Note: Descriptions are shown in the official language in which they were submitted.
1
Metal 30 Printing Method and Metallic 3D Printed Materials
CROSS REFERENCE TO RELATED APPLICATIONS
N/A
FIELD OF THE INVENTION
[0001]
The present invention relates to the 3D printing of metallic materials. More
specifically, the present
invention is concerned with solvent-cast 3D printing of metallic inks,
followed by sintering.
BACKGROUND OF THE INVENTION
[0002]
Metallic structures fabricated by three-dimensional (3D) printing are
progressively more used in medical
(e.g., artificial bones), microwave (e.g., antennas), and microelectro-
mechanical systems (MEMS) fields (e.g.,
sensors and micro-electrodes). These applications benefit from the high
mechanical, electrical and electromagnetic
properties of metals, and the design freedom, mass customization, and ease of
use related to 3D printing. Selective
laser sintering (SLS) and selective laser melting (SLM) are commonly used for
the fabrication of 3D metallic
structures of various sizes. The main techniques use a laser beam to deliver
energy on the surface of a powder bed
in order to activate bonding between the powder particles. The bonds can be
obtained by metallic sintering, metallic
melting and binder melting. Typical materials used are titanium alloys, while
a few studies reported the SLS of steel.
For example, McAlea et al. reported SLS with polymer-coated steel powders
(i.e., 1080 steel, 316 or 420 stainless
steel particles coated with thermoplastic polymer blend) followed by a bronze
infiltration process. The Young's
modulus of manufactured part was 193 GPa.16 These high temperature fabrication
processes require costly laser
systems, a large amount of powder to form the powder bed, and several operator
protection setups. Moreover, the
numerous heat cycles due to repeated laser strikes change the mechanical and
chemical properties of the loose
powder neighboring the structure's span. As a result, some of the powders
cannot be reused. Furthermore, the
mechanical properties of SLS/SLM processed parts are usually limited by
metallurgical defects such as porosities,
cracking, oxide inclusions and loss of alloying elements.
[0003]
Solvent-cast 3D printing is an alternative approach that creates
microstructures by depositing a liquid ink
on a substrate, layer-by-layer, and even in freeform. Inks contain a volatile
solvent that evaporates rapidly after
extrusion from the deposition nozzle, leading to a rigid filamentary
structure. Various ink solutions have been
developed for solvent-cast 3D printing. Ink functions, such as conductivity
and mechanical properties, are created by
adding micro- or nano- fillers including carbon nanotubes (CNTs), nano-clays
and metal microparticles. Woo Jin
Hyun et al. reported the screen printing of 2D filaments with metallic ink
loaded with silver nanoparticles synthesized
from silver nitrate solution. The electrical conductivity of the as-printed
ink filament was 1.8 x S/m.30 Eunji Hong
et al. reported the solvent-cast printing of titanium ink filaments to build
two-dimensional (2D) lattices. The as-printed
2D lattices were rolled into scrolls and heat-treated in a vacuum furnace. The
compression yield strength of the
scrolls after heat-treatments was 735 MPa.31 These two methods provide
structures with good mechanical properties
CA 2976782 2017-08-16
2
or electrical conductivity, but are mainly limited to thin 2D geometries.
Another work by Skylar-Scott et al. presented
a laser-assisted sintering of an ink filament to create 3D freeform metallic
structures.32 While this method is used to
print complex wire-type geometries, it is limited to a few compatible
materials, susceptible to oxidation and porosity,
and is unable to fabricate mechanically strong and highly dense structures.
The low-cost 3D printing of highly dense
metallic structures featuring complex geometries still represent a significant
scientific and technological challenge.
SUMMARY OF THE INVENTION
[0004] In accordance with the present invention, there is provided:
1. A metallic ink for solvent-cast 3D printing, the ink comprising:
= a solution or a gel of a polymer in a volatile solvent, and
= heat-sinterable metallic particles dispersed in the solution or gel,
wherein the particles are present in a particles:polymer weight ratio of more
than about 85:15.
2. The ink of item 1, wherein the particles are present in a carbon
particles:polymer weight ratio of:
= about 86:14, about 87:13, about 88:12, about 89:11, about 90:10, about
91:9, about 92:8, about
93:7, about 94:6, or about 95:5 or more, and/or
= about 99:1, about 98:2, about 97:3, about 96:4, about 95:5, about 94:6,
about 93:7, about 92:8,
about 91:9, or about 90:10, or less.
3. The ink of item 1 or 2, wherein the particles are present in a carbon
particles:polymer weight ratio between
about 90:10 to about 95:5.
4. The ink of any one of items 1 to 3, wherein the particles are present in
a carbon particles:polymer weight ratio
of about 95:5.
5. The ink of any one of items 1 to 4, wherein the heat-sinterable metallic
particles are steel, cast iron, titanium,
silver, copper, zinc, gold, platinum, aluminum, nickel, bronze, or brass
particles.
6. The ink of any one of items 1 to 5, wherein the heat-sinterable metallic
particles are steel particles.
7. The ink of any one of items 1 to 6, wherein the heat-sinterable metallic
particles are microparticles.
8. The ink of any one of items 1 to 7, wherein the heat-sinterable metallic
particles are between about 0.1 pm
and about 100 pm in size.
9. The ink of any one of items 1 to 8, wherein the heat-sinterable metallic
particles are between about 5 pm and
about 50 pm in size.
10. The ink of any one of items 1 to 9, wherein the heat-sinterable
metallic particles are spheroidal.
11. The ink of any one of items 1 to 10, wherein the heat-sinterable
metallic particles are spherical.
12. The ink of any one of items 1 to 11, comprising between about 10 and
about 50 w/w% of the solvent (based
on the total weight of the ink).
13. The ink of any one of items 1 to 12, wherein the polymer is poly(lactic
acid), polystyrene, poly(methyl
acrylate), poly(methyl methacrylate), poly(n-butyl acrylate), poly(2-
hydroxyethyl methacrylate), poly(glycidyl
methacrylate), poly(acrylic acid), poly(N-N-dimethylacrylamide), poly( 1-vinyl
anthracene), poly(2-vinyl
CA 2976782 2017-08-16
3
pyridine), poly(4-vinyl pyridine), poly(N-vinyl carbazole), poly(N-vinyl
carbazole), poly(N-vinyl imidazole),
poly(vinyl benzyl chloride), poly(4-vinyl benzoic acid), poly(vinyl acetate),
polycaprolactone, poly(1144-(4-
butylphenylazo)phenoxyFundecyl methacrylate) (poly(AzoMA)),
poly(ferrocenyldimethylsilane), polyisoprene,
polybutadiene, polyisobutylene, poly propylene glycol, poly(ethylene glycol),
or a polysaccharide, or a mixture
thereof.
14. The ink of any one of items 1 to 13, wherein the polymer is poly(lactic
acid).
15. The ink of any one of items 1 to 14, wherein the solvent is
dichloromethane (DCM), chloroform (CHCI3),
tetrahydrofuran (THF), acetone, methanol (Me0H), ethanol (Et0H), or water.
16. The ink of any one of items 1 to 15, wherein the solvent is
dichloromethane, chloroform, tetrahydrofuran,
acetone, methanol, or ethanol.
17. The ink of any one of items 1 to 16, wherein the solvent is
dichloromethane.
18. The ink of any one of items 1 to 17, wherein the ink further comprises
one or more additive.
19. A 3D printer ink cartridge, the cartridge comprising a container having
an ink outlet, the container comprising
the ink of any one of any one of items 1 to 18.
20. The cartridge of item 19, wherein the cartridge is adapted to be
installed on a 3D printer.
21. The cartridge of item 19 or 20, wherein the cartridge is adapted to be
fitted to a nozzle for delivering the ink,
so that, for ink dispensing, the ink is extruded through the ink outlet and
through the nozzle.
22. The cartridge of any one of items 19 to 21, wherein the cartridge is
designed so that when a pressure is
applied by a 3D printer, the ink is extruded through the ink outlet.
23. A method of method of manufacture of the ink of any one of items 1 to
18, the method comprising the steps
of:
a) providing a solution or a gel of the polymer in the solvent,
b) providing heat-sinterable metallic particles in a particles:polymer
weight ratio of more than about
85:15,
c) dispersing the particles in the solution or gel of the polymer, thereby
producing the ink.
24. The method of item 23, wherein step a) comprises mixing the polymer in
the solvent until the polymer is
dissolved or the gel is formed.
25. The method of item 23 or 24, wherein the polymer concentration of the
solution or gel is:
= about 2 wt%, about 3 wt%, about 4 wt%, about 5 wt%, about 6 wt%, about 7
wt%, about 8 wt%,
about 9 wt%, or about 10 wt% or more and/or
= about 30 wt%, about 35 wt%, about 20 wt%, about 15 wt%, or about 10 wt%
or less,
based on the total weight of the solution or gel.
26. The method of any one of items 23 to 25, wherein the polymer
concentration of the solution or gel is about
20wr/o, based on the total weight of the solution or gel.
27. The method of any one of items 23 to 26, wherein the particles are
dispersed by ball milling in step c).
28. The method of any one of items 23 to 27, further comprising adding
solvent, or removing part of the solvent.
