Note: Descriptions are shown in the official language in which they were submitted.
CA 02976327 2017-08-10
- 1 -
DESCRIPTION
HAIR GROWTH COMPOSITION FOR CUTANEOUS APPLICATION
TECHNICAL FIELD
[0001] The present invention relates to a hair growth composition for
cutaneous application
which can be employed in the field of pharmaceuticals which, even after
applied to the scalp
of users, suppress a stiff feel of their hair to allow for continued smooth
combing.
BACKGROUND ART
[0002] It is well known that when subjected to frequent repetition of
beautification
treatments as by washing, brushing, heating with a dryer, hair colors and
bleaches, the hair
generally undergoes considerable damage to deteriorate and as a result, it
gets dry, even dried
out, causing more split ends, hair breakage, etc. and leading to lower
strength.
[0003] To cope with the drying-out of the hair and other problems that result
from its
deterioration and for the purpose of locking moisture in the hair while
imparting smoothness
and a moist feel to the same, it has been proposed that oils such as silicone
oil or paraffinic
oils, humectants such as low-molecular-weight polyols or glycerin, and a
variety of raw
materials extracted from natural substances should be incorporated as
additives in hair care
cosmetics and hair care compositions for cutaneous application (Non-Patent
Document 1).
Other cases known in the field of hair care cosmetics include: a composition
incorporating
calcium ions, etc. in combination with alcohols or ethers (Patent Document 1);
a composition
incorporating a magnesium compound and a cationic surfactant (Patent Document
2); a
composition incorporating a polyoxyalkylene alkyl glycoside (Patent Document
3); and a
composition incorporating aluminum chloride and magnesium chloride (Patent
Document 4).
However, a concern about hair care compositions for cutaneous application that
incorporate
particular compounds is that if the compounds themselves present a problem to
the comfort
of use, it is necessary to evaluate them individually and no study has been
made to determine
which additive is suitable.
[0004] It should be noted here that the compounds to be used in the present
invention are
CA 02976327 2017-08-10
- 2 -
disclosed in Patent Document 5 and represented by the following formula (1):
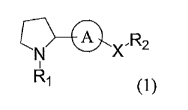
[0005] [Formula 1]
A x,R2
R1
(1)
[wherein Ri is of the following formula (2):
[0006] [Formula 2]
0
F OH
111111 (2)
where ring A is either of the alternatives of the following formula (3):
[0007] [Formula 3]
< 0 jc
Or (3)
where X is either -CH20- or
R2 is a phenyl group or a pyridyl group (the phenyl or pyridyl group may be
substituted by
one to three methoxy groups)] or a pharmaceutically acceptable salt thereof.
CITATION LIST
PATENT LITERATURE
[0008] Patent Document 1: JP H7-2627 A
Patent Document 2: JP H8-53325 A
Patent Document 3: JP 2001-213734 A
Patent Document 4: JP 2010-6778 A
Patent Document 5: pamphlet of WO 2012/124750 Al
CA 02976327 2017-08-10
- 3 -
NON-PATENT LITERATURE
[0009] Non-Patent Document 1: Shin-keshouhingaku (New Science of Cosmetics),
ed. by
Takeo Mitsui, Nanzan-do (May 2006), pp. 440-462.
SUMMARY OF INVENTION
TECHNICAL PROBLEM
[0010] The conventional technology of hair care cosmetics and hair care
compositions for
cutaneous application proves effective to some extent in providing smoothness
and a moist
feel to the hair. However, if compositions containing compounds of formula (1)
are simply
formulated as liquids/solutions for cutaneous application or lotions, the
conventional
technology does not assure adequately sustained effectiveness but provides
only poor
smoothness and other feels of the hair during use; for these and other
reasons, it has been
impossible to prepare products that offer satisfactory comfort of use.
[0011] The present invention has been accomplished in view of this problem and
it aims to
provide a hair growth composition for cutaneous application which, when
containing a
compound represented by the formula (1), features good sustainability of
effects such as
eliminating a feel that the hair gets dried out, imparting smoothness and a
moist feel to the
hair, and suppressing its damage.
SOLUTION TO PROBLEM
[0012] The present inventors conducted an intensive study with a view to
attaining the
above-stated object and found as a result that the aforementioned drawbacks of
the
conventional technology can be eliminated by incorporating a magnesium
compound and/or
a calcium compound and a specific nonionic surfactant in a compound
represented by the
formula (1). This finding has led to the completion of the present invention.
Hereinafter, the
present invention will be explained in detail. Various modes of the present
invention are
shown below.
