Note: Descriptions are shown in the official language in which they were submitted.
CA 02980886 2017-09-25
DESCRIPTION
HIGH-CARBON STEEL WIRE MATERIAL WITH EXCELLENT WIRE DRAWABILITY,
AND STEEL WIRE
Technical Field
[0001]
The present invention relates to a high-carbon steel wire
rod with excellent wire drawability, and a steel wire obtained
by wire drawing of the high-carbon steel wire rod mentioned above.
More particularly, the present invention relates to a high-carbon
steel wire rod produced by hot rolling, which is a raw material
of a high strength steel wire to be used mainly for steel cords,
wire ropes, saw wires and the like.
Background Art
[0002]
There have been known as, a high strength steel wire used
for steel cords, wire rope and the like, for example, piano wires
mentioned in JIS G 3522 (1991) . The piano wires are roughly
classified into three types such as classes A, B, and V, and
examples of the high strength piano wire class B include SWP-class
B having a wire diameter of 0.2 mm and a tensile strength of 2,840
to 3,090 MPa. Generally, pearlite steels such as SWRS82A
mentioned in JIS G 3502 (2004) are used as the raw material of the
piano wire.
1
CA 02980886 2017-09-25
[0003]
A common method for producing a high strength steel wire
is as follows. First, a steel wire rod produced by hot rolling
(also referred to as the rolled wire rod) is placed in a ring shape
on a cooling conveyor, thereby allowing to undergo pearlite
transformation, and then coiled into a coil shape to obtain a wire
rod coil. Then, wire drawing is performed and a steel wire having
desired wire diameter and strength is obtained by making use of
the work hardening function of pearlite. When it is impossible
to be drawn to a desired wire diameter due to working limit of
the steel wire rod, a heat treatment called patenting is applied
between wire drawings. For example, to obtain an extra fine steel
wire having a wire diameter of 0.2 mm, wire drawing and a patenting
treatment are generally performed by repeating several times.
[0004]
To increase the strength of the steel wire, there is a need
to increase the C content of the steel wire rod which is the raw
material. However, a high-carbon steel wire containing 0.90% or
more of C had a problem that proeutectoid cementite is
precipitated in the structure, thus degrading the wire
drawability.
[0005]
Thus, to produce a high-carbon steel wire with excellent
wire drawability, various techniques have been proposed.
[0006]
2
CA 02980886 2017-09-25
For example, Patent Document 1 relates to a wire rod for
a high strength steel wire which is useful as the raw material
of a galvanized steel wire to be used for ropes for a bridge, and
particularly mentions a wire rod for a high strength steel wire,
which is excellent in workability when wire drawing is performed
by so-called cold drawing without subjecting to a heat treatment
after rolling. In Patent Document 1, precipitation of
proeutectoid cementite is suppressed by precipitating fine TiC
near grain boundaries, so that the lower limit of the Ti content
is set at 0.02% or more.
[0007]
Patent Document 2 relates to a small diameter high-carbon
hot-rolled wire rod which is capable of wire drawing at true strain
of 2.2 or more even in an as-hot-rolled state. Specifically,
Patent Document 2 mentions that a steel billet having the Si
content suppressed to 0.50% or less is thinned to a wire rod
diameter of 4.5 mm or less by increasing rolling reduction during
hot rolling, thereby making austenite grains (y grains) finer
leading to acceleration of pearlite transformation, thus making
it possible to prevent precipitation of particles of proeutectoid
ferrite and proeutectoid cementite.
[0008]
Patent Document 3 relates to a deformed wire fora submarine
optical fiber cable in which a wire rod for a high tensile steel
wire is used. Specifically, Patent Document 3 mentions that, by
3
CA 02980886 2017-09-25
using a wire rod in which Si is segregated so as to satisfy the
following inequality expression: Si maximum segregation degree
of cementite/ferrite interface in a range of 30 nm from an
interface between cementite and ferrite to a ferrite phase side
in a pearlite structure (maximum Si concentration in a range of
30 nm from an interface between cementite and ferrite to a ferrite
phase side / Si content of bulk) 1.1,
it is possible to prevent
wire breakage during deformation working.
Prior Art Document
Patent Document
[0009]
Patent Document 1: JP 2014-189855 A
Patent Document 2: JP 2001-181789 A
Patent Document 3: JP 2003-301240 A
Disclosure of the Invention
Problems to be Solved by the Invention
[0010]
However, the above-mentioned techniques of Patent
Documents 1 to 3 respectively have the following problems.
[0011]
First, Patent Document 1 is intended for a wire rod to be
used for a galvanized steel wire, and is not intended fora steel
wire having an extra fine wire diameter of approximately 0.2 mm,
such as a piano wire. Like Patent Document 1, when an extra fine
4
CA 02980886 2017-09-25
steel wire is produced using a wire rod having a large Ti content,
wire breakage during wire drawing becomes remarkable due to Ti
based inclusions. Therefore, it is difficult to apply the
technique of Patent Document 1 to the extra fine steel wire to
be supplied for steel cords.
[0012]
Like Patent Document 2, when using a wire rod having a
diameter of 4.5 mm or less, the productivity is degraded to cause
a problem that wire rods are easily entangled with each other
during the production of a coil.
[0013]
Like Patent Document 3, when using a method in which an Si
concentration difference is applied at an interface between
cementite and ferrite in the pearlite structure, it is impossible
to sufficiently reduce proeutectoid ferrite which is harmful for
the wire drawability. The degree of working carried out in Patent
Document 3 is 82.6% in terms of an area reduction rate even when
summing up wire drawing and cold rolling. Since the area
reduction rate of wire drawing required to an extra fine steel
wire such as a steel cord is larger, it is insufficient to apply
the extra fine steel wire to the above-mentioned applications.
