Note: Descriptions are shown in the official language in which they were submitted.
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
IMPLANTABLE DEVICES AND RELATED METHODS FOR HEART FAILURE
MONITORING
RELATED APPLICATION DATA
[0001] This application claims the benefit of priority of U.S. Provisional
Patent Application
Serial No. 62/115,435, filed February 12, 2015, and titled "Implantable Device
and Related
Methods for Heart Failure Monitoring", this application also claims the
benefit of priority of
U.S. Provisional Patent Application Serial No. 62/157,331, filed May 5, 2015,
and titled "Heart
Failure Monitoring System and Method", and also claims the benefit of priority
of U.S.
Provisional Patent Application No. 62/172,516, filed June 8, 2015, and titled
"Methods and
Apparatus for Monitoring Patient Physiological Status Based On Inferior Vena
Cava Volume".
Each of these applications is incorporated by reference herein in their
entirety.
FIELD OF THE INVENTION
[0002] The present invention generally relates to the field of medical
devices and methods
for monitoring heart health. In particular, the present invention is directed
to an implantable
device for heart failure monitoring, and more particularly for detecting early
warning signs of
acutely decompensated heart failure.
BACKGROUND
[0003] Heart failure is one of the most significant chronic conditions
afflicting adult
populations. In the United States, 5.7 million Americans have heart failure,
with 870,000 new
cases annually. As the population ages, this population is growing, as
approximately 10% of the
population over 80 suffers from heart failure. It is estimated that by 2030 8
million Americans
will have heart failure. The costs of caring for heart failure are over thirty
billion dollars per year.
Twenty billion of this cost is direct medical costs. This expense is expected
to more than double
over the next fifteen years.
[0004] In patients with chronic heart failure, a significant portion of
these costs is due to
hospitalization to manage acutely decompensated heart failure (ADHF). Each re-
hospitalization
can last up to a week, and costs approximately $10,000. ADHF is very often a
result of some
combination of a downturn in the heart's performance and excessive intake of
fluids and/or salt.
This leads to a buildup of fluid in the vascular system. Increased blood
volume in the left atrium
1
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
at higher pressure means higher blood pressure in the lungs, which eventually
leads to fluid
filling the lungs and an inability to breathe. At this stage it is imperative
to hospitalize the patient
to carefully manage them while drugs are delivered to remove the excess
fluids.
[0005] Managing these patients to prevent the need for re-hospitalization
is extremely
challenging. Many non-invasive approaches to monitoring patients have been
tried, such as
weighing patients daily to detect fluid weight gain, having a nurse call them
daily to assess their
health status, and so on. More recently, various implantable monitoring
devices have been tested.
One example is the "CardioMEMS" device of St. Jude Medical, Inc., which is a
wireless
pressure monitor implanted in the pulmonary artery (PA). An external power
supply and receiver
is placed on the patient's chest to charge the implanted sensor and receive
pressure data
measured by it. Other companies are developing their own versions of such PA
pressure
monitors. The money saved by avoiding re-hospitalization can more than pay for
the cost of
such devices.
[0006] It is important to measure the onset of ADHF early enough to give
the patient and/or
caregiver enough time to adjust their behavior, medication, or other factors
to prevent the patient
from ending up with frank congestion and the need for hospitalization. FIG.
47, adapted from the
CardioMEMS website, shows the timeline of physiologic changes leading up to
ADHF requiring
hospitalization. There is clinical evidence that IVC volume variation changes
occur up to several
weeks prior to decompensation.
[0007] In addition to heart failure patients, hemodialysis patients have a
chronic need for
careful volume management. Large volumes of fluid are involved in the
hemodialysis process,
and managing patients so that they don't end up hypovolemic or overloaded with
fluid requires
careful management. A monitor which provided immediate feedback on these
patient's volume
status before, during and after hemodialysis would be very helpful.
[0008] There are other groups of patients who might benefit from such a
monitor. For
example, patients in septic shock or occult shock due to trauma are subject to
hypoperfusion
which can be identified by measuring the degree of collapse of the IVC. While
it may or may not
make sense to implant a device permanently to manage these acute events, if
the patient has
2
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
recurrent episodes of these events or already has such a monitor implanted for
other reasons, the
IVC monitor may be helpful in managing these patients.
[0009] Congestive heart failure is so-named because additional blood volume
backing up
into the lungs causes fluid to seep out of the pulmonary circulation into the
airway passages of
the lungs, causing congestion of the lungs. The patients become short of
breath, and typically
need to be hospitalized and carefully managed while the excess fluid is
removed by a
combination of fluid management and aggressive use of diuretic medications.
[0010] This happens because the left ventricle is not able to pump all of
the volume of blood
returning to the heart from the lungs. Although measurement of left atrial
pressure, typically by
measuring pulmonary artery wedge pressure, is commonly considered the most
direct way to
measure congestion in heart failure, there are other areas where congestion
can be detected.
When additional blood volume is added to the circulatory system, the IVC is
one of the first
places for that added volume to collect. To quote a paper, "In patients with
advanced heart
failure, left ventricular systolic dysfunction causes increased left atrial
pressure. The pressure is
transmitted back through the pulmonary circulation to cause pulmonary artery
hypertension. The
pulmonary artery hypertension can worsen pre-existing right ventricular
dysfunction and
exacerbate tricuspid valve regurgitation, leading to systemic venous
congestion. If venous
congestion and elevated central venous pressure are the hallmarks of heart
failure, then distention
of the inferior vena cava [measured by echocardiography] may be a good
prognostic marker in
patients with decompensated heart failure." (Lee et al, "Prognostic
significance of dilated
inferior vena cava in advanced decompensated heart failure," International
Journal of
Cardiovascular Imaging (2014) 30:1289-1295).
[0011] The diameter of the IVC has also demonstrated correlation with right
atrial pressure,
and it may correlate with renal function and renal sodium retention, which are
also very
important prognostic factors of heart failure. Therefore, increasing IVC
volume and/or pressure
may be a very effective early indicator of worsening heart failure condition.
[0012] However, recent studies have indicated that the variation in IVC
volume over the
respiratory cycle is a more sensitive measurement of fluid overload and/or
heart failure than
simple measurement of average IVC volume, diameter, or pressure. During
inspiration,
3
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
intrathoracic pressure decreases, thereby increasing venous return and causing
collapse of the
IVC. During expiration, intrathoracic pressure increases, decreasing venous
return and causing
an increase in the volume of the IVC.
[0013] Since the IVC typically collapses in the anterior-posterior
direction, some studies
have suggested that the most accurate technique for measuring IVC volume
changes with
ultrasound is to measure the distance from the anterior wall of the IVC to the
posterior wall.
[0014] In applying this measurement to heart failure, at least one study
has suggested that a
variation of less than 15% (measured as maximum anterior-posterior dimension
minus minimum
A-P dimension, divided by the maximum A-P dimension) is indicative of
impending or present
ADHF.
[0015] While vessel dimensions may be measurable using external ultrasound,
magnetic
resonance imaging, computerized axial tomography, or other technologies, these
imaging
procedures must be administered in a hospital or other specialized facility,
do not permit
continuous monitoring, and do not allow for monitoring of the patient at their
home or other
remote location. As a result, the condition of a heart failure patient can
worsen into a critical
state before care providers become aware of it, dramatically increasing the
mortality risk and
cost of treatment for the patient.
[0016] Prior studies of IVC dimensions without implantable devices have
been conducted
using ultrasound imaging. This typically requires a highly trained physician
or ultrasound
technician to manage the ultrasound machine, ensure an appropriate connection
of the transducer
to the skin, position the ultrasound transducer in the appropriate location,
identify the IVC, and
take accurate measurements. This is not something that heart failure patients
or their caregivers
could typically be trained to do predictably and accurately with existing
equipment. Moreover,
these systems typically include large, complex, and expensive pieces of
equipment which are not
suitable for use outside of a specialized medical facility.
[0017] As is understood in the art, there is a long history of implantable
vena cava filters to
catch clots which embolize from the leg veins, catching them and holding them
in the vena cava
until they dissolve in the blood flowing past. The widespread clinical use of
such IVC filters
4
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
demonstrates the safety and feasibility of anchoring an implant in the IVC and
provides useful
teachings as to how aggressively IVC anchors may be shaped, how much radial
force a device
should exert, how strong the elements should be, etc. However, in spite of the
widespread use of
IVC filters over many years, heretofore it has not been suggested that an
implant in the IVC
could be utilized for purposes of monitoring fluid volume or IVC dimensions.
Moreover, even if
a suggestion were made to equip an IVC filter with a sensor for monitoring
vascular dimensions,
such filters would be unsuited to the purpose. The anchoring structures used
to secure IVC filters
constrain the vessel from natural size and shape changes in response to
changes in fluid volume
and would thus limit the usefulness or accuracy of such a device.
SUMMARY OF DISCLOSURE
[0018] Embodiments disclosed herein include an implantable device for
monitoring vascular
lumen diameter, comprising means for detecting lumen diameter at a monitoring
location; an
anchor element configured to securely anchor the device to the vascular lumen
at an anchoring
location with the detecting means positioned at the monitoring location; and
an anchor isolation
structure extending between the detecting means and anchor element, the anchor
isolation
structure having a shape and length specifically configured to substantially
isolate the detecting
means at the sensing location from distortions of the vessel caused by the
anchoring element at
the anchoring location.
[0019] In one alternative embodiment, the detecting means comprises an
active marker
element coupled to an end of the anchor isolation structure opposite the
anchor element; the
anchor element comprises a resilient member moveable between a first,
collapsed configuration
and a second, deployed configuration, wherein the first collapsed
configuration has an overall
diameter sufficiently less than the vascular lumen diameter to permit
deployment of the device
through the vasculature, and the second expanded deployed condition has an
overall diameter
sufficient to securely engage the vessel lumen at the anchoring location; and
the anchor isolation
structure comprises a member having sufficient stiffness to maintain the
active marker element
substantially in contact with the lumen wall without eroding the lumen wall
and with the active
marker element oriented substantially in the direction of the lumen wall
opposite the transducer.
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
[0020] In another alternative embodiment of claim 1, wherein: the detecting
means
comprises an electronics capsule having an ultrasound transducer disposed at
an end opposite the
anchor element, the electronics capsule containing at least power and
communications modules
controlled by a control modules to receive and wirelessly transmit signals
based on inputs from
the ultrasound transducer; the anchor element comprises a looped anchor wire
secured to a
telescoping deployment member moveable between a first collapsed configuration
and a second
deployed configuration, wherein the looped wire in the second deployed
configuration expands
sufficiently to place the electronics capsule against one wall of the lumen
while in contact with a
substantial portion of an internal diameter of the lumen at the anchoring
location; and the anchor
isolation structure comprise a flexible member disposed between the anchor
element deployment
member and the electronics capsule, wherein the anchor isolation structure has
sufficient
stiffness to maintain the electronics capsule against the one wall of the
lumen and a length equal
to approximately 1 to 4 times the undistorted diameter of the lumen at the
anchor location.
[0021] A further alternative embodiment disclosed is a cardiac therapy
system comprising
the implantable monitoring device described above in combination with an
implantable cardiac
therapy device including an implantable housing containing at least power and
control modules;
at least one lead connecting the detecting means with the implantable housing
and modules
contained therein; at least one cardiac therapy delivery element; and at least
one lead connecting
the at least one therapy delivery element to the implantable housing and
modules contained
therein. The control module is configured to execute instructions for
generating a signal with the
detecting means, receiving a signal responsive to the generated signal with
the detecting means,
the received signal being indicative of vessel diameter at the monitoring
location; determining
vena cava diameter at the monitoring location based on the signal; comparing
the determined
diameter to a reference diameter to determine a change in vena cava diameter,
evaluating heart
condition based on the determined change in vena cava diameter; and modulating
cardiac
therapy delivered by the at least one therapeutic device passed on the
evaluated heart condition
[0022] In another disclosed embodiment an implantable device for monitoring
vascular
lumen diameter comprises an electronics capsule having an ultrasound
transducer disposed at
one end, the electronics capsule containing at least power and communications
modules
controlled by a control module to receive and wirelessly transmit signals
based on inputs from
6
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
the ultrasound transducer, the ultrasound transducer configured substantially
in contact with a
lumen wall and to detect lumen diameter at a monitoring location by echo
reflection off an
opposite lumen wall; a looped anchor wire secured to a telescoping deployment
member
moveable between a first collapsed configuration and a second deployed
configuration, wherein
the looped anchor wire in the second deployed configuration expands
sufficiently to place the
electronics capsule against one wall of the lumen while in contact with a
substantial portion of an
internal diameter of the lumen at an anchoring location; and a flexible anchor
isolation member
disposed between the tubular deployment member and the electronics capsule,
wherein the
anchor isolation structure has sufficient stiffness to maintain the
electronics capsule against the
one wall of the lumen, the anchor isolation member having a shape and length
specifically
configured to at least substantially isolate the electronics capsule from
distortions of the vessel
lumen caused by the anchoring looped anchor wire at the anchoring location.
[0023] In a further disclosed embodiment a system for monitoring vascular
lumen diameter
comprises at least two implantable passive marker elements configured to be
implanted in or
through a vascular lumen wall at a monitoring location; an intravascular
delivery device for
delivering and implanting the passive marker elements at the monitoring
location; and detecting
means configured to be positioned outside a patient's body for sensing
position and movement
of the implanted passive marker elements relative to each other.
[0024] Another disclosed embodiment is An implantable device for monitoring
vascular
lumen diameter, comprising an ultrasound transducer configured to be
positioned substantially in
contact with a lumen wall and to detect lumen diameter at a monitoring
location by echo
reflection off an opposite lumen wall; an, electronics capsule containing at
least power and
communications modules controlled by a control module to receive and wireles
sly transmit
signals based on inputs from the ultrasound transducer; an anchoring member
configured to
rotationally and longitudinally immobilize the implantable device at an
anchoring location in the
vascular lumen ; and a flexible anchor isolation member disposed between the
anchoring
member and the ultrasound transducer, wherein the anchor isolation structure
has a shape and
stiffness selected to maintain the ultrasound transducer against the one wall
of the lumen, the
anchor isolation member having a shape and length specifically configured to
at least
7
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
substantially isolate the ultrasound transducer from distortions of the vessel
lumen caused by the
anchoring member at the anchoring location.
[0025] An implantable device for monitoring vascular lumen diameter
according to another
disclosure comprises an ultrasound transducer configured to be positioned
substantially in
contact with a lumen wall and to detect lumen diameter at a monitoring
location by echo
reflection off an opposite lumen wall; an electronics capsule containing at
least power and
communications modules controlled by a control module to receive and wireles
sly transmit
signals based on inputs from the ultrasound transducer; and an anchoring
member configured to
rotationally and longitudinally immobilize the implantable device at an
anchoring location in the
vascular lumen and having a shape and stiffness selected to maintain the
ultrasound transducer
against the one wall of the lumen. The electronics capsule may be mounted
directly to the
anchor element.
[0026] In another aspect of the present disclosure, a method of
continuously monitoring a
diameter of a vascular lumen with an implanted device, comprises implanting at
least one marker
element on a wall of the lumen at a monitoring location; anchoring the marker
element to the
lumen wall at an anchoring location, wherein the anchoring location is spaced
from the
monitoring location by a distance sufficient to isolate the marker element
from distortions of the
lumen wall due to the anchoring; generating a signal in cooperation with the
at least one marker
element indicative of vessel diameter at the monitoring location; receiving
the signal; and
determining vessel diameter based on the signal.
