Note: Descriptions are shown in the official language in which they were submitted.
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
1
CLOSED REACTOR TRANSITIONS BETWEEN METALLOCENE CATALYSTS
FIELD OF THE INVENTION
[mon This disclosure relates to methods for transitioning a gas phase
polymerization reactor
system from one metallocene catalyst to another metallocene catalyst.
BACKGROUND
[0002] In gas phase polymerization, a gaseous stream containing one or more
monomers is
passed through a fluidized bed under reactive conditions in the presence of a
catalyst. A
polymer product is withdrawn from the reactor. Fresh monomer is introduced to
the reactor to
replace the removed polymer product, and unreacted monomer is recycled back to
the reactor.
Process upsets in an ancillary system upstream and/or downstream of the
reactor may require the
polymerization to be shutdown or "killed." The use of multiple catalysts in
the same reactor
system may also require shutdowns, for example, to transition from one
catalyst to another.
[0003] In the case of transitioning between catalysts, it is desirable to
minimize the complexity
and amount of time required for the transition and to minimize the amount of
off-grade resin
product produced. Many transition procedures are directed toward accomplishing
these goals.
However, typical kill procedures still often require the reactor to be opened,
purged of
hydrocarbons, emptied of polymer and catalyst particles, cleaned, and reloaded
with the
removed bed or a new bed to provide a "seedbed" of polymer. This process is
time consuming,
expensive, and allows impurities, such as moisture and air, to enter the
reactor. Such impurities
necessitate another time consuming procedure to remove.
100041 WO 2004/060930 discloses a process for transitioning between a
metallocene and a
Ziegler-Natta polymerization catalyst system. The process requires
discontinuing introduction
of a metallocene catalyst, introducing a catalyst deactivating agent to stop
the metallocene
polymerization reaction, purging the reactor with an inert gas to remove
unreacted deactivating
agent, and then introducing a Ziegler-Natta catalyst to the reactor. =US
2004/0138391 discloses a
process for transitioning between catalyst systems that are incompatible with
each other in a gas
phase fluidized bed reactor containing a fluidized bed of polymer particles.
The process requires
continually passing monomer gases through the polymerization zone,
discontinuing the
introduction of a first catalyst system into the reactor, lowering the height
of the bed of polymer
particles, introducing a second catalyst system into the reactor, and then
increasing the height of
the bed of polymer particles. WO 2011/103280 provides a method for shutting
down and
restarting a gas phase polymerization reactor. The
method involves introducing a
polymerization neutralizer to stop polymerization. The method can also include
stopping
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
2
recovery of a polymer product from the reactor, stopping introduction of a
catalyst and reactor
feed to the reactor, and adjusting the pressure and superficial velocity of
cycle fluid through the
reactor from operating to idling levels.
[0005] Shutdown or transition procedures in gas phase polymerization reactors
are often
accompanied with a buildup of catalyst and polymer on the walls of the
reactor, which is known
as "sheeting." Another common problem is the buildup of catalyst and polymer
on the internal
distribution plate, injection nozzle(s), and/or product discharge nozzle(s),
which is known as
"plugging" or "plate fouling." Sheeting, fouling, and plugging can force a
complete reactor
shutdown for cleaning and removal of the polymer chunks, which could take
several days. This
undermines the efficiency of any process designed to minimize transition time.
[0006] It is particularly difficult to control sheeting, fouling, and plugging
with metallocene
catalysts during reactor shutdowns or transitions because they are known to
exhibit
unpredictable static tendencies. For instance, EP 0 811 638 describes
metallocene catalysts as
exhibiting sudden erratic static charge behavior that can appear even after
long periods of stable
behavior. It has been found that many of the known methods of shutting down or
transitioning a
reactor from one catalyst to another fail to prevent sheeting and the like
with transitions between
metallocene catalysts. There is a need for improved methods for transitioning
a gas phase
polymerization reactor system between metallocene catalysts.
SUMMARY
[0007] A method for transitioning a gas phase polymerization reactor
comprising a fluidized bed
from a first metallocene catalyst to a second metallocene catalyst is
disclosed. The method
comprises first reducing the superficial gas velocity and increasing the
height of the fluidized
bed within the reactor prior to stopping a feed comprising a first metallocene
catalyst. The
method further comprises introducing a first polymerization neutralizer to the
reactor, wherein
the first polymerization reactor does not comprise water, and then introducing
a second
polymerization neutralizer to the reactor, wherein the second polymerization
neutralizer is
different from the first polymerization neutralizer. After this, the method
comprises purging the
reactor with an inert gas and then introducing a feed comprising a second
metallocene catalyst to
the reactor.
[0008] The method disclosed herein is particularly useful with gas phase
polyethylene
polymerization reactors.
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
3
BRIEF DESCRIPTION OF THE DRAWING
[0009] Figure I depicts a schematic of an illustrative gas phase
polymerization system for
making polymers.
DETAILED DESCRIPTION
[00ioj It has been found that metallocene catalysts are particularly sensitive
to certain
compounds typically used as catalyst kill agents or polymerization
neutralizers (these terms are
used interchangeably herein). For example, water is an extremely effective
polymerization
neutralizer for metallocene catalysts. However, it has been found that under
certain
circumstances when water is used as a polymerization neutralizer with
metallocene catalysts
during reactor transitions, the metallocene catalyst can interact with the
water in a manner that
causes severe and rapid sheeting in the reactor. This can potentially lead to
a complete reactor
shutdown so that the reactor can be opened and cleaned, which may take several
days or more,
and undermines any efficiency gained by having a fast transition process.
Loom Due to these issues, there has been a tendency to avoid the use of water
as a
polymerization neutralizer in reactor transitions between metallocene
catalysts. The tendency
has been to look for and use other compounds. In some cases, those compounds
may be less
effective polymerization neutralizers than water. When the polymerization
neutralizer that is
used is less effective, it is possible that residual active metallocene
catalyst may remain in the
reactor during the reactor transition to a new metallocene catalyst. This can
increase the amount
of off-grade product that is produced during the transition and upon start up
with the new
catalyst. It can also increase the time required to complete a catalyst
transition. As such, it is
desirable to be able to use the most effective polymerization neutralizers
available, so long as the
compounds do not lead to other adverse effects on the reactor system or resin
product.
[0012] The methods disclosed herein enable water to be used as a
polymerization neutralizer
with metallocene catalysts, while eliminating the risk that the metallocene
catalyst will interact
with the water and cause a catastrophic event. These methods can enable faster
reactor
transitions with less production of undesirable off-grade product.
[0013] The methods disclosed herein comprise first reducing the superficial
gas velocity and
increasing the height of the fluidized bed within the reactor prior to
stopping a feed comprising a
first metallocene catalyst. Next, a first polymerization neutralizer is added
to the reactor. The
first polymerization neutralizer does not comprise water, as it has been found
that when the first
polymerization neutralizer comprises water the risk of sheeting leading to a
catastrophic event is
substantially increased. The reactor is circulated for a period of time after
introducing the first
polymerization neutralizer to allow the reaction between the first
polymerization neutralizer and
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
4
the metallocene catalyst to take place. After this, a second polymerization
neutralizer is
introduced to the reactor. The second polymerization neutralizer is different
from the first
polymerization neutralizer, and may comprise water in a preferred method. The
reactor is again
circulated for a period of time after introducing the second polymerization
neutralizer.
Following this, the reactor is purged with an inert gas and then a feed
comprising a second
metallocene catalyst may be introduced to the reactor.
[0014] The total amount of polymerization neutralizer (the 'total amount"
meaning the amount
of both the first and second polymerization neutralizer) added to the reactor
should be sufficient
to reduce or completely stop polymerization therein, without interrupting
fluidization within the
reactor. An excess amount of polymerization neutralizer, i.e. an amount
greater than that
necessary to stop polymerization can be used, but more preferably, the amount
added is
sufficient to reduce the rate of polymerization by about 90%, about 95%, about
98%, about 99%,
about 99.9%, about 99.99%, about 99.999%, or 100%. A 99% reduction in the rate
of
polymerization means that polymerization is occurring at only 1% of the
original rate of
polymerization prior to the introduction of the polymerization neutralizer. A
100% reduction in
the polymerization rate means that no polymerization is occurring within the
reactor.