[0013] [1]
A hair growth composition for cutaneous application characterized by
containing the
following (a) to (d):
CA 02976327 2017-08-10
- 4 -
[0014] (a) a compound represented by formula (1):
[Formula 4]
AX-R2
R1
(1)
[wherein Ri is of the following formula (2):
[0015] [Formula 5]
0
OH
1110 (2)
where ring A is either of the alternatives of the following formula (3):
[0016] [Formula 6]
_____________________ <
or 0 (3)
where X is either -CH20- or
R2 is a phenyl group or a pyridyl group (the phenyl or pyridyl group may be
substituted by
one to three methoxy groups)] or a pharmaceutically acceptable salt thereof;
(b) a nonionic surfactant included in either of groups (b-1) to (b-3):
(b-1) one having a polyoxyethylene structure and an HLB value of 9 or more;
(b-2) one having no polyoxyethylene structure but having an HLB value of 10 or
more;
(b-3) a soybean phospholipid which is either hydrogenated or not;
(c) at least one member selected from the group consisting of a magnesium
compound and a
calcium compound;
(d) water.
CA 02976327 2017-08-10
- 5 -
[2]
A hair growth composition for cutaneous application as recited in [1], wherein
the
compound represented by formula (1) in (a) above is (S)-2,2-difluoro-2-(1-
hydroxy-3,3,5,5-
tetramethylcyclohexyl)-1-(2-(5-((pyridin-3-yloxy)methyl)isoxazol-3-
yl)pyrrolidin-1-
yl)ethanone represented by the following formula:
[0017] [Formula 7]
/o
0
0
OH
[3]
A hair growth composition for cutaneous application as recited in [1], wherein
the
compound represented by formula (1) in (a) above is (S)-1-(2-(543,4-
dimethoxyphenoxy)methyl)isoxazol-3-yl)pyrrolidin-1-y1)-2,2-difluoro-2-(1-
hydroxy-3,3,5,5-
tetramethylcyclohexyl)ethanone represented by the following formula:
[0018] [Formula 8]
/N0
0
F
OH = 0\
O-
.
[4]
A hair growth composition for cutaneous application as recited in [1], wherein
the
compound represented by formula (1) in (a) above is (S)-1-(2-(5-((3,4-
CA 02976327 2017-08-10
- 6 -
dimetboxyphenoxy)methyl)-1,3,4-oxadiazol-2-y1)pyrrolidin-1-y1)-2,2-difluoro-2-
(1-hydroxy-
3,3,5,5-tetramethylcyclohexyl)ethanone.
[5]
A hair growth composition for cutaneous application as recited in [1], wherein
the
compound represented by formula (1) in (a) above is (S)-2,2-difluoro-2-(1-
hydroxy-3,3,5,5-
tetramethylcyclohexyl)-1-(2-(5-((3,4,5-trimethoxyphenoxy)methyl)isoxazol-3-
yl)pyrrolidin-
1-yl)ethanone.
[6]
A hair growth composition for cutaneous application as recited in [1], wherein
the
compound represented by formula (1) in (a) above is (S)-2,2-difluoro-2-(1-
hydroxy-3,3,5,5-
tetramethylcyclohexyl)-1-(2-(5-(phenoxymethyl)-1,3,4-oxadiazol-2-y1)pyrrolidin-
1-
yl)ethanone.
[7]
A hair growth composition for cutaneous application as recited in [1], wherein
the
compound represented by formula (1) in (a) above is (S)-2,2-difluoro2-(1-
hydroxy3,3,5,5-
tetramethylcyclohexyl)-1-(2-(5-((pyridin-4-yloxy)methypisoxazol-3-
yl)pyrrolidin-1-
yl)ethanone.
[8]
A hair growth composition for cutaneous application as recited in [1], wherein
the
compound represented by formula (1) in (a) above is a compound represented by
the
following formula:
[0019] [Formula 9]
/0
F
OH
CA 02976327 2017-08-10
- 7 -
[91
A hair growth composition for cutaneous application as recited in any one of
[1] to
[8], which is characterized in that the nonionic surfactant in (b) above is
one or more
members selected from the group consisting of polysorbate 80, a hydrogenated
soybean
phospholipid, polyethylene glycol monostearate, polyoxyethylene stearyl ether,
polyoxyethylene cetyl ether, and polyoxyethylene (20) polyoxypropylene (8)
cetyl ether.
[10]
A hair growth composition for cutaneous application as recited in any one of
[1] to
[9], which is characterized in that the magnesium compound and the calcium
compound in
(c) above are magnesium chloride and/or calcium chloride.
[11]
A hair growth composition for cutaneous application as recited in any one of
[1] to
[10], which is characterized in that the dosage form of the hair growth
composition for
cutaneous application is a liquid for cutaneous application.
[12]
A hair growth composition for cutaneous application as recited in any one of
[1] to
[11], wherein the amount of incorporation of at least one member selected from
the group
consisting of a magnesium compound and a calcium compound in (c) above is
0.001 to
(w/v%) in the total amount of the hair growth composition for cutaneous
application.
[13]
A hair growth composition for cutaneous application as recited in [12],
wherein the
amount of incorporation of at least one member selected from the group
consisting of a
magnesium compound and a calcium compound in (c) above is 0.01 to 5 (w/v%) in
the total
amount of the hair growth composition for cutaneous application.