[0014]
The present invention has been made in light of the foregoing
circumstance, and it is an object of the present invention to
provide a high-carbon steel wire rod with excellent wire
5
CA 02980886 2017-09-25
drawability which can also be applied to extra fine steel wires
such as steel cord, and a steel wire.
Means for Solving the Problems
[0015]
The present invention that can solve the foregoing problems
provides a high-carbon steel wire rod including, in % by mass,
C: 0.90 to 1.3%, Si: 0.4 to 1.2%, Mn: 0.2 to 1.5%, P: more than
0% and 0.02% or less, S: more than 0% and 0.02% or less, Al: more
than 0% and 0.008% or less, Ti: 0 to 0.005%, and N: 0.001 to 0.008%,
with the balance being iron and inevitable impurities, wherein
the structure includes pearlite and proeutectoid cementite, an
area ratio of pearlite is 90% or more relative to the entire
structure, a maximum length of proeutectoid cementite is 15 pm
or less, and a concentration difference between an average of the
Si concentration inside proeutectoid cementite and a maximum
value of the Si concentration inside ferrite that forms a lamellar
structure of pearlite is 0.50 to 3%.
[0016]
Further, in a preferred embodiment, the above-mentioned
high-carbon steel wire includes: in % by mass, at least one
belonging to any one of the following (a) to (d):
(a) B: more than 0% and 0.01% or less,
(b) Co: more than 0% and 1.5% or less,
(c) at least one selected from the group consisting of V: more
6
CA 02980886 2017-09-25
than 0% and 0.5% or less, and Cr: more than 0% and 0.5% or less,
and
(d) at least one selected from the group consisting of Cu: more
than 0% and 0.5% or less, Ni: more than 0% and 0.5% or less, and
Nb: more than 0% and 0.5% or less.
[0017]
A steel wire obtained by wire drawing of the above-mentioned
high-carbon steel wire rod is also included in the scope of the
present invention.
Effects of the Invention
[0018]
The present invention can provide a high-carbon steel wire
rod with excellent wire drawability which can also be applied to
extra fine steel wires such as steel cord.
Brief Description of the Drawings
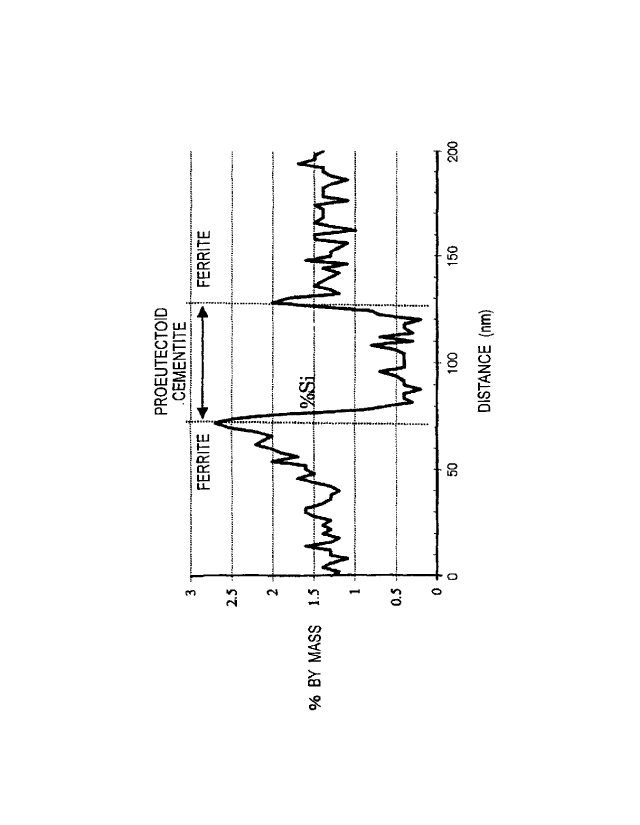
[0019]
Fig. 1 is a diagram showing an Si concentration difference
at an interface between a proeutectoid cementite phase and a
ferrite phase in the sample of test No. 12 in Table 2 of Example.
Mode for Carrying Out the Invention
[0020]
To solve the above problems, the inventors of the present
7
invention have intensively studied using a high-carbon steel
wire rod having the C content of 0.90% or more. As a result, it
has been found that, when applying an Si concentration
difference of 0.50% or more at an interface between proeutectoid
cementite and ferrite that forms a lamellar structure of
pearlite (hereinafter may be simply referred to as ferrite)
(specifically, a concentration difference between an average of
the Si concentration inside proeutectoid cementite, and a
maximum value of the Si concentration inside ferrite is 0.50% or
more), it is possible to suppress the precipitation and growth
of proeutectoid ferrite which is harmful for the wire
drawability, thus completing the present invention.
[0021]
There is also some mention of Si segregation in Patent
Document 3. However, in Patent Document 3, the Si concentration
difference at an interface between cementite (lamellar cementite
that forms a lamellar structure of pearlite) and ferrite in a
pearlite structure is controlled, and the cementite is not.
Therefore, on this point, the invention of Patent Document 3,
and the present invention in which the Si concentration at an
interface between proeutectoid cementite that is not cementite
in the pearlite structure and ferrite is controlled, differ in
structure ofinterest. The cementite in the pearlite structure
is essentially different from proeutectoid cementite, and the
precipitation starting temperature of
8
CA 2980886 2019-03-04
CA 02980886 2017-09-25
proeutectoid cementite is approximately 750 C and is higher than
that of pearlite that precipitates at approximately 590 to 650 C.
