; (2)
obtaining an expected plasma insulin concentration value at a predetermined
next moment based
on a first insulin injection scheme; (3) calculating a supposed injection
value at a current moment
based on a second insulin injection scheme, a current subcutaneous insulin
value, a current
interstitial insulin value, and an expected plasma insulin concentration value
at a next moment; (4)
injecting insulin into an individual using the pump according to the supposed
injection value; and
(5) repeating steps (1) to (4) until the time experienced from the first
injection reaches a lasting
time length of the first insulin injection scheme.
Preferably, when there is no insulin injection, the subcutaneous insulin value
and the interstitial
insulin value are 0.
Preferably, a current subcutaneous insulin value at a current moment is
obtained first, and then a
current interstitial insulin value is obtained based on the current
subcutaneous insulin value.
Preferably, an insulin value refers to a monomeric/dimeric insulin value.
Preferably, a first injection scheme is a long-acting or intermediate-acting
injection scheme.
Preferably, a second injection scheme is a short-acting or rapid-acting
injection scheme.
Preferably, when an individual is injected with insulin using the pump, the
displacement distance
of the pump is monitored in real time. In this way, the injection amount of a
pump can be
accurately controlled. ,
According to another aspect of the present invention, a control unit for
controlling an insulin
injection pump is provided, the control unit comprising: a current
concentration value acquiring
module, which is configured to obtain with a current interstitial insulin
value and a current
subcutaneous insulin value at a current moment; an expected concentration
value acquiring
4
CA 02968480 2017-05-19
module, which is configured to obtain an expected plasma insulin concentration
value at a
predetermined next moment based on a first insulin injection scheme; a
supposed injection value
acquiring module, which is configured to obtain a supposed injection value at
a current moment
based on a second insulin injection scheme, an expected plasma insulin
concentration value at the
next moment, a current subcutaneous insulin value and a current interstitial
insulin value; an
injection instruction sending module, which is configured to send out an
instruction to inject
insulin into the individual using the pump according to the supposed injection
value; and a time
counting module, which is configured to send out a signal to stop injection
when the time
experienced from the first injection reaches the lasting time length of the
first injection scheme.
Preferably, the time counting module is realized through a counter, wherein
when a predetermined
injection times are achieved, the time counting module sends out a signal to
stop injection.
Preferably, a control unit further comprises a displacement transducer for
monitoring a
displacement distance of a pump in real time.
According to a further aspect of the present invention, an injection pump for
injecting insulin is
provided, the injection pump comprising a pump body and the control unit as
stated in the
previous paragraph, wherein the control unit is a'rranged in the pump body.
According to an aspect of the present invention, a method of controlling an
insulin injection pump
is provided. The method comprises: (I) obtaining a first expected plasma
insulin concentration
value of an individual to be injected with insulin at a predetermined first
moment based on a first
insulin injection scheme; (2) calculating an initial supposed injection value
at an initial moment
based on a second insulin injection scheme and the first expected plasma
insulin concentration
value; (3) injecting insulin into an individual using the pump according to
the initial supposed
injection value; (4) obtaining a first moment interstitial insulin value and a
first moment
subcutaneous monomeric/dimeric insulin value at the first moment based on a
second insulin
injection scheme; (5) obtaining a second expected plasma insulin concentration
value at a
predetermined second moment based on the first insulin injection scheme; (6)
calculating a first
supposed injection value at a first moment based on the second insulin
injection scheme, the
second expected plasma insulin concentration value and the first moment
subcutaneous
monomeric/dimeric insulin value and the first moment interstitial insulin
value; (7) injecting
insulin into an individual using the pump according to the first supposed
injection value; and (8)
obtaining a supposed injection value at each moment by iteration based on
steps (4) to (7) and
injecting insulin into the individual using the pump according to the supposed
injection value until
the time experienced from a first injection reaches a lasting time length of
the first insulin
injection scheme.
CA 02968480 2017-05-19
Preferably, an interval between a first momeo and an initial moment is equal
to an interval
between a second moment and the first moment.
Preferably, the time interval between every two injections is fixed.
Preferably, the first injection scheme is a long-acting or intermediate-acting
injection scheme.
Preferably, the second injection scheme is a short-acting or rapid-acting
injection scheme.
