Note: Descriptions are shown in the official language in which they were submitted.
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
SYNERGISTIC COMPOSITIONS AND METHODS FOR MITIGATING SKIN
IRRITATION AND ENHANCING SKIN BARRIER FUNCTION
TECHNICAL FIELD
[0001] One or more embodiments of the present invention provide methods and
compositions for improving skin barrier function and skin hydration. More
particularly, it relates
to compositions containing synergistic combinations of acetyl hexapeptides in
a cosmetically or
pharmaceutically acceptable carrier. Compositions of the invention reduce
inflammation and
protect and heal damaged skin.
BACKGROUND OF THE INVENTION
[0002] Many ingredients in skin care and cosmetic products can cause
skin irritation.
Surfactants such as sodium lauryl sulfate (SLS) are known to be skin
irritants. Retinoid and its
derivatives, can cause severe local irritation manifested as mild erythema and
stratum corneum
peeling of the skin. Topical or systemic use of some skin cleansers and
disinfectants is linked to
skin irritation.
[0003] Ingredients such as benzyol peroxide, alpha-hydroxyl acids and
derivatives thereof,
salicylic acid, natural plant extracts, sunscreen actives, urea, and
preservatives are also known to
cause external skin irritations. Furthermore, skin irritations may be caused
by inherent disease
conditions such as acne, rosacea, atopic dermatitis, and other disease states.
Typical approaches
to reduce irritation include reducing the concentration of the inflammatory
ingredient, use of
alternatives or formulation/delivery approaches, such as encapsulation,
controlled release,
compartmentalization, inclusion of non-irritating excipients. None of the
above has successfully
reduced irritation while retaining efficacy. As a result, there is a need for
anti-irritant substances
to mitigate external skin irritations, or irritations caused by inherent skin
conditions.
[0004] Skin exposure to water and typical cleansers may have a
negative effect on the
stratum corneum (SC) structure and function. Effects include disruption of the
lipid bilayer
architecture to create defects or holes in the barrier. As a result, the
barrier becomes more
permeable, allowing irritants and microorganisms to penetrate into and through
the uppermost
layers of the skin. In cases of severe hand irritation, cracks or fissures
(with or without bleeding)
may develop indicating damage to the dermis. The skin's response to these
damaging effects is
1
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
immediate, but the accelerated efforts to repair the barrier and generate new
stratum corneum
leads to imperfect architecture, when compared to stratum corneum that is
formed during the
normal course of SC replacement. The rapidly-produced SC has poor water
binding properties,
leading to insufficient skin moisture and inadequate desquamation.
[0005] Under normal conditions, there is also a constant loss of SC cells,
as individual units
from the surface of the skin, and new cells move from the bottom of the SC to
the surface,
generally over a period of about 14 days. When skin moisture is too low, the
SC cells come off of
the skin surface as clumps of cells, observed as dry scales. There are
ingredients in most skin
care and cosmetic products that accelerate the loss of SC cells, by affecting
cell viability and/or
by modulating epidermal proliferation. There is a substantial need for
products that protect cell
viability and proliferation, while at the same time decreasing irritation of
the skin cells caused by
exposure to water and typical cleansers.
[0006] Acetyl hexapeptides such as acetyl hexapeptide-3 have been
employed for properties
including anti-wrinkle, collagen boosting, anti-aging, and relaxing of facial
tension. Acetyl
hexapeptide-3 is said to be non-irritating. Some peptides have been described
as capable of
stimulating collagen synthesis, and increasing the hydration of the skin.
[0007] However, specific combinations of acetyl hexapeptides have not
heretofore been
described as providing synergistically enhanced reductions in skin irritation,
nor have they been
described as enhancing skin barrier function and the hydrating efficacy of
lotions.
[0008] Pressure sores, sometimes referred to as bed sores or decubitus
ulcers, may develop if
a person spends a significant amount of time in a wheelchair, regular chair,
or bed. The likelihood
of developing pressure sores is exacerbated if the person is incontinent, is
elderly, has received
radiation therapy, and/or has not been eating well. Conventional incontinence
pads and bandages
can make skin problems worse. Although they may keep bedding and clothing
cleaner, these
products can trap urine, feces, or other possible irritants, keeping them in
constant contact with
the skin. Over time, the skin may break down, and ulcers are likely to
develop.
[0009] Healthcare experts have noted that pressure sores are easier to
prevent than to treat.
Conventional treatment includes multiple steps of applying multiple products
for cleaning, wound
healing, moisturization/hydration of the skin, and barrier protection.
[0010] Many of the current treatments fail to provide adequate skin
protection. Thus, they fail
to eliminate further skin irritation and facilitate the natural healing
process. Others fail to resist
2
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
removal when contacted by clothing and body fluids. Still others fail to
provide acceptable results
within a reasonable time period. Some ointments are difficult to apply and
spread on the damaged
skin. There exists a need in the art for a topical cream formulation that is
easy to apply, physically
stable (i.e. without phase separation), chemically stable, that is well
tolerated by and suitable for
use in individuals with sensitive, reactive, easily irritated or damaged skin.
BRIEF DESCRIPTION OF THE DRAWINGS
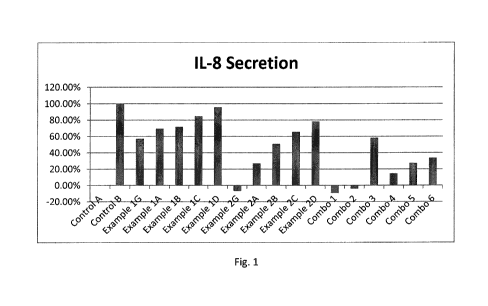
[0011] FIG. 1 is a graphical representation of the effect of
compositions containing
oligopeptides on the irritation response of cells treated with known
irritants, as quantified by
measuring IL-8 secretion.
[0012] FIG. 2 is a graphical representation of the reduction of IL-8
secretion for test samples,
compared to Control B, which contained no oligopeptide.
[0013] FIG. 3 is a graphical representation of the effect of
oligopeptides on cell binding, as
measured by Real-Time Quantitative Reverse Transcription Polymerase Chain
Reaction (qRT-
PCR) for desmoglein (DSG1).
[0014] FIG. 4 is a graphical representation of the effect of
oligopeptides on cell binding, as
measured by qRT-PCR for democollin (DSC3).
[0015] FIG. 5 is a graphical representation of the effect of various
compositions on the
irritation response of cells treated with known irritants, as quantified by
measuring IL-8 secretion,
and on cell viability.
[0016] FIG. 6 is a graphical representation of Involucrin Expression.
[0017] FIG. 7 is a graphical representation of PPAR6 expression.
[0018] FIG. 8 is a graphical representation of ABCA12 expression.
[0019] FIG. 9 is a graphical representation of DSC1 expression.
[0020] FIG. 10 is a graphical representation of APQ3 expression.
[0021] FIG. 11 is a graphical representation of yield stress point for
compositions according
to the present invention and comparative samples.
[0022] FIG. 12 is a graphical representation of thixotropic behavior
for compositions
according to the present invention and comparative samples.
3
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
DETAILED DESCRIPTION OF ILLUSTRATIVE EMBODIMENTS
[0023] In one or more embodiments, compositions of the present
invention include a
combination of at least two oligopeptides in a cosmetically or
pharmaceutically acceptable
carrier.
[0024] Generally, oligopeptides are short chains of amino acid moieties
linked by amide
bonds. Any combination of amino acids may be included.
[0025] In one or more embodiments, the oligopeptides include 12 amino
acid moieties or
less, in other embodiments, 10 amino acid moieties or less, and in other
embodiments, 8 amino
acid moieties or less.
[0026] In one or more embodiments, the peptides include at least 5 amino
acid moieties. In
one or more embodiments, the peptide includes at least 6 amino acid moieties.
In one or more
embodiments, the peptides include from 5 to 6 amino acid moieties. Longer
peptides are believed
to have at least the potential for causing undesirable responses on
application to the human body.
In one or more embodiments, the stereochemistry of at least one of the amino
acids of the peptide
is L-.
[0027] In other embodiments, the oligopeptide may be identified by an
INCI (International
Nomenclature of Cosmetic Ingredients) name, such as acetyl hexapeptide
followed by a
numerical designation. INCI names for suitable oligopeptides include Polmitoyl
Oligopeptide,
Glycerin (and) Aqua (and) Myristoyl Pentapeptide-17, Aqua (and) Butylene
Glycol (and) Oryza
Sativa (Rice) Bran Extract (and) Boswellia Serrata Extract (and) Honey Extract
(and)
Oligopeptide-10 (and) Phenoxyethanol (and) Sodium Benzoate, Oligopeptide-1, rh-
Oligopeptide-
1, rh-Oligopeptide-1 (and) Mannitol (and) Glycerin (and) EDTA (and)
Methylparaben (and)
Ethylparaben, Butylene Glycol (and) Hydrogenated Lecithin (and) Sodium Oleate
(and)
Oligopeptide-68, Hydrolyzed Wheat Protein (and) Palmitoyl Decapeptide-21 (and)
Decapaptide-
22 (and) Oligopeptide-78 (and) Zinc Palmitoyl Nonapeptide-14, Hydrolyzed Wheat
Protein (and)
Palmitoyl Decapeptide-21 (and) Decapeptide-22 (and) Oligopeptide-78 (and) Zinc
Palmitoyl
Nonapeptide-14, and Aqua (and) Rahnella/Soy Protein Ferment (and) Glycerin
(and) Butylene
Glycol (and) Glyceryl Acrylate/Acrylic Acid Copolymer (and) Polysorbate 20
(and) Palmitoyl
Oligopeptide.
4
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
[0028] Oligopeptides are further described in U.S. Published Patent
Application No.
2014/0120141 Al, and co-pending U.S. application number 62/021,310, both of
which are hereby
incorporated by reference.
[0029] In one or more embodiments, compositions of the present
invention include at least
two acetyl hexapeptides. In certain embodiments, a synergistic effect is seen
when two or more
oligopeptides are combined. In other words, the reduction in irritancy when
two or more
oligopeptides are combined is greater than just an additive effect. In one or
more embodiments,
the positive effect on cell regeneration is more than the sum of the
improvement that is seen with
equivalent amounts of the individual components.
[0030] Thus, in one or more embodiments, compositions of the present
invention comprise
two or more oligopeptides. In one or more embodiments, compositions of the
present invention
comprise two or more acetyl hexapeptides. In one or more embodiments,
compositions of the
present invention comprise two or more acetyl hexapeptides, and at least one
of the acetyl
hexapeptides is selected from acetyl hexapeptide-38 and acetyl hexapeptide-46.
In one or more
embodiments, compositions of the present invention comprise acetyl hexapeptide-
38 and acetyl
hexapeptide-46.
[0031] Generally, hexapeptides contain six amino acid moieties. Some
non-limiting examples
of acetyl hexapeptides are acetyl hexapeptide-1, acetyl hexapeptide-7, acetyl
hexapeptide-8,
acetyl hexapeptide-19, acetyl hexapeptide-20, acetyl hexapeptide-22, acetyl
hexapeptide-24,
acetyl hexapeptide-30, acetyl hexapeptide-31, acetyl hexapeptide-37, acetyl
hexapeptide-38,
acetyl hexapeptide-39, acetyl hexapeptide-46, and acetyl hexapeptide-49.
[0032] Acetyl Hexapeptide-1 is reaction product of alanine, arginine,
histidine, leucine,
phenylalanine and tryptophane hexapeptide with acetic acid. Acetyl hexapeptide-
1 is
commercially available, for example from Lucas Meyer Cosmetics as a blend with
glycerin and
water and dextran under the tradename Melitaneg.
[0033] Acetyl hexapeptide-8 is commercially available, for example from
Theraderm
Clinical Skin Care under the tradename Argirelineg.
[0034] Acetyl hexapeptide-30 is commercially available, for example
from Lipotec LLC
under the tradename
[0035] Acetyl hexapeptide-38 is commercially available, for example from
Lipotec LLC as a
blend with butylene glycol and water, under the tradename AdifylineTM.
5
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
[0036] Acetyl hexapeptide-39 is commercially available, for example
from Lipotec LLC
under the tradename Silusyneg.
[0037] Acetyl hexapeptide-46 is commercially available, for example
from Lipotec LLC as a
blend with butylene glycol, water and citric acid, under the tradename
Delisensg.
[0038] Other acetyl hexapeptides are also commercially available.
[0039] In one or more embodiments, the composition includes acetyl
hexapeptide-38 and
acetyl hexapeptide-46.
[0040] In one or more embodiments, compositions of the present
invention include at least
one pentapeptide. Generally, pentapeptides contain five amino acid moieties.
