Note: Descriptions are shown in the official language in which they were submitted.
CA 02979971 2017-09-15
- 1 -
DESCRIPTION
SUSTAINED-RELEASE PHARMACEUTICAL COMPOSITION
[TECHNICAL FIELD]
[0001] The present invention relates to an ophthalmic depot preparation
comprising
benzyl benzoate and/or benzyl alcohol, and polyethylene glycol and/or
dimethylsulfoxide, wherein the volume ratio of benzyl benzoate and/or benzyl
alcohol
to polyethylene glycol and/or dimethylsulfoxide in the ophthalmic depot
preparation
is 75:25 to 25:75, and the total amount of benzyl benzoate and/or benzyl
alcohol and
polyethylene glycol and/or dimethylsulfoxide contained is 50%(w/w) or more.
[0002] The present invention relates to an ophthalmic depot preparation
further
containing a drug, and the drug is contained in the form of a compound
represented by
formula (1):
[Chemical Formula 1]
0
NS H
1
(1)
R2
N
wherein
RI represents a hydrogen atom, halogen atom, hydroxyl group, C1_6 alkyl
group, C16 alkyl group substituted with one or more halogen atoms, C1-6 alkoxy
group,
or C1_6 alkoxy group substituted with one or more halogen atoms, and
R2 represents a hydrogen atom, C1-6 alkyl group, C1_6 alkylcarbonyl group,
or C1_6 alkylcarbonyl group substituted with one or more hydroxyl groups,
or a salt thereof.
LBACKGROUND ART'
[0003] For example, an invasive pharmaceutical agent such as an intravitreal
injection preparation is preferably a preparation in which a drug is gradually
released
from an injected site after having been administered into the body and
subsequently
demonstrates a pharmacological effect over a long period of time from the
viewpoint
CA 02979971 2017-09-15
- 2 -
of reducing the burden on the patient resulting from administration of the
pharmaceutical agent. A known means for realizing this is a preparation in
which a
depot is formed at a site where a pharmaceutical agent is injected and a drug
is
gradually released from that site.
[0004] Non-Patent Document 1 discloses an in situ forming implant that
contains
poly(lactic-co-glycolic acid) (PLGA), and benzyl benzoate, benzyl alcohol,
PEG400,
DMSO or a mixture thereof as a solvent, and granisetron hydrochloride as a
drug, and
describes an examination of the release of the granisetron hydrochloride in a
phosphate buffer. However, Non-Patent Document 1 does not describe an in situ
forming implant that does not contain poly(lactic co-glycolic acid) (PLGA),
and the
use of the in situ forming implant described in Non-Patent Document 1 in the
field of
ophthalmology is also not described.
[0005] Patent Document 1 discloses a preparation that contains PLGA, and
sucrose
acetate isobutyrate (SAIB), and further benzyl benzoate, benzyl alcohol, DMSO
or a
mixture thereof. However, although Patent Document 1 describes a depot
preparation, it does not describe a depot preparation that does not contain
PLGA and
SAIB, and the use of the depot preparation in field of ophthalmology is not
described
or suggested.
[0006] Patent Document 2 discloses a sustained-release preparation for
intramuscular administration that contains DMSO as a solvent, caprylic/capric
triglyceride (Myglyol 812) as an oily mixture, and benzyl benzoate, benzyl
alcohol or
a mixture thereof as a sustained-release component, and a fulvestrant as a
drug.
However, Patent Document 2 does not describe a sustained-release preparation
that
does not contain Myglycol 812, and the use of that sustained-release
preparation in the
field of ophthalmology is also not described.
[0007] These documents do not describe an ophthalmic depot preparation
containing benzyl benzoate and/or benzyl alcohol and polyethylene glycol
and/or
dimethylsulfoxide, wherein the volume ratio of benzyl benzoate and/or benzyl
alcohol
to polyethylene glycol and/or dimethylsulfoxide in the ophthalmic depot
preparation
is 75:25 to 25:75, and the total amount of benzyl benzoate and/or benzyl
alcohol and
polyethylene glycol and/or dimethylsulfoxide contained is 50%(w/w) or more. In
addition, it is also not described that the ophthalmic depot preparation
demonstrates a
pharmacological effect over a long period of time by gradually releasing a
drug by
forming similar depots regardless of the administration site or conditions
around that
site.
[Prior Art Documents]
CA 02979971 2017-09-15
=
=
¨ 3 ¨
[Patent Documents]
[0008] [Patent Document 1] International Publication No. WO 2008/143992
[Patent Document 2] International Publication No. WO 2013/153559
[Non-Patent Documents]
[0009] [Non-Patent Document 1] Turk. J. Pharm. Sci., 7(1), 2010, 49-56
[SUMMARY OF INVENTION]
[TECHNICAL PROBLEM]
[0010] A depot preparation is a preparation which, after administration into a
body,
forms a depot containing a drug and continuously releases the drug. In some
cases,
the formed depot has a randomly unstable shape. Even in such a case, the
preparation
may be used for continuously releasing the drug. However, in order to more
stably
achieve sustained release properties, it is preferred that the formed depot
has a
spherically stable shape. Further, the inventors of the present invention
found that in
a preparation in which a pharmaceutical agent forms a depot, the shape of the
depot
formed differently depending on the difference in administration site or
conditions
around that site (such as in the vitreous body and in the anterior chamber, or
conditions around them), and that the preparation might have no sustained
release
properties when administered into a certain site. An object of the present
invention is
to provide an ophthalmic depot preparation that gradually releases a drug
after having
been administered into the body, the ophthalmic depot preparation being
capable of
similarly forming a depot regardless of the administration site or conditions
around
that site and stably achieving sustained release of the drug.
[SOLUTION TO PROBLEM]
[0011] As a result of extensive studies on solvents that dissolve drugs in
order to
solve the aforementioned problems, the inventors of the present invention
found that,
an ophthalmic depot preparation containing benzyl benzoate and/or benzyl
alcohol
and polyethylene glycol and/or dimethylsulfoxide, wherein the volume ratio of
benzyl
benzoate and/or benzyl alcohol to polyethylene glycol and/or dimethylsulfoxide
in the
ophthalmic depot preparation is 75:25 to 25:75, and the total amount of benzyl
benzoate and/or benzyl alcohol and polyethylene glycol and/or
dimethylsulfoxide
contained is 50')/0(w/w) or more, gradually releases a drug by forming similar
depots
regardless of the administration site or conditions around that site, thereby
leading to
completion of the present invention.
[0012] Namely, the present invention relates to that indicated below.
[0013] (1) An ophthalmic depot preparation containing benzyl benzoate and/or
CA 02979971 2017-09-15
- 4 -
benzyl alcohol and polyethylene glycol and/or dimethylsulfoxide, wherein
the volume ratio of benzyl benzoate and/or benzyl alcohol to polyethylene
glycol and/or dimethylsulfoxide in the ophthalmic depot preparation is 75:25
to 25:75,
and
the total amount of benzyl benzoate and/or benzyl alcohol and polyethylene
glycol and/or dimethylsulfoxide contained is 50%(w/w) or more.
[0014] (2) The ophthalmic depot preparation described in (1) above, further
containing a drug.
[0015] (3) The ophthalmic depot preparation described in (2) above, wherein
the
drug is a compound represented by formula (1):
[Chemical Formula 2]
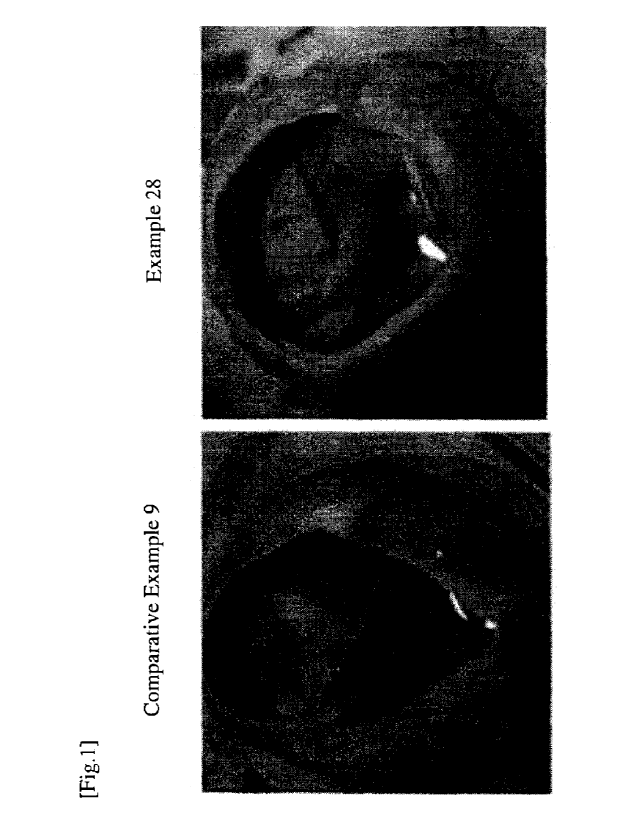
-HR1
(1)
-R2
N
wherein,
RI represents a hydrogen atom, halogen atom, hydroxyl group, C1_6 alkyl
group, C1-6 alkyl group substituted with one or more halogen atoms, C1_6
alkoxy group,
or Ci_6 alkoxy group substituted with one or more halogen atoms, and
R2 represents a hydrogen atom, C1-6 alkyl group, C1_6 alkylcarbonyl group,
or C1_6 alkylcarbonyl group substituted with one or more hydroxyl groups,
or a salt thereof.
[0016] (4) The ophthalmic depot preparation described in (3) above, wherein in
the
formula (1)
RI represents a C1_6 alkoxy group or C1_6 alkoxy group substituted with one
or more halogen atoms, and
R2 represents a C1-6 alkylcarbonyl group or C1-6 alkylcarbonyl group
substituted with one or more hydroxyl groups.
