Note: Descriptions are shown in the official language in which they were submitted.
WO 2016/161010 PCT/US2016/025051
Heavy Chain Constant Regions with Reduced Binding to Fc Gamma Receptors
FIELD OF THE INVENTION
[0002] The present invention resides in the field of recombinant protein
engineering, and
relates to optimized hinge variants of immunoglobulin (Ig) proteins, methods
of engineering
such Ig variants and suitability of such Ig variants in biopharmaceutical
practice.
BACKGROUND
100041 Antibodies of the IgG class are attractive therapeutic agents. IgGs
exist as four
subclasses in humans, IgG1, IgG2, IgG3, and IgG4. The heavy chain constant
(CH) region of IgG
comprises three domains, CH1, CH2, CH3, and a hinge linking CH1 and CH2.
Although the role
of each subclass appears to vary between species, the heavy chain constant
domain is
responsible for various biological effector functions. The human IgG
subclasses mediate several
cellular immune responses through their interaction with Fcy (FcyRs), such as
cell killing,
phagocytosis and opsonization. Such interaction involves binding of at least
functional CH2 and
CH3 domains of a heavy chain constant region to an FcyR on the surface of an
effector cell, such
as a natural killer cell, an activated macrophage or the like. Complement-
mediated lysis can also
be triggered by the interaction of the Fc region with various complement
components.
100051 Effector functions are useful in some antibody therapies, such as
treatment of some
cancers or pathogens, in which effector function is primarily or at least
partially responsible for
killing cancer cells or the pathogen. However, other antibody therapies are
mediated entirely
or predominantly by effector-independent mechanisms, such as inhibiting a
receptor-ligand
interaction or agonizing a receptor. In such therapies, antibody effector
functions serve little or
no useful purpose but can result in undesired inflammation. In such
circumstances, it may be
1
Date Recue/Date Received 2022-07-13
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
advantageous to engineer the Fc receptor binding properties of an antibody so
as to inhibit
some or all of the available effector mechanisms, without substantially
affecting the antibody's
pharmacokinetic properties, immunogenicity and variable regions specificity
and affinity.
[0006] IgG heavy chain constant regions have been mutated in various
positions to test the
effect of amino acids on IgG/FcyR interaction (see e.g. Canfield and Morrison,
J Exp Med 73,
1483-1491 (1991); Chappel et al. JSC 268(33), 25124-31 (1993); and Armour et
al., Eur. J.
Immunol. 29, 2613-24 (1999)). Several amino acid residues in the hinge region
and in the CH2
domain of a heavy chain constant region have been proposed as mediating
binding to Fcy
receptors (see Sarmay et al., Mol Immunol 29, 633-9 (1992); Greenwood et al.,
Eur. J. Immunol,
23(5), 1098 (1993), Morgan et al., Immunology 86, 319 (1995), Stevenson,
Chemical
Immunology, 65, 57-72 (1997)). Glycosylation of a site (N297) in the CH2
domain and variations
in the composition of its carbohydrates also strongly affect the IgG/FcyR
interaction (Stevenson,
Chemical Immunology, 65, 57-72 (1997); Siberil et al, Immunol. Ltrs. 106, 111-
118 (2006)).
[0007] Ala nine residues have usually been the preferred substituent for
replacing a natural
amino acid with an unnatural one so as to reduce function because alanine has
a side chain
without any functional groups. For example, the well-known technique of
alanine-scanning
mutagenesis systematically replaces every natural residue in a protein or
protein domain with
alanine to identify which natural residues contribute primarily to function.
Replacing an amino
acid with a functional group with alanine eliminates the functional group and
its contribution to
binding to any receptor, but the presence of the alanine side chain
substantially preserves
conformation, reducing the potential for immunogenicity or other complexities
due to
conformational changes. An alternative strategy replaces amino acids in the
hinge region of
one IgG isotype with corresponding amino acids from human IgG2 isotype so as
to reduce FcyR
binding without unacceptable conformational changes and consequent
immunogenicity. The
resulting chimeric Fc-containing antibodies include a substitution of EFLG at
positions 232-236
with PVA (see W014/121087).
SUMMARY OF THE CLAIMED INVENTION
[0008] The invention provides an innmunoglobulin heavy chain comprising a
constant
region, wherein positions 233-236 within a hinge domain are G, G, G and
unoccupied; G, G,
2
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
unoccupied, and unoccupied; G, unoccupied, unoccupied, and unoccupied; or all
unoccupied,
with positions numbered by EU numbering. Optionally, the immunoglobulin heavy
chain of
claim 1 that is human IgG4 isotype. Optionally, positions 226-229 are CPPC.
Optionally, the
hinge domain amino acid sequence comprises CPPCPAPGGG-GPSVF (SEQ ID NO:1),
CPPCPAPGG--GPSVF (SEQ ID NO:2), CPPCPAPG---GPSVF (SEQ ID NO:3), or CPPCPAP----
GPSVF
(SEQ ID NO:4). Optionally, the constant region has an amino acid sequences
comprising SEQ ID
NO:5, 6, 7 or 8 or a variant thereof having up to five insertions deletions,
substitutions or
insertions. Optionally, the constant region comprises SEQ ID NO: 5, 6, 7 or 8.
Optionally, the
constant region consists of SEQ ID NO: 5, 6, 7 or 8. Optionally, the
immunoglobulin heavy chain
comprises from N-terminal to C-terminal the hinge domain, a CH2 domain and a
CH3 domain.
Optionally, the immunoglobulin heavy chain comprises from N-terminal to C-
terminal a CH1
domain, the hinge domain, a CH2 domain and a CH3 domain. Optionally, the CH1
region, if
present, remainder of the hinge region, if any, CH2 region and CH3 region are
the same human
isotype. Optionally, the CH1 region, if present, remainder of the hinge
region, if any, CH2
region and CH3 region are human IgG1. Optionally, the CH1 region, if present,
remainder of the
hinge region, if any, CH2 region and CH3 region are human IgG2. Optionally,
the CH1 region if
present, remainder of the hinge region, if any, CH2 region and CH3 region are
human IgG4.
Optionally, the constant region has a CH3 domain modified to reduce binding to
protein A.
Optionally, the immunoglobulin heavy chain of any preceding claim linked at
the N-terminus to
a heavy chain variable region. Optionally, the immunoglobulin heavy chain is
duplexed with an
immunoglobulin light chain. Optionally, the immunoglobulin heavy chain is
duplexed with an
immunoglobulin light chain as a heterodimer comprising two immunoglobulin
heavy chains and
two light chains. The two heavy chains can be the same or different.
Optionally, the
immunoglobulin heavy chain of any preceding claim linked at the N-terminus to
a binding
polypeptide. Optionally, the immunoglobulin heavy chain is linked via a linker
to the binding
polypeptide. Optionally, the binding polypeptide is an extracellular domain.
3
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
BRIEF DESCRIPTION OF FIGURES
[0009] Fig. 1 shows correspondence of numbering schemes in the hinge region
of human
IgG1, IgG2, and IgG4.
[0010] Figs. 2-4 show the wild-type sequences of heavy chain constant
regions of isotypes
IgG1, IgG2, IgG3 and IgG4 with delineation into CH1, hinge, CH2 and CH3
regions.
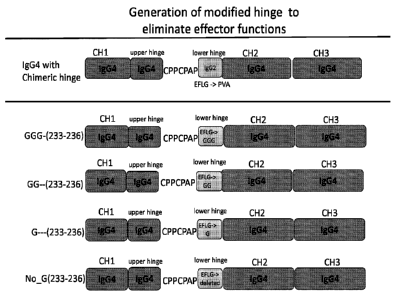
[0011] Fig. 5 is a schematic showing exemplary hinge-modified GGG-(233-
236), GG--(233-
236, G---(233-236) and no_G(233-236) replacement formats compared with a
previously
described chimeric heavy chain constant region, all of human IgG4 isotype.
[0012] Figs. 6-10 show binding of various hinge-modified antibodies of
human IgG4 isotype
(and wild-type IgG1 isotype antibody) to human FcyRI, FcyRIIA, FcyRIIB,
FcyRIIIA, and FcRn.
[0013] Fig. 11A shows hinge-modified antibodies (as recovered from cell
culture
supernatants) are unable to recruit human 1-cells to lyse U937 cells, which
bear FcyRI and
FcyRIIA.
[0014] Fig. 11B also shows hinge-modified antibodies, that have been fully
purified, and
that are unable to recruit human 1-cells to lyse U937 cells bearing FcyRI and
FcyRIIA.
[0015] Figs. 12A-D show that various hinge-modified antibodies show no
significant
activation ofJurkat cells with a luciferase marker when the activation is
dependent on
anchoring of the antibody to HEK cells transformed with FcyRIIA (Fig. 12A,
Fig. 12B) or RIIB (Fig.
12C, Fig. 12D). The activation by the positive control antibody in Fig. 12A
and Fig. 12C is greatly
reduced on competition with a blocking antibody, as in Fig. 12B and Fig. 12D.
[0016] Fig. 13 shows hinge-modified antibodies activating Jurkat cells with
a luciferase
marker when the antibodies are anchored to a plate surface instead of by
attempted binding to
an FcyRIIA or RIIB receptor on the HEK cells.
[0017] Fig. 14 shows hinge-modified antibodies having variable regions that
bind CD20
display reduced antibody-dependent cellular cytotoxicity (ADCC) in the
presence of NK cells
cells engineered to express the higher affinity V allele of FcyRIlla and CD20-
expressing Daudi
cells.
4
CA 02981312 2017-09-28
WO 2016/161010
PCT/US2016/025051
DEFINITIONS
10018]
Antibodies or fusion proteins are typically provided in isolated form. This
means
that an antibody or fusion protein is typically at least 50% w/w pure of
interfering proteins and
other contaminants arising from its production or purification but does not
exclude the
possibility that an antibody or fusion protein is combined with an excess of
pharmaceutical
acceptable carrier(s) or other vehicle intended to facilitate its use.
Sometimes antibodies or
fusion proteins are at least 60, 70, 80, 90, 95 or 99% w/w pure of interfering
proteins and
contaminants from production or purification. Often an antibody or fusion
protein is the
predominant macromolecular species remaining after its purification.
10019] A
basic antibody structural unit is a tetramer of subunits. Each tetramer
includes
two identical pairs of polypeptide chains, each pair having one "light" (about
25 kDa) and one
"heavy" chain (about 50-70 kDa). The amino-terminal portion of each chain
includes a variable
region of about 100 to 110 or more amino acids primarily responsible for
antigen recognition.
This variable region is initially expressed linked to a cleavable signal
peptide. The variable
region without the signal peptide is sometimes referred to as a mature
variable region. Thus,
for example, a light chain mature variable region means a light chain variable
region without
the light chain signal peptide. However, reference to a variable region does
not mean that a
signal sequence is necessarily present; and in fact signal sequences are
cleaved once the
antibodies or fusion proteins of the invention have been expressed and
secreted. A pair of
heavy and light chain variable regions defines a binding region of an
antibody. The ca rboxy-
terminal portion of the light and heavy chains respectively defines light and
heavy chain
constant regions. The heavy chain constant region is primarily responsible for
effector function.
In IgG antibodies, the heavy chain constant region is divided into CH1, hinge,
CH2, and CH3
regions. In IgA, the heavy constant region is divided into CH1, CH2 and CH3.
The CH1 region
binds to the light chain constant region by disulfide and noncovalent bonding.
The hinge region
provides flexibility between the binding and effector regions of an antibody
and also provides
sites for intermolecular disulfide bonding between the two heavy chain
constant regions in a
tetramer subunit. The CH2 and CH3 regions are the primary site of effector
functions and FcRn
binding.
WO 2016/161010 PCT/US2016/025051
[0020] Light chains are classified as either kappa or lambda. Heavy chains
are classified as
y, mu, alpha, delta, or epsilon, and define the antibody's isotype as IgG,
IgM, IgA, IgD and IgE,
respectively. Within light and heavy chains, the variable and constant regions
are joined by a
"1" segment of about 12 or more amino acids, with the heavy chain also
including a "D"
segment of about 10 or more amino acids. (See generally, Fundamental
Immunology (Paul, W.,
ed., 2nd ed. Raven Press, N.Y., 1989), Ch. 7) .
[0021] The mature variable regions of each light/heavy chain pair form the
antibody
binding site. Thus, an intact antibody has two binding sites, i.e., is
divalent. In natural
antibodies, the binding sites are the same. However, bispecific antibodies can
be made in
which the two binding sites are different (see, e.g., Songsivilai and
Lachmann, Clin. Exp.
Immunol., 79:315-321 (1990); Kostelny et al., J. Immunol., 148:1547-53 (1992))
. The variable
regions all exhibit the same general structure of relatively conserved
framework regions (FR)
joined by three hypervariable regions, also called complementarity determining
regions or
CDRs. The CDRs from the two chains of each pair are aligned by the framework
regions,
enabling binding to a specific epitope. From N-terminal to C-terminal, both
light and heavy
chains comprise the domains FR1, CDR1, FR2, CDR2, FR3, CDR3 and FR4. The
assignment of
amino acids to each domain is in accordance with the definitions of Kabat,
Sequences of
Proteins of Immunological Interest (National Institutes of Health, Bethesda,
MD, 1987 and
1991), or Chothia & Lesk, J. Mol. Biol. 196:901-917 (1987); Chothia et al.,
Nature 342:878-883
(1989). Kabat also provides a widely used numbering convention (Kabat
numbering) in which
corresponding residues between different heavy chain variable regions or
between different
light chain variable regions are assigned the same number. Although Ka bat
numbering can be
used for antibody constant regions, the EU index is more commonly used, as is
the case in this
application.