According to another major aspect of the present invention, a control unit for
controlling an
insulin injection pump is provided. The control unit comprises: an expected
plasma insulin
concentration value acquiring module, which is configured to obtain a first
expected plasma
insulin concentration value at a predetermined first moment and a second
expected plasma insulin
concentration value at a predetermined second moment of an individual to be
injected with insulin
based on the first insulin injection scheme, until an N-th expected plasma
insulin concentration
value at a predetermined N-th moment is reached; wherein N refers to the
number of times of
predetermined injections; a current subcutaneous and interstitial insulin
concentration value
acquiring module, which is configured to obtain with a current subcutaneous
insulin value and a
current interstitial insulin value at a current moment; a supposed injection
value acquiring module,
which is configured to obtain a supposed injeotion value at a current moment
based on a second
insulin injection scheme, an expected plasma insulin concentration value at a
next moment, the
current subcutaneous insulin value and the current interstitial insulin value;
an injection instruction
sending module, which is configured to send out an instruction to inject
insulin into an individual
using the pump according to the supposed injection value; and a counting
module, which is
configured to send out a signal to stop injection when the time experienced
from the first injection
reaches the lasting time length of the first injection scheme.
Preferably, a time counting module is achieved through a counter, wherein the
time counting
module sends out a signal to stop injection when predetermined injection times
are accomplished.
Preferably, a control unit further comprises a displacement transducer for
monitoring a
displacement distance of a pump in real time.
According to a further aspect of the present invention, an injection pump for
injecting inulin is
provided, the injection pump comprising a pump body and the control unit as
stated in the
previous paragraph, wherein the control unit is arranged in the pump body.
It should be understood that the technical solution of the present invention
is not only limited to
the abovementioned as listed. The feature orall the abovementioned major
technical solutions
and all the preferable schemes can be combined freely as long as the combined
features are not
conflicting with each other therebetween.
6
CA 02968480 2017-05-19
Brief Description of the Drawings
Figure 1 is a flow chart of a method of corttrolli,ng an injection pump
according to an embodiment
mode of the present invention;
Figure 2 is a curve diagram of plasma concentration variation of a patient
weighed 70kg when
subcutaneously injected with 10IU NPH;
Figure 3 is a curve diagram of plasma concentration variations of pump
injection with rapid-acting
insulin and one shot of injection with 10IU intermediate-acting insulin,
wherein local details are
further displayed in the form of an enlarged view;
Figure 4 is a functional diagram of a control unit for an injection pump
according to an
embodiment mode of the present invention;
Figure 5 is a schematic diagram of an injection pump according to an
embodiment mode of the
present invention;
Figure 6 shows injection amounts and theoretically expected value of an
injection pump every 15
minutes according to an example of the present invention;
Figure 7 is a PK diagram comparing NPH single subcutaneous injection and
successively
subcutaneous injection using an injection pump with a blank control group
according to an
example of the present invention.
The specific embodiments of the present invention are set forth hereinafter in
combination with
the drawings. It should be understood that these embodiment modes are
illustrative rather than
restrictive.
Description of the Embodiments
The present invention is described using some examples in the description,
wherein some
preferable embodiment modes are included. It should be understood that the
scope of protection of
the present invention further comprises other examples.
For example, a system and method may comprise a data signal transmitted via a
network (e.g., a
local area network, a wide area network, an Internet, and their combination,
etc.), an optical fiber
medium, a carrier, a wireless network or the like for communicating with one
or more data
processing units. The data signal can carry any or all data disclosed in the
text. in addition, the
method and system described herein can also be realized on different types of
processing units by
executing program codes comprising instructions on processing subsystems of
these units.
Software program instructions may comprise sburce codes, target codes, machine
codes or other
stored data, which can be operable to enable the processing system to execute
the method and
7
CA 02968480 2017-05-19
operation described herein. Other embodinwnt modes are also feasible, e.g.,
firmware or even
suitable hardware that can execute the method and system as described in this
text is arranged.
Data (e.g., relevance, mapping, data input, data,output, intermediate data
results, final data results
and the like) of the system and method can be stored and executed on one or
more different types
of non-volatile computer readable storage media, wherein the media can be in a
certain location or
distributed in different locations. The media may comprise data storage units
that are implemented
by computers, e.g., different types of storage units and programming
structures (e.g., RAM, ROM,
flash memory, flat files, database, programming data structures, programming
variables, IF-THEN
(or similar) declaration structures, etc.). It should be understood that data
structure describes
format for organizing and storing data, database, programs, memory or other
computer readable
media that can be used by computer programs.
The system and method can be provided on many different types of computer
readable media,
including computer storage mechanical structures (e.g., CD-ROM, disks, RAM,
flash memory,
computer hard disk and the like) which comprise instructions (e.g., software)
to be executed by
processors so as to complete the operation of the method described herein and
realize the system.