[0017] (5) The ophthalmic depot preparation described in (3) above, wherein in
the
formula (1)
RI represents a C1-6 alkoxy group substituted with one or more halogen
atoms, and
CA 02979971 2017-09-15
¨ 5 ¨
R2 represents a C1-6 alkylcarbonyl group substituted with one or more
hydroxyl groups.
[0018] (6) The ophthalmic depot preparation described in (3) above, wherein
the
compound represented by formula (1) is 2-[[[2-[(hydroxyacetyl)amino]-4-
pyridinyl]methyl]thio]-N-[4-(trifluoromethoxy)pheny1]-3-pyridinecarboxamide or
a
salt thereof.
[0019] (7) The ophthalmic depot preparation described in any one of (1) to (6)
above, wherein the average molecular weight of the polyethylene glycol is
within the
range of 90 to 2200.
[0020] (8) The ophthalmic depot preparation described in any one of (1) to (7)
above, wherein the polyethylene glycol is a polyethylene glycol selected from
the
group consisting of PEG400, PEG600, PEG800 and PEG1000.
[0021] (9) The ophthalmic depot preparation described in any one of (1) to (8)
above, wherein the volume ratio of benzyl benzoate and/or benzyl alcohol to
polyethylene glycol and/or dimethylsulfoxide is 60:40 to 35:65.
[0022] (10) The ophthalmic depot preparation described in any one of (1) to
(8)
above, wherein the volume ratio of benzyl benzoate and/or benzyl alcohol to
polyethylene glycol and/or dimethylsulfoxide is 50:50 to 40:60.
[0023] (11) The ophthalmic depot preparation described in any one of (1)
to (10)
above, wherein the total amount of benzyl benzoate and/or benzyl alcohol and
polyethylene glycol and/or dimethylsulfoxide contained is 80%(w/w) or more.
[0024] (12) The ophthalmic depot preparation described in any one of (1)
to (11)
above, wherein the total amount of benzyl benzoate and/or benzyl alcohol
contained is
25`)/0(w/w) to 60%(w/w).
[0025] (13) The ophthalmic depot preparation described in any one of (1) to
(12)
above, wherein the total amount of polyethylene glycol and/or
dimethylsulfoxide
contained is 30%(w/w) to 62%(w/w).
[0026] (14) The ophthalmic depot preparation described in any one of (2) to
(13)
above, which further comprises tocopherol or derivatives thereof.
[0027] (15) The ophthalmic depot preparation described in (14) above, wherein
the
amount of tocopherol or derivatives thereof contained is 0.001%(w/v) to
10%(w/v).
[0028] (16) The ophthalmic depot preparation described in any one of (1) to
(15)
above, which is for administration into the vitreous body or anterior chamber.
[0029] (17) The ophthalmic depot preparation described in any one of (2) to
(16)
above, wherein the drug is contained at 0.001(1/0(w/v) to 30%(w/v).
[0030]
CA 02979971 2017-09-15
- 6 -
(18) The ophthalmic depot preparation described in any one of (2) to (17)
above, which is for prevention and/or treatment of an eye disease.
[0031] (19) The ophthalmic depot preparation described in any one of (1)
to (18)
above, which does not contain poly(lactic-co-glycolic acid) (PLGA).
[0032] (20) The ophthalmic depot preparation described in (1) to (19) above,
which
does not contain a medium-chain triglyceride.
[0033] (21) The ophthalmic depot preparation described in any one of (1) to
(20)
above, which does not contain sucrose acetate isobutyrate (SAIB).
[0034] (22) The ophthalmic depot preparation described in any one of (2) to
(21)
above, which substantially only contains a drug, benzyl benzoate, polyethylene
glycol
and tocopherol or derivatives thereof.
[0035] (23) The ophthalmic depot preparation described in any one of (2) to
(21)
above, which substantially only contains a drug, benzyl benzoate,
dimethylsulfoxide
and tocopherol or derivatices thereof.
[0036] (24) The ophthalmic depot preparation described in any one of (2) to
(21)
above, which substantially only contains a drug, benzyl benzoate and
polyethylene
glycol.
[0037] (25) The ophthalmic depot preparation described in any one of (2) to
(21)
above, which substantially only contains a drug, benzyl benzoate and
dimethylsulfoxide.
[0038] (26) The ophthalmic depot preparation described in (16) above, wherein
the
eye disease is age-related macular degeneration, diabetic retinopathy,
retinopathy of
prematurity, retinal vein occlusion, retinal artery occlusion, polypoidal
choroidal
vasculopathy, retinal angiomatous proliferation, myopic choroidal
neovascularization,
diabetic macular edema, ocular tumor, radiation retinopathy, rubeosis iridis,
neovascular glaucoma or proliferative vitreoretinopathy (PVR), primary open-
angle
glaucoma, secondary open-angle glaucoma, normal tension glaucoma,
hypersecretion
glaucoma, primary closed-angle glaucoma, secondary closed-angle glaucoma,
plateau
iris glaucoma, combined mechanism glaucoma, developmental glaucoma,
corticosteroid glaucoma, exfoliation glaucoma, amyloidotic glaucoma,
neovascular
glaucoma, malignant glaucoma, capsular glaucoma, plateau iris syndrome or high
tension glaucoma.
[0039] (27) The ophthalmic depot preparation described in any one of (1) to
(26)
above, wherein 1 pi, to 1000 }IL is administered at one time.
[0040] (28) The ophthalmic depot preparation described in any one of (1) to
(27)
above, which is administered at an interval of once a week to once every three
years.
CA 02979971 2017-09-15
- 7 -
[0041] (29) The ophthalmic depot preparation described in any one of (1) to
(28)
above, which is for sustained drug release.
[0042] (30) The ophthalmic depot preparation according to any one of (1) to
(29)
above, which is contained in a syringe made of glass, cycloolefin polymer,
polyolefin
or polycarbonate.
[0043] (31) The ophthalmic depot preparation according to any one of (1) to
(29)
above, which is contained in a syringe made of glass, cycloolefin polymer or
polypropylene.
[0044] (32) A method in which a drug contained in an ophthalmic depot
preparation is stabilized by incorporating tocopherol or derivatives thereof
in said
ophthalmic depot preparation,
said ophthalmic depot preparation comprising:
benzyl benzoate and/or benzyl alcohol, and
polyethylene glycol and/or dimethylsulfoxide, wherein,
the volume ratio of benzyl benzoate and/or benzyl alcohol to polyethylene
glycol and/or dimethylsulfoxide in the ophthalmic depot preparation is 75:25
to 25:75,
and
the total amount of benzyl benzoate and/or benzyl alcohol and polyethylene
glycol and/or dimethylsulfoxide contained is 50%(w/w) or more.
[0045] Furthermore, each of the configurations of (1) to (32) above can be
combined by arbitrarily selecting two or more configurations.
[ADVANTAGEOUS EFFECTS OF INVENTION]
[0046] The ophthalmic depot preparation of the present invention is an
ophthalmic
depot preparation that gradually releases a drug after being administered into
the body,
and demonstrates a pharmacological effect over a long period of time by
gradually
and stably releasing the drug by forming similar depots regardless of the
administration site or conditions around that site. Moreover, the ophthalmic
depot
preparation of the present invention has adequate safety as a pharmaceutical.
[BRIEF DESCRIPTION OF DRAWING]
[0047]
[Fig. 1] Photographs showing the shapes of depos formed by the ophthalmic
depot
preparation of the present invention administered into the vitreous body of a
rabbit.
[DESCRIPTION OF EMBODIMENTS]
[0048] The following provides a detailed explanation of the present invention.
[0049] With respect to the ophthalmic depot preparation of the present
invention, a
depot preparation is a preparation for continuously releasing a drug, which
preparation
CA 02979971 2017-09-15
- 8 -
forms depot (mass) after the admenistration into a body or the like. There is
no
particular limitation with respect to the status of the depot preparation and
the
preparation may be in a dissolved state or suspended state, but it is
preferred that the
preparation is in a dissolved state.