[0022] An antibody or fusion protein of the invention is mono-specific if
all of its antigen (or
ligand) binding regions have the same specificity. An antibody or fusion
protein is multispecific
if its antigen binding regions include at least two different specificities.
6
Date Recue/Date Received 2022-07-13
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
10023] A hinge is a region of consecutive amino acid residues that connect
the C-terminus
of the CH1 to the N-terminus of the CH2 domain of an immunoglobulin. In human
IgG1, IgG2
and IgG4, the hinge region runs from residue 216 to 236 by EU numbering.
Residues 231-236
form a lower hinge and residues 216 to 230 form an upper and middle (or core)
hinge. The
demarcation between upper and middle varies by isotype. The upper and middle
hinges of
IgG1, IgG2 and IgG4 are 12-15 consecutive amino acids encoded by a distinct
hinge exon. The
lower hinge includes several N-terminal amino acids of the CH2 domain (encoded
by the CH2
exon) (Brekkeet al. Immunology Today 16(2):85-90 (1995)). IgG3 comprises a
hinge region
consisting of four segments: one upper segment resembling the hinge region of
IgG1, and 3
segments that are identical amino acid repeats unique to IgG3.
[0024] The term "antibody" includes any form of antibody with at least one
binding region
including monovalent fragments, divalent tetrameric units of two heavy chains
and light chains,
and higher order complexes of any of these. An antibody can be mono-specific
in which case all
binding regions have the same specificity or multi-specific in which the
binding sites have at
least two specificities. Antibody fragments typically include a heavy chain
variable region and a
heavy chain constant region and may also include a light chain variable
region. For example, an
antibody fragment can include from N-terminal to C-terminal a light chain
variable region, a
peptide spacer, a heavy chain variable region and a heavy chain constant
region of the
invention. Another fragment includes a heavy chain variable region (the
binding region) and a
heavy chain constant region and no light chain (i.e., a Dab or nanobody).
Likewise, a fusion
protein includes a monomeric or dimeric fusion protein unit, or higher order
complexes.
[0025] A "monoclonal antibody" refers to a preparation of antibody
molecules resulting
from propagation of a single clone consisting essentially of the same antibody
molecules.
Minor differences resulting from spontaneous mutations arising in culture or
posttranslational
processing may be present. A monoclonal antibody composition displays a single
binding
specificity and affinity for a particular epitope. Accordingly, the term
"mouse or murine
monoclonal antibody" refers to antibodies displaying a single binding
specificity which have
variable and constant regions derived from murine or mouse germline
immunoglobulin
sequences.
7
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
[0026] A multispecific antibody typically comprises multiple different
variable domains (two
in the case of bispecific antibody), wherein each variable domain is capable
of specifically
binding to a separate antigen or to a different epitope on the same antigen.
Exemplary
bispecific formats that can be used with disclosed constant regions include
e.g., scFv-based
bispecific formats, IgG-scFv fusions, dual variable domain (DVD)-Ig, Quadroma,
knobs-into-
holes, common light chain (e.g., common light chain with knobs-into-holes,
etc.), CrossMab,
CrossFab, (SEED)body, dual acting Fab (DAF)-IgG, and Mab2 bispecific formats
(see, e.g., Klein et
al. 2012, mAbs 4:6, 1-11, and references cited therein, for a review of the
foregoing formats).
Bispecific antibodies can also be constructed using peptide/nucleic acid
conjugation, e.g.,
wherein unnatural amino acids with orthogonal chemical reactivity are used to
generate site-
specific antibody-oligonucleotide conjugates which then self-assemble into
multimeric
complexes with defined composition, valency and geometry. (See, e.g., Kazane
et al. 2013, J.
Am. Chem. Soc. 9; 135(1 ):340-6 [Epub: Dec. 21, 2012]). Another exemplary
nnultispecific
format that can be used with the disclosed constant regions includes a first
antigen-binding
domain that specifically binds a target molecule, and a second antigen-binding
domain that
specifically binds an internalizing effector protein, wherein such second
antigen-binding
domains are capable of activating and internalizing the effector protein, e.g.
a receptor. (See US
2013/0243775A1.).
[0027] Binding refers to an interaction between at least two entities, or
molecular
structures, such as an antibody-antigen interaction, or an Fc-containing
protein to an FcyR
(wherein the Fc-containing protein is an antibody, Ig, antibody-binding
fragment, or Fc-fusion
protein, e.g. receptor-Fc fusion). For instance, binding affinity typically
corresponds to a KD
value of about 10-7 M or less, such as about 10' M or less, such as about 10-9
M or less when
determined by, for instance, surface plasmon resonance (SPR) technology in a
BlAcore 3000
instrument using the antigen or FcR as the ligand and the antibody, Ig,
antibody-binding
fragment, or Fc-containing protein as the analyte (or antiligand).
Accordingly, an antibody or
fusion protein binds to a target antigen or receptor with an affinity
corresponding to a KD value
that is at least ten-fold lower, such as at least 100 fold lower, for instance
at least 1 ,000 fold
lower, such as at least 10,000 fold lower, for instance at least 100,000 fold
lower than its
8
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
affinity for binding to a non-specific antigen (e.g., BSA, casein). There is
an inverse relationship
between KD and binding affinity, therefore the smaller the KD value, the
higher the affinity.
Thus, the term "lower affinity" relates to a lower ability to form an
interaction and therefore a
larger KD value.
[0028] An epitope is an antigenic determinant capable of specific binding
to an antibody.
Epitopes usually consist of surface groupings of molecules such as amino acids
or sugar side
chains and usually have specific three dimensional structural characteristics,
as well as specific
charge characteristics. Conformational and nonconformational epitopes are
distinguished in
that the binding to the former, but not the latter, is lost in the presence of
denaturing solvents.
The epitope may comprise amino acid residues directly involved in the binding
(also called
immunodonninant component of the epitope) and other amino acid residues, which
are not
directly involved in the binding, such as amino acid residues which are
effectively blocked by
the specific antigen binding peptide (in other words, the amino acid residue
is within the
footprint of the specific antigen binding peptide).
[0029] A humanized antibody is a genetically engineered antibody in which
the CDRs from a
non-human "donor" antibody are grafted into human "acceptor" antibody
sequences (see, e.g.,
Queen, US 5,530,101 and 5,585,089; Winter, US 5,225,539, Carter, US 6,407,213,
Adair, US
5,859,205 6,881,557, Foote, US 6,881,557). The acceptor antibody sequences can
be, for
example, a mature human antibody sequence, a composite of such sequences, a
consensus
sequence of human antibody sequences, or a germline region sequence. Thus, a
humanized
antibody is an antibody having some or all CDRs entirely or substantially from
a donor antibody
and variable region framework sequences and constant regions, if present,
entirely or
substantially from human antibody sequences. Similarly a humanized heavy chain
has at least
one, two and usually all three CDRs entirely or substantially from a donor
antibody heavy chain,
and a heavy chain variable region framework sequence and heavy chain constant
region, if
present, substantially from human heavy chain variable region framework and
constant region
sequences. Similarly a humanized light chain has at least one, two and usually
all three CDRs
entirely or substantially from a donor antibody light chain, and a light chain
variable region
framework sequence and light chain constant region, if present, substantially
from human light
9
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
chain variable region framework and constant region sequences. Other than
nanobodies and
dAbs, a humanized antibody comprises a humanized heavy chain and a humanized
light chain.
A CDR in a humanized antibody is substantially from a corresponding CDR in a
non-human
antibody when at least 85%, 90%, 95% or 100% of corresponding residues (as
defined by Kabat)
are identical between the respective CDRs. The variable region framework
sequences of an
antibody chain or the constant region of an antibody chain are substantially
from a human
variable region framework sequence or human constant region respectively when
at least 85,
90, 95 or 100% of corresponding residues defined by Kabat are identical.
[0030] Although humanized antibodies often incorporate all six CDRs
(preferably as defined
by Kabat) from a mouse antibody, they can also be made with less than all CDRs
(e.g., at least 3,
4, or 5 CDRs from a mouse antibody) (e.g., Pascalis etal., J. Immunol.
169:3076, 2002; Vajdos et
al., Journal of Molecular Biology, 320: 415-428, 2002; lwahashi et al., Mol.
Immunol. 36:1079-
1091, 1999; Tamura et al, Journal of Immunology, 164:1432-1441, 2000).
[0031] A chimeric antibody is an antibody in which the mature variable
regions of light and
heavy chains of a non-human antibody (e.g., a mouse) are combined with human
light and
heavy chain constant regions. Such antibodies substantially or entirely retain
the binding
specificity of the mouse antibody, and are about two-thirds human sequence.
[0032] A veneered antibody is a type of humanized antibody that retains
some and usually
all of the CDRs and some of the non-human variable region framework residues
of a non-
human antibody but replaces other variable region framework residues that may
contribute to
B- or T-cell epitopes, for example exposed residues (Padlan, Mol. Immunol.
28:489, 1991) with
residues from the corresponding positions of a human antibody sequence. The
result is an
antibody in which the CDRs are entirely or substantially from a non-human
antibody and the
variable region frameworks of the non-human antibody are made more human-like
by the
substitutions.
[0033] A human antibody refers to antibodies having variable and constant
regions derived
from human germline immunoglobulin sequences. Such an antibody can be one
produced by a
human or human B-cells, or a transgenic mouse bearing human immunoglobulin
genes, or by
from a phage display, retrovira I display, ribosomal display and the like (see
for instance
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
Hoogenboom et al., J. Mol. Biol. 227, 381 (1991) (phage display), Vaughan et
al., Nature
Biotech 14, 309 (1996) (phage display), Hanes and Plucthau, PNAS USA 94, 4937-
4942 (1997)
(ribosomal display), Parmley and Smith, Gene 73, 305-318 (1988) (phage
display), Scott TIBS 17,
241-245 (1992), Cwirla et al., PNAS USA 87, 6378-6382 (1990), Russell et al.,
Nucl. Acids
Research 21, 1081-1085 (1993), Hogenboom et al., lmmunol. Reviews 130, 43-68
(1992),
Chiswell and McCafferty, TIBTECH 10, 80-84 (1992), and US 5,733,743). Human
antibodies can
include amino acid residues not encoded by human germline immunoglobulin
introduced by
maturation in vivo, such as by somatic mutation or gene rearrangement in vivo.
Human
antibodies can also include a small number of mutations (e.g., up to 10 per
heavy or light chain)
introduced by random or site- specific mutagenesis in vitro).
[0034] A transgenic animal for producing human antibodies refers to a non-
human animal
having a genome comprising one or more human heavy and/or light chain
transgenes or
transchromosomes (either integrated or non-integrated into the animal's
natural genomic DNA)
and which is capable of expressing fully human antibodies or at least
antibodies with fully
human variable regions. For example, a transgenic mouse can have a human light
chain
transgene and either a human heavy chain transgene or human heavy chain
transchromosome,
such that the mouse produces human antibody when immunized with target antigen
and/or
cells expressing the target antigen. The human heavy chain transgene may be
integrated into
the chromosomal DNA of the mouse, as is the case for transgenic mice, for
instance HuMAb
mice, such as HCo7 or HCol2 mice, or the human heavy chain transgene may be
maintained
extrachromosomally, as is the case for transchromosomal KM mice as described
in
W002/43478. Such transgenic and transchromosomal mice (collectively referred
to as
"transgenic mice") are capable of producing multiple isotypes of human
monoclonal antibodies
to a given antigen (such as IgG, IgA, IgM, IgD and/or IgE) by undergoing V-D-J
recombination
and isotype switching. VELOCIMMUNE genetically engineered mice comprise a
replacement of
unrearranged V(D)J gene segments at endogenous mouse loci with human V(D)J
gene
segments. VELOCIMMUNE mice express chimeric antibodies having human variable
domains
and mouse constant domains (see, e.g., U.S. Pat. No. 7,605,237). Most other
reports concern
mice that express fully human antibodies from fully human transgenes in mice
that have
11.
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
disabled endogenous immunoglobulin loci. The VELOCIMMUNE6 mouse includes, in
part,
having a genome comprising human variable regions operably linked to
endogenous mouse
constant region loci such that the mouse produces antibodies comprising a
human heavy chain
variable region and a mouse heavy chain constant region in response to
antigenic stimulation.
The DNA encoding the variable regions of the heavy chains of the antibodies
can be isolated
and operably linked to DNA encoding the human heavy chain constant regions of
the invention.
The DNA can then be expressed in a cell capable of expressing the fully human
heavy chain of
the antibody.
[0035] Several antibody effector functions are mediated at least in part by
Fc receptors
(FcRs), which bind the Fc region of an antibody in the constant domain
(specifically, the CH2
and CH3 domain) of a typical immunoglobulin. There are a number of Fc
receptors which are
specific for the different classes of immunoglobulins, i.e. IgG, IgE, IgA,
IgM, and IgD. The human
IgG Fc receptor family is divided into three groups: FcyRI (CD64), which is
capable of binding IgG
with high affinity, FcyRII (CD32) and FcyRIII (CD16) both of which are low
affinity receptors.
Each FcyR subclass is encoded by two or three genes, and alternative RNA
splicing leads to
multiple transcripts, hence, a broad diversity in FcyR isoforms exists (e.g.