Some non-limiting
examples of p entap epti de s include acetyl p entap epti de-1, pamitoyl p
entap epti de-3, pamitoyl
pentapeptide-4, and myristoyl pentapeptide-17. In one or more embodiments,
compositions of the
present invention comprise pamitoyl pentapeptide-3. In one or more
embodiments, compositions
of the present invention comprise pamitoyl pentapeptide-4. In one or more
embodiments,
compositions of the present invention comprise myristoyl pentapeptide-17.
[0041] Acetyl pentapeptide-1 is commercially available, for example from
Spec Chem Ind.
Under the tradename SpecPed SC-AP1. Pamitoyl pentapeptide-3 is commercially
available, for
example from Spec Chem Ind. Under the tradename SpecPed SC-PP3. Myristoyl
pentapeptide-17
is commercially available, for example from Spec Chem Ind. Under the tradename
SpecPed SC-
MP17.
[0042] The cosmetically or pharmaceutically effective amount of the
peptides of the
invention which should be administered, as well as their dosage, will depend
on numerous
factors, including age, state of the patient, the nature or severity of the
condition, disorder or
disease to be treated and/or cared for, the route and frequency of
administration and of the
particular nature of the peptides to be used.
[0043] In one or more embodiments, compositions of the present invention
comprise at least
an effective amount of the oliopeptide, wherein an effective amount is the
amount that mitigates
skin irritation, enhances skin conditioning, enhances the skin barrier
function, provides hydration,
or enhances healing of damaged skin, when compared to the same composition but
not containing
any oligopeptide. In one or more embodiments, an effective amount of each
oligopeptide is at
least about 0.06 parts per million by weight (ppm), based upon the total
weight of the
6
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
composition. In other embodiments, an effective amount is at least about 0.10
ppm, in other
embodiments at least about 0.12 ppm, based upon the total weight of the
composition.
[0044] In one or more embodiments, the total amount of oligopeptides
in the composition is
at least about 0.12 ppm, in other embodiments, at least about 0.15 ppm, in
other embodiments, at
least about 0.17 ppm, in other embodiments, at least about 0.2 ppm, in other
embodiments, at
least about 0.25 ppm, based upon the total weight of the composition.
[0045] In one or more embodiments, the composition comprises about 100
ppm or less of
each oligopeptide, in other embodiments, about 50 ppm or less, in other
embodiments, about 25
ppm or less, in other embodiments, about 10 ppm or less, in other embodiments,
about 7.5 ppm or
less, in other embodiments, about 5 ppm or less, in other embodiments, about
2.5 ppm or less, in
other embodiments, about 2 ppm or less, in other embodiments, about 1.5 ppm or
less, in other
embodiments, about 1 ppm or less, based upon the total weight of the
composition.
[0046] The total amount of oliopeptides in the composition is not
particularly limited.
Advantageously, due to the synergistic enhancement in efficacy that is
obtained by combining
two or more oligopeptides, the total amount of oligopeptide may be reduced. In
one or more
embodiments, the composition comprises a total of about 1000 ppm or less of
oligopeptides, in
other embodiments, about 800 ppm or less, in other embodiments, about 600 ppm
or less, in other
embodiments, about 500 ppm or less, in other embodiments, about 250 ppm or
less, in other
embodiments, about 200 ppm or less, in other embodiments, about 150 ppm or
less, in other
embodiments, about 100 ppm or less, in other embodiments, about 75 ppm or
less, in other
embodiments, about 50 ppm or less, in other embodiments, about 25 ppm or less,
in other
embodiments, about 20 ppm or less, in other embodiments, about 10 ppm or less,
based upon the
total weight of the composition.
[0047] In one or more embodiments, the composition comprises from
about 0.06 to about 100
ppm of each oligopeptide, based upon the total weight of the composition. In
one or more
embodiments, the composition comprises from about 0.08 to about 50 ppm of each
oligopeptide,
based upon the total weight of the composition. In one or more embodiments,
the composition
comprises from about 0.1 to about 30 ppm of each oligopeptide, based upon the
total weight of
the composition. In one or more embodiments, the composition comprises from
about 0.5 to
about 25 ppm of oligopeptide, based upon the total weight of the composition.
7
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
[0048] The oligopeptides of this invention may have variable solubility
in water. Water-
soluble oligopeptides may be incorporated directly into aqueous compositions.
Water-insoluble
oligopeptides and those with limited water solubility may be solubilized in
cosmetically or
pharmaceutically acceptable solvents such as and not restricted to, ethanol,
propanol,
isopropanol, propylene glycol, glycerin, butylene glycol or polyethylene
glycol or any
combination thereof
[0049] In one or more embodiments, the oligopeptide may be added to the
composition as a
solution or emulsion. In other words, the oligopeptide may be premixed with a
diluent, and
optionally one or more other ingredients, to form an oligopeptide solution or
emulsion, with the
proviso that the diluent does not deleteriously affect the beneficial
properties of the composition.
[0050] Examples of diluents include water, alcohol, or blends of water
and other diluents
such as glycols, ketones, linear and/or cyclic hydrocarbons, triglycerides,
carbonates, silicones,
alkenes, esters such as acetates, benzoates, fatty esters, glyceryl esters,
ethers, amides,
polyethylene glycols, PEG/PPG copolymers, inorganic salt solutions such as
saline, and mixtures
thereof. It will be understood that, when the oligopeptide is premixed to form
an oligopeptide
solution or emulsion, the amount of solution or emulsion that is added to the
composition is
selected so that the amount of oligopeptide falls within the ranges set forth
hereinabove.
[0051] In one or more embodiments, the cosmetically or pharmaceutically
acceptable carrier
may be prepared according to convention known to persons skilled in the art.
For example, the
formulation of cosmetic and pharmaceutical lotion compositions is described in
"Harry's
Cosmeticology," Eighth edition, (2000), Harry, Ralph Gordon, Reiger, Martin
M., ed. Chemical
Publishing Company, which is hereby incorporated by reference.
[0052] The form of the cosmetically or pharmaceutically acceptable
carrier is not particularly
limited, and compositions of the present invention may be formulated as
liquids, lotions, creams,
gels, foams, salves, suspensions, emulsions, and the like. Suitable
formulations are described, for
example, in U.S. Patent Nos. 5,980,970, 8,105,616, U.S. Patent Application
Publication Nos.
2006/0159649 Al, 2009/0041697 Al, 2014/0011894 Al, and 2014/0328769 Al, all of
which are
incorporated by reference herein.
[0053] A wide variety of delivery vehicles may be employed for
compositions according to
the present invention, including pads, bandages, patches, sticks, aerosol
dispensers, pump sprays,
trigger sprays, canisters, and disposable absorbent articles.
8
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
[0054] In one or more embodiments, the compositions of the present
invention may be
formulated as lotions. As is known in the art, lotions include oil-in-water
emulsions as well as
water-in-oil emulsions, oil-water-oil, and water-oil-water. A wide variety of
ingredients may be
present in either the oil or water phase of the emulsion. That is, the lotion
formulation is not
particularly limited.
[0055] Examples of lotion formulations include those containing water
and/or alcohols and
emollients such as hydrocarbon oils and waxes, silicone oils, hyaluronic acid,
vegetable, animal
or marine fats or oils, glyceride derivatives, fatty acids or fatty acid
esters or alcohols or alcohol
ethers, lanolin and derivatives, polyhydric alcohols or esters, wax esters,
sterols, phospholipids
and the like, and generally also emulsifiers (nonionic, cationic or anionic),
although some of the
emollients inherently possess emulsifying properties.
[0056] These same general ingredients may be formulated into a cream
rather than a lotion,
or into gels, or into solid sticks by utilization of different proportions of
the ingredients and/or by
inclusion of thickening agents such as gums, carbomers, or other forms of
hydrophilic colloids.
Very generally, as is known in the art, creams and ointments are typically
spreadable in the range
from room temperature to skin temperature, and lotions and milks are more
flowable within this
temperature.
[0057] In one or more embodiments, the carrier is water-based. In one
or more embodiments,
the carrier contains one or more emulsifiers, one or more vegetal waxes, and
one or more olive
derivatives or extracts.
[0058] In one or more embodiments, a surprising enhancement of the skin
barrier function is
observed when the composition includes a vegetal wax. Advantageously, the
vegetal wax
promotes a liquid crystal network structure to the emulsion.
[0059] In one or more embodiments, a liquid crystal network structure
in the compositions of
the present invention improves the physiological penetration of active
ingredients, increases the
barrier integrity, and aids in functional hydration.
[0060] Without wishing to be bound by theory, it is believed that
carriers including vegetal
wax according to the present invention provide a complex combination of fatty
acids that are
chemically similar to the skin surface lipid composition, and that have the
distinctive property to
self-emulsify in hydrophilic or lipophilic millieus. In one or more
embodiments, the distinctive
complex combination of fatty acids represent a unique biomimetic restructuring
agent endowed
9
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
with the double feature of first restoring and maintaining the integrity of
the skin barrier and then
providing itself the emulsifying base.
[0061] Compositions of the present invention containing a liquid
crystalline carrier provide
liquid crystals with a skin-like fatty acid composition. The highly stable and
dermo-compatible
liquid crystals are similar to the lipids of the cutaneous barrier. In one or
more embodiments, the
liquid crystals act as biomimetic restructuring agents and restore the optimal
integrity of the skin
barrier function and increase the integrity of the stratum corneum barrier
function leading to an
increased and sustained skin hydration.
[0062] The vegetal wax is a vegetable-based liquid crystal promoter
that is believed to
stabilize oil-in-water emulsions and to improve the texture of emulsions. As a
liquid crystal
promoter, the vegetal wax re-organizes the emulsion's microscopic structure,
acting as an
emulsion stabilizing agent. The lamellar liquid crystals produce several bi-
layers that enrobe the
oil droplets, producing an energy layer preventing coalescence.
[0063] In one or more embodiments, the vegetal wax provides the lotion
with sebum-control
benefits. In one or more embodiments, it provides skin-hydration and a unique
texture due to the
high water content of the liquid crystalline structure (water incorporated
between several
bilayers). In one or more embodiments, the vegetal wax influences positively
the delivery of
active ingredients to the skin.
[0064] Examples of vegetal waxes include a blend of cetyl palmitate,
sorbitan palmitate and
sorbitan olivate such as the blend that is available from B&T S.r.l. under the
tradename Oliwax
LC.
[0065] In one or more embodiments, the composition includes at least
one vegetal wax in an
amount of at least about 0.5 wt. %, in other embodiments, at least about 0.75
wt. %, in other
embodiments, at least about 1 wt. %, in other embodiments, at least about 1.5
wt. %, in other
embodiments, at least about 2 wt. %, in other embodiments, at least about 2.5
wt. %, based upon
the total weight of the composition.
[0066] In one or more embodiments, the composition includes at least
one vegetal wax in an
amount of up to about 10 weight percent (wt. %), in other embodiments, up to
about 8 wt. %, in
other embodiments, up to about 5 wt. %, in other embodiments, up to about 3
wt. %, in other
embodiments, up to about 2.5 wt. %, in other embodiments, up to about 2 wt. %,
in other
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
embodiments, up to about 1.5 wt. %, in other embodiments, up to about 1 wt. %,
based upon the
total weight of the composition.
[0067] In one or more embodiments, the composition includes at least
one vegetal wax in an
amount of from about 0.5 to about 10 wt. %, in other embodiments, from about
0.75 to about 8
wt. %, and in other embodiments, based upon the total weight of the
composition.
[0068] In one or more embodiments, the composition further comprises at
least one
derivative or extract of olives. In one or more embodiments, the olive
derivative includes olive
oil. In one or more embodiments, the olive derivative includes a cetearylic
ester derivative and/or
a sorbitan ester derivative. In one or more embodiments, the olive derivative
includes a blend of a
cetearylic ester derivative and a sorbitan ester derivative. In one or more
embodiments, the olive
derivative includes a blend having an INCI designation of Cetearyl Olivate
(and) Sorbitan
Olivate. Cetearyl olivate (and) sorbitan olivate is available from B&T S.r.l.
under the tradename
OliveMg1000. OliveM is sometimes described as an 0/W emulsifier derived from
olive oil. It is
substantially free of polyethylene oxides (PEG).
[0069] In one or more embodiments, it is believed that the cetearylic ester
derivative
stabilizes the liquid crystals. In certain embodiments, it is believed that
the sorbitan ester
derivative enhances the emolliency properties of the composition and/or
provides easier
dispersion for powders. Advantageously, UV filters and pigments may be easily
dispersed at high
percentages. It is believed that the blend of a cetearylic ester derivative
and a sorbitan ester
derivative of olive combines a liquid crystal structure with an oleic
component derived from olive
oil. In one or more embodiments, skin penetration is enhanced, and a soft,
silky smooth after-feel
is obtained. The substantivity of its composition, being very similar to the
human sebum,
provides retention of the skin moisture and increases the active ingredient's
resistance to water
and/or sweat.
