Note: Descriptions are shown in the official language in which they were submitted.
CA 02981867 2017-10-04
WO 2016/164930 PCT/US2016/026998
1
SYSTEM AND METHOD FOR IRREVERSIBLE ELECTROPORATION WITH
THERMALLY CONTROLLED ELECTRODES
Cross Reference to Related Applications
[0001] The present application incorporates by reference the entire
disclosures of U.S.
Provisional Patent Application 62/145,581, filed on April 10, 2015; U.S.
Provisional Patent
Application 62/151,513, filed on April 23, 2015; U.S. Provisional Patent
Application
62,173/538, filed on June 10, 2015; and U.S. Non-Provisional Patent
Application 12/437,843,
entitled Electroporation Device and Method of Use, filed May 8, 2009.
Background
[0002] Irreversible electroporation (IRE) and other electroporation-based
therapies
(EBTs), such as electrochemotherapy and electrogenetherapy, use the delivery
of brief but
intense electric pulses delivered into tissues through a number of electrodes
to treat an intended
treatment zone of tissue. These electric pulses subject cells in tissue to an
electric field, which
alters their native transmembrane potential, and at sufficient strength
results in the creation of
nanoscale defects that facilitate macromolecule transport and disruption of
the membrane's
ability to maintain cellular environment homeostasis. When the strength of the
pulsing protocol
is sufficient, the cell cannot recover from these defects and dies. EBTs
encompass a range of
therapeutic applications that exploit this phenomenon, particularly in regard
to treatment of
diseases in human or animal patients. The invention described herein is
directed towards
irreversible electroporation treatment; however it is conceivable to be
applied to all types of
EBTs.
[0003] The delivery of IRE pulses and the effect of these pulses on tissue
has been
previously described and documented, for example: U.S. Patent 7,765,010, filed
February 6,
SUBSTITUTE SHEET (RULE 26)
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
2
2006, entitled APPARATUS AND METHOD FOR TREATMENT OF BENIGN PROSTATIC
HYPERPLASIA; U.S. Patent 8,048,067, filed December 21, 2004, entitled TISSUE
ABLATION WITH IRREVERSIBLE ELECTROPORATION; U.S. Patent 8,114,070, filed June
24, 2005, entitled METHODS AND SYSTEMS FOR TREATING BPH USING
ELECTROPORATION; U.S. Patent 8,251,986, filed July 10, 2009, entitled METHOD
OF
DESTROYING TISSUE CELLS BY ELECTROPORATION; U.S. Patent 8,282,631, filed
September 20, 2011, entitled TISSUE ABLATION WITH IRREVERSIBLE
ELECTROPORATION; U.S. Patent 8,634,929, filed June 22, 2010, entitled METHOD
FOR
TREATMENT OF NEOPLASTIC CELLS IN THE PROSTATE OF A PATIENT; and U.S.
Patent 9,078,665, filed September 28, 2012, entitled MULTIPLE TREATMENT ZONE
ABLATION PROBE; all of which are hereby incorporated by reference. The
following
reference is related to the subject matter of the present invention and
incorporated herein in its
entirety: U.S. Patent No. 5,951,546, filed September 30, 1997; entitled
"ELECTROSURGICAL
INSTRUMENT FOR TISSUE ABLATION, AN APPARATUS, AND A METHOD FOR
PROVIDING A LESION IN DAMAGED AND DISEASED TISSUE FROM A MAMMAL".
[0004] Current state of the art commercially available IRE treatment
devices do not use
perfusion or other cooling fluid to control the temperature of the tissue or
probe within the
treatment site. While the possibility of using IRE and other EBTs has been
well known as a
means to mitigate thermal damage during a treatment, the actual effect on
treatment outcome in
terms of ablation size and damage due to thermal effects has only been
recently discussed in the
literature, for example Davalos, et al, "IMPLICATIONS AND CONSIDERATIONS OF
THERMAL EFFECTS WHEN APPLYING IRREVERSIBLE ELECTROPORATION TISSUE
ABLATION THERAPY" The Prostate, published by Wiley Periodicals, Inc.; 2015. As
will be
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
3
discussed in more detail below, this invention discloses the use of perfusion
combined with other
key novel aspects of the system to solve problems associated with the
commercially available
IRE treatments options.
[0005] Additional concerns and/or challenges with the current IRE
treatment devices
known and used in the art may include: electrode arcing potential during a
treatment resulting in
a system crash/failure leading to lengthening of overall procedure time and/or
inability to
complete the procedure; unintended rise in temperature adjacent to the probe;
restriction on
certain probe placements due to complexity; difficulty in aligning two or more
monopolar
probes on a parallel axis and maintaining consistent probe insertion depth;
tight tolerances
required in spacing of multiple probe electrodes; difficulty in measuring the
size of the treatment
site when determining treatment parameters; requirement to navigate around
anatomical
obstacles (such as bone, spleen, or other non-target tissue); and
unintentional flexing of the probe
shaft during placement in the treatment site, resulting in misalignment of
electrodes relative to
each other.
[0006] Another problem with current commercially available IRE treatment
systems is
the high number of total pulses delivered for IRE therapies which, depending
on the patient and
tissue conditions, may result in significant cumulative and undesirable
thermal effects. For
example, some pieces of literature in the art, for example Wagstaff PGK, et
al,
"IRREVERSIBLE ELECTROPORATION OF THE PORCINE KIDNEY: TEMPERATURE
DEVELOPMENT AND DISTRIBUTION" Elsevier; 2014, reported that currently accepted
IRE
treatment parameters may result in temperature levels as high as 59 C in
regions between several
pulse pairings at even typical pulse protocols. In addition to thermal damage
which may mitigate
or eliminate the benefits of IRE as a non-thermal procedure, an unintended
temperature rise may
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
4
also change the properties of the tissue and thus alter treatment outcome. In
addition, thermal
issues may add to arcing potential when applying desired treatment parameters.
[0007] A key advantage of the system described herein over other
currently known IRE
treatment devices includes the use of perfusion together with the delivery of
a specific or
customized pulse parameters to achieve clinically acceptable ablation sizes
using a single bipolar
probe while reducing the overall risk of arcing and overall procedure time.
For example, a single
stick bipolar probe of this invention can be used to create the same
clinically acceptable ablation
sizes compared to multiple monopolar probes.
Field of the Invention
[10008] The disclosure generally relates to the systems and method for
delivery of
electrical pulses to treat an intended treatment site. The system may also
comprise of a bipolar
probe with a perfusion system and ability to control pulse parameters.
Summary of the Disclosure
[0009] This disclosure is based upon the concept of temperature
control for IRE therapies
to improve treatment outcome. The intentions of this invention include
eliminating possible
thermal damage or the effects of creating a thermal zone near the treatment
site, improving pulse
stability, reducing arc potential, and facilitating larger IRE ablation zones
by permitting greater
voltages and larger total pulse energy protocols, without causing a
significant increase in targeted
tissue temperature. An additional advantage is confirmation and demonstration
of reducing the
extent of thermally affected tissues as a result of perfusion. Reducing or
eliminating the extent of
thermal damage improves IRE's morbidity profile in therapeutic applications
and further ensures
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
that the bulk of ablated tissue does not include thermal damage to critical
sensitive structures
such as blood vessels, neurovascular bundles, or ductal systems.
[0010] In one aspect of the invention, a medical device is provided
for ablating tissue
cells in a treatment region by irreversible electroporation without thermally
damaging the tissue
5 cells, comprising: a temperature controlled perfusate; an electrode probe
having a perfusate
channel for receiving the temperature controlling perfusate and at least two
electrodes adapted to
apply irreversible electroporation (IRE) pulses to the tissue cells in the
treatment region; a
control device for controlling the IRE pulses to the at least two electrodes
and operable to
provide the temperature controlling perfusate to the perfusate channel of the
probe to maintain
the temperature of the targeted tissue cells between 20 degrees Celsius and 50
degrees Celsius.
[0011] In one aspect of the invention, a medical device is provided
wherein a temperature
controlling perfusate controls an electrical conductivity rise in the tissue
cells sufficiently to
eliminate electrical arcing, but without significantly altering the electric
field distribution and
treatment zone.
[0012] In one aspect of the invention, a medical device is provided wherein
a control
device provides the temperature controlling perfusate to the perfusate channel
to maintain the
temperature of the tissue cells at between 30 degrees Celsius and 45 degrees
Celsius.
[0013] In one aspect of the invention, a medical device is provided
wherein an electrode
probe includes a temperature sensor that measures the temperature of the
targeted tissue cells and
the control circuit adjusts the amount of the temperature controlling
perfusate to the perfusate
channel in real-time based on the monitored temperature.
[0014] In one aspect of the invention, a medical device is provided
comprising a power
distribution unit.
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
6
[0016] In one aspect of the invention, a medical device is provided
comprising a pump
coupled to the control device, wherein the control device controls the pump to
vary the flow rate
of the temperature controlling perfusate.
[0016] In one aspect of the invention, a medical device is provided
comprising a pulse
generator capable of generating the IRE pulses wherein the IRE pulses in one
train between the
two electrodes has a first polarity and the IRE pulses in an adjacent train
has a second polarity
opposite from the first polarity.
[0017] In one aspect of the invention, a medical device is provided
wherein a control
device monitors the current flow through the at least one electrode and
provides the temperature
controlling perfusate to the perfusate channel based on the monitored current
flow.
[0018] In one aspect of the invention, a medical device is provided
wherein a control
device monitors the current flow through the at least one electrode and
provides the temperature
controlling perfusate to the perfusate channel based on the rate of change of
the monitored
current.
[0019] In one aspect of the invention, a medical device is provided wherein
an electrode
probe includes fluid ports along its distal end, wherein the temperature
controlling perfusate is
injected into the target tissue cells through the fluid ports.
[0020] In one aspect of the invention, a medical device is provided
wherein a control
device calculates tissue conductivity based on the current flow through the at
least one electrode.
[0021] In one aspect of the invention, a medical device is provided wherein
a control
device applies a test pulse through the electrode and calculates the tissue
conductivity based on
the current flow from the applied test pulse.
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
7
[0022] In one aspect of the invention, a medical device is provided
comprising a
temperature sensor that senses the temperature of the target region, and a
control device which
calculates tissue conductivity based on the sensed temperature.
[0023] In one aspect of the invention, a medical device is provided
wherein a control
device controls the flow of the temperature controlling perfusate through the
perfusate channel
based on the number of IRE signals, current or the amount of power applied to
the target region.
[0024] In one aspect of the invention, a medical device is provided
comprising a memory
that stores at least one electrical parameter for a plurality of tissue types
and the control device
controls the flow of the temperature controlling perfusate through the
perfusate channel based on
the at least one electrical parameter for the type of tissue cells being
treated.
[0025] In one aspect of the invention, a medical device is provided
comprising: a
pumping device that controls the flow rate of the temperature controlling
perfusate through an
source tube and return tube; wherein the pumping device is controlled by the
control unit.
[0026] In one aspect of the invention, a medical method is provided
for ablating tissue
cells in a treatment region by irreversible electroporation without thermally
damaging the tissue
cells, comprising: applying irreversible electroporation (IRE) signals to the
tissue cells in the
treatment region through at least one electrode of an electrode probe;
providing a temperature
controlling perfusate to a perfusate channel of the electrode probe to
maintain the temperature of
the tissue cells at either 5 degrees Celsius or greater or 50 degrees Celsius
or less.
[0027] In one aspect of the invention, a medical method is provided for
ablating tissue
cells in a treatment region by irreversible electroporation without thermally
damaging the tissue
cells, comprising: applying irreversible electroporation (IRE) signals to the
tissue cells in the
treatment region through at least one electrode of an electrode probe;
providing a temperature
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
8
controlling perfusate to a perfusate channel of the electrode probe to
maintain the temperature of
the tissue cells at 45 degrees Celsius or less.
[0028] In one aspect of the invention, a medical method is provided
comprising a method
of ablating tissue cells in a treatment region by irreversible electroporation
without thermally
damaging the tissue cells wherein the step of providing includes providing the
temperature
controlling perfusate to the perfusate channel to maintain the temperature of
the target tissue
cells at body temperature.
[0029] In one aspect of the invention, a medical method is provided
comprising a method
of ablating tissue cells in a treatment region by irreversible electroporation
without thermally
damaging the tissue cells further comprising: controlling an electrical
conductivity rise in the
tissue cells sufficiently to eliminate electrical arcing with the temperature
controlling perfusate;
the step of eliminating electrical arcing significantly altering the electric
field distribution.