[0050] There are no particular limitations on the drug contained in the
ophthalmic
depot preparation of the present invention, and specific examples thereof
include
tyrosine kinase inhibitors such as Tafetinib, SIM-817378, ACTB-1003,
Chiauranib,
CT-53608, Cinnamon, chim4G8-SDIE, CEP-5214, IMC-1C11, CEP-7055, 34542-
[N-(2-Methoxyethyl)-N-methylamino] ethoxy]-1H-indo1-2-yliquinolin-2(1H)-one,
hF4-3C5, ZK-CDK, IMC-EB10, LS-104, CYC-116, OSI-930, PF-337210, JNJ-
26483327, SSR-106462, R-1530, PRS-050, TG-02, SC-71710, SB-1578, AMG-191,
AMG-820, Sulfatinib, Lucitanib hydrochloride, JNJ-28312141, Ilorasertib, PLX-
5622,
ARRY-382, TAS-115, Tanibirumab, Henatanib, LY-2457546, PLX-7486, FPA-008,
NVP-AEE-788, cgi-1842, RAF-265, MK-2461, SG-00529, Rebastinib, Golvatinib,
Roniciclib, BVT-II, X-82, XV-615, KD-020, Lestaurtinib, Delphinidin,
Semaxanib,
Vatalanib, OSI-632, Telatinib, Alacizumab pegol, ATN-224, Tivozanib, XL-999,
Icrucumab, Foretinib, Crenolanib besylate, R-406, Brivanib, Pegdinetanib, TG-
100572, Olaratumab, Fostamatinib disodium, BMS-690514, AT-9283, MGCD-265,
Quizartinib, ENMD-981693, Famitinib, Anlotinib, Tovetumab, PLX-3397,
Fruquintinib, (-)-Epigallocatechin, Midostaurin, NSC-706456, Orantinib,
Cediranib,
Dovitinib, XL-647, Motesanib, Linifanib, Brivanib, Cediranib, Apatinib,
Fedratinib,
Pacritinib, Ramucirumab, Intedanib, Masitinib, Elemene, Dihydroartemisinin, WS-
1442, Itraconazole, Leflunomide, Dihydroartemisinin, Imatinib, Sorafenib,
Sunitinib,
Dasatinib, Pazopanib, Vandetanib, Axitinib, Regoarfenib, Caboazantinib and
Ponatinib, steroids such as hydrocortisone, triamcinolone, fluocinolone,
dexamethasone and betamethasone, prostaglandin derivatives such as isopropyl
unoprostone, latanoprost, bimatoprost and travoprost, immunosuppressants such
as
cyclosporine, sirolimus and FK506, antiallergic drugs such as azelastine, non-
steroidal
anti-inflammatory drugs such as indomethacin, bromfenac and diclofenac,
angiogenesis inhibitors such as pazopanib, SU5416, lapatanib, ranibizumab and
bevacizumab, circulation ameliorants such as nicardipine and nitrendipine,
antioxidants such as vitamin E, carbonic anhydrase inhibitors such as
acetazolamide
and brinzolamide, 13-receptor blockers such as timolol and carteolol, visual
cycle
modulators such as vitamin A derivatives, neurotrophic factors such as ciliary
neurotropic factor (CNTF) and brain-derived neurotrophic factor (BDNF), growth
factors such as nerve growth factor (NGF) and hepatocyte growth factor (HGF),
CA 02979971 2017-09-15
¨ 9 ¨
aptomers such as pegaptinib, nucleic acid pharmaceuticals such as various
types of
antisense nucleic acids and siRNA, antibody or peptide preparations such as
lucentis,
endoglin antibody and IgG, VEGF inhibitors such as those described in Japanese
Unexamined Patent Publication No. 2006-96739, 2011-37844, 2005-232149, 2006-
273851, 2006-306861 and 2008-266294, compounds having glucocorticoid receptor
binding activity such as those described in Japanese Unexamined Patent
Publication
No. 2007-230993, 2008-074829, 2008-143889, 2008-143890, 2008-143891, 2009-
007344 and 2009-084274, selective glucocorticoid receptor antagonists such as
RU24858, anticancer agents such as fluorouracil, Janus kinase inhibitors such
as
tofacitinib, and protein kinase inhibitors such as ruboxistaurin mesylate.
[0051] Preferable specific examples of drugs contained in the ophthalmic depot
preparation of the present invention include compounds represented by the
aforementioned formula (1) and salts thereof.
[0052] A "halogen atom" refers to fluorine, chlorine, bromine or iodine.
[0053] A "C1.6 alkyl group" refers to a linear or branched alkyl group having
1 to 6
carbon atoms, and is preferably a linear or branched alkyl group having 1 to 4
carbon
atoms. Specific examples include a methyl group, ethyl group, n-propyl group,
n-
butyl group, n-pentyl group, n-hexyl group, isopropyl group, isobutyl group,
sec-butyl
group, tert-butyl group and isopentyl group.
[0054] A "Ci_6 alkoxy group" refers to a group in which the hydrogen atom of a
hydroxyl group is substituted with the aforementioned C1..6 alkyl group.
Specific
examples include a methoxy group, ethoxy group, n-propoxy group, n-butoxy
group,
n-pentoxy group, n-hexyloxy group, isopropoxy group, isobutoxy group, sec-
butoxy
group, tert-butoxy group and isopentyloxy group.
[0055] A "C1_6 alkylcarbonyl group" refers to a group in which the hydrogen
atom
of a formyl group is substituted with the aforementioned C1_6 alkyl group.
Specific
examples include a methylcarbonyl group (acetyl group), ethylcarbonyl group, n-
propylcarbonyl group, n-butylcarbonyl group, n-pentylcarbonyl group, n-
hexylcarbonyl group, isopropylcarbonyl group, isobutylcarbonyl group, sec-
butylcarbonyl group, tert-butylcarbonyl group and isopentylcarbonyl group.
[0056] The phrase "substituted with one or more halogen atoms" as stated in
the
present invention refers to the aforementioned C1_6 alkyl group being
substituted with
one to the maximum substitutable number of halogen atoms. Each halogen atom
may
be the same or different, the case in which the number of halogen atoms is 2
or 3 is
preferable, and the case in which the number of halogen atoms is 3 is
particularly
preferable.
CA 02979971 2017-09-15
¨
[0057] The phrase "substituted with one or more hydroxyl groups" as stated in
the
present invention refers to the aforementioned C1_6 alkyl group being
substituted with
one to the maximum possible substitutable number of hydroxyl groups. The case
in
which the number of hydroxyl groups is 1 or 2 is preferable, and the case in
which the
5 number of hydroxyl groups is 1 is particularly preferable.
[0058] In addition, the drug in the present invention includes
derivatives such as
esters and amides. Specific examples of esters include esters in which a
carboxylic
acid such as acetic acid, propionic acid, isopropionic acid, butyric acid,
isobutyric acid
and pivalic acid is condensed with a hydroxyl group in the drug. Specific
examples
10 of amides include amides in which a carboxylic group such as acetic
acid, propionic
acid, isopropionic acid, butyric acid, isobutyric acid and pivalic acid is
condensed
with an amino group in the drug.
[0059] In addition, the contained drug may be in the form of a hydrate or
solvate.
[0060] In the case the contained drug has geometric isomers, tautomers or
optical
isomers, these isomers are also included within the scope of the present
invention.
[0061] Moreover, in the case the contained drug has crystal polymorphism,
crystal
polymorphs are also included within the scope of the present invention.
[0062] (a) Preferable examples of compounds represented by formula (1) include
compounds, or salts thereof, in which each group is the group indicated below:
[0063] (al) RI represents a C1-6 alkoxy group or C1_6 alkoxy group substituted
with
one or more halogen atoms; and/or
(a2) R2 represents a C1_6 alkylcarbonyl group or C1.6 alkylcarbonyl group
substituted with one or more hydroxyl groups.
[0064] Namely, in compounds represented by formula (1), preferable examples
thereof include compounds, or salts thereof, composed of one or two or more of
each
of the combinations selected from the aforementioned (al) and (a2).
[0065] (b) More preferable examples of compounds represented by formula (1)
include compounds, or salts thereof, in which each group is the group
indicated
below:
[0066] (bl) RI represents a C1_6 alkoxy group substituted with one or more
halogen
atoms; and/or
(b2) R2 represents a C1_6 alkylcarbonyl group substituted with one or more
hydroxyl groups.
[0067] Namely, in compounds represented by formula (1), preferable examples
thereof include compounds, or salts thereof, composed of one or two or more of
each
of the combinations selected from the aforementioned (b 1 ) and (b2). In
addition, the
CA 02979971 2017-09-15
- 11 ¨
conditions for selection thereof can be combined with the conditions of (a).
[0068] (c) The most preferable example of compounds represented by formula (1)
is the compound (2-[[[2-[(hydroxyacetyl)amino]-4-pyridinyllmethyllthiol-N44-
(trifluoromethoxy)phenyl]-3-pyridinecarboxamide), or a salt thereof,
represented by
formula (2).
[0069]
[Chemical Formula 3]
0
1411
N
(2)
OH
N 0
[0070] The compound represented by formula (1), or a salt thereof, contained
in the
ophthalmic depot preparation of the present invention can be produced
according to an
ordinary method in the relevant technical field, such as the method described
in U.S.
Unexamined Patent Application Publication No. 2007/0149574.
[0071] In the ophthalmic depot preparation of the present invention, the
contained
drug may be a salt, and there are no particular limitations thereon provided
it is
allowed as a pharmaceutical. Examples of salts include salts of inorganic
acids, salts
of organic acids, quaternary ammonium salts, salts with halogen ions, salts
with
alkaline metals, salts with alkaline earth metals, metal salts and salts with
organic
amines. Examples of salts of inorganic acids include salts with hydrochloric
acid,
hydrobromic acid, hydroiodic acid, nitric acid, sulfuric acid and phosphoric
acid.
Examples of salts of organic acids include salts with acetic acid, oxalic
acid, fumaric
acid, maleic acid, succinic acid, malic acid, citric acid, tartaric acid,
adipic acid,
gluconic acid, glucoheptonic acid, glucuronic acid, terephthalic acid,
methanesulfonic
acid, alanine, lactic acid, hippuric acid, 1,2-ethanedisulfonic acid,
isethionic acid,
lactobionic acid, oleic acid, gallic acid, pamoic acid, polygalacturonic acid,
stearic
acid, tannic acid, trifluoromethanesulfonic acid, benzenesulfonic acid, p-
toluenesulfonic acid, lauryl sulfate, methyl sulfate, naphthalenesulfonic acid
and
sulfosalicylic acid. Examples of quaternary ammonium salts include salts with
methyl bromate and methyl iodate. Examples of salts with halogen ions include
salts
CA 02979971 2017-09-15
¨ 12 ¨
with chloride ions, bromide ions and iodide ions, examples of salts with
alkaline
metals include lithium, sodium and potassium salts, examples of salts with
alkaline
earth metals include calcium and magnesium salts, and examples of metal salts
include iron and zinc salts. Examples of salts of organic amines include salts
with
triethylenediamine, 2-aminoethanol, 2,2-iminobis(ethanol), 1-deoxy-1-
(methylamino)-
2-D-sorbitol, 2-amino-2-(hydroxymethyl)-1,3-propanediol, procaine and N,N-
bis(phenylmethyl)-1,2-ethanediamine.