CA 2976782 2017-08-16
4
29. The method of any one of items 23 to 28, further comprising adding one
or more additives to:
= the solvent before it is used to form the solution or gel of the polymer,
= the solution or gel of the polymer, and/or
= the ink.
30. The method of item any one of items 23 to 29, further comprising the
step of packaging the ink in a 3D printer
ink cartridge.
31. A method of manufacturing a solvent-cast metallic 3D printed material,
the method comprising the steps of:
a) providing the metallic ink for solvent-cast 3D printing of any one of
items 1 to 18,
b) using a 3D printer, extruding the ink through a nozzle into a controlled
pattern;
c) allowing solvent evaporation, thereby producing a printed material;
d) removing the polymer from the printed material by heating the printed
material to a polymer
degradation temperature or above, thereby leaving the particles arranged into
the controlled pattern;
and
e) heat-sintering the particles, thereby producing the solvent-cast metallic
3D printed material.
32. The method of item 31, wherein step a) includes the method of any one
of items 23 to 30.
33. The method of item 32 or 32, wherein step b) is carried out at about
room temperature.
34. The method of any one of items 31 to 33, wherein step c) is partly or
completely carried out at about room
temperature.
35. The method of any one of items 31 to 34, wherein steps d) and e) are
performed in a single heat treatment.
36. The method of item 35, wherein the heat treatment comprises increasing
the temperature to a sintering
temperature and then holding the temperature at the sintering temperature.
37. The method of item 35 or 36, wherein a heating rate up to a temperature
T between:
= about the polymer degradation temperature and
= up to about 100 C above the polymer degradation temperature,
is lower than a heating rate from the temperature T to the sintering
temperature.
38. The method of item 37, wherein the heating rate up to the temperature T
is from about 1 to about 5 C/min.
39. The method of item 37 or 38, wherein the heating rate from the
temperature T to the sintering temperature is
from about 7 to about 15 C/min.
40. The method of any one of items 31 to 39, wherein a sintering
temperature in step e) is between:
= about 400 C below the melting point of the particles and
= about 200 C below the melting point of the particles.
41. The method of any one of items 31 to 40, wherein a sintering time is
from about 30 minutes to about 12 h.
42. The method of any one of items 31 to 41, wherein a sintering time is
from about 6 h to about 12 h.
43. The method of any one of items 31 to 41, wherein a sintering time is
from about 30 minutes to about 6 h.
44. The method of any one of items 31 to 43, wherein step d) and e) are
carried out in an inert atmosphere.
45. The method of any one of items 31 to 44, further comprising the step f)
of partly or completely filling the pores
CA 2976782 2017-08-16
5
created in-between the particles by the removal of the polymer in step d) with
a second metal or alloy, the
second metal or alloy having a melting point lower than the melting point of
the metal or alloy constituting the
particles.
46. The method of item 45, wherein the second metal or alloy is steel, cast
iron, titanium, silver, copper, zinc,
gold, platinum, aluminum, nickel, bronze, or brass
47. The method of item 45 or 46, wherein the second metal or alloy is
copper.
48. The method of any one of items 45 to 47, wherein step f) comprises
contacting part of the solvent-cast
metallic 3D printed material with the second metal or alloy, the second metal
or alloy being in the molten state,
and allowing the second metal or alloy to diffuse by capillarity into the
pores.
49. The method of any one of items 45 to 48, wherein step f) comprises
placing a piece of the second metal or
alloy on top of the solvent-cast metallic 3D printed material and then heating
at a temperature above the
melting point of the second metal or alloy.
50. The method of any one of items 45 to 49, wherein an excess of the
second metal or alloy is used.
51. The method of any one of items 45 to 50, wherein step f) is carried out
in an inert atmosphere.
52. A solvent-cast metallic 3D printed material, shaped into a controlled
pattern and made of particles of a metal
or alloy peripherally attached to one another
53. The material of item 52, wherein the pores in-between the particles are
completely or partially filled with a
second metal or alloy.
BRIEF DESCRIPTION OF THE DRAWINGS
[0005] In the appended drawings:
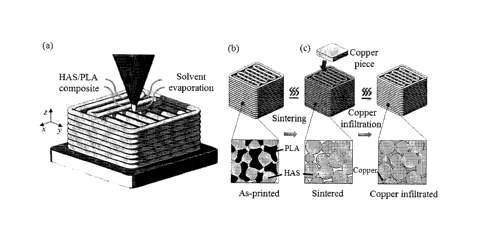
Fig. 1 is a schematic of the fabrication process of a 3D metallic scaffold
combining (a) solvent-cast 3D printing: the
metallic ink is extruded through a micronozzle and the solvent evaporates
right after extrusion at room temperature,
(b) sintering: the as-printed scaffold is heated to burn the polymer away and
sinter the HAS microparticles, and (c)
copper infiltration: the sintered scaffold is heated again with a piece of
copper placed on top of it.
Fig 2 shows the temperature profiles used during sintering and copper
infiltration.
Fig. 3 is a secondary electron micrograph of highly alloyed steel (HAS) powder
particles ( 20 pm).
Fig. 4 show SEM images of as-printed 20-layers scaffolds of different
concentrated inks (85, 90, 95, 98 wt.%) (first
row from left to right) and their close-up view images (middle and bottom
rows).
Fig. 5 show SEM images of sintered 20-layers scaffolds of different
concentrated inks (85, 90, 95, 98 wt.%) (first row
from left to right) and their close-up view images (middle and bottom rows).
Fig. 6 SEM images of copper infiltrated 20-layers scaffolds of different
concentrated inks (90, 95, 98 wt.%) (first row
from left to right) and their close-up view images (middle and bottom rows).
Fig. 7 shows optical images of structures printed with 95 wt.% HAS/PLA ink
through 250pm inner diameter tapered
nozzle, printing speed of 10 mm/s: (a) printing process of a tensile bar
sample (inset: a tensile bar and five 20-layer
scaffolds placed on a Canadian dollar coin), (b) top, side and oblique views
of a 20-layer scaffold, (c) a planetary
CA 2976782 2017-08-16
6
gear with a Canadian 5 cents coin, and (d) an as-printed replica of the
Olympic stadium of Montreal with a
photograph of the stadium for comparison.
Fig. 8 shows optical (left) and SEM images and their close-up views (right) of
20-layer scaffolds printed using 95
wt.% ink and 250 pm inner tapered nozzle, printing speed of 10 mm/s: (a) as-
printed, (b) sintered, and (c) copper
infiltrated. The optical image on the left shows the scaffolds on top of a
Canadian 25-cent coin.
Fig. 9 shows the TGA results of 95 wt.% HAS/PLA scaffold. The temperature was
raised from 20 C to 500 C at a
rate of 1 C/min (the same heating rate as the sintering process). The
degradation of PLA finished before 225 C.
Fig. 10 shows the porosity of the filament in the sintered and copper
infiltrated 20-layer scaffolds for different ink
concentrations, and optical microscope images of the polished cross sections
at 0.5H (scale bar: 50 pm). Error bars
indicate the standard deviations obtained from five samples.
Fig. 11 shows (a) the tensile mechanical response for as-printed, sintered and
copper infiltrated 3D printed tensile
bars and (b) to (e) the tensile fracture surfaces of sintered and copper
infiltrated tensile bars at (b, c) low and (d, e)
high magnification, respectively. Error bars indicate the standard deviations
obtained from three samples.
DETAILED DESCRIPTION OF THE INVENTION
[0006] The present invention relates to solvent-cast 3D printing.
[0007] In solvent-cast 3D printing, an ink containing a volatile solvent is
deposited in a controlled pattern using a
3D printer. A 3D printer is a computer-controlled robot that is able to create
a 3D object, usually from a model
designed by a computer aided design (CAD), by laying down successive thin
layers of a 3D printing ink. To make a
solvent-cast 3D printed structure, the ink is extruded through a moving
micronozzle, thereby depositing the ink in the
desired pattern. Usually, this pattern is multilayered. After extrusion, the
solvent from the ink usually quickly
evaporates (generally at room temperature) producing a solid 3D printed
structure.
[0008] This 3D printed structure can be further modified via sintering
followed or not by metal infiltration.
Metallic Ink for Solvent-Cast 3D Printing
[0009] Turning now to the invention in more details, there is provided a
metallic ink for solvent-cast 3D printing,
the ink comprising:
= a solution or a gel of a polymer in a volatile solvent, and
= heat-sinterable metallic particles dispersed in the solution or gel,
wherein the particles are present in a particles:polymer weight ratio of more
than about 85:15.
[0010] Herein, a "metallic ink for solvent-cast 3D printing" is an ink that
is useful for manufacturing a 3D printed
metallic material by solvent-cast 3D-printing.
[0011] As noted above, the ink comprises the particles and the polymer in a
certain weight ratio range. For
certainty, this weight ratio is expressed as follows: weight ratio = weight of
metallic particles :
weight of polymer. A ratio of 85:15 thus means that the ink comprises 85 wt%
of the particles and 15 wt% of the
polymer, both percentages being based of the total weight of the polymer and
the particles (i.e. excluding the weight
CA 2976782 2017-08-16
7
of the solvent and any other potential additives).
[0012] In embodiments, the particles are present in a carbon
particles:polymer weight ratio of:
= about 86:14, about 87:13, about 88:12, about 89:11, about 90:10, about
91:9, about 92:8, about 93:7, about
94:6, or about 95:5 or more and/or
= about 99:1, about 98:2, about 97:3, about 96:4, about 95:5, about 94:6,
about 93:7, about 92:8, about 91:9,
or about 90:10, or less.