[0015] The total amount or concentration of the polymerization neutralizer
within the reactor
can vary depending on the size of the reactor and the desired time frame for
the polymerization
interruption. For example, the total amount or concentration of the
polymerization neutralizer
within the reactor can be at least 1 part per million by volume ("ppmv"),
about 5 ppmv, about 10
ppmv, about 30 ppmv, about 50 ppmv, about 100 ppmv, about 250 ppmw, about 500
ppmw, or
about 1,000 ppmw, based on the volume of the fluidized bed. In another
example, the total
amount or concentration of the polymerization neutralizer within the reactor
can range from a
low of about 1 ppmv, about 2 ppmv, or about 3 ppmv to a high of about 10 ppmv,
about 30
ppmv, or about 50 ppmv, based on the volume of the fluidized bed.
[0016] The amount of the first polymerization neutralizer that is used may be
represented on a
ppm by weight basis. For example, the amount may be between 5 ppm and 1000
ppm, based on
the weight of the fluidized bed. The amount may range from a low of 5 ppm, 10
ppm, 30 ppm,
50 ppm, 70 ppm, 80 ppm, 90 ppm, 100 ppm, 150 ppm, 250 ppm, or 500 ppm, to a
high of 50
ppm, 60 ppm, 70 ppm, 80 ppm, 90 ppm, 100 ppm, 150 ppm, 250 ppm, 500 ppm, or
1000 ppm,
including any combination of any low or high value recited herein, based on
the weight of the
fluidized bed.
[0017] The amount of the second polymerization neutralizer that is used may
also be
represented on a ppm by weight basis. For example, the amount may be between 5
ppm and
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
1000 ppm, based on the weight of the fluidized bed. The amount may range from
a low of 5
ppm, 10 ppm, 30 ppm, 50 ppm, 70 ppm, 80 ppm, 90 ppm, 100 ppm, 150 ppm, 250
ppm, or 500
ppm, to a high of 50 ppm, 60 ppm, 70 ppm, 80 ppm, 90 ppm, 100 ppm, 150 ppm,
250 ppm, 500
ppm, or 1000 ppm, including any combination of any low or high value recited
herein, based on
the weight of the fluidized bed.
[0018] Polymerization neutralizer can be added to the reactor from any
location or number of
locations within the polymerization system. For example, polymerization
neutralizer can be
introduced directly to the reactor, with the reactor feed, the catalyst feed,
to the cycle fluid, or
any combination thereof. Preferably, polymerization neutralizer is introduced
directly to the
reactor and/or to the cycle fluid.
[0019] Suitable polymerization neutralizers for the first or second
polymerization neutralizer can
include, but are not limited to, one or more Lewis bases such as carbon
monoxide, carbon
dioxide, or any combination thereof. The first polymerization neutralizer can
include carbon
monoxide, carbon dioxide, or a combination thereof, but does not comprise
water. The second
polymerization neutralizer can include carbon monoxide, carbon dioxide, water,
or a
combination thereof. The second polymerization neutralizer preferably
comprises water. For
example, the second polymerization neutralizer can be just water or any
combination of one or
more Lewis bases that includes water. "Water" or "H20" herein refers to water
in any physical
state, including liquid and vapor.
[0020] The recovery of polymer product can be adjusted, i.e., reduced,
increased and/or stopped,
at any time before, after, or at the same time the first polymerization
neutralizer is introduced to
the reactor. For example, recovery of the polymer product can be stopped when
the first
polymerization neutralizer is introduced to the reactor. In another example,
the polymer product
can be stopped within about +/- 1 minute, about +/- 5 minutes, or about +/- 10
minutes of the
time the first polymerization neutralizer is introduced to the reactor.
[0021] The rate the reactor feed is introduced to the reactor can also be
adjusted, i.e., reduced,
increased and/or stopped, at any time before, after, or at the same time the
polymerization
neutralizer is introduced to the reactor. For example, introduction of the
reactor feed can be
stopped when the first polymerization neutralizer is introduced to the
reactor. In another
example, introduction of the reactor feed can be stopped within about +/- 1
minute, about +/- 5
minutes, or about +/- 10 minutes of the time the first polymerization
neutralizer is introduced to
the reactor.
[0022] Each particular component of the reactor feed, e.g. monomer(s), induced
condensing
agents ("ICAs"), hydrogen, and/or inert gases such as nitrogen, can be stopped
at the same time
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
6
or at different times with respect to one another. For example, all components
of the reactor
feed can be stopped at the same time. In another example, for a reactor feed
having monomer(s)
and ICA(s), introduction of the ICA(s) can be stopped prior to introduction of
the first
polymerization neutralizer and introduction of the monomer(s) can be stopped
when or after the
first polymerization neutralizer is introduced to the reactor. In still
another example, for a
reactor feed having monomer(s) and ICA(s), both the ICA(s) and the monomer(s)
can be
stopped before the first polymerization neutralizer is introduced to the
reactor and the
introduction of the ICA(s) can be stopped before the introduction of the
monomer(s) is stopped.
[0023] Further, the rate the catalyst feed is introduced to the reactor can be
adjusted, i.e.,
reduced, increased and/or stopped, at any time before, after, or at the same
time the first
polymerization neutralizer is introduced to the reactor. For example,
introduction of the catalyst
feed can be stopped when the first polymerization neutralizer is introduced to
the reactor. In
another example, introduction of the catalyst feed can be stopped within about
+/- 1 minute,
about +/- 5 minutes, or about +/- 10 minutes of the time the first
polymerization neutralizer is
introduced to the reactor.
[0024] Each particular component of the catalyst feed, e.g. catalyst(s),
activator(s), and/or
additives, can be stopped at the same time or different times with respect to
one another. For
example, all components of the catalyst feed can be stopped at the same time.
In another
example, for a catalyst feed having a first catalyst system and a second
catalyst system,
introduction of the first catalyst system can be stopped prior to introduction
of the first
polymerization neutralizer and introduction of the second catalyst system can
be stopped when
or after the first polymerization neutralizer is introduced to the reactor. In
still another example,
for a catalyst feed having a first catalyst system and a second catalyst
system both the first and
second catalyst systems can be stopped before the first polymerization
neutralizer is introduced
to the reactor and the introduction of the first catalyst system can be
stopped before introduction
of the second catalyst system is stopped.
[0025] As noted above, the methods herein can include idling the reactor for a
period. As part
of the idling procedure, the pressure within the reactor can be adjusted. The
pressure within the
reactor can be adjusted by removing at least a portion of the gases and/or
liquids from within the
reactor or adding gases and/or liquids to the reactor. For example, the
pressure within the
reactor can be reduced by venting or purging at least a portion of the gases
and/or liquids from
within the reactor.
[0026] The idling pressure can be less than or greater than the operating
pressure. Preferably,
the idling pressure is less than the operating pressure. For example, the
normal operating
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
7
pressure within the reactor can range from a low of about 1,000 kPag, about
1,200 kPag, about
1,400 kPag, or about 1,500 kPag to a high of about 1,800 kPag, about 2,200
kPa, about 2,300
kPag, about 2,400 kPag, or about 2,500 kPag. During idling, however, the
pressure can be
reduced to a range having a low of about 500 kPag, about 600 kPag, about 700
kPag, about 800
kPag, about 900 kPag, or about 1,000 kPag to a high of about 600 kPag, about
700 kPag, about
800 kPag, about 900 kPag, about 1,100 kPag, about 1,200 kPag, or about 1,300
kPag, or any
combination of any upper or lower limit recited herein. The pressure within
the reactor can be
reduced by venting or purging at least a portion of the gases and/or liquids
within the reactor
before, when, or after the polymerization neutralizer is introduced to the
reactor.
