Note: Descriptions are shown in the official language in which they were submitted.
84026145
METHOD OF CO-PROCESSING NANOCARBONS IN CARBON BLACK, AND
PRODUCTS THEREFROM
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefits of U.S. Provisional
Application
Nos. 62/133,256 filed on March 13, 2015, and 62/177,212 filed on March 10,
2015.
SUMMARY
[0002] Provided herein is a method of dispersing nanocarbons into
carbon black.
In examples discussed below, nanocarbons, such as nanotubes, graphene,
buckyballs,
nanohorns, etc., and carbon black can be mixed together to facilitate
integration of
nanocarbons and carbon black. The mixture of nanocarbons and carbon black can
assist in dispersion of the nanocarbons into the carbon black, and also assist
in
dispersion of the mixture of the nanocarbons and carbon black within a medium,
such
as an elastomer.
[0003] Also provided herein is a method of dispersing nanocarbons and
carbon
black into a polymer, such as rubber or a thermoplastic. This method can
include pre-
processing the nanocarbons and carbon black into "loosened" aggregates, and
then
combining the loosened aggregates with the polymer. By combining the loosened
aggregates with the polymers, improved properties of the nanocarbon-carbon
black-
polymer product can be achieved.
[0003a] Also provided herein is a method of forming a matrix composition
by:
providing a matrix; providing a co-processed nanocarbon-carbon black
dispersion
consisting essentially of: co-processing nanocarbon agglomerates and carbon
black
agglomerates, comprising: providing nanocarbon agglomerates, wherein
nanocarbons
in the nanocarbon agglomerates have a diameter of less than 50 nm; providing
carbon
black agglomerates; and mixing the nanocarbon agglomerates and the carbon
black
agglomerates such that the nanocarbon agglomerates disperse into: a nanocarbon-
carbon black dispersion with looser agglomerates of nanocarbons and carbon
black; or
a nanocarbon-carbon black dispersion with individualized nanocarbons dispersed
among the carbon black agglomerates; and combining the matrix and the co-
processed
nanocarbons-carbon black dispersion to form the matrix composition.
1
Date Recue/Date Received 2022-04-06
84026145
[0003b] Also provided herein is a method of making a nanocarbon-carbon
black
dispersion, comprising: providing agglomerated nanocarbons; providing carbon
black;
and mixing the agglomerated nanocarbons with carbon black in an essentially
dry state
and applying sufficient shear force for a sufficient time to render the
agglomerate
structure of the nanocarbons unobservable by scanning electron microscope
(SEM) at a
magnification of 100,000x or less.
[0003c] Also provided herein is a method of a dispersion of nanocarbons
in carbon
black, comprising: nanocarbon aggregates with a maximum cross-section or
diameter of
less than 50 nm; and carbon black aggregates, wherein the nanocarbon
aggregates are
dispersed in carbon black aggregates such that individual nanocarbons are
observable
by SEM.
[0003d] Also provided herein is a method of a composition comprising: a
co-
processed material consisting essentially of nanofibers with a diameter of
less than
50 nm and carbon black; and a polymeric matrix material, wherein the co-
processed
material is formed by mixing the nanofibers and carbon black to co-process the
mixture
into individualized nanofibers and/or loosened agglomerates of nanofibers and
carbon
black, and wherein the co-processed material is incorporated within the
polymeric
matrix material.
[0003e] Also provided herein is a method of forming a composition by co-
processing nanocarbon aggregates and carbon black aggregates, comprising:
providing
nanocarbon aggregates with a maximum cross-section or diameter of less than
50nm;
and mixing the nanocarbon aggregates and the carbon black aggregates such that
individual carbon nanotubes release from said nanocarbon aggregates, as
confirmed by
electron microscopy, wherein the co-processing consists essentially of a total
of 100
wt.% of nanocarbon aggregates and carbon black aggregates, wherein
50 wt.% or less nanocarbon aggregates and 50 wt.% or more carbon black
aggregates.
[0003f] Also provided herein is a method of a dispersion of carbon
fibers and
carbon black, comprising: carbon fibers aggregates; and carbon black
aggregates,
wherein the carbon black aggregates disperse carbon fibers in the carbon fiber
aggregates, and wherein each carbon fiber and each carbon fibers aggregate in
the
dispersion consist essentially of carbon nanotubes having a cross-section or a
diameter
of less than 50 nm.
la
Date Recue/Date Received 2022-04-06
84026145
[0003g] Also provided herein is a method of a dispersion of carbon
fibers and
carbon black, comprising: nanocarbon aggregates; and carbon black aggregates,
wherein the carbon black aggregates disperse nanocarbon aggregates in the
carbon
fiber aggregates, wherein the dispersion is free of continuous carbon fibers
with a
diameter of greater than 50 nm, and wherein the nanocarbon aggregates consist
of
multiwalled carbon nanotubes with a diameter less than 50 nm in cross-section
or
diameter.