[14]
A hair growth composition for cutaneous application as recited in any one of
[1] to
[13], wherein the amount of incorporation of the nonionic surfactant in (b)
above is 0.01 to
CA 02976327 2017-08-10
-8-
(w/v%) in the total amount of the hair growth composition for cutaneous
application.
[15]
A hair growth composition for cutaneous application as recited in [14],
wherein the
amount of the nonionic surfactant in (b) above is 0.1 to 5 (w/v%) in the total
amount of the
hair growth composition for cutaneous application.
[16]
A hair growth composition for cutaneous application as recited in any one of
[1] to
[15], wherein the amount of the compound represented by formula (1) is 0.0001
to 20 (w/v%)
in the total amount of the hair growth composition for cutaneous application.
[17]
A hair growth composition for cutaneous application as recited in [16],
wherein the
amount of incorporation of the compound represented by formula (1) is 0.1 to
10 (w/v%) in
the total amount of the hair growth composition for cutaneous application.
ADVANTAGEOUS EFFECTS OF INVENTION
[0020] By means of the present invention, even in the case where a compound
represented
by formula (1) is contained, it becomes possible to provide a hair growth
composition for
cutaneous application which features good sustainability of effects such as
eliminating a feel
that the hair gets dried out, imparting smoothness and a moist feel to the
hair, and
suppressing its damage.
DESCRIPTION OF EMBODIMENTS
[0021] Hereinafter, the constitution of the present invention is described in
detail.
[0022] The present invention is characterized by containing a compound
represented by
formula (1), a magnesium compound and/or a calcium compound, and a nonionic
surfactant
specified above.
[0023] The amount of incorporation of the nonionic surfactant specified above
is preferably
0.01 to 10 (w/v%) in the total amount of the hair growth composition for
cutaneous
application, and the amount of incorporation of the magnesium compound and/or
the calcium
compound is preferably 0.001 to 10 (w/v%) in the total amount of the hair
growth
CA 02976327 2017-08-10
- 9 -
composition for cutaneous application.
[0024] The compound represented by formula (1) can be produced by the
production
method described in the pamphlet of WO 2012/124750 Al.
[0025] The amount of incorporation of the compound represented by formula (1)
is 0.0001
to 20 (w/v%), preferably 0.1 to 10 (w/v%), more preferably 1 to 10 (w/v%), in
the total
amount of the hair growth composition for cutaneous application.
[0026] The nonionic surfactant in (b) above which can be used in the present
invention may
be a nonionic surfactant having a polyoxyethylene structure and an HLB value
of 9 or more,
a nonionic surfactant having no polyoxyethylene structure but having an HLB
value of 10 or
more, or even a soybean phospholipid, hydrogenated or not, for which HLB
cannot be clearly
specified.
[0027] It should be noted that HLB, standing for hydrophile-lipophile balance,
refers to the
balance between lipophilicity and hydrophilicity. The HLB values as mentioned
in the
present invention are obtained by actual measurement based on the
emulsification technique
(see Handbook ¨ Cosmetics/Preparations Raw Materials ¨ Revised Edition, "20.
Cosmetics
and Pharmaceuticals", pp. 854-855 (revised edition published on February 1,
1977 by Nikko
Chemicals Co., Ltd. and NIPPON SURFACTANT INDUSTRIES CO., LTD.))
[0028] Among others, polysorbate 80 (polyoxyethylene sorbitan oleate),
hydrogenated
soybean phospholipid, polyethylene glycol monostearate, polyoxyethylene
stearyl ether,
polyoxyethylene cetyl ether, and polyoxyethylene (20) polyoxypropylene (8)
cetyl ether are
preferred. Among these materials, polysorbate 80 (polyoxyethylene sorbitan
oleate) is
particularly preferred.
[0029] Polysorbate 80 may be exemplified by the trade name "NIKKOL TO- I OM"
manufactured by NIPPON SURFACTANT INDUSTRIES CO., LTD.; the hydrogenated
soybean phospholipid may be exemplified by the trade name "NIKKOL Resinol S-
10M"
manufactured by Nikko Chemicals Co.; the polyethylene glycol monostearate may
be
exemplified by the trade name "NIKKOL MYS-10V" manufactured by Nikko Chemicals
Co.; the polyoxyethylene stearyl ether may be exemplified by the trade name
"NIKKOL BS-
CA 02976327 2017-08-10
- 10 -
4" manufactured by Nikko Chemicals Co.; the polyoxyethylene cetyl ether may be
exemplified by the trade name "NIKKOL BC-5.5" manufactured by Nikko Chemicals
Co.;
the polyoxyethylene (20) polyoxypropylene (8) cetyl ether may be exemplified
by the trade
name "NIKKOL PBC-44" manufactured by Nikko Chemicals Co.; glyceryl
monostearate
may be exemplified by the trade name "NIKKOL MG S-AMV" manufactured by Nikko
Chemicals Co.; sorbitan sesquioleate may be exemplified by the trade name
"NIKKOL SO-
10V" manufactured by Nikko Chemicals Co.; polyoxyethylene oleyl ether may be
exemplified by the trade name "NIKKOL B0-2V" manufactured by Nikko Chemicals
Co.;
lauromacrogol may be exemplified by the trade name "NIKKOL BL-2" manufactured
by
Nikko Chemicals Co.; and polyoxyethylene hardened castor oil may be
exemplified by the
trade name "NIKKOL HCO-10" manufactured by Nikko Chemicals Co.