[0027] In a further embodiment of the method aspects of the present
disclosure, a method of
treating a cardiac condition in a patient comprises implanting a monitoring
device in a vena cava
wall such that at least one active marker element of the monitoring device is
positioned against
the wall at a monitoring location; anchoring the monitoring device to the vena
cava wall at an
anchoring location, wherein the anchoring location is spaced from the
monitoring location by a
distance sufficient to isolate the at least one active marker element from
distortions of the lumen
wall due to the anchoring; implanting at least one therapeutic device
configured to deliver a
cardiac therapy; generating a signal with the at least one active marker
element indicative of
vessel diameter at the monitoring location; receiving the signal; determining
vena cava diameter
8
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
at the monitoring location based on the signal; comparing the determined
diameter to a reference
diameter to determine a change in vena cava diameter, evaluating heart
condition based on the
determined change in vena cava diameter; and modulating cardiac therapy
delivered by the at
least one therapeutic device passed on the evaluated heart condition.
[0028] Another method disclosed herein is a method of monitoring a cardiac
condition of a
patient, comprising implanting an intravascular device in a vena cava with an
anchor element at
an anchoring location, the intravascular device further having a pair of
markers or sensors, the
pair of markers or sensors being positioned in engagement with opposing walls
of the vena cava
at a monitoring location; receiving a signal from the pair of markers or
sensors; and determining
a diameter of the vena cava based on the signal; wherein the implanting
comprises positioning
the anchor element at the anchoring location sufficiently distant from the
monitoring location
such that diameter determinations based on the signal are not substantially
affected by distortion
of the vena cava wall by the anchor element at the anchor location.
[0029] In another disclosed embodiment, a monitoring system comprises an
intravascular
device configured for implantation in a vessel in a body cavity and having
first and second
markers biased or secured against opposing interior walls of the vessel; at
least one anchor
element configured to secure and position the intravascular device on the body
cavity wall; an
anchor isolation structure disposed between the at least one anchor element
and the first and
second markers, the anchor isolation structure having a length specifically
configured to position
the first and second markers sufficiently distant from the monitoring location
such that diameter
calculations derived from the markers are not substantially affected by
distortion of the vena
cava wall by the anchor element at; and an external device adapted to receive
a first signal from
the intravascular device and to calculate a distance between the first and
second markers based
on the signal.
[0030] In a further embodiment an intravascular device comprises an
anchoring structure for
anchoring within the vessel; and first and second arms extending
longitudinally from the
anchoring structure, wherein the first and second markers are coupled to the
first and second
arms, respectively. The first and second arms are configured to bias the first
and second markers
9
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
against the vessel wall and to allow the first and second markers to move
inwardly and outwardly
with physiologic movement of the vessel wall.
[0031] Yet another embodiment is a therapeutic system for treating a
patient comprising a
monitoring system including an intravascular implant for implantation in a
vena cava configured
to monitor a dimension of the vena cava; a transmission device to transmit
data based on the
dimension; and a therapeutic device implanted in the patient and configured to
receive the data
transmitted from the monitoring system, the therapeutic device adapted to
deliver therapy to the
patient based upon the data received.
BRIEF DESCRIPTION OF THE DRAWINGS
[0032] For the purpose of illustrating and exemplifying the claimed
invention, the drawings
show aspects of embodiments of the present disclosure. However, it should be
understood that
the claimed invention is not limited to the precise arrangements and
instrumentalities of the
exemplifying embodiments shown in the drawings, wherein:
FIG. 1 is a schematic illustration of one embodiment of an implantable device
deployed
in the inferior vena cava (IVC) in accordance with the present disclosure.
FIGS. 2A, 2B, 2C and 2D show schematic cross-sections of the IVC and relative
electrode positioning in embodiments described in the present disclosure.
FIG. 3 is a schematic illustration of one disclosed embodiment of an
implantable device,
showing its placement in the vasculature.
FIG. 4 is a schematic illustration of another disclosed embodiment of an
implantable
device, showing its placement in the vasculature.
FIG. 5 is a perspective view of a further alternative embodiment positioned in
a partially
cross-sectioned portion of the IVC.
FIG. 6 is side view of the embodiment shown in FIG. 5 and the partially cross-
sectioned
IVC.
FIG. 7 is an end view of the embodiment of FIG. 5 as viewed in the IVC from
the
superior aspect.
FIG. 8 is an end view of the embodiment of FIG. 5 as viewed in the IVC from
the inferior
aspect.
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
FIG. 9 is a detail of the anchor element of the embodiment of FIG. 5 in a
collapsed
configuration.
FIG. 10 is a detail of the anchor element of the embodiment of FIG. 5 in an
expanded or
deployed condition, shown outside the IVC.
FIGS. 11 and 12 are perspective views illustrating a further alternative
embodiment of
anchoring elements, in deployed and collapsed configurations, respectively.
FIG. 13 is a perspective view illustrating the embodiment of FIGS. 11 and 12
as it may
appear deployed within the IVC (note that for illustration purposes the
orientation is not intended
to be anatomically accurate in this or similar figures).
FIGS. 14 and 15 are perspective views illustrating yet another alternative
embodiment of
an anchor element, in the collapsed and deployed configurations, respectively.
FIG. 16 is a perspective view illustrating the embodiment of FIGS. 14 and 15
as it may
appear deployed within the IVC.
FIG. 17 is a side view illustrating another alternative embodiment of an
implantable IVC
monitor with a stent-like anchor element and electronics capsule.
FIGS. 18 and 19 are perspective views illustrating a further embodiment of an
anchor
element shown in the collapsed and expanded/deployed configurations,
respectively.
FIG. 20 and 21 are perspective views illustrating the embodiment of FIGS. 18
and 19 as
it may appear deployed within in IVCs of different dimensions.
FIG. 22 is a schematic illustration of a further embodiment of implantable
device
positioned in the IVC according to the present disclosure.
FIG. 23 is a schematic illustration of yet another embodiment of implantable
device
positioned in the IVC according to the present disclosure.
FIG. 24 is a schematic illustration of a further embodiment of implantable
device
according to the present disclosure.
FIG. 25 is a schematic illustration of a further embodiment of implantable
device
positioned in the IVC according to the present disclosure.
FIG. 26 is a schematic illustration of another embodiment of implantable
device
positioned in the IVC according to the present disclosure.
FIGS. 27A and 27B are schematic illustrations of a further embodiment of
implantable
device according to the present disclosure.
11
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
FIGS. 28A, 28B and 28C are a series of schematic illustrations showing
delivery and
placement of an embodiment of a device configured for external placement on
the IVC according
to an alternative of present disclosure.
FIG. 29 illustrates another alternative device embodiment deployed in the IVC
[]A-005].
FIG. 30 schematically depicts an alternative embodiment of a system described
in the
present disclosure.
FIG. 31 schematically depicts an embodiment for detection of markers in a
deployed
device using ultrasound.
FIG. 32 schematically depicts an arrangement of markers and two sensors on a
transverse
cross-section of the body in a system according to one disclosed embodiment.
FIG. 33 schematically depicts another alternative embodiment of a system
described in
the present disclosure.
FIG. 34A illustrates placement or injection of a marker between the medial and
adventitial layers of the wall of the IVC according to one exemplary
embodiment disclosed
herein.
FIG. 34B illustrates delivery and adherence of a marker to the outer surface
of the wall of
the IVC in another exemplary embodiment disclosed herein.
FIG. 35 illustrates another exemplary embodiment in which a marker is deployed
through
a delivery catheter that holds the marker between two jaws until it reaches
the distal end of the
delivery catheter, at which point the jaws separate to release the marker.
FIG. 36A illustrates an embodiment of a guidewire coil.
FIG. 36B illustrates a guidewire coil coated with a polymer to permanently
entrap air to
provide echo-reflective characteristics.
FIG. 36C is a close-up or enlarged view of a section of a coiled ribbon marker
with
surface texture configured to increase echo-reflectivity.
FIG. 36D illustrates another embodiment of a marker, which may comprise a
simple
echo-reflective tube such as a sealed tube of air.
FIG. 36E illustrates another embodiment of a marker, in this case a tube of
cast polymer
such as silicone with echo-reflective gas bubbles embedded in the tube wall.
FIGS. 37A, 37B and 37C illustrate various embodiments of markers formed as
particles
in accordance with alternative embodiments disclosed herein.
12
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
FIG. 38 is an enlarged view of a further exemplary embodiment of a marker as
disclosed
herein comprising a gel mixed with marker particles injected into the wall of
the IVC.
FIG. 39A is a cross-sectional view of a particle/marker containing patch
endothelialized
into the IVC wall.
FIG. 39B is a cross-sectional view of an alternative marker patch utilizing a
"Velcro-like"
texture of microneedles or microhooks to adhere to and embed into the IVC
wall.
FIG. 40A illustrates balloon delivery of one or more markers in accordance
with a further
alternative embodiment disclosed herein.
FIG. 40B illustrates another alternative embodiment in which a two-balloon
catheter is
used such that blood flow may be maintained during marker delivery and
placement.
FIG. 41 illustrates an exemplary embodiment of an external
transmitter/receiver
configured to provide more consistent and precise measurements of relative
distance between
IVC two markers as described herein.
FIG. 42 schematically depicts another alternative system employing
communicating
monitoring and therapeutic devices.
FIG. 43 schematically depicts a further alternative system employing direct
communication through the IVC wall.
FIG. 44 schematically depicts yet another alternative system employing
intravascular
leads for direct communication.
FIG. 45 illustrates one exemplary embodiment of a pulmonary artery sensor
being
implanted by a delivery catheter in the pulmonary artery following
implantation of an IVC
monitoring device in the IVC.
FIG. 46 is a block diagram illustrating embodiments for communication and
computerized implementation of various embodiments described herein.
FIG. 47 illustrates a typical timeline of symptoms leading to hospitalization
for ADHF.
DISCLOSURE OF EMBODIMENTS
[0033] Various embodiments disclosed herein are intended to monitor for and
detect
variations in volume and/or pressure of the inferior vena cava (IVC) as an
early warning signal
of the acute severity of heart failure. Implantable IVC monitors, markers and
related systems,
devices and methods as described herein may enable the patient and physician
to take proactive
13
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
steps in time to prevent acute decompensation requiring hospitalization. Such
devices and
methods also may be helpful in managing hemodialysis patients, in whom volume
management
is a chronic challenge. The present disclosure thus describes methods and
devices for measuring
IVC volume and/or pressure more or less continuously, depending on clinical
need, using
various forms of implantable devices.
[0034] In order to measure changes in IVC dimension or volume accurately,
the devices of
the invention must be configured to be secured at the desired location in or
on the vessel without
affecting the natural dilation and constriction of the vessel, or by affecting
it in a way which is
known and predictable so that it can be accounted for. In many of the
embodiments disclosed
herein, implantable monitoring devices include an anchoring member which
secures the device
to the vessel and immobilizes the device both longitudinally and rotationally,
and a sensing or
marking element which responds to vessel expansion and contraction to allow
monitoring of
changes in vessel dimension. It is critical in such embodiments that the
anchoring element not
distort the vessel dimensions being measured by the sensing/marking element.
In preferred
embodiments, the sensing or marking element is isolated from the anchoring
member such that
the vessel can naturally expand and contract at the site of measurement
without significant
constraint. In some embodiments, this isolation comprises a longitudinal
separation of the
sensing/marking element from the anchor member a distance sufficient to
minimize the effects of
the anchor on the vessel motion at the measurement site. In such embodiments
the
sensing/marking element will be coupled to the anchor member by an elongated
connecting
element which has a length and flexibility sufficient to provide the necessary
isolation, which has
sufficient rigidity to maintain the position of the sensing/marking element at
the measurement
site, and which, in many embodiments, has the appropriate shape and resilience
to bias the
sensing/marking element against the wall of the vessel as it moves inward and
outward. Such
connecting elements will also have a length selected to allow the anchor
member to be implanted
in the desired location in the IVC, in preferred embodiments just inferior to
the hepatic veins,
with the sensing/marking element positioned in the IVC between the anchor
member and the
right atrium. In certain exemplary embodiments, such connecting elements will
have a length in
the range of 1 to 4 times the vessel diameter (e.g. 1-8 cm), more desirably 1
to 3 times the vessel
diameter (e.g. 2-6 cm), and preferably 1 to 2 times the vessel diameter (e.g.
2-4 cm). In some
embodiments it will be desirable to provide a longitudinal separation between
the anchor element
14
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
and the marker elements of about 3-5 cm. Also, it may be desirable to position
the anchor
elements somewhat inferior to the renal arteries so that the marker elements
fall between the
renal and hepatic veins. In one preferred embodiment, the marker elements are
positioned at a
monitoring location falling in a region from approximately 2 cm below the
hepatic veins down
to, but not below the renal veins. In other embodiments, instead or in
addition to spatial
separation of the anchor and sensor/marker, isolation may be achieved by a
mechanical coupling
between the anchor and the sensing/marking element which mechanically isolates
movement of
the sensing/marking element from the anchor, such as a spring, hinge, flexible
link, or other type
of isolating coupling.
[0035] Because heart failure patients often receive catheters for
monitoring and treatment
which are inserted through the IVC, preferred embodiments of the invention
will be configured
to allow the placement of catheters and other devices past the location of the
implanted
monitoring device without risk of displacement or compromising its function.
In some
embodiments, the devices of the invention are configured to be anchored to the
vessel wall
without jailing (i.e. extending across) or substantially occluding the vessel
lumen.
[0036] In certain embodiments described herein the monitoring devices of
the invention are
configured to measure vascular dimension in a predetermined direction or along
a predetermined
axis. Such embodiments are configured to facilitate implantation within the
vessel in a position
which enables such directional measurement. In exemplary embodiments, the
devices of the
invention are configured to measure IVC diameter in the anterior-posterior
direction. In such
embodiments the devices are configured to preferentially position and maintain
the sensing or
marking elements against the posterior and/or anterior wall of the IVC
throughout the respiratory
cycle. Exemplary embodiments may further include anchoring elements that
deploy in such a
way as to preferentially position the device in the desired rotational
position in the vessel. For
example, such anchoring elements may have a shape or include features which
take advantage of
the oval cross-sectional shape of the IVC and naturally seat themselves in the
desired rotational
orientation.
[0037] One type of device disclosed herein, as shown, for example, by the
embodiment in
FIG. 1, may have flexible marker elements, such as flexible electrodes, that
lay unobtrusively
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
against the wall of the IVC. Various embodiments of this type of device are
described in more
detail below. As the marker element positions change relative to one another
based on changes
in shape/volume of the IVC, the change may be determined through signals or
feedback
exchanged between the marker elements. For example, as the IVC decreases in
volume, it may
go from being fully inflated with a round shape to a flatter shape. In a
design with a number of
marker elements deployed circumferentially around a cross-section of the IVC,
this means that
certain marker elements may become closer together as the IVC collapses, and
some may move
farther apart. FIGS. 2A, 2B, 2C and 2D schematically illustrate how this
change may occur. As
is seen, the variation in proximity of the marker elements with IVC collapse
depends upon the
orientation of the device relative to the axis of collapse of the IVC. In
FIGS. 2A and 2B, it is
seen that marker elements a and c become closer as the IVC collapses, while
marker elements b
and d move farther apart. In FIGS. 2C and 2D, marker elements a and b and c
and d become
closer as the IVC collapses. Alternatively, a single marker element may be
provided with a
signal type that may be reflected off of the opposite IVC wall. The same
principals apply when a
single marker element is used with a signal reflected off the opposite IVC
wall. In one such
example, the single marker element may be positioned at location a in FIGS. 2A
and 2B, with the
reflected wall being generally at location c, directly across from a.