BRIEF DESCRIPTION OF THE DRAWINGS
[0004] The accompanying drawings, which are incorporated and constitute
a part
of this specification, illustrate example embodiments of the claimed
invention. In the
drawings,
[0005] Figs. 1A-1B are scanning electron microscope (SEM) micrographs
of
carbon nanotubes;
[0006] Figs. 2A-2B are SEM micrographs of carbon black; and
[0007] Figs. 3-16 are SEM micrographs of samples of co-processed
nanocarbons
and carbon black under different conditions.
lb
Date Recue/Date Received 2022-04-06
CA 02979220 2017-09-08
WO 2016/145207
PCT/US2016/021791
DETAILED DESCRIPTION
[0008] The following detailed description refers to the accompanying
drawings. The same reference numbers in different drawings may identify the
same
or similar elements. Also, the following detailed description describes
embodiments
of the invention and is not intended to limit the invention.
[0009] A. Overview
[0010] While not wishing to be bound by any theory, it is believed that
carbon
black within carbon black agglomerates, which has primary particles in the
same size
range as individual nanocarbons in nanocarbon agglomerates, is able to affix
itself
via electrostatic or mechanical forces (because of its irregular structure) to
the
individual nanocarbons. These forces cause the individual nanocarbons to de-
agglomerate from their original nanocarbon aggregates. Once de-agglomerated,
the
individualized nanocarbons are of a particular size that is able to fit within
an
interstitial space between the individual carbon black particles and
agglomerates,
such that the carbon black keeps individual nanocarbons apart from other
individual
nanocarbons. In other words, it is believed that the close contact between the
nanocarbons and carbon black, as well as the shear forces acting within a
small area
provided by physical co-processing, causes the de-agglomeration and
maintenance
of individuality of the individualized nanocarbons.
[0011] B. Nanocarbons
[0012] The term "nanocarbons" is intended to refer to nano-sized carbons,
which can include carbon nanotubes, nanographenic carbons, buckyballs and
nanohorns. Carbon nanotubes are a preferred form of nanocarbon.
[0013] In general, the use of prefix "nano," as used in nanocarbons and
nanographenic carbons, implies that at least one dimension of the material is
less
than 100 nm, and can include material on the size scale of at least one
dimension
being less than 1 micron, less than 0.5 microns, less than 0.2 microns, less
than 100
nm, less than 50 nm, less than 20 nm, or less than 5 nanometers. Nanocarbons
generally also have desirable properties, such as, high surface area and
electrical
conductivity; see, for example, basic properties of carbon nanotubes.
[0014] Nanocarbons can exist in a variety of forms and can be prepared
through the catalytic decomposition of various carbon-containing gases at
metal
2
84026145
surfaces. These include those described in U.S. Patent No. 6,099,965 to
Tennent,
etal. and U.S. Patent No. 5,569,635 to Moy, et al..
[0015] In an embodiment, nanocarbons can be made by catalytic growth from
hydrocarbons or other gaseous carbon compounds, such as CO, mediated by
supported or free floating catalyst particles.
[0016] As produced, nanocarbons may be in the form of discrete
nanocarbons
(i.e., separated individual nanocarbons), aggregates/agglomerates of
nanocarbons
(i.e., dense, entangled nanocarbons), or a mixture of both. Aggregates of
nanocarbons may be dense particulate structures of entangled nanocarbons.
[0017] Aggregates can be formed during the production of nanocarbons,
where the morphology of the aggregate can be influenced by the choice of
catalyst
support. Porous supports with completely random internal texture, e.g., fumed
silica
or fumed alumina, can grow nanocarbons in all directions leading to the
formation of
aggregates.
[0018] As used herein, nanocarbon agglomerates are composed of multiple
nanocarbon aggregates, which adhere to one another or otherwise form a unitary
agglomeration of numerous aggregates. Nanocarbon aggregates can retain their
structure in nanocarbon agglomerates.
[0019] Nanocarbons also differ physically and chemically from other forms
of
carbon such as standard graphite and carbon black. Standard graphite is, by
definition, flat shaped rather than fibrous. Carbon black is an amorphous
structure of
irregular shape, generally characterized by the presence of both sp2 and sp3
bonding. On the other hand, nanocarbons have one or more layers of ordered
graphitic carbon atoms. These differences, among others, make graphite and
carbon black poor predictors of nanocarbon-polymer structure properties.