[0027] If the pressure within the reactor approaches or falls below a desired
idling pressure,
gases and/or liquids can be introduced to the reactor to increase the pressure
therein. For
example, nitrogen can be introduced to the reactor to increase the pressure
within the reactor to a
desired idling pressure. The idling pressure can be less than the operating
pressure, equal to the
operating pressure, or greater than the operating pressure of the reactor.
[0028] Optionally, the idling procedure can also include adjusting the
superficial velocity of
cycle fluid flowing through the reactor can be adjusted from an operating
superficial velocity to
an idling superficial velocity. The pressure drop through the fluidized bed is
equal to or slightly
greater than the weight of the fluidized bed divided by the cross-sectional
area. It is thus
dependent on the geometry of the reactor. To maintain a viable fluidized bed
in the reactor, the
superficial gas velocity through the bed must exceed the minimum flow required
for
fluidization. During operating conditions, preferably the superficial gas
velocity is at least two
times the minimum flow velocity. The operating superficial gas velocity can
range from a low
of about 0.3 m/s, about 0.35 m/s, about 0.4 m/s, or about 0.5 m/s to a high of
about 1 m/s, about
1.4 m/s, about 1.8 m/s, or about 2 m/s. Ordinarily, the superficial gas
velocity does not exceed
1.5 m/s and usually no more than about 0.8 m/s.
[0029] The idling superficial velocity can be less than the operating
superficial velocity. For
example, a reactor with an operating superficial velocity of around 0.8 m/s of
cycle fluid flow
therethrough can be reduced to about 0.60 to about 0.70 m/s or about 0.60 to
about 0.65 m/s
during idling. The superficial velocity can be reduced before, after, and/or
at the same time the
first polymerization neutralizer is introduced to the reactor.
[0030] The use of lower superficial gas velocity during the idling procedure
can aid in
decreasing catalyst entrainment static during idling. Small changes in the
superficial gas
velocity can result in large changes in entrainment static. Thus, reducing or
lowering the
superficial gas velocity during the idling or start up procedures can decrease
the entrainment
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
8
static. Reductions in the entrainment static can lead to reductions in
sheeting and fouling in the
reactor. The level of static in the reactor can be measured using a static
probe, as described in
PCT Publication WO 2008/016478. For example, the level of static in the
reactor may be
measured using an upper static probe located near the top of the fluidized
bed. This upper static
probe may provide a measurement of entrainment static, the static at or near
the top of the
fluidized bed or near the reactor output streams.
[0031] Optionally, the temperature within the reactor can be adjusted during
the transition. The
temperature within the reactor can be reduced, before, after, and/or at the
same time the first
polymerization neutralizer is introduced to the reactor. The idling
temperature of the fluidized
bed can be about 85 C or less, about 83 C or less, about 80 C or less, or
about 77 C or less. The
idling temperature of the fluidized bed can be maintained at a temperature
that can range from
about ambient or "room" temperature to about 79 C, about 82 C, or about 84 C.
Reducing or
stopping the polymerization within the reactor can reduce or eliminate the
heat produced
therefrom, which can reduce the temperature within the reactor. The
temperature within the
reactor can also be reduced and/or maintained by adjusting the temperature of
a heat transfer
medium used to adjust the temperature of the cycle fluid, for example.
[0032] It is advantageous to ensure that the dew point temperature of the gas
composition within
the reactor is at least about 3 C less than the fluidized bed temperature
prior to introducing the
first polymerization neutralizer. Allowing the fluidized bed temperature to
approach too closely
to the dew point temperature of the gas composition during a catalyst
transition can also lead to
sheeting, fouling, and the like. For example, the dew point temperature of the
gas composition
within the reactor may be at least about 5 C, 10 C, 15 C, 20 C, or even 25 C
or more less than
the fluidized bed temperature. This temperature differential may be maintained
for the entire
transition or, for example, through the step of purging the reactor with an
inert gas or until the
second catalyst system is introduced. During the step of purging the reactor
with an inert gas,
the fluidized bed temperature may advantageously be maintained at between 75 C
and 85 C,
78 C and 84 C, 80 C and 83 C, 81 C and 83 C, or be maintained at about 82 C.
[00331 For reactors operating in condensed mode, it is also advantageous to
ensure that the dew
point temperature of the gas composition within the reactor is at least about
3 C, about 5 C, or
about 10 C or more greater than the reactor inlet temperature prior to
introducing the first
polymerization neutralizer. The reactor inlet temperature is typically
measured at the bottom
inlet of the reactor, under the distributor plate.
[0034] If the ambient temperature outside the reactor is cool, e.g. less than
25 C, the fluidized
bed can tend to cool down below a desired idling temperature because of the
reduced or lack of
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
9
heat being generated within the reactor due to a reduced rate or termination
of polymerization.
To compensate for cooling of the fluidized bed, the temperature of the heat
transfer medium can
be increased. However, to avoid plate fouling and/or sheeting or other polymer
accumulation
within the reactor, the temperature of a heat transfer medium used to heat the
cycle fluid
introduced to the reactor can be monitored and controlled. The temperature of
the heat transfer
medium can be maintained at a temperature of less than about 95 C, less than
about 91 C, less
than about 89 C, less than about 85 C, less than about 81 C, less than about
78 C, or less than
about 75 C. Increasing the heat transfer medium beyond about 80 C, about 85 C,
about 90 C,
or about 95 C could lead to plate fouling or other sheeting within the reactor
upon introduction
of the cycle fluid thereto.
[0035] During normal operation, i.e. polymer production, under a given set of
operating
conditions the fluidized bed is maintained at essentially a constant height by
withdrawing a
portion of the bed as polymer product at the rate of formation of the
particulate polymer product.
Since the rate of heat generation during polymerization is directly related to
the rate of product
formation, a measurement of the temperature rise of the fluid across the
reactor (the difference
between inlet cycle fluid temperature and exit cycle fluid temperature) is
indicative of the rate of
particulate polymer formation at a constant fluid velocity if no or negligible
vaporizable liquid is
present in the inlet fluid. The temperature rise of the fluid across the
reactor, i.e. the temperature
of the cycle gas exiting the reactor minus the temperature of the cycle gas
introduced to the
reactor, can be referred to as "DT" or "AT." A normal or typical DT for the
reactor during
polymer production can range from a low of about 5 C, about 10 C, or about 15
C to a high of
about 40 C, about 50 C, or about 55 C.
[0036] During idling of the polymerization system, the DT of the reactor can
range from a low
of about -15 C, about -11 C, or about -8 C to a high of about -4 C, about -2
C, or about 0 C.
The particular DT can depend on the ambient temperature outside the reactor,
the temperature of
the heat transfer medium, the size of the particular reactor, or any
combination thereof In at
least one example, the temperature within the reactor can be allowed to fall
to the surrounding
ambient temperature, i.e. heat exchangers used to cool the cycle fluid during
operation and/or
warm during idling can be bypassed or operated at ambient temperature.
[0037] The height of the fluidized bed during the transition or during idling
of the reactor can
vary. The height of the fluidized bed can be based, at least in part, on the
particular polymer
being produced in the reactor at the time the transition or idling procedure
is initiated, the
particular polymer to be produced next, or a combination thereof. Adjusting
the rate of recovery
of the polymer product can be performed in a manner that adjusts the height of
the fluidized bed
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
to a desired level. For example, the height of the fluidized bed can be
increased by reducing or
stopping the amount of polymer product recovered from the reactor while
polymerization is
continued, i.e. before the first polymerization neutralizer is introduced to
the reactor. In another
example, the height of the fluidized bed can be decreased by increasing the
amount of polymer
product recovered from the reactor, reducing the amount of polymerizable
components
introduced from the feed stream, or a combination thereof.