[0038] In general, marker elements used in embodiments disclosed herein may
be active
marker elements or passive elements. Examples of active marker elements
include ultrasound
transducers, electrodes and inductance coils. Passive marker elements are
generally signal
reflective, such as echo-reflective, which can reflect an ultrasound signal
directed at the marker
elements from outside the body. In an embodiment where the market elements are
comprised of
electrodes, it may be most effective to determine which electrodes are
positioned most directly
on the anterior and posterior walls, and to measure the variation in impedance
between those
electrodes. Alternatively, the system could measure the impedance from each
electrode to each
of the others, and to use the variation in impedances to estimate the change
in shape. Or it may
be equally effective to combine the impedances of all of the opposing
electrode pairs in parallel,
and look at the variation in that single overall impedance reading.
[0039] In certain situations it may alternatively or also be effective to
measure the
longitudinal impedance along the length of the IVC or the superior vena cava
(SVC), or both. As
16
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
the IVC and/or SVC collapses, the cross-sectional area of the IVC and SVC
decreases, which
may lead to a meaningful change in impedance along its length. One exemplary
embodiment
employing this alternative is illustrated in FIG. 35 as described below.
[0040] In addition to simply measuring impedance across the IVC by use of
implantable,
flexible electrodes, there are a number of other ways by which an implantable
device may
measure the variation in shape of the IVC. Such further alternatives include
elements such as
strain gauges or displacement sensors attached to a radial or circumferential
element of the
device, proximity sensors, etc. One exemplary embodiment in this regard is
illustrated in FIG. 24
as described below.
[0041] Fluid pressure sensors may also be useful in measuring variations in
IVC status.
Alternatively, the flow rate through the IVC might be measured using a Doppler
ultrasound
sensor or other sensor. As the volume and cross-sectional area of the IVC
change, the speed of
blood flow through the IVC might change inversely and proportionately,
although blood volume
and flow will also change with changes in posture, exercise level, and so on.
In this approach, it
might be helpful to measure heart rate as well as an indicator of cardiac
output, to normalize flow
rates or to make certain that measurements are only being taken when the
patient is at rest.
Inertial sensors might also be included, to measure posture and motion. MEMS
inertial sensors
have been developed which are tiny and consume very little battery power. It
might also be
helpful to implant a reference pressure sensor or inertial sensor elsewhere in
the body or vascular
system, such as in the leg, to detect posture changes and activity level.
[0042] A further alternative measurement means is to use sonomicrometry.
This involves
tiny piezoelectric crystal sensors which emit tiny sonic signals, which are
then detected by other
sensors and converted to an electrical signal. By analyzing the time between
the transmission and
reception of these signals, the distance between the crystals can be
accurately measured. A
further alternative measurement means is to transmit a sonic vibration into
the IVC, and by
measuring the reflection or resonance of that signal, the overall volume or
dimensions of the IVC
might be determined.
[0043] The choice between these different methods will depend in part on
determining
which ones measure the variation in IVC volume and pressure most consistently
and precisely.
17
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
Minimizing energy consumption is also an essential factor for implantable
devices which are
intended to function for years, unless external power sources are used to
power or re-charge the
device.
[0044] In alternative embodiments, one or more devices may be implanted on
an external
surface of the IVC to detect changes in vascular dimensions. For example, a
single device
having two spaced-apart electrodes, or two separate devices each with its own
electrode, may be
anchored to the outer IVC wall and used to measure impedance between the
electrodes.
Alternatively, a device having a strain gauge may be anchored to the outer
wall to measure
stress, strain, or displacement between two points on the wall. In another
embodiment, a wire
loop or band incorporating a force or displacement sensor may be placed around
the IVC to
detect changes in IVC circumference based upon the change in size or tension
in the loop or
band. Such devices may be miniaturized so as to be delivered using a large-
bore needle or other
low-profile delivery instrument that can be placed through a small puncture in
the thoracic or
abdominal wall and delivered to the desired location on the IVC.
[0045] This monitoring may be performed continuously or intermittently,
depending upon
the desired tradeoff between data intensity and battery life. It might be most
efficient to take
measurements only at night, when the patient is lying down and at rest. It
might be desirable to
intermittently measure IVC dimensions at random, or at specific time
intervals. Although these
intermittent measurements might result in measuring the IVC distention at
random points in the
cardiac and respiratory cycle, over a period of minutes, hours, or days an
effective picture of the
IVC variation may become clear. Alternatively, the device may intermittently
take continuous
measurements over one or more entire cardiac and/or respiratory cycles, to get
an effective
measurement of the maximum and minimum IVC volumes. The difference between
those
minimum and maximum volumes may be an important prognostic indicator. If there
is only a
small variation between minimum and maximum IVC volumes, that may be an
indicator of
congestion.
[0046] Exemplary embodiments shown in the figures will now be described in
more detail
to further illustrate various configurations and designs of the disclosure. As
will be apparent to
persons of ordinary skill in the art based on the teachings herein contained,
different features of
18
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
the various disclosed embodiments may be employed with embodiments other than
those with
which they are specifically shown in the drawings for purposes of
illustration. Given the number
of possible combinations, it is not possible within a concise disclosure to
separately illustrate
each combination of features as would be understood by those skilled in the
art. As non-limiting
examples, each of the different anchor elements shown in FIGS. 5-21,and the
marker
elements shown in FIGS. 1, 3-5, 22-30 may be used together in different
combinations or
individually with each different implant herein.
[0047] As shown in FIG. 1, monitoring device 100 may have several flexible,
insulated
arms 103 that lay passively against the wall W of the IVC. Marker elements 106
(referred to
hereinafter generically as marker elements or by specific marker element type,
such as electrode,
coil or ultrasound element, etc.) may be mounted on arms 103, preferably at an
end spaced from
the body of device 100. In one exemplary embodiment, marker element 106
comprise electrodes
106 mounted on arms 103, and the impedance between the electrodes may be
monitored via
suitable monitoring devices and means as described further below. There may be
as few as two,
three, or four electrodes 106, or there may be many. There also may be more
than two arms 103.
If there are just two arms 103, they may generate the most effective
measurements if positioned
against the anterior and posterior walls of the IVC. Electrodes 106, or other
marker element,
may be arrayed circumferentially around the IVC at one specific cross-section,
or there may be
electrodes at two or more specific cross-sections, or they may be arranged
over the length of the
IVC. Impedances may be measured in a matrix between all of the different
electrodes, or the
system may focus on measuring impedances just between electrodes on opposing
walls, to
measure any collapse of the IVC most efficiently.
[0048] Alternatively, instead of measuring impedance between electrodes,
marker elements
106 may comprise inductance coils that may be located at the ends of arms 103
of device 100 in
FIG. 1. A small current could be delivered to one coil, and the induced
current in the other coil
could be measured to determine the distance between the coils.
[0049] A further alternative embodiment comprises positioning two
ultrasound crystals as
marker elements 106 on opposing arms 103 of device 100. An ultrasonic signal
from one crystal
could then be detected by the opposing crystal, and the diameter of the IVC
could then be
19
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
determined by measuring the time-of-travel between the two crystals.
Alternatively, and as
described further below, a single ultrasound crystal could be positioned on a
single arm of the
device with the crystal acting as both emitter and receiver of an ultrasound
signal such that vessel
diameter could be determined by reflecting a signal against the opposing wall
of the vessel and
measuring time of travel back and forth from the crystal.
[0050] Device 100 may be located entirely within the IVC as shown in FIG.
1. In this
exemplary embodiment, device 100 is held in place by anchor element 109,
comprising a
radially expanding stent, which has hooks 112 that engage the IVC wall W.
Multiple arms 103
extend superiorly along the IVC, and are biased gently outwards to hold
themselves against the
IVC wall W. At the end of arms 103 are marker elements 106, which may be
electrodes as
described above. Device 100 senses changes in impedance between the marker
element
electrodes to measure the degree of distention or collapse of the IVC.
Alternatively or in
addition, device 100 may also include arms 103 extending inferiorly, holding
another set of
marker elements 106 against the IVC wall W in a more inferior position, which
can also be used
to determine the variation in IVC size. Anchor element 109, such as the
illustrated radially
expanding stent, may be made gentle enough so as to not prevent the distention
or collapse of the
IVC. In that case, marker elements 106 (here illustrated as electrodes) may be
mounted directly
on the anchoring element itself. Various similar embodiments disclosed herein,
may be
important to encapsulate the structure and arms of the device in an
electrically insulative
material, so that it doesn't prevent the measurement of IVC cross-section via
impedance
measurements.
[0051] In a further alternative, anchor element 109 (such as, for example,
the stent shown in
FIG. 1 or in FIG. 23) and/or arms 103 may be made of a bioerodable material
which softens over
time, to minimize any effect the structure might have on the natural motion of
the IVC. The
structure of arms 103 (or anchor element 109) may also be designed to
aggressively heal into the
walls of the IVC, to minimize the risk of migration or embolization over time.
Such alternatives
may also be combined, for example, by making the anchor element or arms out of
a bioerodable
material such as poly-l-lactide (PLLA) and covering the struts of the device
with a woven or
braided polyester sleeve or open-cell expanded polytetrafluorethylene (ePTFE).
As the struts
erode over time, they will stimulate a somewhat inflammatory response which
will encourage the
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
fabric to heal into the wall, so that by the time the PLLA structure is gone,
the device will be
well-healed into the wall.
[0052] The IVC is large enough that a low-profile electronics control
housing, such as
capsule 118 in FIG. 1, can be located on an implantable device such as device
100 without
meaningfully occluding blood flow through the IVC. Such an electronics capsule
also may be
configured and dimensioned to be entirely positioned against the walls of the
IVC, so that the
central channel of the IVC remains open and unimpeded for the introduction of
any other
catheter in the future. Electronics capsule 118 may have either a battery or
inductive coil or
both to power the device. Alternatively, or additionally, the device may be
designed to harvest
energy from local environmental sources by including, for example, a
piezoelectric generator to
produce power from the pulsation of the heart. In addition, the electronics
capsule will have
connections to the marker elements and a telemetry circuit to communicate
information to a
controller unit (not shown) outside the patient's body. Preferably the device
includes a wireless
transmitter to transmit sensor data to an external receiver and controller.
The device may be
configured to transmit continuously or at programmed intervals, or to transmit
data upon
interrogation by an external device. It may also have a memory circuit to
store historical sensor
measurements, and a calculation circuit to convert the various sensor
measurements to an
estimate of IVC distention or collapse. Additionally or alternatively, the
device may be
configured to communicate with a wireless-enabled cellular device such as a
smartphone, which
may include software to transmit data via cellular or wireless network to a
remote computer. In
this way, the measured IVC parameters may be automatically transmitted to
healthcare providers
to allow monitoring of the patient's condition. More details of related
control and networking
embodiments are discussed below.
[0053] Device 100, as illustrated in FIG. 1, is shown positioned largely
superior to the renal
veins within the IVC. However, implantable devices as disclosed herein also
may be positioned
partially or entirely inferior to the renal veins, or even within the right
atrium or the superior
vena cava (SVC). Alternatively the devices may have multiple components
implantable in
different locations, such as one component in the IVC, and a second component
in the SVC or
elsewhere.
21
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
[0054] In an alternative embodiment, device 300 may have a secondary
element 321 that
deploys portions of the device within the vascular system much closer to a
point of insertion, or
at another location more easily accessed (for physical access or energy
transfer), as shown in
FIG. 3. In this embodiment, device 300 includes IVC sensing unit 323 (which
may be generally
configured including anchor element(s) and marker element(s) as described with
respect to other
embodiments disclosed herein), with secondary element 321 located remotely
from the sensing
unit 323, and lead 329 connecting and providing communication between the two
units. For
example, an antenna element for telemetry and/or an inductive coil may be
placed in secondary
unit 321 in the subclavian vein or jugular vein. This would make it much
easier to accurately
position an external power source and/or controller antenna 332 close to the
antenna or inductive
coil contained within secondary unit 321. The secondary unit 321 may be held
in place, for
example, using a self-expanding stent or other intraluminal anchor element as
described herein.
[0055] Alternatively, an implantable device according to the present
disclosure may have an
implantable battery and circuitry that can be implanted within the body, but
outside of the
vascular system as shown in FIG. 4. Device 400 comprises IVC sensing unit 423
(which also
may be generally configured as described with respect to other embodiments
disclosed herein)
and implantable controller/battery unit 426 connected to sensing unit 423 by
lead 429 providing
communication there between. There are similarities between placement of
device 400 and the
common placement of pacemakers and defibrillators in an infra-clavicular
pocket. However,
unlike those common devices, lead 429 between the IVC sensing unit 423 and
placement
location of controller/battery unit 426 would not need to traverse any heart
valves, which may
make it a relatively safe and simple connection. Alternatively, IVC sensor 423
may be adapted
to connect to a pacemaker or defibrillator, including additional leads
providing sensing and
stimulation of the heart, for example, as described below in connection with
the embodiment of
FIG. 45.
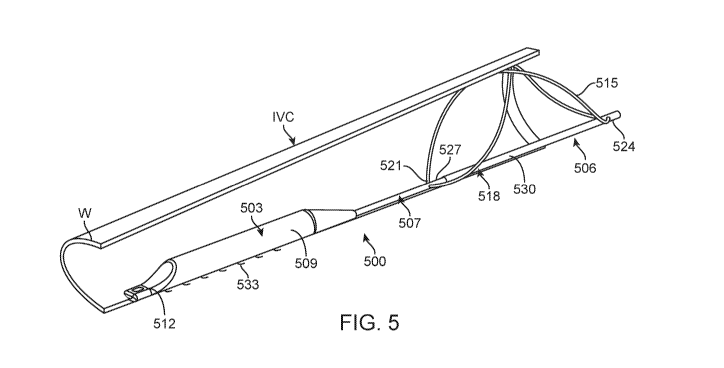
[0056] A further exemplary embodiment is shown in FIGS. 5-10. As shown
therein,
device 500 comprises three major components or assemblies, electronics capsule
503, anchor
element 506 and anchor isolation structure 507 connecting the electronics
capsule and anchor
element. Electronics capsule 503 comprises a sealed housing 509 for containing
control, power
and other alternative functional modules as elsewhere described herein to
provide a self-
22
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
contained, sealed device. Capsule 503 also provides support for marker element
512, which in
the case of device 500 is a single ultrasound marker element positioned at the
inferior end of the
device. Such a marker element may utilize one or more ultrasound crystals to
measure IVC
diameter by emitting an ultrasound pulse, and then detecting the reflection of
that pulse from the
opposing wall of the IVC. Other modes of detection with ultrasound receivers
and/or other
marker element types as described herein may be alternatively employed by
persons of ordinary
skill without departing from the teachings of this disclosure. Electronics
capsule 503 generally
will be provided with the lowest possible profile so as to minimize
obstruction of the lumen
when positioned in the IVC.
[0057] Electronics capsule 503 is connected to anchor element 506 at the
superior end of the
capsule. Anchor element 506 as depicted in this embodiment includes a single
anchor wire 515
configured in a generally figure-eight or double helix shape. Alternatively,
the same
configuration can be provided with two or more wires. Anchor wire 515 is
pinned to telescoping
deployment member 518 at both its inferior end 521 and superior end 524.