[0030] In one aspect of the invention, a medical device is provided
for ablating tissue
cells in a treatment region by irreversible electroporation without thermally
damaging the tissue
cells, comprising: an electrode probe having first and second spaced apart
electrodes; a pulse
generator that generates IRE pulses as follows: a first row of pulses
consisting of a first pulse
train and a second pulse train, the first pulse train consisting of at least
five individual pulses, the
first pulse train having a first polarity, an inter-train delay of at least 2
second, the second pulse
train consisting of at least five individual pulses, the second pulse train
having a second polarity
that is the opposite of the first polarity, an inter-row delay of up to at
least 10 second, and a
second row of pulses consisting of a third pulse train and a fourth pulse
train.
Brief Description of the Drawings
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
9
[0031] Figure 1 shows a functional block diagram of an
electroporation system
contemplated for the current invention.
[0032] Figure 2 shows a perspective view of one embodiment of the
probe.
[0033] Figure 3A shows an exploded view of the probe.
[0034] Figure 3B ¨ Figure 3H show partial side cross-sectional views of the
probe shaft
components at different stages of assembly.
[0035] Figure 4 shows a partial side view of the distal end of the
probe.
[0036] Figure 5 depicts a partial side view of the distal end of the
probe with zone of
arcing potential.
[0037] Figure 6 shows a partial side view of the distal end of the probe
with the predicted
ablation zone.
[0038] Figure 7 shows a partial perspective view of the perfusion
system.
[0039] Figure 8 shows a partial perspective view of the hub of the
perfusion system.
[0040] Figure 9 shows a partial perspective cross-sectional view of
the hub of the
perfusion system.
[0041] Figure 10A shows a partial cross-sectional side view of the
probe handle.
[0042] Figure 10B shows a partial perspective cross-sectional view of
the distal end of
the probe.
[0043] Figure 10C shows a side cross-sectional view of the probe.
[0044] Figure 10D shows a partial side cross-sectional view of fluid path
within the
probe handle.
[0045] Figure 11 depicts a side view of the distal region of another
embodiment of the
probe.
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
[0046] Figure 12 depicts a partial cross-sectional side view of the
distal regions of yet
another embodiment of the probe.
[0047] Figure 13 depicts a functional block diagram of yet another
embodiment of an
electroporation system contemplated of the current invention.
5 [0048] Figure 14 depicts a schematic representation of the
power distribution unit with
controller and generator interfaces.
[0049] Figure 15 shows a table of a simulation using research results
depicting ablation
volumes at varying temperatures contemplated for the current invention.
[0050] Figure 16 shows a line chart of a simulation using volume of
temperature
10 exposure versus temperature threshold for varying perfusate temperatures
contemplated for the
current invention.
[0051] Figure 17 shows a line chart of a simulation using volumes of
exposure versus
perfusate temperature at multiple temperature thresholds contemplated for the
current invention.
[0052] Figure 18 shows a chart of specific parameters of IRE energy
pulse delivery
contemplated for the current invention.
[0053] Figure 19 depicts a flowchart showing the method of IRE
delivery contemplated
for the current invention.
Detailed Description of the Invention
[0054] The present invention can be understood more readily by reference to
the
following detailed description and the examples included therein and to the
figures and their
previous and following description. The drawings, which are not necessarily to
scale, depict
selected preferred embodiments and are not intended to limit the scope of the
invention. The
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
11
detailed description illustrates by way of example, not by way of limitation,
the principles of the
invention.
[0055] The skilled artisan will readily appreciate that the devices
and methods described
herein are merely exemplary and that variations can be made without departing
from the spirit
and scope of the invention. It is also to be understood that the terminology
used herein is for the
purpose of describing particular embodiments only and is not intended to be
limiting.
[0056] As used herein, the term "proximal" denotes the direction
closer to the operator
and the term "distal" denotes the direction closer to (inserted into) the
patient.
[0057] As used herein, the term "perfusate" means a non-corrosive,
sterile physiologic
fluid such as distilled water, saline solution, buffer solution, gas (such as
CO2) such as a
dextrose buffer, or LRS (lactated ringer's solution), Hartmann's solution or
any combination
thereof. The term "perfusion" means circulating or pumping the perfusate so
that the perfusate
enters into the fluid channels within the probe and circulating or pumping the
perfusate through
the probe such that the perfinate is injected, infused or enters into the
tissue within the treatment
zone. Perfusion may also include controlling the temperature or conductivity
of the perfusate,
controlling to internal and surrounding temperatures of the probe, and
infusion of the perfusate
into the tissue such that the perfusate interacts with the cells of the tissue
within the treatment
zone.
[0058] As disclosed herein, the reference to "electrodes" may include
physically discrete
components that serve to deliver the electric pulses, but it can also indicate
individual energized
surface components within a single device, such as a bipolar electrode or an
electrode with
individually energizable surfaces, such as electrically isolated tines or
conducting wires in a
tubular catheter-style device. The latter style of electrodes would
particularly benefit from being
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
12
able to fine tune control of the pulse delivery, and can often times include
more than six
individual surfaces in which to deliver the electric pulses. The electrodes of
this invention may
also be used together with a grounding pad. In one embodiment, the grounding
pad may be
intended to be placed on the surface of the treatment tissue while an
electrode is inserted into or
adjacent to the treatment tissue.
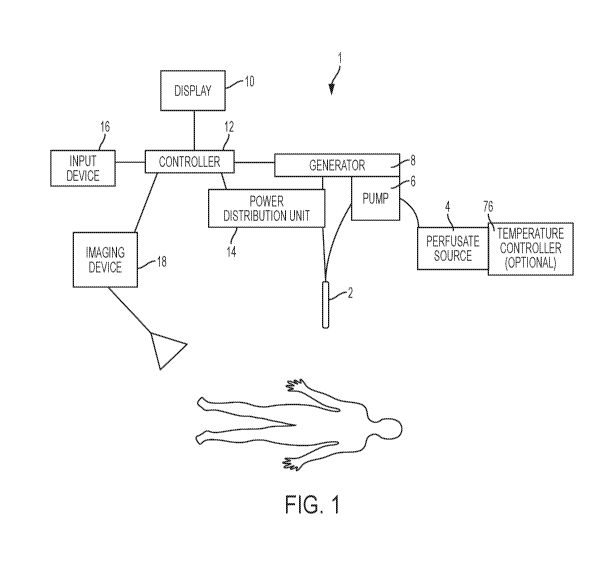
[0059] Referring now to Fig. 1, the system 1 of this disclosure may
include, but is not
limited to, the following elements: a disposable probe 2, a perfusate source
4, a pump 6, an
optional temperature controller 76, a generator 8, a display 10, a controller
12, a power
distribution unit 14, an input device 16, and an imaging device 18. These
various components are
designed to work together and be integrated into a single treatment system.
The treatment system
1 is designed to be used to perform an irreversible electroporation procedure,
however it is
possible that the system 1 may be used for other EBTs. While not all
components of this system
1 or kit may be packaged, shipped, or sold together, it is still understood
that the various
components will work together as a single system 1. For example, it may be
common for the
imaging device 18 to be used with this system 1 to be an ultrasound device,
MRI system, or
another commonly known imaging device that is off the shelf, or otherwise
already used in a
hospital setting. However, the system may be designed such that it can
incorporate such an
imaging device 18 into the system 1, or alternatively it can interface with
the controller 12, such
that the information or feedback received from the imaging device 18 may be
used by the user of
system 1.
[0060] The system 1 may also include one or more probes 2. The probe
2 may be
operably connected to a pump 6 and also connected to a power distribution unit
14 and/or a
generator 8. The probe 2 is intended to deliver therapeutic energy to the
patient. In one
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
13
embodiment, the probe 2 is designed to be inserted into the patient's body
such that the probe 2
is within the desired treatment site. Alternatively, the probe 2 may be placed
on the outside
surface of the patient's body. The probe 2 of this system may include, but is
not limited to, a
bipolar probe having at least two electrodes on the probe 2, multiple
monopolar probes having at
least one electrode, or a single monopolar probe having at least one electrode
on the probe 2 for
use with a grounding pad placed externally on the patient's skin. A perfusate
source 4 may
provide perfusate fluid through the pump 6 to the probe 2. A computer
including a user display
10, input device such as a keyboard 16, and controller 12 may be used to input
instructions
and/or treatment parameters which are transmitted to generator 8 / power
distribution unit 14 to
generate specific pulse trains to the probe 2. An optional imaging device 18,
used to visualize the
treatment area prior to, during and/or after the pulse delivery, may be
separate or integrated with
the system. An optional temperature control unit 76 is in communication with
the probes 2 via a
thermocouple or other sensing component to monitor temperature in and/or
around the probe,
and allow automatic or manual adjustment to the generator 8 parameters and/or
perfusion flow
rate based on the temperature monitoring.
[0061] Referring now to Figs. 2 - 5, one embodiment of this invention
includes a bipolar
probe 2. A major advantage of the bipolar probe 2 of this system is the ease
of use during
placement of the probe 2 prior to treatment, compared with placement of
multiple monopolar
probes. Because the probe 2 is bipolar and incorporates at least two
electrodes 32, 34, the user
only needs to place a single probe 2 into the intended treatment site in order
to achieve clinically
useful ablation volumes, compared to commonly known commercial IRE devices in
which
require placement of multiple monopolar probes. The bipolar probe 2 combined
with the specific
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
14
pulse parameters and infusion of perfusate, both described in more detail
below, result in more
clinically useful treatment outcome with larger ablation zones.
[0062] Proper probe placement is a key aspect of a successful IRE
procedure. The user
must make a decision as to the best placement for the probes relative to the
treatment site. A
current commercially available treatment typically requires anywhere from two
to six monopolar
probes to be placed in the patient. These monopolar probes have a shaft
extending from the
handle to a distal end section where a single monopolar electrode is position.
These single
monopolar probes are commonly placed with a gap of at least lcm and up to 2.5
cm between
each probe. Furthermore, each monopolar probe may have up to a 2 cm active
electrode
exposure length. A problem currently exists in the art related to the
complexity and precision
required when placing these monopolar probes, specifically related to the
current systems
required for alignment of multiple probes relative to each other along an x-
axis, y-axis, and z-
axis. It is common for a user to spend significant amounts of time planning
proper probe
placement and then accurately placing the probes based on the planned location
before the
treatment can even begin. Moreover, ensuring precise probe placement is
critical for a successful
and complete IRE treatment session. For example, if the single monopolar probe
is
mispositioned or misdeployed relative to planned placement and/or misaligned
relative to
another monopolar probe, this may lead to potential complications including
unpredictable
ablation zones; unknown treatment outcomes; and a greater potential for arcing
which may lead
to system failures. Thus, proper probe placement of multiple probes is one of
the most significant
clinical challenges today when conducting an IRE procedure.
(0063] This invention solves a need in the art to simplify probe
placements, thereby
reducing treatment times and potential for unintended complications, saving
the user and hospital
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
time and money, and benefiting the patient by increasing the likelihood of a
successful treatment.
One of the primary benefit of only having to place a single bipolar probe 2
within the treatment
site is a reduction in the overall time required for probe placement planning
and actual
positioning, thereby reducing overall procedure time which saves the
doctor/hospital money and
5 reduces unwanted or unintended risks associated with anesthesia to the
patient. Additionally, the
use a bipolar probe 2 does not require alignment of multiple monopolar probes
in a parallel
arrangement which is often difficult to achieve, leading to incomplete or
unintended ablation
zones. A single bipolar probe is also advantageous in that only a single
puncture is needed, less
imaging is required, as well as providing the user with more flexibility in
probe placement
10 around bone or other non-targeted structures. The bipolar probe 2 of
this system, combined with
the treatment parameters and treatment method described below has been shown
to create a more
predictable, consistent, and larger ablation zone compared ablations created
using multiple
monopolar probes. The use of a single bipolar probe 2 provides more
predictability in the
geometry of the delivery device thereby simplifying the pulse parameter
selection and allows for
15 tight ablation dimension tolerances to be achievable.
[0064] Referring now to Fig. 2, the probe 2 assembly may include a
handle 20 having a
proximal end 22 and a distal end 24, an elongated probe body 26 that extends
from the distal end
24 of the handle towards the treatment zone. The probe body 26 has a proximal
end 28, which
extends into the handle 20 for a selected distance, and a distal end 30. The
probe 2 further
comprises at least two electrodes 32, 34 near the distal end 30 of the probe
body 26. These at
least two electrodes 32, 34 are designed such that they are in a spaced
relationship with one
another along the probe body 26 with an insulated spacer 36 positioned between
electrodes 32,
34. The probe 2 may also include a distal tip 38 which is capable of piercing
through skin and
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
16
other tissue such that the probe may be percutaneously or interoperatively
placed in the treatment
zone. The distal tip 38 may be made of a non-conductive material, or
alternatively in some
embodiments may be made of a conductive material and act as an electrode.
[0065] The electrodes 32, 34 of the probe 2 may be designed such that
they are
independently activated electrodes on the surface of the probe body 26. Each
electrode 32, 34
may be capable of switching between a positive polarity and a negative
polarity during a single
IRE treatment.