FcyRIA (CD64;
FCGR1A, Swiss Prot P12314), FcyRIB (CD64; FCRG1 B), FcyRIIA (CD32; FCGR2A,
Swiss Prot
P12318), FcyRIIB (CD32; FCGR2B, Swiss Prot P31994), FcyRIIC (CD32; FCGR2C),
FcyRIIIA (CD16a;
FCGR3A, Swiss Prot P08637), and FcyRIIIB (CD16b; FCGR3B)). Furthermore, Fc
receptors are
expressed on a variety of cells, including, e.g., B cells, monocytes,
dendritic cells, neutrophils,
and certain lymphocytes. For example, U937 cells, a human monocyte cell line,
express both
FcyRI and FcyRIIA (see e.g., Jones, et al. J Imnnunol 135(5):3348-53 (1985);
and Brooks, et al. J
Exp Med 170:1369-85 (October 1989)).
[0036] Antibody-dependent cellular cytotoxicity or ADCC is an activity to
damage a target
cell when an Fcy receptor-bearing cell (an immune cell or the like) binds to
an Fc portion of a
specific antibody through the Fcy receptor, when the specific antibody has
attached to a cell-
surface antigen of the target cell. Thus, ADCC is a mechanism by which Fc
receptor-positive
effector cells can lyse target cells that have adherent antigen-specific
molecule. The ADCC
activity can be evaluated by for example measuring the fluorescent intensity
using a fluorescent
12
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
dye such as calcein AM (Wako Pure Chemical Industries, Ltd., 349-07201). When
this approach
is employed, the cytotoxic activity (% cell lysis) can be calculated, using
the obtained values,
according to the equation: (A-C)/(B-C)x100, wherein A is a fluorescent value
in each sample, B is
an average fluorescent value of the cells lysed and released into a medium
with Nonidet P-40
having a final concentration of 1 %, and C is an average fluorescent value
when only the
medium was added.
[0037] "Antibody-dependent cellular phagocytosis" or "ADCP" relates to
effector function
that eliminates (or kills) a target cell by engulfing the target cell rather
than inducing cytolysis.
ADCP may be an important in vivo mechanism for killing tumor cells. ADCP can
be measured by
two-color fluorescence flow cytometry methods, for example methods utilizing,
e.g. PKH2
(green fluorescent dye) and phycoerythrin-conjugated (red) monoclonal
antibodies against
different cell surface proteins to differentiate the test cells, thus
determining phagocytic
activity and rate of phagocytosis. Therapeutic strategies that selectively
activate FcyRIla relative
to FcyRIlb may enhance macrophage phagocytic activity (Richards et al. 2008
Mol. Cancer Ther.
7(8):2517-27).
[0038] Complement-directed cytotoxicity or CDC refers to cytotoxic activity
by the
complement system. CDC activity can be measured, for example the target cells,
antibody, and
complement solution (such as baby rabbit complement (Cedarlane Technologies))
are
incubated and are allowed to react, according to standard protocols (NIAID
Manual of Tissue
Typing Techniques 1979-1980, Edited by J.G. Ray, NIH Publication No. NIH-83-
545.) The
cytotoxic activity can be calculated in the same manner as the measurement of
the ADCC
activity. The cytotoxic activity can also be measured using a fluorescent dye
(such as calcein) or
radioactive dyes similarly to the above with respect to ADCC.
[0039] The term "subject" includes human and other mammalian subjects that
receive
either prophylactic or therapeutic treatment.
[0040] Compositions or methods "comprising" one or more recited elements
may include
other elements not specifically recited. For example, a composition that
comprises antibody
may contain the antibody alone or in combination with other ingredients.
13
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
DETAILED DESCRIPTION
I. General
[0041] The invention provides antibody heavy chain constant regions with a
hinge region
modified to reduce binding to Fcy receptors. The modification occurs within
positions 233-236
by EU numbering by replacement of natural residues by glycine(s) and/or
deletion(s). The
inventors unexpectedly found that such modifications in the hinge region of
antibodies can
usefully reduce binding of such antibodies to Fcy receptors to a greater
extent than previous
modifications in this region, and particularly can reduce binding to
background levels for any or
all of FcyRI, FcyRIIA, FcyRIIB, and FcyRIIIA. These modified immunoglobulin
constant regions
can be incorporated into virtually any format of antibody or Fc fusion
protein. Such antibodies
or fusion proteins can be used in methods of treatment, particularly those in
which the
mechanisms of action of the antibody or Fc fusion protein is not primarily or
at all dependent
on effector functions, as is the case when an antibody inhibits a receptor-
ligand interaction or
agonizes a receptor.
II. Heavy Chain Constant Regions
[0042] The invention provides modified immunoglobulin heavy chain regions
in which each
of positions 233-236 by EU number is occupied by G or is unoccupied. Position
236 is
unoccupied in canonical human IgG2 but is occupied by in other canonical human
IgG isotypes.
Positions 233-235 are occupied by residues other than G in all four human
isotypes (see Fig. 1).
Position 233 is not believed to interact directly with Fcy receptors but was
included in the
mutagenesis because removing replacing the wildtype glu residue in IgG1 and
IgG4 or pro
residue in IgG2 in combination with the other mutations would reduce
innmunogenicity. In four
exemplary modified constant regions, positions 233-236 are gly gly gly
unoccupied, gly gly
unoccupied, unoccupied; gly, unoccupied, unoccupied, unoccupied and all
unoccupied (see Fig.
5). These segments can be represented as GGG-, GG--, G--- or ---- with "-"
representing an
unoccupied position.
[0043] The hinge modification within positions 233-236 can be combined with
position 228
being occupied by P. Position 228 is naturally occupied by P in human IgG1 and
IgG2 but is
14
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
occupied by S in human IgG4 and R in human IgG3. An 5228P mutation in an IgG4
antibody is
advantageous in stabilizing an IgG4 antibody and reducing exchange of heavy
chain light chain
pairs between exogenous and endogenous antibodies.
[0044] Preferably positions 226-229 are occupied by C, P. P and C
respectively.
[0045] Exemplary hinge regions have residues 226-236, sometimes referred to
as middle (or
core) and lower hinge, occupied by the modified hinge sequences designated GGG-
(233-236),
GG--(233-236), G---(233-236) and no G(233-236).
hIgG1 CPPCPAPELLGGPSVF
hIgG2 CPPCPAPPVA-GPSVF
hIgG4 CPSCPAPEFLGGPSVF
GGG-(233-236) CPPCPAPGGG-GPSVF (SEQ ID NO:1)
GG--(233-236) CPPCPAPGG--GPSVF (SEQ ID NO:2)
G---(233-236) CPPCPAPG---GPSVF (SEQ ID NO:3)
no_G(233-236) CPPCPAP----GPSVF (SEQ ID NO:4)
[0046] The modified hinge regions described above can be incorporated into
a heavy chain
constant region, which typically include CH2 and CH3 domains, and which may
have an
additional hinge segment (e.g., an upper hinge) flanking the designated
region, and a CH1
region. Such additional constant region segments present are typically of the
same isotype,
preferably a human isotype, although can be hybrids of different isotypes. The
isotype of such
additional human constant regions segments is preferably human IgG4 but can
also be human
IgG1, IgG2, or IgG3 or hybrids thereof in which domains are of different
isotypes. Exemplary
sequences of human IgG1, IgG2 and IgG4 are shown in Figs. 2-4. A constant
region is
considered to be of a designated isotype if it differs from that isotype by no
more than 1, 2, 3,
4, 5, 6, 7, 8, 9 or 10 substitutions, deletions or internal insertions, except
however, that the CH1
domain can be omitted entirely as can the upper hinge region. CH1, CH2 and CH3
domains are
considered to be of IgG1, IgG2 or IgG4 isotype if differing from the CH1, CH2
and CH3 region of
the exemplified sequence by no more than 1, 2, 3, 4 or 5 substitutions,
deletions or internal
insertions. The remainder of a hinge outside the 226-236 region sequences
presented above is
considered to be of IgG1, IgG2 or IgG4 isotype if it differs from the
corresponding part of the
hinge region of the exemplified hinge sequences by no more than 1 or 2
substitutions, deletions
or internal insertions.
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
[0047] Some preferred heavy chain constant regions have amino acid
sequences consisting
or comprising SEQ ID NO. 5, 6, 7 and 8. These heavy chain constant regions
incorporate the
segments SEQ ID NO:1, SEQ ID NO:2, SEQ ID:3 and SEQ ID NO:4 at residues 226-
236 shown
above in an otherwise-human IgG4 isotype. Other preferred constant regions
differ from the
designated SEQ ID NO. at up to 1, 2, 3 ,4 ,5, 6, 7, 8, 9 or 10 positions but
retain at least GGG-,
GG , G or -- at EU positions 232-236 and P at position 228 and preferably
retain of residues
226-236 shown above for SEQ ID NO:1, SEQ ID NO:2, SEQ ID:3 and SEQ ID NO:4.
Variations
from the designated SEQ ID NOS. can represent one or several natural allotypic
or isoallotypic
variations, variations to increase or reduce an effector function such as
complement-mediated
cytotoxicity or ADCC (see, e.g., Winter et al., US Patent No. 5,624,821; Tso
et al., US Patent No.
5,834,597; and Lazar et al., Proc. Natl. Acad. Sci. USA 103:4005, 2006), or to
prolong half-life in
humans (see, e.g., Hinton et al., J. Biol. Chem. 279:6213, 2004), for which
exemplary
substitutions include a Gln at position 250 and/or a Leu at position 428 (EU
numbering. Other
variations can add or remove sites of post-translational modification, such as
N-linked
glycosylation at N-X-S/T motifs. Variations can also include introduction of
knobs (i.e.,
replacement of one or more amino acids with larger amino acids) or holes
(i.e., replacement of
one or more amino acids with smaller amino acids) to promote formation of
heterodimers
between different heavy chains for production of bispecific antibodies.
Exemplary substitutions
to form a knob and hole pair are 1336Y and Y407T respectively (Ridgeway et
al., Protein
Engineering vol.9 no.7 pp.617-621, 1996). Variations can also include
mutations that reduce
protein A interaction (e.g., H435R and Y436F) in the EU numbering system.
Bispecific
antibodies in which one heavy chain has such a variation, and another does
not, can be
separated from their parental antibodies by protein-A affinity chromatography.
For example,
SEQ ID NOS. 9-12 are the same as SEQ ID NOS. 5-8 except for the presence of
H435R and Y436F
mutations. One or more residues from the C-terminus of constant regions,
particularly a C-
terminal lysine on the heavy chain, can be lost as a result of post-
translational modification.
[0048] Other heavy chain constant regions comprise or consist of SEQ ID
NOS. 16-19 and
20-23, which correspond to SEQ ID NOS. 5-8 and 9-12 respectively except that
the former are of
human IgG1 rather than IgG4 isotype. Other heavy chain constant regions
comprise or consist
16
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
of SEQ ID NOS. 24-27 and 28-31, which correspond to SEQ ID NOS. 5-8 and 9-12
respectively
except the former are of human IgG2 rather than IgG4 isotype.
[0049] Other preferred constant regions differ from any of the above
designated SEQ ID
NO. at up to 1, 2, 3, 4 ,5, 6, 7, 8, 9 or 10 positions but retain at least GGG-
, GG- , G or at
EU positions 232-236 and P at position 228 and preferably retain residues 226-
236 shown
above for SEQ ID NO:1, SEQ ID NO:2, SEQ ID:3 and SEQ ID NO:4 in the same
manner as was
discussed for IgG4 isotype constant regions.
[0050] Modified constant regions and antibodies or fusion proteins
incorporating such
constant regions are characterized by reduced affinity for Fcy receptors
compared with isotype
matched controls (wildtype constant regions or antibodies or fusion proteins
incorporating the
same). Binding affinity (Ka) is preferably reduced at least 90, 95 or 99%
compared with the
isotype matched controls. Preferably binding affinity is reduced to background
levels (i.e.,
same signal within experimental error as in a control reaction with an sc-Fv
fragment lacking
any constant region or in which an irrelevant receptor is used in place of
Fcy. Preferably,
affinity is reduced to a background level or at least 90, 95 or 99% for each
of human Fcy
receptors, yRI, yRIIA, yRIIB and yRIIIA.
[0051] Likewise, effector functions dependent on Fcy receptor binding, such
as ADCC or
ADCP are reduced, preferably by at least 90, 95 or 99% or more preferably to
background level.
Such functions include cell killing or phagocytosis, B-cell activation, and
release of inflammatory
mediators, such as cytokines. Some such effects can be quantified by
measurement of EC50,
which refers to the half maximal effective concentration of an antibody which
induces a
response halfway between the baseline and maximum after a specified exposure
time. The ECK,
essentially represents the concentration of an antibody where 50% of its
maximal effect is
observed. Some antibodies or fusion proteins including a modified heavy chain
constant region
of the invention show cytotoxicity of less than 20% cytolysis (i.e. %
cytotoxicity), or less than
10%, or 5%, 4%, 3%, 2%, or even 0% or undetectable cytolysis (cytotoxicity),
as measured in an
in vitro or ex vivo cell killing assay compared with suitable isotype-matched
control antibodies
with a wildtype constant region, optionally, measured at an antibody or fusion
protein
concentration of 10 nM.
17
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
[0052] However, binding affinity of an antibody or fusion protein
incorporating such a
heavy chain constant region is preferably not substantially affected by the
modified constant
region. That is, the binding affinity is typically the same within
experimental error or at least
within a factor of 2 or 3 of a suitable control antibody with a isotype-
matched wild type
constant region. The same is the case for functional properties not dependent
on FcyR binding,
such as ability to inhibit receptor-ligand binding (e.g., EC50), or ability to
agonize a receptor.