Telescoping
deployment member 518 includes inner member 527, which is secured to
electronics capsule
503, through anchor isolation structure 507 and outer member 530. Relative
motion between
inner member 527 and outer member 530 moves anchor wire 515 from a collapsed
position,
shown in FIG. 9, to a deployed or anchoring position, shown in FIG. 10.
[0058] Various actuation mechanisms may be utilized for deploying and
securing anchor
element 506. In one alternative, anchor wire 515 is resilient, with shape-
memory properties
configured to provide a rest state in the deployed configuration. In this
alternative, device 500
may be delivered to the desired location in the IVC via a conventional guide
catheter or other
suitable sheath type delivery device. When position is confirmed as described
below, device 500
is ejected from the delivery catheter or sheath with anchor element 506 self-
deploying upon
ejection.
[0059] In another alternative deployment mechanism, an actuating wire (not
shown) is
removably connected to deployment member 518 at superior end 524 using a
mechanical release
mechanism, for example a screw threaded connection, spring release, hooks or
other such means
known in the art. The actuating wire may be a single or double wire, which may
be coaxial or
23
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
parallel, depending on the mode of actuation. In this alternative, movement of
the actuating wire
effects relative movement of the inner and outer deployment members 527, 530
to deploy anchor
wire 515 from the collapsed configuration to the expanded, deployed
configuration as explained
above. After deployment of the anchor element, the actuating wire is released
from device 500
according to its mode of connection and released to leave the device secured
in the IVC via
anchor element 506.
[0060] As mentioned above, a further feature of this and other embodiments
disclosed
herein is the spacing between the marker element position relative to the
anchor element,
provided by anchor isolation structure 507. In general, it is preferred if the
anchor element is
positioned sufficiently distant from the marker elements so as to not have an
effect upon the IVC
size or shape at or close to the location of measurement due to the anchoring
force imparted to
the IVC wall. Anchor isolation structure 507 ensures the desired positioning,
which may be
approximately 1 to 4 times the IVC diameter as indicated above. In general,
the IVC has a
somewhat oval cross section with a minor axis of the oval extending in the
anterior-posterior
direction and a major axis extending in the lateral-medial direction. It is
thus desirable to
minimize any effect of the device on this natural oval shape at or close to
the point of
measurement.
[0061] The shape of the IVC and possible effect of the anchor element on
the IVC shape is
illustrated, in one possible configuration, in FIGS. 5-8. As shown therein, at
the more inferior
portion of the IVC, proximate marker element 512, the IVC assumes its more
natural oval shape
as best seen in FIG. 7. However, at the superior portion where subjected to
the force of anchor
wire 515 of anchor element 506, the IVC is forced into a more circular shape
as best seen in
FIG. 8. Thus, not only does the anchor element potentially distort the shape
of the IVC, it may
also stiffen the IVC so as not to be as responsive to varying fluid volumes
which may indicate
heart failure risk. Anchor isolation structure reduces or eliminates such
problems as might
otherwise be associated within sensing devices positioned in the IVC.
[0062] In order to achieve accurate measurement with marker element 512
using an anchor
configuration of the type shown in FIGS. 5-10, the entire device, from
deployment member 518
through anchor isolation structure 507 into electronics capsule 503 should be
provided with a
24
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
stiffness sufficient to maintain the electronics capsule (and marker element)
against the wall of
the IVC at one side and yet provide sufficient flexibility (and smoothness) to
avoid damage or
erosion of the IVC wall by contact with device 500 over the remaining lifetime
of the patient.
[0063] As also shown in FIGS. 5-8, it may be most advantageous if the
device, such as
device 500, or other device disclosed herein, is positioned with the
electronics capsule 503, and
more specifically the active marker element (e.g., ultrasound marker element
512), against the
posterior wall of the IVC so as to measure the distance to the anterior wall.
This arrangement
may offer advantages in accuracy and sensitivity in measurements by measuring
along the minor
anterior-posterior axis of the oval IVC shape, and by measuring from the
posterior wall, bony
structures lying behind the posterior wall, which may create artifacts or
other interference with
ultrasound measurements may be avoided. Such positioning may provide for the
greatest
accuracy in measurement of diameter over the respiratory cycle (e.g.,
measurement of diameter
variability vs. static measurement). While a single ultrasound marker element
512 is shown for
device 500, a similar device with more than one ultrasound crystal may be
positioned elsewhere
in the IVC, for example in the center of the IVC, with two crystals measuring
the distance to the
anterior and posterior walls simultaneously. Specific requirements for
positioning and
measurements may be clinically determined based on patient anatomy as
determined by the
procedure provider, and the device to be implanted may be modified according
to the teachings
contained herein to suit those specific patient requirements.
[0064] In general, devices as disclosed herein may be positioned at any
suitable position in
the IVC based on clinical assessment. In one example, the marker element of
the device, such
as an ultrasound crystal, may be disposed at the cranial end of the device,
with the cranial end
then positioned in the IVC between the renal veins and the hepatic veins. In
this case, the anchor
element may be disposed at the opposite, caudal end of the devices and thus
positioned in the
IVC inferior to the renal veins. Also, when positioning the device on the
posterior wall of the
IVC, it may be desirable to ensure that the device is centrally located on the
posterior wall and
oriented at least substantially straight across the minor axis for most
accurate measurements.
Positioning of the device in the IVC may be controlled using convention
catheterization
techniques with observation under fluoroscopy. However, in a device such as
device 500,
marker element 512 may be used to assist in confirming placement by slightly
rotating
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
electronics capsule 503 so as to effectively scan the opposite IVC walls with
the ultrasound
sensor to detect placement position relative to the oval IVC cross-sectional
shape.
[0065] In a further alternative embodiment in FIG. 5, additional anchor
elements may be
provided on electronics capsule 503, such as barbs 533. It is to be noted,
however, that while
barbs 533 are shown in FIG. 5, they are an optional feature. Basic operation
of anchor
element 506 is described above. As anchor element 506 opens, it shortens and
tends to pull back
on electronics capsule 503. Through a linkage between barbs 533 and deployment
member 518,
the relative movement of those two parts during deployment of anchor element
506 may be used
to deploy barbs 533 from the back of electronics capsule 503. Anchor element
506 and
barbs 533 may be positioned to engage the IVC wall in opposition to one
another to reinforce the
anchoring force and security. However, as previously indicated, substantially
the same device
may be alternatively provided without anchor barbs 533, held in place only by
the
collapsible/expandable double helix anchor wire 515 of anchor element 506.
These anchor
structures, as well as further alternative anchor structures described below,
are configured to
achieve secure fixation against both longitudinal and rotational movement
while preferentially
maintaining at least the marker element in the posterior aspect of the IVC,
most preferably
against the posterior IVC wall. The anchor elements described also can be
deployed and
redeployed multiple times during a placement procedure in order to ensure the
most optimum
placement of the device. The shape or configuration of the anchoring wire also
may be adapted
for IVC size and shape using different anchor element configurations as
exemplified by the
following additional alternatives.
[0066] The anchoring elements exemplified herein may take a wide variety of
alternative
shapes, as shown generally in FIGS 11-21. Such alternatives may or may not
utilize one or more
aspects of the "double helix" anchor wire design discussed above
[0067] Alternative anchor element 1100 is shown in FIGS. 11 and 12. Anchor
element 1100 includes two separate wire loops 1103 and 1106 secured to
deployment
member 1109, which is comprised of an inner member 1112 and concentric
telescoping
members 1115 and 1118, which are in turn covered by outer telescoping member
1121. Wire
loop 1103 is secured to inner member 1112 and covered directly by inner
concentric telescoping
26
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
member 115. Second wire loop 1106 is also secured to inner member 1112, either
by access
through an opening in the concentric telescoping members or by an attachment
wire that extends
along the inside of telescoping member 1118 and is secured at the remote end
to inner
member 1112. Alternatively, second wire loop 1106 may be secured directly to
the second
concentric telescoping member 1118. In the collapsed configuration each wire
loop is covered at
least in part by one of the telescoping members. To deploy the anchor element,
the telescoping
members are pulled back, either by self-deployment forces generated by the
wire loops or by
actuation with external means as previously described. FIG. 12 shows anchor
element 1100 in
its fully collapsed state with the anchor wires and concentric telescoping
members 1115, 1118
covered by outer telescoping member 1121. Also shown in FIGS. 11 and 12 is a
further
alternative electronics capsule 1124, which is joined to anchor element 1100
by anchor isolation
structure 1127.
[0068] FIG. 13 illustrates anchor element 1100 and electronics capsule 1124
as it may
appear when deployed within the IVC. Anchor wire loops 1103 and 1106 are
released to extend
outwardly to contact the IVC wall while leaving the central portion of the IVC
unobstructed to
allow access for other procedures and to minimize restriction of blood flow.
[0069] FIGS. 14-16 illustrate another alternative anchor element 1400. In
this embodiment,
mesh sleeve 1403, secured at one end to anchor wire 1405 is deployed over
inner member 1408
to which anchor wire 1405 is secured. Once again, relative movement between
inner
member 1408 and mesh sleeve 1403 controls deployment or collapse of the anchor
wire 1405.
Anchor element 1400 is depicted in FIG. 16 as deployed within the IVC with
wire anchor 1405
engaging the IVC wall.
[0070] FIG. 17 illustrates a collapsible, tubular, stent-like alternative
anchor element 1700.
Anchor element 1700 may be formed of braided wires, welded wires, spiral wound
wire, or
laser-cut tube, and is preferably a resilient self-expanding metal or polymer.
Electronics
capsule 1701 is depicted as attached to one end of the anchor element. Weld or
cold bond 1703
with biocompatible materials may be used to attach the electronics capsule to
the anchor
element. Anchor element 1700 may be deployed through a guide catheter in a
manner similar to
conventional stent deployment. Advantageously, such a tubular anchor element
provides secure
27
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
anchoring in the vessel while leaving the vessel lumen patient to allow
introduction of catheters
and other devices without disruption of the monitoring device.
[0071] FIGS. 18-21 illustrate yet another alternative anchor element 1800
coupled with
electronics capsule 1801. In this embodiment, anchor wire loops 1803 and 1806
in this
embodiment are secured at opposite ends to inner member 1809 and outer member
1812. In this
manner, relative movement between inner member 1809 and outer member 1812
permits
deployment of the anchor wires without a covering sheath. The anchor wires may
be again
collapsed by an opposite relative movement between the inner member and outer
member.
FIGS. 20 and 21 show how anchor element 1800 may be deployed in different
sized IVCs. In
this embodiment, anchor wire loops 1803 and 1806 are relatively longer such
that they may cross
multiple times when less than fully expanded to accommodate smaller size IVCs,
as is apparent
from a comparison of FIGS. 20 and 21.
[0072] As should be apparent to those of ordinary skill in the art, each of
the anchor element
configurations described above includes common features of secure anchoring
with a virtually
unobstructed IVC, even when the anchor elements are fully deployed. By
minimizing or
eliminating obstruction of the IVC, combined with positioning of the anchor
elements remote
from sensing elements and location, embodiments of the present disclosure may
remain
positioned in the IVC over longer periods of time without affecting the
natural tendency of the
IVC to collapse or expand when venous pressure or volume is changed.
[0073] While it is anticipated that in most cases it will be desirable to
maintain an
unobstructed pathway through the IVC as provided by exemplary anchor elements
described
above, in some cases it may be desirable to integrate a monitoring device as
described herein
with an IVC filter, as shown for example in FIG. 22. In addition to monitoring
IVC distention,
device 2200 would trap any clots embolizing from the legs and prevent them
from reaching the
lungs as is understood with respect to IVC filters as stand-alone devices.
Device 2200 includes
electronic capsule 2203 with battery, connections to the sensor, memory,
telemetry, etc., stent-
like anchor element 2206 with anchor members 2209, and flexible arms 2212
supporting marker
element 2215. In this embodiment, marker element 2215 are depicted as
electrodes, which may
be substantially the same as the electrodes described above in connection with
the embodiment
28
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
of FIG. 1. In addition, arms 2218 at the superior end of device 2200 extend
across the lumen of
the IVC and intersect to form a basket to retain any clots which embolize from
the legs.
[0074] FIGS. 23 and 24 show devices 2300 and 2400, respectively, configured
to measure
the longitudinal impedance along the length of the IVC or the superior vena
cava (SVC), or both.
Device 2300 includes anchors 2303 to engage the IVC and secure electronics
capsule 2306. One
electrode 2309 is provided relatively closer to electronics capsule 2306, and
an insulated straight
or spiral wire 2312, which lays against the IVC wall, leads to second
electrode 2315 located
more superiorly in the IVC, right atrium, or SVC. Rather than applying a
simple direct current
voltage between these two electrodes to measure the impedance, it may be more
effective to
apply a particular alternating-current frequency that exhibits a lower
impedance through blood
and a higher impedance across the IVC wall and through other tissues. This
would allow such a
device to measure the variation in IVC volume even more effectively.
Alternatively, a device
may measure a combination of the change in both longitudinal and radial
impedance, to gather
an even more effective measurement of the change in IVC volume.
[0075] FIG. 24 further shows device 2400, shaped similarly to a standard
IVC filter, which
uses the variation in bending of struts 2403 to apply pressure to pressure
sensors 2406 on central
body 2409 of the device. Struts 2403 extend radially outward from body 2409
and have distal
tips 2412 configured to engage and anchor to the wall of the IVC. Struts 2403
have flexibility
and resilience so as to move with the wall as the vessel contracts and
expands, thereby changing
the forces exerted by the struts on sensors 2406. Electronics capsule 2415 is
contained within
body 2409 providing power, control and communication for sensors 2406.
[0076] FIG. 25 shows another embodiment, device 2500, configured similarly
to an IVC
filter. However, in this embodiment, device 2500 is provided with lateral
struts 2503, which are
intended to anchor the device in the IVC, and anterior-posterior struts 2506,
which are intended
to flex with the movement of the anterior and posterior walls of the IVC.
Therefore, the distance
between marker elements 2509, such as sensors or electrodes, on the anterior
and posterior struts
can be measured. As with other embodiments, device 2500 includes electronics
capsule 2512,
which provides structural support for the struts and contains necessary power
and control
functions as elsewhere described.
29
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
[0077] FIG. 26 shows a further alternative embodiment in which device 2600
is comprised
as a stent 2603 on which marker element 2606 are disposed at two different
cross-sections
(lateral-medial and anterior-posterior) of the IVC. Stent 2603 has a
resilient, self-expanding
configuration which will expand and contract with the IVC. Stent 2603 may be a
mesh or woven
structure, a simple wire-form having a zig-zag or sinusoid shape, or a series
of closed or open
cells cut from a tube. Power and control may be provided by integrated power
and data
transmission components in an electronics capsule as previously described, or
marker element
sensors directly powered via external energy delivery means, and transmitting
information
directly to an external module may be provided.
[0078] FIGS. 27A and 27B show another embodiment in which device 2700 is
provided
with two pairs of arms 2703 held in place by a stent structure 2706. Marker
element 2709 (such
as electrodes, ultra sound crystals or other sensors as previously described)
are positioned at the
apex of each pair of arms. These may be oriented in the patient such that one
side of each pair
extends from the anterior wall of the IVC, and one from the posterior wall. As
the IVC collapses
as shown in FIG. 27B, the arms tend to scissor together and the apices holding
marker
elements 2709 move closer together. This change in position may be detected.