[0066] Referring now to Fig. 3A ¨ Fig. 4, additional components of
the probe 2 may
include a perfusate channel 40, a first conductor tube 41, a first electrode
34, a first insulator tube
42, a spacer element 36, a second conductor tube 44, a second electrode 32, a
second insulator
tube 46, a distal tip 38, a first perfusate tube 48, a second perfusate tube
50, a power cable tube
52, and a power cable 54. One embodiment of manufacturing the probe body 26 is
shown in
Figures 3B ¨ 3H.
[0067] Referring first to Figures 3B ¨ 31), the perfusate channel 40
is coaxially placed
inside of the first conductor tube 41. The empty space between the outer wall
of the perfusate
channel 40 and the inner wall of the first conductor tube 41 comprises the
coaxial return lumen
89. The first electrode 34 is securely attached to the distal end of the first
conductor tube 41 by
welding, adhesive, or other known techniques in the art. Next, the distal tip
38 is securely
attached to the distal most end of the first conductor tube 41 by an
interference fit, welding,
adhesive, or other known techniques in the art. The distal tip 38 is connected
to the first
conductor tube 41 such that there is a fluid tight connection to prevent any
perfusate from
escaping out the distal most end of the first conductor tube 41.
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
17
[0068] Referring next to Fig. 3E ¨ 3F, a spacer 36 is coaxially
placed over the first
conductor tube 41. The distal most end 56 of the spacer 36 abuts against the
proximal most end
58 of the first electrode 34. The first insulator tube 42 is then coaxially
placed over the first
conductor tube 41 such that the distal most end 45 of the insulator tube 42
abuts up against the
proximal most end 66 of the spacer 36. As shown in Fig. 3G ¨ 3H, the second
conductor tube 44
is then coaxially placed over the first insulator tube 42. The second
electrode 32 is securely
attached to the distal most end of the second conductor tube 44 by welding,
adhesive, or other
known techniques in the art. The distal most end 64 of the second electrode 32
abuts up against
the proximate most end 66 of the spacer 36. Lastly, the second insulator tube
46 is coaxially
placed over the second conductor 44 such that the distal most end 70 of the
second insulator tube
46 abuts up against the proximate most end 68 of the second electrode 32.
[0069] The probe 2 may be designed to have a relatively constant
outer diameter along
the shaft length such that there is a smooth transition between the outer wall
of the second
insulator tube 46, the second electrode 32, the spacer 36, the first electrode
34 and the proximal
portion of distal tip 38, as shown in Fig. 4. The purpose of this smooth
transition is so that the
probe may have an easy insertion through tissue during placement. A
temperature sensor may be
placed at any place along the probe body, such as near the distal end near the
first electrode, the
second electrode, or the spacer.
[0070] The perfusate channel 40 may be made from such material as
stainless steel or
other non-corrosive metals or rigid materials. The first 41 and second
conductor tubes 44 may be
made from such materials as stainless steel or other non-corrosive metals or
rigid materials. The
first 42 and second insulator tubes 46 may be made from such materials as
polyimide, heat
shrink, or other electrically insulating materials. The spacer 36 may be made
from such material
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
18
as PEEK plastic, ceramic, or other rigid electrically insulating materials.
The distal tip 38 may be
made from such material as PEEK plastic, ceramic, or other rigid electrically
insulating
materials. In an alternative embodiment, the distal tip 38 may be comprised of
a conductive
material, if tip 38 is intended to act as one of the electrodes. The first 48
and second 50 perfusate
tubes may be made from such materials as PVC, PTFE, or other flexible
biocompatible polymer
tubing.
[0071] It is the intention of this system and method described herein
to solve problems
associated with undesirable thermal effects when delivering IRE treatment. The
present
invention achieves an optimal balance of (I) creating the largest ablation
volume possible against
(2) maintaining a threshold temperature in the target area which ensures that
no thermal damage
occurs, especially in those tissue areas adjacent to the active electrodes
where tissue desiccation
is possible. By maintaining this balance between ablation volume and
temperature, the system is
less likely to generate arcing conditions at the electrodes. In one embodiment
the present
invention uses perfusion to control the temperature of the tissue immediately
surrounding the
electrodes within a relatively narrow range of 20 ¨ 45 C, specifically 30 ¨40
C. The upper limit
of this controlled temperature range eliminates the possibility of thermal
damage to the tissue
and other cellular structures, and reduces arcing or sparking between
electrodes, while the lower
limit of this controlled temperature range ensures maximum ablation volume at
the lower tissue
temperatures.
[0072] Advantages of the perfusion of this system include mitigating the
extent of
unintended tissue thermal damage when delivering IRE pulses and preventing
arcing between
electrodes and the resultant generator faulting. Perfused probes result in a
reduction in the bulk
tissue temperature rises and maximum temperatures proximate to the electrodes.
This
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
19
dramatically reduces the extent of unintended thermal damage, including the
variety of thermal
damage that risks morbidity to the sensitive cellular structures.
[0073] One definition of arcing may include a discharge of matter
between two
electrodes that is caused by electrical current ionizing gasses. Arcing occurs
when a medium of
high resistance, low conductivity, exists in the path that current would
normally flow, which is
along the path of least resistance. One possible reason for arcing during an
IRE procedure may
be the result of ionic movement towards the positively charged electrode. Ions
within the soft
tissues are negatively charged or positively charged. Negatively charged ions
will flow towards
the positively charged electrode(s) during the IRE procedure, thus potentially
leaving behind an
empty space or an air gap, at the negatively charged electrode. Positively
charged ions will flow
towards the negatively charged electrode(s) during the IRE procedure, thus
potentially leaving
behind an empty space or air gap, at the positively charged electrode. If more
negatively charged
ions are present in the tissue, there could be a greater possibility for air
pockets to form nearest
the negatively charged electrode. Air pockets increase resistance thus
contributes to likelihood of
arcing.
[0074] If arcing occurs during an IRE procedure it commonly occurs at
the shortest
distances between each electrode, or said another way, it will occur where the
electrodes are
closest together because this is the path of least resistance. As shown in
Fig. 5, the bipolar probe
of this invention would have the highest occurrence of arcing at the distance
72 between the
distal most end 64 of the first electrode 32 and the proximal most end 58 of
the second electrode
34. To mitigate such a risk of arcing at this distance 72, this system uses:
(i) perfusion for
temperature control at distance 72 along probe 2 with greatest risk of arcing;
in combination with
(ii) a specific pulse parameter setting that alternates polarity of the
electrodes 32, 34 throughout a
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
single IRE treatment, which will be discussed in more detail below.
Alternating polarity may
reduce to potential charge in the tissue thereby reducing the arcing
potential.
[0075] Perfusion prevents or reduces the likelihood of the generator
from arcing or
faulting by improving pulse stability and reducing electrical current and
arcing incidence. While
5 electric current and arcing are often correlated, they are in fact two
different modes by which the
electroporation generator can fail. A reduction in voltage delivered to the
tissue also reduces the
power being delivered to the tissue, thereby reducing the likelihood of arcing
potential. Perfused
electrodes address both of these failure modes while increasing the voltages
that can be delivered
to achieve a larger ablation or treatment zone. The probe 2 of this system 1
may include a bipolar
10 electrode probe 2 that, when used together with the system 1 and the
method described below,
can consistently attain clinically useful ablation dimensions with
reduced/eliminated arcing.
Clinically useful ablation dimensions will vary based on tumor modality and
location. As a non-
limiting example, a clinically useful ablation size for a typical liver tumor
may be the size of
greater than 3 cm, but may include the specific treatment zone of at least 5cm
by 3.5 cm, as
15 shown in Figure 6, which is equivalent to the ablation zone achievable
in current commercially
available IRE device which uses at least two monopolar probes.
[0076] Referring now to Figure 7 ¨ Figure 12, the perfusate system 74
will be described
in detail. The perfusate system 74 may be comprised of a perfusate source 4,
an optional
temperature control unit 76, a pump 6, a fluid spike 78 or other attachment
for connecting to a
20 perfusate source 4, a source perfusate tube 80, a return perfusate tube
81, a hub 82, a first
perfusate tube 48, and a second perfusate tube 50. The purpose of the
perfusate system 74 is to
control the temperature of the probe 2 and/or tissue within the treatment
zone. Temperature
control has been found to correlate with arcing potential, so it is within the
scope of this
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
21
invention to prevent potential arcing of the system by using the perfusate
system 74 to control
the temperature of the probe 2 and treatment site during an IRE procedure.
Various components
of the perfusate system 74 may be multi-use such that they can be used for
different patients,
such as the pump 6, temperature controller 76, and even the perfusate source 4
in certain
embodiments. The perfusate system 74 may require a priming sequence that can
be controlled by
the GUI and/or controller 12.
[0077] As will be discussed in more detail below, the temperature of
the perfusate may
vary depending on the type of IRE treatment being performed, the type of
tissue to be treated, or
the specific pulse parameters to be used. The system may optionally include a
temperature
controller 76 that is in communication with the system controller 12 which may
monitor the
temperature levels of the perfusate, probe and/or surrounding tissue, and
automatically adjust
temperature levels to minimize arcing potential which simultaneously maximizes
tissue ablation
volumes. The temperature controller 76 may also heat up the perfusate to body
temperature,
maintain the perfusate temperature at room temperature, and/or cool the
perfusate to any
temperature above freezing. Conversely, if the system is to use room
temperature perfusate then
the temperature controller 76 may not be a required part of the perfusate
system 74.
[0078] The pump 6 may include any number of commercially available
pumps that are
commonly known in the art such as a peristaltic pump, centrifugal pump, roller
pump, piston
driven pump, or other known pumping mechanisms. One advantage of this system
is its compact
footprint. The purpose of this compact design is to allow the users the
greatest amount of
flexibility when it comes to storing, using, and moving this system. Because
this system is
intended to be functional and compact, one embodiment of this system is for
the pump 6 is
assembled together with the generator 8 into a single housing (not shown).
Such a design would
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
22
achieve a compact single box or control unit that could easily be moved and
would not take up a
large footprint inside the hospital or clinical setting. For example, such an
integrated pump and
generator system is described in U.S. Provisional Patent Application Number
62/238,299, filed
October 7, 2015, hereby incorporated by reference.
[0079] As shown in Figure 8, the hub 82 is a junction point in which the
source perfusate
tube 80, the return perfusate tube 81, and the power cables 54 transition into
the first perfusate
tube 48, the second perfusate tube 50, and the power cable tube 52. The
purpose of the hub 82 is
to increase usability and user efficiency of the system. In one embodiment the
power cable tube
52, the first perfusate tube 48 and second perfusate tube 50 are connected or
joined together
during manufacturing, thereby eliminating multiple loose cables and tubing
extending probe
proximal end of the handle 20. The power cables 54 may be comprised of a first
power cable 84
and a second power cable 86. The first power cable 84 may be connected to the
generator 8 or
other power source and provides a conduit for the flow of electrical current
to the first electrode.
The second power cable 86 may be connected to the generator 8 or other power
source and
provides a conduit for the flow of electrical current to the second electrode.
The first power cable
84 and the second power cable 86 may be aligned within hub 82 so that both
power cables 54
extend within and co-axially along the power cable tube 52. The source
perfusate tube 80 is in
fluid communication with the spike 78 and the perfusate source 4. The source
perfusate tube 80
will be placed within the pump head 88. In one embodiment, the pump 6 is a
peristaltic pump as
known in the art and the source perfirsate tube 80 is aligned on top of the
rollers of the pump
head 88. In this embodiment, the return perfusate tube 81 would not be aligned
within the pump
head 88 but rather routed around the pump head 88, as shown in Figure 7, and
back to either a
waste container (not shown) or the perfusate source so that used perfirsate
can be reused.
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
23
[0080] Within the hub 82 the source perfusate tube 80 is aligned with
and connected to
the first perfusate tube 48 so that the source perfusate tube 80 is in fluid
communication with the
first perfusate tube 48. Also within the hub 82 the second perfusate tube 50
is aligned with and
connected to the return perfusate tube 81 so that the second perfusate tube 50
and return
perfusate tube 81 are in fluid communication. The first power cable 84 and the
second power
cable 86 may be twisted, combined, or otherwise connected together to form the
power cables 54
that extend within the power cable tube 52.
[0081] The flow of perfusate within this system will depend on if it
is an "open" system
or a "closed" system. For example, a "closed" system is one which the
perfusate only circulates
inside of the probe and does not enter into the tissue within the treatment
zone. Conversely, an
"open" system refers to a perfusion system in which the perfusate is directly
injected or infused
into the tissue within the treatment zone. Embodiments of both an "open" and
"closed" system
are described in more detail below.