[0053] Immunogenicity of modified constant regions or antibodies or fusion
proteins
incorporated modified constant regions compared with isotype matched controls
can be
assessed in vitro from dendrocyte maturation or T-cell proliferation on
challenge (Gaitonde et
al., Methods Mol. Biol. 2011;716:267-80) or in vivo by comparing incidence of
reactive
antibodies against administered antibodies between populations. The
immunogenicity of
modified constant regions or antibodies or fusion proteins incorporating the
modified constant
is preferably not significantly different from the isotype matched controls or
not worse than 2,
3, or 5 fold greater than the isotype matched control. Likewise,
pharmacokinetic parameters
such as Cmax, Caverage, area under the curve and half-life are preferably not
significantly
different or at least not lower by a factor of no more than 2, 3 or 5 that
isotype matched
controls. Such parameters can be measured in a mouse such as described in the
Examples, in
other animal model or a human. Substantial retention of such PK parameters
provides an
indication that modified constant regions or antibodies or fusion proteins
incorporating them
have not undergone substantial conformational changes triggering enhanced
removal
mechanisms.
Antibodies and Fusion Proteins
[0054] The modified heavy chain constant regions described above can be
incorporated
into antibodies or other fusion proteins. For example, for expression of a
monospecific
antibody, a modified heavy chain constant region is expressed fused to a heavy
chain variable
region and together with a light chain including a light chain variable region
and a light chain
constant region. The heavy and light chain bind to one another via the CH1
region of the heavy
chain and light chain constant region to a form a heterodimer. Two
heterodimers then pair by
association of hinge, CH2 and CH3 regions of the IgG heavy chain to form a
tetramer unit, as is
18
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
the case for a conventional antibody. For expression of a bispecific antibody,
a modified heavy
constant region is expressed fused to each of two heavy chain variable regions
of different
target specificities. The heavy chains can each assembly with a co-expressed
light chain and the
heavy chain-light chain complexes form heterodimers in which both heavy chains
are present.
The light chain variable regions can be the same (see e.g., US 20100331527A1)
or different
within a unit.
[0055] The modified constant regions can be used with any type of
engineered antibody
including chimeric, humanized, veneered or human antibodies. The antibody can
be a
monoclonal antibody or a genetically engineered polyclonal antibody
preparation (see US
6,986,986).
[0056] For fusion protein proteins, a modified constant region is expressed
linked to a
heterologous polypeptide. A heterologous polypeptide in a fusion protein is a
polypeptide not
naturally linked to an immunoglobulin constant region. Such a polypeptide can
be a full-length
protein or any fragment thereof of sufficient length to retain specific
binding to the antigen
bound by the full-length protein. For example, a heterologous polypeptide can
be a receptor
extracellular domain or ligand thereto. The heterologous polypeptide provides
a binding region
at the N-terminus of the constant region and is sometimes referred to simply
as a binding
region. The IgG CH1 region is not typically included in the constant region
for fusion proteins.
The upper hinge region is sometimes omitted or replaced by a synthetic linker
peptide.
Exemplary receptor proteins whose extracellular domains can be combined with
modified
heavy chain constant regions of the invention are known in the art (see e.g.
Klinkert, et al. J
Neuroimmunol. 72(2): 163-8 (1997); Milligan et al., Curr Pharm. Des. 10(17):
1989-2001 (2004);
and Schwache & Muller-Newen, Eur. J Cell Biol. 91 (6-7):428-34 (2012), doi:
10.1016/j.ejcb.201
1 .07.008. Epub 201 1 Sep 29).
[0057] The binding region of a fusion protein can be any of the types of
binding regions
used in other fusion proteins produced to date (among others).
[0058] A multi-specific antibody or fusion protein can include binding
specificities for an
antigen on a target (e.g., a cancer cell or pathogen) and for an antigen on an
effector cell (e.g.,
CD3 on a T-cell). Such a multi-specific complex forms a bridge between the
target cell and
19
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
effector cell and promotes cytotoxic or opsonization activity of the effector
cell. A multi-
specific antibody or fusion protein can additionally or alternatively include
binding specificities
for two different antigens on the same target (e.g., a cancer cell or
pathogen). Such an
antibody or fusion protein can have greater selective toxicity to the target
cell than an antibody
or fusion protein with specificity for a single antigen. Other multi-specific
antibodies or fusion
proteins include binding regions for both a receptor and its ligand or counter-
receptor. Such
antibodies or fusion proteins can exert greater inhibition than antibodies or
fusion proteins
binding receptor or ligand/counterreceptor alone. Any of these specificities
and others can be
combined in the same multi-specific complex.
[0059] Antibodies or fusion proteins can also be chemically modified by
covalent
conjugation to a polymer to, for instance, further increase their circulating
half-life. Exemplary
polymers, and methods to attach them to peptides, are illustrated in for
instance US 4,766,106,
US 4,179,337, US 4,495,285 and US 4,609,546. Additional illustrative polymers
include
polyoxyethylated polyols and polyethylene glycol (PEG) (e.g., a PEG with a
molecular weight of
between about 1,000 and about 40,000, such as between about 2,000 and about
20,000, e.g.,
about 3,000-12,000 g/mol).
[0060] Antibodies or fusion proteins can be radiolabeled antibody for
either diagnostic or
therapeutic purposes. Examples of radioisotopes include 3H, 14C, 15N, 355,
90y, 99TC, and 1251, 1311,
18611e, and 225AC. Methods for preparing radiolabeled amino acids and
antibodies or fusion
proteins containing them are known (see for instance Junghans et al., in
Cancer Chemotherapy
and Biotherapy 655-686 (2nd edition, Chafner and Longo, eds., Lippincott Raven
(1996)) and US
4,681 ,581, US 4,735,210, US 5,101 ,827, US 5,102,990 (US RE35,500), US
5,648,471 and US
5,697,902. For example, a radioisotope may be conjugated by a chloramine T
method. Other
detectable markers include an enzyme, a chromophore, or a fluorescent label.
[0061] Antibodies or fusion proteins can be conjugated to a toxic agent.
Toxic agents can
be cytotoxic or cytostatic. Some example of toxic agents include antitubulin
agents, auristatins,
DNA minor groove binders, DNA replication inhibitors, alkylating agents (e.g.,
platinum
complexes such as cis-platin, mono(platinum), bis(platinum) and tri-nuclear
platinum
complexes and carboplatin), anthracyclines, antibiotics, antifolates,
antimetabolites,
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
chemotherapy sensitizers, duocarmycins, camptothecins, etoposides, fluorinated
pyrimidines,
ionophores, lexitropsins, nitrosoureas, platinols, pre-forming compounds,
purine
antimetabolites, puromycins, radiation sensitizers, steroids, taxanes,
topoisomerase inhibitors,
vinca alkaloids, or the like.
IV. Antibody expression
10062] Nucleic acids encoding antibody chains or fusion proteins can be
made by solid state
synthesis, PCR amplification of overlapping oligonucleotide fragments or site-
directed
nnutagenesis of existing nucleic acids. Such nucleic acids are expressed in an
expression vector.
Vectors can be configured to encode a modified heavy chain constant region
and/or human
light chain constant region such that they can be expressed as fusions with
inserted heavy chain
and light chain variable regions or a heterologous polypeptide.
[0063] The origin of replication and expression control elements (promoter,
enhancer,
signal peptide and so forth) in a vector can be configured for use in
different cell types, such as
bacteria, yeast or other fungi, insect cells, and mammalian cells. Mammalian
cells are a
preferred host for expressing nucleotide segments encoding antibodies or
fusion proteins of
the invention (see Winnacker, From Genes to Clones, (VCH Publishers, NY,
1987)). A number of
suitable host cell lines capable of secreting intact heterologous proteins
have been developed
in the art, and include CHO cell lines, various COS cell lines, HeLa cells,
HEK293 cells, L cells, and
non-antibody-producing myelomas including Sp2/0 and NSO. Preferably, the cells
are
nonhuman. Preferably, an antibody or fusion protein of the invention is
expressed from a
monoclonal cell line.
[0064] Expression vectors for these cells can include expression control
sequences, such as
an origin of replication, a promoter, an enhancer (Queen et al., lmmunol. Rev.
89:49 (1986)),
and necessary processing information sites, such as ribosome binding sites,
RNA splice sites,
polyadenylation sites, and transcriptional terminator sequences. Preferred
expression control
sequences are promoters derived from endogenous genes, cytomegalovirus, SV40,
adenovirus,
bovine pa pillomavirus, and the like. See Co et al., J. lmmunol. 148:1149
(1992).
[0065] Cells are transfected with one or more vectors encoding the antibody
or fusion
protein to be expressed. For a multi-chain antibody, the heavy and light
chains can be
21.
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
expressed on the same or separate vectors. For expression of multi-specific
complexes, the
DNA encoding the components of the complexes (i.e., different antibodies or
fusion proteins)
can be on the same or different vectors.
[0066] Antibody or fusion protein chains are expressed, processed to remove
signal
peptides, assembled and secreted from host cells. Antibodies or fusion
proteins can be
purified from cell culture supernatants by conventional antibody purification
methods. If the
hybrid constant region includes an IgG portion, then the purification can
include a
chromatography step using protein A or protein G as the affinity reagent.
Conventional
antibody purification procedures, such as ion exchange, hydroxyapatite
chromatograph or HPLC
can also be used (see generally, Scopes, Protein Purification (Springer-
Verlag, NY, 1982)).
V. Applications
[0067] Although antibodies and fusion proteins incorporating modified heavy
chain
constant regions of the invention can be generally used in methods of
treatment or diagnosis,
they are particularly useful in situations in which the mechanism of action of
the antibody or
fusion protein is entirely or at least predominantly independent of effector
functions. Such is
the case for example when the therapeutic objective is not to kill a target
cell, but to inhibit or
activate a cell surface molecule on its surface without triggering
cytotoxicity. Another setting in
which reduced binding to Fc receptors is desirable is when the antibody is
bispecific, and its
desired therapeutic properties arise from the different binding specificities.
For example, a
common usage of bispecific antibodies is to combine a tumor targeting
specificity with a T cell
activating specificity to trigger tumor-specific T cell killing. In this case,
if the Fc portion binds to
an Fc receptor, then potentially that could trigger undesirable killing of
cells bearing Fc
receptors by T cells, or killing of T cells by Fc receptor-bearing cells such
as natural killer cells or
macrophages. Another setting in which lack of effector functions can be
advantageous is
inhibiting aggregation of peptides that contribute to pathogenesis, such as in
amyloidogenic
disease. A further setting is in vivo diagnosis, in which an antibody or
fusion protein is intended
to bind to a target but not result in clearing the target or cells bearing the
target.
22
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
VI. Targets
10068] Antibodies or fusion proteins incorporating a modified heavy chain
constant region
may be directed to any number of cellular target proteins. The antibodies or
fusion proteins
are particularly useful for surface-bound target proteins. The desired
response can be, for
example, clearing of a target or cell or virus bearing the same, signal
transduction through a
receptor, e.g., inducing a poptosis, inhibiting a receptor binding to a ligand
or counterreceptor,
or internalization of an antibody or fusion protein conjugated to a toxic
agent. Antibodies or
fusion proteins can be made to the same targets as existing commercial
antibodies or fusion
proteins or can be derivatized versions of commercial antibodies or fusion
proteins in which the
existing constant region has been replaced by a modified constant region of
the present
invention.
10069] Targets of interest include growth factor receptors (e.g., FGFR,
HGFR, PDGFR, EFGR,
NGFR, and VEGFR) and their ligands. Other targets are G-protein receptors and
include
substance K receptor, the angiotensin receptor, a and 13 adrenergic receptors,
the serotonin
receptors, and PAF receptor. See, e.g., Gilman, Ann. Rev. Biochem. 56:625 649
(1987). Other
targets are CD (cluster of differentiation markers). Other targets include ion
channels (e.g.,
calcium, sodium, and potassium channels), muscarinic receptors, acetylcholine
receptors, GABA
receptors, glutamate receptors, and dopamine receptors (see Harpold, U.S. Pat.
No. 5,401,629
and U.S. Pat. No. 5,436,128). Other targets are adhesion proteins such as
integrins, selectins,
and immunoglobulin superfamily members (see Springer, Nature 346:425 433
(1990). Osborn,
Cell 62:3 (1990); Hynes, Cell 69:11 (1992)). Other targets are cytokines, such
as interleukins IL-1
through about IL-37 to-date, tumor necrosis factors, interferon, and, tumor
growth factor beta,
colony stimulating factor (CSF) and granulocyte monocyte colony stimulating
factor (GM-CSF).
See Human Cytokines: Handbook for Basic & Clinical Research (Aggrawal et
al. eds.,
Blackwell Scientific, Boston, Mass. 1991). Other targets are amyloidogenic
peptides, such as
Abeta, alpha-synuclein or prion peptide. Other targets are hormones, enzymes,
and
intracellular and intercellular messengers, such as, adenyl cyclase, guanyl
cyclase, and
phospholipase C. Target molecules can be human, mammalian or bacterial. Other
targets are
23
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
antigens, such as proteins, glycoproteins and carbohydrates from microbial
pathogens, both
viral and bacterial, and tumors.