[0038] It can be desirable to have differing transition or idling fluidized
bed heights depending
on the particular polymer within the reactor. During normal operation the
reactor can be
operated such that the height of the fluidized bed ranges from a low of about -
1 m, about 0 m, or
about 0.2 m to a high of about 0.5 m, about 1 m, or about 1.5 m relative to
the neck of the
reactor. The "neck" refers to the junction or connection between a cylindrical
section and a
transition section of the reactor. During idling of the reactor the height of
the fluidized bed can
fall or decrease if, for example, the superficial velocity of the cycle fluid
flowing through the
fluidized bed is reduced. As such, it can also be desirable to raise or
increase the fluidized bed
height prior to introducing the first polymerization neutralizer. Prior to
introducing the first
polymerization neutralizer to the reactor and/or stopping the reactor feed
and/or the catalyst feed
the height of the fluidized bed can be adjusted to about 0 m, about 0.5 m,
about 1 m, about 1.25
m, about 1.4 m, about 1.5 m, about 1.6 m, about 1.75 m, about 2 m, or about
2.5 m above the
neck of the reactor, or to within a range of any upper or lower value recited
herein.
[0039] The reactor can remain idle for any desired period of time, i.e.
continued circulation of
the gases therethrough to maintain a reduced or non-polymerizing fluidized bed
therein. The
period of time the reactor can be maintained at or in an idled state can range
from a few minutes
or hours to days or even weeks.
[0040] The reactor can also be circulated for a period of time during or after
any step of the
method. For example, the reactor may be circulated for at least 10 minutes, 15
minutes, 20
minutes, 30 minutes, 60 minutes, 120 minutes, or 150 minutes or more after
introducing the first
polymerization neutralizer. The reactor may also be circulated for at least 10
minutes, 15
minutes, 20 minutes, 30 minutes, 60 minutes, 120 minutes, or 150 minutes or
more after
introducing the second polymerization neutralizer.
[0041] Following introduction of the first and second polymerization
neutralizer, and idling or
circulating the reactor for a period if desired, the reactor is purged with an
inert gas. The time
required for this purge may be 1 hour or more, 2 hours or more, 3 hours or
more, or 4 hours or
more. The purge reduces the concentration of polymerization neutralizer within
the reactor
system. This reduction may be done until the amount of the first
polymerization neutralizer
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
11
comprises less than 500 ppm, 100 ppm, 50 ppm, 30 ppm, 20 ppm, 10 ppm, 5 ppm,
or 1 ppm
based on the weight of the fluidized bed. This reduction may also be done
until the amount of
the second polymerization neutralizer comprises less than 500 ppm, 100 ppm, 50
ppm, 30 ppm,
20 ppm, 10 ppm, 5 ppm, or 1 ppm based on the weight of the fluidized bed. The
concentration
of polymerization neutralizer can be reduced by venting a portion of the cycle
gas from the
reactor. Nitrogen or other inert gases can be introduced to the reactor to
maintain a desired
volume of cycle gas and pressure within the polymerization system. The reactor
feed can also
be introduced in addition to or in lieu of the inert gases. Additionally,
prior to or during the
restart procedure, the amount of hydrocarbon within the fluidized bed may be
reduced to less
than 20, less than 15, less than 10, less than 5, less than 4, less than 3,
less than 2, or less than 1
mol% of the fluidized bed.
[0042] After the purge, a reactor restart procedure can be initiated. The
restart procedure can
include re-introducing the reactor feed, reintroducing the catalyst feed,
adjusting the rate gases
are removed from the reactor via the vent or purge line, adjusting the
superficial velocity of the
gases or cycle fluid through the reactor, adjusting the temperature of the
heat transfer medium
used to adjust the temperature of the cycle fluid, adjusting the pressure
within the reactor, re-
starting recovery of the polymer product, and/or adjusting the height of the
fluidized bed within
the reactor. The reintroduction of the catalyst and the reactor feed, the vent
recovery rate,
adjusting the temperature, pressure, and superficial gas velocity within the
reactor, restarting
polymer product recovery, adjusting the temperature of the heat transfer
medium, and/or the
height of the fluidized bed can occur in any order or sequence.
[0043] The order or sequence of re-starting the reactor can generally follow
the order of
reducing the concentration of polymerization neutralizer within the reactor,
restarting the reactor
feed, adjusting the height of the fluidized bed within the reactor, restarting
the catalyst feed, and
restarting polymer product recovery. The pressure can be adjusted during
introduction of the
reactor feed, e.g. as the reactor feed is introduced to the reactor the
pressure therein can increase.
If the introduction of the reactor feed alone is insufficient to increase the
pressure to the desired
pressure, nitrogen or other non-reactive gases can be added thereto. The
temperature can be
adjusted or maintained at a temperature of from about 80 C to about 90 C, e.g.
85 C for any
desired period of time. When the catalyst is introduced to the reactor and the
concentration of
polymerization neutralizer has been sufficiently reduced polymerization can
begin. The heat
generated from the polymerization after restarting can increase the
temperature within the
reactor. To reduce or maintain a desired temperature within the reactor, the
temperature of the
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
12
heat transfer medium can be adjusted such that the cycle fluid has a desired
temperature prior to
introduction to the reactor.
[0044] The reactor feed can be reintroduced at a rate less than a normal
operating rate, equal to
the normal operating rate, or greater than the normal operating rate. The
rates of various
components of the reactor feed, e.g. monomer(s), ICA(s), and/or hydrogen can
be reintroduced
at varying rates. Reintroduction of each component in the reactor feed can
begin at the same
time or different times with respect to one another. For a reactor feed that
includes ethylene,
hexene, isopentane, and hydrogen, the reintroduction of each component can be
started at
different times. For example, reintroduction of the hexene can be started,
which can be followed
by the ethylene, which can be followed by the isopentane, which can then be
followed by the
hydrogen. In another example, the hexene, ethylene, and isopentane can all be
introduced at
about the same time, which can be followed by the reintroduction of hydrogen.
In still another
example, the introduction of hexene and ethylene can be started, which can be
followed by the
isopentane and they hydrogen.
[0045] The reintroduction of each component of the reactor feed can be
continuous or
intermittent. The reintroduction of one or more components of the reactor feed
can be
continuous and the reintroduction of one or more components of the reactor
feed can be
intermittent. Depending on the particular polymer being produced in the
reactor, the particular
amount and rate each component is reintroduced can vary during restart and
operation of the
polymerization system.
[0046] As the reactor feed and/or inert gases are reintroduced to the reactor,
the pressure within
the reactor can be increased to an operating pressure or a pressure
intermediate the transition or
idling pressure and the operating pressure. For example, if the transition or
idling pressure
ranges from about 600 kPag to about 800 kPag and the desired operating
pressure ranges from
about 2,000 kPag to about 2,400 kPag the pressure within the reactor can be
increased to an
intermediate pressure of from about 1,700 kPag to about 1,900 kPag by the
reintroduction of the
reactor feed and/or inerts. Once polymerization has restarted, i.e. after
reintroduction of the
catalyst feed and the reactor feed have been restarted and the concentration
of the
polymerization neutralizer has sufficiently been decreased, the rate of
introducing the reactor
feed can be adjusted to the desired operating rates. In another example, the
rate of introduction
for the reactor feed can be brought to desired operating rates rather than a
rate intermediate
idling and normal production.
[0047] The superficial velocity of the cycle gas through the reactor can be
maintained at the
transition or idling rate, adjusted to the operational rate, or adjusted to a
rate intermediate the
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
13
transition or idling rate and the operational rate. Gases or fluid from within
the polymerization
system can be removed via the vent to maintain a desired reactor pressure
and/or to adjust the
concentration of one or more components, e.g., monomer(s), ICA(s), hydrogen,
and the like.
Once the desired pressure and flow rates "restart rates" for each component of
the reactor feed
are reached the catalyst can be reintroduced to the reactor.
[0048] The rate of catalyst initially reintroduced to the reactor can be less
than the normal
operating rate. The initial rate of catalyst feed reintroduced can range from
a low of about 15%,
about 20% or about 25% to a high of about 35%, about 40%, or about 50% of the
normal
operating rate. For example, if catalyst were introduced at a rate of 10 kg/hr
during normal
operation, the amount of catalyst reintroduced upon restarting can be about
2.7 kg/hr, about 3
kg/hr, about 3.3 kg/hr, or about 3.7 kg/hr. Once polymerization ("light off')
has begun within
the reactor, the rate of catalyst introduction can be increased to normal
operating rates.