[0082] Referring now to Figs. 7 ¨ 10D, the first embodiment is a
closed system in which
the perfusate is circulated within the probe body and is not introduced into
the surrounding
tissue. The purpose of a closed perfusate system in which the perfusate is
circulated and
contained within the probe 2 is so the continuous circulation of perfusate may
control the
temperature of the probe 2 and/or electrodes 32, 34 at the hottest or highest
temperature points,
thereby reducing sudden current inflections and/or arcing. Therefore, the
temperature of the
perfusate used with the closed system will directly control the temperature at
which the probe 2
is intended to be maintained.
[0083] In one embodiment, the flow of the perfusate is contained
within the probe 2 and
designed to be a closed system. The probe 2 may be manufactured with the first
perfusate tube
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
24
48 and second perfusate tube 50 already assembled with the handle 20 so that
the user does not
need to make any fluid connections between the handle 20 and the perfusate
tubing 48, 50. The
flow of perffisate in the closed system of this embodiment originates with the
perfusate source 4.
The perfusate source 4 may be a bag of normal saline or any other perfusate.
First, the user may
prime the perfusate system by placing the spike 78 in the perfusate source 4
to begin the flow of
perfusate. The source perfusate tubing 80 may then be placed on the pump head
88. The return
perfusate tubing 81 may be routed in a channel located external to the pump
head 88 such that
the pump rollers or other pumping mechanism does not compress the return
perfusate tubing 81.
Once the pump 6 is activated, the pump head 88 will force the perfusate to
flow from the
perfusate source 4 into the source perfusate 80 tubing. The perfusate will
continue to flow
through the source tubing 80 and then transition into the first perfusate tube
48 within the
junction point in the hub 82.
[0084] Referring specifically to FIG. 10A, in the handle 20 of the
probe 2, the proximate
end of the perfusate channel 40 is connected to and in fluid communication
with the distal most
end of the first perfusate tube 48. The perfusate will continue to flow
through the first perfusate
tube 48 and then enter into the lumen 51 of the perfusate channel 40; further
illustrated by arrows
in embodiment of Fig. 10D. The perfusate will then flow the entire length of
the perfusate
channel 40 and exit through the open distal end of the channel 40 entering
into the lumen 86 of
the first conductor tube 41, as shown in Fig. 10B. The distal most end 60 of
the first conductor
tube 41 is enclosed by the distal tip 38 as described above; such that the
connection between the
distal most end 60 of the first conductor tube 41 and the distal tip 38
prevents any perfusate from
escaping outside of the probe 2 and into the tissue within the treatment zone.
Since the pump 6 is
continuously pumping perfusate into the system, this constant pumping force
will circulate the
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
perfusate contained within the lumen 86 of the first conductor tube 41 and
force the perfusate to
co-axially flow back up the first conductor tube through the coaxial return
lumen 89 defined by
the outer wall of the first channel 40 and the inner wall of the first
conductor tube 41. This return
lumen 89 extends the entire length of the probe shaft.
5 [0085] As shown in FIG. 10C ¨ 10D, the proximal most end of the
first conductor tube
41 is in fluid communication with the second perfusate tube 50 within the
handle 20 of the probe
2. As the perfusate is forced to flow into the return lumen 89 it will
continue to flow along the
length of the first conductor tube 41 in a distal to proximal direction into
the second perfusate
tube 50; further illustrated by arrows embodiment of Fig. 10D. The perfusate
will continue to
10 flow through the second perfusate tube 50 and then transition into the
return perfusate tube 81
within the junction point in the hub 82, as shown in Figure 9. The return
perfusate tube 81
bypasses the pump head 88 and therefore the perfusate will be passively flowed
into either a
waste container (not shown) or back into the perfusate source 4 to be
recirculated through the
probe's fluid channels.
15 [0086] Referring now to Figs. 11 - 12, other embodiments of the
probe 2 are shown that
include open or infusion designs. The purpose of the open perfusate embodiment
is for the
perfusate to pass through the probe 2 at a distal location and be infused or
injected directly into
the tissue. When the perfinate interacts directly with the tissue it may alter
several aspects or
physical properties of the tissue including, but not limiting to, the
osmolarity, conductivity, and
20 temperature of the interstitial space, as well as any secondary effects
of targeted solutes within
the perfusate such as drugs, immune antigens, or other cytoactive compounds.
[0087] Infusing perfusate through the probe and into the surrounding
tissue may be one
embodiment for solving the problem of arcing. Whereas a closed perfusion
system solves the
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
26
arcing problem by temperature control, the open perfusion system may solve the
arcing problem
by filling the air gaps created within the tissue during an IRE treatment with
the perfusate, which
is more conductive than air. It may be common for air bubbles and gaps to form
during IRE
procedures. The existence of air bubbles within the ablated tissues regions
include, but are not
limited to, the following: (a) the introduction of air when inserting the
probe into the tissues or
(b) the procedure itself, which can generate gasses as a result of the high
voltages (electrolysis)
produced during the procedures. For example, when electrical current passes
through water, the
H20 molecule decomposes into 02 and H2 gasses. Electrolysis can occur in
numerous other
fluids as well. Air is generally highly resistive and could thus lead to
arcing if it exists between
the positive and negative electrodes during an IRE procedure that utilizes a
monopolar probe or a
bipolar probe. Filling these air gaps with a conductive substance, such as a
perfusate, could
potentially decrease the possibility of arcing during an IRE procedure by
lowering the resistance
of the tissue immediately adjacent to the electrodes.
[0088] As seen in Fig. 11, one embodiment of the open perfusion
system includes the
probe 2 having a series of infusion ports 90 along the spacer element 36
between the electrodes
32, 34. The infusion ports 90 may be holes, ports, pressure responsive slits,
or other openings
known in the art. The number of infusion ports 90 may vary depending on how
much perfusate is
desired to be injected or infused into the tissue. As seen in Fig. 12, the
probe 2 of yet another
embodiment for an open perfusion system has an infusion lumen 92 that run the
length of the
probe 2. In one embodiment, the infusion lumen 92 may be between the outer
wall of the first
conductor tube 41 and the inner wall of the first insulator tube 42. As the
perfusate is pumped
through the probe it travels down the infusion lumen 92 and will be infused or
injected through
the infusion ports 90 that are along the spacer element 36. In an alternative
embodiment (not
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
27
shown), there are no infusion ports along the spacer element. Rather, there is
an infusion lumen
between the inner wall of the second conductor tube 32 and the outer wall of
the first insulator
tube 42, and there is an infusion gap (not shown) between the distal most ends
of the second
conductor tube 32 and the first insulator tube 41. The perfusate of this
embodiment would flow
through the infusion gap and exit the probe through the infusion gap, thereby
infusing or
injecting perfusate into the tissue within the treatment zone.
[0089] In yet another embodiment, the infusion ports 90 are located
at the edges of the
spacer adjacent to the closest edges of the electrodes where arcing is most
likely to occur.
(00901 The delivery or circulation of perfusate may be controlled by
a combination of the
pump 6 and control unit 12. The user may be able to input 16 various
parameters of the perfusion
through the graphical user interface (hereafter "GUI"), which is viewable on
the display 10, and
in turn is controlled by the control unit 12. The control unit 12 may be
programmed to
automatically adjust various parameters or settings of the pump 6 based on
user inputted
parameter thresholds, which in turn will control the flow, or lack thereof, of
the perfusate. It is
within the conception of this invention that various parameters of the
perfusate may be
controlled, changed, altered, or otherwise affected. Such parameters of the
perfusate may
include, but are not limited to, the following: the matter state (gas /
liquid); the electrical
conductivity; osmolality or concentration of the perfusate; thermal
conductivity; heat capacity;
temperature of perfusate; flow rate of perfusate through system; delivery of
perfusate during only
certain pulse sets / trains; and timing of the perfusate (before, after, or
during an IRE pulse
delivery or treatment), thereby maximizing ablation zone size and mitigate
late-onset thermal
damage.
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
28
[0091] The system may allow the user to select various options for
when to deliver
perfusate. For example, the user may be able to input or select on the GUI
from various
"perfusate delivery" options. Such options, which are described below in more
detail, may be
preset options on the system or may be added / customized by the user. When
the user selects a
perfusate option the control unit then triggers the pump to deliver perfusate
based on user
requested settings, such as at a particular flow rate, at a particular time
during the procedure, or
at a certain temperature threshold.
[0092] In one embodiment, the control unit 12 triggers the pump 6 to
deliver perfusate
only during delivery of the IRE pulses, but does not perfuse during inactive
states of the pulse
protocol. In this embodiment, the control unit 12 may trigger the pump 6 to
flow perfusate when
the electrode(s) are in an active sequence state, but not during deliberate
pauses in phased pulse
delivery or not during delays between pulse trains, such as during the 3.5 s
delay between trains
of 10 pulses as will be described in more detail below.
[0093] In yet another embodiment, the control unit 12 triggers the
pump 6 to deliver
perfusate only when the temperature of the electrode has reached a threshold.
The user may be
able to select on the GUI either an upper threshold or lower threshold of
temperature. The upper
threshold may be the temperature setting above which thermal damage to the
tissue may occur or
has been deemed to cause increases in electric conductivity and electric
current that risk
exceeding the electric current specifications of the electroporation pulse
generator, or risk
inducing an arc. The lower threshold may be the temperature setting below
which insufficient
ablation volumes are produced; where the temperature being too low risks
adversely affecting the
ablation zone due to redistribution of conductivity and electric field; or
where the lower
temperature risks instability in the pulse. For example, too sharp of a
contrast between the cooled
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
29
electrode and warmed tissue may cause irregular electrical current behavior
and increased
likelihood of arcing.
[0094] In yet another embodiment, the control unit 12 triggers the
pump 6 to deliver
perfusate only when the electrical current of the pulse has reached a
predetermined threshold.
For example, perfusate may be delivered only to reduce the tissue and
electrode conductivity in
order to reduce electrical current. Moreover, the predetermined threshold may
be an absolute
level which is detected or otherwise sensed by the system that suggests
current will soon exceed
specifications of the electroporation generator, or suggests that there is
considerable risk for
electrical arcing. As a non-limiting example, if the system detects that
arcing only occur once
the current exceeds 35 A, the current threshold can be set at or just below
this amperage value.
Once the system detects the current threshold has been reached, the perfusate
flow is
automatically triggered or modified to maintain the detected current below the
preset critical
threshold. The predetermined threshold may be a relative value based on a pre-
pulse low-voltage
electrical current, or the predetermined threshold may be a relative value
based on initial electric
currents for therapeutic pulses.
[0095] In yet another embodiment, the control unit 12 triggers the
pump 6 to deliver
perfusate only when the electrical current displays an unstable waveform
suggestive of an
impending arc due to oscillations of electric current, at least two- or multi-
plateau waveforms, or
a sudden rise in current within but at the end duration of a pulse.
[0096] Another key aspect of this invention is the ability to control the
temperature of the
perfusate for either an open system or a closed system. In one embodiment, the
system of this
invention is able to control the temperature of the perfusate as it relates to
the impact that such a
temperature change would have on the delivered pulse parameters, and in turn
the effects of the
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
delivered IRE pulses on the tissue. It has been found that room temperature
and body
temperature perfusate result in clinically acceptable ablation sizes and use
less power than
chilled perfusate, which may result in a lower likelihood of potential arcing.
[0097] The temperature of the temperature control perfusate can be
active or passive.
5 Passive temperature controlled perfusion is when a large amount of the
perfusate at a set
temperature is stored in a reservoir 4, such that any perfusate coming back
into the reservoir 4
will not significantly change the temperature inside the reservoir 4. The
control unit 12 will
monitor the reservoir 4 temperature and warn the user if the temperature rises
above the
threshold high temperature. Alternatively, the control unit may automatically
adjust temperature
10 levels based on the user-defined threshold. Active temperature
controlled perfusion is when the
perfusate temperature is monitored by the control unit 12 using a temperature
sensor (not shown)
on the probe inside the tissue and near the electrode, and if it rises or
falls below the set
temperature levels, the control unit 12 can activate a temperature control
device associated with
the perfusate reservoir to automatically adjust the temperature of the
perfusate.
15 [0098] Examples of controlling the temperature of the
perfitsate may include, but are not
limited to, (i) changing the temperature of the perfusate relative to ambient
body temperature
throughout the procedure; (ii) dynamically changing the perfusate temperature
during the
procedure, such as starting with a lower temperature perfusate and ending the
procedure with a
higher temperature perfusate or starting with higher temperature perfusate and
ending with a
20 lower temperature; (iii) independently controlling temperature of
perfusate for each electrode
and/or for each probe if multiple monopolar probes are being used; (iv)
setting the temperature
of perfusate based on the tissue type being treated; and (v) controlling the
temperature of
perfusate based on comparison of the real-time temperature of the electrodes
to a pre-set
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
31
temperature threshold, e.g., utilizing an algorithm to initiate cooling
perfusate when/if the
electrodes reach are preset temperature, such as 45 C. The temperature of the
perfusate may be
either chilled (about 10 C); ambient or room temperature (about 20 C); or
body temperature
(about 37 C).
