Note: Descriptions are shown in the official language in which they were submitted.
CA 02971095 2017-06-14
WO 2016/105571
PCT/US2015/000475
METHOD AND APPARATUS OF MONITORING A PATIENT FOR MOTOR
MANIFESTATIONS RELATED TO SEIZURE ACTIVITY
CROSS-REFERENCE TO RELATED APPLICATION
[0001] This application claims priority to U.S. Provisional Patent Application
No.
62/096,331 filed December 23, 2014 and U.S. Provisional Patent Application No.
62/149,434
filed April 17, 2015. The application also claims priority to U.S. Application
No. 14/920,665
filed October 22, 2015. The disclosures of each of the aforementioned
applications are herein
fully incorporated by reference.
BACKGROUND
[0002] A seizure may be characterized as abnormal or excessive synchronous
activity
in the brain. At the beginning of a seizure, neurons in the brain may begin to
fire at a
particular location. As the seizure progresses, this firing of neurons may
spread across the
brain, and in some cases, many areas of the brain may become engulfed in this
activity.
Seizure activity in the brain may cause the brain to send electrical signals
through the
peripheral nervous system activating different muscles of the body.
[0003] Techniques designed for studying and monitoring seizures have typically
relied upon electroencephalography (EEG), which characterizes electrical
signals using
electrodes attached to the scalp or head region of a seizure-prone individual
or seizure
patient. In EEG, electrodes may be positioned so as to measure such activity;
that is,
electrical activity originating from neuronal tissue. Alternatively,
electromyography (EMG)
may be used for seizure detection. In EMG, an electrode may be placed on or
near the skin,
over a muscle, to detect electrical activity resulting from muscle fiber
activation.
[0004] Detecting an epileptic seizure using EEG typically requires attaching
many
electrodes and associated wires to the head and using amplifiers to monitor
brainwave
activity. The multiple EEG electrodes may be very cumbersome and generally
require some
technical expertise to apply and monitor. Furthermore, confirming a seizure
may require
observation in an environment provided with video monitors and video recording
equipment.
Unless used in a staffed clinical environment, such equipment may not be
intended to
determine if a seizure is in progress, but rather provide a historical record
of the seizure after
the incident. Such equipment is usually meant for hospital-like environments
where a video
camera recording or caregiver's observation may provide corroboration of the
seizure, and is
typically used as part of a more intensive care regimen such as a hospital
stay for patients
1
CA 02971095 2017-06-14
WO 2016/105571
PCT/US2015/000475
who experience multiple seizures. Upon discharge from the hospital, a patient
may be sent
home, often with little further monitoring.
[0005] Ambulatory devices for diagnosis of seizures are generally EEG-based,
but
because of the above shortcomings those devices are not designed or suitable
for long-term
home use or daily wearability. Other seizure alerting systems may operate by
detecting
motion of the body, usually the extremities. Such systems may generally
operate on the
assumption that while suffering a seizure, a person will move erratically and
violently. For
example, accelerometers may be used to detect violent extremity movements.
However,
depending upon the type of seizure, this assumption may or may not be true.
Electrical
signals sent from the brain during some seizures may be transmitted to many
muscles
simultaneously, which may result in muscles fighting each other and
effectively canceling out
violent movement. In other words, the muscles may work to make the person
rigid rather than
cause actual violent movement. Thus, some seizures may not be consistently
detected with
accelerometer-based detectors.
[0006] Ambulatory devices for diagnosis of seizures are generally not suited
to grade
seizures based on intensity, nor are they suited to differentiate seizure-
related signals based
on event type. Rather, different types of seizures and related events may
often be grouped
together. Accordingly, ambulatory devices for seizure detection may be ill-
suited to
customize responses for different types of detected seizure events. In
addition, other
ambulatory devices may not be ideally suited for cost-effective monitoring of
some patients.
For example, using current ambulatory devices, caregivers may misdiagnose some
conditions, including, some that may benefit from condition-specific
therapies. For example,
some events, such as psychogenic or non-epileptic seizure events, may be
grouped together
with generalized tonic-clonic seizure events. Statistical analysis of event
signals and pattern
recognition methods may encourage effective diagnosis of some commonly
misdiagnosed
conditions. However, other ambulatory detection systems are generally not
configured to
provide organized statistical information to caregivers or process data for
identification of
specific activity patterns as may be used to medically or surgically manage a
patient's care.
[0007] Accordingly, there is a need for epileptic seizure detection methods
and
apparatuses that can be used in non-institutional or institutional
environments without many
of the cumbersome electrodes to the head or extremities. There is further a
need for detection
methods that are suited to grade seizures by type and/or intensity, identify
specific patterns of
seizure or seizure-related activity, and customize alarms so as to provide
robust and cost
effective patient care. There is also a need for monitoring systems that
organize medical data
2
CA 02971095 2017-06-14
WO 2016/105571
PCT/US2015/000475
within databases to help medically and surgically manage patient care.
SUMMARY
[0008] In some embodiments, a method or apparatus of monitoring a patient for
seizure activity may include identifying peaks included among a collected EMG
signal and
calculating one or more peak property values for the identified peaks. The
property values
may be compared to qualification thresholds, the qualification thresholds
including at least
one qualification threshold associated with individual peaks and at least one
aggregate
qualification threshold associated with a group of peaks. Peaks meeting the
qualification
thresholds may be deemed to be qualified. And, once peaks are qualified, a
level of qualified
peak activity may be calculated and used in initiating a system response.
[0009] In some embodiments, a method or apparatus of monitoring a patient for
seizure activity may include collecting an EMG signal and identifying peaks of
elevated
EMG signal. Peak data may be searched for one or more groups of peaks
indicative of seizure
activity by constructing various test groupings of peaks, calculating one or
more property
values for each of the test groupings, and comparing the property values to
one or more
threshold property values. Peaks may be qualified if they are part of at least
one test grouping
that meets at least one of the threshold property values, and from qualified
peaks or bursts a
level of activity may be determined and compared to a threshold level of
activity for initiating
a system response.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] Fig. 1 illustrates embodiments of a method for qualifying peak data.
[0011] Fig. 2 illustrates model EMG data including a set of peaks and several
groups
constructible from the set of peaks.
[0012] Fig. 3 illustrates additional model EMG data.
[0013] Fig. 4 illustrates additional model EMG data.
[0014] Fig. 5 illustrates a bar distribution graph associated with model EMG
data.
[0015] Fig. 6 illustrates embodiments of methods for combining groups of
peaks.
[0016] Fig. 7 illustrates two groups of peaks in a model EMG data set.
[0017] Fig. 8 illustrates embodiments of a method for detecting or searching
for
seizure patterns in EMG data.
[0018] Fig. 9 illustrates embodiments of a seizure detection system.
[0019] Fig. 10 illustrates embodiments of a detection unit.
3
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
[0020] Fig. 11 illustrates embodiments of a base station.
DETAILED DESCRIPTION
[0021] The apparatuses and
methods described herein may be used to detect
abnormal motor muscle activity, including, for example, seizure activity, and
timely alert
caregivers if activity is identified. The apparatuses may include sensors
disposed on, near, or
underneath the skin of a patient or attached to a patient's clothing and may
be configured for
measurement of muscle electrical activity using EMG. In some embodiments,
apparatuses
herein may include one or more processors suitable to receive an EMG signal
and process the
signal to detect seizure activity. Detection of seizures using EMG electrodes
is further
described in, for example, Applicant's U.S. Patent Nos. 8,983,591 and
9,186,105, Applicant's
U.S. Patent Application Nos. 14/920,665 and 14/816,924, Applicant's
International
Applications PCT/US14/61783 and PCT/US14/68246, and Applicant's U.S.
Provisional
Patent Application Nos. 61/875,429, 61/894,793, 61/910,827, 61/969,660,
61/979,225,
62/001,302, 62/032,147, 62/050,054, 62/096,331, and 62/149,434 the disclosures
of each of
which are herein fully incorporated by reference. Some of the methods
disclosed in the
aforementioned references describe detection of peaks of elevated EMG signal
amplitude and
qualification of peaks most likely to be related to seizure activity. Peak
detection methods,
including some which may be used in some of the embodiments herein, are
described, for
example, in detail in Applicant's U.S. Application No. 14/920,665.
[0022] In this disclosure,
methods of detecting and qualifying peaks in a collected
EMG signal are also described. In addition, embodiments are described where
groups of
peaks may be constructed from among an initial group of peaks. Test groups of
peaks may,
for example, be compared against various qualification criteria or thresholds
to facilitate
qualification of peaks, in aggregate or combination, that may be related to
seizure activity.
Some of those embodiments may be particularly useful in detection or
identification of peak
combinations related to particular patterns of motor manifestations,
including, for example,
patterns associated with seizure activity that may otherwise fail to be
identified in data that
may be noisy or where data quality may be intermittent or sporadic. In
addition to
embodiments for real-time patient monitoring, embodiments herein may also be
used to
organize collected EMG data for use in databases of stored medical data.
[0023] In some embodiments,
methods of monitoring a patient may include
testing various groupings of peaks and searching for one or more groups that
meet one or
more qualification criteria. Qualification criteria may be associated with and
tailored to
4
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
identify particular patterns of seizure activity. In some embodiments where
multiple groups
of peaks may be constructed, groups may be combined in various ways to
determine an
appropriate response. For example, several groupings of peaks may be
constructed each of
which may, in some cases, independently meet a qualification criterion. An
overall level of
qualified peak activity may then be determined using procedures as further
described herein.
For example, in some embodiments, all peaks that are members of at least one
group of peaks
that meets qualification may be included in determining an overall level of
qualified peak
activity. An overall level of qualified peak activity may then be compared to
one or more
thresholds. Based on that comparison, an appropriate response may be
initiated.
[0024] In some embodiments,
methods of monitoring a patient may include
testing multiple groups of peaks and searching for one or more groups that
best fit one or
more qualification criteria. For example, one or more groups may be selected
which are
characterized as having property values that meet qualification with greatest
confidence and
which may be most strongly correlated with seizure-related activity. In some
embodiments,
one or more property values for a group of peaks may be minimized or maximized
in order to
select or find peak groupings that may be related to seizure activity with
high confidence or
to find peak groupings most likely to be related to true physiological
activity of a patient and
not biased by inadvertent inclusion of peak data from sources of noise.
[0025] In some embodiments,
qualification of peak data may be executed in steps
or stages. For example, peaks may be initially qualified, peaks that fail
initial qualification
removed from further qualification, and remaining peaks organized in one or
more groups.