[0030] The amount of incorporation of the nonionic surfactant in (b) above
which is to be
used in the present invention is 0.01 to 10 (w/v%), preferably 0.1 to 5
(w/v%), in the total
amount of the hair growth composition for cutaneous application. If the amount
of interest is
less than 0.01 (w/v%), smoothness as an index for the comfort of use is
impaired; if the
amount of interest exceeds 10 (w/v%), the comfort of use as final product is
impaired.
[0031] The magnesium compound and the calcium compound that are to be used in
the
present invention may be exemplified by halides or complex halides, oxides or
associated
compounds of oxides, sulfides, nitrides, carbides, oxoates, organic magnesium
compounds,
organic calcium compounds, and so on, including hydrates. Among others, (i)
magnesium
halides and (ii) magnesium oxoates are preferred as magnesium compounds. For
specific
illustration, preferred examples of (i) are magnesium chloride, magnesium
bromide,
magnesium iodide, and so on; preferred examples of (ii) are magnesium sulfate,
magnesium
nitrate, and magnesium carbonate. In the group consisting of (i) and (ii),
magnesium chloride
is particularly preferred.
[0032] As for the calcium compound, calcium chloride is preferred.
[0033] In the case where hydrates of the magnesium compound or the calcium
compound
are used, the amount of their incorporation will be indicated, unless
otherwise noted, as
CA 02976327 2017-08-10
- 11 -
calculated for anhydride.
[0034] The amount of incorporation of the magnesium compound and/or the
calcium
compound which is to be used in the present invention is 0.001 to 10 (w/v%),
preferably 0.01
to 5 (w/v%), in the total amount of the hair growth composition for cutaneous
application. If
the amount of interest is less than 0.001 (w/v%), the effect of incorporating
the magnesium
compound and/or the calcium compound will not be exhibited; if the amount of
interest
exceeds 10 (w/v%), not only the comfort of use will be impaired but also the
stability will
decrease, which is by no means preferable.
[0035] In addition to the above-mentioned components, the hair growth
composition for
cutaneous application of the present invention permits the addition of various
materials,
depending on the object, within those quantitative and qualitative ranges
which will not be
deleterious to the intended effects of the present invention and they include,
for example:
polyhydric alcohols; oils; nonionic surfactants other than those listed in (b)
above; anionic
surfactants; amphoteric surfactants; high molecular weight compounds such as
cationic,
anionic, nonionic, and amphoteric high molecular weight compounds; chemicals
such as
polysaccharides, chelatants, antioxidants, UV absorbers, antiseptics, and
vitamins; and
perfumes.
[0036] Polyhydric alcohols that may be used include glycerin, 1,3-butylene
glycol,
propylene glycol, dipropylene glycol, polyethylene glycol, and so on.
[0037] As nonionic surfactants other than those listed in (b) above, the
following may be
used: lipophilic nonionic surfactants including sorbitan fatty acid esters
such as sorbitan
monooleate, sorbitan monoisostearate, sorbitan monolaurate, sorbitan
monopalmitate,
sorbitan monostearate, sorbitan sesquioleate, sorbitan trioleate, diglycerol
sorbitan penta-2-
ethylhexanoate, and diglycerol sorbitan tetra-2-ethylhexanoate; glycerin
polyglycerin fatty
acids such as mono-cottonseed oil fatty acid glycerin, monoerucic acid
glycerol, sesquioleic
acid glycerol, glyceryl monostearate, a,a'-glyceryl oleate pyroglutamate, and
glyceryl
monostearate malate; propylene glycol fatty acid esters such as propylene
glycol
monostearate; as well as hardened castor oil derivatives and glyceryl alkyl
ethers; POE
CA 02976327 2017-08-10
- 12 -
sorbitan fatty acid esters such as POE sorbitan monooleate, POE sorbitan
monostearate, and
POE sorbitan tetraoleate; POE sorbite fatty acid esters such as POE sorbite
monolaurate,
POE sorbite monooleate, POE sorbite pentaoleate, and POE sorbite monostearate;
POE
glyceryl fatty acid esters such as POE glyceryl monostearate, POE glyceryl
monoisostearate,
and POE glyceryl triisostearate; POE fatty acid esters such as POE monooleate,
POE
distearate, POE monodioleate, and ethylene glycol distearate; POE alkyl ethers
such as POE
lauryl ether, POE oleyl ether, POE stearyl ether, POE behenyl ether, POE 2-
octyldodecyl
ether, and POE cholestanol ether; POE alkyl phenyl ethers such as POE octyl
phenyl ether,
POE nonyl phenyl ether, and POE dinonyl phenyl ether; pluaronic type compounds
such as
Pluronic; POE = POP alkyl ethers such as POE = POP cetyl ether, POE = POP 2-
decyltetradecyl
ether, POE = POP monobutyl ether, POE = POP hydrogenated lanolin, POE = POP
glycerin
ether; tetra-POE = tetra-POP ethylenediamine condensates such as Tetronic; POE
hardened
castor oil hardened castor oil derivatives such as POE castor oil, POE
hardened castor oil,
POE hardened castor oil monoisostearate, POE hardened castor oil
triisostearate, POE
hardened castor oil monopyroglutamate monoisostearate diester, and POE
hardened castor oil
maleate; POE beeswax = lanolin derivatives such as POE sorbite beeswax;
alkanolamides such
as coconut oil derived diethanolamide, lauric acid monoethanolamide, and fatty
acid
isopropanolamide; as well as POE propylene glycol fatty acid esters, POE
alkylamines, POE
fatty acid amides, sucrose fatty acid esters, POE nonyl phenyl formaldehyde
condensates,
alkylethoxydimethylamine oxides, trioleyl phosphoric acid, and so on. The
amount of the
nonionic surfactant exemplified hereinabove may be less than 0.5 wt%,
specifically less than
0.1 wt%, more specifically less than 0.01 wt%, relative to the total amount of
the hair growth
composition for cutaneous application.