Preferably the rate of catalyst introduction increased over a period of time.
For example, the rate
of catalyst introduction can be increased over a period of time of about 0.5
hours, about 1 hour,
about 2 hours, about 3 hours, about 4 hours, about 5 hours, or about 6 hours.
[0049] The height of the fluidized bed can be adjusted from a transition or
idling height to a
restart height that can be less than or greater than the transition or idling
height. The particular
height of the fluidized bed upon restart can depend, at least in part, on the
particular polymer to
be produced within the reactor once polymerization begins. For example, the
height of the
fluidized bed can be increased by introducing a polymer to the fluidized bed.
If the reactor is
idling and it is desired to increase the height of the fluidized bed, polymer
granules, beads,
flakes, or the like can be introduced to the reactor, thereby increasing the
height of the fluidized
bed. In another example, the height of the fluidized bed can be increased by
delaying restart of
polymer product recovery after polymerization has been restarted.
[0050] Once introduction of the catalyst has been restarted and polymerization
has been
restarted, polymer product recovery can be restarted. The rate of product
recovery can be less
than the normal operating rate and can increase as the amount of polymer
production increases.
The production of polymer product can increase as the rate of introducing the
catalyst and the
reactor feed increases. The production of polymer product can also be
increased as the
concentration of modifying gaseous and/or liquid components such as ICAs in
the reactor feed
increase within the reactor. Another way to adjust the height of the fluidized
bed can be to delay
recovery of the polymer product until the desired fluidized bed height is
reached or to increase
the rate of polymer product recovery if it is desired to decrease the height
of the bed.
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
14
LOOM] The time required to restart the reactor from idling to normal operating
conditions can
range from a low of about 1 hour, about 3 hours, about 5 hours, or about 7
hours to a high of
about 10 hours, about 15 hours, about 20 hours, about 25 hours, or about 30
hours. During
restart of the reactor, other typical or normal operating conditions and
monitoring procedures
can be ran under "modified" or "moderated" conditions or values for a period
of time after
polymerization is restarted. For example, if the polymerization system is
operated in condensed
mode, a common technique for monitoring the reactor can include monitoring a
stickiness
control parameter ("dMRT") such as a reduced melt initiation temperature or
"dMIT" value,
which can provide an estimate as to the degree of polymer stickiness within
the reactor.
Moderated startup or restart conditions can include operating the reactor at a
dMIT of about 0 C
or a dMIT within about +/- 1 C, about +/- 1.5 C, or about +/- 2 C for a period
of time when the
normal dMIT ranges from about 5 C to about 10 C. Another "modified" restart
condition can
include operating the polymerization system at a level or concentration of
ICAs ranging from a
low of about 8.5 mol%, about 9 mol%, or about 9.5 mol% to a high of about 10.5
mol%, about
11 mol%, or about 11.5 mol% when a desired normal level would be greater.
[0052] The reactor feed can include any polymerizable hydrocarbon of
combination of
hydrocarbons. For example, the reactor feed can be any olefin monomer
including substituted
and unsubstituted alkenes having two to 12 carbon atoms, such as ethylene,
propylene, 1-butene,
1-pentene, 1-hexene, 1-heptene, 1-octene, 4-methylpent-1-ene, 1-decene, 1-
dodecene, 1-
hexadecene, and the like. The reactor feed can also include non-hydrocarbon
gas(es) such as
nitrogen and/or hydrogen. The reactor feed can enter the reactor at multiple
and different
locations. For example, monomers can be introduced into the fluidized bed in
various ways
including direct injection through a nozzle (not shown) into the fluidized
bed. The polymer
product can thus be a homopolymer or a copolymer, including a terpolymer,
having one or more
other monomeric units. For example, a polyethylene product could include at
least one or more
other olefin(s) and/or comonomer(s).
[0053] The reactor feed can also include the one or more modifying components
such as one or
more induced condensing agents ("ICAs"). Illustrative ICAs include, but are
not limited to,
propane, butane, isobutane, pentane, isopentane, hexane, isomers thereof,
derivatives thereof,
and combinations thereof As discussed and described above, the ICAs can be
introduced to
provide a reactor feed to the reactor having an ICA concentration ranging from
a low of about 1
mol%, about 5 mol%, or about 10 mol% to a high of about 25 mol%, about 35
mol%, or about
45 mol%. Typical concentrations of the ICAs can range from about 14 mol%,
about 16 mol%,
or about 18 mol% to a high of about 20 mol%, about 22 mol%, or about 24 mol%.
The reactor
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
feed can include other non-reactive gases such as nitrogen and/or argon.
Further details
regarding ICAs are described in U.S. Patent Nos. 5,352,749; 5,405,922; 5,436,
304; and
7,122,607; and WO Publication No. 2005/113615(A2).
[0054] The catalyst feed can include any metallocene or single-site catalyst,
catalyst system, or
combination of catalysts and/or catalyst systems. For example, at least one of
or both of the the
first metallocene catalyst and the second metallocene catalyst may comprise a
biscyclopentadienyl or a bisindenyl transition metal compound.
[0055] The term "catalyst system" includes at least one "catalyst component"
and at least one
"activator," alternately at least one co-catalyst. The catalyst system can
also include other
components, such as supports, and is not limited to the catalyst component
and/or activator
alone or in combination. The catalyst system can include any number of
catalyst components in
any combination as described, as well as any activator in any combination as
described.
[0056] The term "catalyst component" includes any compound that, once
appropriately
activated, is capable of catalyzing the polymerization or oligomerization of
olefins. Preferably,
the catalyst component includes at least one Group 3 to Group 12 atom and
optionally at least
one leaving group bound thereto. The term "leaving group" refers to one or
more chemical
moieties bound to the metal center of the catalyst component that can be
abstracted from the
catalyst component by an activator, thereby producing the species active
towards olefin
polymerization or oligomerization. Suitable activators are described in detail
below.
[0057] As used herein, in reference to Periodic Table "Groups" of Elements,
the "new"
numbering scheme for the Periodic Table Groups are used as in the CRC Handbook
of
Chemistry and Physics (David R. Lide, ed., CRC Press 81st ed. 2000).
[0058] Suitable metallocene catalyst compounds can include, but are not
limited to,
metallocenes described in U.S. Patent Nos.: 7,179,876; 7,169,864; 7,157,531;
7,129,302;
6,995,109; 6,958,306; 6,884748; 6,689,847; 5,026,798; 5,703,187; 5,747,406;
6,069,213;
7,244,795; 7,579,415; U.S. Patent Application Publication No. 2007/0055028;
and WO
Publications WO 97/22635; WO 00/699/22; WO 01/30860; WO 01/30861; WO 02/46246;
WO
02/50088; WO 04/022230; WO 04/026921; and WO 06/019494.
[0059] As used herein, the terms "activator" refers to any compound or
combination of
compounds, supported or unsupported, which can activate a catalyst compound or
component,
such as by creating a cationic species of the catalyst component. For example,
this can include
the abstraction of at least one leaving group (the "X" group in the single
site catalyst compounds
described herein) from the metal center of the catalyst compound/component.
Activators can
include Lewis acids such as cyclic or oligomeric poly(hydrocarbylaluminum
oxides) and so
84111646
16
called non-coordinating activators ("NCA") (alternately, "ionizing activators"
or "stoichiometric
activators"), or any other compound that can convert a neutral metallocene
catalyst component
to a metallocene cation that is active with respect to olefin polymerization.
Illustrative Lewis
acids include, but are not limited to, aluminoxane (e.g., methylaluminoxane
"MAO"), modified
aluminoxane (e.g., modified methylaluminoxane "MMAO" and/or
tetraisobutyldialuminoxane
"TIBAO"), and alkylaluminum compounds. Ionizing activators (neutral or ionic)
such as tri (n-
butyl)ammoniwn tetrakis(pentalluorophenyl)boron may be also be used.