The groups may then be qualified based on comparison of group property values
to aggregate
property thresholds.
[0026] In some embodiments,
properties and/or qualification threshold values
may be selected for use in routines in order to provide or enhance selectivity
for identification
of particular patterns of seizure activity including, for example, clonic-
phase seizure activity,
psychogenic non-epileptic seizure activity, other activity patterns, and
combinations thereof.
Where reference is made to a routine that may be selective for identification
of a specific
activity, the routine may provide a positive response in a patient
experiencing that activity,
but the routine may provide a negative response in the absence of such
activity, even if other
types of seizure activity may be present. Therefore, execution of one or more
selective
routines may encourage identification of a particular type of seizure
activity, such as one or
more particular part or portion of a seizure. Routines herein that may be
configured for
detection of clonic-phase activity may be used to differentiate detected
clonic-phase activity
5
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
from other phases of seizure activity, including, for example, tonic-phase
portions of seizure
activity. Some of those routines may additionally be configured to
differentiate detected
clonic-phase activity from other activity commonly confused with the clonic
phase of a
generalized tonic-clonic seizure, including, for example, activity resulting
from non-epileptic
psychogenic seizure events.
[0027] At a high level,
procedures for qualification of peaks may include
comparison of various peak properties to one or more qualification thresholds.
For example,
if, for a peak, one or more peak property values meet one or more
qualification thresholds, a
qualification criterion may be deemed satisfied, and the peak may then be
referred to as a
qualified peak. Where a peak is qualified as meeting a criterion selective of
clonic-phase
seizure activity, the peak may also be referred to as a qualified-clothe-phase
burst.
[0028] Some qualification
procedures may operate on individual peaks. For
example, certain properties of a peak such as its height, area, or duration
width may be
defined without including data from other peaks. Therefore, individual values
for the property
may be calculated for each peak in a group and compared to appropriate
qualification
thresholds. Properties of individual peaks may include, for example, peak
height, peak area,
signal-to-noise ratio (SNR) (e.g., a ratio of peak amplitude to estimates of
uncertainty in peak
amplitude as may be measured or estimated from background regions of signal),
duration
width, duration of intervening periods of lesser signal on either side of a
trailing or leading
edge of a peak, other properties of individual peaks, and combinations
thereof.
[0029] In some embodiments,
peaks may be compared against qualification
thresholds selected from a group of qualification thresholds including a
minimum duration
width, maximum duration width, minimum signal-to-noise ratio (SNR), minimum
duration of
one or more intervening periods of lesser signal on either side of a peak,
maximum duration
of one or more intervening periods on either side of a peak, and/or
combinations thereof. An
intervening period may be defined by the duration length of a region of signal
stability or
lesser signal amplitude than elevated portions of a peak (e.g., low signal
variability, RMS
noise or signal magnitude) which may, for example, be marked by the distance
between a
peak edge and a nearby region of signal increase in magnitude or decrease in
signal stability.
In some embodiments, a signal-to-noise ratio for a peak may be calculated
using amplitude
data for the peak and an estimate or calculation of signal noise. Noise may,
for example, be
determined by calculating or estimating a level of variation or uncertainty in
a baseline signal
(e.g., uncertainty in measurement of signal amplitude, height, or area that
may result from
fluctuations in EMG data for a region not included in the peak) which may, for
example, be
6
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
determined from data collected on either side of a peak or from a separately
measured portion
of an EMG signal such as a portion collected when a patient is at rest. To
calculate noise, for
example, signal may be collected and signal variability may be directly
measured.
Alternatively, noise may, for example, be estimated from a signal magnitude
and an estimate
of variability expected from variations typical of a signal of that magnitude
as predicted by
one or more model functions, including for example, a normal distribution
model function. In
some embodiments, an estimate of variations or uncertainty in a baseline
signal or noise may
be selected or calculated during one or more system calibration routines.
[0030] In some embodiments, a
peak may be qualified by meeting a threshold
SNR, by meeting a minimum threshold for peak duration width of about 25 to
about 75
milliseconds, and by meeting a maximum threshold for peak duration width of
about 250
milliseconds to about 500 milliseconds activity. In some embodiments, a peak
may be
qualified based on the presence of an intervening sequence of lesser signal on
either side of a
peak of about 50 milliseconds to about 300 milliseconds. Other embodiments of
peak
qualification, including those that may operate on individual peaks, are
further described in
various others of Applicant's copending applications incorporated by reference
herein.
[0031] Some properties of peak
data may be calculated for more than one peak.
And, in some embodiments herein, procedures for qualification of peaks may
include
comparison of a plurality of peaks to one or more qualification thresholds.
That is, a plurality
of peaks may be selected together as a group, an aggregate property value for
the group of
peaks determined, and the aggregate property value compared to one or more
associated
thresholds. If accurately characterized, aggregate properties of a plurality
of peaks may be
highly selective for seizure activity. However, many aggregate properties may
be biased by
the inadvertent inclusion of data from noise sources and/or biased from
inadvertent exclusion
of desired data associated with relevant physiological events. Many of the
methods herein
address this concern. For example, some embodiments herein may be used to find
particular
combinations of peaks indicative of seizure activity from among noisy data.
[0032] A qualification
threshold value related to a property of a group of peaks
may be referred to as an aggregate qualification threshold value. For example,
included
among aggregate qualification threshold values that may be used to qualify a
plurality of
peaks are minimum and/or maximum rates of peak repetition, thresholds for
variations in
duration of times between peaks, other aggregate property thresholds, and
combinations
thereof. Aggregate qualification of groups of peaks is further described in
Applicant's
copending U.S. Application 14/920,665 incorporated herein by reference.
7
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
[00331 In some embodiments, a
group of peaks may be qualified against a
threshold value for minimum repetition rate of peaks of about 1 peak per
second and a
threshold value for maximum repetition rate of peaks of about 7 peaks per
second. In some
embodiments, for example, if a greater number or lesser number of peaks than
bounded by
the above thresholds is present over an appropriate interval (e.g., an
appropriate interval to
scale a number of peaks as a peak rate), it may be deemed that the peaks may
not meet
qualification.
[0034] Included among various
metrics for characterizing variation in duration of
times between peaks is an average deviation percentage. However, other metrics
for
characterizing variability of peak timing such as standard deviation, average
deviation or
percentage deviation values may also be used. Any of the aforementioned
metrics may be
calculated and may be used as aggregate property values comparable to
aggregate property
threshold values as described herein. In some embodiments, a group of peaks
may be
qualified if an average deviation percentage value for time between peaks is
greater than
about 1% or about 5%. That is, an aggregate property threshold value of
minimum average
deviation percentage may, in some embodiments, be between about 1% to about
5%. In some
embodiments, a group of peaks may be qualified if an average deviation
percentage value for
time between peaks is less than about 40% or about 50%. That is, an aggregate
property
threshold value of maximum average deviation percentage may, in some
embodiments, be
between about 40% to about 50%. Routines for determining variations in
duration of times
between peaks are further explained in greater detail in various others of
Applicant's
copending applications incorporated herein by reference.
[0035] In some embodiments, a
procedure for peak qualification may include an
initial qualification step based on one or more criteria as described above
(e.g., criteria based
on individual peaks), removal of peaks that fail the initial qualification,
and another
qualification step based on calculation of one or more aggregate property
values for
remaining peaks (e.g., all peaks that meet the initial qualification). For
example, peaks may
be identified, some peaks removed from overall qualification (e.g., peaks may
be removed
because the peaks are too narrow or too wide), and then remaining peaks
qualified if the
remaining peak data as a whole meets one or more aggregate threshold criteria.
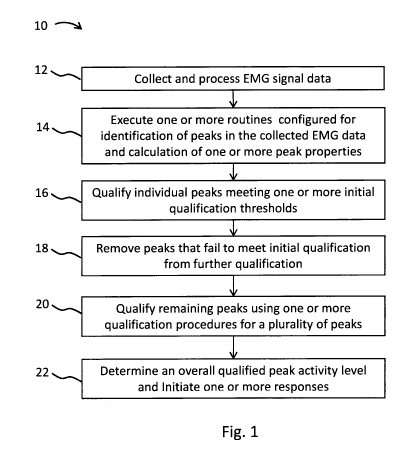
[0036] Figure 1 illustrates
embodiments of a method 10 for qualification of data
including an initial qualification step based on one or more criteria of
individual peaks and
further qualification of data based on one or more criteria for a plurality of
peaks in a group.
[0037] In step 12, EMG signal
may be collected and processed. The signal may be
8
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
processed using various techniques as may be used to improve detection of
seizure activity
and/or to condition the signal data for further analysis. For example,
collected signal may be
processed using one or more low-pass, high-pass, notch, or other filters to
improve
discrimination of seizure signals from other signals including sources of
noise. In some
embodiments, signal may be processed by removing high frequency signal
components, such
as by removing components above about 120 Hz or about 240 Hz. In some
embodiments, one
or more frequency bands may be isolated from a collected EMG signal. Isolation
of one or
more frequency bands may include use of one or more filters and/or execution
of one or more
other procedures for isolation of signal data, including, for example,
execution of a Fourier
transform. In some embodiments, a plurality of frequency bands may be isolated
from a
collected EMG signal, and a 1-squared statistical value may be calculated from
the isolated
signal data. In some embodiments, signal may be conditioned to prepare the
data for
processing using one or more peak detection programs, or signal may be
conditioned for
wavelet analysis. For example, signal data may be rectified, smoothed, and/or
both.
[0038] In step 14, one or more
routines may be executed that may be configured
for identification of peaks in the collected EMG signal. Identification of
peaks may, for
example, include identifying portions of EMG data or portions of smoothed EMG
data where
curvature of the data changes (e.g., inflection or other critical points may
be identified in the
data). Trailing and/or leading edges of one or more peaks may be determined,
which may be
used to define temporal boundaries of peaks. Various peak characteristics or
properties may
then be determined. For example, one or more peak widths, peak height values,
peak areas, or
other peak properties may be calculated. Reference may be made to a peak
amplitude, which
as used herein may refer to either of a peak area or peak height unless where
further
specified. If peaks are successfully qualified, those properties may, for
example, be used to
determine a level of qualified peak activity.