In embodiments, the particles are present in a particles:polymer weight ratio
of about 86:14, about 87:13, about
88:12, about 89:11, about 90:10, about 91:9, about 92:8, about 93:7, about
94:6, about 95:5, about 96:4, about 97:3,
about 98:2, or about 99:1. In preferred embodiments, the particles are present
in a particles:polymer weight ratio
between about 90:10 to about 95:5, more preferably about 95:5.
[0013] Hererin, "heat-sinterable metallic particles" are particles made of
one or more metal or alloy that are
capable of being sintered by heat. Heat sintering is a process of compacting
and forming a solid structure made of a
material by heating without melting the material to the point of liquefaction.
Hence, sintering involves the heating of
the material to a temperature near, but not reaching, its melting point for a
time sufficient for the material to become
compact and form a solid mass. When applied to the present heat-sinterable
metallic particles, sintering results in
the fusion of the particles via "necks" formed between the particles. In other
words, the particles do not coalesce, but
rather peripherally attach to one another.
[0014] Non-limiting examples of heat-sinterable metallic particles include
particles of the metal of groups 3
(including the lanthanides and the actinides) to 16 of the periodic table and
their alloys. Of note, the elements B, C,
Si, Ge, N, P, As, Sb, 0, S, Se, Te, F, Cl, Br, and I, while being part of
groups 12 to 16 of the periodic table are not
metals and thus are not "metals of groups 3 to 16 of the periodic table". More
specific examples of metals and alloys
include steel, cast iron, titanium, silver, copper, zinc, gold, platinum,
aluminum, nickel, bronze, and brass. Preferably,
the heat-sinterable metallic particles are particles of steel or titanium,
more preferably steel. A preferred steel is a
high-alloy steel (HAS), i.e. a steel containing more than about 4 w/w% of
alloyants other than carbon.
[0015] The heat-sinterable metallic particles are preferably
microparticles. Herein, microparticles are particles
between about 0.1 pm and about 200 pm and in size. Preferably, the heat-
sinterable metallic particles have
preferably a size between about 0.1 pm and about 100 pm, more preferably
between about 5 and about 50 pm, and
most preferably with a size of about 20 pm. Typically, smaller particles are
preferred to minimize clogging in fine
nozzles, however they may require higher extrusion pressures during printing.
Of note, when using larger particles,
the nozzle used for 3D printing must have an inner diameter large enough to
accommodate the particles and also the
surface of the 3D printed material may be rougher.
[0016] The heat-sinterable metallic particles can be of any shape regular
or irregular. Preferably, the particles
are spheroidal, and more preferably spherical. Indeed, smooth
spheroidal/spherical shapes tend to minimize the
area/volume ratio, which reduces the friction between the particles and
facilitates the extrusion. Herein, the terms
"spheroidal" and "spherical" are not limited to perfectly spheroidal/spherical
particles, but rather also encompass
particles that present irregularities while being substantially
spheroidal/spherical in shape.
CA 2976782 2017-08-16
8
[0017] The polymer is a polymer that is soluble or that forms a gel,
preferably at room temperature, in the solvent.
In embodiments, the polymer is poly(lactic acid), polystyrene, poly(methyl
acrylate), poly(methyl methacrylate),
poly(n-butyl acrylate), poly(2-hydroxyethyl methacrylate), poly(glycidyl
methacrylate), poly(acrylic acid), poly(N-N-
dimethylacrylamide), poly(1-vinyl anthracene), poly(2-vinyl pyridine), poly(4-
vinyl pyridine), poly(N-vinyl carbazole),
poly(N-vinyl carbazole), poly(N-vinyl imidazole), poly(vinyl benzyl chloride),
poly(4-vinyl benzoic acid), poly(vinyl
acetate), polycaprolactone, poly(11-[4-(4-butylphenylazo)phenoxy]-undecyl
methacrylate) (poly(AzoMA)),
poly(ferrocenyldimethylsilane), polyisoprene, polybutadiene, polyisobutylene,
poly propylene glycol, poly(ethylene
glycol), or a polysaccharide, such as chitosan, or a mixture thereof.
[0018] Polysaccharides, and in particular chitosan, are typically in the
form of a gel in the ink of the present
invention, while the other polymers mentioned above are typically in the form
of solutions.
[0019] In preferred embodiments, the polymer is poly(lactic acid). Herein,
the term "poly(lactic acid)" refers to a
poly(lactic acid) homopolymer or a mixture thereof. The poly(lactic acid)
homopolymers include those derived from
d-lactic acid, I-lactic acid, or a mixture thereof. Poly(lactic acid) is
typically prepared by the catalyzed ring-opening
polymerization of the dimeric cyclic ester of lactic acid, which is referred
to as "lactide." Poly(lactic acid) may also be
made by living organisms such as bacteria or isolated from plant matter that
include corn, sweet potatoes, and the
like. Poly(lactic acid) made by such living organisms may have higher
molecular weights than those made
synthetically. In preferred embodiment, the poly(lactic acid) is that sold
under number PLA 40320 by Natureworks
LLC. This polymer is preferably used in the form of a solution in the ink of
the present invention.
[0020] In alternative preferred embodiments, the polymer is chitosan.
Chitosan is produced commercially by
deacetylation of chitin, which is the structural element in the exoskeleton of
crustaceans (such as crabs and shrimp)
and cell walls of fungi. The degree of deacetylation (%DD) can vary and, in
commercial chitosans, ranges from 60 to
100%. On average, the molecular weight of commercially produced chitosan
ranges from a few thousand to several
hundred thousand Daltons. Chitosan is preferably used in the form of a gel in
the ink of the present invention.
[0021] The solvent may be any volatile solvent capable of dissolving the
polymer or forming a gel of the polymer
as well as being capable of dispersing particles without reacting with the
particles, even at temperatures involved in
sintering of the particles. In preferred embodiments, the solvent is
dichloromethane (DCM), chloroform (CHCI3),
tetrahydrofuran (THF), acetone, methanol (Me0H), ethanol (Et0H) or water.
[0022] In embodiments where the polymer (for example poly(lactic acid)) is
used in the form of a solution, the
solvent is preferably dichloromethane, chloroform, tetrahydrofuran, acetone,
methanol, or ethanol, more preferably
dichloromethane.
[0023] In alternative embodiments where the polymer (for example chitosan)
is used in the form of a gel, the
solvent is preferably water.
[0024] Higher solvent concentrations in the ink yield thinner inks, while
lower concentrations yield thicker inks that
dry more quickly. Thicker inks that dry more quickly tend to retain better
their shape after 30 printing. Hence, a
skilled person will adjust the solvent content to obtain an ink with the
desired performances, i.e. and ink that is thin
enough to be extruded through the nozzle of a 30 printer, while being think
enough to hold its shape after extrusion.
CA 2976782 2017-08-16
9
In embodiments, the ink comprises between about 10 and about 50 w/w% solvent
(based on the total weight of the
ink).
[0025] In embodiments, the ink further comprises one or more additives. Non-
limitative examples of such
additives include:
= glycerol (with a view to conferring flexibility to the 3D printed
structure),
= pigments to change the color of the ink,
= short carbon fibers, fiberglass, and/or boron nitride to change the
mechanical properties of the ink, and/or
= carbon black spheres, graphene, or metal nanowires such as silver,
copper, and/or nickel nanowires to
change the electrical properties of the ink.
[0026] Preferred additives are metal nanowires such as silver, copper,
and/or nickel nanowires.
[0027] Other examples of additives include acids and bases, preferably
acids. Preferably, the acids and bases
are used when water is the solvent for the polymer (preferably chitosan) in
the ink. In such cases, the acids and
bases change the pH and/or the rheological properties (in particular, the
viscosity) of the ink. In particular, acids
decrease both the pH and the viscosity of chitosan hydrogels. The acids and
bases are preferably weak acids and
bases. Weak bases and acids are defined as bases and acids that do not ionize
fully in an aqueous solution.
Typically, weak acids have a pKa between about -2 and about 12, preferably
between about 2 and about 8, and more
preferably between about 3 and about 6.5. Typically, weak bases have a pKb
between about -2 and about 13,
preferably between about -2 and about 2, and the base has more preferably a
pKb of about 0.2 These acids and
bases are preferably organic. These acids and bases are preferably non-toxic.
Non-limiting examples of acids
include acetic acid, lactic acid, citric acid as well as mixtures thereof. A
preferred acid is acetic acid alone or together
with one or more other acids such as lactic acid and/or citric acid.
Preferably, the total acid concentration ranges
from about 40 to about 90 wt% (based on the total weigh of the solvent and the
acid(s)). Preferably, the solvent for
the ink is water and comprises 70 vol% acetic acid alone or together with 10
vol% lactic acid and 3 wt% citric acid,
the vol`)/0 being based on the total volume of the water and acids and the wt%
being based on the total weight of the
water and acids.
[0028] In particular embodiments, the ink comprises a gel of chisotan in
water (hydrogel) containing one or more
non-toxic acids, preferably 70 vol% acetic acid alone or together with 10 vol%
lactic acid and 3 wt% citric acid. In
such cases, the ink could be used to print biomaterials and materials for
biomedical applications as well as any other
electrically conductive materials for which the use of toxic solvents is not
allowed or is undesirable.