[0099] Referring now to Figs. 13 ¨ 14, another key aspect of this invention
is the control
unit 12 that controls the pulse parameter settings, the generator 8 used to
deliver the electric
pulses, and the integration of the other system components used to generate,
control, display,
deliver, and monitor the electrical pulses. While current commercially
available EBT pulse
generators 8 are restricted to ¨3400 V, they are also unable to manage more
than 50 Amperes of
electrical current during each pulse. In addition, typical electrode
geometries and pulse delivery
protocols often result in physical conditions where larger voltages will
result in arcing in the
tissue. As previously discussed, arcing can cause problems with operation of
the generator 8 and
impede the successful delivery of energy into the tissue. Arcing problems may
cause an
automatic shutdown of the generator 8, aborted treatment procedure, and/and
increased
procedure time needed to reboot the generator 8 after a shutdown. The improved
generator 8 of
this system solves these and other problems known in the art.
[00100] The controller 12 may be able to provide the user with real-
time feedback and
pulse parameter control. The controller may include computer program storage
or software 96,
which further comprises various treatment control options 98, data storage
100, a CPU 94, power
source 104 and memory 102. The controller 12 is designed to assist a user in
planning,
executing, monitoring, storing, retrieving, and reviewing the results of an
IRE medical treatment
procedure. For example, in one embodiment of this system the controller 12
provides a GUI
interface on display 10 which allows the user to select various treatment
control 98 options, such
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
32
as the tissue type to be treated and/or the size of desired ablation zone.
Other treatment control
98 options that the GUI provides may include customization of various pulse
parameters of the
pulses to be delivered, including, but not limited to, the pulse length,
number of pulses per train,
number of pulse trains, length between each pulse, length between each train,
or the overall
length of pulse delivery. The controller 12 may also be connected to a power
source 104 or have
an internal power source (such as a battery). Moreover, additional software 96
may be stored in
the data storage component of the controller 12 to provide the user with 3D
reconstructions of
the treatment site and with overlay of predictive ablation zones on the
display such that the user
to be able to better formulate and execute a treatment plan.
[00101] In another aspect of the improved generator 8 of this invention,
the generator
includes an automatic recharge feature in which the system recharges after
each pulse is
delivered. This design eliminates the voltage decay which occurs over a pulse
train seen in
current generators and provides a more consistent voltage delivery that better
matches the user
input. The generator 8 may also be capable of generating or alternating
polarity between pulses
(bipolar pulses) and /or between pulse trains by using additional capacitors.
Another advantage
of the generator 8 may be to eliminate a hard shutdown after an arc has
occurred, thereby
allowing IRE procedures to progress without system data loss. It may also be
conceivable to
allow the user to view real-time pulse metric data, such as voltage, current,
and/or resistance.
[00102] As described above, an advantage of this system is to provide
a compact device
with a small footprint. In addition to integrating the pump 6 within the
generator 8, as described
above, it may be possible to also integrate an ECG synchronization device (not
shown), which
are commonly known and used in current [RE procedures, into the generator 8
housing.
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
33
[00103] The generator 8 may be designed to support up to 12 probes,
either monopolar or
bipolar probes with optional RFID readable technology integrated therein. The
RFID technology
may be used for detecting the probe type, identifying probe configuration,
confirming single use
of each probe, confirming of correct connection, and prohibiting the use of
probes not
compatible or not intended to be used with the generator.
[00104] The generator 8 may also integrate a means to measure the
endpoint of a
procedure. There is a current need in the art for the pulse metrics, such as
voltage, current, and
resistance, to serve as valuable indicators to the progression of IRE; both
from the intra-pulse
IRE data as well as the inherent tissue properties after exposure to a set of
IRE pulses. For
example, a need in the art currently exists for a clinically acceptable IRE
system that can indicate
potential issues that may require intervention during an IRE treatment to
prevent superseding the
capacity of the generator and arcing, as well as to display or notify the user
of the extent and
thoroughness of electroporation, particularly in the ablation zone. While
current commercially
available IRE systems do not provide these pulse data in real-time, the
improved system of this
invention solves the need of deriving this information from the pulses in real-
time during
treatment delivery. By doing so, those skilled in the art of delivering IRE
treatments can prevent
potentially problematic conditions, make necessary adjustments, preventing
issues that may
diminish optimum therapy delivery. One purpose of this invention is to provide
the user with the
ability to determine if either the IRE pulse strength is too low or too high,
if the system is at risk
of arcing, and as well as indicating when the tissue between a given pair of
electrodes has been
completely electroporated or treatment is complete. Since the current IRE
treatment devices do
not provide the user with the ability to be notified or even visualize when
the endpoint of a
procedure has been successfiilly reached, one embodiment would be to use a low
voltage
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
34
measurement system integrated into the generator 8 to monitor for procedure
endpoint. For
example, the generator 8 could be built with two capacitor banks or two
circuits, one for high
voltage and one for low voltage. Low voltage measurements may be separately
monitored during
treatment to detect conductivity changes in the tissue as a result of high
voltage pulse delivery.
Alternatively, another embodiment could be an AC spectroscopy or an AC
frequency sweep to
compile real-time conductivity changes in the targeted tissue.
[00105] The system display 10 may be a standard display 10 that is
currently known in the
art. The display 10 may also be a tablet, smart phone or other portable
computer capable of
wirelessly connecting to the controller over Wi-Fl or other wireless means.
The display 10 may
also be used together with an imaging device 18, such as an ultrasound device,
to provide the
user with the ability to scale the image so they can place the tumor in
context of the patient's
body. Additionally, the system may be comprised of multiple screens or
multiple displays 10.
For example, a first screen may display 10 the current, resistance, or
treatment parameters, and a
second screen may have a user GUI and/or ultrasound/M:RI/CT image.
[00106] As part of the pre-planning process, the system may also be able to
import CT
scans or other imaging device output images and overlay these images with the
modeled electric
fields (current, electrical field distribution) used to predict tumor volume.
[00107] The system may also include a power distribution unit 14 to
control the energy
delivery of IRE electric pulses. The power distribution unit 14 is intended to
solve the need in the
art for an IRE system with an enhanced power distribution of the electric
pulses and provide
necessary real-time electrical pulse data. This may include being able to use
more than the six
controllable electric pulse outputs of current IRE systems. Additionally,
there is also a need for
an IRE system with the ability to alternate the polarity of the pulses at
determined intervals or in
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
response to therapy behavior, such as arcing occurring with pulse delivery,
which can at times be
eliminated by alternating the polarity; reduce total charge delivered; reduce
electrochemical
effects of pH imbalance; and mitigate discrete gas element formation from
electrolysis.
Moreover, current state of the art IRE generators are only able to energize
two electrodes at a
5 time, with one serving as the anode and the other as the cathode. If more
than two electrodes are
required for a treatment, commercial generators currently will sequentially
alter what the two
energized electrodes are, requiring exponentially higher numbers of total
electric pulses as the
number of electrodes and thus combinations of electrode pairs increase. This
may create
problems with requiring significant time increases to treat larger tumors that
require more
10 electrodes; which at times can restrict the practical utility of IRE
therapy or result in incomplete
treatment due to the time constraints for delivering therapy to an
anesthetized patient. In
addition, there are many instances where more effective IRE therapy delivery
may require
energizing several or many electrodes simultaneously, as explained in more
detail below. The
present invention enables activating any number of electrodes to serve as
either the positive or
15 negative portion of the pulse pair.
[00108] Current commercial systems for clinical applications of IRE
pulses are only
display electric pulse metrics after completion of an entire procedure, and
are limited to
delivering electric pulses through a total of six outputs or probes. However,
there are many
clinical cases that may require greater than six outputs or probes to serve as
anodes or cathodes
20 throughout the duration of a procedure, due to either large ablation
volume requirements or
complex tumor geometries. This includes the utilization of greater than six,
single pole
electrodes used in an array, as well as specialized electrodes which may
contain a large number
of individual contact surfaces for the delivery of IRE electric pulses. This
system solves these
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
36
unmet needs in the art through the use of a power distribution unit 14. As
seen in Figure 14, the
power distribution unit 14 may be connected to the generator 8 and/or control
unit 12 with
standard connections known in the art, which in turn is connected to the
display 10 as shown in
Fig. 13, such that the system is capable of monitoring and displaying the
electric pulse metrics
during delivery in real-time, which may offer beneficial information and
feedback to improve
clinical applications of IRE protocols. In another embodiment, the power
distribution unit 14
may be incorporated into the same housing as the generator 8 to achieve a
smaller overall system
footprint.
[00109] In one embodiment, the power distribution unit 14 may consist
of an ammeter
104, a high-voltage voltmeter 106, an array of switches 108 that have three
positions (off, on-
positive, on-negative), and a series of probe outputs 110. The power
distribution unit 14 may
receive the electric pulse signal from the generator 8 through designated
positive input 112 and
negative input 114. The positive input 112 is connected to a positive
distribution node 116 and
the positive terminal of the voltmeter 106. The negative input 114 is
connected through ammeter
1.5 104 to a negative terminal 118, which is connected to a negative
distribution node 120 and the
negative terminal of the voltmeter 106. The positive distribution node 116 is
then connected to
the positive terminal of each switch 108. The negative distribution node 120
is then connected to
the negative terminal of each switch 108.
[00110] In one embodiment, the generator 8 may be set to deliver
energy to each of the
outputs in the same manner. For example, a first output may always be positive
and connect to
the positive input 112 of the power distribution unit 14, while a second
output may always be
negative and connect to the negative input 114 of the power distribution unit
14. When an
electric pulse is delivered, the electrical energy goes through the power
distribution unit 14, and
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
37
any switches 108 set to positive positively energizing any probe connected to
that probe outputs.
The electrodes connected to the negative pole of the switch 108 then return
how much voltage is
left after the pulses have been delivered to the tissue back to the power
distribution unit 14. The
negative signal then returns to the negative distribution node 120, runs
through the ammeter 104,
and then returns back to the generator 8. The voltmeter 106 measures the
voltage drop between
the positive and negative signals to measure the total voltage delivered to
the tissue. The outputs
from the voltmeter 106 and ammeter 104 are then sent to the controller 12
and/or display 10 so
both the voltage and current pulse metrics delivered to the tissue can be
visualized in real-time.
The controller 12 may include software 96 to compute calculations of these
signals so they can
be used to determine the tissue resistance or conductance, as well as numerous
other parameters
resulting from the electric pulse metrics.
[00111] In another embodiment, the power distribution unit 14 may also
include
components and circuitry (not shown) to measure real-time feedback parameters,
including, but
not limited to, resistance; impedance, frequency, and specific impedance.
These measured
parameters may then be depicted on the display 10 to allow the user to
integrate this information
into the treatment planning. In addition, in yet another embodiment, the power
distribution unit
14 may receive pulse energy from at least two inputs, such as a positive and a
ground, and
facilitates individual control of how the electrical voltage from the
generator is distributed
among electrodes. The invention is therefore unlimited in the number of
electrodes that in can
distribute the power to over the course of a procedure. Furthermore, the power
distribution unit
14 together with the controller 12 and generator 8 may also enable complex
distribution patterns
and algorithms to fine tune ablation volumes / geometries of multiple
electrodes serving as a
positive or ground simultaneously. This may have benefits for shaping the
electric field
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
38
distribution, as well as decreasing the overall IRE procedure time by
permitting pulse delivery
between several electrodes simultaneously, rather than serially, when the
total distributed electric
current is maintained within the constraints of the generator 8. This decrease
in overall procedure
time would be a significant advantage over the currently commercially
available IRE treatment
systems.
(00112] In another embodiment, the power distribution unit 14 may also
include a metric
measuring functionality. For example, the ammeter 104 may be replaced with a
Hall Effect probe
(not shown), so as to measure the electric current without directly
interfering with the pulse
signal. Alternatively, a unique ammeter may be placed on the negative signal
connection
between the switch and the node for each individual switch, so as to measure
the electric current
of each negative electrode separately. Moreover, the high-voltage voltmeter
106 may be replaced
with a basic voltmeter placed on a voltage divider circuit. The voltage
divider circuit is of much
higher resistance than the tissue (1M-MCI, versus tissue, which is hundreds of
SI), and thus
inducing negligible effect on the strength of the pulses delivered to the
tissue. A correction factor
is calculated by the controller for the divider circuit based on the
resistances of the three resistors
in the voltage divider circuit to determine the true delivered voltage to the
tissue based on the
measured voltage drop across the resistor. An accurate voltage is required to
attain accurate
resistance measures.