Further, a
trisperfluorophenyl boron metalloid precursor may be used. Any of those
activators/precursors
can be used alone or in combination with the others. There are a variety of
methods for
preparing aluminoxane and modified aluminoxanes known in the art.
[0060] The catalyst compositions can include a support material or carrier. As
used herein, the
terms "support" and "carrier" are used interchangeably and are any support
material, including a
porous support material, for example, talc, inorganic oxides, and inorganic
chlorides. The
catalyst component(s) and/or activator(s) can be deposited on, contacted with,
vaporized with,
bonded to, or incorporated within, adsorbed or absorbed in, or on, one or more
supports or
carriers. Other support materials can include resinous support materials such
as polystyrene,
functionalized or crosslinked organic supports, such as polystyrene divinyl
benzene polyolefins
or polymeric compounds, zeolites, clays, or any other organic or inorganic
support material and
the like, or mixtures thereof.
[0061] Inorganic oxides supports can include Group 2, 3, 4, 5, 13 or 14 metal
oxides. The
preferred supports include silica, which may or may not be dehydrated, fumed
silica, alumina,
silica-alumina and mixtures thereof. Other useful supports include magnesia,
titania, zirconia,
magnesium chloride, montmorillonite, phyllosilicate, zeolites, talc, clays,
and the like. Also,
combinations of these support materials may be used, for example, silica-
chromium, silica-
alumina, silica-titania and the like. Additional support materials may include
those porous
acrylic polymers described in EP 0 767 184.
[0062] The polymer product(s) produced in the reactor can be or include any
type of polymer or
polymeric material. For example, the polymer product can include homopolymers
of olefins
(e.g., homopolymers of ethylene), and/or copolymers, terpolymers, and the like
of olefins,
particularly ethylene, and at least one other olefin. Illustrative polymers
can include, but are not
limited to, polyolefins, polyamides, polyesters, polycarbonates, polysulfones,
polyacetals,
polylactones, acrylonitrile-butadiene-styrene polymers, polyphenylene oxide,
polyphenylene
sulfide, styrene-acrylonitrile polymers, styrene maleic anhydride, polyimides,
aromatic
polyketones, or mixtures of two or more of the above. Suitable polyolefins can
include, but are
Date Recue/Date Received 2022-09-12
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
17
not limited to, polymers comprising one or more linear, branched or cyclic C2
to C40 olefins,
preferably polymers comprising propylene copolymerized with one or more C3 to
C40 olefins,
preferably a C3 to C20 alpha olefin, more preferably C3 to Cio alpha-olefins.
More preferred
polyolefins include, but are not limited to, polymers comprising ethylene
including but not
limited to ethylene copolymerized with a C3 to C40 olefin, preferably a C3 to
C20 alpha olefin,
more preferably propylene and or butene.
[0063] Preferred polymers include homopolymers or copolymers of C2 to C40
olefins, preferably
C2 to C20 olefins, preferably a copolymer of an alpha-olefin and another
olefin or alpha-olefin
(ethylene is defined to be an alpha-olefin for purposes of this invention).
Preferably, the
polymers are or include homo polyethylene, homo polypropylene, propylene
copolymerized
with ethylene and or butene, ethylene copolymerized with one or more of
propylene, butene or
hexene, and optional dienes. Preferred examples include thermoplastic polymers
such as ultra
low density polyethylene, very low density polyethylene ("VLDPE"), linear low
density
polyethylene ("LLDPE"), low density polyethylene ("LDPE"), medium density
polyethylene
("MDPE"), high density polyethylene ("HDPE"), polypropylene, isotactic
polypropylene, highly
isotactic polypropylene, syndiotactic polypropylene, random copolymer of
propylene and
ethylene and/or butene and/or hexene, elastomers such as ethylene propylene
rubber, ethylene
propylene diene monomer rubber, neoprene, and blends of thermoplastic polymers
and
elastomers, such as for example, thermoplastic elastomers and rubber toughened
plastics.
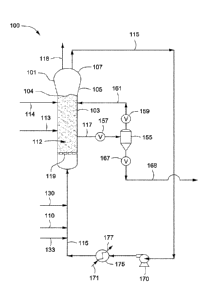
[0064] Figure 1 depicts a flow diagram of an illustrative gas phase
polymerization system 100
for making polymers, according to one or more embodiments. The polymerization
system 100
can include a reactor 101 in fluid communication with one or more discharge
tanks 155 (only
one shown), compressors 170 (only one shown), and heat exchangers 175 (only
one shown).
The polymerization system 100 can also include more than one reactor 101
arranged in series,
parallel, or configured independent from the other reactors, each reactor
having its own
associated discharge tanks 155, compressors 170, and heat exchangers 175, or
alternatively,
sharing any one or more of the associated discharge tanks 155, compressors
170, and heat
exchangers 175. For simplicity and ease of description, the polymerization
system 100 will be
further described in the context of a single reactor train.
[0065] The reactor 101 can include a cylindrical section 103, a transition
section 105, and a
velocity reduction zone or dome 107. The cylindrical section 103 is disposed
adjacent the
transition section 105. The transition section 105 can expand from a first
diameter that
corresponds to the diameter of the cylindrical section 103 to a larger
diameter adjacent the dome
107. As mentioned above, the location or junction at which the cylindrical
section 103 connects
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
18
to the transition section 105 is referred to as the "neck" or the "reactor
neck" 104. The dome 107
has a bulbous shape. One or more cycle fluid lines 115 and vent lines 118 can
be in fluid
communication with the top head 107. The reactor 101 can include the fluidized
bed 112 in
fluid communication with the top head 107.
[0066] In general, the height to diameter ratio of the cylindrical section 103
can vary in the
range of from about 2:1 to about 5:1. The range, of course, can vary to larger
or smaller ratios
and depends, at least in part, upon the desired production capacity and/or
reactor dimensions.
The cross-sectional area of the dome 107 is typically within the range of from
about 2 to about 3
multiplied by the cross-sectional area of the cylindrical section 103.
[0067] The velocity reduction zone or dome 107 has a larger inner diameter
than the fluidized
bed 112. As the name suggests, the velocity reduction zone 107 slows the
velocity of the gas
due to the increased cross-sectional area. This reduction in gas velocity
allows particles
entrained in the upward moving gas to fall back into the bed, allowing
primarily only gas to exit
overhead of the reactor 101 through the cycle fluid line 115. The cycle fluid
recovered via line
115 can contain less than about 10% wt, less than about 8% wt, less than about
5% wt, less than
about 4% wt, less than about 3% wt, less than about 2% wt, less than about 1%
wt, less than
about 0.5% wt, or less than about 0.2% wt of the particles entrained in
fluidized bed 112.
[0068] The reactor feed via line 110 can be introduced to the polymerization
system 100 at any
point. For example, the reactor feed via line 110 can be introduced to the
cylindrical section
103, the transition section 105, the velocity reduction zone 107, to any point
within the cycle
fluid line 115, or any combination thereof. Preferably, the reactor feed 110
is introduced to the
cycle fluid in line 115 before or after the heat exchanger 175. In the Figure,
the reactor feed via
line 110 is depicted entering the cycle fluid in line 115 after the heat
exchanger 175. The
catalyst feed via line 113 can be introduced to the polymerization system 100
at any point.
Preferably the catalyst feed via line 113 is introduced to the fluidized bed
112 within the
cylindrical section 103.