[0070] Some examples of commercial antibodies and their targets include
alemtuzumab
(CD52); rituximab (CD20); trastuzumab (Her/neu); nimotuzumab, cetuximab
(EGFR);
bevacizumab (VEGF); palivizumab (RSV); abciximab (Gpllb/Illa); infliximab,
adalimumab,
certolizumab, golimumab (TNF-alpha); baciliximab, daclizumab (IL-2);
omalizumab (IgE);
gemtuzumab (CD33); natalizumab (VLA-4); vedolizumab (alpha4beta7); belimumab
(BAFF);
otelixizumab, teplizumab (CD3); ofatumumab, ocrelizumab (CD20); epratuzumab
(CD22);
alemtuzumumab (CD52); eculizumab (C5); canakimumab (IL-1beta); mepolizumab (IL-
5);
reslizumab, tocilizumab (IL-6R); ustekinumab, briakinumab (IL-12, 23).
Examples of commercial
fusion proteins include etanercept which binds TNF-alpha, alefacept (LFA3-Fc
fusion which
binds CD2), TACI-Fc fusion which binds BAFF and APRIL, abatacept (CTLA-4-Fc
which binds CD80
and CD86), and romiplostim (a peptide analog of thrombopoietin fused to Fc).
Any of the
commercial antibodies or fusion protein can be modified to replace the
existing heavy chain
constant region with a modified heavy chain constant region of the invention.
Alternatively, a
modified heavy chain constant region can be linked to other antibodies with
the same target
specificity (e.g., as determined by a competition assay) as any of the above
commercial
antibodies or fusion proteins.
VII. Methods of Treatment and Pharmaceutical Compositions
[0071] The antibodies and fusion proteins of the invention can also be used
for suppressing
various undesirable immune responses including those for the same therapies in
which the
commercial antibodies mentioned above have been used.
[0072] One category of immune disorders treatable by antibodies or fusion
proteins of the
invention is transplant rejection. When allogeneic cells or organs (e.g.,
skin, kidney, liver, heart,
lung, pancreas and bone marrow) are transplanted into a host (i.e., the donor
and donee are
different individual from the same species), the host immune system is likely
to mount an
immune response to foreign antigens in the transplant (host-versus-graft
disease) leading to
destruction of the transplanted tissue. The antibodies or fusion proteins are
useful, inter alia,
to block alloantigen-induced immune responses in the donee.
24
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
[0073] A related use for antibodies or fusion proteins of the present
invention is in
modulating the immune response involved in "graft versus host" disease (GVHD).
GVHD is a
potentially fatal disease that occurs when immunologically competent cells are
transferred to
an allogeneic recipient. In this situation, the donor's immunocompetent cells
may attack tissues
in the recipient. Tissues of the skin, gut epithelia and liver are frequent
targets and may be
destroyed during the course of GVHD. The disease presents an especially severe
problem when
immune tissue is being transplanted, such as in bone marrow transplantation;
but less severe
GVHD has also been reported in other cases as well, including heart and liver
transplants.
[0074] A further situation in which immune suppression is desirable is in
treatment of
autoimmune diseases such as type 1 diabetes, Crohn's disease, ulcerative
colitis, multiple
sclerosis, stiff man syndrome, rheumatoid arthritis, myasthenia gravis and
lupus
erythematosus. In these diseases, the body develops a cellular and/or humoral
immune
response against one of its own antigens leading to destruction of that
antigen, and potentially
crippling and/or fatal consequences. Autoimmune diseases are treated by
administering one of
the antibodies or fusion proteins of the invention. Other immune disorders
treatable by
antibodies or fusion proteins including modified constant regions of the
invention include
asthma, allergies, celiac disease, psoriasis, and uveitis. Celiac disease,
psoriasis and uveitis are
autoimmune diseases.
[0075] The antibodies or fusion proteins of the invention can be used for
treating cancers in
which a target antigen to which the antibody or fusion protein is expressed.
The methods can
be used to treat solid tumors, and particularly hematological malignancies,
such as leukemia
(e.g., T cell large granular lymphocyte leukemia), lymphoma (Hodgkin's or Non-
Hodgkin's), or
multiple myeloma. Solid tumors include skin (e.g., melanoma), ovarian,
endometrial, bladder,
breast, rectum, colon, gastric, pancreatic, lung, thymus, kidney and brain.
Killing of cancer cells
can result from mechanism independent of FcyR bindings, such as by induction
of apoptosis,
inhibition of a receptor-ligand interaction or by action of a conjugated
cytotoxic moiety.
[0076] The antibodies or fusion protein can also be used for treatment of
pathogenic
infections, such as viral, bacterial, protozoan or fungal infection. Likewise
killing can occur by
mechanism independent of FcyR binding such as by inhibiting an interaction
between a
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
pathogen and a cell giving other elements of the immune system an opportunity
to kill the
pathogen or by action of a linked radionucleotide or toxin.
[0077] Antibodies or fusion proteins are administered in an effective
regime meaning a
dosage, route of administration and frequency of administration that delays
the onset, reduces
the severity, inhibits further deterioration, and/or ameliorates at least one
sign or symptom of
a disorder. If a subject is already suffering from a disorder, the regime can
be referred to as a
therapeutically effective regime. If the subject is at elevated risk of the
disorder relative to the
general population but is not yet experiencing symptoms, the regime can be
referred to as a
prophylactically effective regime. In some instances, therapeutic or
prophylactic efficacy can be
observed in an individual subject relative to historical controls or past
experience in the same
subject. In other instances, therapeutic or prophylactic efficacy can be
demonstrated in a
preclinical or clinical trial in a population of treated subjects relative to
a control population of
untreated subjects.
[0078] Exemplary dosages for an antibody or fusion protein are 0.01-20, or
0.5-5, or 0.01-1,
or 0.01-0.5 or 0.05-0.5 mg/kg body weight (e.g., 0.1, 0.5, 1, 2, 3, 4 or 5
mg/kg) or 10-1500 mg as
a fixed dosage. The dosage depends on the condition of the subject and
response to prior
treatment, if any, whether the treatment is prophylactic or therapeutic and
whether the
disorder is acute or chronic, among other factors.
[0079] Administration can be parenteral, intravenous, oral, subcutaneous,
intra-arterial,
intracranial, intrathecal, intraperitoneal, topical, intranasal or
intramuscular. Administration
into the systemic circulation by intravenous or subcutaneous administration is
preferred.
Intravenous administration can be, for example, by infusion over a period such
as 30-90 min.
[0080] The frequency of administration depends on the half-life of the
antibody or fusion
protein in the circulation, the condition of the subject and the route of
administration among
other factors. The frequency can be daily, weekly, monthly, quarterly, or at
irregular intervals
in response to changes in the subject's condition or progression of the
disorder being treated.
An exemplary frequency for intravenous administration is between weekly and
quarterly over a
continuous cause of treatment, although more or less frequent dosing is also
possible. For
26
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
subcutaneous administration, an exemplary dosing frequency is daily to
monthly, although
more or less frequent dosing is also possible.
[0081] The number of dosages administered depends on whether the disorder
is acute or
chronic and the response of the disorder to the treatment. For acute disorders
or acute
exacerbations of chronic disorders between 1 and 10 doses are often
sufficient. Sometimes a
single bolus dose, optionally in divided form, is sufficient for an acute
disorder or acute
exacerbation of a chronic disorder. Treatment can be repeated for recurrence
of an acute
disorder or acute exacerbation. For chronic disorders, an antibody can be
administered at
regular intervals, e.g., weekly, fortnightly, monthly, quarterly, every six
months for at least 1, 5
or 10 years, or the life of the subject.
[0082] Pharmaceutical compositions for parenteral administration are
preferably sterile
and substantially isotonic and manufactured under GMP conditions.
Pharmaceutical
compositions can be provided in unit dosage form (i.e., the dosage for a
single administration).
Pharmaceutical compositions can be formulated using one or more
physiologically acceptable
carriers, diluents, excipients or auxiliaries. The formulation depends on the
route of
administration chosen. For injection, antibodies can be formulated in aqueous
solutions,
preferably in physiologically compatible buffers such as Hank's solution,
Ringer's solution, or
physiological saline or acetate buffer (to reduce discomfort at the site of
injection). The
solution can contain formulatory agents such as suspending, stabilizing and/or
dispersing
agents. Alternatively antibodies can be in lyophilized form for constitution
with a suitable
vehicle, e.g., sterile pyrogen-free water, before use.
[0083] Treatment with antibodies of the invention can be combined with
other treatments
effective against the disorder being treated. For treatment of immune
disorders, conventional
treatments include mast cell degranulation inhibitors, corticosteroids,
nonsteroidal anti-
inflammatory drugs, and stronger anti-inflammatory drugs such as azathioprine,
cyclophosphamide, leukeran, FK506 and cyclosporine. Biologic anti-inflammatory
agents, such
as Tysabri (natalizumab) or Humira (adalimumab), can also be used. When used
in treating
cancer, the antibodies of the invention can be combined with chemotherapy,
radiation, stem
cell treatment, surgery or treatment with other biologics such as Herceptin
(trastuzumab)
27
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
against the HER2 antigen, Avastin (bevacizumab) against VEGF, or antibodies
to the EGF
receptor, such as (Erbitux , cetuximab), and Vectibix (pa nitumumab).
Chemotherapy agents
include chlorambucil, cyclophosphamide or melphalan, carboplatinum,
daunorubicin,
doxorubicin, idarubicin, and mitoxantrone, methotrexate, fludarabine, and
cytarabine,
etoposide or topotecan, vincristine and vinblastine. For infections, treatment
can be in
combination with antibiotics, anti-vira Is, anti-fungal or anti-protozoan
agents or the like.
VIII. Other applications
10084] The antibodies or fusion proteins can be used for detecting their
target molecule in
the context of clinical diagnosis or treatment or in research. For example,
the antibodies can be
used to detect a cancer-related antigen as an indication a subject is
suffering from an immune
mediated disorder amenable to treatment. The antibodies can also be sold as
research reagents
for laboratory research in detecting targets and their response to various
stimuli. In such uses,
antibodies or fusion proteins can be labeled with fluorescent molecules, spin-
labeled
molecules, enzymes or radioisotypes, and can be provided in the form of kit
with all the
necessary reagents to perform the assay. The antibodies or fusion protein can
also be used to
purify their target antigens e.g., by affinity chromatography.
[0085] Presence of labeled antibodies or fusion may be detected in vivo for
diagnosis
purposes. In one embodiment, diagnosis comprises: a) administering to a
subject an effective
amount of a labeled antibody or fusion protein; b) waiting for a time interval
following
administration for permitting labeled antibody or fusion protein to
concentrate at sites where
antigen may be detected and to allow for unbound labeled antibody to be
cleared to
background level; c) determining a background level; and d) detecting the
labeled antibody or
fusion protein in the subject, such that detection of labeled antibody above
the background
level is indicative that the subject has the disease or disorder, or is
indicative of the severity of
the disease or disorder. In accordance with such embodiment, the antibody or
fusion protein is
labeled with an imaging moiety suitable for detection using a particular
imaging system known
to those skilled in the art. Background levels may be determined by various
methods known in
the art, including comparing the amount of labeled antibody detected to a
standard value
previously determined for a particular imaging system. Methods and systems
that may be used
28
WO 2016/161010 PCT/US2016/025051
in the diagnostic methods of the invention include, but are not limited to,
computed
tomography (CT), whole body scan such as positron emission tomography (PET),
magnetic
resonance imaging (MRI), and sonography.
[0086]
If different versions of a sequence are associated with an accession
number at different times, the version associated with the accession number at
the effective
filing date of this application is meant. The effective filing date means the
earlier of the actual
filing date or filing date of a priority application referring to the
accession number if applicable.
Likewise if different versions of a publication, website or the like are
published at different
times, the version most recently published at the effective filing date of the
application is
meant unless otherwise indicated. Any feature, step, element, embodiment, or
aspect of the
invention can be used in combination with any other unless specifically
indicated otherwise.
Although the present invention has been described in some detail by way of
illustration and
example for purposes of clarity and understanding, it will be apparent that
certain changes and
modifications may be practiced within the scope of the appended claims.
Examples:
Example 1: Expression of Hinge-Modified Antibodies
[0087] Several of the antibodies described in the following examples are
bispecific
antibodies in which one arm is that of an anti-hCD3 antibody and the other arm
is from an anti-
hCD20 antibody as described by W02014047231.
[0088] Antibody 1, 9F7_,VH_IgG4_,GGG-(233-236), was made by site-directed
mutagenesis
TM
using QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent
Technologies, Inc.;
Catalog#210518) following the manufacturer's protocol, and starting with a
chimeric IgG4 CH
region (which contained the modifications 5228P and ELLG233-236PVA; see
W02014047231).
After sequence confirmation, the coding region was moved to a parent vector
using Xho1-Notl
restriction sites to avoid any mutations in the non-coding region, and
subsequently generating
29
Date Recue/Date Received 2022-07-13
WO 2016/161010 PCT/US2016/025051
a vector construct with the hinge-modified IgG4 CH, having the hinge
modifications of SEQ ID
NO:1.
[0089] The anti-CD3(9F7 VH; see "L2K" based on W02004/106380) variable
region nucleic
acid sequence was amplified and introduced into the same plasmid as the hinge-
modified IgG4
5228P, GGG-(233-236) and the sequence was confirmed using PCR. The final
plasmid was used
to produce Antibody 1 using standard cell culture methodologies for isolating
antibodies.
[0090] Antibody 2, 9F7_VH_IgG4 _GG--(233-236), was made analogously to
Antibody 1 by
using site directed mutagenesis to generate a vector construct with the hinge
modified IgG4 CH
with GG¨(233-236) (SEQ ID NO:2). The anti-CD3(9F7 VH; see W02004/106380)
variable region
nucleic acid sequence was amplified and introduced into the same plasmid using
standard
molecular biology methods. Ab 2 was isolated using standard methodologies.