[0020] One form of nanocarbon is a carbon nanotube. The terms "carbon
nanotube," "fibril," "nanofibers," and "nanotube" are used interchangeably to
refer to
single wall (Le., only a single graphene layer parallel to the nanotube axis)
and/or
multi-wall (i.e., more than one graphene layer more or less parallel to the
nanotube
axis) carbon nanotubes, which may additionally be functionalized or have an
outer
3
Date Recue/Date Received 2022-04-06
84026145
layer of less structured amorphous carbon (note, other forms of nanocarbons
can
also be functionalized if desired).
[0021] Carbon nanotubes have an elongated structure with a cross-section
(e.g., angular fibers having edges) or a diameter (e.g., rounded) of, for
example for
multi-wall carbon nanotubes, less than 100 nm, preferably less than 50 nm,
more
preferably less than 20 nm; or, for example for single wall nanotubes, less
than 5
nanometers. Other types of carbon nanotubes are also known, such as fishbone
fibrils (e.g., wherein the graphene sheets are disposed in a herringbone
pattern with
respect to the nanotube axis), "buckytubes," etc.
[0022] Aggregates of carbon nanotubes may resemble the morphology of bird
nest ("BN"), cotton candy ("CC"), combed yarn ("CY"), open net ("ON"), or
other
conformations. Carbon nanotubes may also be grown on a flat support, attached
by
one end to the support and parallel to each other, forming a "forest"
structure.
[0023] Individual carbon nanotubes in aggregates may be oriented in a
particular direction (e.g., as in "CC," "CY," and "ON" aggregates) or may be
non-
oriented (i.e., randomly oriented in different directions, for example, as in
"BN"
aggregates). "BN" structures may be prepared as disclosed in U.S. Patent No.
5,456,897. "BN" agglomerates are tightly packed with typical densities of
greater
than 0.08 g/cc, for example, 0.12 g/cc. Transmission electron microscopy
("TEM")
reveals no true orientation for carbon nanotubes formed as "BN" agglomerates.
Patents describing processes and catalysts used to produce "BN" agglomerates
include U.S. Patent Nos. 5,707,916 and 5,500,200.
[0024] Figs. lA and 1B are SEM micrographs of carbon nanotubes. As
illustrated in Figs. 1A and 1B, carbon nanotubes (BN type in Fig. 1A and CC
type in
Fig. 1B) show carbon nanotube agglomerate structures. As made carbon nanotube
agglomerates have not been successfully de-agglomerated in a dry state.
Rather,
de-agglomeration in this context indicates either the creation of substantial
numbers
of individualized tubes or the essentially complete absence of the as-made
agglomerates. Even de-agglomeration in a liquid phase can require the use of
4
Date Recue/Date Received 2022-04-06
84026145
substantial energy sources, such as ultrasound. See U.S. Patent No. 5,691,054.
[0025] On the other hand, "CC," "ON," and "CY" agglomerates have lower
density, typically less than 0.1 g/cc, for example, 0.08 g/cc and their TEMs
reveal a
preferred orientation of the nanotubes. U.S. Patent No. 5,456,897 describes
the
production of these oriented agglomerates from catalyst supported on planar
supports. "CY" may also refer generically to aggregates in which the
individual
carbon nanotubes are oriented, with "CC" aggregates being a more specific, low
density form of "CY" aggregates.
[0026] Carbon nanotubes are distinguishable from commercially available
so
called "continuous carbon fibers" (i.e., commercially available, larger than
nanotube-
sized carbon fibers). For instance, the diameter of continuous carbon fibers,
which is
always greater than 1.0 micron and typically 5 to 7 microns, is also far
larger than
that of carbon nanotubes, which is usually less than 1.0 micron. Due to their
smaller
size, carbon nanotubes often have increased conductivity than carbon fibers
for the
same amount provided as additive to polymers.
[0027] Carbon nanotubes, as used herein, may be used in their as-made
agglomerated form, or they may be pre-treated by, for example, mortar and
pestle,
ball mill, rod mill, hammer mill, etc. to reduce the maximum size of the
agglomerates.
Additionally, the as-made nanotubes maybe washed in a strong acid or strong
base
to dissolve any catalyst and support from which the carbon nanotubes are
grown,
such as, for example, phosphoric acid.