The computer components, software modules, functions, data storage and data
structures
described herein can be directly or indirectly connected to one another to
allow data flow required
in the work. It should be further understood that the module or processor not
only comprises such
code units that execute software operation's, bilt also can be realized as a
subprogram unit, as a
software functional unit of codes, as an object (in an object-oriented normal
form), as an applet, in
a computer script language, or as another type of computer codes. The software
components
and/or functions can be located on a single computer or distributed on a
plurality of computers
according to different conditions.
It should be understood that the components used in the present description
and mentioned in the
claims can be in a single form or in a plural form. Similarly, "in ..." as
used in the present
description and mentioned in the claims comprises two meanings, i.e., "in ..."
and "on ..." unless
specifically indicated in the context. Finally, "and" and "or" as used in the
present description and
mentioned in the claims comprise two meanings, i.e., connective and separated
unless specifically
indicated in the context. They can be used interchangeably. The term
"exclusive or" can be used to
indicate the condition of using the meaning of separation only.
Figure 1 presents a flow chart of a method of controlling an injection pump
according to an
embodiment mode of the present invention. As illustrated in the figure, the
method includes five
steps. First of all, a preparation concentration value at a current first
location and a preparation
concentration value at a current second location of an individual to be
injected with a preparation
,
at a current moment are obtained in step 101. Then, in step 102, an expected
concentration value
8
CA 02968480 2017-05-19
at a predetermined next moment is obtained based on a first injection scheme.
Next, in step 103, a
supposed injection value at a current moment is obtained based on a second
injection scheme, an
expected concentration value at a next moment, a preparation concentration
value at a current first
location and a preparation concentration value at a current second location.
Further, in step 104, a
,
preparation is injected into an individual using the pump according to the
supposed injection value.
Next, in step 105, it is determined as to whether or not the time experienced
from the first
injection reaches a lasting time length of the first injection scheme. If yes,
it comes to an end; if no,
it comes back to step 101 to execute the process again successively, i.e.,
steps 101 to 104 are
repeated until the time experienced from the first injection reaches the
lasting time length of the
first injection scheme. Herein, the so-called lasting time length of the first
injection scheme refers
to the lasting time length in which the preparation takes effect when
injection is executed
according to the first injection scheme.
It should be understood that in other embodiments of the present invention, it
is possible to only
obtain a preparation concentration value at a first location or at a second
location, rather than
obtain both preparation concentration values at a first location and a second
location.
In a preferred embodiment mode, the first location can be a subcutaneous
location, and the second
location can be interstitice. The first injection scheme is a long-acting
injection scheme, and the
second injection scheme is a rapid-acting injection scheme, with insulin as
the preparation.
In particular, with respect to insulin injection, the present invention can be
transformed into
rapid-acting injection scheme that is suitable" for injection using an
injection pump based on
pharmacokinetic principles according to conventional prescriptions of insulin,
in which clinical
prescription schemes and clinical experience of insulin acquired by doctors
are fully utilized to
increase accuracy and reliability of the prescriptions. The method of using
the pump is simplified,
the time of setting and adjusting dosage is reduced, and the time spent on
using insulin for doctors
and patients is saved. Therefore, insulin is taken as an example hereinafter
to describe a
particularly specific embodiment mode of the present invention.
At present, there are various types of insulin that are used clinically. The
usual mode of
administration is subcutaneous injection, but the effect features during
action differ significantly.
The short-acting insulin (RI) takes effect from 15 to 60 minutes after
injection, the rapid-acting
insulin from 10 to 15 minutes, the intermediate-acting (NPII, lent) insulin
from 2.5 to 3 hours, and
the long-acting (ultralente, glargine) insulin from 2 to 3 hours, wherein the
effect can last for 20 to
30 hours. The major factor that causes differing time of action lies in the
difference in the process
of subcutaneous absorption of insulin, which process generally comprises the
following stages: in
subcutaneous tissues, crystalline insulin is dissolved into hexamer which can
be depolymerized
9
=
CA 02968480 2017-05-19
into dimer/monomer. The latter can be absorbed into blood after entering into
interstice
subcutaneously. Each stage of absorption follows a first-order kinetic mode.
In this example, it is set that a conventional therapeutic solution is 10IU
NPH/day. Moreover, the
patient weighs 70kg, and rapid-acting insulin, e.g., NovoRapid from Novo
Nordisk, is used in an
insulin pump.
First of all, the pharmacokinetic features of intermediate-acting insulin NPH
(e.g., Novolin from
Novo Nordisk) are calculated.