[0091] Antibody 3, 9F7_VH_IgG4_G---(233-236), was made analogously to
Antibody 1 by
using site directed mutagenesis to generate a vector construct with the hinge
modified IgG4 CH
with G¨(233-236) (SEQ ID NO:3). The anti-CD3 (9F7 VH; see W02004/106380)
variable region
nucleic acid sequence was amplified and introduced into the same plasmid using
standard
molecular biology methods. Ab 3 was isolated using standard methodologies.
[0092] Antibody 4, 9F7_VH_IgG4_No_G(233-236) , was made analogously to
Antibody 1 by
using site directed mutagenesis to generate a vector construct with the hinge
modified IgG4 CH
with No_G(233-236) (SEQ ID NO:4). The a nti-CD3(9F7 VH; see W02004/106380)
variable region
nucleic acid sequence was amplified and introduced into the same plasmid. Ab 4
was isolated
using standard methodologies.
[0093] Antibody 5, 3E39_VH_IgG4 _GGG-(233-236), was made by site-directed
mutagenesis
using QuikChangTem Lightning Site-Directed Mutagenesis Kit (Agilent
Technologies, Inc.;
Catalog#210518) following the manufacturer's protocol, and starting with a
chimeric IgG4 CH
region (which contained the modifications S228P and ELLG233-236PVA; see
W02014047231).
Antibody 5 is a monospecific, bivalent anti-CD20 antibody, and the anti-CD20
variable region
nucleic acid sequence (389 VH) was isolated using standard methodologies as
described in
W02014047231.
Date Recue/Date Received 2022-07-13
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
[0094] Antibody 6, 369_VH_IgG4 _GG--(233-236), was made analogously to
Antibody 5 by
using site directed mutagenesis to generate a vector construct with the hinge
modified IgG4 CH
with GG--(233-236) (SEQ ID NO:2). The anti-CD20 (369 VH; see W02014047231)
variable
region nucleic acid sequence was isolated using standard methodologies.
[0095] Antibody 7, 369_VH_IgG4 _G---(233-236), was made analogously to
Antibody 5 by
using site directed mutagenesis to generate a vector construct with the hinge
modified IgG4 CH
with G---(233-236) (SEQ ID NO:3). The anti-CD20 (369 VH; see W02014047231)
variable region
nucleic acid sequence was isolated using standard methodologies.
[0096] Antibody 8, 369_VH_IgG4 _No_G(233-236), was made analogously to
Antibody 5 by
using site directed mutagenesis to generate a vector construct with the hinge
modified IgG4 CH
with No_G(233-236) (SEQ ID NO:4). The anti-CD20 (369 VH; see W02014047231)
variable
region nucleic acid sequence was isolated using standard methodologies.
[0097] Antibody 9, anti-transmembrane (TM) protein variable domains
(B6H12.2, obtained
from BioXCell, Cat. No. 6E0019-1) were cloned in to a plasmid containing the
hinge-modified
IgG4 5228P, GGG-(233-236) nucleic acid, by methods similarly described for
Antibody 1.
[0098] Antibody 10, B6H12.2_VH_IgG4_PVA was made according to protocols
described
herein, having a chimeric IgG4(5228P and ELLG233-236PVA) Fc domain.
[0099] Antibody 11, anti-FELD1_VH_IgG4_PVA antibody, is isotype matched to
Antibody 10,
having a chimeric IgG4(5228P and ELLG233-236PVA) Fc domain.
[00100] Control Antibody A (Lot- L2), 9F7_VH_IgG4, is an antiCD3 (9F7 VH; see
W02004/106380) human IgG4 isotype antibody (except having a CH3* mutation 435R
and
436F, designated the star mutation- see U520100331527A1).
[00101] Control Antibody B (Lot-L2), 9F7_VH_IgG1, is an antiCD3 (9F7 VH; see
W02004/106380) human IgG1 isotype antibody.
[00102] Control Antibody C: 9F7_Vb(369_VH_IgG4, was made according to the
protocols
described in W02014047231. Control Ab C is a bispecific antiCD3xanti-CD20
monoclonal
antibody having a wild-type IgG4 heavy chain (except one arm has the star
mutation in the CH3
region for ease of bispecific antibody isolation).
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
[00103] Control Antibody D (Lot- L5): 9F7_VLx3B9_VH_IgG4_PVA, was made
according to
the protocols described in W02014047231. Control Ab D is a bispecific anti-
CD3xanti-CD20
monoclonal antibody having a modified IgG4 heavy chain [chimeric IgG4(5228P
and ELLG233-
236PVA), except one heavy chain has the star mutation in the CH3 region for
ease of bispecific
antibody isolation].
[00104] Control Antibody E: Anti-FelD1 monoclonal antibody binds a feline
antigen with no
cross-reactivity to human CD20 or CD3. This IgG1 non-specific antibody control
was obtained by
methods described in PCT Publication No. W02013/166236, published on November
7, 2013.
[00105] Control Antibody F: is small batch (supernatant) preparation of 9F7
(anti-CD3) with
the chimeric IgG4 Fc (5228P and ELLG233-236PVA) (See also W02014047231).
[00106] Control Ab G: 9F7_VH_IgG4_PVA was made according to the protocols
described in
W02014047231 (Lot#2, purified). Control Ab G is a monospecific anti-CD3
monoclonal antibody
having a modified IgG4 heavy chain [chimeric IgG4(5228P and ELLG233-236PVA),
except one
heavy chain has the star mutation in the CH3 region for ease of bispecific
antibody isolation].
[00107] Control Ab H (Lot # 02-091210): 389_VH_IgG1 is an anti-CD20 (3B9
VH; see
W02004/106380) human IgG1 isotype antibody.
[00108] Control Ab I (Lot #01-110607): 369_VH_IgG4 is an anti-CD20 (3B9 VH;
see
W02004/106380) human IgG4 isotype antibody.
[00109] Control AbJ (Lot # L1): 9F7_VK x3B9_VH_IgG4_PVA was made according
to the
protocols described in W02014047231. Control AbJ is a bispecific anti-CD3xanti-
CD20
monoclonal antibody having a modified IgG4 heavy chain [chimeric IgG4 (5228P
and ELLG233-
236PVA), except one heavy chain has the star mutation in the CH3 region for
ease of bispecific
antibody isolation].
Example 2: Loss of affinity to Fcy receptors
[00110] The hinge-modified antibodies (i.e. GGG-, GG--, G--- or no-G hinge
replacement;
Antibodies 1-4) and various control antibodies were tested for binding
affinity to Fcy receptors
by surface plasmon resonance (SPR). The controls included Control Ab D
(antiCD3xantiCD20-
sIgG4 (5228P and ELFG233-236PVA), Control Ab B (antiCD3, human IgG1 isotype),
and Control
Ab A (antiCD3, human IgG4 isotype).
32
WO 2016/161010 PCT/US2016/025051
[00111] Briefly SPR experiments were performed at 25 C on a BiacoremT200
instrument
employing a carboxymethyl dextran-coated (CM-5) chip. A mouse monoclonal anti-
penta-
histidine antibody (GE Healthcare) was immobilized on the surface of the CM-5
sensor chip
using standard amine-coupling chemistry. 140RU-376RU of His-tagged human or
monkey FcyR
proteins were captured on the anti-penta-histidine amine-coupled CM-5 chip (or
in the case of
FcRn, about 155-299 RU of FcRn mutant contructs were immobilized on a high
density anti-myc
coated Biacore chip) and stock solutions of antibodies were injected at 20
plimin for 2.5 min
over the captured proteins and serially diluted. mAb binding response was
monitored and, for
low affinity receptors, steady-state binding equilibrium was calculated.
Kinetic association (ka)
and dissociation (kd) rate constants were determined by processing and fitting
the data to a 1:1
binding model using Scrubber 2.0 curve fitting software. Binding dissociation
equilibrium
constants (KD) and dissociative half-lives (t112) were calculated from the
kinetic rate constants
as: Kr, (M) = kd / ka; and tin (min) = (In2/(60*kd). Some KDs were derived
using the steady state
equilibrium dissociation constant; NB = no binding observed.
[00112] An anti-CD3 antibody designated 9F7 having a heavy chain constant
region including
hinge segments designated SEQ ID NO:1, SEQ ID NO:2, SEQ ID:3 or SEQ ID NO:4 at
residues
226-236, and of human IgG4 isotype, was tested for binding affinity to human
FcyRI, RIIA, RIIB
and RIII. Controls included the same antibody with wildtype IgG1 or IgG4
isotypes, and the
same antibody with IgG4 isotype with a different modification of the hinge
region (i.e. the hinge
modification has positions 226-236 occupied by CPPCPAPPVA-, the same sequences
as in
human IgG2, therefore referred to as chimericFc). In the IgG4 chimeric Fc
format, the
remaining segments of the constant region are human IgG4. In the IgG1 chimeric
Fc format,
the CH1 and CH3 segments are human IgG1 and CH2 is human IgG4.
[00113] The data show binding is reduced to background levels in all of the
hinge-modified
Antibodies 1-4 to each of human FcyRI, IIA, IIB and III. By contrast, binding
of the IgG1 and IgG4
chimericFc antibodies is reduced to background levels for FcyRI and FcyIII,
but is still significant
to FcyRIIA and RIB. All of the hinge-modified Antibodies 1-4 maintain binding
to FcRn,
comparable to IgG4 and chimeric hinge IgG4 formats. See Figs. 6-10.
33
Date Recue/Date Received 2022-07-13
WO 2016/161010 PCT/US2016/025051
Example 3: Cytotoxicity Analysis
[00114] U937 cells are a monocyte cell line expressing FcyRI and FcyRIIA.
U937 cells were
used as a positive killer effector control in the following cytotoxicity
assay. As such, the ability of
antibodies with chimeric CH regions to kill U937 cells via Fc/FcyR
interactions was tested.
Calcein killing assays were carried out using the following protocol: Human
and cynomolgus
Peripheral Blood Mononuclear Cells (PBMCs) were isolated over Ficoll-PaquelGE
Healthcare
Life Sciences) or via Lymphocyte-Mammal density cell separation media
(Cedarlane
Laboratories), respectively. The isolated PBMCs were activated over a course
of several days
with media containing recombinant human IL-2 (30U/m1) and T-cell activation
beads (anti-
CD3/CD28 for human PBMC, anti-CD2/CD3/CD28 for cynomolgus PBMC). Activated T-
cells were
isolated from the PBMCs by centrifugation, then resuspended in 1 ml media. The
magnetized
beads were removed from the T-cells. Target cells (U937) were labeled with
calcein, then
washed and followed by incubation (10,000 cells per well) with 15-fold serial
dilutions of
purified Ab/sup and immortal CD8+ human T-cells (100,000 cells/well) for 3 hr
at 37C
(effector:target ratio ¨ 10:1) Following incubation plates were centrifuged
and the supernatant
transferred to black clear bottom plates for fluorescence analysis. Each EC50,
defined as the
molar concentration of antibody that induces 50% cytotoxicity, was determined
using Prism
(GraphPadThiSoftwa re, San Diego, CA). Values were calculated using a 4-
parameter non-linear
regression analysis (Fig. 11A).
[00115] The above experiments were performed with crude extracts of the
antibodies in cell
culture supernatants (Fig. 11A). Analogous experiments were done with the same
antibodies
purified over standard affinity columns or using the method described in Davis
et al. (see
U52010/0331527) for bispecific antibodies (Fig. 11B).
[00116] The data show that all of the hinge-modified antibodies 1-4 had only
background
levels of cytotoxicity as was also the case for the IgG4 chime ricFc antibody.
IgG1 and IgG4
wildtype antibodies showed strong cytotoxicity due to their ability to
interact with FcyRI and
FcyRIIA. See Fig. 11A and Fig. 11B.
34
Date Recue/Date Received 2022-07-13
WO 2016/161010
PCT/US2016/025051
Example 4: Activation of Jurkat Cells
[00117] This example tests whether an antibody can activate Jurkat cells (T
cell leukemia cell
line) transformed with an NFAT-luciferase construct that acts as marker of
activation.
Activation requires an antibody to anchor on a HEK293 cells expressing FcyRIIA
or FcyRIIB.
[00118] Jurkat/NFAT-Luc cells (50,000/well) were incubated with target cells
(50,000/well) in
the presence of serial dilutions of different antibodies with (Fig. 12B, 12D)
or without (Fig. 12A,
12C) antibody to an irrelevant antigen (FelD1)(1.5 mg/m1) for 4h at 37C. One-
Glo (Promega)
was added to measure luciferase activity.
[00119] The data show that all of the hinge-modified Antibodies 1-4 had only
background
levels of activation. See. Figs. 12A¨ 12D. Accordingly, all hinge-modified
Antibodies 1-4 lack
ability to bind to FcyRIIA or IIB on HEC cells.
[00120] A positive control antibody of wildtype human IgG4 antibody showed
strong
activation that was reduced to near background levels by the anti-FelD1
antibody, which
competes for an anchoring site on HEK293.
[00121] To show that the lack of activation of Jurkat cells resulted from
dampening the FcyR
binding ability of hinge-modified antibodies rather than an impaired ability
of the antibodies to
bind their CD3 target, an assay was performed with the antibodies cross-linked
to a plate
surface. Maxisorp plates were coated with a 2-fold serial dilution of
different Abs starting at
10nM overnight at 4 C. Next day 50,000 Jurkat NFAT Luc cells were added per
well and media
to make up the total volume to 100u1/welland incubate at 37 C for 5 hours.