[0028] Another form of nanocarbon is nanographenic carbon. The term
"nanographenic carbons" is intended to refer to nano-sized carbons, which can
include carbons having nanoscale and graphenic structure. For example,
nanographenic carbons can include graphite of a nanoscopic scale, but would
not
include graphite of macroscopic scale. One type of nanographenic carbon,
graphene, or graphite nanoparticles, can be described as one or more sheets of
graphitic carbon. For example, graphene can include a single sheet of
graphitic
carbon, or nanoplatelets with a few sheets of carbon. Graphene can be on the
same
order of size as carbon nanotubes, as mentioned above, with a structure having
a
dimension in one direction of less than 1 micron, less than 0.5 microns, less
than 0.2
Date Recue/Date Received 2022-04-06
84026145
microns, less than 100 nm, less than 50 nm, less than 20 nm, or less than 5
nanometers.
[0029] Another form of nanocarbon is a buckyball. Buckyballs, also known
as
buckminsterfullerenes, are carbons arranged in a spherical structure
resembling a
ball. Buckyballs are made of 60 carbon atoms and have a dimension on the order
of
1 to 2 nm.
[0030] Another form of nanocarbon is a nanohorn. Nanohorns are horn-
shaped aggregates of stacks of graphene sheets. Carbon nanotubes, both single
and multi-wall are included within the category of nanohorns, as they are made
of
one or more graphene sheets. Nanohorns also have a structure having a
dimension
in one direction of less than 1 micron, less than 0.5 microns, less than 0.2
microns,
less than 100 nm, less than 50 nm, less than 20 nm, or less than 5 nanometers.
[0031] C. Carbon black
[0032] The term "carbon black" is intended to include a carbon powder
with
carbon aggregates of various sizes. In general, carbon black aggregates can be
difficult to disperse due to its strong attractions between adjacent
particles. Due to
the difficulty in dispersing carbon black particles from carbon black
aggregates,
carbon black particles have been subjected to similar treatment to nanocarbons
for
dispersion, such as shear mixing within a medium, dry shearing, and wet
shearing,
as mentioned above concerning nanocarbons.
[0033] Carbon blacks are named according to an ASTM standard used by all
manufacturers. Carbon blacks can also be characterized by their porosity.
Carbon
black porosity is discussed in Porosity in Carbons, Patrick, J.W. ed., Halsted
Press
1995.
[0034] Discussion of dispersion of carbon black agglomerates can be
found,
for example, in the literature. See Pomchaitawarda etal., "Investigation of
the
dispersion of carbon black agglomerates of various sizes in simple-shear
flows,"
Chem. Eng. Sci. 58 (2003), pp. 1859¨ 1865.
[0035] Figs. 2A and 2B are SEM micrographs of carbon black from different
sources. Fig. 2A is Cabot Sterling 1120 carbon black as supplied from Cabot
Corporation, Boston, MA at 100,000x magnification. Fig. 2B is Continental
Carbon
N330 as supplied from Continental Carbon Company, Houston, TX at 200,000x
6
Date Recue/Date Received 2022-04-06
84026145
magnification. As shown in Fig. 2A and 2B, the carbon black as supplied are
aggregated.
[0036] D. Co-processed Nanocarbons and Carbon Black
[0037] Co-processed nanocarbons and carbon black were prepared at varying
concentrations, as well as using different methods to facilitate co-
processing. By
co-processing nanocarbons and carbon black, dispersion of nanocarbon
aggregates
into carbon black aggregates can be observed. Specifically, co-processing can
result in looser nanocarbon aggregates and individualized nanocarbons, as
further
discussed below.
[0038] In embodiments provided below, compositions of the mixtures of
nanocarbons and carbon black can vary from 0.001 wt.% to 99.999 wt.%
nanocarbons (with 99.999 wt.% to 0.001 wt.% carbon black). For example, 2 wt.%
to 50 wt.% nanocarbons can provide dispersion of nanocarbons in carbon black,
such as 50 wt.% or less nanocarbon aggregates and 50 wt.% or more carbon black
aggregates, 30 wt.% or less nanocarbon aggregates and 70 wt.% or more carbon
black aggregates, or 10 wt.% or less nanocarbon aggregates and 90 wt.% or more
carbon black aggregates. As another example, 5 wt.% to 50 wt.% nanocarbons can
provide individualization of nanocarbons in carbon black.
[0039] In U.S. Patent No. 8,771,630 Wu et al. describe graphene and a
method for the preparation of graphene. In this patent, graphene dispersion is
discussed, as is graphene generally.