3D Printer Ink Cartridge
[0029] In another aspect, the present invention provides a 3D printer ink
cartridge, the cartridge comprising a
container having an ink outlet, the container comprising the ink as described
in the previous section.
[0030] In embodiments, the cartridge is adapted to be installed on a 3D
printer.
[0031] In embodiments, the cartridge is adapted to be fitted to a nozzle
for delivering the ink, so that, for ink
dispensing, the ink is extruded through the ink outlet and through the nozzle.
CA 2976782 2017-08-16
10
[0032] In embodiments, the cartridge is designed so that when a pressure is
applied by a 3D printer, the ink is
extruded through the ink outlet.
Method of Manufacture of an Ink for Solvent-Cast 3D Printing
[0033] In another aspect, the present invention provides a method of
manufacture of the above ink for solvent-
cast 3D printing, the method comprising the steps of:
a) providing a solution or a gel of a polymer in a solvent,
b) providing heat-sinterable metallic particles in a particles:polymer
weight ratio of more than about 85:15,
c) dispersing the particles in the solution or gel of the polymer, thereby
producing the ink.
[0034] In this method, the ink, the polymer, the solvent, the solution or
gel, the particles, their concentrations,
their preferred embodiments, etc. are as described above.
[0035] As the polymer is soluble in the solvent or can form a gel with the
solution, the solution or gel in step a)
can be prepared simply by mixing the polymer in the solvent until the polymer
is dissolved or the gel is formed. In
embodiments, the polymer concentration of this solution or gel is:
= about 2 wt%, about 3 wt%, about 4 wt%, about 5 wt%, about 6 wt%, about 7
wt%, about 8 wt%, about 9
wt%, or about 10 wt% or more and/or
= about 30 wt%, about 35 wt%, about 20 wt%, about 15 wt%, or about 10 wt%
or less,
based on the total weight of the solution or gel. In preferred embodiments
where the polymer is PLA, the polymer
concentration of this solution or gel is about 20 wt%, based on the total
weight of the solution or gel. In preferred
embodiments where the polymer is chitosan, the polymer concentration of this
solution or gel is about 4 wt%, based
on the total weight of the solution or gel.
[0036] The dispersion of the particles in step c) can be effected by any
dispersion technique known to the skilled
person. No matter which dispersion technique is used, it should be carried out
with sufficient energy and for sufficient
time so that particles are dispersed in the solution or gel. In preferred
embodiments, dispersion is achieved ball
milling for example for a few minutes.
[0037] In embodiments, the method further comprises the step of adding
solvent, or removing part (for example
by partial evaporation) of the solvent. This allows adjusting the solvent
content in the ink to achieve a desired ink
viscosity. Preferred solvent contents are as noted in the previous section.
[0038] In embodiments, the method further comprises, the step of adding one
or more additives to the solvent
before it is used to form a solution or gel of the polymer, to the solution or
gel of the polymer or to the ink. The step
at which this additive is added and how it is mixed may vary depending on the
additive. In preferred embodiment,
the additive is added to the solution or gel of polymer before step c), and
mixed in the ink during step c). In
alternative embodiments, the additive is mixed into the ink after step c).
When the additive is a base or acid, it is
preferably added to the solvent before it is used to produce the solution or
gel of the polymer (i.e. prior to step a)).
[0039] In embodiments, the method further comprises, the step of packaging
the ink in a 3D printer ink cartridge.
CA 2976782 2017-08-16
11
Method of Manufacturing a Solvent-Cast Metallic 30 Printed Material
[0040] In another aspect, the present invention provides a method of
manufacturing a solvent-cast metallic 3D
printed material, the method comprising the steps of:
a) providing the above described metallic ink for solvent-cast 3D printing
containing a polymer and heat-
sinterable metallic particles;
b) using a 3D printer, extruding the ink through a nozzle into a controlled
pattern;
c) allowing solvent evaporation, thereby producing a printed material;
d) removing the polymer from the printed material by heating the printed
material to a polymer degradation
temperature or above, thereby leaving the particles arranged into the
controlled pattern; and
e) heat-sintering the particles, thereby producing the solvent-cast metallic
3D printed material.
[0041] Herein, a "controlled pattern" refers to a pattern with a controlled
morphology, such as that obtained by 3D
printing from a model. Controlled patterns do not include random pattern such
as those obtained by simple
extrusion, electrospinning or other such methods. However, controlled patterns
include porous patterns, fully-filled
patterns, interlocked patterns and overhung patterns as well as patterns
involving so-called freeform printing, i.e.
patterns including one or more structures printed in the vertical direction
with no adjacent supporting layers (e.g. a
column). The controlled pattern is typically a layered pattern.
[0042] In embodiments of this method, providing step a) includes the method
of manufacture of a solvent-cast 3D
printing ink described in the previous section.
[0043] The speed of the extrusion (in step b)) depends on many interrelated
ink- and printer-related factors.
These factors include the inner diameter of the nozzle, the applied pressure,
the displacement speed of the nozzle,
the volatility of the solvent, particle concentration, and the viscosity of
the ink. For any given ink and desired nozzle
diameter, the remaining printer-related factors are adjusted to allow
successful deposition into the desired pattern.
Exemplary 3D printing conditions include:
= an applied pressure between about 0.2 and about 4.2 MPa,
= a displacement speed of the nozzle ranging from about 0.3 to about 10
mm/sec, and/or
= an inner diameter of the nozzle ranging from about 100 pm to about 410
pm.
[0044] In embodiments, step b) is advantageously carried out at about room
temperature.
[0045] It is to be understood that solvent evaporation (in step c)
typically begins as soon as the ink is extruded
out of the nozzle in step b). In embodiments, the solvent has completely
evaporated from 3D printed material before
heating in step d), i.e. step c) is entirely carried out at about room
temperature. In other embodiments, some residual
solvent evaporates from the 3D printed material during the heating involved in
step d), i.e. part of step c) is carried
out at about room temperature and then the remaining of step c) is carried at
a temperature above room
temperature.
[0046] Step c) results in solid particles/polymer composite material
disposed in the controlled pattern.
[0047] In step d), the polymer is removed from the 3D printed material by
heating the material at or above the
polymer degradation temperature. The polymer degradation temperature will vary
depending on the nature of the
CA 2976782 2017-08-16
12
polymer. The polymer degradation temperature of a polymer is a well-known to
skilled persons and readily available
or, failing that, can be easily determined by heating the polymer and
observing the temperature at which it degrades.
For example, the polymer degradation of PLA is about 225 C.
[0048] In step e), the particles (resulting from step d)) are heat-
sintered. In other words, the particles are heated
to a sintering temperature for a time sufficient for the particles to
peripherally become attached to one another thus
forming a solid mass. The sintering temperature is a temperature at which
sintering occurs. It will vary according to
the nature of the particles. The sintering temperature of a metal or alloy is
well-known to skilled persons and readily
available or, failing that, can be easily determined by heating the metal or
alloy and observing the temperature at
which sintering is observed.
[0049] Typically, the sintering temperature is a temperature approaching,
but not reaching, the melting point of
the particles. For example, the sintering temperature may be as low as about
400 C below the melting point of the
particles or as high as about 200 C below the melting point. The sintering
time (i.e. the length of time at which the
materiel is held at the sintering temperature) will be adjusted according to
the desired properties for the solvent-cast
metallic 3D printed material produced. If a more porous material is desired,
especially in view of carrying out optional
step f) below, the sintering time may be shorter, for example from about 30
minutes to about 6 h, preferably about lh.
If a more robust material is desired, the sintering time may be longer, for
example from about 6 h to about 12 h,
preferably about 6h.
[0050] It should be noted the polymer degradation temperature is lower than
the sintering temperature. Also, if
desired, steps d) and e) can advantageously be performed by a single heat
treatment in which the temperature is
increased to sintering temperature (thus passing the polymer degradation
temperature) and then held at the sintering
temperature. Typically, there is no need to stabilize the temperature and/or
hold the temperature at or around the
polymer degradation temperature. In embodiments, the heating rate will vary
during the heat treatment, for example
being slower up until about the polymer degradation temperature is reached and
being faster afterwards until the
sintering temperature is reached. For example, the heating rate up to about
the polymer degradation temperature or
above (e.g. up to about 100 C above the polymer degradation temperature) may
be from about 1 to about 5 C/min,
preferably about 1 C/min. The heating rate thereafter, up to the sintering
temperature may be from about 7 to about
15 C/min, preferably about 10 C/min. Then, the sintering temperature may be
held for the sintering time discussed
above.
[0051] After step c), the printed material comprises the particles and the
polymer arranged into the controlled
pattern (potentially together with one or more additives as described above).
The removal of the polymer in step d)
results a material comprises the particles arranged into the controlled
pattern (potentially together with one or more
of the additives that have not been removed by the heating). After step e),
similarly to after step d), the material
comprises the particles arranged into the controlled pattern (potentially
together with one or more of the additives that
have not been removed by the heating). However, the particles are now
peripherally attached to one another thus
forming a solid structure and thus yielding the desired solvent-cast metallic
3D printed material. As noted above, the
particles do no coalesce. Therefore, pores created in-between the particles by
the removal of the polymer will
CA 2976782 2017-08-16
13
remain in the solvent-cast metallic 3D printed material resulting from step
e).