The process of subcutaneous absorption of NPH is described using differential
equations:
= lic,NPH(1)+11h,A1PH4)+14m,N11-10 (1)
=aNl'HUiotal,N111(t) (2)
Att,110 =- kegs NPH C N140 tic,NPA (3)
xh(t) = -(k1+ ka)Xy(1) kcrys,NIWC N110+ Uh,N110 (4)
xdn(t) = -02+k,dxd,,,(1)+krxh(t) (-uni,Nr4) (5)
-(k3+kdjx,(t)+k2xdõ,(t) (6)
1(t) = -nl(t)+k3M (7)
Therein,
a total rate (mU/min) of injecting intermediate-acting insulin NPH;
ticvpll(t): a rate (mU/min) of injecting crystalline NPI I insulin;
uh,mw(t): a rate (mU/min) of injecting hexameric NPH insulin;
u,,,Npy(t): a rate (mU/min) of injecting monomeric/dimeric NPH insulin;
CiNpH: a ratio of crystalline insulin to total amount of NPH in NPH;
Cmw(t):an amount (mU) of subcutaneously crystalline NM;
itc,,,,Npll: a rate (min-I) of dissolving NPIl crystals;
xh: an amount (mU) of hexameric insulin in subcutaneous tissues;
10: a rate (min') of depolymerizing hexamers;
CA 02968480 2017-05-19
k2: a rate (min-1) of transporting dimeric/monomeric insulin from
subcutaneously to interstitially;
kd: a rate (min-1) of consumption of hexamers, dimers and monomers owing to
diffusion;
xdm: an amount (mU) of hexameric insulin;
k3: a rate (min-1) of transporting insulin from interstitially to plasma;
kdj: a rate of consumption or loss interstitially, e.g., 0.0029 (min-1);
1(1): a plasma insulin concentration (mU/L);
n: a rate of removing plasma insulin, e.g., 0.16 (min-1);
V,: a volume of insulin plasma distribution, e.g., 0.1421 (liter/kg);
Int,: a weight (kg), e.g., 70kg;
Analytical solutions of plasma NPH concentration IN with respect to time I can
be obtained by
solving the abovementioned equation set:
=exp(-(4*t)/25)*((1022699*exp((27*t)/200))/163961000 +
(50681673*exp((793*t)/5000))/4936028500 - (2182714053*exp((953*0/ 1
0000))/494945696200 -
(4449021*exp((1511*t)/10000))/327887000 + 1682730209/1141906519000) (8)
The insulin concentration in plasma at any moment after one shot of injection
of the
intermediate-acting insulin can be obtained according to the equation above.
Figure 2 illustratively
shows a curve of plasma concentration variation of a patient weighed 70kg when
subcutaneously
,
injected with 10IU NPH.
Next, the pharmacokinetic features of rapid-acting insulin are calculated.
The process of subcutaneous absorption of rapid-acting insulin is described
using
differential equations:
Ulotal,mono(t) Umoidt) (9)
xam(1)= -02+kd)xdm(t) +krxi(t) +um,NpH(t) (5)
xdt) = -(k3d-kdd)xdt) k2xdif(t) (6)
1(0 = -n1(0+ k3M (7)
v,mb
Therein,
11
,
CA 02968480 2017-05-19
Utõ,,Lmõ,,õ(t): a total rate (mU/min) of injecting rapid-acting insulin;
uõ,õ,õ,(t): a rate (mU/min) of injecting monomeric/dimeric rapid-acting
insulin;
With respect to other parameters, reference can be made to the previous text.
The following equations can be obtained by solving the abovementioned equation
set:
xdmi = XdrnO*exp(-(53*0/5000) (10)
x,, ----(20*exp(-(321*)/5000)*((613*x,()/20 (32489* xd0)/5360 +
(32489* xdmo*exp((67*t)/1250))/5360))/613 (11)
=exp(-(4*t)/25)* ((32489*xd0)/7156260 - (613* x,0)19580 + /õ,0 -
exp((479*t)/5000)*((32489*xd,.õ0)/2567440 - (613 *x,0)/9580) +
(32489*xdõ,0*exp((747*0/5000))/4003920) (12)
wherein,
xdõ,0: an amount (mU) of subcutaneous monomeric/dimeric rapid-acting insulin
at the moment of
0;
xdõ,,: an amount (mU) of subcutaneous monomeric/dimeric rapid-acting insulin
at the moment oft;
x,0: an amount (mU) of interstitial rapid-acting insulin at the moment of 0;
x,,: an amount (mU) of interstitial rapid-acting insulin at the moment oft;
a concentration (mU/L) of rapid-acting insulin in plasma at the moment of 0;
1,õ,1: a concentration (mU/L) of rapid-acting insulin in plasma at the moment
oft;
,
The amount of subcutaneous and interstitial insulin and the insulin
concentration in plasma at any
moment after one shot of injection of the rapid-acting insulin can be
respectively calculated
through the abovementioned equations.