[0040] In a paper by Singh, etal., "Polymer-Graphene Nanocomposites:
Preparation, Characterization, Properties, and Applications," Nanocomposites -
New
Trends and Developments, Ebrahimi, F. (Ed.), InTech (2012) Singh etal. further
discuss carbon allotropes, such as graphite, diamond, fullerene, and carbon
nanotube. Singh et al. discuss that "the fabrication of single-layer graphene
is
difficult at ambient temperature ... [because] graphene sheets with a high
surface
area tend to form irreversible agglomerates and restacks to form graphite
through
p-p stacking and Vander Waals interactions." See, p. 38 middle of the first
full
paragraph. Singh etal. further discuss methods of forming graphene. See also
Li, et al., "Processable aqueous dispersions of
7
Date Recue/Date Received 2022-04-06
84026145
graphene nanosheets," Nature Nanotechnology, 3(2), (2008) 101-105; and
Park etal., "Hydrazine-reduction of graphite- and graphene oxide," Carbon 49
(2011) 3019-3023.
[0041] As discussed below, by co-processing nanocarbons in carbon black,
aggregates can be disrupted, and individual nanocarbons can be observed in a
SEM.
[0042] While not wishing to be bound by theory, it is believed that co-
processing of nanocarbons and carbon blacks can lead initially to loosening of
the
nanocarbon aggregates. The loosening of the nanocarbon aggregates can appear
to be "cloud"-like large, loosened aggregates that can be observable in a SEM.
Further processing can convert these loosened aggregates into individual
nanocarbons, which can also be observed in a SEM. These loosened aggregates
can have a nanocarbon to nanocarbon distance greater than that of the starting
material's nanocarbon to nanocarbon distance. For example, in carbon nanotube
loosened aggregates, carbon nanotubes can be separated by a distance of about
10
nanotubes or about 100nm, as observed in the samples discussed below.
[0043] For example, in carbon nanotube aggregates, these "cloud"-like co-
processed carbon nanotube-carbon black may be differentiated from the starting
as-
made carbon nanotube aggregates by the separation of the carbon nanotubes from
other carbon nanotubes within a carbon nanotube-carbon black aggregate.
[0044] Co-processing of nanocarbons and carbon black may be carried out
in
a dry state or a wet state. Dry state co-processing may be preferred as the
process
may require fewer steps due to the addition and removal of liquid. Wet state
co-
processing, on the other hand, may be preferred if the nanocarbons, carbon
black, or
both are provided in wetted form. For example, if carbon nanotubes and carbon
black are provided in wetted form, co-processing in a wet state may require
fewer
steps, and may be preferable.
[0045] In the case of wet pre-processing, the nanocarbon and carbon black
may be added to the liquid in any order or the liquid may be added to the
nanocarbon and carbon black again in any order. The quantity of liquid
employed
may range from 0.10 lbs. to 100 lbs. of liquid per lb. of mixed solids
depending on
8
Date Recue/Date Received 2022-04-06
CA 02979220 2017-09-08
WO 2016/145207
PCT/US2016/021791
the type of pre-processing equipment to be used. Any liquid may be used, but
water
is a preferred liquid. Organic liquids as well as supercritical media, such as
supercritical 002, may also be used. After pre-processing most of the added
liquid
may be readily removed, such as via decanting, from the processed solids.
Final
liquid removal is preferably by volatilizing residual liquid from the solids.
[0046] Dry co-processing can be carried out in any type of equipment or
combination of such equipment used for intimately mixing dry powders, such as
ball
mills, both tumbling and stirred, rod mills, mortar and pestles, Banbury
mixers, two
and three roll mills, Waring blenders and similar stirred equipment, both with
and
without the presence of added media.
[0047] Wet co-processing can employ any of the types of equipment used for
dry pre-processing as well as jet mills, including microfluidizers, and
agitated vessels
of any sort with any type of impeller.
[0048] Depending on the intended use of the nanocarbon-carbon black
mixture the individualization step may take place in the co-processing step
just
described or it may occur in a subsequent compounding step in the presence of
polymer or other material. This compounding step may be carried out in any of
the
known types of equipment used for compounding additives into polymers
including
extruders, such as twin screw extruders and single screw extruders, Banbury
mixers,
Brabender mixers, two and three roll mills, etc.
[0049] Also in embodiments provided below, dispersion methods, such as
physical mixing can be utilized to disperse nanocarbons in carbon black. For
example, mortar and pestle (hand or motorized), shakers (with or without media
added), and tumblers (with or without media) are discussed below in the
embodiments, but other mechanical means can be also be used.
[0050] Table summarizes some examples of co-processing nanocarbons and
carbon blacks. As shown in the Table below, the nanocarbon source and
morphology, the carbon black and morphology, the ratio of nanocarbon to carbon
black, the type of equipment, the mixing parameters, such as time, intensity,
etc.,
can affect the resulting co-processed nanocarbon-carbon black products.