[0039] In some embodiments,
processing of EMG signal and/or peak detection
(steps 12, 14) may include execution of smoothing techniques (e.g., moving
average filter,
Savitzky-Golay filter, Gaussian filter, Kaiser Window, various wavelet
transforms, and the
like), baseline correction processes (e.g., monotone minimum, linear, loss
normalization,
moving average of minima, and the like) and peak-finding criteria (SNR,
detection/intensity
threshold, slopes of peaks, local maximum, shape ratio, ridge lines, model
based criterion,
peak width, and the like) and may involve processing of rectified or
unrectified data.
[0040] In some embodiments,
signal may be processed and peaks detected
(steps 12, 14) without loss or significant loss of temporal resolution of a
collected signal. For
9
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
example, data may be smoothed, integrated or both, but levels of processing
may be
appropriate to detect and qualify peaks as associated with clonic-phase
activity. For example,
protocols for smoothing and integration of data may be selected in order to
maintain temporal
resolution suitable for reliable detection of whether a peak meets selected
duration width
thresholds that are indicative of clonic-phase activity.
[0041] In step 16, peak data
may be initially qualified against one or more
qualification thresholds associated with individual peaks. As described above,
various
qualification thresholds may be used to qualify individual peaks. For example,
in some
embodiments, peaks may be qualified by meeting a threshold SNR and by meeting
a
minimum threshold for peak duration width of about 25 milliseconds to about 75
milliseconds and a maximum threshold for peak duration width of about 250
milliseconds to
about 500 milliseconds.
[0042] In step 18, peaks that
fail to meet initial qualification may be removed.
Accordingly, step 18 may act as an initial screen to filter peaks from being
included in other
qualification steps. In step 20, remaining peaks that were not removed
following step 18 may
be qualified by calculating one or more aggregate properties of a plurality of
peaks and
comparison of property values to one or more aggregate property thresholds.
For example,
any of the above aggregate properties of peaks, including, for example,
repetition rate or
variability in times between peaks, may be used to qualify a plurality of
peaks together as a
qualified group.
[0043] Once peak data is
qualified (e.g., qualified as including some number of
qualified peaks), a level of qualified peak may then be determined. For
example, following
qualification of remaining peaks (as shown in the step 20), a level of
activity may be
determined and compared to an activity level threshold. As shown in step 22, a
comparison of
an activity level to a threshold activity level may be executed. Based on that
comparison
and/or other information, at least in some embodiments, a decision on whether
to initiate one
or more responses may be made. For example, a qualified peak count or rate may
be
determined and compared to a threshold qualified peak count or rate in order
to determine if a
response, including, for example, an alarm response, is warranted. In some
embodiments, a
response may include logging a positive detection of seizure activity, and if
several
consecutive or nearby positive detections are made, an alarm response may be
initiated. In
some embodiments, other responses or actions, in addition or as an alternative
to an alarm
response, may be initiated based, at least in part, on a level of qualified
peak activity. For
example, if a certain rate of qualified peak count is identified in a part of
a collected EMG
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
signal, the part may be flagged or marked as a possible seizure event, the
determined
qualified peak rate may further be linked to that part of the collected signal
data. The data and
other associated qualified peak metrics may then be stored and included in a
searchable
database of medical data.
100441 Steps in the method 10
may be executed within one or more time windows
that may be of the same or different durations. For example, data may be
collected and
processed (step 12) over some number of suitable collection time windows as
may be
appropriate to maintain a desired temporal resolution for a monitoring system.
Steps 14, 16,
and 18 may generally operate as individual peaks are detected or at other
suitable times so
that those steps may be complete before remaining peaks are processed in step
20. Timing
windows for group qualification (step 20) and for determining overall
qualified peak activity
levels and response initiation (step 22) may be adjusted for different
routines. Generally,
qualification time windows applied in step 20 and response windows applied in
step 22 may
be suitable in duration length in order to gather and analyze a desired amount
of statistical
data in order to reliably detect the presence of a seizure or seizure-related
pattern. For
example, in some embodiments, qualification time windows and response time
windows may
last for a duration of about 1 second to about 10 seconds or longer in some
cases. For
example, some patterns related to recovery from seizure activity or patterns
indicative of non-
epileptic psychogenic seizure events may be detected by examining signal
collected over at
least somewhat longer time scales than may be used for detection of initial
seizure activity.
100451 In some embodiments,
all remaining peaks may be qualified together in a
given window for qualification in step 20. For example, all remaining peaks
within a
qualification time window may meet or fail to meet an aggregate threshold
value. Thus, all of
those remaining peaks may be qualified and counted as qualified peaks or all
of the
remaining peaks may fail to meet the aggregate threshold value thereby failing
overall
qualification. Accordingly, those peaks may not be counted towards an overall
level of
qualified peak activity in step 22.
[0046] In some embodiments,
aggregate threshold values may be selected to
accommodate for some probability that one or more physiological events may be
missed
within a qualification time window. For example, aggregate qualification
thresholds may be
broad enough so that even if some number of physiological events (e.g.,
individual
physiological events that tend to produce a seizure-related peak) fail to be
detected, that other
appropriately detected events do not fail aggregate qualification. For
example, if, in a series
of 8 adjacent physiological events associated with a burst of muscle activity
in the clonic
11
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
phase of a seizure, 7 peaks are detected, but a peak detection program fails
to identify one
event, the missing event may not skew the aggregate peak data so much that all
peaks will
fail aggregate qualification.
[0047] In some embodiments, a
qualified peak activity level may be calculated in
a response window that may extend over some number of adjacent or overlapping
qualification time windows. For example, a response window may extend over a
time frame
that includes several qualification time windows. In some embodiments,
successive or
adjacent response time windows and/or qualification time windows may, for
example, be
overlapped to avoid or minimize latency between detection of seizure activity
and initiation
of a response as understood in the art. Response windows may also be tailored
for a given
pattern. For example, two or more routines may run simultaneously, the
routines being
configured or optimized for detection of different patterns and set to analyze
data in response
windows of different duration lengths.
[0048] Therefore, it should be
understood that qualification time windows and
response time windows may conveniently be of the same duration, but this need
not be the
case. For example, in some embodiments, a qualification time window may last
about 2
seconds and adjacent qualification time windows may overlap for about 0.5
seconds. In each
qualification time window, peaks may be qualified and some number of qualified
peaks
determined. A level of qualified peak activity may, for example, be determined
at the
completion of each qualification time window and may, for example, include
data from a
preceding group of three qualification time windows (e.g., 5 seconds of data).
If a calculated
level of qualified peak activity exceeds a threshold, an appropriate response
may be initiated.
Because more than one qualification time window may be included in calculation
of a
qualified peak activity level, a risk that one or more spurious peaks may
eliminate all peak
data from aggregate qualification (step 20) may accordingly be reduced.
[0049] As described above, in
some embodiments, qualified peak activity
thresholds and/or other qualification thresholds may be broad enough so that
even if some
number of physiological events fail to be detected (or if a limited number of
erroneous peaks
are incorrectly counted), an alarm or other appropriate response may still be
initiated. In some
embodiments, an estimate of an actual number of peaks resulting from
physiological
manifestations of motor activity during a seizure may be made, and that number
may be
processed to have peaks that may be unrelated to seizure activity, such as
noise spikes,
removed. Because spurious events may be removed, thresholds for alarm
initiation may, in
=
some embodiments, be centered more narrowly around an expected range,
including, for
12
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
example, a range based closely on numbers of physiological events typically
expected during
a seizure. In some embodiments, thresholds for real-time detection of seizure
events may be
configured to improve sensitivity for seizures, but for other purposes,
including, for example,
meta data creation and/or linking of peak statistical information to collected
data (e.g., for
archiving in a searchable database), processing routines including different
criteria may be
applied. In some embodiments, routines for removing peaks expected to be
spurious may be
executed as part of archiving or retrieving data from a searchable database.
Therefore, in
some embodiments, some routines or combination of routines, including some
that may have
different threshold settings, may be executed as part of real-time seizure
detection methods,
data archiving methods as may be used in creation of searchable databases, or
both.
[0050] In some embodiments of
methods for real-time seizure monitoring and/or
methods of organization of data for a database, one or more test groups of
peaks may be
constructed, each of the one or more test groups of peaks being a subset group
of an original
group of peaks. As used herein, a subset of peaks or subset group of peaks is
a portion of
another set of peaks if every peak among the subset of peaks or subset group
of peaks is a
member of the other set. In some embodiments, one or more aggregate property
values may
be determined for each of multiple test groups that are a subset of an
original set of peaks.
Some of those embodiments may further determine if any of the subset test
groups possess an
aggregate property value that meets an aggregate property value threshold. For
example, a
test group of peaks constructible from an original set of peaks may include
some number of
consecutive peaks in the original peak set. For example, Figure 2 illustrates
various
combinations or groupings of peaks that may be used as test (coups of peaks
and calculations
that may be included as part of a qualification procedure.
[0051] In some embodiments,
test group construction may be integrated together
with the method 10. For example, as an alternative to embodiments of method 10
wherein a
processor may only calculate one or more aggregate property values for all
remaining peaks
in step 20, one or more test groups of peaks may be constructed from a
remaining group of
peaks and one or more aggregate properties for each of the one or more test
groups may be
compared against one or more aggregate property thresholds.
[0052] Referring again to
Figure 2, model data 24 is shown. The model data 24
may, for example, be produced in a scenario where initial qualification of
peaks (as may, in
some embodiments, be executed as described in step 16) and removal of peaks
(as may, in
some embodiments, be executed as described in step 18) provide 8 remaining
peaks (xi, x2,
..., x8) within a time interval. Test groups may be created from those 8
remaining peaks. For
13
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
example, peaks may be ordered based on a time stamp of when they were
identified, and a
first test group, including the first 4 consecutive peaks (xi, x2, x3, x4) in
the time interval
shown in Figure 2, may be created. As part of qualification, it may be
determined if one or
more aggregate property values for that first test group meets one or more
aggregate property
threshold values. More generally, in some embodiments, some routines may
determine if one
or more aggregate property values for a set of test groups, each test group
member of the set
including some number of consecutive peaks (such as four or more) in the
original set of
peaks from which the test groups were created, meets one or more aggregate
property
threshold values. Referring back to the specific example for the model data
24, other test
groups may also be created, and again aggregate property values may be
determined and
tested against thresholds. For example, a next group of 4 consecutive peaks
(x2, x3, Li, x5) in
the time interval may be constructed and used in a second aggregate property
value
calculation. In that next group, for example, peak x5 was added and peak xi
was removed.