[00113] Additional embodiments of the power distribution unit 14 may
include a double
pole double throw switch placed between the distribution nodes 116, 120, but
internal to the
voltmeter and ammeter sections. This switch is connected to the positive and
negative portions
on both ends of the switch, but with connections on opposite ends leading to
the distribution
nodes 116, 120. This enables the switch to function as a rapid switch to
reverse the polarity being
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
39
sent to all active electrodes at once. A fuse may be integrated into the
circuit on the positive end
just after the input of the power distribution unit 114. This fuse is fast-
acting, and is able to
trigger in the instance of a high current condition. This would enable the
fuse to trigger and cease
energy delivery to the tissue prior to any arc issues being encountered by
generator 8. The power
distribution unit 14 may include an oscilloscope to monitor pulse metrics in
real-time.
Additionally, a switch may be introduced to the positive side of the signal
between the voltmeter
and positive distribution node 116. The switch is a single pole double throw
switch. When the
switch is in one position, the signal continues to the positive distribution
node 116. When the
switch is in a second position, the system moves through a relatively high
impedance resistor,
effectively dropping the voltage delivered to the tissue. This enables
delivery of lower voltages
to the tissue than current commercially available electroporation generator
may be capable of
delivering. Additionally, this may also allow for rapid high voltage and low
voltage pulse
delivery without charging the capacitor bank, eliminating the need for a
second capacitor bank or
charging / discharging delays. The use of low voltage pulses includes
determination of baseline
tissue properties and tissue properties in the absence of any engaged
electroporation
phenomenon. An additional resistor may be added and an adjustable
potentiometer, enabling
control to tune exactly how much the voltage will be dropped before reaching
the positive
distribution node 116 and electrodes. The voltmeter 106 may be placed after
the switch to enable
measuring what the effective voltage delivered to the tissue. Adjustable
resistance potentiometers
may be placed on the positive connection to each switch individually. Such an
arrangement
would enable the user to fine tune which electrode connections receive
larger/smaller voltages
based on the electrode geometry and tissue conditions of the targeted region.
Some separations
may be larger and require larger voltages than closer electrodes, among many
other possibilities
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
where this would be useful. Lastly, the power distribution unit may also be
integrated directly
into the generator, before the generator's 8 conventional outputs. In this
way, the switching
system is controlled from the generator 8.
[00114] The method of using this system to treat tissue will now be
described. The method
5 may include reducing the temperature of the affected tissues as a way to
reduce local tissue
conductivity, both over baseline in general, as well as relative to what
conductivities would be
encountered for higher temperature tissues. One key inventive concept of this
system and the
method of using the system is controlling the temperature rise of the
treatment zone and its
correlated electric conductivity, thereby making it possible to deliver higher
voltages reliably
10 into tissue without the risk of arcing or exceeding 50 A (which is the
current limits on
commercially available IRE generators), thus enabling greater electric field
distributions and
affected volumes.
[00115] In one preferred embodiment, the method of using the single
stick bipolar system
probe may include perfusion of internally circulated room temperature
perfusate within the probe
15 together with the pulse parameters of five pulses per train; an inter-
cycle delay of two second
between pulses, ranging between 0.5 and 3 seconds depending on the patient's
heart beat; a set of
two trains per row; and a ten second delay between each row. This combination
of perfusion
together with the specific pulse parameter set has been found to achieve
clinically acceptable
ablation zones using a single stick bipolar probe while reducing the potential
for unstable electric
20 current and/or arcing potential between electrodes.
(00116] In a situation where the temperature of the electrode and/or
tissue is controlled via
perfusion, either directly infusing perfusate into the treatment site or
indirectly by internally
circulating peifusate within the probe, the dependence of electrical
conductivity on temperature
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
41
is altered, thus affecting the electric conductivity of the tissue relative to
its conventional
behavior in response to the Joule heating from the IRE pulses. It is possible
that the high number
of total pulses delivered for a commercially available IRE device may result
in significant
cumulative thermal effects, with temperatures shown to reach levels as high as
59 C in regions
between several pulse pairings at typical pulse protocols. It has been found
that temperature rise
may alter the properties of the tissue within the treatment zone and thus
alter treatment procedure
outcomes. For example, when the temperature rises during an IRE procedure are
not evenly
distributed this may result in heterogeneous conductivity distribution which
may significantly
alter the electric field distribution in the tissue within the treatment site.
Using perfusion the
method of use may include reducing the resistance of the hottest regions of
the tissue or the
probe 2, which are typically adjacent to the point at which the electrodes 32,
34 are closest
together, reducing the bulk tissue conductance, and thereby reducing the
overall electric current
delivered for a given electroporation pulse voltage. Perfusion makes the
electrical field
distribution more homogeneous by leveling / balancing tissue conductivity
within the target area.
Advantageously, reducing the overall electric current delivered for a given
electroporation pulse
voltage may enable greater voltages to be delivered to the treatment zone,
thus increasing the
ablation volume of IRE treatment using just a single stick bipolar probe.
[00117] While unstable electric current and arcing between electrodes
are often correlated
they are in fact two different modes by which an IRE generator or system can
fail during a
treatment leading to an overall reduction in voltage delivered to the tissue,
thereby resulting in an
incomplete or failed treatment procedure. Perfusion of the electrodes may
address unstable
electric current and arcing between electrodes, thus allowing for an overall
increase in the
voltages that can be delivered to the treatment zone. It has been discovered
and described in
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
42
more detail below, that perfusion of a bipolar probe using ambient or room
temperature perfusate
may increase general pulse electric current stability and reduce sudden spikes
in current, thereby
reducing the potential for arcing between electrodes. Moreover, it has been
found that perfusion
via internally circulating room temperature perfusate within the bipolar probe
reduces
temperature at the hottest portions of each electrode and adjacent the tissue
within the treatment
zone resulting in a reduction of sudden inflections and arcing between
electrodes. Therefore,
perfusion of a single stick bipolar probe may exhibit a reduction in the bulk
tissue temperature
rises and maximum temperatures, dramatically reducing the extent of thermal
damage including
the variety of thermal damage that risks morbidity to the sensitive structures
within the treatment
zone while still achieving clinically significant irreversible electroporation
of cells.
[00118] This system may provide for the ability to control and alter
the temperature of the
perfusate in real-time during the procedure, or to use a room temperature
perfilsate that does not
require any active temperature control. Multiple studies were performed using
this system to
determine the effect of different temperature perfusate and the impact it had
on achieving the
desired goal of enabling larger electric field distribution size and affected
volumes. As will be
described below, the method of using this system was tested with perfusion
that was
continuously cooled or chilled, at ambient room temperature, and also
maintained at body
temperature.
[00119] In a first experimental study, a perfused bipolar electrode,
similar to the probe
described above, of dimension lOmm x 7mm x lOmm (energized, insulating
separation,
energized) was inserted into either room temperature potato or liver. Three
temperature solutions
were perfused to determine possible effects of perfusion: chilled perfusate (8
C), room
temperature perfusate (21 C), and body temperature perfusate (37 C). The three
temperature-
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
43
controlled and perfused probes were compared with a non-perfused probe, such
as is
commercially available. The perfusate was 0.9% NaCl solution was used as the
perfusate. A
maximum perfusion setting was used on a peristaltic pump to deliver perfusate
to the probe
during pulse delivery. Voltages applied to the electrodes were varied to
determine the maximum
permissible voltage for each perfusate without arcing.
[00120] The temperature-controlled probes showed maximum temperatures
equivalent to
their respective perfusate temperature in both potato and liver, suggesting
the perfusion rate and
perfusate fluid was sufficient to maintain the electrode at the targeted
temperature throughout the
duration of the procedure. In potato, the maximum temperature reached by the
ambient, meaning
no perfusate was used, probe with no perfusion was 62 C, despite it starting
with an initial
temperature of room temperature (rise of approximately 40 C). In liver, the
maximum
temperature reached from the ambient electrode was 65 C, giving an increase of
¨35 C above its
baseline of the tissue.
[00121] In potato, 2000 V was applied The body temperature perfused
probe and non-
perfused probe showed equivalent trends of rise in electric current, while
room temperature
probe and chilled probe showed reductions in electrical current of 10% and
20%, respectively. In
liver, all three temperature controlled and perfused probes showed less
electrical current
delivered during the procedure compared to the non-perfusate probe, with
maximum reductions
in current of 47% for body temperature perfused to probe, 52% for room
temperature probe, and
65% for chilled temperature perfused compared to the non-perfused probe.
[00122] All three temperatures controlled and perfused probes showed a
reduced
likelihood for electrical arcing compared to the non-perfused probe. In
potato, average number of
arcing events over the entire pulse duration was < 1 for all three temperature
controlled and
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
44
cooled probes; while it was 3.75 for the non-perfused probe. In liver, arcs
were only noted in
non-perfused probe (3.5 average) and chilled perfusate probe (1.5 average),
suggesting that body
temperature and / or room temperature perfusate may be more stable at
preventing arcs in the
tissue even if their electric current is found to be higher.
[00123] In potato, there appeared to be no significant correlation between
ablation zone
size and perfusate temperature, or relative to the non-perfused probe. This
would suggest that the
changes in perfusion may be used without an effect on ablation zone. As
general trends, the non-
perfused electrode performed the worst (smallest lesion) while the body
temperature perfused
probe showed the largest ablation area.
[00124] This first experimental study found all three temperature
controlled perfused
probe significantly reduced the number of arcs and maximum temperatures
reached in the tissues
compared with a non-perfused probe. Below is a table summarizing the results
of the first
experimental study.
pm 25] Table 1: Comparing Temperature Controlled Perfusion probe with
Non-Perfused
probe.
Type of Perfusate Max. Temp % of Current Reduction Ave. number
of Arcs
(Electrode (Liver) relative to Non- (Liver)
Surface) perfused
None 65 C 3.5
(Non-perfused)
Chilled 8 C 65% 1 . 5
8 C
Room Temperature 21 C 52%
21 C
Body Temperature 37 C 47%
37 C
[00126] In a second experimental study, the effects of chilled
perfusate that having a
temperature of approximately 10 C was further compared with a system that has
no perfusate.
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
Voltages applied to the electrodes were varied to determine the maximum
permissible voltage
for perfusate vs. non-perfusate. It should be noted that all simulations use
dynamic conductivity
in the sense that conductivity is changing as a result of rising temperature.
However, the
terminology used here will delineate between static and dynamic conductivity
simulations based
5 on whether electroporation-based conductivity rise is included in the
model, while temperature is
always considered. The results of this study are shown in Table 2 below.
[00127] Table 2: Chilled Perfusate vs. No Perfusate.
Conductivity Cooling Electrical Total Volume Overall
Overall
Condition Current E> 500 V/cm Volume
Volume
T > 50 C T > 70
C
Static None 44.68 A 10.64 cm3 6.64 cm3
2.66 cm
Static 10 C 14.07 A 5.15 cm3 0 cm3 0 cm3
Dynamic =None 89.1 A 14.8 cm3 8.01 cm3
3.80 cm3
Dynamic 10 C 23.9 A 8.04 cm3 0 cm3
0 cm3
[00128] Based on the data in Table 2, the test data clearly showed
that the non-perfused
10 electrode causes very high temperature changes in the tissue, with
volumes of 6.64 cm3 and 2.66
cm3 reaching temperatures above 50 C and 70 C, respectively, for the static,
as well as 8.01
cm' and 3.80 cm3 for the dynamic conductivity model. However, there is much
less temperature
rise for the perfused probes using a chilled perfusate, with no volume of the
tissue reaching
temperatures above these thresholds at all. This suggests that the chilled
perfusate would render
15 minimal or no thermal damage. The use of chilled perfusate was shown to
reduce the electrical
current by 69% and 73% for static and dynamic conductivity simulations,
respectively. This
indicates that significantly higher voltage electric pulses can be used while
remaining below the
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
46
50A threshold for commercially available generators when chilled perfusate is
incorporated into
the system.
[00129] Although chilled perfusate reduces the electric current and
temperatures that the
tissue is exposed to, it also affects the conductivity and thus electric field
distribution through the
tissue. For the conditions examined, the predicted ablation zone decreases by
52% and 46% for
static and dynamic simulation conditions, respectively; with the predominant
effect occurring in
the ablation zone diameter rather than its length.
[00130] The use of chilled perfusate together with the above described
system may likely
allow higher voltages to be used by reducing the electric current and extent
of unintended
thermal damage to tissue immediately surrounding the electrodes. However, for
the same
voltage, the ablation zone may be smaller and less spherical (for certain
electrode embodiments).
Thus, it is desirable for the present invention to optimize the balance
between benefits on
electrical behavior, while minimizing the extent of reduced ablation volume
and minimizing the
possibility of arcing. If this balance can be achieved, the use of perfusate
with the present system
should enable larger ablations to be achieved, with more consistent and
reliable energy delivery
that may be more readily applied repeatedly across numerous clinical
scenarios.