Alternatively,
strain gage type sensors or other angle detection may be used to detect the
change in angle from
compression alone or in combination with the change in distance sensors.
Embodiments such as
device 2700 can be configured such that the sensors move relative to each
other a distance
greater than the actual movement of the IVC wall, thereby magnifying the
change in the distance
from the anterior to posterior walls.
[0079] Device embodiments as described herein may be delivered into the IVC
from a
variety of locations. The subclavian or cephalic vein is the normal route of
introduction of
pacemaker and defibrillator leads, so that these leads can be attached to the
pacemaker itself,
which is placed in an infra-clavicular pocket just below the subclavian vein.
Embodiments
disclosed herein may be similarly delivered from the subclavian vein, cephalic
vein or the
jugular vein, or the femoral vein. Other access points to the venous
circulation may also be used.
[0080] One exemplary delivery method for certain embodiments disclosed
herein is to have
the device to be delivered compressed into a catheter, with a cover sleeve
over the device. A
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
guidewire lumen within the catheter would allow a guidewire to be positioned
under fluoroscopy
or ultrasound guidance into the IVC, and then the delivery catheter would be
advanced over that
guidewire into the appropriate location. Once appropriate location is
confirmed, the cover sleeve
would be retracted, allowing the device to self-expand against the walls of
the IVC. Under
appropriate clinical indications, disclosed devices may be delivered at the
bedside under
ultrasound imaging guidance, without the need for fluoroscopy.
[0081] If the device has an electronic lead, the lead may take advantage of
all of the designs,
materials, and techniques that have been used to optimize pacemaker leads.
This lead may extend
to a secondary fixation element within the circulatory system, as shown in
FIG. 3, or it may
extend out of the circulatory system to an implantable element as shown in
FIG. 4.
[0082] As a source of power, embodiments described herein may include an
inductance coil
to power the sensors on the device using a power source from outside the body.
Externally
powered devices may also include a small battery or capacitor to maintain
steady power to the
sensors. An external power source could be in the form of a pendant which
hangs from a
necklace around the patient's neck, or a module which is kept in a shirt
pocket, strapped around
the patient's chest or abdomen, clipped to the patient's belt, or other
locations proximate to the
implanted device. It could also be kept at the patient's bedside or under
their mattress or pillow,
so it can deliver power, take measurements, download data, etc. each evening
while the patient is
sleeping.
[0083] Given the available cross-sectional area of the IVC and the low
power requirements
of current implantable device circuitry, embodiments of devices described
herein, including a
long-term battery and circuitry, may be safely implanted in the IVC without
disrupting blood
flow. The diameter of the delivery catheter for such a device may be as large
as 24-30 French
size (8-10mm) if delivered via the femoral vein. The overall implanted device
or structural
elements also may be used as an antenna to enhance transmission of this data
outside of the
body, especially, for example, if the device has a stent-like body or multiple
metal arms.
[0084] In yet another alternative embodiment, a sensing device may be
implanted on the
outside of the IVC as shown, for example, in FIGS. 28A-C. In this embodiment,
device 2800 is
configured to be implanted around the outside of the IVC and thus includes two
resilient arms
31
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
2803 extending from electronics capsule 2806. Marker elements 2809 are
disposed at the ends
or elsewhere along resilient arms 2803. Arms 2803 may be, for example, a
coated, resilient
flexible material or a tubular insulator with a wire inside. Device 2800 may
be placed via an
otherwise conventional laparoscopic procedure. A right posterolateral access
to the abdomen,
just inferior and/or posterior to the liver, should allow the surgeon to
advance to the IVC. In this
manner a resilient loop device such as device 2800 could be wrapped around the
IVC, as shown
in FIG. 28B. FIG. 28C shows one embodiment of delivery device 2815 containing
straightened
device 2800. Delivery device 2815 may comprise a tubular member such as a
catheter or trocar
with a pushing element for delivery and position of device 2800 around the
IVC.
[0085] In a further alternative embodiment, a marker element as elsewhere
described herein
may be implanted against one side of the external wall of the IVC, held in
place by sutures, clips,
adhesives, or other mechanical attachment means. Such an external sensor type
element could
measure IVC cross-sectional area via mechanical, sonic, impedance, or other
means.
Marker Element Embodiments with External Activation
[0086] Embodiments described above focus on implantable systems with
electronics to
measure IVC dimensions as well as other physiologically important data, and
then transmit that
information to a receiver located outside the patient's body. Embodiments
described hereinafter
include devices, systems, and methods for measuring the IVC employing external
instruments to
measure IVC dimensions in communication with implanted marker devices,
potentially without a
need for more complex implants, or implanted active measurement devices and/or
the need to
transmit measurement data out of the body. Such devices, systems and methods
may include
passive elements, which are used in conjunction with external instruments for
calculating and
communicating IVC dimensions. More specifically, disclosed systems may have
one, two or
more markers that would allow an instrument outside the body to easily measure
IVC
dimensions without the need for sophisticated training or human analysis. Such
systems may use
portable, and relatively inexpensive instruments to take the measurements.
[0087] In one disclosed embodiment, shown in FIG. 29, device 2900,
including two or more
passive elements 2903, is configured to be implanted in the IVC. Passive
elements 2903 are
themselves configured to reflect a signal directed towards them from outside
the body. Such
32
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
passive element reflectors may be made of a metal such as Nitinol, or they may
be made from
any other echoreflective material (or other suitable biocompatible material
reflective of the signal
employed). Passive elements 2903 are connected by anchor isolation structures
2906 to anchor
structure 2909, exemplified here as a stent-like structure, which may be made
of Nitinol or other
resilient material biased into engagement with the IVC wall. Other anchor
elements as described
herein may be alternatively employed.
[0088] It will be appreciated by persons skilled in the art that the
depiction in FIG. 29, as in
other figures presented in this disclosure, is not at a particular scale.
Connecting elements 2906
may be suitably elongated to allow the anchoring structure to be placed in a
position spaced
upstream or downstream from the location of the markers so that the anchoring
structure does
not affect the natural geometry and movement of the IVC where it is measured
by the passive
elements as previously described. Passive elements 2903 may be gently biased
outwardly
against the anterior and posterior walls of the IVC to maintain contact
therewith.
[0089] In other embodiments employing passive marker elements, the passive
elements may
be mounted directly to an anchoring structure such as a stent, and not
separated therefrom by
connecting elements. Alternatively the marker elements may be stapled,
screwed, sutured, or
otherwise fastened to the IVC wall. Passive elements such as shown in FIG. 29
may be
fenestrated, including grooves, channels, holes, depressions or the like to
accelerate the ingrowth
of IVC wall tissue over them. The passive elements may also have a surface
texture or coating
to enhance reflection of the signals. For example, the surface may have a
series of grooves or
depressions whose walls were at right angles or other selected angles relative
to each other to
more effectively reflect those signals. Alternatively, such grooves, channels
or other features
may be arranged on each passive element in unique patterns which make them
more clearly
identifiable and differentiable from each other and from surrounding
structures by an external
detection instrument. In other embodiments, the passive elements may be of
known size, but
oriented at different known angles relative to each other, such as in
orthogonal directions (e.g. at
least one in circumferential direction and one in the axial direction), so
calculating the length and
orientation of the reflected signal can determine the location of each passive
element in three
dimensions.
33
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
[0090] It is anticipated that within a few months of their implantation,
the passive elements
as of the types described herein would be fully healed into the IVC wall. The
posterior passive
element may be somewhat larger than or offset from the anterior passive
element, so that one
does not shield the other regardless of where the reading/detecting instrument
is held against the
anterior abdomen.
[0091] A reading instrument for use with passive elements as described
above may
comprise a generally conventional ultrasound signal generator and receiver
which would be held
against the anterior abdomen or thorax outside the body to detect the device
in the IVC, as
schematically depicted in FIGS. 30 and 31. In this exemplary embodiment,
ultrasound system
3000 includes handheld probe 3003 connected to table top control console 3006
and display
3009. Advantageously, ultrasound system 3000 would not need to image the IVC,
although
optionally may do so. Thus, ultrasound probe 3003 could be provided as a
single crystal,
intermittently delivering a pulse and measuring the time-of-travel until the
reflected echo is
detected by the same crystal. Ultrasound probe 3003 transmits a sonic signal,
as shown, for
example, in FIG. 31, through the body wall BW and the receiver records the
reflection of that
signal from the two passive elements 2903 elements on device 2900 implanted
within the IVC.
System 3000 may differentiate the two passive elements in any of various ways,
including
through differences in their relative distance away, size, shape, patterns of
fenestrations, echo-
reflective coatings or other features. By a time-of-travel calculation, for
example, the relative
spacing of the two passive elements could be calculated. By measuring this
distance many times
per second, an accurate assessment of the variation in IVC dimensions could be
made.
[0092] System 3000 may be programmed to look for the appropriate number of
signals from
the passive elements within the appropriate time period after it transmits a
signal. This would
minimize the risk of it tracking inappropriate signal reflections from other
sources or anatomical
structures such as the spine. In use, probe 3003 is held against the anterior
abdomen and gently
reoriented until it receives an effective echo from all of the passive
elements. At this point
system 3000 emits an audible signal, shows a green light, or uses other
indicator means to
confirm to the patient that the instrument is appropriately positioned. System
3000 may also
include a strap around the patient's body that could be tightened to hold the
device in place, or
34
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
the patient could hold it in place manually, or use tape, adhesive, or other
means to hold it in
place while readings are taken.
[0093] In further alternative embodiments, instead of or accompanying
passive elements,
more active elements may be included with an implantable device such as device
2900. Such
more active elements may comprise piezoelectric or other crystals which absorb
incoming sonic
signals and re-transmit these signals back to the receiver. Such embodiments
may further
comprise active elements powered by an externally delivered magnetic,
electrical, or ultrasonic
field. In such active embodiments, active marker elements, which may be also
be schematically
depicted by elements 2903 in FIG. 29, could then emit a signal that allows the
external
instrument to determine position more exactly. In further examples, each
active marker element
may include an inductance coil or other means to gather energy from a variable
external electric
or magnetic field; a capacitor or other means to store that energy; and then a
piezoelectric crystal
to emit an ultrasound signal, along with the appropriate circuitry to manage
these elements.
Such externally powered active marker elements need not be overly complex,
firing an
ultrasound signal whenever they are sufficiently charged or excited.
Alternatively, the external
system may send a triggering signal to tell each marker element when to fire,
or to make them all
fire simultaneously.
[0094] In embodiments employing active, ultrasound-emitting marker
elements, one or
more external ultrasound receivers may be arrayed on the body surface to
detect the emitted
signals. Again using time-of-travel calculations as will be appreciated by
persons skilled in the
art, the precise location of the active marker elements within the body could
be determined. If the
active marker elements fired simultaneously, then one ultrasound receiver
located on the anterior
abdominal wall could be enough to accurately measure the anterior-posterior
dimension of the
IVC. If they did not fire simultaneously, then more than one receiver may be
necessary. The
external sensors may be arrayed in a way that maximizes the precision of the
anterior-posterior
measurement. FIG. 32 shows a transverse cross-section of the body, and one
arrangement of two
external sensors 3203. The distance from implanted active marker elements
2903, within the
IVC, to sensor 3203A on the patient's anterior abdomen will vary directly as
the anterior-
posterior (A-P) IVC dimensions change, while the distance from implanted
active marker
elements 2903 to sensor 3203B on the patient's side will change little. By
analyzing the
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
difference in the time it takes for ultrasound signal from each active marker
element to reach
sensor A versus sensor B, the A-P dimensions of the IVC can be calculated. As
long as at least
two sensors are used (for example, one on the anterior wall of the IVC and one
on the posterior
wall), their relative motion could be measured quite accurately, cancelling
out other motions
such as the rise and fall of the abdominal wall during respiration. Note that
elements 2903 may
also be implanted on an external surface of the IVC as depicted in the
embodiment of FIGS.
28A-C, or may be passive elements if transducers are used instead of sensors
3203.
[0095] Other methods of determining position from passive or active marker
elements also
may be employed. For example, in one embodiment, the marker elements may
comprise one or
more small magnets implanted against the IVC wall using device embodiments
described herein
above, and a sensitive magnetometer could be used to detect the position and
motion of the
magnet(s). A SQUID magnetometer (Superconducting Quantum Interference Device)
could be
used to very sensitively measure the variation in location of an implanted
magnet, although this
device may require a cooling apparatus to bring even a high-temperature SQUID
to a
temperature where it becomes superconducting. Other types of magnetometers
could also be
used.
[0096] A further alternative embodiment may comprise the implantation of
marker
elements, such as containing ultrasound crystals, that show up very brightly
on an ultrasound
image. Automated image analysis software within the ultrasound system can then
automatically
detect marker element positions and record them. One embodiment of this
approach would be to
implant a stent with Nitinol arms that can be easily identified by an
ultrasound imaging system
using automated software. An implanted device similar to that shown in FIG. 29
could include a
stent implanted caudal to the renal veins, and two or more metal arms that
extend cranial to the
renal veins. For example, one arm could be positioned along the anterior wall
of the IVC and
another along the posterior wall. A transverse cross-sectional ultrasound
image of the IVC
cranial to the renal veins will cross these arms, and they will show up as
clear marks on the
ultrasound image. Image-analysis software could then identify those marks,
track them, and
measure the variation in IVC dimensions automatically. To make these
measurements
consistent, the patient or caregiver could be trained to hold an ultrasound
imaging transducer on
the patient's abdomen in a specific location. Tattoos or other markings on the
skin could be used
36
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
to identify a consistent location from which measurements are to be taken. In
one embodiment,
as shown in FIG. 33, a wearable detection system 3300 includes ultrasound
probe 3303 that may
be fastened in place on the patient via strap 3306 and buckle 3309. Ultrasound
probe 3303 may
also include windows 3312 mounted on body contacting tabs 3315,or other
indicators, that
would be placed over or adjacent the location mark on the body to ensure
proper location of
probe 3303. Ultrasound probe 3303 may communicate wirelessly with an external
device 3318,
such as a cell phone, which controls the probe, displays the measured data,
and transmits it to
other systems or cell phones. Alternatively the ultrasound transducer could be
shaped so that the
transducer is reliably positioned a certain distance from various anatomical
points, such as a
distance from the bottom of the rib cage.
[0097] A further embodiment is a system that monitors IVC dimensions
without implanted
elements. For example, a portable, external ultrasound system could comprise a
processor and
software that analyzes a reasonably consistent ultrasound image of the abdomen
to automatically
identify the IVC. This software then automatically identifies the anterior and
posterior walls of
the IVC within that image, and continuously or periodically measures and
records the variation
in IVC dimensions over time. In such an embodiment, the system would include
an emitter and
receiver that can be secured to the patient, or that can be positioned at one
or more marked
locations on the patient's skin, so that measurements are taken from a
consistent location.
Preferably such a system is contained in a lightweight, compact housing,
battery-powered, and
small enough to be worn by the patient or easily held by the patient during
measurements.
System 3300 as shown in FIG. 33 may also be used in this embodiment.
[0098] As a further method of simplifying the identification of the IVC and
appropriate
positioning of the probe, a three-dimensional ultrasound map of most of the
patient's abdomen
can be stored in the ultrasound system's memory when the patient first begins
using the system.