Further test groupings may be created and associated calculations determined
as shown in
Figure 2. For example, for model data 24, a series of 5 consecutive groups may
be created
and aggregate property values determined and tested against an appropriate
threshold for each
created group.
100531 In some embodiments,
test groups may, for example, be created by
incrementally selecting a next peak present in an interval and keeping the
number of peaks in
the test group the same. In some embodiments, aggregate property value
calculations may be
executed consecutively or in some other order so that all combinations of test
groups in an
interval that may include some number of consecutive peaks are considered. In
each
calculation, an aggregate property value for the constructed test group may,
for example, be
determined and compared to a threshold aggregate property value.
100541 In some embodiments,
test groups including other numbers of peaks (e.g.,
different than 4) may be constructed. Test group construction may include
scanning a time
stamped list of peaks in order of when they were detected and selecting some
consecutive
number of peaks to be included in a test group. The list may then be
incremented and a next
group among the list selected. For example, in Fig. 2, the groups 1-5 may be
constructed by
incrementing an ordered list in single units. In some embodiments, a list of
peaks may be
incremented in other desired units. Once a set of test groups is constructed,
property values
for members among the set of test groups may be calculated, such as in a
convenient order,
and peaks may be identified that meet qualification for at least one of the
test groups. For
example, in some embodiments, a peak may be deemed qualified if it is part of
at least one
14
CA 02971095 2017-06-14
WO 2016/105571
PCT/US2015/000475
test group that meets qualification. For example, peaks in a window may be
treated as
described above in groups of 4 or some other suitable number such as about 4
to about 8
peaks. Such procedures may, for example, be used to limit a number of
calculations executed
in processing data. In addition, it may be more convenient to write protocols
executed by a
computer processor to construct test groups including some pre-selected
number.
[0055] In some
embodiments, in addition to use of a certain pre-selected number
of peaks for creation of test groups, other test groups may be created
including test groups
including other numbers of peaks or including test groups that include a range
of peak
numbers. Accordingly, another set of calculations of aggregate property values
may be
executed. For example, one aggregate property value may be calculated for each
group. More
than one aggregate property value may also be calculated for each group.
[0056] To
identify particular patterns, different combinations of aggregate
properties and aggregate property thresholds may be applied. Continuing with
the example
herein where groupings of 4 consecutive peaks are considered, aggregate
property values
may next be determined for groups of 5 consecutive peaks. In some embodiments,
groups of
peaks may be limited to those that may fit within a preselected time window.
In other
embodiments, one or more test group of peaks may wrap or extend into an
adjacent window.
Appropriate rules may be established for specific routines to deal with test
groups that may
extend into an adjacent window. Referring to the example in Figure 2, and
where only
combinations of consecutive peaks that fit in the time window are considered,
such as for
ease in explanation, to consider all combinations of test groups that include
5 consecutive
peaks, 4 test groups may be created. That is, a first group of 5 consecutive
peaks in this
example may include the peaks (xi, x2, x3, x4, x5). Other groups, including a
last group in the
set (when iterating forward in the time stamped data in single peak
increments) including the
peaks (x4, x5, x6, x7, x8), may be created.
100571 In some
embodiments, a group of peaks ranging from some minimum
number of peaks (e.g., a chosen minimum number to qualify as a group with a
suitable
number of peaks to perform desired calculations ¨ such as a number to reliably
calculate a
standard deviation for times between peaks) to the total number of peaks
identified in a time
window may be examined. For example, selecting 4 peaks as a minimum number of
peaks
that may be suitable to accurately qualify peaks using a certain aggregate
property, if over a 2
second period of time 8 peaks were identified, various groupings including a
number of 4, 5,
6, 7, and 8 peaks per group may be constructed and included in qualification
calculations. In
that case, by way of example only, a total of 15 groups may be constructed.
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
[00581 In some embodiments,
test groups may consist of peak groups including
only consecutive peaks in a group of peaks from which test groups may be
constructed. In
other embodiments, test groups may include consecutive peaks, non-consecutive
peaks (e.g.,
peak combinations where one or in some cases more than one intervening peak
that is
excluded from the test group), or both.
[00591 For example, Figure 3
shows model data 26 and various groups of peaks
that may be constructed. Model data 26 may, for example, be produced in a
scenario where
initial qualification of peaks (as may, in some embodiments, be executed as
described in step
16) and removal of peaks (as may, in some embodiments, be executed as
described in step
18) provide 5 remaining peaks (A, B, C, D, and E) within a time interval.
Groups may be
constructed from that group of remaining peaks. In the model data 26, a group
of peaks (A,
B, C, D, and E) is shown. The peak C may be a peak that is an artifact of
noise and unrelated
to the desired physiological signal intended for measurement in EMG, but may
have failed to
be removed by other processing methods. Groupings including non-consecutive
peaks may
work under an assumption that a spurious peak failed to be removed in initial
qualification or
in other protocols, and a procedure may test whether excluding a peak from a
group of peaks
results in identification of a group of peaks that may be qualified to be
indicative of seizure or
seizure-related activity. For example, when removing peak C, an expected
pattern or group of
peaks may result. For example, such a pattern may be characterized by meeting
both of an
aggregate threshold for repetition rate and variability of times between
peaks. And, the peak
combination (A, B, D, E) may be deemed to meet a criterion for a seizure
pattern the seizure
pattern, the seizure pattern being identified by removing one spurious peak
from an initial
group of peaks.
[00601 For some patients,
particularly, if, for example, contact between the skin
and electrodes has become poor, a condition that may, at least in some cases,
be the result of
excessive sweating and/or other conditions that may increase in likelihood as
a patient
transitions towards a seizure or during seizure recovery, one or more peaks
not directly
related to clonic-phase activity may fail to be removed by initial
qualification or by other
screening means. In that event, improved protocols for examining whether
spurious peaks are
present in a given data set may be advantageous for some patients and/or in
some situations.
Construction of test groups of non-consecutive peaks may meet the
aforementioned need for
improved protocols.
[00611 In some embodiments, if
a number of peaks is removed from data as part
of peak qualification, other requirements for alarm initiation or emergency
alarm initiation
16
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
may be used to minimize false positive detections. Or, a response may be
different if peaks
have been removed in order to meet an activity level threshold. For example, a
detection
protocol may identify a seizure related pattern by removing some number of
peaks from a
particularly noisy data set. The system may then initiate one or more warning
protocols,
including those that may or may not contact a caregiver or patient (e.g., the
one or more
protocols may terminate passively), and then examine data for one or more
corroborating
events, detection of which may improve confidence in seizure detection. Some
procedures
that remove peak data may also be executed as part of data archiving. For
example, one set of
routines may be used to initiate one or more alarm protocols. Another set of
routines,
including routines configured to remove suspect or erroneous noise peaks and
look for
seizure patterns, may be configured to isolate or find peak patterns
indicative of true
physiological activity and not biased by inadvertent inclusion of peak data
from sources of
noise.
[0062] In some embodiments,
protocols for removing spurious peaks may work
by removing one or more peaks without specific consideration for how likely it
may be that
the peak is erroneous or without specific consideration for whether a removed
peak is more
or less likely to be spurious than other peaks in a peak set. For example, in
some
embodiments, peaks may be removed from a remaining group of peaks serially or
in some
convenient order to test different combinations, but the order in which peaks
are removed
may not be related to any specific property of a given remaining peak.
However, in some
embodiments, decisions or algorithms for removing peaks may be based on other
factors. For
example, qualification of peaks by determining an aggregate property for a
plurality of peaks
(e.g., as described in step 20) may include statistical processing to
determine whether any
peaks among the plurality should be removed because one or more peak
properties indicates
that the peak may be an outlier. For example, it may be found that including a
given peak in a
group inordinately biases a property value (or aggregate property value) such
as variability
for times between peaks. For example, a spurious peak may produce one or more
data points
in a property value calculation that bias group data in a manner greater than
predicted for a
normally or otherwise distributed data population. And, by removing that given
peak from a
group of peaks data that may otherwise fail qualification, a subset peak set
may then be well
qualified. Any of various mathematical techniques for processing data to
determine the
presence of outliers may, in some embodiments, be executed.
[0063] In some embodiments,
test groupings of peaks may be made from a group
of peaks that is itself a subset of another group. Embodiments where test
groups may be
17
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
constructed from other groups that are themselves subsets of another group of
peaks may be
understood in reference to Figure 4. Figure 4 shows a model set of peaks 28.
Model set of
peaks 28 may, for example, be produced in a scenario where initial
qualification of peaks (as
may, in some embodiments, be executed as described in step 16) and removal of
peaks (as
may, in some embodiments, be executed as described in step 18) provide 10
remaining peaks
(A, B,..., J) within a time interval. A group of peaks 30, including the peaks
(C, D, E, F and
G), which is a subset of the remaining peaks (A, B,..., J) is shown. The group
of peaks 30
may be constructed from the model set of peaks 28 as described, for example,
in reference to
Figure 2. For example, the group of peaks 30 may be constructed by scanning a
time stamped
list of a model set of peaks 28 in order of when they were detected and
selecting some
consecutive number of peaks to be included in a subset group. The list may
then be
incremented to identify a plurality of groups, including the subset group 30
shown in Figure
4. Other groups of peaks (e.g., groups 32, 34, 36, 38, and 40) may then be
constructed from
the subset group 30. For example, the aforementioned groups (32, 34, 36, 38,
and 40) may be
constructed by removing serially one peak from the group 30.
[0064] While serial removal of peaks from the subset group 30 may produce a
limited number of test groups, other subset groups may be produced from model
set of peaks
28. Those other subset groups may each serve as a basis for creation of test
groups.
Generally, based on constructing a set of subset groups, and then executing
serial removal of
one or more peaks from members of the set of subset groups, a significant
number of test
groupings may be constructed. Accordingly, the methods herein may be effective
at searching
a significant number of peak combinations and finding patterns of activity
from noisy data. In
some embodiments, only a suitable number of peaks, such as one peak, may be
removed from
a first subset group. In some cases, a maximum ratio of peaks that may be
removed during
75 construction of test groups may be defined in an algorithm. For example,
in some
embodiments, a maximum allowed ratio of removed peaks to peaks in a group
remaining
after peak removal may be about 1:5 to about 1:10. By selecting an appropriate
number of
peaks in a constructed subset group (e.g., 5 peaks as shown in Fig. 4) and/or
a suitable
number of peaks excluded or removed from those subset groups to generate test
groups,
multiple test groups may be constructed thereby increasing the chance that a
particular
pattern of peaks is constructed that will meet qualification. However, the
number of possible
peaks excluded from test groupings may be controlled. Accordingly, risk of
false detection of
a pattern may also be limited. And, therefore, in some embodiments, procedures
may be
made that are suitable for searching noisy data, but still minimizing risk of
false positive
18
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
detection, a problem that may otherwise be present if test groups are made
randomly and/or
using other protocols that may allow indiscriminate peak exclusion. Adverse
consequences of
false positive detection may be more problematic in methods associated with
real-time
detection of abnormal muscle activity. However, those concerns are not the
same for a
caregiver reviewing or searching data in a database. Accordingly, in some
embodiments, it
may be advantageous to execute different procedures for construction of test
groups as may
be used in organizing data for inclusion in a searchable database than used in
real-time
monitoring. For example, more aggressive algorithms for peak removal may be
applied when
including data in a searchable database.