[0038] As anionic surfactants, the following may be used: base materials for
soap; fatty acid
soaps such as sodium laurate and sodium palmitate; higher alkyl sulfate salts
such as sodium
lauryl sulfate and potassium lauryl sulfate; alkyl ether sulfate salts such as
POE
triethanolamine lauryl sulfate and sodium POE lauryl sulfate; N-acyl
sarcosinates such as
sodium lauroylsarcosinate; higher fatty acid amide sulfonic acid salts such as
sodium N-
CA 02976327 2017-08-10
- 13 -
myristoyl-N-methytaurine, sodium coconut oil fatty acid methyltauride, and
sodium lauryl
methyltauride; phosphate salts such as sodium POE oleyl ether phosphate and
POE stearyl
ether phosphoric acid; sulfosuccinic acid salts such as sodium di-2-
ethylhexylsulfosuccinate,
sodium monolauroyl monoethanolamide polyoxyethylene sulfosuccinate, and sodium
lauryl
polypropylene glycol sulfosuccinate; alkylbenzene sulfonic acid salts such as
sodium linear
dodecylbenzene sulfonate, triethanolamine linear dodecylbenzene sulfonate, and
linear
dodecylbenzene sulfonic acid; N-acylglutamate salts such as monosodium N-
lauroylglutamate, disodium N-stearoylglutamate, and monosodium N-myristoyl-L-
glutamate;
higher fatty acid ester sulfuric acid salts such as sodium hardened coconut
oil fatty acid
glycerin sulfate; sulfated oils such as Turkey red oil; as well as POE alkyl
ether carboxylic
acids, POE alkylallyl ether carboxylic acid salts, a-olefinsulfonic acid
salts, higher fatty acid
ester sulfonic acid salts, secondary alcohol sulfonate salts, higher fatty
alkylolamide sulfate
salts, sodium lauroyl monoethanolamide succinate, and di-triethanolamine N-
palmitoylaspartate. The amount of the anionic surfactant may, for example, be
less than
0.5 wt%, specifically less than 0.1 wt%, more specifically less than 0.01 wt%,
relative to the
total amount of the hair growth composition for cutaneous application.
[0039] As amphoteric surfactants, the following may be used: imidazoline-based
amphoteric surfactants such as 2-undecyl-N,N,N-(hydroxyethylcarboxymethyl)-2-
imidazoline sodium and 2-cocoy1-2-imidazolinium hydroxide-1-carboxyethyloxy-2-
sodium
salt; betaine-based amphoteric surfactants such as 2-heptadecyl-N-
carboxymethyl-N-
hydroxyethylimidazolinium betaine, lauryldimethylaminoacetic acid betaine,
alkyl betaine,
amido betaine, and sulfobetaine; and amino acid salts such as N-lauroyl-P-
alanine and N-
stearyl-f3-alanine. The amount of the amphoteric surfactant may, for example,
be less than
0.5 wt%, specifically less than 0.1 wt%, more specifically less than 0.01 wt%,
relative to the
total amount of the hair growth composition for cutaneous application.
[0040] As cationic surfactants, the following may be used:
poly(dimethyldiallylammnoum
halide)-type cationic polymers, dimethyldiallylammonium halide/acrylamide
copolymerized
cationic polymers, or quaternary nitrogen-containing cellulose ether, or the
condensation
CA 02976327 2017-08-10
- 14 -
product of polyethylene glycol, epichlorohydrin, propyleneamine and
talloylamine obtained
from tallow fatty acids, or cationized vinylpyrrolidone/dimethylaminoethyl
methacrylate
copolymers. The poly(dimethyldiallylammnoum halide)-type cationic high
molecular weight
compounds may include the product commercially available from Merck & Co.,
Inc., USA
under the trade name Merquat 100. The dimethyldiallylammonium
halide/acrylamide
copolymer-type cationic polymers may include Merquat 550 (Merck & Co., Inc.