Therefore, it is considered that proeutectoid cementite which is
harmful for the wire drawability cannot be sufficiently reduced
by the technique of Patent Document 3. Patent Document 3 also
mentions that it is effective to set a rate of blast cooling after
rolling of the wire rod at 1 to 10 C/second so as to efficiently
segregate Si to the above-mentioned interface, and blast cooling
at approximately 7 C/second is performed in all Examples.
However, in the below-mentioned sample No.6 in Table 2 in which
rolling was performed under the cooling conditions mentioned
above, the Si concentration difference defined by the present
invention could not be achieved and a maximum length of
proeutectoid cementite increased, leading to degradation of the
wire drawability.
[0022]
Next, a description will be made of the steel wire rod of
the present invention.
[0023]
First, components in the steel of the steel wire rod
according to the present invention are as follows. Unit of each
component is 96 by mass unless otherwise specified.
[0024]
C: 0.90 to 1.3%
Carbon (C) is effective in increasing the strength, and the
9
CA 02980886 2017-09-25
strength of the steel wire after cold working increases with the
increase of the C content. To achieve desired strength of 4,000
MPa or more, the lower limit of the C content is set at 0.90% or
more, preferably 0.93% or more, and more preferably 0.95% or more.
Any excessive C content, however, cannot achieve sufficient
reduction of proeutectoid cementite which is harmful for the wire
drawability, thus degrading the wire drawability. Therefore,
the upper limit of the C content is set at 1.3% or less, and
preferably 1.25% or less.
[0025]
Si: 0.4 to 1.2%
Silicon (Si) is an effective deoxidizing agent and has not
only the effect of reducing oxide based inclusions in the steel,
but also the effect of increasing the strength of the steel wire
rod. As mentioned later, Si also has the effect of suppressing
the growth of proeutectoid cementite. To effectively exhibit
these effects, the lower limit of the Si content is set at 0.4%
or more, preferably 0.45% or more, more preferably more than 0.50%,
and still more preferably 0.55% or more. Addition of excessive
Si accelerates the embrittlement during wire drawing, thus
degrading twisting properties of the drawn wire rod. Therefore,
the upper limit of the Si content is set at 1.2% or less, and
preferably 1.15% or less.
[0026]
Mn: 0.2 to 1.5%
CA 02980886 2017-09-25
Manganese (Mn) has the effect of extremely improving the
hardenability of the steel, thus lowering the transformation
temperature during blast cooling, leading to increased strength
of the pearlite structure. To effectively exhibit these effects,
the lower limit of the Mn content is set at 0.2% or more, and
preferably 0.3% or more. However, Mn is an element which easily
segregates into the center of the wire rod and addition of
excessive Mn excessively enhances the hardenability of a Mn
segregation portion, which may form a supercooled structure such
as martensite. Therefore, the upper limit of the Mn content is
set at 1.5% or less, preferably 1.0% or less, and more preferably
0.95% or less.
[0027]
P: more than 0% and 0.02% or less
Phosphorus (P) is contained as impurities, and segregates
in the prior austenite grain boundary to thereby cause
embrittlement, leading to steel billet cracking and degradation
of fatigue-resistant characteristics of the steel wire after wire
drawing. Therefore, to prevent these harmful influences, the
upper limit of the P content is set at 0.02% or less, and preferably
0.018% or less. It is difficult to set the lower limit of the
P content at 0% in view of industrial production.
[0028]
S: more than 0% and 0.02% or less
Like P, sulfur (S) is contained as impurities, and
11
CA 02980886 2017-09-25
segregates in the prior austenite grain boundary to thereby cause
embrittlement, leading to steel billet cracking and degradation
of fatigue-resistant characteristics of the steel wire after wire
drawing. Therefore, to prevent these harmful influences, the
upper limit of the S content is set at 0.02% or less, and preferably
0.018% or less. It is difficult to set the lower limit of the
S content at 0% in view of industrial production.
(0029]
Al: more than 0% and 0.008% or less
Aluminum (Al) is contained as impurities, and forms Al based
inclusions such as A1203 to thereby increase a wire breakage ratio
during wire drawing. Therefore, to ensure sufficient wire
drawability, the upper limit of the Al content is set at 0.008%
or less, and preferably 0.006% or less. It is difficult to set
the lower limit of the Al content at 0% in view of industrial
production.
[0030]
Ti: 0 to 0.005%
Titanium (Ti) is contained as impurities, and forms Ti based
inclusions such as TIN to thereby increase a wire breakage ratio
during wire drawing. Therefore, to ensure sufficient wire
drawability, the upper limit of the Ti content is set at 0.005%
or less, and preferably 0.003% or less.
[0031]
N: 0.001 to 0.008%
12
CA 02980886 2017-09-25
=
N is solid-soluted in the steel to thereby cause strain aging
during wire drawing, thus degrading the toughness of the steel
wire. Therefore, to prevent these harmful influences, the upper
limit of the N content is set at 0.008% or less, and preferably
0.007% or less. The lower the N content is, the better, and the
lower limit of the N content is set at 0.001% or more, and
preferably 0.0015% or more, in view of industrial production.
[0032]
The steel wire rod of the present invention contains
components mentioned above, the balance being iron and inevitable
impurities.
[0033]
To improve properties such as strength, toughness, and
ductility, the steel wire rod of the present invention can further
include the following selective elements.