10069] The cycle fluid via line 115 can be compressed in the compressor 170
and then passed
through the heat exchanger 175 where heat can be exchanged between the cycle
fluid and a heat
transfer medium. For example, during notinal operating conditions a cool or
cold heat transfer
medium via line 171 can be introduced to the heat exchanger 175 where heat can
be transferred
from the cycle fluid in line 115 to produce a heated heat transfer medium via
line 177 and a
cooled cycle fluid via line 115. In another example, during idling of the
reactor 101 a warm or
hot heat transfer medium via line 171 can be introduced to the heat exchanger
175 where heat
can be transferred from the heat transfer medium to the cycle fluid in line
115 to produce a
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
19
cooled heat transfer medium via line 117 and a heated cycle fluid via line
115. The terms "cool
heat transfer medium" and "cold heat transfer medium" refer to a heat transfer
medium having a
temperature less than the fluidized bed 112 within the reactor 101. The terms
"warm heat
transfer medium" and "hot heat transfer medium" refer to a heat transfer
medium having a
temperature greater than the fluidized bed 112 within the reactor 101. The
heat exchanger 175
can be used to cool the fluidized bed 112 or heat the fluidized bed 112
depending on the
particular operating conditions of the polymerization system 100, e.g. start-
up, normal operation,
idling, and shut down. Illustrative heat transfer mediums can include, but are
not limited to,
water, air, glycols, or the like. It is also possible to locate the compressor
170 downstream from
the heat exchanger 175 or at an intermediate point between several heat
exchangers 175.
[0070] After cooling, all or a portion of the cycle fluid via line 115 can be
returned to the reactor
101. The cooled cycle fluid in line 115 can absorb the heat of reaction
generated by the
polymerization reaction. The heat transfer medium in line 171 can be used to
transfer heat to the
cycle fluid in line 115 thereby introducing heat to the polymerization system
100 rather than
removing heat therefrom. The heat exchanger 175 can be of any type of heat
exchanger.
Illustrative heat exchangers can include, but are not limited to, shell and
tube, plate and frame,
U-tube, and the like. For example, the heat exchanger 175 can be a shell and
tube heat
exchanger where the cycle fluid via line 115 can be introduced to the tube
side and the heat
transfer medium can be introduced to the shell side of the heat exchanger 175.
If desired,
several heat exchangers can be employed, in series, parallel, or a combination
of series and
parallel, to lower or increase the temperature of the cycle fluid in stages.
[0071] Preferably, the cycle gas via line 115 is returned to the reactor 101
and to the fluidized
bed 112 through fluid distributor plate ("plate") 119. The plate 119 is
preferably installed at the
inlet to the reactor 101 to prevent polymer particles from settling out and
agglomerating into a
solid mass and to prevent liquid accumulation at the bottom of the reactor 101
as well to
facilitate easy transitions between processes which contain liquid in the
cycle stream 115 and
those which do not and vice versa. Although not shown, the cycle gas via line
115 can be
introduced into the reactor 101 through a deflector disposed or located
intermediate an end of
the reactor 101 and the distributor plate 119.
[0072] The catalyst feed via line 113 can be introduced to the fluidized bed
112 within the
reactor 101 through one or more injection nozzles (not shown) in fluid
communication with line
113. The catalyst feed is preferably introduced as pre-formed particles in one
or more liquid
carriers (i.e. a catalyst slurry). Suitable liquid carriers can include
mineral oil and/or liquid or
gaseous hydrocarbons including, but not limited to, propane, butane,
isopentane, hexane,
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
heptane octane, or mixtures thereof. A gas that is inert to the catalyst
slurry such as, for
example, nitrogen or argon can also be used to carry the catalyst slurry into
the reactor 101. In
one example, the catalyst can be a dry powder. In another example, the
catalyst can be dissolved
in a liquid carrier and introduced to the reactor 101 as a solution. The
catalyst via line 113 can
be introduced to the reactor 101 at a rate sufficient to maintain
polymerization of the
monomer(s) therein.
[0073] Fluid via line 161 can be separated from a polymer product recovered
via line 117 from
the reactor 101. The fluid can include unreacted monomer(s), hydrogen, ICA(s),
and/or inerts.
The separated fluid can be introduced to the reactor 101. The separated fluid
can be introduced
to the recycle line 115 (not shown). The separation of the fluid can be
accomplished when fluid
and product leave the reactor 101 and enter the product discharge tanks 155
(one is shown)
through valve 157, which can be, for example, a ball valve designed to have
minimum
restriction to flow when opened. Positioned above and below the product
discharge tank 155
can be conventional valves 159, 167. The valve 167 allows passage of product
therethrough.
For example, to discharge the polymer product from the reactor 101, valve 157
can be opened
while valves 159, 167 are in a closed position. Product and fluid enter the
product discharge
tank 155. Valve 157 is closed and the product is allowed to settle in the
product discharge tank
155. Valve 159 is then opened permitting fluid to flow via line 161 from the
product discharge
tank 155 to the reactor 101. Valve 159 can then be closed and valve 167 can be
opened and any
product in the product discharge tank 155 can flow into and be recovered via
line 168. Valve
167 can then be closed. Although not shown, the product via line 168 can be
introduced to a
plurality of purge bins or separation units, in series, parallel, or a
combination of series and
parallel, to further separate gases and/or liquids from the product. The
particular timing
sequence of the valves 157, 159, 167, can be accomplished by use of
conventional
programmable controllers which are well known in the art.
[0074] Another preferred product discharge system which can be alternatively
employed is that
disclosed in U.S. Patent No. 4,621,952. Such a system employs at least one
(parallel) pair of
tanks comprising a settling tank and a transfer tank arranged in series and
having the separated
gas phase returned from the top of the settling tank to a point in the reactor
near the top of the
fluidized bed.
[0075] The reactor 101 can be equipped with one or more vent lines 118 to
allow venting the
bed during start up, idling, and/or shut down. The reactor 101 can be free
from the use of
stirring and/or wall scraping. The cycle line 115 and the elements therein
(compressor 170, heat
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
21
exchanger 175) can be smooth surfaced and devoid of unnecessary obstructions
so as not to
impede the flow of cycle fluid or entrained particles.
[0076] The conditions for polymerizations vary depending upon the monomers,
catalysts,
catalyst systems, and equipment availability. The specific conditions are
known or readily
derivable by those skilled in the art. For example, the temperatures can be
within the range of
from about -10 C to about 140 C, often about 15 C to about 120 C, and more
often about 70 C
to about 110 C. Pressures can be within the range of from about 10 kPag to
about 10,000 kPag,
such as about 500 kPag to about 5,000 kPag, or about 1,000 kPag to about 2,200
kPag, for
example.
[0077] As discussed and described above, various systems and/or methods can be
used to
monitor and/or control the degree or level of fouling within the reactor 101.
For example, if the
polymerization system 100 is operated in condensed mode, a common technique
for monitoring
the polymerization can include monitoring a stickiness control parameter
("dMRT") such as a
reduced melt initiation temperature or "dM1T" value, which can provide an
estimate as to the
degree of polymer stickiness within the reactor 101. Another method for
monitoring
polymerization can include estimating acoustic emissions within the reactor
101, which can also
provide an estimate as to the degree of polymer stickiness within the reactor
101.
[0078] The recovery of the polymer product via line 117 and introduction of
the reactor feed via
line 110 and the catalyst feed via line 113 can be reduced or stopped before,
after, or
simultaneously with the introduction of polymerization neutralizer via line
130. For example,
recovery of the polymer product via line 117 and introduction of the reactor
feed via line 110
and the catalyst feed via line 113 can be stopped when the polymerization
neutralizer via line
130 is introduced to the polymerization system 100.
[0079] The pressure within the reactor can be reduced from an operating
pressure to an idling
pressure by removing at least a portion of the gases within the reactor 101
(the "reactor gases")
via line 118. In addition to removing at least a portion of the reactor gases
via vent line 118, the
amount of polymerizable and/or modifying gases can also be reduced. Should the
pressure fall
below a desired idling pressure or the pressure within the reactor 101 should
be increased
nitrogen or other inert gases via line 133 can be introduced thereto.
[0080] Prior to introducing polymerization neutralizer via line 130 to the
reactor 101 the
concentration of the polymerizable and/or modifying components within the
reactor 101 such as
monomers and/or ICAs can be reduced via the vent line 118, while
polymerization continues
within the reactor 101. The concentration of ICAs within the reactor can be
reduced via vent
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
22
line 118 to a concentration intermediate an idling concentration and a normal
operating
concentration via vent line 118.