[0072] In the ophthalmic depot preparation of the present invention, although
there
are no particular limitations on the content of the contained drug provided it
is an
adequate amount for demonstrating a desired pharmacological effect, it is
preferably
0.001%(w/v) to 30%(w/v), more preferably 0.01%(w/v) to 25%(w/v), even more
preferably 0.1%(w/v) to 20%(w/v), still more preferably 0.5%(w/v) to 15%(w/v),
particularly preferably 1%(w/v) to 12%(w/v), and most preferably 1%(w/v),
1.5%(w/v), r/o(w/v), 2.5%(w/v), 3%(w/v), 3.5%(w/v), 4 /0(w/v), 5 /0(w/v),
6%(w/v),
7%(w/v), 8%(w/v), 9%(w/v), 10%(w/v), 11%(w/v) or 12%(w/v). %(w/v) denotes the
mass (g) of the component (i.e., the drug in this paragraph) in 100 mL of the
ophthalmic depot preparation of the present invention. The same is applied
unless
otherwise particularly specified.
[0073] The benzyl benzoate contained in the ophthalmic depot preparation of
the
present invention is a compound represented by the chemical formula:
PhCO2CH2Ph.
[0074] The benzyl alcohol contained in the ophthalmic depot preparation of the
present invention is a compound represented by the chemical formula: PhCH2OH.
[0075] In the ophthalmic depot preparation of the present invention, the
content of
benzyl benzoate and/or benzyl alcohol is preferably 15%(w/w) to 75%(w/w), more
preferably 20%(w/w) to 70%(w/w), even more preferably 25%(w/w) to 60%(w/w),
even still more preferably 27%(w/w) to 55`)/0(w/w), particularly preferably
30%(w/w)
to 50%(w/w) and most preferably 351)/0(w/w) to 48%(w/w). %(w/v) denotes the
mass
(g) of the component (i.e., benzyl benzoate and/or benzyl alcohol in this
paragraph) in
100 g of the ophthalmic depot preparation of the present invention. The same
is
applied unless otherwise particularly specified.
[0076] The polyethylene glycol (PEG) contained in the ophthalmic depot
preparation of the present invention is a polyether obtained by polymerizing
ethylene
glycol, is represented by the general formula: HO(CH2CH20)õH, and n represents
the
degree of polymerization. A commercially available product or that produced in
accordance with an ordinary method in the relevant technical field can be used
for the
polyethylene glycol (PEG).
CA 02979971 2017-09-15
- 13 -
[0077] In the ophthalmic depot preparation of the present invention, the
average
molecular weight of the polyethylene glycol is preferably 90 to 2200, more
preferably
100 to 2000, even more preferably 150 to 1500, forther more preferably 200 to
1300,
particularly preferably 300 to 1200, more particularly 360 to 1100 and most
preferably
400 to 1000. Specific examples of the polyethylene glycol include PEG100,
PEG200,
PEG300, PEG400, PEG600, PEG800 and PEG1000.
[0078] In the ophthalmic depot preparation of the present invention, the
content of
the polyethylene glycol is preferably 15%(w/w) to 75%(w/w), more preferably
20%(w/w) to 70%(w/w), even more preferably 30%(w/w) to 62')/0(w/w),
particularly
preferably 40%(w/w) to 60%(w/w) and most preferably 43%(w/w) to 57%(w/w).
[0079] The dimethylsulfoxide (DMSO) contained in the ophthalmic depot
preparation of the present invention is a compound represented by the chemical
formula: CH3SOCH3.
[0080] In the ophthalmic depot preparation of the present invention, the
content of
the dimethylsulfoxide is preferably 15%(w/w) to 75%(w/w), more preferably
20%(w/w) to 70')/0(w/w), even more preferably 30%(w/w) to 62%(w/w),
particularly
preferably 40%(w/w) to 60%(w/w) and most preferably 431)/0(w/w) to 57%(w/w).
[0081] The total weight of the benzyl benzoate and/or benzyl alcohol and
polyethylene glycol and/or dimethylsulfoxide contained in the ophthalmic depot
preparation of the present invention is 50%(w/w) or more and preferably
80%(w/w) or
more of the total weight of the ophthalmic depot preparation. In addition, the
total
weight of the benzyl benzoate and/or benzyl alcohol and polyethylene glycol
and/or
dimethylsulfoxide contained in the ophthalmic depot preparation of the present
invention is preferably 60%(w/w) to 99.99%(w/w), more preferably 70%(w/w) to
99.99%(w/w), even more preferably 80 /0(w/w) to 99.5 /0(w/w), particularly
preferably
85')/0(w/w) to 99.3%(w/w) and most preferably 90%(w/w) to 99 /0(w/w).
[0082] The volume ratio of the benzyl benzoate and/or benzyl alcohol to
polyethylene glycol and/or dimethylsulfoxide contained in the ophthalmic depot
preparation of the present invention is 75:25 to 25:75, preferably 60:40 to
35:65, more
preferably 50:50 to 40:60 and most preferably 45:55 to 40:60. Furthermore,
volume
is calculated with the volume of benzyl benzoate and/or benzyl alcohol and
polyethylene glycol and/or dimethylsulfoxide at 25 C and 1 atm.
[0083] An additive can be used as necessary in the ophthalmic depot
preparation of
the present invention.
[0084] When additives are incorporated into the ophthalmic depot preparation
of
the present invention, the amounts of the additives can be appropriately
adjusted
CA 02979971 2017-09-15
¨ 14 ¨
depending on the types or the like of the additives, and the total amount of
the
additives is preferably 0.0001%(w/v) to 30%(w/v), more preferably 0.001%(w/v)
to
25%(w/v), still more preferably 0.01%(w/v) to 20%(w/v), particularly
preferably
0.1%(w/v) to 15%(w/v) and most preferably 1%(w/v) to 10%(w/v).
[0085] To the ophthalmic depot preparation of the present invention, as
additives
which can be used for pharmaceuticals, for example, surfactants, buffering
agents,
tonicity agents, stabilizers, preservatives, antioxidants, high molecular
weight
polymers and solvents can be added, if necessary.
[0086] Examples of surfactants able to be used as pharmaceutical additives
that can
be incorporated in the ophthalmic depot preparation of the present invention
include
cationic surfactants, anionic surfactants and nonionic surfactants. Examples
of
anionic surfactants include phospholipids, and examples of phospholipids
include
lecithin. Examples of cationic surfactants include alkylamine salts,
alkylamine
polyoxyethylene adducts, fatty acid triethanolamine monoester salts,
acylaminoethyl
diethylamine salts, fatty acid polyamine condensates, alkyltrimethylammonium
salts,
dialkyldimethylammonium salts, alkyldimethylbenzylammonium salts,
alkylpyridinium salts, acylaminoalkyl ammonium salts, acylaminoalkyl
pyridinium
salts, diacyloxyethylammonium salts, alkylimidazolines, 1-acylaminoethy1-2-
alkylimidazolines and 1-hydroxyethy1-2-alkylimidazolines. Examples of
alkyldimethylbenzylammonium salts include benzalkonium chloride and
cetalkonium
chloride. Examples of nonionic surfactants include polyoxyethylene fatty acid
esters,
polyoxyethylene sorbitan fatty acid esters, polyoxyethylene hydrogenated
castor oil,
polyoxyethylene castor oil, polyoxyethylene polyoxypropylene glycol, sucrose
fatty
acid esters and vitamin E-TPGS.
[0087] Examples of polyoxyethylene fatty acid esters include Polyoxyl 40
stearate.
[0088] Examples of polyoxyethylene sorbitan fatty acid esters include
Polysorbate
80, Polysorbate 60, Polysorbate 40, polyoxyethylene sorbitan monolaurate,
polyoxyethylene sorbitan trioleate and Polysorbate 65.
[0089] Various polyoxyethylene hydrogenated castor oils having different
degrees
of polymerization of ethylene oxide can be used for the polyoxyethylene
hydrogenated castor oil, and the degree of polymerization of the ethylene
oxide is
preferably 10 to 100, more preferably 20 to 80, particularly preferably 40 to
70 and
most preferably 60. Specific examples of polyoxyethylene hydrogenated castor
oils
include polyoxyethylene (10) hydrogenated castor oil, polyoxyethylene (40)
hydrogenated castor oil, polyoxyethylene (50) hydrogenated castor oil and
polyoxyethylene (60) hydrogenated castor oil.
CA 02979971 2017-09-15
¨ 15 ¨
[0090] Various polyoxyethylene castor oils having different degrees of
polymerization of ethylene oxide can be used for the polyoxyethylene castor
oil, and
the degree of polymerization of the ethylene oxide is preferably 5 to 100,
more
preferably 20 to 50, particularly preferably 30 to 40 and most preferably 35.
Specific
examples of polyoxyethylene castor oils include Polyoxyl 5 castor oil,
Polyoxyl 9
castor oil, Polyoxyl 15 castor oil, Polyoxyl 35 castor oil and Polyoxyl 40
castor oil.
[0091] Examples of polyoxyethylene polyoxypropylene glycols include
polyoxyethylene (160) polyoxypropylene (30) glycol, polyoxyethylene (42)
polyoxypropylene (67) glycol, polyoxyethylene (54) polyoxypropylene (39)
glycol,
polyoxyethylene (196) polyoxypropylene (67) glycol and polyoxyethylene (20)
polyoxypropylene (20) glycol.
[0092] Examples of sucrose fatty acid esters include sucrose stearate.
[0093] Vitamin E-TPGS is also called tocopherol polyethylene glycol 1000
succinate.
[0094] When a surfactant is incorporated into the ophthalmic depot preparation
of
the present invention, the amount of the surfactant can be appropriately
adjusted
depending on the type or the like of the surfactant, and the amount of the
surfactant is
preferably 0.001%(w/v) to 10%(w/v), more preferably 0.01%(w/v) to 5`)/0(w/v),
still
more preferably 0.05%(w/v) to 3%(w/v) and most preferably 0.1%(w/v) to 2
/0(w/v).