[0070] It is believed that the blend of a cetearylic ester derivative and a
sorbitan ester
derivative of olive operates by forming liquid crystals in emulsions, by
placing itself at the
interface of a two phase system in a preferential direction, placing the polar
head into the aqueous
phase and the nonpolar tail into the lipidic phase. In one or more
embodiments, the postmicellar
organization of the blend of a cetearylic ester derivative and a sorbitan
ester derivative of olive in
water is the typical structure of a liquid crystal reticule, where the bilayer
micelles create a
multilayer lamellar formation.
11
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
[0071] In one or more embodiments, emulsions that are formulated with
the blend of a
cetearylic ester derivative and a sorbitan ester derivative of olive appear
very shiny and light and
have an original, fresh and silky touch, even if they contain high percentages
of lipids. The blend
of a cetearylic ester derivative and a sorbitan ester derivative of olive,
promoting the formation of
this reticular structure inside the emulsion, allows the formulation to
contain quite large amounts
of natural and polar lipids without affecting the final stability of the
emulsion. In one or more
embodiments, up to about 25 wt. % of the emulsion may be natural and/or polar
lipids.
[0072] In one or more embodiments, the composition includes at least
one olive derivative or
extract in an amount of at least about 0.1 wt. %, in other embodiments, at
least about 0.25 wt. %,
in other embodiments, at least about 0.5 wt. %, in other embodiments, at least
about 0.75 wt. %,
in other embodiments, at least about 1 wt. %, in other embodiments, at least
about 1.5 wt. %, in
other embodiments, at least about 2 wt. %, in other embodiments, at least
about 2.5 wt. %, based
upon the total weight of the composition.
[0073] In one or more embodiments, the composition includes at least
one olive derivative or
extract in an amount of up to about 20 wt. %, in other embodiments, up to
about 18 wt. %, in
other embodiments, up to about 15 wt. %, in other embodiments, up to about 10
wt. %, in other
embodiments, up to about 8 wt. %, in other embodiments, up to about 5 wt. %,
in other
embodiments, up to about 3 wt. %, in other embodiments, up to about 2 wt. %,
in other
embodiments, up to about 1 wt. %, in other embodiments, up to about 0.5 wt. %,
based upon the
total weight of the composition.
[0074] In one or more embodiments, the composition includes at least
one olive derivative or
extract in an amount of from about 0.1 to about 20 wt. %, in other
embodiments, from about 0.5
to about 15 wt. %, based upon the total weight of the composition.
[0075] In one or more embodiments, the lotion compositions include
olive oil.
[0076] In one or more embodiments, the composition includes at least one
olive oil in an
amount of at least about 0.1 wt. %, in other embodiments, at least about 0.25
wt. %, in other
embodiments, at least about 0.5 wt. %, in other embodiments, at least about
0.75 wt. %, in other
embodiments, at least about 1 wt. %, in other embodiments, at least about 1.5
wt. %, in other
embodiments, at least about 2 wt. %, in other embodiments, at least about 2.5
wt. %, based upon
the total weight of the composition.
12
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
[0077] In one or more embodiments, the composition includes at least
one olive oil in an
amount of up to about 20 wt. %, in other embodiments, up to about 18 wt. %, in
other
embodiments, up to about 15 wt. %, in other embodiments, up to about 10 wt. %,
in other
embodiments, up to about 8 wt. %, in other embodiments, up to about 5 wt. %,
in other
embodiments, up to about 3 wt. %, in other embodiments, up to about 2 wt. %,
in other
embodiments, up to about 1 wt. %, in other embodiments, up to about 0.5 wt. %,
based upon the
total weight of the composition.
[0078] In one or more embodiments, the composition includes at least
one olive oil in an
amount of from about 0.1 to about 20 wt. %, in other embodiments, from about
0.5 to about 15
wt. %, based upon the total weight of the composition.
[0079] In one or more embodiments, the carrier includes one or more
emulsifiers. Examples
of emulsifiers include glycerol esters, in particular glycerol esters of a-
hydroxycarboxylic acids
and saturated fatty acids. Specific examples include glyceryl stearate. In one
or more
embodiments, the total amount of the glycerol esters in the composition is
advantageously chosen
from the range from about 0.1 to about 10.0 wt. %, in one or more embodiments,
from about 0.5
to about 6.0 wt. %, based on the total weight of the composition.
[0080] In one or more embodiments, the carrier comprises a cold process
formulation aid. In
one or more embodiments, the carrier comprises at least one wax selected from
the group
consisting of natural waxes and synthetic waxes and at least one cationic
polymer. In one or more
embodiments, the carrier comprises one or more C12 -C18 fatty acid-C2 -05
polyol esters such
as glyceryl monostearate, ethylene glycol monostearate and polyethylene glycol
distearate.
Examples of polyethylene glycol di stearates include PEG-150 di stearate.
Examples of cationic
polymers include polyquaternium polymers, such as polyquaternium-37. The
carrier may further
comprise one or more fatty alcohols. Examples of fatty alcohols include
cetearyl alcohol. Cold
process formulation aids are further described in U.S. Patent Application
Publication Nos.
2011/0250148, 2011/0250151, 2014/0094558, and 2014/0336308, all of which are
incorporated
by reference herein.
[0081] Compositions or the present invention may further comprise one
or more of a wide
range of optional ingredients, with the proviso that they do not deleteriously
affect the beneficial
properties of the composition. The Personal Care Products Council
International Cosmetic
Ingredient Dictionary and Handbook, Fifteenth Edition 2014, and the 2007 CTFA
International
13
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
Buyer's Guide, both of which are incorporated by reference herein in their
entirety, describe a
wide variety of non-limiting cosmetic and pharmaceutical ingredients commonly
used in the skin
care industry, that are suitable for use in the compositions of the present
invention. Examples of
optional ingredients, classified by function, include: abrasives, anti-acne
agents, anticaking
agents, antioxidants, binders, biological additives, bulking agents, chelating
agents, chemical
additives; colorants, cosmetic astringents, cosmetic biocides, denaturants,
drug astringents,
emulsifiers, external analgesics, film formers, fragrance components,
humectants, opacifying
agents, plasticizers, preservatives (sometimes referred to as antimicrobials),
propellants, reducing
agents, skin bleaching agents, skin-conditioning agents (emollient,
miscellaneous, and occlusive),
skin protectants, solvents, surfactants, foam boosters, hydrotropes,
solubilizing agents,
suspending agents (nonsurfactant), sunscreen agents, ultraviolet light
absorbers, detackifiers, and
viscosity increasing agents (aqueous and nonaqueous). Examples of other
functional classes of
materials useful herein include solubilizing agents, sequestrants, and
keratolytics, topical active
ingredients, deposition enhancers, humectants, moisturizing esters,
emulsifying agents, silicone
glycols, miscellaneous skin conditioners, thickeners, and/or antimicrobial
agents.
[0082]
Optionally, compositions of the present invention may include one or more
pharmacological and/or antibiotic agents, with the proviso that the
pharmacological and/or
antibiotic ingredient does not deleteriously affect the skin barrier function
or skin conditioning
properties of the composition. Examples of such agents include, but are not
limited to, antifungal
agents, antiviral agents, antimicrobial agents, and antiparasitic agents. In
one or more
embodiments, one or more antimicrobial agents are included. Examples of
antimicrobial agents
include, but are not limited to, triclosan, also known as 5-chloro-2(2,4-
dichlorophenoxy) phenol
(PCMX) and available from Ciba-Geigy Corporation under the tradename IRGASANg;
chloroxylenol, also known as 4-chloro-3,5-xylenol, available from Nipa
Laboratories, Inc. under
the tradenames NIPACIDE MX or PX; hexetidine, also known as
5-amino-1,3-bis(2-ethylhexyl)-5-methyl-hexahydropyrimidine; chlorhexidine
salts including
chl orhexi dine gluconate and the salts
of
N,N"-Bi s(4-chloropheny1)-3,12-diimino- 2,4, 11,14-
tetraazatetradecanediimidi amide;
2-bromo-2-nitropropane-1; 3 -di ol, benzalkonium chloride; cetylpyridinium
chloride;
alkylbenzyldimethylammonium chlorides; iodine; phenol, bisphenol, diphenyl
ether, phenol
derivatives, povidone-iodine including polyvinylpyrrolidinone-iodine;
parabens; hydantoins and
14
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
derivatives thereof, including 2,4-imidazolidinedione and derivatives of 2,4-
imidazolidinedione
as well as dimethylo1-5,5-dimethylhydantoin (also known as DMDM hydantoin or
glydant);
phenoxy ethanol ; cis isomer of 1-(3-chloroally1)-3,5,6-triaza- 1 -
azoniaadamantane chloride, also
known as quaternium-15 and available from Dow Chemical Company under the
tradename
DOWCILTM 200; diazolidinyl urea; benzethonium chloride; methylbenzethonium
chloride;
glyceryl laurate, transition metal compounds such as silver, copper,
magnesium, zinc compounds,
hydrogen peroxide, chlorine dioxide, anilides, bisguanidines, a blend of
biostatic and fungistatic
agents having the INCI name caprylhydroxamic acid (and) propanediol, and
mixtures thereof In
one or more embodiments, the composition comprises from about 0.05 to about 3
wt. %, in other
embodiments, from about 0.07 to about 2.5 wt. %, in other embodiments, from
about 0.09 to
about 1 wt. %, in other embodiments, from about 0.1 to about 0.75 wt. %, in
other embodiments,
from about 0.15 to about 0.5 wt. %, of at least one antimicrobial agents,
based upon the total
weight of the composition.
[0083] In one or more embodiments, the composition may include at least
one antibiotic.
Examples of antibiotics include asaminoglycoside antibiotics, cephalosporins,
carbapenems,
quinolone, macrolide antibiotics, penicillins, sulfonamides, tetracyclines,
oxazolidinones,
lipopeptides, gemifloxacin, ketolides, clindamycin, metronidazole, vancomycin,
rifabutin,
rifampin, nitrofurantoin, chloramphenicol, erythromycin, gentamicin,
vancomycin, ciproflaxin,
doxycycline, minocycline, isoniazid, ethambutol, clofazimine,
fluoroquinolones, pyrazinamide,
streptomycin, ofloxacin, ganciclovir, azithromycin, clarithromycin, dapsone,
ampicillin,
amphotericin B, ketoconazole, fluconazole, pyrimethamine, sulfadiazine,
lincomycin, acyclovir,
trifluorouridine, pentamidine, atovaquone, paromomycin, diclazaril, acyclovir,
trifluorouridine,
foscarnet, sparfloxacin, and pharmaceutically acceptable salts and hydrates
thereof
[0084] In one or more embodiments, compositions of the present
invention may further
include one or more probiotics and/or prebiotics. In one or more embodiments,
the one or more
probiotics include one or more skin commensal microorganisms which positively
affect the skin
microbiota. For example, the one or more probiotics can include microorganisms
that positively
affect the skin surface environment, e.g., by altering the pH or inhibiting
growth of pathogenic
microorganisms. In one or more embodiments, the one or more probiotics can
include one or
more microorganisms naturally found on the skin surface of the individual. In
one or more
embodiments, the one or more probiotics can include one or more microorganism
that are not
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
naturally found on the skin surface of the individual, but positively affect
the skin surface
environment. In one or more embodiments, the one or more probiotics can
include one or more
engineered microorganisms. For example, the one or more probiotics can include
a
microorganism genetically engineered to have a property that positively
affects the skin surface
environment, e.g., by synthesizing and excreting an inhibitor of pathogenic
microorganisms. See,
e.g., Martin et al. (2013) Microbial Cell Factories, 12:71, which is
incorporated herein by
reference. In one or more embodiments, the probiotic comprises live probiotic
microorganisms.
In one or more embodiments, the probiotics may be included in a live form,
dead form, semi-
active or in deactivated form and fragments or fractions originating from the
microorganism
either live or dead (e.g., as a lyophilized powder). In one or more
embodiments, the probiotic
includes culture supernatants of the microorganisms.
[0085] In one or more embodiments, the one or more probiotics include
one or more bacterial
probiotics. See, e.g., U.S. Pat. No. 8,557,560, U.S. Patent Application
Publication Nos.
2011/0274676 Al, 2014/0037688 Al, Schrezenmeir & De Vrese (2001) Am. J. Clin.
Nutr.
73(suppl):3615-3645, and Gueniche et al. (2009) Exp. Dermatol. 19:e 1 -e8, all
of which are
incorporated herein by reference.