[0065] In some embodiments,
procedures for generating test groupings may
include removal of peaks from an initial group of peaks wherein the removal of
peaks is
executed in a logical order or pattern. For example, a method of monitoring a
patient may
include construction of test groups wherein test groups most likely to be
associated with a
physiological pattern are tested first or ranked with higher confidence. For
example, a
procedure may order peaks based on one or more peak characteristics. Based on
that
ordering, a list of candidate peaks most likely to be spurious (e.g., not
related to physiological
activity) may be generated. For example, a group of peaks may be ordered based
on duration
widths and that ordering may be used to identify that the duration widths of a
majority of
peaks are clustered around a central range, but one or more peaks may be
characterized as
having a duration width outside of that central range. Peaks outside of the
most common
range may be removed, first removed in generation of test groupings, or test
groups created
from such removal may be ranked at higher confidence than other test groups.
In some
embodiments, test groupings may be constructed by removing one or more peaks
at either
end of a peak characteristic ordering. For example, the highest or lowest
value in an ordering
of one or more peak characteristics may be selected for removal.
[0066] For example, Figure 5
shows a model bar graph characterizing the
distribution of peaks identified in a collection time period and shows bars
52, 54. Peaks
associated with bars 52 and 54 are characterized as having a minimum property
value 52 and
a maximum property value 54 among a group of identified peaks. Peaks
associated with those
bars 52, 54 may be indicative of peaks with property values at the edge of
initial qualification
thresholds. As shown in Figure 5, most of the peaks tend to be clustered
towards greater
values of peak duration. And, the peak associated with bar 52 shows the
largest deviation
from other peaks in the group. Therefore, that peak may be removed from the
set of peaks or
removed first in selection of a test group. Test groups produced by removing
the peak
19
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
associated with bar 52 may be viewed with higher confidence than other test
groups
associated with removal of other peaks. An aggregate property value for the
subset of
remaining peaks may then, for example, be determined. In some embodiments, a
mean value
for the initial group of peaks may be determined and a deviation value from
the mean
determined for each individual member among the initial group of peaks. Test
groups may be
made by removing peaks in order of those peaks identified as having the
highest deviation
from that mean. For example, as shown in Figure 5, the peak associated with
bar 52 may have
the highest deviation from a mean value and may be most likely to be erroneous
or related to
noise in the collection window. Therefore, that peak may be removed or removed
first in a
protocol for generating test groups of peaks.
[0067] In some embodiments, a
routine may remove peaks from another group of
peaks based on how well peaks in the group meet an initial qualification
condition. For
example, a routine may assign an initial certainty value to peaks and test
whether removing
one or more peaks facilitates qualification and the identification of a
seizure-related pattern.
To minimize calculation resources or to provide other metrics of overall
certainty, a routine
may generally test or first test if removal of peaks with the lowest certainty
value results in
identification of an identifiable seizure pattern (e.g., a group of peaks that
meet an aggregate
property value threshold). In some embodiments, once a seizure related pattern
is identified,
computing or signal transmission resources may be allocated to most
efficiently execute one
or more system operations. For example, warning or emergency messages may be
issued as
part of alarm initiation protocol, and further construction of test groups or
transmission of
data associated with test groups (e.g., as may be sent from a remote device to
a managing
device or base station) may cease or only he executed after completion of
other operations,
such as those most critical to make sure emergency care is provided to a
patient. Certainty
values of peaks may, in some embodiments, be based on patient or patient
demographic
values or based on agreement with other peaks collected during a given time
window. In
some embodiments, certainty values for peaks may be based on a combination of
metrics
including, for example, SNR, width and amplitude as described in more detail
in Applicant's
U.S. Patent 8,983,591incorporated herein by reference.
[0068] For any given time
interval during a monitoring session, a number of
detected peaks or initially qualified peaks may be determined, and for a
majority of times
within the monitoring session those numbers may generally be low. For example,
even if a
random noise event is erroneously identified as a peak, most time windows may
only include
a limited number of such events. Furthermore, normal movements unrelated to a
seizure may
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
generally not produce peaks or initially qualified peaks at a high rate. That
is, using
procedures herein for initial qualification of peaks, peak data from most
motor manifestations
associated with non-seizure movements may be removed.
[0069] In some embodiments, if
a certain number of peaks or initially qualified
peaks is identified, an algorithm may attempt to fit remaining peak data to a
seizure pattern.
For example, in some embodiments, only if a suitable number of peaks to
generate test
groupings is identified may test groups of peaks be constructed. For example,
remaining
peaks following step 18 in method 10 may, for a majority of intervals in a
monitoring session,
be low and accordingly a method may not need to execute further calculations
associated
with construction of test groups or execute multiple calculations of aggregate
property values.
Accordingly, in some embodiments, operations associated with test group
generation and
property value calculations may only be executed when necessary, and
computational
resources dedicated to those calculations and remote transmission of that data
may further be
limited. In some embodiments, including embodiments where operations
associated with test
group construction may be executed on one processor and then transmitted to
another
processor for execution of peak construction and/or comparison to multiple
patterns,
protocols for selective execution of test group construction may be applied.
[0070] In some embodiments,
certain computations described herein, such as test
group construction, may be automated, executed with any suitable processor
among the
various components described herein (e.g., detection units and/or base
stations), and may
only be executed if a threshold number of peaks or initially qualified peaks
are found within a
given time window, a condition that may generally be limited particularly if
time windows,
initial qualification protocols, and requisite remaining peak numbers (needed
to invoke
further calculations) are suitably chosen such as described in various
embodiments herein.
[0071] In some of the
embodiments herein, in a certain time period such as a
response window, more than one group of peaks may be qualified. For example,
such a
scenario may arise when multiple peaks are identified from EMG data collected
over a
response window, multiple test groups are construed over that time period, and
different
test groups are found to meet qualification. In some embodiments, peaks among
different test
groups may be distinct. In other embodiments, test groups may sometimes
include peaks that
are members of more than one group. And, once qualified groups of peaks are
identified,
those groups may be combined and used to calculate an overall level of
qualified peak
activity. Various methods of combining groups of peaks, determining levels of
qualified peak
activity, and initiating one or more system responses based on levels of
qualified peak
21
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
activity may be executed in the embodiments described herein. For example,
techniques are
described herein for calculating overall levels of peak activity where groups
include only
distinct peaks. Other techniques may be applied where individual peaks may be
separately
qualified in more than one group.
[0072] Figure 6 illustrates
embodiments of a method 60 for combining groups of
peaks. For example, as may be understood in reference to the model set of
peaks 70 (shown
in Figure 7), it may be found that when including the first 4 peaks therein in
a first test group
72 that the first test group 72 may pass qualification. Thus, the first test
group 72 may also be
referred to as a first group of qualified peaks. A second test group of peaks
74 may likewise
pass qualification and may be identified as a second group of qualified peaks.
Accordingly,
the model set of peaks 70 may be found to include 8 total qualified peaks
present as two
distinct groups 72 and 74. In this scenario, the two groups include distinct
peaks. That is, no
peak is a member of both groups. It should also be understood that the groups
72, 74 may be
qualified even if including all 8 peaks may result in qualification failure.
For example, if all
peaks in the model set of peaks 70 were qualified by calculating a repetition
rate for the
peaks, the data set may be biased by the relatively long gap 75, which may be
present if, for
example, a peak finding routine failed to detect one or more abnormal motor
manifestations
during a seizure.
[0073] Referring again to Fig.
6 and Fig. 8, in step 62, it may be identified that the
two groups 72, 74 meet qualification, and one or more techniques may then be
used to
combine the two groups. For example, in step 64, distinct groups of qualified
peaks, such as
groups 72, 74, may be combined by summing each of the peaks in the two
distinct groups. An
overall level of qualified peak activity may then be calculated. For example,
for the set of
peaks 70, where the groups 72 and 74 are separately qualified, 8 qualified
peaks may be
combined together and used to calculate an overall level of qualified peak
activity. In step 66,
a decision may be made if the overall activity level is above a threshold. For
example, if a
threshold activity level is exceeded, it may be determined that an alarm
should be initiated.
[0074] In some embodiments,
combining of more than one group of qualified
peaks (step 64) may include comparing one or more property values for
different qualified
groups. For example, peak statistics may be calculated for each group of
peaks, and a degree
of similarity or difference for various peak statistics between the groups may
be determined.
Notably, for some patterns a degree of similarity between groups of peaks may
be expected.
For example, if the groups of peaks are part of an intermediate portion of the
clonic phase of
a generalized tonic-clonic seizure, the duration of periods adjacent an
elevated peak portion
22
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
may generally be of similar duration. Accordingly, if two or more groups of
peaks show
similarity in this statistical metric, it may be deemed that the groups in
combination match an
expected pattern for intermediate portions of the clonic phase. For other
patterns, a degree of
difference in the aforementioned metric may be expected between groups of
peaks collected
over time. For example, normal progression out of the clonic phase of a
seizure may be
recognized if peaks across two or more groups in a response window are
characterized by an
increase in the duration of periods adjacent an elevated peak portion.
[0075] In some embodiments,
combining of more than one group of qualified
peaks (step 64) may include determination of one or more pooled property
values based on
peak data from among the various groups. For example, two or more groups of
peaks may be
separately qualified, and a pooled property value may then be calculated to
determine a
relationship, such as a degree of similarity, between the two or more groups.
In some
embodiments, if a pooled property value for a plurality of groups meets a
threshold value,
e.g. if the pooled property value is within a specified minimum and/or maximum
threshold
value, separate qualified peak groups may be combined, and an overall level of
qualified peak
activity may then be determined. Therefore, in some embodiments, independently
qualified
peak groups may be subject to a further step of qualification before
determining an overall
peak activity value.