From that point, optimal positioning of the probe and its two-dimensional
slice within that three-
dimensional volume can be defined. Then, when the patient is imaged in a
subsequent
measurement, the ultrasound system can compare the image to the three-
dimensional map,
determine where the image is relative to the desired slice, and indicate to
the person doing the
image to move the probe cranially, caudally, medially, or laterally to reach
the optimal position.
37
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
[0099] In all of the above-mentioned marker element-based embodiments,
external
components of such systems are preferably configured to report the IVC
dimensions to the
patient, as well as wirelessly transmit that information to the patient's
doctor or other people
monitoring the patient's health. Since they are external to the body, size is
less critical, and such
components could have more significant batteries to allow communication with
the physician or
other monitors via a Bluetooth , WiFi , cellphone, or other communications
modality as
described in more detail later in this disclosure. The ultrasound
receiving/transmitting element
of any of these less-invasive IVC monitoring systems could be configured to be
worn
continuously by the patient, or it could be used for a period of minutes once
or more per day. All
of the external components maybe contained in a single housing, or could be
broken up into two
or more separate units. For example, in system 3000, shown in FIG. 30,
ultrasound
receiver/transmitter probe 3003 may be connected either wirelessly or by cable
to console 3006
adapted to provide user control functions, perform calculations, store and
display information,
and communicate with cell phones or the Internet via wireless networks.
Console 3006 may
include a CPU, memory device, and other components for input, communication
and storage as
described in further detail below in connection with FIG. 46. In this way, a
transmitter/receiver
probe may be compact, lightweight and wearable (as in FIG. 33), while the
control console
could be a larger tabletop unit.
Injectable and other Passive Marker Embodiments
[00100] Embodiments discussed above primarily encompass active marker
element-based
embodiments and more passive marker elements that may be fastened to the IVC
wall by various
embodiments of anchor elements or other similar suitable means. In further
alternative
embodiments, as described herein below, marker elements are placed or injected
into or within
the IVC wall. In some clinical situations, instead of a marker element that is
fastened to the
inner or outer wall of the IVC, such placement may be easier or preferable.
[00101] In one such injectable-type embodiment, as shown in FIGS. 34A and
34B, a
relatively small guiding catheter 3403 may be introduced into the IVC, and
through that catheter
a needle or blade 3406 may be introduced. Under ultrasound or fluoroscopic
guidance,
needle 3406 may be directed towards the appropriate wall of the IVC, and
inserted into the wall.
38
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
Needle/blade/catheter 3406 may have a shoulder or hilt a selected distance
from its distal tip to
engage the wall surface so as to limit the depth of penetration of the needle
or blade. The needle
or blade 3406 may be configured to create a pocket or flap in the IVC wall
into which
biocompatible or resorbable substance 3409 containing marker elements 3412 may
be placed or
injected. System 3400 may be further configured to deliver marker elements
3412 into the
middle of the IVC wall, such as between the medial and adventitial layers of
the IVC, or between
the intimal and medial layers. System 3400 may also be configured to deliver
marker
elements 3412 through the thickness of the IVC wall to the exterior so that
marker elements 3412
would adhere against the outer surface of the IVC as shown, for example, in
FIG. 34B.
[00102] Injectable marker elements may be, for example, a flexible wire,
ribbon, or
guidewire segment, which could be advanced easily through a catheter or needle
into or against
the IVC wall. Such a marker element could be attached to a delivery wire or
catheter, and
removed or repositioned as needed. The marker element would only be detached
from the
delivery system once appropriate positioning had been confirmed. System 3500,
shown in
FIG. 35, uses coiled wire marker element 3503, which is deployed using
delivery catheter 3506.
Delivery catheter 3506 holds marker element 3503 with two jaws 3509 until the
marker element
reaches the distal end of the delivery catheter 3506, at which point the jaws
separate to release
the marker element. Marker element 3503 also may be released via a threaded
connection, an
interlocking mechanism, an electrolytic or other soluble connection, or other
such release
mechanisms as are known in the art.
[00103] Examples of embodiments of guidewire segments for use in such
injectable marker
element embodiments are shown in FIGS. 36A-E. Such segments may have a surface
texture
optimized to reflect the ultrasound or other signal and may be metallic, such
as platinum,
titanium, gold, or other material, or it may be a polymer. A polymer
embodiment may be molded
with appropriate echo-reflective surfaces and radiopaque markings.
Alternatively, such a
guidewire marker element may comprise a section of hollow wire, sealed on each
end and filled
with air or echo-reflective fluid. A wire or ribbon may include barbs, scales,
or other features to
inhibit it from backing out of the IVC wall or migrating completely through
the IVC wall.
FIG. 36A shows a simple guidewire coil 3603. FIG. 36B shows a guidewire coil
3606 coated
with polymer 3607 to permanently entrap air creating a highly echo-reflective
marker. FIG. 36C
39
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
shows a close-up of a coiled ribbon marker element 3609 with surface texture
3612 to enhance
its echo-reflectivity. FIG. 36D shows marker element 3615 formed as a sealed
tube of air.
FIG. 36E shows marker element 3618, formed as a tube of cast polymer such as
silicone which
has been emulsified prior to curing, entrapping many tiny echo-reflective gas
bubbles 3621. Gas
bubbles 3621 can be of a particular gas to minimize any absorption through the
walls of the
polymer over time. Tubular or coil devices delivered through a catheter may be
provided with a
selectively releasable retention mechanism such that placement may first be
confirmed before the
device is released from the catheter. One such mechanism is illustrated in
FIG. 36E, which
includes threaded connector 3624 at one end, configured to cooperate with a
threaded release
mechanism in the delivery catheter. Once appropriate positioning has been
confirmed, the
delivery catheter may be unscrewed, leaving the polymer tube permanently in
place.
[00104] Alternatively, injectable marker elements may comprise a number of
small echo-
reflective beads or particles, as shown in FIGS. 37A-C, which may be injected
into the wall of
the IVC or into the peri-adventitial space against the outside of the IVC.
Such injectable marker
particles may comprise spheres of gas similar to commonly used air bubbles for
temporary
ultrasound imaging, except they would be encased in surrounding shells of a
permanent or semi-
permanent material such as silicone or other polymers. These bubbles form
spherical reflectors
for ultrasound signals. The injectable marker particles may alternatively be
shaped with
pyramidal indentations with 90 degree angles which will reflect signals very
effectively, similar
to radar reflectors used on sailboats. Injectable marker particles may be
metallic, such as
titanium, or they could be a polymer such as PEEK (polyetheretherketone).
[00105] FIG. 37A shows injectable marker particles 3703 with random jagged
echo-reflective
shapes. FIG. 37B shows hollow spherical injectable particles 3706. FIG. 37C
shows alternative
injectable marker particles 3709 with molded or shaped configurations having
echo-reflective
indentations 3712. Such injectable marker particles may be of any size from a
few microns, to
hundreds of microns, or the maximum size which will pass through the delivery
catheter. The
size might be particularly selected to maximize the reflection of signals of
specific frequencies.
Nanoparticle-based technologies also may be employed to provide such
particles.
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
[00106] During delivery, injectable marker particles as described above may
be suspended in
a fluid such as saline, or a gel such as certain formulations of polyethylene
glycol (PEG). PEG is
already used in vessel walls for vascular closure applications, such as the
MYNXtm device from
Access Closure. Depending upon their formulation, materials such as PEG can be
resorbed over
the course of weeks or months, leaving the marker particles permanently in
place. Alternatively,
a permanent polymer could be injected which can be injected as a liquid, but
then hardens in
place. This polymer may have marker particles suspended within it, or the
polymer itself could
be the marker. One example of a biocompatible polymer which could be used for
this is
urethane methacrylate. FIG. 38 shows a close-up of a urethane methacrylate gel
3803 mixed
with marker particles 3806 injected into the wall W of the IVC.
[00107] Another alternative embodiment may comprise securing or sticking
marker
particles 3903 to the inner walls of the IVC with a material 3906 or texture
that encourages the
marker elements to grow into the IVC wall as shown, for example in FIGS. 39A
and 39B. Fibrin
is one example of a biocompatible material that is known to adhere to blood
vessel walls, and to
endothelialize in place. Other biocompatible, bioabsorbable adhesives could be
used. Marker
particles 3903 may be mixed into a fibrin patch and placed as desired in or
against the IVC wall.
After the patch is endothelialized and the fibrin absorbed, the marker
particles remain in the
vessel wall. FIG. 39A shows a cross-sectional view of patch 3906, containing
marker
particles 3903, which has endothelialized into the vessel wall, but patch 3906
itself has not yet
been resorbed. Alternatively, a marker patch could be designed with a "Velcro-
like" texture of
microneedles or microhooks 3909 as shown in FIG. 39B, which embed into the IVC
wall and
may remain in place permanently.
[00108] Marker elements 4003 designed to be applied to an inner surface of
the IVC wall
may be delivered to the IVC wall by mounting them on the outside of an
inflatable balloon 4006,
introducing that balloon into the IVC, and inflating it to press the marker
elements against the
IVC wall as shown in FIG. 40A. Marker elements 4003 may be relatively long and
slender, to
minimize the overall diameter of the combined marker elements and delivery
system. Various
marker elements described herein for adherence to or embedding in the IVC wall
may be applied
using this technique, such as, e.g., as shown in FIG. 39A or 39B. Before or
during delivery, it
will be important to confirm that marker elements 4003 are aligned with the
anterior and
41
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
posterior walls of the IVC, which may be accomplished, for example, by using
radiopaque
markers and fluoroscopy. Delivery balloon 4006 may have wings 4009 to cover
marker
elements 4003 during delivery, but which unfold or retract to expose the
marker elements as the
balloon is inflated. Alternatively, a cover sheath may be provided over the
markers to hold them
in place on the balloon during introduction. This cover sheath would then be
withdrawn shortly
before expansion of the balloon to deploy the markers. In a further
alternative, two-balloon
catheter 4012 may be used as shown in FIG. 40B, to permit blood flow through
space 4015 so
that blood flow would not be interrupted during the delivery process.
[00109] Sensing and position/measurement detection using injectable-type
marker elements
as described in the embodiments above may be accomplished by a variety of
means or systems.
For example, injectable marker elements may be designed to reflect ultrasound
energy. Since the
ultrasound signal is being used to measure distance rather than imaging, the
signal may be
provided at a relatively low power. Since the IVC is located deep in the
abdomen, and higher-
frequency signals attenuate rapidly in human tissue, it may be preferable to
use a relatively low
frequency, perhaps in the range of 200KHz-2MHz, although the frequency might
be higher or
lower in practice. The anterior-posterior dimension of the IVC could be
measured simply by
measuring the additional time it takes the signal to reflect off the posterior
marker element and
return to the system monitor, compared to the time it takes to reflect off the
anterior marker
element. Since the speed of sound in human soft tissue is approximately 1540
meters/second, if
the A-P dimension of the IVC is approximately 20mm in an average human
patient, the posterior
reflection will return to the monitor approximately 26 microseconds after the
anterior reflection.
Each additional millimeter of dimension will add approximately 1.3
microseconds to the
differential.
[00110] It should be noted that one important measurement may be the
percentage variation
in that anterior-posterior dimension. Even if the absolute dimensional
measurement is not
accurate, the percentage variation should still be accurate. For example, if
the monitor is 15
degrees to one side of the anterior-posterior alignment of the marker
elements, the maximum
measured absolute A-P dimension may be reduced by [one minus the cosine of 15
degrees], or
3.4%. But the minimum measured A-P dimension should be similarly reduced, so
the overall
percentage change should be minimal. Similarly, any movement of the abdominal
wall, for
42
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
instance with respiration, should not affect the differential in the time it
takes for the two
reflected signals to return to the system monitor.
[00111] Given the relatively low power needed for such a simple distance
measurement, the
monitor device may be of simple design and obtain a good signal even with a
very low-power
signal, which should maximize both the safety and battery life of the device.
However, if more
accurate distance measurements are desired, an external transmitter/ receiver
can be configured
to provide consistent, precise measurement of the relative distance of the two
marker elements.
In one exemplary embodiment, shown in FIG. 41, external handset 4103 comprises
two
emitter/receiver pairs 4106 mounted a fixed distance apart on handle 4109.
Each
emitter/receiver pair 4106 is mounted on a contact pad 4712 configured to
engage the patient's
skin. Each emitter transmits a signal toward the implanted IVC markers, which
reflect the
signals back toward the handset for reception by the receivers. In this way
the distance to each
IVC marker may be calculated by triangulation to obtain a very precise
measurement.
Optionally, the two emitters can transmit signals at different frequencies to
eliminate
interference.
Multi-Sensor Monitoring Systems
[00112] While pulmonary artery (PA) pressure measurement mentioned in the
Background
above holds some promise as an approach to heart failure monitoring, it is
believed that IVC
volume measurement may present a more accurate and early indication of heart
failure.
However, the combination of IVC volume measurement with PA pressure monitoring
may
provide an even more comprehensive picture of disease progression.
[00113] The systems of the present disclosure may therefore include both an
IVC volume
monitor along with a PA pressure monitor, and/or other sensors for measuring
symptoms related
to heart failure. The multiple monitors/sensors may be coupled together by
either a wired or
wireless connections to allow data transmission between them, or they could
operate completely
independently. In preferred embodiments, the IVC monitor and the PA monitor
will both
communicate with a single data receiver outside the patient's body.
Alternatively one of the
monitors could transmit data to the other, from which it could be transmitted
to an external
receiver. Additionally, a power supply integrated into one of the two monitors
could deliver
43
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
power to the other of the monitors, or a separately implanted power supply
could be connected to
each monitor. The system could also include a controller/data analyzer that
analyzed the data
received from each monitor and used the combined data to determine the extent
of or change in
the patient's disease, and whether to set off an alarm or transmit a
notification to the patient or
health professional.
[00114] One exemplary embodiment of such a system is system 4200, shown in
FIG. 42.
System 4200 may include a first delivery catheter (not shown) for implanting
IVC volume
monitoring device 4203 in the IVC, and second delivery catheter 4206 for
implanting pressure
sensor 4209 in the pulmonary artery. Each of these catheters could be
introduced through a
single introducer 4212 positioned in a peripheral vein such as a femoral or
iliac vein.
Alternatively, system 4200 may utilize a single delivery catheter carrying
both IVC
monitor 4203 and PA pressure monitor 4209. A single catheter arrangement
allows monitoring
of implants to be delivered serially (either PA first or IVC first) from a
single catheter in a single
intervention. Other sensors that could be included in a multi-sensor system
such as system 4200,
to provide additional data related to heart failure include a respiratory rate
monitor, cardiac
rhythm monitor, arterial or venous blood pressure monitor, blood oxygen
saturation sensor, or
cardiac output monitor.
44
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
Closed-Loop Therapy System Embodiments
[00115] This disclosure has heretofore described various embodiments,
devices and methods
for using the size, relative size, and variation in size of the IVC to detect
the early onset of acute
decompensation in heart failure. With the information provided by these
devices and methods,
various actions may be taken by patients, caregivers and physicians to
diagnose or treat the
disease. In still further embodiments, the IVC monitoring system may be
expanded to provide
for closed-loop control of a number of different therapeutic interventional
systems. By sensing
the onset of an event of acute heart failure decompensation and then
triggering an intervention
that may reverse, minimize or eliminate the episode of decompensation,
significant suffering or
death of the patient potentially may be avoided, and the health care system
will save significant
financial and human resources.