[0086] In one or more embodiments, the one or more bacterial probiotics
include one or more
of Firmicutes, Actinobacteria, Bacteriodetes, Proteobacteria, or
Cyanobacteria. In one or more
embodiments, the one or more bacterial probiotics include one or more of
Corynebacteria,
Propionibacteria, Micrococci, or Staphylococci. In one or more embodiments,
the one or more
bacterial probiotics include non-lactic acid and/or lactic acid producing
bacteria (LAB) and can
include Bacteroides, Bifidobacterium, and Lactobacillus. In one or more
embodiments, the one or
more bacterial probiotics include certain strains of Aerococcus, E. coli,
Bacillus, Enterococcus,
Fusobacterium, Lactococcus, Leuconostoc, Melissacoccus, Micrococcus,
Oenococcus,
Sporolactobacillus, Streptococcus, Staphylococcus, Saccharomyces, Pediococcus,
Peptostreptococcus, Proprionebacterium, and Weissella. A wide variety of
strains of bacteria are
available from the ATCC, Manassas, Va. In one or more embodiments, the one or
more
probiotics include one or more non-pathogenic strains of pathogenic bacteria.
[0087] In one or more embodiments, the one or more probiotic may
include a bacterial strain
that inhibits a second bacterial strain, e.g., by out competing for resources
or by inhibiting the
growth of the second bacterial stain. In one or more embodiments, the one or
more probiotics
16
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
include skin commensal microorganism Staphylococcus epidermidis. For example,
Staphylococcus epidermidis may be used as a probiotic to modulate growth of
pathogenic
bacteria on the skin surface by producing microbial peptides that inhibit
Staphylococcus aureus
biofilm formation and/or by producing lanthionine-containing antibacterial
peptides, e.g.,
bacteriocins, which are known to exhibit antibacterial properties toward
certain species of
harmful bacteria, e.g., Streptococcus aureus and Streptococcus pyogenes. For
example,
Staphylococcus epidermidis may be used as a probiotic to stimulate the immune
system by
influencing the innate immune response of keratinocytes through Toll-like
receptor ("TLR")
signaling. For example, Staphylococcus epidermidis may be used as a probiotic
to inhibit the
action of more virulent microorganisms such as Staphylococcus aureus by
occupying receptors
on a host cell that also bind the virulent microorganism. See, e.g., Orrice &
Segre (2011) Nat.
Rev. Microbiol. 9:244-53, which is incorporated herein by reference.
[0088] In one or more embodiments, the one or more probiotics can
include skin commensal
microorganism Propionibacterium acnes. For example, Propionibacterium acnes
can be used as a
probiotic to consume skin oil and to produce byproducts such as short-chain
fatty acids and
propionic acid known to help maintain a healthy skin barrier.
[0089] In one or more embodiments, the one or more treatment agents
include one or more
prebiotics. In one or more embodiments, the one or more prebiotics are agents
that promote the
survival and/or growth of microorganisms of interest on the skin surface of
the individual. In one
or more embodiments, the one or more prebiotics include at least one of
galacto-oligosaccharides,
fructo-oligosaccharides, inulin, or lactulose. In one or more embodiments, the
one or more
prebiotics include one or more of iron, biotin, nicotinic acid, D-pantothenic
acid, pyridoxal,
pyridoxamine dihydrochloride, thiamin hydrochloride, valine, arginine,
galactose, mannose,
fructose, sucrose, lactose, or maltose. In one or more embodiments, the one or
more prebiotics
include one or more of plant derived prebiotics, e.g., derived from acacia
gum, konjac, chicory
root, Jerusalem artichoke, asparagus, and dandelion greens. See, e.g., U.S.
Patent Application
Publication NO. 2013/0115317 Al; and Bateni et al. (2013) Am. J. Dermatology
Venereology
2:10-14, both of which are incorporated herein by reference.
[0090] The composition may further comprise one or more zinc compounds.
Examples of
zinc compounds include aluminum zinc oxide, ammonium silver zinc aluminum
silicate,
ethylene/zinc acrylate copolymer,
lactobacillus/milk/calcium/phosphorus/magnesium/zinc
17
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
ferment, lactobacillus/milk/manganese/zinc ferment lysate, luminescent zinc
sulfide,
magnesium/aluminum/zinc/hydroxide/carbonate, porphyridium/zinc ferment,
saccharomyces/zinc
ferment, saccharomyces/zinc/iron /germanium/copper /magnesium /silicon
ferment,
saccharomyces/zinc/magnesium/calcium/germanium/selenium ferment,
silicon
/titanium/cerium/zinc oxides, sodium zinc cetyl phosphate, sodium zinc
histidine
dithiooctanamide, zinc acetate, zinc acetylmethionate, zinc adenosine
triphosphate, zinc
ascorbate, zinc aspartate, zinc borate, zinc borosilicate, zinc carbonate,
zinc carbonate hydroxide,
zinc cerium oxide, zinc chloride, zinc citrate, zinc coceth sulfate, zinc coco-
sulfate, zinc
cysteinate, zinc dibutyldithiocarbamate, zinc DNA, zinc formaldehyde
sulfoxylate, zinc
glucoheptonate, zinc gluconate, zinc glutamate, zinc glycinate, zinc
glycyrrhetinate, zinc
hexametaphosphate, zinc hydrolyzed collagen, zinc lactate, zinc laurate, zinc
magnesium
aspartate, zinc myristate, zinc neodecanoate, zinc oxide, zinc palmitate, zinc
PCA, zinc
pentadecene tricarboxylate, zinc peroxide, zinc phenolsulfonate, zinc
picolinate, zinc pyrithione,
zinc ricinoleate, zinc rosinate, zinc salicylate, zinc silicates, zinc
stearate, zinc sulfate, zinc
sulfide, zinc thiosalicylate, zinc undecylenate, zinc undecylenoyl hydrolyized
wheat protein, and
zinc zeolite.
[0091]
It will be understood that recommended amounts of zinc compounds for
achieving
good barrier properties are about 20 wt. % or higher. Advantageously, as a
result of the
surprising synergistic effect of the combination of oligopeptides, as well as
the liquid crystal
carrier, beneficial skin-conditioning and barrier effects are achieved with
compositions
containing little or no zinc. Therefore, in one or more embodiments, the total
amount of zinc
compounds may be limited. In one or more embodiments, the total amount of zinc
compounds in
the composition may be less than about 2 wt. %, in other embodiments, less
than about 1 wt. %,
in other embodiments, less than about 0.5 wt. %, in other embodiments, less
than about 0.1 wt. %,
in other embodiments, less than about 0.05 wt. %, based upon the total weight
of the composition.
In one or more embodiments, the composition is devoid of zinc compounds.
[0092]
In one or more embodiments, the amount of zinc oxide may be less than about
2 wt.
%, in other embodiments, less than about 1 wt. %, in other embodiments, less
than about 0.5 wt.
%, in other embodiments, less than about 0.1 wt. %, in other embodiments, less
than about 0.05
wt. %, based upon the total weight of the composition. In other words, the
amount of zinc oxide
18
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
may be from about zero to about 2 wt. %, based upon the total weight of the
composition. In one
or more embodiments, the composition is devoid of zinc oxide.
[0093] In certain embodiments, the composition comprises one or more
humectants.
Examples of humectants include propylene glycol, hexylene glycol, 1,4-
dihydroxyhexane, 1,2,6-
hexanetriol, sorbitol, butylene glycol, propanediols, such as methyl propane
diol, dipropylene
glycol, triethylene glycol, glycerin (glycerol), polyethylene glycols,
ethoxydiglycol, polyethylene
sorbitol, glycolic acid, glycolate salts, lactate salts, urea, hydroxyethyl
urea, alpha-hydroxy acids,
such as lactic acid, sodium pyrrolidone carboxylic acid, hyaluronic acid,
chitin, and combinations
thereof.
[0094] Examples of polyethylene glycol humectants include PEG-4, PEG-6, PEG-
7, PEG-8,
PEG-9, PEG-10, PEG-12, PEG-14, PEG-16, PEG-18, PEG-20, PEG-32, PEG-33, PEG-40,
PEG-
45, PEG-55, PEG-60, PEG-75, PEG-80, PEG-90, PEG-100, PEG-135, PEG-150, PEG-
180, PEG-
200, PEG-220, PEG-240, and PEG-800.
[0095] In one or more embodiments, the composition includes at least
one humectant in an
amount of at least about 0.001 wt. %, in other embodiments, at least about
0.002 wt. %, in other
embodiments, at least about 0.005 wt. %, in other embodiments, at least about
0.01 wt. %, in
other embodiments, at least about 0.02 wt. %, in other embodiments, at least
about 0.05 wt. %, in
other embodiments, at least about 0.1 wt. %, in other embodiments, at least
about 0.2 wt. %, in
other embodiments, at least about 0.5 wt. %, in other embodiments, at least
about 0.7 wt. %, in
other embodiments, at least about 1 wt. %, in other embodiments, at least
about 1.5 wt. %, in
other embodiments, at least about 2 wt. %, based upon the total weight of the
composition.
[0096] In one or more embodiments, the composition includes at least
one humectant in an
amount of up to about 20 wt. %, in other embodiments, up to about 15 wt. %, in
other
embodiments, up to about 10 wt. %, in other embodiments, up to about 8 wt. %,
in other
embodiments, up to about 5 wt. %, in other embodiments, up to about 3 wt. %,
based upon the
total weight of the composition.
[0097] Advantageously, as a result of the surprising synergistic effect
of the combination of
oligopeptides, as well as the liquid crystal carrier, beneficial skin-
conditioning effects are
achieved with compositions containing relatively low amounts of humectants. In
one or more
embodiments, the total amount of humectants in the composition may be less
than about 2 wt. %,
in other embodiments, less than about 1 wt. %, in other embodiments, less than
about 0.5 wt. %,
19
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
in other embodiments, less than about 0.1 wt. %, in other embodiments, less
than about 0.05 wt.
%, based upon the total weight of the composition. In one or more embodiments,
the composition
is devoid of any of the above humectants.
[0098] In one or more embodiments, the amount of glycerin may be less
than about 2 wt. %,
in other embodiments, less than about 1 wt. %, in other embodiments, less than
about 0.5 wt. %,
in other embodiments, less than about 0.1 wt. %, in other embodiments, less
than about 0.05 wt.
%, based upon the total weight of the composition. In one or more embodiments,
the composition
is devoid of glycerin. It is believed that one of the reasons that
compositions of the present
invention have better aesthetics is due to the lower amount of total raw
materials that are required
to produce a product with effective skin-conditioning benefits.
[0099] In these or other embodiments, the composition comprises one or
more conditioning
or moisturizing esters that are not olive derivatives. Examples include cetyl
myristate, cetyl
myristoleate, and other cetyl esters, diisopropyl sebacate, and isopropyl
myristate.
[00100] In one or more embodiments, the composition includes at least one
conditioning or
moisturizing ester in an amount of up to about 10 % by weight, in other
embodiments, up to
about 5 wt. %, in other embodiments, up to about 2 wt. %, in other
embodiments, up to about 1
wt. %, based upon the total weight of the composition.
[00101] In one or more embodiments, the composition includes at least one
conditioning or
moisturizing ester in an amount of at least about 0.001 wt. %, in other
embodiments, at least
about 0.002 wt. %, in other embodiments, at least about 0.005 wt. %, in other
embodiments, at
least about 0.01 wt. %, in other embodiments, at least about 0.02 wt. %, in
other embodiments, at
least about 0.05 wt. %, in other embodiments, at least about 0.1 wt. %, in
other embodiments, at
least about 0.2 wt. %, in other embodiments, at least about 0.5 wt. %, in
other embodiments, at
least about 0.7 wt. %, in other embodiments, at least about 1 wt. %, based
upon the total weight
of the composition.
[00102] In another embodiment each ester that is included is present in an
amount of from
about 0.5 to about 5 % by weight, in another embodiment from about 1 to about
2 % by weight,
based upon the total weight of the composition.
[00103] On the other hand, as a result of the surprising synergistic
effect of the combination of
oligopeptides, as well as the liquid crystal carrier that includes a vetegetal
wax and an olive
derivative or extract, additional moisturizing esters are not required, or may
be present in
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
relatively low amounts. In one or more embodiments, the total amount of
moisturizing esters that
are not olive-derived in the composition may be less than about 1 wt. %, in
other embodiments,
less than about 0.5 wt. %, in other embodiments, less than about 0.1 wt. %, in
other embodiments,
less than about 0.05wt. %, based upon the total weight of the composition. In
one or more
embodiments, the composition is devoid of moisturizing esters that are not
olive-derived.