[0076] For example, two peak
groups separated in time may be independently
qualified, and by pooling property values together it may be estimated whether
the two
groups may reliably be treated as part of the same portion of a seizure. For
example, in two
separate duration intervals of an intermediate portion of the clonic phase of
a seizure, two
peak groups may be measured, but in an intervening section of data collected
at times
between the two intervals, data may be noisy, and that section of data may not
produce a
useful peak group. However, those two peak groups may each be separately
qualified, and if
pooling the data together indicates that the trains are each part of an
intermediate part of the
clonic phase, those two separate groups may be combined to determine an
overall qualified
peak activity level. Accordingly, that overall qualified peak activity level
may be used in
determining an appropriate response.
[0077] A pooled property value
may refer to a property value calculated by
including data from two or more peak groups weighted according to peak numbers
and
degrees of freedom. For example, in some embodiments, a property value for a
group of
peaks may be a variability of times between peaks as may be characterized as a
standard
deviation. To calculate a pooled property value for two groups, a first
standard deviation
23
CA 02971095 2017-06-14
WO 2016/105571
PCT/US2015/000475
value Si may be calculated for one group of peaks where ni is the sample size
of the group. A
second standard deviation value 52 for the second group of peaks may also be
calculated
where n2 is the sample size of that second group. A pooled standard deviation
value including
data from the two groups of peaks may then be calculated as follows:
Soodeco = Sqrt[[ (n1-1)S12 + (n2-1)S22]/[(ni -1)+(n2-1)-1]] Equation 1
More generally, for any number of groups of peaks ni, n2 ... nk with standard
deviation value
Si, S2. ....5k a pooled value may be calculated as follows:
S(pooled)=Sqra(ni -1)S12 +(n2-1 )S22 +...+(nk-1)Sk2]/[(ni-1)+(n2-1)+... (11k-
1)]]
Equation 2
[0078] In some
embodiments, a pooled value may serve as a check to verify that
groups of peaks may logically be considered part of the same portion of a
seizure. For
example, generally in one part of a clonic phase, peak rates may be maintained
within certain
bounds even if there is a general lowering of peak rate during later stages of
the clonic phase.
And, by pooling data from multiple groups of separately detected and qualified
peak groups,
sporadically detected peak data in a noisy signal may still be combined with
confidence.
[0079] Figure 8
illustrates embodiments of a method 80 for collecting EMG
signal, identifying peak data in the EMG signal, and evaluating whether one or
more seizure
or seizure-related motor manifestation patterns may be present. In some
embodiments,
method 80 may be executed in real-time as a patient monitoring strategy and
EMG signal
may be directly collected. In other embodiments, method 80 may be executed
following
signal collection, including, for example, when adding data to or searching
data in a medical
database. The various classes of embodiments are described, in the
alternative, in step 82. For
example, in step 82, EMG signal may be collected and peaks may be identified
in the
collected EMG signal. Alternatively, previously collected signal data may be
accessed such
as by downloading a portion of signal data.
[0080] In some
embodiments, EMG signal may be collected, peaks detected, and
an initial filter or screening of those peaks may be made before searching the
data for the
presence of one or more patterns. For example, individual peaks may be
screened against one
or more properties of the individual peaks prior to executing other
operations. For example,
in some embodiments, peak screening operations, such as step 16 and step 18 of
method 10,
24
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
may be executed as part of step 82. In some embodiments, where method 80 is
executed from
existing EMG data, an operator of a database may, for example, choose one or
more patients
and/or one or more time ranges of collected data. A program may subject the
data to peak
detection, or peaks already identified may be downloaded or accessed for
further processing.
[0081] In step 84, method 80
may include searching identified peaks for one or
more groups of peaks indicative of one or more seizure or seizure-related
activity patterns. In
some embodiments, step 84 may include two parts. For example, in a first part,
an initial
group of identified peaks may be subjected to test group construction. That
is, multiple subset
test groups may be made from the initial group of identified peaks. Second,
property values
of test groups may be compared to property value thresholds suitable to
qualify a test group
as indicative of a given pattern.
[0082] In some embodiments
herein, various motor manifestation patterns may be
detected, including, for example, patterns associated with epileptic seizure
activity,
progression throughout a generalized-tonic-clonic seizure, non-epileptic
psychogenic seizure
activity, and combinations thereof. In some embodiments, patterns of activity
associated with
post-ictal motor movements may also be identified. To detect or search for a
pattern, peak
data, including, for example, different groups of peaks constructed from an
original group,
may be compared to one or more qualification thresholds suitable to defme the
pattern.
Patterns may also be defined by protocols used for test group construction.
For example,
some patterns may be identified using different algorithms for test group
construction. For
example, algorithms may be defined with rules that allow or do not allow for
application of
test groups with excluded peaks or that define a maximum number or ratio of
excluded peaks.
[0083] In some embodiments,
more than one pattern may be searched or
identified in a collected EMG signal. For example, in some embodiments, a
first seizure
pattern may be characterized by detection of a threshold qualified peak number
of at least 8
qualified peaks included among no more than one test group. In another
example, a seizure
pattern may be characterized by detection of a threshold qualified peak number
of at least 12
qualified peaks included among no more than two test groups. In another
example, a seizure
pattern may be characterized by detection of a threshold qualified peak number
of at least 15
qualified peaks included among no more than three test groups. The
aforementioned patterns
may also be defined by one or more qualification thresholds or pooled property
thresholds.
For example, peaks in the aforementioned patterns may be qualified if groups
of peaks
exhibit a peak rate of greater than a minimum peak rate of about 2 peaks per
second and less
than a maximum peak rate of about 7 peaks per second. In some embodiments, the
peaks may
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
be defined by alternate or additional aggregate qualification or other
qualification thresholds.
For example, peaks in the aforementioned patterns may be qualified against a
minimum
threshold peak duration width of about 100 milliseconds and a maximum
threshold peak
duration width of about 300 milliseconds, an aggregate average deviation
percentage value
for duration of times between peaks of between about 3% and about 40%, or
both.
[0084] In some embodiments, a seizure pattern may be characterized by
detection
of a threshold qualified peak number of at least 8 qualified peaks to at least
12 qualified
peaks. The pattern may further be characterized by qualification thresholds or
pooled
property thresholds of a peak rate of greater than a minimum rate of about 5
peaks per second
and less than a maximum rate of about 7 peaks per second. The aforementioned
pattern may
sometimes be part of a group of patterns. For example, another pattern may
identify peaks in
a range of between about 4 to about 6 peaks per second. Still another pattern
may identify
peaks in a range of between about 2 to about 5 peaks per second. Another
pattern may
identify peaks in a range of between about 1 to about 3 peaks per second.
Still another pattern
may identify peaks in a range of between about 0.2 to about 1 peak per second.
Other
qualification thresholds, including, for example, minimum or maximum values
for a required
duration width of elevated portions of a peak, a required duration of one or
more intervening
periods of lesser signal on either side of elevated portions of a peak, a
required average
deviation percentage value for duration of times between peaks, and/or
combinations thereof
may also be included.
[0085] A particularly useful pattern may be defined where peaks are
presented at
a relatively low rate, such as less than about 3 peaks per second, and where
thresholds of
duration width of elevated portions of a peak and threshold duration width of
periods of
lesser signal on either side of a peak are used in peak qualification.
Generally, such low
repetition rates may be associated with times where a patient is recovering,
either normally or
abnormally, from the clonic phase of a seizure. For example, particular
patterns, or
combinations of patterns. may In defined that organize data so that it may be
identified
whether a patient generally or always transition out of a seizure with a
sudden or gradual
change in repetition rate of peaks. Patterns may further identify whether a
change in
repetition rate of peaks is linked with a certain change in the duration width
of elevated
portions of peaks, duration width of lesser portions of signals between
elevated portions of
peaks, or ratio between elevated portions of peaks to lesser portions of
signals between
elevated portions of peaks. For example, if a patient tends to transition out
of a seizure
without experiencing an expected rate of increase in a duration width of
lesser portions of
26
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
signals between elevated portions of peaks that pattern may be flagged as
possibly indicative
of psychogenic non-epileptic seizure activity. In addition, qualification of
groups based on a
distribution of times between peak patterns may be created that show either of
a gradual
progression out of the seizure or a more sporadic pattern. Changes in
amplitude of peaks may
further be considered.
[0086] In some embodiments,
more than one pattern indicative of seizure or
seizure-related activity may be detected during a monitoring session for a
patient. For
example, in step 84, it may be determined that one seizure pattern is
identified and later
another pattern may be identified. Combinations of seizure patterns may be
used to gain
information that may bp difficult to gather from other patterns or simpler
patterns. In a related
manner, in some embodiments, some simpler patterns herein may act as building
blocks for
more complicated patterns. For example, using the aforementioned patterns a
more
complicated pattern may be defined. Some of those patterns may last for
significant durations
extending throughout more than one part of the clonic phase of a seizure,
including transition
out of the clonic phase.
[0087] In some embodiments,
different patterns that may be associated with a
common clinical diagnosis may be grouped together. Accordingly, where one or
more of
those patterns is identified, the significance of that pattern may be flagged
and presented to a
caregiver. For example, generally, where transition out of a seizure is sudden
and not
accompanied by a change in duration of lesser periods on either side of peaks,
such a pattern
may be flagged as possibly related to an occurrence of psychogenic non-
epileptic seizure
activity. Patterns indicative of this behavior may be flagged even if they
differ in other
characteristics, such as average repetition rate or average duration of lesser
periods on either
side of peaks.
[0088] It should be understood
that some patterns may include property value
thresholds associated with individual peaks in addition to aggregate property
thresholds. For
example, it may be useful to compare peak data to individual property value
thresholds as
part of an initial screening used for generation of an initial peak set that
may then be
subjected to test group construction. It may also be useful to compare peaks
during or after
this initial screen to one or more individual property values. For example,
during different
parts of a seizure, some properties of individual peaks may be expected to
change.
Accordingly, the best screen for generating test groups suitable for use in
examining one part
of a seizure may not be the same as for generating test groups suitable for
examining other
parts of a seizure. Accordingly, in some embodiments, an initial broad screen
of individual
27
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
peak properties may be applied in order to define an initial group of peaks
for test group
construction. Further processing may include determining whether peaks in a
test group
possess individual property values that may be more stringent than applied in
the initial
screening of peaks.