[0095] A buffer able to be used as a pharmaceutical additive can be
incorporated in
the ophthalmic depot preparation of the present invention. Examples of buffers
include phosphoric acid or salts thereof, boric acid or salts thereof, citric
acid or salts
thereof, acetic acid or salts thereof, carbonic acid or salts thereof,
tartaric acid or salts
thereof, c-aminocaproic acid or salts thereof and trometamol. Examples
of
phosphates include sodium phosphate, sodium dihydrogen phosphate, disodium
hydrogen phosphate, potassium phosphate, potassium dihydrogen phosphate and
dipotassium hydrogen phosphate, examples of borates include borax, sodium
borate
and potassium borate, examples of citrates include sodium citrate and disodium
citrate,
examples of acetates include sodium acetate and potassium acetate, examples of
carbonates include sodium carbonate and sodium bicarbonate, and examples of
tartrates include sodium tartrate and potassium tartrate.
[0096] When a buffering agent is incorporated into the ophthalmic depot
preparation of the present invention, the amount of the buffering agent can be
appropriately adjusted depending on the type or the like of the buffering
agent, and the
amount of the buffering agent is preferably 0.001%(w/v) to 10%(w/v), more
preferably 0.01%(w/v) to 5%(w/v), still more preferably 0.0Y/o(w/v) to
1370(w/v) and
CA 02979971 2017-09-15
- 16 -
most preferably 0.1%(w/v) to 2%(w/v).
[0097] A tonicity agent able to be used as a pharmaceutical additive can
be suitably
incorporated in the ophthalmic depot preparation of the present invention.
Examples
of tonicity agents include ionic tonicity agents and nonionic tonicity agents.
Examples of ionic tonicity agents include sodium chloride, potassium chloride,
calcium chloride and magnesium chloride, and examples of nonionic tonicity
agents
include glycerin, propylene glycol, sorbitol and mannitol.
[0098] When a tonicity agent is incorporated into the ophthalmic depot
preparation
of the present invention, the amount of the tonicity agent can be
appropriately
adjusted depending on the type or the like of the tonicity agent, and the
amount of the
tonicity agent is preferably 0.001 /0(w/v) to 10%(w/v), more preferably
0.01%(w/v) to
5%(w/v), still more preferably 0.05%(w/v) to 3%(w/v) and most preferably
0.1%(w/v)
to 2%(w/v).
[0099] A stabilizer able to be used as a pharmaceutical additive can be
suitably
incorporated in the ophthalmic depot preparation of the present invention.
Examples
of stabilizers include edetic acid, sodium edetate and sodium citrate.
[0100] When a stabilizer is incorporated into the ophthalmic depot preparation
of
the present invention, the amount of the stabilizer can be appropriately
adjusted
depending on the type or the like of the stabilizer, and the amount of the
stabilizer is
preferably 0.001 /0(w/v) to 10%(w/v), more preferably 0.01%(w/v) to 5 /0(w/v),
still
more preferably 0.05 /0(w/v) to 3%(w/v) and most preferably 0.1%(w/v) to
2%(w/v).
[0101] A preservative able to be used as a pharmaceutical additive can be
suitably
incorporated in the ophthalmic depot preparation of the present invention.
Examples
of preservatives include benzalkonium chloride, benzalkonium bromide,
benzethonium chloride, sorbic acid, potassium sorbate, methyl paraoxybenzoate,
propyl paraoxybenzoate and chlorobutanol.
[0102] When a preservative is incorporated into the ophthalmic depot
preparation
of the present invention, the amount of the preservative can be appropriately
adjusted
depending on the type or the like of the preservative, and the amount of the
preservative is preferably 0.0001%(w/v) to 10%(w/v), more preferably
0.001%(w/v)
to 5%(w/v), still more preferably 0.005%(w/v) to 3%(w/v) and most preferably
0.01%(w/v) to 2%(w/v).
[0103] An antioxidant able to be used as a pharmaceutical additive can be
suitably
incorporated in the ophthalmic depot preparation of the present invention.
Examples
of antioxidants include ascorbic acid, tocopherol, dibutylhydroxytoluene,
butylhydroxyanisole, sodium erythorbate, propyl gallate and sodium sulfite or
CA 02979971 2017-09-15
- 17 -
derivatives thereof, from the viewpoint of higher degree of stabilization of
the drug
(for example, (24[[2-
[(hydroxyacetypamino]-4-pyridinyllmethyllthiol-N44-
(trifluoromethoxy)pheny1]-3-pyridinecarboxamide) or a salt thereof),
tocopherol or
derivatives thereof are particularly preferred. Examples
of tocopherol and
derivatives thereof include vitamin E, a-tocopherol, 13-tocopherol, y-
tocopherol,
tocopherol, and their acetic esters, succinic esters, and their d isomer, 1
isomer, dl
isomers.
[0104] When an antioxidant is incorporated into the ophthalmic depot
preparation
of the present invention, the amount of the antioxidant can be appropriately
adjusted
depending on the type or the like of the antioxidant, and the amount of the
antioxidant
is preferably 0.001 /0(w/v) to 10%(w/v), more preferably 0.01%(w/v) to
5%(w/v), still
more preferably 0.05%(w/v) to 3%(w/v) and most preferably 0.1%(w/v) to
2%(w/v).
[0105] A high molecular weight polymer able to be used as a pharmaceutical
additive can be suitably incorporated in the ophthalmic depot preparation of
the
present invention. Examples of high molecular weight polymers include methyl
cellulose, ethyl cellulose, hydroxymethyl cellulose, hydroxyethyl cellulose,
hydroxypropyl cellulose, hydroxyethyl methyl cellulose, hydroxypropyl methyl
cellulose, carboxymethyl cellulose, sodium carboxymethyl cellulose,
hydroxypropyl
methyl cellulose acetate succinate, hydroxypropyl methyl cellulose phthalate,
carboxymethylethyl cellulose, cellulose acetate phthalate,
polyvinylpyrrolidone,
polyvinyl alcohol, carboxyvinyl polymer and polyethylene glycol.
[0106] When a high molecular weight polymer is incorporated into the
ophthalmic
depot preparation of the present invention, the amount of the high molecular
weight
polymer can be appropriately adjusted depending on the type or the like of the
high
molecular weight polymer, and the amount of the high molecular weight polymer
is
preferably 0.001%(w/v) to 10%(w/v), more preferably 0.01%(w/v) to 5%(w/v),
still
more preferably 0.05%(w/v) to 3%(w/v) and most preferably 0.1%(w/v) to
2%(w/v).
[0107] A solvent able to be used as a pharmaceutical additive can be suitably
incorporated in the ophthalmic depot preparation of the present invention.
Examples
of solvents include N-methylpyrrolidone, N,N-dimethylacetoamide and ethanol.
[0108] When a solvent is incorporated into the ophthalmic depot preparation of
the
present invention, the amount of the solvent can be appropriately adjusted
depending
on the type or the like of the solvent, and the amount of the solvent is
preferably
0.1%(w/v) to 20%(w/v), more preferably 0.5%(w/v) to 15%(w/v), still more
preferably 1%(w/v) to 10%(w/v) and most preferably 2%(w/v) to 5%(w/v).
[0109] A specific mode of the ophthalmic depot preparation of the present
CA 02979971 2017-09-15
- 18 -
invention is an ophthalmic depot preparation substantially containing only the
compound represented by formula (1) or a salt thereof, benzyl benzoate and
polyethylene glycol.
[0110] Another specific mode of the ophthalmic depot preparation of the
present
invention is an ophthalmic depot preparation substantially containing only the
compound represented by formula (1) or a salt thereof, benzyl benzoate and
dimethylsulfoxide.
[0111] Another specific mode of the ophthalmic depot preparation of the
present
invention is an ophthalmic depot preparation substantially containing only the
compound represented by formula (1) or a salt thereof, benzyl benzoate,
polyethylene
glycol and tocopherol or derivatives thereof.
[0112] Another specific mode of the ophthalmic depot preparation of the
present
invention is an ophthalmic depot preparation substantially containing only the
compound represented by formula (1) or a salt thereof, benzyl benzoate,
dimethylsulfoxide and tocopherol or derivatives thereof.
[0113] Another specific mode of the ophthalmic depot preparation of the
present
invention is an ophthalmic depot preparation substantially containing only the
compound represented by formula (2) of a salt thereof, benzyl benzoate and
polyethylene glycol.
[0114] Another specific mode of the ophthalmic depot preparation of the
present
invention is an ophthalmic depot preparation substantially containing only the
compound represented by general formula (2) or a salt thereof, benzyl benzoate
and
dimethylsulfoxide.
[0115] Another specific mode of the ophthalmic depot preparation of the
present
invention is an ophthalmic depot preparation substantially containing only the
compound represented by formula (2) of a salt thereof, benzyl benzoate,
polyethylene
glycol and tocopherol or derivatives thereof.
[0116] Another specific mode of the ophthalmic depot preparation of the
present
invention is an ophthalmic depot preparation substantially containing only the
compound represented by formula (2) of a salt thereof, benzyl benzoate,
dimethylsulfoxide and tocopherol or derivatives thereof.
[0117] The ophthalmic depot preparation of the present invention can be
administered orally or parenterally. There are no particular limitations on
the dosage
form of the ophthalmic depot preparation of the present invention provided it
is able
to be used as a pharmaceutical. Examples of dosage forms include oral
preparations
such as liquids and suspensions and parenteral forms such as injections,
transfusion
CA 02979971 2017-09-15
¨ 19 ¨
fluids, nasal drops, ear drops and eye drops. Examples preferably include
ophthalmic
injections and eye drops, more preferably ophthalmic injections, and most
preferably
injections for administration into the vitreous body, anterior chamber or
subconjunctival administration. These dosage forms can be produced in
accordance
with ordinary methods in the relevant technical field.