Next, an injection amount of an insulin pump at each time point is calculated.
The amount of injecting rapid-acting insulin with a pump can be calculated by
a cyclic iterative
method to analyze the equation set composed of equations (10) to (12)
according to the
concentration values of the intermediate-acting insulin NPH at each time
point. Supposing that the
pump injects once every three minutes, the specific calculation method is as
follow:
(a) it is calculated that the plasma concentration of intermediate-acting
insulin within three
minutes is 1N3 0.07987mU/L according to equation (8);
12
,
CA 02968480 2017-05-19
(b) the amount of rapid-acting insulin in each location at the moment of 0 is
obtained; supposing
that the rapid-acting insulin (lo is injected at the moment of 0, then the
amount of the
instantaneously subcutaneous rapid-acting insulin when insulin U0 is injected
at the moment of 0
is x,,,,,,o+=U0; at this moment, the rapid-acting insulin has not yet arrived
in the interstice and the
plasma, so x,0=0 in the interstice, and 1m=0 in plasma;
(c) if it is desired that the rapid-acting insulin concentration in the 3rd
minute reaches the
intermediate-acting insulin level at this moment, then 6,3=43=0.07987mU/L. If
xthn(7=U0, x1o=0,
1õ70=0, /õ,3=0.07987mU/L are substituted into equation (12), and a value of
3min is assigned tot, a
following equation can be obtained:
0.07987mU/L
exp(-(4*3)/25)*((32489* xdm0+)/7 156260-(613* 0)/9580+0-exp((479*3)/5000)*
432489* xdr,o+)/
2567440-(613*0)/9580) (32489*xdm0+*exp((747*3)/5000))/4003920)
In this equation, only xdri3O+ is unknown, the equation is solved to obtain
xthno+-343.994mU,
Uo=xd7o+=343.994mU, i.e., the injection amount of insulin at moment 0 is
343.994mU;
(d) the amount of rapid-acting insulin at each location in the 3rd minute is
sought; it is recorded
that the subcutaneous rapid-acting insulin before injection for the second
time using the pump in
the 3'd minute is in an amount of xdõ,3-, it can be obtained that xtho-
=333.227mU according to
equation (10); it is recorded that the amount of interstitial rapid-acting
insulin in the 3rd minute is
x,3, and it is obtained that x,3=9.789mU according to equation (1 1 ); and it
can be derived from step
(c) that /õ,3=0.079874mU/L;
(e) according to equation (8), it is calculated that the plasma concentration
of the
intermediate-acting insulin in the 6th minute is 1N6=0.2605mU/L;
,
(f) let the amount of injecting rapid-acting insulin in the 3rd minute be U3,
then
xdõ,3+¨Xrint3-+U3=333.227mU+U3; it can be derived from step (d) that
x,3=9.789mU,
473=0.079874mU/L; it is calculated from step (e) that the plasma concentration
of rapid-acting
insulin after 3 minutes (i.e., in the 6th minute) should be
/õ,6=/v6=0.2605mU/L. The plasma insulin
concentration in the 6th minute is calculated on the basis of the amount of
rapid-acting insulin at
each location in the 3rd minute, and substituted into equation (12) to obtain
the equation
0.2605mU/L=exp(-(4*3 )/25)* ((32489* (333 .227mU+U3))/7156260-(613* 9.789m
U)/9580+
0.079874mU/L-exp((479*3)/5000)*((32489* (333 .227m U+
U3))/2567440-(613*9.789mU)/9580)+(32489*(333.227mU+ U 3)+*exp((7 47*3)/5000))/
4003920)
13
,
CA 02968480 2017-05-19
in which only U3 is unknown and the equation is solved to obtain U3=20.2026mU,
i.e., 20.2026mu
should be injected in the 3441 minute, and xdõ,3+=xdõ,3-+U3=353.4296mU after
injection;
(g) steps (d) to (f) are repeated to calculate the injection amount of the
insulin pump every three
minutes. Reference can be made to Table 1, wherein the injection amount of the
rapid-acting
insulin of the insulin pump at each time point is displayed.
Table 1 Injection Amount (mU) of Rapid-acting Insulin of an Insulin Pump at
Each Time
Point
0 0 12 I, 18 21 M 17 31 41 A 22 44
.64 A it A 2>
W' M, MA 11.40 40,1 AM AO MIZ AO; UM UM nm 26.71 MO DM Mist UA MM
I MA AM MU ,, AN am au an MA MA 44.141
AA ma am UM MM MA ad.A.