[00104] In any of the embodiments described above, the composition may be a
gel, cream,
lotion, ointment, and the like, and may also contain one or more thickening
agents. Examples of
thickeners include stearyl alcohol, cationic hydroxy ethyl cellulose (Ucare;
JR30), hydroxy
propyl methyl cellulose, hydroxy propyl cellulose (Klucel), chitosan
pyrrolidone carboxylate
(Kytamer), behenyl alcohol, zinc stearate, and emulsifying waxes, including
but not limited to
Incroquat and Polawax. Other thickening and/or gelling agents suitable for
incorporation into the
anti-irritant gels, creams, lotions or ointments described herein include, for
example, an addition
polymer of acrylic acid, a resin such as Carbopolg ETD 2020, guar gum, acacia,
acrylates/steareth-20 methacrylate copolymer, agar, algin, alginic acid,
ammonium acrylate co-
polymers, ammonium alginate, ammonium chloride, ammonium sulfate, amylopectin,
attapulgite,
bentonite, C9-15 alcohols, calcium acetate, calcium alginate, calcium
carrageenan, calcium
chloride, caprylic alcohol, carbomer 910, carbomer 934, carbomer 934P,
carbomer 940, carbomer
941, carboxymethyl hydroxyethyl cellulose, carboxymethyl hydroxypropyl guar,
carrageenan,
cellulose, cellulose gum, cetearyl alcohol, cetyl alcohol, corn starch, damar,
dextrin, dibenzlidine
sorbitol, ethylene dihydrogenated tallowamide, ethylene diolamide, ethylene
distearamide,
gelatin, guar gum, guar hydroxypropyltrimonium chloride, hectorite, hyaluronic
acid, hydrated
silica, hydroxybutyl methylcellulose, hydroxyethylcellulose, hydroxyethyl
ethylcellulose,
hydroxyethyl stearamide-MIPA, isocetyl alcohol, isostearyl alcohol, karaya
gum, kelp, lauryl
alcohol, locust bean gum, magnesium aluminium silicate, magnesium silicate,
magnesium
trisilicate, methoxy PEG-22/dodecyl glycol copolymer, methylcellulose,
microcrystalline
cellulose, montmorillonite, myristyl alcohol, oat flour, oleyl alcohol, palm
kernel alcohol, pectin,
PEG-2M, PEG-5M, polyacrylic acid, polyvinyl alcohol, potassium alginate,
potassium
aluminium polyacrylate, potassium carrageenan, potassium chloride, potassium
sulfate, potato
starch, propylene glycol alginate, sodium acrylate/vinyl alcohol copolymer,
sodium
carboxymethyl dextran, sodium carrageenan, sodium cellulose sulfate, sodium
chloride, sodium
polymethacylate, sodium silicoaluminate, sodium sulfate, stearalkonium
bentotnite,
21
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
stearalkonium hectorite, stearyl alcohol, tallow alcohol, TEA-hydrochloride,
tragacanth gum,
tridecyl alcohol, tromethamine magnesium aluminium silicate, wheat flour,
wheat starch, xanthan
gum, abietyl alcohol, acrylinoleic acid, aluminum behenate, aluminum
caprylate, aluminum
dilinoleate, aluminum salts, such as distearate, and aluminum isostearates,
beeswax, behenamide,
butadiene/acrylonitrile copolymer, C29-70 acid, calcium behenate, calcium
stearate, candelilla
wax, carnauba, ceresin, cholesterol, cholesterol hydroxystearate, coconut
alcohol, copal,
diglyceryl stearate malate, dihydroabietyl alcohol, dimethyl lauramine oleate,
dodecanoic
acid/cetearyl alcohol/glycol copolymer, erucamide, ethylcellulose, glyceryl
triacetyl
hydroxystearate, glyceryl tri-acetyl ricinolate, glycol dibehenate, glycol di-
octanoate, glycol
distearate, hexanediol distearate, hydrogenated C6-14 olefin polymers,
hydrogenated castor oil,
hydrogenated cottonseed oil, hydrogenated lard, hydrogenated menhaden oil,
hydrogenated palm
kernel glycerides, hydrogenated palm kernel oil, hydrogenated palm oil,
hydrogenated
polyisobutene, hydrogenated soybean oil, hydrogenated tallow amide,
hydrogenated tallow
glyceride, hydrogenated vegetable glyceride, hydrogenated vegetable oil, Japan
wax, jojoba wax,
lanolin alcohol, shea butter, lauramide, methyl dehydroabietate, methyl
hydrogenated rosinate,
methyl rosinate, methylstyrene/vinyltoluene copolymer, microcrystalline wax,
montan acid wax,
montan wax, myristyleicosanol, myristyloctadecanol, octadecene/maleic
anhyrdine copolymer,
octyldodecyl stearoyl stearate, oleamide, oleostearine, ouricury wax, oxidized
polyethylene,
ozokerite, paraffin, pentaerythrityl hydrogenated rosinate, pentaerythrityl
tetraoctanoate,
pentaerythrityl rosinate, pentaerythrityl tetraabietate, pentaerythrityl
tetrabehenate,
pentaerythrityl tetraoleate, pentaerythrityl tetrastearate, ophthalmic
anhydride/glycerin/glycidyl
decanoate copolymer, ophthalmic/trimellitic/glycols copolymer, polybutene,
polybutylene
terephthalate, polydipentene, polyethylene, polyisobutene, polyisoprene,
polyvinyl butyral,
polyvinyl laurate, propylene glycol dicaprylate, propylene glycol dicocoate,
propylene glycol
diisononanoate, propylene glycol dilaurate, propylene glycol dipelargonate,
propylene glycol
distearate, propylene glycol diundecanoate, PVP/eiconsene copolymer,
PVP/hexadecene
copolymer, rice bran wax, stearlkonium bentonite, stearalkonium hectorite,
stearamide,
stearamide DEA-distearate, stearamide DIBA-stearate, stearamide MEA-stearate,
stearone,
stearyl erucamide, stearyl stearate, stearyl stearoyl stearate, synthetic
beeswax, synthetic wax,
trihydroxystearin, triisononanoin, triisostearin, tri-isostearyl trilinoleate,
trilaurin, trilinoleic acid,
22
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
trilinolein, trimyristin, triolein, tripalmitin, tristearin, zinc laurate,
zinc myristate, zinc
neodecanoate, zinc rosinate, and mixtures thereof.
[00105] Examples of thickeners/rheology modifiers include associative
polymers. Associative
polymers include non-ionic polymeric thickeners. In one or more embodiments,
the associative
polymer includes a hydrophilic backbone and hydrophobic end groups. In one or
more
embodiments, the non-ionic polymer includes urethane-based and polyether
polyol-based
associative thickeners.
[00106] Typical amounts of the above thickeners are from about 0.6 to about 2
wt.%, based
upon the total weight of the composition.
[00107] In one or more embodiments, the composition may be thickened with
polyacrylate
thickeners such as those conventionally available and/or known in the art.
Examples of
polyacrylate thickeners include carbomers, acrylates/C 10-30 alkyl acrylate
crosspolymers,
copolymers of acrylic acid and alkyl (C5 -C10) acrylate, copolymers of acrylic
acid and maleic
anhydride, and mixtures thereof Polyacrylate thickeners are further described
in U.S. Patent
Application Publication No. 2010/0317743 Al, which is hereby incorporated by
reference.
[00108] In one or more embodiments, strong acids and other ingredients that
may attack the
peptide bonds in the oligopeptide may be limited. In one or more embodiments,
the amount of
protein denaturants is limited. In one or more embodiments, elevated
temperatures are avoided.
[00109] Advantageously, ingredients that are typically required for
barrier and/or hydration
products may be limited, or eliminated altogether, which may lead to improved
rheology,
aesthetics, and/or stability. In one or more embodiments, the compositions
according to the
present invention include from zero up to about 2 wt. % dimethicone. In one or
more
embodiments, the total amount of dimethicones in the composition is less than
about 2 wt. %, in
other embodiments, less than about 1.5 wt. %, in other embodiments, less than
about 1 wt. %, in
other embodiments, less than about 0.5 wt. %, in other embodiments, less than
about 0.1 wt. %, in
other embodiments, less than about 0.05 wt. %, based upon the total weight of
the composition.
In one or more embodiments, the composition is devoid of dimethicones.
[00110] In one or more embodiments, the amount of petrolatum may be limited.
More
specifically, in one or more embodiments, the total amount of petrolatum in
the composition may
be less than about 2 wt. %, in other embodiments, less than about 1 wt. %, in
other embodiments,
less than about 0.5 wt. %, in other embodiments, less than about 0.1 wt. %, in
other embodiments,
23
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
less than about 0.05 wt. %, based upon the total weight of the composition. In
one or more
embodiments, the composition is devoid of petrolatum.
[00111] In one or more embodiments, the amount of mineral oil may be limited.
More
specifically, in one or more embodiments, the total amount of mineral oil in
the composition may
be less than about 2 wt. %, in other embodiments, less than about 1 wt. %, in
other embodiments,
less than about 0.5 wt. %, in other embodiments, less than about 0.1 wt. %, in
other embodiments,
less than about 0.05 wt. %, based upon the total weight of the composition. In
one or more
embodiments, the composition is devoid of mineral oil.
[00112] It is also contemplated that ingredients identified throughout
this specification,
including but not limited to the oligopeptides, may be individually or
combinatorially
encapsulated for delivery to a target area such as skin. Non-limiting examples
of encapsulation
techniques include the use of liposomes, vesicles, and/or nanoparticles (e.g.,
biodegradable and
non-biodegradable colloidal particles comprising polymeric materials in which
the ingredient is
trapped, encapsulated, and/or absorbed--examples include nanospheres and
nanocapsules) that
can be used as delivery vehicles to deliver the ingredient to skin.
Encapsulation is further
described in U.S. Pat. Nos. 6,387,398, 6,203,802, and 5,411,744, all of which
are incorporated by
reference herein. Encapsulation of the oligopeptides may allow the use of
ingredients that would
otherwise be limited, such as strong acids and ethanol.
[00113] The composition may be prepared by simply mixing the components
together. The
order of addition is not particularly limited, but may advantageously be
selected based upon the
solubility of the various ingredients.
[00114] In one or more embodiments, the composition may be prepared by
combining at
elevated temperature water, at least one emulsifier, a vegetal wax, and one or
more olive
derivatives or extracts, and mixing until a homogeneous mixture is obtained.
In one or more
embodiments, the elevated temperature is about 82 C.
[00115] After the mixture has cooled to about 52 C or less, an
additional olive derivative or
extract may be added, along with other optional ingredients, if desired.
[00116] After the mixture has cooled to about 38 C or less, the oligomeric
peptides may be
added, along with other optional ingredients such as preservatives, if
desired.
[00117] Mixing is continued until a homogenous mixture is obtained.
24
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
[00118] In one or more embodiments, the compositions of the present invention
are smooth
and uniform, and easy to spread, leaving a uniform residue on skin. In one or
more embodiments,
the compositions are stable and pass standard laboratory stability test
methods. In one or more
embodiments, compositions were stable at 4, 25, 40 and 50 C for at least
three months. In one or
more embodiments, compositions were also stable under freeze ¨ thaw test
conditions.
[00119] Sensory aesthetics deals with evaluating cosmetic preparations
on the basis of sensory
impressions. A sensory assessment of a cosmetic is made by reference to
visual, olfactory and
haptic impressions. Haptic impressions include sensations of the sense of
touch, which relate
primarily to structure and consistency of the product, i.e., texture. Texture
is an aesthetic property
of cosmetic products that is very important for the consumer, but which can
only be
quantitatively measured with difficulty. Texture is generally understood to
mean those properties
of a cosmetic which relate to the structure of the preparation, and are
perceived by the sense of
touch.
[00120] A method of sensory analysis that is often used in research and
development is the
difference test. A sample is compared to a control sample, and differences are
perceived. The use
of groups of trained test persons, screening the testers from one another, and
statistical evaluation
of the data all help to counter the inherent subjectivity of the sensory
analysis. Advantageously,
this product has a light and easy to spread sensory experience that is desired
by end users. Also
leaves a light film on skin that is perceived as continued protection.
[00121] Compositions of the present invention may also be characterized by
reference to
viscosity and/or rheological properties. In one or more embodiments, the
viscosity may be
expressed as a standard, single-point type viscosity, as measured on a
Brookfield Digital
viscometer at a temperature of about 20 C, using spindle T-D, heliopath, at a
speed of 10 rpm. In
one or more embodiments, the compositions may have a viscosity of from about
2000 to about
120,000 cPs.
[00122] In one or more embodiments, compositions of the present invention may
be
characterized as lotions, having a viscosity of less than about 120,000
centipoise (cPs), in other
embodiments, less than about 100,000, and in other embodiments, less than
about 75,000 cPs. In
one or more embodiments, the lotion compositions may have a viscosity of from
about 3000 to
about 50,000 cPs, in other embodiments, from about 4000 to about 30,000 cPs.
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
[00123] In one or more embodiments, compositions of the present invention may
be
characterized as serum, having a viscosity of from about 2000 to about 3000
cPs.
[00124] In one or more embodiments, compositions of the present invention may
be
characterized as creams, having a viscosity of from about 30,000 to about
100,000 cPs, in other
embodiments from about 50,000 to about 80,000 cPs.