9
CA 02979220 2017-09-08
WO 2016/145207
PCT/US2016/021791
[0051] Table
Sample Figure Composition Device Time
1 Not shown 10% graphene in Motorized mortar and pestle 30 min.
N330 (M&P)
2 Not shown 5% graphene in Steel tumbler with 4 hrs. at
N330 Polyamide (PA) 12 media 120 rpm
3 3 10% Ground CC Shaker 4 hrs.
+ 1120
4 4 10% Ground CC Hand M&P 1 hr.
+ 1120
5 10% CC + 1120 Shaker 1 hr.
6 6 10% BN + 1120 Plastic tumbler with PA 12 4 hrs.
7 7 10% Ground CC "
+ 1120
8 8A, 8B 30% Ground CC Hand M&P 30 min.
+ 1120
9 9 5% BN + 1120 Plastic tumbler with PA 12 "
10 2% BN + 1120 "
Li
11 11 5% CC + N330 "
12 Not shown 5% BN + 1120 Ceramic "rod mill" 2 hrs. at
60 rpm
13 Not shown 10% BN + N330 Steel tumbler with PA 12 4 hrs. at
120 rpm
14 Not shown 10% BN + N330 Teflon tumbler with steel 2 hrs. at
media 120 rpm
12 5% CC + N330 "
Li
16 13 30% BN + N330 "
17 14 10% BN + N330 Waring blender 10 min.
18 15 10% BN + N330 Sample 17, motorized M&P 10 min.
19 Not shown 10% BN + N330 Brabender mixer 1 hr.
16 10% BN + N330 Wet in 3 roll mill 5 passes
CA 02979220 2017-09-08
WO 2016/145207
PCT/US2016/021791
[0052] Sample 1 is formed from 10% graphene in N330 with 0.30g of as-
received xGnP graphene nanoplatelets (Grade M, XG Sciences, Inc.) is mixed
with
2.70g of carbon black N330 (Columbian Chemicals Co.). The mixture is ground
with
a motorized mortar and pestle (Model: Retsch, Brinkmann, Type: RMO) for 30
min.
[0053] Sample 2 is formed from 5% graphene in N330 by mixing 0.10g of as-
received xGnP graphene nanoplatelets (Grade M, XG Sciences, Inc.) with 1.90g
of
carbon black N330 (Columbian Chemicals Co.). The mixture is loaded to a
tumbler
made from steel pipe equipped with a baffle along with lOg of PA12 (polyamide
12)
granules (2-6mm OD) as grinding media and tumbled with a roller (Model: Tru-
Square Metal Products) at 120rpm for 4 hrs.
[0054] Examination of the samples 1 and 2 in a SEM reveals that the
graphene nanoplatelets are well dispersed in carbon black. Individual
nanoplatelets
can be observed in each of samples 1 and 2 within the SEM.
[0055] Sample 3 is formed by combining of 0.10g carbon nanotubes (CC
conformation; previously ground in a Fitzpatrick hammer mill, herein after
referred to
as "ground CC") and 0.90g Cabot Sterling 11 20 carbon black in a stainless
steel
cylinder. The cylinder was shaken at high frequency with a Retsch Brinkmann
Shaker at the setting of 60 for 4 hrs.
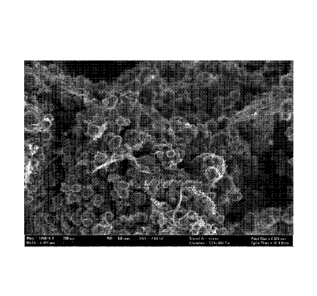
[0056] Fig. 3 is a SEM micrograph of sample 3 at 50,000x magnification
showing numerous individual nanotubes and loosened aggregates. The
agglomerate structure of the carbon black appears to be essentially unchanged.
[0057] Sample 4 is formed by co-processing 0.10g of as made ground carbon
nanotube powder with 0.90g of Cabot Sterling NS 1120 carbon black by hand
grinding with mortar and pestle at room temperature for 1 hr. Samples were
prepared for the SEM by the procedure used in sample 3.
[0058] Fig. 4 is a SEM micrograph of sample 4 at 100,000x magnification of
the co-processed carbon nanotube powder and carbon black. As shown in Fig. 4,
numerous individualized carbon nanotubes are observable because the carbon
nanotubes are dispersed in the carbon black.