USA).
Examples of the condensation product of polyethylene glycol, epichlorohydrin,
propyleneamine and talloylamine or cocoylamine may include the product
commercially
available from Henkel International Co., West Germany under the trade name
Polyquat H.
Examples of the quaternary nitrogen-containing cellulose include the products
commercially
available from Union Carbide Corp., USA under the trade names Polymer JR-400,
Polymer
JR-125, and Polymer JR-30M. Examples of the cationized
vinylpyrrolidone/dimethylaminoethyl methacrylate copolymer include the
products
commercially available from GAF Corp., USA under the trade names Gafquat 755
and
Gafquat 734. The amount of the cationic high molecular weight compound may,
for
example, be less than 0.5 wt%, specifically less than 0.1 wt%, more
specifically less than
0.01 wt%, relative to the total amount of the hair growth composition for
cutaneous
application.
[0041] Examples of the anionic high molecular weight compound include xanthan
gum,
carrageenan, sodium alginate, gum arabic, pectin, carboxyvinyl polymers, and
so on. The
amount of the anionic high molecular weight compound may, for example, be less
than
0.5 wt%, specifically less than 0.1 wt%, more specifically less than 0.01 wt%,
relative to the
total amount of the hair growth composition for cutaneous application.
[0042] Examples of the nonionic high molecular weight compound include
polyvinyl
pyrrolidone, copolymers of vinyl pyrrolidone and vinyl acetate, copolymers of
vinyl
pyrrolidone, vinyl acetate and acrylamino acrylate, hydroxyethyl cellulose,
hydroxypropyl
cellulose, hydroxypropyl methylcellulose, methyl cellulose, dextrin, galactan,
pullulan, and
so on. The amount of the nonionic high molecular weight compound may, for
example, be
CA 02976327 2017-08-10
- 15 -
less than 0.5 wt%, specifically less than 0.1 wt%, more specifically less than
0.01 wt%,
relative to the total amount of the hair growth composition for cutaneous
application.
[0043] The amphoteric high molecular weight compound may be obtained by
copolymerizing dialkyl amino ethyl acrylates, dialkyl amino ethyl
methacrylates, diacetone
acrylamides, etc. with acrylic acid, alkyl acrylates, methacrylic acid, alkyl
methacrylates, etc.
and then rendering the resulting copolymer amphoteric with acetic halides;
examples include
the product commercially available from Mtsubishi Petrochemical Co., Ltd.
under the trade
name YUKA FOAMER AM-75. The amount of the amphoteric high molecular weight
compound may, for example, be less than 0.5 wt%, specifically less than 0.1
wt%, more
specifically less than 0.01 wt%, relative to the total amount of the hair
growth composition
for cutaneous application.
[0044] Examples of the mucopolysaccharide include hyaluronic acid, chondroitin
sulfate,
dermatan sulfate, keratan sulfate, heparan sulfate, and salts thereof, etc.
[0045] As the UV absorber, the following may be used: benzoic acid based
compounds
such as para-aminobenzoic acid (hereinafter abbreviated as PABA), glycerin
PABA, and
ethyldihydroxypropyl PABA; cinnamate-based compounds such as octyl
methoxycinnamate
and 2-ethoxyethyl-p-methoxycinnamate; benzophenone-based compounds such as 2,4-
dihydroxybenzophenone, 2-hydroxy-4-methoxybenzophenone, 2-hydroxy-4-methoxy-4-
methylbenzophenone; and others such as ethyl urocanate, 2-phenyl-5-
methylbenzoxazole, 4-
methoxy-4-t-butyl-dibenzoylmethane, and so on.
[0046] Usable as the antiseptic are alkyl esters of paraoxybenzoic acid,
sodium benzoate,
potassium sorbate, and other antiseptics that are commonly used in cosmetics.
[0047] Other chemicals that may be used are drug components that are also
commonly used
in cosmetics, as exemplified by vitamins such as of the vitamin C family and
vitamin E
family, placenta extract, a variety of amino acids, crude drugs, and anti-
inflammatories.
[0048] The composition of the present invention may be formulated as any
system selected
from among a solubilized system, an emulsified system, a powder dispersed
system, an oil-
water dual phase system, an oil-water-powder tri-phase system, and so on.
CA 02976327 2017-08-10
- 16 -
[0049] For cutaneous application, the composition of the present invention may
assume any
dosage form and can be used as a gel, spray, foam, cream or liquid (e.g.
lotion) product.
[0050] The solvent in liquids may be exemplified by ethanol, water, and 1,3-
butylene
glycol. A lotion, for example, can be prepared by using absolute ethanol in an
amount in the
range of 60 to 80 (w/v%), specifically 62 to 74 (w/v%), relative to the total
amount of the
hair growth composition for cutaneous application, with the remainder being
water to make a
total volume of 100 (mL).