[00131] In a third experimental study, the use of body temperature
perfusate was further
examined. A perfused bipolar probe, similar to the probe described above, with
dimensions of
10x7x10 mm (energized, insulating separation, energized) was used. A total of
19 ablations in
muscle and liver were done to determine the effect of varying active cooling
algorithms on
procedure outcomes. During this in vivo test, body temperature perfusate
resulted in clinically
acceptable ablation size and used less power than chilled perfusate. The use
of body temperature
perfusate (either during delivery or turned on when T> 50 C) resulted in the
largest ablation
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
47
zone diameter, with 3.2 cm relative to 3.0 cm for room temperature perfusate
and 3.1 cm for
continuously cooled perfusate. In addition, the average maximum electrical
current for triggered
body temperature perfusate was 24 A, which is lower than the 33 A and 26 A for
room
temperature and chilled electrodes, respectively. Moreover, the average
incidence of arcs found
for the triggered body temperature perfusate was 2.8 compared to 6.5 for the
room temperature
perfusate and 4.8 for chilled perffisate. This data suggests that continuous
circulation of body
temperature perfusate and ideally triggered body temperature perfusate may
result in greater
reliability for being able to deliver an entire ablation protocol without
exceeding electroporation
generator electric current limits and also with fewer incidence of arcing
compared with non-
perfused systems. The inclusion of the body temperature perffisate markedly
decreased the
volume and extent of thermal damage to the tissue, thus better preserving IREs
unique non-
thermal cell death modality.
[00132] Continuous delivery of body temperature perfusate may provide
a less intense
cooling regimen to attain equivalent benefits on pulse stability and reduced
electrical current,
while providing more reliable energy delivery and higher applied voltages, but
ideally without as
significant of a redistribution in electric conductivity and electric field,
offering larger ablation
zones. The use of body temperature perfusate may result in less arcing and
greater pulse stability
than when using chilled perfusate, despite the additional cooling of the
chilled perfusate.
[00133] A simulation, created with Comsol Multiphysics v3.5, was
conducted to support
the findings from the benchtop and in vivo animal studies, i.e., a marked
increase in ablation
zone when using warmed perfusate or a room temperature perfusate relative to
chilled perfusate.
The results of this simulation are shown in Figures 15 ¨ 17. Importantly, the
use chilled
temperature perfusate, room temperature perfusate and body temperature
perfusate all showed
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
48
notable decreases in electric currents required to achieve clinically
acceptable ablation volumes,
thermally suspicious areas, and arc incidences compared to non-perfused
systems. The objective
of this simulation was to analyze perfusion with varying perfusate
temperatures in order to
determine a crossover point/range where benefits to reduced currents and
thermal damage are
maximized while volume of affected region exposure is maintained at a
clinically level. It was
discovered that the non-perfused or ambient probes had much greater thermal
exposure volumes
compared to all of the temperature controlled perfusate, with progressively
lower exposure
volumes as perfusate temperature decreased. Thermal exposure volume is defined
as that portion
of the total ablated tissue volume subjected to temperatures of 50 C or
greater. Temperatures
reached 70 C, which is relevant for damage to collagen, in greater than 0.1cm3
of tissue perfused
at 50 C or ambient conditions. Volume of thermal exposure to 70 C was less
than 0.1cm3 for all
other perfusate temperatures, showing they should all be negligible in
creating thermal tissue
damage. Even at 50 C perfusion, the exposure volume is only 0.158cm3, which is
still less than
1/10th that of the ambient probe's 1.76 cm3 measurement, as seen in Fig. 15.
Thus any perfusion
temperature at or below 50C should be suitable for maintaining negligible
thermal tissue
damage. Volume of exposure scale changed so there is a crossover between 500
V/cm threshold
and electric current at its scale of 0 ¨ 40 A, as seen in Fig. 17. This
crossover point, where
benefits to reduced currents and thermal damage are maximized while volume of
affected region
exposure is maintained at a clinically level, has been shown to occur between
perfiisate
temperatures of 30-35 C. Therefore, the optimum balance of ablation size gain
with reduced
electric current occurs in the perfusate temperature range of up to 35 C. The
ablation volume
notably increases with perfusate temperature, and thus the maximum perfusate
temperature
should be used, with a cap placed when thermal damage and electric current get
too high. This
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
49
ultimately occurs at the ambient probe growth rate of electric current and all
thermally affected
volumes increase at a faster rate than electric field exposure volume and
minimum diameter, and
must remain within reasonable and practical (actual clinically implemented)
limits. Thus, within
a temperature range of 5-60 C, it has been found that 20-50 C would seem to be
a relatively
ideal point for increasing the ablation size as much as possible while
reducing arcing and thermal
damage. Practically, however, 30-40 C would also seem reasonable with noted
benefit still
present. This may be more practical as many hospitals have equipment that will
raise the fluid
temperature into that range. For perfusate temperatures less than 20 C, the
minimum diameter is
reduced by over 20%.
[00134] The method of using this system also includes the use of a specific
set of IRE
pulse treatment parameters. Traditional pulse parameters may include
delivering between 70 ¨
100 pulses between each pair of electrodes, and then alternating between the
various pairs, if
more than I pair has been inserted. For example, these pulse parameters may
include delivering
multiple trains (or sets) of 70 pulses between each pair of monopolar probes,
with a 1500V/cm of
separation, and each pulse being up to 100 microseconds in length.
Additionally, it is common to
synchronize the delivery of the pulses with an ECG device, as described in
more detail in U.S.
Patent 8,903,488, filed May 28, 2009, entitled SYSTEM AND METHOD FOR
SYNCHRONIZING ENERGY DELIVERY TO THE CARDIAC RHYTHM, which is hereby
incorporate by reference.
[00135] A problem in the art currently exists for traditional pulse
parameters for IRE
treatment, such as these pulse parameters may lead to rise in temperature
adjacent to the
electrodes and/or a rise in potential arcing which may lead to a system
failure. The modulated or
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
cycled pulse parameters of this invention are intended to reduce potentially
dangerous or
problematic temperature rise adjacent to the electrodes and reduce arcing
potential.
[00136] The specific pulse parameters to be used with the above
described system are
intended to modulate or control the specific pulse parameters, thereby
reducing the number of
5 pulses and/or increase delays between pulses during treatment. For
example, modulated pulse
delivery may add deliberate pauses or delays between pulses to allow edema or
tissue-scare
effect normalization, electrolysis, gas dissolution, and/or ionic rebalancing.
The method may
provide a user with the ability to select preset pulse parameters, alter
present pulse parameters, or
provide for customization of pulse parameters. The advantage of modulating the
pulse
10 parameters will be to increase ablation volume, improve tumor response,
decrease IRE-
mitigating temperatures, allow tissue settling, and monitor or adjust pulse
parameters in real-time
during procedure.
[00137] Modulated pulse delivery may extend the duration in which a
cell remains
permeabilized. Increasing a cell's permeability reduces the cell membrane's
ability to maintain a
15 physical barrier between intracellular contents and the surrounding
environment. Tissue regions
experiencing sub-lethal electric fields will undergo pore alteration.
Modulated pulse delivery
enables advantages of maintaining cell poration for a longer period prior to
re-insult, without
costing additional procedural time. The effects of modulated pulse delivery
may include, but are
not limited to: (i) the tissue has the chance to return to baseline
temperatures and electric
20 conductivities before undergoing additional pulses; (2) secondary
physiological responses, such
as edemas, have opportunity to occur and distribute throughout the tissue; (3)
improved pulse
delivery without arcing or increasing arcing potential; and (4) extention of
cell stress periods to
increase lethal effect of pulses being delivered.
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
51
[00138] Referring to Fig. 18, in one embodiment the modulated pulse
parameter timing
algorithm to be used with this system includes a total of 400 pulses being
delivered to the patient.
The algorithm includes a first pulse train consisting of five single pulses.
The voltage of each
pulse may be up to 3000V. The pulse width may be up to 100 msec, with an inter-
pulse delay
dependent on the patient's heart rhythm, but typically between 0.5 and 3.0
seconds. The first
pulse train is of a first polarity, which may be either positive or negative.
The second pulse train
will follow the first pulse train after an inter-train delay of up to 2
seconds. The polarity of the
second pulse train may be of a second polarity, and in this embodiment the
second polarity will
be the opposite of the first polarity. For example, if the first polarity of
the first train is positive
then the second polarity of the second train will be negative. The first and
second trains
combined equal one row of pulses. There is an inter-row delay of up to 10
seconds. After the
inter-row delay, the second row will begin with a third train of five
individual pulses. The third
train of pulses will have a third polarity, and in this embodiment the third
polarity is the same as
the first polarity of the first train. The algorithm runs for a total of 400
pulses, which equals 40
total rows, and 80 total trains, with 40 trains in the first polarity and 40
trains in the second
polarity.
(00139] Modulated or cycled pulse parameters may maintain more
residual heating at low
temperatures (<43 C) but significantly reduce the volume exposed to higher
temperatures as
shown in the Table 3 below.
[00140] Table 3: Effects of modulated pulse parameters on residual heating.
Simulation Number of Volume Volume Volume Volume
Volume
Pulses T > 43 C T > 48 C T > 53 C T 58 C
T > 63 C
Continuous 80 4.54 cm 0.78 cm 0.34 cm 0.145 cm
0.027 cm
Pulses
Cycled 80 5.28 cm 0.53 cm 0.213 cm 0.061 cm
0.002 cm
Pulses
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
52
Continuous 100 5.07 cm 0.89 cm 0.40 cm 0.17 cm
0.040 cm
Pulses
Cycled 100 6.15 cm 0.58 cm 0.23 cm 0.07 cm
0.0025 cm
Pulses
[00141] An in vivo experiment was conducted to test for the optimal
bipolar IRE pulse
parameters to be used with a perfused system as described above. The cycled
pulses include a
delay as discussed below, whereas continuous pulses do not contain such delay.
[00142] Single electrode bipolar IRE was performed in 28 in-vivo pig livers
(total of 78
ablations). First, effects of voltage (2,700-3,000 V), number of pulses,
number repeated cycles
(1-6), and pulse width (70-1001.1sec) were studied. Next, electrical
conductivity was altered by
introduction of hypertonic and hypotonic fluids into the tissue using an open
perfusion system.
Finally, effects of thermal stabilization were assessed using a closed
perfusion system. Treatment
effect was evaluated 2-3 hours post-IRE. Dimensions were compared and
subjected to statistical
analysis.
[00143] The study results demonstrated that by modifying multiple IRE
parameters, one
can achieve a clinically relevant benchmark of 3cm short axis tissue ablation
with a single
bipolar probe. To obtain this result, the study delivered the IRE pulses over
multiple cycles of
application and coupling pulse delivery with altering tissue electrical
conductivity by either
systematically introducing hypotonic solution infusion or internally perfusing
the electrode probe
with perfusate, all of which are designed to increase voltage maximums and
pulse lengths to the
greatest possible without inducing over-current or electrical arcing issues.
[00144] First, the study examined the manipulation of IRE pulses
without any perfusate.
The study demonstrated that multiple cycles of pulses increased the diameter
of the ablation to
2.9 cm. Yet, it was observed that for the bipolar configuration that this set
of parameters
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
53
increased system instability. Specifically, a much greater number of
electrical pulse spikes are
noted, which was attributed to greater electric arcing caused by the higher
electrical fields
encountered. Furthermore, it was demonstrated that the pulse parameters could
result in
enhancement of the ablation effect. Several of the tested tissue modifications
resulted in an
increased frequency of intense arcing and premature generator shut down.