4P! vm WM ,U VW EMI UM UM UN UM MX, MM UM UM UM WM WA MM MM MM
4 101 9.77 31.41 0.12 MAIMS 31.07
98.33 01,424 367.9 60.79 3661 WU AM 23311 19.111
aaaa 3030 33.20me ;au ma ma :am ma MN mu AM UM AO 44' 4731 g÷ MA MU 40.02
, MM nm mn vo pm ,m,no 2520 nm mm 74,705 mm Am AM Mae MM
am mu ma 70.4 24.42 4.0 MA 1$.80 AM AW 24.79 2*41 nv MM
UM MU MM
8882 Mm MA n, nm mit Ali AM MW :2,fr um n, n4, 72w >24.) AM }In
noi
= A
, nm an 2171 UU uu,au am AM MM nDo t,A 4459 30o.õ88
-,188
w 1831 mm MA nx NW Ail NM 31,II_33X6 MMI MS WM MM MM 713.30 MA Vpi
M MU MM 13393, NM MO mm mu mu) um 17.84 mm mo 31.88 VV. PUt Pq Mu 324,,
VA 17A pm vm um 1814 MA Mr MA MA MA MA MA MM MM MM MA KW 21481
-
,! MU no mu om pm om mo mu ma mo 11.12 33.03 MW
AM AW nm an MO 11.51
MA MA MO 14.191422 MA m21_14.1.; Aw med am ow ax aw 13.4 nm 1134 am 1,1.45
- nu, on Mg, MA AV MU 334% 13431 AM UM UM UM 1714 VO VM AA 3717 3142 OM:
46 UA AV 17.22 11.19 mu mu mm um um mu um um *171 2362 am um um am au mml
a ma ua um um um um um MM OM 1903 Ma om mu mn mu am an mm mu am!
42 MO OM 1112 tan 1934 3024* 11.114 19 11 10911 111
SA 4142 090 16 di *78 4144 A 0413. j
9.58 93S 570 416 953 9.114 4231 3.30 9.24, 3.12
9 11 *31 911 807 1.01 8.19 0.116 142 9 Ow 191
8,8 6 1>3 am m sr:* 852. 45* 848 144 8.11
9.37 8.34 3411, 8.31 1123 341? a 16 2.12
EU, 0 493 An ,03. 7111 ?AR 1.14 ".40 ,711 757
20.7 7.1. 047 1423 ,41 ,41
-
0.41 741 730 9 05 7.92 029 023 721* 7.17 7 11
7.11 00! 7742 7.97 699 CA 613 4303 m
17 LA 612 SA 5.74 *7* 10 OM efts s 62 6749
166 4,7,1 6.21 CM 440 6.43 s 40 837 401 4.17
24 624 421 11.24 611 5 *434, 434 6.11 SA 64%
693 6.01 SA 1.96 $.93 5.11 5 Mt 5.21: 693 1201 '
4 A
In order to verify the coincidence of plasma concentrations of one shot of
injecting both
rapid-acting insulin and intermediate-acting insulin using the pump, the
plasma concentrations of
one shot of injecting both the rapid-acting insulin and the intermediate-
acting insulin using the
pump are respectively calculated every six seconds. Results show that the
difference value
therebetween is less than 1% after one minute, and is less than 0.1% after
three minutes. Figure 3
shows both plasma concentration curves, and indicates relatively high
coincidence.
According to the examples above, the injection amounts of the pump at
respective time points can
be calculated so as to realize transformation of a scheme of injecting
intermediate-acting or
long-acting insulin into a scheme of injecting rapid-acting insulin. In other
embodiment modes, it
can be realized that an injection scheme of intermediate-acting or long-acting
insulin is
14
CA 02968480 2017-05-19
transformed into an injection scheme ol hot-actin i: insulin, or that
transformation of other
injection schemes can be realized. Table 2 shows kinetic parameters of various
insulin that are
used for simulation. Table 2 shows kinetic parameters of various types of
insulin products,
wherein the values thereof are medians and can be used for reference.
Table 2 Table of Kinetic Parameters of Various Types of Insulin
Type of Insulin acrys an ex ad m kcrys K1 K2 K3
Source
Rapid-Acting
0 0 1.000 0.0106 0.0613 5
MI
Short-ActingRI 0 0.300 0.700 ¨ 0.0250 0.0089 0.0618 6
Intermediate-
0.9432 0.017 0.040 0.0014 0.0250 0.0089 0.0618 7
Acting N PH
Intermediate-
0.9447 0.017 0.039 0.0037 0250 0.0089 0.0618 8
Acting Lente
Long-Acting
1.0000 0 0 0.0013 0.0018 0.0089
0.0618 9
Ultralente
Long-Acting
0.9462 0.016 0.038 0.0008 0.0084 0.0089 0.0618 10
Glargine
In the abovementioned examples, the amounts of rapid-acting insulin in various
locations can also
be obtained without equations, but measured through proper detection methods
(e.g., ACS
180PL,US); the injection interval can also be set as other suitable values,
e.g., two minutes, five
minutes or ten minutes, rather than 3 minutes.