100u1One-Glo
(Promega) was added to measure luciferase activity. Transfer to opaque white
nunCmplates
before reading luciferase activity. In this experiment all of the hinge-
modified antibodies 1-4
showed similar activation to IgG4 isotype matched controls. See Fig. 13.
Example 5: ADCC assay
[00122] In this
assay, the hinge-modified antibodies have variable regions (3B9_VH) that
bind the cell surface target antigen CD20 (Antibodies 5 through 8, described
above). CD20
positive target cells (Daudi) were labeled with calcein, then washed and
followed by incubation
(10,000 cells per well) with 6-fold serial dilutions of purified antibody and
NK92_CD16V cells
(NK92 cells engineered to express the higher affinity V allele of FcyRIlla at
50,000 cells/well) for
Date Recue/Date Received 2022-07-13
WO 2016/161010 PCT/US2016/025051
4 hr at 37C (effector:target ratio ¨ 5:1). Target cell lysis was determined by
measuring the
calcein fluorescence in the supernatant. Percent cytotoxicity and EC50 were
calculated
analogously to that described in Example 3. Fig. 14 shows the hinge-modified
antibodies do not
mediate ADCC activity (Fig. 14) against Daudi cells.
Example 6: PK assessment of anti-TM monoclonal antibody with modified hinge
[00123] Antibodies having variable domains that bind to a multipass
transmembrane (TM)
protein widely expressed in normal tissues and upregulated in various cancers
were produced
using well known techniques (see US 5,057,604; WO 2011/143624; and WO
97/27873).
[00124] Assessment of the pharmacokinetic (PK) clearance rate: anti-TM
antibody having a
modified hinge (Antibody 9) and a chimeric IgG4 Fc (Antibody 10), as well as
an isotype control
with chimeric IgG4 Fc (Antibody 11) were assessed in C57BL/6 Wild-Type (WT)
mice. For each
anti-TM mAb or isotype control, cohorts of three mice were given a
subcutaneous (s.c.) dose at
1 mg/kg. Mice were bled prior to the dosing and the serum samples were
designated as a pre-
bleed or zero time point. All mice were bled at 6 hours, 1, 3, 7, 10 and 14
days post injection for
PK analysis. Serum fractions from the bleeds were separated and frozen at -80
C until analysis
was conducted.
[00125] Determination of Total Drug Level in Sera by ELISA: Circulating anti-
TM antibody
levels were determined by total human antibody analysis using an ELISA
immunoassay. Briefly,
a goat anti-human IgG polyclonal antibody (Jackson ImmunoResearch, # 109-005-
098) at 1
gg/m1 in PBS was immobilized on 96-well plates overnight and the plates were
blocked with 5%
BSA. The drug containing serum samples in six-dose serial dilutions and the
reference standards
of the respective antibodies in 12-dose serial dilutions were transferred to
the prepared plates
and incubated for one hour. The plate-bound anti-TM antibodies were then
detected using a
goat anti-human IgG polyclonal antibody conjugated with horseradish peroxidase
(Jackson
ImmunoResearch, # 109-035-098). The plates were developed with TMB substrate
(BD
Pharmingen, # 51-2606KC, #15-2607KC) according to manufacturer's recommend
protocol and
signals of optical density (OD) at 450 nm were recorded using a Perkin Elmer
VictorTmX4
Multimode Plate Reader. The anti-TM antibody concentrations in the sera were
calculated
TM
based on the reference standard calibration curve generated using GraphPad
Prism software.
36
Date Recue/Date Received 2022-07-13
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
[00126] C5761./6 WT mice were given a single s.c. dose of 1 mg/kg of Antibody
9, Antibody
or lsotype control (Ab 11). Concentrations of total antibody were determined
at 6 time
points over a 14-day time period. The total anti-TM antibody concentrations
for each antibody
are summarized in Table 1.
Table 1: Serum Antibody Concentrations (Days 0, 0.25, 1,3, 7, and 14)
Total mAb concentration in
mouse serum
Antibody Time (d)
Mean +/- SD
(pg/mL)
Antibody 9 0 ND ND
Antibody 9 0.25 10 0.8
Antibody 9 1 13.3 0.8
Antibody 9 3 10.6 0.7
Antibody 9 7 8.6 1
Antibody 9 14 5.3 0.4
Antibody 9 0 ND ND
Antibody 10 0.25 11.2 1.5
Antibody 10 1 14.4 0.9
Antibody 10 3 11.0 1.6
Antibody 10 7 9.8 0.9
Antibody 10 14 6.4 0.5
Antibody 11 0 ND ND
Antibody 11 0.25 10.1 0.9
Antibody 11 1 12.0 0.4
Antibody 11 3 10 0.6
Antibody 11 7 9 0.8
Antibody 11 14 5.1 0.2
Time = Time in days post single-dose injection; Day = Day of study; SD =
Standard deviation;
ND = Not detected
[00127] The mean concentration versus time profiles show that all three
antibodies achieved
a maximum serum concentration (Cmax) on day 1. Antibody 9, Antibody 10 and
lsotype control
had comparable C. values of 13, 14 and 12 pg/mL, respectively. All three
antibodies exhibited
37
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
a linear elimination with overlapping PK profiles. These PK profiles are
similar to those seen in
previous studies for IgG1 and IgG4(5228P) isotype controls (data not shown).
At day 14,
Antibody 9 and isotype control has average drug levels around 5 p,g/mL while
Antibody 10 had
average drug levels around 6 g/mL.
[00128] The present invention is not to be limited in scope by the specific
embodiments
described herein. Indeed, various modifications of the invention in addition
to those described
herein will become apparent to those skilled in the art from the foregoing
description and the
accompanying figures. Such modifications are intended to fall within the scope
of the
appended claims.
38
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
SEQUENCE LISTING
SEQ ID NO:1 GGG-(233-236)
PPCPAPGGG-GPSVF
SEQ ID NO:2 GG--(233-236)
CPPCPAPGG--GPSVF
SEQ ID NO:3 G---(233-236)
CPPCPAPG---GPSVF
SEQ ID NO:4 No_G-(233-236)
CPPCPAP--GPSVF
SEQ ID NO:5 IgG4 _GGG-(233-236)
ASTKGPSVFP LAPCSRSTSE STAALGCLVK DYFPEPVTVS WNSGALTSGV HTFPAVLQSS GLYSLSSVVT
VPSSSLGTKT YTCNVDHKPS NTKVDKRVES KYGPPCPPCP APGGGGPSVFLFPPKPKDT LMISRTPEVT
CVVVDVSQED PEVQFNWYVD GVEVHNAKTK PREEQFNSTYRVVSVLTVLH QDWLNGKEYK CKVSNKGLPS
SIEKTISKAK GQPREPQVYT LPPSQEEMTK NQVSLTCLVK GFYPSDIAVE WESNGQPENN YKTTPPVLDS
DGSFFLYSRL TVDKSRWQEGNVFSCSVMHE ALHNHYTQKS LSLSLGK
SEQ ID NO:6 IgG4 _GG--(233-236)
ASTKGPSVFP LAPCSRSTSE STAALGCLVK DYFPEPVTVS WNSGALTSGV HTFPAVLQSS GLYSLSSVVT
VPSSSLGTKT YTCNVDHKPS NTKVDKRVES KYGPPCPPCP APGGGPSVFLFPPKPKDT LMISRTPEVT
CVVVDVSQED PEVQFNWYVD GVEVHNAKTK PREEQFNSTYRVVSVLTVLH QDWLNGKEYK CKVSNKGLPS
SIEKTISKAK GQPREPQVYT LPPSQEEMTK NQVSLTCLVK GFYPSDIAVE WESNGQPENN YKTTPPVLDS
DGSFFLYSRL TVDKSRWQEGNVFSCSVMHE ALHNHYTQKS LSLSLGK
SEQ ID NO:7 IgG4 _G---(233-236)
ASTKGPSVFP LAPCSRSTSE STAALGCLVK DYFPEPVTVS WNSGALTSGV HTFPAVLQSS GLYSLSSVVT
VPSSSLGTKT YTCNVDHKPS NTKVDKRVES KYGPPCPPCP APGGPSVFLFPPKPKDT LMISRTPEVT
CVVVDVSQED
PEVQFNWYVD GVEVHNAKTK PREEQFNSTYRVVSVLTVLH QDWLNGKEYK CKVSNKGLPS SIEKTISKAK
39
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
GQPREPQVYT LPPSQEEMTK NQVSLTCLVK GFYPSDIAVE WESNGQPENN YKTTPPVLDS DGSFFLYSRL
TVDKSRWQEGNVFSCSVMHE ALHNHYTQKS LSLSLGK
SEQ ID NO:8 IgG4_No_G-(233-236)
ASTKGPSVFP LAPCSRSTSE STAALGCLVK DYFPEPVTVS WNSGALTSGV HTFPAVLQSS GLYSLSSVVT
VPSSSLGTKT YTCNVDHKPS NTKVDKRVES KYGPPCPPCP APGPSVFLFPPKPKDT LMISRTPEVT
CVVVDVSQED
PEVQFNW'YVD GVEVHNAKTK PREEQFNSTYRVVSVLTVLH QDWLNGKEYK CKVSNKGLPS SIEKTISKAK
GQPREPQVYT LPPSQEEMTK NQVSLTCLVK GFYPSDIAVE WESNGQPENN YKTTPPVLDS DGSFFLYSRL
TVDKSRWQEGNVFSCSVMHE ALHNHYTQKS LSLSLGK
SEQ ID NO:9 IgG4* _GGG-(233-236)
ASTKGPSVFP LAPCSRSTSE STAALGCLVK DYFPEPVTVS WNSGALTSGV HTFPAVLQSS GLYSLSSVVT
VPSSSLGTKT YTCNVDHKPS NTKVDKRVES KYGPPCPPCP APGGGGPSVFLFPPKPKDT LMISRTPEVT
CVVVDVSQED PEVQFNWYVD GVEVHNAKTK PREEQFNSTYRVVSVLTVLH QDWLNGKEYK CKVSNKGLPS
SIEKTISKAK GQPREPQVYT LPPSQEEMTK NQVSLTCLVK GFYPSDIAVE WESNGQPENN YKTTPPVLDS
DGSFFLYSRL TA/DKSRWQEGNVFSCSVMHE ALHNRFTQKS LSLSLGK
SEQ ID NO:10 IgG4* _GG--(233-236)
ASTKGPSVFP LAPCSRSTSE STAALGCLVK DYFPEPVTVS WNSGALTSGV HTFPAVLQSS GLYSLSSVVT
VPSSSLGTKT YTCNVDHKPS NTKVDKRVES KYGPPCPPCP APGGGPSVFLFPPKPKDT LMISRTPEVT
CVVVDVSQED PEVQFNWYVD GVEVHNAKTK PREEQFNSTYRVVSVLTVLH QDWLNGKEYK CKVSNKGLPS
SIEKTISKAK GQPREPQVYT LPPSQEEMTK NQVSLTCLVK GFYPSDIAVE WESNGQPENN YKTTPPVLDS
DGSFFLYSRL TVDKSRWQEGNVFSCSVMHE ALHNRFTQKS LSLSLGK
SEQ ID NO:11 IgG4* _G---(233-236)
ASTKGPSVFP LAPCSRSTSE STAALGCLVK DYFPEPVTVS WNSGALTSGV HTFPAVLQSS GLYSLSSVVT
VPSSSLGTKT YTCNVDHKPS NTKVDKRVES KYGPPCPPCP APGGPSVFLFPPKPKDT LMISRTPEVT
CVVVDVSQED
PEVQFNWYVD GVEVHNAKTK PREEQFNSTYRVVSVLTVLH QDWLNGKEYK CKVSNKGLPS SIEKTISKAK
GQPREPQVYT LPPSQEEMTK NQVSLTCLVK GFYPSDIAVE WESNGQPENN YKTTPPVLDS DGSFFLYSRL
TVDKSRWQEGNVFSCSVMHE ALHNRFTQKS LSLSLGK
CA 02981312 2017-09-28
WO 2016/161010 PCT/US2016/025051
SEQ ID NO:12 IgG4* _No_G-(233-236)
ASTKGPSVFP LAPCSRSTSE STAALGCLVK DYFPEPVTVS WNSGALTSGV HTFPAVLQSS GLYSLSSVVT
VPSSSLGTKT YTCNVDHKPS NTKVDKRVES KYGPPCPPCP APGPSVFLFPPKPKDT LMISRTPEVT
CVVVDVSQED PEVQFNWYVD GVEVHNAKTK PREEQFNSTYRVVSVLTVLH QDWLNGKEYK
CKVSNKGLPS SIEKTISKAK GQPREPQVYT LPPSQEEMTK NQVSLTCLVK GFYPSDIAVE WESNGQPENN
YKTTPPVLDS DGSFFLYSRL TVDKSRWQEGNVFSCSVMHE ALHNRFTQKS LSLSLGK
SEQ ID NOS. 13-15 are wildtype human IgG1, IgG2 and IgG4 as shown in Figs. 2-
4.