[0034]
B: more than 0% and 0.01% or less
Boron (B) has the effect of concentrating on the austenite
grain boundary to thereby prevent the formation of grain boundary
ferrite, thus improving the wire drawability. B also has the
effect of chemically combining with N to form nitrides such as
BN, and suppressing the degradation of the toughness due to
solid-soluted N, thus improving twisting properties. To
effectively exhibit the wire drawability and twisting properties
of the steel wire rod due to the addition of B, the lower limit
13
CA 02980886 2017-09-25
-
of the B content is preferably set at 0.0005% or more. Addition
of excessive B causes cracking during hot rolling as a result of
the precipitation of a compound with Fe (B-constituent), so that
the upper limit of the B content is preferably set at 0.01% or
less, and more preferably 0.008% or less.
[0035]
Co: more than 0% and 1.5% or less
Cobalt (Co) has the effect of accelerating pearlite
transformation to thereby reduce proeutectoid cementite.
Particularly, the wire drawability is accelerated by adding Co,
in addition to Si. To effectively exhibit these functions, the
lower limit of the Co content is preferably set at 0.05% or more,
and more preferably 0.1%. However, Co is a very expensive element
and the effect is saturated even if being added excessively,
resulting in economic waste. Therefore, the upper limit of the
Co content is preferably set at 1.5% or less, more preferably 1.3%
or less, and still more preferably 1% or less.
[0036]
At least one selected from the group consisting of V: more than
0% and 0.5% or less, and Cr: more than 0% and 0.5% or less
Vanadium (V) and chromium (Cr) are elements contributing
to improve the strength of the steel wire rod. These elements
may be added alone or used in combination.
[0037]
Specifically, V has the effect of increasing the strength
14
CA 02980886 2017-09-25
due to the formation of fine carbonitrides, and also can exhibit
the effect of improving twisting properties due to the reduction
of solid-soluted N. To effectively exhibit these effects, the
lower limit of the V content is preferably set at 0.05% or more,
and more preferably 0.1% or more. V is an expensive element and
the effect is saturated even if being added excessively, resulting
in economic waste. Therefore, the upper limit of the V content
is preferably set at 0.5% or less, and more preferably 0.4% or
less.
[0038]
Cr has the effect of making lamellar spacing of pearlite
finer to thereby enhance the strength of the steel wire rod. To
effectively exhibit such effect, the lower limit of the Cr content
is preferably set at 0.05% or more, and more preferably 0.1% or
more. However, the effect is saturated even if being added
excessively, resulting in economic waste. Therefore, the upper
limit of the Cr content is preferably set at 0.5% or less, and
more preferably 0.4% or less.
[0039]
At least one selected from the group consisting of Cu: more than
0% and 0.5% or less, Ni: more than 0% and 0.5% or less, and Nb:
more than 0% and 0.5% or less
All of these elements are elements contributing to improve
the manufacturability and corrosion resistance of the steel wire.
These elements may be added alone or used in combination.
CA 02980886 2017-09-25
4
[0040]
Specifically, copper (Cu) has the effect of being
concentrated on a surface of the steel wire rod to thereby enhance
the peelability of scales, leading to the enhancement of
mechanical descaling (MD) properties. To effectively exhibit
these functions, the lower limit of the Cu content is preferably
set at 0.05% or more. However, blisters occur on a surface of
the steel wire rod when being added excessively, so that the upper
limit of the Cu content is preferably set at 0.5% or less, and
more preferably 0.4% or less.
[0041]
Nickel (Ni) has the effect of enhancing the corrosion
resistance of the steel wire rod. To effectively exhibit such
function, the lower limit of the Ni content is preferably set at
0.05% or more. The effect is saturated even if being added
excessively, resulting in economic waste. Therefore, the upper
limit of the Ni content is preferably set at 0.5% or less, and
more preferably 0.4% or less.
[0042]
Niobium (Nb) has the effect of making crystal grains finer
to thereby enhance the ductility of the wire rod. To effectively
exhibit such function, the lower limit of the Nb content is
preferably set at 0.05% or more. However, the effect is saturated
even if being added excessively, resulting in economic waste.
Therefore, the upper limit of the Nb content is preferably set
16
CA 02980886 2017-09-25
=
at 0.5% or less, and more preferably 0.4% or less.
[0043]
Next, a description will be made of the structure of the
steel wire rod according to the present invention. As mentioned
above, the steel wire rod of the present invention includes
pearlite and proeutectoid cementite, and an area ratio of pearlite
is 90% or more relative to the entire structure, a maximum length
of proeutectoid cementite is 15 pm or less, and a concentration
difference between an average of the Si concentration inside
proeutectoid cementite and a maximum value of the Si concentration
inside ferrite (hereinafter may simply referred to as the Si
concentration difference) is 0.50 to 3%.
[0044]
Area ratio of pearlite relative to the entire structure: 90% or
more
As mentioned above, the steel wire rod of the present
invention includes pearlite and proeutectoid cementite. Since
the low temperature transformation structure, such as bainite or
martensite (may also be referred to as the supercooled structure)
inhibits the wire drawability, an area ratio of the pearlite
structure is set at 90% or more, and preferably 95% or more, so
as to ensure sufficient wire drawability. The upper limit may
be appropriately controlled depending on a relation with
proeutectoid cementite, and is preferably approximately 99 area %
or less.
17
CA 02980886 2017-09-25
[0045]
The steel wire rod of the present invention can include,
in addition to pearlite and proeutectoid cementite, the residual
structure that is inevitably included during production process.
Examples of such residual structure include non-pearlite
structures, such as bainite and proeutectoid ferrite. To
effectively exhibit the functions of the present invention, the
total content of the non-pearlite structure (including
proeutectoid cementite) is preferably controlled to
approximately 10 area% or less relative to the entire structure.