[0118] The ophthalmic depot preparation of the present invention can be
suitably
administered according to the dosage form thereof. For example, in the case of
an
ophthalmic injection, the ophthalmic depot preparation of the present
invention can be
administered into the vitreous body, in the vicinity of the posterior sclera,
around the
orbit or between the sclera and conjunctiva. For example, in the case of
administering an ophthalmic injection into the vitreous body or anterior
chamber,
although there are no particular limitations on the dosage provided it is an
amount
sufficient for demonstrating a desired pharmacological effect, the dosage is
preferably
1 pL to 100 pL, more preferably 5 L to 70 L, even more preferably 10 L to
60 'IL,
particularly preferably 20 1AL to 50 L and most preferably 20 pL, 25 L, 30
pL, 35
L, 40 L, 45 L or 50 L per administration. In the case of administering an
ophthalmic injection subconjunctivally, although there are no particular
limitations on
the dosage provided it is an amount sufficient for demonstrating a desired
pharmacological effect, the dosage is preferably 10 tL to 1000 L, more
preferably 20
I. to 800 L, even more preferably 50 L to 700 L, particularly preferably 100
[IL
to 500 L and most preferably 100 pi-, 200 I., 300 L, 400 I, or 500 L per
administration. The dosage of the drug is preferably 0.001 mg/eye to 30
mg/eye,
more preferably 0.01 mg/eye to 10 mg/eye, even more preferably 0.1 mg/eye to 5
mg/eye, particularly preferably 0.2 mg/eye to 1.6 mg/eye and most preferably
0.2
mg/eye, 0.3 mg/eye, 0.4 mg/eye, 0.5 mg/eye, 0.6 mg/eye, 0.7 mg/eye, 0.8
mg/eye, 1
mg/eye, 1.2 mg/eye, 1.4 mg/eye or 1.6 mg/eye.
[0119] In the case of consecutively administering the ophthalmic depot
preparation
of the present invention into the vitreous body or anterior chamber, although
there are
no particular limitations on administration interval provided it is an amount
sufficient
for demonstrating a desired pharmacological effect, the ophthalmic depot
preparation
of the present invention is preferably administered at an interval of once per
week to
once every three years, more preferably administered at an interval of once
per week,
once every two weeks, once every month, once every two months, once every
three
months, once every four months, once every five months, once every six months,
once
per year, once every two years or once every three years, and most preferably
administered at an interval of once every two months, once every three months,
once
CA 02979971 2017-09-15
¨ 20 ¨
every four months, once every five months, once every six months or once per
year.
In addition, the administration interval can be suitably changed.
[0120] The ophthalmic depot preparation of the present invention is useful as
a
pharmaceutical, and can be used as a prophylactic or therapeutic of eye
diseases such
as age-related macular degeneration, diabetic retinopathy, retinopathy of
prematurity,
retinal vein occlusion, retinal artery occlusion, polypoidal choroidal
vasculopathy,
retinal angiomatous proliferation, myopic choroidal neovascularization,
diabetic
macular edema, ocular tumor, radiation retinopathy, rubeosis iridis,
neovascular
glaucoma, proliferative vitreoretinopathy (PVR), primary open-angle glaucoma,
secondary open-angle glaucoma, normal tension glaucoma, hypersecretion
glaucoma,
primary closed-angle glaucoma, secondary closed-angle glaucoma, plateau iris
glaucoma, combined mechanism glaucoma, developmental glaucoma, steroid
glaucoma, exfoliation glaucoma, amyloidotic glaucoma, neovascular glaucoma,
malignant glaucoma, capsular glaucoma, plateau iris syndrome and high tension
glaucoma. The ophthalmic depot preparation of the present invention can more
preferably be used as a prophylactic or therapeutic of eye diseases such as
age-related
macular degeneration, diabetic retinopathy, primary open-angle glaucoma,
normal
tension glaucoma, primary closed-angle glaucoma and high tension glaucoma.
[0121]
The ophthalmic depot preparation of the present invention can be contained in
a
container used for pharmaceuticals, for example, a hermetic container,
especially an
ampule, vial, syringe or the like. There is no particular limitation with
respect to the
container in which the ophthalmic depot preparation of the present invention
is
contained. Examples of such containers include those made of a cycloolefin
polymer,
glass, polyolefin such as polyethylene and polypropylene, polycarbonate or the
like.
From the viewpoint of the effect of the ophthalmic depot preparation to the
stability of
the container, use of a syringe made of a cycloolefin polymer, polypropylene
or glass
is preferred.
[0122] The ophthalmic depot preparation of the present invention can be stored
at
the temperature of -10 to 30 C, preferably -5 to 30 C, more preferably 0 to
30 C,
for long-term, more than 6 months, preferably more than 1 year, more
preferably 2
years, most preferably 3 years. The remaining ratio of the drug in the
ophthalmic
depot preparation of the present invention during long-term storage is
preferably 90 to
100 %, more preferably 95 to 100 % and most preferably 98 to 100 %.
[0123] The above explanation of the ophthalmic depot preparation of the
present
invention is applied to the method of the present invention for stabilizing
the drug in
CA 02979971 2017-09-15
- 21 -
the ophthalmic depot preparation.
[0124] The method of the present invention for stabilizing the drug in the
ophthalmic depot preparation is a method in which a drug contained in an
ophthalmic
depot preparation is stabilized by incorporating tocopherol or derivatives
thereof in
the preparation,
the preparation comprising:
benzyl benzoate and/or benzyl alcohol, and
polyethylene glycol and/or dimethylsulfoxide, wherein,
the volume ratio of benzyl benzoate and/or benzyl alcohol to polyethylene
glycol and/or dimethylsulfoxide in the ophthalmic depot preparation is 75:25
to 25:75,
and
the total amount of benzyl benzoate and/or benzyl alcohol and polyethylene
glycol and/or dimethylsulfoxide contained is 50%(w/w) or more.
[0125] Although the following indicates preparation examples and test
examples,
these are intended to facilitate a better understanding of the present
invention, and do
not limit the scope of the present invention.
[0126] Preparation Examples
The following indicates typical preparation examples of the present
invention.
[0127] Preparation Example 1
Drug 4g
Benzyl benzoate 45 g
PEG400 55 g
[0128] Preparation Example 2
Drug 4 g
Benzyl benzoate 40 g
PEG400 60 g
[0129] Preparation Example 3
Drug 4g
Benzyl benzoate 45 g
Dimethylsulfoxide 55 g
[0130] Preparation Example 4
Drug 4g
Benzyl benzoate 40 g
Dimethylsulfoxide 60 g
[0131] Preparation Example 5
CA 02979971 2017-09-15
¨ 22 ¨
Drug 4g
Benzyl benzoate 40 g
PEG400 50g
Dimethylsulfoxide 10 g
[0132] Preparation Example 6
Drug 4g
Benzyl alcohol 45 g
PEG400 55 g
[0133] Preparation Example 7
Drug 4g
Benzyl alcohol 40 g
PEG400 60 g
[0134] Preparation Example 8
Drug 4g
Benzyl alcohol 45 g
Dimethylsulfoxide 55 g
[0135] Preparation Example 9
Drug 4g
Benzyl alcohol 40 g
Dimethylsulfoxide 60 g
[0136] Preparation Example 10
Drug 4g
Benzyl alcohol 40 g
PEG400 50g
Dimethylsulfoxide 10 g
[0137] Preparation Example 11
Drug 4g
Benzyl benzoate 45 g
PEG400 55 g
Tocopherol 0.01 g
[0138] Preparation Example 12
Drug 4g
Benzyl benzoate 45 g
PEG400 55 g
Tocopherol 0.1 g
[0139] Preparation Example 13
CA 02979971 2017-09-15
¨ 23 ¨
Drug 4g
Benzyl benzoate 45 g
PEG400 55 g
Tocopherol 0.5 g
[0140] Furthermore, the incorporated amounts of the drug, benzyl benzoate,
benzyl
alcohol, polyethylene glycol and dimethylsulfoxide in the aforementioned
Preparation
Examples 1 to 13 can be suitably adjusted to obtain a desired composition.
[Examples]
[0141] 1. Depot Formation Evaluation Test (1)
Depot formation of the ophthalmic depot preparation of the present
invention not containing a drug was evaluated.
[0142] 1-I. Preparation of Test Preparations
300 pL of polyethylene glycol 400 (Croda) and 700 jiL of benzyl benzoate
(Sigma-Aldrich) were mixed and stirred to prepare the preparation of Example
1.
[0143] The preparations of Example 2, Example 3 and Comparative Example 1
shown in Table 1 were prepared using the same method as the preparation method
of
Example I.
[0144] 1-2. Test Method
5 mL of physiological saline were placed in a glass vial. 50 L of a test
preparation were injected into the physiological saline using a 30G injection
needle
and Hamilton syringe. Following injection, the presence or absence of the
formation
of a depot by the test preparation was confirmed visually. In addition,
similar tests
were carried out using 1% and 2% aqueous hypromellose solutions instead of
physiological saline.
[0145] 1-3. Test Results and Discussion
The test results are shown in Table 1.