[0052] Some shrinkage will typically be observed during step d) because the
polymer is removed and the
particles settle. However, this step nevertheless results in a material
arranged in the same controlled pattern as
steps b) and c) ¨ for example see Fig. 1. Typically, little shrinkage is
observed during step e).
[0053] In embodiments, the method further comprises the step f) of partly
or completely filling the pores created
in-between the particles by the removal of the polymer with a second metal or
alloy. As such, step f) results in a
metal/metal composite. The second metal or alloy has melting point lower than
the melting point of the metal or alloy
of the particles. Non-limiting examples of the second metal or alloy include
metal of groups 3 (including the
lanthanides and the actinides) to 16 of the periodic table and their alloys.
Of note, the elements B, C, Si, Ge, N, P,
As, Sb, 0, S, Se, Te, F, Cl, Br, and I, while being part of groups 12 to 16 of
the periodic table are not metals and thus
are not "metals of groups 3 to 16 of the periodic table". More specific
examples of metals and alloys include steel,
cast iron, titanium, silver, copper, zinc, gold, platinum, aluminum, nickel,
bronze and brass, preferably copper. The
pores created in-between the particles by the removal of the polymer can be
filled with the second metal or alloy, for
example, by contacting part of the solvent-cast metallic 3D printed material,
for example an end, edge or side
thereof, with the second metal or alloy, the second metal or alloy being in
the molten state, and allowing the molten
second metal or alloy to diffuse by capillarity into the pores of the
material. Preferably, the molten second metal or
alloy is placed on top of the solvent-cast metallic 3D printed material and
the gravity force eases the diffusion. This
can be achieved by placing a piece of the second metal or alloy on top of the
solvent-cast metallic 3D printed
material and then heating the assemblage at a temperature above the melting
point of the second metal or alloy.
Preferably, the quantity of second metal or alloy contacted with the solvent-
cast metallic 3D printed material can be
calculated from the porosity and volume of the solvent-cast metallic 3D
printed material and the desired filling level.
When fully filling the pores, the quantity of second metal or alloy can be
slightly in excess (for example about 10 vol%
in excess) of the calculated quantity of second metal or alloy needed to fully
filled the pores.
[0054] Interestingly, the above diffusion method allows filling the pores
of the above-mentioned pores without
significantly filing the macroscopic voids within the solvent-cast metallic 3D
printed material. For example, if two
filaments have been printed adjacent to one another, the pores in-between the
particles within each filament will be
partly or completely filed, but the space between the two filaments will not
be significantly filed ¨ see Fig. 1.
However, sharp corners near interconnecting filaments will then to be filled.
[0055] As noted above, the pores may be completely or partially filled by
the second metal or alloy. Pores that
are more filled result in denser, less porous, metal/metal composite material.
[0056] Steps d), e) and f) can be carried out in air. In embodiments, steps
d), e) and f) are preferably carried out
in an atmosphere that is inert toward (i.e. does not react with) the heated
materials. This can be advantageous when
the metal or alloy when heated reacts with air, which may results in oxidation
of the material or even explosions.
Alternatively, a reactive atmosphere could be used to alter the material (i.e.
the metal or alloy of the particles and/or
the second metal or alloy) in a desirable manner.
CA 2976782 2017-08-16
14
Solvent-Cast Metallic 3D Printed Material
[0057] In another aspect, the present invention provides a solvent-cast
metallic 3D printed material. This material
has been manufactured by the above described method using the above described
ink. Therefore, the above
teachings regarding the metal(s), optional additive(s), etc., including
preferred embodiments thereof, apply to the
material described below.
[0058] This material is shaped into a controlled pattern (described above).
[0059] This material is made of particles of a metal or alloy (described
above) peripherally attached to one
another.
[0060] In embodiments, pores in-between the particles are completely or
partially filled with a second metal or
alloy (described above).
Potential Advantages of the Invention
[0061] In one or more embodiments or aspects, the present invention may
present one or more of the following
advantages.
[0062] The easy-to-implement method of the invention enables lower-cost 3D
printing (compared to SLS and
SLM).
[0063] The invention allows 3D printing of highly dense metallic structures
featuring complex geometries. It can
be used to manufacture high-performance metallic parts.
[0064] The method is highly flexible and various complex 3D structures,
including fully-filled, porous, interlocked
and overhung structures, can be fabricated.
[0065] The method of the invention can be used with diverse metallic
materials.
[0066] The method of the invention involves 3D printing at room
temperature.
[0067] The method of the invention does not involve complex and expensive
equipment. It is cheap and safe.
[0068] The method of the invention can be applied to fabricate small sized
devices (e.g. the artificial bones for
human) in the medical field, benefiting from its high resolution and ability
to fabricate complex microstructures with
desirable mechanical properties.
[0069] In addition, the method is also suitable to fabricate conductive
porous microstructures serving as
electromagnetic shields in the microwave field.
Definitions
[0070] The use of the terms "a" and "an" and "the" and similar referents in
the context of describing the invention
(especially in the context of the following claims) are to be construed to
cover both the singular and the plural, unless
otherwise indicated herein or clearly contradicted by context.
[0071] The terms "comprising", "having', "including', and "containing" are
to be construed as open-ended terms
(i.e., meaning "including, but not limited to") unless otherwise noted.
[0072] Recitation of ranges of values herein are merely intended to serve
as a shorthand method of referring
CA 2976782 2017-08-16
15
individually to each separate value falling within the range, unless otherwise
indicated herein, and each separate
value is incorporated into the specification as if it were individually
recited herein. All subsets of values within the
ranges are also incorporated into the specification as if they were
individually recited herein.
[0073] All methods described herein can be performed in any suitable order
unless otherwise indicated herein or
otherwise clearly contradicted by context.
[0074] The use of any and all examples, or exemplary language (e.g., "such
as") provided herein, is intended
merely to better illuminate the invention and does not pose a limitation on
the scope of the invention unless otherwise
claimed.
[0075] No language in the specification should be construed as indicating
any non-claimed element as essential
to the practice of the invention.
[0076] Herein, the term "about' has its ordinary meaning. In embodiments,
it may mean plus or minus 10% or
plus or minus 5% of the numerical value qualified.
[0077] Unless otherwise defined, all technical and scientific terms used
herein have the same meaning as
commonly understood by one of ordinary skill in the art to which this
invention belongs.
[0078] Other objects, advantages and features of the present invention will
become more apparent upon reading
of the following non-restrictive description of specific embodiments thereof,
given by way of example only with
reference to the accompanying drawings.
DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
[0079] The present invention is illustrated in further details by the
following non-limiting examples.
Example 1 - Solvent-cast based Metal 3D Printing and Secondary Metallic
Infiltration
Summary
[0080] We developed a method to fabricate dense metallic structures by
combining a room temperature 3D
printing and subsequent heat-treatments: sintering and secondary metallic
infiltration. The high flexibility of this
method enabled the fabrication of customized 3D structures, such as fully-
filled, porous, interlocked and overhung
structures. These geometries were printed using a highly concentrated metallic
ink (metallic load up to 98 wt.%)
consisting of highly alloyed steel (HAS) microparticles, polylactic acid (PLA)
and dichloromethane (DCM). In order to
improve the mechanical properties and the electrical conductivity, the as-
printed structures were sintered and
infiltrated by copper in a furnace protected by a mixture of H2 and Ar. The
filament porosity of the copper infiltrated
samples was as low as 0.2%. Mechanical testing and electrical conductivity
measurement on the copper infiltrated
structures reveal that the Young's modulus reached up to -195 GPa and the
electrical conductivity was as high as
1.42 x 106 S/m.
Introduction
CA 2976782 2017-08-16
16
[0081] We describe herein the solvent-cast 3D printing of metallic inks at
room temperature, followed by sintering
and secondary infiltration (see Fig. 1). To demonstrate this concept, a
metallic ink was prepared by mixing spherical
highly alloyed steel (HAS) microparticles, PLA and DCM. In the printing
process (Fig. la), an extrusion device was
employed to extrude the metallic ink. This device included a pressure
dispensing system, a micronozzle and a
syringe barrel that contains the metallic ink. It was mounted on the moving
head of a computer controlled 3-axis
positioning stage, in order to deposit the extruded ink filament layer by
layer on a substrate to create 3D structures.
Right after the ink filament was extruded, the solvent in the metallic ink
evaporated rapidly at room temperature and
the filament became solid. The filament layer was then used as a support for
subsequent filament layers to create a
HAS/PLA composite multi-layer scaffold. In the as-printed scaffold (Fig. 1b),
PLA served as a binder holding the HAS
microparticles together. To directly connect the HAS particles, the polymer
binder was removed by heating the
printed scaffold in a furnace above the polymer degradation temperature. The
temperature was then rapidly raised to
slightly below the melting point of the HAS. At this temperature, sintering
occurred by creating necking links between
neighboring particles. As time elapsed, the necks growth effectively reduced
the size and the number of the pores
within the metallic continuum. Porosity can be reduced to obtain a strong and
conductive filament structure.