[0046]
Maximum Length of Proeutectoid Cementite: 15 pm or less
Proeutectoid cementite precipitating in a plate shape is
the structure which is harmful for the wire drawability, and
disturbs orientation of pearlite colonies of the steel wire rod
and increases wire breakage as a starting point of cracking.
However, proeutectoid cementite having a short maximum length
exert less harmful influences mentioned above. Mechanism due to
such proeutectoid cementite is as mentioned in detail in Patent
Document 1. To ensure sufficient wire drawability, the upper
limit of the maximum length of proeutectoid cementite is set at
15 pm or less, preferably 13 pm or less, and more preferably 10
pm or less. The lower limit of the maximum -length of proeutectoid
cementite is not particularly limited and may be, for example,
approximately 0.1 pm.
18
CA 02980886 2017-09-25
[0047]
Concentration difference between average of Si concentration
inside proeutectoid cementite and maximum value of Si
concentration inside ferrite (Si concentration difference) : 0.50
to 3%
Silicon (Si) is an element which is hardly solid-soluted
in cementite and is discharged to an austenite phase from a
cementite phase when proeutectoid cementite precipitates, and Si
concentration difference is generated at the interface (interface
between proeutectoid cementite and a ferrite phase). The test
results of the inventors revealed that, the more this Si
concentration difference is large, the more the growth of a
proeutectoid cementite phase is suppressed, thus enabling the
reduction of the maximum length of proeutectoid cementite. Si
concentration distribution formed at this time is inherited even
through subsequent pearlite transformation, so that observation
of the structure of the thus produced steel wire rod leads to
confirmation as an Si concentration difference at an interface
between the proeutectoid cementite phase and the ferrite phase
around the proeutectoid cementite phase.
[0048]
For reference, a graph showing an Si concentration
difference in the sample of test No. 12 in Table 2 of Example
mentioned later is shown in Fig. 1. In Fig. 1, an average of the
Si concentration of the proeutectoid cementite phase in the center,
19
CA 02980886 2017-09-25
and a maximum value of the Si concentration of each ferrite phase
existing around the proeutectoid cementite phase are measured,
and a difference therebetween is defined as the Si concentration
difference. The method for measuring the Si concentration will
be mentioned in detail in the columns of Examples mentioned later.
[0049]
In the present invention, the Si concentration difference
calculated as mentioned above is set at 0.50% or more. Whereby,
the maximum length of proeutectoid cementite can be set at 15 pm
or less. The Si concentration difference is preferably 0.6% or
more. The effect mentioned above is saturated even if the Si
concentration difference is excessively formed, so that the upper
limit is set at 3% or less, and preferably 2.8% or less.
[0050]
In the present invention, the Si concentration difference
is generated at an interface between the proeutectoid cementite
phase and ferrite in the pearlite structure, and the Si
concentration difference is not generated at an interface between
the proeutectoid cementite phase and the cementite (lamellar
cementite that forms a lamellar structure of pearlite) phase in
the pearlite structure.
[0051)
description will be made of a preferred method for
producing the above-mentioned the steel wire rod of the present
invention.
CA 02980886 2017-09-25
[0052]
The high-carbon steel wire rod as mentioned in the present
invention is generally produced by the following procedure in
which a steel billet with a predetermined chemical component
adjusted in advance is austenitized by heating and then hot-rolled
into a steel wire rod having a predetermined wire diameter.
[0053]
After hot rolling, the steel wire rod is placed in a ring
shape on a cooling conveyor and then cooled. At this time, the
placing temperature is preferably set at 880 to 980 C. When the
placing temperature is too high or low, scale characteristics may
change, thus exerting an adverse influence on a mechanical
descaling (MD) treatment before wire drawing. The placing
temperature is preferably 900 C or higher and 960 C or lower.
Although the other descaling treatment such as pickling may be
used, it is recommended to control to the placing temperature
within the above range taking the productivity into
consideration.
[0054]
Then, cooling is started at a temperature of 800 C or higher.
The cooling conditions are extremely important so as to control
the desired Si concentration difference within a predetermined
range. There is a need that the entire coil placed in a ring shape
falls within the above-mentioned range of the cooling stop
temperature and holding temperature.
21
CA 02980886 2017-09-25
p
[0055]
Specifically, cooling is performed to the cooling stop
temperature of 480 to 620 C at an average cooling rate of 12 to
60 C/s. At this time, when the average cooling rate is low, the
Si concentration difference generated at a proeutectoid cementite
interface is lost by diffusion of Si atoms, thus failing to obtain
the desired Si concentration difference. Meanwhile, when the
average cooling rate is high, a supercooled structure is formed
and a pearlite area ratio becomes less than 90%. The average
cooling rate is more preferably 15 C/s or more and 55 C/s or less.
[0056]
When the cooling starting temperature is low, precipitation
of proeutectoid cementite starts during being allowed to cool,
thus corresponding to the case where the average cooling rate is
low, so that the Si concentration difference decreases. When the
cooling stop temperature is low, a supercooled structure such as
bainite is formed to thereby decrease the pearlite area ratio.
Meanwhile, when the cooling stop temperature is high, Si atoms
diffuse to thereby decrease the Si concentration difference. The
cooling stop temperature is more preferably 500 C or higher and
600 C or lower.
[0057]
After stopping of cooling, the temperature is raised to the
holding temperature of 590 to 650 C and pearlite transformation
is performed. When the holding temperature is too high, Si atoms
22
CA 02980886 2017-09-25
diffuse to thereby decrease the Si concentration difference.