SEQ ID NO:16 IgG1 GGG
ASTKGPSVFP LAPSSKSTSG GTAALGCLVK DYFPEPVTVS WNSGALTSGV
HTFPAVLQSS GLYSLSSVVT VPSSSLGTQT YICNVNHKPS NTKVDKKVEP
KSCDKTHTCPPCP APGGGG PSVFLFPPKP KDTLMISRTP EVTCVVVDVS
HEDPEVKFNW YVDGVEVHNA KTKPREEQYN STYRVVSVLT VLHQDWLNGK
EYKCKVSNKA LPAPIEKTIS KAKGQPREPQVYTLPPSRDE LTKNQVSLTC
LVKGFYPSDI AVEWESNGQP ENNYKTTPPV LDSDGSFFLY SKLTVDKSRW
QQGNVFSCSV MHEALHNHYT QKSLSLSPGK
SEQ ID NO:17 IgG1 GG
ASTKGPSVFP LAPSSKSTSG GTAALGCLVK DYFPEPVTVS WNSGALTSGV
HTFPAVLQSS GLYSLSSVVT VPSSSLGTQT YICNVNHKPS NTKVDKKVEP
KSCDKTHTCPPCP APGGG PSVFLFPPKP KDTLMISRTP EVTCVVVDVS
HEDPEVKFNW YVDGVEVHNA KTKPREEQYN STYRVVSVLT VLHQDWLNGK
EYKCKVSNKA LPAPIEKTIS KAKGQPREPQVYTLPPSRDE LTKNQVSLTC
LVKGFYPSDI AVEWESNGQP ENNYKTTPPV LDSDGSFFLY SKLTVDKSRW
QQGNVFSCSV MHEALHNHYT QKSLSLSPGK
41.
CA 02981312 2017-09-28
WO 2016/161010
PCT/US2016/025051
SEQ ID NO:18 IgG1 G
ASTKGPSVFP LAPSSKSTSG GTAALGCLVK DYFPEPVTVS WNSGALTSGV
HTFPAVLQSS GLYSLSSVVT VPSSSLGTQT YICNVNHKPS NTKVDKKVEP
KSCDKTHTCPPCP APGG PSVFLFPPKP KDTLMISRTP EVTCVVVDVS
HEDPEVKFNW YVDGVEVHNA KTKPREEQYN STYRVVSVLT VLHQDWLNGK
EYKCKVSNKA LPAPIEKTIS KAKGQPREPQVYTLPPSRDE LTKNQVSLTC
LVKGFYPSDI AVEWESNGQP ENNYKTTPPV LDSDGSFFLY SKLTVDKSRW
QQGNVFSCSV MHEALHNHYT QKSLSLSPGK
SEQ ID NO:19 IgG1 no G
ASTKGPSVFP LAPSSKSTSG GTAALGCLVK DYFPEPVTVS WNSGALTSGV
HTFPAVLQSS GLYSLSSVVT VPSSSLGTQT YICNVNHKPS NTKVDKKVEP
KSCDKTHTCPPCP APG PSVFLFPPKP KDTLMISRTP EVTCVVVDVS
HEDPEVKFNW YVDGVEVHNA KTKPREEQYN STYRVVSVLT VLHQDWLNGK
EYKCKVSNKA LPAPIEKTIS KAKGQPREPQVYTLPPSRDE LTKNQVSLTC
LVKGFYPSDI AVEWESNGQP ENNYKTTPPV LDSDGSFFLY SKLTVDKSRW
QQGNVFSCSV MHEALHNHYT QKSLSLSPGK
SEQ ID NO:20 IgG1* GGG
ASTKGPSVFP LAPSSKSTSG GTAALGCLVK DYFPEPVTVS WNSGALTSGV
HTFPAVLQSS GLYSLSSVVT VPSSSLGTQT YICNVNHKPS NTKVDKKVEP
KSCDKTHTCPPCP APGGGG PSVFLFPPKP KDTLMISRTP EVTCVVVDVS
HEDPEVKFNW YVDGVEVHNA KTKPREEQYN STYRVVSVLT VLHQDWLNGK
EYKCKVSNKA LPAPIEKTIS KAKGQPREPQVYTLPPSRDE LTKNQVSLTC
LVKGFYPSDI AVEWESNGQP ENNYKTTPPV LDSDGSFFLY SKLTVDKSRW
QQGNVFSCSV MHEALHNRFT QKSLSLSPGK
42
CA 02981312 2017-09-28
WO 2016/161010
PCT/US2016/025051
SEQ ID NO:21 IgG1* GG
ASTKGPSVFP LAPSSKSTSG GTAALGCLVK DYFPEPVTVS WNSGALTSGV
HTFPAVLQSS GLYSLSSVVT VPSSSLGTQT YICNVNHKPS NTKVDKKVEP
KSCDKTHTCPPCP APGGG PSVFLFPPKP KDTLMISRTP EVTCVVVDVS
HEDPEVKFNW YVDGVEVHNA KTKPREEQYN STYRVVSVLT VLHQDWLNGK
EYKCKVSNKA LPAPIEKTIS KAKGQPREPQVYTLPPSRDE LTKNQVSLTC
LVKGFYPSDI AVEWESNGQP ENNYKTTPPV LDSDGSFFLY SKLTVDKSRW
QQGNVFSCSV MHEALHNRFT QKSLSLSPGK
SEQ ID NO:22 IgG1* G
ASTKGPSVFP LAPSSKSTSG GTAALGCLVK DYFPEPVTVS WNSGALTSGV
HTFPAVLQSS GLYSLSSVVT VPSSSLGTQT YICNVNHKPS NTKVDKKVEP
KSCDKTHTCPPCP APGG PSVFLFPPKP KDTLMISRTP EVTCVVVDVS
HEDPEVKFNW YVDGVEVHNA KTKPREEQYN STYRVVSVLT VLHQDWLNGK
EYKCKVSNKA LPAPIEKTIS KAKGQPREPQVYTLPPSRDE LTKNQVSLTC
LVKGFYPSDI AVEWESNGQP ENNYKTTPPV LDSDGSFFLY SKLTVDKSRW
QQGNVFSCSV MHEALHNRFT QKSLSLSPGK
SEQ ID NO:23 IgG1* no G
ASTKGPSVFP LAPSSKSTSG GTAALGCLVK DYFPEPVTVS WNSGALTSGV
HTFPAVLQSS GLYSLSSVVT VPSSSLGTQT YICNVNHKPS NTKVDKKVEP
KSCDKTHTCPPCP APG PSVFLFPPKP KDTLMISRTP EVTCVVVDVS
HEDPEVKFNW YVDGVEVHNA KTKPREEQYN STYRVVSVLT VLHQDWLNGK
EYKCKVSNKA LPAPIEKTIS KAKGQPREPQVYTLPPSRDE LTKNQVSLTC
LVKGFYPSDI AVEWESNGQP ENNYKTTPPV LDSDGSFFLY SKLTVDKSRW
QQGNVFSCSV MHEALHNRFT QKSLSLSPGK
43
CA 02981312 2017-09-28
WO 2016/161010
PCT/US2016/025051
SEQ ID NO:24: IgG2 GGG
ASTKGPSVFP LAPCSRSTSE STAALGCLVK DYFPEPVTVS WNSGALTSGV
HTFPAVLQSS GLYSLSSVVT VPSSNFGTQT YTCNVDHKPS NTKVDKTVER
KCCVECPPCP APGGGGPSVF LFPPKPKDTL MISRTPEVTC VVVDVSHEDP
EVQFNWYVDG VEVHNAKTKP REEQFNSTFR VVSVLTVVHQ DWLNGKEYKC
KVSNKGLPAP I EKTISKTKG QPREPQVYTL PPSREEMTKN QVSLTCLVKG
FYPSDISVEW ESNGQPENNY KTTPPMLDSD GSFFLYSKLT VDKSRWQQGN
VFSCSVMHEA LHNHYTQKSL SLSPGK
SEQ ID NO:25: IgG2 GG
ASTKGPSVFP LAPCSRSTSE STAALGCLVK DYFPEPVTVS WNSGALTSGV
HTFPAVLQSS GLYSLSSVVT VPSSNFGTQT YTCNVDHKPS NTKVDKTVER
KCCVECPPCP APGGGPSVF LFPPKPKDTL MISRTPEVTC VVVDVSHEDP
EVQFNWYVDG VEVHNAKTKP REEQFNSTFR VVSVLTVVHQ DWLNGKEYKC
KVSNKGLPAP I EKTISKTKG QPREPQVYTL PPSREEMTKN QVSLTCLVKG
FYPSDISVEW ESNGQPENNY KTTPPMLDSD GSFFLYSKLT VDKSRWQQGN
VFSCSVMHEA LHNHYTQKSL SLSPGK
SEQ ID NO:26 IgG2 G
ASTKGPSVFP LAPCSRSTSE STAALGCLVK DYFPEPVTVS WNSGALTSGV
HTFPAVLQSS GLYSLSSVVT VPSSNFGTQT YTCNVDHKPS NTKVDKTVER
KCCVECPPCP APGGPSVF LFPPKPKDTL MISRTPEVTC VVVDVSHEDP
EVQFNWYVDG VEVHNAKTKP REEQFNSTFR VVSVLTVVHQ DWLNGKEYKC
KVSNKGLPAP I EKTISKTKG QPREPQVYTL PPSREEMTKN QVSLTCLVKG
FYPSDISVEW ESNGQPENNY KTTPPMLDSD GSFFLYSKLT VDKSRWQQGN
VFSCSVMHEA LHNHYTQKSL SLSPGK
44
CA 02981312 2017-09-28
WO 2016/161010
PCT/US2016/025051
SEQ ID NO:27 IgG2 No G
ASTKGPSVFP LAPCSRSTSE STAALGCLVK DYFPEPVTVS WNSGALTSGV
HTFPAVLQSS GLYSLSSVVT VPSSNFGTQT YTCNVDHKPS NTKVDKTVER
KCCVECPPCP APGPSVF LFPPKPKDTL MISRTPEVTC VVVDVSHEDP
EVQFNWYVDG VEVHNAKTKP REEQFNSTFR VVSVLTVVHQ DWLNGKEYKC
KVSNKGLPAP I EKTISKTKG QPREPQVYTL PPSREEMTKN QVSLTCLVKG
FYPSDISVEW ESNGQPENNY KTTPPMLDSD GSFFLYSKLT VDKSRWQQGN
VFSCSVMHEA LHNHYTQKSL SLSPGK
SEQ ID NO:28: IgG2* GGG
ASTKGPSVFP LAPCSRSTSE STAALGCLVK DYFPEPVTVS WNSGALTSGV
HTFPAVLQSS GLYSLSSVVT VPSSNFGTQT YTCNVDHKPS NTKVDKTVER
KCCVECPPCP APGGGGPSVF LFPPKPKDTL MISRTPEVTC VVVDVSHEDP
EVQFNWYVDG VEVHNAKTKP REEQFNSTFR VVSVLTVVHQ DWLNGKEYKC
KVSNKGLPAP I EKTISKTKG QPREPQVYTL PPSREEMTKN QVSLTCLVKG
FYPSDISVEW ESNGQPENNY KTIPPIALDSD GSFFLYSKLT VDKSRWQQGN
VFSCSVMHEA LHNRFTQKSL SLSPGK
SEQ ID NO:29: IgG2* GG
ASTKGPSVFP LAPCSRSTSE STAALGCLVK DYFPEPVTVS WNSGALTSGV
HTFPAVLQSS GLYSLSSVVT VPSSNFGTQT YTCNVDHKPS NTKVDKTVER
KCCVECPPCP APGGGPSVF LFPPKPKDTL MISRTPEVTC VVVDVSHEDP
EVQFNWYVDG VEVHNAKTKP REEQFNSTFR VVSVLTVVHQ DWLNGKEYKC
KVSNKGLPAP I EKTISKTKG QPREPQVYTL PPSREEMTKN QVSLTCLVKG
FYPSDISVEW ESNGQPENNY KTTPPMLDSD GSFFLYSKLT VDKSRWQQGN
VFSCSVMHEA LHNRFTQKSL SLSPGK
CA 02981312 2017-09-28
WO 2016/161010
PCT/US2016/025051
SEQ ID NO:30 IgG2* G
ASTKGPSVFP LAPCSRSTSE STAALGCLVK DYFPEPVTVS WNSGALTSGV
HTFPAVLQSS GLYSLSSVVT VPSSNFGTQT YTCNVDHKPS NTKVDKTVER
KCCVECPPCP APGGPSVF LFPPKPKDTL MISRTPEVTC VVVDVSHEDP
EVQFNWYVDG VEVHNAKTKP REEQFNSTFR VVSVLTVVHQ DWLNGKEYKC
KVSNKGLPAP IEKTISKTKG QPREPQVYTL PPSREEMTKN QVSLTCLVKG
FYPSDISVEW ESNGQPENNY KTTPPMLDSD GSFFLYSKLT VDKSRWQQGN
VFSCSVMHEA LHNRFTQKSL SLSPGK
SEQ ID NO:31 IgG2* No G
ASTKGPSVFP LAPCSRSTSE STAALGCLVK DYFPEPVTVS WNSGALTSGV
HTFPAVLQSS GLYSLSSVVT VPSSNFGTQT YTCNVDHKPS NTKVDKTVER
KCCVECPPCP APGPSVF LFPPKPKDTL MISRTPEVTC VVVDVSH EDP
EVQFNWYVDG VEVHNAKTKP REEQFNSTFR VVSVUTVVHQ DWLNGKEYKC
KVSNKGLPAP IEKTISKTKG QPREPQVYTL PPSREEMTKN QVSLTCLVKG
FYPSDISVEW ESNGQPENNY KTTPPMLDSD GSFFLYSKLT VDKSRWQQGN
VFSCSVMHEA LHNRFTQKSL SLSPGK
46