[0089] Other patterns (e.g.,
in addition to the particular embodiments above) may
also be stored in a database and may also be compared to collected data.
Patterns may be
defined to look at activity over a time window. For example, one pattern may
look for a
group of peaks that exhibit property values typically found near the start of
the clonic phase
of a seizure, and another pattern may look for a group of peaks exhibiting
property values
typically found near the end of a clonic-phase portion of a seizure. Windows
suitable to look
at those different parts of a seizure may be different. For example, windows
may be made
appropriate so that the peak data is limited to relatively brief time windows
(e.g., from about
2 to about 10 seconds). Other patterns may consider data over longer durations
and look for
activity where peak activity changes over time as may, for example, be
expected during
seizure progression. Patterns that consider data over greater periods of time
may look for the
presence of independently qualified groups and look for trends in statistics
of successive or
nearby groups.
[0090] For example, a pattern
may look for a first pattern set with a threshold
typically achieved by activity at the start of a clonic-phase portion of a
seizure and also look
to see if that pattern repeats or if a second pattern associated with later
portions of the clonic
phase may also be present. The first and second patterns may, for example,
have different
thresholds for peak width, peak repetition, peak amplitude or combinations
thereof, and in
some embodiments, a pattern may be selected to see if those characteristics
change or if, for
example, activity terminates without a characteristic change in peak width,
repetition and/or
amplitude. And, in some embodiments, a caregiver may search data for patterns
where
qualified peaks change over time in order to assist the caregiver in
identifying if the patient
may be prone to non-epileptic psychogenic events.
[0091] A variety of systems
may be suitable for collecting large amounts of EMG
and other patient-related data, organizing such data for system optimization,
and initiating an
alarm in response to a suspected seizure or in response to post-seizure motor
manifestations.
Figure 9 illustrates an exemplary embodiment of such a system that may be
configured to
monitor a patient for seizure activity using the methods described herein. In
the embodiment
of Figure 9, a seizure detection system 100 may include an acoustic sensor
108, a video
camera 109, a detection unit 112, a base station 114, and an alert transceiver
116. The
28
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
detection unit 112 may be configured as a portable and wearable device
disposed on or near
(or even attached to) any suitable muscle or muscle groups that may be subject
to motor
manifestations during a seizure. And, in some embodiments, the system 100 may
include any
of various wireless local area network technologies. For example, a detection
unit 112 may
communicate wirelessly to the intemet using WiFi, Bluetooth, or through
another local
network. And, using a local network a detection unit 112 may, in some
embodiments, send
data over the intemet directly or via an intermediate base station 114. In
some embodiments,
a caregiver may be contacted directly through a local network such as WiFi. A
base station
114 may be connected to the intemet wirelessly (such as through a local
network), or may be
linked to the intemet through a hard connection. The detection unit 112 may
comprise one or
more EMG electrodes capable of detecting electrical signals from muscles at or
near the skin
surface of a patient and delivering those electrical EMG signals to a
processor for processing.
The EMG electrodes may be coupled or attached to a patient, and may, in some
embodiments, be implanted within the tissue of a patient near a muscle that
may be activated
during a seizure. Implanted devices may, for example, be particularly amenable
for some
patients where EMG signals may typically be weak, such as patients with
significant adipose
tissue.
[0092] The base station 114
may comprise a computer capable of receiving and
processing EMG signals from the detection unit 112, acoustic data from the
acoustic sensor
108, and/or data from other sensors, determining from the processed signals
whether a
seizure may have occurred, and sending an alert to a caregiver. The alert
transceiver 116 may
be carried by, or placed near, a caregiver to receive and relay alerts
transmitted by the base
station 114 or to the internet. Other components that may be included in the
system 100,
including for example, wireless device 117, 118, storage database 119,
electronic devices for
detecting changes in the integrity of an electrode skin interface, and one or
more
environmental transceivers are also described in Applicant's U.S. Patent
8,983,591 and other
references incorporated herein.
[0093] In using the apparatus
of Figure 9, for example, a patient 120 susceptible
to epileptic seizures may, for example, be resting in bed, or may be at some
other location as
daily living may include, and may have a detection unit 112 in physical
contact with or in
proximity to his or her body. The detection unit 112 may be a wireless device
so that a patient
may be able to get up and walk around without having to be tethered to an
immobile power
source or to a bulkier base station 114. For example, the detection unit 112
may be woven
into a shirt sleeve, may be mounted to an armband or bracelet, or may be an
implanted
29
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
device. In other embodiments, one or more detection units 112 or other sensors
may be
placed or built into a bed, a chair, an infant car seat, or other suitable
clothing, furniture,
equipment and accessories used by those susceptible to seizures. The detection
unit 112 may
comprise a simple sensor, such as an electrode, that may send signals to the
base station 114
for processing and analysis, or may comprise a "smart" sensor having some data
processing
and storage capability. A detection unit 112 may include one or more smart
client
applications. In some embodiments, a simple sensor may be connected via wire
or wirelessly
to a battery-operated transceiver mounted on a belt worn by the person. In
some
embodiments, a detection unit 112 may be configured with a pattern database
and/or include
a processor configured to execute instructions for construction of one or more
subset groups
of peaks from an initial peak set.
[0094] The system 100 may
monitor the patient 120, for example, while resting,
such as during the evening and nighttime hours. If the detection unit 112 on
the patient
detects a seizure, the detection unit 112 may communicate via wire or
wirelessly, e.g., via a
communications network or wireless link, with the base station 114, to a
remote cell phone
117 or other hand held or desktop device 118 via Bluetooth or simultaneously
to a base
station and remote cell phone 117 or other device 118. In some embodiments, a
detection unit
112 may send some signals to the base station device for more thorough
analysis. For
example, in some embodiments, a base station 114 may include a more extensive
database of
patterns suitable for comparison to one or more portions of EMG data.
[0095] In some embodiments,
the detection unit 112 may process and use EMG
signals (and optionally, or in some embodiments, ECG, temperature, orientation
sensors,
saturated oxygen, and/or audio sensor signals) to make an initial assessment
regarding the
likelihood of occurrence of a seizure, and may send those signals and its=
assessment to the
base station 114 for separate processing and confirmation. If the base station
114 confirms
that a seizure is likely occurring, then the base station 114 may initiate an
alarm for
transmission over the network 115 to alert a designated individual by way of
email, text,
phone call, or any suitable wired or wireless messaging indicator. It should
be appreciated
that the detection unit 112 may, in some embodiments, be smaller and more
compact than the
base station 114 and it may be convenient to use a power supply with only
limited strength.
Therefore, it may be advantageous, in some embodiments, to control the amount
of data that
is transferred between the detection unit 112 and the base station 114 as this
may increase the
lifetime of any power supply elements integrated in the detection unit 112. In
some
embodiments, if one or more of the detection unit 112, the base station 114,
or a caregiver,
CA 02971095 2017-06-14
WO 2016/105571 PCT/US2015/000475
e.g., a remotely located caregiver monitoring signals provided from the base
station,
determines that a seizure may be occurring, a video monitor 109 may be
triggered to collect
video information of the patient 120.
[0096] The base station 114,
which may be powered by a typical household power
supply and contain a battery for backup, may have more processing,
transmission and
analysis power available for its operation than the detection unit 112, may be
able to store a
greater quantity of signal history, and evaluate a received signal against
that greater amount
of data. The base station 114 may communicate with an alert transceiver 116
located
remotely from the base station 114, such as in the bedroom of a family member,
or to a
wireless device 117, 118 carried by a caregiver or located at a work office or
clinic. The base
station 114 and/or transceiver 116 may send alerts or messages to designated
people via any
suitable means, such as through a network 115 to a cell phone 117, PDA 118 or
other client
device. The system 100 may thus provide an accurate log of seizures, which may
allow a
patient's physician to understand more quickly the success or failure of a
treatment regimen.
Of course, the base station 114 may simply comprise a computer having
installed a program
capable of receiving, processing and analyzing signals as described herein,
and capable of
transmitting an alert. A base station 114 may include one or more smart client
applications. In
other embodiments, the system 100 may simply comprise, for example, EMG
electrodes as
part of a device configured to transmit signal data to a smartphone, such as
an iPhone,
configured to receive EMG signals from the electrodes for processing the EMG
signals as
described herein using an installed program application. In further
embodiments, so-called
"cloud" computing and storage may be used via network 115 for storing and
processing the
EMG signals and related data. In yet other embodiments, one or more EMG
electrodes may
be packaged together as a single unit with a processor capable of processing
EMG signals as
disclosed herein and sending an alert over a network. In other words, the
apparatus may
comprise a single item of manufacture that may be placed on a patient and that
does not
require a base station or separate transceiver. Or the base station may be a
smartphone or
tablet, for example.
[0097] In the embodiment of
Figure 9, the EMG signal data may be sent to a
remote database 119 for storage. In some embodiments, EMG signal data may be
sent from a
plurality of patients with epilepsy to a central database 119 and "anonymized"
to provide a
basis for establishing and refining generalized "baseline" sensitivity levels
and signal
characteristics of an epileptic seizure. The database 119 and base station 114
may be
remotely accessed via network 115 by a remote computer 113 to allow updating
of detector
31
CA 02971095 2017-06-14
WO 2016/105571
PCT/US2015/000475
unit and/or base station software, and data transmission. And, in some
embodiments, the
remote computer 113 or another computer may also serve to monitor exchange of
data
including alarm signals and EMG signal data between different devices
associated with any
number of designated individuals set to receive the signal. The base station
114 may generate
an audible or visible alarm, as may a remote transceiver 116 or detection unit
112. All
wireless links may be two-way for software, and data transmission and message
delivery
confirmation. The base station 114 may also employ one or all of the messaging
methods
listed above for seizure notification. The base station 114 or detection unit
112 may provide
an "alert cancel" button to terminate an incident warning.
[00981 In some embodiments, a transceiver may additionally be mounted within a
unit of furniture or some other structure, e.g., an environmental unit or
object. If a detection
unit 112 is sufficiently close to that transceiver, such a transceiver may be
capable of sending
data to a base station. Thus, the base station 114 may be aware that
information is being
received from that transducer, and therefore the associated environmental
unit. In some
embodiments, a base station 114 may select a specific template file, e.g.,
such as including
threshold values and other data as described further herein, that is dependent
upon whether or
not it is receiving a signal from a certain transceiver. Thus, for example, if
the base station
114 receives information from a detector 112 and from a transducer that is
associated with a
bed or crib, it may treat the data differently than if the data is received
from a transducer
associated with another environmental unit, such as, for example, clothing
typically worn
while an individual may be exercising or an item close to a user's sink where
for example a
patient may brush his or her teeth. More generally, a monitoring system may,
in some
embodiments, be configured with one or more elements with global positioning
(GPS)
capability, and position information may be used to adjust one or more
routines that may be
used in a detection algorithm. For example, GPS capability may be included
along with or
among one or more microelectromechanical sensor elements included in a
detection unit 112.