Meanwhile, when the holding temperature is too low, a supercooled
structure is generated to thereby decrease the pearlite area ratio.
The holding temperature is more preferably 600 C or higher and
640 C or lower.
[0058]
The steel wire rod of the present invention was obtained
by the procedure mentioned above , and then coiled into a coil shape
to obtain a wire rod coil. Then, wire drawing is performed to
obtain a steel wire having desired wire diameter and strength.
[0059]
A patenting treatment is preferably performed after wire
drawing. An extra fine steel wire having a wire diameter of
approximately 0.2 mm can be obtained by further subjecting to wire
drawing after the patenting treatment. There is no particular
limitation on conditions of the patenting treatment and, for
example, it is possible to employ conditions such as heating
temperature of 950 C and patenting temperature of 600 C. The
patenting treatment may be performed not only once, but also
plural times (for example, 2 to 3 times).
[0060]
The thus obtained steel wire of the present invention has
a high tensile strength such as approximately 4,000 MPa or more.
According to the present invention, a steel wire having a wire
diameter of approximately 0.1 to 0.4 mm is obtained, so that the
23
thus obtained steel wire is suitably used for steel cords,
wire ropes, saw wires and the like.
[0061]
Cancelled
Examples
[0062]
While the present invention will be more specifically
described below by way of Examples, it is to be understood
that the present invention is not limited to the Examples,
and various design variations made in accordance with the
purports described hereinbefore and hereinafter are also
included in the scope of the present invention.
[0063]
Each of steels A to Z (cross-sectional shape: 155 mm x155
mm) shown in Table 1 was heated to a temperature of 1,000 C
and hot-rolled into a predetermined wire diameter of 5.5 mm.
Then, the hot-rolled steel was placed in a ring shape on a
cooling conveyor and allowed to undergo pearlite
transformation while control cooling by blast cooling, and
then coiled into a coil shape to obtain a coil of rolled
material. The cooling conditions after rolling and the wire
rod diameter after rolling are shown in Table
24
CA 2980886 2019-03-04
CA 02980886 2017-09-25
2.
[0064]
Using the thus obtained coil of rolled material, the
following items were measured.
[0065]
Measurement of Pearlite (P) Area Ratio
After cutting off the unsteady part of the end of the coil
of rolled material, the end of the non-defective product was
collected to obtain a specimen having a length of 5 cm. A
micrograph of a transverse section perpendicular to a wire rod
longitudinal direction of the thus obtained specimen was taken
by a scanning electron microscope (SEM). Using the thus obtained
micrograph, an area ratio of a pearlite structure and a
non-pearlite structure was determined by a point counting method.
The point counting method is a method in which the micrograph is
sectioned into meshes and the number of structures existing in
lattice points is counted to thereby easily determine an area
ratio of the structure. Specifically, a micrograph of the center
of the transverse section was taken at a magnification of 4,000
times to fabricate three SEM micrographs. The each micrograph
was sectioned into 100 lattice points and a pearlite area ratio
was determined, and then an average was calculated. An evaluation
area of one SEM micrograph is 868 pm2. The pearlite area ratio
and details of the structure in each specimen are shown in Table
2. The non-pearlite structure detected by the above point
CA 02980886 2017-09-25
counting method (proeutectoid cementite structure, bainite
structure) are also shown in Table 2. In the table, P denotes
a pearlite structure, B denotes a bainite structure, and 0 denotes
proeutectoid cementite.
[0066]
Evaluation of Maximum Length of Proeutectoid Cementite (0)
Using the thus obtained SEM micrograph, a length of each
observed proeutectoid cementite was measured and a maximum length
was determined. The proeutectoid cementite is precipitated in
a plate shape and, when lamellar cementite is divided into
multiple branches, the total value of lengths of each branch was
employed.
[0067]
Measurement of Si Concentration Difference
Using the thus obtained SEM micrograph, regarding the thus
observed proeutectoid cementite, line analysis of the Si
concentration was performed by energy dispersive X-ray
spectrometry (EDX) using a spherical aberration corrected
scanning transmission electron microscope (Cs-STEM)., and then an
Si concentration difference between inside the proeutectoid
cementite phase and a ferrite phase existing around the
proeutectoid cementite phase was determined. Specifically, an
average of the Si concentration of the proeutectoid cementite
phase, and a maximum value of the Si concentration of the ferrite
phase were respectively measured, and then the difference was
26
CA 02980886 2017-09-25
=
defined as the Si concentration difference. A step width of line
analysis was set at 2 nm, and an evaluation length was set at 200
nm.
[0068]
Evaluation of Mechanical Properties of Coil of Rolled Material
After cutting off the unsteady part of the end of the coil
of rolled material, one ring was collected from the coil end of
the non-defective product and then divided into eight samples in
a longitudinal direction. In accordance with JIS Z2201, a tensile
test was performed and a tensile strength TS was measured. An
average of the tensile strength of eight samples in total was
determined, and then TS of the coil of rolled material was
calculated.
[0069]
Evaluation of Wire Drawability
Using the above coil of rolled material, cold wire-drawing
was performed to a predetermined wire diameter at wire drawing
strain in Table 2, and then a tensile strength TS after wire drawing
was determined. Each amount of wire drawing is 200 kg. When wire
breakage occurred during wire drawing, "wire breakage" was filled
in the table.
[0070]
These results are collectively shown in Table 2.