11
CA 02979220 2017-09-08
WO 2016/145207
PCT/US2016/021791
[0059] Sample 5 is formed by co-processing 0.1g of as made CC carbon
nanotubes and 0.90g Cabot Sterling 1120 carbon black under the same conditions
(equipment and time) as sample 3.
[0060] Fig. 5 is a SEM micrograph of sample 5 at 100,000x magnification
showing a loosened aggregate that appears to be a "cloud"-like structure over
a half
a micron long, and about 200nm wide.
[0061] Sample 6 is formed by co-processing 0.1g as made BN carbon
nanotubes and 0.90g Cabot Sterling 1120 carbon black in a plastic tumbler with
irregular spherical (2-6mm OD) PA 12 media therein at 120 rpm for 4 hrs.
[0062] Fig. 6 is a SEM micrograph of sample 6 at 100,000x magnification
showing numerous loosened carbon nanotube aggregates.
[0063] Sample 7 is formed by co-processing 0.1g of as made ground CC
carbon nanotubes and 0.90g Cabot Sterling 1120 carbon black under the same
conditions as sample 6.
[0064] Fig. 7 is a SEM micrograph of sample 7 at 50,000x magnification
showing the presence of loosened aggregates with "cloud"-like structures over
a
micron in length.
[0065] Sample 8 is formed by co-processing 0.3g of as made CC carbon
nanotubes and 0.70g Cabot Sterling 1120 carbon black by hand grinding using a
mortar and a pestle for 30 minutes.
[0066] Figs. 8A-8B are SEM micrographs of sample 8 at different
magnifications. As shown in Fig. 8A, numerous loosened aggregates are present
at
50,000x magnification. As shown in Fig. 8B, numerous individual nanotubes are
shown at 100,000x magnification. As before the structure of the carbon black
aggregate seems unchanged.
[0067] Sample 9 is formed by co-processing 0.05g BN carbon nanotubes with
0.95g Cabot Sterling 1120 carbon black, and sample 10 is formed by co-
processing
0.02g BN carbon nanotubes with 0.98g Cabot Sterling 1120 carbon black using a
plastic tumbler with PA12 media therein at 120 rpm for 4 hrs.
[0068] Figs. 9 and 10 are SEM micrographs of samples 9 and 10,
respectively, illustrate individual nanotubes and loosened aggregates at
100,000x
magnification.
12
CA 02979220 2017-09-08
WO 2016/145207
PCT/US2016/021791
[0069] Sample 11 is formed by co-processing 0.10g CC carbon nanotubes
and 1.90g Continental N330 carbon black by hand grinding using a mortar and
pestle for 30 min. Again, individual nanotubes are observed at 100,000x
magnification, as shown in Fig. 11.
[0070] Sample 12 is formed by co-processing 2.50g of as-made BN carbon
nanotubes with 47.50g of Cabot Sterling 1120 carbon black. The mixture is
loaded
in a ceramic jar together with ceramic rods for tumbling at 60 rpm for 2 hrs
(the
volume of the small marshmallow-like rods is about half of the jar volume).
Shortened nanotubes are observed (not shown).
[0071] Sample 13 is co-processed by tumbling with a steel pipe equipped
with
a baffle. A mixture of 2.0g 10 wt.% BN in carbon black N330 (Columbian) was
charged into the pipe along with lOg of PA12 granules. The tumbler was rolled
at
120 rpm for 4 hours. The SEM image of sample 13, which is not provided,
displayed
a similar set of individual nanotubes as those of Samples 9 and 10 (Figs. 9
and 10)
with no agglomerates observed.
[0072] Sample 14 is co-processed by loading 2.0g of 10 wt.% BN in carbon
black N330 was charged to a Teflon bottle along with stainless steel balls
(1/8' OD)
(the volume of the steel ball is about 50% of the bottle volume) and tumbled
at 120
rpm for 2 hrs. At 100,000x magnification, the presence of individual tubes and
no
agglomerates was observed similar to Figs. 9 and 10, and thus are not
provided.
The carbon black aggregate structure appeared unchanged.
[0073] Sample 15 is co-processed from 0.05g CC carbon nanotubes and
0.95g of carbon black N330 (Columbian) by mixing together in a mortar and
pestle
for 30 minutes.
[0074] Fig. 12 is a SEM micrograph of sample 15 at 198,000x magnification
shows the presence of both individual nanotubes and loosened aggregate
structures
approaching a micron in length. The carbon black aggregate structure appears
unchanged.
[0075] Sample 16 is co-processed from 0.3g of BN carbon nanotubes and
0.7g of carbon black N330 by hand grinding the combination in a mortar and
pestle
for 30 minutes.