However, the sintering process was halted at a porosity favorable for melted
copper infiltration (Fig. 1c). For copper
infiltration, the sintered scaffold was heated again with a piece of copper
(Cubond IP C-437 infiltration copper) placed
on top of it. The melted copper filled the pores within the filament driven
mainly by capillary forces. Melted copper
flowed through the pores within filaments to obtain a highly dense metal/metal
composite.
Experimental
Materials
[0082] 2g of PLA (4032D, Natureworks LLC, glass transition temperature Tg
=50-60 C) was dissolved in 8g of
DCM (Sigma-Aldrich, boiling point=39.6 C) to prepare polymer solutions. After
resting for 24h, the solutions were
sonicated in an ultrasonic bath (Ultrasonic cleaner 8891, Cole-Parmer) for 5
minutes. The metallic inks were
prepared by mixing the polymer solution and HAS microparticles using a ball
mill mixer (8000M Mixer/Mill, SPEX
SamplePrep) for 5 minutes.
3D printing
[0083] The inks were loaded into 3 cc syringes (EFD) attached to a smooth-
flow tapered nozzle (exit inner
diameter = 250 m, EFD). Loaded syringes were mounted on a pressure dispensing
system (HP-7X, EFD), which
was placed on a computer controlled 3-axis positioning stage (I&J2200-4, I&J
Fisnar). The ink was printed on a glass
slide (PN 16004-422, VWR). The scaffolds, tensile bars and Olympic stadium
were printed at a speed of 10 mm/s
and under a pressure around 0.7 MPa using the 95 wt.% concentrated ink. These
structures were designed by a
computer aided design software (i.e. CATIA) and sliced into several layers by
a slicing software (i.e. Cura). Each was
filled by a filament path and the filament path was interpreted to G-code.
Then, the G-code was converted by a
customized python program into a point-to-point program that can be recognized
by the JR Points software to control
the positioning moving stage.
CA 2976782 2017-08-16
17
Sintering and copper infiltration
[0084] The as-printed samples were sintered and copper infiltrated in a
laboratory electric tubular furnace (59256-
P-COM, Lindberg) using a ceramic substrate. A gas mixture of 2.5% H2/ 97.5% Ar
(flow rate=5 ft,/h) was circulated
inside the quartz tube to prevent the oxidation of the samples. The
temperature profiles used during the sintering and
copper infiltration are provided in Fig. 2. Debinding started from 25 C to
300 C with a heating rate of 60 C/h. Then
the temperature was raised up to 1165 C with a heating rate of 600 C/h and
held at 1165 C for 6h for sintering. For
copper infiltration, the temperature was raised up to 1120 C and held for 0.5
h, then cooled down to the room
temperature.
Porosity analysis
[0085] Each sintered and copper infiltrated scaffold was sealed in a resin
(EpoFix Resin, Struers) block before
polishing. The scaffold was polished until reaching 0.2H, 0.5H and 0.8H of the
printed structure (H being the initial
height of the scaffold). The polished cross sections were observed under an
optical microscope (Zeiss Axioplan EL-
Einsatz). For each cross section, five images were taken at different areas of
the cross section and analyzed by an
image analyzing software (Clemex, ST-2000). The porosity was calculated as the
ratio of voids area over the filament
area in the polished cross sections.
Tensile tests
[0086] The tensile bars were printed with 95 wt.% ink and 250 gm tapered
nozzle, of which the average size of
the cross section of the narrow part was -4.0 x 1.8 mm. The filaments were
oriented 00/900 to the tensile direction,
i.e. the filaments from same layer were parallel, while adjacent layers were
orthogonal. The tensile tests were carried
out on a MTS Insight machine with a 50 kN load cell (MTS 569332-01) at a
crosshead speed of 1 mm/min and using
an extensometer (MTS 632.26, C-20). Three specimens for each sample type were
tested.
Electrical conductivity test
[0087] The test samples used for the electrical conductivity measurements
are sintered and copper infiltrated
rods having typical dimensions of 30x1x1 mm. They are printed with the 95 wt.%
ink and the 250 jim tapered nozzle.
The current values of 1 A, 2 A and 3 A is provided by an EMS 150-33-D-RSTL
power supply. A NI 6211 device is
employed for the current and voltage acquisition. The control of the power
supply and the logging of test data are
carried out via a Lab VIEW program. Three specimens of each sample type were
tested.
Results and discussion
Metallic ink recipe
[0088] The HAS powder particles used in this work were fine (5 20 pm) and
had a spherical shape (see Fig. 3).
The PLA solution served as lubrication to assist the extrusion of the HAS
microparticles through the micronozzle. The
solvent was DCM as it efficiently dissolves PLA and rapidly evaporates at room
temperature.
[0089] The HAS particle concentration within the ink affected the
properties of the fabricated structures. To
investigate its influence on as-printed, sintered and copper infiltrated
structures, 20-layer scaffolds were printed using
CA 2976782 2017-08-16
18
HAS/PLA inks with four different concentrations of HAS particles: 85, 90, 95
and 98 wt.%. The inks were named
according to the metal particles weight percentage in the as-printed structure
(solid state with no solvent). In the inks,
the PLA/DCM weight ratio was 1:4, except for 98 wt.% ink, which was 1:9 (see
Table 1 for detailed compositions).
Table 1. Ink formulations created for 3D printing
Ink constituent 85 wt.% (47 vol. /0) 90 wt.% (59 vol. /0) 95 wt.% (75
vol.c/o) 98 wt.% (90 vol.%)
HAS/PLA ink HAS/PLA ink HAS/PLA ink HAS/PLA ink
_____________________ [g1 ________ [g] _______ [g] [gL
PLA 15 10 5 2
DCM 60 40 20 18
HAS microparticles 85 90 95 98
[0090] The 85 wt.% ink contained the highest amount of solvent, and thus
took more time to evaporate. As a
result, the ink transition from liquid to solid state was slower. This was
detrimental to the shape retention of the
extruded filament. The surface of the scaffold printed with the 98 wt.% ink
was the roughest due to the low amount of
PLA (see Fig. 4). The HAS microparticles were covered and bonded by the
polymer. The filaments of all four
scaffolds aligned well. However, as mentioned above the 85 wt.% scaffold
distorted and the surface of the 98 wt.%
scaffold was rough.
[0091] During sintering, 85 wt.% ink printed scaffolds collapse while the
higher concentrated ink successfully
preserved their shape during the sintering (Figs. 5 and 6). After sintering,
the polymer was burned away and the HAS
microparticles were sintered together. The filaments of 90, 95 and 98 wt.%
scaffold keep their shapes and aligned
well, while the 85 wt.% ink printed scaffold collapsed during sintering and
the surface of 98 wt.% scaffold was rough.
Melted copper infiltrated into the sintered filaments. Some excessed copper
was left on the top of the scaffold.
[0092] Since low concentration inks contain more polymer, the structure had
higher risk to flow and collapse
during the heating and removing the polymer binder. Hence, the 95 wt.% ink was
selected for further testing because
it appeared to be the best compromise between having a highly concentrated ink
for dense sintered structures while
ensuring smooth extrusion and low deformation during the solvent evaporation
printing.
3D printing of metallic inks
[0093] Fig. 7 shows various 3D structures fabricated using the above
solvent-cast 3D printing method with the 95
wt.% ink at a printing speed of 10 mm/s, including tensile bars, 20-layer
scaffolds, a planetary gear and a small-scale
replica of Montreal's Olympic stadium. The high flexibility of the technique
enabled the fabrication of customized
structures, such as fully-filled (Fig. 7a), porous (Fig. 7b), interlocked
(Fig. 7c), and overhung structures (Fig. 7d).
[0094] Fig. 7a displays the printing process of a tensile bar. The ink
uniformly and continuously flowed through
the micronozzle under a constant pressure. No significant shape distortion was
observed. The inset of Fig. 7a shows
several scaffolds and the tensile bar samples next to a Canadian dollar
(diameter = 26.5 mm). The fabrication time
for the 20-layer scaffold and the fully-filly tensile bar were -2 min and 30
min, respectively.
[0095] Fig. 7b shows optical images of a 20-layer scaffold from top, side,
and oblique views, respectively. The
CA 2976782 2017-08-16
19
center-to-center distance between neighboring filaments was 0.5 mm and the
layer thickness was 0.2 mm. It was
observed that the top layer of filaments perpendicularly stacked on the
previous layer, by which all subsequent layers
were completely overlapped (from top view). The creation of the complete
overlap implied the accuracy of the
printing. The side view optical image of the structure further shows good
layer superposition. It illustrates,
additionally, that the adjacent layers cohere together.
[0096] A planetary gear consisting of 1 sun gear, 1 ring gear and 4 planet
gears was fabricated. The planetary
gear is shown in Fig. 7c next to a Canadian 5-cent coin (diameter = 21.2 mm).
The diameter of the ring gear was 30
mm. All the parts of the planetary gear were simultaneously printed and the
printed structure required no assembly.
[0097] There is an overhung structure in the replica of the Olympic stadium
showed in Fig. 7d. The overhang part
was printed without any additional supporting structures, and it did not bend
or deform after printing (tower inclination
of -45 ).