[0071]
27
[Table 1A]
Chemical composition (Y0 by mass) *Balance being iron and inevitable
impurities
Steel
C Si Mn Al P S Ti N
B Co Cr V Cu Ni Nb
A 1.10 0.55 0,50 0.003 0.010 0.010 0.0027
B 0.97 0.80 0.40 0.002 0.011 0.006 0.001 0.0040
C 1.05 0.60 0.45 0.002 0.008 0.008 0.001 0.0035 0.0020
D 1.25 0.90 0.48 0.003 0.010 0.010 0.0044 0.0030 0.50
E 1.30 1.20 0.30 0.002 0.010 0.011 0.003 0.0032 0.0015 0.30 0.30
F 0.95 0.70 0.50 0.001 0.007 0.010 0.0044 0.0020
0.15
G 0.98 0.90 0.40 0.001 0.010 0.020 0.002 0.0028 0.0025
0.10 0.05
OD
1-1 1.05 0.60 0.30 0.002 0.020 0.008 0.0048
0.20
1 1.00 0.70 0.50 0.003 0.007 0.010 0.0052 0.0028
0.11
J 1.12 0.66 0.70 0.002 0.008 0.012 0.002 0.0031 0.0080
0.20
K 0.98 0.80 0.70 0.001 0.006 0.008 0.0042 0.0075
0.15
L 1.10 1.15 1.10 0.003 0.010 0.007 0.001 0.0055
M 1.06 0.51 0.60 0.002 0.015 0.011 0.003 0.0036 0.0012
N 0.97 0.90 0.40 0.002
0.008 0.011 0.0031 0.0012
0 1.35 1.10 0.50 0.005 0.010 0.010 0.003 0.0052 0.0034
P 1.00 0.20 0.60 0.003
0.010 0.010 0.0018 0.0070
[0072]
[Table 1B]
Chemical composition (% by mass) *Balance being iron and inevitable impurities
Steel
C Si Mn Al P S Ti N B Co Cr V Cu Ni Nb
Q 1.00 0.40 0.50 0.002 0.008 0.007 0.0045
R 1.05 0.45 0.48 0.001 0.006 0.008 0.001 0.0039
S 1.15 0.90 0.65 0.003 0.010 0.008 0.0031 0.20
T 1.23 1.05 0.35 0.002
0.006 0.011 0.003 0.0028 0.20
U 0.98 0.59 0.50 0.001
0.007 0.010 0.0046 0.10
/
1.02 0.46 0.55 0.001 0.010 0.020 0.002 0.0026 0.08
W 0.98 0.78 0.25 0.002 0.020 0.008 0.0046
0.15
X 0.99 0.55 0.66 0.003 0.007 0.010 0.0051
0.20
S 1.15 0.90 0.65 0.003 0.010 0.008 0.0031 0.20
T 1.23 1.05 0.35 0.002
0.006 0.011 0.003 0.0028 0.20
U 0.98 0.59 0.50 0.001
0.007 0.010 0.0046 0.10
/
1.02 0.46 0.55 0.001 0.010 0.020 0.002 0.0026 0.08
W 0.98 0.78 0.25 0.002 0.020 0.008 0.0046
0.15
X 0.99 0.55 0.66 0.003 0.007 0.010 0.0051
0.20
Y 0.90 0.60 0.30 0.002 0.008 0.005 0.0034
Z 0.93 0.45 0.40 0.003 0.007 0.008 0.0041
_
[0073]
[Table 2A]
Cooling conditions after rolling Properties of rolled
material Wire drawability
Cooling Cooling Rolled
Si -
Average Maximum
Wire Wire
Placing
Test No, Steel starting stop Holding
wire TS p area concentration TS
temperature cooling rate temperature diameter
length of 0 Structure diameter drawing
temperature
temperatureratio difference
( C) ( C) ( Cts) ( C) ( C) (MPa)
(11m) (% by mass) (mm) strain (vrpa)
_
1 A 930 900 15 550 10 620 1 1,40 10 P+B
97% 0.88 2.2 1.83 2,217
6
2 B 910 880 20 570 307 12 P+0
98% 0.96 2.2 1.83 2.067
3 C 940 910 15 550 600 1,357 8 P+B
95% 1.37 2.0 2.02 2,250
Wire
4 C 930 750 16 510 600 1,265 21 P+0
98% 0.38 2.0 2.02
breakage
^
g
Wire
C 940 900 80 530 620 1,602 13 P+B 31% 1.55
2.0 2.02 0
breakage
0
0
0
6
Wire
C 920 880 7 540 610 1,304 17 P+0
97% 0.46 2.0 2.02
breakage
'g
CDWire
7 C 920 880 55 430 610 1,579 12 P+B
48% 1.30 2 0 2.02 0
breakage
0
I
IV
Wire oi
8 C 910 860 13 650 650 1,279 19 P+0
95% 0.41 2.0 2.02
breakage
Wire
9 C 970 850 30 500 560 1,521 11 P+B
74% 1.62 2.0 2.02
breakage
Wire
C 930 850 15 620 680 1,255 22 P+0 96% 0.41
2.0 2.02
breakage
11 D 900 830 35 510 630 5.5 1,467 3
P+B 95% 1.88 1.8 2.23 2,564
12 E 890 800 40 500 630 5.5 1,481 4
P+B 96% 2.10 1.9 2.13 2,570
13 P 910 840 60 480 590 5.5 1,341 9
P--B+0 90% 1.85 2.1 1.93 2,220
14 G 920 880 20 560 610 5.5 1,342 11
P+0 99% 1.79 2.1 1.93 2,222
H 900 820 12 620 650 5.0 1,381 15 P+0 97%
1.29 2.0 1.83 2,234
5
[0074]
[Table 2B]
Cooling conditions after rolling Properties of
rolled material , Wire drawability
Cooling Average
Test Placing
Cooling stop Holding Rolled
Si
Maximum
Wire Wire _
Steel starting cooling
wire TS P area concentration TS
No. temperature
temperature rate temperature temperature
diameter length of 0
Structure ratio difference diameter drawing
strain
( C) ( C) ( C/s) ( C) ( C) (mm) (MPa)
(um) , (% by mass) (mm) (MPa) ..