[00116] It is common practice to use intravenous (IV) diuretics to increase
fluid output for
patients who are admitted to the hospital for acute heart failure
decompensation. Many patients
in heart failure take oral diuretics, but as their heart failure status
deteriorates, diuretics can
become less and less effective when delivered orally. Intravenous or
intramuscular diuretic
delivery remains more effective in these situations. The output from the
sensor/monitor
contemplated herein could be coupled with IV pumps, for example, to control
the dosage of IV
diuretics in the in-patient setting. In this example, the sensor/monitor would
communicate to an
external module, increasing or decreasing dosage of the IV diuretics as
necessary to maintain a
desired IVC status. The external communication module could be a separate
module, which in
turn communicates to the IV pump, or it could be incorporated into the IV pump
directly. Even if
the physician preferred to manually set the initial infusion rate for the
drug, the feedback system
here could serve as an additional safety shut-off, interrupting delivery of
diuretics once the IVC
status reaches the appropriate level.
[00117] Also in clinical practice or in development are wearable or fully
implantable pumps
that can deliver IV or subcutaneous diuretics. To date these have been open-
loop or uncontrolled
systems. For example, Zatarain-Nicolas et al. reported a series of patients
who were implanted
with simple, passive constant-flow elastomeric subcutaneous pumps to deliver
furosemide (a
common diuretic) over time. The sensors discussed herein could be configured
to communicate
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
with a valved version of this type of pump to create a simple closed-loop
system and to deliver
subcutaneous diuretics.
[00118] Alternatively, fully implantable, refillable drug pumps, for
example the Medtronic
SynchroMed pump, are currently used to deliver pain medications. A fully
implantable pump
could be configured to communicate with the IVC sensors and deliver IV or
subcutaneous
diuretics. In one configuration, an implantable pump could be implanted in an
infraclavicular
pouch and the IVC sensor could be introduced into the IVC from the subclavian
vein adjacent to
the pouch. A lead could connect the two elements of the system, so that the
pump uses the data
from the IVC sensor to help determine whether to infuse the drug, and how much
drug to deliver.
The pump could deliver the drug into the infraclavicular pouch, into nearby
muscle tissue, or it
could deliver the drug directly into the vascular system. If it were
delivering the drug directly
into the vascular system, the infusion lumen from the pump to the vascular
system might be
integral with the lead from the IVC sensor, or it might be introduced parallel
to it. The infusion
lumen might be designed with a valve at the distal tip, to minimize the
incidence of clotting or
clogging that might block drug delivery.
[00119] Another class of drugs commonly used to treat heart failure are
inotropes. Inotropes
change the force of muscular contractions. In each of the embodiments
described herein,
diuretics could be exchanged for, or used in conjunction with, inotropic
drugs. For example,
dual chamber drug pumps configured to deliver both diuretic drugs and
inotropic drugs could be
configured to act upon the data generated by and communicated from the sensors
described
herein.
[00120] In addition to directly administering diuretics to the body, drug
pumps and electrical
neuromodulation systems have been contemplated for the control of heart
failure by directly
modulating the activity of the renal nerves. The renal nerves directly
influence the renin
angiotensin system and modulate fluid retention or excretion. The IVC sensors
herein could be
configured to communicate with drug pumps or neuromodulation systems to up- or
down-
regulate the activity of the renal nerves, thus increasing or decreasing the
actions of the renin
angiotensin system.
46
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
[00121] It should be noted that the renin angiotensin system also has
regulatory effects on
other aspects of heart failure decompensation. Notably, the peripheral
vascular system,
specifically vascular tone, is involved in heart failure status. The
modulatory effects of the
closed-loop systems described herein may also act directly on the systems that
control vascular
tone, such as the renin angiotensin system.
[00122] It was described above that the IVC sensor could be configured to
communicate with
an external IV pump. Similarly, the sensor data can be used to control other
external devices to
effect a therapeutic outcome. For example, the external data collection
systems described in
earlier open-loop IVC sensor systems could be configured to contain as part of
that external
system an automatic drug injection device similar to an EpiPen. In one
embodiment the external
data reader can be held in contact with the body while the data from the IVC
sensor is
transmitted. If the data shows that an intervention is required, the external
system containing one
or more automatic injection systems could deploy as a result of the collected
data, injecting for
example subcutaneous furosemide.
[00123] FIG. 43 presents a high-level schematic of such closed-loop system
embodiments,
which may include an IVC monitoring device (passive or active as described
above) and at least
one interventional treatment device, wherein the devices are configured to
communicate with
one another. The IVC monitor and the therapeutic device may communicate as
necessary to
coordinate sensed physiologic data and required intervention in a number of
ways. Any of the
typically used communication protocols may be used, including but not limited
to Bluetooth
protocol, RF communication link, microwave communication link, ultrasound
communication or
the like as further described below.
[00124] In one embodiment, a direct communication can be made through the
wall of the
IVC proximate to, or posterior to the main body of the IVC sensor as shown in
FIG. 44. This
direct communication through the wall of the IVC may, for example, take the
form of a
mechanical grommet 4403 extending laterally from a side wall of anchor element
4406 of
monitoring device 4409. Grommet 4404 is configured to pass through a
penetration in the IVC
wall. The grommet attachment can serve a dual purpose of helping anchor the
sensor to the IVC
and providing a wired communication port to the therapeutic device 4412.
Grommet 4403 may
47
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
be configured to seal with the IVC wall around its periphery, either
mechanically and/or via an
induced healing response, and may include a flange on its outer end to seal
against the exterior
surface of the IVC. Alternatively, a purse-string suture can be used to seal
the vessel wall around
the leads. A further alternative would be to locate the IVC sensor(s) on the
outside of the IVC,
for example as in the embodiment shown in FIG. 28B. If the therapeutic device
is also outside
the vascular system, then no transmural link is necessary. Note that
monitoring device 4409
utilizes marker elements 4415 connected to anchor element 4406 via anchor
isolation
structure 4418.
[00125] In another alternative, exemplified by the embodiment shown in FIG.
45, wired
communication occurs via leads which run intravascularly to allow the IVC
sensor to connect
directly to the therapeutic device. For example, leads 4503 from IVC monitor
4506 can connect
directly to dedicated ports 4509 of the therapeutic device 4512 (for example a
pacing device such
as a biventricular pacemaker). Inputs from IVC monitor 4506 can be programmed
into the
algorithm of the therapeutic device. For example, the inputs can be programmed
into the
biventricular pacing algorithm of a biventricular pacemaker to fine tune the
coordination of the
pacing of the heart. Since biventricular pacers are typically placed in an
infraclavicular pouch
with pacing leads 4515 introduced into the subclavian vein and advanced into
the heart, the IVC
monitor(s) could also be introduced into the subclavian vein on a delivery
catheter and advanced
into the IVC using techniques described above. This example could be
incorporated to modulate
the action of any of the therapeutic devices described below.
[00126] As an alternative to working directly with the therapeutic device
by feeding the IVC
sensor data into the device to modify a treatment algorithm, a separate lead
(or a wireless signal)
emanating from the sensor to modify the actions of a therapeutic device may be
used. For
example, the leads emanating from an IVC sensor could be placed in a position
in which the
signal from the sensor device can interact with the signal of a therapeutic
device such that the
signal from the sensor modifies the action of the therapeutic device. More
specifically, a lead
from a sensor can be placed in proximity to a lead from a pacing device such
that a signal from
the sensor causes a signal to emit from the sensor leads to interfere with or
to augment the signal
from the therapeutic device.
48
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
[00127] Embodiments of the interference mode of action described above may
include
placing a lead from the sensor alongside a lead from a pacemaker or
biventricular pacemaker
such that the signal from the sensor cancels out the signal from the pacer, or
conversely
augments the signal from the pacer to modify or modulate the therapy
delivered. This example
could be incorporated to modulate the action of any of the therapeutic devices
described below.
The integration of sensor data into a closed-loop system may be accomplished
with many
different therapeutic devices and methods currently marketed or in development
for the treatment
of heart failure and its associated comorbidities.
Closed-Loop Systems With Spinal Cord Stimulation
[00128] Spinal Cord Stimulation (SCS) has been tested to treat heart
failure by modulating
the balance of sympathetic and parasympathetic activity in the body. This
typically involves the
surgical implantation of an implantable pulse generator (IPG) or
neurostimulator, with electrodes
which are placed near the spinal cord to deliver a series of low-energy
electrical impulses. The
IPG is typically implanted in the abdomen near the spine.
[00129] It may be appropriate to adjust the delivery, frequency, or
intensity of these electrical
impulses to match the severity of the patient's heart failure status.
Therefore, it may be
appropriate to link the IVC monitor and the SCS into a closed-loop system.
Since the IPG is very
close to the IVC near the posterior wall of the abdomen, it may be appropriate
to surgically
implant sensors in the IVC which are connected via leads directly to the IPG.
Alternatively,
wireless markers could be implanted in the IVC and the IPG could wireles sly
sense the distance
of the markers to determine the volume status of the patient, similar to the
previously described
external monitoring device.
[00130] The healthcare provider could then program this closed-loop system
based on an
algorithm which might have the SCS impulses turned down or completely off when
the patient's
status is relatively healthy, with increasing intensity of SCS impulses if the
patient's condition
deteriorated. This system could also integrate additional physiologic
information such as heart
rate, respiration rate, physical activity, etc. into its calculations. It
could also wirelessly
communicate with external devices, which communicate the patient's and
system's status to the
patient, a physician, or other caregiver.
49
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
[00131] One exemplary application of this device would be to monitor the
heart failure status
of patients with chronic heart failure. It may very sensitively measure a
patient's trend toward
fluid overload, and may do so well in advance of an episode of acute
decompensated heart
failure. This would give the patient, physician, nurse, or other caregiver
time to adjust fluid
intake, increase diuretic medications or take other steps to reduce the
patient's fluid status. The
external module might include an alarm which tells the patient to get out of
bed and sleep more
upright or to go directly to the hospital, if the perceived risk of fluid
overload is extremely high.
[00132] Another exemplary application of disclosed devices is to manage a
patient during an
episode of hospitalization for acute decompensated heart failure. Even though
a patient may
spend several days in the hospital receiving intravenous diuretics, reduced
fluid intake, and even
aquapheresis (dialysis to reduce fluid volume), it is quite possible that the
patient leaves the
hospital with excess fluid. In these situations, embodiments described may be
usefully applied to
titrate the diuresis process and to assess when the patient should be
discharged. Further, as this
new parameter of IVC distention and variability is studied more extensively
based on the
teachings of the present disclosure, it may prove to be an important
prognostic indicator for a
number of other conditions and situations other than management of heart
failure, dialysis, and
patients in shock.
[00133] As noted in connection with various embodiments described herein,
one or more
aspects and embodiments may be conveniently implemented using one or more
machines (e.g.,
one or more computing devices that are utilized as a user computing device for
electronic
medical information or documents, one or more server devices, etc.) programmed
according to
the teachings of the present disclosure, as will be apparent to those of
ordinary skill in the art.
[00134] This device may have electronic circuitry that stores data which
may then be
communicated to an outside monitor via a telemetry system. This information
could then be
further processed for presentation to the patient, giving a simple indication
of their risk level or
recommended level of drug intake, or diet and activity recommendations. This
information could
also be forwarded to the patient's physician, so that they can monitor the
patient's condition and
communicate with the patient as appropriate.
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
[00135] This information could also be forwarded via the internet or other
means to the
company which manufactures or sells the device, so that the company can
continue to optimize
the algorithms which use the raw data to determine the patient's risk level.
It may be most
effective to have the external monitor forward all of the raw data to the
company, since the
company will have the most up-to-date and optimized algorithms for analyzing
data, and then
send the processed information back to the patient and their physician. The
company might also
have the most secure data storage means for storing all historical information
for each specific
patient, so that the data analysis algorithm can be further optimized for each
specific patient.
[00136] Embodiments disclosed herein may also be used for measuring
dimensions of other
body elements besides the IVC. Sensors could be placed in the heart, for
example placing them
in the left ventricle via catheter-based delivery. They could also be placed
on the surface of the
heart, within the pericardium via a subxyphoid access. These sensors could be
used to directly
monitor the heart's activity. As another example, sensors could be placed on
or in the bladder to
monitor bladder conditions. In patients who need to self-catheterize to drain
their bladders, it
may be useful to have an automated warning of when the bladder was full.
[00137] For all of the above-mentioned embodiments that have markers or
sensors implanted
into the IVC, these markers or sensors may heal into the IVC wall over time.
Therefore, the
stent, anchor, or other elements that hold the markers in place may not need
to be permanent.
Therefore, it may be desirable to make the stent or anchor bioerodable, so
that after a period of
time, there is no longer a stent in the IVC. The specific duration of the
anchoring elements can be
varied from weeks to years through material selection, formulation, and
processing. This would
eliminate a foreign body, and it would also render the IVC more flexible,
allowing it to more
naturally collapse or expand. Other bioabsorbable vascular elements have
already been made
from materials such as Poly-L-Lactide. These materials have less springiness
than Nitinol, so the
stent design may need to be modified. For example, the stent could be made
with circumferential
elements that ratchet open to apply pressure to the IVC to hold the stent in
place. The delivery
catheter for this bioabsorbable stent might include a balloon to actively
expand the stent against
the IVC.
51
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
[00138] Embodiments described in this disclosure so far have focused
primarily on volume
changes in the IVC. As the patient inhales, thoracic pressure drops slightly,
increasing the flow
of blood from the IVC into the right atrium (RA). As the patient exhales,
thoracic pressure
increases slightly, decreasing the flow of blood into the right atrium. This
leads to a variation in
blood volume in the IVC over the respiratory cycle. This variation in blood
volume is necessarily
correlated with a slight variation in the relative pressure between the IVC
and the RA. As an
alternative or adjunct to IVC volume measurement, a measurement of the
relative variation in
fluid pressure between the IVC and right atrium may provide a useful
indication of blood
volume. An implant with two pressure sensors arrayed along a single lead could
be implanted
from the femoral vein, jugular vein, or subclavian vein and anchored in
position so that one
pressure sensor is in the RA and one in the IVC. Embodiments described above
having different
configurations for IVC monitors (wireless, externally powered, powered by
electronics deployed
within the IVC or RA, powered from an infraclavicular implant, etc.) may
alternatively or
additionally employ such a measurement of relative variation in fluid
pressure.
System and, Control and Communication Hardware and Software Aspects of
Disclosed
Embodiments
[00139] Appropriate software coding can readily be prepared by skilled
programmers based
on the teachings of the present disclosure, as will be apparent to those of
ordinary skill in the
software arts. Aspects and implementations discussed above employing software
and/or
software modules may also include appropriate hardware for assisting in the
implementation of
the machine executable instructions of the software and/or software module.
[00140] Such software may be a computer program product that employs a
machine-readable
storage medium. A machine-readable storage medium may be any medium that is
capable of
storing and/or encoding a sequence of instructions for execution by a machine
(e.g., a computing
device) and that causes the machine to perform any one of the methodologies
and/or
embodiments described herein. Examples of a machine-readable storage medium
include, but
are not limited to, a magnetic disk, an optical disc (e.g., CD, CD-R, DVD, DVD-
R, etc.), a
magneto-optical disk, a read-only memory "ROM" device, a random access memory
"RAM"
device, a magnetic card, an optical card, a solid-state memory device, an
EPROM, an EEPROM,
52
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
and any combinations thereof. A machine-readable medium, as used herein, is
intended to
include a single medium as well as a collection of physically separate media,
such as, for
example, a collection of compact discs or one or more hard disk drives in
combination with a
computer memory. As used herein, a machine-readable storage medium does not
include
transitory forms of signal transmission.