[0146]
[Table 1]
Comparative
Example 1 Example 2 Example 3
Example 1
Benzyl benzoate 700 !IL 500 pi. 300 [IL 100 111
PEG400 300 p1 500 pi, 700 pi., 900 pi,
Physiological saline 0 0 0 X
1% aqueous
Depot 0 0 0 X
formation hypromellose solution
2% aqueous
0 0 0 0
hypromellose solution
0: Spherical depot formed
CA 02979971 2017-09-15
¨ 24 ¨
X: Depot not formed
[0147]
[Table 2]
Example 4 Example 5 Example 6 Comparative
Example 2
Benzyl benzoate 700 }IL 500 [IL 300 [IL 100pL
PEG200 300 jiL 500 }11_, 700 .1, 900 pL
Physiological saline 0 0 0 X
Depot
1% aqueous
0 0 0 X
formation hypromellose solution
2% aqueous
0 0 0 0
hypromellose solution
0: Spherical depot formed
X: Depot not formed
[0148]
[Table 3]
Comparative
Example 7 Example 8 Example 9
Example 3
Benzyl benzoate 700 111_, 500 111, 300 1., 100 1.d.,
PEG1000 300 L 500L 700 [tL 9001.1L
Physiological saline 0 0 0
Depot
1% aqueous
0 0 0
formation hypromellose solution
2% aqueous
0 0 0
hypromellose solution
0: Spherical depot formed
¨: Solidified after preparation prepared
[0149]
[Table 4]
Example Example Comparative
10 11 Example 4
Benzyl benzoate 500 [IL 300 pt 100 pt
DMSO 500 jiL 700p1. 900 I,
Physiological saline 0 0 X
1% aqueous
Depot 0 0 X
formation hypromellose solution
2% aqueous
0 0 0
hypromellose solution
0: Spherical depot formed
X: Depot not formed
[0150] As indicated in Tables 1 to 4, the preparations of Examples 1 to 11
formed
depots of similar spherical shapes even after having been injected into
solutions
CA 02979971 2017-09-15
¨ 25 ¨
having different viscosities. On the other hand, although the preparations of
Comparative Examples 1, 2 and 4 formed spherical depots in 2% aqueous
hypromellose solution, they did not form depots in physiological saline and 1%
aqueous hypromellose solution. In addition, since the preparation of
Comparative
Example 3 solidified following preparation, it was unable to be injected with
a syringe.
On the basis of the above, the ophthalmic depot preparation of the present
invention
was confirmed to form similar spherical depots regardless of the
administration site or
conditions around that site.
[0151] 2. Depot Formation Evaluation Test (2)
Depot formation of the ophthalmic depot preparation of the present
invention that contained a drug was evaluated.
[0152] 2-1. Preparation of Test Preparations
The compound represented by formula (2) (2-[[[2-[(hydroxyacetypamino]-
4-pyridinyl]methyl]thio]-N-14-(trifluoromethoxy)phenyl]-3-pyridinecarboxamide,
hereinafter to also be referred to as Compound A) was prepared according to
the
method described in U.S. Unexamined Patent Application Publication No.
2007/0149574. 2.75 mL of polyethylene glycol 400 (Croda) were added to 0.16 g
of
Compound A and stirred and after the Compound A was dissolved, 2.25 mL of
benzyl
benzoate (Sigma-Aldrich) were added and stirred to prepare the preparation of
Example 12.
[0153] The preparations of Example 13, Example 14 and Comparative Example 5
shown in Table 5 were prepared using the same method as the preparation method
of
Example 12.
[0154] 2-2. Test Method
5 mL of physiological saline were placed in a glass vial. 50 pt of a test
preparation were injected into the physiological saline using a 30G injection
needle
and Hamilton syringe. Following injection, the presence or absence of the
formation
of a depot by the test preparation was confirmed visually. In addition,
similar tests
were carried out using 1% and 2% aqueous hypromellose solutions instead of
physiological saline.
[0155] 2-3. Test Results and Discussion
The test results are shown in Table 5.
[0156]
[Table 5]
Example Example Example Comparative
12 13 14 Example 5
CA 02979971 2017-09-15
¨ 26 ¨
Compound A 0.16 g 0.16 g 15 mg 0.16
g
Benzyl benzoate 2.25 mL 2.25 mL
Benzyl alcohol 0.25 mL
PEG400 2.75 mL 0.25 mL 5 mL
Dimethylsulfoxide 2.75 mL -
Physiological saline 0 0 0
1% aqueous
Depot 0 0 0 X
formation hypromellose solution
2% aqueous
0 0 0 0
hypromellose solution
0: Spherical depot formed
: Depot not formed
[0157] As shown in Table 5, the preparations of Examples 12 to 14 formed
similar
spherical depots even after having been injected into solutions having
different
viscosities. On the other hand, although the preparation of Comparative
Example 5
formed a spherical depot in 2% aqueous hypromellose solution, it did not form
a depot
in physiological saline and 1% aqueous hypromellose solution. On the basis of
the
above, the ophthalmic depot preparation of the present invention was confirmed
to
form similar spherical depots regardless of the administration site or
conditions
around that site.
[0158] 3. Depot Formation Evaluation Test (3)
Depot formation of the ophthalmic depot preparation of the present
invention that contained a drug was evaluated.
[0159] 3-1. Preparation of Test Preparations
The preparations of Examples 15 to 21 shown in Table 6 were prepared
using the same method as the preparation method of Example 12.
[0160] 3-2. Test Method
5 mL of physiological saline were placed in a glass vial. 50 uL of a test
preparation were injected into the physiological saline using a 30G injection
needle
and Hamilton syringe. Following injection, the presence or absence of the
formation
of a depot by the test preparation was confirmed visually.
[0161] 3-3. Test Results and Discussion
The test results are shown in Table 6.
[0162]
[Table 6]
Example Example Example Example Example Example Example
15 16 17 18 19 20 21
CA 02979971 2017-09-15
¨ 27 ¨
Compound A 12.5 mg 12.5 mg 12.5 mg 12.5 mg 12.5 mg 80 mg 80 mg
Benzyl benzoate 214 1.iL 333 uL 500 L. 750 1. 1167 1_, 600 fiL 700 uL
PEG400 500 L 500 pL 500 uL 500 p.L 500 uL
Dimethylsulfoxide 400 uL 300
pL
Depot Physiolog
0 0 0 0 0 0 0
formation ical saline
0: Spherical depot formed
[0163] As shown in Table 6, differing from the preparation of Comparative
Example 5 described above, the preparations of Examples 15 to 21 formed
similar
spherical depots after having been injected into physiological saline. On the
basis of
the above, the ophthalmic depot preparation of the present invention was
confirmed to
form similar spherical depots regardless of the administration site or
conditions
around that site.
[0164] 4. Drug Sustained Release Evaluation Test
Drug retention rates were confirmed in depots formed by the ophthalmic
depot preparation of the present invention that contained a drug.
[0165] 4-1. Preparation of Test Preparations
The preparations of Examples 22 to 25 and Comparative Example 6 having
the composition ratios shown in Table 7 were prepared using the same method as
the
preparation method of Example 12.
[0166] 4-2. Test Method
5 mL of physiological saline were placed in a glass vial. 20 L, or 50 L of
a test preparation were injected into the physiological saline using a 30G
injection
needle and Hamilton syringe. About 1 hour later, the depot formed following
injection was recovered and the amounts of Compound A in the depot and in the
residual liquid were quantified using high-performance liquid chromatography
(HPLC) to calculate the retention rate (1)/0) of Compound A in the depot. In
addition,
similar tests were carried out for Example 23 and Comparative Example 6 using
1%
and 2% aqueous hypromellose solutions instead of physiological saline.
[0167] 4-3. Test Results and Discussion
The results of the tests consisting of injection into physiological saline are
shown in Table 7, the results of the tests consisting of injection into 1%
aqueous
hypromellose solution are shown in Table 8, and the results of the test
consisting of
injection into 2% aqueous hypromellose solution are shown in Table 9.
[0168]
[Table 7]
Example Example Example Example Comparative
22 23 24 25 Example 6
CA 02979971 2017-09-15
- 28 -
Compound A 2.5 g 2.5 g 2.5 g 2.5 g 2.5 g
Benzyl benzoate 70 mL 55 mL 70 mL 55 mL
PEG400 30 mL 45 mL 40 mL 100 mL
Dimethylsulfoxide 30 mL
dl-a-Tocopherol 5 mL
Depot formation 0 0 0 0 X
Concentration of
Compound A in 23.71 21.65 24.96 22.24 0.01
depot (mg/mL)
Concentration of
Compound A in
Adminis 1.30 0.79 0.48 0.03 25.23
residual liquid
tration
of 50ttL (mg/mL)
Retention rate of
Compound A in 94.8% 96.5% 98.1% 99.9% 0%
depot
Elution rate into
physiological 5.2% 3.5% 1.9% 0.1% 100%
saline
Depot formation 0 0
Concentration of
Compound A in 22.75 23.34 0.01
depot (mg/mL)
Concentration of
Compound A in
Adminis 0.75 0.76 22.76
residual liquid
tration N.D. N.D.
(mg/mL)
of 20ttL
Retention rate of
Compound A in 96.8% 96.8% 0%
depot
Elution rate into
physiological 3.2% 3.2% 100%
saline
0: Spherical depot formed
X: Depot not formed
N.D.: No data
[0169]
[Table 8]
Example Comparative
23 Example 6
Compound A 2.5 g 2.5 g
Benzyl benzoate 55 mL
PEG400 45 mL 100 mL
Depot formation 0
Concentration of Compound A in
16.95 0.04
Administration depot (mg/mL)
of 501.IL Concentration of Compound A in
5.08 24.82
residual liquid (mg/mL)
Retention rate of Compound A in 76.9% 0.2%
CA 02979971 2017-09-15
- 29 -
depot
Elution rate into 1% aqueous
23.1% 99.8%
hypromellose solution
0: Spherical depot formed
X: Depot not formed
[0170]
[Table 9]
Example Comparative
23 Example 6
Compound A 2.5 g 2.5 g
Benzyl benzoate 55 mL
PEG400 45 mL 100 mL
Depot formation 0 0
Concentration of Compound A
19.64 23.51
in depot (mg/mL)
Concentration of Compound A
Administration 0.48 1.36
of 50p.L in residual liquid (mg/mL)
Retention rate of Compound A
97.6% 94.5%
in depot
Elution rate into 2% aqueous
2.4% 5.5%
hypromellose solution
0: Spherical depot formed
[0171] As shown in Tables 7, 8 and 9, the preparations of Examples 22 to 25
formed similar spherical depots when injected into physiological saline, and
the
retention rate of Compound A in the depots was 90% or more. In addition, the
preparation of Example 23 formed a similar spherical depot even after having
been
injected into 1% aqueous hypromellose solution and 2% aqueous hypromellose
solution, and the retention rate of Compound A in the depot was 70% or more.