16 1 910 810 25 530 620 5.5 1,326 12 P
100% 1.66 2.1 1.93 2,146
_ _
17 J 920 850 13 610 630 5.5 1,389 13 P4-0
95% 1.34 2.0 2.02 2,303
i
_______________________________________________________________________________
________________________
18 K 930 890 30 600 650 5.0 1,334 11 P
100% 1.76 2.0 1.83 2,109
,
19 L 930 900 22 590 630 5.0 1,376 12 P
100% 2.70 2.0 1.83 2,176
g 20 M 950 900 40 520
600 5.5 1,346 9 P+B 94% 0.67 2.0 2.02 2,232 0
0
0
0
0
21 N 930 910 42 520 590 5.5 1,378 0
P+B 97% , 1.54 2.0 2.02 2,285 c
co
0
22
Wire 0
0 900 880 22 550 600 5.5 1,416 31
1140 97% 2.10 2.2 1.83
t,..)
breakage .,
,
.
0
F-A
Wire
23 P 900 860 16 560 600 5.5 1,267 26 P+0
96% 0.35 2.2 1.83
breakage01
11,
24 Q 910 880 18 570 590 5.0 1,311 8 P+0
98% 0.55 2.0 1.83 2,073
_ . _
25 R 900 880 20 560 600 4.5 1,342 9 P+0
97% 0.60 1.8 L83 2,122
_
26 S 920 880 12 580 600 5.5 1,451 9 P
98% 1.22 2.2 1.83 2,294
27 T 910 890 14 560 600 5.5 1,423 11 P+0
97% 1.32 2.4 1.66 2,154
. _
28 U 920 880 19 580 590 5.5 1,411 10 P
99% 0.89 2.3 1.74 2,181
29 V 930 870 15 560 600 5.5 1,398 7 P
97% 0.98 2.2 1.83 2,210
30 X 910 890 16 550 610 5.5 1,422 5 P
98% 0.68 2.0 2.02 2,358
,
. ,
_
31 Y 920 900 15 570 610 5.5 1,397 5
P 99% 0.96 2.0 2.02 2,331
_ .
32 Z 910 890 17 550 600 5.5 1,452 7
P 98% 0.57 2.0 2.02 2,409
CA 02980886 2017-09-25
a
[0075]
These results can be explained by the following
consideration.
[0076]
The samples of tests Nos. 1 to 3, 11 to 21, and 24 to 32
are examples that satisfy the requirements of the present
invention, and satisfactory wire drawability was confirmed
without causing wire breakage. Particularly, regarding all
samples of tests Nos. 3, 11 to 14, 16 to 18, 20, and 21 in which
steels C to G, I to K, M, and N, each containing B, in Table 1
are used, wire drawing could be performed to high wire drawing
strain without causing wire breakage. Of these, regarding
samples of tests Nos. 11 and 12 in which steels D and E, each
containing Co in addition to B, in Table 1 are used, wire drawing
could be performed to higher wire drawing strain range (2.13 or
more).
[0077]
To the contrary, examples mentioned below have the
following defects.
[0078]
Regarding all samples of tests Nos. 4 to 10, steel C that
satisfies the requirements of the present invention in Table I
was used. However, since the production was performed without
satisfying any one of conditions recommended by the present
invention, wire breakage occurred during wire drawing.
32
CA 02980886 2017-09-25
[0079]
Specifically, regarding the sample of test No. 4, because
of low cooling starting temperature, the Si concentration
difference decreased and a maximum length of proeutectoid
cementite increased, and thus wire breakage occurred during wire
drawing.
[0080]
Regarding the sample of test No. 5, because of large average
cooling rate from the cooling starting temperature to the cooling
stop temperature, the pearlite area ratio decreased and thus wire
breakage occurred during wire drawing.
[0081]
Regarding the sample of test No. 6, because of small average
cooling rate from the cooling starting temperature to the cooling
stop temperature, the Si concentration difference decreased and
a maximum length of proeutectoid cementite increased, and thus
wire breakage occurred during wire drawing.
[0082]
Regarding the sample of test No. 7, because of low cooling
stop temperature, the pearlite area ratio decreased and thus wire
breakage occurred during wire drawing.
[0083]
Regarding the sample of test No. 8, because of high cooling
stop temperature, the Si concentration difference decreased and
a maximum length of proeutectoid cementite increased, and thus
33
CA 02980886 2017-09-25
4 4
wire breakage occurred during wire drawing.
[0084]
Regarding the sample of test No. 9, because of low holding
temperature, the pearlite area ratio decreased and thus wire
breakage occurred during wire drawing.
[0085]
Regarding the sample of test No. 10, because of high holding
temperature, the Si concentration difference decreased and a
maximum length of proeutectoid cementite increased, and thus wire
breakage occurred during wire drawing.
[0086]
Regarding the sample of test No. 22, since steel 0 having
large C content in Table 1 was used, a maximum length of
proeutectoid cementite increased and thus wire breakage occurred
during wire drawing.
[0087]
Regarding the sample of test No. 23, since steel P having
small Si content in Table 1 was used, the Si concentration
difference decreased and a maximum length of proeutectoid
cementite increased, and thus wire breakage occurred during wire
drawing.
34