[00125] In one or more embodiments, compositions according to the present
invention are
pourable at room temperature, i.e. a temperature in the range of from about 20
to about 25 C. In
one or more embodiments, the lotion formulations are viscous enough to hold a
shape or not flow
for a desired period of time. In other embodiments, compositions of the
present invention are
creams or ointments, and are not pourable and do not flow at room temperature
and will not
conform to a container when placed into the container at room temperature.
[00126] The term yield stress point is understood as meaning the
smallest shear stress above
which a plastic material behaves in rheological terms like a liquid. The yield
stress point may be
used to indicate the amount of shear stress that is needed to initiate flow,
relating to the ability to
both pump and spread the product. The yield stress point may be determined by
plotting a flow
curve.
[00127] Flow curves of the compositions according to the invention may be
prepared using an
SR-2000 from Rheometric Scientific (now TA-Instruments), StressTec High
Resolution Research
Rheometer, or the like. This instrument is a shear-stress-controlled rheometer
with an air-bearing
transducer. The measurement system consists of a parallel plate measurement
system (so-called
plate/plate arrangement) where the lower plate can be temperature controlled.
The measurement
is carried out at a measuring temperature of 25 C. In one or more
embodiments, the
measurement method includes a linear shear stress-time slope with a strain
rate of 40 Pa/min
starting at 0 Pa.
[00128] Advantageously, compositions of the present invention become less
viscous when
relatively low amount of stress is applied. Thus, the compositions are easily
spreadable. In one or
more embodiments, compositions of the present invention may be characterized
in that the flow
curve indicates a significant drop in viscosity at a stress of about 10
pascals (Pa), in other
embodiments, at a stress of about 50 Pa, in other embodiments, at a stress of
about 75 Pa, and in
other embodiments, at a stress of about 100 Pa. By significant drop is meant a
rather sudden drop,
as opposed to a gradual decline.
26
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
[00129] In one or more embodiments, the yield stress point is less than
about 1000 Pa, at 1 s -1
at about 20 0C, in other embodiments, the yield stress point is less than
about 500 Pa, in other
embodiments, less than about 100 Pa, in other embodiments, less than about 50
Pa, at 1 s -1 at
about 20 C.
[00130] In one or more embodiments, the compositions of the present invention
are
thixotropic. Another way to express this feature is to say that the
compositions exhibit a
thixotropic rheology. Thixotropy is a time-dependent shear thinning property.
In general, the
viscosity of thixotropic compositions decreases when shear forces are applied.
Certain gels or
fluids that are viscous under static conditions will flow, i.e. become less
viscous, over time when
stress is applied. The compositions then take a fixed amount of time to return
to a more viscous
state.
[00131] Advantageously, compositions of the present invention recover quickly
when the
stress is removed. In one or more embodiments, the shear-thinning effect is
substantially
reversible. Thus, in one or more embodiments, compositions of the present
invention exhibit a
first viscosity under static conditions, a second, reduced viscosity when
shear force is applied,
and then, when the shear force is removed, the viscosity of the composition
returns to the first
viscosity, or to a viscosity that is substantially the same as the first
viscosity.
[00132] In one or more embodiments, the second viscosity is at least 25 % of
the first
viscosity, in other embodiments, the second viscosity is at least 50 % of the
first viscosity, in
other embodiments, at least 75 % of the first viscosity, when a stress of up
to about 10 to about
100 Pa is applied as described above. In one or more embodiments, the second
viscosity is at
least 85 % of the first viscosity, in other embodiments, the second viscosity
is at least 90 % of the
first viscosity, in other embodiments, at least 95 % of the first viscosity,
in other embodiments, at
least 98 % of the first viscosity, when a stress of up to about 10 to about
100 Pa is applied as
described above. Stated another way, the reduction in viscosity in relation to
stress applied at a
stress of about 100 Pa is less than about 75 %, in other embodiments, less
than about 50 %, in
other embodiments, less than about 25 %, based upon the original viscosity of
the composition. In
one or more embodiments, the reduction in viscosity in relation to stress
applied at a stress of
about 10 to about 100 Pa is less than about 20%, in other embodiments, less
than about 10%, in
other embodiments, less than about 5 %, based upon the original viscosity of
the composition.
27
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
[00133] In one or more embodiments, the composition is topically applied to
skin. In one or
more embodiments, the composition may be topically applied to an affected skin
area in a
predetermined or as-needed regimen. In one or more embodiments, the
composition is included
as part of a skin cleansing or sanitizing regimen.
[00134] Advantageously, in one or more embodiments, lotions according to the
present
invention provide protection against irritants. Irritants may include chemical
irritants, biological
irritants, such as result from incontinence. In one or more embodiments,
lotions according to the
present invention enhance the healing of damaged skin. Damaged skin may be the
result of
environmental factors, aging, or disease. In one or more embodiments, lotions
according to the
present invention prevent pressure ulcers.
[00135] Thus, the present invention further provides a method for
reducing the irritancy
potential of a skin cleanser or sanitizer. The method includes the step of
combining a skin
cleanser or sanitizer composition with one or more oligopeptides prior to form
a less irritating
skin cleanser or sanitizer composition. The method includes the further step
of contacting the skin
with the less irritating composition for a period sufficient to cleanse and/or
sanitize the skin. In
one or more embodiments, when the amount of skin irritation is measured, as
for example by
testing the IL-8 secretion, the amount of skin irritation is reduced, compared
to when the method
is repeated but using the same skin cleanser or sanitizer composition without
any oligopeptide. In
one or more embodiments, compositions containing two or more oligopeptides
provide a
synergistic reduction of the skin irritation potential of the compositions.
[00136] Advantageously, in one or more embodiments, skin cleansers and
sanitizers
containing one or more oligopeptide according to the present invention enhance
the skin barrier
function, when compared to the same skin cleanser or sanitizer but not
containing one or more
oligopeptide according to the present invention.
[00137] Thus, the present invention further provides a method for the
treatment of the skin
comprising the step of contacting the skin with a cosmetically or
pharmaceutically acceptable
amount of the compositions described above. Advantageously, the skin condition
is improved
after contact with the composition. In one or more embodiments, when the skin
barrier function is
assessed, as for example by testing the skin for cell adhesion proteins, the
amount of skin barrier
function is improved, compared to when the method is repeated but using the
same composition
without the two or more oligopeptides. In one or more embodiments,
compositions containing
28
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
two or more oligopeptides, a vegetal wax and a derivative or extract of olives
provide a
synergistic enhancement of the skin barrier function.
[00138] Advantageously, compositions and methods of the present invention may
be useful to
treat a variety of skin conditions that result in inflammation or erythema.
For example,
inflammation or erythema can result from external causes such as sun or wind
burn or irritating
soaps or cleansers. It is also known that inflammation and erythema can be
caused from inherent
conditions such as rosacea, atopic dermatitis, or allergic skin reactions.
[00139] In one or more embodiments, the compositions of the present invention
may be
formulated as a spray cleansing lotion. General characteristics of spray
products are further
described in U.S. Patent Application Publication No. 2010/0239624 Al, which is
incorporated by
reference herein. Advantageously, compositions of the present invention are
well suited for spray
applications, because they are relatively thin compared to compositions
containing zinc
compounds. For the same reason, compositions of the present invention are also
useful in glide
gel and roll on products.
[00140] In one or more embodiments, the invention further provides wipes or
other fibrous
structures comprising the compositions as described herein. Suitable wipes and
fibrous structures
are described, for example, in U.S. Patent Application Publication Nos.
2010/0239624 Al and
2013/0004602 Al, both of which are incorporated by reference herein. In one or
more
embodiments, compositions of the present invention are imbedded in diapers,
wipes, tissues,
and/or bandages.
[00141] In one or more embodiments, the compositions of the present invention
may be
employed to cover and protect skin wounds or ulcers, such as pressure ulcers.
Advantageously,
the skin cover is easily applied, does not need to be removed, protects the
skin from irritants and
contaminants, and similarly, irritants cannot get trapped under the cover.
[00142] The present invention provides a method of cleaning and treating
decubitus ulcers. A
patient in need of treatment of one or more decubitus ulcers is identified.
The compositions of the
present invention are applied to the skin area containing the decubitus ulcer.
Advantageously, the
composition provides cleaning to the one or more decubitus ulcers, provides
wound healing, and
provides a moisture barrier. In one or more embodiments, compositions
according to the present
invention prevent infection and accelerate healing.
29
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
[00143] In general, compositions may be assessed for sensory aesthetic
characteristics by an
expert sensory panel, for example as described in U.S. Patent Application
Publication No.
2006/0159649 Al, which is hereby incorporated by reference. The '649
publication also
describes methods for assessing rheological characteristics, TEWL, in vitro
skin retention test,
controlled application dryness test with wash protocols, and skin hydration
(corneometer).
[00144] Compositions of the present invention have many advantages, including
the
following. Because the compositions do not have a base odor like petrolatum-
based products,
compositions may be fragrance-free, or may more easily be formulated with a
pleasing fragrance.
Rheological properties allow for ease of application with minimum contact and
minimum skin
tearing. The synergistic improvement in skin hydration and barrier repair make
the compositions
useful for dry skin and dry lip repair, soothing, healing skin cracking, acne,
UV damage, aging,
cuts and burns. The compositions provide a barrier against pollutants,
irritants, infection, blisters,
pressure ulcers, chaffing, diaper rash, tough soils.
[00145] In order to demonstrate the practice of the present invention, the
following examples
have been prepared and tested. The examples should not, however, be viewed as
limiting the
scope of the invention. The claims will serve to define the invention.
EXAMPLES
Testing Methods ¨ Part 1
IL-* ELISA
[00146] Interleukin 8 (IL-8) is a chemokine and proinflammatory cytokine
produced by
macrophages and other cell types such as epithelial cells. It is secreted from
keratinocytes in skin
in response to inflammatory stimuli. IL-8 is secreted and is an important
mediator of the immune
reaction in the innate immune system response. IL-8 overexpressed is a
biomarker of skin
irritation.
[00147] For Control A, human dermal keratinocytes are left untreated. No
irritation is
expected, and therefore Control A provides a baseline. For Control B, IL-8 is
induced in human
dermal keratinocytes by applying a surfactant mixture that is a combination of
sodium laureth
sulfate and polyquaternium-10. For all other samples, the human dermal
keratinocytes are co-
treated with the surfactant mixture and a composition containing the
ingredient of interest.
Decreased 11-8 expression reflects the ingredient's anti-irritation activity.
CA 02980759 2017-09-22
WO 2016/161074 PCT/US2016/025193
[00148] In order to carry out the test method, an assay kit was
employed that was obtained
from R&D Systems: Human CXCL8/IL-8 Quantikine ELISA Kit.
[00149] The following steps were followed: 1. Bring all reagents and
samples to room
temperature before use. 2. Prepare all reagents, standard dilutions, and
samples. 3. Remove
excess microplate strips from the plate frame, return them to the foil pouch
containing the
desiccant pack, and reseal. 4. Add 100 [IL of Assay Diluent to each well. 5.
Add 50 [EL of
Standard, control, or sample to each well. Cover with a plate sealer, and
incubate at room
temperature for 2 hours. 6. Aspirate each well and wash, repeating the process
3 times for a total
of 4 washes. 7. Add 100 [EL of Conjugate to each well. Cover with a new plate
sealer, and
incubate at room temperature for 1 hour. 8. Aspirate and wash 4 times. 9. Add
200 [IL Substrate
Solution to each well. Incubate at room temperature for 30 minutes, making
sure to protect the
wells from the light. 10. Add 50 [IL of Stop Solution to each well. The liquid
was removed from
the well and, using a colorimeter, absorbance was measured at 450 nanometers
(nm) within 30
minutes. Wavelength correction was set to 540 nm or 570 nm.
MTT Assay
[00150] The MTT assay is a colorimetric assay for assessing cell
viability, cell proliferation,
and/or cytotoxicity. NAD(P)H-dependent cellular oxidoreductase enzymes may,
under defined
conditions, reflect the number of viable cells present. These enzymes are
capable of reducing the
tetrazolium dye MTT 3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyltetrazolium
bromide to insoluble
formazan, which has a purple color. MTT assay can also be used to measure
cytotoxicity (loss of
viable cells) or cytostatic activity (shift from proliferative to resting
status) of potential medicinal
agents and toxic materials.
[00151] Controls A and B described above for the IL-8 Assay were also
employed in this test.
The mitigating effect of the test samples on the effect of Control B on the
keratinocytes was
measured. More specifically, while Control B has a negative effect on cell
viability, cell
proliferation, and/or cytotoxicity, this mitigation of this negative effect
was determined by
measuring the reduction of MTT.
[00152] The following steps were followed; Once the liquid was removed
from the wells for
the IL-8 Assay described above, 100 p1/well of 0.5 mg/ml of MTT in phenol red-
free DMEM
(cell culture medium) was added into each of the 96-well plates. After
incubating 1 hour at 37 C,
all liquid was removed (MTT solution) from the wells of the culture plate.