Sintering and secondary metallic infiltration
[0098] To improve the mechanical properties and electrical conductivity,
the as-printed metal/polymer composite
structures were converted to metal and metal composites by sintering and
copper infiltration. These heat-treatments
were carried out in a laboratory electrical furnace. The heating and cooling
rates were adjusted according to the
temperature profiles (shown in Fig. 2). To prevent oxidation of the samples
during the heat-treatments, a mixture of
H2 and Ar continuously flowed inside the quartz tube of the furnace.
[0099] Fig. 8 presents an optical and SEM images of the as-printed,
sintered and copper infiltrated 20-layer
scaffolds printed with 95 wt.% ink and 250 pm tapered nozzle. The three types
of scaffolds were placed next to each
other on a Canadian dollar. The as-printed scaffold had a dark gray color
which turned to light gray after sintering.
The sintered scaffold shrunk to - 84.9% 0.6% of the initial size, because
the PLA was removed and the HAS
particles were brought closer together. The copper infiltrated scaffold turned
to brown red and its final dimension was
reduced to - 88.6% 0.8% of the as-printed scaffold. The shrinkage observed
for the copper infiltrated was slightly
less compared to the sintered one probably due to either the shorter duration
of the sintering or the extra thickness of
copper. No oxidation was observed on the surface of the three scaffolds. No
significant distortion happened to the
scaffolds after sintering and copper infiltration. The SEM images of the as-
printed filaments showed HAS particles
were covered and linked together by the PLA binder (Fig. 8a and Fig. 1b).
[00100] Before sintering, a debinding step was required to remove the PLA
within the structure. The degradation
temperature of the PLA in the samples was around 225 C, which was
investigated by thermogravimetric analysis
(TGA) on 95 wt.% HAS/PLA sample. The temperature was raised from 20 C to 500
C at a rate of 1 C/min (the
same heating rate as the sintering process). As can be seen in Fig. 9, the
degradation of PLA finished before at
temperature of 225 C was reached. The debinding temperature was thus set at
300 C to ensure the complete
degradation of PLA.
[00101] After debinding, the structure integrity was held by the friction
forces between the HAS microparticles. The
low heating rate (60 C/h) facilitated the PLA to fully degrade, and prevented
the structures from collapsing due to the
rapid disappearance of PLA binder. After debinding, the temperature was raised
to 1165 C and maintained for 6h.
CA 2976782 2017-08-16
20
This sintering temperature was set slightly lower than the melting point of
HAS for the particles to connect through
the apparition of necks between them. The size and amount of the necks
increased gradually, which densified the
sintered structure and reduced the pores. A long duration of 6 hours was set
to ensure adequate sintering of the HAS
particles and obtain a denser structure. In the sintered scaffold (Fig. lc and
Fig. 8b), the PLA was completely gone
and the HAS particles directly connected with each other through the necks.
[00102] If the structure was prepared for copper infiltration, the sintering
duration at 1165 C was limited to 1 hour.
This shorter sintering duration left more pores for melted copper to flow
through the filaments. A piece of copper was
placed on top of the sintered structures inside the furnace (Fig. 1c). The
furnace was heated at 1120 C (see Fig. 2).
This temperature was higher than the melting point of copper (1085 C), but
lower than the previously used sintering
temperature. The amount of the copper was calculated by the porosity and the
volume of the filaments in the sintered
scaffolds. 10 vol.% extra copper was added to ensure that all the pores within
the filaments were filled. Mainly driven
by capillary forces, the melted copper filled the pores within the filaments
of the structure and the sharp corners near
connecting filaments (Fig. 8c).
Porosity analysis of sintered and copper infiltrated samples
[00103] The filament porosity of sintered and copper infiltrated structures
affects the mechanical properties of the
structures. To investigate the porosity, each scaffold was polished until
reaching three different height positions of
0.2H, 0.5H, and 0.8H (H being the initial .height of the scaffold). Fig. 10
shows a scheme of polishing positions,
representative optical microscope images of the polished cross sections, and
the average value of porosities of the
sintered (6h) and copper infiltration scaffolds. The first three columns
represent the porosities of 90, 95, and 98 wt.%
sintered scaffolds, which were 10.4% 4.4%, 12.1% 5.3% and 12.4% 2.0%,
respectively. The porosities of 90,
95 and 98 wt.% copper infiltrated scaffolds were extremely low and ranged from
0.2% to 0.3%. These are presented
as the three columns on the right. This data is also shown in Table 2.
Table 2. Porosity analysis results of 90, 95 and 98 wt.% sintered (6h) and
copper infiltrated 20-layer scaffolds.
Porosity 90 wt.% 95 wt.% 98 wt.%
Sintered 10.4 % 4.4%
12.1 % 5.3% 12.4 % 2.0%
Copper infiltration 0.3 % 0.3% 0.2 % 0.1% 0.2 % 0.2%
[00104] The microscope image of the polished cross section for each type of
scaffold is displayed above each of
the column. In the sectional images of sintered scaffolds, we observe that the
copper only filled the pores inside the
filament, while no large amount copper was outside the filament. Thus, the
infiltration flow was limited within the
porous filaments rather than in the empty space between filaments (i.e.
interfilamentous pores). The pores had
similar sizes and were uniformly distributed within the filament. It is noted
that the pores in 98 wt.% sintered scaffold
were greater in quantity but smaller in size compared to the others, which
resulted in the smaller error bars. The
difference of measured porosities among the three types of sintered scaffolds
was lower than 2% despite their
different metal to polymer ratios. This can be explained by the densification
of the structure at the debinding stage.
CA 2976782 2017-08-16
21
The polymer was removed and the adjacent HAS particles were brought to similar
distances before the sintering was
completed. For the copper infiltrated scaffolds, it is observed from the
microscope images that the melted copper
almost completely filled the porous HAS filaments.
Electrical properties of sintered and copper infiltrated samples
[00105] To assess the electrical properties of fabricated structures, the
conductivities of sintered and copper
infiltrated rods were measured using the four-point probe technique. The
conductivity of the sintered samples was
(6.24 0.18) x 106 S/m, which is 45% of that of the bulk stainless steel (1.4
x 106 S/m 39. The relatively lower value
is attributed to the pores in the sintered structures. The copper infiltrated
sample had a conductivity of (1.42 0.32) x
106 S/m. As copper is more conductive than steel, the conductivity of the
sample was further improved after
infiltration with copper.
Mechanical characterization of as-printed, sintered and copper infiltrated
samples
[00106] Tensile tests were carried out on as-printed, sintered and copper
infiltrated tensile bars to evaluate their
mechanical properties. Fig. 11 shows the tensile curves, optical images of
representative tested bars and SEM
observations of the tensile fracture surfaces. The Young's modulus E, Ultimate
Tensile Strength (UTS) and
Elongation (%) were determined from the tensile curves presented in Fig. 11a.
See Table 3 for more details on the
tensile results.
Table 3. Tensile test results of 95 wt.% as-printed, sintered and copper
infiltrated tensile bars compared with: (1)
Wrought stainless stee138, (2) Nitrogen alloyed, high strength, medium
elongation, sintered at 1290 C (2350 F) in
dissociated ammonia39, (3) PM steel containing 0.8% carbon and 2% copper39,
and (4) Copper infiltrated steel
containing 0.8% carbon39.
UTS Elongation
[GPal [MPa] [%]
As-printed sample 3.1 0.3
28.0 3.0 1.45 0.10
Sintered sample 196 16 485
70 0.47 0.06
Copper infiltrated sample 195 16 511
57 0.77 0.07
Wrought stainless steel: SS-316 (1) 193 515 30
PM steel stainless steel: SS-316N2-38 (2) 140 480 13
PM steel FC-0208-60 (3) 155 520 <1
Cu infiltrated PM steel: FX-2008-60 (4) 145 550 1
[00107] The E modulus increased (by -63 times) from 3.1 GPa for the as-printed
bars to 196 GPa and 195 GPa
after sintering and copper infiltration, respectively. The stiffness achieved
is an indication that sintering and copper
infiltration are effective and that strong metallic bonds between individual
particles are created. The high modulus of
the 6h sintered bars was attributed to the dense HAS microstructure. Besides,
the copper infiltrated bars were only
sintered for 1h creating a porous and more compliant HAS microstructure.
However, since the pores were
subsequently filled with copper, the effective modulus raised to the same
level as the sintered bars. The E modulus
obtained after sintering and copper infiltration was similar to those of
wrought and cast steels.39 The UTS also
CA 2976782 2017-08-16
22
increased (by 17- 18 times) from 28 MPa for the as-printed bars to 485 MPa and
511 MPa after sintering and copper
infiltration, respectively. These UTS values are similar to those obtained for
carbon steels, stainless steels, tools
steels and highly-alloyed cast irons.39 The rather low ductility of the
sintered and copper infiltrated tensile bars was
partly explained by the composition of the HAS material. The large volume
fraction of carbides results in elongation
at break lower than 1 %. Such elongation values are also typical of some tool
steels, highly alloyed cast irons
(elongation 1 ¨ 10%)39 and many Powder Metallurgy (PM) steel parts (0¨ 3y0)40.
[00108] The SEM images of tensile fracture surfaces (Fig. 11b, c) show the
internal structure of the sintered and
copper infiltrated tensile bars. The tensile fracture always occurred at the
interface of the filaments oriented
transverse to the tensile directions, as the stress was maximal at this point.