EXAMPLES
[0051] Hereinafter, the present invention is described in further detail by
means of
Examples, which are by no means intended to limit the scope of the present
invention.
[0052] [Method of preparation]
A lotion was prepared as an example of the hair growth composition for
cutaneous
application. The lotion is obtained from suitable amounts of a compound of
formula (4), a
magnesium compound and/or calcium compound, a nonionic surfactant of (I))
above, ethanol
and water by mixing them under agitation.
[0053] The compound represented by formula (4) is identified below:
[0054] [Formula 10]
/ 0
0
0
OH
111111
(4)
[0055] [Testing methods]
The test and evaluation methods adopted in the respective Examples are
described
below.
[0056] Evaluating the stiff feel and resistance to combing after application:
Using hair
models for the training of beauticians, about 1 mL of the preparation was
applied to 25 cm2
CA 02976327 2017-08-10
- 17 -
of hair and after letting the hair dry by itself for 8 hours at room
temperature, "stiff feel" was
evaluated by a sensory test checking how the directly applied area felt on
touch whereas
"resistance to combing" was evaluated by a sensory test checking for the
resistance that
developed upon combing the hair in the applied area with a plastic comb.
[0057] Evaluation was carried out based on the following four-score rating.
[0058] 4: None; 3: Some; 2: Moderate; 1: Positive
Lotions were prepared from the recipes shown in Tables 1 to 5, which also show
the
results of evaluation of stiff feel and resistance to combing.
[0059] [Table 1]
Comparative Example
Component name 1 2 3 4 5 6
Compound of formula (4) 5 5 5 5 5 5
Aluminum chloride - 3 - - -
Lithium chloride - - 3 - -
Magnesium bromide - - - 3 -
Sodium chloride - - - - 3 -
Potassium chloride - - - - 3
Absolute ethanol 74 74 74 74 74 74
Water bal. bal. bal. bal. bal. bal.
Total amount 100mL 100mL 100mL 100mL 100mL 100mL
Sensory evaluation (numerals
refer to evaluation scores)
Stiff feel 1 2 2 2 N.T. N.T.
Resistance to combing 1 1 2 2 N.T. N.T.
Unless otherwise noted, the unit of the numerals for the respective components
in
each recipe is w/v%.
The amount of incorporation of each metal salt compound is calculated for
anhydride.
The samples of Comparative Examples 5 and 6 are labeled N.T. because they did
not dissolve to form applicable preparations.
The number of tests for stiff feel and resistance to combing was n = 7 in
Comparative Examples 1, 3 and 4, and n = 8 in Comparative Example 2.
[0060]
Product name Comparative
Example 7-73
Component name NIKKOL HLB 7 8 9 10 11 12 13
14 15 16 17 18 P
cr
_
Compound of formula (4)_------- 5 5 5 _ 5 5 5 5 5
5 5 5 5 cl-
_ _
Polysorbate 80 TO-10M 15 2 - - - - - -
_ - - _ tN)
,
Hydrogenated soybean phospholipid S-10M - - - 1 - - -
, - - - - - _
_
Glycerin monostearate MGS-AMV 4 - - -_ 2 - - -
- _ - - -
Polyethylene glycol monostearate MYS-10V 11 - - - - 2
. - - , - - -
Sorbitan sesquioleate SO-15MV 3.7 - - - - - 2 - -
_ - - -
Polyoxyethylene stearyl ether BS-4V 9 - - _ - - -
1 - _ - - -
_
Polyoxyethylene oleyl ether B0-2V 7.5 - - - - - - -
1 - - - _
_
Lauromacrogol BL-2 9.5 - - - - . - _ - -
1 -
_
Polyoxyethylene cetyl ether BC-5.5 10.5 - - - - - -
- - - 1 - -
_
_
Polyoxyethylene (20) PBC-44 12.5 - - - - - - -
. - 1 -
polyoxypropylene (8) cetyl ether
Polyoxyethylene hardened castor oil HCO-10 6.5 - - - - -
- - - _ - - 1
P
Absolute ethanol .õ/ 74 74 74 74 74 74
74 74 74 ' 74 74 74 .
Water ,------- bal. bal. bal. bal. bal.
bal. bal. bal. , bal. bal, bal. bal. "
-.,
Total amount õ.----- 100mL 100mL 100mL 100mL 100mL 100mL 100mL
100mL 100mL 100mL 100mL 100mL .
,..
Sensory evaluation
_
(numerals refer to evaluation scores) Z
,--,
oc
"
,--
Stiff feel __/- 1 1 N.T. N.T. 1 1 1
1 1 1 1 1
,
Resistance to combing _,..----- 2 1 N.T. N.T. 2 2
1 1 1 1 = 1 2 .
00
,
,--
-
CA 02976327 2017-08-10
- 19 -
Unless otherwise noted, the unit of the numerals for the respective components
in
each recipe is w/v%.
The NIKKOL series in the components refers to product names.