Then 100 [L1 of DMSO
31
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
was added to each well to completely dissolve the purple product. Absorption
was measured
using a plate reader at 550 nm wavelength.
Cell-cell Junction
[00153] Tight Junctions are the closely associated areas of two cells
whose membranes join
together forming a virtually impermeable barrier to fluid. A desmosome is a
cell structure
specialized for cell-to-cell adhesion. A type of junction complex, they are
localized spot-like
adhesions randomly arranged on the lateral sides of plasma membranes.
Desmosomes are
molecular complexes of cell adhesion proteins and linking proteins that attach
the cell surface
adhesion proteins to intracellular keratin cytoskeletal filaments.
[00154] The cell adhesion proteins of the desmosome, desmoglein (DSG) and
desmocollin
(DSC), are members of the cadherin family of cell adhesion molecules. They are
biomarker of
skin tight junctions. In particular, DSG1 is a biomarker for cell binding, the
higher the
expression, the better skin cell-cell junction and the better skin barrier
function will be. DSC3 is
a protein in humans that is encoded by the D SC3 gene, the higher the
expression, the better skin
cell-cell junction and the better skin barrier function will be.
[00155] In the present method, keratinocytes were treated with the
sample compositions in a
6-well plate overnight. After washing with cold phosphate-buffered saline
(PBS), total RNAs
were prepared from each well. Real-Time Quantitative Reverse Transcription PCR
(qRT-PCR)
was performed to detect the target genes (DSC1 and DSG3) expression level
using a One-step
TaqMang RT-PCR kit (Life Technologies).
Test Results
[00156] Aqueous solutions of acetyl hexapeptide-46 and acetyl
hexapeptide-38 were prepared
by dilution to achieve the concentrations shown in Table 1. Acetyl hexapeptide-
46 was obtained
from Lipotec under the tradename Delisens.Tm DelisensTm is a proprietary blend
of butylene
glycol, water, citric acid and acetyl hexapeptide-46, containing 0.025 wt. %
acetyl hexapeptide-
46. Acetyl hexapeptide-38 was obtained from Lipotec under the tradename
Adifyline.
Adifyline Tm is a proprietary blend of butylene glycol, water and acetyl
hexapeptide-38,
containing 0.05 wt. % acetyl hexapeptide-38.
[00157] The concentration of acetyl hexapeptide shown in the following
table represents the
concentration of the active ingredient. Thus, for example, in preparing
Example 1A, 2 grams of
Adifyline was mixed with 98 g deionized water to prepare a solution that was
2 wt % Adifyline
32
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
and 10 parts per million by weight (ppm) acetyl hexapeptide-38, based upon the
total weight of
the solution.
[00158]
The samples were tested for IL-8 secretion as described in the test method
above.
That is, for Control A, human dermal keratinocytes were left untreated. For
Control B, IL-8 a
surfactant mixture that was a combination of sodium laureth sulfate and
polyquaternium-10. For
all other samples, the human dermal keratinocytes were co-treated with the
surfactant mixture
and a composition containing the ingredient of interest. Decreased 11-8
expression reflects the
ingredient's anti-irritation activity. The results are summarized in Table 2
and shown graphically
in Figure 1. As can be seen in Figure 1, both acetyl hexapeptide-38 and acetyl
hexapeptide-46
reduce the IL-8 secretion, when compared to Control B.
[00159]
The amount of the reduction in IL-8 secretion for the test samples
(subtracting from
100% for Control B) is summarized in Table 2 and shown graphically in Figure
2. It can be seen
that the combination of acetyl hexapeptide-46 and acetyl hexapeptide-38
produce an enhanced
reduction in IL-8 secretion, particularly for the higher concentrations.
Table 1
Example Adifyline Acetyl Hexapeptide- Delisens
Acetyl Hexapeptide-
Wt.% 38 Wt.% 46
PPm PPm
1G 3 15
1A 2 10
1B 1 5
1C 0.5 2.5
1D 0.2 1
2G 3 7.5
2A 2 5
2B 1 2.5
2C 0.5 1.25
2D 0.2 0.5
Combo 1 3 15 3 7.5
Combo 2 2 10 2 5
Combo 3 1 5 1 2.5
Combo 4 1 5 2 5
Combo 5 0.5 2.5 2 5
Combo 6 0.2 1 2 5
Table 2
33
CA 02980759 2017-09-22
WO 2016/161074 PCT/US2016/025193
Example IL-8 secreted
Reduction of IL- Standard
8 Secretion Deviation
Compared to
Control B
Control A 0.00% ____ 2.00%
Control B 100% ____ 16.70%
1G 57.20% 42.80% 4.40%
1A 69.60% 30.40% 18.60%
1B 71.70% 28.30% 6.60%
1C 84.90% 15.10% 9.60%
1D 95.80% 4.20% 11.90%
2G -6.90% 106.90% 1.10%
2A 27.10% 72.90% 2.70%
2B 50.90% 49.10% 4.60%
2C 65.60% 34.40% 5.00%
2D 78.20% 21.80% 4.00%
Combo 1 -9.40% 109.40% 1.10%
Combo 2 -4.10% 104.10% 1.10%
Combo 3 58.30% 41.70% 6.10%
Combo 4 14.90% 85.10% 0.40%
Combo 5 27.40% 72.60% 3.60%
Combo 6 33.70% 66.30% 2.60%
[00160] Samples containing various amounts of acetyl hexapeptide-38
and/or acetyl
hexapeptide-46 were tested for cell-cell junction, as described above.
Solution of Vitamin D3 and
keratinocyte growth medium (KGM) were used for comparison. The results are
summarized in
Tables 3-4 below, and graphically represented in Figures 3 - 4. It can be seen
that compositions
containing acetyl hexapeptide-38 and/or acetyl hexapeptide-46 enhanced cell-
cell junction when
compared to Vitamin D3 and KGM.
34
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
Table 3
Example Composition Conc. DSC3
(PPm) %
VitD3 109
KGM 100
3A Acetyl hexapeptide-38 15 160
3B Acetyl hexapeptide-38 7.5 205
3C Acetyl hexapeptide-38 5 210
3D Acetyl hexapeptide-46 7.5 230
3E Acetyl hexapeptide-46 5 377
3F Acetyl hexapeptide-46 2.5 265
Combo 2 Acetyl hexapeptide-38 + -46 5 + 2.5 254
Combo 3 Acetyl hexapeptide-38 + -46 10 + 5 188
Table 4
Example Composition Conc. DSG1
(PPm) %
VitD3 65
KGM 100
3B Acetyl hexapeptide-38 7.5 144
3C Acetyl hexapeptide-38 5 178
3E Acetyl hexapeptide-46 5 162
3F Acetyl hexapeptide-46 2.5 199
[00161] Samples were prepared and tested as described above, except
that pentapeptides were
employed instead of hexapeptides. The concentrations of the samples tested are
shown below, as
well as the test results. The concentration refers to the parts per million by
weight of active in the
sample tested.
Table 5
Example Concentration IL-8 secreted Reduction of IL- MTT
Pentapeptide 8 Secretion
(PPm) Compared to
Control B
Control A ---- 0.00% ---- 100
Control B ---- 100% ---- 57
4A 1 67 33 80
4B 5 59 41 81
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
4C 10 58 42 82
4D 15 64 36 78
4E 20 85 15 75
5A 0.2 47 53 81
5B 0 10 90 54
5C 2 -3 100+ 37
5D 2.5 -6 100+ 32
5E 3 0 100 21
5F 3.5 21 79 12
6A 5 6 94 97
6B 15 5 95 94
6C 25 9 91 65
6D 35 6 94 44
6E 40 3 97 32
[00162] It can be seen that compositions containing the tested
pentapeptides produce an
enhanced reduction in IL-8 secretion, and that they produce an enhanced cell-
cell junction when
compared to Vitamin D3 and KGM.
Testing Methods ¨ Part 2
[00163] FIG. 5 is a graphical representation of the effect of various
compositions on the
irritation response of cells treated with known irritants, as quantified by
measuring IL-8 secretion,
and on cell viability. FIG. 6 is a graphical representation of Involucrin
Expression ¨
Keratinocytes (KC) differentiation. FIG. 7 is a graphical representation of
PPAR6 expression ¨
ceramides related biomarker. FIG. 8 is a graphical representation of ABCA12
expression ¨ fatty
acid related biomarker. FIG. 9 is a graphical representation of DSC1
expression ¨ cell-cell
junction biomarker. FIG. 10 is a graphical representation of APQ3 expression ¨
skin water
channel.
IL-8 ELISA
[00164] Interleukin 8 (IL-8) is a chemokine and proinflammatory cytokine
produced by
macrophages and other cell types such as epithelial cells. It is secreted from
keratinocytes in skin
in response to inflammatory stimuli. IL-8 is secreted and is an important
mediator of the immune
reaction in the innate immune system response. IL-8 overexpressed is a
biomarker of skin
irritation.
36
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
[00165] For Control A, human dermal keratinocytes are left untreated. No
irritation is
expected, and therefore Control A provides a baseline. For Control B, IL-8 is
induced in human
dermal keratinocytes by applying a surfactant mixture that is a combination of
sodium laureth
sulfate and polyquaternium-10. For all other samples, the human dermal
keratinocytes are co-
treated with the surfactant mixture and a composition containing the
ingredient of interest.
Decreased 11-8 expression reflects the ingredient's anti-irritation activity.
[00166] In order to carry out the test method, an assay kit was employed that
was obtained
from R&D Systems: Human CXCL8/IL-8 Duoset ELISA Development Kit.
[00167] The following steps were followed: 1. Coat ETA high binding 96-
well plate with IL-8
capture antibody overnight at room temperature 2. Prepare all reagents,
standard dilutions, and
samples. Warm up to room temperature. 3. Aspirate and wash the coated plate
with 350 I/well
of washing buffer 4 times, then adding 300 I/well of blocking solution
incubating 1 hour at room
temperature. 4. Repeat Aspirate and wash (4 times) step. 5. Add 100 [EL of
Standard, control, or
sample to each well. Cover with a plate sealer, and incubate at room
temperature for 2 hours. 6.
Aspirate each well and wash, repeating the process 3 times for a total of 4
washes. 7. Add 100 [IL
of detection IL-8 antibody to each well. Cover with a new plate sealer, and
incubate at room
temperature for 2 hour. 8. Aspirate and wash 4 times. 9. Add 100 [IL Biotin-
Strepavidin
conjugate to each well, incubating 20 minutes at room temperature. 10.
Aspirate and wash 4
times. 11. Add 100 I substrate Solution to each well. Incubate at room
temperature for 20
minutes, making sure to protect the wells from the light. 12. Add 50 [IL of
Stop Solution to each
well. Data collected using a colorimeter, absorbance was measured at 450
nanometers (nm)
within 30 minutes. Wavelength correction was set to 570 nm.
MTT Assay
[00168] The MTT assay is a colorimetric assay for assessing cell
viability, cell proliferation,
and/or cytotoxicity. NAD(P)H-dependent cellular oxidoreductase enzymes may,
under defined
conditions, reflect the number of viable cells present. These enzymes are
capable of reducing the
tetrazolium dye MTT 3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyltetrazolium
bromide to insoluble
formazan, which has a purple color. MTT assay can also be used to measure
cytotoxicity (loss of
viable cells) or cytostatic activity (shift from proliferative to resting
status) of potential medicinal
agents and toxic materials.
37
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
[00169] Controls A and B described above for the IL-8 Assay were also employed
in this test.
The mitigating effect of the test samples on the effect of Control B on the
keratinocytes was
measured. More specifically, while Control B has a negative effect on cell
viability, cell
proliferation, and/or cytotoxicity, this mitigation of this negative effect
was determined by
measuring the reduction of MTT.
[00170] The following steps were followed; once the liquid was removed from
the wells for
the IL-8 Assay described above, 100 p1/well of 0.5 mg/ml of MTT in phenol red-
free DMEM
(cell culture medium) was added into each of the 96-well plates. After
incubating 1 hour at 37 C,
all liquid was removed (MTT solution) from the wells of the culture plate.
Then 100 pi of DMSO
was added to each well to completely dissolve the purple product. Absorption
was measured
using a plate reader at 550 nm wavelength.
Cell-cell Junction
[00171] Tight Junctions are the closely associated areas of two cells
whose membranes join
together forming a virtually impermeable barrier to fluid. A desmosome is a
cell structure
specialized for cell-to-cell adhesion. A type of junction complex, they are
localized spot-like
adhesions randomly arranged on the lateral sides of plasma membranes.
Desmosomes are
molecular complexes of cell adhesion proteins and linking proteins that attach
the cell surface
adhesion proteins to intracellular keratin cytoskeletal filaments.