[0081] The superficial velocity of the cycle fluid introduced via line 115 to
the reactor 101 can
be adjusted from an operational superficial velocity to an idling superficial
velocity by
controlling the rate of introduction to the reactor 101. For example, the flow
rate of the cycle
fluid in line 115 can be reduced via one or more valves (not shown) to provide
a cycle fluid flow
through the reactor 101 at a reduced superficial velocity.
[0082] After the polymerization neutralizer via line 130 has been introduced
to the reactor 101
and introduction of the reactor feed via line 110, the catalyst feed via line
113 have been reduced
or stopped and the pressure and superficial velocity have been adjusted from
to an idling
pressure and superficial velocity, the reactor 101 can be maintained at these
conditions for a
period of time. After the period of time, a reactor restart procedure can be
initiated.
[0083] During idling of the reactor 101, the concentration of the
polymerization neutralizer
introduced via line 130 can be monitored. Should the concentration fall below
a desired idling
concentration then additional polymerization neutralizer via line 130 can be
introduced to the
reactor 101. The concentration of the polymerization neutralizer can be
monitored via one or
more gas chromatographs or other detection equipment in a lab or in fluid
communication with
the polymerization system.
[0084] The restart procedure can also include adjusting the height of the
fluidized bed 112 from
an idling height to a restart height. The restart height of the fluidized bed
112 can be greater
than or less than the height of the fluidized bed during idling. To increase
the height of the
fluidized bed 112 polymer granules, beads, flakes, or the like can be
introduced via line 114 to
the reactor 101, thereby increasing the height of the fluidized bed 112. To
decrease the height of
the fluidized bed 112 polymer product can be removed via line 117.
100851 The concentration of polymerization neutralizer within the reactor 101
can be reduced
via vent line 118. The reactor feed via line 110 can be increased or restarted
before, after, or
during the reduction in the concentration of the polymerization neutralizer.
The reactor feed via
line 110 can include the same polymerizable and/or modifying components as
before initiation
of the reactor idling procedure or the reactor feed via line 110 can include
different
polymerizable and/or modifying components. The pressure within the reactor 101
can be
adjusted from the idling pressure to the operating pressure or a pressure
intermediate the idling
pressure and the operating pressure. For example, the pressure within the
reactor 101 can be
increased by introducing the reactor feed via line 110 and/or inert gases via
line 133. In another
example, the pressure within the reactor 101 can be decreased by removing
reactor gases via
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
23
vent line 118. The superficial velocity of the reactor gases through the
reactor 101 can be
adjusted from the idling superficial velocity to the operating superficial
velocity or a superficial
velocity intermediate the idling superficial velocity and the operating
superficial velocity.
[0086] Polymerization within the reactor can be restarted once the
concentration of the
polymerization neutralizer has been reduced a sufficient amount. Increasing or
restarting
introduction of the catalyst feed via line 113 can increase the polymerization
within the reactor.
The catalyst feed via line 113 can be the same catalyst feed or a different
catalyst feed than was
introduced to the reactor 101 prior to initiating the reactor idling
procedure.
[0087] The recovery of the polymer product via line 117 can be increased or
restarted before,
when, or after polymerization is restarted within the reactor 101. The
recovery of the polymer
product via line 117 can be restarted at a normal operating recovery rate for
the desired polymer
being produced within the reactor or to a product recovery rate intermediate
the normal
operating recovery rate and the idling recovery rate.
[0088] The temperature within the reactor 101 can be adjusted from an idling
temperature to an
operating temperature as introduction of the reactor feed via line 110 and the
catalyst feed via
line 113 is restarted and polymerization is restarted within the reactor. The
temperature of the
cycle fluid in line 115 can be adjusted via the heat exchanger 175 to provide
a cycle gas at a
desired temperature. The temperature of the reactor 101 can be adjusted to a
normal operating
temperature for the polymer product being produced within the reactor 101 or
the temperature
can be adjusted to a temperature intermediate the normal operating temperature
and the idling
temperature.
EXAMPLES
[0089] To provide a better understanding of the foregoing discussion, the
following non-limiting
examples are provided. Although the examples are directed to specific
embodiments, they are
not to be viewed as limiting the invention in any specific respect. All parts,
proportions and
percentages are by weight unless otherwise indicated.
[0090] MI (12) can be measured in accordance with ASTM D-1238-E (at 190 C,
2.16 kg
weight). Density can be measured according to ASTM D-105.
[0091] The following example illustrates an experimental closed reactor
transition procedure
carried out in a commercial scale gas phase fluidized bed polyethylene
reactor. The
polymerization reactor had a cylindrical section 103, a transition section
105, and a velocity
reduction section 107, according to one or more embodiments discussed and
described herein
with reference to Figure 1.
CA 02981724 2017-10-03
WO 2016/164451 PCT/US2016/026201
24
[0092] Prior to starting the transition, the reactor was operating in
condensed mode using
XCATTm HP-100 Catalyst, a biscyclopentadienyl zirconium-based metallocene
catalyst
available from Univation Technologies. Before introducing any polymerization
neutralizer the
superficial gas velocity within the reactor was reduced to about 0.70 m/s, the
bed level was
allowed to increase to about 1.4 meters above the neck of the reactor, and
then the catalyst feed
was stopped.
[0093] It was ensured that the dew point temperature of the gas composition
within the reactor
was at least 3 C less than the fluidized bed temperature and at least 3 C
greater than the reactor
inlet temperature. Following this, CO was injected into the reactor at an
amount of between
about 60-100 ppmw, based on the weight of the fluidized bed, and the feed
streams of monomer,
comonomer, condensing agent, and hydrogen to the reactor were stopped. The
reactor was
allowed to circulate for about 30 minutes to ensure good contact between the
CO and the
catalyst. Next, H20 was injected into the reactor at an amount of between
about 80-150 ppmw,
based on the weight of the fluidized bed, and the reactor was again circulated
for about 1 hour.
The bed level decreased to slightly below the neck of the reactor.
[0094] A nitrogen purge was introduced into the reactor. The purge was
continued for about 4
hours, maintaining a bed temperature of between about 82 C and 85 C and
pressure of about 8.6
kg/cm2. The superficial gas velocity within the reactor was maintained at
between about 0.61 to
0.64 m/s while purging, and the purge was done until the residual amount of CO
was less than 5
PPm, the residual amount of H2O was less than 10 ppm, and the residual amount
of
hydrocarbons was less than about 2 mol%.
[0095] Concentrations of monomer, comonomer, condensing agent, and hydrogen
were then
increased to appropriate levels, until the total pressure within the fluidized
bed was between
about 17 and 19.5 kg/cm2 and the partial pressure of ethylene monomer was
around 1.10
kg/cm2. The bed level was allowed to increase slightly to between about 0 to 1
m above the
neck of the reactor. The new catalyst, XCATTm EZ-100 Catalyst, a bisindenyl
zirconium-based
metallocene catalyst available from Univation Technologies, was then
introduced to the reactor
at a feed rate of about 60% the targeted steady state feed rate. The target
superficial gas velocity
during introduction of the new catalyst was about 0.68 to 0.72 m/s. It was
observed that the
polyethylene reaction started again within about 1 hour of introducing the
XCATTm EZ-100
Catalyst feed. In total, the entire transition was completed in about 14
hours. The transition did
not result in any observable degree of sheeting or fouling within the reactor,
and there was no
drop in pressure observed across the distributor plate of the reactor on start-
up or on reaching
steady state.
84111646
[0096] All numerical values are "about" or "approximately" the indicated
value, and take into
account experimental error and variations that would be expected by a person
having ordinary
skill in the art.
[0097]
[0098] While the foregoing is directed to embodiments of the present
invention, other and
further embodiments of the invention may be devised without departing from the
basic scope
thereof, and the scope thereof is determined by the claims that follow.
Date Recue/Date Received 2022-09-12