In a further example, the displacement distance of a pump can be further
monitored in real time
using, for example, a displacement transducer, thereby monitoring an injection
amount to achieve
the purpose of accurate injection.
In some other embodiment modes of the present invention, the injection schemes
of other
preparations can also be transformed to obtain injections schemes that are
more beneficial for use
in the injection pumps, thereby providing great convenience to patients.
CA 02968480 2017-05-19
Figure 4 shows a functional diagram of a control unit for an injection pump
according to an
embodiment mode of the present invention. As illustrated in the figure, a
control unit 200
comprises a current concentration value acquiring module 201, an expected
concentration value
acquiring module 202, a supposed injection value acquiring module 203, an
injection instruction
sending module 204 and a time counting module 205. An example of using insulin
as a
preparation is made again. The current concentration value module 201 is used
to acquire a current
subcutaneous insulin value and a current interstitial insulin value at a
current moment of an
individual to be injected with a preparation. The expected concentration value
acquiring module
202 acquires an expected plasma insulin concentration value at a predetermined
next moment
based on a first insulin injection scheme. The supposed injection value
acquiring module 203
acquires a supposed injection value at a current moment based on a second
insulin injection
scheme, an expected plasma insulin concentration value at a next moment, a
current subcutaneous
insulin value and a current interstitial insulin value. The injection
instruction sending module 204
sends out an instruction to inject a preparation into an individual using the
pump according the
supposed injection value. The time counting module 205 counts the time, and
sends out a signal to
stop injection when the time from the first injection reaches a lasting time
length of the first
injection scheme.
The time counting module 205 can be realized through, e.g., a system clock of
the control unit. It
can also be realized through, for example, a counter; then in such
circumstance, when the number
of times of predetermined injections is reached, the counter sends out a
signal to stop injection. In
some embodiment modes, let the time interval between each injection be
unchanged, then the
number of times of predetermined injections can be obtained using a division
method simply
based on the duration of the first injection scheme.
It should be understood that Figure 4 only divides each module of control unit
200 functionally
rather than dividing the control unit physically. In fact, the functions of
some modules might be
integrated into a chip, or even the entire control unit is only embodied as a
chip or integrated as a
part in a chip.
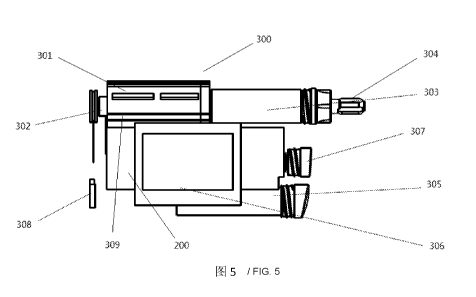
Figure 5 illustratively presents an injection pum'p according to an embodiment
mode of the present
invention. As illustrated in the figure, the pump body of an injection pump
300 comprises a rod
valve push 301, a motor assembly 302, an injection cavity 303 and an injection
head 304. The
motor assembly 302 is arranged at the rear end of the rod valve push 301 to
provide power to
drive the rod valve push 301. The injection cavity 303 is arranged at the
front end of the rod valve
push 301, wherein a preparation is stored, and when the rod valve push 301 is
subjected to a
pressure, the preparation is injected outwardly from an injection head 304 at
the front end of the
injection cavity 303. A displacement transducer 309 can be arranged below the
rod valve push 301
to monitor the displacement distance of a pump. An injection pump 300 further
comprises a
16
CA 02968480 2017-05-19
battery cavity 305 below the pump body and some other electronic components,
e.g., a liquid
crystal panel 306 arranged in the middle for information interaction with a
user, and e.g., a USB
interface 307 arranged at one side of the liquid crystal panel 306 as a
communication interface.
The injection pump 300 further comprises an alarm component 308 that is
detachably connected
to send out alarms in special conditions. A control unit 200 in the form of a
control panel is
arranged inside the injection pump 300. The electronic components mentioned
above can be
arranged as members separated from the control unit 200 in space, or can be
integrated into a
control panel as a part of the control unit 200.