The indication of the hydrogenated soybean phospholipid is abbreviated from
NIKKOL Resinol S-10M.
The samples of Comparative Examples 9 and 10 are labeled N.T. because they did
not dissolve to form applicable preparations.
The number of tests for stiff feel and resistance to combing was n = 1.
[0061] [Table 3]
Product name Comparative
Example
Compound name NIKKOL HLB 19 20 21 22 23
Compound of formula (4) /---- 5 5 5 5 5
Magnesium chloride - 3 3 3 3
Sorbitan sesquioleate SO-15MV 3.7 0.5 - - -
Polyoxyethylene oleyl ether B0-2V 7.5 - 0.5 - -
Lauromacrogol BL-2 9.5 - - 0.5 -
Polyoxyethylene hardened HCO-10 6.5 - - - - 0.5
castor oil 10
Absolute ethanol - 74 74 74 74 74
Water _,-----. bal. bal. bal.
bal. bal.
Total amount ,..--------- 100mL 100mL 100mL 100mL 100mL
Sensory evaluation (numerals
refer to evaluation scores) /
Stiff feel ..õ,------ 1 2 2 2 2
Resistance to combing ¨ 1 2 2 2 2
Unless otherwise noted, the unit of the numerals for the respective components
in
each recipe is w/v%.
The amount of incorporation of each metal salt compound is calculated for
anhydride.
The NIKKO L series in the components refers to product names.
The number of tests for stiff feel and resistance to combing was n = 7.
[0062]
CA 02976327 2017-08-10
' - 20 -
[Table 4]
Comparative Example
Component name 24 25
Compound of formula (4) 5 5
ct-Cyclodextrin 2 -
P-Cyclodextrin - 2
Absolute ethanol 74 74
Water bal. bal.
Total amount 100mL 100mL
Sensory evaluation (numerals refer to evaluation scores)
Stiff feel 1 1
Resistance to combing 2 2
Unless otherwise noted, the unit of the numerals for the respective components
in
each recipe is w/v%.
The number of tests for stiff feel and resistance to combing was n = 1.
[0063] [Table 5]
Product name Example
Component name NIKKOL HLB 1 2 3 4 5 6
Compound of formula (4) ---1. 5 5 5 5 5 5
Magnesium chloride ,----- 3 3 3 3 3 3
Polysorbate 80 TO-10M 15 0.5 - - -
Hydrogenated soybean S-10M - - - 0.5 - -
phospholipid _
Polyethylene glycol MYS-10V 11- - 0.5 -
monostearate
Polyoxyethylene stearyl BS-4V 9- - - - 0.5 -
ether
Polyoxyethylene cetyl BC-5.5 10.5 - - - - 0.5 -
ether ,
Polyoxyethylene (20) PBC-44 12.5- - - - -
0.5
polyoxypropylene (8)
cetyl ether
Absolute ethanol ..,...-------' 74 74 74 74 74
74
Water ..õ------ bal. bal. bal. bal. bal. bal.
Total amount õ,...-------4 100mL 100mL 100mL 100mL 100mL 100mL
Sensory evaluation
(numerals refer to
/
evaluation scores)
Stiff feel ---<õ.. 3 3 3 3 3 3
Resistance to combing _---- 2 3 2 3 2 2
Unless otherwise noted, the unit of the numerals for the respective components
in
each recipe is w/v%.
The amount of incorporation of each metal salt compound is calculated for
anhydride.
CA 02976327 2017-08-10
- 21 -
The NIKKO L series in the components refers to product names.
The indication of the hydrogenated soybean phospholipid is abbreviated from
NIKKOL Resinol S-10M.
The number of tests for stiff feel and resistance to combing was n = 7.
[0064] [Preparation examples]
To make the preparations in accordance with the present invention, the amount
of
incorporation of the compound of formula (4) may be freely changed in Example
1. To be
more specific, the compound of formula (4) may be incorporated in three
different amounts,
2.5, 5 and 10 (w/v%) to prepare lotions at three concentrations of compound
(4).
[0065] In Example 1, a lotion may be prepared by incorporating 6.4 (w/v%) of
magnesium
chloride in hexahydrate form.
[0066] In Example 1, the amount of incorporation of polysorbate 80 may be set
at three
values, 0.5, 1 and 2 (w/v%), to prepare lotions at three concentrations of
polysorbate 80.
[0067] In Example 1, 1,3-butylene glycol may be further added and the amount
of its
incorporation may be set at three different values, 0.5, 1 and 2 (w/v%), to
prepare lotions at
three concentration of 1,3-butylene glycol.
[0068] In Example 1, absolute ethanol as solvent may be adjusted to fall
within the range of
62 to 74 (w/v%), with the balance being water to prepare lotions each having a
total volume
of 100 (mL).
INDUSTRIAL APPLICABILITY
[0069] The hair growth composition for cutaneous application in accordance
with the
present invention contains the compound represented by formula (1) and has
such an
advantage that even after it is applied to the scalp of a user, the stiff feel
of the user's hair is
suppressed and continued smooth combing is assured.