This also explains the lower ef value
compared to bulk stainless steel (68.2%). The high magnification SEM images
(Fig. 11d, e) reveal the details of
tensile fracture surface. There were visible pores, under SEM, within the
filaments of sintered tensile bars. In
addition, the filament from adjacent layers are firmly bound, while some
filaments from the same layer are detached.
This is the main reason why the ductility of the samples is lower compared to
the bulk stainless steel.
Conclusions
[00109] In summary, we develop a method of fabrication of fully-dense 3D
metallic structures consisting of the
solvent-cast 3D printing and the following heat-treatments. The Young's
modulus of fabricated structures was up to
195 GPa and approached typical values for similar bulk material while the
conductivity was 1.62 x 106 S/m, which is
superior to the structures fabricated by most commercial 3D printing
techniques. 41-43
Example 2 ¨ Inks containing Chitosan
[00110] In a manner similar to Example 1, we produced an ink containing
chitosan (90% deacetylated, weight
average molecular weight = 207 kDa, from Biolog in Germany) as the polymer
instead of PLA. The chitosan (CHI)
was provided as a gel in water comprising with 80 vol/vol% acetic acid (AA),
the % being based on the total volume
of the water.
[00111] The ink comprised 0.8g CHI per 10mL AA aqueous solution and comprised
the HAS microparticles of
Example in a HAS:CHI solution weight ratio of 6.5: 1.
[00112] These inks were successfully solvent-cast 3D printed with an applied
pressure of 0.6 to 1.2 MPa, a
platform speed of 5 mm/s using nozzles of 200 and 250 microns.
Example 3 ¨ Inks containing copper particles
[00113] In a manner similar to Example 1, we produced inks containing 20pm
copper particles instead of HAS
particles.
[00114] The inks comprised 20 w/e/0 of PLA/solvent (DCM) and 90 w/w% of the
copper particles.
[00115] These inks were successfully solvent-cast 3D printed using anapplied
pressure of 0.7¨ 1.4 MPa, a speed
of 10 mm/s, and a nozzle of 250 microns.
[00116] The polymer was then removed from the 3D printed material, which was
then successfully sintered.
CA 2976782 2017-08-16
23
[00117] The scope of the claims should not be limited by the preferred
embodiments set forth in the examples, but
should be given the broadest interpretation consistent with the description as
a whole.
CA 2976782 2017-08-16
24
REFERENCES
[00118] The present description refers to a number of documents, the content
of which is herein incorporated by
reference in their entirety. These documents include, but are not limited to,
the following:
1. M. Elahinia, N. S. Moghaddam, M. T. Andani, A. Amerinatanzi, B. A. Bimber,
R. F. Hamilton, Prog. Mater.
Sc., 2016, 83, 630-663.
2. D. Hong, D. T. Chou, 0. I. Velikokhatnyi, A. Roy, B. Lee, I. Swink, I.
lssaev, H. A. Kuhn, P. N. Kumta, Acta
Biomater., 2016,45, 375-386.
3. S. Dadbakhsh, M. Speirs, H. J. Van, J. P. Kruth, MRS Bull., 2016,
41(10), 765-774.
4. J. Hu, M. F. Yu, Science, 2010, 329(5989), 313-316.
5. C. Ladd, J. H. So, J. Muth, M. D. Dickey, Adv Mater, 2013, 25(36), 5081-
5085.
6. Y. S. Rim, S. H. Bae, H. Chen, N. De Marco, Y. Yang, Adv Mater, 2016,
DOI: 10.1002/adma.201505118
7. B. Y. Ahn, E. B. Duoss, M. J. Motala, X. Guo, S. I. Park, Y. Xiong, J.
Yoon, R. G. Nuzzo, J. A. Rogers, J. A.
Lewis, Science, 2009, 323(5921), 1590-1593.
8. J. Lessing, A. C. Glavan, S. B. Walker, C. Keplinger, J. A. Lewis, G. M.
Whitesides, Adv. Mater., 2014,
26(27), 4677-4682.
9. M. G. Mohammed, R. Kramer, Adv. Mater., 2017, 29(19), DOI:
10.1002/adma.201604965
10. L. Thijs, K. Kempen, J. P. Kruth, J. Van Humbeeck, Acta Mater., 2013,
61(5), 1809-1819.
11. S. Hong, J. Yeo, G. Kim, D. Kim, H. Lee, J. Kwon, H. Lee, P. Lee, S. H.
Ko, ACS Nano, 2013, 7(6), 5024-
5031.
12. D. D. Gu, W. Meiners, K. Wissenbach, R. Poprawe, Int. Mater. Rev., 2012,
57(3), 133-164.
13. L. Thijs, F. Verhaeghe, T. Craeghs, J. Van Humbeeck, J. P. Kruth, Acta
Mater., 2010, 58(9), 3303-3312.
14. D. Gu, Y. C. Hagedorn, W. Meiners, G. Meng, R. J. S. Batista, K.
Wissenbach, R. Poprawe, Acta Mater.,
2012, 60(9), 3849-3860.
15. S. Van Bael, Y. C. Chai, S. Truscello, M. Moesen, G. Kerckhofs, H. Van
Oosterwyck, J. P. Kruth, J.
Schrooten, Acta Biomater., 2012, 8(7), 2824-2834.
16. K. McAleal, Materials and applications for the SLS selective laser
sintering process. Proceedings of the 7th
International Conference on Rapid Prototyping, 1997, pp 23-33
17. V. Seyda, N. Kaufmann, C. Emmelmann, Phys. Procedia, 2012, 39, 425-431.
18. H. Gong, K. Rafi, H. Gu, G. J. Ram, T. Starr, B. Stucker, Mater. Des.,
2015, 86, 545-554.
19. E. 0. T. Olakanmi, R. F. Cochrane, K. W. Dalgarno, Mater. Sci., 2015, 74,
401-477.
20. J. A. Lewis, G. M. Gratson, Mater. Today, 2004, 7(7), 32-39.
21. D. Therriault, S. R. White, J. A. Lewis, Nat. Mater., 2003, 2(4), 265-271.
22. D. Therriault, R. F. Shepherd, S. R. White, J. A. Lewis, Adv. Mater.,
2005, 17(4), 395-399.
23. S. Z. Guo, F. Gosselin, N. Guerin, A. M. Lanouette, M. C. Heuzey, D.
Therriault, Small, 2013, 9(24), 4118-
4122.
CA 2976782 2017-08-16
25
24. K. Chizari, M. A. Daoud, A. R. Ravindran, D. Therriault, Small, 2016,
12(44), 6076-6082.
25. S. Z. Guo, M. C. Heuzey, D. Therriault, Langmuir, 2014, 30(4), 1142-1150.
26. S. Bodkhe, G. Turcot, F. P. Gosselin, D. Therriault, ACS AppL Mater.
Interfaces, 2017, DOI:
10.1021/acsami.7b04095
27. G. Postiglione, G. Natale, G. Griffini, M. Levi, S. Turn, Composites, Part
A, 2015, 76, 110-114.
28. B. G. Compton, J. A. Lewis, Adv. Mater., 2014, 26(34), 5930-5935.
29. B. Y. Ahn, D. Shoji, C. J. Hansen, E. Hong, D. C. Dunand, J. A. Lewis,
Adv. Mater., 2010, 22(20), 2251-
2254.
30. W. J. Hyun, S. Lim, B. Y. Ahn, J. A. Lewis, C. D. Frisbie, L. F. Francis,
ACS AppL Mater. Interfaces, 2015,
7(23), 12619-12624.
31. E. Hong, B. Y. Ahn, D. Shoji, J. A. Lewis, D. C. Dunand, Adv. Eng. Mater.,
2011,13(12), 1122-1127.
32. M. A. Skylar-Scott, S. Gunasekaran, J. A. Lewis, Proc. Natl. Acad. Sc.,
2016, 201525131.
33. L. L. Lebel, B. Aissa, M. A. E. Khakani, D. Therriault, Adv. Mater., 2010,
22(5), 592-596.
34. I. W. Chen, X. H. Wang, Nature, 2000, 404(6774), 168-171.
35. P. Beaulieu, Ph.D Thesis, Polytechnique Montreal, 2012
36. K. I. Winey, R. A. Vaia, MRS bull., 2007, 32(04), 314-322.
37. L. Francis, Aerial view of the Montreal's
Olympic stadium,
http://francislepine.photosheltercomiimaqe/100005ivrD3WYZ28, (accessed May
2017)
38. D. Peckner, Handbook of stainless steels.1977
39. J. R. Davis, K. M. Mills, S. R. Lampman, Metals handbook. Vol. 1.
Properties and selection: Irons, steels,
and high-performance alloys. ASM International, Materials Park, Ohio 44073,
USA, 1990, 1063.
40. R. G. P. I. Molding, I. Pressing, Metal Powder Industries Federation,
Princeton, NJ, 1990, 3-22.
41. M. M. Dewidar, K. A. Khalil, J. K. Lim, Trans. Nonferrous Met. Soc. China,
2007, 17(3), 468-473.
42. M. M. Dewidar, K. W. Dalgarno, C. S. Wright, Proc. Inst. Mech. Eng., Part
B, 2003, 217(12), 1651-1663.
43. K. Kempen, E. Yasa, L. Thijs, J. P. Kruth, J. Van Humbeeck, Phys.
Procedia, 2011, 12, 255-263.
CA 2976782 2017-08-16