[00991 The embodiment of Figure 9 may be configured to be minimally intrusive
to
use while sleeping or minimally interfere in daily activities, may require a
minimum of
electrodes such as one or two, may require no electrodes to the head, may
detect a seizure
with motor manifestations, may alert one or more local and/or remote sites of
the presence of
a seizure, and may be inexpensive enough for home use.
[01001 Figure 10 illustrates an embodiment of a detection unit 112 or
detector. The
detection unit 112 may include EMG electrodes 122, and may also include, in
some
embodiments, ECG electrodes 124. The detection unit 112 may further include
amplifiers
32
CA 02971095 2017-06-14
WO 2016/105571
PCT/US2015/000475
with leads-off detectors 126. In some embodiments, one or more leads-off
detectors may
provide signals that indicate whether the electrodes are in physical contact
with the person's
body, or otherwise too far from the person's body to detect muscle activity,
temperature,
brain activity or other patient phenomena. The detection unit 112 may further
include one or
elements 128, such as solid state microelectromechanical (MEMS) structures,
configured for
detection of position and/or orientation of the detection unit 112. For
example, an element
128 may include one or more micromachined inertial sensors such as may include
one or
more gyroscopes, accelerometers, magnetometers or combinations thereof.
[0101] The detection unit 112 may further include a temperature sensor 130 to
sense
the person's temperature and one or more orientation or position sensitive
elements 128.
Other sensors (not shown) may be included in the detection unit, as well, such
as
accelerometers, microphones, and oximeters. Signals from electrodes 122 and
124,
temperature sensor 130, orientation and/or position sensors 128 and other
sensors may be
provided to a multiplexor 132. The multiplexor 132 may be part of the
detection unit 112 or
may be part of the base station 114 if the detection unit 112 is not a smart
sensor. The signals
may then be communicated from the multiplexor 132 to one or more analog-to-
digital
converters 134. The analog-to-digital converters may be part of the detection
unit 112 or may
be part of the base station 114. The signals may then be communicated to one
or more
microprocessors 136 for processing and analysis as disclosed herein. The
microprocessors
136 may be part of the detection unit 112 or may be part of the base station
114. The
detection unit 112 and/or base station 114 may further include memory of
suitable capacity.
The microprocessor 136 may communicate signal data and other information using
a
transceiver 138. Communication by and among the components of the detection
unit 112
and/or base station 114 may be via wired or wireless communication.
[0102] Of course, the exemplary detection unit of Figure 10 may be differently
configured. Many of the components of the detector of Figure 10 may be in base
station 114
rather than in the detection unit 112. For example, the detection unit may
simply comprise an
EMG electrode122 in wireless communication with a base station 114. In such an
embodiment, A-D conversion and signal processing may occur at the base station
114. If an
ECG electrode 124 is included, then multiplexing may also occur at the base
station 114.
[0103] In another example, the detection unit 112 of Figure 10 may comprise an
electrode portion having one or more of the EMG electrode 122, ECG electrode
124 and
temperature sensor 130, in wired or wireless communication with a small belt-
worn
transceiver portion. The transceiver portion may include a multiplexor 132, an
A-D converter
33
CA 02971095 2017-06-14
WO 2016/105571
PCT/US2015/000475
134, microprocessor 136, transceiver and other components, such as memory and
I/0 devices
(e.g., alarm cancel buttons and visual display).
[0104] Figure 11 illustrates an embodiment of a base station 114 that may
include one
or more microprocessors 140, a power source 142, a backup power source 144,
one or more
I/O devices 146, and various communications means, such as an Ethernet
connection 148 and
transceiver 150. The base station 114 may have more processing and storage
capability than
the detection unit 112, and may include a larger electronic display for
displaying EMG signal
graphs for a caregiver to review EMG signals in real-time as they are received
from the
detection unit 112 or historical EMG signals from memory. The base station 114
may process
EMG signals and other data received from the detection unit 112. If the base
station 114
determines that a seizure is likely occurring, it may send an alert to a
caregiver via transceiver
150.
[0105] Various devices in the apparatus of FIGS. 9-11 may communicate with
each
other via wired or wireless communication. The system 100 may comprise a
client-server or
other architecture, and may allow communication via network 115. Of course,
the system 100
may comprise more than one server and/or client. In other embodiments, the
system 100 may
comprise other types of network architecture, such as a peer-to-peer
architecture, or any
combination or hybrid thereof.
[0106] Generally, the devices of a seizure detection system may be of any
suitable
type and configuration to accomplish one or more of the methods and goals
disclosed herein.
For example, a server may comprise one or more computers or programs that
respond to
commands or requests from one or more other computers or programs, or clients.
The client
devices may comprise one or more computers or programs that issue commands or
requests
for service provided by one or more other computers or programs, or servers.
The various
devices in Figure 9 may be servers or clients depending on their function and
configuration.
Servers and/or clients may variously be or reside on, for example, mainframe
computers,
desktop computers, PDAs, smartphones (such as Apple's iPhoneTM, Motorola's
AtrixTM 4G,
Motorola's DroidTM, Samsung's Galaxy STM, Samsung's Galaxy NoteTM, and
Research In
Motion's Blackberry Tm devices), tablets (such as Sony's XperiaTm, Samsung's
Galaxy TabTm,
and Amazon Kindle) netbooks, portable computers, portable media players with
network
communication capabilities (such as Microsoft's Zune HDTM and Apple's iPod
TouchTm
devices), cameras with network communication capabilities, smartwatches,
wearable
computers, and the like.
[0107] A computer may be any device capable of accepting input, processing the
34
CA 02971095 2017-06-14
WO 2016/105571
PCT/US2015/000475
input according to a program, and producing output. A computer may comprise,
for example,
a processor, memory and network connection capability. Computers may be of a
variety of
classes, such as supercomputers, mainframes, workstations, microcomputers,
PDAs and
smartphones, according to the computer's size, speed, cost and abilities.
Computers may be
stationary or portable, and may be programmed for a variety of functions, such
as cellular
telephony, media recordation and playback, data transfer, web browsing, data
processing,
data query, process automation, video conferencing, artificial intelligence,
and much more.
[0108] A program may comprise any sequence of instructions, such as an
algorithm,
whether in a form that can be executed by a computer (object code), in a form
that can be
read by humans (source code), or otherwise. A program may comprise or call one
or more
data structures and variables. A program may be embodied in hardware or
software, or a
combination thereof. A program may be created using any suitable programming
language,
such as C, C++, Java, Perl, PHP, Ruby, SQL, and others. Computer software may
comprise
one or more programs and related data. Examples of computer software include
system
software (such as operating system software, device drivers and utilities),
middleware (such
as web servers, data access software and enterprise messaging software),
application software
(such as databases, video games and media players), firmware (such as device
specific
software installed on calculators, keyboards and mobile phones), and
programming tools
(such as debuggers, compilers and text editors).
[0109] Memory may comprise any computer-readable medium in which information
can be temporarily or permanently stored and retrieved. Examples of memory
include various
types of RAM and ROM, such as SRAM, DRAM, Z-RAM, flash, optical disks,
magnetic
tape, punch cards, EEPROM. Memory may be virtualized, and may be provided in,
or across
one or more devices and/or geographic locations, such as RAID technology. An
1/0 device
may comprise any hardware that can be used to provide information to and/or
receive
information from a computer. Exemplary I/0 devices include disk drives,
keyboards, video
display screens, mouse pointers, printers, card readers, scanners (such as
barcode, fingerprint,
iris, QR code, and other types of scanners), RFID devices, tape drives, touch
screens,
cameras, movement sensors, network cards, storage devices, microphones, audio
speakers,
styli and transducers, and associated interfaces and drivers.
[0110] A network may comprise a cellular network, the Internet, intranet,
local area
network (LAN), wide area network (WAN), Metropolitan Area Network (MAN), other
types
of area networks, cable television network, satellite network, telephone
network, public
networks, private networks, wired or wireless networks, virtual, switched,
routed, fully
CA 02971095 2017-06-14
WO 2016/105571
PCT/US2015/000475
connected, and any combination and subnetwork thereof. The network may use a
variety of
network devices, such as routers, bridges, switches, hubs, repeaters,
converters, receivers,
proxies, firewalls, translators and the like. Network connections may be wired
or wireless,
and may use multiplexers, network interface cards, modems, IDSN terminal
adapters, line
drivers, and the like. The network may comprise any suitable topology, such as
point-to-
point, bus, star, tree, mesh, ring and any combination or hybrid thereof.
[0111] Wireless technology may take many forms such as person-to-person
wireless,
person-to stationary receiving device, person-to-a-remote alerting device
using one or more
of the available wireless technology such as ISM band devices, WiFi,
Bluetooth, cell phone
SMS, cellular (CDMA2000, WCDMA, etc.), WiMAX, WLAN, and the like.
[0112] Communication in and among computers, I/0 devices and network devices
may be accomplished using a variety of protocols. Protocols may include, for
example,
signaling, error detection and correction, data formatting and address
mapping. For example,
protocols may be provided according to the seven-layer Open Systems
Interconnection model
(OSI model), or the TCP/IP model.
[0113] Although the disclosed method and apparatus and their advantages have
been
described in detail, it should be understood that various changes,
substitutions and alterations
can be made herein without departing from the invention as defined by the
appended claims.
Moreover, the scope of the present application is not intended to be limited
to the particular
embodiments of the process, machine, manufacture, composition, or matter,
means, methods
and steps described in the specification. For example, any feature described
for one
embodiment may be used in any other embodiment. Use of the word "include," for
example,
should be interpreted as the word "comprising" would be, i.e., as open-ended.
As one will
readily appreciate from the disclosure, processes, machines, manufacture,
compositions of
matter, means, methods, or steps, presently existing or later to be developed
that perform
substantially the same function or achieve substantially the same result as
the corresponding
embodiments described herein may be utilized. Accordingly, the appended claims
are
intended to include within their scope such processes, machines, manufacture,
compositions
of matter, means, methods or steps.
36