On
the other hand, the preparation of Comparative Example 6 did not form a depot
even
when injected into physiological saline and 1% aqueous hypromellose solution,
and
Compound A was released into the residual liquid without being retained in the
depot.
Especially, in the case of the 1% aqueous hypromellose solution, when the
syringe
was taken out from the 1% aqueous hypromellose solution, the preparation of
Comparative Example 6 was stringy (exhibited stringiness). This fact was
regarded
as one of the reasons of the failure to form a spherical depot. Such
stringiness was
not perceived in the the preparation of Examples 23 which formed a spherical
depot.
On the basis of the above, the ophthalmic depot preparation of the present
invention
was confirmed to form similar spherical depots and gradually release a drug
regardless
of the administration site or conditions around that site.
[0172] 5. Drug Dissolution Performance Evaluation Test
The drug dissolution performance of the ophthalmic depot preparation of the
CA 02979971 2017-09-15
¨ 30 ¨
present invention was examined.
[0173] 5-1. Preparation of Test Preparations
400 tiL of dimethylsulfoxide (Gaylord Chemical) were added to 0.08 g of
Compound A, and after stirred and dissolved, 600 !IL of benzyl benzoate (Sigma-
Aldrich) were added, stirred and dissolved to prepare the preparation of
Example 26.
[0174] Preparations of Example 27 and Comparative Examples 7 and 8 having the
composition ratios shown in Table 10 were prepared using the same method as
the
preparation method of Example 26.
[0175] 5-2. Test Method
Dissolution of the test preparations was confirmed visually.
[0176] 5-3. Test Results and Discussion
The test results are shown in Table 10.
[0177]
[Table 10]
Example Example Comparative Comparative
26 27 Example 7 Example 8
Compound A 0.08 g 0.08 g 0.08 g 0.08 g
Benzyl benzoate 600 i_tL 700 tL 800 L. 1000 11.1_,
Dimethylsulfoxide 400 ptL 300 i.t.L 200 1.tL 0 iL
Solubility 0 0 X X
0: Completely dissolved
X: Not completely dissolved
[0178] As shown in Table 10, in the preparations of Examples 26 and 27
Compound A was completely dissolved. On the other hand, in the preparations of
Comparative Examples 7 and 8, Compound A was not completely dissolved. On the
basis of the above, the ophthalmic depot preparation of the present invention
was
confirmed to be capable of adequately dissolving a drug.
[0179] 6. Depot Formation Evaluation Test in an Animal
The ophthalmic depot preparation of the present invention containing a drug
was administered into the vitreous of an animal and the depot formation was
evaluated
in vivo.
[0180] 6-1. Preparation of Test Preparations
55 mL of polyethylene glycol 400 (Croda) was added to Compound A 5 g to
dissolve Compound A by stirring. Preparation of Example 28 was prepared
further
by adding dl-a-tocopherol (Merck) 0.2 mL and then by adding benzyl benzoate
(Merck) to a total volume of 100 mL. Preparation of Comparative Example 9 as
shown in Table 11 was prepared using the same method as the preparation method
of
CA 02979971 2017-09-15
¨ 31. ¨
Example 28.
[0181]
[Table 11]
Example 28 Comparative Example 9
Compound A 5 g 5 g
PEG400 55 mL q. s.
Benzyl benzoate q. s.
dl-a-Tocopherol 0.2 mL
Total volume 100 mL 100 mL
[0182] 6-2. Test Method
Test preparations were intravitreally injected into white rabbits (20 1AL/eye)
using a 30 gauge needle and, after 4 hours from the administration, eyes were
enucleated. With respect to each of the enucleated eyes, an incision was made
in the
pars plana to remove the tissues of the anterior segment (such as lens, cornea
and the
like), and an image of the state of the depot formed in the eye was taken with
a camera.
[0183] 6-3. Test Results and Discussion
The test results are shown in Fig 1.
[0184] As shown in Fig 1, the preparation of Example 28 formed a spherical
depot
with a sharply defined interface between the vitreous body and depot. On the
other
hand, the preparation of Comparative Example 9 formed an depot with an ill-
defined
interface between the vitreous body and depot, and a randomly unstable shape.
On
the basis of the above, the ophthalmic depot preparation of the present
invention was
confirmed to form a spherical depot even when administered into the vitreous
body.
[0185] 7. Syringe Compatibility Test
The compatibility of the ophthalmic depot preparation of the present
invention with various syringes was evaluated.
[0186] 7-1. Preparation of a Test Preparation
A preparation of Example 29 as shown in Table 12 was prepared using the
same method as the preparation method of Example 28.
[0187]
[Table 12]
Example 29
PEG400 q. s.
Benzyl benzoate 50 mL
dl-a-Tocopherol 0.2 mL
Total Volume 100 mL
[0188] 7-2. Test Method
A 23G injection needle was attached to the syringes shown in Table 13, and
the syringe was filled with approximately 90% of the volume of syringe of the
CA 02979971 2017-09-15
¨ 32 ¨
preparation of Example 29. The injection needle was replaced with a 30G
injection
needle, and the cavity space formed in the injection needle was filled with
the
preparation. The syringe was stored at 25 C or 60 C for 6 hours. After 6
hours,
the state of the syringe was visually observed.
[0189] 7-3. Test Results and Discussion
The test results are shown in Table 13.
[0190]
[Table 13]
External Syringe Supplier Temperature / Time Results
material
MERIT MEDICAL 60 C / 6 hours Cracks
generated
(CUSTOM KIT 0.02 25 C / 6 hours Cracks
generated
Polycarbonate
mL Medallion Sword
Handle)
MERIT MEDICAL (1 60 C / 6 hours No change
in the
Cycloolefin mL Medallion Sword syringe
polymer Handle) 25 C / 6 hours No change
in the
syringe
TOP (SENSYTEC 60 C / 6 hours No change
in the
Pol SYRINGE) syringe
ypropylene
25 C / 6 hours No change
in the
syringe
Hamilton (100 tL 60 C / 6 hours No change
in the
Glass Hamilton Syringe) syringe
25 C / 6 hours No change
in the
syringe
As shown in Table 13, when the preparation of Example 29 was used in a
syringe made of a cycloolefin polymer, polypropylene or glass, no change was
perceived in the syringe. On the other hand, when this preparation was used in
a
syringe made of a polycarbonate, cracks were generated in the syringe. It was
suggested that when the ophthalmic depot preparation of the present invention
was
stored for a long period of time in a prefilled syringe, a syringe made of a
cycloolefin
polymer, polypropylene or glass was more suitable than a syringe made of a
polycarbonate.
[0191] 8. Drug Stability Test
The stability of a drug (compound A) in the ophthalmic depot preparation of
the present invention was evaluated.
[0192] 8-1. Preparation of a Test Preparation
30.8 g of polyethylene glycol 400 (NOF CORPORATION) was added to
Compound A 1.25 g to dissolve Compound A by stirring. Pharmaceutical
CA 02979971 2017-09-15
- 33 -
composition D was prepared further by adding dl-a-tocopherol (BASF) 0.5 mL and
then by adding benzyl benzoate (Sigma-Aldrich) to a total volume of 50 mL.
1.59mL of the pharmaceutical composition was filled in a 2 mL glass vial
(Wheaton,
internal content 2.92 mL) and then sealed with rubber stopper to provide
preparation
of Example 30.
[0193] Preparation of Examples 31 to 36 as shown in Tables 14 and 15 were
prepared using the same method as the preparation method of Example 30. The
filling rate was calculated by the volume of pharmaceutical composition in the
container / the inner volume of the container.
[0194] 8-2. Test Method
With respect to each of the preparations of Examples 30 to 36, the amount
of compound A contained in the preparation after the storage of 4 weeks at the
temperature of 40 C and relative humidity of 20 % was determined by high-
performance liquid chromatography (HPLC), and the remaining ratio of compound
A
was calculated.
[0195] 8-3. Test Results and Discussion
The test results are shown in Tables 14 and 15.
[0196]
[Table 14]
Example Example Example Example Example
30 31 32 33 34
Compound A 1.25 g 1.25 g 1.25 g 1.25 g 1.25 g
PEG400 30.8 g 30.8 g 30.8 g 30.8 g 30.8 g
Benzyl benzoate q.s. q.s. q.s. q.s. q.s.
dl-a-tocopherol 0.5 mL 0.25 mL 0.1 mL 0.01 mL 0.001 mL
Total volume 50 mL 50 mL 50 mL 50 mL 50 mL
Filling ratio 54% 54% 54% 54% 54%
Remaining ratio
of compound A
97.6 97.6 97.2 98.0 98.4
(%)
(40 C /4 weeks)
[0197]
[Table 15]
Example 35 Example 36
Compound A 0.96 g 0.96 g
PEG400 16.5 mL 16.5 mL
Benzyl benzoate 12 mL 13.5 mL
dl-a-tocopherol 1.5 mL
Filling ratio 54% 54%
Remaining ratio of compound A
98.7 95.4
(%)
CA 02979971 2017-09-15
,
- 34 -
(40 C /4 weeks)
[0198]
As shown in Tables 14 and 15, in the preparation of Example 36 containing
no dl-a-tocopherol, the remaining ratio of compound A was 95 % or more, and in
each
of the preparations of Examples 30 to 35 each containing dl-a-tocopherol, the
remaining ratio of compound A was 97 % or more, which was higher than the
former.
On the basis of the above, it was suggested that compound A was further
stabilized
when tocopherol or derivatives thereof were contained in the ophthalmic depot
preparation of the present invention.