Example 1:
The present invention is applied to an SD rat used for experiment. The
injection accuracy of the
injection pump in an animal experiment is studied in the present invention,
feasibility of the
present invention is further discussed by comparing the pharmacokinetic curve
between the
injection accuracy and subcutaneous injection of insulin.
Method: SD rats are used and STZ is used for once large dose injection into an
abdominal cavity.
After one week, a blood glucose level is detected. If it is greater than
11.6mmol/L, then it is
prompted that a TI DM rat model is successfully established. The T1DM rats are
randomly
divided into an NPH single subcutaneous injection group, an RI injection pump
continuous
subcutaneous injection group, and a blank control group. During the 4-hour
experiment, sampling
is conducted at each blood collection point and insulin concentration thereof
is detected,
respectively. The injection amount of the injection pump is also measured
every 15 minutes
according to the scheme and compared with the theoretical expected value.
I. Animals in Experiments
1.1 The strain comes from T1DM diabetic SD rats purchased from Nanjing
BetterBiotechnology
Co., Ltd.
1.2 Gender and Week Age: male, 7-8 weeks.
1.3 The feeding environment is at the temperature between 20 and 25 C, in a
humidity between 40%
and 70% or so. The rats eat and drink fkelY, an the rat cage is cleaned
regularly.
2. Injection Pumps to be Tested
There are totally 8 injection pumps used in the experiment, which are encoded
with P001 to P008
and produced by Shanghai Zensun Science and Technology Development Co., Ltd.
3. Experimental Materials
17
CA 02968480 2017-05-19
,
Novolin R Cartridge 100IU/ml, Novo Nordisk (China) Pharmaceutical Co., Ltd;
Novolin N
Cartridge 10011J/ml, Novo Nordisk (China) Pharmaceutical Co., Ltd.
4. Experimental Method
4.1 Preparation of a T1DM Rat Model
After ambrosia for 24 hours of SD rats that weigh 200g, the dosage of 70mg/kg
is respectively
administered to each group through once abdominal injection.
Model Forming Standard: the rat tail venous blood glucose value is measured
using a glucometer
after 72 hours from administration, a random glucose value equal to or greater
than 11.6mmol/L
serves as a model forming standard for diabetic rats. The random glucose value
that is actually
measured after molding is equal to or greater than 20mmol/L.
4.2 Grouping and Administration
After the model is successfully established, Ti DM rats are randomly divided
into NPH single
subcutaneous injection group, an injection pump using group, and a blank
control group. As for
the NPH single subcutaneous injection group, Novolin N is used for single
subcutaneous injection,
and administered in a dose of 51U/kg; in the RI injection pump continuously
subcutaneous
injection group, an NPH sample of Novolin R in the kinetic progress is
administered for
subcutaneously continuous pumping; the black control group is administered and
injected with
normal saline using the pump for subcutaneously continuous pumping.
4.3 Measurement of Insulin
Blood samples are collected and the insulin amounts are detected 30 minutes,
45 minutes, 60
minutes, 75 minutes, 90 minutes, 105 minutes, 120 minutes, 135 minutes, 150
minutes, 180
minutes and 240 minutes after treatment by administration. A Roche
electrochemical
luminescence instrument cobas e601 is used for insulin detection, which is
produced by a Roche
company of diagnosis products and gauged by Guangzhou Kingmed Medical
Detection Center.
5. Experimental Results
The injection amounts of the injection pump every 15 minutes in the experiment
are substantially
consistent with theoretical expectations, see Figure 6. As compared with NPH
single subcutaneous
injection, the continuously subcutaneous injection group using an RI injection
pump shows the
reliability in the process of controlling in vivo metabolic process of insulin
from in vitro
administration, the peak reaching time and peak reaching concentration of both
conditions being
substantially consistent; the blank control group proves that the mechanical
results of the injection
pump would not produce any impact on insulin variation, see Figure 7.
18
CA 02968480 2017-05-19
e
6. Conclusion
After using the injection pump of the present patent, the curves of insulin in
vivo concentration
can be basically controlled as time changes.
The present invention is set forth above through specific embodiment modes. It
should be
understood that the abovementioned descriptions are descriptive rather than
restrictive. For
example, the features of the abovementioned contents can be used solely, or
can be used in any
combination as long as they do not go beyond the scope of the present
invention. Moreover,
without departing from the spirit of the present invention, amendments can be
made to the
embodiment modes to adapt to specific circumstances. Although the specific
elements and process
in the text define various embodiment modes, they are not restrictive but
demonstrative. By
reading the abovementioned description, many other examples would be obvious
to persons
skilled in the art. Thus, the scope of the present invention should be
determined by considering the
claims as well as the entirely equivalent scope covered by such kind of
claims.
,
19