Particularly, the study
increased IRE pulse length above the recommended 70 sec to 100 sec. While
this
modification did result in an increase in the short axis diameter of ablation
effect from 2.6cm to
2.9cm, it was accompanied by increasing electrical spikes at the end of the
IRE pulse. Although
in an attempt to eliminate heat and gas build up surrounding the electrode by
increasing the time
between cycles of IRE application from 50 to 100 seconds, this alone could not
eliminate the
electrical instability. Summary of these results are shown in Table 4 below:
[00145]
Table 4: Multiple repeat cycles with electrode tip exposure of 7 mm / 8mm
of
insulation / 7mm of tip exposure:
Voltage Number Pulse Delay
# of System Treatment Treatmen Treatmen
Range of Length Pulse trials Crash Duration t Width
t Length
(V) Pulses ( sec) Duration
/0 (min.) (cm)
(cm)
(sec)
2,700V 90 70 50 5 0
13.2 3.8 2.6 0.3 4.5 0.4
2,700 V 50 '100-70* 100 10 40 19.2 3.6 2.4 0.1
4.6 0.2
3,000 V 50 100-70* 100 10 50 18.4 + 3.3 2.9 + 0.1
5.0 0.4
*starts with 100 sec and gradually reduce to 70 usec to prevent arcing and
system crash
[00146] =Next, the study attempted and succeeded in increasing system
stability by infusing
hypotonic distilled water during IRE application. This change in electrical
conductivity and
increased stability of the system, with no arcing or crashes. Yet, there was a
decrease in ablation
size thus failing to meet a primary objective of creating large treatment
zones. To account for
these opposing effects, it was hypothesized that the perfusion of fluid in the
tissue flushes out
CA 02981867 2017-10-04
WO 2016/164930 PCT/US2016/026998
54
microbubbles created by the high intensity electrical field. Summary of these
results are shown
in Table 5 and Table 6 below:
[00147]
Table 5: Summary of ablation sizes for high (100%) and low (10-25%)
concentration of saline infusion into tissue surrounding the electrode having
exposed tip 5-15
mm and insulation 5-8 mm:
Fluid Voltage # of Pulse N= System
Rounds Treatment Width Length
Type (V) Pulses Length Crash Complete Duration (cm)
(cm)
(psec)
(min)
100% 2,700¨ 2.2 4.6
Normal 3,000 50 100 3 100 1 7.0
0.1 0.2 0.1
, Saline
10- 2.2 4.7
25% 3,000 50 100 3 66 2 11.3 0.3
0.2 0.3
Normal
Saline
[00148]
Table 6: Results of ablation sizes for infusion of distilled water ("DW"),
comparing constant flow vs. limited infusion into tissue surrounding the
electrode having
exposed tip 5-15 nun and insulation 5-8 mm:
Fluid Voltage # of Pulse N= High
System Treatment Width Length
Type (V) Pulses Length Current Crash
(,)/0 Duration (cm) (cm)
(usec) (min)
DW 2,700 ¨
Constant 3,000 50 100 3 0 0
11.4 3.4 2.3 0.1 3.8 1.1
Flow*
DW 2,700-
Constant 3,000 50 100 8 0 0
12.7 2.3 2.7 0.2 5.0 0.1
Flow
DW 2,700-
Alternate 3,000 50 100 4 75 25
13.1 1.1 3.1 0.3 5.1 0.5
Flow**
DW 2,700-
Alternate 3,000 50 100 12 66.7 50
12.8 0.2 3.1 0.3 5.3 0.6
Flow***
*5mm exposure and insulation
**Audible popping triggered
***Only while on for last 4 sets
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
[00149]
Finally, the study tested an internally perfused electrode probe design
that would
reduce the inherent electrical conductivity rise of the tissue by mitigating
tissue heating at the
tissue-electrode interface. This strategy indeed did have the desired effect
of permitting stable
IRE application of sufficient duration and intensity to reliably produce 3cm
short-axis diameter
5 treatment effect. The study found that the best results were seen when
stabilizing the tissue
properties with a warmer perfusate. Unlike known thermal ablation techniques,
such as RF or
microwave ablation, where cooler perfusate temperatures result in clinically
better results, this
study discovered that internal perfusion of the probe using body temperature
perfusate resulted in
clinically acceptable larger treatment zones. This likely results from an
optimal balance between
10 mitigating microbubble formation and/or excessive tissue electric
conductivity rise to reduce
likelihood of arcing, but not to the extent where it pronouncedly alters the
electric field
distribution to shrink one of the ablation dimensions. This is likely due to
the redistribution in
electric conductivity and thus lethal electric field distribution when the
tissue nearest the
electrode is 'over-cooled'. Thus, optimal results seem to occur when perfusion
is sufficient to
15 control electrical conductivity rise enough to eliminate electrical
arcing, but not so much that it
dramatically alters the electric field distribution and treatment zone.
Therefore, this study
confirmed a key difference between IRE and thermally-dependent ablation
modalities in that an
electrode probe that is internally perfused with warm perfusate will treat
significant targeted
volumes without inducing protein denaturation due to temperature rise. Summary
of these results
20 are shown in Table 7 below:
(00150]
Table 7: Results of ablation size for closed perfusion, comparing distilled
water at
4-10 C vs. 37 C vs. no fluid.
Fluid Type Voltage Treatment N::: System Ablation
Ablation
(V) Duration Crash % Width (cm)
Length
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
56
(min)
(cm)
Cooled DW 2,700- 18 - 23 4 0 2.3 0.1
(4-10 C) 3,000
Warm DW 2,700- 18 - 23 4 0 3.1 0.1
4.5 1.3
(37 C) 3,000
No Fluid 2,700- 18 - 20 3 66 3.0 0.1
5.0
3,000
*system crashed and this data point was not recorded
[00151] Delivering 3,000 V at 7011sec for a single 90 pulse cycle
yielded 3.8 0.4 x
2.0 0.3cm of ablation. Applying 6 cycles of energy increased the ablation to
4.5 0.4 x
2.6 0.3cm (p<0.001). Further increasing pulse lengths to 1001.1sec (6 cycles)
further increased
ablation to 5.0 0.4 x 2.9 0.3cm (p<0.001), but resulted in electric spikes and
system crashes in
40-50% of cases. Increasing tissue electrical conductivity via hypertonic
solution instillation in
surrounding tissues increased the frequency of generator crashes, whereas
continuous instillation
of distilled water eliminated this arcing phenomenon, but reduced ablation to
2.3 0.1cm.
Controlled instillation of distilled water when electrical arcing was
suspected (using from audible
popping as a trigger) produced ablations of 5.3 0.6 x 3.1 0.3cm without
crashes. Finally,
3.1 0.1cm short-axis ablation was achieved without system crashes with
internal electrode probe
perfusion at 37 C vs. 2.3 0.1cm with 4-10 C perfusion with no system crashes
(p<0.001). This
study confirms the potential utility of using a paradigm of IRE treatment
based upon a single
applicator bipolar electrode configuration. Most notably, it was demonstrated
that by modifying
is multiple IRE parameters, one can achieve a clinically relevant benchmark
of 3cm short axis
tissue ablation with a single electrode probe insertion. The IRE paradigms to
enable this include
delivering the pulses over multiple cycles of application and coupling pulse
delivery with
altering tissue electrical conductivity by either systematically introducing
hypotonic solution
infusion or internally perfusing the electrode with body temperature
perfusate, all of which are
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
57
designed to enable increasing voltage and pulse length to the greatest
possible without inducing
over-current or electrical arcing issues.
[00152] The method of this system may also include the use of a
treatment monitoring
system to customize pulse parameters in real-time during a treatment. The
intent is to monitor the
effects of the pulses being delivered to ensure the parameters are strong
enough to achieve
desired ablation sizes but also remain below key thresholds that will include
exceeding 50 A
(cause arcing) or raising temperature surrounding the electrodes above 43 C.
This device may
be used to treat a wide variety of tissue types and tissue parameters.
[00153] Examples of metrics to be measured may include, but are not
limited to, low
current which would indicate insufficient energy and that voltage may need to
be increased; high
current which would indicate that the user can lower voltage if current is
approaching 50 A;
current drifting higher which is a precursor to arcing and will indicate to
the user to lower
voltage to prevent unwanted arcs; an unstable current which is indicated by
waveforms peaking
near the end of the train; alerting the user to lengthen the pulse or reduce
voltage; or satisfactory
ablation which is when a particular electrode pair is identified as achieving
satisfactory ablation
and that the pair can be removed from the protocol thereby eliminating
redundant pulses and
saying time.
[00154] The method of a treatment monitoring system may include
intraprocedural
monitoring which will help assist the user in delivering ideal pulse
parameters to maintain
complete and effective pulse delivery when treating different tissue types as
well as throughout
the entire procedure as tissue parameters change. Examples of intraprocedural
monitoring
systems include the use of a Hall Effects probe to provide real-time electric
current data and may
be used to guide and inform procedures and parameters decisions. Another
embodiment would
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
58
be tracking a higher resolution understanding of procedure can be accomplished
by breaking the
pulse protocols down to delivery pulses in smaller quantities (such as 10 ¨ 40
pulses per train
rather than 70 ¨ 100 pulses/train) at one time. Decisions by user to adjust
the pulse parameters
may be done prior to proceeding with the next pulse sequence.
[00155] Referring now to Fig. 19, the steps for the method of using the
system will be
explained in detail. First, the user may prime the perfusion system 200 and
connect the perfusion
tubing, perfusion pump and any other perfusion components that may be
required. The user may
then activate the IRE energy delivery device 202 by powering on the various
components of the
system. After connecting the probe to the generator, the user may insert the
probe into the
treatment site 204, optionally using the imaging system for guidance. The GUI
may then prompt
the user to set specific pulse parameters 206 which may include, but are not
limited to, the tissue
type to be treated and the desired ablation zone. Based on the selected pulse
parameters, the
controller will automatically calculate the required parameters to achieve the
desired settings.
The GUI may next prompt the user to select if cardio sync 208 is to be used.
If yes 210, the
cardio sync device 214 will first generate a synchronization signal 216, then
receive a
synchronization signal 218, send information to the GUI 220, and then send the
signal to
delivery treatment energy 222. Alternatively, a test pulse may be sent to
determine if the pulse
parameters are satisfactory. If no cardio sync is to be used 212, or after the
cardio sync device
214 has sent the signal to delivery treatment energy 222, the IRE energy
pulses may be delivered
to the patient 224. In certain embodiments of this method, it may be possible
for intraprocedural
monitoring for arc potential 226. If such monitoring is done 230, the system
may monitor
parameters to determine if an arc is likely to occur 232, and if it is 234 the
GUI then may be
triggered to go back and require the user to reset the pulse parameters 206.
Conversely, if no 228
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
59
intraprocedural monitoring is being done or if arcing is not likely 236, then
the method may
optionally provide for a series of steps including, but not limited to,
treatment end point
confirmation 238, review of pulse delivery settings 240, and/or track ablation
242. Lastly, the
procedure will end 244 and the method has been completed.
[00156] This device and method of use is intended to be used in various
tissue types. The
insertion of the probe 2 can be percutaneous, laparoscopic, endoscopic, as
well as through
natural orifices, including insertions related to orifice translumenal
endoscopic surgery. One of
ordinary skill in the art will recognize that other tissue types can be
treated as well, including, but
not limited to, digestive, skeletal, muscular, nervous, endocrine,
circulatory, reproductive,
vascular, integumentary, lymphatic, adipose, urinary, and soft tissue. The
energy delivery probe
2 can be suitable for treatment of conditions for various tissues, volumes,
sizes and locations,
including small to medium sized tissue volumes, and tissue volumes that are in
close proximity
to other non-targeted structures, such as, but not limited to, neuronal
structures, vascular
structures, duct structures, and collagen-rich structures. Non-limiting
examples of tissue masses
to which the devices of the present application are applicable include benign
tissue masses such
as benign prostate hyperplasia (BPH) and uterine fibroids, as well as benign
or malignant masses
such as cancers and tumors of various tissue types, including, but not limited
to, breast, brain,
prostate, uterine, lung, liver, kidney, brain, head/neck, bone, stomach,
colon, and pancreas. The
method can also be used to target singly or in combination tissues that are
benign, malignant,
cancerous, neoplastic, preneoplastic, or tumorous.
[00157] One example of infected tissue that can be treated using IRE
and this system is
infected bone, or osteomyelitis. Bone infections can be extremely difficult to
treat. Typically,
bone infections can be treated using surgical procedures. The bone can be
accessed by variety of
CA 02981867 2017-10-04
WO 2016/164930
PCT/US2016/026998
procedures, such as through the skin. After the bone is surgically cleaned
out, the remaining bone
defect(s) is treated with a large dose of antibiotics via a non-resorbable
bone cement to eradicate
any bacterial cells in the bone and bloodstream. After this, subsequent
surgery is required for
removal and replacement with a bone graft or an absorbable mix of synthetic
bone substitute.
5 After a bone cleaning and replacements, the bone is typically not strong
enough to bear weight.
Bone rebuilding techniques can involve bone grafting or bone transport.
Antibiotic treatment is
then administered through an intravenous catheter. These treatment procedures
have the
attendant disadvantages mentioned above. Instead of using the above-described
extensive,
painful, and expensive procedures, this system may use IRE to treat bone
infection. In one
1.0 aspect, sufficient electrical pulse parameters can be selected, as
described herein above, to
irreversibly electroporate infected cells that are present within or along
bone. In one aspect, the
single bipolar probe described herein can be inserted into a target tissue
surrounding an infected
bone, and sufficient electrical pulse parameters could be selected to
adequately irreversibly
electroporate an infected bone mass. In one embodiment, an outer layer of bone
could be treated
15 to remove infected cells. When infected tissue of a bone is irreversibly
electroporated, such
target bone tissue could include muscle and/or vessels which could be acutely
necrosed.
However, in time, the critical cellular and/or vascular structures could grow
back so that no long
term harmful consequences would occur.