13
CA 02979220 2017-09-08
WO 2016/145207
PCT/US2016/021791
[0076] Fig. 13 is a
SEM micrograph of sample 16 at 200,000x magnification.
Individualized nanotubes are prominent and no aggregates are seen. Carbon
black
aggregate structure appears unchanged.
[0077] Sample 17 is
co-processed by adding 5g of as made BN powder and
45g of N330 carbon black to a Waring Blender (Model: Blender 7012G, made by
Waring Commercial, Torrington, CT) and processing at the lowest speed for 10
minutes. Density was measured as 0.39 g/cc.
[0078] Fig. 14 is a
SEM micrograph of sample 17 at 100,000x magnification
and shows individualized carbon nanotubes in a carbon black aggregate
structure,
which appears unchanged.
[0079] Sample 18
was co-processed by first processing 5g of as made BN
powder and 45 g of N330 carbon black together in a Waring blender for 10
minutes
at low speed (as was done in sample 17). The resulting mixture was then
transferred to a motorized mortar and pestle and further processed for 10, 20
and 30
minutes to finalize the co-processed material. Tap density falls in 10 minutes
to 0.31
g/cc. at 20 minutes of processing a sample is withdrawn and prepared for
microscopy as previously.
[0080] Fig. 15 is a
SEM micrograph of sample 18 after 20 minutes of further
processing at 200,000x magnification showing the presence of individual
nanotubes
in a carbon black aggregate structure, which appears unchanged.
[0081] Sample 19
was co-processed by lightly mixing with a spatula 10 wt.%
as made BN nanotubes and 90wt. /0 N330 carbon black. 19 g of the mixture is
transferred to the twin screw mixing head of a Brabender mixer (Model: Plasti-
Corder
DR-2052-K13, manufactured by C.W. Brabender Instruments, Inc., So. Hackensack,
NJ) designed to simulate the action of a Banbury-type mixer. The mixture is
processed at 100 rpm for one hour. Tap density falls to 0.28 g/cc. Both
individualized nanotubes and loosened aggregates are observed. As sample 19
appeared similar to sample 18 (Fig. 14), sample 19's SEM micrograph is not
provided.
[0082] Sample 20 is
prepared by mixing 5.0g of the 10 wt.% BN/N330 mix
prepared in sample 19 above with 7g of deionized water to form a wet paste
material. The wet paste material is passed five times through a three roll
mill (Model:
14
CA 02979220 2017-09-08
WO 2016/145207
PCT/US2016/021791
Keith 27502, manufactured by Keith Machinery Corp., Lindenhurst, NY), which
results in a thin film, which in turn is dried at 100 C in vacuum oven.
[0083] Fiq. 16 is a SEM micrograph of sample 20 at 100,000x magnification,
which shows the presence of individual nanotubes.
[0084] As shown herein, co-processed nanocarbons and carbon black can
provide loosened aggregates and/or individualized nanocarbons, such as
individualized carbon nanotubes. These loosened aggregates and/or
individualized
nanocarbons can be used to provide improved dispersion of nanocarbons and/or
carbon black in a matrix, such as polymers or other materials. Suitable matrix
materials include polymers, both organic and inorganic, metals, ceramics, and
other
non-polymer matrices, such as asphalt, cement, or glass. Example polymers
include
thermosets, such as vulcanizeable rubber, polyurethanes, epoxy resins,
polyimides,
etc., or thermoplastics, such as polyolefins, acrylics, nylons,
polycarbonates, etc.
Combining polymers with the co-processed nanocarbons and carbon black can
provide improved properties in polymers, such as improved modulus, elongation,
etc.
[0085] One of ordinary skill in the art would expect the presence of
individualized nanotubes and the absence of large tight aggregates to lead to
composites with improved properties, such as tensile modulus, toughness,
hardness,
durometer, resistance to explosive delamination, tear resistance, relaxation
time,
etc., without sacrifice of elongation to break.
[0086] In addition to the co-processed nanocarbons and carbon black in a
matrix, additional additives, such as inert fillers and active agents, can
also be
provided. For example, inert fillers, such as glass, pumice, etc., and/or
active
agents, such as vulcanization activators, release agents, antioxidants, inks
or other
colorants, etc., can be added to the co-processed nanocarbons and carbon black
in
a matrix before, during, or after the nanocarbon-carbon black loosened
aggregates
are added to the matrix.
[0087] While the invention has been described in detail with reference to
preferred embodiments thereof, it will be apparent to those skilled in the art
that
variations and modifications can be made, and equivalents employed without
departing from the scope of the appended claims.