[00172] The cell adhesion proteins of the desmosome, desmoglein (DSG) and
desmocollin
(DSC), are members of the cadherin family of cell adhesion molecules. They are
biomarker of
skin tight junctions. In particular, DSG1 is a biomarker for cell binding, the
higher the
expression, the better skin cell-cell junction and the better skin barrier
function will be. DSC3 is
a protein in humans that is encoded by the D SC3 gene, the higher the
expression, the better skin
cell-cell junction and the better skin barrier function will be.
[00173] In the present method, keratinocytes were treated with the sample
compositions in a 6-
well plate overnight. After washing with cold phosphate-buffered saline (PBS),
total RNAs were
prepared from each well. Real-Time Quantitative Reverse Transcription PCR (qRT-
PCR) was
performed to detect the target genes (DSC1 and DSG3) expression level using a
One-step
TaqMang RT-PCR kit (Life Technologies).
38
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
Test Results
Preparation of Test Compositions
[00174] Water was heated to 180 F. glyceryl stearate, Oliwax LC, OliveM 1000
were added
with stirring. Heat was held at 180 F for 15 minutes. The mixture was allowed
to slowly cool to
about 125 F, and then olive oil and an emollient (INCI name Diheptyl Succinate
(and) Capryloyl
Glycerin/Sebacic Acid Copolymer, available as LexFeel Natural) was added. When
the mixture
cooled to below 100 F, the oligopeptides Adyfyline and Delisens, and a
preservative blend were
added. The mixture was mixed until homogenous (about 20 to 30 minutes).
[00175] A base lotion composition was prepared according to the method
described above,
except omitting the Adifyline and Delisens. The base lotion is denoted as
Example 7. Example 8
was prepared according to the method described above, except omitting the
Delisens. Example 8
contained contained 1 wt. % Adifyline. Example 9 was prepared according to the
method
descrived above, except omitting the Adifyline. Example 9 contained 1 wt.
%Delisens. Example
10 was prepared according to the method described above, and contained 1 wt. %
each of
Adifyline and Delisens. Acetyl hexapeptide-46 was obtained from Lipotec under
the tradename
Delisens.' Delisens' is a proprietary blend of butylene glycol, water, citric
acid and acetyl
hexapeptide-46, containing 0.025 wt. % acetyl hexapeptide-46. Acetyl
hexapeptide-38 was
obtained from Lipotec under the tradename Adifyline. Adifyline Tm is a
proprietary blend of
butylene glycol, water and acetyl hexapeptide-38, containing 0.05 wt. % acetyl
hexapeptide-38.
[00176] The samples were diluted in water and then tested for IL-8
secretion as described in
the test method above. That is, for Control A, human dermal keratinocytes were
left untreated.
For Control B, IL-8 a surfactant mixture that was a combination of sodium
laureth sulfate and
polyquaternium-10. For all other samples, the human dermal keratinocytes were
co-treated with
the surfactant mixture and a composition containing the ingredient of
interest. Decreased 11-8
expression reflects the ingredient's anti-irritation activity. The results are
summarized in Table 6
and shown graphically in Figure 5.
Table 6
Example # Component Dilution IL-8 Inhibition (%) MTT
Control A Medium 100 %
100 %
Control B PMA 0.0 % 73 %
Example 7 Base Lotion 1/100 64 % 89 %
Example 8 Base Lotion + 1 wt. % 1/100 84 % 84 %
39
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
Adifyline (5 ppm acetyl
hexapeptide-38)
Example 9 Base Lotion + 1 wt. % 1/100 116 % 83 %
Delisens (2.5 ppm acetyl
hexapeptide-46)
Example 10 Base Lotion + 1 wt. % 1/100 48 % 86 %
Adifyline (5 ppm acetyl
hexapeptide-38) and 1 wt.
% Delisens (2.5 ppm
acetyl hexapeptide-46)
[00177] From Figure 5 and Table 6, it can be seen that although the acetyl
hexapeptides didn't
individually show any significant anti-irritation activity and even slightly
caused irritation, in
certain circumstances, the combination showed surprising improvement.
Skin Barrier
[00178] An in vitro model was employed, using monolayer human dermal
keratinocytes
culture (KGM). Two controls were tested: the medium and inflammatory cytokines
interleukin
(IL-1b).
[00179] Skin Barrier Biomarkers: ABCA12, Involucrin, PPAR6. Different
concentrations of
ingredients were used to treat the keratinocyte for 24 hours. Cells were
collected and total RNA
prepared from the treated cells. Real-time RT-PCT was used to detect different
barrier function
related biomarker' s gene expression level. Comparative Benchmark: Vitamin D3
(cholecalciferol) in three different concentrations.
[00180] Peroxisome proliferator-activated receptors (PPARs) are ligand
activated nuclear
receptors. Three PPAR subtypes have been identified: alpha, delta and gamma.
[00181] ABCA12 belongs to a group of genes called the ATP-binding cassette
family, which
makes proteins that transport molecules across cell membranes.
[00182] Involucrin is a protein component of human skin and in humans is
encoded by the
IVL gene. In binding the protein loricrin, involucrin contributes to the
formation of a cell
envelope that protects corneocytes in the skin. Involucrin is a highly
reactive, soluble,
transglutaminase substrate protein present in keratinocytes of epidermis and
other stratified
squamous epithelia. It first appears in the cell cytosol, but ultimately
becomes cross-linked to
membrane proteins by transglutaminase thus helping in the formation of an
insoluble envelope
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
beneath the plasma membrane functioning as a glutamyl donor during assembly of
the cornified
envelope.
[00183] Involucrin is synthesised in the stratum spinosum and cross
linked in the stratum
granulosum by the transglutaminase enzyme that makes it highly stable. Thus it
provides
structural support to the cell, thereby allowing the cell to resist invasion
by micro-organisms.
[00184] Results are shown in the Tables below.
Table 7
Example # Dilution Involucrin (%) PPARd %
ABCA12 %
KGM 100% 100% 100%
Example 7 1/1000 191% 135% 196%
Example 8 1/1000 211% 162% 240%
Example 9 1/1000 96% 99% 93%
Example 10 1/100 353% 180% 334%
[00185] From Figure 6 and Table 7, it can be seen that although the acetyl
hexapeptides didn't
individually show any significant promotion of KC differentiation, the
combination showed
surprising improvement.
[00186] From Figure 7 and Table 7, it can be seen that only the combination of
acetyl
hexapeptides showed significant stimulation of PPARd expression in KC. Example
3 appeared to
reduce PPARd expression in KC.
[00187] From Figure 8 and Table 7, it can be seen that the combination of
acetyl hexapeptides
show a significant increase in ABCA12 expression in KC, while the individual
samples seemed
to inhibit ABCA 12 expression in KC.
Table 8
Example # Dilution DSC1 (%) APQ3 %
KGM 100% 100%
Example 7 1/1000 279% 625%
Example 8 1/1000 406% 674%
Example 9 1/1000 200% 33%
Example 10 1/100 623 % 1030 %
[00188] From Figure 9, it can be seen that the combination of acetyl
hexapeptides significantly
stimulated DSC1 expression to improve skin cell junction.
41
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
[00189] From Figure 10, it can be seen that, although acetyl hexapeptide-46
does not
individually stimulate APQ3 expression in KC, the combined acetyl hexapeptides
show
surprising improvement.
[00190] It can be seen that overall, the composition containing a
combination of acetyl
hexapeptides has the best efficacy in anti-irritation, skin barrier (KC
differentiation, lipids
production and cell-cell junction) and skin hydration (water channel).
Competitive Rheology Profile of Barrier Creams
[00191] Yield Stress Point was used to determine the amount of shear
stress needed to initiate
flow, relating to the ability to both pump and spread a product.
[00192] Example 11 was a composition according to the present invention. More
specifically,
the Example 11 contained glyceryl stearate, cetearyl olivate, sorbitan
olivate, cetyl palmitate,
sorbitan palmitate, olive oil, heptyl undecylenate, butylene glycol, acetyl
hexapeptide-38, citric
acid, acetyl hexapeptide-49, phenoxyethanol, and ethylhexyl glycerin.
[00193] Example 12 was a commercially available lotion containing aloe
barbadensis leaf
juice, ascorbic acid, ascorbyl palmitate, c12-c13 pareth-3, c12-c13 pareth-23,
carthamus
tinctorius seed oil, cetyl dimethicone, cholecalciferol, citric acid, citrus
aurantium dulcis peel oil,
citrus grandis peel oil, citrus tangerina peel oil, cyclopentasiloxane,
diazolidinyl urea,
dimethiconol, divinyldimethicone/dimethicone copolymer, glycine,
hydroxytyrosol,l-proline,l-
taurine, methylparaben, methylsulfonylmethane, n-acetyl-l-cysteine,
niacinamide, olea europaea
fruit oil, peg-8, peg/ppg-18/18 dimethicone, propylene glycol, propylparaben,
pyridoxine hcl,
retinyl palmitate, sodium chloride, tocopherol, vanillin, water, and zea mays
oil, sold under the
tradename NutrashieldTM by Medline Industries, Inc.
[00194] Example 13 was a commercially available ointment containing 0.44 wt. %
menthol,
20.6 wt. % zinc oxide, and also containing calamine, chlorothymol, glycerin,
lanolin, phenol,
sodium bicarbonate, and thymo, sold under the tradename CalmoseptineTM by
Calmoseptine, Inc.
[00195] Example 14 was a commercially available lotion containing 3.5 wt. %
calamine, 0.2
wt. % menthol, 69 wt. % white petrolatum, 20 wt. % zinc oxide, and also
containing Aloe
Barbadensis Leaf Juice, Ascorbic Acid, Ascorbyl Palmitate, Carthamus
Tinctorius (Safflower)
Seed Oil, Cholecalciferol, Citric Acid, Citrus Aurantium Dulcis Peel Oil,
Citrus Grandis Peel Oil,
Glycine, Helianthus Annuus (Sunflower) Seed Oil, Hydroxytyrosol, L-proline, L-
taurine,
Methylparaben, Modified Corn Starch, Methylsulfonymethane, N-acetyl-L-
cysteine,
42
CA 02980759 2017-09-22
WO 2016/161074
PCT/US2016/025193
Niacinamide, Olea Europaea (Olive) Fruit Oil, PEG-8, Pyridoxine Hydrochloride,
Retinyl
PaImitate, Tapioca Starch Polymethylsilsesquioxane, Tocopherol, Vanillin, and
Zea Mays (Corn)
Oil, and sold under the tradename CalazimeTM by Medline Industries, Inc.
[00196] The Yield Stress Point comparison for Examples 11-14 is shown in
Figure 13. It can
be seen that Example 11 requires about 100 pascals (Pa) to initiate flow. Once
flow has started,
the product is very shear-sensitive and needs very little additional stress to
keep the lotion
flowing. The lotion is easy to spread, compared to Examples 12-14.
[00197] In comparison, Example 12 (Nutrashield), requires slightly more
than 10 Pa of stress
to start the product to flow, but is not as shear-sensitive as Example 11. In
order to spread the
lotion of Example 12, consistently increased stress is required.
[00198] The Yield Stress data for Examples 13 (Calmoseptine) and 14
(Calazime) indicate that
these products are very viscous and require almost 10 times the amount of
stress in order to
initiate spreading. Additionally, Example 13 (Calmoseptine) requires
consistently increased stress
to continue applying this product. The Yield Stress assessment of Example 14
(Calazime)
indicates that this product has a stress transition phase. Once the product
has reached about 5000
Pa, the viscosity increases, showing that it also has shear thickening
properties. As stress is
increased, in order to spread this product, it becomes tacky and is less
spreadable.
Thixotropy
[00199] A thixotropic comparison of Examples 11-14 is shown in Figure 14 It
can be seen that
Example 11 becomes less viscous when very little stress is applied, and when
stress is removed,
the product recovers quickly to its original, viscous state.
[00200] Thixotropic assessment of Example 12 (Nutrashield) indicates
that once stress is
applied, the sample is extremely shear sensitive. When stress is applied, the
product has no
elasticity, and does not return to original state, but is irreversibly
altered.
[00201] Thixotropic assessment of Examples 13 (Calmoseptine) and 14
(Calazime) indicates
that the products are very viscous and require relatively high amounts of
stress to start flow. Once
stress is applied and then removed, the samples have a very slow recovery and
may not return to
the original starting state. This is evidenced by the about 80% reduction in
viscosity of the
Examples 13 and 14, compared to the about 40% reduction in viscosity for
Example 11.
43
CA 02980759 2017-09-22
WO 2016/161074 PCT/US2016/025193
[00202] Various modifications and alterations that do not depart from the
scope and spirit of
this invention will become apparent to those skilled in the art. This
invention is not to be duly
limited to the illustrative embodiments set forth herein.
44