[00141] Such software may also include information (e.g., data) carried as
a data signal on a
data carrier, such as a carrier wave. For example, machine-executable
information may be
included as a data-carrying signal embodied in a data carrier in which the
signal encodes a
sequence of instruction, or portion thereof, for execution by a machine (e.g.,
a computing device)
and any related information (e.g., data structures and data) that causes the
machine to perform
any one of the methodologies and/or embodiments described herein.
[00142] Examples of a computing device include, but are not limited to, an
electronic book
reading device, a computer workstation, a terminal computer, a server
computer, a handheld
device (e.g., a tablet computer, a smartphone, etc.), a web appliance, a
network router, a network
switch, a network bridge, any machine capable of executing a sequence of
instructions that
specify an action to be taken by that machine, and any combinations thereof.
In one example, a
computing device may include and/or be included in a kiosk.
[00143] FIG. 46 shows a diagrammatic representation of one embodiment of a
computing
device in the exemplary form of a computer system 4600 within which a set of
instructions for
causing a control system to perform any one or more of the aspects and/or
methodologies of the
present disclosure may be executed, such as a control system that may be
embodied by or
implemented in accordance with one or more components of: any one or more of
the IVC
sensors and/or monitors and/or associated components disclosed herein;
electronics capsule 118
of FIG. 1; electronics capsule 503 of FIG. 5; electronics capsule 1124 of FIG.
11; electronics
capsule 1701 of FIG. 17; electronics capsule 1801 of FIG. 18; electronics
capsule 2306 of
FIG. 23; electronics capsule 2512 of FIG. 25; electronics capsule 2806 of FIG.
28A-C;
console 3006 and/or ultrasound receiver/transmitter probe 3003 of FIG. 30;
wearable detection
system 3300 and/or external device 3318 of FIG. 33; external handset 4103 of
FIG. 41;
system 4200 of FIG. 42; one or more components of the systems of FIGS. 43
and/or 44; and/or
53
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
IVC monitor 4506 of FIG. 45, among others. It is also contemplated that
multiple computing
devices may be utilized to implement a specially configured set of
instructions for causing one or
more of the devices to perform any one or more of the aspects and/or
methodologies of the
present disclosure. Computer system 4600 includes a processor 4604 and a
memory 4608 that
communicate with each other, and with other components, via a bus 4612. Bus
4612 may
include any of several types of bus structures including, but not limited to,
a memory bus, a
memory controller, a peripheral bus, a local bus, and any combinations
thereof, using any of a
variety of bus architectures.
[00144] Memory 4608 may include various components (e.g., machine-readable
media)
including, but not limited to, a random access memory component, a read only
component, and
any combinations thereof. In one example, a basic input/output system 4616
(BIOS), including
basic routines that help to transfer information between elements within
computer system 4600,
such as during start-up, may be stored in memory 4608. Memory 4608 may also
include (e.g.,
stored on one or more machine-readable media) instructions (e.g., software)
4620 embodying
any one or more of the aspects and/or methodologies of the present disclosure.
In another
example, memory 4608 may further include any number of program modules
including, but not
limited to, an operating system, one or more application programs, other
program modules,
program data, and any combinations thereof.
[00145] Computer system 4600 may also include a storage device 4624.
Examples of a
storage device (e.g., storage device 4624) include, but are not limited to, a
hard disk drive, a
magnetic disk drive, an optical disc drive in combination with an optical
medium, a solid-state
memory device, and any combinations thereof. Storage device 4624 may be
connected to
bus 4612 by an appropriate interface (not shown). Example interfaces include,
but are not
limited to, SCSI, advanced technology attachment (ATA), serial ATA, universal
serial bus
(USB), IEEE 1394 (FIREWIRE), and any combinations thereof. In one example,
storage
device 4624 (or one or more components thereof) may be removably interfaced
with computer
system 4600 (e.g., via an external port connector (not shown)). Particularly,
storage device 4624
and an associated machine-readable medium 4628 may provide nonvolatile and/or
volatile
storage of machine-readable instructions, data structures, program modules,
and/or other data for
computer system 4600. In one example, software 4620 may reside, completely or
partially,
54
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
within machine-readable medium 4628. In another example, software 4620 may
reside,
completely or partially, within processor 4604.
[00146] Computer system 4600 may also include an input device 4632. In one
example, a
user of computer system 4600 may enter commands and/or other information into
computer
system 4600 via input device 4632. Examples of an input device 4632 include,
but are not
limited to, an alpha-numeric input device (e.g., a keyboard), a pointing
device, a joystick, a
gamepad, an audio input device (e.g., a microphone, a voice response system,
etc.), a cursor
control device (e.g., a mouse), a touchpad, an optical scanner, a video
capture device (e.g., a still
camera, a video camera), a touchscreen, and any combinations thereof. Input
device 4632 may
be interfaced to bus 4612 via any of a variety of interfaces (not shown)
including, but not limited
to, a serial interface, a parallel interface, a game port, a USB interface, a
FIREWIRE interface, a
direct interface to bus 4612, and any combinations thereof. Input device 4632
may include a
touch screen interface that may be a part of or separate from display 4636,
discussed further
below. Input device 4632 may be utilized as a user selection device for
selecting one or more
graphical representations in a graphical interface as described above.
[00147] A user may also input commands and/or other information to computer
system 4600
via storage device 4624 (e.g., a removable disk drive, a flash drive, etc.)
and/or network interface
device 4640. A network interface device, such as network interface device
4640, may be utilized
for connecting computer system 4600 to one or more of a variety of networks,
such as
network 4644, and one or more remote devices 4648 connected thereto. Examples
of a network
interface device include, but are not limited to, a network interface card
(e.g., a mobile network
interface card, a LAN card), a modem, and any combination thereof. Examples of
a network
include, but are not limited to, a wide area network (e.g., the Internet, an
enterprise network), a
local area network (e.g., a network associated with an office, a building, a
campus or other
relatively small geographic space), a telephone network, a data network
associated with a
telephone/voice provider (e.g., a mobile communications provider data and/or
voice network), a
direct connection between two computing devices, and any combinations thereof.
A network,
such as network 4644, may employ a wired and/or a wireless mode of
communication. In
general, any network topology may be used. Information (e.g., data, software
4620, etc.) may be
communicated to and/or from computer system 4600 via network interface device
4640. In
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
some embodiments, one or more cloud computing services, "software as a
service" services,
"storage as a service" services, and/or distributed networks or components,
among others, may
be used to receive, store, and/or provide data and/or execute software in
accordance with aspects
of the present disclosure, as will be understood by those of ordinary skill in
the relevant art after
reading this disclosure in its entirety.
[00148] Computer system 4600 may further include a video display adapter
4652 for
communicating a displayable image to a display device, such as display device
4636. Examples
of a display device include, but are not limited to, a liquid crystal display
(LCD), a cathode ray
tube (CRT), a plasma display, a light emitting diode (LED) display, and any
combinations
thereof. Display adapter 4652 and display device 4636 may be utilized in
combination with
processor 4604 to provide graphical representations of aspects of the present
disclosure. In
addition to a display device, computer system 4600 may include one or more
other peripheral
output devices including, but not limited to, an audio speaker, a printer, and
any combinations
thereof. Such peripheral output devices may be connected to bus 4612 via a
peripheral interface
4656. Examples of a peripheral interface include, but are not limited to, a
serial port, a USB
connection, a FIRE WIRE connection, a parallel connection, and any
combinations thereof.
[00149] Device embodiments disclosed herein also may measure other
physiologic data, and
integrate that data in its reporting and analysis. It might be used at
different times to treat
different conditions. For example, the IVC diameter and its variation will be
significantly
different while the patient is standing in comparison to when the patient is
sitting, prone, or
supine. Therefore, the IVC monitor can also be used to track patient activity.
Also, electrodes
can be placed on the IVC element itself and/or on the leads leading to the
device, in order to
monitor, record, and communicate the heart's electrical activity.
[00150] Although a primary indication described for embodiments disclosed
herein is the
management of heart failure, embodiments and information collected thereby may
be used for
management of other conditions as well. For example, it could also be
simultaneously used to
manage blood volume in patients undergoing dialysis, providing direct feedback
to dialysis
machines to modulate total fluid volume delivered or removed. It could
similarly be used to
56
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
communicate with IV pumps to manage re-hydration for patients who have acute
episodes of
shock.
[00151] As described above, embodiments may be connected to a drug pump or
stimulator to
modulate the renal nerves, due to the multiple indirect effects the renal
nerves have on heart
failure. If a device of such embodiments is also monitoring heart rhythm
status as well and
detected an episode of atrial fibrillation, it could be programmed to modulate
the renal nerves in
that situation as well. Afferent renal nerves are known to increase systemic
sympathetic tone, and
increased systemic sympathetic tone increases the risk of atrial fibrillation,
so temporary
denervation of the renal nerves might cause the episode of atrial fibrillation
to terminate.
Use of IVC volume measurement in dialysis patients
[00152] Volume management in dialysis patients can be particularly
challenging, since the
kidneys are not providing normal volume homeostasis. Dialysis patients
typically increase their
fluid volume between dialysis sessions. Since the kidneys are not making
urine, excess volume
needs to be removed during dialysis, along with the other waste products which
dialysis filters
out. However, most of the excess volume is in the cells and interstitial
volume, not in the
circulatory system. It takes time, typically more than an hour, for that
volume to re-enter the
circulatory system as other fluid is removed from the blood. The excess volume
should not be
removed too quickly, as that would lead to excessive hemoconcentration and
potentially
dangerously low blood pressure. Excessive hemoconcentration can cause
myocardial stunning
and other significant dangers. Moreover, it may be difficult and impractical
for the care provider
managing the dialysis process to track the patient's blood volume continuously
during the
dialysis session. Therefore, excess fluid is typically removed very gradually
over the course of a
dialysis session. This reduces the rate at which intracellular and
extracellular fluid returns to the
vascular system, and makes the overall dialysis session less efficient. An
efficient and effective
method to measure circulating blood volume real-time during dialysis is
needed.
[00153] Secondly, it is important to remove as much volume as is safely
possible over the
course of the complete dialysis session. As mentioned, fluid volume builds up
in patients
between dialysis sessions. This leads to many potential clinical issues,
including high blood
pressure, fluid in the lungs, fibrosis, and heart failure. If the patients
leave each dialysis session
57
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
with a minimum of fluid in their systems, that increases the chances that they
will not be
overloaded with fluid by the time they return for their next session. However,
determining
whether the patient is euvolemic (has the right fluid volume) or is hyper- or
hypo-volemic is
challenging with current techniques. An effective method to measure final
blood volume in
dialysis patients at the end of dialysis sessions is therefore also an
important need.
[00154] At present, blood concentration during dialysis is often measured
using devices such
as Fresenius' Critline or Intelomedics' CVinsight. These systems measure the
patient's
hematocrit and blood oxygenation to calculate the fluid volume removed from
the patient.
Assessing euvolemia at the end of dialysis may be done using `BioImpedance
measurement',
which gives an indication of extracellular and intracellular fluid volume in
the patient. These
measurements are imperfect, but are the best available at this time.
[00155] Measurement of a dialysis patient's volume status using ultrasound
imaging of the
IVC has been studied. It gives an important physiologic reading of the
patient's volume status,
one which relates directly to whether the patient is truly euvolemic. However,
it is user-
dependent, technology-sensitive, and difficult. It also gives only a single-
point measurement, and
is completely impractical as a method of continuously measuring volume status.
[00156] For all of these reasons, IVC volume measurement systems embodied
in the present
disclosure provide an improved method of managing the dialysis patient's
volume status.
Described systems provide for continuous monitoring and give a true
measurement of whether
the patient is hyper-, hypo-, or euvolemic, and can be used to guide therapy.
The IVC markers
described herein, when implanted on or in the IVC wall, allow a patient's
fluid volume to be
monitored before, during or after a dialysis session. Because dialysis is
typically conducted in a
specialized facility staffed by healthcare providers, readings could be taken
by trained
professionals to ensure accuracy. In addition, with the patient being immobile
in a chair or bed
during dialysis, an ultrasound probe may be attached to the patient's skin to
provide continuous
monitoring of the IVC markers during the dialysis session. The IVC volume
monitor may be
connected directly to the dialysis machine, for closed-loop volume management.
For example, an
appropriate minimum blood volume may be established by the healthcare
provider; for example,
as an average 40% variation in IVC dimension over the respiratory cycle. The
dialysis machine
58
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
could then be programmed to reduce the blood volume at a measured, but
relatively rapid pace
until that volume is reached, and then to maintain that volume over the rest
of the dialysis
session. This approach would maximize the intracellular and extracellular
fluid that is removed
from the patient, while preventing the patient from risk of myocardial
stunning, lightheadedness,
or the other dangers of hypovolemia. It may be possible to shorten the
dialysis session slightly as
a result. At the end of the dialysis session, the IVC monitor would reconfirm
that the patient was
appropriately euvolemic before ending the session.
[00157] All of this discussion of volume management in dialysis patients
applies equally to
the management of heart failure patients who are having volume removed using
an aquapheresis
system such as the CHF Solutions Aquadex, or when using aggressive diuretics.
It is desirable to
remove the excess volume as quickly as possible, but also to allow time for
the excess volume in
tissue to gradually return to the bloodstream, so that overall blood volume
does not drop too low,
nor the blood become too concentrated.
Measurement of volume status in other vessels besides the IVC
[00158] The discussion of the various alternative embodiments above is
generally made in
the context of measurement of volume in the IVC. However, these embodiments
also apply to
and may be used for similar measurements in the superior vena cava (SVC),
right atrium, or
other vessels. The variation in IVC volume over the respiratory cycle has been
well documented
in studies using ultrasound imaging. The mild valsalva effect of respiration
causes a slight
variation in thoracic pressure, which modulates the flow of blood from the IVC
(in the abdomen)
into the right atrium (in the thorax). Therefore, this variation may be more
pronounced in the
IVC than in other vessels. IVC variation may also be more sensitive to
variations in right atrial
pressure, which may vary less in patients with marked volume overload causing
tricuspid
regurgitation or reduced right ventricular filling volume. However, very
sensitive measurement
systems might measure similar variations in vessels that are more accessible
for the placement of
markers and/or for placement of a measurement device. The subclavian veins,
jugular veins, and
femoral veins, among others, are all potential vessels for measurement of
volume and volume
variation that could provide similar information about a patient's blood
volume and/or heart
failure status. However, the teachings of this disclosure are not so limited
and may be applied by
59
CA 02976465 2017-08-11
WO 2016/131020 PCT/US2016/017902
persons of ordinary skill to any vein in the body, using the sensitive
measurement techniques
described herein. Further features, considerations and embodiments are
described below, which
may be incorporated singly or multiply into one or more embodiments described
above. For
example, discussed above is the use of the sensor/monitor input to modify the
action of cardiac
pacemakers to better control the heart and circulatory system. Another
embodiment or method
of treating heart failure, and more specifically the fluid overload associated
with heart failure, is
through chemical, neural, hormonal, or electrical manipulation of the renin-
angiotensin system
and the degree to which the kidneys excrete or retain water.
[00159] Exemplary embodiments have been disclosed above and illustrated in
the
accompanying drawings. It will be understood by those skilled in the art that
various changes,
omissions and additions may be made to that which is specifically disclosed
herein without
departing from the spirit and scope of the present disclosure.